Abstract
Nitrogen fixation, the enzymatic conversion of atmospheric N (N 2) to ammonia (NH 3), is a microbially mediated process by which “new” N is supplied to N-deficient water bodies. Certain bloom-forming cyanobacterial species are capable of conducting N 2 fixation; hence, they are able to circumvent N limitation in these waters. However, this anaerobic process is highly sensitive to oxygen, and since cyanobacteria produce oxygen in photosynthesis, they are faced with a paradoxical situation, where one critically important (for supporting growth) biochemical process is inhibited by another.
N 2-fixing cyanobacterial taxa have developed an array of biochemical, morphological, and ecological adaptations to minimize the “oxygen problem”; however, none of these allows N 2 fixation to function at a high enough efficiency so that it can supply N needs at the ecosystem scale, where N losses via denitrification, burial, and advection often exceed the inputs of “new” N by N 2 fixation. As a result, most marine and freshwater ecosystems exhibit chronic N limitation of primary production. Under conditions of perpetual N limitation, external inputs of N from human sources (agricultural, urban, and industrial) play a central role in determining ecosystem fertility and, in the case of N overenrichment, excessive primary production or eutrophication. This points to the importance of controlling external N inputs (in addition to traditional phosphorus controls) as a means of ensuring acceptable water quality and safe water supplies.
Nitrogen fixation, the enzymatic conversion of atmospheric N 2 to ammonia (NH 3) is a microbially-mediated process by which “new” nitrogen is supplied to N-deficient water bodies. Certain bloom-forming cyanobacterial species are capable of conducting N 2 fixation; hence they are able to circumvent nitrogen limitation in these waters. However, this anaerobic process is highly sensitive to oxygen, and since cyanobacteria produce oxygen in photosynthesis, they are faced with a paradoxical situation, where one critically-important (for supporting growth) biochemical process is inhibited by another. Diazotrophic cyanobacterial taxa have developed an array of biochemical, morphological and ecological adaptations to minimize the “oxygen problem”; however, none of these allows N 2 fixation to function at a high enough efficiency so that it can supply N needs at the ecosystem scale, where N losses via denitrification, burial and advection often exceed the inputs of “new” N by N 2 fixation.
As a result, most marine and freshwater ecosystems exhibit chronic N-limitation of primary production. Under conditions of perpetual N limitation, external inputs of N from human sources (agricultural, urban, industrial) play a central role in determining ecosystem fertility and in the case of N-overenrichment, excessive primary production, or eutrophication. This points to the importance of controlling external N inputs (in addition to traditional phosphorus controls) as a means of ensuring acceptable water quality and safe water supplies.
Keywords: cyanobacteria, nitrogen fixation, freshwater, marine
Nitrogen fixation, the biochemical conversion of “inert” atmospheric N (N 2) to biologically available ammonia (NH 3), is a microbially mediated process of global significance because it provides “new” N to aquatic ecosystems in which biological production is often controlled by N availability 1, 2. N 2 fixation is an anaerobic process carried out by specific prokaryotes, including heterotrophic and chemolithotrophic bacteria and some cyanobacteria (blue-green algae) 3. The process likely evolved during the oxygen (O 2)-devoid Precambrian period some 2+ billion years ago 4, 5. Of the N 2-fixing microbial taxa, the cyanobacteria are of particular biogeochemical and ecological interest because they were also the first O 2-evolving photosynthetic organisms on Earth 6; their proliferation during this period is thought to be an evolutionary “milestone” because it led to the generation of an O 2-rich atmosphere, a prerequisite for the evolution of O 2-requiring fungi, bacteria, animals, and higher plant species on our planet 6.
Ironically, the development of an O 2-rich atmosphere, hydrosphere, and pedosphere constituted a formidable biochemical challenge for the cyanobacteria because, while they were capable of fixing N 2, the process had to be confined to an O 2-free micro-environment 7. This requirement posed a serious dilemma, especially for aquatic cyanobacteria, because they require illuminated conditions in surface waters, but the high ambient O 2 levels produced by photosynthesis in these waters also represents an environmental barrier to O 2-sensitive N 2 fixation. Over their long evolutionary history, cyanobacteria have developed biochemical and structural adaptations as well as biotic associations in order to optimize N 2 fixation while relying on oxygenic photosynthesis to provide energy and organic carbon (C) compounds to support metabolism and growth. The adaptions include (1) confining N 2 fixation to night-time when photosynthesis is “turned off”, (2) forming colonies and aggregates to reduce illumination and form low-O 2 “microzones”, (3) participating as endosymbionts in biological associations, and (4), forming heterocysts (non-photosynthetic, O 2-free cells) in some filamentous taxa, which allows N 2 fixation to proceed while receiving photo-reductant and organic C through photosynthesis from adjacent cells 8.
These are all remarkably clever adaptations to a modern-day oxic biosphere, which help circumvent the “O 2 problem” 6. From an ecosystem perspective, they have allowed N 2-fixing species to provide biologically available N from the vast reservoir of atmospheric N 2. However, on the ecosystem scale, recent N budget analyses indicate that N 2 fixation inputs fall far short of meeting ecosystem requirements when biologically available N inputs (from terrestrial and atmospheric sources) and losses (via denitrification, sedimentation and burial, and advection) are considered 9– 11. As a result, freshwater, estuarine, and marine systems are often chronically N deficient 11– 17. Pervasive N limitation has many implications for ecosystem function, especially when excessive external nutrient inputs lead to accelerating primary production (eutrophication), harmful algal blooms, and excessive O 2 consumption (hypoxia). If chronic N-limited conditions prevail in water bodies and N 2 fixation cannot meet ecosystem N requirements, then external N inputs often supply N to support eutrophication and its unwanted symptoms. From a management perspective, this means that the growing global glut of N inputs from agricultural, urban, and industrial sources 14, 18– 20 needs to be controlled, in addition to the broadly accepted phosphorus (P) input constraints, in order to protect our waterways and water supplies.
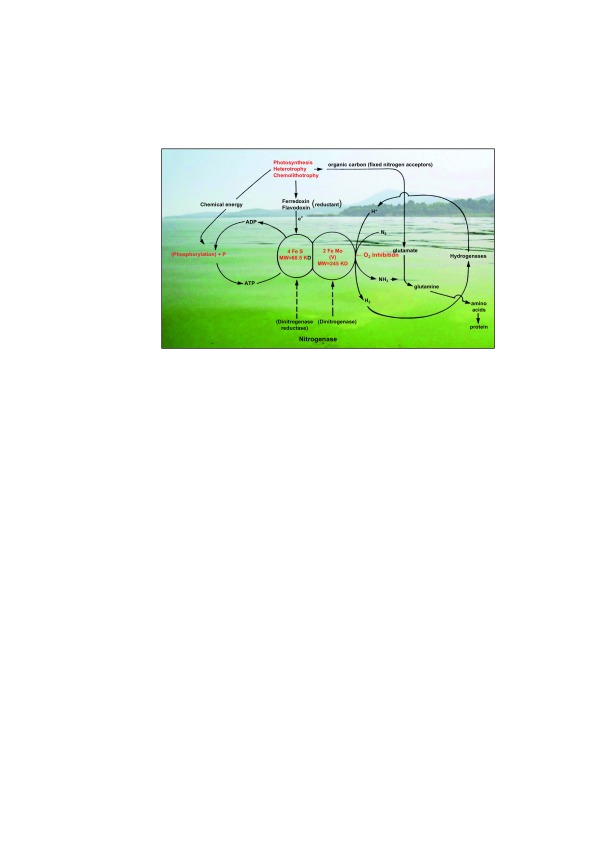
Why does N 2 fixation fall short of meeting ecosystem demands? Apparently, this process does not operate at sufficient rates in a modern-day, oxic world to compensate for losses via burial, export, and denitrification, even though it is protected and optimized by the various biological adaptations mentioned above. It is counteracted at larger scales by biogeochemical processes, such as denitrification, that run in the opposite direction (NO 3 → N 2). The N 2-fixing process is an energy-demanding one, requiring 16 ATP molecules to fix one molecule of N 2 3. In cyanobacteria, this energy demand has to be met by photosynthesis, while in non-photosynthetic bacteria, organic matter and redox reactions serve as energy sources 3. In highly productive (eutrophic), turbid waters where cyanobacteria and bacteria thrive, the availability of photosynthetically active radiation (PAR: 400–700 nm) is often restricted, causing a radiant energy deficit and suboptimal N 2 fixation rates. Secondly, cyanobacteria taxa that dominate in eutrophic waters often accumulate as thick surface “blooms”, in part to circumvent light limitation in subsurface waters 11. High rates of photosynthesis in such blooms lead to O 2 supersaturation, often in excess of 200% saturation 21. These ambient O 2 levels inhibit N 2 fixation in situ, even in heterocystous taxa 22, 23. Thirdly, N 2 fixation requires high levels of P (to support the energetics, e.g. ATP formation and nucleic acid production) and metals, most prominently iron (Fe), which is a co-factor in the enzyme complex nitrogenase 3. In highly oxygenated surface waters, Fe occurs as the insoluble and biologically unavailable Fe 3+ ion that may lead to Fe-limited conditions 24. Lastly, wind-induced turbulence and vertical mixing can reduce N 2 fixation potential by disrupting colonies and aggregates and enhancing inward diffusion of O 2 ( Figure 1) 25 and deepening the mixed layer, reducing light availability.
Figure 1. The nitrogen fixing process, as mediated by cyanobacteria (utilizing oxygenic photosynthesis as an energy and carbon source) as well as heterotrophic and chemolithotrophic microorganisms, in eutrophic surface waters.
Potential environmental controls, including phosphorus (P) and iron (Fe) availability, energy sources, and dissolved oxygen inhibition, are shown in red. The background photo is of an O 2-supersaturated (during daytime) cyanobacterial surface bloom in Lake Taihu, China. Photograph by H. Paerl.
Thus, while N 2 fixation converts inert N 2 into biologically available NH 3 to support aquatic fertility in a remarkable fashion, it faces multiple constraints and limitations in aquatic environments, especially in surface waters, which are often N limited. Geochemists, some limnologists, and a few oceanographers have assumed that as long as P and Fe are readily available, N 2 fixation should make up for an N deficit, given the unlimited supply of N 2 available 26, 27. However, this assumed linear stoichiometric relationship is not straightforward. Major environmental factors constrain this process, preventing it from functioning at optimal rates and supplying complete ecosystem N requirements 8, 11. As a result, much of the world’s marine and freshwater environments remain chronically N deficient. In practical (management) terms, this limitation means that external inputs of N play a key role in providing adequate and excessive fertility (eutrophication) of many freshwater and most marine ecosystems 11, 15, 16. Tremendous increases in anthropogenically generated bioavailable N in the form of synthetic (Haber process) fertilizers, agricultural, industrial, and urban wastes, and N 2 emissions (as both oxides and reduced forms of N) far overshadow biological fixation of N 2 in providing available N to receiving waters. Effective future management and protection of our fresh and marine waters will depend on the control of external inputs of both N and P 11, 27 instead of depending on the more traditional approach of controlling P inputs without N restrictions 28.
Acknowledgements
I appreciate the helpful comments by my colleagues W. Gardner, M. McCarthy, and J.T. Scott and appreciate the technical assistance from A.R. Joyner.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
James Cotner, University of Minnesota, St. Paul, MN, 55108, USA
Justin Chaffin, Stone Laboratory, Ohio State University, Put-in-Bay, OH, USA
Funding Statement
This work was partially supported by the National Science Foundation (DEB 9815495; CBET 0826819, 1230543; and Dimensions of Biodiversity 1240851).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Howarth RW, Marino R, Cole JJ: Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 2. Biogeochemical controls. Limnol Oceanogr. 1988;33(4 part 2):688–701. 10.4319/lo.1988.33.4part2.0688 [DOI] [Google Scholar]
- 2. Karl D, Michaels A, Bergman B, et al. : Dinitrogen fixation in the world’s oceans. Biogeochemistry. 2002;57(1):47–98. 10.1023/A:1015798105851 [DOI] [Google Scholar]
- 3. Postgate J: Nitrogen Fixation.Cambridge University Press,1998. Reference Source [Google Scholar]
- 4. Raymond J, Siefert JL, Staples CR, et al. : The natural history of nitrogen fixation. Mol Biol Evol. 2004;21(3):541–54. 10.1093/molbev/msh047 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Latysheva N, Junker VL, Palmer WJ, et al. : The evolution of nitrogen fixation in cyanobacteria. Bioinformatics. 2012;28(5):603–6. 10.1093/bioinformatics/bts008 [DOI] [PubMed] [Google Scholar]
- 6. Knoll AH: Life on a young planet: the first three billion years of evolution on earth.Princeton University Press, Princeton,2003. Reference Source [Google Scholar]
- 7. Gallon JR: Reconciling the incompatible: N 2 fixation And O 2. New Phytol. 1992;122(4):571–609. 10.1111/j.1469-8137.1992.tb00087.x [DOI] [Google Scholar]
- 8. Paerl HW: Physiological Ecology and Regulation of N 2 Fixation in Natural Waters.In: Marshall KC editor. Adv Microb Ecol.Boston, MA: Springer US;1990;11:305–344. 10.1007/978-1-4684-7612-5_8 [DOI] [Google Scholar]
- 9. Scott JT, McCarthy MJ: Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol Oceanogr. 2010;55(3):1265–70. 10.4319/lo.2010.55.3.1265 [DOI] [Google Scholar]
- 10. Grantz EM, Haggard BE, Scott JT: Stoichiometric imbalance in rates of nitrogen and phosphorus retention, storage, and recycling can perpetuate nitrogen deficiency in highly-productive reservoirs. Limnol Oceanogr. 2014;59(6):2203–16. 10.4319/lo.2014.59.6.2203 [DOI] [Google Scholar]; F1000 Recommendation
- 11. Paerl HW, Scott JT, McCarthy MJ, et al. : It Takes Two to Tango: When and Where Dual Nutrient (N & P) Reductions Are Needed to Protect Lakes and Downstream Ecosystems. Environ Sci Technol. 2016. 10.1021/acs.est.6b02575 [DOI] [PubMed] [Google Scholar]
- 12. Dugdale RC: Nutrient limitation in the sea: dynamics, identification, and significance. Limnol Oceanogr. 1967;12(4):685–95. 10.4319/lo.1967.12.4.0685 [DOI] [Google Scholar]
- 13. Elser JJ, Bracken ME, Cleland EE, et al. : Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett. 2007;10(12):1135–42. 10.1111/j.1461-0248.2007.01113.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Paerl HW, Piehler MF: Nitrogen and Marine Eutrophication.In DG Capone, M. Mulholland and E. Carpenter (Eds.), Nitrogen in the Marine Environment.Academic Press, Orlando.2008;2:529–567. 10.1016/B978-0-12-372522-6.00011-6 [DOI] [Google Scholar]
- 15. Conley DJ, Paerl HW, Howarth RW, et al. : Ecology. Controlling eutrophication: nitrogen and phosphorus. Science. 2009;323(5917):1014–5. 10.1126/science.1167755 [DOI] [PubMed] [Google Scholar]
- 16. Lewis WM, Wurtsbaugh WA, Paerl HW: Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environ Sci Technol. 2011;45(24):10300–5. 10.1021/es202401p [DOI] [PubMed] [Google Scholar]
- 17. Dodds W, Smith V: Nitrogen, phosphorus, and eutrophication in streams. Inland Waters. 2016;6:155–64. 10.5268/IW-6.2.909 [DOI] [Google Scholar]; F1000 Recommendation
- 18. Galloway JN, Cowling EB, Seitzinger SP, et al. : Reactive Nitrogen: Too Much of a Good Thing? Ambio. 2002;31(2):60–3. 10.1579/0044-7447-31.2.60 [DOI] [PubMed] [Google Scholar]
- 19. US EPA: Reactive Nitrogen in the United States: An Analysis of Inputs, Flows, Consequences, and Management Options. A Report of the EPA Science Advisory Board.EPA-SAB-11-013. Unites States of America Environmental Protection Agency: Washington, DC,2011. Reference Source [Google Scholar]
- 20. Glibert PM, Maranger R, Sobota DJ: The Haber Bosch-harmful algal bloom (HB-HAB) link. Environ Res Lett. 2014;9(10):105001 10.1088/1748-9326/9/10/105001 [DOI] [Google Scholar]
- 21. Paerl HW, Webb KL, Weibe WJ: Nitrogen fixation in waters.In WJ Broughton (ed.), The Ecology of Nitrogen Fixation.Oxford Univ Press, Oxford,1981;193–241. [Google Scholar]
- 22. Paerl HW, Kellar PE: Nitrogen-fixing anabaena: physiological adaptations instrumental in maintaining surface blooms. Science. 1979;204(4393):620–2. 10.1126/science.204.4393.620 [DOI] [PubMed] [Google Scholar]
- 23. Kellar PE, Paerl HW: Physiological adaptations in response to environmental stress during an N 2-fixing Anabaena bloom. Appl Environ Microbiol. 1980;40(3):587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hyenstrand P, Rydin E, Gunnerhed M: Response of pelagic cyanobacteria to iron additions--enclosure experiments from Lake Erken. J Plankton Res. 2000;22(6):1113–26. 10.1093/plankt/22.6.1113 [DOI] [Google Scholar]
- 25. Moisander PH, Hench JL, Kononen K, et al. : Small-scale shear effects on heterocystous cyanobacteria. Limnol Oceanogr. 2002;47(1):108–19. 10.4319/lo.2002.47.1.0108 [DOI] [Google Scholar]
- 26. Tyrrell T: The relative influences of nitrogen and phosphorus on oceanic primary production. Nature. 1999;400:525–31. 10.1038/22941 [DOI] [Google Scholar]
- 27. Paerl HW, Otten TG: Duelling 'CyanoHABs': unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N 2 -fixing harmful cyanobacteria. Environ Microbiol. 2016;18(2):316–24. 10.1111/1462-2920.13035 [DOI] [PubMed] [Google Scholar]
- 28. Schindler DW, Hecky RE, Findlay DL, et al. : Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci U S A. 2008;105(32):11254–8. 10.1073/pnas.0805108105 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation