Summary
The rising incidence of obesity and associated metabolic diseases has increased the urgency in understanding all aspects of adipose tissue biology. This includes the function of adipocytes, how adipose tissue expands in obesity, and how expanded adipose tissues in adults can impact physiology. Here, we highlight the growing appreciation for the importance of de novo adipocyte differentiation to adipose tissue expansion in adult humans and animals. We detail recent efforts to identify adipose precursor populations that contribute to the physiological postnatal recruitment of white, brown, and beige adipocytes in mice, and summarize new data that reveal the complexity of adipose tissue development in vivo.
Function of Adipocytes in Adult Physiology
White Adipocytes
Eukaryotes stores excess energy in the form of intracellular triglycerides. Triglyceride storage in vertebrates is largely confined to specialized, dedicated cells, called white adipocytes (fat cells). The white adipocyte is characterized by the presence of a large uni-locular lipid droplet that functions as a safe storage compartment for triglycerides. During times of caloric excess, white adipocytes sequester free fatty acids (FFAs) from the circulation and store them as triglycerides. When there is a demand for energy, such as during a prolonged fast, triglycerides are hydrolyzed into glycerol and FFAs that are released into the circulation and subsequently utilized as fuel.
White adipose tissue (WAT) can be found throughout the body but is invariably organized into anatomically distinct “depots” (Fig. 1). In general, white adipose appears in subcutaneous regions, or within the intra-abdominal area (Shen et al., 2003). Several subcutaneous white adipose depots serve a mechanical function in providing support and cushioning to surrounding organs. In the heel pad, adipocytes are embedded in a dense network of collagen fibers for mechanical support. The orbital fat is a semifluid adipose layer that lines the bony orbit to cushion the eyeball. The subcutaneous fat directly below the skin serves as a layer of thermal insulation. Moreover, bona fide adipose tissue in various intra-abdominal regions juxtaposes several organs such as the kidney, heart, and intestine.
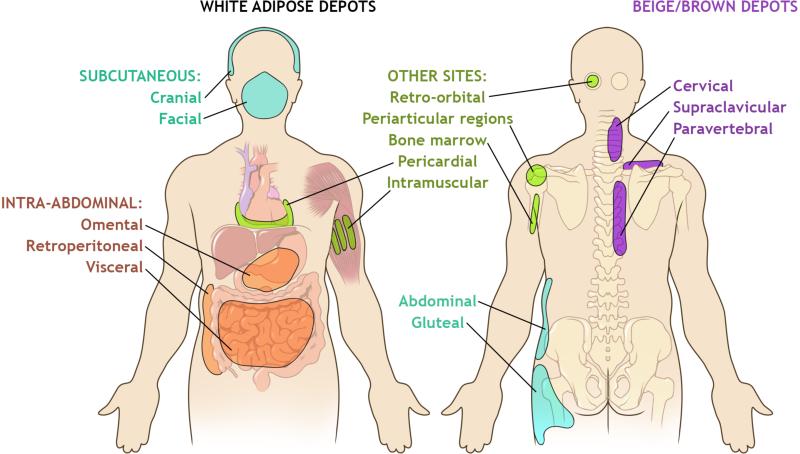
Figure 1. Distribution of White and Brown/Beige Adipose Tissue in Adults.
White adipose tissue (WAT) is organized into distinct depots, classified by location as subcutaneous or intra-abdominal. The major subcutaneous WAT includes the abdominal, gluteal, and femoral depots. Other white subcutaneous depots include the cranial and facial adipose tissue. Intra-abdominal adipose tissue is located within the peritoneum and includes the omental, retroperitoneal, and visceral (mesenteric) fat. White adipocytes also accumulate in other locations, including behind the eye (retro-orbital), around joints (periarticular), in bone marrow, surrounding the heart (pericardial), and within the skeletal muscle (intramuscular). Brown adipose tissue (BAT) in adults exists as a heterogeneous tissue, containing brown and beige adipocytes interspersed with white adipocytes. BAT depots are located in the cervical, supraclavicular, and paravertebral regions in adults.
Fetal development of white adipose tissue begins with a dense mass of blood vessels surrounded by mesenchymal stem cells (Poissonnet et al., 1984). Human adipose tissue appears during the 14th-17th week of gestation as a cluster of fat lobules first in the head and neck, then the trunk, and later in the limbs (Poissonnet et al., 1984). The precise developmental origin of WAT during fetal development is still unclear; however, lineage tracing in mice indicates that subcutaneous and intra-abdominal depots emanate from distinct lineages (Chau et al., 2014). The specific origin may vary from depot to depot. For instance, in mice, craniofacial, but not peripheral, subcutaneous WAT depots originate from neuroectoderm rather than mesodermal structures (Billon and Dani, 2012). Fat mass in humans expands during the first year of birth through an expansion of cell size and number (Knittle et al., 1979; Spalding et al., 2008). Later in adulthood, adipocytes turnover at a rate of 10% per year and adipocyte number remains relatively stable regardless of BMI or weight loss (Spalding et al., 2008).
Over the past two decades, our understanding of adipose tissue has expanded dramatically. The adipose tissue was long considered as merely a storage reservoir; however, now, it is recognized as an active endocrine organ capable of regulating systemic nutrient balance and energy homeostasis (Neumann et al., 2016; Ouchi et al., 2011; Rosen and Spiegelman, 2014). Adipose tissue secretes hormones and cytokines, termed “adipokines,” that regulate systemic glucose homeostasis, lipid metabolism, inflammation, and many other physiological events (Deng and Scherer, 2010). The essential roles of adipose tissue in energy balance become increasingly apparent in individuals that lack functional adipose tissue (lipodystrophy) and in individuals that have excess fat cells (obesity). Both of these extremes lead to dyslipidemia, insulin resistance, and inflammation and are associated with an altered adipokine profile (Barroso et al., 1999; Haque et al., 2002).
Brown Adipocytes
Eutherian mammals also contain “brown” adipocytes whose principle function beyond nutrient homeostasis is to convert chemical energy into heat. Brown adipocytes are characterized by their multilocular lipid droplet appearance and high mitochondrial content (Cannon and Nedergaard, 2004). The thermogenic function of brown adipocytes is mediated by the specific expression of uncoupling protein 1 (UCP1) (Klingenberg, 1999). UCP1 is a transport protein that sits within the inner membrane of mitochondria and facilitates a proton leak across the inner membrane (Fedorenko et al., 2012). This dissipates the electrochemical gradient that has been generated via the electron transport chain, thereby uncoupling oxidative metabolism from ATP synthesis. Heat production occurs as the biochemical reactions involved in mitochondrial fuel oxidation are subsequently accelerated. The activity of brown adipocytes is regulated by the sympathetic nervous system via β3-adrenergic signaling (Lowell and Flier, 1997). This pathway triggers the activation of UCP1 as well as a transcriptional program that leads to further UCP1 expression. Lineage tracing reveals that brown adipocytes, but not most white adipocytes, derive from a Myf5+/Pax7+ lineage, and thus share a common developmental origin with skeletal muscle (Seale et al., 2008).
Brown adipose tissue (BAT) plays an important role in lipid metabolism but likely evolved as a mechanism for mammals to defend against hypothermia (Cannon and Nedergaard, 2004). As such, rodents housed in standard conditions have copious amounts of BAT at the time of birth and maintain these depots throughout life. The most prominent BAT depot in mice is found in the interscapular region. This depot is also present in human infants but largely involutes with age (Aherne and Hull, 1966; Lidell et al., 2013). Initially, it was widely believed that adult BAT was limited to individuals with pheochromocytoma, an adrenal tumor characterized by excess catecholamine secretion, and in outdoor workers in Northern Europe with chronic exposure to cold temperatures (Huttunen et al., 1981; Ricquier et al., 1982). A series of studies published in 2008 showed that this was not the case. 8F-fluoro-2-deoxy-d-glucose positron emission tomography computed tomography (18F-FDG-PET) imaging verified the existence of BAT as an active, energy-consuming organ in most non-obese adults (Cypess et al., 2009; Virtanen et al., 2009). In adults, thermogenic BAT is located in the supraclavicular, cervical, paravertebral, and perirenal regions and the amount of BAT is higher in lean verses obese individuals (Figure 1) (van Marken Lichtenbelt et al., 2009). Importantly, human supraclavicular BAT can be activated in response to chronic cold exposure and contributes to nutrient homeostasis (Chondronikola et al., 2014; Chondronikola et al., 2016).
Beige Adipocytes
It has long been recognized that WAT depots of cold exposed rodents can undergo extensive remodeling and adopt a thermogenic phenotype, elicited by the emergence of “brown-like” energy-burning adipocytes (Loncar, 1991). However, lineage-tracing reveals that most UCP1+ cells within WAT depots are not derived from a Myf5+ lineage, suggesting a developmental origin distinct from the brown adipocytes formed during the fetal period (Sanchez-Gurmaches and Guertin, 2014; Seale et al., 2008). These cells activate UCP1 upon cold exposure and exhibit a multilocular lipid droplet phenotype (Long et al., 2014); however, they appear to be molecularly and likely functionally distinct from brown adipocytes present in brown adipose depots. Wu et al. characterized UCP1+ adipocytes differentiated from WAT-derived clonal precursor cell lines and revealed that these cells exhibit properties of both brown and white adipocytes; thus the term “beige adipocytes” was born (Wu et al., 2012). Beige adipocytes may not be fully dependent on UCP1 for thermogenesis as UCP1-independent mechanisms in these cells are becoming unveiled (Kazak et al., 2015).
Humans appear to have both classical brown fat cells and beige adipocytes. Human infant interscapular UCP1+ brown fat expresses ZIC1, a molecular marker of rodent interscapular classical brown adipocytes (Lidell et al., 2013). Kajimura and colleagues performed gene expression profiling on adult supraclavicular BAT and derived clonal cell lines to reveal that this depot more closely resembles murine beige adipose tissue (Sharp et al., 2012; Shinoda et al., 2015). However, Cypess et al. isolated multiple human neck fat samples from superficial to deep and showed that deep neck fat more closely resembles classical brown fat by appearance and molecular signatures (Cypess et al., 2013). The discrepancy between these two studies in adults is likely due to the heterogeneity of BAT, as brown adipocytes are dispersed throughout the depot and more densely packed deeper in the neck. In fact, Cypess et al. showed the superficial BAT indeed has a closer resemblance to murine beige adipose tissue. The ability to induce “browning” of WAT in rodents is protective against obesity and can reverse insulin resistance in metabolic syndrome (Seale et al., 2011; Shao et al., 2016). Some of these effects may be mediated by the thermogenic capacity of these cells; others may be mediated by an endocrine function. Therefore, tremendous effort is now placed on developing strategies to recruit beige adipocytes from precursors or stimulate activity of existing brown and beige fat in humans.
Physiological Recruitment of Adipocytes in Adults: Links to Metabolic Disease
Subcutaneous vs Visceral Expansion
White adipose tissue has a remarkable capacity to expand as the demand for energy storage increases. WAT expansion is a physiologically appropriate response to caloric excess; however, obesity is associated with increased risk for diabetes, insulin resistance, and cardiovascular disease. Interestingly, not all obese individuals develop metabolic disease (Appleton et al., 2013; Denis and Obin, 2013). Factors outside of BMI and overall WAT mass are thus the drivers of metabolic syndrome. Tremendous emphasis is now placed on identifying better clinical correlates between obesity and its associated diseases.
Such efforts have revealed that body distribution is one of best predictors of metabolic health in obesity (Karpe and Pinnick, 2015; Lee et al., 2013). Obese individuals who preferentially store excess adiposity in the intra-abdominal compartment are at a greater risk than those who accumulate adipose tissue in the subcutaneous regions. There is a tremendous sexual dimorphism in the anatomical distribution of fat tissue (Karastergiou et al., 2012; Palmer and Clegg, 2015). In women, adipose accumulation occurs preferentially in subcutaneous regions; in men visceral WAT expansion is more readily apparent. The role of sex hormones such as estrogen in this patterning is now well established (Davis et al., 2013; Palmer and Clegg, 2015).
One possible reason for the detrimental effects of visceral adipose accumulation is the location of the depot itself. Lipids and metabolites can drain directly into the portal circulation and affect liver function (Rytka et al., 2011). However, the observation that anatomically distinct adipocytes are developmentally distinct raises the possibility that they are also functionally and intrinsically distinct. Whole adipose depot transplantation experiments in mice indicate that factors intrinsic to these depots determine their effect on glucose homeostasis (Tran et al., 2008). A number of studies collectively demonstrate that anatomically distinct adipocytes, in isolation, are functionally unique, differing in their ability to undergo lipolysis, lipogenesis, and activate thermogenic programs (Lee et al., 2013; Macotela et al., 2012; Morgan-Bathke et al., 2015; Wu et al., 2012). Thus, an emerging hypothesis is that anatomically distinct WAT depots likely represent distinct “mini-organs.” A better understanding of how these distinct depots arise and expand during development may lead to therapeutic strategies to alter body fat distribution.
Remodeling of WAT depots: Adipocyte Hypertrophy vs. Adipocyte Hyperplasia
In response to caloric excess, individual WAT depots expand through the enlargement of existing adipocytes (adipocyte hypertrophy) or through the formation of new adipocytes (adipocyte hyperplasia) (Hirsch and Han, 1969). Several analyses of WAT from obese individuals reveal that adipocyte size and number correlate well with the risk for metabolic syndrome, independent of BMI (Gustafson et al., 2015; Hardy et al., 2011; Kloting et al., 2010). WAT from patients with features of metabolic syndrome exhibits a striking pathological phenotype. These adipose depots are characterized by hypertrophic adipocytes, hypoxia, fibrosis, and inflammation (Gustafson et al., 2015; Kloting and Bluher, 2014; Lee et al., 2010; Sun et al., 2011; Sun et al., 2013). This phenotype also correlates well with lower serum adiponectin levels and ectopic lipid deposition into non-adipocytes (Kloting et al., 2010). The precise timing in which all of these events occurs is still somewhat unclear; however, the prevailing model (Fig. 2) proposes that the inability of depots to expand through hyperplasia, combined with inadequate angiogenesis, leads to overloaded and hypoxic adipocytes. As adipocytes reach a maximal storage capacity, cell death can occur, leading to the influx of immune cells and activation of inflammation. Triglyceride spillover and subsequent accumulation of lipid species (diacylglycerols and ceramides) into non-adipose organs, including the liver and skeletal muscle, can trigger local and systemic insulin resistance (Chaurasia and Summers, 2015; Perry et al., 2014). On the contrary, WAT depots from metabolically healthy individuals are characterized by the presence of numerous and relatively smaller adipocytes, as well as a greater blood vessel density (Corvera and Gealekman, 2014; Kloting et al., 2010). Analysis of adipocyte size and number in adults before and after weight gain revealed that females expand lower-body subcutaneous depots through adipocyte hyperplasia while males expand these same depots through hypertrophy (Tchoukalova et al., 2010). Furthermore, human adipose tissue explants isolated from subcutaneous WAT of insulin resistant obese subjects have impaired angiogenic potential (Gealekman et al., 2011).
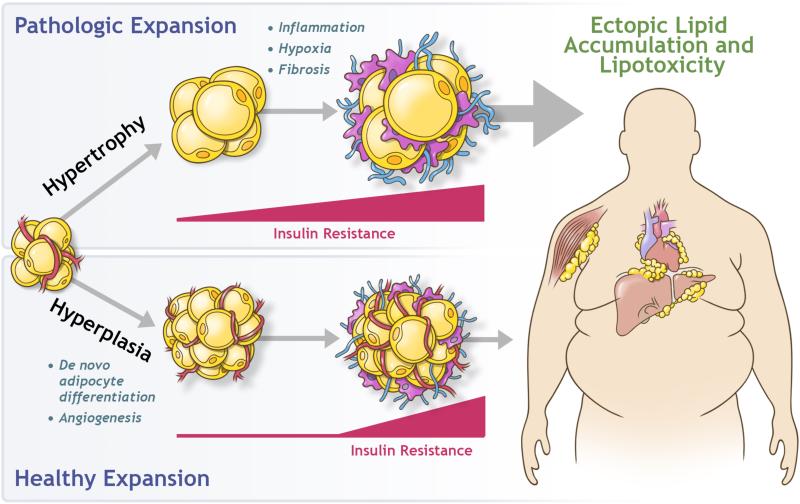
Figure 2. White Adipose Tissue Expansion in Obesity.
Expansion of white adipose tissue in response to caloric excess occurs through the enlargement of existing adipocytes (hypertrophy) and/or through an increase in adipocyte number (hyperplasia). Pathologic expansion through hypertrophy of adipocytes is associated with inflammation, hypoxia, and fibrosis, with early onset of insulin resistance. Adipocyte dysfunction leads to the deleterious spillover of lipids into non-adipose organs, termed lipotoxicity. Healthy expansion through hyperplasia of adipose tissue occurs through the recruitment of preadipocytes and de novo adipocyte differentiation. This occurs alongside with angiogenesis and prevents or delays the onset of both insulin resistance and ectopic lipid accumulation.
The recent development of new genetic lineage tracing systems have now led to a better understanding of how the individual adipose depots expand in mice during obesity (Wang et al., 2014a). Scherer and colleagues developed an elegant “pulse-chase” lineage-tracing model, termed the “AdipoChaser” mouse (Wang et al., 2013). This model allows for adipocyte hyperplasia to be assessed qualitatively and quantitatively (Fig. 3A). Using this model, Wang et al. revealed that epididymal adipose expansion in adult male mice fed a high fat diet (HFD) for 8 weeks occurs through both adipocyte hypertrophy and hyperplasia (Fig. 3B). Surprisingly, the inguinal (subcutaneous) depot in these same animals expands almost exclusively through cellular hypertrophy (Wang et al., 2013). Jeffery et al. and Kim et al. reached similar conclusions utilizing a tamoxifen-based inducible genetic lineage tracing system (Adiponectin-CreERT2) and stable isotopic labeling, respectively (Jeffery et al., 2015; Kim et al., 2014). More recently Jeffery et al. performed lineage tracing studies in female mice and revealed that adipocyte hyperplasia occurs in both the perigonadal WAT and, to some extent, in the in inguinal WAT (Jeffery et al., 2016). All together, these data reveal for the first time the presence of depot- and sex-selective mechanisms for adipose tissue expansion, and importantly, provide novel approaches to track adipogenesis in vivo.
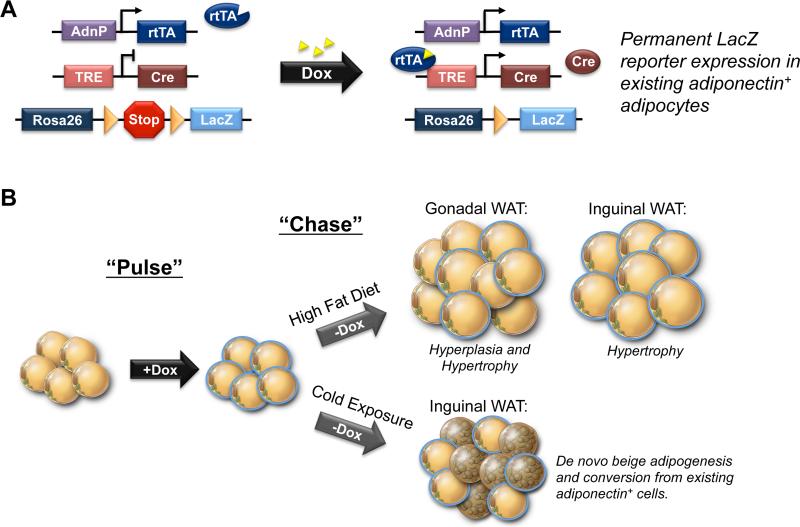
Figure 3. Tracking Adipogenesis with the AdipoChaser Mouse Model.
A) AdipoChaser mice utilize a combination of three published transgenic lines: 1) transgenic mice expressing the “tet-on” transcription factor rtTA under the control of the Adiponectin gene promoter, (“Adn-rtTA”), 2) a tet-responsive CRE (TRE-Cre) line that can be activated with rtTA in the presence of doxycycline, and 3) Rosa26 Reporter mice expressing β-galactosidase (LacZ) from the Rosa26 locus in a Cre dependent manner (Rosa26-loxP-STOP-loxP-LacZ). In the absence of doxycycline (Dox), there is no reporter expression in mature adipocytes. Upon treatment with doxycycline, rtTA activates the TRE promoter to induce Cre expression, and Cre protein will subsequently eliminate the floxed transcriptional stop cassette and permanently turn on reporter gene expression in every mature adipocyte present during doxycycline exposure. Any new adipocytes that develop after removal of Dox are derived from precursors, through de novo adipogenesis. B) “Pulse-Chase” lineage tracing using the AdipoChaser model has now been used to reveal depot-specific mechanisms of adipose expansion in obesity and the origin of inguinal beige adipocytes recruited upon cold exposure. Gonadal white adipose tissue (WAT) expands through enlargement of existing adipocytes (blue adipocytes) and by de novo adipogenesis (unlabeled adipocytes). Inguinal WAT mass expands only by adipocyte hypertrophy. Upon cold exposure, multilocular beige adipocytes emerge through de novo beige adipogenesis (unlabeled beige adipocytes) as well as via a conversion of existing adiponectin+ mature adipocytes (blue beige adipocytes).
Physiological Recruitment of Thermogenic Adipocytes
The abundance of brown and beige adipocytes is also heavily regulated in adult animals. In mice, prolonged cold exposure leads to an expansion in mass of classical BAT depots, characterized by brown adipocyte hyperplasia and hypertrophy. Beige adipocytes accumulate most frequently and rapidly in the subcutaneous inguinal WAT depot; however, long-term cold exposure can lead to a “browning” of other WAT depots to a certain extent. Activation of the sympathetic activation via β3-adrenegic receptors is the most potent driver of beige cell recruitment. However, exercise, also associated with sympathetic activation, can induce beige adipocyte accumulation (Bostrom et al., 2012; Rao et al., 2014; Stanford et al., 2015).
Beige adipocytes have been linked to a number of physiologic states characterized by a systemically increased metabolic rate. Interestingly, in the setting of cancer-associated cachexia, active beige adipocytes have been observed and suggested to expand in response to parathyroid hormone-related protein/parathyroid hormone signaling (Holmes, 2014; Kir et al., 2016; Kir et al., 2014; Petruzzelli et al., 2014). Additionally, increased beige adipocyte accumulation in the gonadal WAT of mice has been observed after gastric bypass surgery, potentially contributing to metabolic enhancements beyond a decrease in food intake (Neinast et al., 2015). Thus, beige adipose accumulation, at least in rodents, may play a broader role in physiology than expected.
Studies in humans show mild cold exposure activates non-shivering thermogenesis through activation of BAT but not browning of WAT (van der Lans et al., 2013). This suggests a more robust stimulus is needed to induce beige adipogenesis. Recent studies on burn trauma victims provided evidence of browning of WAT in characteristically white depots in humans (Patsouris et al., 2015; Sidossis et al., 2015). Subcutaneous depots isolated from these patients exhibit multilocular adipocytes, an increase in UCP1 mRNA and protein, and enhanced mitochondrial respiration. Burn trauma is a unique model associated with adrenergic stress and hypermetabolic status. Destruction of the skin barrier compromises heat retention and thus may require increased thermogenesis to maintain body temperature. These studies show beiging of human subcutaneous WAT is possible and provides evidence that browning of WAT may be a therapeutic possibility.
A number of pharmacological and physiological stimuli can trigger beige adipocyte accumulation; however, in most cases the actual cellular origin of the UCP1+ adipocytes has remained unclear. In principle, beige adipocytes can accumulate via de novo differentiation from resident precursors, or via a conversion of mature white adipocytes into multilocular UCP1+ cells. In multiple studies, we have employed the AdipoChaser model in a pulse-chase lineage tracing experiment to investigate the origin of beige adipocytes induced by cold exposure/adrenergic signaling (Shao et al., 2016; Vishvanath et al., 2015; Wang et al., 2013). Upon stimulation with a β3-adrenergic receptor agonist or cold exposure, UCP1+ cells appear rapidly and arise predominantly, but not exclusively, by de novo beige adipogenesis. Lee et al. also performed an analogous experiment utilizing Adiponectin-CreERT2 mice for adipocyte lineage tracing. However, the authors observed the opposite pattern (Lee et al., 2015b). In their experiments, all UCP1+ cells arose from a conversion of white adipocytes existing prior to cold exposure. One possible explanation lies in the technical approach. Tamoxifen can linger inside the depots for up to two months following injection; this can confound lineage-tracing results as Cre may remain active (Ye et al., 2015). However, another possibility lies in the history of the animals used for experiments. Rosenwald et al. tracked the UCP1 lineage using UCP1-CreERT2 animals (Rosenwald et al., 2013). With their model the authors were able to map the fate of UCP1+ adipocytes as animals transition from cold temperatures back to thermoneutral conditions. They found that beige adipocytes have the capacity to revert to a white adipocyte phenotype; the cells lose UCP1 expression and become unilocular. These same adipocytes can then revert back to UCP1+ cells upon re-exposure to cold. It is tempting to refer to this inter-conversion of phenotypes as “transdifferentiation;” however, this phenotype switching may simply reflect beige adipocytes in active and inactive thermogenic states. As such, Kajimura et al. have proposed a model in which at least two general mechanisms lead to the natural formation of beige adipocytes during cold exposure: 1) de novo differentiation from precursors, and 2) activation of “dormant” unilocular beige adipocytes upon cold exposure (Kajimura et al., 2015).
Adipocyte Precursors in Adult Animals
The growing appreciation for the role of de novo adipocyte differentiation in adults has sparked a renewed interest in understanding the process of adipogenesis. Much of our knowledge of the cellular aspects of adipocyte differentiation stem from studies of immortalized cell lines. In particular, the 3T3-L1 fibroblast cell line has been the standard model system to identify regulators of adipogenesis and fat cell function. In the 1970's, Green and colleagues originally derived 3T3-L1 cells as a clonal subline of Swiss 3T3 mouse embryonic fibroblasts. 3T3-L1 cells are viewed as being a “determined” cell line; the cells are morphologically indistinguishable from less- or nonadipogenic fibroblasts, but are highly competent to undergo adipogenesis in response to a hormonal/pharmacological cocktail consisting of dexamethasone, iso-butyl-methylxanthine, and insulin (generally referred to as “DMI medium”). The 3T3-L1 cell line has been a powerful model for many critical aspects of adipocyte biology. Importantly, the function of nearly every critical transcription factor controlling adipogenesis, including the master regulatory protein Pparγ, has been identified through analysis in 3T3 cells (Chawla and Lazar, 1994; Tontonoz et al., 1994). However, it is widely recognized that there are potential limitations to the use of immortalized cells lines. The precise relationship between these cultured preadipocytes and adipose progenitors found in adult and fetal animals in vivo remains uncertain. Importantly, 3T3-L1 adipocytes are white adipocyte-like; however, to what extent they resemble any particular depot-specific adipocyte found in vivo remains unclear.
Studies from the 1970's first revealed that adherent fibroblast-like cells within cultures of the adipose stromal vascular fraction (SVF) could be propagated and differentiated into mature adipocytes in vitro using largely the same conditions utilized for immortalized cells (Poznanski et al., 1973; Van et al., 1976; Van Robin and Roncari, 1977). Cells within the adherent SVF, however, can also give rise to other osteoblasts, myoblasts, and chondrocytes (Cawthorn et al., 2012). As described in many reviews, this has led to an expanded nomenclature to describe adherent SVF as “adipose stem cells” or “mesenchymal stem cells” (Cawthorn et al., 2012). Importantly, these in vitro SVF-derived adipocytes molecularly resemble the adipocytes found in their depot of origin. Adherent stromal cells from human and mouse WAT give rise to adipocytes in culture that molecularly and functionally resemble adipocytes located in their tissue of origin; this suggests that at least some of the depot-specific properties of anatomically distinct white fat tissues are programmed at the level of the preadipocyte (Macotela et al., 2012; Tchkonia et al., 2006).
There are numerous limitations and potential caveats associated with the sole reliance on cultured SVF cells as a model of adipose precursors. First, it is not clear if only certain subpopulations of adipose precursor cells may be selected, and how these cells may change during the initial growth of these cultures. Importantly, the precise cell type that these precursors represent in vivo, and their native location within the adipose tissue cannot be ascertained entirely with this model. As such, in the paragraphs below we summarize recent efforts to identify and characterize native adipose precursors, with a particular focus on populations contributing to adipocyte hyperplasia in adult animals.
CD24+ and CD24− Pdgfrα+ Adipose Precursor cells
Some of the earliest attempts to purify or enrich for adipose precursors from the SVF were made in studies of human adipose tissue. Sengenes et al. demonstrated that the CD34+; CD31− fraction of the human adipose SVF enriched for the cells capable of undergoing adipocyte differentiation in vitro. To date, this selection strategy remains one of the most widely used approaches to study human adipose precursors. Several years ago, Friedman and colleagues developed a robust strategy to extract distinct adipose precursor populations from mouse adipose depots (Rodeheffer et al., 2008). This prospective approach combines antibodies raised against combinations of known stem cell cell-surface proteins and fluorescent-activated cell sorting (FACS) to selectively purify APCs from freshly isolated adipose SVF. Two distinct APC populations were identified. Both populations were devoid of endothelial and hematopoietic markers (CD31 and CD45, respectively), positive for common mesenchymal stem cell marks, Sca1, CD34, and CD29, but distinguishable by the expression of CD24. Both the CD31−;CD45−;CD29+;Sca1+;CD34+;CD24+ (herein CD24+ APCs) and the CD31−;CD45-;CD29+;Sca1+;CD34+;CD24− (herein CD24− APCs) APCs exhibit a robust capacity for differentiation in vitro; however, their ability to undergo adipogenesis in vivo differ. CD24+, but not CD24−, APCs are capable of reconstituting a functional WAT depot when transplanted into residual gondal WAT of lipodsytrophic animals. However, when transplanted outside the natural WAT microenvironment, CD24−, but not CD24+, APCs exhibit adipogenic potential (Berry and Rodeheffer, 2013). Berry and Rodeheffer have proposed a model in which the CD24+ APCs represent a more primitive progenitor population with the capacity to expand and differentiate when placed in its natural microenvironment, while CD24− APC represent a more committed “preadipocyte” population. In accordance with this hypothesis, CD24+ APCs give rise to CD24− APCs in vivo during development and activate lineage-selective genes such as Pparγ2 and C/ebpα (Berry and Rodeheffer, 2013).
Both the CD24+ and CD24− APC population uniformly express platelet-derived growth factor receptor alpha (Pdgfrα) (Berry and Rodeheffer, 2013). Pdgfrα is a cell surface tyrosine kinase receptor for the family of platelet-derived growth factors (Pdgf). Pdgfrα is expressed broadly in the paraxial mesoderm during fetal development and is commonly found in mesenchymal cells in various adult tissues (Takakura et al., 1997). Importantly, lineage tracing approaches reveal that adipocytes descend from cells that express Pdgfrα at some stage during their genesis; this includes adipocytes formed during development as well as new fat cells formed in adult animals (Berry and Rodeheffer, 2013). Clonal analyses indicate that ~70-80% of Pdgfrα+; CD31−; CD45− cells are capable of adipogenesis in vitro (Joe et al., 2010; Lee et al., 2012; Uezumi et al., 2010). Isolating APCs on the basis of Pdgfrα expression has become a convenient method to purify adipogenic cells.
The identification and isolation of CD24+ and CD24− APCs is a major methodological advance; however, subsequent characterization of these cells has also provided an important conceptual advance. Functional analysis of these two populations reveals the existence of hierarchical populations of adipose precursors, exhibiting different levels of commitment. Importantly, as described in more detail below, CD24+ APCs appear to at least contribute to white adipocyte hyperplasia associated with diet-induced obesity in mice (Jeffery et al., 2015) (Fig. 4). It remains unclear, however, to what extent adipogenesis is initiated from the CD24+ vs. the CD24− population in the setting of obesity, and whether the contribution of these CD24+ cells is essential for healthy adult WAT expansion in obesity. Functional manipulation of these cells in vivo, facilitated by specific genetic tools that target this population, is still needed.
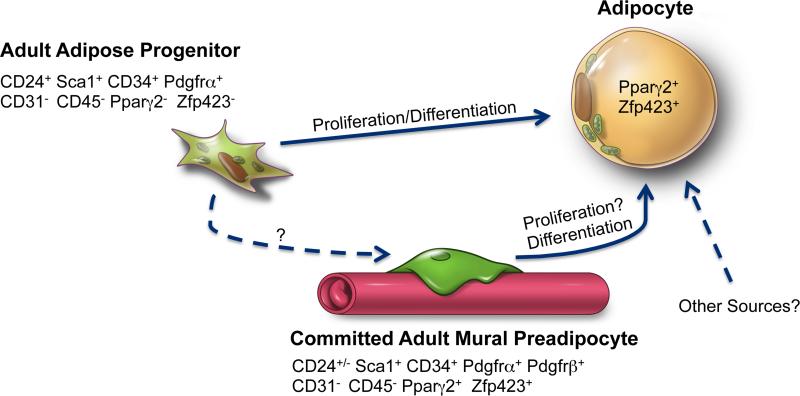
Figure 4. Adipose Precursors Contributing to WAT Hyperplasia in Obesity.
Recent pulse-chase lineage tracing studies reveal multiple adipocyte precursor populations involved in white adipose tissue (WAT) expansion in adult mice undergoing high-fat diet feeding (HFD). BrdU labeling experiments indicate that a stem cell-like population of adipose progenitor cells (CD24+; Sca1+; CD34+; Pdgfrα+; CD31−; CD45−) undergo rapid proliferation in response to HFD and differentiate into white adipocytes. The precise location(s) of these cells within the depot remain unclear. The abundance of highly committed population of perivascular (mural) preadipocytes (Pdgfrβ+; Pdgfrα+; CD34+; Sca1+; Zfp423+; Pparγ2+; CD24+/−; CD31−; CD45−) increases upon HFD feeding. Genetic lineage tracing reveals mural Pdgfrβ+ cells contribute to adipocyte hyperplasia in this setting. Whether mural preadipocytes proliferate prior to differentiation is unclear as is whether the more primitive adult adipose progenitor passes through the perivascular stage during differentiation into the mature adipocyte. Other precursor populations that contribute to adipocyte hyperplasia in obesity may exist.
Perivascular adipose precursors
The key advantage to the cell sorting strategy described above is the ability to utilize the approach in any rodent model or strain. A significant limitation, however, lies in the inability to easily localize these cells in their native environment. Another approach to identifying precursor populations is to localize the expression of key functional regulators. In particular, defining precursor populations of various developmental stages on the basis of transcription factor gene expression has been particularly useful in the study of myogenesis, hematopoiesis, and pancreatic endocrine cell development.
Several years ago, Graff and colleagues took this approach to localize and isolate adipose precursors residing in the adipose depots of adult mice. Tang et al. utilized various reporter models in which GFP or LacZ expression is directed to Pparγ-expressing cells. Of note, subsets of WAT peri-endothelial cells expressing the mural cell marker, Pdgfrβ, also express Pparγ (Tang et al., 2008). This observation was significant since adipose progenitors have long been thought to reside in or near the vasculature of adipose tissue and have been often referred to as specialized pericytes (Cinti et al., 1984; Napolitano, 1963; Wasserman, 1960). Graff and colleagues demonstrated that these cells were proliferating Sca1+; CD34+ precursors that robustly differentiated into adipocytes. Contemporaneously, other groups have clearly demonstrated that perivascular cells isolated from mouse or human adipose tissue have mesenchymal stem cell-like properties and the ability to differentiate into adipocytes (Amos et al., 2008; Crisan et al., 2008; Traktuev et al., 2008; Zannettino et al., 2008; Zimmerlin et al., 2010). Furthermore, adipose precursors targeted by some Fabp4 (aP2) promoter-driven Cre transgenic lines reside in the mural cell compartment of adipose tissue (Shan et al., 2013).
More recent work from our group studying the function and expression of the C2H2 transcription factor, Zfp423, has both confirmed and extended the hypothesis of a perivascular origin of adipogenesis. A role for Zfp423 in the adipose lineage was first suggested following a genome-wide expression screen of clonally derived adipogenic vs. non-adipogenic cell lines. Zfp423 mRNA and protein levels are enriched in 3T3 clonal fibroblast cell lines with a high propensity for adipocyte differentiation in vitro. Subsequent gain and loss of function studies on Zfp423 in cells indicate that this transcription factor functions upstream of Pparγ and is required for adipogenesis (Gupta et al., 2010). We developed a BAC transgenic mouse model in which GFP expression is driven by a large fragment of the Zfp423 locus (Zfp423GFP reporter mice). Using this reporter mouse, we have localized the expression of GFP to a distinct subset of endothelial and peri-endothelial cells in neonatal BAT and adult WAT depots (Gupta et al., 2012). Non-endothelial Zfp423+ stromal cells expressed Pdgfrβ, Pdgfrα, CD34, and Sca1, but were mostly devoid of CD24 expression. Isolation and comparison of Zfp423+; Pdgfrβ+ and Zfp423−; Pdgfrβ+ mural cells revealed that the expression of Pparγ and other adipose lineage selective genes were enriched in Zfp423+ mural cells (Vishvanath et al., 2015). This suggests that this population at least overlaps with the mural cell population examined by Graff and colleagues. Both Zfp423+ and Zfp423− mural cell populations exhibit colony-forming unit potential; however, Zfp423+ mural cells appear more committed to undergo adipocyte differentiation in vitro. Zfp423+ mural cells isolated from gonadal WAT do not require DMI medium; these cells can differentiate with high efficiency in media containing relatively low serum (2% FBS) and insulin.
The identification of perivascular adipose precursors is consistent with historical observations that primitive adipocytes are associated with the vasculature. Genetic lineage analyses using a Pdgfrβ-Cre mouse strain have supported this hypothesis, indicating that adipocytes descend from cells once expressing Pdgfrβ (Tang et al., 2008). However, use of this Cre line, or any constitutively active Cre line, can be limiting in a number of ways. When bred to a Cre-dependent Rosa26R reporter strain, reporter expression is subsequently activated in Pdgfrβ-expressing cells and all descending cells, regardless of whether they continue to express Pdgfrβ. Therefore, it becomes difficult to directly assess whether adipogenesis originates from cells actively expressing Pdgfrβ during development. Moreover, the lack of temporal control over Cre activity prevents the use of these strains to determine the contribution of Pdgfrβ-expressing cells to adipocyte hyperplasia under specific postnatal physiological conditions.
To circumvent these issues we recently derived a novel doxycycline-inducible mural cell lineage tracking system that we termed the “MuralChaser” mouse (Vishvanath et al., 2015). Using this approach, we identified several mural cell-derived adipocytes in epididymal WAT formed during the high-fat diet feeding-period (Vishvanath et al., 2015). In agreement with the AdipoChaser studies described above, adipocyte hyperplasia was not observed in the inguinal WAT depot of male mice. These data provide direct genetic evidence that perivascular preadipocytes contribute to adipocyte hyperplasia in obesity (Fig. 4). This is in line with a contemporaneous study from Olson and colleagues in which the authors followed the fate of tdTomato-labeled nestin+ cells in the adipose tissue (Nestin-Cre; Rosa26RtdTomato) (Iwayama et al., 2015). The authors demonstrated that Nestin-Cre activity is limited to pericytes and adventitial cells within adipose depots of lean mice; however, upon high-fat diet feeding tdTomato+ gonadal adipocytes appear, suggesting they originated from perivascular nestin+ cells. It is currently unclear whether the adipogenic capacity of perivascular preadipocytes cells is critical for healthy WAT expansion in the setting of obesity. Ultimately, functional manipulation of adipogenic signaling pathways in Pdgfrβ+ cells will help clarify the importance of this population.
Pref-1+ cells
The perivascular localization of committed Zfp423+/Pparγ+ preadipocytes, along with the lineage tracing results described above, strongly suggest a mural cell origin of adipogenesis in adult animals. However, other recent studies reveal the presence of non-perivascular adipose precursor populations during fetal development. In particular, Sul and colleagues utilized a genetic reporter mouse guided by the Pref-1 promoter to localize preadipocytes. Pref-1 (or DLK-1) is an EGF-repeat-containing protein that inhibits adipocyte differentiation (Hudak and Sul, 2013). Unlike Zfp423 and Pparγ, Pref-1 expression diminishes as preadipocytes undergo terminal differentiation. As such, Sul and colleagues reasoned that Pref-1/GFP expression identifies the most primitive of adipose precursors. In their recent study, Hudak et al. reported that fetal and adult Pref-1+ stromal cells exhibit adipogenic potential but do not express Zfp423 and Pparγ at the moment of isolation (Hudak et al., 2014). Instead, these cells are enriched for CD24 mRNA and express Pdgfrα. These data suggest that the Pref-1+ cells isolated represent a more primitive precursor population, likely overlapping with the CD24+ APCs described above. Interestingly, Pref-1+ precursors do not express mural cell markers such as Pdgfrβ or α-smooth muscle actin (αSMA).
Hudak et al also revealed Pref1+ precursors first appear in the dorsal mesenteric region around E10.5 of mouse development and give rise to inguinal and gonadal WAT development (Hudak et al., 2014). Mice lacking Pref1+ cells during fetal development are born relatively lipodystrophic, indicating that these precursors are in fact functionally required for normal WAT development. This study did not report Pref1+ lineage tracing results under obesogenic conditions in adult mice; however, loss of Pref1+ cells in adult animals prevented the normal expansion of WAT depots following high-fat diet feeding. This suggests that Pref1+ cells undergo adipogenesis and contribute to adipocyte hyperplasia associated with high-fat diet feeding. However, inguinal WAT expansion was also attenuated in mice lacing Pref1+ cells; this depot usually expands predominantly by adipocyte hypertrophy rather than hyperplasia. This suggests that other functions of these cells critical for the health of adipose tissue may exist. Functional manipulation of adipogenic signaling pathways in Pref1+ cells will further clarify whether the adipogenic capacity of these cells, or rather these cells per se, is critical for WAT remodeling.
Brown and Beige Adipocyte Precursors in Adults
Recently, Seale and colleagues have developed a prospective approach for isolating brown and beige adipose precursors (Wang et al., 2014b). Seale et al. previously discovered that classical brown adipocytes arise from cells expressing Myf5 at some point during fetal development (Seale et al., 2008). Building on this observation, along with the fact that brown adipocytes emanate from Pdgfrα+ cells, Wang et al. isolated and characterized Pdgfrα+; Myf5-lineage cells as fetal brown adipose precursors that express CD34, CD24, but not Sca1. Importantly, expression of Ebf2, a brown and beige adipocyte determination factor, was enriched in adipogenic Myf5-lineage cells vs. myogenic Myf5-lineage cells (Rajakumari et al., 2013; Wang et al., 2014b). Wang et al. utilized Ebf2-GFP reporter mice to isolate Ebf2+ and Ebf2− Pdgfrα+ cells from brown adipose tissue. Indeed, Ebf2/GFP expression can distinguish committed brown Pdgfrα+ adipose precursors from Pdgfrα+ precursors that differentiate in white adipocytes.
The origin of beige precursors in adult WAT is now of tremendous interest. Wu et al. revealed that clonal cell lines derived from cultured inguinal WAT SVF differentiate into either white or thermogenic beige adipocytes, suggesting the presence of committed beige precursors within the adult adipose stromal compartment (Wu et al., 2012). However, selective markers for the prospective isolation of native beige cell precursors from adipose tissues have been elusive. Ebf2 also drives the formation of beige adipocytes; therefore, Wang et al. also examined whether Ebf2 expression can identify beige adipose precursors in the inguinal WAT depot of mice. They clearly demonstrate that cold exposure increases the frequency of Ebf2+;Pdgfrα+ cells in the inguinal WAT, but not gonadal WAT, depot. Ebf2+;Pdgfrα+ cells isolated from cold-exposed mice differentiate in Ucp1+ adipocytes, further suggesting that these cells represent native beige adipose precursors (Wang et al., 2014b).
The isolation of Ebf2+;Pdgfrα+ cells now affords the opportunity to elucidate signals and mechanisms leading to beige adipogenesis in adult mice. Importantly, a number of critical questions remain regarding the exact identity of these precursors and their relationship to white adipocyte precursors. In particular, it remains unclear if Ebf2+ and Ebf2− Pdgfrα+ cells represent distinct mesenchymal cell types/lineages that diverged much earlier in development. Alternatively, beige and white adipocytes may originate from bipotent/multipotent Pdgfrα+ progenitors to give rise to Ebf2+ and Ebf2− preadipocytes. Clonal analyses of Pdgfrα+ cells in vitro indicate a capacity for Pdgfrα+ cells to differentiate into both UCP1+ and UCP1− cells, suggesting the presence of bipotent cells (Lee et al., 2012). However, Daquing et al. revealed that selective ablation of Pdgfrβ+ mural cells led to a loss of white adipocytes and a robust increase in the abundance of beige adipocytes in WAT (Daquinag et al., 2015). The authors propose that white and beige adipocyte precursors are distinct.
Recent lineage tracing studies have begun to shed insight into the origin of beige adipocytes. Spiegelman and colleagues revealed that beige adipocytes express a smooth muscle-like gene program, much like how classical brown adipocytes exhibit a skeletal muscle-like gene signature. This prompted Long et al. to perform lineage-tracing studies using Myh11-CreERT2 mice (Long et al., 2014). Indeed, at least a subpopulation of beige adipocytes formed in cold-exposed mice originates from Myh11+ cells. Interestingly, labeled interscapular brown adipocytes cannot be found in these same cold-exposed animals, supporting the notion that brown and beige adipose precursors are likely distinct. However, whether white adipocytes formed in association with high-fat diet feeding can originate from Myh11+ cells has not been reported. Functional studies are also in support of a lineage relationship between beige adipocytes and smooth muscle cells. Farmer and colleagues revealed that myocardin-related transcription factor A (MRTFA) deficient stromal vascular cells exhibit less of a smooth muscle-like phenotype and more readily undergo beige adipogenesis (McDonald et al., 2015).
Additional lineage tracing studies from Graff and colleagues as well as from our group suggest that the pool of beige adipocyte precursors may be heterogeneous, and multiple distinct populations of beige preadipocytes may even exist. We utilized the MuralChaser model and a “pulse-chase” labeling approach to follow the fate of adipose Pdgfrβ+ cells during cold exposure (Vishvanath et al., 2015). Interestingly, after 7 days of cold exposure, we were unable to find beige adipocytes which differentiated from labeled Pdgfrβ+ precursors. De novo beige cell differentiation occurs during this period (Wang et al., 2013); however, these data suggest that beige adipogenesis is initiated from cells not actively expressing Pdgfrβ at the time of labeling. However, after two weeks of cold exposure, labeled beige adipocytes are readily apparent, although present at relatively low frequency. These data suggest that multiple waves of beige adipogenesis may occur, each drawing upon somewhat distinct precursor populations. Graff and colleagues also reported a temporal contribution of Myh11+ precursor cells to beige adipocyte recruitment; beige cells originating from the Myh11-expressing precursors only appeared after two weeks of cold exposure (Berry et al., 2016). Interestingly, pulse-chase lineage tracing using a α-smooth muscle actin (αSMA)-driven reporter system demonstrated that most beige adipocytes formed following more acute cold exposure (within 7 days) originated from αSMA+ precursors (Berry et al., 2016). All of these data are consistent with the hypothesis that beige adipocyte precursors are smooth muscle-like; however, the beige precursor pool appears to be heterogeneous. Future studies carefully localizing the aforementioned Ebf2+ precursor population before and after cold exposure may help shed more insight into the cellular identity of beige precursors.
Potential non-mesenchymal origins of adipocytes
Lineage tracing studies suggest that non-mesenchymal populations may also serve as adipocyte precursors in some settings. Tran et al. revealed that endothelial-like cells within capillary sprouts of explanted WAT are able to differentiate into adipocytes (Tran et al., 2012). The authors provided lineage-tracing evidence that adipocytes descend from cells expressing the endothelial protein, VeCadherin, at some stage of development (Cdh5-Cre lineage tracing). However, this result was not confirmed when using a different Rosa26 reporter strain (Berry and Rodeheffer, 2013). Additional lineage tracing studies will be needed to resolve the discrepancy in results. Importantly, identification and purification of the putative endothelial adipose precursor will be needed to advance this hypothesis and determine if/when adipocytes descend from bona fide endothelial cells under physiological conditions in vivo.
A number of studies have now suggested that circulating bone marrow-derived cells may differentiate into adipocytes under certain conditions. In particular, Klemm and colleagues demonstrated the production of bona fide adipocytes from bone marrow-derived progenitor cells in the major fat depots of mice (Majka et al., 2010). In these experiments, Bone marrow progenitor (BMP)-derived adipocytes preferentially accumulated in visceral fat depots of mice. High-fat feeding or thiazolidinedione treatment enhanced the accumulation of BMP-derived adipocytes. Most recently, Gavin et al and Ryden et al. demonstrated the presence of BMP-derived adipocytes in human subjects undergoing bone marrow transplantation (Gavin et al., 2016; Ryden et al., 2015). In should be pointed out that some lineage tracing studies have not supported the notion of a myeloid origin of adipocytes, at least during normal development. However, it is certainly plausible that under specific conditions, such as bone marrow irradiation and transplantation, these BMPs give rise to a subset of adipocytes and contribute to adipose expansion or remodeling.
Chau et al. made the intriguing discovery that murine visceral adipose depots descend from cells expressing Wilms’ tumor (Wt1) (Chau et al., 2014). The authors observed that gonadal, mesenteric, and four other visceral fat WAT depots (perirenal, epicardial, retroperitoneal and omental) appearing postnatally descend from cells once expressing Wt1 during development. However, Wt1-expressing cells do not contribute to the development of inguinal WAT and BAT. In the developing gonadal WAT depot, Wt1 expression is observed in the associated mesothelial layer. The mesothelium is a monolayer of epithelial cells of mesodermal origin that line the visceral serosa. Wt1-expressing mesothelial cells express accepted markers of adipose precursors (CD29, CD34, Sca1). Furthermore, explanted cultures of developing gonadal WAT exposed to adipogenic culture conditions give rise to adipocytes from Wt1+ cells. This is in line with earlier studies demonstrating that mesothelial cells in culture can differentiate into adipocytes (Lachaud et al., 2013; Lansley et al., 2011). Fate-mapping approaches demonstrate that epicardial fat descends from embryonic epicardium progenitors expressing Wt1 and mesothelin. Furthermore, cells of the epicardium (mesothelium of the heart) in adult animals give rise to epicardial adipocytes following myocardial infarction (Liu et al., 2014). Chau et al also provided evidence to suggest that postnatal adipogenesis may originate, in part, from mesothelial precursors. However, the presence of Wt1 in fibroblast-like adipose precursors makes it difficult to draw definitive conclusions (Cohen et al., 2014; Gupta et al., 2012). Future studies using more specific and/or additional mesothelial-selective Cre lines should aid in clarifying the contribution of putative mesothelial precursors to adipocyte hyperplasia in adults.
Regulation of Adipocyte Precursors and Adipogenesis in Adult Mice
Adipose precursors are regulated in a sex and depot-dependent manner
The ability to study native adipose precursors and track adipogenesis in vivo now affords the opportunity to gain insight into the mechanisms governing adipose tissue expansion and remodeling. Studies, so far, have revealed that the regulation of adipogenesis in mice, and probably all mammals, is far more complicated than appreciated from studies of 3T3-L1 cells or even primary cell adipogenesis. As described above, adipocyte hyperplasia occurs in obese mice in a depot-dependent manner, with gonadal, but not inguinal, WAT precursors undergoing differentiation in males. This result was surprising as it has been widely appreciated that inguinal WAT-derived stromal vascular cells or purified precursors readily undergo adipogenesis in vitro. In females, differentiation from WAT precursors occurs in both gonadal and inguinal WAT, an effect that may be dependent in part on estrogen signaling. This suggests that the ultimate ability of adipose precursors to differentiate in vivo is dependent on the local microenvironment. Jeffery et al. showed that transplantation of male subcutaneous adipose progenitors into the subcutaneous WAT of female mice led to high-fat diet induced proliferation of the progenitor cells. However, no proliferation was observed when the same cells were transplanted into subcutaneous WAT of male mice. These data provide direct evidence that proliferation of adipose progenitors during obesity is indeed regulated by the local microenvironment of the depot (Jeffery et al., 2016).
A number of the precursor populations described above are regulated in a depot-dependent manner in mice (Jeffery et al., 2015; Joe et al., 2009; Macotela et al., 2012; Vishvanath et al., 2015). In particular, considerable insight has been gained through quantitative analysis of CD24+ progenitors in obese male mice. Jeffery et al. revealed that gonadal, but not inguinal, adipose CD24+ precursors undergo proliferation upon a switch from chow to high-fat diet feed. Remarkably, this burst in proliferation occurs rapidly, within the first few days of the switch to high-fat diet feed. Actual adipocyte hyperplasia occurs after 4-5 weeks of high-fat diet feeding; however, these data indicate that the initial precursor activation is triggered by diet, rather than obesity per se. These data are intriguing, as it has long been postulated that adipocyte hyperplasia is triggered by hypertrophic, or “exhausted”, adipocytes. Along these lines, Finucane et al. made the interesting observation that the fatty acid composition of the high-fat diet impacted adipose expansion in mice (Finucane et al., 2015). Adipose tissue in animals fed a high-fat diet feed enriched in monounsaturated fatty acids appeared remarkably hypercellular as to those tissues from animals fed a saturated fatty-acid rich diet, despite equal degrees of adipose tissue expansion. This hyperplastic expansion correlates with improved insulin sensitivity. It remains unclear as to whether/which dietary lipids act directly on adipose precursors to stimulate precursor proliferation, or whether hormonal/incretin signals play a role. Also unclear is whether the same signals that initiate precursor proliferation also stimulate entry into the adipocyte differentiation program.
Using the Zfp423GFP reporter mouse, we have found that the frequency of Zfp423+ perivascular preadipocytes is higher in the gonadal WAT depot than inguinal WAT depot of lean young adult mice (Vishvanath et al., 2015). This correlates with the potential of these depots to expand by adipocyte hyperplasia under obesogenic conditions. Moreover, the frequency of these perivascular preadipocytes increases after animals are switched to a high-fat diet; this increase occurs prior to onset of actual adipocyte differentiation and occurs more robustly in the gonadal WAT depot. It is currently unclear whether Zfp423+; Pdgfrβ+ cells are replicating during this period or whether Zfp423 expression is being induced in additional mural cells. Nevertheless, this preadipocyte population appears to be regulated in a depot-dependent manner. The relatively lower frequency of Zfp423+ mural cells in inguinal WAT suggests that this population may be limiting for the hyperplastic potential of this depot; however, whether increasing the abundance of this preadipocyte population or driving Zfp423/Pparγ expression in mural cells can lead to inguinal adipocyte hyperplasia needs to be investigated.
Also of interest is the observation that the frequency of Zfp423+; Pdgfrβ+ cells is sex-dependent. In female mice, the abundance of Zfp423+ mural cells is similar between iWAT and gWAT. Upon high-fat diet feeding, the abundance of these cells increases in both depots, albeit the absolute number of cell per depot remains lower than in males. Given the importance of estrogen receptor signaling in adipose tissue remodeling, it appears likely that hormonal signaling plays a role in regulating precursor activity (Davis et al., 2013; Karastergiou et al., 2012; Palmer and Clegg, 2015). This issue is potentially of clinical significance given the sexual dimorphism in adipose distribution in humans. Further insight into these mechanisms through use of the lineage-tracing systems will undoubtedly shed light into the regulation of body fat distribution.
Fetal vs. Adult Adipose Precursors and Their Regulatory Mechanisms
Tremendous insight into mechanisms regulating adipocyte differentiation and function has come from studies of cellular models of differentiation. In particular, the array of transcription factors and their target genes that are activated by the addition of DMI to cultured 3T3-L1 preadipocytes has been elucidated over the course of the past twenty years (Farmer, 2006). At the core of this transcriptional network is the aforementioned Pparγ, as well as the C/ebp family of transcription factors. Furthermore, several signaling pathways leading to the activation or repression of these transcription factors and adipogenesis have been unveiled, most notably BMP signaling, Tgfβ signaling, and WNT signaling (Cristancho and Lazar, 2011).
Excellent reviews have detailed and cataloged the long list of factors involved in regulating in vitro adipogenesis (Farmer, 2006). However, it is now apparent that white, brown, and beige adipogenesis originate from distinct preadipocytes and that the commitment to a white or thermogenic adipocyte fate is controlled by the presence of lineage-selective transcription factors. Furthermore, anatomically distinct white adipocytes (i.e. visceral vs. subcutaneous) seem to originate from distinct precursors of which are programmed to adopt a visceral or subcutaneous adipocyte phenotype. Adding to this complexity are recent observations that individual adipose depots may be heterogenous, with adipocytes arising from multiple origins (Sanchez-Gurmaches and Guertin, 2014; Sanchez-Gurmaches et al., 2016). Sanchez-Gurmaches et al. performed elegant quantitiatve lineage tracing studies that demonstrate that Myf5-Cre and Pax3-Cre lines target subsets of white adipocytes in various WAT depots of mice. The implication of these results is that within individual WAT depots, white adipocytes may be heterogeneous in origin, arising from multiple lineages. Moreover, a recent study by Graff and colleagues indicates that the initial development of adipose depots during the fetal and early postnatal period relies on precursors distinct from those that contribute to tissue maintenance and remodeling in adulthood (Jiang et al., 2014). Specifically, Jiang et al. revealed that αSMA+ adipose precursors are specified early in development but differentiate into adipocytes in adult animals. Precursors contributing to the initial formation of adipocytes during the fetal period appear to be αSMA− and do not lie in a classic mural position. Furthermore, fetal adipose precursors appear to be molecularly distinct from adult precursors. Hong et al. revealed identified proliferating perilipin+; adiponectin+ precursors within the fetal developing inguinal WAT depot (Hong et al., 2015). In adults, perilipin and adiponectin expression is almost exclusively confined to the mature adipocyte.
The concept of adipocyte and preadipocyte heterogeneity raises the question as to how these distinct precursors are triggered to differentiate and whether developmentally distinct white adipocytes are functionally distinct. Insight into regulation of adipocyte differentiation in vivo is now arising through genetic manipulation of putative regulatory factors in engineered mouse models. Emerging from these studies is a growing recognition that the mechanisms controlling adipocyte differentiation are more complex than they appear in the 3T3 models. In particular, recent studies indicate that the regulatory mechanisms required for adipose tissue formation during fetal/early postnatal development are distinct from those required in adult animals for the recruitment of new adipocytes. Jeffery et al. revealed that adipose depots from Pdgfrα-Cre; Akt2loxP/loxP animals appear normal at birth, indicating that Akt2 itself was dispensible for adipose tissue development. However, the adipose depots of adult Pdgfrα-Cre; Akt2loxP/loxP animals fed a HFD failed expand to by adipocyte hyperplasia. This suggests that Akt2 is required for the process of adipocyte differentiation only in postnatal animals (Jeffery et al., 2015).
Direct evidence supporting this concept comes from a contemporaneous study by Wang et al in which the temporal requirements of C/ebpα in the adipose lineage were explored using a doxycycline-inducible gene ablation model (AdiponectinrtTA; TRE-Cre; C/ebpαloxP/loxP) (Wang et al., 2015). C/ebpα, an upstream regulator of Pparγ, is critical for the terminal differentiation and maintenance of the adipocyte phenotype in vitro. In fact, C/ebpα and Pparγ together co-occupy a significant portion of the cis-regulatory elements that govern the adipocyte transcriptome. However, somewhat surprisingly, adipose depots from young adult AdiponectinrtTA; TRE-Cre; C/ebpαloxP/loxP animals exposed to doxycycline during fetal development appear histologically normal. This indicates that C/ebpα is dispensable for the maturation and maintenance of adipose tissue formed during fetal and early postnatal development. However, when C/ebpα inactivation is triggered in adult mice, white, but not beige, adipogenesis is impaired. In particular, C/ebpα is required for intramuscular adipogenesis triggered by chemical-induced muscle injury, WAT regeneration following triggered apoptosis (caspase 8 activation), and for the expansion of WAT associated with high-fat diet feeding or leptin deficiency. However, beige cell recruitment stimulated by cold exposure does not appear to be impacted by C/ebpα ablation in the adipose lineage. These results, along with the various aforementioned studies, illustrate the complexity of adipose development in vivo, and highlight the need to study adipose tissue development in various physiological contexts.
Activation of Beige and Brown Precursors in Adult Mice
It is now clear that de novo beige adipogenesis plays an important role in the recruitment of new beige adipocytes upon cold exposure and activation of β-adrenergic signaling. Likewise, de novo brown adipocyte differentiation contributes to BAT recruitment associated with cold adaptation. β1-adrenergic receptor activation, rather than β3-receptor signaling, triggers brown adipogenesis in this setting. Pdgfrα+ brown preadipocytes express the β1 adrenergic receptor; proliferation and differentiation of these cells could be due, in part, to the direct action of catecholamine signaling on these cells (Lee et al., 2015b). However, β3-adrenergic receptor expression appears largely confined to the mature adipocytes; how beige precursors are activated to differentiate has thus been largely unclear. A number of studies in recent years now suggest that catecholamine-triggered beige adipogenesis is coordinated and facilitated by the innate immune system. Work from Chawla and Artis unveil an adipose type-2 innate immune response driven by the sympathetic nervous system in response to cold exposure. In this proposed model, ILC2s, eosinophils, and macrophages, produce cytokines such as IL-13, IL-4, and Met-enkephalin, all of which act directly on adipose precursors to drive proliferation and/or differentiation (Brestoff et al., 2015; Lee et al., 2015a; Qiu et al., 2014). This mechanism also appears operative in exercise-induced beiging. Rao et al. reported that the exercise-induced myokine, Meteorin-like, stimulates beige adipocyte accumulation in inguinal WAT through an eosinophil/macrophage dependent mechanism (Rao et al., 2014). Thus, these data support the notion that adipose inflammation is not always a pathological feature of unhealthy WAT remodeling, but also a facilitator of “healthy” remodeling events.
Sorting Out the Adipose Lineage: Future Directions and Challenges
It is now abundantly clear that the development and regulation of the adipose lineage is far more complex than previously recognized. In retrospect, this is perhaps not surprising. Adipose tissue is present in various locations throughout the body. WAT has a remarkable capacity to expand and this expansion occurs in many different ways. As described above, in the past eight years several adipose precursor populations have been described. At first glance, these different studies may appear conflicting, particularly as it relates to the location of these precursors and the cell types that they represent. In our view, most of these data are not mutually exclusive. First and foremost, there is likely significant overlap in the precursor populations described here. Furthermore, most in the field have grown accustomed to thinking of the adipose life cycle in terms of “preadipocytes” and “mature adipocytes”. Stable populations with intermediate phenotypes may exist. The exact precursor population drawn upon to undergo adipogenesis may depend on the setting (sex, location, age, physiological trigger, etc.). Moreover, regulatory mechanisms controlling adipocyte differentiation appear to vary from depot to depot and in different physiological settings. The contribution of epigenetic regulation to adipose precursor biology in vivo is now being highlighted by several recent studies (Borengasser et al., 2013; Yang et al., 2016; Yang et al., 2013). Going forward, additional studies utilizing specific genetic tools will be required to test the importance of these different precursor populations and identify specific genetic and epigenetic mechanisms controlling their cellular fate. In the process, a clearer understanding of the link between adult adipogenesis and metabolic disease will be revealed, as well as potential therapeutic strategies to combat these disorders.
Here, we have described the importance of adipogenesis to adipose depot expansion and cellular origin of new adipocytes appearing in these tissues. However, as depicted in Figure 1, bona fide adipocytes can accumulate within the parenchyma of other tissues/organs. The importance of the cells is also certain, albeit still being defined. Excellent reviews and original studies on the contribution of local adipose precursors and adipocytes to bone, skeletal muscle, and dermal remodeling have recently emerged (Alexander et al., 2015; Kruglikov and Scherer, 2016; Scheller et al., 2016). The contribution of adipose lineage cells to other organs will undoubtedly be an area of active investigation going forward. A challenge, however, will be in developing new tools to genetically manipulate these anatomically distinct adipocytes and precursor populations.
It is clear that the mouse has been a tremendously valuable tool in modeling adipose tissue development and the features of healthy and unhealthy adipose tissue expansion in adults. However, it is critical to remember that the mouse and human differ significantly with respect to some aspects of adipose biology. In particular, the representative subcutaneous and visceral depots commonly studied in mouse models (inguinal WAT and perigonadal WAT) are not the same depots examined in clinical studies of white and beige adipose. In fact, mice have relatively small omental WAT. Going forward, coordinated efforts between clinical and basic scientists will be needed to reveal the properties of adult white and thermogenic adipocytes, the mechanisms of adipose expansion in adults, and potential therapeutic strategies to target the adipose lineage and combat metabolic disease.
Highlights.
De novo adipocyte differentiation occurs in various postnatal physiological settings
Recent studies reveal precursors for white, brown, and beige adipocytes in adult mice
Committed preadipocytes reside in the adipose tissue vasculature as mural cells
Adipose precursors are regulated in obesity in a sex and depot-dependent manner
The exact requirement of recently identified precursor populations remains unclear
Acknowledgements
There are a considerable number of outstanding and important studies related to adipose tissue remodeling and adipose precursors. We apologize to our colleagues for not being able to discuss them all here. We thank members of the Touchstone Diabetes Center at UT Southwestern for useful discussion. R.K.G. is supported by NIDDK R01 DK104789 and C.H is supported by the NIH NIGMS training grant T32 GM008203.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicting interests statement. The authors declare that they have no competing financial interests.
References
- Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. The Journal of pathology and bacteriology. 1966;91:223–234. doi: 10.1002/path.1700910126. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Kasza I, Yen CL, Reeder SB, Hernando D, Gallo RL, Jahoda CA, Horsley V, MacDougald OA. Dermal white adipose tissue: a new component of the thermogenic response. J Lipid Res. 2015;56:2061–2069. doi: 10.1194/jlr.R062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Peirce SM. IFATS collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008;26:2682–2690. doi: 10.1634/stemcells.2008-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, Adams RJ. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36:2388–2394. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Berry DC, Jiang Y, Graff JM. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun. 2016;7:10184. doi: 10.1038/ncomms10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. 2012;8:55–66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- Borengasser SJ, Zhong Y, Kang P, Lindsey F, Ronis MJ, Badger TM, Gomez-Acevedo H, Shankar K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology. 2013;154:4113–4125. doi: 10.1210/en.2012-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. Journal of lipid research. 2012;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B, Summers SA. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol Metab. 2015;26:538–550. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Chawla A, Lazar MA. Peroxisome proliferator and retinoid signaling pathways co-regulate preadipocyte phenotype and survival. Proc Natl Acad Sci U S A. 1994;91:1786–1790. doi: 10.1073/pnas.91.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Borsheim E, Porter C, Saraf MK, Annamalai P, Yfanti C, Chao T, Wong D, Shinoda K, et al. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab. 2016 doi: 10.1016/j.cmet.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Cigolini M, Bosello O, Bjorntorp P. A morphological study of the adipocyte precursor. J Submicrosc Cytol. 1984;16:243–251. [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta. 2014;1842:463–472. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquinag AC, Tseng C, Salameh A, Zhang Y, Amaya-Manzanares F, Dadbin A, Florez F, Xu Y, Tong Q, Kolonin MG. Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death Differ. 2015;22:351–363. doi: 10.1038/cdd.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KE, M DN, Sun K, W MS, J DB, J AZ, Zeve D, L DH, D WC, L MG, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–242. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Annals of the New York Academy of Sciences. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis GV, Obin MS. ‘Metabolically healthy obesity’: origins and implications. Mol Aspects Med. 2013;34:59–70. doi: 10.1016/j.mam.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane OM, Lyons CL, Murphy AM, Reynolds CM, Klinger R, Healy NP, Cooke AA, Coll RC, McAllan L, Nilaweera KN, et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1beta secretion and insulin resistance despite obesity. Diabetes. 2015;64:2116–2128. doi: 10.2337/db14-1098. [DOI] [PubMed] [Google Scholar]
- Gavin KM, Gutman JA, Kohrt WM, Wei Q, Shea KL, Miller HL, Sullivan TM, Erickson PF, Helm KM, Acosta AS, et al. De novo generation of adipocytes from circulating progenitor cells in mouse and human adipose tissue. FASEB J. 2016;30:1096–1108. doi: 10.1096/fj.15-278994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab. 2015;26:193–200. doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. The Journal of clinical endocrinology and metabolism. 2002;87:2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- Hardy OT, Perugini RA, Nicoloro SM, Gallagher-Dorval K, Puri V, Straubhaar J, Czech MP. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg Obes Relat Dis. 2011;7:60–67. doi: 10.1016/j.soard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J, Han PW. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J Lipid Res. 1969;10:77–82. [PubMed] [Google Scholar]
- Holmes D. Metabolism: WAT browning--key feature of cancer-associated cachexia. Nat Rev Endocrinol. 2014;10:578. doi: 10.1038/nrendo.2014.134. [DOI] [PubMed] [Google Scholar]
- Hong KY, Bae H, Park I, Park DY, Kim KH, Kubota Y, Cho ES, Kim H, Adams RH, Yoo OJ, et al. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development. 2015;142:2623–2632. doi: 10.1242/dev.125336. [DOI] [PubMed] [Google Scholar]
- Hudak CS, Gulyaeva O, Wang Y, Park SM, Lee L, Kang C, Sul HS. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8:678–687. doi: 10.1016/j.celrep.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak CS, Sul HS. Pref-1, a gatekeeper of adipogenesis. Front Endocrinol (Lausanne) 2013;4:79. doi: 10.3389/fendo.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. European journal of applied physiology and occupational physiology. 1981;46:339–345. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, Olson LE. PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev. 2015;29:1106–1119. doi: 10.1101/gad.260554.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17:376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Pena R, Church CD, Colman L, Berry R, Rodeheffer MS. The Adipose Tissue Microenvironment Regulates Depot-Specific Adipogenesis in Obesity. Cell Metab. 2016;24:142–150. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 2014;9:1007–1022. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, Patwari P, Steinhauser ML. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20:1049–1058. doi: 10.1016/j.cmet.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, Hodin RA, Spiegelman BM. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab. 2016;23:315–323. doi: 10.1016/j.cmet.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Uncoupling protein--a useful energy dissipator. J Bioenerg Biomembr. 1999;31:419–430. doi: 10.1023/a:1005440221914. [DOI] [PubMed] [Google Scholar]
- Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277–287. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. The Journal of clinical investigation. 1979;63:239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov IL, Scherer PE. Dermal Adipocytes: From Irrelevance to Metabolic Targets? Trends Endocrinol Metab. 2016;27:1–10. doi: 10.1016/j.tem.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaud CC, Pezzolla D, Dominguez-Rodriguez A, Smani T, Soria B, Hmadcha A. Functional vascular smooth muscle-like cells derived from adult mouse uterine mesothelial cells. PLoS One. 2013;8:e55181. doi: 10.1371/journal.pone.0055181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley SM, Searles RG, Hoi A, Thomas C, Moneta H, Herrick SE, Thompson PJ, Newman M, Sterrett GF, Prele CM, et al. Mesothelial cell differentiation into osteoblast- and adipocyte-like cells. J Cell Mol Med. 2011;15:2095–2105. doi: 10.1111/j.1582-4934.2010.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:371–376. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015a;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 2015b;29:286–299. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang X, Oh JH, Lin RZ, Duan S, Yu Y, Yang R, Qiu J, Melero-Martin JM, Pu WT, et al. Epicardium-to-fat transition in injured heart. Cell Res. 2014;24:1367–1369. doi: 10.1038/cr.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncar D. Convertible adipose tissue in mice. Cell and tissue research. 1991;266:149–161. doi: 10.1007/BF00678721. [DOI] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annual review of medicine. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci U S A. 2010;107:14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell. 2015;160:105–118. doi: 10.1016/j.cell.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Bathke M, Chen L, Oberschneider E, Harteneck D, Jensen MD. Sex and depot differences in ex vivo adipose tissue fatty acid storage and glycerol-3-phosphate acyltransferase activity. Am J Physiol Endocrinol Metab. 2015;308:E830–846. doi: 10.1152/ajpendo.00424.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano L. The Differentiation of White Adipose Cells. An Electron Microscope Study. J Cell Biol. 1963;18:663–679. doi: 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF, Aguirre V, Gupta RK, Clegg DJ. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab. 2015;4:427–436. doi: 10.1016/j.molmet.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E, Junker S, Schett G, Frommer K, Muller-Ladner U. Adipokines in bone disease. Nat Rev Rheumatol. 2016;12:296–302. doi: 10.1038/nrrheum.2016.49. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, Amini-Nik S, Jeschke MG. Burn Induces Browning of the Subcutaneous White Adipose Tissue in Mice and Humans. Cell reports. 2015;13:1538–1544. doi: 10.1016/j.celrep.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early human development. 1984;10:1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- Poznanski WJ, Waheed I, Van R. Human fat cell precursors. Morphologic and metabolic differentiation in culture. Lab Invest. 1973;29:570–576. [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricquier D, Nechad M, Mory G. Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. The Journal of clinical endocrinology and metabolism. 1982;54:803–807. doi: 10.1210/jcem-54-4-803. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Ryden M, Uzunel M, Hard JL, Borgstrom E, Mold JE, Arner E, Mejhert N, Andersson DP, Widlund Y, Hassan M, et al. Transplanted Bone Marrow-Derived Cells Contribute to Human Adipogenesis. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011;60:56–63. doi: 10.2337/db10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung CM, Guertin DA. Emerging Complexities in Adipocyte Origins and Identity. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow Adipose Tissue: Trimming the Fat. Trends Endocrinol Metab. 2016;27:392–403. doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Liu W, Kuang S. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB J. 2013;27:277–287. doi: 10.1096/fj.12-211516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C, Vishvanath L, MacPherson KA, Spurgin SB, Sun K, Holland WL, et al. Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab. 2016 doi: 10.1016/j.cmet.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG, Imielinska C, Ross R, Heymsfield SB. Adipose tissue quantification by imaging methods: a proposed classification. Obesity research. 2003;11:5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015;21:389–394. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura N, Yoshida H, Ogura Y, Kataoka H, Nishikawa S. PDGFR alpha expression during mouse embryogenesis: immunolocalization analyzed by whole-mount immunohistostaining using the monoclonal anti-mouse PDGFR alpha antibody APA5. J Histochem Cytochem. 1997;45:883–893. doi: 10.1177/002215549704500613. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. The Journal of clinical investigation. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Van RL, Bayliss CE, Roncari DA. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976;58:699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Robin L, Roncari DA. Isolation of fat cell precursors from adult rat adipose tissue. Cell and tissue research. 1977;181:197–203. doi: 10.1007/BF00219980. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, Gupta RK. Pdgfrbeta Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Scherer PE, Gupta RK. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. J Lipid Res. 2014a;55:605–624. doi: 10.1194/jlr.R046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Jiang L, Shao M, Ye R, Zhu Y, Gordillo R, Ali A, Lian Y, Holland WL, et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol. 2015;17:1099–1111. doi: 10.1038/ncb3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, Seale P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A. 2014b;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman F. Electron microscopic investigation of the surface structures of the fat cell and of their changes during depletion of cell. Z Zellforsch Microskop Anat Abt Histochem. 1960;52:778–787. doi: 10.1007/BF00336627. [DOI] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, Berim A, Zhang S, Wang S, Wang B, et al. AMPK/alpha-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016 doi: 10.1016/j.cmet.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QY, Liang JF, Rogers CJ, Zhao JX, Zhu MJ, Du M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes. 2013;62:3727–3735. doi: 10.2337/db13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Wang QA, Tao C, Vishvanath L, Shao M, McDonald JG, Gupta RK, Scherer PE. Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metab. 2015;4:771–778. doi: 10.1016/j.molmet.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]