Abstract
Objective
Overweight/obesity and anti-citrullinated protein antibodies (ACPA) increase RA risk. We investigated the relationship between body mass index (BMI) and ACPA, tested for an interaction between BMI and ACPA for RA risk, and examined effects of BMI and ACPA on time to RA diagnosis.
Design
Within the Nurses’ Health Studies, blood samples were collected before diagnosis from medical record-confirmed incident RA cases and matched controls. Multiplex assays measured seven ACPA subtypes (biglycan, clusterin, enolase, fibrinogen, histone 2A, histone 2B, vimentin). Logistic regression analyses tested the association of BMI and ACPA and for a multiplicative interaction between BMI groups (≥25 vs. <25 kg/m2) and ACPA positivity (≥2 vs. <2 subtypes), adjusting for age, smoking, alcohol use, and HLA-shared epitope. In case-only analyses, log-rank tests compared time from blood draw to RA onset by cross-classified BMI/ACPA status.
Results
Among 255 pre-RA cases and 778 matched controls, 15.7% vs. 2.1% (p<0.001) had ≥2 ACPA and 49.4% vs. 40.2% (p<0.01) were overweight/obese. Continuous BMI was not associated with presence of ≥2 ACPA (OR per kg/m2 unit BMI: 1.03 [95% CI 0.97-1.09]). However, there was a multiplicative interaction between elevated BMI and presence of ≥2 ACPA for RA risk (p=0.027). Women with BMI ≥25 kg/m2 and ≥2 ACPA had OR 22.7 (95% CI [6.64-77.72]) for RA. Median time to RA differed by BMI/ACPA group (overall log-rank p<0.001) and was shortest among women with ≥2 ACPA and BMI ≥25 kg/m2 (45.0 months, IQR [17.5-72.5]) and longest in women with <2 ACPA and BMI <25 kg/m2 (125.0 months, IQR [72.0-161.0]) (pairwise log-rank p=0.002).
Conclusion
Elevated BMI and presence of ACPA interacted to increase RA risk. Time to RA onset was shortest among overweight/obese women with ≥2 ACPA.
Keywords: rheumatoid arthritis, autoantibodies, epidemiology, obesity, ACPA
INTRODUCTION
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease that primarily affects synovial joints, causing pain, swelling, decreased function, bony erosions and ultimately joint deformity. During the pre-clinical phase, autoantibodies, cytokines, and chemokines may be elevated for up to 14 years prior to symptoms [1-7]. Among individuals with genetic risk factors, this loss of self-tolerance without clinically apparent disease may follow an exposure to one or more environmental factors [8, 9]. Synovitis develops subsequently, due to unknown factors. Given the accumulating evidence of this prolonged period of pre-clinical inflammation and autoimmunity, there is growing interest in potential environmental factors related to the induction of RA.
Elevated body mass index (BMI) has been associated with increased RA risk in several past studies; some have observed a differential effect by sex, with elevated risk among women but not men [10-14]. Recent meta-analyses of BMI’s effect on RA risk reported that BMI ≥25 kg/m2 (the World Health Organization definition of overweight/obese) significantly increased RA risk by 15%, compared to BMI <25 kg/m2; BMI ≥30 kg/m2 (obesity) significantly increased RA risk by 21% to 31% compared to normal BMI [15-17]. Obesity may increase RA risk via systemic inflammation, as adipose tissue secretes pro-inflammatory adipokines [18]. Adipokines from visceral fat collections in particular are associated with elevated levels of systemic inflammation [19-21]. In many observational studies, overweight and obese individuals without RA have had higher levels of inflammatory biomarkers, including C-reactive protein (CRP), tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1), than have people of normal weight suggesting independent associations between obesity and inflammation [18, 19, 22, 23].
Anti-citrullinated protein antibodies (ACPA) are autoantibodies targeted against proteins that have undergone post-translational modification of arginine to citrulline, catalyzed by peptidylarginine deiminase (PAD) enzymes [24]. ACPAs may be detectable in the serum more than ten years before the development of RA, and the number of distinct types of ACPAs increases in the years leading up to RA diagnosis, likely reflecting epitope spread [25-29]. ACPA positivity is strongly related to subsequent RA risk [30, 31]. In a nested case-control study in the Nurses’ Health Studies, the presence of one or more ACPA in a blood sample was associated with a five-fold increased risk of future RA [31]. ACPAs are associated with genetic and environmental RA risk factors, including the HLA-shared epitope (HLA-SE) and smoking, both in RA patients and asymptomatic individuals [31-35]. ACPAs to vimentin and enolase have been shown to synergistically interact with HLA-DRB1 to increase risk of seropositive RA [35, 36].
As elevated BMI and ACPA are both associated with RA risk, particularly in women, and because BMI involves systemic inflammation, we aimed to determine the relationship between these two risk factors among asymptomatic women in a nested-case control study. We also investigated whether BMI was related to a genetic risk factor for RA, the HLA-SE. We then sought to investigate whether an interaction existed between elevated BMI and ACPA, or between elevated BMI and HLA-SE, such that the risk of RA was synergistically elevated in the presence of both risk factors. Lastly, among women who later developed RA, we examined the joint effects of BMI and ACPA on the time between blood sample collection and RA diagnosis (referred to as “time to RA”).
MATERIALS AND METHODS
Study design and population
We conducted a nested case-control study among women in the Nurses’ Health Studies, which are large prospective cohorts of female nurses living in the United States. The Nurses’ Health Study (NHS) enrolled 121,700 women aged 30-55 years living in 11 states at the time of enrollment in 1976. Nurses’ Health Study II (NHSII) enrolled 116,430 women aged 25-42 years living in 14 states at the time of enrollment in 1989. All participants completed questionnaires by mail at baseline and in follow-up every two years regarding the development of new diseases, lifestyle factors, and health behaviors.
Approximately one-fourth of subjects donated a one-time blood sample, collected between 1989-90 in the NHS (27%) and 1996-1999 in the NHSII (25%). Our nested case-control study included pre-RA cases and matched controls among women from the NHS/NHSII who had donated a blood sample. For this study, women were followed from cohort inception through May 30, 2012 (36 years in the NHS) and May 30, 2011 (22 years in the NHSII).
Identification of cases
Methods for RA case identification and validation have been previously reported [4, 37]. In brief, self-reported incident connective tissue disease after the blood draw was first elicited via biannual mailed questionnaires. Self-reporters then completed the Connective Tissue Screening Questionnaire [38]. If positive, medical records were obtained and reviewed in detail by two rheumatologists for the 1987 American College of Rheumatology (ACR) classification criteria for RA [39], including documentation of rheumatoid factor (RF) and/or anti-cyclic citrullinated peptide (anti-CCP) by commercial assay at the time of diagnosis. Since some women were diagnosed with RA prior to the clinical use of the anti-CCP test, we defined seropositive as being positive for either RF or anti-CCP by medical record review. The month and year of diagnosis were recorded based on medical record review. Women with prevalent RA at the blood draw were not included in the study.
Matched controls
For each case, we matched three controls within the same cohort who donated a blood sample and had not reported RA or other rheumatic disease at the time of case identification. Matching factors at time of blood draw were age (±1 year), menopausal status and post-menopausal hormone use, time of blood collection, and fasting status, as in prior work [40, 41]. Women were excluded from being controls if they had self-reported RA that was not confirmed upon medical record review, or if they had self-reported another connective tissue disease, whether confirmed or not, at the time of blood draw. Women who reported low back pain or osteoarthritis, without another connective tissue disease, were allowed to be controls.
Laboratory methods
Anti-citrullinated protein antibodies (ACPA)
The development of multiplex ACPA assays was previously described in detail [25]. In brief, synovium-specific protein antigens (citrullinated peptides and native non-citrullinated peptides) were conjugated to spectrally-distinct beads using the BioPlex multiplex assay platform (Bio-Rad Laboratories, Hercules, CA, USA) for analysis on the Luminex 200 instrument (Luminex, Austin, TX, USA) [26]. Pre-established control serum samples with high, medium, low, or no reactivity were performed on each plate as internal controls.
Serum from each pre-RA case and matched controls was added to the bead mix, and the reactivity of ACPAs was measured in raw fluorescent intensity units. Fifteen antibodies against citrullinated proteins, all of which passed quality control, were included in this study. Background reactivity to eight non-citrullinated native proteins was also measured among pre-RA cases and controls. Inter-batch coefficients of variation were 2.2% to 9.1%. We defined a positive assay as having raw fluorescent intensity units ≥3 standard deviations (SD) above the mean fluorescent intensity units among controls, as previously described [31].
The 15 ACPAs analyzed were grouped into seven ACPA subtypes by proteins: biglycan (1 epitope), clusterin (2 epitopes), enolase (1 epitope), fibrinogen (5 epitopes), histone 2A (2 epitopes), histone 2B (2 epitopes), and vimentin (2 epitopes). Presence of ACPA against one or more epitope(s) within a given subtype was deemed positive for that subtype. For each subject we summed the number of different ACPA subtypes present, ranging from zero to seven. We first studied the relationship between BMI and number of ACPA subtypes as a continuous count. Our analyses then included ACPA as a binary variable, <2 ACPA subtypes present vs. ≥2 subtypes present, to permit us to investigate an interaction between elevated BMI and presence of ACPA on RA risk. We chose ≥2 ACPA subtypes to define positivity since the prevalence of ACPAs in controls using this cutoff was similar to the 1-2% prevalence of ACPA observed in healthy subjects in other population-based studies [42, 43].
HLA-shared epitope
Classical HLA-SE alleles were tested by the American Red Cross as previously described [31, 41]. Shared epitopes alleles tested were: HLA-DRB1*0401, *0404, *0405, *0408, *0101, *0102, *1001 or *09. For each woman, HLA-SE was recorded as absent (zero alleles), present (one or two alleles), or missing.
Exposures and covariates
BMI was calculated in kg/m2 using self-reported weight from the questionnaire cycle immediately prior to the blood draw and height from the enrollment questionnaire. We dichotomized BMI <25 vs. ≥25 kg/m2 based on the World Health Organization definition of normal vs. overweight/obese [15], as well as literature supporting this as a clinically important cutoff for RA risk [10, 11, 17, 44, 45]. Subjects were also cross-classified into four groups according to their BMI (<25 vs. ≥25 kg/m2) and ACPA status (<2 vs. ≥2 subtypes).
Covariates selected a priori for inclusion in multivariable models were smoking (continuous pack-years) and cumulative average alcohol use (continuous grams/day) from the questionnaire cycle prior to blood draw, as well as HLA-SE (absent, present, or missing), given that each of these has been shown to be related to ACPA-positive RA [12, 32, 46-50]. In 1988 (NHS) and 1989 (NHSII), participants were asked whether they had a physical examination in the past two years. This variable was included as an indicator of healthcare utilization, as increased healthcare utilization could potentially lead to earlier RA diagnosis. As our conditional logistic regression models were conditioned on the matching factors, they were not additionally adjusted for matched covariates.
Statistical methods
Characteristics of pre-RA cases and matched controls at the blood draw, including the prevalence of ACPA subtypes, were described and compared using univariable conditional logistic regression models to account for the matched design. Characteristics of pre-RA cases at RA diagnosis were summarized using descriptive statistics.
We investigated the cross-sectional relationship between continuous BMI (kg/m2) and number of ACPA subtypes (zero to seven) using Spearman’s correlation coefficient. We also tested for a relationship between BMI and ACPA positivity (≥2 subtypes) using logistic regression models to calculate odds ratios (OR) and 95% confidence intervals (CI) for ≥2 ACPA, adjusting for age at blood draw, smoking, alcohol intake and HLA-SE. We performed multivariable conditional logistic regression models to estimate ORs and 95% CIs for the risk of RA, including BMI, ≥2 ACPA, and a multiplicative interaction between BMI (≥25 vs. <25 kg/m2) and ACPA (≥2 vs. <2 subtypes), adjusting for smoking, alcohol intake, HLA-SE, and physical exam in the past two years.
To test whether elevated BMI was related to the presence of HLA-SE, we used linear regression models to estimate the age-adjusted and then multivariable-adjusted effect (β coefficient [standard error]) of ≥1 HLA-SE allele on BMI in kg/m2. Logistic regression models calculated OR (95% CI) for BMI ≥25 kg/m2 based on HLA-SE status (≥1 vs. 0 alleles), adjusting for age at blood draw, smoking, alcohol intake and ACPA. Conditional logistic regression models then tested for a multiplicative interaction between BMI (≥25 vs. <25 kg/m2) and HLA-SE (≥1 vs. 0 alleles) for RA risk, adjusting for smoking, alcohol intake, ACPA, and physical exam in the past 2 years.
In pre-RA case-only analyses, we evaluated the median time to RA diagnosis in each of four cross-classified BMI/ACPA groups. Overall and pairwise log-rank tests were used to assess differences in the time between the blood draw and RA diagnosis across these groups.
Analyses were performed using SAS (v 9.3, Cary, NC, USA). We considered a two-sided p value of <0.05 as statistically significant in all analyses. All aspects of this study were approved by the Partners HealthCare Institutional Review Board.
RESULTS
Pre-RA cases and matched controls
Two hundred and fifty-five pre-RA cases (166 in NHS, 89 in NHSII) were matched to 778 controls on characteristics at the blood draw. Both pre-RA cases and controls were predominantly White, but the two groups had a few expected differences (Table 1). At the time of blood draw, ≥2 ACPA were present in 15.7% of pre-RA cases and 2.1% of controls (p<0.001). Nearly 50% of pre-RA cases were overweight/obese, compared to 40.2% of controls (p<0.01). Among pre-RA cases, median BMI was 22.3 kg/m2 (IQR 21.0-23.8) among those with BMI <25 kg/m2, and 28.2 kg/m2 (IQR 26.3-31.0) among those with BMI ≥25 kg/m2. Among controls, median BMI was 22.3 kg/m2 (IQR 20.9-23.5) among those with BMI <25 kg/m2, and 28.2 kg/m2 (IQR 26.2-30.9) among those with BMI ≥25 kg/m2. Pre-RA cases had a greater number of pack-years of smoking (p=0.01) and less alcohol use (p<0.01) compared to controls. One or more HLA-SE alleles were present in 56.0% of pre-RA cases and 42.5% of controls (p<0.001). Each of the seven ACPA subtypes was observed more often in pre-RA cases than in controls at the time of blood draw (Table 2). Among all subjects, antibodies against citrullinated (cit) proteins were detected more often than antibodies against non-citrullinated (non-cit) proteins: vimentin (5.2% cit vs. 1.6% non-cit), fibrinogen (7.7% cit vs. 1.1% non-cit), histone 2B (3.4% cit vs. 1.7% non-cit), and histone 2A (2.4% cit vs. 1.7% non-cit).
Table 1.
Characteristics of 1033 women from the Nurses’ Health Study and Nurses’ Health Study II in a nested case-control study of rheumatoid arthritis, at the time of blood draw [1989-90 (NHS) or 1996-99 (NHSII)]
| Characteristics | Pre-RA Cases (n=255) |
Matched Controls (n=778) |
p value |
|---|---|---|---|
| Age at blood draw, years | 51.4 (8.0) | 51.4 (8.0) | matching factor |
| White | 250 (98.0%) | 758 (97.4%) | 0.45 |
| ≥2 ACPA subtypes present | 40 (15.7%) | 16 (2.1%) | <0.001 |
| BMI ≥25 | 126 (49.4%) | 313 (40.2%) | <0.01 |
| Smoking, pack-years | 12.3 (16.1) | 9.4 (15.7) | 0.01 |
| Cumulative average alcohol, g/day | 4.0 (5.3) | 5.5 (8.6) | <0.01 |
| ≥1 HLA-SE allele present* | 139 (56.0%) | 284 (42.5%) | <0.001 |
Presented as mean (SD) or n (%). P values are from univariable conditional logistic regression models. ACPA: anti-citrullinated protein antibody. BMI: body mass index.
HLA-SE: HLA-shared epitope (missing in 117 women). Presented as % of 248 pre-RA cases and 668 controls
Table 2.
Prevalence of ACPA subtypes among 1033 women from the Nurses’ Health Study and Nurses’ Health Study II in a nested case-control study, at the time of blood draw [1989-90 (NHS) or 1996-99 (NHSII)]
| ACPA subtype* |
Pre-clinical RA Cases (n=255) |
Matched Controls (n=778) |
p value |
|---|---|---|---|
| Biglycan | 12 (4.7%) | 7 (0.9%) | <0.001 |
| Clusterin | 26 (10.2%) | 13 (1.7%) | <0.001 |
| Enolase | 6 (2.4%) | 4 (0.5%) | 0.02 |
| Fibrinogen | 45 (17.7%) | 34 (4.4%) | <0.001 |
| Histone 2A | 14 (5.5%) | 11 (1.4%) | 0.001 |
| Histone 2B | 22 (8.6%) | 13 (1.7%) | <0.001 |
| Vimentin | 38 (14.9%) | 16 (2.1%) | <0.001 |
|
| |||
| ≥1 ACPA | 59 (23.1%) | 59 (7.6%) | <0.001 |
| ≥2 ACPAs | 40 (15.7%) | 16 (2.1%) | <0.001 |
P values are from univariable conditional logistic regression models.
For each ACPA subtype, antibodies against the following number of epitopes were tested. Subjects testing positive for antibody against one or more epitope were considered positive for that ACPA subtype: biglycan (1), clusterin (2), enolase (1), fibrinogen (5), histone 2A (2), histone 2B (2), vimentin (2)
The mean age for cases at RA diagnosis was 60.3 years (SD 9.9) (Table 3). Mean duration between blood draw and diagnosis (time to RA) was 106.9 months (SD 63.0) among all cases, though cases with BMI <25 kg/m2 had a longer mean time to RA (118.1 months [SD 63.6]) and those with BMI ≥25 kg/m2 had a shorter mean time to RA (95.4 months [SD 60.4]). RA cases otherwise had similar characteristics of their RA presentation, regardless of BMI category. Approximately two-thirds were seropositive by chart review at the time of diagnosis. The vast majority had symmetric arthritis involving the hands.
Table 3.
Characteristics of 255 rheumatoid arthritis cases from the Nurses’ Health Study and Nurses’ Health Study II in a nested case-control study, at the time of diagnosis*
| All cases (n=255) |
BMI <25 kg/m2
(n=129) |
BMI ≥25 kg/m2
(n=126) |
|
|---|---|---|---|
| Mean age at RA diagnosis, years | 60.3 (9.9) | 61.9 (9.4) | 58.7 (10.2) |
| Mean time from blood draw to RA diagnosis, months |
106.9 (63.0) | 118.1 (63.6) | 95.4 (60.4) |
| Seropositive (RF and/or CCP positive)** | 158 (62.0%) | 79 (61.2%) | 79 (62.7%) |
| Hand arthritis | 251 (98.4%) | 126 (97.7%) | 125 (99.2%) |
| Symmetric arthritis | 250 (98.0%) | 126 (97.7%) | 124 (98.4%) |
| Morning stiffness >1 hour | 194 (76.1%) | 97 (75.2%) | 97 (77.0%) |
| Erosions | 53 (20.8%) | 28 (21.7%) | 25 (19.8%) |
| Rheumatoid nodules | 24 (9.4%) | 10 (7.8%) | 14 (11.1%) |
| Self-reported physical exam in past 2 years+ | 105 (41.2%) | 58 (45.0%) | 47 (37.3%) |
Presented as mean (SD) or %. RA: rheumatoid arthritis. RF: rheumatoid factor. CCP: cyclic citrullinated peptide antibody.
All RA cases had at least 4 criteria of the 1987 ACR criteria present.
Seropositivity was determined by medical record review at time of RA diagnosis. Some RA cases were diagnosed before the clinical use of CCP.
Assessed in 1988 (NHS) and 1989 (NHSII)
Relationship between BMI and ACPA positivity
We did not observe a significant association between continuous BMI and continuous number of ACPA subtypes among all nested case-control subjects (r=−0.015, p=0.63). We also did not detect a significant association between increasing BMI and the presence of ≥2 ACPA in age-adjusted (OR 1.04, 95%CI [0.98-1.10]) or multivariable-adjusted (OR 1.03, 95%CI [0.97-1.09]) models among all subjects (Table 4). However, among pre-RA cases only we detected a significant association between continuous BMI and ACPA positivity in age-adjusted (OR 1.09, 95%CI [1.02-1.16]) and multivariable-adjusted (OR 1.07, 95%CI [1.00-1.15]) models.
Table 4.
Logistic regression models for the presence of ≥2 ACPA according to continuous or categorical BMI among women from the Nurses’ Health Study and Nurses’ Health Study II in a nested case-control study of rheumatoid arthritis, at the time of blood draw
| Predictor | Age-adjusted OR (95% CI) |
Multivariable-adjusted OR (95% CI)* |
|---|---|---|
| Among all 1033 subjects | ||
|
| ||
| Continuous BMI, kg/m2 | 1.04 (0.98-1.10) | 1.03 (0.97-1.09) |
| BMI categories | ||
| <25 kg/m2 | 1.00 (ref) | 1.00 (ref) |
| ≥25 kg/m2 | 1.28 (0.75-2.19) | 1.22 (0.70-2.11) |
|
| ||
| Among 255 pre-RA cases | ||
|
| ||
| Continuous BMI, kg/m2 | 1.09 (1.02-1.16) | 1.07 (1.00-1.15)** |
| BMI categories | ||
| <25 kg/m2 | 1.00 (ref) | 1.00 (ref) |
| ≥25 kg/m2 | 1.67 (0.84-3.32) | 1.68 (0.81-3.51) |
Unconditional logistic regression models for ACPA positivity adjusted for age at blood draw, HLA-shared epitope, smoking, alcohol use
p=0.049
Relationship between BMI, ACPA, and RA risk
Women with ≥2 ACPA had an eight-fold increased RA risk, compared to women with <2 ACPA, in the conditional logistic regression model (OR 8.05, 95% CI [4.43-14.66]). The odds of RA were greater than six-fold elevated (OR 6.65, 95% CI [3.56-12.43]) in the presence of ≥2 ACPA in a model additionally adjusting for smoking, alcohol use, BMI, HLA-SE, and physical exam in the past two years. BMI ≥25 kg/m2 (vs. <25) was also associated with increased RA risk: age-adjusted OR 1.49 (95% CI 1.11-1.99) and multivariable-adjusted OR 1.35 (95% CI 0.99-1.84).
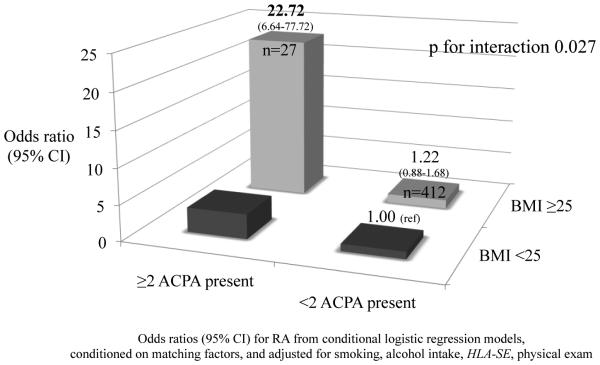
In stratified models, women with BMI ≥25 kg/m2 and ≥2 ACPA had 23-fold increased odds of RA compared to the reference group of women with BMI <25 kg/m2 and <2 ACPA (OR 22.72, 95%CI [6.64-77.72]) (Figure 1). We observed three-fold increased odds for RA among women with BMI <25 kg/m2 and ≥2 ACPA, compared to the reference group (OR 3.44, 95%CI [1.53-7.74]). We observed a significant multiplicative interaction between BMI and ACPA for RA risk (p for interaction 0.027).
Figure 1.
Multiplicative interaction between ACPA status (≥2 ACPA) and BMI ≥25 on risk of RA among 1033 women (NHS and NHSII) in a nested case-control study of RA
Relationship between BMI and HLA-SE positivity
Among 916 subjects with HLA-SE measured, the presence of ≥1 HLA-SE allele was not related to increasing BMI in kg/m2 in age-adjusted (β 0.28 [standard error 0.30], p=0.35) or multivariable-adjusted (β 0.22 [standard error 0.30], p=0.45) linear regression models. Similarly, we observed no relationship between the presence of ≥1 HLA-SE allele (OR 0.98, 95% CI [0.75-1.27]) and BMI ≥25 kg/m2 in the age-adjusted logistic regression model. In a multivariable-adjusted model, ≥1 HLA-SE allele was associated with an OR 0.96 (95%CI 0.74-1.26) for BMI ≥25 kg/m2. Among pre-RA cases only (n=248 with HLA-SE measured) we did not detect a significant association between presence of ≥1 HLA-SE allele and continuous BMI in age-adjusted (β 1.13 [standard error 0.61], p=0.07) or multivariable-adjusted (β 0.85 [standard error 0.61], p=0.16) linear regression models.
Relationship between BMI, HLA-SE and RA risk
The presence of HLA-SE significantly increased RA risk, with OR 1.75 (95% CI 1.30-2.37) for RA in age-adjusted and OR 1.53 (95% CI 1.11-2.12) in multivariable-adjusted conditional logistic regression models. However, we did not observe a multiplicative interaction between BMI and HLA-SE in RA risk (p for interaction 0.92).
Time to RA diagnosis among pre-RA cases
Among the 255 pre-RA cases, time to RA diagnosis differed by BMI/ACPA group (overall log-rank p<0.001) (Table 5). Women with BMI ≥25 kg/m2 and ≥2 ACPA progressed to diagnosis the fastest with a median time to RA 45.0 months (IQR [17.5-72.5]). The longest time to RA was among women with BMI <25 kg/m2 and <2 ACPA, with a median of 125.0 months (IQR [72.0-161.0]).
Table 5.
Time from blood draw to RA diagnosis among the 255 cases who developed RA within the nested case-control study, by cross-classified ACPA and BMI group
| Group | N | Median age at blood draw, years (IQR) |
Median age at RA diagnosis, years (IQR) |
Median time from blood draw to RA diagnosis, months (IQR) |
Pairwise log- rank p value* |
|---|---|---|---|---|---|
| A <2 ACPA, BMI <25 | 113 | 51.0 (47.0- 58.0) |
61.0 (55.0- 69.0) |
125.0 (72.0- 161.0) |
A vs. B 0.49 A vs. C 0.71 A vs. D 0.002 |
| B <2 ACPA, BMI ≥25 | 102 | 48.5 (45.0- 58.0) |
57.0 (52.0- 68.0) |
102.0 (62.0- 152.0) |
B vs. C 0.84 B vs. D 0.89 |
| C ≥2 ACPA, BMI <25 | 16 | 51.5 (48.0- 56.0) |
60.0 (56.0- 68.0) |
107.5 (41.0- 174.5) |
C vs. D 0.001 |
| D ≥2 ACPA, BMI ≥25 | 24 | 49.0 (44.5- 57.0) |
54.5 (48.0- 60.5) |
45.0 (17.5-72.5) |
P values are from pairwise log-rank tests comparing time to RA diagnosis between cross-classified ACPA and BMI groups
In pairwise comparisons of time to RA between each of these groups, we observed a significant difference between women with <2 ACPA and BMI <25 kg/m2 and those with ≥2 ACPA and BMI ≥25 kg/m2 (pairwise log-rank p=0.002). Among women with ≥2 ACPA, time to RA diagnosis was also significantly different based on BMI <25 vs. ≥25 kg/m2 (pairwise log-rank p=0.001).
DISCUSSION
In this case-control study nested within the NHS/NHSII, being overweight or obese was more common among pre-RA cases than matched controls. However, we observed no cross-sectional association between elevated BMI and the presence of ≥2 ACPA among all women. Within the pre-RA cases, elevated BMI was associated with ≥2 ACPA in a multivariable-adjusted model. We detected a multiplicative interaction between elevated BMI and ≥2 ACPA associated with RA risk among all participants, with a 23-fold risk of RA if both risk factors occurred together. These findings suggest that the presence of both elevated BMI and ACPA has a synergistic effect on RA risk. Moreover, among the pre-RA cases, we observed that women with both risk factors progressed to clinical development of RA the most rapidly.
We were interested to investigate elevated BMI, a potentially modifiable risk factor, since this may stimulate the development of ACPAs as smoking is thought to do, perhaps due to citrullination in the inflammatory milieu of adipose tissue [51]. This hypothesis was based upon reports of a murine model in which expression of PAD enzymes was detected in macrophage collections within mammary gland adipose tissue [52]. PAD enzymes and citrullinated histones have been found in adipose breast tissue from obese women as well [53]. In our study, the lack of a cross-sectional relationship between BMI and presence of ACPA among all subjects makes it less likely that excess adipose tissue promotes the development of ACPA, although we did observe an association between BMI and ACPA among pre-RA cases. Among seropositive (anti-CCP and/or RF) patients with early arthralgias, those with elevated BMI were more likely to develop classifiable RA than those with normal BMI [54]. It is possible but unlikely that the relationship between elevated BMI and the development of ACPA differs at other time points prior to RA.
A possible explanation for the multiplicative interaction between BMI and ACPA is that, in the context of already having ACPA, overweight and obesity foster systemic inflammation which hastens RA pathogenesis. In murine models, for example, immunization with neutrophil-derived citrullinated histones has been shown to be arthritogenic, but only in the setting of underlying inflammation [55]. Given the nested case-control study design, we were unable to conduct a mediation analysis to further investigate this hypothesis.
We did not find an association between the HLA-SE and elevated BMI. We also did not observe an interaction between the presence of ≥1 HLA-SE allele and elevated BMI upon RA risk. This suggests that elevated BMI does not affect RA in the same way as smoking, which has been shown to increase the risk of RA in particular among those carrying HLA-SE alleles [35, 41, 56].
In analyses including pre-RA cases only, both BMI and ACPA status were associated with decreased time to diagnosis, and women with BMI ≥25 kg/m2 and ≥2 ACPA had the shortest interval between blood draw and diagnosis. Deane et al. developed a model predicting time from blood draw to RA among pre-RA subjects from a predominantly male military population [7]. In this model, increasing cytokine/chemokine count and age (by decade) were inversely related to time to RA onset. Our observation of shorter time to diagnosis among ACPA positive women with BMI ≥25 kg/m2 may be explained by higher cytokine/chemokine levels in the setting of obesity, which could trigger or accelerate RA pathogenesis in those who already have ACPAs. We investigated the possibility that increased healthcare utilization among obese women could have led to surveillance bias, in which their RA was diagnosed at earlier stages, but we found no evidence that heavier women had increased use of routine physical exams, and adjustment for this healthcare utilization did not affect our results.
Two or more ACPA subtypes were present in 15.7% of pre-RA cases and 2.1% of controls, with a positive assay defined as previously published [31]. These percentages are similar to other recent RA nested case-control studies, including the prospective European cohort study (EPIC), in which the prevalence of each of three ACPA subtypes was 6-18% among pre-RA cases and 2% among controls [42].
We were unable to perform subgroup analyses due to small numbers of women with each individual ACPA subtype. Since many women were diagnosed prior to the widespread clinical availability of the anti-CCP test, we also could not perform stratified analyses based on this test. We had relatively small numbers of women in each BMI category, and thus our confidence intervals were wide. While we did not adjust for inflammatory cytokine concentrations, inflammatory cytokines likely are mediators on the causal pathway between elevated BMI and RA onset. Another potential limitation is that these analyses were not controlled for all pre- RA comorbidities and medications, which could conceivably be related to both obesity and risk of ACPA-positive RA. Additionally, our study included only U.S. women, the vast majority of whom were White with mean age 60 years at RA diagnosis. It is unlikely that the biologic mechanisms of RA differ in other female populations, although differences by sex, age, and socioeconomic status are possible.
In conclusion, in this large nested case-control study with a wide range of follow-up after blood draw and prospective exposure data collected prior to blood draw and RA diagnosis, we found that BMI and ACPA interacted in predicting RA risk and together shortened the time to diagnosis. As our understanding of RA pathogenesis grows, these findings may provide important insights into prevention, screening, and treatment. Obesity is a potentially modifiable risk factor. Thus, the ability of weight loss interventions to reduce RA risk, particularly among individuals who are already ACPA positive, may be tested in the near future. Further investigation into the mechanisms underlying the interaction between BMI and ACPA in RA is needed.
Acknowledgements
The authors would like to thank the participants of the Nurses’ Health Studies; the Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School; and the funding sources supporting this work: NIH AR066109, AR052403, AR049880, AR059073, AR047782, AR061362, AR066953, AR069688, AR007530, CA186107, CA176726, CA49449 and CA67262. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors All authors have revised the manuscripts for important intellectual content and have approved this version of the article for publication. SKT, JC, EA, JAS, EWK, and KHC provided substantial contributions to study design and interpretation of data. SKT and JC performed data analyses. WHR, JS, and NL provided significant input on ACPA assay interpretation. SKT drafted the paper.
Competing interests SKT, JC, EVA, WHR, JS, NL, JAS, EWK, KHC: no conflicts of interest
REFERENCES
- [1].Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against Cyclic Citrullinated Peptide and Iga Rheumatoid Factor Predict the Development of Rheumatoid Arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- [2].Nielen MM, van Schaardenburg D, Reesink HW, Twisk JW, van de Stadt RJ, van der Horst-Bruinsma IE, et al. Simultaneous Development of Acute Phase Response and Autoantibodies in Preclinical Rheumatoid Arthritis. Ann Rheum Dis. 2006;65(4):535–7. doi: 10.1136/ard.2005.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW. Comparison of Threshold Cutpoints and Continuous Measures of Anti-Cyclic Citrullinated Peptide Antibodies in Predicting Future Rheumatoid Arthritis. J Rheumatol. 2009;36(4):706–11. doi: 10.3899/jrheum.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Karlson EW, Chibnik LB, Tworoger SS, Lee IM, Buring JE, Shadick NA, et al. Biomarkers of Inflammation and Development of Rheumatoid Arthritis in Women from Two Prospective Cohort Studies. Arthritis Rheum. 2009;60(3):641–52. doi: 10.1002/art.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, Ronnelid J, et al. Antibodies of Igg, Iga and Igm Isotypes against Cyclic Citrullinated Peptide Precede the Development of Rheumatoid Arthritis. Arthritis Res Ther. 2010;13(1):R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S. Up-Regulation of Cytokines and Chemokines Predates the Onset of Rheumatoid Arthritis. Arthritis Rheum. 2010;62(2):383–91. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- [7].Deane KD, O'Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, et al. The Number of Elevated Cytokines and Chemokines in Preclinical Seropositive Rheumatoid Arthritis Predicts Time to Diagnosis in an Age-Dependent Manner. Arthritis Rheum. 2010;62(11):3161–72. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Majka DS, Holers VM. Can We Accurately Predict the Development of Rheumatoid Arthritis in the Preclinical Phase? Arthritis Rheum. 2003;48(10):2701–5. doi: 10.1002/art.11224. [DOI] [PubMed] [Google Scholar]
- [9].Leslie D, Lipsky P, Notkins AL. Autoantibodies as Predictors of Disease. J Clin Invest. 2001;108(10):1417–22. doi: 10.1172/JCI14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wesley A, Bengtsson C, Elkan AC, Klareskog L, Alfredsson L, Wedren S, et al. Association between Body Mass Index and Anti-Citrullinated Protein Antibody-Positive and Anti-Citrullinated Protein Antibody-Negative Rheumatoid Arthritis: Results from a Population-Based Case-Control Study. Arthritis Care Res (Hoboken) 2013;65(1):107–12. doi: 10.1002/acr.21749. [DOI] [PubMed] [Google Scholar]
- [11].Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen CY, Awosogba JA, et al. Being Overweight or Obese and Risk of Developing Rheumatoid Arthritis among Women: A Prospective Cohort Study. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, Obesity, Alcohol Consumption, and the Risk of Rheumatoid Arthritis. Epidemiology. 1994;5(5):525–32. [PubMed] [Google Scholar]
- [13].Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, et al. Blood Transfusion, Smoking, and Obesity as Risk Factors for the Development of Rheumatoid Arthritis: Results from a Primary Care-Based Incident Case-Control Study in Norfolk, England. Arthritis Rheum. 1997;40(11):1955–61. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- [14].Turesson C, Bergstrom U, Pikwer M, Nilsson JA, Jacobsson LT. A High Body Mass Index Is Associated with Reduced Risk of Rheumatoid Arthritis in Men, but Not in Women. Rheumatology (Oxford) 2015 doi: 10.1093/rheumatology/kev313. [DOI] [PubMed] [Google Scholar]
- [15].World Health Organization Overweight and Obesity. 2015 Nov 20; http://www.who.int/gho/ncd/risk_factors/overweight_text/en/
- [16].Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body Mass Index and the Risk of Rheumatoid Arthritis: A Systematic Review and Dose-Response Meta-Analysis. Arthritis Res Ther. 2015;17:86. doi: 10.1186/s13075-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feng J, Chen Q, Yu F, Wang Z, Chen S, Jin Z, et al. Body Mass Index and Risk of Rheumatoid Arthritis: A Meta-Analysis of Observational Studies. Medicine (Baltimore) 2016;95(8):e2859. doi: 10.1097/MD.0000000000002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gualillo O, Gonzalez-Juanatey JR, Lago F. The Emerging Role of Adipokines as Mediators of Cardiovascular Function: Physiologic and Clinical Perspectives. Trends Cardiovasc Med. 2007;17(8):275–83. doi: 10.1016/j.tcm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- [19].Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The Implication of Obesity and Central Fat on Markers of Chronic Inflammation: The Attica Study. Atherosclerosis. 2005;183(2):308–15. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [20].Fried SK, Bunkin DA, Greenberg AS. Omental and Subcutaneous Adipose Tissues of Obese Subjects Release Interleukin-6: Depot Difference and Regulation by Glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- [21].Brestoff JR, Artis D. Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell. 2015;161(1):146–60. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in Inflammation and Metabolic Disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arkema EV, Lu B, Malspeis S, Karlson EW, Costenbader KH. Monocyte Chemotactic Protein-1 Elevation Prior to the Onset of Rheumatoid Arthritis among Women. Biomark Med. 2015;9(8):723–9. doi: 10.2217/BMM.15.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hunt L, Emery P. Defining Populations at Risk of Rheumatoid Arthritis: The First Steps to Prevention. Nat Rev Rheumatol. 2014;10(9):521–30. doi: 10.1038/nrrheum.2014.82. [DOI] [PubMed] [Google Scholar]
- [25].Wagner CA, Sokolove J, Lahey LJ, Bengtsson C, Saevarsdottir S, Alfredsson L, et al. Identification of Anticitrullinated Protein Antibody Reactivities in a Subset of Anti-Ccp-Negative Rheumatoid Arthritis: Association with Cigarette Smoking and Hla-Drb1 'Shared Epitope' Alleles. Ann Rheum Dis. 2015;74(3):579–86. doi: 10.1136/annrheumdis-2013-203915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody Epitope Spreading in the Pre-Clinical Phase Predicts Progression to Rheumatoid Arthritis. PLoS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, et al. Duration of Preclinical Rheumatoid Arthritis-Related Autoantibody Positivity Increases in Subjects with Older Age at Time of Disease Diagnosis. Ann Rheum Dis. 2008;67(6):801–7. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bos WH, Nielen MM, Dijkmans BA, van Schaardenburg D. Duration of Pre-Rheumatoid Arthritis Anti-Cyclic Citrullinated Peptide Positivity Is Positively Associated with Age at Seroconversion. Ann Rheum Dis. 2008;67(11):1642. doi: 10.1136/ard.2007.085456. [DOI] [PubMed] [Google Scholar]
- [29].Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific Autoantibodies Precede the Symptoms of Rheumatoid Arthritis: A Study of Serial Measurements in Blood Donors. Arthritis Rheum. 2004;50(2):380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- [30].Bos WH, Wolbink GJ, Boers M, Tijhuis GJ, de Vries N, van der Horst-Bruinsma IE, et al. Arthritis Development in Patients with Arthralgia Is Strongly Associated with Anti-Citrullinated Protein Antibody Status: A Prospective Cohort Study. Ann Rheum Dis. 2010;69(3):490–4. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- [31].Arkema EV, Goldstein BL, Robinson W, Sokolove J, Wagner CA, Malspeis S, et al. Anti-Citrullinated Peptide Autoantibodies, Human Leukocyte Antigen Shared Epitope and Risk of Future Rheumatoid Arthritis: A Nested Case-Control Study. Arthritis Res Ther. 2013;15(5):R159. doi: 10.1186/ar4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yahya A, Bengtsson C, Lai TC, Larsson PT, Mustafa AN, Abdullah NA, et al. Smoking Is Associated with an Increased Risk of Developing Acpa-Positive but Not Acpa-Negative Rheumatoid Arthritis in Asian Populations: Evidence from the Malaysian Myeira Case-Control Study. Mod Rheumatol. 2012;22(4):524–31. doi: 10.1007/s10165-011-0544-2. [DOI] [PubMed] [Google Scholar]
- [33].Balandraud N, Picard C, Reviron D, Landais C, Toussirot E, Lambert N, et al. Hla-Drb1 Genotypes and the Risk of Developing Anti Citrullinated Protein Antibody (Acpa) Positive Rheumatoid Arthritis. PLoS One. 2013;8(5):e64108. doi: 10.1371/journal.pone.0064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Terao C, Ohmura K, Ikari K, Kawaguchi T, Takahashi M, Setoh K, et al. Effects of Smoking and Shared Epitope on the Production of Anti-Citrullinated Peptide Antibody in a Japanese Adult Population. Arthritis Care Res (Hoboken) 2014;66(12):1818–27. doi: 10.1002/acr.22385. [DOI] [PubMed] [Google Scholar]
- [35].Kokkonen H, Brink M, Hansson M, Lassen E, Mathsson-Alm L, Holmdahl R, et al. Associations of Antibodies against Citrullinated Peptides with Human Leukocyte Antigen-Shared Epitope and Smoking Prior to the Development of Rheumatoid Arthritis. Arthritis Res Ther. 2015;17:125. doi: 10.1186/s13075-015-0638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mahdi H, Fisher BA, Kallberg H, Plant D, Malmstrom V, Ronnelid J, et al. Specific Interaction between Genotype, Smoking and Autoimmunity to Citrullinated Alpha-Enolase in the Etiology of Rheumatoid Arthritis. Nat Genet. 2009;41(12):1319–24. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- [37].Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do Breast-Feeding and Other Reproductive Factors Influence Future Risk of Rheumatoid Arthritis? Results from the Nurses' Health Study. Arthritis Rheum. 2004;50(11):3458–67. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- [38].Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A Connective Tissue Disease Screening Questionnaire for Population Studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- [39].Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- [40].Costenbader KH, Chang SC, De Vivo I, Plenge R, Karlson EW. Genetic Polymorphisms in Ptpn22, Padi-4, and Ctla-4 and Risk for Rheumatoid Arthritis in Two Longitudinal Cohort Studies: Evidence of Gene-Environment Interactions with Heavy Cigarette Smoking. Arthritis Res Ther. 2008;10(3):R52. doi: 10.1186/ar2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-Environment Interaction between Hla-Drb1 Shared Epitope and Heavy Cigarette Smoking in Predicting Incident Rheumatoid Arthritis. Ann Rheum Dis. 2010;69(1):54–60. doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fisher BA, Cartwright AJ, Quirke AM, de Pablo P, Romaguera D, Panico S, et al. Smoking, Porphyromonas Gingivalis and the Immune Response to Citrullinated Autoantigens before the Clinical Onset of Rheumatoid Arthritis in a Southern European Nested Case-Control Study. BMC Musculoskelet Disord. 2015;16(1):331. doi: 10.1186/s12891-015-0792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hensvold AH, Frisell T, Magnusson PK, Holmdahl R, Askling J, Catrina AI. How Well Do Acpa Discriminate and Predict Ra in the General Population: A Study Based on 12 590 Population-Representative Swedish Twins. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208980. [DOI] [PubMed] [Google Scholar]
- [44].Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, et al. Environmental Risk Factors Differ between Rheumatoid Arthritis with and without Auto-Antibodies against Cyclic Citrullinated Peptides. Arthritis Res Ther. 2006;8(4):R133. doi: 10.1186/ar2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Harpsoe MC, Basit S, Andersson M, Nielsen NM, Frisch M, Wohlfahrt J, et al. Body Mass Index and Risk of Autoimmune Diseases: A Study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843–55. doi: 10.1093/ije/dyu045. [DOI] [PubMed] [Google Scholar]
- [46].Turesson C, Matteson EL. Genetics of Rheumatoid Arthritis. Mayo Clin Proc. 2006;81(1):94–101. doi: 10.4065/81.1.94. [DOI] [PubMed] [Google Scholar]
- [47].Di Giuseppe D, Orsini N, Alfredsson L, Askling J, Wolk A. Cigarette Smoking and Smoking Cessation in Relation to Risk of Rheumatoid Arthritis in Women. Arthritis Res Ther. 2013;15(2):R56. doi: 10.1186/ar4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Criswell LA, Merlino LA, Cerhan JR, Mikuls TR, Mudano AS, Burma M, et al. Cigarette Smoking and the Risk of Rheumatoid Arthritis among Postmenopausal Women: Results from the Iowa Women's Health Study. Am J Med. 2002;112(6):465–71. doi: 10.1016/s0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- [49].Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking Intensity, Duration, and Cessation, and the Risk of Rheumatoid Arthritis in Women. Am J Med. 2006;119(6):503. doi: 10.1016/j.amjmed.2005.09.053. e1-9. [DOI] [PubMed] [Google Scholar]
- [50].Lu B, Solomon DH, Costenbader KH, Karlson EW. Alcohol Consumption and Risk of Incident Rheumatoid Arthritis in Women: A Prospective Study. Arthritis Rheumatol. 2014;66(8):1998–2005. doi: 10.1002/art.38634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Kitas GD. Obesity in Rheumatoid Arthritis. Rheumatology (Oxford) 2011;50(3):450–62. doi: 10.1093/rheumatology/keq266. [DOI] [PubMed] [Google Scholar]
- [52].Mohanan S, Horibata S, McElwee JL, Dannenberg AJ, Coonrod SA. Identification of Macrophage Extracellular Trap-Like Structures in Mammary Gland Adipose Tissue: A Preliminary Study. Front Immunol. 2013;4:67. doi: 10.3389/fimmu.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and Increased Aromatase Expression Occur in the Breast Tissue of Obese Women with Breast Cancer. Cancer Prev Res (Phila) 2011;4(7):1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].de Hair MJ, Landewe RB, van de Sande MG, van Schaardenburg D, van Baarsen LG, Gerlag DM, et al. Smoking and Overweight Determine the Likelihood of Developing Rheumatoid Arthritis. Ann Rheum Dis. 2013;72(10):1654–8. doi: 10.1136/annrheumdis-2012-202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sohn DH, Rhodes C, Onuma K, Zhao X, Sharpe O, Gazitt T, et al. Local Joint Inflammation and Histone Citrullination in a Murine Model of the Transition from Preclinical Autoimmunity to Inflammatory Arthritis. Arthritis Rheumatol. 2015;67(11):2877–87. doi: 10.1002/art.39283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kim K, Jiang X, Cui J, Lu B, Costenbader KH, Sparks JA, et al. Interactions between Amino Acid-Defined Major Histocompatibility Complex Class Ii Variants and Smoking in Seropositive Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(10):2611–23. doi: 10.1002/art.39228. [DOI] [PMC free article] [PubMed] [Google Scholar]