Abstract
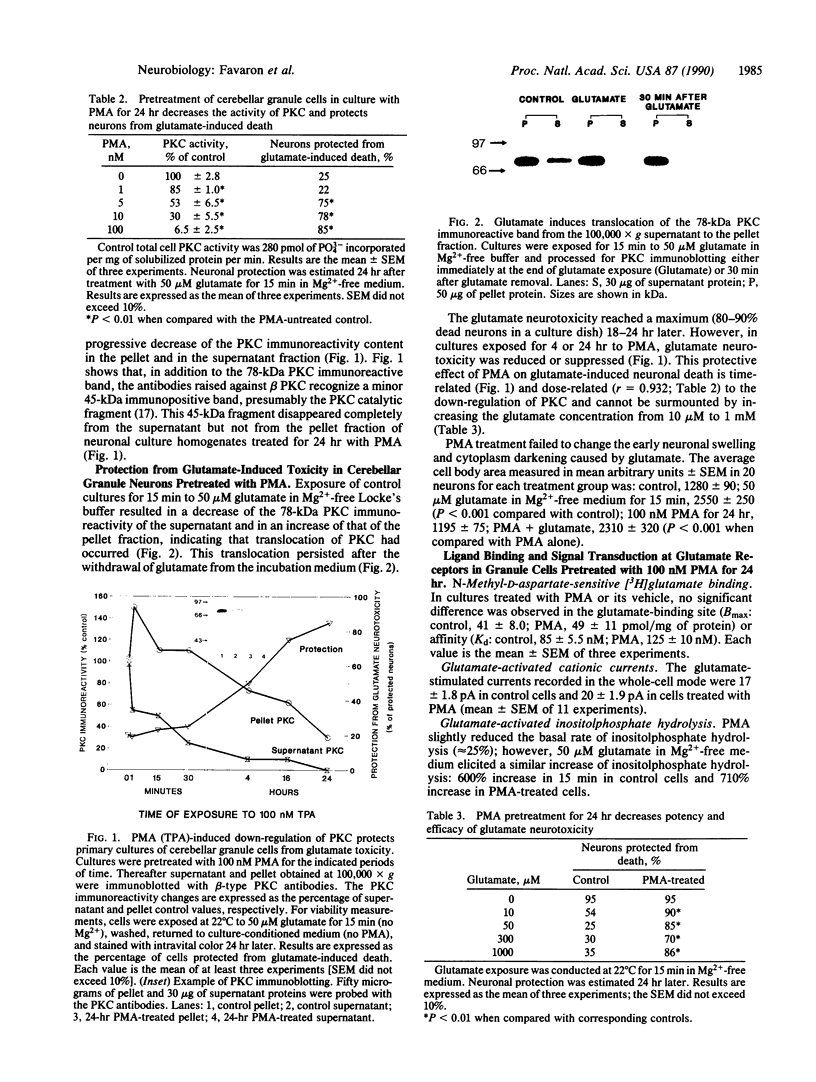
Exposing primary cultures of cerebellar granule neurons to 100 nM phorbol 12-myristate 13-acetate (PMA) for 24 hr decreases the Ca2+/phosphatidylserine/diolein-dependent protein kinase C (PKC; ATP:protein phosphotransferase, EC 2.7.1.37) by approximately 90% in the 100,000 x g supernatant and pellet fractions of neuronal culture homogenates. Immunoblot analysis of the homogenates with polyclonal antibodies raised against either the beta-type PKC peptide or total rat brain PKC reveals a virtual loss of 78-kDa PKC immunoreactivity in the supernatant and a marked decrease of PKC immunoreactivity in the pellet. Exposure of the cultures to 50 microM glutamate for 15 min (no Mg2+) induces the translocation of supernatant PKC immunoreactivity to the pellet. Such translocation persists after glutamate withdrawal and is followed by a progressive increase in neuronal death, which begins 2 hr later. Neuronal death approaches completion in about 24 hr. PMA-induced down-regulation of PKC decreases glutamate-elicited neurotoxicity. Yet, the culture exposure to 100 nM PMA fails to decrease the high-affinity binding of [3H]glutamate to neuronal membranes and does not reduce glutamate-induced activation of ionotropic or metabolotropic receptors (assayed as total membrane current measured in whole-cell voltage-clamped neurons, 45Ca2+ uptake in intact monolayers, inositolphospholipid hydrolysis, and transcriptional activation and translation of c-fos mRNA). Moreover, the immediate cell-body swelling and activation of spectrin proteolysis elicited by glutamate remain unchanged. On the other hand, PMA-induced PKC down-regulation reduces any increase in 45Ca2+ uptake or Ca2(+)-dependent proteolysis (measured as spectrin degradation) after glutamate withdrawal. These results support the view that PKC translocation is operative in glutamate-induced destabilization of cytosolic ionized Ca2+ homeostasis and neuronal death.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ase K., Berry N., Kikkawa U., Kishimoto A., Nishizuka Y. Differential down-regulation of protein kinase C subspecies in KM3 cells. FEBS Lett. 1988 Aug 29;236(2):396–400. doi: 10.1016/0014-5793(88)80064-4. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988 Oct;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Wadman W. J., Hockberger P. E., Wong R. K. Sustained dendritic gradients of Ca2+ induced by excitatory amino acids in CA1 hippocampal neurons. Science. 1988 Apr 29;240(4852):649–653. doi: 10.1126/science.2452481. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaron M., Manev H., Alho H., Bertolino M., Ferret B., Guidotti A., Costa E. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7351–7355. doi: 10.1073/pnas.85.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Cunha-Melo J. R., Beaven M. A., Huang K. P. Differential down-regulation of protein kinase C isozymes. J Biol Chem. 1989 Mar 5;264(7):4238–4243. [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Nakabayashi H., Young W. S., 3rd, Huang K. P. Immunocytochemical localization of protein kinase C isozymes in rat brain. J Neurosci. 1988 Dec;8(12):4734–4744. doi: 10.1523/JNEUROSCI.08-12-04734.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L. Immunochemical characterization of rat brain protein kinase C. J Biol Chem. 1986 Nov 5;261(31):14781–14787. [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manev H., Favaron M., Guidotti A., Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989 Jul;36(1):106–112. [PubMed] [Google Scholar]
- Manev H., Favaron M., Vicini S., Guidotti A., Costa E. Glutamate-induced neuronal death in primary cultures of cerebellar granule cells: protection by synthetic derivatives of endogenous sphingolipids. J Pharmacol Exp Ther. 1990 Jan;252(1):419–427. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Novelli A., Reilly J. A., Lysko P. G., Henneberry R. C. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988 Jun 7;451(1-2):205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A., Miyamoto M., Kudo Y. Neuronal death in vitro: parallelism between survivability of hippocampal neurones and sustained elevation of cytosolic Ca2+ after exposure to glutamate receptor agonist. Exp Brain Res. 1988;73(3):447–458. doi: 10.1007/BF00406601. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Patrone M., Michetti M., Horecker B. L. Activation of neutrophil calpain following its translocation to the plasma membrane induced by phorbol ester or fMet-Leu-Phe. Biochem Biophys Res Commun. 1989 Apr 28;160(2):737–743. doi: 10.1016/0006-291x(89)92495-9. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Takimoto M., Iadarola M., Holbrook N., Levens D. Distinct factors bind the AP-1 consensus sites in gibbon ape leukemia virus and simian virus 40 enhancers. J Virol. 1989 Apr;63(4):1737–1742. doi: 10.1128/jvi.63.4.1737-1742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B. L., Mehegan J. P., Jacobowitz D. M., Robey F., Iadarola M. J. Rat brain protein kinase C: purification, antibody production, and quantification in discrete regions of hippocampus. J Neurochem. 1989 Jan;52(1):215–221. doi: 10.1111/j.1471-4159.1989.tb10919.x. [DOI] [PubMed] [Google Scholar]
- Schramm M., Eimerl S., Costa E. Serum and depolarizing agents cause acute neurotoxicity in cultured cerebellar granule cells: role of the glutamate receptor responsive to N-methyl-D-aspartate. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1193–1197. doi: 10.1073/pnas.87.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R., Noszek J. C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988 Jun;1(4):279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Siman R., Noszek J. C., Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989 May;9(5):1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely A. M., Barbaccia M. L., Alho H., Costa E. In primary cultures of cerebellar granule cells the activation of N-methyl-D-aspartate-sensitive glutamate receptors induces c-fos mRNA expression. Mol Pharmacol. 1989 Apr;35(4):401–408. [PubMed] [Google Scholar]
- Vaccarino F., Guidotti A., Costa E. Ganglioside inhibition of glutamate-mediated protein kinase C translocation in primary cultures of cerebellar neurons. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8707–8711. doi: 10.1073/pnas.84.23.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke R., Levy R. Detection of T and B cell antigens hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J Histochem Cytochem. 1980 Aug;28(8):771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]
- Weiss S., Ellis J., Hendley D. D., Lenox R. H. Translocation and activation of protein kinase C in striatal neurons in primary culture: relationship to phorbol dibutyrate actions on the inositol phosphate generating system and neurotransmitter release. J Neurochem. 1989 Feb;52(2):530–536. doi: 10.1111/j.1471-4159.1989.tb09152.x. [DOI] [PubMed] [Google Scholar]
- Wroblewski J. T., Nicoletti F., Fadda E., Costa E. Phencyclidine is a negative allosteric modulator of signal transduction at two subclasses of excitatory amino acid receptors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5068–5072. doi: 10.1073/pnas.84.14.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]