Abstract
Lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1) is an endothelial receptor for oxidized LDL. Increased expression of LOX-1 has been demonstrated in atherosclerotic lesions and diabetic vasculopathy. In this study, we investigate the expression of LOX-1 receptor in sickle cell disease (SCD) vasculopathy. Expression of LOX-1 in brain vascular endothelium is markedly increased and LOX-1 gene expression is upregulated in cultured human brain microvascular endothelial cells by incubation with SCD erythrocytes. Also, the level of circulating soluble LOX-1 concentration is elevated in the plasma of SCD patients. Increased LOX-1 expression in endothelial cells is potentially involved in the pathogenesis of SCD vasculopathy. Soluble LOX-1 concentration in SCD may provide a novel biomarker for risk stratification of sickle cell vascular complications.
Keywords: Sickle cell disease, Vasculopathy, Lectin-like oxidized low-density lipoprotein receptor (LOX-1), Endothelium, Adhesion molecule
1. Introduction
Sickle cell disease (SCD) is associated with significant morbidity and mortality due, in large measure, to vascular injury and occlusion [1]. Apart from extensive hemolysis, SCD is characterized by recurrent vaso-occlusive episodes or “crises” manifested by severe bone pain, acute chest syndrome, priapism, and stroke, as well as chronic irreversible damage to the heart, brain, lungs, skin, kidneys, spleen and femoral heads [2]. Cumulative acute on chronic tissue injury results in significant morbidity and early mortality.
Inflammation and abnormal adhesion of sickle red blood cells (RBCs), leukocytes and platelets to the vascular endothelium are postulated to play a central role in the pathogenesis of vasculopathy associated with sickle cell disease (SCD) [3,4]. Endothelial cells in SCD display vasoconstrictive, proinflammatory and prothrombotic changes [1,5,6]. Sickle RBCs may initiate damage through activation of the endothelium mediated by cell surface adhesion molecules such as vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), platelet endothelial cellular adhesion molecule (PECAM-1; CD31), E-selectin, and P-selectin [7,8]. Levels of the soluble forms of these adhesion molecules are elevated in patients with SCD, especially during crisis [9], which may lead to cellular aggregates in the postcapillary venule and vaso-occlusive painful crisis [10].
Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) has been identified as a major receptor on endothelial cells for oxidized low-density lipoprotein (ox-LDL) as well as for the surface phosphatidylserine of senescent RBCs [11,12]. LOX-1 can bind and internalize ox-LDL [11], senescent RBCs [13], apoptotic cells [13], activated platelets [14] and neutrophils [15]. This, in turn, induces endothelial damage and promotes the atherogenic process [16,17]. Enhanced expression of LOX-1 is associated with and has been implicated in atherosclerosis and diabetic vascular disease [16,18–20]. High levels of soluble LOX-1 were observed in patients with acute stroke [21]. The pathogenesis of sickle cell vasculopathy shares some of the pathological features seen during the initiation of atherosclerosis. Based on our previous observations concerning endothelial dysfunction initiated through the LOX-1 receptor [16], we hypothesize that LOX-1 may be an important mediator in the pathophysiology of sickle cell vaso-occlusive events.
2. Material and methods
2.1. Patient recruitment and study design
The University of California Davis Institutional Review Board approved the study and a written informed consent was obtained from all patients or from their parents or guardians. Sickle cell anemia (HbSS) was confirmed by hemoglobin electrophoresis and high performance liquid chromatography (HPLC). The comparator group (HbAA) included healthy individuals with normal hemoglobin electrophoresis recruited at the same institution, matched for age, sex, and ethnicity. Pediatric (ages 6–18 y) and adult (ages 27–58 y) patients were recruited from the Sickle Cell Clinic at the University of California Davis Medical Center (UCDMC) [22]. SCD patients on chronic transfusion, those treated with hydroxyurea in the past two weeks, with a history of malignancy, diabetes mellitus, systemic arterial hypertension, other vascular or connective tissue disorders, and pregnancy were excluded from the study. Venous blood samples (10 ml) were collected in sodium citrate, and platelet-free plasma was immediately separated and stored frozen at −20 °C.
2.2. RBC binding assay
Human coronary endothelial cells (HCECs) were purchased from Clonetics. Human brain microvascular endothelial cells (HBMVECs —ACBRI 376) and culture media were purchased from Cell Systems. Cells were cryopreserved at second passage and cultured at <5 passages for all studies. Chinese hamster ovary (CHO) cells stably expressing human LOX-1 (hLOX-1-CHO) and wild-type CHO-K1 cells were maintained as described previously [13]. Sickle red blood cells (RBCs) from SCD patients and normal controls were collected, washed three times with PBS and re-suspended at 20% hematocrit in PBS containing 0.1% glucose. Some normal RBCs were heat-damaged by incubating them at 49.5 °C for 20 min. RBCs (adjusted to a hematocrit of 1%) were incubated with HCEC, CHO-K1 cells, or hLOX-1-CHO cells in culture media at 37 °C for 3 h. Unbound RBCs were removed by extensive gentle washing with media, and the cells were fixed with PBS containing 4% formaldehyde and stained with Giemsa [13].
2.3. Real-time quantitative RT-PCR assay for LOX-1 mRNA
Human brain microvascular endothelial cells (HBMVECs) were cultured with Complete Classic Medium Kit with Serum and Culture Boost (Cell System) to confluency. Cells were incubated with RBCs (isolated from SCD patients or normal controls) for 15 min to 18 h and washed 3 times with medium. RNA was isolated using the RNeasy kit (QIAGEN) according to the manufacturer’s instruction. cDNAs were prepared using QuantiTech Reverse Transcription Kit (QIAGEN). Quantitative real-time PCR to detect LOX-1 gene expression was performed using SYBR Green incorporation on Stratagene Mx3005 system using the following human LOX-1 primer pair: forward primer 5′-GCGACTCTAGGGGTCCTTTG-3′, reverse primer 5′-GTGAGTTAGGTTTGCTTGCTCT-3′; Quantitation of mRNA expression was performed by the comparative Ct method, and normalized to the human Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) gene using the following primer pair: forward primer 5′-CTGGGCTACACTGAGCACC-3′, and reverse primer 5′-AAGTGGTCGTTGAGGGCAATG-3′.
2.4. Immunofluorescent and immunohistochemistry staining
For immunofluorescent staining: cultured HBMVECs were incubated with RBCs (isolated from SCD patients or normal controls) for 6 h, washed 3 times with medium, fixed with 4% paraformaldehyde for 20 min at room temperature, blocked with 20% donkey serum for 30 min at RT, then incubated with primary antibodies mouse anti-human CD31(pre-diluted, Dako) and chicken anti-human LOX-1(10 μg/ml, HUC52) overnight at 4 °C, followed by incubation with secondary antibodies Cy3-donkey anti-mouse and FITC-donkey anti-chicken (1:500, Jackson ImmunoResearch Lab) for 1 h at RT. Nuclei were stained with DAPI and images were acquired using a Keyence microscope. Immunohistochemistry staining of LOX-1 was performed on formalin-fixed paraffin-embedded human tissues collected from an autopsy of a sickle cell disease patient who died in sickle cell crisis and a non-sickle cell disease control autopsy. The slides were immuno-stained after heat-induced antigen retrieval using rabbit polyclonal antibody to human LOX-1 (5 μg/ml, Novus Biologicals CO, USA) as previous described [18].
2.5. Soluble LOX-1 measurement
Plasma circulating soluble LOX-1 (sLOX-1) levels were measured using a commercially available enzyme-linked immunosorbent assay kit (Adipo Bioscience; Santa Clara, California) as described previously [12,23].
3. Results and discussion
3.1. Evidence of red blood cell binding to LOX-1 on endothelial cells in vitro
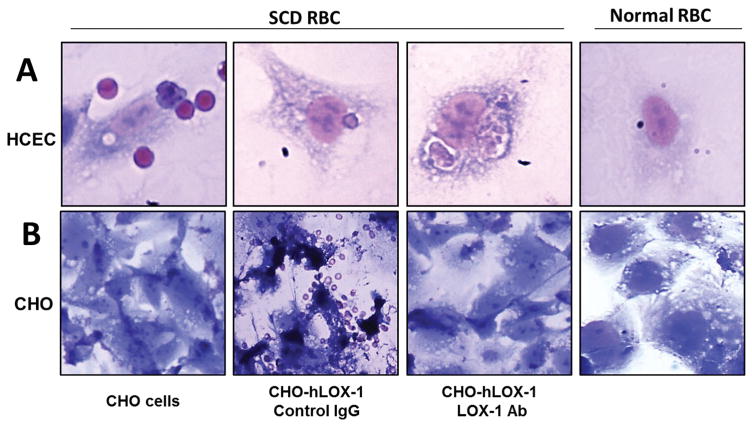
Adhesion and phagocytosis of sickle RBCs was noted in cultured human coronary endothelial cells (HCECs; Fig. 1A). Also, overexpression of LOX-1 in CHO cells resulted in binding SCD RBCs, which was blocked by LOX-1 antibody (Fig. 1B). Our findings indicate that sickle RBCs adhere to endothelial cells as well as to CHO cells engineered to express human LOX-1. Under in vivo conditions during sickle cell crisis, hypoxia results in ATP depletion and hemoglobin S polymerization, leading to red blood cell sickling and exposure of phosphatidylserine (PS) on the outer surface of the plasma membrane [10].
Fig. 1.
A: Binding, internalization and phagocytosis of sickle erythrocytes by cultured human coronary endothelial cells (HCECs). B: Overexpression of LOX-1 in CHO cells enables binding sickle erythrocyte which is blocked by LOX-1 antibody.
3.2. Evidence of LOX-1 gene expression in endothelial cells induced by sickle cells in vitro
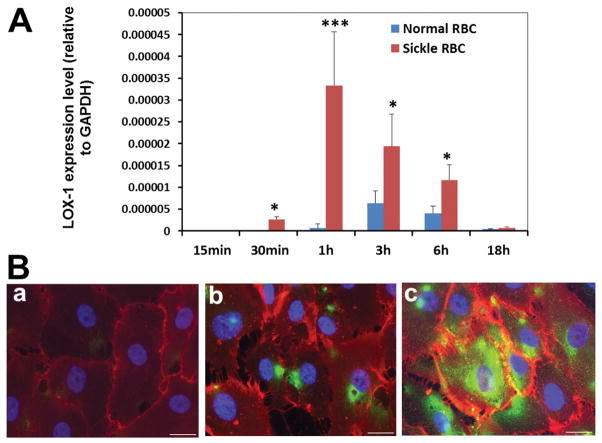
LOX-1 mRNA expression in human endothelial cells was significantly increased following incubation with sickle RBC compared with normal RBC (Fig. 2A). Expression of LOX-1 protein was also prominently up-regulated in endothelial cells incubated with sickle RBC (Fig. 2B). Expression of upregulated LOX-1 could be a marker for endothelial dysfunction.
Fig. 2.
Sickle red blood cells induce LOX-1 gene expression in human brain microvascular endothelial cells (HBMVECs). A: HBMVECs were incubated with sickle RBC or normal RBC for 15 min–18 h. The expression of LOX-1 mRNA was assessed using real-time PCR. Data are presented as the means ± standard deviation (SD), differences between sickle RBC and normal RBC incubation groups at each time point were analyzed using Student’s t-test (***P value < 0.001, *P value < 0.05. n = 3 in each group; representative results of three independent experiments). Other experiments were carried out using heat-damaged normal RBCs. Heat damaging control RBCs did not increase expression of LOX-1 in HBMVEC over non-damaged RBCs (data not shown). B: Immunofluorescent staining of cultured HBMVECs for LOX-1 (green) and endothelium marker CD31 (red) ; (a) non-treated HBMVECs, (b) HBMVECs treated with normal RBC for 6 h, (c) HBMVECs treated with SCD RBC for 6 h (scale bar = 20 μm).
3.3. Detection of soluble LOX-1 (sLOX-1) in SCD patient plasma
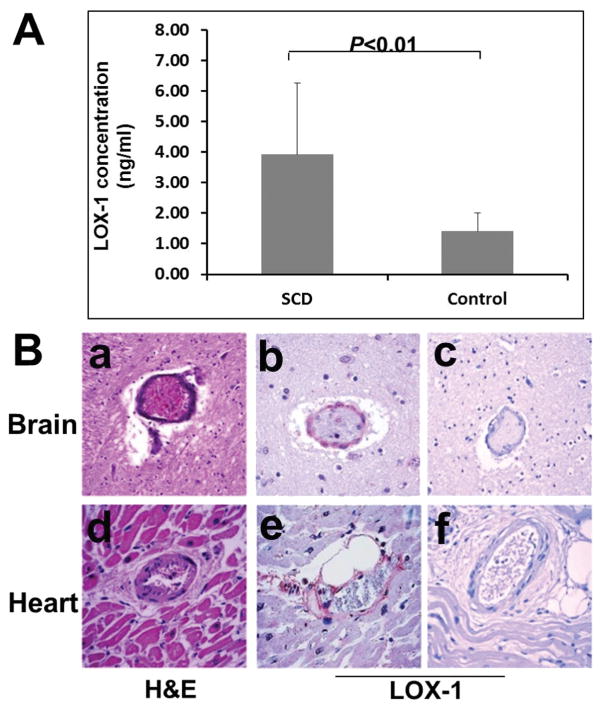
Plasma samples from 22 SCD patients and 9 healthy control subjects were assayed for sLOX-1. The concentration of circulating sLOX-1 protein in plasma of SCD patients (3.92 ± 2.35 ng/ml) was significantly higher (p = 0.0039) than in control healthy subjects (1.40 ± 0.60 ng/ml) (Fig. 3A). It is possible that the plasma concentration of sLOX-1 correlates with clinical status such as impending sickle crisis. Investigation of this question lay beyond the scope of the present study but will be explored in future studies, since it is possible that changes in circulating sLOX-1 levels may serve as a harbinger of impending sickle cell crisis.
Fig. 3.
In vivo expression of LOX-1 in SCD patients. A: Measurement of circulating soluble LOX-1 (sLOX-1) concentrations by sandwich ELISA assay in SCD patient plasma and normal control plasma. Data are presented as means ± SD, difference between SCD and normal control was analyzed using Student’s t-test (n = 22 in SCD group and n = 9 in control group). B: Increased expression of LOX-1 in brain vascular endothelial cells of SCD patient. (a) H&E stain of midbrain autopsy tissue from a SCD patient showed thrombus formation and occlusion of the vessels, (b) IHC staining of the same SCD patient’s brain for LOX-1 showed increased expression of LOX-1 in vascular endothelial cells, (c) the expression of LOX-1 protein is almost undetectable by immunostaining in the control non-SCD brain tissue of a patient who died from trauma.
3.4. Evidence of LOX-1 protein expression in endothelial cells in vivo
Autopsy findings of a SCD patient (40 year-old female) who died in painful vaso-occlusive crisis (VOC) revealed histological features of vaso-occlusive microthrombi formation. H&E stain of midbrain tissue showed thrombus formation and occlusion of vessels. Expression of LOX-1 in brain vascular endothelial cells was confirmed by immunohistochemical staining, whereas the expression of LOX-1 was not detected in normal control brain tissue (Fig. 3B). Similar results were obtained in SCD heart tissue (data not shown). Vaso-occlusive damage caused by blockage of the vessel associated with positive staining of LOX-1 on endothelial cells suggests a possible causative link between SCD vasculopathy and the increased expression of LOX-1. Control brain tissue was obtained from an autopsy of a patient (35 year-old female) who died from trauma.
Sickle cell disease (SCD) is a single gene disorder characterized by mutant hemoglobin-S, causing chronic intravascular hemolysis and vascular dysfunction [1]. Clinical features of SCD may range from life-threatening stroke, acute chest syndrome, and frequent vaso-occlusive episodes to long-term complications, such as avascular necrosis, leg ulcers, and retinopathy. Inflammation and abnormal adhesion of sickle red blood cells (RBCs), leukocytes and platelets to the vascular endothelium are postulated to play a central role in the pathogenesis of vasculopathy associated with SCD. Dysfunctional endothelial cells in the SCD vaso-occlusive process display vasoconstriction, pro-inflammatory and pro-thrombotic changes. Both the induction of endothelial adhesion molecule expression and subsequent endothelial adherence of sickle erythrocytes may play significant roles in the pathophysiology of SCD-related complications, and reduction in adhesion molecule expression may provide a new approach to treatment [5].
The LOX-1 scavenger receptor is now classified as a proatherogenic pro-inflammatory molecule that initiates multiple signaling cascades to activate expression of diverse genes that regulate endothelial cell activation, proliferation and apoptosis [12,16]. Vascular disease in SCD is fundamentally an inflammatory state that arises through a cascade of events from activation of the endothelium, adherence of blood cells, and reperfusion injury, leading ultimately to irreversible organ damage [9]. As an adhesion molecule expressed by endothelial cells, LOX-1 protein can serve as a tether that attaches sickle red blood cells to vascular endothelial cells. Through signal amplification and a cascade of ensuing intracellular events, engagement of LOX-1 could then lead to endothelial activation and a pro-inflammatory state which would result in increased expression of other adhesion molecules for sickle cells, platelets as well as white blood cells [16]. It is noteworthy that in our experiments using HBMVECs, expression of LOX-1 peaked after 1 h of incubation with SCD RBCs. A recent study reported increased expression of LOX-1 in cerebral vasculature after subarachnoid hemorrhage, which may play a role in the pathogenesis of vasospasm [24]. Given that primary hemorrhagic stroke is one of the most devastating neurologic complications of sickle cell disease [25], LOX-1 mediated vasospasm may also be a contributing factor in sickle cell disease vasculopathy leading to stroke. In addition, a recent study reported higher levels of sLOX-1 in patients with acute stroke compared with controls [26].
In the current study, we demonstrate that LOX-1 is an adhesion molecule for sickle RBCs and may therefore play a role in the pathogenesis of SCD vasculopathy. We also show that sickle RBCs can induce expression of LOX-1 in endothelial cells in vitro; Finally, LOX-1 expression is elevated in SCD vascular lesions and increased circulating levels of sLOX-1 may be indicative of SCD vasculopathy. Studies of sLOX-1 in SCD could provide new insights in sickle cell pathophysiology, and lead to novel therapeutic strategies for sickle cell patients with vascular complications.
Acknowledgments
This study was supported in part by the University of California Davis Health System Vision (FL16MCX) grant and Bayer Pharma AG Grants4Targets (2014-08-1145) (M. Chen).
Footnotes
Authorship contributions
M.C. and R.G. were involved in the study’s conception, design, data collection, analysis and interpretation, and writing of the manuscript. T.W. was also involved in aspects of the conception design, interpretation and supervision of the study. H.Q., X.L., D.N., H.A., L.O. and A.J. conducted the experiments, and collected and analyzed the data. H.Q. assisted in compiling the data for writing of the manuscript. T.S. provided the LOX-1 overexpression CHO stable cell line and LOX-1 antibodies. M.C. and R.G. provided overall supervision of the study. All authors reviewed the content and approved the submission of the manuscript.
Conflict of interest disclosure
The authors declare no competing financial interests.
References
- 1.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11(2):129–151. [PubMed] [Google Scholar]
- 2.Archer N, Galacteros F, Brugnara C. Clinical trials update in sickle cell anemia. Am J Hematol. 2015;90(10):934–950. doi: 10.1002/ajh.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wun T, et al. Activated monocytes and platelet-monocyte aggregates in patients with sickle cell disease. Clin Lab Haematol. 2002;24(2):81–88. doi: 10.1046/j.1365-2257.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 4.Owusu-Ansah A, et al. Inflammatory targets of therapy in sickle cell disease. Transl Res. 2016;167(1):281–297. doi: 10.1016/j.trsl.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebbel RP. Perspectives series: cell adhesion in vascular biology. Adhesive interactions of sickle erythrocytes with endothelium. J Clin Invest. 1997;99(11):2561–2564. doi: 10.1172/JCI119442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoppe CC. Inflammatory mediators of endothelial injury in sickle cell disease. Hematol Oncol Clin North Am. 2014;28(2):265–286. doi: 10.1016/j.hoc.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Kaul DK, Finnegan E, Barabino GA. Sickle red cell-endothelium interactions. Microcirculation. 2009;16(1):97–111. doi: 10.1080/10739680802279394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pakbaz Z, Wun T. Role of the hemostatic system on sickle cell disease pathophysiology and potential therapeutics. Hematol Oncol Clin North Am. 2014;28(2):355–374. doi: 10.1016/j.hoc.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato GJ, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130(6):943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L314–L324. doi: 10.1152/ajplung.00252.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawamura T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 12.Sawamura T, Wakabayashi I, Okamura T. LOX-1 in atherosclerotic disease. Clin Chim Acta. 2015;440:157–163. doi: 10.1016/j.cca.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Oka K, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95(16):9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakutani M, Masaki T, Sawamura T. A platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc Natl Acad Sci U S A. 2000;97(1):360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honjo M, et al. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci U S A. 2003;100(3):1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipo-protein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95(1):89–100. doi: 10.1016/s0163-7258(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 17.Stancel N, et al. Interplay between CRP, atherogenic LDL, and LOX-1 and its potential role in the pathogenesis of atherosclerosis. Clin Chem. 2016;62(2):320–327. doi: 10.1373/clinchem.2015.243923. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, et al. Increased expression of lectin-like oxidized low density lipoprotein receptor-1 in initial atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2000;20(4):1107–1115. doi: 10.1161/01.atv.20.4.1107. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, et al. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and AGE. Biochem Biophys Res Commun. 2001;287(4):962–968. doi: 10.1006/bbrc.2001.5674. [DOI] [PubMed] [Google Scholar]
- 20.Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediat Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokota C, et al. High levels of soluble lectin-like oxidized low-density lipoprotein receptor-1 in acute stroke: an age- and sex-matched cross-sectional study. J Atheroscler Thromb. 2016 doi: 10.5551/jat.32466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung AT, et al. Comparison of real-time microvascular abnormalities in pediatric and adult sickle cell anemia patients. Am J Hematol. 2010;85(11):899–901. doi: 10.1002/ajh.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue N, et al. LOX index, a novel predictive biochemical marker for coronary heart disease and stroke. Clin Chem. 2010;56(4):550–558. doi: 10.1373/clinchem.2009.140707. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda N, et al. Role of oxidized LDL and lectin-like oxidized LDL receptor-1 in cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 2014;121(3):621–630. doi: 10.3171/2014.5.JNS132140. [DOI] [PubMed] [Google Scholar]
- 25.Strouse JJ, et al. Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics. 2006;118(5):1916–1924. doi: 10.1542/peds.2006-1241. [DOI] [PubMed] [Google Scholar]
- 26.Yokota C, Sawamura T, Watanabe M, Kokubo Y, Fujita Y, Kakino A, Nakai M, Toyoda K, Miyamoto Y, Minematsu K. High levels of soluble lectin-like oxidized low-density lipoprotein receptor-1 in acute stroke: an age- and sex-matched cross-sectional study. J Atheroscler Thromb. 2016 Mar;29 doi: 10.5551/jat.32466. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]