Abstract
Objective:
To describe better the motor phenotype, molecular genetic features, and clinical course of GNAO1-related disease.
Methods:
We reviewed clinical information, video recordings, and neuroimaging of a newly identified cohort of 7 patients with de novo missense and splice site GNAO1 mutations, detected by next-generation sequencing techniques.
Results:
Patients first presented in early childhood (median age of presentation 10 months, range 0–48 months), with a wide range of clinical symptoms ranging from severe motor and cognitive impairment with marked choreoathetosis, self-injurious behavior, and epileptic encephalopathy to a milder phenotype, featuring moderate developmental delay associated with complex stereotypies, mainly facial dyskinesia and mild epilepsy. Hyperkinetic movements were often exacerbated by specific triggers, such as voluntary movement, intercurrent illnesses, emotion, and high ambient temperature, leading to hospital admissions. Most patients were resistant to drug intervention, although tetrabenazine was effective in partially controlling dyskinesia for 2/7 patients. Emergency deep brain stimulation (DBS) was life saving in 1 patient, resulting in immediate clinical benefit with complete cessation of violent hyperkinetic movements. Five patients had well-controlled epilepsy and 1 had drug-resistant seizures. Structural brain abnormalities, including mild cerebral atrophy and corpus callosum dysgenesis, were evident in 5 patients. One patient had a diffuse astrocytoma (WHO grade II), surgically removed at age 16.
Conclusions:
Our findings support the causative role of GNAO1 mutations in an expanded spectrum of early-onset epilepsy and movement disorders, frequently exacerbated by specific triggers and at times associated with self-injurious behavior. Tetrabenazine and DBS were the most useful treatments for dyskinesia.
De novo GNAO1 mutations (MIM 139311) were initially identified in children with early-onset epileptic encephalopathy (EOEE) and severe developmental delay,1–3 with later development of dyskinetic movement disorders1,4–6 and in children exhibiting developmental delay and severe dyskinesia without seizures.7,8
GNAO1 encodes a subclass (Gαo) of the Gα subunit of heterotrimeric guanine nucleotide-binding proteins and is highly expressed in the brain and involved in the regulation of neuronal excitability and neurotransmission. Mutations in GNAO1 and other G-protein subunits (GNAL), adenylyl cyclase (ADCY5), cyclic nucleotide phosphodiesterase (PDE10A), and G protein–coupled receptor (GPCR) (GPR88)9 in early-onset movement disorders, support the notion that disruption of the G-protein-cAMP pathway axis is a key contributor to the pathophysiology of dystonia and chorea. Although the pathophysiologic mechanisms underlying such genetic movement disorders remain yet to be elucidated, impaired modulation or transduction of transmembrane signaling, presynaptic autoinhibitory effects, and altered neuronal excitability are all putative disease mechanisms that might explain the co-occurrence of multiple neurologic manifestations such as epilepsy and hyperkinesia.
Here, we report 7 newly identified patients with GNAO1 mutations, with the aim of characterizing in detail the clinical phenotype, response to treatment and outcome, as well as associated brain MRI features.
METHODS
Molecular genetic testing.
Patients 1–3, 6, and 7 were studied using whole-exome/genome (WES/WGS) sequencing. For patient 1, WES was performed on a SOLiD 5500XL sequencing platform (Life Technologies, Foster City, CA). Short reads were mapped against the GRCh37/hg19 human assembly by means of LifeScope software (Life Technologies). Variants were detected using the Haplotype Caller software package of the Genome Analysis Toolkit (GATK) suite and filtered to include only variants covered by at least 10 reads with mapping quality values exceeding 30. For patients 2, 3, and 6, WGS was performed with Illumina TruSeq DNA PCR-Free Sample Preparation kit on an Illumina Hiseq 2500 sequencing platform (Illumina Inc., San Diego, CA). For patient 3, WGS was performed as part of the National Institute for Health Research Bioresource Rare Disease Project. A minimum coverage of 15X (95% of the genome) was obtained, with an average coverage of ∼30X. Reads were aligned using Isaac aligner (version 01.14) (Illumina Inc., Great Chesterford, UK) and mapped against the GRCh37 hg19 human assembly. Single nucleotide variants and insertions/deletions were identified using Isaac variant caller. Patients 4 and 5 were studied using a Haloplex panel (Agilent Technologies, Santa Clara, CA) of 95 epilepsy genes. Variants were called and annotated using the GATK10 toolkit and the ANNOVAR tool.11 Patient 7 was analyzed by WES using Nextera Rapid Capture Exome, Illumina NextSeq platform (Illumina Inc.) with an average coverage depth of 100–130X. An end-to-end in-house bioinformatics pipeline including base calling, primary filtering of low-quality reads and probable artefacts, and annotation of variants was applied.
For all patients, variants reported as validated polymorphisms with a minor allele frequency >0.01 (1%) in publicly available human variation resources (including dbSNP144, NHLBI GO Exome Sequencing Project, UK10, 1000 Genomes, Exome Variant Server [EVS], and Exome Aggregation Consortium [ExAC] browser) and variants present in in-house control individuals were filtered out. In silico prediction of the mutation pathogenicity was performed using ANNOVAR and the dbNSFP database (v3.0a) (sites.google.com/site/jpopgen/dbNSFP). Putative causative variants were validated by Sanger sequencing and investigated in the probands' parents to determine whether the mutations were inherited or had occurred de novo.
Standard protocol approvals, registrations, and patient consents.
Written informed consent to disclose clinical information, neuroimaging, and video footage was obtained from all parents/guardians of the 7 participants. Informed consent for genetic testing was obtained according to the local institutional policy.
RESULTS
Genetic analysis.
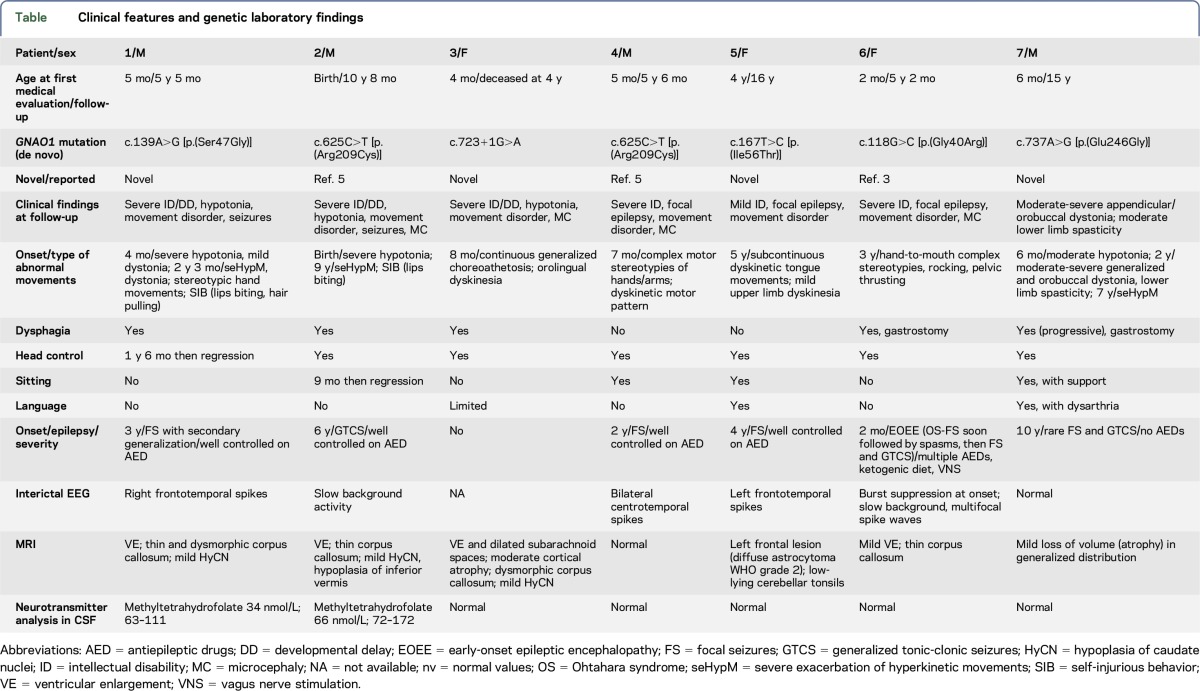
We identified 7 patients carrying 6 different GNAO1 (GenBank accession number NM_020988) mutations; all the variants occurred de novo and 4 are previously unreported (table, figure 1).
Table.
Clinical features and genetic laboratory findings
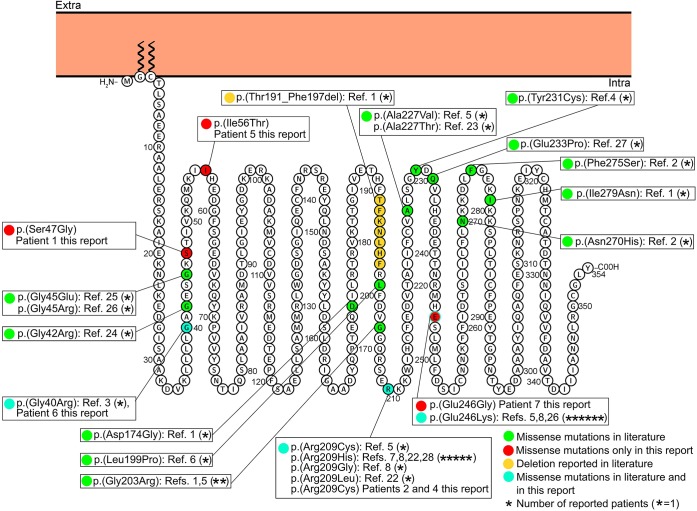
Figure 1. Membrane topology modeling of GNAO1.
Membrane topology was predicted using the Protter online tool29 (P09471, GNAO_HUMAN). Known GNAO1 missense mutations are indicated in green, the deletion is in yellow. Mutations found in this report and already described in the literature are indicated in light blue, novel mutations in red.
In patient 1, we detected the novel c.139A>G [p.(Ser47Gly)] missense variant, located in the N-terminal domain of the protein. In patients 2 and 4, we identified the c.625C>T [p.(Arg209Cys)] missense variant. This variant has previously been reported in 1 patient with developmental delay, intellectual disability, and severe chorea, associated with the later onset of complex partial seizures.5 In patient 3, we observed the novel c.723+1G>A intronic variant, and in patient 5, the novel c.167T>C [p.(Ile56Thr)] missense variant, located in the N-terminal domain of the protein. In patient 6, we detected the c.118G>C [p.(Gly40Arg)] missense mutation, located in the N-terminal domain of the protein. A different mutation leading to the same amino acid change (c.118G>A [p.(Gly40Arg)]) has previously been described in a patient with infantile-onset epilepsy.3 In patient 7, we identified the novel c.737A>G [p.(Glu246Gly)] missense variant. A different nucleotide change in the same amino acid position, c.736G>A [p.(Glu246Lys)], had been reported as disease causing in patients exhibiting developmental delay, intellectual disability, and a paroxysmal movement disorder.5,8
The 5 missense variants are predicted to be deleterious using the dbNSFP database and are not reported in the 1000 Genomes, ExAC, and EVS databases. The splicing mutation c.723+1G>A was predicted to cause the loss of a splice donor site using Mutation Taster and Berkeley Drosophila Genome Project.
Clinical features and neurologic investigations.
Clinical features, as obtained by history taking, direct clinical examination, and evaluation of video footage, neuroimaging, and genetic findings are detailed in the table, in figure 2 and in videos 1–5 at Neurology.org/ng. Within the cohort, we identified 4 boys and 3 girls. Six of the patients are alive, currently aged 5–16 years.
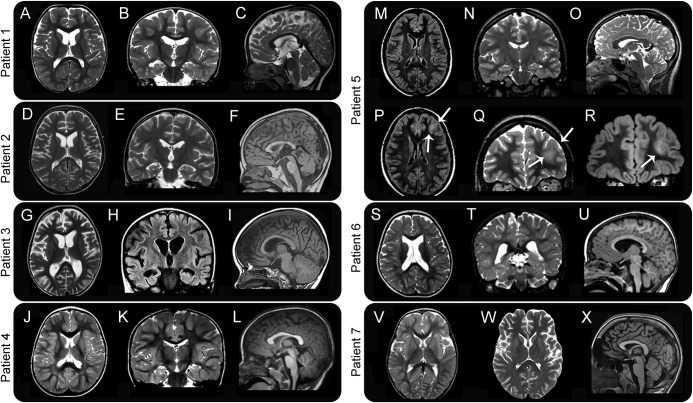
Figure 2. MRI characteristics of 7 patients with GNAO1 mutations.
For patients 1–6, a set of 3 images including an axial (A, D, G, J, M, and S), coronal (B, E, H, K, N, and T), and sagittal midline (C, F, I, L, O, and U) sequences is shown. For patient 5, in addition, 1 axial (P) and 2 coronal (Q and R) images are shown to illustrate better the neoplastic lesion in the left frontal lobe (arrows). For patient 7, axial images of 2 different investigations (V and W) and a sagittal image are shown to illustrate better the progression of atrophic changes. Images are at 1.5–3T. Images A–E, G, J, K, N, O, Q, S, T, and W are T2 weighted. Images F, I, L, U, and X are T1 weighted. Images H, M, P, and R are acquired as fluid attenuation inversion recovery (FLAIR) sequences. Patient 1: axial and coronal images show mild ventricular enlargement in the frontal horns, with concomitant hypoplasia of the caudate nuclei. The sagittal section shows a thin corpus callosum. Patient 2: axial and coronal images show mild ventricular enlargement in the frontal horns, with mild hypoplasia of the caudate nuclei, likewise seen in patient 1. The sagittal section shows a thin corpus callosum and a hypoplastic inferior vermis. Patient 3: axial and coronal images show moderate cortical atrophy, with ventricular enlargement and small caudate nuclei. The sagittal image shows a dysmorphic corpus callosum. Patient 4: no structural abnormality is visible on MRI. Patient 5: MRI shows an isolated abnormality (arrows) involving the anterolateral aspect of the left frontal lobe, including the cortex and underlying white matter, with increased signal intensity on both T2 and FLAIR images. Histopathology, after surgical removal of the lesion, showed characteristics consistent with a diffuse astrocytoma (WHO grade 2). Patient 6: axial and coronal images show dilated lateral ventricles; the sagittal cut shows a thin corpus callosum. Patient 7: the first axial MRI at age 2 (V) is normal, but a follow-up study at age 16 (W) shows loss of brain volume in generalized distribution. No abnormalities in the brainstem and cerebellum are visible in the sagittal image at age 16 (X).
The onset of abnormal hyperkinetic movements ranged from 4 months to 9 years of age (median 2 years). Within the cohort, there was considerable clinical heterogeneity with regard to motor semiology and severity. Profound hypotonia preceded the onset of abnormal movements in patients 1, 2, and 7, who later developed dystonia, followed by severe episodes of paroxysmal choreoathetosis. Gradual development of facial dyskinesia was also reported in these patients (videos 1, 2, and 5). Patient 3 experienced continuous generalized choreoathetosis and facial dyskinesia. At the age of 4, because of repeated and prolonged infective episodes, status dystonicus developed, requiring intensive care, with sedation, ventilation, tracheostomy, gastrostomy, and metabolic support. MRI showed progressive cerebral atrophy. Status dystonicus persisted, with rise in creatine kinase (CK) and renal failure, leading to multisystemic deterioration and death. Patient 4 exhibited complex motor stereotypies and a dyskinetic motor pattern (video 3). Patient 5 manifested almost continuous orolingual dyskinesia. She also had mild dyskinetic movements of the upper limbs (video 4). Patient 6 exhibited complex rocking, staring, back arching, and pelvic thrusting movements, associated with hand-to-mouth complex stereotypies.
Several triggers were reported to elicit or exacerbate abnormal movements, including emotion (all patients), intercurrent illnesses (patients 1, 2, and 7), high temperature (patient 1), intention, and purposeful movements (patients 1 and 7). Attacks frequently presented in clusters, lasting minutes (patient 1), hours or weeks (patient 2), or months (patient 7) (videos 1, 2, and 5). Such exacerbations were pharmacoresistant to standard therapies. Patients 1, 2, and 7 also exhibited associated autonomic instability, sweating, dehydration, and rise in CK, frequently requiring hospitalization and intensive care and, often, administration of anesthetic agents (patients 2 and 7). Worsening of baseline dystonia with hyperkinetic movements and loss of fine motor skills were reported in patient 7 after each episode. One such exacerbation determined a 3-month admission to the intensive care unit and anesthetic agents. The emergency placement of a deep brain stimulator (DBS) device bilaterally into the globus pallidus interna was transformative and resulted in almost complete remission of the pronounced hyperkinesia, although residual generalized dystonia persisted.
Six patients (1, 2, 4–7) exhibited epilepsy, with age of seizure onset ranging from 2 months to 10 years. While seizures were well controlled by medication in 4 patients, patient 7 presented rare generalized tonic-clonic seizures and focal dyscognitive seizures and patient 6 had a history of EOEE followed by drug-resistant focal epilepsy with dyscognitive seizures. Patient 3 never had seizures and died at age 4 years.
Additional clinical features were also reported in some patients. Patients 1 and 2 exhibited mild self-injurious behavior (lip biting, hair pulling). Patient 7 has a history of significant anxiety, requiring treatment with fluoxetine, sertraline, and risperidone.
In our cohort, diagnostic metabolic investigations were unremarkable, including CSF neurotransmitters, except for the reduction of CSF 5-methyltetrahydrofolate levels in patients 1 and 2. In both patients, calcium folinate administration was started but did not lead to discernible clinical improvement. Brain MRI (figure 2) was unremarkable in patients 4 and 7, while inconsistent changes were detected in the remaining patients (microcephaly, macrocephaly, progressive cerebral atrophy with dilated ventricles and subarachnoid spaces, caudate volume loss, and dysmorphic corpus callosum). In 1 patient (patient 5), a focal lesion involving the gray and white matter of the left frontal lobe was identified and confirmed as a diffuse astrocytoma (WHO grade 2) after surgical removal.
DISCUSSION
Through its activation by GPCRs, Gαo is involved as a modulator or transducer in several transmembrane signaling pathways.10,12,13 Gαo also mediates the widespread presynaptic autoinhibitory effect of many neurotransmitters (via α2 adrenoreceptors, M2/M4 muscarinic, μ/δ opioid, GABAB, adenosine A1, or endocannabinoid CB1 receptors) through a reduction of sensitivity to membrane depolarization and a direct inhibitory effect on the vesicle fusion process.14 Furthermore, Gαo indirectly activates G protein–coupled inwardly rectifying K+ channels that mediate neuronal excitability through a slower self-inhibitory postsynaptic potential.15
Gαo-deficient mice (Gnao1−/−) have occasional seizures, severe impairment of motor-control, hyperalgesia, and behavioral abnormalities with early postnatal lethality.16,17 Although heterozygous Gnao1 knockout mice do not manifest seizures, a gain-of-function knock-in mutant murine model (Gnao1 Gly184Ser/+) has severe seizures with markedly increased frequency of interictal epileptiform discharges and sudden premature death. Animal model data thus suggest that pathogenic monoallelic GNAO1 mutations in humans may also result in a gain-of-function effect. Moreover, impaired protein localization and decreased GNAO1-mediated inhibition of calcium currents by norepinephrine compared to the wild type have been showed in in vitro functional expression systems.18 GNAO1 has also been implicated in the etiology of brain tumors, such as ependymoma and glioblastoma multiforme.19,20
De novo GNAO1 mutations were originally first reported in Ohtahara syndrome and EOEE in 4 children, associated with abnormal movements in 2.1 Since this initial description, 30 additional patients have been reported with an emerging phenotype characterized by neurodevelopmental delay with an early onset of a hyperkinetic movement disorder, inconsistently associated with epilepsy. The epilepsy phenotype of the 7 patients we studied ranged from EOEE to mild drug-sensitive epilepsy, whose onset could precede or follow that of the movement disorder, which was certainly a prominent feature. The occurrence of stereotypies (previously reported in 2 patients with EOEE)4,5 and characteristic paroxysmal exacerbations, associated often with clear triggers, may both be considered as 2 further important discerning clinical features of GNAO1 encephalopathy.
As previously reported, standard investigations appear to be unyielding in patients with GNAO1-related disease. Two of our patients had low levels of CSF 5-methyltetrahydrofolate, although this was not consistently seen in the cohort. Brain MRI (figure 2) showed a combination of minor features in most, including a thin corpus callosum with dilated ventricles in 4, as previously reported8 as well as hypoplastic caudate nuclei in 3 and a diffuse astrocytoma (WHO grade II) in the oldest patient (patient 5). The latter finding raises concerns about the possible role of the Gly40Arg mutation in tumorigenesis, in view of the reported role of GNAO1 in promoting oncogenic transformation.19,21 A somatic GNAO1 mutation (p.Arg243Hys) has been identified in breast carcinomas where it promotes oncogenic transformation by rendering the Gα subunit constitutively activated and enhancing signaling pathways responsible for neoplastic transformation.21 Although GNAO1 was identified as part of the human plasma proteome, its high abundance was suggested to promote cancer cell viability via proapoptotic protein interference.20 Larger series and longer follow-up data will be fundamental to determine whether patients carrying specific GNAO1 mutations are at higher risk of developing tumors.
Twenty GNAO1 mutations, 19 missense and 1 deletion, have been previously reported (figure 1).1–8,22–28 The only reported deletion was described in a patient with a severe phenotype, including Ohtahara syndrome, developmental delay, and severe intellectual disability, who died secondary to respiratory-tract complications.1 The 7 patients we are reporting harbored 6 different mutations, one of which was evident in 2 patients (figure 1). Two of the 6 mutations have been reported previously [p.(Arg209Cys) and p.(Gly40Arg)],3,5 while 4 are novel [p.(Ser47Gly), c.723+1G>A, p.(Ile56Thr), and p.(Glu246Gly)]. The c.723+1G>A splicing mutation, likely resulting in abnormal mRNA splicing, was found in patient 3, who died at 4 years of age and exhibited the most severe phenotype. The p.(Arg209Cys) mutation falls within the switch II domain, which is important for guanidine nucleotide-dependent regulation of downstream effectors and is highly conserved across vertebrate species.5 The Arg209 amino acidic residue represents a mutation hotspot since, including our series, 10 patients carrying mutations affecting this residue have been reported. All these patients presented developmental delay and chorea or dystonia and 3 had seizures. The p.(Glu246Gly) mutation, although novel, affects an amino acid residue that represents a second mutation hotspot since 7 patients with mutations affecting this residue have been reported (including our own), all presenting with developmental delay and chorea, with only patient 7 developing late childhood–onset seizures. The Arg209 and Glu246 residues form a salt bridge that is important for the stabilization of the Gα-containing complexes, mainly in GTP-bound active state.5 Hence, the variants involving these residues should disrupt their interaction, resulting in destabilization of the Gα-containing complexes.5 In our study, the identification of 2 additional patients harboring mutations involving the Arg209 amino acid residue and 1 additional patient harboring a mutation involving the Glu246 amino acid residue confirm that these residues are GNAO1 mutation hotspots.
Tetrabenazine was the most effective drug in the baseline management of the severe involuntary movements in patients 1 and 7; its positive effect was previously described in 6 patients8 treated in association with neuroleptics (risperidone and haloperidol). In addition, a recently reported patient harboring the c.626G>A (p.Arg209His) exhibited an initial significant improvement with tetrabenazine and subsequent response to trihexyphenidyl.28 Emergency DBS, during a severe prolonged exacerbation, was life saving in patient 7. Three patients with GNAO1 mutations had previously been reported whose hyperkinetic exacerbations, usually preceded by illness, clearly improved after DBS insertion into the globus pallidus.7,27 Our report provides further evidence that DBS should be promptly considered in all the patients with sustained intractable movement disorder due to GNAO1 mutations.
Long-term outcome of early-onset GNAO1-related disease remains yet to be determined. We would suggest that prognosis of these patients should remain guarded, as 3 previously reported patients1,8 and 1 in this series have died in childhood. Furthermore, 2 reported patients have experienced definite motor regression in the early stages of disease.7,8 Identification of more patients will certainly aid delineating this newly identified disorder. Given the ever-growing disease spectrum, GNAO1 mutations should be considered in the differential diagnosis for patients with unexplained paroxysmal/nonparoxysmal early-onset hyperkinetic movement disorders, especially in the context of neurodevelopmental delay with or without epilepsy.
Supplementary Material
GLOSSARY
- CK
creatine kinase
- DBS
deep brain stimulation
- EOEE
early-onset epileptic encephalopathy
- EVS
Exome Variant Server
- ExAC
Exome Aggregation Consortium
- GATK
Genome Analysis Toolkit
- GPCR
G protein–coupled receptor
- WES
whole-exome sequencing
- WGS
whole-genome sequencing
Footnotes
Supplemental data at Neurology.org/ng
AUTHOR CONTRIBUTIONS
Study concept and design: R. Guerrini and V. Leuzzi. Patient collection: F.R. Danti, S. Galosi, M. Montomoli, V. Leuzzi, M.A. Kurian, R. Guerrini, N. Mahant, A. McTague, T. McShane, S.S. Mohammad, J. Ng, D.C. Russell, R. Samanta, U. Shah, and G. Vadlamani. Mutation screening and data analysis: F.R. Danti, M. Romani, E. Parrini, C. Bianchini, E.M. Valente, R. Guerrini, M.A. Kurian, A. McTague, K.J. Carss, and F.L. Raymond, NIHR Bioresource Rare Diseases Consortium. Drafting of the manuscript: F.R. Danti, M.A. Kurian, and R. Guerrini. Critical revision of the manuscript for important intellectual content: M.A. Kurian, V. Leuzzi, E.M. Valente, and R. Guerrini. Obtained funding: F.L. Raymond, M.A. Kurian, and R. Guerrini.
STUDY FUNDING
This work was partly supported by the European Research Council Starting Grant 260888 (to E.M.V.), the EU seventh Framework Programme (FP7) under the project DESIRE grant N602531 (to R.G.), and by The National Institute for Health Research England (NIHR) for the NIHR BioResource–Rare Diseases project (grant RG65966) (to F.L.R.). M.A.K. is funded by a Wellcome Intermediate Clinical Fellowship (WT098524MA) and receives funding from Rosetrees Trust and Great Ormond Street Hospital Children's Charity.
DISCLOSURE
Dr. Danti, Dr. Galosi, Dr. Romani, Dr. Montomoli, Dr. Carss, Dr. Raymond, Dr. Parrini, Ms. Bianchini, and Dr. McShane report no disclosures. Dr. Dale has served on a scientific advisory board for Queensland Children's Medical Institute Research; has received speaker honoraria from Biogen Idec and Bristol-Myers Squibb; has served on the editorial boards of MSARD, Neurology® Neuroimmunology & Neuroinflammation; and the European Journal of Paediatric Neurology; receives publishing royalties from Biogen Idec (honoraria in 2008) and Bristol-Myers Squibb (in 2015); and has received research support from NHMRC and Multiple Sclerosis Research Australia. Dr. Mohammad has received travel funding from the Movement Disorders Society and has received research support from NHMRC. Dr. Shah and Dr. Mahant report no disclosures. Ms. Ng has received research support from MRC and Great Ormond Street Hospital Children's Charity Rosetrees Trust. Ms. McTeague has received research support from MRC. Dr. Samanta and Dr. Vadlamani report no disclosures. Dr. Valente has received a speaker honorarium from Teva; has served on the editorial board of Pediatric Research; and has received research support from the Italian Ministry of Health, European Community, the European Research Council, the Italian Ministry of University and Research, and Telethon Foundation Italy. Dr. Leuzzi reports no disclosures. Dr. Kurian has received speaker honoraria for 2 Recordati courses. Dr. Guerrini has received travel funding and honoraria for Advisory Board activities from Eisai Inc, Novartis, and Zogenix; has received travel funding from UCB; has served on the editorial boards of Epilepsia, Progress in Epileptic Disorders, Neuropediatrics, the Journal of Child Neurology, Seizure, BMC Medical Genetics, Topics in Epilepsy, the Journal of Pediatric Epilepsy, Epileptic Disorders, the European Neurological Journal, Neurology®, and the Journal of Embryology & Developmental Biology; receives publishing royalties from Cambridge University Press, Lippincott Williams & Wilkins, John Libbey Eurotext, and Oxford University Press; and has received research support from the European Union, Tuscany Region Research Department, EC, Italian Ministry of Health and Tuscany Region, and the Pisa Foundation. Go to Neurology.org/ng for full disclosure forms.
REFERENCES
- 1.Nakamura K, Kodera H, Akita T, et al. . De Novo mutations in GNAO1, encoding a Galphao subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am J Hum Genet 2013;93:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EuroEPINOMICS-RES Consortium, Epilepsy Phenome/Genome Project, Epi4K Consortium. De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet 2014;95:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law CY, Chang STL, Cho SY, et al. . Clinical whole-exome sequencing reveals a novel missense pathogenic variant of GNAO1 in a patient with infantile-onset epilepsy. Clin Chim Acta 2015;451:292–296. [DOI] [PubMed] [Google Scholar]
- 4.Talvik I, Moller RS, Vaher M, et al. . Clinical phenotype of de novo GNAO1 mutation: case report and review of literature. Child Neurol Open 2015;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saitsu H, Fukai R, Ben-Zeev B, et al. . Phenotypic spectrum of GNAO1 variants: epileptic encephalopathy to involuntary movements with severe developmental delay. Eur J Hum Genet 2016;24:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcé-Grau A, Dalton J, López-Pisón J, et al. . GNAO1 encephalopathy: further delineation of a severe neurodevelopmental syndrome affecting females. Orphanet J Rare Dis 2016;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni N, Tang S, Bhardwaj R, Bernes S, Grebe TA. Progressive movement disorder in brothers carrying a GNAO1 mutation responsive to deep brain stimulation. J Child Neurol 2016;31:211–214. [DOI] [PubMed] [Google Scholar]
- 8.Ananth AL, Robichaux-Viehoever A, Kim YM, et al. . Clinical course of six children with GNAO1 mutations causing a severe and distinctive movement disorder. Pediatr Neurol 2016;59:81–84. [DOI] [PubMed] [Google Scholar]
- 9.Alkufri F, Shaag A, Abu-Libdeh B, Elpeleg O. Deleterious mutation in GPR88 is associated with chorea, speech delay, and learning disabilities. Neurol Genet 2016;2:e64 doi: 10.1212/NXG.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holz GG, Rane SG, Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature 1986;319:670–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Wang K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat Protoc 2015;10:1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature 2009;459:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straiker AJ, Borden CR, Sullivan JM. G-protein alpha subunit isoforms couple differentially to receptors that mediate presynaptic inhibition at rat hippocampal synapses. J Neurosci 2002;22:2460–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamid E, Church E, Wells CA, Zurawski Z, Hamm HE, Alford S. Modulation of neurotransmission by GPCRs is dependent upon the microarchitecture of the primed vesicle complex. J Neurosci 2014;34:260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci USA 2009;106:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valenzuela D, Han X, Mende U, et al. . G alpha(o) is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proc Natl Acad Sci USA 1997;94:1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang M, Gold MS, Boulay G, et al. . Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci USA 1998;95:3269–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehrl JM, Sahaya K, Dalton HM, et al. . Gain-of-function mutation in Gnao1: a murine model of epileptiform encephalopathy (EIEE17)? Mamm Genome 2014;25:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Ramírez M, Hernández-Jiménez AJ, Guerrero-Guerrero A, et al. . Genomics and epigenetics: a study of ependymomas in pediatric patients. Clin Neurol Neurosurg 2016;144:53–58. [DOI] [PubMed] [Google Scholar]
- 20.Zupancic K, Blejec A, Herman A, et al. . Identification of plasma biomarker candidates in glioblastoma using an antibody-array-based proteomic approach. Radiol Oncol 2014;48:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Marcos M, Ghosh P, Farquhar MG. Molecular basis of a novel oncogenic mutation in GNAO1. Oncogene 2011;30:2691–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menke LA, Engelen M, Alders M, Odekerken VJ, Baas F, Cobben JM. Recurrent GNAO1 mutations associated with developmental delay and a movement disorder. J Child Neurol 2016; 31:1598–1601. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Cai T, Jiang Y, et al. . Genes with de novo mutations are shared by four neuropsychiatric disorders discovered from NPdenovo database. Mol Psychiatry 2016;21:298. [DOI] [PubMed] [Google Scholar]
- 24.Zhu X, Petrovski S, Xie P, et al. . Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. Genet Med 2015;17:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gawlinski P, Posmyk R, Gambin T, et al. . PEHO syndrome may represent phenotypic expansion at the severe end of the early-onset encephalopathies. Pediatr Neurol 2016;60:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helbig KL, Farwell Hagman KD, Shinde DN, et al. . Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med 2016;18:898–905. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz S, Turhan T, Ceylaner S, Gökben S, Tekgul H, Serdaroglu G. Excellent response to deep brain stimulation in a young girl with GNAO1-related progressive choreoathetosis. Childs Nerv Syst 2016;32:1567–1568. [DOI] [PubMed] [Google Scholar]
- 28.Dhamija R, Mink JW, Shah BB, Goodkin HP. GNAO1-associated movement disorder. Mov Disord Clin Pract 2016;3:615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014;30:884–886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.