Abstract
Background
There are few established risk factors for gallbladder cancer beyond gallstones. Recent studies suggest a higher risk with high body mass index (BMI), an indicator of general heaviness, but evidence from other body size measures is lacking.
Methods
Associations of adult BMI, young adult BMI, height, adult weight gain, waist circumference (WC), waist-height ratio (WHtR), hip circumference (HC), and waist-hip ratio (WHR) with gallbladder cancer risk were evaluated. Individual-level data from 1,878,801 participants in 19 prospective cohort studies (14 studies had circumference measures) were harmonized and included in this analysis. Multivariable Cox proportional hazards regression estimated hazard ratios (HR) and 95% confidence intervals (CI).
Results
After enrollment, 567 gallbladder cancer cases were identified during 20.1 million person-years of observation, including 361 cases with WC measures. Higher adult BMI (per 5 kg/m2, HR: 1.24; 95%CI: 1.13–1.35), young adult BMI (per 5 kg/m2, HR: 1.12; 95%CI: 1.00–1.26), adult weight gain (per 5 kg, HR: 1.07; 95%CI: 1.02–1.12), height (per 5cm, HR: 1.10; 95%CI: 1.03–1.17), WC (per 5cm, HR: 1.09; 95%CI: 1.02–1.17), WHtR (per 0.1 unit, HR: 1.24; 95%CI: 1.00–1.54), and HC (per 5cm, HR: 1.13; 95%CI: 1.04–1.22), but not WHR (per 0.1 unit, HR: 1.03; 95%CI: 0.87–1.22), were associated with higher risks of gallbladder cancer and results did not differ meaningfully by sex or other demographic/lifestyle factors.
Conclusions
These findings indicate that measures of overall and central excess body weight are associated with higher gallbladder cancer risks.
Impact
Excess body weight is an important, and potentially preventable, gallbladder cancer risk factor.
Introduction
Gallbladder cancer etiology is poorly understood with only a few, mostly non-modifiable, established risk factors, including older age, female sex, abnormal pancreatic-biliary junction, and history of cholesterol gallstones (1). Identifying modifiable risk factors for gallbladder cancer is hindered by its rarity and poor prognosis. In more-developed areas, such as the United States, Australia and Western Europe, incidence rates are 1-to-2 cases per 100,000 persons each year whereas in certain high risk populations, such as Mapuche Indians in South America, incidence rates exceed 20/100,000 (2). Overall 5-year relative survival is approximately 18% for US adults diagnosed with gallbladder cancer and the overall median survival time is 3–7 months (3). The poor prognosis is due, in part, to the lack of specific symptoms for the disease. Early-staged gallbladder cancers are uncommon and are typically only detected incidentally during cholecystectomy for gallstones but only 1–3% of patients with gallstones will ever develop gallbladder cancer (4).
Because excess body weight is a risk factor for gallstones and several other digestive system cancers (e.g., colorectum, liver and pancreas) (5–9), it is a plausible risk factor for gallbladder cancer. The 2015 World Cancer Research Fund’s Continuous Update Project (CUP) on gallbladder cancer concluded that body fatness, as defined by high body mass index (BMI), is a ‘probable’ risk factor for gallbladder cancer (10). The CUP identified eight prospective cohort studies (11–18) that contributed to dose-response meta-analyses and reported that each 5 kg/m2 increase in BMI was associated with a 25% higher risk of gallbladder cancer. Of those eight studies, four provided relative risks (RR) for BMI that were not statistically significant (11, 12, 14, 15) and two included biliary system cancer mortality as the main outcome (14, 18). Waist circumference, an indicator of central adiposity that might be more etiologically-relevant to cancers of the digestive system, has been evaluated by only one relatively small study (76 cases) that reported higher risks with increasing waist circumference (11).
Because the evidence-base for overall body fatness (based on BMI) and gallbladder cancer risk is considered probable and not convincing, and because risk estimates for indicators of central adiposity and other non-BMI measures of body size are especially rare, we conducted a pooled analysis of data from 19 prospective cohort studies based in the U.S., Europe, Australia, and Asia to investigate associations of BMI (at enrollment during adulthood and recalled from young-adulthood), height, adult weight gain, waist circumference, waist-height ratio, hip circumference, and waist-hip ratio with gallbladder cancer risk.
Materials and Methods
Study Population
All member studies of the NCI Cohort Consortium (http://epi.grants.cancer.gov/Consortia/cohort.html) with body size data were invited to participate and 19 prospective cohort studies were included in this analysis: Physicians’ Health Study (PHS); NIH-AARP Diet and Health Study (NIH-AARP); Agricultural Health Study (AHS); Breast Cancer Detection Demonstration Project Follow-Up Study (BCDDP); Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO); Women’s Health Study (WHS); New York University Women’s Health Study (NYUWHS); Cancer Prevention Study-II Nutrition Cohort (CPS-II); Iowa Women’s Health Study (IWHS); California Teachers’ Study (CTS); European Prospective Investigation into Cancer and Nutrition (EPIC); Melbourne Collaborative Cohort Study (MCCS); Cohort of Swedish Men (COSM); Swedish Mammography Cohort (SMC); The Sister Study (SISTER); Shanghai Men’s Health Study (SMHS); Shanghai Women’s Health Study (SWHS); Vitamins and Lifestyle Study (VITAL); and Women’s Lifestyle and Health Study (WLH). Participants gave written, informed consent at enrollment or consent was implied from the return of questionnaires. All studies were approved by the institutional review boards of their host centers.
All studies submitted de-identified, participant-level data from their entire cohort study to the data coordinating center. Data were centrally harmonized and pooled for analyses. Prior to exclusions, participant-level data were provided for 2,213,174 men and women. The following exclusions were applied: missing age at study entry, or baseline age less than 18 years, or older than 85 years (n=5,501); less than 1 year of follow-up time (n=51,399); missing BMI (n=147.552); BMI less than 15 kg/m2 or greater than 60 kg/m2 (n=2,110); missing height (n=26,698); height less than 122 cm or greater than 244 cm (n=137); and prevalent cancer at baseline (n=100,976). Data from 1,878,801 participants comprised the analytic cohort.
Gallbladder cancer diagnoses (International Classification of Diseases, 10th version (ICD-10): C23.9(19)) were verified after enrollment by linking to state/provincial/federal cancer or death registries and/or medical record abstraction.
Exposures
Height and weight were self-reported in most cohorts and directly measured in others (MCCS, SMHS, SWHS, EPIC, SISTER); BMI was calculated as weight (kg) divided by height-squared (m2) and categorized according to World Health Organization criteria (20): underweight (15<18.5 kg/m2), normal weight (18.5<25 kg/m2), overweight (25<30 kg/m2), and obese (≥30 kg/m2). Obesity was additionally stratified as classes I (30–34.9 kg/m2), II (35–39.9 kg/m2), and III (≥40 kg/m2). Young-adult BMI was available from 10 of the cohort studies (NIH-AARP, AHS, COSM, CPS-II, IWHS, MCCS, PLCO, SMC, VITAL, and WLH), derived from recalled weight at ages 18–21 years, and categorized as above for adult BMI. Height, in centimeters (cm), was categorized into four groups for women (<160, 160<165, 165<170 and ≥ 170) and men (<170, 170<175, 175<180, and ≥180). Adult weight gain was estimated by subtracting young adult weight from baseline weight, both in kg, and categorized as: any weight loss, weight stable (0 kg change) or weight gain of ≤ 5, weight gain of 6–10, weight gain of 11–15, weight gain of 16 to 20, and weight gain of ≥21.
Waist circumference and hip circumference were measured by trained staff (EPIC, MCCS, NYUWHS, SISTER, SMHS, SWHS) or self-measured by participants who were given instructions on the protocol (NIH-AARP, BCDDP, COSM, CTS, IWHS, CPS-II (waist circumference only), WLH, and SMC). The remaining five cohort studies did not collect waist circumference or hip circumference data. Waist circumference and hip circumference were available at baseline enrollment for COSM, IWHS, MCCS, SISTER, SMC, SMHS, SWHS, and WLH whereas NIH-AARP, BCDDP, CPS-II (waist circumference only), CTS, EPIC, and NYUWHS collected these data 1 to 8 years after baseline. Participants with waist or hip circumference measures below 50cm or above 190cm were excluded from the relevant analysis (n=1329 and n=345 were excluded from waist and hip circumference analyses, respectively). Waist circumference, in cm, was categorized in four pre-defined groups (women: 50–<70, 70–<80, 80–<90, and 90–<191; men: 50–<90, 90–<100, 100–<110, and 110–<191). Hip circumference, in cm, was also categorized in four pre-defined groups (women: 50–<90, 90–<100, 100–<110, and 110–<191; men: 50–<95, 95–<105, 105–<115, and 115–<191). Waist-height ratio was calculated by dividing waist by height, both in cm, and categorized as <0.45, 0.45–<0.50, 0.50–<0.55, and ≥0.55 for women and <0.50, 0.50–<0.55, 0.55–<0.60, and ≥0.60 for men. Waist-hip ratio was calculated by dividing waist circumference by hip circumference, both in cm, and categorized into four groups for women (<0.75, 0.75–<0.80, 0.80–<0.85, and ≥0.85) and men (<0.90, 0.90–<0.95, 0.95–<1.00, and ≥1.00).
Smoking was defined according to baseline cigarette smoking status and categorized as never, former, current or missing. Alcohol consumption was defined as non-drinker and, among persons who consumed alcohol, in categories of grams per day (grams/day: <10, 10–<20, 20–<30, and 30+), or missing. Race was self-identified and categorized as white, black/African American, and all other races including those who did not report race. Physical activity was categorized into study-specific quintiles or missing. Education was categorized as less than high school, high school graduate, some college, college graduate or more, or missing. Sex (men, women) and history of gallstones (yes, no) were defined as binary variables. Missing data were treated with an indicator variable.
Statistical Analysis
Cox proportional hazards regression models estimated hazard ratios (HR) and 95% confidence intervals (CIs) for the associations of body size variables with gallbladder cancer risk. Follow-up time for both BMI measures and height began on the date of enrollment when height and weight were first reported, whereas follow-up time for waist circumference, hip circumference, waist-height circumference, and waist-hip ratio analyses began on the date waist/hip circumference was evaluated. Cases that were diagnosed after baseline but before the time of waist/hip circumference assessment were excluded from those analyses. Studies that did not collect waist/hip circumference data were omitted from the respective analyses. All statistical models were analyzed from a pooled cohort of the combined studies with individual-level data. Initially, Cox models included only baseline age, study, and sex as covariates. Subsequently, more comprehensive models included age, study, sex, alcohol consumption, race, education, physical activity and smoking status. An additional more comprehensively-adjusted model also included personal history of gallstones. Waist circumference, waist-height ratio, hip circumference and waist-hip ratio are presented with and without adjustment for BMI. Adult weight gain statistical models included young adult BMI. Linear models estimated associations of continuous body size measures (per unit increase and per 1 standard deviation (SD)) with gallbladder cancer risk. Wald tests assessed linear trends.
Sensitivity analyses excluded gallbladder cancers that were diagnosed in the first two and five years after baseline to evaluate potential bias from pre-diagnosis weight loss due to disease progression. Sensitivity analyses also evaluated the impact of excluding participants who were diagnosed with gallstones at baseline. Two-stage individual participant meta-analyses explored potential heterogeneity of HRs across studies for continuous body size measures. Meta-analysis methods also evaluated potential heterogeneity according to region of study origin (i.e., North America (NIH-AARP, AHS, BCDDP, CPS-II, CTS, NYUWHS, PHS, PLCO, SISTER, VITAL, and WHS), Europe (i.e., COSM, EPIC, SMC, and WLH), Asia (i.e., SMHS and SWHS), and Australia (i.e., MCCS)) and BMI-assessment method (i.e., self-reported versus directly measured weight and height) for the association between adult BMI and gallbladder cancer risk.
Interaction terms with the main exposures (continuous terms) and time tested the proportional hazards assumption of the Cox models. No interactions were observed. Restricted cubic splines evaluated potential non-linearity of the associations for body size measures with gallbladder cancer risk. All p-values were two-sided; p-values less than 0.05 were considered statistically significant. SAS software was used for all statistical analyses (SAS Institute, Inc., Cary, NC, version 9.4).
Results
In this analysis of 1.88 million adults enrolled in 19 prospective cohort studies, 567 gallbladder cancers occurred during 20.1 million person-years of observation. For analyses of waist circumference/waist-height ratio and hip circumference, 361 and 318 cases were identified, respectively. Table 1 shows baseline characteristics of participants: mean age was 56.7 years, mean BMI at baseline was 26.1 kg/m2, mean waist circumference was 86.5 cm, 71% reported any alcohol intake, and 15.6% were current smokers.
Table 1.
Summary of cohort studies included in the Rare Cancer Collaboration (Gallbladder Cancer)
| Study Name (Acronym) | Gender | Baseline cohort sample size |
Gallbladder cancer case |
Baseline Age |
Baseline BMI (kg/m2) |
Baseline BMI ≥30 kg/m2 |
WC (cm)* | Baseline WC Men: ≥110 cm Women: ≥90 cm* |
Current cigarette smoker* |
Alcohol intake (grams/day) among drinkers* |
Any alcohol intake* |
History of Gallstones* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | Mean (SD) | Mean (SD) | % | Mean (SD) | % | % | Mean (SD) | % | % | ||
| NIH-AARP Diet and Health Study (NIH-AARP) | Women | 191,306 | 57 | 61.3 (5.4) | 26.9 (5.6) | 23.3 | 84.6 (13.4) | 30.7 | 14.5 | 8.5 (20.9) | 70.6 | 13.7 |
| Men | 296,183 | 53 | 61.5 (5.4) | 27.3 (4.2) | 21.4 | 97.9 (11.0) | 13.0 | 11.0 | 22.9 (51.1) | 78.9 | 6.5 | |
|
| ||||||||||||
| Agricultural Health Study (AHS) | Women | 21,643 | 4 | 46.7 (12.0) | 25.9 (4.9) | 18.6 | -- | -- | 10.1 | 2.9 (6.1) | 55.6 | -- |
| Men | 20,464 | 4 | 47.4 (13.0) | 27.5 (4.1) | 23.4 | -- | -- | 14.3 | 8.3 (14.6) | 67.5 | -- | |
|
| ||||||||||||
| The Breast Cancer Detection Demonstration Project (BCDDP) | Women | 37,793 | 8 | 61.2 (8.0) | 25.1 (4.6) | 13.2 | 81.9 (11.8) | 21.2 | 12.8 | 8.0 (14.2) | 48.9 | 12.4 |
|
| ||||||||||||
| Cohort of Swedish Men (COSM) | Men | 42,790 | 9 | 60.0 (9.6) | 25.8 (3.4) | 10.1 | 96.0 (10.1) | 9.0 | 24.7 | 15.4 (23.5) | 91.3 | 11.3 |
|
| ||||||||||||
| Cancer Prevention Study-II (CPS-II) | Women | 80,354 | 43 | 62.1 (6.6) | 25.6 (4.7) | 15.7 | 86.3 (13.0) | 35.1 | 8.6 | 9.0 (13.1) | 52.4 | 17.1 |
| Men | 71,304 | 21 | 63.9 (6.1) | 26.4 (3.7) | 14.4 | 98.8 (10.1) | 12.7 | 9.1 | 17.1 (21.6) | 65.7 | 9.0 | |
|
| ||||||||||||
| California Teachers’ Study (CTS) | Women | 103,811 | 21 | 51.4 (13.5) | 24.8 (5.0) | 13.9 | 81.7 (13.0) | 23.6 | 5.0 | 11.3 (9.7) | 66.7 | 6.4 |
|
| ||||||||||||
| European Prospective Investigation into Cancer and Cancer and Nutrition (EPIC) | Women | 254,169 | 61 | 50.4 (10.7) | 25.5 (4.6) | 15.2 | 81.2 (11.5) | 21.4 | 20.2 | 9.6 (12.0) | 83.6 | 9.2 |
| Men | 143,357 | 24 | 51.7 (10.1) | 26.5 (3.7) | 15.5 | 95.1 (10.3) | 8.1 | 29.9 | 21.8 (23.7) | 93.4 | 4.2 | |
|
| ||||||||||||
| Iowa Women’s Health Study (IWHS) | Women | 37,506 | 54 | 61.5 (4.2) | 26.1 (4.9) | 18.5 | 69.4 (10.9) | 4.9 | 14.7 | 8.9 (13.1) | 43.6 | -- |
|
| ||||||||||||
| Melbourne Collaborative Cohort Study (MCCS) | Women | 22,197 | 17 | 54.3 (8.6) | 26.8 (4.9) | 22.3 | 80.1 (11.8) | 20.0 | 9.0 | 12.4 (14.2) | 57.6 | 12.2 |
| Men | 15,537 | 4 | 54.9 (8.8) | 27.2 (3.6) | 19.1 | 93.5 (10.0) | 6.2 | 14.8 | 24.7 (25.3) | 81.3 | 4.7 | |
|
| ||||||||||||
| New York University Women’s’ Health Study (NYUWHS) | Women | 13,211 | 4 | 50.2 (8.7) | 24.9 (4.6) | 12.7 | 75.1 (11.7) | 10.8 | 18.0 | 13.3 (14.4) | 42.0 | 5.0 |
|
| ||||||||||||
| Physicians’ Health Study (PHS) | Men | 28,108 | 7 | 54.7 (9.7) | 25.1 (3.0) | 6.2 | -- | -- | 9.2 | -- | -- | 3.7 |
|
| ||||||||||||
| Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) | Women | 68,905 | 22 | 62.5 (5.4) | 27.1 (5.5) | 24.9 | -- | -- | 9.5 | 5.6 (14.0) | 99.9 | 16.7 |
| Men | 68,964 | 15 | 62.7 (5.3) | 27.6 (4.2) | 23.4 | -- | -- | 11.5 | 16.5 (33.1) | 99.9 | 7.5 | |
|
| ||||||||||||
| The Sister Study (SISTERS) | Women | 47,551 | 3 | 55.0 (9.0) | 27.8 (6.2) | 29.6 | 86.3 (14.7) | 36.0 | 8.3 | 6.8 (10.0) | 95.4 | 14.5 |
|
| ||||||||||||
| Swedish Mammography Cohort (SMC) | Women | 33,718 | 32 | 61.3 (9.1) | 25.0 (4.0) | 10.6 | 83.6 (10.7) | 26.4 | 23.6 | 6.9 (10.2) | 83.5 | 19.7 |
|
| ||||||||||||
| Shanghai Men’s Health Study (SMHS) | Men | 60,885 | 19 | 54.8 (9.7) | 23.7 (3.1) | 2.6 | 85.1 (8.7) | 0.5 | 58.7 | 35.4 (32.3) | 33.4 | 7.5 |
|
| ||||||||||||
| Shanghai Women’s Health Study (SWHS) | Women | 74,460 | 57 | 52.1 (9.1) | 24.0 (3.4) | 5.2 | 77.9 (8.8) | 10.4 | 2.4 | 10.4 (13.9) | 1.9 | 11.3 |
|
| ||||||||||||
| Vitamins and Lifestyle Study (VITAL) | Women | 30,842 | 7 | 60.7 (7.4) | 27.2 (5.8) | 25.3 | -- | -- | 7.6 | 9.4 (13.1) | 57.9 | -- |
| Men | 30,866 | 4 | 60.6 (7.3) | 27.6 (4.4) | 23.9 | -- | -- | 8.9 | 17.4 (21.8) | 70.1 | -- | |
|
| ||||||||||||
| Women’s Health Study (WHS) | Women | 38,686 | 10 | 54.2 (7.0) | 26.0 (5.1) | 18.2 | -- | -- | 13.1 | 8.6 (11.1) | 56.6 | 9.9 |
|
| ||||||||||||
| Women’s Lifestyle and Health Study (WLH) | Women | 44,191 | 7 | 40.2 (5.8) | 23.5 (3.6) | 5.8 | 77.0 (9.3) | 9.6 | 20.9 | 4.1 (4.5) | 86.2 | -- |
|
| ||||||||||||
| All Women | Women | 1,100,343 | 407 | 55.4 (10.7) | 25.8 (5.0) | 17.1 | 81.3 (12.5) | 22.3 | 13.2 | 8.6 (14.0) | 66.5 | 12.1 |
| N (%) missing | -- | -- | -- | -- | -- | 36.2 | 36.2 | 1.6 | 5.9 | 5.9 | 20.4 | |
|
| ||||||||||||
| All Men | Men | 778,458 | 160 | 58.6 (9.0) | 26.7 (4.0) | 17.3 | 95.1 (11.2) | 9.2 | 19.0 | 21.3 (38.6) | 77.7 | 6.7 |
| N (%) missing | -- | -- | -- | -- | -- | 44.8 | 44.8 | 2.1 | 10.0 | 10.0 | 14.0 | |
|
| ||||||||||||
| All Combined | All | 1,878,801 | 567 | 56.7 (10.1) | 26.1 (4.7) | 17.2 | 86.5 (13.8) | 17.4 | 15.6 | 14.2 (28.4) | 71.0 | 9.8 |
| N (%) missing | -- | -- | -- | -- | -- | 39.7 | 39.7 | 1.8 | 7.6 | 7.6 | 17.8 | |
Abbreviations: BMI, body mass index; WC, waist circumference
Among non-missing responders
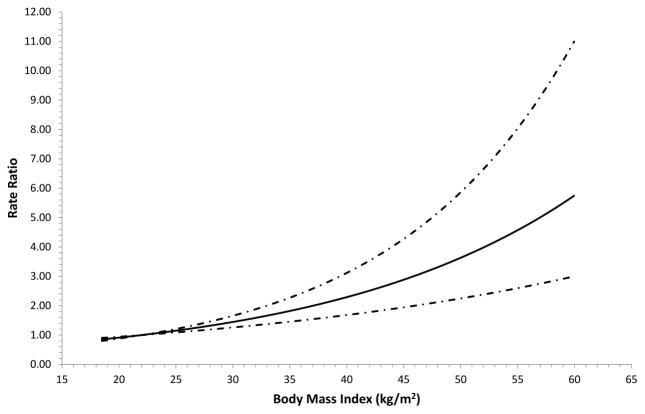
The overall and sex-specific associations between adult BMI and gallbladder cancer risk are shown in Table 2. Compared with a normal adult BMI at baseline, overweight, class I obesity, class II obesity, and class III obesity were associated with 27%, 53%, 86% and 131% higher risks of gallbladder cancer, respectively, after adjusting for age, sex, study, race, physical activity, education, smoking, alcohol and gallstones. There was no indication that risks differed meaningfully by sex (p-interaction: 0.89). There was no statistically significant evidence of between-study heterogeneity for adult BMI (I2: 0%; p-value: 0.49, Supplemental Figure 1). HRs for continuous adult BMI from both the pooled cohort approach (Table 2) and from the two-stage individual participant meta-analysis (Supplemental Figure 1) yielded similar results. Restricted cubic spline analyses supported a linear association (Figure 1; p-value for linearity: <0.0001; p-value for non-linearity: 0.95).
Table 2.
Associations of Body Mass Index, Adult Weight Gain, and Height with Gallbladder Cancer
| BMI (kg/m2) | All
|
Women
|
Men
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case* | Minimally- Adjusted RR (95% CI)† |
Multivariable- Adjusted RR1 (95% CI)‡ |
Multivariable- Adjusted RR2 (95% CI)§ |
Case* | Minimally- Adjusted RR (95% CI)† |
Multivariable- Adjusted RR1 (95% CI)‡ |
Multivariable- Adjusted RR2 (95% CI)§ |
Case* | Minimally- Adjusted RR (95% CI)† |
Multivariable- Adjusted RR1 (95% CI)‡ |
Multivariable- Adjusted RR2 (95% CI)§ |
|
| Baseline BMI | ||||||||||||
| <18.5 | 8 | 1.20 (0.59–2.43) | 1.19 (0.59–2.43) | 1.21 (0.60–2.47) | 6 | 1.04 (0.46–2.36) | 1.06 (0.47–2.39) | 1.07 (0.47–2.43) | 2 | 2.13 (0.51–8.89) | 2.00 (0.48–8.36) | 2.03 (0.49–8.50) |
| 18.5–<25 | 200 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 159 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 41 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 25–<30 | 226 | 1.36 (1.12–1.64) | 1.29 (1.07–1.57) | 1.27 (1.04–1.54) | 147 | 1.31 (1.05–1.65) | 1.24 (0.99–1.56) | 1.21 (0.97–1.52) | 79 | 1.53 (1.04–2.24) | 1.49 (1.01–2.19) | 1.46 (0.99–2.16) |
| 30–<35 | 91 | 1.76 (1.37–2.26) | 1.60 (1.24–2.06) | 1.53 (1.18–1.98) | 59 | 1.56 (1.16–2.12) | 1.41 (1.04–1.92) | 1.35 (0.99–1.83) | 32 | 2.37 (1.47–3.83) | 2.16 (1.33–3.52) | 2.11 (1.30–3.44) |
| 35–<40 | 29 | 2.26 (1.52–3.35) | 1.99 (1.33–2.96) | 1.86 (1.25–2.78) | 25 | 2.38 (1.56–3.65) | 2.11 (1.37–3.26) | 1.97 (1.28–3.05) | 4 | 1.68 (0.60–4.75) | 1.45 (0.51–4.11) | 1.39 (0.49–3.96) |
| ≥40 | 13 | 2.94 (1.67–5.18) | 2.50 (1.41–4.43) | 2.31 (1.30–4.09) | 11 | 2.84 (1.53–5.25) | 2.47 (1.32–4.62) | 2.28 (1.22–4.26) | 2 | 3.48 (0.83–14.5) | 2.74 (0.65–11.6) | 2.61 (0.62–11.0) |
| <18.5 | 8 | 1.20 (0.59–2.43) | 1.19 (0.59–2.43) | 1.21 (0.60–2.47) | 6 | 1.05 (0.46–2.36) | 1.06 (0.47–2.40) | 1.08 (0.48–2.43) | 2 | 2.13 (0.51–8.89) | 2.00 (0.48–8.36) | 2.03 (0.49–8.50) |
| 18.5–<25 | 200 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 159 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 41 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 25–<30 | 226 | 1.36 (1.12–1.65) | 1.29 (1.07–1.57) | 1.27 (1.04–1.54) | 147 | 1.31 (1.05–1.65) | 1.24 (0.99–1.56) | 1.21 (0.96–1.52) | 79 | 1.53 (1.04–2.24) | 1.49 (1.01–2.19) | 1.46 (0.99–2.16) |
| ≥30 | 133 | 1.92 (1.54–2.41) | 1.72 (1.37–2.17) | 1.64 (1.30–2.07) | 95 | 1.82 (1.40–2.36) | 1.62 (1.24–2.12) | 1.54 (1.17–2.01) | 38 | 2.31 (1.46–3.66) | 2.08 (1.30–3.33) | 2.02 (1.26–3.24) |
| Per 5 kg/m2|| | 1.31 (1.21–1.43) | 1.26 (1.16–1.37) | 1.24 (1.13–1.35) | 1.31 (1.20–1.44) | 1.27 (1.15–1.40) | 1.24 (1.13–1.37) | 1.31 (1.09–1.59) | 1.24 (1.02–1.50) | 1.22 (1.01–1.49) | |||
| p-value for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.005 | 0.0312 | 0.0431 | |||
| p-interaction with sex | 0.99 | 0.98 | 0.89 | |||||||||
| Per Std Dev|| | 1.28 (1.19–1.39) | 1.24 (1.14–1.34) | 1.21 (1.12–1.31) | 1.29 (1.18–1.40) | 1.24 (1.14–1.36) | 1.22 (1.12–1.34) | 1.28 (1.08–1.53) | 1.22 (1.02–1.45) | 1.20 (1.01–1.44) | |||
| Young Adult BMI | ||||||||||||
| <18.5 | 38 | 0.85 (0.60–1.19) | 0.83 (0.59–1.17) | 0.83 (0.59–1.18) | 28 | 0.75 (0.50–1.13) | 0.74 (0.49–1.10) | 0.74 (0.49–1.11) | 10 | 1.24 (0.63–2.44) | 1.20 (0.61–2.35) | 1.20 (0.61–2.36) |
| 18.5–<25 | 222 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 163 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 59 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 25–<30 | 29 | 1.29 (0.87–1.90) | 1.25 (0.84–1.84) | 1.24 (0.84–1.83) | 15 | 1.24 (0.73–2.11) | 1.18 (0.70–2.02) | 1.18 (0.69–2.00) | 14 | 1.36 (0.76–2.45) | 1.35 (0.75–2.43) | 1.34 (0.75–2.42) |
| ≥30 | 7 | 1.92 (0.90–4.08) | 1.77 (0.83–3.77) | 1.75 (0.82–3.73) | 4 | 1.59 (0.59–4.29) | 1.45 (0.54–3.93) | 1.44 (0.53–3.90) | 3 | 2.74 (0.86–8.79) | 2.60 (0.81–8.36) | 2.57 (0.80–8.26) |
| Per 5 kg/m2|| | 1.13 (1.02–1.25) | 1.12 (1.00–1.26) | 1.12 (1.00–1.26) | 1.18 (0.95–1.47) | 1.15 (0.91–1.45) | 1.15 (0.91–1.45) | 1.12 (0.97–1.29) | 1.12 (0.97–1.30) | 1.12 (0.96–1.30) | |||
| p-value for trend | 0.0175 | 0.0445 | 0.0531 | 0.1385 | 0.2349 | 0.2502 | 0.1375 | 0.1285 | 0.1399 | |||
| p-interaction with sex | 0.67 | 0.85 | 0.86 | |||||||||
| Per Std Dev|| | 1.07 (1.01–1.14) | 1.07 (1.00–1.14) | 1.07 (1.00–1.14) | 1.10 (0.97–1.25) | 1.08 (0.95–1.24) | 1.08 (0.95–1.23) | 1.06 (0.98–1.16) | 1.07 (0.98–1.16) | 1.07 (0.98–1.16) | |||
| Adult Weight Change (kg) # | ||||||||||||
| Lost weight | 26 | 1.12 (0.66–1.91) | 1.11 (0.65–1.90) | 1.12 (0.66–1.90) | 17 | 0.81 (0.44–1.49) | 0.82 (0.44–1.53) | 0.82 (0.44–1.53) | 9 | 3.44 (1.05–11.3) | 3.18 (0.95–10.6) | 3.18 (0.95–10.6) |
| Gained 0 to 5 | 31 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 27 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 4 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Gained 6 to 10 | 50 | 1.23 (0.78–1.92) | 1.19 (0.76–1.87) | 1.19 (0.76–1.87) | 37 | 1.06 (0.65–1.74) | 1.03 (0.63–1.70) | 1.03 (0.63–1.70) | 13 | 2.38 (0.78–7.30) | 2.33 (0.76–7.17) | 2.33 (0.76–7.17) |
| Gained 10 to 15 | 43 | 1.09 (0.69–1.73) | 1.04 (0.65–1.65) | 1.03 (0.65–1.65) | 32 | 0.98 (0.58–1.63) | 0.93 (0.56–1.56) | 0.93 (0.55–1.56) | 11 | 1.92 (0.61–6.06) | 1.84 (0.58–5.82) | 1.83 (0.58–5.78) |
| Gained 16 to 20 | 44 | 1.29 (0.82–2.06) | 1.20 (0.76–1.91) | 1.19 (0.75–1.90) | 32 | 1.16 (0.70–1.95) | 1.09 (0.65–1.82) | 1.08 (0.64–1.82) | 12 | 2.28 (0.73–7.11) | 2.08 (0.67–6.51) | 2.06 (0.66–6.45) |
| Gained ≥21 | 96 | 1.68 (1.12–2.54) | 1.50 (0.99–2.27) | 1.48 (0.97–2.25) | 61 | 1.35 (0.86–2.14) | 1.22 (0.76–1.95) | 1.20 (0.75–1.93) | 35 | 3.76 (1.32–10.7) | 3.17 (1.10–9.14) | 3.13 (1.08–9.03) |
| Per 5 kg | 1.07 (1.02–1.12) | 1.07 (1.02–1.12) | 1.07 (1.02–1.12) | 1.07 (1.02–1.13) | 1.07 (1.01–1.13) | 1.07 (1.01–1.13) | 1.06 (0.98–1.15) | 1.05 (0.97–1.15) | 1.05 (0.96–1.14) | |||
| p-value for trend | 0.0032 | 0.0054 | 0.0072 | 0.009 | 0.0149 | 0.0182 | 0.1675 | 0.2454 | 0.2667 | |||
| p-interaction with sex | 0.96 | 0.94 | 0.93 | |||||||||
| Per Std Dev|| | 1.19 (1.06–1.34) | 1.18 (1.05–1.33) | 1.18 (1.05–1.33) | 1.20 (1.05–1.38) | 1.19 (1.03–1.37) | 1.19 (1.03–1.37) | 1.16 (0.94–1.44) | 1.14 (0.91–1.43) | 1.13 (0.91–1.42) | |||
| Height (cm) | ||||||||||||
| M <170, W <160 | 167 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 137 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 30 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| M 170–<175, W 160–<165 | 137 | 1.00 (0.79–1.27) | 1.06 (0.83–1.34) | 1.06 (0.83–1.34) | 110 | 1.04 (0.80–1.35) | 1.11 (0.86–1.45) | 1.12 (0.86–1.45) | 27 | 0.84 (0.49–1.45) | 0.87 (0.51–1.49) | 0.86 (0.50–1.48) |
| M 175–<180, W 165–<170 | 143 | 1.21 (0.95–1.54) | 1.31 (1.03–1.68) | 1.31 (1.03–1.68) | 102 | 1.24 (0.94–1.64) | 1.37 (1.04–1.82) | 1.38 (1.04–1.82) | 41 | 1.09 (0.65–1.83) | 1.15 (0.68–1.95) | 1.14 (0.67–1.93) |
| M 180+, W 170+ | 120 | 1.23 (0.94–1.61) | 1.37 (1.05–1.80) | 1.37 (1.05–1.79) | 58 | 1.16 (0.83–1.61) | 1.32 (0.94–1.83) | 1.32 (0.94–1.84) | 62 | 1.27 (0.76–2.10) | 1.36 (0.81–2.27) | 1.34 (0.80–2.24) |
| Per 5 cm | 1.07 (1.00–1.13) | 1.10 (1.03–1.17) | 1.10 (1.03–1.17) | 1.04 (0.96–1.12) | 1.07 (0.99–1.16) | 1.07 (0.99–1.16) | 1.13 (1.01–1.25) | 1.14 (1.03–1.27) | 1.14 (1.02–1.27) | |||
| p-value for trend | 0.049 | 0.0043 | 0.0046 | 0.3755 | 0.0718 | 0.0701 | 0.0318 | 0.0155 | 0.0179 | |||
| p-interaction with sex | 0.24 | 0.23 | 0.23 | |||||||||
| Per Std Dev|| | 1.13 (1.00–1.28) | 1.20 (1.06–1.36) | 1.20 (1.06–1.36) | 1.07 (0.92–1.25) | 1.15 (0.99–1.34) | 1.15 (0.99–1.35) | 1.26 (1.02–1.56) | 1.30 (1.05–1.61) | 1.30 (1.05–1.61) | |||
Abbreviations: BMI, body mass index; RR, relative risk; CI, confidence interval
Some counts do not add to totals because of missing data
Adjusted for age, sex, and study
Adjusted for age, sex, study, race, physical activity, education, smoking, and alcohol
Adjusted for age, sex, study, race, physical activity, education, smoking, alcohol, and gallstones
All adult weight change models additionally adjust for young adult BMI
Continuous BMI models exclude those <18.5 kg/m2
Figure 1.
Restricted cubic spline analysis of body mass index and risk of gallbladder cancer in the Rare Cancer Collaboration. The solid line indicates the hazard ratio while the dashed line indicates 95% confidence intervals.
There was evidence supporting a positive association between young adult BMI (modelled as a continuous measure) and gallbladder cancer risk (HR: 1.12, per 5 kg/m2) although the prevalence of obesity was lower than at baseline enrollment, as expected, and the sex-specific obese categories contained few cases (Table 2). Adult weight gain also was positively associated with risk (HR: 1.07, per 5 kg). The continuous model for height showed a 10% increased risk with each 5 cm increase. There was no evidence of statistically significant interactions for sex and young adult BMI, height or adult weight gain (all p-values for interaction ≥ 0.23) or of between-study heterogeneity for young adult BMI (I2: 0%; p-value: 0.72, Supplemental Figure 2), height (I2: 28%; p-value: 0.13, Supplemental Figure 3) or adult weight gain (I2: 6%; p-value: 0.39, Supplemental Figure 4). Restricted cubic spline analyses confirmed linear associations of young adult BMI, adult weight gain and height with gallbladder cancer risk and demonstrated no evidence of non-linearity (all p-values for linearity: <0.0001; all p-values for non-linearity:≥ 0.30).
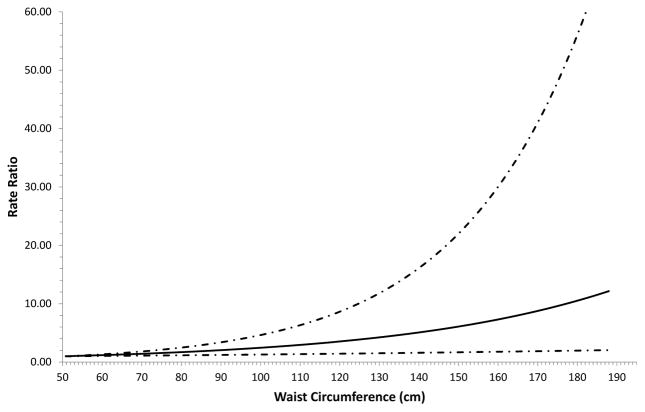
Associations of waist circumference, waist-height ratio, hip circumference, and waist-hip ratio overall and by sex with gallbladder cancer risk are shown in Table 3. Although sample sizes were smaller for the waist- and hip-circumference-related measures than for the weight- and height-related measures, statistically significant positive associations were identified for continuous measures of waist circumference (HR: 1.09, per 5 cm), waist-height ratio (HR: 1.24, per 0.1) and hip circumference (HR: 1.13, per 5 cm). Waist-hip ratio was not statistically significantly associated with risk. Associations were similar when stratified by sex (all p-values for interaction: ≥0.34). There was no statistically significant evidence of between-study heterogeneity for waist circumference (I2: 9%; p-value: 0.36, Supplemental Figure 5), waist-height ratio (I2: 35%; p-value: 0.11, Supplemental Figure 6), hip circumference (I2: 16%; p-value: 0.29, Supplemental Figure 7), or waist-hip ratio (I2: 0%; p-value: 0.88, Supplemental Figure 8). Restricted cubic spline analyses supported linear associations of waist circumference (Figure 2; p-value for linearity: <0.0001; p-value for non-linearity: 0.62), waist-height ratio (p-value for linearity: <0.0001; p-value for non-linearity: 0.76), hip circumference (p-value for linearity: <0.0001; p-value for non-linearity: 0.97) and waist-hip ratio (p-value for linearity: <0.0001; p-value for non-linearity: 0.13) with gallbladder cancer risk.
Table 3.
Associations of Waist Circumference, Waist to Height Ratio, Hip Circumference, and Waist to Hip Ratio with Gallbladder Cancer
| All
|
Women
|
Men
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case* | Minimally- Adjusted RR (95% CI)† |
Multivariable- Adjusted RR1 (95% CI)‡ |
Multivariable- Adjusted RR2 (95% CI)§ |
Case* | Minimally- Adjusted RR (95% CI)† |
Multivariable- Adjusted RR1 (95% CI)‡ |
Multivariable- Adjusted RR2 (95% CI)§ |
Case* | Minimally- Adjusted RR (95% CI)† |
Multivariable- Adjusted RR1 (95% CI)‡ |
Multivariable- Adjusted RR2 (95% CI)§ |
|
| Waist Circumference (cm) | ||||||||||||
| M <90, W <70 | 59 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 36 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 23 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| M 90–<100, W 70–<80 | 99 | 1.30 (0.92–1.82) | 1.26 (0.90–1.78) | 1.25 (0.87–1.80) | 73 | 1.42 (0.93–2.18) | 1.37 (0.89–2.10) | 1.38 (0.88–2.16) | 26 | 1.07 (0.59–1.92) | 1.04 (0.58–1.87) | 0.78 (0.40–1.53) |
| M 100–<110, W 80–<90 | 110 | 1.87 (1.31–2.66) | 1.72 (1.21–2.46) | 1.68 (1.11–2.55) | 87 | 1.93 (1.26–2.98) | 1.73 (1.12–2.67) | 1.77 (1.08–2.91) | 23 | 1.80 (0.96–3.37) | 1.69 (0.90–3.19) | 1.17 (0.54–2.55) |
| M 110+, W 90+ | 93 | 2.45 (1.68–3.55) | 2.08 (1.42–3.05) | 2.03 (1.23–3.35) | 79 | 2.46 (1.57–3.85) | 2.02 (1.28–3.19) | 2.09 (1.16–3.77) | 14 | 2.79 (1.36–5.75) | 2.46 (1.17–5.13) | 1.83 (0.68–4.92) |
| Per 5 cm | 1.12 (1.08–1.17) | 1.10 (1.05–1.15) | 1.09 (1.02–1.17) | 1.12 (1.06–1.17) | 1.09 (1.03–1.14) | 1.09 (1.02–1.18) | 1.15 (1.04–1.26) | 1.12 (1.02–1.24) | 1.08 (0.94–1.24) | |||
| p-value for trend | <0.0001 | 0.0001 | 0.0076 | <0.0001 | 0.0015 | 0.0157 | 0.0051 | 0.0191 | 0.2981 | |||
| p-interaction with sex | 0.62 | 0.47 | 0.46 | |||||||||
| Per Std Dev | 1.38 (1.22–1.55) | 1.29 (1.14–1.46) | 1.27 (1.07–1.52) | 1.36 (1.19–1.55) | 1.26 (1.09–1.45) | 1.28 (1.05–1.57) | 1.46 (1.12–1.90) | 1.38 (1.05–1.81) | 1.23 (0.83–1.82) | |||
| Waist to Height Ratio | ||||||||||||
| M <0.50, W <0.45 | 71 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 60 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 11 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| M 0.50–<0.55, W 0.45–<0.50 | 85 | 1.25 (0.90–1.74) | 1.20 (0.86–1.67) | 1.15 (0.81–1.63) | 56 | 1.15 (0.78–1.69) | 1.09 (0.74–1.60) | 1.05 (0.70–1.57) | 29 | 1.66 (0.82–3.36) | 1.63 (0.81–3.30) | 1.50 (0.67–3.36) |
| M 0.55–<0.60, W 0.50–<0.55 | 100 | 1.78 (1.28–2.48) | 1.62 (1.16–2.26) | 1.47 (1.00–2.17) | 72 | 1.65 (1.13–2.41) | 1.45 (0.99–2.13) | 1.38 (0.89–2.13) | 28 | 2.36 (1.16–4.82) | 2.25 (1.10–4.61) | 1.72 (0.70–4.26) |
| M 0.60+, W 0.55+ | 105 | 2.00 (1.43–2.81) | 1.67 (1.18–2.37) | 1.42 (0.90–2.27) | 87 | 1.90 (1.30–2.76) | 1.52 (1.03–2.25) | 1.38 (0.82–2.32) | 18 | 2.44 (1.12–5.31) | 2.22 (1.01–4.90) | 1.51 (0.52–4.37) |
| Per 0.1 | 1.42 (1.23–1.63) | 1.30 (1.12–1.50) | 1.24 (1.00–1.54) | 1.41 (1.21–1.64) | 1.27 (1.08–1.50) | 1.29 (1.02–1.63) | 1.46 (1.04–2.05) | 1.37 (0.97–1.94) | 1.06 (0.63–1.80) | |||
| p-value for trend | <0.0001 | 0.0005 | 0.0497 | <0.0001 | 0.0032 | 0.0371 | 0.0289 | 0.0751 | 0.8187 | |||
| p-interaction with sex | 0.83 | 0.71 | 0.72 | |||||||||
| Per Std Dev | 1.30 (1.17–1.45) | 1.22 (1.09–1.36) | 1.18 (1.00–1.39) | 1.30 (1.15–1.45) | 1.20 (1.06–1.36) | 1.21 (1.01–1.45) | 1.33 (1.03–1.72) | 1.27 (0.98–1.65) | 1.05 (0.70–1.56) | |||
| Hip Circumference (cm) | ||||||||||||
| M <95, W <90 | 55 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 42 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 13 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| M 95–<105, W 90–<100 | 109 | 1.70 (1.14–2.54) | 1.67 (1.12–2.49) | 1.66 (1.09–2.53) | 84 | 2.12 (1.31–3.43) | 2.05 (1.26–3.32) | 2.11 (1.25–3.55) | 25 | 0.98 (0.49–1.96) | 0.98 (0.49–1.96) | 0.78 (0.35–1.70) |
| M 105–<115, W 100–<110 | 93 | 2.15 (1.39–3.32) | 2.00 (1.29–3.09) | 1.92 (1.17–3.17) | 69 | 2.15 (1.28–3.63) | 1.95 (1.15–3.30) | 2.01 (1.09–3.69) | 24 | 2.44 (1.17–5.07) | 2.33 (1.11–4.88) | 1.54 (0.63–3.78) |
| M 115+, W 110+ | 61 | 3.52 (2.20–5.64) | 2.93 (1.82–4.73) | 2.74 (1.48–5.08) | 54 | 3.77 (2.18–6.52) | 3.04 (1.74–5.32) | 3.24 (1.55–6.75) | 7 | 3.50 (1.31–9.35) | 3.01 (1.11–8.17) | 1.96 (0.58–6.68) |
| Per 5 cm | 1.17 (1.11–1.23) | 1.13 (1.07–1.20) | 1.13 (1.04–1.22) | 1.16 (1.09–1.22) | 1.12 (1.05–1.19) | 1.14 (1.05–1.24) | 1.22 (1.07–1.38) | 1.19 (1.04–1.35) | 1.10 (0.92–1.30) | |||
| p-value for trend | <0.0001 | <0.0001 | 0.0021 | <0.0001 | 0.0000 | 0.0028 | 0.0024 | 0.0095 | 0.3044 | |||
| p-interaction with sex | 0.49 | 0.35 | 0.34 | |||||||||
| Per Std Dev | 1.37 (1.23–1.53) | 1.30 (1.16–1.45) | 1.28 (1.09–1.50) | 1.35 (1.20–1.52) | 1.27 (1.12–1.44) | 1.31 (1.10–1.56) | 1.50 (1.15–1.95) | 1.42 (1.09–1.86) | 1.21 (0.84–1.73) | |||
| Waist to Hip Ratio | ||||||||||||
| M <0.90, W <0.75 | 43 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 27 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 16 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| M 0.90–<0.95, W 0.75–<0.80 | 94 | 1.48 (1.03–2.13) | 1.42 (0.99–2.05) | 1.35 (0.94–1.96) | 70 | 1.56 (1.00–2.43) | 1.47 (0.94–2.30) | 1.43 (0.91–2.24) | 24 | 1.35 (0.71–2.58) | 1.31 (0.69–2.50) | 1.13 (0.58–2.21) |
| M 0.95–<1.00, W 0.80–<0.85 | 81 | 1.34 (0.92–1.96) | 1.23 (0.84–1.81) | 1.11 (0.75–1.64) | 66 | 1.40 (0.89–2.21) | 1.26 (0.80–1.99) | 1.17 (0.74–1.87) | 15 | 1.23 (0.60–2.54) | 1.14 (0.55–2.37) | 0.83 (0.38–1.81) |
| M 1.00+, W 0.85+ | 99 | 1.65 (1.13–2.40) | 1.43 (0.98–2.09) | 1.19 (0.80–1.79) | 85 | 1.69 (1.08–2.64) | 1.43 (0.91–2.24) | 1.27 (0.79–2.03) | 14 | 1.69 (0.80–3.60) | 1.49 (0.70–3.20) | 0.96 (0.42–2.20) |
| Per 0.1 | 1.19 (1.03–1.37) | 1.12 (0.96–1.31) | 1.03 (0.87–1.22) | 1.17 (0.99–1.38) | 1.09 (0.92–1.30) | 1.03 (0.85–1.25) | 1.26 (0.97–1.66) | 1.22 (0.89–1.66) | 1.04 (0.70–1.53) | |||
| p-value for trend | 0.0197 | 0.1496 | 0.7076 | 0.0655 | 0.3229 | 0.7456 | 0.0876 | 0.2102 | 0.8482 | |||
| p-interaction with sex | 0.63 | 0.45 | 0.42 | |||||||||
| Per Std Dev | 1.18 (1.03–1.36) | 1.12 (0.96–1.30) | 1.03 (0.88–1.22) | 1.16 (0.99–1.37) | 1.09 (0.92–1.29) | 1.03 (0.86–1.24) | 1.25 (0.97–1.63) | 1.21 (0.90–1.64) | 1.04 (0.71–1.51) | |||
Abbreviations: RR, relative risk; CI, confidence interval
Some counts do not add to totals because of missing data
Adjusted for age, sex, and study
Adjusted for age, sex, study, race, physical activity, education, smoking, alcohol, and gallstones
Adjusted for age, sex, study, race, physical activity, education, smoking, alcohol, gallstones, and BMI
Figure 2.
Restricted cubic spline analysis of waist circumference and risk of gallbladder cancer in the Rare Cancer Collaboration. The solid line indicates the hazard ratio while the dashed line indicates 95% confidence intervals.
When analyses were restricted to studies and participants that had both BMI and waist circumference in the individual-level data that included all participants, gallbladder cancer risks were similarly elevated for each 1 SD unit increase in waist circumference (HR: 1.28; 95% CI: 1.13 to 1.46) and BMI (HR: 1.21; 95% CI: 1.09 to 1.34), when modelled separately. When BMI and waist circumference were included in the same model, both HRs were attenuated and no longer statistically significant (waist circumference HR: 1.21; 95% CI: 0.99 to 1.50; BMI HR: 1.06; 95% CI: 0.89 to 1.27).
In sensitivity analyses, the main study findings were not materially different after excluding gallbladder cancers that occurred in the first two and five years after baseline and after excluding participants who reported history of gallstones (data not shown). No strong evidence for geographic heterogeneity was detected for continuous adult BMI and gallbladder cancer risk (i.e., North America, HR: 1.25; 95% CI: 1.12 to 1.38; Europe, HR: 1.12; 95% CI: 0.91 to 1.37; Asia, HR: 1.18; 95%CI: 0.84 to 1.67; Australia, HR: 1.85; 95% CI: 1.32 to 2.59; p-value for heterogeneity: 0.09). Studies with self-reported versus directly measured height and weight yielded relatively similar results (i.e., self-reported BMI, per 5 kg/m2, HR: 1.22; 95% CI: 1.10–1.35; directly measured BMI, per 5 kg/m2, HR: 1.30; 95% CI: 1.10–1.54; p-value for heterogeneity: 0.53).
Discussion
In this large prospective analysis of 1.88 million adults enrolled in 19 cohort studies, greater BMI (both at middle age and during young adulthood), adult weight gain, height, waist circumference, waist-height ratio, and hip circumference were all consistently associated with higher risks of gallbladder cancer. Results for waist-hip ratio generally suggested an increased risk, consistent with the other anthropometric measures, but the results were not statistically significant. Restricted cubic spline analyses supported linear associations for all anthropometric measures with gallbladder cancer risk, indicating dose-response associations throughout the ranges of body size measures observed in this study. The main study results were consistent when stratified by sex and they were not materially different in statistical models that included many confirmed and potential risk factors for gallbladder cancer, including sex, smoking, alcohol, race, education and history of cholesterol gallstones. The main study results were robust after a series of sensitivity analyses, including individual participant meta-analyses and when excluding cases that occurred in the first 5 years of follow-up.
Studies regarding BMI and gallbladder cancer risk have been generally hampered by small numbers of outcomes and the related issues of limited statistical power and imprecise risk estimates: of the 12 prospective cohort studies on this topic in the literature (11–13, 15–17, 21–26), six identified fewer than 100 cases (11, 12, 17, 22, 23, 25) and while most studies reported HRs above one, many studies were not statistically significant (11, 12, 15, 23, 25). With data from 567 gallbladder cancer cases, this study makes an important contribution toward confirming the association between high BMI and this rare and highly fatal cancer. The HR identified in this study for obese BMI and gallbladder cancer risk (HR: 1.64) is similar in magnitude to results from individual large, prospective cohort studies (13, 16, 21, 26) and to results from a recent meta-analysis (HR: 1.62) (27). Additionally, this study identified similar HRs for linear BMI and gallbladder cancer risk when stratified by sex, similar to the conclusion reached by the recent CUP (10), but somewhat in contrast to earlier reports that suggested the association was higher for women than men (27–29). Since gallbladder cancer is more common in women than in men (by approximately 2-fold, typically), it is plausible that the earlier studies compared with the more recent, larger studies lacked sufficient statistical power to detect a meaningful association for men.
We are not aware of any epidemiologic studies on young adult BMI as a risk factor for gallbladder cancer; therefore, our finding of higher risk with obese levels of BMI during young adulthood is novel but requires replication in other large, prospective studies. This finding may highlight the importance of early life energy excess with gallbladder cancer etiology. We identified a moderate association between adult weight gain and gallbladder cancer risk: only one previous cohort study assessed adult weight gain with gallbladder cancer risk (11) and reported that average weight gain (in kg) per year from age 20 years onward was not statistically significantly associated with risk, although only 37 gallbladder cancer cases were identified in the cohort, so statistical power to detect an association was limited.
Taller height was associated with higher risk of gallbladder cancer in this study, whereas in one previous large prospective cohort study (16) height was not associated with gallbladder cancer risk. The Million Women Study collaboration reported an association between height and cancer risk overall (30), consistent with this study for gallbladder cancer, but that study did not report results specifically for gallbladder cancer and it is unlikely that the overall result was materially affected from what would have been very few gallbladder cancer cases.
Prospective studies on waist and hip circumference-related measures and gallbladder cancer risk are especially rare, with only one published study to date (11) that reported each 5 cm increase in waist and hip circumferences were associated with 17% and 18% higher risks of gallbladder cancer risk, respectively, and the results were statistically significant despite a relatively small number of cases (n=76). Likewise, a 0.1 increase in the waist-hip ratio was associated with a non-statistically significant 33% higher risk of gallbladder cancer (11). With over 300 prospectively identified gallbladder cancer cases with reported waist- and hip-circumference related measures, our study adds considerably to the sparse literature on central adiposity and gallbladder cancer risk, although further research from additional large, prospective cohort studies is still warranted.
From the statistical models that included mutual adjustment of BMI and waist circumference, some of the risk imparted by these variables is likely shared since both of the main effect associations were attenuated to the null and were no longer statistically significant, although the HR for BMI decreased appreciably more than did the HR for waist circumference. Obesity increases risk of cholesterol gallstones and other gallbladder diseases (31), and gallstones, in turn, are a major risk factor for gallbladder cancer (4). Thus, gallstones might lie on the causal pathway between obesity and gallbladder cancer risk for some men and women; but when history of gallstones at baseline was included in the statistical models, there was no appreciable change to the HRs for obesity. In addition, when persons with a history of gallstones at baseline were excluded, the results were not materially different (data not shown). More work is needed to define the mechanisms that connect general and central obesity to gallbladder cancer risk. Some plausible mechanisms to explain this link may include localized inflammation and the ensuing damage that occurs to gallbladder epithelial tissue over time which for some men and women may lead to gallbladder cancer.
The current study’s strengths include its large sample size, prospective study design, inclusion of cohort studies from several regions of the world, long follow-up, and inclusion of harmonized data on many confirmed and plausible gallbladder cancer risk factors. Several limitations of this study should be also considered, particularly regarding the reliance by most studies on self-reported height and weight. Cross-sectional studies suggest that self-reported BMI is slightly lower than directly measured BMI, especially at obese levels of BMI (32); under-reporting of BMI may inflate associations for overweight BMI and gallbladder cancer risk and simultaneously underestimate the association for obese BMI. Good-to-excellent agreement has been reported for self-reported and directly measured values of height and weight, however, in studies with participants who shared similar demographic characteristics to this study (33, 34) and it is reassuring that the main associations for adult BMI and gallbladder cancer risk were similar for studies with directly measured versus self-reported height and weight. Six studies in this study had interviewer-measured waist and hip circumference data whereas eight studies had these data from participant-measurements. The validity of self-measured versus interviewer-measured waist and hip circumferences is generally quite high, with correlations coefficients of 0.84 to 0.9 (35). Nonetheless, if circumference-related measures are more measurement-error prone than height and weight, then studies of body circumference measures and disease outcomes would tend to underestimate the true associations compared to studies that rely on height and weight. Further, waist-hip ratio tends to show weaker correlations between self-measured and interviewer-measured indices, suggesting that it is more prone to measurement error than other body size variables (35, 36). This potential measurement error may explain, at least in part, our null result for waist-hip ratio and gallbladder cancer risk. We did not have access to updated risk factor information in this pooling project study even though some individual cohort studies collected updated risk factor information during follow-up. For factors that change over time, including body weight and circumference-related measures, this limitation likely causes underestimation of the true associations. Another limitation in this study is the lack of data on cholecystectomy (i.e., gallbladder removal); although it is unclear what effect, if any, this omission would have on the HRs in this study. Five cohort studies did not collect circumference related measures and other studies only collected this information after their initial baseline enrollment, thus we had fewer case numbers for these measures than for the height and weight related analyses.
In conclusion, this pooled cohort analysis of individual-level data from 19 prospective cohort studies identified higher risks of gallbladder cancer with indicators of general and central obesity and height. Because gallbladder cancer has such a poor prognosis with so few established risk factors, additional studies are required to identify further primary prevention opportunities for this disease.
Supplementary Material
Acknowledgments
Grant support:
The Agricultural Health Study (AHS) was funded by the Intramural Program of the NIH, National Cancer Institute (Z01 P010119) and the National Institute of Environmental Health Sciences (Z01 ES 049030-11).
The Breast Cancer Detection Demonstration Project (BCDDP) Follow-up Study has been supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study-II (CPS-II) cohort.
The California Teachers Study (CTS) was supported by National Cancer Institute grants R01 #CA 77398 and K05 CA136967 (awarded to Leslie Bernstein).
The coordination of the European Prospective Investigation into Cancer and Nutrition (EPIC) is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society, Denmark; Ligue Contre le Cancer, France; Institut Gustave Roussy, France; Mutuelle Générale de l’Education Nationale, France; Institut National de la Santé et de la Recherche Médicale, France; Deutsche Krebshilfe, Germany, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research, Germany; Hellenic Health Foundation, Greece; Italian Association for Research on Cancer; National Research Council, Italy; Dutch Ministry of Public Health, Welfare and Sports, the Netherlands; Netherlands Cancer Registry, the Netherlands; LK Research Funds, the Netherlands; Dutch Prevention Funds, the Netherlands; Dutch ZON (Zorg Onderzoek Nederland), the Netherlands; World Cancer Research Fund, London, UK; Statistics Netherlands, the Netherlands; European Research Council, Norway; Health Research Fund, Regional Governments of Andalucía, Asturias, Basque Country, Murcia (project no. 6236) and Navarra, ISCIII RETIC (RD06/0020/0091), Spain; Swedish Cancer Society, Sweden; Swedish Scientific Council, Sweden; Regional Government of Skåne and Västerbotten, Sweden; Cancer Research United Kingdom; Medical Research Council, United Kingdom; Stroke Association, United Kingdom, British Heart Foundation, United Kingdom; Department of Health, Food Standards Agency, United Kingdom; and Wellcome Trust; United Kingdom.
The Iowa Women’s Health Study (IWHS) is supported by a grant from the National Cancer Institute (R01 CA39742).
The Melbourne Collaborative Cohort Study (MCCS) receives core funding from the Cancer Council Victoria and is additionally supported by grants from the Australian NHMRC (209057, 251533, 396414, and 504715).
The NYU Women’s Health Study (NYUWHS) is supported by grant R01 CA 098661 and Center grant CA 016087 from the National Cancer Institute and by Center grant ES 0002
The NIH-AARP Diet and Health Study (NIH-AARP) was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
The Physicians’ Health Study (PHS) was supported by grants CA 97193, CA 34944, CA 40360, HL 26490, and HL 34595 from the National Institutes of Health.
The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial is supported by contracts from the National Cancer Institute.
The Sister Study (SISTER) was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005).
The Swedish Mammography Cohort (SMC) was supported by the Swedish Research Council, Swedish Council for Working Life and Social Research and the Swedish Cancer Foundation.
The Shanghai Men’s Health Study (SMHS) was supported by grants (R01 CA082729 and UM1 CA173640) from the National Institutes of Health.
The Shanghai Women’s Health Study (SWHS) was supported by grants R37 CA070867 and UM1 CA182910 from the National Cancer Institute and in part by the National Cancer Institute intramural program (N02 CP1101066).
VITamin and Lifestyle (VITAL) study: Dr. White was supported by the National Institutes of Health grant K05-CA154337 (National Cancer Institute and Office of Dietary Supplements).
The Women’s Health Study (WHS) was supported by CA047988, HL043851, HL080467, and HL099355.
The Women’s Lifestyle and Health (WLH) project was supported by the Swedish Research Council (grant number 521-2011-295) and a Distinguished Professor Award at Karolinska Institutet to Hans-Olov Adami, grant number: 2368/10-221.
Footnotes
Disclosures: The authors report that they have nothing to disclose.
References
- 1.Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder Cancer in the 21st Century. J Oncol. 2015;2015:967472. doi: 10.1155/2015/967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Jan 14, 2014. [website] [Google Scholar]
- 3.American Cancer Society. Cancer Treatment and Survivorship Facts & Figures 2014–2015. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 4.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coe PO, O’Reilly DA, Renehan AG. Excess adiposity and gastrointestinal cancer. Br J Surg. 2014;101:1518–31. doi: 10.1002/bjs.9623. discussion 31. [DOI] [PubMed] [Google Scholar]
- 6.Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Beane Freeman LE, et al. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults. Cancer Res. 2016;76:6076–83. doi: 10.1158/0008-5472.CAN-16-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Arch Intern Med. 2010;170:791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell PT, Cotterchio M, Dicks E, Parfrey P, Gallinger S, McLaughlin JR. Excess body weight and colorectal cancer risk in Canada: associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1735–44. doi: 10.1158/1055-9965.EPI-06-1059. [DOI] [PubMed] [Google Scholar]
- 9.Campbell PT, Jacobs ET, Ulrich CM, Figueiredo JC, Poynter JN, McLaughlin JR, et al. Case-Control Study of Overweight, Obesity, and Colorectal Cancer Risk, Overall and by Tumor Microsatellite Instability Status. J Natl Cancer Inst. 2010;102:391–400. doi: 10.1093/jnci/djq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project: Diet, Nutrition, Physical Activity and Gallbladder Cancer. 2015. [Google Scholar]
- 11.Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. 2013;132:645–57. doi: 10.1002/ijc.27645. [DOI] [PubMed] [Google Scholar]
- 12.Ishiguro S, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Risk factors of biliary tract cancer in a large-scale population-based cohort study in Japan (JPHC study); with special focus on cholelithiasis, body mass index, and their effect modification. Cancer Causes Control. 2008;19:33–41. doi: 10.1007/s10552-007-9067-8. [DOI] [PubMed] [Google Scholar]
- 13.Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123:1892–6. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 14.Fujino Y Japan Collaborative Cohort Study for Evaluation of C. Anthropometry, development history and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pac J Cancer Prev. 2007;8(Suppl):105–12. [PubMed] [Google Scholar]
- 15.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF., Jr Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–9. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 16.Engeland A, Tretli S, Austad G, Bjorge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control. 2005;16:987–96. doi: 10.1007/s10552-005-3638-3. [DOI] [PubMed] [Google Scholar]
- 17.Kuriyama S, Tsubono Y, Hozawa A, Shimazu T, Suzuki Y, Koizumi Y, et al. Obesity and risk of cancer in Japan. Int J Cancer. 2005;113:148–57. doi: 10.1002/ijc.20529. [DOI] [PubMed] [Google Scholar]
- 18.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. International Classification for Diseases. 9. Geneva: 1985. [Google Scholar]
- 20.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva, Switzerland: WHO; 1998. Report of a WHO consultation on obesity. [PubMed] [Google Scholar]
- 21.Bhaskaran K, Douglas I, Forbes H, Dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolk A, Gridley G, Svensson M, Nyren O, McLaughlin JK, Fraumeni JF, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 23.Hemminki K, Li X, Sundquist J, Sundquist K. Obesity and familial obesity and risk of cancer. Eur J Cancer Prev. 2011;20:438–43. doi: 10.1097/CEJ.0b013e32834761c0. [DOI] [PubMed] [Google Scholar]
- 24.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF., Jr Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 25.Machova L, Cizek L, Horakova D, Koutna J, Lorenc J, Janoutova G, et al. Association between obesity and cancer incidence in the population of the District Sumperk, Czech Republic. Onkologie. 2007;30:538–42. doi: 10.1159/000108284. [DOI] [PubMed] [Google Scholar]
- 26.Borena W, Edlinger M, Bjorge T, Haggstrom C, Lindkvist B, Nagel G, et al. A prospective study on metabolic risk factors and gallbladder cancer in the metabolic syndrome and cancer (Me-Can) collaborative study. PloS one. 2014;9:e89368. doi: 10.1371/journal.pone.0089368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan W, Gao M, Liu N, Zhang G, Xu T, Cui W. Body Mass Index and Risk of Gallbladder Cancer: Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2015;7:8321–34. doi: 10.3390/nu7105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 29.Larsson SC, Wolk A. Obesity and the risk of gallbladder cancer: a meta-analysis. Br J Cancer. 2007;96:1457–61. doi: 10.1038/sj.bjc.6603703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V, et al. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12:785–94. doi: 10.1016/S1470-2045(11)70154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aune D, Norat T, Vatten LJ. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol. 2015;30:1009–19. doi: 10.1007/s10654-015-0081-y. [DOI] [PubMed] [Google Scholar]
- 32.Shields M, Gorber SC, Tremblay MS. Effects of measurement on obesity and morbidity. Health Rep. 2008;19:77–84. [PubMed] [Google Scholar]
- 33.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring) 2007;15:188–96. doi: 10.1038/oby.2007.504. [DOI] [PubMed] [Google Scholar]
- 34.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5:561–5. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 35.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Spencer EA, Roddam AW, Key TJ. Accuracy of self-reported waist and hip measurements in 4492 EPIC-Oxford participants. Public Health Nutr. 2004;7:723–7. doi: 10.1079/phn2004600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.