Summary
Background
Improving survival and extending the longevity of life for all populations requires timely, robust evidence on local mortality levels and trends. The Global Burden of Disease 2015 Study (GBD 2015) provides a comprehensive assessment of all-cause and cause-specific mortality for 249 causes in 195 countries and territories from 1980 to 2015. These results informed an in-depth investigation of observed and expected mortality patterns based on sociodemographic measures.
Methods
We estimated all-cause mortality by age, sex, geography, and year using an improved analytical approach originally developed for GBD 2013 and GBD 2010. Improvements included refinements to the estimation of child and adult mortality and corresponding uncertainty, parameter selection for under-5 mortality synthesis by spatiotemporal Gaussian process regression, and sibling history data processing. We also expanded the database of vital registration, survey, and census data to 14 294 geography–year datapoints. For GBD 2015, eight causes, including Ebola virus disease, were added to the previous GBD cause list for mortality. We used six modelling approaches to assess cause-specific mortality, with the Cause of Death Ensemble Model (CODEm) generating estimates for most causes. We used a series of novel analyses to systematically quantify the drivers of trends in mortality across geographies. First, we assessed observed and expected levels and trends of cause-specific mortality as they relate to the Socio-demographic Index (SDI), a summary indicator derived from measures of income per capita, educational attainment, and fertility. Second, we examined factors affecting total mortality patterns through a series of counterfactual scenarios, testing the magnitude by which population growth, population age structures, and epidemiological changes contributed to shifts in mortality. Finally, we attributed changes in life expectancy to changes in cause of death. We documented each step of the GBD 2015 estimation processes, as well as data sources, in accordance with Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER).
Findings
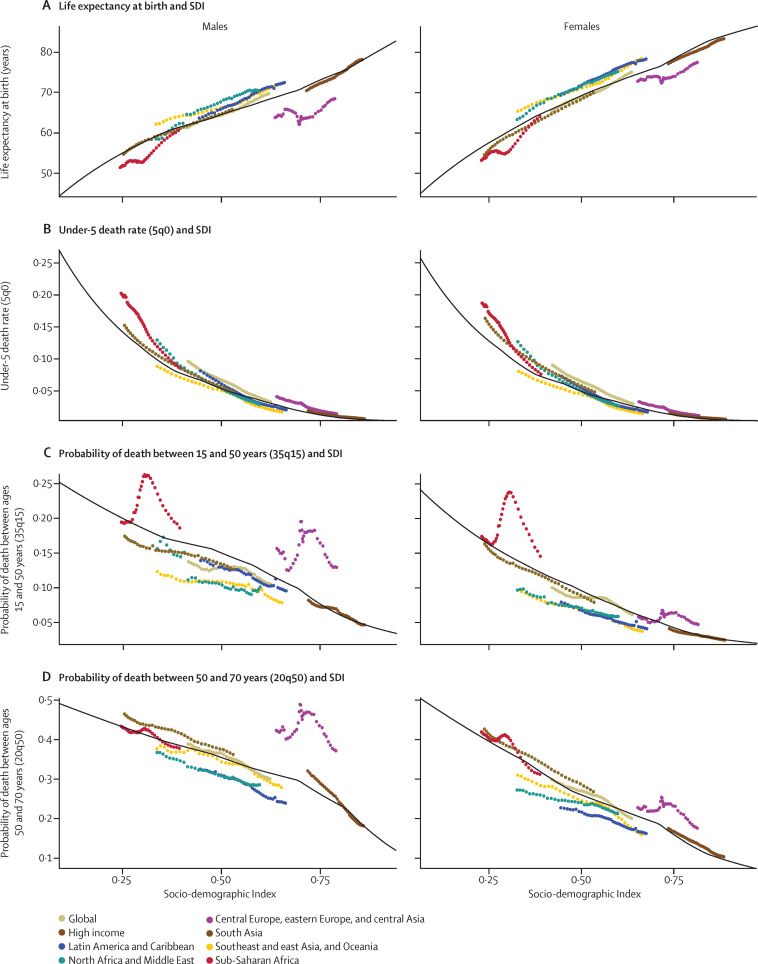
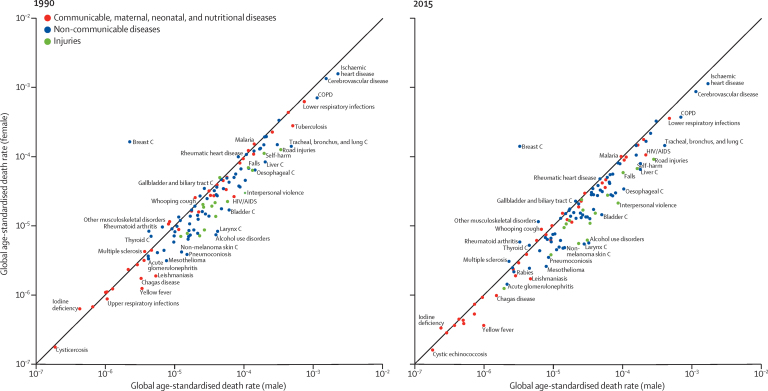
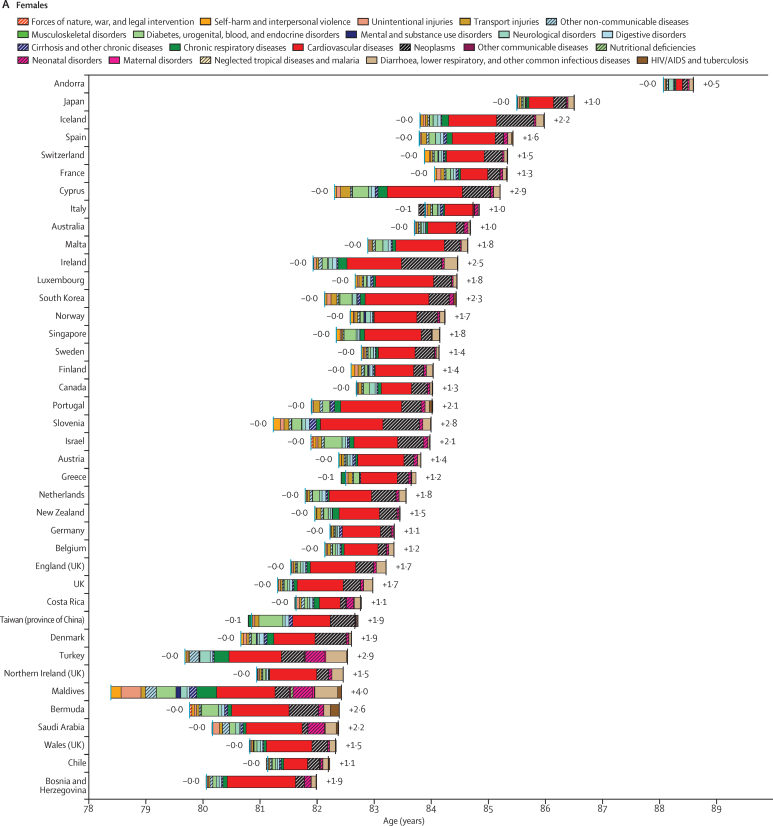
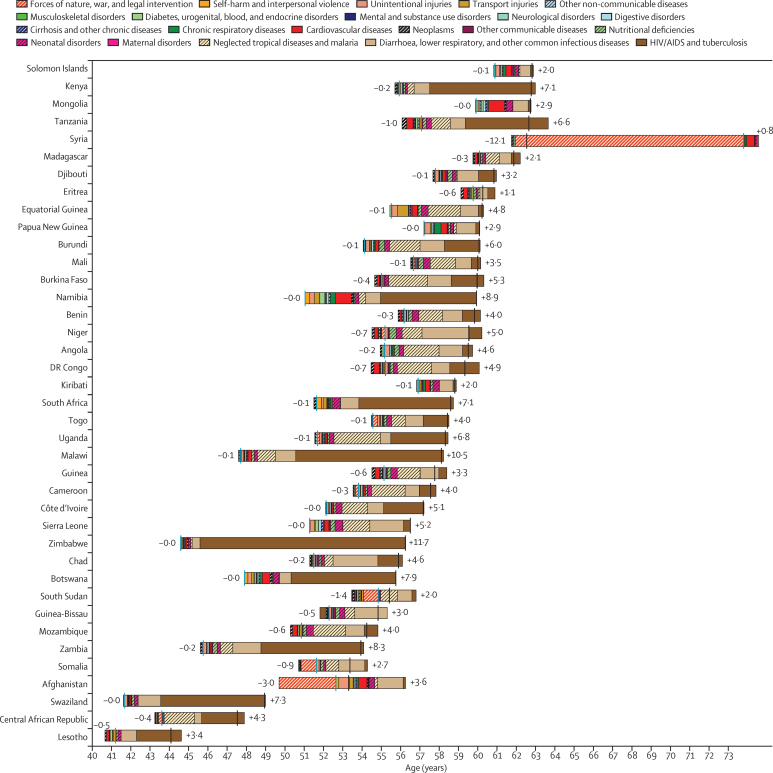
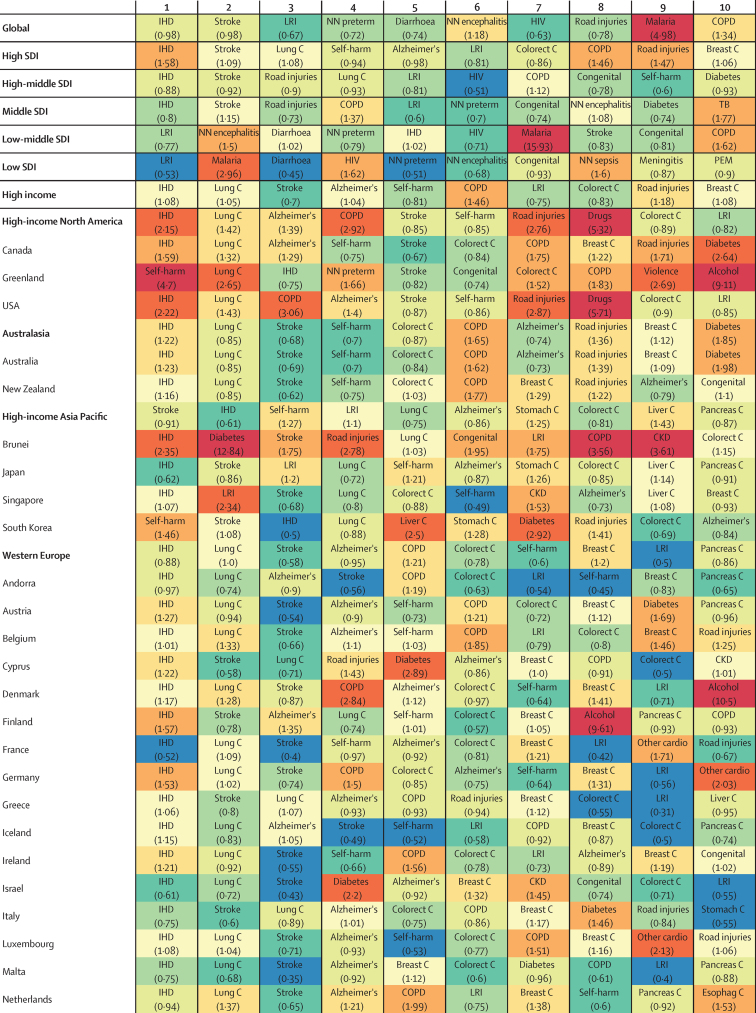
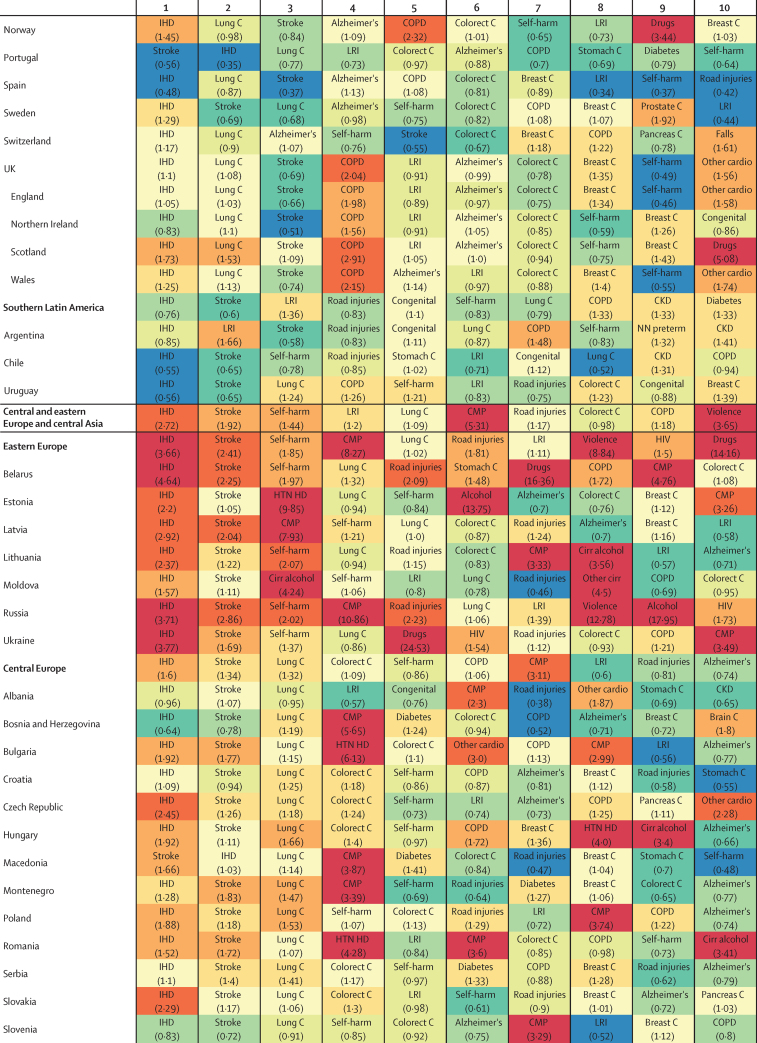
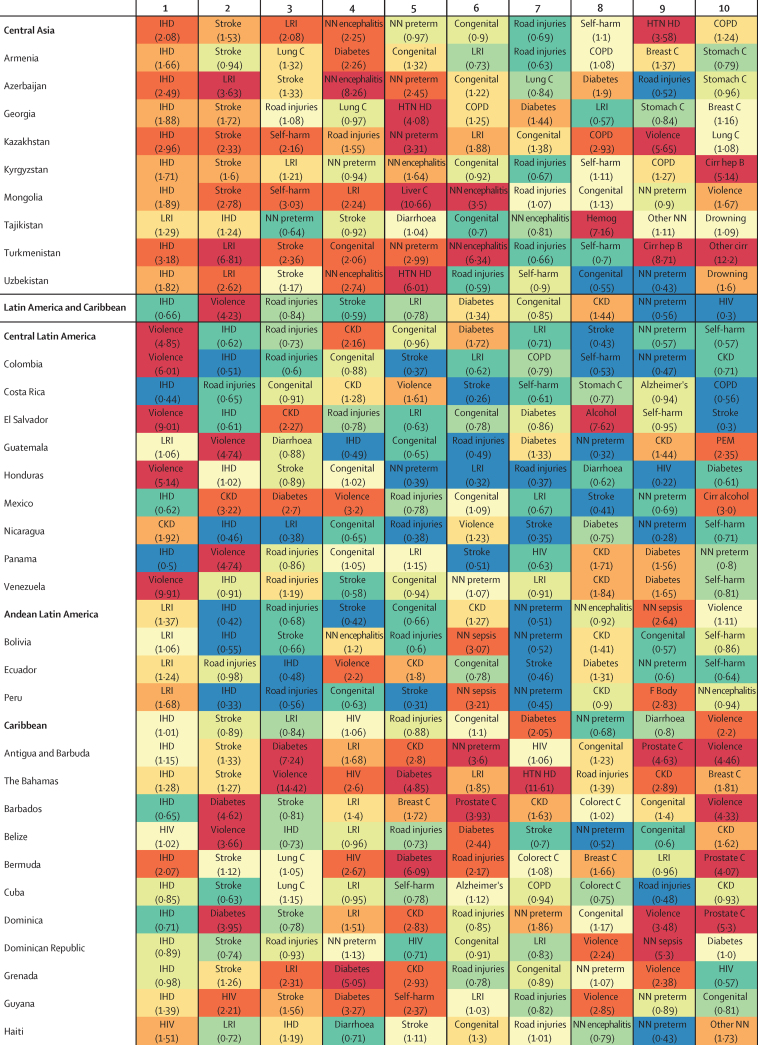
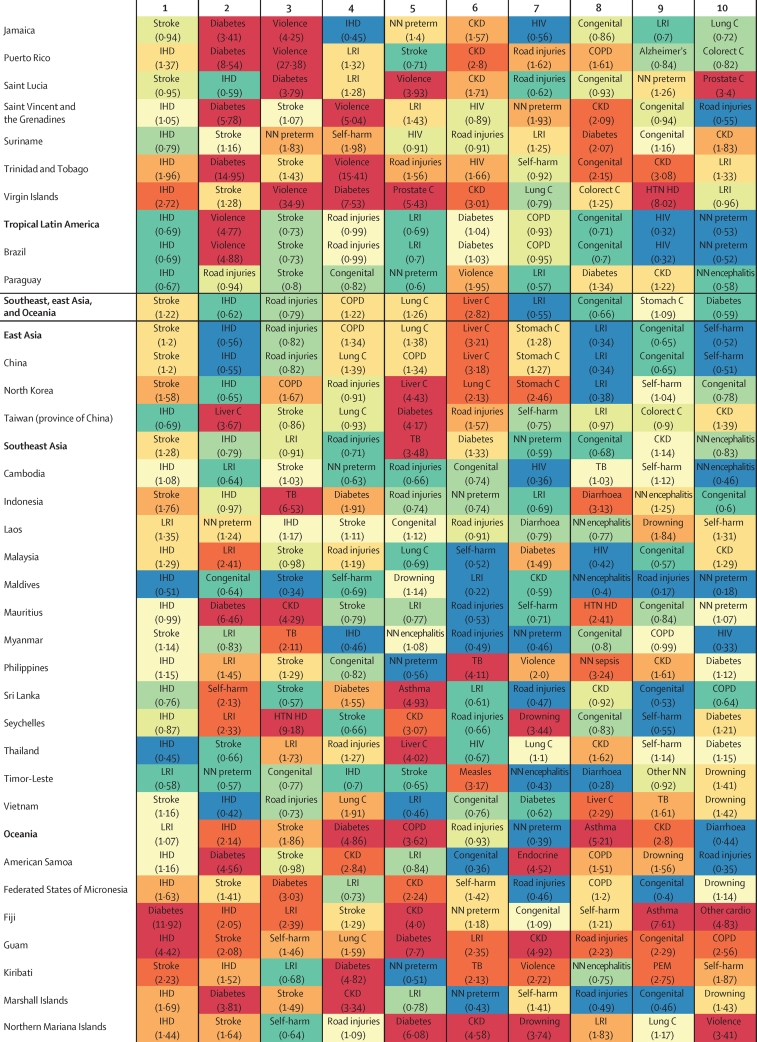
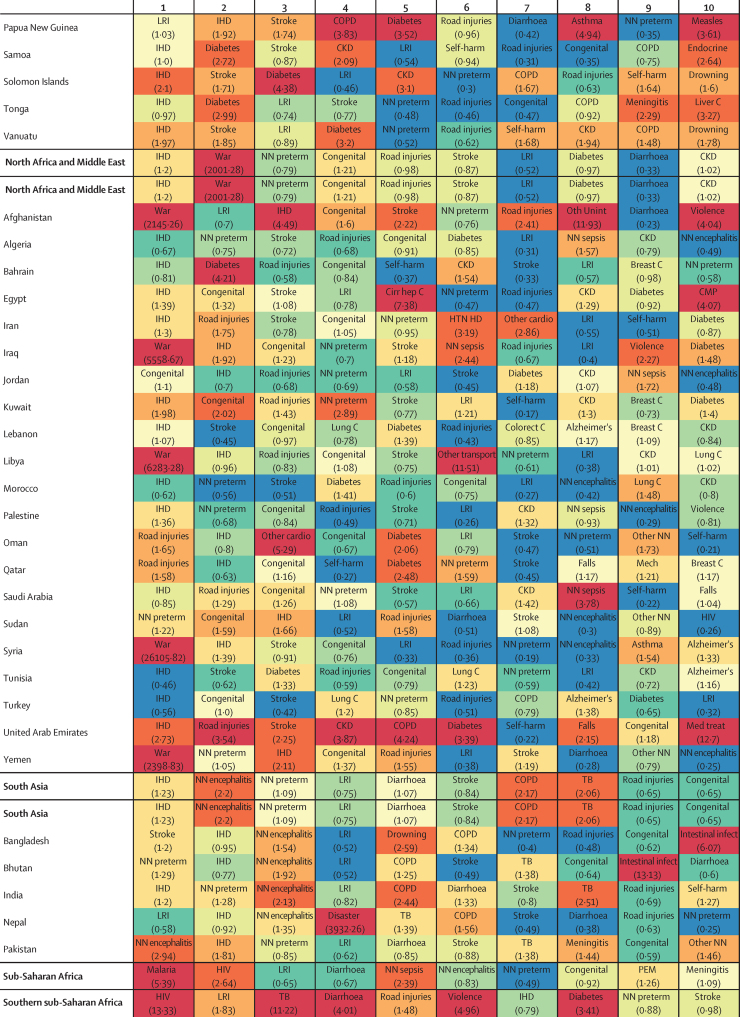
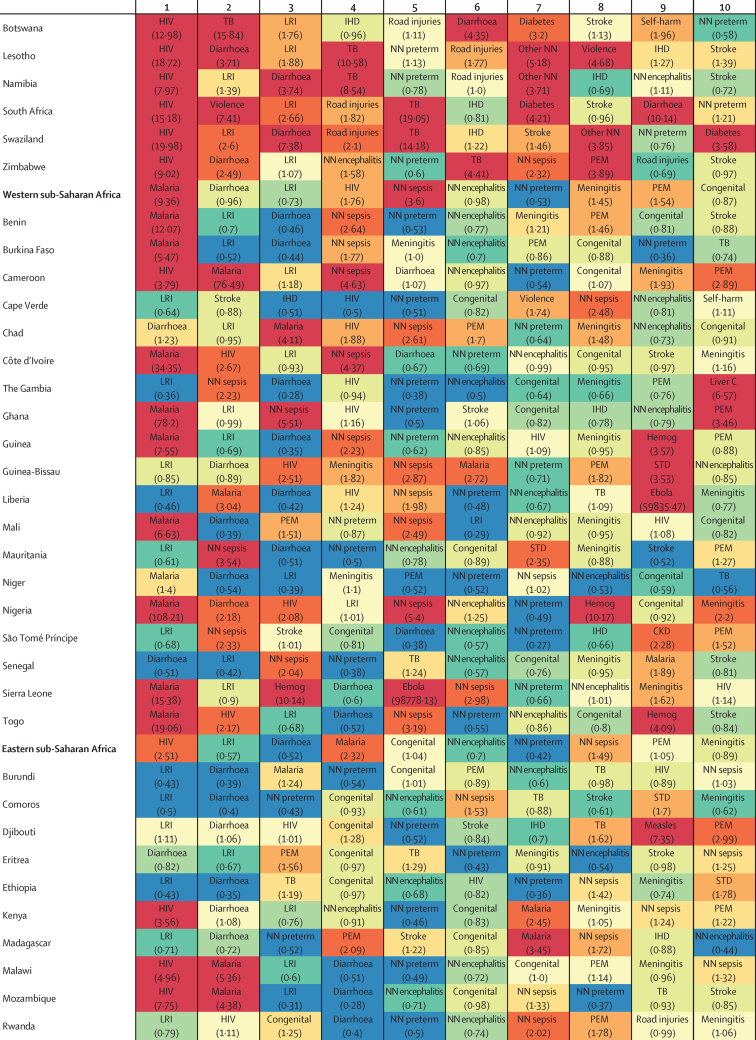
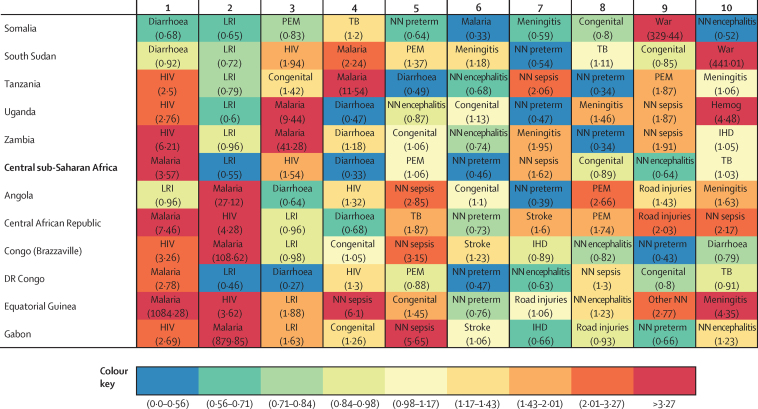
Globally, life expectancy from birth increased from 61·7 years (95% uncertainty interval 61·4–61·9) in 1980 to 71·8 years (71·5–72·2) in 2015. Several countries in sub-Saharan Africa had very large gains in life expectancy from 2005 to 2015, rebounding from an era of exceedingly high loss of life due to HIV/AIDS. At the same time, many geographies saw life expectancy stagnate or decline, particularly for men and in countries with rising mortality from war or interpersonal violence. From 2005 to 2015, male life expectancy in Syria dropped by 11·3 years (3·7–17·4), to 62·6 years (56·5–70·2). Total deaths increased by 4·1% (2·6–5·6) from 2005 to 2015, rising to 55·8 million (54·9 million to 56·6 million) in 2015, but age-standardised death rates fell by 17·0% (15·8–18·1) during this time, underscoring changes in population growth and shifts in global age structures. The result was similar for non-communicable diseases (NCDs), with total deaths from these causes increasing by 14·1% (12·6–16·0) to 39·8 million (39·2 million to 40·5 million) in 2015, whereas age-standardised rates decreased by 13·1% (11·9–14·3). Globally, this mortality pattern emerged for several NCDs, including several types of cancer, ischaemic heart disease, cirrhosis, and Alzheimer's disease and other dementias. By contrast, both total deaths and age-standardised death rates due to communicable, maternal, neonatal, and nutritional conditions significantly declined from 2005 to 2015, gains largely attributable to decreases in mortality rates due to HIV/AIDS (42·1%, 39·1–44·6), malaria (43·1%, 34·7–51·8), neonatal preterm birth complications (29·8%, 24·8–34·9), and maternal disorders (29·1%, 19·3–37·1). Progress was slower for several causes, such as lower respiratory infections and nutritional deficiencies, whereas deaths increased for others, including dengue and drug use disorders. Age-standardised death rates due to injuries significantly declined from 2005 to 2015, yet interpersonal violence and war claimed increasingly more lives in some regions, particularly in the Middle East. In 2015, rotaviral enteritis (rotavirus) was the leading cause of under-5 deaths due to diarrhoea (146 000 deaths, 118 000–183 000) and pneumococcal pneumonia was the leading cause of under-5 deaths due to lower respiratory infections (393 000 deaths, 228 000–532 000), although pathogen-specific mortality varied by region. Globally, the effects of population growth, ageing, and changes in age-standardised death rates substantially differed by cause. Our analyses on the expected associations between cause-specific mortality and SDI show the regular shifts in cause of death composition and population age structure with rising SDI. Country patterns of premature mortality (measured as years of life lost [YLLs]) and how they differ from the level expected on the basis of SDI alone revealed distinct but highly heterogeneous patterns by region and country or territory. Ischaemic heart disease, stroke, and diabetes were among the leading causes of YLLs in most regions, but in many cases, intraregional results sharply diverged for ratios of observed and expected YLLs based on SDI. Communicable, maternal, neonatal, and nutritional diseases caused the most YLLs throughout sub-Saharan Africa, with observed YLLs far exceeding expected YLLs for countries in which malaria or HIV/AIDS remained the leading causes of early death.
Interpretation
At the global scale, age-specific mortality has steadily improved over the past 35 years; this pattern of general progress continued in the past decade. Progress has been faster in most countries than expected on the basis of development measured by the SDI. Against this background of progress, some countries have seen falls in life expectancy, and age-standardised death rates for some causes are increasing. Despite progress in reducing age-standardised death rates, population growth and ageing mean that the number of deaths from most non-communicable causes are increasing in most countries, putting increased demands on health systems.
Funding
Bill & Melinda Gates Foundation.
Introduction
Comparable information about deaths and mortality rates broken down by age, sex, cause, year, and geography provides a starting point for informed health policy debate. However, generating meaningful comparisons of mortality involves addressing many data and estimation challenges, which include reconciling marked discrepancies in cause of death classifications over time and across populations; adjusting for vital registration system data with coverage and quality issues; appropriately synthesising mortality data from cause-specific sources, such as cancer registries, and alternative cause of death identification tools, such as verbal autopsies; and developing robust analytical strategies to estimate cause-specific mortality amid sparse data.1, 2, 3, 4, 5, 6 The annual Global Burden of Disease (GBD) analysis provides a standardised approach to addressing these problems, thereby enhancing the capacity to make meaningful comparisons across age, sex, cause, time, and place.
Research in context.
Evidence before this study
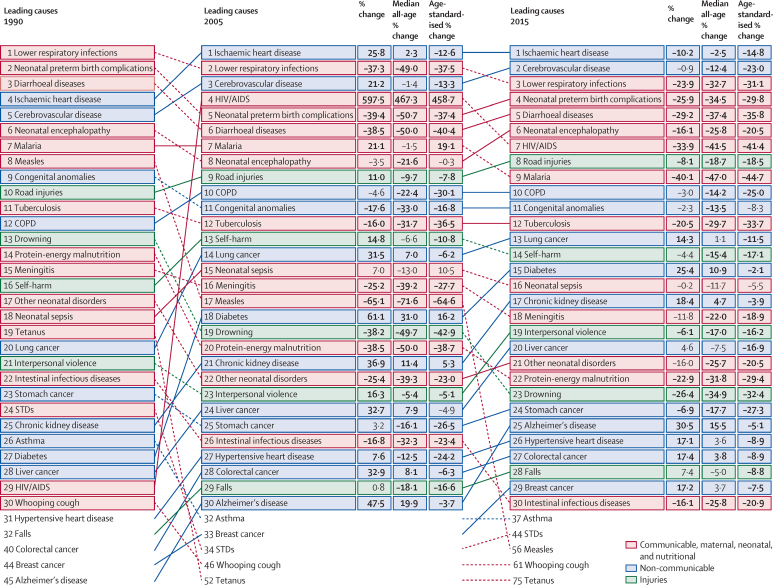
In 2012, the Global Burden of Disease 2010 study was published, providing results from the first complete revision of the Global Burden of Disease (GBD) since the first assessment in 1993. The study reported on mortality and causes of death between 1990 and 2010 in 187 countries. In response to demand for up-to-date information on the health of populations to inform health policy debates, annual updates of the GBD study are now prepared, with the first of these, the GBD 2013 study, published in 2015. For the first time, collaborative teams undertook subnational assessments for China, Mexico, and the UK as part of this study.
Added value of this study
The GBD 2015 assessment of mortality and causes of death provides new and more robust evidence on the health of populations worldwide through the inclusion of subnational data from an expanded group of countries, including Brazil, India, Japan, Kenya, Saudi Arabia, South Africa, Sweden, and the USA, in addition to updates for China, Mexico, and the UK. This study complies with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) recommendations. Estimation of mortality levels, patterns, and distribution for several new causes, including Ebola virus disease, further disaggregations of carcinoma and leukaemia, motor neuron disease, and mortality attributable to environmental heat and cold exposure have been added for the GBD 2015 study. Furthermore, this analysis extends the concept of sociodemographic status first reported in GBD 2013, with important changes to computational methods, resulting in a new Socio-demographic Index (SDI) for a more robust positioning of countries and territories on the development continuum.
Implications of all the available evidence
This study provides the most comprehensive assessment to date of patterns and levels of mortality worldwide, expanding on previous analyses by further investigating the main determinants of epidemiological patterns and trends across geographies and over time. The GBD 2015 study entails a complete reanalysis of trends for each cause of death from 1990 to 2015; the time series published here supersedes the results of the GBD 2013 study. The expansion of geographic units, from 296 in GBD 2013 to 519 for GBD 2015, is envisaged to continue so as to sustain comparability over time and across all geographies. The comparison of estimates of observed mortality levels with patterns expected based on the SDI provides an in-depth understanding of national health challenges and priority areas for intervention.
Previous iterations of the GBD study showed substantial reductions in under-5 mortality, largely driven by decreasing rates of death from diarrhoeal diseases, lower respiratory infections, malaria, and, in several countries, neonatal conditions and malnutrition.7, 8, 9, 10, 11 Non-communicable diseases (NCDs) and injuries claimed increasingly more lives throughout the world, although age-standardised death rates fell for many causes and countries.7 Examination of epidemiological convergence among high-income, middle-income, and low-income countries showed the importance of evaluating both absolute and relative changes in mortality, as solely focusing on absolutes can mask rising relative inequality among certain age groups and causes. The GBD 2015 study expands on these analyses by further evaluating the drivers of epidemiological patterns across countries and over time. Such mortality trends are generally shaped by a combination of factors, including changes in income per capita, educational attainment, fertility, shifts in clinical care and health system responsiveness, emergent health threats such as disease outbreaks or increasing rates of obesity, and geography-specific health contexts. An in-depth understanding of national health gains and priority areas for intervention can be provided by comparing estimates of expected mortality patterns. These results are of particular importance amid debates on financing and policy options for the newly adopted Sustainable Development Goals, which include both ambitious targets for maternal and child health and a much broader health agenda also encompassing NCDs and injuries.
The GBD 2010 study presented results for 187 countries, encompassing all those with a population greater than 50 000 in the year 2000.12 In the GBD 2013 study, collaborative teams produced subnational assessments for the UK, Mexico, and China, expanding the number of geographies included in the GBD analysis to 296.7, 13, 14, 15 The value of such subnational assessments to local decision makers16 has driven further geographical disaggregation for GBD 2015 including in Brazil, India, Japan, Kenya, Saudi Arabia, South Africa, Sweden, and the USA, in addition to updates for China, Mexico, and the UK. The expansion of the geographical units in the GBD studies will continue in a way that will sustain the comparability over time for the period 1990 to present and across all geographic entities.
As with all revisions of the GBD, the GBD 2015 study provides an update for the entire time series from 1990 to 2015 based on newly identified data sources released or collected since GBD 2013. In response to published commentaries and unpublished seminars and communications about GBD methods, various methodological refinements have been implemented.17, 18 Additionally, in the GBD 2015 cycle, a major effort towards data and code transparency has been made. As with each GBD cycle, the full time series published here supersedes previous GBD studies. This detailed assessment of causes of death allows the exploration of key questions including what are the leading causes of deaths in each geography, which causes are increasing or decreasing, what is the expected pattern of change in causes of death with the epidemiological transition and how does this expected pattern over time diverge across geographies.
Methods
Overview
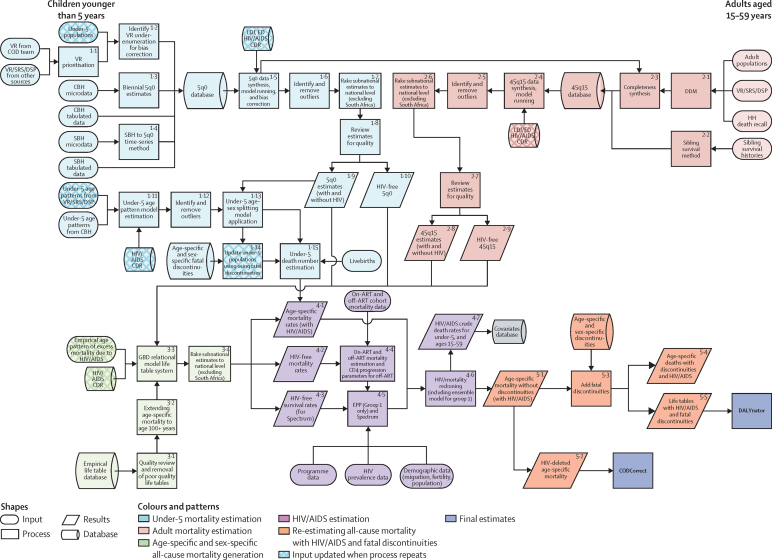
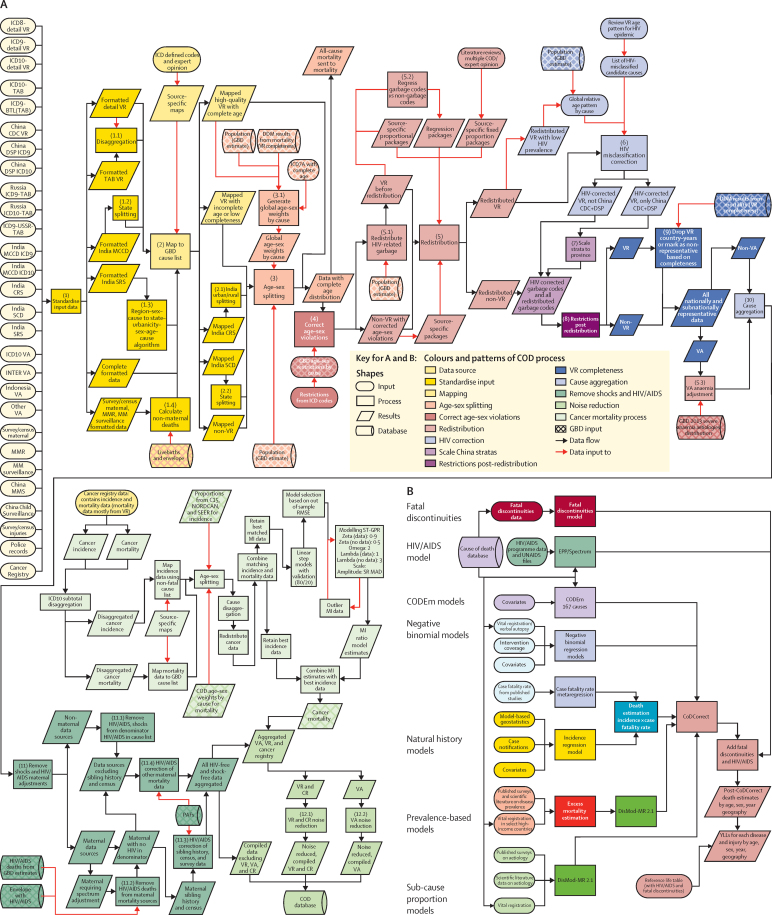
GBD employs various analytical tools and a diverse set of data sources to generate comparable estimates of deaths and mortality rates broken down by age, sex, cause, year, and geography. Multiple publications show more detail on the various aspects of the methods.7, 8, 12, 19 Part 1 of the methods appendix (pp 4–51) is a structured and succinct explanation of each step. Figure 1 shows all of the inputs, analytical processes, and outputs from the analysis of all-cause mortality and HIV/AIDS mortality, included because of its important effects on all-cause mortality in countries with large HIV epidemics, and figure 2 does the same for cause-specific mortality. Each input or process is numbered for reference, with part 2 of the methods appendix (pp 52–70) providing explanation for each step. The GBD analytical approach to estimation is guided by standardised solutions to some general analytical problems: inconsistent case definitions or coding over time or across geographies; missing data; conflicting data for the same year and geography; and population groups (eg, the poor, minorities, and vulnerable groups) who are often missed in administrative data sources. In this Article, we provide only a very high-level summary. This analysis adheres to the new Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) proposed by the World Health Organization (WHO) and others, which includes recommendations on documentation of data sources, estimation methods, and statistical analysis.20 Table 1 shows the precise ways in which we have adhered to each element of the GATHER agreement.
Figure 1.
Estimation of all-cause mortality by age and sex and HIV/AIDS incidence, prevalence, and mortality for GBD 2015
Data and analyses are indicated by shape and the flow chart is colour coded by major estimation component. The process depicted is performed twice to bring in updated under-5 population estimates and crude death rates due to HIV/AIDS. The inputs that are updated in the second run of the process are shown by patterned boxes in this flow chart. Because of the very large and changing effects of HIV/AIDS on all-cause mortality in several countries with large HIV epidemics and limited data on all-cause mortality, the estimation of HIV/AIDS and all-cause mortality are closely linked and are presented jointly here. GBD=Global Burden of Disease. 5q0=probability of death from birth to age 5 years. 45q15=probability of death from age 15 to 60 years. ART=antiretroviral therapy. CBH=complete birth histories. CDR=crude death rate. COD=causes of death. DSP=disease surveillance points. ED=educational attainment in years per capita above age 15 years and mother's educational attainment in years per capita for children younger than 5 years. EPP=Estimation and Projection Package. HIV CDR=crude death rate due to HIV/AIDS. LDI=lagged distributed income per capita. SBH=summary birth history. SRS=Sample Registration System. VR=vital registration.
Figure 2.
Development of the GBD 2015 cause of death database
Figure shows (A) different strategies used to model different causes and to (B) combine them into a consistent set of cause-specific deaths for each location, age, sex, and year. Data and analytical processes are indicated by shape and the flow chart is colour coded by major estimation component. GBD=Global Burden of Disease. BTL=basic tabulation list. CDC=Center for Disease Control and Prevention. COD=cause of death. CODEm=Cause Of Death Ensemble model. CR=cancer registry. CRS=civil registration system. DSP=disease surveillance points. ICD=International Classification of Diseases. MI=mortality/incidence ratio. MCCD=medical certification of causes of death. MM=maternal mortality. MMR=maternal mortality ratio. MMS=maternal mortality surveillance. PAF=population-attributable fraction. SCD=survey of causes of death. SEER=Surveillance, Epidemiology, and End Results Program. SRS=Sample Registration System. SR MAD=super-region median average deviation. ST-GPR=spatiotemporal Gaussian process regression. VA=verbal autopsy. VR=vital registration. YLL=years of life lost.
Table 1.
GATHER checklist with description of compliance and location of information in the GBD 2015 mortality and causes of death study
| GATHER checklist item | Description of compliance | Reference | |
|---|---|---|---|
| Objectives and funding | |||
| 1 | Define the indicators, populations, and time periods for which estimates were made | Narrative provided in paper and methods appendix describing indicators, definitions, and populations | Main text (Methods—Geographic units, GBD cause list, Time periods) and methods appendix (pp 4–70) |
| 2 | List the funding sources for the work | Funding sources listed in paper | Summary (Funding) |
| Data inputs | |||
| For all data inputs from multiple sources that are synthesised as part of the study | |||
| 3 | Describe how the data were identified and how the data were accessed | Narrative description of data seeking methods provided | Main text (Methods) and methods appendix (pp 4–283) |
| 4 | Specify the inclusion and exclusion criteria; identify all ad-hoc exclusions | Narrative about inclusion and exclusion criteria by data type provided | Main text (Methods) and methods appendix (pp 4–283) |
| 5 | Provide information on all included data sources and their main characteristics; for each data source used, report reference information or contact name or institution, population represented, data collection method, years of data collection, sex and age range, diagnostic criteria or measurement method, and sample size, as relevant | An interactive, online data source tool that provides metadata for data sources by component, geography, cause, risk, or impairment has been developed | Online data citation tools |
| 6 | Identify and describe any categories of input data that have potentially important biases (eg, based on characteristics listed in item 5) | Summary of known biases by cause included in methods appendix | Methods appendix (pp 4–283) |
| For data inputs that contribute to the analysis but were not synthesised as part of the study | |||
| 7 | Describe and give sources for any other data inputs | Included in online data source tool | Online data citation tools |
| For all data inputs | |||
| 8 | Provide all data inputs in a file format from which data can be efficiently extracted (eg, a spreadsheet as opposed to a PDF), including all relevant metadata listed in item 5; for any data inputs that cannot be shared due to ethical or legal reasons, such as third-party ownership, provide a contact name or the name of the institution that retains the right to the data | Downloads of input data available through online tools, including data visualisation tools and data query tools; input data not available in tools will be made available upon request | Online data visualisation tools, data query tools, and the Global Health Data Exchange |
| Data analysis | |||
| 9 | Provide a conceptual overview of the data analysis method; a diagram may be helpful | Flow diagrams of the overall methodological processes, as well as cause-specific modelling processes, have been provided | Main text (Methods, Figure 1, Figure 2) and methods appendix (pp 4–287) |
| 10 | Provide a detailed description of all steps of the analysis, including mathematical formulae; this description should cover, as relevant, data cleaning, data pre-processing, data adjustments and weighting of data sources, and mathematical or statistical models | Flow diagrams and corresponding methodological write-ups for each cause, as well as the demographics and causes of death databases and modelling processes, have been provided | Main text (Methods, Figure 1, Figure 2) and methods appendix (pp 4–287) |
| 11 | Describe how candidate models were evaluated and how the final models were selected | Provided in the methodological write-ups | Methods appendix (pp 71–283) |
| 12 | Provide the results of an evaluation of model performance, if done, as well as the results of any relevant sensitivity analysis | Provided in the methodological write-ups | Methods appendix (pp 71–283) |
| 13 | Describe methods for calculating uncertainty of the estimates; state which sources of uncertainty were, and were not, accounted for in the uncertainty analysis | Provided in the methodological write-ups | Methods appendix (pp 71–283) |
| 14 | State how analytic or statistical source code used to generate estimates can be accessed | Access statement provided | Code is provided in an online repository |
| Results and discussion | |||
| 15 | Provide published estimates in a file format from which data can be efficiently extracted | GBD 2015 results are available through online data visualisation tools, the Global Health Data Exchange, and the online data query tool | Main text, methods appendix, and online data tools (data visualisation tools, data query tools, and the Global Health Data Exchange) |
| 16 | Report a quantitative measure of the uncertainty of the estimates (eg, uncertainty intervals) | Uncertainty intervals are provided with all results | Main text, methods appendix, and online data tools (data visualisation tools, data query tools, and the Global Health Data Exchange) |
| 17 | Interpret results in light of existing evidence; if updating a previous set of estimates, describe the reasons for changes in estimates | Discussion of methodological changes between GBD rounds provided in the narrative of the Article and methods appendix | Main text (Methods and Discussion) and methods appendix (pp 4–287) |
| 18 | Discuss limitations of the estimates; include a discussion of any modelling assumptions or data limitations that affect interpretation of the estimates | Discussion of limitations provided in the narrative of the main paper, as well as in the methodological write-ups in the methods appendix | Main text (Limitations) and methods appendix (pp 4–283) |
GBD 2015=Global Burden of Disease 2015 Study. GATHER=Guidelines for Accurate and Transparent Health Estimates Reporting.
Geographic units
We have organised geographies into a set of hierarchical categories: seven super-regions; 21 regions nested within the seven super-regions; and 195 countries and territories nested within the 21 regions (table 2). Details on the classification of each geographical unit into each level of this hierarchy are provided in the methods appendix (pp 670–83). Compared with GBD 2013, we have added seven territories—American Samoa, Bermuda, Greenland, Guam, the Northern Mariana Islands, Puerto Rico, and the Virgin Islands—because of the availability of high-quality vital registration data. These territories were not previously included in the national totals of the USA, UK, or Denmark, and were included only in GBD 2013 regional totals. We have further disaggregated data for selected countries or territories into subnational units: 26 states and one district for Brazil, 34 provinces and municipalities for China, 31 states and union territory groupings for India that include 62 rural and urban units, 47 prefectures for Japan, 47 counties for Kenya, 32 states and districts for Mexico, 13 regions for Saudi Arabia, nine provinces for South Africa, two regions for Sweden, 13 regions for the UK (Northern Ireland, Scotland, Wales, England, and nine subregions of England), and 51 states and districts for the USA. At the first subnational unit level, we have 256 geographic units. Subnational level 1 geographies in the GBD 2015 analysis include countries that have been subdivided into the first subnational level, such as states or provinces. The subnational level 2 category applies only to India and England. In this Article we present national, territory, and previously published subnational units in the UK.13
Table 2.
Number of geographies and causes at each hierarchical level for GBD 2015
| Number of geographies | ||
|---|---|---|
| Geographical levels | ||
| Super-region | 7 | |
| Regions | 21 | |
| Nations and territories | 195 | |
| Subnational level 1 | 480 | |
| Subnational level 2 | 519 | |
| Cause levels | ||
| Level 1 | ||
| Total causes | 3 | |
| YLD causes | 3 | |
| YLL causes | 3 | |
| Level 2 | ||
| Total | 21 | |
| YLD | 21 | |
| YLL | 21 | |
| Level 3 | ||
| Total causes | 166 | |
| YLD causes | 161 | |
| YLL causes | 144 | |
| Level 4 | ||
| Total causes | 261 | |
| YLD causes | 256 | |
| YLL causes | 200 | |
Nations and territories includes countries, territories, and non-sovereign states. Subnational level 1 includes countries that, in the GBD analysis, have been subdivided into the first subnational level such as states or provinces. Subnational level 2 applies only to India and England. In India, states have been divided into urban and rural units. England has been divided into nine regions. For each level, the number of geographies includes the geographies at that level plus the number of most-detailed geographies at each higher level such that at each level of the hierarchy, all geographies create a collectively exhaustive and mutually exclusive set covering the world. Likewise, the GBD cause list is mutually exclusive and collectively exhaustive. The three Level 1 GBD causes consist of communicable, maternal, neonatal, and nutritional disorders; non-communicable diseases; and injuries. Level 2 causes consist of 21 cause groups, such as neoplasms and cardiovascular diseases. Levels 3 and 4 consist of disaggregated causes, such as liver cancer and cerebrovascular disease (Level 3), and liver cancer due to hepatitis C and ischaemic stroke (Level 4). GBD=Global Burden of Disease. YLD=years lived with disability. YLL=years of life lost.
GBD cause list
The GBD cause list is the crucial organising framework for the analysis of causes of death and premature mortality, as well as disease incidence and prevalence and years lived with disability.21 The GBD cause list has evolved during the 25 years of the GBD study to become a list of causes that have public health and medical care importance either because they are major causes of lost health or because of policy relevance.7, 21, 22, 23, 24 Because different levels of cause aggregation are appropriate for different purposes and users, the GBD cause list is organised hierarchically (table 2). At each level of the cause hierarchy, the set of causes is mutually exclusive and collectively exhaustive.21 At the first level of the cause list, there are three broad causes: communicable, maternal, neonatal, and nutritional diseases; NCDs; and injuries. At the second level of the hierarchy, these three causes are broken down into 21 cause groups such as neoplasms (cancers) or cardiovascular diseases. Levels 3 and 4 of the cause list provide more disaggregated causes. Based on policy interest and by approval of the GBD Scientific Council, we have added eight causes to the GBD cause list: Ebola virus disease, motor neuron disease, environmental heat and cold exposure, squamous-cell carcinoma, acute lymphoid leukaemia, chronic lymphoid leukaemia, acute myeloid leukaemia, and chronic myeloid leukaemia. Bulimia nervosa has also been added as a cause of death. In total, there are now three causes at Level 1, 21 at Level 2, 166 at Level 3, and 261 at Level 4. Some causes, such as acne, medication overuse headache, and cutaneous leishmaniasis, are not considered causes of death according to the rules of the International Classification of Diseases (ICD), so the number of causes included in this analysis of causes of death is three at Level 1, 21 at Level 2, 144 at Level 3, and 200 at Level 4. The full GBD cause list, including those for which we estimate deaths, is available in the methods appendix (pp 684–90).
Time periods
Because of the greater availability of data on all-cause mortality than cause-specific mortality, the all-cause mortality analysis for GBD 2015 covered 1970 to 2015. The cause of death analysis of GBD 2015 covered 1980 to 2015. A complete set of age-specific, sex-specific, cause-specific, and geography-specific death numbers and rates were generated. We present results covering different periods. However, for the main global and national results, we have focused on trends in the past decade, from 2005 to 2015, and detailed findings in 2015. Data visualisation tools are available online and provide results for each year from 1990 to 2015.
All-cause mortality and HIV/AIDS mortality
Because of the very large and changing effects of HIV/AIDS on all-cause mortality in several countries with large HIV epidemics and scarce data on all-cause mortality, especially in eastern and southern Africa,11 the estimation of HIV/AIDS mortality and all-cause mortality are closely linked and presented jointly in figure 1. We divided the estimation effort into five distinct components: estimation of under-5 mortality rate (5q0); estimation of the adult mortality rate (45q15); age-specific mortality estimation; HIV/AIDS mortality estimation; and addition of the effects of events such as wars, pandemics, and disasters, which can cause abrupt discontinuities in death numbers (fatal discontinuities). Because of the interdependencies in the estimation of HIV/AIDS incidence, prevalence, and mortality and all-cause mortality, the estimation steps shown in figure 1 were repeated, with the HIV/AIDS crude death rates produced in step 4.7 used as covariates in steps 1.5, 1.11, 2.4, and 3.3 in the flow diagram.
Under-5 mortality estimation
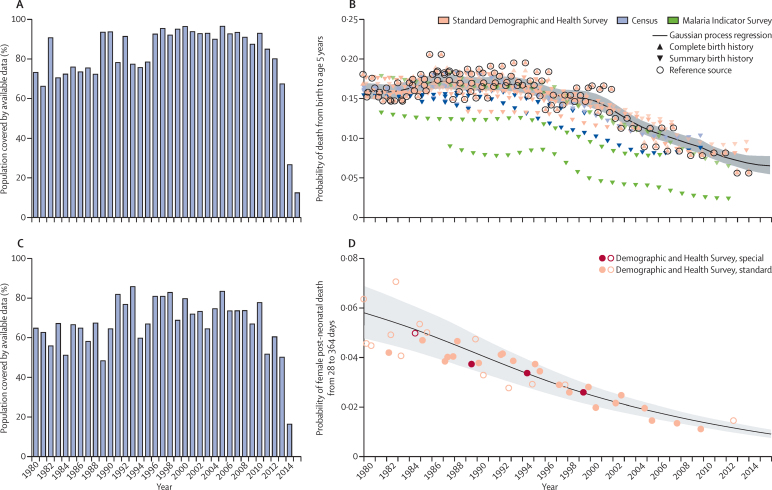
Seven types of primary data contributed to the estimation process (oval shapes in figure 1). The most important set of inputs were the data for estimating the overall level of under-5 mortality (5q0) that were obtained from vital registration systems, surveys, and censuses. Figure 3A provides information about the proportion of the 519 geographies included in the analysis for which data were available in each year from 1980 to 2015. Because of lags in reporting of both vital registration data and the release of household survey or census data, the availability of data was much lower for 2014 and 2015 than for previous years. Different data types, such as summary or complete birth histories, were processed to yield estimates for each year of the under-5 death rate; country-specific and year-specific details of the measurements are provided in the methods appendix (pp 4–19). Figure 3B shows the nature of the data and estimation process for under-5 mortality using the example of Zambia, as well as the uncorrected and bias-adjusted datapoints for each source. We used spatiotemporal Gaussian process regression to synthesise the sources and simultaneously correct for biases in specific source types.8 Bias corrections were made by comparison to reference sources, which for Zambia were the Demographic and Health Surveys. Further details of this estimation process are provided in the methods appendix (pp 4–19).
Figure 3.
Examples of under-5 mortality data availability and estimation
(A) Percentage of global under-5 population covered by under-5 mortality data for each year, 1980–2015. The percentage of under-5 population covered was calculated by dividing the population of children aged 0–4 years in locations covered by available under-5 mortality data by the total global under-5 population. Because of lags in reporting of both vital registration data and the release of household survey or census data, the availability of data was much lower for 2014 and 2015 than for previous years. (B) Country-specific example of data and under-5 mortality estimates in Zambia, 1980–2015. The black line shows Gaussian process regression fit with 95% uncertainty interval shown in grey. Black circles denote reference data. Triangles denote complete birth history data. Inverted triangles denote summary birth history data. Transparent symbols are the data post-adjustment for non-sampling error. Hollow shapes represent data identified as outliers. (C) Percentage of global under-5 population covered by under-5 age-specific and sex-specific data for each year, 1980–2015. The percentage of under-5 population covered was calculated by dividing the population of children aged 0–4 years in locations covered by available under-5 age-specific and sex-specific data by the total global under-5 population. Because of lags in reporting both vital registration data and the release of household survey or census data, the availability of data was much lower for 2014 than for previous years, and no data existed for 2015. (D) Country-specific example of probability of female post-neonatal mortality in Bangladesh, 1980–2015. Standard Demographic and Health Surveys generally include large population samples and standard sets of questions. Special Demographic and Health Surveys can survey smaller, more targeted populations, such as women who have given birth. The black line shows probability of death, with 95% uncertainty interval shown in grey. Solid circles represent data sources. Hollow circles represent outliers. The post-neonatal period is 28–364 days.
Because there are many sources for measuring under-5 mortality, such as summary birth histories from censuses and surveys, that do not provide sex and specific age group detail, we first estimated under-5 mortality and then split it into mortality for four age groups: early neonatal (0–6 days), late neonatal (7–28 days), post-neonatal (29–364 days), and ages 1–4 years. Splitting into these age groups was based on a statistical model using the analysis of available data that provide breakdowns by age and sex. Figure 3C shows the availability by country–year of data used to build the model to estimate mortality for specific age–sex groups younger than age 5 years. We modelled the ratio of male-to-female probability of death from birth to age 5 years as a function of both sexes' combined under-5 mortality rate and country and regional random effects. We further disaggregated sex-specific probability of death between birth and age 5 by modelling the ratio between age-and-sex-specific probability of deaths in the early neonatal, late neonatal, post-neonatal, and 1–4 year age groups and sex-specific probability of death between birth and age 5 years. This model allowed for the association between these age-and-sex-specific probabilities and the under-5 death rate to be non-linear, and included other covariates consisting of the death rate due to HIV/AIDS in children younger than 5 years, average years of schooling among females of reproductive age, and country and regional random effects. More details, including the equations are provided in the methods appendix (p 18). Figure 3D shows an example of the empirical fit for the post-neonatal period for Bangladesh. This model was applied to all countries to generate the under-5 estimates for each geography–year.
With the estimated mortality by detailed age group, we generated both deaths and population estimates for the respective age groups for each location, sex, and year.
Adult mortality estimation
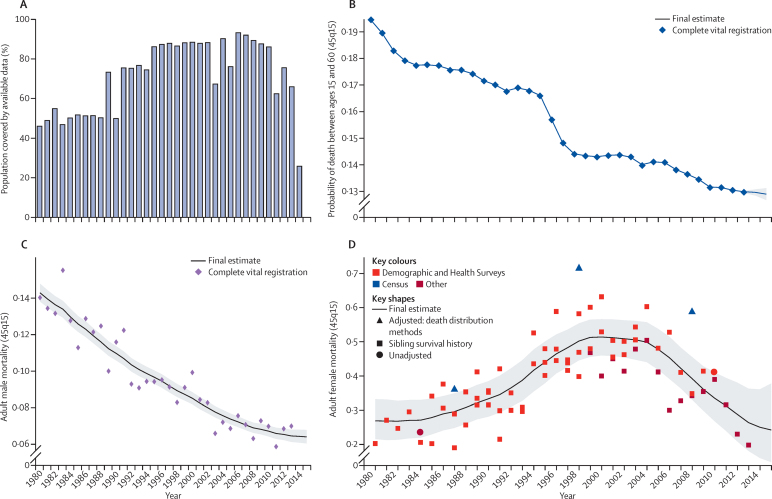
Measurements of adult mortality (45q15) were mainly derived from vital registration data and household surveys that ask about the birth and death of siblings.25 In a smaller set of cases, information was obtained from censuses or surveys about household deaths in a defined interval before the interview. Figure 4A shows the number of geographies for which data in each year were available for adult mortality estimation. Vital registration data were assessed for completeness with death distribution methods optimised for performance.26, 27 We generated a best estimate of the completeness of vital registration in each geography over time by combining estimated completeness of registration for under-5 deaths with the results for different intercensal periods of the application of three death distribution methods. These sources were combined by use of spatiotemporal Gaussian process regression—details are provided in the methods appendix (p 21). Data from sibling histories were corrected for known biases, including selection bias, zero reporter bias, and recall bias.7, 25 Our sibling history method can also deal with data sparsity in many sibling survival modules (ie, sibling history questions and variables from surveys). The predictive validity of the sibling history analytical methods has been assessed with simulated data and shown to be unbiased.25 Additionally, we compared estimates of adult mortality rates from sibling survival data with completeness-adjusted vital registration data in countries from which both sources are available and found no systematic biases from sibling survival method (methods appendix p 292).7, 25, 26 We synthesised vital registration data corrected for completeness and adjusted sibling history data into a best time series estimate of adult 45q15 using spatiotemporal Gaussian process regression. Examples of the application of these steps in three types of settings are shown in figure 4.
Figure 4.
Examples of adult mortality data availability and estimation
(A) Percentage of global adult population covered by adult mortality data from vital registration systems, sibling survival surveys, sample registration systems, or censuses, 1980–2015. The percentage of available data was calculated by dividing the population of adults aged 15–59 years in locations covered by available adult mortality data by the total global population aged 15–49 years. Because of lags in reporting both vital registration data and the release of household survey or census data, the availability of data was much lower for 2014 than for previous years, and no data existed for 2015. Country-specific examples of (B) vital registration data and adult male mortality (45q15) estimation for a country with complete vital registration and large population (USA), 1980–2015; (C) vital registration data and adult male mortality (45q15) estimation for a country with complete vital registration and small population (Iceland), 1980–2015; and (D) sibling survival data and adult female mortality (45q15) estimation (Malawi), 1980–2015. Black line shows final estimates of adult mortality among males or females in each country, with 95% uncertainty interval shown in grey. Squares show sibling survival histories. 45q15=probability of death from age 15 years to 60 years.
The spatiotemporal Gaussian process regression method used to fit the model to the available data included lag distributed income per capita, educational attainment, and the estimated HIV/AIDS death rate as covariates. Because the estimation of the HIV/AIDS death rate used the estimate of HIV-free mortality rate by age and sex as an input, the entire estimation loop was repeated once, which dealt with this interconnection. Step 2.9 in figure 1 deals with situations in which an inconsistency exists between the spatiotemporal Gaussian process regression-estimated adult mortality rate and the separately estimated crude death rate due to HIV/AIDS. When the HIV/AIDS death rate as estimated from the natural history model is too high compared with demographic sources, there is a risk that HIV-free death rates are depressed to implausibly low levels. In step 2.9, we scaled the HIV/AIDS crude death rate by imposing a maximum proportion of deaths that can be attributed to HIV/AIDS, as shown in our version of UNAIDS' Spectrum model, which estimates HIV/AIDS prevalence and deaths by age and sex. Our adult mortality estimation is for ages 15–60 years (45q15), but other adult age groups that can be calculated for other purposes include 35q15 (ages 15–50 years, corresponding to the reproductive age period), and 20q50 (ages 20–70 years).
Age-specific mortality
In demographic estimation, measures of child mortality, adult mortality, or both are used alongside a model life table system to predict age-specific mortality.27, 28, 29, 30 The UN mostly still uses the Coale-Demeny model life tables, which were based on 192 empirical tables gathered before 1963, and in a few cases they use the 33-year-old UN Model Life Table for Developing Countries.31, 32 Murray and colleagues33 developed the Modified Logit Model Life Table system that is used by WHO to estimate age-specific mortality, which captures a much wider range of age patterns of mortality through the year 2000. The GBD approach uses three inputs to generate age-specific mortality: 5q0, 45q15, and a relevant empirical reference pattern of mortality by age.7 The reference in the GBD system was selected on the basis of empirical age patterns that are closest to the population in space and time.7 The reference was developed with a database of 16 507 age patterns of mortality from settings that meet explicit inclusion criteria as described in the methods appendix (pp 34–42). Table 3 shows a summary of the availability of empirical age–sex patterns of mortality in the GBD database.
Table 3.
Distribution of empirical life tables by GBD super-region and decade, 1950–2015
| 1950–59 | 1960–69 | 1970–79 | 1980–89 | 1990–2000 | 2000–14 | |
|---|---|---|---|---|---|---|
| Central Europe, eastern Europe, and central Asia | 61 | 145 | 240 | 386 | 477 | 555 |
| High income | 434 | 498 | 611 | 2481 | 2399 | 3056 |
| Latin America and Caribbean | 56 | 170 | 280 | 879 | 948 | 1416 |
| North Africa and Middle East | 2 | 5 | 16 | 27 | 32 | 61 |
| South Asia | 45 | 145 | ||||
| Southeast and east Asia and Oceania | 3 | 30 | 76 | 171 | 148 | 310 |
| Sub-Saharan Africa | 2 | 60 | 282 | |||
| Total | 556 | 848 | 1223 | 3946 | 4109 | 5825 |
Numbers show available empirical life tables in the GBD 2015 database. All life tables included in the database meet quality inclusion criteria whereby the observed age-specific mortality rate in an empirical life table conforms to the age pattern of mortality as described by the Gompertz–Makeham law of mortality and that observed in countries with high-quality vital and civil registration systems. GBD=Global Burden of Disease.
To account for the effect of HIV/AIDS on the age pattern of mortality, the GBD model life table system for locations affected by HIV/AIDS and without high-quality vital registration data used a two-step process whereby we first estimated an HIV-free age pattern of mortality assuming that deaths due to HIV/AIDS were removed. This was accomplished by use of the HIV-free and without-fatal-discontinuity 5q0 and 45q15 estimates, crude death rates due to HIV/AIDS in age groups 0–4 and 15–59 years, and the methods detailed in the methods appendix (p 44), which reconcile the potential disconnect between HIV/AIDS mortality implied in the spatiotemporal Gaussian process regression estimates of all-cause mortality and those estimated by the Estimation and Project Package (EPP)-Spectrum. We then added the excess mortality due to HIV/AIDS to specific age groups to match the with-HIV/AIDS 5q0 and 45q15 by using the estimated age pattern of excess mortality due to HIV/AIDS for generalised and concentrated epidemics. These age patterns of excess mortality were based on ICD-10-coded vital registration data from various countries, including high-income countries with good-quality vital registration data and other middle-income nations that are affected by HIV/AIDS such as South Africa, Thailand, and Trinidad and Tobago. A list of country–years for which we obtained the empirical age pattern of HIV/AIDS excess mortality rate is shown in the methods appendix (p 313).
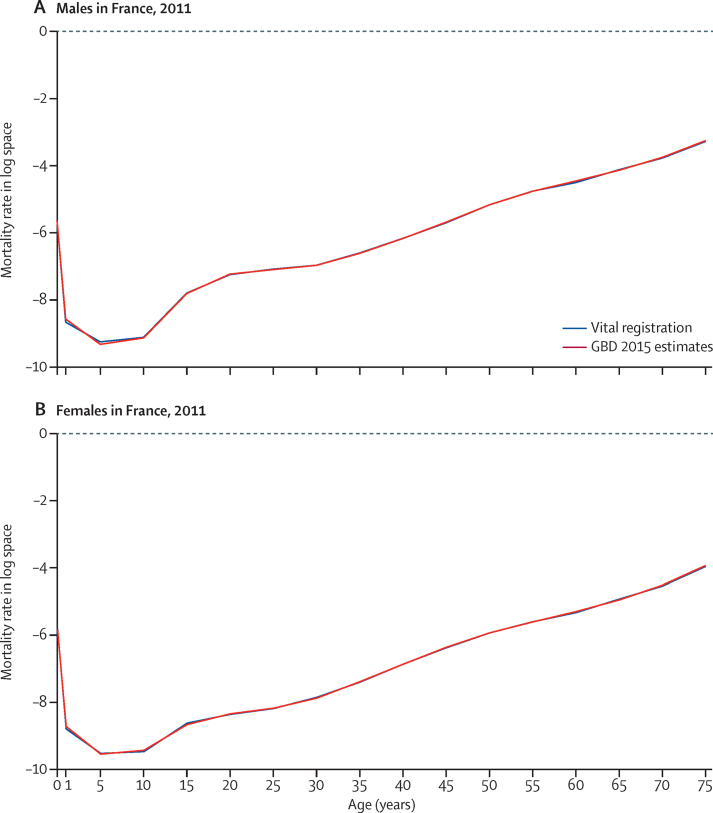
Figure 5 shows examples of the life table system estimates of age-specific mortality compared with observed patterns for males and females in France in 2011. There was a very close fit between the estimated age-specific mortality and the observed mortality.
Figure 5.
Age-specific mortality estimation with GBD life table method versus observed data excluded from the model
Country-specific examples for (A) males and (B) females in France, 2011. The red line shows the GBD 2015 life table system estimates of age-specific mortality rates from birth through age 75 years in log space, compared with observed age-specific mortality (blue line). Year 2011 selected for illustration purposes. GBD=Global Burden of Disease.
HIV/AIDS estimation
Because HIV/AIDS estimation is so closely connected to all-cause mortality estimation, we discuss HIV/AIDS estimation separately here rather than in the later section about estimating other causes of death. We divided geographies into two broad groups: countries with larger epidemics and incomplete or non-existent vital registration systems and the remaining geographies. For the first group of geographies for which we had necessary information about the transmission of HIV/AIDS among adults and children and other programme information, we fitted a modified version of EPP-Spectrum11, 34 to the data on prevalence collated by UNAIDS from antenatal clinic serosurveillance and household surveys. EPP-Spectrum is a natural history model of the HIV/AIDS epidemic that has two distinct components. In the EPP component, data on the prevalence of HIV are used to back-estimate incidence of HIV. In the Spectrum component, the estimated incidence and a set of assumptions are used to estimate prevalence and deaths by age and sex. These assumptions are informed by published or unpublished cohort studies on the initial CD4 distribution of new HIV infections, rates of decline in CD4 counts, death rates on and off antiretroviral therapy (ART) differentiated by age, sex, and CD4 count, and prevention of mother-to-child transmission (PMTCT) coverage data, as well as other demographic assumptions, such as the HIV-free death rate. We have modified EPP-Spectrum to enhance the internal consistency between EPP and Spectrum and to more accurately reflect published cohort data on CD4 progression and death rates on and off ART.
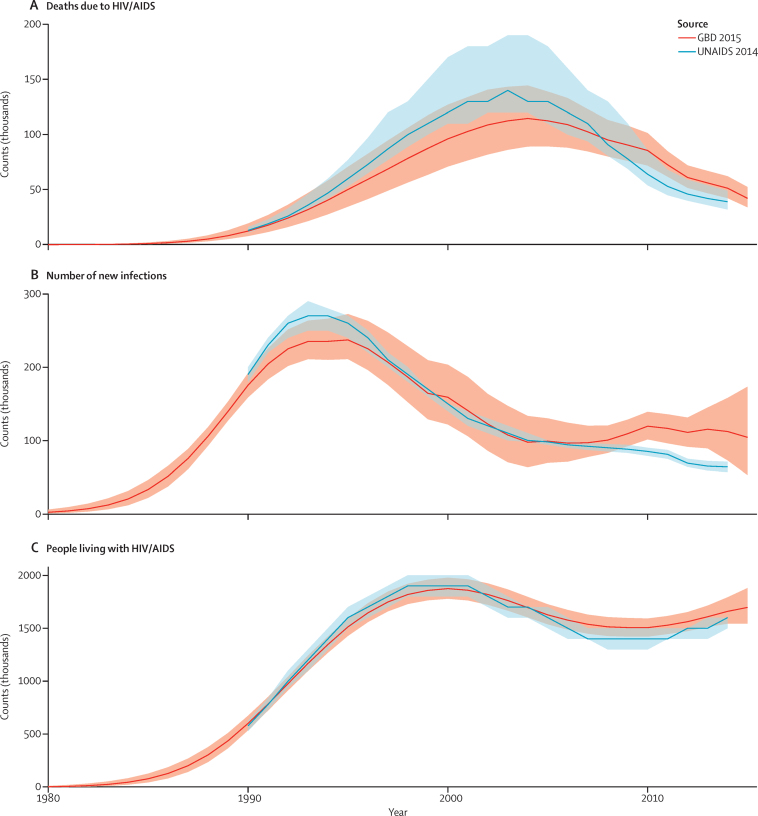
At this point in the estimation process for the first group of geographies, we generated two estimates of HIV/AIDS. One is informed by available data on all-cause mortality and the statistically related association between all-cause mortality and the HIV/AIDS crude death rate; the other is the EPP-Spectrum natural history model. In some locations, these estimates can be quite different. Given the inherent uncertainties in both methods, for GBD 2015 we have adopted an ensemble model which is the average of HIV/AIDS deaths for each age–sex–year from the two approaches. Figure 6 shows the results of this process using Zimbabwe as an example for incidence, prevalence, and deaths from HIV/AIDS. For comparison, we provide prevalence data from surveys and the UNAIDS estimates from their 2014 round of estimation.35, 36
Figure 6.
Comparisons of GBD 2015 estimates and UNAIDS 2014 estimates for Zimbabwe
Country-specific example comparing estimates of deaths due to HIV/AIDS (A), new HIV infections (B), and people living with HIV/AIDS (C) in Zimbabwe from GBD 2015 and UNAIDS 2014. Curves show the estimation process of a particular country and highlight the differences in results from the GBD and UNAIDS analysis of the same prevalence data. Numbers are reported in thousands. Uncertainty intervals are shown in red and blue shading. GBD=Global Burden of Disease. UNAIDS=The Joint United Nations Programme on HIV and AIDS.
For the second group of geographies, we estimated mortality due to HIV/AIDS on the basis of vital registration data if available. Estimates of incidence and prevalence for HIV were based on calibrating Spectrum to match the observed numbers of HIV/AIDS deaths after accounting for under-registration of the vital registration system. This calibration method is based on tracking incidence cohorts through Spectrum and adjusting incidence to fit the observed deaths for that cohort in each year in a specific age group (methods appendix p 44). Using the methods discussed in the age-specific mortality section, with-HIV/AIDS all-cause mortality was estimated for these countries. Depending on the subgroup categorisation within the second group of geographies (methods appendix pp 44–45), we generated the HIV/AIDS-specific mortality either by applying spatiotemporal Gaussian process regression to HIV/AIDS cause-specific data from the vital registration system if the quality of vital registration was deemed high (methods appendix pp 21–26) or by using cohort incidence bias-adjusted mortality estimates from Spectrum.
Fatal discontinuities
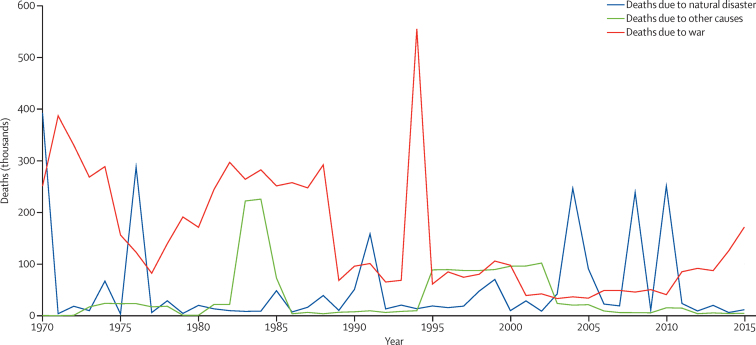
The fifth stage of estimation (figure 1) re-estimates all-cause mortality by incorporating the effects of HIV/AIDS and fatal discontinuities. To incorporate fatal discontinuities from natural disasters (eg, the 2011 Japan earthquake and tsunami), wars, pandemics, wildfires (eg, the Australian bushfires in 2009), or major transportation accidents (eg, the Al Ayyat train accident in Egypt in 2002), we used death counts reported in a wide range of international databases such as the International Disaster Database, the Uppsala Conflict Data Program, the International Institute for Strategic Studies Armed Conflict Databases, the Robert S Strauss Center, and various internet sources for more recent events such as the ongoing Syrian and Yemeni conflicts (databases are listed in the methods appendix [p 270], and additional sources are downloadable from the online source tool).37, 38, 39, 40 When multiple sources for the same fatal discontinuity event exist, we prioritised data from vital registration systems if it had the highest estimate, and gave least priority to data from internet searches. We constructed uncertainty on the basis of high and low estimates when available. We generated regional and cause-specific uncertainty intervals in relative terms and applied them to fatal discontinuities when only the mean estimate was provided by a specific source. The fatal discontinuity section of the methods appendix (pp 270–73) provides more detail on how we assigned fatal discontinuity deaths to different GBD causes as appropriate and how we applied a cause-specific age–sex splitting model to arrive at age-and-sex-specific deaths due to specific fatal discontinuity events.
Given that all-cause mortality analysis requires estimates of crude death rates from HIV/AIDS as initial inputs and that the ensemble model changes the HIV/AIDS-specific and with-HIV/AIDS age-specific mortality rates, the all-cause mortality and HIV/AIDS estimation processes were performed twice. Crude death rates due to HIV/AIDS and under-5 population estimates were generated in the first run of the processes and propagated the second run of the processes to make the HIV/AIDS-specific and all-cause mortality processes more consistent.
Causes of death estimation
The GBD cause list relies on categorical attribution of deaths to a single underlying cause in accordance with the principles outlined in the ICD. The core principle of the ICD is to assign each death to only the underlying cause of death; ie, the cause that initiated the series of events leading to death. We used the ICD principle of underlying cause of death for the primary tabulations in this Article. Data from vital registration sources, verbal autopsy studies, and other sources all adhere to the same principle that one death can only have one cause. The categorical attribution of causes of death differs from a counterfactual approach, which answers the question “in the absence of the disease of interest how many deaths would not have occurred?”, similar to how we estimate burden due to risk factors in GBD. The categorical attribution of causes of death also differs from excess mortality in people with a disease followed up over time in a cohort study or through linkage of a disease registry to vital registration data. The excess mortality in such studies might contain deaths that are assigned as the underlying cause, those that are causally related to the disease, and those that are due to confounding, such as by a common underlying risk that predisposes to the disease but where there are also additional pathways to death. These counterfactual and excess mortality relationships are important and need to be quantified by considering the underlying risk, such as elevated fasting plasma glucose.
Figure 2 shows the steps in the estimation of causes of death, which are divided into seven categories: cause of death database development (figure 2A), Cause of Death Ensemble modelling (CODEm), negative binomial models for rare causes, natural history models, subcause proportion models, prevalence-based models, and CodCorrect (figure 2B). For each component, we discuss the steps, with more extensive detail provided in the methods appendix (pp 52–283). Details about the modelling of HIV/AIDS and fatal discontinuities are also described in detail in the methods appendix (pp 255–64, 270–73).
Cause of death database development
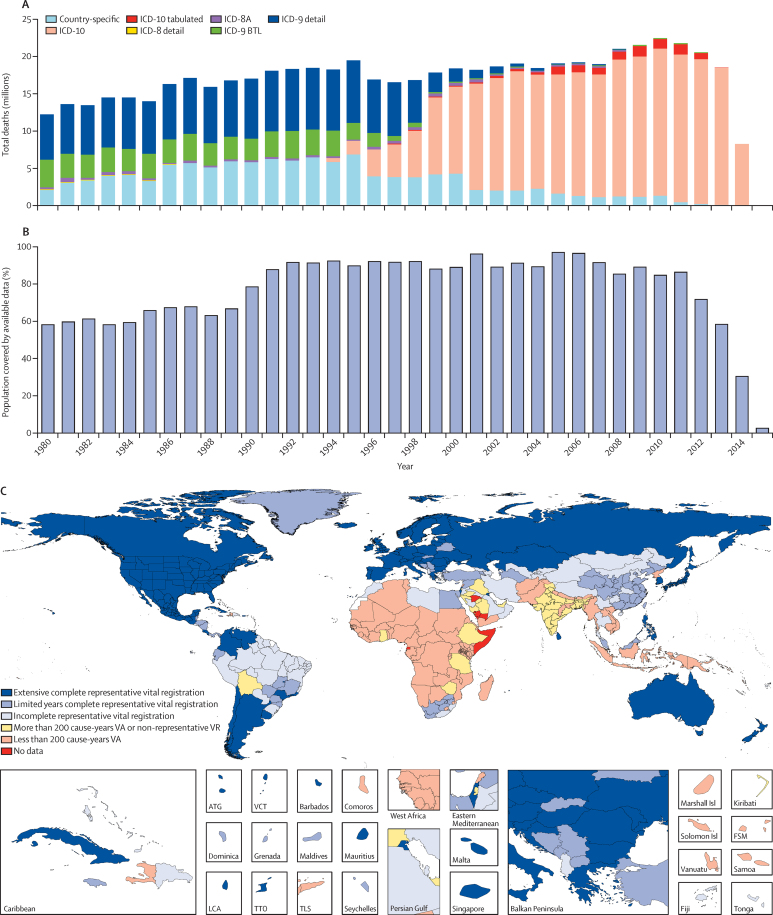
Figure 2A shows the detailed steps from data inputs and processing to the finalisation of the cause of death database. The methods appendix (pp 52–70) includes details on each step. Cause of death data collected through vital registration systems are available from governments and coded to different variants of the ICD including various national ICD variants. Multiple sources were used in addition to vital registration data, including verbal autopsy data, cancer registries, maternal mortality surveillance, census and survey data on maternal death, census and survey data on selected injuries, and police records for some injuries. Figure 2A shows how each type of data were processed to deal with the challenges of different coding schemes, different age group reporting, variation in certification, misclassification of HIV/AIDS deaths, misclassification of maternal HIV/AIDS deaths, and incorporation of population-based cancer registry data. The first and second steps in the cause of death database development were standardisation of multiple data formats to a single GBD standard, then the mapping of each ICD or verbal autopsy variant to the GBD cause map. Figure 7A shows the number of deaths captured for each year in the GBD causes of death database by coding version. In step 3, we split a small subset of data reported in non-GBD-standard age formats into GBD age categories using the global relative age pattern of mortality for each cause as estimated from the pooled data that provide full age detail. In step 4, based on expert judgment, some causes were not allowed for certain age–sex groups, for example, male uterine cancer.
Figure 7.
Availability and quality of cause of death data in the GBD 2015 database
(A) Total deaths with a WHO-standard death certificate available in the GBD 2015 cause of death database classified by the variant of the International Classification of Diseases used for reporting. Cause of death data have been reported in national variants of ICD-8, ICD-9, and ICD-10 during the interval 1980–2015. Because of lags in reporting of both vital registration data and the release of household survey or census data, the availability of data was much lower for 2014 than for previous years and no data existed for 2015. (B) Percentage of global population covered by cause-specific data in the cause of death database for GBD 2015, 1980–2015; the percentage of available data was calculated by dividing the population of locations covered by available cause-specific data by the total global population. This figure is computed using vital registration, verbal autopsy, maternal, cancer, and injury sources. (C) Overall classification of each GBD subnational level 1 geography by availability and quality of cause of death data for the period 1980 to 2015. Countries have been assigned on the basis of the available time series of data into one of six categories. The figure uses GBD subnational level 1 geographies because subnational level 2 cannot be easily seen on a map. Extensive complete representative vital registration was defined as 25 total years or more of vital registration data with an estimated 95% completeness or above. All geographies that do not meet the threshold for extensive complete representative vital registration are classified as one of the following: limited years of complete representative vital registration, defined as 5 years or more of vital registration data with an estimated 95% completeness or above; incomplete representative vital registration, defined as at least 1 year of vital registration data with an estimated 70% completeness or above; more than 200 cause-years VA or non-representative VR, defined as more than 200 cause-years of verbal autopsy or at least 1 year of vital registration with an estimated 50% completeness or above; less than 200 cause-years of VA; or no data. Cause-years are defined as the number of years for each cause for which data are available. GBD=Global Burden of Disease. ICD=International Classification of Diseases. BTL=basic tabulation list. VA=verbal autopsy. VR=vital registration. ATG=Antigua and Barbuda. VCT=Saint Vincent and the Grenadines. LCA=Saint Lucia. TTO=Trinidad and Tobago. TLS=Timor-Leste. FSM=Federated States of Micronesia.
In step 5, deaths assigned to causes that cannot be underlying causes of death (ie, garbage coded) were reassigned to their likely underlying cause of death.4, 7 These redistribution algorithms are based on three approaches. For some garbage codes, such as senility or old age, deaths were proportionately reassigned to all causes that are not garbage codes for a country–age–sex–year. For HIV/AIDS in many countries, deaths from HIV/AIDS have been misclassified as opportunistic infections, tuberculosis, cancer, digestive diseases, and immune deficiencies. In step 6, using methods developed by Birnbaum and colleagues,41 these deaths were identified and reclassified as HIV/AIDS in select countries with evidence of misclassification. In step 7, data from the China Center for Disease Control and Prevention (CDC) vital registration system were re-weighted to take into account potential selection bias caused by a larger fraction of deaths being captured in hospital than out of hospital in some locations.14 Step 8 ensured that the process of redistributing garbage codes or identifying misclassified HIV/AIDS deaths would not assign deaths to causes in an age–sex–country–year that violated age–sex or other restrictions.
Step 9 excluded vital registration sources that were less than 50% complete in a given geography from the database, because of the potential for selection bias in highly incomplete sources. Sources estimated to be 50–70% complete were identified as non-representative, which was information that we used in the building of the cause of death statistical model to increase the estimated data variance for these datapoints. All included sources were corrected to be 100% complete by multiplying the cause fraction in a source for a country–age–sex–year by the estimate of all-cause mortality for that country–age–sex–year. Step 10 aggregated causes of death from most to least detailed levels of the GBD hierarchy, ensuring deaths for a given cause were representative of all branches of the hierarchy that fall beneath it. In step 11, deaths due to HIV/AIDS and various types of fatal discontinuities were removed before cause fractions were computed. Because of the very large effects of fatal discontinuities, such as wars and natural disasters in some cases, and the impact of HIV/AIDS in countries with large epidemics, we converted cause fractions to be cause fractions excluding HIV/AIDS and fatal discontinuities in the denominator. Deaths from HIV/AIDS and the fatal discontinuities were added back during the final stages of the modelling process. Because many sources on maternal mortality identify deaths during pregnancy and the post-partum period and not maternal deaths, the separation of HIV/AIDS deaths during pregnancy and HIV/AIDS deaths aggravated by pregnancy was more complicated (methods appendix p 66).
Figure 7B provides information about the fraction of the 519 geographies in the analysis for which cause of death data were available in each year from 1980 to 2015 for any cause, including maternal death and injuries. Data availability by geography–year by cause is shown in the methods appendix (pp 318–401). To facilitate understanding of the range of quality and availability of data for each geography, we classified geographies into six categories: extensive complete representative vital registration (vital registration data that are 95% complete and cover more than 25 years); moderate data (vital registration data that are 95% complete but cover fewer than 25 years); incomplete representative vital registration (all other geographies with some representative vital registration data); extensive verbal autopsy and other sources (covering more than 20% of cause-years); limited verbal autopsy or other data (all others with some data available); and no data for any cause (methods appendix pp 691–710). Figure 7C shows this designation for each class of country.
CODEm
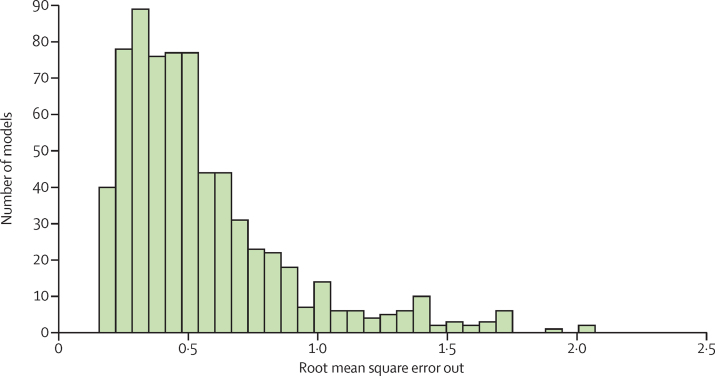
Figure 2B shows the analytical flow chart for modelling different causes of death and combining them into internally consistent estimates of cause-specific mortality that sum to all-cause mortality with uncertainty levels. 167 individual causes of death were modelled using CODEm. Developed for GBD 2010,5 CODEm tests a large number of model specifications, comparing different functional forms and permutations of relevant covariates for each cause of death. Models that met requirements for direction and significance of the regression coefficients were then evaluated for out-of-sample predictive validity through multiple iterations of cross-validation testing. We then combined these models into an ensemble, weighting them such that top performing models (in terms of out-of-sample prediction error on levels and trends) contributed the most to the final prediction. Out-of-sample predictive validity testing was also used to select the psi parameter that determines the number of models and their weight in the final ensemble (figure 8).
Figure 8.
Distribution of out-of-sample model performance for CODEm models used for GBD 2015
Model performance was assessed by use of the root mean square error of the ensemble model predictions of the log of the age-specific death rates for a cause assessed with 15% of the data held out from the statistical model building. The figure shows the distribution of root mean square error across the set all models for all causes. Model performance varies substantially across causes. GBD=Global Burden of Disease. CODEm=cause of death ensemble modelling.
For each cause of death, we ran independent CODEm models by sex and for countries with extensive complete vital registration representation and all other countries. We included all datapoints for the other categories of geographies, whereas for countries with extensive complete vital registration representation, we included only datapoints from those countries, so that heterogeneous data from other countries did not inflate the uncertainty interval.
Negative binomial models
For ten causes of death, the number of events are so low, including many zero counts in countries with high income per capita or high educational attainment, that CODEm out-of-sample predictive validity testing was unstable. For these rare causes of death, which included other intestinal infectious diseases, upper respiratory infections, diphtheria, varicella and herpes zoster, malaria, schistosomiasis, cysticercosis, cystic echinococcosis, ascariasis, and iodine deficiency, we used negative binomial regression to develop simple models to predict deaths. More details are available in the methods appendix (pp 185–200; negative binomial models).
Natural history models
For some causes, deaths are rarely recorded in either vital registration data or verbal autopsy data. Partly, this is because of the geographical location of the deaths or because of the potential for systematic bias in vital registration data or verbal autopsy data. For 14 causes, we have developed natural history models in which incidence and case-fatality rates are modelled separately and combined to yield estimates of cause-specific mortality. We developed natural history models for typhoid fever, paratyphoid fever, whooping cough, measles, visceral leishmaniasis, African trypanosomiasis, yellow fever, syphilis (congenital), and acute hepatitis A, B, C, and E. Additionally, for malaria in sub-Saharan Africa, we have used a natural history model based on the incidence estimated by the Malaria Atlas Project and age–sex-specific case-fatality rates estimated from available data. Further details on the development of these natural history models are available in the methods appendix (pp 201–26; natural history models).
Subcause proportion models
For meningitis, maternal disorders, liver cancer, cirrhosis, and chronic kidney disease, we estimated detailed causes for each of these cause groupings by modelling the proportion of the cause grouping (parent cause) due to each of the component causes. We used this approach because the available data on the specific causes can come from sources other than vital registration, such as end-stage renal disease registries, or from too few places to model the death rates directly. For these causes, the parent cause was first estimated with CODEm and the fraction of the parent due to each component cause for each age–sex–geography–year was generally estimated with DisMod-MR 2.1, a Bayesian meta-regression method developed for the GBD studies.42, 43 Details for each cluster of causes analysed in this way are shown in the methods appendix (pp 233–52; subcause proportion models).
Prevalence-based models
For Alzheimer's disease and other dementias and atrial fibrillation and flutter, there is evidence of marked changes over time in the propensity of individuals who completed death certificates to list these causes as underlying causes of death.44, 45 These changes created increases in the reported death rates. Conversely, prevalence surveys do not show a matching increase in age-specific disease prevalence. Garbage code redistribution algorithms used in the development of the cause of death database have so far not accurately captured this shift over time in the certification of underlying causes of death. For these two causes, we based our estimates on prevalence surveys and estimates of excess mortality based on deaths certified in countries with the greatest proportion of deaths allocated to the correct underlying cause of death in recent years. In both cases, more detail is available in the methods appendix (pp 227–32; prevalence-based models). We developed models for prevalence and excess mortality using DisMod-MR 2.1.
CodCorrect
Depending on the specific data availability and details of individual causes, we adopted different modelling strategies for each cause. We generated a set of underlying cause of death estimates, with uncertainty intervals, that equalled all-cause mortality, with uncertainty intervals, for each age–sex–year–geography and cause and all-cause mortality at the individual draw level.24 In CodCorrect, for each draw from the posterior distribution of each cause, the sum of cause-specific estimates is rescaled to equal the draw from the all-cause distribution (methods appendix p 285).
Pathogen counterfactual analysis
We used a counterfactual analysis approach to estimate aetiology-specific population attributable fraction for mortality due to lower respiratory infections and diarrhoeal diseases. This approach involved analysing changes in mortality on the basis of the estimated prevalence of each pathogen and relative risk of developing disease given pathogen exposure.
The prevalence of each pathogen in diarrhoeal cases was extracted from a systematic literature review and modelled with DisMod-MR 2.1. The odds ratios of an episode of diarrhoea given exposure to the pathogen were estimated from a reanalysis of the Global Enteric Multicentre Study (GEMS) that used the TaqMan Array Card (TAC), which is based on a quantitative polymerase chain reaction diagnostic (qPCR).46, 47 We attributed mortality to all pathogens, even if the odds ratio was not significant in all age groups. We corrected the estimated prevalence for each pathogen on the basis of conventional laboratory techniques, such as bacterial culture or enzyme-linked immunosorbent assay (ELISA), to be consistent with the new qPCR method. Cholera mortality was estimated by modelling the under-reporting to the WHO cholera case notification system and applying this correction factor to estimate the number of cholera cases and deaths (methods appendix p 281). The incidence and mortality of Clostridium difficile was modelled with natural history and incidence data in DisMod-MR 2.1.
We estimated attributable mortality due to respiratory syncytial virus and influenza with a similar approach to that for diarrhoea. We used a counterfactual approach whereby the prevalence in patients with lower respiratory infection was extracted from a systematic literature review and modelled with DisMod-MR 2.1. The odds ratios of lower respiratory infections given pathogen presence were obtained from a meta-analysis by Shi and colleagues.48 We adjusted the population attributable fraction for lower respiratory infection mortality due to respiratory syncytial virus and influenza for the relative case-fatality rate of viral to bacterial pneumonia episodes by age. Haemophilus influenzae type b and pneumococcal pneumonia (Streptococcus pneumonia) were estimated with a vaccine probe approach whereby the attributable fraction was calculated as the ratio of vaccine efficacy against non-specific pneumonia to vaccine efficacy against pathogen-specific and serotype-specific pneumonia. Studies that report vaccine efficacy against vaccine-type invasive pneumococcal disease were adjusted for the relative efficacy against vaccine-type clinical pneumococcal pneumonia using a uniform distribution of uncertainty around this ratio.49, 50
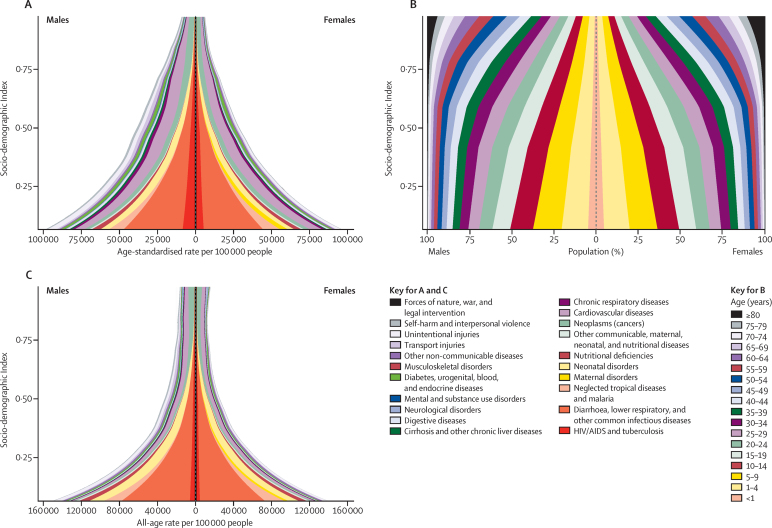
Socio-demographic Index and epidemiological transition analysis
In this Article, we built on GBD 201351 concepts by improving the interpretability of sociodemographic status and characterising and describing this relationship in more detail for years of life lost due to premature mortality (YLLs), as well as highlighting changes in age-standardised death rates, population age structure, and YLL rates. We have made two important changes to the GBD 2013 computation. First, we have used only lag-dependent income per capita, average educational attainment in the population over age 15 years, and the total fertility rate. We excluded the mean age of the population because it is directly affected by death rates. Second, we have applied the methods used to compute the Human Development Index to generate an interpretable scale, resulting in the Socio-demographic Index (SDI).52 The Human Development Index method weights each component equally and rescales each component on a zero-to-one scale with zero being the lowest value observed in the time period 1980 to 2015 and 1 being the highest value observed. The final composite SDI value is the geometric mean of each of the components. The SDI ranges from 0·060 in Mozambique in 1987 to 0·978 in Washington, DC, USA, in 2015. The correlation of the SDI with the sociodemographic status principal component analysis used in GBD 2013 was 0·982. The very high correlation is because the principal component analysis yields weights that are nearly equal across components. The advantage of the index is that 1 can be interpreted as the level of SDI at which a geography has the highest observed log income per capita and educational attainment and lowest fertility rate. We tested whether alternative lags of the components of SDI would provide a better predictor of outcomes such as life expectancy and age-specific probabilities of death. Using lag distributed income per capita, educational attainment, and the total fertility rate in the current year was the most predictive of these mortality outcomes (methods appendix p 286).
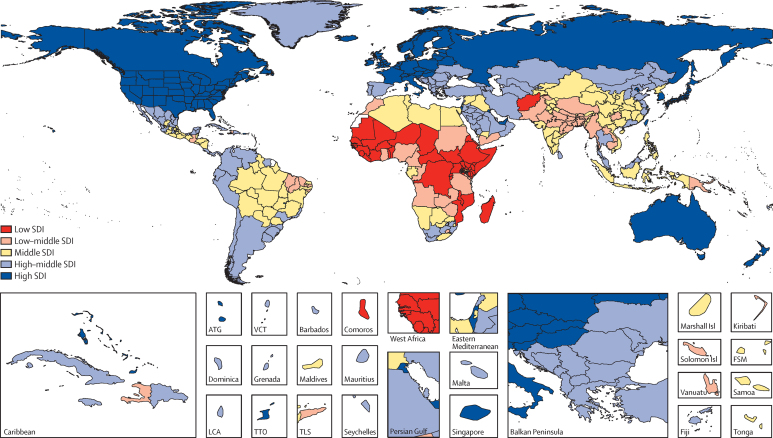
To report on aggregate results, we divided geographies into SDI quintiles in 2015. Quintile cutoffs were based on the entire distribution of geography–years from 1980 to 2015, excluding populations smaller than 1 million. Figure 9 shows a map of the SDI level in 2015 categorised into five groups including subnational geographies. Because SDI includes educational attainment and the total fertility rate, some countries which have very high income, such as Saudi Arabia, are classified in the second quintile of SDI because of lower educational attainment and higher fertility rates.53
Figure 9.
SDI quintiles by GBD subnational level 1 geography, 2015
SDI is calculated for each geography as a function of lag-dependent income per capita, average educational attainment in the population older than age 15 years, and the total fertility rate. SDI units are interpretable; a zero represents the lowest level of income per capita and educational attainment and highest total fertility rate observed during 1980–2015, whereas a one represents the highest income per capita and educational attainment and lowest total fertility rate observed in the same period. Cutoffs on the SDI scale for the quintiles have been selected on the basis of examination of the entire distribution of geographies 1980–2015. GBD=Global Burden of Disease. SDI=Socio-demographic Index. ATG=Antigua and Barbuda. VCT=Saint Vincent and the Grenadines. LCA=Saint Lucia. TTO=Trinidad and Tobago. TLS=Timor-Leste. FSM=Federated States of Micronesia.
To capture the average relationships for each age–sex–cause group, we used spline regression of death rates on SDI (methods appendix pp 285–86). To ensure a coherent set of estimated death rates for Levels 1, 2, and 3 in the GBD cause hierarchy for each level of SDI, the Level 2 death rates were rescaled such that for each age–sex–cause bin, the sum of Level 2 death rates equalled the Level 1 death rate. This procedure was repeated for Level 3 and Level 2 causes. These rates were used as the expected death rates by age–sex–cause and SDI. Various summary measures have been computed on the basis of the age–sex–cause-specific predictions based on SDI, including age-standardised death rates, age-standardised YLL rates, and life expectancy at birth.
To further characterise how patterns of crude death rates and death numbers change with SDI, we have computed the average population age structure associated with each SDI level. These population age structures have then been used to estimate how crude death rates and death numbers by cause are expected to change with rising SDI.
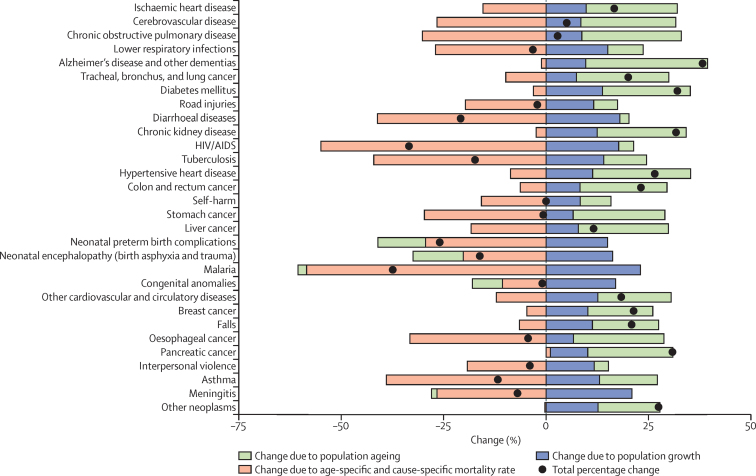
Decomposition of changes in global deaths
To analyse the drivers of change in the numbers of deaths by cause or geography, we decomposed change from 2005 to 2015 into three explanatory components: change due to growth of the total population; change in the population structure by age or sex; and change in age-specific, sex-specific, and cause-specific rates. We refer to all changes in age-specific, sex-specific, and cause-specific death rates not explained by demographic change (population growth and ageing) as the epidemiological change. The observed change in the total number of deaths equals the net change of these three components.
Decomposition analyses for 1980 to 2015 and 2000 to 2015 are shown in the results appendix (pp 6–7). The decomposition analysis uses methods developed in demographic research by Das Gupta.54 As an example, we describe our approach to decomposition for the 2005 to 2015 period. We used counterfactual scenarios to calculate two different sets of numbers for death. In the first scenario, for population growth, the number of deaths in 2015 was the number expected if the total population increased from 2005 as observed, but the age–sex-specific population structure and rates of death were the same in 2015 as in 2005. In the second scenario, for population growth and ageing, the number of deaths in 2015 was the number expected according to the 2015 age–sex-specific population structure, but with the age–sex-specific rates of death held constant to 2005. The difference between the number of deaths observed in 2005 and those estimated for 2015 with the population growth scenario is the change in the number of deaths exclusively from population growth. The difference between the scenario for population growth alone and the scenario for population growth and ageing is the change in the number of deaths exclusively attributable to population ageing.
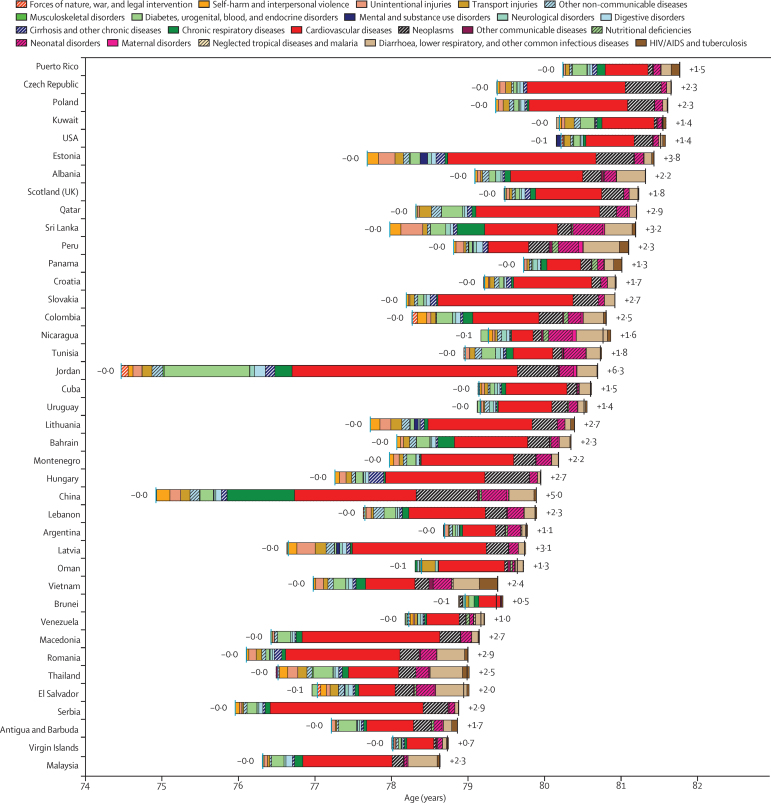
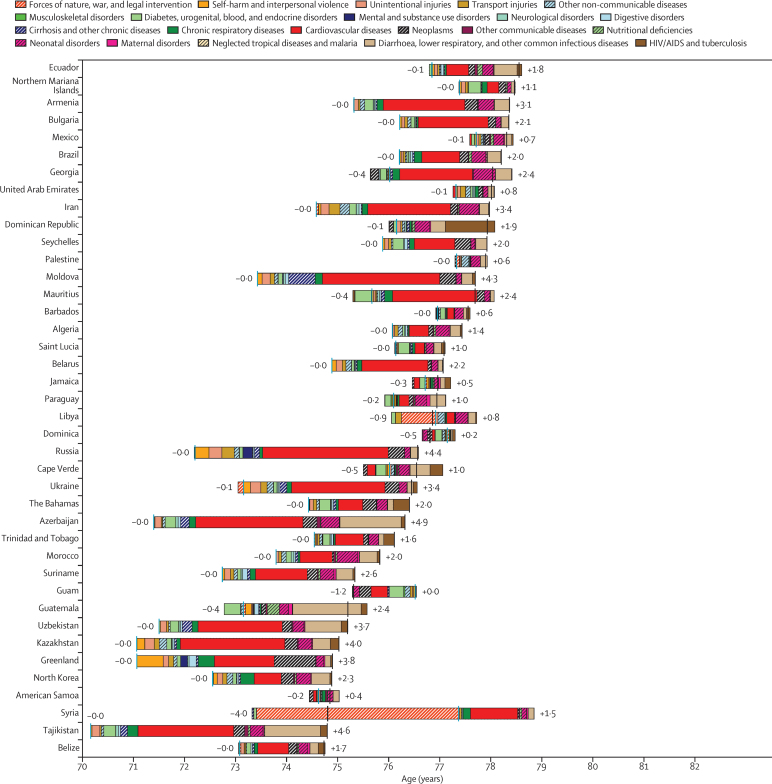
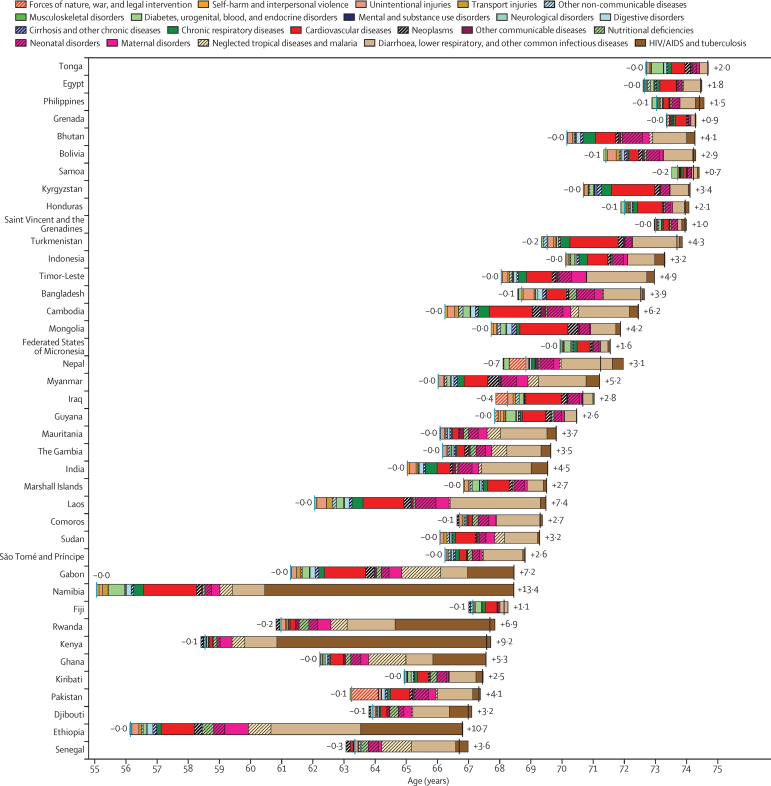
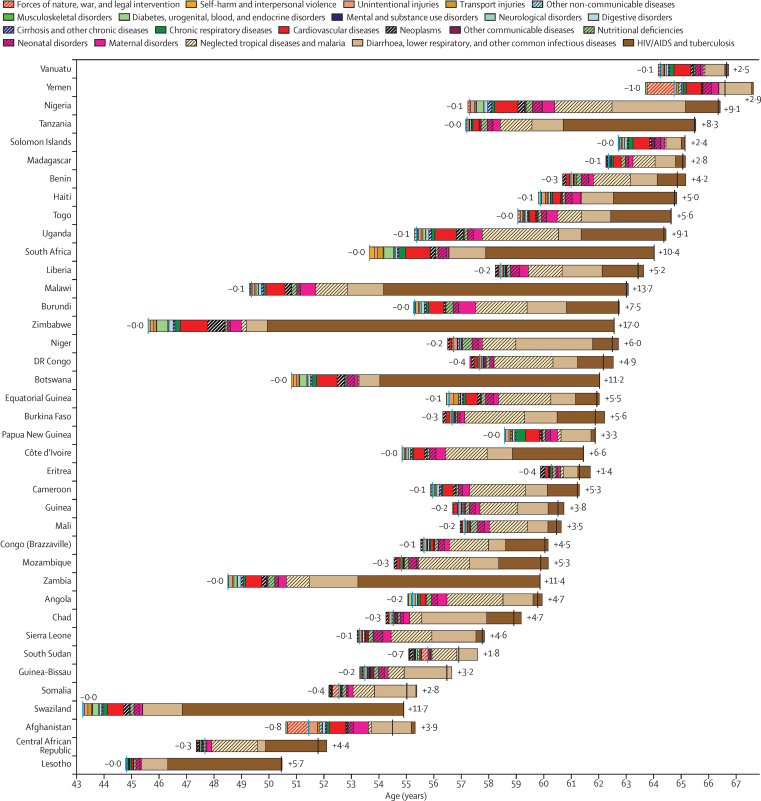
Attribution of changes in life expectancy to changes in causes of death
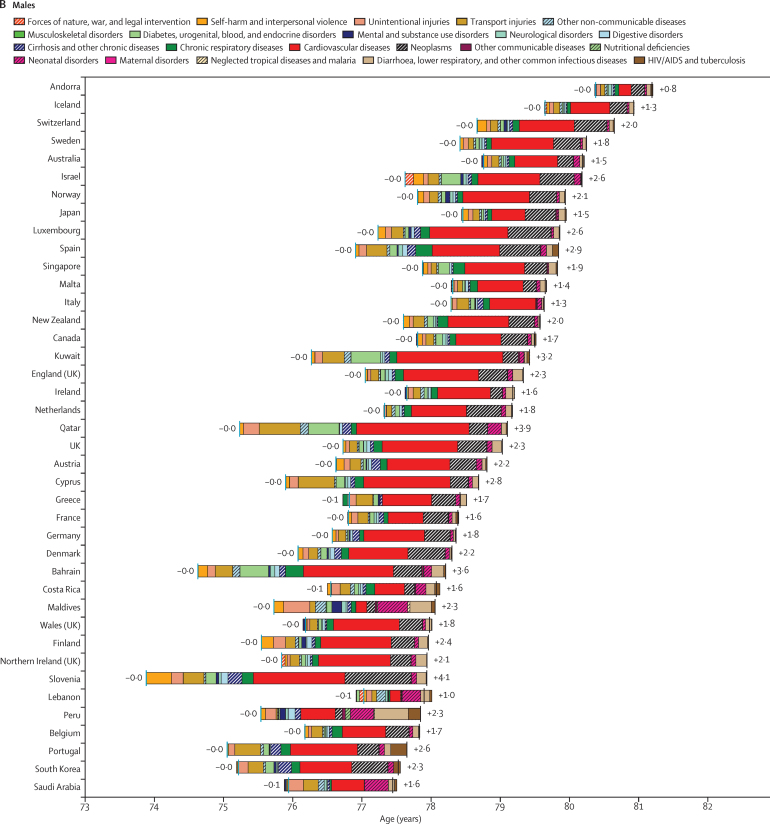
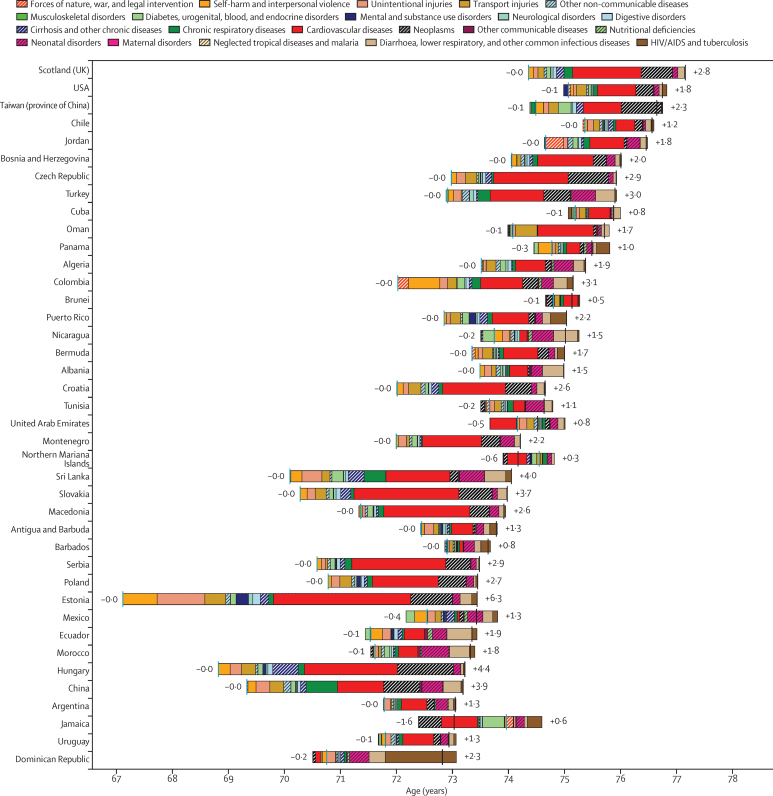
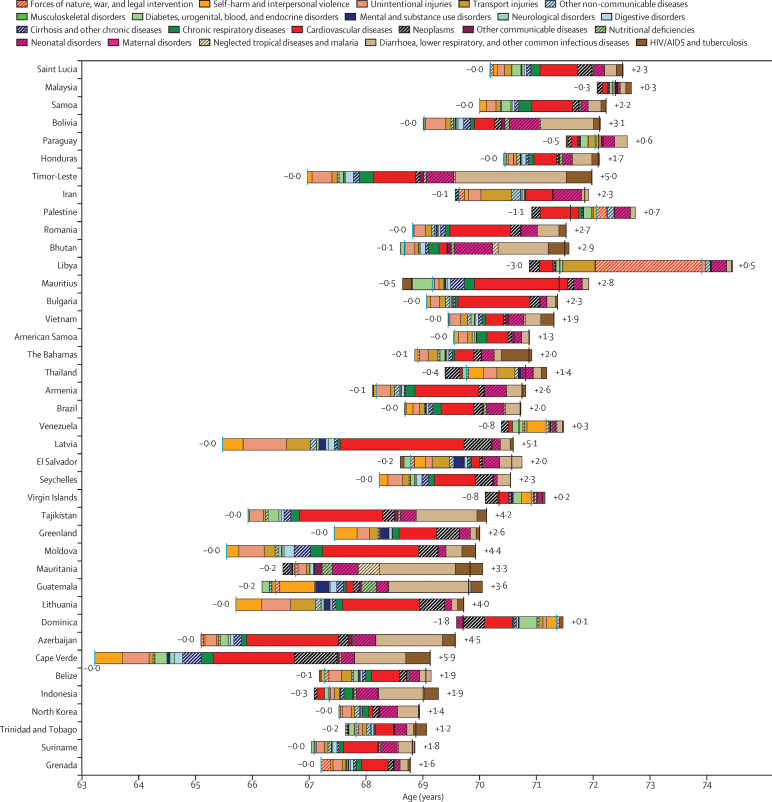
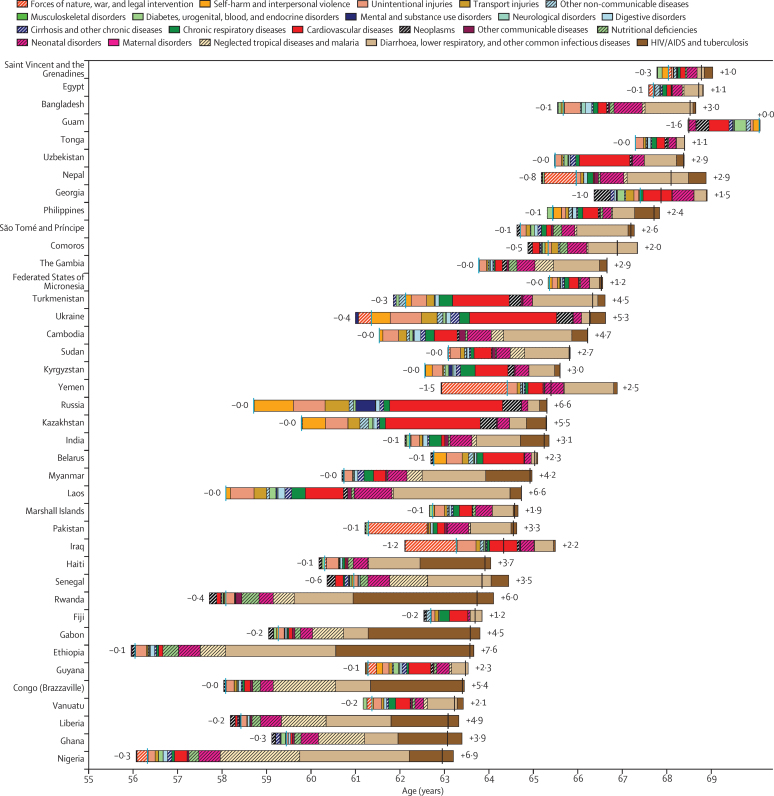
When considering the estimated levels and changes in all-cause and cause-specific mortality rates for each geographical area covered by GBD 2015, it is important to understand the relative contribution of changes in mortality due to each cause to the overall changes in life expectancy at birth during the same period. To examine the changes in life expectancy at birth between 2005 and 2015, we have applied the state-of-the-art life expectancy cause-specific decomposition method developed by Beltran-Sanchez, Preston, and Canudas-Romo.55
YLL computation
We computed YLLs using the standard GBD methods whereby each death is multiplied by the normative standard life expectancy at each age. The normative standard life expectancy at birth is 86·59 years, which is based on the lowest observed death rates for each 5-year age group in populations larger than 5 million. For GBD 2015, we computed age-standardised mortality rates and YLL rates from the updated world population age standard developed for GBD 2013.7 Details of the GBD world population age standard are available in the methods appendix (pp 286–287 and 314).
Uncertainty analysis
To account for uncertainties that arise from sample sizes of data, adjustments to sources of all-cause mortality, model specifications in spatiotemporal Gaussian process regression and model life table systems, and cause-specific model specifications and estimation, we have estimated uncertainty intervals in key steps of the all-cause mortality and cause-specific mortality estimation processes. We have produced 1000 draws of all mortality metrics, including under-5 mortality rate, adult mortality rate, age-specific mortality rate and envelope, and cause-specific mortality rates and death numbers for each location by sex for all years covered by each analytical step from the posterior distribution in the estimation process. This allowed the quantification and propagation of uncertainty into the final quantities of interest. Because of computational time limitations, we have not propagated uncertainty in covariates used in cause of death models, nor have we been able to propagate uncertainty in garbage code redistribution algorithms into the final results. Our tests on the estimation of under-5 mortality rates show that the incorporation of uncertainty for included first stage model covariates such as crude death rate due to HIV/AIDS does not have a significant impact on the final estimates (data not shown).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
Global life expectancy and mortality
Global life expectancy at birth increased by 10·2 years, rising from 61·7 years (95% uncertainty interval [UI] 61·4–61·9) in 1980 to 71·8 years (71·5–72·2) in 2015 (table 4), equating to an average gain of 0·29 years per year. By 2015, male life expectancy had risen by 9·4 years, increasing from 59·6 years (59·3–60·0) in 1980 to 69·0 years (68·6–69·4), whereas female life expectancy improved by 11·1 years, climbing from 63·7 years (63·3–64·1) to 74·8 years (74·4–75·2). On average, an additional 0·27 and 0·32 years of life were gained per year for males and females, respectively, since 1980. Global gains in life expectancy were generally gradual but steady, although catastrophic events, including the Rwandan genocide and North Korean famines, and escalating mortality due to HIV/AIDS, had worldwide effects on longevity. Slower gains were achieved for life expectancy at 50 years, or the average number of additional years of life 50 year olds can anticipate at a given point in time. On average, 50-year-old females saw an increase of 4·5 additional years of life since 1980, and 50-year-old males experienced an increase of 3·5 years. Annual estimates of life expectancy, by sex and geography are shown in the results appendix (pp 36–47).
Table 4.
Global life expectancy at birth and at age 50, age-standardised death rates, age-standardised YLL rate, and total deaths, by sex, 1980–2015
|
Life expectancy at birth (years) |
Life expectancy at age 50 (years) |
Age-standardised death rate (per 100 000) |
Age-standardised YLL rate (per 100) |
Total deaths (millions) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Both sexes | |
| 1980 | 59·6 (59·3–60·0) | 63·7 (63·3–64·1) | 23·1 (22·9–23·2) | 26·4 (26·2–26·7) | 1536·1 (1513·0–1558·5) | 1194·6 (1172·4–1217·9) | 49·8 (49·0–50·5) | 40·8 (40·1–41·6) | 23·5 (23·2–23·9) | 21·6 (21·2–22·0) | 45·2 (44·6–45·7) |
| 1981 | 59·9 (59·6–60·2) | 64·1 (63·7–64·4) | 23·1 (22·9–23·3) | 26·5 (26·3–26·8) | 1525·0 (1502·7–1547·1) | 1177·5 (1155·9–1199·6) | 49·1 (48·4–49·8) | 40·0 (39·3–40·7) | 23·7 (23·4–24·1) | 21·7 (21·3–22·1) | 45·5 (44·9–46·0) |
| 1982 | 60·3 (59·9–60·6) | 64·5 (64·1–64·9) | 23·2 (23·0–23·4) | 26·7 (26·5–26·9) | 1499·7 (1478·4–1521·2) | 1155·8 (1134·3–1177·4) | 48·1 (47·4–48·7) | 39·1 (38·4–39·7) | 23·8 (23·5–24·1) | 21·7 (21·3–22·1) | 45·5 (45·0–46·1) |
| 1983 | 60·5 (60·1–60·9) | 64·7 (64·3–65·2) | 23·3 (23·1–23·5) | 26·7 (26·5–26·9) | 1486·1 (1463·7–1507·7) | 1146·6 (1125·7–1167·7) | 47·4 (46·7–48·2) | 38·5 (37·8–39·2) | 24·0 (23·6–24·4) | 22·0 (21·6–22·4) | 46·0 (45·5–46·6) |
| 1984 | 60·8 (60·4–61·2) | 65·1 (64·6–65·5) | 23·4 (23·2–23·5) | 26·8 (26·6–27·0) | 1472·0 (1451·3–1493·7) | 1132·5 (1112·4–1153·3) | 46·7 (46·0–47·5) | 37·7 (37·0–38·4) | 24·2 (23·9–24·6) | 22·1 (21·8–22·5) | 46·4 (45·8–47·0) |
| 1985 | 61·2 (60·9–61·6) | 65·5 (65·1–65·9) | 23·4 (23·3–23·6) | 26·9 (26·7–27·1) | 1453·9 (1434·0–1474·9) | 1116·4 (1097·3–1135·7) | 45·6 (45·0–46·3) | 36·8 (36·2–37·4) | 24·3 (24·0–24·7) | 22·2 (21·9–22·6) | 46·6 (46·0–47·1) |
| 1986 | 61·7 (61·3–62·0) | 66·0 (65·6–66·4) | 23·6 (23·4–23·8) | 27·1 (26·9–27·3) | 1428·4 (1409·1–1450·0) | 1091·5 (1073·4–1111·2) | 44·5 (43·9–45·2) | 35·7 (35·2–36·3) | 24·3 (24·0–24·7) | 22·1 (21·8–22·5) | 46·5 (45·9–47·0) |
| 1987 | 62·0 (61·6–62·3) | 66·3 (65·9–66·7) | 23·7 (23·5–23·9) | 27·2 (27·0–27·4) | 1411·7 (1392·5–1432·2) | 1077·6 (1059·9–1097·0) | 43·8 (43·2–44·4) | 35·0 (34·5–35·6) | 24·5 (24·1–24·8) | 22·3 (21·9–22·6) | 46·7 (46·2–47·3) |
| 1988 | 62·1 (61·7–62·4) | 66·5 (66·2–66·9) | 23·7 (23·5–23·8) | 27·2 (27·0–27·4) | 1409·9 (1389·3–1430·2) | 1068·2 (1051·0–1087·0) | 43·5 (42·8–44·1) | 34·5 (34·0–35·0) | 24·9 (24·5–25·2) | 22·5 (22·1–22·9) | 47·3 (46·8–47·9) |
| 1989 | 62·4 (62·0–62·7) | 66·9 (66·5–67·2) | 23·6 (23·5–23·8) | 27·3 (27·1–27·5) | 1401·9 (1380·9–1422·3) | 1055·5 (1039·0–1073·2) | 42·8 (42·2–43·4) | 33·8 (33·3–34·3) | 25·1 (24·7–25·4) | 22·6 (22·3–23·0) | 47·7 (47·2–48·2) |
| 1990 | 62·5 (62·2–62·8) | 67·1 (66·7–67·4) | 23·7 (23·5–23·9) | 27·4 (27·2–27·6) | 1381·1 (1359·0–1401·6) | 1035·1 (1019·1–1052·0) | 42·4 (41·8–43·0) | 33·2 (32·8–33·7) | 25·3 (24·9–25·7) | 22·6 (22·3–23·0) | 47·9 (47·4–48·5) |
| 1991 | 62·6 (62·3–62·9) | 67·3 (66·9–67·6) | 23·8 (23·6–24·0) | 27·5 (27·3–27·7) | 1371·9 (1349·3–1393·1) | 1025·1 (1010·2–1041·2) | 42·1 (41·4–42·7) | 32·8 (32·4–33·3) | 25·6 (25·2–26·0) | 22·7 (22·4–23·1) | 48·3 (47·8–48·8) |
| 1992 | 62·8 (62·5–63·1) | 67·5 (67·2–67·8) | 23·8 (23·7–24·0) | 27·6 (27·4–27·7) | 1363·9 (1342·2–1384·4) | 1016·1 (1001·2–1032·0) | 41·6 (41·0–42·2) | 32·3 (31·9–32·8) | 25·8 (25·4–26·2) | 22·9 (22·5–23·2) | 48·7 (48·2–49·2) |
| 1993 | 62·8 (62·5–63·1) | 67·6 (67·3–67·9) | 23·8 (23·6–24·0) | 27·5 (27·3–27·7) | 1367·1 (1347·1–1386·3) | 1016·0 (1001·6–1031·0) | 41·5 (40·9–42·1) | 32·0 (31·6–32·5) | 26·3 (25·9–26·6) | 23·2 (22·8–23·5) | 49·4 (48·9–49·9) |
| 1994 | 62·6 (62·2–63·0) | 67·7 (67·4–68·0) | 23·8 (23·6–24·0) | 27·6 (27·4–27·7) | 1375·7 (1353·5–1399·0) | 1014·1 (999·2–1029·5) | 42·0 (41·2–43·0) | 31·9 (31·5–32·4) | 27·0 (26·5–27·5) | 23·5 (23·2–23·8) | 50·5 (49·9–51·2) |
| 1995 | 63·1 (62·8–63·4) | 68·0 (67·7–68·3) | 23·9 (23·8–24·1) | 27·7 (27·5–27·8) | 1351·0 (1333·4–1368·4) | 1000·7 (987·4–1014·9) | 40·9 (40·4–41·4) | 31·3 (30·9–31·7) | 26·8 (26·5–27·2) | 23·5 (23·2–23·9) | 50·4 (49·9–50·9) |
| 1996 | 63·4 (63·1–63·6) | 68·3 (68·0–68·5) | 24·1 (24·0–24·3) | 27·9 (27·7–28·0) | 1330·2 (1314·1–1347·0) | 986·6 (974·0–999·9) | 40·2 (39·7–40·7) | 30·8 (30·5–31·2) | 26·9 (26·6–27·2) | 23·6 (23·3–23·9) | 50·4 (50·0–50·9) |
| 1997 | 63·7 (63·4–63·9) | 68·5 (68·3–68·8) | 24·3 (24·2–24·4) | 28·0 (27·9–28·1) | 1312·0 (1296·7–1327·7) | 975·1 (962·9–986·9) | 39·6 (39·1–40·0) | 30·4 (30·0–30·7) | 27·0 (26·6–27·3) | 23·6 (23·3–23·9) | 50·6 (50·1–51·1) |
| 1998 | 63·9 (63·6–64·1) | 68·8 (68·5–69·0) | 24·4 (24·3–24·5) | 28·1 (28·0–28·2) | 1301·7 (1286·8–1316·9) | 966·6 (954·7–978·8) | 39·1 (38·7–39·5) | 29·9 (29·6–30·3) | 27·2 (26·8–27·5) | 23·8 (23·5–24·1) | 50·9 (50·5–51·4) |
| 1999 | 64·0 (63·7–64·2) | 68·9 (68·7–69·1) | 24·4 (24·3–24·6) | 28·1 (28·0–28·3) | 1297·1 (1282·1–1312·1) | 963·9 (952·1–976·1) | 38·8 (38·4–39·2) | 29·6 (29·3–30·0) | 27·6 (27·2–27·9) | 24·1 (23·8–24·4) | 51·6 (51·2–52·1) |
| 2000 | 64·2 (64·0–64·4) | 69·1 (68·9–69·4) | 24·5 (24·4–24·6) | 28·2 (28·1–28·3) | 1284·9 (1270·1–1299·5) | 954·8 (943·4–966·3) | 38·3 (37·9–38·7) | 29·2 (28·8–29·6) | 27·9 (27·5–28·2) | 24·3 (24·0–24·6) | 52·1 (51·7–52·6) |
| 2001 | 64·4 (64·2–64·7) | 69·4 (69·1–69·6) | 24·6 (24·5–24·7) | 28·3 (28·2–28·4) | 1272·5 (1258·1–1286·7) | 944·1 (932·9–955·5) | 37·7 (37·3–38·1) | 28·7 (28·4–29·1) | 28·1 (27·8–28·4) | 24·5 (24·2–24·8) | 52·6 (52·1–53·1) |
| 2002 | 64·6 (64·4–64·9) | 69·6 (69·4–69·8) | 24·6 (24·5–24·8) | 28·4 (28·2–28·5) | 1265·8 (1251·2–1280·3) | 933·4 (923·0–944·4) | 37·2 (36·8–37·7) | 28·2 (27·9–28·6) | 28·5 (28·2–28·9) | 24·7 (24·4–25·0) | 53·2 (52·7–53·7) |
| 2003 | 65·0 (64·8–65·2) | 70·0 (69·7–70·2) | 24·8 (24·7–24·9) | 28·5 (28·4–28·7) | 1240·0 (1225·5–1254·6) | 915·4 (905·3–925·9) | 36·4 (36·0–36·8) | 27·5 (27·2–27·9) | 28·6 (28·2–28·9) | 24·7 (24·4–25·0) | 53·3 (52·9–53·8) |
| 2004 | 65·3 (65·1–65·5) | 70·3 (70·1–70·5) | 25·0 (24·8–25·1) | 28·7 (28·6–28·9) | 1216·5 (1202·2–1231·6) | 895·9 (885·5–906·9) | 35·8 (35·3–36·2) | 26·9 (26·6–27·3) | 28·7 (28·4–29·1) | 24·7 (24·4–25·0) | 53·5 (53·0–54·0) |
| 2005 | 65·7 (65·5–65·9) | 70·7 (70·5–71·0) | 25·1 (25·0–25·3) | 28·9 (28·8–29·0) | 1195·0 (1180·9–1209·1) | 878·1 (868·3–888·2) | 34·9 (34·5–35·3) | 26·1 (25·8–26·4) | 28·9 (28·5–29·2) | 24·8 (24·5–25·1) | 53·6 (53·1–54·1) |
| 2006 | 66·2 (65·9–66·4) | 71·2 (71·0–71·5) | 25·4 (25·3–25·5) | 29·2 (29·1–29·3) | 1163·8 (1150·2–1176·9) | 852·4 (842·9–862·3) | 33·8 (33·4–34·2) | 25·2 (24·9–25·5) | 28·7 (28·4–29·1) | 24·6 (24·3–24·8) | 53·3 (52·8–53·7) |
| 2007 | 66·6 (66·3–66·8) | 71·7 (71·5–72·0) | 25·6 (25·4–25·7) | 29·4 (29·3–29·5) | 1141·3 (1127·7–1154·8) | 830·4 (820·5–840·6) | 33·0 (32·6–33·4) | 24·4 (24·1–24·7) | 28·8 (28·4–29·2) | 24·5 (24·2–24·8) | 53·3 (52·8–53·7) |
| 2008 | 66·8 (66·6–67·1) | 72·1 (71·9–72·4) | 25·7 (25·6–25·8) | 29·6 (29·5–29·7) | 1127·2 (1112·9–1140·8) | 814·0 (803·9–823·4) | 32·5 (32·0–32·9) | 23·7 (23·4–24·1) | 29·1 (28·7–29·5) | 24·5 (24·2–24·8) | 53·6 (53·1–54·1) |
| 2009 | 67·3 (67·0–67·6) | 72·6 (72·4–72·9) | 25·9 (25·7–26·0) | 29·9 (29·7–30·0) | 1103·4 (1090·0–1116·6) | 791·7 (782·3–801·5) | 31·5 (31·1–31·9) | 22·9 (22·6–23·2) | 29·1 (28·7–29·5) | 24·4 (24·1–24·7) | 53·5 (52·9–54·0) |
| 2010 | 67·5 (67·2–67·8) | 72·9 (72·6–73·2) | 26·0 (25·8–26·1) | 30·0 (29·9–30·2) | 1091·6 (1077·2–1105·6) | 777·8 (767·4–788·0) | 31·0 (30·6–31·5) | 22·4 (22·0–22·7) | 29·5 (29·1–29·9) | 24·5 (24·2–24·8) | 54·0 (53·4–54·6) |
| 2011 | 68·0 (67·7–68·3) | 73·4 (73·1–73·8) | 26·2 (26·0–26·3) | 30·3 (30·1–30·4) | 1068·1 (1053·8–1083·1) | 756·1 (745·4–767·2) | 30·1 (29·7–30·5) | 21·5 (21·2–21·9) | 29·5 (29·0–29·9) | 24·4 (24·0–24·7) | 53·8 (53·3–54·4) |
| 2012 | 68·3 (68·0–68·6) | 73·9 (73·5–74·2) | 26·3 (26·1–26·5) | 30·5 (30·3–30·6) | 1051·9 (1037·1–1067·9) | 739·9 (728·9–751·9) | 29·4 (29·0–29·9) | 20·8 (20·5–21·2) | 29·7 (29·2–30·2) | 24·4 (24·0–24·8) | 54·1 (53·5–54·7) |
| 2013 | 68·6 (68·2–68·9) | 74·2 (73·9–74·6) | 26·4 (26·2–26·6) | 30·6 (30·4–30·8) | 1037·9 (1021·4–1055·4) | 725·8 (714·1–738·9) | 28·8 (28·3–29·3) | 20·2 (19·9–20·6) | 30·0 (29·5–30·5) | 24·5 (24·1–25·0) | 54·5 (53·8–55·1) |
| 2014 | 68·8 (68·4–69·1) | 74·5 (74·1–74·9) | 26·5 (26·3–26·6) | 30·7 (30·5–30·9) | 1029·7 (1012·5–1048·3) | 715·8 (703·8–729·6) | 28·4 (27·9–28·9) | 19·8 (19·4–20·2) | 30·4 (29·9–31·0) | 24·8 (24·3–25·2) | 55·2 (54·4–55·9) |
| 2015 | 69·0 (68·6–69·4) | 74·8 (74·4–75·2) | 26·6 (26·4–26·8) | 30·9 (30·7–31·1) | 1018·6 (1000·4–1037·1) | 703·4 (691·0–717·8) | 27·9 (27·4–28·5) | 19·3 (18·9–19·7) | 30·9 (30·3–31·5) | 24·9 (24·5–25·5) | 55·8 (55·0–56·6) |
Data in parentheses are 95% uncertainty intervals. Age-standardised rates are standardised using the GBD world population standard. YLLs=years of life lost. GBD=Global Burden of Disease.
Global mortality trends showed a 16·4% (95% UI 14·3–18·5) increase in total deaths between 1990 and 2015, whereas age-standardised rates of mortality fell by 28·5% (27·3–29·8) during this time. The trend was similar from 2005 to 2015, with total deaths increasing by 4·1% (2·6–5·6) and age-standardised death rates decreasing by 17·0% (15·8–18·1). In 2015, 55·8 million deaths (55·0 million to 56·6 million) occurred worldwide, an increase of 7·9 million deaths since 1990 and 2·2 million deaths since 2005 (table 4). From 1990 to 2015, total deaths rose by 21·9% (19·1–24·8) for males and 10·3% (7·6–13·1) for females, whereas age-standardised death rates fell by 26·2% (24·5–27·9) for males and 32·1% (30·4–33·8) for females. In 2015, 30·9 million (30·3 million to 31·5 million) males and 24·9 million (24·5 million to 25·5 million) females died, representing an increase of 5·6 million male deaths and 2·3 million female deaths since 1990. Differences in total deaths by sex widened over time, with increasingly more males dying than females; this gap grew from 2·7 million in 1990 to 4·1 million in 2005 and 6·0 million in 2015.
Age-standardised rates of YLLs per 100 population, a measure of premature mortality, fell 34·1% (95% UI 32·5–35·6) for males and 42·1% (40·6–43·5) for females between 1990 and 2015. Notably, the pace of decline in YLL rates was faster from 2005 to 2015 (19·9%, 95% UI 18·3–21·5 for males and 26·3%, 24·6–27·9 for females) than from 1990 to 2005 (17·7%, 16·2–19·2 for males and 21·4%, 20·0–22·7 for females).
Evolution of global and super-region life expectancy, probabilities of death, and SDI
The differences between observed life expectancy and mortality rates and those expected on the basis of SDI show the complex interactions between gains in SDI and improved health over time. Figure 10 summarises the trends in observed and expected life expectancy or mortality at the global level and for each GBD super-region from 1980 to 2015. Some regions have higher than expected levels, whereas others have lower levels than expected.
Figure 10.
Co-evolution of life expectancy and probabilities of death with SDI globally and for GBD super-regions, 1980 to 2015
(A) Life expectancy at birth and SDI; (B) under-5 death rate (5q0) and SDI; (C) probability of death between 15 and 50 years of age (35q15) and SDI; and (D) probability of death between 50 and 70 years of age (20q50) and SDI. Coloured lines show global and super-region values. Each point in a line represents 1 year, starting at 1980 and ending at 2015. In all super-regions, SDI has increased year on year so progress in SDI is associated with later years for a given super-region. Black lines show trajectories expected for each geography on the basis of SDI alone. GBD=Global Burden of Disease. SDI=Socio-demographic Index. 5q0=probability of death from birth to age 5 years. 35q15=probability of death from age 15 years to 50 years. 20q50=probability of death from age 50 years to 70 years.
By 2015, global life expectancy had increased faster than expected based on changes in SDI for both sexes, equating to an increase of an additional 3·06 years for males and 2·78 years for females (figure 10A); however, before 2005, gains in life expectancy were lower than expected, particularly for females. Observed life expectancy consistently exceeded expected levels over time in southeast and east Asia and Oceania; Latin America and the Caribbean; and north Africa and the Middle East. Furthermore, for the latter two super-regions, gains for male life expectancy improved following the 1980s and 1990s, when observed levels of longevity were closer to expected life expectancy based on SDI. By contrast, observed life expectancy was generally lower than expected based on SDI in high-income countries and central Europe, eastern Europe, and central Asia. For high-income countries, however, observed male life expectancy converged with expected levels around 2005, whereas the gap between observed and expected life expectancy based on SDI widened for females in this super-region. In south Asia, where average SDI more than doubled between 1980 and 2015, observed male life expectancy consistently met or slightly exceeded expected levels, whereas female life expectancy gradually moved closer to expected levels based on SDI. Amid its escalating HIV/AIDS epidemic, sub-Saharan Africa recorded widening gaps between observed and expected life expectancies for both sexes between 1988 and 1999. From 2001 to 2015, during which the region's average SDI rose by 31%, observed life expectancy quickly increased, particularly among females, nearing expected levels.
Overall, global and regional trends for observed under-5 mortality steadily moved closer to expected levels, based on rising SDI, and in some super-regions, such as Latin America, the Caribbean, and north Africa and the Middle East, observed rates of under-5 mortality became lower than expected (figure 10B). Substantial progress occurred in sub-Saharan Africa, with the gap between observed and expected 5q0 decreasing from 0·055 in 1980 to 0·006 in 2015. Observed under-5 mortality was consistently lower than expected, given rising SDI, in southeast Asia, east Asia, and Oceania, whereas the opposite was seen for central and eastern Europe and central Asia, with observed under-5 mortality exceeding expected levels from 1980 to 2015. With the exception of south Asia, super-region under-5 mortality trends did not substantially differ by sex. Observed levels of male under-5 mortality in south Asia gradually neared expected rates of under-5 mortality over time, whereas female under-5 mortality remained above expected levels between 1980 and 2000; by 2015, however, this gap had narrowed considerably.
Regional trends for observed and expected 35q15, which represents the probability of dying between the ages of 15 and 50 years, were much more variable than life expectancy at birth or 5q0 (figure 10C). The 35q15 age band corresponds to the reproductive period; for the analysis of changes in mortality with SDI, we include results for these age groups because of their very strong association with mortality from HIV/AIDS during the reproductive age period. Except for three super-regions (high income; sub-Saharan Africa; and central Europe, eastern Europe, and central Asia), observed levels of 35q15 remained lower than would be expected based on SDI between 1980 and 2015. However, relative trends, in terms of proximity to expected levels of 35q15 over time and by sex, shifted considerably. Although observed rates of female 35q15 were lower than expected from 1980 to 2015 in three super-regions (Latin America and the Caribbean; north Africa and the Middle East; and southeast and east Asia and Oceania), each super-region registered improvements in 35q15 over time and moved closer to expected levels by 2015. In sub-Saharan Africa, observed 35q15 for both sexes increased to well above expected levels between 1988 and 2000, a trend largely attributable to HIV/AIDS. By 2004, however, gains in SDI quickened in sub-Saharan Africa, and observed 35q15 began to fall closer to expected levels at a similar pace. Central and eastern Europe and central Asia experienced the most divergent patterns for 35q15 by sex. For males in this super-region, observed 35q15 remained far above expected levels of mortality based on SDI from 1980 to 2015, but observed 35q15 climbed between 1986 and 1994. This rapid rise in observed male mortality between the ages of 15 and 50 years, relative to SDI, occurred in tandem with the collapse of the Soviet Union and the widespread economic hardships that followed. The gap between observed and expected male 35q15 began to gradually narrow during the late 1990s, corresponding with rises in SDI; nonetheless, gains stalled by 1999. For females in central and eastern Europe and central Asia, observed 35q15 closely followed expected levels from 1980 to 1990, after which observed mortality jumped and remained higher than expected 35q15, based on SDI, through to 2015.
Results were similarly heterogeneous for observed and expected trends for 20q50, or the probability of dying between the ages 50 and 70 years, particularly by sex and rising SDI (figure 10D). First, based on gains in SDI alone, expected levels of 20q50 differed substantially by sex. For males, expected reductions for 20q50 were quite gradual relative to improvements in SDI, until the 80th percentile, after which expected 20q50 steeply fell. For females, expected 20q50 followed a fairly linear trend with rising SDI. Two regions—Latin America and the Caribbean and north Africa and the Middle East—experienced observed levels of 20q50 that were lower than expected from 1980 to 2015 for both sexes; however, for females in north Africa and the Middle East, observed 20q50 shifted closer to expected levels of mortality after 1999. After largely following expected rates of 20q50 from 1989 to 1998, southeast and east Asia and Oceania saw observed male 20q50 drop below expected levels. Observed female 20q50 generally remained lower than expected for this super-region, although observed levels approached expected rates from 2000 to 2003 before declining again. Although south Asia recorded large gains in SDI over time, from an average of the 25th percentile in 1980 to the 54th in 2015, observed rates of 20q50 remained higher than expected for both sexes over time. Aside from a jump in observed 20q50 rates between 1995 and 2007, in sub-Saharan Africa, observed 20q50 for both sexes mainly followed the expected rates given rising SDI. Similar to the results for 35q15, observed levels of male and female 20q50 in central and eastern Europe and central Asia followed a dissonant pattern over time. For males, although observed 20q50 surpassed expected rates between 1980 and 2015, observed 20q50 escalated from 1986 to 1994, rapidly increasing the gap between observed and expected rates of mortality for several years. The difference between observed and expected 20q50 widened for females in central and eastern Europe and central Asia during this time, albeit with a much smaller magnitude of change. Notably, observed levels of male 20q50 consistently exceeded expected rates for high-income countries between 1980 and 1997 before converging. Conversely, observed 20q50 for females in high-income countries remained higher than expected from 1980 to 2015.
Global causes of death
Table 5 shows the global estimates of total deaths and age-standardised death rates by cause for 2005 and 2015, as well as the percentage change in mortality from 2005 to 2015. Annual mortality estimates from 1990 to 2015 and more detailed age–sex results can be viewed online.
Table 5.
Global deaths in 2005 and 2015 for all ages and both sexes combined and age-standardised death rates, with percentage change between 2005 and 2015 for 249 causes
|
All age deaths (thousands) |
Age-standardised mortality rate (per 100 000) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2015 | Percentage change, 2005–15 | 2005 | 2015 | Percentage change, 2005–15 | ||||
| All causes | 53 618·5(53 139·8 to 54 075·8) | 55 792·9 (54 984·1 to 56 640·3) | 4·1 (2·6 to 5·6) | 1024·0 (1015·1 to 1032·6) | 850·1 (838·3 to 862·4) | −17·0 (−18·1 to −15·8) | |||
| Communicable, maternal, neonatal, and nutritional diseases (Group 1 causes) | 14 023·9 (13 734·8 to 14 335·3) | 11 263·6 (10 922·7 to 11 594·5) | −19·7 (−21·6 to −17·8) | 226·2 (221·3 to 231·6) | 159·3 (154·4 to 163·9) | −29·6 (−31·3 to −27·9) | |||
| HIV/AIDS and tuberculosis | 3139·5 (2938·6 to 3469·8) | 2305·2 (2092·7 to 2578·0) | −26·6 (−30·1 to −23·0) | 51·6 (48·0 to 57·4) | 31·9 (28·8 to 35·9) | −38·2 (−41·2 to −35·2) | |||
| Tuberculosis | 1347·6 (1152·9 to 1658·7) | 1112·6 (909·8 to 1392·8) | −17·4 (−24·4 to −11·3) | 24·2 (20·8 to 29·9) | 16·0 (13·1 to 20·1) | −33·8 (−39·6 to −28·7) | |||
| HIV/AIDS | 1791·9 (1703·8 to 1886·6) | 1192·6 (1130·8 to 1270·3) | −33·4 (−36·2 to −30·0) | 27·4 (26·0 to 28·8) | 15·8 (15·0 to 16·9) | −42·1 (−44·6 to −39·1) | |||
| HIV/AIDS—tuberculosis | 351·8 (281·7 to 400·4) | 211·7 (161·9 to 245·0) | −39·8 (−44·3 to −34·4) | 5·4 (4·4 to 6·2) | 2·8 (2·2 to 3·3) | −48·2 (−52·1 to −43·5) | |||
| HIV/AIDS resulting in other diseases | 1440·1 (1349·6 to 1546·7) | 980·8 (914·7 to 1063·6) | −31·9 (−35·6 to −27·7) | 21·9 (20·5 to 23·6) | 13·0 (12·1 to 14·1) | −40·6 (−43·8 to −36·9) | |||
| Diarrhoea, lower respiratory, and other common infectious diseases | 5773·1 (5548·3 to 6004·8) | 4959·8 (4711·6 to 5179·4) | −14·1 (−17·1 to −11·0) | 99·3 (95·5 to 103·1) | 73·2 (69·5 to 76·4) | −26·3 (−28·7 to −23·8) | |||
| Diarrhoeal diseases | 1657·2 (1565·0 to 1756·1) | 1312·1 (1233·6 to 1391·3) | −20·8 (−26·1 to −15·4) | 28·1 (26·7 to 29·6) | 19·1 (18·0 to 20·2) | −32·2 (−36·5 to −27·7) | |||
| Intestinal infectious diseases | 208·6 (118·0 to 344·1) | 178·5 (100·9 to 293·7) | −14·4 (−20·7 to −8·7) | 3·0 (1·7 to 5·0) | 2·4 (1·4 to 4·0) | −20·3 (−26·1 to −15·0) | |||
| Typhoid fever | 172·9 (94·6 to 293·2) | 148·8 (81·9 to 249·7) | −14·0 (−20·6 to −8·1) | 2·5 (1·4 to 4·3) | 2·0 (1·1 to 3·4) | −19·8 (−25·7 to −14·0) | |||
| Paratyphoid fever | 33·9 (15·6 to 65·1) | 29·2 (13·7 to 56·3) | −14·1 (−21·8 to −6·2) | 0·5 (0·2 to 0·9) | 0·4 (0·2 to 0·8) | −20·3 (−27·4 to −13·2) | |||
| Other intestinal infectious diseases | 1·8 (0·6 to 3·3) | 0·6 (0·3 to 1·2) | −64·0 (−75·5 to −43·9) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −67·7 (−77·7 to −51·2) | |||
| Lower respiratory infections | 2828·5 (2628·6 to 2965·8) | 2736·7 (2500·3 to 2860·8) | −3·2 (−6·9 to 0·4) | 51·7 (47·9 to 54·1) | 41·6 (38·0 to 43·5) | −19·5 (−22·3 to −16·9) | |||
| Upper respiratory infections | 3·8 (3·3 to 4·2) | 3·1 (2·8 to 3·5) | −18·0 (−28·5 to −5·2) | 0·1 (0·1 to 0·1) | 0·0 (0·0 to 0·1) | −32·2 (−40·4 to −22·0) | |||
| Otitis media | 3·9 (3·5 to 4·3) | 3·2 (2·9 to 3·7) | −17·7 (−27·3 to −4·5) | 0·1 (0·1 to 0·1) | 0·0 (0·0 to 0·1) | −27·8 (−38·5 to −14·5) | |||
| Meningitis | 407·7 (351·1 to 457·1) | 379·2 (322·7 to 444·7) | −7·0 (−15·4 to 5·2) | 6·3 (5·5 to 7·1) | 5·2 (4·5 to 6·1) | −17·2 (−24·3 to −6·7) | |||
| Pneumococcal meningitis | 112·1 (93·3 to 135·2) | 112·9 (93·4 to 141·8) | 0·7 (−8·7 to 13·8) | 1·7 (1·5 to 2·1) | 1·6 (1·3 to 1·9) | −10·8 (−18·4 to 0·7) | |||
| Haemophilus influenzae type b meningitis | 110·6 (88·3 to 135·8) | 71·5 (56·7 to 91·8) | −35·4 (−43·6 to −24·7) | 1·7 (1·3 to 2·0) | 1·0 (0·8 to 1·3) | −41·1 (−48·3 to −31·4) | |||
| Meningococcal meningitis | 74·3 (58·7 to 91·8) | 73·3 (58·0 to 93·2) | −1·3 (−13·1 to 15·4) | 1·1 (0·9 to 1·4) | 1·0 (0·8 to 1·3) | −11·6 (−21·8 to 3·0) | |||
| Other meningitis | 110·6 (94·9 to 128·6) | 121·5 (101·8 to 144·2) | 9·8 (1·1 to 22·0) | 1·8 (1·5 to 2·1) | 1·7 (1·4 to 2·0) | −4·6 (−11·8 to 5·9) | |||
| Encephalitis | 147·0 (135·3 to 163·0) | 149·5 (137·6 to 167·0) | 1·7 (−4·7 to 8·1) | 2·4 (2·2 to 2·7) | 2·1 (1·9 to 2·4) | −11·7 (−17·1 to −6·4) | |||
| Diphtheria | 5·6 (3·0 to 11·0) | 2·1 (1·1 to 4·7) | −61·3 (−85·0 to −2·0) | 0·1 (0·0 to 0·2) | 0·0 (0·0 to 0·1) | −64·2 (−86·2 to −9·2) | |||
| Whooping cough | 99·6 (36·8 to 226·3) | 58·7 (20·3 to 126·6) | −41·0 (−77·9 to 65·1) | 1·4 (0·5 to 3·3) | 0·8 (0·3 to 1·7) | −45·1 (−79·4 to 53·8) | |||
| Tetanus | 108·0 (90·9 to 151·1) | 56·7 (48·2 to 80·0) | −47·5 (−54·6 to −39·0) | 1·7 (1·4 to 2·4) | 0·8 (0·7 to 1·1) | −53·2 (−59·7 to −45·9) | |||
| Measles | 293·7 (110·6 to 611·4) | 73·4 (26·1 to 161·4) | −75·0 (−84·5 to −58·8) | 4·2 (1·6 to 8·8) | 1·0 (0·4 to 2·2) | −76·7 (−85·5 to −61·6) | |||
| Varicella and herpes zoster | 9·6 (8·5 to 11·0) | 6·4 (5·4 to 7·8) | −33·5 (−44·4 to −19·2) | 0·2 (0·2 to 0·2) | 0·1 (0·1 to 0·1) | −45·8 (−54·7 to −34·7) | |||
| Neglected tropical diseases and malaria | 1298·5 (1082·7 to 1509·1) | 843·1 (669·9 to 1019·7) | −35·1 (−43·6 to −26·7) | 19·5 (16·3 to 22·6) | 11·5 (9·1 to 13·9) | −41·3 (−48·9 to −33·8) | |||
| Malaria | 1167·0 (952·1 to 1378·1) | 730·5 (555·8 to 904·0) | −37·4 (−47·0 to −27·8) | 17·4 (14·2 to 20·6) | 9·9 (7·5 to 12·3) | −43·1 (−51·8 to −34·7) | |||
| Chagas disease | 7·5 (7·2 to 7·8) | 8·0 (7·5 to 8·6) | 7·7 (0·1 to 15·9) | 0·1 (0·1 to 0·2) | 0·1 (0·1 to 0·1) | −16·5 (−22·4 to −10·3) | |||
| Leishmaniasis | 23·1 (14·8 to 33·2) | 24·2 (17·1 to 32·5) | 4·9 (−8·5 to 21·5) | 0·3 (0·2 to 0·5) | 0·3 (0·2 to 0·4) | −7·2 (−18·7 to 7·1) | |||
| Visceral leishmaniasis | 23·1 (14·8 to 33·2) | 24·2 (17·1 to 32·5) | 4·9 (−8·5 to 21·5) | 0·3 (0·2 to 0·5) | 0·3 (0·2 to 0·4) | −7·2 (−18·7 to 7·1) | |||
| African trypanosomiasis | 14·2 (7·6 to 23·1) | 3·5 (1·8 to 5·7) | −75·3 (−81·4 to −67·9) | 0·2 (0·1 to 0·4) | 0·0 (0·0 to 0·1) | −78·4 (−83·7 to −72·0) | |||
| Schistosomiasis | 7·8 (7·0 to 8·9) | 4·4 (3·8 to 4·9) | −44·2 (−52·7 to −35·6) | 0·1 (0·1 to 0·2) | 0·1 (0·1 to 0·1) | −55·8 (−62·7 to −48·8) | |||
| Cysticercosis | 0·6 (0·5 to 0·7) | 0·4 (0·3 to 0·5) | −37·0 (−43·5 to −28·0) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −47·7 (−53·1 to −40·2) | |||
| Cystic echinococcosis | 1·8 (1·7 to 1·9) | 1·2 (1·1 to 1·3) | −32·6 (−35·7 to −29·4) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −44·6 (−47·0 to −42·1) | |||
| Dengue | 12·3 (8·6 to 15·1) | 18·4 (11·8 to 22·7) | 48·7 (15·1 to 90·9) | 0·2 (0·1 to 0·2) | 0·3 (0·2 to 0·3) | 34·0 (4·4 to 70·9) | |||
| Yellow fever | 6·3 (1·3 to 16·9) | 5·1 (1·1 to 14·2) | −18·8 (−33·5 to −0·8) | 0·1 (0·0 to 0·2) | 0·1 (0·0 to 0·2) | −25·7 (−39·2 to −9·4) | |||
| Rabies | 32·1 (28·0 to 36·1) | 17·4 (14·8 to 20·6) | −45·8 (−52·2 to −39·0) | 0·5 (0·4 to 0·6) | 0·2 (0·2 to 0·3) | −52·9 (−58·4 to −47·3) | |||
| Intestinal nematode infections | 3·8 (3·3 to 4·3) | 2·7 (2·4 to 3·1) | −28·5 (−36·9 to −19·7) | 0·1 (0·1 to 0·1) | 0·0 (0·0 to 0·0) | −34·7 (−42·3 to −26·7) | |||
| Ascariasis | 3·8 (3·3 to 4·3) | 2·7 (2·4 to 3·1) | −28·5 (−36·9 to −19·7) | 0·1 (0·1 to 0·1) | 0·0 (0·0 to 0·0) | −34·7 (−42·3 to −26·7) | |||
| Ebola virus disease | 0·0 (0·0 to 0·0) | 5·5 (4·4 to 6·6) | 32 659·1 (32 659·1 to 32 659·1) | 0·0 (0·0 to 0·0) | 0·1 (0·1 to 0·1) | 28 636·1 (28 636·1 to 28 636·1) | |||
| Other neglected tropical diseases | 21·9 (14·0 to 27·7) | 21·8 (12·7 to 27·6) | −0·6 (−14·5 to 15·9) | 0·4 (0·2 to 0·4) | 0·3 (0·2 to 0·4) | −13·0 (−24·9 to 1·1) | |||
| Maternal disorders | 350·8 (327·9 to 376·6) | 275·3 (243·8 to 315·5) | −21·5 (−30·3 to −10·7) | 5·1 (4·7 to 5·5) | 3·6 (3·2 to 4·1) | −29·1 (−37·1 to −19·3) | |||
| Maternal haemorrhage | 99·7 (87·6 to 113·0) | 83·1 (67·0 to 101·5) | −16·6 (−28·8 to −3·2) | 1·4 (1·3 to 1·6) | 1·1 (0·9 to 1·3) | −25·0 (−35·9 to −12·9) | |||
| Maternal sepsis and other maternal infections | 24·7 (20·4 to 29·9) | 17·9 (13·4 to 23·9) | −27·7 (−41·7 to −10·8) | 0·4 (0·3 to 0·4) | 0·2 (0·2 to 0·3) | −35·0 (−47·6 to −19·8) | |||
| Maternal hypertensive disorders | 63·7 (54·9 to 74·3) | 46·9 (37·1 to 59·6) | −26·4 (−36·7 to −13·1) | 0·9 (0·8 to 1·1) | 0·6 (0·5 to 0·8) | −32·9 (−42·5 to −20·9) | |||
| Maternal obstructed labour and uterine rupture | 26·9 (22·1 to 32·0) | 23·1 (17·2 to 30·0) | −13·9 (−27·4 to 1·5) | 0·4 (0·3 to 0·5) | 0·3 (0·2 to 0·4) | −22·5 (−34·5 to −8·5) | |||
| Maternal abortion, miscarriage, and ectopic pregnancy | 41·2 (34·7 to 49·6) | 31·7 (24·6 to 39·7) | −23·1 (−33·9 to −11·1) | 0·6 (0·5 to 0·7) | 0·4 (0·3 to 0·5) | −30·7 (−40·4 to −19·8) | |||
| Indirect maternal deaths | 38·2 (31·6 to 45·4) | 30·8 (23·1 to 40·5) | −19·4 (−32·4 to −1·9) | 0·6 (0·5 to 0·7) | 0·4 (0·3 to 0·5) | −27·1 (−38·8 to −11·1) | |||
| Late maternal deaths | 7·9 (5·3 to 11·5) | 6·7 (4·3 to 10·0) | −15·1 (−26·2 to −1·1) | 0·1 (0·1 to 0·2) | 0·1 (0·1 to 0·1) | −23·3 (−33·3 to −10·6) | |||
| Maternal deaths aggravated by HIV/AIDS | 2·8 (1·8 to 3·8) | 2·3 (1·4 to 3·3) | −15·8 (−33·1 to 8·0) | 0·0 (0·0 to 0·1) | 0·0 (0·0 to 0·0) | −24·8 (−40·4 to −3·7) | |||
| Other maternal disorders | 45·7 (39·0 to 53·9) | 32·7 (26·3 to 40·5) | −28·4 (−37·1 to −18·3) | 0·7 (0·6 to 0·8) | 0·4 (0·3 to 0·5) | −35·3 (−43·3 to −26·2) | |||
| Neonatal disorders | 2653·5 (2583·4 to 2728·1) | 2163·2 (2095·1 to 2232·5) | −18·5 (−20·4 to −16·4) | 37·3 (36·4 to 38·4) | 28·8 (27·9 to 29·7) | −22·8 (−24·6 to −20·9) | |||
| Neonatal preterm birth complications | 1088·0 (1010·9 to 1217·7) | 805·8 (736·2 to 898·6) | −25·9 (−31·3 to −20·6) | 15·3 (14·2 to 17·1) | 10·7 (9·8 to 12·0) | −29·8 (−34·9 to −24·8) | |||
| Neonatal encephalopathy (birth asphyxia and trauma) | 882·8 (800·7 to 974·3) | 740·4 (667·6 to 829·2) | −16·1 (−23·8 to −8·0) | 12·4 (11·3 to 13·7) | 9·9 (8·9 to 11·0) | −20·5 (−27·8 to −12·8) | |||
| Neonatal sepsis and other neonatal infections | 352·3 (252·0 to 465·7) | 351·7 (249·2 to 459·1) | −0·2 (−16·2 to 20·3) | 5·0 (3·5 to 6·6) | 4·7 (3·3 to 6·1) | −5·5 (−20·6 to 13·9) | |||
| Haemolytic disease and other neonatal jaundice | 68·3 (42·9 to 106·1) | 45·1 (30·1 to 67·3) | −34·0 (−47·9 to −17·1) | 1·0 (0·6 to 1·5) | 0·6 (0·4 to 0·9) | −37·6 (−50·6 to −21·6) | |||
| Other neonatal disorders | 262·0 (190·3 to 343·0) | 220·2 (167·6 to 276·8) | −16·0 (−34·1 to 5·6) | 3·7 (2·7 to 4·8) | 2·9 (2·2 to 3·7) | −20·5 (−37·7 to −0·1) | |||
| Nutritional deficiencies | 460·8 (380·3 to 541·6) | 405·7 (331·7 to 495·6) | −11·9 (−22·9 to 0·6) | 7·8 (6·5 to 9·0) | 5·9 (4·8 to 7·2) | −24·3 (−32·9 to −14·3) | |||
| Protein-energy malnutrition | 379·7 (314·3 to 457·1) | 323·2 (264·9 to 400·8) | −14·9 (−27·3 to −0·2) | 6·4 (5·3 to 7·6) | 4·7 (3·9 to 5·8) | −26·3 (−35·8 to −14·5) | |||
| Iodine deficiency | 2·1 (1·6 to 2·9) | 2·0 (1·5 to 2·7) | −3·1 (−31·4 to 40·7) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −18·4 (−41·6 to 15·7) | |||
| Iron-deficiency anaemia | 48·4 (31·9 to 63·1) | 54·2 (35·1 to 72·9) | 12·1 (−2·1 to 28·0) | 0·8 (0·5 to 1·1) | 0·8 (0·5 to 1·0) | −5·1 (−16·8 to 7·8) | |||
| Other nutritional deficiencies | 30·7 (21·7 to 43·7) | 26·3 (20·7 to 33·6) | −14·1 (−33·1 to 1·1) | 0·6 (0·4 to 0·8) | 0·4 (0·3 to 0·5) | −30·0 (−45·1 to −18·7) | |||
| Other communicable, maternal, neonatal, and nutritional diseases | 347·7 (291·2 to 419·8) | 311·3 (257·9 to 372·7) | −10·5 (−15·6 to −4·6) | 5·6 (4·7 to 6·6) | 4·4 (3·6 to 5·2) | −21·5 (−26·0 to −16·6) | |||
| Sexually transmitted diseases excluding HIV | 135·5 (81·2 to 207·2) | 108·0 (64·6 to 165·7) | −20·3 (−28·3 to −12·4) | 2·0 (1·2 to 3·0) | 1·5 (0·9 to 2·2) | −26·1 (−33·7 to −19·0) | |||
| Syphilis | 134·1 (79·9 to 205·7) | 106·8 (63·4 to 164·6) | −20·3 (−28·5 to −12·4) | 1·9 (1·2 to 3·0) | 1·4 (0·9 to 2·2) | −26·1 (−33·8 to −18·8) | |||
| Chlamydial infection | 0·2 (0·1 to 0·2) | 0·2 (0·1 to 0·2) | −5·3 (−17·0 to 10·6) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −23·5 (−32·9 to −11·3) | |||
| Gonococcal infection | 0·8 (0·7 to 0·9) | 0·7 (0·5 to 0·8) | −15·7 (−26·7 to −4·7) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −31·3 (−40·1 to −22·6) | |||
| Other sexually transmitted diseases | 0·4 (0·3 to 0·4) | 0·3 (0·2 to 0·4) | −16·8 (−27·6 to −6·1) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −31·7 (−40·3 to −23·0) | |||
| Hepatitis | 123·0 (118·6 to 127·6) | 105·8 (100·7 to 110·8) | −14·0 (−17·9 to −10·0) | 2·1 (2·0 to 2·2) | 1·5 (1·4 to 1·6) | −28·0 (−31·1 to −24·7) | |||
| Acute hepatitis A | 16·9 (10·9 to 23·1) | 11·2 (6·9 to 15·9) | −34·0 (−43·5 to −24·2) | 0·2 (0·2 to 0·3) | 0·2 (0·1 to 0·2) | −39·4 (−47·9 to −30·4) | |||
| Hepatitis B | 71·4 (62·0 to 80·1) | 65·4 (56·4 to 73·7) | −8·4 (−14·1 to −2·2) | 1·3 (1·1 to 1·4) | 0·9 (0·8 to 1·1) | −26·4 (−30·9 to −21·6) | |||
| Hepatitis C | 2·8 (0·6 to 6·4) | 2·5 (0·5 to 5·9) | −9·8 (−24·4 to 8·6) | 0·1 (0·0 to 0·1) | 0·0 (0·0 to 0·1) | −29·6 (−41·0 to −15·1) | |||
| Acute hepatitis E | 31·9 (23·0 to 42·3) | 26·7 (18·5 to 36·6) | −16·4 (−26·1 to −6·6) | 0·5 (0·4 to 0·7) | 0·4 (0·3 to 0·5) | −26·3 (−34·3 to −17·9) | |||
| Other infectious diseases | 89·1 (61·3 to 102·3) | 97·5 (61·3 to 112·9) | 9·4 (−2·3 to 23·2) | 1·5 (1·1 to 1·7) | 1·4 (0·9 to 1·6) | −6·4 (−16·8 to 5·2) | |||
| Non-communicable diseases | 34 835·6 (34 441·3 to 35 277·1) | 39 804·2 (39 210·8 to 40 452·2) | 14·3 (12·6 to 16·0) | 719·1 (711·9 to 727·3) | 624·7 (615·8 to 634·5) | −13·1 (−14·3 to −11·9) | |||
| Neoplasms | 7492·8 (7378·4 to 7616·5) | 8764·6 (8591·1 to 8945·6) | 17·0 (14·8 to 19·3) | 149·2 (147·1 to 151·7) | 134·3 (131·6 to 137·0) | −10·0 (−11·6 to −8·3) | |||
| Lip and oral cavity cancer | 110·2 (107·6 to 112·9) | 146·0 (141·6 to 150·6) | 32·5 (28·5 to 37·0) | 2·2 (2·1 to 2·2) | 2·2 (2·1 to 2·3) | 1·4 (−1·6 to 4·8) | |||
| Nasopharynx cancer | 55·8 (45·9 to 58·8) | 63·0 (51·1 to 67·0) | 12·8 (5·1 to 19·4) | 1·0 (0·8 to 1·1) | 0·9 (0·7 to 1·0) | −10·9 (−16·8 to −5·7) | |||
| Other pharynx cancer | 51·9 (50·5 to 53·2) | 64·4 (61·6 to 67·1) | 24·1 (18·4 to 29·7) | 1·0 (1·0 to 1·0) | 1·0 (0·9 to 1·0) | −5·0 (−9·3 to −0·6) | |||
| Oesophageal cancer | 459·3 (445·1 to 474·2) | 439·0 (422·6 to 456·9) | −4·4 (−9·0 to 0·7) | 9·2 (8·9 to 9·5) | 6·7 (6·5 to 7·0) | −26·8 (−30·3 to −22·9) | |||
| Stomach cancer | 824·5 (806·8 to 843·0) | 818·9 (795·5 to 843·7) | −0·7 (−3·7 to 2·6) | 16·7 (16·3 to 17·0) | 12·7 (12·4 to 13·1) | −23·8 (−26·0 to −21·4) | |||
| Colon and rectum cancer | 675·5 (664·9 to 688·2) | 832·0 (811·7 to 854·5) | 23·2 (20·6 to 26·0) | 14·0 (13·8 to 14·2) | 13·0 (12·7 to 13·4) | −6·7 (−8·7 to −4·6) | |||
| Liver cancer | 726·7 (636·0 to 762·2) | 810·5 (749·7 to 862·8) | 11·5 (5·9 to 20·4) | 13·9 (12·3 to 14·6) | 12·1 (11·2 to 12·9) | −13·1 (−17·4 to −6·7) | |||
| Liver cancer due to hepatitis B | 263·1 (224·4 to 282·5) | 265·3 (241·0 to 290·5) | 0·8 (−5·6 to 12·5) | 4·8 (4·1 to 5·1) | 3·8 (3·5 to 4·2) | −20·2 (−25·2 to −11·5) | |||
| Liver cancer due to hepatitis C | 137·8 (126·1 to 146·3) | 167·1 (153·9 to 177·9) | 21·3 (17·3 to 26·2) | 2·8 (2·6 to 3·0) | 2·6 (2·4 to 2·8) | −7·4 (−10·4 to −3·8) | |||
| Liver cancer due to alcohol use | 194·5 (168·8 to 208·3) | 245·2 (225·1 to 266·6) | 26·1 (18·5 to 37·1) | 3·8 (3·3 to 4·1) | 3·7 (3·4 to 4·0) | −3·1 (−8·6 to 4·8) | |||
| Liver cancer due to other causes | 131·3 (114·1 to 141·6) | 132·9 (119·8 to 144·4) | 1·2 (−4·2 to 9·7) | 2·5 (2·2 to 2·7) | 2·0 (1·8 to 2·2) | −21·1 (−25·2 to −15·0) | |||
| Gallbladder and biliary tract cancer | 124·5 (120·8 to 127·8) | 140·5 (131·4 to 147·2) | 12·9 (7·3 to 18·2) | 2·6 (2·5 to 2·7) | 2·2 (2·1 to 2·3) | −14·7 (−19·0 to −10·6) | |||
| Pancreatic cancer | 314·6 (310·1 to 319·0) | 411·6 (403·6 to 420·7) | 30·8 (28·3 to 33·6) | 6·5 (6·4 to 6·6) | 6·4 (6·3 to 6·6) | −0·9 (−2·8 to 1·2) | |||
| Larynx cancer | 93·1 (90·9 to 95·7) | 105·9 (102·7 to 109·5) | 13·8 (10·5 to 17·5) | 1·8 (1·8 to 1·9) | 1·6 (1·6 to 1·7) | −12·8 (−15·3 to −10·0) | |||
| Tracheal, bronchus, and lung cancer | 1434·5 (1406·5 to 1463·5) | 1722·5 (1673·7 to 1772·7) | 20·1 (16·7 to 24·0) | 29·0 (28·5 to 29·6) | 26·6 (25·9 to 27·4) | −8·1 (−10·7 to −5·2) | |||
| Malignant skin melanoma | 47·0 (38·7 to 58·6) | 59·8 (47·6 to 72·7) | 27·2 (20·0 to 32·6) | 0·9 (0·8 to 1·1) | 0·9 (0·7 to 1·1) | −1·8 (−7·1 to 2·4) | |||
| Non-melanoma skin cancer | 36·3 (35·4 to 37·1) | 51·9 (49·9 to 53·8) | 42·9 (37·4 to 47·8) | 0·8 (0·7 to 0·8) | 0·8 (0·8 to 0·9) | 7·6 (3·4 to 11·1) | |||
| Squamous-cell carcinoma | 36·3 (35·4 to 37·1) | 51·9 (49·9 to 53·8) | 42·9 (37·4 to 47·8) | 0·8 (0·7 to 0·8) | 0·8 (0·8 to 0·9) | 7·6 (3·4 to 11·1) | |||
| Breast cancer | 439·8 (418·8 to 461·9) | 533·6 (502·2 to 553·1) | 21·3 (14·9 to 27·2) | 8·5 (8·1 to 8·9) | 7·9 (7·5 to 8·2) | −6·8 (−11·5 to −2·5) | |||
| Cervical cancer | 225·4 (213·9 to 237·8) | 238·6 (225·3 to 252·4) | 5·8 (−0·5 to 13·8) | 4·2 (4·0 to 4·4) | 3·5 (3·3 to 3·7) | −17·7 (−22·5 to −11·5) | |||
| Uterine cancer | 81·8 (78·5 to 85·4) | 89·9 (86·1 to 94·3) | 10·0 (3·8 to 17·4) | 1·6 (1·6 to 1·7) | 1·4 (1·3 to 1·4) | −16·1 (−20·8 to −10·7) | |||
| Ovarian cancer | 133·8 (130·8 to 138·6) | 161·1 (156·5 to 166·5) | 20·4 (16·5 to 24·4) | 2·6 (2·6 to 2·7) | 2·4 (2·3 to 2·5) | −7·9 (−10·8 to −4·9) | |||
| Prostate cancer | 277·4 (230·8 to 348·9) | 365·9 (303·5 to 459·6) | 31·9 (28·2 to 35·4) | 6·1 (5·1 to 7·7) | 6·0 (5·0 to 7·6) | −1·7 (−4·5 to 0·8) | |||
| Testicular cancer | 8·6 (8·3 to 9·1) | 9·4 (8·8 to 9·9) | 8·4 (1·3 to 14·4) | 0·1 (0·1 to 0·1) | 0·1 (0·1 to 0·1) | −8·9 (−14·7 to −3·9) | |||
| Kidney cancer | 104·5 (102·4 to 106·9) | 136·9 (133·0 to 141·3) | 31·0 (27·0 to 34·6) | 2·1 (2·1 to 2·1) | 2·1 (2·0 to 2·2) | 0·4 (−2·6 to 3·2) | |||
| Bladder cancer | 150·6 (147·9 to 153·2) | 188·0 (182·8 to 192·7) | 24·8 (21·4 to 28·3) | 3·2 (3·2 to 3·3) | 3·0 (2·9 to 3·1) | −6·4 (−9·0 to −3·8) | |||
| Brain and nervous system cancer | 190·4 (173·3 to 201·2) | 228·8 (209·5 to 244·7) | 20·1 (12·7 to 27·2) | 3·5 (3·2 to 3·6) | 3·3 (3·0 to 3·6) | −3·6 (−9·3 to 1·8) | |||
| Thyroid cancer | 25·5 (24·4 to 27·6) | 31·9 (28·9 to 33·2) | 24·8 (14·8 to 31·0) | 0·5 (0·5 to 0·6) | 0·5 (0·5 to 0·5) | −4·8 (−12·2 to 0·0) | |||
| Mesothelioma | 23·2 (22·7 to 23·8) | 32·4 (31·2 to 33·4) | 39·6 (34·4 to 44·3) | 0·5 (0·5 to 0·5) | 0·5 (0·5 to 0·5) | 7·8 (3·6 to 11·6) | |||
| Hodgkin lymphoma | 25·4 (22·7 to 29·7) | 23·9 (21·8 to 29·0) | −6·0 (−10·6 to −1·3) | 0·5 (0·4 to 0·5) | 0·3 (0·3 to 0·4) | −23·9 (−27·7 to −20·1) | |||
| Non-Hodgkin's lymphoma | 179·3 (160·6 to 191·6) | 231·4 (195·7 to 243·8) | 29·0 (18·1 to 35·2) | 3·5 (3·1 to 3·7) | 3·5 (3·0 to 3·7) | 0·0 (−8·0 to 4·4) | |||
| Multiple myeloma | 77·5 (75·6 to 79·7) | 101·1 (97·7 to 104·1) | 30·5 (26·3 to 34·5) | 1·6 (1·6 to 1·6) | 1·6 (1·5 to 1·6) | −1·3 (−4·4 to 1·7) | |||
| Leukaemia | 303·5 (297·3 to 311·8) | 353·5 (344·6 to 363·1) | 16·5 (13·5 to 19·6) | 5·6 (5·5 to 5·7) | 5·3 (5·1 to 5·4) | −5·7 (−7·9 to −3·3) | |||
| Acute lymphoid leukaemia | 97·5 (90·4 to 108·0) | 110·5 (101·2 to 118·4) | 13·3 (7·1 to 19·0) | 1·6 (1·5 to 1·8) | 1·6 (1·4 to 1·7) | −3·0 (−8·1 to 1·7) | |||
| Chronic lymphoid leukaemia | 51·5 (49·3 to 53·9) | 60·7 (57·9 to 64·6) | 17·9 (12·5 to 23·4) | 1·1 (1·0 to 1·1) | 1·0 (0·9 to 1·0) | −10·1 (−14·0 to −6·1) | |||
| Acute myeloid leukaemia | 119·0 (110·5 to 126·7) | 147·1 (137·3 to 157·0) | 23·5 (19·3 to 27·8) | 2·2 (2·1 to 2·3) | 2·2 (2·1 to 2·3) | −0·4 (−3·5 to 2·7) | |||
| Chronic myeloid leukaemia | 35·4 (33·4 to 38·2) | 35·2 (33·4 to 37·7) | −0·7 (−4·8 to 3·8) | 0·7 (0·6 to 0·7) | 0·5 (0·5 to 0·6) | −22·0 (−25·3 to −18·5) | |||
| Other neoplasms | 292·1 (270·9 to 302·6) | 372·2 (335·9 to 392·1) | 27·4 (21·4 to 32·6) | 5·5 (5·1 to 5·7) | 5·5 (5·0 to 5·8) | 1·2 (−3·6 to 5·3) | |||
| Cardiovascular diseases | 15 933·7 (15 732·1 to 16 161·6) | 17 921·0 (17 590·5 to 18 276·8) | 12·5 (10·6 to 14·4) | 338·1 (333·8 to 342·9) | 285·5 (280·2 to 291·2) | −15·6 (−16·9 to −14·2) | |||
| Rheumatic heart disease | 333·2 (313·1 to 349·5) | 319·4 (297·3 to 337·3) | −4·1 (−8·2 to −0·1) | 6·4 (6·0 to 6·7) | 4·8 (4·5 to 5·1) | −24·7 (−27·9 to −21·6) | |||
| Ischaemic heart disease | 7648·4 (7551·5 to 7774·3) | 8917·0 (8751·6 to 9108·8) | 16·6 (14·6 to 18·6) | 163·1 (160·8 to 165·6) | 142·1 (139·5 to 145·2) | −12·8 (−14·2 to −11·4) | |||
| Cerebrovascular disease | 6020·9 (5920·2 to 6127·1) | 6326·1 (6175·2 to 6492·9) | 5·1 (2·7 to 7·5) | 127·9 (125·8 to 130·1) | 101·0 (98·6 to 103·6) | −21·0 (−22·8 to −19·2) | |||
| Ischaemic stroke | 2760·8 (2682·7 to 2837·5) | 2978·0 (2880·8 to 3068·8) | 7·9 (5·2 to 10·6) | 61·3 (59·5 to 62·9) | 48·9 (47·3 to 50·4) | −20·2 (−22·1 to −18·1) | |||
| Haemorrhagic stroke | 3260·1 (3169·2 to 3359·8) | 3348·2 (3240·9 to 3500·1) | 2·7 (−0·6 to 6·4) | 66·7 (64·9 to 68·7) | 52·1 (50·4 to 54·5) | −21·9 (−24·3 to −19·0) | |||
| Hypertensive heart disease | 760·5 (711·9 to 823·7) | 962·4 (873·6 to 1024·5) | 26·5 (17·5 to 32·3) | 16·2 (15·2 to 17·5) | 15·4 (13·9 to 16·4) | −4·9 (−11·9 to −0·6) | |||
| Cardiomyopathy and myocarditis | 328·8 (315·8 to 338·8) | 353·7 (339·5 to 370·6) | 7·6 (3·8 to 11·4) | 6·4 (6·2 to 6·6) | 5·4 (5·2 to 5·7) | −16·1 (−18·9 to −13·2) | |||
| Atrial fibrillation and flutter | 142·8 (117·3 to 172·0) | 195·3 (159·5 to 236·2) | 36·8 (34·0 to 39·6) | 3·4 (2·8 to 4·1) | 3·3 (2·7 to 4·0) | −3·4 (−4·9 to −1·9) | |||
| Aortic aneurysm | 134·8 (131·7 to 138·3) | 168·2 (163·6 to 172·8) | 24·8 (19·5 to 28·5) | 2·9 (2·8 to 3·0) | 2·7 (2·6 to 2·8) | −6·6 (−10·4 to −3·8) | |||
| Peripheral vascular disease | 38·1 (36·7 to 39·6) | 52·5 (49·7 to 55·7) | 37·7 (30·1 to 46·5) | 0·9 (0·8 to 0·9) | 0·9 (0·8 to 0·9) | −0·3 (−5·9 to 6·3) | |||
| Endocarditis | 68·8 (60·4 to 74·7) | 84·9 (74·7 to 93·0) | 23·4 (18·5 to 28·2) | 1·4 (1·2 to 1·5) | 1·3 (1·2 to 1·4) | −4·6 (−8·1 to −1·1) | |||
| Other cardiovascular and circulatory diseases | 457·4 (446·7 to 470·6) | 541·4 (521·7 to 561·2) | 18·4 (14·0 to 22·5) | 9·6 (9·3 to 9·8) | 8·6 (8·3 to 8·9) | −10·3 (−13·5 to −7·4) | |||
| Chronic respiratory diseases | 3709·1 (3631·6 to 3796·0) | 3795·5 (3683·9 to 3910·4) | 2·3 (−0·8 to 5·4) | 79·0 (77·3 to 80·9) | 61·0 (59·3 to 62·8) | −22·8 (−25·1 to −20·4) | |||
| Chronic obstructive pulmonary disease | 3100·5 (2997·7 to 3194·9) | 3188·3 (3083·8 to 3292·5) | 2·8 (−0·6 to 6·9) | 67·0 (64·8 to 69·0) | 51·7 (50·0 to 53·4) | −22·9 (−25·4 to −20·0) | |||
| Pneumoconiosis | 31·9 (28·4 to 36·3) | 36·1 (31·5 to 40·7) | 13·2 (6·4 to 22·4) | 0·7 (0·6 to 0·8) | 0·6 (0·5 to 0·7) | −14·4 (−19·5 to −7·7) | |||
| Silicosis | 10·2 (9·4 to 11·4) | 10·4 (9·4 to 11·7) | 2·0 (−9·0 to 15·4) | 0·2 (0·2 to 0·2) | 0·2 (0·1 to 0·2) | −21·8 (−30·2 to −11·8) | |||
| Asbestosis | 2·8 (2·0 to 3·2) | 3·6 (2·5 to 4·2) | 28·4 (17·6 to 39·5) | 0·1 (0·0 to 0·1) | 0·1 (0·0 to 0·1) | −2·1 (−10·2 to 6·2) | |||
| Coal workers pneumoconiosis | 2·7 (2·4 to 3·0) | 2·5 (2·2 to 2·9) | −7·3 (−21·5 to 6·4) | 0·1 (0·1 to 0·1) | 0·0 (0·0 to 0·0) | −29·9 (−40·4 to −19·6) | |||
| Other pneumoconiosis | 16·1 (13·3 to 19·7) | 19·5 (15·8 to 23·2) | 21·2 (11·0 to 33·5) | 0·3 (0·3 to 0·4) | 0·3 (0·3 to 0·4) | −9·4 (−16·9 to −0·2) | |||
| Asthma | 449·9 (362·1 to 518·0) | 397·1 (363·0 to 438·7) | −11·7 (−21·2 to 3·4) | 8·8 (7·1 to 10·2) | 6·1 (5·6 to 6·7) | −31·3 (−38·9 to −19·4) | |||
| Interstitial lung disease and pulmonary sarcoidosis | 80·4 (61·2 to 92·8) | 121·8 (94·1 to 135·2) | 51·5 (37·9 to 60·5) | 1·7 (1·3 to 2·0) | 2·0 (1·5 to 2·2) | 14·1 (4·1 to 20·9) | |||
| Other chronic respiratory diseases | 46·4 (33·9 to 55·6) | 52·1 (38·0 to 61·0) | 12·2 (1·4 to 23·0) | 0·8 (0·6 to 1·0) | 0·8 (0·6 to 0·9) | −5·9 (−13·8 to 2·5) | |||
| Cirrhosis and other chronic liver diseases | 1171·7 (1131·9 to 1236·7) | 1292·1 (1239·9 to 1371·7) | 10·3 (7·0 to 13·7) | 21·6 (20·9 to 22·8) | 18·8 (18·0 to 19·9) | −13·1 (−15·6 to −10·5) | |||
| Cirrhosis and other chronic liver diseases due to hepatitis B | 341·4 (316·2 to 374·1) | 371·1 (341·6 to 410·0) | 8·7 (4·7 to 12·7) | 6·3 (5·9 to 6·9) | 5·4 (5·0 to 6·0) | −14·6 (−17·5 to −11·6) | |||
| Cirrhosis and other chronic liver diseases due to hepatitis C | 287·4 (268·1 to 307·8) | 325·6 (301·8 to 349·9) | 13·3 (10·4 to 16·5) | 5·4 (5·0 to 5·8) | 4·8 (4·4 to 5·1) | −11·7 (−13·9 to −9·3) | |||
| Cirrhosis and other chronic liver diseases due to alcohol use | 310·1 (289·4 to 333·4) | 347·9 (322·7 to 374·6) | 12·2 (8·4 to 16·7) | 5·6 (5·2 to 6·0) | 5·0 (4·6 to 5·3) | −11·6 (−14·5 to −8·2) | |||
| Cirrhosis and other chronic liver diseases due to other causes | 232·8 (217·6 to 254·6) | 247·5 (230·3 to 272·2) | 6·3 (3·4 to 9·8) | 4·3 (4·0 to 4·7) | 3·6 (3·4 to 4·0) | −14·8 (−17·1 to −12·2) | |||
| Digestive diseases | 1113·2 (1081·1 to 1186·7) | 1203·0 (1150·5 to 1270·0) | 8·1 (3·7 to 12·6) | 22·0 (21·4 to 23·4) | 18·5 (17·7 to 19·5) | −16·1 (−19·3 to −12·7) | |||
| Peptic ulcer disease | 294·3 (278·0 to 322·0) | 267·5 (249·4 to 290·0) | −9·1 (−15·3 to 0·0) | 5·8 (5·5 to 6·3) | 4·1 (3·8 to 4·4) | −29·3 (−34·1 to −22·1) | |||
| Gastritis and duodenitis | 49·9 (40·2 to 60·9) | 49·7 (41·8 to 60·1) | −0·5 (−10·9 to 12·4) | 1·0 (0·8 to 1·2) | 0·8 (0·6 to 0·9) | −23·0 (−31·0 to −12·9) | |||
| Appendicitis | 49·9 (42·9 to 56·4) | 50·1 (41·2 to 56·0) | 0·5 (−9·0 to 10·7) | 0·9 (0·8 to 1·0) | 0·7 (0·6 to 0·8) | −17·0 (−24·5 to −9·0) | |||
| Paralytic ileus and intestinal obstruction | 233·4 (210·7 to 272·0) | 264·3 (242·4 to 307·4) | 13·2 (7·7 to 19·7) | 4·6 (4·1 to 5·3) | 4·0 (3·7 to 4·7) | −11·4 (−15·5 to −6·6) | |||
| Inguinal, femoral, and abdominal hernia | 56·6 (40·0 to 63·8) | 59·8 (41·1 to 69·2) | 5·8 (−3·1 to 16·8) | 1·1 (0·8 to 1·3) | 0·9 (0·6 to 1·1) | −18·1 (−25·2 to −9·3) | |||
| Inflammatory bowel disease | 42·6 (38·3 to 47·3) | 47·4 (43·5 to 51·7) | 11·2 (2·9 to 18·8) | 0·9 (0·8 to 0·9) | 0·7 (0·7 to 0·8) | −14·6 (−20·4 to −9·1) | |||
| Vascular intestinal disorders | 84·3 (79·0 to 91·2) | 105·8 (99·0 to 114·3) | 25·6 (20·4 to 30·5) | 1·8 (1·7 to 2·0) | 1·7 (1·6 to 1·8) | −6·1 (−10·0 to −2·7) | |||
| Gallbladder and biliary diseases | 96·4 (91·1 to 103·1) | 111·7 (105·3 to 120·0) | 15·9 (10·2 to 21·3) | 2·0 (1·9 to 2·1) | 1·8 (1·7 to 1·9) | −11·7 (−15·8 to −7·7) | |||
| Pancreatitis | 109·6 (103·0 to 117·2) | 132·7 (122·9 to 144·0) | 21·1 (14·3 to 28·1) | 2·0 (1·9 to 2·2) | 1·9 (1·8 to 2·1) | −3·9 (−9·2 to 1·5) | |||
| Other digestive diseases | 96·3 (86·5 to 116·2) | 114·0 (101·9 to 131·1) | 18·3 (8·1 to 28·9) | 2·0 (1·8 to 2·4) | 1·8 (1·6 to 2·0) | −9·5 (−16·6 to −2·1) | |||
| Neurological disorders | 1671·0 (1445·9 to 1889·8) | 2258·9 (1937·8 to 2574·4) | 35·2 (33·2 to 37·2) | 38·7 (33·1 to 44·1) | 37·6 (32·2 to 43·0) | −2·7 (−3·7 to −1·6) | |||
| Alzheimer's disease and other dementias | 1380·8 (1152·7 to 1599·4) | 1908·2 (1586·7 to 2229·1) | 38·2 (36·2 to 40·1) | 33·2 (27·6 to 38·7) | 32·3 (26·9 to 37·8) | −2·7 (−3·7 to −1·7) | |||
| Parkinson's disease | 82·4 (80·0 to 85·6) | 117·4 (113·9 to 121·3) | 42·4 (37·3 to 46·9) | 1·9 (1·8 to 2·0) | 2·0 (1·9 to 2·0) | 4·2 (0·5 to 7·6) | |||
| Epilepsy | 119·0 (111·9 to 127·6) | 124·9 (119·3 to 131·0) | 5·0 (−2·1 to 12·3) | 1·9 (1·8 to 2·0) | 1·7 (1·6 to 1·8) | −9·2 (−15·1 to −3·3) | |||
| Multiple sclerosis | 16·5 (14·9 to 18·1) | 18·9 (17·3 to 20·0) | 14·8 (7·2 to 20·7) | 0·3 (0·3 to 0·3) | 0·3 (0·2 to 0·3) | −9·7 (−15·3 to −5·1) | |||
| Motor neuron disease | 27·6 (26·8 to 28·8) | 35·2 (33·9 to 36·7) | 27·6 (20·6 to 31·1) | 0·5 (0·5 to 0·6) | 0·5 (0·5 to 0·6) | −1·2 (−6·5 to 1·6) | |||
| Other neurological disorders | 44·8 (43·6 to 47·8) | 54·3 (53·0 to 57·5) | 21·3 (17·5 to 24·5) | 0·8 (0·8 to 0·8) | 0·8 (0·8 to 0·8) | −1·0 (−4·1 to 1·7) | |||
| Mental and substance use disorders | 305·9 (293·5 to 314·9) | 324·9 (308·6 to 337·4) | 6·2 (1·9 to 10·4) | 5·1 (4·9 to 5·3) | 4·5 (4·3 to 4·7) | −12·6 (−16·0 to −9·2) | |||
| Schizophrenia | 19·1 (17·9 to 20·0) | 16·9 (15·9 to 18·0) | −11·4 (−18·8 to −2·9) | 0·3 (0·3 to 0·4) | 0·2 (0·2 to 0·3) | −29·2 (−34·9 to −22·9) | |||
| Alcohol use disorders | 157·4 (147·4 to 163·3) | 137·5 (131·5 to 144·0) | −12·6 (−16·7 to −7·0) | 2·7 (2·5 to 2·8) | 1·9 (1·8 to 2·0) | −29·2 (−32·4 to −24·7) | |||
| Drug use disorders | 128·8 (124·0 to 133·6) | 169·9 (152·1 to 179·2) | 31·8 (20·4 to 39·4) | 2·1 (2·0 to 2·2) | 2·3 (2·1 to 2·5) | 11·5 (2·0 to 17·7) | |||
| Opioid use disorders | 94·2 (90·5 to 99·7) | 122·1 (109·5 to 129·7) | 29·6 (18·2 to 37·2) | 1·5 (1·5 to 1·6) | 1·7 (1·5 to 1·8) | 10·0 (0·5 to 16·5) | |||
| Cocaine use disorders | 7·4 (5·0 to 7·9) | 11·1 (8·5 to 12·2) | 49·7 (33·6 to 75·4) | 0·1 (0·1 to 0·1) | 0·1 (0·1 to 0·2) | 26·4 (12·9 to 48·0) | |||
| Amphetamine use disorders | 7·3 (4·3 to 8·2) | 12·2 (8·4 to 14·2) | 67·5 (25·6 to 118·9) | 0·1 (0·1 to 0·1) | 0·2 (0·1 to 0·2) | 42·3 (7·2 to 85·6) | |||
| Other drug use disorders | 19·9 (18·6 to 22·8) | 24·5 (22·7 to 27·3) | 23·0 (12·7 to 32·1) | 0·3 (0·3 to 0·4) | 0·3 (0·3 to 0·4) | 2·6 (−5·6 to 9·5) | |||
| Eating disorders | 0·6 (0·4 to 0·8) | 0·7 (0·5 to 0·9) | 7·7 (0·8 to 17·3) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −4·4 (−10·2 to 3·5) | |||
| Anorexia nervosa | 0·6 (0·4 to 0·7) | 0·6 (0·4 to 0·8) | 5·6 (−1·2 to 14·1) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −6·0 (−11·8 to 1·1) | |||
| Bulimia nervosa | 0·0 (0·0 to 0·1) | 0·1 (0·0 to 0·1) | 33·2 (19·1 to 58·1) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | 14·6 (3·3 to 34·2) | |||
| Diabetes, urogenital, blood, and endocrine diseases | 2635·3 (2534·5 to 2716·0) | 3409·3 (3287·5 to 3516·5) | 29·4 (26·1 to 32·7) | 52·9 (51·0 to 54·3) | 52·9 (51·0 to 54·5) | −0·1 (−2·3 to 2·3) | |||
| Diabetes mellitus | 1150·2 (1120·9 to 1176·8) | 1519·0 (1470·3 to 1576·0) | 32·1 (27·7 to 36·3) | 23·6 (23·0 to 24·2) | 23·7 (23·0 to 24·6) | 0·4 (−2·7 to 3·6) | |||
| Acute glomerulonephritis | 12·7 (9·9 to 14·4) | 11·8 (7·8 to 13·3) | −6·9 (−23·8 to 2·0) | 0·2 (0·2 to 0·3) | 0·2 (0·1 to 0·2) | −25·3 (−38·9 to −18·2) | |||
| Chronic kidney disease | 937·7 (866·2 to 970·8) | 1234·9 (1131·7 to 1282·4) | 31·7 (27·7 to 35·6) | 19·0 (17·7 to 19·7) | 19·2 (17·7 to 20·0) | 1·2 (−1·9 to 4·0) | |||
| Chronic kidney disease due to diabetes mellitus | 299·4 (278·7 to 314·2) | 417·8 (388·7 to 441·4) | 39·5 (35·4 to 43·5) | 6·1 (5·7 to 6·4) | 6·5 (6·1 to 6·9) | 6·4 (3·3 to 9·3) | |||
| Chronic kidney disease due to hypertension | 408·5 (377·1 to 427·6) | 549·5 (501·6 to 575·6) | 34·5 (30·0 to 38·7) | 8·4 (7·8 to 8·8) | 8·7 (7·9 to 9·1) | 2·4 (−0·9 to 5·5) | |||
| Chronic kidney disease due to glomerulonephritis | 205·6 (184·9 to 217·9) | 237·7 (212·6 to 255·9) | 15·6 (10·9 to 20·2) | 4·0 (3·6 to 4·2) | 3·6 (3·3 to 3·9) | −9·0 (−12·6 to −5·4) | |||
| Chronic kidney disease due to other causes | 24·2 (20·2 to 28·6) | 30·0 (25·0 to 35·2) | 23·9 (17·8 to 30·2) | 0·5 (0·4 to 0·6) | 0·5 (0·4 to 0·5) | −2·2 (−6·9 to 2·5) | |||
| Urinary diseases and male infertility | 201·1 (188·2 to 215·7) | 261·7 (243·2 to 277·6) | 30·1 (23·7 to 36·6) | 4·2 (3·9 to 4·5) | 4·1 (3·8 to 4·4) | −1·3 (−6·1 to 3·6) | |||
| Interstitial nephritis and urinary tract infections | 149·8 (139·3 to 160·9) | 196·4 (181·5 to 211·0) | 31·1 (25·3 to 37·3) | 3·2 (2·9 to 3·4) | 3·1 (2·9 to 3·4) | −1·1 (−5·5 to 3·7) | |||
| Urolithiasis | 14·9 (12·5 to 18·1) | 16·1 (14·0 to 20·3) | 7·8 (0·0 to 21·2) | 0·3 (0·3 to 0·4) | 0·2 (0·2 to 0·3) | −16·5 (−22·6 to −5·8) | |||
| Other urinary diseases | 36·4 (30·8 to 42·7) | 49·2 (41·2 to 55·4) | 35·1 (20·4 to 49·9) | 0·7 (0·6 to 0·9) | 0·8 (0·6 to 0·9) | 4·2 (−7·1 to 15·3) | |||
| Gynaecological diseases | 7·6 (6·1 to 8·6) | 7·9 (5·9 to 9·2) | 2·9 (−10·1 to 24·5) | 0·1 (0·1 to 0·2) | 0·1 (0·1 to 0·1) | −17·4 (−28·0 to −1·0) | |||
| Uterine fibroids | 2·1 (1·2 to 2·7) | 2·3 (1·2 to 3·0) | 9·5 (−12·5 to 34·7) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −13·3 (−30·5 to 5·8) | |||
| Polycystic ovarian syndrome | 0·7 (0·2 to 1·3) | 0·6 (0·2 to 1·0) | −17·9 (−37·0 to 10·1) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −30·9 (−47·0 to −7·9) | |||
| Endometriosis | 0·0 (0·0 to 0·1) | 0·1 (0·0 to 0·1) | 24·2 (−0·6 to 59·4) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | 5·7 (−15·2 to 35·4) | |||
| Genital prolapse | 1·1 (0·6 to 1·8) | 0·9 (0·6 to 1·5) | −19·5 (−36·0 to 12·9) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | −39·0 (−51·1 to −15·9) | |||
| Other gynaecological diseases | 3·6 (2·7 to 4·5) | 4·0 (3·0 to 4·8) | 9·9 (−5·8 to 34·4) | 0·1 (0·0 to 0·1) | 0·1 (0·0 to 0·1) | −10·0 (−23·1 to 9·9) | |||
| Haemoglobinopathies and haemolytic anaemias | 215·5 (173·5 to 274·0) | 226·9 (177·2 to 306·2) | 5·3 (−9·4 to 24·1) | 3·6 (2·9 to 4·5) | 3·2 (2·5 to 4·3) | −9·8 (−21·2 to 5·1) | |||
| Thalassaemias | 19·7 (16·5 to 23·3) | 16·8 (13·9 to 20·2) | −14·5 (−27·7 to 2·9) | 0·3 (0·3 to 0·4) | 0·2 (0·2 to 0·3) | −23·6 (−34·1 to −10·3) | |||
| Sickle cell disorders | 108·3 (78·5 to 159·8) | 114·8 (78·3 to 183·2) | 6·0 (−20·1 to 40·4) | 1·6 (1·2 to 2·3) | 1·6 (1·1 to 2·5) | −2·7 (−26·0 to 28·3) | |||
| Glucose-6-phosphate dehydrogenase deficiency | 27·5 (23·5 to 32·1) | 33·0 (28·0 to 38·9) | 19·9 (11·6 to 29·2) | 0·4 (0·4 to 0·5) | 0·5 (0·4 to 0·5) | 1·2 (−5·9 to 8·7) | |||
| Other haemoglobinopathies and haemolytic anaemias | 60·0 (53·2 to 66·5) | 62·3 (54·7 to 71·1) | 3·9 (−3·4 to 10·2) | 1·2 (1·1 to 1·3) | 1·0 (0·9 to 1·1) | −19·6 (−25·6 to −15·0) | |||
| Endocrine, metabolic, blood, and immune disorders | 110·5 (108·1 to 113·5) | 147·3 (142·5 to 151·7) | 33·2 (28·0 to 37·2) | 2·1 (2·1 to 2·2) | 2·2 (2·2 to 2·3) | 5·6 (1·7 to 8·7) | |||
| Musculoskeletal disorders | 76·2 (69·2 to 80·5) | 90·1 (82·5 to 94·6) | 18·2 (10·8 to 24·3) | 1·5 (1·4 to 1·6) | 1·4 (1·3 to 1·5) | −8·3 (−13·5 to −3·8) | |||
| Rheumatoid arthritis | 26·5 (23·2 to 29·3) | 30·0 (27·0 to 34·6) | 13·2 (5·0 to 23·5) | 0·6 (0·5 to 0·6) | 0·5 (0·4 to 0·5) | −14·0 (−20·0 to −6·4) | |||
| Other musculoskeletal disorders | 49·7 (45·5 to 53·1) | 60·1 (53·1 to 63·5) | 20·9 (11·5 to 27·0) | 1·0 (0·9 to 1·0) | 0·9 (0·8 to 1·0) | −5·0 (−11·5 to −0·5) | |||
| Other non-communicable diseases | 726·6 (626·8 to 869·3) | 744·6 (667·8 to 811·6) | 2·5 (−10·6 to 11·7) | 10·9 (9·4 to 13·0) | 10·2 (9·2 to 11·1) | −6·5 (−17·7 to 1·5) | |||
| Congenital anomalies | 634·2 (535·9 to 773·4) | 627·8 (567·3 to 694·4) | −1·0 (−15·4 to 9·0) | 9·2 (7·8 to 11·2) | 8·4 (7·6 to 9·3) | −8·0 (−21·1 to 1·1) | |||
| Neural tube defects | 76·5 (56·7 to 107·0) | 64·6 (47·9 to 83·4) | −15·5 (−34·9 to 4·4) | 1·1 (0·8 to 1·5) | 0·9 (0·6 to 1·1) | −20·5 (−38·8 to −1·7) | |||
| Congenital heart anomalies | 319·0 (267·4 to 378·1) | 303·3 (268·8 to 335·4) | −4·9 (−18·9 to 6·5) | 4·6 (3·9 to 5·4) | 4·1 (3·6 to 4·5) | −11·4 (−24·4 to −0·9) | |||
| Cleft lip and cleft palate | 3·3 (2·6 to 3·8) | 1·3 (1·1 to 1·7) | −59·0 (−66·4 to −50·1) | 0·0 (0·0 to 0·1) | 0·0 (0·0 to 0·0) | −61·3 (−68·3 to −52·9) | |||
| Down's syndrome | 26·6 (17·9 to 42·7) | 26·5 (19·1 to 36·9) | −0·2 (−25·0 to 30·6) | 0·4 (0·3 to 0·6) | 0·4 (0·3 to 0·5) | −10·1 (−31·7 to 16·9) | |||
| Other chromosomal abnormalities | 20·6 (9·8 to 49·3) | 22·7 (12·9 to 40·5) | 10·3 (−21·8 to 41·0) | 0·3 (0·1 to 0·7) | 0·3 (0·2 to 0·5) | 3·3 (−26·6 to 31·3) | |||
| Other congenital anomalies | 188·3 (158·7 to 231·9) | 209·4 (188·9 to 241·3) | 11·2 (−3·7 to 24·5) | 2·8 (2·3 to 3·4) | 2·8 (2·6 to 3·3) | 2·7 (−10·6 to 14·6) | |||
| Skin and subcutaneous diseases | 71·6 (48·1 to 90·4) | 97·6 (66·6 to 128·8) | 36·2 (29·5 to 46·4) | 1·5 (1·0 to 1·8) | 1·5 (1·0 to 2·0) | 3·7 (−1·6 to 12·5) | |||
| Cellulitis | 12·6 (7·8 to 17·4) | 16·9 (10·4 to 23·0) | 34·2 (22·2 to 48·2) | 0·3 (0·2 to 0·3) | 0·3 (0·2 to 0·3) | 3·5 (−5·5 to 14·1) | |||
| Pyoderma | 30·9 (21·3 to 43·5) | 44·1 (31·1 to 62·8) | 42·7 (33·0 to 53·3) | 0·6 (0·4 to 0·8) | 0·7 (0·5 to 1·0) | 12·5 (4·7 to 21·4) | |||
| Decubitus ulcer | 25·1 (14·5 to 30·7) | 32·4 (19·3 to 40·0) | 29·3 (20·4 to 42·4) | 0·6 (0·3 to 0·7) | 0·5 (0·3 to 0·7) | −5·7 (−12·6 to 4·4) | |||
| Other skin and subcutaneous diseases | 3·1 (2·2 to 4·2) | 4·2 (3·0 to 5·8) | 35·4 (26·9 to 46·0) | 0·1 (0·0 to 0·1) | 0·1 (0·0 to 0·1) | 5·7 (−1·2 to 14·2) | |||
| Sudden infant death syndrome | 20·8 (16·9 to 33·7) | 19·2 (15·9 to 27·5) | −7·9 (−23·0 to 8·9) | 0·3 (0·2 to 0·5) | 0·3 (0·2 to 0·4) | −13·5 (−27·7 to 2·3) | |||
| Injuries | 4759·0 (4451·4 to 4893·1) | 4725·1 (4398·5 to 4905·2) | −0·7 (−4·3 to 3·5) | 78·6 (73·5 to 80·8) | 66·2 (61·5 to 68·7) | −15·8 (−18·7 to −12·4) | |||
| Transport injuries | 1494·1 (1444·8 to 1550·6) | 1466·6 (1394·8 to 1536·5) | −1·8 (−7·4 to 3·3) | 24·2 (23·4 to 25·0) | 20·2 (19·3 to 21·2) | −16·2 (−20·8 to −11·8) | |||
| Road injuries | 1392·5 (1341·0 to 1446·0) | 1361·7 (1294·0 to 1428·1) | −2·2 (−7·8 to 2·6) | 22·5 (21·7 to 23·3) | 18·8 (17·9 to 19·7) | −16·4 (−21·1 to −12·3) | |||
| Pedestrian road injuries | 581·4 (546·3 to 631·3) | 560·6 (525·8 to 617·1) | −3·6 (−10·7 to 2·8) | 9·6 (9·1 to 10·5) | 7·8 (7·4 to 8·6) | −18·6 (−24·6 to −13·2) | |||
| Cyclist road injuries | 63·4 (58·7 to 68·9) | 58·7 (54·4 to 64·4) | −7·5 (−15·2 to 1·2) | 1·0 (1·0 to 1·1) | 0·8 (0·8 to 0·9) | −21·9 (−28·5 to −14·6) | |||
| Motorcyclist road injuries | 245·7 (215·2 to 263·8) | 257·1 (230·6 to 290·9) | 4·6 (−4·7 to 16·5) | 3·8 (3·4 to 4·1) | 3·5 (3·1 to 3·9) | −8·9 (−17·0 to 1·6) | |||
| Motor vehicle road injuries | 483·1 (445·9 to 536·6) | 464·2 (417·8 to 508·3) | −3·9 (−9·3 to 2·1) | 7·7 (7·1 to 8·5) | 6·4 (5·7 to 7·0) | −17·2 (−21·8 to −12·1) | |||
| Other road injuries | 18·9 (13·1 to 22·5) | 21·1 (14·0 to 24·8) | 11·3 (−5·4 to 34·9) | 0·3 (0·2 to 0·4) | 0·3 (0·2 to 0·3) | −4·5 (−18·7 to 14·7) | |||
| Other transport injuries | 101·5 (93·2 to 114·0) | 104·9 (90·5 to 127·2) | 3·3 (−6·4 to 14·7) | 1·7 (1·5 to 1·9) | 1·4 (1·2 to 1·8) | −12·6 (−20·7 to −3·2) | |||
| Unintentional injuries | 1887·3 (1701·0 to 1969·6) | 1838·7 (1634·6 to 1939·1) | −2·6 (−5·7 to 3·2) | 32·3 (29·1 to 33·6) | 26·5 (23·6 to 28·0) | −17·8 (−20·4 to −13·4) | |||
| Falls | 436·1 (402·3 to 451·2) | 527·2 (467·8 to 554·5) | 20·9 (14·6 to 27·2) | 8·6 (7·9 to 8·9) | 8·1 (7·2 to 8·5) | −5·5 (−10·1 to −0·8) | |||
| Drowning | 403·1 (349·2 to 424·7) | 323·8 (285·8 to 347·5) | −19·7 (−23·6 to −14·2) | 6·3 (5·4 to 6·6) | 4·5 (4·0 to 4·8) | −28·5 (−31·8 to −23·8) | |||
| Fire, heat, and hot substances | 195·2 (161·4 to 209·0) | 176·0 (145·1 to 189·6) | −9·9 (−14·8 to −2·5) | 3·3 (2·7 to 3·5) | 2·5 (2·1 to 2·7) | −23·5 (−27·7 to −17·7) | |||
| Poisonings | 100·8 (71·4 to 117·4) | 86·4 (58·6 to 101·0) | −14·3 (−24·1 to −1·1) | 1·6 (1·2 to 1·9) | 1·2 (0·8 to 1·4) | −26·7 (−34·3 to −16·2) | |||
| Exposure to mechanical forces | 202·6 (175·4 to 214·0) | 200·6 (157·6 to 216·7) | −1·0 (−11·7 to 7·0) | 3·3 (2·8 to 3·5) | 2·8 (2·2 to 3·0) | −15·1 (−24·1 to −8·9) | |||
| Unintentional firearm injuries | 32·7 (23·8 to 35·6) | 32·0 (23·3 to 35·1) | −2·1 (−7·1 to 3·3) | 0·5 (0·4 to 0·6) | 0·5 (0·3 to 0·5) | −17·0 (−20·9 to −12·7) | |||
| Unintentional suffocation | 34·9 (27·1 to 39·4) | 35·6 (25·6 to 40·3) | 2·0 (−11·6 to 15·8) | 0·6 (0·4 to 0·6) | 0·5 (0·4 to 0·6) | −9·2 (−21·2 to 2·5) | |||
| Other exposure to mechanical forces | 135·0 (113·9 to 144·3) | 133·0 (101·1 to 145·1) | −1·5 (−13·6 to 8·5) | 2·2 (1·9 to 2·3) | 1·9 (1·4 to 2·0) | −16·2 (−26·2 to −8·3) | |||
| Adverse effects of medical treatment | 97·3 (74·1 to 107·8) | 99·8 (77·3 to 109·0) | 2·5 (−2·6 to 9·3) | 1·7 (1·4 to 1·9) | 1·5 (1·1 to 1·6) | −15·6 (−19·2 to −11·4) | |||
| Animal contact | 104·4 (67·1 to 114·9) | 94·0 (55·8 to 132·3) | −9·9 (−19·7 to 18·4) | 1·7 (1·1 to 1·9) | 1·3 (0·8 to 1·8) | −22·5 (−31·0 to 1·6) | |||
| Venomous animal contact | 88·2 (54·1 to 98·6) | 79·6 (44·2 to 115·9) | −9·8 (−21·0 to 20·5) | 1·4 (0·9 to 1·6) | 1·1 (0·6 to 1·6) | −22·3 (−32·1 to 3·7) | |||
| Non-venomous animal contact | 16·2 (13·1 to 18·7) | 14·4 (11·4 to 16·4) | −10·8 (−19·7 to 10·9) | 0·3 (0·2 to 0·3) | 0·2 (0·2 to 0·2) | −23·5 (−30·6 to −5·8) | |||
| Foreign body | 145·7 (118·7 to 175·9) | 151·6 (132·6 to 169·8) | 4·1 (−4·7 to 13·6) | 2·5 (2·1 to 3·0) | 2·2 (1·9 to 2·4) | −12·3 (−18·4 to −6·1) | |||
| Pulmonary aspiration and foreign body in airway | 116·6 (93·0 to 148·1) | 124·0 (105·5 to 143·2) | 6·3 (−4·9 to 15·7) | 2·0 (1·7 to 2·5) | 1·8 (1·5 to 2·1) | −10·8 (−18·5 to −4·6) | |||
| Foreign body in other body part | 29·0 (18·1 to 43·2) | 27·6 (20·3 to 34·4) | −5·0 (−22·7 to 11·2) | 0·5 (0·3 to 0·7) | 0·4 (0·3 to 0·5) | −18·8 (−34·3 to −6·2) | |||
| Environmental heat and cold exposure | 53·4 (39·9 to 57·2) | 45·2 (33·5 to 49·5) | −15·4 (−21·1 to −9·1) | 0·9 (0·7 to 1·0) | 0·7 (0·5 to 0·7) | −31·3 (−35·8 to −26·6) | |||
| Other unintentional injuries | 148·8 (141·4 to 157·7) | 134·2 (124·8 to 147·8) | −9·8 (−16·7 to −1·6) | 2·4 (2·3 to 2·5) | 1·9 (1·7 to 2·0) | −22·3 (−28·2 to −15·4) | |||
| Self-harm and interpersonal violence | 1253·0 (1125·6 to 1288·8) | 1236·7 (1130·1 to 1287·9) | −1·3 (−5·7 to 3·0) | 20·3 (18·2 to 20·9) | 17·0 (15·5 to 17·7) | −16·3 (−20·1 to −12·6) | |||
| Self-harm | 827·6 (725·4 to 855·5) | 828·1 (745·8 to 868·7) | 0·1 (−6·2 to 6·1) | 13·7 (12·0 to 14·2) | 11·5 (10·3 to 12·1) | −16·3 (−21·5 to −11·2) | |||
| Interpersonal violence | 425·3 (388·7 to 439·6) | 408·6 (370·5 to 431·5) | −3·9 (−7·0 to −0·1) | 6·5 (6·0 to 6·8) | 5·5 (5·0 to 5·8) | −16·4 (−19·1 to −13·1) | |||
| Assault by firearm | 162·9 (142·8 to 168·8) | 173·1 (149·3 to 183·2) | 6·3 (2·4 to 11·0) | 2·4 (2·1 to 2·5) | 2·3 (2·0 to 2·4) | −6·0 (−9·4 to −1·8) | |||
| Assault by sharp object | 104·4 (97·5 to 110·7) | 89·5 (83·3 to 97·3) | −14·3 (−18·8 to −9·1) | 1·6 (1·5 to 1·7) | 1·2 (1·1 to 1·3) | −25·6 (−29·4 to −21·2) | |||
| Assault by other means | 158·0 (143·1 to 167·5) | 145·9 (129·6 to 159·7) | −7·6 (−12·6 to −1·5) | 2·5 (2·3 to 2·6) | 2·0 (1·8 to 2·2) | −20·6 (−24·8 to −15·4) | |||
| Forces of nature, war, and legal intervention | 124·7 (82·5 to 166·5) | 183·1 (100·0 to 263·8) | 46·8 (−14·2 to 120·5) | 1·9 (1·3 to 2·6) | 2·4 (1·3 to 3·5) | 27·4 (−25·5 to 90·7) | |||
| Exposure to forces of nature | 90·8 (53·0 to 128·0) | 11·8 (7·2 to 16·4) | −87·0 (−87·9 to −86·1) | 1·4 (0·8 to 2·0) | 0·2 (0·1 to 0·2) | −88·5 (−89·3 to −87·6) | |||
| Collective violence and legal intervention | 33·8 (25·5 to 43·0) | 171·3 (88·1 to 251·1) | 406·0 (236·0 to 524·5) | 0·5 (0·4 to 0·7) | 2·3 (1·2 to 3·3) | 347·5 (192·4 to 455·8) | |||
Data in parentheses are 95% uncertainty intervals.
Broadly, communicable, maternal, neonatal, and nutritional diseases, known as Group 1 causes for GBD, accounted for 20·2% (95% UI 19·7–20·7) of global deaths in 2015 (11·3 million, 95% UI 10·9 million to 11·6 million), NCDs caused 71·3% (70·9–72·0) of deaths (39·8 million, 39·2 million to 40·5 million), and injuries resulted in 8·5% (7·9–8·5) of deaths (4·7 million, 4·4 million to 4·9 million). Between 2005 and 2015, Group 1 causes saw significant reductions for both total deaths (decrease of 19·7% [17·8–21·6]) and age-standardised rates (decrease of 29·6% [27·9–31·3]). For NCDs, total deaths rose by 14·3% (12·6–16·0), an increase of 5·0 million deaths (4·4 million to 5·6 million) since 2005, but age-standardised rates decreased from 719·1 deaths (711·9–727·3) per 100 000 in 2005 to 624·7 deaths (615·8–634·5) per 100 000 in 2015 (decrease of 13·1%, 11·9–14·3). Injuries caused about 4·7 million deaths in both 2005 and 2015, but the age-standardised rates due to injuries significantly declined during this time, decreasing by 15·8% (12·4–18·7) from 78·6 deaths (73·5–80·8) per 100 000 in 2005 to 66·2 deaths (61·5–68·7) per 100 000 in 2015.
Communicable, maternal, neonatal, and nutritional diseases
Marked reductions in total deaths and age-standardised death rates were achieved for many of the world's most important communicable diseases. Total HIV/AIDS deaths fell 33·4% (95% UI 30·0–36·2), from 1·8 million (95% UI 1·7 million to 1·9 million) in 2005 to 1·2 million (1·1 million to 1·3 million) in 2015, and age-standardised death rates dropped even more rapidly (reduction of 42·1%, 39·1–44·6). Globally, HIV/AIDS mortality peaked in 2005, underscoring the continued expansion of ART and PMTCT. Malaria deaths decreased by 37·4% (27·8–47·0), falling to 730 500 (555 800–904 000) in 2015. Age-standardised death rates due to malaria fell slightly more rapidly (43·1%, 34·7–51·8) during this time; nonetheless, this rate of decline only partly represents the sustained gains against malaria, given that mortality peaked in 2003, claiming 1·2 million lives (1·0 million to 1·4 million) that year. Age-standardised death rates due to diarrhoeal diseases fell 32·2% (27·7–36·5) from 2005 to 2015, although total deaths fell more slowly (20·8%, 15·4–26·1) to 1·3 million deaths (1·2 million to 1·4 million). Other communicable diseases that had significant reductions in mortality included tetanus (decreased by 47·5% [95% UI 39·0–54·6], to 56 700 deaths [48 200–80 000]), measles (decreased by 75·0% [58·8–84·5], to 73 400 deaths [26 100–161 400]), and African trypanosomiasis (decreased by 75·3% [67·9–81·4], to 3510 deaths [1790–5660]).
Amid these gains, less pronounced progress occurred for several communicable diseases, and fatalities climbed rapidly for others, such as Ebola virus disease. Tuberculosis, which killed fewer people than HIV/AIDS in 2005 (1·3 million, 95% UI 1·2 million to 1·7 million), essentially matched HIV/AIDS's toll by 2015, causing 1·1 million deaths (0·91 million to 1·4 million). Deaths due to tuberculosis decreased by 17·4% (11·3–24·4) between 2005 and 2015; however, age-standardised tuberculosis death rates dropped by 33·8% (28·7–39·6). Total mortality due to lower respiratory infections remained fairly constant from 2005 to 2015 (between 2·8 million and 2·7 million deaths), although age-standardised death rates fell by 19·5% (16·9–22·3); a similar trend was observed for meningitis. Deaths due to hepatitis and age-standardised death rates decreased (deaths fell by 14·0% [10·0–17·9], to 106 000 [101 000–111 000], and death rates fell by 28·0% [24·7–31·1]), which was mainly driven by significant reductions in deaths due to acute hepatitis A (decrease of 34·0% [24·2–43·5], to 11 000 [7000–16 000]) since 2005. Mortality due to other types of hepatitis improved less rapidly. Dengue deaths increased by 48·7% (15·1–90·9), resulting in 18 400 deaths (11 800–22 700) in 2015, and Chagas disease, which largely affects populations in Latin America, claimed 8000 lives (7500–8600) that year. Deaths due to leishmaniasis increased, albeit not significantly, between 2005 and 2015, causing 24 200 deaths (17 100–32 500) in 2015. The peak of the west African Ebola virus disease outbreak occurred in 2014, causing 12 800 deaths (10 300–15 300) that year. In 2015, 5500 people (4400–6600) died from Ebola virus disease, mainly in Guinea, Liberia, and Sierra Leone.
Among the leading causes of global maternal mortality, most showed significant reductions in both total deaths and age-standardised death rates between 2005 and 2015. Deaths due to maternal haemorrhage decreased by 16·6% (95% UI 3·2–28·8), claiming 16 600 (3300–29 800) fewer lives in 2015, and deaths due to abortion, miscarriage, and ectopic pregnancies dropped by 23·1% (11·1–33·9), to 32 000 (25 000–40 000); age-standardised death rates fell by 25·0% (12·9–35·9) for maternal haemorrhage and by 30·7% (19·8–40·4) for abortion, miscarriage, and ectopic pregnancies. For neonatal disorders, total deaths fell by 18·5% (16·4–20·4) and age-standardised death rates fell by 22·8% (−24·6 to −20·9) from 2005 to 2015, to 2·2 million (2·1 million to 2·2 million). Preterm birth complications caused 282 200 (215 000–353 500) fewer deaths in 2015 than in 2005 (reduction of 25·9%, 20·6–31·3) and age-standardised rates dropped by 29·8% (24·8–34·9). Total deaths and age-standardised death rates due to neonatal encephalopathy also decreased significantly during this time, albeit at a more moderate pace. Overall, these trends probably reflect a combination of decreasing fertility rates, improved maternal care, and safer delivery practices in many settings.
Notably less progress occurred for nutritional deficiencies, which caused 405 700 deaths (95% UI 331 700–495 600) in 2015. In 2015, iron-deficiency anaemia led to 54 200 deaths (35 100–72 900) and protein-energy malnutrition caused 323 200 deaths (264 900–400 800); in combination, nutritional deficiencies accounted for 3·6% (2·7–4·1) of lives lost to Group 1 disorders. Age-standardised death rates significantly decreased for nutritional deficiencies (decreased by 24·3%, 14·3–32·9).
Non-communicable diseases
In 2015, the leading causes of NCD deaths were cardiovascular disease (17·9 million, 95% UI 17·6 million to 18·3 million), cancers (8·8 million, 8·6 million to 8·9 million), and chronic respiratory diseases (3·8 million, 3·7 million to 3·9 million). The global death toll due to cancers increased by 17·0% (95% UI 14·8–19·3) between 2005 and 2015, although age-standardised rates of death fell by 10·0% (8·3–11·6). Tracheal, bronchus, and lung cancer (total deaths 1·7 million, 1·7 million to 1·8 million) were the leading causes of cancer deaths, and also had the highest age-standardised death rate (26·6 deaths [25·9–27·4] per 100 000) among cancers in 2015. For several cancers, total deaths increased by 20% or more between 2005 and 2015, including tracheal, bronchus, and lung cancer (20·1% [16·7–24·0], to 1·7 million deaths [1·7 million to 1·8 million); colon and rectum cancer (23·2% [20·6–26·0], to 832 000 deaths [811 700–854 500]); malignant skin melanoma (27·2% [20·0–32·6], to 59 800 deaths [47 600–72 700]); pancreatic cancer (30·8% [28·3–33·6], to 411 600 deaths [403 600–420 700]); and prostate cancer (31·9% [28·2–35·4], to 365 900 deaths [303 500–459 600]). Breast and ovarian cancers, which largely, if not exclusively, affect females, caused significantly more deaths in 2015 than in 2005 (breast cancer increased by 21·3% [14·9–27·2], to 534 000 deaths [502 000–553 000]; ovarian cancer increased by 20·4% [16·5–24·4], to 161 000 deaths [157 000–167 000]); however, age-standardised death rates for both cancers significantly declined during this time (breast cancer decreased by 6·8% [2·5–11·5] and ovarian cancer decreased by 7·9% [4·9–10·8]). The largest reductions in death rates from 2005 to 2015 were recorded for oesophageal cancer, which fell by 26·8% (22·9–30·3) and Hodgkin's lymphoma, which fell by 23·9% (20·1–27·7). At the same time, significant increases occurred in age-standardised death rates due to non-melanoma skin cancer (increased by 7·6%, 3·4–11·1) and mesothelioma (increased by 7·8%, 3·6–11·6).
Global cardiovascular disease deaths rose by 12·5% (95% UI 10·6–14·4) between 2005 and 2015, whereas age-standardised rates of death due to cardiovascular disease fell 15·6% (14·2–16·9). These reductions were largely driven by declining mortality rates due to cerebrovascular disease (ie, stroke; decreased by 21·0%, 19·2–22·8) since 2005. Globally, deaths due to ischaemic heart disease increased by 16·6% (14·6–18·6) from 2005 to 2015 to 8·9 million deaths (8·8 million to 9·1 million), whereas age-standardised mortality rates for ischaemic heart disease decreased at a more moderate pace (fell by 12·8%, 11·4–14·2). Ischaemic heart disease and stroke accounted for 15·2 million deaths (15·0 million to 15·6 million) in 2015, equating to 85·1% (84·7–85·5) of all deaths due to cardiovascular disease that year. Among respiratory conditions, age-standardised death rates fell by 22·9% (20·0–25·4) for chronic obstructive pulmonary disease (COPD) and by 31·3% (19·4–38·9) for asthma; total deaths due to these causes did not significantly differ from 2005 to 2015. By contrast, for interstitial lung disease and pulmonary sarcoidosis, significant increases occurred in total deaths, which rose by 51·5% (37·9–60·5) to 121 800 deaths (94 100–135 200), and age-standardised rates, which rose by 14·1% (4·1–20·9) from 2005 to 2015.
Mortality patterns were similar for other leading NCD causes of death. Age-standardised mortality rates decreased for all subtypes of cirrhosis, yet total deaths increased to 1·3 million in 2015 (95% UI 1·2 million to 1·4 million). Total mortality also increased from 2005 to 2015 for diabetes, which rose by 32·1% (95% UI 27·7–36·3), to 1·5 million deaths (1·5 million to 1·6 million), and chronic kidney disease, which rose by 31·7% (27·7–35·6), to 1·2 million deaths (1·1 million to 1·3 million); by contrast, changes in age-standardised death rates due to diabetes and chronic kidney disease were not statistically significant. Chronic kidney disease due to diabetes mellitus caused significantly more deaths in 2015 than in 2005 (an increase of 39·5% [35·4–43·5], to 418 000 deaths [389 000–441 000]), and age-standardised death rates also rose 6·4% (3·3–9·3). Global deaths due to Alzheimer's disease and other dementias increased by 38·2% (36·2–40·1), to 1·9 million deaths (1·6 million to 2·2 million), which was largely driven by population ageing, given that age-standardised mortality decreased by 2·7% (1·7–3·7). Notably, both total deaths and age-standardised death rates due to alcohol use disorders significantly dropped from 2005 to 2015, falling by 12·6% (7·0–16·7), to 138 000 deaths (131 000–144 000), and 29·2% (24·7–32·4), respectively. However, drug use disorders claimed increasingly more lives, resulting in a rise of 31·8% (20·4–39·4; rising to 170 000 deaths, 152 000–179 000) since 2005. Deaths due to opioid use disorders accounted for 71·9% (69·5–73·3) of these drug-related deaths in 2015, increasing by 29·6% (18·2–37·2) to a total of 122 100 deaths (109 500–129 700) that year.
Injuries
In 2015, transport injuries caused 1·5 million deaths (95% UI 1·4 million to 1·5 million), unintentional injuries resulted in 1·8 million (1·6 million to 1·9 million), and intentional injuries, including self-harm and interpersonal violence, led to 1·2 million (1·1 million to 1·3 million). Although total deaths did not significantly change between 2005 and 2015, age-standardised death rates fell by 16·2% (11·8–20·8) for transport injuries, 17·8% (13·4–20·4) for unintentional injuries, and 16·3% (12·6–20·1) for intentional injuries. Age-standardised death rates for road injuries and most types of road injuries, including pedestrian, cyclist, and motor vehicle injuries, significantly decreased from 2005 to 2015. Notably, deaths due to falls increased by 20·9% (14·6–27·2) between 2005 and 2015 (to 527 000 deaths, 468 000–555 000), which probably reflects global shifts in ageing rather than a rise in injury risk given that age-standardised death rates fell by 5·5% (0·8–10·1). For drowning, there were significant reductions in both total deaths (decreased by 19·7% [14·2–23·6], to 324 000 deaths [286 000–347 000]) and age-standardised death rates (decreased by 28·5%, 23·8–31·8). Total deaths due to self-harm and interpersonal violence remained relatively unchanged since 2005, but age-standardised deaths fell by 16·3% (11·2–21·5) and 16·4% (13·1–19·1), respectively. Assault by firearms, which accounted for 42·4% (40·4–43·9) of all interpersonal violence deaths, claimed 173 100 lives (149 300–183 200) in 2015, and in contrast with all other causes of interpersonal violence, total deaths significantly increased since 2005 (rose by 6·3%, 2·4–11·0).
Mortality trends due to natural disasters and war were highly irregular (figure 11). By decade, the numbers of war deaths were higher in the 1970s and 1980s, then fell in the 1990s and in the first decade of the 21st century. Conversely, between 2010 and 2015, mortality due to war (collective violence and legal intervention) increased, rising to 171 300 (88 100–251 100) in 2015. More than 40·6% (34·8–45·1) of these deaths occurred in Syria and Yemen (70 000 deaths, 33 000–107 000). These numbers of war fatalities remain much lower than those recorded in 1993 and 1994, when more than 626 000 lives were lost to the Rwandan genocide, the Iraq civil war, ongoing armed conflict in Bosnia and Herzegovina, and other occurrences of collective violence; nonetheless, the rising number of casualties in the Middle East represents the largest increase in war deaths since 1995. Because of deaths in Afghanistan, Iraq, Syria, and Yemen, war deaths have increased during 2014–15. Between 2004 and 2010, natural disasters claimed thousands of lives, including 226 000 from the Indonesian earthquake and tsunami in 2004; 74 700 from earthquakes in India and Pakistan, as well as 1870 from Hurricane Katrina in the USA in 2005; 87 900 from an earthquake in China and 138 000 from a cyclone in Myanmar in 2008; and 223 000 from the earthquake in Haiti in 2010. In 2015, natural disasters caused 11 800 deaths (7160–16 400), mainly due to the Nepal earthquake and floods in India.
Figure 11.
Global deaths due to fatal discontinuities by cause group for each year, 1980–2015
Numbers shown are total deaths. Fatal discontinuities are events that lead to abrupt changes in deaths in a geography. The causes for these fatal discontinuities include wars, natural disasters, industrial accidents, large transport accidents, epidemics, famines, or other injuries.
In general, age-standardised death rates for males and females by cause are highly correlated at the global level (figure 12). For most causes, male age-standardised death rates are higher than for females. Death rates are notably higher in males for many cancers including tracheal, bronchus, and lung, liver, oesophageal, bladder, and larynx cancer, and mesothelioma. Male age-standardised death rates are also higher for many injuries including road injuries, self-harm, falls, and interpersonal violence. For a small number of causes, female age-standardised rates are higher than for males, including breast cancer, rheumatic heart disease, gallbladder and biliary cancer, whooping cough, rheumatoid arthritis, thyroid cancer, and multiple sclerosis, and other musculoskeletal disorders.
Figure 12.
Global age-standardised death rates for males versus females, by GBD cause Level 3, 1990 and 2015
The y-axis and x-axis are shown on a log scale to enable comparisons between males and females spanning a wide range of values. Black lines show where death rates are identical for males and females. Causes that only affect one sex, including maternal disorders, chlamydia, cervical, uterine, ovarian, prostate, testicular cancers, and gynaecological diseases are not shown. GBD=Global Burden of Disease. COPD=chronic obstructive pulmonary disease. C=cancer.
Decomposition analysis of changes in global mortality
Drivers of global changes in mortality—population growth, ageing, and changes to age-standardised rates of cause-specific mortality—varied substantially by cause from 2005 to 2015 (figure 13). Among the leading 30 causes of death worldwide, changes in total death tolls ranged from a reduction of 37·4% (95% UI 27·8–47·0) for malaria to an increase of 38·2% (36·2–40·1) for Alzheimer's disease and other dementias. Population growth accounted for increases in numbers of deaths across all 30 causes, but its contribution ranged from less than 9% for several types of cancer, including lung cancer and liver cancer, to 22·9% for malaria. In fact, for malaria, as well as a subset of other Group 1 causes (ie, preterm birth complications, neonatal encephalopathy, and meningitis), population growth was the only factor that hindered further reductions in mortality. Population ageing led to increasing mortality for most causes, but its relative contribution ranged from less than 4% for interpersonal violence and diarrhoeal diseases to 29·6% for Alzheimer's disease and other dementias. Shifts in population age structures also accounted for more than 23% of increased mortality due to cardiovascular disease (ischaemic heart disease and stroke), 24·2% for COPD, and at least 20% for several types of cancer (eg, pancreatic cancer and oesophageal cancer). Conversely, for some causes, namely neonatal conditions and causes that largely affect children, such as malaria, population ageing contributed to decreasing levels of mortality. Except for pancreatic cancer, changes in age-specific and cause-specific rates of death drove reductions in deaths due to the 28 other leading causes. Declines attributable to changes in age-specific and cause-specific mortality rates markedly differed, with several causes experiencing reductions of more than 40% (eg, malaria [58·2%], HIV/AIDS [54·8%], tuberculosis [41·9%], and diarrhoeal diseases [41·0%]) and others showing much smaller decreases (eg, chronic kidney disease [2·4%] and diabetes [3·1%]). Notably, patterns were less distinct for most injuries because population growth and shifts in age-specific and cause-specific mortality rates had relatively similar contributions to changes in deaths due to road injuries, self-harm, and interpersonal violence. Falls were the exception, with a combination of population growth and ageing mainly driving its rising death toll.
Figure 13.
Global decomposition of changes in leading 30 causes of death, 2005 to 2015
Changes due to population growth, population ageing, and changes in age-specific mortality rates are shown. Causes are reported in descending order by total number of deaths for all ages and both sexes combined in 2015. The black circle shows the overall median percentage change in global deaths from 2005 to 2015. Causes with increases in overall death rates have a circle to the right of the zero, whereas a circle to the left of the zero denotes causes with decreases in overall death rates. The contributions of population growth, ageing, and change in age-specific death rates sum to the total change in numbers of deaths.
Global YLLs
From 2005 to 2015, changes in the relative ranks of YLLs, emphasised the increasing complexity of global mortality patterns (figure 14). The top three causes of YLLs—ischaemic heart disease, stroke (which includes both ischaemic and haemorrhagic stroke), and lower respiratory infections—all saw reductions in age-standardised rates between 2005 and 2015, but changed minimally in rankings. In 2005, the causes ranked fourth to eighth were all communicable diseases (HIV/AIDS [fourth], diarrhoeal diseases [sixth], and malaria [seventh]) or neonatal disorders (preterm birth complications [fifth] and neonatal encephalopathy [eighth]). By 2015, both total and age-standardised rates of YLLs had significantly decreased for all of these causes, but their relative rankings did not substantially change; the exceptions were HIV/AIDS, which fell to seventh, and malaria, which dropped to ninth. Road injuries, COPD, and congenital anomalies, ranked as ninth, tenth, and 11th leading causes of YLLs in 2005, remained largely the same in terms of ranks in 2015, with only road injuries moving up by one spot to rank eighth. YLLs due to road injuries fell significantly between 2005 and 2015, both in terms of total YLLs, which decreased by 8·1% (95% UI 3·3–13·3), and age-standardised rates, which decreased by 18·5% (14·3–23·1). Among the top ten leading causes, premature mortality due to malaria decreased the most, with total YLLs falling by 40·1% (29·4–50·2) and age-standardised rates dropping 44·7% (34·9–54·1).
Figure 14.
Leading 30 Level 3 causes of global YLLs for both sexes combined for 1990, 2005, and 2015, with percent change in number of YLLs, and all-age and age-standardised rates
Causes are connected by lines between time periods. For the time periods 1990 to 2005 and 2005 to 2015, three measures of change are shown: percent change in the number of YLLs, percent change in the all-age YLL rate, and percent change in the age-standardised YLL rate. Statistically significant changes are shown in bold. YLLs=years of life lost. COPD=chronic obstructive pulmonary disease. STDs=sexually transmitted diseases excluding HIV. An interactive version of this figure is available online at http://vizhub.healthdata.org/gbd-compare.
More pronounced shifts in YLL ranks and percentage changes between 2005 and 2015 occurred beyond the leading 11 causes, particularly for several NCDs. Total YLLs due to diabetes rose 25·4% (95% UI 20·4–30·0) and diabetes advanced from being ranked 18th to 15th. Similar increases occurred for chronic kidney disease (18·4% [13·8–23·1] for total YLLs and rising from 21st to 17th) and Alzheimer's disease and other dementias (30·5% [28·6–32·4] for total YLLs and increasing from 30th to 25th). Several types of cancer, including colon and rectum cancer, breast cancer, pancreatic cancer, and brain cancer, showed significant increases in total YLLs and relative ranks by 2015; however, none of these cancers had significant increases in age-standardised rates of YLLs. Among NCDs, only asthma (ranked 32nd in 2005 and 37th in 2015) and rheumatic heart disease (ranked 41st in 2005 and 43rd in 2015) had significant reductions for both total YLLs and age-standardised rates. By contrast, larger declines for total YLLs and age-standardised rates occurred for several leading Group 1 causes, including tuberculosis (20·5% [14·9–26·0] and 33·7% [29·1–38·3], respectively); protein-energy malnutrition (22·9% [4·8–38·1] and 29·4% [13·0–43·0], respectively); and most notably, measles (75·0% [58·9–84·4] and 76·7% [61·8–85·5], respectively). In general, changes in total YLLs and age-standardised rates due to injuries suggested reduced levels of premature mortality; the main exception was early deaths due to war, which climbed from the 92nd leading cause of YLLs in 2005 to 38th in 2015, and increased more than 350% for total YLLs and age-standardised rates of early death. Additional comparisons for changes in YLLs across different years between 1990 and 2015 can be explored online.
Causes of child death
Globally, under-5 deaths decreased by 27·2% (95% UI 25·2–29·0) between 2005 and 2015, reaching 5·8 million deaths (95% UI 5·6 million to 6·0 million). Group 1 causes, which include communicable, maternal, neonatal, and nutritional conditions, led to 80·8% (79·5–82·3; 4·7 million deaths, 4·6 million to 4·8 million) of under-5 deaths, NCDs caused 13·8% (12·6–14·9; 804 000 deaths, 733 000–868 000), and injuries accounted for 5·4% (4·6–6·0; 313 000 deaths, 265 000–348 000) of deaths. Of the selected causes shown in table 6, neonatal disorders, which can affect children beyond the neonatal period (ie, infants younger than 1 month), caused 37·2% (36·0–38·3) of under-5 deaths, equating to 2·2 million deaths (2·1 million to 2·2 million) in 2015; these causes included preterm birth complications, neonatal encephalopathy, neonatal sepsis, and other neonatal disorders. 62 Group 1 causes of under-5 deaths were lower respiratory infections (12·1% [11·2–13·1], 703 000 deaths [651 000–763 000]); diarrhoeal diseases (8·5% [7·7–9·5], 499 000 deaths [447 000–558 000]); malaria (8·1% [5·7–10·7], 474 000 deaths [333 000–624 000]); nutritional deficiencies (3·3% [2·6–4·3], 193 000 deaths [147 000–248 000]); and meningitis (3·0% [2·3–3·9], 173 000 deaths [137 000–229 000]). Congenital anomalies, which include congenital heart anomalies, led to 8·5% (7·7–9·5) of under-5 deaths in 2015 (497 000 deaths, 444 000–555 000), and other NCDs, such as sudden infant death syndrome, accounted for 9·0% (8·1–9·9) of under-5 deaths that year (522 000 deaths, 470 000–580 000).
Table 6.
Selected causes of global child deaths for both sexes combined in 2005 and 2015, with percentage change between 2005 and 2015
|
Neonates age <1 month |
Children age 1–59 months |
Under-5 totals |
|||||
|---|---|---|---|---|---|---|---|
| 2015 (thousands) | Percentage change, 2005–15 | 2015 (thousands) | Percentage change, 2005–15 | 2015 (thousands) | Percentage change, 2005–15 | ||
| All causes | 2621·5 (2562·0–2680·8) | −20·3 (−21·8 to −18·7) | 3199·4 (3093·9–3309·8) | −32·1 (−34·2 to −29·7) | 5820·9 (5673·0–5965·2) | −27·2 (−29·0 to −25·2) | |
| Communicable, maternal, neonatal, and nutritional diseases | 2331·6 (2272·8–2394·0) | −21·6 (−23·2 to −19·9) | 2371·7 (2267·7–2473·5) | −37·1 (−39·9 to −34·4) | 4703·4 (4569·9–4845·5) | −30·3 (−32·4 to −28·1) | |
| HIV/AIDS | .. | .. | 88·9 (84·3–93·7) | −51·9 (−54·2 to −49·6) | 88·9 (84·3–93·7) | −51·9 (−54·2 to −49·6) | |
| Diarrhoeal diseases | 44·0 (38·6–50·6) | −38·5 (−46·3 to −29·1) | 454·9 (404·4–510·2) | −33·9 (−42·4 to −23·4) | 498·9 (447·5–557·6) | −34·3 (−42·3 to −24·9) | |
| Intestinal infectious diseases | .. | .. | 42·2 (22·5–73·2) | −20·0 (−29·8 to −8·9) | 42·2 (22·5–73·2) | −20·0 (−29·8 to −8·9) | |
| Lower respiratory infections | 152·9 (140·4–166·6) | −35·9 (−40·8 to −30·7) | 551·0 (502·2–600·5) | −37·1 (−43·0 to −30·9) | 703·9 (651·4–763·0) | −36·9 (−42·0 to −31·6) | |
| Meningitis | 25·8 (18·3–35·9) | −15·6 (−31·9 to 9·3) | 147·3 (117·1–196·0) | −17·9 (−32·0 to 4·9) | 173·1 (137·1–228·9) | −17·6 (−31·0 to 4·0) | |
| Whooping cough | .. | .. | 54·5 (18·8–117·0) | −41·0 (−77·8 to 63·5) | 54·5 (18·8–117·0) | −41·0 (−77·8 to 63·5) | |
| Tetanus | 19·9 (17·0–23·5) | −57·7 (−64·2 to −50·0) | 5·6 (4·1–7·8) | −55·2 (−66·4 to −39·3) | 25·5 (21·8–30·9) | −57·2 (−63·8 to −49·1) | |
| Measles | .. | .. | 62·6 (22·4–135·8) | −75·1 (−84·5 to −59·6) | 62·6 (22·4–135·8) | −75·1 (−84·5 to −59·6) | |
| Malaria | 13·9 (8·9–19·8) | −55·9 (−67·8 to −41·1) | 460·2 (324·1–604·9) | −42·3 (−54·1 to −29·0) | 474·1 (333·3–623·7) | −42·8 (−54·6 to −29·4) | |
| Neonatal preterm birth complications | 765·9 (700·0–854·3) | −25·9 (−31·5 to −20·5) | 39·9 (32·7–48·3) | −25·9 (−39·3 to −8·4) | 805·8 (736·2–898·6) | −25·9 (−31·3 to −20·6) | |
| Neonatal encephalopathy (birth asphyxia and trauma) | 707·8 (638·4–789·7) | −16·3 (−23·8 to −8·0) | 32·6 (24·8–43·0) | −11·9 (−34·0 to 16·4) | 740·4 (667·6–829·2) | −16·1 (−23·8 to −8·0) | |
| Neonatal sepsis and other neonatal infections | 336·3 (237·4–441·5) | −0·5 (−16·9 to 20·7) | 15·4 (10·0–20·6) | 7·8 (−18·0 to 40·6) | 351·7 (249·2–459·1) | −0·2 (−16·2 to 20·3) | |
| Other neonatal disorders | 180·0 (133·9–229·4) | −16·4 (−35·1 to 6·2) | 40·3 (29·2–52·3) | −14·1 (−39·2 to 19·9) | 220·2 (167·6–276·8) | −16·0 (−34·1 to 5·6) | |
| Nutritional deficiencies | .. | .. | 192·8 (147·2–248·1) | −24·3 (−40·4 to −4·1) | 192·8 (147·2–248·1) | −24·3 (−40·4 to −4·1) | |
| Syphilis | 31·5 (17·5–49·2) | −28·4 (−34·5 to −21·5) | 59·0 (32·9–95·7) | −16·5 (−28·7 to −5·4) | 90·5 (50·6–144·5) | −21·1 (−30·4 to −12·5) | |
| Other communicable diseases | 53·6 (39·3–75·5) | −30·9 (−44·1 to −16·4) | 124·7 (108·2–141·5) | −21·7 (−30·2 to −10·3) | 178·3 (151·7–211·3) | −24·7 (−32·3 to −16·2) | |
| Non-communicable diseases | 267·4 (234·3–290·8) | −7·1 (−18·4 to 0·8) | 537·2 (488·1–592·6) | −8·0 (−18·0 to 2·9) | 804·5 (733·8–868·9) | −7·7 (−17·3 to 1·0) | |
| Congenital anomalies | 242·6 (213·6–263·6) | −6·0 (−18·4 to 2·5) | 254·0 (223·6–297·4) | −0·4 (−17·2 to 13·7) | 496·6 (444·4–554·6) | −3·2 (−17·8 to 7·6) | |
| Sudden infant death syndrome | 1·9 (1·6–2·6) | −9·7 (−25·5 to 4·9) | 17·3 (14·2–24·9) | −7·7 (−23·1 to 9·7) | 19·2 (15·9–27·5) | −7·9 (−23·0 to 8·9) | |
| Other non-communicable diseases | 22·9 (17·3–37·3) | −17·3 (−29·3 to 3·8) | 265·9 (232·7–315·0) | −14·3 (−25·5 to 1·6) | 288·8 (251·6–348·9) | −14·6 (−25·3 to −0·1) | |
| Injuries | 22·5 (17·0–25·9) | −13·9 (−23·1 to −2·7) | 290·5 (247·2–323·4) | −17·5 (−25·9 to −5·0) | 313·0 (265·2–348·4) | −17·3 (−25·3 to −5·2) | |
| Road injuries | 2·4 (1·7–3·4) | −27·8 (−49·9 to 2·4) | 47·1 (40·9–54·2) | −16·0 (−30·0 to 4·1) | 49·5 (43·0–56·8) | −16·7 (−30·1 to 2·5) | |
| Drowning | 1·4 (0·9–1·9) | −20·3 (−44·4 to 16·9) | 54·7 (43·4–64·3) | −37·1 (−47·0 to −23·9) | 56·1 (44·4–65·7) | −36·8 (−46·6 to −23·8) | |
| Other injuries | 18·7 (14·0–21·7) | −11·2 (−20·8 to 1·6) | 188·7 (155·8–216·0) | −9·8 (−20·2 to 6·2) | 207·4 (170·2–236·6) | −9·9 (−19·9 to 5·3) | |
Data in parenthesis are 95% uncertainty intervals. The selected causes are the major causes of death within each Level 1 group that accounted for deaths in children younger than 5 years. Neonates were defined as children younger than 1 month. Childhood was defined as ages 1–59 months.
Neonatal deaths, which accounted for 45·0% (44·9–45·2) of total under-5 deaths in 2015, decreased by 20·3% (95% UI 18·7–21·8), falling from 3·3 million (3·2 million to 3·3 million) in 2005 to 2·6 million (2·6 million to 2·7 million) in 2015. Neonatal causes, mainly preterm complications and neonatal encephalopathy, accounted for 77·5% (76·0–79·5) of deaths among neonates (766 000 deaths, 700 000–854 000), followed by neonatal sepsis, congenital anomalies, and lower respiratory infections. Tetanus had the largest reduction in neonatal deaths between 2005 and 2015, falling 57·7% (50·0–64·2) to 19 900 deaths (17 000–23 400), followed by malaria, which fell by 55·9% (41·1–67·8) to 13 900 deaths (8 930–19 800). Smaller improvements were achieved in the reduction of neonatal deaths from sepsis and congenital anomalies.
Among children aged 1–59 months, total deaths fell by 32·1% (95% UI 29·7–34·2) from 2005 to 2015, with 1·5 million (1·2 million to 1·8 million) fewer children in this age group dying in 2015. Lower respiratory infections, diarrhoeal diseases, and malaria caused 57·0% (49·7–64·6) of deaths among children aged 1–59 months, leading to a total of 1·8 million deaths (1·6 million to 2·1 million). Other leading causes of death for this age group included congenital anomalies (254 000 deaths, 224 000–297 000), nutritional deficiencies (193 000 deaths, 147 000–248 000), and meningitis (147 000 deaths, 117 000–196 000). Deaths due to measles fell by 75·1% (59·6–84·5), the largest reduction among this age group, to 62 600 deaths (22 400–136 000), followed by tetanus, which fell by 55·2% (39·3–66·4) to 5560 deaths (4140–7760), and HIV/AIDS, which fell by 51·9% (49·6–54·2), to 88 900 deaths (84 300–93 700). Nearly all of these leading causes of death showed some form of reduction from 2005 to 2015; neonatal sepsis and congenital anomalies were exceptions, both of which had, albeit not significant, increases in deaths since 2005.
Lower respiratory infections and diarrhoea by pathogens
In 2015, we estimated that 1·3 million deaths (95% UI 1·2 million to 1·4 million) were caused by diarrhoeal diseases, including 499 000 (447 000–558 000) in children younger than 5 years, representing 8·6% (7·7–9·5) of all deaths in this age group. Reductions in under-5 deaths due to diarrhoeal diseases exceeded the rate of change for all other age groups. Rotaviral enteritis (rotavirus) was the leading cause of diarrhoeal death in children younger than 5 years globally (29·3% [24·6–35·9%], 146 500 deaths [118 000–183 500]) in 2015 followed by cryptosporidiosis (Cryptosporidium; 12·1% [2·8–26·9], 60 400 deaths [13 700–134 500]) and shigellosis (Shigella; 11·0% [5·5–18·7], 54 900 deaths [27 000–94 700]). Rotavirus was also the leading cause of mortality due to diarrhoea in all ages (15·2% [12·9–18·1], 199 200 deaths [165 500–241 200]), followed by Shigella (12·5% [6·4–21·2], 164 300 deaths [85 000–278 700]) and other Salmonella infections (6·9% [2·7–13·9], 90 300 deaths [34 100–183 100]; table 7). Adenovirus was an important cause of mortality due to diarrhoea in children younger than 5 years, accounting for nearly 10% of such deaths in this age group (9·2 [3·3–19·7], 46 000 deaths [16 200–97 700]). Mortality due to Clostridium difficile was the lowest among all diarrhoea causes, but was a major cause of diarrhoea mortality in high-income countries. Moreover, it was the only cause for which the attributable fraction increased from 2005 to 2015 (increased by 36·3%, 11·3–65·6). During this same time period, the only attributable fraction to significantly decrease was for rotavirus (decreased by 14·1%, 6·3–20·5; table 7).
Table 7.
Global counterfactual deaths and population attributable fractions for diarrhoea and lower respiratory infection pathogens for 2005 and 2015, with percentage change between 2005 and 2015
|
Children younger than 5 years |
All ages |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2005 |
2015 |
Percentage change, 2005–15 | 2005 |
2015 |
Percentage change, 2005–15 | |||||
| Deaths (thousands) | Population attributable fractions (%) | Deaths(thousands) | Population attributable fractions (%) | Deaths(thousands) | Population attributable fractions (%) | Deaths (thousands) | Population attributable fractions (%) | |||
| Diarrhoea | ||||||||||
| Cholera | 46·8 (32·2 to 64·5) | 6·2 (4·3 to 8·4) | 28·8 (20·6 to 39·7) | 5·8 (4·1 to 7·9) | −38·4 (−49·9 to −24·3) | 98·7 (70·7 to 130·3) | 6·0 (4·3 to 7·7) | 68·4 (50·4 to 87·1) | 5·2 (3·8 to 6·6) | −30·7 (−38·9 to −21·1) |
| Other Salmonella infections | 60·1 (18·6 to 131·3) | 7·9 (2·5 to 17·6) | 38·5 (12·2 to 84·2) | 7·7 (2·5 to 16·6) | −35·9 (−76·9 to 76·3) | 116·8 (44·3 to 241·9) | 7·0 (2·7 to 14·5) | 90·3 (34·1 to 183·1) | 6·9 (2·7 to 13·9) | −22·7 (−71·8 to 116·1) |
| Shigellosis | 83·0 (42·8 to 147·3) | 10·9 (5·7 to 18·9) | 54·9 (27·0 to 94·7) | 11·0 (5·5 to 18·7) | −33·8 (−68·3 to 40·9) | 195·4 (101·5 to 328·5) | 11·8 (6·2 to 19·6) | 164·3 (85·0 to 278·7) | 12·5 (6·4 to 21·2) | −15·9 (−59·2 to 77·4) |
| Enteropathogenic Escherichia coli infection | 15·2 (0·7 to 41·7) | 2·0 (0·1 to 5·4) | 11·3 (0·7 to 32·0) | 2·3 (0·1 to 6·2) | −26·0 (−85·5 to 283·4) | 16·0 (0·6 to 44·6) | 1·0 (0·0 to 2·6) | 12·0 (0·6 to 34·1) | 0·9 (0·0 to 2·6) | −25·1 (−83·8 to 249·8) |
| Enterotoxigenic Escherichia coli infection | 38·2 (15·7 to 72·5) | 5·0 (2·1 to 9·3) | 23·6 (9·6 to 44·3) | 4·7 (2·0 to 8·9) | −38·1 (−74·0 to 42·7) | 91·8 (40·3 to 167·0) | 5·5 (2·5 to 10·1) | 74·1 (29·9 to 137·9) | 5·6 (2·3 to 10·4) | −19·3 (−66·5 to 91·2) |
| Campylobacter enteritis | 46·1 (10·5 to 94·1) | 6·1 (1·4 to 12·4) | 30·9 (8·3 to 62·5) | 6·2 (1·7 to 12·5) | −32·9 (−68·2 to 48·8) | 52·7 (11·1 to 111·0) | 3·2 (0·7 to 6·8) | 37·5 (6·3 to 81·6) | 2·9 (0·5 to 6·2) | −28·9 (−69·8 to 67·0) |
| Amoebiasis | 26·1 (−37·8 to 173·9) | 3·4 (−4·9 to 22·3) | 15·5 (−32·4 to 102·4) | 3·1 (−6·3 to 20·7) | −40·8 (−467·1 to 881·9 | 79·7 (4·9 to 316·8) | 4·8 (0·3 to 18·8) | 67·9 (5·6 to 236·7) | 5·2 (0·4 to 18·1) | −14·8 (−90·9 to 837·6) |
| Cryptosporidiosis | 78·7 (21·2 to 179·0) | 10·3 (2·9 to 22·8) | 60·4 (13·7 to 134·5) | 12·1 (2·8 to 26·9) | −23·2 (−80·6 to 188·8) | 83·0 (14·5 to 201·5) | 5·0 (0·9 to 11·8) | 64·8 (11·1 to 154·2) | 4·9 (0·8 to 11·6) | −21·9 (−81·6 to 208·8) |
| Rotaviral enteritis | 259·7 (211·2 to 323·5) | 34·2 (29·3 to 41·5) | 146·5 (118·0 to 183·5) | 29·3 (24·6 to 35·9) | −43·6 (−52·1 to −33·0) | 336·1 (281·3 to 403·7) | 20·3 (17·4 to 24·0) | 199·2 (165·5 to 241·2) | 15·2 (12·9 to 18·1) | −40·7 (−48·0 to −32·3) |
| Aeromonas | 11·8 (−72·2 to 90·6) | 1·5 (−9·5 to 11·8) | 7·3 (−48·3 to 59·1) | 1·4 (−9·7 to 12·0) | −37·9 (−96·0 to 287·7) | 67·8 (−10·4 to 189·9) | 4·1 (−0·6 to 11·4) | 56·8 (−4·0 to 151·3) | 4·3 (−0·3 to 11·6) | −16·2 (−112·7 to 475·3) |
| Clostridium difficile | 0·9 (0·8 to 1·1) | 0·1 (0·1 to 0·1) | 0·8 (0·7 to 0·9) | 0·2 (0·1 to 0·2) | −10·5 (−24·0 to 2·3 | 6·7 (5·9 to 7·7) | 0·4 (0·3 to 0·5) | 9·4 (7·9 to 11·5) | 0·7 (0·6 to 0·9) | 40·1 (29·6 to 49·9) |
| Norovirus | 20·7 (5·0 to 46·3) | 2·7 (0·7 to 6·3) | 14·8 (4·2 to 33·7) | 3·0 (0·8 to 6·7) | −28·5 (−71·9 to 86·3) | 36·3 (5·6 to 82·5) | 2·2 (0·3 to 5·0) | 29·7 (4·8 to 67·6) | 2·3 (0·4 to 5·2) | −18·3 (−70·8 to 131·3) |
| Adenovirus | 68·5 (24·8 to 141·9) | 9·0 (3·3 to 18·6) | 46·0 (16·2 to 97·7) | 9·2 (3·3 to 19·7) | −32·8 (−77·2 to 90·1) | 95·2 (35·4 to 191·4) | 5·7 (2·1 to 11·6) | 70·2 (25·4 to 145·4) | 5·4 (2·0 to 10·9) | −26·2 (−74·5 to 110·8) |
| Lower respiratory infections | ||||||||||
| Influenza | 16·3 (9·6 to 26·0) | 1·5 (0·8 to 2·4) | 10·2 (5·7 to 16·8) | 1·4 (0·8 to 2·4) | −37·8 (−44·2 to −31·7) | 81·3 (56·3 to 116·8) | 2·9 (1·9 to 4·2) | 83·1 (55·7 to 122·1) | 3·0 (2·0 to 4·4) | 2·3 (−4·4 to 8·7) |
| Pneumococcal pneumonia | 642·0 (386·2 to 848·6) | 57·6 (35·5 to 74·1) | 393·0 (228·4 to 532·3) | 55·8 (32·5 to 75·0) | −38·8 (−45·7 to −32·1) | 1692·3 (1061·1 to 2245·6) | 59·8 (37·7 to 79·2) | 1517·4 (857·9 to 2183·8) | 55·4 (31·5 to 79·1) | −10·3 (−22·4 to −0·8) |
| Haemophilus influenzae type b | 149·5 (−8·9 to 277·9) | 13·4 (−0·8 to 24·7) | 58·7 (−3·1 to 114·5) | 8·3 (−0·5 to 15·9) | −60·7 (−65·7 to −56·8) | 149·5 (−8·9 to 277·9) | 5·3 (−0·3 to 9·9) | 58·7 (−3·1 to 114·5) | 2·1 (−0·1 to 4·2) | −60·7 (−65·7 to −56·8) |
| Respiratory syncytial virus | 58·4 (33·2 to 97·6) | 5·2 (3·0 to 8·7) | 36·4 (20·4 to 61·5) | 5·2 (2·9 to 8·6) | −37·8 (−44·4 to −30·6) | 95·8 (61·5 to 142·6) | 3·4 (2·2 to 5·1) | 82·0 (53·9 to 117·6) | 3·0 (2·0 to 4·3) | −14·3 (−23·1 to −5·2) |
Data in parentheses are 95% uncertainty intervals. Numbers for each cause represent the reduction in deaths that is estimated to occur if a pathogen were eliminated. Numbers should not be summed across pathogens because of interactions between pathogens.
We estimated that 2·7 million (95% UI 2·5 million to 2·9 million) deaths occurred in 2015 due to lower respiratory infections, of which 704 000 (651 000–763 000) occurred among children younger than 5 years, representing 12·1% of deaths in this age group. From 2005 to 2015, the number of deaths due to lower respiratory infections decreased by 3·25% (−0·45 to 6·94) globally in all age groups, but decreased by 36·9% (31·6–42·0) in children younger than 5 years. Pneumococcal pneumonia and H influenzae type b together accounted for nearly 65% of deaths due to lower respiratory infections in children younger than 5 years (table 7). The attributable fraction of deaths due to lower respiratory infection caused by pneumococcal pneumonia was highest in children younger than 5 years (55·8%, 32·5–75·0) and all ages (55·4%, 31·5–79·1). The percentage of under-5 deaths due to H influenzae type b decreased by 60·7% (56·8–65·7), from 13·4% (−0·8 to 24·7) in 2005 to 8·3% (−0·5 to 15·9) in 2015, with 58 700 deaths (−3130 to 115 000) recorded in 2015. Respiratory syncytial virus (5·2% [2·9–8·6], 36 400 deaths [20 400–61 500]) and influenza (1·4% [0·8–2·4], 10 200 deaths [5700–16 800]) together accounted for less than 10% of deaths due to lower respiratory infections in children younger than 5 years, and the remaining 29% of such deaths in this age group remain unattributed.
Expected changes in disease profile with higher SDI
Figure 15 shows the changes in patterns of premature mortality as they relate to age-standardised rates of YLLs, population age structure, and total YLLs per 100 000 population. With increasing SDI, age-standardised YLL rates narrowed (figure 15A), from a total of 98 742 YLLs per 100 000 for males and 96 381 YLLs per 100 000 for females at low SDI, to a low of 9172 YLLs per 100 000 and 6239 YLLs per 100 000, respectively, at high SDI. The cause composition of YLLs substantially shifted as well. At lower SDI, premature mortality was largely due to communicable diseases that disproportionately affect children, such as diarrhoeal disease and lower respiratory infections, measles, and meningitis, but with increasing SDI, YLLs due to these causes markedly decreased. Age-standardised YLL rates due to HIV/AIDS and tuberculosis, neglected tropical diseases and malaria, neonatal disorders, and maternal disorders also rapidly decreased as SDI increased. Nonetheless, the gains achieved for neglected tropical diseases and malaria with rising SDI were somewhat attenuated because the rates of premature mortality due to dengue and Chagas disease increased. For a subset of NCDs, several causes had reductions in age-standardised YLL rates amid improving SDI, including chronic respiratory diseases; digestive diseases; diabetes, urogenital, blood, and endocrine diseases; and unintentional injuries. However, for other causes, the relationship between increasing SDI and premature mortality was less obvious. Age-standardised YLL rates due to cardiovascular disease gradually increased for both sexes until SDI reached 0·40, after which rates declined slowly until an SDI of 0·7, and more rapidly thereafter. For cancers, age-standardised rates of YLLs rose steadily between SDI levels of 0·60 and 0·72, and then largely plateaued. Notably, changes in age-standardised YLL rates and SDI affected the relative ratio of early death by sex for a subset of causes. Cancers, for example, began to exact a larger toll for males than females at SDI levels of about 0·40; this was mainly associated with rising mortality due to lung cancer. A similar trend occurred for cardiovascular disease, with the age-standardised YLL rates of males exceeding those of females beyond an SDI of 0·27. Injuries generally caused higher rates of age-standardised YLLs among males than females across all levels of SDI, but the largest imbalance occurred between SDI levels of 0·35 and 0·72.
Figure 15.
Expected relationship between age-standardised YLL rates per 100 000 people for the 21 GBD Level 2 causes and SDI (A), the expected relationship between population and SDI (B), and the expected relationship between all-age YLL rates per 100 000 people for the 21 GBD Level 2 causes and SDI (C), by sex
The stacked curves in A and C represent the average relationship between SDI and each cause of YLLs observed across all geographies over the time period 1980 to 2015. In each figure, the y-axis spans from the lowest SDI up to the highest SDI. To the left of the midline are male rates, and the female rates are to the right; higher rates are further from the midline. GBD=Global Burden of Disease. SDI=Socio-demographic Index. YLL=years of life lost.
Increases in SDI had sizeable implications for population age structure (figure 15B), and in combination with age-standardised rates of cause-specific YLLs, these factors shape the magnitude—and types—of early deaths worldwide (figure 15C). At the lowest levels of SDI (where SDI equals 1), 49·8% of the population was younger than 15 years and 0·23% was older than 80 years, whereas 11·3% were younger than 15 years and 9·3% were older than 80 years at the highest levels of SDI (where SDI equals 100). Differences by sex with increasing SDI were minimal, except for in the oldest age groups starting from SDI of 0·40. At highest SDI, the 80 years and older age group consisted of far more females (10·9%) than males (6·2%). Below an SDI of 0·50, Group 1 causes, especially infectious diseases such as diarrhoeal diseases and lower respiratory infections, accounted for most premature mortality (as much as 72·5%, or roughly 141 513 YLLs per 100 000). Between SDI levels of 0·32 and 0·58, premature mortality from communicable causes, nutritional deficiencies, and maternal disorders sharply decreased, whereas YLLs per 100 000 due to NCDs and injuries remained relatively unchanged or slowly increased; notably, as SDI increased, early death due to neonatal disorders decreased at a much slower pace than did other Group 1 causes. At SDI of 0·50 and above, NCDs and injuries accounted for a larger portion of total YLLs per 100 000 than did communicable, maternal, neonatal, and nutritional causes. With further improvements in SDI, YLLs per 100 000 generally plateaued—or even increased for some causes—as population age structures began to shift faster than the falls in age-standardised rates of death. The stark contrast between the absolute and relative causes of YLLs per 100 000 for SDI levels lower than 0·40 and higher than 0·60 accentuates the complex disease profile of the remaining levels of SDI: relatively high levels of premature mortality due to a broad mixture of causes, ranging from neonatal disorders to cardiovascular disease.
Attribution of change in life expectancy to changes in major causes of death
Figure 16 shows changes in life expectancy from 2005 to 2015, as attributable to changes in Level 2 causes of death, for each country, territory, and subnational geography. In 2015, Andorra had the highest life expectancy at birth for males (81·2 years, 95% UI 80·8–81·6) and females (88·4 years, 88·1–88·7), whereas Lesotho experienced the lowest life expectancy at birth for both sexes (44·1 years, 38·6–51·8 for males and 50·4 years, 43·6–58·5 for females). Overall, several countries in sub-Saharan Africa had the largest gains in longevity since 2005. By 2015, Zimbabwe had the fastest progress for both sexes, with life expectancy increasing by 11·7 years (5·5–18·3) to 56·3 years (51·1–62·6) for males and 17·0 years (10·1–23·3) to 62·5 years (56·3–68·3) for females. Furthermore, female life expectancy increased by more than 10 years for eight countries in sub-Saharan Africa (South Africa, Ethiopia, Botswana, Zambia, Swaziland, Namibia, Zimbabwe, and Malawi); this progress was largely attributable to marked reductions in mortality from HIV/AIDS. Death rates due to HIV/AIDs peaked from 2003 to 2005 for many of these countries, after which sizeable gains in prolonged life occurred through to 2015. For some countries, including Laos, decreases in death rates from various communicable diseases, such as diarrhoeal diseases and lower respiratory infections, were related to improved life expectancy, whereas several countries in central and eastern Europe recorded gains in longevity that were mainly associated with relative reductions in cardiovascular disease deaths.
Figure 16.
Attribution of changes in life expectancy at birth to changes in major groups of causes of death, 2005 to 2015
Changes are shown for countries and territories (and subnational units in the UK) for females (A) and males (B). Locations are ordered by decreasing life expectancy at birth in 2015. Blue lines show life expectancy at birth in 2005 and black lines show life expectancy at birth in 2015. Causes to the left of the 2005 life expectancy values reflect causes that contributed to reductions in life expectancy from 2005 to 2015. Causes to the right of the 2005 life expectancy values contributed to increases in life expectancy from 2005 to 2015.
Nonetheless, seven countries and territories had higher life expectancies for both sexes combined (nine for males only, four for females only) in 2005 than in 2015 and many others had minimal progress due to rising numbers of war-related causalities. By 2015, average life expectancy for both sexes fell by 1·3 years (−0·3 to 2·8) in Libya, 1·1 years (−0·2 to 2·4) in Dominica, and 7·3 years (1·8–12·1) in Syria; however, these reductions were far more pronounced among males in these countries, with male life expectancy reduced by 11·3 years (3·7–17·4) in Syria, 2·5 years (0·2–4·9) in Libya, and 1·6 years (−0·3 to 3·6) in Dominica. For Syria and Libya, rising mortality due to war was the main driver of such losses in longevity, whereas NCDs, including cancers and cardiovascular disease, led to reduced male life expectancy in Dominica. Six other geographies also had decreases in male life expectancy since 2005, with losses of 0·9 years (−0·6 to 2·5) in Jamaica, 1·6 years (−1·2 to 4·2) in Guam, 0·5 years (−2·4 to 3·2) in Palestine, 0·4 years (–1·3 to 2·0) in the Northern Mariana Islands, 0·6 years (−0·7 to 2·0) in the Virgin Islands, and 0·5 years (−1·2 to 2·1) in Venezuela. Increased mortality from cancers, cardiovascular disease, diabetes, and chronic kidney disease was associated with reduced life expectancy among males in Jamaica and Guam, whereas increased death rates due to interpersonal violence largely contributed to reduced longevity for males in Venezuela. For several countries and territories, overall life expectancy increased from 2005 to 2015, but heightened mortality due to natural disasters, interpersonal violence, and war offset gains achieved against other causes of death since 2005. In Yemen, for example, male and female life expectancy rose by 1·0 years and 1·9 years, respectively, yet rising war-related deaths resulted in reductions in life expectancy of 1·5 years for males and 1·0 years for females, attenuating further improvement in life expectancy. Similar results emerged for other countries in which war has claimed increasingly more lives, including Afghanistan, Iraq, Somalia, and South Sudan. The 2015 earthquake in Nepal largely contributed to the 0·7 years lost for females and 0·8 years for males; nonetheless, overall life expectancy improved by 2·1 years (−0·4 to 4·6) and 2·4 years (0·0–4·6) for males and females in Nepal, respectively—gains mainly attributable to reductions in mortality from diarrhoeal diseases and lower respiratory infections.
Inequalities in life expectancy by sex generally increased over time. In 2005, the difference between male and female life expectancy was 5·0 years (65·7 years, 95% UI 65·5–65·9 for males and 70·7 years, 70·5–71·0 for females), which widened to 5·8 years in 2015 (69·0 years, 68·3–69·4 for males and 74·8 years, 74·4–75·2 for females). For several countries, including Russia, Estonia, and Latvia, differences between male and female life expectancy narrowed more rapidly; these gains could be attributed to reduced mortality due to cardiovascular diseases, cancer, and injuries. Yet, inequalities in life expectancy grew in many countries and territories, often driven by uneven progress in health by sex and increasing male deaths due to a subset of NCDs. For example, Georgia recorded a widening gap between male and female life expectancy, rising from 8·6 years (7·7–9·6) in 2005 to 10·2 years (8·9–11·4) in 2015. In other places (eg, Syria), rising mortality from interpersonal violence or war disproportionately affected males.
Leading causes of YLLs and deviations from expected levels based on SDI
Distinct, yet notably varied, patterns emerged across and within GBD regions when we compared observed YLLs due to leading causes with the levels of premature mortality expected on the basis of SDI. Figure 17 shows the ratios of observed and expected YLLs for the ten leading causes by geography in 2015, colour coded by the magnitude of differences between observed and expected YLLs.
Figure 17.
Leading ten causes of YLLs with the ratio of observed YLLs to YLLs expected on the basis of SDI in 2015, by location
The ratio of observed YLLs to YLLs expected based on SDI is provided in parentheses for each cause, and cells are colour coded by ratio ranges (calculated to place a roughly equal number of cells into each bin). Shades of blue represent much lower observed YLLs than expected levels based on SDI, whereas red shows that observed YLLs exceed expected levels. SDI=Socio-demographic Index. YLL=years of life lost. IHD=ischaemic heart disease. LRI=lower respiratory infection. NN enceph=neonatal encephalitis. COPD=chronic obstructive pulmonary disease. Congenital=congenital disorders. C=cancer. Alzheimer=Alzheimer's disease and other dementias. HTN HD=hypertensive heart disease. Cirr hepB=cirrhosis due to hepatitis B. NN preterm=neonatal preterm birth complications. CKD=chronic kidney disease. TB=tuberculosis. Intestinal infect=intestinal infectious disease. NN sepsis=neonatal sepsis. Endocrine=endocrine, metabolic, blood, and immune disorders. Other cardio=other cardiovascular diseases. CMP=cardiomyopathies. Haemog=haemoglobinopathies and haemolytic anaemias. Cirr alcohol=cirrhosis due to alcohol use. Violence=interpersonal violence. Alcohol=alcohol use disorders. Other cirr=cirrhosis due to other causes. Cirr hepC=cirrhosis due to hepatitis C. Drugs=drug use disorders. F Body=pulmonary aspiration and foreign body in airway. PEM=protein-energy malnutrition. Mech=exposure to mechanical forces. Other transport=other transport injuries. Med treat=adverse effects of medical treatment. Leish=leishmaniasis. Other NN=other neonatal disorders. Iron=iron-deficiency anaemia. Whooping=whooping cough.
Globally, ischaemic heart disease and stroke were the leading two causes of premature mortality in 2015; 119 countries and territories also had ischaemic heart disease or stroke as the leading cause of YLLs that year. Three geographical regions featured countries that largely diverged from this trend: Latin America and the Caribbean, where interpersonal violence or lower respiratory infections frequently accounted for the most YLLs; north Africa and the Middle East, where war was the primary cause of early death in several countries; and sub-Saharan Africa, where HIV/AIDS or malaria was the leading cause of YLLs in 28 countries. Furthermore, lung cancer consistently ranked among the top three causes of YLLs in high-income countries; road injuries were a major cause of early death in Latin American countries; and neonatal disorders were frequently among leading five causes of YLLs in south Asia and sub-Saharan Africa.
Of the ten leading causes of premature mortality globally, lower respiratory infections resulted in the most countries (122) recording observed YLLs lower than those expected on the basis of SDI. This finding was particularly prevalent in east Asia, where China and North Korea had YLL ratios less than 0·40. Other leading causes for which observed YLLs were much lower than expected included stroke in Andean Latin America and neonatal preterm birth complications in Oceania. By contrast, HIV/AIDS led to the highest discrepancies for observed and expected YLLs, particularly affecting southern sub-Saharan Africa. Diarrhoeal diseases, particularly in southern sub-Saharan Africa, and neonatal encephalopathy due to birth asphyxia and trauma, particularly in southern Asia, also resulted in large differences for observed and expected YLLs.
Regional, country, territory, and selected subnational results
For many high-income countries, observed YLLs due to stroke—a cause consistently among the leading three causes of early death—fell below the levels expected on the basis of SDI in 2015. Spain, France, Malta, and Israel had particularly low ratios for observed versus expected YLLs from stroke, all falling below 0·45; 26 countries, including Portugal, Argentina, and Uruguay had ratios lower than 0·80. A subset of countries, including Japan, South Korea, and Chile, also had lower observed YLLs from ischaemic heart disease than expected on the basis of SDI. Early death due to drug use disorders exceeded expected levels in the USA (5·71), Scotland (5·08), and Norway (3·44), and a similar pattern was found for YLLs due to alcohol use disorders in Denmark (10·50) and Finland (9·61). Within the GBD high-income super-region, Brunei, Greenland, and the USA had some of the largest deviations between observed and expected YLLs across causes. Within the UK, observed YLLs from self-harm and stroke were often substantially lower than expected for most regions, whereas observed levels of premature mortality due to COPD were higher than expected.
Throughout Latin America and the Caribbean, observed YLLs due to interpersonal violence far exceeded those expected on the basis of SDI, with 19 countries and territories recording ratios higher than 3·00. Furthermore, interpersonal violence ranked as the first or second leading cause of early death for seven of 11 countries in central and Tropical Latin America. For these two geographical regions, discrepancies between observed and expected YLLs from interpersonal violence were highest in Venezuela (9·91) and Brazil (4·88), respectively. Observed YLLs were also higher than expected for diabetes, especially in the Caribbean; chronic kidney disease, particularly in Mexico (3·22); and prostate cancer for several Caribbean islands and territories. Although ischaemic heart disease was a leading cause of early death for many Latin American countries, several had ratios of observed and expected YLLs lower than 0·60, including Peru (0·33), Panama (0·50), and Colombia (0·51). Similar results were found for stroke (eg, a ratio of 0·26 for Costa Rica); road injuries (eg, Honduras [0·37] and Cuba [0·48]); and for preterm birth complications (Haiti [0·43] and Guatemala [0·32]).
In 2015, leading causes of early death, as well as their observed and expected levels, were markedly heterogeneous in southeast and east Asia and Oceania. For many countries and territories, lower respiratory infections ranked among the ten leading causes of premature mortality, but observed YLLs fell below the levels expected on the basis of SDI (eg, 0·22 in the Maldives, 0·34 in China, and 0·46 in the Solomon Islands). For others, such as Malaysia and Laos, observed YLLs due to lower respiratory infections were higher than expected. Within the region, 18 countries and territories had ischaemic heart disease as their leading cause of YLLs in 2015, but their ratios of observed versus expected YLLs ranged from less than 0·50 in Thailand to more than 4·00 in Guam. Particularly in Oceania, observed levels of YLLs due to diabetes and chronic kidney disease consistently exceeded expected YLLs, and premature mortality due to liver cancer was higher than expected in Taiwan (province of China), Thailand, China, North Korea, Vietnam, and Tonga. Observed YLLs due to communicable diseases were at least twice as high as expected for some countries, including measles in Papua New Guinea (3·61) and tuberculosis in Indonesia (6·53) and the Philippines (4·11). At the same time, several countries and territories recorded cause-specific YLLs that were substantially lower than expected, such as preterm birth complications in Papua New Guinea (0·35); road injuries in Sri Lanka (0·47) and Samoa (0·31); and self-harm in Malaysia (0·52) and China (0·51).
Patterns of early death in south Asia reflect the region's diversity of countries and their relative stages of development. Although lower respiratory infections, diarrhoeal diseases, and congenital anomalies remained among the leading causes of premature mortality throughout south Asia, observed levels of YLLs were generally lower than those expected on the basis of SDI. However, for most countries in south Asia, observed YLLs due to neonatal encephalopathy were more than twice as high as expected (eg, 2·94 in Pakistan and 2·13 in India). Notably, observed YLLs from intestinal infections, such as typhoid fever, were above expected levels in Bangladesh (6·07). Observed levels of YLLs exceeded expected levels for a subset of NCDs, including ischaemic heart disease in Pakistan (1·81), and COPD in India (2·44). In 2015, an earthquake claimed more than 8700 lives in Nepal, and since the occurrence of earthquakes has minimal linkages to SDI, the ratio for observed and expected YLLs was extremely high for natural disasters. At the same time, Nepal and Bangladesh had substantially fewer than expected YLLs due to preterm birth complications (0·25 and 0·40, respectively). Observed levels of early death from stroke were also lower than expected in Bhutan (0·49) and Nepal (0·49).
In central Europe, eastern Europe, and central Asia, except for a subset of causes and countries, observed YLLs generally met or exceeded the levels expected on the basis of SDI. YLL ratios due to ischaemic heart disease and hypertensive heart disease were more than 2·00 for 17 countries, and observed premature mortality due to cardiomyopathy and myocarditis was at least three times higher than expected levels in Russia (10·86), Latvia (7·93), and Bosnia and Herzegovina (5·65). Alcohol and drug use disorders were among the ten leading causes of early death throughout the region, and observed levels often exceeded expected YLLs (eg, 24·53 for drug use disorders in the Ukraine and 17·95 for alcohol use disorders in Russia). Early death from cirrhosis due to alcohol use was more common than expected in several countries, including Moldova (4·24) and Hungary (3·40), whereas levels of YLLs due to interpersonal violence far exceeded expected levels in Russia (12·78) and Kazakhstan (5·65). Group 1 causes often led to higher levels of observed YLLs than expected in central Asia, such as neonatal encephalopathy in Azerbaijan (8·26) and lower respiratory infections in Turkmenistan (6·81). Notably, several countries had much lower than expected observed YLLs due to road injuries, including Albania (0·38) and Macedonia (0·47).
In north Africa and the Middle East, large discrepancies occurred between observed YLLs and those expected on the basis of SDI, underscoring the region's rapid development and inequalities in wealth. Furthermore, because of the region's escalating rates of war-related mortality, which is not strictly related to SDI, ratios of observed versus expected YLLs from war were extremely high. The United Arab Emirates (UAE) and Afghanistan had the most causes for which observed levels of YLLs exceeded expected YLLs; these causes ranged from ischaemic heart disease to interpersonal violence for Afghanistan, and included chronic kidney disease, COPD, diabetes, and road injuries for the UAE. Many countries in the region recorded substantially lower YLLs than expected for several causes: 13 had ratios less than 0·60 for lower respiratory infections, including Iraq (0·40) and Palestine (0·25); eight countries had ratios less than 0·60 for stroke, including Lebanon (0·45) and Turkey (0·42); and six had ratios less than 0·60 for preterm birth complications, including Egypt (0·47) and Syria (0·19).
For every country in sub-Saharan Africa, the leading cause of early death was one of four communicable diseases—HIV/AIDS, malaria, lower respiratory infection, or diarrhoeal diseases—yet patterns in observed and expected levels of YLLs strikingly differed within the continent. In southern sub-Saharan Africa, HIV/AIDS was the leading cause of premature mortality and resulted in exceedingly more YLLs than expected given SDI; Namibia, with a YLL ratio for HIV/AIDS of 7·97, was the lowest among these countries, whereas Swaziland had the highest ratio (19·98). Early death due to tuberculosis also surpassed expected levels, especially in South Africa (19·05), as did observed YLLs due to violence (eg, 7·41 in South Africa). Observed YLLs due to preterm birth complications were somewhat lower than expected in southern sub-Saharan Africa (eg, 0·58 in Botswana), but such levels were largely surpassed by countries in western and eastern sub-Saharan Africa. In 2015, 13 countries in western sub-Saharan Africa and 14 countries in eastern sub-Saharan Africa had YLL ratios lower than 0·60 for preterm birth complications. In western sub-Saharan Africa, observed premature mortality due to malaria was at least twice as high as expected in 13 of 19 countries in the region, with the highest YLL ratios in Nigeria (108·21) and Ghana (78·2). Neonatal sepsis caused more YLLs than expected based on SDI in several western sub-Saharan African countries, including Ghana (5·51). Despite the fact that the Ebola virus disease epidemic claimed more lives in 2014 than in 2015, Ebola virus disease remained among the leading cause of YLLs in Sierra Leone and Liberia. Although still among the leading causes of early death in western sub-Saharan Africa, YLLs due to lower respiratory infections and diarrhoeal diseases were much lower than expected for most countries, with YLL ratios as low as 0·29 in Mali and 0·28 in The Gambia, respectively. Similar trends emerged for eastern sub-Saharan Africa, although the toll of HIV/AIDS was generally higher; observed levels of premature mortality due to HIV/AIDS were at least five times higher than expected in Zambia (6·21) and Mozambique (7·75). Within eastern sub-Saharan Africa, eight of 15 countries had YLL ratios less than 0·60 for YLLs due to diarrhoeal diseases (eg, Rwanda [0·40] and Uganda [0·47]), emphasising the region's rising SDI. Furthermore, every country in eastern sub-Saharan Africa had observed YLLs due to preterm birth complications that were below expected levels, including Ethiopia (0·36) and Kenya (0·46). Nonetheless, YLL ratios for malaria were quite high for several countries, particularly Zambia (41·28) and Tanzania (11·54). Among countries in central sub-Saharan Africa, malaria resulted in many more YLLs than expected based on SDI, with Angola, Congo (Brazzaville), Equatorial Guinea, and Gabon reporting ratios that exceeded 20·00. Neonatal sepsis consistently caused more early deaths than expected within the region. Observed YLLs due to preterm birth complications fell below expected levels in Angola (0·39), Congo (Brazzaville; 0·43), and the Democratic Republic of the Congo (0·47). Notably, the Democratic Republic of the Congo, which had the world's sixth-highest death toll due to diarrhoeal diseases in 2015, had far fewer YLLs due to diarrhoea than expected (0·27).
Discussion
Main findings
Over the past 35 years, global life expectancy, age-standardised death rates, and age-standardised YLL rates have all substantially improved year on year, with the single exception of 1994 (high mortality from the Rwandan genocide, the Iraq civil war, and ongoing armed conflict in Bosnia and Herzegovina that year was enough to change the trend in global life expectancy). During 2005–15, life expectancy increased in 188 of 195 countries and territories and those increases were faster than expected on the basis of SDI improvements in 120 countries and territories. Such gains were mainly driven by marked reductions in mortality due to HIV/AIDS, malaria, infectious diseases such as lower respiratory infections and diarrhoea, neonatal disorders, cardiovascular disease, and cancers. Running counter to the general improvement, age-standardised death rates for 13 causes increased significantly in the past decade. In addition to these causes with increases, for some leading causes of death such as diabetes and chronic kidney disease, age-standardised death rates have stagnated globally and increased in some countries. Since 2011, global deaths from war have risen massively due to conflicts in Syria, Yemen, and Libya. Furthermore, gains in life expectancy were reversed for a subset of countries, with Syrian men bearing the largest toll (a 12·1 year reduction in life expectancy from 2010 to 2015).
Our analysis of the average relationships between age, sex, and cause-specific mortality and SDI suggest that the epidemiological transition does not affect all causes uniformly. Not all causes of death or YLLs improve as SDI increases. In fact, some causes such as cardiovascular disease and a subset of cancer types, initially tend to increase with rising SDI and then decline. This inverted U pattern might be related to changes in risk factors that initially increase disease incidence and then, with rising SDI, risk factor management and disease treatment might act to reduce age-specific mortality. The change in death numbers and population rates with the epidemiological transition was driven by both expected changes in age-specific rates and the highly regular shifts in population age structure that occur with rising SDI. Previous attempts to characterise the shifts in disease pattern with development56, 57, 58 have not been as comprehensive and have not been based on a comprehensive database such as that provided by GBD. Across causes and within geographical regions, we found huge country-level heterogeneities when observed levels of premature mortality were compared with those expected on the basis of SDI. The average patterns of age-specific mortality associated with a given level of SDI are a useful starting point for benchmarking a country's mortality pattern, but many other factors, including public health programmes, access to medical care, and inequalities could account for deviations from the pattern expected on the basis of SDI alone.
Nearly 40 years ago, Samuel Preston observed a shifting relationship between life expectancy and income per capita;58 in a series of subsequent analyses, the crucial role of technological change has been supported by this changing relationship.58, 59, 60, 61 With global life expectancy or other mortality metrics improving faster than expected on the basis of SDI, this general trend was tempered by stagnant or increasing mortality rates due to a subset of causes such as diabetes, cardiovascular disease, and some types of cancer. The overall shift in life expectancy masked larger gains for children and minimal progress for older adults compared with that expected on the basis of SDI. In fact, figures 10C and 10D show that global progress has been much less impressive for the probability of death from ages 15–50 years than for children and that progress in reducing the probability of death from ages 50–70 years only seems to be noticeable at high levels of SDI. In the younger adult age groups, temporal patterns over the past 25 years were profoundly shaped by the unfolding of the HIV epidemic in eastern and southern sub-Saharan Africa and roll-out of ART and PMTCT and the rise and subsequent fall in adult mortality in eastern Europe and central Asia. This is an important difference from previous notions of the inevitability of technological shifts driving up levels of health faster than expected on the basis of income and education alone.58, 59, 60, 61, 62 This finding comes at a time when two very different views of the future of health can be envisioned: rising threats such as climate change, food insecurity, water shortages, pandemics, human security, continued increases in obesity, or antimicrobial resistance that could undermine past health gains; and the realisation of the huge potential of new medical and public health breakthroughs driven by genomics, nanotechnology, and other technical developments.63, 64, 65, 66, 67, 68 Future health scenario construction will crucially depend on how the balance of these forces is played out.
The analysis of trends in numbers of global deaths by cause broken down into changes due to population growth, population ageing, and changes in age-standardised death rates highlights the critical importance of ageing in changing the mixture of causes of death and disease that health systems will have to manage. Likewise, the marked difference in patterns between age-standardised rates to crude rates of YLLs shows how ageing amplifies the speed of the shifts from communicable, maternal, neonatal, and nutritional diseases to NCDs. The combination of demographic and epidemiological change can also lead to extremely rapid changes in disease profiles in some countries. China is a clear example of having an accelerated change in disease profiles: from 1990 to 2015, the percentage of YLLs due to NCDs increased from 50·0% (48·5–53·0) to 77·3% (76·5–78·1). The potential rapidity of these changes presents challenges to many ministries of health in terms of human resources for health planning and policy formulation. Understanding where this potential is about to unfold will be aided by systematic demographic and epidemiological forecasts grounded in the patterns recorded in GBD.
Communicable diseases
Although our methods for the assessment of HIV/AIDS mortality changed substantially from GBD 2013, our general findings at the global level have remained similar across iterations of GBD.11 With the global epidemic of HIV/AIDS mortality peaking in 2005, at 1·8 million deaths (95% UI 1·7 million to 1·9 million), deaths due to HIV/AIDS had decreased by 2015, dropping to 1·2 million (1·1 million to 1·3 million). Yet despite major progress, especially amid the scale-up of ART and PMTCT in sub-Saharan Africa, HIV/AIDS remains the leading cause of YLLs in 16 countries and among top five causes of mortality in 38 countries. Large numbers of individuals continue to die from HIV/AIDS each year in eastern and southern sub-Saharan Africa, where many countries still experience large-scale epidemics, even with the rapid scale-up of ART. We estimate that 17·8% (13·6–20·6) of these HIV/AIDS deaths in 2015 were due to tuberculosis in HIV-positive people, but little data exist for the immediate causes of other HIV/AIDS deaths. Continued high death rates in sub-Saharan Africa highlight the critical importance of improved quality of care and early initiation of therapy, irrespective of disease progression.69 In the face of calls for the end of HIV/AIDS by 2030, more rapid progress is urgently needed.70, 71, 72, 73 Stagnating development assistance for health for HIV/AIDS programmes amplifies the challenge of reducing HIV/AIDS mortality.74, 75
Our results suggest a notable decrease in malaria mortality in sub-Saharan Africa since 2003, consistent with documented reductions in incidence and prevalence, as well as observed increases in effective treatment and preventive interventions.76 We found that malaria mortality peaked in 2003, which is slightly earlier than reported in previous GBD estimates. However, the timing of peak malaria mortality varied across countries (eg, 2005 in Angola and 2000 in Uganda), which might reflect a combination of factors. Estimates presented here for sub-Saharan Africa are based on an estimation method that maps from all untreated cases to mortality and will be refined in further iterations of GBD (eg, the introduction of an intermediate step of severe malaria).
The population attributable fraction of diarrhoeal aetiologies increased for most aetiologies compared with GBD 2013. This is mainly because of two factors. First, we used the new TAC diagnostic for the detection of pathogens in GEMS compared with the conventional laboratory diagnostic methods used in GBD 2013. The TAC method is more sensitive and specific than the conventional methods, such as bacterial culture or ELISA, which tends to increase the odds ratios used in the attributable fraction estimation by correcting for the pathogen misclassification.77, 78 Second, we used a correction factor to adjust for false negatives and false positives of the prevalence of pathogens in patients with diarrhoea and to make it comparable with the odds ratios from the TAC diagnostic method. We corrected our modelled prevalence estimates for the imperfect sensitivity and specificity of the laboratory diagnostic results compared with TAC because most studies reported diarrhoea based on previous diagnostics. Therefore, the correction for the prevalence of the pathogens widened our uncertainty of the final estimates. Although qPCR is a well established diagnostic for diarrhoeal pathogens, the application of TAC remains a novel approach and further testing of the appropriate cutoffs for continuous measures of pathogen presence is needed.
The attributable fractions for Aeromonas, amoebiasis (Entamoeba histolytica), and H influenzae type b were not significant in children younger than 5 years at the global level. Indeed, this is biologically implausible given that these pathogens do not have a protective effect against mortality. The odds ratios of diarrhoea given the presence of Aeromonas and amoebiasis were not significant in children aged 0–1 years and 1–2 years but were significant in the 2–5-year age group, highlighting that these pathogens might not be significant contributors across all age groups. The attributable fraction for H influenzae type b is based on a meta-analysis of randomised controlled trials of vaccine efficacy where the CI of the pooled estimate was not statistically significant. This is a potential limitation and future analyses could use alternative meta-analytical methods, such as log-log meta-analysis or imposition of a non-negative population attributable fraction prior, to prevent negative population attributable fraction estimates.
Our estimation of the fraction of deaths due to lower respiratory infection attributable to pneumococcal pneumonia relies on data from vaccine efficacy studies that show a decrease in all pneumonia and among invasive (bacteraemic) pneumococcal disease in children and adults. However, at least one study that used a urine antigen test in elderly adults found that the relative vaccine efficacy of pneumococcal conjugate vaccine against invasive pneumococcal disease was 66% greater than against pneumococcal pneumonia.49 For GBD 2015, we corrected the estimated pneumococcal conjugate vaccine efficacy against invasive pneumococcal disease by this ratio in both child and adult age groups. This is a change in our GBD 2015 methods, although no studies have used this diagnostic test to detect non-invasive pneumococcal pneumonia in children. To reflect the uncertainty of this adjustment, we used a uniform distribution around the point estimate of the ratio. This adjustment contributed to an increase in our estimates of pneumococcal pneumonia mortality because the vaccine efficacy against pneumococcal pneumonia could be lower than has previously been estimated.49 Given the much larger attributable fraction after the adjustment was made and wide uncertainty of the final estimates, further studies to confirm and precisely quantify the difference in vaccine efficacy between invasive and non-invasive disease are needed. Data availability limitations, particularly for vaccine efficacy data across age groups, hindered our ability to conduct pathogen attribution analyses for H influenzae type b among populations aged 5 years and older; a similar lack of data made estimating population attributable fractions and pathogen-specific mortality for neonates analytically infeasible.
While mortality rates from most infectious diseases fell, dengue emerged as a notable counterexample. The combination of new data and an improved dengue trend covariate (methods appendix p 82) enabled our model to better capture these trends. Compared with GBD 2013 estimates, our GBD 2015 dengue mortality estimates are higher for the most recent decade, yielding a pronounced upward trend that is more consistent with case reports, GBD 2013 incidence estimates, and expert consensus.79 The increasing geographic range of dengue and, in some areas (eg, Latin America) increasing transmission intensity, contribute to growing concerns about other viruses that are transmitted by Aedes mosquitoes, including the chikungunya and Zika viruses.80 Progress in dengue vaccine development, including the licensure of the CYD-TDV vaccine in 2015,81 is promising, but the eventual impact of such vaccines remains unclear.
In GBD we follow the ICD principles of underlying cause of death. According to this principle, a single underlying cause is assigned to each death. In some cases, somewhat arbitrary rules are used to deal with interactions between pathogens or disease processes—eg, all deaths from opportunistic infections, such as fungal infections, in HIV-positive patients are assigned to HIV/AIDS as the underlying cause. Underlying cause assignment is distinct from the notion of excess mortality, whereby a condition or disease can be associated with increased risk of death from other pathophysiological processes. In some cases, the underlying cause of death rules embedded in the ICD could lead to lower death estimates for some neglected tropical diseases. For example, anaemia is a sequela of various different conditions, some of which are parasitic. Hookworm infections can cause iron-deficiency anaemia,82 which is then recorded as the underlying cause of death. Similarly, infections by Clonorchis spp have been associated with increased risk of cholangiocarcinoma83 and are classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC);84 however, the cause of death will be listed as cholangiocarcinoma and will be captured within the total cancer estimates. Alternative post-hoc analyses might be useful for some of these neglected tropical diseases to characterise the excess mortality that could be related to the disease.
Pandemics were incorporated into GBD as fatal discontinuities in the same way as armed conflicts and natural disasters.7, 85 In late 2013 an epidemic of Ebola virus disease started in west Africa. Slow national and international responses allowed the virus to spread throughout 2014 and to become a Public Health Emergency of International Concern,86 which was eventually brought under control in 2015 after a sustained multilateral effort. These estimates of mortality are of the direct deaths attributable to Ebola virus disease and do not account for the full effects of mechanisms such as the breakdown of health systems or critical infrastructure and their subsequent potential health repercussions.87, 88 Previous estimates of mortality86 combined with these destabilising effects on wider health-care provisioning within the three most affected countries have already resulted in appropriate introspection among the international community on the ability of institutions to cope with pandemic threats.89 Correctly establishing the relative public health importance of pandemic preparedness investments is non-trivial, because even if there are no deaths in a year from a potential pandemic cause, such as pandemic influenza, substantial excess mortality risk remains.90 The need for better surveillance and preparedness infrastructures to help to mitigate the risk posed by potential pandemic infectious diseases has been strongly advocated91 and has been underscored again in 2016 with the rapid spread of Zika virus across the Americas and the declaration of another Public Health Emergency of International Concern.92
In GBD 2015, we have not estimated the burden of drug-resistant tuberculosis as a cause distinct from overall tuberculosis. Nor have we estimated deaths related to drug-resistant bacteria or malaria. The challenge that antimicrobial resistance poses both to current and future health-care systems is becoming increasingly documented and quantified,93 with an estimated 3·3% of new tuberculosis cases and 20% of previously treated cases having multidrug resistance.94 Incorporating the proportion of cases caused by pathogens with drug resistance therefore represents a significant, but increasingly important, objective for future estimates.95 Within the GBD framework, drug resistance might be best assessed by examining antimicrobial resistance as a risk factor rather than estimation of subtypes of each pathogen.
Researchers have long foreseen health effects caused by changes in the environment, with a particular focus on climate change.96 To date, our results do not show these trends being realised. Instead they show a substantial global trend of improvement in SDI and notable improvements in many of the infectious disease causes that would be most sensitive to climate change such as malaria,76 in a process known as the environmentalist's paradox.97 This lack of any association so far could be related to time lags between global environmental change and health outcomes and is widely hypothesised to be at the expense of degradation of ecosystem services.98 It might also simply be much harder to detect changes against the backdrop of rapid improvements that are expected with SDI. The value expected from SDI might therefore help to identify which countries or subnational areas are improving at rates that are slower than expected.
Non-communicable diseases
From 2005 to 2015 age-standardised death rates due to most types of cancer decreased, a reflection of risk factor reduction, as well as improvements in some settings of health systems equipped for early diagnosis and effective treatment of cancers. With respect to YLLs due to cancer, lung cancer remains the leading cause globally and for most countries, with YLLs increasing by almost 15% over the past decade. Given that most cases of lung cancer can be prevented,99 this observation stresses the importance of tobacco and air pollution control. For other types of cancer, the geographic pattern is more diverse and reflects the vast differences in risk factors, as well as health system capacity. Whereas colorectal, breast, and pancreatic cancer are the leading causes of cancer YLLs for most high-income countries, stomach, liver, and oesophageal cancer dominate in countries with low SDI. It is encouraging that global YLLs due to stomach cancer have decreased over the past decade, with the substantial decrease in age-specific death rates counterbalancing population growth and ageing. Oesophageal cancer is the other example for which YLLs decreased from 2005 to 2015, with the decrease in age-specific death rates offsetting the increase due to an ageing and growing population. Translating the observed changes in liver cancer mortality into public health planning and policy requires knowledge of the underlying aetiologies, as risk factors differ substantially between locations. This has become an even higher priority now that effective treatment for hepatitis C is available.100 The increase in numbers of liver cancer deaths due to hepatitis B from 2005 to 2015 was not significant, which contrasted with the significant increases in deaths, often 20–30%, observed for many other types of cancer: this relative progress might be at least partly attributable to hepatitis B vaccination. A similar development for liver cancer due to hepatitis C can be achieved in the coming decades if hepatitis C treatment, which currently has high costs per patient,100 becomes accessible in high prevalence populations.
Data newly available to GBD 2015 improved our understanding of global patterns of cardiovascular disease. High proportions of death due to cardiovascular disease have been observed in Oceania. Previously, very few data sources were available from Kiribati and Fiji,101 but with additional data from Tonga, Guam, American Samoa, and the Northern Mariana Islands, it seems that 27–30% of deaths in this region were due to cardiovascular disease causes. Newly available data from India show that, similar to surveillance data previously available from Bangladesh, cardiovascular disease accounts for a large and increasing proportion of deaths. Updated data from China now show a clear trend toward decreasing risk of cardiovascular disease death in all age groups since 2010, when comprehensive vital registration became available. In GBD 2015 we examined age-standardised YLL rates by level of SDI. YLL rates for ischaemic heart disease were lowest in countries with the lowest SDI when country populations were examined by SDI, rising steadily at higher levels of SDI, and only reduced for populations in the quarter of countries with the highest SDI. This pattern was more pronounced among males than females and differs from that seen for stroke, where YLL rates decrease gradually at higher levels of SDI and then fall steeply for the highest SDI populations. The result is that for overall cardiovascular disease, YLL rates were lowest in both the lowest and highest sociodemographic groups with an increase for those in the middle of the sociodemographic rankings. One hypothesis is that medical care in the highest SDI populations might have increased life expectancy to the point where cardiovascular disease is most prevalent, while people in the lowest sociodemographic group are dying from other conditions before reaching an age where they would develop ischaemic heart disease and stroke.102 In this scenario, people living in countries categorised in the middle range of the sociodemographic rankings are surviving long enough to develop ischaemic heart disease but might not have access to optimal medical or surgical treatment.
Deaths due to cirrhosis increased globally from 2005 to 2015, whereas age-standardised cirrhosis mortality rates fell during the same period. Underlying the global picture, though, are notably distinct regional trends, with substantial reductions in cirrhosis mortality in east Asia and central Europe, for example, and increases in cirrhosis mortality in central Asia and north Africa and the Middle East. These trends largely reflect changes in the major risk factors for the disease, with some cirrhosis risk factors, such as chronic hepatitis B infection, decreasing in the face of widespread vaccination, and other risk factors, such as alcohol consumption and chronic hepatitis C, increasing in some parts of the world.103 The decades-long lag between hepatitis B infection and cirrhosis death, and the increasing use of the hepatitis B vaccine, suggest that the potential effect of vaccination on cirrhosis mortality is only beginning to become apparent, and hepatitis B-attributable cirrhosis deaths should continue to fall. Notably, with nearly a quarter of cirrhosis deaths being due to chronic hepatitis C infection, improvements in blood screening and new short-course oral treatments for hepatitis C have the potential to reduce cirrhosis mortality in the future.103, 104
Globally, total deaths from Alzheimer's disease and other dementias increased by almost 40% from 2005 to 2015. This increase is due to population increases and ageing accompanied by a small but significant decrease in age-standardised death rates from Alzheimer's disease and other dementias, possibly reflecting the reduced burden of cardiovascular disease and the contribution of vascular brain injury to the dementia syndrome.105 A small reduction in mortality rates is consistent with reports of a fall in the prevalence of dementia from two cross-sectional surveys a decade apart in the UK and a decline in incidence of dementia reported from the Framingham Cohort Study in the USA.106, 107 There is also evidence that increased education and healthier lifestyles can reduce disease incidence and delay disease onset,105, 107, 108 partly reflecting the reduced burden of cardiovascular disease and the contribution of vascular brain injury to the dementia syndrome. Although the reductions in rates are welcome news, the substantial increase in the number of deaths from Alzheimer's disease and other dementias and the associated increase in prevalence present challenges for health systems and social support systems that need to address the needs of these patients and their families.
We estimate small numbers of death due to mental disorders as the underlying cause. We include schizophrenia as a cause of death because high-quality vital registration data every year show a stable, small number of deaths from this cause. Many more deaths occur in people with schizophrenia in excess of what would be expected on the basis of general population mortality rates. These deaths are certified and coded to other diseases and injuries as the underlying cause, such as self-harm, unintentional injuries, infectious diseases, substance use, cardiovascular disease, and cancers, for which excess mortality in people with schizophrenia has been reported.109, 110, 111 Most deaths from self-harm can be attributed to underlying mental and substance disorders such as depression, anxiety disorders, schizophrenia, bipolar disorder, and alcohol and drug use disorders, as has been quantified based on GBD 2010 estimates.112 In future iterations of GBD, it might be useful to more systematically and regularly quantify the excess mortality associated with several mental disorders.
The estimate of 1·5 million deaths (95% UI 1·5 million to 1·6 million) due to diabetes, plus 418 000 deaths (389 000–441 000) from chronic kidney disease due to diabetes mellitus, still underestimates the full impact of diabetes on all-cause mortality because of the increased risk of ischaemic heart disease, stroke, and tuberculosis associated with diabetes.113, 114, 115 In the GBD framework, computation of deaths attributable to elevated fasting plasma glucose more comprehensively captures the effects of diabetes and pre-diabetes on mortality—see the GBD comparative risk assessment for 2015.116 Given the strong association between diabetes and obesity and the global rise of obesity, the finding that age-standardised death rates for diabetes, including chronic kidney disease due to diabetes mellitus, are not increasing suggests that other protective factors such as treatment might have an effect.117, 118 Spatial patterns show marked variation in death rates, even in places with relatively similar prevalence such as Mexico and the USA.119 These variations in death rates could be related to variation in cause of death certification, with some medical communities more likely than others to list diabetes as the underlying cause of death, or they might be due to treatment effects. Given the importance of diabetes as a cause of death already, and the likely global rise in prevalence of diabetes, more research is needed to understand the determinants of variation in diabetes death rates.119
In this analysis we have estimated that there were more deaths due to chronic kidney disease than in previous analyses because of improved estimates within countries with large populations such as China, India, and Russia. We have also improved our chronic kidney disease subtype estimation strategy by implementing consistent data source inclusion, as well as estimation strategy across the four subtypes. A further improvement is that we have narrowed the definition of deaths due to “chronic kidney disease other” so that deaths formerly included in this category are now attributed to original disease cause, such as polycystic kidney disease. Thus chronic kidney disease subtype mortality results will differ notably from those of previous analyses. Our results indicate that, globally, deaths from chronic kidney disease increased among both males and females, but age-standardised death rates remained relatively unchanged in the past decade. Likely contributors include an increase in the burden of chronic kidney disease risk factors such as diabetes mellitus and hypertension. In 2015 Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus.120 Unique aetiological contributors, such as those suspected in chronic kidney disease due to other causes, have been shown to cause chronic kidney disease deaths mostly in younger adults in El Salvador and Nicaragua, as well as Sri Lanka.121 Efforts to delay deaths due to end-stage renal disease currently depend on renal replacement therapy in the form of either maintenance dialysis or renal transplantation. These costly interventions require appropriate medical infrastructure, as well as government subsidisation, to be accessible to the general population. Therefore, these interventions are currently accessible mainly to populations in high-income countries.122 If deaths due to chronic kidney disease continue to increase globally, further research into possible ways to prevent chronic kidney disease, such as with population-level or individual-level approaches, is warranted. Given that many chronic kidney disease risk factors overlap with cardiovascular risk factors,123, 124, 125 closer collaboration between these two specialties could foster preventive strategies in the future.
Injuries and fatal discontinuities
Experience and evidence from studies on intervention suggest that there is a potential to reduce road injury deaths through a range of interventions. Drunk driving bans, seat belt laws, road engineering including traffic calming, safety devices in vehicles, speed limits, mandatory helmet usage, bans on mobile phone use while driving, and separation of vulnerable road users from vehicles have all been shown to be effective.126, 127, 128, 129, 130 Progress in reducing road injury can be rapid. From 2005 to 2015, western European countries such as Spain (43·8%, 95% UI 39·9–47·3), Portugal (39·6%, 35·4–43·6), and Switzerland (18·8%, 11·8–25·4), had significant reductions in total deaths. Such rapid decreases indicate that not only are there specific interventions that can work,131, 132 but also that population-level reductions are possible in a short period. A reverse trend is apparent in low-income and middle-income countries, partly because the growth in motorisation and traffic density is outpacing the reductions associated with infrastructural development and levels of law enforcement. This trend is particularly the case for major fast-growing BRICS economies (Brazil, Russia, India, China, and South Africa).133, 134, 135
Global death rates due to interpersonal violence have decreased since 2005, but regional trends were much more diverse. Reductions were largest in Asian and European countries, but rates of deaths due to interpersonal violence in Latin America and southern sub-Saharan Africa remained quite high. Interpersonal violence can often be mitigated or reduced by addressing underlying drivers or risks, such as the accessibility of weapons and use of alcohol and psychoactive drugs.136, 137, 138, 139 In 2015, self-harm was the second-leading cause of death from injury. Nearly half of all self-harm deaths occur in India and China, but the trends in these countries have reversed, decreasing significantly in China but rising in India from 1990 to 2015. Over the past two decades China and India have both experienced rapid economic growth and urbanisation, and therefore the opposing trends might be explained by other factors. Evidence from previous research has shown that a combination of preventive approaches addressing multiple factors, such as increased public awareness, programmes based on behavioural change and coping strategies, physician education, and decreased access to means of self-harm is needed.140, 141, 142, 143
Age-standardised death rates from drowning fell by nearly 30% globally during the past decade, with greatest reductions occurring in China, southeast Asia, central Asia, and eastern and central Europe. The highest rates of death due to drowning in 2015 were in island nations of Oceania and in the Indian Ocean, southeast Asia, Afghanistan, Bangladesh, and sub-Saharan African countries. Globally, drowning is a leading cause of death in children younger than 5 years. There is potential to reduce drowning deaths by preventive measures such as installing barriers, controlling access to water, education, swimming lessons and safe boating practices, and shipping and ferry regulations.144, 145, 146 However, change in access to open water with increasing urbanisation might be a more important factor driving down drowning deaths than specific prevention measures.
For GBD 2015 we systematically collected data on major transport accidents, natural and man-made disasters, wildfires, pandemics, and wars. Although the accuracy of the death numbers due to some of these causes vary by country (ie, they are usually more accurate in stable or higher income countries), they tend to be more reliable than are the estimates for wars. Indeed, it has been challenging to accurately document the number of casualties from wars and deaths resulting from malnutrition, infections, or disruption in health services during wars.147, 148, 149, 150 The challenge is due to scarcity of vital statistics during wars, the increase in refugee populations who are displaced internally or externally, and the fact that surveys can only capture mortality rates among those who have remained in their households during the time of interviews. Unfortunately, these estimates can also be challenging to capture with a future census because refugees might have settled in other countries. For example, it is estimated that more than 3 million Syrians have settled in neighbouring countries or elsewhere.151, 152 Nonetheless, we believe that providing such estimates, even with a wide UI, will draw attention to the devastating effects of war on health and hopefully lead to better methods to estimate morbidity and mortality from wars.
Measurement challenges and opportunities
The assessment of all-cause mortality in GBD 2015 includes important innovations that have substantially increased uncertainty for adult mortality in some countries. We believe this is a much more accurate reflection of our knowledge of age-specific mortality in countries without vital registration or sample registration systems. The most important changes included processing of sibling history data using single years rather than pooling data for 5-year intervals, and the propagation of uncertainty in HIV/AIDS crude death rates used in the first stage of the spatiotemporal Gaussian process regression model into the estimation of 45q15. In addition to these changes, we have greatly improved our parameter selection process for both 5q0 and 45q15 Gaussian process regression to reflect data density and quality of data partly represented by the data variance. Combined, these changes led to an increased uncertainty interval for 45q15. In many countries in Africa, the width of the uncertainty interval for 45q15 more than doubled. The increase in the uncertainty interval for 45q15 directly leads to higher uncertainty intervals for our age-specific mortality rate estimates. This partly helped with the removal of an arbitrary matching algorithm from GBD 2013, which picked the pairs of draws of all-cause mortality and HIV-specific mortality estimates that were consistent. With the widened uncertainty interval in all-cause mortality estimates, we are able to apply the ensemble model to combine draw-level HIV/AIDS mortality estimates from the demographic estimation process and the epidemiological model of EPP-Spectrum for all 1000 draws.
Cause of death data for low SDI countries in sub-Saharan Africa were limited to a small number of mostly local verbal autopsy data often with small sample size. Members of the INDEPTH network of demographic surveillance systems have been collecting verbal autopsy data in some sites for many years.153 In the development of the GBD cause of death database, we have been able to make only limited use of INDEPTH verbal autopsy data collected in multiple sites. Some INDEPTH members like Matlab routinely release physician-certified verbal autopsy data in full detail whereas others have published periodic results in journal articles. In 2015, 22 INDEPTH sites published INTERVA model predictions of individual causes of death for the period 1992 to 2012.153 No primary data from the verbal autopsy interviews have been released to date. Unfortunately, INTERVA model predictions are highly inaccurate. The only validation study comparing INTERVA to an objectively defined cause of death standard at the individual level found it accurately assigned the cause of death in 23·8% of adult deaths, 30·3% of child deaths, and 19·4% of neonatal deaths; at the population level, estimated cause-specific mortality fractions were not better than random guessing.154, 155 This potentially important resource for global health estimation is being underused because of the limitations of current versions of the INTERVA tool for cause ascertainment. Given that injury death assignment might (although there are no objective validation data on this issue) be more plausibly established from INTERVA, we have chosen to use the INTERVA results from INDEPTH for eight types of injuries: transport injuries; falls; drowning; fire, heat, and hot substances; poisonings; venomous animal contact; self-harm; and interpersonal violence. Hopefully, a large number of deaths from INDEPTH verbal autopsies will be assessed using physician-certified verbal autopsy or more robust computer algorithms in the future.
In GBD 2013, for select causes, we developed separate CODEm models for countries with long series of complete vital registration data in order that UI estimation in these settings was not affected by UI in settings with either less complete or lower quality data. For GBD 2015, we standardised this approach. We defined countries with extensive complete vital registration representation as those with vital registration equal to or more than 95% complete for more than 25 years. We also modified the ranges of psi used in testing different ensemble models to be higher, effectively allowing CODEm to evaluate out-of-sample ensembles made up of fewer models. In countries with nearly complete high-quality time series, smaller numbers of models in the ensemble allow the models to follow the data more closely with narrower UIs for many but not all causes.
It is extremely difficult to properly inform national and global policy responses to reduce mortality when available cause of death information is very sparse, outdated, or unreliable. Fortunately, there is now evidence of increased investment and technical support being offered to countries to improve their vital registration systems. Important new partnerships have been formed between major bilateral donors and philanthropic organisations,156 building on earlier efforts of the Health Metrics Network and the Australian development assistance program, AusAID.157, 158 Regional UN-led partnerships, particularly in Africa and the Asia-Pacific region, are also helping governments to understand the essential role of good vital registration systems in development, and advocating for change. The interventions now being offered in many countries include targeted and strategic training of doctors to correctly fill out death certificates that identify the underlying cause of death; improving practices and exploiting information technology advances to more effectively register deaths, consolidate and validate data, and transfer information more efficiently to policy-relevant destinations; and, perhaps most importantly, facilitating the widespread adoption of automated verbal autopsy methods to cost-effectively provide information on causes of death in populations for which these data are unavailable.2 Although the initial focus of these efforts is on improving data systems, a substantial impact on data quality and availability can be reasonably expected within the next 3–5 years.159 This will not only improve the evidence base for guiding local policy decisions, but will also lead to reduced uncertainty in global comparative mortality assessments, such as the GBD study.
WHO led the process of developing new guidelines for reporting on global health estimation.20 For GBD 2015, we invested substantial resources to ensure that the GBD 2015 studies, including this Article, are compliant with the GATHER recommendations. Figure 1, Figure 2 provide detailed workflow diagrams. In appendices and the Global Health Data Exchange, we also provide detailed documentation of all sources used in the analysis. For each step in the workflow diagrams, computer code is either on GitHub, such as CODEm 2015 and DisMod-MR 2.1,160, 161 or available on the IHME website for download. Enhanced transparency and documentation highlight the multiple interconnections between analyses, for example, the strong connections between HIV/AIDS incidence, prevalence, and mortality estimation and all-cause mortality estimation in countries with large epidemics. Across this assessment, there are hundreds of separate analytical steps; for each we documented input data, code, and information on model development and performance.
Limitations
Here we highlight some broad cross-cutting limitations to the GBD mortality and cause of death analysis. The analysis of all-cause mortality in countries without vital registration systems is critically dependent on the validity of sibling history data for measuring adult mortality. Although we show, with appropriate corrections for survivor bias (methods appendix pp 21–24), that sibling history data are unbiased when compared with vital registration data, these comparisons are not available for sub-Saharan Africa, where sibling history data are of key importance. Sibling histories in some African countries might underestimate mortality due to the practice of adoption in some countries, but empirical studies in these settings have not confirmed any consistent underestimation of adult mortality.162
All-cause mortality in settings with vital registration is corrected for under-registration using the three available demographic methods for the detection of under-registration: generalised growth balance, synthetic extinct generations, and a hybrid approach of these two methods. However, these methods, as shown in simulation studies,26 are unbiased but imprecise. To further stabilise our estimates of vital registration completeness over time, we synthesise raw estimates of completeness from death distribution methods and implied child death registration completeness by comparing vital registration to GBD under-5 mortality estimates into one coherent time series. Despite these efforts, estimates of completeness can change between GBD revisions as new census data become available or new surveys are released. We propagate uncertainty into the all-cause mortality in the analysis of completeness, but these UIs might be underestimates in some settings because of the scarcity of available data.
Because of the close connection between the estimation of all-cause mortality and HIV/AIDS incidence, prevalence, and mortality in countries with large epidemics, all limitations pertaining to HIV modelling also apply to our estimates of all-cause mortality. For GBD 2015, we used an ensemble model for HIV in which the demographic model and the natural history model of HIV/AIDS death are combined. Despite these attempts to triangulate on the magnitude of the HIV/AIDS epidemic, we assume that the CD4 progression rate, off-ART death rates, and on-ART death rates for age–sex–CD4 categories are the same throughout sub-Saharan Africa. Because of other factors, such as co-infections with tropical diseases or nutritional status, CD4 progression rates can vary across populations. It is also likely that the quality of and access to ART programmes varies across communities, and thus on-ART death rates might well vary. Because of the strong assumptions made in the natural history models (assumptions also made by UNAIDS163) about the consistency of these parameter values across countries, we might be underestimating the true variation in HIV/AIDS death rates and all-cause mortality.
Our cause of death analysis depends substantially on the validity of medical certification of causes of death and physician-certified verbal autopsies. Although efforts to redistribute garbage codes to likely underlying causes of death help to enhance comparability, our findings are affected by systematic bias in medical certification of causes of death. We see in the rapid increase in certain causes of death on death certificates—such as Alzheimer's and other dementias, or atrial fibrillation—evidence of diagnostic trends that are generally incompatible with time series data even after garbage code redistribution. For these reasons, we used other methods to estimate these particular causes of death. However, these patterns suggest that garbage coding practices not only vary by country but also across time.
Our results depend critically on the validity of our approach to garbage code redistribution. As we further utilised statistical methods to establish redistribution algorithms over the development of the GBD 2015 results, we saw sizeable changes in major causes of death. For example, changes in how left-sided heart failure and right-sided heart failure are analysed substantially changed the number of deaths reassigned to pneumoconiosis, haemoglobinopathies, or COPD. We believe these changes reflect improvements in our methods, but they also show how some causes of death, even though they result in lower absolute levels of mortality, can be profoundly affected by the redistribution of large garbage codes such as heart failure or sepsis. The sensitivity of our findings to how major garbage codes, such as heart failure, sepsis, or ICD-X59 (exposure to unspecified factor), are redistributed emphasises the importance of more systematic research on garbage code practices and improvements in primary data collection to avoid deaths being certified to garbage codes.
We used CODEm to model all causes for which sufficient numbers of deaths are observed in vital registration, sample registration, or verbal autopsy datasets. For these 167 causes, we conducted rigorous out-of-sample validation exercises and documented prediction error and UI coverage. However, for the remaining causes, particularly those modelled with natural history models, the design of validation tests was severely limited by data sparseness. In the absence of better data collection, particularly for causes of death that mostly occur in data-sparse geographies, our options for more robust model validation will remain limited. The analysis of causes of death in India also has important ramifications for global estimates. The three major sources of cause of death information for India are the Medical Certification of Causes of Death (MCCD), largely collected in urban areas; the Survey of Causes of Death (Rural; SCD[R]); and verbal autopsy data collected for deaths recorded in the Sample Registration System (SRS) from 2001 to 2013. All three systems are or were maintained by the Registrar-General of India. Unfortunately, whereas the MCCD and SCD(R) data have been released in considerable age, sex, and cause detail for states in India, the SRS data have been released only for large aggregates and not at the state level, although various academic works using data for 2002–04 have been published for specific causes with varying levels of detail. The rich resource of the SRS verbal autopsy data could be much more informative if the standard WHO table of ICD code, age, and sex were publicly released.
Available data from some countries in sub-Saharan Africa pose substantial challenges for cause of death analysis. Only extremely scarce verbal autopsy data are currently available for large populations. Given substantial gradients in mortality within Nigeria, for example, there is substantial risk of over-interpreting the limited verbal autopsy data. Civil registration data are collected in Nigeria, but the completeness is low and the level of garbage coding is very high. New data for countries in the sub-Saharan African region would substantially narrow a major source of uncertainty in sub-Saharan African causes of death.
It is possible that some of the heterogeneity reported in observed and expected causes of early death in different geographies has been artificially created by variations in data quality and the use of different methods. The GBD group makes extensive efforts to try to reduce the effects of variable data quality, and we have used standardised methods for each cause that are the same for all countries.
Lastly, although our estimates of uncertainty intervals reflect multiple sources of uncertainty, they do not include every possible source of uncertainty. For all-cause mortality, we include uncertainty due to the following: sampling error in the underlying data; non-sampling error associated with particular child or adult mortality data types; HIV/AIDS crude death rate; and reference age pattern of mortality used in the GBD model life table. We do not, however, include uncertainty in the measurement of income per capita or educational attainment, and we do not include uncertainty from the choice of our model specification for the first stage of the spatiotemporal Gaussian process regression analysis. For causes of death, we capture sampling uncertainty, model specification uncertainty through the creation of CODEm ensembles, and non-sampling error from subnational or non-representative datasets. We do not, however, capture uncertainty due to garbage code redistribution, uncertainty in the covariates used in the models, or uncertainty due to cause of death assignment in verbal autopsy data. With each iteration of the GBD we have sought to include more comprehensively different sources of uncertainty, and we expect this evolution to continue in the future.
Comparison of different global health estimates
The GBD 2015 estimates for under-5 deaths due to diarrhoea and lower respiratory infection differ from estimates by other agencies, such as the WHO Department of Evidence, Information, and Research and the Maternal and Child Epidemiology Estimation (MCEE) group (results appendix pp 3985–89).164 In 2015, we estimated the number of under-5 deaths as 5·8 million (95% UI 5·7 million to 6·0 million), which was lower than the MCEE's estimate of 5 945 000 (95% CI 5 707 000–6 395 000) for that year.165 Our estimates of under-5 deaths due to diarrhoea for 2015 were also lower, at 499 000 (447 000–558 000), than those of the MCEE group (526 000), although we attributed more diarrhoeal deaths among neonates. For 2015, our results for under-5 lower respiratory infection deaths were notably lower than the estimates produced by MCEE, especially for children aged 1 to 59 months (ie, 557 000 [488 000–633 000]) from GBD 2015 and 760 000 from MCEE. Based on the latest estimates for aetiologies of diarrhoea and lower respiratory infection produced by the Child Health Epidemiology Research Group (CHERG), which is now known as MCEE,166 we found that our estimates for rotavirus are similar for the year 2010. However, in GBD 2015, the estimates for Cryptosporidium were five times higher than those of CHERG, and the estimates for Shigella were 2·5 times higher. These differences might arise from variations in estimation approaches, as well as the use of various sources and types of data. For example, CHERG generated estimates of pathogen-specific diarrhoea based on the proportion of hospitalised cases of diarrhoea that tested positive for each pathogen,167 whereas the GBD study uses a counterfactual approach that includes odds ratios of disease given exposure and corrects pathogen prevalence estimates using the results from a PCR analysis of samples from GEMS.78 Estimates of deaths due to rotavirus in GBD 2015 and CHERG were similar, which is not surprising given the good diagnostic validity of ELISA for rotavirus. However, GBD 2015 estimates for bacterial pathogens were generally higher, which reflects the relatively low sensitivity and specificity of conventional diagnostic methods for these pathogens.77 For pneumonia, estimates for bacterial aetiologies from GBD 2015 were similar to those from CHERG in terms of total lower respiratory infection deaths and generating similarly large wide uncertainty intervals. However, H influenzae type b estimates were lower than the CHERG results for GBD 2015, and our estimates for under-5 deaths due to pneumococcal pneumonia deaths were higher than the CHERG estimates. These differences probably stem from our efforts to correct for vaccine efficacy against bacteraemic pneumococcal disease to instead represent the efficacy against pneumococcal pneumonia. This correction greatly increases the attributable fraction, and uncertainty, for pneumococcal pneumonia.
IARC last produced cancer estimates by country, age, sex, and cancer site for 2012 (GLOBOCAN).168 GBD and GLOBOCAN definitions are compatible for 25 cancer types. For these cancer sites, the total estimated number of deaths from GLOBOCAN is 7 498 760 in 2012. By comparison, the GBD 2015 estimate was 7 823 429 (7 374 053–8 241 385) for 2012. Worldwide, the largest differences between estimates were for larynx cancer, other pharynx cancers, thyroid cancer, nasopharynx cancer, and myeloma, with differences of 20–85%. GLOBOCAN has been a source for descriptive global cancer epidemiology for many years. However, GBD analyses have some advantages over the past GLOBOCAN estimates. One advantage is that the annual GBD updates allow for the incorporation of new data rapidly. We expect the availability of cancer registry data in low-income and middle-income countries to increase due to the Global Initiative for Cancer Registry Development, which is led by IARC. These data, in addition to increasing the availability of cause of death data, will lead to improvements in cancer estimates, especially for regions with sparse data, and will allow policy makers to adjust health-care strategies in accordance with the latest evidence. The GBD study also provides the unique opportunity for direct comparison of the cancer burden with that of other diseases and therefore for health system investments guided by objective, comprehensive estimates rather than incomplete, unreliable data and advocacy. Furthermore, the GBD 2015 study is compliant with the GATHER guidelines, including reporting uncertainty intervals for each cancer estimate.
A comparison of GBD 2013, GBD 2015, and the two most recent UNAIDS assessments of global HIV/AIDS mortality over time is shown in the results appendix p 4). Although, as noted, GBD methods have changed substantially, our results at the global level are fairly consistent with previous iterations of GBD analyses. UNAIDS, in their latest revision, noticeably changed their assessment of peak global HIV/AIDS mortality from 2·24 million deaths in 2005 to 2·0 million deaths,169 rendering increased consistency between the latest UNAIDS statistics and GBD 2015. Although it is encouraging that thoughtful analyses of the epidemic and its mortality consequences are converging, additional analysis is needed to discern the differences between the estimates from GBD and UNAIDS, and the changes that occurred in the estimation series by GBD or UNAIDS. It is important to understand differences and changes in models and underlying assumptions such as on-ART and off-ART mortality and their effects on the metrics of HIV/AIDS incidence, prevalence, and mortality provided by GBD and UNAIDS. GBD 2015 mostly used epidemiological and programmatic data provided by UNAIDS in its 2015 iteration. The significant difference between GBD and UNAIDS might mark the important difference in assumptions for on-ART and off-ART mortality, CD4 progression ratio, and background mortality rates. In the GBD studies, we have also found that results are highly sensitive to the assumptions of initial CD4 count used in both the analysis of pre-ART cohort data and the initial population distribution of CD4 count for new infections.11
The other major effort to estimate age-specific all-cause mortality and life expectancy other than the annual GBD is the biennial United Nations Population Division assessment published in the World Population Prospects.170 Although the UN calculates estimates for each age–sex–country–year, the UN Population Division publishes data for most metrics only in 5-year intervals. The UN has yet to publish since the introduction of the new WHO GATHER guidelines and, in most cases, the statistical method used to generate estimates of age-specific mortality is unclear for any given country and hard to reproduce with no codes made available. A comparison of GBD 2015 life expectancy estimates and the UN Population Division estimates for the midpoint of 2010–15 is available in the results appendix (p 5). For the 189 countries for which both series provide estimates, GBD 2015 tends to have higher estimates of life expectancy at birth for both males (111 countries) and females (112 countries). The differences are even more prominent for the time period 1980–85, for which GBD life expectancy is higher for 139 countries and territories for males and 134 for females. Although the correlation between the two sets of estimates is 0·94 for males and 0·97 for females for the period 1980 to 2015, there are systematic differences in all four GBD geographical regions in Africa, north Africa and the Middle east, south Asia, and Oceania, for which the correlation coefficient is less than 0·9. In southern sub-Saharan Africa, the UN Population Division estimates are 0·1 year (95% UI −3·9 to 3·6) lower for males and 1·7 years (−6·3 to 3·3 years) lower for females on average in these countries around the peak of the HIV/AIDS epidemic in 2002. This difference exists mainly because their estimates are based on estimated under-5 death rates and model life tables, based on patterns of mortality in the Coale-Demeny model life table system and the United Nations Model Life Tables for Developing Countries, that are in turn based on observations of age patterns of mortality before 1980.31, 32, 170 Unlike UN estimates, GBD estimates of adult mortality rates take into account corrected sibling history data collected in household surveys, which provide direct measurements of adult mortality. The empirical information about adult mortality, used as an entry parameter to GBD's model life table system, certainly helps to improve the accuracy of our mortality age pattern assessment, even in countries affected by the HIV/AIDS epidemic.
Another important difference emerged between the GBD and UN Population Division assessments: mortality and population numbers among youths. For the period 2010–14, the UN Population Division estimates that 6·3 million deaths occurred globally among people aged 5–14 years, whereas GBD estimates this number at 4·6 million.170 The difference is mostly driven by the differences between the Coale-Demeny life tables used by the UN compared with our life tables, which use much more recent empirical patterns of mortality, and different assessments of under-5 mortality rate that come from differences in both treatment of data and data synthesis methods. Hill and colleagues171 reported that, based on complete birth histories in the Demographic and Health Surveys (DHS), which are mostly based in low-income and middle-income countries, mortality among youths might also be much higher than was estimated in GBD 2010. As a data source, long-term recall by mothers of their 5–14-year-old children's deaths, using complete birth histories, has not been validated. We examined the estimates of mortality in India in the 5–14 years age groups from the SRS, which directly measures age-specific mortality in a sample of 7597 villages in rural areas and census enumeration blocks in urban areas in India. We compared these estimates with those from the GBD 2015 and found that estimates from the two series are highly concordant, with a correlation coefficient of 0·95 for the 5–14 years age group and a mean relative difference of −2·2% for the probability of death between the ages of 10 and 14 years in the period 1980 to 2012. Our estimated age pattern of mortality for India is mainly informed by data from the SRS, with exceptions in children younger than 5 years, for whom our under-5 mortality rate data synthesis takes into account under-5 mortality rate estimates from other sources, including the India DHS. As shown in the methods appendix (pp 293–96), for the 5–9 years age group, GBD 2015 estimates of probability of death are mostly consistent with those extracted from the DHS. For the 10–14 years age group, GBD estimates are consistent with both DHS and SRS.
In 2014, WHO published cause-specific mortality estimates at global, regional, and country levels for the years 2000 and 2012.172 WHO used GBD 2010 mortality results for all but 12 cause groups, for which WHO and UN agencies have historically produced estimates.172, 173, 174 These 12 causes are tuberculosis, HIV/AIDS and other sexually transmitted infections, malaria, whooping cough, measles, schistosomiasis, maternal disorders, cancers, alcohol and drug use disorders, epilepsy, conflict and natural disasters, and road traffic accidents.172, 173 WHO has also generated estimates of YLLs, but comparisons of WHO estimates with those of GBD are limited because WHO chose to use the highest projected life expectancy for 2050—91·9 years—rather than the GBD 2010 normative standard life expectancy of 86·0 years for 2010.172 GBD 2010 did not include mortality estimates for the year 2012, so WHO estimates for that year were interpolated.
Conclusion
A key goal, if not the fundamental goal, of a health system is to prolong life, especially healthy life, into old age. To do so, decision makers in health need comprehensive and disaggregated evidence on comparative mortality levels in populations, particularly for causes of death that are largely preventable through political action, either through improving health services or strengthening prevention programmes. Traditionally, this evidence has been limited to the findings of relatively conventional and straightforward mortality analyses. As our results show, more novel approaches can provide much more detailed and systematic descriptions of how survival status and cause of death patterns vary according to measures such as SDI, which are becoming increasingly relevant for policy as overall mortality levels fall. Overall population health is likely to improve more rapidly in places where the relationships between determinants of health and cause-specific mortality patterns are understood—especially areas where addressing of these gradients is a key priority for health and development policy. Our analyses provide important new evidence on where such gradients in survival among populations are greatest, and for which causes of death. Thus, although we found continued reductions in major communicable diseases such as HIV/AIDS and malaria in response to concerted global action, and further, albeit more modest reductions in the risk of death from NCDs and injuries, the comprehensive analysis of the effect of SDI on health, across and among countries, is likely to be much more relevant for accelerating global health progress.
Correspondence to: Prof Christopher J L Murray, 2301 5th Avenue, Suite 600, Seattle, WA 98121, USA cjlm@uw.edu
This online publication has been corrected. The corrected version first appeared at thelancet.com on January 5, 2017
Acknowledgments
Acknowledgments
We thank the countless individuals who have contributed to the Global Burden of Disease Study 2015 in various capacities. The data reported here have been supplied by the United States Renal Data System (USRDS). Data for this research was provided by MEASURE Evaluation, funded by the United States Agency for International Development (USAID). Collection of these data was made possible by USAID under the terms of cooperative agreement GPO-A-00-08-000_D3-00. Views expressed do not necessarily reflect those of USAID, the US Government, or MEASURE Evaluation. Parts of this material are based on data and information provided by the Canadian institute for Health Information. However, the analyses, conclusions, opinions and statements expressed herein are those of the author and not those of the Canadian Institute for Health information. The Palestinian Central Bureau of Statistics granted the researchers access to relevant data in accordance with licence number SLN2014-3-170, after subjecting data to processing aiming to preserve the confidentiality of individual data in accordance with the General Statistics Law–2000. The researchers are solely responsible for the conclusions and inferences drawn upon available data. The following individuals acknowledge various forms of institutional support. Simon I Hay is funded by a Senior Research Fellowship from the Wellcome Trust (#095066), and grants from the Bill & Melinda Gates Foundation (OPP1119467, OPP1093011, OPP1106023 and OPP1132415). Panniyammakal Jeemon is supported by a Clinical and Public Health Intermediate Fellowship from the Wellcome Trust-DBT India Alliance (2015–20). Luciano A Sposato is partly supported by the Edward and Alma Saraydar Neurosciences Fund, London Health Sciences Foundation, London, ON, Canada. George A Mensah notes that the views expressed in this Article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the United States Department of Health and Human Services. Boris Bikbov acknowledges that work related to this paper has been done on the behalf of the GBD Genitourinary Disease Expert Group supported by the International Society of Nephrology (ISN). Ana Maria Nogales Vasconcelos acknowledges that her team in Brazil received funding from Ministry of Health (process number 25000192049/2014-14). Rodrigo Sarmiento-Suarez receives institutional support from Universidad de Ciencias Aplicadas y Ambientales, UDCA, Bogotá, Colombia. Ulrich O Mueller and Andrea Werdecker gratefully acknowledge funding by the German National Cohort BMBF (grant number OIER 1301/22). Peter James was supported by the National Cancer Institute of the National Institutes of Health (Award K99CA201542). Brett M Kissela would like to acknowledge NIH/NINDS R-01 30678. Louisa Degenhardt is supported by an Australian National Health and Medical Research Council Principal Research fellowship. Daisy M X Abreu received institutional support from the Brazilian Ministry of Health (Proc number 25000192049/2014-14). Jennifer H MacLachlan receives funding support from the Australian Government Department of Health and Royal Melbourne Hospital Research Funding Program. Miriam Levi acknowledges institutional support received from CeRIMP, Regional Centre for Occupational Diseases and Injuries, Tuscany Region, Florence, Italy. Tea Lallukka reports funding from The Academy of Finland (grant 287488). No individuals acknowledged received additional compensation for their efforts.
GBD 2015 Mortality and Causes of Death Collaborators
Haidong Wang, Mohsen Naghavi, Christine Allen, Ryan M Barber, Zulfiqar A Bhutta, Austin Carter, Daniel C Casey, Fiona J Charlson, Alan Zian Chen, Matthew M Coates, Megan Coggeshall, Lalit Dandona, Daniel J Dicker, Holly E Erskine, Alize J Ferrari, Christina Fitzmaurice, Kyle Foreman, Mohammad H Forouzanfar, Maya S Fraser, Nancy Fullman, Peter W Gething, Ellen M Goldberg, Nicholas Graetz, Juanita A Haagsma, Simon I Hay, Chantal Huynh, Catherine O Johnson, Nicholas J Kassebaum, Yohannes Kinfu, Xie Rachel Kulikoff, Michael Kutz, Hmwe H Kyu, Heidi J Larson, Janni Leung, Xiaofeng Liang, Stephen S Lim, Margaret Lind, Rafael Lozano, Neal Marquez, George A Mensah, Joe Mikesell, Ali H Mokdad, Meghan D Mooney, Grant Nguyen, Elaine Nsoesie, David M Pigott, Christine Pinho, Gregory A Roth, Joshua A Salomon, Logan Sandar, Naris Silpakit, Amber Sligar, Reed J D Sorensen, Jeffrey Stanaway, Caitlyn Steiner, Stephanie Teeple, Bernadette A Thomas, Christopher Troeger, Amelia VanderZanden, Stein Emil Vollset, Valentine Wanga, Harvey A Whiteford, Timothy Wolock, Leo Zoeckler, Kalkidan Hassen Abate*, Cristiana Abbafati*, Kaja M Abbas*, Foad Abd-Allah*, Semaw Ferede Abera*, Daisy M X Abreu*, Laith J Abu-Raddad*, Gebre Yitayih Abyu*, Tom Achoki*, Ademola Lukman Adelekan*, Zanfina Ademi*, Arsène Kouablan Adou*, José C Adsuar*, Kossivi Agbelenko Afanvi*, Ashkan Afshin*, Emilie Elisabet Agardh*, Arnav Agarwal*, Anurag Agrawal*, Aliasghar Ahmad Kiadaliri*, Oluremi N Ajala*, Ali Shafqat Akanda*, Rufus Olusola Akinyemi*, Tomi F Akinyemiju*, Nadia Akseer*, Faris Hasan Al Lami*, Samer Alabed*, Ziyad Al-Aly*, Khurshid Alam*, Noore K M Alam*, Deena Alasfoor*, Saleh Fahed Aldhahri*, Robert William Aldridge*, Miguel Angel Alegretti*, Alicia V Aleman*, Zewdie Aderaw Alemu*, Lily T Alexander*, Samia Alhabib*, Raghib Ali*, Ala'a Alkerwi*, François Alla*, Peter Allebeck*, Rajaa Al-Raddadi*, Ubai Alsharif*, Khalid A Altirkawi*, Elena Alvarez Martin*, Nelson Alvis-Guzman*, Azmeraw T Amare*, Adeladza Kofi Amegah*, Emmanuel A Ameh*, Heresh Amini*, Walid Ammar*, Stephen Marc Amrock*, Hjalte H Andersen*, Benjamin O Anderson*, Gregory M Anderson*, Carl Abelardo T Antonio*, Atsede Fantahun Aregay*, Johan Ärnlöv*, Valentina S Arsic Arsenijevic*, Al Artaman*, Hamid Asayesh*, Rana Jawad Asghar*, Suleman Atique*, Euripide Frinel G Arthur Avokpaho*, Ashish Awasthi*, Peter Azzopardi*, Umar Bacha*, Alaa Badawi*, Maria C Bahit*, Kalpana Balakrishnan*, Amitava Banerjee*, Aleksandra Barac*, Suzanne L Barker-Collo*, Till Bärnighausen*, Lars Barregard*, Lope H Barrero*, Arindam Basu*, Sanjay Basu*, Yibeltal Tebekaw Bayou*, Shahrzad Bazargan-Hejazi*, Justin Beardsley*, Neeraj Bedi*, Ettore Beghi*, Haileeyesus Adamu Belay*, Brent Bell*, Michelle L Bell*, Aminu K Bello*, Derrick A Bennett*, Isabela M Bensenor*, Adugnaw Berhane*, Eduardo Bernabé*, Balem Demtsu Betsu*, Addisu Shunu Beyene*, Neeraj Bhala*, Ashish Bhalla*, Sibhatu Biadgilign*, Boris Bikbov*, Aref A Bin Abdulhak*, Brian J Biroscak*, Stan Biryukov*, Espen Bjertness*, Jed D Blore*, Christopher D Blosser*, Megan A Bohensky*, Rohan Borschmann*, Dipan Bose*, Rupert R A Bourne*, Michael Brainin*, Carol E G Brayne*, Alexandra Brazinova*, Nicholas J K Breitborde*, Hermann Brenner*, Jerry D Brewer*, Alexandria Brown*, Jonathan Brown*, Traolach S Brugha*, Geoffrey Colin Buckle*, Zahid A Butt*, Bianca Calabria*, Ismael Ricardo Campos-Nonato*, Julio Cesar Campuzano*, Jonathan R Carapetis*, Rosario Cárdenas*, David O Carpenter*, Juan Jesus Carrero*, Carlos A Castañeda-Orjuela*, Jacqueline Castillo Rivas*, Ferrán Catalá-López*, Fiorella Cavalleri*, Kelly Cercy*, Jorge Cerda*, Wanqing Chen*, Adrienne Chew*, Peggy Pei-Chia Chiang*, Mirriam Chibalabala*, Chioma Ezinne Chibueze*, Odgerel Chimed-Ochir*, Vesper Hichilombwe Chisumpa*, Jee-Young Jasmine Choi*, Rajiv Chowdhury*, Hanne Christensen*, Devasahayam Jesudas Christopher*, Liliana G Ciobanu*, Massimo Cirillo*, Aaron J Cohen*, Valentina Colistro*, Mercedes Colomar*, Samantha M Colquhoun*, Cyrus Cooper*, Leslie Trumbull Cooper*, Monica Cortinovis*, Benjamin C Cowie*, John A Crump*, James Damsere-Derry*, Hadi Danawi*, Rakhi Dandona*, Farah Daoud*, Sarah C Darby*, Paul I Dargan*, José das Neves*, Gail Davey*, Adrian C Davis*, Dragos V Davitoiu*, E Filipa de Castro*, Pieter de Jager*, Diego De Leo*, Louisa Degenhardt*, Robert P Dellavalle*, Kebede Deribe*, Amare Deribew*, Samath D Dharmaratne*, Preet K Dhillon*, Cesar Diaz-Torné*, Eric L Ding*, Kadine Priscila Bender dos Santos*, Edem Dossou*, Tim R Driscoll*, Leilei Duan*, Manisha Dubey*, Bruce Bartholow Duncan*, Richard G Ellenbogen*, Christian Lycke Ellingsen*, Iqbal Elyazar*, Aman Yesuf Endries*, Sergey Petrovich Ermakov*, Babak Eshrati*, Alireza Esteghamati*, Kara Estep*, Imad D A Faghmous*, Saman Fahimi*, Emerito Jose Aquino Faraon*, Talha A Farid*, Carla Sofia e Sa Farinha*, André Faro*, Maryam S Farvid*, Farshad Farzadfar*, Valery L Feigin*, Seyed-Mohammad Fereshtehnejad*, Jefferson G Fernandes*, Joao C Fernandes*, Florian Fischer*, Joseph R A Fitchett*, Abraham Flaxman*, Nataliya Foigt*, F Gerry R Fowkes*, Elisabeth Barboza Franca*, Richard C Franklin*, Joseph Friedman*, Joseph Frostad*, Thomas Fürst*, Neal D Futran*, Seana L Gall*, Ketevan Gambashidze*, Amiran Gamkrelidze*, Parthasarathi Ganguly*, Fortuné Gbètoho Gankpé*, Teshome Gebre*, Tsegaye Tsewelde Gebrehiwot*, Amanuel Tesfay Gebremedhin*, Alemseged Aregay Gebru*, Johanna M Geleijnse*, Bradford D Gessner*, Aloke Gopal Ghoshal*, Katherine B Gibney*, Richard F Gillum*, Stuart Gilmour*, Ababi Zergaw Giref*, Maurice Giroud*, Melkamu Dedefo Gishu*, Giorgia Giussani*, Elizabeth Glaser*, William W Godwin*, Hector Gomez-Dantes*, Philimon Gona*, Amador Goodridge*, Sameer Vali Gopalani*, Richard A Gosselin*, Carolyn C Gotay*, Atsushi Goto*, Hebe N Gouda*, Felix Greaves*, Harish Chander Gugnani*, Rahul Gupta*, Rajeev Gupta*, Vipin Gupta*, Reyna A Gutiérrez*, Nima Hafezi-Nejad*, Demewoz Haile*, Alemayehu Desalegne Hailu*, Gessessew Bugssa Hailu*, Yara A Halasa*, Randah Ribhi Hamadeh*, Samer Hamidi*, Jamie Hancock*, Alexis J Handal*, Graeme J Hankey*, Yuantao Hao*, Hilda L Harb*, Sivadasanpillai Harikrishnan*, Josep Maria Haro*, Rasmus Havmoeller*, Susan R Heckbert*, Ileana Beatriz Heredia-Pi*, Pouria Heydarpour*, Henk B M Hilderink*, Hans W Hoek*, Robert S Hogg*, Masako Horino*, Nobuyuki Horita*, H Dean Hosgood*, Peter J Hotez*, Damian G Hoy*, Mohamed Hsairi*, Aung Soe Htet*, Maung Maung Than Htike*, Guoqing Hu*, Cheng Huang*, Hsiang Huang*, Laetitia Huiart*, Abdullatif Husseini*, Inge Huybrechts*, Grace Huynh*, Kim Moesgaard Iburg*, Kaire Innos*, Manami Inoue*, Veena J Iyer*, Troy A Jacobs*, Kathryn H Jacobsen*, Nader Jahanmehr*, Mihajlo B Jakovljevic*, Peter James*, Mehdi Javanbakht*, Sudha P Jayaraman*, Achala Upendra Jayatilleke*, Panniyammakal Jeemon*, Paul N Jensen*, Vivekanand Jha*, Guohong Jiang*, Ying Jiang*, Tariku Jibat*, Aida Jimenez-Corona*, Jost B Jonas*, Tushar Kant Joshi*, Zubair Kabir*, Ritul Kamal*, Haidong Kan*, Surya Kant*, André Karch*, Corine Kakizi Karema*, Chante Karimkhani*, Dimitris Karletsos*, Ganesan Karthikeyan*, Amir Kasaeian*, Marzieh Katibeh*, Anil Kaul*, Norito Kawakami*, Jeanne Françoise Kayibanda*, Peter Njenga Keiyoro*, Laura Kemmer*, Andrew Haddon Kemp*, Andre Pascal Kengne*, Andre Keren*, Maia Kereselidze*, Chandrasekharan Nair Kesavachandran*, Yousef Saleh Khader*, Ibrahim A Khalil*, Abdur Rahman Khan*, Ejaz Ahmad Khan*, Young-Ho Khang*, Sahil Khera*, Tawfik Ahmed Muthafer Khoja*, Christian Kieling*, Daniel Kim*, Yun Jin Kim*, Brett M Kissela*, Niranjan Kissoon*, Luke D Knibbs*, Ann Kristin Knudsen*, Yoshihiro Kokubo*, Dhaval Kolte*, Jacek A Kopec*, Soewarta Kosen*, Parvaiz A Koul*, Ai Koyanagi*, Norun Hjertager Krog*, Barthelemy Kuate Defo*, Burcu Kucuk Bicer*, Andreas A Kudom*, Ernst J Kuipers*, Veena S Kulkarni*, G Anil Kumar*, Gene F Kwan*, Aparna Lal*, Dharmesh Kumar Lal*, Ratilal Lalloo*, Tea Lallukka*, Hilton Lam*, Jennifer O Lam*, Sinead M Langan*, Van C Lansingh*, Anders Larsson*, Dennis Odai Laryea*, Asma Abdul Latif*, Alicia Elena Beatriz Lawrynowicz*, James Leigh*, Miriam Levi*, Yongmei Li*, M Patrice Lindsay*, Steven E Lipshultz*, Patrick Y Liu*, Shiwei Liu*, Yang Liu*, Loon-Tzian Lo*, Giancarlo Logroscino*, Paulo A Lotufo*, Robyn M Lucas*, Raimundas Lunevicius*, Ronan A Lyons*, Stefan Ma*, Vasco Manuel Pedro Machado*, Mark T Mackay*, Jennifer H MacLachlan*, Hassan Magdy Abd El Razek*, Mohammed Magdy Abd El Razek*, Marek Majdan*, Azeem Majeed*, Reza Malekzadeh*, Wondimu Ayele Ayele Manamo*, John Mandisarisa*, Srikanth Mangalam*, Chabila C Mapoma*, Wagner Marcenes*, David Joel Margolis*, Gerard Robert Martin*, Jose Martinez-Raga*, Melvin Barrientos Marzan*, Felix Masiye*, Amanda J Mason-Jones*, João Massano*, Richard Matzopoulos*, Bongani M Mayosi*, Stephen Theodore McGarvey*, John J McGrath*, Martin McKee*, Brian J McMahon*, Peter A Meaney*, Alem Mehari*, Man Mohan Mehndiratta*, Fabiola Mejia-Rodriguez*, Alemayehu B Mekonnen*, Yohannes Adama Melaku*, Peter Memiah*, Ziad A Memish*, Walter Mendoza*, Atte Meretoja*, Tuomo J Meretoja*, Francis Apolinary Mhimbira*, Renata Micha*, Anoushka Millear*, Ted R Miller*, Mojde Mirarefin*, Awoke Misganaw*, Charles N Mock*, Karzan Abdulmuhsin Mohammad*, Alireza Mohammadi*, Shafiu Mohammed*, Viswanathan Mohan*, Glen Liddell D Mola*, Lorenzo Monasta*, Julio Cesar Montañez Hernandez*, Pablo Montero*, Marcella Montico*, Thomas J Montine*, Maziar Moradi-Lakeh*, Lidia Morawska*, Katherine Morgan*, Rintaro Mori*, Dariush Mozaffarian*, Ulrich O Mueller*, Gudlavalleti Venkata Satyanarayana Murthy*, Srinivas Murthy*, Kamarul Imran Musa*, Jean B Nachega*, Gabriele Nagel*, Kovin S Naidoo*, Nitish Naik*, Luigi Naldi*, Vinay Nangia*, Denis Nash*, Chakib Nejjari*, Subas Neupane*, Charles R Newton*, John N Newton*, Marie Ng*, Frida Namnyak Ngalesoni*, Jean de Dieu Ngirabega*, Quyen Le Nguyen*, Muhammad Imran Nisar*, Patrick Martial Nkamedjie Pete*, Marika Nomura*, Ole F Norheim*, Paul E Norman*, Bo Norrving*, Luke Nyakarahuka*, Felix Akpojene Ogbo*, Takayoshi Ohkubo*, Foluke Adetola Ojelabi*, Pedro R Olivares*, Bolajoko Olubukunola Olusanya*, Jacob Olusegun Olusanya*, John Nelson Opio*, Eyal Oren*, Alberto Ortiz*, Majdi Osman*, Erika Ota*, Raziye Ozdemir*, Mahesh PA*, Amanda Pain*, Jeyaraj D Pandian*, Puspa Raj Pant*, Christina Papachristou*, Eun-Kee Park*, Jae-Hyun Park*, Charles D Parry*, Mahboubeh Parsaeian*, Angel J Paternina Caicedo*, Scott B Patten*, George C Patton*, Vinod K Paul*, Neil Pearce*, João Mário Pedro*, Ljiljana Pejin Stokic*, David M Pereira*, Norberto Perico*, Konrad Pesudovs*, Max Petzold*, Michael Robert Phillips*, Frédéric B Piel*, Julian David Pillay*, Dietrich Plass*, James A Platts-Mills*, Suzanne Polinder*, C Arden Pope*, Svetlana Popova*, Richie G Poulton*, Farshad Pourmalek*, Dorairaj Prabhakaran*, Mostafa Qorbani*, Justice Quame-Amaglo*, D Alex Quistberg*, Anwar Rafay*, Kazem Rahimi*, Vafa Rahimi-Movaghar*, Mahfuzar Rahman*, Mohammad Hifz Ur Rahman*, Sajjad Ur Rahman*, Rajesh Kumar Rai*, Zhale Rajavi*, Sasa Rajsic*, Murugesan Raju*, Ivo Rakovac*, Saleem M Rana*, Chhabi L Ranabhat*, Thara Rangaswamy*, Puja Rao*, Sowmya R Rao*, Amany H Refaat*, Jürgen Rehm*, Marissa B Reitsma*, Giuseppe Remuzzi*, Serge Resnikoff*, Antonio L Ribeiro*, Stefano Ricci*, Maria Jesus Rios Blancas*, Bayard Roberts*, Anna Roca*, David Rojas-Rueda*, Luca Ronfani*, Gholamreza Roshandel*, Dietrich Rothenbacher*, Ambuj Roy*, Nawal K Roy*, George Mugambage Ruhago*, Rajesh Sagar*, Sukanta Saha*, Ramesh Sahathevan*, Muhammad Muhammad Saleh*, Juan R Sanabria*, Maria Dolores Sanchez-Niño*, Lidia Sanchez-Riera*, Itamar S Santos*, Rodrigo Sarmiento-Suarez*, Benn Sartorius*, Maheswar Satpathy*, Miloje Savic*, Monika Sawhney*, Michael P Schaub*, Maria Inês Schmidt*, Ione J C Schneider*, Ben Schöttker*, Aletta E Schutte*, David C Schwebel*, Soraya Seedat*, Sadaf G Sepanlou*, Edson E Servan-Mori*, Katya A Shackelford*, Gavin Shaddick*, Amira Shaheen*, Saeid Shahraz*, Masood Ali Shaikh*, Marina Shakh-Nazarova*, Rajesh Sharma*, Jun She*, Sara Sheikhbahaei*, Jiabin Shen*, Ziyan Shen*, Donald S Shepard*, Kevin N Sheth*, Balakrishna P Shetty*, Peilin Shi*, Kenji Shibuya*, Min-Jeong Shin*, Rahman Shiri*, Ivy Shiue*, Mark G Shrime*, Inga Dora Sigfusdottir*, Donald H Silberberg*, Diego Augusto Santos Silva*, Dayane Gabriele Alves Silveira*, Jonathan I Silverberg*, Edgar P Simard*, Abhishek Singh*, Gitanjali M Singh*, Jasvinder A Singh*, Om Prakash Singh*, Prashant Kumar Singh*, Virendra Singh*, Samir Soneji*, Kjetil Søreide*, Joan B Soriano*, Luciano A Sposato*, Chandrashekhar T Sreeramareddy*, Vasiliki Stathopoulou*, Dan J Stein*, Murray B Stein*, Saverio Stranges*, Konstantinos Stroumpoulis*, Bruno F Sunguya*, Patrick Sur*, Soumya Swaminathan*, Bryan L Sykes*, Cassandra E I Szoeke*, Rafael Tabarés-Seisdedos*, Karen M Tabb*, Ken Takahashi*, Jukka S Takala*, Roberto Tchio Talongwa*, Nikhil Tandon*, Mohammad Tavakkoli*, Bineyam Taye*, Hugh R Taylor*, Braden J Te Ao*, Bemnet Amare Tedla*, Worku Mekonnen Tefera*, Margreet Ten Have*, Abdullah Sulieman Terkawi*, Fisaha Haile Tesfay*, Gizachew Assefa Tessema*, Alan J Thomson*, Andrew L Thorne-Lyman*, Amanda G Thrift*, George D Thurston*, Taavi Tillmann*, David L Tirschwell*, Marcello Tonelli*, Roman Topor-Madry*, Fotis Topouzis*, Jeffrey Allen Towbin*, Jefferson Traebert*, Bach Xuan Tran*, Thomas Truelsen*, Ulises Trujillo*, Abera Kenay Tura*, Emin Murat Tuzcu*, Uche S Uchendu*, Kingsley N Ukwaja*, Eduardo A Undurraga*, Olalekan A Uthman*, Rita Van Dingenen*, Aaron van Donkelaar*, Tommi Vasankari*, Ana Maria Nogales Vasconcelos*, Narayanaswamy Venketasubramanian*, Ramesh Vidavalur*, Lakshmi Vijayakumar*, Salvador Villalpando*, Francesco S Violante*, Vasiliy Victorovich Vlassov*, Joseph A Wagner*, Gregory R Wagner*, Mitchell T Wallin*, Linhong Wang*, David A Watkins*, Scott Weichenthal*, Elisabete Weiderpass*, Robert G Weintraub*, Andrea Werdecker*, Ronny Westerman*, Richard A White*, Tissa Wijeratne*, James D Wilkinson*, Hywel C Williams*, Charles Shey Wiysonge*, Solomon Meseret Woldeyohannes*, Charles D A Wolfe*, Sungho Won*, John Q Wong*, Anthony D Woolf*, Denis Xavier*, Qingyang Xiao*, Gelin Xu*, Bereket Yakob*, Ayalnesh Zemene Yalew*, Lijing L Yan*, Yuichiro Yano*, Mehdi Yaseri*, Pengpeng Ye*, Henock Gebremedhin Yebyo*, Paul Yip*, Biruck Desalegn Yirsaw*, Naohiro Yonemoto*, Gerald Yonga*, Mustafa Z Younis*, Shicheng Yu*, Zoubida Zaidi*, Maysaa El Sayed Zaki*, Faiez Zannad*, Diego E Zavala*, Hajo Zeeb*, Berihun M Zeleke*, Hao Zhang*, Sanjay Zodpey*, David Zonies*, Liesl Joanna Zuhlke*, Theo Vos†, Alan D Lopez†, Christopher J L Murray†.
*Authors listed alphabetically. †Joint senior authors.
Affiliations
Institute for Health Metrics and Evaluation (H Wang PhD, Prof M Naghavi PhD, C Allen BA, R M Barber BS, A Carter BS, D C Casey BA, F J Charlson PhD, A Z Chen BS, M M Coates MPH, M Coggeshall BA, Prof L Dandona MD, D J Dicker BS, H E Erskine PhD, A J Ferrari PhD, C Fitzmaurice MD, K Foreman PhD, M H Forouzanfar PhD, M S Fraser BA, N Fullman MPH, E M Goldberg BS, N Graetz MPH, J A Haagsma PhD, Prof S I Hay DSc, C Huynh BA, C O Johnson PhD, N J Kassebaum MD, X R Kulikoff BA, M Kutz BS, H H Kyu PhD, H J Larson PhD, J Leung PhD, Prof S S Lim PhD, M Lind BS, Prof R Lozano MD, N Marquez BS, J Mikesell BS, Prof A H Mokdad PhD, M D Mooney BS, G Nguyen MPH, E Nsoesie PhD, D M Pigott DPhil, C Pinho BA, G A Roth MD, L Sandar BS, N Silpakit BS, A Sligar MPH, R J D Sorensen MPH, J Stanaway PhD, C Steiner MPH, S Teeple BA, B A Thomas MD, C Troeger MPH, A VanderZanden MSc, Prof S E Vollset DrPH, V Wanga MS, Prof H A Whiteford PhD, T Wolock MPH, L Zoeckler BA, T Achoki MD, A Afshin MD, L T Alexander BA, G M Anderson MSEE, B Bell MLIS, S Biryukov BS, J D Blore PhD, A Brown MA, J Brown MAIS, K Cercy BS, A Chew ND, A J Cohen DSc, F Daoud BS, E Dossou BS, K Estep MPA, A Flaxman PhD, J Friedman BA, J Frostad MPH, W W Godwin BS, J Hancock MLS, L Kemmer PhD, I A Khalil MD, J Leung PhD, P Y Liu BA, F Masiye PhD, A Millear BA, M Mirarefin MPH, A Misganaw PhD, M Moradi-Lakeh MD, K Morgan MLIS, M Ng PhD, A Pain MPH, J Quame-Amaglo MA, P Rao MPH, M B Reitsma BS, K A Shackelford BA, P Sur BA, J A Wagner BS, Prof T Vos PhD, Prof A D Lopez PhD, Prof C J L Murray DPhil), Harborview/UW Medicine (R G Ellenbogen MD), Harborview Injury Prevention and Research Center (C N Mock PhD, D A Quistberg PhD), Department of Anesthesiology & Pain Medicine (D A Quistberg PhD), University of Washington, Seattle, WA, USA (Prof B O Anderson MD, C D Blosser MD, N D Futran MD, S R Heckbert MD, P N Jensen PhD, T J Montine PhD, D L Tirschwell MD, D A Watkins MD); Centre of Excellence in Women and Child Health (Prof Z A Bhutta PhD), Aga Khan University, Karachi, Pakistan (M I Nisar MSc); Centre for Global Child Health, The Hospital for Sick Children, Toronto, ON, Canada (Prof Z A Bhutta PhD, N Akseer MSc); School of Public Health (F J Charlson PhD, H E Erskine PhD, A J Ferrari PhD, Prof H A Whiteford PhD, N K M Alam MPH, L D Knibbs PhD, J Leung PhD), School of Dentistry (Prof R Lalloo PhD), University of Queensland, Brisbane, QLD, Australia (H N Gouda PhD, Prof J J McGrath MD); Queensland Centre for Mental Health Research, Brisbane, QLD, Australia (F J Charlson PhD, H E Erskine PhD, Prof H A Whiteford PhD, J Leung PhD); Centre for Control of Chronic Conditions (P Jeemon PhD), Public Health Foundation of India, New Delhi, India (Prof L Dandona MD, R Dandona PhD, G A Kumar PhD); Department of Zoology (P W Gething PhD), NIHR Musculoskeletal Biomedical Research Centre (Prof C Cooper FMedSci), Clinical Trial Service Unit (S C Darby PhD), Nuffield Department of Medicine (A Deribew PhD), Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery (Prof S I HayDSc), University of Oxford, Oxford, UK (R Ali FRCP, D A Bennett PhD, Prof V Jha DM, K Rahimi DM); Department of Public Health, Erasmus MC, University Medical Center, Rotterdam, Netherlands (J A Haagsma PhD); Centre for Research & Action in Public Health, Faculty of Health, University of Canberra, Canberra, ACT, Australia (Y Kinfu PhD); Department of Infectious Disease Epidemiology (H J Larson PhD), London School of Hygiene & Tropical Medicine, London, UK (I D A Faghmous MPH, S M Langan PhD, Prof M McKee DSc, Prof G V S Murthy MD, Prof N Pearce PhD, B Roberts PhD); National Institute of Public Health, Cuernavaca, Mexico (Prof R Lozano MD, I R Campos-Nonato PhD, J C Campuzano PhD, H Gomez-Dantes MSc, I B Heredia-Pi PhD, F Mejia-Rodriguez MD, J C Montañez Hernandez MSc, P Montero MS, M J Rios Blancas MPH, Prof E E Servan-Mori MSc, S Villalpando PhD); National Center for Chronic and Noncommunicable Disease Control and Prevention (L Duan MD, S Liu PhD, Prof L Wang MD, P Ye MPH), Chinese Center for Disease Control, Beijing, China (Prof X Liang MD, Prof S Yu PhD); Center for Translation Research and Implementation Science, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA (G A Mensah MD); Department of Global Health and Population (Prof J A Salomon PhD), Department of Nutrition (A L Thorne-Lyman ScD), Harvard T H Chan School of Public Health (O N Ajala MD, Prof T Bärnighausen MD, I R Campos-Nonato PhD, E L Ding ScD, M S Farvid PhD, G R Wagner MD), Department of Epidemiology, Channing Division of Network Medicine (P James ScD), Brigham & Women's Hospital (P James ScD), Harvard Medical School (M Osman MD, M G Shrime MD), Harvard University, Boston, MA, USA (J R A Fitchett MD); Centre for Disease Burden (Prof S E Vollset DrPH, A K Knudsen PhD), Norwegian Institute of Public Health, Oslo, Norway (C L Ellingsen MD, N H Krog PhD, M Savic PhD); Department of Global Public Health and Primary Care (Prof S E Vollset DrPH, A K Knudsen PhD), University of Bergen, Bergen, Norway (A D Hailu MPH, Prof O F Norheim PhD); Jimma University, Jimma, Ethiopia (K H Abate MS, T T Gebrehiwot MPH, A T Gebremedhin MPH); La Sapienza, University of Rome, Rome, Italy (C Abbafati PhD); Virginia Tech, Blacksburg, VA, USA (Prof K M Abbas PhD); Department of Neurology, Cairo University, Cairo, Egypt (Prof F Abd-Allah MD); School of Public Health, College of Health Sciences (S F Abera MSc), School of Public Health (Y A Melaku MPH), College of Health Sciences (F H Tesfay MPH), Mekelle University, Mekelle, Ethiopia (Prof G Y Abyu MS, A F Aregay MS, B D Betsu MS, A A Gebru MPH, G B Hailu MSc, A Z Yalew MS, H G Yebyo MS); Kilte Awlaelo-Health and Demographic Surveillance Site, Mekelle, Ethiopia (S F Abera MSc); Food Security and Institute for Biological Chemistry and Nutrition, University of Hohenheim, Stuttgart, Germany (S F Abera MSc); Federal University of Minas Gerais, Belo Horizonte, Brazil (D M X Abreu PhD, Prof E B Franca PhD); Infectious Disease Epidemiology Group, Weill Cornell Medical College in Qatar, Doha, Qatar (L J Abu-Raddad PhD); Public Health Promotion Alliance, Osogbo, Nigeria (A L Adelekan MPH); University of Ibadan, Ibadan, Nigeria (A L Adelekan MPH, R O Akinyemi PhD, F A Ojelabi MPH); University of Basel, Basel, Switzerland (Z Ademi PhD, T Fürst PhD); Department of Paediatrics (P Azzopardi MEpi), The Peter Doherty Institute for Infection and Immunity (Prof B C Cowie PhD, K B Gibney FRACP, J H MacLachlan MS), Department of Medicine (A Meretoja PhD), Murdoch Childrens Research Institute (K Alam PhD, P Azzopardi MEpi, R Borschmann PhD, S M Colquhoun PhD, Prof G C Patton MD, R G Weintraub MBBS), Institute of Health and Ageing (Prof C E I Szoeke PhD), Center for Youth Mental Health (L Vijayakumar PhD), Melbourne School of Population and Global Health (Prof A D Lopez PhD), The University of Melbourne, Melbourne, VIC, Australia (Z Ademi PhD, K Alam PhD, M A Bohensky PhD, R Borschmann PhD, S M Colquhoun PhD, Prof H R Taylor AC, R G Weintraub MBBS, Prof T Wijeratne MD); Association Ivoirienne pour le Bien-Être Familial, Abidjan, Côte d'Ivoire (A K Adou MD); University of Extremadura, Cáceres, Spain (Prof J C Adsuar PhD); Direction du District Sanitaire de Haho, Notse, Togo (K A Afanvi MD); Faculte des Sciences de Sante, Universite de Lome, Lome, Togo (K A Afanvi MD); Institution of Public Health Sciences, Stockholm, Sweden (E E Agardh PhD); Dalla Lana School of Public Health (N Akseer MSc, Prof J Rehm PhD), Department of Nutritional Sciences, Faculty of Medicine (A Badawi PhD), Institute of Health Policy, Management and Evaluation (M P Lindsay PhD), Centre for Addiction and Mental Health (S Popova PhD), University of Toronto, Toronto, ON, Canada (A Agarwal BHSc); McMaster University, Hamilton, ON, Canada (A Agarwal BHSc); CSIR Institute of Genomics and Integrative Biology, Delhi, India (A Agrawal PhD); Department of Internal Medicine (A Agrawal PhD), Baylor College of Medicine, Houston, TX, USA (P J Hotez PhD); Department of Clinical Sciences Lund, Orthopedics, Clinical Epidemiology Unit (A Ahmad Kiadaliri PhD), Skane University Hospital, Department of Clinical Sciences Lund (Prof B Norrving PhD), Lund University, Lund, Sweden; Health Services Management Research Center, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran (A Ahmad Kiadaliri PhD); University of Pittsburgh Medical Center, McKeesport, PA, USA (O N Ajala MD); University of Rhode Island, Kingston, RI, USA (A S Akanda PhD); Newcastle University, Newcastle upon Tyne, UK (R O Akinyemi PhD); Department of Epidemiology (T F Akinyemiju PhD), University of Alabama at Birmingham, Birmingham, AL, USA (D C Schwebel PhD, J A Singh MD); Baghdad College of Medicine, Baghdad, Iraq (F H Al Lami PhD); University of Sheffield, Sheffield, UK (S Alabed MS); Washington University in Saint Louis, St Louis, MO, USA (Z Al-Aly MD); Sydney School of Public Health (Prof T R Driscoll PhD), The University of Sydney, Sydney, NSW, Australia (K Alam PhD, Prof A H Kemp PhD, J Leigh PhD, A B Mekonnen MS); Queensland Health, Herston, QLD, Australia (N K M Alam MPH); Ministry of Health, Al Khuwair, Oman (D Alasfoor MSc); King Saud University, Riyadh, Saudi Arabia (S F Aldhahri MD, K A Altirkawi MD); Department of Anesthesiology (A S Terkawi MD), King Fahad Medical City, Riyadh, Saudi Arabia (S F Aldhahri MD); Centre for Public Health Data Science, Institute of Health Informatics (R W Aldridge PhD), Farr Institute of Health Informatics Research (R W Aldridge PhD, A Banerjee DPhil), Institute for Global Health (R W Aldridge PhD), Department of Epidemiology and Public Health (T Tillmann MSc), University College London, London, UK; Department of Preventive and Social Medicine, Faculty of Medicine (M A Alegretti MD), School of Medicine (A V Aleman MD), Faculty of Medicine (F Cavalleri BS), University of the Republic, Montevideo, Uruguay (V Colistro MSc); Debre Markos University, Debre Markos, Ethiopia (Z A Alemu MPH); King Abdullah Bin Abdulaziz University Hospital, Riyadh, Saudi Arabia (S Alhabib PhD); Luxembourg Institute of Health (LIH), Strassen, Luxembourg (A Alkerwi PhD); School of Public Health, University of Lorraine, Nancy, France (Prof F Alla PhD); Department of Public Health Sciences (P Allebeck PhD), Department of Clinical Science, Intervention and Technology (Prof J J Carrero PhD), Department of Neurobiology, Care Sciences and Society (NVS) (S Fereshtehnejad PhD), Department of Medical Epidemiology and Biostatistics (E Weiderpass PhD), Karolinska Institutet, Stockholm, Sweden (R Havmoeller PhD); Ministry of Health, Jeddah, Saudi Arabia (R Al-Raddadi PhD); Charité Universitätsmedizin, Berlin, Germany (U Alsharif MPH); Spanish Observatory on Drugs, Government Delegation for the National Plan on Drugs, Ministry of Health, Social Policy and Equality, Madrid, Spain (E Alvarez Martin PhD); Universidad de Cartagena, Cartagena de Indias, Colombia (Prof N Alvis-Guzman PhD); School of Medicine (A T Amare MPH, Y A Melaku MPH), University of Adelaide, Adelaide, SA, Australia (L G Ciobanu MS, G A Tessema MPH); College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia (A T Amare MPH); University of Cape Coast, Cape Coast, Ghana (A K Amegah PhD, A A Kudom PhD); National Hospital, Abuja, Nigeria (Prof E A Ameh MBBS); Environmental Health Research Center, Kurdistan University of Medical Sciences, Sanandaj, Iran (H Amini MSPH); Department of Epidemiology and Public Health (H Amini MSPH, T Fürst PhD), Swiss Tropical and Public Health Institute, Basel, Switzerland (C K Karema MSc); Ministry of Public Health, Beirut, Lebanon (W Ammar PhD, H L Harb MPH); Oregon Health & Science University, Portland, OR, USA (S M Amrock MD); Center for Sensory-Motor Interaction, Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark (H H Andersen MSc); Department of Health Policy and Administration, College of Public Health (C A T Antonio MD), College of Public Health (E J A Faraon MD), University of the Philippines Manila, Manila, Philippines; Department of Medical Sciences, Uppsala University, Uppsala, Sweden (Prof J Ärnlöv PhD, Prof A Larsson PhD); Dalarna University, Falun, Sweden (Prof J Ärnlöv PhD); School of Medicine, Institute of Microbiology and Immunology (Prof V S Arsic Arsenijevic PhD), Faculty of Medicine (A Barac PhD), University of Belgrade, Belgrade, Serbia; University Children Hospital, Belgrade, Serbia (Prof V S Arsic Arsenijevic PhD); University of Manitoba, Winnipeg, MB, Canada (A Artaman PhD); Department of Medical Emergency, School of Paramedic, Qom University of Medical Sciences, Qom, Iran (H Asayesh PhD); South Asian Public Health Forum, Islamabad, Pakistan (R J Asghar MD); Graduate Institute of Biomedical Informatics, Taipei Medical University, Taipei, Taiwan (S Atique MS); Institut de Recherche Clinique du Bénin, Cotonou, Benin (E F G A Avokpaho MPH); Laboratoire d'Etudes et de Recherche-Action en Santé (LERAS Afrique), Parakou, Benin (E F G A Avokpaho MPH, F G Gankpé MD); Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India (A Awasthi MSc); Wardliparingga Aboriginal Research Unit, South Australian Health and Medical Research Institute, Adelaide, SA, Australia (P Azzopardi MEpi); School of Health Sciences, University of Management and Technology, Lahore, Pakistan (U Bacha PhD); Public Health Agency of Canada, Toronto, ON, Canada (A Badawi PhD); INECO Neurociencias, Rosario, Argentina (M C Bahit MD); Department of Environmental Health Engineering, Sri Ramachandra University, Chennai, India (K Balakrishnan PhD); School of Psychology, University of Auckland, Auckland, New Zealand (S L Barker-Collo PhD); Africa Health Research Institute, Mtubatuba, South Africa (Prof T Bärnighausen MD); Institute of Public Health, Heidelberg University, Heidelberg, Germany (Prof T Bärnighausen MD, S Mohammed PhD); Department of Occupational and Environmental Health (Prof L Barregard MD), Health Metrics Unit (Prof M Petzold PhD), University of Gothenburg, Gothenburg, Sweden; Department of Industrial Engineering, School of Engineering, Pontificia Universidad Javeriana, Bogotá, Colombia (L H Barrero ScD); School of Health Sciences, University of Canterbury, Christchurch, New Zealand (A Basu PhD); Stanford University, Stanford, CA, USA (S Basu PhD); Jhpiego-Ethiopia, Addis Ababa, Ethiopia (Y T Bayou PhD); College of Medicine, Charles R Drew University of Medicine and Science, Los Angeles, CA, USA (Prof S Bazargan-Hejazi PhD); David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA (Prof S Bazargan-Hejazi PhD); Kermanshah University of Medical Science, Kermanshah, Iran (Prof S Bazargan-Hejazi PhD); Oxford University, Ho Chi Minh City, Vietnam (J Beardsley MBChB); College of Public Health and Tropical Medicine, Jazan, Saudi Arabia (N Bedi MD); IRCCS - Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy (E Beghi MD); School of Public Health (K Deribe MPH, A D Hailu MPH), Addis Ababa University, Addis Ababa, Ethiopia (H A Belay PhD, A Z Giref PhD, D Haile MPH, T Jibat MS, W A A Manamo MS, W M Tefera MPH, B D Yirsaw PhD); School of Medicine (K N Sheth MD), Yale University, New Haven, CT, USA (Prof M L Bell PhD, Prof B J Biroscak PhD); University of Alberta, Edmonton, AB, Canada (A K Bello PhD); Internal Medicine Department (Prof I S Santos PhD), University of São Paulo, São Paulo, Brazil (I M Bensenor PhD, Prof P A Lotufo DrPH); Debre Berhane University, Debre Berhan, Ethiopia (A Berhane PhD); Division of Health and Social Care Research (Prof C D A Wolfe MD), King's College London, London, UK (E Bernabé PhD); Haramaya University, Harar, Ethiopia (A S Beyene MPH, M D Gishu MS); Queen Elizabeth Hospital Birmingham, Birmingham, UK (N Bhala DPhil); University of Otago Medical School, Wellington, New Zealand (N Bhala DPhil); Postgraduate Institute of Medical Education and Research, Chandigarh, India (A Bhalla MD); Independent Public Health Consultants, Addis Ababa, Ethiopia (S Biadgilign MPH); Department of Nephrology Issues of Transplanted Kidney, Academician V I Shumakov Federal Research Center of Transplantology and Artificial Organs, Moscow, Russia (B Bikbov MD); University of Iowa Hospitals and Clinics, Iowa City, IA, USA (A A Bin Abdulhak MD); University of South Florida, Tampa, FL, USA (Prof B J Biroscak PhD); Department of Community Medicine (Prof E Bjertness PhD), University of Oslo, Oslo, Norway (A S Htet MPhil); World Bank, Washington, DC, USA (D Bose PhD); Vision & Eye Research Unit, Anglia Ruskin University, Cambridge, UK (Prof R R A Bourne FRCOphth); Danube-University Krems, Krems, Austria (Prof M Brainin PhD); Cambridge Institute of Public Health, Cambridge, UK (Prof C E G Brayne MD); Faculty of Health Sciences and Social Work, Department of Public Health, Trnava University, Trnava, Slovakia (A Brazinova PhD, M Majdan PhD); International Neurotrama Research Organization, Vienna, Austria (A Brazinova PhD); College of Medicine (J Shen PhD), The Ohio State University, Columbus, OH, USA (Prof N J K Breitborde PhD); German Cancer Research Center, Heidelberg, Germany (Prof H Brenner MD, B Schöttker MPH); Mayo Clinic, Rochester, MN, USA (J D Brewer MD); University of Leicester, Leicester, UK (Prof T S Brugha MD); University of California, San Francisco, San Francisco, CA, USA (G C Buckle MD, R A Gosselin MD); Al Shifa Trust Eye Hospital, Rawalpindi, Pakistan (Z A Butt PhD); National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT, Australia (B Calabria PhD, A Lal PhD, Prof R M Lucas PhD); National Drug and Alcohol Research Centre (Prof L Degenhardt PhD), Brien Holden Vision Institute (Prof S Resnikoff MD), School of Optometry and Vision Science (Prof S Resnikoff MD), University of New South Wales, Sydney, NSW, Australia (B Calabria PhD); Telethon Kids Institute, the University of Western Australia, Princess Margaret Hospital for Children, Subiaco, WA, Australia (Prof J R Carapetis PhD); Metropolitan Autonomous University, Mexico City, Mexico (R Cárdenas ScD); University at Albany, Rensselaer, NY, USA (Prof D O Carpenter MD); Colombian National Health Observatory, Instituto Nacional de Salud, Bogotá, Colombia (C A Castañeda-Orjuela MSc); Epidemiology and Public Health Evaluation Group, Public Health Department, Universidad Nacional de Colombia, Bogotá, Colombia (C A Castañeda-Orjuela MSc); Caja Costarricense de Seguro Social, San Jose, Costa Rica (Prof J Castillo Rivas MPH); Universidad de Costa Rica, San Pedro, Montes de Oca, Costa Rica (Prof J Castillo Rivas MPH); Department of Medicine, University of Valencia/INCLIVA Health Research Institute and CIBERSAM, Valencia, Spain (F Catalá-López PhD); Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada (F Catalá-López PhD); Albany Medical College, Albany, NY, USA (J Cerda MD); Cancer Institute, Chinese Academy of Medical Sciences, Beijing, China (W Chen PhD); Clinical Governance Unit, Gold Coast Health, Southport, QLD, Australia (P P Chiang PhD); Crowd Watch Africa, Lusaka, Zambia (M Chibalabala BS); National Center for Child Health and Development, Setagaya, Japan (C E Chibueze PhD, R Mori PhD); Department of Environmental Epidemiology (O Chimed-Ochir MPH), Department of Health Development, Institute of Industrial Ecological Sciences (Y Jiang PhD), Institute of Industrial Ecological Sciences, Department of Environmental Epidemiology (Prof K Takahashi MD), University of Occupational and Environmental Health, Kitakyushu, Japan; University of Zambia, Lusaka, Zambia (V H Chisumpa MPhil, C C Mapoma PhD, F Masiye PhD); University of Witwatersrand, Johannesburg, South Africa (V H Chisumpa MPhil); Seoul National University Medical Library, Seoul, South Korea (J J Choi PhD); Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (R Chowdhury PhD); Bispebjerg University Hospital, Copenhagen, Denmark (Prof H Christensen DMSCi); Christian Medical College, Vellore, India (Prof D J Christopher MD); University of Salerno, Baronissi, Italy (Prof M Cirillo MD); Health Effects Institute, Boston, MA, USA (A J Cohen DSc); Ministerio de Salud Pública, Montevideo, Uruguay (V Colistro MSc); UNICEM, Montevideo, Uruguay (M Colomar MSc); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (Prof C Cooper FMedSci); NIHR Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, UK (Prof C Cooper FMedSci); Mayo Clinic, Jacksonville, FL, USA (L T Cooper MD); WHO Collaborating Centre for Viral Hepatitis, Victorian Infectious Diseases Reference Laboratory, Melbourne, VIC, Australia (Prof B C Cowie PhD); Centre for International Health, Dunedin School of Medicine (Prof J A Crump MD), University of Otago, Dunedin, New Zealand (Prof R G Poulton PhD); Building and Road Research Institute, Kumasi, Ghana (J Damsere-Derry MPH); Walden University, Minneapolis, MN, USA (Prof H Danawi PhD, Prof A H Refaat PhD); Guy's and St Thomas' NHS Foundation Trust, London, UK (Prof P I Dargan FRCP); i3S - Instituto de Investigação e Inovação em Saúde and INEB - Instituto de Engenharia Biomédica (J das Neves PhD), Faculty of Medicine (J Massano MD), EPIUnit - Institute of Public Health (J M Pedro MS), University of Porto, Porto, Portugal; Wellcome Trust Brighton & Sussex Centre for Global Health Research, Brighton, UK (Prof G Davey MD); Public Health England, London, UK (Prof A C Davis PhD, F Greaves PhD, Prof J N Newton FRCP); University of Medicine and Pharmacy Bucharest, Bucharest, Romania (D V Davitoiu PhD); National Institute of Public Health, Mexico City, Mexico (E F de Castro PhD); School of Public Health (P de Jager FCPHM(SA)), University of the Witwatersrand, Johannesburg, South Africa (Prof M Petzold PhD); National Health Laboratory Service, National Institute for Occupational Health, Johannesburg, South Africa (P de Jager FCPHM[SA]); Griffith University, Brisbane, QLD, Australia (Prof D De Leo DSc); University of Colorado School of Medicine and the Colorado School of Public Health, Aurora, CO, USA (R P Dellavalle MD); Brighton and Sussex Medical School, Brighton, UK (K Deribe MPH); KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya (A Deribew PhD); Department of Community Medicine, Faculty of Medicine, University of Peradeniya, Peradeniya, Sri Lanka (S D Dharmaratne MD); Centre for Control of Chronic Conditions (P Jeemon PhD), Public Health Foundation of India, Gurgaon, India (P K Dhillon PhD, P Ganguly MD, D K Lal MD, Prof S Zodpey PhD); Hospital de la Santa Creu i Sant Pau, Barcelona, Spain (C Diaz-Torné MD); Universidade do Estado de Santa Catarina, Florianópolis, Brazil (Prof K P B dos Santos MA); International Institute for Population Sciences, Mumbai, India (M Dubey MPhil, M H U Rahman MPhil, A Singh PhD); Federal University of Rio Grande do Sul, Porto Alegre, Brazil (B B Duncan PhD, C Kieling MD, Prof M I Schmidt MD); University of North Carolina, Chapel Hill, NC, USA (B B Duncan PhD); Eijkman-Oxford Clinical Research Unit, Jakarta, Indonesia (I Elyazar PhD); Arba Minch University, Arba Minch, Ethiopia (A Y Endries MPH); The Institute of Social and Economic Studies of Population, Russian Academy of Sciences, Moscow, Russia (Prof S P Ermakov DSc); Federal Research Institute for Health Organization and Informatics, Ministry of Health of the Russian Federation, Moscow, Russia (Prof S P Ermakov DSc); Ministry of Health and Medical Education, Tehran, Iran (B Eshrati PhD); Arak University of Medical Sciences, Arak, Iran (B Eshrati PhD); Endocrinology and Metabolism Research Center (Prof A Esteghamati MD, N Hafezi-Nejad MD), Digestive Diseases Research Institute (S Fahimi PhD, Prof R Malekzadeh MD, G Roshandel PhD, S G Sepanlou PhD), Non-Communicable Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute (F Farzadfar MD, A Kasaeian PhD, M Parsaeian PhD), Multiple Sclerosis Research Center, Neuroscience Institute (P Heydarpour MD), Hematology-Oncology and Stem Cell Transplantation Research Center (A Kasaeian PhD), Department of Epidemiology and Biostatistics, School of Public Health (M Parsaeian PhD), Sina Trauma and Surgery Research Center (Prof V Rahimi-Movaghar MD), Endocrinology and Metabolism Research Centre (S Sheikhbahaei MD), Tehran University of Medical Sciences, Tehran, Iran (M Yaseri PhD); Department of Health, Manila, Philippines (E J A Faraon MD); University of Louisville, Louisville, KY, USA (T A Farid MD, A R Khan MD); DGS Directorate General of Health, Lisboa, Portugal (C S E S Farinha MSc); Universidade Aberta, Lisboa, Portugal (C S E S Farinha MSc); Federal University of Sergipe, Aracaju, Brazil (Prof A Faro PhD); Harvard/MGH Center on Genomics, Vulnerable Populations, and Health Disparities, Mongan Institute for Health Policy, Massachusetts General Hospital, Boston, MA, USA (M S Farvid PhD); National Institute for Stroke and Applied Neurosciences (V L Feigin PhD), Auckland University of Technology, Auckland, New Zealand (B J Te Ao MPH); Institute of Education and Sciences, German Hospital Oswaldo Cruz, São Paulo, Brazil (Prof J G Fernandes PhD); Centre for Experimental Medicine & Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine & Dentistry, Queen Mary University of London, London, UK (J C Fernandes PhD); Bielefeld University, Bielefeld, Germany (F Fischer MPH); Institute of Gerontology, Academy of Medical Science, Kyiv, Ukraine (N Foigt PhD); Alzheimer Scotland Dementia Research Centre (I Shiue PhD), University of Edinburgh, Edinburgh, UK (Prof F G R Fowkes PhD); James Cook University, Townsville, QLD, Australia (R C Franklin PhD); Department of Infectious Disease Epidemiology (T Fürst PhD), Department of Epidemiology and Biostatistics (F B Piel PhD), Imperial College London, London, UK (F Greaves PhD, Prof A Majeed MD); University of Tasmania, Hobart, TAS, Australia (S L Gall PhD); National Center for Disease Control and Public Health, Tbilisi, Georgia (K Gambashidze MS, A Gamkrelidze PhD, M Kereselidze PhD, M Shakh-Nazarova MS); Indian Institute of Public Health Gandhinagar, Ahmedabad, India (P Ganguly MD, V J Iyer MPH); CHU Hassan II, Fès, Morocco (F G Gankpé MD); The Task Force for Global Health, Decatur, GA, USA (T Gebre PhD); Ludwig Maximilians University, Munich, Germany (A T Gebremedhin MPH); Kilte Awlaelo Health and Demographic Surveillance System, Mekelle, Ethiopia (A A Gebru MPH, G B Hailu MSc); Division of Human Nutrition (J M Geleijnse PhD), Wageningen University, Wageningen, Netherlands (T Jibat MS); Agence de Medecine Preventive, Paris, France (B D Gessner MD); National Allergy Asthma Bronchitis Institute, Kolkata, India (A G Ghoshal DNB); The Royal Melbourne Hospital, Melbourne, VIC, Australia (K B Gibney FRACP); College of Medicine, Howard University, Washington, DC, USA (R F Gillum MD, A Mehari MD); Graduate School of Medicine (S Gilmour PhD, M Inoue MD), School of Public Health (Prof N Kawakami MD), University of Tokyo, Tokyo, Japan (K Shibuya MD); University Hospital of Dijon, Dijon, France (Prof M Giroud MD); Kersa Health and Demographic Surveillance System, Harar, Ethiopia (M D Gishu MS); Heller School for Social Policy and Management (E Glaser PhD), Brandeis University, Waltham, MA, USA (Y A Halasa MS, Prof D S Shepard PhD, E A Undurraga PhD); University of Massachusetts Boston, Boston, MA, USA (Prof P Gona PhD); Instituto de Investigaciones Cientificas y Servicios de Alta Tecnologia - INDICASAT-AIP, Ciudad del Saber, Panamá (A Goodridge PhD); Department of Health and Social Affairs, Government of the Federated States of Micronesia, Palikir, Federated States of Micronesia (S V Gopalani MPH); University of British Columbia, Vancouver, BC, Canada (C C Gotay PhD, Prof N Kissoon MD, J A Kopec PhD, S Murthy MD, F Pourmalek PhD); Division of Epidemiology, Center for Public Health Sciences (A Goto PhD), National Cancer Center, Tokyo, Japan (M Inoue MD); Departments of Microbiology and Epidemiology & Biostatistics, Saint James School of Medicine, The Quarter, Anguilla (Prof H C Gugnani PhD); West Virginia Bureau for Public Health, Charleston, WV, USA (R Gupta MD); Eternal Heart Care Centre and Research Institute, Jaipur, India (R Gupta PhD); Department of Anthropology, University of Delhi, Delhi, India (V Gupta PhD); National Institute of Psychiatry Ramon de la Fuente, Mexico City, Mexico (R A Gutiérrez PhD); Arabian Gulf University, Manama, Bahrain (Prof R R Hamadeh DPhil); Hamdan Bin Mohammed Smart University, Dubai, United Arab Emirates (S Hamidi PhD); University of New Mexico, Albuquerque, NM, USA (A J Handal PhD); School of Medicine and Pharmacology (Prof G J Hankey MD), University of Western Australia, Fremantle, WA, Australia (Prof P E Norman MD); Harry Perkins Institute of Medical Research, Nedlands, WA, Australia (Prof G J Hankey MD); Western Australian Neuroscience Research Institute, Nedlands, WA, Australia (Prof G J Hankey MD); School of Public Health, Sun Yat-sen University, Guangzhou, China (Prof Y Hao PhD); Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, India (S Harikrishnan DM); Parc Sanitari Sant Joan de Déu - CIBERSAM, Sant Boi de Llobregat (Barcelona), Spain (J M Haro MD); Universitat de Barcelona, Barcelona, Spain (J M Haro MD); National Institute for Public Health and the Environment (RIVM), Bilthoven, Netherlands (H B Hilderink PhD); Department of Psychiatry, University Medical Center Groningen (Prof H W Hoek MD), University of Groningen, Groningen, Netherlands (A K Tura MPH); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA (Prof H W Hoek MD); Simon Fraser University, Burnaby, BC, Canada (Prof R S Hogg PhD); BC Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada (Prof R S Hogg PhD); Nevada Division of Public and Behavioral Health, Department of Health and Human Services, Carson City, NV, USA (M Horino MPH); Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan (N Horita MD); Albert Einstein College of Medicine, Bronx, NY, USA (Prof H D Hosgood PhD); Public Health Division, The Pacific Community, Noumea, New Caledonia (D G Hoy PhD); Department of Epidemiology, Salah Azaiz Institute, Tunis, Tunisia (Prof M Hsairi MD); International Relations Division (A S Htet MPhil), Ministry of Health, Nay Pyi Taw, Myanmar (M M T Htike MPH); Department of Epidemiology and Health Statistics, School of Public Health, Central South University, Changsha, China (G Hu PhD); George Washington University, Washington, DC, USA (Prof C Huang PhD); Cambridge Health Alliance, Cambridge, MA, USA (H Huang MD); CHU La Réunion, Saint-Denis, France (L Huiart PhD); Birzeit University, Birzeit, Palestine (A Husseini PhD); International Agency for Research on Cancer (IARC), Lyon, France (I Huybrechts PhD); Ghent University, Ghent, Belgium (I Huybrechts PhD); Institute for Disease Modeling, Bellevue, WA, USA (G Huynh PhD); Aarhus University, Aarhus, Denmark (K M Iburg PhD); National Institute for Health Development, Tallinn, Estonia (K Innos PhD); MCH Division, USAID - Global Health Bureau, HIDN, Washington, DC, USA (T A Jacobs MD); Department of Global and Community Health, George Mason University, Fairfax, VA, USA (K H Jacobsen PhD); School of Public Health (N Jahanmehr PhD), Ophthalmic Epidemiology Research Center (M Katibeh MD), Ophthalmic Research Center (Prof Z Rajavi MD, M Yaseri PhD), Shahid Beheshti University of Medical Sciences, Tehran, Iran; Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia (Prof M B Jakovljevic PhD); University of Aberdeen, Aberdeen, UK (M Javanbakht PhD); Department of Surgery, Virginia Commonwealth University, Richmond, VA, USA (S P Jayaraman MD); Postgraduate Institute of Medicine, Colombo, Sri Lanka (A U Jayatilleke PhD); Institute of Violence and Injury Prevention, Colombo, Sri Lanka (A U Jayatilleke PhD); Centre for Chronic Disease Control, New Delhi, India (P Jeemon PhD, D Prabhakaran DM); George Institute for Global Health India, New Delhi, India (Prof V Jha DM); Tianjin Centers for Disease Control and Prevention, Tianjin, China (G Jiang MD); Department of Ocular Epidemiology and Visual Health, Institute of Ophthalmology Conde de Valencia, Mexico City, Mexico (A Jimenez-Corona PhD); General Directorate of Epidemiology, Ministry of Health, Mexico City, Mexico (A Jimenez-Corona PhD); Department of Ophthalmology, Medical Faculty Mannheim, Ruprecht-Karls-University Heidelberg, Mannheim, Germany (Prof J B Jonas MD); Centre for Occupational and Environmental Health, New Delhi, India (T K Joshi MS); University College Cork, Cork, Ireland (Z Kabir PhD); CSIR - Indian Institute of Toxicology Research, Lucknow, India (R Kamal MSc, C N Kesavachandran PhD); Zhongshan Hospital (J She MD), Department of Nephrology (Z Shen MD), Department of Nephrology, Zhongshan Hospital (H Zhang PhD), Fudan University, Shanghai, China (H Kan MD); King George's Medical University, Lucknow, India (Prof S Kant MD); Epidemiological and Statistical Methods Research Group, Helmholtz Centre for Infection Research, Braunschweig, Germany (A Karch MD); Hannover-Braunschweig Site, German Center for Infection Research, Braunschweig, Germany (A Karch MD); Quality and Equity Health Care, Kigali, Rwanda (C K Karema MSc); Case Western University Hospitals, Cleveland, OH, USA (C Karimkhani MD); Center for Research in Health and Economics, Universitat Pompeu Fabra, Barcelona, Spain (D Karletsos MS); All India Institute of Medical Sciences, New Delhi, India (Prof G Karthikeyan DM, N Naik DM, V K Paul MD, A Roy DM, R Sagar MD, M Satpathy PhD, Prof N Tandon PhD); Oklahoma State University, Tulsa, OK, USA (A Kaul MD); Institut de recherche de l'hôpital de Monttfort, Ottawa, ON, Canada (J F Kayibanda PhD); Institute of Tropical and Infectious Diseases, Nairobi, Kenya (P N Keiyoro PhD); School of Continuing and Distance Education, Nairobi, Kenya (P N Keiyoro PhD); Farr Institute (Prof R A Lyons MD), Swansea University, Swansea, UK (Prof A H Kemp PhD); Alcohol, Tobacco & Other Drug Research Unit (Prof C D Parry PhD), South African Medical Research Council, Cape Town, South Africa (A P Kengne PhD, R Matzopoulos PhD, Prof C S Wiysonge PhD); School of Public Health and Family Medicine (R Matzopoulos PhD), Department of Psychiatry (Prof D J Stein PhD), University of Cape Town, Cape Town, South Africa (A P Kengne PhD, Prof B M Mayosi DPhil, D A Watkins MD); Assuta Hospitals, Assuta Hashalom, Tel Aviv, Israel (Prof A Keren MD); Jordan University of Science and Technology, Irbid, Jordan (Prof Y S Khader ScD); Health Services Academy, Islamabad, Pakistan (E A Khan MPH); College of Medicine (Prof Y H Khang MD), Graduate School of Public Health (Prof S Won PhD), Seoul National University, Seoul, South Korea; New York Medical College, Valhalla, NY, USA (S Khera MD, M Tavakkoli MD); Executive Board of the Health Ministers' Council for Cooperation Council States, Riyadh, Saudi Arabia (Prof T A M Khoja FRCP); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (C Kieling MD); Department of Health Sciences, Northeastern University, Boston, MA, USA (Prof D Kim DrPH); Southern University College, Skudai, Malaysia (Y J Kim PhD); University of Cincinnati, Cincinnati, OH, USA (B M Kissela MD); Department of Preventive Cardiology, National Cerebral and Cardiovascular Center, Suita, Japan (Y Kokubo PhD); Division of Cardiology (D Kolte MD), Brown University, Providence, RI, USA (Prof S T McGarvey PhD); Center for Community Empowerment, Health Policy and Humanities, NIHRD, Jakarta, Indonesia (S Kosen MD); Sher-i-Kashmir Institue of Medical Sciences, Srinagar, India (Prof P A Koul MD); Research and Development Unit, Parc Sanitari Sant Joan de Deu (CIBERSAM), Barcelona, Spain (A Koyanagi MD); Department of Demography and Public Health Research Institute (Prof B Kuate Defo PhD), Department of Social and Preventive Medicine, School of Public Health (Prof B Kuate Defo PhD), University of Montreal, Montreal, QC, Canada; Institute of Public Health, Hacettepe University, Ankara, Turkey (B Kucuk Bicer PhD); Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands (Prof E J Kuipers PhD); Arkansas State University, State University, AR, USA (V S Kulkarni PhD); School of Medicine (G F Kwan MD), Department of Surgery, School of Medicine (S R Rao PhD), Boston University, Boston, MA, USA; Institute of Health Policy and Development Studies, National Institutes of Health, Manila, Philippines (Prof H Lam PhD); Johns Hopkins Bloomberg School of Public Health (J O Lam PhD), Bloomberg School of Public Health (Prof J B Nachega PhD), Johns Hopkins University, Baltimore, MD, USA (B X Tran PhD); Help Me See, Inc, New York, NY, USA (V C Lansingh PhD); Instituo Mexicano de Oftalmologia, Queretaro, Mexico (V C Lansingh PhD); Komfo Anokye Teaching Hospital, Kumasi, Ghana (D O Laryea MD); Department of Zoology, Lahore College for WomenUniversity, Lahore, Pakistan (A A Latif PhD); Instituto Nacional de Epidemiología “Dr Juan H Jara,” Mar del Plata, Argentina (A E B Lawrynowicz MPH); Tuscany Regional Centre for Occupational Injuries and Diseases, Florence, Italy (M Levi PhD); San Francisco VA Medical Center, San Francisco, CA, USA (Y Li PhD); Heart and Stroke Foundation Canada, Ottawa, ON, Canada (M P Lindsay PhD); School of Medicine, Wayne State University, Detroit, MI, USA (Prof S E Lipshultz MD, Prof J D Wilkinson MD); Children's Hospital of Michigan, Detroit, MI, USA (Prof S E Lipshultz MD); Rollins School of Public Health (E P Simard PhD), Emory University, Atlanta, GA, USA (Prof Y Liu PhD, Prof M R Phillips MD, Q Xiao MPH); UnionHealth Associates, LLC, St Louis, MO, USA (L Lo MD); Alton Mental Health Center, Alton, IL, USA (L Lo MD); University of Bari, Bari, Italy (Prof G Logroscino PhD); Aintree University Hospital National Health Service Foundation Trust, Liverpool, UK (Prof R Lunevicius PhD); School of Medicine, University of Liverpool, Liverpool, UK (Prof R Lunevicius PhD); Ministry of Health Singapore, Singapore, Singapore (S Ma PhD); Saw Swee Hock School of Public Health, National University of Singapore, Singapore, Singapore (S Ma PhD); Public Health Department, Northern Region Health Administration, Porto, Portugal (V M Machado MSc); Royal Children's Hospital, Melbourne, VIC, Australia (M T Mackay MBBS, R G Weintraub MBBS); Mansoura Faculty of Medicine, Mansoura, Egypt (H Magdy Abd El Razek MBBCH); Aswan University Hospital, Aswan Faculty of Medicine, Aswan, Egypt (M Magdy Abd El Razek MBBCh); JSI Research and Training, Harare, Zimbabwe (J Mandisarisa PhD); Technical Standards and Safety Authority, Toronto, ON, Canada (S Mangalam MS); Division of Population and Patient Health, King's College London Dental Institute, London, UK (Prof W Marcenes PhD); Perelman School of Medicine (P A Meaney MD), University of Pennsylvania, Philadelphia, PA, USA (D J Margolis PhD, D H Silberberg MD); Children's National Health System, Washington, DC, USA (G R Martin MD); University Hospital Doctor Peset, University of Valencia, Valencia, Spain (J Martinez-Raga PhD); CEU Cardenal Herrera University, Moncada (Valencia), Spain (J Martinez-Raga PhD); University of the East Ramon Magsaysay Memorial Medical Center, Quezon City, Philippines (M B Marzan MSc); Department of Health Sciences, University of York, York, UK (A J Mason-Jones PhD); Hospital Pedro Hispano/ULS Matosinhos, Matosinhos, Portugal (J Massano MD); Alaska Native Tribal Health Consortium, Anchorage, AK, USA (B J McMahon MD); Children's Hospital of Philadelphia, Philadelphia, PA, USA (P A Meaney MD); Janakpuri Superspecialty Hospital, New Delhi, India (Prof M M Mehndiratta DM); Department of Epidemiology and Biostatistics, Institute of Public Health (S M Woldeyohannes MPH), University of Gondar, Gondar, Ethiopia (A B Mekonnen MS, B A Tedla BS, B M Zeleke MD); University of West Florida, Pensacola, FL, USA (P Memiah PhD); Saudi Ministry of Health, Riyadh, Saudi Arabia (Prof Z A Memish MD); College of Medicine, Alfaisal University, Riyadh, Saudi Arabia (Prof Z A Memish MD); United Nations Population Fund, Lima, Peru (W Mendoza MD); Department of Neurology, Helsinki University Hospital, Helsinki, Finland (A Meretoja PhD); Helsinki University Hospital, Comprehensive Cancer Center, Breast Surgery Unit, Helsinki, Finland (T J Meretoja PhD); Department of Public Health, Faculty of Medicine (T Lallukka PhD), University of Helsinki, Helsinki, Finland (T J Meretoja PhD); Ifakara Health Institute, Bagamoyo, Tanzania (F A Mhimbira MS); Friedman School of Nutrition Science and Policy (R Micha PhD, D Mozaffarian MD), Tufts University, Boston, MA, USA (P Shi PhD, G M Singh PhD); Pacific Institute for Research & Evaluation, Calverton, MD, USA (T R Miller PhD); Centre for Population Health, Curtin University, Perth, WA, Australia (T R Miller PhD); University of Salahaddin, Erbil, Iraq (K A Mohammad PhD); Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran (A Mohammadi PhD); Health Systems and Policy Research Unit, Ahmadu Bello University, Zaria, Nigeria (S Mohammed PhD); Madras Diabetes Research Foundation, Chennai, India (V Mohan DSc); Dr Mohan's Diabetes Specialities Centre, Chennai, India (V Mohan DSc); University of Papua New Guinea, Boroko, Papua New Guinea (Prof G L D Mola DPH, FRANCOG); Institute for Maternal and Child Health, IRCCS “Burlo Garofolo,” Trieste, Italy (L Monasta DSc, M Montico Msc, L Ronfani PhD); Department of Community Medicine, Gastrointestinal and Liver Disease Research Center, Preventive Medicine and Public Health Research Center, Iran University of Medical Sciences, Tehran, Iran (M Moradi-Lakeh MD); International Laboratory for Air Quality and Health, Queensland University of Technology, Brisbane, QLD, Australia (L Morawska PhD); Competence Center Mortality-Follow-Up of the German National Cohort (A Werdecker PhD), Federal Institute for Population Research, Wiesbaden, Germany (Prof U O Mueller PhD, R Westerman PhD); Indian Institute of Public Health (Prof G V S Murthy MD), Public Health Foundation of India, Gurgaon, India (P K Dhillon PhD, P Ganguly MD, D K Lal MD, Prof S Zodpey PhD); School of Medical Sciences, University of Science Malaysia, Kubang Kerian, Malaysia (K I Musa MD); Graduate School of Public Health (Prof J B Nachega PhD), Public Health Dynamics Laboratory (A J Paternina Caicedo MD), University of Pittsburgh, Pittsburgh, PA, USA; Department of Psychiatry (Prof C D Parry PhD), Stellenbosch University, Cape Town, South Africa (Prof J B Nachega PhD, Prof S Seedat PhD, Prof C S Wiysonge PhD); Institute of Epidemiology and Medical Biometry, Ulm University, Ulm, Germany (Prof G Nagel PhD, Prof D Rothenbacher MD); University of KwaZulu-Natal, Durban, South Africa (Prof K S Naidoo PhD, Prof B Sartorius PhD); Azienda Ospedaliera Papa Giovanni XXIII, Bergamo, Italy (Prof L Naldi MD, Prof G Remuzzi MD); Suraj Eye Institute, Nagpur, India (V Nangia MD); Institute for Implementation Science in Population Health, School of Public Health, City University of New York, New York, NY, USA (D Nash PhD); Faculty of Medicine, Fez, Morocco (Prof C Nejjari PhD); School of Health Sciences, University of Tampere, Tampere, Finland (S Neupane PhD); KEMRI Wellcome Trust, Kilifi, Kenya (Prof C R Newton MD); Ministry of Health and Social Welfare, Dar es Salaam, Tanzania (F N Ngalesoni MSc); East African Community Health Research Commission, Kigali, Rwanda (J D Ngirabega PhD); Institute for Global Health Innovations, Duy Tan University, Da Nang, Vietnam (Q L Nguyen MD); Institute For Research, Socio-Economic Development and Communication, Yaoundé, Cameroon (P M Nkamedjie Pete MS); National Institute of Public Health, Saitama, Japan (M Nomura PhD); Makerere University, Kampala, Uganda (L Nyakarahuka MPH); Centre for Health Research, Western Sydney University, Sydney, NSW, Australia (F A Ogbo MPH); Teikyo University School of Medicine, Tokyo, Japan (Prof T Ohkubo MD); Universidad Autonoma de Chile, Talca, Chile (Prof P R Olivares PhD); Center for Healthy Start Initiative, Lagos, Nigeria (B O Olusanya PhD, J O Olusanya MBA); Lira District Local Government, Lira Municipal Council, Uganda (J N Opio MPH); University of Arizona, Tucson, AZ, USA (Prof E Oren PhD); IIS-Fundacion Jimenez Diaz-UAM, Madrid, Spain (Prof A Ortiz PhD); YBank, Cambridge, MA, USA (M Osman MD); St Luke's International University, Tokyo, Japan (E Ota PhD); Karabuk University, Karabuk, Turkey (R Ozdemir PhD); JSS Medical College, JSS University, Mysore, Karnataka, India (Mahesh PA DNB); Christian Medical College Ludhiana, Ludhiana, India (J D Pandian DM); University of the West of England, Bristol, UK (P R Pant PhD); Charité University Medicine Berlin, Berlin, Germany (C Papachristou PhD); Department of Medical Humanities and Social Medicine, College of Medicine, Kosin University, Busan, South Korea (E Park PhD); Department of Social and Preventive Medicine, Samsung Biomedical Research Institute, School of Medicine, Sungkyunkwan University, Suwon, South Korea (Prof J Park MPH); Universidad de Cartagena, Cartagena, Colombia (A J Paternina Caicedo MD); Department of Community Health Sciences (Prof S B Patten PhD), University of Calgary, Calgary, AB, Canada (Prof M Tonelli MD); Health Research Centre of Angola, Caxito, Angola (J M Pedro MS); Economics Institute, Belgrade, Serbia (L Pejin Stokic MSc); REQUIMTE/LAQV, Laboratório de Farmacognosia, Departamento de Química, Faculdade de Farmácia, Universidade do Porto, Porto, Portugal (Prof D M Pereira PhD); IRCCS - Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy (M Cortinovis Biotech D, G Giussani Biol D, N Perico MD, Prof G Remuzzi MD); Flinders University, Adelaide, SA, Australia (Prof K Pesudovs PhD, F H Tesfay MPH); Shanghai Jiao Tong University School of Medicine, Shanghai, China (Prof M R Phillips MD); Durban University of Technology, Durban, South Africa (J D Pillay PhD); Exposure Assessment and Environmental Health Indicators, German Environment Agency, Berlin, Germany (D Plass DrPH); Department of Anesthesiology (A S Terkawi MD), University of Virginia, Charlottesville, VA, USA (J A Platts-Mills MD); Department of Public Health, Erasmus University Medical Center, Rotterdam, Netherlands (S Polinder PhD); Brigham Young University, Provo, UT, USA (Prof C A Pope PhD); Department of Community Medicine, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran (M Qorbani PhD); Contech School of Public Health, Lahore, Pakistan (A Rafay MS, Prof S M Rana PhD); Research and Evaluation Division, BRAC, Dhaka, Bangladesh (M Rahman PhD); Hamad Medical Corporation, Doha, Qatar (Prof S U Rahman FCPS); Society for Health and Demographic Surveillance, Suri, India (R K Rai MPH); ERAWEB Program, UMIT, Hall in Tirol, Austria (S Rajsic MD); University of Missouri, Columbia, MO, USA (M Raju PhD); WHO Regional Office for Europe, Copenhagen, Denmark (I Rakovac PhD); Contech International Health Consultants, Lahore, Pakistan (Prof S M Rana PhD); Wonju College of Medicine, Institute for Poverty Alleviation and International Development, Yonsei University, Wonju, South Korea (C L Ranabhat PhD); Schizophrenia Research Foundation, Chennai, India (T Rangaswamy FRCPsych); Suez Canal University, Ismailia, Egypt (Prof A H Refaat PhD); Centre for Addiction and Mental Health, Toronto, ON, Canada (Prof J Rehm PhD); Department of Biomedical and Clinical Sciences L Sacco, University of Milan, Milan, Italy (Prof G Remuzzi MD); Hospital das Clinicas da Universidade Federal de Minas Gerais, Belo Horizonte, Brazil (Prof A L Ribeiro MD); UO Neurologia USL Umbria 1, Città di Castello, Italy (S Ricci FRCPEd);); Medical Research Council Unit, The Gambia, Fajara, The Gambia (A Roca PhD); (ISGlobal) Instituto de Salud Global de Barcelona, Barcelona, Spain (D Rojas-Rueda PhD); Golestan Research Center of Gastroenterology and Hepatology, Golestan University of Medical Sciences, Gorgan, Iran (G Roshandel PhD); Holmusk, Singapore, Singapore (N K Roy MS); Duke-NUS Medical School, Singapore, Singapore (N K Roy MS); Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania (G M Ruhago PhD, B F Sunguya PhD); Queensland Centre for Mental Health Research, The Park Centre for Mental Health, Brisbane, QLD, Australia (S Saha PhD); Ballarat Health Service, Ballarat, VIC, Australia (R Sahathevan PhD); Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia (R Sahathevan PhD); Development Research and Projects Center, Abuja, Nigeria (M M Saleh MPH); Marshall University J Edwards School of Medicine, Huntington, WV, USA (J R Sanabria MD); Case Western Reserve University, Cleveland, OH, USA (J R Sanabria MD); IIS-Fundacion Jimenez Diaz, Madrid, Spain (M D Sanchez-Niño PhD); Institut d'Investigacio Biomedica de Bellvitge (IDIBELL), L'Hospitalet de Llobregat, Spain (L Sanchez-Riera PhD); Universidad Ciencias Aplicadas y Ambientales, Bogotá, Colombia (R Sarmiento-Suarez MPH); Marshall University, Huntington, WV, USA (M Sawhney PhD); Swiss Research Institute of Public Health and Addiction (M P Schaub PhD), University of Zurich, Zurich, Switzerland (H G Yebyo MS); Federal University of Santa Catarina, Florianópolis, Brazil (I J C Schneider PhD, D A S Silva PhD); Institute of Health Care and Social Sciences, FOM University, Essen, Germany (B Schöttker MPH); Hypertension in Africa Research Team (HART), North-West University, Potchefstroom, South Africa (Prof A E Schutte PhD); Alcohol, Tobacco & Other Drug Research Unit (Prof C D Parry PhD), South African Medical Research Council, Potchefstroom, South Africa (Prof A E Schutte PhD); University of Bath, Bath, UK (G Shaddick PhD); Department of Public Health, An-Najah University, Nablus, Palestine (A Shaheen PhD); Tufts Medical Center, Boston, MA, USA (S Shahraz PhD); Independent Consultant, Karachi, Pakistan (M A Shaikh MD); Indian Institute of Technology Ropar, Rupnagar, India (R Sharma MA); Research Institute at Nationwide Children's Hospital, Columbus, OH, USA (J Shen PhD); Shanghai Kidney and Dialysis Institute, Shanghai, China (Z Shen MD); Sri Siddhartha University, Tumkur, India (Prof B P Shetty MD); ISHA Diagnostics, Bangalore, India (Prof B P Shetty MD); Department of Public Health Science, Graduate School, Korea University, Seoul, South Korea (Prof M Shin PhD); Work Organizations, Work Disability Prevention, The Finnish Institute of Occupational Health, Helsinki, Finland (R Shiri PhD, T Lallukka PhD); Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne, UK (I Shiue PhD); Reykjavik University, Reykjavik, Iceland (I D Sigfusdottir PhD); Brasília University, Brasília, Brazil (D G A Silveira MD); Feinberg School of Medicine (J I Silverberg MD), Department of Preventive Medicine (Y Yano MD), Northwestern University, Chicago, IL, USA; Department of Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India (O P Singh PhD); Institute for Human Development, New Delhi, India (P K Singh PhD); Asthma Bhawan, Jaipur, India (V Singh MD); Dartmouth College, Hanover, NH, USA (S Soneji PhD); Stavanger University Hospital, Stavanger, Norway (K Søreide PhD); Instituto de Investigación Hospital Universitario de la Princesa, Universidad Autónoma de Madrid, Madrid, Spain (Prof J B Soriano PhD); Department of Clinical Neurological Sciences, Western University, London, ON, Canada (L A Sposato MD); Department of Community Medicine, International Medical University, Kuala Lumpur, Malaysia (C T Sreeramareddy MD); Attikon University Hospital, Athens, Greece (V Stathopoulou PhD); South African Medical Research Council Unit on Anxiety & Stress Disorders, Cape Town, South Africa (Prof D J Stein PhD); University of California, San Diego, La Jolla, CA, USA (M B Stein MD); Luxembourg Institute of Health, Strassen, Luxembourg (S Stranges PhD); Alexandra General Hospital of Athens, Athens, Greece (K Stroumpoulis PhD); Centre Hospitalier Public du Cotentin, Cherbourg, France (K Stroumpoulis PhD); Indian Council of Medical Research, New Delhi, India (S Swaminathan MD); Departments of Criminology, Law & Society, Sociology, and Public Health, University of California, Irvine, Irvine, CA, USA (Prof B L Sykes PhD); Department of Medicine, University of Valencia, INCLIVA Health Research Institute and CIBERSAM, Valencia, Spain (Prof R Tabarés-Seisdedos PhD); School of Social Work, University of Illinois at Urbana-Champaign, Champaign, IL, USA (K M Tabb PhD); WSH Institute, Ministry of Manpower, Singapore, Singapore (J S Takala DSc); Tampere University of Technology, Tampere, Finland (J S Takala DSc); Ministry of Health, MINSANTE, Yaoundé, Cameroon (R T Talongwa MD); Department of Biology, Colgate University, Hamilton, NY, USA (B Taye PhD); James Cook University, Cairns, QLD, Australia (B A Tedla BS); Addis Ababa City Government, Addis Ababa, Ethiopia (W M Tefera MPH); Netherlands Institute of Mental Health and Addiction, Utrecht, Netherlands (M Ten Have PhD); Outcomes Research Consortium (A S Terkawi MD), Cleveland Clinic, Cleveland, OH, USA (Prof E M Tuzcu MD); University Of Gondar, Gondar, Ethiopia (G A Tessema MPH); Adaptive Knowledge Management, Victoria, BC, Canada (A J Thomson PhD); WorldFish, Penang, Malaysia (A L Thorne-Lyman ScD); Department of Medicine, School of Clinical Sciences at Monash Health (Prof A G Thrift PhD), Monash University, Melbourne, VIC, Australia (B M Zeleke MD); Nelson Institute of Environmental Medicine, School of Medicine, New York University, Tuxedo, NY, USA (Prof G D Thurston ScD); Institute of Public Health, Faculty of Health Sciences, Jagiellonian University Medical College, Kraków, Poland (R Topor-Madry PhD); Faculty of Health Sciences, Wroclaw Medical University, Wroclaw, Poland (R Topor-Madry PhD); Aristotle University of Thessaloniki, Thessaloniki, Greece (Prof F Topouzis PhD); Le Bonheur Children's Hospital, Memphis, TN, USA (Prof J A Towbin MD); University of Tennessee Health Science Center, Memphis, TN, USA (Prof J A Towbin MD); St Jude Children's Research Hospital, Memphis, TN, USA (Prof J A Towbin MD); University of Southern Santa Catarina, Palhoça, Brazil (Prof J Traebert PhD); Hanoi Medical University, Hanoi, Vietnam (B X Tran PhD); Department of Neurology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (T Truelsen DMSc); Servicio Canario de Salud, Santa Cruz de Tenerife, Spain (U Trujillo MD); Haramaya University, Dire Dawa, Ethiopia (A K Tura MPH); Department of Veterans Affairs, Washington, DC, USA (U S Uchendu MD); Department of Internal Medicine, Federal Teaching Hospital, Abakaliki, Nigeria (K N Ukwaja MD); Warwick Medical School, University of Warwick, Coventry, UK (O A Uthman PhD); Joint Research Centre, European Commission, Ispra, Italy (R Van Dingenen PhD); Department of Physics and Atsmopheric Science, Dalhousie University, Halifax, Nova Scotia, Canada (A van Donkelaar PhD); UKK Institute for Health Promotion Research, Tampere, Finland (Prof T Vasankari PhD); University of Brasilia, Brasília, Brazil (Prof A M N Vasconcelos PhD); Raffles Neuroscience Centre, Raffles Hospital, Singapore, Singapore (N Venketasubramanian FRCP); Weill Cornell Medical College, New York, NY, USA (R Vidavalur MD); VHS SNEHA, Chennai, India (L Vijayakumar PhD); University of Bologna, Bologna, Italy (Prof F S Violante MD); National Research University Higher School of Economics, Moscow, Russia (Prof V V Vlassov MD); National Institute for Occupational Safety and Health, Washington, DC, USA (G R Wagner MD); VA Medical Center, Washington, DC, USA (M T Wallin MD); Neurology Department, Georgetown University, Washington, DC, USA (M T Wallin MD); McGill University, Montreal, QC, Canada (S Weichenthal PhD); Department of Research, Cancer Registry of Norway, Institute of Population-Based Cancer Research, Oslo, Norway (E Weiderpass PhD); Department of Community Medicine, Faculty of Health Sciences, University of Tromsø, The Arctic University of Norway, Tromsø, Norway (E Weiderpass PhD); Genetic Epidemiology Group, Folkhälsan Research Center, Helsinki, Finland (E Weiderpass PhD); German National Cohort Consortium, Heidelberg, Germany (R Westerman PhD); Department of Infectious Disease Epidemiology and Modelling (R A White PhD), Norwegian Institute of Public Health, Oslo, Norway (C L Ellingsen MD, N H Krog PhD, M Savic PhD); Western Health, Footscray, VIC, Australia (Prof T Wijeratne MD); Children's Hospital of Michigan, Detroit, MI, USA (Prof J D Wilkinson MD); Centre of Evidence-based Dermatology, University of Nottingham, Nottingham, UK (Prof H C Williams DSc); National Institute for Health Research Comprehensive Biomedical Research Centre, Guy's & St Thomas' NHS Foundation Trust and King's College London, London, UK (Prof C D A Wolfe MD); Ateneo School of Medicine and Public Health, Manila University, Pasig City, Philippines (J Q Wong MD); Royal Cornwall Hospital, Truro, UK (Prof A D Woolf FRCP); St John's Medical College and Research Institute, Bangalore, India (Prof D Xavier MD); Department of Neurology, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China (Prof G Xu PhD); Discipline of Public Health Medicine, School of Nursing and Public Health, University of KwaZulu Natal, Durban, South Africa (B Yakob PhD); Global Health Research Center, Duke Kunshan University, Kunshan, China (Prof L L Yan PhD); Social Work and Social Administration Department and The Hong Kong Jockey Club Centre for Suicide Research and Prevention, University of Hong Kong, Hong Kong, China (Prof P Yip PhD); University of South Australia, Mawson Lakes, SA, Australia (B D Yirsaw PhD); Department of Biostatistics, School of Public Health, Kyoto University, Kyoto, Japan (N Yonemoto MPH); Aga Khan University, East Africa, Nairobi, Kenya (Prof G Yonga MD); Jackson State University, Jackson, MS, USA (Prof M Z Younis DrPH); University Hospital, Setif, Algeria (Prof Z Zaidi PhD); Faculty of Medicine, Mansoura University, Mansoura, Egypt (Prof M E Zaki PhD); Clinical Investigation Centre INSERM (the National Institute for Health and Medical Research), Université de Lorraine, Vandoeuvre les Nancy, France (Prof F Zannad PhD); CHU de Nancy, Vandoeuvre les Nancy, France (Prof F Zannad PhD); Ponce Health Sciences University, Ponce, Puerto Rico (D E Zavala PhD); Leibniz Institute for Prevention Research and Epidemiology, Bremen, Germany (Prof H Zeeb PhD); Shanghai Institute of Kidney Disease and Dialysis, Shanghai, China (H Zhang PhD); Oregon Health and Science University, Portland, OR, USA (D Zonies MD); Red Cross War Memorial Children's Hospital, Cape Town, South Africa (L J Zuhlke PhD)
Contributors
Christopher J L Murray, Alan D Lopez, Mohsen Naghavi, and Haidong Wang prepared the first draft. Alan D Lopez and Christopher J L Murray conceived the study and provided overall guidance. All other authors provided data, developed models, reviewed results, initiated modelling infrastructure, or reviewed and contributed to the report.
Declaration of interests
Carl Abelardo T Antonio reports grants, personal fees and non-financial support from Johnson & Johnson (Philippines). Donald S Shepard acknowledges grant support from Sanofi Pasteur. Dariush Mozaffarian reports ad-hoc honoraria or consulting from Boston Heart Diagnostics, Haas Avocado Board, AstraZeneca, GOED, DSM, and Life Sciences Research Organization; and chapter royalties from UpToDate. Ettore Beghi reports grants from UCB-Pharma, during the conduct of the study; personal fees from Viropharma; grants from GSK, UCB-Pharma, Italian Drug Agency (AIFA), Italian Ministry of Health, EISAI, American ALS Association, and Borgonovo Foundation; and personal fees from Viropharma and GSK. Katherine B Gibney reports grants from CSL. Dan J Stein reports personal fees from Lundbeck, Novartis, AMBRF, Biocodex, Sevier, SUN, and CIPLA; and grants from MRC and NRGF. Peter A Meaney serves as the principal investigator for an investigator-initiated study funded by Horizon pharmaceuticals through a grant to DINORA, a 501c3 entity; is on the steering committee of OMERACT, an international organisation that develops measures for clinical trials and receives arms-length funding from 36 pharmaceutical companies; consults for Savient, Takeda, Regeneron, Iroko, Merz, Bioiberica, Crealta, Allergan, WebMD, and UBM; and is supported by grants from Takeda and Savient. Mark G Shrime has received financial support from the GE Foundation Safe Surgery 2020 project and the Kletjian Foundation, and speaking fees from Ethicon (2014). Walter Mendoza reports that no additional funds were provided for his participation in this paper, but as an official of UNFPA Country Office in Peru, he stresses that his institution does not necessarily endorse the study. Aletta E Schutte is funded by the Medical Research Council of South Africa, and the South African Research Chair Initiative by the National Research Foundation. Charles D A Wolfe acknowledges that the research was funded and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. All other authors declare no competing interests.
Contributor Information
GBD 2015 Mortality and Causes of Death Collaborators:
Haidong Wang, Mohsen Naghavi, Christine Allen, Ryan M Barber, Zulfiqar A Bhutta, Austin Carter, Daniel C Casey, Fiona J Charlson, Alan Zian Chen, Matthew M Coates, Megan Coggeshall, Lalit Dandona, Daniel J Dicker, Holly E Erskine, Alize J Ferrari, Christina Fitzmaurice, Kyle Foreman, Mohammad H Forouzanfar, Maya S Fraser, Nancy Fullman, Peter W Gething, Ellen M Goldberg, Nicholas Graetz, Juanita A Haagsma, Simon I Hay, Chantal Huynh, Catherine O Johnson, Nicholas J Kassebaum, Yohannes Kinfu, Xie Rachel Kulikoff, Michael Kutz, Hmwe H Kyu, Heidi J Larson, Janni Leung, Xiaofeng Liang, Stephen S Lim, Margaret Lind, Rafael Lozano, Neal Marquez, George A Mensah, Joe Mikesell, Ali H Mokdad, Meghan D Mooney, Grant Nguyen, Elaine Nsoesie, David M Pigott, Christine Pinho, Gregory A Roth, Joshua A Salomon, Logan Sandar, Naris Silpakit, Amber Sligar, Reed J D Sorensen, Jeffrey Stanaway, Caitlyn Steiner, Stephanie Teeple, Bernadette A Thomas, Christopher Troeger, Amelia VanderZanden, Stein Emil Vollset, Valentine Wanga, Harvey A Whiteford, Timothy Wolock, Leo Zoeckler, Kalkidan Hassen Abate, Cristiana Abbafati, Kaja M Abbas, Foad Abd-Allah, Semaw Ferede Abera, Daisy M X Abreu, Laith J Abu-Raddad, Gebre Yitayih Abyu, Tom Achoki, Ademola Lukman Adelekan, Zanfina Ademi, Arsène Kouablan Adou, José C Adsuar, Kossivi Agbelenko Afanvi, Ashkan Afshin, Emilie Elisabet Agardh, Arnav Agarwal, Anurag Agrawal, Aliasghar Ahmad Kiadaliri, Oluremi N Ajala, Ali Shafqat Akanda, Rufus Olusola Akinyemi, Tomi F Akinyemiju, Nadia Akseer, Faris Hasan Al Lami, Samer Alabed, Ziyad Al-Aly, Khurshid Alam, Noore K M Alam, Deena Alasfoor, Saleh Fahed Aldhahri, Robert William Aldridge, Miguel Angel Alegretti, Alicia V Aleman, Zewdie Aderaw Alemu, Lily T Alexander, Samia Alhabib, Raghib Ali, Ala'a Alkerwi, François Alla, Peter Allebeck, Rajaa Al-Raddadi, Ubai Alsharif, Khalid A Altirkawi, Elena Alvarez Martin, Nelson Alvis-Guzman, Azmeraw T Amare, Adeladza Kofi Amegah, Emmanuel A Ameh, Heresh Amini, Walid Ammar, Stephen Marc Amrock, Hjalte H Andersen, Benjamin O Anderson, Gregory M Anderson, Carl Abelardo T Antonio, Atsede Fantahun Aregay, Johan Ärnlöv, Valentina S Arsic Arsenijevic, Al Artaman, Hamid Asayesh, Rana Jawad Asghar, Suleman Atique, Euripide Frinel G Arthur Avokpaho, Ashish Awasthi, Peter Azzopardi, Umar Bacha, Alaa Badawi, Maria C Bahit, Kalpana Balakrishnan, Amitava Banerjee, Aleksandra Barac, Suzanne L Barker-Collo, Till Bärnighausen, Lars Barregard, Lope H Barrero, Arindam Basu, Sanjay Basu, Yibeltal Tebekaw Bayou, Shahrzad Bazargan-Hejazi, Justin Beardsley, Neeraj Bedi, Ettore Beghi, Haileeyesus Adamu Belay, Brent Bell, Michelle L Bell, Aminu K Bello, Derrick A Bennett, Isabela M Bensenor, Adugnaw Berhane, Eduardo Bernabé, Balem Demtsu Betsu, Addisu Shunu Beyene, Neeraj Bhala, Ashish Bhalla, Sibhatu Biadgilign, Boris Bikbov, Aref A Bin Abdulhak, Brian J Biroscak, Stan Biryukov, Espen Bjertness, Jed D Blore, Christopher D Blosser, Megan A Bohensky, Rohan Borschmann, Dipan Bose, Rupert R A Bourne, Michael Brainin, Carol E G Brayne, Alexandra Brazinova, Nicholas J K Breitborde, Hermann Brenner, Jerry D Brewer, Alexandria Brown, Jonathan Brown, Traolach S Brugha, Geoffrey Colin Buckle, Zahid A Butt, Bianca Calabria, Ismael Ricardo Campos-Nonato, Julio Cesar Campuzano, Jonathan R Carapetis, Rosario Cárdenas, David O Carpenter, Juan Jesus Carrero, Carlos A Castañeda-Orjuela, Jacqueline Castillo Rivas, Ferrán Catalá-López, Fiorella Cavalleri, Kelly Cercy, Jorge Cerda, Wanqing Chen, Adrienne Chew, Peggy Pei-Chia Chiang, Mirriam Chibalabala, Chioma Ezinne Chibueze, Odgerel Chimed-Ochir, Vesper Hichilombwe Chisumpa, Jee-Young Jasmine Choi, Rajiv Chowdhury, Hanne Christensen, Devasahayam Jesudas Christopher, Liliana G Ciobanu, Massimo Cirillo, Aaron J Cohen, Valentina Colistro, Mercedes Colomar, Samantha M Colquhoun, Cyrus Cooper, Leslie Trumbull Cooper, Monica Cortinovis, Benjamin C Cowie, John A Crump, James Damsere-Derry, Hadi Danawi, Rakhi Dandona, Farah Daoud, Sarah C Darby, Paul I Dargan, José das Neves, Gail Davey, Adrian C Davis, Dragos V Davitoiu, E Filipa de Castro, Pieter de Jager, Diego De Leo, Louisa Degenhardt, Robert P Dellavalle, Kebede Deribe, Amare Deribew, Samath D Dharmaratne, Preet K Dhillon, Cesar Diaz-Torné, Eric L Ding, Kadine Priscila Bender dos Santos, Edem Dossou, Tim R Driscoll, Leilei Duan, Manisha Dubey, Bruce Bartholow Duncan, Richard G Ellenbogen, Christian Lycke Ellingsen, Iqbal Elyazar, Aman Yesuf Endries, Sergey Petrovich Ermakov, Babak Eshrati, Alireza Esteghamati, Kara Estep, Imad D A Faghmous, Saman Fahimi, Emerito Jose Aquino Faraon, Talha A Farid, Carla Sofia e Sa Farinha, André Faro, Maryam S Farvid, Farshad Farzadfar, Valery L Feigin, Seyed-Mohammad Fereshtehnejad, Jefferson G Fernandes, Joao C Fernandes, Florian Fischer, Joseph R A Fitchett, Abraham Flaxman, Nataliya Foigt, F Gerry R Fowkes, Elisabeth Barboza Franca, Richard C Franklin, Joseph Friedman, Joseph Frostad, Thomas Fürst, Neal D Futran, Seana L Gall, Ketevan Gambashidze, Amiran Gamkrelidze, Parthasarathi Ganguly, Fortuné Gbètoho Gankpé, Teshome Gebre, Tsegaye Tsewelde Gebrehiwot, Amanuel Tesfay Gebremedhin, Alemseged Aregay Gebru, Johanna M Geleijnse, Bradford D Gessner, Aloke Gopal Ghoshal, Katherine B Gibney, Richard F Gillum, Stuart Gilmour, Ababi Zergaw Giref, Maurice Giroud, Melkamu Dedefo Gishu, Giorgia Giussani, Elizabeth Glaser, William W Godwin, Hector Gomez-Dantes, Philimon Gona, Amador Goodridge, Sameer Vali Gopalani, Richard A Gosselin, Carolyn C Gotay, Atsushi Goto, Hebe N Gouda, Felix Greaves, Harish Chander Gugnani, Rahul Gupta, Rajeev Gupta, Vipin Gupta, Reyna A Gutiérrez, Nima Hafezi-Nejad, Demewoz Haile, Alemayehu Desalegne Hailu, Gessessew Bugssa Hailu, Yara A Halasa, Randah Ribhi Hamadeh, Samer Hamidi, Jamie Hancock, Alexis J Handal, Graeme J Hankey, Yuantao Hao, Hilda L Harb, Sivadasanpillai Harikrishnan, Josep Maria Haro, Rasmus Havmoeller, Susan R Heckbert, Ileana Beatriz Heredia-Pi, Pouria Heydarpour, Henk B M Hilderink, Hans W Hoek, Robert S Hogg, Masako Horino, Nobuyuki Horita, H Dean Hosgood, Peter J Hotez, Damian G Hoy, Mohamed Hsairi, Aung Soe Htet, Maung Maung Than Htike, Guoqing Hu, Cheng Huang, Hsiang Huang, Laetitia Huiart, Abdullatif Husseini, Inge Huybrechts, Grace Huynh, Kim Moesgaard Iburg, Kaire Innos, Manami Inoue, Veena J Iyer, Troy A Jacobs, Kathryn H Jacobsen, Nader Jahanmehr, Mihajlo B Jakovljevic, Peter James, Mehdi Javanbakht, Sudha P Jayaraman, Achala Upendra Jayatilleke, Panniyammakal Jeemon, Paul N Jensen, Vivekanand Jha, Guohong Jiang, Ying Jiang, Tariku Jibat, Aida Jimenez-Corona, Jost B Jonas, Tushar Kant Joshi, Zubair Kabir, Ritul Kamal, Haidong Kan, Surya Kant, André Karch, Corine Kakizi Karema, Chante Karimkhani, Dimitris Karletsos, Ganesan Karthikeyan, Amir Kasaeian, Marzieh Katibeh, Anil Kaul, Norito Kawakami, Jeanne Françoise Kayibanda, Peter Njenga Keiyoro, Laura Kemmer, Andrew Haddon Kemp, Andre Pascal Kengne, Andre Keren, Maia Kereselidze, Chandrasekharan Nair Kesavachandran, Yousef Saleh Khader, Ibrahim A Khalil, Abdur Rahman Khan, Ejaz Ahmad Khan, Young-Ho Khang, Sahil Khera, Tawfik Ahmed Muthafer Khoja, Christian Kieling, Daniel Kim, Yun Jin Kim, Brett M Kissela, Niranjan Kissoon, Luke D Knibbs, Ann Kristin Knudsen, Yoshihiro Kokubo, Dhaval Kolte, Jacek A Kopec, Soewarta Kosen, Parvaiz A Koul, Ai Koyanagi, Norun Hjertager Krog, Barthelemy Kuate Defo, Burcu Kucuk Bicer, Andreas A Kudom, Ernst J Kuipers, Veena S Kulkarni, G Anil Kumar, Gene F Kwan, Aparna Lal, Dharmesh Kumar Lal, Ratilal Lalloo, Tea Lallukka, Hilton Lam, Jennifer O Lam, Sinead M Langan, Van C Lansingh, Anders Larsson, Dennis Odai Laryea, Asma Abdul Latif, Alicia Elena Beatriz Lawrynowicz, James Leigh, Miriam Levi, Yongmei Li, M Patrice Lindsay, Steven E Lipshultz, Patrick Y Liu, Shiwei Liu, Yang Liu, Loon-Tzian Lo, Giancarlo Logroscino, Paulo A Lotufo, Robyn M Lucas, Raimundas Lunevicius, Ronan A Lyons, Stefan Ma, Vasco Manuel Pedro Machado, Mark T Mackay, Jennifer H MacLachlan, Hassan Magdy Abd El Razek, Mohammed Magdy, Abd El Razek, Marek Majdan, Azeem Majeed, Reza Malekzadeh, Wondimu Ayele Ayele Manamo, John Mandisarisa, Srikanth Mangalam, Chabila C Mapoma, Wagner Marcenes, David Joel Margolis, Gerard Robert Martin, Jose Martinez-Raga, Melvin Barrientos Marzan, Felix Masiye, Amanda J Mason-Jones, João Massano, Richard Matzopoulos, Bongani M Mayosi, Stephen Theodore McGarvey, John J McGrath, Martin McKee, Brian J McMahon, Peter A Meaney, Alem Mehari, Man Mohan Mehndiratta, Fabiola Mejia-Rodriguez, Alemayehu B Mekonnen, Yohannes Adama Melaku, Peter Memiah, Ziad A Memish, Walter Mendoza, Atte Meretoja, Tuomo J Meretoja, Francis Apolinary Mhimbira, Renata Micha, Anoushka Millear, Ted R Miller, Mojde Mirarefin, Awoke Misganaw, Charles N Mock, Karzan Abdulmuhsin Mohammad, Alireza Mohammadi, Shafiu Mohammed, Viswanathan Mohan, Glen Liddell D Mola, Lorenzo Monasta, Julio Cesar Montañez Hernandez, Pablo Montero, Marcella Montico, Thomas J Montine, Maziar Moradi-Lakeh, Lidia Morawska, Katherine Morgan, Rintaro Mori, Dariush Mozaffarian, Ulrich O Mueller, Gudlavalleti Venkata Satyanarayana Murthy, Srinivas Murthy, Kamarul Imran Musa, Jean B Nachega, Gabriele Nagel, Kovin S Naidoo, Nitish Naik, Luigi Naldi, Vinay Nangia, Denis Nash, Chakib Nejjari, Subas Neupane, Charles R Newton, John N Newton, Marie Ng, Frida Namnyak Ngalesoni, Jean de Dieu Ngirabega, Quyen Le Nguyen, Muhammad Imran Nisar, Patrick Martial Nkamedjie Pete, Marika Nomura, Ole F Norheim, Paul E Norman, Bo Norrving, Luke Nyakarahuka, Felix Akpojene Ogbo, Takayoshi Ohkubo, Foluke Adetola Ojelabi, Pedro R Olivares, Bolajoko Olubukunola Olusanya, Jacob Olusegun Olusanya, John Nelson Opio, Eyal Oren, Alberto Ortiz, Majdi Osman, Erika Ota, Raziye Ozdemir, Mahesh PA, Amanda Pain, Jeyaraj D Pandian, Puspa Raj Pant, Christina Papachristou, Eun-Kee Park, Jae-Hyun Park, Charles D Parry, Mahboubeh Parsaeian, Angel J Paternina Caicedo, Scott B Patten, George C Patton, Vinod K Paul, Neil Pearce, João Mário Pedro, Ljiljana Pejin Stokic, David M Pereira, Norberto Perico, Konrad Pesudovs, Max Petzold, Michael Robert Phillips, Frédéric B Piel, Julian David Pillay, Dietrich Plass, James A Platts-Mills, Suzanne Polinder, C Arden Pope, Svetlana Popova, Richie G Poulton, Farshad Pourmalek, Dorairaj Prabhakaran, Mostafa Qorbani, Justice Quame-Amaglo, D Alex Quistberg, Anwar Rafay, Kazem Rahimi, Vafa Rahimi-Movaghar, Mahfuzar Rahman, Mohammad Hifz Ur Rahman, Sajjad Ur Rahman, Rajesh Kumar Rai, Zhale Rajavi, Sasa Rajsic, Murugesan Raju, Ivo Rakovac, Saleem M Rana, Chhabi L Ranabhat, Thara Rangaswamy, Puja Rao, Sowmya R Rao, Amany H Refaat, Jürgen Rehm, Marissa B Reitsma, Giuseppe Remuzzi, Serge Resnikoff, Antonio L Ribeiro, Stefano Ricci, Maria Jesus Rios Blancas, Bayard Roberts, Anna Roca, David Rojas-Rueda, Luca Ronfani, Gholamreza Roshandel, Dietrich Rothenbacher, Ambuj Roy, Nawal K Roy, George Mugambage Ruhago, Rajesh Sagar, Sukanta Saha, Ramesh Sahathevan, Muhammad Muhammad Saleh, Juan R Sanabria, Maria Dolores Sanchez-Niño, Lidia Sanchez-Riera, Itamar S Santos, Rodrigo Sarmiento-Suarez, Benn Sartorius, Maheswar Satpathy, Miloje Savic, Monika Sawhney, Michael P Schaub, Maria Inês Schmidt, Ione J C Schneider, Ben Schöttker, Aletta E Schutte, David C Schwebel, Soraya Seedat, Sadaf G Sepanlou, Edson E Servan-Mori, Katya A Shackelford, Gavin Shaddick, Amira Shaheen, Saeid Shahraz, Masood Ali Shaikh, Marina Shakh-Nazarova, Rajesh Sharma, Jun She, Sara Sheikhbahaei, Jiabin Shen, Ziyan Shen, Donald S Shepard, Kevin N Sheth, Balakrishna P Shetty, Peilin Shi, Kenji Shibuya, Min-Jeong Shin, Rahman Shiri, Ivy Shiue, Mark G Shrime, Inga Dora Sigfusdottir, Donald H Silberberg, Diego Augusto Santos Silva, Dayane Gabriele Alves Silveira, Jonathan I Silverberg, Edgar P Simard, Abhishek Singh, Gitanjali M Singh, Jasvinder A Singh, Om Prakash Singh, Prashant Kumar Singh, Virendra Singh, Samir Soneji, Kjetil Søreide, Joan B Soriano, Luciano A Sposato, Chandrashekhar T Sreeramareddy, Vasiliki Stathopoulou, Dan J Stein, Murray B Stein, Saverio Stranges, Konstantinos Stroumpoulis, Bruno F Sunguya, Patrick Sur, Soumya Swaminathan, Bryan L Sykes, Cassandra E I Szoeke, Rafael Tabarés-Seisdedos, Karen M Tabb, Ken Takahashi, Jukka S Takala, Roberto Tchio Talongwa, Nikhil Tandon, Mohammad Tavakkoli, Bineyam Taye, Hugh R Taylor, Braden J Te Ao, Bemnet Amare Tedla, Worku Mekonnen Tefera, Margreet Ten Have, Abdullah Sulieman Terkawi, Fisaha Haile Tesfay, Gizachew Assefa Tessema, Alan J Thomson, Andrew L Thorne-Lyman, Amanda G Thrift, George D Thurston, Taavi Tillmann, David L Tirschwell, Marcello Tonelli, Roman Topor-Madry, Fotis Topouzis, Jeffrey Allen Towbin, Jefferson Traebert, Bach Xuan Tran, Thomas Truelsen, Ulises Trujillo, Abera Kenay Tura, Emin Murat Tuzcu, Uche S Uchendu, Kingsley N Ukwaja, Eduardo A Undurraga, Olalekan A Uthman, Rita Van Dingenen, Aaron van Donkelaar, Tommi Vasankari, Ana Maria Nogales Vasconcelos, Narayanaswamy Venketasubramanian, Ramesh Vidavalur, Lakshmi Vijayakumar, Salvador Villalpando, Francesco S Violante, Vasiliy Victorovich Vlassov, Joseph A Wagner, Gregory R Wagner, Mitchell T Wallin, Linhong Wang, David A Watkins, Scott Weichenthal, Elisabete Weiderpass, Robert G Weintraub, Andrea Werdecker, Ronny Westerman, Richard A White, Tissa Wijeratne, James D Wilkinson, Hywel C Williams, Charles Shey Wiysonge, Solomon Meseret Woldeyohannes, Charles D A Wolfe, Sungho Won, John Q Wong, Anthony D Woolf, Denis Xavier, Qingyang Xiao, Gelin Xu, Bereket Yakob, Ayalnesh Zemene Yalew, Lijing L Yan, Yuichiro Yano, Mehdi Yaseri, Pengpeng Ye, Henock Gebremedhin Yebyo, Paul Yip, Biruck Desalegn Yirsaw, Naohiro Yonemoto, Gerald Yonga, Mustafa Z Younis, Shicheng Yu, Zoubida Zaidi, Maysaa El Sayed Zaki, Faiez Zannad, Diego E Zavala, Hajo Zeeb, Berihun M Zeleke, Hao Zhang, Sanjay Zodpey, David Zonies, Liesl Joanna Zuhlke, Theo Vos, Alan D Lopez, and Christopher J L Murray
Supplementary Material
New results from the Global Burden of Disease Study 2015 examine causes of death and categorise regions according to the Socio-demographic Index, or ‘SDI’.
References
- 1.Roulson J, Benbow EW, Hasleton PS. Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review. Histopathology. 2005;47:551–559. doi: 10.1111/j.1365-2559.2005.02243.x. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lozano R, Flaxman AD. Using verbal autopsy to measure causes of death: the comparative performance of existing methods. BMC Med. 2014;12:5. doi: 10.1186/1741-7015-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serina P, Riley I, Stewart A. A shortened verbal autopsy instrument for use in routine mortality surveillance systems. BMC Med. 2015;13:302. doi: 10.1186/s12916-015-0528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naghavi M, Makela S, Foreman K, O'Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr. 2010;8:9. doi: 10.1186/1478-7954-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: a necropsy study. Lancet. 2000;355:2027–2031. doi: 10.1016/s0140-6736(00)02349-7. [DOI] [PubMed] [Google Scholar]
- 7.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Liddell CA, Coates MM. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:957–979. doi: 10.1016/S0140-6736(14)60497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray CJL, Lopez AD, the Harvard School of Public Health. World Health Organization. World Bank . The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Published by the Harvard School of Public Health on behalf of the World Health Organization and the World Bank: Distributed by Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- 11.Murray CJL, Ortblad KF, Guinovart C. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Dwyer-Lindgren L, Lofgren KT. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2071–2094. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- 13.Newton JN, Briggs ADM, Murray CJL. Changes in health in England, with analysis by English regions and areas of deprivation, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2257–2274. doi: 10.1016/S0140-6736(15)00195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M, Wang H, Zhu J. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Dantés H, Fullman N, Lamadrid-Figueroa H. Dissonant health transition in the states of Mexico, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2016 doi: 10.1016/S0140-6736(16)31773-1. http://dx.doi.org/S0140-6736(16)31773-1 published online Oct 5. [DOI] [PubMed] [Google Scholar]
- 16.From evidence into action: opportunities to protect and improve the nation's health. Public Health England; 2014. [Google Scholar]
- 17.Bhalla K, Harrison JE. GBD-2010 overestimates deaths from road injuries in OECD countries: new methods perform poorly. Int J Epidemiol. 2015;44:1648–1656. doi: 10.1093/ije/dyv019. [DOI] [PubMed] [Google Scholar]
- 18.Rudan I, Chan KY. Global health metrics needs collaboration and competition. Lancet. 2015;385:92–94. doi: 10.1016/S0140-6736(14)62006-7. [DOI] [PubMed] [Google Scholar]
- 19.Rajaratnam JK, Marcus JR, Levin-Rector A. Worldwide mortality in men and women aged 15–59 years from 1970 to 2010: a systematic analysis. Lancet. 2010;375:1704–1720. doi: 10.1016/S0140-6736(10)60517-X. [DOI] [PubMed] [Google Scholar]
- 20.The GATHER Working Group Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016 doi: 10.1016/S0140-6736(16)30388-9. http://dx.doi.org/10.1016/S0140-6736(16)30388-9 published online June 28. [DOI] [PubMed] [Google Scholar]
- 21.Murray CJ, Ezzati M, Flaxman AD. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 22.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 23.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 24.Lozano R, Naghavi M, Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obermeyer Z, Rajaratnam JK, Park CH. Measuring adult mortality using sibling survival: a new analytical method and new results for 44 countries, 1974–2006. PLoS Med. 2010;7:e1000260. doi: 10.1371/journal.pmed.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray CJL, Rajaratnam JK, Marcus J, Laakso T, Lopez AD. What can we conclude from death registration? Improved methods for evaluating completeness. PLoS Med. 2010;7:e1000262. doi: 10.1371/journal.pmed.1000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill K, You D, Choi Y. Death distribution methods for estimating adult mortality: Sensitivity analysis with simulated data errors. Demogr Res. 2009;21:235–254. [Google Scholar]
- 28.United Nations Population Division | Department of Economic and Social Affairs. http://www.un.org/en/development/desa/population/ (accessed March 2, 2016).
- 29.Introduction to UNICEF's work on statistics and monitoring UNICEF. http://www.unicef.org/statistics/ (accessed March 2, 2016).
- 30.Home - Wittgenstein Centre. http://www.wittgensteincentre.org/en/index.htm (accessed March 2, 2016).
- 31.United Nations. Department of International Economic and Social Affairs . Model life tables for developing countries. United Nations; New York: 1982. [Google Scholar]
- 32.Coale AJ, Demeny PG, the Princeton University. Office of Population Research . Regional model life tables and stable populations. Princeton University Press; Princeton, NJ: 1966. [Google Scholar]
- 33.Murray CJL, Ferguson BD, Lopez AD, Guillot M, Salomon JA, Ahmad O. Modified logit life table system: principles, empirical validation, and application. Popul Stud (Camb) 2003;57:165–182. [Google Scholar]
- 34.Eaton J. Read-epp-spectrum. GitHub. https://github.com/jeffeaton/read-epp-spectrum (accessed March 7, 2016).
- 35.UNAIDS AIDSinfo Online Database. 2014. http://www.aidsinfoonline.org/devinfo/libraries/aspx/Home.aspx (accessed April 13, 2016).
- 36.2014 Progress reports submitted by countries. http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2014countries (accessed April 5, 2016).
- 37.Guha-Sapir D, Below R, Hoyois P. EM-DAT: The CRED/OFDA International Disaster Database. Université Catholique de Louvain. http://www.emdat.be/database (accessed April 5, 2016).
- 38.Department of Peace and Conflict Research Uppsala Conflict Data Program. Uppsala University, Uppsala, Sweden. http://www.pcr.uu.se/research/UCDP/ (accessed April 5, 2016).
- 39.The International Institute for Strategic Studies Armed Conflict Database. London. https://www.iiss.org/en/publications/acd (accessed April 5, 2016).
- 40.The Robert S Strauss Center For International Security and Law. The University of Texas at Austin. https://www.strausscenter.org/ (accessed April 5, 2016).
- 41.Birnbaum JK, Murray CJ, Lozano R. Exposing misclassified HIV/AIDS deaths in South Africa. Bull World Health Organ. 2011;89:278–285. doi: 10.2471/BLT.11.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson HM, Flaxman AD. Meta-regression with DisMod-MR: how robust is the model? Lancet. 2013;381:S110. [Google Scholar]
- 43.Flaxman AD, Vos T, Murray CJL. An integrative metaregression framework for descriptive epidemiology. University of Washington Press; Seattle: 2015. [Google Scholar]
- 44.National Center for Health Statistics (US) 1993 National Mortality Followback Survey. US Dept of Health & Human Services, Public Health Service, Centers for Disease Control; Hyattsville, MD: 1991. [Google Scholar]
- 45.Duncan ME, Pitcher A, Goldacre MJ. Atrial fibrillation as a cause of death increased steeply in England between 1995 and 2010. Europace. 2014;16:797–802. doi: 10.1093/europace/eut388. [DOI] [PubMed] [Google Scholar]
- 46.Kotloff KL, Nataro JP, Blackwelder WC. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Platts-Mills JA, Juma J. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta-analysis. J Glob Health. 2015;5:010408. doi: 10.7189/jogh.05.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonten MJM, Huijts SM, Bolkenbaas M. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 50.Feikin DR, Scott JAG, Gessner BD. Use of vaccines as probes to define disease burden. Lancet. 2014;383:1762–1770. doi: 10.1016/S0140-6736(13)61682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray CJL, Barber RM, Foreman KJ, the GBD 2013 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–2191. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.United Nations Development Programme . Human development report 2015. United Nations; Geneva: 2016. [Google Scholar]
- 53.A hand up: Global progress towards universal education. Issuu. https://issuu.com/ihme/docs/policyreport_ihme_educationalattain (accessed April 1, 2016).
- 54.Das Gupta P. Standardization and decomposition of rates: a user's manual. US Bureau of the Census; Washington, DC: 1993. [Google Scholar]
- 55.Beltrán-Sánchez H, Preston SH, Canudas-Romo V. An integrated approach to cause-of-death analysis: cause-deleted life tables and decompositions of life expectancy. Demogr Res. 2008;19:1323–1350. doi: 10.4054/DemRes.2008.19.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49:509–538. [PubMed] [Google Scholar]
- 57.Bloom DE, Canning D. Policy forum: public health. The health and wealth of nations. Science. 2000;287:1207–1209. doi: 10.1126/science.287.5456.1207. [DOI] [PubMed] [Google Scholar]
- 58.Preston SH. The changing relation between mortality and level of economic development. Popul Stud (Camb) 1975;29:231–248. [PubMed] [Google Scholar]
- 59.Deaton A. Health, Inequality, and Economic Development. J Econ Lit. 2003;41:113–158. [Google Scholar]
- 60.Caldwell JC. Routes to Low Mortality in Poor Countries. Popul Dev Rev. 1986;12:171–220. doi: 10.1111/j.1728-4457.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- 61.Cutler D, Deaton A, Lleras-Muney A. The Determinants of Mortality. J Econ Perspect. 2006;20:97–120. [Google Scholar]
- 62.Howitt P, Darzi A, Yang G-Z. Technologies for global health. Lancet. 2012;380:507–535. doi: 10.1016/S0140-6736(12)61127-1. [DOI] [PubMed] [Google Scholar]
- 63.McMichael AJ, Woodruff RE, Hales S. Climate change and human health: present and future risks. Lancet. 2006;367:859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- 64.Ramsey R, Giskes K, Turrell G, Gallegos D. Food insecurity among adults residing in disadvantaged urban areas: potential health and dietary consequences. Public Health Nutr. 2012;15:227–237. doi: 10.1017/S1368980011001996. [DOI] [PubMed] [Google Scholar]
- 65.Farmer PE. Shattuck Lecture. Chronic infectious disease and the future of health care delivery. N Engl J Med. 2013;369:2424–2436. doi: 10.1056/NEJMsa1310472. [DOI] [PubMed] [Google Scholar]
- 66.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manolio TA, Chisholm RL, Ozenberger B. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267. doi: 10.1038/gim.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahoo SK. Nanotechnology in Health Care. CRC Press; 2012. [Google Scholar]
- 69.Lundgren JD, Babiker AG, Gordin F, the INSIGHT START Study Group Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fast-Track strategy to end the AIDS epidemic by 2030. http://www.unaids.org/en/resources/campaigns/World-AIDS-Day-Report-2014 (accessed March 7, 2016).
- 71.FIRS calls for continued international support to end AIDS epidemic by 2030. Dec 7, 2015. http://www.news-medical.net/news/20151207/FIRS-calls-for-continued-international-support-to-end-AIDS-epidemic-by-2030.aspx (accessed March 7, 2016).
- 72.Lo S, Horton R. AIDS and global health: the path to sustainable development. Lancet. 2015;386:106–108. doi: 10.1016/S0140-6736(15)61040-6. [DOI] [PubMed] [Google Scholar]
- 73.Secretary-General Calls for End to AIDS by 2030, in Message to General Assembly | Meetings Coverage and Press Releases. http://www.un.org/press/en/2015/sgsm16830.doc.htm (accessed March 7, 2016).
- 74.Dieleman JL, Graves C, Johnson E. Sources and focus of health development assistance, 1990–2014. JAMA. 2015;313:2359–2368. doi: 10.1001/jama.2015.5825. [DOI] [PubMed] [Google Scholar]
- 75.Schneider MT, Birger M, Haakendstad A. Tracking development assistance for HIV/AIDS: The international response to a global epidemic. AIDS. 2016 doi: 10.1097/QAD.0000000000001081. published online March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhatt S, Weiss DJ, Cameron E. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J, Gratz J, Amour C. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol. 2013;51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Kabir F, Manneh J. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect Dis. 2014;14:716–724. doi: 10.1016/S1473-3099(14)70808-4. [DOI] [PubMed] [Google Scholar]
- 79.Stanaway JD, Shepard DS, Undurraga EA. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386:243–244. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 81.WHO | Dengue vaccine research. WHO. http://www.who.int/immunization/research/development/dengue_vaccines/en/ (accessed March 2, 2016).
- 82.Jonker FAM, Calis JCJ, Phiri K. Real-time PCR demonstrates Ancylostoma duodenale is a key factor in the etiology of severe anemia and iron deficiency in Malawian pre-school children. PLoS Negl Trop Dis. 2012;6:e1555. doi: 10.1371/journal.pntd.0001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qian M-B, Chen Y-D, Liang S, Yang G-J, Zhou X-N. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect Dis Poverty. 2012;1:4. doi: 10.1186/2049-9957-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer A review of human carcinogens. Lyon, France; Geneva, Switzerland: International Agency for Research on Cancer; Distributed by WHO Press. 2012. http://monographs.iarc.fr/ENG/Monographs/vol100B/index.php (accessed April 13, 2016).
- 85.Haagsma JA, Graetz N, Bolliger I. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev. 2016;22:3–18. doi: 10.1136/injuryprev-2015-041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agua-Agum J, Ariyarajah A, Aylward B, the WHO Ebola Response Team West African Ebola epidemic after one year-slowing but not yet under control. N Engl J Med. 2015;372:584–587. doi: 10.1056/NEJMc1414992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker PGT, White MT, Griffin JT, Reynolds A, Ferguson NM, Ghani AC. Malaria morbidity and mortality in Ebola-affected countries caused by decreased health-care capacity, and the potential effect of mitigation strategies: a modelling analysis. Lancet Infect Dis. 2015;15:825–832. doi: 10.1016/S1473-3099(15)70124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takahashi S, Metcalf CJE, Ferrari MJ. Reduced vaccination and the risk of measles and other childhood infections post-Ebola. Science. 2015;347:1240–1242. doi: 10.1126/science.aaa3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moon S, Sridhar D, Pate MA. Will Ebola change the game? Ten essential reforms before the next pandemic. The report of the Harvard-LSHTM Independent Panel on the Global Response to Ebola. Lancet. 2015;386:2204–2221. doi: 10.1016/S0140-6736(15)00946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2006;368:2211–2218. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 91.Gates B. The next epidemic-lessons from Ebola. N Engl J Med. 2015;372:1381–1384. doi: 10.1056/NEJMp1502918. [DOI] [PubMed] [Google Scholar]
- 92.WHO | WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. WHO. http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/ (accessed March 9, 2016).
- 93.World Health Organization . Antimicrobial resistance: global report on surveillance, 2014. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 94.World Health Organization . Global tuberculosis report 2015. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 95.Shallcross LJ, Howard SJ, Fowler T, Davies SC. Tackling the threat of antimicrobial resistance: from policy to sustainable action. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140082. doi: 10.1098/rstb.2014.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005;438:310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- 97.Whitmee S, Haines A, Beyrer C. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation-Lancet Commission on planetary health. Lancet. 2015;386:1973–2028. doi: 10.1016/S0140-6736(15)60901-1. [DOI] [PubMed] [Google Scholar]
- 98.Rockström J, Steffen W, Noone K. A safe operating space for humanity. Nature. 2009;461:472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 99.Malhotra J, Malvezzi M, Negri E, Vecchia CL, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48:889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 100.Bidell MR, McLaughlin M, Faragon J, Morse C, Patel N. Desirable characteristics of hepatitis C treatment regimens: a review of what we have and what we need. Infect Dis Ther. 2016 doi: 10.1007/s40121-016-0118-x. published online July 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.World Health Organization (WHO) WHO Mortality Database Version February 2014. World Health Organization (WHO); Geneva, Switzerland: 2014. [Google Scholar]
- 102.Olshansky SJ, Ault AB. The fourth stage of the epidemiologic transition: the age of delayed degenerative diseases. Milbank Q. 1986;64:355–391. [PubMed] [Google Scholar]
- 103.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 104.Lawitz E, Mangia A, Wyles D. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 105.Scheltens P, Blennow K, Breteler MMB. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 106.Matthews FE, Arthur A, Barnes LE, the Medical Research Council Cognitive Function and Ageing Collaboration A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 109.Ösby U, Westman J, Hällgren J, Gissler M. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987–2010. Eur J Public Health. 2016 doi: 10.1093/eurpub/ckv245. published online Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fekadu A, Medhin G, Kebede D. Excess mortality in severe mental illness: 10-year population-based cohort study in rural Ethiopia. Br J Psychiatry. 2015;206:289–296. doi: 10.1192/bjp.bp.114.149112. [DOI] [PubMed] [Google Scholar]
- 111.Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature Mortality Among Adults With Schizophrenia in the United States. JAMA Psychiatry. 2015;72:1172–1181. doi: 10.1001/jamapsychiatry.2015.1737. [DOI] [PubMed] [Google Scholar]
- 112.Ferrari AJ, Norman RE, Freedman G. The burden attributable to mental and substance use disorders as risk factors for suicide: findings from the Global Burden of Disease Study 2010. PLoS One. 2014;9:e91936. doi: 10.1371/journal.pone.0091936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Forouzanfar MH, Alexander L, Anderson HR, the GBD 2013 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lönnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014;2:730–739. doi: 10.1016/S2213-8587(14)70109-3. [DOI] [PubMed] [Google Scholar]
- 115.Lim SS, Vos T, Flaxman AD. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gregg EW, Li Y, Wang J. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 118.Dinesh Shah A, Langenberg C, Rapsomaniki E. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1·9 million people. Lancet. 2015;385(suppl 1):S86. doi: 10.1016/S0140-6736(15)60401-9. [DOI] [PubMed] [Google Scholar]
- 119.Vos T, Barber RM, Bell B, the Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garcia-Garcia G, Monteon-Ramos JF, Garcia-Bejarano H. Renal replacement therapy among disadvantaged populations in Mexico: a report from the Jalisco Dialysis and Transplant Registry (REDTJAL) Kidney Int Suppl. 2005;68:S58–S61. doi: 10.1111/j.1523-1755.2005.09710.x. [DOI] [PubMed] [Google Scholar]
- 121.Lunyera J, Mohottige D, Isenburg MV, Jeuland M, Patel UD, Stanifer JW. CKD of Uncertain Etiology: A Systematic Review. Clin J Am Soc Nephrol. 2016;11:379–385. doi: 10.2215/CJN.07500715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomas B, Wulf S, Bikbov B. Maintenance Dialysis throughout the World in Years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–2633. doi: 10.1681/ASN.2014101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xie X, Liu Y, Perkovic V. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 124.Özyilmaz A, de Jong PE, Gansevoort RT. Screening for chronic kidney disease can be of help to prevent atherosclerotic end-organ damage. Nephrol Dial Transplant. 2012;27:4046–4052. doi: 10.1093/ndt/gfs438. [DOI] [PubMed] [Google Scholar]
- 125.Komenda P, Ferguson TW, Macdonald K. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis. 2014;63:789–797. doi: 10.1053/j.ajkd.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 126.Cummings P, Rivara FP, Olson CM, Smith KM. Changes in traffic crash mortality rates attributed to use of alcohol, or lack of a seat belt, air bag, motorcycle helmet, or bicycle helmet, United States, 1982–2001. Inj Prev. 2006;12:148–154. doi: 10.1136/ip.2005.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bunn F, Collier T, Frost C, Ker K, Roberts I, Wentz R. Traffic calming for the prevention of road traffic injuries: systematic review and meta-analysis. Inj Prev. 2003;9:200–204. doi: 10.1136/ip.9.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abu-Zidan FM, Abbas AK, Hefny AF, Eid HO, Grivna M. Effects of seat belt usage on injury pattern and outcome of vehicle occupants after road traffic collisions: prospective study. World J Surg. 2012;36:255–259. doi: 10.1007/s00268-011-1386-y. [DOI] [PubMed] [Google Scholar]
- 129.Staton C, Vissoci J, Gong E. Road traffic injury prevention initiatives: a systematic review and metasummary of effectiveness in low and middle income countries. PLoS One. 2016;11:e0144971. doi: 10.1371/journal.pone.0150150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pérez K, Marí-Dell'Olmo M, Tobias A, Borrell C. Reducing road traffic injuries: effectiveness of speed cameras in an urban setting. Am J Public Health. 2007;97:1632–1637. doi: 10.2105/AJPH.2006.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Traffic general directorate Spanish road safety strategy 2011–2020. Executive summary. http://ec.europa.eu/transport/road_safety/pdf/20160107_estrategico_20 20_006.pdf (accessed April 13, 2016).
- 132.ENSR National road safety strategy. 2008–2015. http://ec.europa.eu/transport/road_safety/pdf/20151210_1_portugal.pdf (accessed April 13, 2016).
- 133.Luoma J, Sivak M, the University of Michigan. Sustainable Worldwide Transportation. University of Michigan. Transportation Research Institute . Road-safety management in Brazil, Russia, India, and China. University of Michigan Transportation Research Institute; Ann Arbor, MI: 2012. [Google Scholar]
- 134.Hyder AA, Vecino-Ortiz AI. BRICS: opportunities to improve road safety. Bull World Health Organ. 2014;92:423–428. doi: 10.2471/BLT.13.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bishai D, Quresh A, James P, Ghaffar A. National road casualties and economic development. Health Econ. 2006;15:65–81. doi: 10.1002/hec.1020. [DOI] [PubMed] [Google Scholar]
- 136.United Nations Office on Drugs and Crime . Global study on homicide 2013: trends, contexts, data. UNODC; Vienna, Austria: 2013. [Google Scholar]
- 137.Butchart A, Mikton C, World Health Organization. United Nations Office on Drugs and Crime. United Nations Development Programme . Global status report on violence prevention, 2014. World Health Organization; Geneva: 2014. [Google Scholar]
- 138.Darke S. The toxicology of homicide offenders and victims: a review. Drug Alcohol Rev. 2010;29:202–215. doi: 10.1111/j.1465-3362.2009.00099.x. [DOI] [PubMed] [Google Scholar]
- 139.Kuhns JB, Exum ML, Clodfelter TA, Bottia MC. The prevalence of alcohol-involved homicide offending: a meta-analytic review. Homicide Stud. 2013 published online July 3. [Google Scholar]
- 140.Cusimano MD, Sameem M. The effectiveness of middle and high school-based suicide prevention programmes for adolescents: a systematic review. Inj Prev. 2011;17:43–49. doi: 10.1136/ip.2009.025502. [DOI] [PubMed] [Google Scholar]
- 141.Hahn RA, Bilukha O, Crosby A, the Task Force on Community Preventive Services Firearms laws and the reduction of violence: a systematic review. Am J Prev Med. 2005;28(suppl 1):40–71. doi: 10.1016/j.amepre.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 142.Motohashi Y, Kaneko Y, Sasaki H, Yamaji M. A decrease in suicide rates in Japanese rural towns after community-based intervention by the health promotion approach. Suicide Life Threat Behav. 2007;37:593–599. doi: 10.1521/suli.2007.37.5.593. [DOI] [PubMed] [Google Scholar]
- 143.Mann JJ, Apter A, Bertolote J. Suicide prevention strategies: a systematic review. JAMA. 2005;294:2064–2074. doi: 10.1001/jama.294.16.2064. [DOI] [PubMed] [Google Scholar]
- 144.WHO . Global report on drowning: preventing a leading killer. World Health Organization; Geneva: 2014. [Google Scholar]
- 145.Wallis BA, Watt K, Franklin RC, Taylor M, Nixon JW, Kimble RM. Interventions associated with drowning prevention in children and adolescents: systematic literature review. Inj Prev. 2015;21:195–204. doi: 10.1136/injuryprev-2014-041216. [DOI] [PubMed] [Google Scholar]
- 146.Leavy JE, Crawford G, Portsmouth L. Recreational drowning prevention interventions for adults, 1990–2012: a review. J Community Health. 2015;40:725–735. doi: 10.1007/s10900-015-9991-6. [DOI] [PubMed] [Google Scholar]
- 147.Burnham G, Lafta R, Doocy S, Roberts L. Mortality after the 2003 invasion of Iraq: a cross-sectional cluster sample survey. Lancet. 2006;368:1421–1428. doi: 10.1016/S0140-6736(06)69491-9. [DOI] [PubMed] [Google Scholar]
- 148.Burnham G, Roberts L. A debate over Iraqi death estimates. Science. 2006;314:1241. doi: 10.1126/science.314.5803.1241b. [DOI] [PubMed] [Google Scholar]
- 149.Tapp C, Burkle FM, Jr, Wilson K. Iraq War mortality estimates: a systematic review. Confl Health. 2008;2:1. doi: 10.1186/1752-1505-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burnham G, Doocy S, Dzeng E, Lafta R, Roberts L. The Human Cost of the War in Iraq. A Mortality Study, 2002–2006. Bloomberg School of Public Health, Johns Hopkins University; School of Medicine, Al Mustansiriya University; Center for International Studies, Massachusetts Institute of Technology; Baltimore, MD; Baghdad, Iraq; Cambridge, MA: 2006. [Google Scholar]
- 151.Syrian Arab Republic. OCHA http://www.unocha.org/syria (accessed April 18, 2016).
- 152.United Nations High Commissioner for Refugees UNHCR: Total number of Syrian refugees exceeds four million for first time. UNHCR. http://www.unhcr.org/559d67d46.html (accessed April 18, 2016).
- 153.Streatfield PK, Khan WA, Bhuiya A. Cause-specific mortality in Africa and Asia: evidence from INDEPTH health and demographic surveillance system sites. Glob Health Action. 2014 doi: 10.3402/gha.v7.25362. published online Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Flaxman AD, Serina PT, Hernandez B, Murray CJL, Riley I, Lopez AD. Measuring causes of death in populations: a new metric that corrects cause-specific mortality fractions for chance. Popul Health Metr. 2015;13:28. doi: 10.1186/s12963-015-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lozano R, Freeman MK, James SL, the Population Health Metrics Research Consortium (PHMRC) Performance of InterVA for assigning causes of death to verbal autopsies: multisite validation study using clinical diagnostic gold standards. Popul Health Metr. 2011;9:50. doi: 10.1186/1478-7954-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bloomberg Philanthropies Launches $100 Million Data for Health Program in Developing Countries Bloomberg Philanthropies. http://www.bloomberg.org/press/releases/bloomberg-philanthropies-launches-100-million-data-health-program-developing-countries/ (accessed April 21, 2016).
- 157.AbouZahr C, de Savigny D, Mikkelsen L, Setel PW, Lozano R, Lopez AD. Towards universal civil registration and vital statistics systems: the time is now. Lancet. 2015;386:1407–1418. doi: 10.1016/S0140-6736(15)60170-2. [DOI] [PubMed] [Google Scholar]
- 158.Lopez AD, Setel PW. Better health intelligence: a new era for civil registration and vital statistics? BMC Med. 2015;13:73. doi: 10.1186/s12916-015-0333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.AbouZahr C, de Savigny D, Mikkelsen L. Civil registration and vital statistics: progress in the data revolution for counting and accountability. Lancet. 2015;386:1373–1385. doi: 10.1016/S0140-6736(15)60173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.ihmeuw/dismod_mr. GitHub. https://github.com/ihmeuw/dismod_mr (accessed April 6, 2016).
- 161.IHME/CODEm-2010. GitHub. https://github.com/IHME/CODEm-2010 (accessed April 6, 2016).
- 162.Helleringer S, Pison G, Kanté AM, Duthé G, Andro A. Reporting errors in siblings' survival histories and their impact on adult mortality estimates: results from a record linkage study in Senegal. Demography. 2014;51:387–411. doi: 10.1007/s13524-013-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stover J, Brown T, Marston M. Updates to the Spectrum/Estimation and Projection Package (EPP) model to estimate HIV trends for adults and children. Sex Transm Infect. 2012;88(suppl 2):i11–i16. doi: 10.1136/sextrans-2012-050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.WHO | Estimates for 2000–2015. WHO. http://www.who.int/healthinfo/global_burden_disease/estimates_child_cod_2015/en/ (accessed March 9, 2016).
- 165.World Health Organization Number of deaths (thousands)-Data by WHO region. Global Health Observatory data repository. http://apps.who.int/gho/data/view.main.CM1300N?lang=en (accessed April 21, 2016).
- 166.JH Bloomberg School of Public Health Maternal Child Epidemiology Estimation. Johns Hopkins Bloomberg School of Public Health. http://www.jhsph.edu/research/centers-and-institutes/institute-for-international-programs/current-projects/maternal-child-epidemiology-estimation/ (accessed April 21, 2016).
- 167.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, the Child Health Epidemiology Reference Group of the World Health Organization and UNICEF Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ferlay J, Soerjomataram I, Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 169.UNAIDS Fact sheet 2015. http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet (accessed April 6, 2016).
- 170.World Population Prospects - Population Division - United Nations http://esa.un.org/unpd/wpp/ (accessed April 5, 2016).
- 171.Hill K, Zimmerman L, Jamison DT. Mortality risks in children aged 5–14 years in low-income and middle-income countries: a systematic empirical analysis. Lancet Glob Health. 2015;3:e609–e616. doi: 10.1016/S2214-109X(15)00044-3. [DOI] [PubMed] [Google Scholar]
- 172.World Health Organization (WHO) WHO methods and data sources for country-level causes of death 2000–2012. WHO; Geneva, Switzerland: 2014. [Google Scholar]
- 173.World Health Organization (WHO) WHO methods and data sources for global burden of disease estimates 2000–2011. WHO; Geneva, Switzerland: 2013. [Google Scholar]
- 174.WHO | Estimates for 2000–2012. WHO. http://www.who.int/healthinfo/global_burden_disease/estimates/en/ (accessed June 11, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
New results from the Global Burden of Disease Study 2015 examine causes of death and categorise regions according to the Socio-demographic Index, or ‘SDI’.