Abstract
Gene electrotransfer is a promising non-viral method of gene delivery. In our in vitro study we addressed open questions about this multistep process: how electropermeabilization is related to electrotransfer efficiency; the role of DNA electrophoresis for contact and transfer across the membrane; visualization and theoretical analysis of DNA-membrane interaction and its relation to final transfection efficiency; and the differences between plated and suspended cells. Combinations of high-voltage and low-voltage pulses were used. We obtained that electrophoresis is required for the insertion of DNA into the permeabilized membrane. The inserted DNA is slowly transferred into the cytosol, and nuclear entry is a limiting factor for optimal transfection. The quantification and theoretical analysis of the crucial parameters reveals that DNA-membrane interaction (NDNA) increases with higher DNA concentration or with the addition of electrophoretic LV pulses while transfection efficiency reaches saturation. We explain the differences between the transfection of cell suspensions and plated cells due to the more homogeneous size, shape and movement of suspended cells. Our results suggest that DNA is either translocated through the stable electropores or enters by electo-stimulated endocytosis, possibly dependent on pulse parameters. Understanding of the mechanisms enables the selection of optimal electric protocols for specific applications.
One of the promising methods for delivery of genetic material into a cell is gene electrotransfer, which uses locally delivered electric pulses (electroporation) to transfer DNA into the cell1,2. The first in vivo gene electrotransfer was demonstrated in the early nineties of the last century by Titomirov3 and by several other independent studies4,5,6,7,8 and has since then been extensively studied9,10,11,12. In contrast to viral vector transfer, the use of electric pulses for gene delivery represents a safer method, which is not hampered by concerns of immunogenicity and pathogenicity13. Different protocols where designed for in vitro and in vivo applications, either employing short hundreds-of-microsecond pulses4, long millisecond pulses7,8,14 or combining high-voltage (10–1000 μs) and low-voltage (10–400 ms) pulses12,15,16,17,18,19. We have recently shown in vitro that20,21 longer electric pulses are optimal for high transfection efficiency but reduce viability, while shorter pulses enable moderate transfection efficiency and preserve viability. For clinical applications it is also crucial to achieve sufficient transfection efficiency in a given target tissue (e.g. tumor, muscle, skin)10,22. Gene electrotransfer has in recent years emerged as the most promising non-viral method for delivery of plasmid DNA (pDNA), oligonucleotides and short RNA molecules in gene therapy for a series of conditions like cancer, autoimmune and inflammatory diseases23. The first successful clinical trials have already been completed11. Recently it has been identified as an ideal method for DNA vaccination for hepatitis, HIV and cancer treatment24,25,26 since electric pulses play a dual role; they enable gene delivery and act as an adjuvant.
However, several papers stress that the mechanisms of gene electrotransfer are still not fully understood10,23,27,28. The current description of the process defines several steps: i) electropermeabilization of the cell membrane, ii) contact of the pDNA with the cell membrane (formation of a complex), iii) translocation across the membrane, iv) transfer to and into the nucleus and gene expression10,18,19,29,30. In addition, new observations of the role of the cytoskeleton and endocytosis have also been published31,32. From the perspective of clinical applications, all of these steps relate to the barriers which must be overcome for sufficient DNA delivery and expression in the target tissue.
The first step is cell membrane electropermeabilization, where electric pulses are applied and the transmembrane voltage is induced due to Maxwell-Wagner polarizations. Above a critical (threshold) transmembrane potential (between 0.2–1 V), a high electric field leads to the formation of hydrophilic pores, thus enabling the transfer of molecules into cells2. Ions and small molecules enter the cells by free diffusion through pores during and after pulse delivery33,34, while the transfer of large pDNA is not governed by diffusion, but is a more complex process15,18,19,29,30,34.
The second step is the interaction of the DNA with the permeabilized cell membrane. It was shown that DNA has to be present in close proximity of the cell membrane at the moment of pulse delivery, enabling contact between the DNA and the electropermeabilized membrane16,17,20,21,29,30,35,36. The DNA-membrane complex formation was first suggested when the effect of divalent cations37,38,39 on electrotransfection was studied. The complex presents binding to the membrane or partial insertion of DNA in the permeabilized cell membrane15,16,19,29,30. The first visualization performed by labeling DNA with fluorescent dye TOTO-141 showed complex formation only on the cathodic side of the cell. In addition, within tissues the extracellular matrix represents another barrier which reduces the amount of DNA interacting with the target cells, by hindering the homogeneous distribution of the injected DNA and by decreased DNA mobility during electric pulses40,41,42. The free diffusion of DNA is almost negligible compared to electrophoretic drag, therefore, the selection of sufficiently high and long pulses is important12,16,17,21,40,43.
The third step is the transfer of DNA into the cells. The process of DNA transfer across the cell membrane has not been directly visualized yet. The DNA enters the cytoplasm several minutes after pulse application41,48; first the contact of the DNA with the permeabilized cell membrane is formed15,19,34,44, then the DNA is either translocated across the cell membrane by an unidentified mechanism20,23, or alternatively, the DNA enters the cells by electric-field-stimulated endocytosis32,45,46. Recently32, it was suggested that endocytosis could play an important role in gene electrotransfer, however, our results do not support this hypothesis47.
The last step for efficient electrotransfer is the intracellular trafficking of the DNA through cytosol and nuclear import. Different cytoplasmic structures hinder DNA mobility inside the cytosol, and foreign DNA is also exposed to DNase activity48,49. It was demonstrated that50 pDNA is probably actively transferred to the nucleus via the tubulin network. Further, during mitosis when the nuclear envelope is disintegrated, the highest electrotransfer efficiency was obtained51. It was shown that plasmids containing a nuclear-localization-sequence (NLS) that enables active transport across nuclear pores increased the gene electrotransfer efficiency50,52,53.
In spite of numerous experimental studies, only a few are combined with a theoretical description of DNA-membrane interaction34,37, the quantification of the number of plasmid DNA54 or the DNA mobility in a complex environment such as tissue40,41,55. In this paper we present a systematic in vitro analysis of all the steps of gene electrotransfer. In addition, the differences between cells in a suspension and plated cells are discussed. The most undefined process is the mode of DNA transfer across the cell membrane. We approached this question by different pulse combinations of high-voltage (HV) and low-voltage (LV) pulses (e.g. HV+LV, LV+HV), enabling separate analysis of electropermeabilization and electrophoresis. Our results provide new insights into the processes important for further development of in vitro gene electrotransfer protocols for biotechnological and biomedical applications.
Methods
Cell cultures, electroporation buffers and plasmid
Chinese hamster ovary CHO cells (European Collection of Cell Cultures) were grown in F12 HAM (Gibco) supplemented with 1 mM L glutamine, 10% fetal bovine serum (PAA, Austria) and antibiotics at 5% CO2 and 37°C. Most of the experiments were performed on cells in the early exponential growth phase (24 h after trypsinization). To evaluate the effect of the stage of the cell culture, cells in the early plato phase (72 h after trypsinization) arrested in the G1 stage of the cell cycle were used. The electroporation buffer was iso-osmolar 10 mM NaH2PO4/Na2HPO4, 1 mM MgCl2 and 250 mM sucrose, pH = 7.2, while for the visualization of the DNA-membrane interaction with TOTO, we used 10 mM KH2PO4/K2HPO4 with the same additives. For all gene electrotransfer experiments, we used plasmid pEGFP-N1 (Clontech Laboratories Inc., Mountain View, CA, USA).
Electric pulse protocols
Three different pulsing protocols, consisting of either high-voltage HV pulses only, low-voltage LV pulses only, or a combination of both HV and LV pulses (HV-LV combinations) were used for both plated cells and cells in suspensions. To generate electric pulses, a CliniporatorTM (Igea, Italy) and a prototype of the electric pulse generator described in Ref. 19 were used. Parallel wire electrodes were used for the plated cells18 and parallel plate electrodes (Eppendorf, Germany) for cell suspension. The distance between the electrodes was 4 mm. All the experiments were repeated at least three times at different dates. In all experiments, the standard HV pulses were 4 × 200 μs pulses, 1 Hz with pulse amplitude U = 400 V (applied electric field EHV = 1 kV/cm), except in experiments where E was varied, and the standard LV pulse was 1 × 100 ms, with pulse amplitude 30 V (ELV = 0.075 kV/cm – LV30), except in experiments where higher LV was also used ELV = 0.137 kV/cm (LV55). In the HV+LV protocol, the LV pulse was applied after the HV, with a lag of 20 ms18,19, while for the LV+HV protocol, the sequence was reversed.
Cell membrane permeabilization
Cell membrane permeabilization was determined by the uptake of 150 μM propidium iodide (PI) (Invitrogene, Germany), added immediately before electroporation. For each experiment, a negative control - cells not exposed to an electric field, and positive control - cells exposed to 1.8 kV/cm (100% permeabilization) were prepared. The fluorescence intensity was determined 3 minutes after electroporation in a microplate reader (Tecan, Austria) at a 535/617 nm (excitation/emission) wavelength. The percentage of electroporated cells was calculated as the relative fluorescence intensity vs. the positive control36.
Viability
For plated cells, viability was determined by a manual cell count under bright field optics on an inverted microscope (Zeiss 200, Axiovert, Germany) at 20× objective magnification. The cell viability was calculated as the ratio between the number of all cells counted in the treated sample and the number of all cells in the control sample18,47. For cell suspensions, viability was determined by clonogenic assay. After electroporation, cells were plated in concentrations of 250 cells per 60 mm Petri dish and grown for six days. The colonies were counted and the viability (%) was determined as the ratio between the number of colonies in the treated sample and the number of all cells in the control sample that were not exposed to electric pulses.
Electrotransfer of plasmid DNA
Plated cells: 5 × 104 cells were seeded in 24 multiwell plates and maintained in culture for 24 h, then the growth media was replaced with a pulsing buffer containing different concentrations of the plasmid DNA (cDNA). After a 2–3 min incubation, samples were electroporated, fetal bovine serum (PAA, Austria) was added (37 μl) and the cells were grown for another 24 h in the culture medium. The next day, the electrotransfer efficiency was determined by fluorescent microscopy (Zeiss 200, Axiovert, Germany, at 488/509 nm). At least 7 images were acquired per parameter for each experiment and the percentage transfection (%TR) was determined as a ratio between the fluorescent cells and the total number of cells counted under bright field optics18. For HV-LV pulsing protocols, the average maximal fluoresce intensity – FLGFP [A.U.] was also determined.
Cells in suspension: cell cultures were trypsinized 24 hours before the experiments. On the day of experiment, a cell suspension of 2.5 × 106 cells/ml was prepared in an electroporation buffer. The optimal cDNA was 40 μg/ml, while sub-optimal cDNA were 10 μg/ml and 5 μg/ml. In addition, we also tested cDNA = 100 μg/ml. The electroporation procedure was the same as for plated cells. Cells were plated in 25 cm2 culture dishes for 24 hours. The next day, we prepared a cell suspension (1 × 106 cells/ml) in phosphate-buffered saline (PBS) and the GFP expression was measured by flow cytometry with a Coulter EPICS Altra flow cytometer (Beckman Coulter Electronics) and with a CyFlow space flow cytometer (Partec). For each sample, 10000 cells were analyzed. The collected data were analyzed using FlowJo (Tree Star) software. From this percentage of transfected cells and average fluorescence intensity were obtained.
Visualization of DNA-membrane interaction and plasmid localization in the cytosol
To visualize DNA interaction with the cell membrane, we stained pEGFP-N1 with 2.3 × 10−4 M TOTO-1 nucleic acid stain (Molecular Probes – Invitrogen, Carlsbad, California, USA) as described in Ref. 29. Cells (1 × 105 cells/ml) were plated in a Labtech chamber for 1 h in a cell culture medium. Then electroporation media with TOTO-labeled DNA (10 μg/μl and cDNA 2 μg/μl to obtain a detectable fluorescence) was added to the cells and different combinations of HV and LV pulses were applied. The HV amplitude was 1.4 kV/cm. The interaction of the DNA with the membrane was determined by fluorescent microscopy (Zeiss 200, Axiovert, Germany) at 100× objective magnification at 514/533 nm. The TOTO fluorescence intensity (FLTOTO) profiles were analyzed (MetaMorph) and the average maximal fluoresce intensity – FLTOTO [A.U.] was obtained from recorded images (at least 5 images per parameter for each experiment). For localization of the plasmid in the cytosol, the plasmid was labeled with rhodamin dye (TM-Rhodamin, Mirus, USA), observed at 100× objective magnification.
Stage of the cell culture
Gene electrotransfer was performed in cell suspensions trypsinized 24 h before the experiment, or on cells cultured for 72 h, to obtain confluent cell cultures in the early plato phase arrested in the G1 phase of the cell cycle. Three independent experiments were performed.
Analytical calculations
Calculation of the permeabilized surface of the cell membrane
If a cell is exposed to an external electric field E, a transmembrane voltage Um is induced on the cell membrane. When Um exceeds the threshold voltage Uc, then the part of the cell membrane where |Um| > Uc is permeabilized2. For a spherical cell of radius R, a well-known form of Um for physiological conditions34,56 is valid:
 |
where θ is the angle that defines the point on the membrane with respect to the field direction. The above equation is valid for spherical cells, while for spheroidal cells a similar equation is valid taking into account also the shape of the cells57. One can define the critical angle θc, as the angle where the transmembrane voltage equals the critical voltage:
 |
while the critical electric field Ec is defined as E where θc = 0, therefore: Ec = Uc/1.5R. From this, the total area exposed to an above-threshold transmembrane voltage – the permeabilized area – can be obtained:
 |
where S0 is total surface of the cell membrane.
Analysis of DNA electrophoresis during HV and LV pulses
Electrophoresis is one of the mechanisms that was shown to be important for the efficient delivery of DNA molecules into cells by electric pulses15,16, especially when plasmid concentration is relatively low18,19. We present the calculation of the average traveled distance - L of a pDNA in aqueous solutions, and from this we estimate the number of DNA molecules available for contact with the permeabilized cell membrane (NDNA) for our in vitro conditions. We have followed the derivation of Zaharoff and Yuan41. Briefly, the electrophoretic force acts on the negatively charged DNA molecules and drags it toward the cathodic side of the cell membrane: F = eeff E, where the effective charge (eeff) depends on the ionic strength of the solution and the length of the plasmid (eeff = 0.066 e per base pair × 4.7 kbp for pEGFP plasmid). We can use the approximation that during the electric pulses we have a steady-state condition41, thus:
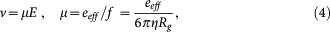 |
where v is velocity of the molecular movement, μ is electrophoretic mobility and f is the Stokes' frictional drag, Rg ≈ 100 nm is the approximate radius of gyration and η = 0.01 g cm−1 s−1 is the viscosity of the medium. In all calculations, E represents the homogeneous electric field strength, which is justified for our geometry of two parallel electrodes. The distance L traveled due to electrophoresis can be thus calculated from the total duration of the electric pulses tE:
 |
Here we have to stress that the mobility μ of a DNA molecule during electric pulses is a complex function of the electric field strength, due to several effects like the elongation and orientation of DNA molecules in the electric field. Therefore, mobility is not the same for HV or LV pulses, however, as already shown41, we can use the approximation of constant μ for our conditions. For a 4.7 kbp supercoiled pEGFP, μ is similar as in Ref. 34: μ = 1.5 × 104 μm2/Vs; where 4.3 kbp pDNA was used, a similar value can be obtained directly from Eq. 4.
Results
Electropermeabilization, electrotransfection and cell viability for different electric field strengths
In Fig. 1A, we present the effect of the electric field strength (E) on the electropermeabilization (%PI positive cells), percentage transfection (%TR) and survival (%viable cells) for a train of four high-voltage pulses (HV) of 200 μs duration and 1 Hz repetition frequency for plated cells and for cells in a suspension. Electropermeabilization increased above a certain electric field threshold (Ec) and electrotransfer occurred only above the Ec. In a suspension, much higher maximal %TR was achieved (70%) compared to plated cells (40%), even though in both cases the maximal electropermeabilization was reached (around 100%). Plated cells were more affected by electric pulses in terms of cell survival, namely viability dropped to 55%, while for cells in suspension it was maintained at approximately 90% at the highest electric fields (>1.4 kV/cm). In Fig. 1B, the %TR is presented with respect to the electropermeabilized membrane area (Eq. 3).
Figure 1.
(A) Effect of electric field strength on the percentage transfection of CHO cells (%TR), viability (%Survival) and electropermeabilization (%PI uptake) for 4 × 200 μs pulses (HV) and 1 Hz repetition frequency. Results are shown for plated cells (closed symbols) and cells in a suspension (open symbols) and are presented as mean ± standard error of at least three independent experiments. (B) Dependence of %TR on the fraction of permeabilized membrane area Sc/S0 as given in Eq. 3, for plated cells and cells in a suspension. The bright shaded surface of the spherical cell represents the permeabilized cell membrane - Sc.
Role of electrophoresis - effect of plasmid concentration on electrotransfer efficiency
We studied electropermeabilization of the cell membrane, electrophoresis and DNA–membrane interaction by applying combinations of HV and LV pulses (HV, LV, HV+LV, LV+HV).
Electropermeabilization for different HV and LV pulses
In order to separate the electrophoretic effect of LV pulses from electropermeabilization, we determined the PI uptake for different combinations of HV and LV pulses on plated cells and two different pulse amplitudes for LV pulses (30 V and 55 V). We obtained a nearly 100% electropermeabilization for the HV, HV+LV30 and HV+LV55 pulsing protocols. No statistically significant PI uptake was obtained with the LV30 pulse, while for the LV55 pulse, PI uptake was 5%. A higher percentage transfection (%TR) was observed for both combinations of HV+LV protocols (HV+ LV33 and HV+ LV55) and approximately 2% of cells were transfected with only the LV55 pulse (results not shown). Therefore, only electrophoretic non-permeabilizing LV30 pulses were used in further experiments with LV pulse.
The effect of plasmid concentrations on the electrotransfection efficiency for different HV and LV pulses – the role of electrophoresis
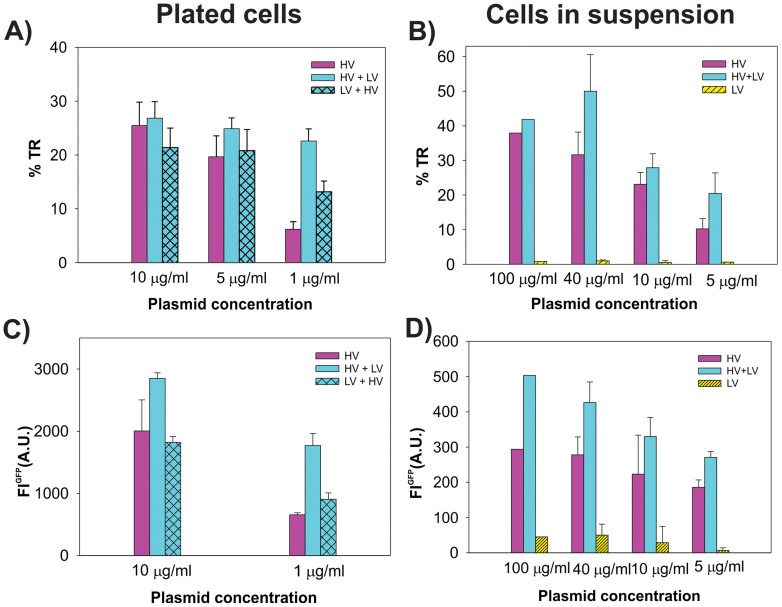
In Fig. 2 the effect of HV and LV pulses on the gene electrotransfer efficiency (%TR and fluorescence intensity of FLGFP) for different cDNA for cells in suspension and plated cells are presented. For cells in suspension (Figs. 1 B and D), the %TR and mean fluorescence intensity FLGFP obtained by flow cytometry are shown. We obtained that at cDNA = 100 μg/ml, no increase in %TR was obtained for HV+LV pulses compared to HV only (Fig. 2B). For all lower plasmid concentrations (cDNA = 40, 10 and 5 μg/ml), an increase was obtained for HV+LV compared to HV pulses (statistically significant only at 5 μg/ml, P = 0.044). For LV pulses only, a negligible GFP expression was obtained. Similar relationships among the pulsing protocols were obtained when analyzing the mean fluorescence intensity FLGFP (Fig. 2D). Again for all concentrations – up to 40 μg/ml, the HV+LV protocol led to a higher expression of GFP. Also at cDNA = 100 μg/ml, there appears to be a different increase of FLGFP for HV+LV vs. HV pulses only.
Figure 2. Effect of different HV-LV protocols on the electrotransfer efficiency for different plasmid concentrations.
Panels A and C plated cells, B and D cells in a suspension, % TR (A and B) and average maximal fluorescence intensity in A.U. Pulse parameters were: HV pulses (4 × 200 μs), 1 Hz and 1 × 100 ms LV EHV = 1 kV/cm (400 V) and ELV = 0.075 kV/cm. The results are presented as mean ± standard error of at least three independent experiments.
In Figs. 2A and C, the results for plated cells are shown, where the percentage transfection (%TR) and fluorescence intensity (FLGFP) were determined by fluorescence microscopy. For HV pulses, the %TR was directly dependent on the plasmid concentration; at cDNA = 10 μg/ml, the % TR was 25%, which dropped to only 6% at cDNA = 1 μg/ml. The HV+LV at cDNA = 1 μg/ml lead to a significant increase (P < 0.001) in the percentage transfection (23%) compared to HV pules only (6%). At 5–10 μg/ml plasmid concentrations, the LV pulse did not significantly affect the %TR (P > 0.05). It is important to note that the optimal plasmid concentration for plated cells was 10 μg/ml, while sub-optimal cDNA were 5 μg/ml and 1 μg/ml.
To further investigate the role of electrophoresis on plated cells, we used an additional pulsing protocol consisting of LV pulse applied before the HV pulses (LV+HV). The LV+HV protocol at cDNA = 1 μg/ml significantly increased the %TR cells compared to HV (P = 0.043), but was still less effective than the HV+LV protocol. With only the LV pulse, a negligible number of transfected cells (less than 1%) was obtained. The average maximal fluorescence intensity FLGFP (Fig. 2C) was highest for HV+LV, compared to the HV (P = 0.026) and LV+HV protocols. The increase of FLGFP for the LV+HV vs. the HV protocol was obtained at cDNA = 1 μg/ml, but it was not statistically significant (P > 0.05), while at cDNA = 10 μg/ml, all three pulsing protocols lead to a similar %TR.
Visualization of DNA-cell membrane interaction and internalization into cytosol
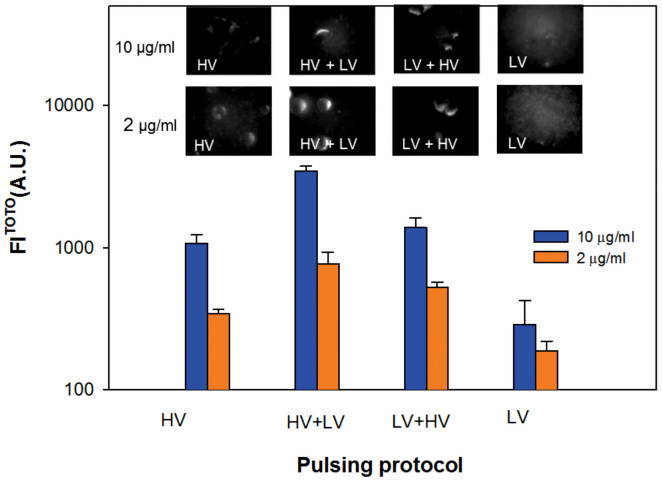
Direct visualization of the DNA-membrane interaction was performed by TOTO-1 labeled pDNA for different HV-LV protocols and different cDNA. For all protocols, DNA interaction with the cell membrane facing the cathode was observed, since DNA is negatively charged. Fluorescent images of treated cells exposed to HV, HV+LV, LV+HV and LV pulses are shown in Fig. 3 A. The images were acquired 2–5 min after pulsation. The fluorescence intensity FLTOTO corresponds to the amount of TOTO-labeled DNA interacting with the permeabilized cell membrane. Along the membrane, spots of high fluorescence intensity were observed for all pulsing protocols.
Figure 3. DNA – membrane interaction for different HV-LV protocols for cDNA = 10 μg/ml and 2 μg/ml.
The average maximal fluorescence intensity FLTOTO is presented as an average ± standard error; the representative images for HV, HV+LV, LV+HV and LV pulses are shown on top. Please note that the scale of fluorescence intensity in the micrographs is adjusted between Min = 100 and Max = 700 A.U. in images with cDNA = 10 μg/μl, and between Min = 100 and Max = 300 A.U. for cDNA = 2 μg/ml. Immediately after the labeled plasmid was added to the cells, different combinations of HV (4 × 200 μs, EHV = 1.4 kV/cm, 1 Hz) and LV pulses (1 × 100 ms, ELV = 0.137 kV/cm) were applied.
The values of FLTOTO (A.U.) were calculated from all recorded images for each pulsing protocol. For HV+LV pulses, an approximately 3× increase in FLTOTO was observed compared to HV pulses only for cDNA = 10 μg/ml and an approximately 2× increase of cDNA = 2 μg/ml (Fig. 3). On average, the FLTOTO of LV+HV was slightly higher compared to HV pulses, but significantly smaller than for the HV+LV protocol. No interaction (the fluorescence intensity was similar to the background) was detected for the non-permeabilizing LV pulse alone. On average, more DNA interaction with the cell membrane and higher FLTOTO was observed for higher amounts of TOTO-labeled DNA (cDNA = 10 μg/ml) compared to sub-optimal cDNA = 1 μg/ml. Further, the entry of pDNA into the cytosol was visualized by rhodamin-labeled DNA. After 10–15 minutes, labeled pDNA was observed inside the cytosol (see Discussion).
DNA entry into the nucleus
The last step and barrier for the successful electrotransfer of DNA is the nuclear import of pDNA. We designed additional experiments using cells in the exponential and plato stage of growth in order to compare the efficiency of gene electrotransfer of cells in the exponential vs. plato phase of cell culture. We obtained a significantly lower percentage transfection %TR for cells in the plato phase (15.5% ± 1.1) compared to cells in the exponential phase (55.7% ± 11.6) for both the HV and HV+LV (17.3 ± 1.4 plato vs. 52.2 ± 10.2 exponential phase) pulsing protocols as shown in Table 1. We should also stress here that our standard experimental protocol was performed on cells in the exponential phase of cell culture, which is also a standard protocol.
Table 1. Efficiency of gene electrotransfer in terms of % transfected cells (%TR) for CHO cells in a suspension in the exponential growth phase vs. cells in the plato phase for the HV (4 × 200 μs, EHV = 1.4 kV/cm, 1 Hz) and HV+LV (HV 4 × 200 μs, EHV = 1.4 kV/cm, LV 1 × 100 ms, ELV = 0.0375 kV/cm) pulsing protocols. The results are presented as mean ± standard error of at least three independent experiments, cDNA = 40 μg/μl.
| %TR PHASE OF CULTURE | HV | HV+LV | LV |
|---|---|---|---|
| exponential | 55.7 ± 11.6 | 52.2 ± 10.2 | 0.3 ± 0.1 |
| plato | 15.5 ± 1.1 | 17.3 ± 1.4 | 0.1 ± 0.0 |
Theoretical analysis
In the following two subsections we: i) calculate the permeabilized surface area of the cell and ii) analyze electrophoresis for different HV-LV pulses and calculate the NDNA – the number of DNA molecules available for contact with the permeabilized surface.
Calculation of the permeabilized surface of the cell membrane
The permeabilized surface Sc of the cell membrane depends on the applied field E and the threshold field Ec58: 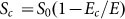 where S0 is total surface of the cell membrane. Thus E determines the area of the membrane which is permeabilized – Sc(E), and consequently the electrotransfer efficiency. In Fig. 1B, dependence of the % of transfected cells on a normalized permeabilized membrane surface area Sc/S0 (Eq. 3) is shown. For plated cells, an approximately linear dependency on the permeabilized surface was obtained, while for cells in a suspension, a non-linear increase of Sc with E was observed.
where S0 is total surface of the cell membrane. Thus E determines the area of the membrane which is permeabilized – Sc(E), and consequently the electrotransfer efficiency. In Fig. 1B, dependence of the % of transfected cells on a normalized permeabilized membrane surface area Sc/S0 (Eq. 3) is shown. For plated cells, an approximately linear dependency on the permeabilized surface was obtained, while for cells in a suspension, a non-linear increase of Sc with E was observed.
Analysis of DNA electrophoresis during HV and LV pulses and estimations of the number of DNA molecules in contact with the permeabilized cell membrane
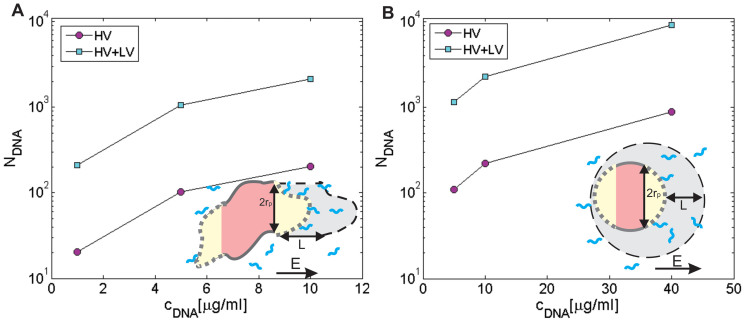
The traveled distance (L) of pDNA during HV and LV pulses due to the electrophoretic force can be obtained from Eq. 5 (see Methods): L = μ E tE. For a single LV pulse (1 × 100 ms, 75 V/cm) we obtain LLV ≈ 11 μm, LHV ≈ 1.2 μm and LHV+LV = 12.2 μm. Thus, for HV+LV pulses the traveled distance is approximately 10× longer compared to HV pulses only (4 × 200 μs, 1 kV/cm). If we assume that the electrophoretic force drags the negatively charged DNA molecules that are at a distance less that L from a cathodic site of a cell, we can estimate the number of DNA molecules – NDNA in the volume V, which are available for contact with the permeabilized part of the cell membrane. The results of the calculations of NDNA for different pulsing protocols are presented in Fig. 4. Thus, if the cDNA is sub-optimal, it is crucial that the DNA is electrophoretically dragged toward the membrane. For plated CHO cells we obtained the following equation for the volume V, from which the DNA molecules are dragged toward the permeabilized part of the cell membrane:
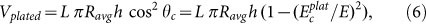 |
where Ravg is average radius of the plated CHO cells36 and the height of a cell is h = 4 μm. For plated cells the equation for the induced transmembrane potential for spheroidal cells is valid57, however, since the cells are oriented randomly, the average long radius Ravg is a valid approximation, since cells oriented with the long axis in parallel with E are first electroporated. For cells in a suspension that are of spherical shapes, the corresponding volume is:
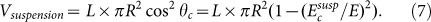 |
From this we can estimate the NDNA available for contact formation for different cDNA:
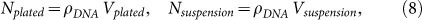 |
where ρDNA is the number density (ρDNA = cDNANA/MrDNA) of DNA for a given plasmid concentration. Thus, from Eqs. 6–8 we can calculate the NDNA in contact with the permeabilized cell membrane as shown in Fig. 4. The NDNA is directly proportional to L and consequently is approx. 10× lower for HV pulses compared to HV+LV pulses (Fig. 4). From our results we can estimate that for efficient transfection in vitro, several tens of molecules have to be in contact with the cell membrane, while for a smaller NDNA < 10 the transfection is very low (Figs. 2 and 4).
Figure 4. Theoretical analysis of DNA accumulation at the cell membrane due to electrophoretic force.
The number of DNA molecules (NDNA) available for contact with the permeabilized part of the cell membrane for different plasmid cDNA for plated cells (A) and cells in a suspension (B) are shown for HV and HV+LV pulses (corresponding transfection efficiencies are shown in Fig 1). The schematic representation shows the calculation of NDNA, where L is the distance traveled due to electrophoresis and rp is the radius of the permeabilized membrane (dotted, yellow area) for plated cells (A) and cells in a suspension (B). The gray shaded region represents the volume V from which DNA molecules are brought in contact with the cell membrane. The strength and length of pulses determine the distance L from which DNA can access the cell membrane (gray) and E determines the area of the membrane which is electropermeabilized.
For cells in a suspension (Fig. 4B), it can be seen that in general a similar relation is obtained: approx. 10× more NDNA are in contact with the membrane obtained for HV+LV vs. HV pulses, which enables more DNA copies to be transferred into the cytosol, leading to a higher fluorescence intensity in agreement with experimental observations for low plasmid concentrations (Fig. 2D). Thus, the strength and length of the electric pulses determine the distance L from which the DNA can access the cell and E determines the area of the membrane which is electropermeabilized -Sc. It is also clear that since NDNA linearly increases with cDNA and that probability of transfection directly depends on NDNA, there is a very strong correlation between the %TR and NDNA until saturation is reached, and the %TR does not increase for higher NDNA.
When comparing plated and cells in a suspension, we observed that in order to reach comparable transfection efficiencies, higher pDNA concentrations had to be used for cells in a suspension. Also, due to smaller sizes, the threshold for electrotransfer was reached at a higher E for cells in suspension compared to plated cells as shown in Ref. 36. Here we extend this study also to HV-LV pulsing combinations. Obviously, electrotransfer of plated cells behaves to some extent differently than with cells in a suspension. The saturation of the %TR for plated cells is already reached at 10 μg/ml compared to cells in suspension, where the highest %TR is reached at 40 μg/ml. Also, cDNA has to be higher for suspensions compared to plated cells. Moreover, in a suspension, a much higher overall maximal %TR can be obtained, both for CHO (up to 70%) and B16 cells (45%) compared to plated cells (38% for CHO and 25% for B16 cells), and also saturation is reached at a higher NDNA for cells in a suspension. The results for B16 cells are presented in Ref. 36.
Calculation of electric energy
One hypothesis can be that the electric energy needed for DNA interaction with the membrane (complex formation)29, is the crucial parameter for electrotransfection, since there exists an energy barrier between the negatively charged DNA and the negatively charged cell membrane. In the most simplified case, we can assume that the electric energy of the pulses We equals the work of the electrophoretic force Ae = Fe × L. Since L = μ E tE we obtain:
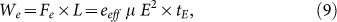 |
where E is the applied electric field strength and tE is total time duration of all pulses. We can thus estimate We of our standard HV (4 × 200 μs, 1 kV/cm), LV (1 × 100 ms, 0.075 kV/cm) and combined HV+LV pulses:
 |
where k = eeff μ.
The relation between electropermeabilization, electrophoresis, DNA-membrane interaction and GFP expression
In Table 2 we summarize the main results related to the different steps (Fig. 5) of electrotransfection. Electropermeabilization was quantified by the %PI of positive cells, DNA-membrane interaction by the analysis of the fluorescence intensity of TOTO-labeled pDNA (FLTOTO), gene expression by the fluorescence intensity of GFP (FLGFP) and the percentage transfection (%TR), viability by the percentage of survived cells (%Survival), electric energy (We) was calculated as defined in Eq. 10 and the number of DNA molecules available for contact with the permeabilized surface (NDNA) was obtained from Eqs. 7 and 8.
Table 2. Quantification of the process related to the different steps of gene electrotransfer: electropermeabilization – PI uptake (%PI), DNA–membrane interaction represented by the fluorescence intensity of TOTO-labeled DNA (FLTOTO), gene expression represented by the fluorescence intensity of GFP (FLGFP), percentage of transfection (%TR), viability (%survival) for cDNA = 10 μg/ml, electric energy obtained from Eq. 10 (We), and estimation of DNA accumulation at the cell membrane – the calculation of number of DNA molecules (NDNA) interacting with the permeabilized cell membrane calculated from Eqs. 7 and 8 for different pulsing protocols: HV: 4 × 200 μs, 1.0 kV/cm, 1 Hz; LV: 1 × 100 ms, 0.075 kV/cm*. All data are given for plated CHO cells; for better readability only mean values are given.
| Pulsing protocol | Permea-bilisation | Interaction | Transfection | Viability | El. energy | Electro-phoresis | |
|---|---|---|---|---|---|---|---|
| %PI | aFLTOTO [A.U.] | FLGFP [A.U.] | %TR | %Survival | We/k = E2 × tE ms×(kV/cm)2 | NDNA | |
| HV | |||||||
| cDNA = 1 μg/ml | 82.9% | 343 | 658 | 6% | 82% | 0.8 | 20 |
| cDNA = 10 μg/ml | 1073 | 2004 | 25% | 200 | |||
| HV+LV | |||||||
| cDNA = 1 μg/ml | 85.9% | 772 | 1769 | 23% | 91% | 1.36 | 250 |
| cDNA = 10 μg/ml | 3469 | 2852 | 27% | 2500 | |||
| LV+HV | |||||||
| cDNA = 1 μg/ml | 85.9%b | 527 | 904 | 13% | 87% | 1.36 | 20–250 |
| cDNA = 10 μg/ml | 1386 | 1819 | 21% | 200–2500b | |||
| LV | |||||||
| cDNA = 1 μg/ml | 1.5% | 187 | 208 | 0.9% | 78% | 0.56 | 0d |
| cDNA = 10 μg/ml | 289c | 258 | 0.3% | ||||
afor observation of DNA–membrane interaction, we used 2 μg/ml DNA and 1.4 kV/cm short-duration pulses instead of 1.0 kV/cm (see the M&M section) and for LV, 0.137 kV/cm instead of 0.075 kV/cm.
bsince LV is applied before HV, it contributes only to accumulation but not to insertion; the %PI is taken from the HV+LV protocol.
csimilar to the background value.
dthe permeabilized surface is zero (see Theoretical section).
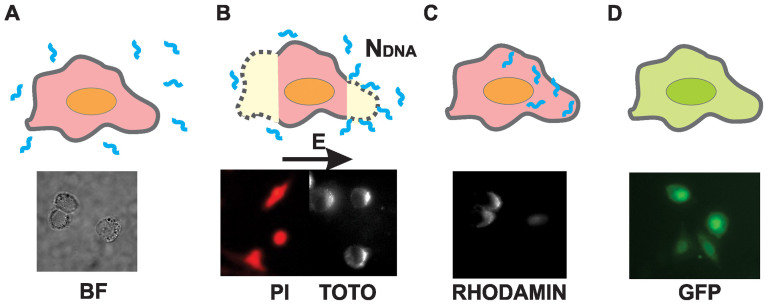
Figure 5. Different steps of gene electrotransfer.
(A) DNA is added to the electroporation buffer, (B) electropermeabilization of the cell membrane and DNA contact/insertion with/into the membrane, (C) transfer across the membrane and into the nucleus, (D) gene expression. Corresponding fluorescence images below: electropermeabilization (PI), interaction of DNA with the membrane (TOTO), transfer into cytoplasm (RHODAMIN) and gene expression (GFP).
No interaction was observed when only a non-permeabilizing LV30 pulse was applied. The detected TOTO fluorescence intensity FLTOTO can be directly related to the NDNA (Table 2), since the number of DNA molecules interacting with the permeabilized membrane is directly proportional to the plasmid concentration (Fig. 3). The HV+LV protocol yielded the highest FLTOTO followed by the LV+HV and HV protocols for all cDNA, in contrast to the %TR, where at optimal cDNA the %TR was similar for all protocols. When we analyze the DNA-membrane interaction (FLTOTO) in relation to the NDNA we can see that there is a direct relation between the theoretically calculated number of DNA molecules and FLTOTO, however, the DNA-membrane interaction is only one of several steps, and other factors such as DNA stability in the cytosol and cell viability are also crucial.
Another aspect is also how the fluorescence intensity of GFP (FLGFP) is related to the NDNA. Indeed, HV+LV pulses consistently resulted in a higher FLGFP compared to HV pulses only, for both plated cells and cells in a suspension (Fig. 2) for all plasmid concentrations. This is also in agreement with other studies (6,14), where for relatively long pulses (8 × 5 ms) FLGFP can be increased even if the maximal %TR is obtained. However, a statistically significant increase of FLGFP for HV+LV pulses compared to HV was obtained only at the lowest cDNA, while at the highest cDNA the difference in FLGFP between the two pulsing protocols was not statistically significant, which also suggests that at some point saturation is reached.
Discussion
Altogether our results confirm that electropermeabilization is a crucial step for efficient gene delivery, in accordance with other reports10,30,34,35,36,59. This was further confirmed by theoretical analyses of the %TR dependence on the fraction of permeabilized membrane surface Sc/S0, where approx. linear dependency was obtained47. However, electrotransfection is a complex process and for cells in suspension the experimental values of %TR deviate from the linear curve (see Fig. 1B); thus, the assumption that Sc/S0 directly correlates with %TR can only be a first approximation.
Further, electrophoresis of the pDNA in an electric field is also an important factor. In order to analyze electropermeabilization and electrophoresis, we used different HV-LV pulsing protocols and different pDNA concentrations (cDNA). We show that HV+LV pulsing protocols were more efficient in terms of percentage transfection (%TR) and fluorescence intensity (FLGFP) compared to HV pulses only, especially for low cDNA. For higher cDNA, saturation in terms of maximal %TR was obtained while FLGFP still increased for high cDNA (see Fig. 2). For in vivo applications, it is important to also analyze the effect of the amplitude of the LV pulse on gene electrotransfer24. We obtained that if LV is above the permeabilization threshold (e.g. LV55), a high %TR for HV+LV pulses can be attributed both to electrophoresis and to increased electropermeabilization due to the LV pulse, while for sub-threshold low-voltage pulses (LV30), LV has only an electrophoretic role.
We extended our previous studies on plated cells and cells in a suspension18,19,36 and analyzed the observed differences. This is relevant since cells in suspension are most often used in vitro, while plated cell are closer to in vivo conditions. A theoretical analysis is presented in Fig. 4, where the results of calculations of the NDNA (the number of DNA molecules that are available for contact with the permeabilized cell membrane) for different pulsing protocols are shown.
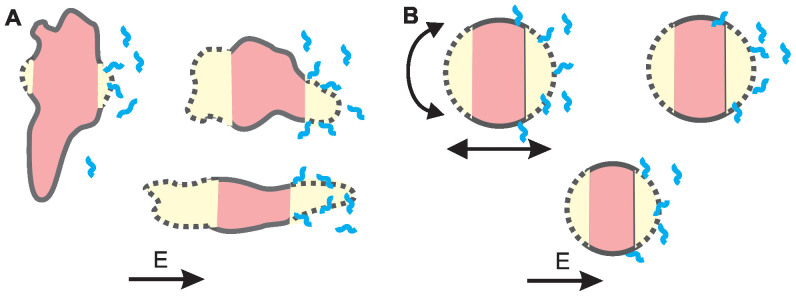
Based on all the results of HV-LV pulses, we confirmed the hypothesis that in conditions where we reach saturation (high NDNA), an additional electrophoretic LV pulse does not increase the %TR, while the GFP expression (FLGFP) can still be increased (see Figs. 2 and 4). Theoretical analysis showed that the strength and length of the electric pulses determine the distance L from which DNA can access the cell, and E also determines the area (Sc) of the membrane which is electropermeabilized. We could adequately explain the differences in transfection for different cDNA, but some data still seemed puzzling. The observed differences in electrotransfection between plated cells and cells in a suspension can be explained by several specific characteristics of the attached cells and suspended cells. This is presented in Fig. 6, where a schematic representation of electropermeabilization and electrotransfection of the plated cells (6A) and cells in a suspension (6B) is shown, and can be analyzed as follows:
different forces acting on cells in a suspension during pulse delivery, like fluid flow and electrophoretic movement due to the cells' negative surface charge, lead to the displacement and rotation of the cells. This leads to permeabilization of a larger area of the cell membrane (see Fig. 6B), which makes more binding sites available for DNA and increases the maximal NDNA as shown in Fig. 4B, thus allowing more DNA molecules to be transferred. It was shown previously30 that cell electropermeabilization and electrotransfer form different sides, enables higher transfection efficiency. Thus, the rotation of suspended cells enables higher transfection (Fig. 6). In addition, for plated cells due to geometry (cells are spread on the surface) smaller surface is available for DNA binding/insertion. This explains why saturation is reached at much higher NDNA values for cells in a suspension compared to plated cells (see Figs. 2 and 4). It also explains partially why the overall maximal %TR can be much higher in a suspension;
with a very low cDNA in a suspension, there is a very low probability of NDNA insertion into the membrane, since there is only a small number of molecules available for contact, and in combination with the movement of cells (displacement and rotation) in a suspension (Fig. 6B), this leads to a very low overall transfection for lower plasmid concentrations. Therefore, the cDNA for suspensions has to be increased compared to plated cells, where cells are attached to the surface and all DNA in the vicinity can form a contact with the membrane. For increasing cDNA and higher NDNA, the transfection efficiency can be significantly increased in a suspension, and saturation is only reached at very high numbers of NDNA (~103), as shown in Fig. 4B. In contrast, with plated cells saturation is already reached at much smaller cDNA and NDNA (~300), as can be seen in Fig. 4A, since the entire permeabilized surface is already occupied by DNA molecules.
the overall maximal %TR in a suspension is much higher than in plated cells (Fig. 1), but this is logical if we take into account that cells in a suspension have a very homogenous distribution of sizes. Thus, with optimal pulses most of the cells are permeabilized and remain viable, enabling good electrotransfer efficiency. On the other hand, plated cells are of different sizes and non-spherical shapes, with large variations in the minimal and maximal diameter, and with different orientations (Fig. 6A)36,57. Therefore, optimal pulses for the transfection of some cells directly lead to the irreversible electroporation or poor transfection of other cells. Thus, saturation for high NDNA and maximal %TR is limited for plated cells, due also to the non-homogeneous distribution of sizes and shapes.
Figure 6. Schematic representation of electrotransfection of plated cells (A) and cells in a suspension (B).
Cells in a suspension are spherical and with a narrow distribution of sizes, while plated cells have large variations in size, orientation and shape. Consequently, for plated cells it is very difficult to achieve pulses that would enable optimal electropermeabilization (yellow) and electrotransfer of all cells, in contrast to cells in suspension where this is possible. Furthermore, cells in a suspension rotate and move during pulses, thus a higher NDNA is needed and therefore a higher cDNA must be used compared to plated cells.
Further, we can assume two hypothesis regarding the most important parameters relevant for efficient electrotransfer; i) the electric energy of the pulses is the crucial parameter for efficient transfection or ii) the number of DNA molecules available for contact with the permeabilized membrane (NDNA) is the most relevant parameter. Based on the presented data and theoretical analysis, we can assume that not We but the accumulation of DNA molecules at the permeabilized membrane surface (NDNA) is the most important factor. NDNA is approximately linearly dependent on the E, tE, cDNA and permeabilized membrane surface Sc:
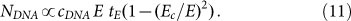 |
The assumption that the electrotransfer efficiency is proportional to NDNA is only an approximation, since these are stochastic processes and consequently the probability of electrotransfer is proportional to the NDNA available for electrotransfer until saturation is reached (explained below). From Table 2, it is clear that We could not be the only factor for efficient gene transfer, since the energy of the LV pulse alone is of the same order of magnitude as the energy of HV pulses, while transfection is negligible for the LV pulse. Electric energy also cannot explain the results obtained for the different cDNA.
On the other hand, NDNA is the parameter that is directly related with experimental results, especially for sub-optimal pDNA concentrations. It can be seen that the NDNA for HV+LV pulses (250) for the lowest pDNA concentration (1 μg/ml) on plated cells is similar to the NDNA for HV pulses only (200) at the highest cDNA = 10 μg/ml, which is in agreement with similar values of %TR (23% and 25%) obtained experimentally (Table 2). Therefore, %TR is directly related to NDNA up to the point where saturation is reached, and an increase in NDNA does not lead to a higher %TR (for plated cells saturation is reached at NDNA ≅ 250). Consequently, we can explain why at cDNA = 10 μg/ml, the HV+LV pulsing protocol (NDNA ≅ 2500) was similarly effective as the HV protocol (NDNA ≅ 250) for plated cells (approx. 25% TR for both protocols). For the LV+HV protocol, this is not so clear since LV is applied before permeabilizing HV pulses, and thus it contributes only to the accumulation but not to the insertion of pDNA into the membrane.
Therefore, the second hypothesis – that the number of DNA molecules interacting with the permeabilized cell membrane is a more relevant parameter – seems more plausible. If we analyze NDNA, it can be seen that electrophoresis is important for low cDNA, where the accumulation of DNA at the membrane is a limiting factor (Table 2), while for higher cDNA the saturation is reached. Therefore, the %TR is directly related to NDNA for sub-optimal plasmid concentrations, while for higher cDNA the %TR does not depend directly on NDNA due to saturation.
If we now summarize the main results in Table 2, we can see that there is a direct relation between the DNA-membrane interaction FLTOTO with the transfection efficiency (FLGFP and %TR) for lower plasmid concentrations. For increased cDNA (10 μg/ml), saturation is reached in terms of maximal %TR and partially for FLGFP. Therefore, at some point even if NDNA interacting with the membrane FLTOTO is increased, the %TR cannot be increased. This clearly shows on one hand that the transfection efficiency is directly related to electropermeabilization, and that it is a stochastic process in which more DNA in contact with the permeabilized part of the membrane enables more molecules to enter. But at some point, there is saturation as the process becomes similar to a chemical process, where there is a limited number of binding sites, naturally leading to saturation.
Another important aspect is also the physiological state of the cell and its viability, which limits transfection efficiency; namely the total yield of transfected cells is lowered since some cells are very effectively transfected while others are in poor physiological condition or die due to extensive membrane damage and the loss of cell homeostasis. Thus, depending on the type of application, the selection of the specific pulsing protocol depends on whether high yield or high loading is needed. Thus, we proposed21 that in in vitro conditions for a high number of copies transferred, it is advisable to use long-duration millisecond pulsing protocols or a combination of HV+LV pulses10,35,43, while for certain biomedical and biotechnological applications where the total yield of transfected cells and/or preserved viability is crucial (e.g. immuno-gene therapy)9, short-duration pulse protocols are more optimal. We have to stress, however, that in vivo the differences between tissue properties also determine the choice of optimal electric pulse parameters.
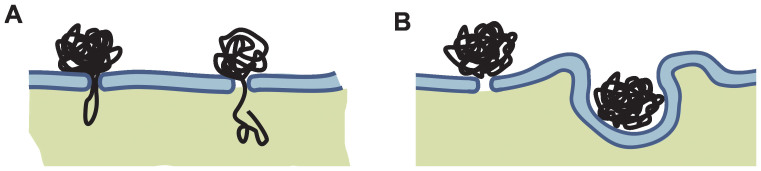
Further, we addressed another open question: how does DNA cross the cell membrane and enter the cytosol? There are two main hypotheses of DNA entry, as schematically presented in Fig. 7 above. The first hypothesis suggests that DNA is first inserted into the permeabilized cell membrane and is then transferred into the cytosol by some unknown mechanism15,16,19,60 (Fig. 7A). Alternatively, the second hypothesis assumes that DNA-membrane complex formation, in combination with exposure to an electric field, triggers endocytotic invagination of the cell membrane (Fig. 7B), followed by the transfer of DNA into the cytosol. However, endocytotic entry has thus far been directly confirmed only in a few reports32,46. No direct observation of DNA transfer across the membrane has been observed; direct visualization only showed21,29,30 that formation of a stable DNA-membrane complex occurs on a time scale of 1 s after EP and that DNA enters in minutes after pulse application29,30. Our results confirmed that DNA enters the cytosol in minutes after pulse delivery as shown in Fig. 5C. However, when we designed a separated study to analyze the role of electrostimulated endocytosis in gene electrotransfer, we have not confirmed this hypothesis47 for our pulsing protocol. Visualization of endocytotic vesicles after the application of our HV pulses showed that the level of endocytosis did not increase above the threshold electric field for electrotransfer, suggesting that electro-endocytosis is not the dominant mechanism for electrotransfer with this protocol. Also in studies21,29,30 where TOTO-labeled pDNA was used for analysis of the interaction with the membrane, no endocytotic uptake was observed – in our experiments fluorescence spots were also observed 15 min after electroporation, only on the cell membrane (results not shown) and not in the cytoplasm.
Figure 7. Two possible hypotheses of DNA entry into the cytoplasm.
(A) DNA is inserted into the permeabilized cell membrane during electric pulses and is translocated inside the cell by a slow process after pulse delivery; (B) DNA interacts with the permeabilized membrane and is consequently endocytosed.
Further, by using HV+LV and the reversed order of LV+HV pulses, we could indirectly analyze the mechanism of how DNA enters the cytosol. However, since a significantly higher transfection efficiency and DNA-membrane interaction was observed for HV+LV compared to the LV+HV protocol (Table 2) at sub-optimal 1 μg/ml cDNA, we propose that the LV pulse applied after HV pulses is crucial for DNA insertion and/or translocation across the cell membrane, and not only for accumulation of DNA at the cell membrane surface. This further supports the hypothesis that DNA is first inserted into the permeabilized membrane and later enters the cytoplasm via translocation across the membrane pores (Fig. 7A).
Taking into account all results of our and other studies, we propose that DNA insertion into the permeabilized membrane during electric pulses is a first and crucial step for later DNA entry via either translocation or electroendocytosis (Fig. 7B), and that the way of DNA entry might also depend on the specific pulsing protocol. The process probably involves DNA interaction with the permeabilized cell membrane and not only the simple contact of the DNA with the membrane. The possible mechanism of DNA translocation through electropores could be a mechanism similar to the Brownian ratchet, which was described for nucleotide translocation through nanopores due to entropic forces19,61,62. So far the most developed theoretical description of electroporation is a model of formation of aqueous pores in the membrane2, and a very consistent and experimentally verified theoretical framework was also presented describing pore formation and resealing63. However, for electrotransfection no such theoretical description exists, thus the mechanism of DNA entry is still an open question.
The last step for successful electrotransfer is the entry of pDNA into the nucleus, since the nuclear envelope is not permeabilized by the standard electroporation pulses commonly used. The majority of plasmids including pDNA, used in our study have the NLS sequence for enhanced transport into the nucleus encoded in SV 40 DLS31,52. In spite of the NLS sequence, better electrotransfer efficiency is obtained in mitotic cells49,51, confirmed also in our experiments. We obtained an approx. 3× times higher %TR for cells in an exponential phase compared to cells in a plato phase (arrested in the G1 phase), as shown in Table 1. This is especially important for clinical applications since most of the somatic cells in tissues are not actively dividing. It was shown that plasmid containing a specific NLS designed for a particular target tissue can significantly improve the transfer efficiency53.
Conclusions
In this paper we integrate an experimental and theoretical analysis of the different steps involved in gene electrotransfer in order to gain new insights into the processes involved. We show that the number of DNA molecules in contact with a permeabilized membrane is governed by the electropermeabilized surface, the electrophoretic force and the pDNA concentration. The inserted DNA is slowly transferred into the cytosol in minutes following the pulses. Nuclear entry can be a limiting factor for in vivo application where cells are not actively dividing, while in vitro cells in the exponential growth phase must be used for optimal transfection. We also explain the differences between the electrotransfer efficiency of suspended and plated cells; the later represent a more relevant system for in vivo applications, while cell suspensions enable a higher transfection yield due to the more homogeneous size, shape and movement of suspended cells.
For different HV-LV pulsing protocols, we analyzed the crucial parameters: from DNA interaction with the permeabilized cell membrane (NDNA) to the observed transfection efficiency (%TR, FLGFP), and their mutual relations. We obtained that NDNA and FLTOTO increase with higher DNA concentrations or with the addition of LV pulses, while %TR and FLGFP both reach saturation. Therefore, direct correlation between interaction and transfection efficiency exists only to a certain point where saturation is reached, due to a limited number of DNA molecules that can interact with the permeabilized surface of the membrane and consequently be transferred.
By understanding the interplay of these parameters, one can design a more optimal electric protocol for a specific application, where either high loading of the plasmid with moderate cell viability or moderate transfection efficiency with preserved viability can be obtained. Further, pDNA concentration is also important: for sub-optimal cDNA (realistic for in vivo conditions), saturation is not reached, thus electrophoresis plays an important role, while for optimal cDNA (high NDNA) a higher transfection yield can be achieved (e.g. in vitro). Finally, as a mechanism of DNA electrotransfer into cells, we propose that after insertion into the permeabilized membrane, DNA is either translocated into the cytoplasm after the pulses by some relatively slow mechanism such as Brownian ratchet, or alternatively it may be transferred by electric-field stimulated endocytosis, or both, where the mechanism probably depends on the choice of pulse parameters.
Acknowledgments
The authors would like to thank Karel Flisar, who developed the pulse generator prototype, which enabled us to switch the time-course of HV and LV pulses, and also Matej Reberšek and Vilko Leben, who were involved in the development of several new high-voltage generators. We also thank Mateja Manček Keber from the National Chemistry Institute in Ljubljana for help with flow cytometry. We also thank our graduated and PhD students Igor Marjanovič, Maruša Stražišar and Saša Haberl, who performed some parts of the experiments and to Duša Hodžič, for preparation of pDNA. We would also like to thank Muriel Golzio and Marie Pierre Rols from CNRS Toulous, France, for their useful suggestions with the TOTO protocol. M.K. would also like to thank Damijan Miklavčič, head of Laboratory of Biocybernetics, for his general support. This work was supported by the Slovenian Research Agency within projects J2-9770, J4-4324, P2-0249, J3-6794 and the MRIC UL IP-0510 Infrastructure Program.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.P. and M.K. wrote the manuscript text, prepared all figures, and designed and performed the experiments. M.P. performed the theoretical calculations and analysis. Both M.P. and M.K. reviewed and approved the manuscript.
References
- Neumann E., Schaeferridder M., Wang Y. & Hofschneider P. Gene-transfer into mouse lyoma cells by electroporation in high electric-fields. EMBO J. 1, 841–845 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J. C. & Chizmadzhev Y. A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 41, 135–160 (1996). [Google Scholar]
- Titomirov A., Sukharev S. & Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta 1088, 131–134 (1991). [DOI] [PubMed] [Google Scholar]
- Heller R. et al. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 389, 225–228 (1996). [DOI] [PubMed] [Google Scholar]
- Aihara H. & Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16, 867–870 (1998). [DOI] [PubMed] [Google Scholar]
- Rols M. P., Delteil C., Golzio M. & Teissie J. In vitro and ex vivo electrically mediated permeabilization and gene transfer in murine melanoma. Bioelectrochem. Bioenerg. 47, 129–134 (1998). [Google Scholar]
- Suzuki T., Shin B. C., Fujikura K., Matsuzaki T. & Takata K. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett 425, 436–440 (1998). [DOI] [PubMed] [Google Scholar]
- Mir L. M., Bureau M. F., Rangara R., Schwartz B. T. & Scherman D. Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. Comptes Rendus Acad. Sci. Ser. Iii-Sci. Vie-Life Sci. 321, 893–899 (1998). [DOI] [PubMed] [Google Scholar]
- Heller R., Lundberg C., Li F., Burcus N. & Shirley S. Effective gene electrotransfer is dependent on the pulse parameters of the applied electric field. Mol. Ther. 21, S41–S41 (2013). [Google Scholar]
- Hojman P. Basic principles and clinical advancements of muscle electrotransfer. Curr. Gene Ther. 10, 128–138 (2010). [DOI] [PubMed] [Google Scholar]
- Daud A. I. et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 26, 5896–5903 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čemazar M. et al. Control by pulse parameters of DNA electrotransfer into solid tumors in mice. Gene Ther. 16, 635–644 (2009). [DOI] [PubMed] [Google Scholar]
- Ferber D. Gene therapy: Safer and virus-free? Science 294, 1638–1642 (2001). [DOI] [PubMed] [Google Scholar]
- Rols M.-P. & Teissié J. Electropermeabilization of mammalian cells to macromolecules: control by pulse duration. Biophys. J. 75, 1415–1423 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenchin V., Sukharev S., Serov S., Chernomordik L. & Chizmadzhev Y. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys. J. 60, 804–811 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S., Klenchin V., Serov S., Chernomordik L. & Chizmadzhev Y. Electroporation and eeectrophoretic DNA transfer into cells - the effect ofDNA interaction with electropores. Biophys. J. 63, 1320–1327 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satkauskas S. et al. Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum. Gene Ther. 16, 1194–1201 (2005). [DOI] [PubMed] [Google Scholar]
- Kandušer M., Miklavčič D. & Pavlin M. Mechanisms involved in gene electrotransfer using high- and low-voltage pulses — An in vitro study. Bioelectrochem. 74, 265–271 (2009). [DOI] [PubMed] [Google Scholar]
- Pavlin M., Flisar K. & Kandušer M. The role of electrophoresis in gene electrotransfer. J. Membr. Biol. 236, 75–79 (2010). [DOI] [PubMed] [Google Scholar]
- Kandušer M. & Pavlin M. [Gene electrotransfer: From understanding the mechanisms to optimization of parameters in tissues.] Advances in Planar Lipid Bilayers and Liposomes [Iglič, A. (ed.)] [77–104] (Elsevier, Amsterdam, 2012). [Google Scholar]
- Haberl S. et al. Effect of different parameters used for in vitro gene electrotransfer on gene expression efficiency, cell viability and visualization of plasmid DNA at the membrane level. J. Gene Med. 15, 169–181 (2013). [DOI] [PubMed] [Google Scholar]
- Zupanič A., Čorović S., Miklavčič D. & Pavlin M. Numerical optimization of gene electrotransfer into muscle tissue. Biomed. Eng. Online 9, 66 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme G. J., Glinka Y., Khan A. S. & Draghia-Akli R. Electroporation-enhanced nonviral gene transfer for the prevention or treatment of immunological, endocrine and neoplastic diseases. Curr. Gene Ther. 6, 243–273 (2006). [DOI] [PubMed] [Google Scholar]
- Sallberg M., Frelin L. & Weiland O. DNA vaccine therapy for chronic hepatitis C virus (HCV) infection: immune control of a moving target. Expert Opin. Biol. Ther. 9, 805–815 (2009). [DOI] [PubMed] [Google Scholar]
- Low L. et al. DNA Vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum. Gene Ther. 20, 1269–1278 (2009). [DOI] [PubMed] [Google Scholar]
- Van Drunen Littel-van den Hurk S. & Hannaman D. Electroporation for DNA immunization: clinical application. Expert Rev. Vaccines 9, 503–517 (2010). [DOI] [PubMed] [Google Scholar]
- Golzio M. et al. Observations of the mechanisms of electromediated DNA uptake-from vesicles to tissues. Curr. Gene Ther. 10, 256–266 (2010). [DOI] [PubMed] [Google Scholar]
- Andre F. M. & Mir L. M. Nucleic acids electrotransfer in vivo: mechanisms and practical aspects. Curr. Gene Ther. 10, 267–280 (2010). [DOI] [PubMed] [Google Scholar]
- Golzio M., Teissié J. & Rols M.-P. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc. Natl. Acad. Sci. 99, 1292–1297 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurie C. et al. Electro-mediated gene transfer and expression are controlled by the life-time of DNA/membrane complex formation. J. Gene Med. 12, 117–125 (2010). [DOI] [PubMed] [Google Scholar]
- Vaughan E. & Dean D. Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol. Ther. 13, 422–428 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C. et al. Cholesterol implications in plasmid DNA electrotransfer: Evidence for the involvement of endocytotic pathways. Int. J. Pharm. 423, 134–143 (2012). [DOI] [PubMed] [Google Scholar]
- Kinosita K. & Tsong T. Voltage-Induced Conductance in Human-Erythrocyte Membranes. Biochim. Biophys. Acta. 554, 479–497 (1979). [DOI] [PubMed] [Google Scholar]
- Wolf H., Rols M.-P., Boldt E., Neumann E. & Teissie J. Control by pulse parameters of electric field-mediated gene-transfer in mammalian cells. Biophys. J. 66, 524–531 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehl J. et al. In vivo electroporation of skeletal muscle: threshold, efficacy and relation to electric field distribution. Biochim. Biophys. Acta-Gen. Subj. 1428, 233–240 (1999). [DOI] [PubMed] [Google Scholar]
- Marjanovič I., Haberl S., Miklavčič D., Kandušer M. & Pavlin M. Analysis and comparison of electrical pulse parameters for gene electrotransfer of two different cell lines. J. Membr. Biol. 236, 97–105 (2010). [DOI] [PubMed] [Google Scholar]
- Neumann E., Kakorin S., Tsoneva I., Nikolova B. & Tomov T. Calcium-mediated DNA adsorption to yeast cells and kinetics of cell transformation by electroporation. Biophys. J. 71, 868–877 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T. & Tsong T. Study of mechanisms of electric-field-induced DNA transfection 5. Effects of DNA topology on surface binding, cell uptake, expression, and integration into host chromosomes of DNA in the mammalian cell. Biophys. J. 65, 1684–1689 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberl S., Miklavčič D. & Pavlin M. Effect of Mg ions on efficiency of gene electrotransfer and on cell electropermeabilization. Bioelectrochem. 79, 265–271 (2010). [DOI] [PubMed] [Google Scholar]
- Zaharoff D. A., Barr R. C., Li C. Y. & Yuan F. Electromobility of plasmid DNA in tumor tissues during electric field-mediated gene delivery. Gene Ther. 9, 1286–1290 (2002). [DOI] [PubMed] [Google Scholar]
- Zaharoff D. A. & Yuan F. Effects of pulse strength and pulse duration on in vitro DNA electromobility. Bioelectrochem. 62, 37–45 (2004). [DOI] [PubMed] [Google Scholar]
- Haberl S. & Pavlin M. Use of collagen gel as a three-dimensional in vitro model to study electropermeabilization and gene electrotransfer. J. Membr. Biol. 236, 87–95 (2010). [DOI] [PubMed] [Google Scholar]
- André F. M. et al. Efficiency of high- and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. Hum. Gene Ther. 19, 1261–1272 (2008). [DOI] [PubMed] [Google Scholar]
- Xie T., Sun L. & Tsong T. Study of mechanisms of electric field-induced DNA transfection. 1. DNA entry by surface binding and diffusion through membrane pores. Biophys. J. 58, 13–19 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U. et al. Mechanisms of Electrostimulated Uptake of Macromolecules into Living Cells. Naturwissenschaften 77, 543–545 (1990). [DOI] [PubMed] [Google Scholar]
- Wu M. & Yuan F. Membrane binding of plasmid DNA and endocytic pathways are involved in electrotransfection of mammalian cells. PLoS ONE 6, e20923 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlin M., Pucihar G. & Kandušer M. The role of electrically stimulated endocytosis in gene electrotransfer. Bioelectrochem. 83, 38–45 (2012). [DOI] [PubMed] [Google Scholar]
- Lechardeur D., Verkman A. & Lukacs G. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv. Drug Deliv. Rev. 57, 755–767 (2005). [DOI] [PubMed] [Google Scholar]
- Yorifuji T., Tsuruta S. & Mikawa H. The effect of cell synchronization on the efficiency of stable gene-transfer by electroporation. FEBS Lett. 245, 201–203 (1989). [DOI] [PubMed] [Google Scholar]
- Miller A. M. & Dean D. A. Cell-specific nuclear import of plasmid DNA in smooth muscle requires tissue-specific transcription factors and DNA sequences. Gene Ther. 15, 1107–1115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio M., Teissié J. & Rols M.-P. Cell synchronization effect on mammalian cell permeabilization and gene delivery by electric field. Biochim. Biophys. Acta BBA-Biomembr. 1563, 23–28 (2002). [DOI] [PubMed] [Google Scholar]
- Lam A. P. & Dean D. A. Progress and prospects: nuclear import of nonviral vectors. Gene Ther. 17, 439–447 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. L., Zimmer W. E. & Dean D. A. Smooth muscle-specific gene delivery in the vasculature based on restriction of DNA nuclear import. Exp. Biol. Med. 233, 840–848 (2008). [DOI] [PubMed] [Google Scholar]
- Maucksch C. et al. Transgene expression of transfected supercoiled plasmid DNA concatemers in mammalian cells. J. Gene Med. 11, 444–453 (2009). [DOI] [PubMed] [Google Scholar]
- Henshaw J. W., Zaharoff D. A., Mossop B. J. & Yuan F. Electric field-mediated transport of plasmid DNA in tumor interstitium in vivo. Bioelectrochem. 71, 233–242 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan H. P. & Pauly H. Impendance of a suspension of ball-shaped particles with a shell; a model for the dielectric behavior of cell suspensions and protein solutions. Z Naturforsch B. 14B, 125–31 (1959). [PubMed] [Google Scholar]
- Valič B. et al. Effect of electric field induced transmembrane potential on spheroidal cells: theory and experiment. Eur. Biophys. J. 32, 519–528 (2003). [DOI] [PubMed] [Google Scholar]
- Schwister K. & Deuticke B. Formation and properties of aqueous leaks induced in human-erythrocytes by electrical breakdown. Biochim. Biophys. Acta. 816, 332–348 (1985). [DOI] [PubMed] [Google Scholar]
- Bureau M. F. et al. Muscle transfection and permeabilization induced by electrotransfer or pluronic® L64. Paired study by optical imaging and MRI. Biochim. Biophys. Acta BBA - Gen. Subj. 1800, 537–543 (2010). [DOI] [PubMed] [Google Scholar]
- Bureau M. F., Gehl J., Deleuze V., Mir L. M. & Scherman D. Importance of association between permeabilization and electrophoretic forces for intramuscular DNA electrotransfer. Biochim. Biophys. Acta-Gen. Subj. 1474, 353–359 (2000). [DOI] [PubMed] [Google Scholar]
- De Gennes P.-G. Passive entry of a DNA molecule into a small pore. Proc. Natl. Acad. Sci. 96, 7262–7264 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasianowicz J. J., Brandin E., Branton D. & Deamer D. W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. 93, 13770–13773 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulis G. Pore disappearance in a cell after electroporation: Theoretical simulation and comparison with experiments. Biophys. J. 73, 1299–1309 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]