Abstract
Improving physical function and mobility in a continuously expanding elderly population emerges as a high priority of medicine today. Muscle mass, strength/power, and maximal exercise capacity are major determinants of physical function, and all decline with aging. This contributes to the incidence of frailty and disability observed in older men. Furthermore, it facilitates the accumulation of body fat and development of insulin resistance.
Muscle adaptation to exercise is strongly influenced by anabolic endocrine hormones and local load-sensitive autocrine/paracrine growth factors. GH, IGF-I, and testosterone (T) are directly involved in muscle adaptation to exercise because they promote muscle protein synthesis, whereas T and locally expressed IGF-I have been reported to activate muscle stem cells. Although exercise programs improve physical function, in the long-term most older men fail to comply. The GH/IGF-I axis and T levels decline markedly with aging, whereas accumulating evidence supports their indispensable role in maintaining physical function integrity.
Several studies have reported that the administration of T improves lean body mass and maximal voluntary strength in healthy older men. On the other hand, most studies have shown that administration of GH alone failed to improve muscle strength despite amelioration of the detrimental somatic changes of aging. Both GH and T are anabolic agents that promote muscle protein synthesis and hypertrophy but work through separate mechanisms, and the combined administration of GH and T, albeit in only a few studies, has resulted in greater efficacy than either hormone alone. Although it is clear that this combined approach is effective, this review concludes that further studies are needed to assess the long-term efficacy and safety of combined hormone replacement therapy in older men before the medical rationale of prescribing hormone replacement therapy for combating the sarcopenia of aging can be established.
-
I.
Introduction
-
II.
Background
-
III.
The Growth Hormone/IGF-I Axis and Testosterone Secretion and Aging
-
A.
Aging and the somatotropic axis
-
B.
Physiology of aging in the male gonadal axis
-
A.
-
IV.
Clinical Consequences of Declining Growth Hormone and Testosterone in Aging Men
-
A.
Associations between clinical manifestations of aging and sex steroid status
-
B.
Associations between clinical manifestations of aging and GH/IGF-I axis
-
A.
-
V.
Similarities between the Adult GHD Syndrome and Hypogonadism and the Aging Phenotype
-
A.
GHD syndrome and the aging phenotype
-
B.
Hypogonadism and the aging phenotype
-
A.
-
VI.
Effect of Growth Hormone and Testosterone on Men with the GHD Syndrome and Hypogonadism
-
A.
Effects of testosterone on clinically overt hypogonadism in young and middle-aged men
-
B.
Effects of GH on the GHD syndrome
-
A.
-
VII.
Evidence of an Additive Anabolic Action of GH and Testosterone
-
A.
Puberty and GH and testosterone interaction
-
B.
Muscle growth and GH and testosterone interaction
-
C.
Lipolysis and GH and testosterone interaction
-
A.
-
VIII.
Potential Implications of the Anabolic Hormones and VO2max Decline in Older Men
-
A.
Exercise and integrity of anabolic hormonal milieu is required for muscle adaptation
-
B.
Impaired hormone anabolic profile and strenuous exercise in older men
-
C.
Possible implications of GH and testosterone decline in older men
-
A.
-
IX.
Anabolic Intervention in Aging
-
A.
Issues regarding the role of hormone replacement (GH and testosterone) in older men in the light of recent research
-
B.
Clinical trials of testosterone and/or GH administration in older men
-
C.
Conclusions and thoughts of designing future trials on HRT in older men
-
D.
Safety issues of growth hormone and testosterone replacement treatment in older men
-
A.
-
X.
Conclusions and Recommendations
I. Introduction
Improvements in sanitation, health, and social conditions are resulting in a great increase in average life expectancy. In the United States alone, it is expected that the percentage of people older than 65 yr will increase from 12% today to almost 20% in 2030 (1). This will inevitably result in higher numbers of frail or disabled older men because the prevalence of disability increases from less than 4% in those aged 50–60 yr to more than 20% in those aged over 75 yr (2).
Disability is defined as difficulty or dependence in carrying out activities essential to independent living and is assessed by the self-reporting of difficulties or the inability to perform specific tasks as activities of daily living. The Instrumental Activities of Daily Living developed by Nagi (3) refers to tasks essential to household management (4) and measures of physical functioning. Frailty has recently been recognized as a distinct clinical entity and is described as a stage of decreased physiological reserves associated with increased risk of disability. Subsequently, the frailty phenotype has been defined as a clinical syndrome in which three or more of the following criteria are present: unintentional weight loss (10 pounds in the past year), self-reported exhaustion, muscle weakness, slow walking speed, and low physical activity (5). Although there is no consensus regarding the definition of frailty assessed only by physical impairment criteria as presented above, it has been recently validated among participants of the Cardiovascular Health Study, and we will adopt it for the purpose of this review (6).
Both muscle power and aerobic capacity are major determinants of physical performance, and this association has been confirmed in several studies. Aging, on the other hand, is closely associated with a progressive decline in muscle mass (7, 8), strength (9), and aerobic exercise capacity (10), and an increase in body fat (BF). Although these changes could be considered as physiological, they have a detrimental effect and contribute to the incidence of frailty, metabolic disorders, and cardiovascular morbidity and mortality of older men (11).
Thus, the decline of muscle mass and strength, a universal process of aging [for which the term sarcopenia has been coined by Rosenberg (12)], has been linked with falls, fractures, and higher mortality rates (13). Although sarcopenia is common in both men and women, the current review will address the potential role of combination therapy just in men.
The GH/IGF-I axis and testosterone (T) levels (especially biologically available T) have all been reported to decline with aging in such a way that older men may be considered partially GH and T deficient (14, 15). Both GH and T are powerful anabolic agents that promote nitrogen retention, increase muscle mass and bone mass, and promote muscle protein synthesis (16, 17). Conditions of absolute deficiency of GH or T that occur in young men as the GH deficiency (GHD) syndrome or hypogonadism present with alterations in body composition and reduced bone mineral density (BMD), muscle strength and function, and aerobic capacity—changes that resemble those that occur in healthy elderly men (18, 19). The aging-associated decline in GH and/or T secretion may contribute to the detrimental aspects of aging (20, 21). Replacement therapy with GH and T, respectively, in GHD and hypogonadal adults improves and reverses most of these detrimental changes (22, 23). Thus, it was reasoned that treatment with GH and T may confer clinical benefits in older men, and indeed, Rudman et al. (20) in his pioneering study showed that this could happen, but he studied a highly selected group of subjects, and although pivotal, the applicability of his findings remains controversial 20 yr later.
In this review, we present the existing evidence behind the argument that restoration of anabolic hormone profile is necessary to improve or preserve physical function in older men, and we evaluate critically the different studies that have assessed the effects of GH and/or T (alone or in combination) in healthy older men.
II. Background
There is no other tissue that declines more dramatically with aging than skeletal muscle (24). This decline starts in the third decade of life and is associated with an even more striking decline of muscle strength and power, as has been shown in both longitudinal and cross-sectional studies (25, 26). These changes of muscle tissue are qualitative as well as quantitative because there is both a preferential atrophy of fast twitch type II muscle fibers and an impairment of metabolic capacity (27).
Aging is also associated with a progressive decrease in exercise capacity that occurs regardless of physical activity and accelerates with each successive decade (10). The mechanism behind this is unclear, but one possible explanation is accumulating oxidative damage because both mitochondria DNA abundance and ATP production have been shown to decline with aging (28). This, in association with the increase in fatigability that occurs with aging (29), may contribute to reduced physical activity commonly observed in older people (30). Restricted physical activity is a hallmark of aging and is closely associated with progression to frailty and disability (31). It is of great importance for two main reasons. First, by reducing energy expenditure and more specifically exercise energy expenditure (32) and without appropriate dietary change, it may facilitate the accumulation of total fat, visceral fat (VF), and im BF (33), all being strongly associated with an adverse metabolic profile, insulin resistance, and cardiovascular morbidity and mortality (11, 34). Second, and most importantly, restricted physical activity may further compromise the already impaired muscle adaptation to habitual activity and training observed in older men (35).
Muscle adaptation to exercise comprises three main processes. First is muscle protein accretion, which results in expansion of the myofibers, second is the enhancement of mitochondrial function, and third is the proliferation of muscle stem cells, called satellite cells, which provide the necessary myonuclei to sustain muscle hypertrophy. IGF-I is directly involved in two of these processes because it stimulates protein accretion [via the phosphatidylinositol 3-kinase-Akt-mammalian target of rapamycin pathway (36)], whereas a putative splice variant of IGF-I called mechano-growth factor (MGF), locally expressed in response to exercise, activates the muscle satellite cells (37). In a similar fashion, T increases muscle protein synthesis, stimulates satellite cell proliferation, and induces myogenesis while simultaneously inhibiting adipogenesis (38, 39).
Aging is associated with several functional changes of the endocrine system. Daily production of GH starts to decrease from the third decade of life by almost 14% for each passing decade, with a marked attenuation of GH secretory pulse amplitude but not frequency (14, 40). IGF-I levels decrease in parallel with the reduction of GH secretion, and 30% of older people could be considered GHD in that their IGF-I levels are lower than the lower limit of the young adult normal range (41). In a similar but less dramatic fashion, T levels decline with increasing age, and this has been seen in both cross-sectional and longitudinal studies (42). In the recently updated Baltimore Longitudinal Study, the incidence of hypogonadism, defined as total T levels at or below 11.2 nmol/liter, increased from 20% in those aged 60 to 70 yr to more than 50% in those aged over 80 yr (15).
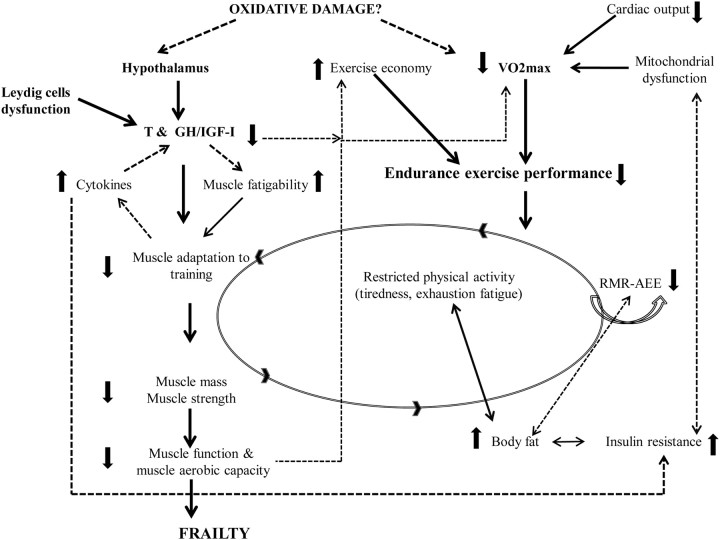
Thus, it appears that both exercise capacity and the anabolic hormone profile necessary for muscle tissue integrity are compromised in older men. Exercise improves muscle function and exercise capacity in healthy older men when a resistance-training program of high intensity and sufficient duration is undertaken (43). The hypertrophic response of muscle to training in older men is blunted when compared with younger counterparts, and this has been attributed (at least in part) to the deficient anabolic hormone profile and locally expressed milieu (35, 44). Recent evidence suggests that in healthy young men, muscle protein synthesis starts to increase in response to intensities of resistance exercise as low as 20% of 1 repetition maximum (1RM). This underlines the importance of exercise and leisure time physical activity in maintaining normal muscle tissue homeostasis (45). The higher levels of cytokines (largely IL-6) recorded in older men, which increase markedly during intense exercise, could inhibit the anabolic stimulus of IGF-I and render this approach catabolic rather than anabolic (46). This may at least partially explain the failure of healthy (previously sedentary) older men to maintain a long-term exercise program (47). Thus, the consistent finding of an improvement in exercise capacity in the two studies measured in well older men after combined treatment with GH and T is potentially of great importance (48, 49). The changes that occur normally with aging may eventually impair physical function to the extent that frailty develops (Fig. 1).
Figure 1.
Aging-related detrimental changes in body systems that lead to physical function decline, with both anabolic hormone milieu and aerobic exercise capacity playing a key role. Solid lines represent well-established findings of aging, and dashed lines represent additional proposed mechanisms that may also contribute to frailty. RMR, Resting metabolic rate; AEE, activity energy expenditure.
III. The Growth Hormone/IGF-I Axis and Testosterone Secretion and Aging
A. Aging and the somatotropic axis
1. Neuroendocrine regulation of GH secretion
GH, the most abundant pituitary hormone, is a single chain polypeptide of 191 amino acids, which is secreted in a pulsatile fashion by the somatotropic cells in the anterior pituitary gland and whose secretion is directly controlled by hypothalamic and peripheral factors acting on the somatotrophs [reviewed by Giustina and Veldhuis (50)]. Three hypothalamic peptides in a fine coordinated interplay regulate pulsatile GH secretion: hypothalamic GHRH, which stimulates GH secretion; somatostatin (SS), which inhibits GH secretion; and ghrelin, recently discovered as the endogenous ligand of previously identified GH secretagogue receptor and suggested to be a powerful regulator of GH secretion in humans (51). The GH secretagogue receptor is distinct from the GHRH receptor (51). Ghrelin is secreted by the stomach but is also expressed in many other tissues, including the pituitary, and was suggested to be a powerful regulator of GH secretion in men in experimental settings (52, 53). It was proposed that ghrelin facilitates a periodic secretory burst of GH by inhibiting nocturnal SS, but so far the exact role under physiological conditions has not been established (54, 55). Thus, Avram et al. (55) have examined ghrelin secretory dynamics over 48 h in the fed and fasted state using frequent (every 10 min) sampling and found no change in ghrelin levels despite a clear secretory burst of GH, whereas Nass et al. (54), using a similar technique, concluded differently in that they found evidence that ghrelin amplified the GH pulses.
GH has direct effects, but many of its actions are mediated through circulating and locally expressed IGF-I (reviewed in Refs. 56 and 57). Circulating IGF-I is largely (∼70%) derived from the liver in response to pituitary GH, whereas IGF-I in turn appears to play an active role in regulating GH secretion through a negative feedback mechanism because infusion of IGF-I rapidly suppresses GH pulsatile secretion in humans (58).
According to the initial somatomedin hypothesis, liver-derived IGF-I was considered as the primary mediator of many of the responses regulated by GH in peripheral tissues (56). This was subsequently modified after gene deletion experiments, which have shown almost normal growth and development in mice completely lacking liver IGF-I and so having undetectable circulating IGF-I levels (59, 60). Consequently, it was proposed that circulating IGF-I and its ternary complex with the acid-labile subunit and IGF binding protein (IGFBP)-3, with which it is circulating in plasma, represent only a dynamic reservoir of GH secretion, and that growth regulation instead occurs mainly by an autocrine/paracrine mode through locally produced IGF-I (56); however, not all agree with this hypothesis.
GH receptors on the other hand have been identified in almost every tissue (61, 62), and GH is the principal regulator of IGF-I expression in tissues (63, 64). This, along with the fact that growth occurs in mice lacking liver IGF-I, indicates that GH has a direct effect on several target tissues such as skeletal muscle, adipose, and bone, possibly by stimulating locally expressed IGF-I (56). Recently, however, a specific role of circulating IGF-I on kidney, prostate, and liver size and cortical bone that could not be replaced by the locally expressed IGF-I has been suggested (65).
IGF-I in turn circulates in plasma bound to IGFBP, the latter being not only a simple carrier of the IGF-I but also able to modulate its action (reviewed in Ref. 66). Six IGFBP have been purified from biological fluids, and their cDNA has been cloned. Two IGFBP merit specific attention. First, IGFBP-3 is the main protein carrier of IGF-I because it carries almost 70% of the IGF-I in the circulation. It forms stable, high molecular mass (∼150 kDa) ternary complexes because it binds to IGF-I and an acid-labile subunit. IGFBP-3 levels do not fluctuate throughout the day, and its production by the liver is closely regulated by GH directly or through IGF-I (67, 68). In contrast, IGFBP-1 is the most dynamic IGFBP and is mainly regulated by insulin, which has been shown immediately to suppress IGFBP-1 transcript levels in liver (69). IGFBP-1 fluctuates widely throughout the day and has a significant strong negative correlation with free IGF-I levels. Aging is characterized by higher BF and thus insulin resistance, which in turn may explain the high IGFBP-1 levels observed in older people (70).
GH stimulates linear growth in children by acting directly and indirectly on the epiphyseal plates of long bones (reviewed in Refs. 71 and 72). GH also has specific anabolic actions, including stimulation of protein synthesis (73–77) and bone accretion (78–81) in both GHD and normal adults.
Acute and short-term administration of IGF-I has been shown similarly to increase protein synthesis (82–84); however, a 1-yr study of the administration of IGF-I in postmenopausal women failed to increase lean body mass (LBM) (85). Regarding carbohydrate metabolism, GH induces insulin resistance (86, 87), whereas IGF-I has potent glucose-lowering effects and increases insulin sensitivity despite suppressing insulin levels (88, 89). Finally, regarding lipid metabolism, GH seems to be a strong lipolytic agent because GH infusion rapidly increases free fatty acid (FFA) and glycerol and promotes FFA oxidation (90, 91), whereas chronic administration of GH has consistently been found to reduce total and abdominal fat mass in GHD patients (16, 22), in young obese men (92, 93), and in healthy older men (48, 49, 94). Conversely, because IGF-I receptor signaling in adipocytes does not appear to be crucial for the development and differentiation of adipose tissue (95), IGF-I has reduced lipolytic effect compared with GH, as has been demonstrated in postmenopausal women and GHD young adults (85, 89, 96). Recent reports have recorded an increase in BF after chronic administration of IGF-I in patients with Laron's syndrome (97). The effects of GH on substrate metabolism in humans have been recently reviewed (98).
2. GH secretion and aging
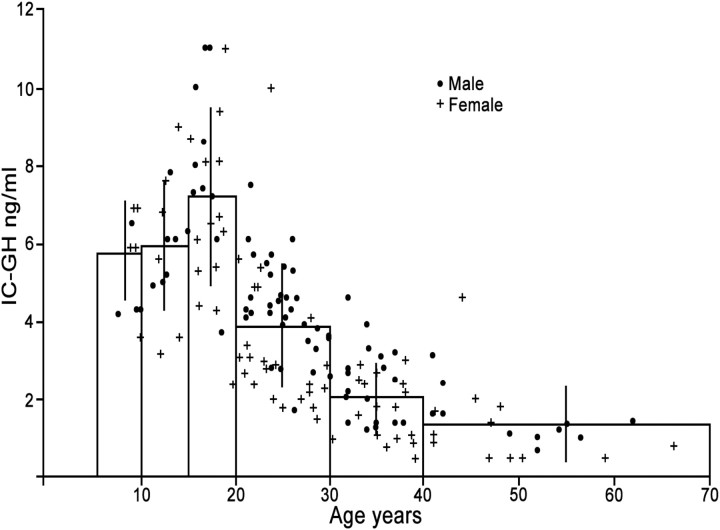
This subject has been reviewed in Refs. 50 and 99. Integrated daily GH (IDGH) secretion (Fig. 2) and IGF-I production decline progressively during adult life (14, 40, 100–103). Consequently, more than 30% of older men have IGF-I levels lower than the young adult reference range (41, 104, 105). GH is secreted almost exclusively through the 10 to 20 daily recorded secretory bursts, with the highest pulses occurring during the period of deep sleep, so that more than 70% of daily GH is secreted during the night (50).
Figure 2.
The relationship between the 24-h integrated GH concentration (IC-GH; y-axis) and age (x-axis) of 89 male and 84 female normal subjects. [Redrawn from Z. Zadik et al.: The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab 60:513–516, 1985 (40), with permission. © The Endocrine Society.]
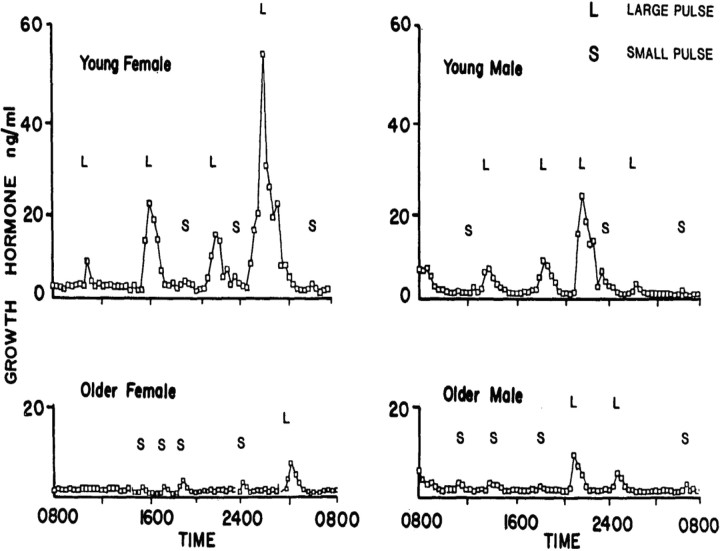
Aging is associated with a significant alteration of GH secretion patterns so that most of the GH is secreted during the day instead and is associated with a steep decline of IDGH secretion (100) (Fig. 3). The latter is affected mainly through decreasing the GH pulse amplitude, whereas the pulse frequency and GH half-life remain the same (14, 106). Accordingly, it has been demonstrated that for each decade of increasing age, IDGH secretion falls by 14%, and in a 70-yr-old man, on average GH secretion has declined by more than 70% (14, 101).
Figure 3.
Serum GH profiles from a young woman, a young man, an older woman, and an older man sampled every 20 min for 24 h. Pulses were categorized as large (L) or small (S) depending on whether the rise was greater or less than three times the threshold criterion for a pulse. [Reproduced from K. Y. Ho et al.: Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64:51–58, 1987 (100), with permission. © The Endocrine Society.]
Increased SS tone, decreased GHRH and ghrelin stimulatory effects, or even increased IGF-I negative feedback have all been proposed as possible causes of the hyposomatotropism of aging (50). The latter mechanism had been excluded by Chapman et al. (107), who showed instead attenuated suppression of GH secretion after IGF-I infusion. Conversely, coadministration of arginine, a presumed SS inhibitor, and GHRH or hexarelin, a synthetic GH-releasing peptide, has been shown to restore the blunted response of GH to GHRH or hexarelin in the elderly, with no differences when compared with the young, which implies an increased SS inhibitory tone in older people (108, 109). Furthermore, Hartman et al. (110) have demonstrated that fasting can increase pulsatile GH secretion in older men to a similar degree of that observed in young men with no relationship to sleep stages. In contrast, others have concluded that reduced GHRH activity rather than increased SS tone is responsible for the decline in GH secretion (111, 112). Of great importance is a study where GHRH and SS were measured directly in the stalk-median eminence of conscious young and aged monkeys. Both decreased GHRH and increased SS pulse frequency and amplitude were recorded (113). In an elegant study, Russell-Aulet et al. (114) administered graded doses of a GHRH antagonist and tried to quantify the endogenous GHRH output in young and older men. The authors in accordance with the previous studies concluded that their results indicated that the fall in GH secretion with aging was due to reduced GHRH activity. Finally, Veldhuis et al. (115), elaborating in a series of studies and stressing the importance of pulsatile GH secretion in exerting its peripheral action (116), postulated that decreased negative feedback of GH and IGF-I may indeed attenuate the renewal of high amplitude GH pulses. Both fat mass and sex hormones had been implicated as possible confounders in attenuating GH secretion with age (14, 101–103).
Of interest, it has been suggested that attenuation of IGF-I feedback inhibition of pulsatile GH secretion is one of the mechanisms (117, 118) through which pharmacological but not physiological doses of T administration may increase GH secretion in adults (119–121). It appears, however, that age is a strong independent predictor of GH secretion because older reproductive-age women were found to have lower IDGH secretion when compared with young women despite having higher estradiol levels (122).
VF, apart from sex steroids, influences GH secretion in young men (123). Because VF increases with aging, it has been suggested that this may well be responsible for the decline of GH secretion observed in older men (103). Although both sex steroids and BF jointly could determine the GH secretion in young men (124), it seems that age per se is the major determinant of the decline of GH secretion with aging (102, 115). In this regard, Holt et al. (102) demonstrated that aerobic fitness and age, rather than BF, predict the GH secretion in response to exercise.
B. Physiology of aging in the male gonadal axis
GnRH is secreted into the hypophyseal portal system in a pulsatile fashion, which in turn elicits pulsatile secretion of LH and FSH by the gonadotrophs of the anterior pituitary. The pulsatile release of GnRH is essential for the pulsatile secretion of LH and FSH because continuous administration of GnRH inhibits gonadotropin release (125). LH in turn interacts with cell membrane receptors on Leydig cells in the testis to stimulate, via a series of intermediate steps, T synthesis (126). Testosterone then directly or indirectly, after conversion to estrogens, exerts a negative feedback at the level of both the pituitary and hypothalamus and thus modulates the pulse generator of GnRH and gonadotroph secretion (127).
Testosterone in plasma is bound strongly to SHBG (60%) and to a lesser degree loosely to albumin, and only 1 to 2% of T circulates freely (128). Of note, SHBG-bound T is not biologically active. SHBG levels increase with age, thus resulting in lower levels of bioavailable T (BioT) (129–131). Aging is associated with a progressive decline of daily T secretion rates and thus reduced plasma T levels (132). For an extensive review of this subject, see Refs. 133 and 134. Both primary and secondary hypogonadism have been suggested as possible causes for the decline of T secretion observed in older men (135). Indeed, the reduced responsiveness of the testis to stimulation by human chorionic gonadotropin, clomiphene, or more recently to pulsatile LH drive, proved a reduced capacity of older men to increase T concentration when compared with young men (136–138). In a recent study, a GnRH antagonist (ganirelix) was administered to block endogenous LH. Older men then had a reduced capacity to stimulate T secretion after pulsatile exogenous LH compared with young men (139, 140), which in turn denotes reduced Leydig cell secretory capacity. On the other hand, it has been shown repeatedly that the pituitary of older men responds to acute or even prolonged (up to 14 d) pulsatile stimulation by GnRH (135, 141). Thus, Mulligan et al. (135), using discrete pulse detection algorithms to analyze the LH concentration series and mathematical deconvolution analysis of the LH pulses, demonstrated that 14 d of pulsatile GnRH administration restored normal pituitary 24-h LH release with normal pulsatile pattern in older men. The authors concluded, in view of their lower T levels, that a combined defect of GnRH release and Leydig cell responsiveness could underlie the lower T levels in older men.
LH pulse amplitude was reported to decline with age and to be the main determinant of lower T concentration commonly seen in aging (142). A series of elaborate studies from the same group revealed the attenuated capacity of hypothalamic GnRH release mechanism, which results in low-amplitude, high-frequency pulses and consequent decreased T levels. This was achieved by selectively blocking the negative feedback on the hypothalamus (either with an aromatase inhibitor or by the administration of ketoconazole) and quantifying the LH response with deconvolution analysis (143, 144).
1. Epidemiology of declining androgen levels in older men
Testosterone and BioT levels have been shown to decline with aging in several cross-sectional (145–149) and longitudinal studies (42, 150–153). Nevertheless, some earlier cross-sectional studies had reported no significant differences in T levels when older men were healthy and fit (138, 154). In longitudinal studies, however, it has been demonstrated consistently that the total T declines with an absolute rate of 0.124 nmol/liter · yr, or otherwise by 0.5 to 1% each year (42, 150, 151). Even higher rates of decline were recorded when free T (FT) was measured (147). In a large longitudinal study with almost 30 yr of follow-up, the prevalence of hypogonadism increased to about 20% of men over 60 yr of age and 30% of men over 70. If the FT index was used as a criterion of hypogonadism, the prevalence increased to more than 60% of those over 70 yr of age (15).
IV. Clinical Consequences of Declining Growth Hormone and Testosterone in Aging Men
We will review briefly in this section the findings of some epidemiological studies that associate the changes in the GH/IGF-I axis and T levels with some of the detrimental changes occurring with increasing age.
A. Associations between clinical manifestations of aging and sex steroid status
1. Cardiovascular diseases (CVD) and the metabolic syndrome
An inverse relationship between T levels and “all cause” and specific CVD mortality and morbidity has been shown in numerous large population-based prospective (155–158) and cross-sectional studies (159). Others, however, could not corroborate this association (160). It has been shown that plasma T and SHBG are inversely associated with VF and that a low T level, together with low SHBG levels, is a strong predictor for the development of metabolic syndrome and diabetes in men (161–163). The association between age, BF, and SHBG levels creates confusion. Obesity is associated with low SHBG levels. Insulin has been reported to inhibit hepatic SHBG production both in vitro and in vivo (164, 165). Thus, the hyperinsulinemia observed in abdominally obese men may indeed suppress the SHBG levels, and this in turn implies an adverse prognosis. On the other hand, whereas in aging there is a progressive accumulation of intraabdominal fat, SHBG levels are increasing (41). This confusing finding may partially be explained by the insulin resistance observed in aging (166). Nevertheless, in a recent cross-sectional study of men older than 70 yr, a strong negative association between insulin resistance and levels of SHBG and T was observed, indicating that insulin probably continues to play a role in SHBG regulation in aging (167). Overall, SHBG and total T are more strongly associated with diabetes and the metabolic syndrome than FT. In longitudinal analysis, only SHBG is independently associated with diabetes risk (168). For a detailed review regarding the association of CVD and androgens, see Refs. 169 and 170.
2. Muscle mass, strength, and physical function
A direct association between T or FT levels and LBM or appendicular body mass or myofibrillar protein synthesis has been consistently reported in several cross-sectional population-based studies (171–174). On the other hand, muscle strength and physical function have been reported to be positively associated with FT or BioT in several, but not all, of the above studies. Hence, in one study no association was found between T and indices of frailty, although a positive association with grip strength was observed (175). In another study, T had a weak association with muscle strength and physical function, which could be explained by the strong association of T and LBM observed in that study (174). Recent longitudinal observational studies of 6- and 4-yr duration have shown a positive association between low FT levels and the decline in physical function, mobility, and occurrence of falls in older men (176, 177). Another study of 3-yr duration, however, could not confirm these findings (178).
3. Bone mineral density
Hypogonadism is a major risk factor for osteoporosis and an increased rate of fractures in young men (179). No clear association between levels of T and BMD in older men could be established, however, because relevant studies have produced conflicting results. Hence, in a large cross-sectional study involving 2447 older men, an association between the prevalence of hypogonadism and that of osteoporosis was recorded (180). This is in accordance with several large cross-sectional studies, which have demonstrated a positive relation between T levels and BMD in older men that was apparent only in univariate analysis. In multivariate analysis, however, including estradiol levels or correcting for confounders, this association was abolished (172, 181–183). In another study, Mellström et al. (184) have reported that FT was an independent positive predictor of BMD in different bone sites and that FT rather than total T below the median was an independent predictor of prevalent osteoporosis-related bone fractures in a cohort of 2900 older men. In contrast, Araujo et al. (185) in a recent cross-sectional study involving 976 men reported that neither T nor FT was associated with BMD, as has also been reported by others (186). Longitudinal studies have also produced conflicting findings, with some reporting that FT is an independent, although weak, predictor of rapid bone loss in older men (180, 187) and others not (188, 189). Conversely, estrogens appear to play an important role in bone loss with aging, even in older men, because estradiol or bioavailable estradiol consistently has been found to be an independent strong predictor of BMD, markers of bone resorption, rapid bone loss, and fracture rates in both cross-sectional and longitudinal studies (181, 183, 187, 189–191). Indeed, a recent large prospective study of 4.6-yr duration reported that low bioavailable estradiol, high SHBG, and low BioT were associated with lower BMD and a faster decline of hip BMD in men (191). Of note, higher SHBG levels have been shown to be independently associated with fracture risk in males (191).
4. Mood, quality of life (QoL), and cognition
Some of the symptoms of hypogonadism, such as decreased libido, low energy, irritability, mood swings, and anxiety, overlap with symptoms commonly seen in depression (192). Recent experimental studies have demonstrated T to increase cortical serotonin 2A receptor-binding densities in the male rat brain and also to have long- and short-term γ-amino-butyric acid-ergic properties, which suggest that T has an important role in modulating behavior and mood (193, 194). Furthermore, differences in depression prevalence between young women and older men have been attributed to differences in sex gonadal steroids (195). In this regard, in the Rancho Bernardo Study, which included men more than 50 yr old, an inverse association was found between the depression inventory score and BioT levels, indicating that low T was associated with low mood (196). In accordance with this, in a recent large cross-sectional study of 3987 older men, a strong negative correlation between FT and depression was also recorded (197). Shores et al. (198), in a prospective study of a 2-yr observational period, reported an increased incidence of diagnosed depressive illness in hypogonadal men older than 45 yr. Seidman et al. (199) detected a negative association of T and dysthymic disorder (a mild form of depression), but not of major depression in older men. The same authors have also suggested that CAG repeat polymorphism of the androgen receptor (AR) gene may be a confounder because low T levels were associated with increased depression only in men with short CAG repeats (200). On the other hand, T'Sjoen et al. (201) failed to demonstrate any significant relation between FT levels and depression scores, even when AR repeats polymorphism was taken into account. Similarly, others in small cross-sectional studies, also including young and middle-aged men, failed to record any relationship (202–204). Nevertheless, T administration has been shown to improve mood in hypogonadal men in several studies (23, 205, 206). Testosterone as a therapeutic tool in depression has produced inconsistent results (207–210). Thus, it appears that low T levels are related to depressive mood, but not to major depressive disorders, and low T levels may contribute to symptoms of low mood in older men. Similarly, T levels have been associated with better cognitive status and memory performance [Blessed Information-Memory-Concentration (BIMC) Test, and the Selective Reminding Test (long-term storage)] in older men in several (211, 212), but not all, of the studies. The associations between T levels and sexual function, cognition, and depression have been reviewed in Ref. 133.
B. Associations between clinical manifestations of aging and GH/IGF-I axis
1. CVD and the metabolic syndrome
Several lines of evidence suggest that IGF-I has an important role in the development of atherosclerosis and CVD (213) because administration of GH to GHD adults has been clearly demonstrated to reduce atherosclerosis risk factors and reverse early atherosclerotic changes (214–217). This beneficial effect of GH may in part be the result of increases in nitric oxide (NO) synthase by IGF-I (218). Indeed, administration of GH in healthy men was shown to increase NO bioavailability and endothelial progenitor cells, the latter being markers of vascular repair (219), whereas an inverse association between IGF-I levels and endothelial dysfunction has been recorded (220). Thus, a cross-sectional study of randomly selected men younger than 60 yr has reported a negative association between IGF-I levels and angiographically documented coronary artery disease (221). Similarly, an inverse association of IGF-I and carotid atherosclerotic lesions and intima media thickness (IMT) has been found in healthy older men (222, 223). Others, however, have produced divergent findings because a positive association between IGF-I and coronary artery disease has been recorded (224, 225), whereas in a cross-sectional study including young men a positive association between IGF-I and IMT has also been shown (226). Several large population-based prospective studies, however, consistently demonstrated that lower IGF-I levels are associated with an increased risk of developing ischemic heart disease, ischemic stroke, and CVD mortality (227–231), whereas Kaplan et al. (232) have found that lower IGF-I levels are associated with nonfatal myocardial infarction. These findings could not be corroborated by recent large cross-sectional studies that report in contrast a positive association of IGF-I levels and ischemic heart disease and CVD mortality (233, 234), whereas Yeap et al. (235) have reported that both lower or higher IGF-I levels are associated with an unfavorable metabolic profile.
Furthermore, lower IGF-I levels appear to correlate with the severity of heart failure in several studies (236, 237), and Vasan et al. (238) in a community-based prospective study have shown that IGF-I levels were inversely related to the risk of congestive heart failure in older people without prior myocardial infarction. GH administration as a therapeutic modality in an ex-Cushing's patient with panhypopituitarism and terminal heart failure produced dramatic improvement in one case study (239) but in trials has produced conflicting results, however, with some studies reporting an improvement (240, 241) of cardiac function, but another did not (242).
2. Muscle mass, strength, and physical function
No clear association has been established between IGF-I levels and measurements of body composition, muscle strength, and physical performance in older men because large epidemiological studies have produced negative findings. In one cross-sectional study involving 349 men and women, an association between IGF-I levels and grip strength or physical function was recorded in overweight (body mass index >30 kg/m2) subjects, but not in normal weight subjects (243). In another study of older persons with mild to moderate functional limitations, no association was recorded between IGF-I and measures of physical function, body composition, or strength (244). Another study involving women older than 70 yr enrolled in the Women's Health and Aging Study, however, found a positive association of IGF-I and muscle strength and physical performance (245). Similarly, Kostka et al. (246) could detect a correlation of muscle power and IGF-I levels in older women, but not in men. Furthermore, data from the Rancho Bernardo Study (247) failed to show any relation between IGF-I levels and LBM or BF in a cohort of 420 men aged 50–90 yr, which in turn confirmed the finding of the Framingham Heart Study that also failed to detect any association between IGF-I and body composition measurements in older men (248). In accordance with this, Schoen et al. (249) could not detect an association between sc fat or VF assessed by computed tomography (CT) scan and IGF-I levels in 267 healthy men aged 55–77 yr.
3. Bone mineral density
Higher BMD and a lower rate of osteoporotic fractures were consistently shown to be associated with higher IGF-I levels in several cross-sectional studies in both men and women (250–253). In a number of rather small cross-sectional studies, a positive association between BMD and IGF-I levels was observed in older men (250, 254, 255). Recently, in corroboration of these findings, Khosla et al. (256), in a cross-sectional study of 269 men aged 21–97 yr, reported that IGF-I is correlated with radius trabecular microstructure because the conversion of thick trabeculae into more numerous, thinner trabeculae was associated with IGF-I levels in young but not older men (where sex steroids were better correlated). Earlier studies could not detect an association of BMD and IGF-I levels in older men (257, 258). Furthermore, several studies that have included both men and women have demonstrated gender differences in association of IGF-I with BMD and osteoporosis. In this regard, Janssen et al. (253) have reported a positive association between IGF-I and BMD but only in men, not women. In contrast, data from the Rancho Bernardo and the Framingham Heart Study reported an association in women only (252, 259). In a similar way, studies that assessed the role of IGF-I gene promoter polymorphism on BMD have produced conflicting findings because in one study, idiopathic osteoporosis in men was associated with homozygosity for a specific allele 192-bp (260). In contrast, in another study homozygosity was associated with a greater rate of bone loss over a 2-yr observation period in women, but not in men (261). Finally, the role of IGF-I levels as predictors of bone fractures was suggested from the results of some cross-sectional studies (262) but not others (263).
4. Mood, QoL, and cognition
The limited amount of data in the literature is conflicting regarding an association of IGF-I and mood or QoL. Hence, Janssen et al. (264), in a cross-sectional study of 218 healthy elderly persons, reported an association between IGF-I levels and perceived QoL, but not with physical function. In another study, Raynaud-Simon et al. (265) could not verify a positive association between self-perceived QoL and IGF-I, whereas Papadakis et al. (266) also reported no association between physical or cognitive function and IGF-I levels.
On the other hand, a positive association has been reported consistently between cognitive performance and IGF-I levels in older men (267–271). Hence, in a cross-sectional study involving 636 men older than 74 yr, IGF-I was independently and positively related to the Mini-Mental State Examination (MMSE) and verbal fluency, and IGFBP-1 was inversely associated with MMSE (269). This has been recently confirmed in a meta-analysis involving 13 studies and a total number of 1981subjects, which reported a relation between IGF-I levels and cognitive function in healthy older men (270). In another prospective study of a cohort of U.S. male physicians, it has been shown that IGF-I levels of middle life may predict better cognitive function in latter life (267). In this regard, it has been shown in a placebo-controlled study that GH administration improves cognitive function in older GHD adults (272).
V. Similarities between the Adult GHD Syndrome and Hypogonadism and the Aging Phenotype
A. GHD syndrome and the aging phenotype
Several reviews have comprehensively presented the symptoms and signs of the GHD syndrome (22, 273, 274). Adults with the GHD syndrome have a 7 to 8% increase in BF with a commensurate decrease in LBM, corresponding to approximately 4 kg (16, 275–278). The increase in BF mostly reflects an accumulation in VF (279, 280). Additionally, reductions in extracellular water, plasma volume, and total blood volume in GHD patients have been reported (281–283). The decline of skeletal muscle mass seen in GHD inevitably results in lower isokinetic torque production and isometric force-generating capacity (18, 284, 285). Cuneo et al. (18) first reported a 35% reduction in isometric quadriceps force per unit muscle mass (assessed by CT scan) when compared with age-matched controls. Janssen et al. (286) reported normal muscle quality. Muscle biopsies produced conflicting reports, with some reporting no changes in muscle fiber type characteristics and percentage (287, 288), others reporting a higher proportion of fast twitch type II muscle fibers (289) and still others reporting a reduction in size of fiber type II similar to that seen in older men (290). As anticipated, whole body and skeletal muscle protein turnover is lower when compared with matched young healthy counterparts (291). Maximal aerobic capacity is reduced in GHD patients to levels comparable with those observed in congestive heart failure, being on average 72–82% of those in matched normal controls (18, 292). Woodhouse et al. (290) have reported that the anaerobic threshold occurred at a higher percentage of maximum rate of O2 consumption (VO2max) (73%) compared with (45–60%) in normal adults. Walking at low and fast speeds requires 83 and 120%, respectively, of the anaerobic threshold, which may explain the increased fatigue of GHD adults.
Numerous studies have shown that BMD at different skeletal sites assessed by dual-energy x-ray absorptiometry (DEXA) scan or quantitative CT is approximately 1 sd below the mean in severe GHD patients of both childhood and adult onset (293–298). GHD per se appears to be the key factor in the osteopenia recorded because no differences in BMD were found between patients with isolated GHD and multiple hormone deficiencies (295).
The age of onset of GHD is a major determinant of the severity of BMD reduction. It has been shown that patients with onset before 30 yr of age are severely osteopenic regardless of the duration of GHD or type of onset, whereas those with onset after 60 yr of age had BMD no different from that of age-matched controls (299). Those with onset in middle age had a BMD reduction of intermediate degree (294), suggesting that both peak BMD and subsequent rates of decline are affected. In this regard, Murray et al. (299), in a cohort of 125 GHD adults divided by age groups, reported BMD Z scores of less than −2.0 in 30% (at lumbar) and 36% (at femoral neck) of patients younger than 30 yr at onset, compared with 14 and 0%, respectively, in patients with onset after 60 yr of age. Thus, it appears that GH in teenage and early adult life is essential for the formation of a normal mature skeleton. BMD is a surrogate marker of bone fractures, and several studies have reported an increased fracture rate in GHD adults when compared with a control population (300–302). Hence, Wüster et al. (300), in a large epidemiological study of more than 3000 GHD patients, clearly demonstrated that the prevalence of osteoporotic fractures was 2.66 times higher than that of the normal population.
It appears that the GHD syndrome may also be considered as a preatherogenic condition because several atherogenic risk factors are clustered in the classical phenotype, which in turn may explain the increased cardiovascular mortality observed in hypopituitary adults (reviewed in Ref. 213). The excess CVD mortality in hypopituitary patients receiving conventional hormone replacement treatment, but not GH, has been demonstrated in several retrospective studies (303–305), and these findings were confirmed by a prospective study, although undertreatment of the T deficiency or overtreatment with other hormones such as cortisol or T4 could also be implicated (306, 307).
Numerous studies have shown that GHD adults have elevated levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) and reduced high-density lipoprotein cholesterol (HDL-C) levels when compared with a reference population (293, 308–310). Recent data from the Kims study including 1289 GHD adults reported that the percentage of patients with TC, LDL-C, and HDL-C levels outside the reference range was 73, 62, and 46%, respectively (311). Lipid concentrations are dependent on very low-density apolipoprotein (VLDL apoB) metabolism, and increased levels of VLDL apoB have been implicated in many hyperlipidemic disorders that predispose to atherosclerosis (312). In this regard, Cummings et al. (313) have reported an increased secretion and reduced catabolism of VLDL apoB in GHD adults when compared with healthy matched controls. Of note, increased secretion of VLDL apoB has also been recorded in older men, possibly because of an increased accumulation of VF (314, 315).
Insulin resistance is another well-described feature of GHD syndrome (316–318). Studies using a hyperinsulinemic euglycemic clamp have demonstrated that GHD patients are insulin resistant when compared with normal controls, whereas Hew et al. (318), by also performing muscle biopsies, have demonstrated that insulin resistance is mainly due to the inhibition of the glucose storage pathway and reduced glycogen synthase activity in peripheral tissues. The latter is in accordance with an earlier report that assessed fuel metabolism by indirect calorimetry and glucose metabolism using D-[3-3H]glucose and suggested that there were reduced stores of glycogen in GHD adults (319).
Endothelial dysfunction characterized by reduced NO bioavailability, an early and probably reversible event in the process of atherosclerosis, is another feature of GHD. Indeed, reduced NO bioavailability (218), increased carotid and brachial artery stiffness (320, 321), reduced aortic compliance (322), impaired flow-mediated brachial artery dilatation, and increased blood pressure (321, 323) have been described in GHD adults. Furthermore, the crude prevalence of the metabolic syndrome was found to be 42% in a large cohort of 2531 GHD patients enrolled in the Hypopituitary Control and Complications Study (324). In this regard, it is not surprising that increased carotid IMT detected by ultrasonography, an early morphological change of atherosclerosis, and atheromatous lesions have been consistently demonstrated in symptom-free GHD patients (325–328). Finally, impaired cardiac function has been found in young GHD adults, which includes reduced left ventricular (LV) mass, a 13% decrease in LV ejection fraction, and abnormal LV diastolic filling (329–331).
B. Hypogonadism and the aging phenotype
Reduced cortical and spine BMD and increased incidence of osteoporosis are well-described features of clinically overt hypogonadism in men (19, 257, 332–336). The latter comprises many clinical conditions that may affect differently both the type and the severity of bone loss (reviewed in Ref. 337). In this regard, Katznelson et al. (19) have reported reduced trabecular spine BMD (assessed by quantitative CT) and spinal BMD but not in radial BMD in men with acquired hypogonadism when compared with eugonadal controls. In another study, it has been shown that the trabecular architecture of the distal tibia, assessed by magnetic resonance microimaging, is greatly disorganized in hypogonadal men (334).
It appears that postpubertally acquired hypogonadism, as in patients with pituitary diseases, anorexia, or chemical castration in prostate cancer treatment, mainly compromises the vertebral BMD (19, 338). On the other hand, congenital or acquired prepubertal hypogonadism such as Klinefelter's syndrome, isolated hypogonadotropic hypogonadism (IHH), or delayed puberty reduces both cortical and trabecular bone (257, 335, 339). Reduced cortical and trabecular BMD were reported in patients with IHH before and even after epiphyseal closure when compared with eugonadal men (340). The fact that similar decrements of both trabecular and cortical BMD of the spine were recorded in hypogonadal patients, regardless of the cause of hypogonadism, which in turn responded with a similar increment of both the trabecular and cortical BMD after T replacement, underlines the causal role of T on bone loss (333).
Studies that assessed the effects of male hypogonadism on bone remodeling have produced conflicting findings, with some suggesting increased bone resorption and formation in line with observations in postmenopausal women (341), whereas others have reported low bone turnover osteoporosis (340).
Although case-control studies have reported an increase in the prevalence of hypogonadism in men with a history of bone fractures (179, 342), prospective studies that clearly define a cause-effect relationship between male hypogonadism and bone fracture are lacking.
There are several pieces of evidence that underscore the importance of estrogens in male bone physiology, such as case reports of patients with rare mutations of the estrogen receptor or aromatase gene who are osteopenic (343, 344), whereas lower bioavailable estrogens appear to be a stronger independent predictor than T of osteoporosis and bone fracture in men (189–191). On the other hand, high SHBG levels were recently reported to be independently associated with increased fracture risk in men in a longitudinal study (345). Accordingly, a recent placebo-controlled study has shown that administration of an aromatase inhibitor to healthy older men for 1 yr has decreased spine BMD compared with placebo, although T levels increased (346). This finding is of importance because it questions the role of nonaromatized androgens or selective AR modulators that do not aromatize as therapeutic modalities in healthy older men.
Testosterone appears to have a crucial and independent role in bone resorption and formation in men because AR are expressed in both osteoclasts and osteoblasts (347). Hence, Leder et al. (348) have compared bone resorption and formation markers in states of androgen and estrogen deficiency, selective estrogen deficiency, and normal estrogen and androgen repletion and in healthy young men after induced hypogonadism, and they demonstrated that T has an independent role in regulating both resorption and formation of bone.
Cross-sectional studies in young and older healthy men have demonstrated that low T levels are associated with an adverse lipid profile, higher inflammatory cytokines, and increased risk of atherosclerosis and carotid atherosclerosis in both healthy older men and diabetic men (223, 349–354). Additionally, an inverse association between T levels and trunk fat, particularly VF, which in turn is strongly related to insulin resistance, has been described (355). Testosterone administration has been shown to decrease VF in obese men, whereas low T levels, by reducing lipolysis, have been postulated to facilitate the accumulation of VF (356). A recent meta-analysis has shown that the prevalence of hypogonadism (defined by a low T level) is much higher in diabetic men (357), whereas another study found that by measuring their FT levels with the equilibrium dialysis method, 33% of type 2 diabetic men were hypogonadal by this definition (358). Furthermore, case-control and prospective studies have demonstrated that low T and low SHBG levels strongly predict the development of type 2 diabetes and of the metabolic syndrome in healthy men (161, 359, 360). It should be noted, however, that no causality between insulin resistance and other variables of the metabolic syndrome could be inferred from these associations because they could be explained by changes in BF. In this regard, accumulation of BF precedes the insulin resistance state observed in patients with prostate cancer after chemical castration (361). In fact, the increased abdominal fat observed in type 2 diabetes could result in insulin resistance, which in turn suppresses SHBG levels and consequently T levels. This could explain the paradox of high SHBG levels being linked to an increased risk of bone fractures mentioned before, whereas low values were linked to the metabolic syndrome and CVD. Introducing into the multivariate analysis confounders such as abdominal BF or LBM may help clarify this. Nevertheless, Yialamas et al. (362) have reported that withdrawal of T replacement treatment in patients with iHH (idiopathic hypogonadotropic hypogonadism) reduced insulin sensitivity before any changes in body composition had occurred, which suggests that T may modulate insulin sensitivity independently of changes in body composition.
In contrast to the well-studied effects of acquired or congenital hypogonadism in young and middle-aged men on bone, the effects on body composition and function have not been thoroughly documented. Loss of libido, erectile dysfunction, and lack of secondary sexual characteristics are usually the main complaints (363).
Congenital or acquired hypogonadism in young or middle-aged men results in an increased BF percentage, reduced LBM, impaired muscle function, and an increased risk of cardiovascular risk factors (19, 353, 364, 365). One study has shown an increased percentage of BF and lower LBM in men with acquired hypogonadism when compared with age-matched normal men (19), whereas in another study, increased levels of LDL-C and TG with similar levels of HDL-C were recorded in patients with acquired hypogonadism (associated or not with hyperprolactinemia) (365). Insulin resistance and type 2 diabetes appear to occur commonly in patients with acquired or congenital forms of hypogonadism (366), and patients with Klinefelter's syndrome have been shown to have increased morbidity and mortality as a result of diabetes (367, 368). A recent study has confirmed these findings, reporting the prevalence of the metabolic syndrome in adult patients with Klinefelter's syndrome to be as high as 44%, compared with 10% in normal controls. These patients were also shown to have lower HDL-C and higher LDL-C and TG, all strongly correlated with truncal obesity assessed by DEXA scan (369). Although hypogonadism appears to be associated with increased BF and an adverse lipid profile, its effect on BF distribution is less well studied. Katznelson et al. (370) assessed BF distribution in acquired hypogonadism by quantitative CT scanning and reported an increase in abdominal sc fat and im adipose tissue but not in VF area, when compared with matched eugonadal men. This is important because it suggests that T may exert a stronger lipolytic effect on im adipose tissue and in sc abdominal or femoral fat rather than in intraabdominal fat and may explain the sexual dimorphism of regional fat distribution (371). This is well illustrated in a study of healthy young men who were artificially rendered hypogonadal and subsequently administered different doses of T. The lower doses, which could not restore a eugonadal profile, were associated with higher increments of mainly sc abdominal and femoral BF, whereas higher supraphysiological doses caused a reduction of sc adipose tissue depots but, interestingly, not that of VF (372). Testosterone has been shown to exert its lipolytic action by modulating lipoprotein lipase (LPL) activity in adipose tissue, with some studies reporting a higher inhibitory effect on LPL activity in abdominal sc fat compared with femoral (373), whereas others produced the opposite results (374). Finally, other well-described signs and symptoms commonly occurring in young or middle-aged hypogonadal men include low energy, mood, and sexual function (375, 376); low hemoglobin concentration and smaller prostate glands (377, 378) are seen in adults with hypogonadism of young onset.
VI. Effect of Growth Hormone and Testosterone on Men with the GHD Syndrome and Hypogonadism
It is of great importance to distinguish and consider studies that assess the effects of T on clinically overt hypogonadism occurring in young or middle-aged men from studies that assess the effect of T in healthy older men with low or low-normal T levels. This is because clinically overt hypogonadism is the result of several well-defined conditions where symptoms and signs can be attributed to the low T levels and the effectiveness of treatment is easily interpretable. Aging however, besides the progressive decline of T secretion presented in Section III.B, is also associated with a gradual decline of all physiological functions, which in turn may act as confounders in the interpretation of T treatment. Thus, by underscoring differences and similarities between these two clinical entities, it may indeed help us to understand the possibilities and limitations of T treatment in healthy older men.
A. Effects of testosterone on clinically overt hypogonadism in young and middle-aged men
Numerous studies have consistently shown that T replacement in young hypogonadal men increases BMD in both the hip and lumbar spine (19, 23, 333, 340, 341, 379–382). Subsequently, in a study where T was administered in the form of transdermal patches for a period of 3 yr in previously untreated hypogonadal men, BMD of the spine increased by 7.7%, whereas that of the femoral trochanter increased by 4% (23). In a second study where T was given in the form of a transdermal gel for a period up to 4 yr, similar increments were recorded (381), whereas im T administration to men with acquired hypogonadism increased trabecular BMD in the lumbar spine by 14% but not in the radius (19). Behre et al. (379) reported that long-term T replacement therapy in hypogonadal men (up to 16 yr) normalized and maintained spinal BMD in the age-specific normal range independently of the mode of T administration, whereas Leifke et al. (333) reported similar improvements in BMD independent of the type of hypogonadism treated. Finkelstein et al. (340) failed to corroborate these findings because they reported greater improvement in BMD in IHH patients with open epiphyses compared with those with fused ones. Although they recorded an improvement of spinal BMD, this did not return to normal. Summarizing, it appears that long-term T replacement improves and even normalizes BMD in young hypogonadal men. It appears that the BMD at baseline and the adequacy of treatment rather than mode of T administration as well as the type of hypogonadism determines the outcome. The highest rate of BMD improvement was recorded during the first year of treatment (379), with the peak reached after 24 months with a greater response observed in the vertebral bone rather than the hip (23, 381).
Considering the effect of T supplementation on bone turnover markers in young hypogonadal men, studies have produced conflicting results, with some reporting a decline of both bone formation and resorption markers (19, 23), while others report a decrease of bone resorption and an increase of bone formation in the first 6 months of treatment, which then plateaus (381, 383, 384).
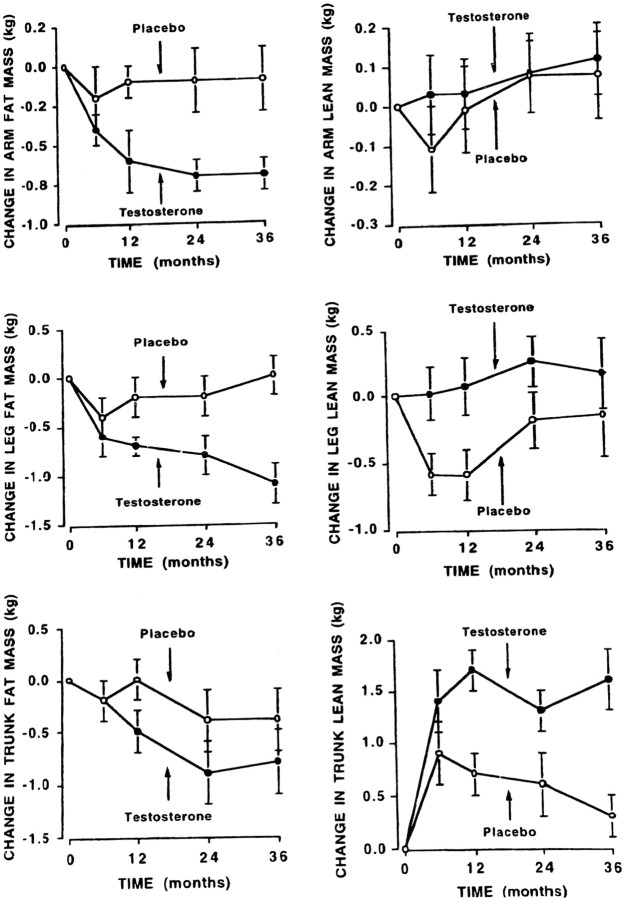
Only a few studies assessed the effect of T on body composition in hypogonadal men. Bhasin et al. (385) administered T enanthate (100 mg/wk im) for 10 wk to seven hypogonadal men and reported an increase of 5 kg in LBM, an increase in muscle strength, and an appropriate increase in thigh muscle size. They could not detect a decrease in BF, and whole body leucine kinetics were unaffected; they explained this by postulating a selective action of T on skeletal muscle mass. Katznelson et al. (19), using im T in a similar dosage in 36 men, reported a 7% increase in LBM and a decrease of 13% in sc fat (but not VF) after 18 months of treatment. In another study, T was administered in the form of a transdermal patch for 3 yr, and LBM increased by 3.1 kg whereas muscle strength and BF did not change significantly (23). A similar finding was reported by Wang et al. (383, 386), who compared the effects of a transdermal patch with that of the gel in a 6-month study. They found an improvement in LBM and strength, whereas BF decreased in the T gel group but not in the T patch group. Similar findings were reported by the same group of authors (384) regarding LBM and muscle strength after 6 months of treatment with sublingual T, but there was no effect on BF. In another study, Wang et al. (381), assessing the long-term effect of T gel administration in 163 hypogonadal men 19 to 68 yr old, reported an increase of LBM by 2 kg at 6 months, which was further increased to 3 kg at 30 months. Muscle strength did not change, whereas changes in BF were significant only in the young hypogonadal patients. The latter suggests that the lipolytic effect of T may be attenuated in older men. There was the anticipated increase of hematocrit from mild anemia to middle normal range hemoglobin and an increase of prostate-specific antigen (PSA) within the normal range; treatment was well tolerated.
Thus, it appears that T treatment in hypogonadal men consistently improves LBM, whereas its effect on BF and muscle strength is less clear. These findings corroborate the results of Brodsky et al. (387), who reported increased whole body and muscle protein synthesis in hypogonadal men after T administration. Additionally, studies that evaluated sexual function, mood, and QoL in hypogonadal men reported a remarkable improvement after T replacement. This occurs as early as d 30 of treatment and then plateaus and remains stable throughout the whole treatment period (23, 205, 206, 381, 384).
The effects of T on lipid profile and vascular function are complex and still a matter of contention (388). Several studies have assessed the effect of T on TC, LDL-C, and TG levels in hypogonadal men or in normal men who were rendered hypogonadal experimentally, with no consistent findings (19, 23, 381, 386, 389–394).
One study where T was administered to hypogonadal men showed an increase in TC and LDL-C (389), five found no changes (23, 381, 386, 391, 392), and two reported a decrease of LDL-C (390, 393). Also, no changes in TC, LDL-C, HDL-C, and TG levels were reported in a study where different doses of T were administered in healthy young men with induced hypogonadism (394). Data regarding the effect of T administration on HDL-C levels are more consistent in hypogonadal males; T was found to decrease HDL-C levels in a dose, duration, and type of treatment-dependent manner (19, 392). In a meta-analysis of 19 studies published between 1987 and 1999, involving only young clinically overt hypogonadal men, Whitsel et al. (395) reported that im administration of an average dosage of 179 ± 13 mg of T every 2 wk for 6 months was associated with a decrease of 4 mg/dl HDL-C with a commensurate decrease of 5 mg/dl of LDL-C levels. These studies usually refer to im T administration, whereas sublingual or transdermal T administration for a period of up to 42 months did not adversely affect the lipid profile (23, 381, 386) in all but one study reported (392).
Testosterone stimulates hepatic lipase activity (396), which in turn has been shown to decrease HDL-C and LDL-C particle size and thus results in a more atherogenic lipid profile (397, 398). On the other hand, in vitro studies suggest that T can intensify reverse cholesterol transport from macrophages and thereby exert an antiatherogenic rather than a proatherogenic effect despite reducing HDL-C levels (399).
The lipolytic action of T decreases with time in parallel with the decrease in the fat depot. Furthermore, T administration reduces insulin resistance in hypogonadal diabetic men and decreases endogenous inflammatory cytokines in hypogonadal men (400, 401). On the other hand, shorter duration studies have reported that T administration impairs endothelium-dependent flow-mediated vasodilatation, a risk factor of atherosclerosis in young hypogonadal men (402, 403).
B. Effects of GH on the GHD syndrome
GH treatment reverses most of the changes associated with the GHD syndrome, with the most striking changes occurring in body composition. Accordingly, LBM has been shown to increase by 2.5–5.5 kg with a concomitant 5% increase of skeletal muscle, whereas BF decreased by 4–6 kg with a 30% decrease in VF (16, 275–278, 650). These changes in body composition are sustained during prolonged (5–10 yr) GH replacement, as has been demonstrated in a series of recent studies (79, 290, 404–407).
Neither isokinetic quadriceps peak torque nor isometric force has been shown to improve after GH treatment in GHD adults in studies where the duration of treatment did not exceed 6 months, although a clear increase in muscle mass was recorded (290, 408). Accordingly, Cuneo et al. (409) have recorded an increase in CSA of thigh muscle and an increase in limb girdle and hip flexors, whereas quadriceps peak torque and isometric force did not change. When GH was used for a period longer than 12 months, however, an increase in muscle strength was consistently found (276, 284, 286, 410, 411). Recent studies of 5–10 yr duration (with no untreated control group for comparison) have reported an increase during the first 5 yr, which almost normalizes muscle strength, and thereafter a preservation of muscle strength and neuromuscular function against the age-related decline (276, 412). GH promotes protein accretion by stimulating amino acid uptake and incorporation into protein. Hence, Russell-Jones et al. (73, 74) in a series of studies have demonstrated an increase in whole body protein synthesis after 3 months of GH treatment in both fasting and postprandial settings. IGF-I likewise promotes protein synthesis in both GHD adults and healthy adults after acute administration (84, 96).
On the other hand, GH has been shown in numerous studies also to increase aerobic capacity in GHD adults (290, 292, 413). GH treatment improves vascular reactivity and the adverse lipid profile commonly occurring in GHD patients (414, 415). A decrease of TC and LDL-C seems to be a uniform finding (reviewed in Ref. 22), whereas HDL-C has been found to increase in some studies (416) but to remain unchanged in other studies (308). These beneficial effects on lipid profiles are maintained with prolonged 5–10 yr of GH treatment (79, 405). The long-term effect of GH administration on insulin sensitivity in GHD adults is controversial, with some reporting an improvement in insulin sensitivity (86, 417, 418) and others not (419, 420). Recent long-term studies of up to 7 yr in duration have, however, consistently demonstrated that GH replacement therapy improves insulin sensitivity and cardiovascular risk factors and may prevent the age-related decline in these risk factors in GHD adults (216, 328, 405, 407, 421). QoL is impaired in many GHD adults and normalizes after GH replacement (422). Several studies have shown persistent treatment benefits being evident years after commencing treatment and that interruption of GH replacement treatment adversely affects the QoL of GHD patients (405, 423–425). For a detailed review of GH replacement therapy in the GHD syndrome, see Refs. 22 and 273.
VII. Evidence of an Additive Anabolic Action of GH and Testosterone
The first evidence for a possible additive anabolic action between the GH/IGF-I axis and T might have been deduced from the observation that at puberty in boys (rather than girls) there is a higher state of anabolism with higher linear growth rates, higher muscle, and bone accretion. Consequently, it may not come as a surprise that androgens in conjunction with GH confer higher anabolic effects than estrogens with GH. It might also be argued that the higher anabolic effect and sexual development seen in boys during puberty is due solely to the androgen spurt. This argument can easily be dismissed because in cases of isolated GHD where the anabolic effect of T is disrupted despite normal T secretion, there is no pubertal growth spurt. Thus, it is the combination of these two hormones that confers the higher anabolic action observed physiologically in men.
A. Puberty and GH and testosterone interaction
Puberty offers plenty of evidence for the close interrelation of these two hormone systems because daily GH secretion more than doubles, mainly as a result of higher GH pulse amplitude, whereas pulse frequency, pulse duration, and GH half-life remain unaffected (426). The highest rates of GH secretion coincide with the highest rates of linear growth during puberty, and relative disorderliness or irregularity of GH secretion (approximate entropy) peaks during this time (427). Finally, in an elegant experiment, Giustina et al. (428) demonstrated how T can modulate and mature the hypothalamic pituitary GH axis because the administration of incremental T doses in hypogonadal prepubertal boys increased daily GH secretion. The action of T in amplifying GH secretion in prepubertal boys and hypogonadal men has been postulated to occur indirectly through conversion of T to estrogens because nonaromatizable androgens do not amplify GH secretion. These findings have been recently confirmed in older men after exposure to high pharmacological doses of exogenous T (119). On the other hand, physiological transdermal T administration did not increase daily GH production in the older people (121, 429). Thus, T amplifies GH secretion in hypogonadal, prepubertal, and older men but not in eugonadal men (430).
Additionally, evidence for an additive action between GH and T might also be inferred from the gender differences in secretory dynamics of GH. Women secrete more daily GH than men and have higher GH peak pulses and larger pulse amplitude (431). Estrogens seem to be responsible for this striking amplification of GH production in women, which doubles in the preovulatory phase in accordance with the elevated estrogen levels in midcycle. IGF-I levels, however, are similar between men and women, and one explanation could be relative resistance to GH action in women at least at the level of the liver (432). Accordingly, the decreased response to GH treatment in GHD women compared with men has been clearly presented in a series of studies (433, 434). Thus, estrogens seem to confer a form of resistance in GH action, whereas androgens seem to facilitate GH/IGF-I action.
B. Muscle growth and GH and testosterone interaction
GH, IGF-I, and T are all strong anabolic agents that promote muscle protein synthesis and hypertrophy (38, 73, 435). Testosterone administration has been shown to increase muscle, prostate, liver, and ovary IGF-I gene expression in animal and human studies (436–438). Urban et al. (436) has shown that im T administration for 1 month in healthy older men increased muscle mRNA IGF-I levels and decreased mRNA levels of IGFBP-4; the latter was reported to be a negative regulator of IGF-I in muscle (439). On the other hand, artificially induced hypogonadism in young men has produced the opposite effects—a decrease of muscle IGF-I gene expression levels and an increase IGFBP-4 mRNA (440).
Androgens have been shown to up-regulate their own receptor (AR) in muscle cells and satellite muscle cells (441), the latter being muscle multipotent stem cells whose activation is imperative for muscle regeneration and adaptation to exercise (37). Sarcopenia may be linked to impaired satellite cell activation, which in turn is regulated by the autocrine/paracrine action of IGF-I and T (442). Intramuscular T has increased muscle AR mRNA levels in healthy older men after 1 month of treatment, but not after 6 months, whereas IGF-I mRNA levels increased at 1 month and remained increased at 6 months (443). Brill et al. (429) did not find an increase of muscle AR mRNA, however, in older men using transdermal T after 1 month. From the above, it seems that T up-regulates its own AR gene expression in muscle and also increases muscle mRNA IGF-I levels. A recent observation of two androgen response elements within the IGF-I upstream promoter gene that act to increase IGF-I expression may well explain these findings and suggest that T acts by amplifying the anabolic actions of GH (444). Testosterone, on the other hand, has been shown to promote muscle growth by a novel early transcriptional program including IGF-I, the putative MGF, and induction of β-catenin (445), which plays an important generic role in the activation of several signal transduction pathways.
GH, on the other hand, may facilitate its benefactor (T in this case) by up-regulating the AR in muscle as shown in a study in artificially induced hypogonadal adults (82).
Additionally, GH administration increases muscle IGF-I gene expression levels, as has been show in GHD patients and healthy older men (82, 290, 429, 446). This effect has been demonstrated after the acute iv administration of GH for a short period of time (447).
Finally, the activation of satellite muscle cells is of foremost importance for muscle adaptation to loading and muscle regeneration (37). Activation of muscle satellite cells leads to proliferation and differentiation and fusion of myoblasts providing the new myonuclei needed (448). IGF-I functioning in an autocrine/paracrine mode is an important mediator of skeletal muscle adaptation (37). In an elegant study, Hameed et al. (446) evaluated the expression of two isoforms of IGF-I in healthy older men at baseline and after GH administration with or without exercise. The first isoform, the putative MGF, is expressed specifically in muscle after muscle loading, whereas IGF-IEa is similar to liver IGF-I. Combined exercise and GH increased MGF and IGF-IEa more than either agent alone, whereas GH alone increased preferentially IGF-IEa and exercise MGF. It appears that exercise and GH/IGF-I axis integrity are paramount for muscle physiological adaptation, which translates into satellite cell activation.
Accordingly, T increases cross-sectional area (CSA) of muscle mass by inducing fiber muscle hypertrophy in both young and older men (38). It was suggested that the hypertrophic response to T administration in muscle is indeed through satellite cell activation (38, 441). Thus, both GH and T have a common target of action, and it seems that one up-regulates the action of the other.
C. Lipolysis and GH and testosterone interaction
VF has been shown to be very sensitive to catecholamine action stimulating lipolysis (449), and its high concentration of GH and AR may play a role in this. Both GH and T facilitate the mobilization of lipids by inhibiting LPL activity mainly in VF and enhancing catecholamine induced lipolysis via β-adrenergic receptors (β1-β2 and β3 stimulatory adrenoreceptor), which are functionally active principally in omental fat (450). It has been shown that in adipocytes from hypophysectomized rats, GH and T have an additive effect on lipolysis because both appear to increase β-adrenergic receptor density, whereas the presence of GH was required for fully expressed lipolytic action of T (451). Testosterone on the other hand, by modulating IGF-I receptor and peroxisome proliferator-activated receptor γ2 expression in preadipocytes, elicits an antiadipogenic effect (452).
VIII. Potential Implications of the Anabolic Hormones and VO2max Decline in Older Men
The decline of physical activity, a universal finding of aging that occurs in humans and other species (453, 454), may contribute to the decline of physical function of aging because both muscle strength and aerobic exercise capacity are strongly associated with levels of physical activity. Consequently, exercise as a possible physiological intervention in counteracting sarcopenia of aging has been extensively studied.
A. Exercise and integrity of anabolic hormonal milieu is required for muscle adaptation
1. Impaired muscle adaptation to exercise in older people
Muscle adaptation to exercise is a multistep process that is modulated by both endocrine anabolic and locally expressed load-sensitive autocrine/paracrine growth factors (reviewed in Ref. 37). In this process, two steps can be clearly distinguished: first, an increase in protein synthesis that results in the expansion of muscle myonuclear domain; and second, the activation of muscle satellite cells. Locally expressed IGF-I appears to play a fundamental role in both of these processes and has been demonstrated to increase protein accretion through the Akt/mammalian target of rapamycin pathway, promoting the formation mainly of fiber type I (fast, high oxidative capacity) myofibers (36). Of considerable significance is that only 14 d of GH infusion has been shown to increase muscle mitochondrial oxidative capacity in healthy young adults (455).
IGF-I on the other hand has a strong mitogenic and myogenic action by promoting satellite cell proliferation (456), differentiation, and eventually fusion of satellite cells that form the new myonuclei needed to support myonuclear domain expansion (37). Furthermore, MGF, a putative splice variant of the IGF-I gene (also known as IGF-IEb), is responsible for the initial hypertrophic response of muscle to training by activating muscle satellite cells (457). This is distinct from the IGF-IEa, the second splice variant also up-regulated in response to exercise. This is the same as the IGF-IEa produced by the liver. These isoforms have been reported to have different roles because they are regulated differently and have different levels of expression and timing after uploading of exercising (457). It was suggested that the putative MGF exerts its action independently of the IGF-I receptor and is required for muscle satellite cell activation, whereas IGF-IEa is required for maintenance of muscle mass hypertrophy (446). Aging is associated with impaired muscle cell activation, which has been proposed to contribute to the sarcopenia of aging (442). Indeed, several studies have shown that mRNA levels of MGF, but not of IGF-IEa, are significantly up-regulated as early as 2 h after an exercise bout in young men, but not in older men (35, 446, 458). Furthermore, Petrella et al. (35) have recently reported that 16 wk of resistance exercise significantly increased the number of new myonuclei formed in young people, but not in older people, and although expression levels of both splice variants increased significantly in young and older people, the increments in the young were at least two times higher. Another study (the only one to date) to assess the effect of GH administration with or without exercise in older men has demonstrated that exercise significantly up-regulates mRNA levels of MGF, and GH up-regulates mRNA levels of IGF-IEa, whereas GH combined with exercise further enhanced MGF transcript levels (446). Moreover, a recent study has shown that both MGF and the generalized IGF-I isoform mRNA levels increase after a single bout of exercise of intensities as low as 60% of 1RM in young people (458). Finally, it has been clearly demonstrated that exercise increases the generalized IGF-I isoform and up-regulates the muscle satellite AR (459, 460) in most but not all of the studies (461).
2. The endocrine role of GH and testosterone on muscle growth
As presented previously, IGF-I is a powerful regulator of muscle growth and cell differentiation, acting locally through an autocrine/paracrine as well as in a classical endocrine fashion (reviewed in Ref. 37). In this regard, locally infused IGF-I has been shown markedly to increase skeletal mass (462). GH on the other hand is the main regulator of the expression of IGF-I in different tissues (63, 64). GH receptor signaling occurs through activation of the Janus-activated kinases (JAK) and signal transducers and activators of transcription (STAT), whereas down-regulation is mediated by a family of cytokine-inducible suppressors of cytokine signaling (SOCS) (reviewed in Ref. 56). GH receptors have been identified in muscle (61), and a recent study has demonstrated that a bolus of GH in young men activates the STAT5b and increases the mRNA levels of IGF-I and SOCS-3 in muscle (463), whereas a STAT5b-specific binding site has been characterized in the IGF-I promoter region that mediated IGF-I gene activation (464). In this regard, administration of GH in hypophysectomized rats has been shown to increase IGF-I levels (465), whereas C2C12 skeletal muscle cells respond rapidly to GH by stimulating tyrosine phosphorylation of the GH receptor and STAT5b and increasing levels of mRNA IGF-I (63). Furthermore, GH administration has been shown to increase muscle IGF-I gene expression in GHD patients (290), in men artificially rendered hypogonadal (82), and in healthy older men (429, 446, 447) in most but not all studies (461). Differences in GH dosage and timing of sampling may explain the discrepancies.
Newer evidence from studies conducted in mice expanded our knowledge of how GH and IGF-I may regulate muscle growth. Hence, Iida et al. (466) have reported that administration of GH to mice acutely increased mRNA levels of both MGF and IGF-IEa, with the former found to increase preferentially in the situation of GHD (lit/lit) mice, whereas in GH-sufficient mice similar increments were found. In another study in mice specifically lacking the skeletal muscle IGF-I receptor, GH failed to induce muscle growth, and consequently it has been postulated that intact IGF-I receptor signaling is required for the action of GH in muscle (467). Another study reported that mice with a skeletal muscle-specific deletion of the Stat5 genes had IGF-I mRNA levels reduced by 60% in muscle tissue and impaired growth (468). This denotes a critical role of GH for the formation of locally produced IGF-I in muscle, which in turn acts through a paracrine/autocrine fashion. Finally, Sotiropoulos et al. (469) have challenged this by demonstrating that GH has a specific role in myoblast fusion independent of that of IGF-I.
Testosterone on the other hand has been shown to promote muscle growth by mainly enhancing im mRNA IGF-I levels (445); however, the role of circulating GH and IGF-I in mediating the effects of T on skeletal muscle has recently been questioned by Serra et al. (470), who reported that IGF-IR signaling in skeletal muscle fibers does not appear to be obligatory for mediating the anabolic effects of T in mice.
The additive effects of GH and T on muscle growth were discussed in Section VII.
From all the data mentioned above, it becomes clear that exercise and the integrity of the anabolic hormonal milieu are of paramount importance for muscle adaptation and that this can be achieved even with rather low training intensities in the young, but not in older people.
B. Impaired hormone anabolic profile and strenuous exercise in older men
1. Strenuous exercise-induced cytokines and their interaction with the GH/IGF-I axis
Exercising muscle has been shown to secrete several cytokines, including IL-6, IL-8, and IL-10, with IL-6 being more extensively investigated (reviewed in Ref. 471). Plasma levels of the proinflammatory cytokine IL-6 increase strikingly after exercise, with the magnitude of the increase related to the intensity and duration of exercise (471). Although both macrophage cells and adipocytes may secrete IL-6, it has been demonstrated that exercising muscle is mainly responsible for the elevated levels of IL-6, and this is not related to muscle injury (472). Increased levels of IL-6, however, have been suggested to have a detrimental effect on muscle growth (473), and in general higher levels of IL-6 were found in frail older men (474) and were negatively associated with reported levels of physical activity, fitness, and IGF-I (475, 476). In addition, higher levels of IL-6 were found in several inflammatory conditions associated with insulin resistance (477, 478). In this regard, it was reported that IL-6 levels rather than TNF or leptin were strongly associated with obesity and insulin resistance (477).
Exercise is the most powerful physiological stimulus of GH secretion. The GH/IGF-I axis response to exercise has been only recently reviewed (479). Age, exercise duration, and intensity have all been reported to be strong predictors of GH response. GH levels peak immediately after exercise, and this has been demonstrated to occur even at exercising intensities well below the lactate threshold (480, 481). Trained or untrained older men have an impaired GH response to exercise (482, 483). IGF-I levels on the other hand have also been found to increase after exercise, but only by a much lesser extent than GH (484). In studies where very vigorous training programs were implemented in prepubertal girls, however, IGF-I levels were found to be suppressed (485–487). There is evidence that suggests an interaction of the GH/IGF-I axis and IL-6, and it was postulated that higher IL-6 levels observed after strenuous exercise may suppress plasma IGF-I levels (487, 488). This hypothesis was explored in a study using IL-6 infusion in healthy older men in doses sufficient to increase IL-6 levels to those observed after intense exercise; this resulted in an increase in GH and IGFBP-1 and a decrease in IGF-I levels (46). There are several pieces of evidence that suggest a convergence of GH/IGF-I and IL-6 signaling, with a common area of interaction in the JAK/STAT pathway. This pathway also participates in cytokine signaling where alteration in the gene expression of members of the SOCS family has an important role (489–491). The latter, as we have mentioned before, is an inhibitor of STAT activity. STAT3 has been recently identified as a mediator of IL-6 signaling (492). In this regard, local IL-6 infusion in rodents resulted in a preferential decrease in myofibrillar proteins and induced a decrease in phosphorylation of STAT5 and an increase by 2-fold of that of STAT3 (493). Supporting these findings, a recent study has shown that a single bout of vigorous exercise significantly activated STAT3 in muscle of young men 2 h after exercise, whereas the expression of SOCS-3 increased 60-fold (494). This is important because it suggests that strenuous exercise in older men may indeed diminish the already impaired endocrine and paracrine/autocrine action of both GH and IGF-I in muscle.
2. Impaired GH response to exercise may be causally linked to diminished training-induced anabolism and insulin resistance of aging: a hypothesis
Interestingly, IL-6 has also been shown to have an antiinflammatory effect by suppressing TNFα levels and to participate in metabolic control pathways during exercise (471). Subsequently, IL-6 has been reported to increase lipolysis and promote FFA oxidation, and recently it has been demonstrated that IL-6 can act as a novel factor that increases endogenous glucose production during exercise (495). It appears that IL-6 might be acting as a hormone, being released by the muscle to signal to the liver to stimulate glucose production when required (495, 496). Glucose on the other hand remains an important energy supply in the early stages of exercise. Glucose availability is dependent on stimulation of glycogenolysis and gluconeogenesis, which will be greater when insulin levels are suppressed. Plasma insulin levels are indeed suppressed during and immediately after exercise (497), thus facilitating glucose production. The majority of evidence supports the notion that IL-6 promotes hepatic insulin resistance by activating SOCS proteins in the liver (498, 499). Furthermore, GH, which is known to induce insulin resistance and its robust increase during exercise, may also play a role in mobilizing glucose (500).
Muscle glucose uptake during and immediately after exercise increases by an insulin-independent mechanism (497). It has been suggested that IL-6 may facilitate glucose uptake by muscle during and immediately after exercise; however, this has been disputed (501, 502). Nevertheless, if this were true, then IL-6 would need to induce insulin resistance at the liver in order to facilitate glucose production while at the same time having insulin-like effects on muscle. The fact that strenuous exercise increase SOCS-3 protein expression by 60-fold in muscle makes this explanation less attractive (494).
IGF-I on the other hand, as its name suggests, has been shown to have insulin-like effects (88). Could the decreased im IGF mRNA levels observed in older people after exercise impede glucose uptake in their exercising muscle? This is an intriguing hypothesis that could explain the dissociated effects of GH and IGF-I and furthermore the link between the insulin resistance of aging and the concomitant decline in the GH/IGF-I axis.
Decreased muscle glucose uptake during exercise may also contribute to insulin resistance in aging. It may contribute to the decreased suppression of protein degradation during exercise and, consequently, impaired protein accretion described in older people (503). Additionally and of similar importance, decreased glucose uptake in exercising muscle may augment the muscle cytokine secretion because muscle glycogen depletion has been shown to be the main stimulus for cytokine secretion to take place.
C. Possible implications of GH and testosterone decline in older men
The observations that IGF-I overexpression in a transgenic model did not prevent muscle atrophy due to acute muscle unloading and that T could not prevent the muscle strength decline during 28 d of bed rest confirms that uploading the muscle mass against gravity is fundamental for preserving its function (504, 505). On the other hand, although it may be argued that circulating anabolic hormones are not necessary for muscle adaptation to exercise, as has been observed in the experimental model using hypophysectomized rats (506), the overwhelming clinical experience has shown that exercise could not replace the administration of GH or T in improving physical function in young GHD or hypogonadal men simply because these patients are effectively unable to exercise to the level required.
The evidence presented in Sections VIII.A and VIII.B may explain the two major drawbacks regarding exercise as an interventional approach for improving physical function in older men. First, improvements in muscle strength and muscle power that have been shown to occur in several studies quickly level off, possibly as a result of impaired muscle hypertrophy because of a diminished anabolic hormone milieu in older people (507, 508). Second, dropouts from training programs are reported to be very high, independent of the design of the exercise intervention (home-based, group-based, educational). The longer the duration of training, the higher the dropout rate recorded. Hence, in a large meta-analysis that included 38 studies of exercise intervention programs in older men, van der Bij et al. (509) concluded that the improvements in physical activity, although present, were small and short lived. Participation seems to decline inevitably and was found to have fallen to less than 30% in the few studies lasting 1 yr. It is possible that the dropout rate is always higher in older people (510). It appears that strenuous exercise may further increase the elevated cytokine levels in the older people that, by inhibiting the already impaired GH/IGF-I axis activity, could further compromise muscle adaptation to training. Thus, the energy-demanding process of protein accretion becomes inefficient. Because there is a close association of feeding and volitional and not volitional exercise (511), it is reasonable to hypothesize that when an organism senses that exercise cannot confer any benefits regarding protein accretion, it slows down to conserve energy.
The essential role of exercise in muscle function presented before could be one explanation of the negative findings regarding improvements in muscle function, despite an increase in muscle CSA in healthy older men after combined treatment with GH and T (48), because newly added muscle requires some time before acquiring strength. This is in keeping with the observation that strength improves in GHD adults usually only after 1 yr of GH replacement, although muscle enlargement occurs within 6 months (410, 411). It may also explain why GH combined with exercise failed to augment the muscle strength recorded with exercise alone in short-term studies, although higher increments in muscle mass were found (512, 513).
It seems that the initial muscle hypertrophy obtained as early as 6 months has to be “uploaded” regularly, as occurs with everyday physical activities, before it gains in power. It appears that this process of activating newly formed muscle needs some time (months to years) even when regular training is undertaken.
1. The importance of the effects of GH combined with testosterone in improving VO2max in healthy older men
A key question arises from the decline in anabolic hormones seen in older men. Are these older men able to respond to hormone replacement as do patients with GHD? Although coadministration of GH and T has been shown to increase muscle mass, can this form of hormone replacement therapy (HRT) preserve or even increase muscle strength and prevent or mitigate the relentless decline in strength that occurs with aging? If so, older people would be able to upload their muscle more often and more intensely and maybe preserve their mobility?
Thus, the improvement in maximal aerobic capacity in older healthy men seen after treatment with GH plus T but not with T or GH alone, as initially reported by Blackman et al. (49) and recently confirmed by Giannoulis et al. (48), is of great importance because it supports the above concept. Differences in study design and baseline VO2max levels may well explain the variable response of maximal aerobic capacity observed in the above studies (9 and 20%, respectively).
It can be argued that the increase of VO2max seen by Giannoulis et al. (48) is mainly due to the increase in skeletal muscle mass. It was only after treatment with GH+T that a significant increase in muscle CSA was recorded; and that, together with the gains in appendicular muscle mass, correlated with changes in VO2max seen during the 6-month period of HRT. GH administration alone does not seem to increase VO2max in older men or women (94, 514). On the other hand, T in general also failed to improve VO2max in healthy older men, even when it was administered in doses high enough to increase skeletal muscle protein synthesis, muscle mass, and strength (436). In addition, other evidence from the literature suggests that T alone does not affect aerobic performance (515), although this is somewhat at odds with the widespread abuse of anabolic steroids in sport (where GH is often/usually used in combination), and it may just be that treatment duration has not been long enough or insufficient doses were used. On the other hand, it was elite athletes who first discovered that GH is a performance-enhancing drug, and GH is most commonly abused together with anabolic steroids (282) because athletes probably find that the combination works. Thus, it appears that the coadministration of GH and T may result in increments of skeletal muscle mass with higher oxidative capacity than seen with T alone. Although it cannot be stated categorically that this is the case, it may be due to the greater anabolic action obtained by combining two anabolic agents in the limited time span of the study. It may be significant in this regard, however, that GH administration alone has been demonstrated to increase skeletal muscle oxidative capacity in GHD men (455).
It has been shown that VO2max is a strong determinant of physical performance and independent living in older men (8, 516) and that the decline of VO2max below a threshold of 18–20 ml/kg · min will compromise physical functional capacity and independent living (517, 518). A decline of VO2max below 18 ml/kg · min would mean an anaerobic ventilatory threshold of approximately 10 ml/kg · min and the onset of fatigue and an inability to perform a task with levels higher than 15 ml/kg · min. Considering that an older man would need approximately 10–12 ml/kg · min to walk a short distance at a slow pace carrying groceries, it is clear that any decline of VO2max below the threshold of 18 ml/kg · will compromise independent living (8, 517). Given the hyperbolic relationship of exercise intensity and time to fatigue (519), it could be argued that small increases in anaerobic threshold could translate into big improvements in the intensity of exercise performed below the anaerobic threshold, before fatigue ensues. Although Giannoulis et al. (48) did not measure the anaerobic threshold, we would expect it to increase in a commensurate fashion as the VO2max. This has been shown first by Cuneo et al. (292) and later confirmed by Woodhouse et al. (290); both reported an improvement in submaximal exercise capacity in GHD adults after GH treatment.
Thus, an improvement of VO2max by 10 to 20% after GH+T (48, 49), which almost offsets the decline per decade of life in those older than 70 yr as demonstrated in the longitudinal (Baltimore) study by Fleg et al. (10), is of great clinical significance for two main reasons. First, by directly increasing their physical functional performance, older men would be able to perform tasks that would previously have required higher intensities of effort or longer duration because these levels of effort are substantially higher than those required for independent living. Second, and of similar importance, the increase of VO2max may in turn increase the daily physical activity (volitional or nonvolitional) in older men. VO2max, defined as the capacity of exercising muscle to extract and use oxygen, is modulated by the training status of individuals and also by their genetic background (520). Similarly, free living daily physical activity has also been shown to be genotype dependent and reflects the daily energy expenditure of volitional and nonvolitional exercise (521). Volitional and mainly nonvolitional exercise (fidgeting) are important determinants of total energy expenditure (∼30%). Total energy expenditure declines with aging largely due to the fall in LBM. The decline of activity energy expenditure seems to be the main reason because it appears that humans slow down as they grow older (454). A positive association was reported between VO2max and free-living nonexercise time physical activity in most but not all studies (522, 523). In a recent study of older men, however, a positive correlation was found between nonexercise physical activity and VO2max (524). Subsequently, it is reasonable to suggest that the increased VO2max observed after GH+T would not only increase exercise capacity but also will ease the fatigue in different tasks and may increase the free-living daily physical activity and probably QoL. This is in reality often quoted by patients with GHD as the greatest benefit of replacement with GH.
In conclusion, physical activity of both higher intensity (volitional) and lower intensity (spontaneous free living) is a prerequisite for muscle adaptation. It can be speculated that these activities will be facilitated by providing older men with a restored anabolic hormone milieu.
IX. Anabolic Intervention in Aging
A. Issues regarding the role of hormone replacement (GH and testosterone) in older men in the light of recent research
1. Symptoms and signs attributed to anabolic hormone level decline in older men
Before presenting the results of various recent studies that have assessed the effects of GH or T in older men, it is helpful to consider some of the issues that surround the possible therapeutic role of HRT in aging men.
At present, neither T nor GH has an established role in either prevention or treatment of the adverse changes in body composition and decline in physical function performance observed in older men. This is reflected in recent guidelines published by The Endocrine Society first for GH (525) and subsequently for T (526). Both guidelines failed to reach a consensus on which older men, if any, would benefit from HRT. In both instances, two main objections were raised. First, GH or T administration in older men failed to demonstrate consistent improvement in muscle function or other measurements of physical performance. Second, a clear casual relationship between declining GH and T levels and signs and symptoms attributed to these low levels has yet to be established. Both of these arguments and the potential danger of serious side effects are the basis of the controversy surrounding HRT in older men (527–530).
Administration of T has been shown to improve physical performance in some (443, 531) but not in all of the studies (21, 48, 49, 532); usually, high rather supraphysiological doses were used, which precluded the clinical utility of these studies. On the other hand, GH treatment is associated with favorable changes in body composition but not usually in physical function (20, 48). Furthermore, early studies, when doses used were in retrospect very high, reported a high incidence of adverse effects (94). Two studies in which GH was given in extremely high doses to healthy older women reported predictably serious adverse effects and a dropout rate of almost 50% in one (533, 534). In a series of studies, Bhasin et al. (17, 535, 536) have demonstrated that the anabolic effects of T are dose-dependent and that older men are as responsive to graded doses of T as young men. In a similar fashion, the magnitude of anabolic effects of GH in older men appears to be dose-dependent, and if anything, the older men are more sensitive to GH than the young. It is of paramount importance for safety reasons alone that effectiveness is shown to occur under a replacement regime that produces what may be considered physiological hormone levels in order for it to be of potential clinical use.
Although normative values for T may differ between laboratories (537), there is a consensus that levels around 300 ng/dl (10.4 nmol/liter) and 6.5 ng/dl (0.225 nmol/liter) for total T and FT, respectively, corresponding to BioT concentrations of around 140 ng/dl (5 nmol/liter), represent the lower limits of the normal range of young healthy men (538). Using the above normative values, Kaufman and Vermeulen (539) reported that 20% of older men (and a slightly higher proportion when FT levels were considered) had abnormally low T levels. Using liquid chromatography tandem mass spectrometry, Wang et al. (540) have reported a higher metabolic clearance rate and daily production rate of T (8.45 ± 1.14 vs. 5.12 ± 0.36 mg/d) for young men when compared with middle-aged men in accordance with earlier studies (541, 542).
We discussed in Section III.A how the integrated daily GH secretion rate had fallen by 70% in 70-yr-old men when compared with 20 yr olds (14) and that 30% of men older than 70 yr had IGF-I levels lower than the lower limit of the young reference range (104, 105).
Despite these unequivocal findings of reduced GH and T secretion in a significant proportion of older men, no clear association between signs and symptoms specifically associated with GHD or T deficiency has yet been found. In fact, case detection questionnaires relying on self-reporting and specifically developed to identify older men with low T levels, such as the Aging Males' Symptoms Scale (543), the Androgen Deficiency in Aging Male (544), and the Massachusetts Male Aging Study Screener (545), have all produced equivocal results (544, 546, 547) and they were found to lack specificity (526). It has been argued that whereas primary and secondary hypogonadism are well-defined clinical entities, this could not apply to older men with low T levels where there are often confounding factors including health, lifestyle, and other comorbidities commonly seen in older men (528). Likewise, population-based surveys of community-dwelling older and middle-aged men using questionnaires directed at sexual and other less specific symptoms, such as tiredness, lethargy, sleep disturbances, and depressed mood, could not find a clear association between symptomatic androgen deficiency and T levels (153, 175, 402, 548–551). Accordingly, Travison et al. (548) in a cohort of men aged 40 to 70 yr found only a weak association between T levels and libido, with a substantial overlap of T levels between those reporting a low or normal libido. In another study in men aged 30 to 70 yr, Araujo et al. (549) found the prevalence of symptomatic androgen deficiency to be 5.6% and to increase with age, and almost half of the men older than 50 with low T levels were asymptomatic. Furthermore, Hall et al. (551) reported that waist circumference and health status appear to be significant confounders of this association. Zitzmann et al. (402), in a cohort study of 434 male patients aged 50–86 yr attending an andrological clinic, could not find a threshold for symptomatic hypogonadism. They found a clustering of symptoms, and different symptoms were associated with different T levels. They reported an increased prevalence of loss of libido and vigor in those with T levels lower than 15 nmol/liter, whereas erectile dysfunction was more prevalent when T levels were lower than 8 nmol/liter. In contrast to these reports, a recent study of men aged 40–79 yr found that the presence of at least three sexual symptoms (poor morning erection, low sexual desire, and erectile dysfunction) was associated with T levels lower than 11 nmol/liter (320 ng/dl) and 6.4 ng/dl of FT (550).
All of these surveys have included middle-aged men, and this was anticipated to strengthen the association of symptoms of hypogonadism with T levels because in middle-aged men, symptoms are more likely to be solely attributed to hypogonadism, which may not be the case in older men. It appears therefore from data obtained from selected cohorts that there is no clear association between symptoms of androgen deficiency and T levels in older men.
In accordance with these findings, similar results were seen between T levels and the frailty phenotype in similar population-based surveys (175, 176, 552). On one hand, results reported from the Massachusetts Male Aging Study could not detect any association between the frailty phenotype (a model comprising a number of components) and T or FT levels, although there was an association between T and some of the components of the model describing the phenotype (grip strength and activity). An association has been found, however, between SHBG levels and frailty (plus the components weight loss, exhaustion, and physical activity) (175). The introduction in the multivariate regression analysis of a measure of androgen sensitivity (the number of CAG repeats in the AR) in a subsequent analysis did not modify these findings (552). On the other hand, Krasnoff et al. (176) in the Framingham Offspring Study reported FT (but not total T) levels to be associated with mobility limitation and physical performance in men with a mean age of 61 yr. In none of these studies was IGF-I measured or the GH/IGF-I axis investigated. We are not aware of similar studies regarding the association of IGF-I levels or the GH/IGF-I axis and the frailty phenotype or symptoms related to GHD in healthy older men.
2. Selecting the older men who may benefit from HRT in view of the new evidence
If both GH and T improve muscle function and physical performance in older men, what accounts for this lack of association between the hormone levels and frailty? This is an important question as expressed by Snyder (553) because it poses a practical dilemma: who are these older men who are most likely going to benefit from HRT?
The evidence presented in Section VII regarding the additive action of GH and T highlights the importance of both the GH/IGF-I axis and T secretion in the ability to undertake normal physical activity and may provide some clues to answering this question. This should be considered together with the fact that coadministration of GH and T in older men improves VO2max and produces highly significant changes in skeletal muscle mass (390, 394). These positive benefits were achieved with what may be considered physiological doses of hormone replacement, they provide some answers to the question presented before, and they open new avenues for exploring the role of HRT in older men.
First, the adverse changes in physical function observed with aging are caused by a progressive decline in all physiological functions, with impairment of GH and T secretion and reduced physical activity being important components. Thus, the symptoms seen in older men, although resembling those of young hypogonadal men, could not be directly linked to low T levels. With the exception of the sexual symptoms, the remaining features are nonspecific and may well be related to the decline in GH secretion and aerobic capacity. In fact, many of the symptoms resemble those that occur in the GHD syndrome in adults (273, 316) and that are relieved by hormone replacement with GH.
This hypothesis has been verified in two recent studies that analyzed data on women aged 70–79 yr from the Women's Health and Aging Studies and reported that the likelihood of frailty increases nonlinearly in relation to the number of physiological abnormal systems, the latter being more predictive of frailty than any individual abnormal system (554). In addition, it was found that the absolute burden of anabolic hormonal deficiencies rather than the type of hormonal deficiency was a strong predictor of frailty (555). The authors concluded, “These analyses suggest generalized endocrine dysfunction in the frailty syndrome.”
Second, baseline T levels are known to be determined by several factors such as overall health status, smoking habit, diet, lifestyle changes, and most importantly an increase in abdominal fat (556–561). A high within-subject variability of baseline T levels has been reported in several studies (562, 563), which further complicates any potential selection process based on a single T measurement.
An important confounder is also the increased SHBG level observed with aging (131), which results in a high total T but lower BioT. Conversely, a low SHBG level and thus total T in overweight elderly men may be associated with normal FT levels. It is of considerable importance that a lack of association between baseline T and IGF-I levels was encountered during screening healthy elderly men by Giannoulis et al. (48), in contrast to the direct relationship described in young men (564). This is in accordance with population-based studies that have included older men and reported even an inverse correlation between IGF-I and T levels in older men (453, 478). In this respect, there was also an inverse correlation between SHBG and IGF-I and IGFBP-3 levels in the study of Giannoulis et al. (48), both potentially useful predictors of GH secretion. This is significant because it underlies the fact that older subjects with apparently normal T levels due to their increased SHBG levels may still be GHD.
Finally, because the metabolic clearance rate of T declines in older people (565), total T is only a crude index of daily T production. It cannot always reflect the active role of the hormone at the cellular level because in contrast to T, SHBG levels increase with age and result in lower BioT levels despite total T values in the normal range. In a similar fashion, baseline IGF-I levels do not directly reflect GH action in peripheral tissues as suggested by the revised somatomedin hypothesis (56). In this regard, IGF-I levels were reported to be an important predictor of 24-h integrated GH secretion in healthy young adults, but not in older men (566).
All the confounders discussed above not only explain the lack of association between baseline T or IGF-I levels and symptoms ascribed to hypogonadism or GHD in older people but also underline the limitation of selecting subjects who could benefit from HRT from a blood test alone. Two important questions were put forward by Snyder (530): Is the decrease in T and GH secretion seen with aging physiological, perhaps conveying benefit or pathological causing harm? Will HRT with GH and T exacerbate the adverse effects to which older people are prone (such as prostate cancer and other malignancy)? These questions can only be answered through long-term (years, not months) clinical trials or possibly (and more realistically) postmarketing surveillance.
B. Clinical trials of testosterone and/or GH administration in older men
1. Introduction
There are numerous reviews and commentaries that present the effects of androgen (192, 567–570) and GH treatment (571–574) in older men. Tables 1–3 summarize the findings of randomized controlled trials (RCT) that have assessed the effects of T, GH, or GH and T combined in older men.
Table 1.
Studies of testosterone therapy (duration 6 months or less) in healthy older men
| First author, year (Ref.) | Study protocol, active treatment, duration, no. of subjects | Main subject characteristics | Main findings and remarks |
|---|---|---|---|
| Ferrando, 2002 (443) | Parallel groups | Healthy older men | High supraphysiological doses of T |
| T enanthate im, weekly in the first month, then every 2 wk | Age, >60 yr | Increase in total and appendicular LBM, decrease in BF, increase in muscle strength | |
| Variable doses (50–400 mg) to maintain nadir T >490 ng/dl | T, <480 ng/dl (17 nmol/liter) | Improvement in skeletal muscle protein turnover | |
| Duration, 6 months | Baseline T, 279–458 ng/dl | No changes in PSA levels | |
| Act, n = 7; PL, n = 5 | |||
| Münzer, 2001 (576); Blackman, 2002 (49); Christmas, 2002 (583); Huang, 2005 (598); Münzer, 2009 (645) | Parallel groups | Community-dwelling healthy older men | Subcutaneous fat decreased; no changes recorded in any of the outcome measurements reported |
| T enanthate im, 100 mg/2 wk | Age, 65–88 yr | ||
| Duration, 6 months | T, <470 ng/dl | ||
| Act, n = 15–21; PL, n = 17 | Baseline T, 440 ± 23 ng/dl | ||
| Giannoulis, 2006 (48, 646); and 2008 (597) | Parallel groups | Community-dwelling healthy older men | No changes in body composition, muscle function, lipid profile, or WBPK |
| T patch, 5 mg/d fixed dose | Age, 65–85 yr | ||
| Duration, 6 months | |||
| Act, n = 23; PL, n = 20 (dropouts, Act, n = 2; PL, n = 4) | Baseline T, 496 ± 63 ng/dl | ||
| Katznelson, 2006 (606) | Parallel groups | Community-dwelling healthy older men | Improvements in QoL after combined Ex and T |
| T patch (5 mg/d) ± Ex | Age, 65–85 yr | ||
| Duration, 12 wk | FT, ≤14.5 pg/ml | ||
| Act T, n = 17; Act T+Ex, n = 17; PL+Ex, n = 19 (dropouts, Act, n = 3; PL, n = 4) | Baseline T, 391 ng/dl (25–512 ng/dl) | ||
| Tenover, 1992 (586) | Crossover | Community-dwelling, healthy older men | |
| T enanthate, 100 mg/wk im | Age, 57–76 yr | Increase in LBM and decrease in markers of bone resorption | |
| Duration, 3 months | T, <400 ng/dl | Increase in PSA and hematocrit levels | |
| n = 13 | Baseline T, 334 ± 14 ng/dl | ||
| Emmelot-Vonk, 2008 (584) | Parallel groups | Community-dwelling healthy older men | Improvements in body composition, but no changes in muscle strength, physical function, BMD, QoL |
| T undecanoate, 80 mg twice daily orally | Age, 60–80 yr | Adverse effect on the lipid profile | |
| Duration, 6 months | T, ≤395 ng/dl (13.7 nmol/liter) | No changes in PSA | |
| Act, n = 120; PL, n = 117 (dropouts, Act, n = 16, PL, n = 14) | Baseline T, 317 ng/dl | ||
| Clague, 1999 (637) | Parallel groups | Community-dwelling healthy older men | Total body mass, hemoglobin, and packed cell volume increased; no effects on strength |
| T enanthate, 200 mg/2 wk im | Age, >60 yr | ||
| Duration, 12 wk | T, <403 ng/dl | ||
| Act, n = 70; PL, n = 7 | Baseline T, 325 ± 49 ng/dl |
All studies are randomized, placebo-controlled, and double blind. Seven trials were identified where T was administered in accepted forms for replacement treatment in healthy subjects and not in older men (aged >60 yr) who were frail or had other associated comorbidities that have reported on outcome measurements related to physical function. Of the 444 subjects included, 219 received T. Act, Active treatment; PL, placebo; Ex, exercise.
Table 2.
Studies of testosterone therapy (duration greater than 12 months) in healthy older men
| First author, year (Ref.) | Study protocol, active treatment, duration, no. of subjects | Main subject characteristics | Main findings and remarks |
|---|---|---|---|
| Sih, 1997 (588) | Parallel groups | Community-dwelling healthy older men | Handgrip strength increased; increased incidence of adverse events |
| T cypionate | Age, 68 ± 6 yr | ||
| Duration, 1 yr | BioT, ≤60 ng/dl | ||
| Act, n = 17; PL, n = 15 (dropouts, Act, n = 7; PL, n = 3) | Baseline T, 294 ± 26 ng/dl | ||
| Snyder, 1999 (21, 585); 2001 (647) | Parallel groups | Community-dwelling healthy older men | No changes in BMD or markers of bone turnover |
| T scrotal patch (6 mg/d fixed dose) | Age, ≥65 yr | Increase in LBM mainly in the trunk, decrease in BF mainly in arms and legs | |
| Duration, 3 yr | T, <475 ng/dl (16.5 nmol/liter) | No changes in muscle strength, physical function, and lipid profile | |
| Act, n = 54; PL, n = 54 (dropouts, Act, n = 4; PL, n = 8) | Baseline T, 367 ± 79 ng/dl | Increase in PSA and hematocrit levels | |
| Nair, 2006 (532); Basu, 2007 (648); Srinivasan, 2010 (649) | Parallel groups | Community-dwelling healthy older men | Marginal increase in total LBM and on BMD at femoral neck only |
| T patch, 5 mg/d fixed dose | Age, ≥60 (mean, 67) yr | No changes in muscle function, BF, VO2max, lipid profile, QoL | |
| Duration, 2 yr | BioT, <103 ng/dl (3.6 nmol/liter) | No increase in PSA or hematocrit levels | |
| Act, n = 27; PL, n = 31 (dropouts, Act, n = 3; PL, n = 1) | Baseline T, 357 (281–464) ng/dl | No improvement in carbohydrate tolerance or postprandial glucose metabolism | |
| Kenny, 2001 (581); 2002 (592) | Parallel groups | Community-dwelling healthy older men | Increased LBM and decreased fat mass with marginal improvements in BMD but no changes in muscle function |
| T patch, 5 mg/d | Age, 65–87 yr | HDL-C levels decreased with no changes in vascular reactivity | |
| Duration, 1 yr | BioT, <128 ng/dl | ||
| Act, n = 34; PL, n = 33 (dropouts, Act, n = 10; PL, n = 13) | Baseline T, 378 ± 173 ng/dl | ||
| Wittert, 2003 (591) | Parallel groups | Community-dwelling healthy older men | Improvements in body composition; no changes in muscle strength |
| T undecanoate, 80 mg twice daily orally | Age, 60–86 yr | Adversely affected the lipid profile, whereas hematocrit increased; no changes in PSA | |
| Duration, 1 yr | FT index (T/SHBG), ≥0.3 to ≤0.5 | ||
| Act, n = 39; PL, n = 37 (dropouts, Act, n = 4; PL, n = 5) | ≥2 symptoms on ADAM questionnaire | ||
| Baseline T, 490 + 130 ng/dl | |||
| Amory, 2004 (582); Page, 2005 (531) | Parallel groups | Age, 65–83 (mean 71) yr | Lumbar spine and hip BMD increased |
| T enanthate im, 200 mg/2wk + FIN orally | T, <350 ng/dl (12.1 nmol/liter) | Total LBM increased and fat mass decreased in trunk, arms and legs, whereas WHR increased; handgrip strength and physical function performance improved, but not ankle or knee strength | |
| Duration, 3 yr | Baseline T, 283 ± 49 ng/dl | PSA and hematocrit increased; lipid profile was not adversely affected | |
| Act T, n = 24; Act T+FIN, n = 22; PL, n = 24 (dropouts, Act T, n = 7; Act T+FIN, n = 7; PL, n = 6) |
All studies are randomized, placebo-controlled, and double blind. Six trials were identified where T was administered in accepted forms for replacement treatment in healthy subjects and not in older men (aged >60 yr) who were frail or had other associated comorbidities that have been reported on outcome measurements related to physical or metabolic function. Of the 413 subjects included, 217 received T. Act, Active treatment; PL, placebo; FIN, finasteride; WHR, waist-hip ratio.
Table 3.
Studies of GH therapy in healthy older men
| First author, year (Ref.) | Study protocol, active treatment, duration, no. of subjects | Main subject characteristics | Main findings and remarks |
|---|---|---|---|
| Papadakis, 1996 (94) | Parallel groups | Healthy older men | LBM increased and BF decreased; no changes in muscle function, VO2max |
| GH, 30 μg/kg 3 times/wk; doses adjusted according to IGF-I levels | Age, 70–85 yr | ||
| Duration, 6 months | IGF-I, <161 ng/ml | ||
| Act, n = 26; PL, n = 29 (dropouts, Act, n = 2; PL, n = 2) | Baseline IGF-I, 75.2 ± 4.5 ng/ml | ||
| Munzer, 2001 (576); Blackman, 2002 (49); Christmas, 2002 (583); Huang, 2005 (598); Münzer, 2009 (645) | Parallel groups | Community-dwelling healthy older men | Increased LBM and decreased total and sc fat; no changes in BMD, VO2max, protein kinetics, or muscle function |
| GH starting dose, 30 reduced to 20 μg/kg, 3 times/wk | Age, 65–88 yr | ||
| Duration, 6 months | IGF-I, <230 ng/ml | ||
| Act, n = 17 to 21; PL, n = 17 (dropouts, Act, n = 1) | Baseline IGF-I, 146 ± 10 ng/ml | ||
| Giannoulis, 2006 (48, 646); 2008 (597) | Parallel groups | Community-dwelling healthy older men | LBM and whole body protein turnover increased |
| GH starting dose, 0.1 mg/d; increased gradually to a mean of 0.54 mg/d | Age, 65–85 yr | No changes in BF, muscle function, VO2max, lipid profile, VLDL metabolism | |
| Target IGF-I, 250 ng/ml | IGF-I, <145 ng/ml | No changes in insulin levels; no glucose intolerance, diabetes, or other adverse events | |
| Duration, 6 months | Baseline IGF-I, 102 ± 5.3 ng/dl | ||
| Act, n = 18; PL, n = 20 (dropouts, Act, n = 2; PL, n = 4) | |||
| Lange, 2001 (595) | Parallel groups | Healthy older men | LBM increased, BF decreased |
| GH increased gradually over 3 wk to 12 μg/kg · d | Age, 74 ± 1 yr | ||
| Duration, 12 wk | Baseline IGF-I, 162 ± 22 ng/ml | ||
| Act, n = 8; PL, n = 8 (dropouts, Act, n = 2) | |||
| Rudman, 1990 (20) | No placebo control study | Healthy older men | Increased LBM, decreased BF, marginal improvement in BMD |
| GH, 30 μg/kg 3 times/wk | Age, 61–81 yr | ||
| Duration, 6 months | IGF-I, <189 ng/ml (350 U/liter) | ||
| Act, n = 12; controls, n = 9 (no treatment was given) | Baseline IGF-I, 162 ± 11.9 ng/ml | ||
| Cohn, 1993 (593) | No placebo control study | Community-dwelling healthy older men | High incidence of adverse events observed when IGF-I levels were above the 75%ile for the young age-specific normal range |
| GH, 30 μg/kg 3 times/wk | Age, >60 yr | ||
| Duration, 6 months | IGF-I, <189 ng/ml | ||
| Act, n = 50; controls, n = 18 (no treatment was given) (dropouts, Act, n = 27; PL, n = 2) | Baseline IGF-I, 165 ± 12.6 ng/dl | ||
| Lange, 2002 (594) | Parallel groups | Community-dwelling healthy older men | Changes in body composition, but muscle strength, power, muscle CSA, fiber size did not change |
| GH ± Ex | Age, 70–82 yr | No additional improvement in muscle strength was observed when GH was co-prescribed with Ex | |
| GH increased gradually over 3wk to 12 μg/kg · d | Baseline IGF-I, 145 ± 14 ng/dl | ||
| Duration, 12 wk | |||
| Act GH, n = 8; Act GH+Ex, n = 8; Ex, n = 8; PL, n = 7 | |||
| Taaffe, 1996 (461); 1994 (607) | Parallel groups | Healthy older men | GH failed to further improve the muscle function, muscle CSA, and fiber size observed after Ex alone |
| 14-wk Ex program followed by 10-wk treatment period | Age, 65–82 yr | ||
| GH, 20 μg/kg · d | Baseline IGF-I, 113 ± 10 ng/ml | ||
| Duration, 10 wk | |||
| Act GH+Ex, n = 10; Ex+PL, n = 8 (dropouts, Act, n = 2) | |||
| Yarasheski, 1995 (512); 1997 (589) | GH ± resistance Ex | Healthy older men | High doses of GH were used, hampered by high incidence of adverse events |
| GH, 12.5–24 μg/kg · d | Age, 67 ± 1 yr | Short-term GH administration in conjunction with Ex program did not confer any additional benefits on muscle function outcomes | |
| Duration, 16 wk | Baseline IGF-I, 106 ± 13 ng/ml | ||
| Act GH+Ex, n = 12–8; PL+Ex, n = 15 to 11 (dropouts, Act GH+Ex, n = 5) |
All studies included are randomized, placebo-controlled and double blind unless otherwise stated. Nine trials were identified where GH was administered in healthy subjects and not in older men (aged >60 yr) who were frail or had other associated comorbidities that were reported on outcome measurements related to physical function. From the 309 subjects included, 169 received GH. Act, Active treatment; PL, placebo; Ex, exercise.
Because the main purpose of this review is to assess the issue of HRT in older men and subsequently its clinical applicability, we will only review RCT where outcome measures (largely surrogate endpoints) of clinical significance relevant to this review were reported. We will also restrict this review to studies where GH or T was administered in well-accepted forms of replacement treatment to older men (aged 60 yr or more) with no major comorbidities. Studies with a high clinical significance but different designs that we believe are of help in the critical evaluation of the literature will also be included. There are several reasons behind this approach.
First, the effects of GH or T administration may be different in middle-aged men when compared with older men. In the former, neither their physiological functions nor their anabolic hormone profile could be assumed to be sufficiently compromised. Thus, the effects of T in middle-aged men occur against a background of almost normal GH secretion and vice versa. Consequently, identifying differences and similarities in the effects of GH or T as single therapeutic agents may provide us with a better understating of their therapeutic potentials and limitation. For example, T or GH administration in middle-aged obese or nonobese men has consistently been shown to reduce intraabdominal VF (93, 562, 575), but not in older men in the few studies so far reported (48, 532, 576). The fact that there is a paucity of studies looking at the use of HRT in older men who are less healthy and those with established frailty is one of the reasons we wrote this review, and we hope to stimulate more interest, debate, and research.
Second, we are assessing effectiveness and potential clinical usefulness, and we have excluded studies of nonaromatized androgens and other anabolic steroids because there are concerns regarding their safety profile (577, 578). We have also excluded studies that have administered dihydrotestosterone, a nonaromatized androgen (579, 580). Finally, we will not include studies where T or GH has been administered to older men with specific diseases. For a comprehensive review on the effects of androgens under these circumstances see Ref. 133.
2. Effects on BMD and bone turnover markers
a. Testosterone.
Testosterone administration in older men has produced marginal improvements in BMD in some studies (532, 581, 582), but not in other studies (21, 583, 584), with both duration and dose of T treatment appearing to be important determinants. This is in contrast to the clear improvements in BMD seen with T replacement in young hypogonadal men described in Section VI.A of this review. Kenny et al. (581) have reported that using the T patch (5 mg/d) for 1 yr in healthy older men resulted in 1.9% increment of BMD between the T (n = 24) and placebo (n = 20) treatment groups. Nair et al. (532), using a similar T regimen for 2 yr, found a marginal but significant improvement in BMD only at the femoral neck in T-treated older men (n = 27) when compared with placebo (n = 31).
On the other hand, Snyder et al. (585) could not detect any difference in BMD between the T-treated (n = 54) and placebo-treated (n = 54) old men in a 3-yr study where T was administered by scrotal patch. Interestingly, however, they reported an inverse association between baseline T levels and the T treatment effect on BMD (in keeping with our earlier discussion in Section IV.A). Statistically significant improvements in BMD were noticed only when men with baseline T levels lower than 300 ng/dl were included in the analysis. Large population studies reported in Section IV.A failed to demonstrate a clear association between T levels and BMD. Amory et al. (582) reported that T esters administered in a somewhat supraphysiological dose (200 mg every 2 wk with or without finasteride for 36 months), increased lumbar spine BMD by 10% and hip BMD by 2.7% in the treated group (n = 24) compared with placebo (n = 24). These increments were positively correlated with the magnitude of the increase in both total T and BioT and were unrelated to pretreatment T levels. Christmas et al. (583) could not detect any changes in BMD in healthy older men when T esters were administered in a lower dose (100 mg every 2 wk) in a study of 6-month duration. Emmelot-Vonk et al. (584) could not detect any significant changes in BMD in a study of 6-month duration where healthy older men with low T levels were randomized to receive 80 mg twice daily of oral T undecanoate (n = 120) or placebo (n = 117).
Concerning markers of bone turnover, studies have produced consistent findings because short-term T administration in older men appears to suppress markers of bone resorption and to increase markers of bone formation, whereas long-term T treatment did not result in significant changes compared with placebo. Tenover (586), in a crossover study of 13 older men with low T levels, recorded a decrease in urinary hydroxyproline excretion after 3 months of T esters (100 mg/wk) but no changes in bone formation markers. In another study, T esters (200 mg every 2 wk) for 3 months in healthy older men increased osteocalcin concentrations in the active treatment group (n = 8) compared with placebo (587). Similarly, Amory et al. (582), in the study described in the previous paragraph using im T (200 mg every 2 wk for 3 yr), reported an increase in bone-specific alkaline phosphate and a decrease of urinary deoxypyridinoline (a marker of bone resorption). On the other hand, Christmas et al. (583), using T doses of 100 mg every 2 wk, did not find any changes in bone turnover markers; neither did others using 1 yr of T transdermal patch or 3 yr of scrotal patch (21, 581). Likewise, Sih et al. (588) could not detect any changes in bone markers between the older men who received T (n = 17) or placebo (n = 15) after im T administration of 200 mg every 2 wk for 1 yr.
In conclusion, T administration in healthy older men for at least 1 yr results in a small but significant improvement in BMD, and this effect is dependent on pretreatment T concentration and T dose.
b. GH and coadministration of GH and testosterone.
There are no published studies that have administered GH to healthy older men for longer than 6 months. There are only four studies that have assessed the effect of GH treatment on bone in older men. In the earliest study, Rudman et al. (20) demonstrated that a high dose of GH (30 μg/kg) in 12 healthy older men for 6 months produced a significant increase of BMD at the lumbar spine (1.6%); however, there was no placebo-treated control group in the study. A crossover study in 10 older men evaluated the effects of GH, T, and GH+T combined on bone turnover markers with a 1-month intervention period followed by a 3-month washout period and reported a significant effect on osteocalcin in the GH and GH+T treatment groups (429). In another study of 16 wk, Yarasheski et al. (589) reported that although markers of bone turnover increased with GH plus resistance exercise training, improvements in BMD were no greater in the GH group than with the placebo. This study demonstrated that bone turnover is stimulated quickly with GH, but the 16-wk treatment period was too short to see anything other than a fall in BMD after GH because we now know that mobilization of bone is stimulated before synthesis (590). The fact that the BMD was not lower in the GH group may well have indicated a hidden positive effect on BMD where the effect of the combined treatments did not result in a fall in BMD.
In the only randomized controlled study, Christmas et al. (583) investigated the combined and separate effects of GH (30 μg/kg three times per week) and im T ester (100 mg every 2 wk) for 6 months in 72 healthy older men. They could not detect any significant changes of BMD in any treatment group, apart from a small decline at the proximal radius after GH+T treatment. This may be explained as the biphasic effect of GH on bone referred to above (590), because markers of bone resorption and formation have been shown to be increased after both GH+T and GH administration.
3. Effects on body composition
a. Testosterone.
Several placebo-controlled studies of 3-month to 3-yr duration have demonstrated that T administration to healthy older men tended to increase LBM; the effect on fat body mass (FBM) is less consistent (48, 49, 532, 586, 588). Differences in pretreatment T levels, study duration, mode and dose of T used, and subsequent posttreatment T concentrations may well explain the discrepant findings.
Administration of im T ester (100 mg/wk) in 13 healthy older men for 3 months significantly increased LBM by 1.8 kg (as measured by the hydrostatic weighting technique) with no changes in FBM (586). In another study, Ferrando et al. (443), using high supraphysiological T doses in a trial lasting 6 months, reported that total and appendicular LBM (measured by DEXA scan) increased significantly by 4.2 and 3 kg, respectively, in the T group (n = 7) when compared with placebo (n = 5). Percentage of FBM decreased significantly by 3.6%, whereas leg muscle volume assessed by magnetic resonance imaging increased. Of note, this is the only placebo-controlled study to show an increase in appendicular LBM in healthy older men, although it was achieved with supraphysiological doses of T. In a third study, administration of T in the form of scrotal patch for a 3-yr period in healthy older men was shown to significantly increase LBM by 1.9 kg and decrease FBM by 3 kg (21) (Fig. 4). Of significance, the increase in LBM occurred as early as 6 months into the study and occurred in the trunk, whereas changes of FBM became evident at 12 months and occurred mainly in the legs and arms (as assessed by DEXA).
Figure 4.
Mean (±se) change from baseline in fat and lean mass of the arms, legs, and trunk, as determined by DEXA, of 108 men over 65 yr of age who were treated with either T or placebo (54 men each). The decrease in fat mass in the arms (P < 0.02) and legs (P < 0.001) and the increase in lean mass of the trunk (P < 0.001) in the T-treated subjects were significantly different from those in the placebo-treated subjects at 36 months. Other changes were not significantly different between the two groups. [Reproduced from P. J. Snyder et al.: Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653, 1999 (21). © The Endocrine Society.]
Page et al. (531) reported that im T ester (200 mg every 2 wk) for 36 months in healthy older men who were randomly assigned to receive T or T plus finasteride increased LBM significantly by 3.7 kg and decreased FBM by 6%. Although T appeared to have equally reduced trunk and leg fat assessed by DEXA scan, it was found at the end of the study that the men treated with T had an increase in their waist-hip ratio when compared with placebo. This suggests that T exerts its lipolytic action mainly on sc and im fat, and not in intraabdominal fat depot. Another study where im injections of T esters were administered to healthy older men for 1 yr in the same manner as above (200 mg every 2 wk) failed to record significant changes of BF when assessed by bioelectrical impedance (588). In a further study in healthy older men, oral T undecanoate 80 mg twice daily for 6 months resulted in significant mean differences for LBM of 1.2 kg and FBM of 1.3 kg (assessed by DEXA scan) between the T-treated group (n = 113) and the placebo group (n = 110), but not of intraabdominal VF measured by ultrasound (586). A similar magnitude of change in LBM and FBM was also observed by Wittert et al. (591), who gave oral T for 12 months to 76 healthy older men with low T levels. Nair et al. (532) observed that T patch therapy in healthy older men with low T levels for a 2-yr period failed to decrease the percentage of FBM in the T-treated group (n = 27) measured by DEXA scan or VF measured by CT scan, when compared with placebo (n = 31); LBM increased significantly, with the mean difference between the two treatment groups being 1.4 kg. The increment in LBM observed could not, however, be attributed to an increase in leg LBM because thigh muscle CSA did not change significantly when compared with placebo, in accordance with the findings reported by Snyder et al. (21). Kenny et al. (592) on the other hand found that T patch administration for 1 yr in healthy older men decreased significantly the percentage of FBM, but changes in LBM assessed by DEXA scan failed (marginally) to reach significance in T-treated men (n = 24) when compared with the placebo group (n = 20).
Giannoulis et al. (48), assessing the effects of GH with and without T on body composition and other functional correlates, randomly assigned 80 healthy older men with lower IGF-I and T levels to receive GH (n = 16) (starting dose, 0.1 mg/d; titrated over 8 wk according to IGF-I levels), T transdermal patch (n = 21), GH+T (n = 16), and placebo (n = 16) for a period of 6 months. They did not detect any significant changes in LBM or FBM (assessed by DEXA scan) in the T-treated group when compared with placebo. No changes were observed in sc fat or VF and sc middle thigh fat or in thigh muscle CSA measured by a CT scan (48). The relatively high baseline T levels and the physiological T administration paradigm by transdermal patch may explain their negative findings. Their results compare well, however, with the findings reported by Blackman et al. (49), the only other placebo-controlled study that reported the effects of GH with or without T in healthy older men. In the Blackman et al. (49) study, the GH starting dose was 30 μg/kg three times per week, whereas T was given im in a dose of 100 mg every 2 wk. No changes were observed in FBM, whereas increases in LBM (measured by DEXA) marginally failed to reach significance after 6 months of T administration to healthy older men (n = 21) when compared with placebo (n = 17). Munzer et al. (576), reporting on the same cohort of patients in another publication, found a significant decline in abdominal sc fat by 7% (but no change in VF) in T-treated men when compared with placebo.
In conclusion, it appears that T administration results in an increase in LBM in healthy older men in a dose-dependent manner. Furthermore, the effects of T on LBM appear principally to occur in the trunk rather than in legs or arms. Conversely, T appears to exert its lipolytic action more on sc appendicular or abdominal fat and not on VF in accordance with what has been shown in young hypogonadal men (presented in Section VI.A) and confirmed by experimental studies (372).
b. GH and coadministration of GH and testosterone.
There are very few placebo-controlled studies investigating the effect of GH on body composition and physical function in healthy older men; consequently, some studies with different experimental designs will be included.
In the first major study of its kind, Rudman et al. (20) showed that administration of GH (30 μg/kg three times per week) to 12 healthy older men for a period of 6 months decreased BF by 14% and increased LBM by 9% as measured by whole body 40K counting. In a further study from the same group involving 62 healthy older men in the GH group and 21 in the placebo group, where GH was given in a similar high dose, they found similar changes in body composition (593).
Papadakis et al. (94) using similar relatively high doses of GH, adjusting the doses according to IGF-I response, observed 12.8 and 4.4% differences in FBM and LBM, respectively, between the GH-treated (n = 26) and placebo (n = 26) groups of healthy older men over 6 months. In a recent study of 12-wk duration, the effects of resistance training and GH were evaluated in 31 healthy older men (594). Measurements were made of body composition, muscle function, muscle thigh CSA, and myosin heavy chain isoforms. LBM increased by 2.4 kg, whereas FBM decreased by 2.2 kg in the GH-treated group, and similar changes were seen in the GH plus exercise group. Muscle CSA, fiber type, and fiber size did not increase after GH, but a substantial increase of myosin heavy chain 2x isoform corresponding to the histochemically determined fiber type IIb was observed. Exercise alone and exercise plus GH increased muscle CSA to the same degree when compared with placebo. Comparable changes in body composition were also observed in another RCT from the same group involving 16 healthy older men who were administered GH in the same doses as before for a 12-wk period and whose body composition was assessed by DEXA scan. LBM increased 3.2 kg, and FBM decreased 3.4 kg, both statistically significant when compared to the placebo group (595).
In a study where 18 healthy older men initially underwent resistance training for 14 wk and were then randomized to GH administration (20 μg/kg · d) or placebo for a further 10-wk intervention, Taaffe et al. (461) did not find an effect of GH on enhancing muscle thigh CSA and muscle fiber size when compared with exercise alone and did not find an increase in im IGF-I mRNA.
Blackman et al. (49), in the study described previously where GH (30 μg/kg three times per wk) and/or T enanthate (100 mg every 2 wk) was administered to 74 healthy older men for 6 months, reported that LBM increased significantly after GH and GH+T by 3.1 and 4.3 kg, respectively; FBM was also reduced significantly in both treatment groups. Munzer et al. (576), in another publication reporting data on these same cohorts, showed that total abdominal fat assessed by magnetic resonance imaging decreased after GH and GH+T but not after T treatment when compared with placebo, whereas sc fat decreased significantly in all treatment groups. VF, however, decreased only after GH and GH+T in a within-group comparison, but not when compared with placebo.
In a similarly designed study performed by Giannoulis et al. (48) where GH (starting dose, 0.1 mg/d, and titrated over 8 wk according to IGF-I levels) and/or T patch (5 mg/d) were administered in a RCT involving 80 healthy older men for 6 months, similar significant changes in body composition were observed after GH or GH+T. Total and appendicular FBM (assessed by DEXA) decreased significantly after GH+T but not after GH alone, whereas small decreases in sc and VF after both GH and GH+T were found, but these changes failed to reach statistical significance. Failure of GH treatment to decrease FBM significantly in this study, in contrast to the studies presented before, could be attributed to the smaller GH dose used because by titrating the GH dose, subjects were exposed to the final GH dose for a shorter period.
LBM increased significantly by 2 kg after GH and by 1.8 kg after GH+T. Interestingly, GH+T but not GH alone significantly increased appendicular LBM by 1.7 kg when compared with placebo, whereas the effects on trunk LBM were similar. Thus, the effect of GH+T on LBM and FBM appears more potent than either GH or T. In accordance with this observation, muscle thigh CSA (as assessed by a CT scan) increased significantly only after combined GH+T treatment (Fig. 5).
Figure 5.
The effects of placebo (P), GH, testosterone (T), and GH plus T (GHT) on appendicular fat mass (top) and appendicular lean mass (bottom). Columns show results at baseline and 6 months. [Reproduced from M. G. Giannoulis et al.: The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab 91:477–484, 2006 (48), with permission. © The Endocrine Society; and http://encore.ulrls.lon.ac.uk/iii/encore/record/C_Rb3127431∼S1?lang=eng.
The finding of a positive interaction between GH and T is of considerable importance because neither GH nor T alone, in physiological doses, was able to increase muscle mass in older men in the few studies reported. Indeed, Lange et al. (594) did not see an increase in muscle CSA in healthy older men after 12-wk GH treatment, whereas Taaffe et al. (461) did not demonstrate an increase in the CSA of muscle fiber of types I and II in exercising men who had been given GH. This is in contrast to the well-described increases in muscle CSA that occur in GHD men after GH treatment. On the other hand, Weissberger et al. (596) have shown an increase in muscle thigh CSA over 14-wk GH treatment preoperatively in older patients who were undergoing elective total hip replacement. This study used a higher dose of GH (0.04 U/kg · d; 13 μg/kg · d) and demonstrates that in a short trial, the effects of GH are dose-dependent.
Similarly, T failed to increase muscle mass in older men when it was administered in what might be judged as physiological doses (21, 48, 532). In a study that reported an increase in muscle volume and leg LBM after 6-month treatment with im T, doses were claimed to be physiological but were rather high for such a claim because trough plasma T levels were maintained “between 17 and 28 nmol/liter” and would be judged too high to be used in long-term replacement therapy (443). In keeping with this interpretation, Sinha-Hikim et al. (38) evaluated the effect of different T doses on muscle fiber types and CSA in healthy older men who were rendered hypogonadal with a long-acting GnRH agonist and observed that only the high supraphysiological doses of 300 and 600 mg/wk im increased type I and II muscle fiber CSA.
In another publication, Giannoulis et al. (597) evaluated the effects of GH and/or T on muscle gene expression and whole body protein kinetics (WBPK) using L-[1-13C]leucine infusion in 24 healthy older men comprising a subgroup of participants of the study described previously (48). In both the GH and GH+T subgroups, whole body protein synthesis (WBPS) and degradation increased, whereas leucine oxidation did not change. In the same study, T alone failed to induce any changes in WBPK. This is in accordance with the study of Huang et al. (598) who also evaluated the effects of GH and/or T on WBPK in 60 healthy older men and showed an increase of WBPS after 6-month treatment with GH+T, whereas the increase observed after GH alone failed to reach significance.
Taking into account that both GH and GH+T have been shown to increase WBPS but that it was only after GH+T that a significant increase in appendicular muscle mass and muscle thigh CSA were found, it might be reasoned that these increments really do reflect skeletal muscle hypertrophy and are of important clinical significance. Thus, the coadministration of GH and T indeed promotes skeletal muscle anabolism, whereas GH alone has a lesser effect and may indeed only affect whole LBM rather than the more important skeletal muscle mass.
GH has been shown unequivocally to increase WBPS in both healthy (76) and GHD adults (73, 599). The effect of GH on skeletal muscle and contractile protein synthesis are less pronounced (600). Welle et al. (601) in a small study could not detect any effect of GH administration for 3 months on myofibrillar protein synthesis in healthy older men (but were also unable to detect an effect on WBPS), whereas Yarasheski et al. (512) reported no changes in muscle protein synthesis in older men when GH was administered in conjunction with exercise. Only Butterfield et al. (513) reported an increase in skeletal muscle protein synthesis after 1 month of GH treatment in older women. Testosterone on the other hand has been shown to increase skeletal muscle protein synthesis in both healthy older men (436, 443) and hypogonadal men (387); the latter, however, occurred with high supraphysiological doses.
These findings further support the initial reasoning for a potential additive effect of GH and T that was presented in Section VII of this review.
In this regard, Sattler et al. (602) have evaluated the effect of T gel in two dosage regimes (5 and 10 mg/d) with or without recombinant human GH in two different doses (3 and 5 μg/kg · d) for 16 wk in 129 healthy older men with artificially induced hypogonadism (Leydig cell clamp). They reported dose-dependent changes in body composition (and strength and aerobic endurance) compared with baseline in all T-treated groups and an additive effect of GH (Fig. 6).
Figure 6.
DEXA-derived changes (mean ± se) in LBM and fat mass for each treatment group (T transdermal gel 5 g, groups A–C; 10 g, groups D–F; rhGH 0 μg/kg · d, groups A and D; 3 μg/kg · d, groups B and E; and 5 μg/kg · d, groups C and F) from baseline to wk 17. A, Increases in total LBM (solid bars) and appendicular lean mass (hatched bars). Changes across groups are significant for linear trend for total lean mass (P = 0.0002) and appendicular lean mass (P = 0.0002). B, Decreases in total BF mass (solid bars) and trunk fat (hatched bars). Changes across groups are significant for linear trend for total fat mass (P = 0.0004) and trunk fat (P = 0.0003). *, Bonferonni adjusted within group changes (P < 0.008). Pairs of treatment groups with different letters (e.g., a vs. b) are significantly different by one-way analysis of covariance with pairwise comparison (Tukey adjusted; P < 0.05). [Reproduced from F. R. Sattler et al.: Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 94:1991–2001, 2009 (602), with permission. © The Endocrine Society.]
In conclusion, GH alone has been shown consistently to increase LBM and to decrease FBM in healthy older men in all of the few studies published. Furthermore, it appears that combined GH+T treatment may be more effective and preferentially affect skeletal muscle mass.
4. Effects on muscle function and physical functional correlates
a. Testosterone.
The current evidence from the literature does not clearly support the argument that T treatment in healthy older men improves muscle and physical function. Placebo-controlled studies have produced inconclusive results. In one study, scrotal patch T administered for 36 months did not increase either knee extension or flexion strength when measured by a dynamometer or hand grip strength or physical function measured by walking and stair climbing (21). In a recently reported study, 2 yr of transdermal T by patch failed to improve muscle strength, VO2max, and QoL in healthy older men (532). In another study, transdermal T patch for 1 yr in healthy older men did not improve leg extension strength measured by 1RM exercise or increase habitual physical activity, as assessed by the Physical Activity Scale for the Elderly (581). A third study involving 6 months of transdermal T patch did not produce any changes in knee extension and flexion at three different angular velocities or on handgrip strength or VO2max (48). Furthermore, im T enanthate (100 mg every 2 wk) given for 6 months did not increase muscle strength in a group of upper and lower body muscles (assessed by 1RM) or VO2max (49). Oral T undecanoate 80 mg twice daily for 6- and 12-month duration had no effect on muscle function (584, 591), whereas functional mobility assessed by the time required to perform different physical tasks also did not change. Finally, 200 mg of im T enanthate every 2 wk for 3 yr increased right but not left hand grip strength and physical function assessed by time modified physical performance test by 4%, while having no effect on isokinetic lower extremity strength measured at both ankles and knees (531). The improvement in physical function contrasts with the studies presented before that failed to demonstrate similar positive findings in healthy elderly men (21, 532).
Higher T increments achieved and possibly the greater length of treatment may well explain these differences. Page et al. (531), attributed the small magnitude of physical function improvement to the “ceiling effect” of the tests used to assess these changes. In this regard, it appears that although a linear relationship exists between muscle strength and time required to perform different tasks as assessed by the physical performance test, there is a threshold above which improvements in strength could not result in improvement in times recorded, which are already near maximally shortened and may well explain the inherent inability of these tests to detect changes in physical function in well-conditioned older men (603, 604).
On a more positive note, 200 mg T cypionate im every 2 wk for 12 months in 17 hypogonadal older men (mean age, 68 yr) increased bilateral grip strength compared with placebo (588). In another study, T enanthate im for 6 months in supraphysiological doses to older men increased 1RM muscle scores but failed to increase VO2max (443). In a recent dose-ranging study, im T enanthate in doses ranging from 25–300 mg/wk for 20 wk resulted in a dose-dependent increase in muscle strength and power but not an improvement in physical function assessed by stair climbing and walking tests (605). Finally, Katznelson et al. (606), in a study where T (5 mg/d) was administered by patch with or without a domestic exercise program for 12 wk, reported an improvement in QoL in the domains of physical functioning, role physical, general health, and social functioning as assessed by the SF-36 questionnaire, when T was combined with exercise but not after T or exercise alone.
In conclusion, it is clear that T treatment alone improves muscle strength but not physical function in a dose-dependent manner in healthy older men. It remains unclear, however, whether clinically meaningful outcomes could be achieved with T doses that could be administered safely.
b. GH and coadministration of GH and testosterone.
On the one hand, studies where GH was given to healthy older adults resulted in consistent findings revealing the inability of GH to improve muscle performance. This was when GH was administered either alone or in conjunction with an exercise program (20, 94, 512, 594, 607). In two studies of 6-month duration, GH failed to increase muscle strength compared with placebo (20, 94). In a series of studies performed in healthy older people (512, 607), administration of GH after a period of intense weight training failed to further improve the strength gains observed after exercise alone, although muscle mass was reported to increase more after GH. Of likely importance, these interventional studies did not exceed 3 months in duration. There is only one study to date that has reported increased muscle bulk and strength after GH in older men (601).
On the other hand, studies that have evaluated the combined effect of GH+T have produced more encouraging results. Thus, administration of GH (starting dose, 30 μg/kg · d, later reduced to 20 μg/kg · d) and im T enanthate 100 mg every 2 wk for 6 months in healthy older men marginally increased 1RM muscle strength (P = 0.05) when compared with placebo. In addition, an improvement in VO2max was recorded, and the changes in both muscle strength and VO2max were directly related to changes of LBM (49). These findings have been confirmed by Giannoulis et al. (48), where GH administration in doses titrated according to IGF-I response combined with the T patch (5 mg/d) for 6 months in healthy older men increased VO2max when compared with placebo, and the increases recorded in LBM and appendicular LBM were also significant when compared with placebo (Fig. 7). In this study, there was evidence of a positive effect on muscle strength where one of the six measurements performed (knee flexion at 120°/sec) significantly improved when compared with placebo. Differences and difficulties in the methods employed to measure isokinetic muscle strength, together with differences in GH dosage between studies, may well explain any discrepancies.
Figure 7.
The effects of placebo (Pl), GH, testosterone (Te), and combined GH and testosterone (GHTe) on LBM (A), fat mass (B), percentage change from 0 to 6 months in the midthigh CSA (C), and VO2max (D). In A, B, and D, solid shading is baseline, and gray columns represent 6-month values. *, P < 0.02; **, P < 0.01; ***, P < 0.001. [Reproduced from M. G. Giannoulis et al.: The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab 91:477–484, 2006 (48), with permission. © The Endocrine Society.]
In conclusion, GH+T, but not GH alone or T alone, appears to improve maximal aerobic capacity and to marginally improve muscle strength, and this can be achieved with near-physiological doses of hormone treatment over a treatment period as short as 6 months. Because VO2max and muscle strength are the strongest determinants of functional performance and independent living in older men, these findings are of potential importance.
5. Effects of testosterone in frail older men
So far there are no published studies on the effects of GH or GH+T in frail older men. In a recent study involving 131 frail elderly men (mean age, 77 yr) who also had a history of bone fracture or osteoporosis and low T levels, Kenny et al. (608) reported that 50 mg/d of T gel treatment for 1 yr increased BMD by 1.4 and 3.2% at the femoral neck and lumbar spine, respectively. At the same time, BMD decreased by 1.3% at mid-radius, and there was a fall in both bone resorption markers (by 6%) and formation markers (by 11%). In addition, total and appendicular LBM increased and BF decreased, but muscle strength and physical function did not change when compared with placebo. The study that was initially designed as a 2-yr intervention was associated with poor treatment adherence and high dropout rate of 53%, however, mainly because of cardiac and prostate events in both treatment groups, and subsequently the analysis of 1-yr data was presented. Srinivas-Shankar et al. (609) in another placebo-controlled study involving 274 frail old men (mean age, 74 yr) with mobility limitations, where T gel was administered (50 mg/d) for 6 months (n = 138), reported an improvement in lower limb muscle strength and QoL when compared with placebo. Physical function has improved only in the subset of subjects who were frailer possibly as a result of the “ceiling effect” of the tests used and described above, whereas the adjustment of T doses to achieve a target range employed may explain the positive finding, contrary to the study reported by Kenny et al. (608). Similar dropout rates were recorded between the treatment and placebo groups, although a slightly higher rate of increased PSA levels and serious adverse events (six vs. three) was found in the active treatment group. In another study where the combined effect of high- or low-intensity exercise with or without im T (100 mg/wk) was evaluated in 71 frail older men over 12 wk, it was reported that there were greater increases in muscle CSA after combined T and exercise treatment, but no differences in muscle function when compared with exercise alone. There were 10 dropouts because of exacerbations of previous heart and pulmonary diseases attributed to exercise (610).
Finally, Basaria et al. (611), in a recent study of 209 frail older men (mean age, 74 yr) with a high prevalence of chronic disease, found that transdermal T gel at a higher dose (100 mg/d) was associated with an increased incidence of serious cardiovascular adverse events in the T group (n = 23) compared with the placebo group (n = 6), which eventually led to the discontinuation of the study. Despite the high prevalence of adverse events, the study did show positive effects of T treatment on strength (leg and chest presses) and in stair climbing.
These consistent findings indicate that frail old men are more susceptible to side effects of T administration and that too high doses of T have been selected for these (and many other) studies. Experience has proved that the older people are very sensitive to hormonal intervention, just as they are to medication—it appears that this is a lesson being learned rather slowly.
Thus, the role of anabolic agents in reversing or halting established frailty of aging, a dynamic catabolic process that involves a decompensated state of muscle adaptive mechanisms, remains questionable. Selecting the optimal dose for a trial is critical. It appears from reviewing the literature that the temptation to use a higher dose to demonstrate effectiveness within the limited time scale of a RCT has too often produced a negative outcome largely attributable to the inappropriate choice of dose. This has been the case for many studies using T or GH in monotherapy. One of the greatest strengths of the study by Giannoulis et al. (48) is that, after reviewing the literature carefully, they selected low and carefully controlled doses of GH and T and adjusted these according to the individual sensitivity of their volunteers. The drop out rate in this trial was about as low as any recorded.
C. Conclusions and thoughts of designing future trials on HRT in older men
Reconsidering the data reviewed and the problems reported when selecting subjects suitable for HRT on the basis of baseline IGF-I and T concentrations, we feel that more attention needs to be placed on using low-dose replacement over a longer period of time rather than to selecting participants on the basis of baseline androgen and GH status. Although we do not know what should be considered physiological replacement, treatment has to be arbitrarily defined from the resulting hormone levels achieved in the blood. A cautious approach that we recommend will be to use doses of GH and/or T that result in average IGF-I and T levels close to or slightly lower than the mean of the young, age-specific, reference range. This in turn is close to the upper limit for the older age-specific reference range, and this should be deemed as a sufficient stimulus for the anabolic effects to be demonstrated. As a matter of fact, exogenous T administration suppresses the endogenous production, and it results in similar increments in plasma T independent of the baseline T levels. In this regard, most but not all of the studies reported to date have used high, rather supraphysiological doses of GH and T replacement.
Longer interventional trials (1 yr and greater) are needed to clarify whether HRT has a place in reversing, preventing, or delaying frailty. The ongoing large-scale interventional trials such as the National Institute of Aging, National Institutes of Health-supported Testosterone Trials (612) may provide some answers.
In conclusion, selecting healthy elderly men with not only low but also low normal baseline levels of T and IGF-I and close monitoring of the HRT to produce individual average hormone concentrations in a predefined target range (into the upper half of the age-specific reference range) appears a well-justified approach.
When planning such HRT, it is important to consider the following points:
-
•.
Intramuscular T esters in doses of 100 mg/wk or 200 mg every 2 wk result in mean serum T concentrations about 50–70% higher than the mean for normal young men (613) but also include peaks and troughs where concentrations of T are way outside the normal reference range. On the other hand, doses of T enanthate of 100 mg every 2 wk or oral T undecanoate 80 mg twice daily may be deemed inadequate.
-
•.
Low doses of T gel preparations (T gel 50) that better mimic the physiological profile should be used rather than high doses (T gel 100) because the latter have been reported to result in T concentrations in the upper physiological range throughout the day (386). The T patch also closely resembles the physiological daily pattern of T, but its use is limited to a degree by the skin irritation caused by the adhesive and the enhancing agent. Nevertheless, irrespective of the T formulation used, T doses should be adjusted to achieve levels as close as possible into the mid-normal range.
-
•.
To date, high and rather supraphysiological doses of GH were used in most of the studies in older people. To minimize possible adverse effects, to increase tolerability, and to assess the effect of GH under truly physiological replacement conditions, it is essential to monitor treatment by measuring IGF-I levels and make appropriate dose adjustments when necessary. One must accept arbitrarily defined “physiological” GH replacement targets, such as providing IGF-I levels close to the upper limit of an age-specific reference range (which will be close to the 50th percentile of IGF-I levels of young men).
D. Safety issues of growth hormone and testosterone replacement treatment in older men
1. GH adverse events
The very long-term effects of GH administration in healthy older men are currently unknown. Some epidemiological studies have shown an association between serum IGF-I level and the occurrence of prostate and breast cancer in the normal population.
On one hand, in a nested case-control study from the Physician's Health Study, a positive association was observed between a single serum IGF-I level and the risk of prostate cancer development after 5 or more years (614). A similar analysis of the Nurses' Health Study showed that IGF-I levels could predict breast cancer in premenopausal but not postmenopausal women (615). Consequent to the coverage of these results in the lay press, there has been concern that GH therapy and its attendant increase in IGF-I could lead to the development of malignancies. This is a statistical association only and as such does not provide any evidence of causality. Because the direct evidence does not indicate GH to be carcinogenic, this statistical link could be what is recognized in the statistical world as a “spurious relationship” or spurious correlation (616).
On the other hand, Colao et al. (330) could not demonstrate any increased risk of prostate malignancy in patients with active or treated acromegaly where GH and IGF-I levels were grossly elevated over many years; in support of this conclusion, it has been long established that acromegalic patients do not die from prostate cancer (617). Also, Olsson et al. (618) recently reported no changes in the progression of pituitary tumors in patients with nonfunctioning pituitary adenomas receiving GH for 10 yr. Furthermore, an increase in the recurrence rates of either intracranial or extracranial tumors was not found in adults with GHD on long-term GH treatment (619). Fradkin et al. (620) did report an increase in leukemia in children treated with GH, but the excess risk could be attributed to the presence of other tumors and/or radiotherapy. There was no increase in leukemia among pediatric patients with idiopathic GHD who received GH (620). In another report (621), mortality from colorectal cancer and Hodgkin's disease was increased in a cohort of 1848 GHD patients who received GH during childhood. The number of cases was small (only two cases of each), however, and treatment regimes differed from modern day dosing regimens. No increased rates of leukemia were reported in this cohort. There are so far no published reports of long-term observational studies in patients with the GHD syndrome treated with GH with respect to the development of malignancies, although all the postmarketing surveillance studies will be watching this carefully, and the fact that no such association has been reported to date weighs heavily in favor of the safety of GH treatment. The Growth Hormone Research Society organized an international consensus workshop in 2001 to discuss the safety of recombinant GH and concluded that there was no good evidence of GH being carcinogenic. They recommend prudence and vigilance with those on long-term treatment because older people may be more susceptible to any putative carcinogen properties (622). There is also the possibility that GH might stimulate growth in an existing but undetected tumor, although there is no evidence to suggest that this has actually occurred.
Older men are more susceptible to GH-related adverse effects, and earlier studies that have administered GH in doses comparable to young GHD adults have reported a high incidence of adverse events (20, 49, 94, 593, 594). Adverse effects appeared to occur early during the study period and were similar to those observed in GHD patients, with fluid retention (varying degrees of pitting leg edema and carpal tunnel syndrome) and arthralgia involving small hand joints being most prevalent. Although most of the symptoms reported were mild and subsided or even disappeared after GH dose reduction, it becomes apparent that the incidence of adverse effects is still somewhat higher than that reported in young GHD patients. The symptoms recorded were largely those predictably attributable to GH action (effects), such as those consequential to sodium retention (ankle edema and carpal tunnel) and arthralgias (growing pains), rather than unexpected side effects. These usually subside spontaneously over 1 or 2 wk or in response to a dose reduction and are in reality indicators of overdosage rather than side effects. In modern regimes of GH treatment developed through experience, the starting dose is always low, and the dose is slowly escalated based on the subject's well-being and the measured IGF-I responses. Individual people differ substantially in their sensitivity to GH (just as they do to insulin), and fixed doses based on body weight (originating from pediatric experience) are now outdated.
With this in mind, Giannoulis et al. (48) in a 6-month study where the individual and combined effects of GH and T were assessed, opted for a generally low dose of HRT and used a GH dose-titrating method to tailor GH dose to the individual's sensitivity. They reported a 41% incidence of “at least one” from a list of possible adverse events in the participants treated with GH (n = 36). The incidence of edema, carpal tunnel syndrome, and arthralgia from that study was 11, 8, and 20%, respectively, whereas no subject developed diabetes. These symptoms were generally minor, transient, or well-tolerated or they subsided in response to a dose reduction, and of greatest significance, they were responsible for no withdrawals from treatment. Many of these symptoms were in fact due to GH therapeutic effects. The safety profile of this study compares favorably not only with studies where GH has been administered in healthy older men but even with studies where GH was administered to young GHD adult men.
Indeed, a recent study of 595 GHD patients comparing the safety and efficacy of two dosage algorithms reported that 44% of patients on low-dose GH (3 μg/kg · d) and 55% on high-dose GH (6 μg/kg · d) reported at least one adverse effect during 6 months of GH treatment (623). A similar trial involving 387 GHD patients and comparing safety and efficacy of an individualized dose-titration regimen with a fixed body weight-based dosing regimen of GH administration reported a 60% frequency of GH-dependent adverse events during 8 months of treatment (624).
GH therapy could be a cause of insulin resistance, as has been shown in studies with GHD adults (419, 420) and has been recorded in older men (49, 593). This is an unwanted and possibly avoidable adverse event (48, 602) because older men are already at higher risk for CVD (625). It seems that insulin sensitivity may improve after 6 to 12 months of GH treatment, and 5 or 7 yr of GH therapy do not adversely affect insulin sensitivity (216, 328, 405, 407, 421). It has been suggested that the initial deterioration in insulin sensitivity is due to increased FFA oxidation because of GH-induced lipolysis, which adversely affects glucose disposal in muscle (626). An inverse relationship between circulating FFA concentrations and insulin sensitivity in GHD adults has been confirmed in several studies using acipimox, a blocker of FFA release (627, 628).
Subsequently, as BF declines, a new steady state is reached, and this may explain the improvement in insulin sensitivity observed after prolong GH administration (628). It appears that by administering low doses of GH, there may still be beneficial effects for insulin sensitivity without going through the first phase of insulin resistance (48, 602). A rare complication reported after GH treatment is gynecomastia or nipple tenderness, mainly in older men, which was reported in some studies (94, 593, 602) but not in others (48, 49).
In conclusion, GH can be administered to healthy older men with a safety profile similar to that seen in GHD men, but so far observations have only been made over a period of 6 months. Dose titration tailors the dose to the sensitivity of the individual and minimizes problems. Many of the earlier recorded adverse events were due to overdosage. As we have stressed, the potential increased susceptibility of older men to any putative carcinogen or mitogenic properties requires vigilance when GH is administered over a longer period, whereas the long-term effects of GH on frail elderly men with associated comorbidities are still unknown.
2. Testosterone adverse events
Several systematic reviews of the literature have presented data regarding the adverse events associated with T administration in men but failed to provide solid evidence regarding the safety of T treatment (388, 629, 630). The short duration and the high heterogeneity of the studies analyzed may explain the inconsistent findings across the studies (526). None of these reviews assessed the adverse affects in older men exclusively. One systematic review recently reported the adverse effects of T treatment in 51 studies where T was administered to men with a wide range of conditions and low or low-normal T levels (388). Testosterone treatment was associated with a significant increase in hemoglobin and hematocrit and a decrease in HDL-C levels, but no treatment effect was reported on PSA levels, prostate cancer, composite prostate outcome, cardiovascular events, or overall mortality. Interestingly, as we have discussed before in this section, T does not appear to affect adversely the lipid profile in healthy older men.
Another meta-analysis of 19 studies of middle-aged and older men reported that the T-treated men were four times more likely to have a hematocrit higher than 50% and a higher combined rate of all prostate events when compared with placebo (odds ratio, 1.79; 95% confidence interval, 1.07–2.95). The individual rates of prostate cancer, increments in PSA levels, and prostate biopsy events did not differ, however, when compared with placebo (629).
A further meta-analysis of 30 studies of both middle-aged and older men with or without associated comorbidities (mainly cardiovascular) failed to show an adverse T effect on blood pressure, glycemia, or lipid profile but reported a trend for increased cardiovascular adverse events (pooled odds ratio, 1.82; 95% confidence interval, 0.78–4.23) (630).
One of the major concerns regarding the administration of T in healthy older men is the development of prostate cancer because case reports have suggested that T-replacement therapy may convert an occult cancer into a clinically apparent lesion (631). Testosterone treatment should not be prescribed to men with clinically evident breast or prostate carcinoma because these tumors are usually androgen sensitive (632).
Despite decades of research, there is no compelling evidence that T has a causative role in prostate cancer. For example, studies using stored frozen plasma samples failed to show a difference in T levels between men in whom prostate cancer developed 7 to 25 yr later and those in whom it did not (633, 634). In addition, a compilation of published prospective studies of T-replacement therapy revealed only five cases of prostate cancer among 461 men (1.1%) followed for 6 to 36 months, a prevalence rate similar to that in the general population. No follow-up data beyond 36 months are available (635). Furthermore, the integrative data of studies that we could identify (as shown in Table 4), where T was administered from 12 to 36 months, revealed three cases of prostate cancer among 219 T-treated men, compared with one case among 194 placebo-treated men, which again appears similar to the age-specific incidence rate of prostate cancer recorded in the general population of 800 new cases in 100,000 patients per year (info.cancerresearchuk.org/cancerstats/types/prostate/). It has been argued that to detect a 30% difference in prostate cancer incidence between placebo- and T-treated subjects, 6000 older men with low T would need to be randomized to each treatment group and would require treatment for an average of 5 yr (636). Several (436, 582, 585, 586, 637) but not all (48, 49, 532, 581, 588, 591) of the studies have shown that T administration in healthy older men can increase PSA levels significantly when compared with placebo. The increments observed do not usually result in PSA levels above the normal upper limit; neither did the incidence of prostate-related events, differences in prostate biopsies, or changes in urine flow differ in T-treated men compared with placebo (526, 581). It should be noted that a substantial increase in PSA level might well indicate that a prostate cancer has developed (638). Consequently, vigilance is still required when T is administered in healthy older men. The recently published guideline from The Endocrine Society on T replacement in men comprehensively addresses this issue (526).
Table 4.
Studies of combined GH and T replacement therapy in healthy older men
| First author, year (Ref.) | Study protocol, active treatment, duration, no. of subjects | Main subject characteristics | Main findings and remarks |
|---|---|---|---|
| Brill, 2002 (429) | Crossover | Healthy older men | LBM increased in all treatment groups, with improvements also noticed in some measurements of physical performance; muscle strength and BF did not change |
| GH, 6.25 μg/kg · d; T patch, 5 mg/d | Age, 68 ± 2.5 yr | ||
| 1-month active treatment alternating with 3-month washout period | T, <450 ng/dl; IGF-I, <200 ng/ml | ||
| n = 10 | |||
| Giannoulis 2006 (48, 646); 2008 (597) | Parallel groups | Shown in Tables 1 and 3 | Total, appendicular, and muscle CSA increased |
| Duration, 6 months | VO2max and one of six measurements of isokinetic muscle strength increased | ||
| Act, n = 19; PL, n = 20 (dropouts, Act, n = 3; PL, n = 4) | Whole body protein turnover also increased | ||
| T patch, 5 mg/d fixed dose | Total and appendicular BF decreased, abdominal fat area did not change; no changes in lipid profile and VLDL metabolism | ||
| GH starting dose, 0.1 mg/d, increased gradually to a mean of 0.54 mg/d | No changes in insulin levels no glucose intolerance, diabetes or other adverse events | ||
| Target IGF-I, 250 ng/ml | |||
| Münzer, 2001 (576); Blackman, 2002 (49); Christmas, 2002 (583); Huang, 2005 (598); Münzer, 2009 (645) | Parallel groups | Shown in Tables 1 and 3 | Total LBM increased and BF decreased; sc fat also decreased, but not VF |
| Duration, 6 months | Muscle strength, VO2max, WBPK increased | ||
| Act, n = 19 to 21; PL, n = 17 (dropouts, Act, n = 1) | Markers of bone turnover increased whereas a marginal decline in BMD on proximal radius was found | ||
| T enanthate, 100 mg/2 wk im | High incidence of adverse effects, mainly glucose intolerance and diabetes | ||
| GH starting dose, 30 μg/kg, reduced to 20 μg/kg, 3 times/wk | |||
| Sattler 2009 (602) | Not placebo controlled | Community-dwelling healthy older men | Total and appendicular LBM increased, total and trunk fat decreased |
| T gel in two doses, 5 and 10 g/d, combined with three different GH doses (0, 0.3, 0.5 μg/kg · d) | Age, 70 ± 4.2 yr | All changes were dose-dependent, with the highest effects recorded when higher dose of combined GH and T was used | |
| Duration, 16 wk | IGF-I, <167 ng/ml | Muscle strength similarly increased only after higher doses of GH and T | |
| Act GH 0.3 + T 5, n = 21; Act GH 0.3 + T 10, n = 21; Act GH 0.5 + T 5, n = 19; Act GH 0.5 + T 10, n = 21(dropouts Act GH 0.3 + T 5, n = 2; Act GH 0.3 + T 10, n = 1; Act GH 0.5 + T 5, n = 2; Act GH 0.5 + T 10, n = 4) | T, <550 ng/dl | Increased incidence of glucose intolerance, diabetes, and high blood pressure |
All studies included are randomized, placebo-controlled and double blind unless otherwise stated. Four trials were identified where GH+T was administered in healthy older men (aged >60 yr) and was reported on outcome measurements related to physical function. A total of 132 subjects received GH+T.
Briefly, it has been advocated that men opting for T treatment should be offered an estimation of prostate cancer risk based on PSA measurement and digital rectal examination at baseline and then use the prostate cancer risk calculator (639) that considers these factors plus additional factors that contribute to prostate cancer risk. Men found to have higher risk should have a urological examination before commencing T treatment despite having PSA levels less than 4 ng/ml. While on treatment, PSA levels should be monitored at 3 to 6 months after the initiation of treatment. An annual increment higher than 1.4 ng/ml should prompt a urological examination. Furthermore, an annual rate of PSA rise greater that 0.4 ng/ml over a 2-yr period should also lead to a urological evaluation (640). Finally, severe symptoms of lower urinary tract obstruction as indicated by an IPSS (International Prostate Symptom Score) of 21 or greater is a relative contraindication to T treatment (636, 641).
Testosterone has been known to stimulate erythropoiesis, possibly by stimulating erythropoietin production (642), whereas suppression by T of serum hepcidin (an iron regulatory peptide) may also contribute to this (643). Polycythemia has been reported to occur in healthy older men mainly after im and oral T administration (443, 582, 584–586, 588, 591, 602, 637), but usually not after transdermal T (48, 49, 532, 581). Nevertheless, it is important to monitor the hematocrit at regular intervals to avoid this potentially serious adverse event.
Pharmacological doses of T may induce or worsen sleep apnea in healthy older men (644); this appears to be an uncommon side effect. Snyder et al. (585) could not detect any change in the respiratory distress index after 36 months of transdermal T.
Finally, an increase in blood pressure and clinically significant edema may seldom occur after T administration in healthy older men (602, 635), but these are potentially serious adverse events that appear to occur most frequently in older patients with preexisting cardiovascular and pulmonary diseases. Indeed, it was necessary to interrupt a recent study where T was prescribed in frail older men because of a high incidence of serious cardiovascular and pulmonary adverse events.
For more details regarding adverse events related to T treatment, see Refs. 42, 635, and 643.
In conclusion, T treatment in healthy older men in near physiological doses does not appear to incur serious adverse events, although long-term safety has not been established, and regular monitoring of PSA and hematocrit levels is required. Conversely, the high incidence of cardiovascular and prostate adverse events in frail elderly men with associated comorbidities in the few studies reported today calls for higher vigilance when T is administered in this category of subjects.
X. Conclusions and Recommendations
In this review, we have highlighted the reduction in secretion of GH and T that appears to be an inevitable feature of aging and is associated with a loss of lean tissue, accumulation of fat, and loss of strength and mobility. We propose that many of these aging-related changes are due to a lack of the essential role of a favorable anabolic hormone milieu (created mainly by GH and T, but supported by other anabolic agents such as dehydroepiandrosterone) and physical activity in maintaining normal body composition and the preservation of physical functional capacity.
We have also summarized the evidence that shows that exercise alone cannot prevent the decline in physical function observed with aging. We believe that the available data are in favor of a partial causative effect from this loss of anabolic milieu in the development of frailty. This hypothesis raises the question of whether or not we can influence this process favorably and thus reduce burdens of frailty for the individual and society. Without successful intervention, this burden is likely to increase considerably in the coming decades.
Both T and GH are powerful anabolic agents. We believe that these two hormones are the most important agents that, in combination with exercise, normally regulate body composition in adult men. The evidence shows that the anabolic effects of T and GH are dose- and time-dependent. In studies of hormone replacement, the literature indicates that the desire to obtain a positive effect within the limited time (and budget) of a research project has often led to the use of too high and unphysiological hormone doses and consequential excess morbidity. There is clear evidence that T delivered into the systemic circulation via injections, or more recently by gels and patches, is more effective than oral preparations. Hormone replacement with T may also be safer when given systemically.
The data also show that T combined with GH is a more effective anabolic treatment regime than either alone. Of considerable interest is the fact that elite athletes abusing these hormones have come to the same conclusion and much earlier1 than we scientists. By combining GH and T, a given anabolic effect is achieved with a smaller dose of each compared with when GH and T are given alone. There may also be an extra effect that may not be achievable with either alone. This also has considerable implications in terms of avoiding side effects and achieving long-term safety. It is also not at all surprising because we know that they act on different metabolic pathways in an additive and possibly even synergistic manner.
We have also provided an explanation as to why short-term intervention studies have often failed to record significant improvement in muscle function. It takes time and use for newly formed muscle to “get up to speed,” and most intervention studies have been too short to allow this to develop. It is of great importance that future studies should be over a number of years because with these interventions we are trying to influence favorably a condition that has been developing over many years. The short-term trials (3–6 months) should be considered more as proof-of-concept studies than efficacy studies into the therapeutic effects of HRT.
The evidence reviewed indicates unequivocally that it is possible to influence at least some components of this aging process favorably by hormone replacement with GH and T. The favorable effects are seen most easily in terms of changes in body composition than in physical function. We have critically discussed the hurdles surrounding the design and methodology of trials assessing the effects of HRT in older men and presented our thoughts on this. We have focused in this review on the effects of HRT in healthy older men because recent data have clearly demonstrated that combined GH and T treatment improves exercise capacity, a prerequisite of muscle function, and thus it was reasoned that such treatment could delay or even prevent the development of frailty. Longer-term studies, possibly in conjunction with physical activity intervention, are urgently needed to demonstrate that these surrogate favorable effects translate into meaningful clinical outcomes.
Frailty is a multisystem, multifactorial condition, the pathogenesis of which is far from clear. The role of HRT in mitigating or even reversing established frailty in older men, although of huge clinical interest, is a topic that needs to be explored in further studies, which this review encourages and hopes to stimulate.
The published data also indicate that the therapeutic interventions with replacement HRT with T and GH in healthy older men is safe, at least over an interval of 3 yr (testosterone) and 6 months (GH and T), providing that moderate doses are used. Safety beyond that time scale will require longer studies than are available today. Although incomplete, the evidence available is generally positive and should encourage rather than discourage future clinical research. The goal of successful mitigation or actual prevention of frailty is so massive that it should encourage a major research priority and effort over the coming years.
Acknowledgments
We thank the editor and reviewers for their considerable help in modifying this manuscript. We are grateful to Dr. Kingsley Micklem (Director of the Medical Informatics Unit, Nuffield Department of Clinical Laboratory Sciences, University of Oxford, Oxford, UK) for redrawing Fig. 2 from the original.
Disclosure Summary: The authors have nothing to declare.
Ben Johnson won a gold medal in the 100 meters in the 1988 Olympic Games in Seoul. It was soon rescinded when he tested positive for anabolic steroids. He subsequently admitted under oath that he took human GH in addition to the anabolic steroids. The first peer-reviewed papers on the effects of GH given to adults did not appear until 1989.
- AR
- Androgen receptor
- BF
- body fat
- BioT
- bioavailable T
- BMD
- bone mineral density
- CSA
- cross-sectional area
- CT
- computed tomography
- CVD
- cardiovascular disease
- DEXA
- dual energy x-ray absorptiometry
- FBM
- fat body mass
- FFA
- free fatty acid
- FT
- free T
- GHD
- GH deficiency or GH-deficient
- HDL-C
- high-density lipoprotein cholesterol
- HRT
- hormone replacement therapy
- IDGH
- integrated daily GH
- IGFBP
- IGF binding protein
- IHH
- isolated hypogonadotropic hypogonadism
- IMT
- intima media thickness
- JAK
- Janus-activated kinases
- LBM
- lean body mass
- LDL-C
- low-density lipoprotein cholesterol
- LPL
- lipoprotein lipase
- LV
- left ventricular
- MGF
- mechano-growth factor
- NO
- nitric oxide
- PSA
- prostate-specific antigen
- QoL
- quality of life
- RCT
- randomized controlled trial
- 1RM
- one repetition maximum
- SOCS
- suppressors of cytokine signaling
- SS
- somatostatin
- STAT
- signal transducers and activators of transcription
- T
- testosterone
- TC
- total cholesterol
- TG
- triglyceride
- VF
- visceral fat
- VLDL apoB
- very low-density apolipoprotein
- VO2max
- maximum rate of O2 consumption
- WBPK
- whole body protein kinetics
- WBPS
- whole body protein synthesis.
References
- 1. 2007. Administration on Aging. Federal Interagency Forum on Aging-Related Statistics. [electronic mail system]. http://www.aoa.gov/AoAroot/Aging_Statistics/Profile/2007/4.aspx
- 2. Adams PF , Dey AN , Vickerie JL. 2007. Summary health statistics for the U.S. population: National Health Interview Survey, 2005. Vital Health Stat 10 233:1–104 [PubMed] [Google Scholar]
- 3. Nagi SZ. 1965. Rehab in the engineering classroom. Rehabil Rec 6:20–21 [PubMed] [Google Scholar]
- 4. Applegate WB , Blass JP , Williams TF. 1990. Instruments for the functional assessment of older patients. N Engl J Med 322:1207–1214 [DOI] [PubMed] [Google Scholar]
- 5. Fried LP , Tangen CM , Walston J , Newman AB , Hirsch C , Gottdiener J , Seeman T , Tracy R , Kop WJ , Burke G , McBurnie MA. 2001. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 6. Bandeen-Roche K , Xue QL , Ferrucci L , Walston J , Guralnik JM , Chaves P , Zeger SL , Fried LP. 2006. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci 61:262–266 [DOI] [PubMed] [Google Scholar]
- 7. Skelton DA , Greig CA , Davies JM , Young A. 1994. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 23:371–377 [DOI] [PubMed] [Google Scholar]
- 8. Cress ME , Meyer M. 2003. Maximal voluntary and functional performance levels needed for independence in adults aged 65 to 97 years. Phys Ther 83:37–48 [PubMed] [Google Scholar]
- 9. Goodpaster BH , Park SW , Harris TB , Kritchevsky SB , Nevitt M , Schwartz AV , Simonsick EM , Tylavsky FA , Visser M , Newman AB. 2006. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 10. Fleg JL , Morrell CH , Bos AG , Brant LJ , Talbot LA , Wright JG , Lakatta EG. 2005. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112:674–682 [DOI] [PubMed] [Google Scholar]
- 11. Calle EE , Thun MJ , Petrelli JM , Rodriguez C , Heath CW. 1999. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 341:1097–1105 [DOI] [PubMed] [Google Scholar]
- 12. Rosenberg IH. 1989. Summary comments. Am J Clin Nutr 50:1231–1233 [Google Scholar]
- 13. Metter EJ , Talbot LA , Schrager M , Conwit R. 2002. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57:B359–B365 [DOI] [PubMed] [Google Scholar]
- 14. Veldhuis JD , Liem AY , South S , Weltman A , Weltman J , Clemmons DA , Abbott R , Mulligan T , Johnson ML , Pincus S. 1995. Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 80:3209–3222 [DOI] [PubMed] [Google Scholar]
- 15. Harman SM , Metter EJ , Tobin JD , Pearson J , Blackman MR. 2001. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- 16. Salomon F , Cuneo RC , Hesp R , Sönksen PH. 1989. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 321:1797–1803 [DOI] [PubMed] [Google Scholar]
- 17. Bhasin S , Storer TW , Berman N , Callegari C , Clevenger B , Phillips J , Bunnell TJ , Tricker R , Shirazi A , Casaburi R. 1996. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335:1–7 [DOI] [PubMed] [Google Scholar]
- 18. Cuneo RC , Salomon F , Wiles CM , Sonksen PH. 1990. Skeletal muscle performance in adults with growth hormone deficiency. Horm Res 33(Suppl 4):55–60 [DOI] [PubMed] [Google Scholar]
- 19. Katznelson L , Finkelstein JS , Schoenfeld DA , Rosenthal DI , Anderson EJ , Klibanski A. 1996. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 81:4358–4365 [DOI] [PubMed] [Google Scholar]
- 20. Rudman D , Feller AG , Nagraj HS , Gergans GA , Lalitha PY , Goldberg AF , Schlenker RA , Cohn L , Rudman IW , Mattson DE. 1990. Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6 [DOI] [PubMed] [Google Scholar]
- 21. Snyder PJ , Peachey H , Hannoush P , Berlin JA , Loh L , Lenrow DA , Holmes JH , Dlewati A , Santanna J , Rosen CJ , Strom BL. 1999. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- 22. Carroll PV , Christ ER , Bengtsson BA , Carlsson L , Christiansen JS , Clemmons D , Hintz R , Ho K , Laron Z , Sizonenko P , Sönksen PH , Tanaka T , Thorne M. 1998. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab 83:382–395 [DOI] [PubMed] [Google Scholar]
- 23. Snyder PJ , Peachey H , Berlin JA , Hannoush P , Haddad G , Dlewati A , Santanna J , Loh L , Lenrow DA , Holmes JH , Kapoor SC , Atkinson LE , Strom BL. 2000. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 85:2670–2677 [DOI] [PubMed] [Google Scholar]
- 24. Rosenberg IH. 1997. Sarcopenia: origins and clinical relevance. J Nutr 127(5 Suppl):990S–991S [DOI] [PubMed] [Google Scholar]
- 25. Lindle RS , Metter EJ , Lynch NA , Fleg JL , Fozard JL , Tobin J , Roy TA , Hurley BF. 1997. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol 83:1581–1587 [DOI] [PubMed] [Google Scholar]
- 26. Candow DG , Chilibeck PD. 2005. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J Gerontol A Biol Sci Med Sci 60:148–156 [DOI] [PubMed] [Google Scholar]
- 27. Hunter GR , Newcomer BR , Weinsier RL , Karapondo DL , Larson-Meyer DE , Joanisse DR , Bamman MM. 2002. Age is independently related to muscle metabolic capacity in premenopausal women. J Appl Physiol 93:70–76 [DOI] [PubMed] [Google Scholar]
- 28. Short KR , Bigelow ML , Kahl J , Singh R , Coenen-Schimke J , Raghavakaimal S , Nair KS. 2005. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwendner KI , Mikesky AE , Holt WS , Peacock M , Burr DB. 1997. Differences in muscle endurance and recovery between fallers and nonfallers, and between young and older women. J Gerontol A Biol Sci Med Sci 52:M155–M160 [DOI] [PubMed] [Google Scholar]
- 30. Crespo CJ , Keteyian SJ , Heath GW , Sempos CT. 1996. Leisure-time physical activity among US adults. Results from the Third National Health and Nutrition Examination Survey. Arch Intern Med 156:93–98 [PubMed] [Google Scholar]
- 31. Gill TM , Allore H , Guo Z. 2003. Restricted activity and functional decline among community-living older persons. Arch Intern Med 163:1317–1322 [DOI] [PubMed] [Google Scholar]
- 32. Frisard MI , Fabre JM , Russell RD , King CM , DeLany JP , Wood RH , Ravussin E. 2007. Physical activity level and physical functionality in nonagenarians compared to individuals aged 60–74 years. J Gerontol A Biol Sci Med Sci 62:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kehayias JJ , Fiatarone MA , Zhuang H , Roubenoff R. 1997. Total body potassium and body fat: relevance to aging. Am J Clin Nutr 66:904–910 [DOI] [PubMed] [Google Scholar]
- 34. Vega GL , Adams-Huet B , Peshock R , Willett D , Shah B , Grundy SM. 2006. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 91:4459–4466 [DOI] [PubMed] [Google Scholar]
- 35. Petrella JK , Kim JS , Cross JM , Kosek DJ , Bamman MM. 2006. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291:E937–E946 [DOI] [PubMed] [Google Scholar]
- 36. Tidball JG. 2005. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol 98:1900–1908 [DOI] [PubMed] [Google Scholar]
- 37. Adams GR. 2002. Invited review: autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol 93:1159–1167 [DOI] [PubMed] [Google Scholar]
- 38. Sinha-Hikim I , Cornford M , Gaytan H , Lee ML , Bhasin S. 2006. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab 91:3024–3033 [DOI] [PubMed] [Google Scholar]
- 39. Singh R , Artaza JN , Taylor WE , Gonzalez-Cadavid NF , Bhasin S. 2003. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144:5081–5088 [DOI] [PubMed] [Google Scholar]
- 40. Zadik Z , Chalew SA , McCarter RJ , Meistas M , Kowarski AA. 1985. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab 60:513–516 [DOI] [PubMed] [Google Scholar]
- 41. Leifke E , Gorenoi V , Wichers C , Von Zur Mühlen A , Von Büren E , Brabant G. 2000. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 53:689–695 [DOI] [PubMed] [Google Scholar]
- 42. Lapauw B , Goemaere S , Zmierczak H , Van Pottelbergh I , Mahmoud A , Taes Y , De Bacquer D , Vansteelandt S , Kaufman JM. 2008. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol 159:459–468 [DOI] [PubMed] [Google Scholar]
- 43. Fiatarone MA , O'Neill EF , Ryan ND , Clements KM , Solares GR , Nelson ME , Roberts SB , Kehayias JJ , Lipsitz LA , Evans WJ. 1994. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330:1769–1775 [DOI] [PubMed] [Google Scholar]
- 44. Conboy IM , Conboy MJ , Wagers AJ , Girma ER , Weissman IL , Rando TA. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764 [DOI] [PubMed] [Google Scholar]
- 45. Kumar V , Selby A , Rankin D , Patel R , Atherton P , Hildebrandt W , Williams J , Smith K , Seynnes O , Hiscock N , Rennie MJ. 2009. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nemet D , Eliakim A , Zaldivar F , Cooper DM. 2006. Effect of rhIL-6 infusion on GH→IGF-I axis mediators in humans. Am J Physiol Regul Integr Comp Physiol 291:R1663–R1668 [DOI] [PubMed] [Google Scholar]
- 47. Kerse N , Peri K , Robinson E , Wilkinson T , von Randow M , Kiata L , Parsons J , Latham N , Parsons M , Willingale J , Brown P , Arroll B. 2008. Does a functional activity programme improve function, quality of life, and falls for residents in long term care? Cluster randomised controlled trial. BMJ 337:a1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giannoulis MG , Sonksen PH , Umpleby M , Breen L , Pentecost C , Whyte M , McMillan CV , Bradley C , Martin FC. 2006. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab 91:477–484 [DOI] [PubMed] [Google Scholar]
- 49. Blackman MR , Sorkin JD , Münzer T , Bellantoni MF , Busby-Whitehead J , Stevens TE , Jayme J , O'Connor KG , Christmas C , Tobin JD , Stewart KJ , Cottrell E , St Clair C , Pabst KM , Harman SM. 2002. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288:2282–2292 [DOI] [PubMed] [Google Scholar]
- 50. Giustina A , Veldhuis JD. 1998. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- 51. Kojima M , Hosoda H , Date Y , Nakazato M , Matsuo H , Kangawa K. 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- 52. Arvat E , Di Vito L , Broglio F , Papotti M , Muccioli G , Dieguez C , Casanueva FF , Deghenghi R , Camanni F , Ghigo E. 2000. Preliminary evidence that ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest 23:493–495 [DOI] [PubMed] [Google Scholar]
- 53. Veldhuis JD , Iranmanesh A , Mielke K , Miles JM , Carpenter PC , Bowers CY. 2006. Ghrelin potentiates growth hormone secretion driven by putative somatostatin withdrawal and resists inhibition by human corticotropin-releasing hormone. J Clin Endocrinol Metab 91:2441–2446 [DOI] [PubMed] [Google Scholar]
- 54. Nass R , Farhy LS , Liu J , Prudom CE , Johnson ML , Veldhuis P , Pezzoli SS , Oliveri MC , Gaylinn BD , Geysen HM , Thorner MO. 2008. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab 93:1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Avram AM , Jaffe CA , Symons KV , Barkan AL. 2005. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J Clin Endocrinol Metab 90:2982–2987 [DOI] [PubMed] [Google Scholar]
- 56. Le Roith D , Bondy C , Yakar S , Liu JL , Butler A. 2001. The somatomedin hypothesis: 2001. Endocr Rev 22:53–74 [DOI] [PubMed] [Google Scholar]
- 57. Kaplan SA , Cohen P. 2007. The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab 92:4529–4535 [DOI] [PubMed] [Google Scholar]
- 58. Hartman ML , Clayton PE , Johnson ML , Celniker A , Perlman AJ , Alberti KG , Thorner MO. 1993. A low dose euglycemic infusion of recombinant human insulin-like growth factor I rapidly suppresses fasting-enhanced pulsatile growth hormone secretion in humans. J Clin Invest 91:2453–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sjögren K , Liu JL , Blad K , Skrtic S , Vidal O , Wallenius V , LeRoith D , Törnell J , Isaksson OG , Jansson JO , Ohlsson C. 1999. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal growth in mice. Proc Natl Acad Sci USA 96:7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yakar S , Liu JL , Stannard B , Butler A , Accili D , Sauer B , LeRoith D. 1999. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96:7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fisker S , Kristensen K , Rosenfalck AM , Pedersen SB , Ebdrup L , Richelsen B , Hilsted J , Christiansen JS , Jørgensen JO. 2001. Gene expression of a truncated and the full-length growth hormone (GH) receptor in subcutaneous fat and skeletal muscle in GH-deficient adults: impact of GH treatment. J Clin Endocrinol Metab 86:792–796 [DOI] [PubMed] [Google Scholar]
- 62. Ballesteros M , Leung KC , Ross RJ , Iismaa TP , Ho KK. 2000. Distribution and abundance of messenger ribonucleic acid for growth hormone receptor isoforms in human tissues. J Clin Endocrinol Metab 85:2865–2871 [DOI] [PubMed] [Google Scholar]
- 63. Sadowski CL , Wheeler TT , Wang LH , Sadowski HB. 2001. GH regulation of IGF-I and suppressor of cytokine signaling gene expression in C2C12 skeletal muscle cells. Endocrinology 142:3890–3900 [DOI] [PubMed] [Google Scholar]
- 64. D'Ercole AJ , Stiles AD , Underwood LE. 1984. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA 81:935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ohlsson C , Mohan S , Sjögren K , Tivesten A , Isgaard J , Isaksson O , Jansson JO , Svensson J. 2009. The role of liver-derived insulin-like growth factor-I. Endocr Rev 30:494–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Firth SM , Baxter RC. 2002. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23:824–854 [DOI] [PubMed] [Google Scholar]
- 67. Zapf J , Hauri C , Waldvogel M , Futo E , Häsler H , Binz K , Guler HP , Schmid C , Froesch ER. 1989. Recombinant human insulin-like growth factor I induces its own specific carrier protein in hypophysectomized and diabetic rats. Proc Natl Acad Sci USA 86:3813–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dai J , Baxter RC. 1994. Regulation in vivo of the acid-labile subunit of the rat serum insulin-like growth factor-binding protein complex. Endocrinology 135:2335–2341 [DOI] [PubMed] [Google Scholar]
- 69. Lee PD , Conover CA , Powell DR. 1993. Regulation and function of insulin-like growth factor-binding protein-1. Proc Soc Exp Biol Med 204:4–29 [DOI] [PubMed] [Google Scholar]
- 70. Wolk K , Larsson SC , Vessby B , Wolk A , Brismar K. 2004. Metabolic, anthropometric, and nutritional factors as predictors of circulating insulin-like growth factor binding protein-1 levels in middle-aged and elderly men. J Clin Endocrinol Metab 89:1879–1884 [DOI] [PubMed] [Google Scholar]
- 71. Lindahl A , Isgaard J , Nilsson A , Isaksson OG. 1986. Growth hormone potentiates colony formation of epiphyseal chondrocytes in suspension culture. Endocrinology 118:1843–1848 [DOI] [PubMed] [Google Scholar]
- 72. Ohlsson C , Isaksson O , Lindahl A. 1994. Clonal analysis of rat tibia growth plate chondrocytes in suspension culture—differential effects of growth hormone and insulin-like growth factor I. Growth Regul 4:1–7 [PubMed] [Google Scholar]
- 73. Russell-Jones DL , Weissberger AJ , Bowes SB , Kelly JM , Thomason M , Umpleby AM , Jones RH , Sönksen PH. 1993. The effects of growth hormone on protein metabolism in adult growth hormone deficient patients. Clin Endocrinol (Oxf) 38:427–431 [DOI] [PubMed] [Google Scholar]
- 74. Russell-Jones DL , Bowes SB , Rees SE , Jackson NC , Weissberger AJ , Hovorka R , Sonksen PH , Umpleby AM. 1998. Effect of growth hormone treatment on postprandial protein metabolism in growth hormone-deficient adults. Am J Physiol 274:E1050–E1056 [DOI] [PubMed] [Google Scholar]
- 75. Lucidi P , Lauteri M , Laureti S , Celleno R , Santoni S , Volpi E , Angeletti G , Santeusanio F , De Feo P. 1998. A dose-response study of growth hormone (GH) replacement on whole body protein and lipid kinetics in GH-deficient adults. J Clin Endocrinol Metab 83:353–357 [DOI] [PubMed] [Google Scholar]
- 76. Mauras N. 1995. Combined recombinant human growth hormone and recombinant human insulin-like growth factor I: lack of synergy on whole body protein anabolism in normally fed subjects. J Clin Endocrinol Metab 80:2633–2637 [DOI] [PubMed] [Google Scholar]
- 77. Buijs MM , Romijn JA , Burggraaf J , De Kam ML , Cohen AF , Frölich M , Stellaard F , Meinders AE , Pijl H. 2002. Growth hormone blunts protein oxidation and promotes protein turnover to a similar extent in abdominally obese and normal-weight women. J Clin Endocrinol Metab 87:5668–5674 [DOI] [PubMed] [Google Scholar]
- 78. Biermasz NR , Hamdy NA , Pereira AM , Romijn JA , Roelfsema F. 2004. Long-term skeletal effects of recombinant human growth hormone (rhGH) alone and rhGH combined with alendronate in GH-deficient adults: a seven-year follow-up study. Clin Endocrinol (Oxf) 60:568–575 [DOI] [PubMed] [Google Scholar]
- 79. Götherström G , Bengtsson BA , Bosaeus I , Johannsson G , Svensson J. 2007. Ten-year GH replacement increases bone mineral density in hypopituitary patients with adult onset GH deficiency. Eur J Endocrinol 156:55–64 [DOI] [PubMed] [Google Scholar]
- 80. Landin-Wilhelmsen K , Nilsson A , Bosaeus I , Bengtsson BA. 2003. Growth hormone increases bone mineral content in postmenopausal osteoporosis: a randomized placebo-controlled trial. J Bone Miner Res 18:393–405 [DOI] [PubMed] [Google Scholar]
- 81. Gillberg P , Mallmin H , Petrén-Mallmin M , Ljunghall S , Nilsson AG. 2002. Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis. J Clin Endocrinol Metab 87:4900–4906 [DOI] [PubMed] [Google Scholar]
- 82. Hayes VY , Urban RJ , Jiang J , Marcell TJ , Helgeson K , Mauras N. 2001. Recombinant human growth hormone and recombinant human insulin-like growth factor I diminish the catabolic effects of hypogonadism in man: metabolic and molecular effects. J Clin Endocrinol Metab 86:2211–2219 [DOI] [PubMed] [Google Scholar]
- 83. Mauras N , Beaufrere B. 1995. Recombinant human insulin-like growth factor-I enhances whole body protein anabolism and significantly diminishes the protein catabolic effects of prednisone in humans without a diabetogenic effect. J Clin Endocrinol Metab 80:869–874 [DOI] [PubMed] [Google Scholar]
- 84. Russell-Jones DL , Umpleby AM , Hennessy TR , Bowes SB , Shojaee-Moradie F , Hopkins KD , Jackson NC , Kelly JM , Jones RH , Sönksen PH. 1994. Use of a leucine clamp to demonstrate that IGF-I actively stimulates protein synthesis in normal humans. Am J Physiol 267:E591–E598 [DOI] [PubMed] [Google Scholar]
- 85. Friedlander AL , Butterfield GE , Moynihan S , Grillo J , Pollack M , Holloway L , Friedman L , Yesavage J , Matthias D , Lee S , Marcus R , Hoffman AR. 2001. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab 86:1496–1503 [DOI] [PubMed] [Google Scholar]
- 86. Fowelin J , Attvall S , Lager I , Bengtsson BA. 1993. Effects of treatment with recombinant human growth hormone on insulin sensitivity and glucose metabolism in adults with growth hormone deficiency. Metabolism 42:1443–1447 [DOI] [PubMed] [Google Scholar]
- 87. Bramnert M , Segerlantz M , Laurila E , Daugaard JR , Manhem P , Groop L. 2003. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab 88:1455–1463 [DOI] [PubMed] [Google Scholar]
- 88. Jacob R , Barrett E , Plewe G , Fagin KD , Sherwin RS. 1989. Acute effects of insulin-like growth factor I on glucose and amino acid metabolism in the awake fasted rat. Comparison with insulin. J Clin Invest 83:1717–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Simpson HL , Jackson NC , Shojaee-Moradie F , Jones RH , Russell-Jones DL , Sönksen PH , Dunger DB , Umpleby AM. 2004. Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J Clin Endocrinol Metab 89:425–432 [DOI] [PubMed] [Google Scholar]
- 90. Hansen TK , Gravholt CH , ØRskov H , Rasmussen MH , Christiansen JS , Jørgensen JO. 2002. Dose dependency of the pharmacokinetics and acute lipolytic actions of growth hormone. J Clin Endocrinol Metab 87:4691–4698 [DOI] [PubMed] [Google Scholar]
- 91. Djurhuus CB , Gravholt CH , Nielsen S , Pedersen SB , Møller N , Schmitz O. 2004. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol Endocrinol Metab 286:E488–E494 [DOI] [PubMed] [Google Scholar]
- 92. Mekala KC , Tritos NA. 2009. Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J Clin Endocrinol Metab 94:130–137 [DOI] [PubMed] [Google Scholar]
- 93. Johannsson G , Mårin P , Lönn L , Ottosson M , Stenlöf K , Björntorp P , Sjöström L , Bengtsson BA. 1997. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82:727–734 [DOI] [PubMed] [Google Scholar]
- 94. Papadakis MA , Grady D , Black D , Tierney MJ , Gooding GA , Schambelan M , Grunfeld C. 1996. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med 124:708–716 [DOI] [PubMed] [Google Scholar]
- 95. Klöting N , Koch L , Wunderlich T , Kern M , Ruschke K , Krone W , Brüning JC , Blüher M. 2008. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes 57:2074–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mauras N , O'Brien KO , Welch S , Rini A , Helgeson K , Vieira NE , Yergey AL. 2000. Insulin-like growth factor I and growth hormone (GH) treatment in GH-deficient humans: differential effects on protein, glucose, lipid, and calcium metabolism. J Clin Endocrinol Metab 85:1686–1694 [DOI] [PubMed] [Google Scholar]
- 97. Laron Z , Ginsberg S , Lilos P , Arbiv M , Vaisman N. 2006. Long-term IGF-I treatment of children with Laron syndrome increases adiposity. Growth Horm IGF Res 16:61–64 [DOI] [PubMed] [Google Scholar]
- 98. Møller N , Jørgensen JO. 2009. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177 [DOI] [PubMed] [Google Scholar]
- 99. Corpas E , Harman SM , Blackman MR. 1993. Human growth hormone and human aging. Endocr Rev 14:20–39 [DOI] [PubMed] [Google Scholar]
- 100. Ho KY , Evans WS , Blizzard RM , Veldhuis JD , Merriam GR , Samojlik E , Furlanetto R , Rogol AD , Kaiser DL , Thorner MO. 1987. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64:51–58 [DOI] [PubMed] [Google Scholar]
- 101. Iranmanesh A , Lizarralde G , Veldhuis JD. 1991. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73:1081–1088 [DOI] [PubMed] [Google Scholar]
- 102. Holt RI , Webb E , Pentecost C , Sönksen PH. 2001. Aging and physical fitness are more important than obesity in determining exercise-induced generation of GH. J Clin Endocrinol Metab 86:5715–5720 [DOI] [PubMed] [Google Scholar]
- 103. Vahl N , Jørgensen JO , Jurik AG , Christiansen JS. 1996. Abdominal adiposity and physical fitness are major determinants of the age associated decline in stimulated GH secretion in healthy adults. J Clin Endocrinol Metab 81:2209–2215 [DOI] [PubMed] [Google Scholar]
- 104. Rudman D , Kutner MH , Rogers CM , Lubin MF , Fleming GA , Bain RP. 1981. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest 67:1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Janssen JA , Stolk RP , Pols HA , Grobbee DE , de Jong FH , Lamberts SW. 1998. Serum free IGF-I, total IGF-I, IGFBP-1 and IGFBP-3 levels in an elderly population: relation to age and sex steroid levels. Clin Endocrinol (Oxf) 48:471–478 [DOI] [PubMed] [Google Scholar]
- 106. Iranmanesh A , Grisso B , Veldhuis JD. 1994. Low basal and persistent pulsatile growth hormone secretion are revealed in normal and hyposomatotropic men studied with a new ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 78:526–535 [DOI] [PubMed] [Google Scholar]
- 107. Chapman IM , Hartman ML , Pezzoli SS , Harrell FE , Hintz RL , Alberti KG , Thorner MO. 1997. Effect of aging on the sensitivity of growth hormone secretion to insulin-like growth factor-I negative feedback. J Clin Endocrinol Metab 82:2996–3004 [DOI] [PubMed] [Google Scholar]
- 108. Arvat E , Gianotti L , Grottoli S , Imbimbo BP , Lenaerts V , Deghenghi R , Camanni F , Ghigo E. 1994. Arginine and growth hormone-releasing hormone restore the blunted growth hormone-releasing activity of hexarelin in elderly subjects. J Clin Endocrinol Metab 79:1440–1443 [DOI] [PubMed] [Google Scholar]
- 109. Ghigo E , Goffi S , Nicolosi M , Arvat E , Valente F , Mazza E , Ghigo MC , Camanni F. 1990. Growth hormone (GH) responsiveness to combined administration of arginine and GH-releasing hormone does not vary with age in man. J Clin Endocrinol Metab 71:1481–1485 [DOI] [PubMed] [Google Scholar]
- 110. Hartman ML , Pezzoli SS , Hellmann PJ , Suratt PM , Thorner MO. 1996. Pulsatile growth hormone secretion in older persons is enhanced by fasting without relationship to sleep stages. J Clin Endocrinol Metab 81:2694–2701 [DOI] [PubMed] [Google Scholar]
- 111. Mulligan T , Jaen-Vinuales A , Godschalk M , Iranmanesh A , Veldhuis JD. 1999. Synthetic somatostatin analog (octreotide) suppresses daytime growth hormone secretion equivalently in young and older men: preserved pituitary responsiveness to somatostatin's inhibition in aging. J Am Geriatr Soc 47:1422–1424 [DOI] [PubMed] [Google Scholar]
- 112. degli Uberti EC , Ambrosio MR , Cella SG , Margutti AR , Trasforini G , Rigamonti AE , Petrone E , Müller EE. 1997. Defective hypothalamic growth hormone (GH)-releasing hormone activity may contribute to declining GH secretion with age in man. J Clin Endocrinol Metab 82:2885–2888 [DOI] [PubMed] [Google Scholar]
- 113. Nakamura S , Mizuno M , Katakami H , Gore AC , Terasawa E. 2003. Aging-related changes in in vivo release of growth hormone-releasing hormone and somatostatin from the stalk-median eminence in female rhesus monkeys (Macaca mulatta). J Clin Endocrinol Metab 88:827–833 [DOI] [PubMed] [Google Scholar]
- 114. Russell-Aulet M , Jaffe CA , Demott-Friberg R , Barkan AL. 1999. In vivo semiquantification of hypothalamic growth hormone-releasing hormone (GHRH) output in humans: evidence for relative GHRH deficiency in aging. J Clin Endocrinol Metab 84:3490–3497 [DOI] [PubMed] [Google Scholar]
- 115. Veldhuis JD , Iranmanesh A , Bowers CY. 2005. Joint mechanisms of impaired growth-hormone pulse renewal in aging men. J Clin Endocrinol Metab 90:4177–4183 [DOI] [PubMed] [Google Scholar]
- 116. Jaffe CA , Turgeon DK , Lown K , Demott-Friberg R , Watkins PB. 2002. Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab 283:E1008–E1015 [DOI] [PubMed] [Google Scholar]
- 117. Veldhuis JD , Keenan DM , Bailey JN , Adeniji A , Miles JM , Paulo R , Cosma M , Soares-Welch C. 2009. Testosterone supplementation in older men restrains insulin-like growth factor's dose-dependent feedback inhibition of pulsatile growth hormone secretion. J Clin Endocrinol Metab 94:246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Veldhuis JD , Evans WS , Iranmanesh A , Weltman AL , Bowers CY. 2004. Short-term testosterone supplementation relieves growth hormone autonegative feedback in men. J Clin Endocrinol Metab 89:1285–1290 [DOI] [PubMed] [Google Scholar]
- 119. Veldhuis JD , Keenan DM , Mielke K , Miles JM , Bowers CY. 2005. Testosterone supplementation in healthy older men drives GH and IGF-I secretion without potentiating peptidyl secretagogue efficacy. Eur J Endocrinol 153:577–586 [DOI] [PubMed] [Google Scholar]
- 120. Muniyappa R , Wong KA , Baldwin HL , Sorkin JD , Johnson ML , Bhasin S , Harman SM , Blackman MR. 2006. Dehydroepiandrosterone secretion in healthy older men and women: effects of testosterone and growth hormone administration in older men. J Clin Endocrinol Metab 91:4445–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Orrego JJ , Dimaraki E , Symons K , Barkan AL. 2004. Physiological testosterone replenishment in healthy elderly men does not normalize pituitary growth hormone output: evidence against the connection between senile hypogonadism and somatopause. J Clin Endocrinol Metab 89:3255–3260 [DOI] [PubMed] [Google Scholar]
- 122. Wilshire GB , Loughlin JS , Brown JR , Adel TE , Santoro N. 1995. Diminished function of the somatotropic axis in older reproductive-aged women. J Clin Endocrinol Metab 80:608–613 [DOI] [PubMed] [Google Scholar]
- 123. Cordido F , Peino R , Peñalva A , Alvarez CV , Casanueva FF , Dieguez C. 1996. Impaired growth hormone secretion in obese subjects is partially reversed by acipimox-mediated plasma free fatty acid depression. J Clin Endocrinol Metab 81:914–918 [DOI] [PubMed] [Google Scholar]
- 124. Paulo RC , Cosma M , Soares-Welch C , Bailey JN , Mielke KL , Miles JM , Bowers CY , Veldhuis JD. 2008. Gonadal status and body mass index jointly determine growth hormone (GH)-releasing hormone/GH-releasing peptide synergy in healthy men. J Clin Endocrinol Metab 93:944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Belchetz PE , Plant TM , Nakai Y , Keogh EJ , Knobil E. 1978. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202:631–633 [DOI] [PubMed] [Google Scholar]
- 126. Conn PM. 1986. The molecular basis of gonadotropin-releasing hormone action. Endocr Rev 7:3–10 [DOI] [PubMed] [Google Scholar]
- 127. Schnorr JA , Bray MJ , Veldhuis JD. 2001. Aromatization mediates testosterone's short-term feedback restraint of 24-hour endogenously driven and acute exogenous gonadotropin-releasing hormone-stimulated luteinizing hormone and follicle-stimulating hormone secretion in young men. J Clin Endocrinol Metab 86:2600–2606 [DOI] [PubMed] [Google Scholar]
- 128. Vermeulen A , Verdonck L. 1968. Studies on the binding of testosterone to human plasma. Steroids 11:609–635 [PubMed] [Google Scholar]
- 129. Pfeilschifter J , Scheidt-Nave C , Leidig-Bruckner G , Woitge HW , Blum WF , Wüster C , Haack D , Ziegler R. 1996. Relationship between circulating insulin-like growth factor components and sex hormones in a population-based sample of 50- to 80-year-old men and women. J Clin Endocrinol Metab 81:2534–2540 [DOI] [PubMed] [Google Scholar]
- 130. Sternfeld B , Liu K , Quesenberry CP , Wang H , Jiang SF , Daviglus M , Fornage M , Lewis CE , Mahan J , Schreiner PJ , Schwartz SM , Sidney S , Williams OD , Siscovick DS. 2008. Changes over 14 years in androgenicity and body mass index in a biracial cohort of reproductive-age women. J Clin Endocrinol Metab 93:2158–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Liu PY , Beilin J , Meier C , Nguyen TV , Center JR , Leedman PJ , Seibel MJ , Eisman JA , Handelsman DJ. 2007. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab 92:3599–3603 [DOI] [PubMed] [Google Scholar]
- 132. Zumoff B , Strain GW , Kream J , O'Connor J , Rosenfeld RS , Levin J , Fukushima DK. 1982. Age variation of the 24-hour mean plasma concentrations of androgens, estrogens, and gonadotropins in normal adult men. J Clin Endocrinol Metab 54:534–538 [DOI] [PubMed] [Google Scholar]
- 133. Kaufman JM , Vermeulen A. 2005. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26:833–876 [DOI] [PubMed] [Google Scholar]
- 134. Liu PY , Iranmanesh A , Nehra AX , Keenan DM , Veldhuis JD. 2005. Mechanisms of hypoandrogenemia in healthy aging men. Endocrinol Metab Clin North Am 34:935–955, ix [DOI] [PubMed] [Google Scholar]
- 135. Mulligan T , Iranmanesh A , Kerzner R , Demers LW , Veldhuis JD. 1999. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol 141:257–266 [DOI] [PubMed] [Google Scholar]
- 136. Tenover JS , Matsumoto AM , Plymate SR , Bremner WJ. 1987. The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J Clin Endocrinol Metab 65:1118–1126 [DOI] [PubMed] [Google Scholar]
- 137. Mulligan T , Iranmanesh A , Veldhuis JD. 2001. Pulsatile iv infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to midphysiological LH drive in the aging male. J Clin Endocrinol Metab 86:5547–5553 [DOI] [PubMed] [Google Scholar]
- 138. Harman SM , Tsitouras PD. 1980. Reproductive hormones in aging men. I. Measurement of sex steroids, basal luteinizing hormone, and Leydig cell response to human chorionic gonadotropin. J Clin Endocrinol Metab 51:35–40 [DOI] [PubMed] [Google Scholar]
- 139. Liu PY , Takahashi PY , Roebuck PD , Iranmanesh A , Veldhuis JD. 2005. Aging in healthy men impairs recombinant human luteinizing hormone (LH)-stimulated testosterone secretion monitored under a two-day intravenous pulsatile LH clamp. J Clin Endocrinol Metab 90:5544–5550 [DOI] [PubMed] [Google Scholar]
- 140. Takahashi PY , Votruba P , Abu-Rub M , Mielke K , Veldhuis JD. 2007. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab 92:3626–3632 [DOI] [PubMed] [Google Scholar]
- 141. Kaufman JM , Giri M , Deslypere JM , Thomas G , Vermeulen A. 1991. Influence of age on the responsiveness of the gonadotrophs to luteinizing hormone-releasing hormone in males. J Clin Endocrinol Metab 72:1255–1260 [DOI] [PubMed] [Google Scholar]
- 142. Veldhuis JD , Urban RJ , Lizarralde G , Johnson ML , Iranmanesh A. 1992. Attenuation of luteinizing hormone secretory burst amplitude as a proximate basis for the hypoandrogenism of healthy aging in men. J Clin Endocrinol Metab 75:707–713 [DOI] [PubMed] [Google Scholar]
- 143. Veldhuis JD , Zwart A , Mulligan T , Iranmanesh A. 2001. Muting of androgen negative feedback unveils impoverished gonadotropin-releasing hormone/luteinizing hormone secretory reactivity in healthy older men. J Clin Endocrinol Metab 86:529–535 [DOI] [PubMed] [Google Scholar]
- 144. Veldhuis JD , Iranmanesh A. 2005. Short-term aromatase-enzyme blockade unmasks impaired feedback adaptations in luteinizing hormone and testosterone secretion in older men. J Clin Endocrinol Metab 90:211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gray A , Feldman HA , McKinlay JB , Longcope C. 1991. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 73:1016–1025 [DOI] [PubMed] [Google Scholar]
- 146. Orwoll E , Lambert LC , Marshall LM , Phipps K , Blank J , Barrett-Connor E , Cauley J , Ensrud K , Cummings S. 2006. Testosterone and estradiol among older men. J Clin Endocrinol Metab 91:1336–1344 [DOI] [PubMed] [Google Scholar]
- 147. Yeap BB , Almeida OP , Hyde Z , Norman PE , Chubb SA , Jamrozik K , Flicker L. 2007. In men older than 70 years, total testosterone remains stable while free testosterone declines with age. The Health in Men Study. Eur J Endocrinol 156:585–594 [DOI] [PubMed] [Google Scholar]
- 148. Simon D , Preziosi P , Barrett-Connor E , Roger M , Saint-Paul M , Nahoul K , Papoz L. 1992. The influence of aging on plasma sex hormones in men: the Telecom Study. Am J Epidemiol 135:783–791 [DOI] [PubMed] [Google Scholar]
- 149. Svartberg J , Midtby M , Bønaa KH , Sundsfjord J , Joakimsen RM , Jorde R. 2003. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. Eur J Endocrinol 149:145–152 [DOI] [PubMed] [Google Scholar]
- 150. Zmuda JM , Cauley JA , Kriska A , Glynn NW , Gutai JP , Kuller LH. 1997. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol 146:609–617 [DOI] [PubMed] [Google Scholar]
- 151. Morley JE , Kaiser FE , Perry HM , Patrick P , Morley PM , Stauber PM , Vellas B , Baumgartner RN , Garry PJ. 1997. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 46:410–413 [DOI] [PubMed] [Google Scholar]
- 152. Feldman HA , Longcope C , Derby CA , Johannes CB , Araujo AB , Coviello AD , Bremner WJ , McKinlay JB. 2002. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 87:589–598 [DOI] [PubMed] [Google Scholar]
- 153. Araujo AB , O'Donnell AB , Brambilla DJ , Simpson WB , Longcope C , Matsumoto AM , McKinlay JB. 2004. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 89:5920–5926 [DOI] [PubMed] [Google Scholar]
- 154. Nieschlag E , Lammers U , Freischem CW , Langer K , Wickings EJ. 1982. Reproductive functions in young fathers and grandfathers. J Clin Endocrinol Metab 55:676–681 [DOI] [PubMed] [Google Scholar]
- 155. Kalme T , Seppälä M , Qiao Q , Koistinen R , Nissinen A , Harrela M , Loukovaara M , Leinonen P , Tuomilehto J. 2005. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J Clin Endocrinol Metab 90:1550–1556 [DOI] [PubMed] [Google Scholar]
- 156. Laughlin GA , Barrett-Connor E , Bergstrom J. 2008. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yeap BB , Hyde Z , Almeida OP , Norman PE , Chubb SA , Jamrozik K , Flicker L , Hankey GJ. 2009. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab 94:2353–2359 [DOI] [PubMed] [Google Scholar]
- 158. Khaw KT , Dowsett M , Folkerd E , Bingham S , Wareham N , Luben R , Welch A , Day N. 2007. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 116:2694–2701 [DOI] [PubMed] [Google Scholar]
- 159. Muller M , Grobbee DE , den Tonkelaar I , Lamberts SW , van der Schouw YT. 2005. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab 90:2618–2623 [DOI] [PubMed] [Google Scholar]
- 160. Araujo AB , Kupelian V , Page ST , Handelsman DJ , Bremner WJ , McKinlay JB. 2007. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med 167:1252–1260 [DOI] [PubMed] [Google Scholar]
- 161. Kupelian V , Page ST , Araujo AB , Travison TG , Bremner WJ , McKinlay JB. 2006. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab 91:843–850 [DOI] [PubMed] [Google Scholar]
- 162. Kupelian V , Hayes FJ , Link CL , Rosen R , McKinlay JB. 2008. Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. J Clin Endocrinol Metab 93:3403–3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chubb SA , Hyde Z , Almeida OP , Flicker L , Norman PE , Jamrozik K , Hankey GJ , Yeap BB. 2008. Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. Eur J Endocrinol 158:785–792 [DOI] [PubMed] [Google Scholar]
- 164. Peiris AN , Stagner JI , Plymate SR , Vogel RL , Heck M , Samols E. 1993. Relationship of insulin secretory pulses to sex hormone-binding globulin in normal men. J Clin Endocrinol Metab 76:279–282 [DOI] [PubMed] [Google Scholar]
- 165. Plymate SR , Matej LA , Jones RE , Friedl KE. 1988. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67:460–464 [DOI] [PubMed] [Google Scholar]
- 166. Yki-Järvinen H , Mäkimattila S , Utriainen T , Rutanen EM. 1995. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab 80:3227–3232 [DOI] [PubMed] [Google Scholar]
- 167. Yeap BB , Chubb SA , Hyde Z , Jamrozik K , Hankey GJ , Flicker L , Norman PE. 2009. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health in Men Study. Eur J Endocrinol 161:591–598 [DOI] [PubMed] [Google Scholar]
- 168. Lakshman KM , Bhasin S , Araujo AB. 2010. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. J Gerontol A Biol Sci Med Sci 65:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wu FC , von Eckardstein A. 2003. Androgens and coronary artery disease. Endocr Rev 24:183–217 [DOI] [PubMed] [Google Scholar]
- 170. Muller M , van der Schouw YT , Thijssen JH , Grobbee DE. 2003. Endogenous sex hormones and cardiovascular disease in men. J Clin Endocrinol Metab 88:5076–5086 [DOI] [PubMed] [Google Scholar]
- 171. Roy TA , Blackman MR , Harman SM , Tobin JD , Schrager M , Metter EJ. 2002. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab 283:E284–E294 [DOI] [PubMed] [Google Scholar]
- 172. van den Beld AW , de Jong FH , Grobbee DE , Pols HA , Lamberts SW. 2000. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 85:3276–3282 [DOI] [PubMed] [Google Scholar]
- 173. Schaap LA , Pluijm SM , Smit JH , van Schoor NM , Visser M , Gooren LJ , Lips P. 2005. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 63:152–160 [DOI] [PubMed] [Google Scholar]
- 174. Araujo AB , Travison TG , Bhasin S , Esche GR , Williams RE , Clark RV , McKinlay JB. 2008. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc 56:2000–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mohr BA , Bhasin S , Kupelian V , Araujo AB , O'Donnell AB , McKinlay JB. 2007. Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc 55:548–555 [DOI] [PubMed] [Google Scholar]
- 176. Krasnoff JB , Basaria S , Pencina MJ , Jasuja GK , Vasan RS , Ulloor J , Zhang A , Coviello A , Kelly-Hayes M , D'Agostino RB , Wolf PA , Bhasin S , Murabito JM. 2010. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 95:2790–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Orwoll E , Lambert LC , Marshall LM , Blank J , Barrett-Connor E , Cauley J , Ensrud K , Cummings SR. 2006. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med 166:2124–2131 [DOI] [PubMed] [Google Scholar]
- 178. Schaap LA , Pluijm SM , Deeg DJ , Penninx BW , Nicklas BJ , Lips P , Harris TB , Newman AB , Kritchevsky SB , Cauley JA , Goodpaster BH , Tylavsky FA , Yaffe K , Visser M. 2008. Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clin Endocrinol (Oxf) 68:42–50 [DOI] [PubMed] [Google Scholar]
- 179. Jackson JA , Riggs MW , Spiekerman AM. 1992. Testosterone deficiency as a risk factor for hip fractures in men: a case-control study. Am J Med Sci 304:4–8 [DOI] [PubMed] [Google Scholar]
- 180. Fink HA , Ewing SK , Ensrud KE , Barrett-Connor E , Taylor BC , Cauley JA , Orwoll ES. 2006. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 91:3908–3915 [DOI] [PubMed] [Google Scholar]
- 181. Center JR , Nguyen TV , Sambrook PN , Eisman JA. 1999. Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J Clin Endocrinol Metab 84:3626–3635 [DOI] [PubMed] [Google Scholar]
- 182. Greendale GA , Edelstein S , Barrett-Connor E. 1997. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 12:1833–1843 [DOI] [PubMed] [Google Scholar]
- 183. Szulc P , Claustrat B , Marchand F , Delmas PD. 2003. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. J Clin Endocrinol Metab 88:5240–5247 [DOI] [PubMed] [Google Scholar]
- 184. Mellström D , Johnell O , Ljunggren O , Eriksson AL , Lorentzon M , Mallmin H , Holmberg A , Redlund-Johnell I , Orwoll E , Ohlsson C. 2006. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 21:529–535 [DOI] [PubMed] [Google Scholar]
- 185. Araujo AB , Travison TG , Leder BZ , McKinlay JB. 2008. Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. J Clin Endocrinol Metab 93:2135–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Scopacasa F , Wishart JM , Need AG , Horowitz M , Morris HA , Nordin BE. 2002. Bone density and bone-related biochemical variables in normal men: a longitudinal study. J Gerontol A Biol Sci Med Sci 57:M385–M391 [DOI] [PubMed] [Google Scholar]
- 187. Khosla S , Melton LJ , Atkinson EJ , O'Fallon WM. 2001. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab 86:3555–3561 [DOI] [PubMed] [Google Scholar]
- 188. Amin S , Zhang Y , Sawin CT , Evans SR , Hannan MT , Kiel DP , Wilson PW , Felson DT. 2000. Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham study. Ann Intern Med 133:951–963 [DOI] [PubMed] [Google Scholar]
- 189. Gennari L , Merlotti D , Martini G , Gonnelli S , Franci B , Campagna S , Lucani B , Dal Canto N , Valenti R , Gennari C , Nuti R. 2003. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab 88:5327–5333 [DOI] [PubMed] [Google Scholar]
- 190. Khosla S , Melton LJ , Atkinson EJ , O'Fallon WM , Klee GG , Riggs BL. 1998. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 [DOI] [PubMed] [Google Scholar]
- 191. LeBlanc ES , Nielson CM , Marshall LM , Lapidus JA , Barrett-Connor E , Ensrud KE , Hoffman AR , Laughlin G , Ohlsson C , Orwoll ES. 2009. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab 94:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Matsumoto AM. 2002. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci 57:M76–M99 [DOI] [PubMed] [Google Scholar]
- 193. Henderson LP , Penatti CA , Jones BL , Yang P , Clark AS. 2006. Anabolic androgenic steroids and forebrain GABAergic transmission. Neuroscience 138:793–799 [DOI] [PubMed] [Google Scholar]
- 194. Sumner BE , Fink G. 1998. Testosterone as well as estrogen increases serotonin 2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res 59:205–214 [DOI] [PubMed] [Google Scholar]
- 195. Almeida OP , Barclay L. 2001. Sex hormones and their impact on dementia and depression: a clinical perspective. Expert Opin Pharmacother 2:527–535 [DOI] [PubMed] [Google Scholar]
- 196. Barrett-Connor E , Von Mühlen DG , Kritz-Silverstein D. 1999. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab 84:573–577 [DOI] [PubMed] [Google Scholar]
- 197. Almeida OP , Yeap BB , Hankey GJ , Jamrozik K , Flicker L. 2008. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry 65:283–289 [DOI] [PubMed] [Google Scholar]
- 198. Shores MM , Sloan KL , Matsumoto AM , Moceri VM , Felker B , Kivlahan DR. 2004. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 61:162–167 [DOI] [PubMed] [Google Scholar]
- 199. Seidman SN , Araujo AB , Roose SP , Devanand DP , Xie S , Cooper TB , McKinlay JB. 2002. Low testosterone levels in elderly men with dysthymic disorder. Am J Psychiatry 159:456–459 [DOI] [PubMed] [Google Scholar]
- 200. Seidman SN , Araujo AB , Roose SP , McKinlay JB. 2001. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol Psychiatry 50:371–376 [DOI] [PubMed] [Google Scholar]
- 201. T'Sjoen GG , De Vos S , Goemaere S , Van Pottelbergh I , Dierick M , Van Heeringen C , Kaufman JM. 2005. Sex steroid level, androgen receptor polymorphism, and depressive symptoms in healthy elderly men. J Am Geriatr Soc 53:636–642 [DOI] [PubMed] [Google Scholar]
- 202. Levitt AJ , Joffe RT. 1988. Total and free testosterone in depressed men. Acta Psychiatr Scand 77:346–348 [DOI] [PubMed] [Google Scholar]
- 203. Kaneda Y , Fujii A. 2002. No relationship between testosterone levels and depressive symptoms in aging men. Eur Psychiatry 17:411–413 [DOI] [PubMed] [Google Scholar]
- 204. Rubin RT , Poland RE , Lesser IM. 1989. Neuroendocrine aspects of primary endogenous depression VIII. Pituitary-gonadal axis activity in male patients and matched control subjects. Psychoneuroendocrinology 14:217–229 [DOI] [PubMed] [Google Scholar]
- 205. Wang C , Alexander G , Berman N , Salehian B , Davidson T , McDonald V , Steiner B , Hull L , Callegari C , Swerdloff RS. 1996. Testosterone replacement therapy improves mood in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab 81:3578–3583 [DOI] [PubMed] [Google Scholar]
- 206. Jockenhövel F , Minnemann T , Schubert M , Freude S , Hübler D , Schumann C , Christoph A , Gooren L , Ernst M. 2009. Timetable of effects of testosterone administration to hypogonadal men on variables of sex and mood. Aging Male 12:113–118 [DOI] [PubMed] [Google Scholar]
- 207. Seidman SN , Spatz E , Rizzo C , Roose SP. 2001. Testosterone replacement therapy for hypogonadal men with major depressive disorder: a randomized, placebo-controlled clinical trial. J Clin Psychiatry 62:406–412 [DOI] [PubMed] [Google Scholar]
- 208. Pope HG , Cohane GH , Kanayama G , Siegel AJ , Hudson JI. 2003. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 160:105–111 [DOI] [PubMed] [Google Scholar]
- 209. Grinspoon S , Corcoran C , Stanley T , Baaj A , Basgoz N , Klibanski A. 2000. Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. J Clin Endocrinol Metab 85:60–65 [DOI] [PubMed] [Google Scholar]
- 210. Sternbach H. 1998. Age-associated testosterone decline in men: clinical issues for psychiatry. Am J Psychiatry 155:1310–1318 [DOI] [PubMed] [Google Scholar]
- 211. Barrett-Connor E , Goodman-Gruen D , Patay B. 1999. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab 84:3681–3685 [DOI] [PubMed] [Google Scholar]
- 212. Beauchet O. 2006. Testosterone and cognitive function: current clinical evidence of a relationship. Eur J Endocrinol 155:773–781 [DOI] [PubMed] [Google Scholar]
- 213. Gola M , Bonadonna S , Doga M , Giustina A. 2005. Clinical review: growth hormone and cardiovascular risk factors. J Clin Endocrinol Metab 90:1864–1870 [DOI] [PubMed] [Google Scholar]
- 214. Bülow B , Hagmar L , Eskilsson J , Erfurth EM. 2000. Hypopituitary females have a high incidence of cardiovascular morbidity and an increased prevalence of cardiovascular risk factors. J Clin Endocrinol Metab 85:574–584 [DOI] [PubMed] [Google Scholar]
- 215. Colao A , Di Somma C , Rota F , Pivonello R , Savanelli MC , Spiezia S , Lombardi G. 2005. Short-term effects of growth hormone (GH) treatment or deprivation on cardiovascular risk parameters and intima-media thickness at carotid arteries in patients with severe GH deficiency. J Clin Endocrinol Metab 90:2056–2062 [DOI] [PubMed] [Google Scholar]
- 216. Colao A , Di Somma C , Spiezia S , Savastano S , Rota F , Savanelli MC , Lombardi G. 2008. Growth hormone treatment on atherosclerosis: results of a 5-year open, prospective, controlled study in male patients with severe growth hormone deficiency. J Clin Endocrinol Metab 93:3416–3424 [DOI] [PubMed] [Google Scholar]
- 217. Pfeifer M , Verhovec R , Zizek B , Prezelj J , Poredos P , Clayton RN. 1999. Growth hormone (GH) treatment reverses early atherosclerotic changes in GH-deficient adults. J Clin Endocrinol Metab 84:453–457 [DOI] [PubMed] [Google Scholar]
- 218. Böger RH , Skamira C , Bode-Böger SM , Brabant G , von zur Muhlen A , Frolich JC. 1996. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest 98:2706–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Thum T , Fleissner F , Klink I , Tsikas D , Jakob M , Bauersachs J , Stichtenoth DO. 2007. Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I. J Clin Endocrinol Metab 92:4172–4179 [DOI] [PubMed] [Google Scholar]
- 220. Perticone F , Sciacqua A , Perticone M , Laino I , Miceli S , Care' I , Galiano Leone G , Andreozzi F , Maio R , Sesti G. 2008. Low-plasma insulin-like growth factor-I levels are associated with impaired endothelium-dependent vasodilatation in a cohort of untreated, hypertensive Caucasian subjects. J Clin Endocrinol Metab 93:2806–2810 [DOI] [PubMed] [Google Scholar]
- 221. Spallarossa P , Brunelli C , Minuto F , Caruso D , Battistini M , Caponnetto S , Cordera R. 1996. Insulin-like growth factor-I and angiographically documented coronary artery disease. Am J Cardiol 77:200–202 [DOI] [PubMed] [Google Scholar]
- 222. Janssen JA , Stolk RP , Pols HA , Grobbee DE , Lamberts SW. 1998. Serum total IGF-I, free IGF-I, and IGFB-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arterioscler Thromb Vasc Biol 18:277–282 [DOI] [PubMed] [Google Scholar]
- 223. van den Beld AW , Bots ML , Janssen JA , Pols HA , Lamberts SW , Grobbee DE. 2003. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol 157:25–31 [DOI] [PubMed] [Google Scholar]
- 224. Fischer F , Schulte H , Mohan S , Tataru MC , Köhler E , Assmann G , von Eckardstein A. 2004. Associations of insulin-like growth factors, insulin-like growth factor binding proteins and acid-labile subunit with coronary heart disease. Clin Endocrinol (Oxf) 61:595–602 [DOI] [PubMed] [Google Scholar]
- 225. Ruotolo G , Båvenholm P , Brismar K , Eféndic S , Ericsson CG , de Faire U , Nilsson J , Hamsten A. 2000. Serum insulin-like growth factor-I level is independently associated with coronary artery disease progression in young male survivors of myocardial infarction: beneficial effects of bezafibrate treatment. J Am Coll Cardiol 35:647–654 [DOI] [PubMed] [Google Scholar]
- 226. Kawachi S , Takeda N , Sasaki A , Kokubo Y , Takami K , Sarui H , Hayashi M , Yamakita N , Yasuda K. 2005. Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 are associated with early carotid atherosclerosis. Arterioscler Thromb Vasc Biol 25:617–621 [DOI] [PubMed] [Google Scholar]
- 227. Juul A , Scheike T , Davidsen M , Gyllenborg J , Jørgensen T. 2002. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 106:939–944 [DOI] [PubMed] [Google Scholar]
- 228. Laughlin GA , Barrett-Connor E , Criqui MH , Kritz-Silverstein D. 2004. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab 89:114–120 [DOI] [PubMed] [Google Scholar]
- 229. Johnsen SP , Hundborg HH , Sørensen HT , Orskov H , Tjønneland A , Overvad K , Jørgensen JO. 2005. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab 90:5937–5941 [DOI] [PubMed] [Google Scholar]
- 230. Friedrich N , Haring R , Nauck M , Lüdemann J , Rosskopf D , Spilcke-Liss E , Felix SB , Dörr M , Brabant G , Völzke H , Wallaschofski H. 2009. Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab 94:1732–1739 [DOI] [PubMed] [Google Scholar]
- 231. Brugts MP , van den Beld AW , Hofland LJ , van der Wansem K , van Koetsveld PM , Frystyk J , Lamberts SW , Janssen JA. 2008. Low circulating insulin-like growth factor I bioactivity in elderly men is associated with increased mortality. J Clin Endocrinol Metab 93:2515–2522 [DOI] [PubMed] [Google Scholar]
- 232. Kaplan RC , McGinn AP , Pollak MN , Kuller LH , Strickler HD , Rohan TE , Cappola AR , Xue X , Psaty BM. 2007. Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. J Clin Endocrinol Metab 92:1319–1325 [DOI] [PubMed] [Google Scholar]
- 233. Schneider HJ , Klotsche J , Saller B , Böhler S , Sievers C , Pittrow D , Ruf G , März W , Erwa W , Zeiher AM , Silber S , Lehnert H , Wittchen HU , Stalla GK. 2008. Associations of age-dependent IGF-I SDS with cardiovascular diseases and risk conditions: cross-sectional study in 6773 primary care patients. Eur J Endocrinol 158:153–161 [DOI] [PubMed] [Google Scholar]
- 234. Andreassen M , Raymond I , Kistorp C , Hildebrandt P , Faber J , Kristensen LØ. 2009. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol 160:25–31 [DOI] [PubMed] [Google Scholar]
- 235. Yeap BB , Chubb SA , Ho KK , Setoh JW , McCaul KA , Norman PE , Jamrozik K , Flicker L. 2010. IGF1 and its binding proteins 3 and 1 are differentially associated with metabolic syndrome in older men. Eur J Endocrinol 162:249–257 [DOI] [PubMed] [Google Scholar]
- 236. Osterziel KJ , Ranke MB , Strohm O , Dietz R. 2000. The somatotrophic system in patients with dilated cardiomyopathy: relation of insulin-like growth factor-1 and its alterations during growth hormone therapy to cardiac function. Clin Endocrinol (Oxf) 53:61–68 [DOI] [PubMed] [Google Scholar]
- 237. Anker SD , Volterrani M , Pflaum CD , Strasburger CJ , Osterziel KJ , Doehner W , Ranke MB , Poole-Wilson PA , Giustina A , Dietz R , Coats AJ. 2001. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol 38:443–452 [DOI] [PubMed] [Google Scholar]
- 238. Vasan RS , Sullivan LM , D'Agostino RB , Roubenoff R , Harris T , Sawyer DB , Levy D , Wilson PW. 2003. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med 139:642–648 [DOI] [PubMed] [Google Scholar]
- 239. Cuneo RC , Wilmshurst P , Lowy C , McGauley G , Sonksen PH. 1989. Cardiac failure responding to growth hormone. Lancet 1:838–839 [DOI] [PubMed] [Google Scholar]
- 240. Fazio S , Sabatini D , Capaldo B , Vigorito C , Giordano A , Guida R , Pardo F , Biondi B , Saccà L. 1996. A preliminary study of growth hormone in the treatment of dilated cardiomyopathy. N Engl J Med 334:809–814 [DOI] [PubMed] [Google Scholar]
- 241. Donath MY , Sütsch G , Yan XW , Piva B , Brunner HP , Glatz Y , Zapf J , Follath F , Froesch ER , Kiowski W. 1998. Acute cardiovascular effects of insulin-like growth factor I in patients with chronic heart failure. J Clin Endocrinol Metab 83:3177–3183 [DOI] [PubMed] [Google Scholar]
- 242. Osterziel KJ , Strohm O , Schuler J , Friedrich M , Hänlein D , Willenbrock R , Anker SD , Poole-Wilson PA , Ranke MB , Dietz R. 1998. Randomised, double-blind, placebo-controlled trial of human recombinant growth hormone in patients with chronic heart failure due to dilated cardiomyopathy. Lancet 351:1233–1237 [DOI] [PubMed] [Google Scholar]
- 243. Onder G , Liperoti R , Russo A , Soldato M , Capoluongo E , Volpato S , Cesari M , Ameglio F , Bernabei R , Landi F. 2006. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab 291:E829–E834 [DOI] [PubMed] [Google Scholar]
- 244. Kiel DP , Puhl J , Rosen CJ , Berg K , Murphy JB , MacLean DB. 1998. Lack of an association between insulin-like growth factor-I and body composition, muscle strength, physical performance or self-reported mobility among older persons with functional limitations. J Am Geriatr Soc 46:822–828 [DOI] [PubMed] [Google Scholar]
- 245. Cappola AR , Bandeen-Roche K , Wand GS , Volpato S , Fried LP. 2001. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab 86:4139–4146 [DOI] [PubMed] [Google Scholar]
- 246. Kostka T , Arsac LM , Patricot MC , Berthouze SE , Lacour JR , Bonnefoy M. 2000. Leg extensor power and dehydroepiandrosterone sulfate, insulin-like growth factor-I and testosterone in healthy active elderly people. Eur J Appl Physiol 82:83–90 [DOI] [PubMed] [Google Scholar]
- 247. Goodman-Gruen D , Barrett-Connor E. 1997. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol 145:970–976 [DOI] [PubMed] [Google Scholar]
- 248. Harris TB , Kiel D , Roubenoff R , Langlois J , Hannan M , Havlik R , Wilson P. 1997. Association of insulin-like growth factor-I with body composition, weight history, and past health behaviors in the very old: the Framingham Heart Study. J Am Geriatr Soc 45:133–139 [DOI] [PubMed] [Google Scholar]
- 249. Schoen RE , Schragin J , Weissfeld JL , Thaete FL , Evans RW , Rosen CJ , Kuller LH. 2002. Lack of association between adipose tissue distribution and IGF-1 and IGFBP-3 in men and women. Cancer Epidemiol Biomarkers Prev 11:581–586 [PubMed] [Google Scholar]
- 250. Rucker D , Ezzat S , Diamandi A , Khosravi J , Hanley DA. 2004. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf) 60:491–499 [DOI] [PubMed] [Google Scholar]
- 251. Salminen H , Sääf M , Ringertz H , Strender LE. 2008. The role of IGF-I and IGFBP-1 status and secondary hyperparathyroidism in relation to osteoporosis in elderly Swedish women. Osteoporos Int 19:201–209 [DOI] [PubMed] [Google Scholar]
- 252. Langlois JA , Rosen CJ , Visser M , Hannan MT , Harris T , Wilson PW , Kiel DP. 1998. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab 83:4257–4262 [DOI] [PubMed] [Google Scholar]
- 253. Janssen JA , Burger H , Stolk RP , Grobbee DE , de Jong FH , Lamberts SW , Pols HA. 1998. Gender-specific relationship between serum free and total IGF-I and bone mineral density in elderly men and women. Eur J Endocrinol 138:627–632 [DOI] [PubMed] [Google Scholar]
- 254. Patel MB , Arden NK , Masterson LM , Phillips DI , Swaminathan R , Syddall HE , Byrne CD , Wood PJ , Cooper C , Holt RI. 2005. Investigating the role of the growth hormone-insulin-like growth factor (GH-IGF) axis as a determinant of male bone mineral density (BMD). Bone 37:833–841 [DOI] [PubMed] [Google Scholar]
- 255. Blain H , Vuillemin A , Blain A , Guillemin F , De Talance N , Doucet B , Jeandel C. 2004. Age-related femoral bone loss in men: evidence for hyperparathyroidism and insulin-like growth factor-1 deficiency. J Gerontol A Biol Sci Med Sci 59:1285–1289 [DOI] [PubMed] [Google Scholar]
- 256. Khosla S , Melton LJ , Achenbach SJ , Oberg AL , Riggs BL. 2006. Hormonal and biochemical determinants of trabecular microstructure at the ultradistal radius in women and men. J Clin Endocrinol Metab 91:885–891 [DOI] [PubMed] [Google Scholar]
- 257. Finkelstein JS , Klibanski A , Neer RM. 1996. A longitudinal evaluation of bone mineral density in adult men with histories of delayed puberty. J Clin Endocrinol Metab 81:1152–1155 [DOI] [PubMed] [Google Scholar]
- 258. Taaffe DR , Cooper CS , Holloway L , Duret C , Marcus R. 1999. Lack of association of anabolic hormone status and muscle strength with regional and whole body bone mineral density in healthy men aged 60–79 years. Aging (Milano) 11:4–11 [PubMed] [Google Scholar]
- 259. Barrett-Connor E , Goodman-Gruen D. 1998. Gender differences in insulin-like growth factor and bone mineral density association in old age: the Rancho Bernardo Study. J Bone Miner Res 13:1343–1349 [DOI] [PubMed] [Google Scholar]
- 260. Rosen CJ , Kurland ES , Vereault D , Adler RA , Rackoff PJ , Craig WY , Witte S , Rogers J , Bilezikian JP. 1998. Association between serum insulin growth factor-I (IGF-I) and a simple sequence repeat in IGF-I gene: implications for genetic studies of bone mineral density. J Clin Endocrinol Metab 83:2286–2290 [DOI] [PubMed] [Google Scholar]
- 261. Rivadeneira F , Houwing-Duistermaat JJ , Vaessen N , Vergeer-Drop JM , Hofman A , Pols HA , Van Duijn CM , Uitterlinden AG. 2003. Association between an insulin-like growth factor I gene promoter polymorphism and bone mineral density in the elderly: the Rotterdam Study. J Clin Endocrinol Metab 88:3878–3884 [DOI] [PubMed] [Google Scholar]
- 262. Sugimoto T , Nishiyama K , Kuribayashi F , Chihara K. 1997. Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J Bone Miner Res 12:1272–1279 [DOI] [PubMed] [Google Scholar]
- 263. Lloyd ME , Hart DJ , Nandra D , McAlindon TE , Wheeler M , Doyle DV , Spector TD. 1996. Relation between insulin-like growth factor-I concentrations, osteoarthritis, bone density, and fractures in the general population: the Chingford study. Ann Rheum Dis 55:870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Janssen JA , Stolk RP , Pols HA , Grobbee DE , Lamberts SW. 1998. Serum free and total insulin-like growth factor-I, insulin-like growth factor binding protein-1 and insulin-like growth factor binding protein-3 levels in healthy elderly individuals. Relation to self-reported quality of health and disability. Gerontology 44:277–280 [DOI] [PubMed] [Google Scholar]
- 265. Raynaud-Simon A , Lafont S , Berr C , Dartigues JF , Baulieu EE , Le Bouc Y. 2001. Plasma insulin-like growth factor I levels in the elderly: relation to plasma dehydroepiandrosterone sulfate levels, nutritional status, health and mortality. Gerontology 47:198–206 [DOI] [PubMed] [Google Scholar]
- 266. Papadakis MA , Grady D , Tierney MJ , Black D , Wells L , Grunfeld C. 1995. Insulin-like growth factor 1 and functional status in healthy older men. J Am Geriatr Soc 43:1350–1355 [DOI] [PubMed] [Google Scholar]
- 267. Okereke OI , Kang JH , Ma J , Gaziano JM , Grodstein F. 2006. Midlife plasma insulin-like growth factor I and cognitive function in older men. J Clin Endocrinol Metab 91:4306–4312 [DOI] [PubMed] [Google Scholar]
- 268. Landi F , Capoluongo E , Russo A , Onder G , Cesari M , Lulli P , Minucci A , Pahor M , Zuppi C , Bernabei R. 2007. Free insulin-like growth factor-I and cognitive function in older persons living in community. Growth Horm IGF Res 17:58–66 [DOI] [PubMed] [Google Scholar]
- 269. Al-Delaimy WK , von Muhlen D , Barrett-Connor E. 2009. Insulin-like growth factor-1, insulin-like growth factor binding protein-1, and cognitive function in older men and women. J Am Geriatr Soc 57:1441–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Arwert LI , Deijen JB , Drent ML. 2005. The relation between insulin-like growth factor I levels and cognition in healthy elderly: a meta-analysis. Growth Horm IGF Res 15:416–422 [DOI] [PubMed] [Google Scholar]
- 271. Rollero A , Murialdo G , Fonzi S , Garrone S , Gianelli MV , Gazzerro E , Barreca A , Polleri A. 1998. Relationship between cognitive function, growth hormone and insulin-like growth factor I plasma levels in aged subjects. Neuropsychobiology 38:73–79 [DOI] [PubMed] [Google Scholar]
- 272. Sathiavageeswaran M , Burman P , Lawrence D , Harris AG , Falleti MG , Maruff P , Wass J. 2007. Effects of GH on cognitive function in elderly patients with adult-onset GH deficiency: a placebo-controlled 12-month study. Eur J Endocrinol 156:439–447 [DOI] [PubMed] [Google Scholar]
- 273. Molitch ME , Clemmons DR , Malozowski S , Merriam GR , Shalet SM , Vance ML , Stephens PA. 2006. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 91:1621–1634 [DOI] [PubMed] [Google Scholar]
- 274. Woodhouse LJ , Mukherjee A , Shalet SM , Ezzat S. 2006. The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr Rev 27:287–317 [DOI] [PubMed] [Google Scholar]
- 275. Hoffman DM , O'Sullivan AJ , Freund J , Ho KK. 1995. Adults with growth hormone deficiency have abnormal body composition but normal energy metabolism. J Clin Endocrinol Metab 80:72–77 [DOI] [PubMed] [Google Scholar]
- 276. Beshyah SA , Freemantle C , Shahi M , Anyaoku V , Merson S , Lynch S , Skinner E , Sharp P , Foale R , Johnston DG. 1995. Replacement treatment with biosynthetic human growth hormone in growth hormone-deficient hypopituitary adults. Clin Endocrinol (Oxf) 42:73–84 [DOI] [PubMed] [Google Scholar]
- 277. De Boer H , Blok GJ , Voerman HJ , De Vries PM , van der Veen EA. 1992. Body composition in adult growth hormone-deficient men, assessed by anthropometry and bioimpedance analysis. J Clin Endocrinol Metab 75:833–837 [DOI] [PubMed] [Google Scholar]
- 278. Bengtsson BA , Edén S , Lönn L , Kvist H , Stokland A , Lindstedt G , Bosaeus I , Tölli J , Sjöström L , Isaksson OG. 1993. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab 76:309–317 [DOI] [PubMed] [Google Scholar]
- 279. Weaver JU , Monson JP , Noonan K , John WG , Edwards A , Evans KA , Cunningham J. 1995. The effect of low dose recombinant human growth hormone replacement on regional fat distribution, insulin sensitivity, and cardiovascular risk factors in hypopituitary adults. J Clin Endocrinol Metab 80:153–159 [DOI] [PubMed] [Google Scholar]
- 280. Snel YE , Doerga ME , Brummer RM , Zelissen PM , Koppeschaar HP. 1995. Magnetic resonance imaging-assessed adipose tissue and serum lipid and insulin concentrations in growth hormone-deficient adults. Effect of growth hormone replacement. Arterioscler Thromb Vasc Biol 15:1543–1548 [DOI] [PubMed] [Google Scholar]
- 281. Christ ER , Cummings MH , Westwood NB , Sawyer BM , Pearson TC , Sönksen PH , Russell-Jones DL. 1997. The importance of growth hormone in the regulation of erythropoiesis, red cell mass, and plasma volume in adults with growth hormone deficiency. J Clin Endocrinol Metab 82:2985–2990 [DOI] [PubMed] [Google Scholar]
- 282. Møller J , Frandsen E , Fisker S , Jørgensen JO , Christiansen JS. 1996. Decreased plasma and extracellular volume in growth hormone deficient adults and the acute and prolonged effects of GH administration: a controlled experimental study. Clin Endocrinol (Oxf) 44:533–539 [DOI] [PubMed] [Google Scholar]
- 283. Janssen YJ , Deurenberg P , Roelfsema F. 1997. Using dilution techniques and multifrequency bioelectrical impedance to assess both total body water and extracellular water at baseline and during recombinant human growth hormone (GH) treatment in GH-deficient adults. J Clin Endocrinol Metab 82:3349–3355 [DOI] [PubMed] [Google Scholar]
- 284. Johannsson G , Grimby G , Sunnerhagen KS , Bengtsson BA. 1997. Two years of growth hormone (GH) treatment increase isometric and isokinetic muscle strength in GH-deficient adults. J Clin Endocrinol Metab 82:2877–2884 [DOI] [PubMed] [Google Scholar]
- 285. Rutherford OM , Jones DA , Round JM , Buchanan CR , Preece MA. 1991. Changes in skeletal muscle and body composition after discontinuation of growth hormone treatment in growth hormone deficient young adults. Clin Endocrinol (Oxf) 34:469–475 [DOI] [PubMed] [Google Scholar]
- 286. Janssen YJ , Doornbos J , Roelfsema F. 1999. Changes in muscle volume, strength, and bioenergetics during recombinant human growth hormone (GH) therapy in adults with GH deficiency. J Clin Endocrinol Metab 84:279–284 [DOI] [PubMed] [Google Scholar]
- 287. Cuneo RC , Salomon F , Wiles CM , Round JM , Jones D , Hesp R , Sönksen PH. 1992. Histology of skeletal muscle in adults with GH deficiency: comparison with normal muscle and response to GH treatment. Horm Res 37:23–28 [DOI] [PubMed] [Google Scholar]
- 288. Bottinelli R , Narici M , Pellegrino MA , Kayser B , Canepari M , Faglia G , Sartorio A. 1997. Contractile properties and fiber type distribution of quadriceps muscles in adults with childhood-onset growth hormone deficiency. J Clin Endocrinol Metab 82:4133–4138 [DOI] [PubMed] [Google Scholar]
- 289. Rutherford OM , Beshyah SA , Schott J , Watkins Y , Johnston DG. 1995. Contractile properties of the quadriceps muscle in growth hormone-deficient hypopituitary adults. Clin Sci (Lond) 88:67–71 [DOI] [PubMed] [Google Scholar]
- 290. Woodhouse LJ , Asa SL , Thomas SG , Ezzat S. 1999. Measures of submaximal aerobic performance evaluate and predict functional response to growth hormone (GH) treatment in GH-deficient adults. J Clin Endocrinol Metab 84:4570–4577 [DOI] [PubMed] [Google Scholar]
- 291. Hoffman DM , Pallasser R , Duncan M , Nguyen TV , Ho KK. 1998. How is whole body protein turnover perturbed in growth hormone-deficient adults? J Clin Endocrinol Metab 83:4344–4349 [DOI] [PubMed] [Google Scholar]
- 292. Cuneo RC , Salomon F , Wiles CM , Hesp R , Sönksen PH. 1991. Growth hormone treatment in growth hormone-deficient adults. II. Effects on exercise performance. J Appl Physiol 70:695–700 [DOI] [PubMed] [Google Scholar]
- 293. Attanasio AF , Lamberts SW , Matranga AM , Birkett MA , Bates PC , Valk NK , Hilsted J , Bengtsson BA , Strasburger CJ. 1997. Adult growth hormone (GH)-deficient patients demonstrate heterogeneity between childhood onset and adult onset before and during human GH treatment. Adult Growth Hormone Deficiency Study Group. J Clin Endocrinol Metab 82:82–88 [DOI] [PubMed] [Google Scholar]
- 294. Lissett CA , Murray RD , Shalet SM. 2002. Timing of onset of growth hormone deficiency is a major influence on insulin-like growth factor I status in adult life. Clin Endocrinol (Oxf) 57:35–40 [DOI] [PubMed] [Google Scholar]
- 295. Kaufman JM , Taelman P , Vermeulen A , Vandeweghe M. 1992. Bone mineral status in growth hormone-deficient males with isolated and multiple pituitary deficiencies of childhood onset. J Clin Endocrinol Metab 74:118–123 [DOI] [PubMed] [Google Scholar]
- 296. Colao A , Di Somma C , Pivonello R , Loche S , Aimaretti G , Cerbone G , Faggiano A , Corneli G , Ghigo E , Lombardi G. 1999. Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab 84:1919–1924 [DOI] [PubMed] [Google Scholar]
- 297. Holmes SJ , Economou G , Whitehouse RW , Adams JE , Shalet SM. 1994. Reduced bone mineral density in patients with adult onset growth hormone deficiency. J Clin Endocrinol Metab 78:669–674 [DOI] [PubMed] [Google Scholar]
- 298. Rosén T , Hansson T , Granhed H , Szucs J , Bengtsson BA. 1993. Reduced bone mineral content in adult patients with growth hormone deficiency. Acta Endocrinol (Copenh) 129:201–206 [DOI] [PubMed] [Google Scholar]
- 299. Murray RD , Columb B , Adams JE , Shalet SM. 2004. Low bone mass is an infrequent feature of the adult growth hormone deficiency syndrome in middle-age adults and the elderly. J Clin Endocrinol Metab 89:1124–1130 [DOI] [PubMed] [Google Scholar]
- 300. Wüster C , Abs R , Bengtsson BA , Bennmarker H , Feldt-Rasmussen U , Hernberg-Ståhl E , Monson JP , Westberg B , Wilton P. 2001. The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. J Bone Miner Res 16:398–405 [DOI] [PubMed] [Google Scholar]
- 301. Vestergaard P , Jørgensen JO , Hagen C , Hoeck HC , Laurberg P , Rejnmark L , Brixen K , Weeke J , Andersen M , Conceicao FL , Nielsen TL , Mosekilde L. 2002. Fracture risk is increased in patients with GH deficiency or untreated prolactinomas—a case-control study. Clin Endocrinol (Oxf) 56:159–167 [DOI] [PubMed] [Google Scholar]
- 302. Rosén T , Wilhelmsen L , Landin-Wilhelmsen K , Lappas G , Bengtsson BA. 1997. Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol 137:240–245 [DOI] [PubMed] [Google Scholar]
- 303. Svensson J , Bengtsson BA , Rosén T , Odén A , Johannsson G. 2004. Malignant disease and cardiovascular morbidity in hypopituitary adults with or without growth hormone replacement therapy. J Clin Endocrinol Metab 89:3306–3312 [DOI] [PubMed] [Google Scholar]
- 304. Besson A , Salemi S , Gallati S , Jenal A , Horn R , Mullis PS , Mullis PE. 2003. Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab 88:3664–3667 [DOI] [PubMed] [Google Scholar]
- 305. Bülow B , Hagmar L , Mikoczy Z , Nordström CH , Erfurth EM. 1997. Increased cerebrovascular mortality in patients with hypopituitarism. Clin Endocrinol (Oxf) 46:75–81 [DOI] [PubMed] [Google Scholar]
- 306. Tomlinson JW , Holden N , Hills RK , Wheatley K , Clayton RN , Bates AS , Sheppard MC , Stewart PM. 2001. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet 357:425–431 [DOI] [PubMed] [Google Scholar]
- 307. Stewart PM , Sheppard MC. 1999. Mortality and hypopituitarism. Growth Horm IGF Res 9(Suppl A):15–19 [DOI] [PubMed] [Google Scholar]
- 308. Cuneo RC , Salomon F , Watts GF , Hesp R , Sönksen PH. 1993. Growth hormone treatment improves serum lipids and lipoproteins in adults with growth hormone deficiency. Metabolism 42:1519–1523 [DOI] [PubMed] [Google Scholar]
- 309. Abdu TA , Neary R , Elhadd TA , Akber M , Clayton RN. 2001. Coronary risk in growth hormone deficient hypopituitary adults: increased predicted risk is due largely to lipid profile abnormalities. Clin Endocrinol (Oxf) 55:209–216 [DOI] [PubMed] [Google Scholar]
- 310. Johansson JO , Landin K , Tengborn L , Rosén T , Bengtsson BA. 1994. High fibrinogen and plasminogen activator inhibitor activity in growth hormone-deficient adults. Arterioscler Thromb 14:434–437 [DOI] [PubMed] [Google Scholar]
- 311. Abs R , Feldt-Rasmussen U , Mattsson AF , Monson JP , Bengtsson BA , Góth MI , Wilton P , Koltowska-Häggström M. 2006. Determinants of cardiovascular risk in 2589 hypopituitary GH-deficient adults—a KIMS database analysis. Eur J Endocrinol 155:79–90 [DOI] [PubMed] [Google Scholar]
- 312. Cummings MH , Watts GF , Umpleby AM , Hennessy TR , Naoumova R , Slavin BM , Thompson GR , Sönksen PH. 1995. Increased hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in NIDDM. Diabetologia 38:959–967 [DOI] [PubMed] [Google Scholar]
- 313. Cummings MH , Christ E , Umpleby AM , Albany E , Wierzbicki A , Lumb PJ , Sönksen PH , Russell-Jones DL. 1997. Abnormalities of very low density lipoprotein apolipoprotein B-100 metabolism contribute to the dyslipidaemia of adult growth hormone deficiency. J Clin Endocrinol Metab 82:2010–2013 [PubMed] [Google Scholar]
- 314. Millar JS , Lichtenstein AH , Cuchel M , Dolnikowski GG , Hachey DL , Cohn JS , Schaefer EJ. 1995. Impact of age on the metabolism of VLDL, IDL, and LDL apolipoprotein B-100 in men. J Lipid Res 36:1155–1167 [PubMed] [Google Scholar]
- 315. Nieves DJ , Cnop M , Retzlaff B , Walden CE , Brunzell JD , Knopp RH , Kahn SE. 2003. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 52:172–179 [DOI] [PubMed] [Google Scholar]
- 316. Cuneo RC , Salomon F , McGauley GA , Sönksen PH. 1992. The growth hormone deficiency syndrome in adults. Clin Endocrinol (Oxf) 37:387–397 [DOI] [PubMed] [Google Scholar]
- 317. Johansson JO , Fowelin J , Landin K , Lager I , Bengtsson BA. 1995. Growth hormone-deficient adults are insulin-resistant. Metabolism 44:1126–1129 [DOI] [PubMed] [Google Scholar]
- 318. Hew FL , Koschmann M , Christopher M , Rantzau C , Vaag A , Ward G , Beck-Nielsen H , Alford F. 1996. Insulin resistance in growth hormone-deficient adults: defects in glucose utilization and glycogen synthase activity. J Clin Endocrinol Metab 81:555–564 [DOI] [PubMed] [Google Scholar]
- 319. Salomon F , Cuneo RC , Umpleby AM , Sönksen PH. 1994. Glucose and fat metabolism in adults with growth hormone deficiency. Clin Endocrinol (Oxf) 41:315–322 [DOI] [PubMed] [Google Scholar]
- 320. Markussis V , Beshyah SA , Fisher C , Parker KH , Nicolaides AN , Johnston DG. 1997. Abnormal carotid arterial wall dynamics in symptom-free hypopituitary adults. Eur J Endocrinol 136:157–164 [DOI] [PubMed] [Google Scholar]
- 321. Smith JC , Evans LM , Wilkinson I , Goodfellow J , Cockcroft JR , Scanlon MF , Davies JS. 2002. Effects of GH replacement on endothelial function and large-artery stiffness in GH-deficient adults: a randomized, double-blind, placebo-controlled study. Clin Endocrinol (Oxf) 56:493–501 [DOI] [PubMed] [Google Scholar]
- 322. Lehmann ED , Hopkins KD , Weissberger AJ , Gosling RG , Sönksen PH. 1993. Aortic distensibility in growth hormone deficient adults. Lancet 341:309. [DOI] [PubMed] [Google Scholar]
- 323. Maison P , Griffin S , Nicoue-Beglah M , Haddad N , Balkau B , Chanson P. 2004. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab 89:2192–2199 [DOI] [PubMed] [Google Scholar]
- 324. Attanasio AF , Mo D , Erfurth EM , Tan M , Ho KY , Kleinberg D , Zimmermann AG , Chanson P. 2010. Prevalence of metabolic syndrome in adult hypopituitary growth hormone (GH)-deficient patients before and after GH replacement. J Clin Endocrinol Metab 95:74–81 [DOI] [PubMed] [Google Scholar]
- 325. Leonsson M , Hulthe J , Oscarsson J , Johannsson G , Wendelhag I , Wikstrand J , Bengtsson BA. 2002. Intima-media thickness in cardiovascularly asymptomatic hypopituitary adults with growth hormone deficiency: relation to body mass index, gender, and other cardiovascular risk factors. Clin Endocrinol (Oxf) 57:751–759 [DOI] [PubMed] [Google Scholar]
- 326. Murata M , Kaji H , Mizuno I , Sakurai T , Iida K , Okimura Y , Chihara K. 2003. A study of carotid intima-media thickness in GH-deficient Japanese adults during onset among adults and children. Eur J Endocrinol 148:333–338 [DOI] [PubMed] [Google Scholar]
- 327. Markussis V , Beshyah SA , Fisher C , Sharp P , Nicolaides AN , Johnston DG. 1992. Detection of premature atherosclerosis by high-resolution ultrasonography in symptom-free hypopituitary adults. Lancet 340:1188–1192 [DOI] [PubMed] [Google Scholar]
- 328. Svensson J , Fowelin J , Landin K , Bengtsson BA , Johansson JO. 2002. Effects of seven years of GH-replacement therapy on insulin sensitivity in GH-deficient adults. J Clin Endocrinol Metab 87:2121–2127 [DOI] [PubMed] [Google Scholar]
- 329. Colao A , Marzullo P , Di Somma C , Lombardi G. 2001. Growth hormone and the heart. Clin Endocrinol (Oxf) 54:137–154 [DOI] [PubMed] [Google Scholar]
- 330. Colao A , Cuocolo A , Di Somma C , Cerbone G , Della Morte AM , Nicolai E , Lucci R , Salvatore M , Lombardi G. 1999. Impaired cardiac performance in elderly patients with growth hormone deficiency. J Clin Endocrinol Metab 84:3950–3955 [DOI] [PubMed] [Google Scholar]
- 331. Jallad RS , Liberman B , Vianna CB , Vieira ML , Ramires JA , Knoepfelmacher M. 2003. Effects of growth hormone replacement therapy on metabolic and cardiac parameters, in adult patients with childhood-onset growth hormone deficiency. Growth Horm IGF Res 13:81–88 [DOI] [PubMed] [Google Scholar]
- 332. Francis RM. 1999. The effects of testosterone on osteoporosis in men. Clin Endocrinol (Oxf) 50:411–414 [DOI] [PubMed] [Google Scholar]
- 333. Leifke E , Körner HC , Link TM , Behre HM , Peters PE , Nieschlag E. 1998. Effects of testosterone replacement therapy on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle area in hypogonadal men. Eur J Endocrinol 138:51–58 [DOI] [PubMed] [Google Scholar]
- 334. Benito M , Gomberg B , Wehrli FW , Weening RH , Zemel B , Wright AC , Song HK , Cucchiara A , Snyder PJ. 2003. Deterioration of trabecular architecture in hypogonadal men. J Clin Endocrinol Metab 88:1497–1502 [DOI] [PubMed] [Google Scholar]
- 335. Bertelloni S , Baroncelli GI , Battini R , Perri G , Saggese G. 1995. Short-term effect of testosterone treatment on reduced bone density in boys with constitutional delay of puberty. J Bone Miner Res 10:1488–1495 [DOI] [PubMed] [Google Scholar]
- 336. Horowitz M , Wishart JM , O'Loughlin PD , Morris HA , Need AG , Nordin BE. 1992. Osteoporosis and Klinefelter's syndrome. Clin Endocrinol (Oxf) 36:113–118 [DOI] [PubMed] [Google Scholar]
- 337. Vanderschueren D , Vandenput L , Boonen S , Lindberg MK , Bouillon R , Ohlsson C. 2004. Androgens and bone. Endocr Rev 25:389–425 [DOI] [PubMed] [Google Scholar]
- 338. Stĕpán JJ , Lachman M , Zvĕrina J , Pacovský V , Baylink DJ. 1989. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metab 69:523–527 [DOI] [PubMed] [Google Scholar]
- 339. Greenspan SL , Neer RM , Ridgway EC , Klibanski A. 1986. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann Intern Med 104:777–782 [DOI] [PubMed] [Google Scholar]
- 340. Finkelstein JS , Klibanski A , Neer RM , Doppelt SH , Rosenthal DI , Segre GV , Crowley WF. 1989. Increases in bone density during treatment of men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 69:776–783 [DOI] [PubMed] [Google Scholar]
- 341. Guo CY , Jones TH , Eastell R. 1997. Treatment of isolated hypogonadotropic hypogonadism effect on bone mineral density and bone turnover. J Clin Endocrinol Metab 82:658–665 [DOI] [PubMed] [Google Scholar]
- 342. Seeman E , Melton LJ , O'Fallon WM , Riggs BL. 1983. Risk factors for spinal osteoporosis in men. Am J Med 75:977–983 [DOI] [PubMed] [Google Scholar]
- 343. Smith EP , Boyd J , Frank GR , Takahashi H , Cohen RM , Specker B , Williams TC , Lubahn DB , Korach KS. 1994. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- 344. Carani C , Qin K , Simoni M , Faustini-Fustini M , Serpente S , Boyd J , Korach KS , Simpson ER. 1997. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95 [DOI] [PubMed] [Google Scholar]
- 345. Cauley JA , Ewing SK , Taylor BC , Fink HA , Ensrud KE , Bauer DC , Barrett-Connor E , Marshall L , Orwoll ES. 2010. Sex steroid hormones in older men: longitudinal associations with 4.5-year change in hip bone mineral density—the Osteoporotic Fractures in Men Study. J Clin Endocrinol Metab 95:4314–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Burnett-Bowie SA , McKay EA , Lee H , Leder BZ. 2009. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab 94:4785–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Colvard DS , Eriksen EF , Keeting PE , Wilson EM , Lubahn DB , French FS , Riggs BL , Spelsberg TC. 1989. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Acad Sci USA 86:854–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Leder BZ , LeBlanc KM , Schoenfeld DA , Eastell R , Finkelstein JS. 2003. Effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab 88:204–210 [DOI] [PubMed] [Google Scholar]
- 349. Fukui M , Kitagawa Y , Nakamura N , Kadono M , Mogami S , Hirata C , Ichio N , Wada K , Hasegawa G , Yoshikawa T. 2003. Association between serum testosterone concentration and carotid atherosclerosis in men with type 2 diabetes. Diabetes Care 26:1869–1873 [DOI] [PubMed] [Google Scholar]
- 350. Tchernof A , Labrie F , Bélanger A , Prud'homme D , Bouchard C , Tremblay A , Nadeau A , Després JP. 1997. Relationships between endogenous steroid hormone, sex hormone-binding globulin and lipoprotein levels in men: contribution of visceral obesity, insulin levels and other metabolic variables. Atherosclerosis 133:235–244 [DOI] [PubMed] [Google Scholar]
- 351. Khaw KT , Barrett-Connor E. 1991. Endogenous sex hormones, high density lipoprotein cholesterol, and other lipoprotein fractions in men. Arterioscler Thromb 11:489–494 [DOI] [PubMed] [Google Scholar]
- 352. Dai WS , Gutai JP , Kuller LH , Laporte RE , Falvo-Gerard L , Caggiula A. 1984. Relation between plasma high-density lipoprotein cholesterol and sex hormone concentrations in men. Am J Cardiol 53:1259–1263 [DOI] [PubMed] [Google Scholar]
- 353. Simon D , Charles MA , Nahoul K , Orssaud G , Kremski J , Hully V , Joubert E , Papoz L , Eschwege E. 1997. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: the Telecom Study. J Clin Endocrinol Metab 82:682–685 [DOI] [PubMed] [Google Scholar]
- 354. Maggio M , Basaria S , Ble A , Lauretani F , Bandinelli S , Ceda GP , Valenti G , Ling SM , Ferrucci L. 2006. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 91:345–347 [DOI] [PubMed] [Google Scholar]
- 355. Seidell JC , Björntorp P , Sjöström L , Kvist H , Sannerstedt R. 1990. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 39:897–901 [DOI] [PubMed] [Google Scholar]
- 356. Kapoor D , Malkin CJ , Channer KS , Jones TH. 2005. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol (Oxf) 63:239–250 [DOI] [PubMed] [Google Scholar]
- 357. Ding EL , Song Y , Malik VS , Liu S. 2006. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295:1288–1299 [DOI] [PubMed] [Google Scholar]
- 358. Dhindsa S , Prabhakar S , Sethi M , Bandyopadhyay A , Chaudhuri A , Dandona P. 2004. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 89:5462–5468 [DOI] [PubMed] [Google Scholar]
- 359. Stellato RK , Feldman HA , Hamdy O , Horton ES , McKinlay JB. 2000. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts Male Aging Study. Diabetes Care 23:490–494 [DOI] [PubMed] [Google Scholar]
- 360. Laaksonen DE , Niskanen L , Punnonen K , Nyyssönen K , Tuomainen TP , Valkonen VP , Salonen R , Salonen JT. 2004. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 27:1036–1041 [DOI] [PubMed] [Google Scholar]
- 361. Smith JC , Bennett S , Evans LM , Kynaston HG , Parmar M , Mason MD , Cockcroft JR , Scanlon MF , Davies JS. 2001. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 86:4261–4267 [DOI] [PubMed] [Google Scholar]
- 362. Yialamas MA , Dwyer AA , Hanley E , Lee H , Pitteloud N , Hayes FJ. 2007. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 92:4254–4259 [DOI] [PubMed] [Google Scholar]
- 363. Bhasin S. 1992. Clinical review 34: androgen treatment of hypogonadal men. J Clin Endocrinol Metab 74:1221–1225 [DOI] [PubMed] [Google Scholar]
- 364. Oppenheim DS , Greenspan SL , Zervas NT , Schoenfeld DA , Klibanski A. 1989. Elevated serum lipids in hypogonadal men with and without hyperprolactinemia. Ann Intern Med 111:288–292 [DOI] [PubMed] [Google Scholar]
- 365. Chauhan AK , Katiyar BC , Misra S , Thacker AK , Singh NK. 1986. Muscle dysfunction in male hypogonadism. Acta Neurol Scand 73:466–471 [DOI] [PubMed] [Google Scholar]
- 366. Pei D , Sheu WH , Jeng CY , Liao WK , Fuh MM. 1998. Insulin resistance in patients with Klinefelter's syndrome and idiopathic gonadotropin deficiency. J Formos Med Assoc 97:534–540 [PubMed] [Google Scholar]
- 367. Swerdlow AJ , Higgins CD , Schoemaker MJ , Wright AF , Jacobs PA. 2005. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab 90:6516–6522 [DOI] [PubMed] [Google Scholar]
- 368. Bojesen A , Juul S , Birkebaek NH , Gravholt CH. 2006. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab 91:1254–1260 [DOI] [PubMed] [Google Scholar]
- 369. Bojesen A , Kristensen K , Birkebaek NH , Fedder J , Mosekilde L , Bennett P , Laurberg P , Frystyk J , Flyvbjerg A , Christiansen JS , Gravholt CH. 2006. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 29:1591–1598 [DOI] [PubMed] [Google Scholar]
- 370. Katznelson L , Rosenthal DI , Rosol MS , Anderson EJ , Hayden DL , Schoenfeld DA , Klibanski A. 1998. Using quantitative CT to assess adipose distribution in adult men with acquired hypogonadism. AJR Am J Roentgenol 170:423–427 [DOI] [PubMed] [Google Scholar]
- 371. Enzi G , Gasparo M , Biondetti PR , Fiore D , Semisa M , Zurlo F. 1986. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr 44:739–746 [DOI] [PubMed] [Google Scholar]
- 372. Woodhouse LJ , Gupta N , Bhasin M , Singh AB , Ross R , Phillips J , Bhasin S. 2004. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab 89:718–726 [DOI] [PubMed] [Google Scholar]
- 373. Ramirez ME , McMurry MP , Wiebke GA , Felten KJ , Ren K , Meikle AW , Iverius PH. 1997. Evidence for sex steroid inhibition of lipoprotein lipase in men: comparison of abdominal and femoral adipose tissue. Metabolism 46:179–185 [DOI] [PubMed] [Google Scholar]
- 374. Mårin P , Odén B , Björntorp P. 1995. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab 80:239–243 [DOI] [PubMed] [Google Scholar]
- 375. Davidson JM , Camargo CA , Smith ER. 1979. Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab 48:955–958 [DOI] [PubMed] [Google Scholar]
- 376. Kwan M , Greenleaf WJ , Mann J , Crapo L , Davidson JM. 1983. The nature of androgen action on male sexuality: a combined laboratory-self-report study on hypogonadal men. J Clin Endocrinol Metab 57:557–562 [DOI] [PubMed] [Google Scholar]
- 377. Jockenhövel F , Vogel E , Reinhardt W , Reinwein D. 1997. Effects of various modes of androgen substitution therapy on erythropoiesis. Eur J Med Res 2:293–298 [PubMed] [Google Scholar]
- 378. Behre HM , Bohmeyer J , Nieschlag E. 1994. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol (Oxf) 40:341–349 [DOI] [PubMed] [Google Scholar]
- 379. Behre HM , Kliesch S , Leifke E , Link TM , Nieschlag E. 1997. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab 82:2386–2390 [DOI] [PubMed] [Google Scholar]
- 380. Greenspan SL , Oppenheim DS , Klibanski A. 1989. Importance of gonadal steroids to bone mass in men with hyperprolactinemic hypogonadism. Ann Intern Med 110:526–531 [DOI] [PubMed] [Google Scholar]
- 381. Wang C , Cunningham G , Dobs A , Iranmanesh A , Matsumoto AM , Snyder PJ , Weber T , Berman N , Hull L , Swerdloff RS. 2004. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 89:2085–2098 [DOI] [PubMed] [Google Scholar]
- 382. Aminorroaya A , Kelleher S , Conway AJ , Ly LP , Handelsman DJ. 2005. Adequacy of androgen replacement influences bone density response to testosterone in androgen-deficient men. Eur J Endocrinol 152:881–886 [DOI] [PubMed] [Google Scholar]
- 383. Wang C , Swerdloff RS , Iranmanesh A , Dobs A , Snyder PJ , Cunningham G , Matsumoto AM , Weber T , Berman N. 2001. Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin Endocrinol (Oxf) 54:739–750 [DOI] [PubMed] [Google Scholar]
- 384. Wang C , Eyre DR , Clark R , Kleinberg D , Newman C , Iranmanesh A , Veldhuis J , Dudley RE , Berman N , Davidson T , Barstow TJ , Sinow R , Alexander G , Swerdloff RS. 1996. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab 81:3654–3662 [DOI] [PubMed] [Google Scholar]
- 385. Bhasin S , Storer TW , Berman N , Yarasheski KE , Clevenger B , Phillips J , Lee WP , Bunnell TJ , Casaburi R. 1997. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82:407–413 [DOI] [PubMed] [Google Scholar]
- 386. Swerdloff RS , Wang C , Cunningham G , Dobs A , Iranmanesh A , Matsumoto AM , Snyder PJ , Weber T , Longstreth J , Berman N. 2000. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab 85:4500–4510 [DOI] [PubMed] [Google Scholar]
- 387. Brodsky IG , Balagopal P , Nair KS. 1996. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab 81:3469–3475 [DOI] [PubMed] [Google Scholar]
- 388. Fernández-Balsells MM , Murad MH , Lane M , Lampropulos JF , Albuquerque F , Mullan RJ , Agrwal N , Elamin MB , Gallegos-Orozco JF , Wang AT , Erwin PJ , Bhasin S , Montori VM. 2010. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 95:2560–2575 [DOI] [PubMed] [Google Scholar]
- 389. Ozata M , Yildirimkaya M , Bulur M , Yilmaz K , Bolu E , Corakci A , Gundogan MA. 1996. Effects of gonadotropin and testosterone treatments on lipoprotein (a), high density lipoprotein particles, and other lipoprotein levels in male hypogonadism. J Clin Endocrinol Metab 81:3372–3378 [DOI] [PubMed] [Google Scholar]
- 390. Zgliczynski S , Ossowski M , Slowinska-Srzednicka J , Brzezinska A , Zgliczynski W , Soszynski P , Chotkowska E , Srzednicki M , Sadowski Z. 1996. Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men. Atherosclerosis 121:35–43 [DOI] [PubMed] [Google Scholar]
- 391. Tan KC , Shiu SW , Pang RW , Kung AW. 1998. Effects of testosterone replacement on HDL subfractions and apolipoprotein A-I containing lipoproteins. Clin Endocrinol (Oxf) 48:187–194 [PubMed] [Google Scholar]
- 392. Dobs AS , Meikle AW , Arver S , Sanders SW , Caramelli KE , Mazer NA. 1999. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 84:3469–3478 [DOI] [PubMed] [Google Scholar]
- 393. Howell SJ , Radford JA , Adams JE , Smets EM , Warburton R , Shalet SM. 2001. Randomized placebo-controlled trial of testosterone replacement in men with mild Leydig cell insufficiency following cytotoxic chemotherapy. Clin Endocrinol (Oxf) 55:315–324 [DOI] [PubMed] [Google Scholar]
- 394. Singh AB , Hsia S , Alaupovic P , Sinha-Hikim I , Woodhouse L , Buchanan TA , Shen R , Bross R , Berman N , Bhasin S. 2002. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab 87:136–143 [DOI] [PubMed] [Google Scholar]
- 395. Whitsel EA , Boyko EJ , Matsumoto AM , Anawalt BD , Siscovick DS. 2001. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med 111:261–269 [DOI] [PubMed] [Google Scholar]
- 396. Sorva R , Kuusi T , Taskinen MR , Perheentupa J , Nikkilä EA. 1988. Testosterone substitution increases the activity of lipoprotein lipase and hepatic lipase in hypogonadal males. Atherosclerosis 69:191–197 [DOI] [PubMed] [Google Scholar]
- 397. Herbst KL , Amory JK , Brunzell JD , Chansky HA , Bremner WJ. 2003. Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wk. Am J Physiol Endocrinol Metab 284:E1112–E1118 [DOI] [PubMed] [Google Scholar]
- 398. Tan KC , Shiu SW , Kung AW. 1999. Alterations in hepatic lipase and lipoprotein subfractions with transdermal testosterone replacement therapy. Clin Endocrinol (Oxf) 51:765–769 [DOI] [PubMed] [Google Scholar]
- 399. Langer C , Gansz B , Goepfert C , Engel T , Uehara Y , von Dehn G , Jansen H , Assmann G , von Eckardstein A. 2002. Testosterone up-regulates scavenger receptor BI and stimulates cholesterol efflux from macrophages. Biochem Biophys Res Commun 296:1051–1057 [DOI] [PubMed] [Google Scholar]
- 400. Kapoor D , Goodwin E , Channer KS , Jones TH. 2006. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 154:899–906 [DOI] [PubMed] [Google Scholar]
- 401. Malkin CJ , Pugh PJ , Jones RD , Kapoor D , Channer KS , Jones TH. 2004. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 89:3313–3318 [DOI] [PubMed] [Google Scholar]
- 402. Zitzmann M , Faber S , Nieschlag E. 2006. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 91:4335–4343 [DOI] [PubMed] [Google Scholar]
- 403. Bernini G , Versari D , Moretti A , Virdis A , Ghiadoni L , Bardini M , Taurino C , Canale D , Taddei S , Salvetti A. 2006. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J Clin Endocrinol Metab 91:1691–1697 [DOI] [PubMed] [Google Scholar]
- 404. Götherström G , Svensson J , Koranyi J , Alpsten M , Bosaeus I , Bengtsson B , Johannsson G. 2001. A prospective study of 5 years of GH replacement therapy in GH-deficient adults: sustained effects on body composition, bone mass, and metabolic indices. J Clin Endocrinol Metab 86:4657–4665 [DOI] [PubMed] [Google Scholar]
- 405. Gibney J , Wallace JD , Spinks T , Schnorr L , Ranicar A , Cuneo RC , Lockhart S , Burnand KG , Salomon F , Sonksen PH , Russell-Jones D. 1999. The effects of 10 years of recombinant human growth hormone (GH) in adult GH-deficient patients. J Clin Endocrinol Metab 84:2596–2602 [DOI] [PubMed] [Google Scholar]
- 406. Götherström G , Elbornsson M , Stibrant-Sunnerhagen K , Bengtsson BA , Johannsson G , Svensson J. 2009. Ten years of growth hormone (GH) replacement normalizes muscle strength in GH-deficient adult. J Clin Endocrinol Metab 94:809–816 [DOI] [PubMed] [Google Scholar]
- 407. Chrisoulidou A , Beshyah SA , Rutherford O , Spinks TJ , Mayet J , Kyd P , Anyaoku V , Haida A , Ariff B , Murphy M , Thomas E , Robinson S , Foale R , Johnston DG. 2000. Effects of 7 years of growth hormone replacement therapy in hypopituitary adults. J Clin Endocrinol Metab 85:3762–3769 [DOI] [PubMed] [Google Scholar]
- 408. Whitehead HM , Boreham C , McIlrath EM , Sheridan B , Kennedy L , Atkinson AB , Hadden DR. 1992. Growth hormone treatment of adults with growth hormone deficiency: results of a 13-month placebo controlled cross-over study. Clin Endocrinol (Oxf) 36:45–52 [DOI] [PubMed] [Google Scholar]
- 409. Cuneo RC , Salomon F , Wiles CM , Hesp R , Sönksen PH. 1991. Growth hormone treatment in growth hormone-deficient adults. I. Effects on muscle mass and strength. J Appl Physiol 70:688–694 [DOI] [PubMed] [Google Scholar]
- 410. Wallymahmed ME , Foy P , Shaw D , Hutcheon R , Edwards RH , MacFarlane IA. 1997. Quality of life, body composition and muscle strength in adult growth hormone deficiency: the influence of growth hormone replacement therapy for up to 3 years. Clin Endocrinol (Oxf) 47:439–446 [DOI] [PubMed] [Google Scholar]
- 411. Rodríguez-Arnao J , Jabbar A , Fulcher K , Besser GM , Ross RJ. 1999. Effects of growth hormone replacement on physical performance and body composition in GH deficient adults. Clin Endocrinol (Oxf) 51:53–60 [DOI] [PubMed] [Google Scholar]
- 412. Svensson J , Sunnerhagen KS , Johannsson G. 2003. Five years of growth hormone replacement therapy in adults: age- and gender-related changes in isometric and isokinetic muscle strength. J Clin Endocrinol Metab 88:2061–2069 [DOI] [PubMed] [Google Scholar]
- 413. Widdowson WM , Gibney J. 2008. The effect of growth hormone replacement on exercise capacity in patients with GH deficiency: a metaanalysis. J Clin Endocrinol Metab 93:4413–4417 [DOI] [PubMed] [Google Scholar]
- 414. Christ ER , Cummings MH , Stolinski M , Jackson N , Lumb PJ , Wierzbicki AS , Sönksen PH , Russell-Jones DL , Umpleby AM. 2006. Low-density lipoprotein apolipoprotein B100 turnover in hypopituitary patients with GH deficiency: a stable isotope study. Eur J Endocrinol 154:459–466 [DOI] [PubMed] [Google Scholar]
- 415. Christ ER , Chowienczyk PJ , Sönksen PH , Russel-Jones DL. 1999. Growth hormone replacement therapy in adults with growth hormone deficiency improves vascular reactivity. Clin Endocrinol (Oxf) 51:21–25 [DOI] [PubMed] [Google Scholar]
- 416. Attanasio AF , Bates PC , Ho KK , Webb SM , Ross RJ , Strasburger CJ , Bouillon R , Crowe B , Selander K , Valle D , Lamberts SW. 2002. Human growth hormone replacement in adult hypopituitary patients: long-term effects on body composition and lipid status—3-year results from the HypoCCS Database. J Clin Endocrinol Metab 87:1600–1606 [DOI] [PubMed] [Google Scholar]
- 417. al-Shoumer KA , Gray R , Anyaoku V , Hughes C , Beshyah S , Richmond W , Johnston DG. 1998. Effects of four years' treatment with biosynthetic human growth hormone (GH) on glucose homeostasis, insulin secretion and lipid metabolism in GH-deficient adults. Clin Endocrinol (Oxf) 48:795–802 [DOI] [PubMed] [Google Scholar]
- 418. Hwu CM , Kwok CF , Lai TY , Shih KC , Lee TS , Hsiao LC , Lee SH , Fang VS , Ho LT. 1997. Growth hormone (GH) replacement reduces total body fat and normalizes insulin sensitivity in GH-deficient adults: a report of one-year clinical experience. J Clin Endocrinol Metab 82:3285–3292 [DOI] [PubMed] [Google Scholar]
- 419. Rosenfalck AM , Maghsoudi S , Fisker S , Jørgensen JO , Christiansen JS , Hilsted J , Vølund AA , Madsbad S. 2000. The effect of 30 months of low-dose replacement therapy with recombinant human growth hormone (rhGH) on insulin and C-peptide kinetics, insulin secretion, insulin sensitivity, glucose effectiveness, and body composition in GH-deficient adults. J Clin Endocrinol Metab 85:4173–4181 [DOI] [PubMed] [Google Scholar]
- 420. Christopher M , Hew FL , Oakley M , Rantzau C , Alford F. 1998. Defects of insulin action and skeletal muscle glucose metabolism in growth hormone-deficient adults persist after 24 months of recombinant human growth hormone therapy. J Clin Endocrinol Metab 83:1668–1681 [DOI] [PubMed] [Google Scholar]
- 421. Jorgensen JO , Vahl N , Nyholm B , Juul A , Moller N , Schmitz O , Skakkebaek NE , Christ , Christiansen JS. 1996. Substrate metabolism and insulin sensitivity following long-term growth hormone (GH) replacement therapy in GH-deficient adults. Endocrinol Metab 3:281–286 [Google Scholar]
- 422. McGauley G , Cuneo R , Salomon F , Sönksen PH. 1996. Growth hormone deficiency and quality of life. Horm Res 45:34–37 [DOI] [PubMed] [Google Scholar]
- 423. Rosilio M , Blum WF , Edwards DJ , Shavrikova EP , Valle D , Lamberts SW , Erfurth EM , Webb SM , Ross RJ , Chihara K , Henrich G , Herschbach P , Attanasio AF. 2004. Long-term improvement of quality of life during growth hormone (GH) replacement therapy in adults with GH deficiency, as measured by questions on life satisfaction-hypopituitarism (QLS-H). J Clin Endocrinol Metab 89:1684–1693 [DOI] [PubMed] [Google Scholar]
- 424. McMillan CV , Bradley C , Gibney J , Healy ML , Russell-Jones DL , Sönksen PH. 2003. Psychological effects of withdrawal of growth hormone therapy from adults with growth hormone deficiency. Clin Endocrinol (Oxf) 59:467–475 [DOI] [PubMed] [Google Scholar]
- 425. Saller B , Mattsson AF , Kann PH , Koppeschaar HP , Svensson J , Pompen M , Koltowska-Häggström M. 2006. Healthcare utilization, quality of life and patient-reported outcomes during two years of GH replacement therapy in GH-deficient adults—comparison between Sweden, The Netherlands and Germany. Eur J Endocrinol 154:843–850 [DOI] [PubMed] [Google Scholar]
- 426. Mauras N , Blizzard RM , Link K , Johnson ML , Rogol AD , Veldhuis JD. 1987. Augmentation of growth hormone secretion during puberty: evidence for a pulse amplitude-modulated phenomenon. J Clin Endocrinol Metab 64:596–601 [DOI] [PubMed] [Google Scholar]
- 427. Veldhuis JD , Metzger DL , Martha PM , Mauras N , Kerrigan JR , Keenan B , Rogol AD , Pincus SM. 1997. Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)-insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J Clin Endocrinol Metab 82:3414–3420 [DOI] [PubMed] [Google Scholar]
- 428. Giustina A , Scalvini T , Tassi C , Desenzani P , Poiesi C , Wehrenberg WB , Rogol AD , Veldhuis JD. 1997. Maturation of the regulation of growth hormone secretion in young males with hypogonadotropic hypogonadism pharmacologically exposed to progressive increments in serum testosterone. J Clin Endocrinol Metab 82:1210–1219 [DOI] [PubMed] [Google Scholar]
- 429. Brill KT , Weltman AL , Gentili A , Patrie JT , Fryburg DA , Hanks JB , Urban RJ , Veldhuis JD. 2002. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab 87:5649–5657 [DOI] [PubMed] [Google Scholar]
- 430. Muniyappa R , Sorkin JD , Veldhuis JD , Harman SM , Münzer T , Bhasin S , Blackman MR. 2007. Long-term testosterone supplementation augments overnight growth hormone secretion in healthy older men. Am J Physiol Endocrinol Metab 293:E769–E775 [DOI] [PubMed] [Google Scholar]
- 431. van den Berg G , Veldhuis JD , Frölich M , Roelfsema F. 1996. An amplitude-specific divergence in the pulsatile mode of growth hormone (GH) secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab 81:2460–2467 [DOI] [PubMed] [Google Scholar]
- 432. Murphy LJ , Friesen HG. 1988. Differential effects of estrogen and growth hormone on uterine and hepatic insulin-like growth factor I gene expression in the ovariectomized hypophysectomized rat. Endocrinology 122:325–332 [DOI] [PubMed] [Google Scholar]
- 433. Drake WM , Rodríguez-Arnao J , Weaver JU , James IT , Coyte D , Spector TD , Besser GM , Monson JP. 2001. The influence of gender on the short and long-term effects of growth hormone replacement on bone metabolism and bone mineral density in hypopituitary adults: a 5-year study. Clin Endocrinol (Oxf) 54:525–532 [DOI] [PubMed] [Google Scholar]
- 434. Burman P , Johansson AG , Siegbahn A , Vessby B , Karlsson FA. 1997. Growth hormone (GH)-deficient men are more responsive to GH replacement therapy than women. J Clin Endocrinol Metab 82:550–555 [DOI] [PubMed] [Google Scholar]
- 435. Fryburg DA , Gelfand RA , Barrett EJ. 1991. Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol 260:E499–E504 [DOI] [PubMed] [Google Scholar]
- 436. Urban RJ , Bodenburg YH , Gilkison C , Foxworth J , Coggan AR , Wolfe RR , Ferrando A. 1995. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol 269:E820–E826 [DOI] [PubMed] [Google Scholar]
- 437. Kamanga-Sollo E , Pampusch MS , Xi G , White ME , Hathaway MR , Dayton WR. 2004. IGF-I mRNA levels in bovine satellite cell cultures: effects of fusion and anabolic steroid treatment. J Cell Physiol 201:181–189 [DOI] [PubMed] [Google Scholar]
- 438. Le H , Arnold JT , McFann KK , Blackman MR. 2006. DHT and testosterone, but not DHEA or E2, differentially modulate IGF-I, IGFBP-2, and IGFBP-3 in human prostatic stromal cells. Am J Physiol Endocrinol Metab 290:E952–E960 [DOI] [PubMed] [Google Scholar]
- 439. Jones JI , Clemmons DR. 1995. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 [DOI] [PubMed] [Google Scholar]
- 440. Mauras N , Hayes V , Welch S , Rini A , Helgeson K , Dokler M , Veldhuis JD , Urban RJ. 1998. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab 83:1886–1892 [DOI] [PubMed] [Google Scholar]
- 441. Sinha-Hikim I , Taylor WE , Gonzalez-Cadavid NF , Zheng W , Bhasin S. 2004. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89:5245–5255 [DOI] [PubMed] [Google Scholar]
- 442. Chakravarthy MV , Davis BS , Booth FW. 2000. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 89:1365–1379 [DOI] [PubMed] [Google Scholar]
- 443. Ferrando AA , Sheffield-Moore M , Yeckel CW , Gilkison C , Jiang J , Achacosa A , Lieberman SA , Tipton K , Wolfe RR , Urban RJ. 2002. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:E601–E607 [DOI] [PubMed] [Google Scholar]
- 444. Wu Y , Zhao W , Zhao J , Pan J , Wu Q , Zhang Y , Bauman WA , Cardozo CP. 2007. Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology 148:2984–2993 [DOI] [PubMed] [Google Scholar]
- 445. Gentile MA , Nantermet PV , Vogel RL , Phillips R , Holder D , Hodor P , Cheng C , Dai H , Freedman LP , Ray WJ. 2010. Androgen-mediated improvement of body composition and muscle function involves a novel early transcriptional program including IGF1, mechano growth factor, and induction of β-catenin. J Mol Endocrinol 44:55–73 [DOI] [PubMed] [Google Scholar]
- 446. Hameed M , Orrell RW , Cobbold M , Goldspink G , Harridge SD. 2003. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Welle S , Thornton C. 1997. Insulin-like growth factor-I, actin, and myosin heavy chain messenger RNAs in skeletal muscle after an injection of growth hormone in subjects over 60 years old. J Endocrinol 155:93–97 [DOI] [PubMed] [Google Scholar]
- 448. Lluís F , Perdiguero E , Nebreda AR , Muñoz-Cánoves P. 2006. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol 16:36–44 [DOI] [PubMed] [Google Scholar]
- 449. Bouchard C , Després JP , Mauriège P. 1993. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev 14:72–93 [DOI] [PubMed] [Google Scholar]
- 450. Bjorntorp P. 1997. Endocrine abnormalities in obesity. Diabetes Rev 5:52–68 [Google Scholar]
- 451. Yang S , Xu X , Björntorp P , Edén S. 1995. Additive effects of growth hormone and testosterone on lipolysis in adipocytes of hypophysectomized rats. J Endocrinol 147:147–152 [DOI] [PubMed] [Google Scholar]
- 452. Dieudonne MN , Pecquery R , Leneveu MC , Giudicelli Y. 2000. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor γ2. Endocrinology 141:649–656 [DOI] [PubMed] [Google Scholar]
- 453. Herndon LA , Schmeissner PJ , Dudaronek JM , Brown PA , Listner KM , Sakano Y , Paupard MC , Hall DH , Driscoll M. 2002. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419:808–814 [DOI] [PubMed] [Google Scholar]
- 454. Black AE , Coward WA , Cole TJ , Prentice AM. 1996. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr 50:72–92 [PubMed] [Google Scholar]
- 455. Short KR , Moller N , Bigelow ML , Coenen-Schimke J , Nair KS. 2008. Enhancement of muscle mitochondrial function by growth hormone. J Clin Endocrinol Metab 93:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Chakravarthy MV , Abraha TW , Schwartz RJ , Fiorotto ML , Booth FW. 2000. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3′-kinase/Akt signaling pathway. J Biol Chem 275:35942–35952 [DOI] [PubMed] [Google Scholar]
- 457. Yang SY , Goldspink G. 2002. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522:156–160 [DOI] [PubMed] [Google Scholar]
- 458. Wilborn CD , Taylor LW , Greenwood M , Kreider RB , Willoughby DS. 2009. Effects of different intensities of resistance exercise on regulators of myogenesis. J Strength Cond Res 23:2179–2187 [DOI] [PubMed] [Google Scholar]
- 459. Singh MA , Ding W , Manfredi TJ , Solares GS , O'Neill EF , Clements KM , Ryan ND , Kehayias JJ , Fielding RA , Evans WJ. 1999. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol 277:E135–E143 [DOI] [PubMed] [Google Scholar]
- 460. Bamman MM , Shipp JR , Jiang J , Gower BA , Hunter GR , Goodman A , McLafferty CL , Urban RJ. 2001. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280:E383–E390 [DOI] [PubMed] [Google Scholar]
- 461. Taaffe DR , Jin IH , Vu TH , Hoffman AR , Marcus R. 1996. Lack of effect of recombinant human growth hormone (GH) on muscle morphology and GH-insulin-like growth factor expression in resistance-trained elderly men. J Clin Endocrinol Metab 81:421–425 [DOI] [PubMed] [Google Scholar]
- 462. Adams GR , McCue SA. 1998. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84:1716–1722 [DOI] [PubMed] [Google Scholar]
- 463. Jørgensen JO , Jessen N , Pedersen SB , Vestergaard E , Gormsen L , Lund SA , Billestrup N. 2006. GH receptor signaling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. Am J Physiol Endocrinol Metab 291:E899–E905 [DOI] [PubMed] [Google Scholar]
- 464. Woelfle J , Chia DJ , Rotwein P. 2003. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem 278:51261–51266 [DOI] [PubMed] [Google Scholar]
- 465. Gosteli-Peter MA , Winterhalter KH , Schmid C , Froesch ER , Zapf J. 1994. Expression and regulation of insulin-like growth factor-I (IGF-I) and IGF-binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology 135:2558–2567 [DOI] [PubMed] [Google Scholar]
- 466. Iida K , Itoh E , Kim DS , del Rincon JP , Coschigano KT , Kopchick JJ , Thorner MO. 2004. Muscle mechano growth factor is preferentially induced by growth hormone in growth hormone-deficient lit/lit mice. J Physiol 560:341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Kim H , Barton E , Muja N , Yakar S , Pennisi P , Leroith D. 2005. Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology 146:1772–1779 [DOI] [PubMed] [Google Scholar]
- 468. Klover P , Hennighausen L. 2007. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology 148:1489–1497 [DOI] [PubMed] [Google Scholar]
- 469. Sotiropoulos A , Ohanna M , Kedzia C , Menon RK , Kopchick JJ , Kelly PA , Pende M. 2006. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci USA 103:7315–7320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Serra C , Bhasin S , Tangherlini F , Barton ER , Ganno M , Zhang A , Shansky J , Vandenburgh HH , Travison TG , Jasuja R , Morris C. 2011. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology 152:193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Pedersen BK , Edward F. 2009. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol 107:1006–1014 [DOI] [PubMed] [Google Scholar]
- 472. Steensberg A , van Hall G , Osada T , Sacchetti M , Saltin B , Klarlund Pedersen B. 2000. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529:237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Goodman MN. 1994. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med 205:182–185 [DOI] [PubMed] [Google Scholar]
- 474. Roubenoff R , Parise H , Payette HA , Abad LW , D'Agostino R , Jacques PF , Wilson PW , Dinarello CA , Harris TB. 2003. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med 115:429–435 [DOI] [PubMed] [Google Scholar]
- 475. Barbieri M , Ferrucci L , Ragno E , Corsi A , Bandinelli S , Bonafè M , Olivieri F , Giovagnetti S , Franceschi C , Guralnik JM , Paolisso G. 2003. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab 284:E481–E487 [DOI] [PubMed] [Google Scholar]
- 476. Visser M , Pahor M , Taaffe DR , Goodpaster BH , Simonsick EM , Newman AB , Nevitt M , Harris TB. 2002. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 57:M326–M332 [DOI] [PubMed] [Google Scholar]
- 477. Kern PA , Ranganathan S , Li C , Wood L , Ranganathan G. 2001. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280:E745–E751 [DOI] [PubMed] [Google Scholar]
- 478. Müller S , Martin S , Koenig W , Hanifi-Moghaddam P , Rathmann W , Haastert B , Giani G , Illig T , Thorand B , Kolb H. 2002. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-α or its receptors. Diabetologia 45:805–812 [DOI] [PubMed] [Google Scholar]
- 479. Gibney J , Healy ML , Sönksen PH. 2007. The growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocr Rev 28:603–624 [DOI] [PubMed] [Google Scholar]
- 480. Pritzlaff CJ , Wideman L , Weltman JY , Abbott RD , Gutgesell ME , Hartman ML , Veldhuis JD , Weltman A. 1999. Impact of acute exercise intensity on pulsatile growth hormone release in men. J Appl Physiol 87:498–504 [DOI] [PubMed] [Google Scholar]
- 481. Giannoulis MG , Boroujerdi MA , Powrie J , Dall R , Napoli R , Ehrnborg C , Pentecost C , Cittadini A , Jørgensen JO , Sonksen PH. 2005. Gender differences in growth hormone response to exercise before and after rhGH administration and the effect of rhGH on the hormone profile of fit normal adults. Clin Endocrinol (Oxf) 62:315–322 [DOI] [PubMed] [Google Scholar]
- 482. Pyka G , Taaffe DR , Marcus R. 1994. Effect of a sustained program of resistance training on the acute growth hormone response to resistance exercise in older adults. Horm Metab Res 26:330–333 [DOI] [PubMed] [Google Scholar]
- 483. Zaccaria M , Varnier M , Piazza P , Noventa D , Ermolao A. 1999. Blunted growth hormone response to maximal exercise in middle-aged versus young subjects and no effect of endurance training. J Clin Endocrinol Metab 84:2303–2307 [DOI] [PubMed] [Google Scholar]
- 484. Schwarz AJ , Brasel JA , Hintz RL , Mohan S , Cooper DM. 1996. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab 81:3492–3497 [DOI] [PubMed] [Google Scholar]
- 485. Nindl BC , Castellani JW , Young AJ , Patton JF , Khosravi MJ , Diamandi A , Montain SJ. 2003. Differential responses of IGF-I molecular complexes to military operational field training. J Appl Physiol 95:1083–1089 [DOI] [PubMed] [Google Scholar]
- 486. Nemet D , Connolly PH , Pontello-Pescatello AM , Rose-Gottron C , Larson JK , Galassetti P , Cooper DM. 2004. Negative energy balance plays a major role in the IGF-I response to exercise training. J Appl Physiol 96:276–282 [DOI] [PubMed] [Google Scholar]
- 487. Eliakim A , Scheett TP , Newcomb R , Mohan S , Cooper DM. 2001. Fitness, training, and the growth hormone→insulin-like growth factor I axis in prepubertal girls. J Clin Endocrinol Metab 86:2797–2802 [DOI] [PubMed] [Google Scholar]
- 488. Lieskovska J , Guo D , Derman E. 2002. IL-6-overexpression brings about growth impairment potentially through a GH receptor defect. Growth Horm IGF Res 12:388–398 [DOI] [PubMed] [Google Scholar]
- 489. Greenhalgh CJ , Metcalf D , Thaus AL , Corbin JE , Uren R , Morgan PO , Fabri LJ , Zhang JG , Martin HM , Willson TA , Billestrup N , Nicola NA , Baca M , Alexander WS , Hilton DJ. 2002. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem 277:40181–40184 [DOI] [PubMed] [Google Scholar]
- 490. Frank SJ. 2001. Growth hormone signalling and its regulation: preventing too much of a good thing. Growth Horm IGF Res 11:201–212 [DOI] [PubMed] [Google Scholar]
- 491. Heinrich PC , Behrmann I , Haan S , Hermanns HM , Müller-Newen G , Schaper F. 2003. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Hirano T , Ishihara K , Hibi M. 2000. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19:2548–2556 [DOI] [PubMed] [Google Scholar]
- 493. Haddad F , Zaldivar F , Cooper DM , Adams GR. 2005. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98:911–917 [DOI] [PubMed] [Google Scholar]
- 494. Trenerry MK , Carey KA , Ward AC , Cameron-Smith D. 2007. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol 102:1483–1489 [DOI] [PubMed] [Google Scholar]
- 495. Febbraio MA , Hiscock N , Sacchetti M , Fischer CP , Pedersen BK. 2004. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53:1643–1648 [DOI] [PubMed] [Google Scholar]
- 496. Febbraio MA , Steensberg A , Keller C , Starkie RL , Nielsen HB , Krustrup P , Ott P , Secher NH , Pedersen BK. 2003. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol 549:607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Wojtaszewski JF , Nielsen JN , Richter EA. 2002. Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol 93:384–392 [DOI] [PubMed] [Google Scholar]
- 498. Senn JJ , Klover PJ , Nowak IA , Zimmers TA , Koniaris LG , Furlanetto RW , Mooney RA. 2003. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 278:13740–13746 [DOI] [PubMed] [Google Scholar]
- 499. Kim JH , Kim JE , Liu HY , Cao W , Chen J. 2008. Regulation of interleukin-6-induced hepatic insulin resistance by mammalian target of rapamycin through the STAT3-SOCS3 pathway. J Biol Chem 283:708–715 [DOI] [PubMed] [Google Scholar]
- 500. Nielsen C , Gormsen LC , Jessen N , Pedersen SB , Møller N , Lund S , Jørgensen JO. 2008. Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab 93:2842–2850 [DOI] [PubMed] [Google Scholar]
- 501. Pedersen BK , Febbraio MA. 2007. Point: interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol 102:814–816 [DOI] [PubMed] [Google Scholar]
- 502. Kim HJ , Higashimori T , Park SY , Choi H , Dong J , Kim YJ , Noh HL , Cho YR , Cline G , Kim YB , Kim JK. 2004. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53:1060–1067 [DOI] [PubMed] [Google Scholar]
- 503. Guillet C , Zangarelli A , Gachon P , Morio B , Giraudet C , Rousset P , Boirie Y. 2004. Whole body protein breakdown is less inhibited by insulin, but still responsive to amino acid, in nondiabetic elderly subjects. J Clin Endocrinol Metab 89:6017–6024 [DOI] [PubMed] [Google Scholar]
- 504. Zachwieja JJ , Smith SR , Lovejoy JC , Rood JC , Windhauser MM , Bray GA. 1999. Testosterone administration preserves protein balance but not muscle strength during 28 days of bed rest. J Clin Endocrinol Metab 84:207–212 [DOI] [PubMed] [Google Scholar]
- 505. Criswell DS , Booth FW , DeMayo F , Schwartz RJ , Gordon SE , Fiorotto ML. 1998. Overexpression of IGF-I in skeletal muscle of transgenic mice does not prevent unloading-induced atrophy. Am J Physiol 275:E373–E379 [DOI] [PubMed] [Google Scholar]
- 506. Goldberg AL. 1967. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol 213:1193–1198 [DOI] [PubMed] [Google Scholar]
- 507. Signorile JF , Carmel MP , Lai S , Roos BA. 2005. Early plateaus of power and torque gains during high- and low-speed resistance training of older women. J Appl Physiol 98:1213–1220 [DOI] [PubMed] [Google Scholar]
- 508. Pyka G , Lindenberger E , Charette S , Marcus R. 1994. Muscle strength and fiber adaptations to a year-long resistance training program in elderly men and women. J Gerontol 49:M22–M27 [DOI] [PubMed] [Google Scholar]
- 509. van der Bij AK , Laurant MG , Wensing M. 2002. Effectiveness of physical activity interventions for older adults: a review. Am J Prev Med 22:120–133 [DOI] [PubMed] [Google Scholar]
- 510. Dolansky MA , Stepanczuk B , Charvat JM , Moore SM. 2010. Women's and men's exercise adherence after a cardiac event. Res Gerontol Nurs 3:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Lenard NR , Berthoud HR. 2008. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 16(Suppl 3):S11–S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Yarasheski KE , Zachwieja JJ , Campbell JA , Bier DM. 1995. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol 268:E268–E276 [DOI] [PubMed] [Google Scholar]
- 513. Butterfield GE , Thompson J , Rennie MJ , Marcus R , Hintz RL , Hoffman AR. 1997. Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am J Physiol 272:E94–E99 [DOI] [PubMed] [Google Scholar]
- 514. Thompson JL , Butterfield GE , Gylfadottir UK , Yesavage J , Marcus R , Hintz RL , Pearman A , Hoffman AR. 1998. Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women. J Clin Endocrinol Metab 83:1477–1484 [DOI] [PubMed] [Google Scholar]
- 515. Fahey TD , Brown CH. 1973. The effects of an anabolic steroid on the strength, body composition, and endurance of college males when accompanied by a weight training program. Med Sci Sports 5:272–276 [PubMed] [Google Scholar]
- 516. Morey MC , Pieper CF , Cornoni-Huntley J. 1998. Physical fitness and functional limitations in community-dwelling older adults. Med Sci Sports Exerc 30:715–723 [DOI] [PubMed] [Google Scholar]
- 517. Arnett SW , Laity JH , Agrawal SK , Cress ME. 2008. Aerobic reserve and physical functional performance in older adults. Age Ageing 37:384–389 [DOI] [PubMed] [Google Scholar]
- 518. Puggaard L. 2005. Age-related decline in maximal oxygen capacity: consequences for performance of everyday activities. J Am Geriatr Soc 53:546–547 [DOI] [PubMed] [Google Scholar]
- 519. Moritani T , Nagata A , deVries HA , Muro M. 1981. Critical power as a measure of physical work capacity and anaerobic threshold. Ergonomics 24:339–350 [DOI] [PubMed] [Google Scholar]
- 520. Bouchard C , Lesage R , Lortie G , Simoneau JA , Hamel P , Boulay MR , Pérusse L , Thériault G , Leblanc C. 1986. Aerobic performance in brothers, dizygotic and monozygotic twins. Med Sci Sports Exerc 18:639–646 [PubMed] [Google Scholar]
- 521. Pérusse L , Tremblay A , Leblanc C , Bouchard C. 1989. Genetic and environmental influences on level of habitual physical activity and exercise participation. Am J Epidemiol 129:1012–1022 [DOI] [PubMed] [Google Scholar]
- 522. Taylor HL , Jacobs DR , Schucker B , Knudsen J , Leon AS , Debacker G. 1978. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31:741–755 [DOI] [PubMed] [Google Scholar]
- 523. Starling RD , Matthews DE , Ades PA , Poehlman ET. 1999. Assessment of physical activity in older individuals: a doubly labeled water study. J Appl Physiol 86:2090–2096 [DOI] [PubMed] [Google Scholar]
- 524. Dvorak RV , Tchernof A , Starling RD , Ades PA , DiPietro L , Poehlman ET. 2000. Respiratory fitness, free living physical activity, and cardiovascular disease risk in older individuals: a doubly labeled water study. J Clin Endocrinol Metab 85:957–963 [DOI] [PubMed] [Google Scholar]
- 525. Goldberg L , Rogol AD , Sonksen PH. 2009. Patient information page. Growth hormone: use and abuse. J Clin Endocrinol Metab 94:2. [DOI] [PubMed] [Google Scholar]
- 526. Bhasin S , Cunningham GR , Hayes FJ , Matsumoto AM , Snyder PJ , Swerdloff RS , Montori VM. 2010. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 95:2536–2559 [DOI] [PubMed] [Google Scholar]
- 527. Anawalt BD. 2010. Guidelines for testosterone therapy for men: how to avoid a mad (t)ea party by getting personal. J Clin Endocrinol Metab 95:2614–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Wu FC. 2007. Commentary: guideline for male testosterone therapy: a European perspective. J Clin Endocrinol Metab 92:418–419 [DOI] [PubMed] [Google Scholar]
- 529. McKinlay JB , Travison TG , Araujo AB , Kupelian V. 2007. Male menopause: time for a decent burial? Menopause 14:973–975 [DOI] [PubMed] [Google Scholar]
- 530. Snyder PJ. 2004. Hypogonadism in elderly men—what to do until the evidence comes. N Engl J Med 350:440–442 [DOI] [PubMed] [Google Scholar]
- 531. Page ST , Amory JK , Bowman FD , Anawalt BD , Matsumoto AM , Bremner WJ , Tenover JL. 2005. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90:1502–1510 [DOI] [PubMed] [Google Scholar]
- 532. Nair KS , Rizza RA , O'Brien P , Dhatariya K , Short KR , Nehra A , Vittone JL , Klee GG , Basu A , Basu R , Cobelli C , Toffolo G , Dalla Man C , Tindall DJ , Melton LJ , Smith GE , Khosla S , Jensen MD. 2006. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- 533. Holloway L , Butterfield G , Hintz RL , Gesundheit N , Marcus R. 1994. Effects of recombinant human growth hormone on metabolic indices, body composition, and bone turnover in healthy elderly women. J Clin Endocrinol Metab 79:470–479 [DOI] [PubMed] [Google Scholar]
- 534. Thompson JL , Butterfield GE , Marcus R , Hintz RL , Van Loan M , Ghiron L , Hoffman AR. 1995. The effects of recombinant human insulin-like growth factor-I and growth hormone on body composition in elderly women. J Clin Endocrinol Metab 80:1845–1852 [DOI] [PubMed] [Google Scholar]
- 535. Bhasin S , Woodhouse L , Casaburi R , Singh AB , Bhasin D , Berman N , Chen X , Yarasheski KE , Magliano L , Dzekov C , Dzekov J , Bross R , Phillips J , Sinha-Hikim I , Shen R , Storer TW. 2001. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281:E1172–E1181 [DOI] [PubMed] [Google Scholar]
- 536. Bhasin S , Woodhouse L , Casaburi R , Singh AB , Mac RP , Lee M , Yarasheski KE , Sinha-Hikim I , Dzekov C , Dzekov J , Magliano L , Storer TW. 2005. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90:678–688 [DOI] [PubMed] [Google Scholar]
- 537. Bhasin S , Zhang A , Coviello A , Jasuja R , Ulloor J , Singh R , Vesper H , Vasan RS. 2008. The impact of assay quality and reference ranges on clinical decision making in the diagnosis of androgen disorders. Steroids 73:1311–1317 [DOI] [PubMed] [Google Scholar]
- 538. Vermeulen A. 2001. Androgen replacement therapy in the aging male—a critical evaluation. J Clin Endocrinol Metab 86:2380–2390 [DOI] [PubMed] [Google Scholar]
- 539. Kaufman JM , Vermeulen A. 1997. Declining gonadal function in elderly men. Baillieres Clin Endocrinol Metab 11:289–309 [DOI] [PubMed] [Google Scholar]
- 540. Wang C , Catlin DH , Starcevic B , Leung A , DiStefano E , Lucas G , Hull L , Swerdloff RS. 2004. Testosterone metabolic clearance and production rates determined by stable isotope dilution/tandem mass spectrometry in normal men: influence of ethnicity and age. J Clin Endocrinol Metab 89:2936–2941 [DOI] [PubMed] [Google Scholar]
- 541. Meikle AW , Smith JA , Stringham JD. 1989. Estradiol and testosterone metabolism and production in men with prostatic cancer. J Steroid Biochem 33:19–24 [DOI] [PubMed] [Google Scholar]
- 542. Ishimaru T , Pages L , Horton R. 1977. Altered metabolism of androgens in elderly men with benign prostatic hyperplasia. J Clin Endocrinol Metab 45:695–701 [DOI] [PubMed] [Google Scholar]
- 543. Heinemann LA , Zimmermann T , Vermeulen A , Thiel C. 1999. A new ‘Aging Male's Symptoms’ (AMS) rating scale. The Aging Male 2:105–114 [Google Scholar]
- 544. Morley JE , Charlton E , Patrick P , Kaiser FE , Cadeau P , McCready D , Perry HM. 2000. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 49:1239–1242 [DOI] [PubMed] [Google Scholar]
- 545. Smith KW , Feldman HA , McKinlay JB. 2000. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol (Oxf) 53:703–711 [DOI] [PubMed] [Google Scholar]
- 546. Tsujimura A , Matsumiya K , Miyagawa Y , Takao T , Fujita K , Takada S , Koga M , Iwasa A , Takeyama M , Okuyama A. 2005. Comparative study on evaluation methods for serum testosterone level for PADAM diagnosis. Int J Impot Res 17:259–263 [DOI] [PubMed] [Google Scholar]
- 547. Spetz AC , Palmefors L , Skobe RS , Strömstedt MT , Fredriksson MG , Theodorsson E , Hammar ML. 2007. Testosterone correlated to symptoms of partial androgen deficiency in aging men (PADAM) in an elderly Swedish population. Menopause 14:999–1005 [DOI] [PubMed] [Google Scholar]
- 548. Travison TG , Morley JE , Araujo AB , O'Donnell AB , McKinlay JB. 2006. The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab 91:2509–2513 [DOI] [PubMed] [Google Scholar]
- 549. Araujo AB , Esche GR , Kupelian V , O'Donnell AB , Travison TG , Williams RE , Clark RV , McKinlay JB. 2007. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 92:4241–4247 [DOI] [PubMed] [Google Scholar]
- 550. Wu FC , Tajar A , Beynon JM , Pye SR , Silman AJ , Finn JD , O'Neill TW , Bartfai G , Casanueva FF , Forti G , Giwercman A , Han TS , Kula K , Lean ME , Pendleton N , Punab M , Boonen S , Vanderschueren D , Labrie F , Huhtaniemi IT. 2010. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 363:123–135 [DOI] [PubMed] [Google Scholar]
- 551. Hall SA , Esche GR , Araujo AB , Travison TG , Clark RV , Williams RE , McKinlay JB. 2008. Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J Clin Endocrinol Metab 93:3870–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552. Travison TG , Shackelton R , Araujo AB , Morley JE , Williams RE , Clark RV , McKinlay JB. 2010. Frailty, serum androgens, and the CAG repeat polymorphism: results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 95:2746–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553. Snyder PJ. 2010. Low testosterone must explain diminished physical performance in the elderly–right? J Clin Endocrinol Metab 95:2634–2635 [DOI] [PubMed] [Google Scholar]
- 554. Chang SS , Weiss CO , Xue QL , Fried LP. 2010. Patterns of comorbid inflammatory diseases in frail older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci 65:407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555. Cappola AR , Xue QL , Fried LP. 2009. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women's Health and Aging studies. J Gerontol A Biol Sci Med Sci 64:243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556. Svartberg J , Jorde R , Sundsfjord J , Bønaa KH , Barrett-Connor E. 2003. Seasonal variation of testosterone and waist to hip ratio in men: the Tromso study. J Clin Endocrinol Metab 88:3099–3104 [DOI] [PubMed] [Google Scholar]
- 557. Travison TG , Araujo AB , Kupelian V , O'Donnell AB , McKinlay JB. 2007. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 92:549–555 [DOI] [PubMed] [Google Scholar]
- 558. Mohr BA , Bhasin S , Link CL , O'Donnell AB , McKinlay JB. 2006. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. Eur J Endocrinol 155:443–452 [DOI] [PubMed] [Google Scholar]
- 559. Brambilla DJ , Matsumoto AM , Araujo AB , McKinlay JB. 2009. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 94:907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Longcope C , Feldman HA , McKinlay JB , Araujo AB. 2000. Diet and sex hormone-binding globulin. J Clin Endocrinol Metab 85:293–296 [DOI] [PubMed] [Google Scholar]
- 561. Wu FC , Tajar A , Pye SR , Silman AJ , Finn JD , O'Neill TW , Bartfai G , Casanueva F , Forti G , Giwercman A , Huhtaniemi IT , Kula K , Punab M , Boonen S , Vanderschueren D. 2008. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 93:2737–2745 [DOI] [PubMed] [Google Scholar]
- 562. Allan CA , Strauss BJ , Burger HG , Forbes EA , McLachlan RI. 2008. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab 93:139–146 [DOI] [PubMed] [Google Scholar]
- 563. Brambilla DJ , O'Donnell AB , Matsumoto AM , McKinlay JB. 2007. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 67:853–862 [DOI] [PubMed] [Google Scholar]
- 564. Erfurth EM , Hagmar LE , Sääf M , Hall K. 1996. Serum levels of insulin-like growth factor I and insulin-like growth factor-binding protein 1 correlate with serum free testosterone and sex hormone binding globulin levels in healthy young and middle-aged men. Clin Endocrinol (Oxf) 44:659–664 [DOI] [PubMed] [Google Scholar]
- 565. Coviello AD , Lakshman K , Mazer NA , Bhasin S. 2006. Differences in the apparent metabolic clearance rate of testosterone in young and older men with gonadotropin suppression receiving graded doses of testosterone. J Clin Endocrinol Metab 91:4669–4675 [DOI] [PubMed] [Google Scholar]
- 566. Clasey JL , Weltman A , Patrie J , Weltman JY , Pezzoli S , Bouchard C , Thorner MO , Hartman ML. 2001. Abdominal visceral fat and fasting insulin are important predictors of 24-hour GH release independent of age, gender, and other physiological factors. J Clin Endocrinol Metab 86:3845–3852 [DOI] [PubMed] [Google Scholar]
- 567. Liu PY , Swerdloff RS , Veldhuis JD. 2004. Clinical review 171: the rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. J Clin Endocrinol Metab 89:4789–4796 [DOI] [PubMed] [Google Scholar]
- 568. Snyder PJ. 2001. Effects of age on testicular function and consequences of testosterone treatment. J Clin Endocrinol Metab 86:2369–2372 [DOI] [PubMed] [Google Scholar]
- 569. Bross R , Javanbakht M , Bhasin S. 1999. Anabolic interventions for aging-associated sarcopenia. J Clin Endocrinol Metab 84:3420–3430 [DOI] [PubMed] [Google Scholar]
- 570. Basaria S , Dobs AS. 2001. Hypogonadism and androgen replacement therapy in elderly men. Am J Med 110:563–572 [DOI] [PubMed] [Google Scholar]
- 571. Nass R , Park J , Thorner MO. 2007. Growth hormone supplementation in the elderly. Endocrinol Metab Clin North Am 36:233–245 [DOI] [PubMed] [Google Scholar]
- 572. Weltman A , Veldhuis JD. 2006. Single and combined effects of growth hormone and testosterone in healthy older men. Horm Res 66(Supp 1):49–57 [Google Scholar]
- 573. Liu H , Bravata DM , Olkin I , Nayak S , Roberts B , Garber AM , Hoffman AR. 2007. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med 146:104–115 [DOI] [PubMed] [Google Scholar]
- 574. Savine R , Sonksen P. 2000. Growth hormone—hormone replacement for the somatopause? Horm Res 53(Suppl 3):37–41 [DOI] [PubMed] [Google Scholar]
- 575. Mårin P , Holmäng S , Jönsson L , Sjöström L , Kvist H , Holm G , Lindstedt G , Björntorp P. 1992. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 16:991–997 [PubMed] [Google Scholar]
- 576. Münzer T , Harman SM , Hees P , Shapiro E , Christmas C , Bellantoni MF , Stevens TE , O'Connor KG , Pabst KM , St Clair C , Sorkin JD , Blackman MR. 2001. Effects of GH and/or sex steroid administration on abdominal subcutaneous and visceral fat in healthy aged women and men. J Clin Endocrinol Metab 86:3604–3610 [DOI] [PubMed] [Google Scholar]
- 577. Thompson PD , Cullinane EM , Sady SP , Chenevert C , Saritelli AL , Sady MA , Herbert PN. 1989. Contrasting effects of testosterone and stanozolol on serum lipoprotein levels. JAMA 261:1165–1168 [PubMed] [Google Scholar]
- 578. Schroeder ET , Singh A , Bhasin S , Storer TW , Azen C , Davidson T , Martinez C , Sinha-Hikim I , Jaque SV , Terk M , Sattler FR. 2003. Effects of an oral androgen on muscle and metabolism in older, community-dwelling men. Am J Physiol Endocrinol Metab 284:E120–E128 [DOI] [PubMed] [Google Scholar]
- 579. Ly LP , Jimenez M , Zhuang TN , Celermajer DS , Conway AJ , Handelsman DJ. 2001. A double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiency. J Clin Endocrinol Metab 86:4078–4088 [DOI] [PubMed] [Google Scholar]
- 580. Kunelius P , Lukkarinen O , Hannuksela ML , Itkonen O , Tapanainen JS. 2002. The effects of transdermal dihydrotestosterone in the aging male: a prospective, randomized, double blind study. J Clin Endocrinol Metab 87:1467–1472 [DOI] [PubMed] [Google Scholar]
- 581. Kenny AM , Prestwood KM , Gruman CA , Marcello KM , Raisz LG. 2001. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 56:M266–M272 [DOI] [PubMed] [Google Scholar]
- 582. Amory JK , Watts NB , Easley KA , Sutton PR , Anawalt BD , Matsumoto AM , Bremner WJ , Tenover JL. 2004. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 89:503–510 [DOI] [PubMed] [Google Scholar]
- 583. Christmas C , O'Connor KG , Harman SM , Tobin JD , Münzer T , Bellantoni MF , Clair CS , Pabst KM , Sorkin JD , Blackman MR. 2002. Growth hormone and sex steroid effects on bone metabolism and bone mineral density in healthy aged women and men. J Gerontol A Biol Sci Med Sci 57:M12–M18 [DOI] [PubMed] [Google Scholar]
- 584. Emmelot-Vonk MH , Verhaar HJ , Nakhai Pour HR , Aleman A , Lock TM , Bosch JL , Grobbee DE , van der Schouw YT. 2008. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 299:39–52 [DOI] [PubMed] [Google Scholar]
- 585. Snyder PJ , Peachey H , Hannoush P , Berlin JA , Loh L , Holmes JH , Dlewati A , Staley J , Santanna J , Kapoor SC , Attie MF , Haddad JG , Strom BL. 1999. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 84:1966–1972 [DOI] [PubMed] [Google Scholar]
- 586. Tenover JS. 1992. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab 75:1092–1098 [DOI] [PubMed] [Google Scholar]
- 587. Morley JE , Perry HM , Kaiser FE , Kraenzle D , Jensen J , Houston K , Mattammal M , Perry HM. 1993. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc 41:149–152 [DOI] [PubMed] [Google Scholar]
- 588. Sih R , Morley JE , Kaiser FE , Perry HM , Patrick P , Ross C. 1997. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 82:1661–1667 [DOI] [PubMed] [Google Scholar]
- 589. Yarasheski KE , Campbell JA , Kohrt WM. 1997. Effect of resistance exercise and growth hormone on bone density in older men. Clin Endocrinol (Oxf) 47:223–229 [DOI] [PubMed] [Google Scholar]
- 590. Ohlsson C , Bengtsson BA , Isaksson OG , Andreassen TT , Slootweg MC. 1998. Growth hormone and bone. Endocr Rev 19:55–79 [DOI] [PubMed] [Google Scholar]
- 591. Wittert GA , Chapman IM , Haren MT , Mackintosh S , Coates P , Morley JE. 2003. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 58:618–625 [DOI] [PubMed] [Google Scholar]
- 592. Kenny AM , Prestwood KM , Gruman CA , Fabregas G , Biskup B , Mansoor G. 2002. Effects of transdermal testosterone on lipids and vascular reactivity in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 57:M460–M465 [DOI] [PubMed] [Google Scholar]
- 593. Cohn L , Feller AG , Draper MW , Rudman IW , Rudman D. 1993. Carpal tunnel syndrome and gynaecomastia during growth hormone treatment of elderly men with low circulating IGF-I concentrations. Clin Endocrinol (Oxf) 39:417–425 [DOI] [PubMed] [Google Scholar]
- 594. Lange KH , Andersen JL , Beyer N , Isaksson F , Larsson B , Rasmussen MH , Juul A , Bülow J , Kjaer M. 2002. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab 87:513–523 [DOI] [PubMed] [Google Scholar]
- 595. Lange KH , Isaksson F , Rasmussen MH , Juul A , Bülow J , Kjaer M. 2001. GH administration and discontinuation in healthy elderly men: effects on body composition, GH-related serum markers, resting heart rate and resting oxygen uptake. Clin Endocrinol (Oxf) 55:77–86 [DOI] [PubMed] [Google Scholar]
- 596. Weissberger AJ , Anastasiadis AD , Sturgess I , Martin FC , Smith MA , Sönksen PH. 2003. Recombinant human growth hormone treatment in elderly patients undergoing elective total hip replacement. Clin Endocrinol (Oxf) 58:99–107 [DOI] [PubMed] [Google Scholar]
- 597. Giannoulis MG , Jackson N , Shojaee-Moradie F , Nair KS , Sonksen PH , Martin FC , Umpleby AM. 2008. The effects of growth hormone and/or testosterone on whole body protein kinetics and skeletal muscle gene expression in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab 93:3066–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598. Huang X , Blackman MR , Herreman K , Pabst KM , Harman SM , Caballero B. 2005. Effects of growth hormone and/or sex steroid administration on whole-body protein turnover in healthy aged women and men. Metabolism 54:1162–1167 [DOI] [PubMed] [Google Scholar]
- 599. Gibney J , Wolthers T , Johannsson G , Umpleby AM , Ho KK. 2005. Growth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary men. Am J Physiol Endocrinol Metab 289:E266–E271 [DOI] [PubMed] [Google Scholar]
- 600. Copeland KC , Nair KS. 1994. Acute growth hormone effects on amino acid and lipid metabolism. J Clin Endocrinol Metab 78:1040–1047 [DOI] [PubMed] [Google Scholar]
- 601. Welle S , Thornton C , Statt M , McHenry B. 1996. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab 81:3239–3243 [DOI] [PubMed] [Google Scholar]
- 602. Sattler FR , Castaneda-Sceppa C , Binder EF , Schroeder ET , Wang Y , Bhasin S , Kawakubo M , Stewart Y , Yarasheski KE , Ulloor J , Colletti P , Roubenoff R , Azen SP. 2009. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 94:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603. Buchner DM , Larson EB , Wagner EH , Koepsell TD , de Lateur BJ. 1996. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing 25:386–391 [DOI] [PubMed] [Google Scholar]
- 604. Kwon IS , Oldaker S , Schrager M , Talbot LA , Fozard JL , Metter EJ. 2001. Relationship between muscle strength and the time taken to complete a standardized walk-turn-walk test. J Gerontol A Biol Sci Med Sci 56:B398–B404 [DOI] [PubMed] [Google Scholar]
- 605. Storer TW , Woodhouse L , Magliano L , Singh AB , Dzekov C , Dzekov J , Bhasin S. 2008. Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc 56:1991–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606. Katznelson L , Robinson MW , Coyle CL , Lee H , Farrell CE. 2006. Effects of modest testosterone supplementation and exercise for 12 weeks on body composition and quality of life in elderly men. Eur J Endocrinol 155:867–875 [DOI] [PubMed] [Google Scholar]
- 607. Taaffe DR , Pruitt L , Reim J , Hintz RL , Butterfield G , Hoffman AR , Marcus R. 1994. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab 79:1361–1366 [DOI] [PubMed] [Google Scholar]
- 608. Kenny AM , Kleppinger A , Annis K , Rathier M , Browner B , Judge JO , McGee D. 2010. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc 58:1134–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609. Srinivas-Shankar U , Roberts SA , Connolly MJ , O'Connell MD , Adams JE , Oldham JA , Wu FC. 2010. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 95:639–650 [DOI] [PubMed] [Google Scholar]
- 610. Sullivan DH , Roberson PK , Johnson LE , Bishara O , Evans WJ , Smith ES , Price JA. 2005. Effects of muscle strength training and testosterone in frail elderly males. Med Sci Sports Exerc 37:1664–1672 [DOI] [PubMed] [Google Scholar]
- 611. Basaria S , Coviello AD , Travison TG , Storer TW , Farwell WR , Jette AM , Eder R , Tennstedt S , Ulloor J , Zhang A , Choong K , Lakshman KM , Mazer NA , Miciek R , Krasnoff J , Elmi A , Knapp PE , Brooks B , Appleman E , Aggarwal S , Bhasin G , Hede-Brierley L , Bhatia A , Collins L , LeBrasseur N , Fiore LD , Bhasin S. 2010. Adverse events associated with testosterone administration. N Engl J Med 363:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612. 2011. The testosterone trial. [electronic mail system]. http://clinicaltrials.gov/ct2/show/NCT00799617
- 613. Snyder PJ , Lawrence DA. 1980. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab 51:1335–1339 [DOI] [PubMed] [Google Scholar]
- 614. Chan JM , Stampfer MJ , Giovannucci E , Gann PH , Ma J , Wilkinson P , Hennekens CH , Pollak M. 1998. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 279:563–566 [DOI] [PubMed] [Google Scholar]
- 615. Hankinson SE , Willett WC , Colditz GA , Hunter DJ , Michaud DS , Deroo B , Rosner B , Speizer FE , Pollak M. 1998. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 351:1393–1396 [DOI] [PubMed] [Google Scholar]
- 616. Simon HA. 1954. Spurious correlation: A causal interpretation. Journal of the American Statistical Association 49:467–479 [Google Scholar]
- 617. Nabarro JD. 1987. Acromegaly. Clin Endocrinol (Oxf) 26:481–512 [DOI] [PubMed] [Google Scholar]
- 618. Olsson DS , Buchfelder M , Schlaffer S , Bengtsson BA , Jakobsson KE , Johannsson G , Nilsson AG. 2009. Comparing progression of non-functioning pituitary adenomas in hypopituitarism patients with and without long-term GH replacement therapy. Eur J Endocrinol 161:663–669 [DOI] [PubMed] [Google Scholar]
- 619. Hatrick AG , Boghalo P , Bingham JB , Ayres AB , Sonksen PH , Russell-Jones DL. 2002. Does GH replacement therapy in adult GH-deficient patients result in recurrence or increase in size of pituitary tumours? Eur J Endocrinol 146:807–811 [DOI] [PubMed] [Google Scholar]
- 620. Fradkin JE , Mills JL , Schonberger LB , Wysowski DK , Thomson R , Durako SJ , Robison LL. 1993. Risk of leukemia after treatment with pituitary growth hormone. JAMA 270:2829–2832 [PubMed] [Google Scholar]
- 621. Swerdlow AJ , Higgins CD , Adlard P , Preece MA. 2002. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959–85: a cohort study. Lancet 360:273–277 [DOI] [PubMed] [Google Scholar]
- 622. Thorner MO. 2001. Critical evaluation of the safety of recombinant human growth hormone administration: statement from the Growth Hormone Research Society. J Clin Endocrinol Metab 86:1868–1870 [DOI] [PubMed] [Google Scholar]
- 623. Kehely A , Bates PC , Frewer P , Birkett M , Blum WF , Mamessier P , Ezzat S , Ho KK , Lombardi G , Luger A , Marek J , Russell-Jones D , Sönksen P , Attanasio AF. 2002. Short-term safety and efficacy of human GH replacement therapy in 595 adults with GH deficiency: a comparison of two dosage algorithms. J Clin Endocrinol Metab 87:1974–1979 [DOI] [PubMed] [Google Scholar]
- 624. Hoffman AR , Kuntze JE , Baptista J , Baum HB , Baumann GP , Biller BM , Clark RV , Cook D , Inzucchi SE , Kleinberg D , Klibanski A , Phillips LS , Ridgway EC , Robbins RJ , Schlechte J , Sharma M , Thorner MO , Vance ML. 2004. Growth hormone (GH) replacement therapy in adult-onset GH deficiency: effects on body composition in men and women in a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab 89:2048–2056 [DOI] [PubMed] [Google Scholar]
- 625. Reaven GM. 1988. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 626. Randle PJ , Garland PB , Hales CN , Newsholme EA. 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789 [DOI] [PubMed] [Google Scholar]
- 627. Segerlantz M , Bramnert M , Manhem P , Laurila E , Groop LC. 2001. Inhibition of the rise in FFA by Acipimox partially prevents GH-induced insulin resistance in GH-deficient adults. J Clin Endocrinol Metab 86:5813–5818 [DOI] [PubMed] [Google Scholar]
- 628. Svensson J , Bengtsson BA. 2003. Growth hormone replacement therapy and insulin sensitivity. J Clin Endocrinol Metab 88:1453–1454 [DOI] [PubMed] [Google Scholar]
- 629. Calof OM , Singh AB , Lee ML , Kenny AM , Urban RJ , Tenover JL , Bhasin S. 2005. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 60:1451–1457 [DOI] [PubMed] [Google Scholar]
- 630. Haddad RM , Kennedy CC , Caples SM , Tracz MJ , Boloña ER , Sideras K , Uraga MV , Erwin PJ , Montori VM. 2007. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc 82:29–39 [DOI] [PubMed] [Google Scholar]
- 631. Curran MJ , Bihrle W. 1999. Dramatic rise in prostate-specific antigen after androgen replacement in a hypogonadal man with occult adenocarcinoma of the prostate. Urology 53:423–424 [DOI] [PubMed] [Google Scholar]
- 632. Goldenberg SL , Bruchovsky N , Gleave ME , Sullivan LD , Akakura K. 1995. Intermittent androgen suppression in the treatment of prostate cancer: a preliminary report. Urology 45:839–844; discussion 844–845 [DOI] [PubMed] [Google Scholar]
- 633. Heikkilä R , Aho K , Heliövaara M , Hakama M , Marniemi J , Reunanen A , Knekt P. 1999. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal study. Cancer 86:312–315 [PubMed] [Google Scholar]
- 634. Morgentaler A. 2006. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol 50:935–939 [DOI] [PubMed] [Google Scholar]
- 635. Rhoden EL , Morgentaler A. 2004. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 350:482–492 [DOI] [PubMed] [Google Scholar]
- 636. Bhasin S , Singh AB , Mac RP , Carter B , Lee MI , Cunningham GR. 2003. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl 24:299–311 [DOI] [PubMed] [Google Scholar]
- 637. Clague JE , Wu FC , Horan MA. 1999. Difficulties in measuring the effect of testosterone replacement therapy on muscle function in older men. Int J Androl 22:261–265 [DOI] [PubMed] [Google Scholar]
- 638. Schröder FH , Kranse R. 2003. Verification bias and the prostate-specific antigen test—is there a case for a lower threshold for biopsy? N Engl J Med 349:393–395 [DOI] [PubMed] [Google Scholar]
- 639. Thompson IM , Ankerst DP , Chi C , Goodman PJ , Tangen CM , Lucia MS , Feng Z , Parnes HL , Coltman CA. 2006. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 98:529–534 [DOI] [PubMed] [Google Scholar]
- 640. Carter HB. 1997. PSA variability versus velocity. Urology 49:305. [PubMed] [Google Scholar]
- 641. Morales A. 1999. Andropause, androgen therapy and prostate safety. The Aging Male 2:81–86 [Google Scholar]
- 642. Coviello AD , Kaplan B , Lakshman KM , Chen T , Singh AB , Bhasin S. 2008. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 93:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643. Bachman E , Feng R , Travison T , Li M , Olbina G , Ostland V , Ulloor J , Zhang A , Basaria S , Ganz T , Westerman M , Bhasin S. 2010. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab 95:4743–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644. Liu PY , Yee B , Wishart SM , Jimenez M , Jung DG , Grunstein RR , Handelsman DJ. 2003. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab 88:3605–3613 [DOI] [PubMed] [Google Scholar]
- 645. Münzer T , Harman SM , Sorkin JD , Blackman MR. 2009. Growth hormone and sex steroid effects on serum glucose, insulin, and lipid concentrations in healthy older women and men. J Clin Endocrinol Metab 94:3833–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646. Giannoulis MG , Jackson N , Shojaee-Moradie F , Sonksen PH , Martin FC , Umpleby AM. 2006. Effects of growth hormone and/or testosterone on very low density lipoprotein apolipoprotein B100 kinetics and plasma lipids in healthy elderly men: a randomised controlled trial. Growth Horm IGF Res 16:308–317 [DOI] [PubMed] [Google Scholar]
- 647. Snyder PJ , Peachey H , Berlin JA , Rader D , Usher D , Loh L , Hannoush P , Dlewati A , Holmes JH , Santanna J , Strom BL. 2001. Effect of transdermal testosterone treatment on serum lipid and apolipoprotein levels in men more than 65 years of age. Am J Med 111:255–260 [DOI] [PubMed] [Google Scholar]
- 648. Basu R , Dalla Man C , Campioni M , Basu A , Nair KS , Jensen MD , Khosla S , Klee G , Toffolo G , Cobelli C , Rizza RA. 2007. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care 30:1972–1978 [DOI] [PubMed] [Google Scholar]
- 649. Srinivasan M , Irving BA , Frye RL , O'Brien P , Hartman SJ , McConnell JP , Nair KS. 2010. Effects on lipoprotein particles of long-term dehydroepiandrosterone in elderly men and women and testosterone in elderly men. J Clin Endocrinol Metab 95:1617–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650. Jørgensen JO , Pedersen SA , Thuesen L , Jørgensen J , Ingemann-Hansen T , Skakkebaek NE , Christiansen JS. 1989. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet 333:1221–12255. [DOI] [PubMed] [Google Scholar]