Abstract
Necrotizing soft tissue infections (NSTI) have been recognized for millennia and continue to impose considerable burden on both patient and society in terms of morbidity, death, and the allocation of resources. With improvements in the delivery of critical care, outcomes have improved, although disease-specific therapies are lacking. The basic principles of early diagnosis, of prompt and broad antimicrobial therapy, and of aggressive debridement have remained unchanged. Clearly novel and new therapeutics are needed to combat this persistently lethal disease. This review emphasizes the pillars of NSTI management and then summarizes the contemporary evidence supporting the incorporation of novel adjuncts to the pharmacologic and operative foundations of managing this disease.
Keywords: : necrotizing fasciitis, necrotizing soft tissue infection, sepsis
Necrotizing soft tissue infections (NSTI) have been recognized for millennia, with reports dating back to notations by Hippocrates in the fifth century BCE: “Many were attacked by the erysipelas all over the body when the exciting cause was a trivial accident…flesh, sinews, and bones fell away in large quantities…there were many deaths” [1]. A potentially life-threatening infection of the neck and floor of the mouth, Ludwig angina, was described by Wilhelm Frederick von Ludwig in 1836 [2]. Genital and perineal region necrotizing infections were first reported in 1764 by Baurienne [3], but they were not referred to as Fournier gangrene until the late 1800s after a series of five male patients were presented in 1883 and 1884 by the French dermatologist and venereologist Jean Alfred Fournier [4,5]. The first large-scale description of necrotizing infections came during the American Civil War when a Confederate Army surgeon, Dr. Joseph Jones, reported 2,642 cases with a mortality rate of 46% [6].
Numerous terms have been used to describe NSTIs, including hospital gangrene, necrotizing erysipelas, suppurative fasciitis, clostridial gangrene, and gas gangrene. Wilson introduced the term necrotizing fasciitis in 1952 to refer to both gas-forming and non–gas-forming necrotizing infections [7]. Because diagnosis and management requires a similar approach regardless of the anatomic location or depth of involvement, necrotizing soft tissue infection has now supplanted necrotizing fasciitis as the preferred term, because it encompasses all forms of this potentially devastating infection.
The NSTIs are characterized as a collection of rapidly advancing, often fatal, infections of the soft tissue compartment (dermis, subcutaneous tissue, superficial fascia, deep fascia, or muscle). Typically, they are not associated with an abscess, but if an abscess is left untreated or is inadequately drained, it may transition to a rapidly progressive, necrotizing infection. Although a recent query of the Nationwide Inpatient Sample (1998–2010) reported a reduced incidence, there are still approximately 3,800–5,800 cases annually in the United States, with a case complication rate of nearly 50% and a case fatality rate of 5%–10% [8].
The basic principles of early diagnosis, prompt and broad antimicrobial therapy, and aggressive, serial, surgical debridement remain the pillars of therapy aimed at reducing morbidity and death [9–12]. Indeed these are the only factors that we, the clinicians, can modify to optimize outcome, and the data are nearly incontrovertible that delay, particularly in operative debridement, is associated with an increased risk of death [13–16]. Unfortunately, these basic principles have remained unchanged for decades, which likely underlies the relatively stagnant and still high and unacceptable mortality rates. Clearly novel and new therapeutics are needed to combat this persistently lethal disease.
In this study, we review the epidemiology, classification, and microbiology of NSTIs and subsequently provide a contemporary perspective of diagnosis and management. We conclude by conducting a systematic review to summarize the contemporary evidence supporting the incorporation of novel adjuncts to the pharmacologic and operative foundations of managing this disease: Hyperbaric oxygen therapy, intravenous immunoglobulin (IVIG), and blood purification.
Methods
We performed a systematic review of the MEDLINE and the Cochrane Database of Systematic Reviews from 2005 to 2015 using specific search strategies. We provide search strings and Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram as Appendix A (see online supplementary material at ftp.liebertpub.com). We restricted articles to adult (age ≥18 years) human data reported in the English language only. We screened articles published between January 1, 1995 and December 31, 2015. We focused on randomized clinical trials (RCTs), meta-analyses, systematic reviews, and clinical practice guidelines. We excluded opinion articles, commentaries, and case series, although we did incorporate observational trials.
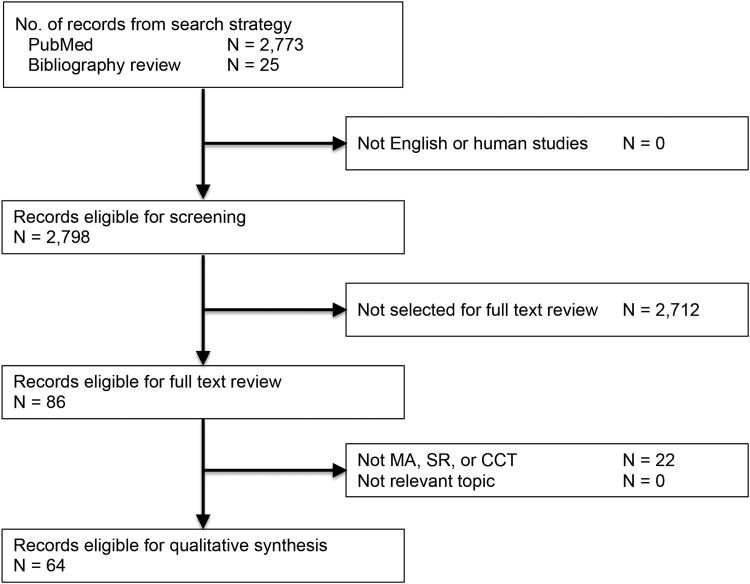
After screening 2,773 titles and abstracts, more articles were identified for full-text review, after which manual review of bibliographies generated 25 additional references. A total of 86 articles were reviewed manually, of which 64 were selected with relevant content (Fig. 1). We selected only articles deemed to provide major advances in the treatment of patients with NSTI. We considered sources of bias in these articles and defined areas of uncertainty as those in which the evidence conflicted. We used a systematic weighting of strength of recommendation and quality of evidence using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) system [17–20].
FIG. 1.
PRISMA flow diagram. MA = 8; SR = 3; CCT (not observational) = 21. Observational trials with controls = 32.
NSTIs
Epidemiology
The NSTIs are rare, with an estimated incidence in the United States to be between 500 and 1,500 cases per year [21]. Using insurance databases, Ellis Simonsen et al. [22] determined the incidence of NSTI to be 0.04 cases per 1,000 person years. Using the Nationwide Inpatient Sample from 2001 and 2004, Endorf et al. [23] found 10,940 patients (0.04%) with NSTI. Males were significantly more likely to have a NSTI (66.3% vs. 63.5%, p < 0.001).
The NSTI patients require extensive intensive care unit (ICU) and hospital resources. In the United Kingdom (UK) between 1995 and 2006, 0.24% of ICU admissions were because of necrotizing fasciitis [24]. In a study of patients admitted to burn centers versus non-burn centers, the average length of stay was 22.1 days for burn centers and 16.0 days for non-burn centers [23]. The cost of care is significant and ranges from $71,000 to $83,000 [23]. The majority of patients with NSTI are treated by surgeons in the community, but increasingly, these patients are being referred to tertiary care hospitals and burn centers for specialized wound and critical care management.
Death from NSTI has decreased over the past 30 years. In the UK between 1995 and 2006, patients with NSTI had a mortality rate of 41.6%. A review of 27 case series between 1980 and 1998 reported an estimated mortality rate of 32% [25]. In 67 studies that included 3,302 patients from 1980–2008, the overall mortality rate was 23.5% [26]. There was only a slight downward trend of 27.8% to 21.7% between those studies published from 1980 to 1999 and the studies from 1999–2008 [26]. A review of NSTI from the National Surgical Quality Improvement Program database found a mortality rate of only 12% [27]. In a study of NSTI at six academic hospitals in Texas between 2004 and 2007, mortality rates varied between 9% and 25% [28].
Comparison between studies is difficult because of the rarity and the heterogeneity of disease presentation. Improvements in outcome continue to require early diagnosis, early and aggressive surgical debridement, early administration of appropriate empiric antibiotic agents that are then tailored to microbiologic identification and antimicrobial susceptibility, and optimization of underlying medical co-morbidities.
Classification
Skin and soft tissue infections are classified in numerous ways and often for specific reasons. For purposes of evaluating new therapeutics for the management of soft tissue infections, the Food and Drug Administration classifies soft tissue infections into two broad categories: Uncomplicated and complicated. Uncomplicated soft tissue infections include superficial infections such as cellulitis, impetiginous lesions, furuncles, and simple abscesses that can be managed with surgical incision alone. Complicated soft tissue infections such as infected ulcers, infected burns, and major abscesses require significant surgical interventions. For clinical therapeutic trials, NSTI is generally an exclusion criterion.
A more useful classification divides soft tissue infections into either non-necrotizing or necrotizing infections. It is important to differentiate necrotizing infections from non-necrotizing infections, because necrotizing infections require aggressive surgical management. Necrotizing infections can be divided further into specific types based on anatomy (e.g., Fournier, Ludwig angina), depth of involvement (e.g., necrotizing adipositis, fasciitis, or myositis), microbial source of infection (types 1, 2, 3), or even a combination of microbial source and depth (i.e., clostridial cellulitis, non-clostridial anaerobic cellulitis).
Microbiology
Three basic microbial sub-types have been described. Type 1 infections are poly-microbial in nature and are the most prevalent form of NSTI, occurring in 55%–75% of NSTIs [16]. Type 2 infections are mono-microbial and are caused by Group A Streptococcus (GAS). Some authors also consider mono-microbial infections from community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) as Type 2 [29]. Type 3 infections are also mono-microbial and are attributed to Clostridium species or rare virulent microbes such as Vibrio vulnificus or Aeromonas species [29,30].
Type 1 infections are a mix of aerobic and anaerobic bacteria and average 4.4 isolates per specimen [31]. The predominant aerobic isolates are Streptococcus species, Staphylococcus species, Enterococcus species, Enterobacteriaceae family (Escherichia coli, Klebsiella species), Pseudomonas species, and Acinetobacter species. Bacteroides species are the most common anaerobic organisms. Clostridium species are common participants in poly-microbial infections but are not necessarily causative of myonecrosis. Fungal species are occasional pathogens [14,31].
Poly-microbial NSTIs tend to occur on the perineum and trunks of immunocompromised patients. Fournier gangrene is a classic example of poly-microbial NSTI. Diabetes mellitus and peripheral vascular disease are common predisposing factors in poly-microbial NSTI [32]. Other predisposing factors include obesity, chronic renal failure, human immunodeficiency virus (HIV) infection, alcohol abuse, abscess, intravenous (IV) drug use, insect bites, recent surgical incisions, and perforation of the gastrointestinal tract [29,33,34]. Interestingly, an inciting event is never identified in 20%–50% of the patients [14,15,35].
Another example of Type 1 NSTI is cervical necrotizing fasciitis. Bacterial penetration into the fascial compartments of the head and neck results in a rapidly progressive gangrenous cellulitis with life-threatening airway obstruction. It is most often associated with an odontogenic infection (78%–90%), but other causes include trauma, tongue piercing, neoplasm, and other parapharyngeal infections [36–39]. Both Ludwig angina (submandibular space infection) and cervical necrotizing fasciitis are usually caused by mouth anaerobes such as Fusobacterium species, anaerobic Streptococcus species, Peptostreptococcus species, Bacteroides species, and spirochetes.
Type 2 NSTIs are caused by GAS (S. pyogenes) either alone or in association with S. aureus. In up to half of patients, Type 2 infections may be accompanied by toxic shock syndrome [40,41]. In contrast to Type 1 NSTI, Type 2 NSTI can occur in any age group and without predisposing medical conditions [14]. Predisposing factors include a history of trauma, IV drug use, surgical procedures, childbirth, burns, exposure to a case, and potentially non-steroidal anti-inflammatory drugs (NSAIDS) [42,43].
Over the past 10 years, MRSA has been seen increasingly as a mono-microbial cause of NSTI. The CA-MRSA that is overwhelmingly associated with NSTI is clone USA300 containing the Panton-Valentine leukocidin cytotoxin [44]. In some communities, this CA-MRSA is responsible for more than 15% of NSTI [43]. Although CA-MRSA most commonly causes necrotizing infection of the subcutaneous tissue and skin, more severe invasive disease such as necrotizing fasciitis and pyomyositis are being reported increasingly [44,45].
Clostridial infections have been classified by some as Type 3 NSTI [30]. Clostridium species are gram-positive, spore-forming, anaerobic rods normally found in soil and the gastrointestinal tract. Clostridial infections are associated classically with trauma or surgery. As surgical technique and wound care have improved, clostridial infections have decreased and are more likely now to be associated with wounds from IV drug abuse [15,46,47]. Clostridium perfringens is the etiology of 70%–80% of clostridial infections [48]. Its local and systemic manifestations are because of the production of potent extracellular toxins. Alpha toxin (a phospholipase C) and theta toxin (perfringolysin) are the two most potent proteins causing hemolysis, microvascular thrombosis, and myonecrosis [48]. Alpha toxin directly inhibits myocardial contractility and indirectly induces systemic cytokine expression, which may contribute to the circulatory collapse commonly seen with this infection [48]. Spontaneous gas gangrene is a rare clostridial infection caused by the hematogenous spread of C. septicum from the gastrointestinal tract in patients with a perforation from colon cancer or diverticulitis [49].
Members of the Vibrionaceae family (V. vulnificus and others) and Aeromonas species, are rare but potentially lethal causes of NSTI. Vibrio vulnificus is endemic to warm coastal waters and raw seafood while Aeromonas species are found in fresh or brackish water, soil, or wood [50–52]. Patients at greatest risk appear to be those with underlying hepatic dysfunction, diabetes mellitus, and other immunocompromised conditions [51,53–55].
The clinical signs and symptoms of V. vulnificus and Aeromonas NSTI are similar. Patients with hemorrhagic bullae, subcutaneous bleeding, purpura, necrosis, and gangrene who present with a fulminating course should raise suspicion of these entities. Clinical history should help differentiate the potential source, because exposure to seawater or shellfish would suggest Vibrio species, while exposure to fresh or brackish water, soil, or wood would point to Aeromonas species. Treatment usually necessitates aggressive debridement, especially in those patients with shock, leukopenia, severe hypoalbuminemia, and underlying chronic illness, especially a combination of hepatic dysfunction and diabetes mellitus [52]. These infections are aggressive, resulting in a high rate of amputation and death.
Mucormycosis (most familiar as zygomycosis) is an uncommon but potentially devastating cause of NSTI. These fungi are ubiquitous and found in soil, manure, plants, and decaying material [56]. Agents most commonly found in human infections are Mucor, Rhizopus, Rhizomucor, Lichtheimia, and Cunninghanmella [57]. Cutaneous mucormycosis is associated with trauma and burn wounds and has mortality rates ranging 38%–80% [58,59]. Patients in whom cutaneous mucormycosis develops from minor breaks in the skin almost always have an underlying disease causing immunosuppression [56,60,61]. Treatment of patients with cutaneous mucormycosis requires aggressive debridement, antifungal therapy, and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and neutropenia. Deferoxamine, which chelates both iron and aluminum, has been associated with promoting tissue invasion by behaving as a siderophore and supplying the organisms with iron [62,63].
Clinical presentation
The presentation of NSTI varies widely, ranging from skin and subcutaneous necrosis to life-threatening sepsis with muscle and fascial involvement. Although necrotizing infection leads to massive destruction of tissues, the initial presentation is not always obvious, because it may involve only the deep tissues in the early phases, leaving the overlying skin appearing normal. This makes the diagnosis of NSTI very difficult. It is important to recognize NSTI early, because delay in the diagnosis and treatment is associated with extensive tissue destruction, limb loss, and death [41,64–67].
Localized pain may be the earliest symptom of NSTI [66, 67]. Pain out of proportion to the physical appearance should raise the suspicion of NSTI. Other clinical manifestations may be edema beyond the area of erythema, skin anesthesia, epidermolysis, and bronzing of the skin (Table 1). Hemorrhagic bullae, crepitus, foul odor, “dishwater” drainage, and dermal gangrene are late manifestations and are usually associated with systemic sepsis and organ dysfunction [68]. An apparent superficial cellulitis that progresses rapidly, fails to respond to standard therapy, or is associated with evolving systemic signs of sepsis must raise the suspicion of a more extensive underlying infection.
Table 1.
Clinical Findings of Necrotizing Soft Tissue Infections
| Early physical findings | Late physical findings |
|---|---|
| Pain out of proportion to examination Erythema Hyperthermia Edema beyond the area of erythema Skin anesthesia Epidermolysis Bronzing of the skin Tachycardia Fever |
Hemorrhagic bullae Foul odor Brownish-tan “dishwater” drainage Dermal gangrene Crepitus Severe pain out of proportion to examination Rapid progression of erythema, edema, pain Systemic inflammatory response syndrome Sepsis Shock and organ failure |
There are multiple risk factors associated with NSTI (Table 2). These include advanced age (>60 years), IV drug use, diabetes mellitus, obesity, malnutrition, congestive heart disease, chronic pulmonary disease, peripheral vascular disease, chronic alcoholism, and immunocompromised states such as malignancy, steroid use, transplantation, and HIV infection [68–70]. Several studies have demonstrated that IV and subcutaneous injection of illicit drugs is the greatest risk factor for NSTI in the urban setting [69,71,72]. Thus, a soft tissue infection in a patient with a recent history of injection should raise the suspicion of more extensive underlying necrotizing infection. It is also important to note that NSTIs do occur in healthy persons as much as 30% of the time [73].
Table 2.
Risk Factors for Necrotizing Soft Tissue Infections
| Advanced age (>60 y) |
| Intravenous drug use |
| Diabetes mellitus |
| Obesity |
| Malnutrition |
| Congestive heart disease |
| Chronic pulmonary disease |
| Peripheral vascular disease |
| Chronic alcoholism |
| Immunocompromised states |
| Malignancy |
| Steroid use |
| HIV infection/AIDS |
| Transplantation |
HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome.
The reported sites of soft tissue infections vary depending on the series [68,69,73]. The most common site of infection is the extremity, followed by perineum and buttocks, trunk, and head and neck. The cause of NSTI is not always obvious and usually involves tissue damage. The reported etiologies are injection of illicit substances, cutaneous infection or ulceration, post-operative infection, peri-rectal abscesses, soft tissue trauma, strangulated hernias, perforated viscus, colostomy site infection, and idiopathic causes [69,74,75]. The association between NSAIDS and NSTI has also been shown [76–78]. It is unclear, however, whether this association is attributable to the immunomodulatory effect of NSAIDS or to the fact that patients with NSTI use NSAIDS to suppress fever and pain. Nevertheless, NSAIDS may mask the usual signs of inflammation and thereby delay the diagnosis.
Diagnosis
Early diagnosis of NSTI is difficult, because early clinical cutaneous manifestations may not be distinguishable from simple superficial infection. There are several methods proposed for the early diagnosis of NSTI. One such method is the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score by Wong et al. [79]. The score was devised retrospectively, based on six common clinical parameters: Serum C-reactive protein (CRP), white blood cell count, hemoglobin concentration, serum sodium, serum creatinine level, and serum glucose level (Table 3). According to the authors, a minimum score of 6 is associated with necrotizing fasciitis, with a positive predictive value of 92% and negative predictive value of 96%. Since the development of the LRINEC score, a few cohort studies attempted to validate its clinical use for the diagnosis of NSTI. In one study, Su et al. [80] reviewed retrospectively 209 patients with a confirmed diagnosis of necrotizing fasciitis and showed that only 100 of 209 (48%) had a LRINEC score ≥6. These authors also demonstrated, however, that the group of patients with LRINEC scores ≥6 had a higher rate of amputation and death compared with the group with LRINEC scores <6. This study suggests that the LRINEC score may be more helpful in prognosticating than in diagnosing NSTI.
Table 3.
The Laboratory Risk Indicator for Necrotizing Fasciitis Score
| Variables, units | Score |
|---|---|
| C-reactive protein, mg/L | |
| <150 | 0 |
| ≥150 | 4 |
| Total white blood cell count, per mm3 | |
| <15 | 0 |
| 15–25 | 1 |
| >25 | 2 |
| Hemoglobin, g/dL | |
| >13.5 | 0 |
| 11–13.5 | 1 |
| <11 | 2 |
| Sodium, mmol/L | |
| ≥135 | 0 |
| 135 | 2 |
| Creatinine, μmol/L | |
| ≤141 | 0 |
| >141 | 2 |
| Glucose, mmol/L | |
| ≤10 | 0 |
| >10 | 1 |
In a more recent cohort study of a much smaller scale (N = 28 patients), the LRINEC score was found to have a sensitivity of 80%, a specificity of 67%, a positive predictive value of 57%, and a negative predictive value of 86% [81]. The sensitivity and specificity reported here are much smaller than those proposed by Wong et al. [79]. Mills et al. [27] reported that leukocytosis and hyponatremia occur simultaneously in only 22% of patients with NSTI, suggesting that the sensitivity of these parameters for the detection of NSTI is low. The major drawback of the LRINEC scoring system is that it is derived from retrospective data and is therefore predisposed to selection bias.
Imaging studies such as conventional radiography and computed tomography (CT) are only helpful if there is gas in the tissue. The presence of gas in the tissue together with a suspicious clinical presentation is pathognomonic of NSTI. Radiographic detection of sub-cutaneous emphysema, however, is seen in only 39% of patients with NSTI [82]. In detecting inflammatory changes, CT is very sensitive but is not necessarily specific for necrotizing infection. In the diagnosis of deep abscesses, however, CT is helpful, especially intra-muscular abscesses that otherwise cannot be diagnosed with physical examination. Magnetic resonance imaging (MRI) with gadolinium contrast enhancement can accurately differentiate between necrotizing infection from non-necrotizing infection [83]. Unfortunately, the time it takes to obtain an MRI scan, especially in an unstable patient with sepsis, who may be undergoing aggressive resuscitation, is difficult to justify. The MRI scan is not always accessible readily in many facilities. Further, an MRI scan requires patient compliance, and any movement by the patient during the study may render a very time-consuming study non-interpretable, thus further delaying diagnosis and treatment.
Bedside tissue biopsy with frozen section has been suggested for the diagnosis of NSTI [84]. The diagnosis is made based on histologic changes that include tissue necrosis, poly-morphonuclear infiltration, fibrinous vascular thrombosis, and sometimes micro-organisms within the destroyed tissue. Although the reported series shows this method to be reliable, the experience is limited. Further, a pathologist is not always available readily at night for frozen section interpretation, and waiting until the next day is not a viable option.
Aspiration of the fluid for the detection of organisms by Gram stain and cultures has also been suggested [85]. The presence of organisms, however, does not necessarily equate to a necrotizing infection and is not as reliable as taking deep tissue samples at the time of surgical exploration. Likewise, failure to obtain fluid is non-diagnostic and does not rule out necrotizing infection. Cultures take too long to make a timely diagnosis of NSTI.
The diagnosis of NSTI, therefore, must be made clinically and surgically. The clinical history and a meticulous physical examination are essential to establish an early suspicion of the disease. Clinical history and physical features that raise the suspicion of NSTI should prompt surgical exploration for a more definitive diagnosis and treatment. Indeed, the data support that a low threshold for operative diagnosis and acceptance of a high incidence of non-therapeutic operative intervention is necessary to optimize sensitivity and ensure that all patients received expeditious care.
Management
The management of NSTI requires aggressive resuscitation, IV antibiotic agents, complete surgical debridement, and supportive care. All aspects of treatment should be started promptly and simultaneously. The single most important aspect of managing NSTI is complete debridement of necrotic and infected tissues. Early operative debridement is the major determinant of outcome [64, 65]. Surgical debridement should never be delayed in the hope of restoring hemodynamic stability before anesthesia induction, because correction of the septic state will not occur until all of the infected and necrotic tissues have been removed.
Surgical management
When NSTI is suspected, surgical exploration is indicated. This involves making an incision over the inflamed and tender area and dissecting down to the fascia. A change in the fascia from a tough and shiny white appearance to a dull gray fascia that can be easily separated from the fat with blunt dissection is indicative of necrotizing infection: “The finger test.” The classically described brownish-tan “dishwater” fluid weeping from the tissues, if present, is also highly suggestive of NSTI. The underlying muscles should also be examined closely by making an incision in the fascia, whether or not the fascia appears normal. All necrotic fascia and muscles, as well as the overlying skin, should be excised. The excision margin should be healthy bleeding tissue.
The patient's ultimate outcome depends on the completeness of surgical debridement. This sometimes means amputation of the affected limb if there is extensive soft tissue loss preventing any reasonable functional recovery or if there is destruction of the major nerves and blood vessels. Under no circumstance should the wounds be closed at the time of surgical debridement, but rather packed for open drainage and re-exploration. Perineal gangrene often involves the scrotum and peri-anal skin. Surgical debridement usually involves excision of the scrotal skin and perineal skin extending to the gluteal region. The testicles are usually spared, and orchiectomy is required rarely.
After adequate surgical debridement, the patient's hemodynamic status should improve significantly. The patient's wound should be re-explored in the operating room within 24 hours to evaluate whether the spread of infection has been stopped and if further debridement is required. All newly identified necrotic tissue should be aggressively debrided. In patients whose clinical condition continues to deteriorate, re-exploration should be considered sooner, because there is a very high likelihood that the initial debridement is inadequate or the infection has spread even further. The goal of surgical therapy is complete debridement with the initial operation. It is not at all uncommon, however, that multiple debridements are required. In one study, 64% of the patients required at least one other debridement [69].
Surgical debridement is usually extensive and involves significant blood loss. Therefore, part of the pre-operative workup should include type and cross match for red blood cells. Many of these patients are coagulopathic, and efforts should be made to correct the coagulopathy to minimize blood loss. Patients are at risk for hypothermia, because a large surface area is often exposed and large areas of skin are excised.
Critical care management
As soon as the diagnosis of NSTI is suspected, immediate fluid resuscitation should begin. Many of these patients are hypovolemic and often have acute kidney injury. Aggressive initial fluid resuscitation will help restore intravascular volume, maintain adequate end-organ perfusion and tissue oxygenation, and limit the adverse effects of end-organ failure. The magnitude of resuscitation is dependent on the individual patient's physiologic status and should be targeted to provide adequate perfusion to the organs. For patients who are in shock or who have underlying cardiac or pulmonary disease, intravascular volume status requires monitoring.
Initial IV antibiotic therapy should be broad enough to cover the diverse and various causative agents (Table 4). High dose penicillin G or ampicillin should be used to cover for potential Clostridium, Streptococcus, and Peptostreptococcus infections. Penicillin G, if chosen, should be given as 18–24 million units per day for an adult. Anaerobes such as Bacteroides, Fusobacterium, and Peptostreptococcus should also be covered with clindamycin or metronidazole. Clindamycin is also effective in treating patients with group A β-hemolytic Streptococcus by suppressing the production of exotoxins [86]. Clindamycin is also the drug of choice for patients allergic to penicillin. Gram-negative coverage can be achieved by adding an aminoglycoside, a third or fourth generation cephalosporin, a fluoroquinolone, aztreonam, or a carbapenem. Alternatively, penicillin or ampicillin can be replaced by piperacillin-tazobactam to include gram-negative coverage.
Table 4.
Necrotizing Soft Tissue Infections—Empiric Antibiotic Regimens
| A. Patients without history of any penicillin or vancomycin allergy, or severe allergy (i.e., urticarial rash or anaphylactic reaction) to cephalosporins | |||||
|---|---|---|---|---|---|
| Drug | Dose | Route | Frequency | 1st dose administration prioritya | Rate of administration 1st dose |
| Piperacillin- tazobactam | 4.5 g | IV | Q6h | 1st | Over 30 min |
| Clindamycin | 900 mg | IV | Q8h | 2nd | Over 30 min |
| Vancomycin | 25 mg/kg × 1, then 15 mg/kg | IV | Q12h | 3rd | 2 h |
| aDo not delay antibiotic administration if 1st priority drug not available; instead begin with next highest priority drug and then give 1st priority drug as soon as possible. | |||||
| Patients without documented type of allergic reaction should be treated as if allergic reaction is severe. Doses reflect standard regimens, adjust accordingly for end-organ dysfunction. | |||||
| IV = intravenous; AKI = acute kidney injury. | |||||
| B. Patients with any history of penicillin or cephalosporin allergy | |||||
|---|---|---|---|---|---|
| Drug | Dose | Route | Frequency | 1st dose administration prioritya | Rate of administration of 1st dose |
| Aztreonamb | 2 g | IV | Q8h | 2nd | Over 60 min |
| Metronidazole | 500 mg | IV | Q6h | 4th | Over 60 min |
| Clindamycin | 900 mg | IV | Q8h | 1st | Over 30 min |
| Vancomycin | 25 mg/kg × 1, then 15 mg/kg | IV | Q12h | 3rd | 2 h |
| aDo not delay antibiotic administration if 1st priority drug not available; instead begin with next highest priority drug and then give 1st priority drug as soon as possible. | |||||
| bLocal susceptibility data for common gram-negatives may guide selection; contemporary guidelines recommend fluoroquinolone or aminoglycoside. | |||||
| Patients without documented type of allergic reaction should be treated as if allergic reaction is severe. Doses reflect standard regimens, adjust accordingly for end-organ dysfunction. | |||||
| IV = intravenous; AKI = acute kidney injury. | |||||
| C. Patients with history of severe vancomycin allergy (i.e., urticarial rash or anaphylactic reaction) | |||||
|---|---|---|---|---|---|
| Drug | Dose | Route | Frequency | 1st dose administration prioritya | Rate of administration of 1st dose |
| Piperacillin- tazobactam | 4.5 g | IV | Q6h | 1st | Over 30 min |
| Clindamycin | 900 mg | IV | Q8h | 2nd | Over 30 min |
| Daptomycinb | 6 mg/kgc (round to nearest 50 mg) | IV | Q24h | 3rd | Over 30 min |
| aDo not delay antibiotic administration if 1st priority drug not available; instead, begin with next highest priority drug and then give 1st priority drug as soon as possible. | |||||
| bObtain baseline and weekly creatine phosphokinase. | |||||
| cUse actual body weight. | |||||
| Patients without documented type of allergic reaction should be treated as if allergic reaction is severe. Doses reflect standard regimens, adjust accordingly for end-organ dysfunction. | |||||
| IV = intravenous. | |||||
| D. For patients with history of severe penicillin, cephalosporin, and vancomycin allergy (i.e., urticarial rash or anaphylactic reaction) | |||||
|---|---|---|---|---|---|
| Drug | Dose | Route | Frequency | 1st dose administration prioritya | Rate of administration 1st dose |
| Aztreonamb | 2 g | IV | Q8h | 2nd | Over 60 min |
| Metronidazole | 500 mg | IV | Q6h | 4th | Over 60 min |
| Clindamycin | 900 mg | IV | Q8h | 1st | Over 30 min |
| Daptomycinc | 6 mg/kgd (round to nearest 50 mg) | IV | Q24h | 3rd | Over 30 min |
| aDo not delay antibiotic administration if 1st priority drug not available; instead, begin with next highest priority drug and then give 1st priority drug as soon as possible. | |||||
| bLocal susceptibility data for common gram-negatives may guide selection; contemporary guidelines recommend fluoroquinolone or aminoglycoside. | |||||
| cObtain baseline and weekly creatine phosphokinase. | |||||
| dUse actual body weight. | |||||
| Patients without documented type of allergic reaction should be treated as if allergic reaction is severe. Doses reflect standard regimens, adjust accordingly for end-organ dysfunction. | |||||
| IV = intravenous. | |||||
| E. Patients with recent history of contact with freshwater or raw seafood, in addition to one of the above regimens. Add: | |||||
|---|---|---|---|---|---|
| Drug | Dose | Route | Frequency | 1st dose admininstration prioritya | Rate of administration 1st dose |
| Doxycycline | 100 mg | IV | Q12h | 1st | Over 60 min |
Do not delay antibiotic administration if 1st priority drug not available; instead, begin with next highest priority drug and then give 1st priority drug as soon as possible.
IV = intravenous.
Selection of antimicrobials that inhibit toxin production should be considered in patients with streptococcal, Clostridium spp., and staphylococcal infections, especially those with evidence of rapidly progressive or severe infections. Clindamycin, erythromycin, or linezolid are potential inhibitory agents, provided that the pathogen is sensitive to the antibiotic. Protein cytotoxins play a significant role in the pathogenesis of staphylococcal infections [87]. Phenol-soluble modulin peptides are staphylococcal-secreted peptides that recruit, activate, and lyse human neutrophils, thereby eliminating a main cellular defense against staphylococcal infection [78]. The β-lactams actually enhance toxin production, while both clindamycin and linezolid inhibit toxin production by suppressing translation, but not transcription of S. aureus toxin genes and directly inhibiting synthesis of group A streptococcal toxins [87]. When patients exhibit signs and symptoms of shock, coagulopathy, and organ failure, antitoxin antimicrobials should be initiated promptly.
Because CA-MRSA necrotizing infections are more prevalent, parenteral anti-MRSA agents should be considered for use in the initial antibiotic regimen in suspected cases. Clinicians should also be aware that not only is there an increase in frequency of MRSA isolates but there is also a shift of the vancomycin minimum inhibitory concentration (MIC). In a study from Houston over a 7-year period, more than a third of MRSA isolates had a MIC greater than or equal to 1 mcg/mL, and nearly a third of isolates had a MIC of 2 mcg/mL [88]. Treatment failure rates for MRSA infections with increased MIC are reported [89, 90].
The antibiotic recommendation for Vibrio and Aeromonas infections is high dose combination therapy that includes a cell wall-active agent (third generation cephalosporins, carbapenems), plus doxycycline (protein synthesis inhibitor) [9].
When invasive mucormycos has been demonstrated, a lipid-based formulation of amphotericin B should be started (less nephrotoxicity than the conventional deoxycholate formulation, but invariably this still occurs especially those with risk factors). Echinocandins have no in vitro activity against the agents of mucormycosis; however certain species do express the target 1,3 beta-D-glucan synthase complex, and there is an in-vitro and observational data supporting the use of echinocandins in combination with amphotericin B for the treatment of these infections [91–93]. Posaconazole, which is a broad-spectrum azole agent with in vitro activity against the zygomycetes, may be considered for combination therapy with amphotericin B, or can be used as step-down therapy for patients who have responded to amphotericin B. It is rarely used as salvage therapy for those who do not respond to or cannot tolerate amphotericin B [94–96].
Antibiotic agents should be adjusted as early as possible to only cover the causative agents once they have been identified. The identification of causative agents and their antibiotic susceptibilities are key information obtained at the time of surgical debridement that will guide antimicrobial therapy.
Supportive care of the patient is extremely important. Nutrition support should be started as soon as the patient can tolerate it. If the patient can take food by mouth, it is the preferred route. If the patient is intubated or is unable to obtain adequate caloric intake orally, enteral feedings via nasogastric or nasoduodenal feeding tube is preferred over parenteral nutrition. When calculating total caloric requirement, a consideration should be given to the fact that these patients will have an increase in caloric and protein demands because of the large protein loss through the open wounds and the hypermetabolic state. Appropriate vitamins such as vitamins A and C and minerals such as zinc should be given to patients with large open wounds to facilitate wound healing. Rehabilitation should be started as soon as the patient is stable and able to participate. Early mobilization will help minimize pulmonary complications and de-conditioning.
Wound management
Once surgical debridement is no longer required, wound care can be performed at the bedside. Because these wounds are so extensive, patients usually require large doses of narcotics for dressing changes. Topical antimicrobial therapy is not necessary and is never a substitute for adequate surgical debridement. Agents such as silver nitrate and silver sulfadiazine will alter the appearance of the wound, making it difficult to be examined. Betadine should not be used on an open wound, because it will cause cell damage and inhibit wound healing. Perineal wounds are especially difficult to manage, because soilage of the wound is frequent. Stool diversion by colostomy is rarely required, however. Meticulous wound care is all that is required even with the most difficult wounds. When the wound is clean, use of a vacuum wound dressing is an option to facilitate wound granulation.
For the majority of the wounds, closure is achieved with simple split thickness skin grafts. More complex wounds should be managed in conjunction with the plastic surgery team. Wound coverage should be performed when the patient is medically stable and the wound is free from infection. It is not necessary to wait for granulation tissue to fill the entire wound bed for split thickness skin grafts, because the grafts will take as long as there is a clean and vascularized bed that is free from infection. Early coverage of the wound is advantageous in that it will help decrease pain associated with dressing changes and decrease metabolic demands.
For perineal wounds that involve the scrotum, the best cosmetic result can be achieved by delayed primary closure of the wound, if it is small. If the wound is too large for primary closure, it should not be allowed to heal by secondary intention because this will lead to contracture deformity of the scrotum. Several scrotal reconstruction methods have been described, including musculocutaneous flap and fasciocutaneous flaps from the thigh and the abdomen [97–99]. Direct split thickness skin grafts over the denuded testicles have also been described to provide good cosmetic and functional outcome, with the additional advantage of being a single stage operation [100,101]. A simple and widely used method is placement of the testicles in subcutaneous pockets in the thigh. It is functionally acceptable, but cosmetically sub-optimal. Many surgeons use this technique for temporary coverage, which can be revised later with other reconstructive methods [100].
Novel adjuncts
Hyperbaric oxygen therapy
Hyperbaric oxygen (HBO) therapy has also been proposed as an adjunctive therapy for NSTI. The HBO therapy involves the administration of oxygen (FiO2 = 1.0) at a pressure exceeding 1 atmosphere absolute (ATA), which results in a dramatic increase in oxygen tension within tissues; three ATA yields an arterial oxygen tension of 300 mm Hg, by contrast to 75 mm Hg under standard conditions [16]. Direct bactericidal activity, enhanced innate immune function, and improved tissue healing have all been proposed as the underlying beneficial biologic mechanisms [12,16]. HBO has been incorporated into previous published guidelines for the management of NSTI [102], although well-conducted trials of the efficacy of HBO in NSTI are lacking.
Should HBO be used in NSTI?
In patients with Type I NSTI, HBO may offer survival benefit (Table 5). Two systematic reviews were identified, both of which were unable to conduct summary estimates because of the poor quality of the studies, and thus could not provide generalized conclusions or recommendations [103, 104]. We identified a total of 12 (n = 12) contemporary observational trials, of which nine (n = 9)[105–113] studies were characterized as incorporating substantial, or being comprised completely of, cases of Type I NSTI [105–116]. Three of these trials [107–109] performed an analysis adjusted for differences in severity of illness and case mix, of which two [107,108] reported improved survival with the use of HBO; the third trial did not observe benefit [109]. Of the unadjusted trials, four reported survival benefit with HBO in uni-variable analysis. A total of 11 additional trials were identified, although not included because of either being of a case series study design (Table 4).
Table 5.
Studies of Hyperbaric Oxygen in Necrotizing Soft Tissue Infections and Sepsis
| Author | Study design | Population | Sample size | Control | Intervention | Adjustment | Mortality or survival (HBO vs. none) |
|---|---|---|---|---|---|---|---|
| Meta-analyses/systematic reviews | |||||||
| Levett et al. (2015) | Meta-analysis | All | n = 673 studies reviewed; 0 studies included | N/A | Not described | N/A | Not described |
| Wang et al. (2003) | Systematic review | All | n = 17 studies for gas gangrene; n = 9 progressive necrotizing infection | N/A | n = 4 observational studies, 13 case series: 2–3 ATM, 4 to 44 tx; N = 6 observational studies, 3 case series: 2–3 ATM, usually 90 min/tx, for 5–7 tx | N/A | For gas gangrene, summary estimates could not be derived; for progressive necrotizing infection, there may be of benefit; in general, very difficult to generate conclusion because of poor quality of studies |
| Observational trials | |||||||
| Shaw et al. (2014) | Retrospective, multiple centers (n = 14), UHC | Predominantly Type I (FG, NF, gas gangrene) | 1,583 | n = 1,466 | n = 117, not described | Yes, multi-variable | In-hospital survival: aOR 10.6 (5.2–25.1); 95% vs. 88%, p = 0.028 |
| Li et al. (2014) | Retrospective | Type I (FG) | 28 | n = 12 | n = 16, 2.5 ATM, 90–120 min/tx, twice daily | None | In-hospital survival: 14/16 (87.5%) vs. 8/12(66%), p < 0.05 |
| Massey et al. (2012) | Retrospective | Mixed as extremity and perineum | 80 | n = 48 | n = 32, 2.8 ATM, 45 min/tx, 1–8 treatments | None | In-hospital survival: 84.4% vs. 81.2%, p = 0.77; no difference by location in perineum or extremity |
| Mehl et al. (2010) | Retrospective, single center | Predominantly Type I (FG, NF, gas gangrene) | 40 | n = 14 | n = 26, 2.0–2.8 ATM, 120 min/tx, 6–20 tx | None | In-hospital survival: 23/26 (88.5%) vs. 9/14 (64.3%), p = 0.07 |
| Hassan et al. (2010) | Retrospective, single center | Not determined | 67 | n = 38 | n = 29 | None | NS |
| Wolf et al. (2010) | Retrospective, single center | Head and neck | 17 | n = 4 | n = 13, not described | None | In-hospital: 17/17(100%) for entire cohort |
| George et al. (2009) | Retrospective, comparison of 2 centers | Type I (FG, mixed, poly-microbial) in 36%; Type II (strep) in 28%; Type III (clostridial in 5 patients) | 78 | n = 30 | n = 48, 3 ATM, 90 min/tx, 3 tx/d then 2 tx/d, until infection resolved | Yes, multi-variable | 44/48 (91.7%) vs. 26/30 (86.7%), p = 0.48; aOR 0.98 (0.18–5.42) |
| Krenk et al. (2007) | Retrospective, single center | Head and neck | 19 | n = 7 | n = 11, 2.8 ATM, 90 min/tx, 2–3 dives/day, until no necrosis | None | In-hospital survival: 11/11 (1005) vs. 2/8(25%), p < 0.001 |
| Ayan et al. (2005) | Retrospective, single center | Type 1 (FG) | 41 | n = 23 | n = 18, 2.5 ATM, 90 min/d, 3–10 d of treatment | None | In-hospital survival: 18/18 (100%) vs. 14/23 (61%), p = 0.013 |
| Mindrup et al. (2005) | Retrospective, single center | Type 1 (FG) | 42 | n = 16 | n = 26, 2.4–3 ATM, 30–90 min/tx, 1–3 dives/day, 2–26 treatments | None | In-hospital survival: 19/26 (72.1%) vs. 14/16 (87.5%), p = 0.44 |
| Sugihara et al. (2004) | Retrospective, single center | Not described, assumed to be all types | 23 | n = 9 | n = 14, 2.5 ATM, 90 min/tx, unknown daily frequency, until symptoms resolved | None | Not described |
| Wilkinson et al. (2004) | Retrospective, single center | Type I (FG, mixed, poly-microbial) | 44 | n = 11 | n = 33, 2.8 ATM, 60 min/tx, 3 tx/d then 2 tx/d, until infection resolved | Yes, multi-variable | In-hospital survival: 31/33 (94%) vs. 7/11 (64%), p = 0.03, univariable; aOR 8.9 (1.3–58) |
| Hollabaugh et al. (1998) | Retrospective, single center | Predominantly Type I (FG, NF, gas gangrene) | 26 | n = 12 | n = 12, 2.4 ATM, 90 min/tx, 2 tx/d for at least 7 d, then until therapy terminated | None | 13/14 (93%) vs. 7/12 (58%), p = 0.04; aOR 11, no 95% CI or p value provided |
| Shupak et al. (1997) | Retrospective, single center | ? | 37 | n = 12 | n = 25; 2.5 ATM, 45 min/t, 2 t/d, until infection resolved | None | In-hospital survival: 9/25 (67%) vs. 3/12 (75%), p = 0.71 |
HBO = hyperbaric oxygen; NA = not available; ATM = atmospheres; UHC = __________; FG = Fournier gangrene; NF = necrotizing fasciitis; aOR = adjusted odds ratio; CI = confidence interval.
We did not identify any contemporary trials that focused on Type II NSTI. Three retrospective trials incorporated an identifiable population that was diagnosed with streptococcal infections, although significant differences in deaths were not observed [109,113,116]. We did not identify any contemporary trials that focused on Type III (clostridial) NSTI, although a single trial incorporated a small cohort (n = 5) with clostridial infections [109].
Recommendation:
Hyperbaric oxygen may be considered as an adjunct (i.e., secondary treatment) to therapy in cases of Type I (poly-microbial) NSTI, but should not delay the administration of antibiotic agents or expeditious operative debridement (weak, low).
Recommendation:
Hyperbaric oxygen is not recommended as an adjunct (i.e., secondary treatment) to therapy in cases of Type II (streptococcal) NSTI (weak, very low).
Recommendation:
Hyperbaric oxygen is not recommended as an adjunct (i.e., secondary treatment) to therapy in cases of Type III (clostridial) NSTI (weak, very low).
When should HBO be used?
There are little data to guide the timing of or criteria to institute HBO therapy. No identified contemporary study reported clinical indications, aside from a diagnosis of NTSI. Similarly, no study detailed or defined physiologic indications for instituting HBO therapy. One study reported that the greatest benefit was observed in patients with the highest degree of organ injury [107].
Recommendation:
The use of HBO should occur after the administration of antibiotic agents and operative intervention (weak, very low).
How should HBO be administered?
The reported protocols for the administration of HBO therapy varied considerably in administration regimen: Atmospheric pressure (range): 2.0–2.8 atmospheres (ATM); session duration (range): 30–90 minutes per session; number of sessions (range): 2–3 sessions per day; and duration of therapy (range): 1–26 treatments, 3–10 days, until infection resolved [103–116].
There are no data regarding a standardized duration of HBO therapy. Retrospective studies continued HBO therapy for a wide range of sessions/days, reporting in most circumstances that therapy was continued until “the infection resolved.”
Recommendation:
Hyperbaric oxygen should be instituted at approximately 2.5 ATM for 30–90 minutes per session, 2–3 sessions per day as tolerated (strong, low).
Recommendation:
Hyperbaric oxygen should be continued until source control is achieved and physiologic abnormalities are resolving (weak, low).
Are there risks of HBO?
HBO may cause acute brain oxygen toxicity, which presents with seizures. In large prospective trials of the application of HBO to a wide variety of medical conditions, the incidence of this complication was low, 0.03% [117]. A systematic review of HBO use, however, specifically in the context of wounds, reported an incidence of grand mal seizures of 0.7% (23/322) [103].
Pressure-induced (barotraumas) to the middle ear trauma is a recognized complication of HBO, particularly in the intubated patient [118]. In the vast majority of the cases, this barotrauma is self-limiting. Pressure-induced trauma to the inner ears, lungs, sinuses, and teeth has also been reported, although exceedingly rare [118].
Recommendation:
Standardized seizure management should be incorporated into the institutional HBO treatment protocol (strong, low).
Recommendation:
A myringostomy tube should be placed before HBO therapy for all patients receiving mechanical ventilation (strong, low)
IVIG
The use of IVIG for the management of NSTI has been advocated by some authors. The rationale for the use of IVIG is that IVIG provides antibodies that can neutralize the circulating streptococcal exotoxins, thus reducing the toxin-induced tissue necrosis [119,120]. In addition, IVIG may also have an effect on the circulating cytokines, thereby controlling the systemic inflammatory response. There are risks associated with the use of IVIG, including allergic reactions and acute kidney injury, although contemporary studies suggest these events are rare [121,122]. Although there are some retrospective as well as prospective studies showing a potential benefit in the use of IVIG in NSTI, additional studies are required before it can be recommended for routine use in NSTI [123–125].
Should IVIG be used in Type I (poly-microbial) or Type III (clostridial) NSTI?
There were no identified studies of the use of IVIG for Type I NSTI. In our expanded search that included all causes of sepsis, we identified six systematic reviews and meta-analyses of the use of IVIG (Table 5) [126–131]. A reduced risk of death was reported in each study; however, sensitivity analyses restricted to studies of low bias rendered estimates not statistically significant [126,127,129,131].
Only four contemporary placebo-controlled RCT of IVIG for severe sepsis or septic shock were identified, of which none reported a significant reduction in death [122,132–134]. In one sub-group analysis, patients on appropriate antibiotics and who received IVIG exhibited a reduced risk of death [133]. The study by Werdan et al. [122] reported reduced Acute Physiology and Chronic Health Evaluation II score and duration of mechanical ventilation [122].
Seven contemporary retrospective analyses [135–140] were identified, of which three adjusted for differences in severity of illness [135–137]. Only one reported a reduced risk of death [137], though none of these three studies observed differences in catecholamine use, vasopressor-free, ventilator-free days or hospital length of stay [135–137].
Recommendation:
We do not recommend the use of IVIG in cases of Type I or Type III NSTI even in the setting of severe sepsis or septic shock (strong, moderate).
Should IVIG be used in Type II (streptococcal) NSTI?
IVIG may offer survival benefit in patients with Type II NSTI and streptococcal toxic shock syndrome (Tables 6 and 7). We identified one multi-center, double blind, randomized, placebo-controlled trial of IVIG for streptococcal toxic shock syndrome (STSS) [125]. Although IVIG was associated with a reduced risk of death, this did not attain statistical significance. The IVIG was associated with a more rapid resolution of shock and organ failure, however, as defined by Sequential Organ Failure Assessment (SOFA) score [125]. We identified a total of three contemporary prospective observational or national surveillance trials, which focused on GAS infections or STSS [124,141,142]. Two trials adjusted for differences in severity of illness and case mix, and both reported increased adjusted odds of survival with IVIG [124,142]. All studies are limited by relatively low cohort size (N = 21–67 subjects) (Table 6).
Table 6.
Studies of Intravenous Immunoglobulin in Necrotizing Soft Tissue Infections and Sepsis
| Author | Study design | Population | Sample size | Control | Intervention | Adjustment | Death or survival IVIG vs. none |
|---|---|---|---|---|---|---|---|
| STREPTOCOCCAL TOXIC SHOCK SYNDROME | |||||||
| Randomized Controlled Trials | |||||||
| Darenberg et al (2003) | Multicenter, double blind, RCT, placebo controlled | STSS | 21 | Placebo 1% albumin, n = 21 | IVIG endobulin 1g/kg day 1 and 0.5 g/kg for days 2–3, N = 10 | RCT | 28-day survival: 9/10 (90%) vs. 7/11(65%), p = 0.3; similar results when restricted to culture proven GAS |
| Observational Studies | |||||||
| Kaul et al (1999) | Prospective, controlled | STSS | 53 | n = 22 | IVIG initially 0.4 g/kg/d for 5 days, then changed to 2 g/kg once and repeated in 28 h if still unstable, N = 21 | Yes, multivariate | 30-day survival: 14/21 (67%) vs. 11/32 (34%), p = 0.02; OR 7.7 (1.5–14.3); aOR 8.1 (6–45); propensity aOR 10 (1.4–70) |
| Mehta et al (2006) | Population-based surveillance | GAS | 62 | n = 27 | Polyclonal IVIG, N = 35 | No | No difference in use of IVIG between survivors and non-survivors: 57% vs. 56%, p = 1.0 |
| Linner et al (2014) | Swedish nationwide surveillance | STSS | 67 | n = 44 | IVIG 0.5 g/kg for 1–6 d, n = 23 | Yes, multi-variable | 28-day survival: 87% vs. 50%, p < 0.01; aOR for survival 5.6 (1.2–26.9), p = 0.03; for patients with NF: 6.0 (0.4–85.2), p = NS, but n = 19 |
| SEPSIS | |||||||
| Meta-analyses/Systematic Reviews | |||||||
| Alejandria et al (2013) | Systematic review/meta-analysis | Sepsis/severe sepsis/ septic shock | 43 trials | Various | All | In-hospital mortality: 0.81 (0.70–0.93); IgM enriched: 0.66 (0.51–0.85); sensitivity analysis of low bias studies: IVIG+IgM studies 0.97 (0.81–1.15) | |
| Laupland et al (2007) | Systematic review/meta-analysis | Severe sepsis/septic shock | 14 trials | Various | All | In-hospital mortality: 0.66 (0.53–0.83); low bias studies: 0.96 (0.71–1.3) | |
| Kreymann et al (2007) | Systematic review/meta-analysis | Severe sepsis/septic shock | 27 trials, n = 15 on adults | Various | All | In-hospital mortality: 0.79 (0.69–0.90) | |
| Tugeon et al (2007) | Systematic review/meta-analysis | Sepsis/severe sepsis/septic shock | 20 trials | Various | All | In-hospital mortality: 0.74 (0.62–0.89); low bias studies: 0.56 (0.31–1.01) | |
| Neilson et al (2005) | Systematic review/meta-analysis | Severe sepsis/septic shock | 9 trials | Various | Pentaglobulin | OR 0.57 (0.31–0.74) | |
| Pildal et al (2004) | Systematic review/meta-analysis | Sepsis | 20 trials, neonates and adults | Various | Polyclonal IVIG | 0.77 (0.68–0.88); low bias studies: 1.02 (0.84–1.24) | |
| Randomized Controlled Trials | |||||||
| Hamano et al (2013) | Single center RCT | Severe sepsis/septic shock | 79 | IVIG 5 g/d for 3 d, n = 42 | IVIG 15 g/d for 1 d within 24 h, n = 37 | N/A | 28-day survival: NS |
| Werdan et al (2007) | Multicenter, double blind, RCT, placebo controlled | Severe sepsis | 653 | Placebo 5% albumin 12 mg/kg day 0 and 6 mg/kg day 1, n = 332 | IVIG 0.6g/kg day 0 and then 0.3 g/kg day 1, n = 321 | N/A | 28-day survival: 60.7% vs. 62.7%, p = 0.67 |
| Rodriguez et al (2005) | Single center RCT, double blind, placebo controlled | Severe sepsis or septic shock undergoing surgery for abdominal sepsis | 56 | Placebo was 5% albumin, n = 27 | N = 29, IVIG Pentaglobin 7 mL/kg/d for 5 d, n = 29 | N/A | In-hospital survival: 20/28(72.5%) vs. 15/28(52.9%), p = 0.06; OR 2.43 (0.80–7.39), p = 0.06 |
| Tugrul et al (2002) | Single center RCT | Severe sepsis, majority abdominal sepsis | 42 | No placebo, n = 21 | IVIG IgM and IgA-enriched Pentaglobin, 5 mL/kg per day for 3 d, n = 21 | N/A | 28-day survival: 16/21(76.2%) vs. 14/21(66.7%), p = 0.70 |
| Tagami et al (2015) | Japanese inpatient database | Severe sepsis | 8,264 | Propensity matched controls, n = 1045 | IVIG within 48 h 5 g/day for 3 days, n = 1,045 | Yes, multivariable | 28-day mortality: 383/1,045(36.7%) vs. 376/1,045(36.0%); 1.03 (0.86–1.23) |
| Ishida et al (2015) | Retrospective, single center | Severe sepsis | 41 | n = 22 | IVIG 5 g/d for 3 d, n = 19 | None | 28-day survival: 94.7% vs. 81.8%, p = NS |
| Tagami et al (2015) | Japanese inpatient database | Septic shock after emergency laparotomy | 4,919 | Propensity matched controls, n = 1,081 | IVIG within 48 h 5 g/d for 3 d, n = 1081 | Yes, multivariable | 28-day mortality: 221/1,081(20.4%) vs. 209/1,081(19.3%), 1.1 (0.87–1.30), p = NS |
| Cavazzuti et al (2014) | Retrospective, single center | Septic shock | 168 | Controls, n = 76; propensity matched, n = 59 | IgM within 24 h of shock, 20 mg/kg/h for 3 d, n = 92 (n = 59 propensity matched) | Yes, multivariable | 30-day mortality: aOR 0.17 (0.06–0.49), propensity aOR 0.35 (0.14–0.85); 15/59 (25.4%) vs. 27/59 (45.8%), p = 0.021 |
| Yavuz et al (2012) | Retrospective, single center | Severe sepsis | 188 | Controls, n = 62 | N = 56 IgM enriched IVIG, Pentaglobulin 5 g/kg/d for 3 d | No | 28-day mortality: 14/56 (25%) vs. 43/62 (69.4%), p < 0.0001 |
| Berlot et al (2012) | Retrospective, single center | Severe sepsis/septic shock | 129 | Controls, none | N = 129 IgM enriched IVIG, 250 mg/kg day 1, and for 3 d, N = 129 | ICU survivors received IVIG earlier: 23 vs. 63 h, p < 0.05, aOR for ICU death with each 1 h delay: 1.07 (1.01–1.10), p < 0.001 | |
| Buda et al (2005) | Retrospective, single center | Sepsis/severe sepsis/septic shock, post- cardiac surgery | 66 | Controls, N = 44 | IgM enriched IVIG, 5 mL/kg/d for 3 d, N = 22 | No | Death: 5/22 (22.7%) vs. 16/44 (36.3%), p = NS; severe sepsis: 1/15(6.6%) vs. 12/32(37.5%), p = 0.04; aOR 0.11 (0.01–1.01) |
IVIG = intravenous immunoglobulin; RCT = randomized controlled trial; STSS = streptococcal toxic shock syndrome; GAS = group A Streptococcus; OR = odds ratio; aOR = adjusted odds ratio; IgM = immunoglobulin M; IgA = immunoglobulin A.
Table 7.
Clinical Criteria for the Diagnosis of Streptococcal Toxic Shock Syndrome
| Major criteria | Minor criteria (two of more of the following) |
|---|---|
| Hypotension (SBP <90 mm Hg) | Renal impairment (urea or creatinine twice upper limit of normal). In patients with pre-existing renal disease. |
| Coagulopathy: Platelets less than or equal to 100,000/mm3 (less than or equal to 100 × 106/L) or disseminated intravascular coagulation, defined by prolonged clotting times, low fibrinogen level, and the presence of fibrin degradation products. | |
| Liver involvement: Alanine aminotransferase, aspartate aminotransferase, or total bilirubin levels greater than or equal to twice the upper limit of normal for the patient's age. In patients with pre-existing liver disease, a greater than twofold increase over the baseline level. | |
| Acute respiratory distress syndrome: Defined by acute onset of diffuse pulmonary infiltrates and hypoxemia in the absence of cardiac failure or by evidence of diffuse capillary leak manifested by acute onset of generalized edema, or pleural or peritoneal effusions with hypoalbuminemia. | |
| A generalized erythematous macular rash that may desquamate. | |
| Soft-tissue necrosis, including necrotizing fasciitis or myositis, or gangrene. |
A “probable” case meets the clinical case definition in the absence of another identified etiology for illness and with isolation of group A Streptococcus from a non-sterile site.
A “confirmed” case meets the clinical case definition and with isolation of group A Streptococcus from a normal sterile site (e.g., blood or cerebrospinal fluid or joint, pleural or pericardial fluid.
SBP = systolic blood pressure.
Recommendation:
We recommend that IVIG be considered as an adjunct to therapy in cases of Type II (streptococcal) NSTI (strong, moderate).
When should IVIG be used?
Three of the four studies specifically focused on GAS incorporated patients meeting criteria for the diagnosis of toxic shock syndrome (Table 7) [143].
Recommendation:
We recommend the use of IVIG for cases of Type II (STSS) that meet TSS criteria (strong, moderate).
How should IVIG should be dosed and administered?
There is considerable batch-to-batch variation of IVIG in terms of the quantity of neutralizing antibodies, and Level I evidence is lacking. Studies of immunoglobulin for the management of GAS and STSS used poly-clonal IVIG. Initial doses ranged from 0.5–1.0 g/kg daily for up to six days [124,125,141,142].
Recommendation:
In cases of GAS and STSS, we recommend a dose of poly-clonal IVIG of 0.5–1.0 g/kg per day for no more than five days (strong, moderate).
Blood purification (plasmapheresis, plasma exchange)
Blood purification incorporates a host of varying interventions aimed at removing a variety of mediators purported as causal in the pathophysiology of NSTI and sepsis.
Should blood purification be used in NSTI?
We did not identify any studies of the use of blood purification for any type of NSTI (Table 8).
Table 8.
Studies of Blood Purification in Necrotizing Soft Tissue Infection and Sepsis
| Author | Study design | Population | Control | Intervention | Adjustment | Death or survival (blood purification vs. none) |
|---|---|---|---|---|---|---|
| Meta-analyses and Systematic Reviews | ||||||
| Zhou et al. (2013) | Meta-analysis | Sepsis, severe sepsis, septic shock | N = 16 trials | All | N/A | All death: All (16 trials) RR: 0.69 (0.56–0.84), p < 0.001; hemoperfusion (10 trials) RR: 0.63 (0.50–0.80), p < 0.001; plasma exchange (2 trials) RR: 0.63 (0.42–0.96) p = 0.03; hemofiltration alone (4 trials) RR: 1.13 (0.75–1.71) p = 0.56; if exclude PMX RR: 0.89 (0.71–1.13 p = 0.36); HP with PMX RR: 0.57 (0.45–0.72) p < 0.001; without PMX RR: 0.98 (0.66–1.47) p = 0.44 |
| Rimmer et al. (2014) | Systematic review and meta-analysis | Sepsis and septic shock | N = 4 trials; 1 of adults, 2 of children, 1 of both | Plasmafiltration (Reeves et al); plasmapheresis (Busund et al) | N/A | All mortality: 0.63 (0.42–0.96) |
| Borthwick et al. (2013) | Systematic review | Sepsis | N = 3 trials | High volume hemofiltration | N/A | Unable to pool estimates because of heterogeneity; Boussekey (2008) reported 3/9 (33%) vs. 6/10 (60%), NS; Cole (2001) only reported hospital rates of 6/11 (54.5%) but is cross-over and no comparisons possible; Ghani (2006) no difference in 60-d survival or SOFA |
| Randomized Controlled Trials | ||||||
| Livigni et al. (2014) | Multicenter, open label, RCT | Septic shock | n = 96 | CPFA, n = 96 | N/A | Survival to discharge, NS |
| Cruz et al. (2009) EUPHAS Study Group | Multicenter, RCT | Abdominal septic shock | n = 30 | Hemoperfusion PMX B, n = 34 | N/A | 28-d death: 11/34 (32%) vs. 16/30 (53%); HR 0.43 (0.20–0.97); aHR 0.36 (0.16–0.80) |
| Payen et al. (2009) | Multicenter, open label, RCT | Severe sepsis | n = 40 | Hemofiltration, n = 40 | RCT | 14-d KM survival worse with HF: p = 0.10; 28-day survival: 46% vs. 56%, p = 0.49 |
| Vincent et al (2005) | Multicenter, open label, RCT | Severe sepsis or septic shock secondary to intra-abdominal infection | n = 19 | Hemoperfusion PMX B, n = 17 | N/A | 28-d survival: 5/17 (29%) vs. 5/18 (28%), p = 0.75 |
| Peng et al. (2005) | Single center, RCT | Severely burned with sepsis | n = 10 | n = 10, venovenous CRRT | N/A | NS, but no data described |
| Nakamura et al. (2004) | Single center, RCT | Severe sepsis | Controls, n = 50; healthy age matched, n = 70 | Hemoperfusion with PMX B, n = 70 | N/A | Not described |
| Nakamura et al. (2003) | Single center, RCT | Sepsis with MRSA | Controls, n = 10 | Hemoperfusion using PMX B, n = 10 | N/A | In-hospital survival: 8/10 (50%) vs. 2/10(20%), p = 0.007 |
| Reinhart et al (2004) Easy Study Group | Single center, RCT | Severe sepsis/septic shock | n = 76 | LPS adsorption, n = 67 | RCT | 28-d survival: 71.2% vs. 74.3%, p = 0.71 |
| Nakamura et al. (2003) | Single center, RCT | Sepsis with MRSA | Controls, n = 10 | Hemoperfusion with PMX B, n = 15 | N/A | In-hospital survival 53% vs. 20%; RR 0.58 (0.31–1.09) |
| Nakamura et al. (2002) | Single center, RCT | Sepsis with MRSA | HD without sepsis, n = 7; age matched controls, n = 12 | HD with sepsis, hemoperfusion with PMX B, n = 7 | N/A | In-hospital survival: 5/7 (71%) vs. 1/7 (14%), p = 0.03 |
| Cole et al. (2002) | Single center, RCT | Severe sepsis, septic shock | Controls, n = 12 | Continuous venovenous hemofiltration (CVVH), n = 12 | N/A | In-hospital survival: 8/12 vs. 8/12, p = 1.0 |
| Busund et al. (2002) | Single center, RCT | Severe sepsis, septic shock | n = 52 | Plasmapheresis (PE), n = 52 | N/A | 28-d survival: 33.3% vs. 53.8%, p = 0.05; OR 1.46 (1.03–2.12), p = 0.03; aOR 0.41 (0.15–1.09) p = 0.07 |
| Nemoto et al. (2001) | Single center, RCT | Sepsis, severe sepsis, septic shock | Controls, n = 44 | Hemoperfusion with PMX B, n = 54 | N/A | KM 28-d survival: 22/54 (41%) vs. 5/44 (11%), p = 0.002 |
| Zu et al. (2015) | Single center, RCT | Burns with severe sepsis | n = 97 | CVVHDF; n = 98 | N/A | 28-d survival: 80.6% vs. 72.2%, p < 0.05 |
| Reeves et al. (1999) | Multicenter RCT | Sepsis syndrome | n = 18; 13 adults, 3 children | n = 14, continuous plasmapheresis (PE); 9 adults, 5 children | RCT | 14-d survival: 8/14 (57%) vs. 8/16 (50%), p = 0.73; aOR 1.78 (0.20–18.1) |
| Observational Studies | ||||||
| Yaroustovsky et al. (2015) | Prospective | Severe sepsis | n = 20 | LPS+CPFA, n = 20 | No | 28-d survival 65 vs. 35%, p = 0.11 |
| Yaroustovsky et al. (2014) | Retrospective | Severe sepsis | Historical, n = 30 | LPS+HD, n = 26 | No | 28-d survival: 65.5% vs. 33.3%, p = 0.03 |
| Sakamoto et al. (2010) | Prospective | Septic shock | None | DHP-PMS, n = 26; CVVHDF-PMMA, n = 28; CVVHD-PAN, n = 26 | No | All survival: PMMA-CVVHD, 80% vs. other, 45%, p = 0.0196 |
| Huang et al. (2010) | Prospective | Severe sepsis, septic shock | n = 20 | Hemoperfusion using neutral porous resin, n = 24 | RCT | 28-d survival: 13/24 (44.2%) vs. 9/20 (25%), p = 0.47 |
| Asanuma et al. (2004) | Retrospective | Severe sepsis | None | PMX B, n = 17; CHF/CHDF, n = 15; PE+CHDF, n = 3 | No | All survival: 57% vs. 42%, NS (no statistics performed) |
| Nakamura et al. (2004) | Prospective, quasi-randomized | Severe sepsis | Controls, n = 10; healthy controls, n = 20 | Hemoperfusion with PMX B, n = 15 | RCT | In-hospital survival: 80% vs. 40%, p < 0.05 |
| Ronco et al. (2002) | Prospective, crossover | Septic shock | None | PFAD or CVVHDF (10 h treatments), n = 10 | None | Not studied |
| Schmidt et al. (2000) | Prospective | Severe sepsis, septic shock | Controls, n = 24 | CVVHF with plasma exchange, n = 19 | None | All survival: 8/19 (42.1%) vs. 11/24 (45.8%), NS; single organ failure: 4/4 (100%) vs. 3/4 (75%), NS; double organ failure: 5/6 (83%) vs. 7/12 (58%), p < 0.001 |
| Tani et al. (1998) | Prospective | Septic shock | n = 33 | Hemoperfusion with PMX B, n = 37 | None | In-hospitaI survival: 20/37 (54%) vs. 12/33 (36.4%), p < 0.05; > 4 failed organs: 7/21 (33%) vs. 1/13(8%), p < 0.01 |
RR = relative risk; PMX = polymixin; HP = hemoperfusion; SOFA = Sequential Organ Failure Assessment; RCT = randomized controlled trial; HR = hazard ratio; aHR = adjusted hazard ratio; KM = Kaplan Meier; HF = hemofiltration; CCRT = ________; MRSA = methicillin resistant Staphylococcus aureus; HD = hemodialysis; CVVH = continuous venovenous hemofiltration; CVVHDF = continuous venovenous hemodiafiltration; PE = plasmapheresis; LPS = lipopolysaccharide; CPFA = coupled plasma filtration and adsorption; DHP = direct hemoperfusion; PMX = polymyxin B; PMMA = polymethylmethacrylate; PAN = polyacrylonitrile; CHF = continuous hemofiltration; CHDF = continuous hemodiafiltration.
Three meta-analyses evaluated the use of blood purification in patients with sepsis, severe sepsis, and septic shock [144–146]. Two trials identified a reduced risk of death: 0.69 (0.56–0.84) [144] and 0.63 (0.42–0.96) [145]. The former trial included 16 studies and conducted sensitivity analyses of each distinct mechanism of blood purification [144]. This trial observed a loss of statistical significance with hemoperfusion in the absence of polymixin B (PMX B), endotoxin-adsorbing columns [144]. A summary estimate of the two included trials of plasma exchange remained significant: 0.63 (0.42–0.96) [144]. The remaining meta-analysis (n = 3 studies) of high volume hemofiltration was unable to pool estimates because of study heterogeneity [146].
We identified 15 contemporary RCTs, of which only two reported a significant reduction in death [147–161]. The Use of Polymyxin B Hemoperfusion in Abdominal Sepsis (EUPHAS) trial demonstrated improved 28-day survival using hemoperfusion and PMX B: aHR 0.36 (0.16–0.80) [147], as did the study of Nemoto et al. [148]. The EUPHAS investigators also reported improved SOFA scores [147]. Of the remaining RCTs (n = 13), a variety of blood purification methods were used, yet no study observed a significant reduction in death.
We also identified nine observational trials that we did not include in the generation of recommendations because of the substantial data derived from RCT and meta-analyses (Table 8).
Recommendation:
In patients with NSTI and sepsis, severe sepsis, or septic shock, hemoperfusion with PMX B may be considered (strong, moderate).
Recommendation:
In patients with NSTI and sepsis, severe sepsis, or septic shock, plasma exchange may be considered (weak, low).
Recommendation:
In patients with NSTI, we do not recommend hemoperfusion without PMX B, hemofiltration, or hemodialysis (strong, moderate).
When should blood purification be used?
The majority of studies evaluated subjects with severe sepsis or septic shock. In the meta-analysis of Zhou et al. [144], however, a sensitivity analysis suggested a greater reduction in death if blood purification is administered during sepsis, rather than in severe sepsis or septic shock: 0.40 (0.26–0.64).
Recommendation:
In patients with NSTI, we recommend the use of adjuvant blood purification early and before the development of septic shock (weak, high).
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Descamps V, Aitken J, Lee MG. Hippocrates on necrotising fasciitis. Lancet 1994;344:556. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SC. The person behind the eponym: Wilhelm Frederick von Ludwig [1790–1865]. J Oral Pathol Med 1996;25:513–515 [DOI] [PubMed] [Google Scholar]
- 3.Baurieene H. Sur Une Plaie Contuse qui s'est terminee par la sphacele de la scrotum. J Med Chir Pharm 1764;20:251–256 [Google Scholar]
- 4.Fournier JA. Gangrene Foudroyante de la verge. Med Pract 1883;4:589–597 [DOI] [PubMed] [Google Scholar]
- 5.Fournier JA. Etude clinique de la gangrene foudroyante de la verge. Semaine Med 1884;4:69 [Google Scholar]
- 6.Jones J. Surgical memoirs of the war of the rebellion: Investigation upon the nature, causes, and treatment of hospital gangrene as prevailed in the Confederate armies 1861–1865. New York: U.S. Sanitary Commission; 1871 [Google Scholar]
- 7.Wilson B. Necrotizing fasciitis. Am Surg 1952;18:416–431 [PubMed] [Google Scholar]
- 8.Psoinos CM, Flahive JM, Shaw JJ, et al. Contemporary trends in necrotizing soft-tissue infections in the United States. Surgery 2013;153:819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May AK, Stafford RE, Bulger EM, et al. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt) 2009;10:467–499 [DOI] [PubMed] [Google Scholar]
- 10.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10–e52 [DOI] [PubMed] [Google Scholar]
- 11.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis 2014;59:147–159 [DOI] [PubMed] [Google Scholar]
- 12.Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg 2014;51:344–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucca K, Spencer R, Orford N, et al. Early diagnosis and treatment of necrotizing fasciitis can improve survival: An observational intensive care unit cohort study. ANZ J Surg 2013;83:365–370 [DOI] [PubMed] [Google Scholar]
- 14.Wong CH, Chang HC, Pasupathy S, et al. Necrotizing fasciitis: Clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003;85-A:1454–1460 [PubMed] [Google Scholar]
- 15.Anaya DA, McMahon K, Nathens AB, et al. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg 2005;140:151–157 [DOI] [PubMed] [Google Scholar]
- 16.Howell GM, Rosengart MR. Necrotizing soft tissue infections. Surg Infect (Larchmt) 2011;12:185–190 [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ 2008;336:1049–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336:995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744. [DOI] [PubMed] [Google Scholar]
- 21.Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: Diagnosis and management. Clin Infect Dis 2007;44:705–710 [DOI] [PubMed] [Google Scholar]
- 22.Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endorf FW, Klein MB, Mack CD, et al. Necrotizing soft-tissue infections: Differences in patients treated at burn centers and non-burn centers. J Burn Care Res 2008;29:933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George SM, Harrison DA, Welch CA, et al. Dermatological conditions in intensive care: A secondary analysis of the Intensive Care National Audit and Research Centre (ICNARC) Case Mix Programme database. Crit Care 2008;12(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunter OL, Guillamondegui OD, May AK, Diaz JJ. Outcome of necrotizing skin and soft tissue infections. Surg Infect [Larchmt] 2008;9:443–450 [DOI] [PubMed] [Google Scholar]
- 26.May AK. Skin and soft tissue infections. Surg Clin North Am 2009;89:403–420 [DOI] [PubMed] [Google Scholar]
- 27.Mills MK, Faraklas I, Davis C, et al. Outcomes from treatment of necrotizing soft-tissue infections: Results from the National Surgical Quality Improvement Program database. Am J Surg 2010;200:790–796 [DOI] [PubMed] [Google Scholar]
- 28.Kao LS, Lew DF, Arab SN, et al. Local variations in the epidemiology, microbiology, and outcome of necrotizing soft-tissue infections: A multicenter study. Am J Surg 2011;202:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: Current concepts and review of the literature. J Am Coll Surg 2009;208:279–288 [DOI] [PubMed] [Google Scholar]
- 30.Napolitano LM. Severe soft tissue infections. Infect Dis Clin North Am 2009;23:571–591 [DOI] [PubMed] [Google Scholar]
- 31.Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg 2000;179:361–366 [DOI] [PubMed] [Google Scholar]
- 32.Freischlag JA, Ajalat G, Busuttil RW. Treatment of necrotizing soft tissue infections. The need for a new approach. Am J Surg 1985;149:751–755 [DOI] [PubMed] [Google Scholar]
- 33.Piedra T, Martin-Cuesta L, Arnaiz J, et al. Necrotizing fasciitis secondary to diverticulitis. Emerg Radiol 2007;13:345–348 [DOI] [PubMed] [Google Scholar]
- 34.Liu SY, Ng SS, Lee JF. Multi-limb necrotizing fasciitis in a patient with rectal cancer. World J Gastroenterol 2006;12:5256–5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Childers BJ, Potyondy LD, Nachreiner R, et al. Necrotizing fasciitis: A fourteen-year retrospective study of 163 consecutive patients. Am Surg 2002;68:109–116 [PubMed] [Google Scholar]
- 36.Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin 2003;19:55–72 [DOI] [PubMed] [Google Scholar]
- 37.Spitalnic SJ, Sucov A. Ludwig's angina: Case report and review. J Emerg Med 1995;13:499–503 [DOI] [PubMed] [Google Scholar]
- 38.Saifeldeen K, Evans R. Ludwig's angina. Emerg Med J 2004;21:242–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn FB., Jr Ludwig angina. Arch Otolaryngol Head Neck Surg 1999;125:599. [DOI] [PubMed] [Google Scholar]
- 40.Darenberg J, Luca-Harari B, Jasir A, et al. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis 2007;45:450–458 [DOI] [PubMed] [Google Scholar]
- 41.Chelsom J, Halstensen A, Haga T, Hoiby EA. Necrotising fasciitis due to group A streptococci in western Norway: Incidence and clinical features. Lancet 1994;344:1111–1115 [DOI] [PubMed] [Google Scholar]
- 42.Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005;330:830–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eneli I, Davies HD. Epidemiology and outcome of necrotizing fasciitis in children: An active surveillance study of the Canadian Paediatric Surveillance Program. J Pediatr 2007;151:79–84 [DOI] [PubMed] [Google Scholar]
- 44.Young LM, Price CS. Community-acquired methicillin-resistant Staphylococcus aureus emerging as an important cause of necrotizing fasciitis. Surg Infect (Larchmt) 2008;9:469–474 [DOI] [PubMed] [Google Scholar]
- 45.Wallin TR, Hern HG, Frazee BW. Community-associated methicillin-resistant Staphylococcus aureus. Emerg Med Clin North Am 2008;26:431–455 [DOI] [PubMed] [Google Scholar]
- 46.Brett MM, Hood J, Brazier JS, et al. Soft tissue infections caused by spore-forming bacteria in injecting drug users in the United Kingdom. Epidemiol Infect 2005;133:575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura AC, Higa JI, Levin RM, et al. Outbreak of necrotizing fasciitis due to Clostridium sordellii among black-tar heroin users. Clin Infect Dis 2004;38:e87–e91 [DOI] [PubMed] [Google Scholar]
- 48.Stevens DL, Bryant AE. Pathogenesis of Clostridium perfringens infection: Mechanisms and mediators of shock. Clin Infect Dis 1997;25(Suppl 2):S160–S164 [DOI] [PubMed] [Google Scholar]
- 49.Chew SS, Lubowski DZ. Clostridium septicum and malignancy. ANZ J Surg 2001;71:647–649 [DOI] [PubMed] [Google Scholar]
- 50.Chiang SR, Chuang YC. Vibrio vulnificus infection: Clinical manifestations, pathogenesis, and antimicrobial therapy. J Microbiol Immunol Infect 2003;36:81–88 [PubMed] [Google Scholar]
- 51.Ko WC, Lee HC, Chuang YC, et al. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteraemia. J Infect 2000;40:267–273 [DOI] [PubMed] [Google Scholar]
- 52.Tsai YH, Hsu RW, Huang TJ, et al. Necrotizing soft-tissue infections and sepsis caused by Vibrio vulnificus compared with those caused by Aeromonas species. J Bone Joint Surg Am 2007;89:631–636 [DOI] [PubMed] [Google Scholar]
- 53.Gold WL, Salit IE. Aeromonas hydrophila infections of skin and soft tissue: Report of 11 cases and review. Clin Infect Dis 1993;16:69–74 [DOI] [PubMed] [Google Scholar]
- 54.Ko WC, Chuang YC. Aeromonas bacteremia: Review of 59 episodes. Clin Infect Dis 1995;20:1298–1304 [DOI] [PubMed] [Google Scholar]
- 55.Tsai YH, Hsu RW, Huang KC, et al. Systemic Vibrio infection presenting as necrotizing fasciitis and sepsis. A series of thirteen cases. J Bone Joint Surg Am 2004;86-A:2497–2502 [DOI] [PubMed] [Google Scholar]
- 56.Eucker J, Sezer O, Graf B, Possinger K. Mucormycoses. Mycoses 2001;44:253–260 [PubMed] [Google Scholar]
- 57.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis 2005;41:634–653 [DOI] [PubMed] [Google Scholar]
- 58.Cocanour CS, Miller-Crotchett P, Reed RL 2nd, et al. Mucormycosis in trauma patients. J Trauma 1992;32:12–15 [DOI] [PubMed] [Google Scholar]
- 59.Patino JF, Castro D, Valencia A, Morales P. Necrotizing soft tissue lesions after a volcanic cataclysm. World J Surg 1991;15:240–247 [DOI] [PubMed] [Google Scholar]
- 60.Adam RD, Hunter G, DiTomasso J, Comerci G., Jr Mucormycosis: Emerging prominence of cutaneous infections. Clin Infect Dis 1994;19:67–76 [DOI] [PubMed] [Google Scholar]
- 61.Losee JE, Selber J, Vega S, et al. Primary cutaneous mucormycosis: Guide to surgical management. Ann Plast Surg 2002;49:385–390 [DOI] [PubMed] [Google Scholar]
- 62.Boelaert JR, de Locht M, Van Cutsem J, et al. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest 1993;91:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenberg RN, Scott LJ, Vaughn HH, Ribes JA. Zygomycosis (mucormycosis): Emerging clinical importance and new treatments. Curr Opin Infect Dis 2004;17:517–525 [DOI] [PubMed] [Google Scholar]
- 64.Majeski JA, Alexander JW. Early diagnosis, nutritional support, and immediate extensive debridement improve survival in necrotizing fasciitis. Am J Surg 1983;145:784–787 [DOI] [PubMed] [Google Scholar]
- 65.McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 1995;221:558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevens DL, Tanner MH, Winship J, et al. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 1989;321:1–7 [DOI] [PubMed] [Google Scholar]
- 67.Stone HH, Martin JD., Jr Synergistic necrotizing cellulitis. Ann Surg 1972;175:702–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majeski JA, John JF., Jr Necrotizing soft tissue infections: A guide to early diagnosis and initial therapy. South Med J 2003;96:900–905 [DOI] [PubMed] [Google Scholar]
- 69.Bosshardt TL, Henderson VJ, Organ CH., Jr Necrotizing soft-tissue infections. Arch Surg 1996;131:846–852 [DOI] [PubMed] [Google Scholar]
- 70.Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg 1996;224:672–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bangsberg DR, Rosen JI, Aragon T, et al. Clostridial myonecrosis cluster among injection drug users: A molecular epidemiology investigation. Arch Intern Med 2002;162:517–522 [DOI] [PubMed] [Google Scholar]
- 72.McGuigan CC, Penrice GM, Gruer L, et al. Lethal outbreak of infection with Clostridium novyi type A and other spore-forming organisms in Scottish injecting drug users. J Med Microbiol 2002;51:971–977 [DOI] [PubMed] [Google Scholar]
- 73.Ben-Abraham R, Keller N, Vered R, et al. Invasive group A streptococcal infections in a large tertiary center: Epidemiology, characteristics and outcome. Infection 2002;30:81–85 [DOI] [PubMed] [Google Scholar]
- 74.Chapnick EK, Abter EI. Necrotizing soft-tissue infections. Infect Dis Clin North Am 1996;10:835–855 [DOI] [PubMed] [Google Scholar]
- 75.Lille ST, Sato TT, Engrav LH, et al. Necrotizing soft tissue infections: Obstacles in diagnosis. J Am Coll Surg 1996;182:7–11 [PubMed] [Google Scholar]
- 76.Forbes N, Rankin AP. Necrotizing fasciitis and non steroidal anti-inflammatory drugs: A case series and review of the literature. N Z Med J 2001;114:3–6 [PubMed] [Google Scholar]
- 77.Kahn LH, Styrt BA. Necrotizing soft tissue infections reported with nonsteroidal antiinflammatory drugs. Ann Pharmacother 1997;31:1034–1039 [DOI] [PubMed] [Google Scholar]
- 78.Stevens DL. Could nonsteroidal antiinflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome? Clin Infect Dis 1995;21:977–980 [DOI] [PubMed] [Google Scholar]
- 79.Wong CH, Khin LW, Heng KS, et al. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004;32:1535–1541 [DOI] [PubMed] [Google Scholar]
- 80.Su YC, Chen HW, Hong YC, et al. Laboratory risk indicator for necrotizing fasciitis score and the outcomes. ANZ J Surg 2008;78:968–972 [DOI] [PubMed] [Google Scholar]
- 81.Holland MJ. Application of the Laboratory Risk Indicator in Necrotising Fasciitis (LRINEC) score to patients in a tropical tertiary referral centre. Anaesth Intensive Care 2009;37:588–592 [DOI] [PubMed] [Google Scholar]
- 82.Wall DB, de Virgilio C, Black S, Klein SR. Objective criteria may assist in distinguishing necrotizing fasciitis from nonnecrotizing soft tissue infection. Am J Surg 2000;179:17–21 [DOI] [PubMed] [Google Scholar]
- 83.Brothers TE, Tagge DU, Stutley JE, et al. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg 1998;187:416–421 [DOI] [PubMed] [Google Scholar]
- 84.Majeski J, Majeski E. Necrotizing fasciitis: Improved survival with early recognition by tissue biopsy and aggressive surgical treatment. South Med J 1997;90:1065–1068 [DOI] [PubMed] [Google Scholar]
- 85.Lee PC, Turnidge J, McDonald PJ. Fine-needle aspiration biopsy in diagnosis of soft tissue infections. J Clin Microbiol 1985;22:80–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevens DL, Bryant AE, Yan S. Invasive group A streptococcal infection: New concepts in antibiotic treatment. Int J Antimicrob Agents 1994;4:297–301 [DOI] [PubMed] [Google Scholar]
- 87.Stevens DL, Ma Y, Salmi DB, et al. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 2007;195:202–211 [DOI] [PubMed] [Google Scholar]
- 88.Awad SS, Elhabash SI, Lee L, et al. Increasing incidence of methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: Reconsideration of empiric antimicrobial therapy. Am J Surg 2007;194:606–610 [DOI] [PubMed] [Google Scholar]
- 89.Hidayat LK, Hsu DI, Quist R, et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: Efficacy and toxicity. Arch Intern Med 2006;166:2138–2144 [DOI] [PubMed] [Google Scholar]
- 90.Howden BP, Ward PB, Charles PG, et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 2004;38:521–528 [DOI] [PubMed] [Google Scholar]
- 91.Del Poeta M, Schell WA, Perfect JR. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother 1997;41:1835–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Espinel-Ingroff A. Comparison of In vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol 1998;36:2950–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pfaller MA, Marco F, Messer SA, Jones RN. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis 1998;30:251–255 [DOI] [PubMed] [Google Scholar]
- 94.Spanakis EK, Aperis G, Mylonakis E. New agents for the treatment of fungal infections: Clinical efficacy and gaps in coverage. Clin Infect Dis 2006;43:1060–1068 [DOI] [PubMed] [Google Scholar]
- 95.Sun QN, Fothergill AW, McCarthy DI, et al. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 2002;46:1581–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Burik JA, Hare RS, Solomon HF, et al. Posaconazole is effective as salvage therapy in zygomycosis: A retrospective summary of 91 cases. Clin Infect Dis 2006;42:e61–e65 [DOI] [PubMed] [Google Scholar]
- 97.Banks DW, O'Brien DP, 3rd, Amerson JR, Hester TR., Jr Gracilis musculocutaneous flap scrotal reconstruction after Fournier gangrene. Urology 1986;28:275–276 [DOI] [PubMed] [Google Scholar]
- 98.Parkash S, Gajendran V. Surgical reconstruction of the sequelae of penile and scrotal gangrene: A plea for simplicity. Br J Plast Surg 1984;37:354–357 [DOI] [PubMed] [Google Scholar]
- 99.Taguchi H. More than 10 years of experience with reconstructed scrotums. J Urol 1976;116:469–471 [DOI] [PubMed] [Google Scholar]
- 100.Maguina P, Palmieri TL, Greenhalgh DG. Split thickness skin grafting for recreation of the scrotum following Fournier's gangrene. Burns 2003;29:857–862 [DOI] [PubMed] [Google Scholar]
- 101.Vincent MP, Horton CE, Devine CJ., Jr An evaluation of skin grafts for reconstruction of the penis and scrotum. Clin Plast Surg 1988;15:411–424 [PubMed] [Google Scholar]
- 102.Endorf FW, Cancio LC, Klein MB. Necrotizing soft-tissue infections: Clinical guidelines. J Burn Care Res 2009;30:769–775 [DOI] [PubMed] [Google Scholar]
- 103.Wang C, Schwaitzberg S, Berliner E, et al. Hyperbaric oxygen for treating wounds: A systematic review of the literature. Arch Surg 2003;138:272–279 [DOI] [PubMed] [Google Scholar]
- 104.Levett D, Bennett MH, Millar I. Adjunctive hyperbaric oxygen for necrotizing fasciitis. Cochrane Database Syst Rev 2015;1:CD007937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ayan F, Sunamak O, Paksoy SM, et al. Fournier's gangrene: A retrospective clinical study on forty-one patients. ANZ J Surg 2005;75:1055–1058 [DOI] [PubMed] [Google Scholar]
- 106.Mindrup SR, Kealey GP, Fallon B. Hyperbaric oxygen for the treatment of fournier's gangrene. J Urol 2005;173:1975–1977 [DOI] [PubMed] [Google Scholar]
- 107.Shaw JJ, Psoinos C, Emhoff TA, et al. Not just full of hot air: Hyperbaric oxygen therapy increases survival in cases of necrotizing soft tissue infections. Surg Infect (Larchmt) 2014;15:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg 2004;139:1339–1345 [DOI] [PubMed] [Google Scholar]
- 109.George ME, Rueth NM, Skarda DE, et al. Hyperbaric oxygen does not improve outcome in patients with necrotizing soft tissue infection. Surg Infect (Larchmt) 2009;10:21–28 [DOI] [PubMed] [Google Scholar]
- 110.Mehl AA, Nogueira Filho DC, Mantovani LM, et al. Management of Fournier's gangrene: Experience of a university hospital of Curitiba. Rev Col Bras Cir 2010;37:435–441 [DOI] [PubMed] [Google Scholar]
- 111.Li CQ, Gerson S, Snyder B. Case report: Hyperbaric oxygen and MRI findings in radiation-induced optic neuropathy. Undersea Hyperb Med 2014;41:59–63 [PubMed] [Google Scholar]
- 112.Sugihara A, Watanabe H, Oohashi M, et al. The effect of hyperbaric oxygen therapy on the bout of treatment for soft tissue infections. J Infect 2004;48:330–333 [DOI] [PubMed] [Google Scholar]
- 113.Massey PR, Sakran JV, Mills AM, et al. Hyperbaric oxygen therapy in necrotizing soft tissue infections. J Surg Res 2012;177:146–151 [DOI] [PubMed] [Google Scholar]
- 114.Hassan Z, Mullins RF, Friedman BC, et al. Treating necrotizing fasciitis with or without hyperbaric oxygen therapy. Undersea Hyperb Med 2010;37:115–123 [PubMed] [Google Scholar]
- 115.Krenk L, Nielsen HU, Christensen ME. Necrotizing fasciitis in the head and neck region: An analysis of standard treatment effectiveness. Eur Arch Otorhinolaryngol 2007;264:917–922 [DOI] [PubMed] [Google Scholar]
- 116.Wolf H, Rusan M, Lambertsen K, Ovesen T. Necrotizing fasciitis of the head and neck. Head Neck 2010;32:1592–1596 [DOI] [PubMed] [Google Scholar]
- 117.Hampson N, Atik D. Central nervous system oxygen toxicity during routine hyperbaric oxygen therapy. Undersea Hyperb Med 2003;30:147–153 [PubMed] [Google Scholar]
- 118.Plafki C, Peters P, Almeling M, et al. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med 2000;71:119–124 [PubMed] [Google Scholar]
- 119.Norrby-Teglund A, Basma H, Andersson J, et al. Varying titers of neutralizing antibodies to streptococcal superantigens in different preparations of normal polyspecific immunoglobulin G: Implications for therapeutic efficacy. Clin Infect Dis 1998;26:631–638 [DOI] [PubMed] [Google Scholar]
- 120.Norrby-Teglund A, Kaul R, Low DE, et al. Plasma from patients with severe invasive group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J Immunol 1996;156:3057–3064 [PubMed] [Google Scholar]
- 121.Dantal J. Intravenous immunoglobulins: In-depth review of excipients and acute kidney injury risk. Am J Nephrol 2013;38:275–284 [DOI] [PubMed] [Google Scholar]
- 122.Werdan K, Pilz G, Bujdoso O, et al. Score-based immunoglobulin G therapy of patients with sepsis: The SBITS study. Crit Care Med 2007;35:2693–2701 [PubMed] [Google Scholar]
- 123.Darenberg J, Ihendyane N, Sjolin J, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: A European randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003;37:333–340 [DOI] [PubMed] [Google Scholar]
- 124.Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis 1999;28:800–807 [DOI] [PubMed] [Google Scholar]
- 125.Norrby-Teglund A, Ihendyane N, Darenberg J. Intravenous immunoglobulin adjunctive therapy in sepsis, with special emphasis on severe invasive group A streptococcal infections. Scand J Infect Dis 2003;35:683–689 [DOI] [PubMed] [Google Scholar]
- 126.Alejandria MM, Lansang MA, Dans LF, Mantaring JB, 3rd. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev 2013;9:CD001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laupland KB, Kirkpatrick AW, Delaney A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: A systematic review and meta-analysis. Crit Care Med. 2007;35:2686–2692 [PubMed] [Google Scholar]
- 128.Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med 2007;35:2677–2685 [PubMed] [Google Scholar]
- 129.Turgeon AF, Hutton B, Fergusson DA, et al. Meta-analysis: Intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 2007;146:193–203 [DOI] [PubMed] [Google Scholar]
- 130.Neilson AR, Burchardi H, Schneider H. Cost-effectiveness of immunoglobulin M-enriched immunoglobulin (Pentaglobin) in the treatment of severe sepsis and septic shock. J Crit Care 2005;20:239–249 [DOI] [PubMed] [Google Scholar]
- 131.Pildal J, Gotzsche PC. Polyclonal immunoglobulin for treatment of bacterial sepsis: A systematic review. Clin Infect Dis 2004;39:38–46 [DOI] [PubMed] [Google Scholar]
- 132.Hamano N, Nishi K, Onose A, et al. Efficacy of single-dose intravenous immunoglobulin administration for severe sepsis and septic shock. J Intensive Care 2013;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodriguez A, Rello J, Neira J, et al. Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery. Shock 2005;23:298–304 [DOI] [PubMed] [Google Scholar]
- 134.Tugrul S, Ozcan PE, Akinci O, et al. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis (ISRCTN28863830). Crit Care 2002;6:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tagami T, Matsui H, Fushimi K, Yasunaga H. Intravenous immunoglobulin use in septic shock patients after emergency laparotomy. J Infect 2015;71:158–166 [DOI] [PubMed] [Google Scholar]
- 136.Tagami T, Matsui H, Fushimi K, Yasunaga H. Intravenous immunoglobulin and mortality in pneumonia patients with septic shock: An observational nationwide study. Clin Infect Dis 2015;61:385–392 [DOI] [PubMed] [Google Scholar]
- 137.Cavazzuti I, Serafini G, Busani S, et al. Early therapy with IgM-enriched polyclonal immunoglobulin in patients with septic shock. Intensive Care Med 2014;40:1888–1896 [DOI] [PubMed] [Google Scholar]
- 138.Yavuz L, Aynali G, Aynali A, et al. The effects of adjuvant immunoglobulin M-enriched immunoglobulin therapy on mortality rate and renal function in sepsis-induced multiple organ dysfunction syndrome: Retrospective analysis of intensive care unit patients. J Int Med Res 2012;40:1166–1174 [DOI] [PubMed] [Google Scholar]
- 139.Berlot G, Vassallo MC, Busetto N, et al. Relationship between the timing of administration of IgM and IgA enriched immunoglobulins in patients with severe sepsis and septic shock and the outcome: A retrospective analysis. J Crit Care 2012;27:167–171 [DOI] [PubMed] [Google Scholar]
- 140.Buda S, Riefolo A, Biscione R, et al. Clinical experience with polyclonal IgM-enriched immunoglobulins in a group of patients affected by sepsis after cardiac surgery. J Cardiothorac Vasc Anesth 2005;19:440–445 [DOI] [PubMed] [Google Scholar]
- 141.Mehta S, McGeer A, Low DE, et al. Morbidity and mortality of patients with invasive group A streptococcal infections admitted to the ICU. Chest 2006;130:1679–1686 [DOI] [PubMed] [Google Scholar]
- 142.Linner A, Darenberg J, Sjolin J, et al. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: A comparative observational study. Clin Infect Dis 2014;59:851–857 [DOI] [PubMed] [Google Scholar]
- 143.Prevention Centers for Disease Control and Prevention. Streptococcal toxic shock syndrome (STSS) (Streptococcus pyogenese) 2010 Case Definition. Atlanta, GA: CDC, 2010 [Google Scholar]
- 144.Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: A meta-analysis of randomized trials. Crit Care Med 2013;41:2209–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rimmer E, Houston BL, Kumar A, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: A systematic review and meta-analysis. Crit Care 2014;18:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borthwick EM, Hill CJ, Rabindranath KS, et al. High-volume haemofiltration for sepsis. Cochrane Database Syst Rev 2013;1:CD008075. [DOI] [PubMed] [Google Scholar]
- 147.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 2009;301:2445–2452 [DOI] [PubMed] [Google Scholar]
- 148.Nemoto H, Nakamoto H, Okada H, et al. Newly developed immobilized polymyxin B fibers improve the survival of patients with sepsis. Blood Purif 2001;19:361–368 [DOI] [PubMed] [Google Scholar]
- 149.Livigni S, Bertolini G, Rossi C, et al. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: A multicenter randomised controlled clinical trial. BMJ Open 2014;4:e003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: A randomized controlled trial. Crit Care Med 2009;37:803–810 [DOI] [PubMed] [Google Scholar]
- 151.Vincent JL, Laterre PF, Cohen J, et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock 2005;23:400–405 [DOI] [PubMed] [Google Scholar]
- 152.Peng Y, Yuan Z, Li H. Removal of inflammatory cytokines and endotoxin by veno-venous continuous renal replacement therapy for burned patients with sepsis. Burns 2005;31:623–628 [DOI] [PubMed] [Google Scholar]
- 153.Nakamura T, Kawagoe Y, Matsuda T, et al. Effect of polymyxin B-immobilized fiber on blood metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 levels in acute respiratory distress syndrome patients. Blood Purif 2004;22:256–260 [DOI] [PubMed] [Google Scholar]
- 154.Reinhart K, Meier-Hellmann A, Beale R, et al. Open randomized phase II trial of an extracorporeal endotoxin adsorber in suspected Gram-negative sepsis. Crit Care Med 2004;32:1662–1668 [DOI] [PubMed] [Google Scholar]
- 155.Nakamura T, Ushiyama C, Suzuki Y, et al. Hemoperfusion with polymyxin B-immobilized fiber in septic patients with methicillin-resistant Staphylococcus aureus-associated glomerulonephritis. Nephron Clin Pract 2003;94:c33–c39 [DOI] [PubMed] [Google Scholar]
- 156.Nakamura T, Ushiyama C, Suzuki Y, et al. Hemoperfusion with polymyxin B immobilized fibers for urinary albumin excretion in septic patients with trauma. ASAIO J 2002;48:244–248 [DOI] [PubMed] [Google Scholar]
- 157.Cole L, Bellomo R, Hart G, et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med 2002;30:100–106 [DOI] [PubMed] [Google Scholar]
- 158.Busund R, Koukline V, Utrobin U, Nedashkovsky E. Plasmapheresis in severe sepsis and septic shock: A prospective, randomised, controlled trial. Intensive Care Med 2002;28:1434–1439 [DOI] [PubMed] [Google Scholar]
- 159.Reeves JH, Butt WW, Shann F, et al. Continuous plasmafiltration in sepsis syndrome. Plasmafiltration in Sepsis Study Group. Crit Care Med 1999;27:2096–2104 [DOI] [PubMed] [Google Scholar]
- 160.Zu H, Li Q, Huang P, Wang X. Therapeutic value of blood purification and prognostic utilities of early serum procalcitonin, C reactive protein, and brain natriuretic peptide levels in severely burned patients with sepsis. Cell Biochem Biophys 2015;72:259–263 [DOI] [PubMed] [Google Scholar]
- 161.Nakamura T, Ushiyama C, Suzuki Y, et al. Combination therapy with polymyxin B-immobilized fibre haemoperfusion and teicoplanin for sepsis due to methicillin-resistant Staphylococcus aureus. J Hosp Infect 2003;53:58–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.