ABSTRACT
Botrytis cinerea causes pre- and postharvest decay of many fruit and vegetable crops. A survey of German strawberry fields revealed Botrytis strains that differed from B. cinerea in diagnostic PCR markers and growth appearance. Phylogenetic analyses showed that these strains belong to an undescribed species in Botrytis clade 2, named Botrytis fragariae sp. nov. Isolates of B. fragariae were detected in strawberry fields throughout Germany, sometimes at frequencies similar to those of B. cinerea, and in the southeastern United States. B. fragariae was isolated from overwintering strawberry tissue but not from freshly infected fruit. B. fragariae invaded strawberry tissues with an efficiency similar to or lower than that of B. cinerea but showed poor colonization of inoculated nonhost plant tissues. These data and the exclusive occurrence of this fungus on strawberry plants indicate that B. fragariae is host specific and has a tissue preference different from that of B. cinerea. Various fungicide resistance patterns were observed in B. fragariae populations. Many B. fragariae strains showed resistance to one or several chemical classes of fungicides and an efflux-based multidrug resistance (MDR1) phenotype previously described in B. cinerea. Resistance-related mutations in B. fragariae were identical or similar to those of B. cinerea for carbendazim (E198A mutation in tubA), azoxystrobin (G143A in cytB), iprodione (G367A+V368F in bos1), and MDR1 (gain-of-function mutations in the transcription factor mrr1 gene and overexpression of the drug efflux transporter gene atrB). The widespread occurrence of B. fragariae indicates that this species is adapted to fungicide-treated strawberry fields and may be of local importance as a gray mold pathogen alongside B. cinerea.
IMPORTANCE Gray mold is the most important fruit rot on strawberries worldwide and requires fungicide treatments for control. For a long time, it was believed to be caused only by Botrytis cinerea, a ubiquitous pathogen with a broad host range that quickly develops fungicide resistance. We report the discovery and description of a new species, named Botrytis fragariae, that is widely distributed in commercial strawberry fields in Germany and the southeastern United States. It was observed on overwintering tissue but not on freshly infected fruit and seems host specific on the basis of its occurrence and artificial infection tests. B. fragariae has also developed resistance to several fungicides that is caused by mutations similar to those known in B. cinerea, including an efflux-based multidrug resistance. Our data indicate that B. fragariae could be of practical importance as a strawberry pathogen in some regions where its abundance is similar to that of B. cinerea.
KEYWORDS: fungicide resistance, phylogeny
INTRODUCTION
The gray mold fungus Botrytis cinerea Pers. ex Fr. is one of the most important plant pathogens worldwide. It attacks about 1,000 known plant species and causes pre- and postharvest losses of cultivated fruits, vegetables, and ornamental flowers (1). It is the major representative of the genus Botrytis, which currently includes about 30 described species that are mainly necrotrophs (2). DNA sequence-based studies revealed Botrytis to be divided into two phylogenetic clades (3). Clade 1 includes B. cinerea and B. pseudocinerea which infect mostly or exclusively dicotyledonous plants, as well as host-specific species such as B. fabae, B. calthae, B. sinoviticola, and B. californica. Clade 2 is phylogenetically more diverse and consists of host-specific members that infect predominantly monocots but also several dicots (2). DNA sequencing and the use of PCR-based genetic markers have greatly improved genetic studies of gray mold field isolates and contributed to the identification of new Botrytis species such as the recently described species B. californica, B. deweyae, B. prunorum, B. pyriformis, and B. sinoviticola (4–8).
Gray mold is a major disease of strawberries and other soft fruits worldwide. Disease control involves several fungicide applications during bloom and sometimes fruit maturation, imposing strong selection pressure on the fungal population. In recent years, high frequencies of fungicide resistance that threaten the efficacy of chemical control of gray mold have been observed in B. cinerea populations from strawberry fields in Germany, Greece, Italy, and the United States (9–12). In addition to the prevailing target site resistances, drug efflux-mediated multidrug resistance (MDR) phenotypes are widespread in B. cinerea populations (13, 14). The MDR1 phenotype and a more resistant MDR1 variant called MDR1h are caused by overexpression of the ABC-type drug efflux transporter AtrB. The MDR1 and MDR1h phenotypes are likely to reduce fungicide field efficacy because they confer partial resistance to two important botryticides, fludioxonil and cyprodinil (10, 14, 15). Genetic studies revealed a diverse composition of Botrytis populations in German strawberry fields, comprising several genotypes of B. cinerea and minor frequencies of B. pseudocinerea (10, 16; M. Hahn and S. Rupp, unpublished data). In striking contrast to B. cinerea, B. pseudocinerea has almost no documented fungicide resistance, the exception being a low-level intrinsic resistance to fenhexamid (16).
During large-scale monitoring of gray mold populations in German strawberry fields, we observed Botrytis isolates with a growth morphology distinct from that of B. cinerea and B. pseudocinerea, which gave unclear results with PCR primers for these two species. Sequence analysis of five phylogenetically informative genes characterized these isolates as members of a previously undescribed species, designated Botrytis fragariae sp. nov., on account of its main host plant. Further analyses were conducted to perform a detailed morphological description of the new species, to analyze its occurrence in strawberry fields and on wild strawberries, to determine its host and possibly tissue specificity, and to identify fungicide resistances and the underlying mutations.
RESULTS
Discovery, phylogenetic placement, and molecular identification of a new Botrytis species.
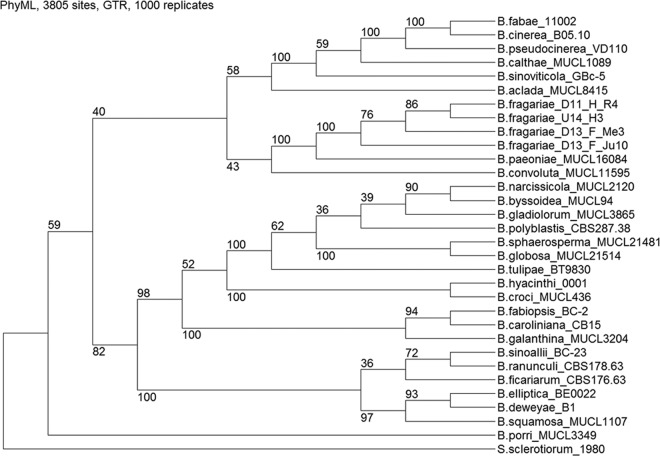
Botrytis strains with growth behavior on MYA agar plates that differed from that of B. cinerea and B. pseudocinerea strains were observed in several strawberry fields. DNA from these Botrytis strains was poorly amplified with mrr1 primers Mrr1-spez-F and Mrr1-spez-R. For three of these strains, a PCR with primers flanking a B. pseudocinerea-specific deletion (see Table S1 in the supplemental material) (16) resulted in a product with a size (ca. 145 bp) larger than that of the product obtained with either B. cinerea (136 bp) or B. pseudocinerea (112 bp; see Fig. S1 in the supplemental material). For phylogenetic placement, four of these unusual strains and three similar strains isolated from strawberry flowers in South Carolina (see below) were selected for sequencing of the hsp60, g3pdh, rpb2, nep1, and nep2 genes (Table 1). A combined tree placed all of the strains into a clade that was clearly separated from all other Botrytis spp., the closest relative being B. paeoniae (Fig. 1). Trees based on each of the five individual gene sequences also separated the strains from all other Botrytis species with high bootstrap values (see Fig. S2).
TABLE 1.
GenBank accession numbers of B. fragariae sequences determined in this study
| Straind | GenBank accession no. of: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| hsp60 | g3pdh | rpb2 | nep1 | nep2 | bos1 | mrr1 | cytB | tubA | |
| U14_P1 | KX429692 | KX429699 | KX429706 | KX429713 | KX429720 | NDa,b | ND | ND | ND |
| U14_G2 | KX429693 | KX429700 | KX429707 | KX429714 | KX429721 | ND | ND | ND | ND |
| U14_H3 | KX429694 | KX429701 | KX429708 | KX429715 | KX429722 | ND | ND | ND | ND |
| D11_H_R4 | KX429695 | KX429702 | KX429709 | KX429716 | KX429723 | KX429730 | KX429733 | KX429739 | KX429741 |
| D13_F_Me3 | KX429696 | KX429703 | KX429710 | KX429717 | KX429724 | ND | KX429734 | KX429740 | ND |
| D13_F_Ju10 | KX429697 | KX429704 | KX429711 | KX429718 | KX429725 | ND | ND | ND | ND |
| D13_H_J2-34 | KX429698 | KX429705 | KX429712 | KX429719 | KX429726 | KX429727 | ND | ND | KX429742 |
| D13_F_Nba1 | ND | ND | ND | ND | ND | KX429728 | KX429735 | ND | ND |
| D13_F_Nba10 | ND | ND | ND | ND | ND | KX429729 | ND | ND | ND |
| D13_F_Ju21 | ND | ND | ND | ND | ND | KX429732 | KX429736 | ND | ND |
| D13_F_Ju26 | ND | ND | ND | ND | ND | NDb | KX429737c | ND | ND |
| D14_F_Ju20 | ND | ND | ND | ND | ND | NDb | KX429738 | ND | ND |
| D14_Bl.427-22 | ND | ND | ND | ND | ND | NDb | NDb,c | ND | ND |
| D14_Bl.427-23 | ND | ND | ND | ND | ND | NDb | NDb,c | ND | ND |
| D15_F_WR3 | ND | ND | ND | ND | ND | KX429731 | NDb | ND | ND |
ND, sequence not determined.
Resistance mutation identified.
Mutation identical to MDR1-related mutation in B. cinerea (22).
D13_F_Ju10 is listed in GenBank as D13_D_F_Ju10, D13_H_J2-34 is listed in GenBank as D13_B_F_HJ_2-34, D13_F_Nba1 is listed in GenBank as D13_T_F_Nba1, and D13_F_Ju26 is listed in GenBank as D13-D-F-Ju26.
FIG 1.
Phylogenetic tree of Botrytis spp., including three strains of B. fragariae from Germany (D11_H_R4, D13_F_Me3, and D13_F_Ju10) and one from South Carolina (U14_H3), based on combined hsp60, g3pdh, rpb2, nep1, and nep2 sequences. Sclerotinia sclerotiorum was used as the outgroup.
On the basis of the sequencing data, their association with strawberries, and several unique phenotypic characters (see below), the seven strains were identified as members of a new species, which was named Botrytis fragariae sp. nov. For rapid preliminary identification of B. fragariae, a PCR-restriction fragment length polymorphism (RFLP) analysis method based on a species-specific sequence polymorphism was developed. A PCR fragment covering g3pdh was digested with the restriction enzyme BsaJI in all of the B. fragariae strains tested, but the BsaJI site was missing from the g3pdh genes of all of the other 29 Botrytis spp. described, including B. cinerea and the closely related species B. paeoniae, on the basis of the available sequence data in the NCBI database (see Fig. S3).
Distribution and host and tissue preference of B. fragariae.
By applying the diagnostic PCR-RFLP for identification of B. fragariae and the indel-based differentiation of B. cinerea and B. pseudocinerea (16), we estimated the relative abundance of the three Botrytis species by analyzing a total of 1,425 isolates from 28 fields (several fields were sampled up to three times) in Germany in different seasons between 2011 and 2015. In nine of the fields, B. fragariae was detected. In four of these fields, 25 to 65% of the isolates recovered belonged to B. fragariae, indicating that the species can be locally abundant (see Fig. S4). Overall, B. cinerea was clearly dominant (90.3%), followed by B. pseudocinerea (7.2%) and B. fragariae (2.5%). Curiously, all of the B. fragariae isolates obtained so far were isolated from overwintering, partly rotten vegetative strawberry tissues and fruit mummies collected before the fungicide treatments but none were from freshly harvested fruit. Among the Botrytis isolates collected before the fungicide treatments (n = 689; 22 fields), 87.1% of the isolates were B. cinerea, followed by B. pseudocinerea (8.6%) and B. fragariae (4.4%). Because of the prevalence of B. fragariae in spring 2013 in Gernsheim (13 of 20 isolates), samplings in that field were repeated. B. fragariae was not found among 20 isolates from moldy fruit in summer 2013 after the treatments but was detected again on leaves (8 of 20 isolates) in spring 2014 (Table 2). In contrast, in 2014, no isolate of B. fragariae was obtained from a total of 88 isolates obtained from three raspberry fields within a 2-km radius of the location of the type strain in Nottensdorf (Table 2). To determine whether B. fragariae occurs on wild strawberries, green and rotten petioles and leaves of Fragaria vesca were collected in November 2016 at four sites in a forest in western Germany. Of the 43 Botrytis isolates recovered, 32 were classified as B. cinerea and 11 were classified as B. pseudocinerea but none were classified as B. fragariae (see Table S3).
TABLE 2.
Phenotypic and genetic characterization of B. fragariae strains isolated from strawberry fields in Germany and the United Statesa
| Field site (time of isolation) | Plant tissue | Strain | Fungicide resistance |
MAT1 locus | Flip | Haplotype | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flub | Fen | Cyp | Carb | Ipr | Bos | Azo | ||||||
| Nottensdorf (autumn 2011) | Fruit mummies | D11_H_R3 | r | S | S | S | R | S | S | 1 | Yes | 1 |
| D11_H_R4c | r | S | S | S | R | S | S | 2 | No | 2 | ||
| D11_H_R5 | r | S | S | S | R | S | R | 1 | Yes | 3 | ||
| D11_H_R8 | r | S | S | R | R | S | R | 1 | Yes | 4 | ||
| D11_H_R11 | r | S | S | S | R | S | S | 2 | Yes | 5 | ||
| Iffezheim (spring 2013) | Flower | D13_HJ2-34 | r | S | S | R | S | S | R | 2 | No | 6 |
| Weiterstadt (spring 2013) | Flower | D13_F_Me3c | r | S | S | S | S | S | R | 2 | Yes | 7 |
| Grafschaft (spring 2012) | Leaf | D12_K_Mai_MU10 | R | S | S | S | R | S | S | NAe | NA | |
| Wittlich-A (spring 2013) | Leaves and stems | D13_F_Nba1 | R | S | S | R | S | S | S | 1 | No | 8 |
| D13_F_Nba9 | R | S | S | S | S | S | S | 1 | No | 9 | ||
| D13_F_Nba10 | r | S | S | R | S | S | S | 1 | No | 10 | ||
| D13_F_Nba12 | r | S | S | R | S | S | S | 1 | No | 10 | ||
| D13_F_Nba13 | R | S | S | S | S | S | S | 1 | No | 9 | ||
| Gernsheim-1 (Spring 2013) | Leaves and stems | D13_F_Ju1 | r | S | S | S | S | S | S | 1 | Yes | 11 |
| D13_F_Ju4 | r | S | S | S | S | S | S | 1 | Yes | 11 | ||
| D13_F_Ju10c | r | S | S | S | S | S | S | 1 | No | 12 | ||
| D13_F_Ju11 | r | S | S | S | S | S | S | 1 | Yes | 11 | ||
| D13_F_Ju13 | r | S | S | S | S | S | S | 1 | Yes | 11 | ||
| D13_F_Ju15 | r | S | S | S | S | S | S | 1 | Yes | 11 | ||
| D13_F_Ju18 | r | S | S | S | S | S | S | 1 | Yes | 11 | ||
| D13_F_Ju19 | r | S | S | S | S | S | S | 1 | No | 12 | ||
| D13_F_Ju21 | R | S | R | S | R | S | S | 1 | No | 13 | ||
| D13_F_Ju23 | r | S | S | S | S | S | S | 1 | No | 12 | ||
| D13_F_Ju26 | R | S | S | S | R | S | S | 2 | No | 14 | ||
| D13_F_Ju28 | r | S | S | S | S | S | S | 1 | Yes | 11 | ||
| D13_F_Ju29 | R | S | R | S | R | S | S | 1 | Yes | 15 | ||
| Gernsheim-2 (Spring 2014) | Leaves and stems | D14_F_Ju12 | R | S | R | S | R | S | S | 1 | Yes | 15 |
| D14_F_Ju13 | R | S | R | S | R | S | S | 1 | Yes | 15 | ||
| D14_F_Ju14 | r | S | S | S | S | S | S | 1 | Yes | 11 | ||
| D14_F_Ju16 | R | S | R | NA | NA | S | R | NA | NA | |||
| D14_F_Ju17 | R | S | R | S | R | S | S | 1 | No | 13 | ||
| D14_F_Ju18 | r | S | S | NA | NA | S | R | 1 | No | |||
| D14_F_Ju19 | R | S | R | NA | NA | S | S | NA | NA | |||
| D14_F_Ju20 | R | S | R | S | R | S | S | 2 | Yes | 16 | ||
| Wittlich-B (Spring 2014) | Leaf | D14_F_Nb3 | r | S | S | R | S | S | R | 2 | Yes | 17 |
| Wennigsen-Deister (Spring 2014) | Leaves and stems | D14_Bl.427-22 | R | S | R | S | R | S | S | NA | NA | |
| D14_Bl.427-23 | R | S | R | S | R | S | S | NA | NA | |||
| Wagshurst (Spring 2015) | Leaf | D15_W_R3 | r | S | R | R | R | S | R | NA | Yes | 18 |
| Pelion, SC, USA (spring 2015) | Flower | U14_P1 | r | S | S | R | S | S | S | NA | NA | |
| Gilbert SC, USA (spring 2015) | Flower | U14_G2 | r | S | S | R | S | S | R | NA | NA | |
| Holly Hill, SC, USA (spring 2015) | Flower | U14_H3 | r | S | S | R | S | S | S | NA | NA | |
The fungicide treatment histories of the fields are shown in Table S4. R, resistant; S, sensitive; NA, not analyzed. Flip, flipper DNA.
r, growth on 0.2 μg/ml fludioxonil; R, growth on 1 μg/ml fludioxonil.
Deposited in the CBS culture collection.
Genetic variability of B. fragariae.
The B. fragariae isolates were analyzed for the occurrence of the two mating type loci, the presence of the DNA transposon flipper (17), and the restriction patterns of PCR fragments generated with primers based on the intergenic region of the ribosomal DNA (IGS) (18). In contrast to B. cinerea, no variability was observed in the IGS-PCR patterns of the B. fragariae strains (not shown). Both mating type loci were observed, with a predominance of MAT1-1 (25 isolates) over MAT1-2 (6 isolates). Nineteen of the 33 strains tested contained flipper DNA. The sequences of two strains were 99 to 100% identical to the B. cinerea flipper DNA sequence (data not shown). When fungicide resistance was scored as equivalent to a genetic marker, 18 of the 38 isolates tested represented different haplotypes. Sequencing of the five genes used for taxonomic classification (Fig. 1; Table 1) revealed that all seven strains were genetically different. No significant genetic differentiation was possible between German and U.S. strains.
Phenotypic characterization of B. fragariae.
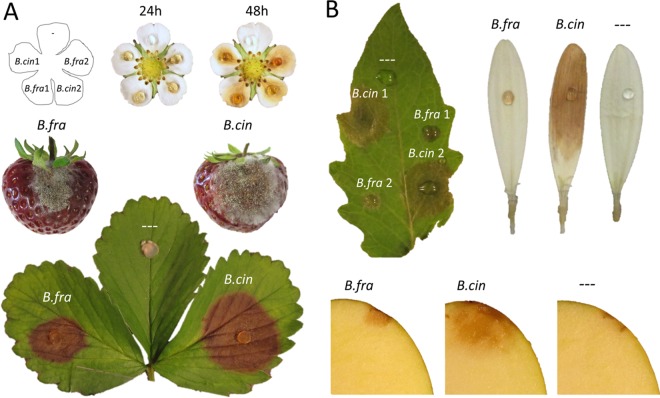
Growth parameters were tested for selected B. fragariae strains and compared to those of B. cinerea. On solid minimal or rich medium, the appearance of colonies was similar for the two species except that the growth of B. fragariae was significantly slower than that of B. cinerea (Fig. 2A; Table 3). When cultures started to sporulate, they turned gray to brown (Fig. 2A). The sporulation yield of B. fragariae on minimal medium was only about half of that of B. cinerea, and the macroconidia were slightly smaller than those of B. cinerea (Table 3). After 4 weeks of incubation in the dark, black sclerotia were produced; their number and size varied considerably between the strains of the two species (Fig. 2A). On minimal and rich agar media, the sporulating mycelium of all B. fragariae strains was flatter and more compact than that of B. cinerea (Fig. 2B). This difference was also evident on infected strawberry tissues (Fig. 2C).
FIG 2.
Growth behavior and morphology of B. fragariae and B. cinerea. (A) Mycelium growth and sclerotium formation. (B) Different heights of aerial mycelia of B. cinerea (B.cin) and B. fragariae (B.fra). (C) Aerial hyphae with conidiophores bearing clusters of macroconidia on strawberry petioles.
TABLE 3.
Growth parameters of selected B. cinerea and B. fragariae strainsa
| Species and strain | Mean radial growth on agar after 72 h (mm) ± SD |
Mean sporulation on agar (105 conidia cm−2) after 9 days ± SD |
Mean conidial length, width (μm) ± SD | ||
|---|---|---|---|---|---|
| GB5 | MA | GB5 | MA | ||
| B. cinerea | |||||
| B05.10 | 8.8 ± 1.3 | 9.9 ± 0.7 | 11.8 ± 3.6 | 12.7 ± 2.6 | 9.9 ± 1.3, 7.0 ± 0.9 |
| D13_F_Ju3 | 6.1 ± 1.9 | 5.0 ± 1.5 | 8.2 ± 6.8 | 7.6 ± 1.4 | 11.9 ± 1.8, 8.5 ± 1.2 |
| D13_F_Me1 | 5.8 ± 0.7 | 8.4 ± 2.2 | 7.1 ± 3.4 | 4.8 ± 1.1 | 9.3 ± 1.2, 7.2 ± 0.9 |
| All | 6.9 ± 2.0 | 7.7 ± 2.6 | 9.0 ± 2.8 | 8.3 ± 5.8 | 10.4 ± 2,2, 7.5 ± 1.2 |
| B. fragariae | |||||
| D11_H_R4H-R4 | 2.6 ± 0.8 | 3.0 ± 0.8 | 3.2 ± 1.2 | 2.4 ± 2.4 | 8.7 ± 1.5, 6.2 ± 0.7 |
| D13_HJ2-34 | 3.5 ± 1.7 | 5.2 ± 2.0 | 3.7 ± 3.5 | 7.0 ± 2.3 | 10.0 ± 2.2, 7.1 ± 1.2 |
| D13_F_Me3 | 3.6 ± 1.6 | 5.5 ± 1.7 | 4.5 ± 1.2 | 1.9 ± 0.9 | 10.2 ± 1.9, 7.2 ± 0.8 |
| D13_F_Ju10 | 2.1 ± 0.5 | 4.4 ± 1.7 | 5.1 ± 2.1 | 7.5 ± 0.3 | 9.3 ± 1.8, 6.0 ± 0.5 |
| All | 3.0 ± 1.4 | 4.5 ± 1.9 | 4.1 ± 1.7 | 5.3 ± 3.3 | 9.6 ± 1.9, 6.6 ± 1.0 |
Growth and sporulation data are the mean values of five and three biological replicates, respectively. Conidial sizes were determined from 100 conidia of each strain. Mean values of growth and sporulation of B. cinerea and B. fragariae are significantly different (P < 0.0001), except for sporulation on MYA. Mean conidial sizes were also different (length, P = 0.013; width, P < 0.001 [two-sided t test]).
Infection behavior of B. fragariae and B. cinerea on different tissues.
The virulence of B. fragariae was studied by artificial inoculation of several known host plants of B. cinerea. On strawberry fruit, B. fragariae showed a somewhat lower infection rate than B. cinerea and both the mycelium development and sporulation of B. fragariae occurred later and were less profuse than those of B. cinerea (Fig. 3; see Fig. S3A). On wounded strawberry leaves inoculated with agar discs containing germinated spores, both species produced expanding lesions but the lesions due to B. fragariae were smaller (P < 0.001) than those due to B. cinerea (see Fig. S3B). On strawberry flower petals, the two species formed necrotic lesions at similar rates (Fig. 3 and 4). On nonstrawberry tissues, B. fragariae strains were much less aggressive than B. cinerea, except for the infections of dog rose petals, in which the two species were comparable. B. cinerea rapidly colonized and formed spreading lesions on tomato leaves, Gerbera and blackberry flower petals, and apple fruit. B. fragariae formed only small primary lesions or sometimes no lesions at all (Fig. 3 and 4). The competitiveness of B. fragariae and B. cinerea was evaluated by mixed-inoculation experiments. On strawberry leaves, B. cinerea strains outcompeted B. fragariae strains in six out of eight experiments in the ability to form mycelium in the spreading lesions. In all experiments with strawberry fruit, B. cinerea dominated over B. fragariae (Table 4).
FIG 3.
Symptoms induced by B. cinerea (B.cin) and B. fragariae (B.fra) on strawberries (A) and the nonhost tissues tomato leaf, Gerbera petals, and apple fruit (B).
FIG 4.
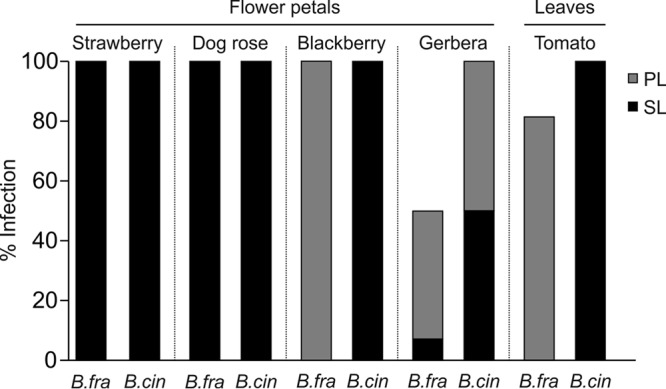
Virulence of B. cinerea (B.cin) and B. fragariae (B.fra) on different plant tissues 72 h after inoculation. On tomato leaves, strawberry petals, and Gerbera petals, five B. cinerea strains and seven B. fragariae strains were analyzed. On dog rose and blackberry petals, two B. cinerea strains and two B. fragariae strains were analyzed. PL, primary localized lesions; SL, secondary expanding lesions. Tests were performed three times with duplicates.
TABLE 4.
Results of mixed-inoculation experiments with B. cinerea and B. fragariae strains on strawberry tissues
| Substrate and B. cinerea strain | B. fragariae strain | Mean % recovery of B. cinerea ± SD (n)a |
|---|---|---|
| Leaves | ||
| B05.10 | D14_F_Ju12 | 90.0 ± 14.1 (5) |
| B05.10 | D14_F_Ju12 | 90.7 ± 14.1 (5) |
| B05.10 | D13_F_Ju29 | 94 ± 12.0 (5) |
| B05.10 | D13_F_Ju29 | 95.0 ± 11.2 (5) |
| D09-A04 | D14_F_Ju12 | 94 ± 12.0 (5) |
| D09-A04 | D14_F_Ju12 | 50 ± 28.9 (5) |
| D09-A04 | D13_F_Ju29 | 100 (5) |
| D09-A04 | D13_F_Ju29 | 48 ± 40.7 (5) |
| All | 83.3 ± 28.4 | |
| Fruit | ||
| B05.10 | D14_F_Ju12 | 64.7 ± 47.4 (14) |
| B05.10 | D14_F_Ju12 | 93.2 ± 8.0 (20) |
| B05.10 | D14_F_Ju12 | 84.5 ± 31.1 (17) |
| B05.10 | D13_F_Me3 | 100 (14) |
| B05.10 | D13_F_Me3 | 72.3 ± 31.5 (22) |
| B05.10 | D13_F_Me3 | 66.9 ± 25.5 (13) |
| All | 80.7 ± 30.3 |
Mean values and standard deviations of two independent experiments are shown. Strains were identified by their resistance to carbendazim (B. cinerea) and to fludioxonil and cyprodinil (B. fragariae).
Fungicide resistance frequencies.
Fungicide sensitivity assays were performed on agar plates containing discriminatory fungicide concentrations to detect resistance to the seven main site-specific fungicides used against Botrytis (10). Of the 38 German and three North American B. fragariae isolates analyzed (Table 2), 30 were resistant to at least one fungicide (excluding the low intrinsic resistance to fludioxonil) and 1 was resistant to four fungicides (see Fig. S4). The fungicide resistance frequencies in nine fields in which all three species were isolated were compared (Fig. 5). While the dominant species, B. cinerea, showed high frequencies of resistance to all five fungicides registered for gray mold control in Germany, all B. pseudocinerea isolates were sensitive, except for one carbendazim-resistant isolate. Some B. fragariae strains were resistant to cyprodinil, azoxystrobin, fludioxonil, iprodione, and carbendazim, but none was resistant to boscalid or fenhexamid.
FIG 5.
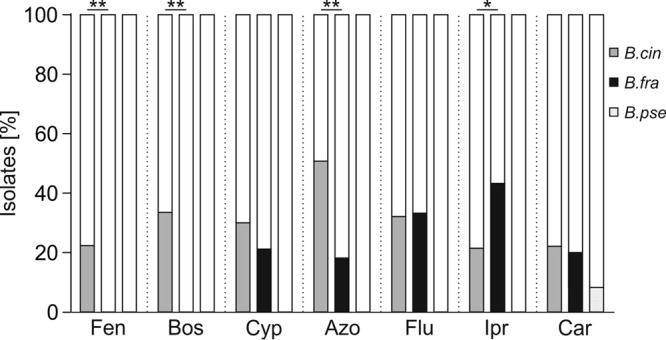
Fungicide resistance frequencies of B. cinerea (B.cin; n = 134; gray bars), B. fragariae (B.fra; n = 33; black bars), and B. pseudocinerea (B.pse; n = 12; light gray bars). Data are from isolates collected in nine German strawberry fields from which all three species were recovered. Significant differences in resistance frequencies between B. fragariae and B. cinerea are indicated by asterisks (*, P < 0.05; **, P < 0.01 [independent-sample t test]). Fen, fenhexamid; Bos, boscalid; Cyp, cyprodinil; Azo, azoxystrobin; Flu, fludioxonil; Ipr, iprodione; Car, carbendazim.
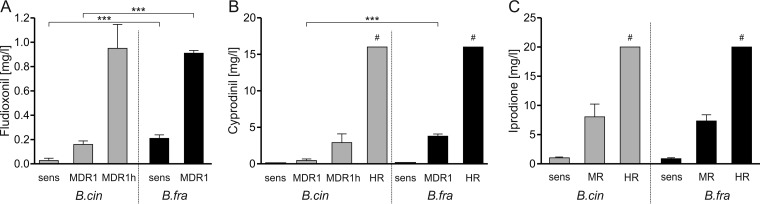
Quantitative evaluation of the sensitivity and resistance of B. fragariae strains to the fungicides fludioxonil, cyprodinil, and iprodione was performed. High levels of resistance of B. cinerea to fludioxonil have not been found in most field environments, but intermediate resistance caused by efflux-mediated MDR type 1 (MDR1) occurs frequently in gray mold populations in fungicide-treated fields in Europe and at lower frequencies in the United States (10, 19). All of the B. fragariae strains showed a sensitivity to fludioxonil approximately 10-fold lower than that of sensitive B. cinerea strains (Fig. 6A) and were able to grow on media containing 0.2 mg of fludioxonil liter−1, a concentration that inhibits the growth of sensitive but not MDR1 B. cinerea strains. Because these strains were sensitive to cyprodinil, the reduced fludioxonil sensitivity of B. fragariae was probably independent of drug efflux-related MDR. However, several B. fragariae strains were able to grow on plates containing 1 mg of fludioxonil liter−1. All of these strains had medium or high resistance to cyprodinil (Fig. 6B) and high resistance to tolnaftate (not shown), strongly indicating an MDR1-like phenotype (10). The fludioxonil resistance levels of these B. fragariae strains were similar to those of B. cinerea strains with the MDR1h phenotype, a more resistant variant of MDR1 (Fig. 6A) (10). The baseline sensitivity of B. fragariae strains to cyprodinil was similar to that of B. cinerea (Fig. 6B). The cyprodinil resistance levels of B. fragariae MDR1 strains were higher than those of B. cinerea MDR1 or MDR1h strains (Fig. 6B). The baseline sensitivities of B. fragariae and B. cinerea to iprodione were similar. Resistant B. fragariae strains could be grouped into medium- and high-resistance strains (Fig. 6C).
FIG 6.
Different fungicide sensitivities (EC50s) of B. cinerea (B.cin) and B. fragariae (B.fra) strains. (A) Fludioxonil. Mean values for sensitive (sens), MDR1, and MDR1h strains (one of each) of B. cinerea and three sensitive and three MDR1 strains of B. fragariae. (B) Cyprodinil. Mean values for sensitive, MDR1, and MDR1h strains of B. cinerea and three sensitive, three MDR1, and three highly resistant (HR; >16 mg liter−1) strains of B. fragariae. (C) Iprodione. Mean values for sensitive (sens; 0.5 to 2.0 mg liter−1), moderately resistant (MR; >2 to 20 mg liter−1), and highly resistant (HR; >20 mg liter−1) strains of B. cinerea (one of each) and three sensitive, three medium resistance, and three high resistance strains of B. fragariae. #, resistant to the highest concentration tested. The strains used are listed in Table S5. ***, significant differences between selected groups of B. cinerea and B. fragariae strains (P < 0.001 by two-tailed t test).
Fungicide resistance mutations.
To identify the mutations responsible for the resistance phenotypes observed, the known target genes of carbendazim (tubA encoding β-tubulin), quinone outside inhibitor (QoI) fungicides (cytB), and iprodione (bos1) were sequenced. All of the carbendazim-resistant strains tested contained the E198A mutation in tubA, and the azoxystrobin-resistant strains contained the G143A mutation in cytB (Table 5). Both mutations are common in fungi resistant to carbendazim and QoI, respectively, including B. cinerea. The bos1 sequences of five highly iprodione-resistant strains and one strain with medium resistance (D11_H_R4) contained two changes in adjacent codons (glycine to alanine at codon 367, valine to phenylalanine at codon 368 [G367A-V368F]). Whereas the double mutation has not been previously described in B. cinerea, mutations at positions 365, 368 (including V368F), and 369 are common in iprodione-resistant B. cinerea strains (20, 21). In medium resistance strain D13_F_Ju21, the observed changes (E62K, G748V) did not correspond to any of the known iprodione resistance-related mutations in B. cinerea (Table 5).
TABLE 5.
Fungicide resistance-related mutations identified in B. fragariae strains and fungicide sensitivity levels of selected strains
| Strain | Azoxystrobin (cytB) | Carbendazim (tubA) | Iprodione (bos1) |
Fludioxonil/MDR1 (mrr1) |
||
|---|---|---|---|---|---|---|
| Mutation | Avg EC50 (mg liter−1) ± SD | Mutation | Avg EC50 (mg liter−1) ± SD | |||
| D11_H_R4 | Sensitivea | Sensitivea | G367A-V368F | 8.35 ± 0.2 | Sensitivea | 0.18 ± 0.04 |
| D13_F_Me3 | G143A | Sensitive | Sensitive | 0.94 ± 0.2 | Sensitivea | 0.24 ± 0.06 |
| D13_HJ2-34 | G143A | E198A | Sensitivea | 0.90 ± 0.3 | Sensitive | 0.20 ± 0.06 |
| D13_F_Nba1 | Sensitive | E198A | Sensitivea | 1.95 ± 0.6 | Sensitivea | NDb |
| D14_F_Nba3 | Sensitive | E198A | Sensitive | ND | Sensitive | ND |
| D13_F_Nba10 | Sensitive | E198A | Sensitivea | 0.79 ± 0.3 | Sensitive | ND |
| D13_F_Ju21 | Sensitive | Sensitive | E62K, G748V | 6.20 ± 2.1 | C588Y | 0.90 ± 0.4 |
| D13_F_Ju26 | Sensitive | Sensitive | G367A-V368F | ND | R632I | 0.86 ± 0.5 |
| D14_F_Ju18 | G143A | Sensitive | Sensitive | ND | Sensitivea | ND |
| D14_F_Ju20 | Sensitive | Sensitive | G367A-V368F | >32 | R632I | 1.01 ± 0.4 |
| D14_Bl.427-22 | Sensitive | Sensitive | G367A-V368F | >32 | R632I | 0.96 ± 0.2 |
| D14_Bl.427-23 | Sensitive | Sensitive | G367A-V368F | ND | R632I | ND |
| D15_F_WR3 | G143A | E198A | G367A-V368F | >32 | C588Y | ND |
| U14_P1 | Sensitive | E198A | Sensitive | ND | Sensitive | ND |
| U14_G2 | G143A | E198A | Sensitive | ND | Sensitive | ND |
| U14_H3 | Sensitive | E198A | Sensitive | ND | Sensitive | ND |
Target gene of sensitive strain sequenced.
ND, not determined.
B. cinerea strains with the MDR1 phenotype harbor gain-of-function mutations in the transcriptional activator Mrr1 (13). Therefore, we sequenced most of the coding region of mrr1 of three B. fragariae strains with partial fludioxonil resistance and two strains with baseline sensitivity to fludioxonil, corresponding to bp 115 to 2411 of B. cinerea B05.10 mrr1. The mrr1 nucleotide sequences of the sensitive strains were identical to one another and encoded a protein with 87% and 84% identity to Mrr1 sequences of B. cinerea and B. cinerea group S strains, respectively (10). The mrr1 sequences of three resistant strains each possessed a single nonsynonymous mutation (Table 5). Three further resistant strains, from which mrr1 was partially sequenced, also contained a single amino acid-changing mutation (not shown). Of these six B. fragariae MDR1 strains, four carried a mutation leading to an R632I exchange, which is identical to a previously described MDR1-related mutation in B. cinerea (19). Two strains had a C588Y exchange, which has not been described before but is located in a region in which many other MDR1-related mutations have been found in B. cinerea (22).
Overexpression of atrB in MDR1 strains.
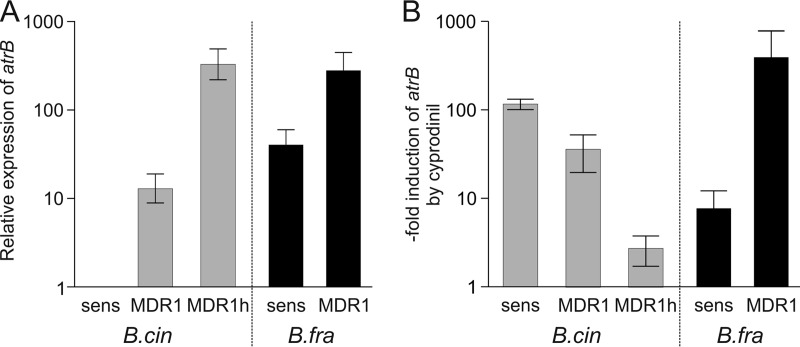
In B. cinerea MDR1 and MDR1h strains, atrB encoding the ABC-type drug efflux transporter is overexpressed in an Mrr1-dependent manner (10, 13). We therefore compared the mRNA levels of atrB in sensitive, MDR1, and MDR1h strains of B. cinerea and B. fragariae. B. cinerea MDR1 strain D06_5-16 and B. fragariae MDR1 strain D14_F_Ju20 both carried the same mutation in mrr1, R632I. For reverse transcription (RT)-PCR, a primer pair suitable for atrB detection in both species was designed. The basal atrB expression levels of sensitive B. fragariae strains were significantly higher than those of sensitive B. cinerea strains. The basal levels of atrB were higher in B. fragariae and B. cinerea MDR1 strains than those of the corresponding sensitive strains and even higher in a B. cinerea MDR1h strain (Fig. 7A). In all B. cinerea strains, atrB expression was strongly upregulated in the presence of cyprodinil, as shown previously (10). Treatment of B. fragariae also resulted in upregulation of atrB, but unexpectedly, the induction factor level was higher in the MDR1 isolate than in the sensitive isolate (Fig. 7B).
FIG 7.
Expression of atrB in B. cinerea (B.cin) and B. fragariae (B.fra) strains with and without the MDR1 and MDR1h phenotypes. (A) Basal transcript levels. The value of the sensitive (sens) B. cinerea strain (B05.10) was set to 1. (B) Increased expression of atrB in B. cinerea and B. fragariae strains after treatment with 1 mg of cyprodinil liter−1 for 30 min. The strains used are listed in Table S5.
TAXONOMY
Formal description of the new species B. fragariae.
On the basis of the molecular, morphological, and host specificity data presented here, the isolates from Fragaria × ananassa (strawberry) represent a new species of Botrytis, i.e., Botrytis fragariae C. Plesken, M. Hahn & R. W. S. Weber sp. nov. (Fig. 2 and 8).
FIG 8.
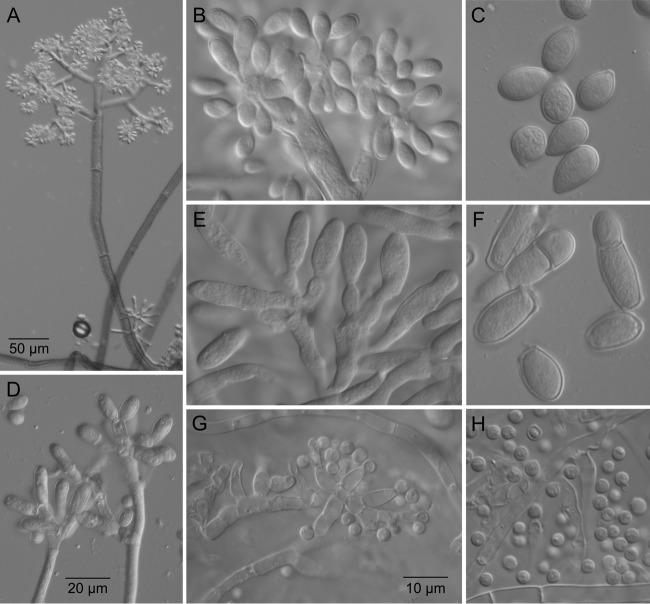
Microscopic features of B. fragariae. (A) Large and small macroconidiophores in direct comparison. (B) Details of conidiogenesis on large macroconidiophores. (C) Conidia produced from large macroconidiophores. (D and E) Small macroconidiophores and details of conidiogenesis. (F) Conidia produced from small macroconidiophores. (G) Microconidiophore. (H) Microconidia. Panels B, C, E, F, G, and H are at the same scale.
MycoBank number 818236.
Etymology: “fragariae” refers to the host plant (Fragaria spp.).
Colonies on malt extract agar growing 2.2 to 3.0 mm in diameter per day (Table 2), colony at first white and later pale gray, developing dark gray to black sclerotia sometimes covered by mycelium, solitary or aggregated. Sclerotia on malt extract agar developing in a scattered fashion within 4 weeks of incubation at 15°C in the dark; hemispherical, convex, sometimes hollow in the center with a concave surface; black, 2- to 6-mm (average, 3-mm) diameter. Conidiophores and conidia produced on strawberry fruit and fruit mummies and on potato dextrose agar in culture. Conidiophores of two kinds: (i) large macroconidiophores determinate, erect, thick walled, smooth, repeatedly septate, brown to subhyaline from base to apex, unbranched or with only one or two branches in the pigmented region, and repeatedly branched in the fertile region near the apex, 1,000 to 1,800 (−2,500) by 10 to 13 μm, tapering only slightly in the fertile region. Conidiogenous cells arising from alternating lateral branches, hyaline, giving rise to botryose clusters of conidia through narrow pores of <1 μm. (ii) Small macroconidiophores hyaline throughout, 4 to 5 μm wide, produced from aerial hyphae, giving rise to irregularly lobed fertile cells producing small botryose clusters of conidia through wide pores of 1.5 to 3.0 μm. Small macroconidiophores often produced earlier than large macroconidiophores. Conidia from large macroconidiophores ellipsoidal to ovoid, hyaline, aseptate, 7.5 to 13.2 by 5.9 to 9.8 μm (average, 9.6 by 6.6 μm). Conidia from small macroconidiophores ellipsoidal to oblong with a blunt base, either aseptate and 7.9 to 18.1 by 4.6 to 8.8 μm (average, 11.8 by 5.9 μm) or one-septate and 13.1 to 25.0 by 5.2 to 8.2 μm (average, 17.3 by 6.4 μm). Elongated two-septate conidia occasionally seen. All macroconidial types capable of germination on potato dextrose agar. Microconidia produced in hyaline droplets from dense clusters of phialides, spherical, smooth walled, except for abscission scar, 3.0 to 3.5 μm in diameter. Apothecia not observed.
Holotype.
Germany, Lower Saxony (53.4872°N, 9.5880°E), isolated from a strawberry (Fragaria × ananassa) fruit mummy in a commercial field after harvest, 7 October 2011, collected by R. W. S. Weber. Holotype CBS 141696 is a culture of D11_H_R4 deposited in the CBS Collection of Fungi (CBS Fungal Biodiversity Centre, Utrecht, The Netherlands).
Isotypes.
D13_F_Me3 (CBS 141697) and D13_F_Ju10 (CBS 141698) are isotypes.
DISCUSSION
A new Botrytis species, B. fragariae, was discovered in strawberry fields in Germany and the southeastern United States. Confirmation of its status as a distinct and previously undescribed species is drawn from morphological and physiological features, its host specificity, and phylogenetic analyses of DNA sequencing data. Morphologically, B. fragariae isolates are distinguished from B. cinerea by a flatter and more compact aerial mycelium. Furthermore, growth of all B. fragariae strains in the presence of 0.2 mg of fludioxonil liter−1 can be taken as a preliminary diagnostic marker of the species. Differential sensitivities to fungicides have been useful as preliminary physiological markers to distinguish B. pseudocinerea (low-level HydR1 resistance to fenhexamid, hypersensitivity to fenpropidin) (16, 23) and B. calthae (higher tolerance to succinate dehydrogenase inhibitor fungicides) (24) from B. cinerea and other related species, although DNA-based methods are required for final identification. A PCR-RFLP analysis method taking advantage of a unique restriction site in g3pdh of B. fragariae was developed. On the basis of five gene sequences, four German and three North American B. fragariae isolates were clearly separated from the most closely related species, B. paeoniae (Fig. 1). On the basis of polymorphic genetic markers and fungicide resistance profiles, 18 haplotypes were distinguished among the 38 B. fragariae isolates tested. Genetic diversity appears to be a hallmark of Botrytis spp., which has been described also for B. cinerea (25–27) and B. calthae (24). The occurrence of both mating types (MAT1-1 and MAT1-2) indicates that sexual reproduction is possible in B. fragariae populations, potentially contributing to the genetic diversity of this species.
B. fragariae was found widely distributed in strawberry fields throughout Germany. Although it was generally much less abundant than B. cinerea, it did contribute a major share of the Botrytis population within individual fields. Furthermore, it was found in North America and seems to be widely distributed along the East Coast of the United States (M. Dowling and G. Schnabel, unpublished data). The availability of a DNA-based screening method permits an analysis of the occurrence and possible origin of B. fragariae with higher precision. Cultivated strawberries are mostly hybrids (Fragaria × ananassa) of the American wild species Fragaria chiloensis and F. virginiana. It is unknown whether wild European strawberries (F. vesca, F. moschata, and F. viridis) are also natural host plants of B. fragariae. Despite extensive searches, we were unable to detect B. fragariae among several hundred isolates from other plant species sampled in the vicinity of strawberry fields with B. fragariae infections between 2012 and 2015, including raspberries and apples (R. W. S. Weber, unpublished data), which are also members of the family Rosaceae. We also failed to isolate B. fragariae from wild strawberries in Germany. One explanation of this could be the co-occurrence of wild strawberries with blackberries, raspberries, and blueberries in the Palatine Forest, all of which are known to be infected by B. cinerea and B. pseudocinerea. In artificial inoculation tests, B. fragariae was unable or barely able to cause expanding lesions on tomato leaves, Gerbera petals, or apple fruit, i.e., tissues that are readily infected by B. cinerea. In contrast, B. fragariae strains caused spreading lesions on strawberry tissues. Together, these data indicate that B. fragariae is a host-specific species on strawberries that coexists alongside B. cinerea and B. pseudocinerea. Other plants that are susceptible to host-specific Botrytis spp. are also attacked by the generalists B. cinerea and B. pseudocinerea. Examples include Caltha palustris, the host of B. calthae (24); broad bean, the host of B. fabae and B. fabiopsis (16, 28); and peony, the host of B. paeoniae (16). All of these host-specific species need to have specific adaptations that confer a selective advantage over B. cinerea and B. pseudocinerea, which are likely to produce larger numbers of airborne conidia from a wider range of colonized host tissues. In artificial inoculation experiments, B. fragariae appeared to be as aggressive on strawberry flowers as B. cinerea, but lesion formation on leaves and fruit was retarded. Similarly, B. fragariae was less competitive than B. cinerea in mixed-inoculation experiments on strawberry leaves and fruit. Therefore, it remains unclear how B. fragariae is able to adapt successfully to the strawberry plant as a host. So far, B. fragariae has been isolated predominantly from dead tissues of overwintering strawberry plants, including fruit mummies, but never from freshly rotting fruit. This indicates that B. fragariae has a tissue preference different from that of B. cinerea, which commonly causes infections of maturing fruit. In a strawberry field in Gernsheim, B. fragariae was found at an elevated frequency in spring 2013, disappeared in summer 2013, and reemerged in spring 2014, indicating that B. fragariae is able to survive in the field by colonizing vegetative tissue. Similar observations were made in a commercial strawberry field in Mullins, SC. Isolates from the same plot collected from flowers and 2 months later from rotting berries revealed that the flowers had been colonized exclusively by B. fragariae and the fruit had been colonized exclusively by B. cinerea. A comparable host tissue preference has been observed in B. pseudocinerea, which was found in French vineyards predominantly on flowers in spring but was less frequent on mature grapes than was the dominant species B. cinerea (29). Ecological specialization by different tissue preference could thus explain the coexistence of these sympatric species on the same host plant.
Mutations in B. fragariae conferring resistance to carbendazim (E198A in β-tubulin) and QoI fungicides (G143A in cytochrome b) were identical to those that are common in resistant strains of B. cinerea and other fungi. Interestingly, the major mutation in iprodione-resistant B. fragariae strains from several fields was a G367A-V368F double mutation in bos1. This mutation has not been found in B. cinerea, which typically has other mutations in the same region of amino acids 365 to 369 (20, 21). Thus, similar but nonidentical steric constraints may determine the probability of these mutations in different Botrytis spp. In all six B. fragariae strains tested, MDR1-related mutations were located in the mrr1 coding region. Four of the strains carried a mutation leading to an amino acid exchange (R632I) previously found in B. cinerea MDR1 strains (19), and two strains carried an as-yet-undescribed change (C588Y). In B. cinerea MDR1 strains, 15 different putative gain-of-function mutations in the transcriptional activator of atrB, Mrr1, have already been identified (22). MDR1 phenotypes in B. cinerea are correlated with the constitutive upregulation of atrB, which encodes an ABC-type efflux transporter. Similarly, a B. fragariae MDR1 strain showed 10-fold overexpression of atrB compared to a sensitive strain. As in B. cinerea, the atrB expression in B. fragariae increased in the presence of cyprodinil, confirming that atrB is similarly regulated in both species. In B. cinerea, the MDR1 phenotype is characterized by resistance factors of 5 to 20 for fludioxonil and cyprodinil (13). The effects of MDR1 on cyprodinil can only be tested in strains that have not acquired other mechanisms of resistance to cyprodinil. All B. fragariae MDR1 strains had medium to high levels of cyprodinil resistance, with resistance factors of at least 260, compared to non-MDR1 strains, which were all cyprodinil sensitive. It remains unclear whether these resistance levels are due only to the mrr1 mutation or also to other types of mutations.
Overall, the resistance frequencies of B. fragariae, B. cinerea, and B. pseudocinerea in the same fields showed significant differences. In agreement with a previous report (16), no resistance to a registered fungicide was found in B. pseudocinerea. While B. cinerea showed medium to high frequencies of resistance to the seven major classes of site-specific gray mold fungicides, in B. fragariae, somewhat lower frequencies of resistance to five fungicides were observed whereas no resistance to fenhexamid or boscalid was found. The intrinsic low-level fludioxonil resistance of B. fragariae and the high resistance levels of MDR1 strains, similar to those of B. cinerea MDR1h strains, could give B. fragariae a selective advantage in commercial strawberry fields when the fungicide Switch, a combination product of fludioxonil and cyprodinil and one of the most effective current botryticides, is repeatedly applied. All of the fields in which B. fragariae MDR1 strains were found had previously been sprayed with Switch. The resistance of B. fragariae to various fungicides, including the benzimidazoles that were introduced in the late 1960s but discontinued for several decades since, indicates that the species has been adapted to commercial strawberry fields for a long time. Being observed in different climatic regions of northern and southern Germany, as well as in the southeastern United States, B. fragariae can be expected to be discovered also in other countries and might be of local importance, together with B. cinerea, in causing tissue damage to strawberry plants.
MATERIALS AND METHODS
Isolation and cultivation of Botrytis strains.
Botrytis strains were obtained from infected strawberry tissue in different ways. Spores from sporulating lesions were directly transferred to malt yeast extract agar (MYA) plates (10 g of malt extract, 4 g of glucose, 4 g of yeast extract, and 15 g of agar per liter, pH 5.5) with sterile forceps or cotton swabs (10). Where necessary, infected tissue was incubated in a humid chamber for several days to allow new sporulation to occur. Alternatively, pieces of petioles, leaves, or inflorescences were placed onto MYA plates containing 0.5% tannic acid to suppress the growth of contaminating fungi. After sporulation, conidia were transferred to fresh MYA and the cultures were tested for the absence of contamination. Pure cultures were obtained by transfer of fresh conidia to MYA. Cultures for further processing were prepared as described previously (10). For long-term storage, cultures were kept in 30% glycerol at −80°C. Mycelial growth tests were performed at 20°C under ambient light conditions. For quantification of sporulation, MYA plates were incubated under Philips Black Light tubes (TL-D 36W, BLB) with a 16-h light, 8-h dark illumination cycle.
For isolation of Botrytis from wild strawberries (F. vesca), whole plants were collected in November 2016 at four sites ca. 0.5 to 1 km apart from each other in the Palatine Forest south of Kaiserslautern, Germany. Green petioles and leaves were cut into pieces with sterile scissors, transferred to MYA with tannic acid, and further treated as described above.
Fungicide sensitivity tests.
The fungicide resistance or sensitivity of the strains was tested on agar plates as described previously (16), by using the following discriminatory fungicide concentrations (per liter): 10 mg of fenhexamid, 0.2 and 1 mg of fludioxonil, 16 mg of cyprodinil, 5 mg of carbendazim, 25 mg of iprodione, 3 mg of boscalid, and 25 mg of azoxystrobin supplemented with 100 mg of salicylhydroxamic acid. Sensitivity to fludioxonil, cyprodinil, and iprodione was quantified as described previously (10), by determining the effective concentration at which a 50% reduction of germ tube growth was achieved (EC50) by using the following concentrations (mg liter−1): 0,0125, 0.025, 0.05, 0.1, 0.2, 0.4, and 0.8 for fludioxonil and cyprodinil and 1, 2, 4, 8, 16, 32, and 64 for all of the fungicides.
Plant infection tests.
For inoculation with spore suspensions, freshly harvested spores were suspended in Gamborg B5 minimal medium with 10 mM sodium phosphate and 25 mM glucose, pH 5.5 (GB5) (30). Leaves from potted strawberry plants (cv. Sonata) cultivated in a phytochamber or open air were inoculated with 5-mm agar discs of GB5 inoculated with 103 spores in 10 μl and incubated for 16 h to allow germination. The agar discs were placed onto detached strawberry leaves wounded by a small cut with a scalpel, the aerial hyphae being in contact with the leaf surface. Strawberry and raspberry fruits from a supermarket were surface sterilized before inoculation by soaking in 1% sodium hypochlorite for 5 min, followed by three washes with sterile water. Fruits were inoculated with 10-μl droplets of GB5 containing 103 conidia. Leaves from tomato (cv. Moneymaker) plants raised in a phytochamber, petals from Gerbera flowers (purchased in a supermarket) and flowers from wild strawberry (F. vesca) plants grown in a private garden were inoculated with 4-μl (flowers) or 10-μl (leaves) droplets of conidia (105 ml−1) suspended in GB5 medium. Inoculated plant tissues were incubated in a humid chamber under ambient laboratory light at 20°C for 72 h unless indicated otherwise.
For mixed-inoculation experiments with fruits, 500 conidia each of B. cinerea (strain B05.10 or D09-A04) and B. fragariae (strain D14_F_Ju12, D13_F_Ju29, or D13_F_Me3) were mixed and applied in 10-μl droplets to the intact fruit surface as described above. After 6 to 7 days of incubation, conidia that formed on the margin of the lesion were removed with wet sterile cotton swabs and transferred into a sterile tube containing a droplet of water. From the resulting suspension, 5-μl aliquots were applied to MYA plates containing discriminatory concentrations of carbendazim (5 mg liter−1) as a resistance marker for B. cinerea strains, fludioxonil (0.2 mg liter−1), or cyprodinil (16 mg liter−1) as resistance markers for B. fragariae strains, and no fungicide as a growth control as described above. For mixed-inoculation experiments with strawberry leaves, 1,000 spores of a 1:1 mixture of a B. cinerea strain and a B. fragariae strain were applied to an agar disc. Preincubation of the agar disc and inoculation of wounded leaves were done as described above. After 5 days of incubation, two peripheral sectors of the infected leaf tissue were transferred to an MYA plate and allowed to sporulate and the conidia were tested for resistance to carbendazim (B. cinerea) or fludioxonil and cyprodinil (B. fragariae) on fungicide-containing plates.
Genetic characterization of Botrytis strains.
PCR analysis and DNA sequencing were performed by using standard techniques with primers shown in Table S1. Genetic differentiation of B. cinerea and B. pseudocinerea was done as described previously (16), with primers BpsID_137F and BpsID_273R. Expression of atrB was analyzed by quantitative RT-PCR as described previously (10), with the following modifications. A 20-ml volume of liquid MY medium was inoculated with 2.5 × 105 spores and incubated overnight under ambient laboratory light at 20°C and 200 rpm. To induce atrB expression, 20 μl of a cyprodinil stock solution (1 mg ml−1 water) was added to the culture for a further 30 min. For isolation of total RNA, the mycelium was collected by centrifugation at 4,000 rpm for 5 min at 4°C and the pellet was rinsed in 650 μl of lysis buffer RA1 containing β-mercaptoethanol (NucleoSpin Plant RNA kit; Macherey-Nagel, Düren, Germany). The mixture was transferred to a 2-ml reaction tube filled with 500 μl of glass beads (0.75 to 1 mm), and the mycelium was disrupted in a Fast-Prep-24 instrument (MP Biomedicals, Solon, OH) by using three pulses of 30 s at setting 6.5 with cooling on ice between the pulses. After centrifugation (30 s at 13,000 rpm), the supernatant was loaded to a NucleoSpin filter, and RNA isolation was performed in accordance with the user manual. RT was done with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Munich, Germany). Quantitative PCR was performed with the MyiQ Real-Time PCR detection system (Bio-Rad Laboratories) and atrB primers optimized for B. fragariae and B. cinerea (atrB_RT_for/atrB_RT_rev). atrB transcript levels were normalized against the expression levels of the reference genes encoding actin and beta-tubulin, and results are shown as relative atrB expression (31). Experiments were performed with three biological replicates (technical triplicates of each).
Genetic diversity of B. fragariae isolates was examined by analyzing the presence or absence of the flipper transposon (17) and the mating type locus MAT1-1 or MAT1-2 (32) with primers shown in Table S1. Differentiation of isolates from B. cinerea groups N and S and B. pseudocinerea was achieved by using the mrr1-specific primers BcinN-in-F/BcinN-in-R and Mrr1-spez-F/Mrr1-spez-R. B. pseudocinerea isolates were identified with primers g2944_137_F/g2944_273_R (16).
Detection of fungicide resistance-related mutations.
Mutations related to fungicide resistance in B. fragariae were detected by sequencing. For detection of the E198A mutation in the tubA gene, which confers resistance to carbendazim, the PCR fragment obtained with primers Bc_Tub_for/Bc_Tub_rev was sequenced. The G143A mutation in cytB conferring resistance to the QoI azoxystrobin was detected by sequencing the PCR product generated with primers Qo13ext/Qo14ext. For detection of mutations related to iprodione resistance, the complete coding region of bos1, including introns, was sequenced. For detection of MDR1-related mutations, most of the mrr1 coding region, corresponding to positions 115 to 2411 in B. cinerea B05.10 mrr1, of two B. fragariae strains with baseline fludioxonil sensitivity, and three strains with partial fludioxonil resistance, was sequenced. Some B. cinerea-specific primers were modified to allow amplification and sequencing of B. fragariae bos1 and mrr1 (see Table S1).
Phylogenetic analyses.
Nucleotide sequences of hsp60, rpb2, g3pdh, nep1, and nep2, commonly used for differentiation of Botrytis spp., were obtained from the NCBI website (see Table S2) and aligned by using the SeaView software (33). Bootstrap frequencies were calculated with 1,000 replicates, and branches with bootstrap frequencies of >70% were considered significant.
Microscopy.
Photomicrographs were taken with an Axio Scope A1 light microscope fitted with 10×, 40×, and 100× Plan-Neofluar objectives and differential interference contrast optics with an ICc 3 digital camera (all from Carl Zeiss, Jena, Germany). Conidial dimensions of B. cinerea (strain B05.10) and B. fragariae (strain D11-H-R4) were measured with the AxioVision software 4.8 by using the 100× objective. Freshly sporulating mycelium after 7 to 10 days of growth on potato dextrose agar (Carl Roth, Karlsruhe, Germany) was used for all microscopic work.
Statistics.
Data were analyzed by unpaired, two-tailed t tests or with an independent-sample t test by using GraphPad Prism 5 for Windows 5.01.
Accession number(s).
The nucleotide sequences generated in this study have been deposited in GenBank under the accession numbers listed in Table 1.
Supplementary Material
ACKNOWLEDGMENTS
We thank Patrick Pattar, Isabel Keller, Julia Schwing, Tim Heyeck, Sylvia Thoms, Juliane Schurig, Franziska Kessler, and Eric Bohn for help with characterization of the strains and Michaela Leroch for fungicide sensitivity assays.
This work was supported by funds from the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support program (grant FKZ 2814705711).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00269-17.
REFERENCES
- 1.Elad Y, Pertot I, Prado AMC, Stewart A. 2016. Plant hosts of Botrytis spp. p 413–486. In Elad Y, Vivier M, Fillinger S (ed), Botrytis, the good, the bad and the ugly. Springer, New York, NY. [Google Scholar]
- 2.Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, Lévesque CA, Li X, Liu JK, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart A, Mortimer PE, Nair PVR, Pawlowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu JC, Yan JY, Zhou N. 2014. One stop shop: backbones trees for important phytopathogenic genera: I (2014). Fungal Divers 67:21–125. doi: 10.1007/s13225-014-0298-1. [DOI] [Google Scholar]
- 3.Staats M, van Baarlen P, van Kan JAL. 2005. Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Mol Biol Evol 22:333–346. doi: 10.1093/molbev/msi020. [DOI] [PubMed] [Google Scholar]
- 4.Saito S, Margosan D, Michailides TJ, Xiao CL. 2016. Botrytis californica, a new cryptic species in the B. cinerea species complex causing gray mold in blueberries and table grapes in California. Mycologia 108:330–343. doi: 10.3852/15-165. [DOI] [PubMed] [Google Scholar]
- 5.Grant-Downton RT, Terhem RB, Kapralov MV, Mehdi S, Rodriguez-Enriquez MJ, Gurr SJ, van Kan JA, Dewey FM. 2014. A novel Botrytis species is associated with a newly emergent foliar disease in cultivated Hemerocallis. PLoS One 9:e89272. doi: 10.1371/journal.pone.0089272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrada EE, Latorre BA, Zoffoli JP, Castillo A. 2016. Identification and characterization of Botrytis blossom blight of Japanese plums caused by Botrytis cinerea and B. prunorum sp. nov. in Chile. Phytopathology 106:155–165. doi: 10.1094/PHYTO-06-15-0143-R. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Yang H, Yu Q, Wu M, Yang L, Zhuang WY, Chen W, Li GQ. 2016. Botrytis pyriformis sp. nov., a novel and likely saprophytic species of Botrytis. Mycologia 108:682–696. doi: 10.3852/15-340. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Zhang J, Wang XD, Yang L, Jiang DH, Li GQ, Hsiang T, Zhuang WY. 2014. Morphological and phylogenetic identification of Botrytis sinoviticola, a novel cryptic species causing gray mold disease of table grapes (Vitis vinifera) in China. Mycologia 106:43–56. doi: 10.3852/13-032. [DOI] [PubMed] [Google Scholar]
- 9.Weber RWS. 2011. Resistance of Botrytis cinerea to multiple fungicides in Northern German small-fruit production. Plant Dis 95:1263–1269. doi: 10.1094/PDIS-03-11-0209. [DOI] [PubMed] [Google Scholar]
- 10.Leroch M, Plesken C, Weber RWS, Kauff F, Scalliet G, Hahn M. 2013. Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl Environ Microbiol 79:159–167. doi: 10.1128/AEM.02655-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Ortuño D, Grabke A, Bryson PK, Amiri A, Li X, Peres N, Schnabel G. 2014. Fungicide resistance profiles in Botrytis cinerea from strawberry fields of seven southern U.S. States. Plant Dis 98:825–833. doi: 10.1094/PDIS-09-13-0970-RE. [DOI] [PubMed] [Google Scholar]
- 12.De Miccolis Angelini RM, Rotolo C, Masiello M, Gerin D, Pollastro S, Faretra F. 2014. Occurrence of fungicide resistance in populations of Botryotinia fuckeliana (Botrytis cinerea) on table grape and strawberry in southern Italy. Pest Manag Sci 70:1785–1796. doi: 10.1002/ps.3711. [DOI] [PubMed] [Google Scholar]
- 13.Kretschmer M, Leroch M, Mosbach A, Walker AS, Fillinger S, Mernke D, Schoonbeek HJ, Pradier JM, Leroux P, de Waard MA. 2009. Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog 5:e1000696. doi: 10.1371/journal.ppat.1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández-Ortuño D, Grabke A, Li X, Schnabel G. 2015. Independent emergence of resistance to seven chemical classes of fungicides in Botrytis cinerea. Phytopathology 105:424–432. doi: 10.1094/PHYTO-06-14-0161-R. [DOI] [PubMed] [Google Scholar]
- 15.Amiri A, Heath SM, Peres NA. 2013. Phenotypic characterization of multifungicide resistance in Botrytis cinerea isolates from strawberry fields in Florida. Plant Dis 97:393–401. doi: 10.1094/PDIS-08-12-0748-RE. [DOI] [PubMed] [Google Scholar]
- 16.Plesken C, Weber RWS, Rupp S, Leroch M, Hahn M. 2015. Botrytis pseudocinerea is a significant pathogen of several crop plants but susceptible to displacement by fungicide-resistant B. cinerea strains. Appl Environ Microbiol 81:7048–7056. doi: 10.1128/AEM.01719-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levis C, Fortini D, Brygoo Y. 1997. Flipper, a mobile Fot1-like transposable element in Botrytis cinerea. Mol Gen Genet 254:674–680. [DOI] [PubMed] [Google Scholar]
- 18.Kretschmer M, Hahn M. 2008. Fungicide resistance and genetic diversity of Botrytis cinerea isolates from a vineyard in Germany. J Plant Dis Protect 115:214–219. doi: 10.1007/BF03356266. [DOI] [Google Scholar]
- 19.Li X, Fernández-Ortuño D, Grabke A, Schnabel G. 2014. Resistance to fludioxonil in Botrytis cinerea isolates from blackberry and strawberry. Phytopathology 104:724–732. doi: 10.1094/PHYTO-11-13-0308-R. [DOI] [PubMed] [Google Scholar]
- 20.Fillinger S, Ajouz S, Nicot PC, Leroux P, Bardin M. 2012. Functional and structural comparison of pyrrolnitrin- and iprodione-induced modifications in the class III histidine-kinase Bos1 of Botrytis cinerea. PLoS One 7:e42520. doi: 10.1371/journal.pone.0042520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabke A, Fernández-Ortuño D, Amiri A, Li X, Peres NA, Smith P, Schnabel G. 2014. Characterization of iprodione resistance in Botrytis cinerea from strawberry and blackberry. Phytopathology 104:396–402. doi: 10.1094/PHYTO-06-13-0156-R. [DOI] [PubMed] [Google Scholar]
- 22.Hahn M, Leroch M. 2015. Multidrug efflux transporters, p 233–250. In Ishii H, Hollomon D (ed), Fungicide resistance in plant pathogens: principles and a guide to practical management. Springer Japan KK, Tokyo, Japan. [Google Scholar]
- 23.Leroux P, Chapeland F, Desbrosses D, Gredt M. 1999. Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Protect 18:687–697. [Google Scholar]
- 24.Plesken C, Westrich LD, Hahn M. 2015. Genetic and phenotypic characterization of Botrytis calthae. Plant Pathol 64:128–134. doi: 10.1111/ppa.12240. [DOI] [Google Scholar]
- 25.Giraud T, Fortini D, Levis C, Lamarque C, Leroux P, LoBuglio Y, Brygoo Y. 1999. Two sibling species of the Botrytis cinerea complex, transposa and vacuma, are found in sympatry on numerous host plants. J Phytopathol 89:967–973. [DOI] [PubMed] [Google Scholar]
- 26.Rowe HC, Kliebenstein DJ. 2007. Elevated genetic variation within virulence-associated Botrytis cinerea polygalacturonase loci. Mol Plant Microbe Interact 20:1126–1137. doi: 10.1094/MPMI-20-9-1126. [DOI] [PubMed] [Google Scholar]
- 27.Amselem J, Cuomo CA, van Kan JAL, Viaud M, Benito EP, Couloux A, Coutinho PM, de Vries RP, Dyer PS, Fillinger S, Fournier E, Gout L, Hahn M, Kohn L, Lapalu N, Plummer KM, Pradier JM, Quévillon E, Sharon A, Simon A, ten Have A, Tudzynski B, Tudzynski P, Wincker P, Andrew M, Anthouard V, Beever RE, Beffa R, Benoit I, Bouzid O, Brault B, Chen Z, Choquer M, Collémare J, Cotton P, Danchin EG, Da Silva C, Gautier A, Giraud C, Giraud T, Gonzalez C, Grossetete S, Güldener U, Henrissat B, Howlett BJ, Kodira C, Kretschmer M, Lappartient A, Leroch M, Levis C, et al. 2011. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet 7:e1002230. doi: 10.1371/journal.pgen.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Wu MD, Li GQ, Yang L, Yu L, Jiang DH, Huang HC, Zhuang WY. 2010. Botrytis fabiopsis, a new species causing chocolate spot of broad bean in central China. Mycologia 102:1114–1126. doi: 10.3852/09-217. [DOI] [PubMed] [Google Scholar]
- 29.Walker AS, Gautier AL, Confais J, Martinho D, Viaud M, Le Pêcheur P, Dupont J, Fournier E. 2011. Botrytis pseudocinerea, a new cryptic species causing grey mould in French vineyards in sympatry with Botrytis cinerea. Phytopathology 101:1433–1445. doi: 10.1094/PHYTO-04-11-0104. [DOI] [PubMed] [Google Scholar]
- 30.Leroch M, Mueller N, Hinsenkamp I, Hahn M. 2015. The signalling mucin Msb2 regulates surface sensing and host penetration via BMP1 MAP kinase signalling in Botrytis cinerea. Mol Plant Pathol 16:787–798. doi: 10.1111/mpp.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Faretra F, Pollastro S. 1993. Genetics of sexual compatibility and resistance to benzimidazole and dicarboximide fungicides in isolates of Botryotinia fuckeliana (Botrytis cinerea) from nine countries. Plant Pathol 42:48–57. [Google Scholar]
- 33.Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.