Abstract
Most species in the Leguminosae (legume family) can fix atmospheric nitrogen (N2) via symbiotic bacteria (rhizobia) in root nodules. Here, the literature on legume-rhizobia symbioses in field soils was reviewed and genotypically characterised rhizobia related to the taxonomy of the legumes from which they were isolated. The Leguminosae was divided into three sub-families, the Caesalpinioideae, Mimosoideae and Papilionoideae. Bradyrhizobium spp. were the exclusive rhizobial symbionts of species in the Caesalpinioideae, but data are limited. Generally, a range of rhizobia genera nodulated legume species across the two Mimosoideae tribes Ingeae and Mimoseae, but Mimosa spp. show specificity towards Burkholderia in central and southern Brazil, Rhizobium/Ensifer in central Mexico and Cupriavidus in southern Uruguay. These specific symbioses are likely to be at least in part related to the relative occurrence of the potential symbionts in soils of the different regions. Generally, Papilionoideae species were promiscuous in relation to rhizobial symbionts, but specificity for rhizobial genus appears to hold at the tribe level for the Fabeae (Rhizobium), the genus level for Cytisus (Bradyrhizobium), Lupinus (Bradyrhizobium) and the New Zealand native Sophora spp. (Mesorhizobium) and species level for Cicer arietinum (Mesorhizobium), Listia bainesii (Methylobacterium) and Listia angolensis (Microvirga). Specificity for rhizobial species/symbiovar appears to hold for Galega officinalis (Neorhizobium galegeae sv. officinalis), Galega orientalis (Neorhizobium galegeae sv. orientalis), Hedysarum coronarium (Rhizobium sullae), Medicago laciniata (Ensifer meliloti sv. medicaginis), Medicago rigiduloides (Ensifer meliloti sv. rigiduloides) and Trifolium ambiguum (Rhizobium leguminosarum sv. trifolii). Lateral gene transfer of specific symbiosis genes within rhizobial genera is an important mechanism allowing legumes to form symbioses with rhizobia adapted to particular soils. Strain-specific legume rhizobia symbioses can develop in particular habitats.
Keywords: Leguminosae, N2 fixation, nodulation, nod genes, lateral gene transfer
1. Introduction
The Leguminosae (Fabaceae, the legume family) is comprised of ca. 19,300 species within 750 genera that occur as herbs, shrubs, vines or trees in mainly terrestrial habitats and are components of most of the world’s vegetation types [1,2,3]. Currently, the legume family is divided into three sub-families, the Caesalpinioideae, Mimosoideae and Papilionoideae [3,4]. Members of the Caesalpinioideae are grouped into four tribes, the Caesalpinieae, Cassieae, Cercideae and Detarieae comprising ca. 170 genera and 2250 species. The Mimosoideae are grouped into two tribes, the Ingeae and Mimoseae with ca. 80 genera and 3270 species, while the Papilionoideae consists of 28 tribes with ca. 480 genera and 13,800 species. However, a new classification of the legumes has been proposed with six sub-families based on the plastid matK gene sequences from ca. 20% of all legume species across ca. 90% of all currently recognized genera [5]. The six sub-families proposed are a re-circumscribed Caesalpinioideae, Cercidoideae, Detarioideae, Dialioideae, Duparquetioideae and Papilionoideae. In this system, the currently recognized Mimosoideae is a distinct clade nested within the re-circumscribed Caesalpinioideae. Species within the Cercidoideae, Detarioideae, Dialioideae and Duparquetioideae do not nodulate [5,6].
Most legume species can fix atmospheric nitrogen (N2) via symbiotic bacteria (general term “rhizobia”) in root nodules, and this can give them an advantage under low soil nitrogen (N) conditions if other factors are favourable for growth [7,8]. Furthermore, N2 fixation by legumes can be a major input of N into natural and agricultural ecosystems [9,10,11,12]. Generally, legume nodules can be classified as indeterminate or determinate in growth [13]. Indeterminate nodules maintain meristematic tissue, while determinate nodules have a transient meristem. Nodule type is dependent on host plant, and legume species that can produce both determinate and indeterminate nodules are rare [14,15]. All genera examined in the Caesalpinioideae and Mimosoideae had indeterminate nodules [13]. Within the Papilionoideae, most tribes had indeterminate nodules, but the Desmodieae, Phaseoleae, Psoraleae and some members of the Loteae show “desmodioid” determinate nodules and the Dalbergieae “aeschynomenoid” determinate nodules [13]. Desmodioid nodules have lenticels, and rhizobia “infected” tissue within them also contains uninfected cells. Aeschynomenoid nodules do not have lenticels, have uniform infected tissue and are always associated with lateral or adventitious roots. Where tested, species within the Desmodieae, Phaseoleae and Psoraleae had ureides as the main N-containing compound transported from nodules, but species in the Dalbergieae and Loteae transported amides/amino acids [13]. Indeterminate nodules have a single or branched apical meristem, and a few genera, such as Lupinus (Genisteae) and Listia (Crotalaria), have “lupinoid” nodules with two or more lateral meristems, which in some cases completely surround the subtending root [16,17]. Generally, indeterminate nodules have a mixture of infected and uninfected cells in the central nodule tissue, but lupinoid nodules, as for aeschynomenoid nodules (Dalbergiae), have uniformly-infected cells.
The nodulation process for almost all legumes studied is initiated by the legume production of a mix of compounds, mainly flavonoids, which induce the synthesis of NodD protein in rhizobia [18,19]. Different legumes produce different types/mixes of compounds. The NodD protein activates the transcription of other genes involved in the nodulation process, including those required to produce Nod factors, the signal molecules produced by the rhizobia and detected by the plant, which induce nodule organogenesis [20]. The nodABC genes encode for the proteins required to make the core Nod factor structure [18]. Nod factors from different rhizobia have a similar structure of a chitin-like N-acetyl glucosamine oligosaccharide backbone with a fatty acyl chain at the non-reducing end, but differ in their length of N-acetyl glucosamine oligosaccharide backbone and the length and saturation of the fatty acid chain. The Nod-factor core is modified by species-specific proteins, which results in various substitutions, including acetylation, glycosylation, methylation and sulfation. Perception of the Nod-factor signal in legumes is mediated by Nod factor receptors, which are plasma membrane localized serine/threonine receptor kinases in the case of the model legumes Lotus japonicus and Medicago truncatula [18,19].
The available data indicate that rhizobia enter the roots of most legume species via root hair infection [13]. Here, rhizobia enter root hairs, and host cell wall material grows around the developing “infection”, forming an infection thread, which grows through the cortex of the root, branching repeatedly. Generally, rhizobia are released from the tips of these infection threads into membrane-bound structures within host cells called symbiosomes where they differentiate into their N2-fixing form known as bacteroids. However, all species examined in the Caesalpinioideae, except herbaceous Chamaecrista spp. and a few species in the Papilionoideae, retain their rhizobia within infection threads [2,13]. Bacteroids vary greatly in their level of differentiation and viability depending on the legume host [13,21]. The process of root hair infection can lead to the formation of either indeterminate or desmodioid determinate nodules. A second mode of rhizobial infection occurs with species in the Dalbergiae (aeschynemonoid nodules) where rhizobia enter roots at the sites of lateral root emergence (“crack” entry), and infection threads are not involved in the infection process [22,23]. Thirdly, for at least some members of the Genisteae (e.g., Lupinus spp.) and Crotalariae (e.g., Listia spp.), rhizobia enter the roots directly through the root epidermis at the junction between epidermal cells, and again, infection threads are not involved in the infection process [17,21,24].
Over the past twenty-five years, DNA-based methods have become increasingly used to characterize rhizobia. In particular, phylogenetic analyses of sequences of the 16S ribosomal RNA (rRNA) gene, a range of “housekeeping” genes and genes involved in the symbiosis have been developed as a “standard approach” [15,25,26]. The 16S rRNA gene sequence on its own can delineate rhizobia at the genus level [27]. The main symbiosis genes studied are the “nif” genes, which encode the subunits of nitrogenase, the rhizobial enzyme that fixes N2, and the “nod” genes, which encode Nod factors that induce various symbiotic responses on legume roots. Specific nod genes have been shown to be major determinants of legume host specificity [28,29]. The nif and nod genes are often carried on plasmids or symbiotic islands, and these genes can be transferred (lateral transfer) between different bacterial species within a genus and more rarely across genera [30,31,32]. Almost all rhizobia tested had nod genes. However, a few Bradyrhizobium strains, which do not possess nodABC genes, can form N2 fixing nodules on particular Aeschynomene spp. [33,34]. Bacterial species from a range of genera in the α-proteobacteria (most commonly Bradyrhizobium, Ensifer (Sinorhizobium), Mesorhizobium and Rhizobium) and two genera in the β-proteobacteria (Burkholderia (Paraburkholderia) and Cupriavidus) can form functional (N2 fixing) nodules on specific legumes (Table 1, Table 2, Table 3 and Table 4). Reports that Achromobacter and Herbaspirillum (β-proteobacteria) produce N2 fixing nodules on Prosopis juliflora and Aspalathus linearis, respectively [35,36] and Pseudomonas (Gammaproteobacteria) produces N2 fixing nodules on Robinia pseudoacacia [37] and Acacia confusa [38] have not been confirmed. Furthermore, for Lotus corniculatus, Geobacillus (phylum Firmicutes), Paenibacillus (Firmicutes) and Rhodococcus (Actinobacteria) were for the first time reported as rhizobial symbionts [39]. These bacterial species had similar nodA gene sequences to Mesorhizobium isolated from the same plants, and it was concluded that the lateral gene transfer of these genes had occurred from the Mesorhizobium. However, lateral gene transfer of symbiosis genes is much less common between than within genera, and this work needs to be independently verified.
Table 1.
Legume-rhizobia symbioses in the legume sub-family Mimosoideae. All species have indeterminate nodules.
| Mimosoideae Tribes and Genera | Rhizobia-Field |
|---|---|
| Ingeae | |
| Acacia auriculiformis | Bradyrhizobium [40,41,42] |
| Acacia mangium | Bradyrhizobium [40,41,43,44], Ochrobactrum [44], Rhizobium [44] |
| Acacia mangium × A. auriculiformis | Bradyrhizobium [43] |
| Acacia mearnsii | Ensifer [45,46] |
| Acacia melanoxylon | Bradyrhizobium [47] |
| Acacia saligna | Bradyrhizobium [42,48], Ensifer [49], Rhizobium [48] |
| Acaciella angustissima | Ensifer [50,51] |
| Calliandra calothyrsis | Ensifer [52], Rhizobium [52] |
| Calliandra grandiflora | Ensifer [53], Mesorhizobium [53], Rhizobium [53] |
| Faidherbia albida | Bradyrhizobium [54] |
| Inga edulis | Bradyrhizobium [55] |
| Inga laurina | Bradyrhizobium [56] |
| Mariosousa acatlensis | Ensifer [57] |
| Senegalia laeta | Ensifer [45] |
| Senegalia macilenta | Ensifer [57], |
| Senegalia senegal | Ensifer [45,46], Rhizobium [45,58], Mesorhizobium [58] |
| Mimoseae | |
| Anadenanthera peregrina | Burkholderia [59] |
| Desmanthus illinoensis | Rhizobium [60] |
| Desmanthus paspalaceus | Mesorhizobium [61], Rhizobium [61] |
| Desmanthus virgatus | Rhizobium [43] |
| Leucaena leucocephala | Ensifer [52,62,63], Mesorhizobium [52,62,63], Rhizobium [52,62,64] |
| Microlobius foetidus | Bradyrhizobium [59], Rhizobium [59] |
| ~50 Mimosa spp. | Burkholderia [65,66,67,68,69,70,71,72,73,74,75,76,77] |
| Mimosa affinis | Rhizobium [72] |
| Mimosa albida, M. biuncifera, M.borealis, M. dysocarpa, M. polyantha, M. tricephala, Mimosa sp. | Ensifer [74], Rhizobium [74] |
| Mimosa asperata | Cupriavidus [78] |
| Mimosa benthamii, M. goldmanii, M. monancistra, M. robusta, M. tequilana | Rhizobium [74] |
| Mimosa borealis, M. lacerata, M. luisana, M. similis | Ensifer [74] |
| Mimosa ceratonia | Rhizobium [72] |
| Mimosa cruenta, M. magentea, M. ramulosa, M. reptans, M. schleidenii | Cupriavidus [79] |
| Mimosa diplotricha | Burkholderia [74], Cupriavidus [65,75,76], Rhizobium [65,72] |
| Mimosa hamata, M. himalayana | Ensifer [77] |
| Mimosa invisa | Rhizobium [70] |
| Mimosa pigra | Burkholderia [68], Cupriavidus [67,69] |
| Mimosa polyantha | Rhizobium [74] |
| Mimosa pudica | Bradyrhizobium [70], Burkholderia [74], Cupriavidus [65,69,72,75,76,77], Rhizobium [69,70] |
| Mimosa skinneri | Burkholderia [74], Rhizobium [74] |
| Mimosa strigillosa | Ensifer [78] |
| Neptunia natans | Allorhizobium [80], Devosia [81] |
| Parapiptadenia pterosperma | Burkholderia [59] |
| Parapiptadenia rigida | Burkholderia [59], Cupriavidus [82], Rhizobium [59] |
| Piptadenia adiantoides, P. flava | Rhizobium [59] |
| Piptadenia gonoacantha, P. paniculata | Burkholderia [59], Rhizobium [59] |
| Piptadenia stipulacea, P. trisperma, P. vividiflora | Burkholderia [59] |
| Prosopis alba | Bradyrhizobium [83], Ensifer [83,84], Mesorhizobium [83,85], Rhizobium [84] |
| Prosopis chilensis | Ensifer [46,86] |
| Prosopis cineraria | Ensifer [87] |
| Prosopis farcta | Ensifer [88], Mesorhizobium [88] |
| Prosopis juliflora | Ensifer [35], Rhizobium [35] |
| Pseudopiptadenia contorta | Burkholderia [59] |
| Stryphnodendron sp. | Bradyrhizobium [59] |
| Vachellia abyssinica | Mesorhizobium [89], Ensifer [90] |
| Vachellia cochliacantha, V. farnesiana, V. pennatula | Ensifer [57] |
| Vachellia gummifera | Ensifer [49] |
| Vachellia horrida | Ensifer [45,49] |
| Vachellia jacquemontii | Ensifer [87,91] |
| Vachellia macracantha | Ensifer [92], Rhizobium [92] |
| Vachellia nubica | Bradyrhizobium [54] |
| Vachellia seyal | Rhizobium [45], Ensifer [90] |
| Vachellia tortilis | Ensifer [45,49,90,93], Mesorhizobium [45,54,89,93], Rhizobium [93] |
| Vachellia xanthophloea | Mesorhizobium [54] |
| Xylia xylocarpa | Bradyrhizobium [40,43] |
Table 2.
Legume-rhizobia symbioses in the inverted repeat lacking clade (IRLC) of the legume sub-family Papilionoideae. All species in the IRLC have indeterminate nodules.
| Papilionoidieae Tribes and Genera | Rhizobia-Field |
|---|---|
| Cicereae | |
| Cicer arietinum | Mesorhizobium [94,95,96,97,98,99,100,101] |
| Cicer canariense | Mesorhizobium [102] |
| Fabeae | |
| Lathyrus aphaca, L. nissolia, L. pratensis | Rhizobium [103] |
| Lathyrus japonicus | Rhizobium [104] |
| Lathyrus odoratus | Rhizobium [105] |
| Lens culinaris | Rhizobium [101,106,107] |
| Pisum sativum | Rhizobium [101,103,107,108] |
| Vicia amoena, V. bungei, V. villosa | Rhizobium [109] |
| Vicia cracca | Rhizobium [103,109,110] |
| Vicia hirsuta | Rhizobium [103,105,110] |
| Vicia faba | Rhizobium [101,103,108,109,111,112,113,114] |
| Vicia multicaulis, V. sylvatica, V. tetrasperma | Rhizobium [110] |
| Vicia sativa | Rhizobium [103,109,115,116,117,118] |
| Vicia sepium | Rhizobium [109,110] |
| Galega | |
| Galega officinalis | Neorhizobium [119,120] |
| Galega orientalis | Neorhizobium [119] |
| Galegeae | |
| Astragalus adsurgense | Ensifer [121], Mesorhizobium [121], Rhizobium [122] |
| Astragalus aksuensis, A. betetovii | Rhizobium [105] |
| Astragalus complanatus | Ensifer [121], Mesorhizobium [121], Rhizobium [122] |
| Astragalus chrysopterus | Rhizobium [122] |
| Astragalus discolor, A. efoliolatus, A. kifonsanicus | Mesorhizobium [121] |
| Astragalus melilotoides | Ensifer [121], Mesorhizobium [121] |
| Astragalus membranaceus | Mesorhizobium [121,123,124] |
| Astragalus mongholicus | Mesorhizobium [124] |
| Astragalus polycladus | Rhizobium [121] |
| Astragalus scaberrimus | Mesorhizobium [121], Rhizobium [122] |
| Biserrula pelecinus | Mesorhizobium [125,126] |
| Carmichaelia australis, C. monroi, | Mesorhizobium [127] |
| Clianthus puniceus | Mesorhizobium [127] |
| Colutea arborescens | Ensifer [128], Mesorhizobium [128,129], Rhizobium [128] |
| Glycyrrhiza eurycarpa | Ensifer [130] |
| Glycyrrhiza glabra | Mesorhizobium [130,131], Rhizobium [130] |
| Glycyrrhiza inflata | Ensifer [130] |
| Glycyrrhiza multiflora | Mesorhizobium [132] |
| Glycyrrhiza pallidiflora | Mesorhizobium [133] |
| Glycyrrhiza uralensis | Mesorhizobium [130,132], Rhizobium [130] |
| Glycyrrhiza sp. | Mesorhizobium [130] |
| Gueldenstaedtia multiflora | Mesorhizobium [132], Rhizobium [132,134] |
| Lessertia annulans, L. capitata, L. diffusa, L. excisa, L. frutescens, L. herbacea, L. microphylla, L. pauciflora | Mesorhizobium [135] |
| Lessertia sp. | Ensifer [136] |
| Montigena novae-zelandiae | Mesorhizobium [137] |
| Oxytropis glabra | Ensifer [109], Mesorhizobium [138], Rhizobium [105,109] |
| Oxytropis kansuenses, O. myriophylla, O. psammocharis | Rhizobium [109] |
| Oxytropis meinshausenii | Rhizobium [105] |
| Oxytropis ochrocephala | Mesorhizobium [109], Rhizobium [109] |
| Oxytropis sp. | Phyllobacterium [109] |
| Sphaerophysa salsula | Ensifer [139], Mesorhizobium [139], Rhizobium [139,140] |
| Swainsona leeana, S. pterostylis | Ensifer [141] |
| Swainsona galegifolia | Mesorhizobium [137] |
| Hedysareae | |
| Alhagi sparsifolia | Mesorhizobium [142] |
| Alhagi toum | Rhizobium [105] |
| Caragana bicolor, C. erinacea | Mesorhizobium, Rhizobium [143] |
| Caragana franchetiana | Mesorhizobium, [143] |
| Caragana intermedia | Bradyrhizobium [143], Mesorhizobium [132,143], Rhizobium [143] |
| Caragana jubata | Rhizobium [105] |
| Caragana microphylla | Mesorhizobium [144] |
| Halimodendron halodendron | Rhizobium [105] |
| Hedysarum coronarium | Rhizobium [120,145] |
| Hedysarum polybotrys | Rhizobium [122], Mesorhizobium [124] |
| Hedysarum scoparium | Rhizobium [122] |
| Hedysarum spinosissimum | Ensifer [118] |
| Onobrychis viciifolia | Phyllobacterium [146] |
| Trifolieae | |
| Medicago archiducis-nicolai | Rhizobium [109] |
| Medicago intertexta | Ensifer [147] |
| Medicago laciniata | Ensifer [147,148,149,150], Neorhizobium [147] |
| Medicago lupulina | Ensifer [109,151] |
| Medicago orbicularis | Ensifer [152] |
| Medicago polymorpha | Ensifer [147], Neorhizobium [147] |
| Medicago rigiduloides | Ensifer [153] |
| Medicago ruthenica | Rhizobium [154] |
| Medicago sativa | Ensifer [109,150,155,156,157,158], Neorhizobium [147], Rhizobium [156] |
| Medicago scutellata | Ensifer [150] |
| Medicago truncatula | Ensifer [149,150,152] |
| Melilotus alba | Ensifer [156], Rhizobium [156] |
| Melilotus indicus, M. messanensis, M. siculus | Ensifer [147] |
| Melilotus officinalis | Ensifer [109,151] |
| Trigonella maritima | Ensifer [118,147] |
| Trifolium | |
| Trifolium fragiferum | Bradyrhizobium [70], Mesorhizobium [70], Rhizobium [70] |
| Trifolium pratense | Phyllobacterium [159], |
| Trifolium repens | Bradyrhizobium [70], Ensifer [70], Rhizobium [70,160] |
Table 3.
Legume-rhizobia symbioses of species in the sub-family Papilionoideae with indeterminate nodules excluding the inverted repeat lacking clade.
| Papilionoideae Tribes (Genera) | Rhizobia-Field |
|---|---|
| Abreae | |
| Abrus precatorius | Ensifer [161] |
| Amorpheae | |
| Amorpha fruticosa | Bradyrhizobium [162], Mesorhizobium [132,151,162] |
| Dalea purpurea | Mesorhizobium [163], Rhizobium [163] |
| Crotalarieae | |
| Aspalathus callosa | Burkholderia [136] |
| Aspalathus ciliaris, A. unifllora | Mesorhizobium [136] |
| Aspalathus linearis | Bradyrhizobium [36], Burkholderia [36], Mesorhizobium [36], Rhizobium [36] |
| Crotalaria comosa, C. hyssopifolia, C. lathyroides | Bradyrhizobium [164] |
| Crotalaria pallida | Bradyrhizobium [70], Burkholderia [70], Rhizobium [70] |
| Crotalaria perrotteti, C. podocarpa | Methylobacterium [164] |
| Crotalaria sp. | Burkholderia [136] |
| Lebeckia ambigua | Burkholderia [165] |
| Listia angolensis | Microvirga [166] |
| Listia bainesii, L. solitudinis, L. listii | Methylobacterium [16] |
| Lotononis laxa, L. sparsifolia | Ensifer [17] |
| Lotononis sp. | Bradyrhizobium, Mesorhizobium [17] |
| Rafnia sp. | Burkholderia [136] |
| Genisteae | |
| Adenocarpus hispanicus | Phyllobacterium [129] |
| Argyrolobium uniflorum | Ensifer [150,157] |
| Argyrolobium sp. | Mesorhizobium [136] |
| Cytisus aeolicus | Bradyrhizobium [167] |
| Cytisus balansae, C. multiflorus, C. striatus | Bradyrhizobium [168] |
| Cytisus laburnum, C. purgans | Bradyrhizobium [129] |
| Cytisus proliferus | Bradyrhizobium [169,170,171,172] |
| Cytisus scoparius | Bradyrhizobium [173,174] |
| Cytisus villosus | Bradyrhizobium [175] |
| Genista hystrix | Bradyrhizobium [168] |
| Genista stenopetula | Bradyrhizobium [170] |
| Genista versicolor | Bradyrhizobium [176] |
| Lupinus albescens | Bradyrhizobium [177,178] |
| Lupinus albus | Bradyrhizobium [172,179,180] |
| Lupinus angustifolius | Bradyrhizobium [172,180] |
| Lupinus honoratus | Ochrobactrum [181] |
| Lupinus luteus | Bradyrhizobium [172,180] |
| Lupinus mariae-josephae | Bradyrhizobium [182,183] |
| Lupinus montanus | Bradyrhizobium [170] |
| Lupinus micranthus | Bradyrhizobium [184] |
| Lupinus polyphyllus | Bradyrhizobium [170,185] |
| Lupinus texensis | Microvirga [166] |
| Lupinus sp. | Bradyrhizobium [172] |
| Retama monosperma | Bradyrhizobium [186] |
| Retama raetam | Bradyrhizobium [187] |
| Retama sphaerocarpa | Bradyrhizobium [168,186,187,188], Phyllobacterium [129] |
| Spartium junceum | Bradyrhizobium [129,167,189], Phyllobacterium [129] |
| Ulex europaeus | Bradyrhizobium [190] |
| Hypocalypteae | |
| Hypocalyptus coluteoides, H. oxalidifolius, H. sophoroides | Burkholderia [191] |
| Indigofereae | |
| Indigofera angustifolia | Burkholderia [136] |
| Indigofera astragalina, I. hirsuta, I. senegalensis, I. tinctoria | Bradyrhizobium [192] |
| Indigofera filifolia | Burkholderia [193] |
| Loteae | |
| Coronilla varia | Mesorhizobium [194], Rhizobium [132,134,194] |
| Ornithopus compressus, O. sativus | Bradyrhizobium [195] |
| Millettieae | |
| Milletia leucantha | Bradyrhizobium [40] |
| Millettia pinnata | Rhizobium [196] |
| Tephrosia capensis | Bradyrhizobium [136] |
| Tephrosia falciformis | Bradyrhizobium [87], Ensifer [87] |
| Tephrosia purpurea | Bradyrhizobium [192], Ensifer [87], Rhizobium [87] |
| Tephrosia villosa | Bradyrhizobium [87,192], Ensifer [87] |
| Tephrosia wallichii | Ensifer [86] |
| Podalyrieae | |
| Cyclopia buxifolia, C. genistoides, C. glabra, C. intemedia, C. longifolia, C. maculata, C. meyeriana, C. pubescens, C. sessiflora, C. subternata | Burkholderia [191] |
| Podalyria burchelli, P. sericea | Burkholderia [136] |
| Podalyria calyptrata | Burkholderia [136,191,193,197] |
| Podalyria pinnata | Burkholderia [193] |
| Virgilia divaricata | Rhizobium [136] |
| Virgilia oroboides | Burkholderia [136,191] |
| Robineae | |
| Gliricidia sepium | Ensifer [52], Rhizobium [52] |
| Robinia pseudocacia | Mesorhizobium [198,199], Rhizobium [105,198] |
| Sesbanieae | |
| Sesbania aculeata, S. grandiflora, S. pachycarpa, Sesbania sp. | Ensifer [45] |
| Sesbania cannabina | Ensifer [45,200,201,202], Neorhizobium [202], Rhizobium [200,202] |
| Sesbania exasperata | Rhizobium [30] |
| Sesbania herbacea | Rhizobium [203] |
| Sesbania punicea | Azorhizobium [136,204], Mesorhizobium [30], Rhizobium [204] |
| Sesbania rostrata | Azorhizobium [205,206], Bradyrhizobium [43], Ensifer [45,161], Rhizobium [43] |
| Sesbania sericea | Mesorhizobium [30], Rhizobium [30] |
| Sesbania sesban | Ensifer [45,52,90], Mesorhizobium [52,54,89], Rhizobium [52,54] |
| Sesbania virgata | Azorhizobium [206], Rhizobium [204] |
| Sophoreae | |
| Sophora alopecuroides | Ensifer [207], Mesorhizobium [207], Phyllobacterium [207], Rhizobium [105,207] |
| Sophora flavescens | Bradyrhizobium [208], Ensifer [208], Mesorhizobium [208], Phyllobacterium [209], Rhizobium [208] |
| Sophora longicarinata, S. microphylla, S. prostrata, S. tetraptera | Mesorhizobium [210] |
| Sophora viciifolia | Mesorhizobium [132] |
| Thermopsideae | |
| Ammopiptanthus nanus, A. mongolicus | Ensifer [211], Neorhizobium [211], Pararhizobium [211], Rhizobium [211] |
| Anagyris latifolia | Mesorhizobium [212] |
| Thermopsis lupinoides | Mesorhizobium [213] |
Table 4.
Legume-rhizobia symbioses of species in the sub-family Papilionoideae with determinate nodules.
| Papilionoideae Tribes and Genera | Rhizobia-Field |
|---|---|
| Dalbergieae | |
| Adesmia bicolor | Rhizobium [214] |
| Aeschynomene afraspera, A. ciliata, A. elaphroxylon, A. scabra, A. sensitiva, A. shimperi | Bradyrhizobium [34] |
| Aeschynomene americana | Bradyrhizobium [34,215] |
| Aeschynomene indica | Bradyrhizobium [34,216] |
| Aeschynomene rudis | Bradyrhizobium [34,217] |
| Arachis duranensis | Bradyrhizobium [218] |
| Arachis hypogaea | Bradyrhizobium [43,219,220,221,222,223,224,225,226,227,228,229], Rhizobium [221,222] |
| Centrolobium paraense | Bradyrhizobium [230,231] |
| Dalbergia baroni, D. louveli, D. madagascariensis, D. maritima, D. monticola, D. purpurascens, Dalbergia sp. | Bradyrhizobium [232] |
| Pterocarpus officinalis | Bradyrhizobium [233] |
| Pterocarpus indicus | Bradyrhizobium [40,43] |
| Zornia glochidiata | Bradyrhizobium [234] |
| Desmodieae | |
| Desmodium caudatum, D. fallax, D. triflorum | Bradyrhizobium [235] |
| Desmodium elegans | Bradyrhizobium [235,236], Pararhizobium [236] |
| Desmodium gangeticum | Bradyrhizobium [235,237] |
| Desmodium heterocarpan | Bradyrhizobium [235,237] |
| Desmodium microphyllum | Bradyrhizobium [235], Mesorhizobium [235], Rhizobium [235] |
| Desmodium oldhami | Rhizobium [236] |
| Desmodium racemosum | Bradyrhizobium [235], Ensifer [235], Rhizobium [235] |
| Desmodium sequax | Bradyrhizobium [235], Ensifer [235], Mesorhizobium [236], Pararhizobium [236], Rhizobium [235,236] |
| Desmodium sinuatum | Rhizobium [238] |
| Kummerowia stipulacea | Bradyrhizobium [151,239], Rhizobium [239] |
| Kummerowia striata | Bradyrhizobium [239], Ensifer [239], Rhizobium [239] |
| Lespedeza bicolor | Bradyrhizobium [240], Ensifer [240], Mesorhizobium [151] Rhizobium [240] |
| Lespedeza capitata, L. cuneata, L. juncea, L. procumbens, L. stipulacea, L. striata | Bradyrhizobium [240] |
| Lespedeza cystobotrya | Ensifer [240], Rhizobium [122] |
| Lespedeza daurica | Bradyrhizobium [240], Ensifer [240], Mesorhizobium [240] |
| Lespedeza davidii | Rhizobium [122] |
| Lespedeza inschanica, L. tomentosa | Ensifer [240] |
| Phaseoleae | |
| Amphicarpaea bracteata, A. edgeworthii | Bradyrhizobium [241] |
| Amphicarpaea trisperma | Rhizobium [132] |
| Bolusafra bituminosa | Burkholderia [136] |
| Cajanus cajan | Bradyrhizobium [242] |
| Canavalia rosea | Ensifer [243] |
| Centrosema pascuorum | Bradyrhizobium [43] |
| Centrosema pubescens | Bradyrhizobium [42] |
| Dipogon lignosus | Burkholderia [15,193] |
| Glycine max | Bradyrhizobium [43,151,244,245,246,247,248,249,250,251], Ensifer [245,246,247,252,253], Rhizobium [254] |
| Glycine soja | Bradyrhizobium [151,255], Ensifer [255], Rhizobium [256] |
| Lablab purpureus | Bradyrhizobium [224,226,227] |
| Neonotonia wightii | Bradyrhizobium [237] |
| Pachyrhizus erosus | Bradyrhizobium [257,258,259], Rhizobium [257] |
| Pachyrhizus ferrugineus, P. tuberosus | Bradyrhizobium [258] |
| Phaseolus lunatus | Bradyrhizobium [260,261,262], Rhizobium [262] |
| Phaseolus vulgaris | Bradyrhizobium [260,263], Burkholderia [264,265], Ensifer [263,266,267,268], Pararhizobium [263], Rhizobium [64,101,151,227,263,266,267,268,269,270,271,272,273,274,275] |
| Pueraria phaseoloides | Bradyrhizobium [276] |
| Rhynchosia aurea | Ensifer [87] |
| Rhynchosia ferulifolia | Burkholderia [277,278] |
| Rhynchosia minima | Bradyrhizobium [192,277] |
| Rhynchosia totta | Bradyrhizobium [277] |
| Vigna angularis | Bradyrhizobium [279], Ensifer [279], Rhizobium [279] |
| Vigna radiata | Bradyrhizobium [280,281], Ensifer [280], Rhizobium [280] |
| Vigna sinensis | Bradyrhizobium [43] |
| Vigna subterranea | Bradyrhizobium [227,282], Burkholderia [282], Rhizobium [282] |
| Vigna unguiculata | Bradyrhizobium [223,227,280,283,284,285], Burkholderia [283], Microvirga [286], Rhizobium [280,283] |
| Psoraleae | |
| Otholobium bracteolatum, O. hirtum, O. virgatum, O. zeyhari, Otholobium sp. | Mesorhizobium [136] |
| Psoralea asarina | Burkholderia [286] |
| Psoralea corylifolia | Ensifer [201] |
| Psoralea pinnata | Bradyrhizobium [193], Burkholderia [287], Mesorhizobium [136,193,287] |
| Loteae | |
| Lotus arabicus, L. arinagensis | Ensifer [157] |
| Lotus bertheloti, L. callis-viridis, L. campylocladus, L. pyranthus | Mesorhizobium [288] |
| Lotus corniculatus | Mesorhizobium [39,288,289,290,291] |
| Lotus creticus | Ensifer [118,157], Mesorhizobium [118,157], Rhizobium [118,157] |
| Lotus frondosus | Mesorhizobium [138], Rhizobium [105] |
| Lotus halophyllus | Ensifer [118] |
| Lotus kunkeli, L. lancerottensis, L. maculatus | Ensifer [292] |
| Lotus sessilifolius | Ensifer [292], Mesorhizobium [288] |
| Lotus tenuis | Mesorhizobium [291,293,294], Rhizobium [105,293] |
| Lotus uliginosus | Bradyrhizobium [295] |
Legume species differ greatly in their specificity for rhizobial symbionts. Galega officinalis (tribe Galegeae) and Hedysarum coronarium (tribe Hedysareae) have been highlighted as being highly specific with respect to their rhizobial symbionts [120,145,296,297]. Both of these species are in the inverted repeat lacking clade (IRLC). The IRLC is marked by the loss of one copy of the inverted region of the plastid genome [298,299]. Almost all genera in the IRLC are temperate; all have indeterminate nodules, and where examined, their bacteroids were terminally differentiated and could not return to their bacterial form [13]. The IRLC contains several important temperate grain (e.g., Pisum sativum and Vicia faba) and forage (e.g., Trifolium spp. and Medicago spp.) legumes. There is evidence that at least some of these crop legumes have a high degree of rhizobial specificity. For example, an analysis of core and symbiotic genes of rhizobia nodulating Vicia faba and Vicia sativa from different continents showed that they belong to a phylogenetically-compact group indicating that these species are restrictive hosts [117]. In contrast, Macroptilium purpureum and the grain legumes Phaseolus vulgaris and Vigna unguiculata in the tribe Phaseoleae are nodulated by rhizobia from different genera across the α- and β-proteobacteria [264,283,300]. The Phaseoleae are of tropical/subtropical origin, have desmodioid determinate nodules with bacteroids, which are not terminally differentiated [2,13].
Here, the literature on legume-rhizobia symbioses in field soils was reviewed and genotypically characterised rhizobia related to the taxonomy of the legumes from which they were isolated. The objectives of the work were to collate data on legume rhizobia symbioses and then assess to what extent legume specificity for rhizobial symbionts is related to legume taxonomy.
2. Framework and Assumptions of Study
The general classification of the Leguminosae follows Lewis et al., 2005 [1], with updates [3,4]. The sub-families Caesalpinioideae, Mimosoideae and Papilionoideae are considered separately. The Papilionoideae is split into those that show indeterminate nodules and those that show determinate nodules. Those that show indeterminate nodules are further split into the IRLC and all other clades.
Nodulating bacteria were classified at the genus level, on the basis of sequences of the 16S rRNA gene (almost all cases), and/or the 16S–23S DNA intergenic spacer region, and/or common house-keeping genes, and/or DNA-DNA hybridisations, and these results are presented in the tables. Sequences for nif and nod genes are considered in the text. Rhizobial genus and species names validated in the International Journal of Systematic and Evolutionary Microbiology were used with one exception: Burkholderia was retained as opposed to using Paraburkholderia [301,302], as a case to reinstate Burkholderia is being prepared by workers in the field. The term symbiovar (sv.) is used when describing rhizobial strains within the same species that differ with respect to the legume species they effectively nodulate [303].
A comprehensive collation of published legume-rhizobia symbioses up until 30 September 2016 was carried out. Articles were collected by searching the Institute for Scientic Information (ISI) Web of Science using each legume genus partnered with each of the rhizobia, Bradyrhizobium, Burkholderia, Cupriavidus, Ensifer, Mesorhizobium, Rhizobium and Sinorhizobium as the keywords. Further searches were carried out on the literature quoted in the selected papers and those listed as quoting the selected papers in the ISI Web of Science. Only data for plants sampled under field conditions, or for plants grown in soils taken from the field, or supplied field soil extracts were used. Bacteria isolated from legume nodules were accepted as rhizobia if they were shown to produce functional (N2 fixing) nodules on inoculation of their original legume host or a species within the original host legume genus under axenic conditions. The range of measurements and visual assessments used as evidence of the occurrence of N2 fixation were accepted. These were acetylene reduction activity, red/pink nodules (evidence of leghaemoglobin and, hence, nodules assumed to be active), increased total plant or shoot dry matter or N content, visually greener (increased chlorophyll) and increased plant vigour. However, it is acknowledged that in some cases, greater growth, vigour and/or greenness could have been caused by plant hormone production by the bacterium [304]. All data obtained for all species are presented with three exceptions. Representative data are presented for Glycine max, Phaseolus vulgaris and Vigna unguiculata due to the large number of publications on these three species.
3. Caesalpinioideae-Rhizobia Symbioses
Of the three legume sub-families, the Caesalpinioideae contains the smallest proportion of nodulated genera with nodulation confirmed for Campsiandra, Chidlowia, Dimorphandra, Erythrophleum, Jacqueshuberia, Melanoxylon, Moldenhauwera and Tachigali in the tribe Caesalpinieae and Chamaecrista in the tribe Cassieae [2,13]. Only two studies have genotypically characterised bacteria confirmed as rhizobia of Caesalpinioideae species. Firstly, five rhizobial isolates from Dimorphandra wilsonii and one from Dimorphandra jorgei sampled in the Cerrado biome in Brazil were Bradyrhizobium [305]. Secondly, 166 rhizobial isolates from Erythrophleum fordii sampled at four sites in the Guangdong and Guangsii Provinces of the southern sub-tropical region of China were also all Bradyrhizobium [306]. In both studies, core and symbiosis gene sequences indicated that there were diverse and novel strains amongst the isolates.
Data are available for bacterial isolates from nodules of other Caesalpinioideae species, but their ability to produce N2 fixing nodules on their legume host under axenic conditions was not tested. Specifically, three isolates from Tachigali versicolor sampled on Barro Colorado Island, Panama, which were not tested on their original host plant, but were shown to nodulate Macroptilium atropurpureum, were Bradyrhizobium [307]. Similarly, strain STM934, stated to be confirmed as Bradyrhizobium, was isolated from nodules of Erythrophleum guineensis growing in natural forests of the Ziama reservation in southeast Guinea and shown to produce functional nodules on Macroptilium atropurpureum [308]. In this case, a re-inoculation experiment was carried out on the original host, but the substrate was non-sterile forest soil. Bradyrhizobium was isolated from and shown to nodulate Chamaecrista sampled in Kakadu National Park, Northern Territory, Australia, but N2 fixation was not reported [309]. Furthermore, there are several reports that Bradyrhizobium inoculum can increase nodulation of Chamaecrista spp. under field conditions in Australia and China [310,311,312]. Thus, the available evidence indicates that Bradyrhizobium spp. are the dominant, possibly exclusive, rhizobial symbionts of legumes in the Caesalpinioideae, but data are limited, and the degree of specificity between legumes in the Caesalpinioideae and their rhizobial symbionts cannot be assessed without further work.
4. Mimosoideae-Rhizobia Symbioses
Rhizobia have been characterized from 15 species across seven genera in the tribe Ingeae and ca. 120 species from 13 genera in the tribe Mimoseae within the sub-family Mimosoideae (Table 1). Bradyrhizobium, Ensifer, Mesorhizobium and Rhizobium were each reported to nodulate species in the Ingeae and the Mimoseae. Furthermore, Ochrobactrum was reported to nodulate Acacia mangium (Ingeae); Allorhizobium and Devosia were reported to nodulate Neptunia natans (Mimoseae); and there are many reports that Cupriavidus and Burkholderia nodulate Mimosa spp. and related species (Mimoseae) (Table 1). In addition, excepting Acacia auriculiformis (Ingeae) and Mimosa pigra (Mimoseae), all species that were examined in three or more separate studies, Acacia mangium, Acacia saligna, Calliandra grandiflora and Senegalia senegal (Ingeae), Leucaena leucocephala, Mimosa diplotricha, Mimosa pudica, Parapiptadenia rigida, Prosopis alba and Vachellia tortilis (Mimoseae), were nodulated by at least three different rhizobial genera. Thus, a range of rhizobial genera, including both α- and β-proteobacteria, can nodulate legume species across the two Mimosoideae tribes, and generally, where tested over different studies, species within the Ingeae and Mimoseae tribes were promiscuous with respect to their rhizobial symbionts.
The Mimosa species examined across studies were the pan-tropical invasive Mimosa diplotricha and Mimosa pudica, and findings for these species appear not to reflect the situation with most Mimosa spp., which are endemic with a restricted range. The evidence indicates that most Mimosa spp. show specificity towards the rhizobial genus depending on their distribution with Burkholderia, Rhizobium/Ensifer and Cupriavidus, the main rhizobial symbiont of endemic Mimosa spp. in central and southern Brazil, central Mexico and southern Uruguay, respectively [73,74,79]. The 16S rRNA and housekeeping gene sequences were diverse for Mimosa Burkholderia symbionts in Brazil, Rhizobium/Ensifer in Mexico and Cupriavidus in Uruguay. For Burkholderia in Brazil and Rhizobium/Ensifer in Mexico, the symbiosis gene sequences were largely congruent with the 16S rRNA and housekeeping gene sequences, indicating that these genes diverged over a long period within Burkholderia without substantial horizontal gene transfer between species [73,74]. For Cupriavidus rhizobia in Uruguay, the nodA gene sequences were not congruent with the housekeeping gene sequences, but grouped together in a cluster [79]. This is strong evidence that the various Cupriavidus species obtained their symbiosis genes via within group lateral gene transfer [32,79,313]. It is not known if endemic Mimosa spp. nodulated by a particular rhizobia genus can form N2-fixing symbioses with Mimosa rhizobia of different genera from outside their region.
5. Papilionoideae-Rhizobia Symbioses
5.1. The IRLC
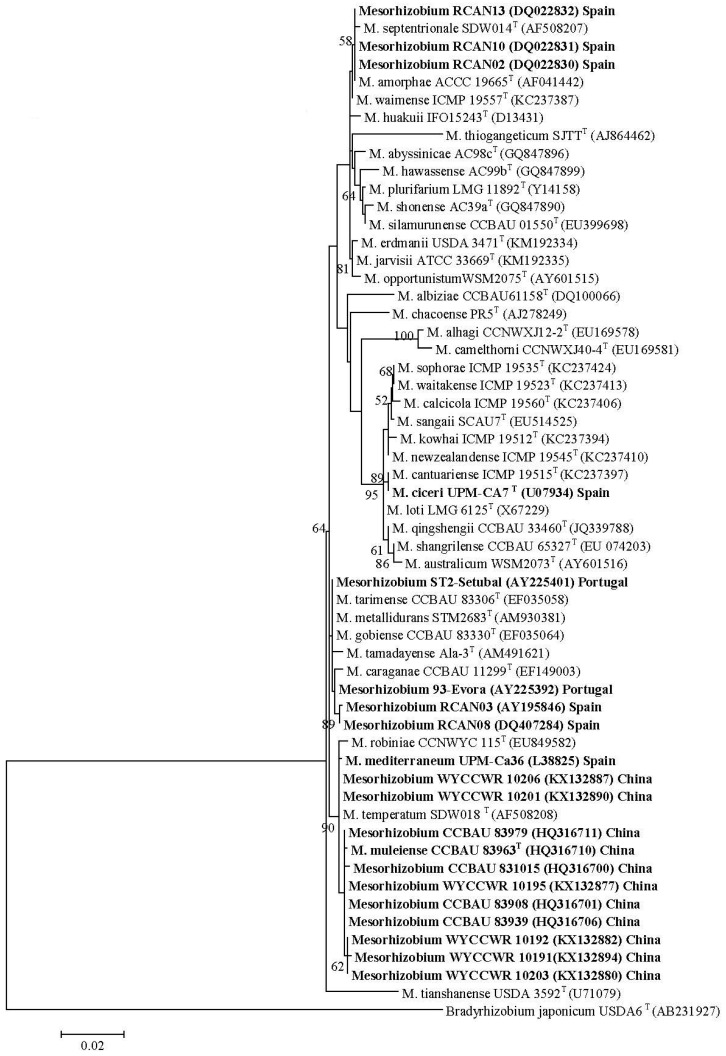
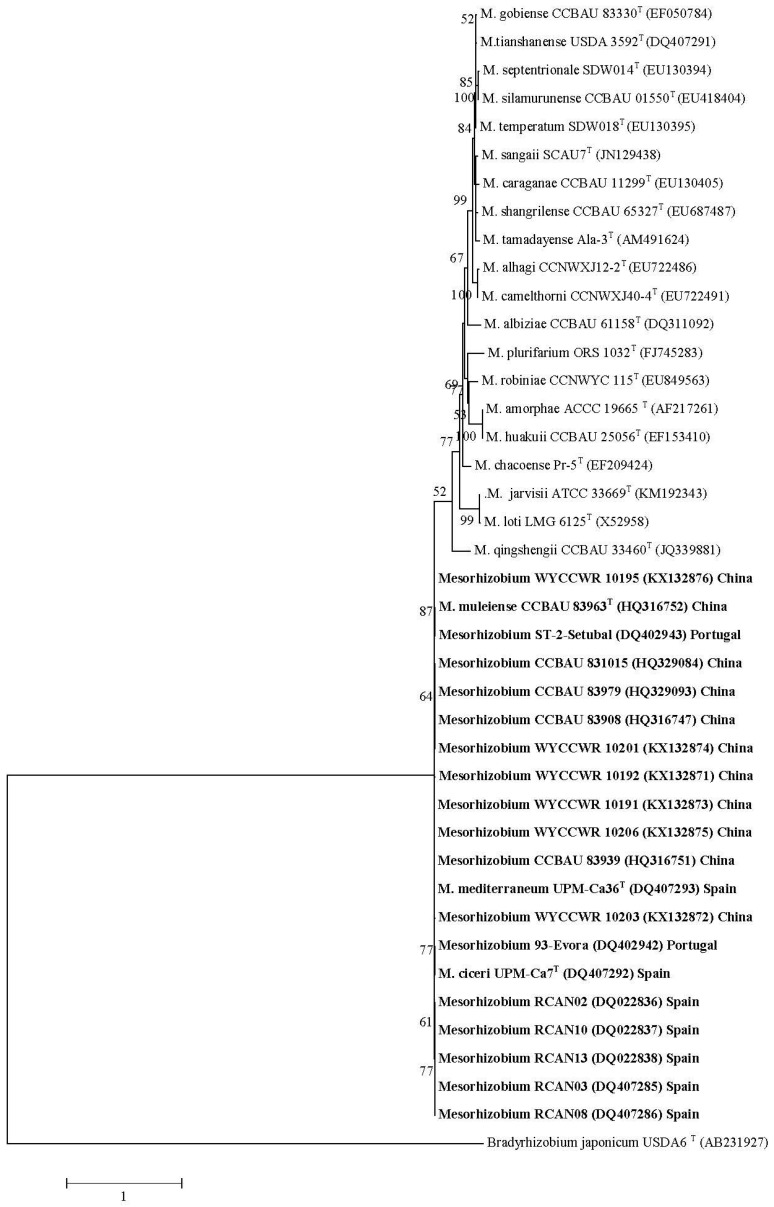
Data are available for 103 species from 27 genera/five tribes in the IRLC with Ensifer, Mesorhizobium and Rhizobium, commonly, and Bradyrhizobium, Neorhizobium and Phyllobacterium, rarely, reported to nodulate species within this clade (Table 2). There are no reports of Burkholderia or Cupriavidus symbionts within the IRLC. Previously, Galega officinalis, Galega orientalis and Hedysarum coronarium within the IRLC clade were reported to only form effective nodules with their respective symbionts Neorhizobium galegeae sv. officinalis, Neorhizobium galegeae sv. orientalis and Rhizobium sullae [120,145,296,297]. The data in Table 2 indicate two other specific relationships between IRLC legumes and rhizobia. Firstly, eight separate studies on Cicer arietinum carried out over different countries and continents reported Mesorhizobium as the only symbiont. The 16S rRNA and housekeeping gene sequences indicated that strains of Mesorhizobium ciceri and M. mediterraneum were common, but not exclusive Mesorhizobium symbionts of Cicer arietinum in most studies outside China, with M. muleiense the main symbiont in Northwest China [100]. Across studies where tested, nifH and nodC gene sequences were similar for all Mesorhizobium isolates shown to produce functional nodules on Cicer arietinum, indicating their specificity towards this legume species and that lateral transfer of these genes had occurred between the different Mesorhizobium spp. [97,99,100].
Secondly, for the tribe Fabeae, seventeen studies across five Lathyrus species, Lens culinaris, Pisum sativum and eleven Vicia spp. reported Rhizobium as the only symbiont. Across these studies, Rhizobium leguminosarum (and where tested, R. leguminosarum sv. viciae) was the most common symbiont with some varieties of Pisum sativum, such as cv. Afghanistan, only nodulated by specific strains of Rhizobium leguminosarum sv. viciae, which occur in soils in their native range in Afghanistan/Turkey [103]. Furthermore, in a study of 154 isolates of 18 Vicia species grown in 16 Chinese provinces, only 17 representative Rhizobium Leguminosarum sv. viciae isolates, from a wide range of potential rhizobia, produced fully-developed, effective (“colour red”) nodules [115]. Thus, a highly specific relationship has developed between species in the Fabeae and R. leguminosarum sv. viciae, but it is not an exclusive relationship, as R. fabeae [116], R. multihospitium [105], R. pisi [111], R. laguerreae [314] and R. anhuiense [108] have been reported to effectively nodulate Fabeae species. However, the nifH and nodC gene sequences of all of these rhizobia showed high similarity, indicating their specificity towards the Fabeae species and that, in this case, lateral gene transfer had occurred between different Rhizobium spp. [32,108].
Within the tribe Trifolieae, 14 out of 16 Medicago/Melilotus spp. had Ensifer as symbiont, but in four cases not exclusively. The most studied species, Medicago sativa (lucerne), was commonly nodulated by Ensifer meliloti, which is the recommended inoculum for this legume crop, but it also had Neorhizobium and Rhizobium symbionts. However, Medicago laciniata and Medicago rigiduloides were found to only nodulate with Ensifer strains sampled in their native range in the Mediterranean Basin. These strains were formally described as Ensifer meliloti sv. medicaginis and Ensifer meliloti sv. rigiduloides, respectively [148,153]. Similarly, different Trifolium spp. have different compatibility with different strains of Rhizobium leguminosarum sv trifolii, which is the recommended inoculum for most Trifolium crops. Trifolium ambiguum, in particular, has been highlighted as only forming N2-fixing nodules with strains of Rhizobium leguminosarum sv trifolii specific to its region of origin (the Caucasus and Eastern Europe) [315].
In relation to other members of the IRLC, Ensifer, Mesorhizobium and Rhizobium were shown to nodulate species within Astragalus, Colutea, Glycyrrhiza, Oxytropis and Sphaerophyceae (Galegeae), Hedysarum (Hedysareae) and Trifolium. Furthermore, Astragalus adsurgense, Astragalus complanatus, Colutea arborescens, Oxytropis glabra and Sphaerophysa salsula (Galegeae), Caragana intermedia (Hedysareae) and Trifolium fragiferum and Trifolium repens were all nodulated by three different rhizobial genera. Thus, for the IRLC, specificity for the rhizobial genus appears to hold at the tribe level for the Fabeae (Rhizobium spp.) and species level for Cicer arietinum (Mesorhizobium spp.). Specificity for rhizobial species or symbiovar holds for Galega officinalis (Neorhizobium galegeae sv. officinalis), Galega orientalis (Neorhizobium galegeae sv. orientalis), Hedysarum coronarium (Rhizobium sullae), Medicago laciniata (Ensifer meliloti sv. medicaginis), Medicago rigiduloides (Ensifer meliloti sv. rigiduloides) and Trifolium ambiguum (Rhizobium leguminosarum sv. trifolii), but it is not a characteristic of all members of the clade.
5.2. Clades with Indeterminate Nodules, Excluding the IRLC
Data are shown for 113 species from 33 genera across 13 Papilionoideae tribes with indeterminate nodules that do not show the IRLC mutation (Table 3). Azorhizobium, Bradyrhizobium, Burkholderia, Ensifer, Mesorhizobium, Methylobacterium, Microvirga, Neorhizobium, Ochrobactrum, Pararhizobium, Phyllobacterium and Rhizobium were all reported to nodulate species within this group. Amorpha fruticosa and Dalea purpurea (Amorpheae), Retama sphaerocarpa and Spartium junceum (Genisteae), Coronilla varia (Loteae), Tephrosia falciformis and Tephrosia villosa (Millettiea), Gliricidia sepium and Robinia pseudoacacia (Robineae) and Sesbania sericea and Sesbania virgata (Sesbanieae) were nodulated by two rhizobial genera. Aspalathus linearis and Crotalaria pallida (Crotalarieae), Tephrosia purpurea (Millettiea), Sesbania cannabina, Sesbania punicea, Sesbania rostrata and Sesbania sesban (Sesbanieae), Sophora alopecuroides and Sophora flavescens (Sophoreae) and Ammopiptanthus nanus and Ammopiptanthus mongolicus (Thermopsideae) were all nodulated by at least three different rhizobial genera. Thus, generally, where tested, Papilionoideae species with indeterminate nodules excluding the IRLC were promiscuous in relation to rhizobial symbiont. Within the Genisteae, Bradyrhizobium was the only symbiont reported for nine Cytisus spp. across ten separate studies and three Genista spp. across three separate studies (Table 3). Furthermore, eight out of 10 Lupinus spp. (Genisteae) across 11 separate studies were nodulated by Bradyrhizobium. These results indicate that Bradyrhizobium may be the main symbionts of Genisteae species, but further work is required to confirm this. Generally, 16S rRNA and housekeeping gene sequences indicate that diverse Bradyrhizobium spp. form N2-fixing nodules on Cytisus spp. and Lupinus spp., but the diversity of their symbiosis genes is dependent on the geographical origin of the legumes. For example, most Bradyrhizobium isolates from native Lupinus spp. in Europe form a distinct lineage, ‘clade 11’, on the basis of their nodA gene sequences [180,195]. Similarly, different Bradyrhizobium spp. associated with native Cytisus villosus in Morocco all showed similar nodC and nifH sequences, which were closely related to those of Bradyrhizobium japonicum sv. genistearum [175]. In contrast, rhizobia sampled from invasive Cytisus scoparius sampled in six states in the United States, differed with respect to housekeeping and symbiosis gene sequences [174]. Specifically, one group of isolates had both housekeeping and symbiosis gene sequences similar to a Bradyrhizobium clade from native legumes in Western North America, but two clades had nifD, nifH and nodC sequences highly similar or identical to a Cytisus scoparius strain isolated in Spain, while their housekeeping genes were similar to American Bradyrhizobium clades. Thus, it appears that Bradyrhizobium ancestrally associated with native North American legumes have acquired symbiosis genes from European Cytisus scoparius Bradyrhizobium symbionts via lateral gene transfer.
The two exceptions to Bradyrhizobium as the rhizobial symbiont of Lupinus spp. were Ochrobactrum [181] and Microvirga [166], both of which are rare as rhizobial symbionts of legumes. The nodD sequence for Ochrobactrum and the nodA sequence for Microvirga indicated that both bacteria obtained their nod genes via horizontal gene transfer from more common rhizobial genera. Microvirga is also the only bacterial genus shown to form N2-fixing nodules on Listia angolensis [17]. Similarly, Listia bainesii has been found to only produce N2-fixing nodules with pink pigmented Methylobacterium [17]. It was suggested that the seasonally waterlogged habitat of Listia spp. may have resulted in the selection of unusual rhizobial symbionts adapted to their environments [17].
In one study in the Cape Floristic Region (CFR) of South Africa, Burkholderia was reported to be the exclusive symbiont of ten Cyclopia spp., Podalyria calyptera and Virgilia oroboides, all species in the Podalyrieae plus three Hypocalyptus spp. (Hypocalypteae) [191]. Burkholderia was confirmed to nodulate Podolyria calyptrata and Virgilia oroboides in the CFR [136,193,197]. The majority of Burkholderia isolates had unique nifH and nodA gene sequences, and the specificity of these symbioses needs testing.
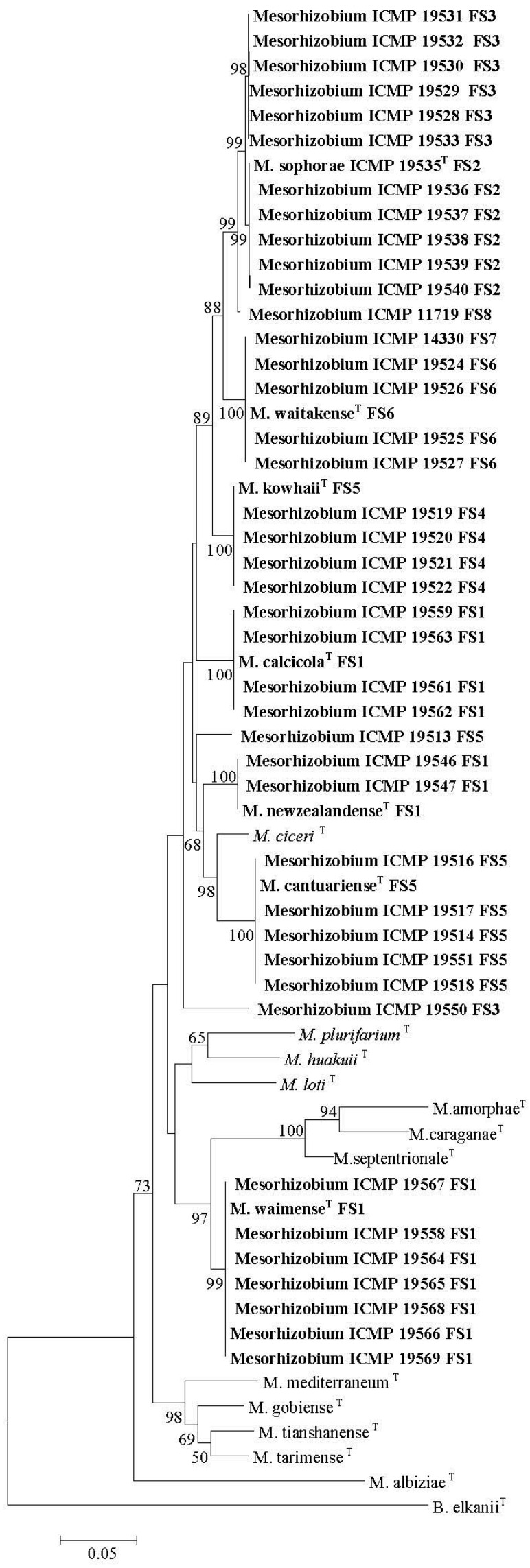
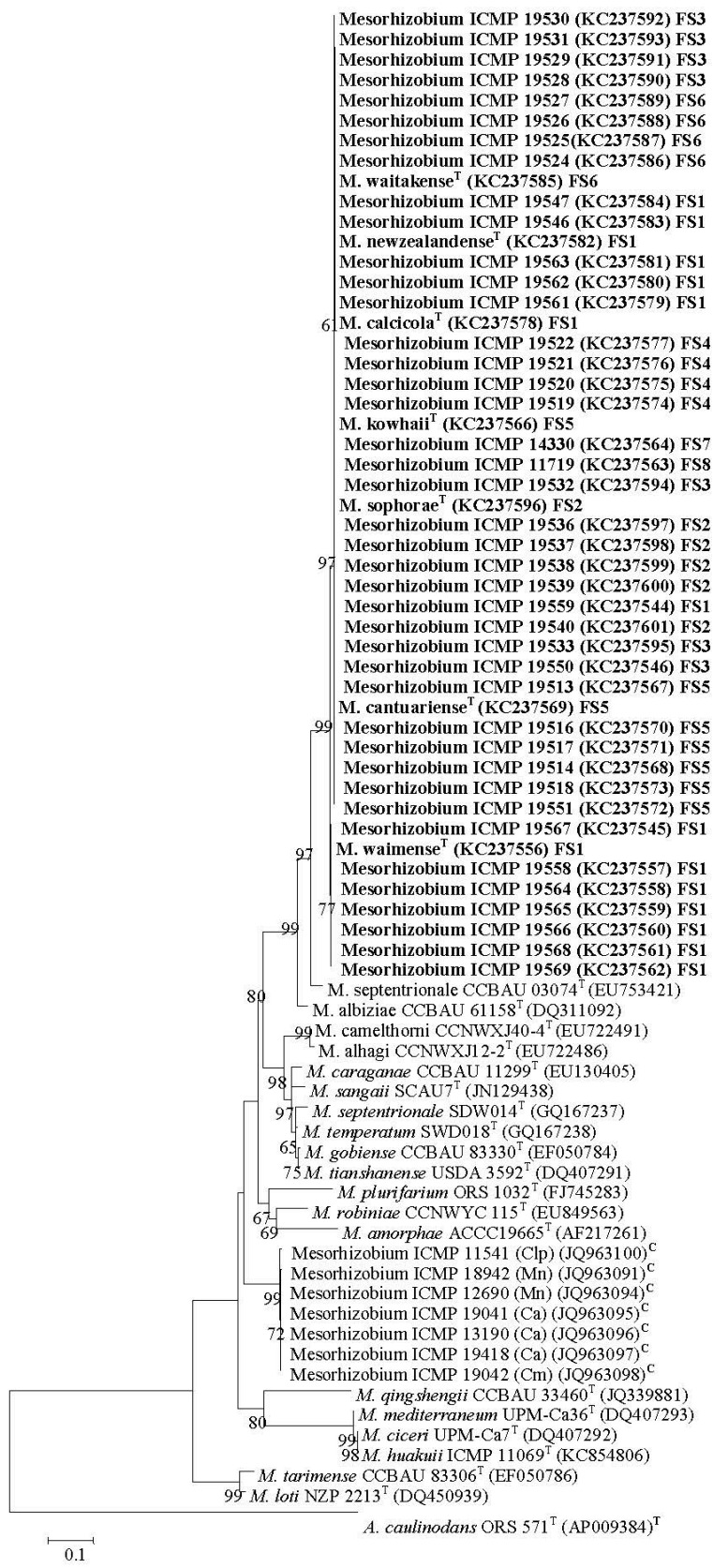
Previously, Sesbania sesban was reported to be highly promiscuous with respect to rhizobial symbionts [31], and the data here indicate that this could be a genus level trait (Table 3). However, the reports that Sophora alopecuroides and Sophora flavescens sampled in China are nodulated by Ensifer, Mesorhizobium, Phyllobacterium and Rhizobium with a wide range of symbiosis gene sequences [207,208] contrasts with the finding that New Zealand (NZ) native Sophora spp. were exclusively nodulated by Mesorhizobium spp. with almost identical unique nodA and nodC gene sequences [210,316,317]. This emphasises that species within the same genus can vary greatly with respect to their specificity for rhizobial symbionts.
5.3. Clades with Determinate Nodules
The Dalbergieae are almost exclusively of tropical/sub-tropical distribution and show an aeschynomenoid determinate nodule structure [2]. Rhizobia have been characterised for 23 species from seven genera in the Dalbergieae, Adesmia, Aeschynomene, Arachis, Centrolobium, Dalbergia, Pterocarpus and Zornia (Table 4). Bradyrhizobium was found to nodulate all species, except Adesmia bicolor (Rhizobium), with Rhizobium also reported for Arachis hypogaea in two studies. Thus, on the data available, the Dalbergieae appear to be primarily nodulated by Bradyrhizobium. For Arachis hypogaea, twelve separate studies reported Bradyrhizobium as a rhizobial symbiont (Table 4). Across these studies, both core and symbiosis gene sequences indicated that Arachis hypogaea was nodulated by a diverse range of Bradyrhizobium spp. and are promiscuous with respect to Bradyrhizobium spp. Excepting Arachis hypogaea, data are limited for rhizobial symbionts of species in the Dalbergieae. However, the unusual ability of specific Bradyrhizobium strains that lack canonical nodABC genes to form N2-fixing nodules on roots and/or stems of particular Aeschynomene spp. is highlighted [34,215].
The closely related tribes Desmodieae, Phaseoleae and Psoraleae are also mainly of tropical/sub-tropical distribution, and with rare exceptions, species within these tribes showed a desmodioid nodule structure [2,15]. Rhizobia have been characterized for 25 species from three genera, Desmodium, Kummerowia and Lespedeza, in the Desmodieae (Table 4). Species from all three genera, Desmodium microphyllum, Desmodium racemosum, Desmodium sequax, Kummerowia striata, Lespedeza bicolor and Lespedeza daurica, were nodulated by rhizobia from three separate genera. Similarly, for 28 species across 14 genera within the Phaseoleae, there was no strong evidence for high specificity for rhizobial symbiont (Table 4). Phaseolus vulgaris and Vigna unguiculata have been highlighted as being promiscuous with respect to their rhizobial symbionts under field conditions. Data in Table 4 show that both species can be nodulated by different rhizobial genera in the α-proteobacteria, as well as Burkholderia in the β-proteobacteria. Across three studies, Phaseolus lunatus was reported to be nodulated by Bradyrhizobium and Rhizobium, while Vigna angularis, Vigna radiata and Vigna subterranea were reported to be nodulated by three separate rhizobial genera. Data are limited for other genera/species within the Phaseoleae with the exception of Glycine max, which is the main grain/oil seed legume grown worldwide, and Glycine soja. Both Glycine spp. were nodulated by Bradyrhizobium, Ensifer and Rhizobium. In the one case where separate studies were carried out on one species within the Psoraleae, Psoralea pinnata was nodulated by Bradyrhizobium, Burkholderia and Mesorhizobium [136,193,287]. Thus, where tested, species within the Desmodieae, Phaseoleae and Psoraleae were promiscuous with respect to their rhizobial symbionts.
Species in the Loteae, which show a desmodoid nodule structure, are of temperate distribution [2]. Data are available for 16 Lotus spp. within the Loteae across 13 separate studies. For all species examined in two or more studies, at least two rhizobia genera were reported as symbionts. Overall, the available data indicate that legume species with a desmodioid determinate nodule structure are promiscuous with respect to their rhizobia symbionts.
6. Legume Specificity for Rhizobial Symbionts
The objectives of the work were to collate data on legume rhizobia symbioses and assess the extent that legume specificity for rhizobial symbiont is related to legume taxonomy. Bradyrhizobium spp. were the exclusive rhizobial symbionts of species in the Caesalpinioideae; but, rhizobia were characterised for only three legume species over two studies, and the degree of specificity between legumes in the Caesalpinioideae and their rhizobial symbionts cannot be assessed without further work. Generally, species within the two Mimosoideae tribes, Ingeae and Mimoseae were promiscuous with respect to their rhizobial symbionts, but Mimosa spp. show specificity towards the rhizobia genus depending on their distribution, with Burkholderia, Rhizobium/Ensifer and Cupriavidus the main rhizobial symbiont of endemic Mimosa spp. in central and southern Brazil, central Mexico and southern Uruguay, respectively [73,74,79]. Papilionoideae species with indeterminate nodules were split into the IRLC and all other clades. A range of species within both groups nodulated with different rhizobia genera, but there was also strong evidence that some species within both groups showed specificity for rhizobial genus or species/symbiovar. Specificity for rhizobial genus appears to hold at the tribe level for the Fabeae (Rhizobium), the genus level for Cytisus (Bradyrhizobium), Lupinus (Bradyrhizobium) and NZ native Sophora spp. (Mesorhizobium) and the species level for Cicer arietinum (Mesorhizobium), Listia bainesii (Methylobacterium) and Listia angolensis (Microvirga). Specificity for rhizobial species/symbiovar appears to hold for Galega officinalis (Neorhizobium galegeae sv. officinalis), Galega orientalis (Neorhizobium galegeae sv. orientalis), Hedysarum coronarium (Rhizobium sullae), Medicago laciniata (Ensifer meliloti sv. medicaginis), Medicago rigiduloides (Ensifer meliloti sv. rigiduloides) and Trifolium ambiguum (Rhizobium leguminosarum sv. trifolii). For Papilionoideae with determinate nodules, the Dalbergieae (aeschynomenoid nodules) were primarily nodulated by Bradyrhizobium, while those in the Desmodieae, Phaseoleae, Psoraleae and Loteae (desmodioid nodules) were promiscuous with respect to rhizobial genus. Thus, on the data available, species in the Papilionoideae that show specificity for rhizobial genus, species or symbiovar have indeterminate nodules and are generally (but not exclusively) of temperate distribution. However, many temperate legumes with indeterminate nodules are promiscuous with respect to the rhizobial genus indicating that high specificity for rhizobial symbiont only occurs under specific conditions [318].
For Mimosa spp., specificity towards rhizobial genus depending on distribution is likely to be at least in part related to the relative occurrence of the potential symbionts in soils of the different regions [74,79]. For example, evidence indicates that Mimosa Cupriavidus symbionts are absent from soils in central and southern Brazil, while Mimosa Burkholderia symbionts are absent from soils in southern Uruguay. This has been related to soil characteristics with low pH favouring Burkholderia over Cupriavidus in Brazil, but high heavy metal content favouring Cupriavidus over Burkholderia in Uruguay [73,74,79]. It was proposed that native Mimosa in the different regions have selected symbiotic bacteria adapted to local conditions, which resulted in the development of highly specific associations [74,79]. However, for this to occur, such rhizobia must be available in the soil. The within genus 16S rRNA and housekeeping gene sequences were diverse for Mimosa symbionts in all regions. For Burkholderia in Brazil and Rhizobium/Ensifer in Mexico, the symbiosis gene sequences were largely congruent with the 16S rRNA and housekeeping gene sequences [73,74]. For Cupriavidus rhizobia in Uruguay, the nodA gene sequences were not congruent with the housekeeping gene sequences, but grouped together in a cluster, indicating that the various species within the group obtained their symbiosis genes via within group lateral gene transfer [79]. Lateral gene transfer is a mechanism whereby rhizobia and non-rhizobial bacteria adapted to local soil conditions could become specific rhizobial symbionts of legumes growing in these soils. The evidence described above indicates that lateral gene transfer of symbiosis genes has been important within the Papilionoideae in relation to the development of specific relationships between the Fabeae and Rhizobium spp., Cytisus and Bradyrhizobium spp., Lupinus and Bradyrhizobium spp., NZ native Sophora and Mesorhizobium spp. and Cicer arietinum and Mesorhizobium spp. Data are presented for Mesorhizobium isolates from Cicer arietinum and NZ native Sophora spp. to emphasise this point.
Firstly, 20 Mesorhizobium isolates from Cicer arietinum, sampled across three countries in five separate studies, showed diverse 16S rRNA sequences, but highly similar nodC sequences (Figure 1A,B). Here, evidence is strong that native Mesorhizobium muleiense adapted to alkaline soils in Gansu and Xinjiang Provinces of China obtained its Cicer arietinum-specific symbiotic genes from Mesorhizobium ciceri or Mesorhizobium mediterraneum introduced together with Cicer arietinum used as a crop [100]. Secondly, 48 isolates from four NZ native Sophora spp. sampled at eight different field sites separated into eight groups and three individual isolates on the basis of their concatenated recA, gln11 and rpoβ gene sequences, but showed almost identical nodC (and nodA [210]) sequences (Figure 2A,B). Seven of the groups have been formally identified as new species [316,317]. This relationship between NZ native Sophora spp. and Mesorhizobium spp. with specific symbiosis gene sequences is highly specific as none of twenty rhizobial isolates from common weed and crop legumes in NZ produced functional nodules on the NZ native Sophora microphylla [190]. Furthermore, Mesorhizobium isolates from Carmichaelia, Clianthus and Montigena, the only other NZ native legume genera, did not nodulate NZ native Sophora microphylla or Sophora tetraptera [127]. Mesorhizobium isolates from Carmichaelia, Clianthus and Montigena had unique nodC sequences different from those of Sophora Mesorhizobium isolates (Figure 2B). However, in some cases, their 16S rRNA, recA and gln11 sequences were similar to those of Sophora Mesorhizobium isolates, emphasizing the importance of the specific symbiosis genes in the NZ Sophora Mesorhizobium symbiosis [137]. Generally, Sophora isolates from the same field site grouped together on concatenated recA, gln11 and rpoβ gene sequences (Figure 2B). This apparent link between housekeeping gene sequences and field site is compatible with the proposal that lateral transfer of symbiosis genes to Mesorhizobium strains adapted to local soil conditions has occurred.
Figure 1.
16S rRNA gene maximum likelihood (ML) tree (ca. 1360 bp) (A) and nodC gene ML tree (ca. 630 bp) (B) of rhizobial strains isolated from Cicer arietinum in Spain, Portugal and China (bold) and selected Mesorhizobium type strains [97,99,100]. Numbers on branches are bootstrap % from 1000 replicates (shown only when ≥50%).
Figure 2.
Concatenated recA, gln11 and rpoβ gene maximum likelihood (ML) tree (ca. 1800 bp) (A) and nodC gene ML tree (ca. 650 bp) (B) of rhizobial strains isolated from New Zealand (NZ) native Sophora spp. (bold) and selected Mesorhizobium type strains. The nodC sequences of isolates from NZ native legumes Clianthus puniceus (Clp), Montigena novozealandiae (Mn), Carmichaelia australis (Ca) and Carmichaelia monroi (Cm) tested on NZ Sophora spp. are shown. Numbers on branches are bootstrap % from 500 replicates (shown only when ≥50%). FS = field site. Modified from Tan et al., 2015 [210].
Some varieties of Pisum sativum, such as cv. Afghanistan, are only nodulated by specific strains of Rhizobium leguminosarum sv. viciae, which occur in soils in their native range in Afghanistan/Turkey [103]. The ability of these strains to nodulate Pisum sativum cv. Afghanistan is controlled by a single recessive gene, sym2 in the plant. The sym2 allele interacts with a specific gene, nodX, present in R. leguminosarum sv. viciae strains able to nodulate cv. Afghanistan [319]. The nodX gene product acetylates a Nod factor, which mediates a specific compatible interaction with this cultivar [320,321]. Furthermore, Medicago laciniata and Medicago rigiduloides were found to only nodulate with Ensifer meliloti strains (E. meliloti sv. medicaginis and E. meliloti sv. rigiduloides, respectively) sampled in their native range in the Mediterranean Basin [148,153], and Trifolium ambiguum only forms N2-fixing nodules with strains of Rhizobium leguminosarum sv. trifolii specific to its region of origin, the Caucasus and Eastern Europe [315]. The mechanisms of these highly specific relationships are not fully understood, but the nodA gene sequences of E. meliloti sv. medicaginis and nodA, nodB and nodC gene sequences of E. meliloti sv. rigiduloides diverged from those of E. meliloti strains, which nodulated other Medicago spp. [148,153], while the ability of certain strains of Rhizobium leguminosarum sv. trifolii to effectively nodulate Trifolium ambiguum, but not Trifolium repens (white clover) appears to be linked to a 111-bp insertion in their nifH/fixA intergenic region [315]. The factors that have resulted in these highly specific relationships are not known. Furthermore, it is not known if these highly specific relationships reflect adaptation of the symbioses and result in greater rates of N2 fixation or more efficient N2 fixation, as would be expected on theoretical grounds [322], and this warrants further study.
7. Conclusions
Overall, the data indicate that lateral gene transfer of specific symbiosis genes within rhizobial genera is an important mechanism allowing legumes to form symbioses with rhizobia adapted to particular soils. It also maintains specificity between legume species and rhizobia species with specific symbiosis genes. Strain-specific legume rhizobia symbioses can develop in particular habitats.
Acknowledgments
Lincoln University, New Zealand provided funds to cover the costs to publish in open access.
Author Contributions
Mitchell Andrews wrote the paper; Morag E. Andrews was responsible for the collation and presentation of data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lewis G.A., Schrire B.B., Mackinder B.C., Lock M.D. Legumes of the World. Kew; Royal Botanic Gardens, London, UK: 2005. [Google Scholar]
- 2.Sprent J.I. Legume Nodulation A Global Perspective. Wiley Blackwell; Chichester, UK: 2009. [Google Scholar]
- 3.LPWG Legume phylogeny and classification in the 21st century: Progress, prospects and lessons for other species-rich clades. Taxon. 2013;62:217–248. [Google Scholar]
- 4.Cardoso D., Pennington R.T., de Queiroz L.P., Boatwright J.S., van Wyk B.-E., Wojciechowski M.F., Lavin M. Reconstructing the deep-branching relationships of the papilionoid legumes. S. Afr. J. Bot. 2013;89:58–75. doi: 10.1016/j.sajb.2013.05.001. [DOI] [Google Scholar]
- 5.LPWG A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon. 2017;66:44–77. [Google Scholar]
- 6.Sprent J.I., Ardley J., James E.K. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytol. 2017 doi: 10.1111/nph.14474. [DOI] [PubMed] [Google Scholar]
- 7.Raven J.A. Why are mycorrhizal fungi and symbiotic nitrogen-fixing bacteria not genetically integrated into plants? Ann. Appl. Biol. 2010;157:381–391. doi: 10.1111/j.1744-7348.2010.00435.x. [DOI] [Google Scholar]
- 8.Andrews M., Raven J.A., Lea P.J. Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 2013;163:174–199. doi: 10.1111/aab.12045. [DOI] [Google Scholar]
- 9.Andrews M., Scholefield D., Abberton M.T., McKenzie B.A., Hodge S., Raven J.A. Use of white clover as an alternative to nitrogen fertilizer for dairy pastures in nitrate vulnerable zones in the UK: Productivity, environmental impact and economic considerations. Ann. Appl. Biol. 2007;151:11–23. doi: 10.1111/j.1744-7348.2007.00137.x. [DOI] [Google Scholar]
- 10.Andrews M., James E.K., Sprent J.I., Boddey R.M., Gross E., dos Reis F.B., Jr. Nitrogen fixation in legumes and actinorhizal plants in natural ecosystems: Values obtained using 15N natural abundance. Plant Ecol. Divers. 2011;4:131–140. doi: 10.1080/17550874.2011.644343. [DOI] [Google Scholar]
- 11.Jackson L.E., Burger M., Cavagnaro T.R. Roots, nitrogen transformations, and ecosystem services. Ann. Rev. Plant Biol. 2008;59:341–363. doi: 10.1146/annurev.arplant.59.032607.092932. [DOI] [PubMed] [Google Scholar]
- 12.Vitousek P.M., Menge D.N.L., Reed S.C., Cleveland C.C. Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philos. Trans. R. Soc. B. 2013;368:20130119. doi: 10.1098/rstb.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprent J.I., Ardley J.K., James E.K. From north to south: A latitudinal look at legume nodulation processes. S. Afr. J. Bot. 2013;89:31–41. doi: 10.1016/j.sajb.2013.06.011. [DOI] [Google Scholar]
- 14.Fernández-López M., Goormachtig S., Gao M., D’Haeze W., van Montagu M., Holsters M. Ethylene-mediated phenotypic plasticity in root nodule development on Sesbania rostrata. Proc. Natl. Acad. Sci. USA. 1998;95:12724–12728. doi: 10.1073/pnas.95.21.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W.Y.Y., Ridgway H.J., James T.K., James E.K., Chen W.-M., Sprent J.I., Young J.P.W., Andrews M. Burkholderia sp. induces functional nodules on the South African invasive legume Dipogon lignosus (Phaseoleae) in New Zealand soils. Microb. Ecol. 2014;68:542–555. doi: 10.1007/s00248-014-0427-0. [DOI] [PubMed] [Google Scholar]
- 16.Yates R.J., Howieson J.G., Reeve W.G., Nandasena K.G., Law I.J., Bräu L., Ardley J.K., Nistelberger H.M., Real D., O’Hara G.W. Lotononis angolensis forms nitrogen fixing, lupinoid nodules with phylogenetically unique, fast growing, pink-pigmented bacteria, which do not nodulate L. bainesii or L. listii. Soil Biol. Biochem. 2007;39:1680–1688. doi: 10.1016/j.soilbio.2007.01.025. [DOI] [Google Scholar]
- 17.Ardley J.K., Reeve W.G., O’Hara G.W., Yates R.J., Dilworth M.J., Howieson J.G. Nodule morphology, symbiotic specificity and association with unusual rhizobia are distinguishing features of the genus Listia within the southern African crotalarioid clade Lotononis s.l. Ann. Bot. 2013;112:1–15. doi: 10.1093/aob/mct095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Yang S., Tang F., Zhu H. Symbiosis specificity in the legume-rhizobial mutualism. Cell Microbiol. 2012;14:334–342. doi: 10.1111/j.1462-5822.2011.01736.x. [DOI] [PubMed] [Google Scholar]
- 19.Downie J.A. Legume nodulation. Curr. Biol. 2014;24:R184–R190. doi: 10.1016/j.cub.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Oldroyd G.E., Downie J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Ann. Rev. Plant Biol. 2008;59:519–546. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- 21.Czernic P., Gully D., Cartieaux F., Moulin L., Guefrachi I., Patrel D., Pierre O., Fardoux J., Chaintreuil C., Nguyen P., et al. Convergent evolution of endosymbiont differentiation in dalbergioid and inverted repeat-lacking clade legumes mediated by nodule specific cysteine rich peptides. Plant Physiol. 2015;169:1254–1265. doi: 10.1104/pp.15.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okubo T., Fukushima S., Minamisawa K. Evolution of Bradyrhizobium–Aeschynomene mutualism: Living testimony of the ancient world or highly evolved state? Plant Cell Physiol. 2012;53:2000–2007. doi: 10.1093/pcp/pcs150. [DOI] [PubMed] [Google Scholar]
- 23.Bianco L. Rhizobial infection in Adesmia bicolor (Fabaceae) roots. Arch. Microbiol. 2014;196:675–679. doi: 10.1007/s00203-014-1004-0. [DOI] [PubMed] [Google Scholar]
- 24.González-Sama A., Lucas M.M., de Felipe M.R., Pueyo J.J. An unusual infection mechanism and nodule morphogenesis in white lupin (Lupinus albus) New Phytol. 2004;163:371–380. doi: 10.1111/j.1469-8137.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- 25.Martens M., Delaere M., Coopman R., de Vos P., Gillis M., Willems A. Multilocus sequence analysis of Ensifer and related taxa. Int. J. Syst. Evol. Microbiol. 2007;57:489–503. doi: 10.1099/ijs.0.64344-0. [DOI] [PubMed] [Google Scholar]
- 26.Peix A., Ramírez-Bahena M.H., Velázquez E., Bedmar E.J. Bacterial associations with legumes. Crit. Rev. Plant Sci. 2015;34:17–42. doi: 10.1080/07352689.2014.897899. [DOI] [Google Scholar]
- 27.Lindström K., Aserse A.A., Mousavi S.A. Evolution and Taxonomy of Nitrogen-Fixing Organisms with Emphasis on Rhizobia. In: de Bruijn F.J., editor. Biological Nitrogen Fixation. 1st ed. Wiley & Sons, Inc.; Hoboken, NJ, USA: 2015. pp. 21–37. [Google Scholar]
- 28.Roche P., Maillet F., Plazanet C., Debellé F., Ferro M., Truchet G., Promé J.-C., Dénarié J. The common nodABC genes of Rhizobium meliloti are host-range determinants. Proc. Natl. Acad. Sci. USA. 1996;93:15305–15310. doi: 10.1073/pnas.93.26.15305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masson-Boivin C., Giraud E., Perret X., Batut J. Establishing nitrogen-fixing symbiosis with legumes: How many rhizobium recipes? Trends Microbiol. 2009;17:10. doi: 10.1016/j.tim.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Vinuesa P., Silva C., Lorite M.J., Izaguirre-Mayoral M.L., Bedmar E.J., Martínez-Romero E. Molecular systematics of rhizobia based on maximum likelihood and Bayesian phylogenies inferred from rrs, atpD, recA and nifH sequences, and their use in the classification of Sesbania microsymbionts from Venezuelan wetlands. Syst. Appl. Microbiol. 2005;28:702–716. doi: 10.1016/j.syapm.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Cummings S.P., Gyaneshwar P., Vinuesa P., Farruggia F.T., Andrews M., Humphry D., Elliott G.N., Nelson A., Orr C., Pettitt D., et al. Nodulation of Sesbania species by Rhizobium (Agrobacterium) strain IRBG74 and other rhizobia. Environ. Microbiol. 2009;11:2510–2525. doi: 10.1111/j.1462-2920.2009.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remigi P., Zhu J., Young J.P.W., Masson-Boivin C. Symbiosis within symbiosis: Evolving nitrogen-fixing legume symbionts. Trends Microbiol. 2016;24:63–75. doi: 10.1016/j.tim.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Giraud E., Moulin L., Vallenet D., Barbe V., Cytryn E., Avarre J.C., Jaubert M., Simon D., Cartieaux F., Prin Y., et al. Legumes symbioses: Absence of Nod genes in photosynthetic bradyrhizobia. Science. 2007;316:1307–1312. doi: 10.1126/science.1139548. [DOI] [PubMed] [Google Scholar]
- 34.Miché L., Moulin L., Chaintreuil C., Contreras-Jimenez J.L., Munive-Hernández J.-A., del Villegas-Hernandez M.C., Crozier F., Béna G. Diversity analyses of Aeschynomene symbionts in tropical Africa and central America reveal that nod-independent stem nodulation is not restricted to photosynthetic bradyrhizobia. Symbiosis. 2010;12:2152–2164. doi: 10.1111/j.1462-2920.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 35.Benata H., Mohammed O., Noureddine B., Abdelbasset B., Abdelmoumen H., Muresu R., Squartini A., El Idrissi M.M. Diversity of bacteria that nodulate Prosopis juliflora in the eastern area of Morocco. Syst. Appl. Microbiol. 2008;31:378–386. doi: 10.1016/j.syapm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Hassen A.I., Bopape F.L., Habig J., Lamprecht S.C. Nodulation of rooibos (Aspalathus linearis Burm. f.), an indigenous South African legume, by members of both the α-proteobacteria and β-proteobacteria. Biol. Fertil. Soils. 2012;48:295–303. doi: 10.1007/s00374-011-0628-3. [DOI] [Google Scholar]
- 37.Shiraishi A., Matsushita N., Hougetsu T. Nodulation in black locust by the γ-proteobacteria Pseudomonas sp. and the β-proteobacteria Burkholderia sp. Syst. Appl. Microbiol. 2010;33:269–274. doi: 10.1016/j.syapm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Huang B., Lv C., Zhao Y., Huang R. A novel strain D5 isolated from Acacia confusa. PLoS ONE. 2012;7:e49236. doi: 10.1371/journal.pone.0049236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ampomah O.Y., Huss-Danell K. Genetic diversity of root nodule bacteria nodulating Lotus corniculatus and Anthyllis vulneraria in Sweden. Syst. Appl. Microbiol. 2011;34:267–275. doi: 10.1016/j.syapm.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Manassila M., Nuntagij A., Kotepong S., Boonkerd N., Teaumroong N. Characterization and monitoring of selected rhizobial strains isolated from tree legumes in Thailand. Afr. J. Biotechnol. 2007;6:1393–1402. [Google Scholar]
- 41.Le Roux C., Tentchev D., Prin Y., Goh D., Japarudin Y., Perrineau M.-M., Duponnois R., Domergue O., de Lajudie P., Galiana A. Bradyrhizobia nodulating the Acacia mangium × A. auriculiformis interspecific hybrid are specific and differ from those associated with both parental species. Appl. Environ. Microbiol. 2009;75:7752–7759. doi: 10.1128/AEM.01887-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helene L.C.F., Delamuta J.R.M., Ribeiro R.A., Ormeño-Orrillo E., Rogel M.A., Martínez-Romero E., Hungria M. Bradyrhizobium viridifuturi sp. nov., encompassing nitrogen-fixing symbionts of legumes used for green manure and environmental services. Int. J. Syst. Evol. Microbiol. 2015;65:4441–4448. doi: 10.1099/ijsem.0.000591. [DOI] [PubMed] [Google Scholar]
- 43.Sinsuwongwat S., Nuntagij A., Shutsrirung A., Nomura M., Tajima S. Characterization of local rhizobia in Thailand and distribution of malic enzymes. Soil Sci. Plant Nutr. 2002;48:719–727. doi: 10.1080/00380768.2002.10409262. [DOI] [Google Scholar]
- 44.Ngom A., Nakagawa Y., Sawada H., Tsukahara J., Wakabayashi S., Uchiumi T., Nuntagij A., Kotepong S., Suzuki A., Higashi S., et al. A novel symbiotic nitrogen-fixing member of the Ochrobactrum clade isolated from root nodules of Acacia mangium. J. Gen. Appl. Microbiol. 2004;50:17–27. doi: 10.2323/jgam.50.17. [DOI] [PubMed] [Google Scholar]
- 45.Lortet G., Méar N., Lorquin J., Dreyfus B., de Lajudie P., Rosenberg C., Boivin C. Nod factor thin-layer chromatography profiling as a tool to characterize symbiotic specificity of rhizobial strains: Application to Sinorhizobium saheli, S. teranga, and Rhizobium sp. strains isolated from Acacia and Sesbania. Mol. Plant Microbe Interact. 1996;9:736–747. doi: 10.1094/MPMI-9-0736. [DOI] [Google Scholar]
- 46.Räsänen L.A., Sprent J.I., Lindström K. Symbiotic properties of sinorhizobia isolated from Acacia and Prosopis nodules in Sudan and Senegal. Plant Soil. 2001;235:193–210. doi: 10.1023/A:1011901706936. [DOI] [Google Scholar]
- 47.Lu J.K., Dou Y.J., Zhu Y.J., Wang S.K., Sui X.H., Kang L.H. Bradyrhizobium ganzhouense sp. nov., an effective symbiotic bacterium isolated from Acacia melanoxylon R. Br. nodules. Int. J. Syst. Evol. Microbiol. 2014;64:1900–1905. doi: 10.1099/ijs.0.056564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marsudi N.D.S., Glenn A.R., Dilworth M.J. Identification and characterization of fast- and slow-growing root nodule bacteria from South-Western Australian soils able to nodulate Acacia saligna. Soil Biol. Biochem. 1999;31:1229–1238. doi: 10.1016/S0038-0717(99)00032-2. [DOI] [Google Scholar]
- 49.Khbaya B., Neyra M., Normand P., Zerhari K., Filali-Maltouf A. Genetic diversity and phylogeny of rhizobia that nodulate Acacia spp. in Morocco assessed by analysis of rRNA genes. Appl. Environ. Microbiol. 1998;64:4912–4917. doi: 10.1128/aem.64.12.4912-4917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lloret L., Ormeño-Orillo E., Rincón R., Martinez-Romero J., Rogel-Hernández M.A., Martinez-Romero E. Ensifer mexicanus sp. nov. a new species nodulating Acacia angustissima (Mill.) Kuntze in Mexico. Syst. Appl. Microbiol. 2007;30:280–290. doi: 10.1016/j.syapm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Rincón-Rosales R., Lloret L., Ponce E., Martínez-Romero E. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico, including Sinorhizobium chiapanecum sp. nov. which has common symbiotic genes with Sinorhizobium mexicanum. FEMS Microbiol. Ecol. 2009;67:103–117. doi: 10.1111/j.1574-6941.2008.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bala A., Giller K.E. Symbiotic specificity of tropical tree rhizobia for host legumes. New Phytol. 2001;149:495–507. doi: 10.1046/j.1469-8137.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- 53.Rincón-Rosales R., Villalobos-Escobedo J.M., Rogel M.A., Martinez J., Ormeño-Orrillo E., Martínez-Romero E. Rhizobium calliandrae sp. nov., Rhizobium mayense sp. nov. and Rhizobium jaguaris sp. nov., rhizobial species nodulating the medicinal legume Calliandra grandiflora. Int. J. Syst. Evol. Microbiol. 2013;63:3423–3429. doi: 10.1099/ijs.0.048249-0. [DOI] [PubMed] [Google Scholar]
- 54.Odee D.W., Haukka K., McInroy S.G., Sprent J.I., Sutherland J.M., Young J.P.W. Genetic and symbiotic characterization of rhizobia isolated from tree and herbaceous legumes grown in soils from ecologically diverse sites in Kenya. Soil Biol. Biochem. 2002;34:801–811. doi: 10.1016/S0038-0717(02)00009-3. [DOI] [Google Scholar]
- 55.Leblanc H.A., McGraw R.L., Nygren P., Le Roux C. Neotropical legume tree Inga edulis forms N2-fixing symbiosis with fast-growing Bradyrhizobium strains. Plant Soil. 2005;275:123–133. doi: 10.1007/s11104-005-0808-8. [DOI] [Google Scholar]
- 56.Da Silva K., de Meyer S.E., Rouws L.F.M., Farias E.N.C., dos Santos M.A.O., O’Hara G., Ardley J.K., Willems A., Pitard R.M., Zilli J.E. Bradyrhizobium ingae sp. nov., isolated from effective nodules of Inga laurina grown in Cerrado soil. Int. J. Syst. Evol. Microbiol. 2014;64:3395–3401. doi: 10.1099/ijs.0.063727-0. [DOI] [PubMed] [Google Scholar]
- 57.Toledo I., Lloret L., Martinez-Romero E. Sinorhizobium americanus sp. nov., a new Sinorhizobium species nodulating native Acacia spp. in Mexico. Syst. Appl. Microbiol. 2003;26:54–64. doi: 10.1078/072320203322337317. [DOI] [PubMed] [Google Scholar]
- 58.Fall D., Diouf D., Ourarhi M., Faye A., Abdelmounen H., Neyra M., Sylla S.N., Missbah el Idrissi M. Phenotypic and genotypic characteristics of Acacia Senegal (L.) Willd. root-nodulating bacteria isolated from soils in the dryland part of Senegal. Lett. Appl. Microbiol. 2008;47:85–97. doi: 10.1111/j.1472-765X.2008.02389.x. [DOI] [PubMed] [Google Scholar]
- 59.Bournaud C., de Faria S.M., dos Santos J.M.F., Tisseyre P., Silva M., Chaintreuil C., Gross E., James E.K., Prin Y., Moulin L. Burkholderia species are the most common and preferred nodulating symbionts of the Piptadenia group (Tribe Mimoseae) PLoS ONE. 2013;8:e63478. doi: 10.1371/journal.pone.0063478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beyhaut E., Tlusty B., van Berkum P., Graham P.H. Rhizobium giardinii is the microsymbiont of Illinois bundleflower (Desmanthus illinoensis (Michx.) Mcmillan) in Midwestern prairies. Can. J. Microbiol. 2006;52:903–907. doi: 10.1139/w06-051. [DOI] [PubMed] [Google Scholar]
- 61.Fornasero L.V., del Papa M.F., López J.L., Albicoro F.J., Zabala J.M., Toniutti M.A., Pensiero J.F., Lagares A. Phenotypic, molecular and symbiotic characterization of the rhizobial symbionts of Desmanthus paspalaceus (Lindm.) Burkart that grow in the province of Santa Fe, Argentina. PLoS ONE. 2014;9:e104636. doi: 10.1371/journal.pone.0104636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang E.T., Martínez-Romero J., Martínez-Romero E. Genetic diversity of rhizobia from Leucaena leucocephala nodules in Mexican soils. Mol. Ecol. 1999;8:711–724. doi: 10.1046/j.1365-294X.1999.00608.x. [DOI] [Google Scholar]
- 63.Xu K.W., Penttinen P., Chen Y.X., Chen Q., Zhang X. Symbiotic efficiency and phylogeny of the rhizobia isolated from Leucaena leucocephala in arid-hot river valley area in Panxi, Sichuan, China. Appl. Microbiol. Biotchnol. 2013;97:783–793. doi: 10.1007/s00253-012-4246-2. [DOI] [PubMed] [Google Scholar]
- 64.López-López A., Rogel-Hernández M.A., Barois I., Ortis Ceballos A.I., Martínez J., Ormeño-Orrillo E., Martínez-Romero E. Rhizobium grahamii sp. nov., from nodules of Dalea leporina, Leucaena leucocephala and Clitoria ternatea, and Rhizobium mesoamericanum sp. nov., from nodules of Phaseolus vulgaris, siratro, cowpea and Mimosa pudica. Int. J. Syst. Evol. Microbiol. 2012;62:2264–2271. doi: 10.1099/ijs.0.033555-0. [DOI] [PubMed] [Google Scholar]
- 65.Chen W.-M., Moulin L., Bontemps C., Vandamme P., Béna G., Boivin-Masson C. Legume symbiotic nitrogen fixation by β-proteobacteria is widespread in nature. J. Bacteriol. 2003;185:7266–7272. doi: 10.1128/JB.185.24.7266-7272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen W.-M., de Faria S.M., Straliotto R., Pitard R.M., Simões-Araùjo J.L., Chou J.-H., Chou Y.-J., Barrios E., Prescott A.R., Elliott G.N., et al. Proof that Burkholderia strains form effective symbioses with legumes: A study of novel Mimosa-nodulating strains from South America. Appl. Environ. Microbiol. 2005;71:7461–7471. doi: 10.1128/AEM.71.11.7461-7471.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen W.-M., James E.K., Chou J.-H., Sheu S.-Y., Yang S.-Z., Sprent J.I. β-Rhizobia from Mimosa pigra, a newly discovered invasive plant in Taiwan. New Phytol. 2005;168:661–675. doi: 10.1111/j.1469-8137.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- 68.Barrett C.F., Parker M.A. Prevalence of Burkholderia sp. nodule symbionts on four mimosoid legumes from Barro Colorado Island, Panama. Syst. Appl. Microbiol. 2005;28:57–65. doi: 10.1016/j.syapm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Barrett C.F., Parker M.A. Coexistence of Burkholderia, Cupriavidus, and Rhizobium sp. nodule bacteria on two Mimosa spp. in Costa Rica. Appl. Environ. Microbiol. 2006;72:1198–1206. doi: 10.1128/AEM.72.2.1198-1206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X.Y., Wang E.T., Li Y., Chen W.X. Diverse bacteria isolated from root nodules of Trifolium, Crotalaria and Mimosa grown in the subtropical regions of China. Arch. Microbiol. 2007;188:1–14. doi: 10.1007/s00203-007-0209-x. [DOI] [PubMed] [Google Scholar]
- 71.Parker M.A., Wurtz A.K., Paynter Q. Nodule symbiosis of invasive Mimosa pigra in Australia and in ancestral habitats: A comparative analysis. Biol. Invasions. 2007;9:127–138. doi: 10.1007/s10530-006-0009-2. [DOI] [Google Scholar]
- 72.Elliott G.N., Chou J.-H., Chen W.-M., Bloemberg G.V., Bontemps C., Martínez-Romero E., Velázquez E., Young J.P.W., Sprent J.I., James E.K. Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environ. Microbiol. 2009;11:762–778. doi: 10.1111/j.1462-2920.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 73.Bontemps C., Elliott G.N., Simon M.F., dos Reis F.B., Jr., Gross E., Lawton R.C., Neto N.E., Loureiro M.deF., de Faria S.M., Sprent J.I., et al. Burkholderia species are ancient symbionts of legumes. Mol. Ecol. 2010;19:44–52. doi: 10.1111/j.1365-294X.2009.04458.x. [DOI] [PubMed] [Google Scholar]
- 74.Bontemps C., Rogel M.A., Wiechmann A., Mussabekova A., Moody S., Simon M.F., Moulin L., Elliott G.N., Lacercat-Didier L., Dasilva C., et al. Endemic Mimosa species from Mexico prefer α-proteobacterial rhizobial symbionts. New Phytol. 2016;209:319–333. doi: 10.1111/nph.13573. [DOI] [PubMed] [Google Scholar]
- 75.Liu X.Y., Wu W., Wang E.T., Zhang B., Macdermott J., Chen W.X. Phylogenetic relationships and diversity of β-rhizobia associated with Mimosa species grown in Sishuangbanna, China. Int. J. Syst. Evol. Microbiol. 2011;61:334–342. doi: 10.1099/ijs.0.020560-0. [DOI] [PubMed] [Google Scholar]
- 76.Liu X.Y., Wei S., Wang F., James E.K., Guo X.Y., Zagar C., Xia L.G., Dong X., Wang Y.P. Burkholderia and Cupriavidus spp. are the preferred symbionts of Mimosa spp. in Southern China. FEMS Microbiol. Ecol. 2012;80:417–426. doi: 10.1111/j.1574-6941.2012.01310.x. [DOI] [PubMed] [Google Scholar]
- 77.Gehlot H.S., Tak N., Kaushik M., Mitra S., Chen W.-M., Poweleit N., Panwar D., Poonar N., Parihar R., Tak A., et al. An invasive Mimosa in India does not adopt the symbionts of its native relatives. Ann. Bot. 2013;112:179–196. doi: 10.1093/aob/mct112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andam C.P., Mondo S.J., Parker M.A. Monophyly of nodA and nifH genes across Texan and Costa Rican populations of Cupriavidus nodule symbionts. Appl. Environ. Microbiol. 2007;73:4686–4690. doi: 10.1128/AEM.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Platero R., James E.K., Rios C., Iriarte A., Sandes L., Zabaleta M., Battistoni F., Fabiano E. Novel Cupriavidus strains isolated from root nodules of native Uruguayan Mimosa species. Appl. Environ. Microbiol. 2016;82:3150–3164. doi: 10.1128/AEM.04142-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Lajudie P., Laurent-Fulele E., Willems A., Torck U., Coopman R., Collins M.D., Kersters K., Dreyfus B., Gillis M. Allorhizobium undicola gen. nov., sp. nov., nitrogen-fixing bacteria that efficiently nodulate Neptunia natans in Senegal. Int. J. Syst. Bacteriol. 1998;48:1277–1290. doi: 10.1099/00207713-48-4-1277. [DOI] [PubMed] [Google Scholar]
- 81.Rivas R., Velázquez E., Willems A., Vizcaíno N., Subba-Rao N.S., Mateos P.F., Gillis M., Dazzo F.B., Martínez-Molina E. A new species of Devosia that forms a unique nitrogen-fixing root-nodule symbiosis with the aquatic legume Neptunia natans (L.f.) Druce. Appl. Environ. Microbiol. 2002;68:5217–5222. doi: 10.1128/AEM.68.11.5217-5222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taulé C., Zabaleta M., Mareque C., Platero R., Sanjurjo L., Sicardi M., Frioni L., Battistoni F., Fabiano E. New β-proteobacterial Rhizobium strains able to efficiently nodulate Parapiptadenia rigida (Benth.) Brenan. Appl. Environ. Microbiol. 2012;78:1692–1700. doi: 10.1128/AEM.06215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diaz L.C., González P., Rubio E., Melchiorre M. Diversity and stress tolerance in rhizobia from Parque Chaqueño region of Argentina nodulating Prosopis alba. Biol. Fertil. Soils. 2013;49:1153–1165. doi: 10.1007/s00374-013-0814-6. [DOI] [Google Scholar]
- 84.Iglesias O., Rivas R., García-Fraile P., Abril A., Mateos P.F., Martinez-Molina E., Velázquez E. Genetic characterization of fast-growing rhizobia able to nodulate Prosopis alba in North Spain. FEMS Microbiol. Lett. 2007;277:210–216. doi: 10.1111/j.1574-6968.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 85.Velázquez E., Igual J.M., Willems A., Fernández M.P., Muñoz E., Mateos P.F., Abril A., Toro N., Normand P., Cervantes E., et al. Mesorhizobium chacoense sp. nov., a novel species that nodulates Prosopis alba in the Chaco Arido region (Argentina) Int. J. Syst. Evol. Microbiol. 2001;51:1011–1021. doi: 10.1099/00207713-51-3-1011. [DOI] [PubMed] [Google Scholar]
- 86.Nick G., de Lajudie P., Eardly B.D., Suomalainen S., Paulin L., Zhang X., Gillis M., Lindström K. Sinorhizobium arboris sp. nov. and Sinorhizobium kostiense sp. nov., isolated from leguminous trees in Sudan and Kenya. Int. J. Syst. Bacterial. 1999;49:1359–1368. doi: 10.1099/00207713-49-4-1359. [DOI] [PubMed] [Google Scholar]
- 87.Gehlot H.S., Panwar D., Tak N., Tak A., Sankhla I.S., Poonar N., Parihar R., Shekhawat N.S., Kumar M., Tiwari R., et al. Nodulation of legumes from the Thar desert of India and molecular characterization of their rhizobia. Plant Soil. 2012;357:227–243. doi: 10.1007/s11104-012-1143-5. [DOI] [Google Scholar]
- 88.Fterich A., Mahdhi M., Caviedes M.A., Pajuelo E., Rivas R., Rodriguez-Llorente I.D., Mars M. Characterization of root-nodulating bacteria associated to Prosopis farcta growing in the arid regions of Tunisia. Arch. Microbiol. 2011;193:385–397. doi: 10.1007/s00203-011-0683-z. [DOI] [PubMed] [Google Scholar]
- 89.Degefu T., Wolde-meskel E., Frostegård Å. Multilocus sequence analyses reveal several unnamed Mesorhizobium genospecies nodulating Acacia species and Sesbania sesban trees in Southern regions of Ethiopia. Syst. Appl. Microbiol. 2011;34:216–226. doi: 10.1016/j.syapm.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Degefu T., Wolde-meskel E., Frostegård Å. Phylogenetic multilocus sequence analysis identifies seven novel Ensifer genospecies isolated from a less-well-explored biogeographical region in East Africa. Int. J. Syst. Evol. Microbiol. 2012;62:2286–2295. doi: 10.1099/ijs.0.039230-0. [DOI] [PubMed] [Google Scholar]
- 91.Sankhla I.S., Tak N., Meghwal R.R., Choudhary S., Tak A., Rathi S., Sprent J.I., James E.K., Gehlot H.S. Molecular characterization of nitrogen fixing microsymbionts from root nodules of Vachellia (Acacia) jacquemontii, a native legume from the Thar Desert of India. Plant Soil. 2017;410:21–40. doi: 10.1007/s11104-016-2838-9. [DOI] [Google Scholar]
- 92.Cordero I., Ruiz-Díez B., de la Peña T.C., Balaguer L., Lucas M.M., Rincón A., Pueyo J.J. Rhizobial diversity, symbiotic effectiveness and structure of nodules of Vachellia macracantha. Soil Biol. Biochem. 2016;96:39–54. doi: 10.1016/j.soilbio.2016.01.011. [DOI] [Google Scholar]
- 93.Ba S., Willems A., de Lajudie P., Roche P., Jeder H., Quatrini P., Neyra M., Ferro M., Promé J.-C., Gillis M., et al. Symbiotic and taxonomic diversity of rhizobia isolated from Acacia tortilis sp. subsp. raddiana in Africa. Syst. Appl. Microbiol. 2002;25:130–145. doi: 10.1078/0723-2020-00091. [DOI] [PubMed] [Google Scholar]
- 94.Nour S.M., Fernandez M.P., Normand P., Cleyet-Marel J.-C. Rhizobium ciceri sp. nov., consisting of strains that nodulate chickpeas (Cicer arietinum L.) Int. J. Syst. Bacteriol. 1994;44:511–522. doi: 10.1099/00207713-44-3-511. [DOI] [PubMed] [Google Scholar]
- 95.Aouani M.E., Mhamdi R., Jebara M., Amarger N. Characterization of rhizobia nodulating chickpea in Tunisia. Agronomie. 2001;21:577–581. doi: 10.1051/agro:2001147. [DOI] [Google Scholar]
- 96.Maâtallah J., Berraho E.B., Muñoz S., Sanjuan J., Lluch C. Phenotypic and molecular characterization of chickpea rhizobia isolated from different areas of Morocco. J. Appl. Microbiol. 2002;93:531–540. doi: 10.1046/j.1365-2672.2002.01718.x. [DOI] [PubMed] [Google Scholar]
- 97.Rivas R., Laranjo M., Mateos P.F., Oliveira S., Martinez-Molina E., Velázquez E. Strains of Mesorhizobium amorphae and Mesorhizobium tianshanense, carrying symbiotic genes of common chickpea endosymbiotic species, constitute a novel biovar (ciceri) capable of nodulating Cicer arietinum. Lett. Appl. Microbiol. 2007;44:412–418. doi: 10.1111/j.1472-765X.2006.02086.x. [DOI] [PubMed] [Google Scholar]
- 98.Ben Romdhane S., Trabelsi M., Aouani M.E., de Lajudie P., Mhamdi R. The diversity of rhizobia nodulating chickpea (Cicer arietinum) under water deficiency as a source of more efficient inoculants. Soil Biol. Biochem. 2009;41:2568–2572. doi: 10.1016/j.soilbio.2009.09.020. [DOI] [Google Scholar]
- 99.Zhang J.J., Lou K., Jin X., Mao P.H., Wang E.T., Tian C.F., Sui X.H., Chen W.F., Chen W.X. Distinctive Mesorhizobium populations associated with Cicer arietinum L. in alkaline soils of Xinjiang, China. Plant Soil. 2012;353:123–134. doi: 10.1007/s11104-011-1014-5. [DOI] [Google Scholar]
- 100.Zhang J., Yang X., Guo C., de Lajudie P., Singh R.P., Wang E., Chen W. Mesorhizobium muleiense and Mesorhizobium gsp. Nov. are symbionts of Cicer arietinum L. in alkaline soils of Gansu, Northwest China. Plant Soil. 2017;410:103–112. doi: 10.1007/s11104-016-2987-x. [DOI] [Google Scholar]
- 101.Zahran H.H., Chahboune R., Moreno S., Bedmar E.J., Abdel-Fattah M., Yasser M.M., Mahmoud A.M. Identification of rhizobial strains nodulating Egyptian grain legumes. Int. Microbiol. 2013;16:157–163. doi: 10.2436/20.1501.01.190. [DOI] [PubMed] [Google Scholar]
- 102.Armas-Carpote N., Pérez-Yépez J., Martínez-Hidalgo P., Garzón-Machado V., del Arco-Aguilar M., Velázquez E., Léon-Barrios M. Core and symbiotic genes reveal nine Mesorhizobium genospecies and three symbiotic lineages among the rhizobia nodulating Cicer canariense in its natural habitat (La Palma, Canary Islands) Syst. Appl. Microbiol. 2014;37:140–148. doi: 10.1016/j.syapm.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 103.Mutch L.A., Young J.P.W. Diversity and specificity of Rhizobium leguminosarum biovar viciae on wild and cultivated legumes. Mol. Ecol. 2004;13:2435–2444. doi: 10.1111/j.1365-294X.2004.02259.x. [DOI] [PubMed] [Google Scholar]
- 104.Aoki S., Kondo T., Prévost D., Nakata S., Kajita T., Itó M. Genotypic and phenotypic diversity of rhizobia isolated from Lathyrus japonicus indigenous to Japan. Syst. Appl. Microbiol. 2010;33:383–397. doi: 10.1016/j.syapm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Han T.X., Wang E.T., Wu L.J., Chen W.F., Gu J.G., Gu C.T., Tian C.F., Chen W.X. Rhizobium multihospitium sp. nov., isolated from multiple legume species native of Xinjiang, China. Int. J. Syst. Evol. Microbiol. 2008;58:1693–1699. doi: 10.1099/ijs.0.65568-0. [DOI] [PubMed] [Google Scholar]
- 106.Rashid M.H., Schäfer H., Gonzalez J., Wink M. Genetic diversity of rhizobia nodulating lentil (Lens culinaris) in Bangladesh. Syst. Appl. Microbiol. 2012;35:98–109. doi: 10.1016/j.syapm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 107.Riah N., Béna G., Djekoun A., Heulin K., de Lajudie P., Laguerre G. Genotypic and symbiotic diversity of Rhizobium populations associated with cultivated lentil and pea in sub-humid and semi-arid regions of Eastern Algeria. Syst. Appl. Microbiol. 2014;37:368–375. doi: 10.1016/j.syapm.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y.J., Zheng W.T., Everall I., Young J.P.W., Zhang X.X., Tian C.F., Sui X.H., Wang E.T., Chen W.X. Rhizobium anhuiense sp. nov., isolated from effective nodules of Vicia faba and Pisum sativum. Int. J. Syst. Evol. Microbiol. 2015;65:2960–2967. doi: 10.1099/ijs.0.000365. [DOI] [PubMed] [Google Scholar]
- 109.Kan F.L., Chen Z.Y., Wang E.T., Tian C.F., Sui X.H., Chen W.X. Characterization of symbiotic and endophytic bacteria isolated from root nodules of herbaceous legumes grown in Qinghai-Tibet plateau and in other zones of China. Arch. Microbiol. 2007;188:103–115. doi: 10.1007/s00203-007-0211-3. [DOI] [PubMed] [Google Scholar]
- 110.Ampomah O.Y., Huss-Danell K. Genetic diversity of rhizobia nodulating native Vicia spp. in Sweden. Syst. Appl. Microbiol. 2016;39:203–210. doi: 10.1016/j.syapm.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 111.Santillana N., Ramírez-Bahena M.H., García-Fraile P., Velázquez E., Zúñiga D. Phylogenetic diversity based on rrs, atpD, recA genes and 16S–23S intergenic sequence analyses of rhizobial strains isolated from Vicia faba and Pisum sativum in Peru. Arch. Microbiol. 2008;189:239–247. doi: 10.1007/s00203-007-0313-y. [DOI] [PubMed] [Google Scholar]
- 112.Saïdi S., Chebil S., Gtari M., Mhamdi R. Characterization of root-nodule bacteria isolated from Vicia faba and selection of plant growth promoting isolates. World J. Microbiol. Biotechnol. 2013;29:1099–1106. doi: 10.1007/s11274-013-1278-4. [DOI] [PubMed] [Google Scholar]
- 113.Youseif S.H., Abd El-Megeed F.H., Ageez A., Cocking E.C. Saleh, S.A. Phylogenetic multilocus sequence analysis of native rhizobia nodulating faba bean (Vicia faba L.) in Egypt. Syst. Appl. Microbiol. 2014;37:560–569. doi: 10.1016/j.syapm.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 114.Xu K.W., Zou L., Penttinen P., Wang K., Heng N.N., Zhang X.P., Chen Q., Zhao K., Chen Y.X. Symbiotic effectiveness and phylogeny of rhizobia isolated from faba bean (Vicia faba L.) in Sichuan hilly areas, China. Syst. Appl. Microbiol. 2015;38:515–523. doi: 10.1016/j.syapm.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 115.Lei X., Wang E.T., Chen W.F., Sui X.H., Chen W.X. Diverse bacteria isolated from root nodules of wild Vicia species grown in temperate region of China. Arch. Microbiol. 2008;190:657–671. doi: 10.1007/s00203-008-0418-y. [DOI] [PubMed] [Google Scholar]
- 116.Tian C.F., Wang E.T., Wu L.J., Han T.X., Chen W.F., Gu C.T., Gu J.G., Chen W.X. Rhizobium fabae sp. nov., a bacterium that nodulates Vicia faba. Int. J. Syst. Evol. Microbiol. 2008;58:2871–2875. doi: 10.1099/ijs.0.2008/000703-0. [DOI] [PubMed] [Google Scholar]
- 117.Alvarez-Martínez E.R., Valverde Á., Ramírez-Bahena M.H., García-Fraile P., Tejedor C., Mateos P.F., Santillana N., Zúñiga D., Peix A., Velázquez E. The analysis of core and symbiotic genes of rhizobia nodulating Vicia from different continents reveals their common phylogenetic origin and suggests the distribution of Rhizobium leguminosarium strains together with Vicia seeds. Arch. Microbiol. 2009;191:659–668. doi: 10.1007/s00203-009-0495-6. [DOI] [PubMed] [Google Scholar]
- 118.Rejili M., Mahdhi M., Fterich A., Dhaoui S., Guefrachi I., Abdeddayem R., Mars M. Symbiotic nitrogen fixation of wild legumes in Tunisia: Soil fertility dynamics, field nodulation and nodules effectiveness. Agric. Ecosyst. Environ. 2012;157:60–69. doi: 10.1016/j.agee.2012.01.015. [DOI] [Google Scholar]
- 119.Radeva G., Jurgens G., Niemi M., Nick G., Suominen L., Lindström K. Description of two biovars in the Rhizobium galegae species: Biovar orientalis and biovar officinalis. System. Appl. Microbiol. 2001;24:192–205. doi: 10.1078/0723-2020-00029. [DOI] [PubMed] [Google Scholar]
- 120.Liu W.Y.Y., Ridgway H.J., James T.K., Premaratne M., Andrews M. Characterisation of rhizobia nodulating Galega officinalis (goat’s rue) and Hedysarum coronarium (sulla) NZ Plant Prot. 2012;65:192–196. [Google Scholar]
- 121.Chen W., Sun L., Lu J., Bi L., Wang E., Wei G. Diverse nodule bacteria were associated with Astragalus species in arid region of northwestern China. J. Basic Microbiol. 2015;55:121–128. doi: 10.1002/jobm.201300209. [DOI] [PubMed] [Google Scholar]
- 122.Wei G.H., Zhang Z.X., Chen C., Chen W.M., Ju W.T. Phenotypic and genetic diversity of rhizobia isolated from nodules of the legume genera Astragalus, Lespedeza and Hedysarum in northwestern China. Microbial. Res. 2008;163:651–662. doi: 10.1016/j.micres.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 123.Zhao C.T., Wang E.T., Zhang Y.M., Chen W.F., Sui X.H., Chen W.X., Liu H.C., Zhang X.X. Mesorhizobium silamurunense sp. nov., isolated from root nodules of Astragalus species. Int. J. Syst. Evol. Microbiol. 2012;62:2180–2186. doi: 10.1099/ijs.0.031229-0. [DOI] [PubMed] [Google Scholar]
- 124.Yan H., Ji Z.J., Jiao Y.S., Wang E.T., Chen W.F., Guo B.L., Chen W.X. Genetic diversity and distribution of rhizobia associated with the medicinal legumes Astragalus spp. and Hedysarum polybotrys in agricultural soils. Syst. Appl. Microbiol. 2016;39:141–149. doi: 10.1016/j.syapm.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 125.Nandasena K.G., O’Hara G.W., Tiwari R.P., Yates R.J., Howieson J.G. Phylogenetic relationships of three bacterial strains isolated from the pasture legume Biserrula pelecinus L. Int. J. Syst. Evol. Microbiol. 2001;51:1983–1986. doi: 10.1099/00207713-51-6-1983. [DOI] [PubMed] [Google Scholar]
- 126.Nandasena K.G., O’Hara G.W., Tiwari R.P., Willems A., Howieson J.G. Mesorhizobium australicum sp. nov. and Mesorhizobium opportunistum sp. nov., isolated from Biserrula pelecinus L. in Australia. Int. J. Syst. Evol. Microbiol. 2009;59:2140–2147. doi: 10.1099/ijs.0.005728-0. [DOI] [PubMed] [Google Scholar]
- 127.Tan H.W., Weir B.S., Carter N., Heenan P.B., Ridgway H.J., James E.K., Sprent J.I., Young J.P.Y., Andrews M. Rhizobia with 16S rRNA and nifH similar to Mesorhizobium huakuii but novel recA, gln11, nodA and nodC genes are symbionts of New Zealand Carmichaelinae. PLoS ONE. 2012;7:e47677. doi: 10.1371/journal.pone.0047677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ourarhi M., Abdelmoumen H., Guerrouj K., Benata H., Muresu R., Squartini A., El Idrissi M.M. Colutea arborescens is nodulated by diverse rhizobia in Eastern Morocco. Arch. Microbiol. 2011;193:115–124. doi: 10.1007/s00203-010-0650-0. [DOI] [PubMed] [Google Scholar]
- 129.Ruiz-Díez B., Fajardo S., Puertas-Mejía M.A., del Felipe M.R., Fernández-Pascual M. Stress tolerance, genetic analysis and symbiotic properties of root-nodulating bacteria isolated from Mediterranean leguminous shrubs in Central Spain. Arch. Microbiol. 2009;191:35–46. doi: 10.1007/s00203-008-0426-y. [DOI] [PubMed] [Google Scholar]
- 130.Li L., Sinkko H., Montonen L., Wei G., Lindström K., Räsänen L.A. Biogeography of symbiotic and other endophytic bacteria isolated from medicinal Glycyrrhiza species in China. FEMS Microbiol. Ecol. 2012;79:46–68. doi: 10.1111/j.1574-6941.2011.01198.x. [DOI] [PubMed] [Google Scholar]
- 131.Wei G.H., Yang X.-Y., Zhang Z.-X., Yang Y.-Z., Lindström K. Strain Mesorhizobium sp. CCNWGX035: A stress-tolerant isolate from Glycyrrhiza glabra displaying a wide host range of nodulation. Pedosphere. 2008;18:102–112. doi: 10.1016/S1002-0160(07)60108-8. [DOI] [Google Scholar]
- 132.Tan Z.Y., Wang E.T., Peng G.X., Zhu M.E., Martínez-Romero E., Chen W.X. Characterization of bacteria isolated from wild legumes in the north-western regions of China. Int. J. Syst. Bacteriol. 1999;49:1457–1469. doi: 10.1099/00207713-49-4-1457. [DOI] [PubMed] [Google Scholar]
- 133.Chen W., Wang E., Wang S., Li Y., Chen X., Li Y. Characteristics of Rhizobium tianshanense sp. nov., a moderately and slowly growing root nodule bacterium isolated from an arid saline environment in Xinjiang, People’s Republic of China. Int. J. Syst. Bacteriol. 1995;45:153–159. doi: 10.1099/00207713-45-1-153. [DOI] [PubMed] [Google Scholar]
- 134.Tan Z.Y., Kan F.L., Peng G.X., Wang E.T., Reinhold-Hurek B., Chen W.X. Rhizobium yanglingense sp. nov., isolated from arid and semi-arid regions in China. Int. J. Syst. Evol. Microbiol. 2001;51:909–914. doi: 10.1099/00207713-51-3-909. [DOI] [PubMed] [Google Scholar]
- 135.Gerding M., O’Hara G.W., Bräu L., Nandasena K., Howieson J.G. Diverse Mesorhizobium spp. with unique nodA nodulating the South African legume species of the genus Lessertia. Plant Soil. 2012;358:385–401. doi: 10.1007/s11104-012-1153-3. [DOI] [Google Scholar]
- 136.Lemaire B., Dlodlo O., Chimphango S., Stirton C., Schrire B., Boatwright J.S., Honnay O., Smets E., Sprent J., James E.K., et al. Symbiotic diversity, specificity and distribution of rhizobia in native legumes of the Core Cape Subregion (South Africa) FEMS Microbiol. Ecol. 2015;91:1–17. doi: 10.1093/femsec/fiu024. [DOI] [PubMed] [Google Scholar]
- 137.Tan H.W., Heenan P., Ridgway H., Andrews M. The New Zealand alpine endemic Montigena novae-zelandiae (Fabaceae) shares rhizobial symbionts with Carmichaelia and Clianthus. N. Z. J. Bot. 2013;51:297–307. doi: 10.1080/0028825X.2013.831360. [DOI] [Google Scholar]
- 138.Han T.X., Han L.L., Wu L.J., Chen W.F., Sui X.H., Gu J.G., Wang E.T., Chen W.X. Mesorhizobium gobiense sp. nov. and Mesorhizobium tarimense sp. nov., isolated from wild legumes growing in desert soils of Xinjiang, China. Int. J. Syst. Evol. Microbiol. 2008;58:2610–2618. doi: 10.1099/ijs.0.2008/000125-0. [DOI] [PubMed] [Google Scholar]
- 139.Deng Z.S., Zhao L.F., Kong Z.Y., Yang W.Q., Lindström K., Wang E.T., Wei G.H. Diversity of endophytic bacteria within nodules of the Sphaerophysa salsula in different regions of Loess Plateau in China. FEMS Microbiol. Ecol. 2011;76:463–475. doi: 10.1111/j.1574-6941.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 140.Xu L., Shi J.F., Zhao P., Chen W.M., Qin W., Tang M., Wei G.H. Rhizobium sphaerophysae sp. nov., a novel species isolated from root nodules of Sphaerophysa salsula in China. Antonie van Leeuwenhoek. 2011;99:845–854. doi: 10.1007/s10482-011-9559-0. [DOI] [PubMed] [Google Scholar]
- 141.Yates R.J., Howieson J.G., Nandasena K.G., O’Hara G.W. Root-nodule bacteria of indigenous legumes in the north-west of Westen Australia and their interaction with exotic legumes. Soil Biol. Biochem. 2004;36:1319–1329. doi: 10.1016/j.soilbio.2004.04.013. [DOI] [Google Scholar]
- 142.Wei G., Chen W., Young J.P.W., Bontemps C. A new clade of Mesorhizobium nodulating Alhagi sparsifolia. Syst. Appl. Microbiol. 2009;32:8–16. doi: 10.1016/j.syapm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 143.Lu Y.L., Chen W.F., Wang E.T., Guan S.H., Yan X.R., Chen W.X. Genetic diversity and biogeography of rhizobia associated with Caragana species in three ecological regions of China. Syst. Appl. Microbiol. 2009;32:351–361. doi: 10.1016/j.syapm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 144.Guan S.H., Chen W.F., Wang E.T., Lu Y.L., Yan X.R., Zhang X.X., Chen W.X. Mesorhizobium caraganae sp. nov., a novel rhizobial species nodulated with Caragana spp. in China. Int. J. Syst. Evol. Microbiol. 2008;58:2646–2653. doi: 10.1099/ijs.0.65829-0. [DOI] [PubMed] [Google Scholar]
- 145.Squartini A., Struffi P., Döring H., Selenska-Pobell S., Tola E., Giacomini A., Vendramin E., Velázquez E., Mateos P.F., Martinez-Molina E., et al. Rhizobium sullae sp. nov. (formerly “Rhizobium hedysari”), the root-nodule microsymbiont of Hedysarum coronarium L. Int. J. Syst. Evol. Microbiol. 2002;52:1267–1276. doi: 10.1099/00207713-52-4-1267. [DOI] [PubMed] [Google Scholar]
- 146.Baimiev A.K., Baimiev A.K., Gubaidullin I.I., Kulikova O.L., Chemeris A.V. Bacteria closely related to Phyllobacterium trifolii according to their 16S rRNA gene are discovered in the nodules of Hungarian sainfoin. Genetika. 2007;43:587–590. doi: 10.1134/S1022795407050146. [DOI] [PubMed] [Google Scholar]
- 147.El Batanony N.H., Castellano-Hinojosa A., Correa-Galeote D., Bedmar E.J. The diversity of rhizobia nodulating the Medicago, Melilotus and Trigonella inoculation group in Egypt is marked by the dominance of two genetic types. Symbiosis. 2015;67:3–10. doi: 10.1007/s13199-015-0365-8. [DOI] [Google Scholar]
- 148.Del Villegas M.C., Rome S., Mauré L., Domergue O., Gardan L., Bailly X., Cleyet-Marel J.-C., Brunel B. Nitrogen-fixing sinorhizobia with Medicago laciniata constitute a novel biovar (bv. medicaginis) of S. meliloti. Syst. Appl. Microbiol. 2006;29:526–538. doi: 10.1016/j.syapm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 149.Badri Y., Zribi K., Badri M., Huguet T., van Berkum P., Aouani M.E. Comparison of rhizobia that nodulate Medicago laciniata and Medicago truncatula present in a single Tunisian arid soil. Can. J. Microbiol. 2007;53:277–283. doi: 10.1139/w06-130. [DOI] [PubMed] [Google Scholar]
- 150.Mnasri B., Badri Y., Saïdi S., de Lajudie P., Mhamdi R. Symbiotic diversity of Ensifer meliloti strains recovered from various legume species in Tunisia. Syst. Appl. Microbiol. 2009;32:583–592. doi: 10.1016/j.syapm.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 151.Wang H., Man C.X., Wang E.T., Chen W.X. Diversity of rhizobia and interactions among the host legumes and rhizobial genotypes in an agricultural-forestry ecosystem. Plant Soil. 2009;314:169–182. doi: 10.1007/s11104-008-9716-z. [DOI] [Google Scholar]
- 152.Rome S., Fernandez M.P., Brunel B., Normand P., Cleyet-Marel J.-C. Sinorhizobium medicae sp. nov., isolated from annual Medicago spp. Int. J. Syst. Bacteriol. 1996;46:972–980. doi: 10.1099/00207713-46-4-972. [DOI] [PubMed] [Google Scholar]
- 153.Gubry-Rangin C., Béna G., Cleyet-Merel J.-C., Brunel B. Definition and evolution of a new symbiovar, sv. rigiduloides, among Ensifer meliloti efficiently nodulating Medicago species. Syst. Appl. Microbiol. 2013;36:490–496. doi: 10.1016/j.syapm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 154.Van Berkum P., Beyene D., Bao G., Campbell T.A., Eardly B.D. Rhizobium mongolense sp. nov. is one of three rhizobial genotypes identified which nodulate and form nitrogen-fixing symbioses with Medicago ruthenica (L. Ledebour) Int. J. Syst. Bacteriol. 1998;48:13–22. doi: 10.1099/00207713-48-1-13. [DOI] [PubMed] [Google Scholar]
- 155.Yan A.M., Wang E.T., Kan F.L., Tan Z.Y., Sui X.H., Reinhold-Hurek B., Chen W.X. Sinorhizobium meliloti associated with Medicago sativa and Melilotus spp. in arid saline soils in Xinjiang, China. Int. J. Syst. Evol. Microbiol. 2000;50:1887–1891. doi: 10.1099/00207713-50-5-1887. [DOI] [PubMed] [Google Scholar]
- 156.Bromfield E.S.P., Tambong J.T., Cloutier S., Prévost D., Laguerre G., van Berkum P., Tran Thi T.V., Assabgui R., Barran L.R. Ensifer, Phyllobacterium and Rhizobium species occupy nodules of Medicago sativa (alfalfa) and Melilotus alba (sweet clover) grown at a Canadian site without a history of cultivation. Microbiology. 2010;156:505–520. doi: 10.1099/mic.0.034058-0. [DOI] [PubMed] [Google Scholar]
- 157.Merabet C., Martens M., Mahdi M., Zakhia F., Sy A., Le Roux C., Domergue O., Coopman R., Bekki A., Mars M., et al. Multilocus sequence analysis of root nodule isolates from Lotus arabicus (Senegal), Lotus creticus, Argyrolobium uniflorum and Medicago sativa (Tunisia) and description of Ensifer numidicus sp. nov. and Ensifer garamanticus sp. nov. Int. J. Syst. Evol. Microbiol. 2010;60:664–674. doi: 10.1099/ijs.0.012088-0. [DOI] [PubMed] [Google Scholar]
- 158.Djedidi S., Yokoyama T., Tomooka N., Ohkama-Ohtsu N., Risal C.P., Abdelly C., Sekimoto H. Phenotypic and genetic characterization of rhizobia associated with alfalfa in the Hokkaido and Ishigaki regions of Japan. Syst. Appl. Microbiol. 2011;34:453–461. doi: 10.1016/j.syapm.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 159.Valverde A., Velázquez E., Fernández-Santos F., Vizcaíno N., Rivas R., Mateos P.F., Martínez-Molina E., Igual J.M., Willems A. Phyllobacterium trifolii sp. nov., nodulating Trifolium and Lupinus in Spanish soils. Int. J. System. Evol. Microbiol. 2005;55:1985–1989. doi: 10.1099/ijs.0.63551-0. [DOI] [PubMed] [Google Scholar]
- 160.Zhang J.J., Jing X.Y., de Lajudie P., Ma C., He P.X., Singh R.P., Chen W.F., Wang E.T. Association of white clover (Trifolium repens L.) with rhizobia of sv. trifolii belonging to three genomic species in alkaline soils in North and East China. Plant Soil. 2016;407:417–427. doi: 10.1007/s11104-016-2899-9. [DOI] [Google Scholar]
- 161.Ogasawara M., Suzuki T., Mutoh I., Annapurna K., Arora N.K., Nishimura Y., Maheshwari D.K. Sinorhizobium indiaense sp. nov. and Sinorhizobium abri sp. nov. isolated from tropical legumes, Sesbania rostrata and Abrus precatorius, respectively. Symbiosis. 2003;34:53–68. [Google Scholar]
- 162.Wang E.T., van Berkum P., Sui X.H., Beyene D., Chen W.X., Martínez-Romero E. Diversity of rhizobia associated with Amorpha fruticosa isolated from Chinese soils and description of Mesorhizobium amorphae sp. nov. Int. J. Syst. Bacteriol. 1999;49:51–65. doi: 10.1099/00207713-49-1-51. [DOI] [PubMed] [Google Scholar]
- 163.Tlusty B., van Berkum P., Graham P.H. Characteristics of the rhizobia associated with Dalea spp. in the Ordway, Kellogg-Weaver Dunes, and Hayden prairies. Can. J. Microbiol. 2005;51:15–23. doi: 10.1139/w04-107. [DOI] [PubMed] [Google Scholar]
- 164.Sy A., Giraud E., Jourand P., Garcia N., Willems A., de Lajudie P., Prin Y., Neyra M., Gillis M., Boivin-Masson C., et al. Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. J. Bacteriol. 2001;183:214–220. doi: 10.1128/JB.183.1.214-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Howieson J.G., de Meyer S.E., Vivas-Marfisi A., Ratnayake S., Ardley J.K., Yates R.J. Novel Burkholderia bacteria isolated from Lebeckia ambigua—A perennial suffrutescent legume of the fynbos. Soil Biol. Biochem. 2013;60:55–64. doi: 10.1016/j.soilbio.2013.01.009. [DOI] [Google Scholar]
- 166.Ardley J.K., Parker M.A., de Meyer S.E., Trengove R.D., O’Hara G.W., Reeve W.G., Yates R.J., Dilworth M.J., Willems A., Howieson J.G. Microvirga lupini sp. nov., Microvirga lotononidis sp. nov. and Microvirga zambiensis sp. nov. are α proteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate legume hosts. Int. J. Syst. Evol. Microbiol. 2012;62:2579–2588. doi: 10.1099/ijs.0.035097-0. [DOI] [PubMed] [Google Scholar]
- 167.Cardinale M., Lanza A., Bonnì M.L., Marsala S., Puglia A.M., Quatrini P. Diversity of rhizobia nodulating wild shrubs of Sicily and some neighbouring islands. Arch. Microbiol. 2008;190:461–470. doi: 10.1007/s00203-008-0394-2. [DOI] [PubMed] [Google Scholar]
- 168.Rodríguez-Echeverría S., Pérez-Fernández M.A., Vlaar S., Finnan T. Analysis of the legume-rhizobia symbiosis in shrubs from central western Spain. J. Appl. Microbiol. 2003;95:1367–1374. doi: 10.1046/j.1365-2672.2003.02118.x. [DOI] [PubMed] [Google Scholar]
- 169.Vinuesa P., Rademaker J.L.W., de Bruijn F.J., Werner D. Genotypic characterization of Bradyrhizobium strains nodulating endemic woody legumes of the Canary Islands by PCR-restriction fragment length polymorphism analysis of genes encoding 16S rRNA (16S rDNA) and 16S–23S rDNA intergenic spacers, repetitive extragenic palindromic PCR genomic fingerprinting, and partial 16S rDNA sequencing. Appl. Environ. Microbiol. 1998;64:2096–2104. doi: 10.1128/aem.64.6.2096-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vinuesa P., Léon-Barrios M., Silva C., Willems A., Jarabo-Lorenzo A., Pérez-Galdona R., Werner D., Martínez-Romero E. Bradyrhizobium canariense sp. nov., an acid-tolerant endosymbiont that nodulates endemic genistoid legumes (Papilionoideae: Genisteae) from the Canary Islands, along with Bradyrhizobium japonicum bv. genistearum, Bradyrhizobium genospecies alpha and Bradyrhizobium genospecies beta. Int. J. Syst. Evol. Microbiol. 2005;55:569–575. doi: 10.1099/ijs.0.63292-0. [DOI] [PubMed] [Google Scholar]
- 171.Jarabo-Lorenzo A., Velázquez E., Pérez-Galdona R., Vega-Hernández M.C., Martínez-Molina E., Mateos P.F., Vinuesa P., Martínez-Romero E., Léon-Barrios M. Restriction fragment length polymorphism analysis of 16S rDNA and low molecular weight RNA profiling of rhizobial isolates from shrubby legumes endemic to the Canary Islands. System. Appl. Microbiol. 2000;23:418–425. doi: 10.1016/S0723-2020(00)80073-9. [DOI] [PubMed] [Google Scholar]
- 172.Jarabo-Lorenzo A., Pérez-Galdona R., Donate-Correa J., Rivas R., Velázquez E., Hernández M., Temprano F., Martínez-Molina E., Ruiz-Argüeso T., Léon-Barrios M. Genetic diversity of Bradyrhizobial populations from diverse geographic origins that nodulate Lupinus spp. and Ornithopus spp. System. Appl. Microbiol. 2003;26:611–623. doi: 10.1078/072320203770865927. [DOI] [PubMed] [Google Scholar]
- 173.Kalita M., Malek W., Kaznowski A. Analysis of genetic relationship of Sarothamnus scoparius microsymbionts and Bradyrhizobium sp. by hybridization in microdilution wells. J. Biosci. Bioeng. 2004;97:158–161. doi: 10.1016/S1389-1723(04)70185-1. [DOI] [PubMed] [Google Scholar]
- 174.Horn K., Parker I.M., Malek W., Rodríguez-Echeverria S., Matthew A.P. Disparate origins of Bradyrhizobium symbionts for invasive populations of Cytisus scoparius (Leguminosae) in North America. FEMS Microbiol. Ecol. 2014;89:89–98. doi: 10.1111/1574-6941.12335. [DOI] [PubMed] [Google Scholar]
- 175.Chahboune R., Barrijal S., Moreno S., Bedmar E.J. Characterization of Bradyrhizobium species isolated from root nodules of Cytisus villosus grown in Morocco. Syst. Appl. Microbiol. 2011;34:440–445. doi: 10.1016/j.syapm.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 176.Cobo-Díaz J.F., Martínez-Hidalgo P., Fernández-González A.J., Martínez-Molina E., Toro N., Velázquez E., Fernández-López M. The endemic Genista versicolor from sierra nevada national park in Spain is nodulated by putative new Bradyrhizobium species and a novel symbiovar (sierranevadense) Syst. Appl. Microbiol. 2014;37:177–185. doi: 10.1016/j.syapm.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 177.Stroschein M.R.D., Eltz F.L.F., Antoniolli Z.I., Lupatini M., Vargas L.K., Giongo A., Pontelli M.P. Symbiotic efficiency and genetic characteristics of Bradyrhizobium sp. strain UFSM LA 1.3 isolated from Lupinus albescens (H. et Arn) Sci. Agric. 2010;67:702–706. doi: 10.1590/S0103-90162010000600012. [DOI] [Google Scholar]
- 178.Granada C.E., Beneduzi A., Lisboa B.B., Turchetto-Zolet A.C., Vargas L.K., Passaglia L.M.P. Multilocus sequence analysis reveals taxonomic differences among Bradyrhizobium sp. symbionts of Lupinus albescens plants growing in arenized and non-arenized areas. Syst. Appl. Microbiol. 2015;38:323–329. doi: 10.1016/j.syapm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 179.Velázquez E., Valverde A., Rivas R., Gomis V., Peix A., Gantois I., Igual J.M., León-Barrios M., Willems A., Mateos P.F., et al. Strains nodulating Lupinus albus on different contintents belong to several new chromosomal and symbiotic lineages within Bradyrhizobium. Antonie van Leeuwenhoek. 2010;97:363–376. doi: 10.1007/s10482-010-9415-7. [DOI] [PubMed] [Google Scholar]
- 180.Stępkowski T., Żak M., Moulin L., Króliczak J., Golińska B., Narożna D., Safronova V.I., Mądrzak C.J. Bradyrhizobium canariense and Bradyrhizobium japonicum are the two dominant rhizobium species in root nodules of lupin and serradella plants growing in Europe. Syst. Appl. Microbiol. 2011;34:368–375. doi: 10.1016/j.syapm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 181.Trujillo M.E., Willems A., Abril A., Planchuelo A.-M., Rivas R., Ludeña D., Mateos P.F., Martínez-Molina E., Velázquez E. Nodulation of Lupinus albus by strains of Ochrobactrum lupini sp. nov. Appl. Environ. Microbiol. 2005;71:1318–1327. doi: 10.1128/AEM.71.3.1318-1327.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sánchez-Cañizares C., Rey L., Durán D., Temprano F., Sánchez-Jiménez P., Navarro A., Polajnar M., Imperial J., Ruiz-Argüeso T. Endosymbiotic bacteria nodulating a new endemic lupine Lupinus mariae-josephi from alkaline soils in Eastern Spain represent a new lineage within the Bradyrhizobium genus. Syst. Appl. Microbiol. 2011;34:207–215. doi: 10.1016/j.syapm.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 183.Durán D., Rey L., Sánchez-Cañizares C., Navarro A., Imperial J., Ruiz-Argueso T. Genetic diversity of indigenous rhizobial symbionts of the Lupinus mariae-josephae endemism form alkaline-limed soils within its area of distribution in Eastern Spain. Syst. Appl. Microbiol. 2013;36:128–136. doi: 10.1016/j.syapm.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 184.Bourebaba Y., Durán D., Boulila F., Ahnia H., Boulila A., Temprano F., Palacios J.M., Imperial J., Ruiz-Argüeso T., Rey L. Diversity of Bradyrhizobium strains nodulating Lupinus micranthus on both sides of the Western Mediterranean: Algeria and Spain. Syst. Appl. Microbiol. 2016;39:266–274. doi: 10.1016/j.syapm.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 185.Ryan-Salter T.P., Black A.D., Andrews M., Moot D.J. Identification and effectiveness of rhizobial strains that nodulate Lupinus polyphyllus. Proc. NZ Grassland Assoc. 2014;76:61–66. [Google Scholar]
- 186.Guerrouj K., Ruíz-Díez B., Chahboune R., Ramírez-Bahena M.-H., Abdelmoumen H., Quiñones M.A., El Idrissi M.M., Velázquez E., Fernández-Pascual M., Bedmar E.J., Peix A. Definition of a novel symbiovar (sv. retamae) within Bradyrhizobium retamae sp. nov., nodulating Retama sphaerocarpa and Retama monosperma. Syst. Appl. Microbiol. 2013;36:218–223. doi: 10.1016/j.syapm.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 187.Farida B., Géraldine D., Abdelghani B., Djellali B., Said B., Gisèle L. Retama species growing in different ecological-climatic areas of northeastern Algeria have a narrow range of rhizobia that form a novel phylogenetic clade within the Bradyrhizobium genus. Syst. Appl. Microbiol. 2009;32:245–255. doi: 10.1016/j.syapm.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 188.Rodríguez-Echeverría S., Moreno S., Bedmar E.J. Genetic diversity of root nodulating bacteria associated with Retama sphaerocarpa in sites with different soil and environmental conditions. Syst. Appl. Microbiol. 2014;37:305–310. doi: 10.1016/j.syapm.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 189.Quatrini P., Scaglione G., Cardinale M., Caradonna F., Puglia A.M. Bradyrhizobium sp. nodulating the Mediterranean shrub Spanish broom (Spartium junceum L.) J. Appl. Microbiol. 2002;92:13–21. doi: 10.1046/j.1365-2672.2002.01485.x. [DOI] [PubMed] [Google Scholar]
- 190.Liu W.Y.Y. Ph.D. Thesis. Lincoln University; Lincoln, New Zealand: 2014. Characterisation of Rhizobia and Studies on N2 Fixation of Common Weed Legumes in New Zealand. [Google Scholar]
- 191.Beukes C.W., Venter S.N., Law I.J., Phalane F.L., Steenkamp E.T. South African papilionoid legumes are nodulated by diverse Burkholderia with unique nodulation and nitrogen-fixation loci. PLoS ONE. 2013;8:e68406. doi: 10.1371/journal.pone.0068406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Doignon-Bourcier F., Sy A., Willems A., Torck U., Dreyfus B., Gillis M., de Lajudie P. Diversity of bradyrhizobia from 27 tropical Leguminosae species native of Senegal. System. Appl. Microbiol. 1999;22:647–661. doi: 10.1016/S0723-2020(99)80018-6. [DOI] [PubMed] [Google Scholar]
- 193.Lemaire B., Chimphango S.B.M., Stirton C., Rafudeen S., Honnay O., Smets E., Chen W.-M., Sprent J., James E.K., Muasya A.M. Biogeographical patterns of legume-nodulating Burkholderia spp.: From African fynbos to continental scales. Appl. Environ. Microbiol. 2016;82:5099–5115. doi: 10.1128/AEM.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang W., Kong Z., Chen W., Wei G. Genetic diversity and symbiotic evolution of rhizobia from root nodules of Coronilla varia. Syst. Appl. Microbiol. 2013;36:49–55. doi: 10.1016/j.syapm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 195.Stępkowski T., Moulin L., Krzyżańska A., McInnes A., Law I.J., Howieson J. European origin of Bradyrhizobium populations infecting lupins and serradella in soils of Western Australia and South Africa. Appl. Environ. Microbiol. 2005;71:7041–7052. doi: 10.1128/AEM.71.11.7041-7052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kesari V., Ramesh A.M., Rangan L. Rhizobium pongamiae sp. nov. from root nodules of Pongamia pinnata. Biomed. Res. Int. 2013;2013:165198. doi: 10.1155/2013/165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lemaire B., van Cauwenberghe J., Verstraete B., Chimphango S., Stirton C., Honnay O., Smets E., Sprent J., James E.K., Muasya A.M. Characterization of the papilionoid-Burkholderia interaction in the Fynbos biome: The diversity and distribution of β-rhizobia nodulating Podalyria calyptrata (Fabaceae, Podalyrieae) Syst. Appl. Microbiol. 2016;39:41–48. doi: 10.1016/j.syapm.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 198.Ulrich A., Zaspel I. Phylogenetic diversity of rhizobial strains nodulating Robinia pseudoacacia L. Microbiology. 2000;146:2997–3005. doi: 10.1099/00221287-146-11-2997. [DOI] [PubMed] [Google Scholar]
- 199.Mierzwa B., Wdowiak-Wróbel S., Malek W. Phenotypic, genomic and phylogenetic characteristics of rhizobia isolated from root nodules of Robinia pseudoacacia (black locust) growing in Poland and Japan. Arch. Microbiol. 2009;191:697–710. doi: 10.1007/s00203-009-0500-0. [DOI] [PubMed] [Google Scholar]
- 200.Chen W.-M., Lee T.-M. Genetic and phenotypic diversity of rhizobial isolates from sugarcane-Sesbania cannabina-rotation fields. Biol. Fertil. Soils. 2001;34:14–20. doi: 10.1007/s003740100346. [DOI] [Google Scholar]
- 201.Wang Y.C., Wang F., Hou B.C., Wang E.T., Chen W.F., Sui X.H., Chen W.X., Li Y., Zhang Y.B. Proposal of Ensifer psoraleae sp. nov., Ensifer sesbaniae sp. nov., Ensifer morelense comb. nov. and Ensifer americanum comb. nov. Syst. Appl. Microbiol. 2013;36:467–473. doi: 10.1016/j.syapm.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 202.Li Y., Li X., Liu Y., Wang E.T., Ren C., Liu W., Xu H., Wu H., Jiang N., Li Y., Zhang X., Xie Z. Genetic diversity and community structure of rhizobia nodulating Sesbania cannabina in saline-alkaline soils. Syst. Appl. Microbiol. 2016;39:195–202. doi: 10.1016/j.syapm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 203.Wang E.T., van Berkum P., Beyene D., Sui X.H., Dorado O., Chen W.X., Martínez-Romero E. Rhizobium huautlense sp. nov., a symbiont of Sesbania herbacea that has a close phylogenetic relationship with Rhizobium galegae. Int. J. Syst. Bacteriol. 1998;48:687–699. doi: 10.1099/00207713-48-3-687. [DOI] [PubMed] [Google Scholar]
- 204.Blanco A.R., Csukasi F., Abreu C., Sicardi M. Characterization of rhizobia from Sesbania species native to seasonally wetland areas in Uruguay. Biol. Fertil. Soils. 2008;44:925–932. doi: 10.1007/s00374-008-0275-5. [DOI] [Google Scholar]
- 205.Dreyfus B., Garcia J.L., Gillis M. Characterisation of Azorhizobium caulinodans gen. nov., sp. nov., a stem nodulating nitrogen-fixing bacterium isolated from Sesbania rostrata. Int. J. Syst. Bacteriol. 1988;38:89–98. doi: 10.1099/00207713-38-1-89. [DOI] [Google Scholar]
- 206.De Moreira F.M.S., Cruz L., de Faria S.M., Marsh T., Martínez-Romero E., de Pedrosa F.O., Pitard R.M., Young J.P.W. Azorhizobium doebereinerae sp. nov. microsymbiont of Sesbania virgata (Caz.) Pers. Syst. Appl. Microbiol. 2006;29:197–206. doi: 10.1016/j.syapm.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 207.Zhao L., Deng Z., Yang W., Cao Y., Wang E., Wei G. Diverse rhizobia associated with Sophora alopecuroides grown in different regions of Loess Plateau in China. Syst. Appl. Microbiol. 2010;33:468–477. doi: 10.1016/j.syapm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 208.Jiao Y.S., Liu Y.H., Yan H., Wang E.T., Tian C.F., Chen W.X., Guo B.L., Chen W.F. Rhizobial diversity and nodulation characteristics of the extremely promiscuous legume Sophora flavescens. Mol. Plant Microbe Interact. 2015;28:1338–1352. doi: 10.1094/MPMI-06-15-0141-R. [DOI] [PubMed] [Google Scholar]
- 209.Jiao Y.S., Yan H., Ji Z.J., Liu Y.H., Sui X.H., Zhang X.X., Wang E.T., Chen W.X., Chen W.F. Phyllobacterium sophorae sp. nov., a symbiotic bacterium isolated from root nodules of Sophora flavescens. Int. J. Syst. Evol. Microbiol. 2015;65:399–406. doi: 10.1099/ijs.0.067017-0. [DOI] [PubMed] [Google Scholar]
- 210.Tan H.W., Heenan P.B., de Meyer S.E., Willems A., Andrews M. Diverse novel mesorhizobia nodulate New Zealand native Sophora species. Syst. Appl. Microbiol. 2015;38:91–98. doi: 10.1016/j.syapm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 211.Zhao L., Wang X., Huo H., Yuan G., Sun Y., Zhang D., Cao Y., Xu L., Wei G. Phylogenetic diversity of Ammopiptanthus rhizobia and distribution of rhizobia associated with Ammopiptanthus mongolicus in diverse regions of Northwest China. Microb. Ecol. 2016;72:231–239. doi: 10.1007/s00248-016-0759-z. [DOI] [PubMed] [Google Scholar]
- 212.Donate-Correa J., Léon-Barrios M., Hernández M., Pérez-Galdona R., del Arco-Aguilar M. Different Mesorhizobium species sharing the same symbiotic genes nodulate the shrub legume Anagyris latifolia. Syst. Appl. Microbiol. 2007;30:615–623. doi: 10.1016/j.syapm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 213.Ampomah O.Y., Huss-Danell K. Nodulation of Thermopsis lupinoides by a Mesorhizobium huakuii strain with a unique nodA gene in Kamtchatka, Russia. Appl. Environ. Microbiol. 2011;77:5513–5516. doi: 10.1128/AEM.00622-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bianco L., Angelini J., Fabra A., Malpassi R. Diversity and symbiotic effectiveness of indigenous rhizobia-nodulating Adesmia bicolor in soils of Central Argentina. Curr. Microbiol. 2013;66:174–184. doi: 10.1007/s00284-012-0260-y. [DOI] [PubMed] [Google Scholar]
- 215.Noisangiam R., Teamtisong K., Tittabutr P., Boonkerd N., Toshiki U., Minamisawa K., Teaumroong N. Genetic diversity, symbiotic evolution, and proposed infection process of Bradyrhizobium strains isolated from root nodules of Aeschynomene americana L. in Thailand. Appl. Environ. Microbiol. 2012;78:6236–6250. doi: 10.1128/AEM.00897-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Van Berkum P., Eardly B.D. The aquatic budding bacterium Blastobacter denitrificans is a nitrogen-fixing symbiont of Aeschynomene indica. Appl. Environ. Microbiol. 2002;68:1132–1136. doi: 10.1128/AEM.68.3.1132-1136.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Montecchia M.S., Kerber N.L., Pucheu N.L., Perticari A., García A.F. Analysis of genomic diversity among photosynthetic stem-nodulating rhizobial strains from Northeast Argentina. Syst. Appl. Microbiol. 2002;25:423–433. doi: 10.1078/0723-2020-00123. [DOI] [PubMed] [Google Scholar]
- 218.Chen J.Y., Gu J., Wang E.T., Ma X.X., Kang S.T., Huang L.Z., Cao X.P., Li L.B., Wu Y.L. Wild peanut Arachis duranensis are nodulated by diverse and novel Bradyrhizobium species in acid soils. Syst. Appl. Microbiol. 2014;37:525–532. doi: 10.1016/j.syapm.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 219.Urtz B.E., Elkan G.H. Genetic diversity among Bradyrhizobium isolates that effectively nodulate peanut (Arachis hypogaea) Can. J. Microbiol. 1996;42:1121–1130. doi: 10.1139/m96-144. [DOI] [PubMed] [Google Scholar]
- 220.Yang J.K., Xie F.L., Zou J., Zhou Q., Zhou J.C. Polyphasic characteristics of bradyrhizobia isolated from nodules of peanut (Arachis hypogaea) in China. Soil Biol. Biochem. 2005;37:141–153. doi: 10.1016/j.soilbio.2004.06.016. [DOI] [Google Scholar]
- 221.Taurian T., Ibañez F., Fabra A., Aguilar O.M. Genetic diversity of rhizobia nodulating Arachis hypogaea L. in Central Argentinean soils. Plant Soil. 2006;282:41–52. doi: 10.1007/s11104-005-5314-5. [DOI] [Google Scholar]
- 222.El-Akhal M.R., Rincón A., Arenal F., Lucas M.M., El Mourabit N., Barrijal S., Pueyo J.J. Genetic diversity and symbiotic efficiency of rhizobial isolates obtained from nodules of Arachis hypogaea in northwestern Morocco. Soil Biol. Biochem. 2008;40:2911–2914. doi: 10.1016/j.soilbio.2008.08.005. [DOI] [Google Scholar]
- 223.Steenkamp E.T., Stępkowski T., Przymusiak A., Botha W.J., Law I.J. Cowpea and peanut in southern Africa are nodulated by diverse Bradyrhizobium strains harboring nodulation genes that belong to the large pantropical clade common in Africa. Mol. Phylogenet. Evol. 2008;48:1131–1144. doi: 10.1016/j.ympev.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 224.Chang Y.L., Wang J.Y., Wang E.T., Liu H.C., Sui X.H., Chen W.X. Bradyrhizobium lablabi sp. nov., isolated from effective nodules of Lablab purpureus and Arachis hypogaea. Int. J. Syst. Evol. Microbiol. 2011;61:2496–2502. doi: 10.1099/ijs.0.027110-0. [DOI] [PubMed] [Google Scholar]
- 225.Muñoz V., Ibañez F., Tonelli M.L., Valetti L., Anzuay M.S., Fabra A. Phenotypic and phylogenetic characterization of native peanut Bradyrhizobium isolates obtained from Córdoba, Argentina. Syst. Appl. Microbiol. 2011;34:446–452. doi: 10.1016/j.syapm.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 226.Wang R., Chang Y.L., Zheng W.T., Zhang D., Zhang X.X., Sui X.H., Wang E.T., Hu J.Q., Zhang L.Y., Chen W.X. Bradyrhizobium arachidis sp. nov., isolated from effective nodules of Arachis hypogaea grown in China. Syst. Appl. Microbiol. 2013;36:101–105. doi: 10.1016/j.syapm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 227.Grönemeyer J.L., Kulkarni A., Berkelmann D., Hurek T., Reinhold-Hurek B. Rhizobia indigenous to the Okavango region in Sub-Saharan Africa: Diversity, adaptations, and host specificity. Appl. Environ. Microbiol. 2014;80:7244–7257. doi: 10.1128/AEM.02417-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li Y.H., Wang R., Zhang X.X., Young J.P.W., Wang E.T., Sui X.H., Chen W.X. Bradyrhizobium guangdongense sp. nov. and Bradyrhizobium guangxiense sp. nov., isolated from effective nodules of peanut. Int. J. Syst. Evol. Microbiol. 2015;65:4655–4661. doi: 10.1099/ijsem.0.000629. [DOI] [PubMed] [Google Scholar]
- 229.Chen J., Hu M., Ma H., Wang Y., Wang E.T., Zhou Z., Gu J. Genetic diversity and distribution of bradyrhizobia nodulating peanut in acid-neutral soils in Guangdong Province. Syst. Appl. Microbiol. 2016;39:418–427. doi: 10.1016/j.syapm.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 230.Baraúna A.C., da Silva K., Pereira G.M.D., Kaminski P.E., Perin L., Zilli J.E. Diversity and nitrogen fixation efficiency of rhizobia isolated from nodules of Centrolobium paraense. Pesq. Agropecu. Bras. 2014;49:296–305. doi: 10.1590/S0100-204X2014000400008. [DOI] [Google Scholar]
- 231.Zilli J.E., Baraúna A.C., da Silva K., de Meyer S.E., Farias E.N.C., Kaminski P.E., da Costa I.B., Ardley J.K., Willems A., Camacho N.N., et al. Bradyrhizobium neotropicale sp. nov., isolate from effective nodules of Centrolobium paraense. Int. J. Syst. Evol. Microbiol. 2014;64:3950–3957. doi: 10.1099/ijs.0.065458-0. [DOI] [PubMed] [Google Scholar]
- 232.Rasolomampianina R., Bailly X., Fetiarison R., Rabevohitra R., Béna G., Ramaroson L., Raherimandimby M., Moulin L., de Lajudie P., Dreyfus B., et al. Nitrogen-fixing nodules from rose wood legume trees (Dalbergia spp.) endemic to Madagascar host seven different genera belonging to α- and β-proteobacteria. Mol. Ecol. 2005;14:4135–4146. doi: 10.1111/j.1365-294X.2005.02730.x. [DOI] [PubMed] [Google Scholar]
- 233.Le Roux C., Muller F., Bouvet J.-M., Dreyfus B., Béna G., Galiana A., Bâ A.M. Genetic diversity patterns and functional traits of Bradyrhizobium strains associated with Pterocarpus officinalis Jacq. in Caribbean islands and Amazonian forest (French Guiana) Microb. Ecol. 2014;68:329–338. doi: 10.1007/s00248-014-0392-7. [DOI] [PubMed] [Google Scholar]
- 234.Gueye F., Moulin L., Sylla S., Ndoye I., Béna G. Genetic diversity and distribution of Bradyrhizobium and Azorhizobium strains associated with the herb legume Zornia glochidiata sampled from across Senegal. Syst. Appl. Microbiol. 2009;32:387–399. doi: 10.1016/j.syapm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 235.Gu J., Wang E.T., Chen W.X. Genetic diversity of rhizobia associated with Desmodium species grown in China. Lett. Appl. Microbiol. 2007;44:286–292. doi: 10.1111/j.1472-765X.2006.02071.x. [DOI] [PubMed] [Google Scholar]
- 236.Xu K.W., Zou L., Penttinen P., Zeng X., Liu M., Zhao K., Chen C., Chen Y.X., Zhang X. Diversity and phylogeny of rhizobia associated with Desmodium spp. in Panxi, Sichuan, China. Syst. Appl. Microbiol. 2016;39:33–40. doi: 10.1016/j.syapm.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 237.Delamuta J.R.M., Ribeiro R.A., Ormeño-Orrillo E., Parma M.M., Melo I.S., Martínez-Romero E., Hungria M. Bradyrhizobium tropiciagri sp. nov. and Bradyrhizobium embrapense sp. nov., nitrogen-fixing symbionts of tropical forage legumes. Int. J. Syst. Evol. Microbiol. 2015;65:4424–4433. doi: 10.1099/ijsem.0.000592. [DOI] [PubMed] [Google Scholar]
- 238.Chen W.-X., Tan Z.-Y., Gao J.-L., Li Y., Wang E.-T. Rhizobium hainanense sp. nov., isolated from tropical legumes. Int. J. Syst. Bacteriol. 1997;47:870–873. doi: 10.1099/00207713-47-3-870. [DOI] [PubMed] [Google Scholar]
- 239.Lin D.X., Man C.X., Wang E.T., Chen W.X. Diverse rhizobia that nodulate two species of Kummerowia in China. Arch. Microbiol. 2007;188:495–507. doi: 10.1007/s00203-007-0271-4. [DOI] [PubMed] [Google Scholar]
- 240.Yao Z.Y., Kan F.L., Wang E.T., Wei G.H., Chen W.X. Characterization of rhizobia that nodulate legume species of the genus Lespedeza and description of Bradyrhizobium yuanmingense sp. nov. Int. J. Syst. Evol. Microbiol. 2002;52:2219–2230. doi: 10.1099/00207713-52-6-2219. [DOI] [PubMed] [Google Scholar]
- 241.Parker M.A., Doyle J.L., Doyle J.J. Comparative phylogeography of Amphicarpaea legumes and their root-nodule symbionts in Japan and North America. J. Biogeogr. 2004;31:425–434. doi: 10.1046/j.0305-0270.2003.01030.x. [DOI] [Google Scholar]
- 242.Araujo J., Díaz-Alcántara C.-A., Velázquez E., Urbano B., González-Andrés F. Bradyrhizobium yuanmingense related strains form nitrogen-fixing symbiosis with Cajanus cajan L. in Dominican Republic and are efficient biofertilizers to replace N fertilization. Sci. Hort. 2015;192:421–428. doi: 10.1016/j.scienta.2015.06.009. [DOI] [Google Scholar]
- 243.Chen W.-M., Lee T.-M., Lan C.-C., Cheng C.-P. Characterization of halotolerant rhizobia isolated from root nodules of Canavalia rosea from seaside areas. FEMS Microbiol. Ecol. 2000;34:9–16. doi: 10.1111/j.1574-6941.2000.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 244.Xu L.M., Ge C., Cui Z., Li J., Fan H. Bradyrhizobium liaoningense sp. nov., isolated from the root nodules of soybeans. Int. J. Syst. Bacteriol. 1995;45:706–711. doi: 10.1099/00207713-45-4-706. [DOI] [PubMed] [Google Scholar]
- 245.Barcellos F.G., Menna P., da Batista J.S.S., Hungria M. Evidence of horizontal transfer of symbiotic genes from a Bradyrhizobium japonicum inoculant strain to indigenous diazotrophs Sinorhizobium (Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian savannah soil. Appl. Environ. Microbiol. 2007;73:2635–2643. doi: 10.1128/AEM.01823-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Appunu C., Sasirekha N., Prabavathy V.R., Nair S. A significant proportion of indigenous rhizobia from India associated with soybean (Glycine max L.) distinctly belong to Bradyrhizobium and Ensifer genera. Biol. Fertil. Soils. 2009;46:57–63. doi: 10.1007/s00374-009-0405-8. [DOI] [Google Scholar]
- 247.Zhang Y.M., Li Y., Jr., Chen W.F., Wang E.T., Tian C.F., Li Q.Q., Zhang Y.Z., Sui X.H., Chen W.X. Biodiversity and biogeography of rhizobia associated with soybean plants grown in the North China Plain. Appl. Environ. Microbiol. 2011;77:6331–6342. doi: 10.1128/AEM.00542-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jaiswal S.K., Anand A., Dhar B., Vaishampayan A. Genotypic characterization of phage-typed indigenous soybean Bradyrhizobia and their host range symbiotic effectiveness. Microb. Ecol. 2012;63:116–126. doi: 10.1007/s00248-011-9950-4. [DOI] [PubMed] [Google Scholar]
- 249.Tang J., Bromfield E.S.P., Rodrigue N., Cloutier S., Tambong J.T. Microevolution of symbiotic Bradyrhizobium populations associated with soybeans in east North America. Ecol. Evol. 2012;2:2943–2961. doi: 10.1002/ece3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang J.Y., Wang R., Zhang Y.M., Liu H.C., Chen W.F., Wang E.T., Sui X.H., Chen W.X. Bradyrhizobium daqingense sp. nov., isolated from soybean nodules. Int. J. Syst. Evol. Microbiol. 2013;63:616–624. doi: 10.1099/ijs.0.034280-0. [DOI] [PubMed] [Google Scholar]
- 251.Ribeiro R.A., Ormeño-Orrillo E., Dall’Agnol R.F., Graham P.H., Martinez-Romero E., Hungria M. Novel Rhizobium lineages isolated from root nodules of the common bean (Phaseolus vulgaris L.) in Andean and Mesoamerican areas. Res. Microbiol. 2013;164:740–748. doi: 10.1016/j.resmic.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 252.Peng G.X., Tan Z.Y., Wang E.T., Reinhold-Hurek B., Chen W.F., Chen W.X. Identification of isolates from soybean nodules in Xinjiang Region as Sinorhizobium xinjiangense and genetic differentiation of S. xinjiangense from Sinorhizobium fredii. Int. J. Syst. Evol. Microbiol. 2002;52:457–462. doi: 10.1099/00207713-52-2-457. [DOI] [PubMed] [Google Scholar]
- 253.Li Q.Q., Wang E.T., Chang Y.L., Zhang Y.Z., Zhang Y.M., Sui X.H., Chen W.F., Chen W.X. Ensifer sojae sp. nov., isolated from root nodules of Glycine max grown in saline-alkaline soils. Int. J. Syst. Evol. Microbiol. 2011;61:1981–1988. doi: 10.1099/ijs.0.025049-0. [DOI] [PubMed] [Google Scholar]
- 254.Alam F., Bhuiyan M.A.H., Alam S.S., Waghmode T.R., Kim P.J. Effect of Rhizobium sp. BARIRGm901 inoculation on nodulation, nitrogen fixation and yield of soybean (Glycine max) genotypes in gray terrace soil. Biosci. Biotechnol. Biochem. 2015:1660–1668. doi: 10.1080/09168451.2015.1044931. [DOI] [PubMed] [Google Scholar]
- 255.Wu L.J., Wang H.Q., Wang E.T., Chen W.X., Tian C.F. Genetic diversity of nodulating and non-nodulating rhizobia associated with wild soybean (Glycine soja Sieb. & Zucc.) in different ecoregions of China. FEMS Microbiol. Ecol. 2011;76:439–450. doi: 10.1111/j.1574-6941.2011.01064.x. [DOI] [PubMed] [Google Scholar]
- 256.Zhao L., Fan M., Zhang D., Yang R., Zhang F., Xu L., Wei X., Shen Y., Wei G. Distribution and diversity of rhizobia associated with wild soybean (Glycine soja Sieb. & Zucc.) in Northwest China. Syst. Appl. Microbiol. 2014;37:449–456. doi: 10.1016/j.syapm.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 257.Fuentes J.B., Abe M., Uchiumi T., Suzuki A., Higashi S. Symbiotic root nodule bacteria isolated from yam bean (Pachyrhizus erosus) J. Gen. Appl. Microbiol. 2002;48:181–191. doi: 10.2323/jgam.48.181. [DOI] [PubMed] [Google Scholar]
- 258.Rodríguez-Navarro D.N., Camacho M., Leidi E.O., Rivas R., Velázquez E. Phenotypic and genotypic characterization of rhizobia from diverse geographical origin that nodulate Pachyrhizus species. Syst. Appl. Microbiol. 2004;27:737–745. doi: 10.1078/0723202042369839. [DOI] [PubMed] [Google Scholar]
- 259.Ramírez-Bahena M.H., Peix A., Rivas R., Camacho M., Rodríguez-Navarro D.N., Mateos P.F., Martínez-Molina E., Willems A., Velázquez E. Bradyrhizobium pachyrhizi sp. nov. and Bradyrhizobium jicamae sp. nov., isolated from effective nodules of Pachyrhizus erosus. Int. J. Syst. Evol. Microbiol. 2009;59:1929–1934. doi: 10.1099/ijs.0.006320-0. [DOI] [PubMed] [Google Scholar]
- 260.López-López A., Negrete-Yankelevich S., Rogel M.A., Ormeño-Orrillo E., Martínez J., Martínez-Romero E. Native bradyrhizobia from Los Tuxtlas in Mexico are symbionts of Phaseolus lunatus (Lima bean) Syst. Appl. Microbiol. 2013;36:33–38. doi: 10.1016/j.syapm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 261.Durán D., Rey L., Mayo J., Zúñiga-Dávila D., Imperial J., Ruiz-Argüeso T., Martínez-Romero E., Ormeño-Orrillo E. Bradyrhizobium paxllaeri sp. nov. and Bradyrhizobium icense sp. nov., nitrogen-fixing rhizobial symbionts of Lima bean (Phaseolus lunatus L.) in Peru. Int. J. Syst. Evol. Microbiol. 2014;64:2072–2078. doi: 10.1099/ijs.0.060426-0. [DOI] [PubMed] [Google Scholar]
- 262.Matsubara M., Zúñiga-Dávila D. Phenotypic and molecular differences among rhizobia that nodulate Phaseolus lunatus in the Supe valley in Peru. Ann. Microbiol. 2015;65:1803–1808. doi: 10.1007/s13213-015-1054-9. [DOI] [Google Scholar]
- 263.Wang L., Cao Y., Wang E.T., Qiao Y.J., Jiao S., Liu Z.S., Zhao L., Wei G.H. Biodiversity and biogeography of rhizobia associated with common bean (Phaseolus vulgaris L.) in Shaanxi Province. Syst. Appl. Microbiol. 2016;39:211–219. doi: 10.1016/j.syapm.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 264.Talbi C., Delgado M.J., Girard L., Ramirez-Trujillo A., Caballero-Mellado J., Bedmar E.J. Burkholderia phymatum strains capable of nodulating Phaseolus vulgaris are present in Moroccan soils. Appl. Environ. Microbiol. 2010;76:4587–4591. doi: 10.1128/AEM.02886-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Dall’Agnol R.F., Plotegher F., Souza R.C., Mendes I.C., dos Reis Junior F.B., Béna G., Moulin L., Hungria M. Paraburkholderia nodosa is the main N2-fixing species trapped by promiscuous common bean (Phaseolus vulgaris L.) in the Brazilian “Cerradão”. FEMS Microbiol. Ecol. 2016;92:fiw108. doi: 10.1093/femsec/fiw108. [DOI] [PubMed] [Google Scholar]
- 266.Mhamdi R., Laguerre G., Aouani M.E., Mars M., Amarger N. Different species and symbiotic genotypes of field rhizobia can nodulate Phaseolus vulgaris in Tunisian soils. FEMS Microbiol. Ecol. 2002;41:77–84. doi: 10.1111/j.1574-6941.2002.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 267.Mnasri B., Mrabet M., Laguerre G., Aouani M.E., Mhamdi R. Salt-tolerant rhizobia isolated from a Tunisian oasis that are highly effective for symbiotic N2-fixation with Phaseolus vulgaris constitute a novel biovar (bv. mediterranense) of Sinorhizobium meliloti. Arch. Microbiol. 2007;187:79–85. doi: 10.1007/s00203-006-0173-x. [DOI] [PubMed] [Google Scholar]
- 268.Mnasri B., Saïdi S., Chihaoui S.-A., Mhamdi R. Sinorhizobium americanum symbiovar mediterranense is a predominant symbiont that nodulates and fixes nitrogen with common bean (Phaseolus vulgaris L.) in a Northern Tunisian field. Syst. Appl. Microbiol. 2012;35:263–269. doi: 10.1016/j.syapm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 269.Martínez-Romero E., Segovia L., Mercante F.M., Franco A.A., Graham P., Pardo M.A. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int. J. System. Bacteriol. 1991;41:417–426. doi: 10.1099/00207713-41-3-417. [DOI] [PubMed] [Google Scholar]
- 270.Amarger N., Macheret V., Laguerre G. Rhizobium gallicum sp. nov. and Rhizobium giardinii sp. nov., from Phaseolus vulgaris nodules. Int. J. Syst. Bacteriol. 1997;47:996–1006. doi: 10.1099/00207713-47-4-996. [DOI] [PubMed] [Google Scholar]
- 271.Mostasso L., Mostasso F.L., Dias B.G., Vargas M.A.T., Hungria M. Selection of bean (Phaseolus vulgaris L.) rhizobial strains for the Brazilian Cerrados. Field Crops Res. 2002;73:121–132. doi: 10.1016/S0378-4290(01)00186-1. [DOI] [Google Scholar]
- 272.Valverde A., Igual J.M., Peix A., Cervantes E., Velázquez E. Rhizobium lusitanum sp. nov. a bacterium that nodulates Phaseolus vulgaris. Int. J. System. Evol. Microbiol. 2006;56:2631–2637. doi: 10.1099/ijs.0.64402-0. [DOI] [PubMed] [Google Scholar]
- 273.Wang F., Wang E.T., Wu L.J., Sui X.H., Li Y., Jr., Chen W.X. Rhizobium vallis sp. nov., isolated from nodules of three leguminous species. Int. J. Syst. Evol. Microbiol. 2011;61:2582–2588. doi: 10.1099/ijs.0.026484-0. [DOI] [PubMed] [Google Scholar]
- 274.De Ribeiro P.R.A., dos Santos J.V., da Costa E.M., Lebbe L., Assis E.S., Louzada M.O., Guimarães A.A., Willems A., de Moreira F.M.S. Symbiotic efficiency and genetic diversity of soybean bradyrhizobia in Brazilian soils. Agric. Ecosyst. Environ. 2015;212:85–93. doi: 10.1016/j.agee.2015.06.017. [DOI] [Google Scholar]
- 275.Cao Y., Wang E.-T., Zhao L., Chen W.-M., Wei G.-H. Diversity and distribution of rhizobia nodulated with Phaseolus vulgaris in two ecoregions of China. Soil Biol. Biochem. 2014;78:128–137. doi: 10.1016/j.soilbio.2014.07.026. [DOI] [Google Scholar]
- 276.Sarr P.S., Araki S., Begoude D.A., Yemefack M., Manga G.A., Yamakawa T., Htwe A.Z. Phylogeny and nitrogen fixation potential of Bradyrhizobium species isolated from the legume cover crop Pueraria phaseoloides (Roxb.) Benth. in Eastern Cameroon. Soil Sci. Plant Nutr. 2016;62:13–19. doi: 10.1080/00380768.2015.1086279. [DOI] [Google Scholar]
- 277.Garau G., Yates R.J., Deiana P., Howieson J.G. Novel strains of nodulating Burkholderia have a role in nitrogen fixation with papilionoid herbaceous legumes adapted to acid, infertile soils. Soil Biol. Biochem. 2009;41:125–134. doi: 10.1016/j.soilbio.2008.10.011. [DOI] [Google Scholar]
- 278.De Meyer S.E., Cnockaert M., Ardley J.K., Trengove R.D., Garau G., Howieson J.G., Vandamme P. Burkholderia rhynchosiae sp. nov., isolated from Rhynchosia ferulifolia root nodules. Int. J. Syst. Evol. Microbiol. 2013;63:3944–3949. doi: 10.1099/ijs.0.048751-0. [DOI] [PubMed] [Google Scholar]
- 279.Han L.L., Wang E.T., Lu Y.L., Zhang Y.F., Sui X.H., Chen W.F., Chen W.X. Bradyrhizobium spp. and Sinorhizobium fredii are predominant in root nodules of Vigna angularis, a native legume crop in the subtropical region of China. J. Microbiol. 2009;47:287–296. doi: 10.1007/s12275-009-0001-5. [DOI] [PubMed] [Google Scholar]
- 280.Zhang Y.F., Wang E.T., Tian C.F., Wang F.Q., Han L.L., Chen W.F., Chen W.X. Bradyrhizobium elkanii, Bradyrhizobium yuanmingense and Bradyrhizobium japonicum are the main rhizobia associated with Vigna unguiculata and Vigna radiata in the subtropical region of China. FEMS Microbiol. Lett. 2008;285:146–154. doi: 10.1111/j.1574-6968.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 281.Risal C.P., Djedidi S., Dhakal D., Ohkama-Ohtsu N., Sekimoto H., Yokoyama T. Phylogenetic diversity and symbiotic functioning in mungbean (Vigna radiata L. Wilczek) bradyrhizobia from contrast agro-ecological regions of Nepal. Syst. Appl. Microbiol. 2012;35:45–53. doi: 10.1016/j.syapm.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 282.Onyango B., Anyango B., Nyunja R., Koech P.K., Skilton R.A., Stomeo F. Morphological, genetic and symbiotic characterization of root nodule bacteria isolated from Bambara groundnuts (Vigna subterranea L. Verdc) from soils of Lake Victoria basin, western Kenya. J. Appl. Biol. Biotechnol. 2015;3:1–10. [Google Scholar]
- 283.Guimarães A.A., Jaramillo P.M.D., Nóbrega R.S.A., Florentino L.A., Silva K.B., de Moreira F.M.S. Genetic and symbiotic diversity of nitrogen-fixing bacteria isolated from agricultural soils in the Western Amazon by using cowpea as the trap plant. Appl. Environ. Microbiol. 2012;78:6726–6733. doi: 10.1128/AEM.01303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bejarano A., Ramírez-Bahena M.-H., Velázquez E., Peix A. Vigna unguiculata is nodulated in Spain by endosymbionts of Genisteae legumes and by a new symbiovar (vignae) of the genus Bradyrhizobium. Syst. Appl. Microbiol. 2014;37:533–540. doi: 10.1016/j.syapm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 285.Silva F.V., de Meyer S.E., Simões-Araújo J.L., da Barbé T.C., Xavier G.R., O’Hara G., Ardley J.K., Rumjanek N.G., Willems A., Zilli J.E. Bradyrhizobium manausense sp. nov., isolated from effective nodules of Vigna unguiculata grown in Brazilian Amazonian rainforest soils. Int. J. Syst. Evol. Microbiol. 2014;64:2358–2363. doi: 10.1099/ijs.0.061259-0. [DOI] [PubMed] [Google Scholar]
- 286.Radl V., Simões-Araújo J.L., Leite J., Passos S.R., Martins L.M.V., Xavier G.R., Rumjanek N.G., Baldani J.I., Zilli J.E. Microvirga vignae sp. nov., a root nodule symbiotic bacterium isolated from cowpea grown in semi-arid Brazil. Int. J. Syst. Evol. Microbiol. 2014;64:725–730. doi: 10.1099/ijs.0.053082-0. [DOI] [PubMed] [Google Scholar]
- 287.Kanu S.A., Dakora F.D. Symbiotic nitrogen contribution and biodiversity of root-nodule bacteria nodulating Psoralea species in the Cape Fynbos, South Africa. Soil Biol. Biochem. 2012;54:68–76. doi: 10.1016/j.soilbio.2012.05.017. [DOI] [Google Scholar]
- 288.Lorite M.J., Donate-Correa J., del Arco-Aguilar M., Galdona R.P., Sanjuán J., Léon-Barrios M. Lotus endemic to the Canary Islands are nodulated by diverse and novel rhizobial species and symbiotypes. Syst. Appl. Microbiol. 2010;33:282–290. doi: 10.1016/j.syapm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 289.Jarvis B.D.W., van Berkum P., Chen W.X., Nour S.M., Fernandez M.P., Cleyet-Marel J.C., Gillis M. Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, and Rhizobium tianshanense to Mesorhizobium gen. nov. Int. J. Syst. Bacteriol. 1997;47:895–898. doi: 10.1099/00207713-47-3-895. [DOI] [Google Scholar]
- 290.Lorite M.J., Muñoz S., Olivares J., Soto M.J., Sanjuán J. Characterization of strains unlike Mesorhizobium loti that nodulate Lotus spp. in saline soils of Granada, Spain. Appl. Environ. Microbiol. 2010;76:4019–4026. doi: 10.1128/AEM.02555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Marcos-García M., Menéndez E., Cruz-González X., Velázquez E., Mateos P.F., Rivas R. The high diversity of Lotus corniculatus endosymbionts in soils of northwest Spain. Symbiosis. 2015;67:11–20. doi: 10.1007/s13199-015-0368-5. [DOI] [Google Scholar]
- 292.Léon-Barrios M., Lorite M.J., Donate-Correa J., Sanjuán J. Ensifer meliloti bv. lancerottense establishes nitrogen-fixing symbiosis with Lotus endemic to the Canary Islands and shows distinctive symbiotic genotypes and host range. Syst. Appl. Microbiol. 2009;32:413–420. doi: 10.1016/j.syapm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 293.Estrella M.J., Muñoz S., Soto M.J., Ruiz O., Sanjuán J. Genetic diversity and host range of rhizobia nodulating Lotus tenuis in typical soils of the Salado River basin (Argentina) Appl. Environ. Microbiol. 2009;75:1088–1098. doi: 10.1128/AEM.02405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sannazzaro A.I., Bergottini V.M., Paz R.C., Castagno L.N., Menéndez A.B., Ruiz O.A., Pieckenstain F.L., Estrella M.J. Comparative symbiotic performance of native rhizobia of the Flooding Pampa and strains currently used for inoculating Lotus tenuis in this region. Antonie van Leeuwenhoek. 2011;99:371–379. doi: 10.1007/s10482-010-9502-9. [DOI] [PubMed] [Google Scholar]
- 295.Lorite M.J., Videira e Castro I., Muñoz S., Sanjuán J. Phylogenetic relationship of Lotus uliginosus symbionts with bradyrhizobia nodulating genistoid legumes. FEMS Microbiol. Ecol. 2012;79:454–464. doi: 10.1111/j.1574-6941.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- 296.Lindström K. Rhizobium galegae, a new species of legume root nodule bacteria. Int. J. Syst. Bacteriol. 1989;39:365–367. doi: 10.1099/00207713-39-3-365. [DOI] [Google Scholar]
- 297.Franche C., Lindström K., Elmerich C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil. 2009;321:35–59. doi: 10.1007/s11104-008-9833-8. [DOI] [Google Scholar]
- 298.Wojciechowski M.F., Lavin M., Sanderson M.J. A phylogeny of legumes (Leguminosae) based on analysis of the plastid MATK gene resolves many well-supported subclades within the family. Am. J. Bot. 2004;91:1846–1862. doi: 10.3732/ajb.91.11.1846. [DOI] [PubMed] [Google Scholar]
- 299.Schwarz E.N., Ruhlman T.A., Sabir J.S.M., Hajirah N.H., Alharbi N.S., Al-Malki A.L., Bailey C.D., Jansen R.K. Plastid genome sequences of legumes reveal parallel inversions and multiple losses of rps16 in papilionoids. J. Syst. Evol. 2015;53:458–468. doi: 10.1111/jse.12179. [DOI] [Google Scholar]
- 300.Martínez-Romero E. Diversity of Rhizobium-Phaseolus vulgaris symbiosis: Overview and perspectives. Plant Soil. 2003;252:11–23. doi: 10.1023/A:1024199013926. [DOI] [Google Scholar]
- 301.Oren A., Garrity G.M. List of new names and new combinations previously effectively, but not validly, published. Int. J. Syst. Evol. Microbiol. 2015;65:2017–2025. doi: 10.1099/ijs.0.000317. [DOI] [PubMed] [Google Scholar]
- 302.Dobritsa A.P., Samadpour M. Transfer of eleven Burkholderia species to the genus Paraburkholderia and proposal of Caballeronia gen. nov., a new genus to accommodate twelve species of Burkholderia and Paraburkholderia. Int. J. Syst. Evol. Microbiol. 2016;66:2836–2846. doi: 10.1099/ijsem.0.001065. [DOI] [PubMed] [Google Scholar]
- 303.Rogel M.A., Ormeño-Orrillo E., Martinez-Romero E. Symbiovars in rhizobia reflect bacterial adaptation to legumes. Syst. Appl. Microbiol. 2011;34:96–104. doi: 10.1016/j.syapm.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 304.Andrews M., Hodge S., Raven J.A. Positive plant microbial interactions. Ann. Appl. Biol. 2010;157:317–321. doi: 10.1111/j.1744-7348.2010.00440.x. [DOI] [Google Scholar]
- 305.Fonseca M.B., Peix A., de Faria S.M., Mateos P.F., Rivera L.P., Simões-Araujo J.L., França M.G.C., dos Isaias R.M.S., Cruz C., Velázquez E., et al. Nodulation in Dimorphandra wilsonii Rizz. (Caesalpinioideae), a threatened species native to the Brazilian Cerrado. PLoS ONE. 2012;7:e49520. doi: 10.1371/journal.pone.0049520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yao Y., Wang R., Lu J.K., Sui X.H., Wang E.T., Chen W.X. Genetic diversity and evolution of Bradyrhizobium populations nodulating Erythrophleum fordii, an evergreen tree indigenous to the southern subtropical region of China. Appl. Environ. Microbiol. 2014;80:6184–6194. doi: 10.1128/AEM.01595-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Parker M.A. Divergent Bradyrhizobium symbionts on Tachigali versicolor from Barro Colorado Island, Panama. Syst. Appl. Microbiol. 2000;23:585–590. doi: 10.1016/S0723-2020(00)80034-X. [DOI] [PubMed] [Google Scholar]
- 308.Diabate M., Munive A., de Faria S.M., Ba A., Dreyfus B., Galiana A. Occurrence of nodulation in unexplored leguminous trees native to the West African tropical rainforest and inoculation response of native species useful in reforestation. New Phytol. 2005;166:231–239. doi: 10.1111/j.1469-8137.2005.01318.x. [DOI] [PubMed] [Google Scholar]
- 309.Lafay B., Burdon J.J. Molecular diversity of legume root-nodule bacteria in Kakadu National Park, Northern Territory, Australia. PLoS ONE. 2007;3:e277. doi: 10.1371/journal.pone.0000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Michalk D.L., Zhi-Kai H. Grassland improvement in subtropical Guangdong province, China. 1. Evaluation of pasture legumes. Trop. Grassl. 1994;28:129–138. [Google Scholar]
- 311.Keller K.R. Mutualistic rhizobia reduce plant diversity and alter community composition. Oecologia. 2014;176:1101–1109. doi: 10.1007/s00442-014-3089-1. [DOI] [PubMed] [Google Scholar]
- 312.Australian Centre for International Agricultural Research Forages fact sheet. [(accessed on 19 June 2016)]; Available online: www.tropicalforages.info.
- 313.Sullivan J.T., Ronson C.W. Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proc. Natl. Acad. Sci. USA. 1998;95:5145–5149. doi: 10.1073/pnas.95.9.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Saidi S., Ramírez-Bahena M.H., Santillana N., Zúñiga D., Álvarez-Martínez E.R., Peix A., Mhamdi R., Velázquez E. Rhizobium laguerreae sp. nov. nodulates Vicia faba on several continents. Int. J. Syst. Evol. Microbiol. 2014;64:242–247. doi: 10.1099/ijs.0.052191-0. [DOI] [PubMed] [Google Scholar]
- 315.Miller S.H., Elliot R.M., Sullivan J.T., Ronson C.W. Host-specific regulation of symbiotic nitrogen fixation in Rhizobium leguminosarum biovar trifolii. Microbiology. 2007;153:3184–3195. doi: 10.1099/mic.0.2007/006924-0. [DOI] [PubMed] [Google Scholar]
- 316.De Meyer S.E., Tan H.W., Heenan P.B., Andrews M., Willems A. Mesorhizobium waimense sp. nov. isolated from Sophora longicarinata root nodules and Mesorhizobium cantuariense sp. nov. isolated from Sophora microphylla root nodules in New Zealand. Int. J. Syst. Evol. Microbiol. 2015;65:3419–3426. doi: 10.1099/ijsem.0.000430. [DOI] [PubMed] [Google Scholar]
- 317.De Meyer S.E., Tan H.W., Andrews M., Heenan P.B., Willems A. Mesorhizobium calcicola sp. nov., Mesorhizobium waitakense sp. nov., Mesorhizobium sophorae sp. nov., Mesorhizobium newzealandense sp. nov. and Mesorhizobium kowhaii sp. nov. isolated from Sophora root nodules in New Zealand. Int. J. Syst. Evol. Microbiol. 2016;66:786–795. doi: 10.1099/ijsem.0.000796. [DOI] [PubMed] [Google Scholar]
- 318.Perret X., Staehelin C., Broughton W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Bio. Rev. 2000;64:180–201. doi: 10.1128/MMBR.64.1.180-201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Davis E.O., Evans I.J., Johnston A.W. Identification of nodX, a gene that allows Rhizobium leguminosarum biovar viciae strain TOM to nodulate Afghanistan peas. Mol. Gen. Genet. 1988;212:531–535. doi: 10.1007/BF00330860. [DOI] [PubMed] [Google Scholar]
- 320.Firmin J.L., Wilson K.E., Carlson R.W., Davies A.E., Downie J.A. Resistance to nodulation of cv. Afghanistan peas is overcome by nodX, which mediates an O-acetylation of the Rhizobium leguminosarum lipo-oligosaccharide nodulation factor. Mol. Microbiol. 1993;10:351–360. doi: 10.1111/j.1365-2958.1993.tb01961.x. [DOI] [PubMed] [Google Scholar]
- 321.Geurts R., Heidstra R., Hadri A.E., Downie J.A., Franssen H., van Kammen A., Bisseling T. Sym2 of pea is involved in a nodulation factor-perception mechanism that controls the infection process in the epidermis. Plant Physiol. 1997;115:351–359. doi: 10.1104/pp.115.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ehinger M., Mohr T.J., Starcevich J.B., Sachs J.L., Porter S.S., Simms E.L. Specialisation-generalisation trade off in a Bradyrhizobium symbiosis with wild legume hosts. BMC Ecol. 2014;14:8. doi: 10.1186/1472-6785-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]