Abstract
The obligatory role of follicle-stimulating hormone (FSH) in normal development and function of ovarian antral follicles is well recognized, but its function in preantral growth is less clear. The specific objective of this study was to investigate the response, in culture, to FSH of mouse preantral follicles of increasing size, focusing particularly on growth rate and gene expression. Preantral follicles were mechanically isolated from ovaries of C57BL/6 mice, 12 to 16 days postpartum, and single follicles cultured for up to 96 hours in medium alone (n = 511) or with recombinant human FSH 10 ng/mL (n = 546). Data were grouped according to initial follicle diameter in 6 strata ranging from <100 to >140 μm. Follicles of all sizes grew in the absence of FSH (P < 0.01, paired t test). All follicles grew at a faster rate (P < 0.0001) in the presence of 10 ng/mL FSH but larger follicles showed the greatest change in response to FSH. Even the smallest follicles expressed FSH receptor messenger RNA (mRNA). FSH-induced growth was inhibited by KT5720, an inhibitor of protein kinase A (PKA), implicating the PKA pathway in FSH-induced follicle growth. In response to FSH in vitro, FSH receptor mRNA (measured by quantitative polymerase chain reaction) was reduced (P < 0.01), as was Amh (P < 0.01), whereas expression of StAR (P < 0.0001) and the steroidogenic enzymes Cyp11a1 (P < 0.01) and Cyp19 (P < 0.0001) was increased. These results show heterogeneous responses to FSH according to initial follicle size, smaller follicles being less FSH dependent than larger preantral follicles. These findings strongly suggest that FSH has a physiological role in preantral follicle growth and function.
Follicle-stimulating hormone (FSH) is essential for antral follicle development in the mammalian ovary, but its role in preantral growth is unclear. Depletion of FSH in genetically hypogonadotropic mice (1, 2), following hypophysectomy (3, 4) or by pharmacological suppression of FSH (5, 6) in mice or sheep, results in depletion of antral follicles and arrest of follicle development in the late preantral stages. Similarly, in mice with mutation of the FSH receptor gene (Fshr), follicle development does not progress beyond the multilayered preantral stage (2, 7, 8). In humans, the rarely reported inactivating mutations of either the β subunit of FSH (9–11) or the FSHR gene (10) are associated with a lack of mature antral follicles, estrogen deficiency and amenorrhea.
There is also plentiful evidence that FSH can influence the development of preantral follicles both in vivo and using in vitro culture of ovarian tissue explants or isolated, multilayered preantral follicles. FSH administered in vivo to mouse (1), rat (5) or sheep (6) promotes growth and enhances survival of preantral follicles. Similar effects have been noted when FSH is added to cultures of preantral follicles derived from mouse (12–17), sheep (18), and human (19). FSH binding sites have been demonstrated in preantral follicles in ovaries of rodents (20) and sheep (21), whereas FSHR gene expression has been shown in rodent (18, 22) and in human (23, 24) preantral follicles from the primary stage onwards. It is clear, therefore, that preantral follicles are capable of responding to FSH but the question remains: how important is FSH in the physiology of growth of preantral follicles?
In a key study of follicle development in the sheep, Dufour et al. (4) quantified populations of preantral follicles in the chronically hypophysectomized ewe and found that the number of activated, small preantral follicles was significantly reduced in the absence of FSH. A similar effect of hypophysectomy on preantral follicle depletion was noted in the mouse (3), and these 2 studies provide evidence that FSH might indeed have a physiological role in maintaining the health and promoting the growth and development of preantral follicles. Despite this intriguing insight into the role of FSH in promoting follicle activation and growth, very little is known about the dynamics of FSH action in preantral follicles, particularly with regard to the relationship between follicle size and responsiveness to FSH. Further, there are few data regarding the effect of FSH on gene expression in small preantral follicles. The specific objective of this study was to investigate the response to FSH of mouse preantral follicles, in culture, over a range of follicle diameters, with respect to effects on rates of growth, (including effects on cell proliferation and markers of apoptosis) and on gene expression.
Materials and Methods
Animals and tissue collection
Whole ovaries were collected from female C57BL/6 mice (Harlan Olac Ltd, Bicester, Oxon, UK) aged between 12 and 21 days postpartum (day of birth = 0 days postpartum), as previously described (17). Mice were housed in accordance with the Animals (Scientific Procedures) Act of 1986 and associated Codes of Practice. Briefly, ovaries were dissected in Liebovitz’s L-15 medium (Gibco, ThermoFisher Scientific, Paisley, UK) supplemented with 1% [weight-to-volume (w/v)] bovine serum albumin (BSA; Sigma, Sigma-Aldrich Ltd, Poole, Dorset, UK). Day 12 and day 21 ovaries were fixed immediately in 10% neutral buffered formalin (Sigma), dehydrated in a graded series of ethanol, embedded in paraffin, and serially sectioned (5 µm) for morphological analysis of follicle size and developmental stage according to previous classifications (25). Briefly, follicles with a single layer of flattened pregranulosa cells were termed primordial; those with a mixture of cuboidal and flattened granulosa cells (GCs), transitional; a single layer of entirely cuboidal GCs, primary; the onset of a second layer of GCs, primary plus; a complete second layer, secondary; and the onset of a third layer, secondary plus. Follicles with more than 3 layers of GCs were termed multilayered. Two sections each from ovaries from day 12 (n = 4) and day 21 (n = 4) pups were analyzed. All follicles containing a sharply demarcated oocyte nuclear membrane were measured. Follicle diameter (as defined by the basal lamina surrounding the GCs) was calculated from the mean of 2 perpendicular measurements using the image-analysis program Lucia (Nikon UK). These measurements were used to estimate the stage of development of isolated follicles.
Isolation and culture of mouse preantral follicles
Preantral follicles were mechanically isolated from day 14 to 17 ovaries using 29-gauge insulin needles or acupuncture needles as previously described (17, 26). Follicles were placed in 96 well plates (one ovary per plate) containing α-minimal essential medium (Invitrogen) supplemented with 0.1% (w/v) BSA, 75 µg/mL penicillin, 100 µg/mL streptomycin sulfate, and ITS (5 µg/mL insulin, 5 µg/mL transferrin and 5 ng/mL sodium selenite, Sigma). A single follicle was placed in each well containing 100 µL medium, supplemented with phosphate-buffered saline vehicle alone or a specified concentration of recombinant human FSH (rhFSH; National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, Dr. A. F. Parlow, Torrance, CA). Cultures were maintained under humid conditions at 37°C with 5% CO2 for 24, 72, or 96 hours.
For investigation of signaling via the PKA pathway, isolated follicles were cultured as above in either 0.1 µm or 1 µm of the PKA inhibitor KT5720 (Tocris Bioscience, Bristol, UK) with or without 10 ng/mL rhFSH for 72 hours. Further studies were carried out using 8-bromoadenosine 3′,5′-cyclic monophosphate (8-br-cAMP, Sigma), a stable cAMP analog, at concentrations of 0.05, 0.5, and 1 mM in culture medium.
Measurement of follicles
Follicles were photographed at 24-hour intervals. Follicle area was measured using ImageJ (1.45s; http://imagej.nih.gov/ij). Images of follicles at consecutive time points, as well as measurements, were imported into a custom-made database (FileMaker Pro 11.v2; http://www.filemaker.com) to enable easy inspection and analysis of follicles during development. Follicles with a central spherical oocyte and intact layer of GCs were selected for culture. At the end of culture (72 or 96 hours), follicles were excluded from analysis if their oocyte became misshapen or was extruded from the follicle or if the follicle underwent atresia (darkened GCs). Follicles were analyzed based on their diameter at the start of culture.
Immunofluorescence
At the end of culture, follicles were fixed in 10% neutral buffered formalin (Sigma) and processed for immunofluorescence analysis of GC apoptosis [terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL), cleaved caspase-3] and proliferation (Ki67), as previously described (26). Antibodies are listed in Table 1. Briefly, follicles were set in 2% (w/v) low melting point agarose (Sigma), embedded in paraffin and sectioned (5 µm). Sections were dewaxed and rehydrated before boiling in citrate buffer (10 mM citric acid, pH 6.0). Potential nonspecific interactions were blocked with 10% (w/v) goat serum and 4% (w/v) BSA (Sigma). Sections were incubated with rat anti-Ki67 (1:50; M7249, Dako) or rabbit anticleaved caspase-3 (1:200; 9664, Cell Signaling) overnight at 4°C. The latter were subsequently exposed a 1:9 ratio of TUNEL enzyme:label (Roche) for 60 minutes at 37°C after phosphate-buffered saline washes. Sections were incubated with secondary antibodies (1:200; Alexa488 or 555, Invitrogen) for 60 minutes before mounting with Prolong Gold medium containing 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). Images were recorded using a Leica inverted SP-5 confocal microscope. The number of nuclei which were labeled just with DAPI, and those positive for the marker of interest (Ki67, TUNEL or caspase), were counted in 1 section from each follicle and the % of positive cells calculated.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog Number, or Name of Source | Species Raised in Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Ki67 | CD14, MKI67 | Anti-Ki67 | Dako UK Ltd, M7249 | Rat, monoclonol | 1 in 50 |
| Cleaved caspase-3 | Large fragment (17/19 kDa) resulting from cleavage adjacent to Asp175 | Anticleaved caspase-3 | Cell Signaling Technology, 9664 | Rabbit, monclonol | 1 in 200 |
Quantitative PCR
To examine expression of Fshr in follicles of different sizes, freshly isolated preantral follicles were sorted into 4 size ranges (75 to 90 µm, 91 to 110 µm, 111 to 130 µm, and >130 µm), and samples containing 5 follicles were frozen in liquid nitrogen. Total RNA was isolated from follicle samples using RNeasy microcolumns, which include a DNase digestion step (Qiagen, Crawley, West Sussex, UK). The entire RNA sample was concentrated using vacuum centrifugation and converted to complementary DNA using random primers and SuperScript III reverse transcription kit in accordance with supplied guidelines (Life Technologies). Quantitative polymerase chain reaction (qPCR) was performed on 384-well plates using an Applied Biosystems 7900HT Fast instrument. For each reaction, complementary DNA (1µl) was added to 400-nM primers (Table 2), KAPA SYBR FAST mastermix with ROX (Labtech International) and nuclease free water. Reactions were prepared in duplicate and were subjected to an initial 95°C for 3 minutes, followed by 32 cycles of 95°C for 3 seconds, 60°C for 20 seconds, 72°C for 1 second, and 77°C for 10 seconds. A melting curve analysis of products was performed to ensure consistent and specific amplification. Expression was normalized to the internal reference Atp5b (PrimerDesign, Southampton, UK), which was stably expressed across all samples. Fold changes relative to the 91- to 110-µm–size group (5 samples with consistent values) were calculated using the 2-Δ-Δ-cycle threshold method (27).
Table 2.
Primers Used for PCR Assays
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Fshr | ACAACTGTGCATTCAACGGAAC | GACCTGGCCCTCAACTTCTT |
| Kitl1 | GATTCCAGAGTCAGTGTCAC | CCAGTATAAGGCTCCAAAAGCAA |
| Kitl2 | CTTGTCAAAACCAAGGAGATCTGCG | CTTTGCGGCTTTCCCTTTCTC |
| Ccnd2 | CCCGACTCCTAAGACCCATC | CCACTTCAGCTTACCCAACA |
| Cdkn1b | CGGTGCCTTTAATTGGGTCT | CTTCTTGGGCGTCTGCTC |
| Bmp15 | GAGAACCGCACGATTGGAG | AGTTCGTATGCTACCTGGTTTG |
| Ar | ATTCCTGGATGGGACTGATG | GCCCATCCACTGGAATAATG |
| Star | AAGAACAACCCTTGAGCACCT | CTCCCTGCTGGATGTAGGAC |
| Cyp19 | ATCCACACTGTTGTGGGTGA | ACTCGAGCCTGTGCATTCTT |
| Gja1 | GTGGCCTGCTGAGAACCTAC | GAGCGAGAGACACCAAGGAC |
| Amh | GGGGCACACAGAACCTCT | GCACCTTCTCTGCTTGGTTG |
| Gdf9 | TCACCTCTACAATACCGTCCGG | GAGCAAGTGTTCCATGGCAGTC |
| Cyp11a1 | CTGGGCACTTTGGAGTCAGT | AGGACGATTCGGTCTTTCTTC |
| Inha | CTCCCAGGCTATCCTTTTCC | TGGCCGGAATACATAAGTGA |
To examine the effect of FSH on gene expression, follicles were cultured for 24 hours in the presence and absence of 10ng/mL FSH, before pooling (n = 5) into the size ranges described above. qPCR for genes of interest (Table 2) was performed as described above, using cycle conditions of an initial 95°C for 3 minutes, followed by 32 cycles of 95°C for 5 seconds, 57°C for 20 seconds, and 72°C for 3 seconds. Fold changes relative to untreated follicles (0 ng/mL FSH) were calculated using the 2-Δ-Δ-cycle threshold method.
In vitro knockdown of Fshr in preantral follicles
Size-matched preantral follicles were mechanically isolated from mice aged 15 to 16 days as described above. Single follicles were cultured in individual wells in 96 well plates with 1 µm Accell small interfering RNA (siRNA) designed to target exon 8 of the mouse Fshr transcript (GCGAUAACAAUAAUUUGGA; A-062874-17; ThermoScientific). Control follicles were exposed to Accell Nontargeting siRNA (D-001910-01; ThermoScientific) or Accell Red Nontargeting siRNA, containing a DY-547 label (D-001960-01; ThermoScientific). After 24 hours, 10 ng/mL rhFSH was added to all groups, with the exception of an additional control group. Follicles were maintained in culture for another 96 hours and were photographed daily using light microscopy for assessment of growth as described above. Fshr messenger RNA (mRNA) expression was reduced by 50% relative to nontargeting controls (data not shown). Some of the follicles exposed to labeled siRNA were incubated in 5 µm DRAQ5 nuclear stain (Abcam; Cambridge, UK) for 10 min before imaging in chamber slides using a Leica inverted SP5 confocal laser-scanning microscope (Leica Microsystems, Wetzlar, Germany) to visualize penetration of siRNA into the follicle.
Statistics
Follicle area was measured for each follicle at the start of culture (0 hours), and every 24 hours thereafter, up to 72 or 96 hours. Area was measured to increase accuracy of cumulative growth curves, as the GCs of some follicles escaped from the basal lamina after several days in culture, leading to an asymmetrical outline. For ease of comparison with previously published data, follicle diameter was calculated from follicle area [2 × (√Area/π)]. Follicles were grouped in 6 size “bins” according to initial diameter at 0 hours: <100 µm, 100 to 109.99 µm, 110 to 119.99 µm, 120 to 129.99 µm, 130 to 139.99 µm, and ≥140 µm in diameter. Relative area was calculated for each follicle between 0 hours (baseline) and 24 hours, 48 hours, 72 hours, and 96 hours (area timex/area time0) and cumulative growth curves generated. Data for each size bin were normally distributed with 1 exception (110 to 119.99 µm + FSH). The overall growth of follicles in the presence and absence of FSH, with or without 8-br-cAMP, was compared using linear regression (Prism 6 for Mac OS X, version 6.0a; www.graphpad.com). The effect of FSH treatment on cumulative growth of follicles was analyzed at each time point using paired Student t test or analysis of variance (ANOVA) with appropriate post hoc tests as described in figure legends (Prism 6). In all cases, a probability value (P) less than 0.05 was considered statistically significant.
Results
Classification of isolated preantral follicles and response to FSH in culture
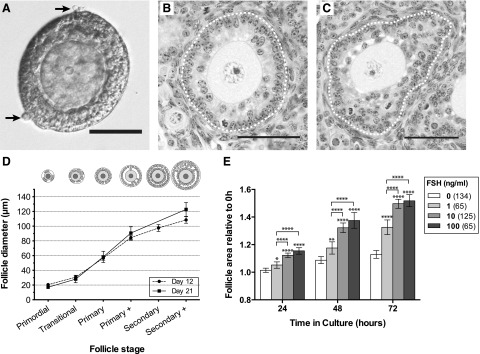
The follicles that were manually isolated from ovaries of 15- to 17-day-old mice in these studies ranged between 70 and 150 µm in diameter. The follicles were cleanly isolated, with only a few theca cells adherent to the basal lamina [Fig. 1(A)]. Based on measurements of follicles from histological sections taken on days 12 and 21 [Fig. 1(B) and 1(C)], follicles of 70 µm have at least 1 full layer of cuboidal GCs, which is equivalent to a primary follicle beginning to form a second layer [Fig. 1(D)]. Follicles with diameters of 100 µm approximate to the secondary stage with 2 complete layers of GCs; therefore, preantral follicles with diameters greater than 100 µm will exhibit more than 2 layers [Fig. 1(D)].
Figure 1.
Relationship between follicle stage and diameter, and response to FSH. (A) Light micrograph of a freshly isolated follicle (∼110 µm) with 2 layers of GCs visible, surrounded by a basal lamina and occasional theca cells (arrow). Scale bar = 50 µm. (B, C) Histological sections of day 12 mouse ovary stained with hematoxylin and eosin. Scale bars = 50 µm. (B) Primary plus stage follicle with second layer of GCs forming, ∼80 µm in diameter. (C) Secondary plus stage follicle developing multiple layers of GCs, ∼115 µm diameter. Dotted white line marks basal lamina. (D) Follicle diameter of follicles at successive developmental stages in 12- and 21-days-postpartum mice. Follicles were measured in a formalin-fixed, paraffin-embedded ovary. Values are mean ± 95% confidence interval. (E) Response of follicles to increasing concentrations of FSH. Relative follicle areas (area at timex/area at time0, where tx = 24, 48, or 72 hours) were compared at each time point using 1-way ANOVA with a Tukey’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Numbers in parentheses are numbers of follicles.
Isolated follicles (70 to 150 µm) were assessed for their ability to respond to increasing concentrations of FSH. When all follicles were considered independent of initial size, 1 ng/mL FSH promoted significant growth increase compared with controls (0 ng/mL FSH) after 24 hours (P < 0.05) and subsequent time points [Fig. 1(E)]. Follicles exposed to 10 or 100 ng/mL exhibited an even greater increase in size relative to controls at all time points (P < 0.0001). Follicles exposed to 10 ng/mL (equivalent to 67 mIU/mL) grew at a similar rate to follicles exposed to 100 ng/mL. This concentration (10 ng/mL) was therefore chosen as the minimum dose required to elicit a maximal FSH-induced growth response in preantral follicles.
Fsh receptor (Fshr) expression in preantral follicles
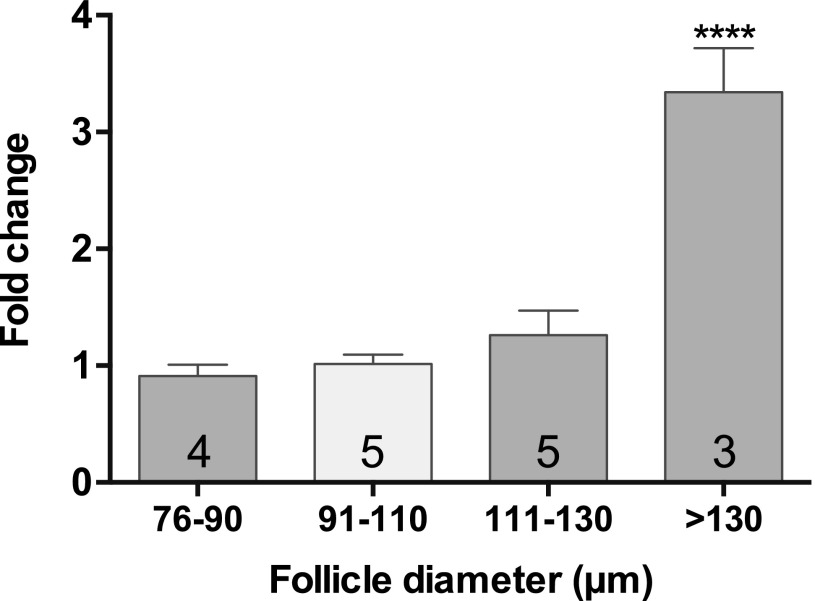
Fshr mRNA expression was measureable by qPCR in follicles of 75 to 90 µm in diameter (Fig. 2). Follicles of this size are developing a second layer of GCs [Fig. 1(D)]. Levels did not change significantly between follicles of this size range and those up to 130 µm. Expression significantly increased (P < 0.001) by approximately threefold in follicles >130 µm (>2 layers of GC) relative to follicles <130 µm (Fig. 2).
Figure 2.
Expression of Fshr in isolated preantral follicles. Comparison of abundance of Fshr in isolated follicles of increasing diameter (5 to 7 follicles per sample; number in bars = number of samples). Transcripts were normalized to Atp5b, and fold changes were calculated relative to expression in the 91- to 110-µm diameter group. Values are mean fold changes ± SEM. Fshr mRNA was detectable in follicles in each size stratum. Relative levels of Fshr in different sized follicles were compared using ANOVA with a Tukey’s multiple comparisons test. ****P ≤ 0.0001.
Size-dependent response of cultured preantral follicles to FSH
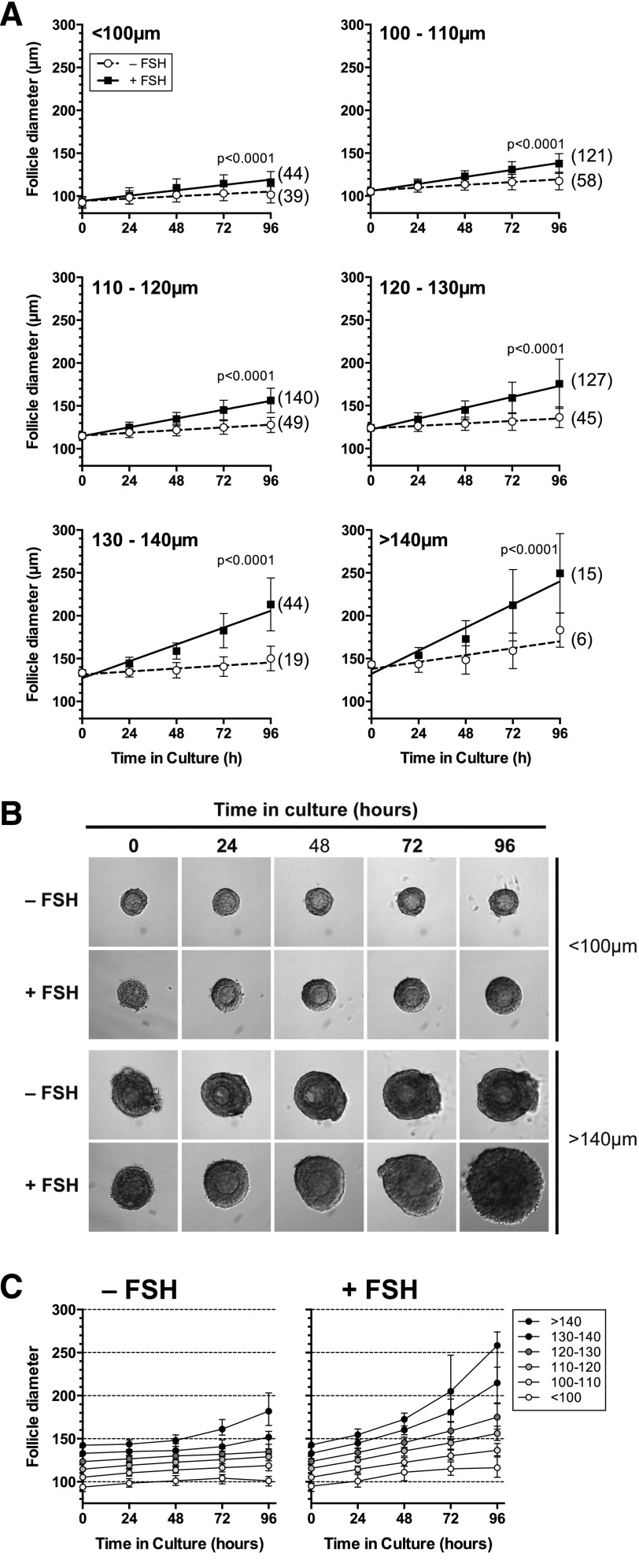
Following 96 hours in vitro, in both the absence and presence of 10 ng/mL FSH, follicles of all sizes were significantly larger than they were at the start of culture (paired t test; P < 0.01, Table 3). FSH stimulated growth of follicles over 72 or 96 hours, including those with an initial diameter smaller than 100 µm [Linear regression, Fig. 3(A) and 3 (B)]. Follicles with an initial diameter <130 µm exhibited a linear rate of growth over 96 hours in the presence of FSH, whereas larger follicles (>130 µm) began to increase in size in an exponential manner over the same period [Fig. 3(C)]. In the absence of FSH, follicles <130 µm grew significantly in the absence of FSH in the first 24 hours, by comparison, follicles >130 did not grow significantly during the same period (Table 3). This suggested that smaller follicles were less responsive to FSH than larger follicles, and this was examined in more detail.
Table 3.
More Sustained Growth in the Presence of FSH
| Initial Diameter | 0 to 24 | 24 to 48 | 48 to 72 | 72 to 96 | 0 to 96 |
| – FSH | |||||
| <100 | <0.0001 | NS | NS | NS | <0.0001 |
| 100 to 110 | <0.0001 | 0.0008 | 0.02 | NS | <0.0001 |
| 110 to 120 | <0.0001 | 0.004 | 0.0184 | NS | <0.0001 |
| 120 to 130 | <0.0001 | NS | NS | NS | <0.0001 |
| 130 to 140 | NS | NS | NS | NS | <0.0001 |
| >140 | NS | NS | NS | NS | <0.0001 |
| + FSH (10 ng/mL) | |||||
| <100 | <0.0001 | <0.0001 | NS | NS | <0.0001 |
| 100 to 110 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 110 to 120 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 120 to 130 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 130 to 140 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 |
| >140 | <0.0001 | 0.0072 | 0.0048 | NS | 0.0052 |
| Statistics | ANOVA with a Tukey's multiple comparisons test | Paired t test 0 h vs 96 h | |||
Follicles of all sizes were significantly larger at 96 hours than they were at the start of culture, with or without FSH, but growth was more sustained in the presence of FSH.
Abbreviation: NS, not significant.
Figure 3.
Effect of FSH (10 ng/mL) on in vitro growth of follicles of different initial sizes. (A) Growth in vitro of preantral follicles of increasing size. Values are means and standard deviation, lines are regression slopes. Slopes were compared using linear regression. (B) Morphology of the smallest (<100 µm) and largest (>140 µm) preantral follicles cultured over 96 hours. (C) Growth curves (follicle diameter) for follicles of increasing follicle size. Values are mean and 95% confidence interval. In the absence of FSH, follicles of <120 µm reduced their growth after the first 24 hours, whereas larger follicles maintained or accelerated their growth. In the presence of FSH, follicles of <100 µm showed slower growth after the first 24 hours in vitro. Follicles of between 100 and 120 µm in diameter exhibited linear growth, whereas those >120 µm at the outset exhibited accelerating growth.
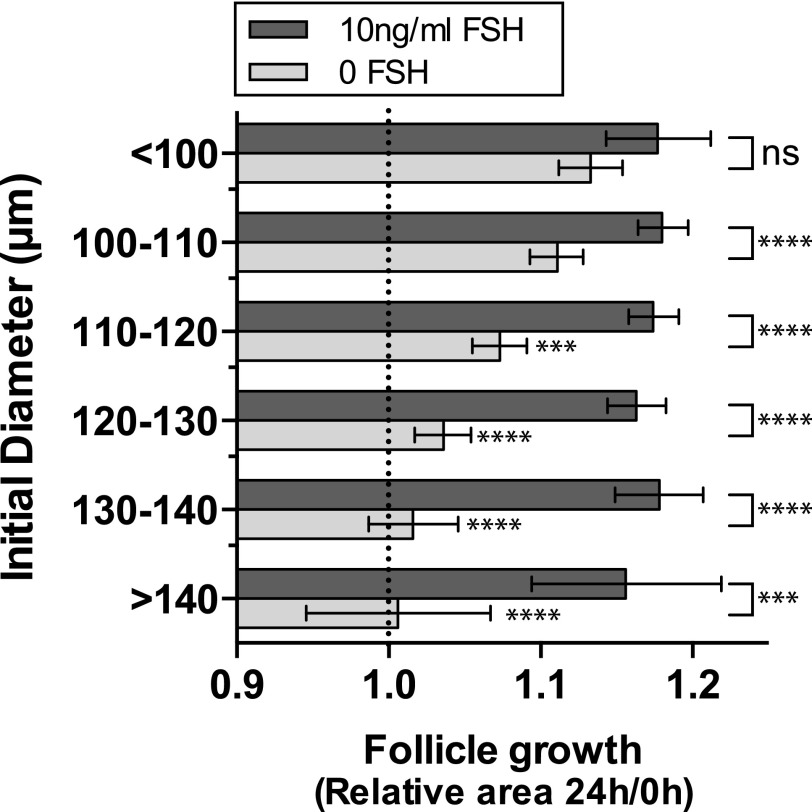
Closer examination of follicle growth during the first 24 hours of culture showed that with increasing initial follicle size, the ability of follicles to grow in the absence of FSH decreased [Fig. 4]. In contrast, in the presence of FSH, follicles of all sizes grew to a similar extent in the first 24 hours, suggesting overall a greater dependency on FSH with increasing follicle size.
Figure 4.
Dependence of larger follicles on FSH. In the presence of FSH follicles of all sizes grow to a similar extent (1-way ANOVA, not significant [ns]). In the absence of FSH (pale bars), larger follicles do not grow as much as smaller follicles during the first 24 hours of culture, suggesting an increasing need for FSH as follicles get larger (ANOVA, each size bin compared with <100 µm diameter, Dunnett’s multiple comparisons test—significantly less growth for follicles >110 µm indicated by asterisks next to pale gray bars). Relative follicle areas (area at time24 hours/area at time0) were compared for each initial follicle diameter in the presence and absence of FSH, using 1-way ANOVA with a Sidak’s multiple comparisons test (6 comparisons). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Values are means ± 95% confidence interval. Dotted, vertical line indicates no change in follicle diameter over the first 24 hours.
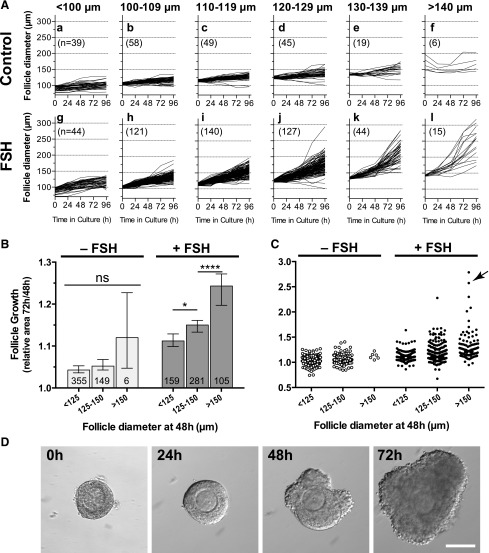
Another notable finding was that, with increasing time in culture, follicles exposed to FSH showed a wider distribution of follicle diameter, demonstrating a heterogeneous response to FSH [Fig. 5(A)]. In the absence of FSH, larger follicles (>140 µm) showed little further growth [Fig. 5(A)(f)]. In contrast, in the presence of FSH, the growth of follicles >120 µm diverged considerably, showing that these follicles had marked heterogeneity in their response to FSH [Fig. 5(A)(j–l)]. We went on to explore the response to FSH of follicles spanning a wider size range, during the 48- to 72-hour culture period. At 48 hours, follicles cultured in the absence of FSH ranged in diameter from 83 to 186 µm, and in the presence of FSH, from 83 to 214 µm. Over the subsequent 24 hours, the growth of follicles of all diameters did not differ significantly in the absence of FSH [Fig. 5(B)]. In contrast, in the presence of FSH, larger follicles grew significantly more than smaller, showing that responsiveness to FSH increased with size [Fig. 5(B)]. Importantly, with FSH, a small proportion of follicles showed accelerated growth [Fig. 5(C; arrowed) and 5(D)].
Figure 5.
Increased heterogeneity of follicle growth in the presence of FSH. (A) Growth curves (diameter) for individual follicles of increasing size in the absence [A(a–f)] and presence [A(g–l)] of FSH. Follicles were cultured for 96 hours; numbers in parentheses are number of follicles. (B) Comparison of growth of small (<125 µm), medium (125 to 150 µm), and large (>150 µm) follicles from 48 to 72 hours in the absence (n = 510) and presence (n = 546) of FSH. Values are median with 95% confidence interval. Numbers in the bars are number of follicles. Median values were compared using Kruskal-Wallis with a Dunn’s multiple comparisons test. (C) Scattergram showing a subpopulation of larger follicles exhibiting accelerated growth in the presence of FSH, between 48 and 72 hours (arrowed). (D) Growth of the individual follicle arrowed in (C), showing accelerated growth between 48 and 72 hours.
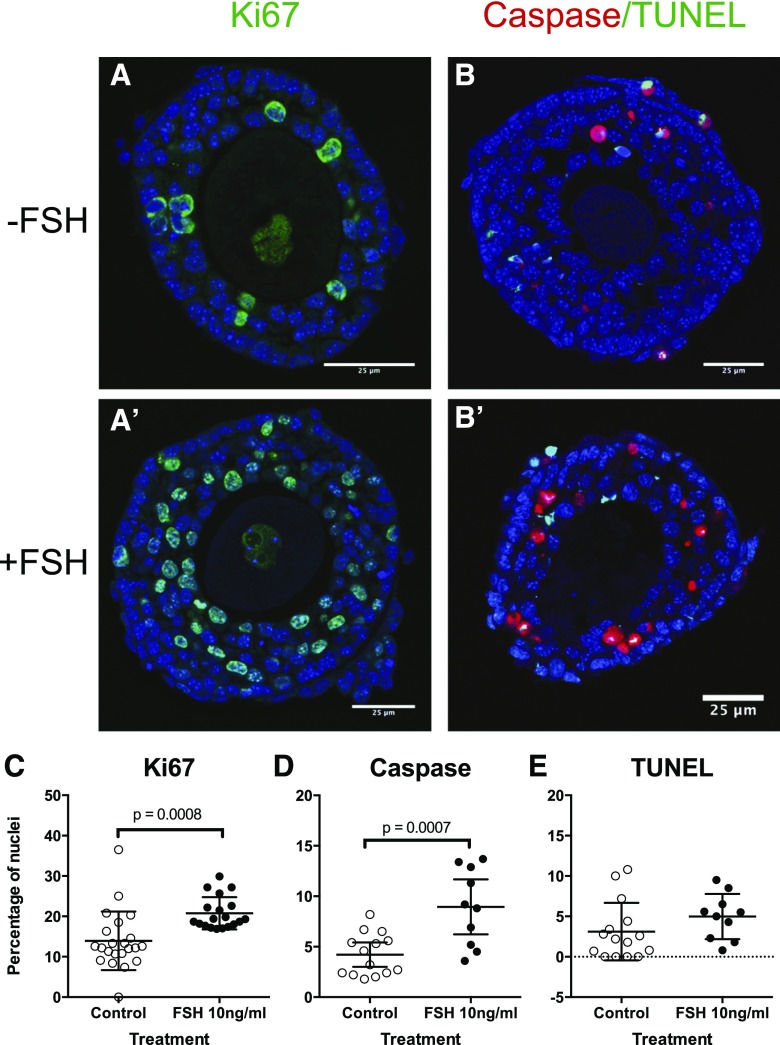
In line with the observation of growth and survival of follicles in culture, even without FSH stimulation, we noted that expression of Ki67 (a marker of cell proliferation) was evident whether or not FSH was present, although there were significantly more Ki67-positive nuclei in follicles cultured with FSH (Fig. 6). A few apoptotic cells were present with or without FSH, as evidenced by expression of TUNEL and caspase-3 labeling. Follicles exposed to FSH had a significantly higher proportion of active caspase-3 positive cells compared with controls (P < 0.001), whereas the proportion of TUNEL positive cells was similar between the 2 groups [Fig. 6(E)].
Figure 6.
Maintenance of follicle health in vitro in both the absence and presence of FSH. (A and A′) Representative images of Ki67 immunolabeling (green) of follicles following 96 hours in culture in the (A) absence (–FSH) and (A′) presence (A +10 ng/mL FSH). DAPI labeled nuclei, with no Ki67, are blue. (B and B′) Dual caspase (red) and TUNEL (green) labeling of follicles cultured for 96 hours in the (B) absence (–FSH) and (B′) presence (+10 ng/mL FSH). The percentage of positive nuclei in each treatment was quantified for (C) Ki67, (D) caspase, and (E) TUNEL, and compared between control and +FSH using an unpaired t test. *P < 0.05. Values are means and 95% confidence interval.
Effect of reduced Fshr on preantral follicle growth
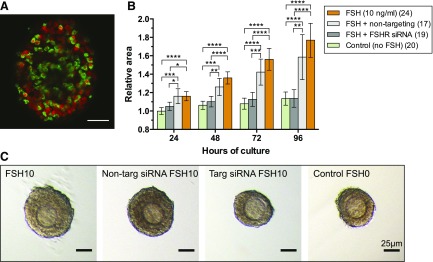
To assess the specific requirement for Fshr in FSH-induced follicle growth, RNA-interference (siRNA) was used to knockdown expression of the receptor in isolated, cultured follicles. The follicles in the 4 treatment groups were of similar initial size (ANOVA, not significant): control (no FSH), median 116 µm (range 98 to 133 µm); 10ng/mL FSH, 114 µm (95 to 124); siRNA, 113 µm (91 to 132); nontargeting siRNA, 113 µm (95 to 129). Preantral follicles cultured in the presence of a labeled (DY-547) nontargeting siRNA revealed penetrance of siRNA into the cytoplasm of many GCs [Fig. 7(A)]. Follicles preincubated for 24h in siRNA targeting the Fshr transcript and then exposed to FSH, grew at a similar rate to control follicles that were not exposed to either [Fig. 7(B) and 7(C)]. In the presence of FSH, follicles exposed to Fshr siRNA were significantly smaller than follicles exposed to nontargeting siRNA (control) at every time point [P < 0.05; Fig. 7(B) and 7(C)], showing that FSH was stimulating follicle growth via Fshr.
Figure 7.
Knockdown of Fshr with siRNA inhibits follicle growth. (A) siRNA (green) is taken up from culture medium. Nuclei are red. (B) siRNA targeted to Fshr significantly reduces follicle growth in the presence of FSH. Relative follicle areas (area at timex/area at time0) were compared using 1-way ANOVA with a Tukey’s multiple comparisons test (6 comparisons). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Values are means ± 95% confidence interval. Nontargeting siRNA did not significantly reduce follicle growth in the presence of FSH. (C) Follicles cultured in the presence and absence of targeted and nontargeted siRNA remain healthy. Scale bars are 25 µm.
Effect of FSH on gene expression in cultured preantral follicles
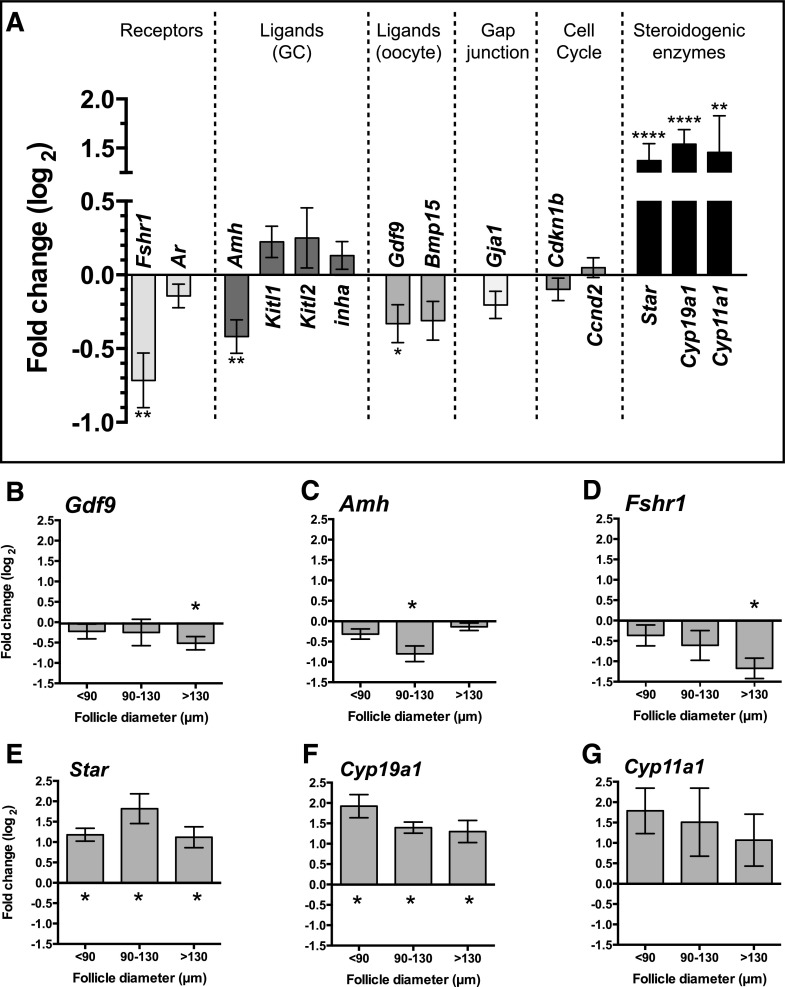
Preantral follicles from a range of sizes cultured for 24 hours in the presence or absence of 10 ng/mL FSH were assayed by qPCR to determine expression levels of a range of candidate genes. FSH exposure was associated with a substantial reduction in the level of Fshr, Amh and Gdf9 compared with untreated controls (P < 0.05), whereas no change in expression was found for Ar, Kitl1, Kitl2, Inha, Bmp15, Gja1, Cddkn1b and Ccnd2 (P > 0.05). By comparison, transcript levels of the steroidogenic enzymes Star, Cyp19a1, and Cyp11a1 were all elevated in follicles exposed to FSH [P < 0.01; Fig. 8(A)].
Figure 8.
Fold change in expression of candidate genes in preantral follicles from 3 initial size groups (<90 µm, 90 to 130 µm, and >130 µm in diameter) cultured in FSH (10 ng/mL). Fold changes (log2) for FSH treated follicles are presented relative to untreated follicles (value = 0). (A) Overall fold change in follicles of all sizes. (B–G) Fold change in follicles where significant differences were detected in follicles of a particular size. Gene expression in the absence and presence of 10 ng/mL FSH was compared for each gene using an unpaired t test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
When follicles were assayed according to initial size, Gdf9 and Fshr were reduced in the larger group only (>130 µm) when treated with FSH (P < 0.05), whereas Amh was reduced in follicles measuring 90 to 130 µm only. Expression of Star and Cyp19a1 were increased in preantral follicles exposed to FSH regardless of size (P < 0.05), whereas considerable variability in was found in Cyp11a1 expression [P > 0.05; Fig. 8(B–H)].
FSH and the PKA pathway
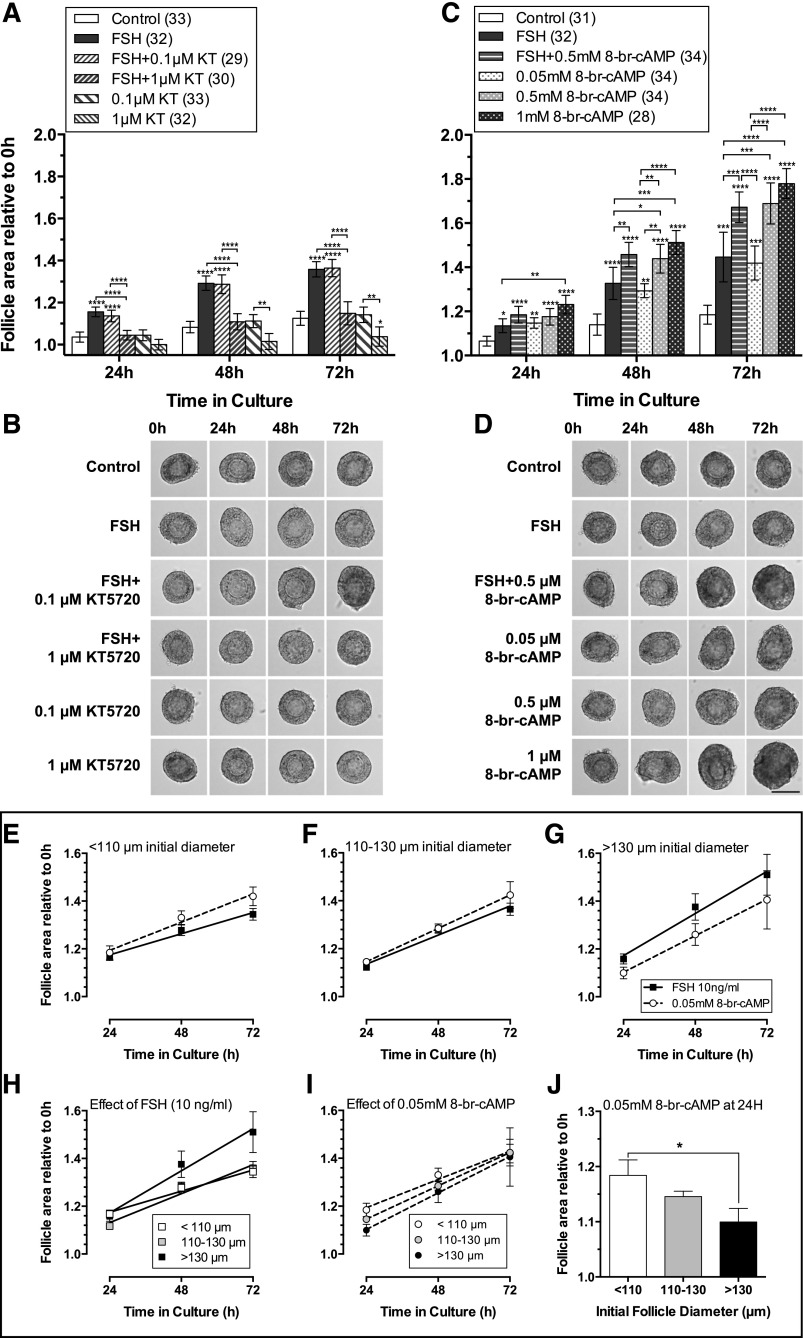
To test whether FSH acts via the PKA pathway to stimulate preantral follicle growth, follicles were cultured in the presence and absence of FSH and the specific PKA pathway inhibitor KT5720. The lowest concentration of KT5720 (0.1 µm) had little effect on FSH-stimulated follicle growth. However, 1 µm KT5720 significantly inhibited FSH-stimulated follicle growth to levels similar to control [Fig. 9(A)], with no deleterious effect on follicle health [Fig. 9(B)]. Follicle growth was reduced below control levels in response to 1 µm KT5720, alone, again with no signs of follicle atresia [Fig. 9(B)], suggesting inhibition of PKA signaling from other, endogenous, stimuli.
Figure 9.
Inhibition of the PKA pathway with KT5720 (KT, panel A) reduced follicle growth, whereas treatment with the cAMP analog, 8-br-cAMP (C) stimulated follicle growth. (A) Response of preantral follicles to vehicle control medium, FSH (10 ng/mL), FSH + 0.1 µm KT5720, FSH + 1µM KT5720, and 0.1 and 1 µm KT5720 alone. FSH-stimulated growth was reversed with 1 µm KT5720. There was no significant difference in the distribution of initial follicle size between treatments (Kruskal-Wallis). The relative area of follicles in different treatments (area at timex/area at time0, where timex = 24, 48, or 72 hours) was compared at each time point using 1-way ANOVA with a Tukey’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Values are means and 95% confidence interval. Numbers in parentheses are numbers of follicles. (B) Growth of similarly sized follicles (between 110 and 120 µm) diameter, in the treatments described in (A), scale as in (D). Follicles in all treatments remained healthy throughout the 72-hour culture period, as shown by morphology and absence of atresia (darkening of GCs). (C) Response of preantral follicles to control medium (vehicle alone), FSH (10 ng/mL), FSH + 0.5 mM 8-bromo-cAMP, and 0.05, 0.5 and 1 mM 8-bromo-cAMP alone. Statistical analysis, values, error bars, numbers in parentheses and P values as in (A). (D) Growth of similarly sized follicles (between 110 and 120 µm) diameter, in the treatments described in (C). Scale bar = 100 µm. Follicles remained healthy in all treatments. (E–J) Response of follicles of different sizes to 0.05 mM 8-bromo-cAMP and 10 ng/mL FSH. 8-bromo-cAMP (0.05 mM) stimulated follicle growth to a similar extent to 10 ng/mL FSH, and therefore was used as a positive control for PKA pathway activity.
We went on to explore how follicles responded to PKA stimulation, using the cAMP analog 8-br-cAMP. The lowest concentration of 8-br-cAMP alone (0.05 mM) stimulated follicle growth to a similar extent to the effect of 10 ng/mL FSH [Fig. 9(C) and 9(D)]. We therefore used this concentration to compare consistent, stable cAMP stimulation of PKA pathway activity to stimulation by FSH in follicles of various sizes [Fig. 9(E–G)]. Small follicles (<110 µm initial diameter) grew more in 8-br-cAMP than in FSH [Fig. 9(E)], while, conversely, large follicles (>130 µm initial diameter) grew more in FSH than 8-br-cAMP [Fig. 9(G)]. In medium-sized follicles (110 to 130 µm) the growth trajectories were similar [Fig. 9(F)]. By the end of the culture period [72 hours, Fig. 9(I)], the response to 8-br-cAMP was similar in follicles of all sizes, with follicle area increasing by around 1.4 times [Fig. 9(I)]. As expected, large follicles (>130 µm) grew more in the presence of FSH than small follicles [<110 µm; P = 0.07, Fig. 9(H)]. Intriguingly, the initial response (i.e. within 24 hours) to 8-br-cAMP differed depending on initial follicle diameter. Smaller follicles (<110 µm) were more responsive to 0.05 mM 8-br-cAMP, growing significantly more in the first 24 hours than did larger follicles [>130 µm, Fig. 9(J)].
Discussion
In this systematic study of the effects of FSH on isolated preantral follicles in culture, we have shown that follicle growth is maintained throughout the 96 hours of culture in the absence of FSH, but that addition of FSH causes a substantial increase in the rate of growth. In the first 24 hours of culture, FSH does not significantly stimulate growth of follicles smaller than 100 µm, suggesting that follicles up to the secondary stage are not responsive to FSH. However, FSH does stimulate these follicles over a longer time period, most likely due to them reaching a key threshold size. The observation that FSH stimulates follicles larger than 100 µm within 24 hours suggests that this is the threshold size, and that the secondary stage of follicle development (2 layers of GCs) marks the onset of FSH responsiveness during preantral development. Fshr gene expression is detectable, even in the smallest follicles, but increases significantly at 130 µm (with 3 layers of GCs), and as follicles further increase in size, they become increasingly responsive to FSH, as marked by increased growth. Indeed, the growth trajectory of a small subset of follicles accelerates dramatically, leading to marked heterogeneity of follicle size, which is not observed in the absence of FSH. During late antral follicle development, heterogeneity of follicle size and differing sensitivity to FSH play a key role in selection of a dominant follicle. Here, we have shown that this heterogeneity starts at the multilayered preantral follicle stage, with follicles having differing growth trajectories, likely due to varying levels of Fshr expression.
Follicles larger than 140 µm in diameter show little growth in the absence of FSH, suggesting that FSH is becoming essential. Follicles were only cultured for a maximum of 96 hours, so this does not preclude a slow rate of growth over a longer time period. In addition, the follicles were cleanly dissected, with minimal adherent theca or stroma. These follicles therefore lacked any growth factor stimulation from these cell compartments, with the major growth factor involvement being GC-specific AMH and KL and oocyte specific GDF9.
Follicle growth was accompanied by protein expression of the cell proliferation marker Ki67, which was increased with exposure to FSH. Here, we confirm that FSH is not essential for preantral growth, as even after a 96-hour culture in the absence of FSH GC proliferation was observed, and there was no increase in apoptosis. Surprisingly, in view of the well-documented actions of FSH as a survival factor (28), the proportion of caspase-positive GCs was significantly higher in the presence of FSH (Fig. 6). Similar stimulation of both proliferation and apoptosis was observed previously in follicles cultured in the presence of BMP15 and GDF9 together (26), suggesting an increase in cell turnover may be induced as a self-regulatory mechanism to prevent excessive follicle growth. The observed increase in apoptosis in the presence of FSH may be due to inhibition of antiapoptotic factors or stimulation of proapoptotic factors by FSH. The former is a possibility, as expression of Gdf9 was downregulated by FSH in the current study, and GDF9 has been shown to be antiapoptotic in large rat preantral follicles (29). Indeed, in the same study, substantial apoptosis was noted in follicles >200 µm in the presence of 10 ng/mL FSH alone (the same concentration as in our study).
Total inhibition of FSH-stimulated follicle growth by the PKA inhibitor KT5720 (1 µm) strongly suggests that FSH action in preantral follicles is predominantly via the PKA pathway. The reduction in follicle growth to below control levels by 1 µm KT5720 alone implies that there are other endogenous factors stimulating follicle growth that act via the same pathway. On microscopy, the absence of darkening of GC and oocyte, as well as maintenance of smooth spherical shape of both oocyte and follicle in the presence of inhibitor (which was used at a 10-fold lower concentration than that generally used in other studies), indicate that the decreased growth was very unlikely to be due to toxicity. We went on to explore whether use of a cell permeable, phosphodiesterase resistant, stable cAMP analog could recapitulate FSH action. Incubation of follicles in both FSH and 8-br-cAMP resulted in greater growth than FSH alone, whereas incubation of follicles in increasing concentrations of 8-br-cAMP alone resulted in increased growth in a dose response manner. The lowest concentration of 8-br-cAMP used (0.05 mM) had a similar stimulatory effect to 10 ng/mL FSH, so we went on to compare follicle growth stimulated by FSH (acting via Fshr) to growth that was constantly and directly stimulated by 8-br-cAMP. Interestingly, 8-br-cAMP had a greater effect than FSH in small follicles, but was less stimulatory than FSH in large follicles, strongly supporting the evidence that Fshr, and hence PKA signaling, increases with follicle size. Closer analysis of the effect of 8-br-cAMP on follicles after 24 hours of exposure showed that follicles of different sizes had varying responses to 8-br-cAMP. Small follicles (<110 µm diameter) grew relatively more than larger follicles (>130 µm) in the presence of 8-br-cAMP. The mechanisms underlying this are unclear, but raise the possibility of other, endogenous factors that contribute to follicle growth via the PKA pathway. Involvement of the PKA pathway in FSH-stimulated growth and differentiation has previously been demonstrated in GCs from antral follicles (30) but not, to our knowledge, in isolated preantral follicles.
In this study, we chose a single dose of FSH (10 ng/mL) that we had previously shown to be effective in inducing optimal follicle growth (17). Kreeger et al. (15) examined dose-related effects of FSH on preantral follicles in a 3-dimensional, alginate-based culture system, but in that study, small and large follicles were grouped together and thus, the influence of initial follicle size on the responsiveness to FSH was not explored. They did, however, report that these follicles were steroidogenically active, producing both progesterone and estradiol that could be detected in the culture medium (15). Our data on gene expression of Cyp11a1, Cyp19a1, and Star are consistent with their findings.
Previous reports of FSH-induced gene expression have been largely confined to studies of antral follicles or mature GCs (30–33). Roy and Greenwald (12) showed, 20 years ago, that FSH induced DNA synthesis in preantral follicles. Skory et al. (34) undertook an extensive microarray analysis of gene expression in large (150 to 180 µm) preantral follicles, followed by qPCR of key genes, but all follicles were cultured in the presence of FSH and the focus here was on the effects of time in culture on gene expression rather than the action of FSH or the impact of follicle size. Fatehi et al. (35) studied growth factor gene expression in mouse preantral follicles in relation to the effects of vitrification but, once again, neither the effects of follicle size nor FSH on gene expression were considered. As mentioned above, our finding of FSH-induced expression of Cyp11a1, Cyp19a1, and Star reflect previous observations regarding steroidogenic capacity of preantral follicles. However, it is intriguing that, in our studies, we were able to show that even the smallest follicles that were cultured showed FSH-dependent expression of genes encoding key steroidogenic enzymes.
Our finding that FSH treatment was associated with suppression of Amh gene expression in preantral follicles was intriguing. In situ hybridization studies have shown that expression of AMH RNA in rat ovary is highest in large preantral and small antral follicles and negligible in large antral follicles (36, 37). It may be that as FSH simulates follicle growth, follicles are more rapidly reaching a stage where AMH levels are coincidentally decreasing. Furthermore, during FSH induction of ovulation, rising serum levels of FSH are associated with declining concentrations of AMH. Interestingly, we observed an inhibitory effect of FSH on expression of Gdf9, which was in contrast to our previous findings (no effect) (17). However, this action may have been masked in that study by grouping together preantral follicles of different sizes. In the current study, the inhibition of Gdf9 expression was only substantial in follicles >130 μm, at the time when Fshr expression is significantly upregulated. FSH-induced downregulation of Fshr is, however, a well-recognized phenomenon (38, 39).
In conclusion, preantral follicles acquire FSH receptors and are responsive to FSH stimulation in terms of growth and gene expression. There is marked heterogeneity of response, according to the initial follicle diameter. Thus, although (as demonstrated in this study) follicles can progress through the preantral stages without FSH, our data confirm the notion of FSH responsiveness in preantral follicles and support the concept of a physiological role for FSH in preantral follicle growth and function.
Acknowledgments
M.F. is now with the Academic Unit of Reproductive and Developmental Medicine, University of Sheffield, Sheffield, United Kingdom. M.L. is now with Biomedical Sciences, University of Reading, Reading, United Kingdom.
Acknowledgments
Supported by Biotechnology and Biological Sciences Research Council UK grants BB/F000014/1 (J.M.) and BB/H00002X/1 (M.F.), Medical Research Council Programme Grant G0802782 (S.F, K.H, M.L.), MRC Project Grant MR/M012638/1 (S.F., K.H.) and The Genesis Research Trust.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- DAPI
- 4′,6-diamidino-2-phenylindole
- FSH
- follicle-stimulating hormone
- GC
- granulosa cell
- mRNA
- messenger RNA
- PKA
- protein kinase A
- qPCR
- quantitative polymerase chain reaction
- rhFSH
- recombinant human FSH
- siRNA
- small interfering RNA
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
References
- 1.Halpin DM, Charlton HM. Effects of short-term injection of gonadotrophins on ovarian follicle development in hypogonadal (hpg) mice. J Reprod Fertil. 1988;82(1):393–400. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Balla A, Danilovich N, Sairam MR. Developmental and molecular aberrations associated with deterioration of oogenesis during complete or partial follicle-stimulating hormone receptor deficiency in mice. Biol Reprod. 2003;69(4):1294–1302. [DOI] [PubMed] [Google Scholar]
- 3.Wang XN, Greenwald GS. Hypophysectomy of the cyclic mouse. I. Effects on folliculogenesis, oocyte growth, and follicle-stimulating hormone and human chorionic gonadotropin receptors. Biol Reprod. 1993;48(3):585–594. [DOI] [PubMed] [Google Scholar]
- 4.Dufour J, Cahill LP, Mauléon P. Short- and long-term effects of hypophysectomy and unilateral ovariectomy on ovarian follicular populations in sheep. J Reprod Fertil. 1979;57(2):301–309. [DOI] [PubMed] [Google Scholar]
- 5.McGee EA, Perlas E, LaPolt PS, Tsafriri A, Hsueh AJ. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol Reprod. 1997;57(5):990–998. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BK, Telfer EE, Webb R, Baird DT. Ovarian autografts in sheep as a model for studying folliculogenesis. Mol Cell Endocrinol. 2000;163(1-2):131–139. [DOI] [PubMed] [Google Scholar]
- 7.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95(23):13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141(5):1795–1803. [DOI] [PubMed] [Google Scholar]
- 9.Matthews CH, Borgato S, Beck-Peccoz P, Adams M, Tone Y, Gambino G, Casagrande S, Tedeschini G, Benedetti A, Chatterjee VK. Primary amenorrhoea and infertility due to a mutation in the beta-subunit of follicle-stimulating hormone. Nat Genet. 1993;5(1):83–86. [DOI] [PubMed] [Google Scholar]
- 10.Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21(5):551–583. [DOI] [PubMed] [Google Scholar]
- 11.Kottler ML, Chou YY, Chabre O, Richard N, Polge C, Brailly-Tabard S, Chanson P, Guiochon-Mantel A, Huhtaniemi I, Young J. A new FSHbeta mutation in a 29-year-old woman with primary amenorrhea and isolated FSH deficiency: functional characterization and ovarian response to human recombinant FSH. Eur J Endocrinol. 2010;162(3):633–641. [DOI] [PubMed]
- 12.Roy SK, Greenwald GS. Follicular development through preantral stages: signalling via growth factors. J Reprod Fertil Suppl. 1996;50:83–94. [PubMed] [Google Scholar]
- 13.Cortvrindt R, Smitz J, Van Steirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12(4):759–768. [DOI] [PubMed] [Google Scholar]
- 14.Spears N, Murray AA, Allison V, Boland NI, Gosden RG. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fertil. 1998;113(1):19–26. [DOI] [PubMed] [Google Scholar]
- 15.Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73(5):942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi N, Orisaka M, Cao M, Kotsuji F, Leader A, Sakuragi N, Tsang BK. Growth differentiation factor-9 mediates follicle-stimulating hormone-thyroid hormone interaction in the regulation of rat preantral follicular development. Endocrinology. 2009;150(12):5566–5574. [DOI] [PubMed] [Google Scholar]
- 17.Fenwick MA, Mansour YT, Franks S, Hardy K. Identification and regulation of bone morphogenetic protein antagonists associated with preantral follicle development in the ovary. Endocrinology. 2011;152(9):3515–3526. [DOI] [PubMed] [Google Scholar]
- 18.Cecconi S, Barboni B, Coccia M, Mattioli M. In vitro development of sheep preantral follicles. Biol Reprod. 1999;60(3):594–601. [DOI] [PubMed] [Google Scholar]
- 19.Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S, Hardy K. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14(6):1555–1562. [DOI] [PubMed] [Google Scholar]
- 20.Kishi H, Greenwald GS. Autoradiographic analysis of follicle-stimulating hormone and human chorionic gonadotropin receptors in the ovary of immature rats treated with equine chorionic gonadotropin. Biol Reprod. 1999;61(5):1171–1176. [DOI] [PubMed] [Google Scholar]
- 21.Monniaux D, de Reviers MM. Quantitative autoradiographic study of FSH binding sites in prepubertal ovaries of three strains of rats. J Reprod Fertil. 1989;85(1):151–162. [DOI] [PubMed] [Google Scholar]
- 22.O’Shaughnessy PJ, Dudley K, Rajapaksha WR. Expression of follicle stimulating hormone-receptor mRNA during gonadal development. Mol Cell Endocrinol. 1996;125(1–2):169–175. [DOI] [PubMed] [Google Scholar]
- 23.Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82(11):3748–3751. [DOI] [PubMed] [Google Scholar]
- 24.Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Müllerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92(3):1034–1040. [DOI] [PubMed] [Google Scholar]
- 25.Da Silva-Buttkus P, Jayasooriya GS, Mora JM, Mobberley M, Ryder TA, Baithun M, Stark J, Franks S, Hardy K. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J Cell Sci. 2008;121(23):3890–3900. [DOI] [PubMed] [Google Scholar]
- 26.Fenwick MA, Mora JM, Mansour YT, Baithun C, Franks S, Hardy K. Investigations of TGF-β signaling in preantral follicles of female mice reveal differential roles for bone morphogenetic protein 15. Endocrinology. 2013;154(9):3423–3436. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 28.Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137(4):1447–1456. [DOI] [PubMed] [Google Scholar]
- 29.Orisaka M, Orisaka S, Jiang JY, Craig J, Wang Y, Kotsuji F, Tsang BK. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol. 2006;20(10):2456–2468. [DOI] [PubMed] [Google Scholar]
- 30.Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18(9):1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu S, Rushdi S, Zumpe ET, Mamers P, Healy DL, Jobling T, Burger HG, Fuller PJ. FSH-regulated gene expression profiles in ovarian tumours and normal ovaries. Mol Hum Reprod. 2002;8(5):426–433. [DOI] [PubMed] [Google Scholar]
- 32.Amsterdam A. Novel genes regulated by gonadotropins in granulosa cells: new perspectives on their physiological functions. Mol Cell Endocrinol. 2003;202(1–2):133–137. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez F, Romero S, Smitz J. Oocyte and cumulus cell transcripts from cultured mouse follicles are induced to deviate from normal in vivo conditions by combinations of insulin, follicle-stimulating hormone, and human chorionic gonadotropin. Biol Reprod. 2011;85(3):565–574. [DOI] [PubMed] [Google Scholar]
- 34.Skory RM, Bernabé BP, Galdones E, Broadbelt LJ, Shea LD, Woodruff TK. Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth. Mol Reprod Dev. 2013;80(2):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fatehi R, Ebrahimi B, Shahhosseini M, Farrokhi A, Fathi R. Effect of ovarian tissue vitrification method on mice preantral follicular development and gene expression. Theriogenology. 2014;81(2):302–308. [DOI] [PubMed] [Google Scholar]
- 36.Hirobe S, He WW, Lee MM, Donahoe PK. Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology. 1992;131(2):854–862. [DOI] [PubMed] [Google Scholar]
- 37.Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136(11):4951–4962. [DOI] [PubMed] [Google Scholar]
- 38.Passos MJ, Vasconcelos GL, Silva AW, Brito IR, Saraiva MV, Magalhães DM, Costa JJ, Donato MA, Ribeiro RP, Cunha EV, Peixoto CA, Campello CC, Figueiredo JR, van den Hurk R, Silva JR. Accelerated growth of bovine preantral follicles in vitro after stimulation with both FSH and BMP-15 is accompanied by ultrastructural changes and increased atresia. Theriogenology. 2013;79(9):1269–1277. [DOI] [PubMed] [Google Scholar]
- 39.Manna PR, Pakarainen P, Rannikko AS, Huhtaniemi IT. Mechanisms of desensitization of follicle-stimulating hormone (FSH) action in a murine granulosa cell line stably transfected with the human FSH receptor complementary deoxyribonucleic acid. Mol Cell Endocrinol. 1998;146(1–2):163–176. [DOI] [PubMed] [Google Scholar]