Abstract
The current classification of both diabetes and antidiabetes medication is complex, preventing a treating physician from choosing the most appropriate treatment for an individual patient, sometimes resulting in patient-drug mismatch. We propose a novel, simple systematic classification of drugs, based on their effect on adenosine monophosphate-activated protein kinase (AMPK). AMPK is the master regular of energy metabolism, an energy sensor, activated when cellular energy levels are low, resulting in activation of catabolic process, and inactivation of anabolic process, having a beneficial effect on glycemia in diabetes. This listing of drugs makes it easier for students and practitioners to analyze drug profiles and match them with patient requirements. It also facilitates choice of rational combinations, with complementary modes of action. Drugs are classified as stimulators, inhibitors, mixed action, possible action, and no action on AMPK activity. Metformin and glitazones are pure stimulators of AMPK. Incretin-based therapies have a mixed action on AMPK. Sulfonylureas either inhibit AMPK or have no effect on AMPK. Glycemic efficacy of alpha-glucosidase inhibitors, sodium glucose co-transporter-2 inhibitor, colesevelam, and bromocriptine may also involve AMPK activation, which warrants further evaluation. Berberine, salicylates, and resveratrol are newer promising agents in the management of diabetes, having well-documented evidence of AMPK stimulation medicated glycemic efficacy. Hence, AMPK-based classification of antidiabetes medications provides a holistic unifying understanding of pharmacotherapy in diabetes. This classification is flexible with a scope for inclusion of promising agents of future.
KEY WORDS: Adenosine monophosphate-activated protein kinase, classification, diabetes, drug
Introduction
Diabetes has become a global pandemic with India along with China, constituting the diabetes capital of the globe (178.8 million patients; present-day estimates).[1] Specific challenges associated with diabetes in the developing world are the high prevalence of both prediabetes and diabetes,[2,3,4] younger age of onset of Type-2 diabetes mellitus (T2DM), with significantly rapid rates of disease progression (prediabetes to diabetes conversion rate in India, China, Finland, and the USA being 14–18%, 11%, 6%, and 2.5%, respectively).[4,5,6,7] Increased prevalence of obesity, metabolic syndrome, and sedentary lifestyles, coupled with genetic predisposition, is believed to contribute to this increased burden of diabetes in the developing world.[7,8]
Complexity in Pathophysiology and Classification of Diabetes
Till recently, the term “ominous octet,” coined by DeFronzo, has been used to exemplify the complexity of the disease.[9] Ominous octet highlights the multifaceted pathogenesis of diabetes, characterized by varying degree of contribution of pancreatic beta cell dysfunction/loss, pancreatic alpha cell (glucagon) over activity, hepatic insulin resistance (IR) and glucose overproduction, decreased disposal of glucose at muscles and adipose tissue (IR), increased lipolysis, impaired incretin effect, increased glucose resorption in the kidneys, and central neurotransmitter dysfunction in the development of diabetes.[9,10] Recent data have further highlighted the importance of central dopamine dysfunction, hypovitaminosis-D, renin-angiotensinogen system (RAS) over-activity and hypogonadism (especially in males) in the pathogenesis of diabetes, giving rise to the concept of “dirty dozen in diabetes.”[7,10,11,12,13]
The current classification of diabetes into T1DM, T2DM, latent onset autoimmune diabetes of adults (LADA; also known as Type 1.5 diabetes) does not take into account the above-mentioned multiple interlinked pathways in the disease pathogenesis.[14] Further certain variants of diabetes such as malnutrition-related diabetes mellitus, ketosis-resistant diabetes of the youth, and Flatbush diabetes have not been accounted for.[15]
Challenges in Classification of Antidiabetes Medications
The pharmacology of diabetes is complex. The past two decades have seen an upsurge in the number of antidiabetes medications. While earlier drugs could easily be classified as secretagogues (sulfonylureas, repaglinide, and nateglinide) or sensitizers (metformin, thiazolidinediones (TZDs), this simple taxonomy did not work for newer molecules such as alpha-glucosidase inhibitors. The development of incretin-based therapy brought a fresh challenge to the systematic of diabetes pharmacotherapeutics, and this was further compounded by the approval of drugs as varied as bromocriptine, colesevelam, and sodium glucose co-transporter-2 inhibitors (SGLT2i). While these pharmaceutical advances are welcome and provide greater choice to both physician and patient, they pose certain challenges as well.
The current classification of antidiabetes medication does not provide a contemporary, holistic, mechanistic, comparative, and systematic classification to easily analyze these available drugs. These prevent the treating physician from choosing the most appropriate therapeutic option for an individual patient, and hence sometimes resulting in a patient drug mismatch. In most cases, drug-related complications occur because of incomplete understanding of the mechanism of action of the molecule, and its indications, leading to incorrect patient selection, e.g. euglycemic ketoacidosis with SGLT2i.[16,17,18]
Adenosine monophosphate-activated protein kinase-based classification of diabetes pharmacotherapy
We propose the use of a novel, simple systematic classification of drugs, based on their effect on adenosine monophosphate-activated protein kinase (AMPK). This article intends to elaborate on this classification system, highlighting the impact of traditional as well as novel antidiabetes medications on AMPK. Antidiabetes medications have been classified as stimulators, inhibitors, mixed action, possible action, and no action on AMPK activity. This listing of drugs makes it easier for students and practitioners to analyze drug profiles and match them with patient requirements. It also facilitates choice of rational combinations, with complementary modes of action.
Adenosine monophosphate-activated protein kinase as regulator of metabolism
Alternation in biological pathways involved in energy metabolism is perhaps the single most important underlying unifying mechanism explaining this increased occurrence of dysglycemia.[19] One such important pathway is that of AMPK, a nutrient sensing serine/threonine kinase, which is increasingly being called the master regular of energy metabolism.[19] It seems that drugs which modulate metabolic disorders, including diabetes and obesity, may have an indirect or direct effect on AMPK.
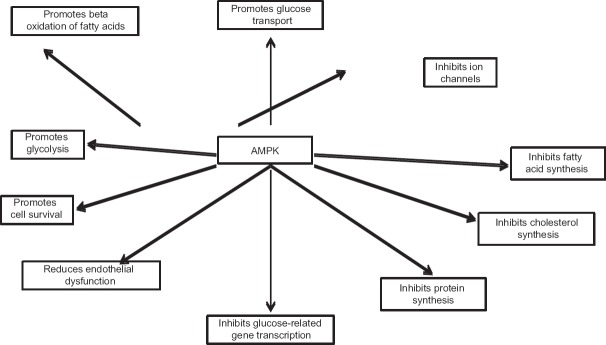
AMPK is an energy sensor, activated when cellular energy levels are low, namely high intracellular adenosine monophosphate (AMP) to adenosine triphosphate (ATP) ratio, resulting in activation of metabolic pathways which result in ATP generation, and downregulation of metabolic pathways which need ATP consumption.[20,21] This results in net energy conservation and homeostasis restoration.[20,21] Hence, glucose entry into cells (translocation of glucose transporter type [GLUT]-4), mitochondrial fatty acid oxidation (through inhibition of enzyme acetyl-coenzyme-A carboxylase), and glycolysis are upregulated and pathways involving triglyceride, cholesterol, glycogen and protein synthesis, gluconeogenesis and lipolysis are downregulated.[20,21,22,23] Insulin secretion from pancreatic beta cells is inhibited.[21] All of these metabolic alterations, resulting from peripheral AMPK activation, have beneficial effects in diabetes. It is important to highlight that central (hypothalamic) activation of AMPK results in increased food intake, an expected response to starvation and energy deficiency state.[24] AMPK act as the gateway for various hormones involved in carbohydrate, lipid, and protein metabolism, and it is not surprising that AMPK dysregulation has been demonstrated to have a central role in the genesis of IR and T2DM.[20,21] Figure 1 summarizes the main biologic functions of AMPK.
Figure 1.
Biologic functions of adenosine monophosphate-activated protein kinase
Homeostatic regulators of adenosine monophosphate-activated protein kinase
Phosphorylation of the Th172 residue in the activation loop of alpha subunit of AMPK is the single most important mechanism of AMPK activation.[25,26,27] The three main upstream kinases responsible for this phosphorylation are liver kinase B1 (LKB1), calmodulin-mediated kinase kinase b (CaMKKb), and transforming growth factor-b activated kinase 1 (TAK1).[25,26,27] Second, AMP binding to the gamma-subunit of AMPK also leads to allosteric activation of AMPK, by resisting the breakdown of activated phosphorylated AMPK by phosphatases.[28]
LKB1 is also a tumor suppressor gene, and its loss of function mutation has been reported in Peutz–Jeghers syndrome.[29] CaMKKb is activated by increased cytosolic calcium level.[30] TAK1 is a member of the mitogen-activated protein kinase family, activated by interleukin-1, transforming growth factor-b, toll-like receptors, CD40, and B-cell receptors.[31]
Adenosine monophosphate-activated protein kinase pathophysiology in different organ systems
Muscle and adipose tissue
In skeletal muscles, cardiac muscles, and adipose tissue, AMPK stimulates fatty acid oxidation, mitochondrial biogenesis, GLUT4 translocation, and glucose uptake, while inhibiting protein synthesis, gluconeogenesis, and fatty acid and cholesterol synthesis.[32] Stimulation of b-oxidation results in the generation of acetyl CoA, which then enters the Kreb's cycle to generate ATP, the primary objective of the body in the energy deficient state. AMPK, through inhibition of the mammalian target of rapamycin (mTOR) pathway, inhibits protein synthesis, an anabolic process needing ATP consumption.[33] AMPK also initiates autophagy, a catabolic process that is activated during starvation or stress, which may result in decreased fat and muscle mass over a long period. Autophagy is characterized by digestion of cellular macromolecules and organelles by engulfing them in double-membrane vesicles (autophagosomes), which fuse with the lysosomes to hydrolyze its contents.[34]
Pancreas
The net effect of AMPK activation is inhibition of insulin secretion from pancreatic B-cells, which is both a direct effect AMPK activation and indirectly through decreased IR at liver, muscle, and adipose tissue.[32] Increased glucose disposal due to decreased IR leads to decreased circulating levels of glucose, which also has an inhibitory effect on insulin release from pancreatic B-cells.
Liver
AMPK activation leads to decreased hepatic IR, leading to decreased hepatic glucose output, a result of both inhibition of gluconeogenesis and glycogenolysis.[32] AMPK, by phosphorylating and inhibiting the enzymes acetyl CoA carboxylase 1 (ACC1) and hydroxymethylglutaryl CoA reductase, the rate-limiting steps in the synthesis of fatty acids and cholesterol, respectively, inhibits these ATP (energy)-consuming processes.[35,36] ACC1 is responsible for conversion of acetyl-CoA to malony-CoA. Malonyl-CoA inhibits carnitine palmitoyltransferase 1 on mitochondrial outer membrane, an enzyme essential for enabling activated long chain fatty acids to enter the mitochondrion for metabolism via the b-oxidation pathway.[37] Hence, inhibition of ACC1 by AMPK not only leads to a direct inhibition of lipogenesis but also leads to an indirect activation of lipolysis, a result of decreased malonyl-CoA. AMPK, through inhibition of the activity of sterol regulatory element-binding protein-1 (SREBP1), downregulates the expression of fatty acid synthase and ACC1, hence an additional mechanism of inhibition of lipogenesis.[38]
Hypothalamus
As highlighted previously, AMPK can increase food intake via activating signaling in the hypothalamus.[31,32] This is an expected physiologic response in an energy-deficient state when the body tries to restore energy balance by increased intake of food (fuel for ATP generation).
Adenosine monophosphate-activated protein kinase in different disease/metabolic states
AMPK downregulation is believed to have a central role in the development of diabetes, through decreased beta-oxidation of fatty acids, increased circulating levels of fatty acids leading to impaired insulin secretion and action (lipotoxicity) and decreased glucose disposal into muscles and adipose tissue secondary to decreased GLUT4 translocation, leading to worsening of hyperglycemia. AMPK activators have been shown to improve IR and glucose uptake in rodent models of the metabolic syndrome.[29,39]
AMPK overactivity has been implicated in the development of neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease, and Huntington's disease.[29,40,41] Mitochondrial dysfunction is common to all the three disorders. Increased mitochondrial dysfunction leads to increased anaerobic glycolysis leading to increased lactate levels, which is a potent stimulator of AMPK activity.[42] Wolff–Parkinson–White (WPW) syndrome, a primary disorder of cardiac rhythm abnormality, is caused by mutations in the PRKAG2 gene, which codes for the AMPKg2 subunit, leading to loss of function mutation of AMPK.[43] This results in cardiac hypertrophy, preexcitation, and conduction abnormalities, the principle features of WPW syndrome.[29,43] AMPK has been implicated both in the suppression as well as promotion of cancer cell growth in different cancers through its complex interlinked regulation of different metabolic pathways.[29,44]
Antidiabetes medications that activate adenosine monophosphate-activated protein kinase
Metformin
Metformin, a biguanide, a derivative from French Lilac plant, is globally accepted as the first-line therapy for uncomplicated T2DM across different guidelines.[45] Glycated hemoglobin (HbA1c) reduction with metformin is comparable to other oral antidiabetes agents (1–2%). Pleotropic benefits attributed to metformin included mild weight reduction, reduction in cardiovascular morbidity and mortality and decreased risk of certain cancers.[46,47] Insulin-sensitizing property of metformin, inhibition of hepatic gluconeogenesis, and increased glucose uptake in muscle and adipose tissue partially have been attributed to indirect activation of AMPK through inhibition of complex-I of mitochondrial respiratory chain.[48] This inhibition results in the switching of metabolism from aerobic to anaerobic glycolysis leading to increased AMP: ATP ratio, and hence AMPK activation.[49] Antagonizing glucagon signaling at liver in an AMPK independent mechanism of insulin-sensitizing action of metformin.[50] Metformin antagonizes glucagon signaling in liver through cyclic AMP and protein kinase A.[50]
Thiazolidinediones
TZDs are another class of insulin sensitizers acting primarily through the nuclear hormone receptor peroxisome proliferator-activated receptor-gamma (PPARγ). The major agents of this class include pioglitazone and rosiglitazone. Studies have demonstrated TZDs to exert a part of their antihyperglycemic effect through AMPK activation.[51] AMPK activation due to TZDs is also indirect as observed with metformin. TZDs stimulate adiponectin release through PPARγ activation.[52] Increased adiponectin has a direct stimulatory effect on AMPK through its signaling via adipoR1 receptor.[53] In addition, TZDs also inhibit complex 1 of the mitochondrial respiratory chain resulting in increased cellular AMP: ATP ratio, leading to AMPK activation.[54] Apart from its insulin-sensitizing effect at muscles, liver, and adipose tissues, TZDs also improve endothelial function and reduce systemic inflammation.[55]
Antidiabetes medications that have mixed action on adenosine monophosphate-activated protein kinase
Glucagon-like peptide-1 receptor agonists
Glucagon-like peptide-1 (GLP-1) is an endogenous incretin molecule secreted from L-cells in ileum. GLP1a (exenatide and liraglutide) are synthetic long-acting agents, which are resistant to breakdown by dipeptidyl peptidase-4 (DPP-4) enzyme unlike endogenous GLP-1, hence need to administer once/twice a day.[56] Beneficial effects of GLP-1a in diabetes are through accentuation of food-mediated insulin release from pancreas (incretin effect), inhibition of glucagon release from pancreas, delaying gastric emptying (promoting satiety), stimulating satiety in brain (thus reducing food intake), increasing peripheral insulin sensitivity, and possibly beta cell regenerative effect.[56,57] Insulin-sensitizing effect of GLP1a at least in part can be explained through AMPK activation.[58,59] Increased AMPK mRNA and protein expression has been documented through increased Thr172 phosphorylation in exenatide-treated hepatocytes and high fat-fed mice, resulting in improved glycemic control along with improvement in dyslipidemia and systemic inflammation.[57,59] Another mechanism of indirect activation of AMPK by GLP1a is through activation of calcium/calmodulin-dependent protein kinase kinase-β (CAMKKβ).[60] Liraglutide has been demonstrated to exert a strong anti-inflammatory effect on human aortic endothelial cells through increased intracellular Ca2+, which activates CAMKKβ, in turn leading to activation of AMPK.[60] The third mechanism of indirect activation of AMPK by GLP1a is through upregulation of sirtuin-1 (SIRT1).[61] In vitro and in vivo studies have demonstrated an ameliorative effect of exenatide on nonalcoholic fatty liver disease (NAFLD) through upregulation of SIRT1 and AMPK.[61] AMPK-mediated insulin-sensitizing effect of GLP1a has been documented only at liver, muscle, and endothelium, but not adipose tissue.[62]
It is interesting to note that the enhancement of beta-cell proliferation by liraglutide has been mediated, partially through its action on AMPK/mTOR signaling.[63] Liraglutide increases cellular ATP levels, leading to inhibition of AMPK phosphorylation, which leads to enhanced mTOR activity, which in turn protects beta cells from glucolipotoxicity induced apoptosis.[63] Thus, liraglutide, and exenatide act as selective site-dependent AMPK agonist/antagonists. In general, it has an AMPK agonist action at all tissues except pancreas where it protects beta cells through AMPK inhibition.
Dipeptidyl peptidase-4 enzyme inhibitors
There is some evidence that the beta-cell protective and anti-inflammatory effects of DPP-4 inhibitors (DPP-4i) are mediated via AMPK activation.[58,59] DPP4i have been demonstrated to have an ameliorative effect on NAFLD in ob/ob mice through indirect activation of AMPK, via increased circulating levels of adiponectin and increased expression of PPARα/microsomal triglyceride transfer protein.[64] Both GLP1a and DPP4i (linagliptin, liraglutide, and sitagliptin) have been demonstrated to ameliorate lipopolysaccharide-induced hypotension and endothelial dysfunction in endotoxemic rats through AMPK activation.[65]
Antidiabetes medications that inhibit adenosine monophosphate-activated protein kinase
Sulfonylureas
Glimepiride, a third-generation sulfonylurea, apart from its insulin secretagogue action, has been demonstrated to also improve IR through activation of PPARγ.[66] Glimepiride has been demonstrated not to have any effect on 5-aminoimidazole-4-carboxamide ribonucleotide-induced phosphorylation of AMPK.[66] Metformin and sitagliptin treatment has been associated with increased adiponectin levels, whereas glimepiride therapy has been associated with decreased adiponectin levels.[67] This decreased adiponectin levels with use of glimepiride, may explain its lack of effect/inhibitory effect on AMPK, as adiponectin-mediated activation has been well demonstrated (vide supra).[67] Metformin, but not sulfonylurea gliclazide, has been demonstrated to activate AMPK and in turn inhibit the activity of the enzyme ACC in human adipose tissue.[68]
Antidiabetes medication that may have an adenosine monophosphate-activated protein kinase-dependent mechanism of action
Alpha glucosidase inhibitor
Data evaluating the relationship between Alpha-glucosidase inhibitor (AGI) use and AMPK activity are scant. Miglitol, an AGI, has been demonstrated to protect against endothelial cells damage under oxidative stress, through AMPK activation and endothelial nitric oxide synthase (eNOS) phosphorylation.[69] This AMPK activation and eNOS phosphorylation have been demonstrated to inhibit endothelial cell apoptosis and mitochondrial superoxide production, respectively.[69]
Sodium glucose co-transporter-2 inhibitor
As of today, no data are available evaluating the impact of use of SGLT2i on AMPK activity. However, studies have shown that postischemic hyperglycemia exacerbates cerebral ischemia, neuronal injury and death through activation of cerebral sodium-glucose transporter type 1 (SGLT1) function, which happens through AMPK activation.[70] In heart, studies have shown that SGLT1 knockout in mice with the PRKAG2 Thr400Asn mutation (implicated in the development of WPW syndrome) attenuates the structural and clinical phenotype of cardiomyopathy associated with WPW syndrome.[71] Hence, this link between SGLT1 and AMPK at brain and heart suggests the urgent need for studies to evaluate the link between SGLT2 and AMPK. It is highly probable that one of the mechanisms of decreasing IR with use of SGLT2i may be through AMPK activation.
Colesevelam
Colesevelam, a nonabsorbable bile acid sequestrant, approved as a cholesterol-reducing agent since 2000, has been observed to reduce blood glucose and HbA1c (≈0.5%).[72] Colesevelam has been observed to be beneficial in improving glycemic control in T2DM, as a single agent as well as in combination with metformin, sulfonylureas, pioglitazone, and insulin.[72,73,74,75] However, the mechanism of this antihyperglycemic effect of colesevelam is poorly understood. It causes a small reduction in acute glucose absorption.[76] It also has a mild incretin effect to stimulate GLP-1 secretion from L-cells in ileum.[76,77] Colesevelam use results in amore hydrophilic bile acid pool due to an increase of cholic acid synthesis, which results in activation of farnesoid X receptor (FXR) and TGR5 (a G-protein-coupled membrane receptor) in liver, contributing to improvement in glucose homeostasis.[73] Hepatic FXR activation induces the expression of the orphan nuclear receptor short heterodimer protein, which leads to decreased activity of SREBP-1, which results in decreased lipogenesis and increased lipolysis.[73,74] However, the detailed pathway of how FXR activation leads to decreased SREBP-1 activity is not known. It is likely that AMPK activation may have a role, which needs further evaluation.
Bromocriptine
A dosage of quick-release bromocriptine, taken within 2 h of waking up daily in the morning, is an approved agent for managing T2DM. It is noted for its mild antihyperglycemic effects with low risk for hypoglycemia. Randomized controlled trials have demonstrated significant decline in both fasting, postprandial blood glucose levels along with HbA1c.[78] The therapeutic effects of bromocriptine are most likely to be centrally mediated with results in decreased IR and increased glucose uptake in peripheral tissues especially skeletal muscles and adipose tissue.[77,78]
Central dopamine concentrations are diminished in T2DM patients, especially in those with obesity, which leads to increased sympathetic activity.[79] Increased sympathetic activity and RAS activation are well known to be associated with the development of T2DM. Early morning administration of bromocriptine is believed to increase hypothalamic dopaminergic tone resulting in reduced sympathetic nervous activity, decreased hepatic glucose output, glucose intolerance, and IR.[78,79] The impact of bromocriptine on central and peripheral AMPK activity has not been evaluated. There is an urgent need for studies evaluating the impact of bromocriptine on AMPK activity.
Investigational agents for managing diabetes based on their adenosine monophosphate-activated protein kinase activation properties
Berberine
Derived from Berberis plant, this compound has been used in traditional Chinese and Korean medicine for managing diabetes as well as different infections.[80] Animal studies have demonstrated berberine use to be associated with improved glucose tolerance, reduced body weight, increased expression of both the insulin receptor and low-density lipoprotein receptor, with a favorable impact on lipid profile (reduced low-density lipoprotein cholesterol and triglycerides).[81,82] Human studies have demonstrated glycemic efficacy of berberine to be comparable to that of metformin.[83] Berberine has been demonstrated to upregulate AMPK through inhibiting complex I of the mitochondrial respiratory chain, similar to that of metformin.[84] Berberine acutely activates AMPK activity in both adipocytes and myocytes, resulting in increased GLUT4 translocation, reduced lipid content in adipocytes; increased expression of genes involved in lipid oxidation and decreased expression of genes involved in lipid synthesis.[82]
Salicylates
Salicylates are currently under investigation as promising agents of the future for managing diabetes.[29] Salicylates have been demonstrated to activate AMPK by binding itself in the cleft between the kinase domain of the a-subunit and the CBM domain of the b-subunit.[85]
Resveratrol
Resveratrol, a polyphenol found in red wine, has been demonstrated to stimulate AMPK activity in hepatocytes, muscle cells, and neurons, secondary to increase in AMP levels due to inhibition of the mitochondrial F1 ATPase.[86,87,88,89] AMPK activation results in increased mitochondrial biogenesis along with increased glucose uptake in muscle and adipose tissue.[90] Resveratrol is believed to be responsible for the beneficial effects of red wine on oxidative stress and IR. Improvements in insulin sensitivity along with decreased circulating markers of aging were observed with resveratrol treatment of animals on high-fat diet.[91]
Conclusion
To summarize, metformin and glitazones are pure stimulators of AMPK. Incretin-based therapies have a mixed action on AMPK. Sulfonylureas either inhibit AMPK or have no effect on AMPK. Glycemic efficacy of AGIs, SGLT2i, colesevelam, and bromocriptine may also involve AMPK activation, which warrants further evaluation. Berberine, salicylates, and resveratrol are newer promising agents in the management of diabetes, having well-documented evidence of AMPK stimulation-medicated glycemic efficacy. Hence, AMPK-based classification of antidiabetes medications is important as it provides a simple unifying understanding of the complex pharmacotherapy in diabetes. This form of classification is flexible with a scope for inclusion of promising agents of the future in the management of diabetes. This classification allows for a more rational choice of antidiabetes medication, either alone or in combination.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. [Last accessed on 2016 May 01]. Available from: http://www.diabetesatlas.org . [Google Scholar]
- 2.Dutta D, Maisnam I, Shrivastava A, Sinha A, Ghosh S, Mukhopadhyay P, et al. Serum Vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J Med Res. 2013;138:853–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Dutta D, Choudhuri S, Mondal SA, Mukherjee S, Chowdhury S. Urinary albumin: Creatinine ratio predicts prediabetes progression to diabetes and reversal to normoglycemia: Role of associated insulin resistance, inflammatory cytokines and low Vitamin D. J Diabetes. 2014;6:316–22. doi: 10.1111/1753-0407.12112. [DOI] [PubMed] [Google Scholar]
- 4.Dutta D, Mukhopadhyay S. Comment on Anjana et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai urban rural epidemiology study (CURES) Diabetes Care. 2015;38:1441–1448. doi: 10.2337/dc14-2814. Diabetes Care 2015;38:e146. [DOI] [PubMed] [Google Scholar]
- 5.Dutta D, Mukhopadhyay S. Intervening at prediabetes stage is critical to controlling the diabetes epidemic among Asian Indians. Indian J Med Res. 2016;143:401–4. doi: 10.4103/0971-5916.184281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta D, Mondal SA, Choudhuri S, Maisnam I, Hasanoor Reza AH, Bhattacharya B, et al. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: An open label randomized prospective study from Eastern India. Diabetes Res Clin Pract. 2014;103:e18–23. doi: 10.1016/j.diabres.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Dutta D, Mondal SA, Kumar M, Hasanoor Reza AH, Biswas D, Singh P, et al. Serum fetuin-A concentration predicts glycaemic outcomes in people with prediabetes: A prospective study from eastern India. Diabet Med. 2014;31:1594–9. doi: 10.1111/dme.12539. [DOI] [PubMed] [Google Scholar]
- 8.Dutta D, Choudhuri S, Mondal SA, Maisnam I, Reza AH, Ghosh S, et al. Tumor necrosis factor alpha -238G/A (rs 361525) gene polymorphism predicts progression to type-2 diabetes in an Eastern Indian population with prediabetes. Diabetes Res Clin Pract. 2013;99:e37–41. doi: 10.1016/j.diabres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalra S, Ayyar V, Unnikrishnan AG. Adrenergic India: Managing its diabetes. Indian J Endocrinol Metab. 2011;15(Suppl 1):S1–2. doi: 10.4103/2230-8210.83046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalra S, Kalra B, Agrawal N, Kumar S. Dopamine: The forgotten felon in type 2 diabetes. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:61–5. doi: 10.2174/187221411794351842. [DOI] [PubMed] [Google Scholar]
- 12.Kalra S. Recent advances in pathophysiology of diabetes: Beyond the dirty dozen. J Pak Med Assoc. 2013;63:277–80. [PubMed] [Google Scholar]
- 13.Kalra S, Chawla R, Madhu SV. The dirty dozen of diabetes. Indian J Endocrinol Metab. 2013;17:367–9. doi: 10.4103/2230-8210.111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR, 3rd, Aguilar RB. The time is right for a new classification system for diabetes: Rationale and implications of the ß-cell-centric classification schema. Diabetes Care. 2016;39:179–86. doi: 10.2337/dc15-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitabchi AE. Ketosis-prone diabetes – a new subgroup of patients with atypical type 1 and type 2 diabetes? J Clin Endocrinol Metab. 2003;88:5087–9. doi: 10.1210/jc.2003-031656. [DOI] [PubMed] [Google Scholar]
- 16.Dutta D, Kalra S. Sodium glucose transporter 2 (sglt2) inhibitors: Current status in clinical practice. J Pak Med Assoc. 2014;64:1203–6. [PubMed] [Google Scholar]
- 17.Kalra S, Gupta Y, Patil S. Sodium-glucose cotransporter-2 inhibition and the insulin: Glucagon ratio: Unexplored dimensions. Indian J Endocrinol Metab. 2015;19:426–9. doi: 10.4103/2230-8210.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalra S, Sahay R, Gupta Y. Sodium glucose transporter 2 (SGLT2) inhibition and ketogenesis. Indian J Endocrinol Metab. 2015;19:524–8. doi: 10.4103/2230-8210.157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–51. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 20.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–72. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–78. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 22.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 24.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro . J Biol Chem. 2006;281:25336–43. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 25.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32(Suppl 4):S55–9. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 26.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 27.Green MF, Anderson KA, Means AR. Characterization of the CaMKKß-AMPK signaling complex. Cell Signal. 2011;23:2005–12. doi: 10.1016/j.cellsig.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landström M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–9. doi: 10.1016/j.biocel.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Krishan S, Richardson DR, Sahni S. Adenosine monophosphate-activated kinase and its key role in catabolism: Structure, regulation, biological activity, and pharmacological activation. Mol Pharmacol. 2015;87:363–77. doi: 10.1124/mol.114.095810. [DOI] [PubMed] [Google Scholar]
- 30.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res. 2012;53:2490–514. doi: 10.1194/jlr.R025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 33.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi KS. Autophagy and cancer. Exp Mol Med. 2012;44:109–20. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi A, Evans JL, Iverson AJ, Nordlund AC, Watts TD, Witters LA. Identification of an isozymic form of acetyl-CoA carboxylase. J Biol Chem. 1990;265:1502–9. [PubMed] [Google Scholar]
- 36.Habegger KM, Hoffman NJ, Ridenour CM, Brozinick JT, Elmendorf JS. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology. 2012;153:2130–41. doi: 10.1210/en.2011-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bremer J. Carnitine in intermediary metabolism. The biosynthesis of palmitylcarnitine by cell subfractions. J Biol Chem. 1963;238:2774–9. [PubMed] [Google Scholar]
- 38.Hardie DG. Regulation of fatty acid synthesis via phosphorylation of acetyl-CoA carboxylase. Prog Lipid Res. 1989;28:117–46. doi: 10.1016/0163-7827(89)90010-6. [DOI] [PubMed] [Google Scholar]
- 39.Song XM, Fiedler M, Galuska D, Ryder JW, Fernström M, Chibalin AV, et al. 5-aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia. 2002;45:56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer's disease. J Neurosci. 2007;27:824–31. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim MA, Selak MA, Xiang Z, Krainc D, Neve RL, Kraemer BC, et al. Reduced activity of AMP-activated protein kinase protects against genetic models of motor neuron disease. J Neurosci. 2012;32:1123–41. doi: 10.1523/JNEUROSCI.6554-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JL, Merl D, Peterson CW, Wu J, Liu PY, Yin H, et al. Lactic acidosis triggers starvation response with paradoxical induction of TXNIP through MondoA. PLoS Genet. 2010;6:e1001093. doi: 10.1371/journal.pgen.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gollob MH, Roberts R. AMP-activated protein kinase and familial Wolff–Parkinson–White syndrome: New perspectives on heart development and arrhythmogenesis. Eur Heart J. 2002;23:679–81. doi: 10.1053/euhj.2001.2954. [DOI] [PubMed] [Google Scholar]
- 44.Bonini MG, Gantner BN. The multifaceted activities of AMPK in tumor progression – why the “one size fits all” definition does not fit at all? IUBMB Life. 2013;65:889–96. doi: 10.1002/iub.1213. [DOI] [PubMed] [Google Scholar]
- 45.Standards of medical care in diabetes – 2016: Summary of revisions. Diabetes Care. 2016;39(Suppl 1):S4–5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 46.Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–72. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 48.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 49.Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241–53. doi: 10.2147/DMSO.S43731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–60. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 52.Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and independent pathways. J Biol Chem. 2006;281:8748–55. doi: 10.1074/jbc.M505649200. [DOI] [PubMed] [Google Scholar]
- 53.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 54.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: A common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–9. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 55.Yau H, Rivera K, Lomonaco R, Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr Diab Rep. 2013;13:329–41. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 56.Hwang JI, Yun S, Moon MJ, Park CR, Seong JY. Molecular evolution of GPCRs: GLP1/GLP1 receptors. J Mol Endocrinol. 2014;52:T15–27. doi: 10.1530/JME-13-0137. [DOI] [PubMed] [Google Scholar]
- 57.Baggio LL, Drucker DJ. Harnessing the therapeutic potential of glucagon-like peptide-1: A critical review. Treat Endocrinol. 2002;1:117–25. doi: 10.2165/00024677-200201020-00005. [DOI] [PubMed] [Google Scholar]
- 58.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–97. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 59.Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC, et al. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS One. 2012;7:e31394. doi: 10.1371/journal.pone.0031394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krasner NM, Ido Y, Ruderman NB, Cacicedo JM. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One. 2014;9:e97554. doi: 10.1371/journal.pone.0097554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu F, Li Z, Zheng X, Liu H, Liang H, Xu H, et al. SIRT1 mediates the effect of GLP-1 receptor agonist exenatide on ameliorating hepatic steatosis. Diabetes. 2014;63:3637–46. doi: 10.2337/db14-0263. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka K, Masaki Y, Tanaka M, Miyazaki M, Enjoji M, Nakamuta M, et al. Exenatide improves hepatic steatosis by enhancing lipid use in adipose tissue in nondiabetic rats. World J Gastroenterol. 2014;20:2653–63. doi: 10.3748/wjg.v20.i10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013;39:71–9. doi: 10.1016/j.peptides.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Ohyama T, Sato K, Yamazaki Y, Hashizume H, Horiguchi N, Kakizaki S, et al. MK-0626, a selective DPP-4 inhibitor, attenuates hepatic steatosis in ob/ob mice. World J Gastroenterol. 2014;20:16227–35. doi: 10.3748/wjg.v20.i43.16227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steven S, Hausding M, Kröller-Schön S, Mader M, Mikhed Y, Stamm P, et al. Gliptin and GLP-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res Cardiol. 2015;110:6. doi: 10.1007/s00395-015-0465-x. [DOI] [PubMed] [Google Scholar]
- 66.Inukai K, Watanabe M, Nakashima Y, Takata N, Isoyama A, Sawa T, et al. Glimepiride enhances intrinsic peroxisome proliferator-activated receptor-gamma activity in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;328:484–90. doi: 10.1016/j.bbrc.2004.12.190. [DOI] [PubMed] [Google Scholar]
- 67.Saad MI, Kamel MA, Hanafi MY. Modulation of adipocytokines production and serum NEFA level by metformin, glimepiride, and sitagliptin in HFD/STZ diabetic rats. Biochem Res Int 2015. 2015 doi: 10.1155/2015/138134. 138134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyle JG, Logan PJ, Jones GC, Small M, Sattar N, Connell JM, et al. AMP-activated protein kinase is activated in adipose tissue of individuals with type 2 diabetes treated with metformin: A randomised glycaemia-controlled crossover study. Diabetologia. 2011;54:1799–809. doi: 10.1007/s00125-011-2126-4. [DOI] [PubMed] [Google Scholar]
- 69.Aoki C, Suzuki K, Yanagi K, Satoh H, Niitani M, Aso Y. Miglitol, an anti-diabetic drug, inhibits oxidative stress-induced apoptosis and mitochondrial ROS over-production in endothelial cells by enhancement of AMP-activated protein kinase. J Pharmacol Sci. 2012;120:121–8. doi: 10.1254/jphs.12108fp. [DOI] [PubMed] [Google Scholar]
- 70.Yamazaki Y, Ogihara S, Harada S, Tokuyama S. Activation of cerebral sodium-glucose transporter type 1 function mediated by post-ischemic hyperglycemia exacerbates the development of cerebral ischemia. Neuroscience. 2015;310:674–85. doi: 10.1016/j.neuroscience.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Ramratnam M, Sharma RK, D’Auria S, Lee SJ, Wang D, Huang XY, et al. Transgenic knockdown of cardiac sodium/glucose cotransporter 1 (SGLT1) attenuates PRKAG2 cardiomyopathy, whereas transgenic overexpression of cardiac SGLT1 causes pathologic hypertrophy and dysfunction in mice. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000899. pii: e000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: The clinical evidence. Diabetes Obes Metab. 2010;12:384–92. doi: 10.1111/j.1463-1326.2009.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levy P. Review of studies on the effect of bile acid sequestrants in patients with type 2 diabetes mellitus. Metab Syndr Relat Disord. 2010;8(Suppl 1):S9–13. doi: 10.1089/met.2010.0087. [DOI] [PubMed] [Google Scholar]
- 74.Prawitt J, Staels B. Bile acid sequestrants: Glucose-lowering mechanisms. Metab Syndr Relat Disord. 2010;8(Suppl 1):S3–8. doi: 10.1089/met.2010.0096. [DOI] [PubMed] [Google Scholar]
- 75.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 76.Henry RR, Aroda VR, Mudaliar S, Garvey WT, Chou HS, Jones MR. Effects of colesevelam on glucose absorption and hepatic/peripheral insulin sensitivity in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:40–6. doi: 10.1111/j.1463-1326.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thulé PM. Mechanisms of current therapies for diabetes mellitus type 2. Adv Physiol Educ. 2012;36:275–83. doi: 10.1152/advan.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, et al. Bromocriptine: A novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000;23:1154–61. doi: 10.2337/diacare.23.8.1154. [DOI] [PubMed] [Google Scholar]
- 79.Defronzo RA. Bromocriptine: A sympatholytic, d2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011;34:789–94. doi: 10.2337/dc11-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tillhon M, Guamán Ortiz LM, Lombardi P, Scovassi AI. Berberine: New perspectives for old remedies. Biochem Pharmacol. 2012;84:1260–7. doi: 10.1016/j.bcp.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 81.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 82.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 83.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–7. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–65. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–63. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–22. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Birnbaum MJ. Activating AMP-activated protein kinase without AMP. Mol Cell. 2005;19:289–90. doi: 10.1016/j.molcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 90.Park CE, Kim MJ, Lee JH, Min BI, Bae H, Choe W, et al. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med. 2007;39:222–9. doi: 10.1038/emm.2007.25. [DOI] [PubMed] [Google Scholar]
- 91.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]