Abstract
Mutations in the guanine nucleotide-binding protein (G protein), α activating activity polypeptide O (GNAO1) gene have recently been described in 6 patients with early infantile epileptic encephalopathies. In the present study, we report the phenotype and the clinical course of a 4-year-old female with an epileptic encephalopathy (Ohtahara syndrome) and profound intellectual disability due to a de novo GNAO1 mutation (c.692A>G; p.Tyr231Cys). Ohtahara syndrome is a devastating early infantile epileptic encephalopathy that can be caused by mutations in different genes, now also including GNAO1. The mutation was found using a targeted next generation sequencing gene panel and demonstrates targeted sequencing as a powerful tool for identifying mutations in genes where only a few de novo mutations have been identified.
Keywords: early infantile epileptic encephalopathy, ohtahara syndrome, GNAO1 mutation, next generation sequencing gene panel
Mutations in the guanine nucleotide-binding protein (G protein), α activating activity polypeptide O (GNAO1) gene have been identified in breast carcinomas,1 hepatocellular carcinomas,2 and in 6 patients with early infantile epileptic encephalopathies.3,4 Epileptic encephalopathies are a group of disorders characterized by severe and progressive cognitive and behavioral impairments that most likely are caused or worsened by epileptic activity.5 Ohtahara syndrome is one of the most severe and earliest forms of epileptic encephalopathies and is characterized by tonic spasms in the neonatal period, seizure intractability, and a suppression-burst pattern on electroencephalography (EEG).6 Recently, de novo mutations in several other genes (ARX, SCN2A, STXBP1, KCNQ2, etc) have been reported in individuals with Ohtahara syndrome.7–10
GNAO1 encodes the α subunit of the heterotrimeric guanine nucleotide-binding proteins (G proteins). G proteins are a large family of signal-transducing molecules composed of α, β, and γ subunits. Members of the G protein family have been characterized most extensively on the basis of the α subunit, which binds the guanine nucleotide and is capable of hydrolyzing guanosine-5-triphosphate as well as interacting with specific receptor and effector molecules. The α subunits decoded by GNAO1 are extremely abundant in brain tissue, suggesting important roles in brain function. Mice lacking Gαo show multiple neurological abnormalities, including generalized tremor, occasional seizures, severe motor-control impairment, hyperalgesia, and behavioral abnormalities with early postnatal lethality.11,12
In vitro functional expression studies showed that 3 of the previously published mutations in GNAO1 cause impaired protein localization to the plasma membrane, and electrophysiological analysis showed a decreased GNAO1-mediated inhibition of calcium currents by norepinephrine compared to the wild type. The findings suggest that aberrant GNAO1 signaling can cause multiple neurodevelopmental phenotypes, including epileptic encephalopathy and involuntary movements.13 In the present study, we report the phenotype and clinical course of a 4-year-old female with a de novo GNAO1 (c.692A>G; p.Tyr231Cys) mutation (OMIM 615473, EIEE17).
Case Report
The present patient was the third child of nonconsanguineous healthy parents of Estonian ancestry. At gestation week 28, hypotrophia of the fetus was diagnosed; however, the girl was born at term with a birth weight of 4630 g and a head circumference of 38 cm. The neonatal period was complicated by poor feeding. In addition, unspecified stereotypic jerks in the lower extremities were noticed. According to the mother’s description, the child developed epileptic spasm-like episodes at the age of 2½ months. At the age of 3 months, she was admitted to the Department of Paediatric Neurology of Children’s Clinic of Tartu University Hospital with a suspected seizure disorder: She had myoclonic seizures, tonic spasms, and tonic–clonic seizures. On admission, she was developmentally delayed with no eye contact. She was opistotonic and had a spastic muscle tone most severe in the lower extremities. Babinski reflex was bilaterally positive. She had subtle dysmorphic features (Figure 1), including a broad nasal bridge, deep philtrum, high palate, and her head circumference was 39 cm (−25 percentile).
Figure 1.
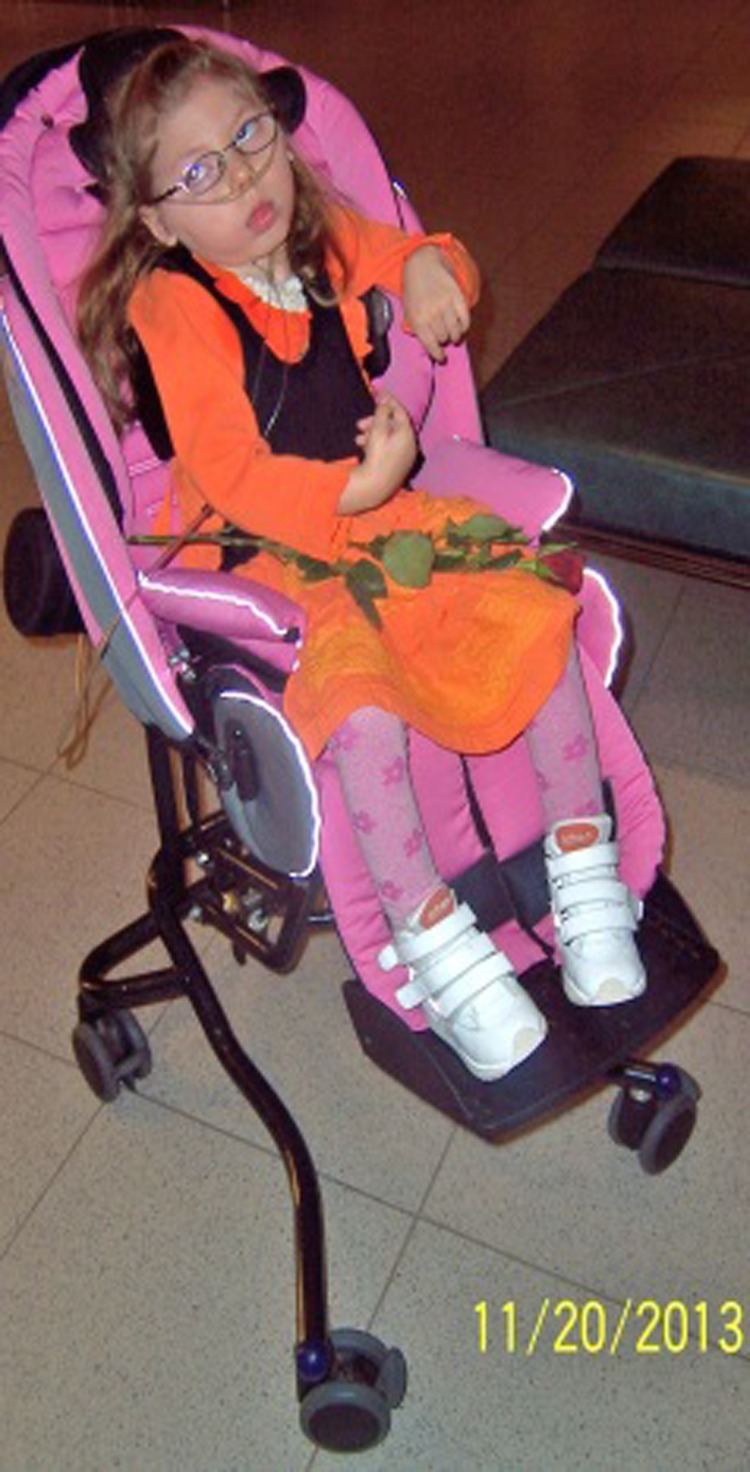
Phenotype with subtle dysmorphic features.
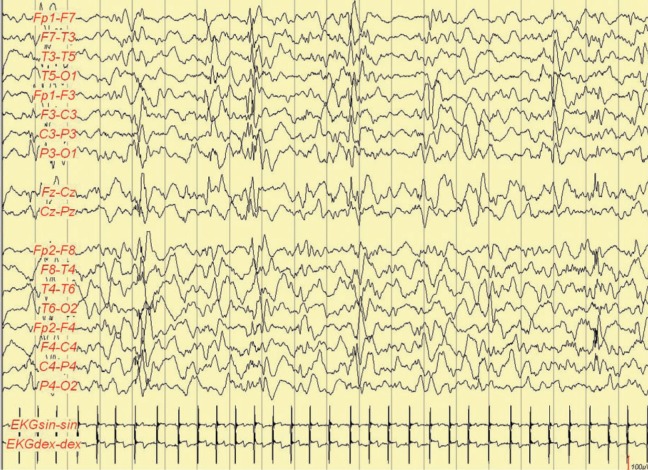
Electroencephalography at the age of 3 months showed a modified burst-suppression pattern. Interictal EEG when awake showed background activity with 1.5 to 4.5 Hz, up to 150 µV, delta activity mixed with high-voltaged (up to 250 µV) spike waves and sharp waves, and bilateral asynchronous and synchronous bursts in the posterior areas. At sleep, there were bilateral high-voltaged (up to 360 µV) irregular slow wave bursts mixed with multifocal spikes and sharp waves (duration in deeper sleep <1 seconds) alternately with low-voltaged (20-30 µV) periods (duration up to 2 seconds; Figure 2). In some stages, 150 to 200 µV delta activity mixed with multifocal sharp waves and spikes. Ictally myoclonias, tonic spasms, and myoclonias following tonic or tonic–clonic seizures were present (Figure 3). Considering the clinical symptoms and EEG findings, Ohtahara syndrome was suspected.
Figure 2.
Electroencephalography (EEG) at the age of 3 months. At sleep bilateral high voltaged up to 360 µV irregular slow wave bursts mixed with multifocal spikes and sharp waves (duration <1 second) alternately with low-voltaged (20-30 µV) periods (duration up to 2 seconds).
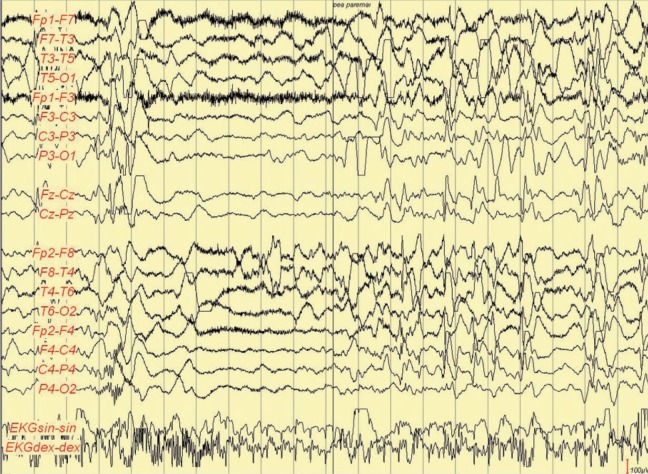
Figure 3.
Ictal electroencephalography (EEG) at the age of 3 months. Myoclonia followed by tonic–clonic seizure.
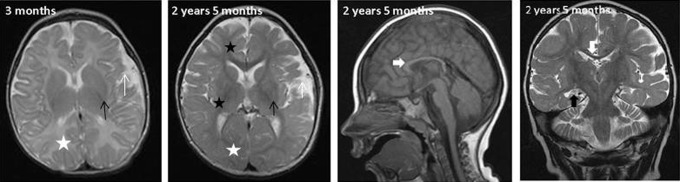
Magnetic resonance imaging (MRI; Figure 4) at the age of 3 months showed normal lateral ventricles but asymmetrical subarachnoidal spaces in temporal regions (white arrow). Some delay was seen in myelinization in the anterior part of internal capsule and splenium of corpus callosum, however, normal amount of white matter (white asterix). Corpus callosum was generally short and thin, and basal ganglia showed normal size (black arrow) and signal intensity. The MRI was performed later twice (at the age of 1.2 years and 2 years 5 months) with generally decreased amount of white matter (white asterix) and size of basal ganglia (black arrow) with some increase in signal intensity in external capsule and periventricular white matter (black asterix) on T2-weighted images. Some delay in myelinization was still visible. At the age of 1.2 years, the myelinization was at the level of 8 to 10 months, at the age of 2 years 5 months, some progression in myelinization had occurred, but the myelinization was still at the level of 1.5 years. At the age of 2 years and 5 months, the side ventricles were normal in size, but progression of enlargement of the left-side temporal subarachnoidal space (white arrow) was documented without signs of migration failure. Short and thin corpus callosum (white bold arrow) and hippocampus (black bold arrow) atrophy without significant progression between 1.2 and 2 years 5 months were noted. Pathologic metabolites were not found on spectroscopy. On magnetic resonance angiography, hypoplastic left vertebral artery was identified.
Figure 4.
Magnetic resonance imaging (MRI) findings.
During the course of the disease, the patient had multiple seizure types, including myoclonic jerks, focal seizures with secondary generalization, epileptic spasms, and generalized tonic–clonic seizures. Her seizure frequency had been fluctuating from 10 to 15 seizures per day to a few per month. She has been treated with several different antiepileptic drugs, including phenobarbitone, vigabatrin, and lamotrigine and also with different combinations of these medications without any sufficient effect. At the age of 6 months, she had stereotypic waving movements of the hands, and at the age of 4 years, she developed series of nonepileptic spasm-like episodes. Psychomotor development has been delayed from the birth, however, some progress has been observed. She achieved occasional eye contact at the age of 6 months, head control was obtained at the age of 9 months, and at the age of 1 year she had communicative smile. By the age of 2 years, she became oxygen dependent due to multiple pneumonias, several bronchitises, food aspirations, and asthma.
Burst-suppression pattern in EEG disappeared at the age of 9 months. The EEG showed slow activity 2 to 3 Hz with some theta bursts when awake and asymmetrical slowing 1 to 2 Hz in left side with multifocal interictal epileptiform discharges in sleep. Last EEG at the age of 4 years showed general slowing with interictal epileptiform discharges in sleep in the left hemisphere.
On the last examination at 4 years of age, she had obtained head control for a short time and was able to pull herself to an upright seating position, which she could not maintain. She was able to turn herself from supine to one side. She had spastic tetraparesis (legs > hands), more pronounced on the right side. Her head growth had been slow since the age of 3 months, and at the age of 4 years, her head circumference was 46.5 cm (−2 standard deviation). She had profound global developmental delay without any spoken language. She was able to keep a short-term eye contact, observe her surroundings for some minutes. She responded with a proper smile when being spoken to. No adequate control over salivation.
Over the years, extensive metabolic workup was performed, but all tests were normal. Chromosomal analysis showed normal karyotype 46, XX, copy number variant analyses were normal. At the age of 4 years, gene panel of epileptic encephalopathies was performed. A written informed consent was obtained from parents for genetic testing and for publication of a case report.
Methods
We collected the DNA samples from the girl and her parents. Genomic DNA from the family was extracted from EDTA anticoagulated blood samples using standard methods. To identify the disease-causing gene, targeted next generation sequencing of 40 different genes associated with childhood epilepsy was performed. Targeted next generation sequencing libraries were prepared from 20 ng of template DNA using the Ion AmpliSeq library 2.0 kit and primers for the 40 genes (Life Technologies, USA) following the manufacturer’s instructions. Sequencing adaptors with index sequences (barcodes) that enable sample multiplexing were ligated to the amplicons using the Ion Xpress barcode adaptor kit (Life Technologies). The library DNA was clonally amplified onto the IonSpheres (Life Technologies) by emulsion polymerase chain reaction following the manufacturer’s protocol, and IonSpheres were isolated by breaking the emulsion using an Ion OneTouch system. Enrichment of IonSpheres was achieved using the IT OneTouch ES (Life Technologies) and the Ion OneTouch 200 template kit following the manufacturer’s protocol. Enriched IonSpheres were sequenced on an Ion 314 Chip using the sequencing kit (Life Technologies) per the manufacturer’s instructions. Segregation analysis was carried out using conventional Sanger sequencing.
Results
Targeted next generation sequencing of 40 genes associated with early-onset epilepsy was performed. A heterozygous missense variation at c.692A>G; p.Tyr231Cys in exon 6 of GNAO1 was detected. The amino acid change was predicted to be pathogenic by PolyPhen-2 and Sift altering a highly evolutionary conserved amino acid. The mutation has not been observed in the 1000Genomes project, Exome Variant Server, or dbSNP (The Single Nucleotide Polymorphism Database), and segregation analysis revealed that the mutation occurred de novo in the girl.
Discussion
In the present study, we describe a 4-year-old girl with an epileptic encephalopathy (Ohtahara syndrome), severe developmental delay, intellectual disability, and absence of speech (EIEE17). We have identified a likely damaging genetic variation in the GNAO1 gene using a targeted next generation sequencing gene panel. The role of GNAO1 in EIEE was recently identified by Nakamura et al3 who described de novo heterozygous missense GNAO1 mutations in 4 patients with severe intellectual disability and motor developmental delay. In addition, 2 patients with a de novo GNAO1 mutation were recently identified by exome sequencing of a cohort of patients with infantile spasms.4 The present patient is hence the seventh case described with an epileptic encephalopathy due to a mutation in GNAO1.
There are some similarities, as well as differences, between the present patient and the patients described previously3,4 (Table 1). Three of the published cases were diagnosed as having Ohtahara syndrome. In the present patient, Ohtahara syndrome was suspected at the age of 3 months. However, it is important to underline that at the age of 9 months, the burst-suppression pattern in the EEG disappeared in the presented case—being evaluated as one of the important symptoms of Ohtahara syndrome. During the course of the disease, the present patient had multiple seizure types including myoclonic seizures, focal seizures with secondary generalization, epileptic spasms, and generalized tonic and tonic–clonic seizures. In comparison, all 4 patients described by Nakamura et al3 and 1 girl from the EuroEPINOMICS4 cohort developed only tonic seizures. These observations underline that several diagnostic features are age dependent and/or disease course dependent. Furthermore, in addition to the alterations in the epileptic seizure type, other clinical symptoms have also changed during the 4 years of follow-up. Interestingly, the child has obtained some abilities, for example, short-term eye contact and the ability to push herself up for a moment. The patients described by Nakamura et al3 had additional movement disorders including dystonia and chorea and athetosis, while the patient of this study developed nonepileptic spasms-like movements in her legs at the age of 4 years, which could be of a subcortical origin. The patients described by the EuroEPINOMICS Consortium4 did not have any movement disorders but presented with profound hypotonia. Helbig14 underlined that this observation could indicate that Gαo is also involved in other systems and that the phenotype may well extend beyond an epileptic encephalopathy. Two of the patients described by Nakamura et al3 died from respiratory tract obstruction at the age of 3 and 11 months, respectively, while the present patient became oxygen dependent at the age of 2 years after several respiratory infections.
Table 1.
Basic Clinical Data of Our Patient and 3 Patients Described by Nakamura et al3 and 2 Patients by EuroEPINOMICS4 Cohort.
| The Patient of this Study | Individual 13 | Individual 23 | Individual 33 | Individual 4 3 | Individual 54 | Individual 64 | |
|---|---|---|---|---|---|---|---|
| Gender | Female | Female | Female | Female | Female | Female | Female |
| Age | 4 years 9 months | 13 years | 4 years 1 month | Died at 11 month | 8 years | 3 years | 9 years |
| Mutation | c.692A>G (p.Tyr231Cys) | c.836T>A (p.Ile279Asn) | c.521A>G (p.Asp174Gly) | c.572_592del (p.Thr191_Phe197 del) | c.607 G>A (p.Gly203Arg) | c.808>C (p.Asn270His) | c.824T>C (p.Phe278Ser) |
| Inheritance | De novo | De novo | De novo, somatic mosaic | De novo | De novo | De novo | De novo |
| DG | OS | OS | OS | OS | EE | EE | EE |
| Initial symptom | Myoclonic jerks 1 mo | Tonic sz at 4 days | Tonic sz at 29 days | Tonic sz at 2 weeks | Opisthotonic posture | Sz at the age of 3 months | Sz at the age of 3 days |
| Initial EEG | Modified SBP at 3 months | SBP at 4 days | SBP at 29 days | SBP at 2 weeks | Spike and slow wave complex at 5 years | Hypsarrythmia | First EEG normal, later BSP |
| Course of seizures | Spasms, tonic, tonic–clonic, myoclonic sz | Tonic seizures at 5 years | Series of tonic seizures at 9 months | Tonic seizure at 10 months | Focal seizure, tonic sz at 5 years | Sz free since the age of 5 months | Daily tonic sz |
| Course of EEG First EEG at 3 Mo | Hypsarrhythmia in awake BSP in sleep at 7 months; slowing of background activity with multifocal IED at 9 months | Multifocal sharp waves at 1 year, 4 months; SBP at 5 years, 6 months | Hypsarrhythmia at 3 months; diffuse spike and slow wave complex at 1 year 7 months; sharp waves at frontal lobe at 3 years 9 months | Hypsarrhythmia at 4 months | Not done | Slow background no epileptic activity | Hypsarrythmia, multifocal discharges |
| Involuntary movement | Stereotypic spasm-like movements | – | – | Dystonia | Severe chorea, athetosis | – | – |
| Seizure control | Intractable (4-6 times per day) | Intractable (2-3 times per day) | Intractable (0-2 times per day) | Intractable | Intractable (several times per day) | Off the medication | Daily tonic seizures |
Abbreviations: OS, ohtahara syndrome; EE, epileptic encephalopathy; SBP, suppression-burst pattern; sz, seizures; EEG, electroencephalography; IED, interictal epileptiform discharge; BSP, burst-supression pattern; DG, diagnosis.
By identifying a de novo GNAO1 mutation in a patient with a severe epileptic encephalopathy, the present report demonstrates the ability of targeted sequencing as a powerful tool to identify mutations in genes where only a few de novo mutations have been identified. We recommend that targeted sequencing or whole-exome sequencing should be performed in children with early infantile epileptic encephalopathies, which may lead to the discovery of rare disease-causing genetic variations,15 as in the present case. Unfortunately, this is not always possible due to high costs of these investigations, but on the other hand, the early etiological diagnosis might reduce unnecessary investigations and might lead to a targeted treatment reducing side effects from the trial-and-error treatment approach. Taking into account the changing of the symptoms during course of the disease, the long-term follow-up will eventually benefit from knowing the basis of disease and its prognosis. Furthermore, it plays an important role in the process of informing the parents about the cause of the disease and is of utmost importance in genetic counseling.
Acknowledgments
Authors would like to thank the family of the patients of the study for extremely nice cooperation. Authors are thankful to the Triin Laisk-Podar for the revision of the manuscript.
Footnotes
Author Contribution: Patient referral and patient’s clinical information were provided by IT, and TT; patient’s MRI was reviewed by PI; electroencephalography by UV, sequencing was carried out and interpreted by RM, LL, and HD. Manuscript was written by IT and MV, and cowritten and critically revised by TT; all authors contributed to and approved the final version of the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the EuroEPINOMICS grant SARLA 11091E. I. Talvik, U. Vaher, and T. Talvik received support from the EuroEPINOMICS grant SARLA 11091E.
Ethical approval: This study was approved by the Research Ethics Committee of the University of Tartu and the Ethics Committee of Western Sealand.
References
- 1. Garcia-Marcos M, Ghosh P, Farquhar MG. Molecular basis of a novel oncogenic mutation in GNAO1. Oncogene. 2011;30(23):2691–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pei X, Zhang J, Wu L, et al. The down-regulation of GNAO1 and its promoting role in hepatocellular carcinoma. Biosci Rep. 2013;33(5):e00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakamura K, Kodera H, Akita T, et al. De Novo mutations in GNAO1, encoding a Galphao subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am J Hum Genet. 2013;93(3):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. EuroEPINOIMICS-RES Consortium, Epilepsy Phenomke? Genome Project, and Epi4 K Consortium. De novo mutations in synaptic transmission genes including DNM1 cause epileptic encepühalopathies. Am J Hum Genet. 2014;95(4):360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dulac O. Epileptic encephalopathy. Epilepsia. 2001;42(suppl 3):23–26. [DOI] [PubMed] [Google Scholar]
- 6. Ohtahara S, Yamatogi Y. Ohtahara syndrome: with special reference to its developmental aspects for differentiating from early myoclonic encephalopathy. Epilepsy Res. 2006;70(suppl 1):S58–S67. [DOI] [PubMed] [Google Scholar]
- 7. Saitsu H, Kato M, Koide A, et al. Whole exome sequencing identifies KCNQ2 mutations in Ohtahara syndrome. Ann Neurol. 2012;72(2):298–300. [DOI] [PubMed] [Google Scholar]
- 8. Saitsu H, Kato M, Mizuguchi T, et al. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat Genet. 2008;40(6):782–788. [DOI] [PubMed] [Google Scholar]
- 9. Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71(1):15–25. [DOI] [PubMed] [Google Scholar]
- 10. Kato M, Saitoh S, Kamei A, et al. A longer polyalanine expansion mutation in the ARX gene causes early infantile epileptic encephalopathy with suppression-burst pattern (Ohtahara syndrome). Am J Hum Genet. 2007;81(2):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valenzuela D, Han X, Mende U, et al. G alpha(o) is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proc Natl Acad Sci USA. 1997;94(5):1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang M, Gold MS, Boulay G, et al. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci U S A. 1998;95(6):3269–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kehrl JM, Sahaya K, Dalton HM, et al. Gain-of-function mutation in Gnao1: a murine model of epileptiform encephalopathy (EIEE17)? Mamm Genome. 2014;25(5-6):202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helbig I. G proteins, GNAO1 mutations and Ohtahara syndrome, in Beyond the ion channel—the blog of the ILAE Genetics Commission; 2013. Web site http://epilepsygenetics.net/2013/09/08/g-proteins-gnao1-mutations-and-ohtahara-syndrome/. Accessed September 08, 2014.
- 15. Boycott KM, Vanstone MR, Bulman DE, MacKenzie AE. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet. 2013;14(10):681–691. [DOI] [PubMed] [Google Scholar]