Abstract
Although liver fatty acid binding protein (FABP1, L-FABP) is not detectable in brain, Fabp1 gene ablation (LKO) markedly increases endocannabinoids (EC) in brains of male mice. Since the brain EC system of females differs significantly from that of males, it was important to determine if LKO differently impacted the brain EC system. LKO did not alter brain levels of arachidonic acid (ARA)-containing ECs, i.e arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG), but decreased non-ARA-containing N-acylethanolamides (OEA, PEA) and 2-oleoylglycerol (2-OG) that potentiate the actions of AEA and 2-AG. These changes in brain potentiating EC levels were not associated with: i) a net decrease in levels of brain membrane proteins associated with fatty acid uptake and EC synthesis; ii) a net increase in brain protein levels of cytosolic EC chaperones and enzymes in EC degradation; or iii) increased brain protein levels of EC receptors (CB1, TRVP1). Instead, the reduced or opposite responsiveness of female brain EC levels to loss of FABP1 (LKO) correlated with intrinsically lower FABP1 level in livers of WT females than males. These data show that female mouse brain endocannabinoid levels were unchanged (AEA, 2-AG) or decreased (OEA, PEA, 2-OG) by complete loss of FABP1 (LKO).
Keywords: female, mouse, FABP1, gene ablation, brain, endocannabinoid
INTRODUCTION
The endogenous cannabinoid receptor (CB) agonists (i.e. endocannabinoids, EC) N-arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG) are both synthesized from arachidonic acid (ARA)-esterified to phospholipids (1, 2). Unlike other tissues, however, ability of the brain to synthesize ARA is not sufficient to meet needs and thus brain ARA is derived primarily from plasma (3, 4). However, plasma ARA availability for brain uptake is limited by high hepatic clearance (5, 6). Hepatic ARA clearance is associated with high hepatic levels of liver fatty acid binding protein (FABP1), a cytosolic protein that not only binds ARA with high affinity (7, 8) but also facilitates ARA uptake (9–12). Recent findings with male mice have shown that ablation of FABP1, a protein not found in brain (13–15), markedly increases serum ARA availability for brain uptake concomitant with increasing brain levels of ARA, AEA and 2-AG (16, 17).
Although most animal studies of the EC system have been performed with male rodents, increasing evidence indicates that the EC system of female humans and rodents differs significantly from that of their male counterparts (18–22). For example, females have a higher pain sensitivity threshold and are more susceptible to cannabinoid antinociception (18, 21, 23, 24). At the same time females are also more susceptible to developing cannabinoid abuse and dependence, while having more severe withdrawal, and are more likely to relapse than males (18). Female brains have fewer CB1 receptor binding sites, but their CB1 receptors are more efficient as compared to those in males (21, 23). Female rat brain hypothalamus and pituitary have higher AEA and 2-AG levels than those of males (21, 23), consistent with higher blood ARA levels in females as compared to blood ARA levels to males (25, 26).
Taken together, the above findings suggested that the brain EC system of females may respond differently to ablation of FABP1 from that observed with male Fabp1 gene ablated mice. Therefore, this possibility was examined using female WT and Fabp1 gene ablated mice to determine the potential impact of its ablation on brain: 1) levels of ARA-containing ECs, i.e. AEA and 2-AG; 2) levels of non-ARA containing N-acylethanolamides and 2-monoacylglycerols; and 3) protein levels and expression of proteins in the endocannabinoid system. The data show that the brain EC system of female mice was not altered (i.e. AEA, 2-AG) or decreased (OEA, PEA, 2-OG) in response to Fabp1 gene ablation that was opposite of changes previously shown in males (17). This correlated with livers of WT female mice exhibiting significantly lower basal FABP1 levels than those of WT males.
MATERIALS AND METHODS
Mice
Female inbred C57BL/6NCr mice were from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). Female Fabp1 gene ablated (LKO) mice on the same C57BL/6NCr background were backcrossed to C57BL/6NCr to the N10 generation (27). Mice were fed a standard rodent chow mix [5% calories from fat; D8604 Teklad Rodent Diet, Teklad Diets (Madison, WI)] and water ad libitum. Mice were housed in barrier cages on ventilated racks at 12-hr light/dark cycle in a temperature controlled facility (25°C), sentinel monitored quarterly, and confirmed free of all known rodent pathogens. At age 8 wk, WT and Fabp1 gene ablated female mice were placed on a defined phytol-free (28–33), phytoestrogen-free (34, 35) control chow to avoid dietary complications due to their potential impact on hepatic FABP1 level and/or the EC system. After 4 weeks on the phytol-free, phytoestrogen-free diet the mice were fasted overnight followed by brain removal/flash freezing and storage at −80°C. Mouse experimental protocols were approved by the Institutional Animal Care and Use Committee at Texas A&M University.
Extraction and Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of Brain N-Acylethanolamide (NAE) and 2-Monoacylglycerol (2-MG)
Arachidonoylethanolamide (AEA), oleoylethanolamide (OEA), palmitoylethanolamide (PEA), n-3 docosahexaenoylethanolamide (DHEA), n-3 eicosapentaenoylethanolamide (EPEA), 2-arachidonoylglycerol (2-AG), 2-oleoylglycerol (2-OG), 2-palmitoylglycerol (2-PG), AEA-d4, OEA-d2, PEA-d4, DHEA-d4, EPEA-d4, and 2-AG-d8 were purchased from Cayman Chemical (Ann Arbor, MI). All solvents and reagents were highest grade available commercially. Frozen mouse brain (100–200 mg wet weight) was homogenized in 1.0 mL of ice-cold homogenization buffer containing 2000 pg each of AEA-d4, OEA-d2, PEA-d4, DHEA-d4, EPEA-d4, and 2-AG-d8. Lipids were extracted from mouse brain essentially as described in (36), reconstituted in 100 μL of ice-cold methanol, purged with nitrogen, and stored at −80°C until analysis by liquid chromatography-mass spectrometry (LC-MS). The NAEs (AEA, OEA, PEA, DHEA, EPEA) in the brain lipid extract were resolved, identified, and quantified in the Texas A&M University Protein Chemistry Laboratory (Dr. Larry Dangott, Director) essentially as described in (37) and modified as in (17). Likewise, the 2-MGs (2-AG, 2-OG, and 2-PG) in the brain lipid extract were resolved, identified and quantified in the Protein Chemistry Laboratory basically as in (38) and as modified in (17). Brain NAE and 2-MG levels are expressed as pmol/g wet weight and nmol/g wet weight, respectively.
Antibodies and Proteins for Western Blotting
Rabbit polyclonal anti-SCP2 was prepared as described in (39). Caveolin-1 (CAV1; 610060) polyclonal anti-rabbit antibody was from BD Transduction Laboratories (Lexington, KY). Fatty acid transport protein 1 (FATP-1; sc-25541) polyclonal anti-rabbit, fatty acid binding protein-3 (FABP3; sc-58275) monoclonal anti-mouse, fatty acid binding protein-7 (FABP7; sc-30088) polyclonal anti-rabbit, FABP1 (sc-16064) polyclonal anti-mouse, N-acylethanolamide hydrolyzing acid amidase (NAAA; sc-100470) monoclonal anti-mouse, and β-Actin (sc-47778) monoclonal anti-mouse were from Santa Cruz Biotech (Santa Cruz, CA). Fatty acid binding protein-5 (FABP5; RD181060100) antibody was from BioVendor R&D (Asheville, NC). Fatty acid translocase/cluster of differentiation 36/thrombospondin receptor (FAT/CD36; RDI-M1537db) monoclonal anti-mouse antibody was from Research Diagnostics (Flanders, NJ). Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; MAB374) monoclonal anti-mouse antibody was from Millipore (Billerica, MA). Diacylglycerol lipase α (DAGLα; 13626 Cell Signaling, Danvers, MA) and 2-monoacylglycerol lipase (MAGL; 310212) polyclonal antibodies were from Cayman Chemical Co (Ann Arbor, MI). Cytochrome C oxidase 4 (COX4, ab16056) polyclonal anti-rabbit, antibody to cannabinoid receptor-1 (CB1; AB172970), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, AB8245), fatty acid amide hydrolase (FAAH, AB54615), and N-acylphosphatidylethanolamide phospholipase D (NAPEPLD; AB95397) were from Abcam (Cambridge, MA). Antibody to transient receptor potential cation channel subfamily V member 1 (TRVP-1: 75j-254) was from Antibodies Inc. (Davis, CA). For quantitative Western blotting, recombinant protein standards were purified and delipidated as described in the following cited papers: murine FABP1 (7, 40), murine acyl-CoA binding protein (ACBP) (41, 42), and human sterol carrier protein-2 (SCP-2) (43–45).
Brain Protein Levels of Enzymes and Other Proteins in the Endocannabinoid System
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis was performed on brain post-nuclear supernatants (PNS) as described earlier (17, 46, 47). Brain proteins were resolved by 12% Tris-SDS-PAGE gel, transferred to 0.2 μm nitrocellulose membrane (162-0112, BioRad Laboratories, Hercules, CA), blocked with 3% gelatin for 1 hr, and incubated overnight with select primary antibodies followed by species-specific Horseradish Peroxidase (HRP) or Alkaline Phosphatase (AP) conjugated secondary antibodies for 1–2 hr. After rinsing nitrocellulose membrane three times for 5 min in TBST (10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween 20), the HRP conjugated antibodies were exposed to the Super Signal West Pico chemiluminescent substrate (34077, Pierce, Rockford, IL) or Immuno-star HRP substrate (Bio-Rad, Hercules, CA). Images were obtained with an Image Quant LAS 4000 mini (GE Healthcare Life Sciences, Marlborough, MA) or C-DiGiT scanner (Li-COR, Lincoln, NE). AP-conjugated antibodies were exposed to BCIP/NBT solution (B6404, Sigma Aldrich) and images obtained with an Epson Perfection V700 Photo scanner (Long Beach, CA). Proteins were quantified by densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD). Relative protein levels normalized to GAPDH or β-actin internal gel-loading controls and representative cropped Western blot images are inserted into figure panels similarly as in earlier publications in which individual Western blots are separated by a white line/space (48–52). Quantitative Western blotting of FABP1 was performed using a standard curve with recombinant murine FABP1 as in (53–56). Images of the blots were taken by Epson Perfection V700 Photo scanner (Long Beach, CA) and quantified by densitometric analysis with ImageJ software (NIH, Bethesda, MD) as described earlier (57).
QrtPCR Reagents for Analyzing Brain mRNAs of Genes in the Endocannabinoid System
TaqMan® RNA-to-CT™ 1-Step PCR Master Mix Reagent kit was purchased from Life Technologies™ (Carlsbad, CA). The following gene-specific TaqMan® PCR probes and primers were obtained from Life Technologies™ (Carlsbad, CA) to determine brain mRNA levels of: G protein coupled receptor kinase-2 (Adrbk2, Mm00622042_m1); cannabinoid receptor-1 (Cnr1, Mm01212171_s1); cannabinoid receptor-2 (Cnr2, Mm02620087_s1); diacylglycerol lipase α (Dagla, Mm00813830_m1); diacylglycerol lipase β (Daglb, Mm00523381_m1); fatty acid amide hydrolase (Faah, Mm00515684_m1); 2-monoacylglycerol lipase (Mgll, Mm00449274_m1); fatty acid binding protein-3 (Fabp3, Mm02342494_m1); fatty acid binding protein-5 (Fabp5, Mm00783731_s1); fatty acid binding protein-7 (Fabp7, Mm01246302_m1); N-acylethanolamide hydrolyzing acid amidase (Naah, Mm01341699_m1); N-acylphosphatidylethanolamide phospholipase D (Napepld, Mm00724596_m1); transient receptor potential cation channel subfamily V member 1 (Trvp-1, Mm01246302_m1).
mRNA Extraction and QrtPCR to Determine mRNA Levels of Genes in the Brain Endocannabinoid System
Brain total RNA was isolated and purified with the RNeasy mini kit (Qiagen, Valencia, CA) using the manufacturer’s standard protocol. Concentration and quality of mRNA were determined by a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA). Samples were stored at −80°C. QrtPCR expression patterns were analyzed with an ABI PRISM 7000 sequence detection system (Applied Biosystems®, Foster City, CA) using TaqMan® RNA-to-CT™ 1-Step PCR Master Mix Reagent kit, gene-specific TaqMan PCR probes and primers. The thermal cycler protocol was as follows: 48°C for 30 min, 95°C for 10 min, 95°C for 0.15 min and 60°C for 1.0 min, repeated a total of 40 cycles. TaqMan® gene expression assays to determine brain mRNA transcript levels of the genes listed above. Two replicates of each sample reaction (20 μL total volume each) were performed on 96 well plates (Applied Biosystems®, Foster City, CA). The threshold cycle from each well was established with ABI Prism 7000 SDS software (Applied Biosystems®, Foster City, CA) and QrtPCR data normalized to the housekeeping gene 18S RNA for mRNA. Expression of Adrbk2, Arrb2, Cnr1, Cnr2, Dagla, Daglb, Faah, Mgll, Fabp3, Fabp5, Fabp7, Naah, Nape-pld, and Trvp-1 were relative to the control female mouse group.
Brain Cytokine Levels
Mouse LINCOplex kit (MADPK-71K) and mouse LINCOplex kit (MADPCYT-72K) from LINCO Research (St. Charles, MO) were used to determine brain levels of insulin, resistin, leptin, adiponectin, monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), interleukin-6 (IL-6), and tumor necrosis factor α (TNFα) according to the manufacturer’s instructions. Samples were detected with a Luminex 100IS microsphere analyzer (Luminex Corp., Austin, TX) and analyzed with Luminex 100 version 2.1 software supplied by the manufacturer using 5-parameter data reduction.
Statistical Analysis
Values represent the mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls post-hoc test. Statistical significance was assigned to values with p < 0.05.
RESULTS
Fabp1 Gene Ablation (LKO) Differentially Impacts Brain Levels of Arachidonic Acid (ARA)-containing versus non-ARA-containing Endocannabinoids (EC)
Brain contains three major classes of endocannabinoids: i) ARA-containing (2-AG ≫ AEA) ECs are the major endogenous ligand activators of cannabinoid (CB) receptors (4, 38, 58–62); ii) Non-ARA-containing ‘potentiating’ ECs (OEA, PEA, 2-OG, and/or 2-PG) that enhance the activity of ARA-containing ECs by increasing their affinities for CB receptors or decreasing their enzymatic degradation (63–68); iii) Non-ARA-containing antagonistic ECs (DHEA, EPEA) that displace ARA from membrane phospholipids and decrease ARA containing phospholipid synthesis to thereby lower AEA and 2-AG production (69).
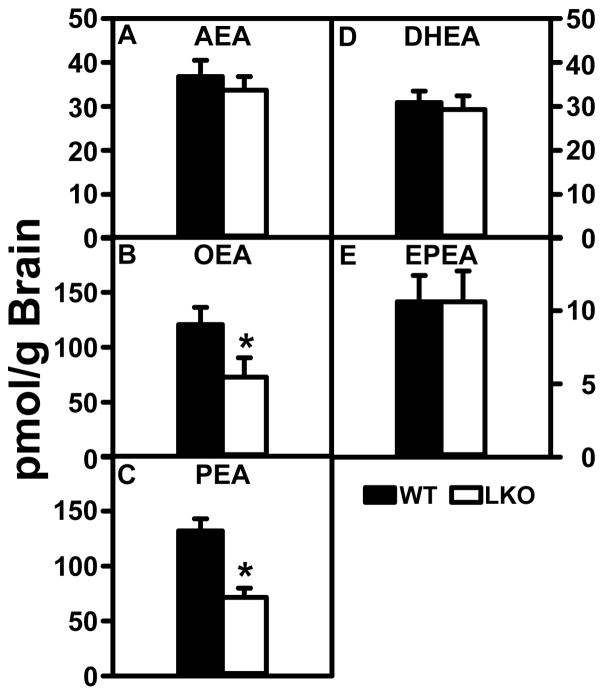
Arachidonoylethanolamide (AEA) levels were not different between groups (Fig. 1A), levels of potentiating endocannabinoids OEA and PEA were nearly 2-fold higher in the WT than LKO mice (Fig. 1B, C). LKO differentially impacted brain levels of potentiating, but not antagonistic non-ARA containing, ECs. LKO did not significantly alter brain levels of AEA (Fig 1A) or 2-AG (Fig. 2A). In contrast, brain levels of potentiating OEA and PEA (Fig. 1B, C) and 2-OG (Fig. 2B) were decreased. In contrast, LKO did not significantly alter the brain levels of antagonistic DHEA or EPEA (Fig. 1D, E).
FIGURE 1.
Impact of FABP1 gene ablation (LKO) on brain N-acylethanolamide (NAE) levels. Female WT and LKO (8 wk old) were fed phytol-free, phytoestrogen-free control diet for 4 weeks, fasted overnight, brains removed/flash frozen and stored at −80°C, and NAEs extracted for resolution, identification and quantitation by LC-MS analysis as described in Materials and Methods to determine content of: (A) arachidonoylethanolamide (AEA), (B) oleoylethanolamide (OEA), (C) palmitoylethanolamide (PEA), (D) docosahexaenoylethanolamide (DHEA), and (E) eicosapentaenoylethanolamide (EPEA). Data represent the mean ± SEM (n = 8); *, p < 0.05 for LKO vs WT.
FIGURE 2.
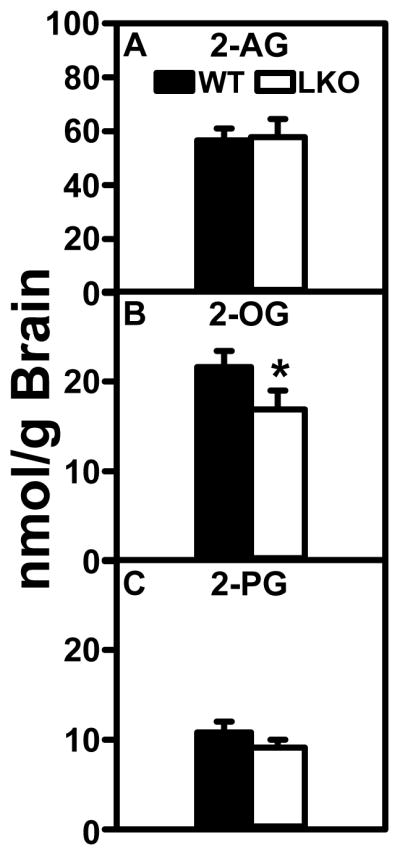
Effect of FABP1 gene ablation (LKO) on brain 2-monoacylglycerol (2-MG) levels. All conditions were as in legend to Fig. 1 except that LC-MS analysis was used to quantify 2-monoacylglyerols as described in Materials and Methods: (A) 2-arachidonoylglycerol (2-AG), (B) 2-oleoylglycerol (2-OG), and (C) 2-palmitoylglycerol (2-PG). Data represent the mean ± SEM (n = 8); *, p < 0.05 for LKO vs WT.
Consistent with the literature, WT brain levels of the antagonistic DHEA (Fig 1D) and even more so EPEA (Fig 1E) were lower. WT brain levels of the other major ARA-containing EC, i.e. 2-arachidonoylglycerol (2-AG) (Fig. 2A) were 3 orders of magnitude higher than those observed for AEA, but WT brain levels of the potentiating 2-monoacylglycerols (2-MGs) 2-OG and 2-PG (Fig. 2B, C) were 2–4 fold lower than those of 2-AG (Fig. 2A). Nevertheless the WT brain levels of 2-OG and 2-PG (Fig. 2B, C) were still markedly higher than those of AEA (Fig. 1A).
Fabp1 Gene Ablation (LKO) Does Not Affect Brain Protein Levels of Membrane Fatty Acid Transport/Translocase Proteins
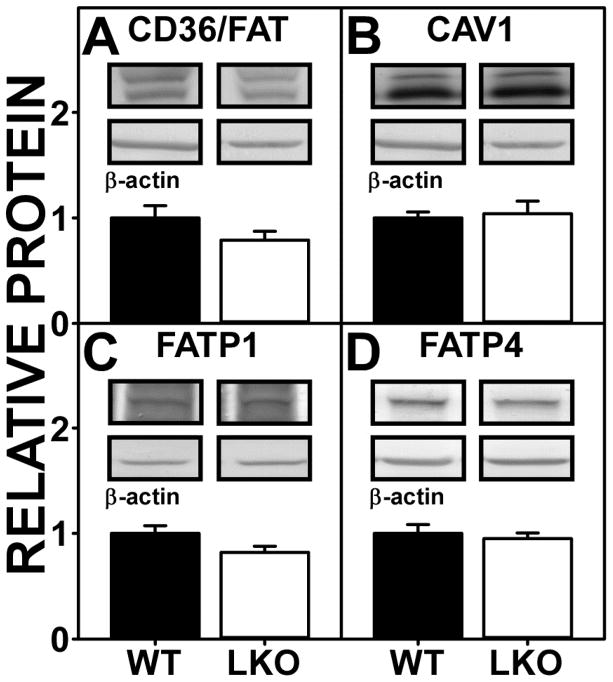
WT brain contains several membrane associated proteins (CD36/FAT, CAV1, FATP1 and FATP4) that facilitate translocation/uptake of long chain fatty acids such as ARA as well as other non-ARA fatty acids (e.g. palmitic or oleic acid) (3). As shown by Western blotting, LKO did not alter expression of CD36/FAT, CAV1, FATP1, or FATP4 (Fig 3A–D). The lower levels of OEA, PEA, and 2-OG in LKO brain (Fig. 1,2) did not correlate with decreased levels of membrane fatty acid uptake proteins.
FIGURE 3.
FABP1 gene ablation (LKO) impact on protein levels of brain membrane proteins involved in fatty acid uptake. Female WT and LKO mice (8 wk old) were fed phytol-free, phytoestrogen-free control chow for 4 weeks, overnight fasted, brains removed/flash frozen and stored at −80°C, and aliquots of brain homogenate proteins examined by SDS-PAGE and subsequent Western blot analysis as described in Materials and Methods. (A) CD36/FAT, (B) CAV1, (C) FATP1, and (D) FATP4. Insets show representative Western blot images of the respective protein (upper blot) and the gel-loading control protein β-Actin (lower blot). Relative protein levels were normalized to gel-loading control protein; values were compared to WT set to 1. Data represent the mean ± SEM (n = 7); *, p < 0.05 for LKO vs WT.
Impact of Fabp1 Gene Ablation (LKO) on Brain Levels of Proteins Involved in NAE and 2-MG Synthesis and Degradation
Brain levels of NAEs and 2-MGs are determined in part both by synthetic enzymes in the plasma membrane (NAPEPLD and DAGLα) and degradative membrane enzymes (FAAH, NAAA, MAGL) localized in intracellular sites (70–72). Thus, it was important to examine if LKO-induced alteration in brain EC levels was attributable to altered levels of these key enzymes.
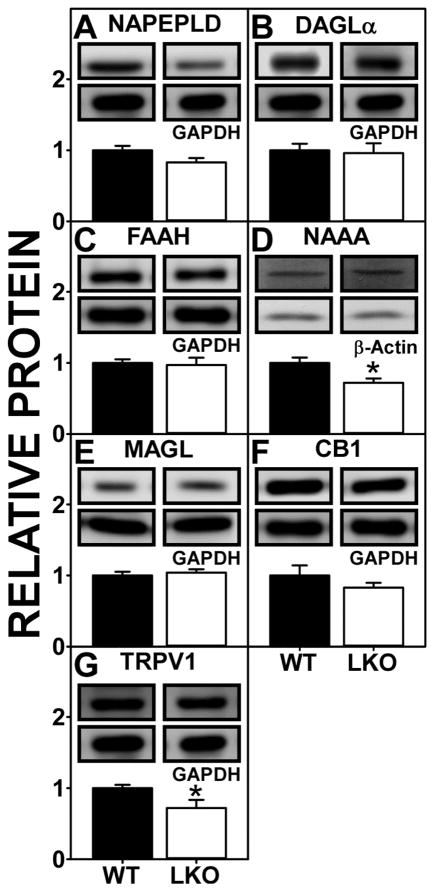
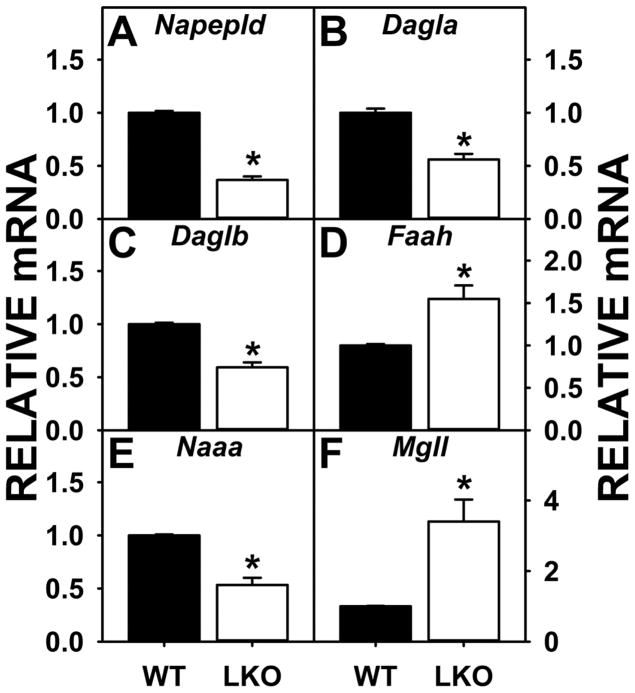
Western blotting showed that Fabp1 gene ablation did not alter expression of the NAE synthetic enzyme NAPEPLD (Fig. 4A) or the 2-MG synthetic enzyme DAGLα (Fig. 4B). With regards to the NAE degradative enzymes, LKO did not alter that of the major one, i.e. FAAH (Fig. 4C), but decreased that of NAAA (Fig. 4D). Protein levels of the 2-MG degradative enzyme MAGL were not altered by LKO (Fig 4E). Finally, Western blotting showed that LKO did not alter protein levels of the AEA and 2-AG receptor CB1 (Fig. 4F), but did reduce protein levels of TRVP1 in brain (Fig. 4G).
FIGURE 4.
Impact of FABP1 gene ablation (LKO) on protein levels of brain proteins involved in endocannabinoid synthesis and degradation and associated receptors. All conditions were as in legend to Fig. 3 except that Western blot analysis was performed to determine protein levels of (A) NAPEPLD, (B) DAGLα, (C) FAAH, (D) NAAA, (E) MAGL, (F) CB1, and (G) TRVP1. Insets show representative Western blot images of the respective protein (upper blot) and the gel-loading control protein (GAPDH or β-Actin, lower blot). Relative protein levels were normalized to the gel-loading control protein; values were compared to WT set to 1. Data represent the mean ± SEM (n = 7); *, p < 0.05 for LKO vs WT.
Impact of Fabp1 Gene Ablation (LKO) on Brain Levels of Cytosolic NAE and 2-MG ‘Chaperone’ Proteins
Due to their highly hydrophobic nature, not only ARA but even more so NAEs and 2-MGs, require cytosolic ‘chaperone’ proteins for intracellular transport/targeting to metabolic organelles. These roles are served by the brain cytosolic FABPs 3, 5, and 7 (13, 46, 73–79) and SCP-2 (17, 45, 80, 81). Therefore, it was important to determine the impact of LKO on brain proteins levels of these lipidic ligand ‘chaperones’.
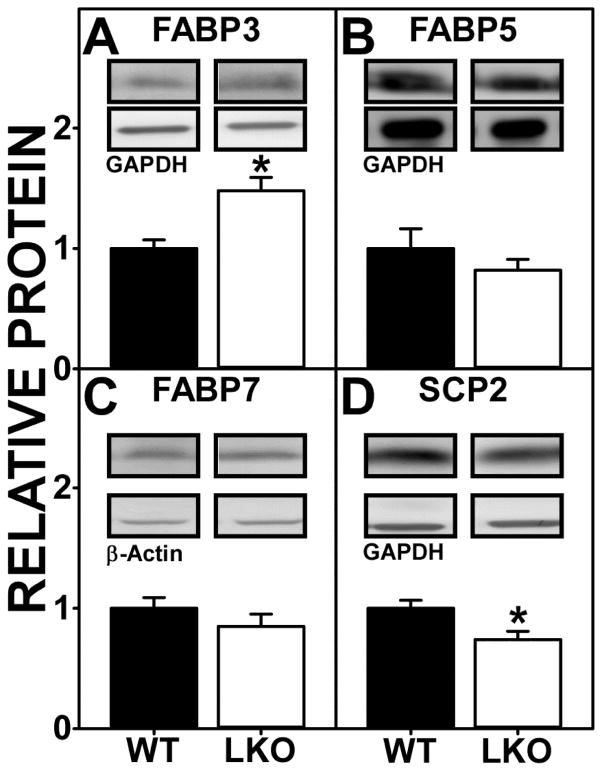
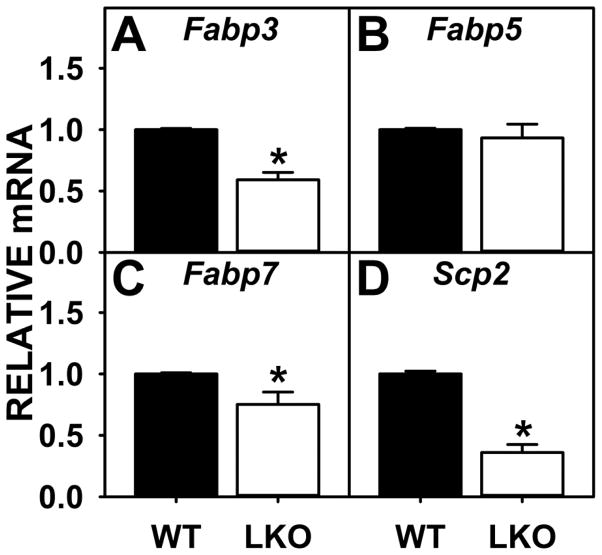
As shown by Western blotting, LKO differentially impacted the expression of the cytosolic ‘chaperone’ proteins. Brain protein level of FABP3 was significantly increased by LKO (Fig. 5A). Concomitantly, brain protein levels of the other ‘chaperones’ were either significantly decreased, e.g. SCP-2 (Fig. 5D) or did not change (FABP5, FABP7) (Fig 5. B, C).
FIGURE 5.
FABP1 gene ablation (LKO) alters protein levels of brain cytosolic ‘chaperone’ endocannabinoid binding proteins. All conditions were as in legend to Fig. 3 except that Western blot analysis was performed to determine protein levels of (A) FABP3, (B) FABP5, (C) FABP7, and (D) SCP-2. Insets are representative Western blot images of the respective protein (upper blot) and gel-loading control (GAPDH or β-Actin, lower blot). Relative protein levels were normalized to the gel-loading control protein; values were compared to WT set to 1. Data represent the mean ± SEM (n = 7); *, p < 0.05 for LKO vs WT.
Ablation/inhibition of cytosolic ‘chaperones’ is known to decrease NAE and 2-MG targeting for degradation which in turn increases their level (38, 47, 79). Since LKO decreased brain levels of non-ARA NAEs and 2-MGs, this would suggest that the concomitant upregulation of FABP3 may have exerted a larger impact than downregulation of the other cytosolic ‘chaperones’ which were either decreased or unchanged.
Role of Transcriptional Regulation on the Impact of Fabp1 Gene Ablation (LKO) on Brain Protein Levels of Proteins and Enzymes in the Endocannabinoid System
LKO-induced changes in protein levels of some, but not most, brain proteins were attributable in part to altered mRNA levels. The decreased protein level of the NAE degradative enzyme NAAA (Fig. 4D) was consistent with decreased Naaa mRNA level (Fig. 6E). LKO-induced decreased or unaltered protein levels of brain cytosolic ‘chaperones’ such as FABP5 and SCP2 (Fig. 5B, D) was consistent with decreased or unaltered Fabp5 and Scp2 mRNAs (Fig. 7B, D).
FIGURE 6.
Effect of FABP1 gene ablation (LKO) on brain levels of mRNAs encoding proteins for endocannabinoid synthesis and degradation. Female WT and LKO mice (8 wk old) were fed phytol-free, phytoestrogen-free control chow for 4 weeks, overnight fasted, brains removed/flash frozen and stored at −80°C, and aliquots of brain homogenate used for qrtPCR to determine mRNA levels of (A) Napepld, (B) Dagla, (C) Daglb, (D) Faah, (E) Naaa, and (F) Mgll as described in Materials and Methods. Levels of mRNA were normalized to an internal control (18S RNA); values were compared to WT set to 1. Data represent the mean ± SEM (n = 7); *, p < 0.05 for LKO vs WT.
FIGURE 7.
FABP1 gene ablation (LKO) alters brain levels of mRNAs encoding cytosolic ‘chaperone’ endocannabinoid binding proteins. All conditions were as in legend to Fig. 6 except that qrtPCR was performed to determine mRNA levels of (A) Fabp3, (B) Fabp5, (C) Fabp7, and (D) Scp-2 as described in Materials and Methods. Levels of mRNA were normalized to an internal control (18S RNA); values were compared to WT set to 1. Data represent the mean ± SEM (n = 6); *, p < 0.05 for LKO vs WT.
In contrast, other brain EC system protein levels did not correlate with the respective mRNAs in LKO mice. The protein levels of the synthetic enzymes NAPEPLD and DAGLα were unaltered (Fig. 4A, B) despite significantly decreased Napepld and Dagl mRNA levels (Fig. 6A–C). The protein levels of the degradative enzymes FAAH and MAGL were unaltered (Fig 4C, E) despite increased Faah and Mgll mRNA levels (Fig. 6D, F). The protein level of FABP3 was increased (Fig. 5A) but the Fapb3 mRNA decreased (Fig. 7A). Finally, the protein level of FABP7 was unchanged (Fig 5C); however, FABP7 mRNA level was decreased in LKO (Fig 7C).
Hepatic FABP1 Expression is Sexually Dimorphic
Since LKO did not alter brain AEA and 2-AG levels in females (Fig. 1A, 2A), but significantly increased that in males (AEA: MWT = 15 ± 2 pmol/g brain, MLKO = 24 ± 2 pmol/g brain; 2-AG: MWT = 16 ± 2 nmol/g brain, MLKO = 44 ± 3 nmol/g brain) (17), the possibility that this might be attributed at least in part to sex-differences in hepatic FABP1 expression in WT mice was examined by quantitative Western blotting using a standard curve with purified recombinant murine FABP1 as described in Materials and Methods. As shown in multiple separate experiments, FABP1 was more highly expressed in livers of male than female mice fed a phytol-free, phytoestrogen-free diet (Fig 8).
FIGURE 8.
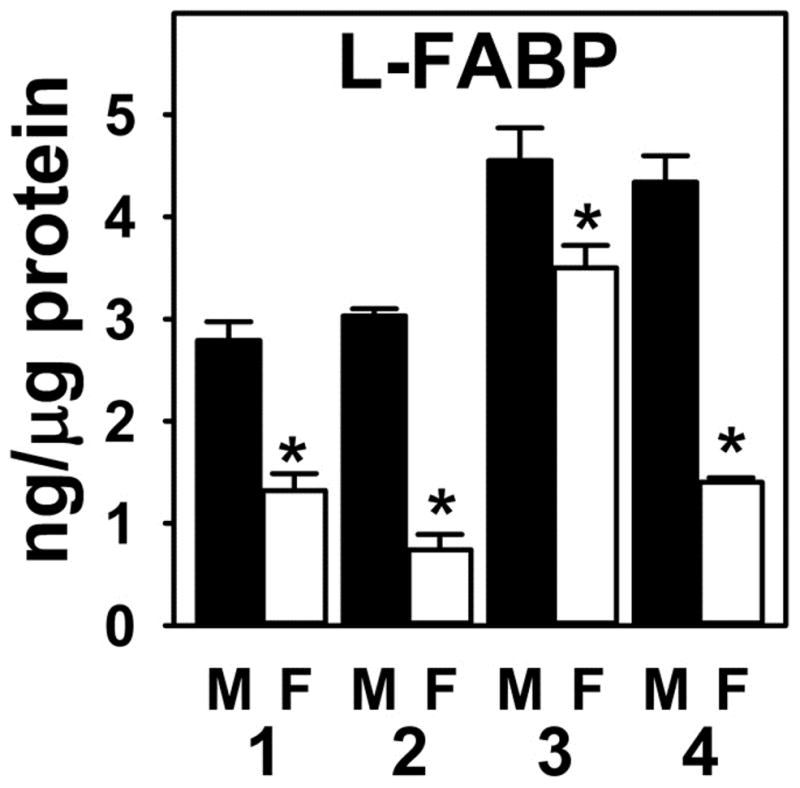
Hepatic FABP1 expression is sexual dimorphic. C57BL/6N male and female mice (8 wk old) were fed phytol-free, phytoestrogen-free control chow for 4 weeks, overnight fasted, livers removed and frozen at −80°C. Quantitative Western blotting was performed on livers to determine FABP1 protein level compared to standard curve of pure recombinant FABP1 as described (53–56). FABP1 levels (ng L-FABP/μg total protein) are shown from four separate experiments, each presented as mean ± SEM (n = 3–10); *, p < 0.05 for LKO vs WT.
FABP1 Gene Ablation (LKO) Impact on Brain Inflammatory Cytokine Levels
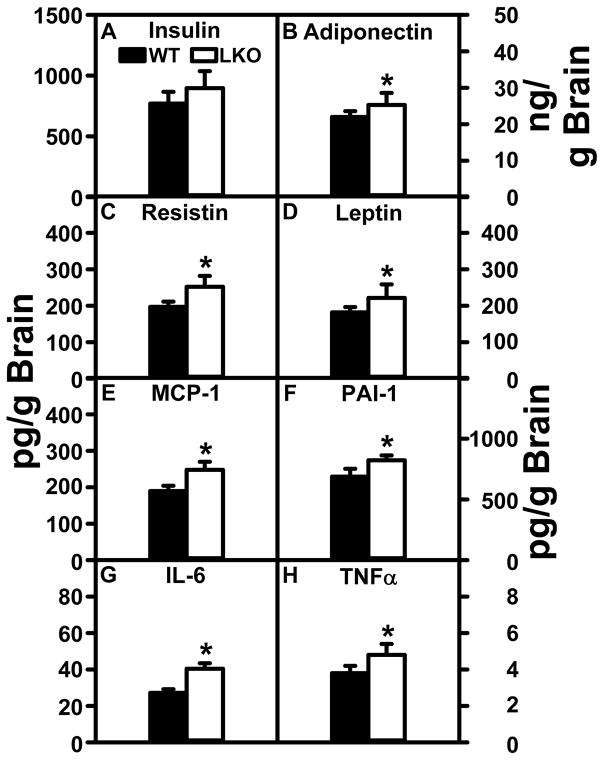
LKO had no significant impact on brain concentrations of insulin (Fig. 9A). LKO did modestly increase brain levels of inflammatory cytokines MCP-1 (Fig. 9E), PAI-1 (Fig. 9F), IL-6 (Fig. 9G), and TNFα (Fig. 9H). LKO also increased brain levels of adiponectin (Fig. 9B), resistin (Fig. 9C), and leptin (Fig. 9D); however, these cytokines are not normally associated with inflammation in the brain. Taken together, the lack of major changes in inflammatory cytokine levels correlated with the lack of change in brain AEA and 2-AG levels in LKO mice.
FIGURE 9.
Impact of FABP1 gene ablation on brain cytokine levels. Brain homogenate levels of (A) insulin, (B) adiponectin, (C) resistin, (D) leptin, (E) MCP-1, (F) PAI-1, (G) IL-6, and (H) TNFα were quantified as described in Materials and Methods. Data represent the mean ± SEM (n = 8); *, p < 0.05 for LKO vs WT.
DISCUSSION
Behavioral and other studies suggest considerable sexual dimorphism in the brain endocannabinoid (EC) system of both humans and rodents (18–23). However, little is known concerning the molecular details on which these differences are based—especially with regards to factors outside the brain that influence brain endocannabinoid levels. For example, liver fatty acid binding protein (FABP1) is not detectable in brain (13–15), but its ablation (LKO) in male mice markedly increases brain levels of arachidonic acid (ARA)-containing EC (e.g. AEA: MWT = 15 pmol/g brain vs MLKO = 24 pmol/g; 2-AG: MWT = 16 nmol/g vs MLKO = 44 nmol/g) and non-ARA-containing EC (e.g. OEA: MWT = 72 pmol/g brain vs MLKO = 190 pmol/g; PEA: MWT = 80 pmol/g vs MLKO = 150 pmol/g; 2-OG: MWT = 5 nmol/g vs MLKO = 18 nmol/g) (16, 17). Whether a similar effect is seen in the female brain EC system is unknown. The studies presented herein with female LKO mice presented new insights into the impact of sexual dimorphic FABP1 expression on the brain EC system.
First, there are a number of known differences in the brain EC system between males and females. For example, ARA-containing EC (AEA, 2-AG) levels were near 40 pmol/g and 60 nmol/g brain, respectively, in female brains (shown herein)—several fold higher than those observed in the brains of male mice (AEA, 15 pmol/g brain; 2-AG, 16 nmol/g brain) (16, 17). Consistent with these findings, female rat brain hypothalamus and pituitary have higher AEA and 2-AG levels than those of males (21, 23). In the rat, the higher AEA and 2-AG level in female brain is attributed to the higher plasma availability of ARA in females (25, 26). This is important because most brain ARA is derived from plasma for uptake into brain and rapid esterification into phospholipids from which AEA and 2-AG are derived (3, 4). Finally, the markedly higher levels of AEA and 2-AG in brains of female vs male mice correlated with the significantly lower basal FABP1 levels in livers of female vs male mice (shown herein). The possibility that lower hepatic FABP1 levels in females contributed to higher brain EC levels is supported by earlier studies showing that: i) FABP1 has high affinity for ARA (7, 9, 17, 82); ii) native FABP1 isolated from liver is preferentially enriched with endogenously-bound ARA (8); iii) hepatic FABP1 concentration is at least 20-fold higher than that of all the FABPs (FABP 3, 5, 7) in brain combined (83–90); iv) FABP1 overexpression enhances ARA uptake (9–12).
In contrast, very little is known with respect to differences in the non-ARA containing ECs between males and females. Although brain can synthesize sufficient non-ARA fatty acids such as oleic acid and palmitic acid needed for incorporation into phospholipids from which non-ARA-containing EC are derived (3, 4), brain can take up non-ARA fatty acids from the blood (3, 74, 91). Thus, it was difficult to predict a priori the net impact of sex on brain levels of non-ARA-containing ECs. The data presented herein showed that female brain basal levels of non-ARA containing ECs (OEA, PEA, 2-OG, and 2-PG near 125 pmol/g brain, 135 pmol/g, 22 nmol/g, and 11 nmol/g, respectively) were signficantly higher than those in male brains (OEA, 70 pmol/g brain; PEA, 80 pmol/g; 2-OG, 5 nmol/g; 2-PG, 6 nmol/g) (16, 17). This may be attributed at least in part by: i) females’ lower hepatic FABP1 level; ii) FABP1 also binding non-ARA fatty acids with high affinity, albeit less than that for ARA (7, 92–94); iii) non-ARA fatty acids palmitic acid and oleic acid comprising the most common, i.e. 10% and 30% respectively, endogenously-bound fatty acids in native FABP1 isolated from liver (8); iv) enhancement of non-ARA fatty acid uptake by FABP1 overexpression and in direct proportion to FABP1 level in cloned human HepG2 liver cells (10, 12, 95–98).
Second, although Fabp1 gene ablation (LKO) markedly increased brain levels of both ARA-containing (AEA: WT = 15 pmol/g brain, LKO = 24 pmol/g; 2-AG: WT = 16 nmol/g brain, LKO = 44 nmol/g) and non-ARA containing (OEA: WT = 72 pmol/g, LKO = 190 pmol/g; PEA: WT = 80 pmol/g, LKO = 150 pmol/g, 2-OG: WT = 5 nmol/g, LKO = 18 nmol/g; 2-PG: WT = 7 nmol/g, LKO = 9 nmol/g) ECs in males (16, 17), its impact in female brain was not known. The data presented herein showed for the first time that (LKO) did not alter brain levels of AEA or 2-AG in females, while the levels of the non-ARA containing ECs (OEA, PEA, 2-OG) were decreased by 20–50%. While this was consistent with the already much lower level of hepatic FABP1 in WT females as compared to WT males, there is a paucity of literature regarding the impact of hepatic FABP1 level on sex differences in the brain EC system.
Third, the FABP1 gene ablation induced decreases in brain ECs were not attributable to marked alteration in proteins levels of: i) plasma membrane proteins for fatty acid uptake in brain; ii) membrane enzymes in synthesis/degradation of non-ARA-containing ECs; or iii) protein levels of cytosolic chaperones that would enhance non-ARA-containing EC cytosolic transport and targeting for degradation. Furthermore, the lack of compensatory changes in brain EC system proteins in response to FABP1 gene ablation was not attributable to lack of changes in respective mRNA levels—many of which were significantly altered. While the lack of correlation between brain EC system protein levels and mRNA transcripts is not known, a similar lack of correlation in liver EC system protein levels and mRNAs has been attributed to specific micro RNAs (miRNAs) that inhibit mRNA translation (99).
With regards to physiological impact of these findings on brain function, an important function of endocannabinoids such as AEA is on analgesia (38). Lower hepatic FABP1 level in females (shown herein) vs males (16, 17) correlate with females having higher brain levels of AEA and potentiating OEA and PEA (enhancers of AEA activity on CB receptors). Higher AEA level in brains of females is associated with lower sensitivity to pain as compared to males (18, 21, 23, 24). Conversely, elevated liver FABP1 levels in human lipid disorders such as obesity (102), alcoholic fatty liver disease (AFLD) (103, 104), and nonalcoholic fatty liver disease (NAFLD) (105–108) are associated with increased pain sensitivity in obesity (118–120), AFLD (121, 122), and NAFLD (123) reported in these lipid disorders. While expression of a SNP in the human FABP1 gene coding region results in a T94A substitution also increases hepatic total FABP1 and is associated with NAFLD (109–111), another relatively common SNP in the human FABP1 gene promoter region (rs2919872) decreases FABP1 promoter transcriptional activity to decrease FABP1 (110). However, the impact of these SNPs on pain sensitivity is not known. Resolving this issue is important, especially since the SNP leading to the FABP1 T94A variant is highly prevalent in the human population, occurring with 26–38% minor allele frequency and 8.3±1.9% homozygosity (MAF for 1000 genomes in NCBI dbSNP database; ALFRED database) (109, 112–117). Taken together these studies would suggest that FABP1 reduction or Fabp1 gene ablation may impact pain sensitivity much less in females than males—a possibility to be tested in future studies beyond the scope of the present investigation.
Another major physiological effect regulated by brain endocannabinoids is the desire for food intake. Elevated AEA increases desire for food intake (124), while increased OEA, PEA, or 2-OG decrease the desire for food intake (69, 125, 126). Thus, the female brain’s higher AEA level (shown herein) as compared to that in brain of males (16, 17) would suggest higher food intake by females. Conversely, the female brain’s higher OEA, PEA and less so 2-OG content would tend to decrease food intake. The overall net effect led to less food intake in females versus males in control chow fed mice (127–131). With regards to the impact of loss of FABP1, LKO did not alter female brain AEA level, but decreased OEA and PEA by about 50%, and less so 2-OG (shown herein). As a result of the unaltered AEA and much smaller difference in potentiating ECs, the LKO female mice had unaltered or only slightly altered control chow food intake (127, 128, 131, 132).
In summary, wild-type mouse brain EC levels in females (shown herein) differed significantly from those of males (16, 17). This differential level of endocannabinoids adds a new level of understanding of our previously published studies demonstrating a reduction in food intake in female mice compared to males (127–131). Our studies further extend the impact of sex-differences on the content of endocannabinoids in the brain, demonstrating a higher level in female mice as compared to male mice. Finally, female brain EC levels were much less responsive to Fabp1 gene ablation (shown herein) as compared to their male FABP1 gene ablated counterparts (16, 17). This diminution of responsiveness of female brain EC levels to loss of FABP1 was associated with intrinsically lower FABP1 level in livers of WT females than males. This was in marked contrast to males wherein lower brain EC levels correlated with higher liver FABP1 such that loss of FABP1 upon ablation markedly increased brain EC levels (16, 17), approaching the levels observed in female brains.
Summary Statement.
Brain AEA and 2-AG levels of female mice are resistant to the impact of hepatic Fabp1 gene ablation.
Acknowledgments
The work presented herein was supported in part by the US Public Health Service/National Institutes of Health Grant R25 OD016574 (S.C., A.B.K.), Merial Veterinary Scholars Program, CVM (S.C., A.B.K.), and DA035949 (M.K.). The authors acknowledge ThermoFisher Scientific for use of the Exactive Orbitrap mass spectrometer.
Abbreviations
- ACBP
acyl-CoA binding protein
- ARA
arachidonic acid
- AEA, anandamide
arachidonoylethanolamide
- 2-AG
2-arachidonoylglycerol
- CB1, Cnr1
cannabinoid receptor-1
- CB2, Cnr2
cannabinoid receptor-2
- DAGL-α, Dagla
diacylglycerol lipase α
- DHEA
docosahexaenoylethanolamide
- EPEA
eicosapentaenoylethanolamide
- EC
endocannabinoid
- FAAH, Faah
fatty acid amide hydrolase
- FABP1, L-FABP
liver fatty acid binding protein-1
- FABP-3, Fabp3
fatty acid binding protein-3
- FABP-5, Fabp5
fatty acid binding protein-5
- FABP-7, Fabp7
fatty acid binding protein-7
- FAT/CD36
fatty acid translocase/thrombospondin receptor
- FATP-1
fatty acid transport protein-1
- FATP-4
fatty acid transport protein-4
- LKO
Fabp1 gene ablated mouse on C57BL/6NCr background
- GPCR
G protein coupled receptor
- GRK-2, Adrbk2
G protein coupled receptor kinase-2
- LCFA
long chain fatty acid
- LCFA-CoA
long chain fatty acyl-CoA
- 2-MG
2-monoacylglycerol
- MGL, Mgll
2-monoacylglycerol lipase
- NAAA, Naaa
N-acylethanolamide-hydrolyzing acid amidase
- NAE
N-acylethanolamide
- NAPE
N-acylphosphatidylethanolamide
- NAPE-PLD, Nape-pld
N-acylphosphatidylethanolamide phospholipase-D
- OEA
oleoylethanolamide
- 2-OG
2-oleoylglycerol
- PEA
palmitoylethanolamide
- 2-PG
2-palmitoyl glycerol
- SCP-2, Scp2
sterol carrier protein-2
- TRVP-1, vanilloid receptor-1, Trvp-1
transient receptor potential cation channel subfamily V member 1
- WT
wild-type C57BL/6NCr mouse
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: Experiments were designed, performed, and analyzed by the following: GGM and LJD for Figures 1and 2 and GGM for Figure 9; SC, DL, and KKL for Figures 3, 4D, 5A, C, D, 6, 7, 8; XP and MK for Figures 4A, B, C, E, F, G and 5B. FS, ABK, MK, and EJM conceived and coordinated the study and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
References
- 1.Muccioli GG. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discovery Today. 2010;15:474–483. doi: 10.1016/j.drudis.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 2.D’Addario C, Di Francesco A, Pucci M, Agro AF, Maccarrone M. Epigenetic mechanisms and endocannabinoid signaling. FEBS J. 2013;280:1905–1917. doi: 10.1111/febs.12125. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell RW, Hatch GM. Fatty acid transport into the brain: of fatty acid fables and lipid tails. Prost Leukot Essen Fatty Acids. 2011;85:293–302. doi: 10.1016/j.plefa.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Bazinet RP, Laye S. PUFA and their metabolites in brain function and disease. Nature Reviews Neuroscience. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 5.Havel RJ, Felts JM, Van Duyne CM. Formation and fate of endogenous triglycerides in blood plasma of rabbits. J Lipid Res. 1962;3:297–308. [Google Scholar]
- 6.Kohout M, Kohoutova B, Heimberg M. The regulaton of hepatic triglyceride metabolism by free fatty acids. J Biol Chem. 1971;246:5067–5074. [PubMed] [Google Scholar]
- 7.Frolov A, Cho TH, Murphy EJ, Schroeder F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry. 1997;36:6545–6555. doi: 10.1021/bi970205t. [DOI] [PubMed] [Google Scholar]
- 8.Murphy EJ, Edmondson RD, Russell DH, Colles SM, Schroeder F. Isolation and characterization of two distinct forms of liver fatty acid binding protein from the rat. Biochim Biophys Acta. 1999;1436:413–425. doi: 10.1016/s0005-2760(98)00150-7. [DOI] [PubMed] [Google Scholar]
- 9.McIntosh AL, Huang H, Atshaves BP, Wellburg E, Kuklev DV, Smith WL, Kier AB, Schroeder F. Fluorescent n-3 and n-6 very long chain polyunsaturated fatty acids: three photon imaging and metabolism in living cells overexpressing liver fatty acid binding protein. J Biol Chem. 2010;285:18693–18708. doi: 10.1074/jbc.M109.079897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy EJ, Prows DR, Jefferson JR, Schroeder F. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim Biophys Acta. 1996;1301:191–198. doi: 10.1016/0005-2760(96)00024-0. [DOI] [PubMed] [Google Scholar]
- 11.Murphy EJ, Prows DR, Jefferson JR, Incerpi S, Hertelendy ZI, Heiliger CE, Schroeder F. Cis-parinaric acid uptake in L-cells. Arch Biochem Biophys. 1996;335:267–272. doi: 10.1006/abbi.1996.0507. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder F, Jefferson JR, Powell D, Incerpi S, Woodford JK, Colles SM, Myers-Payne S, Emge T, Hubbell T, Moncecchi D, Prows DR, Heyliger CE. Expression of rat L-FABP in mouse fibroblasts: role in fat absorption. Mol Cell Biochem. 1993;123:73–83. doi: 10.1007/BF01076477. [DOI] [PubMed] [Google Scholar]
- 13.Owada Y, Abdelwahab SA, Kitanaka N, Sakagami H, Takano H, Sutigani Y, Sugawara M, Kawashima H, Kiso Y, Mobarakeh JI, Yanai K, Kaneko K, Sasaki H, Kato H, Saino-Saito S, Matsumoto N, Akaike N, Noda T, Kondo H. Altered emotional behavioral responses in mice lacking brain type FABP gene. Eur J Neurosci. 2006;24:175–187. doi: 10.1111/j.1460-9568.2006.04855.x. [DOI] [PubMed] [Google Scholar]
- 14.Myers-Payne S, Fontaine RN, Loeffler AL, Pu L, Rao AM, Kier AB, Wood WG, Schroeder F. Effects of chronic ethanol consumption on sterol transfer protein in mouse brain. J Neurochem. 1996;66:313–320. doi: 10.1046/j.1471-4159.1996.66010313.x. [DOI] [PubMed] [Google Scholar]
- 15.Avdulov NA, Chochina SV, Myers-Payne S, Hubbell T, Igbavboa U, Schroeder F, Wood WG. Expression and lipid binding of sterol carrier protein-2 and liver fatty acid binding proteins: differential effects of ethanol in vivo and in vitro. In: Riemersma RAARKRWaWR., editor. Essential Fatty Acids and Eicosanoids: Invited Papers from the Fourth International Congress. American Oil Chemists Society Press; Champaign, IL: 1998. pp. 324–327. [Google Scholar]
- 16.Schroeder F, McIntosh AL, Martin GG, Huang H, Landrock D, Chung S, Landrock KK, Dangott LJ, Li S, Kaczocha M, Murphy EJ, Atshaves BP, Kier AB. Fatty acid binding protein-1 (FABP1) and the human FABP1 T94A variant: Roles in the endocannabinoid system and dyslipidemias. (2016) Lipids. 2016 Apr 27; doi: 10.1007/s11745-016-4155-8. doi:10.1007.s11745-016-4156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin G, Chung S, Landrock D, Landrock KK, Huang H, Dangott LJ, Peng X, Kaczocha M, Seeger DR, Murphy EJ, Golovko MY, Kier AB, Schroeder F. FABP1 gene ablation impacts brain endocannabinoid system in male mice. J Neurochem. 2016 doi: 10.1111/jnc.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in endocannabinoid system? Life Sci. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fattore L, Fratta W. Themed Issue: Cannabinoids Review. How important are sex differences in cannabinoid action? Br J Pharmacol. 2010;160:544–548. doi: 10.1111/j.1476-5381.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llorente R, Llorente-Berzal A, Petrosino S, Marco EM, Guaza C, Prada C, López-Gallardo M, Di Marzo V, Viveros MP. Gender-dependent cellular and biochemical effects of maternal deprivation on the hippocampus of neonatal rats: a possible role for the endocannabinoid system. Develop Neurobiology. 2008;68:1334–1347. doi: 10.1002/dneu.20666. [DOI] [PubMed] [Google Scholar]
- 21.Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behavioral Neurosci. 2011;5 doi: 10.3389/fnbeh.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubino T, Parolaro D. Sex-dependent vulnerability to Cannabis abuse in adolescence. Front Behavioral Neurosci. 2015;6:1–5. doi: 10.3389/fpsyt.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, Di Marzo V, Ramos JA, Fernández-Ruiz JJ. Sex steroid influence on CB1 receptor mRNA and endocannabinoid levels in anterior pituitary gland. Biochem Biophy Res Comm. 2000;270:260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Sanz G, Sasso O, Guijarro A, Piomelli D. Pharmacological characterization of the peripheral FAAH inhibitor URB937 in female rodents: interaction with the Abcg2 transporter in the blood-placenta barrier. Br J Pharmacol. 2012;167:1620–1628. doi: 10.1111/j.1476-5381.2012.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 2011;94:1914S–1919S. doi: 10.3945/ajcn.110.000893. [DOI] [PubMed] [Google Scholar]
- 26.Lohner S, Fekete K, Marosvolgyi T, Desci T. Gender differences in long chain PUFA status: systematic review of 51 publications. Ann Nutr Metab. 2013;62:98–112. doi: 10.1159/000345599. [DOI] [PubMed] [Google Scholar]
- 27.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- 28.Ellinghaus P, Wolfrum C, Assmann G, Spener F, Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein-2-/sterol carrier protein x-deficient mice. J Biol Chem. 1999;274:2766–2772. doi: 10.1074/jbc.274.5.2766. [DOI] [PubMed] [Google Scholar]
- 29.Hanhoff T, Benjamin S, Borchers T, Spener F. Branched-chain fatty acids as activators of peroxisome proliferators. Eur J Lip Sci Technol. 2005;107:716–729. [Google Scholar]
- 30.Fuchs M, Hafer A, Muench C, Kannenberg F, Teichmann S, Scheibner J, Stange EF, Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J Biol Chem. 2001;276:48058–48065. doi: 10.1074/jbc.M106732200. [DOI] [PubMed] [Google Scholar]
- 31.Hanhoff T, Wolfrum C, Ellinghaus P, Seedorf U, Spener F. Pristanic acid is activator of PPARalpha. Eur J Lipid Sci. 2001;103:75–80. [Google Scholar]
- 32.Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F. Phytanic acid is ligand and transcriptional activator of murine liver fatty acid binding protein. J Lipid Res. 1999;40:708–714. [PubMed] [Google Scholar]
- 33.Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KWA, Wanders RJA, Maeda N, Assmann G. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes and Development. 1998;12:1189–1201. doi: 10.1101/gad.12.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thigpen JE, Setchell KD, Ahlmark KB, Kocklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab An Science. 1999;49:530–536. [PubMed] [Google Scholar]
- 35.Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phytoestrogen content of rodent diets. Envron Health Persp. 1999;107:A182–A183. doi: 10.1289/ehp.107-1566530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is invovled in ethanol prference and its age-dpendent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jian W, Edom R, Weng N, Zannikos P, Zhang Z, Wang H. Validation and application of an LC-MS/MS method for quantitation of three fatty acid ethanolamides as biomarkers for fatty acid hydrolase inhibitionin human placenta. J Chrom B. 2010;878:1687–1699. doi: 10.1016/j.jchromb.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Kaczocha M, Rebecchi MJ, Ralph BP, Teng YHG, Berger WT, Galbavy W, Elmes MW, Glaser ST, Wang L, Rizzo RC, Deutsch DG, Ojima I. Inhibition of fatty acid binding protein elevates brain anandamide levels and produces analgesia. PLoS ONE. 2014;9:e94200. doi: 10.1371/journal.pone.0094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atshaves BP, Petrescu A, Starodub O, Roths J, Kier AB, Schroeder F. Expression and Intracellular Processing of the 58 kDa Sterol Carrier Protein 2/3-Oxoacyl-CoA Thiolase in Transfected Mouse L-cell Fibroblasts. J Lipid Res. 1999;40:610–622. [PubMed] [Google Scholar]
- 40.Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BW, Pai PJ, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein (L-FABP) gene ablated mice. Am J Physiol. 2009;297:G1053–G1065. doi: 10.1152/ajpgi.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao H, Zhou M, McIntosh A, Schroeder F, Kier AB. Acyl CoA binding protein and cholesterol differentially alter fatty acyl CoA utilization by microsomal acyl CoA: cholesterol transferase. J Lipid Res. 2003;44:72–83. doi: 10.1194/jlr.m200191-jlr200. [DOI] [PubMed] [Google Scholar]
- 42.Frolov AA, Schroeder F. Acyl coenzyme A binding protein: conformational sensitivity to long chain fatty acyl-CoA. J Biol Chem. 1998;273:11049–11055. doi: 10.1074/jbc.273.18.11049. [DOI] [PubMed] [Google Scholar]
- 43.Matsuura JE, George HJ, Ramachandran N, Alvarez JG, Strauss JFI, Billheimer JT. Expression of the mature and the pro-form of human sterol carrier protein 2 in Escherichia coli alters bacterial lipids. Biochemistry. 1993;32:567–572. doi: 10.1021/bi00053a023. [DOI] [PubMed] [Google Scholar]
- 44.Martin GG, Hostetler HA, McIntosh AL, Tichy SE, Williams BJ, Russell DH, Berg JM, Spencer TA, Ball JA, Kier AB, Schroeder F. Structure and function of the sterol carrier protein-2 (SCP-2) N-terminal pre-sequence. Biochem. 2008;47:5915–5934. doi: 10.1021/bi800251e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frolov A, Cho TH, Billheimer JT, Schroeder F. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J Biol Chem. 1996;271:31878–31884. doi: 10.1074/jbc.271.50.31878. [DOI] [PubMed] [Google Scholar]
- 46.Kaczocha LM, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaczocha M, Glaser ST, Maher T, Clavin B, Hamilton J, O’Rourke J, Rebecchi M, Puopolo M, Owada Y, Thanos PK. Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mechanisms. Molecular Pain. 2015;11:52. doi: 10.1186/s12990-015-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin GG, Atshaves BP, Landrock KK, Landrock D, Storey SM, Howles PN, Kier AB, Schroeder F. Ablating L-FABP in SCP-2/SCP-x null mice impairs bile acid metabolism and biliary HDL-cholesterol secretion. Am J Physiol Gastrointest and Liver Phys. 2014;307:G1130–G1143. doi: 10.1152/ajpgi.00209.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrescu AD, Huang H, Martin GG, McIntosh AL, Storey SM, Landrock D, Kier AB, Schroeder F. Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARa regulated b-oxidative enzymes. Am J Physiol Gastrointest and Liver Phys. 2013;304:G241–G256. doi: 10.1152/ajpgi.00334.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H, McIntosh AL, Martin GG, Petrescu AD, Landrock K, Landrock D, Kier AB, Schroeder F. Inhibitors of fatty acid synthesis induce PPARa-regulated fatty acid b-oxidative enzymes: synergistic roles of L-FABP and glucose. PPAR Research. 2013;2013:1–22. doi: 10.1155/2013/865604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Loss of intracellular lipid binding proteins differentially impacts saturated fatty acid uptake and nuclear targeting in mouse hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2012;303:G837–G850. doi: 10.1152/ajpgi.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Intracellular cholesterol binding proteins enhance HDL-mediated cholesterol uptake in cultured primary mouse hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2012;302:G824–G839. doi: 10.1152/ajpgi.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am J Physiol. 2005;288:C543–C558. doi: 10.1152/ajpcell.00359.2004. [DOI] [PubMed] [Google Scholar]
- 54.Atshaves BP, McIntosh AL, Landrock D, Payne HR, Mackie J, Maeda N, Ball JM, Schroeder F, Kier AB. Effect of SCP-x gene ablation on branched-chain fatty acid metabolism. Am J Physiol. 2007;292:939–951. doi: 10.1152/ajpgi.00308.2006. [DOI] [PubMed] [Google Scholar]
- 55.Atshaves BP, McIntosh AL, Martin GG, Landrock D, Payne HR, Bhuvanendran S, Landrock K, Lyuksyutova OI, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Overexpression of sterol carrier protein-2 differentially alters hepatic cholesterol accumulation in cholesterol-fed mice. J Lipid Res. 2009;50:1429–1447. doi: 10.1194/jlr.M900020-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res. 2004;45:812–830. doi: 10.1194/jlr.M300408-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Atshaves BP, McIntosh AL, Payne HR, Gallegos AM, Landrock K, Maeda N, Kier AB, Schroeder F. Sterol carrier protein-2/sterol carrier protein-x gene ablation alters lipid raft domains in primary cultured mouse hepatocytes. J Lipid Res. 2007;48:2193–2211. doi: 10.1194/jlr.M700102-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Wood JT, Williams JS, Makriyannis A. Dietary DHA supplementation alters select physiological endocannabinoid system metabolites in brain and plasma. J Lipid Res. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoyl hydrolysis produces cannabinoid behavioral effects. Nature Chemical Biology. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlosburg JE, Blankman JI, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature Neuroscience. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, O’Neal ST, Walentiny DM, Wiley JL, Cravatt BF, Lichtman AH. In vivo characterization of the highly selective inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014;171:1392–1407. doi: 10.1111/bph.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nomura DK, Morrison BAE, Blankman JI, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho WSV, Barrett DAR. Entourage effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxaton to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smart D, Jonsson KO, Vanvoorde S, Lambert DM, Fowler CJ. Entourage effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br J Pharmacol. 2002;136:452–458. doi: 10.1038/sj.bjp.0704732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piomelli S, Seaman C. Mechanism of red blood cell aging: relationship of cell density and cell age. Am J Hematology. 1993;42:46–52. doi: 10.1002/ajh.2830420110. [DOI] [PubMed] [Google Scholar]
- 66.Franklin A, Parmentier-Batteur S, Walter L, Greenbert DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neuroscience. 2003;23:7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R. An entoruage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharm. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- 68.Mechoulam R, Fride E, Hanus L, Sheskin T, Bisogno T, Di Marzo V, Bayewitch M, Vogel Z. Anandamide may mediate sleep induction. Nature. 1997;389:25–26. doi: 10.1038/37891. [DOI] [PubMed] [Google Scholar]
- 69.Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Fatty acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int J Endocrinol ID. 2013;361895:1–11. doi: 10.1155/2013/361895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-AG, another cannabinoid receptor ligand. FEBS Lett. 1989;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- 71.Di Marzo V, Bisogno T, Sugiura T, Melck D, De Petrocellis L. The novel endogenous cannabinoid 2-AG is inactivated by neuronal- and basophil-like cells: connections with anandamide. Biochem J. 1998;331:15–19. doi: 10.1042/bj3310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blankman JL, Simon GM, Cravatt BF. Comprehensive profile of brain enzymes that hydrolyze endocannabinoid 2-arachidonoylglycerol. Chemistry and Biology. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu S, Levi L, Casadesus G, Kunos G, Noy N. Fatty acid binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator activated receptor b/d (PPARb/d) in the brain. J Biol Chem. 2014;289:12748–12758. doi: 10.1074/jbc.M114.559062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy EJ, Owada Y, Kitanaka N, Kondo H, Glatz JFC. Brain arachidonic acid incorportion is decreased in heart FABP gene ablated mice. Biochem. 2005;44:6350–6360. doi: 10.1021/bi047292r. [DOI] [PubMed] [Google Scholar]
- 75.Owada Y. Fatty acid binding protein: localization and functional significance in brain. Tohoku J Exp Med. 2008;214:213–220. doi: 10.1620/tjem.214.213. [DOI] [PubMed] [Google Scholar]
- 76.Moulle VSF, Cansell C, Luquet S, Cruciani-Guglielmacci C. Multiple roles of fatty acid handling proteins in brain. Frontiers in Physiology. 2012;3:1–6. doi: 10.3389/fphys.2012.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elmes MW, Kaczocha M, Berger WT, Leung KN, Ralph BP, Wang L, Sweeney JM, Miyauchi JT, Tsirka SE, Ojima I, Deutsch DG. Fatty acid binding proteins are intracellular carriers for delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) J Biol Chem. 2015;290:8711–8721. doi: 10.1074/jbc.M114.618447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG. Fatty acid binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem. 2012;287:3415–3424. doi: 10.1074/jbc.M111.304907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaczocha M. Ph D Thesis. Stony Brook University; 2009. Role of fatty acid binding proteins and FAAH-2 in endocannabinoid uptake and inactivation. [Google Scholar]
- 80.Frolov A, Miller K, Billheimer JT, Cho TC, Schroeder F. Lipid specificity and location of the sterol carrier protein-2 fatty acid binding site: A fluorescence displacement and energy transfer study. Lipids. 1997;32:1201–1209. doi: 10.1007/s11745-997-0154-5. [DOI] [PubMed] [Google Scholar]
- 81.Liedhegner ES, Vogt CD, Sem DS, Cunninham CW, Hillard CJ. Sterol carrier protein-2: binding protein for endocannabinoids. Mol Neurobiol. 2014;50:149–158. doi: 10.1007/s12035-014-8651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schroeder F, Jolly CA, Cho TH, Frolov AA. Fatty acid binding protein isoforms: structure and function. Chem Phys Lipids. 1998;92:1–25. doi: 10.1016/s0009-3084(98)00003-6. [DOI] [PubMed] [Google Scholar]
- 83.Myers-Payne SC, Hubbell T, Pu L, Schnutgen F, Borchers T, Wood WG, Spener F, Schroeder F. Isolation and characterization of two fatty acid binding proteins from mouse brain. J Neurochem. 1996;66:1648–1656. doi: 10.1046/j.1471-4159.1996.66041648.x. [DOI] [PubMed] [Google Scholar]
- 84.Pu L, Igbavboa U, Wood WG, Roths JB, Kier AB, Spener F, Schroeder F. Expression of Fatty Acid Binding Proteins Is Altered in Aged Mouse Brain. Molecular and Cellular Biochemistry. 1999;198:69–78. doi: 10.1023/a:1006946027619. [DOI] [PubMed] [Google Scholar]
- 85.Pu L, Annan RS, Carr SA, Frolov A, Wood WG, Spener F, Schroeder F. Isolation and identification of a native fatty acid binding protein form mouse brain. Lipids. 1999;34:363–373. doi: 10.1007/s11745-999-0374-8. [DOI] [PubMed] [Google Scholar]
- 86.Owada Y, Yoshimoto T, Kondo H. Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. Journal of Chemical Neuroanatomy. 1996;12:113–122. doi: 10.1016/s0891-0618(96)00192-5. [DOI] [PubMed] [Google Scholar]
- 87.Schnutgen F, Borchers T, Muller T, Spener F. Heterologous expression and characterization of mouse brain fatty acid binding protein. Biol Chem Hoppe-Seyler. 1996;377:211–215. doi: 10.1515/bchm3.1996.377.3.211. [DOI] [PubMed] [Google Scholar]
- 88.Bennett E, Stenvers KL, Lund PK, Popko B. Cloning and characterization of a cDNA encoding a novel fatty acid binding protein from rat brain. J Neurochem. 1994;63:1616–1624. doi: 10.1046/j.1471-4159.1994.63051616.x. [DOI] [PubMed] [Google Scholar]
- 89.Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 90.Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- 91.Chen CT, Domenichiello AF, Trepanier MO, Liu Z, Masoodi M, Bazinet RP. Low levels of EPA in rat brain phospholipids are maintained via multiple redundant mechanisms. J Lipid Res. 2013;54:2410–2422. doi: 10.1194/jlr.M038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang H, McIntosh AL, Martin GG, Landrock K, Landrock D, Gupta S, Atshaves BP, Kier AB, Schroeder F. Structural and functional interaction of fatty acids with human liver fatty acid binding protein (L-FABP) T94A variant. FEBS J. 2014;281:2266–2283. doi: 10.1111/febs.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nemecz G, Hubbell T, Jefferson JR, Lowe JB, Schroeder F. Interaction of fatty acids with recombinant rat intestinal and liver fatty acid-binding proteins. Arch Biochem Biophys. 1991;286:300–309. doi: 10.1016/0003-9861(91)90044-j. [DOI] [PubMed] [Google Scholar]
- 94.Nemecz G, Jefferson JR, Schroeder F. Polyene fatty acid interactions with recombinant intestinal and liver fatty acid binding proteins. J Biol Chem. 1991;266:17112–17123. [PubMed] [Google Scholar]
- 95.Prows DR, Murphy EJ, Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-Cells. Lipids. 1995;30:907–910. doi: 10.1007/BF02537481. [DOI] [PubMed] [Google Scholar]
- 96.Murphy EJ. L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L-cells. Am J Physiol. 1998;275:G244–G249. doi: 10.1152/ajpgi.1998.275.2.G244. [DOI] [PubMed] [Google Scholar]
- 97.McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res. 1999;40:1371–1383. [PubMed] [Google Scholar]
- 98.Wolfrum C, Buhlman C, Rolf B, Borchers T, Spener F. Variation of liver fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and antisense RNA affects the rate of fatty acid uptake. Biochim Biophys Acta. 1999;1437:194–201. doi: 10.1016/s1388-1981(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 99.Mukhopadyay B, Liu J, Osei-Hylaman D, Kunos G. Transcriptional regulation of the cannabinoid receptor-1 expression in the liver by retinoid acid via retinoid acid receptor-g. J Biol Chem. 2010;285:19002–19011. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang J, Teng Z, Tang YP, Chen C. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregualtion of miR-188-3p in a mouse model of Alzheimer’s disease. J of Neuroscience. 2014;34:1419–1433. doi: 10.1523/JNEUROSCI.1165-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed type hypersensitivity response by triggering IL-10 production and consequent miRNA induction. PLoS ONE. 2014;9:e93954. doi: 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morrow FD, Allen CE, Martin RJ. Intracellular fatty acid-binding protein: Hepatic levels in lean and obese rats. Fed Proc. 1979;38:280. [Google Scholar]
- 103.Pignon J-P, Bailey NC, Baraona E, Lieber CS. Fatty acid-binding protein: a major contributor to the ethanol-induced increase in liver cytosolic proteins in the rat. Hepatology. 1987;7:865–871. doi: 10.1002/hep.1840070512. [DOI] [PubMed] [Google Scholar]
- 104.Gyamfi MA, He L, French SW, Damjanov I, Wan YJY. Hepatocyte retinoid X receptor alpha-dependent regulation of lipid homeostasis and inflammatory cytokine expression contributes to alcohol-induced liver injury. J Pharm Exp Ther. 2008;324:443–453. doi: 10.1124/jpet.107.132258. [DOI] [PubMed] [Google Scholar]
- 105.Yang SY, He XY, Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem. 1987;262:13027–13032. [PubMed] [Google Scholar]
- 106.Higuchi N, Kato M, Tanaka M, Miyazaki M, Takao S, Kohjima M, Kotoh K, Enjoji M, Nakamuta M, Takayanagi R. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP, and L-FABP in non-alcoholic fatty liver disease. Exp and Ther Med. 2011;2:1077–1081. doi: 10.3892/etm.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Charlton M, Viker K, Krishnan A, Sanderson S, Veldt B, Kaalsbeek AJ, Kendrick M, Thompson G, Que F, Swain J, Sarr M. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49:1375–1384. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest and Liver Phys. 2007;294:G27–G38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- 109.Peng XE, Wu YL, Lu QQ, Ju ZJ, Lin X. Two genertic variants in FABP1 and susceptibility to non-alcoholic fatty liver disease in a Chinese population. Gene. 2012;500:54–58. doi: 10.1016/j.gene.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 110.Peng X-E, Wu Y-L, Zhu Y, Huang R-D, Lu Q-Q, Lin X. Association of a human FABP1 gene promoter region polymorphism with altered serum triglyceride levels. PLoS ONE. 2015:1–13. doi: 10.137/journal.pone..0139417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McIntosh AL, Huang H, Storey SM, Landrock K, Landrock D, Petrescu AD, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human FABP1 T94A variant impacts fatty acid metabolism and PPARa activation in cultured human female hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2014;307:G164–G176. doi: 10.1152/ajpgi.00369.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robitaille J, Brouillette C, Lemieux S, Perusse L, Gaudet D, Vohl MC. Plasma concentrations of apolipoprotein B are modulated by a gene-diet interaction effect between the L-FABP T94A polymorphism and dietary fat intake in French-Canadian men. Mol Gen and Metab. 2004;82:296–303. doi: 10.1016/j.ymgme.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 113.Fisher E, Weikert C, Klapper M, Lindner I, Mohlig M, Spranger J, Boeing H, Schrezenmeir J, Doring F. L-FABP T94A is associated with fasting triglycerides and LDL-cholesterol in women. Mol Gen and Metab. 2007;91:278–284. doi: 10.1016/j.ymgme.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Weikert MO, Loeffelholz Cv, Roden M, Chandramouli V, Brehm A, Nowotny P, Osterhoff MA, Isken F, Spranger J, Landau BR, Pfeiffer A, Mohlig M. A Thr94Ala mutation in human liver fatty acid binding protein contributes to reduced hepatic glycogenolysis and blunted elevation of plasma glucsoe levels in lipid-exposed subjects. Am J Physiol Endocrinol Metab. 2007;293:E1078–E1084. doi: 10.1152/ajpendo.00337.2007. [DOI] [PubMed] [Google Scholar]
- 115.Yamada Y, Kato K, Oguri M, Yoshida T, Yokoi K, Watanabe S, Metoki N, Yoshida H, Satoh K, Ichihara S, Aoyagi Y, Yasunaga A, Park H, Tanaka M, Nozawa Y. Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int J Mol Med. 2008;21:801–808. [PubMed] [Google Scholar]
- 116.Bu L, Salto LM, De Leon KJ, De Leon M. Polymorphisms in fatty acid binding protein 5 show association with type 2 diabetes. Diabetes Res Clin Prac. 2011;92:82–91. doi: 10.1016/j.diabres.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mansego ML, Martinez F, Martinez-Larrad MT, Zabena C, Rojo G, Morcillo S, Soriguer F, Martin-Escudero JC, Serrano-Rios M, Redon J, Chaves FJ. Common variants of the liver fatty acid binding protein gene influence the risk of Type 2 Diabetes and insulin resistance in Spanish population. PLoS ONE. 2012;7:e31853. doi: 10.1371/journal.pone.0031853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399–408. doi: 10.2147/JPR.S55598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Janke EA, Collins A, Kozak AT. Overview of the relationship between pain and obesity: What do we know? Where do we go next? J Rehab Res Dev. 2007;44:245–262. doi: 10.1682/jrrd.2006.06.0060. [DOI] [PubMed] [Google Scholar]
- 120.McKendall MJ, Haier RJ. Pain sensitivity and obesity. Psychiatry Res. 1983;8:119–125. doi: 10.1016/0165-1781(83)90099-9. [DOI] [PubMed] [Google Scholar]
- 121.Jackson P, Gleeson D. Alcoholic liver disease. Cont Ed in Anaesthesia, Crit Care and Pain. 2010;10:66–71. [Google Scholar]
- 122.Bouneva I, Abou-Assi S, Heuman DM, Mihas AA. Alcoholic liver disease. Hospital Physician. 2003 Oct;2003:31–38. [Google Scholar]
- 123.Kneeman JM, Misdraji J, Corey KC. Secondary causes of nonalcoholic liver disease. Therapeutic Advances in Gastroenterology. 2012;5:199–207. doi: 10.1177/1756283X11430859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alswat KA. The role of endocannabinoid system in fatty liver disease and therapeutic potential. Saudi Journal of Gastroenterology. 2015;19:144–151. doi: 10.4103/1319-3767.114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maccarrone M, Gasperi V, Catani MV, Diep TI, Dainese E, Hansen HS, Avigliano L. The endocannabinoid system and its relevance to nutrition. Annu Rev Nutr. 2010;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. [DOI] [PubMed] [Google Scholar]
- 126.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–E1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 127.Atshaves BP, McIntosh AL, Kier AB, Schroeder F. High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene ablated mice. Lipids. 2010;45:97–110. doi: 10.1007/s11745-009-3379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver Fatty Acid Binding Protein GeneAblated Female Mice Exhibit Increased AgeDependent Obesity. J Nutr. 2008;138:1859–1865. doi: 10.1093/jn/138.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol Cell Biochem. 2009;324:101–115. doi: 10.1007/s11010-008-9989-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem J. 2005;391:549–560. doi: 10.1042/BJ20050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am J Physiol. 2006;290:G36–G48. doi: 10.1152/ajpgi.00510.2004. [DOI] [PubMed] [Google Scholar]
- 132.McIntosh AL, Atshaves BP, Landrock D, Landrock KK, Martin GG, Storey SM, Kier AB, Schroeder F. Liver fatty acid binding protein gene-ablation exacerbates weight gain in high fat fed female mice. Lipids. 2013;48:435–448. doi: 10.1007/s11745-013-3777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]