Abstract
Meat and vitamin B3 – nicotinamide – intake was high during hunter-gatherer times. Intake then fell and variances increased during and after the Neolithic agricultural revolution. Health, height, and IQ deteriorated. Low dietary doses are buffered by ‘welcoming’ gut symbionts and tuberculosis that can supply nicotinamide, but this co-evolved homeostatic metagenomic strategy risks dysbioses and impaired resistance to pathogens. Vitamin B3 deficiency may now be common among the poor billions on a low-meat diet. Disease transitions to non-communicable inflammatory disorders (but longer lives) may be driven by positive ‘meat transitions’. High doses of nicotinamide lead to reduced regulatory T cells and immune intolerance. Loss of no longer needed symbiotic ‘old friends’ compounds immunological over-reactivity to cause allergic and auto-immune diseases. Inhibition of nicotinamide adenine dinucleotide consumers and loss of methyl groups or production of toxins may cause cancers, metabolic toxicity, or neurodegeneration. An optimal dosage of vitamin B3 could lead to better health, but such a preventive approach needs more equitable meat distribution. Some people may require personalised doses depending on genetic make-up or, temporarily, when under stress.
Keywords: Diet, hyper-vitaminosis B3, nicotinamide, tryptophan, disease transitions, health inequality, hygiene hypothesis, environmental enteropathy, Parkinson, metabolic syndrome, cancer, allergies, pellagra
No civilisation can be sound or stable which has at its base this mass of stunted life.
Poverty, Seebohm Rowntree, 1900.
It must first, however, be generally believed with Sydenham, that our chronic maladies are of our own making.
Thomas Beddoes, 1800.
Introduction
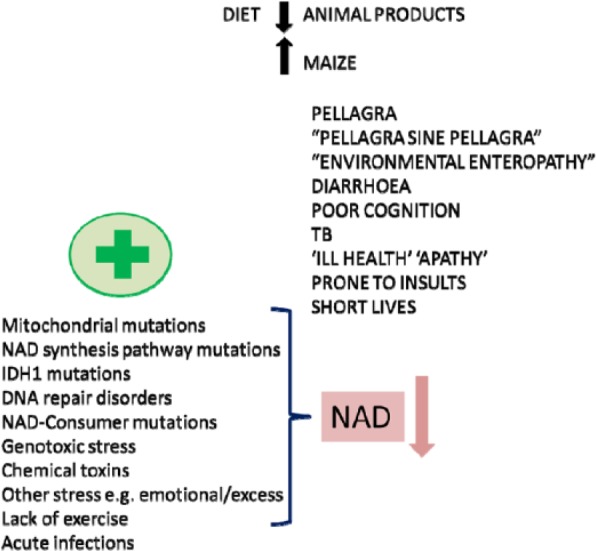
Meat transitions have been a feature of human history. Ever since the evolution of agriculture, there have been marked variances of meat intake between rich and poor nations and individuals within social classes, and therefore, oscillations of key components such as nicotinamide that when severely deficient causes pellagra.1–5 Nicotinamide and its derivatives nicotinamide adenine dinucleotide (NAD) and reduced nicotinamide adenine dinucleotide (NAD(H)) are intrinsic to mitochondrial function, and levels can be high, optimal, or low: these can involve manageable change through homeostasis or use of symbionts (or when short-lived, can be a beneficial hormetic shock). However, if nicotinamide supplies are very low, this can lead to energy decline with loss of high-energy molecules and disease often through protein modifications – as is seen with pellagra and similar to those seen with some mitochondrial and other mutations.6,7 Nicotinamide adenine dinucleotide is an important ‘food signal’ to all organisms and, often mediated by serotonin, has defining effects on development, circadian rhythms, gene regulation through chromatin remodelling, and reproductive and other behaviour.8,9 NADH as a cofactor performs more than 500 key dehydrogenase and other reactions, excluding NAD reactions that are important to metabolism, (stem-cell) development, repair, and longevity, but then is consumed – so it requires constant replenishment ultimately from dietary precursors.10
Much has been written about modern maladaptations relative to our long hunter-gatherer days. These mismatches usually concentrate on loss of fibre or excessive sugar or fructose or gluten in recent affluent diets.5,11–23 We argue that a high risk of too little nicotinamide may be the most important difference between diets in the past 10 000 years and those in the Palaeolithic. During that long time, as we (co)evolved much of our nutrigenome, microbiome, and ‘meat cultures’, variances were usually ‘feasts or famines’ (or fatal as meat then provided most of the calories) rather than chronic shortages over lifetimes. The first Neolithic agricultural revolution and the second ‘Green revolution’ 50 years ago may have, as an unintended consequence, reduced meat and micronutrients for those in poverty. Both revolutions increased cereal availability reducing hunger but not the ‘hidden hunger’ of micronutrient deficiency in something of a ‘Faustian’ bargain.24
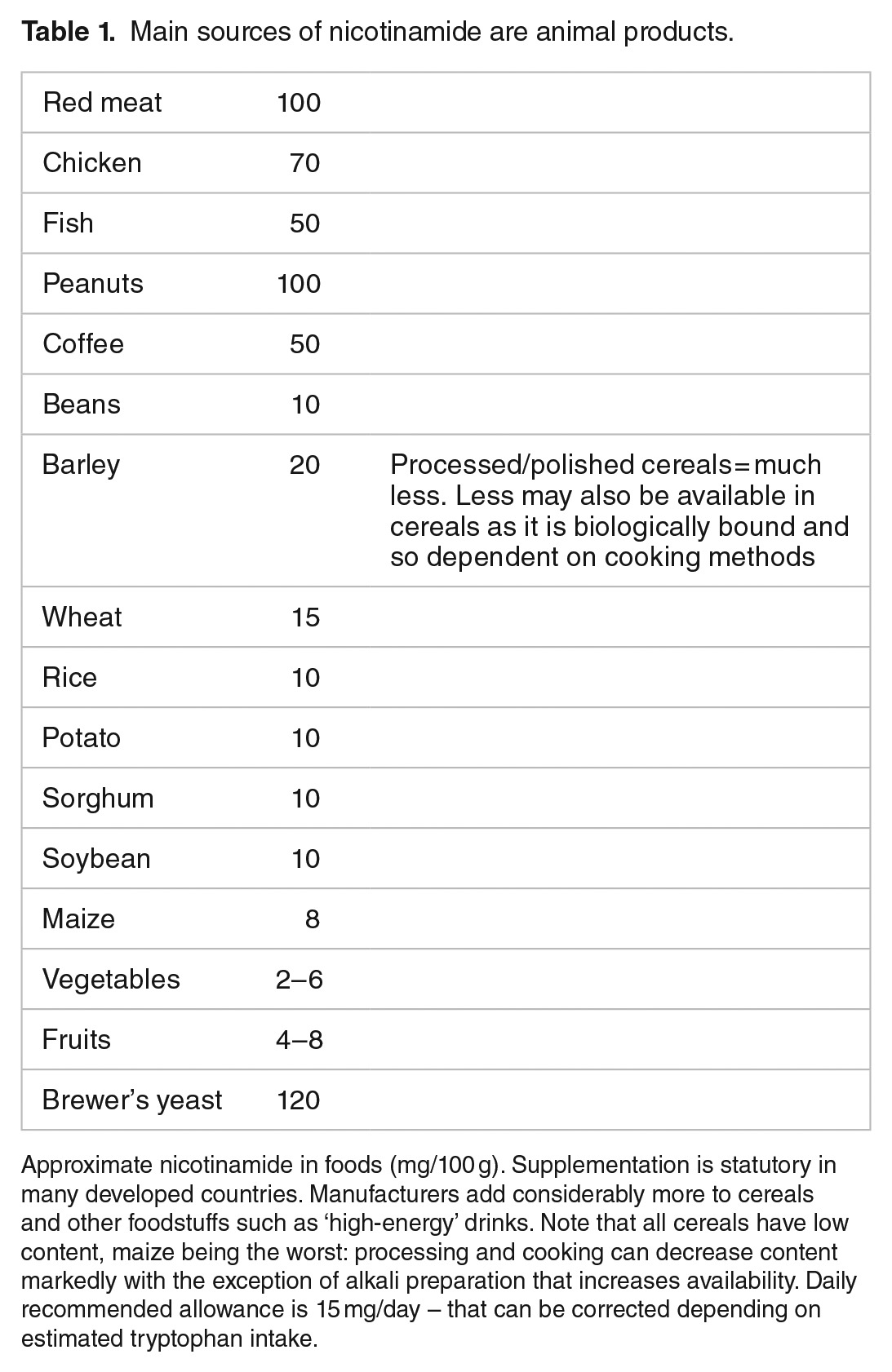
Nicotinamide’s approximate concentrations in foods are shown in Table 1.25,26 Milk contains average amounts of nicotinamide but high amounts of the potent nicotinamide riboside. Culturally acquired cooking methods ‘nixtamalisation’ can improve availability of nicotinamide from the bound form niacytin: the microbiome can contribute as many bacterial symbionts can synthesise nicotinamide, though, whether they share all this with other bacteria that cannot, or, with the host is unclear in man, though, must happen in ruminants. The synthetic pathway from tryptophan is inefficient but can supply 1 mg of niacin for 60 mg of tryptophan depending on other factors including pyridoxine and iron or zinc availability and high-fat or leucine diets. Tryptophan is sourced largely from animal products boosting the importance of meat, eggs, and milk. Cereals particularly maize barely increase post-prandial levels of tryptophan, and transport across the blood-brain barrier can be reduced by other amino acids complicating effects on mood and cognition.27–32
Table 1.
Main sources of nicotinamide are animal products.
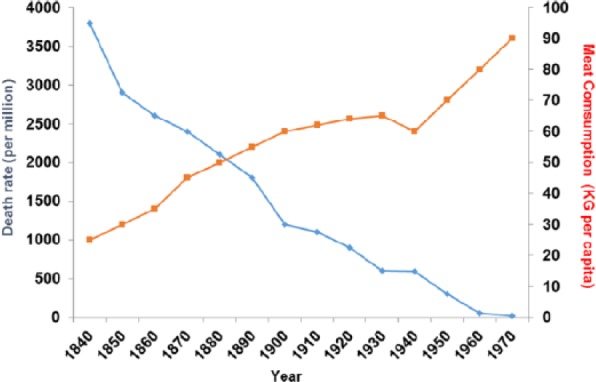
‘Meatification’ of the diet doubled meat intake in the United Kingdom between 1850 and 1960 and has overall increased by 2-fold average intake from 20 to 40 kg per annum per person in 1960 to double that in 2010 (as has nicotinamide intake) and is predicted to rise to 50 kg per annum.33,34 The extremes are striking at 120 kg per annum per person as an average in many rich countries (who also obtain nicotinamide as mandated supplements and manufacturer’s additives) but 20 kg per annum per person in many poor countries with many individuals eating negligible amounts that have to affect a society’s health and industriousness.35,36
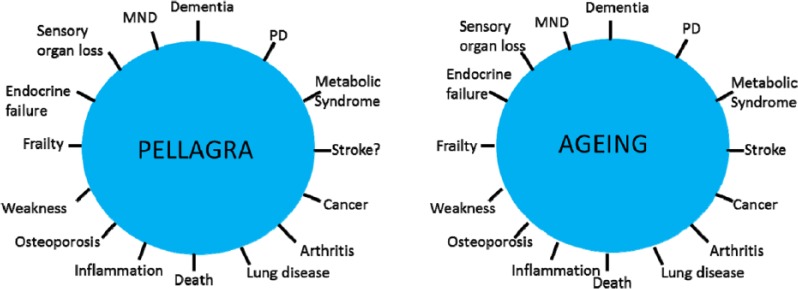
Such individuals must be at risk of the classic meat deficiency disorder particularly when on a poor monophagic cereal diet (usually maize), known as pellagra. Pellagrins can have a diagnostic rash ‘Casal necklace’ triggered by sunlight. The rash, however, is not always present or characteristic (often diagnosed as eczema) – ‘pellagra sine pellagra’.37 Other cardinal features of pellagra such as gut infections and neurodevelopmental and neuropsychiatric syndromes and an inability to deal with stress or an absence of allergies are non-specific, so the diagnosis is bound to be missed (Figure 1). There is no easy biochemical test.
Figure 1.
Pellagra has a very wide phenotype. The parallel with diseases of ageing are striking. NAD/NADH/nicotinamide imbalances may be the treatable underlying common factor to many common diseases whether from dietary deficiency or excess or varying needs from genetic mutation, toxic, anoxic, or other external stresses. NAD indicates nicotinamide adenine dinucleotide.
Hypotheses
We will argue that nobody ever systematically checked that pellagra was eliminated globally (dietary supplementation mainly happened in rich countries). Pellagra may be common and misdiagnosed masquerading as ‘environmental enteropathy’, poor cognition, eczema, or general ill health and a lack of well-being or poor homeostasis when under environmental stress with shortened lives.38,39
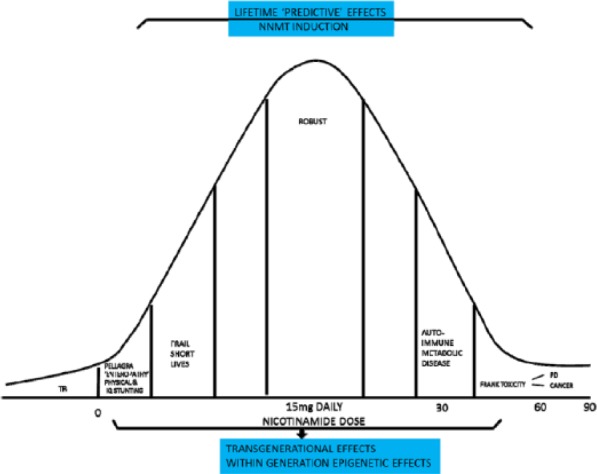
We also argue that environmental insults from trauma, hypoxia, toxins, stress, or mutations (such as in mitochondrial or DNA-repair genes) may require either lifetime or temporary higher doses than normally recommended (15 mg/d).
Controversially, we suggest that many people in rich countries may be on too high a dose. A hyper-vitaminosis B3 state may be common and, like pellagra, have a wide phenotype that includes the metabolic syndrome, several cancers, and some degenerative or neuro-behavioural disorders.40
Furthermore, we propose that the transition from diseases of poverty to diseases of affluence is due to switching the dose of meat/nicotinamide too fast and too far.41,42 The most recent version of the ‘hygiene hypothesis’ concentrates on reductions in symbiotic/commensal biome diversity acquired early in development, not cleanliness during childhood and common pathogens.43–46 We will discuss how a biochemical switch away from the need to produce nicotinamide ‘in house’ from tryptophan, and the related reduced metabolic need for gut symbionts or tuberculosis (TB) on a better diet so that they are no longer ‘welcomed’ by the immune system is a more plausible explanation for the loss of microbial ‘old friends’. This switch of microbiomes reduces tolerogenic instruction to the immune system further encouraging it to over-react to otherwise irrelevant foreign proteins or self-proteins.47
Biochemical Background
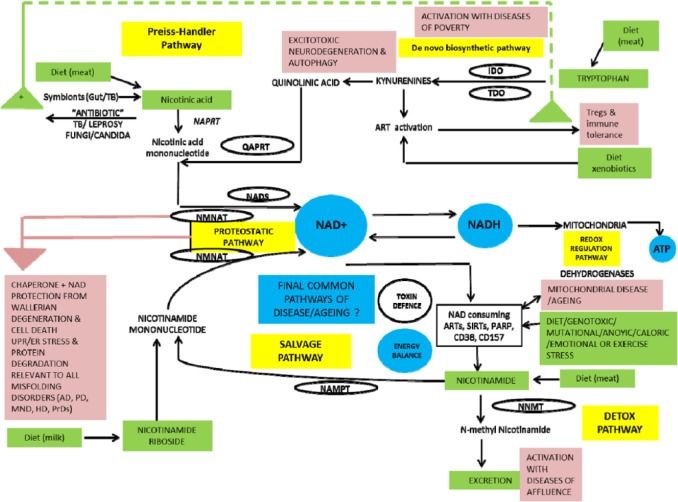
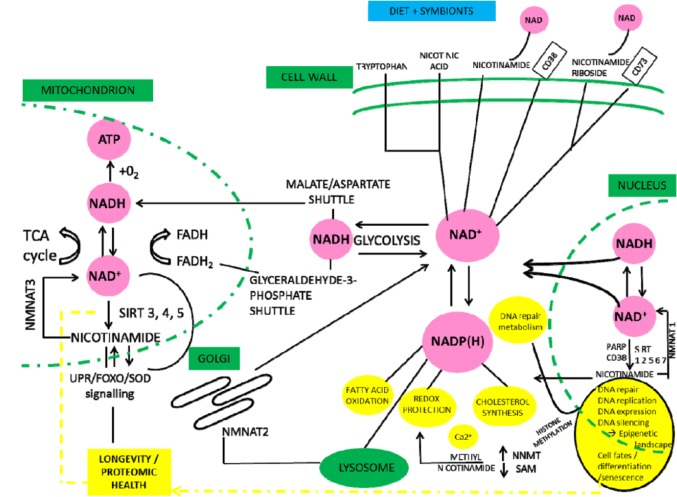
We summarise NAD metabolism in a diagrammatic form (Figures 2 to 4). Nicotinamide adenine dinucleotide has 3 related precursor vitamins – nicotinamide, nicotinic acid, and nicotinamide riboside – that have highly efficient synthetic and recycling ‘salvage’ pathways and one ‘detoxification’ pathway using nicotinamide N-methyltransferase (NNMT).48–54 There is an important backup nicotinamide/NAD ‘de novo’ synthetic pathway from the essential amino acid tryptophan.55 This pathway is crucial to our story as it is closely linked with immunologic tolerance and intolerance.
Figure 2.
Diet supplies nicotinamide, nicotinic acid, and nicotinamide riboside as well as the essential amino acid tryptophan. Tryptophan can be degraded to synthesise nicotinamide when there is dietary stress by the kynurenine ‘de novo’ ‘immune tolerance’ pathway. Symbionts, whether in gut or TB, are a backup source as is ‘autocarnivory’. Salvage pathways are extensive and efficient to conserve NAD/nicotinamide as NAD consumers mean that there is a continuous need for replenishment. Many are involved in repair and disease processes and ageing. Excess nicotinamide can be excreted after a methylation reaction by NNMT. NNMT, indicates nicotinamide N-methyltransferase; TB, tuberculosis.
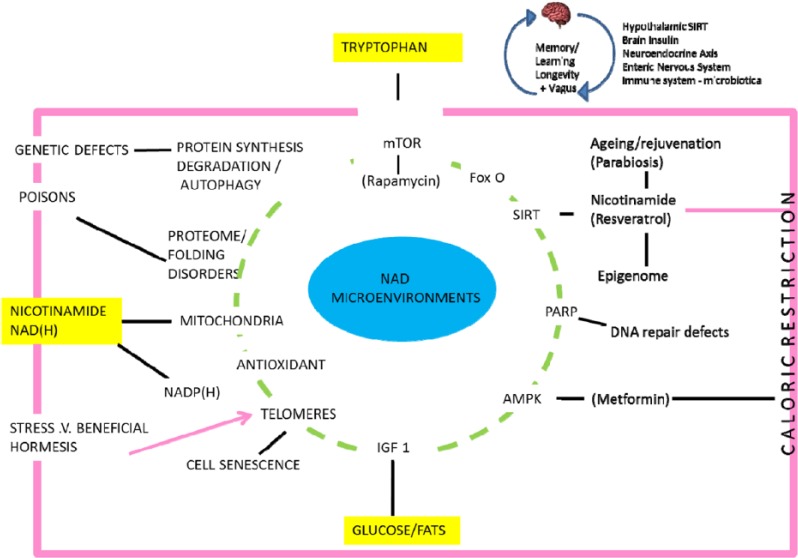
Figure 4.
Nicotinamide/NAD and tryptophan metabolism are linked by the ‘de novo’ pathway. All these pathways have been implicated in diseases of ageing and mechanisms such as proteotoxicity, and, interventions such as caloric restriction, resveratrol, parabiosis and metformin.
Nicotinamide adenine dinucleotide, the central redox coenzyme in cellular metabolism, functions as a hydride group acceptor forming NADH with concomitant oxidation of metabolites derived from carbohydrates, amino acids, and fats. Gluconeogenesis, oxidative phosphorylation, ketogenesis, detoxification, and lipogenesis require reduced cofactors NADH and reduced nicotinamide adenine dinucleotide phosphate (NADPH). Nicotinamide adenine dinucleotide is the consumed substrate of poly(ADP-ribose) polymerases (PARPs), sirtuins (SIRTs), and cyclic adenosine diphosphate (ADP) synthetases. Nicotinamide adenine dinucleotide and NAD/NADH ratios have been described as a master controller of many physiological and repair activities and are themselves under circadian control with much cross-talk with multiple nutrient pathways, whether carbon, nitrogen, or phosphate sensing. Despite extensive recycling, cell stresses that include ageing, toxins, and over-nutrition activate NAD consumption and alongside growth requirements mean that there is a continuous need for a dietary or microbiomic source of NAD precursors.56–58
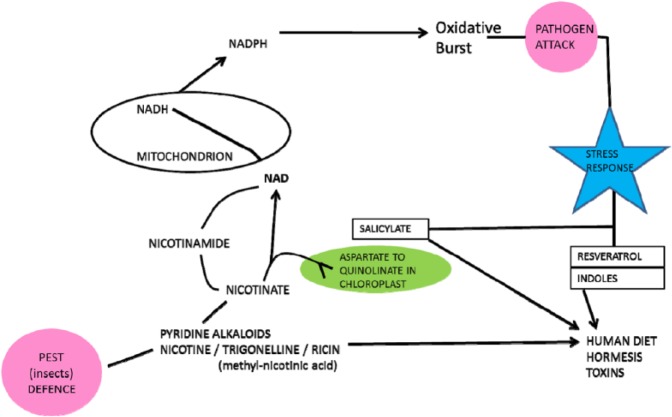
The close relationship between man and meat/milk-providing animals is not surprising. Nicotinamide adenine dinucleotide metabolism is also integral to plant primary energy acquisition from the sun on which all animals depend. Plants also use NAD-dependent oxidative bursts to ward off microbial attack.59 Other deterrants from herbivore attack involve nicotinamide-nicotine metabolism; this is interesting as both nicotine and other stress compounds, such as salicylate and resveratrol, affect our NAD metabolism in both a medicinal and hormetic sense (Figure 5).
Figure 5.
Plant and human metabolisms have active and overlapping reactions involving nicotine and nicotinamide. Stress molecules such as nicotine, salicylate, and resveratrol have medicinal and hormetic actions in man.
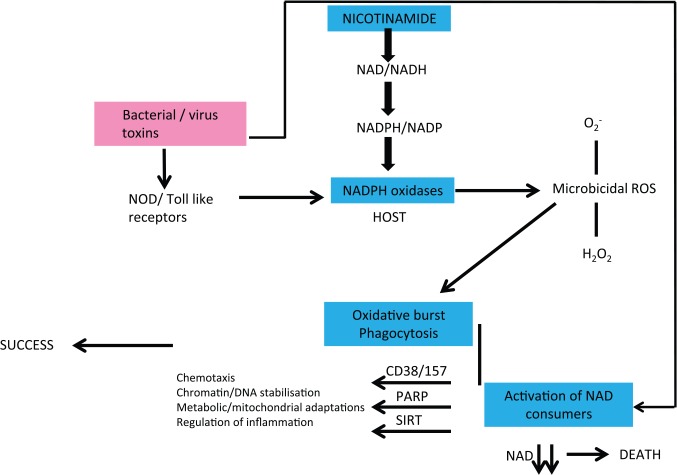
Some hindgut symbionts produce nicotinic acid (that they share) as does TB60–62 (Figure 6).63–65 We have argued that when meat sources are good and shortages short-lived, TB acts as a symbiont farmed for its nicotinamide. The relationship with classic pathogens is different. Many have evolved to be consumers of host NAD, and many of their toxins directly affect NAD consumer pathways66–74 (Figure 7). If the host is moderately deficient in nicotinamide/NAD in the first place, they will be less resilient – if severe, the pathogen may not have enough to survive perhaps explaining the paradoxical relationship between nutrition and infection when very poor nutrition can be protective.75
Figure 6.

A designer symbiont. TB produces nicotinic acid but cannot recycle it to NAD so excretes it to a ‘welcoming’ host that ‘farms’ the organism at some risk of dysbiosis if the dietary dose of nicotinamide is too low. Nicotinamide is a natural and its analogues are some of the artificial anti-tuberculous agents. NAD indicates nicotinamide adenine dinucleotide; TB, tuberculosis.
Figure 7.
Infections and host NAD metabolism are intertwined. Many pathogens evolved to consume host NAD or their toxins lead to host NAD depletion. If the host is NAD deficient, this will exacerbate virulence and death rates. If severely deficient, there may not be enough NAD to allow the pathogen to replicate. Symbionts, by contrast, can improve host NAD levels.
Nicotinamide adenine dinucleotide pathways have been heavily implicated in ageing and many diseases from the neurological to retinal disease to cancer and can restore stem cells.73,76–103 Recent evidence fits with the very wide phenotype of disease seen with pellagra. The cellular response to NAD deficiency (and excesses) across cell lines is heterogeneous and may explain these broad phenotypes suggesting that NAD/NADH upsets might be a common underlying cause of many diseases.104
Pathways – Over- and Under-Nutrition
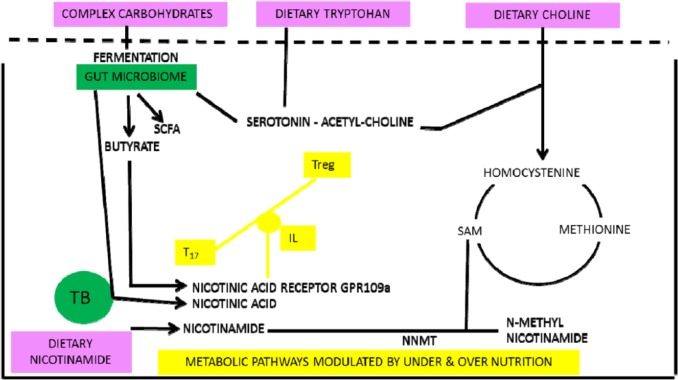
Over- and under-nutrition are bad for health as demonstrated by ‘Waaler’ curves for height, weight, and mortality.105 We propose that they share pathways that intersect with symbionts buffering poor diet and both relate to nicotinamide metabolism (Figure 8). Butyrate (and nicotinic acid) are generated by gut symbionts that include Lactobacillus or Bifidobacteria or Clostridia sp that ferment fibre and other otherwise indigestible complex carbohydrates and are in high concentration in the colon.90,106–110 Butyrate is an endogenous ligand active on the G protein–coupled GPR109A nicotinic acid receptor (expressed on macrophages, dendritic and epithelial cells, and adipocytes); this sends non-redundant signals via interleukins that affect T-cell differentiation favouring Tregs over helper T cells when the diet is poor as does nicotinamide from the diet when it is rich.90,111–118 This signal from the ‘niacin receptor’ reduces inflammation and carcinogenesis and has independently been proposed as a factor in mediating and perhaps moderating diet and disease transitions91,119
Figure 8.
Low-meat/high-fibre diets lead to symbionts that increase nicotinamide levels or produce butyrate. Butyrate is an agonist at the nicotinic acid receptor as well as having epigenetic effects. Both butyrate and nicotinic acid will affect the T-cell balances and immunologic tolerance.
There is much supportive evidence of differences between the microbiome on rich, poor, and traditional hunter-gatherer diets and that the tryptophan pathway is involved in states of malnutrition (or rare but illustrative genetic defects that affect nutrition).120–122 Reliance on the microbiome could mean that those on a poor diet are at risk of broad-spectrum antibiotics temporarily eliminating symbionts and triggering clinical nicotinamide deficiency – pellagra (as can happen with anti-tuberculous therapy).123,124
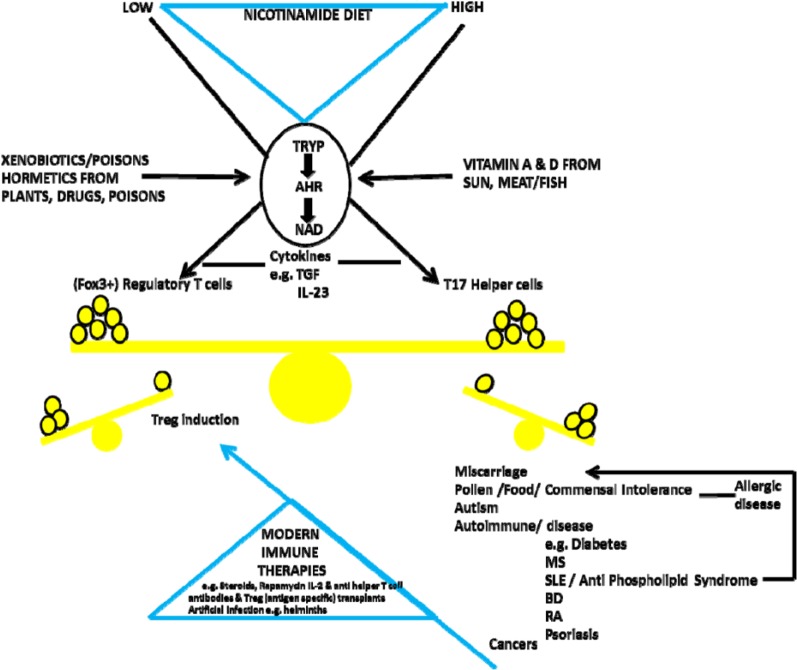
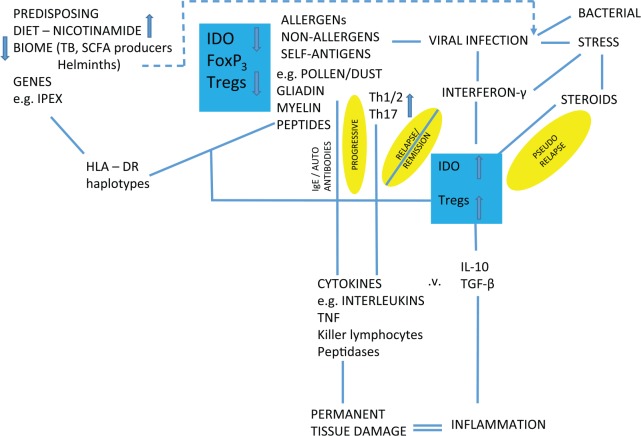
Although many factors have been shown to alter traffic in the ‘de novo’ pathway, particularly infectious diseases, it is important to remember that its primary purpose is the production of nicotinamide/NAD from tryptophan when the diet is inadequate (Figure 9). The consequences are immune tolerance to some useful symbionts. Immune tolerance is mediated by an effect on the balance between Tregs and T17 helper cells – also the target for steroids and many modern immune therapies or artificial infection with helminths. Kynurenine-derived toxins drive auto-carnivory that also releases NAD and tryptophan at the longer term cost of organ damage. The host will be more prone to pathogens, so there will be other severe downsides for the individual.78,125–130 On a high-nicotinamide diet, the opposite may be occurring with immune intolerance to normally harmless antigens.131–135 Although induction of indoleamine 2,3-dioxygenase (IDO) has been described paradoxically (on our theory) in some of these inflammatory or cancerous conditions, there is a difference between a predisposing endo-phenotype and one that is being influenced as the disease develops: we would argue that IDO induction can be compensatory (Figure 10).136–151
Figure 9.
The key switch is the kynurenine ‘de novo’ pathway. When dietary supplies of nicotinamide/NAD are not sufficient, there is Treg-induced tolerance for metabolically useful symbionts but dangers to individual health from dysbioses or pathogens. However, when dietary nicotinamide is high, there is immune intolerance with too few Tregs and an excess of pro-inflammatory T17 cells and many diseases of modernity. Many immunologic therapies from steroids to recent T-cell–targeted approaches or artificial infection try to correct this imbalance. Prevention might be more effective and safer.
Figure 10.
Predisposing phenotype for inflammatory disease driven by high nicotinamide in diet leads to reduced IDO activity. The more immediate triggers to these diseases and the disease process itself may sometimes lead to the apparent paradox of induced IDO as a compensation that may exacerbate or mitigate the disease. Lack of early infections or allergens may be ultimate causes but can act later as proximate triggers. IDO indicates indoleamine 2,3-dioxygenase.
We will now look at some data from the United Kingdom, 1850 to 1950, and across the contemporary world comparing states of under- and over-nutrition and diseases and health markers that are likely to be related to nicotinamide dose, as many are components of pellagra.
Methods
All meat data were collected from ‘Eating meat: evolution, patterns, and consequences’ by Smil152 and The Atlas of Food Who Eats What, Where, and Why by Millstone and Lang.153 IQ and literacy data were from ‘Some British pioneers of social medicine’ by Greenwood154 and ‘National IQS predict differences in scholoastic achievement in 67 countries’ Lynn et al.155 Tuberculosis data were collected from McKeown156 and the Institute for Health Metrics and Evaluation’s Global Burden of Disease (GBD) (2013).157 The Parkinson disease (PD) data were taken from Duvoisin and Schweitzer158 and the Institute for Health Metrics and Evaluation GBD (2013) (http://www.healthdata.org/gbd). All disease data had been corrected for age structure of populations. Cancer data were derived from ‘Mortality in England and Wales from 1848 to 1947’ by Logan159 and the Institute for Health Metrics and Evaluation’s GBD (2013). Diabetes data were taken from The Health of Adult Britain 1841–1994 by Charlton and Murphey160 and from the Institute for Health Metrics and Evaluation’s GBD (2013). Life expectancy data were from ‘Ecological public health: the 21st century’s big idea?’ by Rayner and Lang161 and The Atlas of Health: Mapping the Challenges and Causes of Disease by O’Donovan.162 Height data sets were from Galton’s midparent height revisited by Cole.163
Statistics
Exploratory analysis was conducted on these data to identify relationships between meat consumption and other variables by conducting scatterplots. The correlation between meat consumption and other variables was analysed using the Pearson correlation coefficient. All statistics were conducted using SPSS (version 21).
Results
In summary, and in round terms in the United Kingdom between 1850 and 1900, death rates per million with both sexes combined are as follows: for TB (before any treatment) halved from 7000 to 3500, for dysentery fell by 9/10ths from 150 to 15, whereas cancer (before much effect from smoking, at least in women) increased by 2.5-fold from 700 to 1700, and diabetes increased 4-fold from 50 to 200. The incidence of Parkinson’s disease rose markedly having only been described in 1817. During this period, meat intake almost doubled. Measures of cognitive and physical health such as literacy rates increased as did height. Contemporary data comparing these conditions with average meat intake across countries support these correlations.
More specifically, the reduction in deaths from diarrhoea between 1850 and 1950 in the United Kingdom trended with the rise in meat intake (P = .085) (Figure 11).
Figure 11.
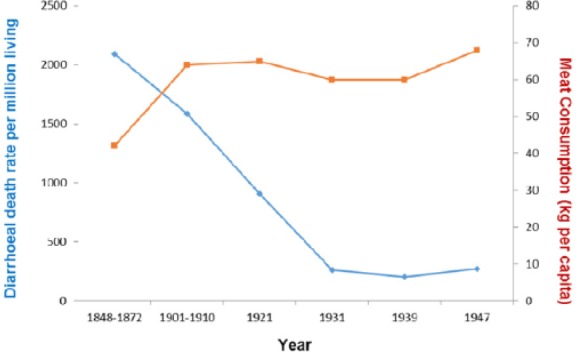
Diarrhoea plotted against meat intake in the United Kingdom, 1850–1950 (r = −0.642; P = .085).
Across the world today, deaths from diarrhoeal diseases correlate strongly with meat consumption P > .0001 (Figure 12).
Figure 12.
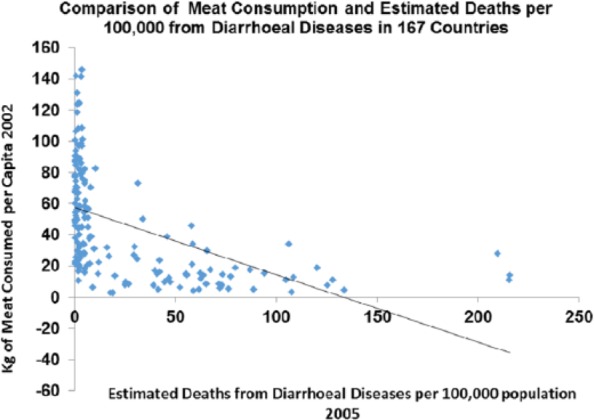
Diarrhoea plotted against meat intake in the contemporary world across nations (r = −0.508; P < .0001).
Literacy rates in the United Kingdom between 1850 and 1900 correlated with the rise in meat consumption P > .001 (Figure 13).
Figure 13.
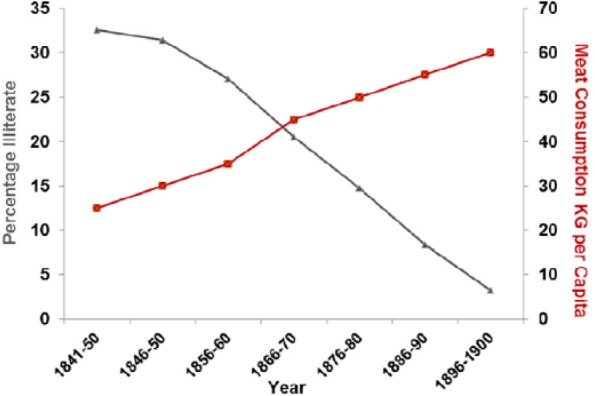
Literacy rates plotted against meat consumption in the United Kingdom, 1850–1900 (r = −0.988; P < .001).
In the contemporary world across nations, literacy rates correlate strongly with meat consumption P > .001 (Figure 14).
Figure 14.
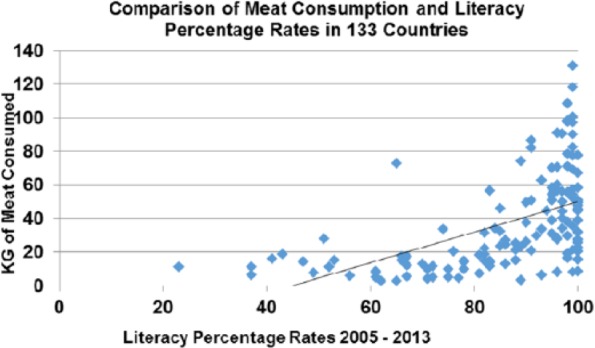
Literacy rates plotted against meat consumption in the contemporary world across nations (r = 0.531; P < .001).
Measured IQ correlates with meat consumption currently across countries P > .001 (Figure 15).
Figure 15.
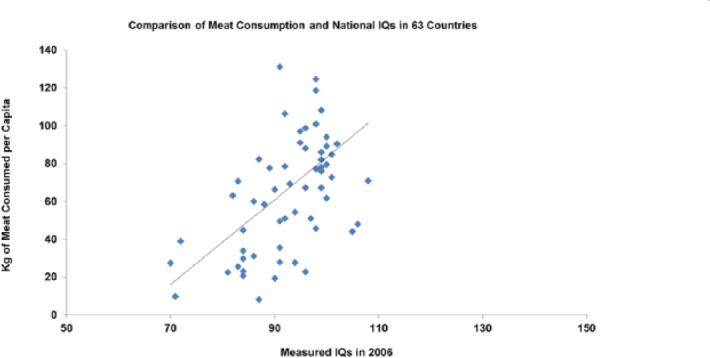
IQ plotted against meat consumption in the contemporary world across nations (r = 0.538; P < .001).
Between 1870 and 1970, boys height increased and correlates with meat intake (P > .001) as does height across nations currently (P > .001) (Figures 16 and 17).
Figure 16.
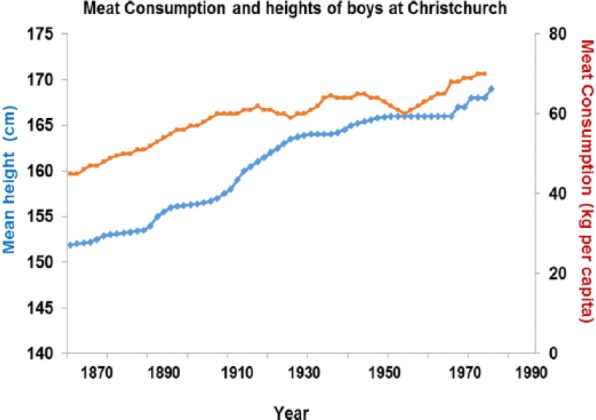
Increased height correlates strongly with higher meat intake (r = 0.934; P < .001).
Figure 17.
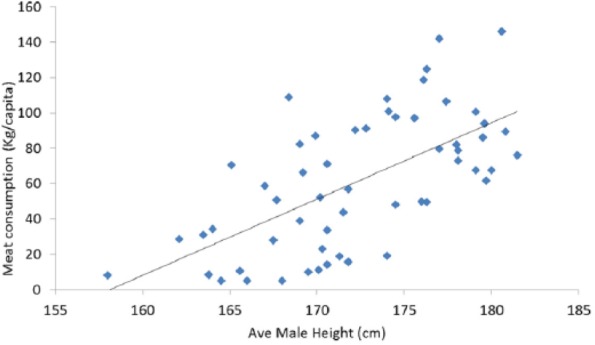
Increased height correlates strongly with higher meat intake (r = 0.635; P < .001).
Between 1850 and 1970, also between 1850 and 1950 (before any drug treatment), TB rates fell dramatically and this correlates with increased meat intake (P > .001). The decline in TB did not happen at the same time even within Europe and was also delayed in other industrial nations such as Japan where improvements in diet particularly of meat were also delayed.164 Reverses are well described under famine conditions165,166 (Figure 18).
Figure 18.
Tuberculosis plotted against meat consumption in the United Kingdom, 1850–1920, before there was any drug therapy (r = −0.958; P < .001).
This correlation is true across countries today where meat consumption correlates strongly with deaths from TB (P > .0001) (Figure 19).
Figure 19.
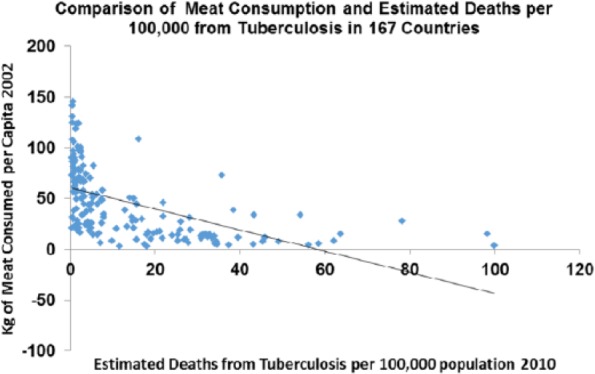
Tuberculosis plotted against meat consumption in the contemporary world across nations (r = −0.545; P < .0001).
Diabetes deaths rose between 1850 and 1950 and correlate with meat intake (P > .05) as they do across the world today (P > .001) (Figures 20 and 21).
Figure 20.
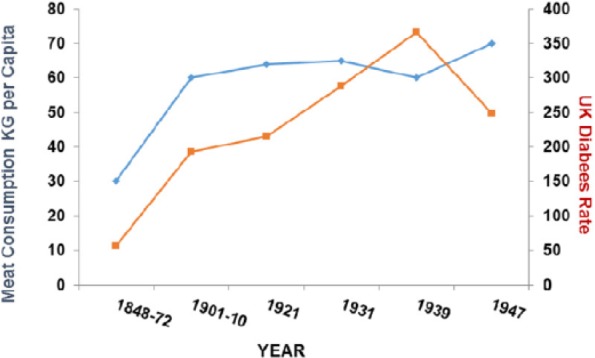
Diabetes plotted against meat consumption in the United Kingdom, 1850–1950 (r = 0.756; P < .05).
Figure 21.
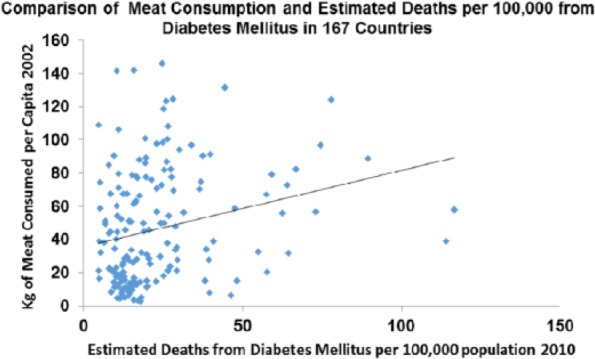
Diabetes plotted against meat consumption in the contemporary world across nations (r = 0.247; P < .001).
Cancer rates were increasing in both sexes in the United Kingdom from 1850 to 1900 before there was much effect from smoking (and none in women) and correlate with meat intake (P > .0001) as they do across the contemporary world (P > .0001) (Figures 22 and 23).
Figure 22.
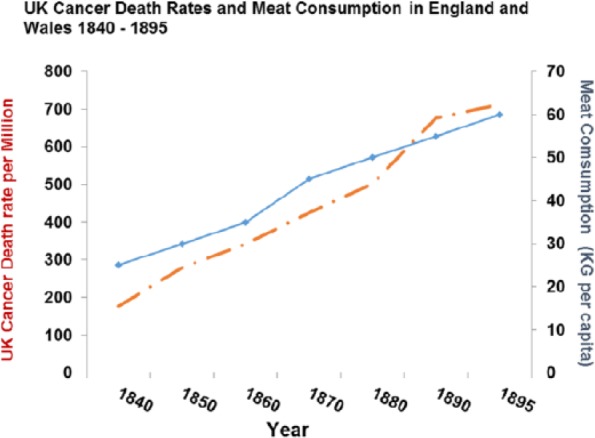
Cancer death rates plotted against meat consumption 1850–1950 (r = 0.981409; P < .0001).
Figure 23.
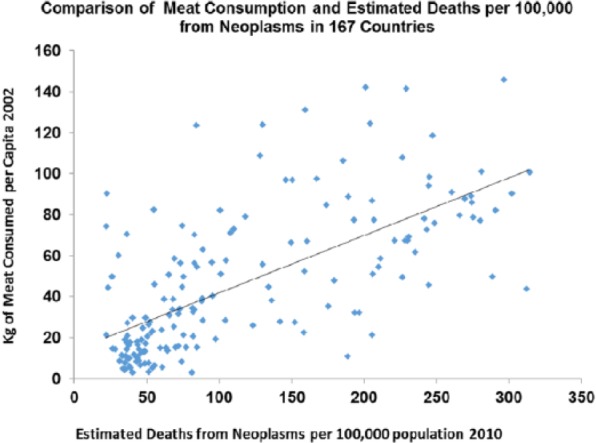
Cancer death rates plotted against meat consumption in the contemporary world across nations (r = 0.667; P < .0001).
Rates for the Parkinson disease were increasing between 1850 and 1960 (P > .001) and correlate with meat intake as they do across the world today (P > .0001) (Figures 24 and 25).
Figure 24.
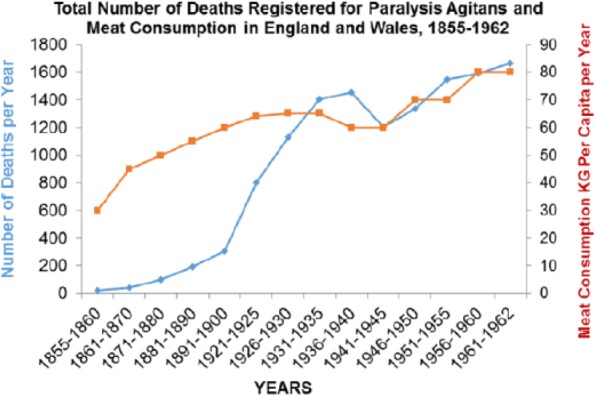
Parkinson disease plotted against meat consumption 1850–1900 (r = 0.842; P < .001).
Figure 25.
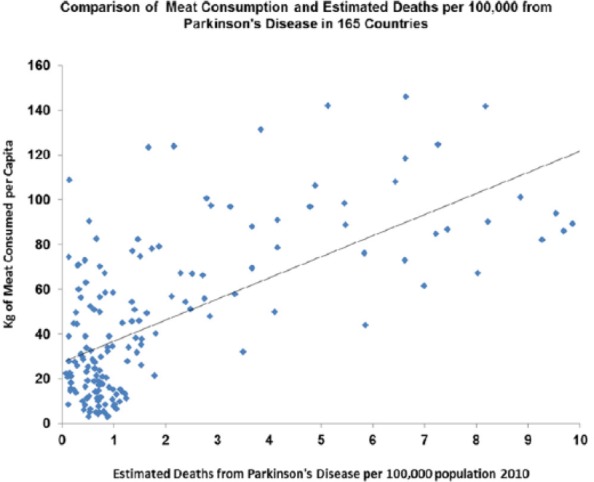
Parkinson disease plotted against meat consumption in the contemporary world across nations (r = 0.842; P < .0001).
The Allergy Epidemic, 1870-Till Now
The recent rise and exact timing of the rise in hay fever and allergic/auto-immune disease are less well-documented numerically – although the facts are not in dispute and average meat intake has also doubled again between 1960 and now, so if they were strong correlations would be obtained.34 Apart from a very few references in ancient literature, allergy first in the form of hay fever was first described in the mid-19th century exactly at the time the TB epidemic was abating in both the United Kingdom and in the United States.167–170 Observers at the time described these conditions and noted that they were on the increase, to begin with among the fashionable wealthy. Allergic disease had been unknown in previous generations (much as they are still unknown in poor African villages today). Increases are being recorded in countries that are getting richer or have had the economic benefits of European re-unification.171–180 Proximate triggers such as grass and ragweed pollen were noted early on, but they do not contain new antigens even if some became more prevalent. Rather paradoxically avoiding the allergen can make matters worse, as recently demonstrated for peanut allergy, so treating the proximate allergen as the actual or preventable cause is problematic.181
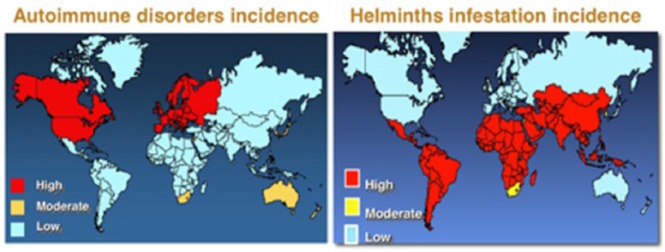
The ultimate cause of the epidemic has been linked by others to the decline of TB using evidence from BCG vaccination and inverse associations with tuberculin responses or other infections.182–185 Current maps of the incidence of infections such as helminths and TB are diametrically opposed to incidences of allergic and auto-immune diseases186 (Figure 26). Reduction in helminth infection, as with TB, is also associated with relief from poverty and poor diet with limited overall impact of antibiotics at least at a population level: as animal parasites, their interaction with host nicotinamide status and biochemistry could be either positive or negative but has not been studied.187–191
Figure 26.
Incidence of helminth infestation and tuberculosis is diametrically opposed with incidence of autoimmune disorders now. In 1850, this map would have looked more homogeneous.
A reasonable proposition as to timing of the allergy epidemic in the northwest is shown192 (Figure 27). Not only numbers but also diversity of auto-immune disease continues to radiate.193 Links with NAD metabolism through CD38 and allergen/pollen NADPH oxidases have been made; the latter links in to oxidative stress and T-cell imbalances that could cause allergic disease but only if Tregs were unbalanced in the first place, prior to exposure to the immediate extrinsic signal.194–200 High tryptophan levels and low IDO activity have also been recorded in allergic disease whether asthma or food allergies.201–203 In addition, the modern outbreak of depression appears, despite cross-cultural issues to be a feature of diseases of affluence, and may influence many of the others and can clearly be related to disturbed tryptophan and serotonin pathways.204,205 The commonest cause of infertility – polycystic ovary syndrome – is also far commoner in developed economies and has been related to the metabolic syndrome and auto-immune disease; this is relevant to the argument we make in our companion article about increasing infertility in high-meat economies.206
Figure 27.
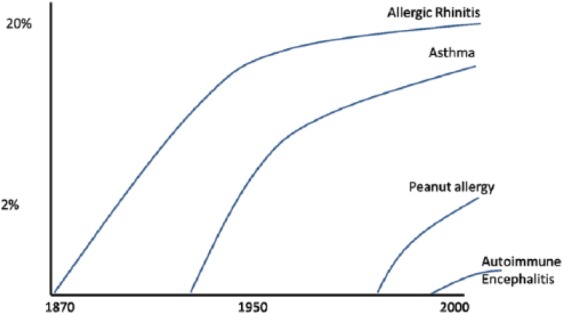
The rapid and sequential rise of allergic and auto-immune disease in the United Kingdom is shown. During this period, meat intake on average doubled again, so if accurate figures were available for the new diseases, strong correlations would be apparent. Incidence and severity may be stabilising for some such as asthma (as is meat intake).
Questions
We now ask a series of questions to further explore and try and convince readers that the dose of vitamin B3 is a significant health issue and that deficiencies and perhaps excesses are commoner than is usually supposed.
Question 1a: was pellagra conquered or is it still endemic in some poor meat areas?
The short answer is that nobody has checked systematically at a clinical or biochemical level. The few studies done in high-risk populations such as refugees in war-torn Africa suggest that it may be common but rarely diagnosed.207–211 Pellagra may be treated inadvertently in alcoholics while restoring thiamine levels with multivitamins for Wernicke encephalopathy.212–215 Indeed, it may genuinely be difficult to separate nicotinamide deficiency from deficits of other micronutrients as seen with tropical neuropathies; even when there are putative (cyanogenic or amino acid) toxins involved, nicotinamide deficiency could play a part in degenerative diseases when on poor monophagic plant diets such as those described on Guam as well as Lathyrism and Konzo.216–219 Some of these conditions including pellagra, like scurvy in the past, can get considered to be ‘badges of dishonour’ (even though captains Columbus and Cook were probably affected) or wilful self-neglect rather than due to chronic food shortages obfuscating calls to action.220
Pellagra happens to those on a very low-meat/milk and maize diet, so many in Africa and Asia must be at risk. The rash is photosensitive, so may not be present as often in those with pigmented skin – earlier epidemics were mainly in poor whites. Diarrhoea and poor mental development are endemic. Kwashiorkor, when first described, was felt by some to be a form of pellagra, but the argument was initially lost to the proponents of calorie or protein deficiency.221–224 The renamed ‘environmental enteropathy’ with gut dysbioses, cognitive impairment, and later ‘epigenetic’ metabolic syndromes may have a closer relationship with a ‘new version’ of pellagra than is generally appreciated.225,226 Pellagra may be masquerading as general ill health and shortened lives with lower than expected IQ and exacerbate both TB and human immunodeficiency virus.227–229
Question 1b: was pellagra sine pellagra conquered or is it still a common cause of poor intellectual development or premature ageing including dementia?
Before the biochemistry and treatment of pellagra were sorted out in the 1940s, it was pointed out that lack of meat in diet had profound effects on health and height, and the clinical manifestations went outside the classic pellagra phenotype.230,231 This view was criticised by vegetarian groups (including Gandhi). We now realise that there is a world of difference between an economically driven poor monophagic vegetarian diet and a voluntary vegetarian diet of good quality that often includes some animal products and supplements – so the latter has few dangers and some advantages.232
During the American and earlier European epidemics of pellagra, often in families with other members diagnosed, there were many with poor physical and mental development. Indeed, a high proportion of both blacks and ‘white trash’ in the Southern states may have been affected. The average IQ of Confederate recruits was in the ‘moron’ category and that was not true for the richer Union recruits. The correlations we now see between IQ/literacy and meat intake may represent nicotinamide deficiency. The dramatic changes in IQ known as the ‘Flynn effect’ as countries become more prosperous may reflect increased nicotinamide intake and a better brain in the first place more capable of learning from a better educational system and less prone to dementia.233–235 Indeed, there is already considerable evidence that nicotinamide could prevent or benefit Alzheimer’s disease and has a role in serious neuropsychiatric disease such as schizophrenia and post-traumatic dementia..236–248 Increased longevity could also represent improving nicotinamide dosage. This is supported by pellagra causing premature ageing and dementia and evidence that NAD levels fall with age given that NAD consumers, whether SIRTs or PARPs, are largely responsible for ageing and repair mechanisms249 (Figure 28).
Figure 28.
A summary of how poor diet can interact with the microbiome and with internal stresses such as mutations and with external stresses. These can all contribute to a single NAD endo-phenotype with multiple clinical phenotypes. NAD indicates nicotinamide adenine dinucleotide.
Question 2: is there a strict recommended dose of nicotinamide or do some people with some genetic mutations require a personalised dosage and do some others temporarily need a boost in their dosage when under stress?
The recommended daily dose of nicotinamide of around 15 mg/day was a reasonable informed ‘guesstimate’ designed to avoid or treat pellagra. However, there are examples of increased needs under some mutational circumstances, such as those causing muscular dystrophy or optic atrophy.250–252 The same may be true of DNA-repair or metabolic defects such as those causing ataxia-telangiectasia, Friedreich ataxia, defects in glutamine synthetase, and some cancers.253–256 Other diseases such as Huntington’s are known to have a disturbed ‘de novo’ pathway and an intervention with nicotinamide could work.257,258
It has been known for a long time that the dose needs to be increased in patients with the carcinoid or Hartnup syndrome who shunt tryptophan to increased serotonin synthesis or have a tryptophan transport defect.259,260 If there is genotoxic stress such as that from chemicals or drugs, sunlight, or even emotional stress, it would be expected that the extra NAD consumption would be easier to support if the dietary dosage was high. There is emerging evidence that nicotinamide dosage can be (neuro)protective under a wide range of environmental insults whether traumatic, anoxic, or toxic suggesting that prophylactic boosting of the dosage or treating soon after the insult may reduce cell damage in a number of organs, including the brain.96,104,236,261–290 Many diseases where the environmental trigger is not known may have an NAD-deficient endo-phenotype. This is suggested by the extraordinarily varied phenotype of pellagra where a wide range of dementias and neuropsychiatric conditions was described including mimics of Creutzfeldt-Jakob disease, PD, motor neuron disease, multiple sclerosis (MS), and cerebellar syndromes alongside migraine and epilepsy. Evidence for such a deficit is already strong for some, such as prion diseases, where there is phenotypic overlap with pellagra.291,292
Question 3: was the change in dosage of nicotinamide the transition factor converting diseases of poverty to diseases of affluence? Did this lead to the loss of ‘old friends’ such as TB allowing a poorly educated immune system to become overactive?
Nicotinamide adenine dinucleotide status is probably crucial to the poor resistance to pathogens seen in poor countries. Many pathogens or their toxins target the host’s NAD system by disrupting NAD consumer pathways, or by secreting NAD glycohydrolase, break down the host’s NAD levels.293 We have also argued that TB and some gut symbionts originally co-evolved to supply nicotinic acid. Such symbionts become dysbiotic and behave as pathogens when the diet is low in meat for protracted periods of time, or if driven to mutate using antibiotics. Nicotinamide was the first anti-tuberculous antibiotic discovered. The largely spontaneous disappearance of TB as countries and diets become richer becomes less of a mystery and is less likely to be related to better hygiene. There are, indeed, good examples of improving sanitation improving water-borne infection such as cholera but having little impact on other infections, until diet and particularly meat intake is addressed.294 In one famous example, improved sanitation caused an increase in mortality as the increased rents led to a decline in food quality.295 A famous other example was rebuilding barracks to no effect on TB rates until the meat intake was increased, and of course, TB becomes rampant during many famines.296
It is notable that TB sanatoria were not closed but were transformed into sanatoria for allergies that first became a problem for the rich on rich diets.15,297–303 A ‘nicotinamide switch’ towards the evolutionary norm of a high-meat diet could be responsible for the switch from TB and other chronic infections, now co-evolved to affect the development of the immune system, to a poorly educated overactive immune system responding to normally innocuous antigens.81,304–310 The biochemical nature of this switch was outlined earlier (Figure 9). The speed at which the switch is turned could be important, with the highest risk within a lifetime or a generation or two for disease of affluence – thereafter, immune adaptations and a different starting point of the microbiome may occur compatible with little evidence that hunter-gatherers had autoimmune/allergic disease (trauma leading to shorter lifespans may have spared them later onset diseases).
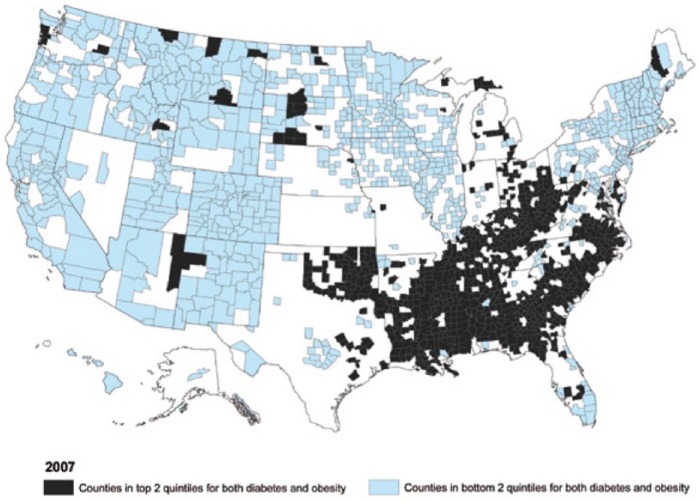
The spread and origin of the obesity epidemic is interesting regarding this as modern maps of the epidemic and obesity, diabetes, and stroke ‘belts’ are strikingly similar to maps of pellagra a century ago suggesting that the switch can manifest over several generation raising future discussion over ‘thrifty’ (and in other contexts ‘non-thrifty’) nicotinamide-related genotypes and phenotypes and developmental plasticity having effects on later disease compatible with the known involvement of nicotinamide, butyrate, and methyl groups in epigenetic modifications 311–316 (Figure 29).
Figure 29.
US map illustrates the obesity epidemic and a diabetic belt. The distribution is almost exactly the same as maps of pellagra a century ago. Has the change from very low levels of nicotinamide to high levels been the crucial inter- and intra-generational change rather than calories or allergens?
Question 4: over and above a rash of immune diseases has the dose of nicotinamide become so high that there is an unrecognised hyper-vitaminosis B3 syndrome?
Nicotinamide is widely viewed as having little serious toxicity at least in the short term.317,318 We suggested nicotinamide toxicity as a causative factor for the Parkinson’s disease, the metabolic syndrome, and some cancers based on direct or indirect measure of high levels of NNMT.53,83,319–333 Too low a dose and nicotinamide can be protective for cancers and Parkinson’s, so we are proposing a double-edged dosage effect.333 As an inducible enzyme, a logical culprit for NNMT overexpression is the dose of nicotinamide – even if other factors such as caloric restriction, stress, and exercise play their part.334,335 Background genetic variation in levels may reflect exposure in earlier generations – certainly, this is true of species as the enzyme is not expressed in herbivores.336 Our original hypothesis for PD was that N-methylnicotinamide resembled the dopaminergic toxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) and so may be toxic at high levels even though beneficial at lower levels. Part of the jigsaw may be that the nicotinamide dose upsets the microbiome that in turn contributes to the proteotoxicity and affects the nicotinic acid receptor.337 Other metabolites may be nephrotoxic.338
Several other plausible mechanisms for nicotinamide toxicity exist whether from excessive inhibition of NAD consumers such as SIRTs or PARPs or consumption of valuable methyl groups depleting the methylome and the epigenome (Figure 30). Recent evidence shows that nicotinamide promotes adipogenesis probably via SIRT inhibition and adipogenic proteins (eg, peroxisome proliferator–activated receptor gamma and FABP4 [fatty acid–binding protein 4]) and is associated with neonatal adiposity – with surprisingly little need for excessive calories or fats.339–342
Figure 30.

The presence of a detoxification pathway suggests that nicotinamide can be toxic. A balance may have been required as NAD consumers evolved to be an important control mechanism that both needs a supply of NAD from nicotinamide and whose enzyme activity is affected by nicotinamide.
Question 5: how hard would it be to moderate the dose of nicotinamide worldwide or personalise dosages?
As dietary manipulations go reducing, the dosage would be easy as supplements could be reduced in rich countries with no behavioural change necessary. Reducing meat intake may also be fairly painless, even if nicotinamide like nicotine has some addictive qualities, as it is now practised by the ‘healthy wealthy’.
Increasing the dose to those really at risk would also be relatively easy and not very expensive if talking about supplements or biofortification of crops. More meat, however, is not as easy as it is expensive but may be necessary if nicotinamide is not the only factor necessary or is actually dangerous on its own as it is a drain on methyl groups. Redistributing meat from those eating too much to those eating too little would improve the health of both the groups.
Conclusions
Hypo- (subclinical/misdiagnosed pellagra) and hyper-vitaminosis B3 (nicotinamide overload) may both be far commoner than has been supposed and have equally wide phenotypes transcending many conventional clinically convenient disease categories. The transition from populations of low to high intake may explain the extraordinary shifts within single generations from Rowntree’s ‘stunted life’ with chronic diarrhoeal infections and TB and disproportionally high death rates from pathogens – to non-communicable diseases. Non-communicable diseases include multiple and expanding allergic, inflammatory, and auto-immune diseases along with the metabolic syndrome, many cancers and neuropsychiatric disease, but in the context of longer lives. Geography per se has little to do with these phenomena as they track much more closely with poverty and affluence and therefore meat intake.343–345 Even the term ‘tropical diseases’ is a misnomer as many of these infections were common in Victorian England and the pellagra-prone southeastern American states a century ago.
Much current effort in rich countries is for personalised precision and genetic medicine usually for people who already have contracted often rare diseases. Understanding these disease transitions may be less eye-catching but offers more potential for low-tech population-based preventive approaches. Personalised NAD-related medicine can be nutritional, rather than glitzy genetic manipulation, as several genetic diseases may be due to genes that originally evolved at times of nicotinamide luxury or thrift.
Predicted tidal waves of dementia may be preventable (too little nicotinamide) as may some neuropsychiatric disease, diabetes and the metabolic syndrome, some cancers, and Parkinson’s disease (too much).346 Nicotinamide overload may not work alone but with other hyper-vitaminosis or deficiency states. One example might be high nicotinamide but low vitamin D in MS compatible with the known epidemiology and genetics implicating little sun but a lot of meat and known involvement of the ‘de novo’ pathway.347–349 It will have been partly iatrogenic if increasing the dose of nicotinamide with supplements also affects caloric hunger and obesity levels and other diseases where NNMT induction has been demonstrated.350,351
Phenotypic diversity may be explained by individual variation whether genetic or from previous environmental exposure and in differential sensitivity of cell types to NAD upsets. Homeostatic responses that fail or have a longer term price whether auto-carnivory, use of symbionts, or inflammatory or cancerous tissue that over-express NNMT or NAD consumer enzymes or induction of IDO, may also be responsible for clinical heterogeneity. New phenotypes may be occurring. Predicted Armageddon as antibiotics rapidly become ineffective from multi-drug resistance may be due to not dealing with the fundamental nutritional cause. Antibiotics are a strategy that encourages mutations and emergent pathogens. Better NAD status will reduce the need for broad-spectrum antibiotics by reducing virulence. Reliance on symbionts (including TB) also means that antibiotic use could trigger pellagra that will probably not be recognised or treated.352–356 When symbionts are relied on too heavily, ‘blooming’ in the gut can lead to dysbiosis and pathogen evolution relevant to ‘environmental enteropathy’ that may be ‘new version’ pellagra88,347,357 (Figure 31).
Figure 31.
An optimal dose of nicotinamide is suggested with trouble at the extremes. Transgenerational effects may be marked. Dysbioses that begin under these circumstances could put the affluent at risk. Within generation effects may be mismatches between early and late life exposure with poor nicotinamide in early life predisposing to the metabolic syndrome later if the dose increases. TB indicates tuberculosis; PD, Parkinson disease.
All our questions could be answered definitively. The variances in environmental exposure could be analysed in more detail epidemiologically and not just using meat intake as a surrogate. Clinical assessments sensitive to the possibility of pellagra in at-risk groups could be made. Joint clinical and biochemical assessments are the likely way forward closely followed by interventional studies.358,359 Biochemical measures have been available for a long time measuring tryptophan levels, or urinary N-methylnicotinamide excretion or NNMT levels are enough to confirm pellagra (or nicotinamide overload) but have rarely been used in the field. Metabolomics could make this more practical. Direct measurement of NAD/NADH or NAD/NADP (nicotinamide adenine dinucleotide phosphate) (known as the ‘niacin number’) ratios could be further developed. Laboratory studies could explore nicotinamide overload further as it has been assumed that NNMT or high N-methylnicotinamide levels are a marker or reaction rather than directly involved in causation.360 The long lifetime nature of this toxicity with doses only 2 to 4 times the upper recommended range would need to be recognised in the experimental design (Figure 32).
Figure 32.
Nicotinamide overdosage is unlikely to be working alone. It may act in concert with other excess dietary factors known to be involved with ageing and pathological pathways and may take many years to express toxicity.
Moderating the dose of nicotinamide to begin with would be easier than most dietary manipulations as supplementation is happening where it is least needed in rich countries.361 Supplementation should be targeted at some 2 billion who are already known to be micronutrient deficient (iron, zinc, iodine, vitamin A, and folate) including the one-fifth of children who are physically or cognitively stunted. The cost has been shown to be low relative to the benefits (16-fold) either at an individual level where many are robbed of future earnings or by population – malnutrition loses 10% of gross domestic product in many parts of Africa and Asia.362–367 Meat subsidies or vouchers or conditional cash transfer systems as part of a meat ‘entitlement’ reducing meat insecurity would make sense as nicotinamide is unlikely to be the only fortification factor involved. Adding nicotinamide to diet without methyl groups could be problematic as they would be lost as excesses of nicotinamide are excreted.
Systems need to be developed that nourish rather than feed, and sometimes this means more meat not less and should be driven by evidence not ideology.361 At first glance, there is a big environmental price to pay for more equitable meat intake. Access is as much of an issue as lack of available meat, so redistribution would mitigate many ecological effects. When combined with the demographic argument we made in the accompanying article, this may all have to be addressed objectively – unnecessary transgenerational disease or high population density is the greater ecological danger to future generations. The diet instinctively followed by the ‘healthy-wealthy’ may be a win-win diet improving health and human capital and be environmentally sustainable (Figure 33).368 In addition, the wealthy who make more effort to exercise in safe environments and virtual hunting as sport, and consequently control weight, may be overcoming not so much evolutionary tendencies to gain weight preparing for famines (thrifty genotypes and phenotypes) but to overcome a fear of exercise as it was previously linked to danger from predators (Figure 34).369–371
Figure 33.
The ‘healthy-wealthy’ have instinctively worked this out. Can lessons learnt influence policy in developing nations?
Figure 34.
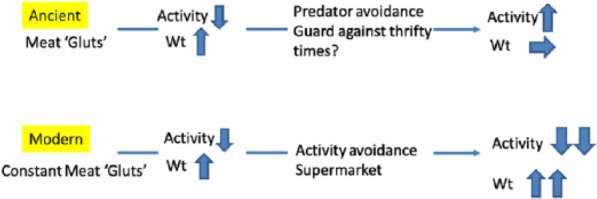
Finally ancient meat gluts lead to an adaptive strategy saving lives from periods of safety not hunting and later stabilising weight. This is now maladaptive unless there are alternative strategies to increase exercise levels. Guarding against thrift may not have been as important as starvation may not have been as common as previously suspected.
Learning from history could help developing countries avoid the trap of the Western diet with lack of exercise and meat intake ‘overshoots’. There is little sign of such wisdom currently as ‘the double burden’ in developing countries reflects a break down in the Engel law whereby poor-to-moderate wealth transitions classically increased the absolute (but not the proportional) amount of income spent on food increasing meat intake.372 This economic transition is now more likely to lead to increased ‘empty calories’ from ultra-processed foods such as sugar-sweetened nicotinamide-enhanced drinks driving the apparent paradox of diseases of poverty and the metabolic syndrome co-existing in developing countries and sometimes in the same individual.
Figure 3.
NAD is central to metabolism and the energy supply. NAD determines cell fates during development. NAD links to the epigenome and genomic expression means close interactions with our environment that in turn supplies NAD.
Acknowledgments
A.C.W. would like to thank Mike Hammond and the Richardson family for their support.
Footnotes
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totalled 288 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by QEHB Charities.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
ACW and LJH analysed the data, agree with manuscript results and conclusions, made critical revisions and approved final version. ACW wrote the first draft of the manuscript. LJH contributed to the writing of the manuscript. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28:S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- 3.Popkin BM, Slining M. New dynamics in global obesity facing low- and middle-income countries. Obes Rev. 2013;14:11–20. doi: 10.1111/obr.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omran AR. The epidemiologic transition theory. A preliminary update. J Trop Pediatr. 1983;29:305–316. doi: 10.1093/tropej/29.6.305. [DOI] [PubMed] [Google Scholar]
- 5.Lindeberg S. Food and Western Disease: Health and Nutrition from an Evolutionary Perspective. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 6.Williams A, Ramsden D. Hydrogen symbioses in evolution and disease. QJM. 2007;100:451–459. doi: 10.1093/qjmed/hcm045. [DOI] [PubMed] [Google Scholar]
- 7.Wallace DC. Mitochondrial DNA variation in human radiation and disease. Cell. 2015;163:33–38. doi: 10.1016/j.cell.2015.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger SL, Sassone-Corsi P. Metabolic signaling to chromatin. Cold Spring Harb Perspect Biol. 2016;8:a019463. doi: 10.1101/cshperspect.a019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mylenko M, Boland S, Penkov S, et al. NAD+ is a food component that promotes exit from dauer diapause in Caenorhabditis elegans. PLoS ONE. 2016;11:e0167208. doi: 10.1371/journal.pone.0167208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 11.Perlmutter D, Loberg K. Grain Brain: The Surprising Truth about Wheat, Carbs, and Sugar – Your Brain’s Silent Killers. Boston, MA: Little, Brown and Company; 2013. [Google Scholar]
- 12.Ungar PS, Teaford MF. Human Diet: Its Origin and Evolution. Westport, CT: Greenwood Publishing Group; 2002. [Google Scholar]
- 13.Larsen CS. Animal source foods and human health during evolution. J Nutr. 2003;133:3893S–3897S. doi: 10.1093/jn/133.11.3893S. [DOI] [PubMed] [Google Scholar]
- 14.Eaton SB, Konner M, Shostak M. Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective. Am J Med. 1988;84:739–749. doi: 10.1016/0002-9343(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 15.Trowell HC, Burkitt DP. Western Diseases, Their Emergence and Prevention. London, England: Edward Arnold; 1981. [Google Scholar]
- 16.Pollard TM. Western Diseases: An Evolutionary Perspective. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 17.Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 18.Trevathan W, Smith EO, McKenna JJ. Evolutionary Medicine. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- 19.Stearns SC. Evolution in Health and Disease. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- 20.Nesse RM. Evolution and Healing: The New Science of Darwinian Medicine. Darlington, UK: J. M. Dent; 1996. [Google Scholar]
- 21.De Vany A. The New Evolution Diet: What Our Paleolithic Ancestors Can Teach Us about Weight Loss, Fitness, and Aging. Emmaus, PA: Rodale Books; 2011. [Google Scholar]
- 22.Clark JL, Speth JD. Zooarchaeology and Modern Human Origins: Human Hunting Behavior During the Later Pleistocene. Dordrecht, Netherlands: Springer; 2013. [Google Scholar]
- 23.Mercer A. Infections, Chronic Disease, and the Epidemiological Transition: A New Perspective. Rochester, NY: University of Rochester Press; 2014. [Google Scholar]
- 24.Evenson RE, Gollin D. Assessing the impact of the green revolution 1960 to 2000. Science. 2003;300:758–762. doi: 10.1126/science.1078710. [DOI] [PubMed] [Google Scholar]
- 25.McDowell LR. Vitamins in Animal and Human Nutrition. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 26.Combs GF, McClung JP. The Vitamins: Fundamental Aspects in Nutrition and Health. Amsterdam, The Netherlands: Elsevier Science; 2016. [Google Scholar]
- 27.Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. 2016;19:55–61. doi: 10.1097/MCO.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 28.Poesen R, Mutsaers HA, Windey K, et al. The influence of dietary protein intake on mammalian tryptophan and phenolic metabolites. PLoS ONE. 2015;10:e0140820. doi: 10.1371/journal.pone.0140820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan Z, Yang Y, Wen Y, et al. Metabolomic analysis of amino acid and fat metabolism in rats with l-tryptophan supplementation. Amino Acids. 2014;46:2681–2691. doi: 10.1007/s00726-014-1823-y. [DOI] [PubMed] [Google Scholar]
- 30.Kumar JS, Subramanian VS, Kapadia R, Kashyap ML, Said HM. Mammalian colonocytes possess a carrier-mediated mechanism for uptake of vitamin B3 (niacin): studies utilizing human and mouse colonic preparations. Am J Physiol Gastrointest Liver Physiol. 2013;305:G207–G213. doi: 10.1152/ajpgi.00148.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:148. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNamara JM, Houston AI, Higginson AD. Costs of foraging predispose animals to obesity-related mortality when food is constantly abundant. PLoS ONE. 2015;1811;10:e014. doi: 10.1371/journal.pone.0141811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiza H, Bente L. Nutrient Content of the US Food Supply, 1909–2004. A Summary Report. Washington, DC: United States Department of Agriculture; 2007. [Google Scholar]
- 34.Pritchard B, Ortiz R, Shekar M. Routledge Handbook of Food and Nutrition Security. Abingdon, UK: Taylor & Francis; 2016. [Google Scholar]
- 35.Pimentel D, Marcia H, Pimentel MS. Food, Energy, and Society. 3rd ed. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 36.Muldrew C. Food, Energy and the Creation of Industriousness: Work and Material Culture in Agrarian England, 1550–1780. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- 37.Harris HF. Pellagra. Los Angeles, USA: Hardpress, BiblioBazaar; 2016. [Google Scholar]
- 38.van den Broek TJ, Kremer BH, Rezende MM, et al. The impact of micronutrient status on health: correlation network analysis to understand the role of micronutrients in metabolic-inflammatory processes regulating homeostasis and phenotypic flexibility. Genes Nutr. 2017;12:5. doi: 10.1186/s12263-017-0553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 40.Williams AC, Ramsden DB. Pellagra: a clue as to why energy failure causes diseases? Med Hypotheses. 2007;69:618–628. doi: 10.1016/j.mehy.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Williams AC, Dunbar RI. Big brains, meat, tuberculosis, and the nicotinamide switches: co-evolutionary relationships with modern repercussions? Int J Tryptophan Res. 2013;6:73. doi: 10.4137/IJTR.S12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams AC, Dunbar RIM. Big brains, meat, tuberculosis and the nicotinamide switches: co-evolutionary relationships with modern repercussions on longevity and disease? Med Hypotheses. 2014;83:79–87. doi: 10.1016/j.mehy.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Bach JF, Chatenoud L. The hygiene hypothesis: an explanation for the increased frequency of insulin-dependent diabetes. Cold Spring Harb Perspect Med. 2012;2:a007799. doi: 10.1101/cshperspect.a007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rook G. The Hygiene Hypothesis and Darwinian Medicine. Basel, Switzerland: Birkhäuser; 2009. [Google Scholar]
- 45.Webley WC, Aldridge KL. Infectious asthma triggers: time to revise the hygiene hypothesis? Trends Microbiol. 2015;23:389–391. doi: 10.1016/j.tim.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Feehley T, Stefka AT, Cao S, Nagler CR. Microbial regulation of allergic responses to food. Semin Immunopathol. 2012;34:671–688. doi: 10.1007/s00281-012-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andoh A. Physiological role of gut microbiota for maintaining human health. Digestion. 2016;93:176–181. doi: 10.1159/000444066. [DOI] [PubMed] [Google Scholar]
- 48.Montserrat-de la Paz S, Naranjo MC, Lopez S, Abia R, Muriana FJ, Bermudez B. Niacin and its metabolites as master regulators of macrophage activation. J Nutr Biochem. 2016;39:40–47. doi: 10.1016/j.jnutbio.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Sauve AA. NAD+ metabolism: bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016;1864:1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petriacq P, Ton J, Patrit O, Tcherkez G, Gakiere B. NAD acts as an integral regulator of multiple defense layers. Plant Physiol. 2016;172:1465–1479. doi: 10.1104/pp.16.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guarente L. CELL METABOLISM. The resurgence of NAD(+) Science. 2016;352:1396–1397. doi: 10.1126/science.aag1718. [DOI] [PubMed] [Google Scholar]
- 52.Preyat N, Leo O. Reassessing the role of NAD as a prosurvival factor. Mol Cell Oncol. 2016;3:e1062591. doi: 10.1080/23723556.2015.1062591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trammell SA, Schmidt MS, Weidemann BJ, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kennedy BE, Sharif T, Martell E, et al. NAD+ salvage pathway in cancer metabolism and therapy. Pharmacol Res. 2016 doi: 10.1016/j.phrs.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 55.Majewski M, Kozlowska A, Thoene M, Lepiarczyk E, Grzegorzewski W. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J Physiol Pharmacol. 2016;67:3–19. [PubMed] [Google Scholar]
- 56.Min SW, Sohn PD, Cho SH, Swanson RA, Gan L. Sirtuins in neurodegenerative diseases: an update on potential mechanisms. Front Aging Neurosci. 2013;5:53. doi: 10.3389/fnagi.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu L, Doreswamy V, Prakash R. The biochemical pathways of central nervous system neural degeneration in niacin deficiency. Neural Regen Res. 2014;9:1509. doi: 10.4103/1673-5374.139475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wyness L. The role of red meat in the diet: nutrition and health benefits. Proc Nutr Soc. 2016;75:227–232. doi: 10.1017/S0029665115004267. [DOI] [PubMed] [Google Scholar]
- 59.Pétriacq P, de Bont L, Tcherkez G, Gakière B. NAD: not just a pawn on the board of plant-pathogen interactions. Plant Signal Behav. 2013;8:e22477. doi: 10.4161/psb.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 61.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 63.Stehr M, Elamin AA, Singh M. Pyrazinamide: the importance of uncovering the mechanisms of action in mycobacteria. Expert Rev Anti Infect Ther. 2015;13:593–603. doi: 10.1586/14787210.2015.1021784. [DOI] [PubMed] [Google Scholar]
- 64.Seiner DR, Hegde SS, Blanchard JS. Kinetics and inhibition of nicotinamidase from Mycobacterium tuberculosis. Biochemistry. 2010;49:9613–9619. doi: 10.1021/bi1011157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aguilar-Ayala DA, Palomino JC, Vandamme P, Martin A, Gonzalez YMJA. Genetic regulation of Mycobacterium tuberculosis in a lipid-rich environment [published online ahead of print October 19, 2016] Infect Genet Evol. doi: 10.1016/j.meegid.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 66.Prunier A-L, Schuch R, Fernández RE, Maurelli AT. Genetic structure of the nadA and nadB antivirulence loci in Shigella spp. J Bacteriol. 2007;189:6482–6486. doi: 10.1128/JB.00525-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cotter PA, DiRita VJ. Bacterial virulence gene regulation: an evolutionary perspective. Annu Rev Microbiol. 2000;54:519–565. doi: 10.1146/annurev.micro.54.1.519. [DOI] [PubMed] [Google Scholar]
- 68.Merdanovic M, Sauer E, Reidl J. Coupling of NAD+ biosynthesis and nicotinamide ribosyl transport: characterization of NadR ribonucleotide kinase mutants of Haemophilus influenzae. J Bacteriol. 2005;187:4410–4420. doi: 10.1128/JB.187.13.4410-4420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leonardo MR, Dailly Y, Clark DP. Role of NAD in regulating the adhE gene of Escherichia coli. J Bacteriol. 1996;178:6013–6018. doi: 10.1128/jb.178.20.6013-6018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edwards RL, Bryan A, Jules M, Harada K, Buchrieser C, Swanson MS. Nicotinic acid modulates Legionella pneumophila gene expression and induces virulence traits. Infect Immun. 2013;81:945–955. doi: 10.1128/IAI.00999-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 72.Mahmoud ME, Fereig R, Nishikawa Y. Involvement of host defense mechanisms against Toxoplasma gondii infection in anhedonic and despair-like behaviors in mice [published online ahead of print January 30, 2017] Infect Immun. doi: 10.1128/IAI.00007-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mesquita I, Varela P, Belinha A, et al. Exploring NAD+ metabolism in host-pathogen interactions. Cell Mol Life Sci. 2016;73:1225–1236. doi: 10.1007/s00018-015-2119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Hara JK, Kerwin LJ, Cobbold SA, et al. Targeting NAD+ metabolism in the human malaria parasite Plasmodium falciparum. PLoS ONE. 2014;9:e94061. doi: 10.1371/journal.pone.0094061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Monogr Ser World Health Organ. 1968;57:3–329. [PubMed] [Google Scholar]
- 76.Oka S, Hsu C, Sadoshima J. Regulation of cell survival and death by pyridine nucleotides. Circ Res. 2012;111:611–627. doi: 10.1161/CIRCRESAHA.111.247932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corradi J, Bouzat C. Understanding the bases of function and modulation of α7 nicotinic receptors: implications for drug discovery. Mol Pharmacol. 2016;90:288–99. doi: 10.1124/mol.116.104240. [DOI] [PubMed] [Google Scholar]
- 78.Hubert S, Rissiek B, Klages K, et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med. 2010;207:2561–2568. doi: 10.1084/jem.20091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hunt NH, Too LK, Khaw LT, et al. The kynurenine pathway and parasitic infections that affect CNS function. Neuropharmacology. 2016;112:389–398. doi: 10.1016/j.neuropharm.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 80.Braidy N, Poljak A, Grant R, et al. Mapping NAD+ metabolism in the brain of ageing Wistar rats: potential targets for influencing brain senescence. Biogerontology. 2014;15:177–198. doi: 10.1007/s10522-013-9489-5. [DOI] [PubMed] [Google Scholar]
- 81.Breda C, Sathyasaikumar KV, Sograte Idrissi S, et al. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci U S A. 2016;113:5435–5440. doi: 10.1073/pnas.1604453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 83.Liu M, Li L, Chu J, et al. Serum N(1)-methylnicotinamide is associated with obesity and diabetes in Chinese. J Clin Endocrinol Metab. 2015;100:3112–3117. doi: 10.1210/jc.2015-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lavado-Roldán A, Fernández-Chacón R. Two for the price of one: a neuroprotective chaperone kit within NAD synthase protein NMNAT2. PLoS Biol. 2016;14:e1002522. doi: 10.1371/journal.pbio.1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mouchiroud L, Houtkooper RH, Moullan N, et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. ADP-ribosylation of P2X7: a matter of life and death for regulatory T cells and natural killer T cells. Curr Top Microbiol Immunol. 2015;384:107–126. doi: 10.1007/82_2014_420. [DOI] [PubMed] [Google Scholar]
- 88.Wang P, Li W-L, Liu J-M, Miao CY. NAMPT and NAMPT-controlled NAD metabolism in vascular repair. J Cardiovasc Pharmacol. 2016;67:474–481. doi: 10.1097/FJC.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 89.Wang SN, Xu TY, Li WL, Miao CY. Targeting nicotinamide phosphoribosyltransferase as a potential therapeutic strategy to restore adult neurogenesis. CNS Neurosci Ther. 2016;22:431–439. doi: 10.1111/cns.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and ‘western-lifestyle’ inflammatory diseases. Immunity. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 91.Jobin C. GPR109a: the missing link between microbiome and good health? Immunity. 2014;40:8–10. doi: 10.1016/j.immuni.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Son MJ, Kwon Y, Son T, Cho YS. Restoration of mitochondrial NAD+ levels delays stem cell senescence and facilitates reprogramming of aged somatic cells. Stem Cells. 2016;34:2840–2851. doi: 10.1002/stem.2460. [DOI] [PubMed] [Google Scholar]
- 93.Wu LE, Sinclair DA. Restoring stem cells – all you need is NAD+ Cell Res. 2016;26:971–972. doi: 10.1038/cr.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin TC, Voorhees JR, Genova RM, et al. Acute axonal degeneration drives development of cognitive, motor, and visual deficits after blast-mediated traumatic brain injury in mice. eNeuro. 2016;3 doi: 10.1523/ENEURO.0220-16.2016. ENEURO.0220-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen A, Martin A, Dalziell R, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol. 2016;175:1073–1075. doi: 10.1111/bjd.14662. [DOI] [PubMed] [Google Scholar]
- 97.Ratajczak J, Joffraud M, Trammell SA, et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun. 2016;7:13103. doi: 10.1038/ncomms13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ming G-F, Wu K, Hu K, Chen Y, Xiao J. NAMPT regulates senescence, proliferation, and migration of endothelial progenitor cells through the SIRT1 AS lncRNA/miR-22/SIRT1 pathway. Biochem Biophys Res Commun. 2016;478:1382–1388. doi: 10.1016/j.bbrc.2016.08.133. [DOI] [PubMed] [Google Scholar]
- 99.Musiek ES, Xiong DD, Patel T, et al. Nmnat1 protects neuronal function without altering phospho-tau pathology in a mouse model of tauopathy. Ann Clin Transl Neurol. 2016;3:434–442. doi: 10.1002/acn3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Srivastava S. Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders. Clin Transl Med. 2016;5:25. doi: 10.1186/s40169-016-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin JB, Kubota S, Ban N, et al. NAMPT-mediated NAD+ biosynthesis is essential for vision in mice. Cell Rep. 2016;17:69–85. doi: 10.1016/j.celrep.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pétriacq P, Ton J, Patrit O, Gakiere B. NAD acts as an integral regulator of multiple defense layers. Plant Physiol. 2016;172:1465–1479. doi: 10.1104/pp.16.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schultz MB, Sinclair DA. Why NAD(+) declines during aging: it’s destroyed. Cell Metab. 2016;23:965–966. doi: 10.1016/j.cmet.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiao Y, Kwong M, Daemen A, et al. Metabolic response to NAD depletion across cell lines is highly variable. PLoS ONE. 2016;11:e0164166. doi: 10.1371/journal.pone.0164166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Waaler HT. Height, weight and mortality. The Norwegian experience. Acta Med Scand Suppl. 1984;679:1–56. doi: 10.1111/j.0954-6820.1984.tb12901.x. [DOI] [PubMed] [Google Scholar]
- 106.Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeraldo P, Hernandez A, Nielsen HB, et al. Capturing one of the human gut microbiome’s most wanted: reconstructing the genome of a novel butyrate-producing, clostridial scavenger from metagenomic sequence data. Front Microbiol. 2016;7:783. doi: 10.3389/fmicb.2016.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung TH, Jeon WM, Han KS. In vitro effects of dietary inulin on human fecal microbiota and butyrate production. J Microbiol Biotechnol. 2015;25:1555–1558. doi: 10.4014/jmb.1505.05078. [DOI] [PubMed] [Google Scholar]
- 109.Ringel-Kulka T, Choi CH, Temas D, et al. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol. 2015;110:1339–1346. doi: 10.1038/ajg.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alvarez-Curto E, Milligan G. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem Pharmacol. 2016;114:3–13. doi: 10.1016/j.bcp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 112.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 116.Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138:666–675. doi: 10.1016/j.jaci.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiotagut-brain axis? Neurochem Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 118.Tao J-H, Cheng M, Tang J-P, Liu Q, Pan F, Li XP. Foxp3, regulatory T cell, and autoimmune diseases. Inflammation. 2016;40:328–339. doi: 10.1007/s10753-016-0470-8. [DOI] [PubMed] [Google Scholar]
- 119.Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Segata N. Gut microbiome: westernization and the disappearance of intestinal diversity. Curr Biol. 2015;25:R611–R613. doi: 10.1016/j.cub.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 121.Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schnorr SL. The diverse microbiome of the hunter-gatherer. Nature. 2015;518:S14–S15. doi: 10.1038/518S14a. [DOI] [PubMed] [Google Scholar]
- 123.Ishii N, Nishihara Y. Pellagra encephalopathy among tuberculous patients: its relation to isoniazid therapy. J Neurol Neurosurg Psychiatry. 1985;48:628–634. doi: 10.1136/jnnp.48.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gupta Y, Shah I. Ethionamide-induced Pellagra. J Trop Pediatr. 2015;61:301–303. doi: 10.1093/tropej/fmv021. [DOI] [PubMed] [Google Scholar]
- 125.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 126.Zelante T, Iannitti RG, Fallarino F, et al. Tryptophan feeding of the IDO1-AhR axis in host-microbial symbiosis. Front Immunol. 2014;5:640. doi: 10.3389/fimmu.2014.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Metz R, Rust S, Duhadaway JB, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McGaha TL, Huang L, Lemos H, et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev. 2012;249:135–157. doi: 10.1111/j.1600-065X.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elkhal A, Rodriguez Cetina Biefer H, Heinbokel T, et al. NAD(+) regulates Treg cell fate and promotes allograft survival via a systemic IL-10 production that is CD4(+) CD25(+) Foxp3(+) T cells independent. Sci Rep. 2016;6:22325. doi: 10.1038/srep22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bedoya SK, Lam B, Lau K, Larkin J., 3rd Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013;2013:986789. doi: 10.1155/2013/986789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lippens C, Duraes FV, Dubrot J, et al. IDO-orchestrated crosstalk between pDCs and Tregs inhibits autoimmunity. J Autoimmun. 2016;75:39–49. doi: 10.1016/j.jaut.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takenaka MC, Quintana FJ. Tolerogenic dendritic cells. Seminars in Immunopathology. 2017;39(2):113–120. doi: 10.1007/s00281-016-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li R, Li H, Sun Q, Liu L, Zhang C, Ren X. Indoleamine 2,3-dioxygenase regulates T cell activity through Vav1/Rac pathway. Mol Immunol. 2017;81:102–107. doi: 10.1016/j.molimm.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 134.de Araújo EF, Medeiros DH, de Lima Galdino NA, Condino-Neto A, Calich VLG, Loures FV. Tolerogenic plasmacytoid dendritic cells control Paracoccidioides brasiliensis infection by inducting regulatory T cells in an IDO-dependent manner. PLoS Pathogen. 2016;12:e1006115. doi: 10.1371/journal.ppat.1006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lovelace MD, Varney B, Sundaram G, et al. Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Front Immunol. 2016;7:246. doi: 10.3389/fimmu.2016.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Byakwaga H, Boum Y, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;210:383–391. doi: 10.1093/infdis/jiu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park G, Choi Y-J, Lee S-E, et al. A paradoxical pattern of indoleamine 2,3-di-oxygenase expression in the colon tissues of patients with acute graft-versus-host disease. Exp Hematol. 2014;42:734–740. doi: 10.1016/j.exphem.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 139.Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alroqi FJ, Chatila TA. T regulatory cell biology in health and disease. Curr Allergy Asthma Rep. 2016;16:1–8. doi: 10.1007/s11882-016-0606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25:708. doi: 10.1097/MOP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AK. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr Med Chem. 2011;18:2257–2262. doi: 10.2174/092986711795656072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ciorba MA, Bettonville EE, McDonald KG, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol. 2010;184:3907–3916. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu H, Oriss TB, Fei M, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci U S A. 2008;105:6690–6695. doi: 10.1073/pnas.0708809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mazzarella G. Effector and suppressor T cells in celiac disease. World J Gastroenterol. 2015;21:7349. doi: 10.3748/wjg.v21.i24.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hardy MY, Tye-Din JA. Coeliac disease: a unique model for investigating broken tolerance in autoimmunity. Clin Transl Immunology. 2016;5:e112. doi: 10.1038/cti.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Christophersen A, Risnes LF, Bergseng E, Lundin KE, Sollid LM, Qiao SW. Healthy HLA-DQ2. 5+ subjects lack regulatory and memory T cells specific for immunodominant gluten epitopes of celiac disease. J Immunol. 2016;196:2819–2826. doi: 10.4049/jimmunol.1501152. [DOI] [PubMed] [Google Scholar]
- 148.Hmida NB, Ahmed MB, Moussa A, et al. Impaired control of effector T cells by regulatory T cells: a clue to loss of oral tolerance and autoimmunity in celiac disease? Am J Gastroenterol. 2012;107:604–611. doi: 10.1038/ajg.2011.397. [DOI] [PubMed] [Google Scholar]
- 149.Marshall EA, Ng KW, Kung SH, et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol Cancer. 2016;15:67. doi: 10.1186/s12943-016-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salazar F, Awuah D, Negm OH, Shakib F, Ghaemmaghami AM. The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs. Sci Rep. 2017;7:43337. doi: 10.1038/srep43337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Braidy N, Grant R. Kynurenine pathway metabolism and neuroinflammatory disease. Neural Regen Res. 2017;12:39. doi: 10.4103/1673-5374.198971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smil V. Eating meat: evolution, patterns, and consequences. Popul Dev Rev. 2002;28:599–639. [Google Scholar]
- 153.Millstone E, Lang T. The Atlas of Food Who Eats What, Where, and Why. London, England: Earthscan; 2008. [Google Scholar]
- 154.Greenwood M. Some British pioneers of social medicine. Br J Soc Med. 1948;2:74. [Google Scholar]
- 155.Lynn R, Meisenberg G, Mikk J, Williams A. National IQS predict differences in scholoastic achievement in 67 countries. J Biosoc Sci. 2007;39:861–874. doi: 10.1017/S0021932007001964. [DOI] [PubMed] [Google Scholar]
- 156.McKeown T. The Origins of Human Disease. Hoboken, NJ: Wiley; 1991. [Google Scholar]
- 157.Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 158.Duvoisin RC, Schweitzer MD. Paralysis agitans mortality in England and Wales, 1855–1962. Br J Prev Soc Med. 1966;20:27–33. [Google Scholar]
- 159.Logan WPD. Mortality in England and Wales from 1848 to 1947: a survey of the changing causes of death during the past hundred years. Popul Stud. 1950;4:132–178. [Google Scholar]
- 160.Charlton J, Murphy M. The Health of Adult Britain 1841–1994. London, England: Stationery Office; 1997. [Google Scholar]
- 161.Lang T, Rayner G. Ecological public health: the 21st century’s big idea? BMJ. 2012;345:17–20. doi: 10.1136/bmj.e5466. [DOI] [PubMed] [Google Scholar]
- 162.O’Donovan D. The Atlas of Health: Mapping the Challenges and Causes of Disease. London, England: Earthscan; 2008. [Google Scholar]
- 163.Cole TJ. Galton’s midparent height revisited. Ann Hum Biol. 2000;27:401–405. doi: 10.1080/03014460050044874. [DOI] [PubMed] [Google Scholar]
- 164.Johnston W. The Modern Epidemic: A History of Tuberculosis in Japan Council on East Asian Studies. Cambridge, MA: Harvard University Press; 1995. [Google Scholar]
- 165.Lumey LH, Van Poppel FW. The Dutch famine of 1944–45: mortality and morbidity in past and present generations. Soc Hist Med. 1994;7:229–246. doi: 10.1093/shm/7.2.229. [DOI] [PubMed] [Google Scholar]
- 166.Keys A, Brožek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation. Vol. 2. USA: University of Minnesota Press; 1950. [Google Scholar]
- 167.Bostock J. Case of a periodical affection of the eyes and chest. Med Chir Trans. 1819;10:161–165. [PMC free article] [PubMed] [Google Scholar]
- 168.Blackley CH. Experimental Researches on the Causes and Nature of Catarrhus Aestivus (Hay-Fever or Hay-Asthma) London, Englan: Baillière, Tindall & Cox; 1873. [Google Scholar]
- 169.Beard GM. Hay-Fever: Or, Summer Catarrh: Its Nature and Treatment. New York, NY: Harper; 1876. [Google Scholar]
- 170.Parnes O. ‘Trouble from within’: allergy, autoimmunity, and pathology in the first half of the twentieth century. Stud Hist Philos Sci. 2003;34:425–454. [Google Scholar]
- 171.Jogi R, Janson C, Bjornsson E, Boman G, Bjorksten B. Atopy and allergic disorders among adults in Tartu, Estonia compared with Uppsala, Sweden. Clin Exp Allergy. 1998;28:1072–1080. doi: 10.1046/j.1365-2222.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 172.Bergmann KC, Heinrich J, Niemann H. Current status of allergy prevalence in Germany: position paper of the Environmental Medicine Commission of the Robert Koch Institute. Allergo J Int. 2016;25:6–10. doi: 10.1007/s40629-016-0092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Krämer U, Schmitz R, Ring J, Behrendt H. What can reunification of East and West Germany tell us about the cause of the allergy epidemic? Clin Exp Allergy. 2015;45:94–107. doi: 10.1111/cea.12458. [DOI] [PubMed] [Google Scholar]
- 174.Perzanowski MS, Ng’ang’a LW, Carter MC, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. 2002;140:582–588. doi: 10.1067/mpd.2002.122937. [DOI] [PubMed] [Google Scholar]
- 175.Scrivener S, Yemaneberhan H, Zebenigus M, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493–1499. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 176.Stevens W, Addo-Yobo E, Roper J, et al. Differences in both prevalence and titre of specific immunoglobulin E among children with asthma in affluent and poor communities within a large town in Ghana. Clin Exp Allergy. 2011;41:1587–1594. doi: 10.1111/j.1365-2222.2011.03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Endara P, Vaca M, Platts-Mills T, et al. Effect of urban vs rural residence on the association between atopy and wheeze in Latin America: findings from a case-control analysis. Clin Exp Allergy. 2015;45:438–447. doi: 10.1111/cea.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Adami AJ, Bracken SJ. Focus: microbiome: breathing better through bugs: asthma and the microbiome. Yale J Biol Med. 2016;89:309. [PMC free article] [PubMed] [Google Scholar]
- 179.Sampson HA. Food allergy: past, present and future. Allergol Int. 2016;65:363–369. doi: 10.1016/j.alit.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 180.Apostolovic D, Tran TAT, Starkhammar M, Sánchez-Vidaurre S, Hamsten C, Van Hage M. The red meat allergy syndrome in Sweden. Allergo J Int. 2016;25:49–54. doi: 10.1007/s40629-016-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 182.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 183.Linehan MF, Nurmatov U, Frank TL, Niven RM, Baxter DN, Sheikh A. Does BCG vaccination protect against childhood asthma? Final results from the Manchester Community Asthma Study retrospective cohort study and updated systematic review and meta-analysis. J Allergy Clin Immunol. 2014;133:688.e14–695.e14. doi: 10.1016/j.jaci.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 184.Briggs N, Weatherhead J, Sastry KJ, Hotez PJ. The hygiene hypothesis and its inconvenient truths about helminth infections. PLoS Negl Trop Dis. 2016;10:e0004944. doi: 10.1371/journal.pntd.0004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Parker W, Ollerton J. Evolutionary biology and anthropology suggest biome reconstitution as a necessary approach toward dealing with immune disorders. Evol Med Public Health. 2013;2013:89–103. doi: 10.1093/emph/eot008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Santiago HC, Nutman TB. Human helminths and allergic disease: the hygiene hypothesis and beyond. Am J Trop Med Hyg. 2016;95:746–753. doi: 10.4269/ajtmh.16-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Humphreys M. How four once common diseases were eliminated from the American South. Health Aff. 2009;28:1734–1744. doi: 10.1377/hlthaff.28.6.1734. [DOI] [PubMed] [Google Scholar]
- 188.Humphries D, Simms BT, Davey D, et al. Hookworm infection among school age children in Kintampo North Municipality, Ghana: nutritional risk factors and response to albendazole treatment. Am J Trop Med Hyg. 2013;89:540–548. doi: 10.4269/ajtmh.12-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Campbell SJ, Nery SV, Doi SA, et al. Complexities and perplexities: a critical appraisal of the evidence for soil-transmitted helminth infection-related morbidity. PLoS Negl Trop Dis. 2016;10:e0004566. doi: 10.1371/journal.pntd.0004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu W, Ross AG, Olveda RM, et al. Risk of human helminthiases: geospatial distribution and targeted control. Int J Infect Dis. 2016;55:131–138. doi: 10.1016/j.ijid.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 191.Papier K, Williams GM, Luceres-Catubig R, et al. Childhood malnutrition and parasitic helminth interactions. Clin Infect Dis. 2014;59:234–243. doi: 10.1093/cid/ciu211. [DOI] [PubMed] [Google Scholar]
- 192.Platts-Mills TA. The allergy epidemics: 1870–2010. J Allergy Clin Immunol. 2015;136:3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koplin JJ, Mills EC, Allen KJ. Epidemiology of food allergy and food-induced anaphylaxis: is there really a Western world epidemic? Curr Opin Allergy Clin Immunol. 2015;15:409–416. doi: 10.1097/ACI.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 194.Deshpande DA, Guedes AG, Lund FE, Subramanian S, Walseth TF, Kannan MS. CD38 in the pathogenesis of allergic airway disease: potential therapeutic targets. Pharmacol Ther. 2017;172:116–126. doi: 10.1016/j.pharmthera.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hosoki K, Boldogh I, Sur S. Innate responses to pollen allergens. Curr Opin Allergy Clin Immunol. 2015;15:79–88. doi: 10.1097/ACI.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Casaca V, Illi S, Klucker E, et al. STAT6 polymorphisms are associated with neonatal regulatory T cells and cytokines and atopic diseases at 3 years. Allergy. 2013;68:1249–1258. doi: 10.1111/all.12220. [DOI] [PubMed] [Google Scholar]
- 197.Schröder PC, Casaca VI, Illi S, et al. IL-33 polymorphisms are associated with increased risk of hay fever and reduced regulatory T cells in a birth cohort. Pediatr Allergy Immunol. 2016;27:687–695. doi: 10.1111/pai.12597. [DOI] [PubMed] [Google Scholar]
- 198.Blankenhaus B, Klemm U, Eschbach M-L, et al. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J Immunol. 2011;186:4295–4305. doi: 10.4049/jimmunol.1001920. [DOI] [PubMed] [Google Scholar]
- 199.Schiering C, Wincent E, Metidji A, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hirsch CS, Rojas R, Wu M, Toossi Z. Mycobacterium tuberculosis induces expansion of Foxp3+ positive CD4 T-cells with a regulatory profile in tuberculin non-sensitized healthy subjects: implications for effective immunization against TB. J Clin Cell Immunol. 2016;7:428. doi: 10.4172/2155-9899.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Robberecht H, De Bruyne T, Hermans N. Effect of various diets on biomarkers of the metabolic syndrome [published online ahead of print December 28, 2016] Int J Food Sci Nutr. doi: 10.1080/09637486.2016.1269726. [DOI] [PubMed] [Google Scholar]
- 202.Gostner JM, Becker K, Kofler H, Strasser B, Fuchs D. Tryptophan metabolism in allergic disorders. Int Arch Allergy Immunol. 2016;169:203–215. doi: 10.1159/000445500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Buyuktiryaki B, Sahiner U, Girgin G, et al. Low indoleamine 2,3-dioxygenase activity in persistent food allergy in children. Allergy. 2016;71:258–266. doi: 10.1111/all.12785. [DOI] [PubMed] [Google Scholar]
- 204.Klerman GL, Weissman MM. Increasing rates of depression. JAMA. 1989;261:2229–2235. [PubMed] [Google Scholar]
- 205.Depression S. The new cross-cultural psychiatry. Soc Sci Med. 1977;11:3–9. doi: 10.1016/0037-7856(77)90138-x. [DOI] [PubMed] [Google Scholar]
- 206.Balen A. Pathogenesis of polycystic ovary syndrome – the enigma unravels? Lancet. 1999;354:966–967. doi: 10.1016/S0140-6736(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 207.Frank GP, Voorend DM, Chamdula A, van Oosterhout JJ, Koop K. Pellagra: a non-communicable disease of poverty. Trop Doct. 2012;42:182–184. doi: 10.1258/td.2012.120155. [DOI] [PubMed] [Google Scholar]
- 208.Seal AJ, Creeke PI, Dibari F, et al. Low and deficient niacin status and pellagra are endemic in postwar Angola. Am J Clin Nutr. 2007;85:218–224. doi: 10.1093/ajcn/85.1.218. [DOI] [PubMed] [Google Scholar]
- 209.van den Briel T, Cheung E, Zewari J, Khan R. Fortifying food in the field to boost nutrition: case studies from Afghanistan, Angola and Zambia. Food Nutr Bull. 2007;28:353–364. doi: 10.1177/156482650702800312. [DOI] [PubMed] [Google Scholar]
- 210.Baquet S, Wuillaume F, Van Egmond K, Ibanez F. Pellagra outbreak in Kuito, Angola. Lancet. 2000;355:1829–1830. doi: 10.1016/S0140-6736(05)73093-2. [DOI] [PubMed] [Google Scholar]
- 211.Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol. 1993;22:504–511. doi: 10.1093/ije/22.3.504. [DOI] [PubMed] [Google Scholar]
- 212.Terada N, Kinoshita K, Taguchi S, Tokuda Y. Wernicke encephalopathy and pellagra in an alcoholic and malnourished patient. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-209412. bcr2015209412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Savvidou S. Pellagra: a non-eradicated old disease. Clin Pract. 2014;4:637. doi: 10.4081/cp.2014.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Delgado-Sanchez L, Godkar D, Niranjan S. Pellagra: rekindling of an old flame. Am J Ther. 2008;15:173–175. doi: 10.1097/MJT.0b013e31815ae309. [DOI] [PubMed] [Google Scholar]
- 215.Teare JP, Hyams G, Pollock S. Acute encephalopathy due to coexistent nicotinic acid and thiamine deficiency. Br J Clin Pract. 1993;47:343–344. [PubMed] [Google Scholar]
- 216.Lee SE. Guam dementia syndrome revisited in 2011. Curr Opin Neurol. 2011;24:517–524. doi: 10.1097/WCO.0b013e32834cd50a. [DOI] [PubMed] [Google Scholar]
- 217.Spencer PS, Palmer VS, Kisby GE. Seeking environmental causes of neurodegenerative disease and envisioning primary prevention. Neurotoxicology. 2016;56:269–283. doi: 10.1016/j.neuro.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 218.de Andraca I, Castillo M, Walter T. Psychomotor development and behavior in iron-deficient anemic infants. Nutr Rev. 1997;55:125–132. doi: 10.1111/j.1753-4887.1997.tb06463.x. [DOI] [PubMed] [Google Scholar]
- 219.Ordunez-Garcia PO, Nieto FJ, Espinosa-Brito AD, Caballero B. Cuban epidemic neuropathy, 1991 to 1994: history repeats itself a century after the ‘amblyopia of the blockade’. Am J Public Health. 1996;86:738–743. doi: 10.2105/ajph.86.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lamb J. Scurvy: The Disease of Discovery. Princeton, NJ: Princeton University Press; 2016. [Google Scholar]
- 221.Trowell H, Muwazi E. Severe and prolonged underfeeding in African children: (The Kwashiorkor Syndrome of Malignant Malnutrition) Arch Dis Child. 1945;20:110. doi: 10.1136/adc.20.103.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating Group Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 223.Grantham-McGregor S, Ani C. Cognition and undernutrition: evidence for vulnerable period. Forum Nutr. 2003;56:272–275. [PubMed] [Google Scholar]
- 224.Grantham-McGregor S, Baker-Henningham H. Review of the evidence linking protein and energy to mental development. Public Health Nutr. 2005;8:1191–1201. doi: 10.1079/phn2005805. [DOI] [PubMed] [Google Scholar]
- 225.Donowitz JR, Haque R, Kirkpatrick BD, et al. Small intestine bacterial overgrowth and environmental enteropathy in Bangladeshi children. mBio. 2016;7:e02102–e02115. doi: 10.1128/mBio.02102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Perlot T, Penninger JM. ACE2-from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866–873. doi: 10.1016/j.micinf.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:1–23. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fu X, Lawson MA, Kelley KW, Dantzer R. HIV-1 Tat activates indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures in a p38 mitogen-activated protein kinase-dependent manner. J Neuroinflammation. 2011;8:88. doi: 10.1186/1742-2094-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bipath P, Levay PF, Viljoen M. The kynurenine pathway activities in a sub-Saharan HIV/AIDS population. BMC Infect Dis. 2015;15:346. doi: 10.1186/s12879-015-1087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Burdge GC, Lillycrop KA. Environment-physiology, diet quality and energy balance: the influence of early life nutrition on future energy balance. Physiol Behav. 2014;134:119–122. doi: 10.1016/j.physbeh.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 231.McCarrison R. Nutrition and Health, Together with Two Earlier Essays. London, England: Faber & Faber; 1936. [Google Scholar]
- 232.Melina V, Craig W, Levin S. Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet. 2016;116:1970–1980. doi: 10.1016/j.jand.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 233.Ceci SJ. How much does schooling influence general intelligence and its cognitive components? A reassessment of the evidence. Dev Psychol. 1991;27:703–722. [Google Scholar]
- 234.Flynn JR. Does Your Family Make You Smarter?: Nature, Nurture, and Human Autonomy. Cambridge, UK: Cambridge University Press; 2016. [Google Scholar]
- 235.Lynn R, Vanhanen T. IQ and Global Inequality. Athens, Greece: Washington Summit Books; 2006. [Google Scholar]
- 236.Wang X, Hu X, Yang Y, Takata T, Sakurai T. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1–9. doi: 10.1016/j.brainres.2016.04.060. [DOI] [PubMed] [Google Scholar]
- 237.Young GS, Kirkland JB. The role of dietary niacin intake and the adenosine-5′-diphosphate-ribosyl cyclase enzyme CD38 in spatial learning ability: is cyclic adenosine diphosphate ribose the link between diet and behaviour? Nutr Res Rev. 2008;21:42–55. doi: 10.1017/S0954422408945182. [DOI] [PubMed] [Google Scholar]
- 238.Wang Y, Zuo M. Nicotinamide improves sevoflurane-induced cognitive impairment through suppression of inflammation and anti-apoptosis in rat. Int J Clin Exp Med. 2015;8:20079. [PMC free article] [PubMed] [Google Scholar]
- 239.Morris MC, Schneider JA, Tangney CC. Thoughts on B-vitamins and dementia. J Alzheimers Dis. 2006;9:429–433. doi: 10.3233/jad-2006-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hoane MR, Akstulewicz SL, Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in rats. J Neurotrauma. 2003;20:1189–1199. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- 241.Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367:31–37. doi: 10.1111/nyas.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Barnes JL, Tian M, Edens NK, Morris MC. Consideration of nutrient levels in studies of cognitive decline. Nutr Rev. 2014;72:707–719. doi: 10.1111/nure.12144. [DOI] [PubMed] [Google Scholar]
- 243.Morris MC, Tangney CC. Diet and prevention of Alzheimer disease diet and prevention of Alzheimer disease. JAMA. 2010;303:2519–2520. doi: 10.1001/jama.2010.844. [DOI] [PubMed] [Google Scholar]
- 244.Morris MC, Evans DA, Bienias JL, et al. Dietary niacin and the risk of incident Alzheimer’s disease and of cognitive decline. J Neurol Neurosurg Psychiatry. 2004;75:1093–1099. doi: 10.1136/jnnp.2003.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yao JK, Dougherty GG, Gautier CH, et al. Prevalence and specificity of the abnormal niacin response: a potential endophenotype marker in schizophrenia. Schizophr Bull. 2016;42:369–376. doi: 10.1093/schbul/sbv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Berger GE, Smesny S, Schäfer MR, et al. Niacin skin sensitivity is increased in adolescents at ultra-high risk for psychosis. PLoS ONE. 2016;11:e0148429. doi: 10.1371/journal.pone.0148429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Xu X, Jiang G. Niacin-respondent subset of schizophrenia – a therapeutic review. Eur Rev Med Pharmacol Sci. 2015;19:988–997. [PubMed] [Google Scholar]
- 248.Messamore E. Niacin subsensitivity is associated with functional impairment in schizophrenia. Schizophr Res. 2012;137:180–184. doi: 10.1016/j.schres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 249.Camacho-Pereira J, Tarragó MG, Chini CC, et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bleeker JC, Houtkooper RH. Sirtuin activation as a therapeutic approach against inborn errors of metabolism. J Inherit Metab Dis. 2016;39:565–572. doi: 10.1007/s10545-016-9939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Greenwald SH, Charette JR, Staniszewska M, et al. Mouse models of NMNAT1-leber congenital amaurosis (LCA9) recapitulate key features of the human disease. Am J Pathol. 2016;186:1925–1938. doi: 10.1016/j.ajpath.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ryu D, Zhang H, Ropelle ER, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med. 2016;8:361ra139. doi: 10.1126/scitranslmed.aaf5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tateishi K, Wakimoto H, Iafrate AJ, et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell. 2015;28:773–784. doi: 10.1016/j.ccell.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lynch DR, Fischbeck KH. Nicotinamide in Friedreich’s ataxia: useful or not? Lancet. 2014;384:474–475. doi: 10.1016/S0140-6736(14)60573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Fang EF, Kassahun H, Croteau DL, et al. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hu L, IbrahimStucki K, Ibrahim M, et al. Secondary NAD+ deficiency in the inherited defect of glutamine synthetase. J Inherit Metab Dis. 2015;38:1075–1083. doi: 10.1007/s10545-015-9846-4. [DOI] [PubMed] [Google Scholar]
- 257.Rosas HD, Doros G, Bhasin S, et al. A systems-level ‘misunderstanding’: the plasma metabolome in Huntington’s disease. Ann Clin Transl Neurol. 2015;2:756–768. doi: 10.1002/acn3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Naia L, Rosenstock TR, Oliveira AM, et al. Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in Huntington’s disease models [published online ahead of print September 2, 2016] Mol Neurobiol. doi: 10.1007/s12035-016-0048-3. [DOI] [PubMed] [Google Scholar]
- 259.Shah GM, Shah RG, Veillette H, Kirkland JB, Pasieka JL, Warner RR. Biochemical assessment of niacin deficiency among carcinoid cancer patients. Am J Gastroenterol. 2005;100:2307–2314. doi: 10.1111/j.1572-0241.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 260.Broer S. The role of the neutral amino acid transporter B0AT1 (SLC6A19) in Hartnup disorder and protein nutrition. IUBMB Life. 2009;61:591–599. doi: 10.1002/iub.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Peng QY, Ai ML, Zhang LN, Zou Y, Ma XH, Ai YH. Blocking NAD(+)/CD38/cADPR/Ca(2+) pathway in sepsis prevents organ damage. J Surg Res. 2016;201:480–489. doi: 10.1016/j.jss.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 262.Park JH, Long A, Owens K, Kristian T. Nicotinamide mononucleotide inhibits post-ischemic NAD+ degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol Dis. 2016;95:102–110. doi: 10.1016/j.nbd.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Long A, Park JH, Klimova N, Fowler C, Loane DJ, Kristian T. CD38 knockout mice show significant protection against ischemic brain damage despite high level poly-ADP-ribosylation. Neurochem Res. 2017;42:283–293. doi: 10.1007/s11064-016-2031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mahmoud YI, Mahmoud AA. Role of nicotinamide (vitamin B3) in acetaminophen-induced changes in rat liver: nicotinamide effect in acetaminophen-damged liver. Exp Toxicol Pathol. 2016;68:345–354. doi: 10.1016/j.etp.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 265.Ogawa K, Fukumoto T, Yoshida M, Matsumoto Y, Shobatake C, Asada H. Eosinophilic annular erythema in a patient with autoimmune pancreatitis: nicotinamide therapy may be beneficial for achieving remission. J Dermatol. 2016;43:1380–1381. doi: 10.1111/1346-8138.13411. [DOI] [PubMed] [Google Scholar]
- 266.Parsons RB, Aravindan S, Kadampeswaran A, et al. The expression of nicotinamide N-methyltransferase increases ATP synthesis and protects SH-SY5Y neuroblastoma cells against the toxicity of Complex I inhibitors. Biochem J. 2011;436:145–155. doi: 10.1042/BJ20101685. [DOI] [PubMed] [Google Scholar]
- 267.Yeung AW, Terentis AC, King NJ, Thomas SR. Role of indoleamine 2,3-di-oxygenase in health and disease. Clin Sci (Lond) 2015;129:601–672. doi: 10.1042/CS20140392. [DOI] [PubMed] [Google Scholar]
- 268.Xie X, Liu H, Wang Y, et al. Nicotinamide N-methyltransferase enhances resistance to 5-fluorouracil in colorectal cancer cells through inhibition of the ASK1-p38 MAPK pathway. Oncotarget. 2016;7:45837–45848. doi: 10.18632/oncotarget.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yue Z, Ma Y, You J, et al. NMNAT3 is involved in the protective effect of SIRT3 in Ang II-induced cardiac hypertrophy. Exp Cell Res. 2016;347:261–273. doi: 10.1016/j.yexcr.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 270.Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 2016;214:103–119. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zou XD, Guo SQ, Hu ZW, Li WL. NAMPT protects against 6-hydroxydopamine-induced neurotoxicity in PC12 cells through modulating SIRT1 activity. Mol Med Rep. 2016;13:4058–4064. doi: 10.3892/mmr.2016.5034. [DOI] [PubMed] [Google Scholar]
- 272.Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mills KF, Yoshida S, Stein LR, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tung C-S, Chang S-T, Huang C-L, Huang NK. The neurotoxic mechanisms of amphetamine: step by step for striatal dopamine depletion. Neurosci Lett. 2017;639:185–191. doi: 10.1016/j.neulet.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 275.Bostom AG, Merhi B, Walker J, Robinson-Bostom L. More than skin deep? Potential nicotinamide treatment applications in chronic kidney transplant recipients. World J Transplant. 2016;6:658. doi: 10.5500/wjt.v6.i4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Forbat E, Al-Niaimi F, Ali F. Use of nicotinamide in dermatology. Clin Exp Dermatol. 2017;42:137–144. doi: 10.1111/ced.13021. [DOI] [PubMed] [Google Scholar]
- 277.Williams PA, Harder JM, Foxworth NE, et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355:756–760. doi: 10.1126/science.aal0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhu X-J, Lin Y-J, Chen W, et al. Physiological study on association between nicotinamide N-methyltransferase gene polymorphisms and hyperlipidemia. BioMed Res Int. 2016;2016 doi: 10.1155/2016/7521942. Article 7521942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Saini JS, Corneo B, Miller JD, et al. Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration [published online ahead of print January 21, 2017] Cell Stem Cell. doi: 10.1016/j.stem.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Monfrecola G, Di Caprio R, Balato N, et al. Nicotinamide reduces COX-2expression in HaCaT keratinocytes after ultraviolet-B irradiation [published online ahead of print January 24, 2017] Br J Dermatol. doi: 10.1111/bjd.15338. [DOI] [PubMed] [Google Scholar]
- 281.Maltos AL, Portari GV, Moraes GV, Monteiro MCR, Vannucchi H, da Cunha DF. Niacin metabolism and indoleamine 2,3-dioxygenase activation in malnourished patients with flaky paint dermatosis. Nutrition. 2015;31:890–892. doi: 10.1016/j.nut.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 282.Thompson BC, Halliday GM, Damian DL. Nicotinamide enhances repair of arsenic and ultraviolet radiation-induced DNA damage in HaCaT keratinocytes and ex vivo human skin. PLoS ONE. 2015;10:e0117491. doi: 10.1371/journal.pone.0117491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kim B, Halliday G, Damian D. Oral Nicotinamide and Actinic Keratosis: A Supplement Success Story Actinic Keratosis. Vol. 46. Berlin, Germany: Karger Publishers; 2014. pp. 143–149. [DOI] [PubMed] [Google Scholar]
- 284.Kim D, Lee G, Huh Y, et al. NAMPT is an essential regulator of RA-mediated periodontal inflammation [published online ahead of print February 1, 2017] J Dent Res. doi: 10.1177/0022034517690389. [DOI] [PubMed] [Google Scholar]
- 285.Liu M, Chu J, Gu Y, et al. Serum N1-methylnicotinamide is associated with coronary artery disease in Chinese patients. J Am Heart Assoc. 2017;6:e004328. doi: 10.1161/JAHA.116.004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fahrmann JF, Grapov DD, Wanichthanarak K, et al. Integrated metabolomics and proteomics highlight altered nicotinamide-and polyamine pathways in lung adenocarcinoma [published online ahead of print January 3, 2017] Carcinogenesis. doi: 10.1093/carcin/bgw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Shi W, Hegeman MA, Dartel DA, et al. Effects of a wide range of dietary nicotinamide riboside (NR) concentrations on metabolic flexibility and white adipose tissue (WAT) of mice fed a mildly obesogenic diet [published online ahead of print February 16, 2017] Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201600878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol. 2013;61:440–446. doi: 10.1016/j.jacc.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 289.Guan Y, Wang S-R, Huang X-Z, et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner [published online ahead of print February 28, 2017] J Am Soc Nephrol. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hershberger KA, Martin AS, Hirschey MD. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol. 2017;13:213–225. doi: 10.1038/nrneph.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhou M, Ottenberg G, Sferrazza GF, et al. Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment. Brain. 2015;138:992–1008. doi: 10.1093/brain/awv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kapas I, Majtenyi K, Toro K, Keller E, Voigtlander T, Kovacs GG. Pellagra encephalopathy as a differential diagnosis for Creutzfeldt-Jakob disease. Metab Brain Dis. 2012;27:231–235. doi: 10.1007/s11011-012-9308-8. [DOI] [PubMed] [Google Scholar]
- 293.Sharma O, O’Seaghdha M, Velarde JJ, Wessels MR. NAD+-glycohydrolase promotes intracellular survival of group A streptococcus. PLoS Pathog. 2016;12:e1005468. doi: 10.1371/journal.ppat.1005468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ashenburg K. The Dirt on Clean: An Unsanitized History. New York, NY: Farrar, Straus and Giroux; 2014. [Google Scholar]
- 295.M’Gonigle GCM, Kirby J. Poverty and Public Health. London, England: Victor Gollancz; 1936. [Google Scholar]
- 296.Helweg-Larsen P, Hoffmeyer H, Kieler J, et al. Famine disease in German concentration camps; complications and sequels, with special reference to tuberculosis, mental disorders and social consequences. Acta Psychiatr Neurol Scand Suppl. 1952;83:1–460. [PubMed] [Google Scholar]
- 297.Dubos RJ, Dubos J. The White Plague: Tuberculosis, Man, and Society. New Brunswick, NJ: Rutgers University Press; 1952. [Google Scholar]
- 298.Anderson W, Mackay IR. Intolerant Bodies: A Short History of Autoimmunity. Baltimore, MD: Johns Hopkins University Press; 2014. [Google Scholar]
- 299.Bynum H. Spitting Blood: The History of Tuberculosis. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 300.Daniel TM. Captain of Death: The Story of Tuberculosis. Rochester, NY: University of Rochester Press; 1997. [Google Scholar]
- 301.Haahtela T, Holgate S, Pawankar R, et al. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. 2013;6:1. doi: 10.1186/1939-4551-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Prescott S. The Allergy Epidemic: A Mystery of Modern Life. Crawley, WA, Australia: UWA Publishing; 2011. [Google Scholar]
- 303.Jackson M. Allergy: The History of a Modern Malady. Islington, UK: Reaktion Books; 2007. [Google Scholar]
- 304.Boaz NT. Evolving Health: The Origins of Illness and How the Modern World Is Making Us Sick. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 305.Inglis B. The Diseases of Civilisation. London, England: Hodder & Stoughton; 1981. [Google Scholar]
- 306.Kozyrskyj AL, Bahreinian S, Azad MB. Early life exposures: impact on asthma and allergic disease. Curr Opin Allergy Clin Immunol. 2011;11:400–406. doi: 10.1097/ACI.0b013e328349b166. [DOI] [PubMed] [Google Scholar]
- 307.Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol. 2015;136:860–865. doi: 10.1016/j.jaci.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 308.Maizels RM. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin Microbiol Infect. 2016;22:481–486. doi: 10.1016/j.cmi.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 309.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 310.Rosenberg E, Zilber-Rosenberg I. The Hologenome Concept: Human, Animal and Plant Microbiota. Berlin, Germany: Springer; 2014. [Google Scholar]
- 311.Rodrigo CP. Current mapping of obesity. Nutr Hosp. 2013;28:21–31. doi: 10.3305/nh.2013.28.sup5.6915. [DOI] [PubMed] [Google Scholar]
- 312.Slack T, Myers CA, Martin CK, Heymsfield SB. The geographic concentration of US adult obesity prevalence and associated social, economic, and environmental factors. Obesity. 2014;22:868–874. doi: 10.1002/oby.20502. [DOI] [PubMed] [Google Scholar]
- 313.Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ. Geographic distribution of diagnosed diabetes in the US: a diabetes belt. Am J Prev Med. 2011;40:434–439. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 314.Neel JV Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’. Am J Hum Genet. 1962;14:353. [PMC free article] [PubMed] [Google Scholar]
- 315.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 316.Gluckman PD, Hanson MA. The Fetal Matrix: Evolution, Development and Disease. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 317.Al-Mohaissen M, Pun S, Frohlich J. Niacin: from mechanisms of action to therapeutic uses. Mini Rev Med Chem. 2010;10:204–217. doi: 10.2174/138955710791185046. [DOI] [PubMed] [Google Scholar]
- 318.Alsheikh-Ali AA, Karas RH. The safety of niacin in the US Food and Drug Administration adverse event reporting database. Am J Cardiol. 2008;101:S9–S13. doi: 10.1016/j.amjcard.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 319.Parsons RB, Smith M-L, Williams AC, Waring RH, Ramsden DB. Expression of nicotinamide N-methyltransferase (EC 2.1. 1.1) in the Parkinsonian brain. J Neuropathol Exp Neurol. 2002;61:111–124. doi: 10.1093/jnen/61.2.111. [DOI] [PubMed] [Google Scholar]
- 320.Parsons RB, Smith SW, Waring RH, Williams AC, Ramsden DB. High expression of nicotinamide N-methyltransferase in patients with idiopathic Parkinson’s disease. Neurosci Lett. 2003;342:13–16. doi: 10.1016/s0304-3940(03)00218-0. [DOI] [PubMed] [Google Scholar]
- 321.Bromberg A, Lerer E, Udawela M, et al. Nicotinamide-N-methyltransferase (NNMT) in schizophrenia: genetic association and decreased frontal cortex mRNA levels. Int J Neuropsychopharmacol. 2012;15:727–737. doi: 10.1017/S1461145711001179. [DOI] [PubMed] [Google Scholar]
- 322.Chen C, Wang X, Huang X, et al. Nicotinamide N-methyltransferase: a potential biomarker for worse prognosis in gastric carcinoma. Am J Cancer Res. 2016;6:649. [PMC free article] [PubMed] [Google Scholar]
- 323.Goldberg RB, Jacobson TA. Effects of niacin on glucose control in patients with dyslipidemia. Mayo Clin Proc. 2008 Apr;83(4):470–478. doi: 10.4065/83.4.470. [DOI] [PubMed] [Google Scholar]
- 324.Goldie C, Taylor AJ, Nguyen P, McCoy C, Zhao X-Q, Preiss D. Niacin therapy and the risk of new-onset diabetes: a meta-analysis of randomised controlled trials. Heart. 2015;102:198–203. doi: 10.1136/heartjnl-2015-308055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 326.Matte JJ, Corrent E, Simongiovanni A, Le Floc’h N. Tryptophan metabolism, growth responses, and postprandial insulin metabolism in weaned piglets according to the dietary provision of niacin (vitamin B) and tryptophan. J Animal Sci. 2016;94:1961–1971. doi: 10.2527/jas.2015-0221. [DOI] [PubMed] [Google Scholar]
- 327.Trammell SA, Brenner C. NNMT: a bad actor in fat makes good in liver. Cell Metab. 2015;22:200–201. doi: 10.1016/j.cmet.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Qi Z, Xia J, Xue X, He Q, Ji L, Ding S. Long-term treatment with nicotinamide induces glucose intolerance and skeletal muscle lipotoxicity in normal chow-fed mice: compared to diet-induced obesity. J Nutr Biochem. 2016;36:31–41. doi: 10.1016/j.jnutbio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 329.Kourtzidis IA, Stoupas AT, Gioris IS, et al. The NAD+ precursor nicotinamide riboside decreases exercise performance in rats. J Int Soc Sports Nutr. 2016;13:32. doi: 10.1186/s12970-016-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kim S-W, Lee J-H, Moon J-H, et al. Niacin alleviates TRAIL-mediated colon cancer cell death via autophagy flux activation. Oncotarget. 2016;7:4356. doi: 10.18632/oncotarget.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kirkland JB. Niacin requirements for genomic stability. Mutat Res. 2012;733:14–20. doi: 10.1016/j.mrfmmm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 332.Liu L, Peritore C, Ginsberg J, Shih J, Arun S, Donmez G. Protective role of SIRT5 against motor deficit and dopaminergic degeneration in MPTP-induced mice model of Parkinson’s disease. Behav Brain Res. 2015;281:215–221. doi: 10.1016/j.bbr.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 333.Williams A, Ramsden D. Nicotinamide: a double edged sword. Parkinsonism Relat Disord. 2005;11:413–420. doi: 10.1016/j.parkreldis.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 334.Chłopicki S, Kurdziel M, Sternak M, et al. Single bout of endurance exercise increases NNMT activity in the liver and MNA concentration in plasma; the role of IL-6. Pharmacol Rep. 2012;64:369–376. doi: 10.1016/s1734-1140(12)70777-6. [DOI] [PubMed] [Google Scholar]
- 335.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 336.Williams AC, Ramsden DB. Nicotinamide homeostasis: a xenobiotic pathway that is key to development and degenerative diseases. Med Hypotheses. 2005;65:353–362. doi: 10.1016/j.mehy.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 337.Sampson Timothy R, Debelius Justine W, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 167:1469.e12–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Lenglet A, Liabeuf S, Bodeau S, et al. N-methyl-2-pyridone-5-carboxamide (2PY) – major metabolite of nicotinamide: an update on an old uremic toxin. Toxins. 2016;8:339. doi: 10.3390/toxins8110339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Jukarainen S, Heinonen S, Rämö JT, et al. Obesity is associated with low NAD+/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. J Clin Endocrinol Metab. 2015;101:275–283. doi: 10.1210/jc.2015-3095. [DOI] [PubMed] [Google Scholar]
- 340.Kannt A, Pfenninger A, Teichert L, et al. Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia. 2015;58:799–808. doi: 10.1007/s00125-014-3490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kraus D, Yang Q, Kong D, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Rappou E, Jukarainen S, Rinnankoski-Tuikka R, et al. Weight loss is associated with increased NAD+/SIRT1 expression but reduced PARP activity in white adipose tissue. J Clin Endocrinol Metab. 2016;101:1263–1273. doi: 10.1210/jc.2015-3054. [DOI] [PubMed] [Google Scholar]
- 343.Gatrell AC, Elliott SJ. Geographies of Health: An Introduction. Hoboken, NJ: Wiley; 2014. [Google Scholar]
- 344.Giampietro M, Mayumi K, Sorman AH. The Metabolic Pattern of Societies: Where Economists Fall Short. Abingdon, UK: Taylor & Francis; 2011. [Google Scholar]
- 345.Glanz K, Sallis JF, Saelens BE, Frank LD. Healthy nutrition environments: concepts and measures. Am J Health Promot. 2005;19:330–333. doi: 10.4278/0890-1171-19.5.330. [DOI] [PubMed] [Google Scholar]
- 346.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Rajda C, Majlath Z, Pukoli D, Vecsei L. Kynurenines and multiple sclerosis: the dialogue between the immune system and the central nervous system. Int J Mol Sci. 2015;16:18270–18282. doi: 10.3390/ijms160818270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Nimmagadda VK, Makar TK, Chandrasekaran K, et al. SIRT1 and NAD+ precursors: therapeutic targets in multiple sclerosis a review. J Neuroimmunol. 2016;304:29–34. doi: 10.1016/j.jneuroim.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Swank RL, Dugan BB. Effect of low saturated fat diet in early and late cases of multiple sclerosis. Lancet. 1990;336:37–39. doi: 10.1016/0140-6736(90)91533-g. [DOI] [PubMed] [Google Scholar]
- 350.Zhou S-S, Li D, Na-Na C, Zhou Y. Vitamin paradox in obesity: deficiency or excess? World J Diabet. 2015;6:1158. doi: 10.4239/wjd.v6.i10.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Power ML, Schulkin J. The Evolution of Obesity. Baltimore, MD: Johns Hopkins University Press; 2013. [Google Scholar]
- 352.Ewald PW. Evolution of Infectious Disease. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 353.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 354.Goldberg DE, Siliciano RF, Jacobs WR., Jr Outwitting evolution: fighting drug-resistant TB, malaria, and HIV. Cell. 2012;148:1271–1283. doi: 10.1016/j.cell.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Amyes SGB. Magic Bullets, Lost Horizons: The Rise and Fall of Antibiotics. Abingdon, UK: Taylor & Francis; 2001. [Google Scholar]
- 356.Boni MF, Feldman MW. Evolution of antibiotic resistance by human and bacterial niche construction. Evolution. 2005;59:477–491. [PubMed] [Google Scholar]
- 357.Stecher B, Maier L, Hardt W-D. ‘Blooming’in the gut: how dysbiosis might contribute to pathogen evolution. Nat Rev Microbiology. 2013;11:277–284. doi: 10.1038/nrmicro2989. [DOI] [PubMed] [Google Scholar]
- 358.Akalu G, Taffesse S, Gunaratna NS, De Groote H. The effectiveness of quality protein maize in improving the nutritional status of young children in the Ethiopian highlands. Food Nutr Bull. 2010;31:418–430. doi: 10.1177/156482651003100304. [DOI] [PubMed] [Google Scholar]
- 359.Bilan DS, Belousov VV. New tools for redox biology: from imaging to manipulation. Free Radic Biol Med. http://doi.org/10.1016/j.freeradbiomed.2016. 12.004. In press. [DOI] [PubMed]
- 360.Abdellatif M. Sirtuins and pyridine nucleotides. Circ Res. 2012;111:642–656. doi: 10.1161/CIRCRESAHA.111.246546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Friel S, Labonte R, Sanders D. Measuring progress on diet-related NCDs: the need to address the causes of the causes. Lancet. 2013;381:903–904. doi: 10.1016/S0140-6736(13)60669-8. [DOI] [PubMed] [Google Scholar]
- 362.Neidecker-Gonzales O, Nestel P, Bouis H. Estimating the global costs of vitamin A capsule supplementation: a review of the literature. Food Nutr Bull. 2007;28:307–316. doi: 10.1177/156482650702800307. [DOI] [PubMed] [Google Scholar]
- 363.Gross R, Gross U, Lechtig A, Lopez de Romana D. We know much about what to do but little about how to do it: experiences with a weekly multimicronutrient supplementation campaign. Food Nutr Bull. 2006;27:S111–S114. doi: 10.1177/15648265060274S401. [DOI] [PubMed] [Google Scholar]
- 364.Fiedler JL, Sanghvi TG, Saunders MK. A review of the micronutrient intervention cost literature: program design and policy lessons. Int J Health Plann Manage. 2008;23:373–397. doi: 10.1002/hpm.928. [DOI] [PubMed] [Google Scholar]
- 365.Darnton-Hill I. Global burden and significance of multiple micronutrient deficiencies in pregnancy. Nestle Nutr Inst Workshop Ser. 2012;70:49–60. doi: 10.1159/000337421. [DOI] [PubMed] [Google Scholar]
- 366.Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer WH. Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr Bull. 2011;32:S31–S40. doi: 10.1177/15648265110321S105. [DOI] [PubMed] [Google Scholar]
- 367.Bhutta ZA, Salam RA, Das JK. Meeting the challenges of micronutrient malnutrition in the developing world. Br Med Bull. 2013;106:7–17. doi: 10.1093/bmb/ldt015. [DOI] [PubMed] [Google Scholar]
- 368.Grinspoon D. Earth in Human Hands: Shaping Our Planet’s Future. New York, USA: Grand Central Publishing; 2016. [Google Scholar]
- 369.Cordain L, Gotshall RW, Eaton SB. Evolutionary Aspects of exercise Nutrition and Fitness: Evolutionary Aspects, Children’s Health, Programs and Policies. Vol. 81. Berlin, Germany: Karger Publishers; 1997. pp. 49–60. [DOI] [PubMed] [Google Scholar]
- 370.Heymsfield S, Bourgeois B, Thomas D. Assessment of human energy exchange: historical overview. Eur J Clin Nutr. 2016;71:294–300. doi: 10.1038/ejcn.2016.221. [DOI] [PubMed] [Google Scholar]
- 371.J-H Li, Chen W, Zhu X-J, et al. Associations of nicotinamide N-methyltransferase gene single nucleotide polymorphisms with sport performance and relative maximal oxygen uptake [published online ahead of print November 30, 2017] J Sport Sci. doi: 10.1080/02640414.2016.1261176. [DOI] [PubMed] [Google Scholar]
- 372.Engel E. Zeitschrift des Statistischen Bureaus des Königl. Sächs. Ministeriums des Innern. Leipzig, Germany: Hübner; 1855. [Google Scholar]