Abstract
Phylogeographical studies have suggested that several plant species on the Tibetan Plateau (TP) underwent recolonization during the Quaternary and may have had distinct range dynamics in response to the last glacial. To further test this hypothesis and locate the possible historical dispersal routes, we selected 20 plant species from different parts of the TP and modeled their geographical distributions over four time periods using species distribution models (SDMs). Furthermore, we applied the least-cost path method together with SDMs and shared haplotypes to estimate their historical dispersal corridors. We identified three general scenarios of species distribution change during the late Quaternary: the ‘contraction-expansion’ scenario for species in the northeastern TP, the ‘expansion-contraction’ scenario for species in the southeast and the ‘stable’ scenario for widespread species. During the Quaternary, we identified that these species were likely to recolonize along the low-elevation valleys, huge mountain ranges and flat plateau platform (e.g. the Yarlung Zangbo Valley and the Himalaya). We inferred that Quaternary cyclic glaciations along with the various topographic and climatic conditions of the TP could have resulted in the diverse patterns of range shift and dispersal of Tibetan plant species. Finally, we believe that this study would provide valuable insights for the conservation of alpine species under future climate change.
Introduction
Phylogeography has provided a way to understand the demographic history of species, which was primarily affected by periodic climate oscillations during the Quaternary, generating varying degrees of range contraction and expansion [1]. In general, species retreated to refugia during the glacial period, preserving multiple and original haplotypes within these regions [2]. During the postglacial period, several species recolonized in other areas, producing nearly complete haplotype uniformity in the colonized populations [3]. Hence, the range dynamics of species during the Quaternary may have influenced the present-day genetic structures and phylogeographical patterns [4, 5].
Typically, phylogeographers can use mismatch distribution analysis or neutral test to detect whether species underwent recent demographic expansion [6–8], and occasionally to estimate the expansion time based on the mutation rate of DNA sequences [7]. However, the detailed changes in range during the late Quaternary and expansion routes in a spatial context have rarely been identified. Fortunately, the available paleoclimate data and species distribution models (SDMs) have been widely applied to determine the trends in species distribution during the late Quaternary [9–11]. Furthermore, the least cost path method, which is often used in the field of landscape ecology [12–14], has been used to locate past dispersal routes in combination with population genetic data and SDMs [15, 16]. Such integrative calculation would undoubtedly supplement the demographic history of species.
The Tibetan Plateau (TP) has become a hotspot of phylogeographical studies [17–20], owing to its complicated alpine conditions (e.g. climate and topography) and past environmental changes (e.g. Quaternary climate fluctuations). Based on the demographic history of plant taxa inferred from these studies, we found that the changes in distribution were diverse during the late Quaternary. Because there was no formation of large and whole ice sheet during the Last Glacial Maximum (LGM) period on the TP [21], most evidence has shown that the LGM had a limited effect on species distribution [17, 22–24]. Thus, the distributions of numerous species remained stable status throughout the LGM. However, a few species in the northeastern part of the TP, including Juniperus przewalskii [25] and Picea crassifolia [26], still underwent the typical tabula rasa scenario. In contrast, several cold-adapted species (e.g. Picea likiangensis and Taxus wallichiana) in the southeastern TP experienced range expansion during the LGM [10, 11]. Based on the above-mentioned inferences, we thereby recommend three common patterns of range dynamics across the TP: i) a contraction-expansion scenario for species in the northeastern TP; ii) an expansion-contraction scenario for species in the southeastern TP; and iii) a stable scenario for widespread species throughout the TP. This assumption is only based on the phylogeography of a few species; thus, to further verify this speculation, more species and distribution modelling needs to be carried out.
Several plant species on the TP underwent rapid and extensive recolonization events during the Quaternary [27, 28]. We assumed that these species might be susceptible to climatic fluctuations, we thus chose them in this study to i) test whether species in different parts of the TP had distinct range dynamics during the late Quaternary, and ii) identify the possible historical dispersal corridors of plant species on the TP.
Data and methods
Study area
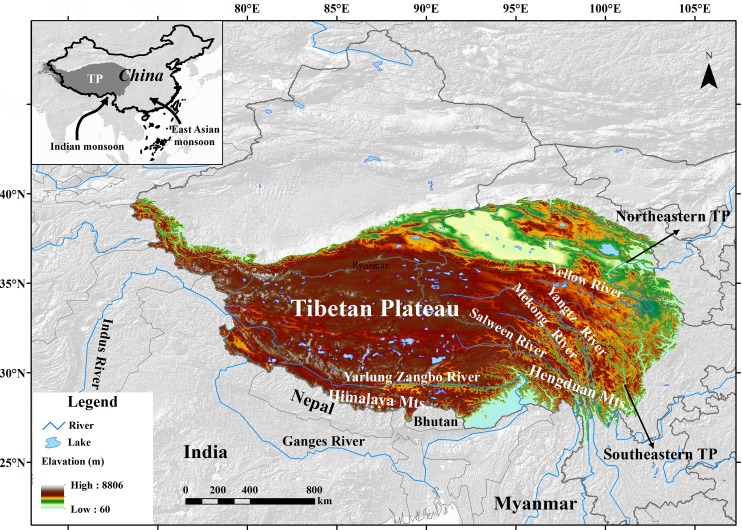
The TP is the largest (c. 2.5×106 km2) and highest plateau in the world with an average elevation of greater than 4000 m [29] (Fig 1). Several major Chinese and Asian rivers originate from the TP, such as the Yangtze River, Yellow River, Mekong River, Salween River, Yarlung Zangbo River, Indus River and Ganges River. These rivers could be important corridors for the dispersal of water vapor, plants and animals [16, 30]. During the summer season each year, both the Indian and East Asian monsoon bring warm and wet air that is critical for the growth and reproduction of plant species on the TP [31]. In the southeastern TP, the East Himalaya and Hengduan Mountains is a key biodiversity hotspot harboring a great number of endemic and alpine species [32], which is regarded as the potential recent origin areas and Quaternary refugia of many temperate plants of East Asia [33–35].
Fig 1. The scope of the Tibetan Plateau and its basic geographical conditions.
In this study, the area of northeastern TP includes the Hehuang Valley, the Qilian Mountains and the Qaidam Basin in Qinghai Province, the Taohe Valley in Gansu Province and the Minshan in northern Sichuan Province; the southeastern TP mainly covers the East Himalaya and Hengduan Mountains.
Species selection and determination of shared haplotypes
To test whether species in different parts of the TP have distinct range dynamics, we chose 20 plant species from three regions of the TP (Table 1), which experienced demographic expansion during the Quaternary. Based on the phylogeographical study of each species, we collected the haplotype composition and geographical coordinate of each population. To ensure the congruence in DNA markers, we mainly selected maternally inherited markers (e.g. cpDNA for angiosperms and mtDNA for gymnosperms). From the spatial haplotype structures of each plant (S1 Fig), we obtained which populations had shared haplotypes.
Table 1. Twenty plant species in different parts of the TP were used in this study.
| Species | Family | DNA Type | Population No. | Haplotype No. | Shared Haplotype No. | Reference |
|---|---|---|---|---|---|---|
| Northeast TP | ||||||
| Juniperus przewalskii | Cupressaceae | cpDNA | 20 | 6 | 5 | [25] |
| Picea crassifolia | Pinaceae | mtDNA | 32 | 9 | 9 | [26] |
| Metagentiana striata | Gentianaceae | cpDNA | 14 | 10 | 2 | [36] |
| Angelica nitida | Apiaceae | cpDNA | 16 | 20 | 5 | [37] |
| Bupleurum smithii | Apiaceae | cpDNA | 22 | 25 | 9 | [38] |
| Southeast TP | ||||||
| Buddleja crispa | Loganiaceae | cpDNA | 23 | 13 | 4 | [39] |
| Picea likiangensis | Pinaceae | mtDNA | 42 | 9 | 7 | [40] |
| Taxus wallichiana | Taxaceae | cpDNA | 43 | 29 | 11 | [11] |
| Spenceria ramalana | Rosaceae | cpDNA | 19 | 17 | 6 | [41] |
| Quercus aquifolioides | Fagaceae | cpDNA | 58 | 36 | 11 | [42] |
| Entire TP | ||||||
| Potentilla fruticosa | Rosaceae | cpDNA | 52 | 54 | 17 | [22] |
| Allium przewalskianum | Liliaceae | cpDNA | 48 | 32 | 13 | [23] |
| Sibiraea angustata | Rosaceae | cpDNA | 38 | 8 | 6 | [43] |
| Hippuris vulgaris | Hippuridaceae | cpDNA | 47 | 8 | 4 | [44] |
| Ranunculus bungei | Ranunculaceae | cpDNA | 44 | 6 | 4 | [45] |
| Pomatosace filicula | Primulaceae | cpDNA | 24 | 7 | 5 | [46] |
| Orinus thoroldii | Poaceae | cpDNA | 28 | 7 | 5 | [47] |
| Rosa sericea | Rosaceae | cpDNA | 61 | 37 | 18 | [27] |
| Stuckenia filiformis | Potamogetonaceae | cpDNA | 54 | 13 | 5 | [48] |
| Anisodus tanguticus | Solanaceae | cpDNA | 32 | 6 | 4 | [28] |
Modelling species distribution
We applied the available bioclimatic and occurrence data to model the species distribution. Based on the WorldClim database (http://www.worldclim.org/) [49], we obtained 19 bioclimatic variables (S1 Table) with a resolution of 30 s (the data from the LGM were resampled to 30 s) for the Last Interglacial (LIG, c. 140–120 ka), the Last Glacial Maximum (LGM, c. 22 ka) and the Mid Holocene (c. 6 ka) based on the Community Climate System Model (CCSM4), and the current conditions (c. 1960–1990). We then clipped these variables according to the approximate species range in ArcGIS 10.1 (Environmental Systems Research Institute, Inc., Redlands, CA, USA). Next, to eliminate highly correlated factors, we carried out an autocorrelation test for 19 clipped variables using the SDMtoolbox v1.1c [50] in ArcGIS 10.1 (ESRI). Consequently, several bioclimatic variables of the four periods with low Spearman’s coefficients (r < 0.75) were retained for subsequent analysis.
The species occurrence data (S2 Table) were mainly collected from published literatures and further supplemented with data from field surveys and online database, including the Global Biodiversity Information Facility (GBIF, http://data.gbif.org) and the Chinese Virtual Herbarium (CVH, www.cvh.org.cn). As spatial clusters of localities in the gathered occurrence data may result in model’s inflation and over-fit towards environmental biases [51–53], we applied a spatially rarefy method to filter the occurrence data [53]. Based on the climatic heterogeneity, localities of occurrence were spatially filtered at 5, 15 and 25 km2 in areas of high, medium and low climatic heterogeneity, respectively. To calculate climatic heterogeneity, we conducted a principal component (PC) analysis of 19 bioclimatic variables and then calculated the mean standard deviation of the first three climate PCs. These analyses were implemented using the SDMtoolbox v1.1c [50]. Finally, the remaining localities combined with low-correlated bioclimatic layers were used to estimate species distribution.
In this study, we used the maximum entropy algorithm in MAXENT v3.3 [9] to model the current and past distributions of 20 species with a convergence threshold of 10−5, 500 iterations, and the localities of occurrence divided into testing data sets and training data sets (25% and 75%, respectively). We then conducted simple statistics on data distribution of range dynamics for each species and different regions in R 3.2.5 [54].
Visualizing the dispersal routes
We identified the dispersal routes of plant species on the TP under the least-cost path (LCP) method using the SDMtoolbox v1.1c [50] in ArcGIS 10.1 (ESRI). (i) We first obtained a dispersal cost layer (resistance layer) by inverting the species distribution layer (1-SDM) and then created a cost distance raster for each sample locality based on the resistance layer. (ii) Using the cost distance raster, we obtained the corridor layers between two localities with shared haplotype. (iii) In contrast to previous studies [12, 55], which generally obtained linear dispersal routes, we applied the categorical LCP method to better represent environmental heterogeneity in dispersal. We classified the value of each corridor layer into four intervals (three cutoff values); if the lowest value of corridor layer was hypothesized to be 1.00, the four intervals were: 1.00 ~ 1.01 (1.00 + 1.00*1%), 1.01 ~ 1.02 (1.00 + 1.00*2%), 1.02 ~ 1.05 (1.00 + 1.00*5%) and 1.05 ~ highest value. These four intervals were then reclassified as new values (5, 2, 1, 0, respectively). (iv) We summed all of the pairwise reclassified corridor layers, and the spatial dispersal routes for each plant were obtained. Finally, based on the routes of 20 species, we identified the possible recolonization pathways of species on the TP during the Quaternary.
Results
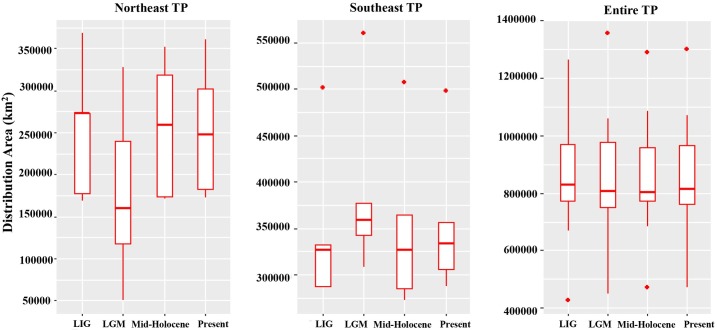
During the late Quaternary, plant species in three regions of the TP had distinct range dynamics (Fig 2, S2 Fig). For the species in the northeastern part, they mainly presented contraction-expansion scenario before and after the LGM. In contrast, the species in the southeastern TP underwent surprising range expansion during the LGM and then contracted to some extent. For the widespread species across the TP, their distribution exhibited almost no change since the LIG.
Fig 2. Boxplots of distribution area of plant species in three regions of the TP over four time periods.
Three types of range changes were shown: 1) ‘contraction–expansion (northeast)’, 2) ‘expansion–contraction (southeast)’ and 3) ‘stable (entire)’. LIG, the Last Interglacial; LGM, the Last Glacial Maximum.
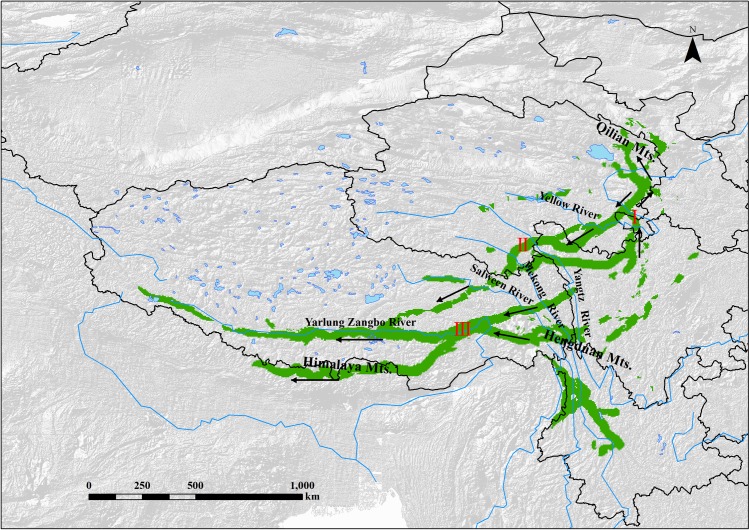
Phylogeographical studies have suggested that at least 20 plant species on the TP experienced recolonization during the Quaternary. To date, we have identified the dispersal routes of each plant (S3 Fig) and predicted several major recolonization paths for species on the TP (Fig 3). These dispersal routes are also supported by fossil data and other evidence (summarized in Table 2). We found that at least 3 dispersal corridors existed, and the Hengduan Mountains (HM) seemed to act as the main dispersal source (i.e. glaciation refugia). These paths included: i) the northward path from the northern part of the HM to the Qilian Mountains; ii) the westward path, in which species started in the HM region and recolonized along the Yarlung Zangbo River or Himalaya Mountains; and iii) the southward path, which went from the northeastern part to the southern part throughout the majority of TP’s platform, converged with the Yarlung Zangbo River, and moved further into the vast western region of the TP.
Fig 3. The possible Quaternary dispersal routes of plant species on the Tibetan Plateau.
Three pathways were shown: i) the northward route, ii) the southward route, and iii) the westward route. Arrows represent the dispersal direction of species.
Table 2. Fossil and other evidence for supporting the existence of dispersal routes.
| Path | Description | Evidence | Source |
|---|---|---|---|
|
Westward path (including the Yarlung Zangbo Valley and the Himilaya) |
Several oryctocoenoses were found in Namling County and Gyirong Basin; | Fossil | [56, 57] |
| The radiation of Tripterospermum, Gentiana and Adoxaceae from the Hengduan Mountains to the western TP; | Phylogenetic analysis | [34, 58, 59] | |
| Several species underwent extensive recolonization events from the Hengduan Mountains to Himalaya; | Phylogeographic analysis | [11, 60] | |
| The great canyon of Yarlung Zangbo River has abundant plants, which is a young floristic region originated from the East Asia flora since the Tertiary; | Floristic analysis | [61] | |
| The species Pedicularis longiflora and Tsuga dumosa underwent westward dispersal along this path; | Least-cost path analysis | [16, 62] | |
| The seed dispersal of Sophora moorcroftiana relied on the wind and waterflow in the midstream of Yarlung Zangbo River. | Seed dispersal experiment | [63] | |
|
Northward path (including the Hehuang Valley and the Qilian Mountains) |
The alternation of grassland and forest in the northeastern TP during the late Quaternary; | Vegetation reconstruction based on pollen | [64, 65] |
| The species Juniperus przewalskii and Picea crassifolia underwent invasion of platform after the last glaciation. | Phylogeographic analysis | [25, 26] | |
|
Southward path |
The species Potentilla fruticosa and Allium przewalskianum underwent extensive recolonization events from the northeastern to the southern TP. | Phylogeographic analysis | [22, 23, 34] |
Discussion
Range dynamics of plants on the TP during the late Quaternary
Within the European and North American continents, contraction-expansion in species distribution was generally predominant [66, 67], owing to the existence of a large and complete ice sheet during the LGM. However, there was no such formation of an ice sheet on the TP [21], and our analysis suggests that the range dynamics of species on the TP were complex during the late Quaternary. It might be argued that these distinct shifts in distribution were associated with the heterogeneous climate and topography across the TP.
In the northeastern TP, the tabula rasa scenario seemed to be dominant, which is consistent with previous phylogeographical studies and pollen fossil records [25, 26, 64]. In this region, the climate is colder and drier than that of the southern TP, and the platform is basically flat; only the eastern part has a series of mountain ranges (e.g. the Qilian Mountains and the Minshan Mountains in the northern HM). During the LGM, according to the rare palaeovegetation on the platform and the climatic characteristics [68, 69], we assumed that platform populations of species became extinct and only marginal populations survived within the eastern mountains. Subsequently, when the warmer and wetter Holocene period came, forest and alpine meadow recolonized on the platform [64]. Therefore, the alternation of vegetation on the platform suggests that the LGM might have had greater effects on species distribution in this region.
In the southeastern TP, the species exhibited an expansion-contraction response during the late Quaternary. We assumed that this response was closely related to regional topographic and climatic features. This region is mainly located within the East Himalaya and Hengduan Mountains, which provide ample altitudinal range (e.g. niche) and stable ecological environment [70]. On the other hand, the Indian monsoon produces a warmer and wetter climate compared to other regions of the QTP [31, 71]. The pollen data indicate that the late Quaternary was characterized by a warm temperate climate [72], and phylogeographical studies suggest that this region acted as the major glaciation refugia for many alpine species on the TP [18, 73]. Hence, during the LGM, suitable habitats could have facilitated the persistence of populations in low-elevation areas, and populations on the top of mountains could have moved downward and expanded into other areas because of the abundant niche in this region. In contrast, during the LIG and Holocene period, the warmer and wetter climate would have driven alpine species upward, resulting in their limited distribution in an isolated area.
For the widespread species on the TP, as discussed above, during the late Quaternary, the populations in the northeastern platform may have contracted to refugia, whereas the populations in the southeastern part may have expanded in range. For the populations in the central and southern TP’s platform, a lot of evidence suggests that the LGM had a limited influence on species distribution of the platform [22, 56]. In addition, there might be several microrefugia (e.g. the Three Rivers’ Headstream Regions) within these platform areas [74, 75]. Therefore, as a whole, widely distributed species maintained stable populations throughout the LGM.
Dispersal routes of plants on the TP
Phylogeographical studies have been able to test which species experienced rapid and extensive range expansion. In this study, using the LCP method, in combination with SDMs and shared haplotypes, we have identified the possible dispersal routes of Tibetan plant species during the Quaternary. Undoubtedly, determining the historical dispersal routes is important to supplement the demographic history of species on the TP.
In this study, we identified three common pathways across the TP. Among the initial locations of these routes, the East Himalaya-Hengduan Mountains seemed to be the dispersal source, which may be associated with this region’s role as the major glaciation refugia [18, 73]. According to historical biogeographic studies, this place was also an important center of origin and radiation of alpine plants, triggering numerous species to migrate into other areas from this region in the Neogene or earlier period [34, 58]. Surprisingly, the migration routes estimated in those studies partially overlapped with the paths during the Quaternary. Thus, it might be argued that species on the TP tend to disperse along several constant routes. We assumed that this could be related to the topographic and monsoon climatic features of the TP. The northward and westward routes identified in this study were basically along the Hehuang Valley and Yarlung Zangbo Valley. Why did species migrate along these valleys? These valleys are not only lower elevation areas (average elevation < 4000 m), but also important vapor channels; in particular, the Yarlung Zangbo Valley is the largest vapor channel of the TP [30]. Hence, with lower topography and strong airflow, these valleys are likely to facilitate the dispersal of seeds and pollen. Additionally, many fossils found in these valleys along with phylogenetic and floristic analysis also support the existence of these corridors (see Table 2). Apart from these two valleys, we predicted that the Yangtze River, Mekong River and Salween River might also be important dispersal pathways. Even not obviously identified in our study, other evidence implied that alpine species would migrate along these valleys during the glacial period [10, 11].
Mountain ridges are another important dispersal corridors, like the Himalaya Mountains in this study, which is a very long-narrow mountainous area. Evidence has shown that the Quaternary glaciations likely had a great impact on plants in this region, resulting in many species becoming extinct [60]. However, because the Himalaya is linked with the Hengduan Mountains, some species could have easily moved to the Himalaya during the postglacial period. In other montane areas around the world, huge mountains (e.g. the Andes) are also regarded as important dispersal corridors [76].
We found that the TP’s platform (average elevation > 4500 m), which has flatter topography, could facilitate long distance dispersal of alpine species, like the southward path in this study. Despite the existence of a series of east-westward mountains across the platform, their relative elevation is actually not so high. Through Barrier analysis, we have also demonstrated that these mountains are not acted as geographical isolations (unpublished data). Thus, several drought-tolerant species (e.g. Potentilla fruticosa and Allium przewalskianum), originating in the northern TP or adjacent desert (e.g. the Tengger Desert), could migrate into the southern Tibet across the entire TP [22, 23].
Conclusions
The plant species on the TP exhibited diverse demographic histories during the late Quaternary. There were at least three types of changes in range. Despite the detailed range shifts are distinct across different parts of the TP, they actually had no significant difference, and as a whole, they remained relatively stable status in response to the last glacial, suggesting that LGM had a limited impact on species distribution. In fact, an earlier glaciation (the Naynayxungla glaciation, 0.72–0.50 Ma) might have had a significant influence. After this glaciation, species were continuously dispersed along the low-elevation valleys (e.g. the Yarlung Zangbo Valley and Hehuang Valley), huge mountain ranges (e.g. the Himalaya) or even the flat plateau platform. Under future climate change, the species on the TP could continue to move along these pathways, and thereby these areas should be paid more attention. Besides, the inferences in this study are just based on 20 species, we believe that more diverse range dynamics and dispersal corridors would be found if many species are involved in future studies.
Supporting information
(DOCX)
(DOCX)
The haplotype compositions of populations for each species were originated form phylogeographical studies (see Table 1). Pie chart corresponds to the sample locations of each species and the diverse colors from green to red are attributed to different chlorotypes or mitotypes.
(RAR)
LIG, the Last Interglacial; LGM, the Last Glacial Maximum.
(RAR)
Warm colors represent higher population connectivity.
(RAR)
Acknowledgments
The authors appreciate two anonymous reviewers for their insightful suggestions, which made the paper much better. We are also very grateful for the guidance of Dr. Jason L. Brown in Southern Illinois University and Dr. Xinghua Sui in Sun Yat-sen University.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Open Foundation of Key Laboratory of Land Surface Pattern and Simulation (CAS), the Strategic Priority Research Program of the Chinese Academy of Sciences (No.XDB03030500) and the National Natural Science Foundation of China (No. 41271068, No.41371120).
References
- 1.Avise JC. Phylogeography: the history and formation of species. Cambridge: Harvard University Press; 2000. [Google Scholar]
- 2.Schönswetter P, Stehlik I, Holderegger R, Tribsch A. Molecular evidence for glacial refugia of mountain plants in the European Alps Mol Ecol. 2005; 14: 3547–3555. doi: 10.1111/j.1365-294X.2005.02683.x [DOI] [PubMed] [Google Scholar]
- 3.Hampe A, Arroyo J, Jordano P, Petit RJ. Rangewide phylogeography of a bird-dispersed Eurasian shrub: contrasting Mediterranean and temperate glacial refugia. Mol Ecol. 2003; 12: 3415–3426. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt G. The genetic legacy of the Quaternary ice ages. Nature. 2000; 405: 907–913. doi: 10.1038/35016000 [DOI] [PubMed] [Google Scholar]
- 5.Hewitt G. Genetic consequences of climatic oscillations in the Quaternary. Philos T R Soc B. 2004; 359: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989; 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers AR, Harpending H. Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol. 1992; 9: 552–569. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997; 147: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006; 190: 231–259. [Google Scholar]
- 10.Li L, Abbott RJ, Liu B, Sun Y, Li L, Zou J, et al. Pliocene intraspecific divergence and Plio-Pleistocene range expansions within Picea likiangensis (Lijiang spruce), a dominant forest tree of the Qinghai-Tibet Plateau. Mol Ecol. 2013; 22: 5237–5255. doi: 10.1111/mec.12466 [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Moeller M, Provan J, Gao L, Poudel RC, Li D. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytol. 2013; 199: 1093–1108. doi: 10.1111/nph.12336 [DOI] [PubMed] [Google Scholar]
- 12.Wang IJ, Savage WK, Bradley Shaffer H. Landscape genetics and least-cost path analysis reveal unexpected dispersal routes in the California tiger salamander (Ambystoma californiense). Mol Ecol. 2009; 18: 1365–1374. doi: 10.1111/j.1365-294X.2009.04122.x [DOI] [PubMed] [Google Scholar]
- 13.Li H, Li D, Li T, Qiao Q, Yang J, Zhang H. Application of least-cost path model to identify a giant panda dispersal corridor network after the Wenchuan earthquake-Case study of Wolong Nature Reserve in China. Ecol Model. 2010; 221: 944–952. [Google Scholar]
- 14.Etherington TR, Holland EP. Least-cost path length versus accumulated-cost as connectivity measures. Landscape Ecol. 2013; 28: 1223–1229. [Google Scholar]
- 15.Chan LM, Brown JL, Yoder AD. Integrating statistical genetic and geospatial methods brings new power to phylogeography. Mol Phylogenet Evol. 2011; 59: 523–537. doi: 10.1016/j.ympev.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 16.Yu H, Zhang Y, Liu L, Qi W, Li S, Hu Z. Combining the least cost path method with population genetic data and species distribution models to identify landscape connectivity during the late Quaternary in Himalayan hemlock. Ecol Evol. 2015; 5: 5781–5791. doi: 10.1002/ece3.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Y, Fu C, Comes HP. Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol Phylogenet Evol. 2011; 59: 225–244. doi: 10.1016/j.ympev.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Duan Y, Hao G, Ge X, Sun H. Evolutionary history and underlying adaptation of alpine plants on the Qinghai-Tibet Plateau. J Syst Evol. 2014; 52: 241–249. [Google Scholar]
- 19.Wen J, Zhang J, Nie Z, Zhong Y, Sun H. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau. Front Genet. 2014; 5: 4 doi: 10.3389/fgene.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favre A, Päckert M, Pauls SU, Jähnig SC, Uhl D, Michalak I, et al. The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biol Rev. 2015; 90: 236–253. doi: 10.1111/brv.12107 [DOI] [PubMed] [Google Scholar]
- 21.Li B, Li J. Map of Quaternary glacier on the Qinghai-Xizang (Tibet) Plateau. Beijing: Science Press; 1991. [Google Scholar]
- 22.Sun Y, Ikeda H, Wang Y, Liu J. Phylogeography of Potentilla fruticosa (Rosaceae) in the Qinghai-Tibetan Plateau revisited: a reappraisal and new insights. Plant Ecol Divers. 2010; 3: 249–257. [Google Scholar]
- 23.Wu L, Cui X, Milne RI, Sun Y, Liu J. Multiple autopolyploidizations and range expansion of Allium przewalskianum Regel. (Alliaceae) in the Qinghai-Tibetan Plateau. Mol Ecol. 2010; 19: 1691–1704. doi: 10.1111/j.1365-294X.2010.04613.x [DOI] [PubMed] [Google Scholar]
- 24.Opgenoorth L, Vendramin GG, Mao K, Miehe G, Miehe S, Liepelt S, Liu J, Ziegenhagen B. Tree endurance on the Tibetan Plateau marks the world’s highest known tree line of the Last Glacial Maximum. New Phytol. 2010; 185: 332–342. doi: 10.1111/j.1469-8137.2009.03007.x [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Chiang T, George M, Liu J, Abbott RJ. Phylogeography of the Qinghai-Tibetan Plateau endemic Juniperus przewalskii (Cupressaceae) inferred from chloroplast DNA sequence variation. Mol Ecol. 2005; 14: 3513–3524. doi: 10.1111/j.1365-294X.2005.02677.x [DOI] [PubMed] [Google Scholar]
- 26.Meng L, Yang R, Abbott RJ, Miehe G, Hu T, Liu J. Mitochondrial and chloroplast phylogeography of Picea crassifolia Kom. (Pinaceae) in the Qinghai-Tibetan Plateau and adjacent highlands. Mol Ecol. 2007; 16: 4128–4137. doi: 10.1111/j.1365-294X.2007.03459.x [DOI] [PubMed] [Google Scholar]
- 27.Gao Y, Zhang Y, Gao X, Zhu Z. Pleistocene glaciations, demographic expansion and subsequent isolation promoted morphological heterogeneity: A phylogeographic study of the alpine Rosa sericea complex (Rosaceae). Sci Rep-UK. 2015; 5: 11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan D, Feng J, Jiang D, Mao K, Duan Y, Miehe G, et al. The Quaternary evolutionary history, potential distribution dynamics, and conservation implications for a Qinghai-Tibet Plateau endemic herbaceous perennial, Anisodus tanguticus (Solanaceae). Ecol Evol. 2016; 7: 1977–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Li B, Zheng D. A discussion on the boundary and area of the Tibetan Plateau in China. Geogr Res. 2002; 21: 1–8. [Google Scholar]
- 30.Lin Z, Wu X. A preliminary analysis about the tracks of moisture transportation on the Qinghai-Xizang Plateau. Geogr Res. 1990; 9: 33–40. [Google Scholar]
- 31.Yao T, Thompson L, Yang W, Yu W, Gao Y, Guo X, et al. Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nat Clim Change. 2012; 2: 663–667. [Google Scholar]
- 32.Zhang D, Ye J, Sun H. Quantitative approaches to identify floristic units and centres of species endemism in the Qinghai-Tibetan Plateau, south-western China. J Biogeogr. 2016; 43: 2465–2476. [Google Scholar]
- 33.Wu Z. The flora of Tibet. Beijing: Science Press; 1988. [Google Scholar]
- 34.Matuszak S, Muellner Riehl AN, Sun H, Favre A. Dispersal routes between biodiversity hotspots in Asia: the case of the mountain genus Tripterospermum (Gentianinae, Gentianaceae) and its close relatives. J Biogeogr. 2016; 43: 580–590. [Google Scholar]
- 35.Zhang J, Meng S, Rao G. Phylogeography of Rhodiola kirilowii (Crassulaceae): A Story of Miocene Divergence and Quaternary Expansion. PLoS ONE. 2014; 9: e112923 doi: 10.1371/journal.pone.0112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S, Wu G, Zhang D, Gao Q, Duan Y, Zhang F, et al. Potential refugium on the Qinghai-Tibet Plateau revealed by the chloroplast DNA phylogeography of the alpine species Metagentiana striata (Gentianaceae). Bot J Linn Soc. 2008; 157: 125–140. [Google Scholar]
- 37.Zhang X, He X. Phylogeography of Angelica nitida (Apiaceae) endemic to the Qinghai-Tibet Plateau based on chloroplast DNA sequences. J Syst Evol. 2013; 51: 564–577. [Google Scholar]
- 38.Zhao C, Ma X, Liang Q, Wang C, He X. Phylogeography of an alpine plant (Bupleurum smithii, Apiaceae) endemic to the Qinghai-Tibetan Plateau and adjacent regions inferred from chloroplast DNA sequence variation. J Syst Evol. 2013; 51: 382–395. [Google Scholar]
- 39.Yue L, Chen G, Sun W, Sun H. Phylogeography of Buddleja crispa (Buddlejaceae) and its correlation with drainage system evolution in southwestern China. Am J Bot. 2012; 99: 1726–1735. doi: 10.3732/ajb.1100506 [DOI] [PubMed] [Google Scholar]
- 40.Zou J, Peng X, Li L, Liu J, Miehe G, Opgenoorth L. Molecular phylogeography and evolutionary history of Picea likiangensis in the Qinghai-Tibetan Plateau inferred from mitochondrial and chloroplast DNA sequence variation. J Syst Evol. 2012; 50: 341–350. [Google Scholar]
- 41.Yue L, Sun H. Montane refugia isolation and plateau population expansion: Phylogeography of Sino-Himalayan endemic Spenceria ramalana (Rosaceae). J Syst Evol. 2014; 52: 326–340. [Google Scholar]
- 42.Du F, Hou M, Wang W, Mao K, Hampe A. Phylogeography of Quercus aquifolioides provides novel insights into the Neogene history of a major global hotspot of plant diversity in south-west China. J Biogeogr. 2017; 44: 294–307. [Google Scholar]
- 43.Duan Y, Gao Q, Zhang F, Li Y, Fu P, Chen S. Phylogeographic analysis of the endemic species Sibiraea angustata reveals a marginal refugium in the Qinghai-Tibet Plateau. Nord J Bot. 2011; 29: 615–624. [Google Scholar]
- 44.Chen J, Du Z, Sun S, Gituru RW, Wang Q. Chloroplast DNA phylogeography reveals repeated range expansion in a widespread aquatic herb Hippuris vulgaris in the Qinghai-Tibetan Plateau and adjacent areas. PLoS ONE. 2013; 8: e60948 doi: 10.1371/journal.pone.0060948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Du Z, Yuan Y, Wang Q. Phylogeography of an alpine aquatic herb Ranunculus bungei (Ranunculaceae) on the Qinghai-Tibet Plateau. J Syst Evol. 2014; 52: 313–325. [Google Scholar]
- 46.Wang G, He X, Miehe G, Mao K. Phylogeography of the Qinghai-Tibet Plateau endemic alpine herb Pomatosace filicula (Primulaceae). J Syst Evol. 2014; 52: 289–302. [Google Scholar]
- 47.Liu Y, Su X, He Y, Han L, Huang Y, Wang Z. Evolutionary history of Orinus thoroldii (Poaceae), endemic to the western Qinghai-Tibetan Plateau in China. Biochem Syst Ecol. 2015; 59: 159–167. [Google Scholar]
- 48.Du Z, Wang Q. Allopatric divergence of Stuckenia filiformis (Potamogetonaceae) on the Qinghai-Tibet Plateau and its comparative phylogeography with S. pectinata in China. Sci Rep-UK. 2016; 6: 20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005; 25: 1965–1978. [Google Scholar]
- 50.Brown JL. SDMtoolbox: a python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol Evol. 2014; 5: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veloz SD. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J Biogeogr. 2009; 36: 2290–2299. [Google Scholar]
- 52.Hijmans RJ. Cross-validation of species distribution models: removing spatial sorting bias and calibration with a null model. Ecology. 2012; 93: 679–688. [DOI] [PubMed] [Google Scholar]
- 53.Boria RA, Olson LE, Goodman SM, Anderson RP. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol Model. 2014; 275: 73–77. [Google Scholar]
- 54.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2016; https://www.R-project.org/. [Google Scholar]
- 55.Vignieri SN. Streams over mountains: influence of riparian connectivity on gene flow in the Pacific jumping mouse (Zapus trinotatus). Mol Ecol. 2005; 14:1925–1937. doi: 10.1111/j.1365-294X.2005.02568.x [DOI] [PubMed] [Google Scholar]
- 56.Shi Y, Li J, Li B. Uplift and environmental changes of Qinghai-Xizang (Tibetan) in the Late Cenozoic. Guangzhou: Guangdong Science and Technology Press; 1998. [Google Scholar]
- 57.Song Z, Liu G. Early Tertiary palynofloral and its significance of palaeogeography from northern and eastern Xizang. Beijing: Science Press; 1982. [Google Scholar]
- 58.Ho T, Liu S. A worldwide monograph of Gentiana. Beijing: Science Press; 2001. [Google Scholar]
- 59.Liang H, Wu Z. On the Taxonomic system, phylogeny and distribution in Adoxaceae. Acta Bot Yunn. 1995; 17: 380–390. [Google Scholar]
- 60.Cun Y, Wang X. Plant recolonization in the Himalaya from the southeastern Qinghai-Tibetan Plateau: Geographical isolation contributed to high population differentiation. Mol Phylogenet Evol. 2010; 56: 972–982. doi: 10.1016/j.ympev.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 61.Sun H, Zhou Z. The characters and origin of the flora from the big bend gorge of Yalutsangpu (Brahmabutra) River, eastern Himalayas. Acta Bot Yunn. 1996; 18: 185–204. [Google Scholar]
- 62.Yu H, Zhang Y, Li S, Qi W. Predicting the dispersal routes of alpine plant Pedicularis longiflora (Orobanchaceae) based on GIS and species distribution models. Chin J Appl Ecol. 2014; 25: 1669–1673. [PubMed] [Google Scholar]
- 63.Liu Z, Zhao W, Li Z. Characteristics of the seed bank of Sophora moorcroftiana population in the middle reach of Yarlung Zangbo River, Tibet. Acta Ecol Sin. 2001; 22: 715–722. [Google Scholar]
- 64.Tang L, Shen C, Kong Z, Wang F, Liu K. Pollen evidence of climate during the Last Glacial Maximum in eastern Tibetan plateau. J Glaciol Geocryol. 1998; 20: 133–140. [Google Scholar]
- 65.Wan H, Tang L, Zhang H, Li C, Pang Y. Pollen record reflects climate changes in eastern Qaidam Basin during 36–18 ka B.P. Quat Sci. 2008; 28: 112–121. [Google Scholar]
- 66.Taberlet P, Fumagalli L, Wust Saucy AG, Cosson JF. Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol. 1998; 7: 453–464. [DOI] [PubMed] [Google Scholar]
- 67.Soltis DE, Morris AB, McLachlan JS, Manos PS, Soltis PS. Comparative phylogeography of unglaciated eastern North America. Mol Ecol. 2006; 15: 4261–4293. doi: 10.1111/j.1365-294X.2006.03061.x [DOI] [PubMed] [Google Scholar]
- 68.Tang L, Shen C. Late Cenozoic vegetational history and climatic characteristics of Qinghai-Xizang Plateau. Acta Micropalaeontol Sin. 1995; 13: 321–338. [Google Scholar]
- 69.Yu G, Chen X, Ni J, Cheddadi R, Guiot J, Han H, et al. Palaeovegetation of China: a pollen data-based synthesis for the mid-Holocene and last glacial maximum. J Biogeogr. 2000; 27: 635–664. [Google Scholar]
- 70.Yan Y, Yang X, Tang Z. Patterns of species diversity and phylogenetic structure of vascular plants on the Qinghai-Tibetan Plateau. Ecol Evol. 2013; 3: 4584–4595. doi: 10.1002/ece3.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.An Z, Kutzbach JE, Prell WL, Porter SC. Evolution of Asian monsoons and phased uplift of the Himalaya-Tibetan plateau since Late Miocene times. Nature. 2001; 411: 62–66. doi: 10.1038/35075035 [DOI] [PubMed] [Google Scholar]
- 72.Harrison SP, Yu G, Takahara H, Prentice IC. Palaeovegetation (Communications arising): diversity of temperate plants in east Asia. Nature. 2001; 413: 129–130. doi: 10.1038/35093166 [DOI] [PubMed] [Google Scholar]
- 73.Yu H, Zhang Y. Advances in phylogeography of alpine plants in the Tibetan Plateau and adjacent regions. Acta Bot Boreali-Occident Sin. 2013; 33: 1268–1278. [Google Scholar]
- 74.Gao Q, Zhang D, Duan Y, Zhang F, Li Y, Fu P, et al. Intraspecific divergences of Rhodiola alsia (Crassulaceae) based on plastid DNA and internal transcribed spacer fragments. Bot J Linn Soc. 2012; 168: 204–215. [Google Scholar]
- 75.Zhang F, Gao Q, Zhang D, Duan Y, Li Y, Fu P, et al. Phylogeography of Spiraea alpina (Rosaceae) in the Qinghai-Tibetan Plateau inferred from chloroplast DNA sequence variations. J Syst Evol. 2012; 50: 276–283. [Google Scholar]
- 76.Luebert F, Weigend M. Phylogenetic insights into Andean plant diversification. Front Ecol Evol. 2014; 2: 27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
The haplotype compositions of populations for each species were originated form phylogeographical studies (see Table 1). Pie chart corresponds to the sample locations of each species and the diverse colors from green to red are attributed to different chlorotypes or mitotypes.
(RAR)
LIG, the Last Interglacial; LGM, the Last Glacial Maximum.
(RAR)
Warm colors represent higher population connectivity.
(RAR)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.