Abstract
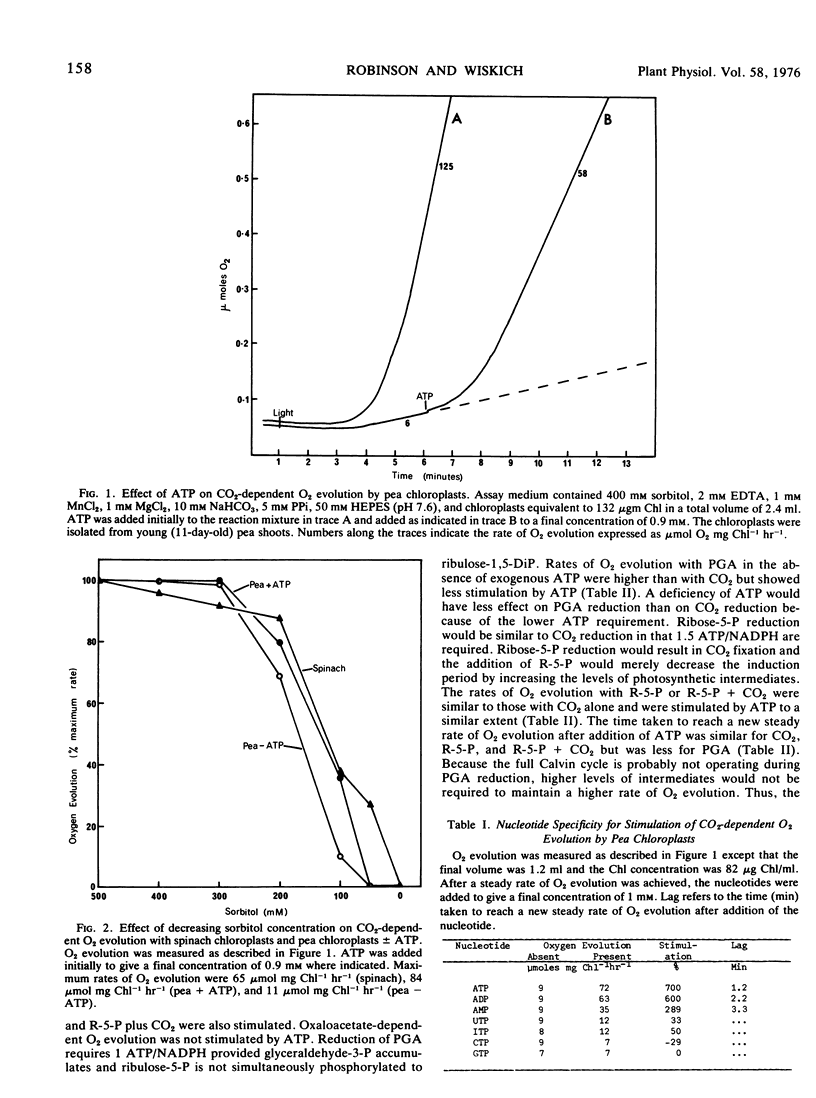
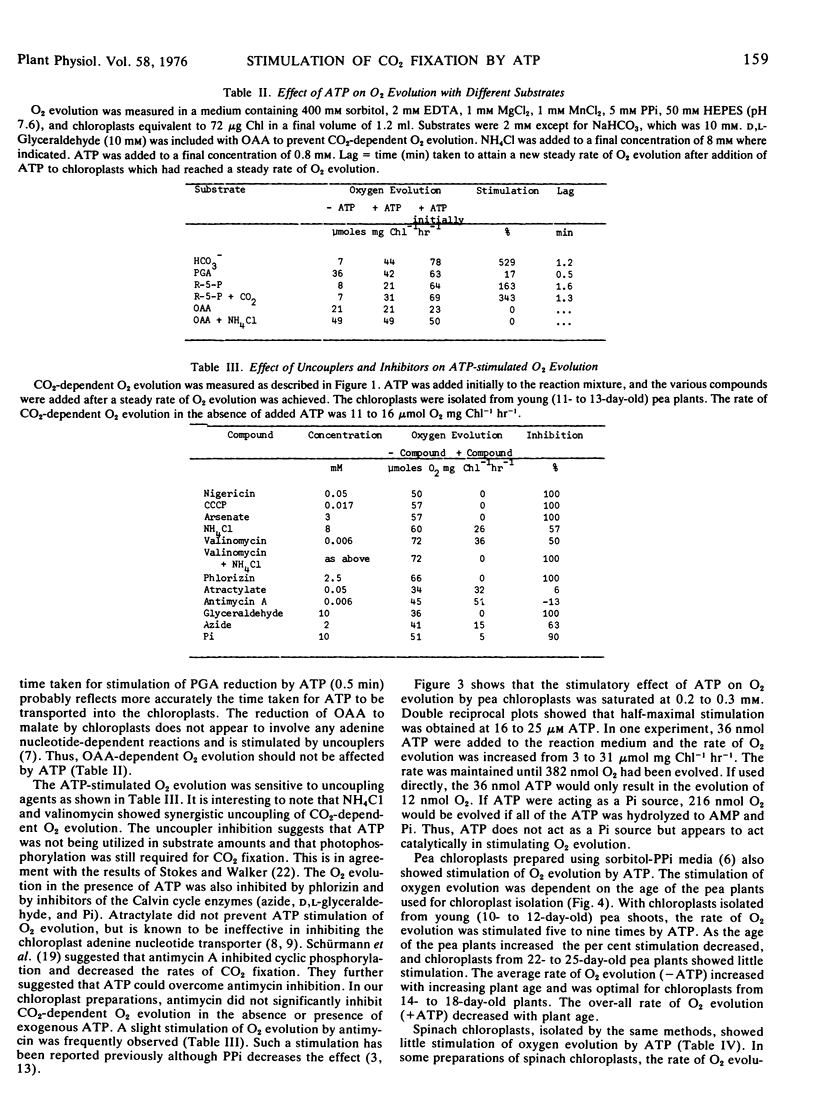
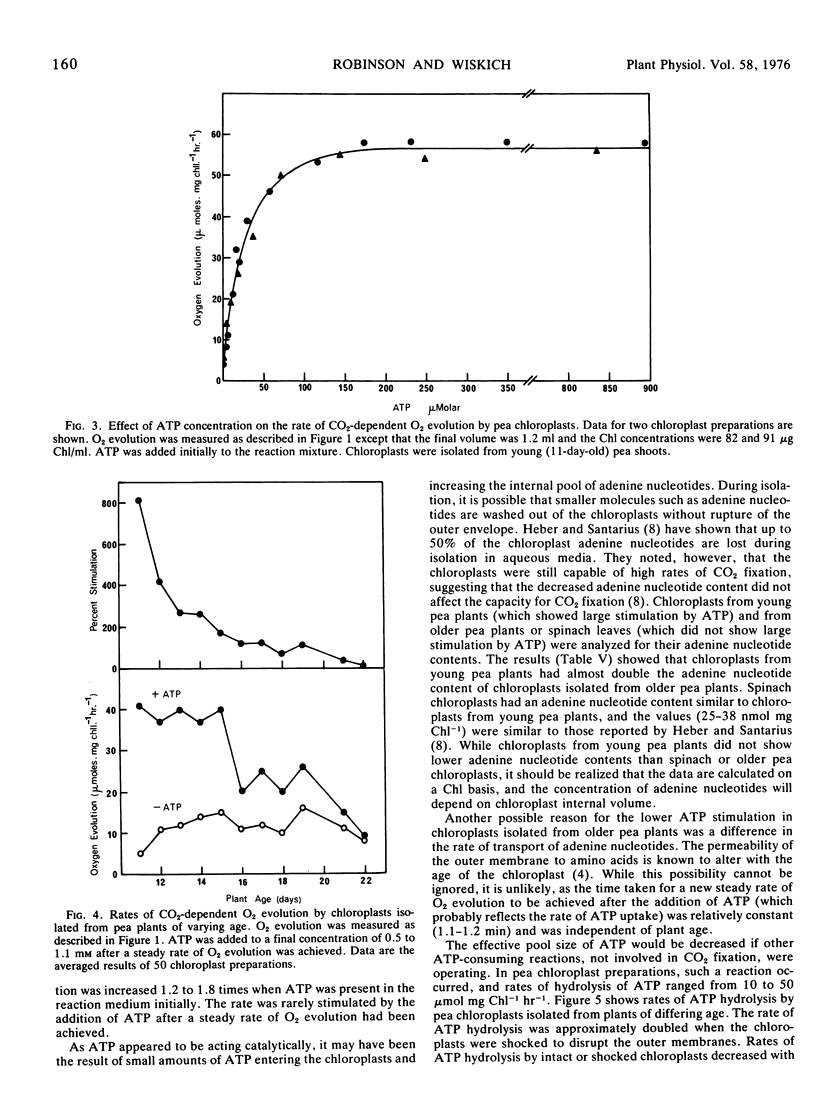
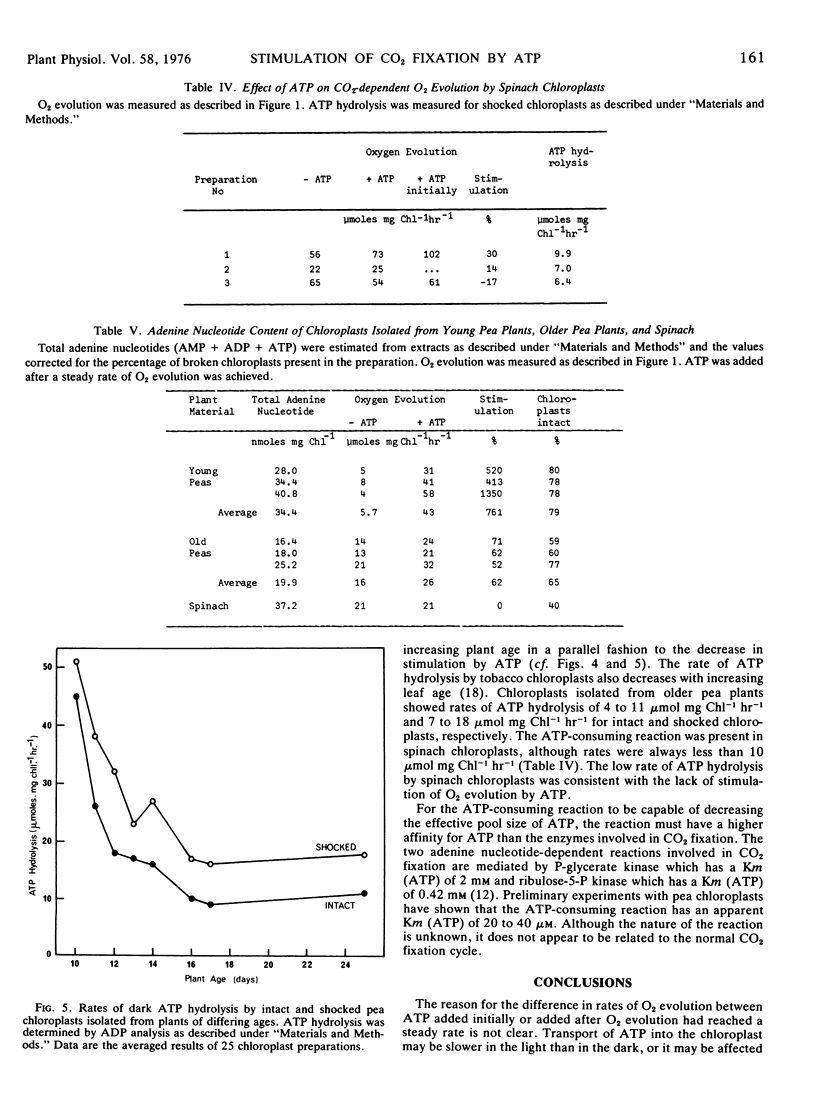
Carbon dioxide-dependent O2 evolution by isolated pea (Pisum sativum var. Massey Gem) chloroplasts was increased two to 12 times by the addition of ATP. O2 evolution was also stimulated by ADP and to a lesser extent by AMP. The ATP effects were not due to broken chloroplasts present in the preparations nor was ATP acting as a phosphate source. We concluded that the adenine nucleotides were acting catalytically. The concentration of ATP required for half-maximum rate of O2 evolution was 16 to 25 μm. The degree to which ATP stimulated O2 evolution depended on the age of pea plants from which the chloroplasts were isolated. Spinach (Spinacia oleracea var. True Hybrid 102) chloroplasts did not show a consistent stimulation of O2 evolution by adenine nucleotides.
The adenine nucleotide content of pea chloroplasts was not lower than that of spinach chloroplasts, but pea chloroplasts which showed a large stimulation of O2 evolution by ATP contained an ATP-hydrolyzing reaction with rates of 10 to 50 μmol ATP hydrolyzed mg chlorophyll−1 hour−1. The rate of the ATP-consuming reaction was much lower in spinach chloroplasts and in chloroplasts from older pea plants which did not show large stimulation of O2 evolution by ATP. We propose that the ATP-consuming reaction, with a high affinity for ATP, decreased the effective size of the ATP pool available for CO2 fixation. Added adenine nucleotides could be transported into the chloroplasts increasing the concentration of internal nucleotides. Calculations showed that the adenine nucleotide transporter on the outer chloroplast membranes could operate at a sufficient rate to produce such an effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champigny M. L., Miginiac-Maslow M. Relations entre l'assimilation photosynthétique de CO 2 et la photophosphorylation des chloroplastes isolés. I. Stimulation de la fixation de CO 2 par l'antimycine A, antagoniste de son inhibition par le phosphate. Biochim Biophys Acta. 1971 Jun 15;234(3):335–343. doi: 10.1016/0005-2728(71)90200-3. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Kirk M. R. Flexibility of coupling and stoichiometry of ATP formation in intact chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):136–150. doi: 10.1016/0005-2728(75)90212-1. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Unspecific permeation and specific uptake of substances in spinach chloroplasts. FEBS Lett. 1970 Apr 2;7(2):139–142. doi: 10.1016/0014-5793(70)80140-5. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. 3. Light activation of the carboxylation reaction. Biochim Biophys Acta. 1968 Jan 15;153(1):227–234. doi: 10.1016/0005-2728(68)90164-3. [DOI] [PubMed] [Google Scholar]
- Miginiac-Maslow M., Champigny M. L. Relationship between the Level of Adenine Nucleotides and the Carboxylation Activity of Illuminated Isolated Spinach Chloroplasts: A Study with Antimycin A. Plant Physiol. 1974 Jun;53(6):856–862. doi: 10.1104/pp.53.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavey D. G., Gibbs M. Photosynthetic enhancement studied in intact spinach chloroplasts. Plant Physiol. 1975 May;55(5):799–802. doi: 10.1104/pp.55.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Stocking C. R. Oxygen evolution and the permeability of the outer envelope of isolated whole chloroplasts. Plant Physiol. 1968 Oct;43(10):1597–1604. doi: 10.1104/pp.43.10.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Schwenn J. D., Lilley R. M., Walker D. A. Inorganic pyrophospatase and photosynthesis by isolated chloroplasts. I. Characterisation of chloroplast pyrophosphatase and its relation to the response to exogenous pyrophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):586–595. doi: 10.1016/0005-2728(73)90218-1. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Buchanan B. B., Arnon D. I. Role of cyclic photophosphorylation in photosynthetic carbon dioxide assimilation by isolated chloroplasts. Biochim Biophys Acta. 1972 Apr 20;267(1):111–124. doi: 10.1016/0005-2728(72)90143-0. [DOI] [PubMed] [Google Scholar]
- TREBST A. V., TSUJIMOTO H. Y., ARNON D. I. Separation of light and dark phases in the photosynthesis of isolated chloroplasts. Nature. 1958 Aug 9;182(4632):351–355. doi: 10.1038/182351a0. [DOI] [PubMed] [Google Scholar]
- Vose J. R., Spencer M. Effects of adenosine triphosphate and inorganic pyrophosphate on carbon dioxide fixation by isolated spinach chloroplasts. Can J Biochem. 1969 Apr;47(4):443–447. doi: 10.1139/o69-069. [DOI] [PubMed] [Google Scholar]
- Walker D. A. Correlation between Photosynthetic Activity and Membrane Integrity in Isolated Pea Chloroplasts. Plant Physiol. 1965 Nov;40(6):1157–1161. doi: 10.1104/pp.40.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Lilley R. M. Autocatalysis in a reconstituted chloroplast system. Plant Physiol. 1974 Dec;54(6):950–952. doi: 10.1104/pp.54.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]


