Abstract
The present work follows a previous report describing the antibacterial activity of silver camphorimine complexes of general formula [Ag(NO3)L]. The synthesis and demonstration of the antifungal and antibacterial activity of three novel [Ag(NO3)L] complexes (named 1, 2 and 3) is herein demonstrated. This work also shows for the first time that the previously studied complexes (named 4 to 8) also exert antifungal activity. The antibacterial activity of complexes was evaluated against Staphylococcus aureus, Pseudomonas aeruginosa, Burkholderia contaminans and Escherichia coli strains, while antifungal activity was tested against the Candida species C. albicans, C. glabrata, C. parapsilosis and C. tropicalis. The antimicrobial activity of the complexes ranged from very high (complex 4) to moderate (complex 6) or low (complex 8), depending on the structural and electronic characteristics of the camphorimine ligands. Notably, the highest antibacterial and anti-Candida activities do not coincide in the same complex and in some cases they were even opposite, as is the case of complex 4 which exhibits a high anti-bacterial and low antifungal activity. These distinct results suggest that the complexes may have different mechanisms against prokaryotic and eukaryotic cells. The antifungal activity of the Ag(I) camphorimine complexes (in particular of complex 1) was found to be very high (MIC = 2 μg/mL) against C. parapsilosis, being also registered a prominent activity against C. tropicalis and C. glabrata. None of the tested compounds inhibited C. albicans growth, being this attributed to the ability of these yeast cells to mediate the formation of less toxic Ag nanoparticles, as confirmed by Scanning Electron Microscopy images. The high antibacterial and anti-Candida activities of the here studied camphorimine complexes, especially of complexes 1 and 7, suggests a potential therapeutic application for these compounds.
Introduction
An increasing number of pathogenic bacterial and fungal strains resistant to existing antibiotics and antifungals have been isolated either from hospitals or the community. This situation is a threat for public health since existing pharmaceuticals become non-effective and in some cases useless to treat infections.
In the case of bacteria, the problem of resistance to antibiotics is particularly severe among members of the so-called ESKAPE group that includes Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacteriaceae [1]. In the case of fungi, the main concerns come from an increasing resistance of the Candida species (namely, C. albicans, C. glabrata, C. tropicalis and C. parapsilosis) to the clinically used antifungals [2,3]. Infections caused by the above-mentioned bacterial and fungal species are emerging and are responsible for high rates of mortality and morbidity, posing serious challenges to the sustainability of healthcare systems [4]. In this scenario, the identification of new compounds with efficacy against emerging pathogens for the development of more effective therapies [5] is a challenge to the academic community and an opportunity to the development of alternative products by the pharmaceutical industry. Silver nitrate and silver sulphadiazine have been efficiently used as components of balms to prevent infections on wounds or burns [6,7]. However, their therapeutic use as antimicrobials has been limited by toxicity at concentrations higher than 1% [8–10]. Albeit the toxicity has to be considered, the emergent potential of silver based species (compounds [11–13] or nanoparticles [14–18]) as antimicrobials challenge the design, synthesis, trial and assessment of the properties of new silver compounds against bacteria and fungi to explore their therapeutic and antiseptic properties (e.g. in water purification properties or on coating of implants surface [19–22]).
In the present study, we focused on the synthesis and evaluation of the antibacterial and antifungal properties of silver camphorimine complexes obtained from silver nitrate and camphor derivatives. The choice of camphor as a precursor for ligands to synthesize new complexes comes from the fact that such as silver nitrate camphor has a long history of pharmacological uses, e.g. as anesthetic, muscular relaxant or insect repellent, either the compound itself or as part of natural products [23–25]. Information on the antimicrobial applications of camphor are scarce, although studies showed that camphorimine derivatives have antiviral properties against HIV [26] or influenza virus [27]. In addition, previous results obtained by us on the assessment of the antibacterial activity of camphorimine Ag(I) complexes showed that some have MIC (minimum inhibitory concentration) values considerably lower than AgNO3 [28]. In this work we have investigated the activity of the three newly synthesized camphorimine Ag(I) complexes 1–3 against the ESKAPE members S. aureus, P. aeruginosa, and E. coli, the B. contaminans IST408 isolate from a Portuguese cystic fibrosis patient [29, 30]. We also report results on the antifungal activity of the Ag(I) camphorimine complexes 1–8 against the Candida species C. albicans, C. glabrata, C. parapsilopsis and C. tropicalis.
Materials and methods
Chemicals and chemical techniques
All the complexes were synthesized under inert gas atmosphere using vacuum / Schlenk techniques. The camphor derivatives were synthesized under air using conventional organic synthesis techniques. Complexes (4–8) [28,31] and ligands (LII, LIV-LVIII) [32,33] were synthesized following published procedures. Camphor, camphorsulfonic acid and the appropriate amines were purchased from Sigma-Aldrich (St. Louis, MO, USA). The solvents were of PA grade and were pre-dried (using the suitable drying agent) [34] and distilled under nitrogen immediately before use. Acetonitrile was from Carlo Erba (France) and THF and all the other solvents from Panreac (Germany). The IR spectra were obtained from KBr pellets using a JASCO FT/IR 4100 spectrometer. NMR spectra (1H, 13C, DEPT and HSQC, HMBC) were obtained from chloroform-d1 or acetonitrile-d3 solutions using Bruker Avance II+ (300 or 400 MHz) spectrometers. NMR chemical shifts are referred to TMS (δ = 0 ppm). Cyclic voltammograms (CV) were obtained from Bu4NBF4/CH3CN (0.10 M) solutions using a three compartments cell, equipped with platinum wire working and secondary electrodes interfaced with a VoltaLab PST050 equipment. The potentials were measured in Volts (± 10 mV) versus SCE at 200 mV/s using (Fe(η5-C5H5)2]0/+ (E1/2 = 0.382 V) as internal reference. Elemental analysis was based on the complete oxidation of the sample (flash combustion) in a EA 1108 CHNS-O Element Analyser from Thermo Scientific (Fisons) (Waltham, MA, USA). The resulting combustion gases passed through a reducing furnace and swept into the chromatographic column by helium (carrier gas), where they were separated and quantitatively detected by appropriated gas analysis procedures using a TCD detector.
Ligands synthesis
(1,2)-1,2-bis((1,4)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)hydrazine, (LI)—(1R)-(+)-Camphor (610 mg; 4.0 mmol) was stirred in etanol/acetic acid (10/0.5 mL) for ca. 1h at RT. Then, hydrazine monohydrate (0.1 mL; 2.0 mmol) was added and the mixture stirred for 72 h at 50°C. Yield 55%. Elem. Anal. (%) for C20H32N2 ½H2O: Found: C, 77.6; N, 9.3; H, 10.7. Calc.: C, 77.7; N, 9.1; H, 10.7. for IR (cm-1): 1670. 1H NMR: (CDCl3, δ ppm): 2.28 (d, J = 18 Hz, 2H), 1.97–1.77 (m, 6H), 1.71 (t, J = 12.0 Hz, 2H), 1.47 (t, J = 12.0 Hz, 2H), 1.22 (t, J = 12.0 Hz, 2H), 1.04 (s, 6H), 0.92 (s, 6H), 0.78 (s, 6H). 13C NMR: (CDCl3, δ ppm): 176.2 (C2), 52.8 (C1), 48.0 (C7), 43.9 (C4), 35.4 (C3), 32.6 (C6), 27.2 (C5), 19.6, 18.8, (C9, C10), 11.2 (C8).
(3,3)-3,3'-([1,1'-biphenyl]-4,4'-diylbis(azanylylidene))bis(1,7,7-trimethylbicyclo [2.2.1] heptan-2-one), (LIII)—Camphorquinone (500 mg; 3.0 mmol) was stirred in EtOH (10 mL) acidified with acetic acid (0.3 mL) at room temperature for 30 minutes. Benzidine (275 mg; 1.5 mmol) was then added and the yellow mixture was stirred at 50°C for 18 hours. After cooling, the solvent was removed under vacuum and the yellow solid was washed with Et2O and n-pentane. Yield 86%. Elem. Anal. (%) for C32H36N2O2: Found: C, 80.0; N, 5.7; H, 7.8. Calc.: C, 80.0; N, 5.8; H, 7.6. IR (cm-1): 1745 (νCO), 1661 (νCN). 1H NMR: (CDCl3, δ ppm): 7.59 (d, J = 8.0 Hz, 4H), 7.03 (d, J = 8.0 Hz, 4H), 2.90 (d, J = 4.0 Hz, 2H), 2.15–2.11 (m, 2H), 1.90–1.86 (m, 2H), 1.73–1.63 (m, 4H), 1.12 (s, 6H), 1.00 (s, 6H), 0.93 (s, 6H). 13C NMR: (CDCl3, δ ppm): 206.7 (C2), 172.1 (C3), 148.8 (Cipso), 137.7 (Cipso), 127.5 (CPh), 121.4 (CPh), 58.1 (C1), 50.4 (C4), 44.8 (C7), 30.3 (C6), 24.6 (C5), 21.2, 17.7, (C9, C10), 9.3 (C8).
Complexes synthesis
[Ag(NO3)(C20H32N2)] (1)–AgNO3 (85 mg, 0.5 mmol) and compound LI (150 mg, 1.0 mmol) were mixed and stirred under vacuum for 30 minutes. THF (10 mL) was then added and the solution was stirred for 72 hours at 40°C. The solvent was fully evaporated and the off-white solid obtained was recrystallized from CH2Cl2 / Et2O. A white solid was obtained which was filtered off solution and dried under vacuum. Yield 84%. Elem. Anal. (%) for AgC20H32N3O3.(½CH2Cl2): Found: C, 47.6; N, 8.5; H, 6.7. Calc.: C, 48.0; N, 8.2; H, 6.5. IR (cm-1): 1655 (υCN); 1385 (υNO3). 1H NMR (CD3CN, δ ppm): 2.17 (s, 4H), 1.88–1.77 (m, 6H), 1.41–1.35 (m, 2H), 1.24–1.18 (m, 2H), 1.01 (s, 6H), 0.93 (s, 6H), 0.76 (s, 6H). 13C NMR (CD3CN, δ ppm): 175.0 (C2), 53.2 (C1), 48.7 (C7), 44.8 (C4), 36.0 (C3), 33.4 (C6), 27.9 (C5), 19.9, 19.0 (C9, C10), 11.7 (C8).
[Ag(NO3)(C26H32N2O2)] (2)–AgNO3 (170 mg, 1.0 mmol) and LII (405 mg, 1.0 mmol) were mixed and stirred under vacuum for 30 minutes. Then, acetonitrile (10 mL) was added and the solution stirred at room temperature for 18 hours. A yellow solid formed that was filtered off solution and washed with Et2O and n-pentane. Yield 86%. Elem. Anal. (%) for AgC26H32N3O5.H2O: Found: C, 52.6; N, 7.0; H, 5.5. Calc.: C, 52.7; N, 7.1; H, 5.8. IR (cm-1): 1748 (υCO); 1661 (υCN); 1384 (υNO3). 1H NMR (CD3CN, δppm): 7.42 (t, J = 8 Hz, 1H), 6.79 (d, J = 8 Hz, 2H), 6.29 (s, 1H), 2.80 (d, J = 4 Hz, 2H), 2.12–2.06 (m, 2H), 1.96–190 (m, 2H), 1.63–1.51 (m, 4H), 1.05 (s, 6H), 0.99 (s, 6H), 0.84 (s, 6H). 13C NMR (CD3CN, δppm): 206.7 (C2), 174.9 (C3), 151.0 (Cipso), 131.2 (CPh), 118.7 (CPh), 113.0 (CPh), 59.0 (C1), 51.4 (C4), 45.4 (C7), 30.8 (C6), 24.4 (C5), 21.3, 17.4 (C9, C10), 9.3 (C8).
[Ag(NO3)(C32H36N2O2)] (3)–AgNO3 (170 mg, 1.0 mmol) and LIII (480 mg, 1.0 mmol) were mixed and stirred under vacuum for 30 minutes. Acetonitrile (10 mL) was then added and the solution was stirred at room temperature for 18 hours. The yellow suspension was filtered off solution and the solid was washed with n-pentane. Yield 81%. Elem. Anal. (%) for AgC32H36N3O5 ½H2O: Found: C, 58.1; N, 6.8; H, 5.6. Calc.: C, 58.3; N, 6.4; H, 5.7. IR (cm-1): 1746 (υCO); 1662 (υCN); 1384 (υNO3). 1H NMR (CD3CN, δppm): 7.68 (d, J = 8 Hz, 4H), 7.04 (d, J = 8 Hz, 4H), 2.87 (d, J = 4 Hz, 2H), 2.17–2.11 (m, 2H), 1.97–191 (m, 2H), 1.70–1.58 (m, 4H), 1.07 (s, 6H), 1.00 (s, 6H), 0.87 (s, 6H). 13C NMR (CD3CN, δppm): 207.2 (C2), 173.8 (C3), 149.7 (Cipso), 138.5 (Cipso), 128.4 (CPh), 122.3 (CPh), 58.9 (C1), 51.4 (C4), 45.4 (C7), 30.9 (C6), 24.8 (C5), 21.2, 17.5 (C9, C10), 9.3 (C8).
Bacterial strains and assessment of antibacterial activity
When in use, Staphylococcus aureus Newman, Pseudomonas aeruginosa 477, Escherichia coli ATCC 25922, and Burkholderia contaminans IST408 were routinely maintained in Lennox Broth (LB) solid medium (Sigma-Aldrich, St. Louis, USA). The antimicrobial activity of the camphorimine Ag(I) complexes were quantified by estimating the MIC based on standard methods [35] as previously described [36]. Briefly, the MIC values of the compounds were determined as follows: overnight grown bacterial cultures (carried out in Mueller-Hinton (MH) broth (Sigma-Aldrich, St. Louis, USA) at 37°C and 250 rev.min-1) were diluted with fresh MH medium to a final optical density of 0.02, measured at 640 nm (OD640) in a Hitachi U-2000 UV/Vis spectrophotometer. From these cell suspensions, 100 μL aliquots were mixed in the wells of 96-wells polystyrene plates with 100 μL of fresh MH supplemented with the compounds under study, previously serially diluted (1:2) from stock solutions of each compound. After incubation for 24 h at 37°C, the OD640 of the cultures were measured using a SpectrostarNano microplate reader (BMG Labtech, Germany).
The MIC values were estimated by fitting the OD640 mean values with a Gompertz modified equation as described before [28]. Mean OD640 values were obtained from a total of at least 4 experiments from 2 independently prepared MICs assays performed as duplicates. Colony-forming units (CFUs) of the bacterial strains in cultures carried out in the presence of concentrations of the compounds below and above the estimated MIC values were assessed by spot inoculating onto the surface of LB solid medium 10 μL aliquots of serially diluted samples.
Candida strains and assessment of antifungal activity
The ability of the camphor-derived Ag(I) complexes (1–8) to inhibit the growth of C. albicans, C. glabrata, C. tropicalis and C. parapsilosis was assessed using the standardized microdilution method recommended by EUCAST (European Committee on Antimicrobial Susceptibility Testing) to determine the MIC50 values, considered to be the concentration of drug that reduced yeast growth by more than 50% the growth registered in drug-free medium [37]. The strains used in this work were C. albicans SC5314, C. glabrata CBS138, C. parapsilosis ATCC 22019 and C. tropicalis ATCC750. Briefly, cells of the different species were cultivated (at 30°C and with 250 rpm orbital agitation) for 17h in YPD growth medium and then diluted in fresh RPMI growth medium (Sigma) to obtain a cell suspension having an OD530nm of 0.05. From these cell suspensions, 100 μL aliquots were mixed in the 96-multiwell polystyrene plates with 100 μL of fresh RPMI medium (control) or with 100 μL of this same medium supplemented with 2000, 1000, 500, 250, 125, 62.5, 31.3, 15.63, 7.81 and 3.91 μg/mL of the different compounds. As a control we have also examined the inhibitory effect of AgNO3 and that of the free ligands. After inoculation, the 96-multiwell plates were incubated without agitation at 37°C for 24h. After that time, cells were re-suspended and the OD530nm of the cultures was measured in a microplate reader. The MIC50 value was taken as being the highest concentration tested at which the growth of the strains was 50% of the value registered in the control lane.
Microscopic characterization of complexes and cells
The physico-chemical characterization of the complexes and the cells before and after incubation in the presence of the complexes was performed using scanning electron microscopy (SEM) with a JEOL-JSM7001F apparatus. The elemental chemical composition was performed by the respective X-ray energy dispersive spectrometer (EDS). To increase the conductivity of the samples they were coated with a thin layer of conductive chromium (Polaron E-5100). The histograms for the diameter distributions of the nanoparticles were produced from SEM images using ImageJ software.
Results and discussion
Synthesis and characterization of the compounds
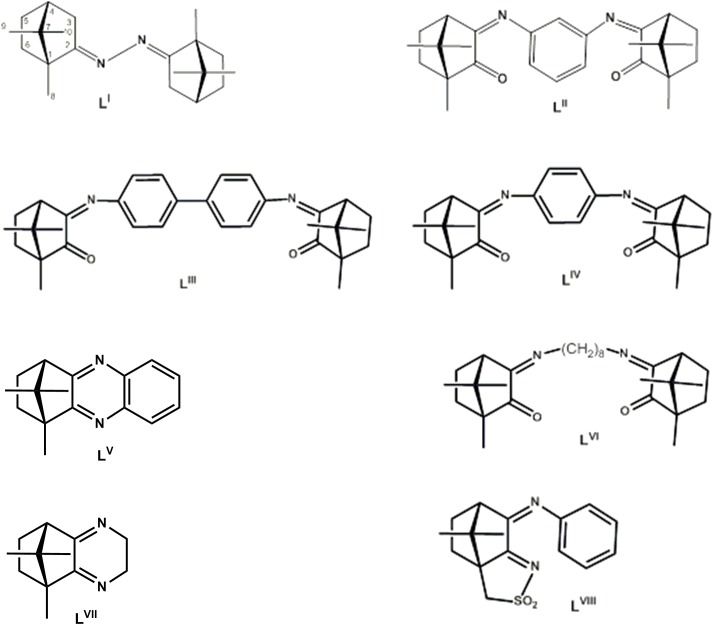
Functionalization of camphor or camphorquinone by condensation with primary amines or hydrazines is a strategy typically used to synthesize compounds that essentially keep the bicyclic structure of camphor with improved ability to coordinate transition metals. This way, several camphorimine derivatives were prepared which were used as ligands (Fig 1) to coordinate silver nitrate. The camphor derivatives LI and LIII are new, while the other camphor compounds (LII, LIV—LVIII) were previously reported [28]. The two new compounds were formulated based on analytical and spectroscopic data (FTIR and NMR). Formulation by elemental analysis is corroborated by observation of just one band in the region assigned to the stretching of the CN and CO double bonds (1750–1600 cm-1) in the IR spectrum of LI (νCN, 1670 cm-1) while in the IR spectrum of LIII two bands (νCN 1661 cm-1; νCO 1745cm-1) are observed, as expected. The NMR spectra evidence in LI, LIII equivalent camphor skeletons and imine (LI, 176.2 ppm, C2; LIII, 172.1 ppm, C3) and ketone (LIII, 206.7 ppm, C2) groups with the right camphor/aromatic linker integration consistent with formulation.
Fig 1. Camphorimine compounds used as ligands.
By reaction of the appropriate camphor derivative (L) with AgNO3, three new complexes [Ag(NO3)L] (L = LI, 1; LII, 2; LIII, 3) were synthesized that were characterized by elemental analysis, FTIR and NMR. The most relevant differences in the spectra of the complexes and the corresponding ligands rely on a band attributable to the NO3 stretching (1384–1385 cm-1) observed in the IR spectra of the complexes and absent in the spectra of the ligands.
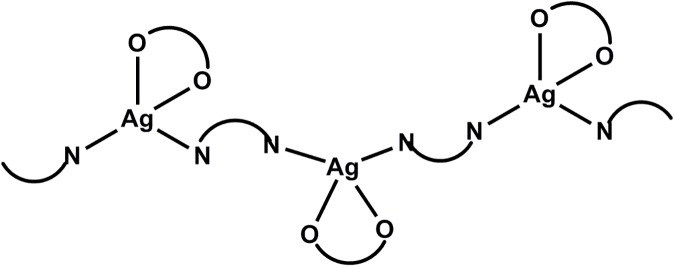
The new compounds (1–3) enlarge the variety of the [Ag(NO3)L]n camphorimine complexes previously synthesized from the ligands LIV- LVIII (4–8), essentially keeping their formulation and structure according to elemental analysis and spectroscopic data, i.e. the camphorimine ligands binding the silver metal through the nitrogen atom of the camphor imine group and the NO3 group through two oxygen atoms within a zig-zag polymeric arrangement (Fig 2) as found by X-ray analysis for 5 [28].
Fig 2. Structural arrangement of compounds [Ag(NO3)L] (L = N͡N), according to crystallographic data obtained for 5 [28].
Redox properties
The redox properties of the complexes and of the ligands were evaluated by cyclic voltammetry. The complexes display one irreversible cathodic process at a potential close to that of AgNO3 reduction (0.18 V) attributed to Ag(I)→Ag(0) reduction (Table 1). Additionally, reversible cathodic and anodic waves at potentials in the range of those of the corresponding free camphorimine compounds (Ligand) are indicative of ligand based processes (Table 1). Adsorption waves, detected on the reverse scan upon reduction, evidence deposition of Ag(0) on the working electrode, thus corroborating silver metal formation upon electron transfer. The potential range of the cathodic process (1–3; 0.049 to 0.14 V, Table 1) is consistent with a facile reduction of the Ag(I) site, as found before for complexes 4–8 [28].
Table 1. Cyclic voltammetry dataa for complexes [Ag(NO3)(L)] (1–3) and ligands LI-LIII.
| Complex | Potential | Ligand | Potential | |||
|---|---|---|---|---|---|---|
| 1 | 0.13 b | - 2.37 | 1.77 | LI | — | 1.60 |
| 2 | 0.14 b | -1.69 | 1.63 | LII | -1.84 | 1.62 |
| 3 | 0.049 b | -1.50 | 1.34 | LIII | -1.72 | 1.31 |
aValues in Volt (±10 mV). Bu4NBF4/CH3CN (0.10M) used as electrolyte and [Fe(η5-C5H5)2]0/+ ( = 0.382 V vs. SCE) as internal reference.
b Generates an adsorption wave.
The slightly lower Ag(I)→Ag(0) potential displayed by 3 compared to 1 and 2 (Table 1) indicates that there is a slightly higher electron density at the Ag(I) site due to electron withdrawing from the biphenyl bicamphor ligand (LIII, Fig 1), as confirmed by the ligand based reduction at the complex occurring at a considerably higher potential than at the free ligand (Table 1). Such pattern renders the Ag(I) site slightly more electron-rich and thus more resistant to reduction, a fact that may be significant on the low antimicrobial activity displayed by 3 (see below). Complex 5 that displays a reduction potential ( = 0.031 V) [28] similar to 3, also displayed low antibacterial and antifungal activities. Data on redox properties of the complexes is still insufficient to draw further conclusions. However, it is worth to highlight that electron transfer could be responsible for formation of AgNPs upon interaction of the camphorimine Ag(I) complexes with C. albicans and the complexes have no antifungal activity (see below).
Antimicrobial activity of the Ag(I) complexes
Antibacterial properties
The antibacterial properties of Ag(I) camphorimine complexes 1–3, the ligands LI-LIII and AgNO3 were assessed based on the determination of the MIC values towards S. aureus, B. contaminans, P. aeruginosa and E. coli using the previously described methods and a modified Gompertz equation to estimate the MIC values [28,35]. No bacterial growth inhibition was detected for the ligands (LI-LIII) in the range of concentrations: 10–1000 μg/mL (LII) and 2–150 μg/mL (LI and LIII) (Supporting information S1 Fig, S2 Fig, and S3 Fig). Lower solubility precluded the use of higher concentrations in the cases of LI and LIII. Low solubility of complex 3 also precluded studies using concentrations higher than 100 μg/mL. These results are consistent with the previously reported bactericidal activity of complexes 4–8 when used in concentrations equal or above the MIC [28].
The analysis of the antibacterial activity of complexes 1, 2 and 3 towards S. aureus, P. aeruginosa, B. contaminans and E. coli (Supporting information S4 Fig, S5 Fig and S6 Fig) shows that complex 1 displays a MIC value lower than AgNO3 for S. aureus Newman. For the other bacterial strains complex 1 displays MIC values slightly higher than AgNO3 (Table 2). All the MIC values estimated for complexes 2 and 3 are considerably higher than those obtained for AgNO3 (Table 2). No colony forming units (CFUs) were detected for concentrations of complexes 1 and 2 equal or above the respective MIC values, demonstrating the bactericidal activity of the complexes. For complex 3, no P. aeruginosa CFUs were detected for concentrations equal or above the estimated MIC value.
Table 2. MIC values (μg/mL)a estimated for complexes [Ag(NO3)L] (L: LI, 1; LII, 2; LIII, 3; LIV, 4; LV, 5; LVI, 6; LVII, 7; LVIII, 8).
|
Compound |
S. aureus Newman |
B. contaminans IST408 |
P. aeruginosa 477 |
E. coli ATCC 25922 |
|---|---|---|---|---|
| 1 | 66 ± 4.8 | 79 ± 4.4 | 56 ± 4.3 | 50 ± 1.0 |
| 2 | 183 ± 3.3 | 144 ± 1.0 | 121 ± 2.1 | 65 ± 1.5 |
| 3 | ˃ 100 | ˃ 100 | 86 ± 6.8 | ˃ 100 |
| 4b | 73 ± 2.3 | 36 ± 2.5 | 19 ± 4.3 | 20 ± 1.3 |
| 5 b | 118 ± 1.7 | 97 ± 1.2 | 68 ± 1.5 | 98 ± 1.3 |
| 6 b | 119 ± 2.9 | 96 ± 1.6 | 105 ± 3.0 | 98 ± 1.3 |
| 7 b | 95 ± 3.3 | 81 ± 4.5 | 39 ± 3.4 | 49 ± 1.5 |
| 8 b | 259 ± 2.6 | 127 ± 4.6 | 138 ± 4.5 | 123 ± 4.0 |
| AgNO3b | 73 ± 1.9 | 74 ± 1.4 | 39 ± 2.0 | 47 ± 1.1 |
a Towards S. aureus, B. contaminans, P. aeruginosa or E. coli. MIC values were estimated by fitting data from cultures optical density (measured at 640 nm) to a modified Gompertz equation (see experimental).
b Data taken from reference [28].
Antifungal properties
The effect of the supplementation of the growth medium with Ag(I) complexes on the growth of the different yeast species under study is shown in S7A Fig to S7D Fig in Supporting information S7 Fig. The MIC50 values determined based on those results are compiled in Table 3. With the exception of LV, none of the ligands inhibited growth of the Candida spp tested (S8A Fig to S8D Fig in Supporting information S8 Fig). Nonetheless, the inhibitory effect of Lv (MIC50 within the range of 500–1000 μg/mL) was considerably smaller than the effect exerted by complex 5 (derived from it), this being particularly evident for C. tropicalis and C. parapsilosis (see S7C Fig and S8C Fig in Supporting information S7 Fig and S8 Fig). Within the range of concentrations tested none of the Ag(I) camphorimine complexes, nor AgNO3, showed efficacy in inhibiting growth of C. albicans (Table 3). This result was further confirmed by the observation that viability of C. albicans cells in the presence of 1000 μg/mL (the highest concentration used in the MIC assay) of all the compounds is identical to the one observed during cultivation in non-supplemented RPMI medium (results not shown).
Table 3. MIC values (μg/mL) for Ag(I) camphor complexes 1–8 and for AgNO3 against C. albicans, C. glabrata, C. tropicalis and C. parapsilosis, based on the EUCAST microdilution method [37].
| Compound | C. albicans | C. glabrata | C. tropicalis | C. parapsilosis |
|---|---|---|---|---|
| 1 | > 1000 | 31.3 | 3.9 | > 1000 |
| 2 | > 1000 | 15.6 | 7.8 | 2.0 |
| 3 | > 1000 | 62.5 | 31.3 | 15.6 |
| 4 | > 1000 | > 1000 | > 1000 | > 500 |
| 5 | > 1000 | 125 | 3.9 | 2.0 |
| 6 | > 1000 | 62.5 | 7.8 | 2.0 |
| 7 | > 1000 | 31.3 | 3.9 | 2.0 |
| 8 | > 1000 | 62.5 | 7.8 | 2.0 |
| AgNO3 | > 1000 | 15.6 | 3.9 | 2.0 |
Results in Table 3 show that the complexes display a degree of efficacy towards the Candida species ranging from non-active (4) to highly active (2). Consistent with the increased resilience of C. glabrata species to antimicrobials [38], the MIC values of all Ag(I) complexes obtained for this species are higher than those obtained for C. tropicalis or C. parapsilosis (Table 3). The inhibitory effect of the compounds on growth of C. glabrata, C. parapsilosis and C. tropicalis is fungistatic, since cellular viability at a concentration of the complexes equal to the MIC50 values was reduced but not fully abrogated (results not shown).
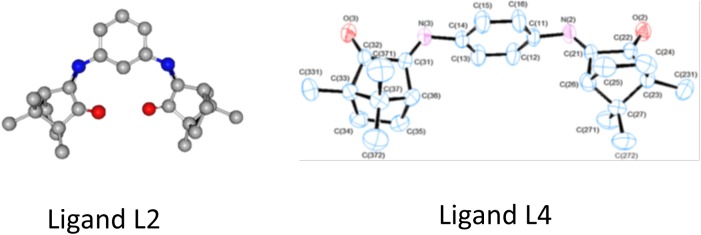
The results in this study reinforce the previously demonstrated potential of Ag(I) camphor complexes to be used as antimicrobials [28]. In this context, it is worth to highlight the high activity exhibited by the Ag(I) camphorimine complexes against the non-albicans Candida species C. parapsilosis and, to a lesser extent, against C. tropicalis. The incidence of infections caused by these species has been increasing due to increase of resistance to currently used antifungals and to their remarkable ability to thrive in indwelling medical devices [39]. It is interesting to observe that the camphorimine Ag(I) complexes 1–8 appear to exert specific and distinct effects against bacteria and Candida cells. For example, complex 4 is highly active against bacteria but less effective in inhibiting Candida spp. growth, while complex 2 is highly active against the Candida spp. (with MIC values identical to those estimated for AgNO3) and one of the less active compounds against the set of bacterial strains tested. Complexes prepared with ligands L2 and L4 essentially differ on the aromatic spacer binding the two camphor moieties at meta or para positions, in the ligands (Fig 1). However, this apparently small difference enforces steric modifications, that according to the results obtained drive the interaction with the biological receptors and consequently to the antimicrobial activities of the complexes. In a former work, camphorimine compounds L2 and L4 were used as ligands to prepare Cu(I) complexes and their anti-proliferative activity against the cancer cell line HT29 was studied [32]. Both complexes displayed high anti-proliferative activity, being the activity of the Cu(I) complex with ligand L2 higher compared to L4. This is consistent with the higher antifungal activity of Ag+ complexed with L2 compared to L4. The geometry of the bicamphor imine ligands L2 and L4 is highly different (Fig 3). When complexed with L2, the metal Ag+ finds a coordination geometry that conceivably provides it a better protection, while when complexed with L4 it is more exposed and prone to multiple interactions. Since the activity of metal complexes with ligand L2 towards eukaryotic cells is higher than when complexed with L4, we speculate that both the geometry of the complexes and the distinct lipid and protein content of eukaryotic and prokaryotic cell membranes might determine the distinct specificity of the compounds.
Fig 3. Spatial structures of ligands L2 and L4, evidencing that in complex 2 the Ag+metal ion finds a coordination geometry that conceivably provides a better protection, while in complex 4 it seems highly exposed to multiple interactions and deactivation due to reduction to Ag(0).
Another relevant point from this study is that complexes for which a MIC value could be determined showed to be bactericidal (when used in concentrations equal or above the MIC) while they are fungistatic. Such behaviour suggests that there are different biological targets in the prokaryotic and eukaryotic cells or, that complexes penetrate fungal and bacterial cells through different pathways, their structural arrangements finely-tuning the reactivity. There are marked differences between the structure of the cell envelope of the tested bacterial strains and Candida species which could sustain different cellular permeability to the different Ag(I) complexes. Further studies are required to deepen the understanding of the mechanisms underlying the specificity of the inhibitory effects exerted by Ag(I) complexes on the growth of Candida spp. and of bacteria.
Although the antibacterial and antifungal activities of the Ag(I) camphorimine complexes indicate their potential as antimicrobials, their feasible use in clinical practice requires the evaluation of their toxicity against mammalian cells.
AgNPs from Ag(I) camphor complexes synthesized by C. albicans
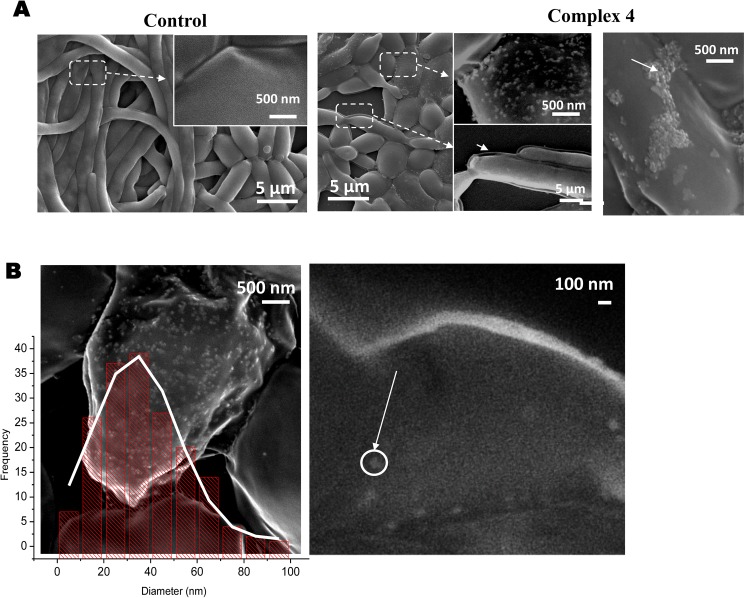
An intriguing point on the study of the antifungal properties of the Ag(I) camphorimine complexes 1–8 is their generalized lack of effect in inhibiting growth of C. albicans. To further examine this issue, cellular suspensions of C. albicans were incubated in the presence or absence of complexes 1 and 4 and afterwards examined by scanning electronic microscopy (SEM). The SEM pictures evidenced significant morphological alterations in C. albicans cells exposed to complexes 1 and 4 and cells cultivated in the absence of the complexes. Cells exposed to complexes 1 and 4 showed a marked reduction of the hyphae formed and alterations at the cell surface, as displayed in Fig 4.
Fig 4. Microscopy images obtained by SEM of C. albicans cells SC5314 when cultivated for 24h in: A1- RPMI growth medium (control); A2- RPMI growth medium supplemented with 125 μg/mL of 4.
Panel A2 shows a magnification of the image evidencing the occurrence of modifications on the cell surface; B- size distribution of the small nanoparticles found at the cell surface of C. albicans.
Small protuberances (at the nanoscale level—Fig 4A2) were detected on the cell surface of C. albicans upon exposure to Ag(I) complexes 1 and 4. The calculated size (33±18 nm) and subsequent elemental analysis by EDS confirmed these nanoparticles to be silver nanoparticles (Fig 3B and results not shown). It is important to stress that no AgNPs were detected on samples of complexes 1 and 4 that had not been exposed to the C. albicans (Supporting information S9 Fig). These observations support Ag(I)→Ag(0) reduction by C. albicans with concomitant formation of silver nanoparticles (AgNPs). Consistently, AgNPs are known to inhibit formation of hyphae and to affect the cell wall structure of C. albicans [16,17], two effects that we have observed to occur in our assays (Fig 4A and 4B). AgNPs have a milder antifungal activity [17,40] than Ag+ and thus the ability of C. albicans to convert the camphorimine Ag(I) complexes into AgNPs is likely to be one of the causes for the lack of activity of complexes 1–8 against C. albicans.
Reports of fungal-mediated synthesis of AgNPs from Ag+ have been published before [41,42]. However, to the best of our knowledge, this is the first time that the widely used C. albicans SC5314 reference strain is reported to synthesize silver nanoparticles.
The ability of C. albicans cells to prompt reduction of Ag(I) to Ag(0) while the other Candida spp. used in this study do not suggest further studies (out of the scope of this work). The mechanism of formation of AgNPs promoted by fungal cells remains to be elucidated, although it has been suggested that it involves the initial adsorption of Ag+ ions to the surface of the fungal cells promoted by electrostatic interactions between carboxylate groups (negatively charged) at the cell wall and the Ag+ ions (positively charged). Electron transfer from the enzymes to the silver ions would then be responsible for their reduction leading to formation of silver particles [17]. In the case of camphorimine complexes, it was shown by cyclic voltammetry (see above) that Ag(I)→Ag(0) reduction occur at potentials 0.14–0.049 V (Table 1) accessible to enzymes. Formation of AgNPs suggests that C. albicans harbors at least one protein able to trigger electron transfer and promote this reduction process. The other Candida species conceivably do not have at their cell surface proteins analogous to those in C. albicans able to mediate Ag+ reduction, thus no formation of AgNPs is detected. An interesting strategy to sensitize C. albicans cells against Ag-based compounds could be to target the activity of these enzymes mediating Ag-reduction.
Conclusions
All the camphorimine [Ag(NO3)L] complexes 1–8 assessed for antimicrobial properties display bactericidal activity against B. contaminans and the bacterial species of the ESKAPE group S. aureus, P. aeruginosa, and E. coli. The low MIC values observed for some of the complexes suggest their potential use as as alternatives to the existing antibiotics. In addition to their bactericidal properties, the complexes (except complex 4) also display fungistatic activity against the three pathogenic Candida species C. tropicalis, C. parapsilosis, and C. glabrata. The camphorimine Ag(I) complexes are especially active against C. parapsilosis, a recognized emergent agent of fungal infections. The fact that complexes behave as bactericidal but fungistatic suggests that they have different targets and/or mechanisms of action in prokaryotic and eukaryotic cells.
None of the complexes is active against C. albicans SC5314 which is able to promote the reduction of the Ag(I) site forming AgNPs as confirmed by SEM.
Since the molecular formulae of the complexes 1–8 essentially differ on the ligand L, their distinct antimicrobial properties have to be based on the characteristic of the camphorimine ligands. In fact, complexes 2 and 4 that just differ on the geometry of the aromatic spacer between the two camphor ligands, display opposite properties, i.e. complex 2 displays low antibacterial and high antifungal properties while complex 4 displays high antibacterial and low antifungal activity. Electron delocalization may one of the parameters responsible for increases antifungal activity in complexes 1, 3, 4 and 5, while no such trend is detected for the antibacterial properties. Further efforts are necessary to fully understand the processes underlying the antimicrobial activities which may be relevant to the development of Ag(I) camphor-derived chemicals, including AgNPs as anti-Candida agents.
Overall, the results now reported show that antifungal and antibacterial properties combine in Ag(I) camphorimine complexes which have potential to be used as alternative therapeutic compounds for the development of new antimicrobials against clinically relevant pathogenic bacterial and Candida species.
Supporting information
(PPT)
(PPT)
(PPT)
(PPT)
(PPT)
(PPT)
(PPTX)
(PPTX)
(TIF)
Acknowledgments
Financial support by FCT-Fundação para a Ciência e Tecnologia through projects UID/QUI/00100/2013, UID/BIO/04565/2013, RECI/QEQ-QIN/0189/2012 and partial contracts PTDC/BIA-MIC/1615/2014); Programa Operacional Regional de Lisboa 2020 (Project N. 007317) and the NMR Network (IST-UTL Node) for facilities are gratefully acknowledged.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support by FCT-Fundação para a Ciência e Tecnologia through projects UID/QUI/00100/2013, UID/BIO/04565/2013, RECI/QEQ-QIN/0189/2012 and partial contracts PTDC/BIA-MIC/1615/2014); Programa Operacional Regional de Lisboa 2020 (Project N. 007317) and the NMR Network (IST-UTL Node) for facilities are gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008; 197:1079–81. doi: 10.1086/533452 [DOI] [PubMed] [Google Scholar]
- 2.Sanguinetti M, Posteraro B, Lass-Florl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015; 58 (Suppl 2):2–13. [DOI] [PubMed] [Google Scholar]
- 3.Chai LY, Denning DW, Warn P. Candida tropicalis in human disease. Crit Rev Microbiol. 2010; 36:282–98. doi: 10.3109/1040841X.2010.489506 20883082 [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007; 20:133–63. doi: 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America Clin. Infect Dis. 2009, 48, 1–12. [DOI] [PubMed] [Google Scholar]
- 6.Klasen HJ. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns. 2000; 26:117–130. [DOI] [PubMed] [Google Scholar]
- 7.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007; 33:139–148. doi: 10.1016/j.burns.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 8.A. E. McRee AE. Therapeutic Review: Silver. J Exotic Pet Med. 2015; 24:240–244. [Google Scholar]
- 9.Drake PL, Hazelwood KJ. Exposure-Related Health Effects of silver and silver Compounds: A Review. Ann. Occup. Hyg. 2005, 49, 575–585. doi: 10.1093/annhyg/mei019 [DOI] [PubMed] [Google Scholar]
- 10.Maillard J-Y, Hartemann P. Silver as an antimicrobial: facts and gaps in knowledge. Critical Rev Microbiol. 2013;39:373–383. [DOI] [PubMed] [Google Scholar]
- 11.Medici S, Peana M, Crisponi G, Nurchi GM, Lachowicz JI, Remelli M, et al. Silver coordination compounds: A new horizon in medicine Coord Chem Rev. 2016; 327–328:349–359. [Google Scholar]
- 12.Rendošová M, Vargová Z, Kuchár J, Sabolová D, Levoča Š, Kudláčová J, et al. New silver complexes with bioactive glycine and nicotinamide molecules–Characterization, DNA binding, antimicrobial and anticancer evaluation J. Inorg. Biochem. 2017; 168:1–12. doi: 10.1016/j.jinorgbio.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Mujahid M, Trendafilova N, Arfa-Kia AF, Rosair G, Kavanagh K, Devereux M, et al. Novel silver(I) complexes of coumarin oxyacetate ligands and their phenanthroline adducts: Biological activity, structural and spectroscopic characterisation. J Inorg Biochem. 2016; 163:53–67. doi: 10.1016/j.jinorgbio.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 14.Lara HH, Romero-Urbina DG, Pierce C, Lopez-Ribot JL, Arellano-Jimenez MJ, Jose-Yacaman M. Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study. J Nanobiotechnology 2015; 13:91 doi: 10.1186/s12951-015-0147-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva S, Pires P, Monteiro DR, Negri M, Gorup LF, Camargo ER, et al. The effect of silver nanoparticles and nystatin on mixed biofilms of Candida glabrata and Candida albicans on acrylic Med Mycol 2013; 51:178–184. doi: 10.3109/13693786.2012.700492 [DOI] [PubMed] [Google Scholar]
- 16.Dorobantu LS, Fallone C, Noble AJ, Veinot J, Ma G, Goss GG, et al. Toxicity of silver nanoparticles against bacteria, yeast, and algae. J Nanopar Res. 2015; 17:172. [Google Scholar]
- 17.Vazquez-Munoz R, Avalos-Borja M, Castro-Longoria E. Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles PLoS One 2014; 9: e108876 doi: 10.1371/journal.pone.0108876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J Adv Res. 2016; 7:7–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fewtrell L. Silver: water disinfection and toxicity. Aberystwyth University, Aberystwyth: 2014. [Google Scholar]
- 20.Brunetto PS, Slenters TV, Fromm KM. In vitro biocompatibility of new silver(I) coordination compound coated-surfaces for dental implant applications. Materials. 2011; 4:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvestry-Rodriguez N, Bright KR, Slack DC, Uhlmann DR, Gerba CP. Silver as a residual disinfectant to prevent biofilm formation in water distribution systems. Appl Environm Microbiol. 2008;74:1639–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fromm KM. Silver coordination compounds with antimicrobial properties. Appl Organometal Chem. 2013; 27:683–687. [Google Scholar]
- 23.Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005; 25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W., Vermaak I., Viljoen A.. Camphor—A fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules. 2013; 18:5434–5454. doi: 10.3390/molecules18055434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obeng-Ofori D, Reichmuth CH, Bekele AJ, Hassanali A. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum, against four stored product beetles. Int J Pest Management.1998;44:203–209. [Google Scholar]
- 26.Justino GC, Pinheiro PF, Roseiro APS, Knittel ASO, Gonçalves J, Justino MC, et al. Camphor-based CCR5 blocker lead compounds–a computational and experimental approach. RSC Adv. 2016; 6:56249–56259. [Google Scholar]
- 27.Sokolova AS, Yarovaya OI, Shernyukov AV, Gatilov YV, Razumova YV, Zarubaev VV, et al. Discovery of a new class of antiviral compounds: Camphor imine derivatives. Eur J Med Chem. 2015; 105:263–273. doi: 10.1016/j.ejmech.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 28.Cardoso JMS, Galvão AM, Guerreiro SI, Leitão JH, Suarez AC, Carvalho MFNN. Antibacterial activity of silver camphorimine coordination polymers. Dalton Trans. 2016; 45:7114–7123. doi: 10.1039/c6dt00099a [DOI] [PubMed] [Google Scholar]
- 29.Richau JA, Leitão JH, Correia M, Lito L, Salgado MJ, Barreto C, et al. Molecular typing and exopolysaccharide biosynthesis of Burkholderia cepacia isolates from a Portuguese cystic fibrosis center. J Clin Microbiol. 2000; 38:1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coutinho CP, Barreto C, Pereira L, Lito L, Cristino JM, Sá-Correia I. Incidence of Burkholderia contaminans at a cystic fibrosis centre with an unusually high representation of Burkholderia cepacia during 15 years of epidemiological surveillance. J. Med Microbiol. 2015; 64:927–935. doi: 10.1099/jmm.0.000094 [DOI] [PubMed] [Google Scholar]
- 31.Cardoso JMS, Correia I, Galvão AM, Marques F, Carvalho MFNN. Synthesis of Ag(I) camphor sulphonylimine complexes and assessment of their cytotoxic properties against cisplatin-resistant A2780cisR and A2780 cell lines. J Inorg Biochem. 2017; 166:55–63. doi: 10.1016/j.jinorgbio.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 32.Fernandes TA, Mendes F, Roseiro APS, Santos I, Carvalho MFNN. Insight into the cytotoxicity of polynuclear Cu(I) camphor complexes. Polyhedron. 2015; 87:215–219. [Google Scholar]
- 33.Fernandes TA, Ferraria A.M, Galvão AM, Botelho do Rego AM, Suárez ACM, Carvalho MFNN. Synthesis, characterization and study of the catalytic properties of Zn(II) camphor derived complexes. J Organometal Chem. 2014; 760:186–196. [Google Scholar]
- 34.Armarego W.L.F., Chai C.L.L.. Purification of Laboratory Chemicals (6ª Ed), 2009, Elsevier, ISBN: 978-1-85617-567-8. [Google Scholar]
- 35.Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. Wayne, PA. Clinical and Laboratory Standards Institute: CLSI M26-A.
- 36.Leitão JH, Sousa SA, Cunha MV, Salgado MJ, Melo-Cristino J, Barreto MC, et al. Variation of the antimicrobial susceptibility profiles of Burkholderia cepacia complex clonal isolates obtained from chronically infected cystic fibrosis patients: a five-year survey in the major Portuguese treatment center. Eur J Clin Microbiol Infect Dis. 2008; 27:1101–1111,. doi: 10.1007/s10096-008-0552-0 [DOI] [PubMed] [Google Scholar]
- 37.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W, Eucast-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect. 2012; 18:E246–247. doi: 10.1111/j.1469-0691.2012.03880.x [DOI] [PubMed] [Google Scholar]
- 38.Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012; 36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x [DOI] [PubMed] [Google Scholar]
- 39.Trofa D, Gacser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008; 21:606–625. doi: 10.1128/CMR.00013-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorobantu LS, Fallone C, Noble AJ, Veinot J, Ma G, Goss GG, et al. Toxicity of silver nanoparticles against bacteria, yeast, and algae. J Nanopar Res. 2015; 17:172. [Google Scholar]
- 41.Rauwel P., Kuunal S., Ferdov S., Rauwel E.. A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv Mater Sci Eng. 2015; Article ID 682749. [Google Scholar]
- 42.Waghmare SR, Mulla MN, Marathe SR, Sonawane KD. Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. 3 Biotech. 2015; 5:33–38. doi: 10.1007/s13205-014-0196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPT)
(PPT)
(PPT)
(PPT)
(PPT)
(PPT)
(PPTX)
(PPTX)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.