Abstract
Mitochondria are the powerhouses of the cell and are involved in essential functions of the cell, including ATP production, intracellular Ca2+ regulation, reactive oxygen species production & scavenging, regulation of apoptotic cell death and activation of the caspase family of proteases. Mitochondrial dysfunction and oxidative stress are largely involved in aging, cancer, age-related neurodegenerative and metabolic syndrome. In the last decade, tremendous progress has been made in understanding mitochondrial structure, function and their physiology in metabolic syndromes such as diabetes, obesity, stroke and hypertension, heart disease. Further, progress has also been made in developing therapeutic strategies, including lifestyle interventions (healthy diet and regular exercise), pharmacological strategies and mitochondria-targeted approaches. These strategies were mainly focused to reduce mitochondrial dysfunction and oxidative stress and to maintain mitochondrial quality in metabolic syndromes. The purpose of our article is to highlight the recent progress on the mitochondrial role in metabolic syndromes and also summarize the progress of mitochondria-targeted molecules as therapeutic targets to treat metabolic syndromes.
Keywords: Metabolic syndrome, Mitochondria, Oxidative stress, Reactive oxygen species, Mitochondria-targeted antioxidants, Cardiovascular disease, Pre-diabetes, Type-2-diabetes, Obesity
Graphical Abstract
1. Introduction
Mitochondria are the intracellular organelles which play a significant role in the cells by metabolizing nutrients and producing the “energy currency” adenosine triphosphate (ATP) and responsible for various processes such as energy metabolism, generation of free radicals and calcium homeostasis, cell survival and death [1, 2]. Their principal function is to synthesize ATP via oxidative phosphorylation (OXPHOS) in concurrence with the oxidation of metabolites by Krebs’s cycle and β-oxidation of fatty acids. Currently, it is appreciated that pathophysiological alterations in mitochondria in aging and many other metabolic disorders are linked with impaired mitochondrial functions such as diminished oxidative capacity and antioxidant defense by the enhanced generation of reactive oxygen species (ROS), reduced OXPHOS, and decreased ATP production. Reduced mitochondrial biogenesis with age may be due to alterations in mitochondrial fission and fusion processes and the inhibition of mitophagy, a process which eliminates dysfunctional mitochondria [3]. ROS are a family of free radicals that includes superoxide anions, hydroxyl, peroxyl radicals and other non-radicals capable of generating free radicals [4]. Although the intracellular generation of ROS per se is an inevitable process, cells possess numerous defense systems to counter it. The overproduction of ROS has been associated with oxidative damage inflicted on lipids, DNA, and proteins. [2, 5]. It is evident from the previous studies that oxidative stress is associated with various pathophysiological conditions involving aging, cancer and age-related metabolic disorders and neurodegenerative diseases [6-16].
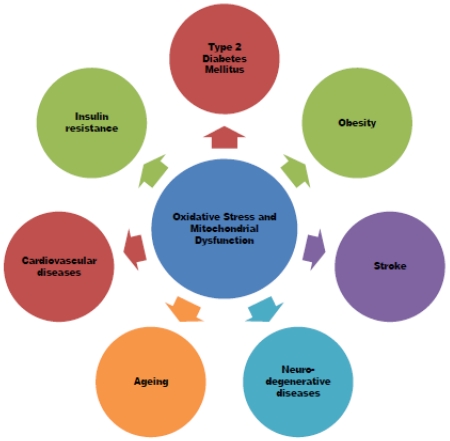
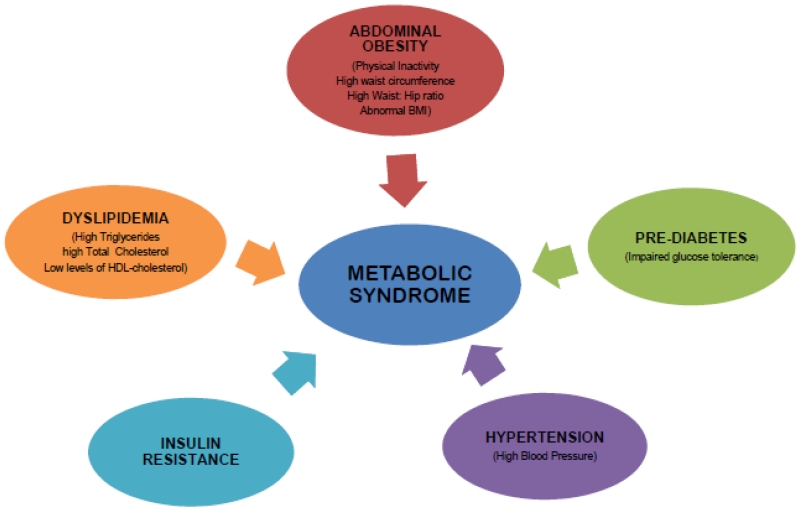
Metabolic syndrome (MetS) is a constellation of many metabolic abnormalities including hypertension, hyperglycemia, abdominal obesity and dyslipidemia represented by low-HDL-Cholesterol and hypertriglyceridemia. These conditions occurred together and increased the risk of type 2 diabetes and cardiovascular diseases (Figure 1). It has been emerged as a major health problem in the modern society, associated with enormous social, personal, and economic burden in the developing and developed world [17-20]. Earlier studies demonstrated the interaction of genetic variants and environmental factors that contribute to the escalating situation of metabolic syndrome [21-24]. Several lines of evidence indicate the role of oxidative stress and mitochondrial dysfunction in the pathogenesis of aging, age-related neurodegenerative and metabolic diseases [5, 12, 13, 16, 25-38]. Nevertheless, the basic mechanisms underlying the pathogenesis of metabolic syndrome remain largely unknown.
Figure 1.
Risk factors associated with metabolic syndrome.
The present review article is focused to overview the basic mechanism of mitochondrial dysfunction and the link between oxidative stress/mitochondrial dysfunction and various components of metabolic syndrome. We specifically focused on heart disease, stroke, diabetes, and obesity, which are intimately related to oxidative damage induced by the enhanced generation of ROS that leads to mitochondrial dysfunction. Then, pharmacologic strategies translated from the bench to bedside will be provided to target mitochondrial dysfunction for the prevention of risk associated with metabolic syndrome.
2. Mitochondria: Structure, function, and pathophysiology
Mitochondria are the double membrane, cytoplasmic organelles which contain their self-replicating genome. Mitochondria perform key biochemical functions essential for metabolic homeostasis and are arbiters of cell death and survival. In eukaryotes, mitochondria generates energy in the form of ATP via oxidative metabolism of nutrients using two major steps, 1) oxidation of NADH or FADH2 produced during the glycolysis, TCA cycle or β-oxidation of fatty acids 2) oxidative phosphorylation to generate ATP. All these processes are regulated by a complex of transcription factors in mitochondria. Each mitochondrion contains 800 to 1000 copies of mtDNA, which are maternally inherited and packaged in high-ordered nucleoprotein structures called nucleoids [39]. Although nucleoids are distributed throughout the mitochondrial matrix, they are often located in the proximity of the cristae, which carry the OXPHOS system. There is a small intermembrane space between the outer and inner mitochondrial membranes. Outer mitochondrial membrane and intermembrane space are relatively more permeable than the inner mitochondrial membrane. In contrast, the inner membrane has much more restricted permeability, contains enzymes involved in the process of electron transport chain and ATP generation. The inner membrane surrounds the mitochondrial matrix, wherein the electrons produced by TCA cycle are taken in by electron transport chain for the production of ATP. An electrochemical gradient generated across the inner membrane drives the process of OXPHOS [40]. Most of the body’s cellular energy (>90%) is produced by mitochondria in the form of ATP via TCA cycle and the electron transport chain (ETC).
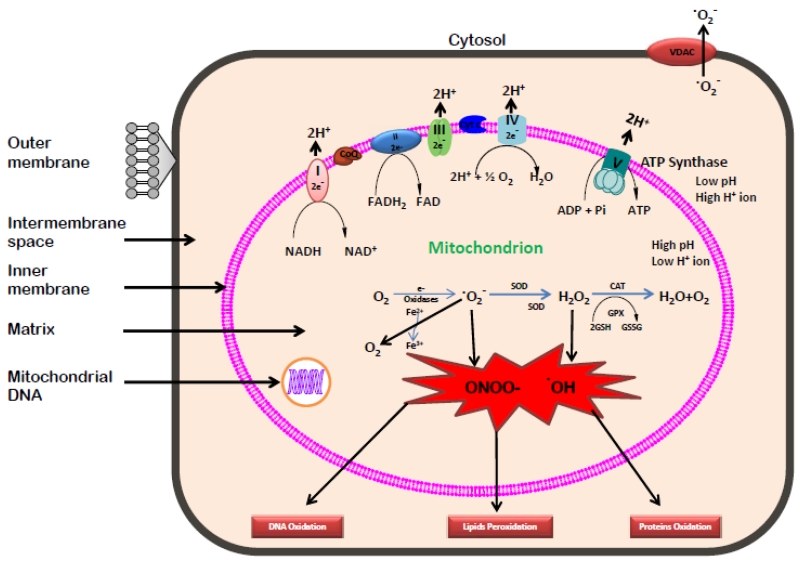
Mitochondrial ETC is composed of five multi-subunit enzyme complexes viz. I, II, III, IV and V located in the inner mitochondrial membrane [41]. The electrons donated by coenzymes, NADH and FADH2 in TCA cycle are accepted and transferred to components of ETC at complex I (NADH ubiquinone reductase) or complex II (Succinate dehydrogenase), and then consecutively to complex III (Ubiquinol-cytochrome c reductase), complex IV (Cytochrome c oxidase) and finally to oxygen through complex V (F0F1 ATP synthase). This transfer of electrons along the electron transport chain is coupled with the transport of protons across the inner membrane, establishing the electrochemical gradient that generated ATP [42]. Mitochondria continuously function to metabolize oxygen and generate ROS (Figure 2). However, either by accident or for a purpose, the flow of electrons through the ETC is an imperfect process in which 0.4 to 4% of oxygen consumed by mitochondria is incompletely reduced and leads to the production of ROS such as superoxide anion (•O2−) designated as “primary” ROS [2, 43]. Excessive generation of superoxide anion further interact with many other compounds and generate “secondary” ROS [32, 44]. It is earlier established that the interactions of hydroxyl radical (•OH) with DNA molecule damages the nitrogenous bases, purine and pyrimidine and deoxyribose backbone of DNA [2]. Also, the overproduction of ROS damage the mitochondrial proteins/enzymes, membranes, and DNA, which leads to the interruption of ATP generation and other essential functions in mitochondria [32, 43]. Besides superoxide anion and hydroxyl radicals, the ETC also generates other reactive species such as nitric oxide (NO) and reactive nitrogen species (RNS). Most of the cellular proteins and glutathione are affected through nitration induced by RNS. Free radicals are fundamental to any biochemical process and are continuously produced in the body. The cells have many ways to counter the effects of oxidative damage induced by ROS, either by directly diminishing the generation of free radicals or by scavenging the free radicals by an array of antioxidants, both enzymatic and non-enzymatic mechanisms.
Figure 2.
Generation of reactive oxygen species in mitochondrion
Several defense mechanisms are used to alleviate the oxidative stress induced by the excessive generation of free radicals in the cells. Enzymatic defense system includes the ameliorative action of various antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), Glutathione Reductase (GR) and glutathione peroxidase (GPx). The other non-enzymatic defenses are the antioxidant compounds which protect the cells against oxidative stress. It includes Vitamin E and C, glutathione (GSH), various carotenoids and flavonoids. Under normal conditions, the overproduction of ROS is restricted in mitochondria to protect the cellular organelle from oxidative damage via enzymatic and non-enzymatic defense systems. On the other hand, when the antioxidant defenses are overwhelmed, there is an overproduction of ROS which then leads to oxidative damage to the proteins, DNA, and lipids in mitochondria [45]. This impaired the enzyme functions in the respiratory chain and ultimately leading to mitochondrial dysfunction, reduced mitochondrial biogenesis and a broad range of pathologic conditions such as aging, various metabolic diseases and neurodegenerative disorders [5, 12, 44, 46-52]. Reduced mitochondrial number and capacity for oxidative phosphorylation in diabetes resulted in impaired mitochondrial biogenesis [53, 54]. Mitochondria plays very critical role in the metabolic processes in mammalian cells and alterations in the mitochondrial structure and function lead to age-related neurodegenerative diseases as demonstrated by various studies in the past [34, 55, 56].
3. Regulation of mitochondrial biogenesis
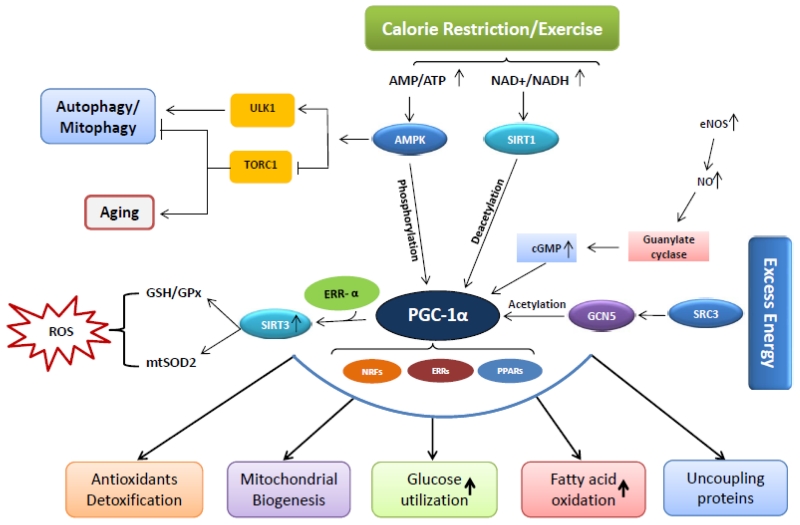
Mitochondrial biogenesis maintains the number and size of mitochondria involving both nuclear and mitochondrial genomes. Several transcription factors are involved in the regulation of mitochondrial biogenesis, mediated by physiologic stimuli including physical exercise, dietary restrictions, temperature, and muscle myogenesis. Peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α), a co-transcriptional regulation factor that regulate the process of mitochondrial biogenesis by interacting with many transcription factors/proteins such as nuclear respiratory factors (NRF-1 and NRF-2), mitochondrial transcription factor A (Tfam), uncoupling proteins (UCP2), PPARs, thyroid hormone, glucocorticoid, oestrogen and oestrogen related receptors (ERR) α and γ [57-59]. NRF-, NRF-2, and Tfam regulate the transcription of the main mitochondrial enzymes and mtDNA synthesis [60]. Besides these transcription factors, there are two important enzymes viewed as metabolic sensors that regulates mitochondrial biogenesis are AMP-activated protein kinase (AMPK) and the mammalian counterpart of silent information regulator 2 (SIRT1). In energy deprived state, AMPK and SIRT1 regulate PGC-1α through phosphorylation and deacetylation, respectively [61]. Figure 3 shows the role of PGC-1α and other transcriptional factors involved in mitochondrial biogenesis. Many experimental and clinical studies revealed the alterations in the morphology and number of mitochondria in heart, skeletal muscles and liver tissues in pathogenic conditions [1, 62-66].
Figure 3.
Pathways involved in mitochondrial biogenesis
4. Mitochondrial dynamics
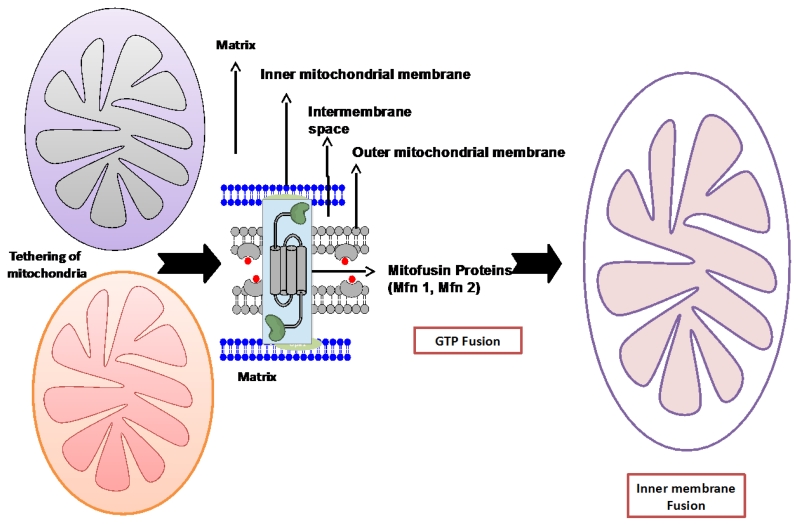
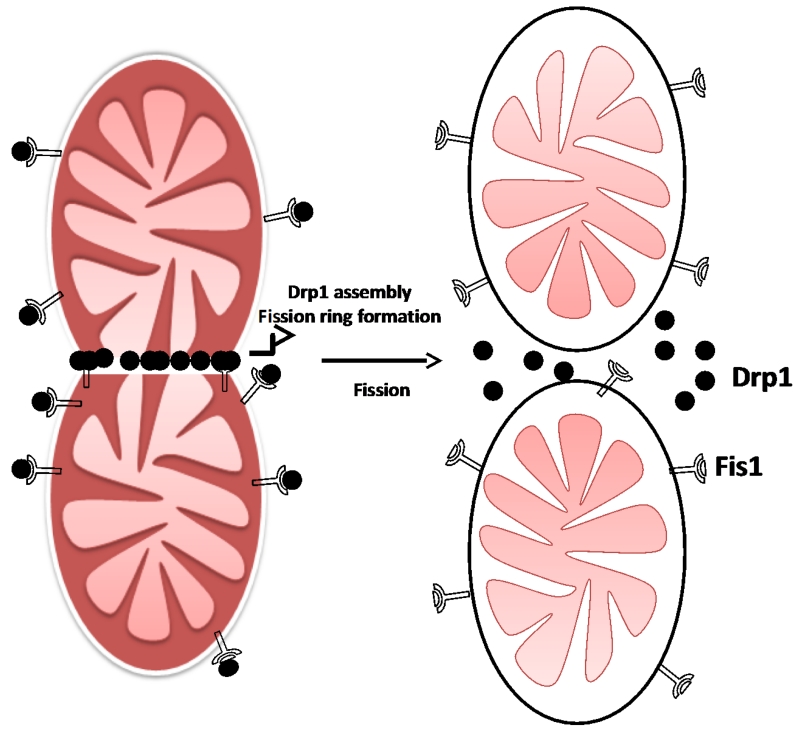
Mitochondrial dynamics is a delicate physiological balance between fission and fusion events of mitochondria, which is essential for their maintenance in the growing cells, regulation of cell death pathway, and removal of damaged mitochondria [67, 68]. These mitochondrial events were first described in budding yeast [69]. Mitochondrial morphology varies tremendously in the cells and tissues in response to external stimuli and availability of nutrients. Mitophagy is an autophagy-lysosome system that removes dysfunctional mitochondria through fusion with lysosomes [70]. Mitochondrial fragmentation occurs in response to nutrient excess and cellular dysfunction escalates the prevalence of obesity, cancer, cardiovascular and neuromuscular disorders[71]. A mitochondrial defect leads to impaired oxidative capacity which then causes the overproduction of ROS. Over the past several years, many key regulators of fusion and fission have been identified. There are three GTPase genes that regulate the process of mitochondrial fusion viz. mitofusin 1 and 2 (Mfn1/2) and optic atrophy1 (Opa1), localized in the outer and inner mitochondrial membranes, respectively [72, 73]. Mfn 1/2 are located on the outer mitochondrial membrane, whereas Opa 1 is localized to the inner mitochondrial membrane (Figure 4). Mitochondrial fission, on the other hand, is regulated by highly conserved two GTPase genes, Fis1 and Drp1, located on outer membrane and in the cytosol, respectively. In the fusion process, mitochondrial content is intermixed and electrical conductivity maintained throughout the mitochondria [74-76]. Disturbed mitochondrial dynamics includes abnormal and/or impaired fission/fusion that occurs in response to nutrient excess and cellular dysfunction, directly impacts mitochondrial function, resulting in an excessive generation of ROS, altered mitochondrial enzymatic activities, impaired calcium homeostasis, diminished ATP production, and overall reduced energy metabolism in mammalian cells. Earlier studies documented the possible role of these mitochondrial alterations in escalation of metabolic and neurodegenerative disorders including aging, cancer, type 2 diabetes, obesity and dementia [34, 36, 56, 77-88]. It also plays a pivotal role in the age-dependent decline in mitochondrial biogenesis. Increased ROS has been documented in the aging process. This increase may be primarily due to enhanced accumulation of mtDNA mutations which in turn could damage the mitochondrial structure and functions, consequently, altering antioxidant defense system, disturbed calcium homeostasis, low ATP generation and ultimately leading to many pathogenic conditions.
Figure 4.
(A). Mitochondrial fusion process
(B). Mitochondrial fission process
5. Oxidative stress and mitochondrial dysfunction
Oxidative stress refers to the imbalance of two opposite and antagonistic forces, production of ROS and antioxidants, wherein the damaging effects of ROS is more powerful compared to the compensatory effect of antioxidants in the cells. Mitochondrial dysfunction is defined as diminished mitochondrial biogenesis, altered membrane potential, and the decrease in mitochondrial number and altered activities of oxidative proteins due to the accumulation of ROS in cells and tissues [89]. Regular metabolism of oxygen generates reactive oxygen species as a by-product. Indeed, mitochondria are the most important source of ROS in most of the mammalian cells [43]. ROS produced in mitochondria during OXPHOS process primarily triggered mitochondrial dysfunction by interacting with mitochondrial and cellular components such as DNA, proteins, lipids, and other molecules [90, 91].
Metabolic syndrome represent a constellation of many risk factors such as obesity, higher blood pressure, abnormal levels of triglycerides and cholesterol etc. which are involved in the higher prevalence of non-communicable diseases. Altered mitochondrial functioning has been implicated in the pathophysiology of type 2 diabetes, obesity, dyslipidemia, and cardiovascular diseases. The imbalance between energy production and its utilization - may leads to defective cell metabolism which is considered as the main culprits of metabolic syndrome [92]. Increased glucose levels enhance overproduction of ROS which leads to morphological changes in mitochondria [93]. Inhibition of insulin signaling pathway caused the accumulation of lipids and free fatty acids (FFA) contributes to the metabolic disorders [94-99]. Also, it is evident that aging, altered mitochondrial biogenesis and decreased antioxidant defense capacity along with genetic factors caused insulin resistance which is the major cause of many metabolic diseases. In the following sections, we have established the link between mitochondrial dysfunction and individual components of metabolic syndrome.
6. Mitochondrial dysfunction and Insulin Resistance
Insulin resistance (IR) is characterized by diminished capacity of the cells to respond to the physiological levels of insulin. Various risk factors including aging, physical inactivity, abdominal obesity, stress etc. contribute to the pathophysiology of insulin resistance. Oxidative stress induced by an excess of ROS in mitochondria might also contribute to the development of insulin resistance [1, 16, 100]. It is evident from previous studies that oxidative stress and mitochondrial dysfunction are involved in the pathophysiology of many metabolic disease states such as insulin resistance, obesity diabetes, and many cardiovascular and neurodegenerative diseases [101-112]. However, it is still not clear whether insulin resistance is the primary cause of mitochondrial dysfunction or vice-versa. Nonetheless, many studies established the association of mitochondrial dysfunction with insulin resistance in various tissues [64, 113-117]. In skeletal muscle, altered mitochondrial functions, reduced ATP synthesis, and increased ROS generation leads to insulin resistance and obesity/diabetes [66, 118, 119]. The imbalance between energy production and its utilization may result in impaired cell metabolism which is considered as the main culprits of metabolic syndrome [92]. Increased glucose levels enhance overproduction of ROS which lead to morphological changes in mitochondria [93]. Inhibition of insulin signaling pathway caused the accumulation of lipids and free fatty acids (FFA) which contributes to insulin resistance and other metabolic disorders [94-99]. Over the past decade, many studies on human subjects and rodents provide evidences for alterations in markers of mitochondrial metabolism in insulin resistant individuals. These observations led to the theory that compromised mitochondrial oxidative functions, particularly in skeletal muscle, causes excess lipid deposition, and development of insulin resistance. Considerable research indicates that decrease in fatty acid oxidation causes the inhibition of insulin signaling, which consequently leads to FFA and insulin resistance and further reduces the mitochondrial oxidative capacity and ATP synthesis in obese and insulin resistance models [92, 100]. Indeed, further clinical research studies are necessary to investigate the role of antioxidants and antioxidant pathways that drive mitochondrial functions and insulin sensitivity in humans.
7. Mechanistic link between diabetes and oxidative stress/mitochondrial dysfunction
Over the past 20 years, there has been an explosive increase in the incidence and prevalence of type 2 diabetes and this increase becomes a major public health problem all over the world. Presently more than 382 million people are suffering from type 2 diabetes, and it is predicted that this number will rise to 438 million in 2030. India, China, and USA are the worst affected countries bearing the major burden of type 2 diabetes in the world. Although many experimental and clinical studies established the role of environmental and genetic factors associated with the pathophysiology of type 2 diabetes but the primary cause of this problem is still unknown. Both pancreatic β-cell dysfunction and Insulin resistance in insulin sensitive tissues including adipocytes, myocytes, and hepatocytes have been implicated in the etiology of diabetes and related complications. Also, recent studies have indicated abnormal mitochondrial dynamics along with overproduction of ROS in diabetic patients [93, 120]. T2DM results from a combination of reduced tissue sensitivity to insulin and inadequate insulin secretion. It usually develops in adults and is thought to stem from a complex interaction between obesity, physical inactivity, diet and genes [121]. A recent study done on diabetic and obese patients established the impaired glucose and lipid homeostasis in skeletal muscle [116]. Increased fat mass leads to several factors that inhibit insulin action including decreased glucose transporter type 4 (GLUT4), increased free fatty acids and other circulating molecules [122, 123]. Due to reduced insulin response in sensitive tissues, excess glucose accumulates leading to chronic hyperglycemia [121].
Several studies have documented the role of mitochondrial dysfunction in the pathophysiology of T2DM [66, 88, 124-128]. Reduced mitochondrial respiration, ATP production and mitochondrial density and mRNA have been reported in the insulin resistance and type 2 diabetic patients [54, 96, 129-132]. A short-term high-calorie diet resulted in increased markers of oxidative stress and a transient increase in OXPHOS enzyme protein expression. The mtDNA is more predominantly susceptible to oxidative damage induced by the excess of ROS during OXPHOS process in mitochondria of the brain [133] in obesity and T2DM. Mitochondrial dysfunction inhibits insulin signaling pathway through the overproduction of ROS and interfering with oxidation of acetyl CoA, consequently resulting in increased lipid and diacylglycerol [1, 48, 66, 134]. Mitochondrial biogenesis is reduced in the condition of diabetes and obesity [116, 135, 136]. PGC-1α also regulates the process of mitochondrial biogenesis [137, 138]. Furthermore, mitochondrial dysfunction seems to play a vital role in the pathophysiology of IR and T2DM and may be considered as a target for therapeutic measures in metabolic diseases.
8. Mechanistic link between obesity and oxidative stress/mitochondrial dysfunction
Currently, obesity has become one of the major global health problems. It is one of the principal components of metabolic syndrome and known to be a major risk factor in the development of many metabolic disorders. Although obesity is caused by the interaction of genetic and environmental factors, the role of obesity in mitochondrial dysfunction has been revealed in many studies. Recent studies demonstrated the role of mitochondrial dysfunction in the pathogenesis of components of metabolic syndrome. There is an overproduction of ROS in adipose tissues with altered activities of NADPH oxidase and antioxidative enzymes in obese mice [139]. Intriguingly, abdominal obesity has been associated with defective mitochondrial biogenesis manifested by impaired mitochondrial dysfunction, oxidative metabolism, low mitochondrial gene expression and reduced ATP generation in rodents and humans [63, 140-142]. Also, mtDNA, respiratory protein, and mtDNA transcription factor A (Tfam) gene expressions were markedly reduced in obese mice. Also altered mitochondrial dynamics plays a pivotal role in mitochondrial dysfunction linked to obesity as evident from reduced expression of mitofusin 2 gene in skeletal muscle [143].
9. Mechanistic link between heart disease and oxidative stress/mitochondrial dysfunction
Cardiovascular diseases (CVD) includes atherosclerosis, ischemic heart disease, cardiomyopathy, cardiac hypertrophy and heart failure are the leading cause of mortality, worldwide. CVD is multifactorial in nature involving the interactions of both environmental and genetic risk factors in cardiovascular diseases. It is evident from previous studies that oxidative stress may be associated with increased mtDNA damage in CAD patients. In the heart, the ROS can be generated in cardiac myocytes, endothelial cells, and neutrophils. In vivo and ex vivo studies have established the role of oxidative stress induced by the excess of ROS in a wide range of cardiovascular diseases [6, 13, 25, 32, 144-148]. The majority of ROS in the heart are generated by uncoupling of mitochondrial ETC complexes I and III [49, 149]. However, there are other mechanisms such as NADPH oxidase, xanthine oxidoreductase, or NOS by which ROS are generated and induce oxidative damage in heart tissue. The excess of ROS leads to cellular injury and declined antioxidant capacity, which seems to be due to defects in mitochondrial functions and mtDNA damage, endothelial dysfunction and altered gene expression [6]. The reduced mitochondrial oxidative capacity contributes to cardiac dysfunction. The ROS are significantly enhanced in failing myocardium [51, 148, 150-152]. Recent findings have established the role of ROS, pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), altered mitochondrial biogenesis and mtDNA damage, structural and morphological changes in mitochondria contributing to the development and progression of heart diseases such as heart failure and cardiac dysfunction [147, 153, 154]. Moreover, ROS stimulate contractile function, activate a variety of enzymes, transcription factors, and induce apoptosis. Thus ROS play a central role in the pathophysiology of cardiovascular diseases.
10. Mechanistic link between stroke and oxidative stress/mitochondrial dysfunction
Stroke is the fourth leading cause of adult disability and mortality in the developing world, associated with social and economic problems [155]. Oxidative stress is one of the contributing factor leading to cellular damage during ischemic brain injury [156]. Mitochondrial dysfunctions have been demonstrated as a key player in the development of brain stroke as evident by reduced ATP production, the starvation of glucose and oxygen to the tissues and influence on cell death pathways [157]. In experimental models of stroke, the diminished supply of glucose and oxygen leads to impaired oxidative metabolism in brain tissue [158]. The resultant oxygen-glucose deprivation in the brain tissue causes the accumulation of reducing intermediates and leads to enhanced ROS formation [156]. During oxidative stress, the excess of ROS alter antioxidant defense mechanism by reducing the scavenging capacity of antioxidant enzymes that could lead to altered mitochondrial functions by interacting with mitochondrial and cellular components such as DNA, proteins, and lipids [91, 159]. In focal ischemia, the damaging action of oxidative stress in necrosis and apoptosis has been explained in previous studies [160, 161]. Peroxynitrite radical also plays a significant role in the pathogenesis of brain stroke. Thus overproduction of ROS in mitochondria significantly induces oxidative damage in the ischemic and post-ischemic brain [157, 162, 163].
11. Pharmacologic strategies to target mitochondrial dysfunction
Mitochondrial stress pathways have been implicated in disease manifestations of mitochondrial dysfunction and could highlight promising therapeutic targets [164-166]. Several lines of evidence imply that mitochondrial dysfunction plays a central role in the pathophysiology of metabolic disorders, age, and age-related neurodegenerative diseases. So targeting mitochondria might be a promising strategy for potential therapeutic purposes in metabolic disorders and age-related neurodegenerative diseases. Following strategies might be employed to diminish the mitochondrial dysfunction caused by excessive generation of ROS that induced oxidative stress.
12. Lifestyle interventions
Mitochondrial dysfunction contributes to severity of many pathological conditions including skeletal muscle atrophy, diabetes, cardiovascular diseases and many metabolic disorders and neurodegenerative diseases. Recent studies indicated that physical activity offers many benefits through improved insulin sensitivity and mitochondrial biogenesis in skeletal muscles in T2D patients. Also, a significant increase in muscle mitochondrial respiration and mitochondrial content, oxidative enzyme activity and mitochondrial density were observed in T2D patients after exercise [61, 167-173]. Exercise stimulates AMPK, leading to activation of PGC 1 by direct phosphorylation of threonine and serine residues [174]. This phosphorylation event may ultimately promote mitochondrial biogenesis. Also, regular exercise triggers many other signalling pathways involved in skeletal muscle mitochondrial biogenesis, dynamics and metabolism in healthy and aged persons [174, 175]. Aging causes loss of muscle mass and structural changes in the neuromuscular components resulting in impaired contractile function. Exercise induces beneficial adaptations that slow down the progression of age-related muscle functional decline. Reduced physical activity and sedentary lifestyle are also the major contributing factors for escalating the prevalence of obesity, type 2 diabetes and many other metabolic disorders [176]. Several studies established the pleiotropic effect of physical exercise on mitochondrial dynamics in aging skeletal muscle [177]. Nutrients control the glucose homeostasis through a complex of PGC-1α and SIRT1 [178]. Reduced energy results increased AMP/ATP ratio and activate AMPK which then regulate PGC-1α through phosphorylation. Calorie restriction or exercise also increases tissue NAD+ content thereby activates SIRT1, which then activates PGC-1α through deacetylation [178]. Nitric oxide also appears to be an important regulator of mitochondrial biogenesis. Previous studies shows that defect in PGC-1α are associated with insulin sensitivity in type 2 diabetic patients [54].
In addition to regular exercise, calorie restriction (CR) without malnutrition is also a promising non-genetic and non-pharmacologic nutritional intervention that prolongs the lifespan of a variety of organisms and helps in the prevention of age-related metabolic disorders [179-181]. However, the molecular mechanisms of calorie restrictions induced benefits in aging and related disorders are still not clear. Indeed, earlier studies reported that CR reduces the over production of ROS and oxidative damage, leading to enhanced mitochondrial function in humans and be an effective remedy for the treatment of obesity and insulin resistance [182-185]. CR has been also been reported to substantially elevate the insulin sensitivity in many species including humans, nonhuman primates, dogs, mice, and rats [186, 187]. Recent studies demonstrated that CR leads to increased Akt2 activity and glucose uptake by insulin-stimulated skeletal muscle in aged rats [188, 189]. Finally, data from various epidemiological, clinical and experimental studies have shown that CR exerts additional beneficial health effects by inhibiting key nutrient-sensing and inflammatory pathways and remains the cornerstone in the prevention and treatment of metabolic disorders [190].
13. Pharmacological interventions
The recent literature revealed that oxidative stress in the cell leads to structural and functional changes in the mitochondria. These mitochondrial changes triggers cell signaling pathways and generate uncontrollable ROS which ultimately leads to organ failure and diseases. Therefore pharmaceutical drugs that can limit the overproduction of ROS in the cells may be the potential therapeutic solution to improve mitochondrial health in a wide range of diseases. Although the molecular mechanisms of mitochondria-mediated diseases are uncertain, oxidative stress induced by overproduction ROS in mitochondria seems to be the major risk for development of metabolic and neurodegenerative diseases. Growing shreds of evidence demonstrated that NAD-dependent deacetylase family (Sirtuins), SIRT1 is involved in many cellular processes including regulation of glucose and lipid metabolism, through insulin signaling in the liver, adipose tissue, and skeletal muscles [191-202]. Newer pharmacologic approaches have been proposed to improve mitochondrial function. Resveratrol, an activator of SIRT1, found in grapes have strong antioxidant properties and improves insulin resistance. Activation of SIRT1 gene protects the cells against inflammation and oxidative stress. It activates PGC-1α that promotes glucose uptake and mitochondrial biogenesis [195, 203, 204]. Mitochondrial fission has been implicated in various metabolic conditions, the inhibitors of mitochondrial fission may be used as therapeutic targets to treat patients with metabolic disorders [205]. Three inhibitors of mitochondrial fission have been identified as Mdivi 1, P110, and Dynasore [206-208] which play an ameliorative role against mitochondrial oxidative stress.
14. Mitochondria-targeted antioxidants
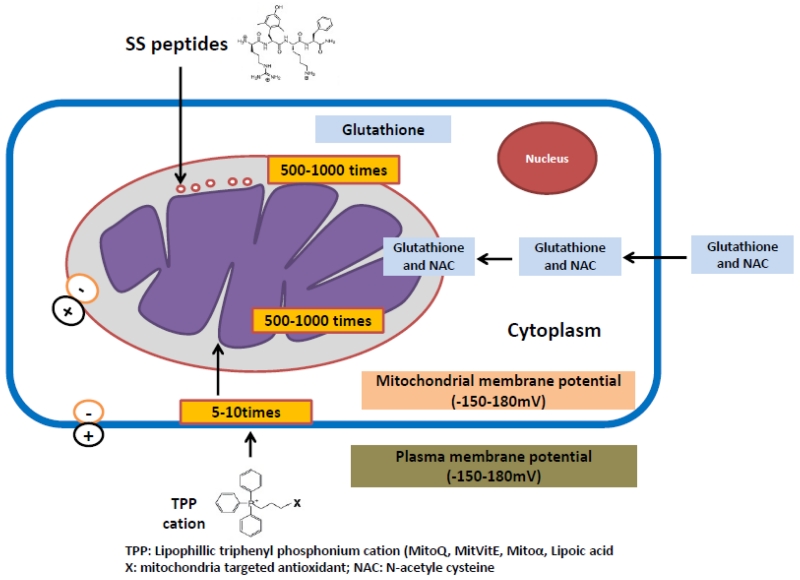
Metabolic syndrome affects 20%-30% of the world’s population. The involvement of oxidative stress and mitochondrial dysfunction in the pathophysiology of metabolic disorders has been widely established. The mitochondria targeted antioxidants therapies could be potential treatment for a number of pathologic conditions including neurodegenerative or metabolic diseases. Recently, in vivo and in vitro studies done in experimental animals and humans, reported ameliorative role of mitochondria targeted antioxidants against metabolic disorders [209-212]. The commonly used vitamins (vitamin E and C) and other chemical compounds with antioxidant properties such as coenzyme Q, a-lipoic acid, N-acetylcysteine (NAC) have been used to reduce the excess generation of ROS in various metabolic conditions [213-215]. In the recent years, antioxidant compounds incorporating ubiquinone (MitoQ) or vitamin E (MitoVit E) specifically targeted to mitochondria have been successively used against mitochondrial dysfunctions [216] (Figure 5). Recent studies have demonstrated that mitochondria-targeted antioxidants are far better in reducing oxidative damage in mitochondria [216-219].
Figure 5.
MitoQ is a potent mitochondria-targeted therapeutic antioxidant in which lipophilic triphenylphosphonium (TPP) cation is bound to ubiquinone antioxidant moiety of the endogenous antioxidant co-enzyme Q10 [220]. The lipophilic nature of TPP-cation enables MitoQ to cross phospholipid bilayers that leads to its accumulation many hundred-fold within mitochondria and reduce ROS in the mitochondria and protect against age-related mitochondrial insult in brain tissue [216, 217, 220]. Recent studies established the defensive action of MitoQ in metabolic syndrome by affecting the redox signaling pathways [221, 222]. These findings support the understanding that increased ROS in mitochondria may contribute to the etiology of a wide variety of diseases [223-226]. Now a day MitoQ has been extensively used as a potential therapeutic molecule for the treatment of neurodegenerative diseases [227-229]. Like MitoQ, MitoVitE, a TPP-conjugated vitamin E is another mitochondria-targeted vitamin E derivative, which can easily pass through the lipid bilayers and accumulate 100-1000-fold within mitochondria [230, 231]. It also protects mitochondria against oxidative damage induced by the excess of ROS in many pathogenic conditions [217, 220, 230]. It also protects mitochondria against oxidative damage induced by the excess of ROS in many pathogenic conditions [217, 220, 230]. MitoVit E has been shown to be 350-fold more potent than untargeted vitamin E in protecting against cell death induced by mitochondrial oxidative damage [219].
15. Conclusions and future perspectives
Mitochondria are the cytoplasmic organelles responsible for cell survival and cell death. The major function of mitochondria is to ‘energy’ adenosine triphosphate (ATP) to the cells by metabolizing nutrients and responsible for cellular processes ranging from energy metabolism, generation of reactive oxygen species and Ca2+ homeostasis, cell survival, and death. Mitochondrial structural and functional changes are reported to involve in aging, cancer, metabolic syndromes, including stroke, ischemia, pre-diabetes, diabetes, obesity, hypertension, dyslipidemia, heart disease, alcohol injury, and neurodegenerative diseases. Recent research revealed that mitochondrial abnormalities, including impaired mitochondrial dynamics, defects in mitochondrial biogenesis, mitochondrial dysfunction, and oxidative stress are primarily involved in metabolic syndromes. Recent research also revealed that maintaining mitochondrial dynamics (fission-fusion balance) and mitochondrial function are necessary to treat patients with metabolic syndromes. To reduce and/or delay the progression of disease in metabolic syndromes, many therapeutic approaches are useful, including – lifestyle intervention (healthy diet & regular exercise), pharmaceutical strategies, and treating patients with mitochondrial-targeted molecules. However, molecular links between metabolic syndromes and mitochondrial structural/functional changes are not well understood. Further genetics and genetic susceptibility to patients with metabolic syndromes, in relation to aging, are poorly understood. The role of epigenetics in patients with metabolic syndromes is unclear. Also, current generalized treatments to patients with metabolic syndromes may not be efficient and work because of body physiology different from population to population. Further research is urgently needed to answer these questions.
Highlights.
Mitochondrial dysfunction and oxidative stress are largely involved in aging, cancer, age-related neurodegenerative and metabolic syndrome.
The overproduction of ROS has been associated with oxidative damage inflicted on lipids, DNA, and proteins.
Mitochondrial stress pathways have been implicated in disease manifestations of mitochondrial dysfunction and could be the promising therapeutic targets for the prevention of metabolic diseases.
Acknowledgements
Work presented in this article is supported by NIH grants AG042178, AG047812 and the Garrison Family Foundation. Dr. Jasvinder Singh Bhatti is financially supported by University Grants Commission, India under Raman Post-Doctoral Research Fellowship in USA [F.No. 5-82/2016 (IC)].
Abbreviations
- ATP
Adenosine Triphosphate
- OXPHOS
Oxidative Phosphorylation
- ROS
Reactive Oxygen Species
- RNS
Reactive Nitrogen Species
- TCA
Tricarboxylic Acid
- IR
Insulin Resistance
- MetS
Metabolic Syndrome
- ETC
Electron Transport Chain
- SOD
Superoxide Dismutase
- GPx
Glutathione Peroxidase
- GSH
Glutathione
- CAT
Catalase
- PGC1α
Peroxisome Proliferator-Activated Receptor Gamma – Coactivator 1 alpha
- TNF-α
Tumor Necrosis Factor-Alpha
- T2DM
Type 2 Diabetes Mellitus
- CR
Caloric Restricted
- MitoQ
Mitochondria-Targeted Quinone
- SS31
Szeto-Schiller Peptide 31
- NAC
N-acetylcysteine
- mtDNA
Mitochondrial DNA
- ERR
Oestrogen Related Receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circulation research. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th ed. Oxford University Press; Oxford: 2007. [Google Scholar]
- [3].Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN, Bobryshev YV. Mitochondrial aging and age-related dysfunction of mitochondria. BioMed research international. 2014;2014:238463. doi: 10.1155/2014/238463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant physiology. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- [6].Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. Journal of hypertension. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- [7].Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clinical chemistry. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- [8].Jenner P. Oxidative stress in Parkinson’s disease. Annals of neurology. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36-28. [DOI] [PubMed] [Google Scholar]
- [9].Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative medicine and cellular longevity. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Current medicinal chemistry. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- [11].Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M. Oxidative Stress in Neurodegenerative Diseases. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nature reviews. Drug discovery. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- [13].Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure, American journal of physiology. Heart and circulatory physiology. 2011;301:H2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- [14].Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, Jacobs DR., Jr. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes care. 2009;32:1302–1307. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, Yongvanit P, Kawanishi S, Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. International journal of molecular sciences. 2015;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life sciences. 2009;84:705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- [17].Schwarz PE, Reimann M, Li J, Bergmann A, Licinio J, Wong ML, Bornstein SR. The Metabolic Syndrome - a global challenge for prevention. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2007;39:777–780. doi: 10.1055/s-2007-990312. [DOI] [PubMed] [Google Scholar]
- [18].Grundy SM. Metabolic syndrome pandemic. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- [19].C. Global Burden of Metabolic Risk Factors for Chronic Diseases Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. The lancet. Diabetes & endocrinology. 2014;2:634–647. doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pan WH, Yeh WT, Weng LC. Epidemiology of metabolic syndrome in Asia. Asia Pacific journal of clinical nutrition. 2008;17(Suppl 1):37–42. [PubMed] [Google Scholar]
- [21].Pollex RL, Hegele RA. Genetic determinants of the metabolic syndrome, Nature clinical practice. Cardiovascular medicine. 2006;3:482–489. doi: 10.1038/ncpcardio0638. [DOI] [PubMed] [Google Scholar]
- [22].Takahara M, Shimomura I. Metabolic syndrome and lifestyle modification. Reviews in endocrine & metabolic disorders. 2014;15:317–327. doi: 10.1007/s11154-014-9294-8. [DOI] [PubMed] [Google Scholar]
- [23].Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metabolic syndrome and related disorders. 2009;7:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- [24].Bhatti GK, Bhadada SK, Vijayvergiya R, Mastana SS, Bhatti JS. Metabolic syndrome and risk of major coronary events among the urban diabetic patients: North Indian Diabetes and Cardiovascular Disease Study-NIDCVD-2. Journal of diabetes and its complications. 2016;30:72–78. doi: 10.1016/j.jdiacomp.2015.07.008. [DOI] [PubMed] [Google Scholar]
- [25].Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, Ohta Y, Sami M, Tachibana T, Ishikawa H, Kurosawa H, Kahn RC, Otsu K, Shirasawa T. Oxidative stress causes heart failure with impaired mitochondrial respiration. The Journal of biological chemistry. 2006;281:33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- [26].Ohta Y, Kinugawa S, Matsushima S, Ono T, Sobirin MA, Inoue N, Yokota T, Hirabayashi K, Tsutsui H. Oxidative stress impairs insulin signal in skeletal muscle and causes insulin resistance in postinfarct heart failure. American journal of physiology. Heart and circulatory physiology. 2011;300:H1637–1644. doi: 10.1152/ajpheart.01185.2009. [DOI] [PubMed] [Google Scholar]
- [27].Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxidants & redox signaling. 2011;15:1911–1926. doi: 10.1089/ars.2010.3739. [DOI] [PubMed] [Google Scholar]
- [28].Ozgen IT, Tascilar ME, Bilir P, Boyraz M, Guncikan MN, Akay C, Dundaroz R. Oxidative stress in obese children and its relation with insulin resistance. Journal of pediatric endocrinology & metabolism: JPEM. 2012;25:261–266. doi: 10.1515/jpem-2011-0397. [DOI] [PubMed] [Google Scholar]
- [29].Poitout V, Tanaka Y, Reach G, Robertson RP. Oxidative stress, insulin secretion, and insulin resistance. Journ Annu Diabetol Hotel Dieu. 2001:75–86. [PubMed] [Google Scholar]
- [30].Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain research. 2010;1359:178–185. doi: 10.1016/j.brainres.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nature chemical biology. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Droge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- [33].Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- [34].Roy M, Reddy PH, Iijima M, Sesaki H. Mitochondrial division and fusion in metabolism. Current opinion in cell biology. 2015;33:111–118. doi: 10.1016/j.ceb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Human molecular genetics. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- [36].Blake R, Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochimica et biophysica acta. 2014;1840:1404–1412. doi: 10.1016/j.bbagen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- [37].Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- [38].Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain research reviews. 2011;67:103–118. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nature reviews. Genetics. 2005;6:815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- [40].Sherratt HS. Mitochondria: structure and function. Revue neurologique. 1991;147:417–430. [PubMed] [Google Scholar]
- [41].Dallner G, Sindelar PJ. Regulation of ubiquinone metabolism. Free radical biology & medicine. 2000;29:285–294. doi: 10.1016/s0891-5849(00)00307-5. [DOI] [PubMed] [Google Scholar]
- [42].Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry. Biokhimiia. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- [43].Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [45].Beckman KB, Ames BN. Endogenous oxidative damage of mtDNA. Mutation research. 1999;424:51–58. doi: 10.1016/s0027-5107(99)00007-x. [DOI] [PubMed] [Google Scholar]
- [46].Turrens JF. Mitochondrial formation of reactive oxygen species. The Journal of physiology. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].James AM, Murphy MP. How mitochondrial damage affects cell function. Journal of biomedical science. 2002;9:475–487. doi: 10.1159/000064721. [DOI] [PubMed] [Google Scholar]
- [48].Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- [49].Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circulation research. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- [50].Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circulation research. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- [51].Tsutsui H, Ide T, Hayashidani S, Suematsu N, Shiomi T, Wen J, Nakamura K, Ichikawa K, Utsumi H, Takeshita A. Enhanced generation of reactive oxygen species in the limb skeletal muscles from a murine infarct model of heart failure. Circulation. 2001;104:134–136. doi: 10.1161/01.cir.104.2.134. [DOI] [PubMed] [Google Scholar]
- [52].Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular medicine. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Current opinion in lipidology. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature genetics. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- [55].Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. Journal of biomedicine & biotechnology. 2006;2006:31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain research. 2011;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovascular research. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- [58].Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays in biochemistry. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annual review of physiology. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- [60].Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. The Journal of physiology. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. Journal of molecular medicine. 2010;88:993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nisoli E, Clementi E, Carruba MO, Moncada S. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circulation research. 2007;100:795–806. doi: 10.1161/01.RES.0000259591.97107.6c. [DOI] [PubMed] [Google Scholar]
- [64].Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clinical science (London, England: 1979) 2008;114:195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- [65].Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- [66].Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science (New York, N.Y.) 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- [67].Hales KG. The machinery of mitochondrial fusion, division, and distribution, and emerging connections to apoptosis. Mitochondrion. 2004;4:285–308. doi: 10.1016/j.mito.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [68].Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends in cell biology. 2002;12:178–184. doi: 10.1016/s0962-8924(01)02246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biological chemistry. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wai T, Langer T. Mitochondrial Dynamics and Metabolic Regulation. Trends in endocrinology and metabolism: TEM. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- [72].Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- [74].Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochimica et biophysica acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Elgass K, Pakay J, Ryan MT, Palmer CS. Recent advances into the understanding of mitochondrial fission. Biochimica et biophysica acta. 2013;1833:150–161. doi: 10.1016/j.bbamcr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- [76].Hoppins S. The regulation of mitochondrial dynamics. Current opinion in cell biology. 2014;29:46–52. doi: 10.1016/j.ceb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- [77].Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochimica et biophysica acta. 2012;1822:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trends in cell biology. 2013;23:64–71. doi: 10.1016/j.tcb.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cho DH, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cellular and molecular life sciences: CMLS. 2010;67:3435–3447. doi: 10.1007/s00018-010-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxidants & redox signaling. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Molecular and cellular biology. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen L, Knowlton AA. Mitochondrial dynamics in heart failure. Congestive heart failure. 2011;17:257–261. doi: 10.1111/j.1751-7133.2011.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Human molecular genetics. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Human molecular genetics. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Reddy PH. Increased mitochondrial fission and neuronal dysfunction in Huntington’s disease: implications for molecular inhibitors of excessive mitochondrial fission. Drug discovery today. 2014;19:951–955. doi: 10.1016/j.drudis.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rocha M, Rovira-Llopis S, Banuls C, Bellod L, Falcon R, Castello R, Morillas C, Herance JR, Hernandez-Mijares A, Victor VM. Mitochondrial dysfunction and oxidative stress in insulin resistance. Current pharmaceutical design. 2013;19:5730–5741. doi: 10.2174/13816128113199990373. [DOI] [PubMed] [Google Scholar]
- [88].Parish R, Petersen KF. Mitochondrial dysfunction and type 2 diabetes. Current diabetes reports. 2005;5:177–183. doi: 10.1007/s11892-005-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Experimental and molecular pathology. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- [90].Harper ME, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, oxidative stress, and mitochondrial uncoupling. Acta physiologica Scandinavica. 2004;182:321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- [91].Hu F, Liu F. Mitochondrial stress: a bridge between mitochondrial dysfunction and metabolic diseases? Cellular signalling. 2011;23:1528–1533. doi: 10.1016/j.cellsig.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gastaldi G, Giacobino JP, Ruiz J. Metabolic syndrome, a mitochondrial disease? Revue medicale suisse. 2008;4:1387–1388. 1390–1381. [PubMed] [Google Scholar]
- [93].Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free radical biology & medicine. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- [94].Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochimica et biophysica acta. 2004;1688:95–101. doi: 10.1016/j.bbadis.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [95].Maassen JA, LM TH, Van Essen E, Heine RJ, Nijpels G, Jahangir Tafrechi RS, Raap AK, Janssen GM, Lemkes HH. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53(Suppl 1):S103–109. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- [96].Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. The Journal of clinical investigation. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance, American journal of physiology. Heart and circulatory physiology. 2007;293:H2009–2023. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- [98].Smith SC., Jr. Multiple risk factors for cardiovascular disease and diabetes mellitus. The American journal of medicine. 2007;120:S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [99].Sowers JR. Insulin resistance and hypertension. American journal of physiology. Heart and circulatory physiology. 2004;286:H1597–1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- [100].Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends in endocrinology and metabolism: TEM. 2008;19:324–330. doi: 10.1016/j.tem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- [101].Chen L, Xu WM, Zhang D. Association of abdominal obesity, insulin resistance, and oxidative stress in adipose tissue in women with polycystic ovary syndrome. Fertil Steril. 2014;102:1167–1174. e1164. doi: 10.1016/j.fertnstert.2014.06.027. [DOI] [PubMed] [Google Scholar]
- [102].Das P, Biswas S, Mukherjee S, Bandyopadhyay SK. Association of Oxidative Stress and Obesity with Insulin Resistance in Type 2 Diabetes Mellitus. Mymensingh Med J. 2016;25:148–152. [PubMed] [Google Scholar]
- [103].Korkmaz GG, Altinoglu E, Civelek S, Sozer V, Erdenen F, Tabak O, Uzun H. The association of oxidative stress markers with conventional risk factors in the metabolic syndrome. Metabolism: clinical and experimental. 2013;62:828–835. doi: 10.1016/j.metabol.2013.01.002. [DOI] [PubMed] [Google Scholar]
- [104].Meigs JB, Larson MG, Fox CS, Keaney JF, Jr., Vasan RS, Benjamin EJ. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diabetes care. 2007;30:2529–2535. doi: 10.2337/dc07-0817. [DOI] [PubMed] [Google Scholar]
- [105].Calo LA, Maiolino G, Naso A, Davis PA. The association of systemic oxidative stress with insulin resistance: mechanistic insights from studies in Bartter’s and Gitelman’s syndromes. Clinical endocrinology. 2015;83:994–995. doi: 10.1111/cen.12817. [DOI] [PubMed] [Google Scholar]
- [106].Mitsuyoshi H, Itoh Y, Sumida Y, Minami M, Yasui K, Nakashima T, Okanoue T. Evidence of oxidative stress as a cofactor in the development of insulin resistance in patients with chronic hepatitis C. Hepatology research: the official journal of the Japan Society of Hepatology. 2008;38:348–353. doi: 10.1111/j.1872-034X.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- [107].Luque-Contreras D, Carvajal K, Toral-Rios D, Franco-Bocanegra D, Campos-Pena V. Oxidative stress and metabolic syndrome: cause or consequence of Alzheimer’s disease? Oxidative medicine and cellular longevity. 2014;2014:497802. doi: 10.1155/2014/497802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Keaney JF, Jr., Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ, Framingham S. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- [109].Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circulation research. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- [110].Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kostis JB, Sanders M. The association of heart failure with insulin resistance and the development of type 2 diabetes. American journal of hypertension. 2005;18:731–737. doi: 10.1016/j.amjhyper.2004.11.038. [DOI] [PubMed] [Google Scholar]
- [112].Reaven GM, Chen YD. Insulin resistance, its consequences, and coronary heart disease. Must we choose one culprit? Circulation. 1996;93:1780–1783. doi: 10.1161/01.cir.93.10.1780. [DOI] [PubMed] [Google Scholar]
- [113].Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- [114].De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. The American journal of pathology. 2009;175:927–939. doi: 10.2353/ajpath.2009.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hojlund K, Mogensen M, Sahlin K, Beck-Nielsen H. Mitochondrial dysfunction in type 2 diabetes and obesity. Endocrinology and metabolism clinics of North America. 2008;37:713–731. x. doi: 10.1016/j.ecl.2008.06.006. [DOI] [PubMed] [Google Scholar]
- [116].Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- [117].Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147:2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- [118].Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- [119].Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. The international journal of biochemistry & cell biology. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- [121].Pirola L, Johnston AM, Van Obberghen E. Modulation of insulin action. Diabetologia. 2004;47:170–184. doi: 10.1007/s00125-003-1313-3. [DOI] [PubMed] [Google Scholar]
- [122].Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. American journal of physiology. Endocrinology and metabolism. 2005;289:E551–561. doi: 10.1152/ajpendo.00116.2005. [DOI] [PubMed] [Google Scholar]
- [123].Leguisamo NM, Lehnen AM, Machado UF, Okamoto MM, Markoski MM, Pinto GH, Schaan BD. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovascular diabetology. 2012;11:100. doi: 10.1186/1475-2840-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Irving BA, Nair KS. Aging and diabetes: mitochondrial dysfunction. Current diabetes reports. 2007;7:249–251. doi: 10.1007/s11892-007-0039-x. [DOI] [PubMed] [Google Scholar]
- [125].Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Current diabetes reports. 2008;8:173–178. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- [126].Mulder H, Ling C. Mitochondrial dysfunction in pancreatic beta-cells in Type 2 diabetes. Molecular and cellular endocrinology. 2009;297:34–40. doi: 10.1016/j.mce.2008.05.015. [DOI] [PubMed] [Google Scholar]
- [127].Schrauwen-Hinderling VB, Roden M, Kooi ME, Hesselink MK, Schrauwen P. Muscular mitochondrial dysfunction and type 2 diabetes mellitus. Current opinion in clinical nutrition and metabolic care. 2007;10:698–703. doi: 10.1097/MCO.0b013e3282f0eca9. [DOI] [PubMed] [Google Scholar]
- [128].Maassen JA. Mitochondrial dysfunction in adipocytes: the culprit in type 2 diabetes? Diabetologia. 2006;49:619–620. doi: 10.1007/s00125-006-0165-z. [DOI] [PubMed] [Google Scholar]
- [129].Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–1599. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- [130].Razak F, Anand SS. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. [DOI] [PubMed] [Google Scholar]; Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. N Engl J Med. 2004;350:664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vascular medicine. 2004;9:223–224. doi: 10.1191/1358863x04vm568xx. [DOI] [PubMed] [Google Scholar]
- [131].Morino K, Petersen KF, Sono S, Choi CS, Samuel VT, Lin A, Gallo A, Zhao H, Kashiwagi A, Goldberg IJ, Wang H, Eckel RH, Maegawa H, Shulman GI. Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes. 2012;61:877–887. doi: 10.2337/db11-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nature genetics. 1992;2:324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- [134].Krebs M, Roden M. Molecular mechanisms of lipid-induced insulin resistance in muscle, liver and vasculature. Diabetes, obesity & metabolism. 2005;7:621–632. doi: 10.1111/j.1463-1326.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- [135].Song J, Oh JY, Sung YA, Pak YK, Park KS, Lee HK. Peripheral blood mitochondrial DNA content is related to insulin sensitivity in offspring of type 2 diabetic patients. Diabetes care. 2001;24:865–869. doi: 10.2337/diacare.24.5.865. [DOI] [PubMed] [Google Scholar]
- [136].Antonetti DA, Reynet C, Kahn CR. Increased expression of mitochondrial-encoded genes in skeletal muscle of humans with diabetes mellitus. The Journal of clinical investigation. 1995;95:1383–1388. doi: 10.1172/JCI117790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- [139].Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wlodek D, Gonzales M. Decreased energy levels can cause and sustain obesity. Journal of theoretical biology. 2003;225:33–44. doi: 10.1016/s0022-5193(03)00218-2. [DOI] [PubMed] [Google Scholar]
- [141].Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- [142].Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- [143].Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- [144].Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and mitochondrial DNA damage in heart failure. Circulation journal: official journal of the Japanese Circulation Society. 2008;72(Suppl A):A31–37. doi: 10.1253/circj.cj-08-0014. [DOI] [PubMed] [Google Scholar]
- [145].Victor VM, Apostolova N, Herance R, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in atherosclerosis: mitochondria-targeted antioxidants as potential therapy. Current medicinal chemistry. 2009;16:4654–4667. doi: 10.2174/092986709789878265. [DOI] [PubMed] [Google Scholar]
- [146].Chen J, Mehta JL. Role of oxidative stress in coronary heart disease. Indian heart journal. 2004;56:163–173. [PubMed] [Google Scholar]
- [147].Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- [148].Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. British heart journal. 1991;65:245–248. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Brown GC, Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion. 2012;12:1–4. doi: 10.1016/j.mito.2011.02.001. [DOI] [PubMed] [Google Scholar]
- [150].Bloemen PG, Van den Tweel MC, Henricks PA, Engels F, Van de Velde MJ, Blomjous FJ, Nijkamp FP. Stimulation of both human bronchial epithelium and neutrophils is needed for maximal interactive adhesion. The American journal of physiology. 1996;270:L80–87. doi: 10.1152/ajplung.1996.270.1.L80. [DOI] [PubMed] [Google Scholar]
- [151].Ide T, Tsutsui H, Kinugawa S, Utsumi H, Takeshita A. Amiodarone protects cardiac myocytes against oxidative injury by its free radical scavenging action. Circulation. 1999;100:690–692. doi: 10.1161/01.cir.100.7.690. [DOI] [PubMed] [Google Scholar]
- [152].Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K, Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circulation research. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- [153].Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. The Journal of biological chemistry. 1992;267:5317–5323. [PubMed] [Google Scholar]
- [154].Zell R, Geck P, Werdan K, Boekstegers P. TNF-alpha and IL-1 alpha inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: evidence for primary impairment of mitochondrial function. Molecular and cellular biochemistry. 1997;177:61–67. doi: 10.1023/a:1006896832582. [DOI] [PubMed] [Google Scholar]
- [155].Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, C. American Heart Association Statistics. S. Stroke Statistics Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature reviews. Neuroscience. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- [157].Vijayan M, Reddy PH. Stroke, Vascular Dementia, and Alzheimer’s Disease: Molecular Links. J Alzheimers Dis. 2016;54:427–443. doi: 10.3233/JAD-160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- [160].Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain research reviews. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [162].Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke; a journal of cerebral circulation. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- [163].Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Reddy PH. Role of mitochondria in neurodegenerative diseases: mitochondria as a therapeutic target in Alzheimer’s disease. CNS spectrums. 2009;14:8–13. doi: 10.1017/s1092852900024901. discussion 16-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Kuzmicic J, Del Campo A, Lopez-Crisosto C, Morales PE, Pennanen C, Bravo-Sagua R, Hechenleitner J, Zepeda R, Castro PF, Verdejo HE, Parra V, Chiong M, Lavandero S. Mitochondrial dynamics: a potential new therapeutic target for heart failure. Revista espanola de cardiologia. 2011;64:916–923. doi: 10.1016/j.recesp.2011.05.018. [DOI] [PubMed] [Google Scholar]
- [167].Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB life. 2008;60:145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Holloway GP. Mitochondrial function and dysfunction in exercise and insulin resistance. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2009;34:440–446. doi: 10.1139/H09-028. [DOI] [PubMed] [Google Scholar]
- [169].Joseph AM, Hood DA. Relationships between exercise, mitochondrial biogenesis and type 2 diabetes. Medicine and sport science. 2014;60:48–61. doi: 10.1159/000357335. [DOI] [PubMed] [Google Scholar]
- [170].Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59:572–579. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–2147. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- [172].Nielsen J, Mogensen M, Vind BF, Sahlin K, Hojlund K, Schroder HD, Ortenblad N. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. American journal of physiology. Endocrinology and metabolism. 2010;298:E706–713. doi: 10.1152/ajpendo.00692.2009. [DOI] [PubMed] [Google Scholar]
- [173].Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714–1721. doi: 10.1007/s00125-010-1764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochimica et biophysica acta. 2014;1840:1276–1284. doi: 10.1016/j.bbagen.2013.11.016. [DOI] [PubMed] [Google Scholar]
- [176].Bhatti JS, Bhatti GK, Joshi A, Rai S, Mastana SS, Ralhan SK, Bansal DD, Tewari R. Identification of the risk factors for the high prevalence of type 2 diabetes and its complications in a Punjabi population: North Indian Diabetes Study: A case-control study. International Journal of Diabetes in Developing Countries. 2007;27 [Google Scholar]
- [177].Barbieri E, Agostini D, Polidori E, Potenza L, Guescini M, Lucertini F, Annibalini G, Stocchi L, De Santi M, Stocchi V. The pleiotropic effect of physical exercise on mitochondrial dynamics in aging skeletal muscle. Oxidative medicine and cellular longevity. 2015;2015:917085. doi: 10.1155/2015/917085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- [179].Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicologic pathology. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. The Journal of nutrition. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- [181].Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS letters. 2011;585:1537–1542. doi: 10.1016/j.febslet.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Barja G. Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev. 2002;1:397–411. doi: 10.1016/s1568-1637(02)00008-9. [DOI] [PubMed] [Google Scholar]
- [183].Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- [184].Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E, C.P. Team Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS medicine. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:1825–1836. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]
- [186].Sharma N, Arias EB, Bhat AD, Sequea DA, Ho S, Croff KK, Sajan MP, Farese RV, Cartee GD. Mechanisms for increased insulin-stimulated Akt phosphorylation and glucose uptake in fast- and slow-twitch skeletal muscles of calorie-restricted rats. American journal of physiology. Endocrinology and metabolism. 2011;300:E966–978. doi: 10.1152/ajpendo.00659.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. The American journal of physiology. 1994;266:E540–547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- [188].Wang H, Arias EB, Cartee GD. Calorie restriction leads to greater Akt2 activity and glucose uptake by insulin-stimulated skeletal muscle from old rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2016;310:R449–458. doi: 10.1152/ajpregu.00449.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Wang H, Sharma N, Arias EB, Cartee GD. Insulin Signaling and Glucose Uptake in the Soleus Muscle of 30-Month-Old Rats After Calorie Restriction With or Without Acute Exercise. The journals of gerontology. Series A, Biological sciences and medical sciences. 2016;71:323–332. doi: 10.1093/gerona/glv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: An update. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Turkmen K, Karagoz A, Kucuk A. Sirtuins as novel players in the pathogenesis of diabetes mellitus. World journal of diabetes. 2014;5:894–900. doi: 10.4239/wjd.v5.i6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Huynh FK, Hershberger KA, Hirschey MD. Targeting sirtuins for the treatment of diabetes. Diabetes management. 2013;3:245–257. doi: 10.2217/dmt.13.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kitada M, Kume S, Kanasaki K, Takeda-Watanabe A, Koya D. Sirtuins as possible drug targets in type 2 diabetes. Current drug targets. 2013;14:622–636. doi: 10.2174/1389450111314060002. [DOI] [PubMed] [Google Scholar]
- [194].Albiero M, Avogaro A, Fadini GP. A perspective on sirtuins in the metabolic syndrome. Metabolic syndrome and related disorders. 2015;13:161–164. doi: 10.1089/met.2015.0005. [DOI] [PubMed] [Google Scholar]
- [195].Kumar S, Lombard DB. Mitochondrial sirtuins and their relationships with metabolic disease and cancer. Antioxidants & redox signaling. 2015;22:1060–1077. doi: 10.1089/ars.2014.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Chalkiadaki A, Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nature reviews. Endocrinology. 2012;8:287–296. doi: 10.1038/nrendo.2011.225. [DOI] [PubMed] [Google Scholar]
- [197].Yu J, Auwerx J. The role of sirtuins in the control of metabolic homeostasis. Annals of the New York Academy of Sciences. 2009;1173(Suppl 1):E10–19. doi: 10.1111/j.1749-6632.2009.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Current opinion in investigational drugs. 2008;9:371–378. [PubMed] [Google Scholar]
- [199].Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- [200].Bindu S, Pillai VB, Gupta MP. Role of Sirtuins in Regulating Pathophysiology of the Heart. Trends in endocrinology and metabolism: TEM. 2016 doi: 10.1016/j.tem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- [201].Tanno M, Kuno A, Horio Y, Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic research in cardiology. 2012;107:273. doi: 10.1007/s00395-012-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Schenk S, McCurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K, Olefsky JM. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. The Journal of clinical investigation. 2011;121:4281–4288. doi: 10.1172/JCI58554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- [204].Parihar P, Solanki I, Mansuri ML, Parihar MS. Mitochondrial sirtuins: emerging roles in metabolic regulations, energy homeostasis and diseases. Experimental gerontology. 2015;61:130–141. doi: 10.1016/j.exger.2014.12.004. [DOI] [PubMed] [Google Scholar]
- [205].Reddy PH. Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. Journal of Alzheimer’s disease: JAD. 2014;40:245–256. doi: 10.3233/JAD-132060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Meuer K, Suppanz IE, Lingor P, Planchamp V, Goricke B, Fichtner L, Braus GH, Dietz GP, Jakobs S, Bahr M, Weishaupt JH. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell death and differentiation. 2007;14:651–661. doi: 10.1038/sj.cdd.4402087. [DOI] [PubMed] [Google Scholar]
- [207].Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Developmental cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. Journal of cell science. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Mailloux RJ. Application of Mitochondria-Targeted Pharmaceuticals for the Treatment of Heart Disease. Current pharmaceutical design. 2016;22:4763–4779. doi: 10.2174/1381612822666160629070914. [DOI] [PubMed] [Google Scholar]
- [210].Silva FS, Simoes RF, Couto R, Oliveira PJ. Targeting Mitochondria in Cardiovascular Diseases. Current pharmaceutical design. 2016 doi: 10.2174/1381612822666160822150243. [DOI] [PubMed] [Google Scholar]
- [211].Ni R, Cao T, Xiong S, Ma J, Fan GC, Lacefield JC, Lu Y, Le Tissier S, Peng T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free radical biology & medicine. 2016;90:12–23. doi: 10.1016/j.freeradbiomed.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].He Q, Harris N, Ren J, Han X. Mitochondria-targeted antioxidant prevents cardiac dysfunction induced by tafazzin gene knockdown in cardiac myocytes. Oxidative medicine and cellular longevity. 2014;2014:654198. doi: 10.1155/2014/654198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Yorek MA. The role of oxidative stress in diabetic vascular and neural disease. Free radical research. 2003;37:471–480. doi: 10.1080/1071576031000083161. [DOI] [PubMed] [Google Scholar]
- [214].Yi X, Maeda N. alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006;55:2238–2244. doi: 10.2337/db06-0251. [DOI] [PubMed] [Google Scholar]
- [215].Mehta K, Van Thiel DH, Shah N, Mobarhan S. Nonalcoholic fatty liver disease: pathogenesis and the role of antioxidants. Nutrition reviews. 2002;60:289–293. doi: 10.1301/002966402320387224. [DOI] [PubMed] [Google Scholar]
- [216].Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. The Journal of biological chemistry. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- [217].Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J, Kalyanaraman B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. The Journal of biological chemistry. 2004;279:37575–37587. doi: 10.1074/jbc.M404003200. [DOI] [PubMed] [Google Scholar]
- [218].Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- [219].Mao G, Kraus GA, Kim I, Spurlock ME, Bailey TB, Zhang Q, Beitz DC. A mitochondria-targeted vitamin E derivative decreases hepatic oxidative stress and inhibits fat deposition in mice. The Journal of nutrition. 2010;140:1425–1431. doi: 10.3945/jn.110.121715. [DOI] [PubMed] [Google Scholar]
- [220].Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. European journal of biochemistry / FEBS. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- [221].Mercer JR, Yu E, Figg N, Cheng KK, Prime TA, Griffin JL, Masoodi M, Vidal-Puig A, Murphy MP, Bennett MR. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/− mice. Free radical biology & medicine. 2012;52:841–849. doi: 10.1016/j.freeradbiomed.2011.11.026. [DOI] [PubMed] [Google Scholar]
- [222].Feillet-Coudray C, Fouret G, Ebabe Elle R, Rieusset J, Bonafos B, Chabi B, Crouzier D, Zarkovic K, Zarkovic N, Ramos J, Badia E, Murphy MP, Cristol JP, Coudray C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Free radical research. 2014;48:1232–1246. doi: 10.3109/10715762.2014.945079. [DOI] [PubMed] [Google Scholar]
- [223].Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- [224].Dare AJ, Bolton EA, Pettigrew GJ, Bradley JA, Saeb-Parsy K, Murphy MP. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox biology. 2015;5:163–168. doi: 10.1016/j.redox.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Galley HF. Bench-to-bedside review: Targeting antioxidants to mitochondria in sepsis. Critical care. 2010;14:230. doi: 10.1186/cc9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free radical biology & medicine. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- [227].Yin X, Manczak M, Reddy PH. Mitochondria-targeted molecules MitoQ and SS31 reduce mutant huntingtin-induced mitochondrial toxicity and synaptic damage in Huntington’s disease. Human molecular genetics. 2016 doi: 10.1093/hmg/ddw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Mao P, Manczak M, Shirendeb UP, Reddy PH. MitoQ, a mitochondria-targeted antioxidant, delays disease progression and alleviates pathogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Biochimica et biophysica acta. 2013;1832:2322–2331. doi: 10.1016/j.bbadis.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Powell RD, Swet JH, Kennedy KL, Huynh TT, Murphy MP, McKillop IH, Evans SL. MitoQ modulates oxidative stress and decreases inflammation following hemorrhage. The journal of trauma and acute care surgery. 2015;78:573–579. doi: 10.1097/TA.0000000000000533. [DOI] [PubMed] [Google Scholar]
- [230].Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annual review of pharmacology and toxicology. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]