A current view of the role and regulation of plant receptor kinases that act as innate immune receptors or an Achilles’ heel targeted by phytopathogens.
Abstract
Receptor-like kinases (RLKs) and Receptor-like proteins (RLPs) play crucial roles in plant immunity, growth, and development. Plants deploy a large number of RLKs and RLPs as pattern recognition receptors (PRRs) that detect microbe- and host-derived molecular patterns as the first layer of inducible defense. Recent advances have uncovered novel PRRs, their corresponding ligands, and mechanisms underlying PRR activation and signaling. In general, PRRs associate with other RLKs and function as part of multiprotein immune complexes at the cell surface. Innovative strategies have emerged for the rapid identification of microbial patterns and their cognate PRRs. Successful pathogens can evade or block host recognition by secreting effector proteins to “hide” microbial patterns or inhibit PRR-mediated signaling. Furthermore, newly identified pathogen effectors have been shown to manipulate RLKs controlling growth and development by mimicking peptide hormones of host plants. The ongoing studies illustrate the importance of diverse plant RLKs in plant disease resistance and microbial pathogenesis.
INTRODUCTION
A long-standing question in plant biology is how plants perceive and ward off attack by numerous potential pathogens. Two parallel paths were taken to address this question in the last century. The first path follows Harold Flor’s famous gene-for-gene hypothesis, which predicts that resistance gene products in the host plant specifically recognize cognate “avirulence” gene products in the pathogen to confer disease resistance. This led to the discovery that plant resistance proteins broadly fall into two classes according to their protein sequences and subcellular localization: cytoplasmic nucleotide binding domain leucine-rich repeat domain-containing receptors (NLRs; Jones et al., 2016) and cell surface-localized receptors. The latter are proteins belonging to large families of receptor-like kinases (RLKs) and receptor-like proteins (RLPs) (Jones and Dangl, 2006). Accordingly, their corresponding avirulence proteins were identified as effector proteins targeted to the plant cytosol or apoplast. The second path follows the findings that microbe-derived and host-derived elicitors, also called pathogen/microbe-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), respectively, can act as danger signals to trigger defenses in plants, which culminated in the identification of RLKs and RLPs as pattern recognition receptors (PRRs) for direct recognition of the patterns (Boller and Felix, 2009). These combined efforts led to the consensus that the plant surveillance system is primarily composed of NLRs and PRRs. Thus, NLRs are responsible for the recognition of cytoplasmic effectors, whereas PRRs are responsible for the detection of apoplastic effectors or patterns.
Unlike animals, which employ receptor tyrosine kinases, seven transmembrane G protein-coupled receptors, and Toll-like receptors at the plasma membrane to perceive growth hormones, environmental signals, and danger signals derived from pathogens, plants rely on RLKs (∼410 in Arabidopsis thaliana) and RLPs (∼170 in Arabidopsis) to fulfill these diverse roles (Shiu and Bleecker, 2003; Shiu et al., 2004; Li et al., 2016a). A plant RLK is analogous to an animal receptor tyrosine kinase and contains an ectodomain (ECD), a single pass transmembrane domain, and a cytoplasmic kinase domain, whereas an RLP is essentially an RLK lacking a cytoplasmic kinase domain. The ECDs of RLKs and RLPs are highly variable, providing means to recognize a wide range of ligands, including steroids, peptides, polysaccharides, and lipopolysaccharides. While some RLKs and RLPs are known to act as PRRs that perceive danger signals (Table 1), others regulate plant growth and development, reproduction, symbiosis, and tolerance to abiotic stresses (Breiden and Simon, 2016).
Table 1. Known and Probable PRRs Involved in Plant Immunity.
| Name | Family | Ligand | Plant | References |
|---|---|---|---|---|
| FLS2 | LRR-RK | flg22 | Arabidopsis | Gómez-Gómez and Boller (2000); Bauer et al. (2001) |
| FLS3 | LRR-RK | flgII-28 | Tomato | Hind et al. (2016) |
| EFR | LRR-RK | elf18 | Arabidopsis | Kunze et al. (2004); Zipfel et al. (2006) |
| PEPR1/2 | LRR-RK | Peps | Arabidopsis | Yamaguchi et al. (2006); Huffaker et al. (2006); Huffaker and Ryan (2007); Krol et al. (2010); Yamaguchi et al. (2010) |
| XA21 | LRR-RK | RaxX21-sY | Rice | Pruitt et al. (2015) |
| DORN1 | Lectin-RK | eATP | Arabidopsis | Choi et al. (2014) |
| LORE | Lectin-RK | LPS | Arabidopsis | Ranf et al. (2015) |
| WAK1 | EGF-Like-RLK | OGs | Arabidopsis | Decreux and Messiaen (2005); Brutus et al. (2010) |
| XPS1 | LRR-RK | xup25 | Arabidopsis | Mott et al. (2016) |
| OsCERK1 | LysM-RLK | Chitin | Rice | Shimizu et al. (2010) |
| CEBiP | LysM-RP | Chitin | Rice | Kaku et al. (2006) |
| LYM1/3 | LysM-RP | PGNs | Arabidopsis | Willmann et al. (2011) |
| LYP4/6 | LysM-RLP | PGNs/chitin | Rice | B. Liu et al. (2012) |
| RLP23 | LRR-RP | nlp20 | Arabidopsis | Bi et al. (2014); Albert et al. (2015) |
| NbCSPR | LRR-RP | csp22 | N. benthamiana | Saur et al. (2016) |
| LeEix1 | LRR RP | Eix | Tomato | Bar et al. (2010) |
| LeEix2 | LRR-RP | Eix | Tomato | Ron and Avni (2004) |
| ReMax/RLP1 | LRR-RLP | eMax | Arabidopsis | Jehle et al. (2013) |
| Ve1 | LRR-RLP | Ave1 | Tomato | de Jonge et al. (2012) |
| Cf-2 | LRR-RLP | Avr2 | Tomato | Dixon et al. (1996); Luderer at al. (2002) |
| Cf-4 | LRR-RLP | Avr4 | Tomato | Joosten et al. (1997); Thomas et al. (1997) |
| Cf-4E | LRR-RLP | Avr4E | Tomato | Takken et al. (1999); Westerink et al. (2004) |
| Cf-9 | LRR-RLP | Avr9 | Tomato | Van den Ackerveken et al. (1992); Jones et al. (1994) |
| Cf-5 | LRR-RLP | Unknown | Tomato | Dixon et al. (1998) |
| RLP30 | LRR-RLP | SCFE1 | Arabidopsis | Wang et al. (2008); Zhang et al. (2013) |
| ELR | LRR-RLP | Elicitin | Potato | Du et al. (2015) |
Known PRRs refer to genetically confirmed receptors whose binding to patterns has been biochemically demonstrated, whereas probable PRRs refer to likely receptors with genetically confirmed roles in pattern recognition, but for which direct binding to patterns remains to be shown.
Pathogenic microbes, including bacteria, fungi, oomycetes, and nematodes, deliver a large number of effector proteins into the plant apoplast or cytosol (Dou and Zhou, 2012). Analyses of these effectors have uncovered a variety of mechanisms that pathogens use for infection and colonization of host plants. Often, these proteins provide means to evade PRR-mediated surveillance by camouflage or blockage of PRR-induced signaling. Others can mimic host growth factors and manipulate specific RLK-mediated host processes for their benefit. On the other hand, perturbation by these effectors imposes a selection pressure on host plants, which ultimately leads to the evolution of NLRs or PRRs recognizing these effectors as danger signals and conferring disease resistance in plants.
A major effort in plant-pathogen interaction research has been to identify PRRs, their corresponding ligands, and mechanisms by which different PRRs activate defenses. While genetic and reverse genetic studies have proved straightforward in the identification of a large number of RLKs and RLPs involved in disease resistance to diverse pathogens, identification of molecular patterns and their corresponding PRRs has been much more challenging. Nonetheless, conventional biochemical studies and genetic analyses have identified important founding members of microbial and plant molecular patterns and their corresponding PRRs. The advent of microbial and plant genomics has enabled the development of novel strategies for the discovery of new microbial molecular patterns and PRRs. Furthermore, molecular genetics, biochemistry, and structural biology studies are uncovering generalities and differences in mode of action among different types of PRRs, how PRRs activate downstream defenses, crosstalk between different receptor kinase pathways, and mechanisms by which various pathogens perturb receptor kinase signaling for pathogenesis.
In this review, we provide an update on PRRs involved in disease resistance and mechanisms by which different types of PRRs recognize ligands to form active receptor complexes. We then discuss how the knowledge gained has led to the development of strategies for identifying PAMPs and PRRs. For PRR-triggered defenses, we focus on early signaling mediated by PRRs. Readers are referred to several excellent reviews on the regulation of downstream signaling, such as transcriptional controls (Bigeard et al., 2015; Couto and Zipfel, 2016; Li et al., 2016b). We also discuss how pathogen effectors interfere with early PRR signaling to inhibit defenses and how pathogen effectors enhance virulence by manipulating RLKs that do not function as PRRs.
RLKs AND RPLs INVOLVED IN PLANT IMMUNITY
Numerous RLKs and RLPs have been shown to function in plant disease resistance, but only a handful of them are confirmed to function as PRRs with known ligands (Wu and Zhou, 2013; Böhm et al., 2014; Zipfel, 2014; Couto and Zipfel, 2016). In the following text, RLKs and RLPs known to directly bind ligands are referred to as RKs and RPs, respectively. An extended list of known PRRs and other RLKs and RLPs involved in plant immunity is presented in Tables 1 and 2.
Table 2. Other RLKs and RLPs Involved in Plant Immunity.
| Name | Family | Function | Plant | References |
|---|---|---|---|---|
| BAK1 | LRR-RLK | PRR coreceptor | Arabidopsis | Chinchilla et al. (2007); Heese et al. (2007); Schulze et al. (2010); Postel et al. (2010); Roux et al. (2011); Bar et al. (2010) |
| SERK4 | LRR-RLK | PRR coreceptor | Arabidopsis | Roux et al. (2011) |
| SISERK1 | LRR-RLK | PRR coreceptor | Tomato | Mantelin et al. (2011) |
| BIR1 | LRR-RLK | Interacts with PRR coreceptor | Arabidopsis | Gao et al. (2009) |
| BIR2 | LRR-RLK | Interacts with PRR coreceptor | Arabidopsis | Halter et al. (2014) |
| SOBIR1 | LRR-RLK | Scaffold for PRR | Arabidopsis, tomato | Gao et al. (2009); Zhang et al. (2013, 2014); Albert et al. (2015); Liebrand et al. (2013) |
| FER | Malectin-RK | RALF receptor, scaffold for PRR | Arabidopsis | Escobar-Restrepo et al. (2007); Kessler et al. (2010); Haruta et al. (2014); Stegmann et al. (2017) |
| CRK28 | Cys-rich RLK | Interacts with PRR | Arabidopsis | Yadeta et al. (2017) |
| IOS1 | LRR-RLP | Interacts with PRR | Arabidopsis | Yeh et al. (2016) |
| PSKR1 | LRR-RK | PSKa receptor | Arabidopsis | Mosher et al. (2013) |
| PSY1R | LRR RLK | PSY1 receptor | Arabidopsis | Mosher et al. (2013) |
| ERECTA | LRR-RLK | Unknown | Arabidopsis | Llorente et al. (2005) |
| SRF3 | LRR-RLK | Unknown | Arabidopsis | Alcázar et al. (2010) |
| XA26 | LRR-RLK | Unknown | Rice | Sun et al. (2004) |
| ds1 | LRR-RLK | Unknown | Sorghum | Kawahigashi et al. (2011) |
| Bti9 | LysM-RLK | Unknown | Tomato | Zeng et al. (2012) |
| SILyk13 | LysM-RLK | Unknown | Tomato | Zeng et al. (2012) |
| THE1 | Malectin-RLK | Unknown | Arabidopsis | Hématy et al. (2007) |
| Pi-d2 | Lec-RLK | Unknown | Rice | Chen et al. (2006) |
| LecRK-I.9 | Lec-RLK | Unknown | Arabidopsis | Gouget et al. (2006); Bouwmeester et al. (2011) |
| LecRK-V.5 | Lec-RLK | Unknown | Arabidopsis | Desclos-Theveniau et al. (2012) |
| LecRK-VI.2 | Lec-RLK | Unknown | Arabidopsis | Singh et al. (2012) |
| NgRLK1 | Lectin-RLK | Interacts with elicitin | N. glutinosa | Kim et al. (2010) |
| LecRK1 | Lectin-RLK | Unknown | N. attenuata | Gilardoni et al. (2011) |
| NbLRK1 | Lectin-RLK | Interacts with elicitin | N. benthamiana | Kanzaki et al. (2008) |
| WAKL22 | EGF-Like-RLK | Unknown | Arabidopsis | Diener and Ausubel (2005) |
| OsWAK1 | EGF-Like-RLK | Unknown | Rice | Li et al. (2009) |
| TaRLK-R1,2,3 | Other | Unknown | Wheat | Zhou et al. (2007) |
| SNC4 | Other | Unknown | Arabidopsis | Bi et al. (2010) |
| LRK10 | S-domain-RLK | Unknown | Wheat | Feuillet et al. (1997) |
| SNC2 | LRR-RLP | Unknown | Arabidopsis | Y. Zhang et al. (2010) |
| RLP52 | LRR-RLP | Unknown | Arabidopsis | Ramonell et al. (2005) |
Includes RLKs/RLPs that regulate plant immunity through an unknown molecular function or function other than PRRs.
PRRs can be classified into different subfamilies according to domains or motifs in their ECDs: leucine-rich repeat (LRR) domain, lysine motifs (LysM), lectin domain, or epidermal growth factor (EGF)-like domain (Wu and Zhou, 2013; Böhm et al., 2014; Zipfel, 2014; Couto and Zipfel, 2016). All known LRR-containing PRRs, including LRR-RKs and LRR-RPs, bind proteins or peptides. For example, the Arabidopsis LRR-RKs FLAGELLIN SENSING2 (FLS2) and EFR recognize a conserved 22-amino acid epitope (flg22) of the N terminus of the bacterial flagellin and a conserved N-terminal epitope (elf18) of the bacterial elongation factor Tu (EF-Tu), respectively (Gómez-Gómez and Boller, 2000; Bauer et al., 2001; Kunze et al., 2004; Zipfel et al., 2006). The Arabidopsis LRR-RKs PEP RECEPTOR1 (PEPR1) and PEPR2 perceive proteinaceous DAMPs called Peps (plant elicitor peptides), which are conserved epitopes of a small family of pro-peptides (PROPEPs) (Huffaker et al., 2006; Huffaker and Ryan, 2007; Krol et al., 2010; Yamaguchi et al., 2010; Z. Liu et al., 2013; Yamada et al., 2016a). PROPEP proteins are produced and released through an unknown mechanism into the extracellular space upon pathogen infection, in a manner comparable to the production and secretion of inflammatory cytokines in animals after pathogen infection (Yamada et al., 2016a). In rice (Oryza sativa), LRR-RK XA21 recognizes RaxX, a highly conserved protein in many Xanthomonas species, to trigger immune responses. RaxX21-sY, a sulfated, 21-amino acid synthetic peptide derived from RaxX, is sufficient for XA21 activation (Pruitt et al., 2015).
Several LRR-RLPs have been shown to recognize proteinaceous patterns. Among the 57 LRR-RLPs encoded by the Arabidopsis genome (Wang et al., 2008), RLP23 specifically binds and recognizes nlp20, a conserved amino acid peptide from necrosis and ethylene-inducing peptide1-like proteins (NLPs), which are widespread proteins among diverse group of microbes, including bacteria, fungi, and oomycetes (Albert et al., 2015). In tomato (Solanum lycopersicum), the LRR-RLPs Cf-2, Cf-4, and Cf-9 confer resistance to the fungal pathogen Cladosporium fulvum by recognizing the effectors Avr2, Avr4, and Avr9, respectively (Dixon et al., 1996; Krüger et al., 2002; Luderer et al., 2002; Rooney et al., 2005). It is not known whether any of these apoplastic effectors directly bind to the RLPs. However, Avr2 is known to inhibit several cysteine proteases, including Rcr3 for virulence, and the Cf-2 protein indirectly recognizes Avr2 likely by sensing the modification of Rcr3 (Rooney et al., 2005; van Esse et al., 2008). In this case, the Avr2-modified Rcr3 is equivalent to a DAMP that activates immunity in plants carrying Cf-2.
LysM-RPs and LysM-RKs represent a major class of receptors for microbial N-acetylglucosamine-containing glycans, including fungal chitin and bacterial peptidoglycan (PGN), which trigger defenses, and Rhizobium- and arbuscular mycorrhiza-derived lipochitooligosaccharides (LCOs), which trigger symbioses (Gust et al., 2012). Three Arabidopsis LysM-RLKs, CHITIN ELICITOR RECEPTOR KINASE1 (CERK1), LysM-CONTAINING RECEPTOR KINASE4 (LYK4), and LYK5, are required for chitin signaling (Miya et al., 2007; Wan et al., 2008, 2012; Petutschnig et al., 2010; Cao et al., 2014), whereas CERK1 and two Arabidopsis LysM-RLPs, LysM DOMAIN-CONTAINING GPI-ANCHORED PROTEIN1 (LYM1) and LYM3, are required for response to PGN (Willmann et al., 2011). Both CERK1 and LYK5 have been shown to bind chitin, with LYK5 reported to have greater affinity (T. Liu et al., 2012; Cao et al., 2014), whereas LYM1 and LYM3 were shown to bind PGN (Willmann et al., 2011). The rice LysM-RP CHITIN OLIGOSACCHARIDE ELICITOR BINDING PROTEIN (CEBiP) and the rice counterpart of CERK1 are essential for chitin-induced defenses in plants (Kaku et al., 2006; Shimizu et al., 2010). CEBiP binds chitin with high affinity, indicating that it is the chitin receptor in rice plants. The perception of chitin by the Arabidopsis LysM-RK LYK5 and rice LysM-RP CEBiP echoes the recent findings that bacterial cold shock protein epitope csp22 is perceived by the tomato LRR-RK CORE and tobacco (Nicotiana tabacum) LRR-RP NbCSPR (Saur et al., 2016; Wang et al., 2016). Interestingly, the peptide hormone CLAVATA3 (CLV3p) is perceived by not only the LRR-RK CLV1, but also by a likely LRR-RP CLV2, although direct ligand binding has been shown only for CLV1 (Soyars et al., 2016). Thus, an RK and an RP can perceive the same ligand with a shared ECD.
Lectin-RLKs are uniquely important for plant disease resistance. The Arabidopsis lectin-RK LORE is a receptor for the lipid A moiety of bacterial lipopolysaccharides (LPSs) (Ranf et al., 2015). Another Lectin-RK, DORN1 (Choi et al., 2014), is a high affinity receptor for ATP (eATP), which may act as a DAMP as it induces cytosolic Ca2+ elevation and mitogen-activated protein kinase (MAPK) activation (Tanaka et al., 2014). It remains to be determined whether eATP contributes to plant immunity in response to pathogens.
LIGAND BINDING AND OLIGOMERIZATION OF PRR RECEPTOR COMPLEXES
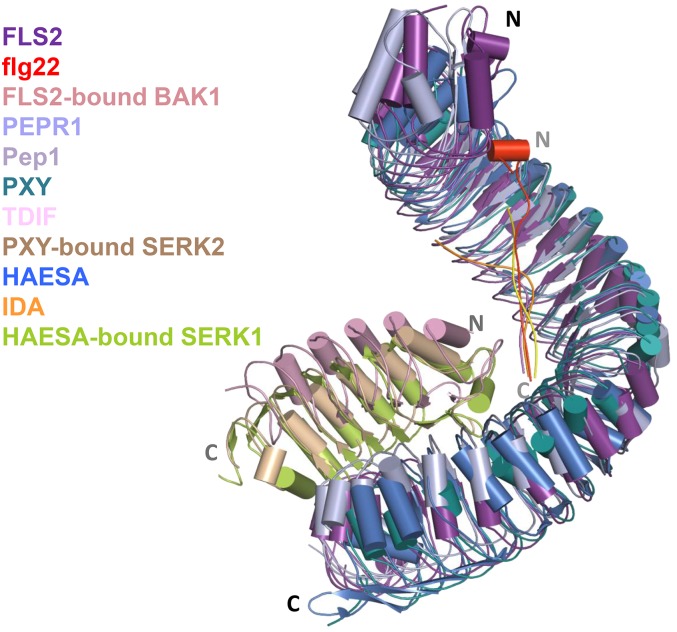
Upon ligand binding, PRRs of the LRR-RK class recruit BRI1-ASSOCIATED RECEPTOR KINASE (BAK1), a LRR-RLK with only five LRRs and member of the SOMATIC EMBRYOGENESIS RECEPTOR KINASES (SERKs) (Figure 1) (Couto and Zipfel, 2016). Thus, the binding of FLS2 to flg22 recruits BAK1 to form a heterodimer, resulting in rapid phosphorylation of both FLS2 and BAK1 (Chinchilla et al., 2007; Heese et al., 2007; Schulze et al., 2010; Roux et al., 2011). Molecular and genetic studies showed that SERKs are also recruited to EFR, PEPRs, and Xa21 upon ligand binding and required for signaling (Roux et al., 2011; Chen et al., 2014; Yamada et al., 2016a). In addition, LRR-RKs for peptide hormones, including tracheary element differentiation inhibitory factor (TDIF), INFLORESCENCE DEFIECIENT IN ABSCISSION (IDA), PHYTOSULFOKINE (PSK), and ROOT GROWTH FACTORS (RGFs), also require SERKs for function (Ma et al., 2016; Zhang et al., 2016). LRR-RKs generally contain many more LRRs (28 for FLS2 and 27 for PEPR1), which provide an interface for peptide binding, than do SERKs. Crystal structures have been solved for the ECDs of FLS2, PEPR1, RGF receptor RGFR1, PSK receptor PSKR1, IDA receptor HAESA, and TDIF receptor PXY (phloem intercalated with xylem) in complex with their respective ligands and/or a SERK ECD (Sun et al., 2013; Tang et al., 2015; Song et al., 2016; Santiago et al., 2016; J. Wang et al., 2015; Zhang et al., 2016). The FLS2-flg22-BAK1 complex showed that flg22 adopts a linear conformation and binds to FLS2 LRR3-16 in the same N-terminal to C-terminal orientation (Figure 1; Sun et al., 2013). Strikingly, Gly-18 of flg22 fits in an “inner-curved loop” between Thr-52 and Val-54 at the N terminus of the BAK1 ECD, and this interaction is further stabilized by hydrogen bonds between flg22 Leu-19 and BAK1 Thr-52 and Val-54. This interaction effectively bridges the dimerization between FLS2 and BAK1, demonstrating that BAK1 is a coreceptor for flg22. FLS2 LRR23-26 additionally interacts with a cluster of bulky BAK1 amino acids, forming a stable complex. The binding of flg22 does not lead to a conformational change in the FLS2. The PEPR1-Pep1, HAESA-IDA-SERK1, PXY-TDIF-SERK2, and RGFR1-RGF1-BAK1 complexes also adopt a conformation strikingly similar to the FLS2-flg22-BAK1 complex (Figure 1; Tang et al., 2015; Santiago et al., 2016; Song et al., 2016; Zhang et al., 2016). The PSKR1-PSK-SERK1 structure showed a different mechanism for ligand binding and receptor complex formation. PSK presents as a β-strand and forms an antiparallel β-sheet conformation with the island domain of PSKR1 ECD (J. Wang et al., 2015). This induces an allosteric change in PXY, allowing the recruitment of SERKs. Taken together, SERKs are likely common coreceptors for many LRR-RKs perceiving proteinaceous ligands.
Figure 1.
Ligand-Induced Heterodimerization between LRR-RKs and SERKs.
Structural alignment of FLS2-flg22-BAK1, PEPR1-Pep1, PXY-TDIF-SERK2, and HAESA-IDA-SERK1 complexes. The structure of FLS2 ECD was used as the template for the alignment. Note that only ECDs were included in the structural studies. N and C denote N and C termini of LRR-RK ECDs and peptides.
Although LRR-RPs do not carry a cytoplasmic kinase domain, they associate with RLKs to transmit the signal to downstream components. The LRR-RLK SUPPRESOR of BIR1 (SOBIR1), originally identified as a component required for autoimmunity caused by loss of function of BAK1-INTERACTING RECEPTOR-LIKE KINASE1 (BIR1) (Gao et al., 2009), functions as a common adaptor for multiple LRR-RP-type PRRs (Gust and Felix, 2014). In tomato, SOBIR1 interacts with a number of RLPs, including Ve1, EIX1, and Cf-4, and plays crucial roles in plant immunity against fungal infection (Liebrand et al., 2013, 2014). RLP23 forms a complex with SOBIR1 in the resting state and recruits BAK1 into the complex after nlp20 recognition to activate immune signaling (Albert et al., 2015). RLP30 associates with SOBIR1, and SCFE1-triggered immune responses are dependent on BAK1 and SOBIR1 (Zhang et al., 2013). In addition, SOBIR1 is required for PG-triggered, RBPG1-mediated immune responses (Zhang et al., 2014), and ELR associates with BAK1 for elicitin recognition (Du et al., 2015). The consensus is that LRR-RPs form a complex with SOBIR1 before ligand binding and recruit BAK1 to form an active receptor complex upon ligand binding.
Chitin binding can induce homodimerization of the LysM-ECDs of CEBiP and CERK1, which is essential for chitin-induced defenses in Arabidopsis (Shimizu et al., 2010; T. Liu et al., 2012; Hayafune et al., 2014). Two competing models have been proposed for chitin-induced dimerization of LysM-ECDs. Chitin oligomers longer than six sugars are required for both triggering defenses and inducing dimerization of CERK1 or CEBiP, whereas only three sugar residues are directly bound to CERK1-ECD in structural analyses (T. Liu et al., 2012). T. Liu et al. (2012) thus proposed a “cross-linking” model in which the two ends of long chitin oligomers each bind a LysM-ECD to form a dimer. In a separate NMR study assisted by synthetic chemistry, N-acetyl groups of alternating sugars were found to be located on two opposite sides of the chitin chain and directly bound by the LysM-ECD (Hayafune et al., 2014). Depleting N-acetyl groups from one side of the chitin chain did not affect chitin binding to a CEBiP-ECD, but abolished dimerization. This led to the proposal of a “sandwich” model in which two CEBiP-ECDs bind the chitin chain, one on each side (Hayafune et al., 2014). However, this does not explain why shorter chitin oligomers do not induce dimerization. Whether CEBiP possesses significantly different affinities toward short and long chitin oligomers remains controversial, in part because different fragments of CEBiP-ECD were used in different studies (Hayafune et al., 2014; S. Liu et al., 2016). Moreover, the sandwich model predicts that five chitin sugars are required for binding to one CEBiP, whereas the recent crystal structure data clearly showed only three are bound (S. Liu et al., 2016). S. Liu et al. (2016) thus proposed a third “sliding model” in which two CEBiP-ECDs slide along a longer chitin chain involving alternating N-acetyl groups from opposite sides for optimum interaction (S. Liu et al., 2016), a model explaining both structural data and N-acetyl depletion experiments.
In rice plants, chitin binding to CEBiP recruits CERK1 to form a CEBiP-CERK1 heterocomplex (Shimizu et al., 2010). In Arabidopsis, chitin treatment was reported to induce CERK1 dimerization only in the presence of LYK5 (Cao et al., 2014). LYK5 is present as homodimer before and after chitin perception. LYK5 kinase activity is not required for chitin signaling, but the intact kinase domain is essential for chitin-induced LYK5/CERK1 association (Cao et al., 2014). How CERK1 and LYK5 work together to recognize chitin remains to be determined. In sum, these findings support that CEBiP and LYK5 are chitin receptors and recruit CERK1 to form active receptor complexes (Figure 1). The requirement of CERK1 for PGN signaling in Arabidopsis (Willmann et al., 2011) and arbuscular mycorrhizal symbiosis in rice (Zhang et al., 2015) suggests that CERK1 is similarly recruited to PGN and LCO receptors. Thus, CERK1 appears to be a universal component for the perception of chitin, PGN, and LCOs. CERK1 may be functionally analogous to BAK1 and may act as a coreceptor for these highly related ligands, a possibility that remains to be tested by structural studies.
INNOVATIVE APPROACHES TO IDENTIFY MICROBIAL PATTERNS/APOPLASTIC EFFECTORS AND THEIR COGNATE RECEPTORS
Identification of microbial patterns, apoplastic effectors, and PRRs through which they activate immunity remains challenging. While most of the microbial patterns and immune-triggering apoplastic effectors reported to date were identified through classical biochemical purification and genetics, these approaches are time-consuming and require extensive expertise. For proteinaceous microbial patterns and apoplastic effectors recognized by host PRRs, their coding genes often display a positive selection on individual amino acids as a result of adaptation. For instance, Xanthomonas campestris campestris isolates frequently accumulate mutations in the flagellin-coding gene that evade detection by FLS2 (Sun et al., 2006). This unique feature can be exploited to rapidly identify new effectors or microbial patterns by interrogating microbial genome sequences. As such, a second 28-amino acid epitope (flgII-28) of bacterial flagellin and Ave1 of Verticillium dahliae were found to be subject to strong positive selection (Cai et al., 2011; de Jonge et al., 2012). These findings directly led to the discovery of flgII-28 as a pattern recognized by LRR-RK FLS3 (see below) and Ave1 as an effector recognized by LRR-RLP Ve1 in tomato plants. Most recently, this approach was applied to identify six new peptides, including xup25, from Pseudomonas syringae that trigger immune responses in Arabidopsis (Mott et al., 2016). This important work further illustrates the power of the comparative genomics approach in revealing novel microbial patterns and in isolating their corresponding PRRs.
Like microbial patterns and pathogen effectors, plant PRRs are also evolving rapidly. FLS2, EFR, FLS3, XPS1, and Xa21 all belong to LRR-RLK XII, a subfamily that has undergone significant gene expansion (Shiu et al., 2004). Functional EFR and Xa21 occur only in Brassicaceae and certain rice cultivars, respectively (Boller and Felix, 2009; Lacombe et al., 2010). Similarly, flgII-28 only elicits immune responses in a few species of the Solanaceae, such as tomato, potato (Solanum tuberosum), and pepper (Capsicum annuum) (Clarke et al., 2013; Hind et al., 2016). Natural variations in flgII-28 responses among tomato varieties were exploited to isolate FLS3 as the flgII-28 receptor. Lineage-specific expansion also appears to occur in LRR-RLP types of PRRs. Ve- and Cf-mediated resistance only occurs in some tomato cultivars, whereas RLP23, RLP30, and ReMax/RLP1 occur only in Brassicaceae species (Jehle et al., 2013; Zhang et al., 2013; Albert et al., 2015). A recent success story is the identification of CORE as a receptor of csp22 (Wang et al., 2016). csp22 sensitivity was observed in many, but not all, Solanaceae species. Exploitation of natural variation among cultivated and wild tomato species allowed the isolation of CORE, which is required for csp22 perception. Transformation of CORE into Arabidopsis, which lacks CORE, confers csp22 sensitivity and disease resistance to P. syringae. Thus, the lineage specificity of PRRs will not only facilitate the future identification of PRRs, but also allow deployment of disease resistance in crop plants by transgenic expression of PRRs from different plant species (Lacombe et al., 2010; Albert et al., 2015).
The discovery of BAK1 as a common coreceptor for LRR-RKs and LRR-RPs is particularly useful for the identification of receptors for protein ligands. csp22-induced defenses require BAK1, suggesting that an LRR-RK or LRR-RP is involved. BAK1 was thus used as a bait to identify the hypothetical PRR in Nicotiana benthamiana that interacts with BAK1 only in the presence csp22 (Saur et al., 2016). Indeed, mass spectrum analysis identified NbCSPR as a novel LRR-RLP associated with BAK1 in csp22-treated tissue. Subsequent genetic analyses supported NbCSPR as a likely LRR-RP for csp22. The findings that both CORE and NbCSPR perceive csp22 suggest that the two types of PRRs evolve independently for the perception of a single PAMP. The approach using BAK1 as a bait is particularly useful in the rapid identification of new PRRs, as BAK1 has been shown to be required for defenses triggered by many pathogens, including aphids (Heese et al., 2007; Chaparro-Garcia et al., 2011; Kørner et al., 2013; Larroque et al., 2013; Prince et al., 2014).
In addition to using BAK1 as bait for receptor identification, recent advances in LRR-ECD ligand complex structures also provide novel means for receptor identification. A PEPR-Pep structure suggests that a ligand-recognition motif consisting of Arg-x-Arg in the ECD of PEPR directly recognizes the C-terminal residue Asn of Pep1. This signature motif is conserved in subfamily XI of the LRR-RLKs in Arabidopsis. Interestingly, several members of this subfamily have been shown to bind peptides ending with an Asn or His. Members of a rather large family of Arabidopsis peptide hormones carry an Asn or His residue at their C termini, suggesting that additional members of the XI subfamily could be receptors for these peptides. Song et al. (2016) tested if the ECD of a previously uncharacterized member of this subfamily could bind a specific peptide from a pool of synthetic peptides. Mass spectrum analysis indicated that this LRR-RK specifically binds RGFs. Subsequent genetic analysis showed that this LRR-RK and four additional members of this clade, namely, RGF RECEPTOR1 (RGFR1) to RGFR5, are required for RGF-regulated root growth. Structural studies showed that the specificity of RGFRs in RGF binding lies in a unique signature motif Arg-x-Gly-Gly that specifically recognizes the sulfated tyrosine in the RGF peptide. This elegant work illustrates how knowledge gained from structural studies can guide future identification of receptor-ligand pairs (Song et al., 2016).
DYNAMIC CONTROL OF PRR COMPLEXES
Besides the coreceptors mentioned above, receptor-like cytoplasmic kinases (RLCKs), are the major components of PRR complexes, which are involved in transducing signals from extracellular ligand perception into downstream signaling by phospho-relay (Wu and Zhou, 2013; Böhm et al., 2014; Macho and Zipfel, 2014; Couto and Zipfel, 2016). Several Arabidopsis RLCKs associate with PRRs and play important roles in PTI. For example, BOTRYTIS-INDUCED KINASE1 (BIK1) (Veronese et al., 2006), a member of Arabidopsis RLCK subfamily VII, associates with FLS2 and BAK1, in the absence of ligand elicitation (Lu et al., 2010; J. Zhang et al., 2010). Upon flg22 elicitation, BAK1 associates with FLS2 and phosphorylates BIK1 (Lu et al., 2010; J. Zhang et al., 2010). BIK1 then dissociates from the PRR complex to activate downstream signaling. In addition, BRASSINOSTEROID-SIGNALING KINASE1 (BSK1), a member of the RLCK XII subfamily and previously reported to be a substrate of BRI1 (Tang et al., 2008), also associates with FLS2 to regulate flg22-induced immune responses (Shi et al., 2013).
Immune receptor complexes are subject to dynamic regulation to allow tight control of the intensity and duration of plant immune responses, as inappropriate or unnecessary activation of PRR-mediated immune responses will lead to developmental and growth defects (Couto and Zipfel, 2016). PRR complexes are actively maintained in a resting state in the absence of patterns. For example, BIR2, a LRR-RLK with a pseudokinase domain, associates with BAK1 to prevent BAK1-FLS2 complex formation in the resting state (Halter et al., 2014). Upon perception of flg22, BIR2 releases BAK1 to facilitate FLS2-BAK1 interaction. BIR2 can be phosphorylated by the BAK1 kinase domain in vitro (Halter et al., 2014). BIR1 is a BIR2-related RLK and functions as a negative regulator in plant immunity (Gao et al., 2009). The bir1 mutant displays constitutive cell death and defense responses, which are partially dependent on BAK1 and SOBIR1. Interestingly, BAK1 and SOBIR1 constitutively associate with each other only in seedlings lacking BIR1, suggesting that BIR1 prevents the interactions between BAK1 and SOBIR1 in the resting state (Y. Liu et al., 2016; Gao et al., 2009).
The phosphorylation of PRR complex components is critical for receptor activation and is subject to tight regulation (Couto and Zipfel, 2016; Couto et al., 2016). In Arabidopsis, several protein phosphatases negatively regulate PRR activation. The Arabidopsis Ser/Thr phosphatase type 2A (PP2A) negatively regulates EFR-mediated defenses by controlling the phosphorylation levels of BAK1 (Segonzac et al., 2014), although it is not clear whether PP2A directly dephosphorylates BAK1. Similarly, the partially redundant Arabidopsis protein phosphatases type 2C (PP2C) POLTERGEIST-LIKE4 (PLL4) and PLL5, associate with EFR to negatively regulate PRR-mediated responses and bacterial resistance (Holton et al., 2015). PLL4 and PLL5 are close homologs of rice XANTHOMONAS RESISTANCE21 (XA21) BINDING PROTEIN15 (XB15), which was previously shown to dephosphorylate XA21 in vitro and negatively affect XA21-mediated immunity to the bacterium Xanthomonas oryzae pv oryzae (Park et al., 2008). These results also indicate conserved regulatory mechanisms for Xa21 and EFR, a notion supported by XA21-EFR domain swapping experiments (Holton et al., 2015; Schwessinger et al., 2015).
Protein accumulation of the PRR immune components is also under tight control. Two closely related Arabidopsis U-box (PUB) family E3 ubiquitin ligases, PUB12 and PUB13, were reported to associate with BAK1 in the resting state. Upon flg22 elicitation, BAK1 phosphorylates PUB12 and PUB13, which then associate with and promote degradation of FLS2 through ubiquitination. Loss-of-function mutations in PUB12 and PUB13 enhance immune responses to flg22 and disease resistance (Lu et al., 2011).
Protein accumulation of PRRs is also affected by endocytosis (Ben Khaled et al., 2015; Frescatada-Rosa et al., 2015; Couto and Zipfel, 2016). flg22 induces FLS2 internalization and degradation in Arabidopsis (Robatzek et al., 2006). Clathrin-dependent endocytosis and subcellular compartmentalization of FLS2 is critical for pattern-triggered immunity and requires flg22-induced recruitment of BAK1 (Chinchilla et al., 2007; Beck et al., 2012; Mbengue et al., 2016). flg22-induced internalization and degradation of FLS2 coincides with signal desensitization, which was proposed to play an important role in turning over ligand-occupied FLS2 by replenishing newly synthesized signal-competent FLS2 during subsequent infections (Smith et al., 2014). PEPR1/2 and EFR are also internalized in a ligand-dependent manner with a similar regulation mechanism (Mbengue et al., 2016; Ortiz-Morea et al. 2016; Yamada et al., 2016a). Similarly, Cf-4- and Cf-9-mediated resistance and endocytosis in tomato plants require ligand-induced recruitment of BAK1, indicating that diverse classes of PRRs share similar requirements for initiation of resistance and endocytosis (Postma et al., 2016). Readers are referred to recent reviews for detailed discussion on the endocytic regulation of plant RKs and RPs (Ben Khaled et al., 2015; Frescatada-Rosa et al., 2015).
Pathogen infection often induces the rearrangement of host plasma membrane constituents and the formation of a membrane microdomain, a compartment distinct from the surrounding membrane in composition, structure, and biological function (Malinsky et al., 2013). Ligand-induced receptor endocytosis leads to plasma membrane compartmentation or microdomain formation. For instance, flg22 induces FLS2 internalization, which forms distinct vesicles underneath the plasma membrane (Robatzek et al., 2006). Consistent with this observation, flg22 induces rapid and profound changes of protein composition in detergent-resistant membranes, i.e., the nonsolubilized fraction of membranes, in which FLS2 is enriched (Keinath et al., 2010). By contrast, FLS2 is depleted in the soluble fraction of membranes, indicting a relocalization of FLS2 (Keinath et al., 2010). Ligand-induced accumulation of PRRs in defined microdomains may play an important role in receptor regulation.
In addition to PRRs, BIK1 protein levels are also finely regulated by protein turnover. Arabidopsis CPK28 constitutively associates with and promotes proteasome-dependent turnover of BIK1 (Monaghan et al., 2014). The cpk28 mutant accumulates higher BIK1 protein levels and displays enhanced PTI responses, whereas overexpression of CPK28 leads to reduced BIK1 protein levels and decreased responses to PAMPs (Monaghan et al., 2014).
Plant heterotrimeric G proteins play important roles in PRR-mediated immunity. Arabidopsis GUANINE NUCLEOTIDEBINDING PROTEIN SUBUNIT-β (AGB1), NUCLEOTIDE BINDING PROTEIN SUBUNIT-γ1 (AGG1), and AGG2 are required for FLS2-, EFR-, and CERK1-mediated immunity as well as cell death and defenses triggered by the bir1 mutation (J. Liu et al., 2013; Lorek et al., 2013; Torres et al., 2013). Recently, it was shown that a heterotrimeric G protein complex, consisting of EXTRALARGE GUANINE NUCLEOTIDE BINDING PROTEIN2 (XLG2), AGB1, and AGG1 or AGG2 associates with the FLS2 complex to stabilize BIK1 and promotes immune signaling (Zhu et al., 2009; Maruta et al., 2015; Liang et al., 2016). It remains unknown which ubiquitin E3 ligase is responsible for BIK1 turnover and whether this E3 is subject to regulation by CPK28 and the heterotrimeric G protein complex.
REGULATION OF DOWNSTREAM SIGNALING BY PRRs
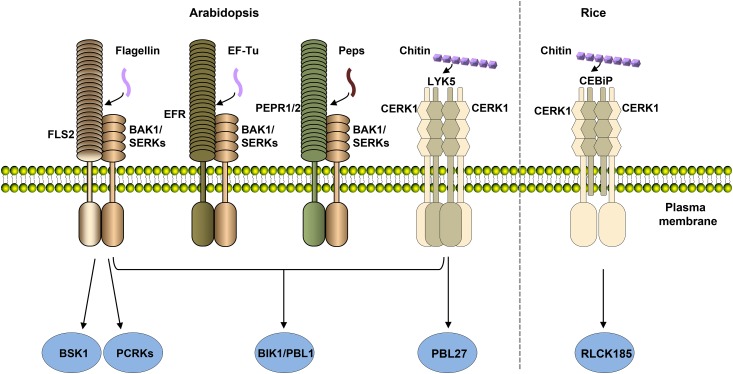
A number of RLCKs have emerged as central components linking PRRs to downstream defenses (Figure 2; Lin et al., 2013a). Among these, BIK1 is a central component integrating signals from multiple PRRs by directly interacting with FLS2, EFR, PEPRs, and CERK1 (Lu et al., 2010; Y. Zhang et al., 2010; Z. Liu et al., 2013). Additional Arabidopsis RLCK VII members, including PBS1-LIKE1 (PBL1), PBL27, PCRKs, and one RLCK XII member, BSK1, have been shown to play a role in pattern-triggered immunity by directly interacting with PRRs (Y. Zhang et al., 2010; Z. Liu et al., 2013; Shi et al., 2013; Shinya et al., 2014; Sreekanta et al., 2015; Kong et al., 2016). The rice RLCK VII members RLCK185 and RLCK176 have also been reported to mediate PGN- and chitin-induced defenses by interacting with CERK1 (Yamaguchi et al., 2013; Ao et al., 2014). Likewise, rice RLCK107 and RLCK118 were reported to interact with XA21, and silencing of these genes compromised XA21-dependent resistance (Zhou et al., 2016).
Figure 2.
RLCKs Differentially Mediate Immune Signaling from Different PRRs.
Different RLCKs known to interact with PRRs are colored in blue. BIK1 and PBL1 directly interact with multiple PRRs or coreceptors, including LRR-RKs (FLS2, EFR, and PEPRs), LRR-RLK (BAK1), and LysM-RLK (CERK1), to mediate immune signaling. The RLCK PBL27 was reported to specifically associate with CERK1 to mediate chitin-triggered immune signaling. In addition, the RLCKs BSK1 and PCRKs are also capable of interacting with FLS2 to mediate flagellin-triggered immune signaling. The rice RLCK185 directly interacts with CERK1 to mediate chitin-triggered signaling. FLS2, EFR, and PEPRs perceive bacteria flagellin, EF-Tu, and endogenous DAPMP Peps, respectively, and recruit coreceptors BAK1/SERKs to form active receptor complexes, whereas the rice LysM-RLP CEBiP and Arabidopsis LysM-RK LYK5 bind chitin and recruit LysM-RLK CERK1 to form active receptor complexes. The activated receptor complexes phosphorylate RLCKs to activate downstream signaling.
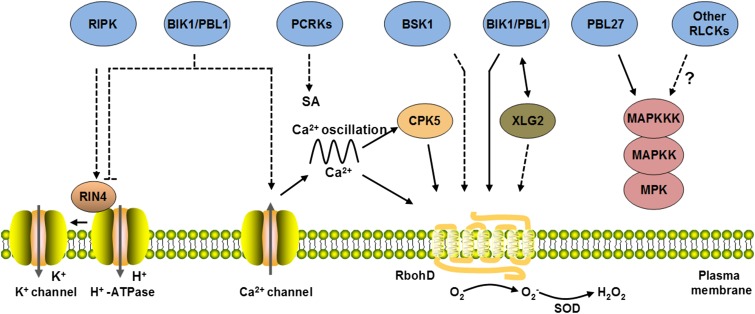
Within minutes, pattern recognition triggers a number of cellular events, including increase of cytoplasmic calcium ([Ca2+]cyt), cation and anion effluxes, extracellular alkalization, production of reactive oxygen species (ROS), and activation of MAP kinase (MPK) cascades (Boller and Felix, 2009). Pharmacological experiments indicated that calcium influx from the apoplast and calcium release from internal stores are required for the pattern-triggered [Ca2+]cyt increase (Klüsener et al., 2002; Thor and Peiter, 2014). Calcium influx is required for ROS burst, cation and anion effluxes, MPK activation, and downstream defense gene expression, suggesting that elevation of [Ca2+]cyt is central for pattern-triggered immunity (Jeworutzki et al., 2010; Segonzac et al., 2011; Ranf et al., 2011). BIK1 and PBL1 are required for flg22-triggered [Ca2+]cyt increase, suggesting that BIK1 and PBL1 directly or indirectly regulate a calcium channel (Figure 3). Identification of the pattern-triggered calcium channel remains a challenging task, but several plasma membrane-localized calcium permeable channels such as GLUTAMATE-LIKE RECEPTORs (GLRs), CYCLIC NUCLEOTIDE GATED CHANNELs (CNGCs), and REDUCED HYPEROSMOLARITY-INDUCED [Ca2+]i INCREASEs (OSCAs) exist in plants (Dodd et al., 2010; Hou et al., 2014; Yuan et al., 2014). For instance, GLRs are required for [Ca2+]cyt increase in the pollen tube during self-incompatibility (Iwano et al., 2015). OSCA1 is required for ([Ca2+]cyt) increase during osmotic stress (Hou et al., 2014; Yuan et al., 2014). CNGC2 has been shown to be a calcium channel for LPS- and Pep-induced calcium influx (Ali et al., 2007; Qi et al., 2010), whereas GLRs have been implicated in calcium burst following flg22, elf18, and chitin treatment (Kwaaitaal et al., 2011). However, a more recent study failed to lend support for GLRs and CNGCs in pattern-induced calcium burst (Thor and Peiter, 2014). During symbiosis, calcium oscillation in the nucleus is controlled by nuclear membrane-localized CNGCs and Ca2+-dependent adenosine triphosphatase (Ca2+-ATPase) (Capoen, et al., 2011; Charpentier et al., 2016). flg22 treatment also leads to calcium oscillation in the guard cell (Thor and Peiter, 2014), suggesting that pattern-induced calcium burst may be similarly controlled by both calcium channels and calcium pumps. Indeed, Arabidopsis Ca2+-ATPase 8 (ACA8) and ACA10 are directly associated with FLS2 and are required for optimum defenses (Frei dit Frey et al., 2012). Future challenges will be to identify the calcium channels involved, to decipher their regulation by BIK1 and PBL1, and to determine how they act together with Ca2+-ATPases to regulate cytosolic calcium oscillation.
Figure 3.
RLCKs Differentially Regulate Downstream Signaling.
RLCKs including BIK1, PBL1, RIPK, PCRKs, BSK1, and PBL27 are colored in blue. BIK1 and PBL1 positively regulate multiple downstream responses including RbohD-dependent ROS burst, [Ca2+]cyt increase, and activation of heterotrimeric G proteins. BIK1 and RIPK are required for phosphorylating distinct residues in RIN4, a plasma membrane proton ATPase-interacting protein, to positively or negatively regulate immunity. Proton efflux further regulates other ion channels, such as K+ channels. BSK1 positively regulates the ROS burst downstream of FLS2. PCRKs positively regulate SA biosynthesis upon flg22 treatment. PBL27 positively regulates MAPKKK5 to specifically mediate chitin-triggered MPK activation. The activation of RbohD also requires [Ca2+]cyt increase and CPK5. Solid lines indicate direct phosphorylations or protein-protein interactions, whereas dashed lines indicate that direct interactions/phosphorylations are unknown. Arrows indicate positive regulation, while T-bars indicate inhibition.
In Arabidopsis, the NADPH oxidase RESPIRATORY BURST OXIDASE HOMOLOG D (RbohD) is essential for pattern-triggered ROS production (Zhang et al., 2007). Pattern-triggered ROS production is required for stomatal closure and callose deposition (Zhang et al., 2007; Mersmann et al., 2010; Macho et al., 2012). BIK1, PBL1, BSK1, and RLCK185 have been shown to positively regulate pattern-triggered ROS production in Arabidopsis and rice (Figure 3; Y. Zhang et al., 2010; Shi et al., 2013; Yamaguchi et al., 2013). RbohD constitutively associates with BIK1 and FLS2 in plants (Kadota et al., 2014; Li et al., 2014). Upon activation by flg22, BIK1 and PBL1 phosphorylate RbohD at multiple sites required for ROS production. However, this phosphorylation is not sufficient for the activation of the NADPH oxidase activity of RbohD, indicating that additional regulation is required. Indeed, CALCIUM-DEPENDENT PROTEIN KINASEs (CPKs), such as CPK5, are necessary to phosphorylate additional sites in RbohD required for activation (Dubiella et al., 2013). Furthermore, RbohD contains EF-hand motifs for calcium ion binding, which likely provide further regulation. XLG2 and PBL13 may also positively or negatively regulate RbohD activity, respectively, although the underlying mechanisms are unknown (Figure 3; Liang et al., 2016; Lin et al., 2015).
It is not known how pattern recognition leads to extracellular alkalinization or what the biological function of extracellular alkalinization is. FERONIA (FER), a lectin RK, interacts with a family of Arabidopsis peptides termed RAPID ALKALINIZATION FACTORs (RALFs) to trigger extracellular alkalinization in plants and plays multiple roles in the regulation of plant growth and development (Pearce et al., 2001; Haruta et al., 2014; Tavormina et al., 2015). The activation of FER by RALF results in the phosphorylation of Arabidopsis plasma membrane H+ ATPase 2 (AHA2) at Ser-899, which inhibits the H+ ATPase activity and increases extracellular alkalinization. Interestingly, flg22 treatment also induces AHA2 Ser-899 phosphorylation, suggesting that the phosphorylation-regulated H+ ATPase activity may be responsible for flg22-induced extracellular alkalinization (Figure 3; Felix et al., 1999; Benschop et al., 2007). Although it is unknown whether AHA2 or other AHAs are required for pattern-triggered immunity, constitutive activation of AHA1 prevents stomatal closure in response to flg22 treatment (Liu et al., 2009). The Arabidopsis RPM1-INTERACTING4 (RIN4) protein originally identified as a protein guarded by the NLR protein RESISTANCE TO PSEUDOMONAS SYRINGAE PV. MACULICOLA1 (RPM1) has been shown to interact with and promote the activity of AHA1 to induce stomatal opening, which favors bacterial pathogen invasion. Interestingly, induced phosphorylation of RIN4 at Thr-21, Ser-160, and Thr-166 by P. syringae effector AvrB enhances AHA activity and promotes stomatal opening (Lee et al., 2015), whereas flg22 treatment specifically induces RIN4 phosphorylation at Ser-141 to enhance FLS2-mediated immunity (Chung et al., 2014), although it remains unknown whether this is associated with a regulation of AHA activity.
Pattern-triggered immunity activates two MPK cascades that play crucial roles in regulating downstream defense gene expression and phytoalexin biosynthesis (Meng and Zhang, 2013). One cascade is composed of MAP Kinase Kinase Kinase 1 (MEKK1), MAP Kinase Kinase 1 (MKK1), MKK2, and MAP Kinase 4 (MPK4). The second MAP kinase cascade is composed of an unknown MAPKKK, MKK4 and MKK5, and MPK3 and MPK6. Increasing evidence supports that PBLs mediate pattern-triggered MPK activation (Figure 3). The X. campestris campestris effector protein AvrAC, a specific inhibitor of BIK1 and related PBL kinases, strongly inhibits MPK3, MPK4, and MPK6 activity in flg22-treated seedlings, suggesting that PBLs collectively are required for MPK activation (Feng et al., 2012). The bik1 pbl1 double mutant is normal in flg22-triggered but impaired in Pep-triggered MPK activation (Feng et al., 2012; Yamada et al., 2016a). A recent report showed that pcrk1 pcrk2 plants are slightly reduced in flg22-induced MPK activation (Kong et al., 2016). PBL27 was shown to interact with CERK1, and the pbl27 mutant was reported to exhibit impaired MPK activation in response to chitin, but elevated MPK activation in response to flg22 (Shinya et al., 2014). These results suggest differential contribution of RLCK family members to MPK activation by different patterns. Recently, MAPKKK5 was reported to be a specific substrate of PBL27 and mediate chitin-triggered MPK activation (Yamada et al., 2016b). Paradoxically, MAPKKK5 appears to negatively impact flg22-triggered MPK activation. MAPKKK5 activation was shown to activate MKK4 and MKK5, which is consistent with the activation of MPK3 and MPK6 (Yamada et al., 2016b). The finding that PBL27 directly links a PRR to MPK activation is potentially exciting. However, it is not clear how MAPKKK5 activates MPK4, which is known to be activated by MEKK1, MKK1, and MKK2. Another MAPKKK, MAPKKK7, has been shown to interact with FLS2 to negatively regulate flg22-triggered MPK activation, but the underlying mechanism remains unknown (Mithoe et al., 2016). Moreover, a bacterial protease called PrpL was shown to trigger MPK activation potentially through an unknown PRR (Cheng et al., 2015). PrpL-triggered MPK activation requires heterotrimeric G proteins AGB1, GPA1, AGG1, and AGG2, although heterotrimeric G proteins are not necessary for MPK activation by known PRRs. One possibility is that different PRRs activate MPKs through distinct mechanisms. It will be important to determine whether additional MAPKKKs are responsible for MPK3 and MPK6 activation by other PRRs. More importantly, the aforementioned RLCK mutants are only partially affected in MPK activation in response to various patterns, suggesting that multiple RLCKs have redundant functions. Higher order mutants for RLCKs and MAPKKKs must be carefully analyzed to test whether different RLCKs and MAPKKKs are recruited by different PRRs for MPK activation.
Salicylate (SA) is a key defense hormone whose biosynthesis is induced in plants upon pathogen infection. SA accumulation is required for resistance at the primary inoculation site (local resistance) and elevated resistance at distal, uninoculated sites, a phenomenon called systemic acquired resistance (SAR) (Fu and Dong, 2013). Pattern-triggered immunity has also been reported to contribute to SAR (Mishina and Zeier, 2007). Application of flg22 and LPS to local leaves induces salicylate accumulation, expression of SAR marker genes PATHOGENESIS RELATED1 (PR1) and FLAVIN-DEPENDENT MONOOXYGENASE1 (FMO1), and disease resistance in systemic leaves (Mishina and Zeier, 2007). The pattern-induced systemic defenses require known SAR components such as SID2, PAD4, and NPR1, indicating that pattern-triggered immunity is integrated into the existing SAR signaling pathway. In addition, PEPRs are required for P. syringae- and pattern-induced PR1 expression and disease resistance in systemic leaves, indicating that PEPRs mediate pattern-induced SAR (Ross et al., 2014). PROPEP2 and PROPEP3 transcripts are induced only in the primary leaves, and local application of Peps can induce SAR, suggesting that the PEPR pathway contributes to the onset of SAR at primary challenge sites. Recently, PCRK1 and PCRK2 were shown to act redundantly to mediate flg22-induced SA accumulation and disease resistance to pathogens (Figure 3; Kong et al., 2016), although it is not known whether the PEPRs are required for PCRK-mediated SA induction. Future work is needed to test whether PEPRs and PCRKs act in the same pathway to regulate SA signaling and SAR.
As discussed above, RLCKs collectively activate multiple signaling pathways downstream of PRRs (Figure 3). Often, loss of function of a RLCK only leads to partial impairment in downstream signaling, suggesting functional redundancy. Different PRRs can recruit both specific and common RLCKs. For example, PEPRs specifically require BIK1 and PBL1, but not other RLCKs (Z. Liu et al., 2013). Likewise, PBL27 was reported to be specifically required for defenses triggered by chitin, but not flg22. On the other hand, BIK1 and PBL1 are required for defenses mediated by FLS2, EFR, PEPRs, and CERK1. In addition, FLS2 has been shown to interact with multiple RLCKs including BIK1, PBL1, PCRKs, and BSK1. Furthermore, different RLCKs may be required for the regulation of different downstream signaling components (Figure 3). Thus, BIK1 and PBL1 are required for flg22-, elf18-, and chitin-triggered [Ca2+]cyt increase and ROS burst, but not MPK activation. PBL27 and PCRKs are required for chitin-triggered MPK activation and flg22-triggered SA accumulation, respectively, but appear to play a minimal role, if any, in ROS production in response to the same pattern. However, this assumption may be overly simplified, as BIK1 and PBL1 have been shown to contribute to Pep-triggered MPK activation (Yamada et al., 2016a), whereas PCRKs have been shown to contribute to flg22-triggered MPK activation (Kong et al., 2016). Given that RLCK VII is a large family containing more than 40 members, many of which may play a role in pattern-triggered defenses, it will be important to empirically test both redundancy and specificity of these RLCKs in different PRR complexes and their role in different downstream signaling pathways.
CROSSTALK BETWEEN RLK-MEDIATED IMMUNE SIGNALING AND GROWTH/DEVELOPMENT
Plants must prioritize immunity versus growth and development according to the presence or absence of pathogen-imposed danger (Belkhadir et al., 2014; Lozano-Durán and Zipfel, 2015). BRI1, an Arabidopsis LRR-RK that perceives the steroid hormones brassinosteroids (BRs), is essential for diverse growth and development processes (Li and Chory, 1997). BRs inhibit PTI responses, and activation of BR signaling increases susceptibility to bacterial pathogens (Lozano-Durán and Zipfel, 2015). The BR signaling pathway shares multiple components with FLS2 pathways. Although BAK1 is a coreceptor for both BRI1 and FLS2, it is not a limiting factor to modulate BR and PAMP signaling (Lozano-Durán et al., 2013). BSK1 associates with both BRI1 and FLS2 and plays a positive role in PTI and BR signaling, but does not appear to function in the crosstalk between of BR and PTI signaling (Tang et al., 2008; Shi et al., 2013). By contrast, BIK1, a positive regulator in the FLS2 pathway, interacts with and phosphorylates BRI1 to negatively regulate BR signaling (Lin et al., 2013b). The transcription factors BRASSINAZOLE RESISTANT1 (BZR1) and HBI1 are also shared components of the BRI1 and FLS2 pathways. BZR1 and HBI1 positively regulate BR signaling but suppress immunity upon BR treatment (Bai et al., 2012; Lozano-Durán et al., 2013; Fan et al., 2014; Malinovsky et al., 2014). A more recent study suggests that the transcriptional cascade formed by BZR1 and HBI1 integrates BR and immunity crosstalk with multiple environmental inputs to fine tune the trade-off between growth and immunity (Lozano-Durán and Zipfel, 2015).
The second example of crosstalk between RLK pathways controlling immune signaling and growth/development is exemplified by the study of FER, which plays multiple roles in the regulation of plant growth and development (Tavormina et al., 2015). Early studies showed that FER-mediated signaling can negatively impact disease resistance and pattern-triggered immune responses (Kessler et al., 2010) and that FER becomes phosphorylated following flg22 treatment (Benschop et al., 2007). The recent finding that RALFs negatively regulate PRR complex formation (Stegmann et al., 2017) provides a mechanism for such crosstalk. A previous study suggested that FLS2 and BAK1 exist in a preformed complex prior to flg22 treatment (Sun et al., 2013). A recent study identified FER as a modulator of flg22, elf18, and chitin signaling, as a loss-of-function fer mutant displays reduced PAMP responses and increased susceptibility to P. syringae (Stegmann et al., 2017). FER is also enriched in microdomains after flg22 treatment (Keinath et al., 2010) and weakly interacts with both FLS2 and BAK1 (Stegmann et al., 2017). The flg22-induced FLS2-BAK1 and elf18-induced EFR-BAK1 interactions are compromised in fer seedlings, indicating that FER promotes ligand-induced dimerization of the receptors and coreceptor (Stegmann et al., 2017). Furthermore, cotreatment of seedlings with RALF23 peptide or overexpression of RALF23 decreases PAMP-induced responses and FLS2-BAK1 and EFR-BAK1 interactions (Stegmann et al., 2017). Together, the study supports that FER can act as a scaffold protein mediating PAMP-induced PRR complex formation and that RALFs actively regulate this process. Similar to the FLS2-FER interaction, the Arabidopsis malectin-like/LRR-RLK IMPAIRED OOMYCETE SUSCEPTIBILITY1 (IOS1) was recently shown to interact with FLS2, EFR, and CERK1 and contributes to pattern-triggered immunity (Yeh et al., 2016). Furthermore, a cysteine-rich RLK, CPK28, was recently shown to interact with FLS2 and positively regulate disease resistance and cell death (Yadeta et al., 2017). A direct interaction between different receptor complexes may facilitate crosstalk between different pathways. It will be interesting to test whether this is a common feature for RKs and RPs.
Another recent example of such crosstalk comes from the study of PSKs. PSKs and PLANT PEPTIDE CONTAINING SULFATED TYROSINE1 (PSY1) are related tyrosine-sulfated peptide hormones that promote root growth and xylem trachea development in plants (Matsubayashi and Sakagami, 2006; Amano et al., 2007). These peptides are perceived by the related LRR-RKs PSK RECEPTORs (PSKRs) and PSY1 RECEPTOR (PSY1R), respectively (Matsubayashi and Sakagami, 2006; Amano et al., 2007). Similar to fer mutants, Arabidopsis mutants lacking PSKR1 or TYROSINE PROTEIN SULFOTRANSFERASE (TPSP), the latter of which is required for sulfation of PSKs, exhibit enhanced defense gene expression in response to elf18 and flg22 and increased disease resistance to P. syringae, indicating a negative role of PSKR1 in pattern-triggered immunity (Igarashi et al., 2012). Similarly, PSKR1 and PSY1R play an additive role in the negative regulation of disease resistance to the fungal pathogen Alternaria brassicicola in addition to P. syringae (Mosher et al., 2013). The pskr1 pskr2 psy1r triple mutant is strongly enhanced in flg22-induced defense gene expression. Conversely, overexpression of PSKs and PSKR1 increases disease susceptibility to the bacterial and fungal pathogens. These findings strongly suggest that PSKR1- and PSY1R-mediated signaling inhibits pattern-triggered immunity.
Several LRR-RLKs, including ERECTA (ER), ER-LIKE1 (ERL1), ERL2, and LRR-RLP TOO MANY MOUTH (TMM), regulate stomatal patterning in response to epidermal pattern factor peptides (EPFs). Recent findings indicate that these proteins have prominent roles in disease resistance. Overexpression of ER lacking the kinase domain dominantly inhibits ER family receptor function, resulting in increased susceptibility to the fungal pathogen Plectosphaerella cucumerina (Jordá et al., 2016). The er erl1 erl2 triple mutant and er erl1 erl2 tmm quadruple mutant also display increased susceptibility to P. cucumerina, indicating that ER, ERL1, ERL2, and TMM contribute to disease resistance. er mutant plants are diminished in defense gene expression in response to the fungal spore extract, suggesting that ER positively regulates plant immunity in response to an unknown PAMP. Overexpression of EPFs fails to affect disease resistance to P. cucumerina, suggesting that ER, ERL1, ERL2, and TMM regulate disease resistance independently of EPF signaling. Future studies are needed to determine whether these RLKs and RLP regulate plant immunity by crosstalk with unknown PRRs that perceive fungal PAMPs or DAMPs.
MANIPULATION OF RLP AND RLK SIGNALING BY PATHOGEN EFFECTORS
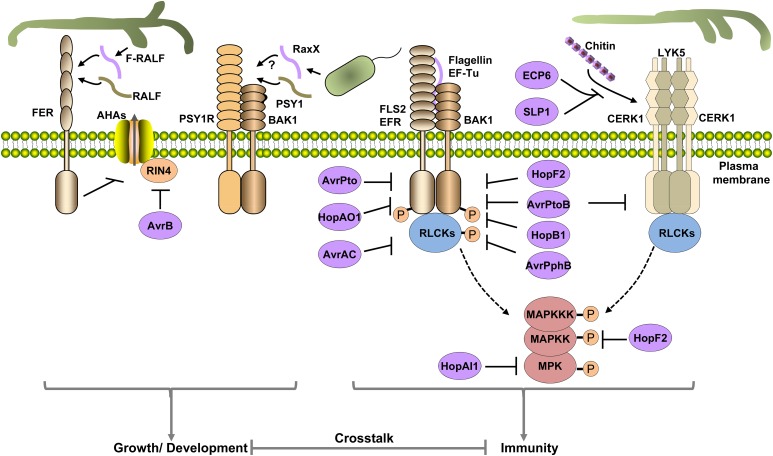
While PRRs are powerful in sensing and triggering effective defenses against potential pathogenic microbes, adapted pathogens have evolved a variety of effectors to evade detection or subvert the pattern-triggered immunity (Figure 4). Many effectors impede PRR functions by targeting microbial patterns, PRRs, or early signaling components of pattern-triggered immunity (see Deslandes and Rivas, 2012; Feng and Zhou, 2012; Dou and Zhou, 2012; Macho and Zipfel, 2015, and references therein). Recently, P. syringae was found to deliver a novel protease called HopB1 to destroy BAK1 (Li et al., 2016c). Perturbation of BAK1 is known to trigger the plant surveillance system and elevates defenses (Yamada et al., 2016b). Surprisingly, HopB1 goes undetected during infection (Li et al., 2016c). Close examination of the biochemical mode of action showed that HopB1 does not cleave normal BAK1. Instead, it only attacks an flg22-induced, phosphorylated form of BAK1. This highly selective action minimized perturbation to the host, allowing evasion of the host surveillance system. The analyses of these effectors not only elucidated mechanisms of pathogenesis of various pathogens, but also advanced our understanding of key signaling mechanisms mediated by PRRs. For instance, the search for virulence targets of the P. syringae effector AvrPphB led to the identification of BIK1 and PBLs as key signaling components downstream of multiple PRRs (Y. Zhang et al., 2010). The investigation of targets of the X. campestris effector AvrAC not only re-enforced the importance of BIK1 in pattern-triggered immunity (Feng et al., 2012), but also led to the identification of PBL2 as a decoy for NLR-mediated recognition of AvrAC (G. Wang et al., 2015). The finding that the P. syringae effector HopAO1 acts as a tyrosine phosphatase targeting EFR and FLS2 helped uncover a key role of Tyr836 phosphorylation in EFR immune activation (Macho et al., 2014).
Figure 4.
Pathogen Effector Proteins Enhance Susceptibility by Interfering with PRR-Mediated Signaling or Mimicking Peptide Hormones.
Different effectors and molecular patterns are colored in purple. The fungal apoplastic effectors ECP6 and SLP1 compete with plant receptors for chitin binding. Bacterial cytoplasmic effectors can block PRR-mediated signaling by physically inhibiting (AvrPto and AvrPtoB) or dephosphorylating (HopAO1) PRR kinases, proteolysis of coreceptors (HopB1), proteolysis (AvrPphB) or uridylylation (AvrAC) of RLCKs, ADP-ribosylation of MAPKK or BAK1 (HopF2), or dephosphorylation of MPKs (HopAI1). AvrB induces phosphorylation of RIN4 to stimulate plasma membrane proton ATPase activity. Fungal apoplastic effector F-RALF mimics plant RALF peptide to induce the FER-mediated signaling pathway, which inhibits PRR-specified immunity through crosstalk. The bacterial protein RaxX may mimic plant peptide PSY1 to stimulate PSY1R-mediated signaling, which is known to inhibit PRR-mediated immunity.
In addition to pathways mediated by PRRs, increasing evidence shows that pathogens also actively manipulate RKs that regulate plant growth and development to increase plant susceptibility (Figure 4). It has long been known that cyst nematodes secrete peptides that are structural and functional mimics of plant CLAVATA3/ENDOSPERM SURROUNDING REGION-related (CLE) peptides (Wang et al., 2001, 2005; Gao et al., 2003; Lu et al., 2009), with the latter being peptide hormones required for meristem maintenance and vascular differentiation (Fiers et al., 2007). The nematode CLE effectors are required for virulence (Patel et al., 2008), apparently by stimulating both the CLV2 and the CLV1 pathways (Replogle et al., 2011, 2013). Similarly, phytopathogenic root-knot nematodes encode peptides similar to plant C-TERMINAL ENCODED PEPTIDEs (CEPs) that are involved in the regulation of cell expansion (Bobay et al., 2013; Tavormina et al., 2015), although their roles in pathogenesis remain unknown. An outstanding question remains how CLE-mediated signaling benefits nematode pathogenesis. It is possible that CLE effectors may reprogram root development for infection and/or feeding. It is equally possible that CLE signaling is engaged in crosstalk with immunity. Indeed, it has been shown that the Arabidopsis coryne (crn) mutant displays an enhanced defense response gene expression in roots (Miwa et al., 2008). Future studies are needed to elucidate the underlying mechanisms for CLE effector-mediated nematode virulence. The CLV1 and CLV2 pathways may be targeted by additional pathogens, as clv1 and clv2 plants are more resistant to Ralstonia solanacearum (Hanemian et al., 2016), although the underlying mechanism remains unknown.
A search of fungal genomes identified numerous RALF-like peptides (Masachis et al., 2016; Thynne et al., 2016). Strikingly, all fungi encoding RALF-like sequences are plant pathogens. Among these, a Fusarium oxysporum RALF-like peptide (F-RALF) has been shown to stimulate extracellular alkalinization in plants. A loss-of-function fer mutant exhibits increased resistance to F. oxysporum (Masachis et al., 2016). Of note, hemitrophic and biotrophic fungal pathogens are known to cause extracellular alkalinization in plants, an important feature of their life styles (Prusky et al., 2001; Prusky and Yakoby, 2003). F. oxysporum mutants lacking F-RALF are unable to induce extracellular alkalinization and are less virulent in plants (Masachis et al., 2016). The F-RALF-induced pH elevation triggers rapid phosphorylation of the fungal MPK Fmk1 essential for invasive hyphal growth (Di Pietro et al., 2001; Masachis et al., 2016). The fungal pathogen may have taken advantage of the crosstalk between FER-mediated signaling and pattern-triggered immunity to promote pathogenesis (Figure 4). Indeed, F. oxysporum F-RALF mutants are less capable of inhibiting pattern-induced defense gene expression (Masachis et al., 2016).
The observation that PSKR1- and PSY1R-mediated signaling negatively impacts pattern-triggered immunity suggests that pathogens could target these proteins for virulence. Indeed, the X. oryzae oryzae peptide RaxX-sY has high levels of amino acid sequence similarity to PSY1 from plants (Pruitt et al., 2015), raising the tantalizing possibility that RaxX evolved to mimic PSY1, thereby suppressing immunity (Figure 4). It will be interesting to determine whether RaxX-sY indeed mimics PSY1 in the regulation of plant growth and development and plant defenses. If so, an outstanding question would be how Xa21 differentiates endogenous PSY1 peptide from RaxX-sY so that defenses do not get activated by the endogenous peptide hormone.
Filamentous pathogens secrete hundreds of effectors, many of which are targeted to the apoplast. It is tempting to speculate that additional effectors inhibit PRRs or stimulate RKs that control growth and development. Indeed, the C. fulvum effector Ave1 is a virulence factor and shares sequence homology with proteins from diverse plant species, suggesting a plant origin of Ave1 (de Jonge et al., 2012). Although the function of these plant proteins is not known, the findings raise the possibility that Ave1 enhances virulence by mimicking these plant proteins. As peptide sequences are often too short for simple BLAST analysis, advanced computational methods will likely uncover additional pathogen effectors that mimic plant peptides.
CONCLUSION
RLKs and RLPs are at the core of the plant early warning system in the wake of pathogen attacks. Biochemical and structural studies are rapidly advancing our understanding of the mechanisms of PRR action in immunity. Ligand-induced recruitment of coreceptors is crucial for LRR- and LysM-containing PRRs. Future studies will test whether this mode of action can be extrapolated to PRRs containing other classes of ECDs. In addition to receptors and coreceptors, PRR complex components are also associated with RLKs, RLCKs, heterotrimeric G proteins, PUBs, and PP2Cs. These proteins allow dynamic and tight regulation of PRR complexes before and after pattern recognition. RLCKs associated with the RK type of PRRs are central for the activation of bifurcating downstream signaling pathways, while it remains to be determined whether this is also true for the RP type of PRRs. Understanding how RLCKs activate downstream signaling components will continue to yield exciting mechanistic insights for plant immunity.
The number of microbial patterns recognized by different plant species is likely to be high. Our understanding of host recognition of microbial patterns is limited by our ability to identify microbial patterns and their corresponding receptors. Nonetheless, proteinaceous microbial patterns are subject to positive selection at individual residues as a result of host-pathogen coevolution. With the help of computational tools, it is now possible to exploit microbial genome sequences to expedite the discovery of microbial patterns. Likewise, PRRs often belong to the RLK and RLP subfamilies that display lineage-specific expansion. This feature not only helps the identification of PRRs, but also allows deployment of disease resistance by cross-species/genus transfer of PRR-coding genes. Indeed, transgenic overexpression of EFR confers elf18 sensitivity and elevated resistance to cereal bacterial pathogens in rice and wheat (Triticum aestivum) plants, which do not encode EFR (Schwessinger et al., 2015; Schoonbeek et al., 2015).
Extensive studies in the past decade have demonstrated that microbial effectors enhance virulence often by directly interfering with PRR-mediated signaling. Emerging evidence indicates that pathogen effectors can also promote virulence by mimicking plant peptide hormones to indirectly interfere with immune signaling through crosstalk between different signaling pathways. The interactions between microbial effectors and signaling pathways mediated by plant RKs will continue to be a fertile ground of investigation.
Supplementary Material
Acknowledgments
We thank Jijie Chai for providing structural alignment of receptor-ligand complexes and apologize to colleagues whose work was not cited due to space limitation. The work was supported by grants from the Chinese Natural Science Foundation (31230007) to J.-M.Z., the Chinese Ministry of Science and Technology (Grant 2015CB910200 to J.-M.Z. and D.T.), and the National Science Fund for Distinguished Young Scholars of China (31525019 to D.T.).
AUTHOR CONTRIBUTIONS
All authors contributed to writing the article.
Footnotes
Articles can be viewed without a subscription.
References
- Albert I., et al. (2015). An RLP23-SOBIR1-BAK1 complex mediates NLP-triggered immunity. Nat. Plants 1: 15140. [DOI] [PubMed] [Google Scholar]
- Alcázar R., García A.V., Kronholm I., de Meaux J., Koornneef M., Parker J.E., Reymond M. (2010). Natural variation at Strubbelig Receptor Kinase 3 drives immune-triggered incompatibilities between Arabidopsis thaliana accessions. Nat. Genet. 42: 1135–1139. [DOI] [PubMed] [Google Scholar]
- Ali R., Ma W., Lemtiri-Chlieh F., Tsaltas D., Leng Q., von Bodman S., Berkowitz G.A. (2007). Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 19: 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano Y., Tsubouchi H., Shinohara H., Ogawa M., Matsubayashi Y. (2007). Tyrosine-sulfated glycopeptide involved in cellular proliferation and expansion in Arabidopsis. Proc. Natl. Acad. Sci. USA 104: 18333–18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Y., Li Z., Feng D., Xiong F., Liu J., Li J.F., Wang M., Wang J., Liu B., Wang H.B. (2014). OsCERK1 and OsRLCK176 play important roles in peptidoglycan and chitin signaling in rice innate immunity. Plant J. 80: 1072–1084. [DOI] [PubMed] [Google Scholar]
- Bai M.Y., Fan M., Oh E., Wang Z.Y. (2012). A triple helix-loop-helix/basic helix-loop-helix cascade controls cell elongation downstream of multiple hormonal and environmental signaling pathways in Arabidopsis. Plant Cell 24: 4917–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M., Sharfman M., Ron M., Avni A. (2010). BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1. Plant J. 63: 791–800. [DOI] [PubMed] [Google Scholar]
- Bauer Z., Gómez-Gómez L., Boller T., Felix G. (2001). Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. J. Biol. Chem. 276: 45669–45676. [DOI] [PubMed] [Google Scholar]
- Beck M., Heard W., Mbengue M., Robatzek S. (2012). The INs and OUTs of pattern recognition receptors at the cell surface. Curr. Opin. Plant Biol. 15: 367–374. [DOI] [PubMed] [Google Scholar]
- Belkhadir Y., Yang L., Hetzel J., Dangl J.L., Chory J. (2014). The growth-defense pivot: crisis management in plants mediated by LRR-RK surface receptors. Trends Biochem. Sci. 39: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Khaled S., Postma J., Robatzek S. (2015). A moving view: subcellular trafficking processes in pattern recognition receptor-triggered plant immunity. Annu. Rev. Phytopathol. 53: 379–402. [DOI] [PubMed] [Google Scholar]
- Benschop J.J., Mohammed S., O’Flaherty M., Heck A.J., Slijper M., Menke F.L.H. (2007). Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol. Cell. Proteomics 6: 1198–1214. [DOI] [PubMed] [Google Scholar]
- Bi D., Cheng Y.T., Li X., Zhang Y. (2010). Activation of plant immune responses by a gain-of-function mutation in an atypical receptor-like kinase. Plant Physiol. 153: 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G., Liebrand T.W., Cordewener J.H., America A.H., Xu X., Joosten M.H. (2014). Arabidopsis thaliana receptor-like protein AtRLP23 associates with the receptor-like kinase AtSOBIR1. Plant Signal. Behav. 9: e27937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigeard J., Colcombet J., Hirt H. (2015). Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 8: 521–539. [DOI] [PubMed] [Google Scholar]
- Bobay B.G., DiGennaro P., Scholl E., Imin N., Djordjevic M.A., Mck Bird D. (2013). Solution NMR studies of the plant peptide hormone CEP inform function. FEBS Lett. 587: 3979–3985. [DOI] [PubMed] [Google Scholar]
- Böhm H., Albert I., Fan L., Reinhard A., Nürnberger T. (2014). Immune receptor complexes at the plant cell surface. Curr. Opin. Plant Biol. 20: 47–54. [DOI] [PubMed] [Google Scholar]
- Boller T., Felix G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60: 379–406. [DOI] [PubMed] [Google Scholar]
- Bouwmeester K., de Sain M., Weide R., Gouget A., Klamer S., Canut H., Govers F. (2011). The lectin receptor kinase LecRK-I.9 is a novel Phytophthora resistance component and a potential host target for a RXLR effector. PLoS Pathog. 7: e1001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiden M., Simon R. (2016). Q&A: How does peptide signaling direct plant development? BMC Biol. 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutus A., Sicilia F., Macone A., Cervone F., De Lorenzo G. (2010). A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. USA 107: 9452–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R., et al. (2011). The plant pathogen Pseudomonas syringae pv. tomato is genetically monomorphic and under strong selection to evade tomato immunity. PLoS Pathog. 7: e1002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Liang Y., Tanaka K., Nguyen C.T., Jedrzejczak R.P., Joachimiak A., Stacey G. (2014). The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. eLife 3: e03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoen W., Sun J., Wysham D., Otegui M.S., Venkateshwaran M., Hirsch S., Miwa H., Downie J.A., Morris R.J., Ané J.M., Oldroyd G.E. (2011). Nuclear membranes control symbiotic calcium signaling of legumes. Proc. Natl. Acad. Sci. USA 108: 14348–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro-Garcia A., Wilkinson R.C., Gimenez-Ibanez S., Findlay K., Coffey M.D., Zipfel C., Rathjen J.P., Kamoun S., Schornack S. (2011). The receptor-like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen phytophthora infestans in Nicotiana benthamiana. PLoS One 6: e16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M., Sun J., Vaz Martins T., Radhakrishnan G.V., Findlay K., Soumpourou E., Thouin J., Véry A.-A., Sanders D., Morris R.J., Oldroyd G.E. (2016). Nuclear-localized cyclic nucleotide-gated channels mediate symbiotic calcium oscillations. Science 352: 1102–1105. [DOI] [PubMed] [Google Scholar]
- Chen X., et al. (2006). A B-lectin receptor kinase gene conferring rice blast resistance. Plant J. 46: 794–804. [DOI] [PubMed] [Google Scholar]
- Chen X., Zuo S., Schwessinger B., Chern M., Canlas P.E., Ruan D., Zhou X., Wang J., Daudi A., Petzold C.J., Heazlewood J.L., Ronald P.C. (2014). An XA21-associated kinase (OsSERK2) regulates immunity mediated by the XA21 and XA3 immune receptors. Mol. Plant 7: 874–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Li J.F., Niu Y., Zhang X.C., Woody O.Z., Xiong Y., Djonović S., Millet Y., Bush J., McConkey B.J., Sheen J., Ausubel F.M. (2015). Pathogen-secreted proteases activate a novel plant immune pathway. Nature 521: 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J.D., Felix G., Boller T. (2007). A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500. [DOI] [PubMed] [Google Scholar]
- Choi J., Tanaka K., Cao Y., Qi Y., Qiu J., Liang Y., Lee S.Y., Stacey G. (2014). Identification of a plant receptor for extracellular ATP. Science 343: 290–294. [DOI] [PubMed] [Google Scholar]
- Chung E.-H., El-Kasmi F., He Y., Loehr A., Dangl J.L. (2014). A plant phosphoswitch platform repeatedly targeted by type III effector proteins regulates the output of both tiers of plant immune receptors. Cell Host Microbe 16: 484–494. [DOI] [PubMed] [Google Scholar]
- Clarke C.R., Chinchilla D., Hind S.R., Taguchi F., Miki R., Ichinose Y., Martin G.B., Leman S., Felix G., Vinatzer B.A. (2013). Allelic variation in two distinct Pseudomonas syringae flagellin epitopes modulates the strength of plant immune responses but not bacterial motility. New Phytol. 200: 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto D., Zipfel C. (2016). Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16: 537–552. [DOI] [PubMed] [Google Scholar]
- Couto D., et al. (2016). The Arabidopsis protein phosphatase PP2C38 negatively regulates the central immune kinase BIK1. PLoS Pathogen. 12: e1005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decreux A., Messiaen J. (2005). Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 46: 268–278. [DOI] [PubMed] [Google Scholar]
- de Jonge R., van Esse H.P., Maruthachalam K., Bolton M.D., Santhanam P., Saber M.K., Zhang Z., Usami T., Lievens B., Subbarao K.V., Thomma B.P. (2012). Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc. Natl. Acad. Sci. USA 109: 5110–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclos-Theveniau M., Arnaud D., Huang T.Y., Lin G.J., Chen W.Y., Lin Y.C., Zimmerli L. (2012). The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 8: e1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes L., Rivas S. (2012). Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci. 17: 644–655. [DOI] [PubMed] [Google Scholar]
- Diener A.C., Ausubel F.M. (2005). RESISTANCE TO FUSARIUM OXYSPORUM 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics 171: 305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro A., García-MacEira F.I., Méglecz E., Roncero M.I. (2001). A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39: 1140–1152. [PubMed] [Google Scholar]
- Dixon M.S., Hatzixanthis K., Jones D.A., Harrison K., Jones J.D. (1998). The tomato Cf-5 disease resistance gene and six homologs show pronounced allelic variation in leucine-rich repeat copy number. Plant Cell 10: 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M.S., Jones D.A., Keddie J.S., Thomas C.M., Harrison K., Jones J.D. (1996). The tomato Cf-2 disease resistance locus comprises two functional genes encoding leucine-rich repeat proteins. Cell 84: 451–459. [DOI] [PubMed] [Google Scholar]
- Dodd A.N., Kudla J., Sanders D. (2010). The language of calcium signaling. Annu. Rev. Plant Biol. 61: 593–620. [DOI] [PubMed] [Google Scholar]
- Dou D., Zhou J.M. (2012). Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe 12: 484–495. [DOI] [PubMed] [Google Scholar]
- Du J., et al. (2015). Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nat. Plants 1: 15034. [DOI] [PubMed] [Google Scholar]
- Dubiella U., Seybold H., Durian G., Komander E., Lassig R., Witte C.P., Schulze W.X., Romeis T. (2013). Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 110: 8744–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo J.M., Huck N., Kessler S., Gagliardini V., Gheyselinck J., Yang W.C., Grossniklaus U. (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317: 656–660. [DOI] [PubMed] [Google Scholar]
- Fan M., Bai M.Y., Kim J.G., Wang T., Oh E., Chen L., Park C.H., Son S.H., Kim S.K., Mudgett M.B., Wang Z.Y. (2014). The bHLH transcription factor HBI1 mediates the trade-off between growth and pathogen-associated molecular pattern-triggered immunity in Arabidopsis. Plant Cell 26: 828–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng F., Zhou J.M. (2012). Plant-bacterial pathogen interactions mediated by type III effectors. Curr. Opin. Plant Biol. 15: 469–476. [DOI] [PubMed] [Google Scholar]
- Feng F., Yang F., Rong W., Wu X., Zhang J., Chen S., He C., Zhou J.M. (2012). A Xanthomonas uridine 5′-monophosphate transferase inhibits plant immune kinases. Nature 485: 114–118. [DOI] [PubMed] [Google Scholar]
- Feuillet C., Schachermayr G., Keller B. (1997). Molecular cloning of a new receptor-like kinase gene encoded at the Lr10 disease resistance locus of wheat. Plant J. 11: 45–52. [DOI] [PubMed] [Google Scholar]
- Fiers M., Ku K.L., Liu C.M. (2007). CLE peptide ligands and their roles in establishing meristems. Curr. Opin. Plant Biol. 10: 39–43. [DOI] [PubMed] [Google Scholar]
- Felix G., Duran J.D., Volko S., Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18: 265–276. [DOI] [PubMed] [Google Scholar]
- Frei dit Frey N., et al. (2012). Plasma membrane calcium ATPases are important components of receptor-mediated signaling in plant immune responses and development. Plant Physiol. 159: 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frescatada-Rosa M., Robatzek S., Kuhn H. (2015). Should I stay or should I go? Traffic control for plant pattern recognition receptors. Curr. Opin. Plant Biol. 28: 23–29. [DOI] [PubMed] [Google Scholar]
- Fu Z.Q., Dong X. (2013). Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 64: 839–863. [DOI] [PubMed] [Google Scholar]
- Gao B., Allen R., Maier T., Davis E.L., Baum T.J., Hussey R.S. (2003). The parasitome of the phytonematode Heterodera glycines. Mol. Plant Microbe Interact. 16: 720–726. [DOI] [PubMed] [Google Scholar]
- Gao M., Wang X., Wang D., Xu F., Ding X., Zhang Z., Bi D., Cheng Y.T., Chen S., Li X., Zhang Y. (2009). Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44. [DOI] [PubMed] [Google Scholar]
- Gilardoni P.A., Hettenhausen C., Baldwin I.T., Bonaventure G. (2011). Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. Plant Cell 23: 3512–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L., Boller T. (2000). FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5: 1003–1011. [DOI] [PubMed] [Google Scholar]
- Gouget A., Senchou V., Govers F., Sanson A., Barre A., Rougé P., Pont-Lezica R., Canut H. (2006). Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis. Plant Physiol. 140: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust A.A., Felix G. (2014). Receptor like proteins associate with SOBIR1-type of adaptors to form bimolecular receptor kinases. Curr. Opin. Plant Biol. 21: 104–111. [DOI] [PubMed] [Google Scholar]
- Gust A.A., Willmann R., Desaki Y., Grabherr H.M., Nürnberger T. (2012). Plant LysM proteins: modules mediating symbiosis and immunity. Trends Plant Sci. 17: 495–502. [DOI] [PubMed] [Google Scholar]
- Halter T., et al. (2014). The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr. Biol. 24: 134–143. [DOI] [PubMed] [Google Scholar]
- Hanemian M., et al. (2016). Arabidopsis CLAVATA1 and CLAVATA2 receptors contribute to Ralstonia solanacearum pathogenicity through a miR169-dependent pathway. New Phytol. 211: 502–515. [DOI] [PubMed] [Google Scholar]
- Haruta M., Sabat G., Stecker K., Minkoff B.B., Sussman M.R. (2014). A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayafune M., et al. (2014). Chitin-induced activation of immune signaling by the rice receptor CEBiP relies on a unique sandwich-type dimerization. Proc. Natl. Acad. Sci. USA 111: E404–E413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese A., Hann D.R., Gimenez-Ibanez S., Jones A.M.E., He K., Li J., Schroeder J.I., Peck S.C., Rathjen J.P. (2007). The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 104: 12217–12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K., Sado P.E., Van Tuinen A., Rochange S., Desnos T., Balzergue S., Pelletier S., Renou J.P., Höfte H. (2007). A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17: 922–931. [DOI] [PubMed] [Google Scholar]
- Hind S.R., et al. (2016). Tomato receptor FLAGELLIN-SENSING 3 binds flgII-28 and activates the plant immune system. Nat. Plants 2: 16128. [DOI] [PubMed] [Google Scholar]
- Holton N., Nekrasov V., Ronald P.C., Zipfel C. (2015). The phylogenetically-related pattern recognition receptors EFR and XA21 recruit similar immune signaling components in monocots and dicots. PLoS Pathog. 11: e1004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C., Tian W., Kleist T., He K., Garcia V., Bai F., Hao Y., Luan S., Li L. (2014). DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 24: 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A., Ryan C.A. (2007). Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proc. Natl. Acad. Sci. USA 104: 10732–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A., Pearce G., Ryan C.A. (2006). An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. USA 103: 10098–10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi D., Tsuda K., Katagiri F. (2012). The peptide growth factor, phytosulfokine, attenuates pattern-triggered immunity. Plant J. 71: 194–204. [DOI] [PubMed] [Google Scholar]
- Iwano M., et al. (2015). Calcium signalling mediates self-incompatibility response in the Brassicaceae. Nat. Plants 1: 15128. [DOI] [PubMed] [Google Scholar]
- Jehle A.K., Lipschis M., Albert M., Fallahzadeh-Mamaghani V., Fürst U., Mueller K., Felix G. (2013). The receptor-like protein ReMAX of Arabidopsis detects the microbe-associated molecular pattern eMax from Xanthomonas. Plant Cell 25: 2330–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeworutzki E., Roelfsema M.R., Anschütz U., Krol E., Elzenga J.T., Felix G., Boller T., Hedrich R., Becker D. (2010). Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca-associated opening of plasma membrane anion channels. Plant J. 62: 367–378. [DOI] [PubMed] [Google Scholar]
- Jones D.A., Thomas C.M., Hammond-Kosack K.E., Balint-Kurti P.J., Jones J.D. (1994). Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 266: 789–793. [DOI] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. (2006). The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Jones J.D.G., Vance R.E., Dangl J.L. (2016). Intracellular innate immune surveillance devices in plants and animals. Science 354: aaf6395. [DOI] [PubMed] [Google Scholar]
- Joosten M.H., Vogelsang R., Cozijnsen T.J., Verberne M.C., De Wit P.J. (1997). The biotrophic fungus Cladosporium fulvum circumvents Cf-4-mediated resistance by producing unstable AVR4 elicitors. Plant Cell 9: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordá L., Sopeña-Torres S., Escudero V., Nuñez-Corcuera B., Delgado-Cerezo M., Torii K.U., Molina A. (2016). ERECTA and BAK1 receptor like kinases interact to regulate immune responses in Arabidopsis. Front. Plant Sci. 7: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y., Sklenar J., Derbyshire P., Stransfeld L., Asai S., Ntoukakis V., Jones J.D.G., Shirasu K., Menke F., Jones A., Zipfel C. (2014). Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54: 43–55. [DOI] [PubMed] [Google Scholar]
- Kaku H., Nishizawa Y., Ishii-Minami N., Akimoto-Tomiyama C., Dohmae N., Takio K., Minami E., Shibuya N. (2006). Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA 103: 11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki H., Saitoh H., Takahashi Y., Berberich T., Ito A., Kamoun S., Terauchi R. (2008). NbLRK1, a lectin-like receptor kinase protein of Nicotiana benthamiana, interacts with Phytophthora infestans INF1 elicitin and mediates INF1-induced cell death. Planta 228: 977–987. [DOI] [PubMed] [Google Scholar]
- Kawahigashi H., Kasuga S., Ando T., Kanamori H., Wu J., Yonemaru J., Sazuka T., Matsumoto T. (2011). Positional cloning of ds1, the target leaf spot resistance gene against Bipolaris sorghicola in sorghum. Theor. Appl. Genet. 123: 131–142. [DOI] [PubMed] [Google Scholar]
- Keinath N.F., Kierszniowska S., Lorek J., Bourdais G., Kessler S.A., Shimosato-Asano H., Grossniklaus U., Schulze W.X., Robatzek S., Panstruga R. (2010). PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J. Biol. Chem. 285: 39140–39149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S.A., Shimosato-Asano H., Keinath N.F., Wuest S.E., Ingram G., Panstruga R., Grossniklaus U. (2010). Conserved molecular components for pollen tube reception and fungal invasion. Science 330: 968–971. [DOI] [PubMed] [Google Scholar]
- Kim Y.T., Oh J., Kim K.H., Uhm J.Y., Lee B.M. (2010). Isolation and characterization of NgRLK1, a receptor-like kinase of Nicotiana glutinosa that interacts with the elicitin of Phytophthora capsici. Mol. Biol. Rep. 37: 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B., Young J.J., Murata Y., Allen G.J., Mori I.C., Hugouvieux V., Schroeder J.I. (2002). Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 130: 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Sun T., Qu N., Ma J., Li M., Cheng Y.T., Zhang Q., Wu D., Zhang Z., Zhang Y. (2016). Two redundant receptor-like cytoplasmic kinases function downstream of pattern recognition receptors to regulate activation of SA biosynthesis. Plant Physiol. 171: 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kørner C.J., Klauser D., Niehl A., Domínguez-Ferreras A., Chinchilla D., Boller T., Heinlein M., Hann D.R. (2013). The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol. Plant Microbe Interact. 26: 1271–1280. [DOI] [PubMed] [Google Scholar]
- Krol E., Mentzel T., Chinchilla D., Boller T., Felix G., Kemmerling B., Postel S., Arents M., Jeworutzki E., Al-Rasheid K.A.S., Becker D., Hedrich R. (2010). Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J. Biol. Chem. 285: 13471–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J., Thomas C.M., Golstein C., Dixon M.S., Smoker M., Tang S., Mulder L., Jones J.D. (2002). A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 296: 744–747. [DOI] [PubMed] [Google Scholar]
- Kunze G., Zipfel C., Robatzek S., Niehaus K., Boller T., Felix G. (2004). The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaaitaal M., Huisman R., Maintz J., Reinstädler A., Panstruga R. (2011). Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochem J. 440: 355–365. [DOI] [PubMed] [Google Scholar]
- Lacombe S., Rougon-Cardoso A., Sherwood E., Peeters N., Dahlbeck D., van Esse H.P., Smoker M., Rallapalli G., Thomma B.P., Staskawicz B., Jones J.D., Zipfel C. (2010). Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat. Biotechnol. 28: 365–369. [DOI] [PubMed] [Google Scholar]
- Larroque M., Belmas E., Martinez T., Vergnes S., Ladouce N., Lafitte C., Gaulin E., Dumas B. (2013). Pathogen-associated molecular pattern-triggered immunity and resistance to the root pathogen Phytophthora parasitica in Arabidopsis. J. Exp. Bot. 64: 3615–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Bourdais G., Yu G., Robatzek S., Coaker G. (2015). Phosphorylation of the plant immune regulator RPM1-INTERACTING PROTEIN4 enhances plant plasma membrane H+-ATPase activity and inhibits flagellin-triggered immune responses in Arabidopsis. Plant Cell 27: 2042–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Meng X., Shan L., He P. (2016b). Transcriptional regulation of pattern-triggered immunity in plants. Cell Host Microbe 19: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhou S.Y., Zhao W.S., Su S.C., Peng Y.L. (2009). A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast disease resistance. Plant Mol. Biol. 69: 337–346. [DOI] [PubMed] [Google Scholar]
- Li J., Chory J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938. [DOI] [PubMed] [Google Scholar]
- Li L., Kim P., Yu L., Cai G., Chen S., Alfano J.R., Zhou J.M. (2016c). Activation-dependent destruction of a co-receptor by a Pseudomonas syringae effector dampens plant immunity. Cell Host Microbe 20: 504–514. [DOI] [PubMed] [Google Scholar]
- Li L., Li M., Yu L., Zhou Z., Liang X., Liu Z., Cai G., Gao L., Zhang X., Wang Y., Chen S., Zhou J.M. (2014). The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338. [DOI] [PubMed] [Google Scholar]
- Li L., Yu Y., Zhou Z., Zhou J.M. (2016a). Plant pattern-recognition receptors controlling innate immunity. Sci. China Life Sci. 59: 878–888. [DOI] [PubMed] [Google Scholar]
- Liang X., Ding P., Lian K., Wang J., Ma M., Li L., Li L., Li M., Zhang X., Chen S., Zhang Y., Zhou J.M. (2016). Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. eLife 5: e13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrand T.W., van den Berg G.C., Zhang Z., Smit P., Cordewener J.H., America A.H., Sklenar J., Jones A.M., Tameling W.I., Robatzek S., Thomma B.P., Joosten M.H. (2013). Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc. Natl. Acad. Sci. USA 110: 10010–10015. Erratum. Proc. Natl. Acad. Sci. USA 110: 13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrand T.W., van den Burg H.A., Joosten M.H. (2014). Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 19: 123–132. [DOI] [PubMed] [Google Scholar]
- Lin W., Lu D., Gao X., Jiang S., Ma X., Wang Z., Mengiste T., He P., Shan L. (2013b). Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc. Natl. Acad. Sci. USA 110: 12114–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Ma X., Shan L., He P. (2013a). Big roles of small kinases: the complex functions of receptor-like cytoplasmic kinases in plant immunity and development. J. Integr. Plant Biol. 55: 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z.J., Liebrand T.W., Yadeta K.A., Coaker G. (2015). PBL13 is a serine/threonine protein kinase that negatively regulates Arabidopsis immune responses. Plant Physiol. 169: 2950–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., et al. (2012). Lysin motif-containing proteins LYP4 and LYP6 play dual roles in peptidoglycan and chitin perception in rice innate immunity. Plant Cell 24: 3406–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ding P., Sun T., Nitta Y., Dong O., Huang X., Yang W., Li X., Botella J.R., Zhang Y. (2013). Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiol. 161: 2146–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Elmore J.M., Fuglsang A.T., Palmgren M.G., Staskawicz B.J., Coaker G. (2009). RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol. 7: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wang J., Han Z., Gong X., Zhang H., Chai J. (2016). Molecular mechanism for fungal cell wall recognition by rice chitin receptor OsCEBiP. Structure 24: 1192–1200. [DOI] [PubMed] [Google Scholar]
- Liu T., Liu Z., Song C., Hu Y., Han Z., She J., Fan F., Wang J., Jin C., Chang J., Zhou J.M., Chai J. (2012). Chitin-induced dimerization activates a plant immune receptor. Science 336: 1160–1164. [DOI] [PubMed] [Google Scholar]
- Liu Y., Huang X., Li M., He P., Zhang Y. (2016). Loss-of-function of Arabidopsis receptor-like kinase BIR1 activates cell death and defense responses mediated by BAK1 and SOBIR1. New Phytol. 212: 637–645. [DOI] [PubMed] [Google Scholar]
- Liu Z., Wu Y., Yang F., Zhang Y., Chen S., Xie Q., Tian X., Zhou J.M. (2013). BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc. Natl. Acad. Sci. USA 110: 6205–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente F., Alonso-Blanco C., Sánchez-Rodriguez C., Jorda L., Molina A. (2005). ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J. 43: 165–180. [DOI] [PubMed] [Google Scholar]
- Lorek J., Griebel T., Jones A.M., Kuhn H., Panstruga R. (2013). The role of Arabidopsis heterotrimeric G-protein subunits in MLO2 function and MAMP-triggered immunity. Mol. Plant Microbe Interact. 26: 991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Durán R., Zipfel C. (2015). Trade-off between growth and immunity: role of brassinosteroids. Trends Plant Sci. 20: 12–19. [DOI] [PubMed] [Google Scholar]
- Lozano-Durán R., Macho A.P., Boutrot F., Segonzac C., Somssich I.E., Zipfel C. (2013). The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. eLife 2: e00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Lin W., Gao X., Wu S., Cheng C., Avila J., Heese A., Devarenne T.P., He P., Shan L. (2011). Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332: 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Wu S., Gao X., Zhang Y., Shan L., He P. (2010). A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA 107: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.W., Chen S., Wang J., Yu H., Chronis D., Mitchum M.G., Wang X. (2009). Structural and functional diversity of CLAVATA3/ESR (CLE)-like genes from the potato cyst nematode Globodera rostochiensis. Mol. Plant Microbe Interact. 22: 1128–1142. [DOI] [PubMed] [Google Scholar]
- Luderer R., Takken F.L., de Wit P.J., Joosten M.H. (2002). Cladosporium fulvum overcomes Cf-2-mediated resistance by producing truncated AVR2 elicitor proteins. Mol. Microbiol. 45: 875–884. [DOI] [PubMed] [Google Scholar]
- Ma X., Xu G., He P., Shan L. (2016). SERKing coreceptors for receptors. Trends Plant Sci. 21: 1017–1033. [DOI] [PubMed] [Google Scholar]
- Macho A.P., Zipfel C. (2015). Targeting of plant pattern recognition receptor-triggered immunity by bacterial type-III secretion system effectors. Curr. Opin. Microbiol. 23: 14–22. [DOI] [PubMed] [Google Scholar]
- Macho A.P., Zipfel C. (2014). Plant PRRs and the activation of innate immune signaling. Mol. Cell 54: 263–272. [DOI] [PubMed] [Google Scholar]
- Macho A.P., Boutrot F., Rathjen J.P., Zipfel C. (2012). Aspartate oxidase plays an important role in Arabidopsis stomatal immunity. Plant Physiol. 159: 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A.P., et al. (2014). A bacterial tyrosine phosphatase inhibits plant pattern recognition receptor activation. Science 343: 1509–1512. [DOI] [PubMed] [Google Scholar]
- Malinovsky F.G., Batoux M., Schwessinger B., Youn J.H., Stransfeld L., Win J., Kim S.K., Zipfel C. (2014). Antagonistic regulation of growth and immunity by the Arabidopsis basic helix-loop-helix transcription factor homolog of brassinosteroid enhanced expression2 interacting with increased leaf inclination1 binding bHLH1. Plant Physiol. 164: 1443–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinsky J., Opekarová M., Grossmann G., Tanner W. (2013). Membrane microdomains, rafts, and detergent-resistant membranes in plants and fungi. Annu. Rev. Plant Biol. 64: 501–529. [DOI] [PubMed] [Google Scholar]
- Mantelin S., Peng H.C., Li B., Atamian H.S., Takken F.L., Kaloshian I. (2011). The receptor-like kinase SlSERK1 is required for Mi-1-mediated resistance to potato aphids in tomato. Plant J. 67: 459–471. [DOI] [PubMed] [Google Scholar]
- Maruta N., Trusov Y., Brenya E., Parekh U., Botella J.R. (2015). Membrane-localized extra-large G proteins and Gbg of the heterotrimeric G proteins form functional complexes engaged in plant immunity in Arabidopsis. Plant Physiol. 167: 1004–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masachis S., Segorbe D., Turrà D., Leon-Ruiz M., Fürst U., El Ghalid M., Leonard G., López-Berges M.S., Richards T.A., Felix G., Di Pietro A. (2016). A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 1: 16043. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y., Sakagami Y. (2006). Peptide hormones in plants. Annu. Rev. Plant Biol. 57: 649–674. [DOI] [PubMed] [Google Scholar]
- Mbengue M., Bourdais G., Gervasi F., Beck M., Zhou J., Spallek T., Bartels S., Boller T., Ueda T., Kuhn H., Robatzek S. (2016). Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc. Natl. Acad. Sci. USA 113: 11034–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Zhang S. (2013). MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51: 12.1–12.22. [DOI] [PubMed] [Google Scholar]
- Mersmann S., Bourdais G., Rietz S., Robatzek S. (2010). Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 154: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina T.E., Zeier J. (2007). Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 50: 500–513. [DOI] [PubMed] [Google Scholar]
- Mithoe S.C., Ludwig C., Pel M.J., Cucinotta M., Casartelli A., Mbengue M., Sklenar J., Derbyshire P., Robatzek S., Pieterse C.M., Aebersold R., Menke F.L. (2016). Attenuation of pattern recognition receptor signaling is mediated by a MAP kinase kinase kinase. EMBO Rep. 17: 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H., Betsuyaku S., Iwamoto K., Kinoshita A., Fukuda H., Sawa S. (2008). The receptor-like kinase SOL2 mediates CLE signaling in Arabidopsis. Plant Cell Physiol. 49: 1752–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya A., Albert P., Shinya T., Desaki Y., Ichimura K., Shirasu K., Narusaka Y., Kawakami N., Kaku H., Shibuya N. (2007). CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 104: 19613–19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan J., Matschi S., Shorinola O., Rovenich H., Matei A., Segonzac C., Malinovsky F.G., Rathjen J.P., MacLean D., Romeis T., Zipfel C. (2014). The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 16: 605–615. [DOI] [PubMed] [Google Scholar]
- Mosher S., Seybold H., Rodriguez P., Stahl M., Davies K.A., Dayaratne S., Morillo S.A., Wierzba M., Favery B., Keller H., Tax F.E., Kemmerling B. (2013). The tyrosine-sulfated peptide receptors PSKR1 and PSY1R modify the immunity of Arabidopsis to biotrophic and necrotrophic pathogens in an antagonistic manner. Plant J. 73: 469–482. [DOI] [PubMed] [Google Scholar]
- Mott G.A., Thakur S., Smakowska E., Wang P.W., Belkhadir Y., Desveaux D., Guttman D.S. (2016). Genomic screens identify a new phytobacterial microbe-associated molecular pattern and the cognate Arabidopsis receptor-like kinase that mediates its immune elicitation. Genome Biol. 17: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Morea F.A., et al. (2016). Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc. Natl. Acad. Sci. USA 113: 11028–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.J., Peng Y., Chen X., Dardick C., Ruan D., Bart R., Canlas P.E., Ronald P.C. (2008). Rice XB15, a protein phosphatase 2C, negatively regulates cell death and XA21-mediated innate immunity. PLoS Biol. 6: e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Hamamouch N., Li C., Hussey R., Mitchum M., Baum T., Wang X., Davis E.L. (2008). Similarity and functional analyses of expressed parasitism genes in Heterodera schachtii and Heterodera glycines. J. Nematol. 40: 299–310. [Google Scholar]
- Pearce G., Moura D.S., Stratmann J., Ryan C.A. Jr (2001). RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. USA 98: 12843–12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petutschnig E.K., Jones A.M., Serazetdinova L., Lipka U., Lipka V. (2010). The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. J. Biol. Chem. 285: 28902–28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel S., Küfner I., Beuter C., Mazzotta S., Schwedt A., Borlotti A., Halter T., Kemmerling B., Nürnberger T. (2010). The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur. J. Cell Biol. 89: 169–174. [DOI] [PubMed] [Google Scholar]
- Postma J., Liebrand T.W., Bi G., Evrard A., Bye R.R., Mbengue M., Kuhn H., Joosten M.H., Robatzek S. (2016). Avr4 promotes Cf-4 receptor-like protein association with the BAK1/SERK3 receptor-like kinase to initiate receptor endocytosis and plant immunity. New Phytol. 210: 627–642. [DOI] [PubMed] [Google Scholar]
- Prince D.C., Drurey C., Zipfel C., Hogenhout S.A. (2014). The leucine-rich repeat receptor-like kinase BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 and the cytochrome P450 PHYTOALEXIN DEFICIENT3 contribute to innate immunity to aphids in Arabidopsis. Plant Physiol. 164: 2207–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt R.N., et al. (2015). The rice immune receptor XA21 recognizes a tyrosine-sulfated protein from a Gram-negative bacterium. Sci. Adv. 1: e1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky D., Yakoby N. (2003). Pathogenic fungi: leading or led by ambient pH? Mol. Plant Pathol. 4: 509–516. [DOI] [PubMed] [Google Scholar]
- Prusky D., McEvoy J.L., Leverentz B., Conway W.S. (2001). Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol. Plant Microbe Interact. 14: 1105–1113. [DOI] [PubMed] [Google Scholar]
- Qi Z., Verma R., Gehring C., Yamaguchi Y., Zhao Y., Ryan C.A., Berkowitz G.A. (2010). Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc. Natl. Acad. Sci. USA 107: 21193–21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonell K., Berrocal-Lobo M., Koh S., Wan J., Edwards H., Stacey G., Somerville S. (2005). Loss-of-function mutations in chitin responsive genes show increased susceptibility to the powdery mildew pathogen Erysiphe cichoracearum. Plant Physiol. 138: 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S., Eschen-Lippold L., Pecher P., Lee J., Scheel D. (2011). Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J. 68: 100–113. [DOI] [PubMed] [Google Scholar]
- Ranf S., Gisch N., Schäffer M., Illig T., Westphal L., Knirel Y.A., Sánchez-Carballo P.M., Zähringer U., Hückelhoven R., Lee J., Scheel D. (2015). A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 16: 426–433. [DOI] [PubMed] [Google Scholar]
- Replogle A., Wang J., Bleckmann A., Hussey R.S., Baum T.J., Sawa S., Davis E.L., Wang X., Simon R., Mitchum M.G. (2011). Nematode CLE signaling in Arabidopsis requires CLAVATA2 and CORYNE. Plant J. 65: 430–440. [DOI] [PubMed] [Google Scholar]
- Replogle A., Wang J., Paolillo V., Smeda J., Kinoshita A., Durbak A., Tax F.E., Wang X., Sawa S., Mitchum M.G. (2013). Synergistic interaction of CLAVATA1, CLAVATA2, and RECEPTOR-LIKE PROTEIN KINASE 2 in cyst nematode parasitism of Arabidopsis. Mol. Plant Microbe Interact. 26: 87–96. [DOI] [PubMed] [Google Scholar]
- Robatzek S., Chinchilla D., Boller T. (2006). Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 20: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M., Avni A. (2004). The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 16: 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney H.C., Van’t Klooster J.W., van der Hoorn R.A., Joosten M.H., Jones J.D., de Wit P.J. (2005). Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2-dependent disease resistance. Science 308: 1783–1786. [DOI] [PubMed] [Google Scholar]
- Ross A., Yamada K., Hiruma K., Yamashita-Yamada M., Lu X., Takano Y., Tsuda K., Saijo Y. (2014). The Arabidopsis PEPR pathway couples local and systemic plant immunity. EMBO J. 33: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M., Schwessinger B., Albrecht C., Chinchilla D., Jones A., Holton N., Malinovsky F.G., Tör M., de Vries S., Zipfel C. (2011). The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J., Brandt B., Wildhagen M., Hohmann U., Hothorn L.A., Butenko M.A., Hothorn M. (2016). Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur I.M., Kadota Y., Sklenar J., Holton N.J., Smakowska E., Belkhadir Y., Zipfel C., Rathjen J.P. (2016). NbCSPR underlies age-dependent immune responses to bacterial cold shock protein in Nicotiana benthamiana. Proc. Natl. Acad. Sci. USA 113: 3389–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonbeek H.J., Wang H.H., Stefanato F.L., Craze M., Bowden S., Wallington E., Zipfel C., Ridout C.J. (2015). Arabidopsis EF-Tu receptor enhances bacterial disease resistance in transgenic wheat. New Phytol. 206: 606–613. [DOI] [PubMed] [Google Scholar]
- Schulze B., Mentzel T., Jehle A.K., Mueller K., Beeler S., Boller T., Felix G., Chinchilla D. (2010). Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 285: 9444–9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., et al. (2015). Transgenic expression of the dicotyledonous pattern recognition receptor EFR in rice leads to ligand-dependent activation of defense responses. PLoS Pathog. 11: e1004809 Erratum. PLoS Pathog. 11: e1004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C., Feike D., Gimenez-Ibanez S., Hann D.R., Zipfel C., Rathjen J.P. (2011). Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol. 156: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C., Macho A.P., Sanmartín M., Ntoukakis V., Sánchez-Serrano J.J., Zipfel C. (2014). Negative control of BAK1 by protein phosphatase 2A during plant innate immunity. EMBO J. 33: 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Shen Q., Qi Y., Yan H., Nie H., Chen Y., Zhao T., Katagiri F., Tang D. (2013). BR-SIGNALING KINASE1 physically associates with FLAGELLIN SENSING2 and regulates plant innate immunity in Arabidopsis. Plant Cell 25: 1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Nakano T., Takamizawa D., Desaki Y., Ishii-Minami N., Nishizawa Y., Minami E., Okada K., Yamane H., Kaku H., Shibuya N. (2010). Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 64: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya T., et al. (2014). Selective regulation of the chitin-induced defense response by the Arabidopsis receptor-like cytoplasmic kinase PBL27. Plant J. 79: 56–66. [DOI] [PubMed] [Google Scholar]
- Shiu S.H., Bleecker A.B. (2003). Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 132: 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu S.H., Karlowski W.M., Pan R., Tzeng Y.H., Mayer K.F., Li W.H. (2004). Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16: 1220–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Kuo Y.C., Mishra S., Tsai C.H., Chien C.C., Chen C.W., Desclos-Theveniau M., Chu P.W., Schulze B., Chinchilla D., Boller T., Zimmerli L. (2012). The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 24: 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.M., Salamango D.J., Leslie M.E., Collins C.A., Heese A. (2014). Sensitivity to Flg22 is modulated by ligand-induced degradation and de novo synthesis of the endogenous flagellin-receptor FLAGELLIN-SENSING2. Plant Physiol. 164: 440–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., et al. (2016). Signature motif-guided identification of receptors for peptide hormones essential for root meristem growth. Cell Res. 26: 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyars C.L., James S.R., Nimchuk Z.L. (2016). Ready, aim, shoot: stem cell regulation of the shoot apical meristem. Curr. Opin. Plant Biol. 29: 163–168. [DOI] [PubMed] [Google Scholar]
- Sreekanta S., Bethke G., Hatsugai N., Tsuda K., Thao A., Wang L., Katagiri F., Glazebrook J. (2015). The receptor-like cytoplasmic kinase PCRK1 contributes to pattern-triggered immunity against Pseudomonas syringae in Arabidopsis thaliana. New Phytol. 207: 78–90. [DOI] [PubMed] [Google Scholar]
- Stegmann M., Monaghan J., Smakowska-Luzan E., Rovenich H., Lehner A., Holton N., Belkhadir Y., Zipfel C. (2017). The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355: 287–289. [DOI] [PubMed] [Google Scholar]
- Sun W., Dunning F.M., Pfund C., Weingarten R., Bent A.F. (2006). Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 18: 764–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Cao Y., Yang Z., Xu C., Li X., Wang S., Zhang Q. (2004). Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 37: 517–527. [DOI] [PubMed] [Google Scholar]
- Sun Y., Li L., Macho A.P., Han Z., Hu Z., Zipfel C., Zhou J.M., Chai J. (2013). Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342: 624–628. [DOI] [PubMed] [Google Scholar]
- Takken F.L., Thomas C.M., Joosten M.H., Golstein C., Westerink N., Hille J., Nijkamp H.J., De Wit P.J., Jones J.D. (1999). A second gene at the tomato Cf-4 locus confers resistance to cladosporium fulvum through recognition of a novel avirulence determinant. Plant J. 20: 279–288. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Choi J., Cao Y., Stacey G. (2014). Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant Sci. 5: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Han Z., Sun Y., Zhang H., Gong X., Chai J. (2015). Structural basis for recognition of an endogenous peptide by the plant receptor kinase PEPR1. Cell Res. 25: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Kim T.W., Oses-Prieto J.A., Sun Y., Deng Z., Zhu S., Wang R., Burlingame A.L., Wang Z.Y. (2008). BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina P., De Coninck B., Nikonorova N., De Smet I., Cammue B.P.A. (2015). The plant peptidome: an expanding repertoire of structural features and biological functions. Plant Cell 27: 2095–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.M., Jones D.A., Parniske M., Harrison K., Balint-Kurti P.J., Hatzixanthis K., Jones J.D. (1997). Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. Plant Cell 9: 2209–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K., Peiter E. (2014). Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol. 204: 873–881. [DOI] [PubMed] [Google Scholar]
- Thynne E., et al. (2016). Fungal phytopathogens encode functional homologues of plant rapid alkalinization factor (RALF) peptides. Mol. Plant Pathol. http://dx.doi.org/10.1111/mpp.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M.A., Morales J., Sánchez-Rodríguez C., Molina A., Dangl J.L. (2013). Functional interplay between Arabidopsis NADPH oxidases and heterotrimeric G protein. Mol. Plant Microbe Interact. 26: 686–694. [DOI] [PubMed] [Google Scholar]
- Van den Ackerveken G.F., Van Kan J.A., De Wit P.J. (1992). Molecular analysis of the avirulence gene avr9 of the fungal tomato pathogen Cladosporium fulvum fully supports the gene-for-gene hypothesis. Plant J. 2: 359–366. [DOI] [PubMed] [Google Scholar]
- van Esse H.P., Van’t Klooster J.W., Bolton M.D., Yadeta K.A., van Baarlen P., Boeren S., Vervoort J., de Wit P.J., Thomma B.P. (2008). The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell 20: 1948–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese P., Nakagami H., Bluhm B., Abuqamar S., Chen X., Salmeron J., Dietrich R.A., Hirt H., Mengiste T. (2006). The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 18: 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J., Tanaka K., Zhang X.C., Son G.H., Brechenmacher L., Nguyen T.H., Stacey G. (2012). LYK4, a lysin motif receptor-like kinase, is important for chitin signaling and plant innate immunity in Arabidopsis. Plant Physiol. 160: 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J., Zhang X.C., Neece D., Ramonell K.M., Clough S., Kim S.Y., Stacey M.G., Stacey G. (2008). A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., et al. (2015). The decoy substrate of a pathogen effector and a pseudokinase specify pathogen-induced modified-self recognition and immunity in plants. Cell Host Microbe 18: 285–295. [DOI] [PubMed] [Google Scholar]
- Wang G., et al. (2008). A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol. 147: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li H., Han Z., Zhang H., Wang T., Lin G., Chang J., Yang W., Chai J. (2015). Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature 525: 265–268. [DOI] [PubMed] [Google Scholar]
- Wang L., Albert M., Einig E., Fürst U., Krust D., Felix G. (2016). The pattern-recognition receptor CORE of Solanaceae detects bacterial cold-shock protein. Nat. Plants; 2: 16185. [DOI] [PubMed] [Google Scholar]
- Wang X., Allen R., Ding X., Goellner M., Maier T., de Boer J.M., Baum T.J., Hussey R.S., Davis E.L. (2001). Signal peptide-selection of cDNA cloned directly from the esophageal gland cells of the soybean cyst nematode Heterodera glycines. Mol. Plant Microbe Interact. 14: 536–544. [DOI] [PubMed] [Google Scholar]
- Wang X., Mitchum M.G., Gao B., Li C., Diab H., Baum T.J., Hussey R.S., Davis E.L. (2005). A parasitism gene from a plant-parasitic nematode with function similar to CLAVATA3/ESR (CLE) of Arabidopsis thaliana. Mol. Plant Pathol. 6: 187–191. [DOI] [PubMed] [Google Scholar]
- Westerink N., Brandwagt B.F., de Wit P.J., Joosten M.H. (2004). Cladosporium fulvum circumvents the second functional resistance gene homologue at the Cf-4 locus (Hcr9-4E ) by secretion of a stable avr4E isoform. Mol. Microbiol. 54: 533–545. [DOI] [PubMed] [Google Scholar]
- Willmann R., et al. (2011). Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc. Natl. Acad. Sci. USA 108: 19824–19829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou J.M. (2013). Receptor-like kinases in plant innate immunity. J. Integr. Plant Biol. 55: 1271–1286. [DOI] [PubMed] [Google Scholar]
- Yadeta K.A., Elmore J.M., Creer A.Y., Feng B., Franco J.Y., Rufian J.S., He P., Phinney B., Coaker G. (2017). A cysteine-rich protein kinase associates with a membrane immune complex and the cysteine residues are required for cell death. Plant Physiol. 173: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., et al. (2016b). The Arabidopsis CERK1-associated kinase PBL27 connects chitin perception to MAPK activation. EMBO J. 35: 2468–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Yamashita-Yamada M., Hirase T., Fujiwara T., Tsuda K., Hiruma K., Saijo Y. (2016a). Danger peptide receptor signaling in plants ensures basal immunity upon pathogen-induced depletion of BAK1. EMBO J. 35: 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., et al. (2013). A receptor-like cytoplasmic kinase targeted by a plant pathogen effector is directly phosphorylated by the chitin receptor and mediates rice immunity. Cell Host Microbe 13: 347–357. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Huffaker A., Bryan A.C., Tax F.E., Ryan C.A. (2010). PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 22: 508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Pearce G., Ryan C.A. (2006). The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl. Acad. Sci. USA 103: 10104–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y.H., Panzeri D., Kadota Y., Huang Y.C., Huang P.Y., Tao C.N., Roux M., Chien H.C., Chin T.C., Chu P.W., Zipfel C., Zimmerli L. (2016). The Arabidopsis malectin-like/LRR-RLK IOS1 is critical for BAK1-dependent and BAK1-independent pattern-triggered immunity. Plant Cell 28: 1701–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F., et al. (2014). OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514: 367–371. [DOI] [PubMed] [Google Scholar]
- Zeng L., Velásquez A.C., Munkvold K.R., Zhang J., Martin G.B. (2012). A tomato LysM receptor-like kinase promotes immunity and its kinase activity is inhibited by AvrPtoB. Plant J. 69: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Lin X., Han Z., Wang J., Qu L.J., Chai J. (2016). SERK family receptor-like kinases function as co-receptors with PXY for plant vascular development. Mol. Plant 9: 1406–1414. [DOI] [PubMed] [Google Scholar]
- Zhang J., et al. (2007). A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1: 175–185. [DOI] [PubMed] [Google Scholar]
- Zhang J., et al. (2010). Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7: 290–301. [DOI] [PubMed] [Google Scholar]
- Zhang L., Kars I., Essenstam B., Liebrand T.W.H., Wagemakers L., Elberse J., Tagkalaki P., Tjoitang D., van den Ackerveken G., van Kan J.A.L. (2014). Fungal endopolygalacturonases are recognized as microbe-associated molecular patterns by the Arabidopsis receptor-like protein RESPONSIVENESS TO BOTRYTIS POLYGALACTURONASES1. Plant Physiol. 164: 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Fraiture M., Kolb D., Löffelhardt B., Desaki Y., Boutrot F.F., Tör M., Zipfel C., Gust A.A., Brunner F. (2013). Arabidopsis receptor-like protein30 and receptor-like kinase suppressor of BIR1-1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell 25: 4227–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Dong W., Sun J., Feng F., Deng Y., He Z., Oldroyd G.E., Wang E. (2015). The receptor kinase CERK1 has dual functions in symbiosis and immunity signalling. Plant J. 81: 258–267. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang Y., Fang B., Gannon P., Ding P., Li X., Zhang Y. (2010). Arabidopsis snc2-1D activates receptor-like protein-mediated immunity transduced through WRKY70. Plant Cell 22: 3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Li S., Deng Z., Wang X., Chen T., Zhang J., Chen S., Ling H., Zhang A., Wang D., Zhang X. (2007). Molecular analysis of three new receptor-like kinase genes from hexaploid wheat and evidence for their participation in the wheat hypersensitive response to stripe rust fungus infection. Plant J. 52: 420–434. [DOI] [PubMed] [Google Scholar]
- Zhou X., et al. (2016). Four receptor-like cytoplasmic kinases regulate development and immunity in rice. Plant Cell Environ. 39: 1381–1392. [DOI] [PubMed] [Google Scholar]
- Zhu H., Li G.J., Ding L., Cui X., Berg H., Assmann S.M., Xia Y. (2009). Arabidopsis extra large G-protein 2 (XLG2) interacts with the Gbeta subunit of heterotrimeric G protein and functions in disease resistance. Mol. Plant 2: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C. (2014). Plant pattern-recognition receptors. Trends Immunol. 35: 345–351. [DOI] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J.D., Boller T., Felix G. (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.