Abstract
Objective
There is a pressing need to elucidate the brain–behavior interactions underlying autism spectrum disorders (ASD) given the marked rise in ASD diagnosis over the past decade. Functional magnetic resonance imaging (fMRI) has begun to address this need, but few fMRI studies have evaluated age-related changes in ASD. Therefore, we conducted a developmental analysis of activation likelihood estimation (ALE) meta-analysis to compare child versus adult ASD fMRI studies. We hypothesized that children and adolescents with ASD (<18 years old) would rely less on prefrontal cortex structures than adults (≥18 years old).
Method
PubMed and PsycInfo literature searches were conducted to identify task-dependent fMRI studies of children or adults with ASD. Then recent GingerALE software improvements were leveraged to perform direct comparisons of child (n =18) versus adult (n =24) studies.
Results
ALE meta-analyses of social tasks showed that children and adolescents with ASD versus adults had significantly greater hyperactivation in the left post-central gyrus, and greater hypoactivation in the right hippocampus and right superior temporal gyrus. ALE meta-analyses of nonsocial tasks showed that children with ASD versus adults had significantly greater hyperactivation in the right insula and left cingulate gyrus, and hypoactivation in the right middle frontal gyrus.
Conclusion
Our data suggest that the neural alterations associated with ASD are not static, occurring only in early childhood. Instead, children with ASD have altered neural activity compared to adults during both social and nonsocial tasks, especially in fronto-temporal structures. Longitudinal neuroimaging studies are required to examine these changes prospectively, as potential targets for brain-based treatments for ASD.
Keywords: autistic disorder, magnetic resonance imaging (MRI)
Autism spectrum disorders (ASD), including autistic disorder, Asperger’s disorder, and pervasive developmental disorder not otherwise specified (PDD-NOS), are among the most common and impairing psychiatric conditions affecting children and adolescents today. In fact, the Centers for Disease Control just published 2008 data estimating the prevalence of ASD as 1 in 88 children—up 78% since 2002.1 Thus, there is a pressing need to elucidate the brain–behavior interactions underlying ASD.
Functional magnetic resonance imaging (fMRI) studies have begun to use cognitive/emotional tasks to probe the neurobiology of the triad of symptoms characteristic of ASD—for example, impaired social interaction; qualitatively impaired communication; and restricted, repetitive, and stereotyped patterns of behavior.2 Brain regions implicated in the pathophysiology of ASD include the prefrontal cortex (PFC), temporal cortex, and parietal cortex.3 However, these fMRI studies have several inherent limitations. First, although ASD is the hallmark neurodevelopmental disorder, few studies have examined developmental changes by directly comparing fMRI neural alterations in children with ASD to adults with ASD. Second, fMRI studies’ power and generalizability are somewhat limited, given their reliance on small samples of well-characterized individuals because of the inherent costs. They also have considerable inter-study and inter-research group variability in the design and administration of these tasks—even when used to study the same cognitive process, such as attention. These limitations could be addressed by large, multi-site studies using identical tasks, scanners, and enrollment procedures, with staff trained to high levels of reliability in ASD assessment and neuroimaging procedures. Yet, the costs of such studies are prohibitive.
Another approach would be to conduct a meta-analysis of ASD fMRI studies. A meta-analysis can leverage the power of large numbers of participants across studies to determine the convergence of brain regions implicated in a disorder with potentially reduced susceptibility to false-positive rates than smaller studies, estimated by some to be as high as 10% to 20%.4 Activation likelihood estimation (ALE) is one of several coordinate-based meta-analysis techniques used to conduct a meta-analysis using spatial coordinates and numbers of participants from published studies to model the voxelwise convergence in activation foci—that is, how likely that region was truly implicated in that illness or process 5–7 ALE allows comparison of results across different cognitive domains and tasks.8,9 Importantly, ALE has been used previously to study ASD participants, including a study by Di Martino et al. that bifurcated fMRI studies published before 2008 into social and nonsocial tasks in accordance with two main facets of ASD, and another, more recent study by Philip et al. that subdivided ASD fMRI studies into six different task types (motor, visual processing, executive processing, auditory/language, basic social skills, and complex cognition).8,10 However, neither study directly compared child to adult studies due to software limitations that prevented such direct comparisons and the limited number of child ASD fMRI studies at the time.
Recently, both limitations have been resolved. 7,11–14 Therefore, we conducted a developmentally oriented ALE meta-analysis directly comparing child to adult studies. Our primary analyses followed the approach of Di Martino et al. approach of dichotomizing studies into social (e.g., theory of mind, face processing, language) and nonsocial tasks (e.g., executive function, reward processing). Secondary analyses aggregated all studies to harness sample size and power. Based on prior work in ASD, we hypothesized that both child and adult ASD studies would implicate fronto-temporo-parietal networks.15–21 However, based on longitudinal neuroimaging studies in typically developing control (TDC) participants, we predicted that children with ASD would demonstrate significantly less reliance on the PFC structures than adults with ASD.22,23
METHOD
As in prior ALE studies, we conducted a literature search for both child (“child”, “autism”, “Asperger”, and “fMRI”) and adult (“adult”, “autism”, “Asperger”, and “fMRI”) populations published through December 2011, limited to English language publications in humans, initially using PubMed and then repeated via PsycInfo.11,13 Studies were included if they met the following criteria: were original reports of task-dependent fMRI experiments; included both ASD and TDC groups; reported data from either children (defined as mean age plus standard deviation <18 years old) or adults (defined as mean age minus standard deviation ≥18 years old); and reported significant between-group differences in neural activation in stereotactic coordinates (Talairach or Montreal Neurological Institute [MNI]). Following the approach of Di Martino et al., our primary ALE meta-analysis dichotomized studies into social and nonsocial tasks.8
Studies were excluded if they met the following exclusion criteria: were review articles; reported non-fMRI neuroimaging data, such as functional connectivity or diffusion tensor imaging; included a mixture of children and adults; or failed to report either between-group differences or stereotactic coordinates.
GingerALE software (version 2.1) developed by the BrainMap Project was used to conduct the meta-analysis. Data not reported in Talairach space (i.e., MNI) was transformed using the icbm2Tal transformation.11,24,25 Pairwise ALE meta-analyses used random effects methodology to evaluate data from child-only or adult-only studies. Finally, we conducted subtraction contrasts directly comparing child versus adult ASD data from pairwise analyses.
Pairwise ALE meta-analyses included the following comparisons: greater activation in children with ASD versus TDC children (ASD-child > TDC-child); greater activation in TDC children versus ASD children (TDC-child > ASD-child); greater activation in adults with ASD versus TDC adults (ASD-adult > TDC-adult); and greater activation in TDC adults versus ASD adults (TDC-adult > ASD-adult). These ALE meta-analyses used random-effects methodology, with ALE values determined by the sample size of each study.
Initial pairwise ALE meta-analyses involved GingerALE software computing the ALE values for each voxel in the brain, performing a test to determine the null distribution of the ALE statistic at each voxel. The current version also empirically determined the full-width half-maximum threshold using an algorithm to model the probability distribution reflective of the “true” location of a reported activation based on the spatial uncertainty associated with each experiment.7 These p values were used to compute thresholds for the ALE map using Nichol’s False Discovery Rate algorithm to control for multiple comparisons with 5,000 p-value permutations and the same minimum cluster size of 200 mm3. 26 Finally, a cluster analysis was performed on the thresholded map, based on this minimum cluster size. Pairwise ALE analyses results were reported at p = .05 whole-brain corrected. Talairach daemon (http:Talairach.org) was used for anatomical locations for significant clusters.27
Subsequent subtraction-type contrasts were used to determine brain regions where children with ASD differed from adults with ASD. 12 This involved direct comparisons of the convergence of where children with ASD differed from TDC vs. the convergence of where adults with ASD differed from TDC. We tested the following: (a) where hyperactivation in children with ASD (relative to TDC children) was significantly greater than that of adults with ASD (relative to TDC adults) ([ASD-child > TDC-child] – [ASD-adult > TDC-adult]); (b) where hypoactivation among children with ASD (relative to TDC children) was significantly greater than hypoactivation among adults with ASD (relative to TDC adults) ([TDC-child > ASD-child] – [TDC-adult > ASD-adult]); (c) where hyperactivation in adults with ASD (relative to TDC adults) was significantly greater than hyperactivation in children with ASD (relative to TDC children) ([ASD-adult > TDC-adult] – [ASD-child > TDC-child]); and (d) where hypoactivation in adults with ASD (relative to TDC adults) was significantly greater than hypoactivation in children with ASD (relative to TDC) ([TDC-adult > ASD-adult] – [TDC-child > ASD-child]).
To address potential errors due to multiple comparisons in these developmental subtraction contrasts, we followed the approach of Eickhoff et al. with permutation of the experiments’ associations from pairwise ALE meta-analyses cluster analyses serving as a statistical tool to estimate the magnitude of the difference.12 These analyses used a threshold of p = .05 and minimum cluster size of 40 mm3 to account for interstudy variability.
RESULTS
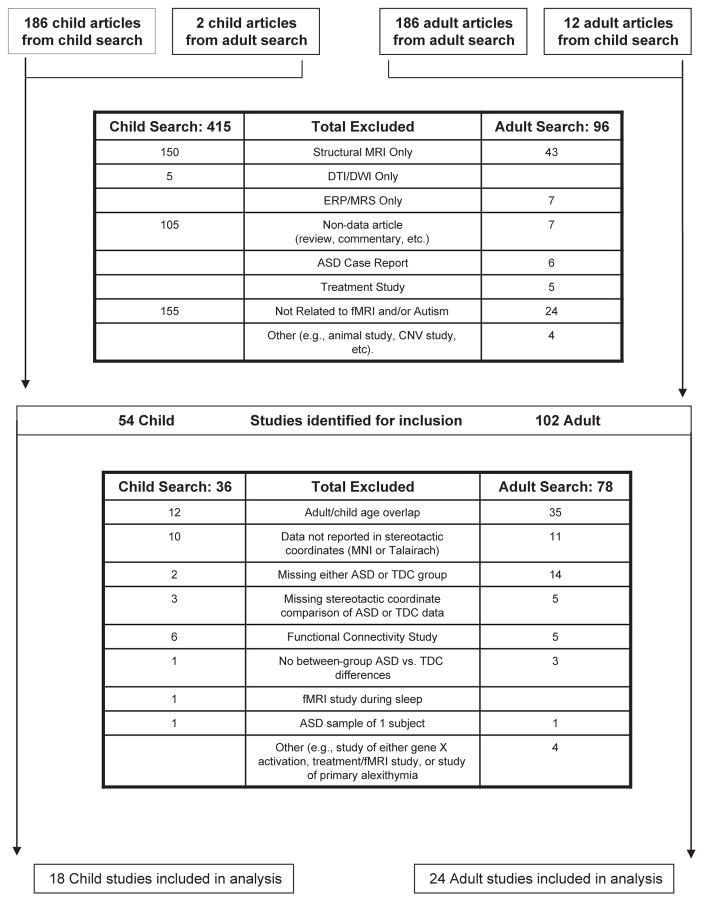
Our literature search yielded 469 child and 198 adult articles. Of these, 18 child and 24 adult articles met eligibility criteria for ALE meta-analysis, including a total of 535 child participants (262 ASD-child, 273 TDC-child) and 604 adult participants (288 ASD-adult, 316 TDC-adult). There was no main effect of mean reported intelligence quotient (IQ) across studies (F = 2.29, p = .09; ASD-child = 100.1 ± 23.8, TDC-child = 107.6 ± 24.5, ASD-adult = 109.8 ± 8.9, TDC-adult = 114.6 ± 5.8). There was no significant difference in mean reported age between ASD and TDC participants in either child or adult studies (ASD-child = 12.95 ± 1.74; TDC-child = 12.97 ± 1.81; p = .97; ASD-adult = 30.55 ± 4.94, TDC-adult = 28.77 ± 4.60; p = 0.23) (Figure 1 and see Table S1, available online).
FIGURE 1.
Flow diagram of child (left) and adult (right) literature search. Note: ASD = autism spectrum disorder; CNV = contingent negative variation; DTI = diffusion tensor imaging; DWI = diffusion weighted imaging; ERP = event-related potential; MNI = Montreal Neurological Institute; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; TDC = typically developing controls.
Social Task Meta-Analyses
ALE meta-analyses of child-only studies using social tasks (n = 11) showed significantly greater activation in ASD-child versus TDC-child studies in the left pre-central gyrus (BA 6). In contrast, we found significantly greater activation in TDC-child versus ASD-child studies in regions including the right superior temporal gyrus, para-hippocampal gyrus, and bilateral amygdala, plus the right fusiform gyrus.
ALE meta-analyses of adult-only studies using social tasks (n = 16) showed significantly greater activation in ASD-adult versus TDC-adult studies in the left superior temporal gyrus (STG; BA 41). We found significantly greater activation in TDC-adults versus ASD-adult studies in the left anterior cingulate gyrus and culmen.
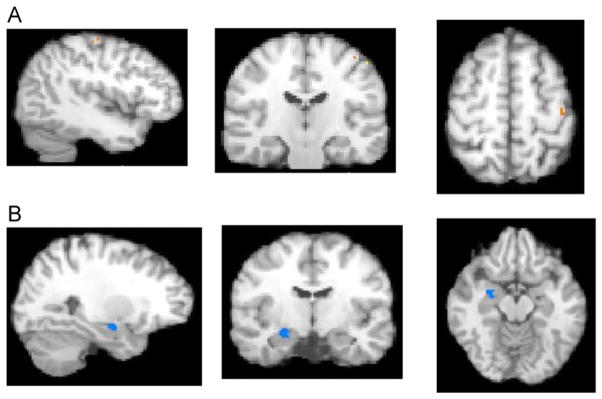
Directly comparing child-only to adult-only social findings showed that the convergence of hyperactivation in ASD children was significantly greater than the convergence of hyperactivation in ASD adults (both versus TDC) in the left post-central gyrus (including clusters in BA 3 and 2). The convergence of hypoactivation in ASD children was significantly greater than the convergence of hypoactivation in ASD adults (both versus TDC) in the right para-hippocampal gyrus/hippocampus and the right superior temporal gyrus (BA 22). There were no significant differences in the convergence of hyperactivation or hypoactivation where ASD-adults were greater than ASD-children (versus TDC) (Table 1 and Figure 2).
TABLE 1.
Activation Likelihood Estimation (ALE) Meta-Analyses Results for Social Tasks Comparing Participants With Autism-Spectrum Disorder (ASD) Versus Typically Developing Controls (TDC)
| Analysis | Side | Brain Region | BA | Talairach | Cluster Size (MM3) | Extrema Value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| ASD-child>TDC-child | L | Pre-central gyrus | 6 | −32 | −12 | 60 | 376 | 0.01 |
| R | Temporal lobe | 21 | 40 | −4 | −12 | 200 | 0.01 | |
| ASD-adult>TDC-adult | L | Superior temporal gyrus | 41 | −56 | −24 | 6 | 312 | 0.01 |
| TDC-child>ASD-child | R | Superior temporal gyrus | 22 | 50 | −14 | −4 | 928 | 0.01 |
| R | Para-hippocampal gyrus/Amygdala | 22 | −10 | −14 | 424 | 0.02 | ||
| L | Amygdala | −22 | −8 | −10 | 384 | 0.02 | ||
| R | Fusiform gyrus | 19 | 28 | −66 | −4 | 368 | 0.02 | |
| R | Superior temporal gyrus | 22 | 48 | −32 | 6 | 256 | 0.01 | |
| L | Precentral gyrus | 44 | −50 | 14 | 10 | 256 | 0.01 | |
| R | Culmen | 24 | −40 | −14 | 216 | 0.01 | ||
| TDC-adult>ASD-adult | L | Anterior cingulate | 32 | −4 | 42 | −2 | 352 | 0.01 |
| L | Culmen | −34 | −54 | −24 | 304 | 0.01 | ||
| Developmental Contrasts | ||||||||
| Greater in ASD-child vs. ASD-adult | L | Post-central gyrus | 3 | −41 | −22 | 55 | 64 | 1.80 |
| L | Post-central gyrus | 2 | −50 | −24 | 50 | 40 | 1.80 | |
| Greater in ASD-adult vs. ASD-child | NS | |||||||
| Reduced in ASD-child vs. ASD-adult | R | Para-hippocampal gyrus/hippocampus | 26 | −11 | −14 | 232 | 2.00 | |
| R | Superior temporal gyrus | 22 | 50 | −13 | 0 | 128 | 1.96 | |
| R | Superior temporal gyrus | 22 | 44 | −19 | −9 | 64 | 2.03 | |
| Reduced in ASD-adult vs. ASD-child | NS | |||||||
Note: BA = Brodmann area; L = left; NS = nonsignificant; R = right.
FIGURE 2.
Results from activation likelihood estimation (ALE) developmental social analyses. Note: (A) Greater in autism spectrum disorder (ASD)–child versus ASD-adult (X = −41, Y = −22, Z = 55; Left post-central gyrus Brodmann area [BA] 3). (B) Reduced in ASD-child versus ASD-adult (X = 26, Y = −11, Z = −14; right para/hippocampus). TDC = typically developing controls.
Nonsocial Task Meta-Analyses
ALE meta-analyses of child-only studies employing nonsocial tasks (N = 7) showed significantly greater activation in ASD-child versus TDC-child studies in the insula bilaterally (BA 13) and the right middle frontal gyrus (BA 46). In contrast, we found significantly greater activation in TDC-child versus ASD-child studies in the right caudate and superior frontal gyrus.
ALE meta-analyses of adult-only studies using nonsocial tasks (N = 8) demonstrated significantly greater activation in ASD-adult versus TDC-adult studies in the right medial frontal gyrus (BA 8) and inferior occipital gyrus (BA 19), as well as the left middle frontal gyrus (BA 11) and anterior cingulate gyrus (BA 32). There were no areas where TDC-adults had significantly greater activation than ASD-adult studies.
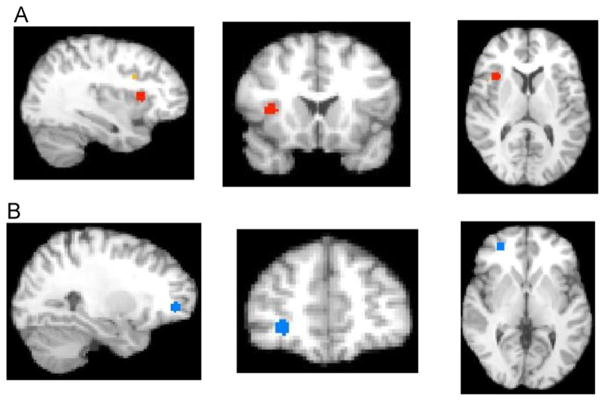
Directly comparing child-only to adult-only nonsocial task findings, we observed that the convergence of hyperactivation in ASD children was significantly greater than the convergence of hyperactivation in ASD adults (both versus TDC) in regions including the right insula (BA 13), right middle frontal gyrus (BA 9 and 46), and left cingulate gyrus (BA 24). The convergence of hypoactivation in ASD children was significantly greater than the convergence of hypoactivation in ASD adults (both versus TDC) in the right middle frontal gyrus (BA 11 and 10). There were no significant in the convergence hyperactivation or hypoactivation where ASD-adults were greater than ASD-children (versus TDC) (Table 2 and Figure 3).
TABLE 2.
Activation Likelihood Estimation (ALE) Meta-Analyses Results for Nonsocial Tasks Comparing Participants With Autism-Spectrum Disorder (ASD) Versus Typically Developing Controls (TDC)
| Analysis | Side | Brain Region | BA | Talairach
|
Cluster Size (mm3) | Extrema Value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| ASD-child>TDC-child | R | Insula | 13 | 34 | 14 | 8 | 464 | 0.02 |
| L | Insula | 13 | −36 | 14 | 4 | 376 | 0.01 | |
| R | Middle frontal gyrus | 46 | 46 | 30 | 20 | 256 | 0.01 | |
| ASD-adult>TDC-adult | R | Medial frontal gyrus | 8 | 8 | 44 | 40 | 480 | 0.02 |
| R | Inferior occipital gyrus | 19 | 38 | −74 | −8 | 360 | 0.01 | |
| L | Middle frontal gyrus | 11 | −28 | 42 | −8 | 240 | 0.01 | |
| L | Anterior cingulate | 32 | 0 | 42 | 10 | 224 | 0.01 | |
| TDC-child>ASD-child | R | Caudate | 12 | 0 | 12 | 448 | 0.02 | |
| R | Superior frontal gyrus | 26 | 44 | 0 | 416 | 0.02 | ||
| TDC-adult>ASD-adult | NS | |||||||
| Developmental Contrasts | ||||||||
| Greater in ASD-child vs. ASD-adult | R | Insula | 13 | 35 | 16 | 9 | 456 | 2.31 |
| L | Cingulate gyrus | 24 | −3 | −1 | 53 | 184 | 1.75 | |
| R | Middle frontal gyrus | 46 | 45 | 29 | 20 | 112 | 2.31 | |
| R | Insula | 13 | 44 | 10 | 10 | 64 | 2.88 | |
| R | Middle frontal gyrus | 9 | 32 | 12 | 26 | 56 | 2.31 | |
| R | Middle frontal gyrus | 46 | 50 | 21 | 21 | 48 | 2.88 | |
| L | Cingulate gyrus | 24 | −10 | −2 | 46 | 40 | 1.75 | |
| Greater in ASD-adult vs. ASD-child | NS | |||||||
| Reduced in ASD-child vs. ASD-adult | R | Middle frontal gyrus | 11 | 27 | 45 | 1 | 416 | 1.67 |
| R | Middle frontal gyrus | 10 | 30 | 48 | 12 | 56 | 1.67 | |
| Reduced in ASD-adult vs. ASD-child | NS | |||||||
Note: BA = Brodmann Area; L = left; NS = nonsignificant; R = right.
FIGURE 3.
Results from activation likelihood estimation (ALE) developmental nonsocial analyses. Note: (A) Greater in autism spectrum disorder (ASD)–child versus ASD-adult (X = 35, Y = 16, Z = 9; right insula Brodmann area [BA] 13). (B) Reduced in ASD-child versus ASD-adult (X =27, Y =45, Z =1; middle frontal gyrus BA 11). TDC =typically developing controls.
DISCUSSION
Our ALE meta-analysis directly comparing child versus adult ASD fMRI studies is an important step in understanding age-related brain activity changes associated with ASD, the hallmark neurodevelopmental disorder. In particular, using ALE meta-analytic methods to leverage data from 535 child and 604 adult participants, our study demonstrated age-related alterations in fMRI neural activation on both social and nonsocial tasks. Thus, our study is important because it suggests that fMRI neural alterations associated with ASD are not static but, rather, change as children become adults. Longitudinal neuroimaging studies are required to confirm the developmental trajectories of the neural alterations associated with ASD as children become adolescents and, ultimately, adults.
Our data align with current models supporting the role for early cerebral overgrowth in the brain–behavior interactions underlying ASD. Specifically, many have demonstrated that children with ASD have larger head circumference than their age-matched TDC peers during the first 2 years of life, with head circumference as an indirect marker for overall brain size.28–30 Furthermore, structural MRI studies have shown that children less than 3 years of age with ASD have enlarged cerebral gray matter and white matter, but not enlarged cerebellums, compared to TDC.31 These findings have been corroborated by Schumann et al.’s longitudinal structural MRI study of ASD, showing significant enlargement of cerebral gray (including frontal, temporal, parietal, and cingulate areas) and cerebral white matter, but no difference in occipital gray matter in 41 ASD and 44 TDC children serially scanned from ages 1.5 to 5 years.32 Post mortem studies by Courchesne et al. align with these findings by showing that ASD children had greater total PFC neuron count and brain weight for age than TDC children 33. In this context, our data are among the first to evaluate developmental alterations in neural functional activity occurring across the lifespan in patients with ASD by directly comparing children to adults. These data suggest that children with ASD have particularly altered functional neural activity compared to adults with ASD, given that children with ASD had significantly greater hyper- and hypoactivation than adults with ASD, whereas the converse was not true—that is, there were no regions where adults with ASD had significantly greater hyper-or hypoactivation than children with ASD—regardless of whether social or nonsocial tasks were used. How early can functional brain changes due to these structural alterations be detected? This remains unknown, although findings from our ALE meta-analyses suggest that they occur before the current minimum typical age for conducting fMRI (around age 7 years). Thus, our data suggest the field must push the lower age bounds of task-dependent fMRI, scanning younger and younger ASD and TDC children, as well as scanning them longitudinally.
Prior ALE meta-analyses of ASD neuroimaging data have implicated fronto-temporal-parietal neurocircuitry in ASD, although ours is perhaps the first to directly compare child versus adult studies. Specifically, the prior ALE meta-analysis by Di Martino et al. that aggregated 39 child and adult fMRI studies published until 2008 found that ASD participants had decreased neural activation compared to TDC participants in the right insula and peri-genual anterior cingulate cortex (ACC) for social tasks, and decreased neural activation in the dorsal ACC during non-social tasks.8 Philip et al. evaluated data from 49 studies parsed into six different task domains: motor, visual processing, executive processing, auditory/language, basic social skills, and complex cognition tasks. They found alterations in frontal-parietal circuitry, along with temporal or basal-ganglia structures, across tasks. They also conducted separate child and adult analyses for auditory/language, basic social, and complex social cognition tasks, but were unable to directly compare these groups via subtraction contrast as in our present study.10 We included fewer studies than either Di Martino et al. or Philip et al., because we wanted to minimize overlap between child and adult studies. Two prior ALE meta-analyses have evaluated structural MRI data, with Cauda et al. reporting increased gray matter in regions including the fusiform gyrus in ASD participants versus TDC, although this study also was unable to evaluate potential developmental effects.34 Nickl-Jockschat et al. used a slightly different approach to conduct a structural MRI ALE meta-analysis, visually exploring the relationship between age and areas of significant volume change from modeled anatomical effects maps for 16 included studies using SPM software.35 Thus, our data address an important need to begin to look at developmental differences in functional neural activity in children and adults with ASD.
Regarding our social task ALE meta-analysis, we note that children with ASD have less activation than adults with ASD in the para-hippocampal gyrus and hippocampus, along with STG. Alteredhippocampal neural function and structure may have some specificity to ASD children, as one study has shown that ASD children had significantly less activation in the hippocampal gyrus than either children with attention-deficit/hyperactivity disorder (ADHD) or TDC participants during a visual attention task.36 Another found corroborated hippocampal alterations associated with ASD, in this case using structural MRI to demonstrate that children with autism—both with and without mental retardation—had larger right hippocampal volume than TDC children, even after controlling for total cerebral volume.37 Still others suggest that these hippocampal abnormalities may play a role in visual abnormalities associated with ASD, including reduced eye contact and avoidance of the emotionally communicative face areas (eyes, mouth).38,39 For example, participants with ASD have aberrant hippocampal–fusiform pathway white matter neural connectivity, as measured by diffusion-tensor tracking,40 and also have significant left versus right hippocampal asymmetry linked to the laterality of visual perception.41 Moreover, Monk et al. demonstrated that participants with ASD had abnormal resting-state task-independent fMRI neural connectivity between the right posterior cingulate and right para-hippocampal gyrus that was, in turn, associated with repetitive, restricted behaviors.21 Thus, our work suggests the need to identify treatments, both medication and psychotherapy, that might engage this circuit as a potential brain-based treatment for social skills deficits in ASD.
Regarding nonsocial tasks, our data indicated that children with ASD have significantly greater hyperactivation in the right insula and middle frontal gyrus (BA 46) and left cingulate gyrus compared to adults with ASD. During nonsocial tasks, the prior ALE meta-analysis by Di Martino et al. demonstrated hypoactivation in the rostral ACC among ASD versus TDC participants, but they were not able to examine child versus adult differences.8 Several studies have shown the insula’s involvement in ASD, including another study by Di Martino et al. showing that greater anticorrelation in resting state fMRI data between the insula and ACC was associated with greater ASD traits as rated on the Social Responsiveness Scale among TDC adults.42 With respect to the middle frontal gyrus, many PFC regions in combination with both temporal and parietal cortex have been implicated in the pathophysiology of ASD.20,43 Such neuroimaging findings have been linked to physical examination findings, such as increased head circumference and post mortem neuropathology, to suggest that early brain overgrowth may play a role in the pathophysiology of ASD.28,30,31,33,44–46 Our data from nonsocial, social, and the aggregate of these tasks seems to align with that body of work.
Albeit highly speculative, our study has several potential clinical implications. First, using ALE meta-analytic methods to aggregate data from 535 child and 604 adult participants, we have improved power and generalizability, to identify candidate brain regions that may differ between children and adults with ASD. Researchers can build on this work, using their particular cognitive or emotional tasks to probe how ASD affects the underlying neurocircuitry and how it changes as patients with ASD age. Such information could, in turn, lead to novel brain-based treatments for ASD, such as the development of computer-assisted cognitive remediation for social skills deficits in ASD that might engage the fusiform gyrus and related networks and improve functioning in ASD youths. Similar approaches—that is, using computer-assisted training to build cognitive or emotional skills shown to be impaired in a particular psychiatric illness—have shown promise in several conditions, including depression and anxiety disorders.47,48 In addition, pharmacoimaging studies, that pair neuroimaging with medication treatment trials, may use these candidate brain regions useful either in identifying novel treatments or in measuring the neural effects of current treatments for ASD. Future studies are also needed to determine whether these alterations are specific to ASD compared to those with other forms of psychopathology, such as disorders whereby irritability may be an associated symptom—that is, ADHD or bipolar disorder.
Our study has several potential limitations inherent in coordinate-dependent meta-analysis, including the possible role of publication bias, as we could include only published studies. Also, using other search terms might have yielded additional studies. To address this concern, we corroborated our initial PubMed search with a separate PsycInfo search, yielding only two additional child and two additional adult studies. Also, we tried to minimize sample overlap and to avoid double-counting manuscripts from the same research group by checking that they either reported data from different paradigms or different participants (e.g., different sample size or demographic variables such as age or IQ). We also excluded studies using neuroimaging methods other than task-based fMRI, such as structural MRI, diffusion tensor imaging, or neural connectivity. The rationale for this was that ALE methods enable meta-analyses within a particular type of imaging, but their validity in cross-platform approaches remains unknown at the present. We also excluded some studies that did not report their data in standardized space coordinates, a requirement for ALE and other CDMA techniques such as multi-level kernel density mapping analysis (MKDA) and signed differential mapping (SDM) 9. With respect to these alternative methodologies, we selected ALE because it allowed us to build on prior ASD studies and to leverage new subtraction contrasts to examine the role of development in fMRI activity. Future studies might use MKDA or SDM, or use mega-analyses—whereby raw neuroimaging data are pooled across studies and sites and reanalyzed—as an alternative meta-analytic approach to examine functional neural activation across studies.49 Such a mega-analysis might benefit from our present ALE study because our study will inform what key brain regions to focus on, thus reducing susceptibility to multiple comparisons problems. Finally, our study uses cross-sectional data, comparing child and adult studies to evaluate potential developmental effects in task-related brain activity in ASD, requiring future corroboration from longitudinal fMRI studies.
We also note that some findings turned out to be significant in the developmental contrasts that were not significantly different in pairwise comparisons—that is, post-central gyrus findings in social task meta-analysis. It is possible that this is a true finding—highlighting a strength of ALE methods to model the foci from published studies as probability distributions the width of which is based on empirical estimates of the spatial uncertainty because of the between-subject and between-template variability of neuroimaging data, and then to determine the convergence of foci reported from different experiments. Moreover, recent improvements now address potential for false-positive results and multiple-comparisons problems by new approaches for correcting the familywise error rate and the cluster-level significance.13 It is also possible that this post-central gyral finding is a type I error. Therefore, we have chosen to focus our discussion on less ambiguous results.
Finally, although some studies note high rates of intellectual disability in children with ASD, we note that the constituent child and adult studies from our meta-analyses included participants whose IQ was average. For example, Charman et al. evaluated an epidemiological sample of 75 children with ASD and found that 55% had an intellectual disability (i.e., IQ < 70), but only 16% had a moderate to severe intellectual disability (i.e., IQ < 50).50 This may be an inherent issue in fMRI studies that require the capacity to understand the instructions related to a cognitive task in an MRI scanner, and to execute the instructions, despite noise from radiofrequency pulses, relatively small spaces, and stimulus–response devices. It is possible that task-independent resting state fMRI studies may be able to partially address this issue, as no task is required although participants must still understand detailed instructions and remain still in the MRI scanner.
In sum, our ALE meta-analysis of task-dependent fMRI data from 535 child and 604 adult participants supports the position that functional neural alterations associated with ASD are not static but, rather, change as children become adults. Building on this work, future longitudinal neuroimaging studies are required to prospectively examine these changes, ultimately leading to a more brain-based approach to the diagnosis and treatment of ASD.
Supplementary Material
FIGURE S1 Results from activation likelihood estimation (ALE) developmental analyses (social and nonsocial tasks merged). Note: (A) Greater in autism spectrum disorder [ASD]–child versus ASD-adult (X = 38, Y = 20, Z = 14; right insula Brodmann area [BA] 13). (B) Reduced in ASD-child versus ASD-adult (X =12, Y =3, Z =12; right caudate). TDC =typically developing controls.
FIGURE S2 Pairwise results from activation likelihood estimation (ALE) developmental analyses (social and nonsocial tasks merged). Note: (A) Autism spectrum disorder (ASD)–child > typically developing controls (TDC)–child (X = 34, Y = 16, Z = 8; right insula Brodmann area [BA] 13). (B) ASD-adult > TDC-adult (X = 22, Y = −4, Z = −12; right para-hippocampal gyrus and amygdala). (C) TDC-child > ASD-child (X = 50, Y = −14, Z = −4; right superior temporal gyrus BA 22). (D) TDC-adult > ASD-adult (X = −10, Y = −46, Z = 0; left culmen).
FIGURE S3 Pairwise results from activation likelihood estimation (ALE) social analyses. Note: (A) Autism spectrum disorder (ASD)–child > typically developing controls (TDC)–child (X = −32, Y = −12, Z = 60; left pre-central gyrus Brodmann area [BA] 6). (B) ASD-adult > TDC-adult (X = −56, Y = −24, Z = 6; left superior temporal gyrus BA 41). (C) TDC-child > ASD-child (X = 50, Y = −14, Z = −4; right superior temporal gyrus BA 22).( D) TDC-adult > ASD-adult (X= −4,Y = 42, Z = −2; left anterior cingulate BA 32).
FIGURE S4 Pairwise results from activation likelihood estimation (ALE) nonsocial analyses. Note: (A) Autism spectrum disorder (ASD)–child > typically developing controls (TDC)–child (X = 34, Y = 14, Z = 8; right insula Brodmann area [BA] 13). (B) ASD-adult > TDC-adult (X = 8, Y = 44, Z = 40; right medial frontal gyrus BA 8). (C) TDC-child > ASD-child (X = 12, Y = 0, Z = 12; right caudate).
TABLE S1 Functional Magnetic Resonance Imaging (fMRI) Articles Included in Meta-Analysis
TABLE S2 Activation Likelihood Estimation (ALE) Meta-Analyses Results for Social and Nonsocial Tasks Merged in Participants With Autism Spectrum Disorder (ASD) Versus Typically Developing Controls (TDC)
Acknowledgments
This work and the Pediatric Mood, Imaging, and Neurodevelopment Program (PediMIND) were partially supported by Bradley Hospital.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Drs. Dickstein, Kim, and Seymour, and Mr. Pescosolido, Ms. Reidy, and Ms. Galvan have received support from the National Institute of Mental Health (NIMH) as part of their work for the PediMIND Program. Dr. Laird and the BrainMap Initiative, and Dr. Di Martino have received support from NIMH. Dr. Barrett has received support from Bradley Hospital.
Contributor Information
Dr. Daniel P. Dickstein, Bradley Hospital’s PediMIND Program and the Alpert Medical School of Brown University
Mr. Matthew F. Pescosolido, Bradley Hospital’s PediMIND Program and the Alpert Medical School of Brown University
Ms. Brooke L. Reidy, Bradley Hospital’s PediMIND Program and the Alpert Medical School of Brown University
Ms. Thania Galvan, Bradley Hospital’s PediMIND Program and the Alpert Medical School of Brown University
Dr. Kerri L. Kim, Bradley Hospital’s PediMIND Program and the Alpert Medical School of Brown University
Dr. Karen E. Seymour, Bradley Hospital’s PediMIND Program and the Alpert Medical School of Brown University
Dr. Angela R. Laird, Florida International University
Dr. Adriana Di Martino, New York University Child Study Center
Dr. Rowland P. Barrett, Bradley Hospital’s Center for Autism and Developmental Disabilities and the Alpert Medical School of Brown University
References
- 1.Prevalence of Autism Spectrum Disorders—Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 3.Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Curr Opin Neurol. 2010;23:124–130. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 6.Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage. 2009;45:S210–S221. doi: 10.1016/j.neuroimage.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philip RC, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev. 2012;36:901–942. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Laird AR, Eickhoff SB, Fox PM, et al. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eickhoff SB, Bzdok D, Laird AR, et al. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effectsin activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- 16.Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monk CS, Peltier SJ, Wiggins JL, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giedd JN, Lenroot RK, Shaw P, et al. Trajectories of anatomic brain development as a phenotype. Novartis Found Symp. 2008;289:101–112. doi: 10.1002/9780470751251.ch9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laird AR, Robinson JL, McMillan KM, et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 29.Dawson G, Munson J, Webb SJ, Nalty T, Abbott R, Toth K. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry. 2007;61:458–464. doi: 10.1016/j.biopsych.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb SJ, Nalty T, Munson J, Brock C, Abbott R, Dawson G. Rate of head circumference growth as a function of autism diagnosis and history of autistic regression. J Child Neurol. 2007;22:1182–1190. doi: 10.1177/0883073807306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazlett HC, Poe M, Gerig G, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 32.Schumann CM, Bloss CS, Barnes CC, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Courchesne E, Mouton PR, Calhoun ME, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 34.Cauda F, Geda E, Sacco K, et al. Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J Neurol Neurosurg Psychiatry. 2011;82:1304–1313. doi: 10.1136/jnnp.2010.239111. [DOI] [PubMed] [Google Scholar]
- 35.Nickl-Jockschat T, Habel U, Michel TM, et al. Brain structure anomalies in autism spectrum disorder–a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp. 2012;33:1470–1489. doi: 10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malisza KL, Clancy C, Shiloff D, et al. Functional magnetic resonance imaging of facial information processing in children with autistic disorder, attention deficit hyperactivity disorder and typically developing controls. Int J Adolesc Med Health. 2011;23:269–277. doi: 10.1515/ijamh.2011.055. [DOI] [PubMed] [Google Scholar]
- 37.Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klin A, Jones W. Attributing social and physical meaning to ambiguous visual displays in individuals with higher-functioning autism spectrum disorders. Brain Cogn. 2006;61:40–53. doi: 10.1016/j.bandc.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Bookheimer SY, Wang AT, Scott A, Sigman M, Dapretto M. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J Int Neuropsychol Soc. 2008;14:922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ. Neuronal fiber pathway abnormalities in autism: an initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J Int Neuropsychol Soc. 2008;14:933–946. doi: 10.1017/S1355617708081381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maltbie E, Bhatt K, Paniagua B, et al. Asymmetric bias in user guided segmentations of brain structures. Neuroimage. 2012;59:1315–1323. doi: 10.1016/j.neuroimage.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Martino A, Shehzad Z, Kelly C, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Via E, Radua J, Cardoner N, Happe F, Mataix-Cols D. Meta-analysis of gray matter abnormalities in autism spectrum disorder: should Asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder? Arch Gen Psychiatry. 2011;68:409–418. doi: 10.1001/archgenpsychiatry.2011.27. [DOI] [PubMed] [Google Scholar]
- 44.Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: a magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maslowsky J, Mogg K, Bradley BP, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2010;20:105–111. doi: 10.1089/cap.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costafreda SG. Pooling FMRI data: meta-analysis, mega-analysis and multi-center studies. Front Neuroinform. 2009;3:33. doi: 10.3389/neuro.11.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP) Psychol Med. 2011;41:619–627. doi: 10.1017/S0033291710000991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Results from activation likelihood estimation (ALE) developmental analyses (social and nonsocial tasks merged). Note: (A) Greater in autism spectrum disorder [ASD]–child versus ASD-adult (X = 38, Y = 20, Z = 14; right insula Brodmann area [BA] 13). (B) Reduced in ASD-child versus ASD-adult (X =12, Y =3, Z =12; right caudate). TDC =typically developing controls.
FIGURE S2 Pairwise results from activation likelihood estimation (ALE) developmental analyses (social and nonsocial tasks merged). Note: (A) Autism spectrum disorder (ASD)–child > typically developing controls (TDC)–child (X = 34, Y = 16, Z = 8; right insula Brodmann area [BA] 13). (B) ASD-adult > TDC-adult (X = 22, Y = −4, Z = −12; right para-hippocampal gyrus and amygdala). (C) TDC-child > ASD-child (X = 50, Y = −14, Z = −4; right superior temporal gyrus BA 22). (D) TDC-adult > ASD-adult (X = −10, Y = −46, Z = 0; left culmen).
FIGURE S3 Pairwise results from activation likelihood estimation (ALE) social analyses. Note: (A) Autism spectrum disorder (ASD)–child > typically developing controls (TDC)–child (X = −32, Y = −12, Z = 60; left pre-central gyrus Brodmann area [BA] 6). (B) ASD-adult > TDC-adult (X = −56, Y = −24, Z = 6; left superior temporal gyrus BA 41). (C) TDC-child > ASD-child (X = 50, Y = −14, Z = −4; right superior temporal gyrus BA 22).( D) TDC-adult > ASD-adult (X= −4,Y = 42, Z = −2; left anterior cingulate BA 32).
FIGURE S4 Pairwise results from activation likelihood estimation (ALE) nonsocial analyses. Note: (A) Autism spectrum disorder (ASD)–child > typically developing controls (TDC)–child (X = 34, Y = 14, Z = 8; right insula Brodmann area [BA] 13). (B) ASD-adult > TDC-adult (X = 8, Y = 44, Z = 40; right medial frontal gyrus BA 8). (C) TDC-child > ASD-child (X = 12, Y = 0, Z = 12; right caudate).
TABLE S1 Functional Magnetic Resonance Imaging (fMRI) Articles Included in Meta-Analysis
TABLE S2 Activation Likelihood Estimation (ALE) Meta-Analyses Results for Social and Nonsocial Tasks Merged in Participants With Autism Spectrum Disorder (ASD) Versus Typically Developing Controls (TDC)