Abstract
Abstract
Cardiovascular diseases are major contributors to global deaths and disability‐adjusted life years, with hypertension a significant risk factor for all causes of death. The endothelium that lines the inner wall of the vasculature regulates essential haemostatic functions, such as vascular tone, circulation of blood cells, inflammation and platelet activity. Endothelial dysfunction is an early predictor of atherosclerosis and future cardiovascular events. We review the prognostic value of obtaining measurements of endothelial function, the clinical techniques for its determination, the mechanisms leading to endothelial dysfunction and the therapeutic treatment of endothelial dysfunction. Since vascular oxidative stress and inflammation are major determinants of endothelial function, we have also addressed current antioxidant and anti‐inflammatory therapies. In the light of recent data that dispute the prognostic value of endothelial function in healthy human cohorts, we also discuss alternative diagnostic parameters such as vascular stiffness index and intima/media thickness ratio. We also suggest that assessing vascular function, including that of smooth muscle and even perivascular adipose tissue, may be an appropriate parameter for clinical investigations.
Linked Articles
This article is part of a themed section on Redox Biology and Oxidative Stress in Health and Disease. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.12/issuetoc
Abbreviations
- ACE
angiotensin‐converting enzyme
- ADMA
asymmetric dimethylarginine
- AGEs
advanced glycation end products
- AOE
antioxidant enzyme
- BH4
tetrahydrobiopterin
- CRP
C‐reactive protein
- eNOS
endothelial nitric oxide synthase
- EPCs
endothelial progenitor cells
- ET‐1
endothelin‐1
- FMD
flow‐mediated dilation
- GCH‐1
GTP cyclohydrolase‐1
- HDAC
histone deacetylase
- HDL
high density lipoprotein
- ICAM
intercellular cell adhesion molecule
- miRNA
microRNA
- Nox
NADPH oxidase catalytic subunit (isoforms 1, 2 and 4)
- PECAM
platelet endothelial cell adhesion molecule
- PEG
polyethylene glycol
- PGIS
prostacyclin synthase
- PVAT
perivascular adipose tissue
- PWV
pulse wave velocity
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species (in the present review mostly represents superoxide and hydrogen peroxide)
- sGC
soluble guanylyl cyclase
- SOD
superoxide dismutase
Tables of Links
| TARGETS | ||
|---|---|---|
| GPCRs a | Angiotensin‐converting enzyme | Serine/threonine kinase 11 |
| AT1 receptor | Arginase | Sirtuin 1 |
| Bradykinin receptors | DNA (cytosine‐5‐)‐methyltransferase 1 | Soluble guanylyl cyclase |
| Catalytic receptors b | Endothelial nitric oxide synthase | Other protein targets d |
| Type IV RTKs | PDE5A | Advanced glycosylation end product‐specific receptor |
| Enzymes c | Phosphodiesterases | |
| AMPK subfamily | Prostacyclin synthase |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,dAlexander et al., 2015a, 2015b, 2015c, 2015d).
Introduction
Risk factors, disease burden and life span
The contribution of different risk factors to the global disease burden and their impact on life expectancy has shifted over the last 20 years from risks for communicable childhood diseases towards those for non‐communicable adulthood diseases that are more frequently observed in the elderly (Lim et al., 2012; Murray et al., 2012). This shift is largely due to demographic changes, improved clinical prevention of childhood mortality, reductions in several preventable causes of death and a lower exposure to some risk factors. Socio‐economic and scientific progress have led to improvements in water quality and sanitation, significant reductions in vitamin A and zinc deficiencies, and to lower exposures to particulate matter in households and the environment. There are large regional differences in the extent of these epidemiological and socio‐economic changes and their impact on the importance of different risk factors, disease burden and mortality (e.g. poverty and childhood diseases continue to be the highest risk factors in sub‐Saharan Africa).
A comparison of estimated deaths and disability‐adjusted life years (= sum of years lived with a disability) between 1990 and 2010 was based on a new calculation model using 67 risk factors for 21 regions (Lim et al., 2012; Murray et al., 2012). In 2010, the global disease burden was largely affected by three leading risk factors, with high blood pressure accounting for 7.0%, tobacco (active and passive) smoking being responsible for 6.3%, and exposure to particulate matter in households accounting for 4.3% of disability‐adjusted life years. In 1990, the primary risk factors for the global disease burden were being underweight during childhood (7.9%), followed by household air pollution from solid fuels (6.8%), and tobacco smoking (6.1%). In 2010, malnutrition (mostly related to diets low in fresh fruits and vegetables or high in sodium) and physical inactivity collectively accounted for 10.0% of global disability‐adjusted life years. As shown in Figure 1, the leading risk factors and diseases accounting for global deaths and disability‐adjusted life years are hypertension (approx. 18 and 7%), ischaemic heart disease (approx. 14 and 5%), tobacco smoking (approx. 12 and 6%) and cerebrovascular disease (approx. 11 and 4%). It is important to note that these are all cardiovascular risk factors and diseases (tobacco smoking, independent of its carcinogenic effects, is a well‐accepted cardiovascular risk factor), thus stressing their significance as the leading modifiable causes of death, particularly in Western societies. The impact of traditional risk factors on the extent and severity of coronary atherosclerosis was identified more than 15 years ago (Wilson et al., 1999). The striking importance of hypertension as a risk factor for cardiovascular and all‐cause mortality was recently demonstrated by the results of the SPRINT trial (Group et al., 2015). These results show that aggressively lowering blood pressure to below 120 mmHg in patients with a high risk of cardiovascular events significantly decreased the incidence of major cardiovascular events and death from any cause despite significantly higher rates of other adverse side effects.
Figure 1.
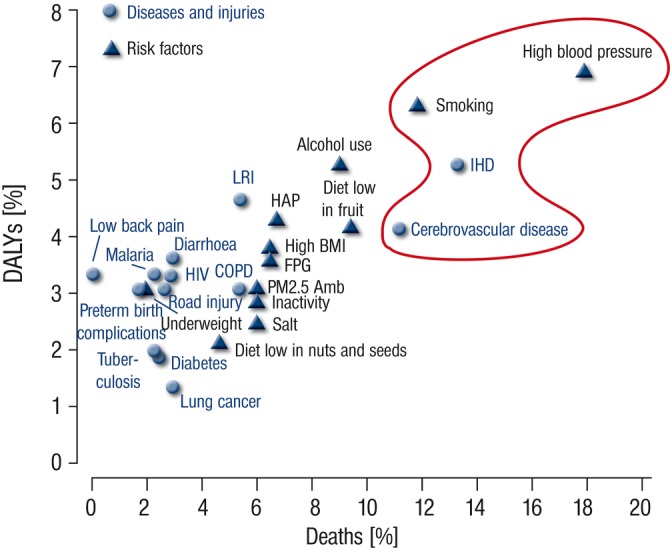
Contribution of the major diseases/injuries and risk factors to global deaths and global disability‐adjusted life years in the year 2010. The top 25 causes of diseases, injuries, and risk factors with respect to their contribution to disability‐adjusted life years and deaths are shown. DALYs = disability‐adjusted life years. IHD = ischaemic heart disease. LRI = lower respiratory infections. COPD = chronic obstructive pulmonary disease. HAP = household air pollution from solid fuels. BMI = body‐mass index. FPG = fasting plasma glucose. PM2.5 Amb = ambient particulate matter pollution. *Tobacco smoking, including second‐hand smoke. †Physical inactivity and low physical activity. Adopted from (Murray et al., 2012). With permission of Elsevier. Copyright © 2012 Elsevier Ltd. All rights reserved.
Cardiovascular risk and endothelial function
Based on the aforementioned data on a major role of cardiovascular disease and risk factors for global deaths and disability‐adjusted life years, physicians and pharmacologists have for several decades searched for a reliable, early predictor of cardiovascular mortality. One of the most promising candidates is the measurement of endothelial function (encompassing production of the different endothelium‐derived messengers that help to control vascular tone, blood flow, immune cell and platelet activity/adhesion, thereby regulating perfusion and/or blood pressure), which also correlates with classical markers of inflammation, obesity and cardiovascular risk such as C‐reactive protein (CRP), adiponectin and brain natriuretic peptide (BNP) (Gonzalez and Selwyn, 2003; Okui et al., 2008; Pauriah et al., 2012). Classic cardiovascular risk factors such as arterial hypertension (Panza et al., 1990), hypercholesterolaemia (Vita et al., 1990), diabetes mellitus (Calver et al., 1992) and chronic smoking (Celermajer et al., 1993) are all associated with endothelial dysfunction. The presence of several risk factors produces synergistic effects on endothelial function as well as the associated cardiovascular prognosis (Munzel et al., 2008). Previous studies confirmed that hypercholesterolaemia or chronic smoking lead to a moderate impairment of endothelial function (reduction in the maximal acetylcholine‐dependent vasodilatation by ~ 30%), whereas the presence of both risk factors caused severe endothelial dysfunction (reduction in the maximal acetylcholine‐dependent vasodilatation by ~ 60%) (Heitzer et al., 1996b).
Endothelial dysfunction is the first clinical correlate of atherosclerosis identified (Panza et al., 1990; Vita et al., 1990). Since most cardiovascular diseases are either related to or are a direct consequence of atherosclerosis, endothelial dysfunction is an early predictor of subsequent cardiovascular events or mortality (Gokce et al., 2002; Gokce et al., 2003) (Figure 2). Patients with peripheral arterial occlusive disease (Anderson et al., 1995; Boger et al., 1998), coronary artery disease (Schachinger et al., 2000; Suwaidi et al., 2000; Heitzer et al., 2001) or heart failure (Heitzer et al., 2005) demonstrate impaired endothelial function. Endothelial dysfunction is also clearly associated with oxidative stress (Heitzer et al., 2001), which is another feature in the development of atherosclerosis (Miller et al., 1998; Harrison et al., 2003; Laufs et al., 2005). Levels of oxidized thiols were also negatively correlated with cumulative survival in a large scale clinical trial of patients undergoing coronary angiography, thus providing strong evidence for a role of oxidative stress in cardiovascular disease‐associated mortality (Patel et al., 2016).
Figure 2.
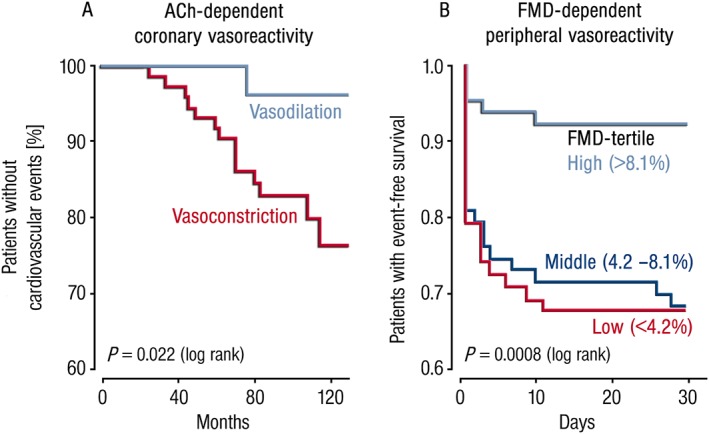
Endothelial function as a prognostic parameter for cardiovascular events and mortality. (A) Kaplan–Meier‐curves for the correlation between acetylcholine (ACh)‐induced coronary vasoreactivity and prognosis of patients with coronary heart disease (n = 147). Patients displaying ACh‐induced vasodilatation had a better prognosis than those with ACh‐dependent vasoconstriction. Cardiovascular events included lethal and non‐lethal myocardial infarction, stroke, coronary and peripheral revascularization and symptoms of unstable Angina pectoris. Redrawn from (Schachinger et al., 2000). With permission of Wolters Kluwer Health, Inc. Copyright © 2000 Wolters Kluwer Health, Inc. All rights reserved. (B) Kaplan–Meier‐curves illustrating the correlation between flow‐mediated dilation (FMD) of capacitance vessels in the forearm and event‐free survival of patients after coronary or peripheral bypass surgery (n = 187). Patients displaying higher FMD values (indicator of endothelial function) had a better prognosis than those with lower FMD values. Cardiovascular events included lethal and non‐lethal myocardial infarction, symptoms of unstable Angina pectoris, atrial fibrillation and increased troponin‐I. Redrawn from (Gokce et al., 2002). With permission of Wolters Kluwer Health, Inc. Copyright © 2002 Wolters Kluwer Health, Inc. All rights reserved.
Prognostic value of endothelial function measurement
At present, the most reliable parameters with prognostic value are circulating markers such as BNP or CRP as well as risk scores that are based on calculations that consider different risk factors/markers such as Framingham Risk Score, Synergy Between PCI With Taxus and Cardiac Surgery Score (Verma et al., 2004; Matsuzawa et al., 2013). During the last 15 years, data from a number of clinical studies support the prognostic importance of coronary and peripheral measurements of endothelial dysfunction not only in patients with coronary artery disease (Schachinger et al., 2000), peripheral arterial occlusive disease (Anderson et al., 1995), arterial hypertension (Perticone et al., 2001), postmenopausal women (Sharma et al., 2011) and heart failure (Heitzer et al., 2005), but also in healthy subjects (Maruhashi et al., 2013; Shechter et al., 2014) (Table 1). Of particular interest is a study that assessed endothelial function (measured by flow‐mediated dilation, FMD) for the prediction of future cardiovascular events in patients undergoing coronary bypass surgery: the results were expressed in tertailes representing the lowest event rate (normal FMD > 8%), an intermediate event rate (FMD = 4‐8%) and the highest event rate (FMD < 4%) (Gokce et al., 2002). Another large cohort study identified the hyperaemic velocity, a stimulus for FMD and also a marker of microvascular function, but not FMD itself, as a prognostic marker for future cardiovascular events (Anderson et al., 2011). Results of the general population‐based Gutenberg Health Study (5,000 individuals) revealed a strong correlation between the biomarker of cardiovascular disease, pro‐ atrial natriuretic peptide (ANP), and non‐invasive measurement of conduit artery and peripheral arterial performance (Schnabel et al., 2012).
Table 1.
Selected studies on the prognostic value of endothelial dysfunction in cardiovascular disease
| Reference | Number of patients | Population * | Method and target vessel | Follow‐up period (months) | ED as (independent) predictor of events |
|---|---|---|---|---|---|
| (Suwaidi et al., 2000) | 157 | Patients w/o significant coronary stenosis | ACh i.c. Coronary resistance vessels | 28 | No statement |
| (Schachinger et al., 2000) | 147 | Patients with coronary heart disease | ACh i.c. Epicardiac capacity vessels | 80 | Yes |
| (Perticone et al., 2001) | 225 | Patients with untreated arterial hypertension | ACh i.a. Resistance vessels of the forearm | 32 | Yes |
| (Heitzer et al., 2001) | 281 | Patients with coronary heart disease | ACh i.a. Resistance vessels of the forearm | 54 | Yes |
| (Halcox et al., 2002) | 308 | Patients with and without coronary heart disease | ACh i.c. Epicardiac capacity vessels and coronary resistance vessels | 46 | Yes |
| (Gokce et al., 2002) | 187 | Patients with future peripheral and coronary bypass surgery | FMD Capacity vessels of the forearm | 1 | Yes |
| (Targonski et al., 2003) | 503 | Patients with no significant coronary stenosis | ACh i.c. Coronary resistance vessels | 16 | Yes |
| (Gokce et al., 2003) | 199 | Patients with peripheral arterial occlusive disease | FMD Capacity vessels of the forearm | 14 | Yes |
| (Brevetti et al., 2003) | 131 | Patients with peripheral arterial occlusive disease | FMD Capacity vessels of the forearm | 23 | Yes |
| (Fichtlscherer et al., 2004) | 198 | Patients with acute coronary syndrome | ACh i.a. Resistance vessels of the forearm | 48 | Yes |
| (Heitzer et al., 2005) | 289 | Patients with mild heart failure (EF 35–50%) | ACh i.a. Resistance vessels of the forearm | 36 | Yes |
| (Anderson et al., 2011) | 1574 | Healthy men | FMD Capacity vessels of the forearm | 60 | Yes (Hyperaemic velocity, a marker of microvascular function) |
| (Yeboah et al., 2012) | 1330 | Individuals with intermediate cardiovascular risk | FMD Capacity vessels of the forearm | 120–144 | No, not independent |
| (Matsuzawa et al., 2013) | 528 | Patients at high‐risk for cardiovascular events | RH‐PAT Resistance vessels of the fingertip | 60 | Yes |
| (Maruhashi et al., 2013) | 5314 | Healthy subjects | FMD Capacity vessels of the forearm | 28 | Yes |
| (Suessenbacher et al., 2013) | 396 | Patients with chest pain | FMD Capacity vessels of the forearm | 144 | No |
| (Shechter et al., 2014) | 618 | Healthy subjects | FMD Capacity vessels of the forearm | 34–77 | Yes |
ACh, acetylcholine; i.a ., intra‐arterial; i.c ., intra‐coronary; EF, ejection fraction; FMD, flow‐mediated dilation; RH‐PAT, reactive hyperaemia‐peripheral arterial tonometry.
Large‐scale clinical trials conducted recently suggest that endothelial function has no significant prognostic value in cohort studies of mostly healthy subjects (Suessenbacher et al., 2013) and is not an independent predictor of cardiovascular events in individuals with intermediate cardiovascular risk (Yeboah et al., 2012). Similarly, results of the Gutenberg Health Study revealed that non‐invasive measurement of vascular function is unlikely to improve the prognostic value of the European Society of Cardiology risk score (Schnabel et al., 2011). However, in patients at high risk of cardiovascular disease, peripheral endothelial dysfunction was significantly correlated with impending cardiovascular events (Matsuzawa et al., 2013). A systematic review of 15 cohort studies indicates a significant correlation of endothelial function measurements using FMD with diagnosed atherosclerosis in patients (Garcia et al., 2012). There are several possible reasons that may account for the large discrepancies reported in endothelial function in various studies: in addition to the nature of the cohorts (healthy versus unwell patients), other variables such as differences in methods (tonometry, plethysmography, FMD), procedures, devices, vascular beds, operator‐dependency of the complex methods should be considered along with the effects of physical exercise, age, body mass index (BMI), blood pressure, gender, and baseline brachial diameter in the case of forearm‐based measurements on endothelial function (Benjamin et al., 2004). Therefore, measurements of intima‐media thickness (Suessenbacher et al., 2013) and stiffness index (Mitchell, 2015) could well be considered better parameters for determining endothelial or more importantly vascular function. Importantly, intima‐media thickness also positively correlates with the levels of oxidized thiols and early atherosclerosis, so providing a link between vascular dysfunction and oxidative stress (Ashfaq et al., 2006).
Physiological role of endothelial (vascular) function
The central role of the cardiovascular system is the transportation of nutrients, biomolecules and signalling molecules, and importantly, transport of gasses to and from all organs, tissues and cells (Pries and Kuebler, 2006; Mikhed et al., 2015a). In addition, the cardiovascular system is an important regulator of host defence by the immune system (Libby et al., 2006; Pries and Kuebler, 2006; Karbach et al., 2014a) and blood haemostasis/coagulation (Arnout et al., 2006; Pries and Kuebler, 2006; Steven et al., 2015). The endothelium, a mono‐layer of cells lining the inner /luminal surface of blood vessels, acts as a barrier to control the exchange of nutrients, biomolecules and messengers with surrounding tissues. The endothelium also prevents the adhesion of immune cells and infiltration of monocytes into the sub‐endothelial space of lesion‐prone areas, an essential step in the development of atherosclerotic plaques (Cheng et al., 2005; Lau and Baldus, 2006). The endothelium controls vascular tone by releasing vasoconstrictors such as endothelin‐1 and vasodilators such as nitric oxide (•NO) (formerly known as endothelium‐derived relaxing factor, EDRF), endothelium‐derived hyperpolarizing factor (EDHF), prostacyclin or natriuretic peptides (Mombouli and Vanhoutte, 1999; Busse and Fleming, 2006; Moncada and Higgs, 2006; Spieker et al., 2006). The endothelium‐derived vasodilators also possess anti‐aggregatory properties, suppress thrombus formation, vascular stenosis (Willerson et al., 1989) and, in the case of the •NO/ cGMP axis, also retard cardiac hypertrophy (Ritchie et al., 2009). More recently, hydrogen sulphide (H2S) was identified as another gaseous endothelium‐derived vasodilator (Yang et al., 2008) that acts in concert with •NO (Cortese‐Krott et al., 2015; Yuan et al., 2015). Endothelium‐derived vasoactive messengers act together with other regulatory systems, which consist of vasoconstrictors such as catecholamines and other vasoactive peptides (e.g. angiotensin II, vasopressin). An imbalance in the formation of these vasoactive messengers is an important determinant in the development of endothelial dysfunction that is often further aggravated by oxidative stress (Munzel et al., 1999; Kahler et al., 2000). There is also evidence that the glycocalyx, which is located on the luminal surface of vascular endothelial cells and consists of proteoglycans, glycosaminoglycans, glycoproteins and glycolipids, plays an essential role in the adhesion of leukocytes and platelets, and accordingly in endothelial function (van den Berg et al., 2006). Importantly, areas of dysfunctional endothelium can be repaired by circulating endothelial progenitor cells (EPCs) and the number as well as quality of EPCs largely affect cardiovascular outcomes (Werner et al., 2005) as well as endothelial function (Hill et al., 2003).
According to more recent data, evaluation of endothelial function in itself may not be sufficient as vascular function is also affected by processes within the smooth muscle (e.g. activity of soluble guanylyl cyclase [sGC]) (Stasch et al., 2006). It is also now recognized that perivascular adipose tissue (PVAT) contributes to vascular homeostasis by producing vasoactive compounds such as adipokines, reactive oxygen species (ROS) and •NO (Brown et al., 2014; Jankovic et al., 2017). It is beyond the scope of this review to address all of these tissues and processes involved in detail and we will mainly focus our discussion on pathways related to endothelial function in health and disease. An excellent overview on open biological and physiological questions in endothelial (dys)function can be found in the editorial “Perspectives in pharmacology of endothelium: from bench to bedside” in the respective special issue (Chlopicki, 2015).
Mechanisms of development of endothelial (vascular) dysfunction
Endothelial (vascular) dysfunction implies impaired production of the different endothelium (smooth muscle, PVAT)‐derived messengers that results in a vasoconstrictor, pro‐inflammatory and pro‐atherothrombotic phenotype leading to an impaired regulation of perfusion and/or vascular tone. Since the development of endothelial (vascular) dysfunction is a multi‐factorial process, we will concentrate on some of its most important components in the following sections (Figure 3). Among the most important vasoactive compounds produced by endothelial cells are •NO, EDHF, prostacyclin and endothelin‐1 since they not only affect vascular tone but also the activity and adhesion of platelets and immune cells to the endothelium (Pries and Kuebler, 2006; Selivanova et al., 2007; Karbach et al., 2014a; Steven et al., 2015). The redox regulation of their production was previously reviewed in detail (Kahler et al., 2000; 2001; Bachschmid et al., 2005; Daiber et al., 2014; Schulz et al., 2014) and is discussed extensively in this virtual collection (Daiber et al., 2017). In brief, endothelial nitric oxide synthase (eNOS) uncoupling, sGC desensitization, nitration and inactivation of prostacyclin synthase (CYP8A1), oxidative activation of the endothelin‐1 system and direct inactivation of •NO by superoxide play important roles in the development of endothelial dysfunction (for review see Forstermann and Munzel, 2006; Daiber et al., 2017, 2014). According to more recent reports, there is also a strong interaction and cross‐regulatory mechanism between these important vasodilators (Hink et al., 2003; Bachschmid et al., 2005) and newly identified mediators such as H2S (Cortese‐Krott et al., 2015; Yuan et al., 2015) and other gasotransmitters such as carbon monoxide (Andreadou et al., 2015). Here, we will discuss new risk factors for endothelial dysfunction besides the classical ones (e.g. hypertension, hyperlipidaemia, diabetes, smoking, alcohol abuse) (Munzel et al., 2008; Ferdinandy et al., 2014).
Figure 3.
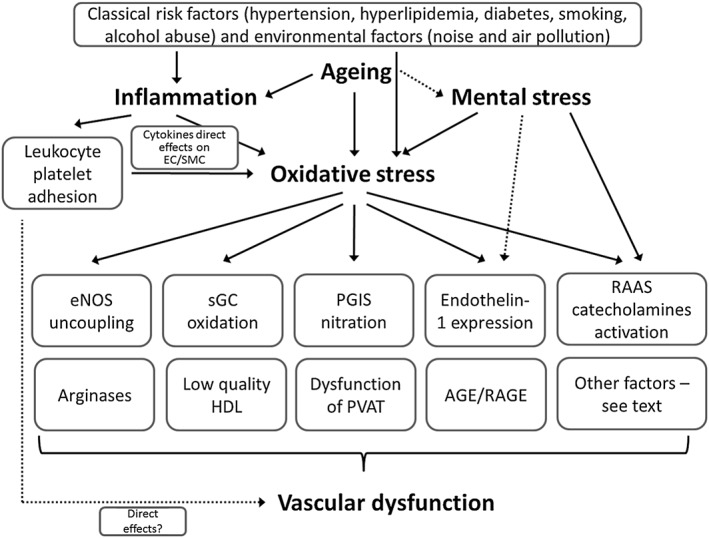
Initiators of endothelial (vascular) dysfunction. Aging, inflammation, mental stress and oxidative stress are strong triggers of endothelial (vascular) dysfunction. It should be noted that to a certain extent the classical risk factors (see top of the scheme) converge at the level of inflammation and oxidative stress to further trigger the down‐stream pathomechanisms. Inflammation can have detrimental effects by direct effects of cytokines on vascular cells leading to activation of secondary sources of ROS, direct leukocyte‐derived ROS formation or effects of infiltrated immune cells on vascular structure and function. Aging and mental stress act via oxidative stress, inflammation but can also directly affect the major triggers of endothelial (vascular) dysfunction such as are eNOS uncoupling, sGC oxidation (maybe also imbalanced phosphodiesterase expression/activity), prostacyclin synthase (PGIS) nitration and inactivation, redox‐triggered endothelin‐1 (ET‐1) signalling, activation of the renin‐angiotensin‐aldosterone system (RAAS) and other stress hormones (catecholamines), and finally, AGE/RAGE signalling. In addition, dysregulated arginase metabolism decreases levels of the eNOS substrate L‐arginine. The quality of high density lipoprotein (HDL) changes under oxidative stress conditions and metabolic disease. Other factors that also contribute to endothelial (vascular) dysfunction are changes in fatty acid metabolism and release of adipokines by perivascular adipose tissue (PVAT) that occurs in some diseases.
Environmental factors: mental stress, noise exposure, air pollution and endothelial (vascular) dysfunction
Mental stress activates the immune system and leads to adverse cardiovascular effects (Marvar and Harrison, 2012a; Marvar et al., 2012b). Mental depression in humans is associated with higher cardiovascular risk and vice versa, as patients with cardiovascular events have more frequent depressive phases (Lippi et al., 2009). Recent studies also suggest that transportation (road, railway, aircraft) noise is a novel cardiovascular risk factor for stroke, myocardial infarction, chronic stable coronary artery disease and arterial hypertension (Sorensen et al., 2011; Raaschou‐Nielsen et al., 2012; Munzel et al., 2014a); this was also supported by a recent meta‐analysis for traffic noise exposure and incidence of ischaemic heart disease (Vienneau et al., 2015). Vascular function studies revealed that night‐time aircraft noise exposure induced endothelial dysfunction, a deterioration of sleep quality, an increase in adrenaline levels and a trend for increased blood pressure in healthy subjects (Schmidt et al., 2013). Importantly, noise‐induced vascular dysfunction was improved by oral administration of L‐ascorbic acid (vitamin C) suggesting that ROS are involved in causing endothelial dysfunction (Schmidt et al., 2013). Another important finding of this study was that previous noise exposure sensitized the vasculature to damage by subsequent exposure to noise, strongly suggesting a lack of tolerance to the cardiovascular impact of noise. A recent study confirmed and extended these findings in patients with established coronary artery disease (Schmidt et al., 2015a). In these patients, night‐time aircraft noise caused a marked degree of endothelial dysfunction, increased blood pressure and worsened sleep quality. Importantly, the deterioration of vascular function was independent of noise sensitivity and annoyance reactions of noise‐exposed subjects.
Likewise, exposure to particulate matter causes endothelial dysfunction both in animals (Ying et al., 2015) and humans (Krishnan et al., 2012). The Heinz Nixdorf Recall Study indicates that environmental stress, e.g. air pollution and traffic noise, are independently associated with atherosclerosis (Arpornchayanon et al., 2013). In summary, environmental factors affect endothelial (vascular) function and are now considered to be novel risk factors for cardiovascular disease (see Figure 1).
Inflammation and endothelial (vascular) dysfunction
According to recent clinical studies, cardiovascular risk is significantly increased in patients with chronic autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and severe psoriasis (Hak et al., 2009; Vena et al., 2010; Soltesz et al., 2011; Murdaca et al., 2012). It is not surprising that psoriasis has been proposed as a risk factor for future cardiovascular events independently of the classical cardiovascular risk factors such as smoking, obesity and diabetes (Mehta et al., 2010). The guidelines of the European League against Rheumatism strongly emphasize the increased cardiovascular risk associated with, for example, inflammatory arthritis (Peters et al., 2010). Chronic inflammation causes a significant increase in the intima‐media thickness and is associated with impaired vascular function in patients with rheumatoid arthritis (Sodergren et al., 2010). Infections (pathogens burden) are negatively correlated with endothelial function, show a positive correlation with the prevalence of coronary artery disease and predispose human subjects to atherosclerosis (Prasad et al., 2002). Impaired endothelial function was observed by measuring FMD in patients with psoriasis (Balci et al., 2009). More recently our group provided insight into the mechanisms of vascular dysfunction caused by inflammation of the skin in an experimental model of psoriasis (Karbach et al., 2014b). These studies demonstrated that overexpression of IL‐17A caused a psoriasis‐like disease inducing infiltration of inflammatory cells and increased oxidative stress and vascular dysfunction (Karbach et al., 2014b).
Further details of the interaction of inflammatory processes and endothelial and vascular dysfunction is extensively reviewed elsewhere (Harrison et al., 2011; Karbach et al., 2014a; Knorr et al., 2014; Steven et al., 2015). It is important to note that oxidative stress is a hallmark of all cardiovascular diseases and that it contributes to endothelial cell activation, priming it for adhesion and infiltration of immune cells as well as activation of these infiltrated immune cells. Endothelial cell activation per se is not harmful since it represents a physiological adaptation to different stimuli and can be considered a response to injury; endothelial activation is also an early step in the development of endothelial (vascular) dysfunction as long as it is not counter‐regulated and returns to a “normal” phenotype (e.g. as seen under chronic inflammatory conditions). Accordingly, there is a persistent low‐grade inflammatory phenotype of the vasculature observed in most cardiovascular diseases.
Aging and endothelial (vascular) dysfunction
The aging process is associated with arterial stiffness and endothelial dysfunction, hallmarks of future cardiovascular events in humans (Ras et al., 2013). The vasculature in the elderly is also more susceptible to atherosclerotic lesions, vascular injury, impaired angiogenesis and calcification (Herrera et al., 2010). As a result, the incidence and frequency of cardiovascular complications such as coronary artery disease or stroke is higher in the elderly (Ras et al., 2013; Savji et al., 2013). Vascular oxidative stress and inflammation are increased during the aging process and may further stimulate each other in a positive feedback fashion (El Assar et al., 2013; Mikhed et al., 2015a). Activated and infiltrated immune cells induce secondary vascular sources of oxidative stress, with further endothelial cell activation that primes the endothelium for the adhesion of more leukocytes and platelets (Karbach et al., 2014a). This immuno‐redox crosstalk significantly contributes to aging‐associated endothelial dysfunction (Mikhed et al., 2015a). In addition to oxidative stress and inflammation, other causes of endothelial dysfunction in the aging vasculature include apoptosis and necrosis of various cell types, possibly including repair cells such as EPCs (Kujoth et al., 2005).
Drug‐induced endothelial (vascular) dysfunction
Multiple reports on drug‐induced endothelial (vascular) dysfunction exist and it would be beyond the scope of this review to mention all of them. We briefly provide some important examples of drug (or radiotherapy)‐induced endothelial dysfunction and refer to a recent review on this topic (Wojcik et al., 2015).
Previous data reported that treatment with anticancer drugs and/or radiotherapy contributes to the development of endothelial dysfunction. Survivors of childhood acute lymphoblastic leukaemia receiving chemotherapy and/or chemotherapy combined with radiation displayed impaired FMD but not nitroglycerin‐mediated dilation (NMD) as compared to healthy subjects (Dengel et al., 2008). Of note, early damage to the endothelium can have prolonged (>20 years) effects in humans. A similar observation was made in lymphoma survivors treated with radiotherapy – this group had a higher burden of atherosclerotic lesions and greater peripheral endothelial dysfunction than the control group (Wethal et al., 2014). Young adult survivors of Hodgkin Lymphoma following radiotherapy displayed impaired endothelial function as measured by peripheral arterial tonometry (PAT) (Zelcer et al., 2010).
Although organic nitrates are among the nitric oxide replacement therapies, most of these drugs induce so‐called nitrate tolerance with chronic administration, a phenomenon that is based on the loss of potency of the nitrovasodilators but also other severe side effects such as oxidative stress and endothelial dysfunction (cross‐tolerance) (Munzel et al., 2013; Daiber and Munzel, 2015). The development of endothelial dysfunction in humans was reported for nitroglycerin and isosorbide mononitrate (to a minor extent also isosorbide dinitrate) treatment, whereas pentaerithrityl tetranitrate may be the only organic nitrate in clinical use that is devoid of these side effects (Munzel et al., 2011). The development of nitroglycerin‐induced endothelial dysfunction is probably due to mechanisms similar to those produced by classical cardiovascular risk factors.
Other factors for endothelial (vascular) dysfunction
As already mentioned, the development of endothelial (vascular) dysfunction is a complex and multifactorial process and not all mechanisms can be adequately discussed here. Another important cause of endothelial dysfunction is the plasma concentration of high density lipoprotein (HDL), since low levels are associated with an increased burden of cardiovascular disease (Kontush, 2014). HDL levels also negatively correlate with markers of oxidative stress (Bencsik et al., 2015). It may also be that the quality of HDL (e.g. content of oxidant and antioxidant proteins) regulates normal endothelial function and prevents cardiovascular diseases (Riwanto and Landmesser, 2013). Of note, the quality of HDL is subject to redox‐driven modifications involving myeloperoxidase and paraoxonase (Huang et al., 2013). HDL from healthy subjects has strong anti‐oxidant effects and also inhibits the activation of NADPH oxidase in endothelial cells, all of which may explain the positive effects of HDL on the •NO/superoxide balance. Intra‐arterial infusion of HDL alleviates endothelial dysfunction in hypercholesterolaemic men (Spieker et al., 2002). Unfortunately, more recent studies clearly demonstrate that HDL from subjects with established coronary artery disease is dysfunctional, thus increasing rather than inhibiting oxidative stress in vascular tissue (Besler et al., 2011).
The impact of alcohol (ethanol) on endothelial function is a dual‐edged sword (Cahill and Redmond, 2012). Epidemiological studies demonstrate that frequent low‐moderate alcohol intake is vasculoprotective, whereas acute binge drinking or chronic alcohol abuse is detrimental, as demonstrated by the development of alcohol‐dependent cardiomyopathy and progression of atherosclerosis in chronic drinkers (Lucas et al., 2005).
DNA damage (e.g. by oxidative stress or irradiation) results in the activation of poly(ADP‐ribose) polymerase (PARP), which in turn leads to inhibition of glyceraldehyde‐3‐phosphate dehydrogenase, diabetic complications such as activation of NFκB, protein kinase C and generation of intracellular advanced glycation end products, all of which contribute to “glycaemic memory” and endothelial dysfunction (Szabo, 2009). Moreover, PARP activation contributes to the destabilization of atherosclerotic plaques, immune cell infiltration, sirtuin 1 inactivation and neointima formation, all of which promote endothelial (vascular) dysfunction (Xu et al., 2014).
PVAT releases important vasoactive compounds, a function that can be dysregulated and so contribute to vascular dysfunction (Brown et al., 2014). Importantly, redox processes play an important role in these alterations (Jankovic et al., 2015). One of these PVAT‐derived factors, adiponectin, is proposed to be a better predictor of endothelial function of coronary arteries than insulin resistance index (HOMA‐R), body mass index (BMI), immuno‐reactive insulin, or triglycerides (Okui et al., 2008). Advanced glycosylation end products (AGEs) and their specific receptor (advanced glycosylation end product‐specific receptor [RAGE]), play an important role in atherosclerosis and determine endothelial function in diabetic patients (Kajikawa et al., 2015). AGE/RAGE signalling is subject to redox regulation and there is crosstalk with oxidative stress pathways (Wautier et al., 2001; Coughlan et al., 2009). The Janus face of bradykinin receptors in the control of vascular homeostasis underlies the clinical use of bradykinin receptor agonists (e.g. for the direct vasodilator effects and beneficial effects on components of the renin‐angiotensin‐aldosterone system (RAAS)) as well as antagonists (e.g. for the control of inflammatory and pain signalling) (Blaes and Girolami, 2013). Dysregulation of arginases represents another trigger for endothelial dysfunction, since arginases compete with eNOS for the substrate L‐arginine and are upregulated under inflammatory conditions and released during haemolysis (Risbano and Gladwin, 2013). There is also evidence for endothelial dysfunction in oncological diseases; however, since most cancer patients receive anticancer therapy directly after diagnosis, it is challenging to distinguish between direct effects of cancer on vascular function and the indirect effects of the (mostly aggressive) anticancer therapies (e.g. chemotherapy, radiotherapy) (Wojcik et al., 2015). Finally, oestrogen signalling has a significant impact on endothelial function as reflected by sex differences in the burden of cardiovascular disease as well as changes in the incidence of endothelial dysfunction in post‐menopausal and pregnant women (Chakrabarti et al., 2014).
Measurement of endothelial function and dysfunction
Besides general clinical signs of endothelial dysfunction, such as erectile dysfunction (Kaiser et al., 2004; Kovacs et al., 2008; Bhatia et al., 2013), which is also a strong indicator of cardiovascular risk (Dong et al., 2011; Vlachopoulos et al., 2013), there are several established techniques to determine endothelial function in vivo (Figure 4). We mainly focus on those techniques that are routinely applied in clinical studies or emerging techniques that may be of relevance for human diagnostics in the future. Some more sophisticated methods, including biochemical markers and bioassays, measurement of endothelial‐derived microparticles and progenitor cells, are discussed elsewhere (Lekakis et al., 2011). A helpful correlation matrix of multiple parameters of endothelial function, arterial stiffness and end‐points of atherosclerosis can be found elsewhere (Frolow et al., 2015).
Figure 4.
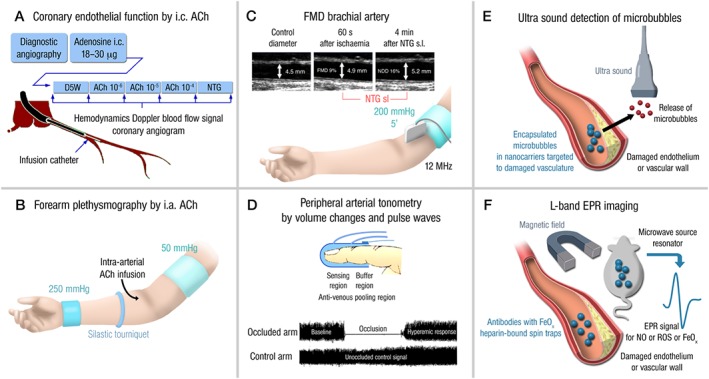
Invasive and non‐invasive methods for the determination of endothelial function. (A) Acetylcholine (ACh)‐dependent vasoreactivity of coronary vessels caused by intra‐coronary (i.c.) ACh infusion. Vasodilatation and stenotic areas are monitored by angiographic imaging and Doppler ultrasound (blood flow). (B) ACh‐dependent vasoreactivity of capacity vessels of the forearm upon intra‐arterial ACh infusion. Vasodilatation is recorded by Doppler ultrasound (diameter and blood flow). (C) Flow‐mediated dilation (FMD) of capacity vessels of the forearm (brachial artery) upon occlusion/ischaemia and reperfusion/hyperaemia. Vasodilatation is recorded by Doppler ultrasound (diameter and blood flow). Maximal vasoreactivity/dilation is determined by sublingual administration of nitroglycerin (NTG). Adapted from (Munzel, 2008). With permission of Georg Thieme Verlag KG Stuttgart. Copyright © 2008, Rights Managed by Georg Thieme Verlag KG Stuttgart • New York. All rights reserved. (D) Peripheral arterial tonometry measures volume changes and pulse waves by a finger probe. Additional details for the determination of pulse wave velocity (PWV) are explained in the text. (E) Ultrasound‐based detection of microbubbles released from nanocarriers upon chemical reaction with ROS or upon destruction of the carriers by ultrasound. The nanocarriers are targeted to damaged endothelium (e.g. by bound antibodies). The released microbubbles are detected by ultrasound. (F) L‐band electron paramagnetic resonance (EPR) spectroscopy can be used for the detection of paramagnetic compounds or particles (usually having an unpaired electron) in whole animals and tissues. Damaged vasculature can be labelled by antibodies linked to iron oxide (EPR active). Likewise, heparin‐bound spin traps are bound to the endothelial surface and allow the detection of nitric oxide or ROS.
Acetylcholine binds to muscarinic receptors on endothelial cells leading to eNOS activation, elevation of vascular •NO production and vasodilatation of blood vessels (Ludmer et al., 1986). The relaxation of coronary vessels upon infusion of acetylcholine can be detected by quantitative coronary angiography or measurement of coronary blood flow (Schachinger et al., 2000). Similarly, acetylcholine infusion can be applied to any peripheral resistance vessel (e.g. in the forearm) and coupled with ultra‐sound measurement of blood flow, which is termed forearm plethysmography (Heitzer et al., 1996a). A non‐invasive technique for measurement of endothelial function in vivo is quantification of flow mediated dilation (FMD) (Ramsey et al., 1996). In this process, a baseline diameter of the brachial artery is first recorded by ultrasound; blood‐flow is then interrupted by inflation of a blood pressure cuff for five minutes, and restoration of blood flow leads to a reactive hyperaemia that is associated with increased release of •NO. The subsequent vasodilation is again detected by ultrasound. Maximal dilation of the brachial artery is induced by sublingual application of nitroglycerin (NMD) and then used for normalization of FMD values. A newer non‐invasive technique to detect endothelial function in humans is peripheral arterial tonometry (PAT), where a finger probe assesses digital volume changes and pulse waves that can be detected after induction of reactive hyperaemia (Bonetti et al., 2003). Other advanced techniques for the diagnosis of endothelial dysfunction are low flow mediated constriction (Gori et al., 2008; Gori et al., 2012) and passive leg movement (PLM) as a potentially practical assessment of systemic vascular function via •NO‐dependent mechanisms (Groot et al., 2015; Hughes and Kruse, 2016). The most traditional technique for the ex vivo assessment of endothelial dysfunction is the isometric tension method as first described in animal tissues by the Nobel laureate Robert Furchgott (Furchgott and Zawadzki, 1980), or in human vessels from bypass surgery (Schulz et al., 2002). An additional novel ex vivo technique to assess endothelial dysfunction is based on the identification of atherosclerotic lesions by Raman spectroscopy (Baranska et al., 2015). The advantages and disadvantages of these methods were discussed in full detail elsewhere (Munzel et al., 2008). Clearly the non‐invasive techniques are easier to apply to a large study population and the risks of side effects (e.g. puncture‐induced bleeding complications) are lower.
The early stages of atherosclerosis are characterized by endothelial dysfunction while the later stages result in arterial stiffness, both of which can be quantified by different techniques. Pulse wave velocity (PWV) has emerged as an established method to examine arterial stiffness and has a strong correlation with cardiovascular outcomes (Reference Values for Arterial Stiffness C, 2010). Applanation tonometry and Doppler ultrasound are also clinical methods available for evaluating global PWV (Lehmann et al., 1993; Nelson et al., 2010; Reference Values for Arterial Stiffness' Collaboration, 2010). However, the gold standard for measuring PWV remains flow meter or catheter‐based pressure probes. Nevertheless, their invasiveness limits their use in clinical studies (Wentland et al., 2014). In recent years magnetic resonance imaging (MRI) has become more accessible and is another minimally‐invasive method to determine PWV (Boese et al., 2000; Dogui et al., 2011; Bar et al., 2015).
A new molecular imaging method for the detection of areas of dysfunctional endothelium is based on the infusion of compounds that release gaseous microbubbles that can be detected by ultrasound (reviewed by Villanueva et al., 2002; Camici et al., 2012). Lipid‐covered decafluorobutane microbubbles linked to specific antibodies [e.g. against P‐selectin, vascular cell adhesion molecule 1 (VCAM‐1)] were used to detect areas of vascular inflammation in obese primates upon ultrasound‐triggered release of the microbubbles (Chadderdon et al., 2014). Another feature of the antibody‐targeted microbubbles is specific drug targeting of inflamed, thrombotic or atherosclerotic endothelium (Tsutsui et al., 2004). In addition to using preformed microbubbles, in situ generated microbubbles from chemical reactions can also be used for ultrasound detection of damaged endothelium. A study in mice used ultrasound to measure the reaction of ROS with liposome‐encapsulated allylhydrazine, a liquid compound, that yields nitrogen and propylene gas that is detected by ultrasound methods (Perng et al., 2012).
Another method that will probably be used in the future is L‐band electron paramagnetic resonance (EPR) spectroscopy for the detection of areas of dysfunctional endothelium in intact animals. Previous studies used iron oxide particles bound to endothelium‐specific antibodies (e.g. anti‐E‐selectin) for the detection of endothelial inflammation (Radermacher et al., 2009). Likewise, heparin‐polynitroxide derivatives bind to the endothelium via heparin‐binding sites and due to their superoxide scavenging potential, can serve as diagnostic and therapeutic tools (Kleschyov et al., 2012; Kleschyov and Sen, 2013). Most of these probes can be used in L‐band and MRI approaches. Combined with the respective spin trap, these techniques have the potential for the specific detection of vascular •NO or ROS formation in isolated tissues and whole animals (Fujii and Berliner, 2004).
Another new diagnostic technique to measure endothelial function could be based on the determination of the glycocalyx by intravital microscopy or by orthogonal polarization spectral imaging (Nieuwdorp et al., 2008). The distance between the endothelium and passing erythrocytes represents the thickness or dimension of the glycocalyx that correlates with levels of classical cardiovascular risk markers such as low density lipoprotein (LDL), HDL, blood glucose and BMI.
Targeting endothelial (vascular) dysfunction
The endothelium, which was initially regarded as a relatively inert cell layer, is now recognized as an important regulator (in concert with the smooth muscle and PVAT) of numerous physiological functions such as coagulation, fibrinolysis, arterial tone and vascular growth. As outlined above, endothelial dysfunction is a key event in the development and progression of atherosclerosis, coronary artery diseases and myocardial ischaemia (Celermajer, 1997). The demonstration that endothelial dysfunction can be reversed raises the possibility of retarding or even preventing the progression of atherosclerosis, while also improving arterial function and decreasing the incidence of cardiovascular events (see Figure 5A for a summary of therapeutic options). Here we mainly focus on those treatment options that are routinely applied or are emerging therapies of likely relevance for patient care in the future.
Figure 5.
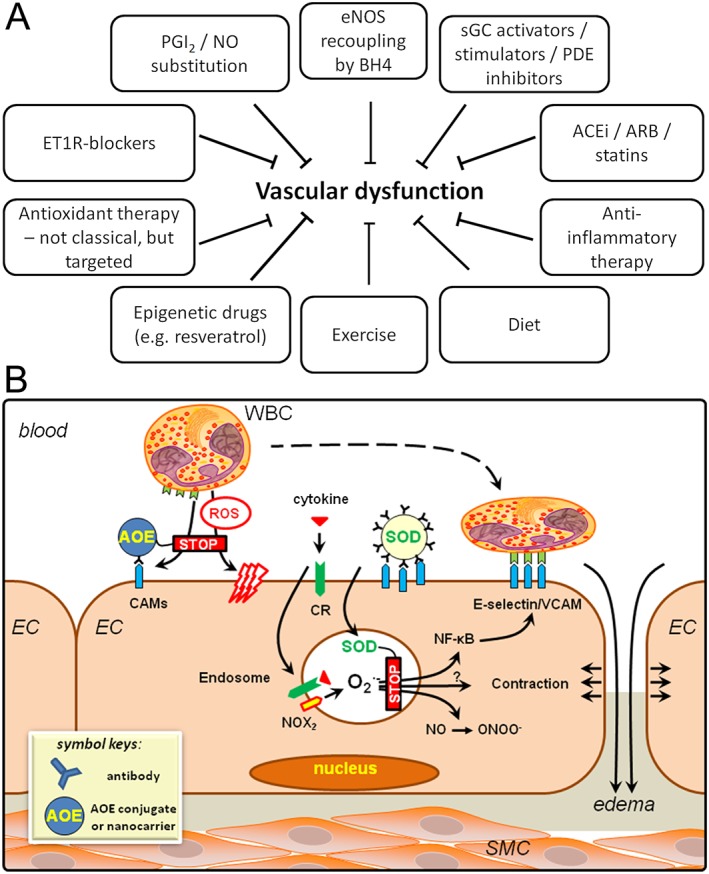
Overview of therapeutic options for the improvement of vascular dysfunction. (A) In addition to currently available cardiovascular drugs, several new therapies are currently in preclinical and clinical studies. Anti‐inflammatory, “repair“ and epigenetic therapy are promising candidates for the future. Exercise and dietary changes are non‐pharmaceutical but highly potent “natural“ alternatives. Classical antioxidant therapy failed in most large clinical trials and might require specific delivery of the antioxidants at sites of inflammation or increased oxidative stress. (B) Targeted antioxidant interventions to alleviate pro‐inflammatory activation and oxidative stress in endothelial cells. Endothelial ROS from activated Nox2 enzyme in endosomes are formed in response to cytokine binding to the receptors and ignite signalling cascade of transcription factor NF‐κB. Targeted delivery of antioxidants, antioxidant enzymes (AOE) and inhibitors of ROS production can be achieved using antibodies and other ligands of endothelial surface determinants including cell adhesion molecules PECAM and ICAM. Surface‐bound targeted AOE intercept extracellular ROS, whereas targeted formulations using the same ligands configured in a way permitting internalization into the ROS‐signalling endosomes allows interception of pro‐inflammatory activation manifested among other characteristics by exposure of inducible cell adhesion molecules – E‐selectin, VCAM‐1 and ICAM‐1 ‐ that can be detected using imaging probes conjugated to the ligands of these molecules.
Established drugs improving endothelial dysfunction
Angiotensin‐converting enzyme (ACE) inhibitors, AT1 receptor (angiotensin II type‐1 receptor) antagonists, statins and many other cardiovascular drugs display pleiotropic indirect antioxidant properties (e.g., inhibition of Nox enzymes and secondary to this prevention of eNOS uncoupling) and anti‐inflammatory effects leading to improved endothelial function (for review see Drexler and Hornig, 1999; Warnholtz and Munzel, 2000; Gori and Munzel, 2011; Steven et al., 2015). Similar beneficial effects were described for endothelin‐1 receptor antagonists (Clozel, 2003; Thorin and Clozel, 2010). Gemfibrozil is a lipid‐lowering drug that also activates sGC in a nitric oxide‐ and haem‐independent fashion, which may explain the more pronounced cardiovascular benefit observed with this drug as compared to other members of the fibrate group of drugs (Sharina et al., 2015).
Antioxidant therapy
Reactive oxygen species (ROS) from different sources play an important role in regulating vascular function (Karbach et al., 2014a; Steven et al., 2015). Superoxide reacts with •NO to form the highly reactive product peroxynitrite (ONOO‐), leading to not only reduced •NO bioavailability but also impaired endothelial enzymatic processes (Munzel et al., 2008). Several studies using vitamin C as an antioxidant therapy have been performed in an attempt to restore endothelial function and thereby reduce cardiovascular disease. Promising results were obtained in studies in which vitamin C was given acutely to patients suffering from classic risk factors for cardiovascular disease such as metabolic syndrome, smoking or hypertension (Takase et al., 2004; Teede et al., 2006; Cangemi et al., 2007; Plantinga et al., 2007). Even more intriguing are results showing a correlation between vitamin C deficiency and cardiovascular outcomes (Heitzer et al., 2001) (see Table 2 for an overview). Large‐scale clinical trials performed to investigate the impact of antioxidants on clinical end‐points and the progression of atherosclerosis have yielded rather disappointing findings, since almost no beneficial effects on cardiovascular outcome were detected (Salonen et al., 2003; Cook et al., 2007; Sesso et al., 2008). In fact, in a study on postmenopausal women with type 2 diabetes, administration of supplemental vitamin C correlated positively with mortality end‐points (Lee et al., 2004).
Table 2.
Effects of anti‐oxidant treatment on endothelial function
| Inclusion criteria | Reference | Antioxidants used | Treatment | Improvement of endothelial function |
|---|---|---|---|---|
| Hypertension | (Solzbach et al., 1997) | Vitamin C | acute, i.v. | Yes |
| (Teede et al., 2006) | Flavonoids | 3 month, oral | No | |
| (Plantinga et al., 2007) | Vitamin C and E | 8 weeks, oral | Yes | |
| Smoking | (Neunteufl et al., 2000) | Vitamin E | 4 weeks, oral | Yes |
| (Mangoni et al., 2002) | Folic acid | 4 weeks, oral | Yes | |
| (Antoniades et al., 2003) | Vitamin C and E | 4 weeks, oral | Yes | |
| (Takase et al., 2004) | Vitamin C and E | 4 weeks, oral | Yes | |
| (Heiss et al., 2005) | Flavonoids | 2 hours, oral | Yes | |
| (Young et al., 2006) | Vitamin C | 12 weeks, oral | Yes | |
| (Heitzer et al., 1996a) | Vitamin C | Acute, i.a. | Yes | |
| Diabetes Type 1 and 2,Hyperglycaemia | (Skyrme‐Jones et al., 2000) | Vitamin E | 12 hours, oral | Yes |
| (Regensteiner et al., 2003) | L‐arginine, Vitamin C and E | 1 week, oral and 1‐week „wash out“ | Yes | |
| (Pena et al., 2004) | Folic acid | 8 weeks, oral | Yes | |
| (Economides et al., 2005) | Vitamin E | 12 month, oral | No (Impairment of FMD) | |
| (Mangoni et al., 2005) | Folic acid | 4 weeks, oral | Yes | |
| (Anderson et al., 2006) | Vitamin C | 2 days, oral | Yes | |
| Kidney failure | (Cross et al., 2003) | Vitamin C | Acute, i.a. | Yes |
| (Ghiadoni et al., 2004) | Vitamin C | 2 hours, oral | Yes | |
| (Nanayakkara et al., 2007) | Vitamin E | 12 month, oral | Yes | |
| Heart failure | (Ellis et al., 2001) | Vitamin C | 2 hours, i.v. | Yes |
| Stable angina | (Tousoulis et al., 2005) | Vitamin C | Acute, i.v. | Yes |
| Healthy older men | (Jablonski et al., 2007) | Vitamin C | Acute, intravenously | Yes |
| Endotoxaemia in healthy subjects | (Aschauer et al., 2014) | Vitamin C | 2 hours, intravenously | Yes |
The reasons for the failure of large clinical trials using oral antioxidant therapy are numerous and include, among others, the limited up‐take of classic oral antioxidants in tissues undergoing oxidative stress, access to intracellular sites of ROS production, the limited reactivity towards specific ROS (e.g. hydrogen peroxide or superoxide) and most importantly, interference with essential physiological ROS signalling (for review see (Chen et al., 2012; Schmidt et al., 2015b)). Since most large‐scale clinical trials on oral antioxidant therapy were either not properly controlled for compliance or for individual basal antioxidant serum levels [compare the different outcome of the EPIC Norfolk study (Khaw et al., 2001)], measuring the antioxidant status of each subject is necessary to determine if benefits occurred only in those with pre‐existing antioxidant deficiencies (Schmidt et al., 2015b).
Anti‐inflammatory therapy
Inflammation is an independent risk factor for the development of cardiovascular disease (Kaptoge et al., 2014). Levels of the acute phase protein CRP are elevated in various inflammatory diseases. The PROVE IT‐TIMI 22 study demonstrated that high CRP levels in patients with acute coronary syndrome predicted death from myocardial infarction (Ridker et al., 2005). There are numerous human and animal studies demonstrating a correlation between inflammatory processes and endothelial dysfunction (for review see (Karbach et al., 2014a; Steven et al., 2015)). Lipopolysaccharide (LPS) from the bacterial cell wall is a strong trigger for inflammation and endothelial (vascular) dysfunction in humans (Becker et al., 2012). This occurs in severe inflammatory conditions, such as septic shock, and also in low‐grade inflammatory diseases such as rheumatoid arthritis (Bergholm et al., 2002; Hansel et al., 2003) or type 2 diabetes (Henry et al., 2004), causing endothelial (vascular) dysfunction and accelerated atherosclerosis. Furthermore, it is now generally agreed that in addition to atherosclerosis, arterial hypertension is also a low‐grade inflammatory disease (Libby, 2002; Wenzel et al., 2011).
Several clinical trials are currently underway to investigate whether anti‐inflammatory treatment improves cardiovascular outcomes e.g. methotrexate therapy (TETHYS trial and CIRT trial) (Everett et al., 2013; Moreira et al., 2013) and blockade of the cytokine IL‐1β with canakinumab for the management of cardiovascular disease (CANTOS trial) (Ridker et al., 2011). Another new anti‐inflammatory therapy could be based on the restoration of a disturbed glycocalyx, which is associated with higher susceptibility to triggers of atherosclerosis and leukocyte/platelet adhesion (Meuwese et al., 2009; Drake‐Holland and Noble, 2012; Tarbell and Cancel, 2016).
Repair therapy with pharmacological agents
Pharmacological targeting of oxidatively impaired eNOS and sGC
There is overwhelming evidence supporting an important pathophysiological role of eNOS dysregulation/uncoupling in several diseases, making targeting of this enzyme, in particular in its uncoupled state, an attractive therapeutic option. So far, eNOS‐directed pharmaceutical attempts have included the development of so‐called eNOS enhancers, compounds that up‐regulate the expression of eNOS at the mRNA and protein level (Fraccarollo et al., 2008; Wohlfart et al., 2008; Frantz et al., 2009). Despite quite favorable results in these preclinical studies an inherent problem may be that sole overexpression of eNOS without up‐regulation of its co‐factor sapropterin (BH4) will ultimately lead to its uncoupling and so worsen disease conditions rather than improving them. However, some eNOS enhancers provide beneficial effects because of a simultaneous up‐regulation of BH4. Unfortunately to this date no clinical data are available to judge the efficacy of eNOS enhancers in patients with cardiovascular disease. In line with this, Channon and co‐workers demonstrated that eNOS overexpression leads to an increase in •NO formation only when the BH4 synthase GTP‐cyclohydrolase 1 (GCH‐1) is also up‐regulated (Crabtree et al., 2009). Overexpression of GCH‐1 or co‐administration of BH4 successfully prevented cardiac hypertrophy by pressure overload and also lowered blood pressure in the salt‐sensitive low‐renin hypertension model (Du et al., 2008; Moens et al., 2008). BH4 supplementation improved cardiac and pulmonary function in a canine model of cardiopulmonary bypass (Szabo et al., 2011). BH4 treatment or treatment with analogues such as folic acid and sepiapterin improved endothelial dysfunction in e.g. chronic smokers and diabetes via recoupling eNOS (Heitzer et al., 2000b; Heitzer et al., 2000a) and in numerous ex vivo studies (Tiefenbacher et al., 2000; Gori et al., 2001) (for review see (Antoniades et al., 2009)). The PHACeT Trial reported benefits of eNOS gene‐enhanced progenitor cell therapy for pulmonary arterial hypertension (Granton et al., 2015).
Recent studies have identified sGC as an important therapeutic target since oxidation of the enzyme at numerous sites impairs activation by •NO. The discovery of compounds such as haem‐dependent sGC stimulators and haem‐independent sGC activators that can stimulate the enzyme in a •NO‐independent manner has led to the development of a clinical programme for the treatment of patients with pulmonary (arterial) hypertension (Ghofrani et al., 2013a), chronic thromboembolic pulmonary hypertension (Ghofrani et al., 2013b) and heart failure (Lapp et al., 2009) (for review see (Evgenov et al., 2006)). The sGC activator cinaciguat normalized vascular oxidative stress and coronary endothelial function in a rat model of myocardial infarction (Korkmaz et al., 2009b), a canine model of cardiopulmonary bypass (Radovits et al., 2011) and prevented endothelial dysfunction of isolated rat aorta when challenged with peroxynitrite (Korkmaz et al., 2013). Inhibitors of phosphodiesterases (PDE) act down‐stream of sGC to increase cGMP levels and are used for multiple indications (for review see (Boswell‐Smith et al., 2006)). A recent meta‐analysis of subjects with type 2 diabetes mellitus (T2DM) suggests a beneficial effect of treatment with a PDE5 inhibitor (sildenafil) on endothelial function (Santi et al., 2015). Likewise, therapy with the PDE5 inhibitor vardenafil prevented cardiovascular dysfunction in a rat model of T1DM (Radovits et al., 2009) and a canine model of cardiopulmonary bypass with hypothermic cardiac arrest (Szabo et al., 2009). Vardenafil also improved endothelial function of isolated rat aorta upon ex vivo challenges with peroxynitrite (Korkmaz et al., 2009a) and hypochlorite (Radovits et al., 2013).
Nitric oxide and other substitution therapies
Nitrovasodilators such as organic nitrates act in •NO substitution therapy and have been used for more than a century for the relief of anginal pain that is caused by stenosis or spasm of the coronary arteries associated with endothelial dysfunction (for review see (Daiber and Munzel, 2015). However, as is the case for eNOS enhancer therapy, chronic substitution of •NO alone does not produce beneficial effects since •NO under oxidative stress conditions rapidly yield the potent oxidant peroxynitrite (Warnholtz et al., 2002) (for review see Gori and Parker, 2004). The target enzyme receptor for •NO, sGC, can also be inhibited under conditions of oxidative stress (Evgenov et al., 2006). Therefore, a combined antioxidant and •NO substitution therapy seems to be a more promising strategy, as shown for pentaerithrityl tetranitrate, the only organic nitrate that improves oxidative stress, nitrate tolerance and endothelial dysfunction (Munzel et al., 2011; 2014b). Inorganic nitrite and nitrate are emerging options for •NO substitution therapy as they have beneficial effects in hypertension, myocardial infarction and acute heart failure (for review see (Bueno et al., 2013; Bailey et al., 2014; Rassaf et al., 2014)). Accumulation of the endogenously produced eNOS inhibitor asymmetric dimethyl arginine (ADMA) under oxidative stress conditions is another trigger of endothelial dysfunction that could be remedied by treatment with high doses of L‐arginine, which directly replaces ADMA at the level of eNOS enzyme (for review see (Sydow and Munzel, 2003)) or by prevention of endothelial ADMA accumulation through activation of the cationic amino acid extrusion transporter (Closs et al., 2012). Another new therapeutic option is supplementation with an H2S donor that not only confers direct antioxidant effects but which also acts synergistically with •NO via different signalling pathways (Cortese‐Krott et al., 2015; Yuan et al., 2015). Prostacyclin substitution, e.g. by iloprost, is not generally used for the treatment of cardiovascular disease but more specifically for the therapy of severe pulmonary hypertension (Galie et al., 2001).
Inhibition of PARP to suppress down‐stream effects of oxidative (or irradiation‐induced) DNA damage
Pharmacological inhibition of PARP prevented irradiation‐induced vascular dysfunction in isolated aortic tissue (Beller et al., 2006), improved endothelial dysfunction in isolated vessels treated with hydrogen peroxide‐ (Radovits et al., 2007a) or hypochlorite‐induced oxidative stress (Radovits et al., 2007c) and reversed cardiac and vascular dysfunction in aged rats (Radovits et al., 2007b).
Epigenetic approaches
There is emerging evidence that epigenetic pathways are redox‐regulated and play a causal role in cardiovascular diseases by directly affecting endothelial function (for reviews see Kim et al., 2013; Mikhed et al., 2015b). Due to their reversibility, epigenetic processes represent ideal targets for therapeutic approaches.
microRNAs
Since their discovery in C. Elegans in the last decade of the 20th century, microRNAs have been of increasing interest in biomedicine, with the cardiovascular field being no exception (Small and Olson, 2011). miRNAs were among the first of the small, non‐coding RNA molecules (<200 nt) to be recognized in regulating gene expression; this ever‐expanding family now includes another group of non‐coding RNAs that are longer (>200 nt) and hence termed long non‐coding RNAs (Uchida and Dimmeler, 2015). MicroRNAs (miRNAs) are short (20‐24 nt) non‐coding RNAs involved in the post‐transcriptional regulation of gene expression by affecting both stability and translation of mRNA. Approximately 2600 miRNAs have been described in humans. More than half of all mRNAs are estimated to be targets of miRNAs, and each miRNA is thought to regulate hundreds of targets (Agarwal et al., 2015). The role of miRNAs in vascular function and atherogenesis has been comprehensively reviewed recently (Zampetaki and Mayr, 2012; Fernandez‐Hernando and Baldan, 2013; Zampetaki et al., 2013; Menghini et al., 2014; Andreou et al., 2015).
In the specific case of vascular/endothelial dysfunction, miRNAs have been grouped according to their roles in general programmes related to either endothelial senescence or endothelial inflammation (Menghini et al., 2014). In the case of vascular aging, up‐regulation of miR‐29, miR‐34, miR‐217 and miR‐146 have been linked to vascular wall alterations or endothelial senescence (Dimmeler and Nicotera, 2013). Targeting of Nox4 by miR‐146a has been suggested to reduce ROS levels, potentially helping to mitigate endothelial dysfunction (Vasa‐Nicotera et al., 2011). In contrast miR‐200 increases oxidative stress and endothelial cell senescence (Magenta et al., 2011). The miRNA miR‐126 is abundantly expressed in endothelial cells (Wei et al., 2013) and is important for vascular integrity (Schober et al., 2014).
Recent work shows promising avenues to explore specific miRNAs as potential therapeutic tools or targets to treat vascular and endothelial dysfunction. An interesting example is posed by miR‐181b, which inhibits NF‐κB activation, vascular inflammation and atherosclerosis in ApoE‐deficient mice that were systemically treated with the miRNA (Sun et al., 2014). In addition, circulating levels of miR‐181b were reduced in patients with inflammatory conditions and in elderly people, suggesting a potential protective role for this miRNA. Similarly, in vivo inhibition of miR‐92a that is associated with low shear stress and pro‐atherogenic stimuli, reduced plaque size in LDL‐receptor null mice (Loyer et al., 2014) and produced beneficial effects in models of vascular injury (Daniel et al., 2014). Nevertheless, other authors reported that perturbation of homeostatic levels of this miRNA alters redox‐regulated responses and endothelial angiogenesis (Zhang et al., 2014; Chen et al., 2015). Overall it is fair to state that we are at the early stages of employing strategies directed towards regulating the levels of endogenous miRNAs for ameliorating vascular dysfunction, with rapid strides being made on an ongoing basis (van Rooij and Olson, 2012).
Histone acetylation/methylation and DNA methylation
Disturbed blood flow associated with atherosclerosis alters endothelial gene expression by inducing DNA (cytosine‐5‐)‐methyltransferase 1 (DNMT1) mediated changes in genome‐wide DNA methylation patterns (Dunn et al., 2014). Treatment with the DNMT inhibitor azacitidine (5‐aza‐2′‐deoxycytidine) restores normal methylation patterns and the expression of mechanosensitive master transcription factors including Homeobox protein A5 (HoxA5) and Kruppel‐like Factor 3 (KLF3) to prevent endothelial dysfunction and atherosclerosis in animals. Likewise, endothelial function is improved by DNMT inhibitors (by abolishing the disturbed flow‐induced silencing of the athero‐protective transcription factor KLF4) in human aortic endothelial cells and adult swine aortas (Jiang et al., 2014) and also by restoring the expression of KLF2in human umbilical vein endothelial cells (HUVECs), which is repressed in dysfunctional endothelium by LDL‐mediated DNA methylation (Kumar et al., 2013). Oxidized LDL (oxLDL) induces acetylation of histone H3 and H4 as well as phosphorylation of histone H3, resulting in the expression of pro‐inflammatory genes in endothelial cells, all of which was prevented by statin therapy (Dje N'Guessan et al., 2009). Methyl‐CpG‐binding domain protein2 (MBD2) negatively regulates eNOS and vascular endothelial growth factor receptor 2 (VEGFR‐2, Type IV RTKs) expression whereas knockdown of MBD2 in HUVECs enhanced angiogenesis, provided protection against H2O2‐induced apoptosis and hind‐limb ischaemic injury in Mbd2‐KO mice (Rao et al., 2011).
Histone acetyl transferase (HAT) p300 plays a role in diabetes and atherosclerosis by up‐regulating the response to hyperglycaemia and shear stress, respectively (Chen et al., 2010; Katsume et al., 2011), with effects on eNOS expression (Chen et al., 2008), NF‐κB acetylation and endothelial inflammation (Zhang et al., 2011). Likewise, the pan‐histone deacetylase (HDAC) inhibitor scriptaid decreases Nox4 expression levels in human endothelial cells to reduce endothelial oxidative stress (Siuda et al., 2012). Hyndman et al. propose HDAC1 inhibition as a method to prevent endothelial dysfunction, as HDAC1 up‐regulation in primary bovine aortic endothelial cells significantly decreased •NO production through deacetylation of eNOS whereas knockdown of HDAC1 or its inhibition by the pan‐HDAC inhibitor trichostatin A (TSA) increased eNOS activity and restored basal •NO production (Hyndman et al., 2014). In contrast, Rössig et al. demonstrated that nonselective HDAC inhibition by trichostatin A, sodium butyrate, and the synthetic benzamide derivative entinostat (MS‐275) reduced expression of eNOS in HUVECs and impaired •NO‐dependent vasorelaxation and angiogenesis (Rossig et al., 2002). HDAC2 indirectly regulates •NO production by suppressing the expression of arginase2 (Arg2), which competes for the eNOS substrate L‐arginine (Ryoo et al., 2008; Pandey et al., 2014). In line with that, HDAC2 overexpression restored •NO production in oxLDL‐treated human aortic endothelial cells and improved vascular relaxation in mouse aortic rings (Pandey et al., 2014). Thus, therapeutic activation of HDAC2 has great promise as a new method for correcting endothelial dysfunction and atherosclerosis.
Resveratrol is a naturally occurring compound (e.g. in grapes) which acts as an activator of SIRT1, a class III HDAC. Several studies confirm that SIRT1 activity promotes eNOS expression/activity and improves endothelial cell survival and function (Lagouge et al., 2006; Mattagajasingh et al., 2007; Zhang et al., 2008). Clinical trials are underway to examine the potential benefits of resveratrol administration in subjects with cardiovascular disorders (Schleithoff et al., 2012; Xia et al., 2017). However, there are still unsolved safety concerns regarding the chronic consumption of high resveratrol doses (Tome‐Carneiro et al., 2013).
Endothelium‐specific antioxidant and anti‐inflammatory drug‐delivery
As outlined above, excessive ROS formation, especially superoxide generation, contributes to endothelial dysfunction, vascular contractility, oedema and a pro‐inflammatory phenotype of vascular cells. Antioxidant therapies including N‐acetyl cysteine (NAC) and antioxidant enzymes (AOE, i.e. catalase and SOD, quenching H2O2 and superoxide O2 .‐, respectively), in theory, can alleviate this pathological mechanism (Bowler et al., 2001). Diverse approaches have been designed to achieve specific delivery of antioxidants. For example, conjugation with polyethylene glycol (PEG), PEG‐containing carriers or liposomes improve the bioavailability of antioxidants and their ability to detoxify ROS. However, encouraging data from in vitro and whole animal studies have not yet translated to clinical practice (Freeman et al., 1985; Barnard et al., 1993) (see also “Antioxidant therapy” section above). Current efforts to prevent oxidative stress have yielded rather mixed outcomes in clinical studies (Bowler et al., 2001). This may be explained by the fact that endothelial ROS are marginally accessible to untargeted antioxidants (Bowler et al., 2001). Most reactive and hence damaging ROS are short‐lived species that act at angstrom to nanometre distances (Parthasarathy et al., 2001), increasing the need for precise antioxidant delivery to quench endothelial ROS (Shuvaev et al., 2011b).
Studies in vitro (Muzykantov et al., 1987; Sakharov et al., 1987) and in vivo established that drugs can be targeted to the endothelium using ligands binding to the angiotensin‐converting enzyme (ACE) (Muzykantov et al., 1996), and cell adhesion molecules such as ICAM‐1 (Atochina et al., 1998) and PECAM (Hood et al., 2011). Further, by varying the configuration of these ligands in drug delivery systems, drugs may be either retained on the luminal surface or delivered into endosomes (Muro et al., 2006; Simone et al., 2009a). Endothelial ligands have been conjugated with antioxidant enzymes (Dziubla et al., 2008; Shuvaev et al., 2011a), as well as with antioxidant‐loaded liposomes (Hood et al., 2012; Howard et al., 2014a), polymeric and non‐polymeric nanocarriers (Dziubla et al., 2005; Simone et al., 2007) and magnetic nanocarriers (Hood et al., 2014).
AOE conjugated to antibodies against ACE, ICAM and PECAM (Ab/AOE), but not untargeted IgG/AOE or PEG/AOE, binds to endothelial cells (Shuvaev et al., 2007; 2011b), protecting them from the toxic effects of extracellular H2O2 (Sweitzer et al., 2003) and O2 ·‐• (Shuvaev et al., 2007), as well as endothelial dysfunction caused by the accumulation of intracellular ROS (Shuvaev et al., 2007). Ab/AOE but not IgG/AOE or PEG/AOE accumulates in endothelial cells after intravascular injection (Muzykantov et al., 1996; Atochina et al., 1998; Shuvaev et al., 2011b). Ab/catalase targeted to PECAM or ACE injected into donor rats attenuated subsequent ischaemic injury in transplanted lungs (Kozower et al., 2003; Nowak et al., 2010), improved oxygenation and pulmonary microcirculation associated with warm lung ischaemia and transplantation in pigs (Preissler et al., 2011) and alleviated lung ischaemia‐reperfusion injury in situ in mice (Shuvaev et al., 2009). Ab/SOD normalized vascular constriction induced by angiotensin II in mice (Shuvaev et al., 2009), inhibited endothelial pro‐inflammatory activation caused by cytokines and potentiated the anti‐inflammatory effects of NO donors (Shuvaev et al., 2011b; Shuvaev et al., 2013). Ab/SOD enters endothelial endosomes and quenches superoxide anion produced in these vesicles by Nox enzymes, thereby inhibiting the NF‐κB pathway (Shuvaev et al., 2011b; 2013).
Encapsulation into poly(ethylene glycol)‐b‐poly(lactic‐co‐glycolic acid) (PEG‐PLGA) carriers protects AOE from proteases (Dziubla et al., 2005; Dziubla et al., 2008), and inhibits lysosomal proteolysis typical of protein conjugates such as Ab/AOE (Muro et al., 2003; Dziubla et al., 2005). Using PEG‐modified AOE and varying the polymeric content allows control of enzyme loading and shape of the nanocarriers (Simone et al., 2009b, 2007, 2009c; Hood et al., 2011). Catalase loaded in PECAM‐targeted Ab/PEG‐PLGA carriers accumulated in the pulmonary vasculature, conferring sustained antioxidant protection (Dziubla et al., 2008). A phospholipid derivative MJ33 (an indirect inhibitor of NADPH‐oxidase) loaded in Ab/liposomes targeted to PECAM accumulated in the endothelial cells, inhibited ROS production and provided more potent protection than non‐targeted counterparts against oxidative stress and inflammation in mice (Hood et al., 2012). Ab/liposomes loaded with EUK‐134, a superoxide dismutase/catalase mimetic, binds to endothelial cells and alleviates endotoxin‐induced lung inflammation in mice (Howard et al., 2014a). PECAM‐targeted nanocarriers loaded with tocopherol and a SOD mimetic alleviated endothelial inflammatory activation (Howard et al., 2014b). Magnetic nanoparticles loaded with AOE (Chorny et al., 2010) targeted to PECAM accumulated in the pulmonary endothelium and alleviated oedema and inflammation in a mouse model of endotoxin‐induced lung injury (Hood et al., 2014).
The preferential targeting of the pulmonary circulation is based on several factors: the pulmonary vasculature is a privileged target tissue for endothelial delivery, because it contains about 20‐25% of the total endothelial surface in the body, receives more than 50% of total cardiac output and is perfused at a relatively slow rate. Due to these factors, agents possessing an affinity for factors expressed on the surface of the endothelium throughout the body (i.e. pan endothelial determinants, such as CD31/PECAM‐1), also accumulate in the lungs after systemic intravascular injections, especially via the intravenous routes that favour first pass through the pulmonary circulation (Han et al., 2012).
In summary, endothelial targeting of antioxidants facilitates anti‐inflammatory mechanisms based on the interception of endothelial ROS, as shown in Figure 5B. This drug delivery strategy may find utility in the management of acute vascular oxidative stress and inflammation.
Impact of dietary changes
Human dietary preferences are important influences on health in modern societies. An injudicious diet represents one of the leading causes of premature death and chronic disease (Katz and Meller, 2014). On the one hand, a single high‐fat meal can transiently impair endothelial function in healthy subjects (Vogel et al., 1997). On the other hand, optimal eating is associated with increased life expectancy and a reduction in lifetime risk of all chronic disease (Katz and Meller, 2014).
The health benefits of dietary patterns such as the Mediterranean, low glycaemic index, moderately low carbohydrate, and vegetarian diets have been widely promoted (Katz and Meller, 2014; Ley et al., 2014). While it is not possible (perhaps also not necessary) to define which diet is best for health, it is nonetheless clear that healthy diet patterns have many common features: limited amounts of refined starches, added sugars, processed foods; reduced intake of certain fats; an emphasis on whole plant foods, with or without lean meats, poultry and seafood (Katz and Meller, 2014). These elements are reflected in the recommendations from major scientific associations (Ley et al., 2014).
The Mediterranean diet, just as one example, reduces the incidence of major cardiovascular events in people with a high cardiovascular risk (Estruch et al., 2013). In a randomized, single‐blind trial involving 180 patients (99 men and 81 women) with metabolic syndrome, the Mediterranean diet (for 2 years) improved endothelial function and reduced the prevalence of metabolic syndrome (Esposito et al., 2004). Interestingly, the health benefits of the Mediterranean diet are likely to persist for a long time. The original improvement in endothelial function from an 8‐week Mediterranean diet intervention is still evident one year after the initial study (Klonizakis et al., 2014).
The favourable effects of the Mediterranean diet on health are thought to result from a higher ratio of omega‐6 and omega‐3 fatty acids, higher intake of fibre, antioxidants and polyphenols (Zamora‐Ros et al., 2013). Both marine omega‐3 fatty acids (Egert and Stehle, 2011) and plant‐derived α‐linolenic acid (Wang et al., 2012; Egert et al., 2014) improve vascular endothelial function in human subjects. Polyphenols are found in virtually all foods of plant origin (Landberg et al., 2012). In particular, the red wine polyphenol resveratrol has received much attention. Indeed, resveratrol improves endothelial function in laboratory animals as well as in human subjects; at least part of the effect is attributable to stimulation of endothelial •NO production (see also chapter “Epigenetic approaches”) (Xia et al., 2014). Despite the relatively poor bioavailability of resveratrol, some beneficial effects can be achieved in humans in vivo with dietary doses of resveratrol (i.e. 5 mg day‐1) (Cai et al., 2015).
In addition to the above cross‐sectional trials, a recent longitudinal cohort study demonstrated that healthy diets attenuate endothelial dysfunction and reduce low‐grade inflammation over a 7‐year period in adults at risk of cardiovascular disease (van Bussel et al., 2015). Food component analyses indicate that the consumption of more lean fish and raw vegetables, and fewer high‐fat dairy products improves endothelial function (van Bussel et al., 2015). A reduction in dietary salt intake (e.g. from 9 g salt day‐1 to 6 g salt day‐1) improves endothelial function in normotensive overweight and obese subjects (Dickinson et al., 2014). In summary, current data suggest that dietary changes positively affect endothelial function.
It is beyond the scope of this review to mention all dietary ingredients that affect endothelial function but it may be useful to mention some of particular interest. Flavanols from cacao have vasculoprotective effects (Balzer et al., 2008; Rassaf et al., 2016) and the polyphenol resveratrol from grapes/red wine markedly improves FMD in multiple clinical studies (Xia et al., 2017). Likewise, nitrate from vegetables improves vascular dysfunction in the elderly (Rammos et al., 2014) and hypercholesterolaemic patients (Velmurugan et al., 2016). Importantly, all of these dietary ingredients improve eNOS function, reduce the burden of oxidative stress or directly generate •NO (see preceding chapters).
Impact of exercise
The landmark study by the Diabetes Prevention Program Research Group in 27 centres (3234 participants) used a randomized, double‐blind, placebo‐controlled design to compare metformin treatment with lifestyle intervention for the prevention or delay of type 2 diabetes in high risk patients (Knowler et al., 2002). Participants undergoing intensive lifestyle intervention (healthy diet, increased physical activity) had significantly improved outcomes (~60% reduction in cumulative incidence of diabetes) compared to those managed with metformin (Knowler et al., 2002). A subsequent study in more than 8,000 same‐sex twins revealed that leisure‐time physical activity provided protection from type 2 diabetes independent of familial and genetic predisposition (Waller et al., 2010). A related study by the same group using a monozygotic co‐twin study revealed that leisure‐time physical activity increased artery lumen diameters (Leskinen et al., 2011). A more recent meta‐analysis examined the effects of exercise (≥ 4 weeks) on FMD using data from 51 clinical trials consisting of 2260 participants with mixed comorbidities (Montero et al., 2014). This meta‐analysis confirmed that exercise (aerobic, resistance or combined) significantly improved FMD, with the intensity of aerobic exercise (but not resistance or combined exercise) related to improved endothelial function in a dose‐related manner. Of note, physical exercise also improved cardiovascular parameters, restored glutathione and reduced CRP levels in patients with depression and subclinical vascular disease (Al Mheid et al., 2016).
The study by Sinoway et al. was one of the first to report that exercise improves endothelial function (measured by changes in forearm blood flow) in the dominant forearm of tennis players (Sinoway et al., 1986). Our current understanding of mechanisms contributing to the beneficial effects of exercise on endothelial function includes increased phosphorylation of eNOS, up‐regulation of superoxide dismutase subtypes, down‐regulation of NADPH oxidase subtypes, reduced uncoupling of eNOS, reduced levels of ADMA, increased circulating EPC, reduced endothelial senescence, improved endothelial repair, increased activation of AMP‐activated protein kinase (AMPK) and its activator proteins such as SIRT1, serine/threonine kinase 11 (LKB1) and calmodulin kinases (regulators of eNOS) (Ross et al., 2015). Plasma levels of ADMA, which normally increase with age, are reduced by aerobic exercise with simultaneous improvements in carotid arterial compliance in postmenopausal women (Tanahashi et al., 2014). Physical activity below the accepted minimal international recommendations is sufficient to alleviate oxidative stress, reduce ADMA levels and improve small artery endothelial function in overweight/obese men and women (Merino et al., 2015).
Efforts are underway to develop “exercise pills” that mimic the effects of physical exercise ostensibly by activating signalling pathways activated by exercise (Goodyear, 2008; Li and Laher, 2015). The currently described candidate exercise pills are unlikely to directly improve endothelial regulation of vascular tone as these agents do not appear to increase shear stress, a key factor for stimulating endothelial function (Johnson et al., 2011).
Conclusions and clinical implications
The prognostic value of endothelial function measurements for risk stratification of patients for future cardiovascular events is under debate, although it is clear that endothelial dysfunction is a hall‐mark of most cardiovascular risk factors and diseases. It is likely that parameters reflecting vascular function more globally (including smooth muscle and perivascular adipose tissue function), such as vascular stiffness index and intima/media thickness ratio, may be better predictors of future cardiovascular events. New diagnostic tools may improve the “imaging” of vascular (endothelial) dysfunction and so herald a new method to regularly predict cardiovascular events. Targeting vascular (endothelial) dysfunction still represents an attractive pharmacological approach due to its central involvement in the progression of most cardio‐ and cerebrovascular diseases as well as inflammatory processes. In addition to the classical cardiovascular drugs that have beneficial effects on vascular (endothelial) function, such as ACE inhibitors and statins, new therapeutic strategies based on site‐specific drug delivery, anti‐inflammatory as well as site‐ and source‐specific antioxidant interventions are being developed. Moreover, physical exercise and healthy nutrition represent a main‐stay in non‐pharmacological cardiovascular therapy. Direct targeting of vascular homeostasis (e.g. •NO/cGMP signalling cascade by •NO donors, PDE inhibitors and other vasodilators) is still not fully explored, but it is emerging that compounds such as sGC activators and stimulators have therapeutic potential. As shown by the REPAIR‐AMI study, the intracoronary administration of bone marrow‐derived cells (containing EPC) with high migratory capacity increases event‐free survival of patients with acute myocardial infarction (Assmus et al., 2014). Finally, epigenetic therapy shows beneficial effects in animal models, but this requires more extensive investigation before it can be transferred to a clinical setting.
Conflict of interest
We certify that there is no conflict of interest with any financial organizations regarding the materials discussed in the manuscript.
Acknowledgements
We are indebted to Margot Neuser for expert graphical assistance. The present work was supported by the European Cooperation in Science and Technology (COST Action BM1203/EU‐ROS to A.D., S.S., A.W. and S.L.), by generous financial support by the “Stiftung Mainzer Herz”, by the Centre of Translational Vascular Biology (CTVB), by “NMFZ” and “Stufe 1” funds from the Johannes Gutenberg University Mainz (to A.D., H.L. and T.M.). A.W. holds a stipend from the International PhD Program on the “Dynamics of Gene Regulation, Epigenetics and DNA Damage Response” from the Institute of Molecular Biology gGmbH, (Mainz, Germany) funded by the Boehringer Ingelheim Foundation.
Daiber, A. , Steven, S. , Weber, A. , Shuvaev, V. V. , Muzykantov, V. R. , Laher, I. , Li, H. , Lamas, S. , and Münzel, T. (2017) Targeting vascular (endothelial) dysfunction. British Journal of Pharmacology, 174: 1591–1619. doi: 10.1111/bph.13517.
Contributor Information
Andreas Daiber, Email: andreas.daiber@bioredox.com.
Thomas Münzel, Email: tmuenzel@uni-mainz.de.
References
- Agarwal V, Bell GW, Nam JW, Bartel DP (2015). Predicting effective microRNA target sites in mammalian mRNAs. eLife 4: e05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Mheid I, Held E, Uphoff I, Martin GS, Dunbar S, Bidulescu A et al. (2016). Depressive Symptoms and Subclinical Vascular Disease: The Role of Regular Physical Activity. J Am Coll Cardiol 67: 232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Evans LM, Ellis GR, Khan N, Morris K, Jackson SK et al. (2006). Prolonged deterioration of endothelial dysfunction in response to postprandial lipaemia is attenuated by vitamin C in Type 2 diabetes. Diabet Med 23: 258–264. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H et al. (2011). Microvascular function predicts cardiovascular events in primary prevention: long‐term results from the Firefighters and Their Endothelium (FATE) study. Circulation 123: 163–169. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D et al. (1995). Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241. [DOI] [PubMed] [Google Scholar]
- Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P (2015). The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol 172: 1587–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou I, Sun X, Stone PH, Edelman ER, Feinberg MW (2015). miRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends Mol Med 21: 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades C, Antonopoulos AS, Bendall JK, Channon KM (2009). Targeting redox signaling in the vascular wall: from basic science to clinical practice. Curr Pharm Des 15: 329–342. [DOI] [PubMed] [Google Scholar]
- Antoniades C, Tousoulis D, Tentolouris C, Toutouza M, Marinou K, Goumas G et al. (2003). Effects of antioxidant vitamins C and E on endothelial function and thrombosis/fibrinolysis system in smokers. Thromb Haemost 89: 990–995. [PubMed] [Google Scholar]
- Arnout J, Hoylaerts MF, Lijnen HR (2006). Haemostasis. Handb Exp Pharmacol (176 Pt 2): 1–41. [DOI] [PubMed] [Google Scholar]
- Arpornchayanon W, Canis M, Ihler F, Settevendemie C, Strieth S (2013). TNF‐alpha inhibition using etanercept prevents noise‐induced hearing loss by improvement of cochlear blood flow in vivo. Int J Audiol 52: 545–552. [DOI] [PubMed] [Google Scholar]
- Aschauer S, Gouya G, Klickovic U, Storka A, Weisshaar S, Vollbracht C et al. (2014). Effect of systemic high dose vitamin C therapy on forearm blood flow reactivity during endotoxemia in healthy human subjects. Vascul Pharmacol 61: 25–29. [DOI] [PubMed] [Google Scholar]
- Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC et al. (2006). The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol 47: 1005–1011. [DOI] [PubMed] [Google Scholar]
- Assmus B, Leistner DM, Schachinger V, Erbs S, Elsasser A, Haberbosch W et al. (2014). Long‐term clinical outcome after intracoronary application of bone marrow‐derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event‐free survival. Eur Heart J 35: 1275–1283. [DOI] [PubMed] [Google Scholar]
- Atochina EN, Balyasnikova IV, Danilov SM, Granger DN, Fisher AB, Muzykantov VR (1998). Immunotargeting of catalase to ACE or ICAM‐1 protects perfused rat lungs against oxidative stress. Am J Physiol 275: L806–L817. [DOI] [PubMed] [Google Scholar]
- Bachschmid M, Schildknecht S, Ullrich V (2005). Redox regulation of vascular prostanoid synthesis by the nitric oxide‐superoxide system. Biochem Biophys Res Commun 338: 536–542. [DOI] [PubMed] [Google Scholar]
- Bailey JC, Feelisch M, Horowitz JD, Frenneaux MP, Madhani M (2014). Pharmacology and therapeutic role of inorganic nitrite and nitrate in vasodilatation. Pharmacol Ther 144: 303–320. [DOI] [PubMed] [Google Scholar]
- Balci DD, Balci A, Karazincir S, Ucar E, Iyigun U, Yalcin F et al. (2009). Increased carotid artery intima‐media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol 23: 1–6. [DOI] [PubMed] [Google Scholar]
- Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M et al. (2008). Sustained benefits in vascular function through flavanol‐containing cocoa in medicated diabetic patients a double‐masked, randomized, controlled trial. J Am Coll Cardiol 51: 2141–2149. [DOI] [PubMed] [Google Scholar]
- Bar A, Skorka T, Jasinski K, Chlopicki S (2015). MRI‐based assessment of endothelial function in mice in vivo. Pharmacol Rep 67: 765–770. [DOI] [PubMed] [Google Scholar]
- Baranska M, Kaczor A, Malek K, Jaworska A, Majzner K, Staniszewska‐Slezak E et al. (2015). Raman microscopy as a novel tool to detect endothelial dysfunction. Pharmacol Rep 67: 736–743. [DOI] [PubMed] [Google Scholar]
- Barnard ML, Baker RR, Matalon S (1993). Mitigation of oxidant injury to lung microvasculature by intratracheal instillation of antioxidant enzymes. Am J Physiol 265: L340–L345. [DOI] [PubMed] [Google Scholar]
- Becker L, Prado K, Foppa M, Martinelli N, Aguiar C, Furian T et al. (2012). Endothelial dysfunction assessed by brachial artery ultrasound in severe sepsis and septic shock. J Crit Care 27: e319–e314. [DOI] [PubMed] [Google Scholar]
- Beller CJ, Radovits T, Seres L, Kosse J, Krempien R, Gross ML et al. (2006). Poly(ADP‐ribose) polymerase inhibition reverses vascular dysfunction after gamma‐irradiation. Int J Radiat Oncol Biol Phys 65: 1528–1535. [DOI] [PubMed] [Google Scholar]
- Bencsik P, Sasi V, Kiss K, Kupai K, Kolossvary M, Maurovich‐Horvat P et al. (2015). Serum lipids and cardiac function correlate with nitrotyrosine and MMP activity in coronary artery disease patients. Eur J Clin Invest 45: 692–701. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF Jr et al. (2004). Clinical correlates and heritability of flow‐mediated dilation in the community: the Framingham Heart Study. Circulation 109: 613–619. [DOI] [PubMed] [Google Scholar]
- Bergholm R, Leirisalo‐Repo M, Vehkavaara S, Makimattila S, Taskinen MR, Yki‐Jarvinen H (2002). Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler Thromb Vasc Biol 22: 1637–1641. [DOI] [PubMed] [Google Scholar]
- Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM et al. (2011). Mechanisms underlying adverse effects of HDL on eNOS‐activating pathways in patients with coronary artery disease. J Clin Invest 121: 2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia T, Kapoor A, Kumar J, Sinha A, Ranjan P, Kumar S et al. (2013). Impaired flow‐mediated vasodilatation in Asian Indians with erectile dysfunction. Asian J Androl 15: 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaes N, Girolami JP (2013). Targeting the ‘Janus face’ of the B2‐bradykinin receptor. Expert Opin Ther Targets 17: 1145–1166. [DOI] [PubMed] [Google Scholar]
- Boese JM, Bock M, Schoenberg SO, Schad LR (2000). Estimation of aortic compliance using magnetic resonance pulse wave velocity measurement. Phys Med Biol 45: 1703–1713. [DOI] [PubMed] [Google Scholar]
- Boger RH, Bode‐Boger SM, Thiele W, Creutzig A, Alexander K, Frolich JC (1998). Restoring vascular nitric oxide formation by L‐arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol 32: 1336–1344. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT et al. (2003). Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol 41: 1761–1768. [DOI] [PubMed] [Google Scholar]
- Boswell‐Smith V, Spina D, Page CP (2006). Phosphodiesterase inhibitors. Br J Pharmacol 147 (Suppl 1): S252–S257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RP, Arcaroli J, Crapo JD, Ross A, Slot JW, Abraham E (2001). Extracellular superoxide dismutase attenuates lung injury after hemorrhage. Am J Respir Crit Care Med 164: 290–294. [DOI] [PubMed] [Google Scholar]
- Brevetti G, Silvestro A, Di Giacomo S, Bucur R, Di Donato A, Schiano V et al. (2003). Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg 38: 374–379. [DOI] [PubMed] [Google Scholar]
- Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT et al. (2014). Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol 34: 1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno M, Wang J, Mora AL, Gladwin MT (2013). Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling, and therapeutics. Antioxid Redox Signal 18: 1797–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Fleming I (2006). Vascular endothelium and blood flow. Handb Exp Pharmacol (176 Pt 2): 43–78. [DOI] [PubMed] [Google Scholar]
- Cahill PA, Redmond EM (2012). Alcohol and cardiovascular disease–modulation of vascular cell function. Nutrients 4: 297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A et al. (2015). Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med 7: 298ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver A, Collier J, Vallance P (1992). Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin‐dependent diabetes. J Clin Invest 90: 2548–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camici PG, Rimoldi OE, Gaemperli O, Libby P (2012). Non‐invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J 33: 1309–1317. [DOI] [PubMed] [Google Scholar]
- Cangemi R, Angelico F, Loffredo L, Del Ben M, Pignatelli P, Martini A et al. (2007). Oxidative stress‐mediated arterial dysfunction in patients with metabolic syndrome: Effect of ascorbic acid. Free Radic Biol Med 43: 853–859. [DOI] [PubMed] [Google Scholar]
- Celermajer DS (1997). Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol 30: 325–333. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J et al. (1993). Cigarette smoking is associated with dose‐related and potentially reversible impairment of endothelium‐dependent dilation in healthy young adults. Circulation 88: 2149–2155. [DOI] [PubMed] [Google Scholar]
- Chadderdon SM, Belcik JT, Bader L, Kirigiti MA, Peters DM, Kievit P et al. (2014). Proinflammatory endothelial activation detected by molecular imaging in obese nonhuman primates coincides with onset of insulin resistance and progressively increases with duration of insulin resistance. Circulation 129: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Morton JS, Davidge ST (2014). Mechanisms of estrogen effects on the endothelium: an overview. Can J Cardiol 30: 705–712. [DOI] [PubMed] [Google Scholar]
- Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM et al. (2012). Free radical biology of the cardiovascular system. Clin Sci (Lond) 123: 73–91. [DOI] [PubMed] [Google Scholar]
- Chen S, Feng B, George B, Chakrabarti R, Chen M, Chakrabarti S (2010). Transcriptional coactivator p300 regulates glucose‐induced gene expression in endothelial cells. Am J Physiol Endocrinol Metab 298: E127–E137. [DOI] [PubMed] [Google Scholar]
- Chen W, Bacanamwo M, Harrison DG (2008). Activation of p300 histone acetyltransferase activity is an early endothelial response to laminar shear stress and is essential for stimulation of endothelial nitric‐oxide synthase mRNA transcription. J Biol Chem 283: 16293–16298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wen L, Martin M, Hsu CY, Fang L, Lin FM et al. (2015). Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA‐92a. Circulation 131: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZJ, Vapaatalo H, Mervaala E (2005). Angiotensin II and vascular inflammation. Med Sci Monit 11: RA194–RA205. [PubMed] [Google Scholar]
- Chlopicki S (2015). Perspectives in pharmacology of endothelium: From bench to bedside. Pharmacol Rep 67: vi–ix. [DOI] [PubMed] [Google Scholar]
- Chorny M, Hood E, Levy RJ, Muzykantov VR (2010). Endothelial delivery of antioxidant enzymes loaded into non‐polymeric magnetic nanoparticles. J Control Release 146: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs EI, Ostad MA, Simon A, Warnholtz A, Jabs A, Habermeier A et al. (2012). Impairment of the extrusion transporter for asymmetric dimethyl‐l‐arginine: A novel mechanism underlying vasospastic angina. Biochem Biophys Res Commun 423: 218–223. [DOI] [PubMed] [Google Scholar]
- Clozel M (2003). Effects of bosentan on cellular processes involved in pulmonary arterial hypertension: do they explain the long‐term benefit? Ann Med 35: 605–613. [DOI] [PubMed] [Google Scholar]
- Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E et al. (2007). A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med 167: 1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese‐Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF et al. (2015). Key bioactive reaction products of the NO/H2S interaction are S/N‐hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A 112: E4651–E4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC et al. (2009). RAGE‐induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 20: 742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree MJ, Tatham AL, Al‐Wakeel Y, Warrick N, Hale AB, Cai S et al. (2009). Quantitative regulation of intracellular endothelial nitric‐oxide synthase (eNOS) coupling by both tetrahydrobiopterin‐eNOS stoichiometry and biopterin redox status: insights from cells with tet‐regulated GTP cyclohydrolase I expression. J Biol Chem 284: 1136–1144. [DOI] [PubMed] [Google Scholar]
- Cross JM, Donald AE, Nuttall SL, Deanfield JE, Woolfson RG, Macallister RJ (2003). Vitamin C improves resistance but not conduit artery endothelial function in patients with chronic renal failure. Kidney Int 63: 1433–1442. [DOI] [PubMed] [Google Scholar]
- Daiber A, Munzel T (2015). Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate‐Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress. Antioxid Redox Signal 23: 899–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Di Lisa F, Oelze M, Kröller‐Schön S, Steven S, Schulz E et al. (2017). Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol 174: 1670–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Oelze M, Daub S, Steven S, Schuff A, Kroller‐Schon S et al. (2014). Vascular Redox Signaling, Redox Switches in Endothelial Nitric Oxide Synthase and Endothelial Dysfunction In: Laher I. (ed). Systems Biology of Free Radicals and Antioxidants. , ednSpringer‐Verlag: Berlin Heidelberg, pp. 1177–1211. [Google Scholar]
- Daniel JM, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W et al. (2014). Inhibition of miR‐92a improves re‐endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res 103: 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengel DR, Ness KK, Glasser SP, Williamson EB, Baker KS, Gurney JG (2008). Endothelial function in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 30: 20–25. [DOI] [PubMed] [Google Scholar]
- Dickinson KM, Clifton PM, Keogh JB (2014). A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin‐1 in a randomised cross_over study in normotensive overweight and obese subjects. Atherosclerosis 233: 32–38. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Nicotera P (2013). MicroRNAs in age‐related diseases. EMBO Mol Med 5: 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dje N'Guessan P, Riediger F, Vardarova K, Scharf S, Eitel J, Opitz B et al. (2009). Statins control oxidized LDL‐mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler Thromb Vasc Biol 29: 380–386.19122173 [Google Scholar]
- Dogui A, Redheuil A, Lefort M, DeCesare A, Kachenoura N, Herment A et al. (2011). Measurement of aortic arch pulse wave velocity in cardiovascular MR: comparison of transit time estimators and description of a new approach. J Magn Reson Imaging 33: 1321–1329. [DOI] [PubMed] [Google Scholar]
- Dong JY, Zhang YH, Qin LQ (2011). Erectile dysfunction and risk of cardiovascular disease: meta‐analysis of prospective cohort studies. J Am Coll Cardiol 58: 1378–1385. [DOI] [PubMed] [Google Scholar]
- Drake‐Holland AJ, Noble MI (2012). Update on the important new drug target in cardiovascular medicine ‐ the vascular glycocalyx. Cardiovasc Hematol Disord Drug Targets 12: 76–81. [DOI] [PubMed] [Google Scholar]
- Drexler H, Hornig B (1999). Endothelial dysfunction in human disease. J Mol Cell Cardiol 31: 51–60. [DOI] [PubMed] [Google Scholar]
- Du YH, Guan YY, Alp NJ, Channon KM, Chen AF (2008). Endothelium‐specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt‐sensitive low‐renin hypertension. Circulation 117: 1045–1054. [DOI] [PubMed] [Google Scholar]
- Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW et al. (2014). Flow‐dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest 124: 3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziubla TD, Karim A, Muzykantov VR (2005). Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release 102: 427–439. [DOI] [PubMed] [Google Scholar]
- Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H et al. (2008). Endothelial targeting of semi‐permeable polymer nanocarriers for enzyme therapies. Biomaterials 29: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economides PA, Khaodhiar L, Caselli A, Caballero AE, Keenan H, Bursell SE et al. (2005). The effect of vitamin E on endothelial function of micro‐ and macrocirculation and left ventricular function in type 1 and type 2 diabetic patients. Diabetes 54: 204–211. [DOI] [PubMed] [Google Scholar]
- Egert S, Stehle P (2011). Impact of n‐3 fatty acids on endothelial function: results from human interventions studies. Curr Opin Clin Nutr Metab Care 14: 121–131. [DOI] [PubMed] [Google Scholar]
- Egert S, Baxheinrich A, Lee‐Barkey YH, Tschoepe D, Wahrburg U, Stratmann B (2014). Effects of an energy‐restricted diet rich in plant‐derived alpha‐linolenic acid on systemic inflammation and endothelial function in overweight‐to‐obese patients with metabolic syndrome traits. Br J Nutr 112: 1315–1322. [DOI] [PubMed] [Google Scholar]
- El Assar M, Angulo J, Rodriguez‐Manas L (2013). Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65: 380–401. [DOI] [PubMed] [Google Scholar]
- Ellis GR, Anderson RA, Chirkov YY, Morris‐Thurgood J, Jackson SK, Lewis MJ et al. (2001). Acute effects of vitamin C on platelet responsiveness to nitric oxide donors and endothelial function in patients with chronic heart failure. J Cardiovasc Pharmacol 37: 564–570. [DOI] [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G et al. (2004). Effect of a mediterranean‐style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 292: 1440–1446. [DOI] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas‐Salvado J, Covas MI, Corella D, Aros F et al. (2013). Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368: 1279–1290.23432189 [Google Scholar]
- Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E et al. (2013). Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 166: 199–207 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP (2006). NO‐independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5: 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R (2014). Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66: 1142–1174. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Hernando C, Baldan A (2013). MicroRNAs and Cardiovascular Disease. Curr Genet Med Rep 1: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtlscherer S, Breuer S, Zeiher AM (2004). Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation 110: 1926–1932. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Munzel T (2006). Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714. [DOI] [PubMed] [Google Scholar]
- Fraccarollo D, Widder JD, Galuppo P, Thum T, Tsikas D, Hoffmann M et al. (2008). Improvement in left ventricular remodeling by the endothelial nitric oxide synthase enhancer AVE9488 after experimental myocardial infarction. Circulation 118: 818–827. [DOI] [PubMed] [Google Scholar]
- Frantz S, Adamek A, Fraccarollo D, Tillmanns J, Widder JD, Dienesch C et al. (2009). The eNOS enhancer AVE 9488: a novel cardioprotectant against ischemia reperfusion injury. Basic Res Cardiol 104: 773–779. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Turrens JF, Mirza Z, Crapo JD, Young SL (1985). Modulation of oxidant lung injury by using liposome‐entrapped superoxide dismutase and catalase. Fed Proc 44: 2591–2595. [PubMed] [Google Scholar]
- Frolow M, Drozdz A, Kowalewska A, Nizankowski R, Chlopicki S (2015). Comprehensive assessment of vascular health in patients; towards endothelium‐guided therapy. Pharmacol Rep 67: 786–792. [DOI] [PubMed] [Google Scholar]
- Fujii H, Berliner LJ (2004). Detection of bioradicals by in vivo L‐band electron spin resonance spectrometry. NMR Biomed 17: 311–318. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376. [DOI] [PubMed] [Google Scholar]
- Galie N, Manes A, Branzi A (2001). Medical therapy of pulmonary hypertension. The prostacyclins. Clin Chest Med 22: 529–537, x. [DOI] [PubMed] [Google Scholar]
- Garcia MM, Lima PR, Correia LC (2012). Prognostic value of endothelial function in patients with atherosclerosis: systematic review. Arq Bras Cardiol 99: 857–865. [DOI] [PubMed] [Google Scholar]
- Ghiadoni L, Cupisti A, Huang Y, Mattei P, Cardinal H, Favilla S et al. (2004). Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol 17: 512–519. [PubMed] [Google Scholar]
- Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC et al. (2013a). Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 369: 330–340. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH et al. (2013b). Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 369: 319–329. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA (2002). Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105: 1567–1572. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO et al. (2003). Predictive value of noninvasively determined endothelial dysfunction for long‐term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 41: 1769–1775. [DOI] [PubMed] [Google Scholar]
- Gonzalez MA, Selwyn AP (2003). Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med 115 (Suppl 8A): 99S–106S. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ (2008). The exercise pill–too good to be true? N Engl J Med 359: 1842–1844. [DOI] [PubMed] [Google Scholar]
- Gori T, Parker JD (2004). Long‐term therapy with organic nitrates: the pros and cons of nitric oxide replacement therapy. J Am Coll Cardiol 44: 632–634. [DOI] [PubMed] [Google Scholar]
- Gori T, Munzel T (2011). Oxidative stress and endothelial dysfunction: therapeutic implications. Ann Med 43: 259–272. [DOI] [PubMed] [Google Scholar]
- Gori T, Burstein JM, Ahmed S, Miner SE, Al‐Hesayen A, Kelly S et al. (2001). Folic acid prevents nitroglycerin‐induced nitric oxide synthase dysfunction and nitrate tolerance: a human in vivo study. Circulation 104: 1119–1123. [DOI] [PubMed] [Google Scholar]
- Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M et al. (2008). Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol 51: 1953–1958. [DOI] [PubMed] [Google Scholar]
- Gori T, Muxel S, Damaske A, Radmacher MC, Fasola F, Schaefer S et al. (2012). Endothelial function assessment: flow‐mediated dilation and constriction provide different and complementary information on the presence of coronary artery disease. Eur Heart J 33: 363–371. [DOI] [PubMed] [Google Scholar]
- Granton J, Langleben D, Kutryk MB, Camack N, Galipeau J, Courtman DW et al. (2015). Endothelial NO‐Synthase Gene‐Enhanced Progenitor Cell Therapy for Pulmonary Arterial Hypertension: The PHACeT Trial. Circ Res 117: 645–654. [DOI] [PubMed] [Google Scholar]
- Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE et al. (2015). The role of nitric oxide in passive leg movement‐induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol 593: 3917–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM et al. (2015). A Randomized Trial of Intensive versus Standard Blood‐Pressure Control. N Engl J Med 373: 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH (2009). Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses' health study. Arthritis Rheum 61: 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA et al. (2002). Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658. [DOI] [PubMed] [Google Scholar]
- Han J, Shuvaev VV, Muzykantov VR (2012). Targeted interception of signaling reactive oxygen species in the vascular endothelium. Ther Deliv 3: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel S, Lassig G, Pistrosch F, Passauer J (2003). Endothelial dysfunction in young patients with long‐term rheumatoid arthritis and low disease activity. Atherosclerosis 170: 177–180. [DOI] [PubMed] [Google Scholar]
- Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H (2003). Role of oxidative stress in atherosclerosis. Am J Cardiol 91: 7A–11A. [DOI] [PubMed] [Google Scholar]
- Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR et al. (2011). Inflammation, immunity, and hypertension. Hypertension 57: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H et al. (2005). Acute consumption of flavanol‐rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 46: 1276–1283. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Just H, Munzel T (1996a). Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94: 6–9. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Krohn K, Albers S, Meinertz T (2000a). Tetrahydrobiopterin improves endothelium‐dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 43: 1435–1438. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T (2001). Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Baldus S, von Kodolitsch Y, Rudolph V, Meinertz T (2005). Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol 25: 1174–1179. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Ylä‐Herttuala S, Luoma J, Kurz S, Munzel T, Just H et al. (1996b). Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia: role of oxidized LDL. Circulation 93: 1346–1353. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S et al. (2000b). Tetrahydrobiopterin improves endothelium‐dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 86: E36–E41. [DOI] [PubMed] [Google Scholar]
- Henry RM, Ferreira I, Kostense PJ, Dekker JM, Nijpels G, Heine RJ et al. (2004). Type 2 diabetes is associated with impaired endothelium‐dependent, flow‐mediated dilation, but impaired glucose metabolism is not; The Hoorn Study. Atherosclerosis 174: 49–56. [DOI] [PubMed] [Google Scholar]
- Herrera MD, Mingorance C, Rodriguez‐Rodriguez R, Alvarez de Sotomayor M (2010). Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA et al. (2003). Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600. [DOI] [PubMed] [Google Scholar]
- Hink U, Oelze M, Kolb P, Bachschmid M, Zou MH, Daiber A et al. (2003). Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J Am Coll Cardiol 42: 1826–1834. [DOI] [PubMed] [Google Scholar]
- Hood E, Simone E, Wattamwar P, Dziubla T, Muzykantov V (2011). Nanocarriers for vascular delivery of antioxidants. Nanomedicine (Lond) 6: 1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood ED, Chorny M, Greineder CF, A SA, Levy RJ, Muzykantov VR (2014). Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials 35: 3708–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood ED, Greineder CF, Dodia C, Han J, Mesaros C, Shuvaev VV et al. (2012). Antioxidant protection by PECAM‐targeted delivery of a novel NADPH‐oxidase inhibitor to the endothelium in vitro and in vivo. J Control Release 163: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MD, Greineder CF, Hood ED, Muzykantov VR (2014a). Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK‐134 alleviates acute pulmonary inflammation. J Control Release 177: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MD, Hood ED, Greineder CF, Alferiev IS, Chorny M, Muzykantov V (2014b). Targeting to endothelial cells augments the protective effect of novel dual bioactive antioxidant/anti‐inflammatory nanoparticles. Mol Pharm 11: 2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wu Z, Riwanto M, Gao S, Levison BS, Gu X et al. (2013). Myeloperoxidase, paraoxonase‐1, and HDL form a functional ternary complex. J Clin Invest 123: 3815–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WE, Kruse NT (2016). A ‘passive’ movement into the future of assessing endothelial dysfunction? J Physiol 594: 1525–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyndman KA, Ho DH, Sega MF, Pollock JS (2014). Histone deacetylase 1 reduces NO production in endothelial cells via lysine deacetylation of NO synthase 3. Am J Physiol Heart Circ Physiol 307: H803–H809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ (2007). High‐dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol 103: 1715–1721. [DOI] [PubMed] [Google Scholar]
- Jankovic A, Korac A, Buzadzic B, Otasevic V, Stancic A, Daiber A et al. (2015). Redox implications in adipose tissue (dys)function‐A new look at old acquaintances. Redox Biol 6: 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic A, Korac A, Buzadzic B, Stancic A, Otasevic V, Ferdinandy P et al. (2017). Targeting the NO/superoxide ratio in adipose tissue: relevance to obesity and diabetes management. Br J Pharmacol 174: 1570–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YZ, Jimenez JM, Ou K, McCormick ME, Zhang LD, Davies PF (2014). Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel‐Like Factor 4 promoter in vitro and in vivo. Circ Res 115: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Mather KJ, Wallace JP (2011). Mechanotransduction of shear in the endothelium: basic studies and clinical implications. Vasc Med 16: 365–377. [DOI] [PubMed] [Google Scholar]
- Kahler J, Mendel S, Weckmuller J, Orzechowski HD, Mittmann C, Koster R et al. (2000). Oxidative stress increases synthesis of big endothelin‐1 by activation of the endothelin‐1 promoter. J Mol Cell Cardiol 32: 1429–1437. [DOI] [PubMed] [Google Scholar]
- Kahler J, Ewert A, Weckmuller J, Stobbe S, Mittmann C, Koster R et al. (2001). Oxidative stress increases endothelin‐1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol 38: 49–57. [DOI] [PubMed] [Google Scholar]
- Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL, Bank AJ (2004). Impaired brachial artery endothelium‐dependent and ‐independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol 43: 179–184. [DOI] [PubMed] [Google Scholar]
- Kajikawa M, Nakashima A, Fujimura N, Maruhashi T, Iwamoto Y, Iwamoto A et al. (2015). Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care 38: 119–125. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A et al. (2014). Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta‐analysis. Eur Heart J 35: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A (2014a). eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr Pharm Des 20: 3579–3594. [DOI] [PubMed] [Google Scholar]
- Karbach S, Croxford AL, Oelze M, Schuler R, Minwegen D, Wegner J et al. (2014b). Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis‐like skin disease. Arterioscler Thromb Vasc Biol 34: 2658–2668. [DOI] [PubMed] [Google Scholar]
- Katsume A, Okigaki M, Matsui A, Che J, Adachi Y, Kishita E et al. (2011). Early inflammatory reactions in atherosclerosis are induced by proline‐rich tyrosine kinase/reactive oxygen species‐mediated release of tumor necrosis factor‐alpha and subsequent activation of the p21Cip1/Ets‐1/p300 system. Arterioscler Thromb Vasc Biol 31: 1084–1092. [DOI] [PubMed] [Google Scholar]
- Katz DL, Meller S (2014). Can we say what diet is best for health? Annu Rev Public Health 35: 83–103. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Bingham S, Welch A, Luben R, Wareham N, Oakes S et al. (2001). Relation between plasma ascorbic acid and mortality in men and women in EPIC‐Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 357: 657–663. [DOI] [PubMed] [Google Scholar]
- Kim GH, Ryan JJ, Archer SL (2013). The role of redox signaling in epigenetics and cardiovascular disease. Antioxid Redox Signal 18: 1920–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschyov AL, Sen VD (2013). Heparin‐polynitroxide derivatives: a platform for new diagnostic and therapeutic agents in cardiovascular disease? Future Med Chem 5: 385–388. [DOI] [PubMed] [Google Scholar]
- Kleschyov AL, Sen V, Golubev V, Munnemann K, Hinderberger D, Lackner KJ et al. (2012). Heparin‐polynitroxides: synthesis and preliminary evaluation as cardiovascular EPR/MR imaging probes and extracellular space‐targeted antioxidants. Eur J Med Chem 58: 265–271. [DOI] [PubMed] [Google Scholar]
- Klonizakis M, Alkhatib A, Middleton G (2014). Long‐term effects of an exercise and Mediterranean diet intervention in the vascular function of an older, healthy population. Microvasc Res 95: 103–107. [DOI] [PubMed] [Google Scholar]
- Knorr M, Munzel T, Wenzel P (2014). Interplay of NK cells and monocytes in vascular inflammation and myocardial infarction. Front Physiol 5: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontush A (2014). HDL‐mediated mechanisms of protection in cardiovascular disease. Cardiovasc Res 103: 341–349. [DOI] [PubMed] [Google Scholar]
- Korkmaz S, Radovits T, Barnucz E, Neugebauer P, Arif R, Hirschberg K et al. (2009a). Dose‐dependent effects of a selective phosphodiesterase‐5‐inhibitor on endothelial dysfunction induced by peroxynitrite in rat aorta. Eur J Pharmacol 615: 155–162. [DOI] [PubMed] [Google Scholar]
- Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S et al. (2009b). Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation 120: 677–686. [DOI] [PubMed] [Google Scholar]
- Korkmaz S, Loganathan S, Mikles B, Radovits T, Barnucz E, Hirschberg K et al. (2013). Nitric oxide‐ and heme‐independent activation of soluble guanylate cyclase attenuates peroxynitrite‐induced endothelial dysfunction in rat aorta. J Cardiovasc Pharmacol Ther 18: 70–77. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Csaszar A, Toth J, Siller G, Farkas A, Tarjan J et al. (2008). Correlation between flow‐mediated dilation and erectile dysfunction. J Cardiovasc Pharmacol 51: 148–153. [DOI] [PubMed] [Google Scholar]
- Kozower BD, Christofidou‐Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC et al. (2003). Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol 21: 392–398. [DOI] [PubMed] [Google Scholar]
- Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG et al. (2012). Vascular responses to long‐ and short‐term exposure to fine particulate matter: MESA Air (Multi‐Ethnic Study of Atherosclerosis and Air Pollution). J Am Coll Cardiol 60: 2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE et al. (2005). Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kumar S, Vikram A, Hoffman TA, Naqvi A, Lewarchik CM et al. (2013). Histone and DNA methylation‐mediated epigenetic downregulation of endothelial Kruppel‐like factor 2 by low‐density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol 33: 1936–1942. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart‐Hines Z, Meziane H, Lerin C, Daussin F et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC‐1alpha. Cell 127: 1109–1122. [DOI] [PubMed] [Google Scholar]
- Landberg R, Naidoo N, van Dam RM (2012). Diet and endothelial function: from individual components to dietary patterns. Curr Opin Lipidol 23: 147–155. [DOI] [PubMed] [Google Scholar]
- Lapp H, Mitrovic V, Franz N, Heuer H, Buerke M, Wolfertz J et al. (2009). Cinaciguat (BAY 58‐2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation 119: 2781–2788. [DOI] [PubMed] [Google Scholar]
- Lau D, Baldus S (2006). Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther 111: 16–26. [DOI] [PubMed] [Google Scholar]
- Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M et al. (2005). Physical Inactivity Increases Oxidative Stress, Endothelial Dysfunction, and Atherosclerosis. Arterioscler Thromb Vasc Biol 25: 809–814. [DOI] [PubMed] [Google Scholar]
- Lee DH, Folsom AR, Harnack L, Halliwell B, Jacobs DR Jr (2004). Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr 80: 1194–1200. [DOI] [PubMed] [Google Scholar]
- Lehmann ED, Parker JR, Hopkins KD, Taylor MG, Gosling RG (1993). Validation and reproducibility of pressure‐corrected aortic distensibility measurements using pulse‐wave‐velocity Doppler ultrasound. J Biomed Eng 15: 221–228. [DOI] [PubMed] [Google Scholar]
- Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J et al. (2011). Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil 18: 775–789. [DOI] [PubMed] [Google Scholar]
- Leskinen T, Usenius JP, Alen M, Kainulainen H, Kaprio J, Kujala UM (2011). Leisure‐time physical activity and artery lumen diameters: a monozygotic co‐twin control study. Scand J Med Sci Sports 21: e208–e214. [DOI] [PubMed] [Google Scholar]
- Ley SH, Hamdy O, Mohan V, Hu FB (2014). Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 383: 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Laher I (2015). Exercise Pills: At the Starting Line. Trends Pharmacol Sci 36: 906–917. [DOI] [PubMed] [Google Scholar]
- Libby P (2002). Inflammation in atherosclerosis. Nature 420: 868–874. [DOI] [PubMed] [Google Scholar]
- Libby P, Aikawa M, Jain MK (2006). Vascular endothelium and atherosclerosis. Handb Exp Pharmacol : 285–306. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H et al. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Montagnana M, Favaloro EJ, Franchini M (2009). Mental depression and cardiovascular disease: a multifaceted, bidirectional association. Semin Thromb Hemost 35: 325–336. [DOI] [PubMed] [Google Scholar]
- Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE et al. (2014). Inhibition of microRNA‐92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res 114: 434–443. [DOI] [PubMed] [Google Scholar]
- Lucas DL, Brown RA, Wassef M, Giles TD (2005). Alcohol and the cardiovascular system: research challenges and opportunities. J Am Coll Cardiol 45: 1916–1924. [DOI] [PubMed] [Google Scholar]
- Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW et al. (1986). Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315: 1046–1051. [DOI] [PubMed] [Google Scholar]
- Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra‐Ferraris G et al. (2011). miR‐200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ 18: 1628–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni AA, Sherwood RA, Swift CG, Jackson SH (2002). Folic acid enhances endothelial function and reduces blood pressure in smokers: a randomized controlled trial. J Intern Med 252: 497–503. [DOI] [PubMed] [Google Scholar]
- Mangoni AA, Sherwood RA, Asonganyi B, Swift CG, Thomas S, Jackson SH (2005). Short‐term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens 18: 220–226. [DOI] [PubMed] [Google Scholar]
- Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y et al. (2013). Relationship between flow‐mediated vasodilation and cardiovascular risk factors in a large community‐based study. Heart 99: 1837–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Harrison DG (2012a). Stress‐dependent hypertension and the role of T lymphocytes. Exp Physiol 97: 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ et al. (2012b). T lymphocytes and vascular inflammation contribute to stress‐dependent hypertension. Biol Psychiatry 71: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K et al. (2013). Peripheral endothelial function and cardiovascular events in high‐risk patients. J Am Heart Assoc 2: e000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB et al. (2007). SIRT1 promotes endothelium‐dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 104: 14855–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM (2010). Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 31: 1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini R, Stohr R, Federici M (2014). MicroRNAs in vascular aging and atherosclerosis. Ageing Res Rev 17: 68–78. [DOI] [PubMed] [Google Scholar]
- Merino J, Ferre R, Girona J, Aguas D, Cabre A, Plana N et al. (2015). Physical activity below the minimum international recommendations improves oxidative stress, ADMA levels, resting heart rate and small artery endothelial function. Clin Investig Arterioscler 27: 9–16. [DOI] [PubMed] [Google Scholar]
- Meuwese MC, Mooij HL, Nieuwdorp M, van Lith B, Marck R, Vink H et al. (2009). Partial recovery of the endothelial glycocalyx upon rosuvastatin therapy in patients with heterozygous familial hypercholesterolemia. J Lipid Res 50: 148–153. [DOI] [PubMed] [Google Scholar]
- Mikhed Y, Daiber A, Steven S (2015a). Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age‐Related Vascular Dysfunction. Int J Mol Sci 16: 15918–15953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhed Y, Gorlach A, Knaus UG, Daiber A (2015b). Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol 5: 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FJ Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL (1998). Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 82: 1298–1305. [DOI] [PubMed] [Google Scholar]
- Mitchell GF (2015). Arterial stiffness: insights from Framingham and Iceland. Curr Opin Nephrol Hypertens 24: 1–7. [DOI] [PubMed] [Google Scholar]
- Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G et al. (2008). Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation 117: 2626–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM (1999). Endothelial dysfunction: from physiology to therapy. J Mol Cell Cardiol 31: 61–74. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs EA (2006). Nitric oxide and the vascular endothelium. Handb Exp Pharmacol (176 Pt 1): 213–254. [DOI] [PubMed] [Google Scholar]
- Montero D, Padilla J, Diaz‐Canestro C, Muris DM, Pyke KE, Obert P et al. (2014). Flow‐mediated dilation in athletes: influence of aging. Med Sci Sports Exerc 46: 2148–2158. [DOI] [PubMed] [Google Scholar]
- Moreira DM, Lueneberg ME, da Silva RL, Fattah T, Mascia Gottschall CA (2013). Rationale and design of the TETHYS trial: the effects of methotrexate therapy on myocardial infarction with ST‐segment elevation. Cardiology 126: 167–170. [DOI] [PubMed] [Google Scholar]
- Munzel T (2008). Endothelial dysfunction: pathophysiology, diagnosis and prognosis. Dtsch Med Wochenschr 133: 2465–2470. [DOI] [PubMed] [Google Scholar]
- Munzel T, Daiber A, Gori T (2011). Nitrate therapy: new aspects concerning molecular action and tolerance. Circulation 123: 2132–2144. [DOI] [PubMed] [Google Scholar]
- Munzel T, Daiber A, Gori T (2013). More answers to the still unresolved question of nitrate tolerance. Eur Heart J 34: 2666–2673. [DOI] [PubMed] [Google Scholar]
- Munzel T, Hink U, Heitzer T, Meinertz T (1999). Role for NADPH/NADH oxidase in the modulation of vascular tone. Ann N Y Acad Sci 874: 386–400. [DOI] [PubMed] [Google Scholar]
- Munzel T, Gori T, Babisch W, Basner M (2014a). Cardiovascular effects of environmental noise exposure. Eur Heart J 35: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Sinning C, Post F, Warnholtz A, Schulz E (2008). Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med 40: 180–196. [DOI] [PubMed] [Google Scholar]
- Munzel T, Meinertz T, Tebbe U, Schneider HT, Stalleicken D, Wargenau M et al. (2014b). Efficacy of the long‐acting nitro vasodilator pentaerithrityl tetranitrate in patients with chronic stable angina pectoris receiving anti‐anginal background therapy with beta‐blockers: a 12‐week, randomized, double‐blind, placebo‐controlled trial. Eur Heart J 35: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaca G, Colombo BM, Cagnati P, Gulli R, Spano F, Puppo F (2012). Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 224: 309–317. [DOI] [PubMed] [Google Scholar]
- Muro S, Cui X, Gajewski C, Murciano JC, Muzykantov VR, Koval M (2003). Slow intracellular trafficking of catalase nanoparticles targeted to ICAM‐1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol 285: C1339–C1347. [DOI] [PubMed] [Google Scholar]
- Muro S, Mateescu M, Gajewski C, Robinson M, Muzykantov VR, Koval M (2006). Control of intracellular trafficking of ICAM‐1‐targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am J Physiol Lung Cell Mol Physiol 290: L809–L817. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C et al. (2012). GBD 2010: design, definitions, and metrics. Lancet 380: 2063–2066. [DOI] [PubMed] [Google Scholar]
- Muzykantov VR, Sakharov DV, Domogatsky SP, Goncharov NV, Danilov SM (1987). Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am J Pathol 128: 276–285. [PMC free article] [PubMed] [Google Scholar]
- Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB (1996). Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci U S A 93: 5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanayakkara PW, van Guldener C, ter Wee PM, Scheffer PG, van Ittersum FJ, Twisk JW et al. (2007). Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on carotid intima‐media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: results from the Anti‐Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Arch Intern Med 167: 1262–1270. [DOI] [PubMed] [Google Scholar]
- Nelson MR, Stepanek J, Cevette M, Covalciuc M, Hurst RT, Tajik AJ (2010). Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clin Proc 85: 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunteufl T, Priglinger U, Heher S, Zehetgruber M, Soregi G, Lehr S et al. (2000). Effects of vitamin E on chronic and acute endothelial dysfunction in smokers. J Am Coll Cardiol 35: 277–283. [DOI] [PubMed] [Google Scholar]
- Nieuwdorp M, Meuwese MC, Mooij HL, Ince C, Broekhuizen LN, Kastelein JJ et al. (2008). Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J Appl Physiol 104: 845–852. [DOI] [PubMed] [Google Scholar]
- Nowak K, Hanusch C, Nicksch K, Metzger RP, Beck G, Gebhard MM et al. (2010). Pre‐ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin‐converting‐enzyme antibodies. Eur J Cardiothorac Surg 37: 859–863. [DOI] [PubMed] [Google Scholar]
- Okui H, Hamasaki S, Ishida S, Kataoka T, Orihara K, Fukudome T et al. (2008). Adiponectin is a better predictor of endothelial function of the coronary artery than HOMA‐R, body mass index, immunoreactive insulin, or triglycerides. Int J Cardiol 126: 53–61. [DOI] [PubMed] [Google Scholar]
- Pandey D, Sikka G, Bergman Y, Kim JH, Ryoo S, Romer L et al. (2014). Transcriptional regulation of endothelial arginase 2 by histone deacetylase 2. Arterioscler Thromb Vasc Biol 34: 1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE (1990). Abnormal endothelium‐dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22–27. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Khan‐Merchant N, Penumetcha M, Khan BV, Santanam N (2001). Did the antioxidant trials fail to validate the oxidation hypothesis? Curr Atheroscler Rep 3: 392–398. [DOI] [PubMed] [Google Scholar]
- Patel RS, Ghasemzadeh N, Eapen DJ, Sher S, Arshad S, Ko YA et al. (2016). Novel Biomarker of Oxidative Stress Is Associated With Risk of Death in Patients With Coronary Artery Disease. Circulation 133: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauriah M, Khan F, Lim TK, Elder DH, Godfrey V, Kennedy G et al. (2012). B‐type natriuretic peptide is an independent predictor of endothelial function in man. Clin Sci (Lond) 123: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena AS, Wiltshire E, Gent R, Hirte C, Couper J (2004). Folic acid improves endothelial function in children and adolescents with type 1 diabetes. J Pediatr 144: 500–504. [DOI] [PubMed] [Google Scholar]
- Perng JK, Lee S, Kundu K, Caskey CF, Knight SF, Satir S et al. (2012). Ultrasound imaging of oxidative stress in vivo with chemically‐generated gas microbubbles. Ann Biomed Eng 40: 2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A et al. (2001). Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196. [DOI] [PubMed] [Google Scholar]
- Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK et al. (2010). EULAR evidence‐based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 69: 325–331. [DOI] [PubMed] [Google Scholar]
- Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S et al. (2007). Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens 20: 392–397. [DOI] [PubMed] [Google Scholar]
- Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA (2002). Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation 106: 184–190. [DOI] [PubMed] [Google Scholar]
- Preissler G, Loehe F, Huff IV, Ebersberger U, Shuvaev VV, Bittmann I et al. (2011). Targeted Endothelial Delivery of Nanosized Catalase Immunoconjugates Protects Lung Grafts Donated After Cardiac Death. Transplantation 92: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Kuebler WM (2006). Normal endothelium. Handb Exp Pharmacol (176 Pt 1): 1–40. [DOI] [PubMed] [Google Scholar]
- Raaschou‐Nielsen O, Andersen ZJ, Jensen SS, Ketzel M, Sorensen M, Hansen J et al. (2012). Traffic air pollution and mortality from cardiovascular disease and all causes: a Danish cohort study. Environ Health 11: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher KA, Beghein N, Boutry S, Laurent S, Vander Elst L, Muller RN et al. (2009). In vivo detection of inflammation using pegylated iron oxide particles targeted at E‐selectin: a multimodal approach using MR imaging and EPR spectroscopy. Invest Radiol 44: 398–404. [DOI] [PubMed] [Google Scholar]
- Radovits T, Lin LN, Zotkina J, Gero D, Szabo C, Karck M et al. (2007a). Poly(ADP‐ribose) polymerase inhibition improves endothelial dysfunction induced by reactive oxidant hydrogen peroxide in vitro. Eur J Pharmacol 564: 158–166. [DOI] [PubMed] [Google Scholar]
- Radovits T, Seres L, Gero D, Berger I, Szabo C, Karck M et al. (2007b). Single dose treatment with PARP‐inhibitor INO‐1001 improves aging‐associated cardiac and vascular dysfunction. Exp Gerontol 42: 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovits T, Korkmaz S, Miesel‐Groschel C, Seidel B, Stasch JP, Merkely B et al. (2011). Pre‐conditioning with the soluble guanylate cyclase activator Cinaciguat reduces ischaemia‐reperfusion injury after cardiopulmonary bypass. Eur J Cardiothorac Surg 39: 248–255. [DOI] [PubMed] [Google Scholar]
- Radovits T, Arif R, Bomicke T, Korkmaz S, Barnucz E, Karck M et al. (2013). Vascular dysfunction induced by hypochlorite is improved by the selective phosphodiesterase‐5‐inhibitor vardenafil. Eur J Pharmacol 710: 110–119. [DOI] [PubMed] [Google Scholar]
- Radovits T, Zotkina J, Lin LN, Bomicke T, Arif R, Gero D et al. (2007c). Poly(ADP‐Ribose) polymerase inhibition improves endothelial dysfunction induced by hypochlorite. Exp Biol Med 232: 1204–1212. [DOI] [PubMed] [Google Scholar]
- Radovits T, Bomicke T, Kokeny G, Arif R, Loganathan S, Kecsan K et al. (2009). The phosphodiesterase‐5 inhibitor vardenafil improves cardiovascular dysfunction in experimental diabetes mellitus. Br J Pharmacol 156: 909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammos C, Hendgen‐Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T (2014). Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol 63: 1584–1585. [DOI] [PubMed] [Google Scholar]
- Ramsey M, Goodfellow J, Bellamy M, Lewis M, Henderson A (1996). Non‐invasive detection of endothelial dysfunction. Lancet 348: 128–129. [DOI] [PubMed] [Google Scholar]
- Rao X, Zhong J, Zhang S, Zhang Y, Yu Q, Yang P et al. (2011). Loss of methyl‐CpG‐binding domain protein 2 enhances endothelial angiogenesis and protects mice against hind‐limb ischemic injury. Circulation 123: 2964–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ras RT, Streppel MT, Draijer R, Zock PL (2013). Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol 168: 344–351. [DOI] [PubMed] [Google Scholar]
- Rassaf T, Ferdinandy P, Schulz R (2014). Nitrite in organ protection. Br J Pharmacol 171: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassaf T, Rammos C, Hendgen‐Cotta UB, Heiss C, Kleophas W, Dellanna F et al. (2016). Vasculoprotective Effects of Dietary Cocoa Flavanols in Patients on Hemodialysis: A Double‐Blind, Randomized, Placebo‐Controlled Trial. Clin J Am Soc Nephrol 11: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reference Values for Arterial Stiffness' Collaboration (2010). Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 31: 2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner JG, Popylisen S, Bauer TA, Lindenfeld J, Gill E, Smith S et al. (2003). Oral L‐arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vasc Med 8: 169–175. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, Libby P (2011). Interleukin‐1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti‐inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 162: 597–605. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH et al. (2005). C‐reactive protein levels and outcomes after statin therapy. N Engl J Med 352: 20–28. [DOI] [PubMed] [Google Scholar]
- Risbano MG, Gladwin MT (2013). Therapeutics targeting of dysregulated redox equilibrium and endothelial dysfunction. Handb Exp Pharmacol 218: 315–349. [DOI] [PubMed] [Google Scholar]
- Ritchie RH, Irvine JC, Rosenkranz AC, Patel R, Wendt IR, Horowitz JD et al. (2009). Exploiting cGMP‐based therapies for the prevention of left ventricular hypertrophy: NO* and beyond. Pharmacol Ther 124: 279–300. [DOI] [PubMed] [Google Scholar]
- Riwanto M, Landmesser U (2013). High‐density lipoproteins and Endothelial Functions: Mechanistic Insights and Alterations in Cardiovascular Disease. J Lipid Res 54: 3227–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D et al. (2012). Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA 308: 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MD, Malone E, Florida‐James G (2015). Vascular Ageing and Exercise: Focus on Cellular Reparative Processes. Oxid Med Cell Longev 2016: 3583956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossig L, Li H, Fisslthaler B, Urbich C, Fleming I, Forstermann U et al. (2002). Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ Res 91: 837–844. [DOI] [PubMed] [Google Scholar]
- Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G et al. (2008). Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res 102: 923–932. [DOI] [PubMed] [Google Scholar]
- Sakharov DV, Muzykantov VR, Domogatsky SP, Danilov SM (1987). Protection of cultured endothelial cells from hydrogen peroxide‐induced injury by antibody‐conjugated catalase. Biochim Biophys Acta 930: 140–144. [DOI] [PubMed] [Google Scholar]
- Salonen RM, Nyyssonen K, Kaikkonen J, Porkkala‐Sarataho E, Voutilainen S, Rissanen TH et al. (2003). Six‐year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation 107: 947–953. [DOI] [PubMed] [Google Scholar]
- Santi D, Giannetta E, Isidori AM, Vitale C, Aversa A, Simoni M (2015). Therapy of endocrine disease. Effects of chronic use of phosphodiesterase inhibitors on endothelial markers in type 2 diabetes mellitus: a meta‐analysis. Eur J Endocrinol 172: R103–R114. [DOI] [PubMed] [Google Scholar]
- Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T et al. (2013). Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol 61: 1736–1743. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM (2000). Prognostic impact of coronary vasodilator dysfunction on adverse long‐term outcome of coronary heart disease. Circulation 101: 1899–1906. [DOI] [PubMed] [Google Scholar]
- Schleithoff C, Voelter‐Mahlknecht S, Dahmke IN, Mahlknecht U (2012). On the epigenetics of vascular regulation and disease. Clin Epigenetics 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M et al. (2015a). Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol 104: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FP, Basner M, Kroger G, Weck S, Schnorbus B, Muttray A et al. (2013). Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J 34: 3508–3514a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HH, Stocker R, Vollbracht C, Paulsen G, Riley DP, Daiber A et al. (2015b). Antioxidants in Translational Medicine. Antioxid Redox Signal 23: 1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M et al. (2011). Noninvasive vascular function measurement in the community: cross‐sectional relations and comparison of methods. Circ Cardiovasc Imaging 4: 371–380. [DOI] [PubMed] [Google Scholar]
- Schnabel RB, Wild PS, Schulz A, Zeller T, Sinning CR, Wilde S et al. (2012). Multiple endothelial biomarkers and noninvasive vascular function in the general population: the Gutenberg Health Study. Hypertension 60: 288–295. [DOI] [PubMed] [Google Scholar]
- Schober A, Nazari‐Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J et al. (2014). MicroRNA‐126‐5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20: 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz E, Wenzel P, Munzel T, Daiber A (2014). Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal 20: 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz E, Tsilimingas N, Rinze R, Reiter B, Wendt M, Oelze M et al. (2002). Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation 105: 1170–1175. [DOI] [PubMed] [Google Scholar]
- Selivanova O, Heinrich UR, Brieger J, Feltens R, Mann W (2007). Fast alterations of vascular endothelial growth factor (VEGF) expression and that of its receptors (Flt‐1, Flk‐1 and Neuropilin) in the cochlea of guinea pigs after moderate noise exposure. Eur Arch Otorhinolaryngol 264: 121–128. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J et al. (2008). Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 300: 2123–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharina IG, Sobolevsky M, Papakyriakou A, Rukoyatkina N, Spyroulias GA, Gambaryan S et al. (2015). The fibrate gemfibrozil is a NO‐ and haem‐independent activator of soluble guanylyl cyclase: in vitro studies. Br J Pharmacol 172: 2316–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA (2011). GLP‐1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One 6: e25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter M, Shechter A, Koren‐Morag N, Feinberg MS, Hiersch L (2014). Usefulness of brachial artery flow‐mediated dilation to predict long‐term cardiovascular events in subjects without heart disease. Am J Cardiol 113: 162–167. [DOI] [PubMed] [Google Scholar]
- Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR (2007). Platelet‐endothelial cell adhesion molecule‐1‐directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther 323: 450–457. [DOI] [PubMed] [Google Scholar]
- Shuvaev VV, Tliba S, Pick J, Arguiri E, Christofidou‐Solomidou M, Albelda SM et al. (2011a). Modulation of endothelial targeting by size of antibody‐antioxidant enzyme conjugates. J Control Release 149: 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M et al. (2011b). PECAM‐targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J 25: 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev VV, Christofidou‐Solomidou M, Bhora F, Laude K, Cai H, Dikalov S et al. (2009). Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther 331: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev VV, Han J, Tliba S, Arguiri E, Christofidou‐Solomidou M, Ramirez SH et al. (2013). Anti‐inflammatory effect of targeted delivery of SOD to endothelium: mechanism, synergism with NO donors and protective effects in vitro and in vivo. PLoS One 8: e77002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone E, Ding BS, Muzykantov V (2009a). Targeted delivery of therapeutics to endothelium. Cell Tissue Res 335: 283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone EA, Dziubla TD, Discher DE, Muzykantov VR (2009b). Filamentous Polymer Nanocarriers of Tunable Stiffness that Encapsulate the Therapeutic Enzyme Catalase. Biomacromolecules 10: 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone EA, Dziubla TD, Colon‐Gonzalez F, Discher DE, Muzykantov VR (2007). Effect of polymer amphiphilicity on loading of a therapeutic enzyme into protective filamentous and spherical polymer nanocarriers. Biomacromolecules 8: 3914–3921. [DOI] [PubMed] [Google Scholar]
- Simone EA, Dziubla TD, Arguiri E, Vardon V, Shuvaev VV, Christofidou‐Solomidou M et al. (2009c). Loading PEG‐catalase into filamentous and spherical polymer nanocarriers. Pharm Res 26: 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinoway LI, Musch TI, Minotti JR, Zelis R (1986). Enhanced maximal metabolic vasodilatation in the dominant forearms of tennis players. J Appl Physiol 61: 673–678. [DOI] [PubMed] [Google Scholar]
- Siuda D, Zechner U, El Hajj N, Prawitt D, Langer D, Xia N et al. (2012). Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res Cardiol 107: 283. [DOI] [PubMed] [Google Scholar]
- Skyrme‐Jones RA, O'Brien RC, Berry KL, Meredith IT (2000). Vitamin E supplementation improves endothelial function in type I diabetes mellitus: a randomized, placebo‐controlled study. J Am Coll Cardiol 36: 94–102. [DOI] [PubMed] [Google Scholar]
- Small EM, Olson EN (2011). Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodergren A, Karp K, Boman K, Eriksson C, Lundstrom E, Smedby T et al. (2010). Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther 12: R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz P, Kerekes G, Der H, Szucs G, Szanto S, Kiss E et al. (2011). Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev 10: 416–425. [DOI] [PubMed] [Google Scholar]
- Solzbach U, Hornig B, Jeserich M, Just H (1997). Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation 96: 1513–1519. [DOI] [PubMed] [Google Scholar]
- Sorensen M, Hvidberg M, Andersen ZJ, Nordsborg RB, Lillelund KG, Jakobsen J et al. (2011). Road traffic noise and stroke: a prospective cohort study. Eur Heart J 32: 737–744. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker LE, Flammer AJ, Luscher TF (2006). The vascular endothelium in hypertension. Handb Exp Pharmacol (176 Pt 2): 249–283. [DOI] [PubMed] [Google Scholar]
- Spieker LE, Sudano I, Hurlimann D, Lerch PG, Lang MG, Binggeli C et al. (2002). High‐density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation 105: 1399–1402. [DOI] [PubMed] [Google Scholar]
- Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H SA, Meurer S et al. (2006). Targeting the heme‐oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven S, Munzel T, Daiber A (2015). Exploiting the Pleiotropic Antioxidant Effects of Established Drugs in Cardiovascular Disease. Int J Mol Sci 16: 18185–18223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suessenbacher A, Dorler J, Wunder J, Hohenwarter F, Alber HF, Pachinger O et al. (2013). Comparison of brachial artery wall thickness versus endothelial function to predict late cardiovascular events in patients undergoing elective coronary angiography. Am J Cardiol 111: 671–675. [DOI] [PubMed] [Google Scholar]
- Sun X, He S, Wara AK, Icli B, Shvartz E, Tesmenitsky Y et al. (2014). Systemic delivery of microRNA‐181b inhibits nuclear factor‐kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E‐deficient mice. Circ Res 114: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A (2000). Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954. [DOI] [PubMed] [Google Scholar]
- Sweitzer TD, Thomas AP, Wiewrodt R, Nakada MT, Branco F, Muzykantov VR (2003). PECAM‐directed immunotargeting of catalase: specific, rapid and transient protection against hydrogen peroxide. Free Radic Biol Med 34: 1035–1046. [DOI] [PubMed] [Google Scholar]
- Sydow K, Munzel T (2003). ADMA and oxidative stress. Atheroscler Suppl 4: 41–51. [DOI] [PubMed] [Google Scholar]
- Szabo C (2009). Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol 156: 713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Radovits T, Veres G, Krieger N, Loganathan S, Sandner P et al. (2009). Vardenafil protects against myocardial and endothelial injuries after cardiopulmonary bypass. Eur J Cardiothorac Surg 36: 657–664. [DOI] [PubMed] [Google Scholar]
- Szabo G, Seres L, Soos P, Gorenflo M, Merkely B, Horkay F et al. (2011). Tetrahydrobiopterin improves cardiac and pulmonary function after cardiopulmonary bypass. Eur J Cardiothorac Surg 40: 695–700. [DOI] [PubMed] [Google Scholar]
- Takase B, Etsuda H, Matsushima Y, Ayaori M, Kusano H, Hamabe A et al. (2004). Effect of chronic oral supplementation with vitamins on the endothelial function in chronic smokers. Angiology 55: 653–660. [DOI] [PubMed] [Google Scholar]
- Tanahashi K, Akazawa N, Miyaki A, Choi Y, Ra SG, Matsubara T et al. (2014). Aerobic exercise training decreases plasma asymmetric dimethylarginine concentrations with increase in arterial compliance in postmenopausal women. Am J Hypertens 27: 415–421. [DOI] [PubMed] [Google Scholar]
- Tarbell JM, Cancel LM (2016). The glycocalyx and its significance in human medicine. J Intern Med. doi: 10.1111/joim.12465. [DOI] [PubMed] [Google Scholar]
- Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Lerman A (2003). Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation 107: 2805–2809. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Giannopoulos D, Dalais FS, Hodgson J, McGrath BP (2006). Randomised, controlled, cross‐over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J Am Coll Nutr 25: 533–540. [DOI] [PubMed] [Google Scholar]
- Thorin E, Clozel M (2010). The cardiovascular physiology and pharmacology of endothelin‐1. Adv Pharmacol 60: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W (2000). Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation 102: 2172–2179. [DOI] [PubMed] [Google Scholar]
- Tome‐Carneiro J, Larrosa M, Gonzalez‐Sarrias A, Tomas‐Barberan FA, Garcia‐Conesa MT, Espin JC (2013). Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des 19: 6064–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D, Xenakis C, Tentolouris C, Davies G, Antoniades C, Crake T et al. (2005). Effects of vitamin C on intracoronary L‐arginine dependent coronary vasodilatation in patients with stable angina. Heart 91: 1319–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui JM, Xie F, Porter RT (2004). The use of microbubbles to target drug delivery. Cardiovasc Ultrasound 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Dimmeler S (2015). Long noncoding RNAs in cardiovascular diseases. Circ Res 116: 737–750. [DOI] [PubMed] [Google Scholar]
- van Bussel BC, Henry RM, Ferreira I, van Greevenbroek MM, van der Kallen CJ, Twisk JW et al. (2015). A healthy diet is associated with less endothelial dysfunction and less low‐grade inflammation over a 7‐year period in adults at risk of cardiovascular disease. J Nutr 145: 532–540. [DOI] [PubMed] [Google Scholar]
- van den Berg BM, Nieuwdorp M, Stroes ES, Vink H (2006). Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep 58 (Suppl): 75–80. [PubMed] [Google Scholar]
- van Rooij E, Olson EN (2012). MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 11: 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa‐Nicotera M, Chen H, Tucci P, Yang AL, Saintigny G, Menghini R et al. (2011). miR‐146a is modulated in human endothelial cell with aging. Atherosclerosis 217: 326–330. [DOI] [PubMed] [Google Scholar]
- Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A et al. (2016). Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double‐blind, placebo‐controlled study. Am J Clin Nutr 103: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vena GA, Vestita M, Cassano N (2010). Psoriasis and cardiovascular disease. Dermatol Ther 23: 144–151. [DOI] [PubMed] [Google Scholar]
- Verma S, Wang CH, Lonn E, Charbonneau F, Buithieu J, Title LM et al. (2004). Cross‐sectional evaluation of brachial artery flow‐mediated vasodilation and C‐reactive protein in healthy individuals. Eur Heart J 25: 1754–1760. [DOI] [PubMed] [Google Scholar]
- Vienneau D, Schindler C, Perez L, Probst‐Hensch N, Roosli M (2015). The relationship between transportation noise exposure and ischemic heart disease: a meta‐analysis. Environ Res 138: 372–380. [DOI] [PubMed] [Google Scholar]
- Villanueva FS, Klibanov A, Wagner WR (2002). Microbubble‐endothelial cell interactions as a basis for assessing endothelial function. Echocardiography 19: 427–438. [DOI] [PubMed] [Google Scholar]
- Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC et al. (1990). Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation 81: 491–497. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos CV, Terentes‐Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI (2013). Prediction of cardiovascular events and all‐cause mortality with erectile dysfunction: a systematic review and meta‐analysis of cohort studies. Circ Cardiovasc Qual Outcomes 6: 99–109. [DOI] [PubMed] [Google Scholar]
- Vogel RA, Corretti MC, Plotnick GD (1997). Effect of a single high‐fat meal on endothelial function in healthy subjects. Am J Cardiol 79: 350–354. [DOI] [PubMed] [Google Scholar]
- Waller K, Kaprio J, Lehtovirta M, Silventoinen K, Koskenvuo M, Kujala UM (2010). Leisure‐time physical activity and type 2 diabetes during a 28 year follow‐up in twins. Diabetologia 53: 2531–2537. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J et al. (2012). Effect of omega‐3 fatty acids supplementation on endothelial function: a meta‐analysis of randomized controlled trials. Atherosclerosis 221: 536–543. [DOI] [PubMed] [Google Scholar]
- Warnholtz A, Munzel T (2000). Why do antioxidants fail to provide clinical benefit? Curr Control Trials Cardiovasc Med 1: 38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnholtz A, Mollnau H, Heitzer T, Kontush A, Moller‐Bertram T, Lavall D et al. (2002). Adverse effects of nitroglycerin treatment on endothelial function, vascular nitrotyrosine levels and cGMP‐dependent protein kinase activity in hyperlipidemic Watanabe rabbits. J Am Coll Cardiol 40: 1356–1363. [DOI] [PubMed] [Google Scholar]
- Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL (2001). Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 280: E685–E694. [DOI] [PubMed] [Google Scholar]
- Wei Y, Nazari‐Jahantigh M, Neth P, Weber C, Schober A (2013). MicroRNA‐126, −145, and −155: a therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol 33: 449–454. [DOI] [PubMed] [Google Scholar]
- Wentland AL, Grist TM, Wieben O (2014). Review of MRI‐based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc Diagn Ther 4: 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S et al. (2011). Lysozyme M‐positive monocytes mediate angiotensin II‐induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381. [DOI] [PubMed] [Google Scholar]
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A et al. (2005). Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007. [DOI] [PubMed] [Google Scholar]
- Wethal T, Nedregaard B, Andersen R, Fossa A, Lund MB, Gunther A et al. (2014). Atherosclerotic lesions in lymphoma survivors treated with radiotherapy. Radiother Oncol 110: 448–454. [DOI] [PubMed] [Google Scholar]
- Willerson JT, Golino P, Eidt J, Campbell WB, Buja LM (1989). Specific platelet mediators and unstable coronary artery lesions. Experimental evidence and potential clinical implications. Circulation 80: 198–205. [DOI] [PubMed] [Google Scholar]
- Wilson SH, Celermajer DS, Nakagomi A, Wyndham RN, Janu MR, Ben Freedman S (1999). Vascular risk factors correlate to the extent as well as the severity of coronary atherosclerosis. Coron Artery Dis 10: 449–453. [DOI] [PubMed] [Google Scholar]
- Wohlfart P, Xu H, Endlich A, Habermeier A, Closs EI, Hubschle T et al. (2008). Antiatherosclerotic effects of small‐molecular‐weight compounds enhancing endothelial nitric‐oxide synthase (eNOS) expression and preventing eNOS uncoupling. J Pharmacol Exp Ther 325: 370–379. [DOI] [PubMed] [Google Scholar]
- Wojcik T, Szczesny E, Chlopicki S (2015). Detrimental effects of chemotherapeutics and other drugs on the endothelium: A call for endothelial toxicity profiling. Pharmacol Rep 67: 811–817. [DOI] [PubMed] [Google Scholar]
- Xia N, Forstermann U, Li H (2014). Resveratrol and endothelial nitric oxide. Molecules 19: 16102–16121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia N, Daiber A, Forstermann U, Li H (2017). Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol 174: 1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Bai P, Little PJ, Liu P (2014). Poly(ADP‐ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev 34: 644–675. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K et al. (2008). H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma‐lyase. Science 322: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xie X, Bai Y, Chen M, Wang X, Zhang X et al. (2015). Exposure to concentrated ambient particulate matter induces reversible increase of heart weight in spontaneously hypertensive rats. Part Fibre Toxicol 12: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Shand BI, McGregor PM, Scott RS, Frampton CM (2006). Comparative effects of enzogenol and vitamin C supplementation versus vitamin C alone on endothelial function and biochemical markers of oxidative stress and inflammation in chronic smokers. Free Radic Res 40: 85–94. [DOI] [PubMed] [Google Scholar]
- Yuan S, Patel RP, Kevil CG (2015). Working with nitric oxide and hydrogen sulfide in biological systems. Am J Physiol Lung Cell Mol Physiol 308: L403–L415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora‐Ros R, Serafini M, Estruch R, Lamuela‐Raventos RM, Martinez‐Gonzalez MA, Salas‐Salvado J et al. (2013). Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: evidence for a mechanism of antioxidant tuning. Nutr Metab Cardiovasc Dis 23: 1167–1174. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Mayr M (2012). MicroRNAs in vascular and metabolic disease. Circ Res 110: 508–522. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Dudek K, Mayr M (2013). Oxidative stress in atherosclerosis: the role of microRNAs in arterial remodeling. Free Radic Biol Med 64: 69–77. [DOI] [PubMed] [Google Scholar]
- Zelcer S, Chen B, Mangel J, Vujovic O, Thiessen‐Philbrook HR, Reider M et al. (2010). Impaired vascular function in asymptomatic young adult survivors of Hodgkin Lymphoma following mediastinal radiation. J Cancer Surviv 4: 218–224. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou M, Qin G, Weintraub NL, Tang Y (2014). MiR‐92a regulates viability and angiogenesis of endothelial cells under oxidative stress. Biochem Biophys Res Commun 446: 952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G et al. (2008). Endothelium‐specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E‐deficient mice. Cardiovasc Res 80: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qiu J, Wang X, Zhang Y, Xia M (2011). AMP‐activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler Thromb Vasc Biol 31: 2897–2908. [DOI] [PubMed] [Google Scholar]


