Abstract
Background: Dyspnea is a common, distressing symptom of cardiopulmonary and neuromuscular diseases. Since the ATS published a consensus statement on dyspnea in 1999, there has been enormous growth in knowledge about the neurophysiology of dyspnea and increasing interest in dyspnea as a patient-reported outcome.
Purpose: The purpose of this document is to update the 1999 ATS Consensus Statement on dyspnea.
Methods: An interdisciplinary committee of experts representing ATS assemblies on Nursing, Clinical Problems, Sleep and Respiratory Neurobiology, Pulmonary Rehabilitation, and Behavioral Science determined the overall scope of this update through group consensus. Focused literature reviews in key topic areas were conducted by committee members with relevant expertise. The final content of this statement was agreed upon by all members.
Results: Progress has been made in clarifying mechanisms underlying several qualitatively and mechanistically distinct breathing sensations. Brain imaging studies have consistently shown dyspnea stimuli to be correlated with activation of cortico-limbic areas involved with interoception and nociception. Endogenous and exogenous opioids may modulate perception of dyspnea. Instruments for measuring dyspnea are often poorly characterized; a framework is proposed for more consistent identification of measurement domains.
Conclusions: Progress in treatment of dyspnea has not matched progress in elucidating underlying mechanisms. There is a critical need for interdisciplinary translational research to connect dyspnea mechanisms with clinical treatment and to validate dyspnea measures as patient-reported outcomes for clinical trials.
Keywords: breathlessness, shortness of breath, respiratory sensation
Contents
Executive Summary
Introduction
Methods
Definition
Neurophysiological Mechanisms
Sources of Sensory Afferent Information
Qualities of Dyspnea
Cerebral Processing of Dyspnea
Opioid Modulation of Dyspnea
The Dyspneic Patient: Connecting Pathophysiology to Neural Mechanism
Dyspnea Measurement
Evaluation and Treatment
Evaluation
Treatment
Research Priorities
Conclusion
Executive Summary
Dyspnea is a common and often debilitating symptom that affects up to 50% of patients admitted to acute, tertiary care hospitals and a quarter of patients seeking care in ambulatory settings. The presence of dyspnea is a potent predictor of mortality, often surpassing common physiological measurements in predicting the clinical course of a patient. Respiratory discomfort may arise from a wide range of clinical conditions, but also may be a manifestation of poor cardiovascular fitness in our increasingly sedentary population. Diagnosis and treatment of the underlying cause of dyspnea is the preferred and most direct approach to ameliorating this symptom, but there are many patients for whom the cause is unclear or for whom dyspnea persists despite optimal treatment.
The purpose of this document is to update the 1999 ATS Consensus Statement on Dyspnea to provide clinicians and investigators with a picture of recent advances in this field with emphasis on the following areas: (1) mechanisms underlying dyspnea, (2) instruments used to measure dyspnea, (3) the clinical approach to the patient who complains of breathlessness, (4) the treatment of dyspnea that persists despite maximal treatment of underlying pathological processes responsible for the breathing discomfort, and (5) topics that should be the focus of future research if we are to make additional advances in our understanding and treatment of this problem.
Major conclusions of the Statement include:
- A wide range of information arising from numerous sensory afferent sources (Table 2) contributes to multiple sensations of dyspnea (Table 3). Specific physiological processes may be linked to corresponding sensory descriptors, the best characterized of which are sensations of work or effort, tightness, and air hunger/unsatisfied inspiration.
- ○ Sensory–perceptual mechanisms underlying sensations of work or effort in breathing are similar to those underlying similar sensations in exercising muscle.
- ○ Tightness is relatively specific to stimulation of airway receptors in conjunction with bronchoconstriction.
- ○ Intensity of air hunger/unsatisfied inspiration is magnified by imbalances among inspiratory drive, efferent activation (outgoing motor command from the brain), and feedback from afferent receptors throughout the respiratory system.
Data from investigations utilizing three-dimensional brain-imaging technology demonstrate that dyspnea activates cortico-limbic structures that also subserve interoceptive awareness and nociceptive sensations such as pain. Opioids, both endogenous and exogenous, may relieve dyspnea by altering central processing of efferent and afferent sensory information.
- As with pain, dyspnea can and should be measured.
- ○ Instruments or sections of instruments (e.g., subscales) pertaining to dyspnea should be classified as addressing domains of sensory–perceptual experience, affective distress, or symptom/disease impact or burden (see Table E1 in the online supplement).
- ○ Clinicians and investigators should be more explicit about the domains being measured.
The evaluation of a patient with dyspnea continues to be dependent on a thorough history and physical examination. In the patient with acute worsening of chronic breathlessness, the clinician must be attuned to the possibility of a new pathophysiological derangement superimposed on a known disorder. No diagnostic test or biomarker correlates closely with changes in dyspnea across all conditions or settings. Specific tests (e.g., spirometry or peak flow, d-dimer, brain natriuretic peptide [BNP], arterial blood gases) have diagnostic utility in specific clinical settings or circumstances.
- The first priority for treatment of the patient with dyspnea is to focus on identifying and relieving the pathologic process leading to the symptom. Once such therapy has been optimized, the clinician should direct attention to persistent physiological derangements amenable to intervention (e.g., hypoxemia, acidemia).
- ○ The contribution of cardiovascular deconditioning to chronic exertional dyspnea should be investigated and pulmonary rehabilitation and exercise training considered for patients with long-standing dyspnea and reduced functional capacity.
- ○ Mechanical and pharmacological interventions targeted at altering afferent sensory information (e.g., inhaled furosemide) or central processing of sensory inputs to the brain (e.g., opioids) demand further investigation.
TABLE 2.
POSSIBLE AFFERENT SOURCES FOR RESPIRATORY SENSATION*
| Source of Sensation | Adequate Stimulus |
| Medullary respiratory corollary discharge | Drives to automatic breathing (hypercapnia, hypoxia, exercise) |
| Primary motor cortex corollary discharge | Voluntary respiratory drive |
| Limbic motor corollary discharge | Emotions |
| Carotid and aortic bodies | Hypercapnia, hypoxemia, acidosis |
| Medullary chemoreceptors | Hypercapnia |
| Slowly adapting pulmonary stretch receptors | Lung inflation |
| Rapidly adapting pulmonary stretch receptors | Airway collapse, irritant substances, large fast (sudden) lung inflations/deflations |
| Pulmonary C-fibers (J-receptors) | Pulmonary vascular congestion |
| Airway C-fibers | Irritant substances |
| Upper airway “flow” receptors | Cooling of airway mucosa |
| Muscle spindles in respiratory pump muscles | Muscle length change with breathing motion |
| Tendon organs in respiratory pump muscles | Muscle active force with breathing motion |
| Metaboreceptors in respiratory pump muscles | Metabolic activity of respiratory pump |
| Vascular receptors (heart and lung) | Distention of vascular structures |
| Trigeminal skin receptors | Facial skin cooling |
| Chest wall joint and skin receptors | Tidal breathing motion |
Reviewed, for example, in References 24–26 and 39–41.
TABLE 3.
DESCRIPTORS FOR AIR HUNGER COMMONLY CHOSEN FROM LISTS
| Urge to breathe (115) | Unsatisfied inspiration (83) |
| Like breath hold (115) | Feeling of suffocation (115) |
| Starved for air (115) | Need for more air (37) |
| Hunger for air (115) | Breath does not go in all the way (37) |
| Breaths felt too small (115) | Cannot get enough air (58) |
Numbers in parentheses indicate reference numbers.
To further our understanding of and ability to treat dyspnea, research efforts must focus on underlying physiological mechanisms contributing to breathing discomfort, must involve larger numbers of subjects, must measure dyspnea directly, and must be explicit with respect to the domain being measured and the outcome variable being assessed. Efforts should be made to further validate and utilize a modest number of measurement tools as endpoints for clinical trials, to facilitate comparison across different studies of dyspnea, and to enhance multidisciplinary approaches to studies of breathing discomfort.
Introduction
Dyspnea is a common problem affecting up to half of patients admitted to acute, tertiary care hospitals (1) and one quarter of ambulatory patients (2, 3). Population-based studies have shown a prevalence of 9 to 13% for mild to moderate dyspnea among community-residing adults (4–6), 15 to 18% among community-residing adults aged 40 years or older (5, 7, 8), and 25 to 37% of adults aged 70 years and older (9). In the United States, “shortness of breath” and “labored or difficult breathing (dyspnea)” account for 3 to 4 million emergency department visits annually (10, 11).
More than 10 years have elapsed since the American Thoracic Society published a consensus statement on mechanisms, assessment, and management of dyspnea (12). Since that time, evidence has emerged that dyspnea is a predictor of hospitalization (13) and mortality in patients with chronic lung disease (14), and in some cases is more closely correlated with 5-year survival than forced expiratory volume in 1 second (FEV1) (15). Dyspnea is also more closely associated with cardiac mortality than angina (16). There has been enormous growth in knowledge about the neurophysiology of dyspnea. In addition, there has been growing interest in the potential use of dyspnea as a patient-reported outcome in clinical trials of pharmacologic and nonpharmacologic interventions in patients with cardiopulmonary disease (17, 18).
Our goal is to identify areas in which new data and experience have altered understanding of: mechanisms underlying dyspnea, instruments used to measure breathing discomfort, the clinical approach to the patient who complains of breathlessness, and the treatment of this often disabling symptom. We believe that this update will help clinicians to improve the care patients receive, and will aid researchers by delineating areas in need of further investigation.
Methods
The writing group was composed of members from the following assemblies of the American Thoracic Society (ATS): Nursing, Sleep and Respiratory Neurobiology, Pulmonary Rehabilitation, Clinical Problems, and Behavioral Science. More than half the members of the writing group were involved in the development of the 1999 consensus statement.
The overall scope of the update was determined by group discussion at the ATS International Conference. Sections were drafted by members of the writing group with relevant expertise. For each section of the statement, a literature review was conducted, focusing as much as possible on empirical studies; systematic reviews and clinical guidelines; and high-quality, theoretically focused, narrative review and expert opinion publications from 1999 onward. Searches were conducted in PubMed and CINAHL databases using “dyspnea OR breathlessness OR respiratory sensation” as primary keywords, with additional search terms as appropriate to each section (e.g., AND [mechanism OR sensory OR afferent] for dyspnea mechanisms; AND [neuroimage* OR positron emission* OR functional magnetic resonance*] for neuroimaging; AND [questionnaire OR scale] for measures; as well as by names of various drugs or treatments for the treatment section). In addition, reference lists were searched (Table 1).
TABLE 1.
METHODS
| Methods Checklist | Yes | No |
| Panel assembly | ||
| • Included experts from relevant clinical and non-clinical disciplines | X | |
| • Included individual who represents views of patients and society at large | X | |
| • Included methodologist with documented expertise | X | |
| Literature review | ||
| • Performed in collaboration with librarian | X | |
| • Searched multiple electronic databases | X | |
| • Reviewed reference lists of retrieved articles | X | |
| Evidence synthesis | ||
| • Applied pre-specified inclusion and exclusion criteria | X | |
| • Evaluated included studies for sources of bias | X | |
| • Explicitly summarized benefits and harms | X | |
| • Used PRISMA* to report systematic review | X | |
| • Used GRADE† to describe quality of evidence | X | |
| Generation of recommendations | ||
| • Used GRADE to rate the strength of recommendations | X | |
See Reference 292.
See Reference 293.
A working draft was submitted to the full writing group in late 2009 and, based on their critiques, was extensively revised by the co-chairs and submitted to the committee in August 2010. Further revisions were agreed upon in an October 2010 conference call, prior to submission for peer review. Based on initial peer review, revisions were completed in Spring 2011 and finalized in a meeting at the 2011 ATS International Conference prior to resubmission.
Definition
We define dyspnea as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity,” as was suggested in the 1999 ATS consensus statement (12). Since that definition was proposed, however, substantial evidence has accrued that (1) distinct mechanisms and afferent pathways are reliably associated with different sensory qualities (notably work/effort, tightness, and air hunger/unsatisfied inspiration), (2) distinct sensations most often do not occur in isolation, and (3) dyspnea sensations also vary in their unpleasantness and in their emotional and behavioral significance.
We also reaffirm the corollary statement that the experience of dyspnea “derives from interactions among multiple physiological, psychological, social, and environmental factors, and may induce secondary physiological and behavioral responses,” (12) but we emphasize strongly that dyspnea per se can only be perceived by the person experiencing it. Perception entails conscious recognition and interpretation of sensory stimuli and their meaning. Therefore, as is the case with pain, adequate assessment of dyspnea depends on self-report.
Because dyspnea is a symptom (i.e., perception of an abnormal or distressing internal state), it must generally be distinguished from signs that clinicians typically invoke as evidence of respiratory distress, such as tachypnea, use of accessory muscles, and intercostal retractions (19–23). Within the definition of dyspnea, we have avoided using terms such as “difficult,” “labored,” or “heavy” breathing because they may or may not characterize the experience of a particular patient at a given point in time. Distinctive sensory qualities are important in considering the physiological mechanisms underlying the breathing discomfort, but the definition of dyspnea should be neutral with respect to any particular quality.
Dyspnea is a complex symptom that potentially warns of a critical threat to homeostasis and thus frequently leads to adaptive responses (such as resting or seeking medical care). Protracted or intractable dyspnea causes suffering and impaired performance and quality of life. For most patients, dyspnea begins with a physiological impairment that leads to the stimulation of pulmonary and extrapulmonary afferent receptors (Table 2) and the transmission of afferent information to the cerebral cortex, where the sensation is perceived as uncomfortable or unpleasant (24–26). Although distinct afferent pathways may be stimulated in some laboratory investigations, clinically, it is more common that multiple afferent inputs contribute to the sensation(s) experienced by patients, and it is unlikely that any laboratory stimulus reproduces exactly what patients perceive (27, 28). There is evidence that different physiological derangements lead to qualitatively different sensations (27, 29–32), and attention to these qualities may be helpful in the evaluation of the cause and treatment of the patient's discomfort.
Having perceived the symptom, the individual evaluates its personal meaning. Depending on the circumstances in which breathing discomfort occurs and the history of similar sensations, breathlessness may be perceived as a threat associated with anxiety, fear, or depression, and it may be viewed as a sign of disease. The individual may avoid activities that precipitate the symptom, thereby becoming more sedentary and increasingly unfit (33). The high prevalence of anxiety and depression in patients with chronic cardiopulmonary diseases likely contributes to the degree of disability associated with dyspnea (34, 35), although highly motivated patients may “work through” breathing discomfort and remain active.
Humans are social animals, and the environment in which they live often has a significant effect on how they perceive their capabilities and their self-concepts. With encouraging support from family, friends, and health care providers, symptoms may not seem so bad, and disabilities may be overcome or ameliorated. Alternatively, social isolation or withdrawal may breed discouragement, frustration, loneliness, or depression, which may further decrease functional performance and quality of life. Without the support to stay active, a patient's expectations may be low, and cardiovascular deconditioning may develop, which leads to additional physiological impairments, worsening symptoms, and declining performance (33).
Neurophysiological Mechanisms
Sensory information from the respiratory system activates regions of the cerebral cortex to produce the perception of dyspnea. We know far less about the neurophysiology of dyspnea than we know about vision, hearing, or even pain. Dyspnea comprises several different uncomfortable respiratory sensations (27, 29, 36–38) that are distinct in the sense that subjects describe them differently, and they can be varied independently with physiological interventions. Thus, we infer that they arise from different sensory mechanisms.
Sources of Sensory Afferent Information
Sensory afferent sources available for respiratory sensation (e.g., reviewed in References 24–26, 39–41) are summarized in Table 2. In addition to traditionally defined sensory afferents, information on the state of respiration is available from respiratory motor areas of the brain, which can send an ascending copy of their descending motor activity to perceptual areas (corollary discharge). The role of corollary discharge has been well described in the limb motor control literature (42–44). The respiratory motor system, however, is unusual in having both automatic (brainstem) and voluntary (cortical) sources of motor command; corollary discharge from these different sources probably gives rise to different sensations.
Qualities of Dyspnea
Work/effort.
From the 1960s through the 1980s, it was widely believed that the sense of respiratory effort/work was responsible for all dyspnea (e.g., see Reference 45), an idea that has been disproved (46–48). Nonetheless, an uncomfortable sense of respiratory “work” and “effort” is commonly reported by patients with conditions such as asthma, chronic obstructive pulmonary disease (COPD), and diseases that impair respiratory muscle performance (27, 31, 32). Respiratory muscle afferents project to the cerebral cortex, and subjects report sensations localized to respiratory muscles when the work of breathing is high (49). Perceptions of work and effort probably arise through some combination of respiratory muscle afferents and perceived cortical motor command or corollary discharge (50). Similar mechanisms give rise to sensations of work and effort from exercising limb muscles (reviewed in References 42 and 51).
During exercise, a variety of physiological adaptations match alveolar ventilation to metabolic demand in healthy, normal persons. As the intensity of exercise increases, individuals generally become aware that breathing requires more work or effort, much as they become aware that exercising limb muscles are working harder. Until the physiological capacity to match ventilation to metabolic demand is approached, afferent mechanoreceptor feedback signals that breathing is appropriate to the prevailing respiratory drive, and breathing distress is minimal (52, 53). As long as sensations of increased work or effort in breathing and the achieved ventilation are consistent with expected responses to exercise, they are not necessarily unpleasant (52, 53), and may not even be the primary reason for stopping. However, breathing discomfort is much greater in patients with cardiopulmonary disease and frequently limits exercise (52).
Perception of breathing effort or work can be produced in the laboratory by external resistive or elastic loads (e.g., 37, 54–57), by volitional hyperpnea (e.g., 54, 58, 59), or by weakening the respiratory muscles via changes in operating length, fatigue, or partial neuromuscular blockade (60–62). With weakened respiratory muscles, the requirement for motor command is increased, and the perception of inspiratory force, effort, and work can be substantially magnified (54, 60, 61, 63), even in the absence of an increase in ventilation. It is likely that simultaneous information from muscle afferents focuses and calibrates sensation from corollary discharge (42, 64). However, there is no evidence, as yet, that experimental alterations adequately reproduce the sensations experienced by patients with cardiopulmonary disease.
Tightness.
“Tightness” is commonly experienced during bronchoconstriction (27, 65–69). Some studies suggest that chest tightness is the dominant experience in the early stages of an asthma attack, but as airway narrowing worsens, patients also report work/effort and air hunger/unsatisfied inspiration (65, 68). Bronchoconstriction gives rise to both a sense of tightness and added physical work of breathing (65, 70); however, blocking pulmonary afferents can diminish tightness (71). In laboratory studies, the relationship between FEV1 and dyspnea intensity in patients with asthma depends on the particular bronchoprovocation agent used, but descriptions of sensory quality are similar (72). In contrast, patients with asthma are more likely to report tightness and less likely to report increased work or effort during methacholine-evoked bronchoconstriction than when exposed to large external resistive loads (68) or during cardiopulmonary exercise testing (73). Mechanical ventilation can eliminate the sense of excessive respiratory work but does not diminish tightness (74). There is also evidence in patients with asthma that perception of increased work/effort does not respond as rapidly as tightness to treatment with nebulized albuterol (67), suggesting that work/effort may be more related to increased respiratory motor output needed to overcome airflow obstruction (e.g., due to inflammation), whereas tightness may be more specifically related to stimulation of airway receptors. Together, these findings suggest that tightness arises from pulmonary afferents rather than being a work-related sensation.
Air hunger/unsatisfied inspiration.
A perception of not getting enough (or of needing more) air, which has been variously labeled as air hunger, unsatisfied inspiration, or an unpleasant urge to breathe, can be induced experimentally by increasing inspiratory drive (e.g., with exercise, hypercapnia, or hypoxia), especially if the capacity to satisfy the increased ventilatory demand is limited. As the demand for ventilation exceeds the capacity to provide it (which occurs only at very high levels of exercise in healthy individuals but is common in patients with cardiopulmonary or neuromuscular disease), a state of imbalance develops between the motor drive to breathe, as sensed via corollary discharge, and afferent feedback from mechanoreceptors of the respiratory system. This becomes increasingly unpleasant and distressing. Various terms have been used to describe this imbalance, including length–tension inappropriateness (75), efferent–reafferent dissociation (76), neuroventilatory dissociation (77), afferent mismatch (78), neuromechanical uncoupling (52, 79, 80), or neuromuscular dissociation (53), but none fully captures the interplay among neurophysiological mechanisms.
Wright and Branscomb (81) proposed the term “air hunger” to describe severe respiratory discomfort evoked by strapping the chest and abdomen with broad adhesive tape during exercise and subsequently by using a device that limited tidal volume and respiratory rate during hypoxia. Other investigators have replicated this effect by corseting the chest during exercise (82, 83), or limiting the volume available at the airway opening (46, 81, 84), giving rise to sensations of air hunger (82), inspiratory difficulty, or unsatisfied inspiration (83). Similar sensations have been reported during symptom-limited exercise testing by patients with restrictive (32) or obstructive (31, 32, 65) lung disease (in particular, when dynamic hyperinflation restricts inspiratory capacity among the latter) (31, 52, 53, 65, 66, 79, 85–88).
Air hunger/unsatisfied inspiration is intensified by stimuli that increase spontaneous ventilatory drive (84, 89, 90), such as hypoxia, hypercapnia, acidosis, and signals arising from exercise-related drive (91, 92), especially if ventilatory response is constrained (52, 53). The preponderance of evidence suggests that information on the spontaneous respiratory motor drive of the brainstem (induced, for example, by hypoxia or exercise) is conveyed to the cerebral cortex as corollary discharge (e.g., 47, 93, 94). When this is not matched by an adequate ventilatory response, individuals perceive air hunger/unsatisfied inspiration. In contrast, increased voluntary motor drive to respiratory muscles, which originates in the cerebral cortex (95, 96), predominantly evokes a perception of respiratory effort (58, 61), which, in healthy volunteers, is not as unpleasant as air hunger (97).
Mechanoreceptors in the lungs, airways, and chest wall provide afferent information about achieved pulmonary ventilation and can inhibit (relieve) air hunger/unsatisfied inspiration (98–103). In animal models of emphysema, pulmonary stretch receptor discharge is decreased (104). Pulmonary stretch receptor activation alone provides potent relief (100), independent of vagal influences on inspiratory drive (105) and changes in alveolar and arterial blood gas concentrations (106, 107). Sensitization of pulmonary mechanoreceptors can reduce dyspnea, offering a potential therapy for intractable dyspnea (55, 108–110). However, further research is needed to determine the clinical importance of these effects.
Evidence that chest wall mechanoreceptor feedback provides relief equal to that produced by pulmonary stretch receptors is equivocal. Some evidence suggests that relief of air hunger from lung and chest expansion is attenuated in lung transplant patients compared with heart transplant patients and healthy subjects, suggesting a greater role for pulmonary stretch receptors than chest wall receptors (103, 111). However, these studies were performed before it was discovered that transplanted lungs are re-innervated quickly, and thus may have overestimated the contribution of chest wall receptors (112).
Unsatisfied inspiration or air hunger is not specific to any particular disease or stimulus. It has been reported in quadriplegic patients in response to increased partial pressure of carbon dioxide (Pco2) (47), decreased tidal volume (100), or methacholine challenge (66), as well as in response to increased Pco2 in normal subjects undergoing neuromuscular blockade (46, 48). In addition, patients with asthma, COPD, interstitial lung disease, and idiopathic hyperventilation are significantly more likely than healthy control subjects to report perceptions of air hunger when recalling perceptions of breathing at the end of exercise (113). A consistent finding across experimental exercise and clinical studies is that patients with a variety of conditions (or normal subjects with chest wall corseting) report greater difficulty and discomfort during inspiration compared with expiration (inspiratory difficulty) (31, 32, 36, 52, 70, 79, 83, 85, 113, 114).
Naïve experimental subjects exposed to a variety of similar stimuli have chosen descriptors listed in Table 3 (37, 58, 83, 115). Although relatively few naïve subjects spontaneously use the terms “air hunger” or “unsatisfied inspiration,” they commonly endorse those terms from a list of descriptors after exposure to appropriate stimuli. Patients with chronic cardiopulmonary disease tend to report sensations such as smothering, suffocating, not enough air, or inability to breathe when breathing is sufficiently distressing to provoke an emergency department visit, but commonly endorse air hunger when presented with a list of descriptors (114, 116).
Unexplained dyspnea is one of the hallmarks of panic disorder (117), and clustering of suffocating, smothering, and air hunger has been observed in patients with panic disorder (118–120) in response to breath-holding and CO2 rebreathing (120) or challenge (119). Panic disorder is more common in patients with COPD than in the general population (121–123), but similar clustering of descriptors has been reported in patients with idiopathic hyperventilation (113) who do not have cardiopulmonary or neuromuscular disease. This suggests that, in susceptible individuals, air hunger may occur even in the absence of reduced ventilatory capacity. Factors such as excessive ventilatory drive or impaired perception of achieved ventilation may play a role. There also may be a role for increased sensitivity to CO2 (which may, in turn, have a component of genetic predisposition) or excessive response to cerebral alkalosis or hypoxia due to hyperventilation-induced hypocapnia (118, 124).
Whatever term is used to characterize this cluster of related descriptors, it is not merely the awareness that breathing has increased, as that is not necessarily uncomfortable (93). Rather, it is a perception that the drive to breathe is not being matched by adequate pulmonary ventilation.
Other qualities of dyspnea.
Qualities of clinical dyspnea are not limited to the foregoing categories. Rather, sensations of effort, tightness, and air hunger are more reliably characterized than others (e.g., rapid or heavy breathing) (36, 69, 97, 114, 125). In addition, the mechanisms described above do not provide a good explanation for dyspnea arising from vascular problems such as congestive heart failure and pulmonary hypertension. It seems plausible that pulmonary vascular receptors (“J-receptors”) (126) play a role in this dyspnea, but only suggestive evidence is available at this time (127, 128). Other neural pathways may be identified in the future.
Summary of dyspnea qualities.
Although multiple distinguishable sensations varying in intensity are central to the definition of dyspnea, these separate sensations seldom, if ever, occur in a pure or isolated fashion in the real world. Multiple uncomfortable sensations are often present in patients (27, 31, 32, 36, 114, 116, 125, 129–133) and together produce the overall perception of dyspnea, as described in The Dyspneic Patient: Connecting Pathophysiology to Neural Mechanism. Even laboratory stimuli designed to be specific generally stimulate more than one afferent pathway. Assessment of perceived sensory qualities with magnitude scales, rather than binary choice (114, 116, 125), may be more sensitive in picking up qualities of sensation, but in general reports of dyspnea are not as consistent as, for example, reports of colors or sounds.
Cerebral Processing of Dyspnea
Neuroimaging.
In recent years, three-dimensional brain mapping techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have been used to infer neural activation from changes in cerebral blood flow. Specific brain regions activated during laboratory-generated dyspnea have been identified in healthy subjects. It is likely that these methods will eventually extend to studies in patients with dyspnea.
These methods yield impressive images, but the design, analysis, and interpretation of studies is complex, and conclusions should be accepted with caution. Inferring dyspnea-related activity can be problematic, as some respiratory interventions, such as acute hypercapnia, can alter cerebral blood flow independently of neural activation. Images depend on comparisons of signals in different states, so that selection of the control condition contributes as much to the image as the test condition. Nonetheless, some important conclusions can be drawn from several studies that have produced similar results using different approaches. A core pattern has emerged: dyspnea activates cortico-limbic structures (134–143) that also subserve interoceptive awareness of homeostatic threats such as thirst and hunger (144–148) or pain (134, 137–140, 149–152). Recent reviews provide a comprehensive analysis of both the power and limitations of these techniques (141, 153).
Using PET imaging of the forebrain, Banzett and coworkers (134) reported that air hunger, induced in healthy subjects by constraining ventilation during constant mild hypercapnia, resulted in a strong activation of the right anterior insular cortex. This study controlled for both the effect of reducing ventilation and for arterial Pco2, a powerful modulator of cerebral blood flow. In another PET study, Peiffer and colleagues (138) used a different stimulus—inspiratory resistive loading—to induce respiratory discomfort in healthy participants, and reported activations in the right anterior insula and cerebellum; however, a difference in arterial Pco2 between experimental and control states was a possible confounding factor.
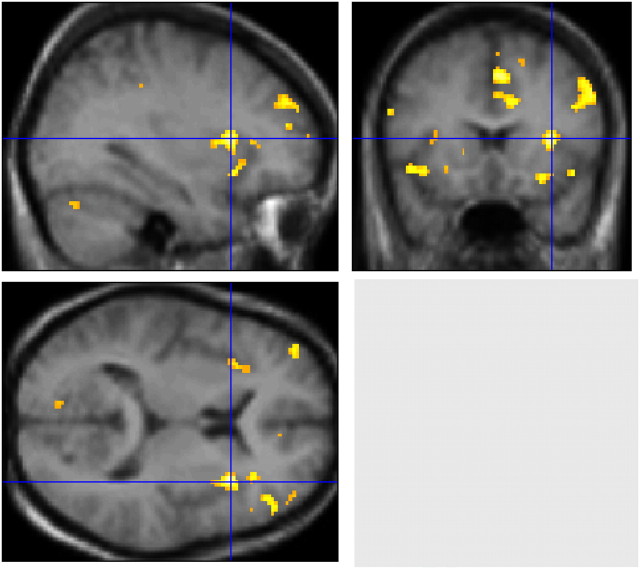
Evans and coworkers (137) used the same mild-hypercapnia/restricted ventilation stimulus as in the initial study by Banzett and colleagues, but used fMRI to image the whole brain (Figure 1). Advantages of fMRI included coverage of the entire brain, greater spatial resolution, and lack of ionizing radiation, permitting control experiments in the same subjects. Evans and coworkers (137) also detected anterior insular activation (R > L) with additional limbic and paralimbic activations, notably in the cingulate gyrus and amygdala. Midline cerebellar activation was also present.
Figure 1.
Functional magnetic resonance images showing cerebral activations correlated with the experience of strong air hunger in healthy subjects. The test condition consisted of low tidal volume controlled ventilation during mild hypercapnia; the baseline comparison condition used the same level of hypercapnia but with high tidal volume (subjects reported little or no discomfort at baseline). The strongest activation is in the right anterior insula, indicated by the blue crosshairs; this activation has been shown in a number of studies. Other activations can be seen in the left anterior insula, anterior cingulate, supplementary motor area, prefrontal cortex, and cerebellum. Not visible in this figure, but reported in the same study was activation of the amygdala. Most of these regions fall in the category of limbic/paralimbic, and overlap with activations seen during pain, thirst, fear, and hunger. Reproduced and adapted with permission from Reference 137.
The cerebral regions activated in these initial dyspnea imaging studies were similar to those seen with somatic pain by other investigators (151, 152, 154), except for the amygdala, which is activated in visceral pain (155, 156). Clearly dyspnea and pain are different, and it seems likely that the activations evoked by dyspnea and pain differ in detail. Imaging both dyspnea and pain in the same subjects with fMRI might provide sufficient added resolution to distinguish differences in the anatomic regions of activation. However, the only such study performed to date (140) failed to identify a difference in location of activation between thermal skin pain and respiratory discomfort induced by brief inspiratory loads.
Interoceptive awareness of homeostatic threats such as thirst (157) and hunger (158) may demand behavioral action (143–148) motivated by emotions such as anxiety and fear (159) that are associated with limbic activations (e.g., in the amygdala and anterior insula) (160). Fear and anxiety also may give rise to or amplify dyspnea. A few investigators have begun to look at the cerebral correlates of the interaction between emotion and respiratory sensation. Increased insular activation has been noted in patients with idiopathic hyperventilation compared with normal, healthy control subjects exposed to repeated transient inspiratory occlusions (161). Viewing photographs that stimulate negative emotions when brief inspiratory loads are applied has been reported to increase activations of the right anterior insula as well as increasing activation of an extension of the amygdala not previously associated with dyspnea (139). In contrast, decreased insular activation has been found after opiate administration (162), in patients with obstructive sleep apnea exposed to inspiratory loads (163), and in patients with congenital central hypoventilation syndrome exposed to hypercapnia (164) or hypoxia (165).
Although neuroimaging studies provide indirect evidence of differential neural activation, they have the potential to help delineate sensory from affective components of dyspnea and improve understanding of the impact of emotional, cognitive, and experiential processes occurring alongside this symptom. Over the long run, results of neuroimaging studies may contribute to developing more effective therapeutic strategies for the dyspneic patient.
Opioid Modulation of Dyspnea
There is good evidence that opioid drugs reduce dyspnea (see Evaluation and Treatment). Laboratory studies in healthy subjects show that opioids reduce the discomfort of air hunger (166) but not the discomfort of work or effort (167). Opioids likely act both by depressing spontaneous respiratory drive (thus reducing corollary discharge), and by modulating cortical activity, as they do in pain. Decreased insular activation in response to breath-holding has been observed after opiate administration (162), and it is possible that further imaging experiments will ascertain the sites of opiate action.
Several studies have examined the possibility that endogenous opioids may modulate dyspnea (168–172). Randomized, double-blind, crossover administration of the opioid antagonist, naloxone versus placebo has been used with healthy subjects during laboratory stimuli (170, 171) and in patients with COPD during exercise (168, 172). Results have been mixed. One study of healthy subjects breathing freely during hypercapnia found a significant increase of both e and “difficulty breathing” with naloxone compared with placebo (170). Another study found significant increases in the breathlessness–o2 regression slope (the primary endpoint) as well as in peak and mean breathlessness ratings throughout high-intensity constant work treadmill exercise with naloxone compared with placebo; other ventilatory parameters and β-endorphin immunoreactivity were equivalent across conditions (172). However, two other studies found no effect of naloxone on respiratory sensation (168, 171). Interpretation and comparisons between these studies are complicated by methodological differences (e.g., sample composition, physiological stimuli, endpoints, naloxone dose, and how dyspnea was measured).
The Dyspneic Patient: Connecting Pathophysiology to Neural Mechanism
To provide an example connecting impaired respiratory function with the neurophysiological mechanisms discussed above, we consider a typical patient with COPD who is not dyspneic at rest but who becomes dyspneic when walking (173). When walking at a normal pace, our patient is likely to experience greater brainstem drive to breathe than a healthy subject. At the same time, increased airflow resistance and alterations in chest wall geometry lead to additional work of breathing. Afferent feedback from proprioceptors and metaboreceptors in the respiratory muscles is perceived as excessive respiratory work or effort. Deconditioning of locomotor muscles leads to increased metabolic byproducts of exercise and, consequently, increased drive to breathe.
Expiratory flow limitation and the accompanying dynamic hyperinflation force the inspiratory muscles to operate at a shorter, disadvantageous sarcomere length. This puts the inspiratory muscles at a mechanical disadvantage, potentially adding a functional restrictive defect over and above the underlying obstructive mechanics (52, 53, 79), and increasing the amount of motor command required for a given ventilation (174). Increased corollary discharge likely gives rise to sensations of respiratory effort (50), especially during inspiration (inspiratory difficulty) (31, 32, 36, 83), air hunger, or both, depending on the source of motor command.
As our patient begins to walk uphill, ventilatory drive increases further. The resulting increase in ventilation produces further dynamic hyperinflation, and the desired tidal volume begins to approach inspiratory capacity, at which point the only way to increase minute ventilation is by increasing breathing frequency, which comes at the cost of greater deadspace ventilation (175) and decreased dynamic compliance. Our patient is no longer able to match ventilation to the prevailing respiratory drive. Pulmonary stretch receptors, and perhaps chest wall proprioceptors, report this shortfall. If asked, our patient would likely use terms that have been grouped under the headings inspiratory difficulty (“Breathing in requires effort”; “My breath does not go in all the way”) and unsatisfied inspiration (“I feel a need for more air” or “I cannot get enough air in”) by O'Donnell and coworkers (83), at least some of which are similar to descriptors grouped by other investigators as air hunger (27, 37). Analogous shortfall of ventilation under laboratory conditions (in the absence of increased respiratory muscle demand) produces a sensation classified as air hunger, but in our patient, sensations of air hunger or unsatisfied inspiration are experienced together with sensations of work or effort, and with increasing awareness that breathing has become unpleasant and distressing (129).
Dyspnea Measurement
As with pain, dyspnea can and should be measured to assess it adequately. In the 1999 statement (12), several validated measures were cataloged, but relatively little attention was paid to which domain(s) or dimension(s) of the symptom a given instrument measured. This may have left an impression that the choice of a dyspnea measure was somewhat arbitrary. In actuality, there are important differences in what instruments measure (e.g., one instrument may ask what breathing feels like, whereas another may ask how distressing it is or how it impacts performance or quality of life), the rating task (i.e., what patients or research subjects are instructed to rate), and whether measurements are real-time or involve recall of a specific episode, some defined interval, or how things usually are.
The number and diversity of dyspnea measures makes any comprehensive critical synthesis difficult, as has been noted in several systematic reviews (176–178). Dorman and colleagues (176) categorized measures as pertaining to “severity of breathlessness,” “descriptions of breathlessness,” and measures of “functional impact or limitations associated with breathlessness” (p. 181). Bausewein and coworkers (177) categorized measures as specific to breathlessness versus multidimensional disease-specific measures of symptoms and other domains (e.g., physical, psychosocial, or spiritual functioning). Johnson and colleagues categorized measures primarily according to whether they were unidimensional or multidimensional (178). Others have suggested categorizations of intensity or severity, situational or functional impact, effects on health-related quality of life or health status, and qualitative descriptors (179, 180).
We propose that instruments or sections of instruments (e.g., subscales) pertaining to dyspnea should be classified as pertaining to domains of sensory–perceptual experience, affective distress, or symptom/disease impact or burden (Table 4). These categories are in alignment with the definition of dyspnea, but are not mutually exclusive. Many instruments measure more than one domain. Greater clarity about what domain(s) an instrument measures will aid clinicians and researchers in selecting measures appropriate to their specific needs.
TABLE 4.
DOMAINS OF DYSPNEA MEASUREMENT
| Domain | Definition | Examples* |
| Sensory–perceptual experience | Measures of what breathing feels like to the patient or research subject. | Single item ratings of intensity (e.g., Borg scale, VAS) |
| Descriptors of specific sensations/clusters of related sensations | ||
| Affective distress | Measures of how distressing breathing feels. Focus can be either immediate (e.g. unpleasantness) or evaluative (e.g., judgments of meaning or consequences). | Single-item ratings of severity of distress or unpleasantness |
| Multi-item scales of emotional responses such as anxiety | ||
| Symptom impact or burden | Measures of how dyspnea/breathlessness affects functional ability, employment (disability), quality of life, or health status. | Unidimensional rating of disability or activity limitation (e.g., MRC scale) |
| Unidimensional or multidimensional ratings of functional ability | ||
| Multidimensional scales of quality of life/health status |
Definition of abbreviations: MRC = Medical Research Council; VAS = visual analog scale.
Specific measures cataloged in Table E1.
Sensory–perceptual measures (what breathing “feels like” to the patient or research participant) include ratings of intensity or sensory quality. Often, though not always, ratings involve one or more separate single-item scales, such as visual analog scales (181–183), Borg ratings (184, 185), Likert-type ratings (186), or numerical (e.g., 0–10) rating scales (187).
Measures of affective distress may use single- or multiple-item scales. Affective distress can pertain to either a perception of immediate unpleasantness or to a cognitive–evaluative response to or judgment about the possible consequences of what is perceived (144, 145, 151, 152). There is experimental evidence that immediate unpleasantness of pain is at least potentially distinguishable from its intensity (188), and results of several studies suggest this also may be the case for dyspnea (97, 139, 189). A few studies have focused on discriminating the affective response from the intensity of respiratory sensation (139, 189–194).
Most real-time dyspnea measures have used a single scale and have not distinguished whether the scale measured intensity, unpleasantness, or distress. Patients rating pain on a single-item scale tend to rate how distressing it is (195), but that is not necessarily the case for subjects rating pain (or dyspnea) in a laboratory. Thus, single-item ratings may contribute to difficulties in translating between laboratory and clinical research findings.
Impact measures (e.g., how breathing affects behaviors, beliefs, or values that matter to patients or society) generally involve multi-item scales, often across multiple dimensions, such as functional performance or disability, quality of life or health status, and psychosocial functioning. Impact measures, although very important, do not directly assess what breathing feels like. Conclusions about changes in dyspnea must be inferred from burden or impact measures, and such inferences may be confounded by changes in other symptoms (e.g., pain, fatigue, depression, or nonspecific emotional distress).
In general, sensory–perceptual measures and ratings of affective distress pertain to the magnitude of perceived sensation (or of the associated unpleasantness or emotional distress) for some specific stimulus condition (e.g., elastic or resistive loads, CO2 or methacholine inhalation) or at some specific moment(s) in time. Specificity of stimuli is more characteristic of controlled experiments than of clinical presentations. The time-frame for ratings may be the present (e.g., right now or continuous real-time ratings), the immediate past (e.g., at the conclusion of an experimental session), or short-term recall of a particular episode (e.g., how breathing felt prior to seeking care).
In contrast, the time-frame for impact measures is commonly either some elapsed interval (e.g., since the last visit to a health care provider or over the past week to month) or an unspecified interval (e.g., an implicit comparison against what is usual for the patient). For some impact/burden measures, item-level scaling pertains to how frequently (e.g., how much of the time) an impact occurs, not how intense it is.
Whatever instrument is used must be adequately described. Adequate description includes specifying what the individual was asked to rate (e.g., sense of unsatisfied inspiration or of breathing work, distress or anxiety, etc.) and the direction and verbal anchors of the scale (e.g., 0 = none; 10 = most imaginable).
To demonstrate the potential utility of the proposed categorization to facilitate such description, we used it to characterize all measures that were cataloged in the original ATS consensus statement (12) or in recent systematic reviews (176–178), and others for which data are available in peer-reviewed publications (Table E1). Inclusion of a measure in the online supplement is not intended as endorsement, nor do we suggest that any particular category or scale type is intrinsically “better” than any other. What matters is that dyspnea is measured when it is possible to do so.
Most measures were developed prior to the publication of the ATS consensus definition (12). Most relate to some part or parts of that definition, but none measures all aspects or domains implied in the definition. Measures should be chosen based on considered judgment about the alignment of questionnaire content with the aspects or domains of dyspnea that are most relevant to clinical or research objectives. We believe that the classification scheme we are recommending will facilitate comparisons across physiological, clinical, and translational studies as well as communication among clinical practitioners, researchers, and patients about which aspects of dyspnea are being measured.
Evaluation and Treatment
Evaluation
From the standpoint of clinical evaluation, there are two major categories of patients with dyspnea: those with new onset of breathing discomfort for whom the underlying cause of dyspnea has not yet been determined; and those with known cardiovascular, respiratory, or neuromuscular disease who are experiencing worsening dyspnea. For the former, evaluation is focused on discovering an underlying abnormality or diagnosis; for the latter, the goal is to discern whether there is deterioration of a known disorder or emergence of a new problem.
The general approach to the evaluation of the patient with dyspnea has not changed significantly from the 1999 ATS statement (12), and is detailed in general textbooks (196). In a patient with new onset of dyspnea, the history and physical examination remain the mainstays of diagnostic evaluation. Among those for whom diagnosis remains elusive and unexplained, specialty referral (e.g., pulmonologist, cardiologist, or multidisciplinary dyspnea clinic) may help identify a potentially treatable underlying cause (197–199). A categorization of mechanisms and clinical conditions associated with dyspnea is shown in Table 5 (196). In most cardiopulmonary diseases, both increased ventilatory demand and altered ventilatory mechanics are present in varying degrees. However, in some nonpathological circumstances (e.g., at altitude or during pregnancy), increased drive predominates.
TABLE 5.
EXAMPLES OF CONDITIONS AND CAUSES OF DYSPNEA GROUPED BY PHYSIOLOGICAL MECHANISM*
| Increased respiratory drive—increased afferent input to respiratory centers |
| Stimulation of pulmonary receptors (irritant, mechanical, vascular)† |
| Interstitial lung disease |
| Pleural effusion (compressive atelectasis) |
| Pulmonary vascular disease (e.g., thromboembolism, idiopathic pulmonary hypertension) |
| Congestive heart failure |
| Simulation of chemoreceptors |
| Conditions leading to acute hypoxemia, hypercapnia, and/or acidemia |
| Impaired gas exchange, e.g., asthma, pulmonary embolism, pneumonia, heart failure‡ |
| Environmental hypoxia, e.g., altitude, contained space with fire |
| Conditions leading to increased dead space and/or acute hypercapnia |
| Impaired gas exchange, e.g., acute, severe asthma, exacerbations of COPD, severe pulmonary edema |
| Impaired ventilatory pump (see below), e.g., muscle weakness, airflow obstruction |
| Metabolic acidosis |
| Renal disease (renal failure, renal tubular acidosis) |
| Decreased oxygen carrying capacity, e.g., anemia |
| Decreased release of oxygen to tissues, e.g., hemoglobinopathy |
| Decreased cardiac output |
| Pregnancy |
| Behavioral factors |
| Hyperventilation syndrome, anxiety disorders, panic attacks |
| Impaired ventilatory mechanics—reduced afferent feedback for a given efferent output (corollary discharge of motor command) |
| Airflow obstruction (includes increased resistive load from narrowing of airways and increased elastic load from hyperinflation) |
| Asthma, COPD, laryngospasm, aspiration of foreign body, bronchitis |
| Muscle weakness |
| Myasthenia gravis, Guillain-Barre, spinal cord injury, myopathy, post-poliomyelitis syndrome |
| Decreased compliance of the chest wall |
| Severe kyphoscoliosis, obesity, pleural effusion |
This adapted table was published in Saunders, Mason RJ, Broaddus VC, Martin TR, King TE, Schraufnagel DE, Murray JF, Nadel JA, Murray and Nadel's Textbook of Respiratory Medicine, Copyright Elsevier 2012. This permission is granted for non-exclusive world rights in all languages. Reproduction of this material is granted for the purpose for which permission is hereby given.
In most cardiopulmonary disease states, a combination of increased respiratory drive and impaired mechanics will be present.
These conditions probably produce dyspnea by a combination of increased ventilatory drive and primary sensory input from the receptors.
Heart failure includes both systolic and diastolic dysfunction. Systolic dysfunction may produce dyspnea at rest and with activity. Diastolic dysfunction typically leads to symptoms primarily with exercise. In addition to the mechanisms noted above, systolic heart failure may also produce dyspnea via metaboreceptors; these are receptors that are postulated to lie in muscles and that are stimulated by changes in the metabolic milieu of the tissue that result when oxygen delivery does not meet oxygen demand.
In addition to laboratory, radiographic, and clinical studies, the words or phrases patients use to describe the quality of the breathing discomfort (Table 3) may provide insight into the underlying pathophysiological mechanisms (27, 29–32, 36). Chest tightness may be relatively specific for dyspnea due to bronchoconstriction (30, 67, 68, 74, 132). Sensations of “air hunger” and “inability to get a deep breath,” which probably represent the combined effects of increased drive to breathe and limited tidal volume, are commonly seen in association with dynamic hyperinflation (31, 86) and other conditions characterized by restrictive mechanics (e.g., heart failure or pulmonary fibrosis). Sensations of effort, suffocation, and rapid breathing have been found to characterize CO2-induced panic attacks in patients diagnosed with panic disorder (119), but are nonspecific (200). There also may be linguistic and cultural differences in how patients characterize their symptoms (201–203), especially symptoms of affective distress (201), and some patients have difficulty grasping the concept of “quality” of their breathing sensations.
Cardiopulmonary exercise tests can be particularly helpful in the evaluation of patients in whom an initial evaluation is unrevealing or patients in whom multiple problems may contribute to dyspnea (199). Identifying nonrespiratory causes of exercise limitation (e.g., leg discomfort, fatigue, or weakness) is important, because they often coexist with breathing discomfort.
For patients with advanced cardiac or pulmonary disease, a recent clinical consensus statement recommended that the intensity of breathlessness be rated by the patient on a regular basis and routinely documented in the patient's medical record (204). Such ratings can be used to guide interdisciplinary care and clinical management in much the same manner as pain ratings are used. Moreover, it was recommended that the distress associated with dyspnea should be assessed along with its perceived meaning and any unmet needs associated with the symptom (204). However, the statement did not recommend any specific rating tool over any other.
There are relatively few blood tests that are necessary in the initial evaluation of the patient with dyspnea. A check of the hematocrit or hemoglobin is important to exclude occult anemia. Reduced oxygen-carrying capacity of the blood is associated with exertional dyspnea and may be an explanation for worsening dyspnea in patients with underlying cardiopulmonary disease. Arterial blood gas measurements may be of value in managing severe, underlying cardiopulmonary disease (e.g., respiratory failure, acute respiratory distress syndrome), but their value is limited in the assessment of dyspnea in patients who are stable.
The d-dimer is a component of the evaluation of patients with suspected pulmonary embolism (205). As with many screening tests, the sensitivity of d-dimer is much greater than its specificity, and its positive predictive value is poor. Thus, its primary value is in rapid identification of patients with low probability of pulmonary embolism, particularly in outpatient settings. There is evidence that its negative predictive value is poor in hospitalized patients, especially after several days of hospitalization, or in patients older than 60 years of age (206).
For patients with acute dyspnea, especially those who come to an emergency department with dyspnea of new onset or uncertain etiology, B-type natriuretic peptide (BNP) or its N-terminal prohormone precursor (NTproBNP) may help in evaluating the possibility of heart failure as the cause of dyspnea. This can expedite evaluation and initiation of appropriate treatment and may contribute to reduced costs and length of stay for those who require hospitalization (207, 208). Routine use of BNP in all patients presenting with acute dyspnea is not recommended (209), especially when admission is highly likely in any event (209). In acute care settings such as an ED, the sensitivity of BNP or NTproBNP is substantially higher than its specificity, and its utility is greatest for ruling out heart failure as a cause of acute dyspnea in patients with a low to intermediate pretest probability of heart failure (210, 211). The utility of serial BNP testing in inpatients or outpatients with known heart failure is uncertain (212–215).
Treatment
In approaching any patient with dyspnea, the initial focus should be on optimizing treatment of the patient's underlying disease, such as the inhaled bronchodilator and corticosteroid regimens of patients with asthma or diuretics and afterload reduction in patients with heart failure. The discussion that follows assumes that treatment of the underlying condition has been optimized (212–219).
In recent years, there have been significant advances in our understanding of the pathophysiology of dyspnea. Unfortunately, our improved understanding of dyspnea, as outlined in this section, has translated to only modest improvements in our ability to bring relief to symptomatic patients. There are currently no FDA-approved treatments for dyspnea per se (as opposed to approval for treatment of diseases in which dyspnea may be a prominent symptom), and even when evidence of efficacy exists, the magnitude of the benefit is variable.
Oxygen.
Although supplemental oxygen improves mortality in chronically hypoxemic patients with COPD, there are conflicting data about its ability to relieve breathlessness (220–224). A beneficial effect of oxygen could be related to changes in chemoreceptor stimulation, the resulting changes in breathing pattern (225, 226), and/or stimulation of receptors related to gas flow through the upper airway (227, 228). Thus, symptomatic benefit may not be confined to patients who meet Medicare guidelines for supplemental oxygen (225, 229). Oxygen therapy may be useful for patients with advanced heart or lung disease, in particular those who are hypoxemic at rest or with minimal activity (204, 212, 213, 224, 230).
Heliox.
As a result of their decreased density, helium-containing gas mixtures reduce the resistance to airflow, which in turn may decrease the work of breathing, reduce the severity of hyperinflation, increase exercise capacity, and decrease dyspnea in patients with obstructive lung disease (231–233). A single study suggests a beneficial effect in patients with lung cancer, although all the patients in the study had coexistent airflow obstruction (234). To date, no study has addressed the effect of long-term heliox use.
Pharmacologic therapy.
Opioids have been the most widely studied agent in the treatment of dyspnea (204, 230, 235). Short-term administration reduces breathlessness in patients with a variety of conditions, including advanced COPD (236, 237), interstitial lung disease (238), cancer (239), and chronic heart failure (240). However, evidence of long-term efficacy is limited and conflicting (241, 242). Opioids are associated with frequent side effects (241, 243), particularly constipation, but clinically significant respiratory depression is uncommon with the doses used to treat dyspnea, even in elderly patients (244, 245). Randomized controlled trials have not found nebulized opioids to be associated with fewer side effects than oral or parenteral opioids (246). Recent evidence-based clinical guidelines (230, 247) recommend that opioids be considered on an individualized basis for palliation of unrelieved dyspnea in patients with advanced cardiopulmonary disease despite otherwise adequate treatment of the underlying disease, with due consideration to patient history, comorbid conditions, and risk for respiratory depression (204, 248).
Nebulized furosemide has been investigated as a novel pharmacologic approach to the treatment of dyspnea. Inhaled furosemide decreases breathlessness induced in normal volunteers (55, 109); the mechanism of the effect is uncertain, but may be mediated by vagal afferents (110). The majority of clinical studies have focused on patients with asthma, and the applicability of those findings to patients with other disorders is uncertain. Two small studies in patients with COPD showed a reduction in dyspnea during constant load exercise (108, 249). A recent small randomized trial in patients with cancer showed no evidence of benefit (250). The role of nebulized furosemide warrants further study, but there are currently insufficient data to support its use in the treatment of dyspnea.
A number of other pharmacologic agents, including anxiolytics (251–253), antidepressants (254), phenothiazines (237, 255), indomethacin (256, 257), inhaled topical anesthetics (258, 259), nitrous oxide (260), and sodium bicarbonate (261), have been found to be ineffective or lack sufficient data to recommend their use (247).
Pulmonary rehabilitation.
Pulmonary rehabilitation is an integral component of the management of patients with chronic lung disease (262, 263). Among the beneficial effects of pulmonary rehabilitation are a reduction in exertional dyspnea during exercise and improved exercise tolerance (262–266), as well as decreases in self-reported dyspnea with activity. The main component of pulmonary rehabilitation responsible for these improvements is exercise (33, 265, 267, 268), but it is less clear whether mechanisms leading to improvement in dyspnea are mainly due to improvements in conditioning, in pacing of activities, desensitization to respiratory sensations or affective distress, or a combination of those effects. Evidence that other components of pulmonary rehabilitation (e.g., education to improve inhaler technique or adherence with medications, pacing activities, or breathing techniques) ameliorate dyspnea independent of exercise is inconsistent, but it is likely that individual characteristics (e.g., motivation) are relevant.
In COPD, pulmonary rehabilitation may result in decreased ventilatory requirements and respiratory rate during ambulation, thereby decreasing risk for developing dynamic hyperinflation. There is evidence that patients with COPD who undergo 6 weeks of exercise training experience comparable small decreases in dyspnea intensity, regardless of whether or not they demonstrate improved exercise capacity (269).
Systematic reviews of randomized trials of inspiratory muscle training (IMT) have shown some reduction of dyspnea intensity and impact in COPD (270), but not in cystic fibrosis (271). However, the number and sample sizes of included trials was small in both reviews (270, 271). A recent evidence-based clinical practice guideline stated that there was insufficient evidence to recommend IMT as a routine component of pulmonary rehabilitation, but that IMT could “be considered in selected patients with COPD who have decreased inspiratory muscle strength and breathlessness despite receiving optimal medical therapy” (262). There also is some evidence that pursed-lip breathing may relieve dyspnea in advanced COPD (247).
Other nonpharmacological approaches.
A number of other strategies have been investigated for their potential role in the relief of breathlessness. Several small studies have shown that chest wall vibration reduces dyspnea in patients with COPD (272–275). However, the timing and site of application of the vibratory stimulus are important variables, and to date there is no commercially available device for delivering chest wall vibration.
Patients with dyspnea often report that movement of cool air reduces breathlessness, and laboratory studies have shown that cold air directed on the face decreases dyspnea induced in healthy individuals (276). However, no large clinical trial has examined the use of fans and/or cool airflow for the relief of dyspnea in patients (247). One small, randomized crossover trial in patients with a variety of disorders demonstrated a small, statistically significant reduction in breathlessness with facial stimulation (277).
Increased respiratory muscle effort, associated with high ventilatory demand relative to respiratory muscle capacity, may contribute to dyspnea in many patients with chronic respiratory disease. By reducing the demand on the respiratory muscles, noninvasive ventilation (NIV) might reduce dyspnea. However, few studies of NIV have used dyspnea as an endpoint. Two studies examining long-term nocturnal use of NIV in patients with severe COPD reported significant improvements in dyspnea ratings (278, 279); whether NIV would have similar effects in patients with other forms of chronic pulmonary or cardiac disease is unknown. The use of NIV during exercise decreases dyspnea and increases exercise tolerance (280, 281), which may facilitate patients’ participation in pulmonary rehabilitation.
Alternative and complementary medicine.
There are minimal data about the effectiveness of alternative and complementary medicine in relieving breathlessness (247). In two small studies in patients with COPD, acupuncture was associated with a significant reduction in breathlessness (282, 283). However, a third study reported no benefit (284), and no benefit was found in a recent study of patients with advanced cancer (285). A related technique, acupressure, led to improvements in dyspnea in patients with COPD (286, 287), but the studies were uncontrolled trials involving only a very small number of subjects. A recent pilot study of yoga training in patients with COPD demonstrated small improvements in dyspnea that were not statistically significant (288). A randomized trial comparing an 8-week intervention combining mindfulness-based stress reduction and relaxation techniques with an attention-control support group found no significant or clinically meaningful differences in dyspnea during a 6-minute-walk test (289). Thus, there are currently insufficient data to recommend acupuncture, acupressure, mindfulness treatment, or yoga for the relief of breathlessness (247).
Research Priorities
Throughout this Statement, we have chosen to consider the problem of dyspnea in a broad context rather than focus on issues pertinent to specific diseases, which are addressed at length in other reviews and related consensus documents. Similarly, our recommendations regarding research priorities for dyspnea also address large themes, which are applicable to dyspnea research regardless of the underlying disease process.
New treatments and larger clinical trials. There have been many advances in the understanding of dyspnea mechanisms since the publication of the 1999 consensus statement, but these have not yet translated into improved therapies. The field is plagued by studies involving small numbers of patients (290) in what are often uncontrolled (or poorly controlled, e.g., not blinded) trials. In particular, there are still no drugs for which relief of dyspnea is an approved indication; rather, drugs are approved for the treatment of diseases in which dyspnea is a prominent symptom. We recommend that research be directed to the development and testing of therapies specifically aimed at underlying mechanisms of dyspnea, and that funding be made available to support large-scale, multi-institutional investigations.
There are far more instruments for measuring dyspnea than there are treatments. This profusion of measures makes it very difficult to compare results across studies and draw evidence-based conclusions. In addition, there is a pressing need for one or more dyspnea measures to be adequately validated as patient-reported outcomes for use as endpoints in clinical trials. Recent guidance on developing patient-reported outcome measures offers grounds for cautious optimism in this area (291). In addition, more effort needs to be directed toward validating translations of existing measures. When new measures are proposed, investigators should provide a clear justification of the reasons why they are needed; specifically, what aspect of dyspnea (e.g., sensory–perceptual, affective distress, etc.) or what patient population will be assessed better with the new instrument than with existing measures? Recent work on developing an observational respiratory distress rating for palliative care patients who are unable to self-report provides an example of such justification (19, 20, 23).
Further research is needed in the areas of neuromodulation, neuroimaging, and central processing of dyspneic sensations and associated unpleasantness and affective distress. There is a need for more rigorously designed and evaluated clinical–translational studies in these areas.
Finally, more than at any time in the past, there is a need for interdisciplinary approaches to research into dyspnea mechanisms and treatments that will accelerate translation of research findings into clinical practice. It is still too often the case that studies of dyspnea mechanisms and treatments are siloed by disease or by specialty or disciplinary focus. Studies of treatments such as pulmonary or cardiac rehabilitation, for example, as well as behavioral treatments that have the potential to be relevant to multiple conditions, have tended to be limited to a particular diagnosis or specialty. Given the difficulty of controlling dyspnea in many patients with chronic medical problems, we encourage increased communication and collaboration between medical and palliative care specialists and among clinicians and researchers across other specialties and disciplines.
Conclusion
Since the publication of the original ATS consensus statement in 1999, there has been substantial progress in research into mechanisms of dyspnea. However, there has been little progress in treatment of dyspnea. Despite advances in the therapy of a number of cardiopulmonary disorders, there are millions of patients who are severely disabled by breathlessness. Efforts to cure disease are the focus of much biomedical research and tend to grab the public's attention. However, the duty to alleviate suffering must remain a top priority. It is our hope that this document summarizes the progress that has been made and what remains to be done to allow our patients to enjoy one of our most primal needs—breathing.
Acknowledgments
This statement was prepared by an ad hoc subcommittee of the ATS Nursing Assembly and the Clinical Problems Assembly.
Members of the subcommittee:
Mark B. Parshall, Ph.D., RN (Co-Chair)
Richard M. Schwartzstein, M.D. (Co-Chair)
Lewis Adams, Ph.D.
Robert B. Banzett, Ph.D.
Jean Bourbeau, M.D.
Peter M. Calverley, M.D.
Audrey G. Gift, Ph.D., RN
Andrew Harver, Ph.D.
Suzanne C. Lareau, RN, M.S.
Donald A. Mahler, M.D.
Harold L. Manning, M.D.
Paula M. Meek, Ph.D., RN
Denis E. O'Donnell, M.D.
The subcommittee acknowledges Virginia Carrieri-Kohlman, Ph.D., RN, FAAN, and the officers and membership of the ATS Nursing Assembly for their support and encouragement. They are grateful to Dr. Karleyton C. Evans for Figure 1 and to the American Physiological Society for permission to use it. The subcommittee also thanks Ms. Anne Mattarrella of the University of New Mexico College of Nursing for invaluable assistance with technical editing, and Ms. Judy Corn of the American Thoracic Society for guidance on ATS documents policies.
Footnotes
This statement has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
References
- 1.Desbiens NA, Mueller-Rizner N, Connors AF, Wenger NS. The relationship of nausea and dyspnea to pain in seriously ill patients. Pain 1997;71:149–156. [DOI] [PubMed] [Google Scholar]
- 2.Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Public Health Nations Health 1964;54:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med 1990;150:1685–1689. [DOI] [PubMed] [Google Scholar]
- 4.Frostad A, Soyseth V, Andersen A, Gulsvik A. Respiratory symptoms as predictors of all-cause mortality in an urban community: a 30-year follow-up. J Intern Med 2006;259:520–529. [DOI] [PubMed] [Google Scholar]
- 5.Bowden J, To T, Abernethy A, Currow D. Predictors of chronic breathlessness: a large population study. BMC Public Health 2011;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currow DC, Plummer JL, Crockett A, Abernethy AP. A community population survey of prevalence and severity of dyspnea in adults. J Pain Symptom Manage 2009;38:533–545. [DOI] [PubMed] [Google Scholar]
- 7.Hawthorne VM, Watt GC, Hart CL, Hole DJ, Smith GD, Gillis CR. Cardiorespiratory disease in men and women in urban Scotland: baseline characteristics of the Renfrew/Paisley (midspan) study population. Scott Med J 1995;40:102–107. [DOI] [PubMed] [Google Scholar]
- 8.Shin C, Lee S, Abbott R, Kim J, Lee S, In K, Kimm K. Relationships between respiratory symptoms and FEV1 in men and women with normal lung function: The Korean Health and Genome Study. Lung 2005;183:301–309. [DOI] [PubMed] [Google Scholar]
- 9.Ho SF, O'Mahony MS, Steward JA, Breay P, Buchalter M, Burr ML. Dyspnoea and quality of life in older people at home. Age Ageing 2001;30:155–159. [DOI] [PubMed] [Google Scholar]
- 10.Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data 2007:1–32. [PubMed] [Google Scholar]
- 11.Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report 2010:1–31. [PubMed] [Google Scholar]
- 12.American Thoracic Society. Dyspnea: mechanisms, assessment, and management. A consensus statement. Am J Respir Crit Care Med 1999;159:321–340. [DOI] [PubMed] [Google Scholar]
- 13.Ong KC, Earnest A, Lu SJ. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest 2005;128:3810–3816. [DOI] [PubMed] [Google Scholar]
- 14.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002;121:1434–1440. [DOI] [PubMed] [Google Scholar]
- 16.Abidov A, Rozanski A, Hachamovitch R, Hayes SW, Aboul-Enein F, Cohen I, Friedman JD, Germano G, Berman DS. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med 2005;353:1889–1898. [DOI] [PubMed] [Google Scholar]
- 17.Pang PS, Cleland JGF, Teerlink JR, Collins SP, Lindsell CJ, Sopko G, Peacock WF, Fonarow GC, Aldeen AZ, Kirk JD, et al. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. Eur Heart J 2008;29:816–824. [DOI] [PubMed] [Google Scholar]
- 18.Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, Andre S, Mark Courtney D, Hasa J, Spinar J, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J 2010;31:832–841. [DOI] [PubMed] [Google Scholar]
- 19.Campbell ML. Psychometric testing of a respiratory distress observation scale. J Palliat Med 2008;11:44–50. [DOI] [PubMed] [Google Scholar]
- 20.Campbell ML. Respiratory distress: a model of responses and behaviors to an asphyxial threat for patients who are unable to self-report. Heart Lung 2008;37:54–60. [DOI] [PubMed] [Google Scholar]
- 21.Lush MT, Janson-Bjerklie S, Carrieri VK, Lovejoy N. Dyspnea in the ventilator-assisted patient. Heart Lung 1988;17:528–535. [PubMed] [Google Scholar]
- 22.Schwartzstein RM, Gold DR. Dyspnea in overweight children: is it asthma? J Allergy Clin Immunol 2009;123:1319–1320. [DOI] [PubMed] [Google Scholar]
- 23.Campbell ML, Templin T, Walch J. A respiratory distress observation scale for patients unable to self-report dyspnea. J Palliat Med 2010;13:285–290. [DOI] [PubMed] [Google Scholar]
- 24.Widdicombe J. Lung afferent activity: implications for respiratory sensation. Respir Physiol Neurobiol 2009;167:2–8. [DOI] [PubMed] [Google Scholar]
- 25.Lee LY. Respiratory sensations evoked by activation of bronchopulmonary c-fibers. Respir Physiol Neurobiol 2009;167:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Undem BJ, Nassenstein C. Airway nerves and dyspnea associated with inflammatory airway disease. Respir Physiol Neurobiol 2009;167:36–44. [DOI] [PubMed] [Google Scholar]
- 27.Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis 1990;142:1009–1014. [DOI] [PubMed] [Google Scholar]
- 28.Williams M, Garrard A, Cafarella P, Petkov J, Frith P. Quality of recalled dyspnoea is different from exercise-induced dyspnoea: an experimental study. Aust J Physiother 2009;55:177–183. [DOI] [PubMed] [Google Scholar]
- 29.Elliott MW, Adams L, Cockcroft A, MacRae KD, Murphy K, Guz A. The language of breathlessness: use of verbal descriptors by patients with cardiopulmonary disease. Am Rev Respir Dis 1991;144:826–832. [DOI] [PubMed] [Google Scholar]
- 30.Killian KJ, Watson R, Otis J, St. Amand TA, O'Byrne PM. Symptom perception during acute bronchoconstriction. Am J Respir Crit Care Med 2000;162:490–496. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 1997;155:109–115. [DOI] [PubMed] [Google Scholar]
- 32.O'Donnell DE, Chau LK, Webb KA. Qualitative aspects of exertional dyspnea in patients with interstitial lung disease. J Appl Physiol 1998;84:2000–2009. [DOI] [PubMed] [Google Scholar]
- 33.Sassi-Dambron DE, Eakin EG, Ries AL, Kaplan RM. Treatment of dyspnea in COPD. Chest 1995;107:724–729. [DOI] [PubMed] [Google Scholar]
- 34.Kunik ME, Roundy K, Veazey C, Souchek J, Richardson P, Wray NP, Stanley MA. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest 2005;127:1205–1211. [DOI] [PubMed] [Google Scholar]
- 35.Williams M, Cafarella P, Olds T, Petkov J, Frith P. Affective descriptors of the sensation of breathlessness are more highly associated with severity of impairment than physical descriptors in people with COPD. Chest 2010;138:315–322. [DOI] [PubMed] [Google Scholar]
- 36.Mahler DA, Harver A, Lentine T, Scott JA, Beck K, Schwartzstein RM. Descriptors of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med 1996;154:1357–1363. [DOI] [PubMed] [Google Scholar]
- 37.Simon PM, Schwartzstein RM, Weiss JW, Lahive K, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable sensations of breathlessness induced in normal volunteers. Am Rev Respir Dis 1989;140:1021–1027. [DOI] [PubMed] [Google Scholar]
- 38.Harver A, Mahler DA, Schwartzstein RM, Baird JC. Descriptors of breathlessness in healthy individuals: distinct and separable constructs. Chest 2000;118:679–690. [DOI] [PubMed] [Google Scholar]
- 39.Eldridge FL, Chen Z. Respiratory sensations: a neurophysiological perspective. : , Adams L, Guz A, Respiratory sensation, 1st ed New York: Marcel Dekker; 1996. pp. 19–68. [Google Scholar]
- 40.Shea SA, Banzett RB, Lansing RW. Respiratory sensations and their role in the control of breathing : , Dempsey J, Pack A, Regulation of breathing, 2nd ed New York: Marcel Dekker; 1995. p. 1219. [Google Scholar]
- 41.Cunningham D, Robbins P, Wolff C. Integration of respiratory responses to changes in alveolar partial pressures of CO2 and O2 and in arterial pH. : , Cherniack NS, Widdicombe JG, Handbook of physiology, section 3: The respiratory system, vol II: Control of breathing, part 2. Washington, DC: American Physiological Society; 1986. pp. 475–528. [Google Scholar]
- 42.Gandevia SC. Roles for perceived voluntary commands in motor control. Trends Neurosci 1987;10:81–85. [Google Scholar]
- 43.McCloskey D. Corollary discharges: motor commands and perception. : , Brooks VB, editor Handbook of physiology: Section 1: The nervous system; vol II: Motor control, part 2. Washington, D.C.: American Physiological Society; 1981. pp. 1415–1447. [Google Scholar]
- 44.Matthews PBC. Where does Sherrington's “muscular sense” originate? Muscles, joints, corollary discharges? Annu Rev Neurosci 1982;5:189–218. [DOI] [PubMed] [Google Scholar]
- 45.Altose M, Cherniack N, Fishman AP. Respiratory sensations and dyspnea. J Appl Physiol 1985;58:1051–1054. [DOI] [PubMed] [Google Scholar]
- 46.Banzett RB, Lansing RW, Brown R, Topulos GP, Yager D, Steele SM, Londoño B, Loring SH, Reid MB, Adams L, et al. 'Air hunger' from increased PCO2 persists after complete neuromuscular block in humans. Respir Physiol 1990;81:1–17. [DOI] [PubMed] [Google Scholar]
- 47.Banzett RB, Lansing RW, Reid MB, Adams L, Brown R. 'Air hunger' arising from increased PCO2 in mechanically ventilated quadriplegics. Respir Physiol 1989;76:53–67. [DOI] [PubMed] [Google Scholar]
- 48.Gandevia SC, Killian K, McKenzie DK, Crawford M, Allen GM, Gorman RB, Hales JP. Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol 1993;470:85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandevia SC, Macefield G. Projection of low-threshold afferents from human intercostal muscles to the cerebral cortex. Respir Physiol 1989;77:203–214. [DOI] [PubMed] [Google Scholar]
- 50.el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol 1986;61:896–905. [DOI] [PubMed] [Google Scholar]
- 51.Cole J. Pride and a daily marathon. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 52.Jensen D, Ofir D, O'Donnell DE. Effects of pregnancy, obesity and aging on the intensity of perceived breathlessness during exercise in healthy humans. Respir Physiol Neurobiol 2009;167:87–100. [DOI] [PubMed] [Google Scholar]
- 53.Scano G, Innocenti-Bruni G, Stendardi L. Do obstructive and restrictive lung diseases share common underlying mechanisms of breathlessness? Respir Med 2010;104:925–933. [DOI] [PubMed] [Google Scholar]
- 54.Killian KJ, Gandevia SC, Summers E, Campbell EJ. Effect of increased lung volume on perception of breathlessness, effort, and tension. J Appl Physiol 1984;57:686–691. [DOI] [PubMed] [Google Scholar]
- 55.Nishino T, Ide T, Sudo T, Sato J. Inhaled furosemide greatly alleviates the sensation of experimentally induced dyspnea. Am J Respir Crit Care Med 2000;161:1963–1967. [DOI] [PubMed] [Google Scholar]
- 56.Lane R, Adams L, Guz A. Is low-level respiratory resistive loading during exercise perceived as breathlessness? Clin Sci 1987;73:627–634. [DOI] [PubMed] [Google Scholar]
- 57.Chapman KR, Rebuck AS. Inspiratory and expiratory resistive loading as a model of dyspnea in asthma. Respiration 1983;44:425–432. [DOI] [PubMed] [Google Scholar]
- 58.Lansing RW, Im BS, Thwing JI, Legedza AT, Banzett RB. The perception of respiratory work and effort can be independent of the perception of air hunger. Am J Respir Crit Care Med 2000;162:1690–1696. [DOI] [PubMed] [Google Scholar]
- 59.O'Donnell DE, D'Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:892–898. [DOI] [PubMed] [Google Scholar]
- 60.Campbell EJM, Gandevia SC, Killian KJ, Mahutte CK, Rigg JRA. Changes in the perception of inspiratory resistive loads during partial curarization. J Physiol 1980;309:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moosavi SH, Topulos GP, Hafer A, Lansing RW, Adams L, Brown R, Banzett RB. Acute partial paralysis alters perceptions of air hunger, work and effort at constant P(CO(2)) and V(E). Respir Physiol 2000;122:45–60. [DOI] [PubMed] [Google Scholar]
- 62.Remmers JE, Brooks J, Tenney SM. Effect of controlled ventilation on the tolerable limit of hypercapnia. Respir Physiol 1968;4:78–90. [DOI] [PubMed] [Google Scholar]
- 63.Gandevia SC, Killian KJ, Campbell EJ. The effect of respiratory muscle fatigue on respiratory sensations. Clin Sci 1981;60:463–466. [DOI] [PubMed] [Google Scholar]
- 64.Lansing RW, Banzett RB. What do paralyzed awake humans feel when they attempt to move? J Mot Behav 1993;25:309–313. [DOI] [PubMed] [Google Scholar]
- 65.Lougheed MD, Fisher T, O'Donnell DE. Dynamic hyperinflation during bronchoconstriction in asthma: Implications for symptom perception. Chest 2006;130:1072–1081. [DOI] [PubMed] [Google Scholar]
- 66.Lougheed MD, Flannery J, Webb KA, O'Donnell DE. Respiratory sensation and ventilatory mechanics during induced bronchoconstriction in spontaneously breathing low cervical quadriplegia. Am J Respir Crit Care Med 2002;166:370–376. [DOI] [PubMed] [Google Scholar]
- 67.Moy ML, Lantin ML, Harver A, Schwartzstein RM. Language of dyspnea in assessment of patients with acute asthma treated with nebulized albuterol. Am J Respir Crit Care Med 1998;158:749–753. [DOI] [PubMed] [Google Scholar]
- 68.Moy ML, Weiss JW, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med 2000;162:451–455. [DOI] [PubMed] [Google Scholar]
- 69.Harver A, Schwartzstein RM, Kotses H, Humphries CT, Schmaling KB, Mullin ML. Descriptors of breathlessness in children with persistent asthma. Chest 2011;139:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lougheed MD, Lam M, Forkert L, Webb KA, O'Donnell DE. Breathlessness during acute bronchoconstriction in asthma: pathophysiologic mechanisms. Am Rev Respir Dis 1993;148:1452–1459. [DOI] [PubMed] [Google Scholar]
- 71.Petit JM, Delhez L. Some recent experimental data on the origin of dyspnea and ventilatory regulation in asthmatics [in French]. Acta Tuberc Pneumol Belg 1970;61:169–186. [PubMed] [Google Scholar]
- 72.Marks GB, Yates DH, Sist M, Ceyhan B, De Campos M, Scott DM, Barnes PJ. Respiratory sensation during bronchial challenge testing with methacholine, sodium metabisulphite, and adenosine monophosphate. Thorax 1996;51:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laveneziana P, Lotti P, Coli C, Binazzi B, Chiti L, Stendardi L, Duranti R, Scano G. Mechanisms of dyspnoea and its language in patients with asthma. Eur Respir J 2006;27:742–747. [DOI] [PubMed] [Google Scholar]
- 74.Binks AP, Moosavi SH, Banzett RB, Schwartzstein RM. “Tightness” sensation of asthma does not arise from the work of breathing. Am J Respir Crit Care Med 2002;165:78–82. [DOI] [PubMed] [Google Scholar]
- 75.Campbell EJM, Howell JBL. The sensation of breathlessness. Br Med Bull 1963;19:36–40. [DOI] [PubMed] [Google Scholar]
- 76.Schwartzstein R, Manning H, Weiss J, Weinberger S. Dyspnea: a sensory experience. Lung 1990;168:185–199. [DOI] [PubMed] [Google Scholar]
- 77.O'Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation: the role of lung hyperinflation. Am Rev Respir Dis 1993;148:1351–1357. [DOI] [PubMed] [Google Scholar]
- 78.Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med 1995;333:1547–1553. [DOI] [PubMed] [Google Scholar]
- 79.O'Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol 2008;105:753–755. [DOI] [PubMed] [Google Scholar]
- 80.O'Donnell DE, Ora J, Webb KA, Laveneziana P, Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol 2009;167:116–132. [DOI] [PubMed] [Google Scholar]
- 81.Wright GW, Branscomb BV. The origin of the sensations of dyspnea? Trans Am Clin Climatol Assoc 1955;66:116–125. [PMC free article] [PubMed] [Google Scholar]
- 82.Harty HR, Corfield DR, Schwartzstein RM, Adams L. External thoracic restriction, respiratory sensation, and ventilation during exercise in men. J Appl Physiol 1999;86:1142–1150. [DOI] [PubMed] [Google Scholar]
- 83.O'Donnell DE, Hong HH, Webb KA. Respiratory sensation during chest wall restriction and dead space loading in exercising men. J Appl Physiol 2000;88:1859–1869. [DOI] [PubMed] [Google Scholar]
- 84.Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol 2003;94:141–154. [DOI] [PubMed] [Google Scholar]
- 85.O'Donnell DE, Hamilton AL, Webb KA. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J Appl Physiol 2006;101:1025–1035. [DOI] [PubMed] [Google Scholar]
- 86.O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1557–1565. [DOI] [PubMed] [Google Scholar]
- 87.O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:770–777. [DOI] [PubMed] [Google Scholar]
- 88.Rock LK, Schwartzstein RM. Mechanisms of dyspnea in chronic lung disease. Curr Opin Support Palliat Care 2007;1:102–108. [DOI] [PubMed] [Google Scholar]
- 89.Lane R, Adams L, Guz A. The effects of hypoxia and hypercapnia on perceived breathlessness during exercise in humans. J Physiol 1990;428:579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lane R, Adams L. Metabolic acidosis and breathlessness during exercise and hypercapnia in man. J Physiol 1993;461:47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dempsey JA, Forster HV, Ainsworth DM. Regulation of hyperpnea, hyperventilation, and respiratory muscle recruitment during exercise. : , Dempsey J, Pack A, Regulation of breathing, 2nd ed New York: Marcel Dekker; 1995. pp. 1065–1134. [Google Scholar]
- 92.Mitchell GS, Babb TG. Layers of exercise hyperpnea: modulation and plasticity. Respir Physiol Neurobiol 2006;151:251–266. [DOI] [PubMed] [Google Scholar]
- 93.Adams L, Lane R, Shea SA, Cockcroft A, Guz A. Breathlessness during different forms of ventilatory stimulation: a study of mechanisms in normal subjects and respiratory patients. Clin Sci 1985;69:663–672. [DOI] [PubMed] [Google Scholar]
- 94.Chen Z, Eldridge FL, Wagner PG. Respiratory-associated thalamic activity is related to level of respiratory drive. Respir Physiol 1992;90:99–113. [DOI] [PubMed] [Google Scholar]
- 95.Ramsay SC, Adams L, Murphy K, Corfield DR, Grootoonk S, Bailey DL, Frackowiak RS, Guz A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. J Physiol 1993;461:85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Colebatch JG, Adams L, Murphy K, Martin AJ, Lammertsma AA, Tochon-Danguy HJ, Clark JC, Friston KJ, Guz A. Regional cerebral blood flow during volitional breathing in man. J Physiol 1991;443:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med 2008;177:1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flume PA, Eldridge FL, Edwards LJ. Role of vagal input in the relief of the distress of breathholding: Normals vs. Pts. With double lung transplants. Am Rev Respir Dis 1993;147:A550. [Google Scholar]
- 99.Hill L, Flack F. The effect of excess of carbon dioxide and of want of oxygen upon the respiration and the circulation. J Physiol 1908;37:77–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett RB. Reduced tidal volume increases 'air hunger' at fixed PCO2 in ventilated quadriplegics. Respir Physiol 1992;90:19–30. [DOI] [PubMed] [Google Scholar]
- 101.Opie L, Smith A, Spalding J. Conscious appreciation of the effects produced by independent changes of ventilation volume and of end-tidal PCO2 in paralysed patients. J Physiol 1959;149:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Flume PA, Eldridge FL, Edwards LJ, Houser LM. Relief of distress of breathholding: separate effects of expiration and inspiration. Respir Physiol 1995;101:41–46. [DOI] [PubMed] [Google Scholar]
- 103.Flume PA, Eldridge FL, Edwards LJ, Mattison LE. Relief of the 'air hunger' of breathholding: a role for pulmonary stretch receptors. Respir Physiol 1996;103:221–232. [DOI] [PubMed] [Google Scholar]
- 104.Tryfon S, Kontakiotis T, Mavrofridis E, Patakas D. Hering-Breuer reflex in normal adults and in patients with chronic obstructive pulmonary disease and interstitial fibrosis. Respiration 2001;68:140–144. [DOI] [PubMed] [Google Scholar]
- 105.Eldridge FL, Chen Z. Respiratory-associated rhythmic firing of midbrain neurons is modulated by vagal input. Respir Physiol 1992;90:31–46. [DOI] [PubMed] [Google Scholar]
- 106.Fowler WS. Breaking point of breath-holding. J Appl Physiol 1954;6:539–545. [DOI] [PubMed] [Google Scholar]
- 107.Flume PA, Eldridge FL, Edwards LJ, Houser LM. The Fowler breathholding study revisited: continuous rating of respiratory sensation. Respir Physiol 1994;95:53–66. [DOI] [PubMed] [Google Scholar]
- 108.Jensen D, Amjadi K, Harris-McAllister V, Webb KA, O'Donnell DE. Mechanisms of dyspnoea relief and improved exercise endurance after furosemide inhalation in COPD. Thorax 2008;63:606–613. [DOI] [PubMed] [Google Scholar]
- 109.Moosavi SH, Binks AP, Lansing RW, Topulos GP, Banzett RB, Schwartzstein RM. Effect of inhaled furosemide on air hunger induced in healthy humans. Respir Physiol Neurobiol 2007;156:1–8. [DOI] [PubMed] [Google Scholar]
- 110.Sudo T, Hayashi F, Nishino T. Responses of tracheobronchial receptors to inhaled furosemide in anesthetized rats. Am J Respir Crit Care Med 2000;162:971–975. [DOI] [PubMed] [Google Scholar]
- 111.Harty HR, Mummery CJ, Adams L, Banzett RB, Wright IG, Banner NR, Yacoub MH, Guz A. Ventilatory relief of the sensation of the urge to breathe in humans: are pulmonary receptors important? J Physiol 1996;490:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Butler JE, Anand A, Crawford MR, Glanville AR, McKenzie DK, Paintal AS, Taylor JL, Gandevia SC. Changes in respiratory sensations induced by lobeline after human bilateral lung transplantation. J Physiol 2001;534:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith J, Albert P, Bertella E, Lester J, Jack S, Calverley P. Qualitative aspects of breathlessness in health and disease. Thorax 2009;64:713–718. [DOI] [PubMed] [Google Scholar]
- 114.Parshall MB. Psychometric characteristics of dyspnea descriptor ratings in emergency department patients with exacerbated chronic obstructive pulmonary disease. Res Nurs Health 2002;25:331–344. [DOI] [PubMed] [Google Scholar]
- 115.Banzett RB, Lansing RW, Evans KC, Shea SA. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol 1996;103:19–31. [DOI] [PubMed] [Google Scholar]
- 116.Parshall MB, Welsh JD, Brockopp DY, Heiser RM, Schooler MP, Cassidy KB. Reliability and validity of dyspnea sensory quality descriptors in heart failure patients treated in an emergency department. Heart Lung 2001;30:57–65. [DOI] [PubMed] [Google Scholar]
- 117.Smoller J, Pollack M, Otto M, Rosenbaum J, Kradin R. Panic anxiety, dyspnea, and respiratory disease: theoretical and clinical considerations. Am J Respir Crit Care Med 1996;154:6–17. [DOI] [PubMed] [Google Scholar]
- 118.Nardi AE, Freire RC, Zin WA. Panic disorder and control of breathing. Respir Physiol Neurobiol 2009;167:133–143. [DOI] [PubMed] [Google Scholar]
- 119.Perna G, Caldirola D, Namia C, Cucchi M, Vanni G, Bellodi L. Language of dyspnea in panic disorder. Depress Anxiety 2004;20:32–38. [DOI] [PubMed] [Google Scholar]
- 120.Rassovsky Y, Abrams K, Kushner MG. Suffocation and respiratory responses to carbon dioxide and breath holding challenges in individuals with panic disorder. J Psychosom Res 2006;60:291–298. [DOI] [PubMed] [Google Scholar]
- 121.Brenes GAP. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosom Med 2003;65:963–970. [DOI] [PubMed] [Google Scholar]
- 122.Giardino ND, Curtis JL, Abelson JL, King AP, Pamp B, Liberzon I, Martinez FJ. The impact of panic disorder on interoception and dyspnea reports in chronic obstructive pulmonary disease. Biol Psychol 2010;84:142–146. [DOI] [PubMed] [Google Scholar]
- 123.Moore MC, Zebb BJ. The catastrophic misinterpretation of physiological distress. Behav Res Ther 1999;37:1105–1118. [DOI] [PubMed] [Google Scholar]
- 124.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 2000;157:493–505. [DOI] [PubMed] [Google Scholar]
- 125.Parshall MB, Carle AC, Ice U, Taylor R, Powers J. Validation of a 3-factor measurement model of dyspnea in hospitalized adults with heart failure. Heart Lung 2012;44:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paintal AS. Sensations from J receptors. News Physiol Sci 1995;10:238–243. [Google Scholar]
- 127.Dehghani GA, Parvizi MR, Sharif-Kazemi MB, Raj H, Anand A, Paintal AS. Presence of lobeline-like sensations in exercising patients with left ventricular dysfunction. Respir Physiol Neurobiol 2004;143:9–20. [DOI] [PubMed] [Google Scholar]
- 128.Raj H, Singh VK, Anand A, Paintal AS. Sensory origin of lobeline-induced sensations: a correlative study in man and cat. J Physiol 1995;482:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nishino T, Isono S, Ishikawa T, Shinozuka N. An additive interaction between different qualities of dyspnea produced in normal human subjects. Respir Physiol Neurobiol 2007;155:14–21. [DOI] [PubMed] [Google Scholar]
- 130.Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: Development and initial testing of the Dyspnoea-12. Thorax 2010;65:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Swigris JJ, Yorke J, Sprunger DB, Swearingen C, Pincus T, du Bois RM, Brown KK, Fischer A. Assessing dyspnea and its impact on patients with connective tissue disease-related interstitial lung disease. Respir Med 2010;104:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yorke J, Russell A-M, Swigris J, Shuldham C, Haigh C, Rochnia N, Hoyle J, Jones PW. Assessment of dyspnea in asthma: validation of the dyspnea-12. J Asthma 2011;48:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yorke J, Swigris J, Russell A-M, Moosavi SH, Ng Man Kwong G, Longshaw M, Jones PW. Dyspnea-12 is a valid and reliable measure of breathlessness in patients with interstitial lung disease. Chest 2011;139:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport 2000;11:2117–2120. [DOI] [PubMed] [Google Scholar]
- 135.Corfield DR, Fink GR, Ramsay SC, Murphy K, Harty HR, Watson JD, Adams L, Frackowiak RS, Guz A. Evidence for limbic system activation during CO2-stimulated breathing in man. J Physiol 1995;488:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corfield DR, Fink GR, Ramsay SC, Murphy K, Harty HR, Watson JD, Adams L, Frackowiak RS, Guz A. Activation of limbic structures during CO2-stimulated breathing in awake man. : , Semple SJG, Adams L, Whipp BJ, Advances in experimental medicine and biology (vol 393: Modeling and control of ventilation). New York: Plenum; 1995. pp. 331–334. [DOI] [PubMed] [Google Scholar]
- 137.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. Bold fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 2002;88:1500–1511. [DOI] [PubMed] [Google Scholar]
- 138.Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med 2001;163:951–957. [DOI] [PubMed] [Google Scholar]
- 139.von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Buchel C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Respir Crit Care Med 2008;177:1026–1032. [DOI] [PubMed] [Google Scholar]
- 140.von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Büchel C. Dyspnea and pain share emotion-related brain network. Neuroimage 2009;48:200–206. [DOI] [PubMed] [Google Scholar]
- 141.Evans KC. Cortico-limbic circuitry and the airways: insights from functional neuroimaging of respiratory afferents and efferents. Biol Psychol 2010;84:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evans KC, Dougherty DD, Schmid AM, Scannell E, McCallister A, Benson H, Dusek JA, Lazar SW. Modulation of spontaneous breathing via limbic/paralimbic-bulbar circuitry: an event-related fMRI study. Neuroimage 2009;47:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Medford N, Critchley H. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct 2010;214:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655–666. [DOI] [PubMed] [Google Scholar]
- 145.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 2003;13:500–505. [DOI] [PubMed] [Google Scholar]
- 146.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- 147.Craig AD. The sentient self. Brain Struct Funct 2010;214:563–577. [DOI] [PubMed] [Google Scholar]
- 148.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci 2004;7:189–195. [DOI] [PubMed] [Google Scholar]
- 149.Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, et al. Brain responses associated with consciousness of breathlessness (air hunger). Proc Natl Acad Sci USA 2001;98:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Parsons LM, Egan G, Liotti M, Brannan S, Denton D, Shade R, Robillard R, Madden L, Abplanalp B, Fox PT. Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air. Proc Natl Acad Sci USA 2001;98:2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000;288:1769–1772. [DOI] [PubMed] [Google Scholar]
- 152.Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv 2002;2:392–403. [DOI] [PubMed] [Google Scholar]
- 153.Logothetis NK. What we can do and what we cannot do with fMRI. Nature 2008;453:869–878. [DOI] [PubMed] [Google Scholar]
- 154.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: a review and meta- analysis (2000). Neurophysiol Clin 2000;30:263–288. [DOI] [PubMed] [Google Scholar]
- 155.Bonaz B, Baciu M, Papillon E, Bost R, Gueddah N, Le Bas JF, Fournet J, Segebarth C. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol 2002;97:654–661. [DOI] [PubMed] [Google Scholar]
- 156.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004;53:1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc Natl Acad Sci USA 1999;96:2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 1999;96:4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Denton D. The primordial emotions: the dawning of consciousness. New York: Oxford University Press; 2006. [Google Scholar]
- 160.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol 2009;35:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jack S, Kemp GJ, Bimson WE, Calverley PMA, Corfield DR. Patterns of brain activity in response to respiratory stimulation in patients with idiopathic hyperventilation (IHV). : , Homma I, Onimaru H, Fukuchi Y, Advances in experimental medicine and biology (vol 669: New frontiers in respiratory control: XIth annual Oxford conference on modeling & control of breathing). New York: Springer; 2010. pp. 341–345. [DOI] [PubMed] [Google Scholar]
- 162.Pattinson KTS, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG. Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci 2009;29:8177–8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Macey KE, Macey PM, Woo MA, Henderson LA, Frysinger RC, Harper RK, Alger JR, Yan-Go F, Harper RM. Inspiratory loading elicits aberrant fMRI signal changes in obstructive sleep apnea. Respir Physiol Neurobiol 2006;151:44–60. [DOI] [PubMed] [Google Scholar]
- 164.Harper RM, Macey PM, Woo MA, Macey KE, Keens TG, Gozal D, Alger JR. Hypercapnic exposure in congenital central hypoventilation syndrome reveals cns respiratory control mechanisms. J Neurophysiol 2005;93:1647–1658. [DOI] [PubMed] [Google Scholar]
- 165.Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, Harper RM. Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic deficits in congenital central hypoventilation syndrome. J Appl Physiol 2005;98:958–969. [DOI] [PubMed] [Google Scholar]
- 166.Banzett RB, Adams L, O'Donnell CR, Gilman SA, Lansing RW, Schwartzstein RM. Using laboratory models to test treatment: morphine reduces dyspnea and hypercapnic ventilatory response. Am J Respir Crit Care Med 2011;184:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Supinski G, Dimarco A, Bark H, Chapman K, Clary S, Altose M. Effect of codeine on the sensations elicited by loaded breathing. Am Rev Respir Dis 1990;141:1516–1521. [DOI] [PubMed] [Google Scholar]
- 168.Kirsch JL, Muro JR, Stansbury DW, Fischer CE, Monfore R, Light RW. Effect of naloxone on maximal exercise performance and control of ventilation in COPD. Chest 1989;96:761–766. [DOI] [PubMed] [Google Scholar]
- 169.Bellofiore S, Di Maria GU, Privitera S, Sapienza S, Milic-Emili J, Mistretta A. Endogenous opioids modulate the increase in ventilatory output and dyspnea during severe acute bronchoconstriction. Am Rev Respir Dis 1990;142:812–816. [DOI] [PubMed] [Google Scholar]
- 170.Akiyama Y, Nishimura M, Kobayashi S, Yoshioka A, Yamamoto M, Miyamoto K, Kawakami Y. Effects of naloxone on the sensation of dyspnea during acute respiratory stress in normal adults. J Appl Physiol 1993;74:590–595. [DOI] [PubMed] [Google Scholar]
- 171.Nishino T, Isono S, Shinozuka N, Ishikawa T. Effects of naloxone on respiratory sensation before and after a removal of severe respiratory stress. Jpn J Physiol 2005;55:117–126. [DOI] [PubMed] [Google Scholar]
- 172.Mahler DA, Murray JA, Waterman LA, Ward J, Kraemer WJ, Zhang X, Baird JC. Endogenous opioids modify dyspnoea during treadmill exercise in patients with COPD. Eur Respir J 2009;33:771–777. [DOI] [PubMed] [Google Scholar]
- 173.Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:622–629. [DOI] [PubMed] [Google Scholar]
- 174.De Troyer A, Leeper JB, McKenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med 1997;155:1335–1340. [DOI] [PubMed] [Google Scholar]
- 175.Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol 1950;2:592–607. [DOI] [PubMed] [Google Scholar]
- 176.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med 2007;21:177–191. [DOI] [PubMed] [Google Scholar]
- 177.Bausewein C, Farquhar M, Booth S, Gysels M, Higginson IJ. Measurement of breathlessness in advanced disease: a systematic review. Respir Med 2007;101:399–410. [DOI] [PubMed] [Google Scholar]
- 178.Johnson MJ, Oxberry SG, Cleland JGF, Clark AL. Measurement of breathlessness in clinical trials in patients with chronic heart failure: the need for a standardized approach. A systematic review. Eur J Heart Fail 2010;12:137–147. [DOI] [PubMed] [Google Scholar]
- 179.Mularski RA, Campbell ML, Asch SM, Reeve BB, Basch E, Maxwell TL, Hoverman JR, Cuny J, Clauser SB, Snyder C, et al. A review of quality of care evaluation for the palliation of dyspnea. Am J Respir Crit Care Med 2010;181:534–538. [DOI] [PubMed] [Google Scholar]
- 180.Dorman S, Jolley C, Abernethy A, Currow D, Johnson M, Farquhar M, Griffiths G, Peel T, Moosavi S, Byrne A, et al. Researching breathlessness in palliative care: consensus statement of the National Cancer Research Institute Palliative Care Breathlessness Subgroup. Palliat Med 2009;23:213–227. [DOI] [PubMed] [Google Scholar]
- 181.Aitken RCB. Measurement of feelings using visual analogue scales. Proc R Soc Med 1969;62:989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gift AG. Visual analogue scales: measurement of subjective phenomena. Nurs Res 1989;38:286–288. [PubMed] [Google Scholar]
- 183.Gift AG. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs 1989;14:323–325. [DOI] [PubMed] [Google Scholar]
- 184.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 1970;2:92–98. [PubMed] [Google Scholar]
- 185.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381. [PubMed] [Google Scholar]
- 186.Guyatt GH, Townsend M, Berman LB, Keller JL. A comparison of Likert and visual analogue scales for measuring change in function. J Chronic Dis 1987;40:1129–1133. [DOI] [PubMed] [Google Scholar]
- 187.Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care 1998;7:200–204. [PubMed] [Google Scholar]
- 188.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277:968–971. [DOI] [PubMed] [Google Scholar]
- 189.Wan L, Van Diest I, De Peuter S, Bogaerts K, Van den Bergh O. Repeated breathlessness experiences induced by hypercapnia: differential effects on intensity and unpleasantness. Chest 2009;135:455–461. [DOI] [PubMed] [Google Scholar]
- 190.Carrieri-Kohlman V, Gormley JM, Eiser S, Demir-Deviren S, Nguyen H, Paul SM, Stulbarg MS. Dyspnea and the affective response during exercise training in obstructive pulmonary disease. Nurs Res 2001;50:136–146. [DOI] [PubMed] [Google Scholar]
- 191.Wilson RC, Jones PW. Differentiation between the intensity of breathlessness and the distress it evokes in normal subjects during exercise. Clin Sci 1991;80:65–70. [DOI] [PubMed] [Google Scholar]
- 192.Carrieri-Kohlman V, Gormley JM, Douglas MK, Paul SM, Stulbarg MS. Differentiation between dyspnea and its affective components. West J Nurs Res 1996;18:626–642. [DOI] [PubMed] [Google Scholar]
- 193.Carrieri-Kohlman V, Donesky-Cuenco D, Park SK, Mackin L, Nguyen HQ, Paul SM. Additional evidence for the affective dimension of dyspnea in patients with COPD. Res Nurs Health 2010;33:4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wan L, Van Diest I, De Peuter S, Bogaerts K, Oyen N, Hombroux N, Van de Woestijne K, Gallego J, Van den Bergh O. Repeated experiences of air hunger and ventilatory behavior in response to hypercapnia in the standardized rebreathing test: effects of anxiety. Biol Psychol 2008;77:223–232. [DOI] [PubMed] [Google Scholar]
- 195.Clark WC, Yang JC, Tsui S-L, Ng K-F, Bennett Clark S. Unidimensional pain rating scales: a multidimensional affect and pain survey (MAPS) analysis of what they really measure. Pain 2002;98:241–247. [DOI] [PubMed] [Google Scholar]
- 196.Schwartzstein RM, Adams L. Dyspnea. : , Mason RJ, Broaddus VC, Martin TR, King TE, Schraufnagel DE, Murray JF, Nadel JA, Murray and Nadel's textbook of respiratory medicine [electronic edition], 5th ed Philadelphia: Saunders; 2010. [Google Scholar]
- 197.Flaherty KR, Wald J, Weisman IM, Zeballos RJ, Schork MA, Blaivas M, Rubenfire M, Martinez FJ. Unexplained exertional limitation: characterization of patients with a mitochondrial myopathy. Am J Respir Crit Care Med 2001;164:425–432. [DOI] [PubMed] [Google Scholar]
- 198.Hekier E, Mandel J. A 22-year-old woman with unexplained dyspnea. Chest 2009;136:867–876. [DOI] [PubMed] [Google Scholar]
- 199.Martinez FJ, Stanopoulos I, Acero R, Becker FS, Pickering R, Beamis JF. Graded comprehensive cardiopulmonary exercise testing in the evaluation of dyspnea unexplained by routine evaluation. Chest 1994;105:168–174. [DOI] [PubMed] [Google Scholar]
- 200.Wilcock A, Crosby V, Hughes A, Fielding K, Corcoran R, Tattersfield AE. Descriptors of breathlessness in patients with cancer and other cardiorespiratory diseases. J Pain Symptom Manage 2002;23:182–189. [DOI] [PubMed] [Google Scholar]
- 201.Han J, Zhu Y, Li S, Chen X, Put C, Van de Woestijne KP, Van den Bergh O. Respiratory complaints in chinese: cultural and diagnostic specificities. Chest 2005;127:1942–1951. [DOI] [PubMed] [Google Scholar]
- 202.Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest 2000;117:935–943. [DOI] [PubMed] [Google Scholar]
- 203.Phankingthongkum S, Daengsuwan T, Visitsunthorn N, Thamlikitkul V, Udompunthuruk S, Vichyanond P. How do Thai children and adolescents describe asthma symptoms? Pediatr Allergy Immunol 2002;13:119–124. [DOI] [PubMed] [Google Scholar]
- 204.Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V, Curtis JR, Manning HL, Mularski RA, Varkey B, Campbell M, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010;137:674–691. [DOI] [PubMed] [Google Scholar]
- 205.van Belle A, Buller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, Kramer MH, Kruip MJ, Kwakkel-van Erp JM, Leebeek FW, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d-dimer testing, and computed tomography. JAMA 2006;295:172–179. [DOI] [PubMed] [Google Scholar]
- 206.Brotman DJ, Segal JB, Jani JT, Petty BG, Kickler TS. Limitations of d-dimer testing in unselected inpatients with suspected venous thromboembolism. Am J Med 2003;114:276–282. [DOI] [PubMed] [Google Scholar]
- 207.Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, Pfisterer M, Perruchoud AP. Use of b-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 2004;350:647–654. [DOI] [PubMed] [Google Scholar]
- 208.Mueller C, Laule-Kilian K, Christ A, Perruchoud A. The use of b-type natriuretic peptide in the management of patients with diabetes and acute dyspnoea. Diabetologia 2006;49:629–636. [DOI] [PubMed] [Google Scholar]
- 209.Schneider HG, Lam L, Lokuge A, Krum H, Naughton MT, De Villiers Smit P, Bystrzycki A, Eccleston D, Federman J, Flannery G, et al. B-type natriuretic peptide testing, clinical outcomes, and health services use in emergency department patients with dyspnea: a randomized trial. Ann Intern Med 2009;150:365–371. [DOI] [PubMed] [Google Scholar]
- 210.Worster A, Balion CM, Hill SA, Santaguida P, Ismaila A, McKelvie R, Reichert SM, McQueen MJ, Booker L, Raina PS. Diagnostic accuracy of BNP and NT-proBNP in patients presenting to acute care settings with dyspnea: a systematic review. Clin Biochem 2008;41:250–259. [DOI] [PubMed] [Google Scholar]
- 211.Korenstein D, Wisnivesky J, Wyer P, Adler R, Ponieman D, McGinn T. The utility of b-type natriuretic peptide in the diagnosis of heart failure in the emergency department: a systematic review. BMC Emerg Med 2007;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Heart Failure Society Of America. Executive summary: HFSA 2010 comprehensive heart failure practice guideline. J Card Fail 2010;16:475–539. [Google Scholar]
- 213.Heart Failure Society Of America. 2010 HFSA comprehensive heart failure practice guideline [Internet]. c2010. [accessed 2011 Dec 30]. Available from: http://www.heartfailureguideline.org/ [DOI] [PubMed]
- 214.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, et al. , American College of Cardiology/American Heart Association Task Force on Practice Guidelines. (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: summary article. Circulation 2005;112:1825–1852. [DOI] [PubMed] [Google Scholar]
- 215.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, et al. , American College of Cardiology/American Heart Association Task Force on Practice Guidelines. (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: summary article. J Am Coll Cardiol 2005;46:1116–1143. [Google Scholar]
- 216.Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley PMA, Chavannes N, Dillard T, Fahy B, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- 217.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet; updated 2011]. c2011. [accessed 2012 Jan 7]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011Dec30.pdf
- 218.Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. Asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. An official American Thoracic Society/European Respiratory Society statement. Am J Respir Crit Care Med 2009;180:59–99. [DOI] [PubMed] [Google Scholar]
- 219.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, et al. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology, developed in collaboration with the Heart Failure Association of the ESC (HFA), and endorsed by the European Society of Intensive Care Medicine (ESICM). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur J Heart Fail 2008;10:933–989. [DOI] [PubMed] [Google Scholar]
- 220.McDonald CF, Blyth CM, Lazarus MD, Marschner I, Barter CE. Exertional oxygen of limited benefit in patients with chronic obstructive pulmonary disease and mild hypoxemia. Am J Respir Crit Care Med 1995;152:1616–1619. [DOI] [PubMed] [Google Scholar]
- 221.Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database Syst Rev 2008;CD004769. [DOI] [PubMed] [Google Scholar]
- 222.McKeon JL, Murree-Allen K, Saunders NA. Effects of breathing supplemental oxygen before progressive exercise in patients with chronic obstructive lung disease. Thorax 1988;43:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Uronis HE, Currow DC, McCrory DC, Samsa GP, Abernethy AP. Oxygen for relief of dyspnoea in mildly- or non-hypoxaemic patients with cancer: a systematic review and meta-analysis. Br J Cancer 2008;98:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Moore RP, Berlowitz DJ, Denehy L, Pretto JJ, Brazzale DJ, Sharpe K, Jackson B, McDonald CF. A randomised trial of domiciliary, ambulatory oxygen in patients with COPD and dyspnoea but without resting hypoxaemia. Thorax 2011;66:32–37. [DOI] [PubMed] [Google Scholar]
- 225.Somfay A, Porszasz J, Lee SM, Casaburi R. Dose–response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J 2001;18:77–84. [DOI] [PubMed] [Google Scholar]
- 226.Swinburn CR, Wakefield JM, Jones PW. Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemia. Clin Sci 1984;67:515–519. [DOI] [PubMed] [Google Scholar]
- 227.Liss HP, Grant BJ. The effect of nasal flow on breathlessness in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988;137:1285–1288. [DOI] [PubMed] [Google Scholar]
- 228.Abernethy AP, McDonald CF, Frith PA, Clark K, Herndon JE, Marcello J, Young IH, Bull J, Wilcock A, Booth S, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010;376:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dean NC, Brown JK, Himelman RB, Doherty JJ, Gold WM, Stulbarg MS. Oxygen may improve dyspnea and endurance in patients with chronic obstructive pulmonary disease and only mild hypoxemia. Am Rev Respir Dis 1992;146:941–945. [DOI] [PubMed] [Google Scholar]
- 230.Qaseem A, Snow V, Shekelle P, Casey DE, Cross JT, Owens DK, for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2008;148:141–146. [DOI] [PubMed] [Google Scholar]
- 231.Eves ND, Petersen SR, Haykowsky MJ, Wong EY, Jones RL. Helium-hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:763–771. [DOI] [PubMed] [Google Scholar]
- 232.Laude EA, Duffy NC, Baveystock C, Dougill B, Campbell MJ, Lawson R, Jones PW, Calverley PM. The effect of helium and oxygen on exercise performance in chronic obstructive pulmonary disease: a randomized crossover trial. Am J Respir Crit Care Med 2006;173:865–870. [DOI] [PubMed] [Google Scholar]
- 233.Palange P, Valli G, Onorati P, Antonucci R, Paoletti P, Rosato A, Manfredi F, Serra P. Effect of heliox on lung dynamic hyperinflation, dyspnea, and exercise endurance capacity in COPD patients. J Appl Physiol 2004;97:1637–1642. [DOI] [PubMed] [Google Scholar]
- 234.Ahmedzai SH, Laude E, Robertson A, Troy G, Vora V. A double-blind, randomised, controlled phase II trial of heliox28 gas mixture in lung cancer patients with dyspnoea on exertion. Br J Cancer 2004;90:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003;327:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Johnson MA, Woodcock AA, Geddes DM. Dihydrocodeine for breathlessness in “pink puffers”. Br Med J (Clin Res Ed) 1983;286:675–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Woodcock AA, Gross ER, Geddes DM. Drug treatment of breathlessness: contrasting effects of diazepam and promethazine in pink puffers. Br Med J (Clin Res Ed) 1981;283:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Allen S, Raut S, Woollard J, Vassallo M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med 2005;19:128–130. [DOI] [PubMed] [Google Scholar]
- 239.Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Ann Intern Med 1993;119:906–907. [DOI] [PubMed] [Google Scholar]
- 240.Johnson MJ, McDonagh TA, Harkness A, McKay SE, Dargie HJ. Morphine for the relief of breathlessness in patients with chronic heart failure—a pilot study. Eur J Heart Fail 2002;4:753–756. [DOI] [PubMed] [Google Scholar]
- 241.Poole PJ, Veale AG, Black PN. The effect of sustained-release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1877–1880. [DOI] [PubMed] [Google Scholar]
- 242.Lorenz KA, Lynn J, Dy SM, Shugarman LR, Wilkinson A, Mularski RA, Morton SC, Hughes RG, Hilton LK, Maglione M, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med 2008;148:147–159. [DOI] [PubMed] [Google Scholar]
- 243.Currow DC, McDonald C, Oaten S, Kenny B, Allcroft P, Frith P, Briffa M, Johnson MJ, Abernethy AP. Once-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage 2011;42:388–399. [DOI] [PubMed] [Google Scholar]
- 244.Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage 1990;5:341–344. [DOI] [PubMed] [Google Scholar]
- 245.Mazzocato C, Buclin T, Rapin C-H. The effects of morphine on dyspnea and ventilatory function in elderly patients with advanced cancer: a randomized double-blind controlled trial. Ann Oncol 1999;10:1511–1514. [DOI] [PubMed] [Google Scholar]
- 246.Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev 2001;CD002066. [DOI] [PubMed] [Google Scholar]
- 247.Marciniuk D, Goodridge D, Hernandez P, Rocker G, Balter M, Bailey P, Ford G, Bourbeau J, O'Donnell DE, Maltais F, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J 2011;18:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lanken PN, Terry PB, DeLisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, Levy M, Mularski RA, Osborne ML, Prendergast TJ, et al. ; ATS End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med 2008;177:912–927. [DOI] [PubMed] [Google Scholar]
- 249.Ong KC, Kor AC, Chong WF, Earnest A, Wang YT. Effects of inhaled furosemide on exertional dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;169:1028–1033. [DOI] [PubMed] [Google Scholar]
- 250.Wilcock A, Walton A, Manderson C, Feathers L, El Khoury B, Lewis M, Chauhan A, Howard P, Bell S, Frisby J, et al. Randomised, placebo controlled trial of nebulised furosemide for breathlessness in patients with cancer. Thorax 2008;63:872–875. [DOI] [PubMed] [Google Scholar]
- 251.Singh NP, Despars JA, Stansbury DW, Avalos K, Light RW. Effects of buspirone on anxiety levels and exercise tolerance in patients with chronic airflow obstruction and mild anxiety. Chest 1993;103:800–804. [DOI] [PubMed] [Google Scholar]
- 252.Man GC, Hsu K, Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest 1986;90:832–836. [DOI] [PubMed] [Google Scholar]
- 253.Simon ST, Higginson IJ, Booth S, Harding R, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev 2010;2010:CD007354 10.1002/14651858.CD007354.pub2. [DOI] [PubMed] [Google Scholar]
- 254.Lacasse Y, Beaudoin L, Rousseau L, Maltais F. Randomized trial of paroxetine in end-stage COPD. Monaldi Arch Chest Dis 2004;61:140–147. [DOI] [PubMed] [Google Scholar]
- 255.Rice KL, Kronenberg RS, Hedemark LL, Niewoehner DE. Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. Br J Dis Chest 1987;81:287–292. [DOI] [PubMed] [Google Scholar]
- 256.Schiffman GL, Stansbury DW, Fischer CE, Sato RI, Light RW, Brown SE. Indomethacin and perception of dyspnea in chronic airflow obstruction. Am Rev Respir Dis 1988;137:1094–1098. [DOI] [PubMed] [Google Scholar]
- 257.O'Neill PA, Stretton TB, Stark RD, Ellis SH. The effect of indomethacin on breathlessness in patients with diffuse parenchymal disease of the lung. Br J Dis Chest 1986;80:72–79. [DOI] [PubMed] [Google Scholar]
- 258.Winning AJ, Hamilton RD, Shea SA, Knott C, Guz A. The effect of airway anaesthesia on the control of breathing and the sensation of breathlessness in man. Clin Sci 1985;68:215–225. [DOI] [PubMed] [Google Scholar]
- 259.Stark RD, O'Neill PA, Russell NJ, Heapy CG, Stretton TB. Effects of small-particle aerosols of local anaesthetic on dyspnoea in patients with respiratory disease. Clin Sci 1985;69:29–36. [DOI] [PubMed] [Google Scholar]
- 260.Nishino T, Isono S, Ide T. A low concentration of nitrous oxide reduces dyspnoea produced by a combination of hypercapnia and severe elastic load. Br J Anaesth 1999;82:14–19. [DOI] [PubMed] [Google Scholar]
- 261.Taguchi N, Ishikawa T, Sato J, Nishino T. Effects of induced metabolic alkalosis on perception of dyspnea during flow-resistive loading. J Pain Symptom Manage 1996;12:11–17. [DOI] [PubMed] [Google Scholar]
- 262.Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary rehabilitation: Joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 2007;131:4S–42S. [DOI] [PubMed] [Google Scholar]
- 263.Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006;173:1390–1413. [DOI] [PubMed] [Google Scholar]
- 264.Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:19–38. [DOI] [PubMed] [Google Scholar]
- 265.Stulbarg MS, Carrieri-Kohlman V, Demir-Deviren S, Nguyen HQ, Adams L, Tsang AH, Duda J, Gold WM, Paul SM. Exercise training improves outcomes of a dyspnea self-management program. J Cardiopulm Rehabil 2002;22:109–121. [DOI] [PubMed] [Google Scholar]
- 266.Bianchi R, Gigliotti F, Romagnoli I, Lanini B, Castellani C, Binazzi B, Stendardi L, Bruni GI, Scano G. Impact of a rehabilitation program on dyspnea intensity and quality in patients with chronic obstructive pulmonary disease. Respiration 2011;81:186–195. [DOI] [PubMed] [Google Scholar]
- 267.Gigliotti F, Coli C, Bianchi R, Romagnoli I, Lanini B, Binazzi B, Scano G. Exercise training improves exertional dyspnea in patients with COPD. Chest 2003;123:1794–1802. [DOI] [PubMed] [Google Scholar]
- 268.O'Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med 1998;157:1489–1497. [DOI] [PubMed] [Google Scholar]
- 269.Mahler DA, Ward J, Mejia-Alfaro R. Stability of dyspnea ratings after exercise training in patients with COPD. Med Sci Sports Exerc 2003;35:1083–1087. [DOI] [PubMed] [Google Scholar]
- 270.Geddes EL, O'Brien K, Reid WD, Brooks D, Crowe J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med 2008;102:1715–1729. [DOI] [PubMed] [Google Scholar]
- 271.Reid WD, Geddes EL, O'Brien K, Brooks D, Crowe J. Effects of inspiratory muscle training in cystic fibrosis: a systematic review. Clin Rehabil 2008;22:1003–1013. [DOI] [PubMed] [Google Scholar]
- 272.Sibuya M, Yamada M, Kanamaru A, Tanaka K, Suzuki H, Noguchi E, Altose MD, Homma I. Effect of chest wall vibration on dyspnea in patients with chronic respiratory disease. Am J Respir Crit Care Med 1994;149:1235–1240. [DOI] [PubMed] [Google Scholar]
- 273.Cristiano LM, Schwartzstein RM. Effect of chest wall vibration on dyspnea during hypercapnia and exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;155:1552–1559. [DOI] [PubMed] [Google Scholar]
- 274.Nakayama H, Shibuya M, Yamada M, Suzuki H, Arakawa M, Homma I. In-phase chest wall vibration decreases dyspnea during arm elevation in chronic obstructive pulmonary disease patients. Intern Med 1998;37:831–835. [DOI] [PubMed] [Google Scholar]
- 275.Fujie T, Tojo N, Inase N, Nara N, Homma I, Yoshizawa Y. Effect of chest wall vibration on dyspnea during exercise in chronic obstructive pulmonary disease. Respir Physiol Neurobiol 2002;130:305–316. [DOI] [PubMed] [Google Scholar]
- 276.Schwartzstein RM, Lahive K, Pope A, Weinberger SE, Weiss JW. Cold facial stimulation reduces breathlessness induced in normal subjects. Am Rev Respir Dis 1987;136:58–61. [DOI] [PubMed] [Google Scholar]
- 277.Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010;39:831–838. [DOI] [PubMed] [Google Scholar]
- 278.Casanova C, Celli BR, Tost L, Soriano E, Abreu J, Velasco V, Santolaria F. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000;118:1582–1590. [DOI] [PubMed] [Google Scholar]
- 279.Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, Ambrosino N. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002;20:529–538. [DOI] [PubMed] [Google Scholar]
- 280.Borel J-C, Verges S, Pepin J-L, Vivodtzev I, Levy P, Wuyam B. Home exercise training with non-invasive ventilation in thoracic restrictive respiratory disorders: a randomised study. Respir Physiol Neurobiol 2009;167:168–173. [DOI] [PubMed] [Google Scholar]
- 281.Keilty SE, Ponte J, Fleming TA, Moxham J. Effect of inspiratory pressure support on exercise tolerance and breathlessness in patients with severe stable chronic obstructive pulmonary disease. Thorax 1994;49:990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jobst K, Chen JH, McPherson K, Arrowsmith J, Brown V, Efthimiou J, Fletcher HJ, Maciocia G, Mole P, Shifrin K, et al. Controlled trial of acupuncture for disabling breathlessness. Lancet 1986;2:1416–1419. [DOI] [PubMed] [Google Scholar]
- 283.Suzuki M, Namura K, Ohno Y, Tanaka H, Egawa M, Yokoyama Y, Akao S, Fujiwara H, Yano T. The effect of acupuncture in the treatment of chronic obstructive pulmonary disease. J Altern Complement Med 2008;14:1097–1105. [DOI] [PubMed] [Google Scholar]
- 284.Lewith GT, Prescott P, Davis CL. Can a standardized acupuncture technique palliate disabling breathlessness: a single-blind, placebo-controlled crossover study. Chest 2004;125:1783–1790. [DOI] [PubMed] [Google Scholar]
- 285.Vickers AJ, Feinstein MB, Deng GE, Cassileth BR. Acupuncture for dyspnea in advanced cancer: a randomized, placebo-controlled pilot trial. BMC Palliat Care 2005;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Maa SH, Gauthier D, Turner M. Acupressure as an adjunct to a pulmonary rehabilitation program. J Cardiopulm Rehabil 1997;17:268–276. [DOI] [PubMed] [Google Scholar]
- 287.Wu HS, Wu SC, Lin JG, Lin LC. Effectiveness of acupressure in improving dyspnoea in chronic obstructive pulmonary disease. J Adv Nurs 2004;45:252–259. [DOI] [PubMed] [Google Scholar]
- 288.Donesky-Cuenco D, Nguyen HQ, Paul S, Carrieri-Kohlman V. Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med 2009;15:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mularski RA, Munjas BA, Lorenz KA, Sun S, Robertson SJ, Schmelzer W, Kim AC, Shekelle PG. Randomized controlled trial of mindfulness-based therapy for dyspnea in chronic obstructive lung disease. J Altern Complement Med 2009;15:1083–1090. [DOI] [PubMed] [Google Scholar]
- 290.Bailey C, Wagland R, Dabbour R, Caress A, Smith J, Molassiotis A. An integrative review of systematic reviews related to the management of breathlessness in respiratory illnesses. BMC Pulm Med 2010;63:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Food and Drug Administration (FDA). Guidance for industry: Patient-reported outcome measures: use in medical product development to support labeling claims [Internet]. c2009. [accessed 2011 Dec 30]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf [DOI] [PMC free article] [PubMed]
- 292.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W-65–W-94. [DOI] [PubMed] [Google Scholar]
- 293.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]