Abstract
Air pollution and climate change are potential drivers for the increasing burden of allergic diseases. The molecular mechanisms by which air pollutants and climate parameters may influence allergic diseases, however, are complex and elusive. This article provides an overview of physical, chemical and biological interactions between air pollution, climate change, allergens, adjuvants and the immune system, addressing how these interactions may promote the development of allergies. We reviewed and synthesized key findings from atmospheric, climate, and biomedical research. The current state of knowledge, open questions, and future research perspectives are outlined and discussed. The Anthropocene, as the present era of globally pervasive anthropogenic influence on planet Earth and, thus, on the human environment, is characterized by a strong increase of carbon dioxide, ozone, nitrogen oxides, and combustion- or traffic-related particulate matter in the atmosphere. These environmental factors can enhance the abundance and induce chemical modifications of allergens, increase oxidative stress in the human body, and skew the immune system toward allergic reactions. In particular, air pollutants can act as adjuvants and alter the immunogenicity of allergenic proteins, while climate change affects the atmospheric abundance and human exposure to bioaerosols and aeroallergens. To fully understand and effectively mitigate the adverse effects of air pollution and climate change on allergic diseases, several challenges remain to be resolved. Among these are the identification and quantification of immunochemical reaction pathways involving allergens and adjuvants under relevant environmental and physiological conditions.
1. Introduction and Motivation
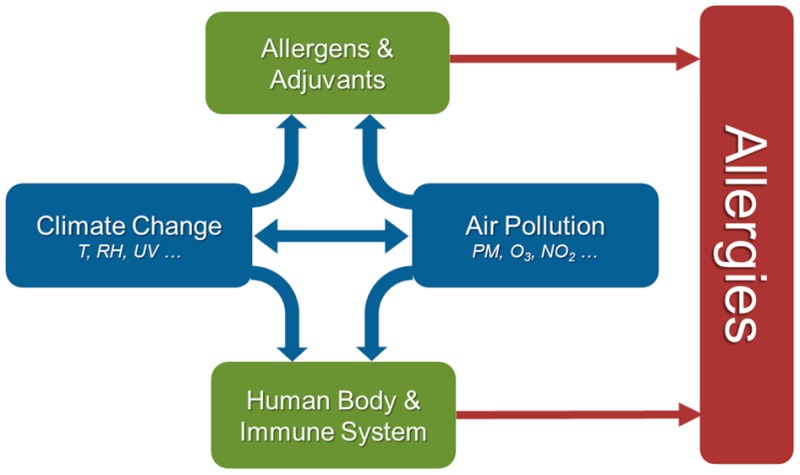
Allergies are hypersensitivities initiated by specific immunologic mechanisms (abnormal adaptive immune responses).1−3 They constitute a major health issue in most modern societies, and related diseases, such as allergic rhinitis, atopic asthma, eczema (atopic dermatitis), and food allergies, have strongly increased during the past decades.4−12 While some of the perceived rise in allergies may be due to improved diagnosis, the prevalence of allergic diseases has genuinely increased with industrialization and with the adoption of a “Western” lifestyle.13 The development of allergies is a complex multifactorial process that involves various factors influencing the body’s predisposition and immune response, and the manifestation of allergic diseases depends on exposure to allergens, adjuvants and other environmental and lifestyle factors (Figure S1 and section S1).3,4,14−16 Among the risk factors for allergic diseases are the genetic predisposition of the individual (referred to as atopy), reduced childhood exposure to pathogens and parasites (“hygiene hypothesis”), diet/nutrition, psychological/social stress, and environmental pollution, including outdoor and indoor air pollutants (ozone, nitrogen oxides, diesel exhaust particles, tobacco smoke, etc.).4,12,17−35 As outlined in Figure 1, climate change and air pollution can influence the bioavailability and potency of allergens and adjuvants in multiple ways, including changes in vegetation cover, pollination and sporulation periods, and chemical modifications. Moreover, climatic conditions and air pollutants may skew physiological processes and the immune system toward the development of allergies, for example, by oxidative stress and inflammation, disruption of protective epithelial barriers, and disturbance of related microbial communities (microbiomes).4,8,35−38
Figure 1.
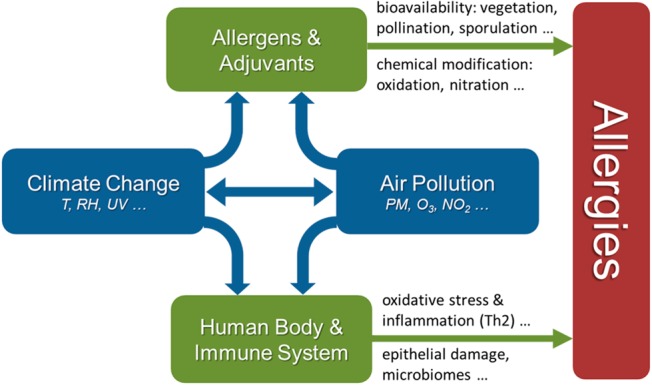
Interplay of air pollution and climate change can promote allergies by influencing the human body and immune system, as well as the abundance and potency of environmental allergens and adjuvants.
The term Anthropocene describes the present era of globally pervasive and steeply increasing anthropogenic/human influence on planet Earth, including the land surface, biosphere and atmosphere.38−44 Human activities have become a driving force that changes many characteristics of our environment such as biodiversity and air quality on local, regional, and global scales, for example, through land use change, agriculture, fossil fuel burning, traffic emissions, and the release of industrial products.38,39,41,43,45−49 While the basic concept of the Anthropocene, as introduced by Nobel laureate Paul J. Crutzen and colleagues,39,44,50 is widely accepted and increasingly used across the sciences and humanities, the actual beginning of the Anthropocene as a new geological epoch is still under investigation and discussion.38,45−47,51−64 The proposed dates range from early human history via the 19th century (industrialization) to the 1960s (nuclear weapon testing and “Great Acceleration”).45−47,58−64 Since the industrialization of the 19th century and especially during the “Great Acceleration” of the 20th century, the primary emission, secondary formation, and concentration of air pollutants like ozone, nitrogen, and sulfur oxides, soot, and a wide range of other reactive trace gases and aerosols have greatly increased relative to preindustrial times, especially in densely populated and industrialized areas but also in agricultural environments and around the globe.38,47,65−69 For example, the average mixing ratios of ozone in continental background air have increased by factors of 2–4 from around 10–20 ppb from the beginning of the 19th century to 30–40 ppb in the 21st century, and the number and mass concentrations of aerosol particles in polluted urban air are typically by 1–2 orders of magnitude higher than in pristine air of remote continental regions (∼102–103 cm–3 and ∼1–10 μg m–3 vs ∼103–105 cm–3 and ∼10–100 μg m–3).38,70
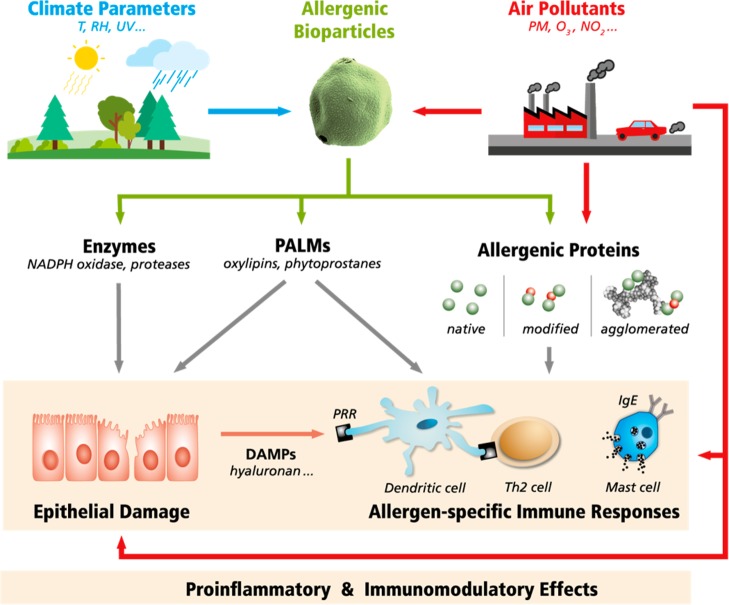
Numerous studies indicate that ozone and air particulate matter have strong effects on human health and mortality as well as on agricultural crop yields.71−80 In view of these findings, it appears unlikely that the strong environmental changes of the Anthropocene would have no effect on the interaction of the human immune system with environmental stimuli, including allergens and adjuvants. Indeed, it seems necessary to address the question whether human activities are creating a hazardous atmosphere that may severely affect public health.35,37,38,81,82Figure 2 illustrates how climate parameters and air pollutants can exert proinflammatory and immunomodulatory effects.8 As detailed in the following sections, both air pollutants and climate parameters can influence the environmental abundance of allergenic bioparticles and the release of allergenic proteins and biogenic adjuvants. Moreover, air pollutants can chemically modify and agglomerate allergenic proteins, and they can act as adjuvants inducing epithelial damage and inflammation.
Figure 2.
Pathways through which climate parameters and air pollutants can influence the release, potency, and effects of allergens and adjuvants: temperature (T), relative humidity (RH), ultraviolet (UV) radiation, particulate matter (PM), ozone and nitrogen oxides (O3, NOx), reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, pollen-associated lipid mediators (PALMs), damage-associated molecular patterns (DAMPs), pattern recognition receptors (PRR), type 2 T helper (Th2) cells, immunoglobulin E (IgE), allergenic proteins (green dots), and chemical modifications (red dots).
Several reviews have addressed the general determinants of allergenicity3−8,83−85 and various environmental risk factors for allergic diseases.4,9,12,34,36,86−101 In this Critical Review, we attempt to summarize, update, and synthesize the different perspectives and most relevant findings reported in earlier reviews and recent research articles addressing the effects of air pollutants and climate parameters on allergies. A central aim of this article is to review and outline both proven and potential effects of the globally pervasive environmental changes that are characteristic for the Anthropocene; a holistic view of environmentally caused changes in the abundance, interaction, and modification of allergens and related substances is provided. Our target audience comprises physical, chemical, and biomedical scientists interested in environmental effects on public health. Sections 2–4 deal with specific environmental processes and air pollutants that are likely to affect the development of allergies in the Anthropocene, that is, in an environment strongly influenced by human activity. Section 5 provides an outlook identifying key questions and promising directions of future research. For orientation of readers not familiar with the basics of allergic sensitization and response, section S1 outlines key features of the immunochemical interactions involved in IgE-mediated allergies (type I hypersensitivities)3−5,14−16,84,102−136 on which this article is mainly focused and which usually involve Th2 cell-mediated inflammation137,138 (Figure S2).
2. Abundance and Release of Allergens and Adjuvants
Environmental allergens are mostly proteins derived from plants, animals, and fungi that can trigger chemical and biological reaction cascades in the immune system leading to allergic sensitization and formation of IgE antibodies (section S1).8,84,103,105−109 Prominent examples are major allergens of birch pollen (Bet v 1), timothy grass pollen (Phl p 1), ragweed (Ambrosia, Amb a 1), molds (Alternaria alternata, Alt a 1, Cladosporium herbarum, Cla h 1, Aspergillus fumigatus, Asp f 1), and dust mites (Der p 1).4,139,140 Besides allergens, also adjuvants and their interaction with the immune system play a critical role in the development of allergies. Here, we use the term adjuvant generically for substances that are promoting pro-allergic immune responses. Adjuvants can trigger the immune system by inducing tissue damage and subsequent enhanced uptake of allergens, by inducing oxidative stress and activation of immune cells, by coexposure with the allergen favoring Th2 responses, or by modification of allergens enhancing their allergic potential. An overview of biogenic and anthropogenic adjuvants, including particulate matter as well as trace gases, and their effects on the immune system is given in Table 1.
Table 1. Biogenic and Anthropogenic Adjuvants with Reported Pro-allergic Effects: (I) Pollen-Associated and Microbial Compounds, Such as Pollen-Associated Lipid Mediators (PALMs), Bacterial Lipopolysaccharides (LPS), and Fungal β-Glucans and (II) Anthropogenic Pollutants and Chemicals Including Air Particulate Matter, Gaseous Oxidants, and Organic Compounds.
| substances | effects |
|---|---|
| (I) Pollen-Associated and Microbial Compounds | |
| proteases | disrupt intracellular adhesion; stimulate protease activated receptors (PAR) inducing inflammation and enhanced IgE production204,447,448 |
| fungal proteases activate TLR4449 | |
| leukotrien-like PALMs | attract and activate innate cells like neutrophils and eosinophils450 |
| phytoprostane PALMs | inhibit IL12 production and enhance IgE production107 |
| NADPH oxidase | ROS production and inflammation451 |
| adenosine | Th2 cytokine profile and inflammation452 |
| flavonoids | modulate immune responses as ligands of allergenic proteins, e.g., a natural ligand of Bet v 1 is a quercetin and binds to the C-terminal helix372,453 |
| the pollen-derived flavonoid isorhamnetin modulates the immunological barrier function of the epithelium454 | |
| bacterial LPS | trigger TLR4 in dose dependent manner, induce a Th2 bias and allergic inflammation455 |
| gram-positive bacteria | induce DC maturation by upregulation of CD80, CD83, and CD86445 |
| fungal β-glucans | activate C-type lectin receptor105 |
| fungal VOC | stimulate inflammatory response456 |
| (II) Anthropogenic Pollutants and Chemicals | |
| air particulate matter (PM) | diesel exhaust particles (DEP) increase Th2 sensitization to coinhaled allergens (IgE isotype switching and production, mast cell and basophil degranulation, cytokine production (e.g., IL-4); exaberates allergic airway responses86,457−462 |
| PM and DEP induce ROS production and inflammation86,463−465 | |
| DEP suppress alveolar macrophage function466,467 | |
| DEP and cigartette smoke can increase thymic stromal lymphopoietin (TSLP) expression in epithelial cells468,469 | |
| DEP induce permeability of epithelial cells; disrupt tight junctions by a ROS-mediated pathway470,471 | |
| PM increase the expression of costimulatory molecules on DCs (MHC class II, CD40, CD80, CD86)86,469 | |
| ultrafine particles (UFP < 100 nm) and DEP alter soluble protein levels (e.g., surfactant protein D, complement protein C3), increase levels of e.g., glycerin-aldehyde-3-phosphate-dehydrogenase (GADPH), manganese superoxide dismutase (MnSOD), or mitochondrial heat shock protein (Hsp 90)472,473 | |
| PM2.5 and DEP activate complement proteins (C3)474,475 | |
| black carbon (BC) and DEP induce epigenetic effects: DNA methylation in genes associated with Th2 polarization476−478 | |
| DEP and cigarette smoke induce epithelial damage, oxidatitive stress, and inflammation460 | |
| prenatal and postnatal exposure to environmental tobacco smoke (EST) is associated with asthma and wheezing34,479,480 | |
| transition metals and other redox-active compounds (organic peroxides, quinones) induce ROS production and inflammation via Fenton-like reactions38,129,309,311,481−483 | |
| colocalization of allergens on gold nanoparticles can facilitate IgE-receptor cross-linking244 | |
| ozone (O3) | cause oxidative stress, airway inflammation, increased airway permeability329,362,368,484 |
| formation of protein ROI (reactive oxygen intermediates) and protein dimers329,362 elevated levels of complement protein C3a485 | |
| degradation of high molecular weight to low molecular weight hyaluronan, which is a DAMP that activates the TLR4 pathway407,486 | |
| nitrogen oxides (NOx = NO + NO2) | nitration of allergens328,329 |
| skew towards Th2 response,487 increase eosinophilic inflammation,488 and enhance airway permeability484 | |
| volatile, semivolatile and low-volatile organic compounds (VOC, SVOC, LVOC) | significant positive association between formaldehyde exposure and childhood asthma272 |
| antimicrobial endocrine disrupting compounds such as parabens and triclosan are associated with allergic sensitization489,490 Bisphenol A can increase Il-4 and IgE levels491 | |
| dermal and pulmonary exposure to indoor VOC elicit irritant and allergic responses270,271 | |
Climate change is influencing vegetation patterns and plant physiology through spatial and temporal changes in temperature and humidity (Figure 1),141−143 and increasing atmospheric carbon dioxide (CO2) affects plant biology by supplying more carbon for photosynthesis, biomass production, and growth (CO2 fertilization).144,145 These factors can influence the spread of invasive plants, the beginning, duration, and intensity of pollination, the fruiting patterns and sporulation of fungi, as well as the allergen content and allergenicity of pollen grains, fungal spores, and other biological aerosol particles (Figure 2).12,90,93,96−98,145−162 Specific examples of climate change effects on allergenic plants and fungi are outlined in Table 2. Climate and land use change are also expected to influence the composition and spread of microbial surface communities (cryptogamic covers), from which allergenic cyanobacteria and other microbial allergens or adjuvants can be emitted to the atmosphere.163−174 Moreover, the frequency and intensity of dust storms are expected to increase,141,175−179 and dust particles are known to carry biological and organic components with pathogenic, allergenic, and adjuvant activity.152,154,180−187 Dust storms have been shown to cause and aggravate respiratory disorders including atopic asthma and allergic rhinitis.181,188−191 So-called “thunderstorm asthma” is characterized by acute asthma exacerbations possibly caused by the dispersion of inhalable allergenic particles derived from plant pollen and fungal spores by osmotic rupture.145,192 On the other hand, climate change-related regional enhancements of outdoor humidity and indoor home dampness may also lead to an increase of respiratory symptoms and atopic asthma induced by allergenic and adjuvant substances from fungi, other microbes, and mite.12,193−196
Table 2. Climate Change Effects on the Abundance and Properties as Reported for Selected Plants and Fungi Emitting Aeroallergens.
| allergenic species | effect of increasing temperature and CO2 concentration |
|---|---|
| Ambrosia artemisiifolia (ragweed) | increased pollen and allergen production, plant migration and spreading157,492−495 |
| changes in pollen transcriptome, changes in allergenic potential, increase in flavonoid metabolites158 | |
| Betula spp. (birch) | earlier pollination start, increased pollen production161,267,496 |
| Phleum pratense L. (timothy grass) | increased pollen production159 |
| Alternaria spp. (mold) | increased spore numbers, decreased allergen per spore146,156,160 |
| Aspergillus fumigatus (mold) | modified allergenicity and Asp f 1 content, increased spore numbers146,155,497 |
| Cladosporium spp. (mold) | increased spore numbers146 |
| Penicillium spp. (mold) | increased spore numbers146 |
Pollen grains generally belong to the coarse fraction of air particulate matter (particle diameters >10 μm), but fungal spores and pollen fragments are also found in fine particulate matter (<2.5 μm; PM2.5), which can penetrate deep into the human respiratory tract and alveolar regions of the lung.152,153,197−203 Allergenic proteins can be released from pollen and spores after cell damage or under humid conditions.204 In particular, pollen rupture due to an osmotic shock during rain can lead to outbreaks of thunderstorm asthma.145,192,205,206 Furthermore, peaks of high concentrations of pollen, fungal spores, and other primary biological aerosol (PBA) particles have been observed at the onset of heavy rain and moist weather conditions;200,207,208 and increased concentrations of free allergen molecules in fine air particulate matter have been observed after rainfall.209 Prominent airborne fungi, such as Cladosporium herbarum, Alternariaalternata, and Aspergillus fumigatus, have been found to release higher amounts of allergens after germination under humid conditions,210 and certain allergens are expressed only following germination.210,211 Air pollutants, such as ozone, nitrogen oxides, and acids, can also interact with PBA particles, damage their envelope, and facilitate the release of allergenic substances, such as cytoplasmic granules from pollen (Figure S3).205,212,213
Besides allergenic proteins, pollen and fungal spores also release other compounds that can act as adjuvants (Table 1). In particular, the release of nonallergenic, bioactive, pollen-associated lipid mediators (PALMs) with pro-inflammatory and immunomodulatory effects can trigger and enhance allergies (Figure 2).8,109,214−217 For example, skin prick tests of pollen allergens elicited larger wheals when tested together with low molecular weight compounds extracted from pollen.218 The release of these substances can be influenced by climatic conditions and air pollution, and significantly higher levels were found for pollen collected near roads with heavy traffic.205 Leukotriene-like PALMs (oxylipins) have the potential to attract and activate innate immune cells like neutrophils and eosinophils.214,217 Other PALMs such as phytoprostanes (lipophilic counterparts of prostaglandins) are water-soluble and can inhibit the production of interleukin 12 (IL-12) by dendritic cells in the lower respiratory tract, thus favoring an allergenic Th2 T cell response.8,215 A recent study showed that the low-molecular-weight fraction of phytoprostane E1 (PPE1) in ragweed pollen extract specifically enhanced IgE production in Th2 primed B cells. It was suggested that pollen-derived nonallergenic substances might be responsible for aggravation of IgE-mediated allergies.219
Fine aerosol particles and a wide range of inorganic, organic and biological substances from both natural and anthropogenic sources (e.g., secondary organic material, sulfuric and nitric acid, microbial compounds) can agglomerate and accumulate on the surface of pollen, fungal spores, and other PBA particles as illustrated in Figure S3.152,205,220−223 An overview of reported air pollutant effects on the allergenic potential of plant pollen and fungal spores is given in Table S1.38,205,221,224−240 Moreover, free allergens and adjuvants can bind to particulate pollutants, such as dust, soot, black/elemental carbon (BC/EC), and diesel exhaust particles (DEP) carrying the allergens and adjuvants into peripheral and deep airways.241−243 The colocalization of allergens and adjuvants on particle surfaces (sorption layers, protein coronas) might also promote allergic sensitization and response by providing multiple/multivalent epitopes that facilitate receptor cross-linking (similar to parasitic organisms, against which IgE is naturally deployed).244,245
During recent years, great progress has been made in the development and application of efficient sampling and measurement methods for bioaerosol particles and components, including microscopic, spectroscopic, mass spectrometric, genomic, and proteomic analyses.152,246−253 These and related advances in measurement and modeling techniques for health and climate relevant air contaminants (aerosols and trace gases) are expected to enable comprehensive characterization and forecasting of allergenic and adjuvant substances, as well as their mixing state in outdoor and indoor air.38,70,254−268 Note that indoor air quality is usually influenced by both outdoor air pollutants (O3, NOx, PM2.5, etc.) and additional pollutants from indoor sources (e.g., formaldehyde and other organic compounds).35,37,265,269−274 The data from ambient and individual monitoring and modeling of aeroallergen and adjuvant exposure can then be applied in epidemiological studies to better understand the risk factors of allergic sensitization and disease.74−76,275−280
Several epidemiological studies and meta-analyses reported that respiratory allergies and atopic dermatitis are associated with exposure to traffic-related air pollution (TRAP), but different results were obtained for different diseases and locations/studies.281−293 TRAP is a complex mixture comprising variable proportions of particulate matter and gaseous pollutants from traffic-related primary emissions, as well as secondary pollutants formed by chemical reactions in the atmosphere.283 Among the pollutants from primary emissions (combustion and noncombustion sources) are road dust, tire and break wear, soot/DEP, BC/EC, metals, polycyclic aromatic hydrocarbons (PAH), and nitrogen oxides (NOx); among the secondary pollutants are ozone, nitrates, and secondary organic aerosols (SOA).38,70,273,283 A recent review concluded that epidemiological studies were restricted by imprecise methods of assessing both TRAP exposure and related health effects.283 Accordingly, several studies called for more comprehensive investigations of TRAP markers, personal exposure, and lifetime outcomes.281,294,295 The application of improved measurement and modeling techniques as outlined above should enable refined epidemiological studies and more targeted testing of hypotheses by resolving different types of TRAP (e.g., freshly emitted DEP vs resuspended road dust; soot and polycyclic hydrocarbons vs trace metals; ozone vs nitrogen oxides; etc.).
3. Chemical Modification of Proteins and Amino Acids
Chemical modification by air pollutants can lead to changes in the structure of protein macromolecules (amino acid oxidation, peptide backbone cleavage, conformational changes, cross-linking, and oligomerization), and affect protein stability and other properties, such as hydrophobicity and acidity of binding sites.296−303 These and other posttranslational protein modifications may induce multiple effects in the molecular interaction of allergens with the immune system: (1) stability effects influencing the accumulation and degradation of allergenic proteins, the duration of exposure times to cellular receptors, and the process of antigen presentation via major histocompatibility complex (MHC) class II;304,305 (2) epitope effects, that is, generation of new epitopes or modification of existing epitopes, changing the binding properties of antibodies and receptors, by direct chemical modification or as a result of conformational changes; (3) adjuvant effects, that is, generation of new adjuvant functions or modification of existing adjuvant functions such as lipid-binding capacities due to modified ligand binding sites; and (4) agglomeration effects, that is, multiplication or shielding of epitopes or adjuvant functions by cross-linking (oligomerization) of allergenic proteins, which may enhance the cross-linking of effector cell receptors (FcεRI) or sterically hinder molecular and cellular interactions.307,308306229
In the atmosphere, reactive oxygen and nitrogen species (ROS/RNS) are generated via photochemistry and gas-phase, heterogeneous, and multiphase reactions involving atmospheric oxidants and aerosol particles. In the human body, ROS/RNS can be formed upon exposure to air pollutants38,309−312 or radiation (UV, X-rays, γ-rays),313 and by regular physiological reactions.314 For example, ROS/RNS are generated during oxidative metabolism as well as in cellular responses to foreign or danger signals (cytokines, xenobiotics, bacterial invasion).315 Low amounts of ROS/RNS are involved in intra- and intercellular redox signaling processes, for example, oxidizing low molecular mass thiols and protein thiols (Figure 3).316,317 An imbalance between oxidants and antioxidants in favor of oxidants (e.g., induced by air pollutants) can lead to irreversible damage of cellular lipids, proteins, nucleic acids, and carbohydrates, eventually resulting in cell death.38,317,318 Rising levels of atmospheric oxidants and air particulate matter may lead to protein modifications in the atmosphere, as well as in the human body because of elevated oxidative stress levels, especially in the epithelial lining fluid (section 4).38 Moreover, air pollutants and climatic stress factors, such as UV radiation, drought, salinity, and temperature extremes, can also induce higher ROS/RNS levels inside plants, which may lead to chemical modification of plant proteins, including allergens.38,142,143 In the course of the Anthropocene, the ambient concentrations of many ROS/RNS have strongly increased because of emissions from traffic and combustion sources, as well as other industrial and agricultural activities like nitrogen fertilization of soils.37,38,82,319,320
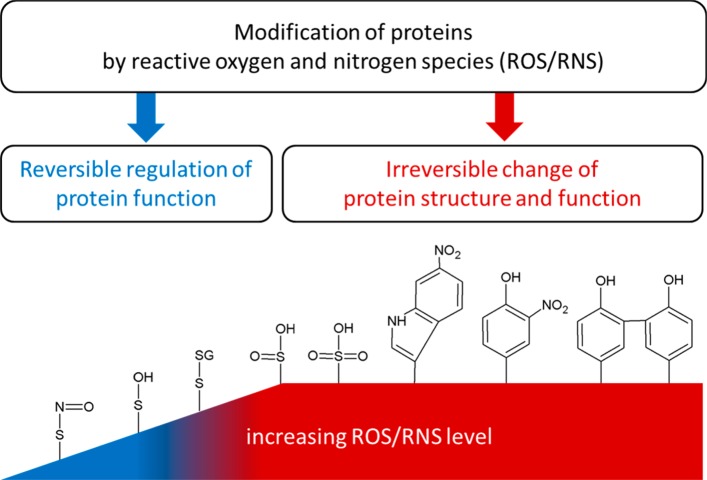
Figure 3.
Upon interaction with reactive oxygen and nitrogen species (ROS/RNS), proteins can undergo a wide range of reversible and irreversible chemical modifications. Among the most commonly formed functional groups and products are S-nitrosothiol (SNO), sulfenic acid (SOH), disulfides with protein thiols or low molecular mass thiols (e.g., with glutathione, SSG), sulfinic acid (SO2H), sulfonic acid (SO3H), nitrotryptophan, nitrotyrosine, and dityrosine. Adapted from ref (317). Copyright 2013 American Chemical Society.
In the following, we focus on irreversible modifications of allergenic proteins, such as oxidation, nitration, and cross-linking (Figure 3) by endogenous and exogenous ROS and RNS, like ozone (O3), hydroxyl radicals (OH), hydrogen peroxide (H2O2), superoxide anion (O2–), nitric oxide (NO), nitrogen dioxide (NO2), nitrous acid (HONO), nitric acid (HNO3), peroxyacetylnitrate (PAN), peroxynitrite (ONOO–), and nitrate radicals (NO3). ROS and RNS can react with oxidation-sensitive amino acids, such as cysteine (Cys), methionine (Met), tryptophan (Trp), tyrosine (Tyr), phenylalanine (Phe), and histidine (His), as well as with aliphatic side chains and the peptide backbone.317,321−324 For example, OH radicals can cause backbone cleavage by abstracting hydrogen atoms from the α-carbon of any amino acid in the polypeptide backbone. Subsequent reactions lead to oxidative degradation of the protein and the formation of amide and carbonyl groups.321,325,326 Oxidation reactions can result in aggregation, fragmentation, and denaturation of proteins.327−329 While oxidative degradation appears likely to reduce the recognition of allergenic proteins, other chemical modifications, such as nitration or cross-linking may enhance the potency of allergens.8,229,306−308,328,330−332
The reaction of proteins with nitrating agents leads mainly to the nitration of the aromatic amino acid tyrosine forming 3-nitrotyrosine (NTyr).333 The addition of the rather bulky NO2 group at the ortho position of the aromatic ring induces a significant shift in the pKa value of the tyrosine residue (Tyr) from ∼10 to ∼7, thus increasing the acidity of the hydroxyl group. These structural and chemical changes of the amino acid can affect the conformation and function of proteins.334,335 For example, the modification of tyrosine residues can influence cell signaling through the important role of receptor tyrosine kinases, which are key regulators of cellular processes.336 Moreover, nitrotyrosine has been reported as a biomarker for oxidative stress, inflammation, and a wide range of diseases.296,301,337,338
Early immunological studies already suggested that dinitrophenyl derivatives of proteins and peptides evade immune tolerance and boost immune responses.339,340 As early as 1934, the allergic reaction to dinitrophenol was described,341 and dinitrophenyl haptens became very popular reagents for the experimental induction of allergies.342−344 Thus, nitrated aromatics and especially nitrophenols can be considered corner stones in the field of allergy research, suggesting that protein nitration by air pollutants might play a role in the development of allergies.330
Indeed, several studies showed enhanced allergenic potentials for nitrated pollen allergens,229,305,306 nitrated fungal allergens,237 and nitrated food allergens.304,345 For example, the most efficiently nitrated tyrosine residue in the food allergen ovalbumin (OVA) is part of human and murine IgE epitopes and also belongs to a human T cell epitope.304 Recent studies suggest that nitration may also affect the allergenic potential and adjuvant activity of α-amylase/trypsin inhibitors (ATIs) from wheat and other gluten-containing grains, which act as aeroallergens in baker’s asthma and are involved in hypersensitivities and chronic inflammation of the gastrointestinal tract.346−351 Nitrated variants of the major birch pollen allergen Bet v 1 induced enhanced levels of specific IgE in murine models, possibly because of the formation of neo-epitopes.229 Nitration of Bet v 1 also increased the presentation of allergen-derived peptides by antigen presenting cells (APC).305 Moreover, increased proteolytic stability, up-regulation of CCL17 (Th2-associated chemokine secreted by dendritic cells, DC), and alterations of T cell proliferation and stimulatory capacities have been observed for nitrated Bet v 1.306 Nitrated proteins also have been observed to modulate the antioxidant levels in murine pneumocytes.352 In a recent study, in vivo fumigation of ragweed pollen with NO2 resulted in an altered proteomic pattern including nitrosylation products and the treated pollen showed higher IgE recognition in immunoblots.239 Enhanced allergenic potential was also observed for Betula pendula, Ostrya carpinifolia, and Carpinus betulus pollen after NO2 exposure (Table S1).236
Reaction product studies and kinetic experiments have shown that environmentally relevant O3 and NO2 concentrations can induce protein nitration on tyrosine residues.237,328−330,333,353−355 This is in line with earlier observations that atmospheric oxidation and nitration processes leads to the formation of nitrophenols and dinitrophenols,356 and that nitration is an important reaction pathway particularly in the atmospheric aqueous phase.357,358 Especially, aromatic amino acids like tyrosine and tryptophan can react with atmospheric nitrating agents, such as ozone/NO2 mixtures or peroxyacetylnitrate (PAN).330,359 Under photochemical smog conditions in polluted urban environments (high O3 and NO2 concentrations), proteins on the surface of aerosol particles can be efficiently nitrated within minutes to hours.328,330 The reaction kinetics also depends strongly on ambient relative humidity: At high relative humidity and especially during aqueous phase processing (when aerosol particles are activated as cloud or fog droplets), nitration may proceed efficiently also within the particle bulk.328,360,361
Mechanistically, the reaction between O3/NO2 and tyrosine involves the formation of long-lived reactive oxygen intermediates (ROI), likely via hydrogen abstraction from the phenolic OH group, yielding tyrosyl radicals (phenoxy radical derivatives of tyrosine) that can further react with NO2 to form nitrotyrosine residues as shown in Figure 4.329,362,363 The two-step protein nitration by air pollutants is similar to the endogenous nitration of proteins by peroxynitrite (ONOO–)298,328,364 formed from nitrous oxide (NO) and superoxide anions (O2–).301,365,366 For endogeneous protein nitration by ONOO–, both radical and electron transfer reaction pathways have been proposed.367 Besides nitration, tyrosyl radicals can also undergo hydroxylation or self-reaction (cross-linking) to form dityrosine derivatives (Figure 4).368
Figure 4.
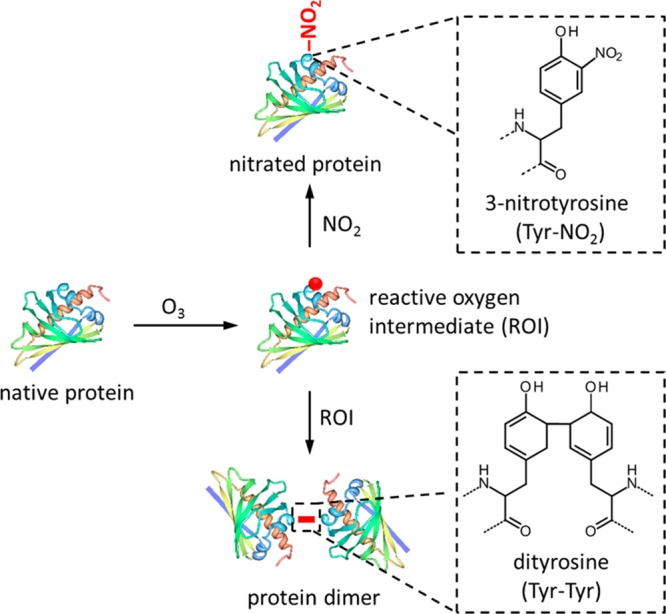
Posttranslational modification of proteins exposed to ozone (O3) and nitrogen dioxide (NO2). The initial reaction with O3 leads to the formation of reactive oxygen intermediates (ROI, tyrosyl radicals), which can further react with each other to form cross-linked proteins (dityrosine) or with NO2 to form nitrated proteins (nitrotyrosine). The shown protein is Bet v 1.0101 (PDB accession code 4A88,370 created with the PDB protein workshop 3.9498), for which nitration and cross-linking were found to influence the immunogenicity and allergenic potential.229,305,306,328 Red dot indicates a tyrosyl radical; red bar indicates dityrosine cross-link.
The site selectivity of protein nitration is influenced by the molecular structure of the protein, the nitrating agent, and the reaction conditions. For example, different preferred reaction sites were observed for the birch pollen allergen Bet v 1, the egg allergen ovalbumin, and bovine serum albumin.304,328,333,354 Upon exposure of Bet v 1 to atmospherically relevant concentrations of O3/NO2 and physiologically relevant concentrations of ONOO–, the preferred sites of nitration were tyrosine residues with high solvent accessibility and within a hydrophobic environment. Accordingly, nitrated tyrosine residues occurred mainly in the C-terminal helix and in the hydrophobic cavity (Figure S4).328 Both are key positions for the binding of specific IgE,369 as well as ligands like fatty acids, cytokines, and flavonoids.370−372 The binding of such ligands may be involved in allergic and inflammatory immune responses by stabilizing Bet v 1 against endo/lysosomal degradation.373 Moreover, nitration-related changes in ligand-binding capacity might influence the interaction of allergenic proteins like Bet v 1 with adjuvant substances like lipopolysaccharide (LPS) and induce a shift from Th1 to Th2 responses, thus resulting in increased allergenicity.306
Dimerization and oligomerization are supposed to have a strong influence on the immunogenicity of allergenic proteins and are common features of major allergens like Bet v 1.307,308 The cross-linking of IgE receptors (FcεRI) on effector cells is a key element of allergic reactions and requires IgE antibody clustering on the cell surface,374,375 which may be facilitated by multivalent allergens, such as oligomers of allergenic proteins providing multiple epitopes of the same kind.122,376 Moreover, cross-linking can make proteins less susceptible to enzymatic proteolysis and influence immune responses.313,373,377 Indeed, immune responses to oligomers and aggregates of certain allergenic proteins were found to be enhanced compared to the monomeric form of the allergenic protein.307,308,378−380 The clustering of allergenic proteins on nanoparticle surfaces (protein coronas) can also modulate allergic respones depending on protein and particle properties.244 Accordingly, the investigation and effects of allergen colocalization on the surface of inhalable ambient particles, such as pollen fragments or soot (DEP), are potentially important research perspectives.
Oxidative protein cross-linking can occur upon (a) tyrosyl radical coupling through dityrosine cross-links, (b) Schiff-base coupling of oxidation-derived protein carbonyl groups with the ε-amino groups of lysine residues, and (c) intermolecular disulfide coupling.381 Recently, protein cross-linking and oligomerization upon exposure to atmospherically relevant concentrations of O3 have been shown to proceed via the formation dityrosine cross-links as outlined in Figure 4, yielding up to ∼10% of dimers, trimers, and higher oligomers of a model protein within minutes to hours of exposure under summer smog conditions.368 Similar reaction mechanisms involving reactive oxygen intermediates may also be responsible for the protein cross-linking observed upon reaction with physiological and synthetic nitrating agents like ONOO– and tetranitromethane, respectively.306,313,382,383 Cross-linking upon reaction with tetranitromethane was suggested to alter the immunogenicity and enhance the allergenicity of Bet v 1 through decreased endolysosomal degradation leading to extended MHC class II antigen presentation.306 On the other hand, oligomerization of allergens induced by modification with glutaraldehyde, that is, formation of glutaraldehyde bridges between nucleophilic amino acid residues (in particular lysine), was suggested to reduce immunogenicity and allergenicity due to delayed allergen uptake and presentation by dendritic cells.384,385
As illustrated in Figure S2, the processes of allergic sensitization and response involve a wide range of interactions between protein molecules dissolved in liquids (blood, lymph, etc.) and embedded in semisolid structures (membranes, cells, tissues), which can be regarded as protein multiphase chemistry.38 Protein reactions with ROS/RNS are generally pH-dependent and yield a mixture of hydroxylated, nitrated, cross-linked, aggregated or degraded products.386−391 To assess immune responses to specific posttranslational modifications of proteins, it is necessary to carefully characterize the investigated samples and avoid artifacts or misinterpretations that might arise from interferences between different reaction products and pathways, for example, nitration vs dimerization or oligomerization of proteins exposed to oxidizing and nitrating agents (Figure 4).
4. Epithelial Surface Interactions
The deposition of particles in the respiratory tract is size-dependent and deposited particles are removed by a number of physical, chemical, and biological clearance processes, including mucociliary movement, endo- and phagocytosis, dissolution, leaching, and protein binding.201 Thus, the first step of an inhaled allergen-carrying particle is evading the mechanical defenses of the respiratory tract and passing, for example, alveolar macrophages, which prevent inappropriate immune activation by removing inhaled allergens via phagocytosis.392−394 The epithelial surface is a protective barrier, which protects the underlying tissue from many inhaled substances. The epithelial cells are covered by a viscous mucosal lining rich in immune cells and soluble components, such as antioxidants, complement proteins, and surfactant proteins.201,395,396 As the epithelium is more than a passive protective barrier, it recruits and activates more specialized immune cells and promotes inflammatory responses,397 allergy is also discussed to be an epithelial barrier disease.15,131,398−400 For example, nasal epithelium is clearly different between healthy and allergic subjects and only in allergic subjects the transport of Bet v 1 is caveolar-mediated.401
Air pollutants interacting with epithelial surfaces can act as adjuvants promoting pro-allergic innate and adaptive immune reactions as outlined in Table 2 and section S1. For example, they can induce inflammation and disrupt epithelial barriers, facilitating the access of allergens to immunogenic effector cells.8,86 In particular, air particulate matter can trigger ROS production through Fenton-like reactions and the activation of macrophages, mitochondria and enzymes related to the oxidant/antioxidant balance (e.g., NADPH oxidase, glutathione peroxidase).309,310,402−405 Additionally, pollution-derived ROS can induce proinflammatory responses by the production of damage associated molecular patterns (DAMPs oxidized phospholipids, hyaluronic acid, etc.) and trigger immune reactions leading to acute or chronic inflammation,29,406 for example, through feedback cycles involving Toll-like receptors (TLR) and other pattern recognition receptors (PRR) (Figure S5).407 Ozone and particulate matter can prime the airways for pro-allergic responses, and TLR signaling plays an important role in pollutant-induced inflammation.408,409 During inflammation, inducible nitric oxide synthase (iNOS) that is mainly expressed in innate immune cells (monocytes, macrophages, dendritic cells) provides high amounts of nitrogen oxide (NO), which can react with superoxide radicals to form peroxynitrite (ONOO–), a central endogenous nitrating agent for proteins.301 In addition, particulate and gaseous pollutants may also drive pro-allergic inflammation through the generation of oxidative stress involving elevated levels of ONOO–.410
As illustrated in Figure 5A, epithelial surfaces are interfaces coupling the atmospheric and the physiological production, cycling, and effects of ROS/RNS.38 Specific interactions of atmospheric ROS/RNS with antioxidants in the epithelial lining fluid are shown in Figure 5B. An increase of ozone from typical background concentration levels (∼30 ppb) to summer smog conditions (>100 ppb) reduces the chemical half-life of antioxidants from days to hours,309 which may be comparable or shorter than the physiological replenishment rates.411 Furthermore, the adjuvant effect of ambient ultrafine particles was correlated with their oxidant potential.412 Major contributors to the redox properties of ambient particles are transition metals, polycyclic aromatic hydrocarbons, and derivatives (PAH, nitro/oxy-PAH), and semiquinones.38,312,412−415 In addition, the deposition of acidic particles may reduce the pH of the epithelial lining fluid (ELF). For healthy people the mean pH is ∼7.4, while in people with diseases (e.g., asthma, acid reflux) it can be as low as ∼4.416,417 Oxidant exposure and changes of pH can alter reaction pathways of antioxidants418 and also decrease the activities of antioxidant-related enzymes in the ELF, which are also reduced in smokers and people suffering from lung diseases.419−421
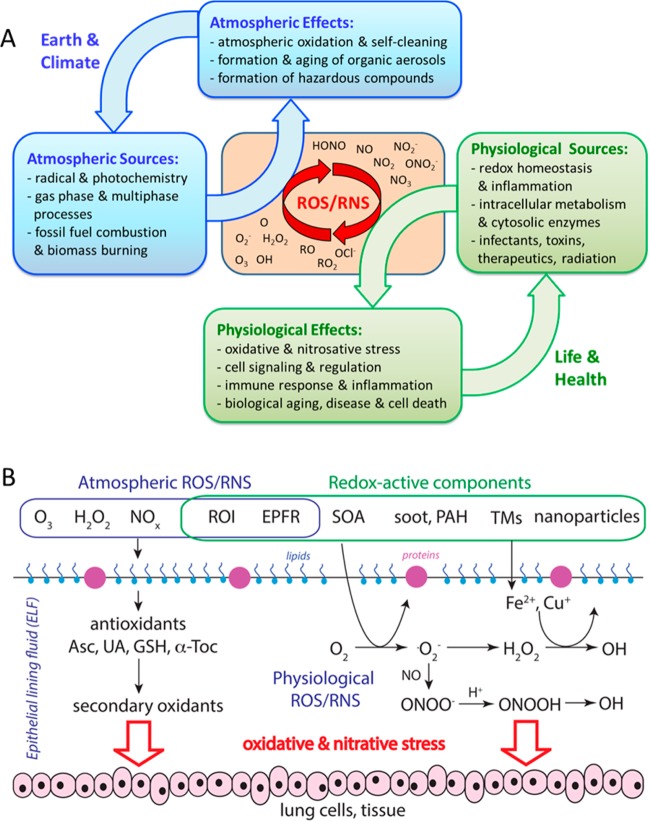
Figure 5.
(A) Sources, effects, and interactions at the interface of atmospheric and physiological chemistry with feedback loops involving Earth System, climate, life, and health. (B) Interactions of atmospheric and physiological ROS/RNS with antioxidants (ascorbate, uric acid, reduced glutathione, α-tocopherol) in the epithelial lining fluid (ELF) of the human respiratory tract. Redox-active components, including reactive oxygen intermediates (ROI), soot, quinones and transition metals can induce ROS formation in vivo, leading to oxidative stress and biological aging. Adapted from ref (38). Copyright 2015 American Chemical Society.
Recent studies yielded chemical exposure-response relations between ambient concentrations of air pollutants and the production rates and concentrations of ROS in the ELF of the human respiratory tract.309 As illustrated in Figure 6, the total concentration of ROS generated by redox-active substances contained in fine particulate matter (PM2.5) deposited in the ELF ranges from ∼10 nmol L–1 under clean conditions up to almost ∼250 nmol L–1 under highly polluted conditions. Thus, the inhalation of PM2.5 can increase ROS concentrations in the ELF to levels that exceed physiological background levels (50–200 nmol L–1) and are characteristic for respiratory diseases.309,422 In addition to the effects of PM2.5, ambient ozone readily saturates the ELF and can enhance oxidative stress by depleting antioxidants and surfactants.309 Ozone also reacts with skin lipids (e.g., squalene) and generates organic compounds (e.g., mono- and dicarbonyls) that can act as irritants.269 These and related organic compounds were found to act as adjuvants in the development of respiratory allergies as well as atopic dermatitis.270,271,423,424 Some air pollutants and chemical reaction products formed at epithelial interfaces are sufficiently long-lived and mobile to diffuse through membranes and interact with the neural, cardiovascular, and immune system networks of the human body.314,425−429 Through these and related physiological interactions involving DAMPs, inflammatory mediators, cytokines, leukocytes etc., oxidative stress and inflammation caused by air pollutants may propagate from the respiratory tract and skin to other parts of the human organism and exert systemic influence on the development of allergies, reaching also the gastrointestinal tract.38,429
Figure 6.
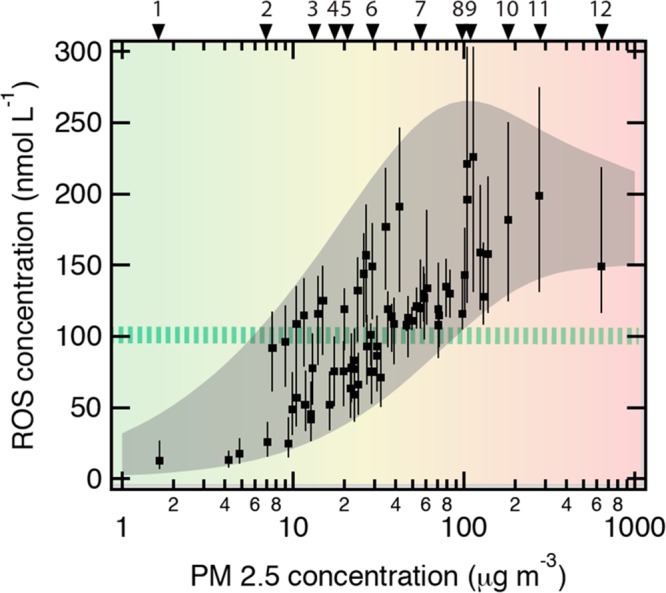
Chemical exposure-response relations between ambient concentrations of fine particulate matter (PM2.5) and the concentration of reactive oxygen species (ROS) in the epithelial lining fluid (ELF) of the human respiratory tract. The green-striped horizontal bar indicates the ROS level characteristic for healthy humans (∼100 nmol L–1). The gray envelope represents the range of aerosol-induced ROS concentrations obtained with approximate upper and lower limit mass fractions of redox-active components observed in ambient PM2.5. The data points represent various geographic locations for which measured or estimated mass fractions are available, including (1) Amazon, Brazil (pristine rainforest air); (2) Edinburgh, UK; (3) Toronto, Canada; (4) Tokyo, Japan; (5) Budapest, Hungary; (6) Hong Kong, China; (7) Milan, Italy; (8) Guangzhou, China; (9) Pune, India; (10) Beijing, China; (11) New Delhi, India; (12) Sumatra, Indonesia (biomass burning/peat fire smoke). Adapted from Lakey, S. J. P.; Berkemeier, T.; Tong, H.; Arangio, A. M.; Lucas, K.; Pöschl, U.; Shiraiwa, M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep.2016, 6, 32916. DOI: 10.1038/srep32916.309 Copyright 2016 Lakey et al.
A wide variety of commensal, symbiotic, and pathogenic microorganisms are found on the epithelial surfaces of the human body, such as the skin, lungs, and the gastrointestinal tract. Recent research suggests that the human microbiome is important to maintain physiological functions and to induce immune regulation by balancing the activities of Th1 and Th2 cells.430−433 Normal microbial colonization in early life can promote tolerance to aeroallergens via induced regulatory T cells.434 The development and composition of the human microbiome are influenced by many factors such as diet, infections, medical treatment, and also environmental factors.435 For example, air pollutants and climatic stress factors may disturb microbial communities through oxidative stress, inflammation, and changes in environmental biodiversity.4,36 Modifications in the composition of the gastrointestinal and lung microbiome can in turn affect the development of allergies in accordance with the “hygiene hypothesis”36,436−440 and may also promote pathogenic species that can contribute to these diseases.4,441−443 Recent studies revealed differences in the structure and composition of microbiota in the lower airways of healthy and asthmatic people: Bacteroidetes, Firmicutes, and Proteobacteria are the most common phyla found in airways of healthy subjects, whereas increased concentrations of pathogenic Proteobacteria, such as Haemophilus, Moraxella, and Neisseria spp., were found in asthma patients.442,443 Moreover, viral infections can exacerbate allergies.31 It is still unclear, however, if these changes are a cause or a consequence of the disease. Moreover, it has been suggested that air pollutants, especially air particulate matter, ingested together with food can trigger and accelerate the development of gastrointestinal inflammatory diseases by altering the gastrointestinal microbiome and immune functions.444 Besides the human microbiome, also microbes associated with allergenic pollen (pollen microbiome) and other aeroallergens may act as adjuvants when deposited on epithelial surfaces.235,445
5. Conclusions and Outlook
As the globally pervasive anthropogenic influence continues to shape planet Earth and the human environment in the Anthropocene, it becomes increasingly important to understand and assess the potential effects of environmental change on human health. The widespread increase of allergies and their complex dependence on multiple influencing factors, including environmental pollution, indicate that allergic diseases are a major challenge with regard to maintaining and improving public health.
Anthropogenic emissions of atmospheric trace substances are affecting air quality and climate on local, regional, and global scales. Changes in atmospheric aerosol composition, oxidant concentrations, and climate parameters can induce chemical modifications of allergens, increase oxidative stress in the human body, and skew the immune system toward allergic reactions. In particular, air pollutants can act as adjuvants and alter the immunogenicity of allergenic proteins, while climate change affects the abundance and properties of bioaerosols as carriers of aeroallergens. The production, release and properties of allergens and adjuvants are subject to various human interferences with the biosphere and climate system, including air pollutant interactions with natural and agricultural vegetation, fertilization and land-use change, as well as plant breeding and genetic engineering.
The following key questions remain to be resolved to understand and mitigate potential effects of air pollution and climate change on the observed increase and future development of allergies:
-
(Q1)
Which air pollutant and climate change effects have the largest potential to influence on the abundance and potency of allergens and adjuvants in the human environment (indoor and outdoor)?
-
(Q2)
Which elements and reaction pathways of the immune system are particularly susceptible to disturbance by air pollutants, and what are the most relevant chemical and physiological mechanisms (adjuvant activity vs allergen modification)?
-
(Q3)
Which environmental and physiological parameters are needed and best suited to account for and assess air pollutant and climate change effects in epidemiological studies of allergic diseases (attribution and prediction of environmental risk factors)?
-
(Q4)
How important are air pollutant and climate change effects relative to other environmental, lifestyle, genetic and epigenetic risk factors for allergic diseases?
Recommendations on how to address these key questions in future research are listed in Table S2, building on and extending suggestions given in related review and perspective articles (e.g., refs (8, 12, 93, and 280)). Beyond addressing the above questions, it appears worthwhile to explore which components of the immune system could be modulated to prevent adverse effects of air pollution, for example, whether therapeutic monoclonal antibodies against relevant cytokines (e.g., IL-4, IL-5, IL-13) or IgE antibodies could make a difference. Further information about ongoing efforts and future perspectives of mitigating the health effects of climate change and air pollution is available from various national and international government agencies, medical institutions and related organizations (e.g., refs (4, 37, and 446)). For efficient scientific progress, it will be important to combine and optimize state-of-the-art methods and results of environmental, immunological and epidemiological studies, tightly coupling physical, chemical, biological, and medical techniques and knowledge. One of the challenges consists in identifying and quantifying the mechanisms and feedback loops of immunochemical reactions in response to environmental influencing factors, including chemical modifications and interactions of allergens and adjuvants under realistic environmental and physiological conditions. For this purpose, the results of laboratory experiments and monitoring networks with improved detection methods for allergens, adjuvants and reactive intermediates should be used to design and inform epidemiological studies targeting the effects of different types and combinations of air pollutants and climate parameters.
Acknowledgments
K.R.-S. acknowledges financial support by the Max Planck Graduate Center with the Johannes Gutenberg University of Mainz (MPGC); C.J.K. acknowledges support by the MPGC and financial support by the German Research Foundation (DFG, grant number KA 4008-1/2); J.F.-N., U.P., and B.W. acknowledge support from the German Research Foundation (DFG FR3641/1-2, FOR 1525 INUIT). N.L.-Y. acknowledges support from the Max Planck Society and from the Weizmann Institute of Science (National Postdoctoral Award Program for Advancing Women in Science). F.L. and S.L. acknowledge financial support by the China Scholarship Council (CSC). A.D. acknowledges funding from the FWF (W-1213) and from the University of Salzburg via the ACNB program. Thanks to M. Trainic, M. Riekert, and S. Benner for support with graphical illustrations. The authors acknowledge stimulating exchange and discussions with Paul J. Crutzen, the members of the Mainz Program for Chemical Allergology (MPCA), the Allergie-Zentrum Rheinland-Pfalz (AZ-RP), and the Mainz Bioaerosol Laboratory (MBAL), as well as numerous colleagues in the scientific communities of the Earth, Environmental, and Life Sciences.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b04908.
Allergic sensitization and response, air pollution effects on the allergenic potential of plant pollen and fungal spores, research activities proposed to address the key questions, essential steps and influencing factors in the development of IgE-mediated allergies, simplified scheme of major processes involved in allergic sensitization and response, scanning electron micrographs of oak and birch pollen, 3D-structure of the major birch pollen allergen Bet v 1.0101, and TLR4-radical cycle of inflammation (PDF)
Author Contributions
○ K.R.-S. and C.J.K. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Johansson S. G. O.; Bieber T.; Dahl R. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004, 113 (5), 832–836. 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- Tanno L. K.; Calderon M. A.; Smith H. E.; Sanchez-Borges M.; Sheikh A.; Demoly P. Dissemination of definitions and concepts of allergic and hypersensitivity conditions. World Allergy Organ. J. 2016, 9 (1), 24. 10.1186/s40413-016-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J.; Tsai M.; Piliponsky A. M. The development of allergic inflammation. Nature 2008, 454 (7203), 445–454. 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkinson N. F. Jr.; Bochner B. S.; Burks A. W.; Busse W. W.; Holgate S. T.; Lemanske R. F. Jr.; O’Hehir R. E.. Middleton’s Allergy Principles and Practice, 8th ed.; Elsevier: 2014; Vol. 1 and 2, pp 1896. [Google Scholar]
- Huby R. D. J.; Dearman R. J.; Kimber I. Why are some proteins allergens?. Toxicol. Sci. 2000, 55 (2), 235–246. 10.1093/toxsci/55.2.235. [DOI] [PubMed] [Google Scholar]
- Palm N. W.; Rosenstein R. K.; Medzhitov R. Allergic host defences. Nature 2012, 484 (7395), 465–472. 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakib F.; Ghaemmaghami A. M.; Sewell H. F. The molecular basis of allergenicity. Trends Immunol. 2008, 29 (12), 633–642. 10.1016/j.it.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Traidl-Hoffmann C.; Jakob T.; Behrendt H. Determinants of allergenicity. J. Allergy Clin. Immunol. 2009, 123 (3), 558–566. 10.1016/j.jaci.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Ring J.; Eberlein-Koenig B.; Behrendt H. Environmental pollution and allergy. Ann. Allergy, Asthma, Immunol. 2001, 87 (6), 2–6. 10.1016/S1081-1206(10)62332-0. [DOI] [PubMed] [Google Scholar]
- Pawankar R.; Baena-Cagnani C.; Bousquet J.; Walter Canonica G.; Cruz A.; Kaliner M.; Lanier B.; Henley K. State of World Allergy Report 2008: Allergy and Chronic Respiratory Diseases. World Allergy Organ. J. 2008, 1 (Suppl 1), S4–S17. 10.1186/1939-4551-1-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen U.; Schmitz R.; Steppuhn H. Prevalence of allergic diseases in Germany. Results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2013, 56 (5–6), 698–706. 10.1007/s00103-012-1652-7. [DOI] [PubMed] [Google Scholar]
- D’Amato G.; Holgate S. T.; Pawankar R. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ. J. 2015, 8, 25. 10.1186/s40413-015-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham-Rowe D. Lifestyle: When allergies go west. Nature 2011, 479 (7374), S2–S4. 10.1038/479S2a. [DOI] [Google Scholar]
- Valenta R.; Hochwallner H.; Linhart B.; Pahr S. Food Allergies: The Basics. Gastroenterology 2015, 148 (6), 1120–1131. 10.1053/j.gastro.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian D.; Hansen S.; Wurtzen P. A. Airway responses towards allergens - from the airway epithelium to T cells. Clin. Exp. Allergy 2015, 45 (8), 1268–1287. 10.1111/cea.12451. [DOI] [PubMed] [Google Scholar]
- Lambrecht B. N.; Hammad H. The airway epithelium in asthma. Nat. Med. 2012, 18 (5), 684–692. 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- Evans H.; Mitre E. Worms as therapeutic agents for allergy and asthma: Understanding why benefits in animal studies have not translated into clinical success. J. Allergy Clin. Immunol. 2015, 135 (2), 343–353. 10.1016/j.jaci.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Ring J.; Kramer U.; Schafer T.; Behrendt H. Why are allergies increasing?. Curr. Opin. Immunol. 2001, 13 (6), 701–708. 10.1016/S0952-7915(01)00282-5. [DOI] [PubMed] [Google Scholar]
- Heinrich J.; Popescu M. A.; Wjst M.; Goldstein I. F.; Wichmann H. E. Atopy in children and parental social class. Am. J. Public Health 1998, 88 (9), 1319–1324. 10.2105/AJPH.88.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W.; Buckley C. E. III; Machamer C. E.; Schlagel G. D.; Yost J. A.; Blessingmoore J.; Levy D. Does hyperimmunoglobulinemia-E protect tropical populations from allergic disease?. J. Allergy Clin. Immunol. 1983, 71 (2), 184–188. 10.1016/0091-6749(83)90097-0. [DOI] [PubMed] [Google Scholar]
- Olesen A. B.; Juul S.; Birkebaek N.; Thestrup-Pedersen K. Association between atopic dermatitis and insulin-dependent diabetes mellitus: a case-control study. Lancet 2001, 357 (9270), 1749–1752. 10.1016/S0140-6736(00)04896-0. [DOI] [PubMed] [Google Scholar]
- Coca A. F.; Cooke R. A. On the classification of the phenomena of hypersensitiveness. J. Immunol. 1923, 8 (3), 163–182. [Google Scholar]
- Holt P. G.; Thomas W. R. Sensitization to airborne environmental allergens: unresolved issues. Nat. Immunol. 2005, 6 (10), 957–960. 10.1038/ni1005-957. [DOI] [PubMed] [Google Scholar]
- Bégin P.; Nadeau K. C. Epigenetic regulation of asthma and allergic disease. Allergy, Asthma, Clin. Immunol. 2014, 10 (1), 27. 10.1186/1710-1492-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring J.; Akdis C.; Lauener R.; et al. Global Allergy Forum and Second Davos Declaration 2013 Allergy: Barriers to cure - challenges and actions to be taken. Allergy 2014, 69 (8), 978–982. 10.1111/all.12406. [DOI] [PubMed] [Google Scholar]
- Portelli M. A.; Hodge E.; Sayers I. Genetic risk factors for the development of allergic disease identified by genome-wide association. Clin. Exp. Allergy 2015, 45 (1), 21–31. 10.1111/cea.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer U.; Koch T.; Ranft U.; Ring J.; Behrendt H. Traffic-related air pollution is associated with atopy in children living in urban areas. Epidemiology (Cambridge, Mass.) 2000, 11 (1), 64–70. 10.1097/00001648-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Martino D. J.; Prescott S. L. Progress in understanding the epigenetic basis for immune development, immune function, and the rising incidence of allergic disease. Curr. Allergy Asthma Rep. 2013, 13 (1), 85–92. 10.1007/s11882-012-0312-1. [DOI] [PubMed] [Google Scholar]
- Peden D. B. Does air pollution really cause allergy?. Clin. Exp. Allergy 2015, 45 (1), 3–5. 10.1111/cea.12414. [DOI] [PubMed] [Google Scholar]
- Miller R. L.; Peden D. B. Environmental Impacts on Immune Responses in Atopy and Asthma. J. Allergy Clin. Immunol. 2014, 134 (5), 1001–1008. 10.1016/j.jaci.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffin J. M.; Kanchongkittiphon W.; Phipatanakul W. Perinatal and early childhood environmental factors influencing allergic asthma immunopathogenesis. Int. Immunopharmacol. 2014, 22 (1), 21–30. 10.1016/j.intimp.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahn U. What drives the allergic march?. Allergy 2000, 55 (7), 591–599. 10.1034/j.1398-9995.2000.00111.x. [DOI] [PubMed] [Google Scholar]
- Krämer U.; Behrendt H.; Dolgner R.; Ranft U.; Ring J.; Willer H.; Schlipkoter H. W. Airway diseases and allergies in East and West German children during the first 5 years after reunification: time trends and the impact of sulphur dioxide and total suspended particles. Int. J. Epidemiol. 1999, 28 (5), 865–873. 10.1093/ije/28.5.865. [DOI] [PubMed] [Google Scholar]
- Castro-Rodriguez J. A.; Forno E.; Rodriguez-Martinez C. E.; Celedon J. C. Risk and Protective Factors for Childhood Asthma: What Is the Evidence?. J. Allergy Clin. Immunol.-Pract. 2016, 4 (6), 1111–1122. 10.1016/j.jaip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J. A.; Alexis N.; Barnes C.; et al. Health effects of air pollution. J. Allergy Clin. Immunol. 2004, 114 (5), 1116–1123. 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Kim B.-J.; Lee S.-Y.; Kim H.-B.; Lee E.; Hong S.-J. Environmental Changes, Microbiota, and Allergic Diseases. Allergy, Asthma Immunol. Res. 2014, 6 (5), 389–400. 10.4168/aair.2014.6.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B.; Holgate S. T. Air pollution and health. Lancet 2002, 360 (9341), 1233–1242. 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Pöschl U.; Shiraiwa M. Multiphase Chemistry at the Atmosphere–Biosphere Interface Influencing Climate and Public Health in the Anthropocene. Chem. Rev. 2015, 115 (10), 4440–4475. 10.1021/cr500487s. [DOI] [PubMed] [Google Scholar]
- Crutzen P. J. Geology of mankind. Nature 2002, 415 (6867), 23–23. 10.1038/415023a. [DOI] [PubMed] [Google Scholar]
- Steffen W.; Crutzen P. J.; McNeill J. R. The Anthropocene: Are humans now overwhelming the great forces of nature. Ambio 2007, 36 (8), 614–621. 10.1579/0044-7447(2007)36[614:TAAHNO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zalasiewicz J.; Williams M.; Steffen W.; Crutzen P. The New World of the Anthropocene. Environ. Sci. Technol. 2010, 44 (7), 2228–2231. 10.1021/es903118j. [DOI] [PubMed] [Google Scholar]
- Zalasiewicz J.; Crutzen P. J.; Steffen W., The Anthropocene. In Geologic Time Scale 2012, Vols 1 & 2, Gradstein F. M., Ogg J. G., Mark Schmitz M., Ogg G., Eds.; Elsevier, 2012; 1033–1040. [Google Scholar]
- Steffen W.; Grinevald J.; Crutzen P.; McNeill J. The Anthropocene: conceptual and historical perspectives. Philos. Trans. R. Soc., A 2011, 369 (1938), 842–867. 10.1098/rsta.2010.0327. [DOI] [PubMed] [Google Scholar]
- Crutzen P. J. Anthropocene man. Nature 2010, 467 (7317), S10. 10.1038/467S10a. [DOI] [PubMed] [Google Scholar]
- Foley S. F.; Gronenborn D.; Andreae M. O.; et al. The Palaeoanthropocene–The beginnings of anthropogenic environmental change. Anthropocene 2013, 3, 83–88. 10.1016/j.ancene.2013.11.002. [DOI] [Google Scholar]
- Lewis S. L.; Maslin M. A. Defining the Anthropocene. Nature 2015, 519 (7542), 171–180. 10.1038/nature14258. [DOI] [PubMed] [Google Scholar]
- Waters C. N.; Zalasiewicz J.; Summerhayes C. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 2016, 351 (6269), aad2622. 10.1126/science.aad2622. [DOI] [PubMed] [Google Scholar]
- Canfield D. E.; Glazer A. N.; Falkowski P. G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330 (6001), 192–196. 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- Heald C. L.; Spracklen D. V. Land Use Change Impacts on Air Quality and Climate. Chem. Rev. 2015, 115 (10), 4476–4496. 10.1021/cr500446g. [DOI] [PubMed] [Google Scholar]
- Crutzen P. J.; Stoermer E. F. The “Anthropocene”. Global Change Newsletter 2000, 41, 17. [Google Scholar]
- Crutzen P. J. The effects of industrial and agricultural practices on atmospheric chemistry and climate during the anthropocene. J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2002, 37 (4), 423–424. 10.1081/ESE-120003224. [DOI] [PubMed] [Google Scholar]
- Crutzen P. J.Atmospheric Chemistry in the “Anthropocene”. In Challenges of a Changing Earth, Proceedings of the Global Change Open Science Conference, Amsterdam, The Netherlands, 10–13 July 2001; Steffen W.; Jäger J.; Carson D. J.; Bradshaw C., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2002; 45–48. [Google Scholar]
- Williams J.; Crutzen P. J. Perspectives on our planet in the Anthropocene. Environ. Chem. 2013, 10 (4), 269–280. 10.1071/EN13061. [DOI] [Google Scholar]
- Suni T.; Guenther A.; Hansson H. C.; et al. The significance of land-atmosphere interactions in the Earth system—iLEAPS achievements and perspectives. Anthropocene 2015, 12, 69–84. 10.1016/j.ancene.2015.12.001. [DOI] [Google Scholar]
- Schäfer S.; Stelzer H.; Maas A.; Lawrence M. G. Earth’s future in the Anthropocene: Technological interventions between piecemeal and utopian social engineering. Earth's Future 2014, 2 (4), 239–243. 10.1002/2013EF000190. [DOI] [Google Scholar]
- Brondizio E. S.; O’Brien K.; Bai X.; et al. Re-conceptualizing the Anthropocene: A call for collaboration. Glob. Environ. Change 2016, 39, 318–327. 10.1016/j.gloenvcha.2016.02.006. [DOI] [Google Scholar]
- Lawrence M. G.; Crutzen P. J. Was breaking the taboo on research on climate engineering via albedo modification a moral hazard or a moral imperative?. Earth’s Future 2017, 5, 136. 10.1002/2016EF000463. [DOI] [Google Scholar]
- Zalasiewicz J.; Waters C. N.; Williams M.; et al. When did the Anthropocene begin? A mid-twentieth century boundary level is stratigraphically optimal. Quat. Int. 2015, 383, 196–203. 10.1016/j.quaint.2014.11.045. [DOI] [Google Scholar]
- Waters C. N.; Zalasiewicz J. A.; Williams M.; Ellis M. A.; Snelling A. M. A stratigraphical basis for the Anthropocene?. Geol. Soc. Spec. Publ. 2014, 395, 1–21. 10.1144/SP395.18. [DOI] [Google Scholar]
- Zalasiewicz J.; Williams M.; Waters C. N., Can an Anthropocene Series be defined and recognized? In Stratigraphical Basis for the Anthropocene; Waters C. N., Zalasiewicz J. A., Williams M., Ellis M., Snelling A. M., Eds.; Geological Society: London, 2014; Vol. 395, 39–53. [Google Scholar]
- Zalasiewicz J.; Williams M.; Haywood A.; Ellis M. The Anthropocene: a new epoch of geological time?. Philos. Trans. R. Soc., A 2011, 369 (1938), 835–841. 10.1098/rsta.2010.0339. [DOI] [PubMed] [Google Scholar]
- Zalasiewicz J.; Williams M.; Steffen W.; Crutzen P. Response to ″The Anthropocene forces us to reconsider adaptationist models of human-environment interactions″. Environ. Sci. Technol. 2010, 44 (16), 6008–6008. 10.1021/es102062w. [DOI] [PubMed] [Google Scholar]
- Steffen W.; Leinfelder R.; Zalasiewicz J.; et al. Stratigraphic and Earth System approaches to defining the Anthropocene. Earth's Future 2016, 4 (8), 324–345. 10.1002/2016EF000379. [DOI] [Google Scholar]
- Williams M.; Zalasiewicz J.; Waters C. N.; et al. The Anthropocene: a conspicuous stratigraphical signal of anthropogenic changes in production and consumption across the biosphere. Earth's Future 2016, 4 (3), 34–53. 10.1002/2015EF000339. [DOI] [Google Scholar]
- Cooper O. R.; Parrish D. D.; Ziemke J. Global distribution and trends of tropospheric ozone: An observation-based review. Elementa-Science of the Anthropocene 2014, 2, 000029 10.12952/journal.elementa.000029. [DOI] [Google Scholar]
- Monks P. S.; Archibald A. T.; Colette A.; et al. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmos. Chem. Phys. 2015, 15 (15), 8889–8973. 10.5194/acp-15-8889-2015. [DOI] [Google Scholar]
- Monks P. S.; Granier C.; Fuzzi S.; et al. Atmospheric composition change - global and regional air quality. Atmos. Environ. 2009, 43 (33), 5268–5350. 10.1016/j.atmosenv.2009.08.021. [DOI] [Google Scholar]
- Pusede S. E.; Steiner A. L.; Cohen R. C. Temperature and Recent Trends in the Chemistry of Continental Surface Ozone. Chem. Rev. 2015, 115 (10), 3898–3918. 10.1021/cr5006815. [DOI] [PubMed] [Google Scholar]
- Andreae M. O. Aerosols before pollution. Science 2007, 315 (5808), 50–51. 10.1126/science.1136529. [DOI] [PubMed] [Google Scholar]
- Seinfeld J. H.; Pandis S. N.. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons, 2016; pp 1152. [Google Scholar]
- Fishman J.; Creilson J. K.; Parker P. A.; Ainsworth E. A.; Vining G. G.; Szarka J.; Booker F. L.; Xu X. An investigation of widespread ozone damage to the soybean crop in the upper Midwest determined from ground-based and satellite measurements. Atmos. Environ. 2010, 44 (18), 2248–2256. 10.1016/j.atmosenv.2010.01.015. [DOI] [Google Scholar]
- Lelieveld J.; Evans J. S.; Fnais M.; Giannadaki D.; Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525 (7569), 367–371. 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Lelieveld J.; Barlas C.; Giannadaki D.; Pozzer A. Model calculated global, regional and megacity premature mortality due to air pollution. Atmos. Chem. Phys. 2013, 13 (14), 7023–7037. 10.5194/acp-13-7023-2013. [DOI] [Google Scholar]
- Brauer M.; Freedman G.; Frostad J.; et al. Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ. Sci. Technol. 2016, 50 (1), 79–88. 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- Brauer M.; Amann M.; Burnett R. T.; et al. Exposure Assessment for Estimation of the Global Burden of Disease Attributable to Outdoor Air Pollution. Environ. Sci. Technol. 2012, 46 (2), 652–660. 10.1021/es2025752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. J.; Cohen A.; Dentener F.; et al. What We Breathe Impacts Our Health: Improving Understanding of the Link between Air Pollution and Health. Environ. Sci. Technol. 2016, 50 (10), 4895–4904. 10.1021/acs.est.5b03827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia A. W.; Pope C. A. 3rd; Dockery D. W.; Wang Y.; Ezzati M.; Dominici F. Effect of air pollution control on life expectancy in the United States: an analysis of 545 U.S. counties for the period from 2000 to 2007. Epidemiology (Cambridge, Mass.) 2013, 24 (1), 23–31. 10.1097/EDE.0b013e3182770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. A. 3rd; Dockery D. W. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manage. Assoc. 2006, 56 (6), 709–742. 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Dockery D. W.; Pope C. A. 3rd; Xu X.; Spengler J. D.; Ware J. H.; Fay M. E.; Ferris B. G. Jr.; Speizer F. E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329 (24), 1753–1759. 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Gao M.; Guttikunda S. K.; Carmichael G. R.; Wang Y. S.; Liu Z. R.; Stanier C. O.; Saide P. E.; Yu M. Health impacts and economic losses assessment of the 2013 severe haze event in Beijing area. Sci. Total Environ. 2015, 511, 553–561. 10.1016/j.scitotenv.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Whitmee S.; Haines A.; Beyrer C.; et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet 2015, 386 (10007), 1973–2028. 10.1016/S0140-6736(15)60901-1. [DOI] [PubMed] [Google Scholar]
- Brunekreef B.; Sunyer J. Asthma, rhinitis and air pollution: is traffic to blame?. Eur. Respir. J. 2003, 21 (6), 913–915. 10.1183/09031936.03.00014903. [DOI] [PubMed] [Google Scholar]
- Breiteneder H.; Mills E. N. C. Plant food allergens - structural and functional aspects of allergenicity. Biotechnol. Adv. 2005, 23 (6), 395–399. 10.1016/j.biotechadv.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Scheurer S.; Toda M.; Vieths S. What makes an allergen?. Clin. Exp. Allergy 2015, 45, 1150–1161. 10.1111/cea.12571. [DOI] [PubMed] [Google Scholar]
- Thomas W. R.; Hales B. J.; Smith W.-A. Structural biology of allergens. Curr. Allergy Asthma Rep. 2005, 5 (5), 388–393. 10.1007/s11882-005-0012-1. [DOI] [PubMed] [Google Scholar]
- Saxon A.; Diaz-Sanchez D. Air pollution and allergy: you are what you breathe. Nat. Immunol. 2005, 6 (3), 223–226. 10.1038/ni0305-223. [DOI] [PubMed] [Google Scholar]
- Kim K. H.; Jahan S. A.; Kabir E. A review on human health perspective of air pollution with respect to allergies and asthma. Environ. Int. 2013, 59, 41–52. 10.1016/j.envint.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Bartra J.; Mullol J.; del Cuvillo A.; Davila I.; Ferrer M.; Jauregui I.; Montoro J.; Sastre J.; Valero A. Air pollution and allergens. J. Invest. Allergol. Clin. Immunol. 2007, 17, 3–8. [PubMed] [Google Scholar]
- Blando J.; Bielory L.; Nguyen V.; Diaz R.; Jeng H. A. Anthropogenic Climate Change and Allergic Diseases. Atmosphere 2012, 3 (1), 200–212. 10.3390/atmos3010200. [DOI] [Google Scholar]
- D’Amato G.; Baena-Cagnani C. E.; Cecchi L.; et al. Climate change, air pollution and extreme events leading to increasing prevalence of allergic respiratory diseases. Multidscip. Respir. Med. 2013, 8, 12. 10.1186/2049-6958-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea K. M.; Truckner R. T.; Weber R. W.; Peden D. B. Climate change and allergic disease. J. Allergy Clin. Immunol. 2008, 122 (3), 443–453. 10.1016/j.jaci.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Beggs P. J.; Bambrick H. J. Is the global rise of asthma an early impact of anthropogenic climate change?. Environ. Health Perspect. 2005, 113 (8), 915–919. 10.1289/ehp.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C. E.; Gamble J. L. Aeroallergens, Allergic Disease, and Climate Change: Impacts and Adaptation. EcoHealth 2009, 6 (3), 458–470. 10.1007/s10393-009-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa M.; Selzle K.; Pöschl U. Hazardous components and health effects of atmospheric aerosol particles: reactive oxygen species, soot, polycyclic aromatic compounds and allergenic proteins. Free Radical Res. 2012, 46 (8), 927–939. 10.3109/10715762.2012.663084. [DOI] [PubMed] [Google Scholar]
- Frank U.; Ernst D. Effects of NO2 and ozone on pollen allergenicity. Front. Plant Sci. 2016, 7, 91. 10.3389/fpls.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato G.; Vitale C.; De Martino A.; et al. Effects on asthma and respiratory allergy of Climate change and air pollution. Multidiscip. Respir. Med. 2015, 10, 39–39. 10.1186/s40248-015-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato M.; Vitale C.; Stanziola A.; Molino A.; Vatrella A.; D’Amato G.. Update on Effects of Climate Changes on Respiratory Allergy. Allergy, Asthma & Immunophysiology: From Genes to Clinical Management, New York, NY, April 26–29, 2014; pp 45–52. [Google Scholar]
- Ziska L. H.; Beggs P. J. Anthropogenic climate change and allergen exposure: The role of plant biology. J. Allergy Clin. Immunol. 2012, 129 (1), 27–32. 10.1016/j.jaci.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Tibbetts J. H. Air Quality and Climate Change: A Delicate Balance. Environ. Health Perspect. 2015, 123 (6), A148–A153. 10.1289/ehp.123-A148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni G.; D’Amato G.; Afferni C. The dangerous liaison between pollens and pollution in respiratory allergy. Ann. Allergy, Asthma, Immunol. 2017, 118 (3), 269–275. 10.1016/j.anai.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Jenerowicz D.; Silny W.; Danczak-Pazdrowska A.; Polanska A.; Osmola-Mankowska A.; Olek-Hrab K. Environmental factors and allergic diseases. Ann. Agric. Environ. Med. 2012, 19 (3), 475–481. [PubMed] [Google Scholar]
- Coombs R. R. A.; Gell P. G. H., The classification of allergic reactions underlying disease. In Clinical Aspects of Immunology; Gell PGH C. R. e., Ed.; Blackwell Science, 1963. [Google Scholar]
- Radauer C.; Bublin M.; Wagner S.; Mari A.; Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121 (4), 847–852. 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Lambrecht B. N.; Hammad H. The immunology of asthma. Nat. Immunol. 2015, 16 (1), 45–56. 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- Lambrecht B. N.; Hammad H. Allergens and the airway epithelium response: Gateway to allergic sensitization. J. Allergy Clin. Immunol. 2014, 134 (3), 499–507. 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Iwasaki A.; Medzhitov R. Regulation of Adaptive Immunity by the Innate Immune System. Science 2010, 327 (5963), 291–295. 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deifl S.; Bohle B. Factors influencing the allergenicity and adjuvanticity of allergens. Immunotherapy 2011, 3 (7), 881–893. 10.2217/imt.11.69. [DOI] [PubMed] [Google Scholar]
- Thomas W. R. Innate affairs of allergens. Clin. Exp. Allergy 2013, 43 (2), 152–163. 10.1111/j.1365-2222.2012.04059.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Casado C.; Díaz-Perales A. Allergen-Associated Immunomodulators: Modifying Allergy Outcome. Arch. Immunol. Ther. Exp. 2016, 64 (5), 339–347. 10.1007/s00005-016-0401-2. [DOI] [PubMed] [Google Scholar]
- Neurath M. F.; Finotto S.; Glimcher L. H. The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 2002, 8 (6), 567–573. 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- Berin M. C.; Sampson H. A. Mucosal immunology of food allergy. Curr. Biol. 2013, 23 (9), R389–R400. 10.1016/j.cub.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V.; Singh A. K.; Akbari O. The Role of Costimulatory Molecules in Allergic Disease and Asthma. Int. Arch. Allergy Immunol. 2010, 151 (3), 179–189. 10.1159/000242355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M.; Koehl J. New insights into the role of the complement pathway in allergy and asthma. Curr. Allergy Asthma Rep. 2005, 5 (5), 362–369. 10.1007/s11882-005-0007-y. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Köhl J. A complex role for complement in allergic asthma. Expert Rev. Clin. Immunol. 2010, 6 (2), 269–277. 10.1586/eci.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye S. G.; Ma C. S.; Brink R.; Deenick E. K. The good, the bad and the ugly - T-FH cells in human health and disease. Nat. Rev. Immunol. 2013, 13 (6), 412–426. 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- Schmudde I.; Laumonnier Y.; Köhl J. Anaphylatoxins coordinate innate and adaptive immune responses in allergic asthma. Semin. Immunol. 2013, 25, 2–11. 10.1016/j.smim.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Pandya P. H.; Wilkes D. S. Complement system in lung disease. Am. J. Respir. Cell Mol. Biol. 2014, 51 (4), 467–473. 10.1165/rcmb.2013-0485TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A.; Assiri A. M.; Broering D. C. Complement mediators: key regulators of airway tissue remodeling in asthma. J. Transl. Med. 2015, 13 (1), 272. 10.1186/s12967-015-0565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis C. A. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat. Med. 2012, 18 (5), 736–749. 10.1038/nm.2754. [DOI] [PubMed] [Google Scholar]
- Pellerin L.; Jenks J. A.; Begin P.; Bacchetta R.; Nadeau K. C. Regulatory T cells and their roles in immune dysregulation and allergy. Immunol. Res. 2014, 58 (2–3), 358–368. 10.1007/s12026-014-8512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman S. C.; Wurzburg B. A.; Tarchevskaya S. S.; Kinet J. P.; Jardetzky T. S. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilon RI alpha. Nature 2000, 406 (6793), 259–266. 10.1038/35018500. [DOI] [PubMed] [Google Scholar]
- Posner R. G.; Savage P. B.; Peters A. S.; Macias A.; DelGado J.; Zwartz G.; Sklar L. A.; Hlavacek W. S. A quantitative approach for studying IgE–FcεRI aggregation. Mol. Immunol. 2002, 38 (16–18), 1221–1228. 10.1016/S0161-5890(02)00067-6. [DOI] [PubMed] [Google Scholar]
- Skoner D. R. Allergic rhinitis: Definition, epidemiology, detection, and pathophysiology, diagnosis. J. Allergy Clin. Immunol. 2001, 108 (1), S2–S8. 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- Greiner A. N.; Hellings P. W.; Rotiroti G.; Scadding G. K. Allergic rhinitis. Lancet 2011, 378 (9809), 2112–2122. 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- Bianchi M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukocyte Biol. 2007, 81 (1), 1–5. 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Thomas W. R. Allergen Ligands in the Initiation of Allergic Sensitization. Curr. Allergy Asthma Rep. 2014, 14 (5), 10. 10.1007/s11882-014-0432-x. [DOI] [PubMed] [Google Scholar]
- Trompette A.; Divanovic S.; Visintin A.; et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009, 457 (7229), 585–588. 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp C. L. Guilt by intimate association: What makes an allergen an allergen?. J. Allergy Clin. Immunol. 2010, 125 (5), 955–960. 10.1016/j.jaci.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L.; Lucas K.; Fortuna C. A.; Chuang C. C.; Best T. M. Molecular Regulation of Toll-like Receptors in Asthma and COPD. Front. Physiol. 2015, 6, 312. 10.3389/fphys.2015.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18 (5), 673–683. 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- Holgate S. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol. Rev. 2011, 242, 205–219. 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- Salimi M.; Barlow J. L.; Saunders S. P.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210 (13), 2939–2950. 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartemes K. R.; Kephart G. M.; Fox S. J.; Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J. Allergy Clin. Immunol. 2014, 134 (3), 671–678.e4. 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernink J. H.; Germar K.; Spits H. The role of ILC2 in pathology of type 2 inflammatory diseases. Curr. Opin. Immunol. 2014, 31, 115–120. 10.1016/j.coi.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Ho J.; Bailey M.; Zaunders J.; Mrad N.; Sacks R.; Sewell W.; Harvey R. J. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin. Exp. Allergy 2015, 45 (2), 394–403. 10.1111/cea.12462. [DOI] [PubMed] [Google Scholar]
- Scanlon S. T.; McKenzie A. N. The messenger between worlds: the regulation of innate and adaptive type-2 immunity by innate lymphoid cells. Clin. Exp. Allergy 2015, 45 (1), 9–20. 10.1111/cea.12464. [DOI] [PubMed] [Google Scholar]
- Wynn T. A. Type 2 cytokines: mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15 (5), 271–282. 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- Woodruff P. G.; Modrek B.; Choy D. F.; Jia G. Q.; Abbas A. R.; Ellwanger A.; Arron J. R.; Koth L. L.; Fahy J. V. T-helper Type 2-driven Inflammation Defines Major Subphenotypes of Asthma. Am. J. Respir. Crit. Care Med. 2009, 180 (5), 388–395. 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroch T. E.; Curin M.; Valenta R.; Swoboda I. Mold Allergens in Respiratory Allergy: From Structure to Therapy. Allergy, Asthma Immunol. Res. 2015, 7 (3), 205–220. 10.4168/aair.2015.7.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radauer C.; Nandy A.; Ferreira F.; et al. Update of the WHO/IUIS Allergen Nomenclature Database based on analysis of allergen sequences. Allergy 2014, 69 (4), 413–419. 10.1111/all.12348. [DOI] [PubMed] [Google Scholar]
- Stocker T. F., Qin D.; Plattner G.-K.; Tignor M.; Allen S.K.; Boschung J.; Nauels A.; Xia Y.; Bex V.; Midgley P.M.. The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC, 2013: Climate Change 2013; Cambridge University Press: Cambridge, United Kingdom, 2013; p 1525. [Google Scholar]
- Gill S. S.; Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48 (12), 909–930. 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Apel K.; Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Ciais P.; Sabine C.; Bala G.. et al. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker T. F., Qin D., Plattner G.-K., Tignor M., Allen S.K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P.M., Ed.; Cambridge University Press: Cambridge, United Kingdom, 2013; pp 465–570. [Google Scholar]
- Cecchi L.; D’Amato G.; Ayres J. G.; et al. Projections of the effects of climate change on allergic asthma: the contribution of aerobiology. Allergy 2010, 65 (9), 1073–1081. 10.1111/j.1398-9995.2010.02423.x. [DOI] [PubMed] [Google Scholar]
- Klironomos J. N.; Rillig M. C.; Allen M. F.; Zak D. R.; Pregitzer K. S.; Kubiske M. E. Increased levels of airborne fungal spores in response to Populus tremuloides grown under elevated atmospheric CO2. Can. J. Bot. 1997, 75 (10), 1670–1673. 10.1139/b97-880. [DOI] [Google Scholar]
- Ciappetta S.; Ghiani A.; Gilardelli F.; Bonini M.; Citterio S.; Gentili R. Invasion of Ambrosia artemisiifolia in Italy: Assessment via analysis of genetic variability and herbarium data. Flora (Jena) 2016, 223, 106–113. 10.1016/j.flora.2016.05.002. [DOI] [Google Scholar]
- Ziska L. H.; McConnell L. L. Climate Change, Carbon Dioxide, and Pest Biology: Monitor, Mitigate, Manage. J. Agric. Food Chem. 2016, 64 (1), 6–12. 10.1021/jf506101h. [DOI] [PubMed] [Google Scholar]
- Ziska L. H.; Tomecek M. B.; Valerio M.; Thompson J. P. Evidence for recent evolution in an invasive species, Microstegium vimineum, Japanese stiltgrass. Weed Res. 2015, 55 (3), 260–267. 10.1111/wre.12138. [DOI] [Google Scholar]
- Ziska L.; Knowlton K.; Rogers C.; et al. Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (10), 4248–4251. 10.1073/pnas.1014107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato G. Effects of climatic changes and urban air pollution on the rising trends of respiratory allergy and asthma. Multidiscip. Respir. Med. 2011, 6 (1), 28–37. 10.1186/2049-6958-6-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres V. R.; Huffman J. A.; Burrows S. M.; et al. Primary biological aerosol particles in the atmosphere: a review. Tellus, Ser. B 2012, 64, 15598. 10.3402/tellusb.v64i0.15598. [DOI] [Google Scholar]
- Despres V. R.; Nowoisky J. F.; Klose M.; Conrad R.; Andreae M. O.; Pöschl U. Characterization of primary biogenic aerosol particles in urban, rural, and high-alpine air by DNA sequence and restriction fragment analysis of ribosomal RNA genes. Biogeosciences 2007, 4 (6), 1127–1141. 10.5194/bg-4-1127-2007. [DOI] [Google Scholar]
- Fröhlich-Nowoisky J.; Kampf C. J.; Weber B.; et al. Bioaerosols in the Earth System: Climate, Health, and Ecosystem Interactions. Atmos. Res. 2016, 182, 346–376. 10.1016/j.atmosres.2016.07.018. [DOI] [Google Scholar]
- Lang-Yona N.; Levin Y.; Dannemiller K. C.; Yarden O.; Peccia J.; Rudich Y. Changes in atmospheric CO2 influence the allergenicity of Aspergillus fumigatus. Glob. Change Biol. 2013, 19 (8), 2381–2388. 10.1111/gcb.12219. [DOI] [PubMed] [Google Scholar]
- Wolf J.; O’Neill N. R.; Rogers C. A.; Muilenberg M. L.; Ziska L. H. Elevated Atmospheric Carbon Dioxide Concentrations Amplify Alternaria alternata Sporulation and Total Antigen Production. Environ. Health Perspect. 2010, 118 (9), 1223–1228. 10.1289/ehp.0901867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H.; Pauling A.; Vogel B. Numerical simulation of birch pollen dispersion with an operational weather forecast system. Int. J. Biometeorol. 2008, 52 (8), 805–814. 10.1007/s00484-008-0174-3. [DOI] [PubMed] [Google Scholar]
- El Kelish A.; Zhao F.; Heller W.; et al. Ragweed (Ambrosia artemisiifolia) pollen allergenicity: SuperSAGE transcriptomic analysis upon elevated CO2 and drought stress. BMC Plant Biol. 2014, 14, 176. 10.1186/1471-2229-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertine J. M.; Manning W. J.; DaCosta M.; Stinson K. A.; Muilenberg M. L.; Rogers C. A. Projected Carbon Dioxide to Increase Grass Pollen and Allergen Exposure Despite Higher Ozone Levels. PLoS One 2014, 9 (11), e111712. 10.1371/journal.pone.0111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzyk I.; Rodinkova V.; Šaulienė I.; et al. Air pollution by allergenic spores of the genus Alternaria in the air of central and eastern Europe. Environ. Sci. Pollut. Res. 2015, 22 (12), 9260–9274. 10.1007/s11356-014-4070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham R. M.; Sparks T. H.; Skjoth C. A.; Head K.; Adams-Groom B.; Smith M. Pollen season and climate: Is the timing of birch pollen release in the UK approaching its limit?. Int. J. Biometeorol. 2013, 57 (3), 391–400. 10.1007/s00484-012-0563-5. [DOI] [PubMed] [Google Scholar]
- Weber R. W.Aerobiology of Outdoor Allergens A2—Adkinson, N. Franklin. In Middleton’s Allergy, 8th ed.; Bochner B. S., Burks A. W., Busse W. W., Holgate S. T., Lemanske R. F., O’Hehir R. E., Eds.; Elsevier: London, 2014; 430–452. [Google Scholar]
- Cohen S. G.; Reif C. B. Cutaneous Sensitization to Blue-Green Algae. J. Allergy 1953, 24 (5), 452–457. 10.1016/0021-8707(53)90047-1. [DOI] [PubMed] [Google Scholar]
- Stewart I.; Webb P. M.; Schluter P. J.; Fleming L. E.; Burns J. W.; Gantar M.; Backer L. C.; Shaw G. R. Epidemiology of recreational exposure to freshwater cyanobacteria - an international prospective cohort study. BMC Public Health 2006, 6 (1), 9310. 10.1186/1471-2458-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genitsaris S.; Kormas K. A.; Moustaka-Gouni M. Airborne algae and cyanobacteria: occurrence and related health effects. Front. Biosci., Elite Ed. 2011, 3 (1), 772–787. 10.2741/285. [DOI] [PubMed] [Google Scholar]
- Petrus M.; Culerrier R.; Campistron M.; Barre A.; Rouge P. First case report of anaphylaxis to spirulin: identification of phycocyanin as responsible allergen. Allergy 2010, 65 (7), 924–925. 10.1111/j.1398-9995.2009.02257.x. [DOI] [PubMed] [Google Scholar]
- Geh E. N.; Ghosh D.; McKell M.; de la Cruz A. A.; Stelma G.; Bernstein J. A. Identification of Microcystis aeruginosa Peptides Responsible for Allergic Sensitization and Characterization of Functional Interactions between Cyanobacterial Toxins and Immunogenic Peptides. Environ. Health Persp. 2015, 123 (11), 1159–1166. 10.1289/ehp.1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pichel F.; Loza V.; Marusenko Y.; Mateo P.; Potrafka R. M. Temperature Drives the Continental-Scale Distribution of Key Microbes in Topsoil Communities. Science 2013, 340 (6140), 1574–1577. 10.1126/science.1236404. [DOI] [PubMed] [Google Scholar]
- Elbert W.; Weber B.; Burrows S.; Steinkamp J.; Buedel B.; Andreae M. O.; Pöschl U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5 (7), 459–462. 10.1038/ngeo1486. [DOI] [Google Scholar]
- Weber B.; Büdel B.; Belnap J.. Biological Soil Crusts: An Organizing Principle in Drylands; Springer International Publishing: Switzerland, 2016; Vol. 226. [Google Scholar]
- Reed S. C.; Coe K. K.; Sparks J. P.; Housman D. C.; Zelikova T. J.; Belnap J. Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nat. Clim. Change 2012, 2 (10), 752–755. 10.1038/nclimate1596. [DOI] [Google Scholar]
- Escolar C.; Martínez I.; Bowker M. A.; Maestre F. T. Warming reduces the growth and diversity of biological soil crusts in a semi-arid environment: implications for ecosystem structure and functioning. Philos. Trans. R. Soc., B 2012, 367 (1606), 3087–3099. 10.1098/rstb.2011.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrenberg S.; Reed S. C.; Belnap J. Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (39), 12116–12121. 10.1073/pnas.1509150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang-Yona N.; Kunert A. T.; Vogel L.. et al. Fresh Water, Marine and Terrestrial Cyanobacteria Display Distinct Allergen Characteristics. 2017, submitted for publication. [DOI] [PubMed] [Google Scholar]
- Stanelle T.; Bey I.; Raddatz T.; Reick C.; Tegen I. Anthropogenically induced changes in twentieth century mineral dust burden and the associated impact on radiative forcing. J. Geophys. Res.-Atmos. 2014, 119 (23), 13526–13546. 10.1002/2014JD022062. [DOI] [Google Scholar]
- McLeman R. A.; Dupre J.; Berrang Ford L.; Ford J.; Gajewski K.; Marchildon G. What we learned from the Dust Bowl: lessons in science, policy, and adaptation. Popul. Environ. 2014, 35 (4), 417–440. 10.1007/s11111-013-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff J. C.; Ballantyne A. P.; Farmer G. L.; et al. Increasing eolian dust deposition in the western United States linked to human activity. Nat. Geosci. 2008, 1 (3), 189–195. 10.1038/ngeo133. [DOI] [Google Scholar]
- Mahowald N. M.; Kloster S.; Engelstaedter S.; et al. Observed 20th century desert dust variability: impact on climate and biogeochemistry. Atmos. Chem. Phys. 2010, 10 (22), 10875–10893. 10.5194/acp-10-10875-2010. [DOI] [Google Scholar]
- Mulitza S.; Heslop D.; Pittauerova D.; et al. Increase in African dust flux at the onset of commercial agriculture in the Sahel region. Nature 2010, 466 (7303), 226–228. 10.1038/nature09213. [DOI] [PubMed] [Google Scholar]
- Esmaeil N.; Gharagozloo M.; Rezaei A.; Grunig G. Dust events, pulmonary diseases and immune system. Am. J. Clin. Exp. Immunl. 2014, 3 (1), 20–29. [PMC free article] [PubMed] [Google Scholar]
- Goudie A. S. Desert dust and human health disorders. Environ. Int. 2014, 63, 101–113. 10.1016/j.envint.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Griffin D. W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007, 20 (3), 459–477. 10.1128/CMR.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg C. A.; Griffin D. W. Aerobiology and the global transport of desert dust. Trends Ecol. Evol. 2006, 21 (11), 638–644. 10.1016/j.tree.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Leski T. A.; Malanoski A. P.; Gregory M. J.; Lin B. C.; Stenger D. A. Application of a Broad-Range Resequencing Array for Detection of Pathogens in Desert Dust Samples from Kuwait and Iraq. Appl. Environ. Microb. 2011, 77 (13), 4285–4292. 10.1128/AEM.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Martinez M. G.; Rodriguez-Cotto R. I.; Ortiz-Rivera M. A.; Pluguez-Turull C. W.; Jimenez-Velez B. D. Linking Endotoxins, African Dust PM10 and Asthma in an Urban and Rural Environment of Puerto Rico. Mediators Inflammation 2015, 2015, 784212. 10.1155/2015/784212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T.; Susuki S.; Kobayashi F.; et al. Phylogenetic analysis of atmospheric halotolerant bacterial communities at high altitude in an Asian dust (KOSA) arrival region, Suzu City. Sci. Total Environ. 2010, 408 (20), 4556–4562. 10.1016/j.scitotenv.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N.; Ichijo T.; Sakotani A.; Baba T.; Nasu M. Global dispersion of bacterial cells on Asian dust. Sci. Rep. 2012, 2, 525. 10.1038/srep00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M.; Yamasaki A.; Burioka N.; et al. Correlation between Asian Dust Storms and Worsening Asthma in Western Japan. Allergol. Int. 2011, 60, 267–275. 10.2332/allergolint.10-OA-0239. [DOI] [PubMed] [Google Scholar]
- Kanatani K. T.; Ito I.; Al-Delaimy W. K.; Adachi Y.; Mathews W. C.; Ramsdell J. W. Desert Dust Exposure Is Associated with Increased Risk of Asthma Hospitalization in Children. Am. J. Respir. Crit. Care Med. 2010, 182 (12), 1475–1481. 10.1164/rccm.201002-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyan K.; Henry W.; Lacaille S.; Laloo A.; Lamsee-Ebanks C.; McKay S.; Antoine R. M.; Monteil M. A. African dust clouds are associated with increased paediatric asthma accident and emergency admissions on the Caribbean island of Trinidad. Int. J. Biometeorol. 2005, 49 (6), 371–376. 10.1007/s00484-005-0257-3. [DOI] [PubMed] [Google Scholar]
- Chang C. C.; Lee I. M.; Tsai S. S.; Yang C. Y. Correlation of Asian dust storm events with daily clinic visits for allergic rhinitis in Taipei, Taiwan. J. Toxicol. Environ. Health, Part A 2006, 69 (3), 229–235. 10.1080/15287390500227415. [DOI] [PubMed] [Google Scholar]
- D’Amato G.; Vitale C.; D’Amato M.; et al. Thunderstorm-related asthma: what happens and why. Clin. Exp. Allergy 2016, 46 (3), 390–396. 10.1111/cea.12709. [DOI] [PubMed] [Google Scholar]
- Mendell M. J.; Mirer A. G.; Cheung K.; Tong M.; Douwes J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environ. Health Perspect. 2011, 119 (6), 748–756. 10.1289/ehp.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer C. G.; Hohmann C.; Thiering E.; et al. Meta-analysis of mould and dampness exposure on asthma and allergy in eight European birth cohorts: an ENRIECO initiative. Allergy 2011, 66 (12), 1570–1579. 10.1111/j.1398-9995.2011.02712.x. [DOI] [PubMed] [Google Scholar]
- Dannemiller K. C.; Gent J. F.; Leaderer B. P.; Peccia J. Indoor microbial communities: Influence on asthma severity in atopic and nonatopic children. J. Allergy Clin. Immunol. 2016, 138 (1), 76–83. 10.1016/j.jaci.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A. E.Indoor Allergens A2—Adkinson, N. Franklin. In Middleton’s Allergy, 8th ed.; Bochner B. S., Burks A. W., Busse W. W., Holgate S. T., Lemanske R. F., O’Hehir R. E., Eds. Elsevier: London, 2014; 453–469. [Google Scholar]
- Elbert W.; Taylor P. E.; Andreae M. O.; Pöschl U. Contribution of fungi to primary biogenic aerosols in the atmosphere: wet and dry discharged spores, carbohydrates, and inorganic ions. Atmos. Chem. Phys. 2007, 7 (17), 4569–4588. 10.5194/acp-7-4569-2007. [DOI] [Google Scholar]
- Fröhlich-Nowoisky J.; Burrows S. M.; Xie Z.; et al. Biogeography in the air: fungal diversity over land and oceans. Biogeosciences 2012, 9 (3), 1125–1136. 10.5194/bg-9-1125-2012. [DOI] [Google Scholar]
- Fröhlich-Nowoisky J.; Pickersgill D. A.; Despres V. R.; Pöschl U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (31), 12814–12819. 10.1073/pnas.0811003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Germann I.; Vogel B.; Vogel H.; Pauling A.; Fröhlich-Nowoisky J.; Pöschl U.; Despres V. R. Quantitative DNA Analyses for Airborne Birch Pollen. PLoS One 2015, 10 (10), e0140949. 10.1371/journal.pone.0140949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G.; Oberdorster E.; Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113 (7), 823–839. 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. E.; Flagan R. C.; Miguel A. G.; Valenta R.; Glovsky M. M. Birch pollen rupture and the release of aerosols of respirable allergens. Clin. Exp. Allergy 2004, 34 (10), 1591–1596. 10.1111/j.1365-2222.2004.02078.x. [DOI] [PubMed] [Google Scholar]
- Taylor P. E.; Flagan R. C.; Valenta R.; Glovsky M. M. Release of allergens as respirable aerosols: A link between grass pollen and asthma. J. Allergy Clin. Immunol. 2002, 109 (1), 51–56. 10.1067/mai.2002.120759. [DOI] [PubMed] [Google Scholar]
- Knutsen A. P.; Bush R. K.; Demain J. G.; et al. Fungi and allergic lower respiratory tract diseases. J. Allergy Clin. Immunol. 2012, 129 (2), 280–291. 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- Behrendt H.; Becker W. M. Localization, release and bioavailability of pollen allergens: the influence of environmental factors. Curr. Opin. Immunol. 2001, 13 (6), 709–715. 10.1016/S0952-7915(01)00283-7. [DOI] [PubMed] [Google Scholar]
- Taylor P. E.; Jonsson H. Thunderstorm asthma. Curr. Allergy Asthma Rep. 2004, 4 (5), 409–413. 10.1007/s11882-004-0092-3. [DOI] [PubMed] [Google Scholar]
- Taylor P. E.; Jacobson K. W.; House J. M.; Glovsky M. M. Links between Pollen, Atopy and the Asthma Epidemic. Int. Arch. Allergy Immunol. 2007, 144 (2), 162–170. 10.1159/000103230. [DOI] [PubMed] [Google Scholar]
- Huffman J. A.; Prenni A. J.; DeMott P. J.; et al. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos. Chem. Phys. 2013, 13 (13), 6151–6164. 10.5194/acp-13-6151-2013. [DOI] [Google Scholar]
- Schäppi G. F.; Suphioglu C.; Taylor P. E.; Knox R. B. Concentrations of the major birch tree allergen Bet v 1 in pollen and respirable fine particles in the atmosphere. J. Allergy Clin. Immunol. 1997, 100 (5), 656–661. 10.1016/S0091-6749(97)70170-2. [DOI] [PubMed] [Google Scholar]
- Green B. J.; Zinovia Mitakakis T. Z.; Tovey E. R. Allergen detection from 11 fungal species before and after germination. J. Allergy Clin. Immunol. 2003, 111 (2), 285–289. 10.1067/mai.2003.57. [DOI] [PubMed] [Google Scholar]
- Sporik R. B.; Arruda L. K.; Woodfolk J.; Chapman M. D.; Plattsmills T. A. E. Environmental exposure to aspergillus-fumigatus allergen (asp-f-i). Clin. Exp. Allergy 1993, 23 (4), 326–331. 10.1111/j.1365-2222.1993.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Motta A. C.; Marliere M.; Peltre G.; Sterenberg P. A.; Lacroix G. Traffic-Related Air Pollutants Induce the Release of Allergen-Containing Cytoplasmic Granules from Grass Pollen. Int. Arch. Allergy Immunol. 2006, 139 (4), 294–298. 10.1159/000091600. [DOI] [PubMed] [Google Scholar]
- Ouyang Y.; Xu Z.; Fan E.; Li Y.; Zhang L. Effect of nitrogen dioxide and sulfur dioxide on viability and morphology of oak pollen. Int. Forum Allergy Rhinol. 2016, 6 (1), 95–100. 10.1002/alr.21632. [DOI] [PubMed] [Google Scholar]
- Gilles S.; Mariani V.; Bryce M.; Mueller M. J.; Ring J.; Behrendt H.; Jakob T.; Traidl-Hoffmann C. Pollen allergens do not come alone: pollen associated lipid mediators (PALMS) shift the human immune systems towards a T(H)2-dominated response. Allergy, Asthma, Clin. Immunol. 2009, 5 (1), 3. 10.1186/1710-1492-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles-Stein S.; Traidl-Hoffmann C. Pollen are more than allergen carriers. Allergologie 2016, 39 (2), 69–76. 10.5414/ALX01815. [DOI] [Google Scholar]
- Gilles S.; Beck I.; Lange S.; Ring J.; Behrendt H.; Traidl-Hoffmann C. Non-allergenic factors from pollen modulate T helper cell instructing notch ligands on dendritic cells. World Allergy Organ. J. 2015, 8, 2. 10.1186/s40413-014-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traidl-Hoffmann C.; Kasche A.; Jakob T.; Huger M.; Plotz S.; Feussner I.; Ring J.; Behrendt H. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J. Allergy Clin. Immunol. 2002, 109 (5), 831–838. 10.1067/mai.2002.124655. [DOI] [PubMed] [Google Scholar]
- Gilles-Stein S.; Beck I.; Chaker A.; et al. Pollen derived low molecular compounds enhance the human allergen specific immune response in vivo. Clin. Exp. Allergy 2016, 46 (10), 1355–1365. 10.1111/cea.12739. [DOI] [PubMed] [Google Scholar]
- Oeder S.; Alessandrini F.; Wirz O. F.; et al. Pollen-derived nonallergenic substances enhance Th2-induced IgE production in B cells. Allergy 2015, 70 (11), 1450–1460. 10.1111/all.12707. [DOI] [PubMed] [Google Scholar]
- Degobbi C.; Lopes F.; Carvalho-Oliveira R.; Munoz J. E.; Saldiva P. H. N. Correlation of fungi and endotoxin with PM2.5 and meteorological parameters in atmosphere of Sao Paulo, Brazil. Atmos. Environ. 2011, 45 (13), 2277–2283. 10.1016/j.atmosenv.2010.12.005. [DOI] [Google Scholar]
- Majd A.; Chehregani A.; Moin M.; Gholami M.; Kohno S.; Nabe T.; Shariatzade M. A. The effects of air pollution on structures, proteins and allergenicity of pollen grains. Aerobiologia 2004, 20 (2), 111–118. 10.1023/B:AERO.0000032950.12169.38. [DOI] [Google Scholar]
- Pöschl U.; Martin S. T.; Sinha B.; et al. Rainforest Aerosols as Biogenic Nuclei of Clouds and Precipitation in the Amazon. Science 2010, 329 (5998), 1513–1516. 10.1126/science.1191056. [DOI] [PubMed] [Google Scholar]
- Ring J.; Buters J.; Behrendt H.. Particulate and Pollen Interactions A2—Adkinson, N. Franklin. In Middleton’s Allergy, 8th ed.; Bochner B. S., Burks A. W., Busse W. W., Holgate S. T., Lemanske R. F., O’Hehir R. E., Eds. Elsevier: London, 2014; 497–507. [Google Scholar]
- Beck I.; Jochner S.; Gilles S.; et al. High Environmental Ozone Levels Lead to Enhanced Allergenicity of Birch Pollen. PLoS One 2013, 8 (11), e80147. 10.1371/journal.pone.0080147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce M.; Drews O.; Schenk M. F.; et al. Impact of Urbanization on the Proteome of Birch Pollen and Its Chemotactic Activity on Human Granulocytes. Int. Arch. Allergy Immunol. 2010, 151 (1), 46–55. 10.1159/000232570. [DOI] [PubMed] [Google Scholar]
- Chehregani A.; Majde A.; Moin M.; Gholami M.; Ali Shariatzadeh M.; Nassiri H. Increasing allergy potency of Zinnia pollen grains in polluted areas. Ecotoxicol. Environ. Saf. 2004, 58 (2), 267–272. 10.1016/j.ecoenv.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Cortegano I.; Civantos E.; Aceituno E.; del Moral A.; Lopez E.; Lombardero M.; del Pozo V.; Lahoz C. Cloning and expression of a major allergen from Cupressus arizonica pollen, Cup a 3, a PR-5 protein expressed under polluted environment. Allergy 2004, 59 (5), 485–490. 10.1046/j.1398-9995.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- Ghiani A.; Aina R.; Asero R.; Bellotto E.; Citterio S. Ragweed pollen collected along high-traffic roads shows a higher allergenicity than pollen sampled in vegetated areas. Allergy 2012, 67 (7), 887–894. 10.1111/j.1398-9995.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- Gruijthuijsen Y. K.; Grieshuber I.; Stocklinger A.; et al. Nitration enhances the allergenic potential of proteins. Int. Arch. Allergy Immunol. 2006, 141 (3), 265–275. 10.1159/000095296. [DOI] [PubMed] [Google Scholar]
- Jin H. J.; Choi G. S.; Shin Y. S.; Kim J. H.; Kim J. E.; Ye Y. M.; Park H. S. The Allergenic Potency of Japanese Hop Pollen Is Increasing With Environmental Changes in Korea. Allergy, Asthma Immunol. Res. 2013, 5 (5), 309–314. 10.4168/aair.2013.5.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Cervera M.; Castells T.; Vega-Maray A.; et al. Effects of air pollution on Cup a 3 allergen in Cupressus arizonica pollen grains. Ann. Allergy, Asthma, Immunol. 2008, 101 (1), 57–66. 10.1016/S1081-1206(10)60836-8. [DOI] [PubMed] [Google Scholar]
- Pöschl U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem., Int. Ed. 2005, 44 (46), 7520–7540. 10.1002/anie.200501122. [DOI] [PubMed] [Google Scholar]
- Kanter U.; Heller W.; Durner J.; et al. Molecular and Immunological Characterization of Ragweed (Ambrosia artemisiifolia L.) Pollen after Exposure of the Plants to Elevated Ozone over a Whole Growing Season. PLoS One 2013, 8 (4), e61518. 10.1371/journal.pone.0061518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini S.; Tedeschini E.; Frenguelli G.; Wopfner N.; Ferreira F.; D’Amato G.; Ederli L. Ozone affects pollen viability and NAD(P)H oxidase release from Ambrosia artemisiifolia pollen. Environ. Pollut. 2011, 159 (10), 2823–2830. 10.1016/j.envpol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obersteiner A.; Gilles S.; Frank U. Pollen-Associated Microbiome Correlates with Pollution Parameters and the Allergenicity of Pollen. PLoS One 2016, 11 (2), e0149545. 10.1371/journal.pone.0149545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuinica L. G.; Abreu I.; Esteves da Silva J. E. Effect of air pollutant NO2 on Betula pendula, Ostrya carpinifolia and Carpinus betulus pollen fertility and human allergenicity. Environ. Pollut. 2014, 186, 50–55. 10.1016/j.envpol.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Lang-Yona N.; Shuster-Meiseles T.; Mazar Y.; Yarden O.; Rudich Y. Impact of urban air pollution on the allergenicity of Aspergillus fumigatus conidia: Outdoor exposure study supported by laboratory experiments. Sci. Total Environ. 2016, 541, 365–371. 10.1016/j.scitotenv.2015.09.058. [DOI] [PubMed] [Google Scholar]
- Ribeiro H.; Duque L.; Sousa R.; Cruz A.; Gomes C.; Esteves da Silva J. E.; Abreu I. Changes in the IgE-reacting protein profiles of Acer negundo, Platanus x acerifolia and Quercus robur pollen in response to ozone treatment. Int. J. Environ. Health Res. 2014, 24 (6), 515–527. 10.1080/09603123.2013.865716. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Elkelish A.; Durner J.; et al. Common ragweed (Ambrosia artemisiifolia L.): allergenicity and molecular characterization of pollen after plant exposure to elevated NO2. Plant, Cell Environ. 2016, 39 (1), 147–164. 10.1111/pce.12601. [DOI] [PubMed] [Google Scholar]
- Sénéchal H.; Visez N.; Charpin D.; et al. A Review of the Effects of Major Atmospheric Pollutants on Pollen Grains, Pollen Content, and Allergenicity. Sci. World J. 2015, 2015, 940243. 10.1155/2015/940243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. B.; Suphioglu C.; Taylor P.; Desai R.; Watson H. C.; Peng J. L.; Bursill L. A. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: Implications for asthma and air pollution. Clin. Exp. Allergy 1997, 27 (3), 246–251. 10.1111/j.1365-2222.1997.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Ormstad H. Suspended particulate matter in indoor air: adjuvants and allergen carriers. Toxicology 2000, 152 (1–3), 53–68. 10.1016/S0300-483X(00)00292-4. [DOI] [PubMed] [Google Scholar]
- Namork E.; Johansen B. V.; Lovik M. Detection of allergens adsorbed to ambient air particles collected in four European cities. Toxicol. Lett. 2006, 165 (1), 71–78. 10.1016/j.toxlet.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Radauer-Preiml I.; Andosch A.; Hawranek T.; Luetz-Meindl U.; Wiederstein M.; Horejs-Hoeck J.; Himly M.; Boyles M.; Duschl A. Nanoparticle-allergen interactions mediate human allergic responses: protein corona characterization and cellular responses. Part. Fibre Toxicol. 2015, 13, 3–3. 10.1186/s12989-016-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L.How the Immune System Works, 3rd ed.; Blackwell Publishing, 2008. [Google Scholar]
- Buters J.; Prank M.; Sofiev M.; et al. Variation of the group 5 grass pollen allergen content of airborne pollen in relation to geographic location and time in season. J. Allergy Clin. Immunol. 2015, 136 (1), 87–U179. 10.1016/j.jaci.2015.01.049. [DOI] [PubMed] [Google Scholar]
- Buters J. T. M.; Thibaudon M.; Smith M.; et al. Release of Bet v 1 from birch pollen from 5 European countries. Results from the HIALINE study. Atmos. Environ. 2012, 55, 496–505. 10.1016/j.atmosenv.2012.01.054. [DOI] [Google Scholar]
- Creer S.; Deiner K.; Frey S.; Porazinska D.; Taberlet P.; Thomas W. K.; Potter C.; Bik H. M. The ecologist’s field guide to sequence-based identification of biodiversity. Methods in Ecology and Evolution 2016, 7, 1008–1018. 10.1111/2041-210X.12574. [DOI] [Google Scholar]
- Lang-Yona N.; Dannemiller K.; Yamamoto N.; Burshtein N.; Peccia J.; Yarden O.; Rudich Y. Annual distribution of allergenic fungal spores in atmospheric particulate matter in the Eastern Mediterranean; a comparative study between ergosterol and quantitative PCR analysis. Atmos. Chem. Phys. 2012, 12 (5), 2681–2690. 10.5194/acp-12-2681-2012. [DOI] [Google Scholar]
- Liu F.; Lai S.; Reinmuth-Selzle K.; Scheel J. F.; Fröhlich-Nowoisky J.; Després V. R.; Hoffmann T.; Pöschl U.; Kampf C. J. Metaproteomic analysis of atmospheric aerosol samples. Anal. Bioanal. Chem. 2016, 408 (23), 6337–6348. 10.1007/s00216-016-9747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. S.; Kimber R. B. E. Innovations in air sampling to detect plant pathogens. Ann. Appl. Biol. 2015, 166 (1), 4–17. 10.1111/aab.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estillore A. D.; Trueblood J. V.; Grassian V. H. Atmospheric chemistry of bioaerosols: heterogeneous and multiphase reactions with atmospheric oxidants and other trace gases. Chem. Sci. 2016, 7, 6604–6616. 10.1039/C6SC02353C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöhlker C.; Huffman J. A.; Pöschl U. Atmos. Meas. Tech. 2012, 5 (1), 37–71. 10.5194/amt-5-37-2012. [DOI] [Google Scholar]
- Iglesias-Otero M. A.; Fernandez-Gonzalez M.; Rodriguez-Caride D.; Astray G.; Mejuto J. C.; Rodriguez-Rajo F. J. A model to forecast the risk periods of Plantago pollen allergy by using the ANN methodology. Aerobiologia 2015, 31 (2), 201–211. 10.1007/s10453-014-9357-z. [DOI] [Google Scholar]
- Laskin A.; Gilles M. K.; Knopf D. A.; Wang B.; China S. Progress in the Analysis of Complex Atmospheric Particles. Annu. Rev. Anal. Chem. 2016, 9 (1), 117–143. 10.1146/annurev-anchem-071015-041521. [DOI] [PubMed] [Google Scholar]
- Marecal V.; Peuch V. H.; Andersson C.; et al. A regional air quality forecasting system over Europe: the MACC-II daily ensemble production. Geosci. Model Dev. 2015, 8 (9), 2777–2813. 10.5194/gmd-8-2777-2015. [DOI] [Google Scholar]
- Noziere B.; Kaberer M.; Claeys M.; et al. The Molecular Identification of Organic Compounds in the Atmosphere: State of the Art and Challenges. Chem. Rev. 2015, 115 (10), 3919–3983. 10.1021/cr5003485. [DOI] [PubMed] [Google Scholar]
- Prank M.; Chapman D. S.; Bullock J. M.; et al. An operational model for forecasting ragweed pollen release and dispersion in Europe. Agric. For. Meteorol 2013, 182-183, 43–53. 10.1016/j.agrformet.2013.08.003. [DOI] [Google Scholar]
- Sujaritpong S.; Dear K.; Cope M.; Walsh S.; Kjellstrom T. Quantifying the health impacts of air pollution under a changing climate—a review of approaches and methodology. Int. J. Biometeorol. 2014, 58 (2), 149–160. 10.1007/s00484-012-0625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesson S. V. M; Skjoth C. A.; Santl-Temkiv T.; Londahl J. Airborne Microalgae: Insights, Opportunities, and Challenges. Appl. Environ. Microbiol. 2016, 82 (7), 1978–1991. 10.1128/AEM.03333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. M.; Saari R. K.; Selin N. E. Air quality resolution for health impact assessment: influence of regional characteristics. Atmos. Chem. Phys. 2014, 14 (2), 969–978. 10.5194/acp-14-969-2014. [DOI] [Google Scholar]
- Tsigaridis K.; Daskalakis N.; Kanakidou M.; et al. The AeroCom evaluation and intercomparison of organic aerosol in global models. Atmos. Chem. Phys. 2014, 14 (19), 10845–10895. 10.5194/acp-14-10845-2014. [DOI] [Google Scholar]
- von Schneidemesser E.; Monks P. S.; Allan J. D.; et al. Chemistry and the Linkages between Air Quality and Climate Change. Chem. Rev. 2015, 115 (10), 3856–3897. 10.1021/acs.chemrev.5b00089. [DOI] [PubMed] [Google Scholar]
- Weschler C. J. Roles of the human occupant in indoor chemistry. Indoor Air 2016, 26 (1), 6–24. 10.1111/ina.12185. [DOI] [PubMed] [Google Scholar]
- Weschler C. Chemistry in indoor environments: 20 years of research. Indoor Air 2011, 21 (3), 205–218. 10.1111/j.1600-0668.2011.00713.x. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Wang G.; Guo S.; Zamora M. L.; Ying Q.; Lin Y.; Wang W.; Hu M.; Wang Y. Formation of Urban Fine Particulate Matter. Chem. Rev. 2015, 115 (10), 3803–3855. 10.1021/acs.chemrev.5b00067. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Duhl T.; Salam M. T.; et al. Development of a regional-scale pollen emission and transport modeling framework for investigating the impact of climate change on allergic airway disease. Biogeosciences 2014, 11 (6), 1461–1478. 10.5194/bg-11-1461-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore A. M.; Dentener F. J.; Wild O.; et al. Multimodel estimates of intercontinental source-receptor relationships for ozone pollution. J. Geophys. Res. 2009, 114 (D04), D04301. 10.1029/2008JD010816. [DOI] [Google Scholar]
- Lakey P. S. J.; Wisthaler A.; Berkemeier T.; Mikoviny T.; Pöschl U.; Shiraiwa M. Chemical kinetics of multiphase reactions between ozone and human skin lipids: Implications for indoor air quality and health effects. Indoor Air 2016, 10.1111/ina.12360. [DOI] [PubMed] [Google Scholar]
- Anderson S. E.; Franko J.; Jackson L. G.; Wells J. R.; Ham J. E.; Meade B. J. Irritancy and allergic responses induced by exposure to the indoor air chemical 4-oxopentanal. Toxicol. Sci. 2012, 127 (2), 371–381. 10.1093/toxsci/kfs102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. E.; Wells J. R.; Fedorowicz A.; Butterworth L. F.; Meade B. J.; Munson A. E. Evaluation of the Contact and Respiratory Sensitization Potential of Volatile Organic Compounds Generated by Simulated Indoor Air Chemistry. Toxicol. Sci. 2007, 97 (2), 355–363. 10.1093/toxsci/kfm043. [DOI] [PubMed] [Google Scholar]
- McGwin G.; Lienert J.; Kennedy J. I. Formaldehyde Exposure and Asthma in Children: A Systematic Review. Environ. Health Perspect. 2010, 118 (3), 313–317. 10.1289/ehp.0901143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson-Pitts B. J.; Pitts J. N.. Chemistry of the Upper and Lower Atmosphere; Academic Press: San Diego, CA, 2000. [Google Scholar]
- Hernandez M. L.; Peden D. B.. Air Pollution: Indoor and Outdoor A2—Adkinson, N. Franklin. In Middleton’s Allergy, 8th ed.; Bochner B. S., Burks A. W., Busse W. W., Holgate S. T., Lemanske R. F., O’Hehir R. E., Eds.; Elsevier: London, 2014; 482–496. [Google Scholar]
- Berger U.; Kmenta M.; Bastl K. Individual pollen exposure measurements: are they feasible?. Curr. Opin. Allergy Clin. Immunol. 2014, 14 (3), 200–205. 10.1097/ACI.0000000000000060. [DOI] [PubMed] [Google Scholar]
- Bastl K.; Kmenta M.; Pessi A.-M.; et al. First comparison of symptom data with allergen content (Bet v 1 and Phl p 5 measurements) and pollen data from four European regions during 2009–2011. Sci. Total Environ. 2016, 548-549, 229–235. 10.1016/j.scitotenv.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Caillaud D.; Martin S.; Segala C.; Besancenot J.-P.; Clot B.; Thibaudon M.; Effects of Airborne Birch Pollen Levels on Clinical Symptoms of Seasonal Allergic Rhinoconjunctivitis. Int. Arch. Allergy Immunol. 2014, 163 (1), 43–50. 10.1159/000355630. [DOI] [PubMed] [Google Scholar]
- Lim S. S.; Vos T.; Flaxman A. D.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380 (9859), 2224–2260. 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmenta M.; Bastl K.; Jaeger S.; Berger U. Development of personal pollen information-the next generation of pollen information and a step forward for hay fever sufferers. Int. J. Biometeorol. 2014, 58 (8), 1721–1726. 10.1007/s00484-013-0776-2. [DOI] [PubMed] [Google Scholar]
- Exley K.; Robertson S.; Pope F. D.; Harrison R. M.; Gant T. W. Workshop on the sources, quantification and health implications of bioaerosols. Am. J. Pharmacol. Toxicol. 2014, 9 (3), 189–199. 10.3844/ajptsp.2014.189.199. [DOI] [Google Scholar]
- Bowatte G.; Lodge C.; Lowe A. J.; Erbas B.; Perret J.; Abramson M. J.; Matheson M.; Dharmage S. C. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 2015, 70 (3), 245–256. 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- Gehring U.; Wijga A. H.; Brauer M.; Fischer P.; de Jongste J. C.; Kerkhof M.; Oldenwening M.; Smit H. A.; Brunekreef B. Traffic-related Air Pollution and the Development of Asthma and Allergies during the First 8 Years of Life. Am. J. Respir. Crit. Care Med. 2010, 181 (6), 596–603. 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- Guarnieri M.; Balmes J. R. Outdoor air pollution and asthma. Lancet 2014, 383 (9928), 1581–1592. 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R.; Islam T.; Shankardass K.; et al. Childhood Incident Asthma and Traffic-Related Air Pollution at Home and School. Environ. Health Perspect. 2010, 118 (7), 1021–1026. 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern V.; Zutavern A.; Cyrys J.; et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am. J. Respir. Crit. Care Med. 2008, 177 (12), 1331–1337. 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- Anderson H. R.; Favarato G.; Atkinson R. W. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Qual., Atmos. Health 2013, 6 (1), 47–56. 10.1007/s11869-011-0144-5. [DOI] [Google Scholar]
- Esposito S.; Galeone C.; Lelii M.; et al. Impact of air pollution on respiratory diseases in children with recurrent wheezing or asthma. BMC Pulm. Med. 2014, 14, 130. 10.1186/1471-2466-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y. Y.; Wilhelm M.; Rull R. P.; English P.; Ritz B. Traffic and outdoor air pollution levels near residences and poorly controlled asthma in adults. Ann. Allergy, Asthma, Immunol. 2007, 98 (5), 455–463. 10.1016/S1081-1206(10)60760-0. [DOI] [PubMed] [Google Scholar]
- Kim J. J.; Smorodinsky S.; Lipsett M.; Singer B. C.; Hodgson A. T.; Ostro B. Traffic-related air pollution near busy roads: the East Bay Children’s Respiratory Health Study. Am. J. Respir. Crit. Care Med. 2004, 170 (5), 520–526. 10.1164/rccm.200403-281OC. [DOI] [PubMed] [Google Scholar]
- Bowatte G.; Lodge C. J.; Knibbs L. D.; et al. Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J. Allergy Clin. Immunol. 2017, 139, 122–129.e1. 10.1016/j.jaci.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Krämer U.; Sugiri D.; Ranft U.; et al. Eczema, respiratory allergies, and traffic-related air pollution in birth cohorts from small-town areas. J. Dermatol. Sci. 2009, 56 (2), 99–105. 10.1016/j.jdermsci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Anderson H. R.; Favarato G.; Atkinson R. W. Long-term exposure to outdoor air pollution and the prevalence of asthma: meta-analysis of multi-community prevalence studies. Air Qual., Atmos. Health 2013, 6 (1), 57–68. 10.1007/s11869-011-0145-4. [DOI] [Google Scholar]
- Devereux G.; Matsui E. C.; Burney P. G. J., Epidemiology of Asthma and Allergic Airway Diseases A2—Adkinson, N. Franklin. In Middleton’s Allergy, 8th ed.; Bochner B. S., Burks A. W., Busse W. W., Holgate S. T., Lemanske R. F., O’Hehir R. E., Eds.; Elsevier: London, 2014; pp 754–789. [Google Scholar]
- Janssen N. A. H.; Hoek G.; Simic-Lawson M.; et al. Black Carbon as an Additional Indicator of the Adverse Health Effects of Airborne Particles Compared with PM10 and PM2.5. Environ. Health Perspect. 2011, 119 (12), 1691–1699. 10.1289/ehp.1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M. How Much, How Long, What, and Where. Proc. Am. Thorac. Soc. 2010, 7 (2), 111–115. 10.1513/pats.200908-093RM. [DOI] [PubMed] [Google Scholar]
- Greenacre S. A. B.; Ischiropoulos H. Tyrosine nitration: Localisation, quantification, consequences for protein function and signal transduction. Free Radical Res. 2001, 34 (6), 541–581. 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003, 305 (3), 776–783. 10.1016/S0006-291X(03)00814-3. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. U. S. A. 2004, 101 (12), 4003–4008. 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza J. M.; Peluffo G.; Radi R. Protein tyrosine nitration - Functional alteration or just a biomarker?. Free Radical Biol. Med. 2008, 45 (4), 357–366. 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Abello N.; Kerstjens H. A. M.; Postma D. S.; Bischoff R. Protein Tyrosine Nitration: Selectivity, Physicochemical and Biological Consequences, Denitration, and Proteomics Methods for the Identification of Tyrosine-Nitrated Proteins. J. Proteome Res. 2009, 8 (7), 3222–3238. 10.1021/pr900039c. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H. Protein tyrosine nitration-An update. Arch. Biochem. Biophys. 2009, 484 (2), 117–121. 10.1016/j.abb.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Jones L. H. Chemistry and Biology of Biomolecule Nitration. Chem. Biol. 2012, 19 (9), 1086–1092. 10.1016/j.chembiol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Radi R. Protein Tyrosine Nitration: Biochemical Mechanisms and Structural Basis of Functional Effects. Acc. Chem. Res. 2013, 46 (2), 550–559. 10.1021/ar300234c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untersmayr E.; Diesner S. C.; Oostingh G. J.; et al. Nitration of the Egg-Allergen Ovalbumin Enhances Protein Allergenicity but Reduces the Risk for Oral Sensitization in a Murine Model of Food Allergy. PLoS One 2010, 5 (12), e14210. 10.1371/journal.pone.0014210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle A. C.; Oostingh G. J.; Mutschlechner S.; Ferreira F.; Lackner P.; Bohle B.; Fischer G. F.; Vogt A. B.; Duschl A. Nitration of the Pollen Allergen Bet v 1.0101 Enhances the Presentation of Bet v 1-Derived Peptides by HLA-DR on Human Dendritic Cells. PLoS One 2012, 7 (2), e31483. 10.1371/journal.pone.0031483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackaert C.; Kofler S.; Horejs-Hoeck J.; et al. The impact of nitration on the structure and immunogenicity of the major birch pollen allergen Bet v 1.0101. PLoS One 2014, 9 (8), e104520. 10.1371/journal.pone.0104520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll I.; Kalkura N.; Shedziankova Y.; et al. Dimerization of the major birch pollen allergen Bet v 1 is important for its in vivo IgE-cross-linking potential in mice. J. Immunol. 2005, 175 (10), 6645–6650. 10.4049/jimmunol.175.10.6645. [DOI] [PubMed] [Google Scholar]
- Rouvinen J.; Janis J.; Laukkanen M. L. Transient Dimers of Allergens. PLoS One 2010, 5 (2), e9037. 10.1371/journal.pone.0009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey S. J. P.; Berkemeier T.; Tong H.; Arangio A. M.; Lucas K.; Pöschl U.; Shiraiwa M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016, 6, 32916. 10.1038/srep32916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgueira S. A.; Lawrence J.; Coull B.; Murthy G. G. K.; Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect. 2002, 110 (8), 749–755. 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier J. G.; McFall A. S.; Richards-Henderson N. K.; Anastasio C. Hydrogen Peroxide Formation in a Surrogate Lung Fluid by Transition Metals and Quinones Present in Particulate Matter. Environ. Sci. Technol. 2014, 48 (12), 7010–7017. 10.1021/es501011w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V.; Fang T.; Xu L.; Peltier R. E.; Russell A. G.; Ng N. L.; Weber R. J. Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM2.5. Environ. Sci. Technol. 2015, 49 (7), 4646–4656. 10.1021/es505577w. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R.; Levine R. L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25 (3–4), 207–218. 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4 (5), 278–286. 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Ray P. D.; Huang B.-W.; Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signalling 2012, 24 (5), 981–990. 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Du Y.; Le W.; Wang K.; Kieffer N.; Zhang J. Redox control of the survival of healthy and diseased cells. Antioxid. Redox Signaling 2011, 15 (11), 2867–2908. 10.1089/ars.2010.3685. [DOI] [PubMed] [Google Scholar]
- Bachi A.; Dalle-Donne I.; Scaloni A. Redox Proteomics: Chemical Principles, Methodological Approaches and Biological/Biomedical Promises. Chem. Rev. 2013, 113 (1), 596–698. 10.1021/cr300073p. [DOI] [PubMed] [Google Scholar]
- Halliwell B. G. J.Free Radicals in Biology and Medicine; Oxford University Press: Oxford, U.K., 2007; pp 851. [Google Scholar]
- Oswald R.; Behrendt T.; Ermel M.; et al. HONO Emissions from Soil Bacteria as a Major Source of Atmospheric Reactive Nitrogen. Science 2013, 341 (6151), 1233–1235. 10.1126/science.1242266. [DOI] [PubMed] [Google Scholar]
- Su H.; Cheng Y.; Oswald R.; et al. Soil Nitrite as a Source of Atmospheric HONO and OH Radicals. Science 2011, 333 (6049), 1616–1618. 10.1126/science.1207687. [DOI] [PubMed] [Google Scholar]
- Roeser J.; Bischoff R.; Bruins A. P.; Permentier H. P. Oxidative protein labeling in mass-spectrometry-based proteomics. Anal. Bioanal. Chem. 2010, 397 (8), 3441–3455. 10.1007/s00216-010-3471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. B.; Leavitt R.; Ongun A.; McManus T. T. Reaction of ozone with amino acids and proteins. Atmos. Environ. 1969, 3 (6), 669–681. 10.1016/0004-6981(69)90024-9. [DOI] [PubMed] [Google Scholar]
- Pryor W. A.; Giamalva D. H.; Church D. F. Kinetics of ozonation. 2. Amino-acids and model compounds in water and comparisons to rates in nonpolar-solvents. J. Am. Chem. Soc. 1984, 106 (23), 7094–7100. 10.1021/ja00335a038. [DOI] [Google Scholar]
- Sharma V. K.; Graham N. J. D. Oxidation of Amino Acids, Peptides and Proteins by Ozone: A Review. Ozone: Sci. Eng. 2010, 32 (2), 81–90. 10.1080/01919510903510507. [DOI] [Google Scholar]
- Garrison W. M. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem. Rev. 1987, 87 (2), 381–398. 10.1021/cr00078a006. [DOI] [Google Scholar]
- Xu G. H.; Chance M. R. Hydroxyl radical-mediated modification of proteins as probes for structural proteomics. Chem. Rev. 2007, 107 (8), 3514–3543. 10.1021/cr0682047. [DOI] [PubMed] [Google Scholar]
- Davies K. J. A. Protein damage and degradation by oxygen radicals 0.1. General-aspects. J. Biol. Chem. 1987, 262 (20), 9895–9901. [PubMed] [Google Scholar]
- Reinmuth-Selzle K.; Ackaert C.; Kampf C. J.; et al. Nitration of the Birch Pollen Allergen Bet v 1.0101: Efficiency and Site-Selectivity of Liquid and Gaseous Nitrating Agents. J. Proteome Res. 2014, 13 (3), 1570–1577. 10.1021/pr401078h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa M.; Selzle K.; Yang H.; Sosedova Y.; Ammann M.; Pöschl U. Multiphase Chemical Kinetics of the Nitration of Aerosolized Protein by Ozone and Nitrogen Dioxide. Environ. Sci. Technol. 2012, 46 (12), 6672–6680. 10.1021/es300871b. [DOI] [PubMed] [Google Scholar]
- Franze T.; Weller M. G.; Niessner R.; Pöschl U. Protein nitration by polluted air. Environ. Sci. Technol. 2005, 39 (6), 1673–1678. 10.1021/es0488737. [DOI] [PubMed] [Google Scholar]
- Kofler S.; Ackaert C.; Samonig M.; et al. Stabilization of the dimeric birch pollen allergen Bet v 1 impacts its immunological properties. J. Biol. Chem. 2014, 289 (1), 540–551. 10.1074/jbc.M113.518795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani A.; Bruschi M.; Citterio S.; Bolzacchini E.; Ferrero L.; Sangiorgi G.; Asero R.; Perrone M. G. Nitration of pollen aeroallergens by nitrate ion in conditions simulating the liquid water phase of atmospheric particles. Sci. Total Environ. 2016, 573, 1589–1597. 10.1016/j.scitotenv.2016.09.041. [DOI] [PubMed] [Google Scholar]
- Walcher W.; Franze T.; Weller M. G.; Pöschl U.; Huber C. G. Liquid- and gas-phase nitration of bovine serum albumin studied by LC-MS and LC-MS/MS using monolithic columns. J. Proteome Res. 2003, 2 (5), 534–542. 10.1021/pr034034s. [DOI] [PubMed] [Google Scholar]
- Hodara R.; Norris E. H.; Giasson B. I.; Mishizen-Eberz A. J.; Lynch D. R.; Lee V. M. Y.; Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration - Diminished binding to lipid vesicles and increased fibril formation. J. Biol. Chem. 2004, 279 (46), 47746–47753. 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- Turko I. V.; Murad F. Protein nitration in cardiovascular diseases. Pharmacol. Rev. 2002, 54 (4), 619–634. 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- Lemmon M. A.; Schlessinger J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141 (7), 1117–1134. 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.; Janocha A. J.; Aronica M. A.; et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J. Immunol. 2006, 176 (9), 5587–5597. 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- Murata M.; Kawanishi S. Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem. Biophys. Res. Commun. 2004, 316 (1), 123–128. 10.1016/j.bbrc.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Eisen H. N.; Carsten M. E.; Belman S. Studies of hypersensitivity to low molecular weight substances 3. The 2,4 Dinitrophenyl group as a determinat in the precipitin reaction. J. Immunol. 1954, 73 (5), 296–308. [PubMed] [Google Scholar]
- Ovary Z.; Benacerraf B. Immunological specificity of secondary response with dinitrophenylated proteins. Exp. Biol. Med. 1963, 114 (1), 72–76. 10.3181/00379727-114-28589. [DOI] [PubMed] [Google Scholar]
- Frumess G. M. Allergic reaction to dinitrophenol - Report of case. J. Am. Med. Assoc 1934, 102, 1219–1220. 10.1001/jama.1934.02750150023009. [DOI] [Google Scholar]
- Parker C. W.; Kern M.; Eisen H. N. Polyfunctional dinitrophenyl haptens as reagents for elicitation of immediate type allergic skin responses. J. Exp. Med. 1962, 115 (4), 789–801. 10.1084/jem.115.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida M.; Amesara R.; Ukai K.; Sakakura Y. Antigen (DNP-AS)-induced rhinitis model in guinea-pigs. Ann. Allergy 1994, 72 (3), 240–244. [PubMed] [Google Scholar]
- Landsteiner K.; Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J. Exp. Med. 1935, 61 (5), 643–656. 10.1084/jem.61.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesner S. C.; Schultz C.; Ackaert C.; et al. Nitration of β-Lactoglobulin but Not of Ovomucoid Enhances Anaphylactic Responses in Food Allergic Mice. PLoS One 2015, 10 (5), e0126279. 10.1371/journal.pone.0126279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorello E. A.; Farioli L.; Conti A.; et al. Wheat IgE-mediated food allergy in European patients: alpha-amylase inhibitors, lipid transfer proteins and low-molecular-weight glutenins. Allergenic molecules recognized by double-blind, placebo-controlled food challenge. Int. Arch. Allergy Immunol. 2007, 144 (1), 10–22. 10.1159/000102609. [DOI] [PubMed] [Google Scholar]
- Junker Y.; Zeissig S.; Kim S.-J.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209 (13), 2395–2408. 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander I.; Rozynek P.; Rihs H. P.; et al. Multiple wheat flour allergens and cross-reactive carbohydrate determinants bind IgE in baker’s asthma. Allergy 2011, 66 (9), 1208–1215. 10.1111/j.1398-9995.2011.02636.x. [DOI] [PubMed] [Google Scholar]
- Zevallos V. F.; Raker V.; Tenzer S.; et al. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2016, 10.1053/j.gastro.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Becker K.; Lucas K.; Bockamp E.; Zevallos V. F.; Ashfaq-Khan M.; Bellinghausen I.; Saloga J.; Schuppan D.; Pöschl U. 28. Mainzer Allergie-Workshop-Abstract: Modulation of innate immune reactions upon interaction of the Toll-like receptor 4 with chemically modified allergens. Allergo Journal 2016, 25 (1), 36–36. 10.1007/s15007-016-1016-y. [DOI] [Google Scholar]
- Ziegler K.; Lucas K.; Bellinghausen I.; Liu F.; Ashfaq-Khan M.; Saloga J.; Schuppan D.; Pöschl U. 29. Mainzer Allergie-Workshop-Abstract: The effect of nitration on the allergenicity of wheat derived alpha amylase trypsin inhibitors. Allergo J. 2017, 26 (1), 48–48. 10.1007/s15007-017-1273-4. [DOI] [Google Scholar]
- Hochscheid R.; Schreiber N.; Kotte E.; Weber P.; Cassel W.; Yang H.; Zhang Y.; Pöschl U.; Müller B. Nitration of Protein Without Allergenic Potential Triggers Modulation of Antioxidant Response in Type II Pneumocytes. J. Toxicol. Environ. Health, Part A 2014, 77 (12), 679–695. 10.1080/15287394.2014.888023. [DOI] [PubMed] [Google Scholar]
- Yang H.; Zhang Y. Y.; Pöschl U. Quantification of nitrotyrosine in nitrated proteins. Anal. Bioanal. Chem. 2010, 397 (2), 879–886. 10.1007/s00216-010-3557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Y.; Yang H.; Pöschl U. Analysis of nitrated proteins and tryptic peptides by HPLC-chip-MS/MS: site-specific quantification, nitration degree, and reactivity of tyrosine residues. Anal. Bioanal. Chem. 2011, 399 (1), 459–471. 10.1007/s00216-010-4280-9. [DOI] [PubMed] [Google Scholar]
- Selzle K.; Ackaert C.; Kampf C. J.; Kunert A. T.; Duschl A.; Oostingh G. J.; Pöschl U. Determination of nitration degrees for the birch pollen allergen Bet v 1. Anal. Bioanal. Chem. 2013, 405 (27), 8945–8949. 10.1007/s00216-013-7324-0. [DOI] [PubMed] [Google Scholar]
- Nojima K.; Fukaya K.; Fukui S.; Kanno S. Studies on photochemistry of aromatic hydrocarbons II: The formation of nitrophenols and nitrobenzene by the photochemical reaction of benzene in the presence of nitrogen monoxide. Chemosphere 1975, 4 (2), 77–82. 10.1016/0045-6535(75)90017-X. [DOI] [Google Scholar]
- Kohler M.; Heeb N. V. Determination of nitrated phenolic compounds in rain by liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Anal. Chem. 2003, 75 (13), 3115–3121. 10.1021/ac0264067. [DOI] [PubMed] [Google Scholar]
- Vione D.; Maurino V.; Minero C.; Pelizzetti E. Aqueous atmospheric chemistry: Formation of 2,4-dinitrophenol upon nitration of 2-nitrophenol and 4-nitrophenol in solution. Environ. Sci. Technol. 2005, 39 (20), 7921–7931. 10.1021/es050824m. [DOI] [PubMed] [Google Scholar]
- Lin J. K.; Chen K. J.; Liu G. Y.; Chu Y. R.; Lin-Shiau S. Y. Nitration and hydroxylation of aromatic amino acid and guanine by the air pollutant peroxyacetyl nitrate. Chem.-Biol. Interact. 2000, 127 (3), 219–236. 10.1016/S0009-2797(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Mikhailov E.; Vlasenko S.; Martin S. T.; Koop T.; Pöschl U. Amorphous and crystalline aerosol particles interacting with water vapor: conceptual framework and experimental evidence for restructuring, phase transitions and kinetic limitations. Atmos. Chem. Phys. 2009, 9 (2), 9491–9522. 10.5194/acp-9-9491-2009. [DOI] [Google Scholar]
- Shiraiwa M.; Ammann M.; Koop T.; Pöschl U. Gas uptake and chemical aging of semisolid organic aerosol particles. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (27), 11003–11008. 10.1073/pnas.1103045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa M.; Sosedova Y.; Rouviere A.; Yang H.; Zhang Y. Y.; Abbatt J. P. D.; Ammann M.; Pöschl U. The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles. Nat. Chem. 2011, 3 (4), 291–295. 10.1038/nchem.988. [DOI] [PubMed] [Google Scholar]
- Sandhiya L.; Kolandaivel P.; Senthilkumar K. Oxidation and Nitration of Tyrosine by Ozone and Nitrogen Dioxide: Reaction Mechanisms and Biological and Atmospheric Implications. J. Phys. Chem. B 2014, 118 (13), 3479–3490. 10.1021/jp4106037. [DOI] [PubMed] [Google Scholar]
- Radi R.; Peluffo G.; Alvarez M. N.; Naviliat M.; Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radical Biol. Med. 2001, 30 (5), 463–488. 10.1016/S0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- Beckman J. S.; Beckman T. W.; Chen J.; Marshall P. A.; Freeman B. A. Apparent hydroxyl radical production by peroxynitrite- implications for endothelial injury from nitric-oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 1990, 87 (4), 1620–1624. 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H.; Zhu L.; Chen J.; Tsai M.; Martin J. C.; Smith C. D.; Beckman J. S. Peroxynitrite-mediated tyrosine nitartion catalyzed by superoxide-dismutase. Arch. Biochem. Biophys. 1992, 298 (2), 431–437. 10.1016/0003-9861(92)90431-U. [DOI] [PubMed] [Google Scholar]
- Grossi L. Evidence of an electron-transfer mechanism in the peroxynitrite-mediated oxidation of 4-alkylphenols and tyrosine. J. Org. Chem. 2003, 68 (16), 6349–6353. 10.1021/jo034089d. [DOI] [PubMed] [Google Scholar]
- Kampf C. J.; Liu F.; Reinmuth-Selzle K.; Berkemeier T.; Meusel H.; Shiraiwa M.; Pöschl U. Protein Cross-Linking and Oligomerization through Dityrosine Formation upon Exposure to Ozone. Environ. Sci. Technol. 2015, 49 (18), 10859–10866. 10.1021/acs.est.5b02902. [DOI] [PubMed] [Google Scholar]
- Hecker J.; Diethers A.; Schulz D.; et al. An IgE epitope of Bet v 1 and fagales PR10 proteins as defined by a human monoclonal IgE. Allergy 2012, 67 (12), 1530–1537. 10.1111/all.12045. [DOI] [PubMed] [Google Scholar]
- Kofler S.; Asam C.; Eckhard U.; Wallner M.; Ferreira F.; Brandstetter H. Crystallographically Mapped Ligand Binding Differs in High and Low IgE Binding Isoforms of Birch Pollen Allergen Bet v 1. J. Mol. Biol. 2012, 422 (1), 109–123. 10.1016/j.jmb.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen J. E.; Wimmer R.; Larsen J. N.; Spangfort M. D.; Otzen D. E. The major birch allergen, Bet v 1, shows affinity for a broad spectrum of physiological ligands. J. Biol. Chem. 2002, 277 (26), 23684–23692. 10.1074/jbc.M202065200. [DOI] [PubMed] [Google Scholar]
- Seutter von Loetzen C.; Hoffmann T.; Hartl M. J.; Schweimer K.; Schwab W.; Rosch P.; Hartl-Spiegelhauer O. Secret of the major birch pollen allergen Bet v 1: identification of the physiological ligand. Biochem. J. 2014, 457 (3), 379–390. 10.1042/BJ20130413. [DOI] [PubMed] [Google Scholar]
- Asam C.; Batista A. L.; Moraes A. H.; et al. Bet v 1-a Trojan horse for small ligands boosting allergic sensitization?. Clin. Exp. Allergy 2014, 44 (8), 1083–1093. 10.1111/cea.12361. [DOI] [PubMed] [Google Scholar]
- Gould H. J.; Sutton B. J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8 (3), 205–217. 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J. Mechanisms of IgE Inflammation. Curr. Allergy Asthma Rep. 2011, 11 (2), 178–183. 10.1007/s11882-011-0179-6. [DOI] [PubMed] [Google Scholar]
- Hlavacek W. S.; Perelson A. S.; Sulzer B.; Bold J.; Paar J.; Gorman W.; Posner R. G. Quantifying aggregation of IgE-Fc epsilon RI by multivalent antigen. Biophys. J. 1999, 76 (5), 2421–2431. 10.1016/S0006-3495(99)77397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J. A. Degradation of oxidized proteins by the 20S proteasome. Biochimie 2001, 83 (3–4), 301–310. 10.1016/S0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. S. Effects of protein aggregates: An immunologic perspective. AAPS J. 2006, 8 (3), E501–E507. 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinghausen I.; Haeringer B.; Lafargue B.; Strand D.; Koenig B.; Decker H.; Saloga J. Allergological implication of the quaternary hexameric structure of the cockroach allergen Per a 3. Clin. Exp. Allergy 2008, 38 (3), 539–548. 10.1111/j.1365-2222.2007.02910.x. [DOI] [PubMed] [Google Scholar]
- Vrtala S.; Fohr M.; Campana R.; Baumgartner C.; Valent P.; Valenta R. Genetic engineering of trimers of hypoallergenic fragments of the major birch pollen allergen, Bet v 1, for allergy vaccination. Vaccine 2011, 29 (11), 2140–2148. 10.1016/j.vaccine.2010.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Free Radical Res. 2006, 40 (12), 1250–1258. 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- Ahmad P.; Moinuddin; Ali A. Peroxynitrite induced structural changes result in the generation of neo-epitopes on human serum albumin. Int. J. Biol. Macromol. 2013, 59 (0), 349–356. 10.1016/j.ijbiomac.2013.04.068. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S.; Schmidt K.; Mayer B. Dityrosine formation outcompetes tyrosine nitration at low steady-state concentrations of peroxynitrite - Implications for tyrosine modification by nitric oxide/superoxide in vivo. J. Biol. Chem. 2000, 275 (9), 6346–6352. 10.1074/jbc.275.9.6346. [DOI] [PubMed] [Google Scholar]
- Heydenreich B.; Bellinghausen I.; Lorenz S.; Henmar H.; Strand D.; Wurtzen P. A.; Saloga J. Reduced in vitro T-cell responses induced by glutaraldehyde-modified allergen extracts are caused mainly by retarded internalization of dendritic cells. Immunology 2012, 136 (2), 208–217. 10.1111/j.1365-2567.2012.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund L.; Henmar H.; Wurtzen P. A.; Lund G.; Hjortskov N.; Larsen J. N. Comparison of allergenicity and immunogenicity of an intact allergen vaccine and commercially available allergoid products for birch pollen immunotherapy. Clin. Exp. Allergy 2007, 37 (4), 564–571. 10.1111/j.1365-2222.2007.02687.x. [DOI] [PubMed] [Google Scholar]
- Beckman J. S. Oxidative Damage and Tyrosine Nitration from Peroxynitrite. Chem. Res. Toxicol. 1996, 9 (5), 836–844. 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Davies K. J. A.; Lin S. W.; Pacifici R. E. PROTEIN damage and degradation by oxygen radicals 0.4. Degradation of denatured protein. J. Biol. Chem. 1987, 262 (20), 9914–9920. [PubMed] [Google Scholar]
- Gunaydin H.; Houk K. N. Mechanisms of Peroxynitrite-Mediated Nitration of Tyrosine. Chem. Res. Toxicol. 2009, 22 (5), 894–898. 10.1021/tx800463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J. A.; Delsignore M. E. Protein damage and degradation by oxygen radicals 0.3. Modification of secondary and tertiary structure. J. Biol. Chem. 1987, 262 (20), 9908–9913. [PubMed] [Google Scholar]
- Prütz W. A.; Mönig H.; Butler J.; Land E. J. Reactions of nitrogen dioxide in aqueous model systems: Oxidation of tyrosine units in peptides and proteins. Arch. Biochem. Biophys. 1985, 243 (1), 125–134. 10.1016/0003-9861(85)90780-5. [DOI] [PubMed] [Google Scholar]
- Dalsgaard T. K.; Nielsen J. H.; Brown B. E.; Stadler N.; Davies M. J. Dityrosine, 3,4-Dihydroxyphenylalanine (DOPA), and Radical Formation from Tyrosine Residues on Milk Proteins with Globular and Flexible Structures as a Result of Riboflavin-Mediated Photo-oxidation. J. Agric. Food Chem. 2011, 59 (14), 7939–7947. 10.1021/jf200277r. [DOI] [PubMed] [Google Scholar]
- Bunn H. J.; Hewitt C. R. A.; Grigg J. Suppression of autologous peripheral blood mononuclear cell proliferation by alveolar macrophages from young infants. Clin. Exp. Immunol. 2002, 128 (2), 313–317. 10.1046/j.1365-2249.2002.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins J. B. Alveolar Macrophages. Am. J. Respir. Crit. Care Med. 2003, 167 (2), 103–104. 10.1164/rccm.2210007. [DOI] [PubMed] [Google Scholar]
- Hussell T.; Bell T. J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14 (2), 81–93. 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- Knowles M. R.; Boucher R. C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002, 109 (5), 571–577. 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnicozzi M.; Sawyer R. T.; Fenton M. J. Innate immunity in allergic disease. Immunol. Rev. 2011, 242, 106–127. 10.1111/j.1600-065X.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- Golebski K.; Roschmann K. I. L.; Toppila-Salmi S.; Hammad H.; Lambrecht B. N.; Renkonen R.; Fokkens W. J.; van Drunen C. M. The multi-faceted role of allergen exposure to the local airway mucosa. Allergy 2013, 68 (2), 152–160. 10.1111/all.12080. [DOI] [PubMed] [Google Scholar]
- Mattila P.; Joenvaara S.; Renkonen J.; Toppila-Salmi S.; Renkonen R. Allergy as an epithelial barrier disease. Clin. Transl. Allergy 2011, 1 (1), 5. 10.1186/2045-7022-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine A. D.; McLean W. H.; Leung D. Y. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011, 365 (14), 1315–1327. 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Gandhi V. D.; Vliagoftis H. Airway Epithelium Interactions with Aeroallergens: Role of Secreted Cytokines and Chemokines in Innate Immunity. Front. Immunol. 2015, 6, 147. 10.3389/fimmu.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenvaara S.; Mattila P.; Renkonen J.; et al. Caveolar transport through allergen Bet v 1 in allergic nasal epithelium of birch pollen patients. J. Allergy Clin. Immunol. 2009, 124 (1), 135–142. 10.1016/j.jaci.2008.11.048. [DOI] [PubMed] [Google Scholar]
- Borcherding J.; Baltrusaitis J.; Chen H.; et al. Iron oxide nanoparticles induce Pseudomonas aeruginosa growth, induce biofilm formation, and inhibit antimicrobial peptide function. Environ. Sci.: Nano 2014, 1 (2), 123–132. 10.1039/c3en00029j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs S. J.; Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol. Med. 1995, 18 (2), 321–336. 10.1016/0891-5849(94)00159-H. [DOI] [PubMed] [Google Scholar]
- Becker S.; Soukup J. M.; Gilmour M. I.; Devlin R. B. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol. Appl. Pharmacol. 1996, 141 (2), 637–648. 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- Zorov D. B.; Juhaszova M.; Sollott S. J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta, Bioenerg. 2006, 1757 (5–6), 509–517. 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Auerbach A.; Hernandez M. L. The effect of environmental oxidative stress on airway inflammation. Curr. Opin. Allergy Clin. Immunol. 2012, 12 (2), 133–139. 10.1097/ACI.0b013e32835113d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K.; Maes M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48 (1), 190–204. 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R. N.; Diaz-Sanchez D.; Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J. Allergy Clin. Immunol. 2012, 129 (1), 14–24. 10.1016/j.jaci.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden D. B. The role of oxidative stress and innate immunity in O3 and endotoxin-induced human allergic airway disease. Immunol. Rev. 2011, 242 (1), 91–105. 10.1111/j.1600-065X.2011.01035.x. [DOI] [PubMed] [Google Scholar]
- Manzo N.; LaGier A.; Slade R.; Ledbetter A.; Richards J.; Dye J. Nitric oxide and superoxide mediate diesel particle effects in cytokine-treated mice and murine lung epithelial cells-- implications for susceptibility to traffic-related air pollution. Part. Fibre Toxicol. 2012, 9 (1), 43. 10.1186/1743-8977-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio A. J.; Turi J. L.; Yang F.; Garrick L. M.; Garrick M. D. Iron homeostasis in the lung. Biol. Res. 2006, 39 (1), 67–77. 10.4067/S0716-97602006000100008. [DOI] [PubMed] [Google Scholar]
- Li N.; Wang M. Y.; Bramble L. A.; Schmitz D. A.; Schauer J. J.; Sioutas C.; Harkema J. R.; Nel A. E. The Adjuvant Effect of Ambient Particulate Matter Is Closely Reflected by the Particulate Oxidant Potential. Environ. Health Perspect. 2009, 117 (7), 1116–1123. 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V.; Rico-Martinez R.; Kotra N.; King L.; Liu J. M.; Snell T. W.; Weber R. J. Contribution of Water-Soluble and Insoluble Components and Their Hydrophobic/Hydrophilic Subfractions to the Reactive Oxygen Species-Generating Potential of Fine Ambient Aerosols. Environ. Sci. Technol. 2012, 46 (20), 11384–11392. 10.1021/es302484r. [DOI] [PubMed] [Google Scholar]
- Gehling W.; Dellinger B. Environmentally Persistent Free Radicals and Their Lifetimes in PM2.5. Environ. Sci. Technol. 2013, 47 (15), 8172–8178. 10.1021/es401767m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinolo M.; Willis M. D.; Zhou S.; Abbatt J. P. D. Connecting the oxidation of soot to its redox cycling abilities. Nat. Commun. 2015, 6, 6812. 10.1038/ncomms7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget-Brown A. O.; Ngamtrakulpanit L.; Smith A.; Bunyan D.; Hom S.; Nguyen A.; Hunt J. F. Normative data for pH of exhaled breath condensate. Chest 2006, 129 (2), 426–430. 10.1378/chest.129.2.426. [DOI] [PubMed] [Google Scholar]
- Ricciardolo F. L. M.; Gaston B.; Hunt J. Acid stress in the pathology of asthma. J. Allergy Clin. Immunol. 2004, 113 (4), 610–619. 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Enami S.; Hoffmann M. R.; Colussi A. J. Acidity enhances the formation of a persistent ozonide at aqueous ascorbate/ozone gas interfaces. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (21), 7365–7369. 10.1073/pnas.0710791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannala V. R.; Bazil J. N.; Camara A. K. S.; Dash R. K. A mechanistic mathematical model for the catalytic action of glutathione peroxidase. Free Radical Res. 2014, 48 (4), 487–502. 10.3109/10715762.2014.886775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley A. R.; Emrani P.; Cassano P. A. Genetic variation and gene expression in antioxidant-related enzymes and risk of chronic obstructive pulmonary disease: a systematic review. Thorax 2008, 63 (11), 956–961. 10.1136/thx.2007.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar N. E.; Reed C. K.; Cox C.; Frampton M. W.; Finkelstein J. N. Ozone, but not nitrogen dioxide, exposure decreases glutathione peroxidases in epithelial lining fluid of human lung. Am. J. Respir. Crit. Care Med. 2000, 162 (4), 1342–1347. 10.1164/ajrccm.162.4.9912041. [DOI] [PubMed] [Google Scholar]
- Corradi M.; Pignatti P.; Brunetti G.; Goldoni M.; Caglieri A.; Nava S.; Moscato G.; Balbi B. Comparison between exhaled and bronchoalveolar lavage levels of hydrogen peroxide in patients with diffuse interstitial lung diseases. Acta. Biomed 2008, 79 (Suppl 1), 73–78. [PubMed] [Google Scholar]
- Ahn K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134 (5), 993–999. 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Kim J.; Kim E. H.; Oh I.; Jung K.; Han Y.; Cheong H. K.; Ahn K. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J. Allergy Clin. Immunol. 2013, 132 (2), 495–498. 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Pacher P.; Beckman J. S.; Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87 (1), 315–424. 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T. B.; Coburn J.; Dao K.; Roque P.; Chang Y. C.; Kalia V.; Guilarte T. R.; Dziedzic J.; Costa L. G. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology 2016, 374, 1–9. 10.1016/j.tox.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.; Georas S.; Alexis N.; Fritz P.; Xia T.; Williams M. A.; Horner E.; Nel A. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J. Allergy Clin. Immunol. 2016, 138 (2), 386–396. 10.1016/j.jaci.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque P. J.; Dao K.; Costa L. G. Microglia mediate diesel exhaust particle-induced cerebellar neuronal toxicity through neuroinflammatory mechanisms. NeuroToxicology 2016, 56, 204–214. 10.1016/j.neuro.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togias A. Systemic effects of local allergic disease. J. Allergy Clin. Immunol. 2004, 113 (1), S8–S14. 10.1016/j.jaci.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Round J. L.; Mazmanian S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9 (5), 313–323. 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L. V.; Littman D. R.; Macpherson A. J. Interactions between the microbiota and the immune system. Science (Washington, DC, U. S.) 2012, 336 (6086), 1268–1273. 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahtela T.; Holgate S.; Pawankar R.; et al. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ. J. 2013, 6 (1), 3. 10.1186/1939-4551-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski I.; von Hertzen L.; Fyhrquist N.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (21), 8334–8339. 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer E. S.; Saglani S.; Trompette A.; Yadava K.; Sherburn R.; McCoy K. D.; Nicod L. P.; Lloyd C. M.; Marsland B. J. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014, 20 (6), 642–647. 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- Shanahan F. The gut microbiota-a clinical perspective on lessons learned. Nat. Rev. Gastroenterol. Hepatol. 2012, 9 (10), 609–614. 10.1038/nrgastro.2012.145. [DOI] [PubMed] [Google Scholar]
- Legatzki A.; Rosler B.; von Mutius E. Microbiome diversity and asthma and allergy risk. Curr. Allergy Asthma Rep. 2014, 14 (10), 466. 10.1007/s11882-014-0466-0. [DOI] [PubMed] [Google Scholar]
- Blázquez A. B.; Berin M. C. Microbiome and food allergy. Transl. Res. 2017, 179, 199–203. 10.1016/j.trsl.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riiser A. The human microbiome, asthma, and allergy. Allergy, Asthma, Clin. Immunol. 2015, 11, 35. 10.1186/s13223-015-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K. D.; Koeller Y. New developments providing mechanistic insight into the impact of the microbiota on allergic disease. Clin. Immunol. 2015, 159 (2), 170–176. 10.1016/j.clim.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura K. E.; Lynch S. V. Microbiota in Allergy and Asthma and the Emerging Relationship with the Gut Microbiome. Cell Host Microbe 2015, 17 (5), 592–602. 10.1016/j.chom.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. R.; Bartlett N. W.; Hussell T.; Openshaw P.; Johnston S. L. The microbiology of asthma. Nat. Rev. Microbiol. 2012, 10 (7), 459–471. 10.1038/nrmicro2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilty M.; Burke C.; Pedro H.; et al. Disordered Microbial Communities in Asthmatic Airways. PLoS One 2010, 5 (1), e8578. 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. J.; Nelson C. E.; Brodie E. L.; et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 2011, 127 (2), 372–381. 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S. Y.; Kaplan G. G.; Madsen K. L. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut microbes 2014, 5 (2), 215–219. 10.4161/gmic.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydenreich B.; Bellinghausen I.; Koenig B.; Becker W. M.; Grabbe S.; Petersen A.; Saloga J. Gram-positive bacteria on grass pollen exhibit adjuvant activity inducing inflammatory T cell responses. Clin. Exp. Allergy 2012, 42 (1), 76–84. 10.1111/j.1365-2222.2011.03888.x. [DOI] [PubMed] [Google Scholar]
- CDC Website: Climate and Health. http://www.cdc.gov/climateandhealth/BRACE.htm.
- Runswick S.; Mitchell T.; Davies P.; Robinson C.; Garrod D. R. Pollen proteolytic enzymes degrade tight junctions. Respirology 2007, 12 (6), 834–842. 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- Reed C. E.; Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J. Allergy Clin. Immunol. 2004, 114 (5), 997–1008. 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Millien V. O.; Lu W.; Shaw J.; et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 2013, 341 (6147), 792–796. 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles S.; Mariani V.; Bryce M.; Mueller M. J.; Ring J.; Jakob T.; Pastore S.; Behrendt H.; Traidl-Hoffmann C. Pollen-derived E1-phytoprostanes signal via PPAR-gamma and NF-kappaB-dependent mechanisms. J. Immunol. 2009, 182 (11), 6653–8. 10.4049/jimmunol.0802613. [DOI] [PubMed] [Google Scholar]
- Boldogh I.; Bacsi A.; Choudhury B. K.; Dharajiya N.; Alam R.; Hazra T. K.; Mitra S.; Goldblum R. M.; Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Invest. 2005, 115 (8), 2169–2179. 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer M.; Alessandrini F.; Gilles S.; et al. Pollen-derived adenosine is a necessary cofactor for ragweed allergy. Allergy 2015, 70 (8), 944–954. 10.1111/all.12642. [DOI] [PubMed] [Google Scholar]
- Berrens L.; de la Cuadra Lopez B. Complement activating agents in allergenic extracts. Inflammation Res. 1997, 46 (11), 455–460. 10.1007/s000110050224. [DOI] [PubMed] [Google Scholar]
- Blume C.; Swindle E. J.; Gilles S.; Traidl-Hoffmann C.; Davies D. E. Low molecular weight components of pollen alter bronchial epithelial barrier functions. Tissue barriers 2015, 3 (3), e1062316. 10.1080/15476286.2015.1062316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth S. C.; Piggott D. A.; Huleatt J. W.; Visintin I.; Herrick C. A.; Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002, 196 (12), 1645–1651. 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar A. A.; Bennett J. W. A common fungal volatile organic compound induces a nitric oxide mediated inflammatory response in Drosophila melanogaster. Sci. Rep. 2014, 4, 3833. 10.1038/srep03833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D.; Garcia M. P.; Wang M.; Jyrala M.; Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J. Allergy Clin. Immunol. 1999, 104 (6), 1183–1188. 10.1016/S0091-6749(99)70011-4. [DOI] [PubMed] [Google Scholar]
- Riedl M. A.; Landaw E. M.; Saxon A.; Diaz-Sanchez D. Initial high-dose nasal allergen exposure prevents allergic sensitization to a neoantigen. J. Immunol. 2005, 174 (11), 7440–7445. 10.4049/jimmunol.174.11.7440. [DOI] [PubMed] [Google Scholar]
- Pandya R. J.; Solomon G.; Kinner A.; Balmes J. R. Diesel exhaust and asthma: Hypotheses and molecular mechanisms of action. Environ. Health Perspect. 2002, 110, 103–112. 10.1289/ehp.02110s1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes T.; Provoost S.; Lanckacker E. A.; Cataldo D. D.; Vanoirbeek J. A.; Nemery B.; Tournoy K. G.; Joos G. F. Mouse models to unravel the role of inhaled pollutants on allergic sensitization and airway inflammation. Respir. Res. 2010, 11, 7. 10.1186/1465-9921-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provoost S.; Maes T.; Joos G. F.; Tournoy K. G. Monocyte-derived dendritic cell recruitment and allergic T(H)2 responses after exposure to diesel particles are CCR2 dependent. J. Allergy Clin. Immunol. 2012, 129 (2), 483–91. 10.1016/j.jaci.2011.07.051. [DOI] [PubMed] [Google Scholar]
- Devouassoux G.; Saxon A.; Metcalfe D. D.; Prussin C.; Colomb M. G.; Brambilla C.; Diaz-Sanchez D. Chemical constituents of diesel exhaust particles induce IL-4 production and histamine release by human basophils. J. Allergy Clin. Immunol. 2002, 109 (5), 847–853. 10.1067/mai.2002.122843. [DOI] [PubMed] [Google Scholar]
- Hiura T. S.; Li N.; Kaplan R.; Horwitz M.; Seagrave J. C.; Nel A. E. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J. Immunol. 2000, 165 (5), 2703–2711. 10.4049/jimmunol.165.5.2703. [DOI] [PubMed] [Google Scholar]
- Hiura T. S.; Kaszubowski M. P.; Li N.; Nel A. E. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J. Immunol. 1999, 163 (10), 5582–5591. [PubMed] [Google Scholar]
- Dick C. A.; Brown D. M.; Donaldson K.; Stone V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhalation Toxicol. 2003, 15 (1), 39–52. 10.1080/08958370304454. [DOI] [PubMed] [Google Scholar]
- Siegel P. D.; Saxena R. K.; Saxena Q. B.; Ma J. K. H.; Ma J. Y. C.; Yin X. J.; Castranova V.; Al-Humadi N.; Lewis D. M. Effect of diesel exhaust particulate (DEP) on immune responses: Contributions of particulate versus organic soluble components. J. Toxicol. Environ. Health, Part A 2004, 67 (3), 221–231. 10.1080/15287390490266891. [DOI] [PubMed] [Google Scholar]
- Yang H. M.; Antonini J. M.; Barger M. W.; Butterworth L.; Roberts J. R.; Ma J. K. H.; Castranova V.; Ma J. Y. C. Diesel exhaust particles suppress macrophage function and slow the pulmonary clearance of Listeria monocytogenes in rats. Environ. Health Perspect. 2001, 109 (5), 515–521. 10.2307/3454711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleck B.; Tse D. B.; Gordon T.; Ahsan M. R.; Reibman J. Diesel Exhaust Particle-Treated Human Bronchial Epithelial Cells Upregulate Jagged-1 and OX40 Ligand in Myeloid Dendritic Cells via Thymic Stromal Lymphopoietin. J. Immunol. 2010, 185 (11), 6636–6645. 10.4049/jimmunol.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.; Buglak N. Convergence of air pollutant-induced redox-sensitive signals in the dendritic cells contributes to asthma pathogenesis. Toxicol. Lett. 2015, 237 (1), 55–60. 10.1016/j.toxlet.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Bayram H.; Devalia J. L.; Sapsford R. J.; Ohtoshi T.; Miyabara Y.; Sagai M.; Davies R. J. The effect of diesel exhaust particles on cell function and release of inflammatory mediators from human bronchial epithelial cells in vitro. Am. J. Respir. Cell Mol. Biol. 1998, 18 (3), 441–448. 10.1165/ajrcmb.18.3.2882. [DOI] [PubMed] [Google Scholar]
- Fukuoka A.; Matsushita K.; Morikawa T.; Takano H.; Yoshimoto T. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin. Exp. Allergy 2016, 46 (1), 142–152. 10.1111/cea.12597. [DOI] [PubMed] [Google Scholar]
- Kang X. D.; Li N.; Wang M. Y.; Boontheung P.; Sioutas C.; Harkema J. R.; Bramble L. A.; Nel A. E.; Loo J. A. Adjuvant effects of ambient particulate matter monitored by proteomics of bronchoalveolar lavage fluid. Proteomics 2010, 10 (3), 520–531. 10.1002/pmic.200900573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G. G.; Nel A. E.; Loo J. A. Nitrotyrosine-modified proteins and oxidative stress induced by diesel exhaust particles. Electrophoresis 2005, 26 (1), 280–292. 10.1002/elps.200406145. [DOI] [PubMed] [Google Scholar]
- Kanemitsu H.; Nagasawa S.; Sagai M.; MORI Y. Complement activation by diesel exhaust particles (DEP). Biol. Pharm. Bull. 1998, 21 (2), 129–132. 10.1248/bpb.21.129. [DOI] [PubMed] [Google Scholar]
- Walters D. M.; Breysse P. N.; Schofield B.; Wills-Karp M. Complement factor 3 mediates particulate matter–induced airway hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2002, 27 (4), 413–418. 10.1165/rcmb.4844. [DOI] [PubMed] [Google Scholar]
- Liu J.; Ballaney M.; Al-alem U.; Quan C.; Jin X.; Perera F.; Chen L. C.; Miller R. L. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol. Sci. 2008, 102 (1), 76–81. 10.1093/toxsci/kfm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer T.; Baccarelli A.; Cantone L.; Coull B.; Maity A.; Lin X.; Schwartz J. Exposure to airborne particulate matter is associated with methylation pattern in the asthma pathway. Epigenomics 2013, 5 (2), 147–154. 10.2217/epi.13.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezza G.; Mazzei F.; Boner A. Epigenetics of allergy. Early Hum. Dev. 2013, 89, S20–S21. 10.1016/S0378-3782(13)70007-0. [DOI] [PubMed] [Google Scholar]
- Vork K. L.; Broadwin R. L.; Blaisdell R. J. Developing asthma in childhood from exposure to secondhand tobacco smoke: Insights from a meta-regression. Environ. Health Perspect. 2007, 115 (10), 1394–1400. 10.1289/ehp.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke H.; Leonardi-Bee J.; Hashim A.; Pine-Abata H.; Chen Y.; Cook D. G.; Britton J. R.; McKeever T. M. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 2012, 129 (4), 735–744. 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- Ni L.; Chuang C.-C.; Zuo L. Fine particulate matter in acute exacerbation of COPD. Front. Physiol. 2015, 6, 294. 10.3389/fphys.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.; Diaz P. T.; Best T. M.; Stimpfl J. N.; He F.; Zuo L. Molecular characterization of redox mechanisms in allergic asthma. Ann. Allergy, Asthma, Immunol. 2014, 113 (2), 137–142. 10.1016/j.anai.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Zuo L.; Otenbaker N. P.; Rose B. A.; Salisbury K. S. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol. Immunol. 2013, 56 (1–2), 57–63. 10.1016/j.molimm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Bayram H.; Rusznak C.; Khair O. A.; Sapsford R. J.; Abdelaziz M. M. Effect of ozone and nitrogen dioxide on the permeability of bronchial epithelial cell cultures of non-asthmatic and asthmatic subjects. Clin. Exp. Allergy 2002, 32 (9), 1285–1292. 10.1046/j.1365-2745.2002.01435.x. [DOI] [PubMed] [Google Scholar]
- Park J.-W.; Taube C.; Joetham A.; et al. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am. J. Respir. Crit. Care Med. 2004, 169 (6), 726–732. 10.1164/rccm.200307-1042OC. [DOI] [PubMed] [Google Scholar]
- Cyphert J. M.; Trempus C. S.; Garantziotis S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevelander M.; Mayette J.; Whittaker L. A.; et al. Nitrogen dioxide promotes allergic sensitization to inhaled antigen. J. Immunol. 2007, 179 (6), 3680–3688. 10.4049/jimmunol.179.6.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty V.; Guillossou G.; Neukirch C.; et al. Repeated nitrogen dioxide exposures and eosinophilic airway inflammation in asthmatics: a randomized crossover study. Environ. Health Perspect. 2014, 122 (8), 850–855. 10.1289/ehp.1307240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J. H.; Matsui E. C.; Wood R. A.; Keet C. A. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J. Allergy Clin. Immunol. 2012, 130 (2), 453–460. 10.1016/j.jaci.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E. M. R.; Todd M.; Dowd J. B.; Aiello A. E. The Impact of Bisphenol A and Triclosan on Immune Parameters in the U.S. Population, NHANES 2003–2006. Environ. Health Perspect. 2011, 119 (3), 390–396. 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. H.; Chung S. W.; Kang B. Y.; Park J.; Lee C. H.; Hwang S. Y.; Kim T. S. Enhanced interleukin-4 production in CD4+ T cells and elevated immunoglobulin E levels in antigen-primed mice by bisphenol A and nonylphenol, endocrine disruptors: involvement of nuclear factor-AT and Ca2+. Immunology 2003, 109 (1), 76–86. 10.1046/j.1365-2567.2003.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne P.; Foster S.; Connolly J.; Bazzaz F.; Epstein P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann. Allergy, Asthma, Immunol. 2002, 88 (3), 279–282. 10.1016/S1081-1206(10)62009-1. [DOI] [PubMed] [Google Scholar]
- Singer B. D.; Ziska L. H.; Frenz D. A.; Gebhard D. E.; Straka J. G. Increasing Amb a 1 content in common ragweed (Ambrosia artemisiifolia) pollen as a function of rising atmospheric CO2 concentration. Funct. Plant Biol. 2005, 32 (7), 667–670. 10.1071/FP05039. [DOI] [PubMed] [Google Scholar]
- Ziska L. H.; Gebhard D. E.; Frenz D. A.; Faulkner S.; Singer B. D.; Straka J. G. Cities as harbingers of climate change: Common ragweed, urbanization, and public health. J. Allergy Clin. Immunol. 2003, 111 (2), 290–295. 10.1067/mai.2003.53. [DOI] [PubMed] [Google Scholar]
- Clot B. Trends in airborne pollen: An overview of 21 years of data in Neuchatel (Switzerland). Aerobiologia 2003, 19 (3–4), 227–234. 10.1023/B:AERO.0000006572.53105.17. [DOI] [Google Scholar]
- Ahlholm J. U.; Helander M. L.; Savolainen J. Genetic and environmental factors affecting the allergenicity of birch (Betula pubescens ssp. czerepanovii Orl. Hamet-Ahti) pollen. Clin. Exp. Allergy 1998, 28 (11), 1384–1388. 10.1046/j.1365-2222.1998.00404.x. [DOI] [PubMed] [Google Scholar]
- Low S. Y.; Dannemiller K.; Yao M.; Yamamoto N.; Peccia J. The allergenicity of Aspergillus fumigatus conidia is influenced by growth temperature. Fungal Biol. 2011, 115 (7), 625–632. 10.1016/j.funbio.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland J. L.; Gramada A.; Buzko O. V.; Zhang Q.; Bourne P. E. The Molecular Biology Toolkit (MBT): a modular platform for developing molecular visualization applications. BMC Bioinf. 2005, 6 (21), 21. 10.1186/1471-2105-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.