Highlights
-
•
Precision farming is measuring and responding to inter and intra-field variability in crops to form a decision support system.
-
•
About 40–70% of N, 80–90% of P and 50–70% of K of the applied fertilizers is lost to the environment causing pollution.
-
•
Nanofertilizers helps in slow and sustained release of agrochemicals resulting in precise dosage to the plants.
-
•
Green synthesized Ag, ZnO and TiO2 NPs are extensively used for plant protection and treatment of diseases.
-
•
Biosensors helps in detecting pesticides in the vegetable crops and form a decision support system for crop commodities.
Keywords: Sustained release, Fertilizers, Nanoparticles, Nutrients, Biosensors
Abstract
Nanotechnology is an interdisciplinary research field. In recent past efforts have been made to improve agricultural yield through exhaustive research in nanotechnology. The green revolution resulted in blind usage of pesticides and chemical fertilizers which caused loss of soil biodiversity and developed resistance against pathogens and pests as well. Nanoparticle-mediated material delivery to plants and advanced biosensors for precision farming are possible only by nanoparticles or nanochips. Nanoencapsulated conventional fertilizers, pesticides and herbicides helps in slow and sustained release of nutrients and agrochemicals resulting in precise dosage to the plants. Nanotechnology based plant viral disease detection kits are also becoming popular and are useful in speedy and early detection of viral diseases. In this article, the potential uses and benefits of nanotechnology in precision agriculture are discussed. The modern nanotechnology based tools and techniques have the potential to address the various problems of conventional agriculture and can revolutionize this sector.
1. Introduction
Nanotechnology has been used in many fields of science like physics, chemistry, pharmaceutical science, material science, medicine and agriculture. The promising results in other fields opened up a lot of scope in the agriculture field also. According to the Directorate-General for internal policies of the European Union; precision agriculture is a farming management concept of measuring and responding to inter and intra-field varying in crops to form a decision support system for whole farm management and to reap the maximum output from the available resources. Now a day, nanotechnology is extensively used in modern agriculture to make true the concept of precision agriculture. Nanotechnology includes nanoparticles having one or more dimensions in the order of 100 nm or less [1]. Nanomaterials find applications in plant protection, nutrition and management of farm practices due to small size, high surface to volume ratio and unique optical properties [2]. A wide range of materials are used to make nanoparticles like metal oxides, ceramics, magnetic materials, semiconductor, quantum dots, lipids, polymers (synthetic or natural), dendrimers and emulsions [3]. Chitosan nanoparticles are being used in agriculture in seed treatment and as biopesticide which helps the plants to fight off fungal infections. The uptake efficiency and effects of nanoparticles on the growth and metabolic functions vary among plants. The concentration of nanoparticles affects processes like germination and growth of the plant [4]. Nanoencapsulation play a vital role in the protection of environment by reducing leaching and evaporation of harmful substances.
The worldwide consumption of pesticides is about two million tonnes per year; out of which 45% is used by Europe alone, 25% is consumed in the USA and 25% in the rest of the world [5]. Careless and haphazard pesticide usage increases pathogen and pest resistance, reduces soil biodiversity, kills useful soil microbes; causes bio magnification of pesticides, pollinator decline and destroys natural habitat of farmer friends like birds [6]. The potential uses and benefits of nanotechnology are enormous. These include insect pest management via formulations of nanomaterial based pesticides and insecticides, increase in agricultural productivity using nanoparticles encapsulated fertilizers for slow and sustained release of nutrients and water. Nanoparticles mediated gene or DNA transfer in plants for the development of insect pest resistant varieties and use of nanomaterial for preparation of different kinds of biosensors would be useful in remote sensing devices required for precision farming are some of the boon of this modern nanotechnology [7]. Traditional strategies such as integrated pest management used in agriculture are insufficient and application of chemical pesticides has adverse effects on animals, useful soil microbes and declines the fertility of soil as well. To combat this problem, development of more effective and non-persistent pesticides such as controlled release formulation is needed [8]. Tools like quantum dots are commonly used in plant pathology successfully for routine monitoring of pathogens and beneficial organisms in a wide range of microorganisms and substrates due to gene profiling of wide microorganisms [9]. Advances in micro fabrication and nanotechnology now play an important role in viral detection and improving the detection limit, operational simplicity and cost-effectiveness of viral diagnostics [10].
2. Biosynthesis of nanoparticles and their use in agriculture
Many chemical methods are available for synthesis of nanoparticles, which use toxic chemicals so the need of the hour is to use environmentally benign, greener and ecofriendly routes. Researchers are looking forward for various biological entities like bacteria, fungi, higher plants, actinomycetes and viruses for nanoparticles synthesis as they can also reduce the salts to corresponding nanoparticles. Different biological sources have been used for the synthesis of nanoparticles and are being used in agriculture for precision farming [11]. Some of them are as follows: silver nanoparticles, zinc oxide nanoparticles, titanium dioxide nanoparticles.
2.1. Silver nanoparticles
Silver nanoparticles have a high surface area and fraction of surface atoms; as a result have high antimicrobial effect as compared to the bulk silver [12]. Antimicrobial property of silver nanoparticles has been used against a broad range of human pathogens [13], [14], [15], [16]. However, the full potential is still to be explored for crop protection. Therefore, there is a growing interest to utilize antimicrobial property of silver nanoparticles for plant disease management [17]. Silver nanoparticles have been experimenting as pesticides to reduce burden of pests from crops. Silver nanoparticles can be synthesized from physical, chemical and biological methods. Owing to requirement of extreme conditions and toxic chemicals in physical and chemical methods, biological methods are widely used. Being single step synthesis and ecofriendly, different researchers have synthesized silver nanoparticles from different sources (plants, bacteria, fungi etc.). These silver nanoparticles have been used to get rid of harmful microorganisms in plants.
Biological synthesis of silver nanoparticles in sizes ranging from 6 to 38 nm from white radish (Raphanus sativus var. aegyptiacus) has been documented. The exposure of the snails and soil matrix to silver nanoparticles in a laboratory experiment reduced the activity and the viability of the land snail (20% of silver nanoparticles treated snails died) as well as the frequency of fungal population in the surrounding soil [18]. Spherical shaped silver nanoparticles in size range of ∼10 to 20 nm using culture supernatant of Serratia sp. BHU-S4 and their effective application for the management of spot blotch disease in wheat have been experimented. Silver nanoparticles exhibited strong antifungal activity against Bipolaris sorokiniana, the spot blotch pathogen of wheat [17]. Effect of silver nanoparticles with diameters of 20 nm on seeds of Fenugreek (Trigonella foenum-graecum) has been carried out. [19]. Different concentrations of silver nanoparticles (0, 10, 20, 30 and 40 μg mL−1) were used and results showed that maximum seed germination (76.11%), speed of germination (4.102), root length (76.94 mm), root fresh weight (2.783) and root dry weight (1.204) at a concentration of 10 μg mL−1. These results revealed that application of silver nanoparticles could be used to significantly enhance seed germination potential, mean germination time, seed germination index, seed vigor index, seedling fresh weight and dry weight.
2.2. Zinc oxide nanoparticles
Zinc deficiency is a most common micronutrient problem that adversely affects agricultural production in alkaline soils with calcium carbonate [20]. The soils with calcium carbonate are a major source of agriculture in arid or Mediterranean environments of the world [21]. The parameters that limit zinc availability to plants in calcium carbonate soils are the alkaline pH, which reduces zinc solubility and the high calcium carbonate (CaCO3) content, which can absorb and precipitate zinc [22], [23]. Zinc oxides (ZnO) and zinc sulphates (ZnSO4·H2O) or (ZnSO4·7H2O) are commonly used as zinc fertilizers to correct deficiency of zinc in soils [24]. However, their applications as fertilizer are limited due to non-availability of zinc to plants. Zinc oxide nanoparticles can overcome this problem by providing more soluble and available form of zinc to plants due to their higher reactivity in comparison to micron- or millimeter-sized zinc particles present in bulk. The use of zinc oxide nanoparticles as zinc fertilizers may increase zinc dissolution and its bioavailability in soils with calcium carbonate. Diffusion of dissolved zinc is the main mechanism for the movement of zinc from fertilizer to the plant roots following the dissolution process [25]. Zinc oxide nanoparticles have shown much better antimicrobial activity than large zinc particles, since the small size less than 100 nm and high surface-to-volume ratio of nanoparticles allows better interaction with bacteria [26]. Zinc oxide nanoparticles have the ability to induce reactive oxygen species (ROS) generation, which can lead to cell death when the antioxidative capacity of the cell is exceeded [27], [28], [29], [30], [31]. Generation of reactive nitrogen species (RNS) and hydrogen peroxide (H2O2) upon exposure to silver and zinc oxide engineered nanoparticles on the duckweed (Spirodela punctuta) suggested that the toxicity of silver and zinc oxide nanoparticles predominantly caused by both the particulates and ionic forms [32]. Zinc nanoparticles have shown to induce free radical formation in wheat, resulting in increased malondialdehyde and lower levels of reduced glutathione [33] and reduced chlorophyll contents [34].
Zinc oxide nanoparticles can be synthesized by chemical and biological methods. Since chemical methods require toxic chemicals, biological methods are becoming popular. Synthesis of zinc oxide nanoparticles from plants is cost effective and eco-friendly. Plant leaf extract has been used commonly for synthesis of zinc oxide nanoparticles. For synthesis of zinc oxide nanoparticles, appropriate concentration of either zinc sulfate heptahydrate (ZnSO4·7H2O) or zinc acetate dehydrate (Zn(CH3COO)2·2H2O) is dissolved in water. Plant leaf extract can be prepared in solvents such as water, ethanol or methanol. By mixing plant extract and zinc sulfate heptahydrate or zinc acetate dehydrate solution at desired pH, zinc oxide nanoparticles are synthesized.
Zinc oxide nanoparticles have been tested in the laboratory as bactericide and fungicide. Zinc oxide nanoparticles using leaf extract of Moringa oleifera in size range from 16 to 20 nm has been synthesized and antimicrobial activity against bacterial strains such as Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Proteus mirabilis, Escherichia coli and fungal strains such as Candida albicans and Candida tropicalis using the agar disc diffusion method has been tested. The maximum zone of inhibition was observed in Staphylococcus aureus (23.8 ± 0.76) as compared to others [35]. Spherical and hexagonal zinc oxide nanoparticles from Parthenium hysterophorus L. have been synthesized by inexpensive, ecofriendly and simple method using different concentrations of 50% and 25% of Parthenium leaf extracts with size 27 ± 5 and 84 ± 2 nm, respectively. These zinc oxide nanoparticles were explored for the size-dependent antifungal activity against plant fungal pathogens i.e. Aspergillus flavus and Aspergillus niger. A maximum zone of inhibition was observed for 27 ± 5 nm size zinc oxide nanoparticles against Aspergillus flavus and Aspergillus niger. Parthenium mediated zinc oxide nanoparticles proved to be good antifungal agents and environment friendly [36]. Spherical shaped zinc oxide nanoparticles with an average size of 23 to 57 nm were prepared by zinc acetate and sodium hydroxide using leaves of Catharanthus roseus (L.) G. Don leaf extracts. The synthesized zinc oxide nanoparticles were evaluated for antibacterial activity against gram negative bacteria Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 15442), gram positive Staphylococcus aureus (ATCC 6538) and Bacillus thuringiensis (ATCC 10792). Bacillus thuringiensis indicated the resistance to zinc oxide nanoparticles followed by Escherichia coli whereas Pseudomonas aeruginosa was more susceptible. This study concluded that zinc oxide nanoparticles might be used as antibacterial formulations against Pseudomonas aeruginosa [37].
2.3. Titanium dioxide (TiO2) nanoparticles
Titanium is a tough, glorious, corrosion resistant metal. Its compound titanium dioxide is a popular photo-catalyst, used in the manufacture of pigments [38]. Titanium stimulates production of more carbohydrates, encouraging growth and photosynthesis rate in plants [39], [40], [41]. Titanium dioxide has shown photo catalytic activity for degradation of pesticides [42]. Photocatalyst property of titanium dioxide has applications in plant protection because it does not form toxic and dangerous compounds hence possess great pathogen disinfection efficiency. Scientists are trying to improve the phytopathogenic disinfection efficiency of titanium dioxide thin films by dye doping and other suitable methods [43].
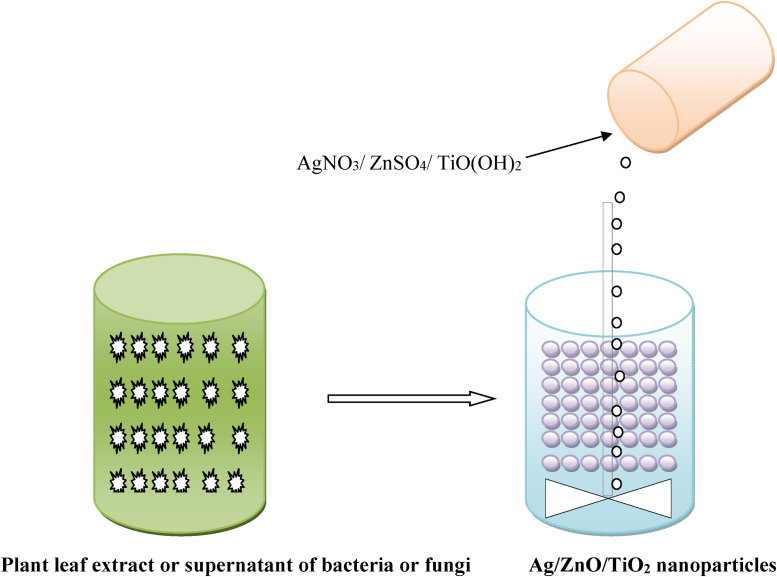
Plants are also the primary choice of researchers for synthesis of titanium dioxide nanoparticles. Spherical shaped, clustered titanium dioxide nanoparticles with an average size of 32.58 nm from the aqueous leaf extract of Psidium guajava have been synthesized [44]. These nanoparticles were tested against bacteria Aeromonas hydrophila (MTCC-1739), Proteus mirabilis (MTCC-442), Escherichia coli (MTCC-1677), Staphylococcus aureus (MTCC-3160) and Pseudomonas aeruginosa (MTCC-4030). The maximum zone of inhibition was observed against Staphylococcus aureus (25 mm) and Escherichia coli (23 mm) when titanium dioxide nanoparticles were used at 20 μg/mL concentration. The synthesized TiO2 nanoparticles showed enhanced antibacterial activity than the standard antibiotic disk, tetracycline which reduced the chances for the development of antibiotics resistance of bacterial species. The aqueous extract from plants and titanium dioxide nanoparticles synthesized from these possessed best antioxidant activity when compared with ascorbic acid. Synthesis of spherical clusters, quite polydispersed titanium dioxide nanoparticles with a size range from 36 to 68 nm by Eclipta prostrata leaf extract has been done successfully [45]. A schematic representation of biological synthesis of silver, zinc oxide and titanium dioxide nanoparticles is shown in Fig. 1.
Fig. 1.
Biological synthesis of silver/zinc oxide/titanium dioxide nanoparticles.
3. Applications of nanotechnology in precision agriculture
3.1. Delivery of fertilizers
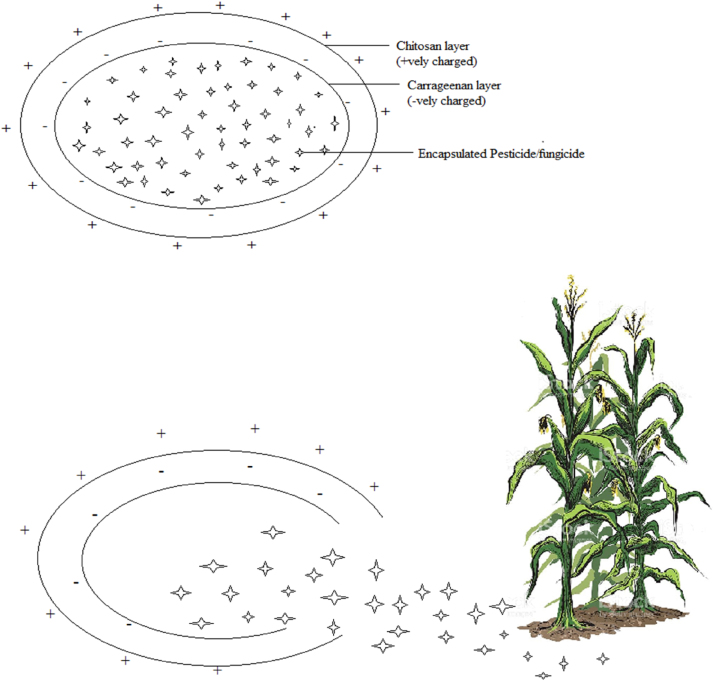
Enormous amounts of fertilizer in the form of ammonium salts, urea, and nitrate or phosphate compounds have increased the food production considerably, but they have many harmful effects on the beneficial soil microflora. Most of the fertilizers are not available to plants due to run-off and cause pollution [46]. Fertilizers coated in nanomaterials can solve this problem. Nano materials have potential contributions in slow release of fertilizers as nanoparticles hold the material more strongly from the plant due to higher surface tension of nanoparticles than conventional surfaces. Moreover, nanocoatings provide surface protection for larger particles [47], [48]. A schematic representation of delivery of pesticides/fungicides/nutrients from nanocoating is shown in Fig. 2.
Fig. 2.
Controlled release of pesticides/fungicides/nutrients from nanocoating.
3.1.1. Chemical fertilizers
The consumption of nitrogen fertilizer in the form of urea has increased manifold (29%) after the green evolution era in India. Increased food production through excess nitrogen application is responsible for 80% of the increase in atmospheric N2O (a greenhouse gas) which causes increased atmospheric temperature and thus contributes to global warming [49]. Chemical fertilizers like urea, diammonium phosphate (DAP) and single superphosphate (SSP) are used in agriculture to meet the shortage of N, P and K in the soil. But most part of these fertilizers are lost as run-off or volatilized. It is estimated that about 40–70% of nitrogen, 80–90% of phosphorus, and 50–70% of potassium of the applied fertilizers is lost to the environment and can’t be absorbed by plant applied causing exchequer loss to the nation and environmental pollution as well [50], [51]. A modern approach lies in the use of nanocoated urea or other chemical fertilizers. The stability of the nanocoating reduces the rate of dissolution of the fertilizer and allows slow, sustained release of coated fertilizer which is more efficiently absorbed by plant roots. Recently, the use of slow release fertilizers has become a new method to save fertilizer consumption in order to minimize environmental pollution [52]. Fertilizers with sulphur nanocoating (≤100 nm layer) are useful as slow release fertilizers because sulfur contents are beneficial especially for sulfur deficient soils [47], [48]. Nanocoated urea and phosphate and their sustained release will be beneficial to meet the soil and crop demands as majority of soil in India is deficient in these macronutrients especially nitrogen. Many natural and synthetic polymers have been used for this sustained release of fertilizers. Biodegradable polymeric chitosan nanoparticles (∼78 nm) showed good results for the slow release behavior of NPK fertilizer [53]. Kaolin and polymeric biocompatible nanoparticles also have potential application in the slow release behavior of fertilizers [46].
Nanofertilizers balances the release of fertilizer nitrogen and phosphorus with the absorption by the plant, thereby preventing the nutrient losses and avoiding unwanted nutrients interaction with microorganisms, water and air [54]. Absorption of nutrients by the plants from soil can be maximized using nanofertilizer. Nanofertilizer encapsulated nanosilica can form a binary films on the cell wall of fungi or bacteria after absorption of nutrients and prevent infections, hence improve plant growth under high temperature and humidity and to improve plant resistance to disease [55]. Silicon-based fertilizers used to increase plant resistance as silicon dioxide nanoparticles can improve seedling growth and root development [56]. To increase food production, TiO2 or titanium that is non-toxic can be used as additives in fertilizers. The additives in fertilizers can increase water retention [54]. Due to the high solubility of many nitrogen fertilizers and their potential vulnerability to leaching and denitrification (especially in the nitrate form); a wide range of slow-release fertilizers (SRFs) and controlled-release fertilizers (CRFs) have been developed using synthetic or biopolymers [57], [58]. The adsorbents zeolite, halloysite, montmorillonite and bentonite nanoclays were used to develop nitrogen fertilizers with controlled release characteristics where nanoclay purification comes to be a costly affair except zeolite [59]. Preparation of chitosan nanoparticles has been reported with polymethacrylic acid (PMAA) for loading NPK fertilizers. The stability of the chitosan-polymethacrylic acid (CS-PMAA) colloidal suspension was found to be higher with the addition of nitrogen and potassium and phosphorus, due to the higher anion charge from the calcium phosphate than the anion charges from the potassium chloride and urea. [60]. The dispersions of CS-PMAA combined with 500 ppm of nitrogen were higher in stability compared with that of phosphorus. Above 500 ppm of nitrogen, a reduction of positive charges occurred in the colloidal dispersion of CS-PMAA due to the presence of negative groups from the urea molecules [54]. For dispersion with potassium, the stability of the solution has been confirmed with the addition of 400 ppm. This showed the presence of Cl− ions (from KCl) which did not affect the stability of colloidal dispersion with the addition of up to 400 ppm [61], [62], [63], [54].
3.1.2. Biofertilizers
Biofertilizers are living, beneficial microorganisms. These include fungal mycorrhizae, Rhizobium, Azotobacter, Azospirillum and blue green algae, phosphate solubilizing bacteria like Pseudomonas and Bacillus [64]. Microorganisms convert organic matter into simple compounds that provide essential nutrients to plants, improve soil fertility, maintain the natural habitat of the soil and increase crop yield. The preparation, storage and method of application are crucial to the success of biofertilizers application [65]. Drawbacks in usage are short shelf life, temperature sensitivity and storage desiccation problems. Potential applications of polymeric nanoparticles for coating of biofertilizer preparations to yield formulations that are resistant to desiccation have been utilized. Water-in-oil emulsion is one of the techniques for storage and distributes microorganisms through liquid formulations [66]. The oil traps the water around the microorganism hence down water evaporation. This is particularly favorable for microorganisms that are susceptive to desiccation. Water-in-oil emulsions also improve both cell viability and release kinetics by addition of substances to the oil and/or aqueous phases. However, sedimentation during storage is one of the major issues of concern. Hydrophobic silica nanoparticles reduced cell sedimentation and improved cell viability by thickening the oil phase during storage [67].
Due to limited availability of land and water resources and development of horticultural crops of Fabaceae, use of silver and gold nanoparticles as a growth promoting materials could be effective [68]. These nanoparticles with natural biofertilizers such as Pseudomonas fluorescens, Bacillus subtilis and Paenibacillus elgii have shown very good growth promotion under in vitro conditions. Hence, needs in very minute amount in comparison to other fertilizers and their costs are manageable as one liter of nano-biofertilizers can be used in several hectares of crops. Several soil microorganisms are present in the rhizosphere zone, especially plant growth promoting rhizobacteria (PGPR) have the best plant growth-promoting activities. The impact of gold nanoparticles on PGPR was investigated viz., Pseudomonas fluorescens, Bacillus subtilis, Paenibacillus elgii, and Pseudomonas putida. No positive or negative impact was observed in Pseudomonas putida to gold nanoparticles. Significant increase was observed in case of P. fluorescens, P. elgii and B. subtilis and hence gold nanoparticles can be exploited as nano-biofertilizers [69]. A list of nanofertilizers tested and their action against plant pathogens is given in Table 1.
Table 1.
Nanofertilizers and their action against plant pathogens.
| Type | Nanofertilizers | Antimicrobial action against plant pathogens | References |
|---|---|---|---|
| Plant growth promoting microorganisms | Ag | Bacillus cereus | [70] |
| Ag | Escherichia coli, Bacillus subtilis and Streptococcus thermophilus | [71] | |
| Ag and TiO2 | Lactobacillus strains | [72] | |
| Ag | Corynebacterium sp. | [73] | |
| Au | Klebsiella pneumoniae | [74] | |
| Ag | Fusarium oxysporium | [75] | |
| Ag | Aspergillus fumigatus | [76] |
3.2. Micronutrient supply
It’s a well-established fact that micronutrients like manganese, copper, boron, iron, molybdenum, zinc etc. are important for the growth and development. A substantial increase of crop yields with the green revolution and new farming practices has progressively decreased the micronutrients of soil like zinc, iron and molybdenum [77]. Foliar application of micronutrients can enhance uptake by the leaves [78]. Nanotechnology can be used to make the availability of micronutrients to plants. Nano formulations of micronutrients can be sprayed on plants or can be supplied to soil for uptake by roots to enhance soil health and vigor [79]. The release of behavior of 1-naphthylacetic acid (an important plant growth hormone) from chitosan nanoparticles has been tested at different pH’s and temperature. The formulation was found to have potential for the slow release of agrochemicals such as hormones [80]. Different nanoparticles have been tested to provide appropriate level of micronutrients in plants.
Iron deficiency is a widespread problem in plants growing mainly in high pH and calcareous soils. Foliar application of iron compounds with the technology of nanoparticles may be a solution to the problem. Effect of spraying of iron oxide nanoparticles on the response of wheat growth, yield and quality has been assessed. The concentrations of iron nano-oxide solution has been used in five levels (0, 0.01%, 0.02%, 0.03% and 0.04%) to check the effect on spike weight, 1000 grain weight, biologic yield, grain yield and grain protein content. Increase in these traits was found as compared to control one [81]. Increase in chlorophyll contents in sub-apical leaves of soybeans in a greenhouse test under hydroponic conditions have been reported using low concentrations of superparamagnetic iron oxide nanoparticles. This study concluded that iron oxide nanoparticles could be used as a source of iron for soybean for reducing chlorotic symptoms of iron deficiency [82]. Foliar application of 500 mg L−1 iron nanoparticles to black-eyed peas significantly increased the number of pods per plant (by 47%), weight of 1000-seeds (by 7%), the iron content in leaves (by 34%), and chlorophyll content (by 10%) over those of the controls. Application of iron nanoparticles also improved crop performance more than that by application of a regular iron salt. These parameters were increased by 28%, 4%, 45%, and 12%, respectively, under the iron nanoparticles treatment compared with these under treatment with iron salt. [83]. In addition, iron nanoparticles significantly improved the beneficial effect of magnesium nanoparticles used as nanofertilizer on black-eyed peas. For an optimal growth, most of the plants generally required 1–5 mg L−1 iron in soil solution [84]. Manganese nanoparticles have been reported to enhance growth of mung bean (Vigna radiata) and photosynthesis [85]. Enhanced growth of mung bean and chickpea (Cicer arietinum) seedlings at low concentrations using zinc-oxide nanoparticles with plant agar method were observed, however a decline in growth rates of roots and shoots were observed beyond optimal concentrations [86].
3.3. Insect pest management
Synthetic agrochemicals have changed the face of agriculture, but it has also developed new challenge in form of insect pest resistance. Nanoparticles have a great promise for the management and control of insect pest of modern agriculture. Insecticidal activity of garlic essential oil against Tribolium castaneum (red flour beetle) has been increased by polyethylene glycol-coated nanoparticles [87]. Using this formulation the control efficacy against adult T. castaneum was calculated about 80% which was presumably due to the slow and sustained release of the active components from the nanoparticles. Applications of different kinds of nanoparticles such as silver nanoparticles, aluminium oxide, zinc oxide and titanium dioxide in the control of rice weevil (caused by Sitophilus oryzae) and grasserie disease in silkworm (caused by Bombyx mori and baculovirus BmNPV(B. mori nuclear polyhedrosis virus) were studied [88]. Das et al. [89] studied the transformation of Bombyx mori nucleopolyhedrovirus by lipophilically coated silica nanoparticle, alumina nanoparticles in the hexagonal close-packed α structure and aspartate capped gold nanoparticle in B. mori cell line using cytopathic effect and plaque reduction assay. A moderate polyhedra roughening was observed for alumina nanoparticles, and no roughening was noticed for gold nanoparticles. When leaves of mulberry (B. mori) were treated with ethanolic suspension of hydrophobic alumino-silicate nanoparticle, a significant decrease in viral load was reported [7]. Insecticidal activity of nanostructured alumina against Sitophilus oryzae L. and Rhyzopertha dominica reported significant mortality after 3 days of continuous exposure to nanostructured alumina-treated wheat. So, commercially available insecticides, inorganic nanostructured alumina may provide a cheap and reliable alternative for control of insect pests [90]. Debnath et al. [91] tested the entomotoxicity of silica nanoparticles against rice weevil Sitophilus oryzae and compared the efficacy with bulk-sized silica (individual particles larger than 1.0 μm). Amorphous silica nanoparticles was found to be highly effective against this insect pest causing more than 90% mortality, indicated the effectiveness of silica nanoparticles to control insect pests. Nano-encapsulation of pesticide allows proper absorption of the chemical into the plants due to slow and sustained release and has a long lasting and persistent effect unlike the normal agrochemicals [92]. Synthetic pesticides have detrimental environmental impacts, but their specificity towards the targeted pests is high. So there is a need to come towards botanical insecticides with the use of nanotechnology to expand the frontiers for nanoparticle-based technologies in pest management.
3.4. Nanofungicides
Fungal diseases among crops cause major loss to the production. Although there are a number of fungicides available commercially, their application causes detrimental effects to plants also. Nanotechnology can play a very important role in solving this problem. Nanoparticles have been experimenting as antifungal agents against pathogenic fungi. Antifungal activity of nanoparticles of zinc oxide (35–45 nm), silver (20–80 nm) and titanium dioxide (85–100 nm) has been tested against Macrophomina phaseolina, a major soil borne pathogen of pulse and oilseed crops. The higher antifungal effect was observed in silver nanoparticles at lower concentrations than zinc oxide and titanium dioxide nanoparticles [93]. Maize treated with nanosilica (20–40 nm) has been screened for resistance against phytopathogens, Fusarium oxysporum and Aspergillus niger as compared with that of bulk silica. Nanosilica-treated plant showed a higher expression of phenolic compounds (2056 and 743 mg/mL respectively) in collected leaf extracts and a low expression of stress-responsive enzymes against these fungi. These results showed significantly higher resistance in maize in treated with nanosilica than with bulk in terms of disease index and expression of total phenols, phenylalanine ammonia lyase, peroxidase and polyphenol oxidase, at 10 and 15 kg/ha. So silica nanoparticles can be used as an alternative potent antifungal agent against phytopathogens [94].
The silver has much higher antifungal activity than that of other metals. This is because silver ions cause the inactivation of cell wall thiol groups of fungal cell wall resulting in disruption of transmembrane, energy metabolism and electron transport chain. Mutations in fungal DNA, dissociation of the enzyme complexes that are essential for the respiratory chain, reduced membrane permeability and cell lysis are also other mechanisms [95]. The efficacy of silver nanoparticles is dependent on particle size and shape and decreases with increasing particle size. It has been found that truncated triangular particle shape showed greater “cidal” effect than spherical and rod shaped particles [96], [97]. Antifungal properties of silver nanoparticles for plant disease management have been utilized [98]. Well diffused and sustained silver nanoparticles solution can act as an excellent fungicide due to good adhesion on bacterial and fungal cell surface [99]. Table 2 shows nanoparticles with their fungicidal action against pathogenic fungi.
Table 2.
Nanoparticles used as fungicides against plant pathogenic fungi.
| Nanoparticles | Fungicidal action against plant pathogenic fungi | References |
|---|---|---|
| Ag | Bipolaris sorokiniana | [100] |
| Bipolaris sorokiniana and Magnaporthe grisea | [101] | |
| Fusarium sp., Alternaria solani, Pythium spinosum, Pythium aphanidermatum, Cylindrocarpon destructans, Cladosporium cucumerinum, Glomerella cingulata, Didymella bryoniae, Stemphylium lycopersici and Monosporascus cannonballus | [102] | |
|
Alternaria alternata, Penicillium digitatum and Alternaria citri |
[103] | |
| Bipolaris sorokiniana | [104] | |
| Candida spp., Aspergillus spp., Fusarium spp | [105] | |
| Cu | Aspergillus flavus, Aspergillus niger, Candida albicans, Staphylococcus aureus, Escherichia coli, Micrococcus luteus, Klebsiella pneumoniae, Pseudomonas aeruginosa | [106] |
| Phoma destructiva, Curvularia lunata, Alternaria alternata and Fusarium oxysporum | [107] | |
| Fusarium equiseti, F. oxysporum, F. oxysporum | [108] | |
| Fusarium sp. | [109] | |
| Cu-chitosan | Alternaria alternata, Macrophomina phaseolina and Rhizoctonia solani | [110] |
| Zn | Aspergillus niger and Fusarium oxysporum | [111] |
| S | Aspergillus niger and Fusarium oxysporum | [112] |
| ZnO | Botrytis cinerea and Penicillium expansum | [113] |
| Aspergillus fumigatus and Candida albicans | [114] | |
| Fusarium oxysporum and Penicilium expansum | [115] |
3.5. Nanoherbicides
Weeds are the biggest threat in agriculture and decline the yield of crop to a greater quantity by using the nutrients which otherwise were available to the crop plants. Eradicating weeds by conventional means are time consuming. There are a number of herbicides available commercially. They kill the weeds in the fields, but also damages crops plants. They are also responsible in decreasing soil fertility and creating soil pollution. Nanoherbicides can play a very important role in removing weeds from crops in an eco-friendly way, without leaving any harmful residues in soil and environment [116]. Encapsulation of herbicide in polymeric nanoparticles also results in environmental safety [117]. Disproportionate use of herbicides for longer period of times leaves residues in soil, which cause damage to succeeding crops [118]. Continuous use of same herbicide for constant period of time cause weeds resistance against same herbicide. Effectiveness of nano zerovalent iron (nano ZVI) has been assessed to dechlorinate herbicide atrazine (2-chloro-4ethylamino-6-isopropylamino-1, 3, 5-triazine) from atrazine-contaminated water and soil [119]. Target specific nanoparticles loaded with herbicide has been developed for delivery in roots of weeds. These molecules enter into the roots system of the weeds, translocate to cells and inhibit metabolic pathways such as glycolysis. This ultimately leads to death of plants [120], [121].
Toxicity of poly(ε-caprolactone) nanocapsules containing ametryn and atrazine against alga Pseudokirchneriella subcapitata and the microcrustacean Daphnia similis has been tested. Herbicides encapsulated in the poly(ε-caprolactone) nanocapsules resulted in lower toxicity to the alga (Pseudokirchneriella subcapitata) and higher toxicity to the microcrustacean (Daphnia similis) as compared to the herbicides alone [122]. A list of nanoparticles used as herbicides against weeds in different vegetable crops is given in Table 3.
Table 3.
Nanoparticles used as herbicides in commercial vegetable crops.
| Sr. No. |
Nanoparticles |
Nanoherbicides used against herbs/weeds |
References |
|---|---|---|---|
| 1. | Silver nanoparticles-chitosan encapsulated paraquate | Eichhornia crassipes | [123] |
| 2. | Ag, Cu, Fe, Zn, Mn | Allium cepa (L.) | [124] |
| 3. | Cu | Cucurbita pepo | [125], [126] |
| 4. | CuO | Raphanus sativus, Lolium perenne and Lolium rigidum | [127] |
| 5. | CuO and ZnO | Fagopyrum esculentum | [128] |
| 6. | Cu | Elodea densa | [129] |
| 7. | CuO and ZnO | Cucumis sativus | [130] |
3.6. Biosensors in precision agriculture
Precision farming has been a long-desired goal to maximize output from crops while minimizing the input of fertilizers, pesticides, herbicides, etc. through monitoring environmental variables and applying targeted action. Precision farming makes use of computers, sensors, global satellite positioning systems and remote sensing devices to measure highly localized environmental conditions and helps in determining whether crops are growing at maximum efficiency or precisely identifying the nature and location of problems. Ultimately, precision farming with the help of smart sensors will allow enhanced productivity in agriculture by providing accurate information thus helping farmers to make better decisions [131].
3.6.1. Normalized difference in vegetative index (NDVI) sensor or green seeker
NDVI is a device which uses light emitting diodes (nano-based) to generate red and near infrared light which are used to calculate NDVI values. Green seeker calculates the normalized difference in vegetative index using red and near infra-red light. It is based on the simple principle that plant chlorophyll absorb red light as an energy source during photosynthesis. Therefore, healthy plants absorb more red lights and reflect larger amounts of near infra-red light than those that are unhealthy and thus give higher NDVI value.
NDVI is calculated using the equation:
| NDVI = NIRreflected − Redreflected/NIRreflected + Redreflected |
Green seeker is an excellent indicator of biomass. This tiny device makes plants speaks up for their nitrogen needs [132].
3.6.2. Nanobiosensors
Nanobiosensors can be productively used in sensing a broad array of agriculture like in fertilizers, herbicide, pesticide, insecticide, moisture and soil pH. Controlled use of biosensors can assist in sustainable agriculture for increasing crop productivity. Precision farming, with the aid of smart sensors, could enhance productivity as this technology ensures better fertilization management, reduced input cost and environment safety. Nanosensors based smart delivery systems could help in the efficient use of natural resources like water, nutrients and agrochemicals by precision farming [133].
The presence of plant viruses, the level of soil nutrients and crop pathogens can be detected by nanosensors [134], [135]. A nanobiosensor based on atomic force microscopy tip functionalized with the acetolactate synthase enzyme was detected for herbicide metsulfuron-methyl (an acetolactate synthase inhibitor) through the acquisition of force curves [136]. Bionanosensors also makes it possible for rapid detection of bacteria and viruses with precise quantification and thereby increasing the food safety for the customer [137]. A highly sensitive organophosphorus pesticide biosensor constructed with the help of surface functionalized carbon nanotubes tailored with amino groups to control the efficient immobilization of acetylcholinesterase onto the surface of glassy carbon electrode and these have been successfully used for the direct analysis of vegetable samples [138]. An acetyl cholinesterase biosensor based on assembly of multiwall carbon nanotubes onto liposome bioreactors was developed for detection of organophosphates pesticides [139]. A highly sensitive acetylcholinesterase biosensor modified with hollow gold nanospheres with the detection limits 0.06 μg/dm3 for chlorpyrifos and 0.08 μg/dm3 for carbofuran was developed [140]. Zn-Se quantum dot immobilized acetylcholinesterase has been used for detection of organophosphate pesticides using graphene-chitosan nanocomposite modified electrode. Organophosphate pesticides have been detected with this biosensor using methyl parathion as a model enzyme inhibitor, with a detection limit of 0.2 nmol/dm3 [141]. For determination of pesticides methylparathion and chlorpyrifos; an electrochemical biosensor based on acetylcholinesterase immobilized on polyaniline precipitated on vertically assembled carbon nanotubes and wrapped with ssDNA was prepared. The pesticides were determined via inhibition of enzyme reaction acetylcholinesterase-acetylcholine that caused small changes of local pH in the surrounding of an electrode surface and the detection limit of the biosensor for both the pesticides was found to be 1&903;10–12 mol/dm3 [142].
Electrochemically functionalized single walled carbon nanotubes (SWCNTs) based nanosensors with metal/metal oxide nanoparticles or nanotubes for gases viz. ammonia, nitrogen oxides, hydrogen sulfide, sulfur dioxide and volatile organics have potential application in monitoring agricultural pollutants and assessment of impacts on living matter or health and in increase of crop productivity [143]. Screening of organophosphorus agents with gold nanoparticles and fluorescence spectroscopy has been reported with high sensitivity (1.0 μmol/dm3) [144].
3.6.3. Quantum dots (QDs)
Quantum dots are far better and rapid than organic fluorescent dyes due to more efficient luminescence, small emission spectra, excellent photostability and tenability according to the particle sizes and material composition. By a single excitation light source, QDs can be excited to all colors due to their broad absorption spectra [145]. Quantum dots have been used to detect pathogens associated with different plant diseases.
Quantum dots-fluorescence resonance energy transfer based sensors have been developed to detect witches’ broom disease of lime caused by Phytoplasma aurantifolia. The immunosensor developed showed a high sensitivity, specificity of 100%, and a detection limit of 5 ca. P. aurantifolia per μL [146]. Rhizomania is the most destructive disease in sugar beet caused by beet necrotic yellow vein virus. Polymyxa betae (Keskin), the only known vector of beet necrotic yellow vein virus, for transmission of the virus to the plants was successfully detected by quantum dots-fluorescence resonance energy transfer-based sensor [147]. To detect organophosphorus pesticides (OPs) in vegetables and fruits; the optical transducer of cadmium telluride semiconductor quantum dots integrated with acetylcholinesterase enzyme by the layer-by-layer assembly technique. This resulted in a highly sensitive biosensor based on enzyme inhibition mechanism with detection limits of 1.05 × 10−11 M for paraoxon and 4.47 × 10−12 M for parathion which was far better than the conventional gas chromatography methods or amperometric biosensors (0.5 nM) [148]. Unique optical property of thiol-stabilized luminescent cadmium telluride (CdTe) quantum dots has been used to detect smethyl parathion at picogram levels [149]. Water-soluble CdTe quantum dots and highly fluorescent silica molecularly imprinted nanospheres embedded cadmium telluride quantum dots have been used as a biosensor for determination of deltmethrin in fruit and vegetable samples [150].
3.6.4. Nanobarcodes
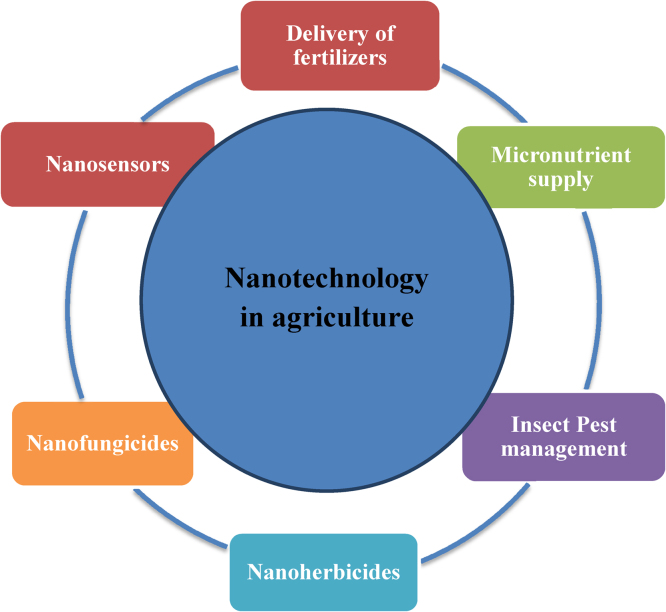
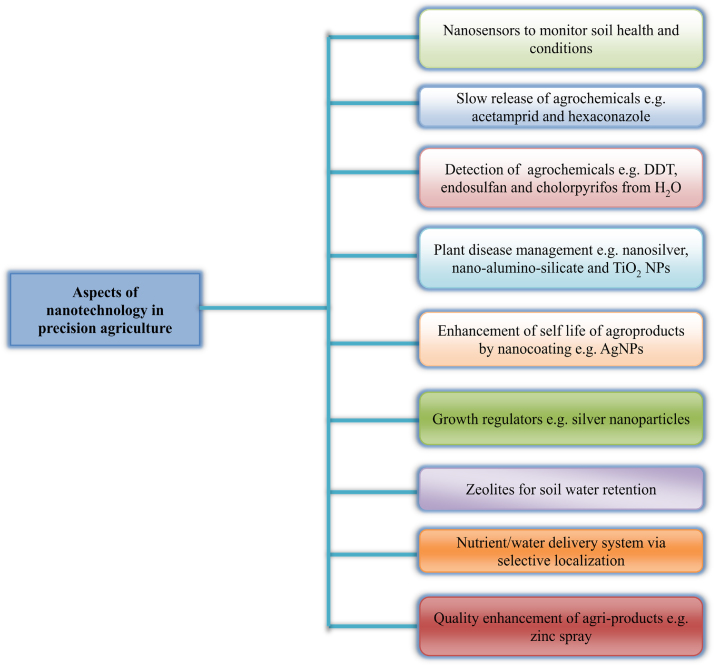
Nanobarcode particles are manufactured via a semi-automated and highly scalable process of electroplating of inert metals such as gold, silver, etc. into templates defining particle diameter and thus resulting nanorods from the templates are released. These nanobarcodes are used as identification tags for multiplexed analysis of gene expression. Nanotechnology assisted advancement in the field of biotechnology has seen improvement in the plant resistance to environmental stresses such as drought, salinity and diseases. Nanotechnology-based gene sequencing is capable of rapid and cost effective identification and utilization of plant gene trait resources [151]. So there are a number of applications of nanotechnology in agriculture which have been shown by schematic representation in Fig. 3. A schematic representation of different aspects of nanotechnology in agriculture is shown in Fig. 4.
Fig. 3.
Schematic representation of applications of nanotechnology in agriculture.
Fig. 4.
Different aspects of nanotechnology in agriculture.
4. Nanotechnology and agribusiness
According to global economic reports, an estimated global agribusiness market in 2010 ranged from about US$ 20.7 billion to US$ 0.98 trillion. By 2020, the market will be estimated to be more than US$ 3.4 trillion [152], [153]. At present the United States of America leads with a 4 year, 3.7 billion USD investments through the National Nanotechnology Initiative (NNI) [154], [155]. The USA is followed by Japan and the European Union and these countries have abundant funds (US$ 750 million and US$ 1.2 billion, including individual country contributions, respectively per year [156]. Today, more than 400 companies in the world are active in nanotechnology research and development and this number is expected to increase to more than 1000 in next 10 years [157]. Different nanoproducts in agriculture have been produced by different organizations and a list of these is given in Table. 4.
Table 4.
Nanotechnology based products and their applications in agriculture.
| Nano-products | Year | Institute | Applications | References |
|---|---|---|---|---|
| Nano-sized nutrients (ZnO and TiO2 nanoparticles) | 2015 | Washington University in St. Louis | Boost in growth and antioxidants in tomatoes | [158] |
| Biodegradable thermoplastic starch (TPS) | 2002 | Pusan National University, Korea | Good tensile strength and lowered water permeability | [159] |
| Hydrolyzed collagen/sodium alginate nanocomposite | 2008 | Sichuan University, Chengdu, Sichuan, China | Preservation of loquat and cherry | [160] |
| Macronutrient fertilizers coated with zinc oxide nanoparticles | 2012 | University of Adelaide, AU, CSIRO Land and Water, AU, Kansas State University, US |
Enhancement of nutrients absorption by plants and the delivery of nutrients to specific sites | [161] |
| Primo MAXX | 2011 | Syngenta, Greensboro, NC, USA | Grass growth regulatory | [162] |
| Nanoemulsion | 2012 | VIT University, INDIA | Neem oil (Azadirachta indica) nanoemulsion as larvicidal agent | [163] |
| Zeolites and Nano-clays | – | Geohumus-Frankfurt, DE |
Water retention and slow release of agrochemicals for proper absorption by the plants | [164] |
| Nanosensors | 2007 | (University of Crete, GR) | Pesticide detection with a liposome-based nano-biosensor | [165] |
| Acetamprid loaded alginate-chitosan nanocapsules | 2015 | GJUS&T Hisar, India |
Improved delivery of agrochemicals in the field, better efficacy, better control of application/dose. | [166] |
5. Toxicity
Nanotechnology has diverse applications in precision agriculture. But toxicity can be a major problem to nanoparticles due to their unique properties. The current phytotoxicity profile of nanoparticles is in preliminary stage. Effects of the unique characteristics of nanoparticles are not well understood; hence more studies on toxicity are required for commercial food crop applications. However, applications of nanoparticles is not always detrimental to plants, there are positive effects also.
Carbon nano-materials such fullerenes, carbon naoparticles, fullerol, and single-walled carbon nanotubes/multiwall carbon nanotubes have been in used in agriculture showing positive and adverse effects. Among positive effects, Tripathi and Sarkar [167] reported enhanced root and shoot growth (10 x) of wheat upon 10 days of exposure to 150 mg/L (200 μl) water soluble carbon nano-dots as compared to controls. Lahiani et al. [168] reported the effects of multiwall carbon nanotubes exposure for 10–11 days at concentrations of 50, 100, and 200 μg/mL on the germination and growth of soybean (Glycine max), corn (Zea mays), and barley (Hordeum vulgare) in agar medium. Upon exposure, nearly 50% in Hordeum vulgare and Glycine max and 90% in Zea mays increase in germination rate were observed as compared to untreated controls. In Glycine max, the root lengths increased up to 26% and for Zea mays; shoot and leaf length were enlarged by 40% and more than threefold, respectively. In addition, multiwall carbon nanotubes internalization was visualized by both Raman Spectroscopy and transmission electron microscopy. Toxicity of carbon nano-materials were found to be largely dependent on their concentrations, growth/exposure conditions, and plant species. Kerfahi et al. [169] investigated the effects of native and functionalized multiwall carbon nanotubes (0–5000 mg/kg) on soil bacteria. They reported that after two weeks, the soil bacterial community composition was affected by the multiwall carbon nanotubes at the highest concentrations. After 8 weeks there was no effect on the bacterial diversity with either type of nanotube. They attributed this early effect to the acidic nature of multiwall carbon nanotubes, caused a decrease in soil pH at higher exposure concentrations and subsequently changed (temporarily) soil bacterial communities.
Among adverse effects, Boonyanitipong et al. [170] studied phytotoxicity of zinc oxide and titanium dioxide nanoparticles on rice (Oryza sativa L.) roots. The three parameters examined were: seed germination percentage, root length and number of roots. The results showed that there was no reduction in the percent seed germination from zinc and titanium dioxide nanoparticles. However zinc oxide nanoparticles showed detrimental effects on rice roots at the early seedling stage. Zinc oxide nanoparticles were found to stunt roots length and reduce the number of roots. Whereas titanium dioxide nanoparticles had no effect on root length. This study showed that direct exposure to specific types of nanoparticles could cause significant phytotoxicity and emphasized the necessity for ecologically responsible disposal of wastes containing nanoparticles and further use in agricultural and environmental systems. Chai et al. [171] studied the effect of metal oxide nanoparticles (ZnO, SiO2, TiO2 and CeO2) on functional bacteria and metabolic profiles in agricultural soil. They treated agricultural soil with ZnO, SiO2, TiO2 and CeO2 nanoparticles at 1.0 mg g−1. The toxicity effect was evaluated by thermal metabolism, the abundance of functional bacteria and enzymatic activity. ZnO and CeO2 nanoparticles were observed to hinder thermogenic metabolism, reduced numbers of soil Azotobacter, P-solubilizing and K-solubilizing bacteria and inhibited enzymatic activities. Whereas TiO2 nanoparticles reduced the population of functional bacteria and enzymatic activity. SiO2 nanoparticles slightly boosted the soil microbial activity. A list of negative and positive effects of nanoparticles on plants is given in Table 5, Table 6.
Table 5.
Adverse effects of nanoparticles on plants.
| Sr. No. | Nanoparticles | Effects | Plants | References |
|---|---|---|---|---|
| 1. | TiO2 | Inhibition in cell growth and nitrogen fixation activity | Anabaena variabilis | [172] |
| 2. | TiO2 | Reduced germination | Triticum aestivum L. var. Pishtaz | [173] |
| 2. | Al | Decreased root length | Lolium perenne | [174] |
| 3. | Al | Reduced germination | Lolium perenne | [174] |
| 4. | Al | Reduced root length | Zea mays, Lactuca sativa | [174] |
| 5. | Ag | Reduced shoot and root length | Triticum aestivum L. | [175] |
| 6. | Ag | Reduced germination | (Hordeum vulgare L., cv. Annabell | [176] |
| 7. | Ag | Decreased mitosis, disturbed metaphase, sticky chromosome, cell wall disintegration and breaks | Allium cepa | [177] |
| 8. | Ag | Reduced shoot length |
Linum usitatissimum L., cv. Electra, Lolium perenne L., cv. Tove |
[176] |
| 9. | Ag | Reduced transpiration | Cucurbita pepo | [178] |
| 10. | Zn | Reduced root growth and elongation |
Zea mays, Cucumis Sativus, Lactuca sativa, Raphanus sativus, Brassica napus, Lolium perenne |
[174] |
| 11. | Cu | Reduced seedling growth | Phaseolus radiatus | [179] |
| 12. | Cu | Reduced biomass and root growth | Cucurbita pepo | [178] |
| 13. | Al2O3 | Reduced root growth | Zea mays, Cucumis sativus, Brassica oleracea, Daucus carota | [180] |
| 14. | Al2O3 | Reduced root length | Zea mays | [174] |
| 15. | CeO2 | Reduced shoot growth | Lycopersicon esculentum | [181] |
| 16. | CeO2 | Reduced shoot growth | Zea mays | [181] |
| 17. | ZnO | Reduced germination | Zea mays | [174] |
Table 6.
Positive effects of nanoparticles on plants.
| Sr. No. | Nanoparticles | Effects | Plants | References |
|---|---|---|---|---|
| 1. | Al | Improved root growth | Raphanus sativus, Brassica napus | [174] |
| 2. | Au | Positive effect on germination index | Cucumis sativus, Lactuca sativa | [182] |
| 2. | CeO2 | Increased root and stem growth | Cucumis sativus, Zea mays | [181] |
| 3. | CeO2 | Increased shoot and root length, biomass, catalase activity in shoots and ascorbate peroxidise activity in roots | Coriandrum sativum L. | [183] |
| 4. | TiO2 | Increased shoot and seedling lengths | Triticum aestivum L. var. Pishtaz | [173] |
| 5. | Ag | Enhanced plant growth and diosgenin synthesis | Trigonella foenum-graecum L. | [184] |
| 6. | ZnO | Improved growth and yield | Arachis hypogaea | [185] |
| 7. | ZnO | Improved shoot-root growth, chlorophyll, total soluble leaf protein content, rhizospheric microbial population and P nutrient-mobilizing enzymes including phytase, acid and alkaline phosphatase |
Cyamopsis tetragonoloba L. |
[186] |
| 8. | SiO2 | Improved seed germination |
Lycopersicum esculentum Mill |
[187] |
6. Conclusion
Nanotechnology has shown great potential in precision agriculture. Nanoparticles with unique properties can be easily synthesized from different biological sources and can be applied in agriculture. Among biological sources, plant extracts (leaves, flower, stem, roots) from diverse range of plant species have been successfully used in synthesizing nanoparticles. Biomolecules present in plant extracts reduce metal ions to nanoparticles in a single-step green synthesis process. This process of green synthesis is environment friendly, easy to perform, can be performed at room temperature without the need of sophisticated instrument and can be easily scaled up or modified according to the needs. In this process the various water soluble plant metabolites (e.g. alkaloids, phenolic compounds, terpenoids) and co-enzymes are reduced to nanoparticles. The nanotechnology based delivery of nanoparticles has given promising results for plant disease resistance, enhanced plant growth and nutrition via site specific delivery of fertilizers and other essential nutrients with the help of controlled release formulations of nanoparticles. Nanoencapsulation can also improve herbicide application by providing better penetration and allowing the slow and sustained release of the active substances. Hence nanotechnology can provide green, efficient and ecofriendly strategy for insect pests management in agriculture. The advanced nanotechnologies tool and techniques can improve the way; agriculture is seen and has the promising future in the upcoming age of agricultural mechanization. Nanoparticles have a great potential as ‘magic bullets’ loaded with herbicides, fungicides, nutrients, fertilizers or nucleic acids and targeting specific plant tissues to release their charge to desired part of plant to achieve the desired results. Biotechnological advances and the rapid and more precise diagnosis tools with use of nanomaterials have a great and promising future for the modern agriculture practices like precision delivery of nutrients and fertilizers and disease diagnosis at an early stage.
References
- 1.Auffan M., Rose J., Bottero J.Y., Lowry G.V., Jolivet J.P., Wiesner M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009;4:634–664. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 2.Ghormade V., Deshpande M.V., Paknikar K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011;29:792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Puoci F., Lemma F., Spizzirri U.G., Cirillo G., Curcio M., Picci N. Polymer in agriculture: a review. Am. J. Agri. Biol. Sci. 2008;3:299–314. [Google Scholar]
- 4.Zheng L., Hong F., Lu S., Liu C. Effect of nano-TiO(2) on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005;104:83–91. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]
- 5.De A., Bose R., Kumar A., Mozumdar S. Worldwide pesticide use. In: De A., Bose R., Kumar A., Mozumdar S., editors. Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles. Springer; India: 2014. pp. 5–6. [Google Scholar]
- 6.Tilman D., Cassman K.G., Matson P.A., Naylor R., Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 7.Rai M., Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012;94:287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- 8.Ragaei M., Sabry A.H. Nanotechnology for insect pest control. Int. J. Sci. Environ. Technol. 2014;3:528–545. [Google Scholar]
- 9.André Lévesque C. Molecular methods for detection of plant pathogens-What is the future. Can. J. Plant Pathol. 2001;24:333–336. [Google Scholar]
- 10.Cheng X., Chen G., Rodrigue W.R. Micro- and nanotechnology for viral detection. Anal. Bioanal. Chem. 2009;393:487–501. doi: 10.1007/s00216-008-2514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal P., Duhan J.S., Gahlawat S.K. Biogenesis of nanoparticles: a review. Afr. J. Biotechnol. 2014;13:2778–2785. [Google Scholar]
- 12.Cho K.H., Park J.E., Osaka T., Park S.G. The study of antimicrobial activity and preservateive effects of nanosilver ingredient. Electrochem. Acta. 2005;51:956–960. [Google Scholar]
- 13.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2354. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 14.Tian J., Wong K.K., Ho C.M., Lok C.N., Yu W.Y., Che C.M., Chiu J.F., Tam P.K. Topical delivery of silver nanoparticles promotes wound healing. Chem. Med. Chem. 2007;2:129–136. doi: 10.1002/cmdc.200600171. [DOI] [PubMed] [Google Scholar]
- 15.Prakash P., Gnanaprakasam P., Emmanuel R., Arokiyaraj S., Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B. 2013;108:255–259. doi: 10.1016/j.colsurfb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Oves M., Khan M.S., Zaidi A., Ahmed A.S., Ahmed F., Ahmad E., Sherwani A., Owais M., Azam A. Antibacterial and cytotoxic efficacy of extracellular silver nanoparticles biofabricated from chromium reducing novel OS4 strain of Stenotrophomonas maltophilia. PLoS One. 2013;8:e59140. doi: 10.1371/journal.pone.0059140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra S., Singh B.R., Singh A., Keswani C., Naqvi A.H., Singh H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS One. 2014;9:e97881. doi: 10.1371/journal.pone.0097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali S.M., Yousef N.M.H., Nafady N.A. Application of biosynthesized silver nanoparticles for the control of land snail Eobania vermiculata and some plant pathogenic fungi. J. Nanomater. 2015;2015 (Article ID 218904, 10 pages) [Google Scholar]
- 19.Hojjat S.S. Impact of silver nanoparticles on germinated fenugreek seed. Int. J. Agric. Crop. Sci. 2015;8:627–630. [Google Scholar]
- 20.Takkar P.N., Walker C.D. The distribution and correction of zinc deficiency. In: Robson A.D., editor. Zinc in Soils and Plants, Development in Plant and Soil Science. Kluwer Academic Publishers; Boston: 1993. pp. 151–165. [Google Scholar]
- 21.Land, FAO and plant nutrient management, ProSoil-Problem soil database, Rome, Italy, 2000. Available from: http://www.fao.org/ag/agl/agll/nrdb/links.jsp?lang=en.
- 22.Alloway B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health. 2009;31:537–548. doi: 10.1007/s10653-009-9255-4. [DOI] [PubMed] [Google Scholar]
- 23.Rashid A., Ryan J. Micronutrient constraints to crop production in soils with Mediterranean-type characteristics: a review. J. Plant Nutr. 2004;27:959–975. [Google Scholar]
- 24.Mortvedt J.J. Crop response to level of water-soluble zinc in granular zinc fertilizers. Fert. Res. 1992;33:249–255. [Google Scholar]
- 25.Gangloff W.J., Westfall D.G., Peterson G.A., Mortvedt J.J. Mobility of organic and inorganic zinc fertilizers in soils. Commun. Soil Sci. Plant Anal. 2006;37:199–209. [Google Scholar]
- 26.Xie Y., He Y., Irwin P.L., Jin T., Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011;77:2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia T., Kovochich M., Brant J., Hotze M., Sempf J., Oberley T., Sioutas C., Yeh J.I., Wiesner M.R., Nel A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 28.Ryter S.W., Kim H.P., Hoetzel A., Park J.W., Nakahira K., Wang X., Choi A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 29.Long T.C., Saleh N., Tilton R.D., Lowry G.V., Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006;40:4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 30.Lovric J., Cho S.J., Winnik F.M., Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem. Biol. 2005;12:1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Lewinski N., Colvin V., Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 32.Thwala M., Musee N., Sikhwivhilu L., Wepener V. The oxidative toxicity of Ag and ZnO nanoparticles towards the aquatic plant Spirodela punctuta and the role of testing media parameters. Environ. Sci. Process. Impacts. 2013;15:1830–1843. doi: 10.1039/c3em00235g. [DOI] [PubMed] [Google Scholar]
- 33.Panda S.K., Chaudhury I., Khan M.H. Heavy metals induce lipid peroxidation and affect antioxidants in wheat leaves. Biol. Plantarum. 2003;46:289–294. [Google Scholar]
- 34.Aarti P.D., Tanaka R., Tanaka A. Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol. Plantarum. 2006;128:186–197. [Google Scholar]
- 35.Elumalai K., Velmurugan S., Ravi S., Kathiravan V., Ashokkumar S. Green synthesis of zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;143:158–164. doi: 10.1016/j.saa.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Rajiv P., Rajeshwari S., Venckatesh R. Bio-fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim. Acta Mol. Biomol. Spectrosc. 2013;12:384–387. doi: 10.1016/j.saa.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 37.Bhumi G., Savithramma N. Biological synthesis of zinc oxide nanoparticles from Catharanthus roseus (l.) G. Don. leaf extract and validation for antibacterial activity. Int. J. Drug Dev. Res. 2014;6:208–214. [Google Scholar]
- 38.Sang L., Zhao Y., Burda C. TiO2 nanoparticles as functional building blocks. Chem. Rev. 2014;114:9283–9318. doi: 10.1021/cr400629p. [DOI] [PubMed] [Google Scholar]
- 39.Owolade O., Ogunleti D., Adenekan M. Titanium dioxide affects disease development and yield of edible cowpea. Electron. J. Environ. Agric. Food Chem. 2008;7:2942–2947. [Google Scholar]
- 40.Khodakovskaya M.V., Lahiani M.H. Nanoparticles and plants: from toxicity to activation of growth. In: Sahu S.C., Casciano D.A., editors. Handbook of Nanotoxicology. Nanomedicine and Stem Cell Use in Toxicology; Wiley: 2014. pp. 121–130. [Google Scholar]
- 41.Chen H., Seiber J.N., Hotze M. ACS select on nanotechnology in food and agriculture: a perspective on implications and applications. J. Agric. Food Chem. 2014;62:1209–1212. doi: 10.1021/jf5002588. [DOI] [PubMed] [Google Scholar]
- 42.Pelaez M., Nolan N.T., Pillai S.C., Seery M.K., Falaras P., Kontos A.G., Dunlop P.S., Hamilton J.W., Byrne J.A., O'Shea K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B: Environ. 2012;125:331–349. [Google Scholar]
- 43.S.J. K.S. Yao , K.C. Li , T.C. Tzeng , C.Y. Cheng , C.Y. Chang , C.Y. Chiu , J.J. Liao , Z.P. Lin Hsu . Fluorescence silica nanoprobe as a biomarker for rapid detection of plant pathogens Y. Yin, X. Wang, Multi-Functional Materials and Structures II, Adv. Mater. Res. vol. 79–82 2009; 513-516.
- 44.Santhoshkumar T., Rahuman A.A., Jayaseelan C., Rajakumar G., Marimuthu S., Kirthi A.V., Velayutham K., Thomas J., Venkatesan J., Kim S.K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014;7:968–976. doi: 10.1016/S1995-7645(14)60171-1. [DOI] [PubMed] [Google Scholar]
- 45.Rajakumar G., Abdul Rahuman A., Priyamvada B., Gopiesh Khanna V., Kishore Kumar D., Sujin P.J. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater. Lett. 2012;68:115–117. [Google Scholar]
- 46.Wilson M.A., Tran N.H., Milev A.S., Kannangara G.S.K., Volk H., Lu G.Q.M. Nanomaterials in soils. Geoderma. 2008;146:291–302. [Google Scholar]
- 47.Brady N.R., Weil R.R., editors. Prentice Hall; New Jersey: 1999. The Nature and Properties of Soils; pp. 415–473. [Google Scholar]
- 48.Santoso D., Lefroy R.D.B., Blair G.J. Sulfur and phosphorus dynamics in an acid soil/crop system. Aust. J. Soil Res. 1995;33:113–124. [Google Scholar]
- 49.Park S., Croteau P., Boering K.A., Etheridge D.M., Ferretti D., Fraser P.J., Kim K.-R., Krumme P.B., Langenfelds R.L., Ommen T.D.V., Steele L.P., Trudinger C.M. Trends and seasonal cycles in the isotopic composition of nitrous oxide since 1940. Nat. Geosci. 2012;5:261–265. [Google Scholar]
- 50.Trenkel M.E. Controlled-release and stabilized fertilizers in agriculture. Int. Fert. Ind. Assoc. (Paris) 1997:234–318. [Google Scholar]
- 51.Ombodi A., Saigusa M. Broadcast application versus band application of polyolefin coated fertilizer on green peppers grown on andisol. J. Plant Nutr. 2000;23:1485–1493. [Google Scholar]
- 52.Wu L., Liu M. Preparation and properties of chitosan coated NPK compound fertilizer with controlled release and water-retention. Carbohydr. Polym. 2008;72:240–247. [Google Scholar]
- 53.Corradini E., Moura M.R., Mattoso L.H.C. A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. eXPRESS Polym. Lett. 2010;4:509–515. [Google Scholar]
- 54.Emadian S.E. Texas A & M Univ; college station, TX: 2017. Physiological Responses of Loblolly Pine (Finustaeda L.) to Silicon and Water Stress; pp. 27–37. (Ph.D. Thesis, Diss. Abst.AAC8815865) [Google Scholar]
- 55.Wang L.J., Wang Y.H., Li M., Fan M.S., Zhang F.S., Wu X.M., Yang W.S., Li T.J. Synthesis of ordered biosilica materials. Chin. J. Chem. 2002;20:107–110. [Google Scholar]
- 56.Hutasoit S., Suada I.K., Susrama I.G.K. Antifungal activity test extract some type of marine life link to Aspergillus flavus and Penicillium sp. E J. Trop. Agroecotechnol. 2013;2:27–38. [Google Scholar]
- 57.Shaviv A. Advances in controlled release of fertilizers. Adv. Agron. 2000;71:1–49. [Google Scholar]
- 58.Subramanian K.S., Tarafdar J.C. Prospects of nanotechnology in Indian farming. Ind. J. Agric. Sci. 2011;81:887–893. [Google Scholar]
- 59.Sharmila R.C. Tamil Nadu Agricultural University; Coimbatore, Tamil Nadu, India: 2010. Nutrient Release Pattern of Nano-Fertilizer Formulations. (Ph.D. Thesis) [Google Scholar]
- 60.Hasaneen M.N.A., Abdel-Aziz H.M.M., El-Bialy D.M.A., Omer A.M. Preparation of chitosan nanoparticles for loading with NPK fertilizer. Afr. J. Biotechnol. 2014;13:3158–3164. [Google Scholar]
- 61.Wu Y., Guo J., Yang W., Wang C., Fu S. Preparation and characterization of chitosan-poly(acrylic acid) polymer magnetic microspheres. Polymer. 2006;47:5287–5294. [Google Scholar]
- 62.de Vasconcelos C.L., Bezerril P.M., dos Santos D.E.S., Dantas T.N.C., Pereira M.R., Fonseca J.L.C. Effect of molecular weight and ionic strength on the formation of polyelectrolyte complexes based on poly(methacrylic acid) and chitosan. Biomacromolecules. 2006;7:1245–1252. doi: 10.1021/bm050963w. [DOI] [PubMed] [Google Scholar]
- 63.de Moura M.R., Aouda F.A., Mattoso L.H.C. Preparation of chitosan nanoparticles using methacrylic acid. J. Colloid Interface Sci. 2008;321:477–483. doi: 10.1016/j.jcis.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Wu S.C., Cao Z.H., Li Z.G., Cheung K.C., Wong M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma. 2005;125:155–166. [Google Scholar]
- 65.Jha M.N., Prasad A.N. Efficacy of new inexpensive cyanobacterial biofertilizer including its shelf-life. World J. Microbiol. Biotechnol. 2006;22:73–79. [Google Scholar]
- 66.Vandergheynst J.S., Scher H., Hong-Yun G. Design of formulations for improved biological control agent viability and sequestration during storage. Ind. Biotechnol. 2006;2:213–219. [Google Scholar]
- 67.Vandergheynst J.S., Scher H.B., Gou H.Y., Schultz D.L. Water in oil emulsions that improve the storage and delivery of the biolarvacide Lagenidium giganteum. Biol. Control. 2007;52:207–229. [Google Scholar]
- 68.Dikshit A., Shukla S.K., Mishra R.K. Lap Lambert Academic Publishing; Germany: 2013. Exploring Nanomaterials with PGPR in Current Agricultural Scenario. [Google Scholar]
- 69.Shukla S.K., Kumar R., Mishra R.K., Pandey A., Pathak A., Zaidi M., Srivastava S.K., Dikshit A. Prediction and validation of gold nanoparticles (GNPs) on plant growth promoting rhizobacteria (PGPR): A step toward development of nano-biofertilizers. Nano. Rev. 2015;4:439–448. [Google Scholar]
- 70.Sunkar S., Valli Nachiyar C. Microbial synthesis and characterization of silver nanoparticles using the endophytic bacterium Bacillus cereus: A novel source in the benign synthesis. Glob. J. Med. Res. 2012;12:43–49. [Google Scholar]
- 71.El-Shanshoury A.E.R.R., ElSilk S.E., Ebeid M.E. Extracellular biosynthesis of silver nanoparticles using Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633 and Streptococcus thermophilus ESh1 and their antimicrobial activities. Nanotechnology. 2011;10:1–7. [Google Scholar]
- 72.Jha A.K., Prasad K. Biosynthesis of metal and nanoparticles using Lactobacilli from yoghurt and probiotic spore tablets. Biotechnol. J. 2010;5:285–291. doi: 10.1002/biot.200900221. [DOI] [PubMed] [Google Scholar]
- 73.Zhang H., Li Q., Lu Y., Sun D., Lin X., Deng X. Biosorption and bioreduction of diamine silver complex by Corynebacterium. J. Chem. Technol. Biotechnol. 2005;80:285–290. [Google Scholar]
- 74.Malarkodi C., Rajeshkumar S., Vanaja M., Paulkumar K., Gnanajobitha G., Annadurai G. Eco-friendly synthesis and characterization of gold nanoparticles using Klebsiella pneumonia. J. Nanostr. Chem. 2013;3:2013. [Google Scholar]
- 75.Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B. 2003;28:313–318. [Google Scholar]
- 76.Bhainsa K.C., D‘Souza S.F. Extracellular biosynthesis of silver nanoparticles using fungus Aspergillus fumigatus. Colloids Surf. B. 2006;47:160–164. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 77.Alloway B.J. Micronutrients and crop production: an introduction. In: Alloway B.J., editor. Micronutrient Deficiencies in Global Crop Production. Springer; 2008. pp. 1–39. [Google Scholar]
- 78.Martens D.C., Westermann D.T. Fertilizer applications for correcting micronutrient deficiencies. In: Alloway B.J., editor. Micronutrient Deficiencies in Global Crop Production. Springer; 1991. pp. 549–553. [Google Scholar]
- 79.Peteu S.F., Oancea F., Sicuia O.A., Constantinescu F., Dinu S. Responsive polymers for crop protection. Polymer. 2010;2:229–251. [Google Scholar]
- 80.Tao S., Pang R., Chen C., Ren X., Hu S. Synthesis, characterization and slow release properties of O-naphthylacetyl chitosan. Carbohydr. Polym. 2012;88:1189–1194. [Google Scholar]
- 81.Bakhtiari M., Moaveni P., Sani B. The effect of iron nanoparticles spraying time and concentration on wheat. Biol. Forum Int. J. 2015;7:679–683. [Google Scholar]
- 82.Ghafariyan M.H., Malakouti M.J., Dadpour M.R., Stroeve P., Mahmoudi M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 2013;47:10645–10652. doi: 10.1021/es402249b. [DOI] [PubMed] [Google Scholar]
- 83.Delfani M., Firouzabadi M.B., Farrokhi N., Makarian H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 2014;45:530–540. [Google Scholar]
- 84.Hoagland D.R., Arnon D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stat. Circ. 1950;347:1–32. [Google Scholar]
- 85.Pradhan S., Patra P., Das S., Chandra S., Mitra S., Dey K.K. Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular biochemical, and biophysical study. Environ. Sci. Technol. 2013;47:13122–13131. doi: 10.1021/es402659t. [DOI] [PubMed] [Google Scholar]
- 86.Mahajan P., Dhoke S.K., Khanna A.S. Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 2011;7:1–7. [Google Scholar]
- 87.Yang F.L., Li S.G., Zhu F., Lei C.L. Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: tenebrionidae) J. Agric. Food Chem. 2009;57:10156–10162. doi: 10.1021/jf9023118. [DOI] [PubMed] [Google Scholar]
- 88.Goswami A., Roy I., Sengupta S., Debnath N. Novel applications of solid and liquid formulations of nanoparticles against insect pests and pathogens. Thin Solid Films. 2010;519:1252–1257. [Google Scholar]
- 89.Das S., Bhattacharya A., Debnath N., Datta A., Goswami A. Nanoparticle-induced morphological transition of Bombyx mori nucleopolyhedrovirus: a novel method to treat silkworm grasserie disease. Appl. Microbiol. Biotechnol. 2013;97:6019–6030. doi: 10.1007/s00253-013-4868-z. [DOI] [PubMed] [Google Scholar]
- 90.Teodoro S., Micaela B., David K.W. Novel use of nano-structured alumina as an insecticide. Pest. Manag. Sci. 2010;66:577–579. doi: 10.1002/ps.1915. [DOI] [PubMed] [Google Scholar]
- 91.Debnath N., Das S., Seth D., Chandra R., Bhattacharya S.C., Goswami A. Entomotoxic effect of silica nanoparticles against Sitophilus oryzae (L.) J. Pest. Sci. 2011;84:99–105. [Google Scholar]
- 92.Scrinis G., Lyons K. The emerging nano-corporate paradigm nanotechnology and the transformation of nature, food and agrifood systems. Int. J. Sociol. Agric. Food. 2007;15:22–44. [Google Scholar]
- 93.Shyla K.K., Natarajan N., Nakkeeran S. Antifungal activity of zinc oxide, silver and titanium dioxide nanoparticles against Macrophomina phaseolina. J. Mycol. Plant Pathol. 2014;44:268–273. [Google Scholar]
- 94.Suriyaprabha R., Karunakaran G., Kavitha K., Yuvakkumar R., Rajendran V., Kannan N. Application of silica nanoparticles in maize to enhance fungal resistance. IET Nanobiotechnol. 2014;8:133–137. doi: 10.1049/iet-nbt.2013.0004. [DOI] [PubMed] [Google Scholar]
- 95.Velmurugan N., Gnana Kumar G., Sub Han S. Synthesis and characterization of potential fungicidal silver nano-sized particles and chitosan membrane containing silver particles. Iran. Polym. J. 2009;18:383–392. [Google Scholar]
- 96.Pal S., Tak Y.K., Song J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panáček A., Kvítek L., Prucek R., Kolář M., Večeřová R., Pizúrová N., Sharma V.K., Nevecna T., Zboril R. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 98.Karimi N., Minaei S., Almassi M., Shahverdi A.R. Application of silver nano-particles for protection of seeds in different soils. Afr. J. Agric. Res. 2012;7:863–1869. [Google Scholar]
- 99.Kim S.W., Kim K.S., Lamsal K., Kim Y.J., Kim S.B., Jung M., Sim S.J., Kim H.S., Chang S.J., Kim J.K., Lee Y.S. An in vitro study of the antifungal effect of silver nanoparticles on oak wilt pathogen Raffaelea sp. J. Microbiol. Biotechnol. 2009;19:760–764. [PubMed] [Google Scholar]
- 100.Mishra S., Singh B.R., Singh A., Keswani C., Naqvi A.H., Singh H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS One. 2014;9:e97881. doi: 10.1371/journal.pone.0097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jo Y.K., Kim B.H., Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009;93:1037–1043. doi: 10.1094/PDIS-93-10-1037. [DOI] [PubMed] [Google Scholar]
- 102.Kim S.W., Jung J.H., Lamasal K., Kim Y.S., Min J.S., Lee Y.S. Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology. 2012;40:53–58. doi: 10.5941/MYCO.2012.40.1.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abdelmalek G.M.A., Salaheldin T.A. Silver nanoparticles as a potent fungicide for Citrus phytopathogenic fungi. J. Nanomed. Res. 2016;3:00065. [Google Scholar]
- 104.Mishra S., Singh B.R., Singh A., Keswani C., Naqvi A.H., Singh H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS One. 2014;9:e97881. doi: 10.1371/journal.pone.0097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xue B., He D., Gao S., Wang D., Yokoyama K., Wang L. Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016;11:1899–1906. doi: 10.2147/IJN.S98339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramyadevi J., Jeyasubramanian K., Marikani A., Rajakumar G., Rahuman A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012;71:114–116. [Google Scholar]
- 107.Kanhed P., Birla S., Gaikwad S., Gade A., Seabra A.B., Rubilar O., Durane N., Rai M. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014;115:13–17. [Google Scholar]
- 108.Bramhanwade K., Shende S., Bonde S., Gade A., Rai M. Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environ. Chem. Lett. 2016;14:229–235. [Google Scholar]
- 109.Viet P.V., Nguyen H.T., Cao T.M., Hieu L.V. Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method. J. Nanomater. 2016;2016 (Article ID 1957612, 7 pages) [Google Scholar]
- 110.Saharan V., Mehrotra A., Khatik R., Rawal P., Sharma S.S., Pal A. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013;62:677–683. doi: 10.1016/j.ijbiomac.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 111.Patra P., Mitra S., Debnath N., Goswami A. Biochemical-, Biophysical-, and microarray-based antifungal evaluation of the buffer-mediated synthesized nano zinc oxide: an in vivo and in vitro toxicity study. Langmuir. 2012;28:16966–16978. doi: 10.1021/la304120k. [DOI] [PubMed] [Google Scholar]
- 112.Choudhury S.R., Ghosh M., Mandal A., Chakravorty D., Pal M., Pradhan S., Goswami A. Surface-modified sulfur nanoparticles: an effective antifungal agent against Aspergillus niger and Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2011;90:733–743. doi: 10.1007/s00253-011-3142-5. [DOI] [PubMed] [Google Scholar]
- 113.He L., Liu Y., Mustapha A., Lin M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011;166:207–215. doi: 10.1016/j.micres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 114.Jasim N.O. Antifungal activity of Zinc oxide nanoparticles on Aspergillus fumigatus fungus & Candida albicans yeast. J. Nat. Sci. Res. 2015;5:23–27. [Google Scholar]
- 115.Yehia R.S., Ahmed O.F. In vitro study of the antifungal efficacy of zinc oxide nanoparticles against Fusarium oxysporum and Penicilium expansum. Afr. J. Microbiol. Res. 2013;7:1917–1923. [Google Scholar]
- 116.Pérez-de-Luque A., Rubiales D. Nanotechnology for parasitic plant control. Pest Manag. Sci. 2009;65:540–545. doi: 10.1002/ps.1732. [DOI] [PubMed] [Google Scholar]
- 117.Kumar S., Bhanjana G., Sharma A., Sarita, Sidhu M.C., Dilbaghi N. Herbicide loaded carboxymethyl cellulose nanocapsules as potential carrier in agrinanotechnology. Sci. Adv. Mater. 2015;7:1143–1148. [Google Scholar]
- 118.Chinnamuthu C., Boopathi P.M. Nanotechnology and agroecosystem. Madras Agric. J. 2009;96:17–31. [Google Scholar]
- 119.Satapanajaru T., Anurakpongsatorn P., Pengthamkeerati P., Boparai H. Remediation of atrazine-contaminated soil and water by nano zerovalent iron. Water Air Soil Poll. 2008;192:349–359. [Google Scholar]
- 120.Nair R., Varghese S.H., Nair B.G., Maekawa T., Yoshida Y., Kumar D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. [Google Scholar]
- 121.Ali M.A., Rehman I., Iqbal A., Din S., Rao A.Q., Latif A., Samiullah T.R., Azam S., Husnain T. Nanotechnology: a new frontier in agriculture. Adv. Life Sci. 2014;1:129–138. [Google Scholar]
- 122.Clemente Z., Grillo R., Jonsson M., Santos N.Z., Feitosa L.O., Lima R. Ecotoxicological evaluation of poly(ε-caprolactone) nanocapsules containing triazine herbicides. J. Nanosci. Nanotechnol. 2014;14:4911–4917. doi: 10.1166/jnn.2014.8681. [DOI] [PubMed] [Google Scholar]
- 123.Namasivayam S.K.R., Aruna A. Gokila Evaluation of silver nanoparticles-chitosan encapsulated synthetic herbicide paraquate (AgNp-CS-PQ) preparation for the controlled release and improved herbicidal activity against Eichhornia crassipes. Res. J. Biotechnol. 2014;9:19–27. [Google Scholar]
- 124.Konotop Y.O., Kovalenko M.S., Ulynets V.Z., Meleshko A.O., Batsmanova L.M., Taran N.Y. Phytotoxicity of colloidal solutions of metal-containing nanoparticles. Cytol. Genet. 2014;48:99–102. [PubMed] [Google Scholar]
- 125.Musante C., White J.C. Toxicity of silver and copper to Cucurbita pepo: Differential effects of nano and bulk-size particles. Environ. Toxicol. 2012;27:510–517. doi: 10.1002/tox.20667. [DOI] [PubMed] [Google Scholar]
- 126.Hawthorne J., Musante C., Sinha S.K., White J.C. Accumulation and phytotoxicity of engineered nanoparticles to Cucurbita pepo. Int. J. Phytorem. 2012;14:429–442. doi: 10.1080/15226514.2011.620903. [DOI] [PubMed] [Google Scholar]
- 127.Atha D.H., Wang H., Petersen E.J., Cleveland D., Halbrook R.D., Jaruga P., Dizdaroglu M., Xing B., Nelson B.C. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 2012;46:1819–1827. doi: 10.1021/es202660k. [DOI] [PubMed] [Google Scholar]
- 128.Lee S., Chung H., Kim S., Lee I. The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water Air Soil Poll. 2013;224:1668. [Google Scholar]
- 129.Nekrasova G.F., Ushakova O.S., Ermakov A.E., Uimin M.A., Byzov I.V. Effects of copper(II) ions and copper oxide nanoparticles on Elodea densa Planch, Russ. J. Ecol. 2011;42:458–463. [Google Scholar]
- 130.Kim S., Lee S., Lee I. Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut. 2012;223:2799–2806. [Google Scholar]
- 131.Cioffi N., Torsi L., Ditaranto N., Sabbatini L., Zambonin P.G., Tantillo G., Ghibelli L., D’Alessio M., Bleve-Zacheo T., Traversa E. Antifungal activity of polymer-based copper nano-composite coatings. Appl. Phys. Lett. 2004;85:2417–2419. [Google Scholar]
- 132.R. Kumar, G.C. Pandey, H.M. Mamrutha, N.R. Nagaraja, K. Venkatesh, http://krishisewa.com/articles/soil-fertility/600-tools-for-nitrogenmanagement.html. (Accessed 17 October, 2016).
- 133.Rai V., Acharya S., Dey N. Implications of nanobiosensors in agriculture. J Biomater Nanobiotchnol. 2012;3:315–324. [Google Scholar]
- 134.Jones P.B.C. Information systems for Biotechnology; Blacksburg, VA: 2006. A Nanotech Revolution in Agriculture and the Food Industry. Available from: http://www.isb.vt.edu/articles/jun0605.html. (Accessed 07 November, 2016) [Google Scholar]
- 135.Brock D.A., Douglas T.E., Queller D.C., Strassmann J.E. Primitive agriculture in a social amoeba. Nature. 2011;469:393–396. doi: 10.1038/nature09668. [DOI] [PubMed] [Google Scholar]
- 136.da Silva A.C.N., Deda D.K., da Róz A.L., Prado R.A., Carvalho C.C., Viviani V., Leite F.L. Nanobiosensors based on chemically modified AFM probes: a useful tool for metsulfuron-methyl detection. Sensors (Basel) 2013;13:1477–1489. doi: 10.3390/s130201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Otles S., Yalcin B. Nano-biosensors as new tool for detection of food quality and safety. Log Forum. 2010;6:67–70. [Google Scholar]
- 138.Yu G.X., Wu W.X., Zhao Q., Wei X.Y., Lu Q. Efficient immobilization of acetylcholinesterase onto amino functionalized carbon nanotubes for the fabrication of high sensitive organophosphorus pesticides biosensors. Biosens. Bioelectron. 2015;68:288–294. doi: 10.1016/j.bios.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 139.Yan J.X., Guan H.N., Yu J., Chi D.F. Acetylcholinesterase biosensor based on assembly of multiwall carbon nanotubes onto liposome bioreactors for detection of organophosphates pesticides. Pest Biochem. Physiol. 2013;105:197–202. [Google Scholar]
- 140.Sun X., Zhai C., Wang X.Y. A novel and highly sensitive acetyl-cholinesterase biosensor modified with hollow gold nanospheres. Bioprocess Biosyst. Eng. 2013;36:273–283. doi: 10.1007/s00449-012-0782-5. [DOI] [PubMed] [Google Scholar]
- 141.Dong J., Zhao H., Qiao F., Liu P., Wang X.D., Ai S.Y. Quantum dot immobilized acetylcholinesterase for the determination of organophosphate pesticides using graphene-chitosan nanocomposite modified electrode. Anal. Methods. 2013;5:2866–2872. [Google Scholar]
- 142.Viswanathan S., Radecka H., Radecki J. Electrochemical biosensor for pesticides based on acetylcholinesterase immobilized on polyaniline deposited on vertically assembled carbon nanotubes wrapped with ssDNA. Biosens. Bioelectron. 2009;24:2772–2777. doi: 10.1016/j.bios.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 143.Sekhon B.S. Nanotechnology in agri-food production: an overview. Nanotechnol. Sci. Appl. 2014;7:31–53. doi: 10.2147/NSA.S39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dasary S.S.R., Rai U.S., Yu H.T., Anjaneyulu Y., Dubey M., Ray P.C. Gold nanoparticle based surface enhanced fluorescence for detection of organophosphorus agents. Chem. Phys. Lett. 2008;460:187–190. doi: 10.1016/j.cplett.2008.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Warad H.C., Ghosh S.C., Thanachayanont C., Dutta J., Hilborn J.G. Highly luminescent manganese doped ZnS quantum dots for biological labeling. Proc. Int. Conf. on Smart Materials/Intelligent Materials (SmartMat ‘04); Chiang Mai, Thailand, 1–3 December; 2004. pp. 203–206. [Google Scholar]
- 146.Rad F., Mohsenifar A., Tabatabaei M., Safarnejad M.R., Shahryari F., Safarpour H., Foroutan A., Mardi M., Davoudi D., Fotokian M. Detection of Candidatus Phytoplasma aurantifolia with a quantum dots fret-based biosensor. J. Plant Pathol. 2012;94:525–534. [Google Scholar]
- 147.Safarpour H., Safarnejad M.R., Tabatabaei M., Mohsenifar A., Rad F., Basirat M., Shahryari F., Hasanzadeh F. Development of a quantum dots FRET-based biosensor for efficient detection of Polymyxa betae. Can. J. Plant Pathol. 2012;34:507–515. [Google Scholar]
- 148.Zheng Z., Zhou Y., Li X., Liu S., Tang Z. Highly-sensitive organophosphorous pesticide biosensors based on nanostructured films of acetylcholinesterase and CdTe quantum dots. Biosens. Bioelectron. 2011;26:3081–3085. doi: 10.1016/j.bios.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 149.Chouhan R.S., Vinayaka A.C., Thakur M.S. Thiol-stabilized luminescent CdTe quantum dot as biological fluorescent probe for sensitive detection of methyl parathion by a fluoro-immune chromatographic technique. Anal. Bioanal. Chem. 2010;397:1467–1475. doi: 10.1007/s00216-009-3433-1. [DOI] [PubMed] [Google Scholar]
- 150.Ge S., Lu J., Ge L., Yan N., Yu J. Development of a novel deltamethrin sensor based on molecularly imprinted silica nanospheres embedded CdTe quantum dots. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011;79:1704–1709. doi: 10.1016/j.saa.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 151.Branton D., Deamer D.W., Marziali A., Bayley H., Benner S.A., Butler T., Di Ventra M., Garaj S., Hibbs A., Huang X., Jovanovich S.B., Krstic P.S., Lindsay S., Ling X.S., Mastrangelo C.H., Meller A., Oliver J.S., Pershin Y.V., Ramsey J.M., Riehn R., Soni G.V., Tabard-Cossa V., Wanunu M., Wiggin M., Schloss J.A. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 2008;10:1146–1153. doi: 10.1038/nbt.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.http://www.bccresearch.com/market-research/nanotechnology/2011nanotechnologyreview-nan047c.html. (Accessed 20 October 2016).
- 153.Hooley G., Piercy N.F., Nicoulaud B. Prentice Hall/Financial Times; London: 2012. Marketing Strategy and Competitive Positioning. (ISBN 9780273740933) [Google Scholar]
- 154.Hirsh S., Schiefer J., Gschwandtner A., Hartmann M. The determinants of firm profitability differences in EU food processing. J. Agric. Econ. 2014;65:703–721. [Google Scholar]
- 155.Banterle A., Cavaliere A., Carraresi L., Stranieri S. Food SMEs face increasing competition in the EU market: marketing management capability is a tool for becoming a price maker. Agribusiness. 2014;30:113–131. [Google Scholar]
- 156.Sodano V., Verneau F. Competition policy and food sector in the European Union. J. Int. Food Agribusiness Mark. 2014;26:155–172. [Google Scholar]
- 157.http://www.bccresearch.com/market-research/nanotechnology/2014-nanotechnology-research-review-report-nan047f.html. (Accessed 20 October, 2016).
- 158.Raliya R., Nair R., Chavalmane S., Wang W.N., Biswas P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics. 2015;12:1584–1594. doi: 10.1039/c5mt00168d. [DOI] [PubMed] [Google Scholar]
- 159.Park H.M., Li X., Jin C.Z., Park C.Y., Cho W.J., Ha C.S. Preparation and properties of biodegradable thermoplastic starch/clay hybrids. Macromol. Mater. Eng. 2002;287:553–558. [Google Scholar]
- 160.Jia L.R., Xia J., Zhou N., Chen W.Y. Preservation of fruits by hydrolyzed collagen/sodium alginate nanoparticles latex. Food Mach. 2008;1:46–50. [Google Scholar]
- 161.Milani N., Hettiarachchi G.M., Kirby J.K., Beak D.G., Stacey S.P., McLaughlin M.J. Fate of zinc oxide nanoparticles coated onto macronutrient fertilizers in an alkaline calcareous soil. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Prasad R., Kumar V., Prasad K.S. Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr. J. Biotechnol. 2014;13:705–713. [Google Scholar]
- 163.Anjali C.H., Sharma Y., Mukherjee A., Chandrasekaran N. Neem oil (Azadirachta indica) nanoemulsion-a potent larvicidal agent against Culex quinquefasciatus. Pest Manag. Sci. 2012;68:158–163. doi: 10.1002/ps.2233. [DOI] [PubMed] [Google Scholar]
- 164.http://www.geohumus.com/us/products.html. (Accessed 20 October, 2016).
- 165.Vamvakaki V., Chaniotakis N.A. Pesticide detection with a liposome-based nano-biosensor. Biosens. Bioelectron. 2007;22:2848–2853. doi: 10.1016/j.bios.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 166.Kumar S., Chauhan N., Gopal M., Kumar R., Dilbaghi N. Development and evaluation of alginate-chitosan nanocapsules for controlled release of acetamiprid. Int. J. Biol. Macromol. 2015;81:631–637. doi: 10.1016/j.ijbiomac.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 167.Tripathi S., Sarkar S. Influence of water soluble carbon dots on the growth of wheat plant. Appl. Nanosci. 2014;5:609–616. [Google Scholar]
- 168.Lahiani M.H., Dervishi E., Chen J., Nima Z., Gaume A., Biris A.S., Khodakovskaya M.V. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces. 2013;5:7965–7973. doi: 10.1021/am402052x. [DOI] [PubMed] [Google Scholar]
- 169.Kerfahi D., Tripathi B.M., Singh D., Kim H., Lee S., Lee J., Adams J.M. Effects of functionalized and raw multi-walled carbon nanotubes on soil bacterial community composition. PLoS One. 2015;10(2015):e0123042. doi: 10.1371/journal.pone.0123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Boonyanitipong P., Kositsup B., Kumar P., Baruah S., Dutta J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinforma. 2011;1:282–285. [Google Scholar]
- 171.Chai H., Yao J., Sun J., Zhang C., Liu W., Zhu M., Ceccanti B. The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull. Environ. Contam. Toxicol. 2015;94:490–495. doi: 10.1007/s00128-015-1485-9. [DOI] [PubMed] [Google Scholar]
- 172.Cherchi C., Gu A.Z. Impact of titanium dioxide nanomaterials on nitrogen fixation rate and intracellular nitrogen storage in Anabaena variabilis. Environ. Sci. Technol. 2010;44:8302–8307. doi: 10.1021/es101658p. [DOI] [PubMed] [Google Scholar]
- 173.Feizi H., Moghaddam P.R., Shahtahmassebi N., Fotovat A. Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol. Trace Elem. Res. 2012;146:101–106. doi: 10.1007/s12011-011-9222-7. [DOI] [PubMed] [Google Scholar]
- 174.Lin D., Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 175.Dimkpa C.O., McLean J.E., Martineau N., Britt D.W., Haverkamp R., Anderson A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013;47:1082–1090. doi: 10.1021/es302973y. [DOI] [PubMed] [Google Scholar]
- 176.El-Temsah Y.S., Joner E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012;27:42–49. doi: 10.1002/tox.20610. [DOI] [PubMed] [Google Scholar]
- 177.Kumari M., Mukherjee A., Chadrasekaran N. Genotoxicity of silver nanoparticle in Allium cepa. Sci. Total. Environ. 2009;407:5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 178.Stampoulis D., Sinha S.K., White J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009;43:9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- 179.Lee W.M., An Y.J., Yoon H., Kwbon H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestrivum): plant agar test for water-insoluble nanoparticles. Environ. Toxico. Chem. 2008;27:1915–1921. doi: 10.1897/07-481.1. [DOI] [PubMed] [Google Scholar]
- 180.Yang L., Watts D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxico. Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 181.Lopez-Moreno M.L., De La Rosa G., Hernandez-Viezcas J.A., Peralta-Videa J.R., Gardea-Torresdey J.L. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food. Chem. 2010;58:3689–3693. doi: 10.1021/jf904472e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Barrena R., Casals E., Colon J., Font X., Sanchez A., Puntes V. Evaluation of the ecotoxicity of model nanoparticles. Chemotherapy. 2009;75:850–857. doi: 10.1016/j.chemosphere.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 183.Morales M.I., Rico C.M., Hernandez-Viezcas J.A., Nunez J.E., Barrios A.C., Tafoya A., Flores-Marges J.P., Peralta-Videa J.R., Gardea-Torresdey J.L. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J. Agric. Food Chem. 2013;61:6224–6230. doi: 10.1021/jf401628v. [DOI] [PubMed] [Google Scholar]
- 184.Jasim B., Thomas R., Mathew J., Radhakrishnan E.K. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.) Saudi Pharm. J. 2016 doi: 10.1016/j.jsps.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Prasad T.N.V.K.V., Sudhakar P., Sreenivasulu Y., Latha P., Munaswamy V., Reddy K.R., Sreeprasad T.S., Sajanlal P.R., Pradeep T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012;35:905–927. [Google Scholar]
- 186.Raliya R., Tarafdar J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.) Agric. Res. 2013;2:48–57. [Google Scholar]
- 187.Siddiqui M.H., Al-Whaibi M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.) Saudi J. Biol. Sci. 2014;21:13–17. doi: 10.1016/j.sjbs.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]