Abstract
Purpose
To identify factors related to who undergoes a prostate biopsy in a screened population and to estimate the impact of biopsy verification on risk factor–prostate cancer associations.
Patients and Methods
Men who were screened regularly from the placebo arms of two large prostate cancer prevention trials (Prostate Cancer Prevention Trial [PCPT] and Selenium and Vitamin E Cancer Prevention Trial [SELECT]) were examined to define incident prostate cancer cohorts. Because PCPT had an end-of-study biopsy, prostate cancer cases were categorized by a preceding prostate-specific antigen/digital rectal examination prompt (yes/no) and noncases by biopsy-proven negative status (yes v no). We estimated the association of risk factors (age, ethnicity, family history, body mass index, medication use) with prostate cancer and quantified differences in risk associations across cohorts.
Results
Men 60 to 69 years of age, those with benign prostatic hyperplasia, and those with a family history of prostate cancer were more likely, and those with a higher body mass index (≥ 25), diabetes, or a smoking history were less likely, to undergo biopsy, adjusting for age and longitudinal prostate-specific antigen and digital rectal examination. Medication use, education, and marital status also influenced who underwent biopsy. Some risk factor estimates for prostate cancer varied substantially across cohorts. Black (v other ethnicities) had odds ratios (ORs) that varied from 1.20 for SELECT (community screening standards, epidemiologic-like cohort) to 1.83 for PCPT (end-of-study biopsy supplemented with imputed end points). Statin use in SELECT provided an OR of 0.65 and statin use in in PCPT provided an OR of 0.99, a relative difference of 34%.
Conclusion
Among screened men enrolled in prostate cancer prevention trials, differences in risk factor estimates for prostate cancer likely underestimate the magnitude of bias found in other cohorts with varying screening and biopsy recommendations and acceptance. Risk factors for prostate cancer derived from epidemiologic studies not only may be erroneous but may lead to misdirected research efforts.
INTRODUCTION
Prostate cancer is ubiquitous in aging men. Taking the lives of approximately 27,500 men annually, it is the second most common cause of cancer death in men.1 Strategies to control prostate cancer include prevention, screening, and improved treatment. Because of the prevalence of prostate cancer in the general population and the lower rate of high-risk, high-grade cancer, cancer prevention and early detection must incorporate disease risk factors into implementation strategies.
Risk factors are often identified in epidemiologic studies, and conclusions are then implemented, population-wide, without confirmatory trials.2 Challenges to epidemiologic studies include the high prevalence of biopsy-detectable prostate cancer in aging men and the fact that prostate cancer is usually asymptomatic until metastatic. As a result, if a risk factor, even one unassociated with prostate cancer, is incorporated into clinical practice, it will be found to increase cancer risk because men with the risk factor will be more likely to undergo screening; thereafter, screen-positive men will be more likely to be recommended for and undergo biopsy. Ultimately, men with the risk factor will then be more likely to be diagnosed with prostate cancer.
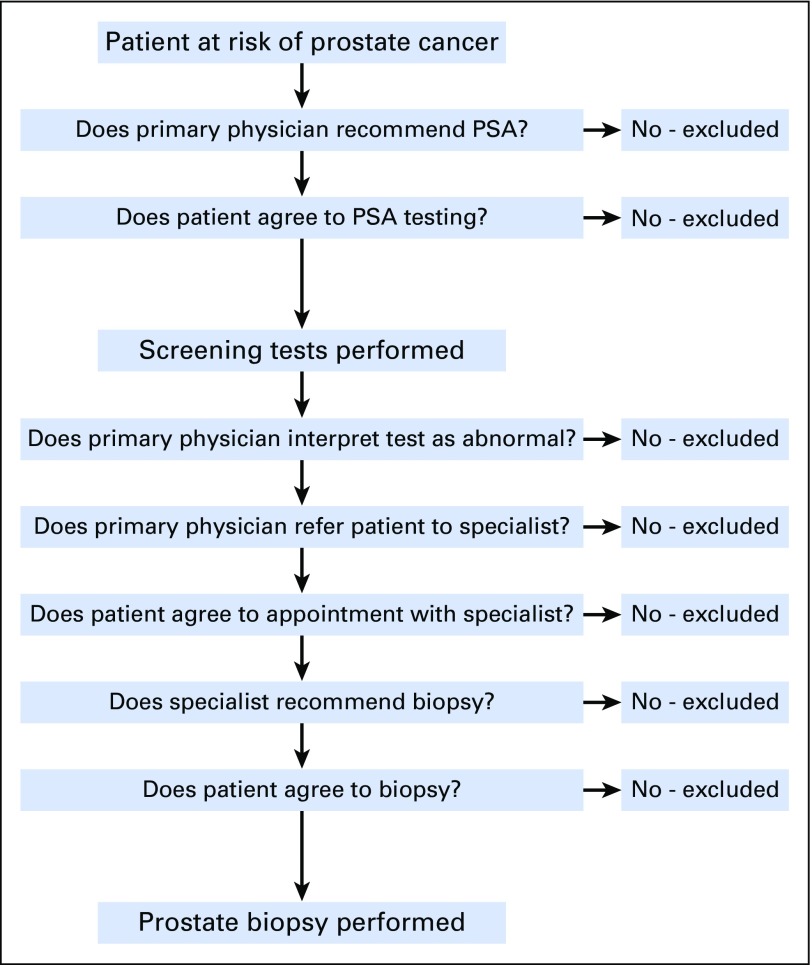
Figure 1 represents the numerous steps necessary for a man to be detected with prostate cancer in a screening setting. In the absence of any one of these steps, the diagnosis might not be made.
Fig 1.
Steps required for a man to undergo a prostate biopsy. PSA, prostate-specific antigen.
Using two large, prospective, cancer prevention trials, in one of which intensive disease ascertainment was conducted, we first sought to identify the factors related to which men are more likely to undergo prostate biopsy and second, to explore the impact of biopsy detection bias on commonly reported prostate cancer risk factors.
PATIENTS AND METHODS
The Prostate Cancer Prevention Trial (PCPT) included an end-of-study (EOS) biopsy for all men regardless of prostate-specific antigen (PSA) levels or digital rectal examination (DRE), thereby minimizing detection bias. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) incorporated community standards for screening and biopsy; there were no study requirements for biopsy. However, longitudinal PSA and DRE data, information about conducted biopsies, and prostate cancer diagnoses were collected. We defined cohorts across the two trials allowing comparisons of odds ratios (ORs). Details of the four cohorts follow. The comparisons provide insights into the impact of biopsy detection bias on prostate cancer risk estimates.
PCPT
PCPT randomly assigned 18,880 eligible men to placebo or finasteride, between 1994 and 1997, with a primary end point of period prevalence of prostate cancer after 7 years. Details of trial design and outcomes are published elsewhere.3-5 Men 55 years of age or older with a normal DRE, PSA level ≤ 3.0 ng/mL, no clinically significant coexisting conditions, and an American Urologic Association symptom score < 20 were randomly assigned. Participants were screened annually by centrally monitored PSA level and with DRE. Those with a PSA level ≥ 4.0 ng/mL were recommended for biopsy, and recommendations were followed up for outcomes. After 7 years, participants not diagnosed with prostate cancer were requested to undergo an EOS biopsy regardless of PSA level or DRE results. A biopsy-assessed end point was anticipated for 60% of participants, and that rate was attained.4,5
Because roughly one half of prostate cancers were identified at the EOS biopsy at 7 years, a time-to-event analysis was not appropriate for evaluating risk factor associations with biopsy or prostate cancer. Instead, logistic regression was used to evaluate the period prevalence of prostate cancer at 7 years. For PCPT analyses, we included only those men randomly assigned to the placebo arm because finasteride reduced prostate cancer risk. We defined three subsets of men from PCPT:
PCPT cohort 1 (n = 8,052): Observational cohort with screening and biopsy recommendations. Men with either a for-cause prostate cancer diagnosis or those who survived the 7 years of the trial were included regardless of whether they had an EOS biopsy. The outcome for this group was prostate cancer detected for cause (ie, elevated PSA level ≥ 4.0 ng/mL or abnormal DRE, biopsy centrally prompted) within 7 years of study entry. To mimic a typical observational cohort study, EOS prostate cancers (normal PSA level and DRE) were considered noncases.
PCPT cohort 2 (n = 5,823): More complete disease ascertainment. Men who had a known prostate cancer end point were included: a prostate cancer diagnosis either for cause or at EOS, and men with a negative EOS biopsy at 7 years; thus, both cases and control subjects were biopsy proven (primary PCPT analysis approach).5 Results from cohort 2 analysis are in the Appendix, online only.
PCPT cohort 3 (n = 5,823): Estimate of true cancer rate. We developed a multivariate logistic regression model to predict who had a study end point, and then used the inverse of the predicted probability of having an end point and assigned weights to the cohort 2 observations to account for those who did not have an end point. Inverse probability weighting (IPW) methods similar to those described in Redman et al6 were used. Details on IPW methodology are included in the Appendix.
SELECT
SELECT was a phase III randomized trial testing whether selenium and vitamin E alone and/or in combination prevent prostate cancer. From 2001 to 2004, 35,533 men (34,888 analyzable) were randomly assigned to receive vitamin E, selenium, both active agents, or two placebos. SELECT enrolled men age 55 years or older (50 years or older for blacks), with no previous prostate cancer diagnosis, PSA level ≤ 4 ng/mL, and a normal DRE. At the time of study closure in 2008, median follow-up was 5.5 years; trial design and results have been published previously.2,7 For these analyses, we included only those participants (n = 8,228) randomly assigned to both placebo supplements, to avoid confounding by study supplementation, and excluded those enrolled previously in PCPT. Unlike in PCPT, prostate cancer screening was not required in SELECT; participants underwent annual PSA assessment and DRE and received biopsy recommendations according to local standard of care and the participant’s preference. Each year, approximately 85% of SELECT participants had PSA testing, and 70% underwent DRE. Although the data are from a randomized clinical trial, the SELECT cohort more resembles the men who would typically be seen in an observational cohort and more closely approximates biopsy practices seen in the general population. To make the SELECT cohort as comparable as possible to the PCPT cohort, we used a dichotomous prostate cancer outcome over a 5-year period (period prevalence) with the cutoff chosen to maximize the inclusion of participants despite their having 2 fewer years of follow-up (n = 6,276) than those in PCPT.
All participants provided written informed consent in accordance with institutional and federal guidelines.
Analysis Methods
To identify factors that may be related to receipt of prostate biopsy, but not necessarily prostate cancer, associations of covariates with time to biopsy in SELECT were estimated using Cox regression models. This analysis allowed us to include a broader cohort of SELECT participants regardless of follow-up duration. Time was defined from date of random assignment to first biopsy, regardless of cancer outcome. Participants were censored at last contact date if no biopsy was performed. Associations of potential risk factors with time to first biopsy were adjusted for age at study entry, and the Cox model allowed us to use PSA level and DRE status as time-dependent covariates before biopsy, reflecting the change in screening status over time.
Associations of risk factors with prostate cancer outcomes at 5 years (SELECT) or 7 years (PCPT cohorts 1 to 3) were estimated using a logistic regression model adjusted for age, ethnicity, family history, and PSA level at study entry. ORs and 95% CIs were reported. PCPT cohort 3 (with EOS biopsy and IPW), with minimal biopsy detection bias, was considered the gold standard, and the percentage of differences in cohort ORs relative to PCPT cohort 3 were reported. All analyses used SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Table 1 displays the characteristics of PCPT cohorts 1, 2, and 3 and the SELECT cohort. Because of focused recruitment, more minorities were enrolled in SELECT. A higher PSA level was allowed at study entry in SELECT (≤ 4.0 ng/mL), relative to PCPT (≤ 3.0 ng/mL). Men in SELECT had a higher body mass index (BMI) and more urologic symptoms, fewer were married, and diabetes was more prevalent. Statin use was higher in SELECT, paralleling the increase in statin use in the United States between the opening of PCPT in 1993 and of SELECT in 2001.8
Table 1.
Baseline Descriptive Statistics
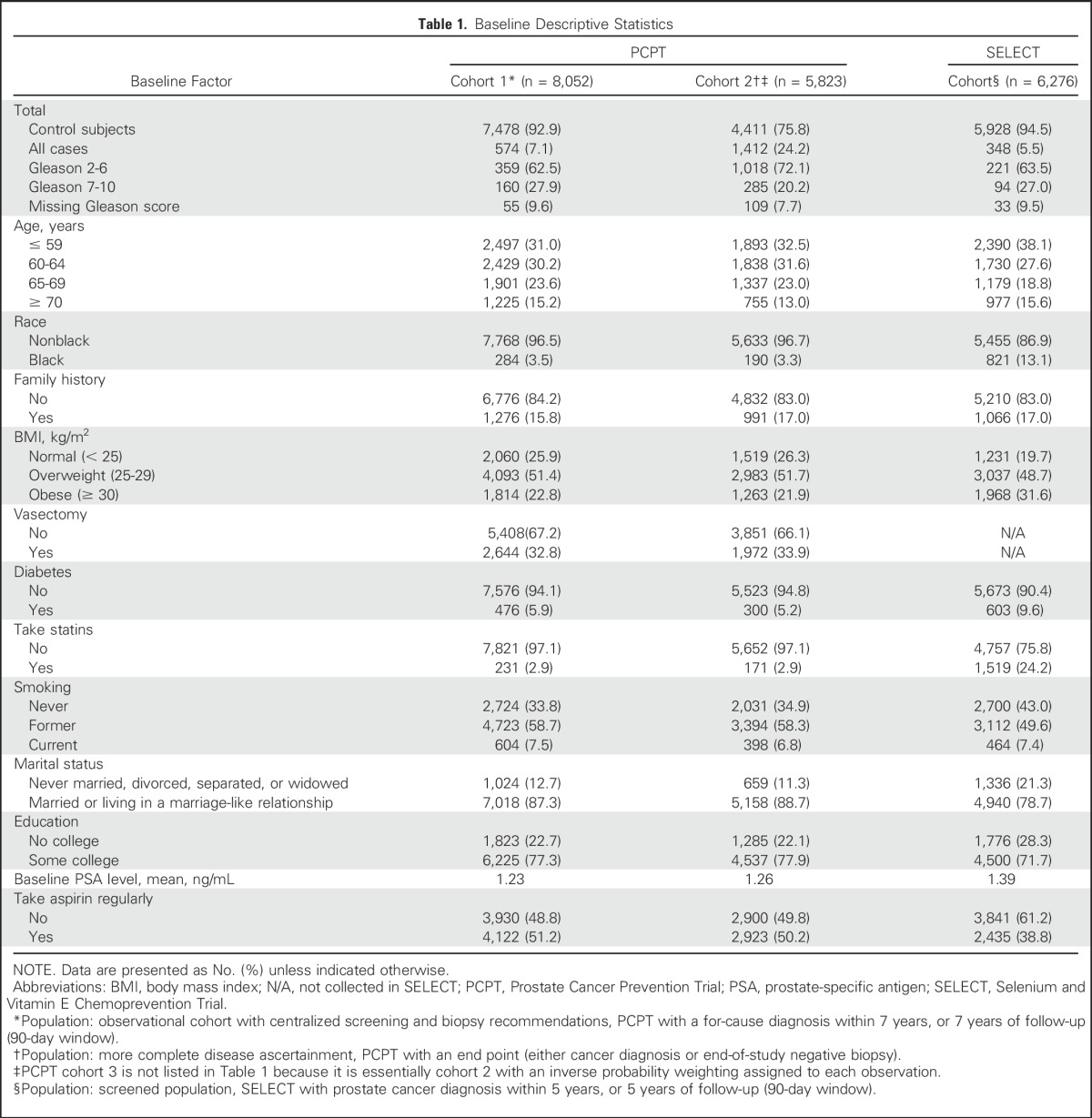
Table 2 provides hazard ratios for time to biopsy in the SELECT cohort. After accounting for their age, PSA level, and DRE status, we found that younger or married men, those with a family history of prostate cancer, or those with benign prostatic hyperplasia were more likely to undergo biopsy. Those with a higher BMI (≥ 25), those with diabetes, and previous or current smokers were less likely to undergo biopsy. Men taking aspirin or statins at baseline or during the trial and those having some college education were more likely to undergo a biopsy. Black men in SELECT had the same likelihood of prostate biopsy as men of other ethnicities, conditional on PSA level and DRE status. Thus, among men who agreed to participate in a prostate cancer prevention trial and who underwent substantial PSA screening and annual clinic visits, the decision to recommend (and accept) prostate biopsy, even after accounting for age and PSA/DRE history, was substantially influenced by other factors, some of which could be related to prostate cancer.
Table 2.
Association of Covariates with Risk of Biopsy in SELECT (n = 8,228)
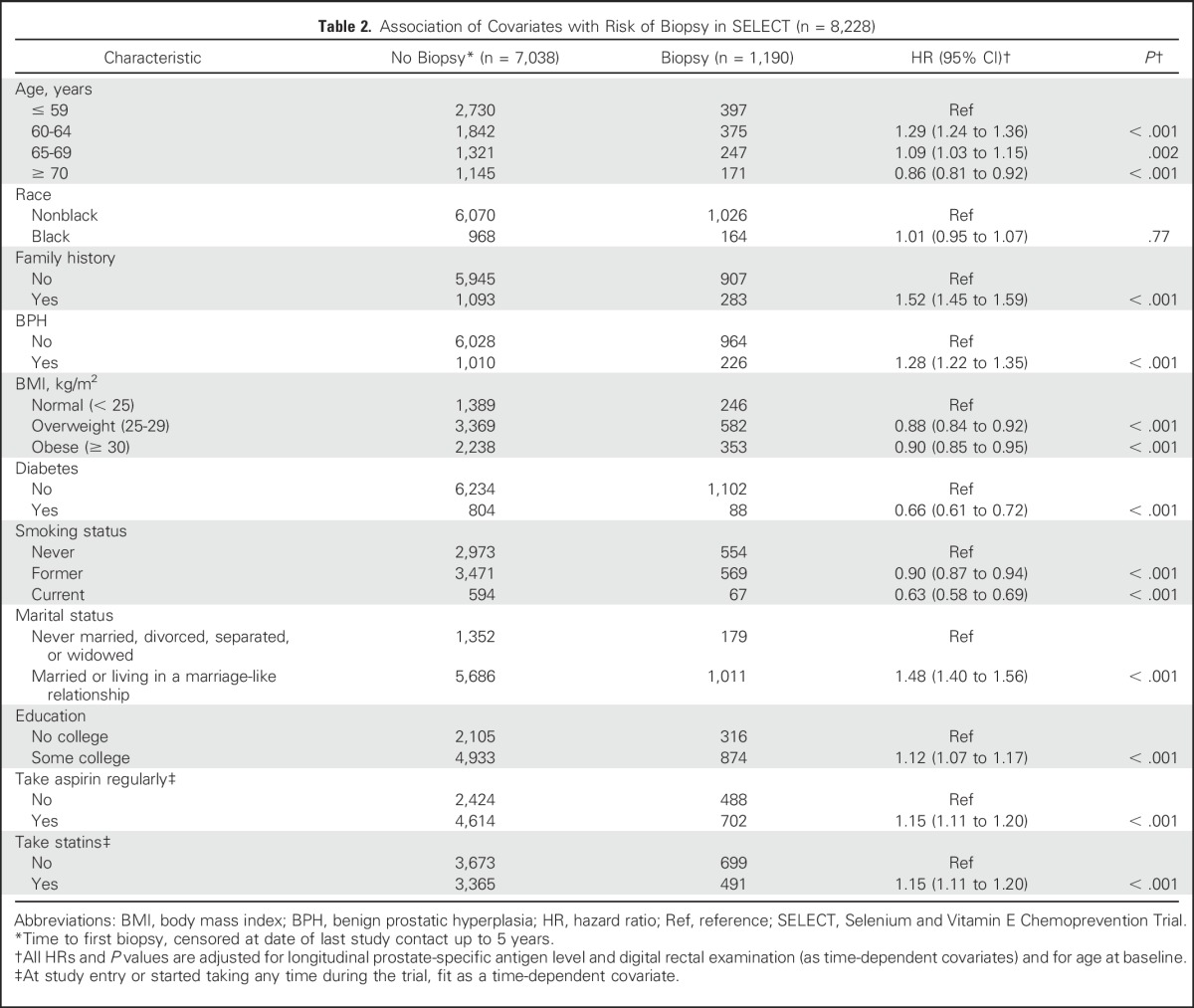
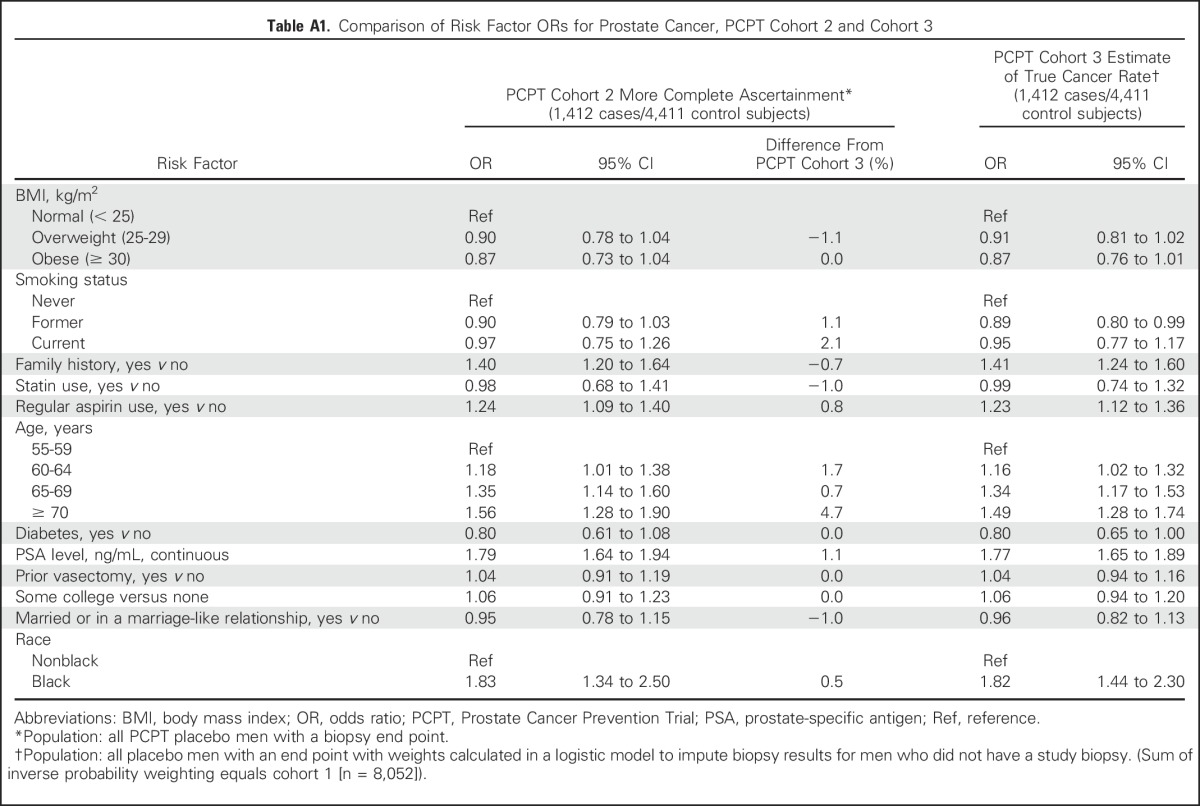
Table 3 displays associations of potential risk factors with prostate cancer from a multivariate logistic model in the SELECT cohort and PCPT cohorts 1 and 3. (Cohort 2 is reported in Appendix Table A1, online only).
Table 3.
Comparison of ORs of Risk Factors and Prostate Cancer Across Cohorts in PCPT and SELECT
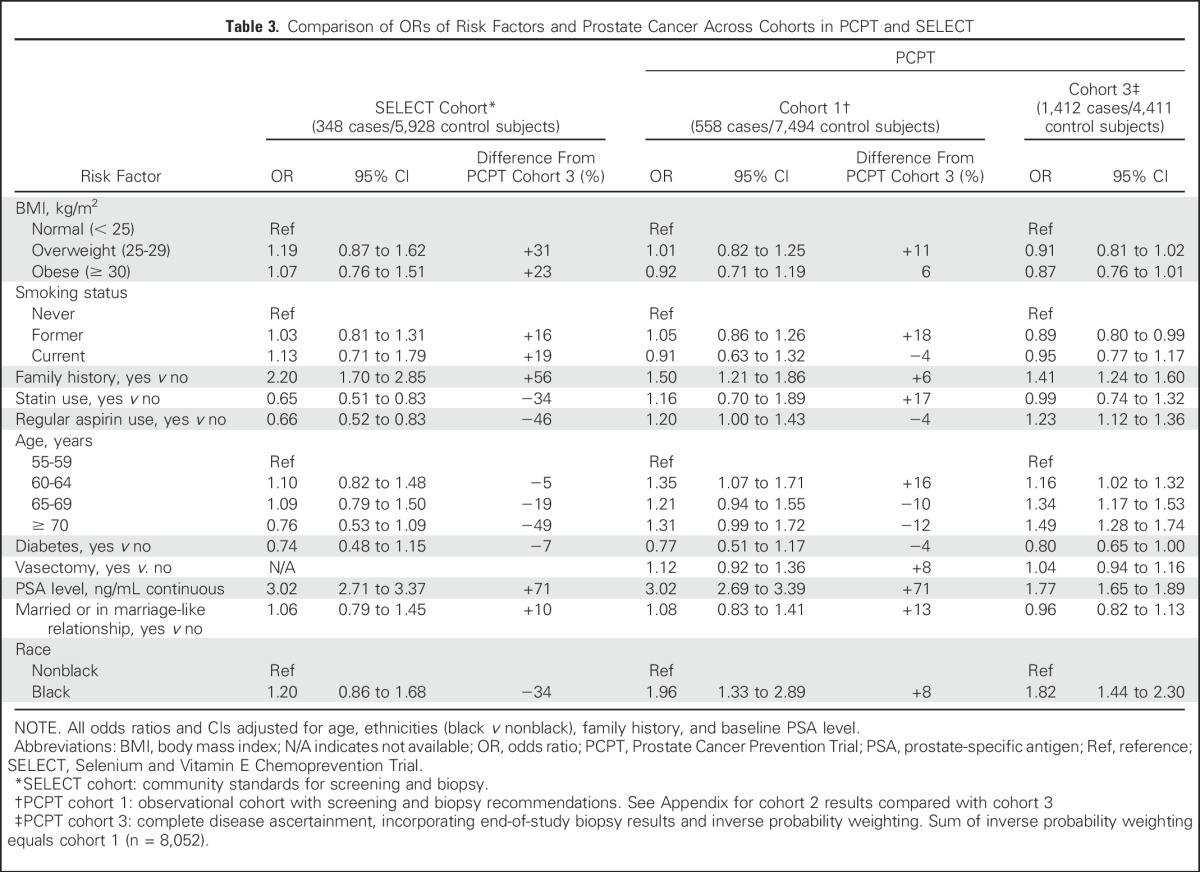
A BMI of 25 to 29 (v < 25) in the SELECT cohort (community practice/epidemiologic-like study) was associated with a 19% increase in the odds of prostate cancer (OR, 1.19); by comparison, no difference in the odds (OR, 1.01) was seen in PCPT cohort 1 (population screening), and there was a 9% reduction in the odds (OR, 0.91) of cancer in PCPT cohort 3 (accounting for biopsy bias). In relative terms, the estimated SELECT BMI OR is 31% larger than the PCPT OR in cohort 3. A similar trend was seen for baseline PSA level because men with higher PSA values were more likely to undergo biopsy. A unit increase in PSA level at baseline in SELECT provided an OR of 3.02, but accounting for annual PSA screening, a study-mandated biopsy, and the probability of having a biopsy in PCPT (cohort 3), the PSA level OR was 1.77, a relative difference of 69%.
A family history of prostate cancer in a first-degree relative (yes/no) provides a point estimate OR of 2.20 for the SELECT cohort, but like PSA level, the association moved closer to the null in PCPT cohort 3 (PCPT cohort 3 OR, 1.41), a relative difference of 56%. The likely explanation is illustrated in Table 2: men with a family history of prostate cancer are significantly more likely to undergo biopsy, even when controlling for PSA and DRE findings. Because rates of screening, recommendations for biopsy, and acceptance of biopsy were likely further related to measures such as family history and PSA level, the true associations between these factors and prostate cancer are likely overestimated in the absence of study-mandated biopsies.
In the SELECT cohort, intermediate age ranges (60 to 69 years) were associated with the greatest odds of prostate cancer, and older age (≥ 70 years) was associated with a substantial reduction in odds (OR, 0.76) compared with those younger than 60 years of age. By comparison, an examination of PCPT cohort 3 with biopsy verification showed a stepwise increased odds of prostate cancer with each increment in age group. This is likely explained, as seen in Table 2, by the stepwise reduction in the likelihood of biopsy from ages 60 to 64 years, to ≥ 70 years. Other factors exhibiting significant differences between the SELECT and PCPT cohorts were statins (35% odds reduction of prostate cancer in SELECT v no association in PCPT cohort 3; OR, 0.99) and aspirin use (34% odds reduction in SELECT v 20% and 23% increases in odds in PCPT cohorts 1 and 3, respectively). A final difference was the 20% increased odds of prostate cancer among black men in SELECT that was substantially higher in PCPT cohorts 1 and 3 (OR, 1.96 and 1.82, respectively). Risk factor ORs that were based on the subset with a prostate biopsy end point and no imputation (cohort 2) were similar to those of PCPT cohort 3, which used imputed end points (Appendix). Those who had a biopsy end point in PCPT had a risk of prostate cancer similar to that of those who did not. The EOS biopsy minimized detection bias, despite the lack of compliance by some men (Appendix Table A1).
DISCUSSION
Although there have been reports of factors associated with biopsy compliance in PCPT9 and Reduction by Dutasteride of Prostate Cancer Events study (REDUCE),10,11 to our knowledge, this is the first systematic evaluation of how biases related to prostate cancer ascertainment may affect our understanding of disease risk factors. The potential for such bias is substantial because, if all men underwent prostate biopsy, at least 15% would be diagnosed with cancer.12 As such, if a random risk factor were selected, for example, blue eye color, and it then led to an increased risk of biopsy in men with blue eyes, blue eyes would be proven to increase cancer risk.
Although some of the differences in risk factor associations across cohorts may be, in part, a result of random variation, our study has identified profound differences in the characteristics of men who undergo prostate biopsy and those who do not. The clinical steps required to be ascertained (ie, to undergo prostate biopsy) are enumerated in Figure 1. If any of the steps along this clinical continuum do not occur, the patient will not undergo biopsy and, even if he has an asymptomatic, high-grade cancer, he would not be diagnosed with prostate cancer. Thus, factors that increase the likelihood of progression along this continuum will increase the risk of a prostate cancer diagnosis. Men participating in SELECT and PCPT were also probably much more likely to undergo PSA testing, receive a biopsy recommendation, and agree to have the biopsy, compared with the general US population. Furthermore, if differences similar to those we observed in rates of biopsy are also operational in terms of a man’s decision or a physician’s recommendation to have a PSA test, our observations may significantly underestimate the true degree of bias.13,14
Many epidemiologic studies have suggested that statins, vasectomy, and aspirin affect prostate cancer risk.14-16 For vasectomy, we observed a 12% increase in the odds of prostate cancer in PCPT cohort 1, as opposed to only a 4% increase when a component of the biased detection is removed. Vasectomy status was not collected in SELECT. One explanation for this bias is that vasectomies are often performed by urologists, who may be more likely to recommend PSA testing and biopsy. In addition, men with a vasectomy may be more willing to undergo a prostate biopsy. Similarly, if men who take supplements or other preventive agents (eg, statins) undergo screening/biopsy in a systematically different fashion than those who do not, major biases in epidemiologic studies will result. Without detailed data about screening and biopsy verification, it is not possible to tease out whether there is a biopsy detection bias or a true association of a risk factor with prostate cancer, or a combination of both. Adjusting for PSA screening alone is not adequate.
The overarching implication of our data is obvious: evidence from observational studies suggesting that certain factors reduce/increase the risk of prostate cancer may be seriously flawed because of detection bias. Publication of these observations without careful attention to propensity-to-screen and propensity-to-biopsy bias can have major negative impacts at several levels. First, patients may choose an intervention (eg, aspirin) to reduce prostate cancer risk when the intervention may truly have no effect on cancer risk but may increase other disease risk (eg, stroke, hemorrhage). A second unfortunate result is that precious research resources may be directed to preclinical and clinical studies, only to find the original observation to be flawed.
Detection bias may also play a role in prevention and screening studies. In PCPT, the EOS biopsy was included to minimize detection bias between treatment arms. By comparison, the PLCO screening study compared annual PSA screening with community standard screening and ultimately found no reduction in prostate cancer mortality with annual testing. It was noted that at least one half of the men observed who underwent the community standard screening also underwent PSA testing; a recent update suggested that as many as 90% of men in the control group underwent PSA testing.17 Our study’s observations suggest that men who opted to undergo regular PSA testing and biopsy in the community standard arm were fundamentally different from those who had no PSA testing. If men with a higher risk of prostate cancer and prostate cancer death (eg, family history, older, black, and other risk factors) were more commonly tested and underwent a biopsy more often, the study outcome would be substantially biased to the null: the ultimate study outcome.
Limitations of our study include the generalizability of the results observed from men who consented to a prostate cancer prevention trial relative to other cohorts of men. However, these trials had more rigorous prostate cancer screening and biopsy adoption than expected in other observational studies of risk factors; our results thus likely underestimate detection biases. In addition, SELECT follow-up was shorter than that of PCPT, so the cohorts for the two trials were based on different intervals of time (5 v 7 years of follow-up). Not all men in PCPT had an EOS biopsy, but because the risk factor ORs for cohort 2, using only men with a study end point, and cohort 3, weighting for missing end points, are nearly identical, we conclude that a missing EOS biopsy in PCPT was not related to the risk of prostate cancer.
Our analysis and conclusions are not designed to address population-based PSA screening and biopsy; such screening should be based on individual risk factors and health considerations and should include careful assessment of the risks and possible benefits.18 The EOS PCPT biopsy in all participants, a unique study feature required to eliminate detection bias, allowed an evaluation of this bias but also resulted in the diagnosis of many low-risk prostate cancers. Better methods are needed for identifying consequential prostate cancers in order to avoid unnecessary biopsies. These data provide testimony that past and current screening and biopsy practice has likely led to biased conclusions regarding prostate cancer risk factors. Should higher-risk populations and higher-risk thresholds be adopted universally, it is possible that these biases may be mitigated by the greater risks of high-grade disease. Our data call into question previous observations related to prostate cancer risk. Future prevention and early-detection studies should collect comprehensive screening and biopsy data and control for these factors.
Our observations may not be limited to prostate cancer. Evidence suggests that other tumors (melanoma, thyroid, and breast) have a significant reservoir of asymptomatic, indolent disease.19 Because detection testing (eg, skin cancer screening, thyroid examination or ultrasound, mammography) and subsequent biopsy would follow the same pathway as that illustrated in Figure 1, it is likely that conclusions regarding risk factors or preventive strategies for other tumors may suffer from similar confounds. We encourage further study of this area to develop a better understanding of the true relationship between risk factors and cancer.
Appendix
Additional Method Details for PCPT Cohort 3: Predicting Prostate Cancer if Everyone Had an End Point
It may be that the men who did not have a prostate cancer end point evaluated had a different risk of prostate cancer than those who did have an end point. We assumed that there were measured study covariates, which both explain the differences between men with and without end points and are related to the risk of prostate cancer (Rubin DB: Biometrika 63:581-592, 1976). Under this assumption, for two men with similar covariate values such as age and family history of prostate cancer, one with an end point evaluated and one without, the outcome data from the man with the evaluated end point inform the cancer status for the man without the end point evaluation. An approach that uses this assumption and can be used to estimate the prevalence of prostate cancer is the inverse probability of censoring weighted estimation (Robins JM, et al: Boston, MA, Birkhäuser, 1992).
This involves a two-step process: (1) estimate the probability of having an end point evaluated conditional on covariates; and (2) estimate the probability of cancer, given the probability estimated in the first step. The probability of cancer is estimated by the weighted average of cancers among men with an observed end point, using the inverse of the probabilities from the first step as weights.
To estimate the probability of having an end point evaluated in the first step, logistic regression was used. To model the predicted probabilities, we chose study covariates related to both (1) having the study end point and (2) having a diagnosis of prostate cancer. The baseline covariates that were included in these analyses were age, ethnicity/race, PSA value, and family history of prostate cancer. Covariates measured after random assignment that were included in this analysis were interim biopsy prompts that were based on prostate-specific antigen levels or digital rectal examination and ever having a negative biopsy result during follow-up and before the end of study. The weights were then calculated as the inverse of the fitted (predicted) probabilities for men with an end point evaluated.
The final analysis included all men in the placebo arm who had a prostate cancer end point known at the end of the trial (the same population as that of cohort 2) but with weights associated with each record, so the sum of the weights equaled all eligible men on the placebo arm. To account for the estimation of the weights, 500 bootstrap samples of observed data were constructed. The analysis was repeated on each data set, and the variance of the estimates was calculated as the mean of the variances over all samples.
Table A1.
Comparison of Risk Factor ORs for Prostate Cancer, PCPT Cohort 2 and Cohort 3
Footnotes
Supported by National Institutes of Health, National Cancer Institute Grants UM1 CA182883, P30 CA065174, U01 CA086402, U10 CA37429.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00006392.
See accompanying editorial on page 4310
AUTHOR CONTRIBUTIONS
Conception and design: Catherine M. Tangen, Phyllis J. Goodman, Jeannette M. Schenk, M. Scott Lucia, Ian M. Thompson Jr
Collection and assembly of data: Catherine M. Tangen, Phyllis J. Goodman, M. Scott Lucia
Data analysis and interpretation: Catherine M. Tangen, Phyllis J. Goodman, Cathee Till, Jeannette M. Schenk, Ian M. Thompson Jr
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Biases in Recommendations for and Acceptance of Prostate Biopsy Significantly Affect Assessment of Prostate Cancer Risk Factors: Results From Two Large Randomized Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Catherine M. Tangen
No relationship to disclose
Phyllis J. Goodman
No relationship to disclose
Cathee Till
Research Funding: Genentech (I)
Patents, Royalties, Other Intellectual Property: Patent application for CD20-targeted CAR T cells (I)
Jeannette M. Schenk
No relationship to disclose
M. Scott Lucia
Stock or Other Ownership: 3DBiopsy
Consulting or Advisory Role: Genomic Health
Patents, Royalties, Other Intellectual Property: Multiexcitatin diagnostic system and methods for classification of tissue (US No. 8406858 B2)
Ian M. Thompson Jr
Consulting or Advisory Role: Exosome Diagnostics, Magforce
Patents, Royalties, Other Intellectual Property: I have been involved in the establishment of a new company, NanoTX Therapeutics, to commercialize a novel therapy for glioblastoma for our cancer center. I am on the board of directors and the company has intellectual property developed by our cancer center. I have several patents with colleagues involving novel biomarkers for cancer and two devices for sexual dysfunction and urinary incontinence. There are no revenues at this time, and our university Intellectual Property office is working with industry to determine if these can be commercialized.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman PJ, Tangen CM, Crowley JJ, et al. Implementation of the Prostate Cancer Prevention Trial (PCPT) Control Clin Trials. 2004;25:203–222. doi: 10.1016/j.cct.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Goodman PJ, Thompson IM, Jr, Tangen CM, et al. The Prostate Cancer Prevention Trial: Design, biases and interpretation of study results. J Urol. 2006;175:2234–2242. doi: 10.1016/S0022-5347(06)00284-9. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 6.Redman MW, Tangen CM, Goodman PJ, et al. Finasteride does not increase the risk of high-grade prostate cancer: A bias-adjusted modeling approach. Cancer Prev Res (Phila) 2008;1:174–181. doi: 10.1158/1940-6207.CAPR-08-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 8.Bansal D, Undela K, D’Cruz S, et al. Statin use and risk of prostate cancer: A meta-analysis of observational studies. PLoS One. 2012;7:e46691. doi: 10.1371/journal.pone.0046691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gritz ER, Arnold KB, Moinpour CM, et al. Factors associated with adherence to an end-of-study biopsy: Lessons from the Prostate Cancer Prevention Trial (SWOG-Coordinated Intergroup Study S9217) Cancer Epidemiol Biomarkers Prev. 2014;23:1638–1648. doi: 10.1158/1055-9965.EPI-14-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer S, Sun S, Howard LE, et al. Baseline subject characteristics predictive of compliance with study-mandated prostate biopsy in men at risk of prostate cancer: Results from REDUCE. Prostate Cancer Prostatic Dis. 2016;19:202–208. doi: 10.1038/pcan.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho T, Howard LE, Vidal AC, et al. Smoking and risk of low- and high-grade prostate cancer: Results from the REDUCE study. Clin Cancer Res. 2014;20:5331–5338. doi: 10.1158/1078-0432.CCR-13-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 13.Nijs HGT, Essink-Bot ML, DeKoning HJ, et al. Why do men refuse or attend population-based screening for prostate cancer? J Public Health Med. 2000;22:312–316. doi: 10.1093/pubmed/22.3.312. [DOI] [PubMed] [Google Scholar]
- 14.Hayat Roshanai A, Nordin K, Berglund G. Factors influencing primary care physicians’ decision to order prostate-specific antigen (PSA) test for men without prostate cancer. Acta Oncol. 2013;52:1602–1608. doi: 10.3109/0284186X.2012.762998. [DOI] [PubMed] [Google Scholar]
- 15.Vidal AC, Howard LE, Moreira DM, et al. Aspirin, NSAIDs, and risk of prostate cancer: Results from the REDUCE study. Clin Cancer Res. 2015;21:756–762. doi: 10.1158/1078-0432.CCR-14-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui MM, Wilson KM, Epstein MM, et al. Vasectomy and risk of aggressive prostate cancer: A 24-year follow-up study. J Clin Oncol. 2014;32:3033–3038. doi: 10.1200/JCO.2013.54.8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med. 2016;374:1795–1796. doi: 10.1056/NEJMc1515131. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, Jr, Leach RJ, Ankerst DP. Focusing PSA testing on detection of high-risk prostate cancers by incorporating patient preferences into decision making. JAMA. 2014;312:995–996. doi: 10.1001/jama.2014.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]