Abstract
Amines such as 1,2,3,4-tetrahydroisoquinoline undergo redox-neutral annulations with 2-alkylquinoline-3-carbaldehydes as well as the corresponding 4-alkyl isomers and pyridine analogues. These processes involve dual C–H bond functionalization. Acetic acid is used as a cosolvent and acts as the sole promoter of these transformations.
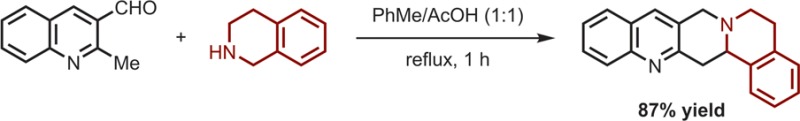
As part of our continuing efforts to develop methods for the redox-neutral α-C–H bond functionalization of amines,1,2 we have reported on a range of transformations that achieve amine annulation (Scheme 1).3−5 Specifically, aldehydes with a pendent nucleophilic site undergo condensations with amines in reactions that combine reductive N-alkylation with concurrent oxidative α-C–H bond functionalization.6 While a broad range of polycyclic amines have been accessed with this general method, in all cases, the nucleophilic site has been limited to rather activated systems including anilines,3a,3c,3d phenols,3e thiophenols,3f electron-rich aromatics,3b malonates,3h nitroalkanes,3g and ketones.3i Here, we report the first examples of redox annulations that involve alkyl azaarenes as relatively nonactivated nucleophiles.7
Scheme 1. Redox Annulations of Amines.
At least in part due to the prevalence of the pyridine nucleus in bioactive materials,8 the functionalization of the alkyl group in 2-alkyl azaarenes has drawn significant attention in recent years.9 The possibly earliest report on “Condensations of Methylated Quinolines and Pyridines” dates back to 1883 and describes zinc chloride promoted reactions of quinaldine and picoline with phthalic anhydride and benzaldehyde.10 Recent developments include transition metal, Lewis acid, and Brønsted acid catalyzed variants in addition to additive-free reactions and catalytic enantioselective versions.11
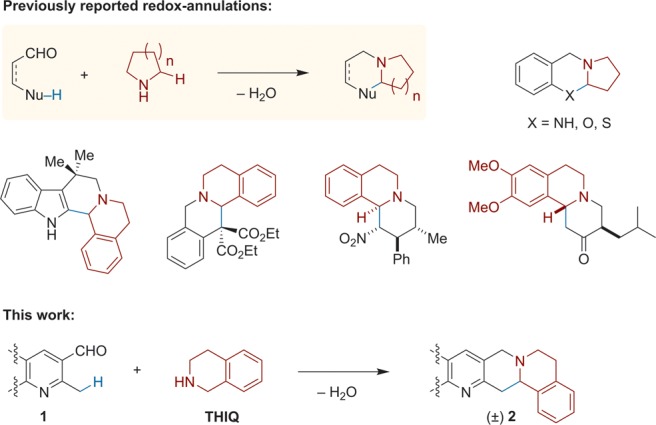
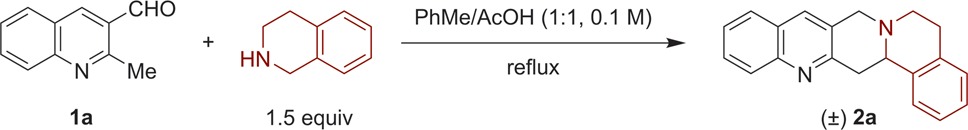
We evaluated the title reaction under a range of conditions using 2-methylquinoline-3-carbaldehyde (1a) and 1,2,3,4-tetrahydroisoquinoline (THIQ) as model substrates (Table 1). The optimized conditions call for using a 1:1 mixture of toluene and acetic acid as the reaction medium at a 0.1 M substrate concentration. Reflux of 1a and THIQ (1.5 equiv) in this mixture for 1 h provided desired product 2a in 87% yield. In the absence of acetic acid, or when using catalytic amounts of carboxylic acids, only complex reaction mixtures were observed (entries 2–4). Upon increasing the amount of the acetic acid promoter, the yield of 2a increased gradually with concurrent reduction in reaction time (entries 5–8). An increase in acetic acid beyond the 1:1 mixture with toluene led to a slight drop in yield (entry 9). However, it is notable that the reaction can be conducted in pure acetic acid as the solvent (entry 10). A reduction in yield was also observed at higher substrate concentration (entry 11), whereas little change was observed under more diluted conditions (entry 12).
Table 1. Reaction Developmenta.
| entry | deviation from optimized conditions | time (h) | yield (%) |
|---|---|---|---|
| 1 | noneb | 1 | 87 |
| 2 | no AcOH | 25 | complex |
| 3 | 20 mol % of AcOH in PhMe | 2 | complex |
| 4 | 20 mol % of BzOH in PhMe | 2 | complex |
| 5 | 5 equiv of AcOH in PhMe | 3 | 30 |
| 6 | 10 equiv of AcOH in PhMe | 3 | 38 |
| 7 | 20 equiv of AcOH in PhMe | 1.5 | 57 |
| 8 | PhMe/AcOH = 3:1c | 1.5 | 84 |
| 9 | PhMe/AcOH = 1:3d | 3.5 | 80 |
| 10 | AcOH as solvente | 3.5 | 73 |
| 11 | 0.2 M conc | 1 | 71 |
| 12 | 0.05 M conc | 1.5 | 87 |
Reactions were performed on a 0.2 mmol scale. All yields correspond to isolated yields.
Corresponds to 87.5 equiv of AcOH.
44 equiv of AcOH.
131 equiv of AcOH.
175 equiv of AcOH.
The scope of the amine annulation with alkyl azaarenes is outlined in Scheme 2.12 2-Methylquinoline-3-carbaldehydes possessing a range of electron-withdrawing or electron-donating substituents on different ring-positions of the quinoline core readily underwent annulation with THIQ to provide products 2 in consistently good yields. Replacement of the methyl group in 1a with benzyl or ethyl was also tolerated. These reactions were moderately diastereoselective. Replacement of the aldehyde in 1a with a methyl ketone also allowed for the synthesis of the corresponding annulation product. 2-Alkylpyridine-3-carboxaldehydes, which are typically less reactive than their corresponding quinoline counterparts, also participated in annulation reactions with THIQ. These reactions were performed in acetic acid as the only solvent. In addition, amines other than THIQ underwent reactions with 1a. Finally, 1-phenyl-THIQ and the corresponding 1,2,3,4-tetrahydro-β-carboline participated in redox annulations with 1a to provide products possessing a tetrasubstituted stereogenic center at the site of C–C bond formation.
Scheme 2. Scope of the Redox Annulation.
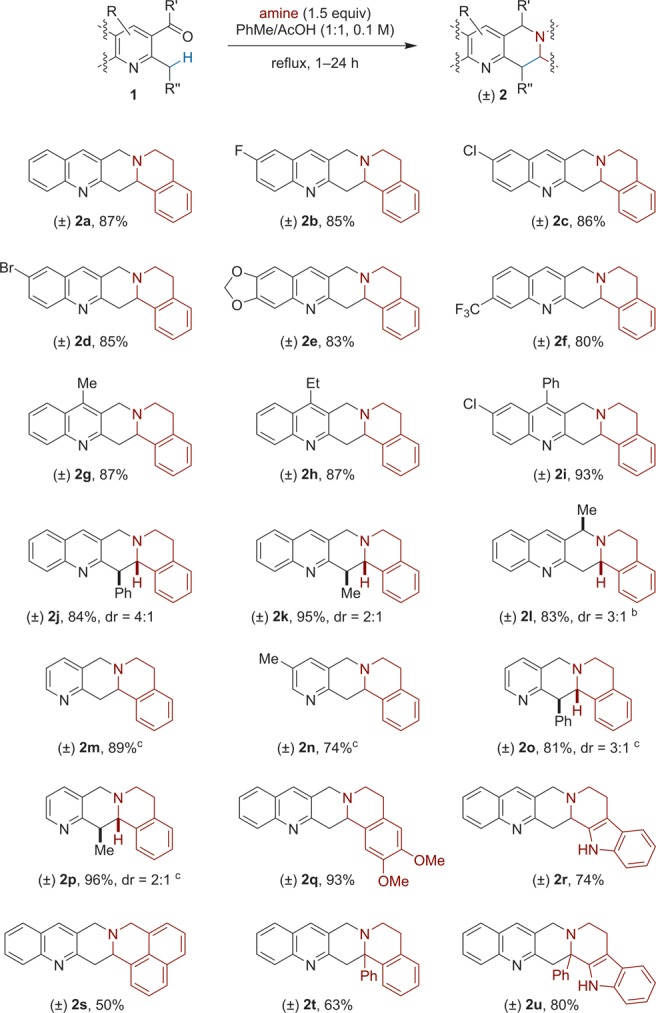
Reactions were performed on a 0.5 mmol scale. All yields correspond to isolated yields.
20 equiv of acetic acid in PhMe.
AcOH was used as the solvent.
Compared to 2-alkyl azaarenes, the corresponding 4-alkyl azaarenes are less activated. In fact, few previous reports have targeted this class of compounds with regard to alkyl group functionalization.11b,11v Gratifyingly, conditions optimized for 1a and its analogues proved to be suitable for the redox annulation of 4-methyl azaarenes (Scheme 3). Interestingly, 4-methylpyridine-3-carboxaldehyde provided higher yields than the corresponding quinoline derivative.
Scheme 3. Redox Annulation with 4-Methylazaarenes.
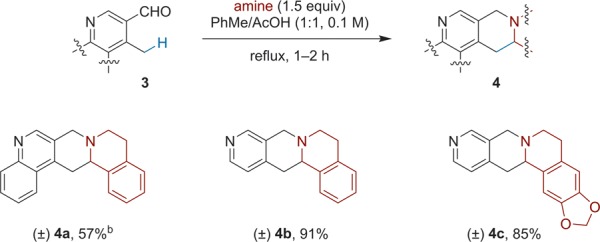
Reactions were performed on a 0.5 mmol scale. All yields correspond to isolated yields.
AcOH was used as the solvent.
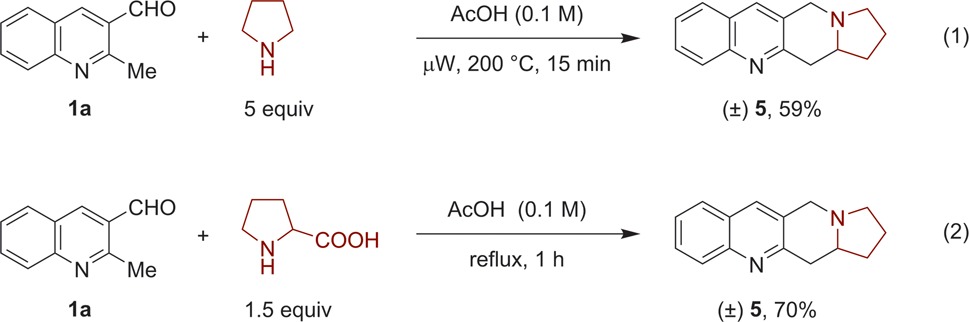
The reaction of 1a with pyrrolidine, an amine that is less reactive than THIQ in most redox reactions,3−5 required modified reaction conditions (5 equiv of amine) and elevated temperatures (eq 1). Product 5 was obtained in 59% yield. We have previously shown that decarboxylative variants of certain amine α-C–H bond functionalization reactions can offer advantages with regard to reaction setup and product yields.3b,3d,13,14 Indeed, decarboxylative condensation of 1a with proline in acetic acid provided product 5 with an improved yield of 70% (eq 2).
 |
1 |
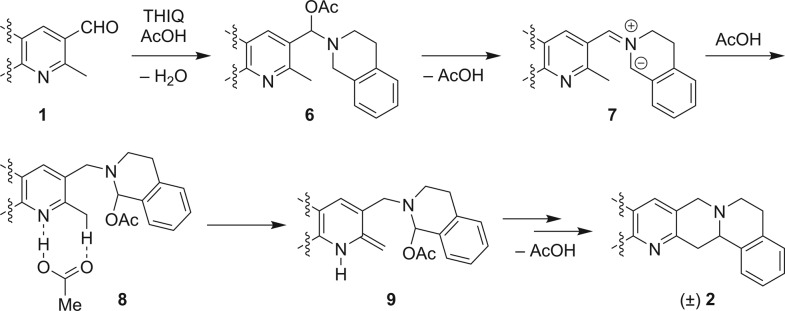
The overall mechanism of the redox annulation likely shares many features with previously reported redox transformations (Scheme 4).6 Accordingly, acetic acid promoted condensation of 1a and THIQ is expected to give rise to the initial formation of N,O-acetal 6. This species can undergo loss of AcOH to form azomethine ylide intermediate 7. The latter reengages AcOH to form a regioisomeric N,O-acetal that can interact with acetic acid via 8.11l,11q Acetic acid promoted tautomerization to proposed intermediate 9 is followed by ring closure with loss of AcOH to ultimately form product 2.
Scheme 4. Proposed Mechanism.
In summary, we have achieved redox annulations of amines with various alkyl azaarenes. Acetic acid acts as the sole promoter of these reactions, which proceed with dual C–H bond functionalization.
Acknowledgments
Financial support from the NIH-NIGMS (Grant No. R01GM101389) is gratefully acknowledged. We thank Dr. Tom Emge (Rutgers University) for X-ray crystallographic analysis and Dr. Wazo Myint (Rutgers University) for assistance with NMR assignments.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b01047.
The authors declare no competing financial interest.
Supplementary Material
References
- Selected reviews on amine C–H functionalization, including redox-neutral approaches:; a Murahashi S.-I. Angew. Chem., Int. Ed. Engl. 1995, 34, 2443. 10.1002/anie.199524431. [DOI] [Google Scholar]; b Matyus P.; Elias O.; Tapolcsanyi P.; Polonka-Balint A.; Halasz-Dajka B. Synthesis 2006, 2006, 2625. 10.1055/s-2006-942490. [DOI] [Google Scholar]; c Campos K. R. Chem. Soc. Rev. 2007, 36, 1069. 10.1039/B607547A. [DOI] [PubMed] [Google Scholar]; d Murahashi S.-I.; Zhang D. Chem. Soc. Rev. 2008, 37, 1490. 10.1039/b706709g. [DOI] [PubMed] [Google Scholar]; e Li C.-J. Acc. Chem. Res. 2009, 42, 335. 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]; f Jazzar R.; Hitce J.; Renaudat A.; Sofack-Kreutzer J.; Baudoin O. Chem. - Eur. J. 2010, 16, 2654. 10.1002/chem.200902374. [DOI] [PubMed] [Google Scholar]; g Yeung C. S.; Dong V. M. Chem. Rev. 2011, 111, 1215. 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]; h Pan S. C. Beilstein J. Org. Chem. 2012, 8, 1374. 10.3762/bjoc.8.159. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Mitchell E. A.; Peschiulli A.; Lefevre N.; Meerpoel L.; Maes B. U. W. Chem. - Eur. J. 2012, 18, 10092. 10.1002/chem.201201539. [DOI] [PubMed] [Google Scholar]; j Zhang C.; Tang C.; Jiao N. Chem. Soc. Rev. 2012, 41, 3464. 10.1039/c2cs15323h. [DOI] [PubMed] [Google Scholar]; k Jones K. M.; Klussmann M. Synlett 2012, 2012, 159. 10.1055/s-0031-1290117. [DOI] [Google Scholar]; l Peng B.; Maulide N. Chem. - Eur. J. 2013, 19, 13274. 10.1002/chem.201301522. [DOI] [PubMed] [Google Scholar]; m Platonova A. Y.; Glukhareva T. V.; Zimovets O. A.; Morzherin Y. Y. Chem. Heterocycl. Compd. 2013, 49, 357. 10.1007/s10593-013-1257-6. [DOI] [Google Scholar]; n Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 113, 5322. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Girard S. A.; Knauber T.; Li C.-J. Angew. Chem., Int. Ed. 2014, 53, 74. 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]; p Haibach M. C.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5010. 10.1002/anie.201306489. [DOI] [PubMed] [Google Scholar]; q Wang L.; Xiao J. Adv. Synth. Catal. 2014, 356, 1137. 10.1002/adsc.201301153. [DOI] [Google Scholar]; r Vo C.-V. T.; Bode J. W. J. Org. Chem. 2014, 79, 2809. 10.1021/jo5001252. [DOI] [PubMed] [Google Scholar]; s Seidel D. Org. Chem. Front. 2014, 1, 426. 10.1039/c4qo00022f. [DOI] [PMC free article] [PubMed] [Google Scholar]; t Qin Y.; Lv J.; Luo S. Tetrahedron Lett. 2014, 55, 551. 10.1016/j.tetlet.2013.11.051. [DOI] [Google Scholar]; u Seidel D. Acc. Chem. Res. 2015, 48, 317. 10.1021/ar5003768. [DOI] [PMC free article] [PubMed] [Google Scholar]; v Beatty J. W.; Stephenson C. R. J. Acc. Chem. Res. 2015, 48, 1474. 10.1021/acs.accounts.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected reviews on various types of redox-neutral transformations:; a Burns N. Z.; Baran P. S.; Hoffmann R. W. Angew. Chem., Int. Ed. 2009, 48, 2854. 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]; b Mahatthananchai J.; Bode J. W. Acc. Chem. Res. 2014, 47, 696. 10.1021/ar400239v. [DOI] [PubMed] [Google Scholar]; c Ketcham J. M.; Shin I.; Montgomery T. P.; Krische M. J. Angew. Chem., Int. Ed. 2014, 53, 9142. 10.1002/anie.201403873. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Huang H.; Ji X.; Wu W.; Jiang H. Chem. Soc. Rev. 2015, 44, 1155. 10.1039/C4CS00288A. [DOI] [PubMed] [Google Scholar]
- a Zhang C.; De C. K.; Mal R.; Seidel D. J. Am. Chem. Soc. 2008, 130, 416. 10.1021/ja077473r. [DOI] [PubMed] [Google Scholar]; b Zhang C.; Das D.; Seidel D. Chem. Sci. 2011, 2, 233. 10.1039/C0SC00432D. [DOI] [Google Scholar]; c Dieckmann A.; Richers M. T.; Platonova A. Y.; Zhang C.; Seidel D.; Houk K. N. J. Org. Chem. 2013, 78, 4132. 10.1021/jo400483h. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Richers M. T.; Deb I.; Platonova A. Y.; Zhang C.; Seidel D. Synthesis 2013, 45, 1730. 10.1055/s-0033-1338852. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Richers M. T.; Breugst M.; Platonova A. Y.; Ullrich A.; Dieckmann A.; Houk K. N.; Seidel D. J. Am. Chem. Soc. 2014, 136, 6123. 10.1021/ja501988b. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Jarvis C. L.; Richers M. T.; Breugst M.; Houk K. N.; Seidel D. Org. Lett. 2014, 16, 3556. 10.1021/ol501509b. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Kang Y.; Chen W.; Breugst M.; Seidel D. J. Org. Chem. 2015, 80, 9628. 10.1021/acs.joc.5b01384. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Ma L.; Seidel D. Chem. - Eur. J. 2015, 21, 12908. 10.1002/chem.201501667. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Chen W.; Seidel D. Org. Lett. 2016, 18, 1024. 10.1021/acs.orglett.6b00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected examples of related intermolecular redox transformations from our laboratory:; a Ma L.; Chen W.; Seidel D. J. Am. Chem. Soc. 2012, 134, 15305. 10.1021/ja308009g. [DOI] [PubMed] [Google Scholar]; b Das D.; Sun A. X.; Seidel D. Angew. Chem., Int. Ed. 2013, 52, 3765. 10.1002/anie.201300021. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Das D.; Seidel D. Org. Lett. 2013, 15, 4358. 10.1021/ol401858k. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chen W.; Kang Y.; Wilde R. G.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5179. 10.1002/anie.201311165. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Chen W.; Seidel D. Org. Lett. 2014, 16, 3158. 10.1021/ol501365j. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Chen W.; Wilde R. G.; Seidel D. Org. Lett. 2014, 16, 730. 10.1021/ol403431u. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Zhu Z.; Seidel D. Org. Lett. 2016, 18, 631. 10.1021/acs.orglett.5b03529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Examples of related redox reactions by others:; a Zheng L.; Yang F.; Dang Q.; Bai X. Org. Lett. 2008, 10, 889. 10.1021/ol703049j. [DOI] [PubMed] [Google Scholar]; b Zheng Q.-H.; Meng W.; Jiang G.-J.; Yu Z.-X. Org. Lett. 2013, 15, 5928. 10.1021/ol402517e. [DOI] [PubMed] [Google Scholar]; c Lin W.; Cao T.; Fan W.; Han Y.; Kuang J.; Luo H.; Miao B.; Tang X.; Yu Q.; Yuan W.; Zhang J.; Zhu C.; Ma S. Angew. Chem., Int. Ed. 2014, 53, 277. 10.1002/anie.201308699. [DOI] [PubMed] [Google Scholar]; d Haldar S.; Mahato S.; Jana C. K. Asian J. Org. Chem. 2014, 3, 44. 10.1002/ajoc.201300233. [DOI] [Google Scholar]; e Rahman M.; Bagdi A. K.; Mishra S.; Hajra A. Chem. Commun. 2014, 50, 2951. 10.1039/c4cc00454j. [DOI] [PubMed] [Google Scholar]; f Li J.; Wang H.; Sun J.; Yang Y.; Liu L. Org. Biomol. Chem. 2014, 12, 2523. 10.1039/c3ob42431f. [DOI] [PubMed] [Google Scholar]; g Lin W.; Ma S. Org. Chem. Front. 2014, 1, 338. 10.1039/c4qo00058g. [DOI] [Google Scholar]; h Mahato S.; Haque M. A.; Dwari S.; Jana C. K. RSC Adv. 2014, 4, 46214. 10.1039/C4RA05045B. [DOI] [Google Scholar]; i Shao G.; He Y.; Xu Y.; Chen J.; Yu H.; Cao R. Eur. J. Org. Chem. 2015, 2015, 4615. 10.1002/ejoc.201500526. [DOI] [Google Scholar]; j Haldar S.; Roy S. K.; Maity B.; Koley D.; Jana C. K. Chem. - Eur. J. 2015, 21, 15290. 10.1002/chem.201502297. [DOI] [PubMed] [Google Scholar]; k Cheng Y.-F.; Rong H.-J.; Yi C.-B.; Yao J.-J.; Qu J. Org. Lett. 2015, 17, 4758. 10.1021/acs.orglett.5b02298. [DOI] [PubMed] [Google Scholar]; l Yi F.; Su J.; Zhang S.; Yi W.; Zhang L. Eur. J. Org. Chem. 2015, 2015, 7360. 10.1002/ejoc.201501102. [DOI] [Google Scholar]; m Hu G.; Chen W.; Ma D.; Zhang Y.; Xu P.; Gao Y.; Zhao Y. J. Org. Chem. 2016, 81, 1704. 10.1021/acs.joc.5b02625. [DOI] [PubMed] [Google Scholar]; n Zhou S.; Tong R. Chem. - Eur. J. 2016, 22, 7084. 10.1002/chem.201601245. [DOI] [PubMed] [Google Scholar]; o Kumar M.; Kaur B. P.; Chimni S. S. Chem. - Eur. J. 2016, 22, 9948. 10.1002/chem.201601222. [DOI] [PubMed] [Google Scholar]; p Zheng K.-L.; Shu W.-M.; Ma J.-R.; Wu Y.-D.; Wu A.-X. Org. Lett. 2016, 18, 3526. 10.1021/acs.orglett.6b01369. [DOI] [PubMed] [Google Scholar]; q Huang J.; Li L.; Xiao T.; Mao Z.-w.; Zhou L. Asian J. Org. Chem. 2016, 5, 1204. 10.1002/ajoc.201600338. [DOI] [Google Scholar]; r Yan J.-M.; Bai Q.-F.; Xu C.; Feng G. Synthesis 2016, 48, 3730. 10.1055/s-0035-1562551. [DOI] [Google Scholar]; s Rong H.-J.; Cheng Y.-F.; Liu F.-F.; Ren S.-J.; Qu J. J. Org. Chem. 2017, 82, 532. 10.1021/acs.joc.6b02562. [DOI] [PubMed] [Google Scholar]; t Du Y.; Yu A.; Jia J.; Zhang Y.; Meng X. Chem. Commun. 2017, 53, 1684. 10.1039/C6CC08996H. [DOI] [PubMed] [Google Scholar]
- For detailed discussions on the mechanisms of these transformations, see refs1u and (3c,3e−3g) and the following report:Ma L.; Paul A.; Breugst M.; Seidel D. Chem. - Eur. J. 2016, 22, 18179. 10.1002/chem.201603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During the preparation of this manuscript, a related report appeared in which aluminum triflate (30 mol %) was used as a catalyst. The substrate scope appears to be more limited:Li J.; Qin C.; Yu Y.; Fan H.; Fu Y.; Li H.; Wang W. Adv. Synth. Catal. 2017, 359, x. 10.1002/adsc.201601423. [DOI] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. J. Med. Chem. 2014, 57, 10257. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Selected reviews:; a Best D.; Lam H. W. J. Org. Chem. 2014, 79, 831. 10.1021/jo402414k. [DOI] [PubMed] [Google Scholar]; b Yang L.; Huang H. Chem. Rev. 2015, 115, 3468. 10.1021/cr500610p. [DOI] [PubMed] [Google Scholar]; c Vanjari R.; Singh K. N. Chem. Soc. Rev. 2015, 44, 8062. 10.1039/C5CS00003C. [DOI] [PubMed] [Google Scholar]
- a Jacobsen E.; Reimer C. L. Ber. Dtsch. Chem. Ges. 1883, 16, 2602. 10.1002/cber.188301602196. [DOI] [Google Scholar]; See also:; b Baurath H. Ber. Dtsch. Chem. Ges. 1887, 20, 2719. 10.1002/cber.188702002123. [DOI] [Google Scholar]
- Selected recent examples of alkyl azaarene C–H functionalization:; a Qian B.; Guo S.; Shao J.; Zhu Q.; Yang L.; Xia C.; Huang H. J. Am. Chem. Soc. 2010, 132, 3650. 10.1021/ja910104n. [DOI] [PubMed] [Google Scholar]; b Duez S.; Steib A. K.; Manolikakes S. M.; Knochel P. Angew. Chem., Int. Ed. 2011, 50, 7686. 10.1002/anie.201103074. [DOI] [PubMed] [Google Scholar]; c Trost B. M.; Thaisrivongs D. A.; Hartwig J. J. Am. Chem. Soc. 2011, 133, 12439. 10.1021/ja205523e. [DOI] [PubMed] [Google Scholar]; d Yan Y.; Xu K.; Fang Y.; Wang Z. J. Org. Chem. 2011, 76, 6849. 10.1021/jo2008934. [DOI] [PubMed] [Google Scholar]; e Rueping M.; Tolstoluzhsky N. Org. Lett. 2011, 13, 1095. 10.1021/ol103150g. [DOI] [PubMed] [Google Scholar]; f Komai H.; Yoshino T.; Matsunaga S.; Kanai M. Org. Lett. 2011, 13, 1706. 10.1021/ol200217y. [DOI] [PubMed] [Google Scholar]; g Qian B.; Xie P.; Xie Y.; Huang H. Org. Lett. 2011, 13, 2580. 10.1021/ol200684b. [DOI] [PubMed] [Google Scholar]; h Qian B.; Shi D.; Yang L.; Huang H. Adv. Synth. Catal. 2012, 354, 2146. 10.1002/adsc.201200285. [DOI] [Google Scholar]; i Liu J.-Y.; Niu H.-Y.; Wu S.; Qu G.-R.; Guo H.-M. Chem. Commun. 2012, 48, 9723. 10.1039/c2cc35309a. [DOI] [PubMed] [Google Scholar]; j Best D.; Kujawa S.; Lam H. W. J. Am. Chem. Soc. 2012, 134, 18193. 10.1021/ja3083494. [DOI] [PubMed] [Google Scholar]; k Wang F.-F.; Luo C.-P.; Wang Y.; Deng G.; Yang L. Org. Biomol. Chem. 2012, 10, 8605. 10.1039/c2ob26604k. [DOI] [PubMed] [Google Scholar]; l Niu R.; Xiao J.; Liang T.; Li X. Org. Lett. 2012, 14, 676. 10.1021/ol2030982. [DOI] [PubMed] [Google Scholar]; m Komai H.; Yoshino T.; Matsunaga S.; Kanai M. Synthesis 2012, 44, 2185. 10.1055/s-0031-1291041. [DOI] [Google Scholar]; n Guan B.-T.; Wang B.; Nishiura M.; Hou Z. Angew. Chem., Int. Ed. 2013, 52, 4418. 10.1002/anie.201208867. [DOI] [PubMed] [Google Scholar]; o Jin J.-j.; Wang D.-c.; Niu H.-y.; Wu S.; Qu G.-r.; Zhang Z.-b.; Guo H.-m. Tetrahedron 2013, 69, 6579. 10.1016/j.tet.2013.05.135. [DOI] [Google Scholar]; p Gao X.; Zhang F.; Deng G.; Yang L. Org. Lett. 2014, 16, 3664. 10.1021/ol501422k. [DOI] [PubMed] [Google Scholar]; q Zhu Z.-Q.; Bai P.; Huang Z.-Z. Org. Lett. 2014, 16, 4881. 10.1021/ol502402s. [DOI] [PubMed] [Google Scholar]; r Fu S.; Wang L.; Dong H.; Yu J.; Xu L.; Xiao J. Tetrahedron Lett. 2016, 57, 4533. 10.1016/j.tetlet.2016.08.065. [DOI] [Google Scholar]; s Meazza M.; Tur F.; Hammer N.; Jørgensen K. A. Angew. Chem., Int. Ed. 2017, 56, 1634. 10.1002/anie.201611306. [DOI] [PubMed] [Google Scholar]; t Bai X.; Zeng G.; Shao T.; Jiang Z. Angew. Chem., Int. Ed. 2017, 56, 3684. 10.1002/anie.201700190. [DOI] [PubMed] [Google Scholar]; u Liu X.-J.; You S.-L. Angew. Chem., Int. Ed. 2017, 56, 4002. 10.1002/anie.201700433. [DOI] [Google Scholar]; v Suzuki H.; Igarashi R.; Yamashita Y.; Kobayashi S. Angew. Chem., Int. Ed. 2017, 56, 4520. 10.1002/anie.201611374. [DOI] [PubMed] [Google Scholar]
- Selected reports on alternate syntheses and biological evaluation of structurally related compounds:; a Shiozawa A.; Ichikawa Y.; Ishikawa M.; Kogo Y.; Kurashige S.; Miyazaki H.; Yamanaka H.; Sakamoto T. Chem. Pharm. Bull. 1984, 32, 995. 10.1248/cpb.32.995. [DOI] [PubMed] [Google Scholar]; b Shiozawa A.; Ichikawa Y.; Komuro C.; Ishikawa M.; Furuta Y.; Kurashige S.; Miyazaki H.; Yamanaka H.; Sakamoto T. Chem. Pharm. Bull. 1984, 32, 3981. 10.1248/cpb.32.3981. [DOI] [PubMed] [Google Scholar]; c Clark R. D.; Repke D. B.; Berger J.; Nelson J. T.; Kilpatrick A. T.; Brown C. M.; MacKinnon A. C.; Clague R. U.; Spedding M. J. Med. Chem. 1991, 34, 705. 10.1021/jm00106a036. [DOI] [PubMed] [Google Scholar]; d Prokai-Tatrai K.; Zoltewicz J. A.; Kem W. R. Tetrahedron 1994, 50, 9909. 10.1016/S0040-4020(01)89606-X. [DOI] [Google Scholar]; e Gatta F.; Giudice M. R. D.; Mustazza C. J. Heterocycl. Chem. 1996, 33, 1807. 10.1002/jhet.5570330642. [DOI] [Google Scholar]; f Shah U.; Lankin C. M.; Boyle C. D.; Chackalamannil S.; Greenlee W. J.; Neustadt B. R.; Cohen-Williams M. E.; Higgins G. A.; Ng K.; Varty G. B.; Zhang H.; Lachowicz J. E. Bioorg. Med. Chem. Lett. 2008, 18, 4204. 10.1016/j.bmcl.2008.05.069. [DOI] [PubMed] [Google Scholar]; g Gómez E.; Marco-Contelles J.; Soriano E.; Jimeno M. L. Tetrahedron 2009, 65, 9224. 10.1016/j.tet.2009.09.013. [DOI] [Google Scholar]
- a Zhang C.; Seidel D. J. Am. Chem. Soc. 2010, 132, 1798. 10.1021/ja910719x. [DOI] [PubMed] [Google Scholar]; b Das D.; Richers M. T.; Ma L.; Seidel D. Org. Lett. 2011, 13, 6584. 10.1021/ol202957d. [DOI] [PubMed] [Google Scholar]; c Kang Y.; Seidel D. Org. Lett. 2016, 18, 4277. 10.1021/acs.orglett.6b02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected related reports:; a Cohen N.; Blount J. F.; Lopresti R. J.; Trullinger D. P. J. Org. Chem. 1979, 44, 4005. 10.1021/jo01336a066. [DOI] [Google Scholar]; b Bi H.-P.; Teng Q.; Guan M.; Chen W.-W.; Liang Y.-M.; Yao X.; Li C.-J. J. Org. Chem. 2010, 75, 783. 10.1021/jo902319h. [DOI] [PubMed] [Google Scholar]; c Yang D.; Zhao D.; Mao L.; Wang L.; Wang R. J. Org. Chem. 2011, 76, 6426. 10.1021/jo200981h. [DOI] [PubMed] [Google Scholar]; d Kaboudin B.; Karami L.; Kato J. Y.; Aoyama H.; Yokomatsu T. Tetrahedron Lett. 2013, 54, 4872. 10.1016/j.tetlet.2013.06.129. [DOI] [Google Scholar]; e Manjappa K. B.; Jhang W.-F.; Huang S.-Y.; Yang D.-Y. Org. Lett. 2014, 16, 5690. 10.1021/ol5027574. [DOI] [PubMed] [Google Scholar]; f Samala S.; Singh G.; Kumar R.; Ampapathi R. S.; Kundu B. Angew. Chem., Int. Ed. 2015, 54, 9564. 10.1002/anie.201504429. [DOI] [PubMed] [Google Scholar]; g Dighe S. U.; K. S. A. K.; Srivastava S.; Shukla P.; Singh S.; Dikshit M.; Batra S. J. Org. Chem. 2015, 80, 99. 10.1021/jo502029k. [DOI] [PubMed] [Google Scholar]; h Tang M.; Tong L.; Ju L.; Zhai W.; Hu Y.; Yu X. Org. Lett. 2015, 17, 5180. 10.1021/acs.orglett.5b02484. [DOI] [PubMed] [Google Scholar]; i Jin Z.-n.; Jiang H.-j.; Wu J.-s.; Gong W.-z.; Cheng Y.; Xiang J.; Zhou Q.-Z. Tetrahedron Lett. 2015, 56, 2720. 10.1016/j.tetlet.2015.04.018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.