Brassicaceae hydathode anatomy affects infection by the adapted vascular pathogen Xanthomonas campestris pv campestris.
Abstract
Hydathodes are water pores found on leaves of a wide range of vascular plants and are the sites of guttation. We report here on the detailed anatomy of cauliflower (Brassica oleracea) and Arabidopsis (Arabidopsis thaliana) hydathodes. Hydathode surface presents pores resembling stomata giving access to large cavities. Beneath, the epithem is composed of a lacunar and highly vascularized parenchyma offering a direct connection between leaf surface and xylem vessels. Arabidopsis hydathode pores were responsive to ABA and light similar to stomata. The flg22 flagellin peptide, a well-characterized elicitor of plant basal immunity, did not induce closure of hydathode pores in contrast to stomata. Because hydathodes are natural infection routes for several pathogens, we investigated hydathode infection by the adapted vascular phytopathogenic bacterium Xanthomonas campestris pv campestris (Xcc), the causal agent of black rot disease of Brassicaceae. Microscopic observations of hydathodes six days postinoculation indicated a digestion of the epithem cells and a high bacterial multiplication. Postinvasive immunity was shown to limit pathogen growth in the epithem and is actively suppressed by the type III secretion system and its effector proteins. Altogether, these results give a detailed anatomic description of Brassicaceae hydathodes and highlight the efficient use of this tissue as an initial niche for subsequent vascular systemic dissemination of Xcc in distant plant tissues.
During their life, plants need a continuous water flow to supply stems and leaves with water, organic molecules, and inorganic ions. The transport of these substances from the roots to the leaves is performed through the xylem and mostly enabled by the combined action of root pressure and transpiration. When transpiration decreases (e.g. darkness, high CO2, drought, etc.), xylem sap transport mostly relies on root pressure and results in guttation of fluid from the leaf margin at hydathodes (Grunwald et al., 2003). Guttation and hydathodes are observed in a wide range of vascular plants belonging to dicot and monocot lineages as well as in pteridophytes and some aquatic plants (Chen and Chen, 2005). For instance, in Brassicaceae, hydathodes are found along the leaf margin, at the junction of adaxial and abaxial surfaces and in front of the vascular terminations (Kawamura et al., 2010). In such epithemal hydathodes, guttation is considered as a passive process (Haberlandt, 1914). Fluids are proposed to exude via aqueous pores present in hydathode epidermis, which open to a group of thin-walled and small cells with abundant intercellular spaces called epithem. The epithem connects directly with the vasculature of the plant. Guttation fluid contains minerals (e.g. calcium, potassium, iron, and magnesium), sugars (e.g. Glc, Gal, and Fru), and amino acids (e.g. Asp, Gln, and His; Goatley and Lewis, 1966; Pilot et al., 2004). Besides auxin biosynthesis (Aloni et al., 2003; Scarpella et al., 2006; Wang et al., 2011; Baylis et al., 2013), physiological roles of hydathodes are mostly unknown. Yet, analyses of their anatomy and physiology suggest an active and selective role in solutes exchange (Lagarde et al., 1996; Shibagaki et al., 2002; Pilot et al., 2003; Evert, 2006; Sutton et al., 2007). Interestingly, pathogenesis-related proteins are enriched in guttation fluids of healthy barley plants relative to total leaf extracts (Grunwald et al., 2003). These results suggest that hydathodes could build one of the multiple plant immune layers mounted against vascular pathogens such as Xanthomonas axonopodis pv dieffenbachiae, Xanthomonas oryzae pv oryzae, or Xanthomonas campestris pv campestris (Xcc), which have been reported to preferentially infect hydathodes (Russell, 1898; Hugouvieux et al., 1998; Niño-Liu et al., 2006; Bhat et al., 2010). Intraspecific variations in the tropism for stomata or hydathodes are observed that can result in distinct diseases. For instance, strains of pathovars oryzae and oryzicola of X. oryzae enter rice leaves by hydathodes or stomata causing bacterial leaf blight and bacterial leaf streak, respectively. Similarly, strains of pathovar raphani of X. campestris enter plant leaves through stomata, only causing bacterial leaf spot when pathovar campestris enters hydathodes and invades the xylem to cause black rot disease. Some Pseudomonas syringae strains appear to enter mostly by stomata but can in some cases also initiate vascular infection from the hydathodes (Yu et al., 2013). Yet, the underlying bacterial genetic determinants for such differential behaviors remain to be identified (Bogdanove et al., 2011).
Among plant immune layers (reviewed in Jones and Dangl, 2006), so-called preinvasive immunity limits entry of microbes and pathogens inside plant tissues (Sawinski et al., 2013). For instance, the cuticular wax limits pathogen adhesion on leaf surface (Marcell and Beattie, 2002; Serrano et al., 2014). Microbes also harbor conserved microbe- or pathogen-associated molecular patterns (MAMP/PAMP) molecules (e.g. chitin, flagellin, lipopolysaccharides, peptidoglycan, etc.), which perception induces stomatal closure to restrict microbial access to leaf mesophyll (Melotto et al., 2006; Gudesblat et al., 2009a; Sawinski et al., 2013). Some pathogens such as P. syringae (Melotto et al., 2006) or Xcc (Gudesblat et al., 2009b) produce molecules that are able to reopen stomata to efficiently enter the plant. Besides inducing stomatal closure, MAMP/PAMP perception also elicits an effective postinvasive basal immunity referred to as PAMP-triggered immunity (PTI), which efficiently limits the colonization of tissues, microbial multiplication, and disease symptom development (Jones and Dangl, 2006). A key feature of phytopathogens compared to regular microbes is their ability to suppress multiple layers of plant immunity. Phytopathogenic bacteria block PTI thanks to effector (T3E) proteins injected inside plant cells using their type 3 secretion (T3S) system resulting in effector-triggered susceptibility (Jones and Dangl, 2006). In most Gram-negative phytopathogenic bacteria, these T3S systems and their T3E proteins are collectively essential for pathogen virulence (Büttner and Bonas, 2002; Block et al., 2008; Kay and Bonas, 2009). Yet, the specific detection of T3E by plant NOD-like receptors may trigger rapid immune responses (effector-triggered immunity, or ETI), which limit pathogen spread and multiplication (Jones and Dangl, 2006).
X. campestris species encompasses Brassicaceae-infecting Xanthomonas (Vauterin et al., 1995), which are further subdivided in three pathovars: raphani (Xcr, mesophyll pathogen), incanae, and campestris (vascular pathogens; Fargier and Manceau, 2007). Xcc is the causal agent of black rot disease, the most serious bacterial disease of Brassica crops (e.g. cabbages, mustard, or radish) worldwide (Williams, 1980; Vicente and Holub, 2013). Importantly, Xcc is also a natural pathogen of the wild model plant Arabidopsis (Arabidopsis thaliana; Kniskern et al., 2007). Xcc can be transmitted by infected seeds or crop debris in soil, water splashes, tools, or adjacent infected plants (weeds or crops; Cook et al., 1952; Williams, 1980; Kuan et al., 1986; Dzhalilov and Tiwari, 1995; Köhl and Wolf, 2005). Hallmark for black rot disease are the formation of V-shaped lesions at the leaf margin, which reflect its predominant entry via hydathodes and the blackening of the veins highlighting vascular infection (Bretschneider et al., 1989; Buell, 2002). Epiphytic Xcc can also enter plant vessels directly through wounds (Williams, 1980) but seems unable to enter stomata of mature Arabidopsis leaves (Hugouvieux et al., 1998). Once inside the leaf xylem vessels, the bacteria multiply and move systemically to upper leaves and inflorescences (Sutton and Williams, 1970; Akimoto-Tomiyama et al., 2014). Xcc pathogenicity relies on pathogenicity determinants important for adhesion to the plant, the invasion of plant apoplast, the dampening of plant immunity, and also the uptake of nutrients (reviewed in Büttner and Bonas, 2010). For instance, Xcc produces large amounts of extracellular polysaccharides (xanthan) or lipopolysaccharides to protect itself from environmental stresses and promote its epiphytic survival and in planta growth (Dow et al., 1995, 2003; Silipo et al., 2005; Torres et al., 2007). Xcc also presents protein secretion systems, two of which are major pathogenicity determinants: Xcc type II secretion (T2S) systems (xps and xcs) export proteins such as proteases or cell-wall-degrading enzymes from the bacterial periplasm to the extracellular space. Only xps has been described as important for pathogenicity (Qian et al., 2005). The T3S system and its 20 to 30 T3E proteins directly injected inside plant cells are essential for Xcc pathogenicity (Arlat et al., 1991; Guy et al., 2013a; Roux et al., 2015). These T3E proteins are likely important to suppress PTI induced by Xcc PAMPs such as peptidoglycan (Erbs et al., 2008), enigmatic MAMP of Xanthomonas (Jehle et al., 2013), and lipo-oligosaccharides (Proietti et al., 2014). To date, the only known T3E capable of triggering ETI is AvrAC/XopAC, which is indirectly recognized in some Arabidopsis ecotypes by the NOD-like receptor complex ZAR1/RKS1 upon modification of the PBL2 decoy kinase by AvrAC (Xu et al., 2008; Guy et al., 2013b; Wang et al., 2015). All these analyses bypassed hydathode infection and likely ignored some important pre- and postinvasive immune mechanisms occurring in natural infections.
In this study, we provide a thorough description of healthy cauliflower (Brassica oleracea) and Arabidopsis hydathodes as well as hydathodes infected by the Brassicaceae-adapted Xcc bacteria. These results evidenced the importance of both postinvasive plant immunity at hydathodes and Xcc type III secretion machinery in the suppression of this immunity. Thus, the molecular dialog established between plant cells and bacteria at hydathodes determines the outcome of the interaction.
RESULTS
Cauliflower Hydathodes Are Covered with Pores Resembling Stomata
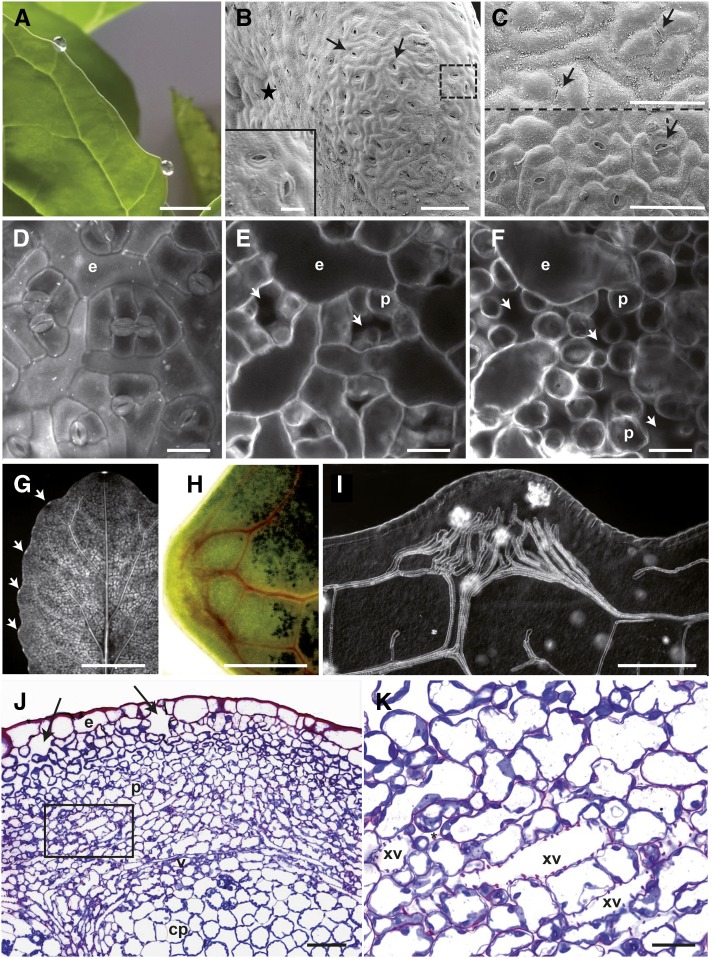
Guttation could be observed on cauliflower leaves (Brassica oleracea var botrytis cv Clovis) at high humidity in darkness (Fig. 1A). These droplets accumulate at the leaf margin in front of veins in structures called hydathodes. In order to determine the surface anatomy of this tissue, cauliflower leaf margins were observed by cryo-electron scanning microscopy revealing the presence of protrusions of ∼500 µm diameter (0.19 mm2 ± 0.05) slightly oriented toward the abaxial face of the leaf (Fig. 1B; Supplemental Table S1). The hydathode epidermis has a very reduced wax layer on its surface (Fig. 1C) and is covered by pores that resemble stomata present on the leaf blade. Though pores appeared significantly more open for hydathode pores than stomata, guard cells sizes were similar (Supplemental Table S1). Hydathode pore density was also comparable to the stomatal density on the abaxial leaf epidermis (∼200/mm2; Supplemental Table S1). Yet, hydathode epidermal cell area was twice as large as found on the leaf blade. Therefore, the hydathode epidermis is characterized by large epidermal cells and pores morphologically indistinguishable from stomata.
Figure 1.
General organization of cauliflower hydathodes (B. oleracea var botrytis) cultivar Clovis. A, Macroscopic view of guttation droplets exuding through hydathodes in the prolongation of lateral veins. Scale bar = 1 cm. B and C, Fresh cryoprepared samples observed by cryoscanning electron microscopy. The adaxial faces are marked with a black star. Scale bars = 100 µm. B, Front view of a hydathode showing numerous pores (dashed arrows). The insert is a magnification over two hydathode pores corresponding to the area indicated by a dashed square. Scale bar = 20 µm. C, View of the adaxial part of the leaf blade (top) and of the hydathode (bottom). Note the reduced wax ornamentation on the hydathode protrusion compared to the leaf blade. Arrows indicate stomata or hydathode pores. D to F, Confocal planes of a series in z dimension of the abaxial face at hydathode. Scale bars = 25 µm. The sample was clarified and stained with calcofluor (0.01%). One hundred micrometers separate the surface (D) from the deepest plane (F). D, Visualization of numerous pores and large epidermal cells (e). E and F, Below the pore, large chambers (arrows) are delimited by small parenchyma cells of the epithem. G, Macroscopic fluorescence imaging of a detached leaf fed overnight with calcofluor by the petiole. Staining of the vasculature and bright fluorescent hydathodes (arrows) are observed. Scale bar = 4 cm. H, Bright-field microscopy of a hydathode from a detached leaf fed overnight by its petiole with Congo red. Scale bar = 200 µm. I, Observation in Nomarsky of a clarified hydathode sample. Note the numerous xylem vessel endings and complex ramification of the vasculature at the hydathode. Scale bar = 200 µm. J and K, Bright-field microscopy of thin paradermal sections from resin-embedded hydathode. J, General view of the tissular organization. The epithem composed of parenchyma (p) cells is covered by an epidermal layer (e). Note the large chambers (arrows) beneath the hydathode pores. The cortical parenchyma (cp) below the veins (v) is composed of large parenchyma cells. Scale bar = 100 µm. K, Magnification of the rectangle indicated in J to better visualize the xylem vessels within the epithem. Note the numerous wide intercellular spaces (*). Scale bar = 50 µm.
Hydathodes of Arabidopsis (ecotype Col-0) were subjected to a comparable analysis and, besides their reduced dimensions and the presence of apical trichomes compared to cauliflower, revealed a similar surface organization to cauliflower (Supplemental Fig. S1, A–C; Supplemental Table S2). It was previously shown in Arabidopsis that the MUTE gene was required for the differentiation of both stomata and hydathode pores, suggesting that hydathode pores and stomata might have a common developmental origin (Pillitteri et al., 2008). In order to test this hypothesis further, the expression of stomata-specific markers was followed at hydathode pores (Supplemental Fig. S2). Interestingly, reporter expression of E1728 Gal4:GFP and FAMAp:YFP-YFP transgenic lines could be observed in Arabidopsis hydathode pores, suggesting a common identity with stomata guard cells. Thus, both Arabidopsis and cauliflower hydathodes are protrusions of the leaf margin covered with pores similar to stomata.
Cauliflower Hydathodes Are Highly Vascularized Multilayer Tissues
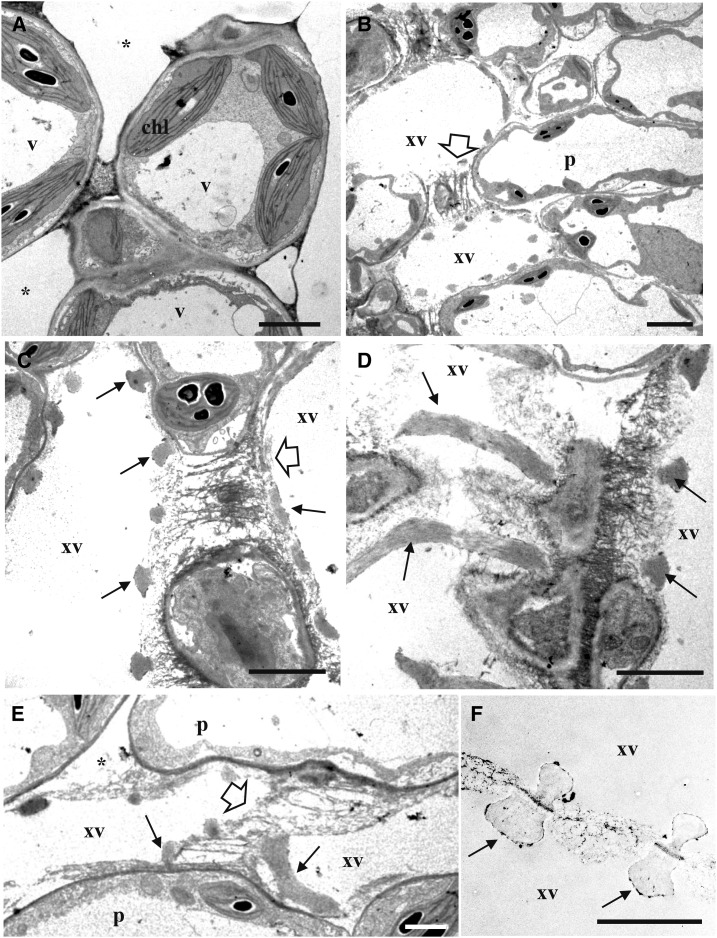
We next wanted to describe the inner ultrastructure of cauliflower hydathodes. To this end, confocal microscopy of clarified hydathodes stained with calcofluor was first used (Fig. 1, D–F). Large cavities also known as “water cavities” could be detected below the hydathode pores as well as large intercellular space within a parenchyma composed of small globular cells. These parenchyma cells were three to four times smaller in area compared to the cortical parenchyma (Supplemental Table S1). Interestingly, petiole-fed stains such as calcofluor or Congo red were quickly taken up by the leaf and accumulated strongly in the vasculature and hydathodes (Fig. 1, G and H). This suggests that hydathode parenchyma is tightly connected to the xylem. The observation of clarified hydathode by Nomarsky pointed out the high vascularization of the hydathode with more than 30 ± 5 xylem terminations on average (Supplemental Table S1). These observations were further confirmed by thin sections of fixed and embedded hydathodes (Fig. 1, J and K). The presence of large cavities below the hydathode pores was confirmed. The parenchyma made of small cells tightly associated to xylem tubes defined a tissue called epithem. The veins clearly separate this epithem from the cortical parenchyma made of larger cells. Transmission electron microscopy of ultra-thin sections of epithem stained with periodic acid-thiocarbohydrazide-silver proteinate reaction (PATAg) revealed the tight association between the parenchyma cells and xylem vessels and the presence of large intercellular space within the tissue (Fig. 2). In particular, the xylem-to-xylem cell wall appeared very fibrillar and loose similar to the one observed outside hydathodes (Fig. 2F). Once again, the inner anatomy of Arabidopsis hydathodes was very comparable to that of cauliflower (Supplemental Fig. S1, D and E; Supplemental Table S2). Yet, the extent of vascularization was lower (13 ± 4 xylem terminations/hydathode) in agreement with the reduced size of the tissue. In conclusion, cauliflower and Arabidopsis are typical epithemal hydathodes, i.e. a tissue highly vascularized by numerous xylem vessels irrigating a small-cell parenchyma.
Figure 2.
Observations of the epithem of cauliflower hydathodes by transmission electron microscopy. Ultrathin paradermal sections were stained with PATAg to visualize polysaccharides. A, The epithem, below the epidermal layer, is composed of vacuolated (v) parenchyma cells (p) with chloroplasts (chl) containing heavily stained starch granules. Note the presence of large intercellular spaces (*). B to E, Visualization of xylem vessels (xv) within the epithem. Each xylem vessel exhibit annular thickenings (arrows). A loose fibrillar matrix is observed between adjacent xylem vessels (white arrow) and between vessels and adjacent epithem cells (*). F, A comparable fibrillar matrix is also observed between xylem vessels outside the hydathode tissue. Scale bars = 3 µm.
Arabidopsis Hydathode Pores Are Responsive to Abiotic Stimuli
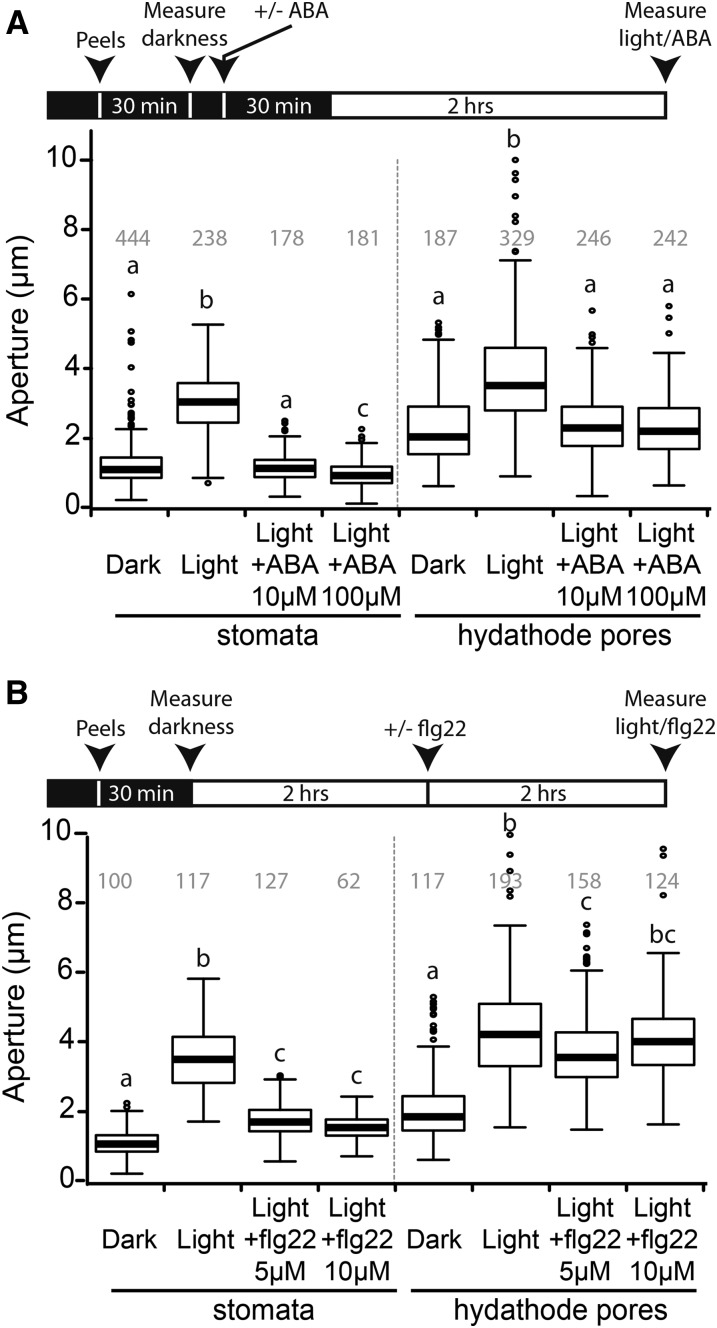
While stomata are well known to modify their aperture in response to abiotic stimuli such as light, CO2 or the plant hormone abscisic acid (ABA), hydathode pores were so far reported to be unable to regulate their aperture (Stevens, 1959; Pillitteri and Dong, 2013; Hossain et al., 2016). We thus measured the pore apertures on epidermal peels of Arabidopsis ecotype Col-0 for stomata and hydathode pores in response to light and ABA. In darkness, hydathode pores were more open than stomata (Fig. 3, A and B): 1.3 ± 0.6 µm and 2.4 ± 1.0 µm for stomata and hydathode pores, respectively. After 2 h light treatment, both hydathode pores and stomata opened, and this aperture could be efficiently blocked by 10 or 100 µm ABA pretreatment (Fig. 3A). These results demonstrate that Arabidopsis hydathode pores are as responsive as stomata to abiotic stimuli but are more open than stomata in all conditions tested (nonparametric Kruskal-Wallis test, P < 0.01).
Figure 3.
Arabidopsis hydathode pores are responsive to light and ABA but fail to close in response to flg22 peptide treatment. Box plot representations of aperture (µm) of stomata and hydathode pores. At least three independent experiments were performed except for 5 and 10 µm flg22 treatment on stomata (two and one independent experiments, respectively). Number of measured pores are indicated in gray. Statistical groups were determined using a nonparametric Kruskal-Wallis test (P < 0.001) and are indicated by different letters. A, Apertures were measured on epidermal peels after 30 min incubation in darkness. Peels were then preincubated for 30 min in darkness with 0 to 100 µm ABA followed by a 2-h light incubation prior to aperture measurements. B, Apertures were measured on epidermal peels after 30 min incubation in darkness. Peels were then preincubated for 2 h in light. Peels were further incubated for 2 h with 0 to 10 µm flg22 prior to measurements.
Hydathodes Are Preferential Entry Points for Xcc
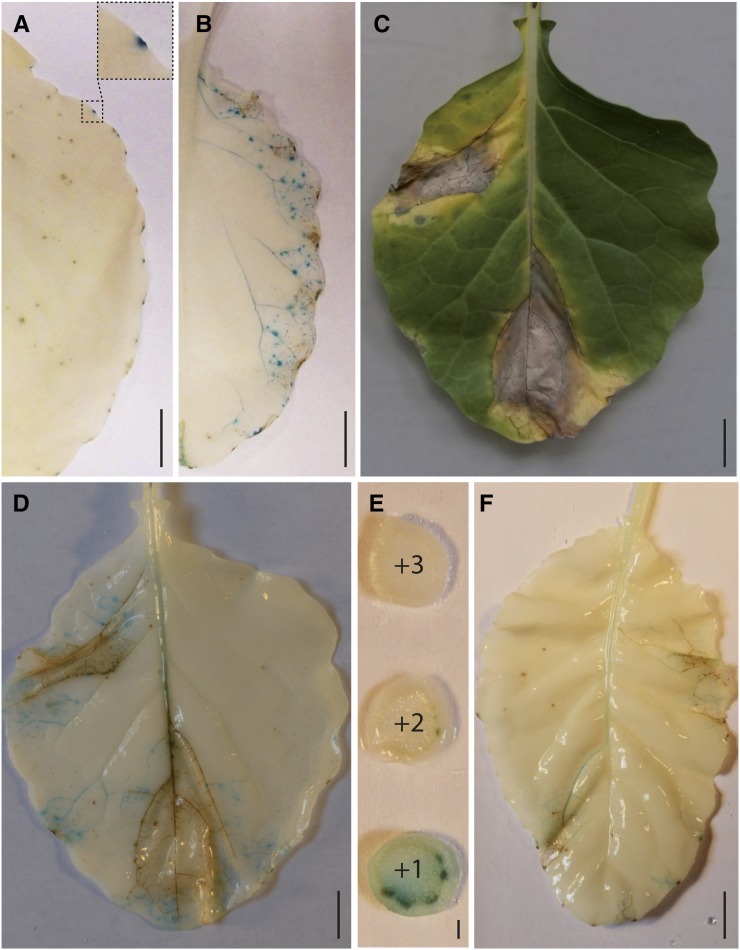
Hydathodes are known to be entry points for several vascular pathogens, including Xcc, the causal agent of black rot disease of Brassicaceae, such as cauliflower or Arabidopsis. In order to be able to track bacterial entry, multiplication, and spread in planta, a GUS-GFP translational fusion was stably inserted in Xcc strain 8004 chromosome under the control of a strong constitutive promoter. Cauliflower leaves were dip-inoculated in a suspension of the virulent Xcc strain 8004::GUS-GFP and stained for GUS activity (Fig. 4). At 6 d postinoculation (dpi), GUS staining was detected in hydathodes only, indicating that the bacteria failed to enter stomata but could accumulate in hydathodes (Fig. 4A). At 13 dpi, brown staining appeared at hydathodes, and GUS staining was observed in the vasculature in addition to more focal staining (Fig. 4B). At 16 dpi, large necrotic lesions without GUS staining were observed, as well as a GUS staining of the vasculature that reached the midvein of the leaf (Fig. 4, C and D). This indicates that necrotic lesions do not contain viable bacteria and that Xcc is capable of systemic infection. Xcc was indeed detected in the vasculature of stem sections situated above the infected leaf (Fig. 4E) and the noninoculated leaf just above (Fig. 4F). Staining in the midvein was observed as well as in hydathodes suggesting that, during systemic infection, Xcc also accumulates in hydathodes and resumes infection from hydathodes again. Frequent hydathode browning was observed. A bioluminescent Xcc strain LMG568::lux (Meyer et al., 2005) was also used to follow leaf infection in a nondestructive manner (Supplemental Fig. S3). Hydathode infection could be observed as early as 3 dpi as well as progressive accumulation of Xcc in the vasculature. In conclusion, we could establish efficient hydathode infection in lab conditions, observe typical black rot disease symptoms mimicking those observed in natural infection settings, and develop the resources to track Xcc accumulation in the course of systemic infection.
Figure 4.
X. campestris pv campestris is a systemic vascular pathogen entering the plant leaf through hydathodes. Xcc strain 8004::GUS-GFP was inoculated by dipping and tracked in plant tissues by GUS staining (blue) of infected leaves. GUS activity was visualized after destaining leaves in alcohol. A, Xcc is localized at leaf margin into hydathodes only, 6 d after dip inoculation (the squared zone presents an enlargement of an infected hydathode). B, Xcc spreads all along the leaf vasculature 13 d after dip inoculation. C, Typical V-shaped lesions and necrotic symptoms on a leaf 16 d after dip inoculation. D, The absence of staining in necrotic areas indicates that no living Xcc are to be found in dead tissues. The leaf is the same as in C. E, Xcc follows the vasculature. Bacteria can be found in the vasculature of the stem internodes 16 d after dip inoculation. Internode sections in the ascending order (+1 to +3) starting immediately above the dip-inoculated leaf. F, Noninoculated leaf situated above the +1 internode showing systemic bacterial multiplication (blue) 16 d after dip inoculation of the leaf situated immediately below internode +1. Scales bars = 1 cm in A to D and F and 1 mm in E.
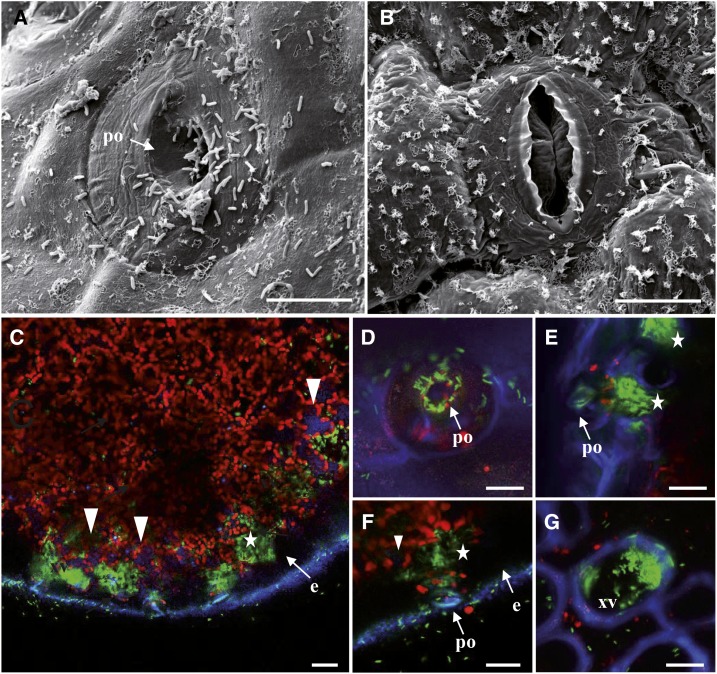
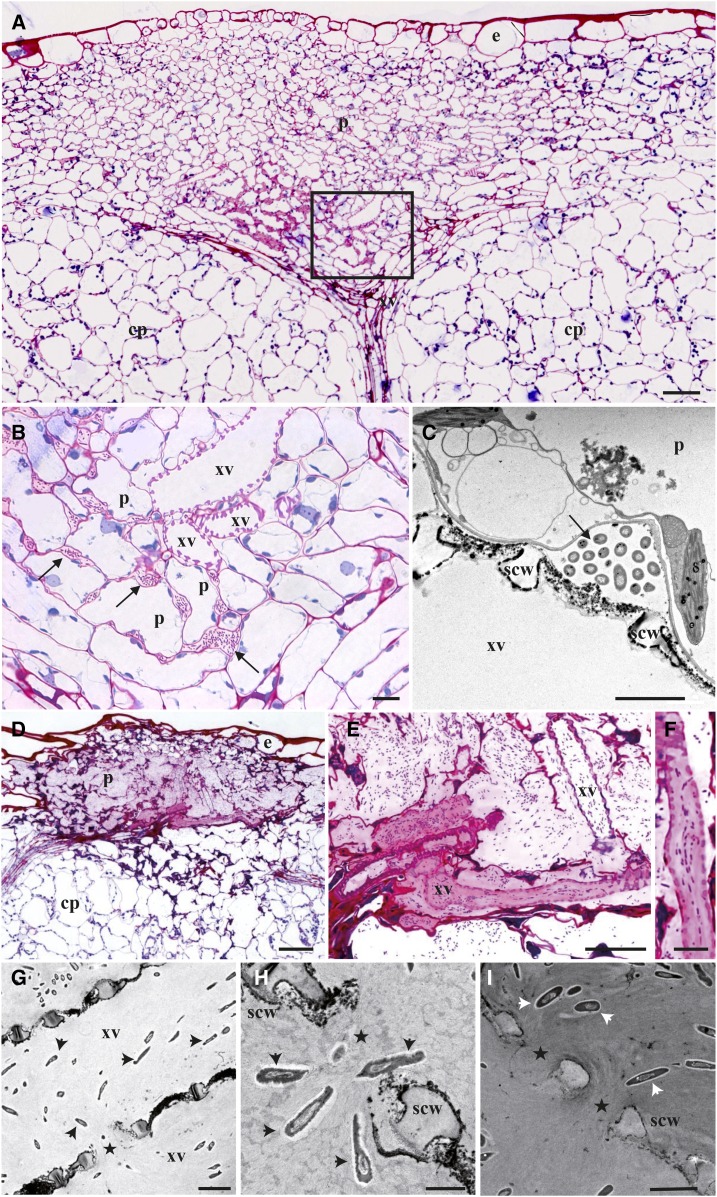
A microscopic analysis of hydathode infection was then conducted upon dip inoculation using Xcc strain 8004::GUS-GFP (Fig. 5). Many bacteria could be observed near and on hydathode pores at 3 dpi by scanning electron microscopy compared to none at stomata (Fig. 5, A and B). The difference in wax content at the leaf surface is similar to that observed previously (Fig. 1C). Confocal microscopy was used to track bacteria inside leaf tissues at 3 dpi (Fig. 5, C–G). Bacteria accumulated around hydathode pore’s openings (Fig. 5D) and in cavities located below as a result of bacterial entry and multiplication (Fig. 5, E and F). Bacteria could also be observed at 6 dpi inside the xylem vessels of the main leaf rib (Fig. 5G). At 3 dpi, hydathode ultrastructure observed in thin sections appeared globally unaffected by infection when compared to Figure 1J (Fig. 6, A–C). Surprisingly, bacteria were not observed near the outer epidermis but mostly found deep in the epithem, in intercellular space close to the xylem vessels where microcolonies started to form. Transmission electron microscopy of ultra-thin sections of epithem stained with PATAg showed that the primary cell wall of xylem vessels was not breached yet and no bacteria were observed in the vasculature (Fig. 6C). These observations highlight the progress made by Xcc cells between epithem cells. At 6 dpi, the epithem was highly colonized by bacteria, and its parenchyma cell walls were almost totally dissolved (Fig. 6, D–F). Numerous bacteria were found aligned in xylem vessels along the presumed direction of the flow. A pink fuchsine-reactive matrix was correlated with increased bacterial densities. Transmission electron microscopy of epithem stained with PATAg revealed the loss of the primary cell wall of xylem vessels, leaving only their spiral secondary cell wall appositions (scw; Fig. 6G). Bacterial entry by these xylem openings could be visualized (Fig. 6H). PATAg-reactive matrix around bacteria appeared nonrandomly oriented, as if it was leaking out of xylem vessels or inversely (Fig. 6, H and I). These observations revealed the extent of epithem degradation at 6 dpi and xylem colonization by degradation of the xylem primary cell wall.
Figure 5.
Cauliflower infected by Xcc strain 8004::GUS-GFP. A and B, Typical scanning electron micrographs of a pore at the hydathode’s surface (A) and a stomata at the leaf surface (B) 3 dpi by transient dipping in the bacterial suspension. Scale bars = 10 µm. A, Note the presence of a large number of bacteria rods inside the hydathode pore (po) and at the surface of the epidermal layer. B, Bacteria are not observed near stomata of the leaf blade. Note the numerous wax ornamentations on the epidermis. C to G, Confocal images of cauliflower infected by GFP expressing bacteria at 3 dpi (C–F) and 6 dpi (G). Images are maximal projections computed from 15 to 25 confocal planes acquired in the z dimension (increment of 0.5 µm). Scale bars = 20 µm. C, Paradermal optical section (parallel to the epidermis) of an infected hydathode at 3 dpi. GFP-labeled bacteria are mainly located in large pockets (white stars) beneath the epidermis (e). Note the absence of bacteria within the epidermal cells (e) and the faint blue autofluorescence inside some parenchyma cells (white arrowheads). D, Visualization of GFP-labeled Xcc at the hydathode pore level (comparable to scanning electron micrograph in A). E to F, Detailed localization of GFP-labeled bacteria in large pockets (white stars) beneath the pore (po) of the hydathode. G, Detection of GFP-labeled bacteria in a xylem vessel (xv) in a transversal section of the mid rib.
Figure 6.
Infection of cauliflower hydathode by Xcc strain 8004::GUS-GFP results in near-complete epithem degradation. Paradermal sections of infected hydathodes were observed by optical or electron microscopy at 3 (A–C) and 6 dpi (D–I). A, General view of an infected hydathode at 3 dpi shows little impact on epithemal parenchyma (p) organization and limited colonization of intercellular space, in vicinity to xylem vessels (xv). Epidermis (e) and cortical parenchyma (cp) are indicated. Scale bar = 50 µm. B, Detail of the localization of bacteria within the hydathode. Close up of the boxed area shown in A. Arrows indicate sites of bacterial accumulation. Scale bar = 10 µm. C, Transmission electron micrograph of bacteria between epithemal parenchyma cells (p) and xylem vessels (xv) of infected hydathode (3 dpi). The sections are treated with PATAg for the visualization of polysaccharides. Xylem vessels are characterized by the presence of lignified scw thickenings along the thin PATAg-reactive primary cell wall. Arrows indicate sites of bacterial accumulation. Scale bar = 2 µm. D, General view of an infected hydathode at 6 dpi illustrating a highly degraded ultrastructure. Note the differential staining between the infected hydathode area and the other tissues (pink stained area between the epidermis and the cortical parenchyma [cp]). Scale bar = 50 µm. E and F, Details of the localization of bacteria within the hydathode at 6 dpi. Scale bars = 20 and 10 µm, respectively. F, Note the alignment of rod-shaped bacteria in a xylem vessel of the hydathode. G to I, Transmission electron micrograph of bacteria (arrowheads) in the xylem vessels of infected hydathode (6 dpi). Scale bars = 2 µm. All the sections are treated with PATAg. In xylem vessels, bacteria are surrounded by a PATAg-stained matrix and are observed within degraded primary cell wall area (stars) between lignin thickenings (scw; H and I).
Hydathodes Do Not Mount a Detectable Preinvasive Immunity against Xcc
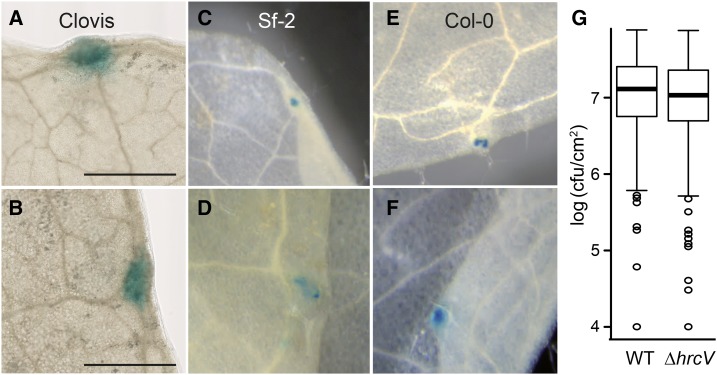
We investigated whether hydathode pore’s closure upon Xcc PAMP perception might build a first layer of preinvasive immunity as described for the stomata-P. syringae interaction. In preliminary observations, cauliflower hydathode pores appeared to lack selectivity for limiting entry of Xcc 8004::GUS-GFP or other inert particles (Supplemental Movie 1). Flagellin-derived PAMP flg22 peptide failed to elicit hydathode pore’s closure compared to bona fide stomata when applied to Arabidopsis epidermal peels (Fig. 3B). In order to test if stomata and hydathode pore closure might restrict Xcc entry inside leaf tissue, Arabidopsis mutants with constitutively opened stomata were dip inoculated with a compatible Xcc strain (8004::GUS-GFP∆avrAC; Supplemental Fig. S4, A and B). We selected the Arabidopsis lox1-2 mutant, in which stomata fail to respond to flagellin treatment but still respond to abiotic stimulations (Montillet et al., 2013), and the slac1-2 mutant, in which stomata do not respond to any environmental nor biotic signals (Negi et al., 2008; Montillet et al., 2013). Infection was exclusively observed from hydathodes of lox1-2 and slac1-2 plants (Supplemental Fig. S4A), suggesting that infection cannot be initiated from stomata. In those conditions, the lox1-2 mutant, in contrast to slac1-2, supported a significantly increased bacterial growth at 6 dpi (Supplemental Fig. S4A). Yet, lox1-2 mutants were also significantly more susceptible to Xcc upon direct wound inoculation at 6 dpi (Supplemental Fig. S4C), suggesting that LOX1 might also contribute to postinvasive immunity against Xcc. On the bacterial side, we tested the importance of the T3S system for hydathode colonization. To this end, we constructed in Xcc strain 8004 a deletion of the HrcV gene. HrcV encodes a core component of the T3S system, which was previously shown to be essential for T3E secretion and the virulence of Xanthomonas including Xcc (Arlat et al., 1991; Bonas et al., 1991; Rossier et al., 1999). In order to focus on bacterial entry and not multiplication, cauliflower or Arabidopsis leaves were immersed in a bacterial suspension (8004::GUS-GFP versus 8004::GUS-GFP∆hrcV) for 4 and 24 h, respectively, essentially as described (Hugouvieux et al., 1998) and directly stained for GUS activity or processed to measure bacterial populations (Fig. 7). In this setup, the amount of bacteria available for entry is nonlimiting, and a 1-d incubation only permits a limited bacterial multiplication. In these conditions, the T3S mutant did not show a significantly reduced accumulation in both susceptible (Cauliflower Clovis or Arabidopsis ecotype Sf-2) or resistant (Arabidopsis ecotype Col-0) plants. No mesophyll colonization could be detected. Finally, the proportion of infected cauliflower hydathodes was measured by sampling individual hydathodes 3 d post-dip inoculation with Xcc 8004 or 8004∆hrcV. Ninety-seven percent of hydathodes were infected with the 8004∆hrcV strain, versus 98% for the wild-type strain (n = 114). These results indicate that the T3S system is not required for bacterial entry inside hydathodes. In conclusion, these experiments suggest that hydathodes fail to block or prevent Xcc ingress via the hydathode pores and thus do not mount a strong preinvasive immunity against Xcc.
Figure 7.
Xcc type III secretion system is not required to enter hydathodes. A, B, and G, Detached leaves of B. oleracea var botrytis (cultivar Clovis) were incubated in a solution of Xcc strain 8004::GFP-GUS (wild type; A) or 8004ΔhrcV::GFP-GUS (∆hrcV; B) for 4 h. C to F, Attached leaves from Arabidopsis ecotype Sf-2 (C and D) or ecotype Col-0 (E and F) were incubated in a solution of wild type or ∆hrcV Xcc strains (D and F) for 24 h. Leaves were stained for GUS activity (blue) and destained in ethanol. Scale bar = 1 mm. G, Box plot representation of bacterial populations (cfu/cm2) were determined in individual hydathodes (cv Clovis) 4 h after immersion in a solution of wild-type (WT) or ∆hrcV Xcc strains. Three independent experiments were performed, and more than 25 hydathodes sampled per condition and per experiment. Populations were not statistically different using a nonparametric Kruskal-Wallis test (P < 0.05)
Hydathodes Mount a Postinvasive Immunity against Xcc
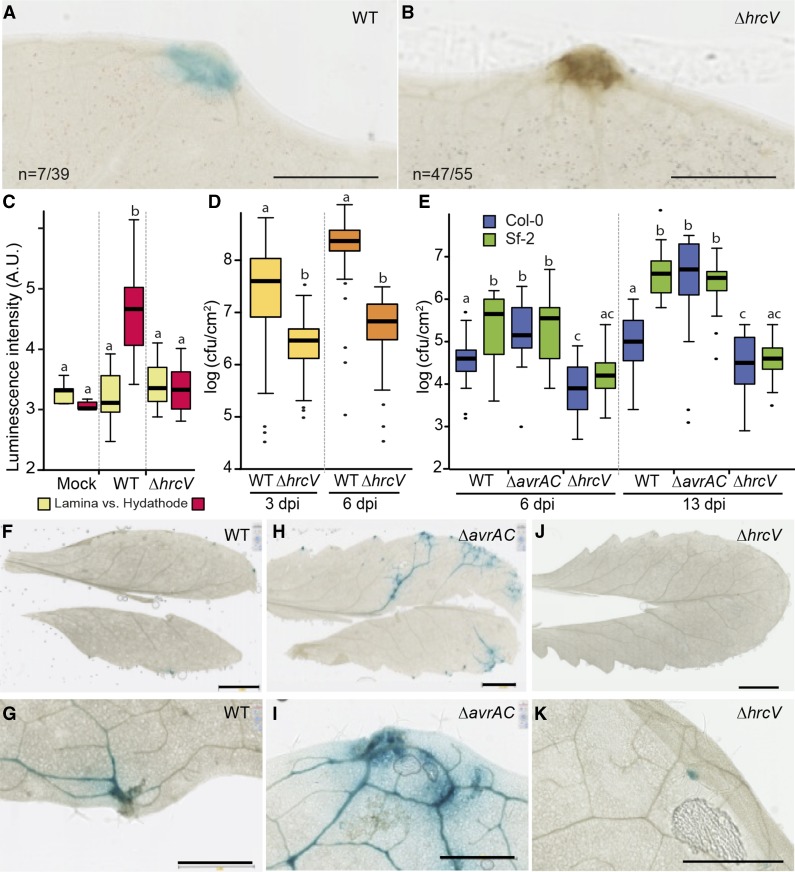
Postinvasive immune responses were investigated in both cauliflower and Arabidopsis hydathodes. We previously mentioned that a brown staining at infected cauliflower hydathodes was sometimes observed (Fig. 4F). At 3 dpi, a simple destaining of the tissues in ethanol revealed a brown staining at hydathodes, which was all the more pronounced when an 8004::GUS-GFP∆hrcV T3S mutant strain was inoculated compared to a wild-type strain (Fig. 8, A and B). This observation suggests that the T3S system suppresses some kind of necrotic response in infected hydathodes. Biophoton imaging emitted upon plant lipid oxidation was also used to visualize plant stress and immune responses (Bennett et al., 2005; Birtic et al., 2011). Interestingly, a significant photon emission was measured at 6 dpi in hydathodes with a wild-type Xcc, but not with a T3S mutant or mock inoculation (Fig. 8C; Supplemental Fig. S5). This observation suggests that light emission can be used as a marker for successful hydathode infection in a stain-/label-free system. We next tested Xcc wild-type or mutants (∆avrAC or ∆hrcV) strain 8004::GUS-GFP in planta multiplication and colonization of cauliflower and Arabidopsis tissues in compatible or incompatible interactions. In a compatible interaction with cauliflower, the hrcV mutant had a reduced growth at 6 dpi compared to wild type (Fig. 8D). Similarly, the susceptible Arabidopsis ecotype Sf-2 sustained lower growth of the hrcV mutant and no accumulation of Xcc was detected outside hydathodes (Fig. 8, E, J, and K). These results indicate that the T3S is required for in planta multiplication and vascular spread. We also investigated whether avrAC ETI might be observed upon hydathode infection. The resistant ecotype Col-0 was dip inoculated with wild-type or ∆avrAC mutant strains of Xcc. While the avirulent wild-type strain showed reduced multiplication and vascularization, the ∆avrAC mutant showed enhanced multiplication and vascular spread (Fig. 8, E–I). Thus, avrAC-dependent ETI restricts pathogen growth upon hydathode infection. These results indicate that the hydathode engages active postinvasive immune responses, which depend on Xcc T3S system and its effectors to control the disease.
Figure 8.
Xcc induces ETI and PTI responses in cauliflower and Arabidopsis hydathodes. A and B, Cauliflower leaves (Clovis cultivar) were dipped in a bacterial solution of Xcc strain 8004::GUS-GFP (wild type [WT]; A) and 8004::GUS-GFP∆hrcV (∆hrcV; B). Two dpi, GUS staining (blue) was performed and leaf cleared in ethanol prior to imaging. The proportion of necrotic hydathodes (brown) is given. One representative experiment out of three is shown. Scale bar = 1 mm. C, Box plot representation of the intensity of spontaneous photon emission (arbitrary units [A.U.]) was measured on lamina or hydathodes of infected cauliflower leaves at 6 dpi. Statistical groups were determined using a parametric Tukey test (P < 0.01) and are indicated by different letters. D, Box plot representation of bacterial titers (cfu/cm2) determined in individual hydathodes (1.77-mm2 leaf discs) of cauliflower 3 and 6 dpi. Three independent experiments were performed and more than 25 hydathodes sampled per condition and per experiment. Statistical groups were determined using a nonparametric Kruskal-Wallis test (P < 0.01) and are indicated by different letters. E to K, Bacterial multiplication and leaf colonization of Arabidopsis leaves (Col-0 or Sf-2 ecotypes) infected by dip inoculation with Xcc strain 8004::GUS-GFP wild type or mutant for the avrAC (∆avrAC) or hrcV (∆hrcV) genes. (E) Box plot representation of bacterial titers (cfu/cm2) determined on entire leaves at 6 and 13 dpi. F to K, Visualization of Arabidopsis ecotype Col-0 leaf colonization and bacterial multiplication by the wild type (F and G), ∆avrAC (H and I), and ∆hrcV (J and K) Xcc strains at 10 dpi. G, I, and K are enlargements of F, H, and J, respectively. Scale bars = 5 mm in F, H, and J and 1 mm in G, I, and K. Four independent experiments were performed. Statistical groups were determined using a nonparametric Kruskal-Wallis test (P < 0.01) and are indicated by different letters.
DISCUSSION
We presented here a detailed anatomy of healthy and infected hydathodes in Brassicaceae and demonstrated that Arabidopsis hydathode pores do respond to abiotic stimuli but not to flg22 treatment. We also show that preinvasive immunity at hydathodes or stomata has little effect of bacterial entry in plant tissues. We finally demonstrate that postinvasive immunity is mounted against Xcc in the epithem and suppressed by the T3S machinery.
A Detailed Cytological Atlas of Brassicaceae Hydathodes
Anatomy of hydathodes is poorly documented, even in Arabidopsis where no comprehensive histological studies have been reported. Yet our results are consistent with fragmental results previously published (Meier, 1934). The most striking feature of hydathode surface is the presence of pores that look very much like leaf stomata. This suggests that hydathode pores might share developmental and physiological pathways with stomata. In agreement, the Arabidopsis MUTE gene controls differentiation of both stomata and hydathode pores (Pillitteri et al., 2008) and both reporters of stomatal development tested here were also expressed in hydathode pores. As for their physiology, hydathode pores were always more open than stomata but fully responsive to ABA and light. These results contrast with previous reports on Arabidopsis and other plant systems (e.g. Caltha palustris, Colocasia esculenta, and Ficus formosana) where hydathodes are qualitatively presented as nonresponsive to environmental cues (Stevens, 1959; Chen and Chen, 2005; Pillitteri and Dong, 2013; Hossain et al., 2016). Because hydathodes are constantly supplied in guttation fluid, this aqueous microclimate may affect hydathode pore responses similar to stomata of the inner rosette of Arabidopsis, which are also less responsive to abiotic stimuli, probably as a result of reduced priming at high relative humidity (Pantin et al., 2013). It is thus tempting to speculate that the peculiar environment of the hydathode also limits pore priming. Despite the lack of transcriptomics approaches of hydathodes, the activity of most promoters in the epithem highlights the very strong transcriptional activity of this tissue. Some genes, such as MIR164A (Nikovics et al., 2006), are specifically expressed in the epithem, indicating that a particular transcriptional program is established in this tissue. We also observed a discrete activity of the CYCLIN B1 promoter in hydathodes of mature leaves (Supplemental Fig. S1F), suggesting that some meristematic activities may perdure in the epithem. Yet, hydathode physiology remains very enigmatic, and thorough transcriptomic, proteomic, and metabolomic approaches are needed to advance our understanding of the biology of this neglected plant tissue.
The Tropism of Xcc for Hydathodes Is Not Solely Determined by Stomatal Preinvasive Immunity
A differential tropism of certain bacterial pathogens for hydathodes or stomata can be observed at the intraspecific level (Vauterin et al., 1995; Hugouvieux et al., 1998; Niño-Liu et al., 2006). For Xcc, the bacteria appear to be unable to infect via stomata even after a high density dip inoculation of the entire leaf surface (Fig. 4). Several hypotheses could be proposed to explain such behavior and the observed accumulation of Xcc at hydathode pores but not stomata (Fig. 5, A and B). First, adhesion of bacteria might be easier to hydathodes, since lower wax accumulation was observed (Fig. 1C). Thus, rain or guttation droplets from upper leaves could result in a preferential accumulation of bacteria at leaf periphery. Second, Xcc might have a particular tropism for hydathodes, which could be due to a particular microclimate or the presence of attractant molecules. Repeated guttation may deposit such molecules or attractants at the surface of hydathodes. This scenario is reminiscent of Brassicaceae-adapted caterpillars, which are attracted by glucosinolates in contrast to other caterpillars (Verschaffelt, 1910; Blau et al., 1978). Glucosinolates are Brassicaceae-specific secondary metabolites important for immunity against herbivores and bacterial, oomycete, and fungal pathogens (Bednarek et al., 2009; Clay et al., 2009; Schlaeppi and Mauch, 2010; Bednarek, 2012). Third, Xcc might be poorly mobile on dry surfaces. Thus, Xcc may not be able to enter stomata in absence of a water continuum, while successful in entering hydathodes filled with liquids (Cook et al., 1952). Yet, little is known about both adhesion and motility of Xcc versus Xcr in the course of infection.
No Clear Evidence for the Existence of a Preinvasive Immunity at Hydathode Pores
Besides these hypotheses, bacteria may face differential preinvasive immunities at hydathode pores and stomata that could in part explain the differential Xcc/Xcr infectious behaviors. Stomata are known to close in response to PAMPs and restrict pathogen entry as described for P. syringae (Melotto et al., 2006). Interestingly, Arabidopsis hydathode pores did not respond to treatment by bioactive flg22 in contrast to stomata (Fig. 3B). This result suggests a potential insensitivity of such pores to PAMPs and could explain why Xcc enters hydathode pores but not stomata. Though FLS2 has been shown to be expressed in the hydathode and stomata (Beck et al., 2014), it is not known whether guard cells of hydathode pores express FLS2, thus providing a possible explanation for their insensitivity to flg22. To test this hypothesis, lox1 open-stomata leaves were inoculated with wild-type Xcc strain. The lox1 mutant was previously shown to support an increased growth of P. syringae attributed to an increased bacterial invasion of the mesophyll (Montillet et al., 2013). We also observed a slightly higher in planta growth of Xcc upon hydathode infection and higher symptoms upon wound inoculation (Supplemental Fig. S4C). Yet, the slight increased susceptibility of lox1 mutants upon wound inoculation is indicative of other LOX1 functions in postinvasive hydathode immunity as previously described in pepper or Arabidopsis against Xanthomonas euvesicatoria, P. syringae, Hyaloperonospora arabidopsidis, and Alternaria brassicicola (Hwang and Hwang, 2010). Besides, slac1 open-stomata mutants showed no significant increased susceptibility to Xcc after dip inoculation. Thus, the main infection route for Xcc remains the hydathode even when stomata are wide open. Because Xcc flagellin evades recognition by FLS2 and several Xcc EF-Tu variants are not recognized by Brassicaceae-specific EFR receptor (Sun et al., 2006; Guy et al., 2013a; Roux et al., 2015), it would be important to test whether other known Xanthomonas PAMPs such as enigmatic MAMP of Xanthomonas, peptidoglycans, and lipo-oligosaccharides induce closure of stomata or hydathode pores. To date, cultures and culture supernatants of Xcc were shown to first promote stomatal closure (Gudesblat et al., 2009b) and to later revert stomatal closure similar to Pseudomonas coronatine phytotoxin (Melotto et al., 2006; Gudesblat et al., 2009b). Stomatal reopening was attributed to the diffusible small factor (DSF) quorum-sensing molecule produced by Xcc, which could help Xcc to gain access to both epithem and mesophyll. Yet, in seminatural infection settings, Xcc fails to enter stomata and multiply in leaf mesophyll. Thus, there might be insufficient accumulation of DSF or any unknown DSF-dependent molecule during the epiphytic growth of Xcc to promote stomatal aperture. While the importance of DSF in the regulation of Xcc pathogenicity is well established (Dow et al., 2003; Ryan et al., 2015; Deng et al., 2016), the biological relevance of DSF for the regulation of stomata/hydathode pores aperture during plant infection needs to be clarified. In conclusion, hydathodes do not appear to mount any or very limited preinvasive immunity against Xcc.
Postinvasive Immunity at Hydathodes Restricts Infection by Xcc
Once inside the hydathode, Xcc cells find their way between the large intercellular spaces between epithemal cells toward plant vasculature where they gather as soon as 3 dpi (Fig. 6). It is not known how bacteria reach this location. Xcc distribution could result from a combination of active processes of adhesion, motility, and chemotropism, which are reported to contribute to Xcc pathogenicity (Kamoun and Kado, 1990; Dow et al., 2003; Torres et al., 2007; Akimoto-Tomiyama et al., 2014). Obviously, liquid flow within the hydathode can be important (see Supplemental Movie 1) so that the inward flow of the guttation fluid within the epithem might also carry bacteria toward xylem vessels, which could act as sieves. Such processes would have to be experimentally addressed in future studies together with the biological importance of plant cell wall degrading enzymes. Plant cell wall degrading enzymes could facilitate this migration and digest the primary cell wall of epithemal and xylem vessels during the process of colonization. While the Hrp T3S machinery was dispensable for hydathode colonization (Fig. 7), injection of T3E proteins was needed for optimal growth inside the epithem of cauliflower or Arabidopsis (Fig. 8). Furthermore, the T3S machinery was also important to limit ROS accumulation in the hydathode. These results indicate that the epithem mounts an immune response against Xcc, which is dampened by the T3S system and its T3E proteins. This situation is per se very similar to the responses observed at the mesophyll with other bacterial mesophyll pathogens of the Xanthomonas, Ralstonia, or Pseudomonas genus. Finally, the avrAC T3E gene confers strong avirulence to Xcc upon hydathode infection. These observations indicate that hydathodes mount potent PTI and ETI immune responses, which are actively suppressed by bacterial pathogenicity determinants leading to effector-triggered susceptibility.
CONCLUSION
Compared to some of the pioneer studies (Meier, 1934; Bretschneider et al., 1989), our study brings a deeper and more comprehensive view of hydathode ultrastructure, its physiology, and its infection by Xcc. Despite its immune responses against Xcc, hydathodes do not appear to be such hostile environments, and bacteria rapidly reach extremely high titers in this niche (105 cfu/hydathode at 3 dpi). While mesophyll-colonizing pathogens have to fight for moisture and food (Xin et al., 2016; Yamada et al., 2016), water and nutrients are both likely unlimited in hydathodes for Xcc. Indeed, the epithem is continuously perfused with fresh sap composed of scarce but steady levels of nutrients. Xcc, as many other phytopathogens, is fully adapted to scavenge those carbohydrates, amino acids, organic acids, or minerals at high affinity in planta (e.g. Blanvillain et al., 2007). Future experiments will now have to thoroughly evaluate the biological importance of the known Xcc pathogenicity determinants during hydathode colonization and the contribution of hydathodes to plant physiology and immunity.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Growth Conditions
The Xanthomonas campestris pv campestris strains used in this study are derived from strain 8004 (Daniels et al., 1984) and ATCC33913/LMG568 (da Silva et al., 2002). We also used the 8004ΔavrAC/xopAC mutant derivative (ΔXC_1553; Guy et al., 2013b). Xcc LMG568::lux expressing the lux operon has been previously described (Meyer et al., 2005). Xcc cells were grown in MOKA-rich medium (4 g/L yeast extract, 8 g/L casamino acids, 2 g/L K2HPO4, and 0.3 g/L MgSO4,7H2O; Blanvillain et al., 2007) or in MME minimal medium [8 g/L casamino acids, 10.5 g/L K2HPO4, 4.5 g/L KH2PO4, 1 g/L (NH4)2SO4, and 0.3 g/L MgSO4,7H2O; Arlat et al., 1991] at 28°C under shaking. Escherichia coli strain TG1 or DH10B were grown on Luria-Bertani medium at 37°C. For solid media, agar was added at a final concentration of 1.5% (w/v). The following antibiotics were used: kanamycin (50 μg⋅mL−1), rifampicin (50 μg⋅mL−1), and spectinomycin (40 µg⋅mL−1).
Construction of X. campestris pv campestris Strain 8004 Expressing GUS and GFP
A 1.35-kb genomic sequence of Xcc strain 8004 (chromosomal position 4,034,196 to 4,035,544) was cloned into pK18mobsacB suicide plasmid derivative (Schäfer et al., 1994) with a multicloning site in its middle, giving pK18-GI. A transcriptional fusion of GUS and GFP (variant S65T and F64L; Zhang et al., 2009) genes placed under the control of the strong constitutive promoter pTac and T7 terminator was inserted in the multicloning site of pK18-GI, so that the resulting construct pK18-GUS-GFP could mediate chromosomal integration of the pTac::GUS-GFP at position 4,034,944 in Xcc strain 8004. Cloning details are available upon request. Plasmids were introduced into E. coli by electroporation. pK18-GUS-GFP was introduced into Xcc strain 8004 or mutant derivatives by triparental mating as described (Figurski and Helinski, 1979; Ditta et al., 1980) giving 8004::GUS-GFP and derivatives. Chromosomal integration of GUS-GFP was selected as described (Schäfer et al., 1994) and verified by PCR (primer details available upon request) and monitoring of GUS activity or GFP fluorescence. Constitutive GUS activity was verified in the different mutant background in both MOKA and MME media at exponential and stationary phase. Pathogenicity of 8004::GUS-GFP did not differ from the wild-type strain 8004 on Arabidopsis (Arabidopsis thaliana) and cauliflower (Brassica oleracea; data not shown).
Construction of the X. campestris pv campestris mutant Strain 8004ΔhrcV
Sequences flanking the HrcV gene (XC_3013) were amplified from genomic DNA from Xcc strain 8004 using primers 5′-tttggtctcAAGGTACGTCACTGCCGCCGCAGTGCTG-3′/5′-tttggtctcACATAGCCATCTCCTAGCAGGGCAGCG-3′ and 5′-tttggtctcATATGTGCGCAAGCCACCGGTCCGCCAT-3′/5′-tttggtctcACTTGCCCAGTGGTTCCAGGGCGCGCAG-3′ and cloned into the GoldenGate-compatible suicide vector p∆13 (Guy et al., 2013a) as described (Engler et al., 2008), giving p∆3013. p∆3013 was introduced into Xcc strain 8004 by triparental mating as described (Figurski and Helinski, 1979; Ditta et al., 1980) giving 8004∆hrcV. hrcV deletion was selected as described (Schäfer et al., 1994) and verified by PCR (primer details available upon request).
Plant Material, Growth Conditions, and Infection Tests
Cauliflower (B. oleracea var botrytis) cultivar Clovis was grown under greenhouse conditions. Arabidopsis accession Col-0 or Sf-2 were grown under short-day conditions (9 h light; 22°C; relative humidity 70%). Arabidopsis Col-0 plants expressing reporters of stomatal cell identity or function were obtained: E1728 Gal4:GFP (Gardner et al., 2009) and FAMAp:YFP-YFP (gift of Diego Wengier and Dominique Bergmann, Stanford University, Stanford, CA; based on Ohashi-Ito and Bergmann [2006]). The Col-0 Arabidopsis mutants lox1-2 (Montillet et al., 2013) and slac1-2 (Negi et al., 2008) with altered stomatal responses to biotic or abiotic stimuli were used.
Xcc inoculations were performed on 4-week-old plants. Bacteria were harvested from overnight cultures in MOKA by centrifugation (4,000g, 10 min) and suspended in 1 mm MgCl2. Dip inoculation was performed by dipping leaves (second true leaf for cauliflower; entire rosette for Arabidopsis) for 15 s inside the bacterial solution (108 cfu⋅mL−1) containing SILWET L-77 (0.02%). After inoculation, plants were placed for 24 h in miniature greenhouses with a clear plastic cover and kept at 100% relative humidity and then returned to regular growth chamber conditions (9 h light; 22°C; relative humidity 70%). Alternatively, Xcc pathogenicity was assayed by wound inoculation of the main leaf vein of 4-week-old Arabidopsis plants with a bacterial suspension at 5⋅107 cfu/mL essentially as described (Meyer et al., 2005). Disease indices were scored 6 or 8 dpi: 0 to 1, no symptoms; 1 to 2, weak chlorosis; 2 to 3, strong chlorosis; 3 to 4, necrosis.
Determination of In Planta Bacterial Populations
Leaf tissue was surface sterilized with a solution of commercial sodium hypochlorite bleach (10% v/v) and Tween 80 (0.02% v/v) for 1 min and rinsed three times with deionized water. For Arabidopsis, bacteria were extracted from entire leaves (four plants per strain and ecotype; two leaves per plant). Leaves were scanned to determine their total area prior to grinding. For B. oleracea, bacteria were extracted from single hydathodes sampled with a punch of 1.5 mm diameter (three leaves per strain, one leaf per plant, 12 hydathode per leaf). Infected plant tissues were homogenized with a bead grinder in 1 mm MgCl2. Serial dilutions of the homogenates were performed and spotted on MOKA plates containing rifampicin and pimaricin (30 µg/mL). The plates were incubated at 28°C for 36 h, and colonies were counted in spots containing 1 to 30 colonies. Experiments were performed at least three times.
GUS Staining of Infected Leaf Material
Infected leaf material were harvested, surface sterilized as described above, vacuum infiltrated (10 min, three times) with a substrate-detergent solution (1 mm 5-bromo-4-chloro-3-indolyl β-d-glucuronide, 0.2% Triton X-100 [v/v], 2 mm potassium ferricyanide [K3FeCN6], 2 mm potassium ferrocyanide [K4FeCN6], and 50 mm sodium phosphate buffer, pH 7.2), incubated overnight at 37°C and fixed and destained in 80% ethanol. Healthy material was processed similarly though not sterilized prior to staining. Samples were rehydrated and mounted between slide and coverslip with a Mowiol solution (2.4 g Mowiol 4-88, 6 g glycerol, 6 mL water, and 12 mL of 0.2 m Tris-HCl, pH 8.5) diluted 1:2 (v/v) in distilled water. Slides were incubated overnight at 37°C to enable Mowiol polymerization and imaged with the digital slide scanner NanoZoomer 2.0-HT C9600 (Hamamatsu).
In Vivo Imaging and Microscopy
Fresh samples (leaf disks of 0.5–1 cm in diameter) were mounted in water on a glass slide and covered with a coverslip. Images were acquired in bright field using an inverted microscope (DMIRBE; Leica) equipped with a color CCD camera (DFC300 FX; Leica).
Confocal images were acquired with a laser scanning confocal microscope (Leica SP2 AOBS). Sample disks were mounted as indicated above. A 488-nm ray line of an argon laser was used for the excitation of GFP, and the emitted fluorescence was collected between 505 and 540 nm. A 405-nm diode laser and a 633-nm HeNe laser for excitation were used to collect the autofluorescence between 410 and 460 nm to depict the cell contours and between 650 and 700 nm for the chloroplasts, respectively. Semithin sections (80–100 µm in thickness) performed with a vibratome (VT1000S; Leica) were also mounted in water and confocal image acquired using the 405-nm diode laser to depict the distribution of blue autofluorescence within the leaf blade. A 514-nm ray line of an argon laser was used for the excitation of YFP, and the emitted fluorescence was collected between 520 and 560 nm.
For luminescence, plants infected with Xcc strain 568::lux expressing the Photorhabdus luminescens lux operon (Winson et al., 1998; Meyer et al., 2005) were placed in a dark box, and images of entire leaf were captured using a C9100-13 EMCCD camera (Hamamatsu) with a 16-mm Cosmicar lens. Luciferase-mediated photons were acquired over appropriate time period (close to 60 s) using Hamamatsu Imaging Software. Biophotons (ultraweak photon emission) were collected using the same camera setup for 1800-s acquisition time. Intensity profiles were obtained using Image-Pro Plus software (Media Cybernetics). Reference lines (not passing through the hydathode) were first drawn to define the baseline for the intensity profile along the same-length line passing through a hydathode.
Measurement of Stomatal and Hydathode Pore Apertures
The abaxial side of leaves of 4- to 5-week-old Arabidopsis Col-0 plants was stuck on coverslips and peeled. Peels were submersed in petri dishes containing 10 mm MES/Tris, pH 6.0, and 30 mm KCl at 23°C. To test ABA-mediated inhibition of light-induced stomatal aperture, peels were first kept for 30 min in darkness. Epidermal peels were then transferred in the same buffer supplemented with 10 or 100 µm ABA or the equivalent dose of ethanol for 2 h under light (250 µmol⋅m−2⋅s−1). Ethanol used for ABA stock solutions did not exceed 0.1% (v/v) final concentration. In order to assay for flg22-induced stomatal closure, epidermal peels were first exposed to light for 2 h (250 µmol⋅m−2⋅s−1) at 23°C. Peels were then treated with 0 to 10 µm Flg22 (NH2-QRLSTGSRINSAKDDAAGLQIA-COOH) for 2 h under light. Stomatal apertures were measured with an optical microscope (Nikon; Optiphot) fitted with a camera lucida and a digitizing table (Houston Instrument) linked to a computer as described (Leonhardt et al., 1997). Each data point represented the mean of at least 50 stomatal apertures. Each experiment was repeated at least twice.
Observations of Clarified Samples
Samples were treated in an aqueous solution of chlorate hydrate (45 g of chloral hydrate and 9.3 mL of a solution 60% glycerol in 7.6 mL water) for 1 to 3 weeks. They were mounted in the same solution and images of hydathodes acquired in Nomarsky (DIC) using an inverted microscope (DMIRBE; Leica) equipped with a color CCD camera (DFC300 FX; Leica). Some samples were rinsed in water and bathed in an aqueous solution of calcofluor (0.01% [w/v]) for cell wall staining. Confocal images were then captured using the xyz acquisition mode of a laser scanning confocal microscope (SP2AOBS; Leica). The 405-nm diode laser was used for excitation, and the emitted fluorescence was collected between 410 and 500 nm. Images were then analyzed using Image-Pro Plus software (Media Cybernetics).
Scanning Electron Microscopy
Samples were fixed as described above, dehydrated in ethanol series, and then critical-point dried with liquid CO2, attached with double side tape to metal stubs, grounded with conductive silver paint, and sputter coated with platinum. For cryo-observations, fresh samples were plunged into pasty nitrogen slusher pot of workstation (PrepDek). Slusher pot evacuated by primary pump transformed liquid nitrogen (−196°C) inside to pasty nitrogen (−210°C, approximately). Sample transfer to column mounted preparation chamber PP3000T (Quorum Technologies) is realized by rod cryotransfer. Sublimation was realized to 95°C. Images were acquired with a scanning electron microscope (SEM Quanta 250 FEG FEI) at 5 kV with a working distance of 1 cm. For cryoprepared samples, the temperature was maintained below −140°C during the image acquisition.
Resin Embedded Material and Microscopies
Hydathodes (2–3 mm2) were fixed under vacuum for 30 min with 2.5% glutaraldehyde in 0.2 mm sodium cacodylate buffer (pH 7.2) containing 0.1% Triton X-100 and then, at the atmospheric pressure, for 1 h in the same solution without Triton X-100. Samples were rinsed in the same cacodylate buffer and postfixed for 1 h at room temperature with 2% osmium tetroxide (OsO4) in the buffer. Samples were dehydrated in a series of aqueous solutions of increasing ethanol concentrations (25, 50, 70, 95, and 100%, 1 h each) and then infiltrated stepwise in Epon using a microwave apparatus (Leica EM AMW). Resin was finally polymerized for 48 h at 60°C. From embedded material, thin (0.5 μm in thickness) or ultra-thin (80–90 nm thickness) sections were prepared using an UltraCut E ultramicrotome equipped with a diamond knife (Reichert-Leica).
For bright-field microscopy, thin section were mounted on glass slides and stained in a 1% borax solution containing 0.1% toluidine blue and 0.2% methylene blue, rinsed in water and then in an aqueous solution of 0.07% of basic fuchsin. For transmission electron microscopy, ultrathin sections were collected on gold grids and submitted to the periodic acid-thiocarbohydrazide-silver proteinate reaction (PATAg). For PATAg staining, sections were floated on a 1% (w/v) aqueous solution of periodic acid for 30 min at room temperature, rinsed twice in distilled water for 2 h and treated overnight at 4°C with a 20% aqueous solution of acetic acid containing 0.2% thiocarbohydrazide. Sections were washed in solutions of decreasing concentrations of acetic acid and finally in water, floated in a 1% (w/v) aqueous solution of silver proteinate for 30 min in the dark, washed in water and air-dried before observation. Images were obtained using an AxioPlan Imaging microscope equipped with a color CCD camera (Zeiss) or a Hitachi-HT-7700 (Japan) transmission electron microscope operating at 80 kV.
Statistical Analysis
Statistical significance of the results was calculated using ANOVA model followed by a parametric Tukey’s post hoc test at a 95% confidence limit (Yandell, 1997) or a nonparametric Kruskal-Wallis test at a 99% confidence limit. Normality of model residual error was assessed using a Shapiro and Wilk test (Royston, 1995) and homoscedasticity was verified using the Levene test (Hines and Hines, 2000).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. General organization of hydathodes of Arabidopsis thaliana ecotype Col-0.
Supplemental Figure S2. Stomatal lineage markers are expressed in hydathode pores of 4-week-old Col-0 Arabidopsis.
Supplemental Figure S3. Visualization of Xcc strain LMG568::lux by bioluminescence detection during cauliflower leaf infection.
Supplemental Figure S4. Open stomata mutants lox1-2 and slac1-2 do not show increased susceptibility to infection by virulent 8004::GUS-GFP∆avrAC strain.
Supplemental Figure S5. Cauliflower hydathodes spontaneously emit photons upon infection with Xcc.
Supplemental Table S1. Cytological properties of hydathodes versus leaf blade of cauliflower (Brassica oleracea var botrytis cv Clovis).
Supplemental Table S2. Cytological properties of hydathodes versus leaf blade in leaves of Arabidopsis ecotype Col-0.
Supplemental Movie 1. Movie showing Xcc cells strain 8004::GUS-GFP (green) and unknown particles (red) entering a cauliflower hydathode pore (cultivar Clovis).
Supplementary Material
Acknowledgments
We thank Claudine Zischek for constructing pK18-GUS-GFP, Marie-Françoise Jardinaud for performing statistical analyses, Olivier Catrice for technical assistance, and Nathalie Aoun for thoroughly proofreading the manuscript. We also thank Alex Webb (University of Cambridge, UK) for contributing Arabidopsis reporter lines E1728::GFP and Dominique Bergmann and Diego Wengier (Stanford University, CA) for the gift of FAMAp:YFP-YFP seeds.
Glossary
- MAMP
microbe-associated molecular patterns
- PAMP
pathogen-associated molecular patterns
- PTI
PAMP-triggered immunity
- ETI
effector-triggered immunity
- scw
secondary cell wall
- DSF
diffusible small factor
Footnotes
This work was supported by a PhD grant from the French Ministry of National Education and Research to A.C. The Laboratoire des Interactions Plantes-Microorganismes is part of the French Laboratory of Excellence project (TULIP ANR-10-LABX-41; ANR-11-IDEX-0002-02).
References
- Akimoto-Tomiyama C, Furutani A, Ochiai H (2014) Real time live imaging of phytopathogenic bacteria Xanthomonas campestris pv. campestris MAFF106712 in ‘plant sweet home’. PLoS One 9: e94386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R, Schwalm K, Langhans M, Ullrich CI (2003) Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216: 841–853 [DOI] [PubMed] [Google Scholar]
- Arlat M, Gough CL, Barber CE, Boucher C, Daniels MJ (1991) Xanthomonas campestris contains a cluster of hrp genes related to the larger hrp cluster of Pseudomonas solanacearum. Mol Plant Microbe Interact 4: 593–601 [DOI] [PubMed] [Google Scholar]
- Baylis T, Cierlik I, Sundberg E, Mattsson J (2013) SHORT INTERNODES/STYLISH genes, regulators of auxin biosynthesis, are involved in leaf vein development in Arabidopsis thaliana. New Phytol 197: 737–750 [DOI] [PubMed] [Google Scholar]
- Beck M, Wyrsch I, Strutt J, Wimalasekera R, Webb A, Boller T, Robatzek S (2014) Expression patterns of flagellin sensing 2 map to bacterial entry sites in plant shoots and roots. J Exp Bot 65: 6487–6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P. (2012) Sulfur-containing secondary metabolites from Arabidopsis thaliana and other Brassicaceae with function in plant immunity. ChemBioChem 13: 1846–1859 [DOI] [PubMed] [Google Scholar]
- Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, et al. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106 [DOI] [PubMed] [Google Scholar]
- Bennett M, Mehta M, Grant M (2005) Biophoton imaging: A nondestructive method for assaying R gene responses. Mol Plant Microbe Interact 18: 95–102 [DOI] [PubMed] [Google Scholar]
- Bhat NA, Syeed N, Bhat KA, Mir SA (2010) Pathogenicity and host range of Xanthomonas campestris pv. campestris—incitant of black rot of crucifers. J Phytol 2: 1–5 [Google Scholar]
- Birtic S, Ksas B, Genty B, Mueller MJ, Triantaphylidès C, Havaux M (2011) Using spontaneous photon emission to image lipid oxidation patterns in plant tissues. Plant J 67: 1103–1115 [DOI] [PubMed] [Google Scholar]
- Blanvillain S, Meyer D, Boulanger A, Lautier M, Guynet C, Denancé N, Vasse J, Lauber E, Arlat M (2007) Plant carbohydrate scavenging through tonB-dependent receptors: A feature shared by phytopathogenic and aquatic bacteria. PLoS One 2: e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau PA, Feeny P, Contardo L, Robson DS (1978) Allylglucosinolate and herbivorous caterpillars: A contrast in toxicity and tolerance. Science 200: 1296–1298 [DOI] [PubMed] [Google Scholar]
- Block A, Li G, Fu ZQ, Alfano JR (2008) Phytopathogen type III effector weaponry and their plant targets. Curr Opin Plant Biol 11: 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove AJ, Koebnik R, Lu H, Furutani A, Angiuoli SV, Patil PB, Van Sluys MA, Ryan RP, Meyer DF, Han SW, et al. (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J Bacteriol 193: 5450–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonas U, Schulte R, Fenselau S, Minsavage GV, Staskawicz BJ, Stall RE (1991) Isolation of a gene cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol Plant Microbe Interact 4: 81–88 [Google Scholar]
- Bretschneider KE, Gonella MP, Robeson DJ (1989) A comparative light and electron microscopical study of compatible and incompatible interactions between Xanthomonas campestris pv. campestris and cabbage (Brassica oleracea). Physiol Mol Plant Pathol 34: 285–297 [Google Scholar]
- Buell CR. (2002) Interactions between Xanthomonas species and Arabidopsis thaliana. Arabidopsis Book 1: e0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D, Bonas U (2002) Getting across—bacterial type III effector proteins on their way to the plant cell. EMBO J 21: 5313–5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D, Bonas U (2010) Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev 34: 107–133 [DOI] [PubMed] [Google Scholar]
- Chen C-C, Chen Y-R (2005) Study on laminar hydathodes of Ficus formosana (Moraceae) I. Morphology and ultrastructure. Bot Bull Acad Sin 46: 205–215 [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AA, Walker JC, Larson RH (1952) Studies on the disease cycle of black rot of crucifers. Phytopathology 42: 162–167 [Google Scholar]
- da Silva AC, Ferro JA, Reinach FC, Farah CS, Furlan LR, Quaggio RB, Monteiro-Vitorello CB, Van Sluys MA, Almeida NF, Alves LM, et al. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417: 459–463 [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Barber CE, Turner PC, Sawczyc MK, Byrde RJ, Fielding AH (1984) Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J 3: 3323–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Wu J, Yin W, Li P, Zhou J, Chen S, He F, Cai J, Zhang LH (2016) Diffusible signal factor family signals provide a fitness advantage to Xanthomonas campestris pv. campestris in interspecies competition. Environ Microbiol 18: 1534–1545 [DOI] [PubMed] [Google Scholar]
- Ditta G, Stanfield S, Corbin D, Helinski DR (1980) Broad host range DNA cloning system for gram-negative bacteria: Construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA 77: 7347–7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow JM, Crossman L, Findlay K, He YQ, Feng JX, Tang JL (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc Natl Acad Sci USA 100: 10995–11000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow JM, Osbourn AE, Wilson TJ, Daniels MJ (1995) A locus determining pathogenicity of Xanthomonas campestris is involved in lipopolysaccharide biosynthesis. Mol Plant Microbe Interact 8: 768–777 [DOI] [PubMed] [Google Scholar]
- Dzhalilov FS, Tiwari RD (1995) Soil and cabbage plant debris as infection sources of black rot. Arch Phytopathol Pflanzenschutz 29: 383–386 [Google Scholar]
- Engler C, Kandzia R, Marillonnet S (2008) A one pot, one step, precision cloning method with high throughput capability. PLoS One 3: e3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs G, Silipo A, Aslam S, De Castro C, Liparoti V, Flagiello A, Pucci P, Lanzetta R, Parrilli M, Molinaro A, et al. (2008) Peptidoglycan and muropeptides from pathogens Agrobacterium and Xanthomonas elicit plant innate immunity: Structure and activity. Chem Biol 15: 438–448 [DOI] [PubMed] [Google Scholar]
- Evert RF, Eichhorn SE (2006). Esau's Plant Anatomy, Ed 3 John Wiley & Sons, Hoboken, NJ, pp 447–472 [Google Scholar]
- Fargier E, Manceau C (2007) Pathogenicity assays restrict the species Xanthomonas campestris into three pathovars and reveal nine races within X. campestris pv. campestris. Plant Pathol 56: 805–818 [Google Scholar]
- Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76: 1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Baker AJ, Assie JM, Poethig RS, Haseloff JP, Webb AA (2009) GAL4 GFP enhancer trap lines for analysis of stomatal guard cell development and gene expression. J Exp Bot 60: 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goatley JL, Lewis RW (1966) Composition of guttation fluid from rye, wheat, and barley seedlings. Plant Physiol 41: 373–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald I, Rupprecht I, Schuster G, Kloppstech K (2003) Identification of guttation fluid proteins: The presence of pathogenesis-related proteins in non-infected barley plants. Physiol Plant 119: 192–202 [Google Scholar]
- Gudesblat GE, Torres PS, Vojnov AA (2009a) Stomata and pathogens: Warfare at the gates. Plant Signal Behav 4: 1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Torres PS, Vojnov AA (2009b) Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol 149: 1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy E, Genissel A, Hajri A, Chabannes M, David P, Carrère S, Lautier M, Roux B, Boureau T, Arlat M, et al. (2013a) Natural genetic variation of Xanthomonas campestris pv. campestris pathogenicity on Arabidopsis revealed by association and reverse genetics. MBio 4: e00538–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy E, Lautier M, Chabannes M, Roux B, Lauber E, Arlat M, Noël LD (2013b) xopAC-triggered immunity against Xanthomonas depends on Arabidopsis receptor-like cytoplasmic kinase genes PBL2 and RIPK. PLoS One 8: e73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt G. (1914) Physiological Plant Anatomy. Macmillan and Co., London [Google Scholar]
- Hines WG, Hines RJ (2000) Increased power with modified forms of the Levene (Med) test for heterogeneity of variance. Biometrics 56: 451–454 [DOI] [PubMed] [Google Scholar]
- Hossain MB, Matsuyama N, Kawasaki M (2016) Hydathode morphology and role of guttation in excreting sodium at different concentrations of sodium chloride in eddo. Plant Prod Sci 19: 528–539 [Google Scholar]
- Hugouvieux V, Barber CE, Daniels MJ (1998) Entry of Xanthomonas campestris pv. campestris into hydathodes of Arabidopsis thaliana leaves: A system for studying early infection events in bacterial pathogenesis. Mol Plant Microbe Interact 11: 537–543 [DOI] [PubMed] [Google Scholar]
- Hwang IS, Hwang BK (2010) The pepper 9-lipoxygenase gene CaLOX1 functions in defense and cell death responses to microbial pathogens. Plant Physiol 152: 948–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle AK, Lipschis M, Albert M, Fallahzadeh-Mamaghani V, Fürst U, Mueller K, Felix G (2013) The receptor-like protein ReMAX of Arabidopsis detects the microbe-associated molecular pattern eMax from Xanthomonas. Plant Cell 25: 2330–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kamoun S, Kado CI (1990) Phenotypic switching affecting chemotaxis, xanthan production, and virulence in Xanthomonas campestris. Appl Environ Microbiol 56: 3855–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura E, Horiguchi G, Tsukaya H (2010) Mechanisms of leaf tooth formation in Arabidopsis. Plant J 62: 429–441 [DOI] [PubMed] [Google Scholar]
- Kay S, Bonas U (2009) How Xanthomonas type III effectors manipulate the host plant. Curr Opin Microbiol 12: 37–43 [DOI] [PubMed] [Google Scholar]
- Kniskern JM, Traw MB, Bergelson J (2007) Salicylic acid and jasmonic acid signaling defense pathways reduce natural bacterial diversity on Arabidopsis thaliana. Mol Plant Microbe Interact 20: 1512–1522 [DOI] [PubMed] [Google Scholar]
- Köhl J, Wolf VDJ (2005) Alternaria brassicicola and Xanthomonas campestris pv. campestris in Organic Seed Production of Brassicae: Epidemiology and Seed Infection. Plant Research International, Wageningen, The Netherlands
- Kuan TL, Minsavage GV, Schaad NW (1986) Aerial dispersal of Xanthomonas campestris pv. campestris from naturally infected Brassica campestris. Plant Dis 70: 409 [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C (1996) Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 9: 195–203 [DOI] [PubMed] [Google Scholar]
- Leonhardt N, Marin E, Vavasseur A, Forestier C (1997) Evidence for the existence of a sulfonylurea-receptor-like protein in plants: Modulation of stomatal movements and guard cell potassium channels by sulfonylureas and potassium channel openers. Proc Natl Acad Sci USA 94: 14156–14161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcell LM, Beattie GA (2002) Effect of leaf surface waxes on leaf colonization by Pantoea agglomerans and Clavibacter michiganensis. Mol Plant Microbe Interact 15: 1236–1244 [DOI] [PubMed] [Google Scholar]
- Meier D. (1934) A cytologycal study of the early infection stages of the black rot of cabbage. Bull Torrey Bot Club 61: 173–190 [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Meyer D, Lauber E, Roby D, Arlat M, Kroj T (2005) Optimization of pathogenicity assays to study the Arabidopsis thaliana-Xanthomonas campestris pv. campestris pathosystem. Mol Plant Pathol 6: 327–333 [DOI] [PubMed] [Google Scholar]
- Montillet JL, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M, Garcia AV, Douki T, Bigeard J, Laurière C, et al. (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11: e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Nikovics K, Blein T, Peaucelle A, Ishida T, Morin H, Aida M, Laufs P (2006) The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18: 2929–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niño-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol Plant Pathol 7: 303–324 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC (2006) Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 18: 2493–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin F, Renaud J, Barbier F, Vavasseur A, Le Thiec D, Rose C, Bariac T, Casson S, McLachlan DH, Hetherington AM, et al. (2013) Developmental priming of stomatal sensitivity to abscisic acid by leaf microclimate. Curr Biol 23: 1805–1811 [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Bogenschutz NL, Torii KU (2008) The bHLH protein, MUTE, controls differentiation of stomata and the hydathode pore in Arabidopsis. Plant Cell Physiol 49: 934–943 [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Dong J (2013) Stomatal development in Arabidopsis. Arabidopsis Book 11: e0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot G, Gaymard F, Mouline K, Chérel I, Sentenac H (2003) Regulated expression of Arabidopsis shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol Biol 51: 773–787 [DOI] [PubMed] [Google Scholar]
- Pilot G, Stransky H, Bushey DF, Pratelli R, Ludewig U, Wingate VP, Frommer WB (2004) Overexpression of GLUTAMINE DUMPER1 leads to hypersecretion of glutamine from hydathodes of Arabidopsis leaves. Plant Cell 16: 1827–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti S, Giangrande C, Amoresano A, Pucci P, Molinaro A, Bertini L, Caporale C, Caruso C (2014) Xanthomonas campestris lipooligosaccharides trigger innate immunity and oxidative burst in Arabidopsis. Plant Physiol Biochem 85: 51–62 [DOI] [PubMed] [Google Scholar]
- Qian W, Jia Y, Ren SX, He YQ, Feng JX, Lu LF, Sun Q, Ying G, Tang DJ, Tang H, et al. (2005) Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res 15: 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier O, Wengelnik K, Hahn K, Bonas U (1999) The Xanthomonas Hrp type III system secretes proteins from plant and mammalian bacterial pathogens. Proc Natl Acad Sci USA 96: 9368–9373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B, Bolot S, Guy E, Denancé N, Lautier M, Jardinaud MF, Fischer-Le Saux M, Portier P, Jacques MA, Gagnevin L, et al. (2015) Genomics and transcriptomics of Xanthomonas campestris species challenge the concept of core type III effectome. BMC Genomics 16: 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. (1995) Remark AS R94: A remark on algorithm AS-181: The W-test for normality. Appl Stat 44: 547–551 [Google Scholar]
- Russell HL. (1898) A bacterial rot of cabbage and allied plants. Univ of Wis Agric Exp Sta Bull 8: 1–39 [Google Scholar]
- Ryan RP, An SQ, Allan JH, McCarthy Y, Dow JM (2015) The DSF family of cell-cell signals: An expanding class of bacterial virulence regulators. PLoS Pathog 11: e1004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawinski K, Mersmann S, Robatzek S, Böhmer M (2013) Guarding the green: Pathways to stomatal immunity. Mol Plant Microbe Interact 26: 626–632 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145: 69–73 [DOI] [PubMed] [Google Scholar]
- Schlaeppi K, Mauch F (2010) Indolic secondary metabolites protect Arabidopsis from the oomycete pathogen Phytophthora brassicae. Plant Signal Behav 5: 1099–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Coluccia F, Torres M, L’Haridon F, Métraux JP (2014) The cuticle and plant defense to pathogens. Front Plant Sci 5: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29: 475–486 [DOI] [PubMed] [Google Scholar]
- Silipo A, Molinaro A, Sturiale L, Dow JM, Erbs G, Lanzetta R, Newman MA, Parrilli M (2005) The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. J Biol Chem 280: 33660–33668 [DOI] [PubMed] [Google Scholar]
- Stevens ABP. (1959) The structure and development of the hydathodes of Caltha palustris L. New Phytol 55: 339–345 [Google Scholar]
- Sun W, Dunning FM, Pfund C, Weingarten R, Bent AF (2006) Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 18: 764–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton T, Baumann U, Hayes J, Collins NC, Shi B-J, Schnurbusch T, Hay A, Mayo G, Pallotta M, Tester M, et al. (2007) Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318: 1446–1449 [DOI] [PubMed] [Google Scholar]
- Sutton JC, Williams PH (1970) Comparison of extracellular polysaccharides of Xanthomonas campestris from culture and from infected cabbage leaves. Can J Bot 48: 645–651 [Google Scholar]
- Torres PS, Malamud F, Rigano LA, Russo DM, Marano MR, Castagnaro AP, Zorreguieta A, Bouarab K, Dow JM, Vojnov AA (2007) Controlled synthesis of the DSF cell-cell signal is required for biofilm formation and virulence in Xanthomonas campestris. Environ Microbiol 9: 2101–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauterin L, Hoste B, Kersters K, Swings J (1995) Reclassification of Xanthomonas. Int J Syst Bacteriol 45: 472–489 [Google Scholar]
- Verschaffelt E. (1910) The cause determining the selection of food in some herbivorous insects. Proc Royal Acad Amsterdam 13: 536–542 [Google Scholar]
- Vicente JG, Holub EB (2013) Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol Plant Pathol 14: 2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Roux B, Feng F, Guy E, Li L, Li N, Zhang X, Lautier M, Jardinaud MF, Chabannes M, et al. (2015) The decoy substrate of a pathogen effector and a pseudokinase specify pathogen-induced modified-self recognition and immunity in plants. Cell Host Microbe 18: 285–295 [DOI] [PubMed] [Google Scholar]
- Wang W, Xu B, Wang H, Li J, Huang H, Xu L (2011) YUCCA genes are expressed in response to leaf adaxial-abaxial juxtaposition and are required for leaf margin development. Plant Physiol 157: 1805–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PH. (1980) Black rot: A continuing threat to world crucifers. Plant Dis 64: 736–742 [Google Scholar]
- Winson MK, Swift S, Hill PJ, Sims CM, Griesmayr G, Bycroft BW, Williams P, Stewart GS (1998) Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol Lett 163: 193–202 [DOI] [PubMed] [Google Scholar]
- Xin XF, Nomura K, Aung K, Velásquez AC, Yao J, Boutrot F, Chang JH, Zipfel C, He SY (2016) Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539: 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RQ, Blanvillain S, Feng JX, Jiang BL, Li XZ, Wei HY, Kroj T, Lauber E, Roby D, Chen B, et al. (2008) AvrAC(Xcc8004), a type III effector with a leucine-rich repeat domain from Xanthomonas campestris pathovar campestris confers avirulence in vascular tissues of Arabidopsis thaliana ecotype Col-0. J Bacteriol 190: 343–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Saijo Y, Nakagami H, Takano Y (2016) Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 354: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Yandell B. (1997) Practical Data Analysis for Designed Experiments. Chapman & Hall, London [Google Scholar]
- Yu A, Lepère G, Jay F, Wang J, Bapaume L, Wang Y, Abraham AL, Penterman J, Fischer RL, Voinnet O, et al. (2013) Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc Natl Acad Sci USA 110: 2389–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Callaway EM, Jones JB, Wilson M (2009) Visualisation of hrp gene expression in Xanthomonas euvesicatoria in the tomato phyllosphere. Eur J Plant Pathol 124: 379–390 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.