Solar UV-B inhibits leaf growth in maize by suppressing cell proliferation, a response mediated through a decrease in GAs in the growth zone.
Abstract
Ultraviolet-B (UV-B) radiation affects leaf growth in a wide range of species. In this work, we demonstrate that UV-B levels present in solar radiation inhibit maize (Zea mays) leaf growth without causing any other visible stress symptoms, including the accumulation of DNA damage. We conducted kinematic analyses of cell division and expansion to understand the impact of UV-B radiation on these cellular processes. Our results demonstrate that the decrease in leaf growth in UV-B-irradiated leaves is a consequence of a reduction in cell production and a shortened growth zone (GZ). To determine the molecular pathways involved in UV-B inhibition of leaf growth, we performed RNA sequencing on isolated GZ tissues of control and UV-B-exposed plants. Our results show a link between the observed leaf growth inhibition and the expression of specific cell cycle and developmental genes, including growth-regulating factors (GRFs) and transcripts for proteins participating in different hormone pathways. Interestingly, the decrease in the GZ size correlates with a decrease in the concentration of GA19, the immediate precursor of the active gibberellin, GA1, by UV-B in this zone, which is regulated, at least in part, by the expression of GRF1 and possibly other transcription factors of the GRF family.
To reach its final size and shape, leaf development needs to be tightly coordinated at the cellular level, which involves the regulation of cell proliferation, cell expansion, and maturation (Piazza et al., 2005; Tsukaya, 2006). In Arabidopsis (Arabidopsis thaliana), cell proliferation is observed throughout the developing leaf blade; at a certain point, the cell cycle is arrested at the tip of the leaf and a mitotic arrest front moves toward the base of the organ (Donnelly et al., 1999; Andriankaja et al., 2012). Once cells cease to divide, they begin to enlarge, and cell expansion becomes the driving force regulating organ size (Piazza et al., 2005; Tsukaya, 2006). In this way, in dicots such as Arabidopsis, cell proliferation and expansion are separated in time (Beemster et al., 2005). On the other hand, in monocots like maize (Zea mays), proliferating and expanding cells largely coexist and are primarily separated in space. Four developmental phases can be differentiated in these species.ally with time, with a nearly constant cell length from the base to the tip of the young lamina; the leaf is considered as a unique compartment, called the DEZ (for dividing and elongating zone), with coordinated dividing and elongating cells. During the second phase, a new zone develops formed by a flux of elongating cells without division, the elongation zone (EZ). Therefore, the leaf at this stage comprises a growth zone (GZ) that includes both the DEZ and the EZ. These two phases cooccur before leaf emergence. During the third phase, the GZ has a fixed length, and mature cells leave this zone, creating the mature zone that increases in length with time. Finally, the GZ gets smaller and eventually disappears, resulting in a leaf composed mainly of mature cells. This event marks the end of the fourth phase (Granier and Tardieu, 2009). The rate of growth during the third phase, also called the stationary state, largely determines final leaf length (Baute et al., 2016). During this phase, the growth of a given leaf comprises dividing, expanding, and mature cells that are found at increasing distance from the leaf base. In this phase, the rate of growth is constant and the GZ has a fixed length, so kinematic analysis of cell division and expansion can be performed at a single time point (Granier and Tardieu, 2009); while for non-steady-state situations, cell length distributions need to be analyzed for at least three time points (Beemster and Baskin, 1998).
Growth inhibition is one of the most consistent plant responses to UV-B exposure; this radiation, both as part of the solar spectrum in the field and from UV-B lamps in controlled environments, affects leaf growth in a wide range of species (Ballaré et al., 2001; Searles et al., 2001; Flint et al., 2004). Different studies have been performed trying to understand this effect, but there are only a few reports focusing on how the dynamics of cell division and cell expansion are affected by UV-B radiation. Existing data on the effects of UV-B on cell proliferation and cell expansion are contradictory, probably reflecting differences in experimental conditions. Acute, stress-inducing UV-B conditions usually inhibit cell proliferation, while both cell proliferation and expansion are affected by lower doses and/or chronic UV-B treatments (Staxen and Bornman, 1994; Nogués et al., 1998; Laakso et al., 2000; Hopkins et al., 2002; Hofmann et al., 2003; Kakani et al., 2003; Hectors et al., 2007, 2010; Wargent et al., 2009; Robson and Aphalo, 2012). For example, the number of epidermal cells at maturity decreased after UV-B exposure in some species (Gonzalez et al., 1998; Hopkins et al., 2002); on the contrary, in lettuce (Lactuca sativa) leaves, UV-B reduced cell expansion rate and final leaf size (Wargent et al., 2009). In Arabidopsis, a number of UV-induced morphological changes have been described, including decreases in rosette diameter and inflorescence height (Hectors et al., 2007). In this species, one of the most well-characterized physiological responses mediated by the UV-B photoreceptor UVR8 is the inhibition of hypocotyl growth (Kliebenstein et al., 2002). In addition, UVR8 controls leaf morphogenesis (Wargent et al., 2009). However, there are other UV-B-specific responses that occur independently of UVR8; some are related to UV-B-induced DNA damage, as the accumulation of photodimers can activate DNA damage response pathways that result in cell cycle arrest or programmed cell death in stem cells of the root apical meristem (Curtis and Hays, 2007; Furukawa et al., 2010). For example, Biever et al. (2014) demonstrated that UV-B-induced hypocotyl growth inhibition in etiolated Arabidopsis seedlings also can occur independently of UVR8 but as a consequence of UV-B absorption by DNA, which can then induce an arrest in the cell cycle. Moreover, mutants in the nucleotide excision repair DNA repair system show a very dramatic effect on Arabidopsis growth under UV-B (Gardner et al., 2009; Biever et al., 2014).
Recently, we demonstrated that the exposure of Arabidopsis developing leaves to UV-B doses that cause the accumulation of DNA damage inhibits leaf expansion as a consequence of an early end of the proliferative phase of leaf development (Casadevall et al., 2013). A single UV-B radiation treatment in growth chamber conditions with irradiances similar to those found in nature inhibited cell proliferation in developing leaves; this inhibition is regulated at least in part by the miR396-mediated repression of transcription factors from the growth-regulating factors (GRFs; Casadevall et al., 2013). Also earlier, we reported that the exposure of maize plants to elevated UV-B doses causes a similar inhibition of leaf growth (Casati and Walbot, 2003).
In this work, we used the experimental possibilities offered by the maize leaf as a model system (Avramova et al., 2015b) to perform an integrated analysis of the response of maize leaves to solar UV-B doses. To this end, we analyzed the cellular basis of the growth phenotype and performed a genome-wide transcriptome analysis, allowing us to zoom in on hormone homeostasis at the metabolite level and providing evidence for the genetic basis of the response by means of an analysis of mutant and transgenic lines, demonstrating the involvement of GRFs and GA signaling.
RESULTS
UV-B Effect on Maize Leaf Growth
A well-documented effect of UV-B exposure on plants is the reduction in biomass (Bornman and Teramura, 1993); however, the underlying process affected depends on the species and the treatments employed. As described in the introduction, we demonstrated previously that the exposure of Arabidopsis developing leaves to UV-B in controlled growth chamber conditions using UV-B lamps inhibits leaf expansion as a consequence of an early inhibition of cell proliferation (Casadevall et al., 2013). In this work, we investigated the effect of natural solar UV-B radiation on leaf growth of maize plants, a monocot species. Plants were grown outdoors in Rosario, Argentina, during summer time, covered with different plastic filters to screen UV-B. Solar UV-B radiation was removed to produce the minus-UV-B treatment using polyester (PE) filters, which absorb UV-B without significantly affecting UV-A or visible radiation (transmitting on average 1.57 µmol m−2 UV-B and 36 µmol m−2 UV-A; Casati and Walbot, 2003). The UV-B-exposed plants were covered using a cellulose acetate (CA) filter that transmits most radiation from sunlight, including UV-B (resulting on average in 6.75 µmol m−2 UV-B and 36 µmol m−2 UV-A), to control for differences in wind or humidity under the plastic sheeting.
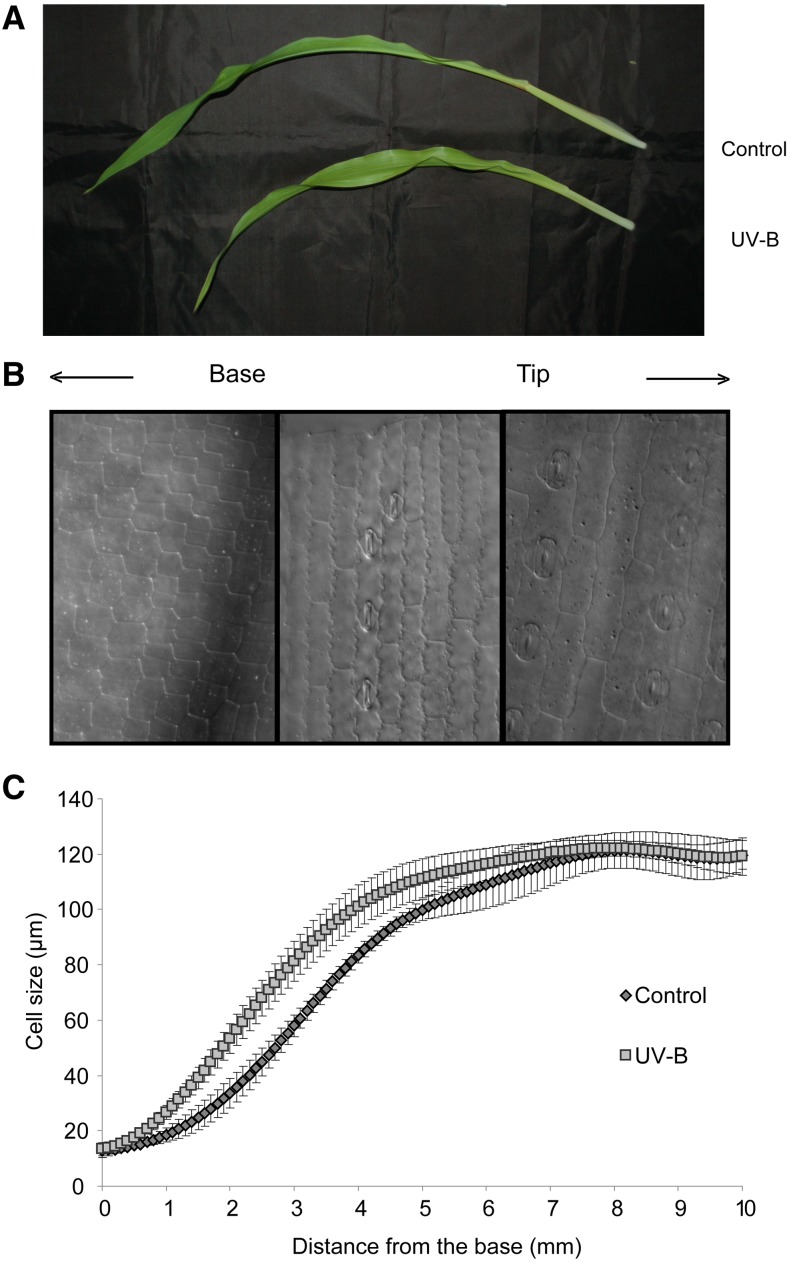
Supplemental Figure S1 shows that UV-B radiation from sunlight affects total plant growth in different maize genotypes. Plants grown without UV-B under a PE filter are taller than UV-B-exposed plants grown under a CA filter at different stages of plant development. Thus, we analyzed the effect of sunlight UV-B radiation in leaf 4 by measuring its growth from leaf emergence (about 1 month after sowing) to leaf maturity. The treatment did not cause any visual symptoms of leaf stress. Interestingly, even cyclobutane pyrimidine dimers (CPD) accumulation in DNA of mature leaf tissue, which is a very early and sensitive measure of UV-B damage, was similar in both groups of plants; it was significantly lower than the levels measured in Arabidopsis leaves irradiated for 4 h of UV-B using UV-B lamps, a UV-B treatment used previously to analyze growth inhibition in Arabidopsis (Supplemental Fig. S2; Casadevall et al., 2013). These results demonstrate that DNA repair mechanisms and/or other UV-B protection processes were active in maize leaves under the conditions assayed in our experiments. However, solar UV-B triggered a 13% reduction of the final leaf length in plants grown under the CA filter (Fig. 1A; Table I); this was due to a 30% reduction in leaf elongation rate during the first 3 d after emergence (Table I).
Figure 1.
Effects of UV-B radiation on leaf elongation and epidermal cell length. A, Representative image of leaf 4 at 12 d after emergence from plants that were grown in the presence (UV-B) or absence (Control) of solar UV-B. B, Representative image of different cells from the base to the tip of leaf 4. C, Cell length profiles of files adjacent to stomatal rows in the abaxial epidermis measured for control and UV-B-exposed plants. The data were obtained using leaf 4 from 12 plants for which the length of all cells in the basal 100 mm of two cell files adjacent to stomatal files was measured in relation of position. To enable averaging across leaves, the data were smoothed and interpolated into 50-mm spaced data points as described in “Materials and Methods.” For each condition, the average (n = 5) and corresponding se are shown at each position.
Table I. Solar UV-B effects on cell division and cell expansion parameters.
Average values (n = 5) and corresponding se are shown. NS, Not significant.
| Growth Parameters | Control | UV-B | UV-B/C Ratio | Student’s t Test |
|---|---|---|---|---|
| Final leaf length (cm) | 46.1 ± 4.5 | 40.0 ± 5.7 | 0.87 | 0.02 |
| Leaf elongation rate (mm h−1) | 2.7 ± 0.3 | 1.9 ± 0.6 | 0.70 | 0.009 |
| Mature cell size (μm) | 121 ± 18 | 122 ± 8 | 1.01 | NS |
| Cell production (cells h−1) | 24 ± 4 | 15 ± 5 | 0.63 | 0.010 |
| DEZ size (mm) | 38 ± 4 | 25 ± 2 | 0.65 | <0.001 |
| Size of cells leaving the DEZ (μm) | 76 ± 15 | 67 ± 20 | 0.88 | NS |
| No. of cells in the DEZ | 1,614 ± 338 | 965 ± 196 | 0.60 | 0.002 |
| Cell cycle duration (h) | 46 ± 5 | 46 ± 12 | 1.00 | NS |
| Average cell division rate (μm μm−1 h−1) | 0.030 ± 0.007 | 0.016 ± 0.008 | 0.54 | 0.035 |
| Residence time in the EZ (h) | 17 ± 4 | 30 ± 9 | 1.79 | 0.006 |
| GZ size (mm) | 81 ± 8 | 69 ± 10 | 0.84 | 0.04 |
| No. of cells in the GZ | 2,030 ± 440 | 1,413 ± 293 | 0.69 | 0.02 |
| Residence time in the DEZ (h) | 489 ± 60 | 454 ± 112 | 0.93 | NS |
| No. of cells in the EZ | 415 ± 137 | 448 ± 144 | 1.07 | NS |
| EZ size (mm) | 44 ± 9 | 44 ± 9 | 1.01 | NS |
| Average cell expansion rate (μm μm−1 h−1) | 0.028 ± 0.009 | 0.023 ± 0.012 | 0.83 | NS |
Kinematic Analysis
To understand the cellular basis of the growth reduction caused by solar UV-B radiation, we performed a kinematic analysis on leaf 4 growth at steady-state growth (72 h after leaf emergence) of control and UV-B-exposed plants. To this end, we measured epidermal cell length along the leaf longitudinal axis. The cell length profiles of both control and UV-B-exposed plants had a similar sigmoidal shape (Fig. 1). At the leaf base, both groups of plants have dividing cells with an average length of 16 µm. Toward the leaf tip, cells lose their ability to divide and only expand, reaching their mature size (Fig. 1B). In UV-B-exposed leaves, essentially the same pattern spans at a shorter distance from the leaf base (Fig. 1C). Nevertheless, the mature cell length reached by both groups of plants was identical (Fig. 1; Table I). On the other hand, the cell production rate of UV-B-exposed plants was reduced by 37% compared with control plants (Table I). The reduced cell production could be a consequence of leaves from UV-B-exposed plants having a lower number of dividing cells or, alternatively, because dividing cells have a longer cell cycle. In order to analyze this, we estimated the size of leaf 4 DEZ by staining nuclei and recording the positions of the most distal mitotic figures. We found that the DEZ ended at 24.5 mm from the base of the leaf in UV-B-exposed plants, whereas in control plants, the DEZ ended at 37.8 mm (Table I). Despite differences in the size of the DEZ, the size of cells leaving the DEZ was not affected; therefore, the DEZ of leaf 4 from UV-B-exposed plants contained 40% fewer cells compared with that from control plants (Table I). The cell cycle duration, on the other hand, was unaffected by the treatment (Table I). Therefore, we can conclude that the reduced cell production in leaf 4 induced by UV-B radiation is entirely due to a lower number of dividing cells and a smaller DEZ. Finally, as reported previously for the inhibition of leaf elongation by cold stress (Rymen et al., 2007) and drought stress (Avramova et al., 2015a), the average cell expansion rate was 20% lower in cells from UV-B-exposed plants, but the time in the EZ was 80% higher (30.32 versus 16.93 h; Table I). Thus, the longer residence time in the EZ compensated for the lower expansion rates, resulting in mature cells with the same size as in control plants (Table I).
UV-B Radiation Effect on the Maize GZ Transcriptome
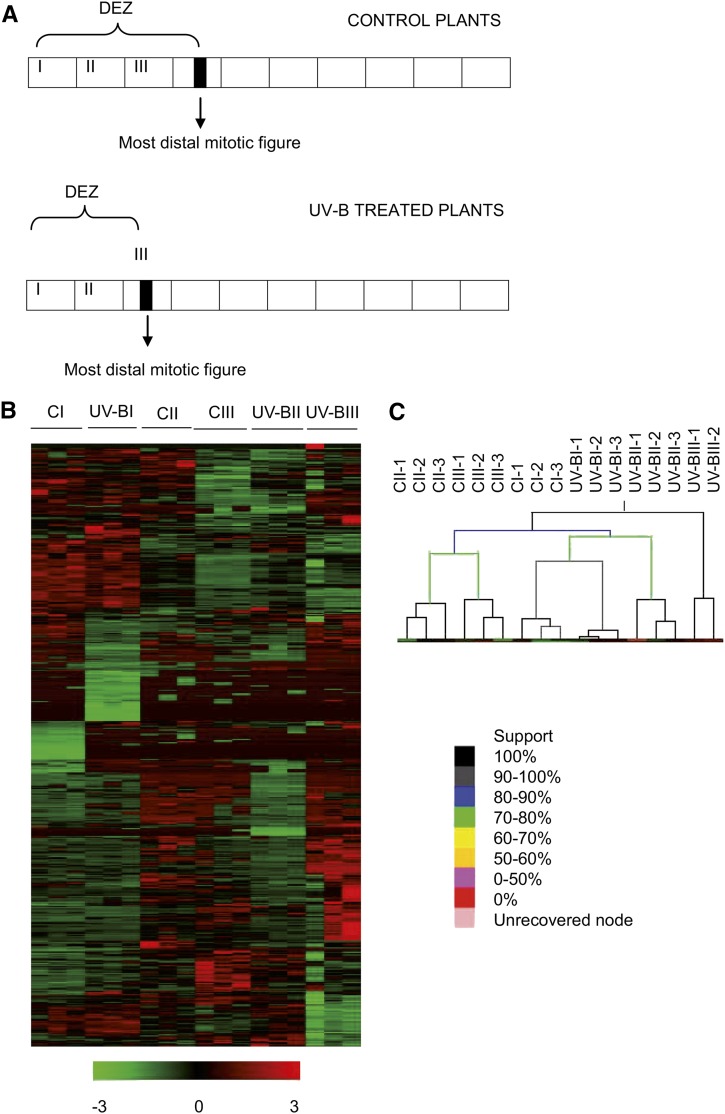
To identify the molecular changes underlying the smaller size of the DEZ in maize UV-B-exposed leaves, we conducted RNA sequencing (RNA-seq) experiments using Illumina technology. From each treatment (control and UV-B), we collected samples from the leaf GZ (located at 0–1 cm [segment I], 1–2 cm [segment II], and 2–3 cm [segment III] from the base of the leaf) during the steady-state growth of leaf 4 as shown in Figure 2. For both treatments, segments I and II corresponded to the DEZ, segment III from control leaves corresponded to the DEZ, while segment III from UV-B-exposed leaves included cells from the DEZ and the EZ (Fig. 2A). For each segment, we then determined the relative transcript abundance by RNA-seq using three independent biological replicates.
Figure 2.
Analysis of global gene expression differences between different leaf segments corresponding to the GZ from UV-B-exposed and control maize leaves. A, Schematic representation of maize leaves from control and UV-B-exposed plants during steady-state growth or the third developmental phase (Granier and Tardieu, 2009), which was used in the RNA-seq experiments. The first three basal 10-mm segments (segments I, II, and III) of leaf 4 at 3 d after leaf emergence from 12 plants per group were collected; all segments correspond to the GZ. The arrows indicate the most distal mitotic image from each leaf corresponding to the different treatments. B, Hierarchical clustering of all expressed transcripts from independent biological samples from segments I, II, and III (46,436 transcripts) as shown in A from UV-B-exposed and control (C) leaf 4. Values are expressed as mean-centered log2 expression values. The log2 scale of color values is provided at bottom. C, Sample tree of the three segments from UV-B-exposed and control treatments with support strength indication. The color scale is provided at bottom.
Hierarchical clustering of differentially expressed genes showed that, over all, reproducibility between biological replicates was high (Fig. 2B). One notable exception was one of the UV-B-treated segment III samples, which we eliminated from further data analysis. Of the 62,892 genes annotated in the maize genome (B73 RefGen_v3; MaizeSequence.org), 46,436 were expressed in all replicate samples for at least one treatment/section combination. Of these, the expression levels of 2,805 transcripts (6%) was significantly affected by the UV-B treatment across all zones or showed a significant interaction between the leaf zone and the treatment, indicating that they were affected differently in different zones (FDR < 0.05 and log fold change > 0.75). To see the global effect of the treatments on the transcriptome, we performed a support tree clustering. The results show that replicate samples always group together. Moreover, the tree structure shows that the transcriptome of segment I from control plants was more similar to that from the same segment from UV-B-exposed leaves than to those from segment II or III of control plants (Fig. 2C). This indicates that differences between developmental stages are dominant over the effect of the treatment. Nevertheless, differences due to the UV-B treatment also were clear (Fig. 2B). Interestingly, the transcriptome from segment II from control leaves was more similar to that from segment III from the same treatment than to that from segment II from UV-B-irradiated plants, showing that, for these samples, the treatment effect was more pronounced than the position effect (Fig. 2C), possibly because here the treatment effect is already partly combined with a developmental effect, reflecting the shortening of the DEZ. Consequently, segment III from UV-B-treated plants, which due to the shortening of the DEZ should combine the strongest developmental difference with an effect of UV-B, indeed showed the most distinct transcriptome (Fig. 2C). It is important to note that none of the leaf segments used in our experiments received natural sunlight, as they were located well inside the whorl of mature leaves at the moment of collection. Therefore, differential expression due to UV-B irradiation must be transmitted by signals from the exposed tissues.
Global Changes in Gene Expression
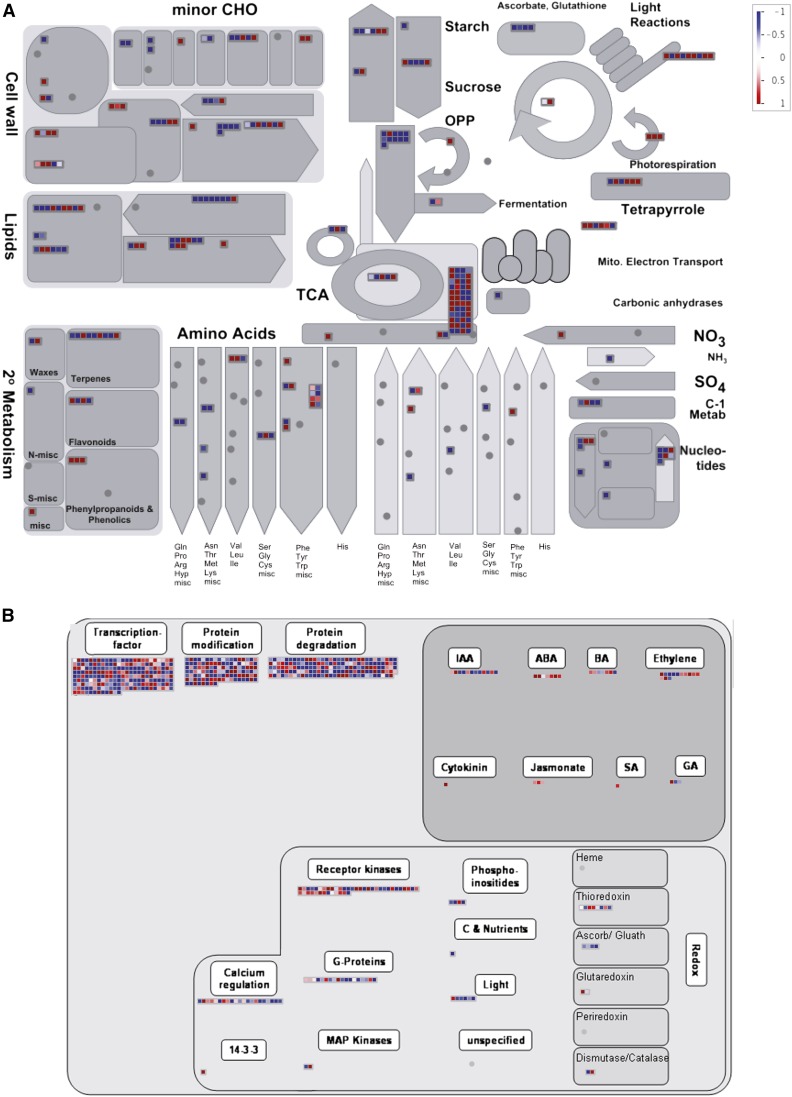
Global transcriptome changes in the three analyzed GZ segments induced by UV-B radiation were first analyzed using PageMan. The results presented in Figure 3 show that differentially expressed genes coding proteins of the photosynthetic light reactions and of the photorespiration pathway were predominantly up-regulated by UV-B. Interestingly, as mentioned above, the tissues analyzed were not exposed directly to light, so photosynthetic activity was probably very low in these leaf sections. On the other hand, transcripts for different enzymes involved in secondary metabolism were higher expressed in leaf tissues from UV-B-exposed plants; in particular enzymes of the phenylpropanoid, flavonoid, and terpenoid metabolism, proteins encoded by these transcripts were probably induced to provide plants with protective compounds against UV-B. Transcripts for stress response proteins also were mainly up-regulated by UV-B; however, mRNAs for enzymes in the metabolism of ascorbic acid and glutathione were down-regulated (Fig. 3B). Moreover, our data show that UV-B radiation also induced the expression of enzymes that participate in cell wall degradation and modification but also regulated the expression of enzymes of other metabolic pathways, such as tricarboxylic acid cycle, lipid, amino acid, and nucleotide pathways (Fig. 3A).
Figure 3.
Global transcriptome changes in leaf 4 GZ by UV-B radiation analyzed using PageMan. A, Transcripts encoding proteins in primary and secondary metabolism. B, Transcripts encoding proteins in regulatory pathways. Transcripts that are down-regulated by UV-B are represented by blue boxes, while up-regulated transcripts are represented by red boxes. The color scale is shown in the top right corner. CHO, carbohydrate; TCA, tricarboxylic acid cycle; OPP, oxidative pentose phosphate pathway.
A number of transcripts that encode transcription factors from different families, enzymes that participate in protein degradation, and signaling proteins were differentially regulated by UV-B. Interestingly, the expression of genes encoding enzymes that participate in hormone signaling pathways also was altered by UV-B in the GZ (Fig. 3B). Given that the response in the GZ must be triggered by a long-distance signal from mature, exposed leaves, these proteins could have a role in determining a smaller meristem size in leaves of UV-B-exposed plants.
Patterns of Expression Changes after UV-B Exposure in Different Leaf Segments
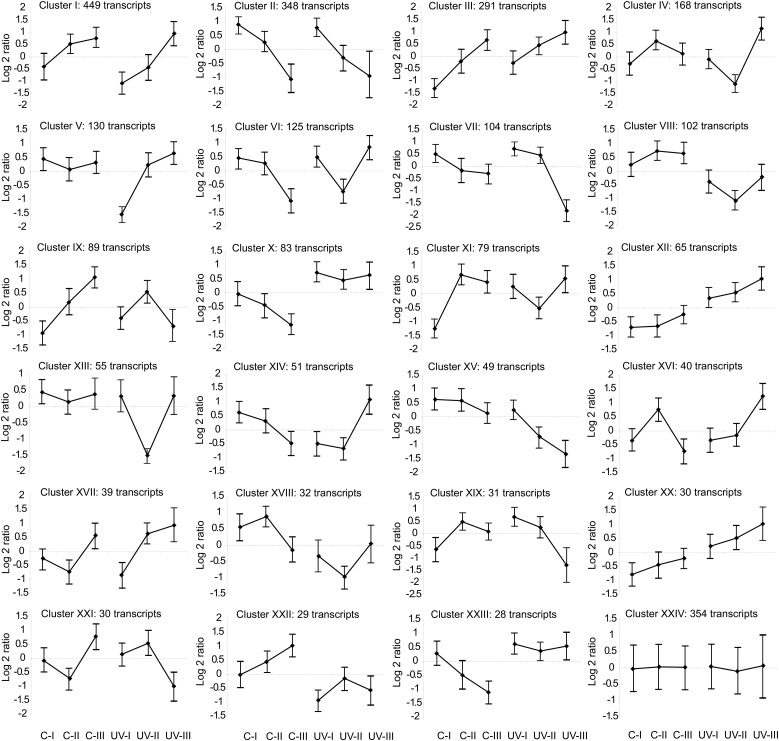
We grouped transcripts that were significantly changed either by the UV-B treatment (differential treatment) or by the interaction of the treatment with the leaf zone (differential interaction; Supplemental Table S1) according to the similarity of expression patterns by quality threshold (QT) clustering, resulting in 23 clusters (+1 nonassigned; Fig. 4). These clusters were analyzed in detail. The largest cluster (I) includes transcripts for which expression levels increase with distance from the leaf base. UV-B lowers the expression in the first two sections but enhances it in the third. Transcripts for several proteins that regulate and participate in cell cycle transitions, and that are required during cell division, are overrepresented in this cluster (Supplemental Table S1). For example, several cyclophilins, proteins from the cytoskeleton and that participate in microtubule organization like actin and tubulin, spindle checkpoint proteins, histones, and transcription factors with a role in development, such as NAC domain-containing proteins and TEOSINTE BRANCHED1, are represented in this cluster. Although the pattern of expression of transcripts in clusters II and IV is different from that of cluster I, these two clusters also include genes that show higher expression in segment II from control plants than from UV-B-irradiated leaves (Fig. 4). A cyclin-dependent kinase inhibitor, cell division cycle proteins, cyclophilins, and transcription factors that regulate cell cycle transitions such as several E2Fs (Gutierrez et al., 2002) are in cluster II; while transcripts for a cyclin-dependent protein kinase, other cyclophilins, and transcription factors similar to Arabidopsis AINTEGUMENTA and ASYMMETRIC LEAVES2, with roles in development and cell proliferation (Mizukami and Fischer, 2000; Luo et al., 2012), are in cluster IV. Interestingly, transcripts for the growth-regulating transcription factors GRF1, GRF2, GRF14, and GRF15 are concentrated in cluster II. In Arabidopsis, some transcription factors of this family are repressed by UV-B, and as a consequence, leaf growth is inhibited (Casadevall et al., 2013). In this way, transcripts that are required for cell division and proliferation are repressed by UV-B in the GZ, and this may explain the smaller size of the DEZ in UV-B-exposed leaves.
Figure 4.
Analysis of gene expression profiles across different leaf segments corresponding to the GZ from UV-B-exposed and control maize leaves. Cluster analysis is shown for transcripts that show significant differential expression between treatments or for the interaction of the treatment with the developmental stage (2,801 total transcripts) by QT clustering. Values are log2 mean-centered gene expression levels. Each graph displays the mean pattern of expression of the transcripts in the cluster in black; error bars represent the sd of average expression. The number of transcripts in each cluster is shown at the top left corner of each cluster.
Transcripts in clusters V and XIII also are interesting, because they have a similar expression pattern in all segments in the two light conditions, but they show a significant down-regulation in segment I (cluster V) or segment II (cluster XIII) by UV-B. A cyclinA1;1 and several transcription factors with roles in development and cell proliferation are members of cluster V, and transcripts encoding proteins with similarity to AUXIN RESPONSE FACTOR2 and ANGUSTIFOLIA, also related to cell proliferation (Nelissen et al., 2015), are in cluster XIII (Supplemental Table S1). Cluster VI includes transcripts that show similar expression levels in segments I and II from control leaves but significantly decrease their expression in segment III; this decrease in expression is observed in segment II from UV-B-exposed leaves. One transcript in this cluster is ANGUSTIFOLIA3, a transcriptional coactivator that interacts with GRFs and chromatin-remodeling complexes, regulating cell proliferation and leaf growth (Nelissen et al., 2015). Again, the repression of the expression of these proteins by UV-B may contribute to the phenotype observed in the kinematic analysis.
Inversely, several transcripts are enhanced in UV-B-exposed leaves compared with control leaves. Clusters X, XII, and XX include transcripts that are induced in each segment from UV-B-irradiated plants compared with the corresponding segment in control plants (Fig. 4). Several transcription factors are in cluster X, which includes transcripts that show decreased expression with distance from the leaf base under control conditions but are highly induced by UV-B in all three segments, such as zinc finger and WRKY transcription factors, and proteins that participate in hormone signaling (Supplemental Table S1). Interestingly, a putative DNA repair enzyme with similarity to ERCC1 from Arabidopsis (Hefner et al., 2003) is in this cluster, although the analyzed leaf segments are not exposed directly to UV-B and the exposed parts of leaf 4 do not show detectable accumulation of CPDs after exposure (Supplemental Fig. S2). Cluster XII, which includes transcripts that show low expression levels under control conditions but are highly induced by UV-B in all three segments, comprises TASSEL SEED1, which encodes a lipoxygenase involved in the synthesis of jasmonate in maize (Nemchenko et al., 2006); and cluster XX, with a very similar expression pattern to cluster XII, comprises a TCP transcription factor that also could participate in the regulation of leaf development (Koyama et al., 2010). These or other transcripts in the three clusters may be involved in the repression of the expression of transcripts in clusters I, II, IV, V, and XIII, which may be necessary for cell division in the DEZ.
Finally, different transcripts encoding enzymes that participate in hormone pathways are included in several clusters (clusters I–VII, X, XII, XVII, XVIII, XXIII, and XXIV; Fig. 4). For example, specific transcripts for enzymes in the abscisic acid (ABA), ethylene, and jasmonate pathways are up-regulated by UV-B in several of these clusters, consistent with their roles in stress responses; while certain transcripts that encode proteins in the GA, auxin, and cytokinin pathways, consistent with their role as growth stimulators, are down-regulated by this radiation (Fig. 4; Supplemental Table S1).
Hormone Profiles after UV-B Exposure in Maize Leaf 4
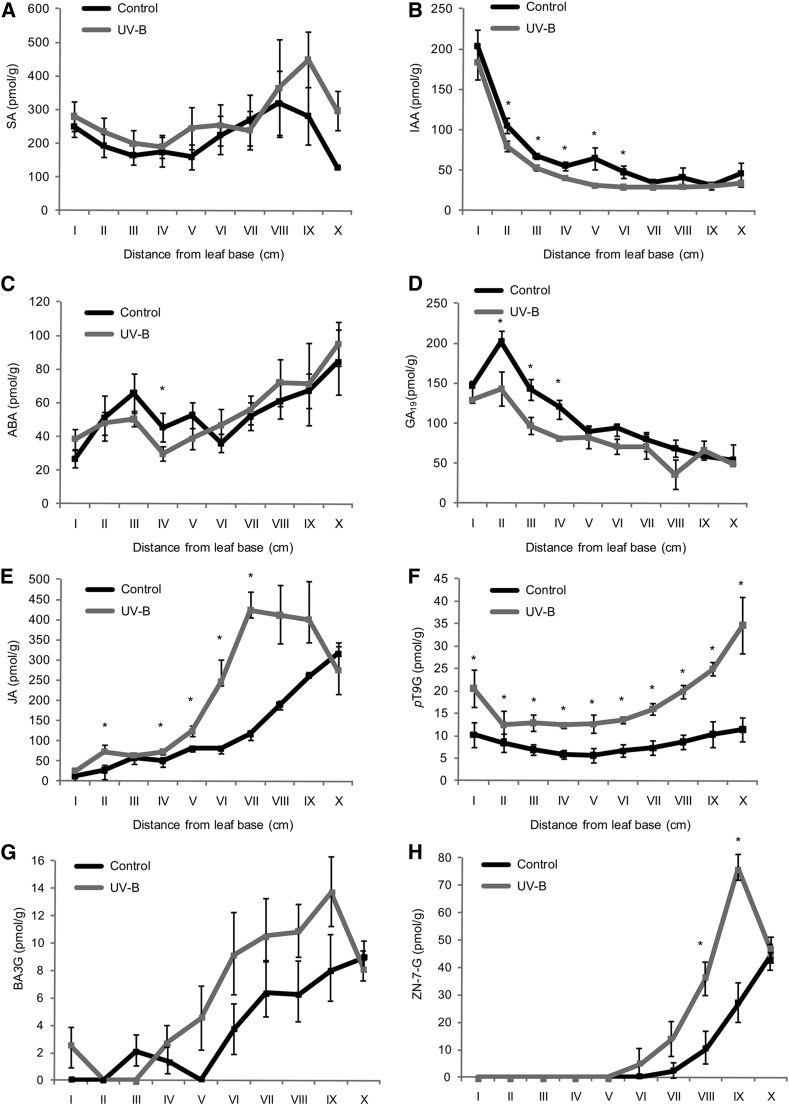
As shown, transcripts encoding proteins in hormone pathways are differentially regulated by UV-B in maize leaf segments I to III (Figs. 3 and 4). Plant hormones play essential roles in the spatial control of the division and expansion processes in the maize leaf (Nelissen et al., 2012) and may well play a role in the long-distance signaling induced by UV-B radiation. Thus, to investigate the effect of UV-B radiation in plant hormone profiles along the leaf, we quantified the endogenous hormone concentrations in the different leaf 4 segments, from I to X, from UV-B and control plants to correlate them with the spatial distribution of cell division and expansion in the growing maize leaf in the two different conditions. The concentrations of salicylic acid (SA), auxins (indole-3-acetic acid [IAA]), ABA, GAs, jasmonic acid (JA), and aromatic and isoprenoid cytokinins, such as paratopolin 9-glucoside (pT9G), 6-benzyladenine 3-glucoside (BA3G; an aromatic cytokinin), and zeatin nucleotide 7-glucoside (ZN-7-G; an isoprenoid cytokinin), varied significantly across the developmental gradient (Fig. 5). However, only a subset of them showed significant differences by UV-B in certain leaf segments. For example, IAA levels were lower in leaf segments II to VI from UV-B-exposed plants, while JA was significantly higher in segments II to VII excluding segment III from the same leaves. The concentration of the aromatic cytokinin pT9G was significantly higher in all leaf segments in irradiated plants, levels of the isoprenoid cytokinin ZN-7-G also were increased by UV-B in segments VIII and IX, and BA3G reflected only the shortening of the GZ. Unfortunately, bioactive GAs (GA1 and GA4), which peak at the transition between the DEZ and the EZ (Nelissen et al., 2012), could not be detected in our analysis. However, GA19, which is an earlier intermediate of the bioactive GA1 synthesis and shows a similar pattern of concentration along the maize leaves to GA1, peaking at the transition between the DEZ and the EZ (Nelissen et al., 2012; Fig. 5D), was decreased significantly by UV-B in segments II, III, and IV, which correspond to the GZ. In this way, variations in GA19 levels by UV-B correlate with the observed changes in the GZ size caused by this radiation.
Figure 5.
Hormone concentrations in the division, expansion, and mature zones of a maize leaf under control conditions and after UV-B exposure. Endogenous concentrations are shown for SA (A), auxin (IAA; B), ABA (C), GA19 (D), JA (E), and the cytokinins pT9G (F), BA3G (G), and ZN-7-G (H). Values are averages ± se (n = 3). Statistical significance was analyzed using Student’s t test with P < 0.05; differences from the control are marked with asterisks.
The Inhibition of Leaf Growth by UV-B Requires the Activity of GRF1, Which Modulates the Levels of GAs in the GZ
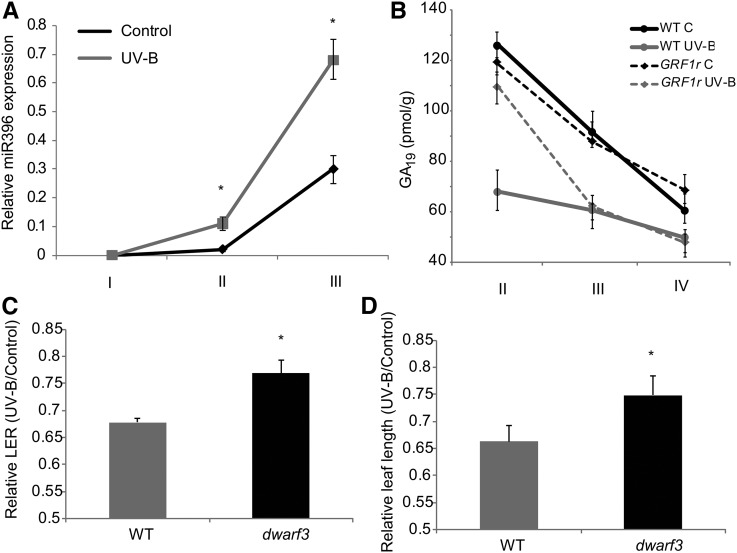
As shown above, the growth-regulating transcription factors GRF1, GRF2, GRF14, and GRF15 are repressed by UV-B in the maize leaf GZ (Supplemental Table S1). Some transcription factors of this family are posttranscriptionally regulated by miR396; this miRNA is essential for cell proliferation inhibition by UV-B in Arabidopsis, and its levels are increased after exposure in this species (Casadevall et al., 2013). Under our experimental conditions, miR396 also is induced by UV-B in the maize GZ (Fig. 6A). Therefore, we analyzed the effect of UV-B in leaf growth in transgenic maize plants expressing a miR396-resistant version of GRF1 under the control of its own promoter (GRF1r; Nelissen et al., 2015). A kinematic analysis of leaf 4 from GRF1r plants at steady-state growth was done in control and UV-B-exposed plants. Simultaneously, the assay was done using plants from the same genetic background not expressing the transgene (B103). Table II and Supplemental Table S2 show that leaf growth and leaf elongation rate were less inhibited by UV-B in GRF1r than in wild-type plants. This lower inhibition of leaf growth is because the decrease in the DEZ size of leaf 4 in GRF1r plants by UV-B is lower than in leaf 4 of the wild-type plants, having more transgenic dividing cells after exposure than wild-type leaves, as reported previously in Arabidopsis (Casadevall et al., 2013). In both groups of plants, the mature cell size and the cell cycle duration were similar, both under control conditions and after UV-B exposure (Table II; Supplemental Table S2). Interestingly, in contrast to the wild type, the EZ size was increased significantly by UV-B in leaf 4 of the GRF1r plants; this may be because the residence time of cells in the EZ in these plants was higher than that of wild-type plants. Together, these results suggest that GRF1 participates in the regulation of maize leaf growth inhibition by UV-B radiation; however, this process also requires other factors or regulatory pathways, as the growth of GRF1r leaf 4 under UV-B conditions is still impaired.
Figure 6.
A, Relative expression of miR396 across leaf segments corresponding to the GZ from UV-B-exposed and control maize leaves by reverse transcription-quantitative PCR. Data show means ± se of at least three independent experiments. Statistical significance was analyzed using Student’s t test with P < 0.05; differences from the control are marked with asterisks. B, Endogenous concentrations of GA19 in different leaf segments corresponding to the GZ from GRF1r and wild-type (WT) plants from the same genetic background (B103) under control conditions (C) and after UV-B exposure. Values are averages ± se (n = 3). C and D, Relative leaf elongation rate (LER; C) and leaf length (D) of wild-type and dwarf3 mutant leaves from UV-B-irradiated versus control plants grown in the absence of UV-B. Statistical significance was analyzed using Student’s t test with P < 0.05; differences from the control are marked with asterisks.
Table II. Comparison of the UV-B effects on cell division and cell expansion parameters in wild-type and GRF1r plants.
Values shown represent the ratios of average values from UV-B-exposed versus control samples (n = 5). NS, Not significant.
| Growth Parameters | Wild Type | GRF1r | GRF1r/Wild Type | Student’s t Test |
|---|---|---|---|---|
| Final leaf length | 0.68 | 0.88 | 1.29 | 0.02 |
| Leaf elongation rate | 0.72 | 0.76 | 1.04 | 0.04 |
| Mature cell size | 1.09 | 0.97 | 0.81 | NS |
| Cell production | 0.63 | 0.85 | 1.48 | 0.03 |
| DEZ size | 0.69 | 0.84 | 1.22 | 0.03 |
| Size of cells leaving the DEZ | 0.91 | 0.86 | 0.95 | NS |
| No. of cells in the DEZ | 0.70 | 0.89 | 1.27 | 0.05 |
| Cell cycle duration | 1.01 | 1.13 | 1.12 | NS |
| Average cell division rate | 0.73 | 0.80 | 1.09 | NS |
| Residence time in the EZ | 1.72 | 2.08 | 1.21 | 0.01 |
| GZ size | 0.87 | 1.26 | 1.44 | <0.001 |
| No. of cells in the GZ | 0.79 | 1.11 | 1.41 | 0.02 |
| Residence time in the DEZ | 1.19 | 1.24 | 1.05 | NS |
| No. of cells in the EZ | 0.99 | 1.25 | 1.26 | NS |
| EZ size | 0.91 | 1.75 | 1.92 | <0.001 |
| Average cell expansion rate | 0.65 | 0.52 | 0.66 | 0.02 |
Several reports showed that miR396/GRFs exert their effect on organ growth at least in part by regulating the expression of genes of the GA metabolism (Liu et al., 2009; Hewezi et al., 2012; Vercruyssen et al., 2015). Thus, to investigate if the lower decrease in the DEZ size by UV-B measured in leaf 4 of the GRF1r plants can be related to changes in GA levels in this zone, GA19 levels were quantified in segments I to IV from wild-type and GRF1r leaf 4 from control and UV-B-exposed plants. While GA19 levels decrease significantly by UV-B in segment II from wild-type leaves, the concentration of this hormone was unaffected in the same leaf segment from GRF1r leaves (Fig. 6B). However, in segments III and IV, GA19 levels under control conditions differ significantly from the levels in leaves exposed to UV-B in both groups of plants. To test the hypothesis that the role of GRF1 in the response to UV-B is mediated by GA, we analyzed the maize dwarf3 mutant, which is defective in the conversion of ent-kaurenoic acid to GA12 early in GA biosynthesis and, as a consequence, has very low levels of GA1 (Fujioka et al., 1988; Nelissen et al., 2012). Interestingly, similar to GRF1r plants, this mutant indeed shows a strong reduction of leaf growth response to UV-B (Fig. 6, C and D). Therefore, the modulation of levels of GAs in the DEZ controlled by the activity of GRF1 (and possibly by other transcription factors of this family) is one of the pathways that regulate leaf growth in maize plants exposed to UV-B.
DISCUSSION
In this work, we demonstrate that UV-B levels present in solar radiation inhibit maize leaf growth without causing any other visible stress symptoms or measurable accumulation of DNA damage. The observed decrease in leaf growth is a consequence of a reduction in cell production, as the division zone is shorter in UV-B-irradiated leaves. This decrease in the DEZ size correlates with a decrease in GA concentration by UV-B in this zone, which is regulated by the expression of GRF1 and possibly other transcription factors of the GRF family. UV-B also has been shown to inhibit cell expansion in several species (Gonzalez et al., 1998; Ruhland and Day, 2000; Ruhland et al., 2005; Wargent et al., 2009). In our experiments, the decrease in final leaf length and in leaf elongation rate in steady-state growth is not due to the reduction of mature cell size. The cell length profiles of both control and UV-B-exposed plants were similar, with shorter cells near the base of the leaf and longer cells toward the tip. However, in UV-B-exposed plants, the whole pattern was positioned at a shorter distance from the leaf base, primarily because the transition from proliferation to expansion occurred closer to the leaf base.
Growth inhibition caused by other abiotic factors or other stresses often is due to a combination of both reduced cell production and mature cell size (Granier et al., 2000; West et al., 2004), while our results show a specific effect only on cell production. In this way, the cell production rate of the GZ from UV-B-exposed leaves was 37% lower than that from control leaves. Interestingly, and in contrast with what was reported previously for cold stress (Rymen et al., 2007), the cell cycle duration was similar in UV-B-irradiated and control leaves (Table I); thus, the reduced cell production in leaf 4 by UV-B radiation is due to the lower number of dividing cells in the GZ as a consequence of the smaller DEZ. Cell cycle duration generally is constant across a wide range of experimental conditions (Baskin, 2000), and the results presented in our work are in agreement with those reported in response to soil compaction (Beemster et al., 1996) and drought (Tardieu and Granier, 2000; Sharp et al., 2004; Avramova et al., 2015a), where the decrease in the GZ was because of the lower number of dividing cells. Taken together, these results demonstrate that similar phenotypes observed for diverse environmental conditions may be the consequence of different mechanisms and regulatory pathways involved. Therefore, we propose that there are specific UV-B-inducible mechanisms regulating cell proliferation.
To identify the major pathways involved in this response and putative candidate genes that may be related to the molecular changes underlying the lower number of proliferating cells in maize UV-B-exposed leaves, we conducted RNA-seq experiments using different segment regions of the GZ to capture transcriptional gradients that could be associated with developmental transitions. Our experiments identified that the expression levels of 2,801 transcripts varied between the three leaf regions analyzed either by the UV-B treatment or by the interaction between the leaf zone and the treatment, which corresponds to 6% of the expressed transcripts. When global transcript changes in the GZ of leaf 4 were analyzed, we found that several transcripts encoding enzymes of different primary and secondary metabolic pathways were differentially regulated by UV-B radiation. Interestingly, although the leaf region analyzed is not exposed directly to solar radiation, levels of some photosynthetic and photorespiration mRNAs were found to be controlled by UV-B, suggesting that the photosynthetic machinery is already formed in these young stages prior to emergence, as reported previously (Li et al., 2010), and this activity seems to be regulated not only by photosynthetically active radiation but also by UV-B. Also, as reported previously in directly exposed maize leaves (Casati and Walbot, 2003), mRNAs for secondary metabolism enzymes were higher expressed in the GZ from UV-B-exposed plants; phenylpropanoids in general and flavonoids in particular have been reported several times to be UV-B inducible in order to protect the plants by absorbing UV-B radiation (Falcone Ferreyra et al., 2012). Again, this result is also very interesting, as the GZ is not exposed directly to UV-B radiation; despite this, flavonoids also have antioxidant properties, so maybe these compounds could still be required in shielded tissues to cope with oxidative stress conditions that may be the result of exposure. Alternatively, as proposed above, shielded leaves may activate some UV-B adaptation mechanisms prior to emergence. Different hypotheses may be raised about the fact that the samples used in our experiments did not directly receive natural sunlight but showed transcriptome and hormone changes induced by UV-B exposure. We cannot rule out that some UV-B may reach the meristematic region of the leaf; alternatively, UV-B may act to inhibit polar auxin transport, resulting in a lower auxin concentration in UV-B-exposed leaves in the GZ, as shown in Figure 5. Several genes involved in auxin signaling and metabolism showed altered expression patterns in our transcriptome analysis in the maize GZ by UV-B (Supplemental Tables S1 and S3); however, none of these genes are associated specifically with auxin transport. On the other hand, flavonoids may serve as inhibitors of auxin transport as well as photoprotectants (Kuhn et al., 2011). Therefore, an increase in transcripts encoding enzymes in the flavonoid pathway in the GZ by UV-B, such as chalcone isomerase and flavonol synthase I (Supplemental Table S3), suggests that flavonoids may be signals that could act by inhibiting auxin transport away from the GZ.
Moreover, our analysis shows that some transcripts for stress response proteins also were increased by UV-B, also suggesting that stress metabolism is active in the GZ in these tissues after exposure.
On the other hand, transcripts for enzymes in the GA, auxin, cytokinin, ABA, ethylene, and jasmonate pathways were regulated by UV-B in the GZ. Leaf size and development are regulated by hormones; so at least some of the proteins encoded by these transcripts could participate in the inhibition of cell proliferation by UV-B in our experiments. In agreement with these results, it was reported previously that, in the maize leaf base, auxin and cytokinin concentrations were highest in dividing tissues, while GAs showed a peak at the transition zone between the division and the expansion zones (Nelissen et al., 2012). As demonstrated by Nelissen et al. (2012), one mechanism that specifically controls the size of the DEZ in maize leaves involves the location of a peak of active GAs at the transition to the EZ. Although we were not able to detect any bioactive GA, GA19, which is a precursor of active GA1 and shows a similar pattern of concentration along the maize leaf to GA1 (Nelissen et al., 2012), also peaked at the transition between the DEZ and the EZ in our experiments, and it was decreased significantly by UV-B in the GZ. In this way, variations in GA19 levels correlated with the changes in the GZ size by UV-B measured in the kinematic analysis, suggesting that these changes may be responsible, at least in part, for the inhibition of leaf growth. Also, the facts that several genes of the GA metabolism were UV-B regulated, and that the dwarf3 mutant showed a lower inhibition of leaf growth by UV-B (Fig. 6, C and D), suggest that this mechanism may at least partly explain the observed growth phenotype.
Cluster analysis according to the similarity of expression patterns by QT clustering resulted in 24 clusters (Fig. 4). A subset of them (e.g. clusters I, II IV, V, and XIII) include genes that show higher expression in the DEZ from control plants than from UV-B-irradiated leaves; these include transcripts that are required for cell division and proliferation. Transcripts for the growth-regulating transcription factors GRF1, GRF2, GRF14, and GRF15 are included in cluster II. In Arabidopsis, some transcription factors in this family are repressed by UV-B, and as a consequence, leaf growth is inhibited (Casadevall et al., 2013). We demonstrated previously that miR396 was up-regulated by UV-B radiation in Arabidopsis proliferating tissues and that this induction was correlated with a decrease in GRF1, GRF2, and GRF3 transcripts. In maize, miR396 also was up-regulated by UV-B in the GZ (Fig. 6). The induction of miR396 resulted in the inhibition of cell proliferation in Arabidopsis, and transgenic plants expressing an artificial target mimic directed against miR396 to decrease the endogenous miRNA activity or plants expressing miR396-resistant copies of several GRFs were less sensitive to this inhibition (Casadevall et al., 2013). Maize plants expressing a miR396-resistant copy of GRF1 also were less sensitive to leaf growth inhibition by UV-B (Table II; Supplemental Table S2). The lower inhibition of maize leaf growth in GRF1r plants was because the decrease in the DEZ size by UV-B was lower than that of leaf 4 of wild-type plants, having more transgenic dividing cells remaining after exposure than the wild-type leaves, as reported previously in Arabidopsis (Casadevall et al., 2013).
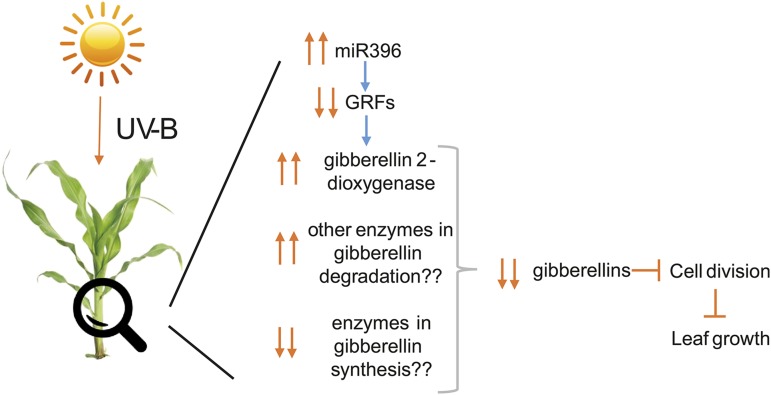
miR396 and GRF transcription factors have only been shown recently to participate in the control of phytohormone-related genes. For example, using a grf1grf2grf3 triple mutant and two miRNA-resistant forms of AtGRF1 and AtGRF3, Hewezi et al. (2012) found that more than 60 transcripts involved in phytohormone signaling pathways showed altered expression in at least one of these plants, including genes involved directly in the biosynthesis of bioactive GAs. According to these data, miR396 appears to act as a strict repressor of the GA pathways. For example, in the grf1grf2grf3 triple mutant, the expression of AtGA3ox1 and an AtGA20oxidase, two genes involved in GA biosynthesis, is inhibited; while the expression of AtGA2ox8, a GA 2-β-dioxygenase that catalyzes the GA catabolism of bioactive GAs or their precursors, is increased (Hewezi et al., 2012). Transcripts for a putative GA 2-β-dioxygenase are included in cluster III in our experiments (Supplemental Table S1). In this cluster, genes whose expression levels increase with distance from the leaf base but are higher expressed in UV-B-irradiated leaves are included. In this way, the up-regulation of AtGA2ox8 inversely correlates with the down-regulation of GRF1, GRF2, GRF14, and GRF15 (Fig. 4). Thus, an up-regulation of miR396 in the maize GZ by UV-B could decrease GRF levels, which in turn may down-regulate the expression of GA biosynthetic genes and increase catabolic transcript levels, such as GA2ox. This would result in lower concentrations of GAs in the GZ of exposed plants, inducing a decrease in GZ size by UV-B (Fig. 7).
Figure 7.
Model of maize leaf growth inhibition by UV-B mediated by miR396/GRFs and GAs.
On the other hand, other proteins with a role in cell cycle progression also were identified to be UV-B down-regulated in the GZ (Fig. 4). Examples are several cyclins and a cyclin kinase inhibitor; cyclins are primary regulators of the activity of cyclin-dependent kinases, which are known to play critical roles in controlling eukaryotic cell cycle progression (Barrôco et al., 2003). Moreover, several cell division control proteins (such as CDC48B proteins) were identified; CDC proteins target primarily cell cycle regulators for proteolysis, and their gene function is required at G1/S and G2/M transitions during mitosis (Baek et al., 2011). Putative cyclophilins also were found. In Arabidopsis, mutations in the cyclophilin FKBP70 lead to tumor growth in plants, indicating its role in cell division and cell differentiation, while mutations in FKBP42 displayed a drastic reduction of cell elongation (Faure et al., 1998; Geisler et al., 2003). Interestingly, two different putative E2F transcription factors are in cluster II of Figure 4. The E2F transcription factors are key components of the cyclin/retinoblastoma/E2F pathway that control cell cycle transitions in multicellular organisms (Gutierrez et al., 2002). The activation of the E2F family of transcription factors is negatively regulated by the retinoblastoma-related (RBR) protein; phosphorylation of RBR by cyclin-dependent kinases/cyclin complexes releases RBR repression of E2F, and these transcription factors activate the expression of genes necessary for the progression of the cell cycle. Together, other genes participating in cell cycle progression, through their regulation by GRF transcription factors and GA levels, or alternatively through other activation pathways, also may participate in the regulation of leaf elongation under UV-B conditions.
In conclusion, solar UV-B exposure in maize causes leaf growth inhibition as a consequence of an inhibition of cell proliferation. This response is accompanied by specific changes in the expression of genes that encode proteins that regulate cell cycle transitions and leaf development, including GRF transcription factors and genes for hormone synthesis, degradation, and signaling, and by a decrease of GAs in the GZ. We provide evidence that the inhibition of maize growth by UV-B is mediated by transcription factors of the GRF family, some of them probably regulated by miR396, which modify GA levels at the GZ. Despite this, this process also requires other factors or regulatory pathways. Our results show that transcriptional regulation of cell cycle-, development-, and hormone-associated genes plays a central role in the inhibition of cell production and growth under UV-B exposure conditions in maize that could be added to the regulatory cascade (Fig. 7), ultimately resulting in reduced leaf growth.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Maize (Zea mays) plants from the B73 inbred line were used in the experiments. GRF1r transgenic seeds (B103 background) and dwarf3 mutant seeds were obtained from the Inzé laboratory (Nelissen et al., 2012, 2015). UV treatments were done outdoors during the summer of 2013 in Rosario, Argentina (32° latitude), using different plastic filters to screen UV-B. Solar UV-B radiation was removed to produce the minus-UV-B treatment using PE filters (100-μm clear PE plastic; Tap Plastics). This PE filter absorbs UV-B without significantly affecting UV-A or visible radiation (Casati and Walbot, 2003). To control for differences in wind or humidity under plastic sheeting, CA sheeting was used (100-μm extra-clear CA plastic; Tap Plastics); the CA sheeting transmits most radiation from sunlight and is designated as the full UV treatment. For this experiment, 1 × 2 m of each plastic sheet was draped over 0.5- × 1.4-m wooden frames that were erected outdoors; the excess plastic was stapled to the sides of the frames to reduce shading. The north and south sides were left open to allow air to circulate. However, 50-cm-long curtains of the same plastic were made on the east and west sides to avoid early morning and late afternoon UV exposure. The frames were maintained about 30 cm above the plant canopy during the course of the experiments. Plants were grown under the specified conditions for approximately 40 d, until leaf 4 reached its final length. Measurements of incoming UV-B radiation were recorded at noon using a UV-B/UV-A radiometer (UV203 A+B radiometer; Macam Photometrics), and photosynthetic active radiation was measured with a quantum sensor and light meter (QSL-100; Biospherical Instruments). The average photosynthetic active radiation at noon was 1,500 µmol m−2 s−1, and UV-A radiation was 36 µmol m−2 s−1 in both groups; while the average UV-B radiation was 1.57 µmol m−2 s−1 in the control group and 6.75 µmol m−2 s−1 in the UV-B-exposed group. The experiment was repeated three times during the summer of 2013; climatological data of the period when the experiments were done were obtained from the Instituto Nacional de Tecnología Agropecuaria (http://siga2.inta.gov.ar/en/datoshistoricos/) and are shown in Supplemental Table S4. Arabidopsis (Arabidopsis thaliana) plants were exposed for 4 h to UV-B radiation from UV-B bulbs (Bio-Rad ChemiDoc XRS UV-B lamps, 302-nm maximum spectra; catalog no. 1708097) in a growth chamber at 100 µmol m−2 s−1 photosynthetic active radiation at plant height using fluora lamps (OSRAM L36W/77) and standard fluorescent tubes (Sylvania F38W/T8/154) and a 16/8-h photoperiod. UV-B lamps were covered with CA filters and placed 30 cm above the plants in order to exclude only UV-C but not remove UV-B and UV-A radiation from the spectrum. The UV intensities measured were 9 and 2.92 µmol m−2 s−1 for UV-B and UV-A, respectively; UV readings include the ambient chamber lamps. Control plants (without supplemental UV-B radiation) were exposed for the same period of time to the light sources described above but covered with PE filters.
Growth Analysis
To calculate leaf elongation rate, we measured the length of leaf 4, from leaf emergence (about 1 month after sowing) to maturity, using the soil level as a reference point. All measurements were done at noon using 20 plants per treatment. To determine the final length of leaf 4, we measured leaf length until it was constant for 3 consecutive days, typically about 12 d after leaf emergence.
To determine cell length profiles, we collected the basal 100 mm of leaf 4 at 3 d after leaf emergence, during steady-state growth or the third developmental phase (Granier and Tardieu, 2009), from 12 plants per treatment, and prepared 10-mm segments for microscopy, as described previously (Fiorani et al., 2000). Segments were kept in absolute ethanol for 48 h and then transferred to lactic acid. Samples were mounted on microscopy slides using lactic acid as a mounting medium, and then successive images were taken using an optical microscope (BH-2; Olympus) and a digital camera (Sight DS-Fi1; Nikon). For samples of meristematic cells, 40× objectives were used, while for elongating and mature cells, 20× objectives were used. ImageJ software was used to determine cell length. The raw data obtained for individual leaves were smoothed and interpolated at an interval of 50 mm using the smoothing function LocPoly algorithm (Rymen et al., 2010) of the R statistical package (R Foundation for Statistical Computing), which allowed averaging between leaves and comparison between treatments.
To estimate meristem size, we isolated the basal 50 mm of leaf 4 at 3 d after leaf emergence during steady-state growth from six plants per group. Samples were placed in 3:1 (v/v) absolute ethanol:acetic acid for fixation of cell walls and clearing of chlorophyll, rinsed in a buffer containing 50 mm NaCl, 5 mm EDTA, and 10 mm Tris-HCl, pH 7, and nuclei were stained by incubating the samples in the dark for 5 min in the same buffer solution containing 4′,6-diamino-phenylindole at a concentration of 1 μg mL−1. Fluorescent nuclei were observed with a microscope equipped with an epifluorescent condenser (Olympus BH2 attached to a Nikon DS-Fi1 camera), and the images obtained were used to measure leaf meristem size, defined as the distance between the base of the leaf and the most distal mitotic cell.
To calculate the kinematic parameters, we followed the procedure described by Rymen et al. (2010) and visually demonstrated by Sprangers et al. (2016).
DNA Damage Analysis
The induction of CPD was determined using an assay described in detail previously (Stapleton et al., 1993). Monoclonal antibodies specific to CPDs (TDM-2) were from Cosmo Bio. Maize leaf samples (0.1 g) that correspond to the mature zone (Granier and Tardieu, 2009; Sprangers et al., 2016) were collected 3 d after leaf emergence, during steady-state growth, from UV-B-exposed and control leaves at noon. Leaf samples from 4-week-old Arabidopsis plants that were either exposed for 4 h to UV-B radiation from UV-B bulbs or kept under control conditions were collected immediately after the treatment. Samples were immersed immediately in liquid nitrogen and stored at −80°C. A total of 1.5 μg of the extracted DNA by a modified cetyl-trimetyl-ammonium bromide method was denatured in 0.3 m NaOH for 10 min and 6-fold dot blotted onto a nylon membrane (Perkin-Elmer Life Sciences). DNA from Arabidopsis plants that were irradiated with UV-B lamps during 4 h was used as a control. The membrane was incubated for 2 h at 80°C and then blocked in Tris-buffered saline (TBS; 20 mm Tris-HCl, pH 7.6, and 137 mm NaCl) containing 5% dried milk for 1 h at room temperature or overnight at 4°C. The blot was then washed with TBS and incubated with TDM-2 (1:2,000 in TBS) overnight at 4°C with agitation. Unbound antibody was washed away, and secondary antibody (Bio-Rad) conjugated to alkaline phosphatase (1:3,000) was added. The blot was then washed several times followed by the addition of the detection reagents nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate. Quantification was achieved by densitometry of the dot blot using ImageQuant software version 5.2. The DNA concentration was fluorometrically determined using the Qubit dsDNA Assay Kit (Invitrogen) and checked on 1% (w/v) agarose gels after quantification.
RNA Extraction
Total RNA was isolated from the first three 10-mm leaf segments from the basal 30 mm of leaf 4 at 3 d after leaf emergence, during steady-state growth (Granier and Tardieu, 2009; Sprangers et al., 2016), from each treatment. Samples were obtained from three different biological replicates. For RNA extraction, the TRIzol reagent (Invitrogen) was used as described in the manufacturer’s protocol. Then, 0.5 to 1 mg of total RNA was incubated with RNase-free DNase I (1 unit mL−1) following the protocol provided by the manufacturer to remove possible genomic DNA.
RNA-Seq Experiments
Prior to library preparation, RNA quality and integrity were assessed using a gel cartridge on a QIAxcel platform (Qiagen). Library preparation was done using the TruSeq Stranded mRNA sample preparation 96 rcx kit (Illumina) following the low-sample protocol according to Illumina guidelines. Briefly, approximately 2.5 µg of total RNA was diluted and purified using RNA purification beads targeting the poly(A) tail of the mRNA and subsequently was fragmented by means of the enzymes provided in the kit. After cDNA synthesis, adenylation of the 3′ ends and ligation of the adaptors were employed. Adaptors were ligated in 12-plex formations, allowing the pooling of 12 samples after PCR enrichment of the library. Subsequently, the library was quantified using PicoGreen dye (Life Technologies) as described in the manufacturer’s protocol. Thereafter, groups of 12 samples were pooled at equal concentrations to create eight pools. In order to accurately quantify the concentration in nanomolar of our pools, the Kapa SYBR FAST Universal qPCR Kit (Kapa Biosystems) for Illumina sequencing was used to quantify the number of amplifiable molecules in the pools and the Bioanalyzer (Agilent Technologies) to determine the average fragment size of our pools. These measurements allowed optimizing the flow cell clustering and proceeded with the run. The Illumina HiSeq 1500 was used in high-throughput mode together with the TruSeq SBS Kit to sequence the samples in a 50-cycle pair-end run. Following the analysis of these runs, rapid run was performed on the samples having yielded below 5 million reads. The rapid runs were performed on the same pools as in the first sequencing round. Data from these rapid runs were added to the high-throughput data. RNA-seq data were deposited in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE 95858. Accession numbers for all genes are shown in Supplemental Table S1.
Statistical Analysis
Transcriptome analysis was performed by means of CLC Genomics Workbench version 6 using the maize cv B73 sequence (RefGen V3; http://www.maizegdb.org) as the reference genome. After trimming the Illumina adaptors from the reads, they were mapped against the reference genome. The expression values were calculated based on reads per kilobase of exon model per million mapped reads values (Mortazavi et al., 2008). The expression values were normalized by scaling to 106 reads per sample.
Genes for which no counts were found for all three replicates for at least one of the position/treatment combinations were considered not to be expressed and were removed from further analysis. Expression data were log transformed, whereby 0 expression values were replaced by the lowest non-0 in that sample, corresponding to 1 count, effectively leading to an underestimation of the differences between samples with none and those with several counts. Statistical analysis for the effect of UV-B and differences between zones was conducted by means of two-way ANOVA using MultiExperiment Viewer 4.9.0. The obtained P values were corrected for multiple testing for each contrast separately by means of the FDR (Benjamini and Hochberg, 1995) using only genes for which expression was detected in all three replicates of at least one treatment. FDR < 0.05 in combination with a log fold change of 0.75 was used to select significantly affected genes. The samples were compared by hierarchical clustering (Eisen et al., 1998) and clustered using Support Trees (Graur and Li, 2000). Expression patterns of differentially expressed genes were visualized and clustered using QT clustering (Heyer et al., 1999). All clustering was performed in MultiExperiment Viewer 4.9.0.
Extraction of Acid Hormones
Plant material was homogenized by grinding and extracted for 16 h in 80% (v/v) methanol at −20°C. [C613]Phenyl-IAA (100 pmol; Cambridge Isotope Laboratories), 150 pmol of D6-ABA ([2H6](+)-cis,trans-ABA [OlChemIm]), 100 pmol of [2H4]SA (OlChemIm), 100 pmol of dehydro-JA (OlChemIm), 20 pmol of [2H2]GA1 (OlChemIm), 20 pmol of [2H2]GA3 (OlChemIm), 20 pmol of [2H2]GA4 (OlChemIm), 20 pmol of [2H2]GA7 (OlChemIm), 20 pmol of [2H2]GA8 (OlChemIm), 20 pmol of [2H2]GA9 (OlChemIm), 20 pmol of [2H2]GA12 (OlChemIm), and 20 pmol of [2H2]GA19 (OlChemIm) were added as internal standards. After a purification step removing pigments on a C18 cartridge (Bond Elut C18 6 cc, 500 mg; Agilent) in 80% (v/v) methanol, the extract was diluted and acidified with 6% (v/v) formic acid so that hormones bind to the C18 cartridge. To elute the hormones, diethyl ether was used. The residual water was removed, and the ether phase was evaporated under a stream of N2 gas (Turbovac LV Evaporator). After methylation with diazomethane (Schlenk and Gellerman, 1960), the samples were dried under N2 gas and dissolved in 15 µL of 100% (v/v) hexane for gas chromatography-mass spectrometry analysis of SA and JA. The remaining samples after JA and SA analysis were dried and redissolved in 50 µL of 10% (v/v) methanol for liquid chromatography-mass spectrometry analysis of IAA and ABA.
SA and JA Analysis
Gas chromatography-mass spectrometry was performed using a Waters Micromass Quattro micro gas chromatograph (Waters): a triple quadrupole with an integrated Agilent 6890N gas chromatography oven and using an electron-impact ion source, positive ion mode of 70 eV, collision energy of 10 eV, interchannel delay of 10 ms, and interscan delay of 10 ms. The gas chromatography column used was a 15-m × 0.25-mm Agilent J&W DB-5ms, film thickness of 0.25 µm (Agilent Technologies), injection volume of 10 µL, carrier gas was helium, and flow rate of 1 mL min−1. The oven started isothermally at 50°C for 2 min, increased linearly to 300°C at a rate of 25°C min−1, and then 300°C was held for 3 min. The diagnostic transitions used for the quantification of SA and JA in MRM mode are 152 > 120 m/z for Me-SA, 156 > 124 m/z for D4-Me-SA, 224 > 151 m/z for Me-JA, and 226 > 153 m/z for dihydro-Me-JA (dwell time, 0.01 s).
IAA, ABA, and GA Analysis
GAs, IAA, and ABA were analyzed by ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS) after methylation (Acquity TQD; Waters) using a 6-µL injection by partial loop (Acquity BEHC18, 1.7-µm column; Waters), a column temperature of 30°C, flow of 400, solvent gradient of 0 to 2 min 95:5 10% (v/v) methanol in 1 mm NH4OAc:methanol; 2- to 4-min linear gradient until 10:90 10% (v/v) methanol in 1 mm NH4OAc:methanol; and 4- to 6-min isocratic 10:90 10% (v/v) methanol in 1 mm NH4OAc:methanol. MS conditions were as follows: polarity MS ES(+), capillary 2 kV, cone 20 V; collision energy, 20 eV; source temperature, 120°C; desolvation temperature, 450°C; cone gas flow, 50 L h−1; desolvation gas flow, 750 L h−1; collision gas flow, 0.19 mL min−1. The diagnostic ions used for the quantification of IAA and ABA were as follows: 190 > 130 m/z for Me-IAA, 196 > 136 m/z for Me-C13-IAA, 279 > 173 m/z for Me-ABA, and 285 > 179 m/z for d6-Me-ABA (dwell time, 0.02 s). The diagnostic transitions used for the quantification of the GAs were as follows: 382 > 333 m/z for D2-GA1-Me, 380 > 331 m/z for GA1-Me, 380 > 241 m/z for D2-GA3-Me, 378 > 239 m/z for GA3-Me, 366 > 317 m/z for D2-GA4-Me, 364 > 315 m/z for GA4-Me, 362 > 285 m/z for GA5-Me, 364 > 315 m/z for D2-GA7-Me, 362 > 313 m/z for GA7-Me, 398 > 349 m/z for D2-GA8-Me, 396 > 347 m/z for GA8-Me, 350 > 301 m/z for D2-GA9-Me, 348 > 299 m/z for GA9-Me, 399 > 300 m/z for D2-GA12-Me, 397 > 298 m/z for GA12-Me, 393 > 300 m/z for D2-GA19-Me, 391 > 298 m/z for D2-GA19-Me, and 364 > 315 m/z for GA20-Me (dwell time, 0.01 s). Methanol and water used for mass spectrometry were ultra-performance liquid chromatography grade from Biosolve. Data are expressed in pmol per g fresh weight.
Extraction of Neutral Hormones
Plant material was homogenized by grinding and extracted 16 h in 80% (v/v) methanol at −20°C. [2H7]N6-Benzyladenine (d-BA), [2H7]N6-benzyladenosine (d-BAR), [2H7]N6-benzyladenine-9-glucoside (d-BA9G), [15N4]meta-topolin (15N-mT), [15N4]ortho-topolin (15N-oT), [2H3]dihydro-zeatin (d-DHZ), [2H3]dihydro-zeatin riboside (d-DHZR), [2H6]N6-isopentenyladenine (d-iP), [2H6]N6-isopentenyladenosine (d-iPR), [2H5]trans-zeatin-7-glucoside (d-tZ7G), [2H5]trans-zeatin-9-glucoside (d-tZ9G), [2H5]trans-zeatin-O-glucoside (d-tZOG), [2H5]trans-zeatin-O-glucoside riboside (d-tZROG), [2H6]N6-isopentenyladenine-7-glucoside (d-iP7G), and [2H6]N6-isopentenyladenine-9-glucoside (d-iP9G [10 pmol each; OlChemIm]) were added as internal standards. After centrifugation at 20,000g for 15 min at 4°C using a 5810R centrifuge and an FA-45-30-11 rotor (Eppendorf), the supernatant was passed over a C18 cartridge (500 mg; Varian) to retain pigments and consecutively filtered (Chromafil Xtra PA-20/25, 0.20 µm, Φ 25 mm; MN). Samples were dried in a Speed-Vac (Christ RNC2-25 vacuum concentrator with KNF N860.3FT.40.18 pump) and redissolved in 50 µL of 10% (v/v) methanol for analysis.
Cytokinin Analysis
Isoprenoid cytokinins were analyzed by UPLC-MS/MS using a 6-µL injection by partial loop (Acquity BEHC18, 1.7 µm column; Waters), column temperature of 30°C, flow of 400, solvent gradient was 0 to 0.5 min 95:5 10% (v/v) methanol in 1 mm NH4OAc:methanol; 0.5- to 3-min linear gradient until 75:25 10% (v/v) methanol in 1 mm NH4OAc:methanol; 3- to 5-min isocratic 75:25 10% (v/v) methanol in 1 mm NH4OAc:methanol; 5- to 6-min linear gradient until 5:95 10% (v/v) methanol in 1 mm NH4OAc:methanol; 6- to 6.5-min isocratic 5:95 10% (v/v) methanol in 1 mm NH4OAc:methanol. MS conditions were as follows: polarity MS ES(+), capillary 2 kV, cone 20 V; collision energy, 20 eV; source temperature, 120°C; desolvation temperature, 400°C; cone gas flow, 20 L h−1; desolvation gas flow, 800 L h−1; collision gas flow, 0.22 mL min−1.
The diagnostic ions used for quantification for the isoprenoid cytokinins were as follows: 225 > 136 m/z for D3-DHZ, 222 > 136 m/z for DHZ, 220 > 136 m/z for Z, 357 > 225 m/z for D3-DHZR, 354 > 222 m/z for DHZR, 352 > 220 m/z for ZR, 372 > 210 m/z for D6-iP, 366 > 204 m/z for iP, 342 > 210 m/z for D6-iPR, 336 > 204 m/z for iPR, 387 > 225 m/z for D5-Z7G, D5-Z9G, and D5-ZOG, 384 > 222 m/z for DHZ7G, DHZ9G, and DHZOG, 382 > 220 m/z for Z7G, Z9G, and ZOG, 519 > 387 m/z for D5-tZROG, 516 > 384 m/z for DHZROG, 514 > 382 m/z for ZROG, 372 > 210 m/z for D6-iP7G and D6-iP9G, and 366 > 204 m/z for iP7G and iP9G.
Aromatic cytokinins were analyzed by UPLC-MS/MS (Waters) using a 6-µL injection by partial loop (Acquity BEHC18, 1.7 µm column; Waters), column temperature of 40°C, flow of 550, solvent gradient was 0 to 1 min 100:0 10% (v/v) methanol in 1 mm NH4OAc:methanol; 1- to 5-min linear gradient until 72:28 10% (v/v) methanol in 1 mm NH4OAc:methanol; 5- to 5.5-min linear gradient until 0:100 10% (v/v) methanol in 1 mm NH4OAc:methanol; 5.5- to 6-min isocratic 100% (v/v) methanol. MS conditions were as follows: polarity MS ES(+), capillary 1.78 kV, cone 20 V; collision energy, 12 eV; source temperature, 120°C; desolvation temperature, 400°C; cone gas flow, 20 L h−1; desolvation gas flow, 800 L h−1; collision gas flow, 0.22 mL min−1.
The diagnostic transitions used for quantification for the aromatic cytokinins were as follows: 233 > 98 m/z for D7-BA, 226 > 91 m/z for BA, 365 > 233 m/z for D7-BAR, 358 > 226 m/z for BAR, 395 > 233 m/z for D7-BA9G, 388 > 226 m/z for BA9G, BA7G, and BA3G, 246 > 107 m/z for 15N-mT, 242 > 107 m/z for mT, 247 > 141 for 15N-oT, 242 > 136 m/z for oT and pT, 256 > 121 for Me-m,o-T, 374 > 242 m/z for o,m,p-TR, 404 > 242 m/z for o,m,p -T-G, and 418 > 256 m/z for Me-mT-G. Methanol and water used for mass spectrometry were ultra-performance liquid chromatography grade from Biosolve. Data were expressed as pmol g−1 fresh weight.
Small RNA Analysis
RNA was extracted using TRIzol reagent (Invitrogen), and miR396 levels were determined by stem-loop reverse transcription-quantitative PCR, as described previously (Casadevall et al., 2013).
Accession Numbers
Sequence data from this article can be found in the GenBank data libraries under the accession numbers shown in Supplemental Table S1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Plant height after the UV-B treatment in B73 and B103 inbreds.
Supplemental Figure S2. CPD levels in the DNA of Arabidopsis and maize leaves.
Supplemental Table S1. List of transcripts that show significant differential expression in response to the UV-B treatment.
Supplemental Table S2. Solar UV-B effect on cell division and cell expansion parameters in wild type B103 and GRF1r maize plants or the interaction between the treatment and the developmental stage grouped by expression patterns obtained by QT clustering.
Supplemental Table S3. List of transcripts that show significant differential expression in response to the UV-B treatment or the interaction between the treatment and the developmental stage related to auxin and flavonoid metabolism grouped by expression patterns obtained by QT clustering.
Supplemental Table S4. Climatological data of the period of the experiments.
Supplementary Material
Acknowledgments
We thank H. Nelissen and D. Inzé for generously providing GRF1r and dwarf3 seed stocks as well as J. Palatnik and U.P. Chorostecki for help in RNA-seq analysis and interpretation.
Glossary
- DEZ
dividing and elongating zone
- EZ
elongation zone
- GZ
growth zone
- PE
polyester
- CA
cellulose acetate
- RNA-seq
RNA sequencing
- FDR
false discovery rate
- QT
quality threshold
- ABA
abscisic acid
- SA
salicylic acid
- IAA
indole-3-acetic acid
- JA
jasmonic acid
- pT9G
paratopolin 9-glucoside
- BA3G
6-benzyladenine 3-glucoside
- ZN-7-G
zeatin nucleotide 7-glucoside
- TBS
Tris-buffered saline
- UPLC-MS/MS
ultra-performance liquid chromatography-mass spectrometry
Footnotes
This work was supported by FONCyT (grant nos. PICT 2013-268 and PICT 2015-157 to P.C.), the Interuniversity Attraction Poles from the Belgian Federal Science Policy Office, the FWO (grant nos. G0D0514N and G0B6716N to G.T.S.B.), the European Molecular Biology Organization (short-term fellowship to J.F.), the Erasmus Mundus EADICII program (to R.C.), and the GOA (research grant from the University of Antwerp to M.N.C.).
Articles can be viewed without a subscription.
References
- Andriankaja M, Dhondt S, De Bodt S, Vanhaeren H, Coppens F, De Milde L, Mühlenbock P, Skirycz A, Gonzalez N, Beemster GTS, et al. (2012) Exit from proliferation during leaf development in Arabidopsis thaliana: a not-so-gradual process. Dev Cell 22: 64–78 [DOI] [PubMed] [Google Scholar]
- Avramova V, AbdElgawad H, Zhang Z, Fotschki B, Casadevall R, Vergauwen L, Knapen D, Taleisnik E, Guisez Y, Asard H, et al. (2015a) Drought induces distinct growth response, protection, and recovery mechanisms in the maize leaf growth zone. Plant Physiol 169: 1382–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramova V, Sprangers K, Beemster GTS (2015b) The maize leaf: another perspective on growth regulation. Trends Plant Sci 20: 787–797 [DOI] [PubMed] [Google Scholar]
- Baek GH, Kim I, Rao H (2011) The Cdc48 ATPase modulates the interaction between two proteolytic factors Ufd2 and Rad23. Proc Natl Acad Sci USA 108: 13558–13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL, Rousseau MC, Searles PS, Zaller JG, Giordano CV, Robson TM, Caldwell MM, Sala OE, Scopel AL (2001) Impacts of solar ultraviolet-B radiation on terrestrial ecosystems of Tierra del Fuego (southern Argentina): an overview of recent progress. J Photochem Photobiol B 62: 67–77 [DOI] [PubMed] [Google Scholar]
- Barrôco RM, De Veylder L, Magyar Z, Engler G, Inzé D, Mironov V (2003) Novel complexes of cyclin-dependent kinases and a cyclin-like protein from Arabidopsis thaliana with a function unrelated to cell division. Cell Mol Life Sci 60: 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. (2000) On the constancy of cell division rate in the root meristem. Plant Mol Biol 43: 545–554 [DOI] [PubMed] [Google Scholar]
- Baute J, Herman D, Coppens F, De Block J, Slabbinck B, Dell’Acqua M, Pè ME, Maere S, Nelissen H, Inzé D (2016) Combined large-scale phenotyping and transcriptomics in maize reveals a robust growth regulatory network. Plant Physiol 170: 1848–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI (1998) Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116: 1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, De Veylder L, Vercruysse S, West G, Rombaut D, Van Hummelen P, Galichet A, Gruissem W, Inzé D, Vuylsteke M (2005) Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol 138: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Masle J, Williamson RE, Farquhar GD (1996) Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L.): kinematic analysis of leaf elongation. J Exp Bot 47: 1663–1678 [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57: 289–300 [Google Scholar]
- Biever JJ, Brinkman D, Gardner G (2014) UV-B inhibition of hypocotyl growth in etiolated Arabidopsis thaliana seedlings is a consequence of cell cycle arrest initiated by photodimer accumulation. J Exp Bot 65: 2949–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornman JF, Teramura AH (1993) Effects of ultraviolet-B radiation on terrestrial plants. In Young AR, Björn LO, Moan J, Nultsch W, eds, Environmental UV Photobiology. Plenum Press, New York, pp 427–471 [Google Scholar]
- Casadevall R, Rodriguez RE, Debernardi JM, Palatnik JF, Casati P (2013) Repression of growth regulating factors by the microRNA396 inhibits cell proliferation by UV-B radiation in Arabidopsis leaves. Plant Cell 25: 3570–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati P, Walbot V (2003) Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content. Plant Physiol 132: 1739–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Hays JB (2007) Tolerance of dividing cells to replication stress in UVB-irradiated Arabidopsis roots: requirements for DNA translesion polymerases eta and zeta. DNA Repair 6: 1341–1358 [DOI] [PubMed] [Google Scholar]
- Donnelly P, Bonetta D, Tsukaya H, Dengler R, Dengler N (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215: 407–419 [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone Ferreyra ML, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure JD, Vittorioso P, Santoni V, Fraisier V, Prinsen E, Barlier I, Van Onckelen H, Caboche M, Bellini C (1998) The PASTICCINO genes of Arabidopsis thaliana are involved in the control of cell division and differentiation. Development 125: 909–918 [DOI] [PubMed] [Google Scholar]
- Fiorani F, Beemster GTS, Bultynck L, Lambers H (2000) Can meristematic activity determine variation in leaf size and elongation rate among four Poa species? A kinematic study. Plant Physiol 124: 845–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint SD, Searles PS, Caldwell MM (2004) Field testing of biological spectral weighting functions for induction of UV-absorbing compounds in higher plants. Photochem Photobiol 79: 399–403 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Gaskin P, Macmillan J, Phinney BO, Takahashi N (1988) Qualitative and quantitative analyses of gibberellins in vegetative shoots of normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 seedlings of Zea mays L. Plant Physiol 88: 1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Curtis MJ, Tominey CM, Duong YH, Wilcox BW, Aggoune D, Hays JB, Britt AB (2010) A shared DNA-damage-response pathway for induction of stem-cell death by UVB and by gamma irradiation. DNA Repair 9: 940–948 [DOI] [PubMed] [Google Scholar]
- Gardner G, Lin C, Tobin EM, Loehrer H, Brinkman D (2009) Photobiological properties of the inhibition of etiolated Arabidopsis seedling growth by ultraviolet-B irradiation. Plant Cell Environ 32: 1573–1583 [DOI] [PubMed] [Google Scholar]
- Graur D, Li WH (2000) Fundamentals of Molecular Evolution, Ed 2 Sinauer Associates, Sunderland, MA [Google Scholar]
- Geisler M, Kolukisaoglu HU, Bouchard R, Billion K, Berger J, Saal B, Frangne N, Koncz-Kalman Z, Koncz C, Dudler R, et al. (2003) TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol Biol Cell 14: 4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Mepsted R, Wellburn AR, Paul ND (1998) Non-photosynthetic mechanisms of growth reduction in pea (Pisum sativum L.) exposed to UV-B radiation. Plant Cell Environ 21: 23–32 [Google Scholar]
- Granier C, Inzé D, Tardieu F (2000) Spatial distribution of cell division rate can be deduced from that of p34(cdc2) kinase activity in maize leaves grown at contrasting temperatures and soil water conditions. Plant Physiol 124: 1393–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Tardieu F (2009) Multi-scale phenotyping of leaf expansion in response to environmental changes: the whole is more than the sum of parts. Plant Cell Environ 32: 1175–1184 [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Ramirez-Parra E, Castellano MM, del Pozo JC (2002) G(1) to S transition: more than a cell cycle engine switch. Curr Opin Plant Biol 5: 480–486 [DOI] [PubMed] [Google Scholar]
- Hectors K, Jacques E, Prinsen E, Guisez Y, Verbelen JP, Jansen MAK, Vissenberg K (2010) UV radiation reduces epidermal cell expansion in leaves of Arabidopsis thaliana. J Exp Bot 61: 4339–4349 [DOI] [PubMed] [Google Scholar]
- Hectors K, Prinsen E, De Coen W, Jansen MAK, Guisez Y (2007) Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol 175: 255–270 [DOI] [PubMed] [Google Scholar]
- Hefner E, Preuss SB, Britt AB (2003) Arabidopsis mutants sensitive to gamma radiation include the homologue of the human repair gene ERCC1. J Exp Bot 54: 669–680 [DOI] [PubMed] [Google Scholar]
- Hewezi T, Maier TR, Nettleton D, Baum TJ (2012) The Arabidopsis microRNA396-GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection. Plant Physiol 159: 321–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer LJ, Kruglyak S, Yooseph S (1999) Exploring expression data: identification and analysis of coexpressed genes. Genome Res 9: 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann RW, Campbell BD, Bloor SJ, Swinny EE, Markham KR, Ryan KG, Fountain DW (2003) Responses to UV-B radiation in Trifolium repens L.: physiological links to plant productivity and water availability. Plant Cell Environ 26: 603–612 [Google Scholar]
- Hopkins L, Bond MA, Tobin AK (2002) Ultraviolet-B radiation reduces the rates of cell division and elongation in the primary leaf of wheat (Triticum aestivum L. cv Maris Huntsman). Plant Cell Environ 25: 617–624 [Google Scholar]
- Kakani VG, Reddy KR, Zhao D, Mohammed AR (2003) Effects of ultraviolet-B radiation on cotton (Gossypium hirsutum L.) morphology and anatomy. Ann Bot (Lond) 91: 817–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Lim JE, Landry LG, Last RL (2002) Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol 130: 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2010) TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell 22: 3574–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn BM, Geisler M, Bigler L, Ring C (2011) Flavonols accumulate asymmetrically and affect auxin transport in Arabidopsis. Plant Physiol 156: 585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso K, Sullivan JH, Huttunen S (2000) The effects of UV-B radiation on epidermal anatomy in loblolly pine (Pinus taeda L.) and Scots pine (Pinus sylvestris L.). Plant Cell Environ 23: 461–472 [Google Scholar]
- Li P, Ponnala L, Gandotra N, Wang L, Si Y, Tausta SL, Kebrom TH, Provart N, Patel R, Myers CR, et al. (2010) The developmental dynamics of the maize leaf transcriptome. Nat Genet 42: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Liu D, Song Y, Chen Z, Yu D (2009) Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plant 136: 223–236 [DOI] [PubMed] [Google Scholar]
- Luo L, Ando S, Sasabe M, Machida C, Kurihara D, Higashiyama T, Machida Y (2012) Arabidopsis ASYMMETRIC LEAVES2 protein required for leaf morphogenesis consistently forms speckles during mitosis of tobacco BY-2 cells via signals in its specific sequence. J Plant Res 125: 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Fischer RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci USA 97: 942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628 [DOI] [PubMed] [Google Scholar]
- Nelissen H, Eeckhout D, Demuynck K, Persiau G, Walton A, van Bel M, Vervoort M, Candaele J, De Block J, Aesaert S, et al. (2015) Dynamic changes in ANGUSTIFOLIA3 complex composition reveal a growth regulatory mechanism in the maize leaf. Plant Cell 27: 1605–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Van Lijsebettens M, Kamiya Y, Inzé D, Beemster GTS (2012) A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Curr Biol 22: 1183–1187 [DOI] [PubMed] [Google Scholar]
- Nemchenko A, Kunze S, Feussner I, Kolomiets M (2006) Duplicate maize 13-lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. J Exp Bot 57: 3767–3779 [DOI] [PubMed] [Google Scholar]
- Nogués S, Allen DJ, Morison JIL, Baker NR (1998) Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiol 117: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza P, Jasinski S, Tsiantis M (2005) Evolution of leaf developmental mechanisms. New Phytol 167: 693–710 [DOI] [PubMed] [Google Scholar]
- Robson TM, Aphalo PJ (2012) Species-specific effect of UV-B radiation on the temporal pattern of leaf growth. Physiol Plant 144: 146–160 [DOI] [PubMed] [Google Scholar]
- Ruhland CT, Day TA (2000) Effects of ultraviolet-B radiation on leaf elongation, production and phenylpropanoid concentrations of Deschampsia antarctica and Colobanthus quitensis in Antarctica. Physiol Plant 109: 244–251 [Google Scholar]
- Ruhland CT, Xiong FS, Clark WD, Day TA (2005) The influence of ultraviolet-B radiation on growth, hydroxycinnamic acids and flavonoids of Deschampsia antarctica during springtime ozone depletion in Antarctica. Photochem Photobiol 81: 1086–1093 [DOI] [PubMed] [Google Scholar]
- Rymen B, Coppens F, Dhondt S, Fiorani F, Beemster GTS (2010) Kinematic analysis of cell division and expansion. Methods Mol Biol 655: 203–227 [DOI] [PubMed] [Google Scholar]
- Rymen B, Fiorani F, Kartal F, Vandepoele K, Inzé D, Beemster GTS (2007) Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol 143: 1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk H, Gellerman JL (1960) Esterification of fatty acids with diazomethane on a small scale. Anal Chem 32: 1412–1414 [Google Scholar]
- Searles PS, Flint SD, Caldwell MM (2001) A meta analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 127: 1–10 [DOI] [PubMed] [Google Scholar]
- Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55: 2343–2351 [DOI] [PubMed] [Google Scholar]
- Sprangers K, Avramova V, Beemster GTS (2016) Kinematic analysis of cell division and expansion: quantifying the cellular basis of growth and sampling developmental zones in Zea mays leaves. J Vis Exp 118: e54887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton AE, Mori T, Walbot V (1993) A simple and sensitive antibody-based method to measure UV-induced DNA damage in Zea mays. Plant Mol Biol Rep 11: 230–236 [Google Scholar]
- Staxen I, Bornman JF (1994) A morphological and cytological study of Petunia hybrida exposed to UV-B radiation. Physiol Plant 91: 735–740 [Google Scholar]
- Tardieu F, Granier C (2000) Quantitative analysis of cell division in leaves: methods, developmental patterns and effects of environmental conditions. Plant Mol Biol 43: 555–567 [DOI] [PubMed] [Google Scholar]
- Tsukaya H. (2006) Mechanism of leaf-shape determination. Annu Rev Plant Biol 57: 477–496 [DOI] [PubMed] [Google Scholar]
- Vercruyssen L, Tognetti VB, Gonzalez N, Van Dingenen J, De Milde L, Bielach A, De Rycke R, Van Breusegem F, Inzé D (2015) GROWTH REGULATING FACTOR5 stimulates Arabidopsis chloroplast division, photosynthesis, and leaf longevity. Plant Physiol 167: 817–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargent JJ, Moore JP, Roland Ennos A, Paul ND (2009) Ultraviolet radiation as a limiting factor in leaf expansion and development. Photochem Photobiol 85: 279–286 [DOI] [PubMed] [Google Scholar]
- West G, Inzé D, Beemster GTS (2004) Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol 135: 1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.