Abstract
Global climate change is expected to affect waterborne enteric diseases, yet to date there has been no comprehensive, systematic review of the epidemiological literature examining the relationship between meteorological conditions and diarrheal diseases. We searched PubMed, Embase, Web of Science and the Cochrane Collection for studies describing the relationship between diarrheal diseases and four meteorological conditions that are expected to increase with climate change: ambient temperature, heavy rainfall, drought, and flooding. We synthesized key areas of agreement and evaluated the biological plausibility of these findings, drawing from a diverse, multidisciplinary evidence base. We identified 141 articles that met our inclusion criteria. Key areas of agreement include a positive association between ambient temperature and diarrheal diseases, with the exception of viral diarrhea; and an increase in diarrheal disease following heavy rainfall and flooding events. Insufficient evidence was available to evaluate the effects of drought on diarrhea. There is evidence to support the biological plausibility of these associations, but publication bias is an ongoing concern. Future research evaluating whether interventions, such as improved water and/or sanitation access, modify risk would further our understanding of the potential impacts of climate change on diarrheal diseases and aid in the prioritization of adaptation measures.
Keywords: Climate, Weather, Diarrhea, Climate Change, Ambient Temperature, Heavy Rainfall, Flooding, Drought, Social Vulnerability
Graphical Abstract

INTRODUCTION
Global climate change has the potential to severely impact human health worldwide, including diarrheal diseases,1 which are highly affected by environmental drivers such as water availability.2 The Intergovernmental Panel on Climate Change (IPCC) predicts that global surface temperature change for the end of the 21st century is likely to exceed 1.5°C relative to the period from 1850–1900, and in some scenarios is likely to exceed 2°C. Changes in the global water cycle also are expected, with increasing contrast in precipitation between wet and dry regions and between wet and dry seasons, although there may be regional exceptions.3
Because of the projected impacts of climate change on hydrological systems, waterborne enteric diseases are among the primary expected health impacts of climatic shifts.1, 4, 5 Understanding the impact of meteorological phenomena on enteric diseases is critical because even relatively small proportional increases in risk for diarrhea represent substantial overall impacts to the global burden of disease. Diarrheal diseases account for 10–12% of all deaths in children under five years old,6, 7 and an estimated 1.4–1.9 million deaths worldwide.7, 8 In addition to mortality, diarrheal disease can impair growth and cognitive development, and increase susceptibility to other infectious and chronic diseases,9 which may also exacerbate individual and community vulnerability to climate change.
Despite the importance of this topic, to date there has been no comprehensive, systematic review of the literature examining the relationship between climatic variables and patterns of diarrheal diseases. Other authors have reviewed associations between temperature and all-cause diarrhea,10, 11 meteorological variables and specific diarrheal pathogens,e.g., 12, 13–16 extreme weather events and waterborne diseases,17 and climatic influences on pathogens in the environment.18 However, these have been non-systematic reviews, focused on only one pathogen, and/or focused on specific geographical regions only. Thus, there is a need to review and synthesize the body of data that has accumulated on this topic, using rigorous methods following established guidelines,19–21 to better understand and interpret the epidemiological patterns reported in the literature, inform climate change adaptation and mitigation measures, and direct future research on the topic.
In this paper, we present the results of a systematic review of epidemiological associations between diarrhea and ambient temperature, heavy rainfall, flooding, and drought. We chose these meteorological conditions because of the strong evidence that these conditions are increasing due to climate change, and because of prior publications suggesting associations with diarrheal diseases. We summarize key areas of agreement, as well as gaps in the literature. In a related paper we carried out a meta-analysis of the subset of these studies specifically related to ambient temperature and diarrhea.22 Here we also evaluate the biological plausibility of each key finding, presenting a conceptual framework that describes mechanisms to explain the observed exposure-response relationships. We conclude by providing examples of how the insights from this conceptual framework can be applied, including in the evaluation of adaptation strategies.
METHODS
Systematic Search
Articles were identified through a comprehensive search of the literature and by reviewing references from 54 review articles identified through the search process. Briefly, we searched PubMed, Embase, Web of Science, and The Cochrane Collection on 26 November 2013 for the health outcome terms “diarrhea*” or “diarrhoea*”, paired with climate or meteorological terms: “climate change”, “temperature”, “rain*”, “precipitation”, “flood*”, “drought*,” and “sea surface temperature”.
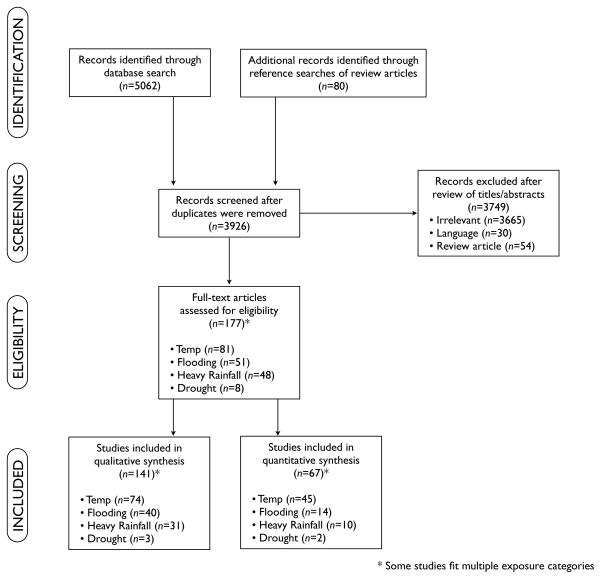
Two independent reviewers considered all articles for inclusion. Articles were deemed to meet inclusion criteria if they included: 1) human health outcome data; 2) all-cause diarrhea, pathogen-specific diarrhea, gastroenteritis, or diarrhea and vomiting as outcomes; and 3) temperature, heavy rainfall, flooding, or drought as exposures of interest related to rates of diarrheal diseases. Only English-language articles were included. Case reports were excluded. Meta-analyses and reviews were included if they provided a novel analysis of the data from the studies included. We decided a priori to focus on articles evaluating heavy rainfall rather than average rainfall because of previous work suggesting that this is the primary rainfall exposure influencing rates of diarrheal diseases.23–25 While our search originally included sea surface temperature, we decided to exclude this category because all 16 articles identified focused on cholera, limiting the generalizability of such findings to other diarrheal diseases. Duplicate reports were handled by selecting the study with the most complete dataset and analysis. Following initial article review, the two reviewers discussed and resolved differences in inclusion decisions, consulting with the other study authors as necessary. Figure 1 shows the PRISMA diagram19 of the selection process. For each article that met our inclusion criteria, one reviewer extracted relevant data (including exposure, outcome, location of study, study duration, study population) and at least one other person verified the extracted information.
Figure 1.
PRISMA diagram of the study selection process.
Evaluating the quality of the evidence
We used three approaches to assess the quality of the evidence: 1) evaluating the weight of evidence, including study design and strength and consistency of observed relationships; 2) evaluating risk of bias; and 3) exploring biological plausibility of the relationships.
Weight of Evidence
Studies were classified as quantitative epidemiological studies, non-quantitative epidemiological studies or outbreak reports. A study was classified as quantitative if the authors presented the results of a statistical analysis. To account for potential publication bias, we did not require studies to present actual point estimates, allowing us to include studies that reported whether or not significant associations were detected by the particular analytical method applied. When studies presented the results of multiple analyses, we followed the Cochrane guidelines20 and selected the analysis that the authors indicated as the final or main analysis (typically a fully adjusted model), and did not include supplementary exploratory studies. However, when analyses were stratified by exposure, location, or another risk factor, and a single unified estimate was not presented, we included each of the stratified analyses in our summary. We limited temperature studies to those that examined associations between temperature and diarrheal diseases at a one-month or finer resolution.
Quantitative studies were classified as showing positive or negative associations if the analysis allowed rejection of the null hypothesis (p<0.05 or 95% confidence interval did not include the null). Studies were classified as showing a neutral association if the null hypothesis could not be rejected. Outbreak reports were not eligible to be classified as quantitative studies because these studies generally report on single events with a noted association between a meteorological event and diarrheal disease, and therefore are subject to publication bias. Meta-analysis was conducted on a subset of studies of temperature and diarrhea that met more rigorous reporting and design criteria; this analysis has been described elsewhere.22 For studies of heavy rainfall, flooding and drought meta-analysis was not appropriate, according to the Cochrane Collaboration Handbook, because of the disparate analytical methods and exposure definitions applied in these studies.20 The approach used here allows us to present a semi-quantitative summary of the studies we identified, in order to allow inclusion of the widest range of studies and to avoid selection bias that would result from restricting studies to those with a single exposure and outcome definition.
Following the recommendations of others,21 we evaluated the strength and consistency of reported relationships between each exposure of interest and diarrheal diseases within each study type and, subsequently, between study types. Quantitative epidemiological studies were considered stronger evidence sources than non-quantitative epidemiological studies and outbreak reports. Evidence of a dose-response relationship, consistency across study types and dissimilar populations, and large effect sizes were considered factors that increased confidence in the observed associations. Evidence of bias, imprecision in effect estimates and unexplained inconsistencies in the observed associations were considered factors that decreased confidence. We classified the body of evidence for each association as providing high, moderate, low or very low confidence to draw conclusions, adapting previously outlined categories.21, 26
Risk of bias
Publication bias was a primary concern, particularly because the evidence consisted of observational epidemiological studies and outbreak reports. Because we did not perform meta-analysis (for the reasons described above), standard methods to assess publication bias such as assessment of funnel plot asymmetry were not appropriate. Instead, we compared the findings of quantitative epidemiological studies that presented multiple analyses to those that presented a single estimate. We hypothesized that if there were a tendency to publish “significant” (or non-null) findings, we would be more likely to find non-significant findings in manuscripts that presented multiple estimates (since one “significant” finding among several analyses may be seen as sufficient for publication). We also summarized the location of studies, to examine whether some areas were over- or under-represented in the literature.
Biological plausibility
To evaluate the plausibility of associations reported in the epidemiological articles included in the review, we took advantage of the detail provided in these articles and also drew on other evidence from the literature to develop a conceptual diagram that details potential causal pathways between meteorological conditions and diarrheal disease outcomes.
RESULTS
Weight of Evidence
We identified a total of 141 articles that met our inclusion criteria. Details of each of these studies are provided by meteorological exposure in the Supplemental Material (Tables S1–4). In Table 1 we summarize the evidence of key findings on the relationship between diarrhea and each of our exposures of interest, and in Table S5 we summarize the results reported in the 67 articles that provided a statistical analysis of the association between meteorological exposures and diarrhea. The majority of studies focusing on temperature, heavy rainfall and flooding reported a significant positive association between the exposure variable and diarrhea. Below we review epidemiological patterns observed for each of the exposure categories.
Table 1. Summary of evidence of key findings regarding the relationship between diarrhea and ambient temperature, heavy rainfall, flooding, and drought.
Confidence categories selected based on guidelines presented by Rooney et al. (2014) and Guyatt et al. (2008). High confidence: The true effect is likely to be reflected in the observed relationship. Additional research is very unlikely to change our confidence in the relationship; Moderate confidence: The true effect may be reflected in the observed relationship. Additional research may change the estimated association; Low confidence: The true effect may be different from the observed association. Additional research is likely to have an important impact on the estimated association; Very low confidence: The true effect is highly likely to be different from the observed association. Additional research is very likely to have an important impact on the estimated association.
| Key findings | Weight of evidence | Notes on the quality of evidence | Confidence |
|---|---|---|---|
| AMBIENT TEMPERATURE | |||
| Higher temperatures are associated with elevated rates of diarrhea |
|
Range of exposure definitions, timescales of analyses, and analytical approaches | Moderate/High* |
| Associations between temperature and diarrhea vary by taxonomic category of diarrheal disease agent | Of the quantitative associations reported:
|
More evidence for bacteria and viral pathogens than for protozoan pathogens. Over-representation of certain geographic regions. |
High |
| HEAVY RAINFALL | |||
| Heavy rainfall events are associated with elevated rates of diarrhea |
|
All evidence for all-cause diarrhea or bacterial diarrhea pathogens; no studies on protozoan or viral pathogens. Wide variety of exposure and outcome definitions, data sources, and analytical approaches Implicit publication bias for outbreak reports |
Moderate |
| Heavy rainfall following dry periods are associated with elevated rates of diarrhea |
|
Inconsistent definitions of dry periods | Moderate |
| FLOODING | |||
| Flooding is associated with elevated rates of diarrhea |
|
Most quantitative evidence is for allcause diarrhea; very few analyses of specific pathogens. Implicit publication bias for outbreak reports |
Moderate |
| DROUGHT | |||
| Drought is associated with an increase in diarrheal diseases |
|
Inadequate evidence base. Difficulties defining drought due to its prolonged nature |
Very low |
Confidence in the overall positive relationship of all-cause diarrhea and ambient temperature is considered moderate due to the differential relationships observed by taxonomic category of diarrheal disease agent. However, confidence is high for the positive relationship between bacterial diarrhea and ambient temperature.
Temperature
We identified 74 articles that examined the relationship between ambient temperature and diarrheal diseases (Table S112–16, 27–95), including 45 articles with a total of 82 statistical analyses. These articles included data collected between 1950–2009. Authors used a range of exposure definitions including average, maximum, and minimum temperatures over daily, weekly, biweekly, and monthly resolutions, and considered a variety of different temporal lags between exposure and outcome. These studies were conducted in a variety of settings. Despite this heterogeneity, there was consistency in the observed relationships. In most of the quantitative analyses (65%), there was a significant positive association between temperature and diarrhea, including 69% of analyses of all-cause diarrhea and 79% of analyses of bacterial pathogens. However, in most analyses of viral diarrheal pathogens (71%), primarily rotavirus, there was a negative association between ambient temperature and diarrhea. Only five analyses in three articles considered protozoan diarrheal pathogens; three of these analyses (60%) described a positive association with temperature.
Several studies examined the impact of temperature shifts associated with El Niño events on diarrhea, allowing examination of temperature-diarrhea relationships outside the range of normal temperature variation for a particular location. Ambient temperature increases during El Niño events in Peru were associated with increased diarrhea among children in a cohort study31 and increased pediatric diarrhea admissions in children.79, 96
Heavy Rainfall
We identified 31 articles describing the relationship between heavy rainfall and diarrhea that met our inclusion criteria, including 10 articles with a total of 14 statistical analyses, and 18 studies reporting on an outbreak following a heavy rainfall event (Tables S2a23–25, 39, 43, 46, 50, 81, 97–101 and S2b102–123). These articles included data collected between 1910–2009. Articles used a wide variety of definitions of heavy rainfall, outcome definitions, data sources, and analytical approaches, which makes it difficult to compare results across studies. While there is heterogeneity in observed effects, some general trends are apparent. Of the articles that met our quantitative inclusion criteria, a significant positive association between heavy rainfall and diarrhea was noted in most (71%) of these (Table 1, Table S5). None of the quantitative studies evaluated viral or protozoan diarrhea.
Several long-term analyses of nationwide datasets reported significant associations between heavy rainfall and outbreaks of waterborne diseases in the USA,24, 124 Canada,25 and the UK,100 and between heavy rainfall and hospital admissions for diarrhea in Taiwan.39 More location-specific studies of associations between heavy rainfall and diarrhea have found mixed results. In some cases, positive associations have been observed, such as higher observed diarrhea rates during peak monsoon rainfall months in the Philippines97 and Bangladesh,43 and increased diarrhea with increases in maximum rainfall in Ghana.81 Two studies (in Bangladesh46 and the USA98) observed no association between heavy rainfall and diarrhea, although the US study found that any rainfall four days prior was significantly associated with an 11% increase in acute gastrointestinal illness (AGI) visits. A common theme among several studies, from a range of settings (the Philippines,97 Ecuador,23 Swaziland,109 UK,100, 120, 122 USA,121 and Japan123), found that heavy rainfall after a dry period was associated with increased diarrhea. The study in Ecuador also found the converse, i.e., that heavy rainfall following a wet period was protective against diarrhea.
Our search also resulted in several studies reporting on outbreak investigations, ranging in size from just a few cases up to hundreds of thousands, again from a range of settings (Turkey,102 UK,104, 106, 120 Canada,105, 107, 117 USA,110, 111, 113, 114, 121 Japan,123 Swaziland109), citing heavy rainfall immediately preceding the outbreak as a presumptive cause of contamination of water supplies.
Flooding
We identified 40 articles on the relationship between flooding and diarrhea that met our inclusion criteria, including 14 articles with a total of 25 statistical analyses, and 15 studies that reported on an outbreak following a flood (Table S3a58, 125–148 and S3b149–162). These articles included data collected between 1985–2011. Of the articles that met our quantitative inclusion criteria, a significant positive association was noted in most (76%) of these (Table 1, Table S5). Most of the quantitative analyses evaluated all-cause diarrhea, thus it is difficult to generalize quantitative trends by taxa.
In the non-outbreak studies with a comparison group (Table S3a), several authors report higher diarrhea rates during flood periods versus pre-flood or non-flood period in the same year 125, 132, 138, 146, 148 or versus comparable time periods in non-flood years.10, 127, 129, 130, 132, 133, 135, 141, 144, 148, 163 Many studies also report higher diarrhea rates in flood-affected compared to unaffected groups,58, 138, 139, 142, 145, 147 although two studies did not detect such a difference.134, 137 Some studies report exposure-response effects, with increasing contact with flood waters140, 146 or flood depth139 associated with increased risk of diarrhea. In reviews of large national databases, positive associations were noted between flood days and diarrhea incidence in China,131 and in Finland the most common causes of waterborne disease outbreaks were floods and surface runoff.136 Several articles report changes in the percentage of diarrhea cases attributable to particular pathogens during or after floods, including increased detection of pathogenic E. coli,126, 141 and V. cholera;132, 141 and both increased141 and decreased132 detection of rotavirus.
Table S3b details articles that reported on outbreaks of diarrheal diseases following floods, or rates of clinical diarrhea following flooding events. In these studies, flood-related outbreaks were reported for cholera159 or acute watery diarrhea,161, 164 enterotoxigenic E. coli (ETEC),159 rotavirus,149, 152 and norovirus.153, 160,156
Drought
Our search identified only three articles examining the relationship between drought and diarrheal diseases, only two of which met our quantitative inclusion criteria (Table 1; Table S4109, 165, 166). These articles included data collected between 1978–2002.
Burr et al.165 found a dose-response relationship between drought-related water restrictions (hours per day without access to municipal water) and rates of childhood diarrhea and vomiting in Wales. Effler et al.109 document the first reported outbreak of E. coli O157 in Swaziland and South Africa, noting that the outbreak was preceded by heavy rainfall following three months of drought. The authors suggest that stress on livestock during the drought and heavy livestock use of traditionally exclusively human water sources, concentrated pathogens, facilitated human exposure, and allowed pathogens to be distributed broadly when the first heavy rains arrived. De Sherbinin166 found no significant correlation between diarrhea and drought in Africa, defining drought as precipitation <75% of the median for the region occurring for at least three months between 1980–2000.
Risk of bias
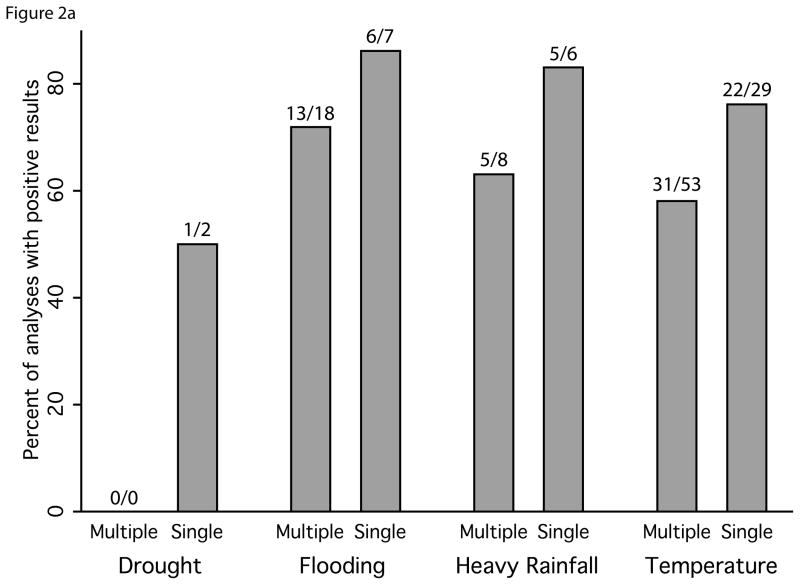
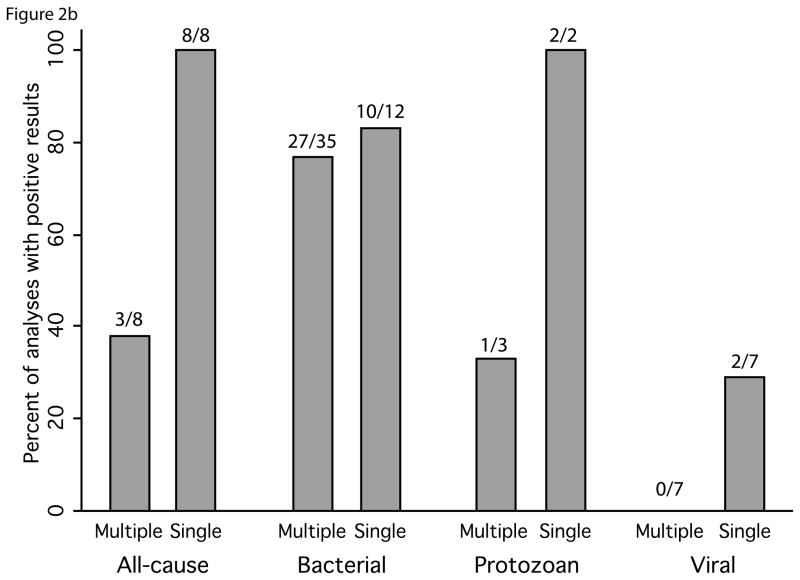
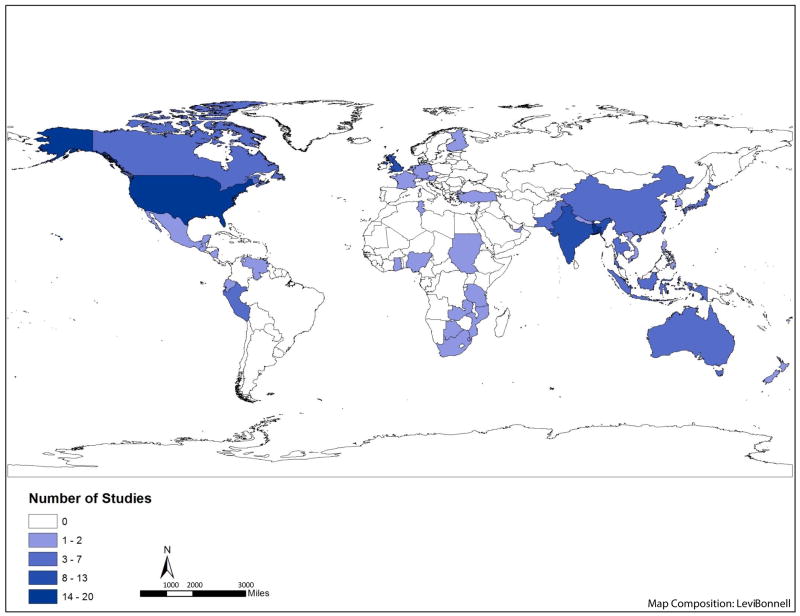
We observed evidence of publication bias in our review. Studies that reported multiple estimates were approximately twice as likely to include results showing no effect or an effect in the direction contrary to the prevailing literature compared to manuscripts reporting a single analysis (16% and 32% of estimates from studies with single and multiple estimates, respectively) (Figure 2). We also observed geographic over-representation from certain areas: while studies were identified from all regions of the world (Figure 3), of the 141 studies we reviewed, 45% were from four countries: Bangladesh (20), United States (17), the United Kingdom (13) and India (13). Additionally, smaller outbreaks were more commonly reported upon in developed countries.
Figure 2.
Comparison of the associations between diarrhea and exposures of interest presented in manuscripts with a single quantitative analysis to manuscripts with multiple analyses. Percentage of positive results shown for a) drought, flooding, heavy rainfall and temperature; and b) for subsets of temperature papers by type of pathogen reported on in the study. Number of analyses with reported significant positive associations over the total number of analyses is shown above each bar.
Figure 3.
Map showing all studies included in the systematic review by country. The 8 studies that included 6 or more countries were excluded from the map. This includes 3 global studies, 2 European studies, 2 African studies and 1 South Asian study. World shapefile is from the GADM database of Global Administrative Area.
Biological plausibility
Examination of biological plausibility of the observed relationships is discussed below.
DISCUSSION
Weight of Evidence
We observed a predominant trend for positive relationships between diarrhea and meteorological conditions that are expected to increase with climate change, with the exception of the relationship between ambient temperature and viral diarrhea. Confidence in these relationships ranged from very low (for the relationship between drought and all-cause diarrhea) to high (for the relationship between ambient temperature and bacterial diarrhea). Our systematic review uncovered the most articles focusing on the effect of temperature on diarrhea, perhaps because temperature can be measured continuously, whereas heavy rainfall, flooding, and drought are episodic and less predictable events. Definitions for heavy rainfall, flooding, and drought are often much less concrete than those of temperature, creating a less unified literature for those exposures, particularly where sophisticated modeling is conducted. Most of the studies of temperature effects were time-series analyses comparing long-term temperature datasets with diarrhea surveillance or hospital records datasets. For both heavy rainfall and flooding, studies could be divided into those that provided a systematically collected dataset with a robust comparison group versus those that reported on outbreaks. While we did not include the outbreak studies in our quantitative analysis because they are inherently subject to publication bias (only outbreaks with a noted association with meteorological conditions are captured), they still provide useful information, so we included them in the qualitative review.
The epidemiological literature on drought and diarrhea was particularly sparse. This may reflect difficulties in precisely defining drought, the timescale over which droughts occur, and outcomes reported for drought events (i.e., famine or malnutrition instead of diarrhea). Stanke et al. highlight the inherent difficulties in documenting the health effects of drought,167 due to lack of standardized definitions,168 complexity of determining when droughts begin and end, the accumulation and persistence of effects over time, and the indirect effects on health related to mediating circumstances such as loss of livelihoods.. In addition to extensive periods of extreme drought, changes in the duration and/or extent of seasonal dry conditions may also affect waterborne diseases, but our search was not designed to capture these effects.
Risk of Bias
We observed evidence of reporting bias in our review. When we restricted our summary of the quantitative studies to those with a single estimate, the proportion of studies finding a positive relationship between temperature, heavy rainfall, and flooding all increased (Figure 2). This suggests that there may be considerable reporting bias – studies that reported multiple estimates were more likely to include results showing no effect or an effect in the direction contrary to the prevailing literature. We also found evidence of over-representation of certain geographic regions in the studies reviewed: 45% of the studies were from four countries and smaller outbreaks were more commonly reported upon in developed countries. The geographic representation of studies may be of particular concern for flooding, heavy rainfall, and drought because infrequent events may bias the geography further toward places where robust surveillance programs are already in place. Our decision to include only English language articles might have contributed to the lack of geographic representation of studies.
Biological Plausibility
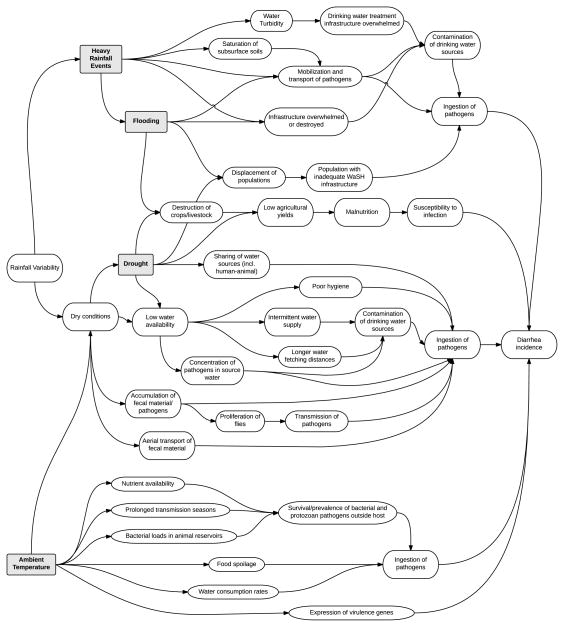
In order to evaluate the plausibility of associations reported in the epidemiological articles included in the review, we developed a conceptual diagram illustrating potential causal pathways between meteorological conditions and diarrheal disease outcomes (Figure 4). This framework takes advantage of the rich detail provided in the articles included in the review and draws on other evidence from the literature, to explore potential biophysical and behavioral explanatory mechanisms for these relationships. Note that the figure focuses on the mechanisms leading to positive relationships, as a way to illustrate the overall trends observed in this review. In some cases the relationship will be negative, for example as often is observed for ambient temperature and viral diarrhea; this is not captured in the figure but is discussed in the text below. Below we describe plausible mechanisms associated with the observed patterns for each exposure category.
Figure 4.
Conceptual diagram illustrating mechanisms by which meteorological conditions expected to be impacted by climate change could potentially increase the risk of diarrheal diseases. All arrows lead to increases in the items listed. Note that the diagram does not show possible mechanisms for how the same factors could potentially decrease the risk of diarrheal diseases.
Temperature
The patterns observed in the relationship between temperature and diarrheal diseases highlight the importance of distinguishing between taxonomic categories of pathogens when considering the mechanisms involved in this relationship. Microorganisms differ in their rates of environmental survival and reproduction outside of the host, because of differential sensitivity to changes in heat, moisture, oxygen, light and nutrients.169–172
The details of environmental conditions differentially affecting bacteria, protozoa, and virus survival outside of the host may explain the contrasting patterns observed for these different types of pathogens. Protozoans form spores, and even E. coli experience biphasic lifestyles, with host-independent and host-associated phases.173 In terms of pathogen survival, increased die-off occurs with increasing temperatures outside of the host for bacteria,170, 173 protozoa,174, 175 and viruses.176 However, in groundwater, the evidence that temperature affects pathogen survival is more consistent for viruses than for bacteria.176 Temperature-based inactivation may be more complex for bacteria. Inactivation rates of fecal coliform bacteria, which may replicate in aquatic environments at higher temperatures given adequate nutrient levels,177 may be affected by a combination of die-off, reproduction, predation by larger organisms, competition for nutrients, bacterial antagonism, enzymatic or chemical inactivation, and phage lysis.176 Increased yields of bacteria from drinking water delivery systems have been recorded during warmer, summer months.178 In addition, increased temperatures may increase bacterial and protozoan pathogen loads in animal reservoirs, and may prolong transmission seasons.169
Temperature may also affect expression of virulence genes in bacterial pathogens. For bacterial pathogens of mammals, elevated temperature can signal successful infection of a host, and lead to expression of bacterial virulence genes that continue as long as the bacteria remain at the elevated temperature.179, 180 Growth temperature is considered the most important environmental stimulus for the activation of virulence gene expression in Shigella spp., which have been shown to be induced at 37 °C and repressed at 30 °C.180
Host factors directly or indirectly influenced by meteorological conditions may also explain the observed epidemiological patterns. For example, Pitzer et al.181 suggest that peaks in viruses in cooler periods may be most strongly influenced by demographics and human behavioral factors. Interestingly, some articles noted seasonal differences in observed relationships,30, 64, 96, 182 which supports the idea that seasonal factors not directly related to environmental conditions influence the observed patterns. Higher temperatures may also increase the probability of food spoilage,40, 63, 183 and concurrent temperature-related changes in food storage and consumption practices may affect disease risk. Water consumption rates may also increase during warmer times of the year, increasing probability of pathogen ingestion in regions with poor quality drinking water.
Heavy Rainfall & Flooding
While ambient temperature may impact pathogen survival and host behavior, both heavy rainfall and flooding can directly affect transport of pathogens, and can affect the existing water and sanitation infrastructure, altering human exposure patterns. Severe flooding can also lead to population displacement, with a variety of resulting health impacts.184, 185
Transport of pathogens as a result of heavy rainfall occurs in multiple ways. If pathogens from human and/or animal excreta are present in soils and on environmental surfaces or subsurfaces, rainfall can mobilize these pathogens and transport them into surface waters or to other areas of a village, exposing individuals to pathogens. It is well documented that water in general and floodwaters specifically can spread pathogens within watersheds.186, 187 Heavy rainfall can also lead to resuspension of pathogens in sediment,188 or mobilization of pathogens in soils.189 Heavy rainfall events can cause contamination of groundwater,e.g., 112 and can lead to saturation of the subsurface,105, 110 which facilitates water transport of pathogens into surface or groundwater.
Several articles document increases in diarrhea or outbreaks following dry periods, suggesting an interaction between heavy rainfall events and the accumulation of pathogens in the environment.23, 97, 100, 109, 120, 122, 123 This is consistent with the results of Carlton et al.,23 who found that heavy rainfall was associated with increased risk of diarrhea following dry periods but decreased risk following wet periods. Potential time-dependent mechanisms explaining these interactions have been described by Levy et al.14 and Moors et al.190 The “concentration effect” occurs when dry conditions allow for the accumulation of microorganisms in the environment. Precipitation can cause a short-term “runoff effect,” via flushing of pathogens into surface water, resuspension of pathogens in sediment, and sewage overflows. When this occurs over longer time periods, the “dilution effect” may act, with higher flows diluting microbial concentrations. The dilution effect may be especially salient in the case of flooding, where heavy rainfall may initially distribute accumulated contaminants but then dilute contaminants after the initial flushing has occurred.
Evidence of dose-response effect related to explicit contact with floodwaters was noted in several studies, including flooding explicitly in the subject’s house or yard,146 increasing association with increasing flood depth,139 explicit skin contact with flood water,140 and direct contact with floodwaters.160
Many articles discuss animal sources of contamination.102, 113, 115, 121, 122 Factors affecting pathogen transport in runoff, such as slope, vegetation, flow-rate, infiltration rate, and rainfall intensity have been studied in agricultural settings.18 Heavy rainfall can promote manure-borne oocyst transport of protozoan pathogens,18 and may lead to splashing of manure-amended soils onto fresh produce.191 Exposure to zoonotic pathogens can also cause illness through recreational exposure.115
An examination of relative rates of specific pathogens in flood versus non-flood periods can provide insight into which pathogens are associated with flooding. Several such analyses have been carried out in Bangladesh, with studies finding variable results: both cholera and ETEC associated with floods;159 an initial peak in the incidence of rotavirus coinciding with flooding followed by a decline immediately after the flood receded;152 increases in the proportion of rotavirus diarrhea, rotavirus in older children, percentage of mixed rotavirus infection cases, and an abrupt change in epidemic strains coinciding with the spread of floods;149 cholera playing a primary role in flood-related diarrhea epidemics, but rotavirus and ETEC also contributing to the epidemics;141 cholera as the most common cause of diarrhea during flood years, with rotavirus more common in non-flood years.132
Heavy rainfall and flooding can also affect pathogen transmission via impacts on sanitation and/or drinking water treatment infrastructure. Several articles document or discuss heavy rainfall causing contamination of drinking water sources102, 104–107, 110, 112–114, 116, 117, 122, 123, 151 or food products.108 Floods can overwhelm water systems, causing backflows that lead to contamination of groundwater and other drinking water sources.e.g., 156 Furthermore, several studies reported on dose-response relationships between consumption of municipal drinking water after a heavy rainfall and gastrointestinal illness.103, 104, 107, 117, 121 Resuspension of sediments by heavy rainfall events can lead to high turbidity levels in source waters, which can overwhelm drinking water treatment infrastructure.104, 111, 116, 192 High turbidity levels in source waters have been associated with elevated rates of gastrointestinal illness in the United States and elsewhere.193–197
Many human factors can modify the associations between diarrheal diseases and heavy rainfall or flooding. For example, rainfall effects on diarrhea can vary by local practices such as fecal sludge application in agricultural communities.81
Beyond the effects of heavy rainfall, floods can lead to broader effects. Floods may destroy crops and kill livestock, which may lead to longer term effects associated with malnutrition.9 Flooding can also displace populations to temporary or permanent communities with inadequate infrastructure. For example, there were several reports of outbreaks of diarrheal diseases amongst Hurricane Katrina evacuees,153, 154 and this was cited as an explanation for the elevated diarrhea and cholera rates observed in Bangladesh after a severe flood in 1998.133
Drought
Despite the paucity of epidemiological data available, there are many plausible mechanisms for an association between dry conditions and/or drought and diarrheal diseases. Biophysical effects include the accumulation and concentration of fecal contamination in water sources under dry conditions,190, 198, 199 which may increase the probability of human contact with pathogens. As mentioned above, dry conditions may lead to a build-up of a fecal film on surfaces – causing a pulse of pathogens when rains occur, potentially increasing aerial transport of fecal material due to soil drying,190 and also potentially leading to increases in fly abundance.182 On the other hand, droughts are typically periods of immobilization and inactivation of microorganisms of fecal origin, due to lack of hydrologic connection between surface soils and subsurface aquifers, as well as inactivation in the water column due to higher temperatures and irradiation.188
Effects may also be associated with water supply conditions, such as the dose-response association between water restrictions and rates of childhood diarrhea observed by Burr et al.165 Drought conditions may cause intermittent water supply, which can lead to intrusion into drinking water distribution system pipes.200, 201 Decreases in rainfall may increase the fraction of wastewater in surface water,190 which can lead to consumption of lower quality water due to increases in the concentration of pathogens in both drinking and irrigation water sources. Dry conditions can also increase sharing of water sources between human and animal populations, as observed in Swaziland.109 However, during dry seasons or under drought conditions, people may switch to less contaminated water sources such as bottled or well water. Pinfold et al. document shifts in drinking water sources in the rainy versus dry seasons in Thailand.76 Water fetching distances, which have been associated with increased water contamination, diarrhea and impaired growth,202, 203 may increase with decreasing water availability. Hygiene can also be impaired by decreased water availability.190
Indirect effects of drought include malnutrition, associated with increased susceptibility to diarrheal diseases, due to negative effects of drought on agricultural crops, livestock, and fisheries. A review on the health impacts of drought cite malnutrition and its associated implications for morbidity and mortality as “the most obvious and best recognized health impact of drought.”167 Displacement of populations may also occur as a consequence of prolonged drought conditions, which may lead to an increase in the population with inadequate water, sanitation, and hygiene (WaSH) conditions.
Implications for population health in a changing climate
It is evident from our review of the literature that both biophysical and social phenomena determine the impact of meteorological conditions on diarrheal diseases. The extent to which a population experiences health impacts from climatic conditions depends not only upon the severity of the meteorological exposure, but also on factors such as water and sanitation infrastructure, healthcare access, and available resources with which to intervene to prevent increased disease burden. The IPCC recognizes this, stating that “vulnerability encompasses a variety of concepts and elements including sensitivity or susceptibility to harm and lack of capacity to cope and adapt.”204
Still, our review reveals that even in developed countries that have more extensive water and sanitation infrastructure, elevated temperature, heavy rainfall, and flooding can impact risk of diarrheal diseases. In the United States, the largest documented waterborne disease outbreak was associated with water treatment plant failure during a spring runoff event,116 and significant associations between heavy rainfall and outbreaks of waterborne diseases have been documented.24 A recent review of extreme water-related weather events and waterborne disease outbreaks found that in both developing and developed countries, the most common cause of outbreaks was contamination of the water source through heavy rainfall (55.2% of studies) and flooding (52.9% of studies). The authors of this review note that while in developing countries this was usually associated with untreated water, in developed countries, in the majority of cases, this was associated with contamination of a treated water source.17
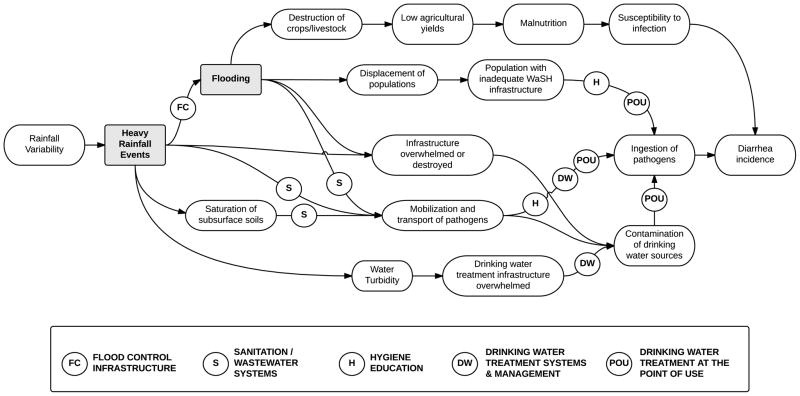
Understanding the mechanisms involved in exposure – disease response relationships, as illustrated in Figure 4, can provide insights into where to intervene to prevent transmission of waterborne disease agents, under both present day conditions and future climate scenarios. In Figure 5 we illustrate this for the example of heavy rainfall and flooding. The effects of these events may be minimized through prevention of contamination of water systems, by controlling floods from occurring (“Flood Control Infrastructure”), or by reducing the presence of pathogens in the environment available for transport through adequate disposal of fecal material (“Sanitation/Wastewater Systems”). Should contamination of the environment and/or drinking water sources occur, prevention of human ingestion of pathogens may occur through efforts to improve hygiene (“Hygiene Education”) and/or through household drinking water treatment (“Drinking Water Treatment at the Point of Use”), as well as provision of drinking water treatment systems and adaptive management of those systems in order to prevent drawing from turbid water sources (“Drinking Water Treatment Systems & Management”).
Figure 5.
Conceptual diagram for heavy rainfall and flooding illustrating how interventions along the causal pathway may interrupt transmission of diarrheal pathogens.
Limitations
Different types of data and approaches to analysis limited our ability to combine quantitative estimates across the different studies included in the review. In a separate manuscript, we compiled a meta-analysis for studies considering temperature as an exposure. In that manuscript, we address issues such as timescale of analysis, treatment of lags, different exposure definitions (e.g., mean vs. maximum vs. minimum temperature), and stratify by pathogen.22 In the present analysis, we made an a priori decision to focus on articles reporting on the effects of heavy rainfall, so we did not capture studies reporting on the effects of rainfall defined in other ways, such as average precipitation. Similarly, we decided not to include sea surface temperature as a primary exposure of interest because this literature was exclusively focused on cholera, primarily in Bangladesh; this removed some sophisticated analyses from consideration.99, 205–208 We limited our review to articles published in English only, which narrows the scope of this review, and may have decreased our ability to collect the broadest possible sample of reports on these exposures. However, only 29 of 3,952 articles captured in our initial search (which was not restricted by language) were rejected on the basis of language. Many of these reports were considered irrelevant because review of titles and abstracts determined that they did not explore the relationship between diarrhea and the meteorological exposures.
Future research needs
Our assessment of the mechanisms underlying the relationships between meteorological conditions and diarrheal diseases reveals complex causal pathways. While studies evaluating simple exposure-disease associations are a necessary first step, epidemiological studies in this area should move towards more sophisticated analyses that evaluate the proposed causal pathways and the potential impacts of interventions to reduce risk. Specifically, we identify three priority research areas. First, there is a need to evaluate the ways in which factors known to affect diarrhea risk such as demographics and water and sanitation infrastructure modify relationships between meteorological exposures and diarrhea incidence. The social factors that determine a community’s ability to cope with and respond to climate variability must be made explicit in any study of climate and health. This is especially the case for waterborne diseases, where infrastructure is so clearly critical to reduction in disease burden.209 In addition, it may be important to evaluate the combined effects of climate change and land use change on diarrheal disease.210 Studies that evaluate changes in climate-disease relationships by modifiable risk factors (such as access to safe drinking water or vaccination coverage), will improve our ability to identify vulnerable populations and opportunities to intervene to prevent negative consequences.
Second, there is a need to evaluate differences in the relationship between meteorological variables and diarrheal diseases by causative pathogen. Our findings here and elsewhere14, 22, 211 suggest that the relationship between temperature and diarrheal diseases varies by pathogen. We suspect that the relationships between diarrhea and heavy rainfall, flooding, and drought may also depend on the causative pathogen, as exposure pathways and environmental survival can vary considerably by pathogen. However, few studies examined the relationships between heavy rainfall, flooding, or drought and pathogen-specific diarrhea. Researchers should make attempts to go beyond examinations of all-cause diarrhea, and evaluate specific pathogens whenever possible. Classifying diarrhea by causative pathogen is more resource intensive than measuring all-cause diarrhea, however prior studies have demonstrated this is possible even in remote and/or low-resource settings.e.g., 212–214 Taxa- or even pathogen-specific estimates of the relationship between meterological conditions and diarrheal agents would help guide public health practice with respect to climate change adaptation efforts and vaccine development prioritization.
Third, there is a need to evaluate the concurrent impacts of multiple meteorological exposures. A common theme that emerged from this review is that the effects of heavy rainfall on diarrhea were magnified after dry periods, suggesting that models should incorporate antecedent conditions. Analyses that incorporate combined effects and/or interactions between temperature and rainfall are also needed. To address these research needs, non-linear models such as threshold models may be appropriate in these cases, as may mathematical models that can evaluate mechanistic assumptions against sparse observed data.
CONCLUSION
We observed a predominant trend for positive relationships between diarrhea and temperature, heavy rainfall, flooding, and drought, meteorological conditions that are expected to increase with climate change. The exception to this trend was the relationship between ambient temperature and viral diarrhea, which was primarily negative. These trends occurred in both developing and developed countries. Our review also uncovered synergistic relationships between drought and heavy rainfall in promoting diarrheal diseases.. Epidemiological studies were limited for drought, and few studies of pathogen-specific diarrhea were observed for heavy rainfall, flooding, or drought, thereby limiting conclusions.
This review and conceptual framework underscore the importance of considering basic approaches to improving public health, including civil and environmental engineering and behavior change efforts, in climate change adaptation efforts. In 2012, an estimated 842 000 diarrhea deaths were caused by inadequate water, sanitation and hygiene in low- and middle-income settings.215 While diarrheal disease burden has been declining globally, climate change has the potential to slow progress in reducing the burden of diarrheal diseases, particularly disease burden linked to unsafe WaSH conditions.216 Future research to evaluate the ability of such measures to reduce the deleterious health impacts of climate change is needed. However, given the high current baseline rates of these diseases and the potential for increases in diarrheal disease risk under future climate conditions, investing in the prevention of diarrheal diseases through improvements in WaSH systems and other proven diarrhea prevention strategies should be a global priority.
Supplementary Material
Table S1: Articles included in the systematic review of the relationship between ambient temperature and diarrheal diseases.
Table S2: Articles included in the systematic review of the relationship between heavy rainfall and diarrheal diseases; a) Systematically collected datasets, b) Outbreak reports.
Table S3: Articles included in the systematic review of the relationship between flooding and diarrheal diseases; a) Studies with an explicitly defined comparison group, b) Outbreak reports.
Table S4: Articles included in the systematic review of the relationship between drought and diarrheal diseases
Table S5: Summary of quantitative associations between diarrhea and drought, flooding, heavy rainfall and temperature reported in 67 articles with quantitative estimates.
Acknowledgments
FUNDING
This work was supported by grants from the National Institutes of Health [Fogarty International Center grant# R21TW009032; and National Institute of Allergy and Infectious Diseases grant # K01AI103544, both to K.L.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
LITERATURE CITED
- 1.Smith KR, Woodward A, Campbell-Lendrum D, Chadee DD, Honda Y, Liu Q, Olwoch JM, Revich B, Sauerborn R. Human health: impacts, adaptation, and co-benefits. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL, editors. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge and New York: 2014. pp. 709–754. [Google Scholar]
- 2.Eisenberg JN, Desai MA, Levy K, Bates SJ, Liang S, Naumoff K, Scott JC. Environmental determinants of infectious disease: a framework for tracking causal links and guiding public health research. Environ Health Perspect. 2007;115(8):1216–23. doi: 10.1289/ehp.9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IPCC. Intergovernmental Panel on Climate Change. Switzerland: 2013. Climate Change 2013. The Physical Science Basis. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Summary for Policymakers. [Google Scholar]
- 4.Patz JA, Frumkin H, Holloway T, Vimont DJ, Haines A. Climate change: challenges and opportunities for global health. JAMA. 2014;312(15):1565–80. doi: 10.1001/jama.2014.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luber G, Knowlton K, Balbus J, Frumkin H, Hayden M, Hess J, McGeehin M, Sheats N, Backer L, Beard CB, Ebi KL, Maibach E, Ostfeld RS, Wiedinmyer C, Zielinski-Gutiérrez E, Ziska L. Chapter 9: Human Health. In: Melillo JM, Richmond TC, Yohe GW, editors. Climate Change Impacts in the United States: The Third National Climate Assessment. U.S. Global Change Research Program; Washington, D.C: 2014. [Google Scholar]
- 6.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Number of deaths. WORLD by cause. http://apps.who.int/gho/data/node.main.CODWORLD?lang=en 11/26/13.
- 9.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut--a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10(4):220–9. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell-Lendrum D, Woodruff R. Comparative risk assessment of the burden of disease from climate change. Environmental Health Perspectives. 2006;114(12):1935–1941. doi: 10.1289/ehp.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolstad EW, Johansson KA. Uncertainties Associated with Quantifying Climate Change Impacts on Human Health: A Case Study for Diarrhea. Environ Health Perspect. 2011;119(3):299–305. doi: 10.1289/ehp.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagai JS, Castronovo DA, Monchak J, Naumova EN. Seasonality of cryptosporidiosis: A meta-analysis approach. Environ Res. 2009;109(4):465–478. doi: 10.1016/j.envres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagai JS, Sarkar R, Castronovo D, Kattula D, McEntee J, Ward H, Kang G, Naumova EN. Seasonality of rotavirus in South Asia: a meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0038168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy K, Hubbard AE, Eisenberg JNS. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38(6):1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naumova EN, Jagai JS, Matyas B, DeMaria A, Jr, MacNeill IB, Griffiths JK. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol Infect. 2007;135(2):281–92. doi: 10.1017/S0950268806006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovats RS, Edwards SJ, Charron D, Cowden J, D’Souza RM, Ebi KL, Gauci C, Gerner-Smidt P, Hajat S, Hales S, Hernandez Pezzi G, Kriz B, Kutsar K, McKeown P, Mellou K, Menne B, O’Brien S, van Pelt W, Schmid H. Climate variability and campylobacter infection: an international study. Int J Biometeorol. 2005;49(4):207–14. doi: 10.1007/s00484-004-0241-3. [DOI] [PubMed] [Google Scholar]
- 17.Cann KF, Thomas DR, Salmon RL, Wyn-Jones AP, Kay D. Extreme water-related weather events and waterborne disease. Epidemiol Infect. 2013;141(4):671–86. doi: 10.1017/S0950268812001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterk A, Schijven J, de Nijs T, Husman AMD. Direct and Indirect Effects of Climate Change on the Risk of Infection by Water-Transmitted Pathogens. Environ Sci Technol. 2013;47(22):12648–12660. doi: 10.1021/es403549s. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Green S. The Cochrane Collaboration. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] [Google Scholar]
- 21.Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. 2014;122(7):711–8. doi: 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlton EJ, Woster AP, DeWitt P, Goldstein RS, Levy K. A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlton EJ, Eisenberg JN, Goldstick J, Cevallos W, Trostle J, Levy K. Heavy rainfall events and diarrhea incidence: the role of social and environmental factors. Am J Epidemiol. 2014;179(3):344–52. doi: 10.1093/aje/kwt279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curriero FC, Patz JA, Rose JB, Lele S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am J Public Health. 2001;91(8):1194–9. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas KM, Charron DF, Waltner-Toews D, Schuster C, Maarouf AR, Holt JD. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975 – 2001. Int J Environ Health Res. 2006;16(3):167–80. doi: 10.1080/09603120600641326. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander KA, Blackburn JK. Overcoming barriers in evaluating outbreaks of diarrheal disease in resource poor settings: assessment of recurrent outbreaks in Chobe District, Botswana. BMC Public Health. 2013;13(775) doi: 10.1186/1471-2458-13-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali M, Kim DR, Yunus M, Emch M. Time Series Analysis of Cholera in Matlab, Bangladesh, during 1988–2001. J Health Popul Nutr. 2013;31(1):11–19. doi: 10.3329/jhpn.v31i1.14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atchison CJ, Tam CC, Hajat S, van Pelt W, Cowden JM, Lopman BA. Temperature-dependent transmission of rotavirus in Great Britain and The Netherlands. Proc R Soc [Biol] 2010;277(1683):933–942. doi: 10.1098/rspb.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandyopadhyay S, Kanji S, Wang LM. The impact of rainfall and temperature variation on diarrheal prevalence in Sub-Saharan Africa. Appl Geogr. 2012;33(1):63–72. [Google Scholar]
- 31.Bennett A, Epstein LD, Gilman RH, Cama V, Bern C, Cabrera L, Lescano AG, Patz J, Carcamo C, Sterling CR, Checkley W. Effects of the 1997–1998 El Nino episode on community rates of diarrhea. Am J Public Health. 2012;102(7):e63–9. doi: 10.2105/AJPH.2011.300573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bern C, Hernandez B, Lopez MB, Arrowood MJ, De Merida AM, Klein RE. The contrasting epidemiology of Cyclospora and Cryptosporidium among outpatients in Guatemala. Am J Trop Med Hyg. 2000;63(5–6):231–235. [PubMed] [Google Scholar]
- 33.Bhandari GP, Gurung S, Dhimal M, Bhusal CL. Climate change and occurrence of diarrheal diseases: evolving facts from Nepal. J Nepal Health Res Counc. 2012;10(22):181–6. [PubMed] [Google Scholar]
- 34.Bi P, Cameron AS, Zhang Y, Parton KA. Weather and notified Campylobacter infections in temperate and sub-tropical regions of Australia: an ecological study. J Infection. 2008;57(4):317–23. doi: 10.1016/j.jinf.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Britton E, Hales S, Venugopal K, Baker MG. Positive association between ambient temperature and salmonellosis notifications in New Zealand, 1965–2006. Aust N Z J Public Health. 2010;34(2):126–9. doi: 10.1111/j.1753-6405.2010.00495.x. [DOI] [PubMed] [Google Scholar]
- 36.Chai JY, Kim NY, Guk SM, Park YK, Seo M, Han ET, Lee SH. High prevalence and seasonality of cryptosporidiosis in a small rural village occupied predominantly by aged people in the Republic of Korea. Am J Trop Med Hyg. 2001;65(5):518–522. doi: 10.4269/ajtmh.2001.65.518. [DOI] [PubMed] [Google Scholar]
- 37.Chakravarti A, Broor S, Natarajan R, Setty VS, Mittal SK. Epidemiological and clinical characteristics of acute diarrhoea in children due to human rotavirus. J Trop Pediatr. 1992;38(4):192–3. doi: 10.1093/tropej/38.4.192. [DOI] [PubMed] [Google Scholar]
- 38.Checkley W, Epstein L, Gilman R, Figueroa D, Cama R, Patz J, Black R. Effects of El Nino and ambient temperature on pediatric diarrhea in Lima, Peru. Epidemiology. 2000;11(4):S123–S123. [Google Scholar]
- 39.Chou WC, Wu JL, Wang YC, Huang H, Sung FC, Chuang CY. Modeling the impact of climate variability on diarrhea-associated diseases in Taiwan (1996–2007) Sci Total Environ. 2010;409(1):43–51. doi: 10.1016/j.scitotenv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 40.D’Souza RM, Becker NG, Hall G, Moodie KB. Does ambient temperature affect foodborne disease? Epidemiology. 2004;15(1):86–92. doi: 10.1097/01.ede.0000101021.03453.3e. [DOI] [PubMed] [Google Scholar]
- 41.D’Souza RM, Hall G, Becker NG. Climatic factors associated with hospitalizations for rotavirus diarrhoea in children under 5 years of age. Epidemiol Infect. 2008;136(1):56–64. doi: 10.1017/S0950268807008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean AG, Jones TC. Seasonal gastroenteritis and malabsorption at an American military base in the Philippines. I. Clinical and epidemiologic investigations of the acute illness. Am J Epidemiol. 1972;95(2):111–27. doi: 10.1093/oxfordjournals.aje.a121376. [DOI] [PubMed] [Google Scholar]
- 43.Dewan AM, Corner R, Hashizume M, Ongee ET. Typhoid Fever and Its Association with Environmental Factors in the Dhaka Metropolitan Area of Bangladesh: A Spatial and Time-Series Approach. PLoS Negl Trop Dis. 2013;7(1) doi: 10.1371/journal.pntd.0001998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espejo RT, Calderon E, Gonzalez N, Salomon A, Martuscelli A, Romero P. Presence of two distinct types of rotavirus in infants and young children hospitalized with acute gastroenteritis in Mexico City, 1977. J Infect Dis. 1979;139(4):474–7. doi: 10.1093/infdis/139.4.474. [DOI] [PubMed] [Google Scholar]
- 45.Fleury M, Charron DF, Holt JD, Allen OB, Maarouf AR. A time series analysis of the relationship of ambient temperature and common bacterial enteric infections in two Canadian provinces. Int J Biometeorol. 2006;50(6):385–91. doi: 10.1007/s00484-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 46.Glass RI, Becker S, Huq MI, Stoll BJ, Khan MU, Merson MH, Lee JV, Black RE. Endemic cholera in rural Bangladesh, 1966–1980. Am J Epidemiol. 1982;116(6):959–70. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- 47.Gomwalk NE, Umoh UJ, Gosham LT, Ahmad AA. Influence of Climatic Factors on Rotavirus Infection among Children with Acute Gastroenteritis in Zaria, Northern Nigeria. J Trop Pediatr. 1993;39(5):293–297. doi: 10.1093/tropej/39.5.293. [DOI] [PubMed] [Google Scholar]
- 48.Haffejee IE, Moosa A. Rotavirus studies in Indian (Asian) South African infants with acute gastro-enteritis: I. Microbiological and epidemiological aspects. Ann Trop Paediatr. 1990;10(2):165–72. doi: 10.1080/02724936.1990.11747425. [DOI] [PubMed] [Google Scholar]
- 49.Hall GV, Hanigan IC, Dear KBG, Vally H. The influence of weather on community gastroenteritis in Australia. Epidemiol Infect. 2011;139(6):927–936. doi: 10.1017/S0950268810001901. [DOI] [PubMed] [Google Scholar]
- 50.Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque ASG, Hayashi T, Sack DA. Association between climate variability and hospital visits for non-cholera diarrhoea in Bangladesh: effects and vulnerable groups. Int J Epidemiol. 2007;36(5):1030–1037. doi: 10.1093/ije/dym148. [DOI] [PubMed] [Google Scholar]
- 51.Hashizume M, Armstrong B, Wagatsuma Y, Faruque ASG, Hayashi T, Sack DA. Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol Infect. 2008;136(9):1281–1289. doi: 10.1017/S0950268807009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashizume M, Faruque ASG, Wagatsuma Y, Hayashi T, Armstrong B. Cholera in Bangladesh: Climatic components of seasonal variation. Epidemiology. 2010;21(5):706–710. doi: 10.1097/EDE.0b013e3181e5b053. [DOI] [PubMed] [Google Scholar]
- 53.Hu W, Mengersen K, Fu SY, Tong S. The use of ZIP and CART to model cryptosporidiosis in relation to climatic variables. Int J Biometeorol. 2010;54(4):433–40. doi: 10.1007/s00484-009-0294-4. [DOI] [PubMed] [Google Scholar]
- 54.Hu W, Tong S, Mengersen K, Connell D. Weather variability and the incidence of cryptosporidiosis: comparison of time series poisson regression and SARIMA models. Ann Epidemiol. 2007;17(9):679–88. doi: 10.1016/j.annepidem.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Huang DS, Guan P, Guo JQ, Wang P, Zhou BS. Investigating the effects of climate variations on bacillary dysentery incidence in northeast China using ridge regression and hierarchical cluster analysis. BMC Infect Dis. 2008;8(130) doi: 10.1186/1471-2334-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ijaz MK, Alharbi S, Uduman SA, Cheema Y, Sheek-Hussen MM, Alkhair ARA, Shalabi AG, Ijaz SS, Bin-Othman SA, Sattar SA, Liddle LF. Seasonality and prevalence of rotavirus in Al-Ain, United Arab Emirates. Clin Diagn Virol. 1994;2(6):323–329. doi: 10.1016/0928-0197(94)90002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Islam MS, Sharker MA, Rheman S, Hossain S, Mahmud ZH, Islam MS, Uddin AM, Yunus M, Osman MS, Ernst R, Rector I, Larson CP, Luby SP, Endtz HP, Cravioto A. Effects of local climate variability on transmission dynamics of cholera in Matlab, Bangladesh. Trans R Soc Trop Med Hyg. 2009;103(11):1165–70. doi: 10.1016/j.trstmh.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Katsumata T, Hosea D, Wasito EB, Kohno S, Hara K, Soeparto P, Ranuh IG. Cryptosporidiosis in Indonesia: A hospital-based study and a community-based survey. Am J Trop Med Hyg. 1998;59(4):628–632. doi: 10.4269/ajtmh.1998.59.628. [DOI] [PubMed] [Google Scholar]
- 59.Kelly-Hope LA, Alonso WJ, Thiem VD, Anh DD, Canh DG, Lee H, Smith DL, Miller MA. Geographical distribution and risk factors associated with enteric diseases in Vietnam. Am J Trop Med Hyg. 2007;76(4):706–712. [PubMed] [Google Scholar]
- 60.Kelly-Hope LA, Alonso WJ, Thiem VD, Canh DG, Anh DD, Lee H, Miller MA. Temporal trends and climatic factors associated with bacterial enteric diseases in Vietnam, 1991–2001. Environ Health Perspect. 2008;116(1):7–12. doi: 10.1289/ehp.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimura K, Rai SK, Rai G, Insisiengmay S, Kawabata M, Karanis P, Uga S. Study on cyclospora cayetanensis associated with diarrheal disease in Nepal and Loa PDR. The Southeast Asian J Trop Med Public Health. 2005;36(6):1371–6. [PubMed] [Google Scholar]
- 62.Konno T, Suzuki H, Katsushima N, Imai A, Tazawa F, Kutsuzawa T, Kitaoka S, Sakamoto M, Yazaki N, Ishida N. Influence of temperature and relative humidity on human rotavirus infection in Japan. J Infect Dis. 1983;147(1):125–8. doi: 10.1093/infdis/147.1.125. [DOI] [PubMed] [Google Scholar]
- 63.Kovats RS, Edwards SJ, Hajat S, Armstrong BG, Ebi KL, Menne B. The effect of temperature on food poisoning: a time-series analysis of salmonellosis in ten European countries. Epidemiol Infect. 2004;132(3):443–53. doi: 10.1017/s0950268804001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lake IR, Bentham G, Kovats RS, Nichols GL. Effects of weather and river flow on cryptosporidiosis. J Water Health. 2005;3(4):469–74. doi: 10.2166/wh.2005.048. [DOI] [PubMed] [Google Scholar]
- 65.Lama JR, Seas CR, Leon-Barua R, Gotuzzo E, Sack RB. Environmental temperature, cholera, and acute diarrhoea in adults in Lima, Peru. J Health, Popul Nutr. 2004;22(4):399–403. [PubMed] [Google Scholar]
- 66.Lee WT, Lin PC, Lin LC, Chen HL, Yang RC. Salmonella/rotavirus coinfection in hospitalized children. Kaohsiung J Med Sci. 2012;28(11):595–600. doi: 10.1016/j.kjms.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lloyd SJ, Kovats RS, Armstrong BG. Global diarrhoea morbidity, weather and climate. Climate Res. 2007;34(2):119–127. [Google Scholar]
- 68.Lopman B, Armstrong B, Atchison C, Gray J, Host J. Weather and Virological Factors Drive Norovirus Epidemiology: Time-Series Analysis of Laboratory Surveillance Data in England and Wales. PLoS One. 2009;4(8) doi: 10.1371/journal.pone.0006671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luque Fernandez MA, Bauernfeind A, Jimenez JD, Gil CL, El Omeiri N, Guibert DH. Influence of temperature and rainfall on the evolution of cholera epidemics in Lusaka, Zambia, 2003–2006: analysis of a time series. Trans R Soc Trop Med Hyg. 2009;103(2):137–43. doi: 10.1016/j.trstmh.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Matsuda F, Ishimura S, Wagatsuma Y, Higashi T, Hayashi T, Faruque ASG, Sack DA, Nishibuchi M. Prediction of epidemic cholera due to Vibrio cholerae O1 in children younger than 10 years using climate data in Bangladesh. Epidemiol Infect. 2008;136(1):73–79. doi: 10.1017/S0950268807008175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCormick BJJ, Alonso WJ, Miller MA. An exploration of spatial patterns of seasonal diarrhoeal morbidity in Thailand. Epidemiol Infect. 2012;140(7):1236–1243. doi: 10.1017/S0950268811001919. [DOI] [PubMed] [Google Scholar]
- 72.Mitui MT, Chan PKS, Nelson EAS, Leung TF, Nishizono A, Ahmed K. Co-dominance of G1 and emerging G3 rotaviruses in Hong Kong: A three-year surveillance in three major hospitals. J Clin Virol. 2011;50(4):325–333. doi: 10.1016/j.jcv.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Onozuka D, Hashizume M, Hagihara A. Effects of weather variability on infectious gastroenteritis. Epidemiol Infect. 2010;138(2):236–243. doi: 10.1017/S0950268809990574. [DOI] [PubMed] [Google Scholar]
- 74.Paredes-Paredes M, Okhuysen PC, Flores J, Mohamed JA, Padda RS, Gonzalez-Estrada A, Haley CA, Carlin LG, Nair P, DuPont HL. Seasonality of diarrheagenic Escherichia coli pathotypes in the US students acquiring diarrhea in Mexico. J Travel Med. 2011;18(2):121–125. doi: 10.1111/j.1708-8305.2010.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phukan AC, Patgiri DK, Mahanta J. Rotavirus associated acute diarrhoea in hospitalized children in Dibrugarh, north-east India. Indian J Pathol Microbiol. 2003;46(2):274–8. [PubMed] [Google Scholar]
- 76.Pinfold JV, Horan NJ, Mara DD. Seasonal effects on the reported incidence of acute diarrhoeal disease in northeast Thailand. Int J Epidemiol. 1991;20(3):777–86. doi: 10.1093/ije/20.3.777. [DOI] [PubMed] [Google Scholar]
- 77.Purohit SG, Kelkar SD, Simha V. Time series analysis of patients with rotavirus diarrhoea in Pune, India. J Diarrhoeal Dis Res. 1998;16(2):74–83. [PubMed] [Google Scholar]
- 78.Ram S, Khurana S, Khurana SB, Sharma S, Vadehra DV. Seasonal fluctuations in the occurrence of enteroinvasive Escherichia coli diarrhoea. Indian J Med Res. 1990;91:258–62. [PubMed] [Google Scholar]
- 79.Salazar-Lindo E, Pinell-Salles P, Maruy A, Chea-Woo E. El Nino and diarrhoea and dehydration in Lima, Peru. Lancet. 1997;350(9091):1597–1598. doi: 10.1016/S0140-6736(05)64013-5. [DOI] [PubMed] [Google Scholar]
- 80.Sarkar R, Kang G, Naumova EN. Rotavirus Seasonality and Age Effects in a Birth Cohort Study of Southern India. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seidu R, Stenstrom TA, Lofman O. A comparative cohort study of the effect of rainfall and temperature on diarrhoeal disease in faecal sludge and non-faecal sludge applying communities, Northern Ghana. J Water Clim Change. 2013;4(2):90–102. [Google Scholar]
- 82.Singh RBK, Hales S, de Wet N, Raj R, Hearnden M, Weinstein P. The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environ Health Perspect. 2001;109(2):155–159. doi: 10.1289/ehp.01109155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soenarto Y, Sebodo T, Ridho R, Alrasjid H, Rohde JE, Bugg HC, Barnes GL, Bishop RF. Acute diarrhea and rotavirus infection in newborn babies and children in Yogyakarta, Indonesia, from June 1978 to June 1979. J Clin Microbiol. 1981;14(2):123–9. doi: 10.1128/jcm.14.2.123-129.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Speelmon EC, Checkley W, Gilman RH, Patz J, Calderon M, Manga S. Cholera incidence and El Nino-related higher ambient temperature. JAMA. 2000;283(23):3072–4. [PubMed] [Google Scholar]
- 85.Sumi A, Rajendran K, Ramamurthy T, Krishnan T, Nair GB, Harigane K, Kobayashi N. Effect of temperature, relative humidity and rainfall on rotavirus infections in Kolkata, India. Epidemiol Infect. 2013;141(8):1652–1661. doi: 10.1017/S0950268812002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sutra S, Srisontrisuk S, Panpurk W, Sutra P, Chirawatkul A, Snongchart N, Kusowon P. The pattern of diarrhea in children in Khon Kaen, northeastern Thailand: I. The incidence and seasonal variation of diarrhea. The Southeast Asian J Trop Med Public Health. 1990;21(4):586–93. [PubMed] [Google Scholar]
- 87.Tam CC, Rodrigues LC, O’Brien SJ, Hajat S. Temperature dependence of reported Campylobacter infection in England, 1989–1999. Epidemiol Infect. 2006;134(1):119–25. doi: 10.1017/S0950268805004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Torres BV, Ilja RM, Esparaza J. Epidemiological aspects of rotavirus infection in hospitalized Venezuelan children with gastroenteritis. Am J Trop Med Hyg. 1978;27(3):567–72. doi: 10.4269/ajtmh.1978.27.567. [DOI] [PubMed] [Google Scholar]
- 89.Trabelsi A, Fodha I, Chouikha A, Fredj MB, Mastouri M, Ben Abdelaziz A, Sfar T, Essoussi AS, Jaoua S, Steele AD. Rotavirus Strain Diversity in the Centre Coast of Tunisia from 2000 through 2003. J Infect Dis. 2010;202:S252–S257. doi: 10.1086/653580. [DOI] [PubMed] [Google Scholar]
- 90.Traerup SLM, Ortiz RA, Markandya A. The Costs of Climate Change: A study of cholera in Tanzania. Int J Environ Res Public Health. 2011;8(12):4386–4405. doi: 10.3390/ijerph8124386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Utsalo SJ, Eko FO, Antiaobong EO. Features of Cholera and Vibrio-Parahaemolyticus Diarrhea Endemicity in Calabar, Nigeria. Eur J Epidemiol. 1992;8(6):856–860. doi: 10.1007/BF00145332. [DOI] [PubMed] [Google Scholar]
- 92.Woodward WE, Hirschhorn N, Sack RB, Cash RA, Brownlee I, Chickadonz GH, Evans LK, Shepard RH, Woodward RC. Acute diarrhea on an Apache Indian reservation. Am J Epidemiol. 1974;99(4):281–90. doi: 10.1093/oxfordjournals.aje.a121613. [DOI] [PubMed] [Google Scholar]
- 93.Workman SN, Sobers SJ, Mathison GE, Lavoie MC. Human Campylobacter-associated enteritis on the Caribbean island of Barbados. Am J Trop Med Hyg. 2006;74(4):623–627. [PubMed] [Google Scholar]
- 94.Zhang Y, Bi P, Hiller JE. Weather and the transmission of bacillary dysentery in Jinan, northern China: A time-series analysis. Public Health Rep. 2008;123(1):61–66. doi: 10.1177/003335490812300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Bi P, Hiller JE. Climate variations and Salmonella infection in Australian subtropical and tropical regions. Sci Total Environ. 2010;408(3):524–530. doi: 10.1016/j.scitotenv.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 96.Checkley W, Epstein LD, Gilman RH, Figueroa D, Cama RI, Patz JA, Black RE. Effect of El Nino and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. Lancet. 2000;355(9202):442–50. doi: 10.1016/s0140-6736(00)82010-3. [DOI] [PubMed] [Google Scholar]
- 97.Adkins HJ, Escamilla J, Santiago LT, Ranoa C, Echeverria P, Cross JH. Two-year survey of etiologic agents of diarrheal disease at San Lazaro Hospital, Manila, Republic of the Philippines. J Clin Microbiol. 1987;25(7):1143–7. doi: 10.1128/jcm.25.7.1143-1147.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drayna P, McLellan SL, Simpson P, Li SH, Gorelick MH. Association between rainfall and pediatric emergency department visits for acute gastrointestinal illness. Environ Health Perspect. 2010;118(10):1439–1443. doi: 10.1289/ehp.0901671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque ASG, Hayashi T, Sack DA. The effect of rainfall on the incidence of cholera in Bangladesh. Epidemiology. 2008;19(1):103–110. doi: 10.1097/EDE.0b013e31815c09ea. [DOI] [PubMed] [Google Scholar]
- 100.Nichols G, Lane C, Asgari N, Verlander NQ, Charlett A. Rainfall and outbreaks of drinking water related disease and in England and Wales. J Water Health. 2009;7(1):1–8. doi: 10.2166/wh.2009.143. [DOI] [PubMed] [Google Scholar]
- 101.Said B, Wright F, Nichols GL, Reacher M, Rutter M. Outbreaks of infectious disease associated with private drinking water supplies in England and Wales 1970–2000. Epidemiol Infect. 2003;130(3):469–79. [PMC free article] [PubMed] [Google Scholar]
- 102.Aksoy U, Akisu C, Sahin S, Usluca S, Yalcin G, Kuralay F, Oral AM. First reported waterborne outbreak of cryptosporidiosis with Cyclospora co-infection in Turkey. Euro Surveill. 2007;12(7):E070215 4. doi: 10.2807/esw.12.07.03142-en. [DOI] [PubMed] [Google Scholar]
- 103.Anderson AD, Heryford AG, Sarisky JP, Higgins C, Monroe SS, Beard RS, Newport CM, Cashdollar JL, Fout GS, Robbins DE, Seys SA, Musgrave KJ, Medus C, Vinje J, Bresee JS, Mainzer HM, Glass RI. A waterborne outbreak of Norwalk-like virus among snowmobilers-Wyoming, 2001. J Infect Dis. 2003;187(2):303–6. doi: 10.1086/346239. [DOI] [PubMed] [Google Scholar]
- 104.Atherton F, Newman CP, Casemore DP. An outbreak of waterborne cryptosporidiosis associated with a public water supply in the UK. Epidemiol Infect. 1995;115(1):123–31. doi: 10.1017/s0950268800058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Auld H, MacIver D, Klaassen J. Heavy rainfall and waterborne disease outbreaks: the Walkerton example. J Tox Env Heal A. 2004;67(20–22):1879–87. doi: 10.1080/15287390490493475. [DOI] [PubMed] [Google Scholar]
- 106.Bridgman SA, Robertson RM, Syed Q, Speed N, Andrews N, Hunter PR. Outbreak of cryptosporidiosis associated with a disinfected groundwater supply. Epidemiol Infect. 1995;115(3):555–66. doi: 10.1017/s0950268800058726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.CCDR. Waterborne outbreak of gastroenteritis associated with a contaminated municipal water supply Walkerton Ontario, May–June 2000. Can Commun Dis Rep. 2000;26(20):170–3. [PubMed] [Google Scholar]
- 108.Doyle A, Barataud D, Gallay A, Thiolet JM, Le Guyaguer S, Kohli E, Vaillant V. Norovirus foodborne outbreaks associated with the consumption of oysters from the Etang de Thau, France, December 2002. Euro Surveill. 2004;9(3):24–6. doi: 10.2807/esm.09.03.00451-en. [DOI] [PubMed] [Google Scholar]
- 109.Effler P, Isaacson M, Arntzen L, Heenan R, Canter P, Barrett T, Lee L, Mambo C, Levine W, Zaidi A, Griffin PM. Factors contributing to the emergence of Escherichia coli O157 in Africa. Emerg Infect Dis. 2001;7(5):812–819. doi: 10.3201/eid0705.017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fong TT, Mansfield LS, Wilson DL, Schwab DJ, Molloy SL, Rose JB. Massive microbiological groundwater contamination associated with a waterborne outbreak in Lake Erie, South Bass Island, Ohio. Environ Health Perspect. 2007;115(6):856–64. doi: 10.1289/ehp.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fox KR, Lytle DA. Milwaukee’s crypto outbreak: Investigation and recommendations. J Am Water Works Ass. 1996;88(9):87–94. [Google Scholar]
- 112.Gelting R, Sarisky J, Selman C, Otto C, Higgins C, Bohan PO, Buchanan SB, Meehan PJ. Use of a systems-based approach to an environmental health assessment for a waterborne disease outbreak investigation at a snowmobile lodge in Wyoming. Int J Hyg Environ Health. 2005;208(1–2):67–73. doi: 10.1016/j.ijheh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 113.Goodman RA, Buehler JW, Greenberg HB, McKinley TW, Smith JD. Norwalk gastroenteritis associated with a water system in a rural Georgia community. Arch Environ Health. 1982;37(6):358–60. doi: 10.1080/00039896.1982.10667591. [DOI] [PubMed] [Google Scholar]
- 114.Hejkal TW, Keswick B, Labelle RL, Gerba CP, Sanchez Y, Dreesman G, Hafkin B, Melnick JL. Viruses in a Community Water-Supply Associated with an Outbreak of Gastroenteritis and Infectious-Hepatitis. J Am Water Works Ass. 1982;74(6):318–321. [Google Scholar]
- 115.Ihekweazu C, Barlow M, Roberts S, Christensen H, Guttridge B, Lewis D, Paynter S. Outbreak of E. coli O157 infection in the south west of the UK: risks from streams crossing seaside beaches. Euro Surveill. 2006;11(4):128–30. [PubMed] [Google Scholar]
- 116.Mac Kenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE, Kazmierczak JJ, Addiss DG, Fox KR, Rose JB, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. New England J Med. 1994;331(3):161–7. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 117.Millson M, Bokhout M, Carlson J, Spielberg L, Aldis R, Borczyk A, Lior H. An outbreak of Campylobacter jejuni gastroenteritis linked to meltwater contamination of a municipal well. Can J Public Health. 1991;82(1):27–31. [PubMed] [Google Scholar]
- 118.O’Reilly CE, Bowen AB, Perez NE, Sarisky JP, Shepherd CA, Miller MD, Hubbard BC, Herring M, Buchanan SD, Fitzgerald CC, Hill V, Arrowood MJ, Xiao LX, Hoekstra RM, Mintz ED, Lynch MF, Outbreak Working G. A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clin Infect Dis. 2007;44(4):506–12. doi: 10.1086/511043. [DOI] [PubMed] [Google Scholar]
- 119.Patil SB, Deshmukh D, Dixit JV, Damle AS. Epidemiological investigation of an outbreak of acute diarrheal disease: A shoe leather epidemiology. J Global Infect Dis. 2011;3(4):361–365. doi: 10.4103/0974-777X.91060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith HV, Patterson WJ, Hardie R, Greene LA, Benton C, Tulloch W, Gilmour RA, Girdwood RW, Sharp JC, Forbes GI. An outbreak of waterborne cryptosporidiosis caused by post-treatment contamination. Epidemiol Infect. 1989;103(3):703–15. doi: 10.1017/s0950268800031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vogt RL, Sours HE, Barrett T, Feldman RA, Dickinson RJ, Witherell L. Campylobacter enteritis associated with contaminated water. Ann Intern Med. 1982;96(3):292–6. doi: 10.7326/0003-4819-96-3-292. [DOI] [PubMed] [Google Scholar]
- 122.Willocks L, Crampin A, Milne L, Seng C, Susman M, Gair R, Moulsdale M, Shafi S, Wall R, Wiggins R, Lightfoot N. A large outbreak of cryptosporidiosis associated with a public water supply from a deep chalk borehole. Outbreak Investigation Team. Commun Dis Public Health. 1998;1(4):239–43. [PubMed] [Google Scholar]
- 123.Yamamoto N, Urabe K, Takaoka M, Nakazawa K, Gotoh A, Haga M, Fuchigami H, Kimata I, Iseki M. Outbreak of cryptosporidiosis after contamination of the public water supply in Saitama Prefecture, Japan, in 1996. Kansenshogaku zasshi. 2000;74(6):518–26. doi: 10.11150/kansenshogakuzasshi1970.74.518. [DOI] [PubMed] [Google Scholar]
- 124.Rose JB, Daeschner S, Easterling DR, Curriero FC, Lele S, Patz JA. Climate and waterborne disease outbreaks. J Am Water Works Ass. 2000;92(9):77–87. [Google Scholar]
- 125.Biswas R, Pal D, Mukhopadhyay SP. A community based study on health impact of flood in a vulnerable district of West Bengal. Indian J Public Health. 1999;43(2):89–90. [PubMed] [Google Scholar]
- 126.Bokhari H, Shah MA, Asad S, Akhtar S, Akram M, Wren BW. Escherichia coli Pathotypes in Pakistan from Consecutive Floods in 2010 and 2011. Am J Trop Med Hyg. 2013;88(3):519–525. doi: 10.4269/ajtmh.12-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Campanella N. Infectious diseases and natural disasters: the effects of Hurricane Mitch over Villanueva municipal area, Nicaragua. Public Health Rev. 1999;27(4):311–9. [PubMed] [Google Scholar]
- 128.CDC. Morbidity and mortality associated with Hurricane Floyd--North Carolina, September–October 1999. MMWR Morb Mortal Wkly Rep. 2000;49(17):369–72. [PubMed] [Google Scholar]
- 129.CDC. Rapid health response assessment, and surveillance after a tsunami--Thailand 2004–2005. MMWR Morb Mortal Wkly Rep. 2005;54(3):61–4. [PubMed] [Google Scholar]
- 130.Chhotray GP, Pal BB, Khuntia HK, Chowdhury NR, Chakraborty S, Yamasaki S, Ramamurthy T, Takeda Y, Bhattacharya SK, Nair GB. Incidence and molecular analysis of Vibrio cholerae associated with cholera outbreak subsequent to the super cyclone in Orissa, India. Epidemiol Infect. 2002;128(2):131–138. doi: 10.1017/s0950268801006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ding GY, Zhang Y, Gao L, Ma W, Li XJ, Liu J, Liu QY, Jiang BF. Quantitative Analysis of Burden of Infectious Diarrhea Associated with Floods in Northwest of Anhui Province, China: A Mixed Method Evaluation. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harris AM, Chowdhury F, Begum YA, Faruque AS, Svennerholm AM, Harris JB, Ryan ET, Cravioto A, Calderwood SB, Qadri F. Shifting Prevalence of Major Diarrheal Pathogens in Patients Seeking Hospital Care during Floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am J Trop Med Hyg. 2008;79(6):107–107. [PMC free article] [PubMed] [Google Scholar]
- 133.Hashizume M, Wagatsuma Y, Faruque ASG, Hayashi T, Hunter PR, Armstrong B, Sack DA. Factors determining vulnerability to diarrhoea during and after severe floods in Bangladesh. J Water Health. 2008;6(3):323–332. doi: 10.2166/wh.2008.062. [DOI] [PubMed] [Google Scholar]
- 134.Joshi PC, Kaushal S, Aribam BS, Khattri P, D’Aoust O, Singh MM, Marx M, Guha-Sapir D. Recurrent floods and prevalence of diarrhea among under five children: observations from Bahraich district, Uttar Pradesh, India. Glob Health Action. 2011;4 doi: 10.3402/gha.v4i0.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kondo H, Seo N, Yasuda T, Hasizume M, Koido Y, Ninomiya N, Yamamoto Y. Post-flood--infectious diseases in Mozambique. Prehosp Disaster Med. 2002;17(3):126–33. doi: 10.1017/s1049023x00000340. [DOI] [PubMed] [Google Scholar]
- 136.Miettinen IT, Zacheus O, von Bonsdorff CH, Vartiainen T. Waterborne epidemics in Finland in 1998–1999. Water Science Technol. 2001;43(12):67–71. [PubMed] [Google Scholar]
- 137.Milojevic A, Armstrong B, Hashizume M, McAllister K, Faruque A, Yunus M, Streatfield PK, Moji K, Wilkinson P. Health Effects of Flooding in Rural Bangladesh. Epidemiology. 2012;23(1):107–115. doi: 10.1097/EDE.0b013e31823ac606. [DOI] [PubMed] [Google Scholar]
- 138.Mondal NC, Biswas R, Manna A. Risk factors of diarrhoea among flood victims: a controlled epidemiological study. Indian J Public Health. 2001;45(4):122–7. [PubMed] [Google Scholar]
- 139.Reacher M, McKenzie K, Lane C, Nichols T, Kedge I, Iversen A, Hepple P, Walter T, Laxton C, Simpson J Lewes Flood Action Recovery T. Health impacts of flooding in Lewes: a comparison of reported gastrointestinal and other illness and mental health in flooded and non-flooded households. Commun Dis Public Health. 2004;7(1):39–46. [PubMed] [Google Scholar]
- 140.Schnitzler J, Benzler J, Altmann D, Mucke I, Krause G. Survey on the population’s needs and the public health response during floods in Germany 2002. J Public Health Manag Pract. 2007;13(5):461–464. doi: 10.1097/01.PHH.0000285197.23932.3e. [DOI] [PubMed] [Google Scholar]
- 141.Schwartz BS, Harris JB, Khan AI, Larocque RC, Sack DA, Malek MA, Faruque ASG, Qadri F, Calderwood SB, Luby SP, Ryan ET. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg. 2006;74(6):1067–1073. [PMC free article] [PubMed] [Google Scholar]
- 142.Setzer C, Domino ME. Medicaid outpatient utilization for waterborne pathogenic illness following Hurricane Floyd. Public Health Rep. 2004;119(5):472–8. doi: 10.1016/j.phr.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Siddique AK, Islam Q, Akram K, Mazumder Y, Mitra A, Eusof A. Cholera epidemic and natural disasters; where is the link. Trop Geogr Med. 1989;41(4):377–82. [PubMed] [Google Scholar]
- 144.Sur D, Dutta P, Nair GB, Bhattacharya SK. Severe cholera outbreak following floods in a northern district of West Bengal. Indian J Med Res. 2000;112:178–82. [PubMed] [Google Scholar]
- 145.Vollaard AM, Ali S, van Asten HA, Widjaja S, Visser LG, Surjadi C, van Dissel JT. Risk factors for typhoid and paratyphoid fever in Jakarta, Indonesia. JAMA : J Am Med Assoc. 2004;291(21):2607–15. doi: 10.1001/jama.291.21.2607. [DOI] [PubMed] [Google Scholar]
- 146.Wade TJ, Sandhu SK, Levy D, Lee S, LeChevallier MW, Katz L, Colford JM. Did a severe flood in the midwest cause an increase in the incidence of gastrointestinal symptoms? Am J Epidemiol. 2004;159(4):398–405. doi: 10.1093/aje/kwh050. [DOI] [PubMed] [Google Scholar]
- 147.Waring S, Zakos-Feliberti A, Wood R, Stone M, Padgett P, Arafat R. The utility of geographic information systems (GIS) in rapid epidemiological assessments following weather-related disasters: Methodological issues based on the Tropical Storm Allison experience. Int J Hyg Environ Health. 2005;208(1–2):109–116. doi: 10.1016/j.ijheh.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 148.Woodruff BA, Toole MJ, Rodrigue DC, Brink EW, Mahgoub ES, Ahmed MM, Babikar A. Disease Surveillance and Control after a Flood - Khartoum, Sudan, 1988. Disasters. 1990;14(2):151–163. [Google Scholar]
- 149.Ahmed MU, Urasawa S, Taniguchi K, Urasawa T, Kobayashi N, Wakasugi F, Islam A, Sahikh HA. Analysis of Human Rotavirus Strains Prevailing in Bangladesh in Relation to Nationwide Floods Brought by the 1988 Monsoon. J Clin Microbiol. 1991;29(10):2273–2279. doi: 10.1128/jcm.29.10.2273-2279.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ahmed Z, Khan AA, Nisar N. Frequency of infectious diseases among flood affected people at district Rajanpur, Pakistan. Pak J Med Sci. 2011;27(4):866–869. [Google Scholar]
- 151.Bhunia R, Ghosh S. Waterborne cholera outbreak following Cyclone Aila in Sundarban area of West Bengal, India, 2009. Transs R Soc Trop Med Hyg. 2011;105(4):214–219. doi: 10.1016/j.trstmh.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 152.Bingnan FN, Unicomb L, Rahim Z, Banu NN, Podder G, Clemens J, Vanloon FPL, Rao MR, Malek A, Tzipori S. Rotavirus-Associated Diarrhea in Rural Bangladesh - 2-Year Study of Incidence and Serotype Distribution. J Clin Microbiol. 1991;29(7):1359–1363. doi: 10.1128/jcm.29.7.1359-1363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.CDC. Norovirus outbreak among evacuees from hurricane Katrina--Houston, Texas September 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1016–8. [PubMed] [Google Scholar]
- 154.CDC. Infectious disease and dermatologic conditions in evacuees and rescue workers after Hurricane Katrina--multiple states, August–September 2005. MMWR. 2005;54(38):961–4. [PubMed] [Google Scholar]
- 155.CDC. Surveillance for illness and injury after hurricane Katrina--New Orleans Louisiana September 8–25, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1018–21. [PubMed] [Google Scholar]
- 156.Kukkula M, Arstila P, Klossner ML, Maunula L, Bonsdorff CH, Jaatinen P. Waterborne outbreak of viral gastroenteritis. Scand J Infect Dis. 1997;29(4):415–8. doi: 10.3109/00365549709011840. [DOI] [PubMed] [Google Scholar]
- 157.Kunii O, Nakamura S, Abdur R, Wakai S. The impact on health and risk factors of the diarrhoea epidemics in the 1998 Bangladesh floods. Public Health. 2002;116(2):68–74. doi: 10.1038/sj.ph.1900828. [DOI] [PubMed] [Google Scholar]
- 158.Paul BK, Rahman MK, Rakshit BC. Post-Cyclone Sidr illness patterns in coastal Bangladesh: an empirical study. Nat Hazards. 2011;56(3):841–852. [Google Scholar]
- 159.Qadri F, Khan AI, Faruque ASG, Begum YA, Chowdhury F, Nair GB, Salam MA, Sack DA, Svennerholm AM. Enterotoxigenic Escherichia coli and Vibrio cholerae diarrhea, Bangladesh, 2004. Emerg Infect Dis. 2005;11(7):1104–1107. doi: 10.3201/eid1107.041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schmid D, Lederer I, Much P, Pichler AM, Allerberger F. Outbreak of norovirus infection associated with contaminated flood water, Salzburg, 2005. Euro Surveill. 2005;10(6):E050616 3. doi: 10.2807/esw.10.24.02727-en. [DOI] [PubMed] [Google Scholar]
- 161.Siddique AK, Baqui AH, Eusof A, Zaman K. 1988 floods in Bangladesh: pattern of illness and causes of death. J Diarrhoeal Dis Res. 1991;9(4):310–4. [PubMed] [Google Scholar]
- 162.World Health Organization. Epidemic-prone disease surveillance and response after the tsunami in Aceh Province Indonesia. Wkly Epidemiol Rec. 2005;80(18):160–4. [PubMed] [Google Scholar]
- 163.CDC. Morbidity and mortality associated with Hurricane Floyd--North Carolina September–October 1999. MMWR Morb Mortal Wkly Rep. 2000;49(17):369–72. [PubMed] [Google Scholar]
- 164.CDC. Early warning disease surveillance after a flood emergency--Pakistan 2010. MMWR Morb Mortal Wkly Rep. 2012;61(49):1002–7. [PubMed] [Google Scholar]
- 165.Burr ML, Davis AR, Zbijowski AG. Diarrhoea and the drought. Public Health. 1978;92(2):86–7. doi: 10.1016/s0033-3506(78)80034-1. [DOI] [PubMed] [Google Scholar]
- 166.de Sherbinin A. The Biophysical and Geographical Correlates of Child Malnutrition in Africa. Popul Space Place. 2011;17(1):27–46. [Google Scholar]
- 167.Stanke C, Kerac M, Prudhomme C, Medlock J, Murray V. Health effects of drought: a systematic review of the evidence. PLoS Curr. 2013;5 doi: 10.1371/currents.dis.7a2cee9e980f91ad7697b570bcc4b004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wilhite DA, Glantz MH. Understanding the Drought Phenomenon: The Role of Definitions. Water Int. 1985;10(3):111–120. [Google Scholar]
- 169.Lal A, Hales S, French N, Baker MG. Seasonality in human zoonotic enteric diseases: a systematic review. PloS One. 2012;7(4):e31883. doi: 10.1371/journal.pone.0031883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Murphy C, Carroll C, Jordan KN. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J Appl Microbiol. 2006;100(4):623–32. doi: 10.1111/j.1365-2672.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 171.Roszak DB, Colwell RR. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51(3):365–79. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rzezutka A, Cook N. Survival of human enteric viruses in the environment and food. FEMS Microbiol Rev. 2004;28(4):441–53. doi: 10.1016/j.femsre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 173.van Elsas JD, Semenov AV, Costa R, Trevors JT. Survival of Escherichia coli in the environment: fundamental and public health aspects. ISME J. 2011;5(2):173–83. doi: 10.1038/ismej.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alum A, Absar IM, Asaad H, Rubino JR, Ijaz MK. Impact of environmental conditions on the survival of cryptosporidium and giardia on environmental surfaces. Interdiscip Perspect Infect Dis. 2014;2014:210385. doi: 10.1155/2014/210385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Peng X, Murphy T, Holden NM. Evaluation of the effect of temperature on the die-off rate for Cryptosporidium parvum oocysts in water, soils, and feces. Appl Environ Microbiol. 2008;74(23):7101–7. doi: 10.1128/AEM.01442-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.John DE, Rose JB. Review of factors affecting microbial survival in groundwater. Environ Sci Technol. 2005;39(19):7345–56. doi: 10.1021/es047995w. [DOI] [PubMed] [Google Scholar]
- 177.Hernández-Delgado EA, Toranzos GA. In-Situ Replication Studies of Somatic and Male-Specific Coliphages in a Tropical Pristine River. Water Sci Technol. 1995;31(5–6):247–250. [Google Scholar]
- 178.Kelly JJ, Minalt N, Culotti A, Pryor M, Packman A. Temporal variations in the abundance and composition of biofilm communities colonizing drinking water distribution pipes. PloS One. 2014;9(5):e98542. doi: 10.1371/journal.pone.0098542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shapiro RS, Cowen LE. Thermal control of microbial development and virulence: molecular mechanisms of microbial temperature sensing. mBio. 2012;3(5) doi: 10.1128/mBio.00238-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Konkel ME, Tilly K. Temperature-regulated expression of bacterial virulence genes. Microbes Infect. 2000;2(2):157–66. doi: 10.1016/s1286-4579(00)00272-0. [DOI] [PubMed] [Google Scholar]
- 181.Pitzer VE, Viboud C, Lopman BA, Patel MM, Parashar UD, Grenfell BT. Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence. J R Soc Interface. 2011;8(64):1584–93. doi: 10.1098/rsif.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alexander KA, Carzolio M, Goodin D, Vance E. Climate change is likely to worsen the public health threat of diarrheal disease in Botswana. Int J Environ Res Public Health. 2013;10(4):1202–30. doi: 10.3390/ijerph10041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tirado MC, Clarke R, Jaykus L, McQuatters-Gollop A, Frank JM. Climate change and food safety: A review. Food Res Int. 2010;43:1745–1765. [Google Scholar]
- 184.Ahern M, Kovats RS, Wilkinson P, Few R, Matthies F. Global health impacts of floods: epidemiologic evidence. Epidemiol Rev. 2005;27:36–46. doi: 10.1093/epirev/mxi004. [DOI] [PubMed] [Google Scholar]
- 185.Alderman K, Turner LR, Tong S. Floods and human health: a systematic review. Environ Int. 2012;47:37–47. doi: 10.1016/j.envint.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 186.Dorner SM, Anderson WB, Slawson RM, Kouwen N, Huck PM. Hydrologic modeling of pathogen fate and transport. Environ Sci Technol. 2006;40(15):4746–4753. doi: 10.1021/es060426z. [DOI] [PubMed] [Google Scholar]
- 187.Ferguson C, Husman AMD, Altavilla N, Deere D, Ashbolt N. Fate and transport of surface water pathogens in watersheds. Crit Rev Env Sci Tec. 2003;33(3):299–361. [Google Scholar]
- 188.Jofre J, Blanch AR, Lucena F. Water-Borne Infectious Disease Outbreaks Associated with Water Scarcity and Rainfall Events. In: Sabater S, Barcelo D, editors. Water Scarcity in the Mediterranean: Perspectives Under Global Change. 8. Springer-Verlag; Berlin: 2010. pp. 147–159. [Google Scholar]
- 189.Jean JS, Guo HR, Chen SH, Liu CC, Chang WT, Yang YJ, Huang MC. The association between rainfall rate and occurrence of an enterovirus epidemic due to a contaminated well. J Appl Microbiol. 2006;101(6):1224–31. doi: 10.1111/j.1365-2672.2006.03025.x. [DOI] [PubMed] [Google Scholar]
- 190.Moors E, Singh T, Siderius C, Balakrishnan S, Mishra A. Climate change and waterborne diarrhoea in northern India: Impacts and adaptation strategies. Sci Total Environ. 2013;468–469(Suppl):S139–51. doi: 10.1016/j.scitotenv.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 191.Liu C, Hofstra N, Franz E. Impacts of climate change on the microbial safety of pre-harvest leafy green vegetables as indicated by Escherichia coli O157 and Salmonella spp. Int J Food Microbiol. 2013;163(2–3):119–28. doi: 10.1016/j.ijfoodmicro.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 192.Hurst AM, Edwards MJ, Chipps M, Jefferson B, Parsons SA. The impact of rainstorm events on coagulation and clarifier performance in potable water treatment. Sci Total Environ. 2004;321(1–3):219–30. doi: 10.1016/j.scitotenv.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 193.Egorov AI, Naumova EN, Tereschenko AA, Kislitsin VA, Ford TE. Daily variations in effluent water turbidity and diarrhoeal illness in a Russian city. Int J Environ Health R. 2003;13(1):81–94. doi: 10.1080/0960312021000071567. [DOI] [PubMed] [Google Scholar]
- 194.Schwartz J, Levin R. Drinking water turbidity and health. Epidemiology. 1999;10(1):86–90. doi: 10.1097/00001648-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 195.Schwartz J, Levin R, Goldstein R. Drinking water turbidity and gastrointestinal illness in the elderly of Philadelphia. J Epidemiol Community Health. 2000;54(1):45–51. doi: 10.1136/jech.54.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schwartz J, Levin R, Hodge K. Drinking water turbidity and pediatric hospital use for gastrointestinal illness in Philadelphia. Epidemiology. 1997;8(6):615–20. doi: 10.1097/00001648-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 197.Tinker SC, Moe CL, Klein M, Flanders WD, Uber J, Amirtharajah A, Singer P, Tolbert PE. Drinking water turbidity and emergency department visits for gastrointestinal illness in Atlanta, 1993–2004. J Expo Sci Environ Epidemiol. 2010;20(1):19–28. doi: 10.1038/jes.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Moran P, Nhandara C, Hove I, Charimari L, Katito C, Bradley M, Williams MA. Contamination of traditional drinking water sources during a period of extreme drought in the Zvimba communal lands, Zimbabwe. Cent Afr J Med. 1997;43(11):316–21. [PubMed] [Google Scholar]
- 199.Shehane SD, Harwood VJ, Whitlock JE, Rose JB. The influence of rainfall on the incidence of microbial faecal indicators and the dominant sources of faecal pollution in a Florida river. J Appl Microbiol. 2005;98(5):1127–36. doi: 10.1111/j.1365-2672.2005.02554.x. [DOI] [PubMed] [Google Scholar]
- 200.Besner M, Prévost M, Regli S. Assessing the public health risk of microbial intrusion events in distribution systems: Conceptual model, available data, and challenges. Water Res. 2011;45:961–979. doi: 10.1016/j.watres.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 201.Hunter PR, Zmirou-Navier D, Hartemann P. Estimating the impact on health of poor reliability of drinking water interventions in developing countries. Sci Total Environ. 2009;407(8):2621–4. doi: 10.1016/j.scitotenv.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 202.En WL, Gan GL. Factors Associated with Use of Improved Water Sources and Sanitation among Rural Primary Schoolchildren in Pursat Province, Cambodia. Southeast Asian J Trop Med Public Health. 2011;42(4):1022–1031. [PubMed] [Google Scholar]
- 203.Pickering AJ, Davis J. Freshwater availability and water fetching distance affect child health in sub-Saharan Africa. Environ Sci Technol. 2012;46(4):2391–7. doi: 10.1021/es203177v. [DOI] [PubMed] [Google Scholar]
- 204.IPCC. Summary for policymakers. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL, editors. Climate Change 2014: Impacts, Adaptation, and Vulnerability. In Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2014. pp. 1–32. [Google Scholar]
- 205.Emch M, Yunus M, Escamilla V, Feldacker C, Ali M. Local population and regional environmental drivers of cholera in Bangladesh. Environ Health. 2010;9 doi: 10.1186/1476-069X-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hashizume M, Chaves LF, Faruque ASG, Yunus M, Streatfield K, Moji K. A Differential Effect of Indian Ocean Dipole and El Nino on Cholera Dynamics in Bangladesh. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hashizume M, Faruque ASG, Terao T, Yunus M, Streatfield K, Yamamoto T, Moji K. The indian ocean dipole and cholera incidence in Bangladesh: A time-series analysis. Environ Health Perspect. 2011;119(2):239–244. doi: 10.1289/ehp.1002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque ASG, Colwell R. Climate and infectious disease: Use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc Natl Acad Sci USA. 2000;97(4):1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cutler D, Miller G. The role of public health improvements in health advances: the twentieth-century United States. Demography. 2005;42(1):1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- 210.Patz JA, Olson SH, Uejio CK, Gibbs HK. Disease emergence from global climate and land use change. Medical Clinics of North America. 2008;92(6):1473–91. doi: 10.1016/j.mcna.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 211.Philipsborn R, Ahmed SM, Brosim BJ, Levy K. Climatic Drivers of Diarrheagenic Escherichia coli: A systematic review and meta-analysis. J Inf Dis. 2016 doi: 10.1093/infdis/jiw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Eisenberg JN, Cevallos W, Ponce K, Levy K, Bates SJ, Scott JC, Hubbard A, Vieira N, Endara P, Espinel M, Trueba G, Riley LW, Trostle J. Environmental change and infectious disease: how new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proc Natl Acad Sci USA. 2006;103(51):19460–5. doi: 10.1073/pnas.0609431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 214.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER Investigators MEN. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob Health. 2015;3(9):e564–75. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pruss-Ustun A, Bartram J, Clasen T, Colford JM, Jr, Cumming O, Curtis V, Bonjour S, Dangour AD, De France J, Fewtrell L, Freeman MC, Gordon B, Hunter PR, Johnston RB, Mathers C, Mausezahl D, Medlicott K, Neira M, Stocks M, Wolf J, Cairncross S. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop Medi Int Health. 2014;19(8):894–905. doi: 10.1111/tmi.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hodges M, Belle JH, Carlton EJ, Liang S, Li H, Luo W, Freeman MC, Liu Y, Gao Y, Hess JJ, Remais JV. Delays in reducing waterborne and water-related infectious diseases in China under climate change. Nat Clim Change. 2014;4:1109–1115. doi: 10.1038/nclimate2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Articles included in the systematic review of the relationship between ambient temperature and diarrheal diseases.
Table S2: Articles included in the systematic review of the relationship between heavy rainfall and diarrheal diseases; a) Systematically collected datasets, b) Outbreak reports.
Table S3: Articles included in the systematic review of the relationship between flooding and diarrheal diseases; a) Studies with an explicitly defined comparison group, b) Outbreak reports.
Table S4: Articles included in the systematic review of the relationship between drought and diarrheal diseases
Table S5: Summary of quantitative associations between diarrhea and drought, flooding, heavy rainfall and temperature reported in 67 articles with quantitative estimates.