Abstract
Background
Insulin-like growth factor-1(IGF-1) has a multitude of effects besides cell growth and metabolism. Reports also indicate anti-inflammatory and antioxidative effects. The concentrations of IGF-1 decrease with age and during inflammation. As selenium and coenzyme Q10 are involved in both the antioxidative defense and the inflammatory response, the present study aimed to examine the effects of supplementation with selenium and coenzyme Q10 on concentrations of IGF-1 and its binding protein IGFBP-1 in a population showing reduced cardiovascular mortality following such supplementation.
Methods
215 elderly individuals were included and given the intervention for four years. A clinical examination was performed and blood samples were taken at the start and after 48 months. Evaluations of IGF-1, the age adjusted IGF-1 SD score and IGFBP-1 were performed using group mean values, and repeated measures of variance.
Findings
After supplementation with selenium and coenzyme Q10, applying group mean evaluations, significantly higher IGF-1 and IGF-1 SD scores could be seen in the active treatment group, whereas a decrease in concentration could be seen of the same biomarkers in the placebo group.
Applying the repeated measures of variance evaluations, the same significant increase in concentrations of IGF-1 (F = 68; P>0.0001), IGF-1 SD score (F = 29; P<0.0001) and of IGFBP-1 (F = 6.88; P = 0.009) could be seen, indicating the effect of selenium and coenzyme Q10 also on the expression of IGF-1 as one of the mechanistic effects of the intervention.
Conclusion
Supplementation with selenium and coenzyme Q10 over four years resulted in increased levels of IGF-1 and the postprandial IGFBP-1, and an increase in the age-corrected IGF-1 SD score, compared with placebo. The effects could be part of the mechanistic explanation behind the surprisingly positive clinical effects on cardiovascular morbidity and mortality reported earlier. However, as the effects of IGF-1 are complex, more research on the result of intervention with selenium and coenzyme Q10 is needed.
Introduction
Circulating insulin-like growth factor-1 (IGF-1) is mainly produced in the liver as a response to stimulus from the growth hormone [1], but is also produced locally in different tissues in the body. It is a 70 amino acid polypeptide with a weight of 7.6 kDa [2], and there are six binding proteins that modulate the effects of IGF-1 [3]. It has anabolic effects in adults, with pleiotropic effects on both cell growth and metabolism. Of the multitude of effects described, it has been reported that overexpression of IGF-1 diminishes myocyte necrosis and apoptosis in myocardial infraction in a mouse model [4]. It has also been demonstrated that there is reduced cardiac myocyte atrophy as a result of ischemia in those with high levels of IGF-1 [5]. IGF-1 also has anti-inflammatory effects and decreases the concentration of pro-inflammatory cytokines [6, 7]. In the normal population there is a well-known decrease of the IGF-1 levels with increasing age [8]. Therefore,
In patients with ischemic heart disease, the amount of coronary calcium deposits has been reported to be inversely associated with the level of IGF-1 [9], and in several studies a low level of IGF-1 has been associated with increased risk of ischemic heart disease or myocardial infarction, as seen in both human and animal models [10–12]. In elderly men, however, Carlzon et al. demonstrated increased risk of cardiovascular events both in those with high and low IGF-1 levels [13].
Scharin Täng et al. reported interesting data from a mouse model indicating that a greater cardiac left ventricular volume and decreased cardiac function could be demonstrated in those with induced absence of IGF-1 [14]. In humans, Andreassen et al. found that low levels of IGF-1 could act as a predictor for heart failure progression and cardiovascular mortality [15]. This has also been demonstrated by others [16, 17].
Thus, there is a clear clinical association between decreased concentration of IGF-1 and disease also in the elderly, as can be seen in the literature [10, 15, 17–21]. As described in the literature, the IGF-binding protein-1 (IGFBP-1) is also associated with cardiovascular disease [17,18]. In previous studies we have shown that supplementation with selenium and coenzyme Q10 in an elderly healthy Swedish population reduced cardiovascular mortality, improved cardiac function [22], and decreased oxidative stress[23] and inflammation [24]. A possible mechanism behind these effects may be an influence on IGF concentrations. As our study population had a mean age of 78 years and the IGF concentration decreases with age [25], we hypothesized that those randomized to selenium and coenzyme Q10 would preserve their high IGF concentration, whereas the placebo group would have a decrease in IGF concentration.
The aim of this sub study was to investigate a possible influence of supplementation with selenium and coenzyme Q10 for four years on IGF-1 and IGFBP-1 in an elderly healthy Swedish population.
Material and methods
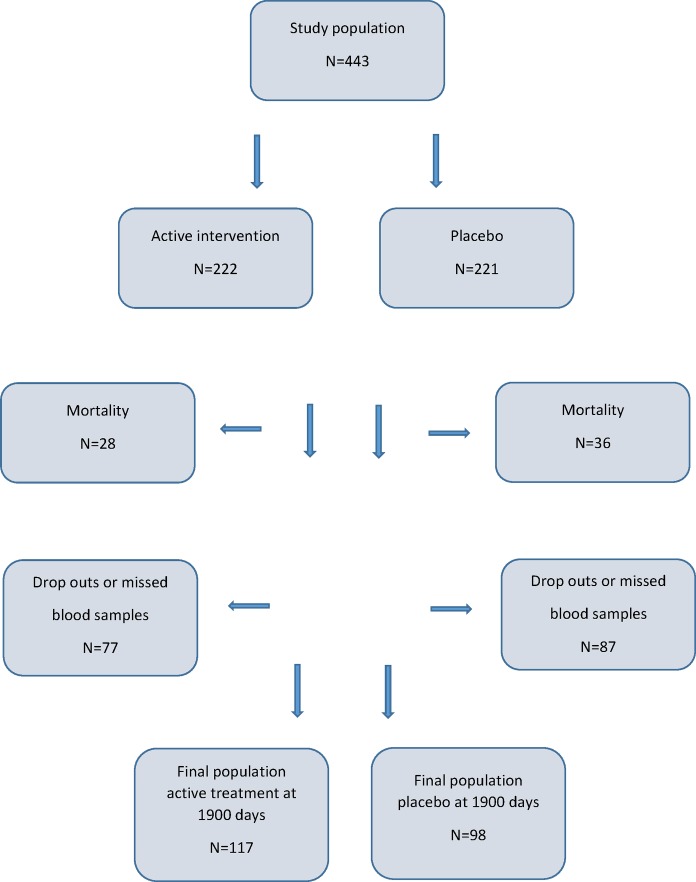
The design of the main study has been published elsewhere [22]. In brief, 443 elderly healthy participants living in a rural municipality in the south of Sweden were randomized to dietary supplementation of 200 mg/day of coenzyme Q10 capsules (Bio-Quinon 100 mg B.I.D, Pharma Nord, Vejle, Denmark) and 200 μg/day of organic selenium yeast tablets (SelenoPrecise 200 μg, Pharma Nord, Vejle, Denmark), or a similar placebo. The study supplementation was taken in addition to regular medication. The participants were examined by one of three experienced cardiologists. A new clinical history was recorded, and a clinical examination was performed, including resting blood pressure, assessment of New York Heart Association functional class (NYHA class) as well as ECG and Doppler- echocardiography. Echocardiographical examinations were performed with the participant in the left lateral position. The ejection fraction (EF) readings were categorized into four classes with interclass limits placed at 30%, 40% and 50% [26, 27]. Normal systolic function was defined as EF≥ 50%, while severely impaired systolic function was defined as EF< 30%.
As the intervention time was unusually long (48 months) only 221 participants completed the study, 64 died during the total intervention time, and 129 (29.1%) decided not to complete the study. The reasons for the latter have been presented in detail in the main publication, but the main reason was that there were too many tablets to take [22]. The first participant was included in January 2003, and the last participant concluded the study in February 2010. Out of the total study population, 215 participants were analyzed regarding IGF-1, the age-corrected values of IGF-1 (IGF-1 SD) based on the standard deviation of the mean value based on 247 healthy individuals [28, 29], and the IGF-binding protein 1 (IGFBP-1), which is presented in this report. Of these, 117 had previously been randomized to active treatment (supplementation with selenium and Q10) and 98 to placebo. Thirteen participants missed delivering the final blood samples for the IGF-1 evaluation.
Written, informed consent was obtained from all patients. The study was approved by the Regional Ethical Committee in Linköping, Sweden (Diary no. 03–176), and conforms to the ethical guidelines of the 1975 Declaration of Helsinki. This study was registered at Clinicaltrials.gov, and has the identifier NCT01443780
Biochemical analyses
All blood samples were obtained while the patients were at rest in a supine position. The blood samples were collected in plastic vials containing EDTA (ethylenediamine tetracetic acid). The vials were placed on ice before chilled centrifugation at 3000g, and then frozen at -70°C. No sample was thawed more than twice.
IGF-1 concentrations in plasma were determined by an in-house RIA after separation of the different IGFs from IGFBPs by acid ethanol extraction and cryoprecipitation. To minimize interference from the remaining IGFBPs, des (1–3) IGF-1 was used as a radioligand [30]. The intra- and inter-assay coefficients of variation (CV) were 4% and 11%, respectively. Plasma concentrations of IGF-1 are age-dependent, decreasing with age, thus IGF-1 values are also expressed as standard scores (SD scores) calculated from the regression of the values of two populations of healthy adult subjects [29, 31–34].
IGFBP-1 concentrations were determined by an in-house RIA using the method of Póvoa et al. [35]. The sensitivity of the RIA was 3 μg/L and the intra- and inter-assays CV were 3% and 10%, respectively.
Statistical methods
Descriptive data are presented as percentages or mean ± standard deviation (SD). A student’s unpaired two-sided t-test was used for continuous variables and the Chi-square test was used for analysis of one discrete variable. As the dataset demonstrated a slight non-Gaussian distribution, the dataset was transformed in order to obtain a normal distribution, which was controlled through Kolmogorov-Smirnov´s test. Transformed data were used in the t-test evaluations as this evaluation is more sensitive to a non-normal distribution of data. As the ANOVA algorithm can handle a slight non-Gaussian distribution, non-transformed data were applied in the repeated measures of variance evaluation.
Evaluation of the effects of treatment were based both on group mean values, but also on use of a repeated measures of variance analysis where the values of the individual participant were identified during the two different measured time points. P-values < 0.05 were considered significant, based on a two-sided evaluation. All data were analyzed using standard software (Statistica v. 13.2, Dell Inc, Tulsa, OK).
Results
Study population
The study population of this study consisted of 215 individuals, of which 117 had been given active supplementation consisting of selenium and coenzyme Q10 combined, and 98 individuals had been randomized to placebo (Table 1, Fig 1).
Table 1. Basal characteristics of the study population divided into active treatment and placebo, as compared to the total study population.
| Active treatment | P-value | Placebo | Total study population | |
|---|---|---|---|---|
| n | 117 | 98 | 443 | |
| Age years mean (SD) | 76.1 (3.1) | 0.96 | 76.2 (3.1) | 77.1 (3.5) |
| History | ||||
| Smokers n (%) | 8 (6.8) | 0.53 | 9 (7.7) | 41 (9.3) |
| Diabetes n (%) | 20 (17.1) | 0.96 | 17 (14.5) | 95 (21.4) |
| Hypertension n (%) | 81 (69.2) | 0.61 | 71 (72.4) | 326 (73.6) |
| IHD n (%) | 22 (18.8) | 0.64 | 16 (16.3) | 100 (22.6) |
| NYHA class I n (%) | 72 (61.5) | 0.61 | 57 (58.2) | 226 (51.0) |
| NYHA class II n (%) | 28 (23.9) | 0.27 | 30 (30.6) | 125 (28.2) |
| NYHA class III n (%) | 17 (14.5) | 0.34 | 10 (10.2) | 88 (19.9) |
| NYHA class IV n (%) | 0 | 0 | 0 | |
| Unclassified NYHA n (%) | 0 | 1 | 4 (1.0) | |
| Medications | ||||
| ACEI n (%) | 16 (13.7) | 0.90 | 14 (14.3) | 89 (20.1) |
| ARB n (%) | 3 (2.6) | 7 (7.1) | 23 (5.2) | |
| Betablockers n (%) | 42 (36.0) | 0.73 | 33 (33.7) | 153 (34.5) |
| Digitalis n (%) | 5 (4.3) | 1 (1.0) | 22 (5.0) | |
| Diuretics n (%) | 37 (31.6) | 0.87 | 33 (33.7) | 158 (35.7) |
| Statins n (%) | 27 (23.1) | 0.40 | 18 (18.4) | 96 (21.7) |
| Examinations | ||||
| EF<40% n (%) | 7 (6.0) | 0.53 | 4 (4.1) | 33 (7.4) |
Note: ARB; Angiotension receptor blockers; EF: Ejection fraction; IHD; Ischemic heart disease; NYHA: New York Heart Association functional class
Fig 1. CONSORT flow chart of the study.
Of the study population, 113 were females, and 102 were males. In the population, about 71% had been diagnosed with hypertension, about 18% had ischemic heart disease, 17% had diabetes, and 5% had an impaired systolic cardiac function defined as EF<40%. The different covariates were well balanced between the active versus the placebo group, and it could also be seen that the subgroup analyzed was representative of the original study population consisting of 443 individuals (Table 1).
At start of the study we were able to demonstrate a trend for higher IGF-1 levels in those already on treatment with ACE-inhibitors, or AII blockers (t = 1.83; P = 0.068), a result that could be expected to be significant if a larger study group had been included. This concurs with results from Maggio et al. including elderly patients on treatment with ACE-inhibitors [36].
Evaluations of the IGF-1, IGF-1 SD and IGFBP-1 levels have been performed in relation to basal serum selenium concentration, which varied from 21.4 μg/L Se to 158 μg/L Se, with a median of 67.1 μg/L Se. No significant associations could be found.
IGF-1 and intervention with selenium and coenzyme Q10
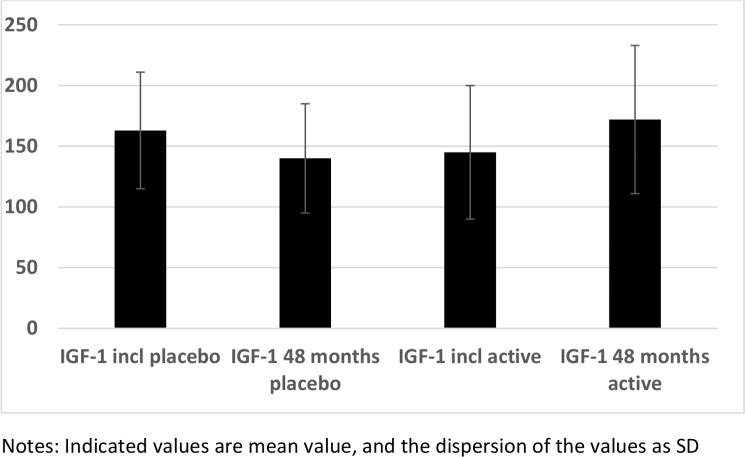
At the start of the intervention the serum levels of IGF-1 in the subgroups of those given selenium and coenzyme Q10 combined were not significantly different from values in those given the placebo (t = 1.82; P = 0.06) (Fig 2). However, at the end of the intervention period a significantly higher serum level of IGF-1 could be seen in the active treatment group compared with the placebo group (183 vs. 166 microgram/L; t = 5.78; P<0.0001). A significant increase in the serum IGF-1 was observed in the active treatment group (from 154 to 183 microgram/L; t = 4.38; P<0.0001), whereas a decrease was seen in the placebo group (166 vs. 144 microgram/L; t = 3.43; P = 0.0007).
Fig 2. IGF-1 in the active and the placebo groups at the inclusion compared to after 48 months.
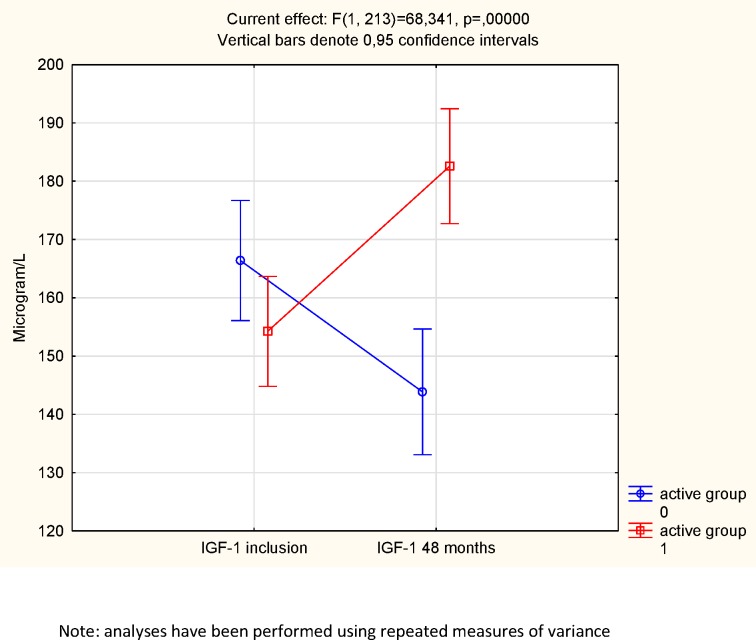
However, as group mean values do not necessarily express the individual change of levels, repeated measures of variance were also performed. Applying repeated measures of variance, using group (active and placebo) and follow-up (baseline and 48 months) a significant effect on serum levels of IGF-1 (i.e. group*follow-up interaction),F = 68; P<0.0001 could be seen, favoring those receiving selenium and coenzyme Q10. Thus, an increase in IGF-1 concentration in those receiving the active substance and a decrease in those given the placebo could be seen (Fig 3.)
Fig 3. IGF-1 concentration in the active treatment and the placebo groups at the start and at the end of the intervention.
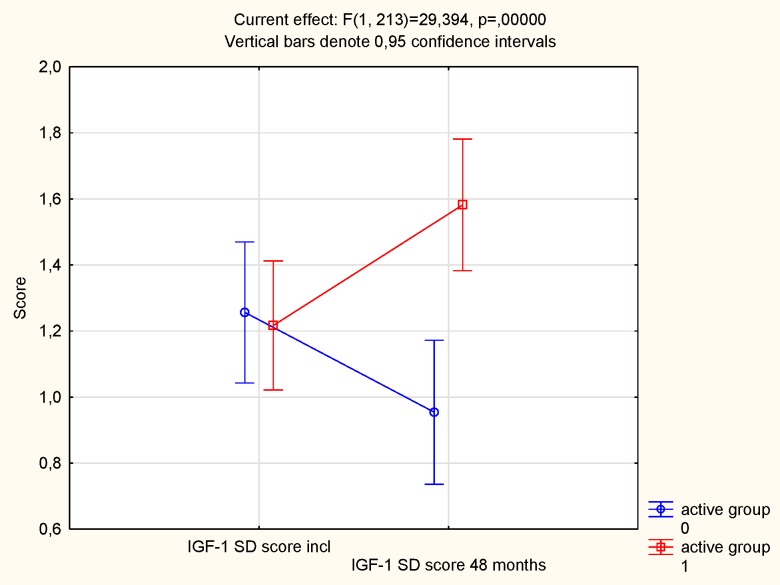
Applying an age-corrected score of IGF-1, the IGF-1 SD, the same result was obtained; at the start of the intervention no statistical difference in score between the active treatment group and the placebo group could be seen (t = 0.06; P = 0.96) (Fig 4). However, at the end of the intervention, a highly significant difference could be noted between the groups, with a higher score in the active treatment group (t = 3.11; P = 0.002). In the active treatment group, a tendency to increase in score was noted during the intervention (from 1.22 to 1.58; P = 0.05), whereas a non-significant decrease was noted in the placebo group (from 1.26 to 0.95; P = 0.16). Applying the repeated measure of variance methodology, as reported above, a highly significant effect on IGF-1 SD could be demonstrated (F = 29; P<0.0001)(Fig 5.).
Fig 4. IGF-1 SD score in the active and the placebo groups at inclusion compared to after 48 months.
Fig 5. IGF-1 SD score at inclusion on the active treatment and in the placebo groups at the start and at the end of the intervention.
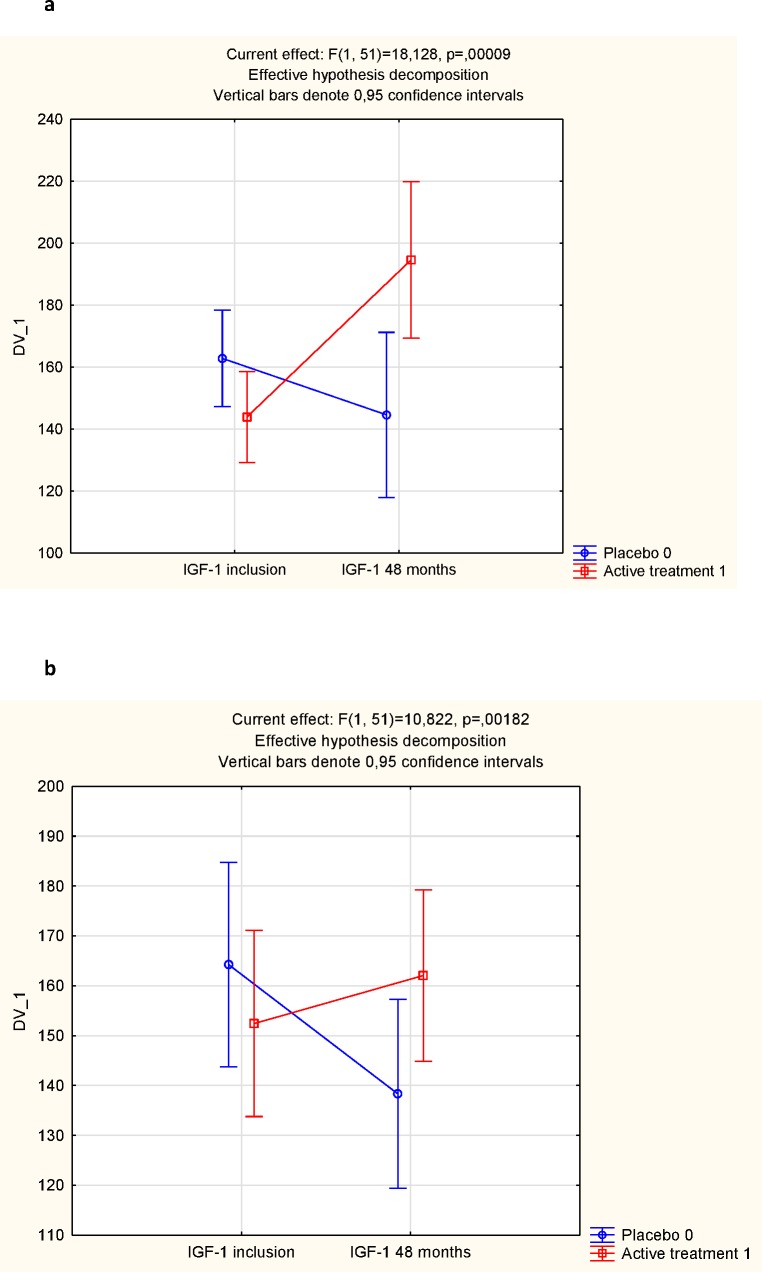
In order to evaluate the influence of the basal serum selenium concentration on the response to supplementation of selenium and coenzyme Q10 as seen in the concentration of IGF-1, we performed evaluations of the subgroup having the lowest quartile of basal selenium concentration, and compared the response of IGF-1 with those having the highest quartile (Fig 6A and 6B). It could be seen that the response to supplementation with selenium on IGF-1 concentration was significantly higher in those where there was a selenium deficiency compared with those with no, or little deficiency. This is in line with previous reports.
Fig 6. Response to IGF-1 of supplementation with selenium and coenzyme Q10 in those with a basal selenium concentration in the lowest quartile (Fig 6a), and in those with a basal selenium concentration in the highest quartile (Fig 6b).
Estimated active form of IGF-1
In the literature, the use of the ratio of IGF-1/IGFBP-1 as a measure of the active form of IGF-1 has been proposed [37]. A decline in this ratio could be reported in the placebo group during the intervention time (9.72 to 6.31; t = 2.37; P = 0.019), whereas in the active treatment group a trend towards a decline during the intervention time could be seen (10.13 to 8.97; t = 2.00; P = 0.05), and comparing the ratios of IGF-1/IGFBP-1 between the active and the placebo groups after the intervention a significant difference could be reported (8.97 versus 6.31; t = 2.18; P = 0.04).
IGF-binding protein-1 and intervention with selenium and coenzyme Q10
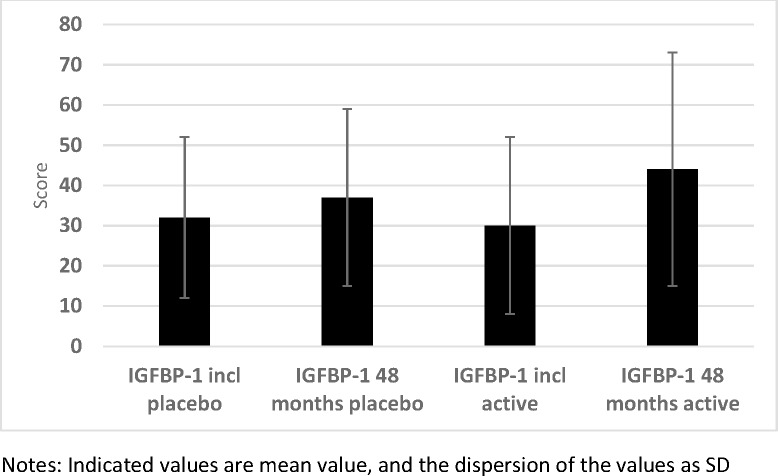
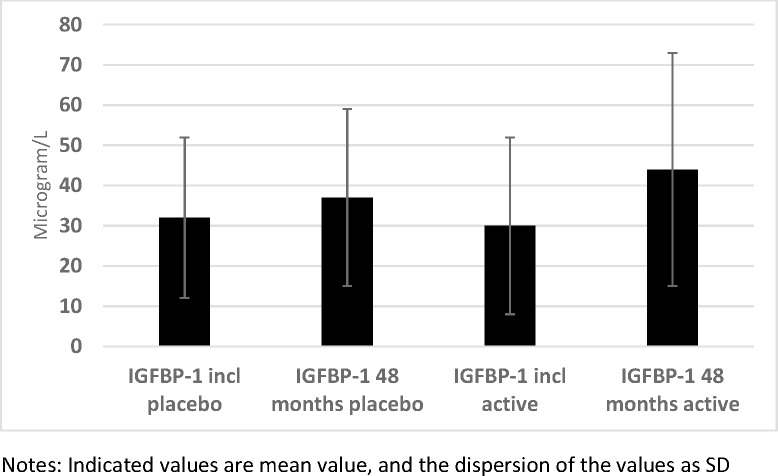
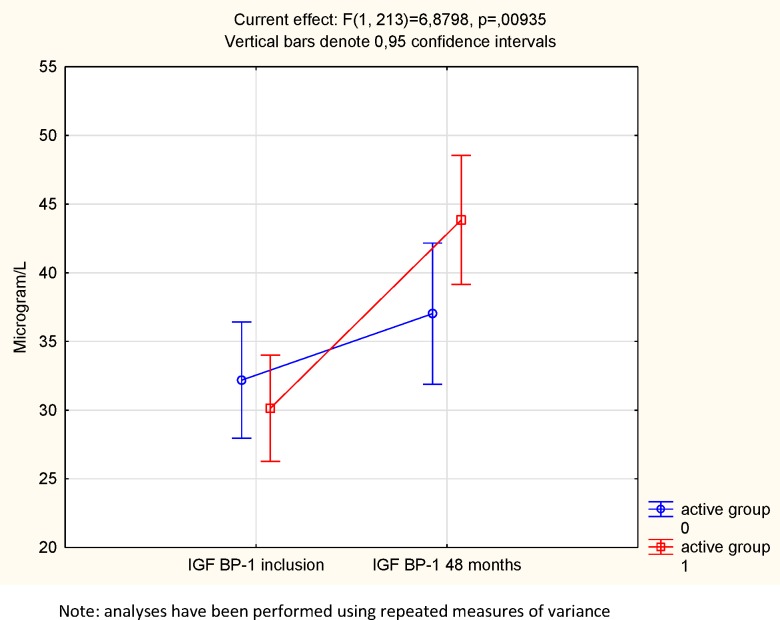
The plasma concentrations of one of the six IGF-binding proteins, IGFBP-1, were determined 2–3 hours after a meal. At the start of the intervention, no statistical difference in mean concentrations between the active treatment group, and the placebo group could be noted (30 vs. 32 microgram/L; P = 0.25) (Fig 7). At the end of the intervention, no significant difference could be noted (44 vs. 37 microgram/L; P = 0.45). However, applying the repeated measure of variance methodology, a significant difference could be demonstrated (F = 6.88; P = 0.009), with a higher increase in the IGFBP-1 concentration in the active group compared with the active treatment group (Fig 8).
Fig 7. IGFBP-1 in the active and the placebo groups at inclusion compared with after 48 months.
Fig 8. IGFBP-1 concentration in the active treatment and the placebo groups at the start and at the end of the intervention.
We also examined the participants having a concentration of IGFBP-1 in the highest quartile (Q4) and compared the number in the active treatment group with that of the placebo group. No difference in the numbers of participants in Q4 could be seen at the start of the intervention (24/98 versus 29/117: P = 0.96). However, after the intervention, compared to the placebo group a significantly higher number of participants could be seen in Q4 of IGFBP-1 from the active treatment group (16/98 versus 36/117; χ2 = 6.07; P = 0.014).
Discussion
In the present study we found raised concentrations of IGF-1 and IGF-binding protein-1 in the participants receiving the active intervention, upon the follow-up investigation of the population at the end of the study period. In contrast, we observed decreasing concentrations in those receiving the placebo, which was as expected in this group of aged Swedish individuals [8]. The increase in IGF-1in the supplemented group compared to the placebo group may be connected to the earlier reported decrease in both inflammatory activity and oxidative stress [6] possibly caused by supplemental selenium [38].
As the intervention time in our study was unusually long, four years, and the study population consisted of elderly individuals with a relatively low basal selenium status (41), we hypothesized that there would be a decrease in the levels of IGF-1 in the placebo group and preserved levels in those given active supplementation. In our post hoc analysis, using repeated measurement analyses and an age-corrected dataset, we found raised concentrations of IGF-1 and IGF-binding protein-1 resulting from the active intervention in contrast to the decreasing levels in those receiving the placebo.
In the literature, several pleiotropic effects of IGF-1 have been reported, including antiapoptotic effects [39], and antioxidative effects that could delay atherosclerosis [40, 41]. It has also been shown that IGF-1 increases the circulating number of endothelial progenitor cells that might correct the age-dependent deterioration of the cells [42]. The raised levels of IGF-1 resulting from the intervention, might decrease inflammation and oxidative stress, and promote formation of nitric oxide, and thus relax arterial smooth muscle cells, reduce platelet adhesion and decrease the level of oxidized LDL cholesterol [43–45]. Also, important information was presented by Lohr et al. showing an inverse relationship between IGF-1 and the inflammation biomarker hsCRP [46].
However, upon relating IGF-1, IGF-1 SD and IGFBP-1 levels at baseline to the basal levels of selenium, no significant correlations were seen. Here, it should be noted that the present population was relatively homogenous with respect to selenium status, with median serum selenium of 67.1 μg/L, all individual values being below a proposed optimum of 90 μg/L [47]. In contrast to our finding, Maggio et al. have shown an independent and positive association between selenium levels and IGF-1 in an elderly community-living population comparable to the population examined in the present study, but with a higher selenium status, mean 113 μg/L [48]. By using a mouse model and low doses of selenite, Ren et al. observed both an increased growth and expression of serum IGF-1R and antiapoptotic effects. With higher toxic doses of selenite, growth inhibition, decreased IGF-1R and apoptosis were seen. Similarly, low doses of selenite induced IGF-1R in osteoblasts in vitro whereas with high doses the opposite occurred [49]. Here, the positive effect of selenium might be mediated via an effect on the IGF-1R rather than changes in IGF-1 levels.
When expressing the free and active fraction of IGF-1 as the ratio IGF-1/IGFBP-1, we found that the intervention caused a significantly attenuated decline in the active form of IGF-1. IGFBP-1 was determined after a meal, and we expected its postprandial concentrations to be low due to suppressed expression following the insulin response [33, 50]. The higher IGFBP-1 levels seen in those treated with CoQ10 and selenium suggest a decreased insulin response to the meal due to improved insulin sensitivity. The increased IGF-1 levels induce improved insulin sensitivity in accordance with previous studies showing that antioxidants can improve insulin sensitivity [51, 52]. Our results indicating increased insulin sensitivity are interesting in relation to previous concerns about the possibility that selenium supplementation should cause an increased risk of diabetes [53]. In the present study population, plasma selenium remained well below the upper limit of the considered optimal zone (EFSA, 2014). Furthermore, no increased risk for diabetes (HR:1.09; 95% CI: 0.99–1.20) was reported in one of the biggest meta-analyses (n = 20.294) evaluating the risk for diabetes during selenium supplementation, and also including participants from the US, [54]. Moreover, improved insulin sensitivity may also reduce mortality in old people [55]. This supports the results presented above indicating that selenium and coenzyme Q10 have fundamental effects in the different areas where IGF-1 exerts its actions. In European populations with low basal selenium values our observations suggest that combined supplementation with selenium and coenzyme Q10 may evoke a multitude of effects, including increased IGF-1 and improved insulin sensitivity, which might be among the underlying mechanisms for the positive clinical health effects reported previously.
Clinical implications
The present subgroup analysis is one of the various efforts to better understand the mechanisms behind the positive clinical results, which include better health-related quality of life [56], better cardiac function[22], and reduced cardiovascular mortality [22] resulting from the intervention with selenium and coenzyme Q10 previously reported. Thus, the analysis provides important data for the clinician, at least in areas with a low basal selenium intake in the general population. Of particular interest here is that the intervention resulted in reduced cardiovascular mortality after 10 years, despite the intervention period lasting only fours [57]. We have also reported less inflammatory activity and less oxidative stress as a result of the intervention, as seen in the biomarkers sP-selectin and hs-CRP [24] and the biomarkers copeptin, and MR-proADM, respectively [23].
It is tempting to argue that in areas with low selenium content in the soil, or where residents have low daily intake of selenium, a moderate supplementation with selenium and coenzyme Q10 will have positive effects, including effects on the levels of IGF-1 in the elderly, as part of the total clinical response, reported above.
Limitations and strengths
This is a small post hoc study including elderly participants; thus it is not possible at present to extrapolate our results into other age strata. Also, the population was ethnically homogenous, so caution should be used when interpreting the results.
The strength of the report is that the study population was extensively evaluated, and the positive results regarding IGF-1 accord very well with the positive clinical and laboratory results reported earlier.
Conclusions
IGF-1 has important functions in both cell growth and metabolism, but also in inflammation and antioxidative defense. A decrease in IGF-1 has been reported with increased age, and also in advanced cardiovascular disease.
From our studies on an elderly healthy Swedish population, we have reported in previous publications that more than 90% had suboptimal selenium status [58]. The present evaluation shows that intervention with selenium and coenzyme Q10 resulted in increased levels of IGF-1, as well as in the age-corrected value of IGF-1, and also in the postprandial IGF-binding protein-1 indicative of improved insulin sensitivity. These findings may represent important information on the mechanisms behind the positive clinical effects on cardiovascular morbidity and mortality of supplementation with selenium and coenzyme Q10.
Supporting information
(DOCX)
Data Availability
Under Swedish Law, the authors cannot share the data underlying this study and cannot do any further research than what is specified in the ethical permissions application. For inquires on the data, researchers should first reach out to the owner of the database, the University of Linköping. Please reach out to the corresponding author with requests and for assistance with data requests. If the university approves the request, researchers can submit an application to the Regional Ethical Review Board for the specific research question that the researcher wants to examine.
Funding Statement
Part of the analysis costs was supported by grants from Family Erling-Persson Foundation, Medical Research Council, Pharma Nord Aps, Denmark, the County Council of Östergötland, Linköping University. The selenium tablets and coenzyme Q10 capsules were kindly donated by Pharma Nord Aps. The funding organizations had no role in the design, management, analysis, interpretation of the data, preparation, review or approval of the manuscript.
References
- 1.Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96(12):7088–92. ; PubMed Central PMCID: PMCPMC22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141(12):4436–41. doi: 10.1210/endo.141.12.7825 . [DOI] [PubMed] [Google Scholar]
- 3.Kelley KM, Oh Y, Gargosky SE, Gucev Z, Matsumoto T, Hwa V, et al. Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int J Biochem Cell Biol. 1996;28(6):619–37. . [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100(8):1991–9. doi: 10.1172/JCI119730 ; PubMed Central PMCID: PMCPMC508388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulze PC, Fang J, Kassik KA, Gannon J, Cupesi M, MacGillivray C, et al. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Circ Res. 2005;97(5):418–26. doi: 10.1161/01.RES.0000179580.72375.c2 . [DOI] [PubMed] [Google Scholar]
- 6.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2684–90. doi: 10.1161/ATVBAHA.107.156257 . [DOI] [PubMed] [Google Scholar]
- 7.Succurro E, Andreozzi F, Sciacqua A, Hribal ML, Perticone F, Sesti G. Reciprocal association of plasma IGF-1 and interleukin-6 levels with cardiometabolic risk factors in nondiabetic subjects. Diabetes Care. 2008;31(9):1886–8. doi: 10.2337/dc08-0553 ; PubMed Central PMCID: PMCPMC2518365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67(5):1361–9. ; PubMed Central PMCID: PMCPMC370702. doi: 10.1172/JCI110164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannavo S, Marini F, Curto L, Torre ML, de Gregorio C, Salamone I, et al. High prevalence of coronary calcifications and increased risk for coronary heart disease in adults with growth hormone deficiency. J Endocrinol Invest. 2011;34(1):32–7. doi: 10.3275/7076 . [DOI] [PubMed] [Google Scholar]
- 10.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106(8):939–44. . [DOI] [PubMed] [Google Scholar]
- 11.Dobrucki LW, Tsutsumi Y, Kalinowski L, Dean J, Gavin M, Sen S, et al. Analysis of angiogenesis induced by local IGF-1 expression after myocardial infarction using microSPECT-CT imaging. J Mol Cell Cardiol. 2010;48(6):1071–9. doi: 10.1016/j.yjmcc.2009.10.008 ; PubMed Central PMCID: PMCPMC2866767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schutte AE, Conti E, Mels CM, Smith W, Kruger R, Botha S, et al. Attenuated IGF-1 predicts all-cause and cardiovascular mortality in a Black population: A five-year prospective study. Eur J Prev Cardiol. 2016;23(16):1690–9. doi: 10.1177/2047487316661436 . [DOI] [PubMed] [Google Scholar]
- 13.Carlzon D, Svensson J, Petzold M, Karlsson MK, Ljunggren O, Tivesten A, et al. Both low and high serum IGF-1 levels associate with increased risk of cardiovascular events in elderly men. J Clin Endocrinol Metab. 2014;99(11):E2308–16. doi: 10.1210/jc.2014-1575 ; PubMed Central PMCID: PMCPMC4258605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scharin Tang M, Redfors B, Lindbom M, Svensson J, Ramunddal T, Ohlsson C, et al. Importance of circulating IGF-1 for normal cardiac morphology, function and post infarction remodeling. Growth Horm IGF Res. 2012;22(6):206–11. doi: 10.1016/j.ghir.2012.09.002 . [DOI] [PubMed] [Google Scholar]
- 15.Andreassen M, Kistorp C, Raymond I, Hildebrandt P, Gustafsson F, Kristensen LO, et al. Plasma insulin-like growth factor I as predictor of progression and all cause mortality in chronic heart failure. Growth Horm IGF Res. 2009;19(6):486–90. doi: 10.1016/j.ghir.2009.03.003 . [DOI] [PubMed] [Google Scholar]
- 16.Arcopinto M, Isgaard J, Marra AM, Formisano P, Bossone E, Vriz O, et al. IGF-1 predicts survival in chronic heart failure. Insights from the T.O.S.CA. (Trattamento Ormonale Nello Scompenso CArdiaco) registry. Int J Cardiol. 2014;176(3):1006–8. doi: 10.1016/j.ijcard.2014.07.003 . [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S, Tamura T, Ono K, Horiuchi H, Kimura T, Kita T, et al. Insulin-like growth factor axis (insulin-like growth factor-I/insulin-like growth factor-binding protein-3) as a prognostic predictor of heart failure: association with adiponectin. Eur J Heart Fail. 2010;12(11):1214–22. doi: 10.1093/eurjhf/hfq166 . [DOI] [PubMed] [Google Scholar]
- 18.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89(1):114–20. doi: 10.1210/jc.2003-030967 . [DOI] [PubMed] [Google Scholar]
- 19.Maggio M, Cattabiani C, Lauretani F, Bandinelli S, De Vita F, Dall'Aglio E, et al. Insulin-like growth factor-1 bioactivity plays a prosurvival role in older participants. J Gerontol A Biol Sci Med Sci. 2013;68(11):1342–50. doi: 10.1093/gerona/glt045 ; PubMed Central PMCID: PMCPMC3805296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117(5):312–7. doi: 10.1016/j.amjmed.2004.02.049 . [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi H, Komamura K, Choraku M, Hirono A, Takamori N, Tamura K, et al. Impact of serum insulin-like growth factor-1 on early prognosis in acute myocardial infarction. Intern Med. 2008;47(9):819–25. . [DOI] [PubMed] [Google Scholar]
- 22.Alehagen U, Johansson P, Bjornstedt M, Rosen A, Dahlstrom U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol. 2013;167(5):1860–6. doi: 10.1016/j.ijcard.2012.04.156 . [DOI] [PubMed] [Google Scholar]
- 23.Alehagen U, Aaseth J, Johansson P. Less increase of copeptin and MR-proADM due to intervention with selenium and coenzyme Q10 combined: Results from a 4-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Biofactors. 2015;41(6):443–52. doi: 10.1002/biof.1245 . [DOI] [PubMed] [Google Scholar]
- 24.Alehagen U, Lindahl TL, Aaseth J, Svensson E, Johansson P. Levels of sP-selectin and hs-CRP Decrease with Dietary Intervention with Selenium and Coenzyme Q10 Combined: A Secondary Analysis of a Randomized Clinical Trial. PLoS One. 2015;10(9):e0137680 doi: 10.1371/journal.pone.0137680 ; PubMed Central PMCID: PMCPMC4574282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Copeland KC, Colletti RB, Devlin JT, McAuliffe TL. The relationship between insulin-like growth factor-I, adiposity, and aging. Metabolism. 1990;39(6):584–7. . [DOI] [PubMed] [Google Scholar]
- 26.Jensen-Urstad K, Bouvier F, Hojer J, Ruiz H, Hulting J, Samad B, et al. Comparison of different echocardiographic methods with radionuclide imaging for measuring left ventricular ejection fraction during acute myocardial infarction treated by thrombolytic therapy. Am J Cardiol. 1998;81(5):538–44. . [DOI] [PubMed] [Google Scholar]
- 27.van Royen N, Jaffe CC, Krumholz HM, Johnson KM, Lynch PJ, Natale D, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77(10):843–50. . [DOI] [PubMed] [Google Scholar]
- 28.Hilding A, Brismar K, Degerblad M, Thoren M, Hall K. Altered relation between circulating levels of insulin-like growth factor-binding protein-1 and insulin in growth hormone-deficient patients and insulin-dependent diabetic patients compared to that in healthy subjects. J Clin Endocrinol Metab. 1995;80(9):2646–52. doi: 10.1210/jcem.80.9.7545695 . [DOI] [PubMed] [Google Scholar]
- 29.Unden AL, Elofsson S, Knox S, Lewitt MS, Brismar K. IGF-I in a normal population: relation to psychosocial factors. Clin Endocrinol (Oxf). 2002;57(6):793–803. . [DOI] [PubMed] [Google Scholar]
- 30.Bang P, Eriksson U, Sara V, Wivall IL, Hall K. Comparison of acid ethanol extraction and acid gel filtration prior to IGF-I and IGF-II radioimmunoassays: improvement of determinations in acid ethanol extracts by the use of truncated IGF-I as radioligand. Acta Endocrinol (Copenh). 1991;124(6):620–9. . [DOI] [PubMed] [Google Scholar]
- 31.Soderberg S, Ahren B, Eliasson M, Dinesen B, Brismar K, Olsson T. Circulating IGF binding protein-1 is inversely associated with leptin in non-obese men and obese postmenopausal women. Eur J Endocrinol. 2001;144(3):283–90. . [DOI] [PubMed] [Google Scholar]
- 32.Unden AL, Elofsson S, Brismar K. Gender differences in the relation of insulin-like growth factor binding protein-1 to cardiovascular risk factors: a population-based study. Clin Endocrinol (Oxf). 2005;63(1):94–102. doi: 10.1111/j.1365-2265.2005.02306.x . [DOI] [PubMed] [Google Scholar]
- 33.Wolk K, Larsson SC, Vessby B, Wolk A, Brismar K. Metabolic, anthropometric, and nutritional factors as predictors of circulating insulin-like growth factor binding protein-1 levels in middle-aged and elderly men. J Clin Endocrinol Metab. 2004;89(4):1879–84. doi: 10.1210/jc.2003-031349 . [DOI] [PubMed] [Google Scholar]
- 34.Hilding A, Hall K, Wivall-Helleryd IL, Saaf M, Melin AL, Thoren M. Serum levels of insulin-like growth factor I in 152 patients with growth hormone deficiency, aged 19–82 years, in relation to those in healthy subjects. J Clin Endocrinol Metab. 1999;84(6):2013–9. doi: 10.1210/jcem.84.6.5793 . [DOI] [PubMed] [Google Scholar]
- 35.Povoa G, Roovete A, Hall K. Cross-reaction of serum somatomedin-binding protein in a radioimmunoassay developed for somatomedin-binding protein isolated from human amniotic fluid. Acta Endocrinol (Copenh). 1984;107(4):563–70. . [DOI] [PubMed] [Google Scholar]
- 36.Maggio M, Ceda GP, Lauretani F, Pahor M, Bandinelli S, Najjar SS, et al. Relation of angiotensin-converting enzyme inhibitor treatment to insulin-like growth factor-1 serum levels in subjects >65 years of age (the InCHIANTI study). Am J Cardiol. 2006;97(10):1525–9. doi: 10.1016/j.amjcard.2005.11.089 ; PubMed Central PMCID: PMCPMC2646084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boquist S, Ruotolo G, Skoglund-Andersson C, Tang R, Bjorkegren J, Bond MG, et al. Correlation of serum IGF-I and IGFBP-1 and -3 to cardiovascular risk indicators and early carotid atherosclerosis in healthy middle-aged men. Clin Endocrinol (Oxf). 2008;68(1):51–8. doi: 10.1111/j.1365-2265.2007.02998.x . [DOI] [PubMed] [Google Scholar]
- 38.Maggio M, De Vita F, Lauretani F, Butto V, Bondi G, Cattabiani C, et al. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients. 2013;5(10):4184–205. doi: 10.3390/nu5104184 ; PubMed Central PMCID: PMCPMC3820068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnqvist HJ. The role of IGF-system in vascular insulin resistance. Horm Metab Res. 2008;40(9):588–92. doi: 10.1055/s-0028-1082325 . [DOI] [PubMed] [Google Scholar]
- 40.Payne JA, Reckelhoff JF, Khalil RA. Role of oxidative stress in age-related reduction of NO-cGMP-mediated vascular relaxation in SHR. Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R542–51. doi: 10.1152/ajpregu.00056.2003 . [DOI] [PubMed] [Google Scholar]
- 41.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8(3–4):572–81. doi: 10.1089/ars.2006.8.572 . [DOI] [PubMed] [Google Scholar]
- 42.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100(3):434–43. doi: 10.1161/01.RES.0000257912.78915.af . [DOI] [PubMed] [Google Scholar]
- 43.Tsukahara H, Gordienko DV, Tonshoff B, Gelato MC, Goligorsky MS. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994;45(2):598–604. . [DOI] [PubMed] [Google Scholar]
- 44.Hasdai D, Rizza RA, Holmes DR, Jr., Richardson DM, Cohen P, Lerman A. Insulin and insulin-like growth factor-I cause coronary vasorelaxation in vitro. Hypertension. 1998;32(2):228–34. . [DOI] [PubMed] [Google Scholar]
- 45.Napoli R, Guardasole V, Matarazzo M, Palmieri EA, Oliviero U, Fazio S, et al. Growth hormone corrects vascular dysfunction in patients with chronic heart failure. J Am Coll Cardiol. 2002;39(1):90–5. . [DOI] [PubMed] [Google Scholar]
- 46.Lohr J, Grotevendt A, Nauck M, Volzke H, Wallaschofski H, Friedrich N. Relation of insulin-like growth factor-I and IGF binding protein 3 with markers of inflammation: results of a population-based study. Clin Endocrinol (Oxf). 2014;80(1):148–54. doi: 10.1111/cen.12241 . [DOI] [PubMed] [Google Scholar]
- 47.Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, et al. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr. 2010;92(3):525–31. Epub 2010/06/25. doi: 10.3945/ajcn.2010.29642 ; PubMed Central PMCID: PMC2921536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maggio M, Ceda GP, Lauretani F, Bandinelli S, Dall'Aglio E, Guralnik JM, et al. Association of plasma selenium concentrations with total IGF-1 among older community-dwelling adults: the InCHIANTI study. Clin Nutr. 2010;29(5):674–7. doi: 10.1016/j.clnu.2010.03.012 ; PubMed Central PMCID: PMCPMC3963695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren G, Ali T, Chen W, Han D, Zhang L, Gu X, et al. The role of selenium in insulin-like growth factor I receptor (IGF-IR) expression and regulation of apoptosis in mouse osteoblasts. Chemosphere. 2016;144:2158–64. doi: 10.1016/j.chemosphere.2015.11.003 . [DOI] [PubMed] [Google Scholar]
- 50.Conover CA, Lee PD, Kanaley JA, Clarkson JT, Jensen MD. Insulin regulation of insulin-like growth factor binding protein-1 in obese and nonobese humans. J Clin Endocrinol Metab. 1992;74(6):1355–60. doi: 10.1210/jcem.74.6.1375600 . [DOI] [PubMed] [Google Scholar]
- 51.Karimi P, Farhangi MA, Sarmadi B, Gargari BP, Zare Javid A, Pouraghaei M, et al. The Therapeutic Potential of Resistant Starch in Modulation of Insulin Resistance, Endotoxemia, Oxidative Stress and Antioxidant Biomarkers in Women with Type 2 Diabetes: A Randomized Controlled Clinical Trial. Ann Nutr Metab. 2016;68(2):85–93. doi: 10.1159/000441683 . [DOI] [PubMed] [Google Scholar]
- 52.Castro MC, Massa ML, Arbelaez LG, Schinella G, Gagliardino JJ, Francini F. Fructose-induced inflammation, insulin resistance and oxidative stress: A liver pathological triad effectively disrupted by lipoic acid. Life Sci. 2015;137:1–6. doi: 10.1016/j.lfs.2015.07.010 . [DOI] [PubMed] [Google Scholar]
- 53.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect. 2009;117(9):1409–13. doi: 10.1289/ehp.0900704 ; PubMed Central PMCID: PMCPMC2737018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao S, Zhang A, Huang S. Selenium supplementation and the risk of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Endocrine. 2014;47(3):758–63. doi: 10.1007/s12020-014-0298-7 . [DOI] [PubMed] [Google Scholar]
- 55.Schernthaner G, Schernthaner-Reiter MH, Schernthaner GH. EMPA-REG and Other Cardiovascular Outcome Trials of Glucose-lowering Agents: Implications for Future Treatment Strategies in Type 2 Diabetes Mellitus. Clin Ther. 2016;38(6):1288–98. doi: 10.1016/j.clinthera.2016.04.037 . [DOI] [PubMed] [Google Scholar]
- 56.Johansson P, Dahlstrom O, Dahlstrom U, Alehagen U. Improved Health-Related Quality of Life, and More Days out of Hospital with Supplementation with Selenium and Coenzyme Q10 Combined. Results from a Double Blind, Placebo-Controlled Prospective Study. J Nutr Health Aging. 2015;19(9):870–7. doi: 10.1007/s12603-015-0509-9 . [DOI] [PubMed] [Google Scholar]
- 57.Alehagen U, Aaseth J, Johansson P. Reduced Cardiovascular Mortality 10 Years after Supplementation with Selenium and Coenzyme Q10 for Four Years: Follow-Up Results of a Prospective Randomized Double-Blind Placebo-Controlled Trial in Elderly Citizens. PLoS One. 2015;10(12):e0141641 doi: 10.1371/journal.pone.0141641 ; PubMed Central PMCID: PMCPMC4666408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alehagen U, Alexander J, Aaseth J. Supplementation with Selenium and Coenzyme Q10 Reduces Cardiovascular Mortality in Elderly with Low Selenium Status. A Secondary Analysis of a Randomised Clinical Trial. PLoS One. 2016;11(7):e0157541 doi: 10.1371/journal.pone.0157541 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Under Swedish Law, the authors cannot share the data underlying this study and cannot do any further research than what is specified in the ethical permissions application. For inquires on the data, researchers should first reach out to the owner of the database, the University of Linköping. Please reach out to the corresponding author with requests and for assistance with data requests. If the university approves the request, researchers can submit an application to the Regional Ethical Review Board for the specific research question that the researcher wants to examine.