A closer look using approach-based meta-analysis shows conflicting evidence of the causal and associative role of CA in CP.
Abstract
CONTEXT:
Chorioamnionitis (CA) has often been linked etiologically to cerebral palsy (CP).
OBJECTIVES:
To differentiate association from risk of CA in the development of CP.
DATA SOURCES:
PubMed, Cochrane Library, Embase, and bibliographies of original studies were searched by using the keywords (chorioamnionitis) AND ((cerebral palsy) OR brain).
STUDY SELECTION:
Included studies had to have: (1) controls, (2) criteria for diagnoses, and (3) neurologic follow-up. Studies were categorized based on: (1) finding incidence of CP in a CA population, or risk of CP; and (2) incidence of CA in CP or association with CP.
DATA EXTRACTION:
Two reviewers independently verified study inclusion and extracted data.
RESULTS:
Seventeen studies (125 256 CA patients and 5 994 722 controls) reported CP in CA. There was significantly increased CP inpreterm histologic chorioamnionitis (HCA; risk ratio [RR] = 1.34, P < .01), but not in clinical CA (CCA). Twenty-two studies (2513 CP patients and 8135 controls) reported CA in CP. There was increased CCA (RR = 1.43, P < .01), but no increase in HCA in preterm CP. Increased HCA was found (RR = 4.26, P < .05), as well as CCA in term/near-term CP (RR = 3.06, P < .01).
CONCLUSIONS:
The evidence for a causal or associative role of CA in CP is weak. Preterm HCA may be a risk factor for CP, whereas CCA is not. An association with term and preterm CP was found for CCA, but only with term CP for HCA.
Cerebral palsy (CP) is the most common motor disability in childhood,1 with a reported prevalence of 1.5 to 4 per 1000 live births.2,3 The lifetime cost for all patients with CP is estimated to be $11.5 billion.4 The etiology of CP is complex and multifactorial. One of the key pathogenetic mechanisms is perinatal inflammation. Chorioamnionitis (CA) is a common manifestation of perinatal inflammation and is treated aggressively in the peripartum period. For this article, we have used CA interchangeably with the new nomenclature of “intrauterine inflammation or infection or both” (triple-I).5
As a potentially severe condition in pregnancy, clinical CA (CCA) is a syndrome often diagnosed by the presence of maternal fever (temperature >37.8°C) accompanied by ≥2 of the following criteria: (1) uterine tenderness; (2) malodorous vaginal discharge; (3) fetal tachycardia (heart rate >160 beats per minute); (4) maternal tachycardia (heart rate >100 beats per minute); and (5) maternal leukocytosis (leukocyte count >15 000 cells/mm3).6,7 On the other hand, histologic CA (HCA) is a pathologic diagnosis requiring acute morphologic criteria of diffuse infiltration of neutrophils into the chorioamniotic membranes. Although HCA is generally considered to represent the presence of intraamniotic infection, acute HCA can occur with “sterile intraamniotic inflammation” in the absence of detectable microorganisms.8
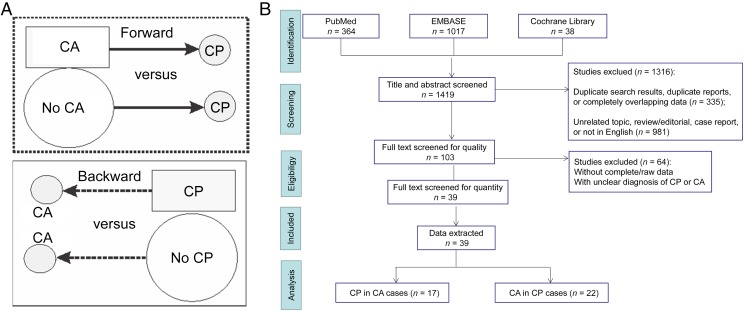
It has long been held that CA is among the key risk factors of CP, and there have been several meta-analyses and systematic reviews showing either CCA or HCA, or both, as risk factors for CP.9–12 Some have grouped preterm and term gestation cases together for analysis.9,11 Part of the confusion for clinicians is that some of the included studies restricted the investigation to culture results,13 or made no mention of CP in CA cases or controls.14 Confusion also arises from an absence of criteria for the diagnosis of CA,15,16 the imprecise categorization of patients into CCA and HCA, and combined analysis in different patient cohorts.10 Previous reviews and meta-analyses have treated studies employing forward and backward approaches with the same weight, namely, determining the rate of CP in patients with CA (forward) as similar to studies describing how many CP patients had a past history of CA (backward).9–11 Note that the terms “forward” and “backward” are not equivalent to “prospective” and “retrospective,” respectively, because one could use a forward approach in a retrospective cohort study, and a prospective study of HCA would be hard to conduct because of the intrinsic delayed nature of diagnosis. In the forward approach, the rate of CP is compared in a cohort of CA with patients without a diagnosis of CA (Fig 1A). In the backward approach, the rate of CA is compared in a cohort of CP with patients without CP (Fig 1A). In addition to taking into consideration that an updated analysis is required (the previous meta-analyses were some years ago9–11), this review also focused on the quality of each enrolled study and whether the studies had ruled out defined postnatal etiologic factors (such as child abuse or other injuries and infections that occurred after birth) that contribute to CP, herein labeled as “postnatal causes.” This review is aimed at the clinician who would like to know the risk of CP in CA patients separately from the association of CA in CP patients and to determine the risk with categorization of CA as CCA or HCA and gestational age. We found that studies of preterm infants using forward approaches demonstrated that HCA is a risk factor for CP, and the association was also found in studies of near-term/term infants that used a backward approach. No evidence of CCA as a risk factor for CP was borne out from studies using a forward approach, but an association was found in studies of both preterm and term infants using a backward approach.
FIGURE 1.
A, Comparison between forward (dotted rectangle) and backward approaches (solid rectangle). B, Flow diagram of the current study (based on the PRISMA 2009 flow diagram).
Methods
Sources
We searched PubMed, the Cochrane Library, and Embase for relevant case-control or cohort studies to evaluate the relationship between CP and CA. Queries included articles published from January 1, 1960 to September 30, 2016 in peer-reviewed publications (including abstracts). Keywords used were (chorioamnionitis) AND ((cerebral palsy) OR (brain)). We also hand-searched bibliographies of original studies, reviews (including meta-analyses), and relevant conference abstracts and contacted some investigators directly. The last search was conducted on October 8, 2016. No review protocol exists.
Study Selection
Using the methods of Meta-analysis of Observational Studies in Epidemiology (MOOSE)17 and PRISMA, 2 authors (L.M. and Z.S.) independently selected relevant studies, assessed study quality by means of quality assessment of case-control studies18 or observational cohort and cross-sectional studies,19 and extracted data. Questionable studies were confirmed by discussion with a third author (S.T.). Included studies met all of the following criteria: (1) clinical cohort (with a control group) or case-control study investigating the relationship between CA and CP; (2) an explanation of diagnosis and outcomes; (3) had neurologic follow-up information especially for CP; and (4) a quality of good or fair according to published criteria.18,19 Studies were excluded from consideration if: (1) they did not show all of the above information; (2) had no patient numbers available; and (3) were not written in English.
Data Extraction
We extracted data about study design and methods, inclusion and exclusion criteria, patient (mother, fetus, or newborn) characteristics, treatments (steroids, antibiotics, tocolytics, mechanical ventilation, NICU admission, etc), patient outcomes, and follow-up information. For primary outcomes, we estimated the relationship between CA and CP in term and preterm infants. Statistical analysis was conducted by using Review Manager version 5.0 software (Cochrane Collaboration). The Mantel-Haenszel model was used for dichotomous data. The pooled risk ratios (RRs) and 95% confidence intervals (CIs) were determined by either a fixed-effects or random-effects model, depending on which was most conservative. We used a forest plot to illustrate the relative strength of treatment effects in multiple quantitative scientific studies addressing the same question.20 Statistical between-study heterogeneity was assessed by I2 test21 and χ2 test. Publication bias was assessed by funnel plot.22,23 An asymmetric funnel indicated a relationship between treatment effect and study size, suggesting the possibility of either publication bias or a systematic difference between smaller and larger studies (“small study effects”) or the use of an inappropriate effect measure. Differences between groups were assessed on the basis of the χ2 statistic. For all tests done, statistical significance was achieved if the 2-tailed P value was <.05 (for overall effect of CP or CA conditions, or for the heterogeneity test).24
Results
Based on our search strategy, 1419 studies were identified. After screening with preset inclusion and exclusion criteria, there were 17 studies analyzing the incidence of CP in the CA population (125 256 CA patients and 5 994 722 controls)25–41 and 22 studies analyzing the incidence of CA in the CP population (2513 CP patients and 8135 controls)42–63 (flow diagram in Fig 1B; characteristic information in Tables 1 and 2).
TABLE 1.
Characteristics of Enrolled Studies Using a Forward Approach (CP in CA Cases)
| Studies Enrolled | Country | Study Period | wk/g | CP/CA, n/N | Mortality Before Discharge, n/N | Singleton/Twin | CP Diagnosed Age, y | Study Type by Original Authors | Study Quality | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| CCA preterm | ||||||||||
| Bashir et al25 2016 | Canada | 1995–2007 | <1250 g | 23/130: 37/437 | NA | NA | 3 | Retrospective | Good | Bronchopulmonary dysplasia cohort |
| Mendez-Figueroa et al26 2015 | United States | 1997–2004 | 23–36 | 9/220: 78/2336 | 16/220: 99/2336 | Both | 2 | Second RCT | Good | Treated with antenatal steroid or MgSO4 |
| Nasef et al27 2013-1 | Canada | 2007–2008 | <30 | 2/23: 9/96 | 4/33: 25/146 | NA | 1.5 | Retrospective cohort | Good | HCA + CCA included |
| Botet et al28 2011 | Spain | 2004–2006 | <1500 g | 5/103: 11/106 | NA | Both | 2 | Case-control | Good | |
| Allan et al29 1997 | United States | 1989–1992 | 600–1250 g | 10/53: 26/322 | NA | Both | 1.5 | Second RCT | Good | |
| Gray et al30 1997 | Australia | 1988–1990 | 24–29 | 1/12: 8/110 | 4/16: 58/173 | Both | 2 | Cohort | Good | |
| CCA mixed gestation | ||||||||||
| Bear and Wu31 2016 | United States | 1991–2001 | Mixed | 642/118 578: 7831/5 899 926 | NA | NA | ≥5 | Retrospective cohort | Good | Postnatally caused CP excluded |
| Trønnes et al32 2014 | Norway | 1967–2001 | 23–43 | 69/4195: 3082/88 125 | NA | Both | Unknown, possibly >4 | Prospective cohort | Fair | CA + fever or sepsis |
| HCA preterm | ||||||||||
| Huetz et al33 2016 | France | 2008–2011 | 24–34 | 4/57: 13/219 | 6/57: 16/219 | Singleton | 2 | Retrospective cohort | Good | |
| Miyazaki et al34 2016 | Japan | 2003–2007 | 22–34 | 64/661: 101/1522 | 129/1235: 221/2843 | Singleton | 3–3.5 | Retrospective cohort | Good | |
| Miyazaki et al35 2015: 1 | Japan | 2003–2007 | 22–34 | 21/249: 50/575 | 32/438: 70/1089 | Singleton | 3–3.5 | Retrospective cohort | Good | Antenatal steroid, data included in ref 34 |
| Miyazaki et al35 2015: 2 | Japan | 2003–2007 | 22–34 | 15/194: 72/906 | 65/402: 173/1645 | Singleton | 3–3.5 | Retrospective cohort | Good | No antenatal steroid, data included in ref 34 |
| Pappas et al36 2014: 1 | United States | 2006–2008 | <27 | 23/473: 23/512 | 477/910: 423/1014 | Both | 1.5–2 | Retrospective cohort | Good | |
| Nasef et al27 2013: 2 | Canada | 2007–2008 | <30 | 2/61: 9/96 | 15/95: 25/146 | NA | 1.5 | Retrospective cohort | Good | HCA + CCA excluded |
| Soraisham et al37 2013 | Canada | 2000–2006 | <29 | 25/197: 12/187 | 46/270: 41/259 | Both | 2.5–3.5 | Retrospective cohort | Good | |
| Watterberg et al38 2007: 1 | United States | 2001–2003 | 500 g–1 kg | 7/55: 8/52 | NA | Both | 1.5–2 | Second RCT | Good | Postnatal saline placebo, ventilated |
| Watterberg et al38 2007: 2 | United States | 2001–2003 | 500 g–1 kg | 7/57: 6/46 | NA | Both | 1.5–2 | Second RCT | Good | Postnatal steroid, ventilated |
| Polam et al39 2005 | United States | 1997–2000 | 22–29 | 9/102: 5/75 | 37/186: 38/251 | NA | 1–2 | Case-control | Good | |
| Kent et al40 2005: 1 | Australia | 1996–2001 | <30 | 3/9: 1/31 | 4/14: 8/47 | NA | 1–3 | Cohort | Good | No antenatal steroid |
| Kent et al40 2005: 2 | Australia | 1996–2001 | <30 | 5/49: 5/77 | 4/58: 8/101 | NA | 1–3 | Cohort | Good | Antenatal steroid |
| CCA and HCA preterm | ||||||||||
| Pappas et al36 2014: 2 | United States | 2006–2008 | <27 | 17/209: 23/512 | 215/466: 423/1014 | Both | 1.5–2 | Retrospective cohort | Good | |
| CCA or HCA preterm | ||||||||||
| Fung et al41 2003 | Australia | 1997–2001 | <28 or <1000 g | 3/12: 5/43 | 34/105: 74/283 | NA | 2 | Prospective cohort | Good |
NA, not available.
TABLE 2.
Characteristics of Enrolled Studies Using a Backward Approach (CA in CP Cases)
| Studies Enrolled | Country | Study Period | wk/g | CA/CP | Singleton/Twin | CP Diagnosed Age | Study Type by Original Authors | Study Quality | Notes |
|---|---|---|---|---|---|---|---|---|---|
| CCA in preterm CP | |||||||||
| Accordino et al42 2016: 1 | Italy | 2006–2012 | 24–34 | 2/26: 6/142 | Singleton | 1 y | Retrospective | Good | PPROM and PTL |
| Manuck et al43 2014 | United States | 1997–2004 | <34 | 62/459: 161/1312 | Both | 2 y | Second RCT | Good | Same cohort as in ref 25 |
| Skrablin et al44 2008 | Croatia | 1999–2001 | <37 | 7/35: 3/35 | Both | >2 y | Case-control | Good | |
| Neufeld et al45 2005: 1 | United States | 1987–1999 | <37 | 33/247: 9/180 | Singleton | ≤6 y | Case-control | Fair | |
| Takahashi et al46 2005 | Japan | 1990–1998 | 22–33 | 3/30: 17/150 | Both | 4 y | Retrospective cohort | Good | |
| Vigneswaran et al47 2004: 1 | Australia | 1984–1994 | <1500 g | 10/82: 27/207 | Singleton | 5 y | Case-control | Good | Minimal CP excluded |
| Nelson et al48 2003: 1 | United States | 1988–1994 | <32 | 20/64: 29/107 | Singleton | 4 y | Case-control | Good | Postnatally caused CP excluded |
| Jacobsson et al49 2002: 1 | Sweden | 1983–1990 | <37 | 16/148: 19/296 | Both | ≥4 y | Case-control | Good | Postnatally caused CP excluded |
| Gray et al50 2001 | Australia | 1989–1996 | 24–27 | 9/30: 26/120 | Both | ≥2 y | Case-control | Good | |
| Matsuda et al51 2000: 1 | Japan | 1992–1996 | 26–30 | 6/22: 19/170 | Singleton | >2 y | Case-control | Fair | Not matched |
| Redline et al52 2000: 1 | United States | 1983–1991 | <1500 g | 17/60: 7/59 | Singleton | 20 mo | Case-control | Good | |
| Yoon et al53 2000: 1 | Korea | 1993–1995 | ≤35 | 2/14: 7/109 | Singleton | 3 y | Cohort | Good | |
| Wilson-Costello et al54 1998 | United States | 1983–1991 | <1500 g | 11/50: 6/50 | Singleton | 20 mo | Case-control | Good | |
| O’Shea et al55 1998a | United States | 1978–1989 | 500–1500 g | 21/160: 10/80 | Both | 1 y | Case-control | Fair | Postnatally caused CP excluded |
| O’Shea et al56 1998b: 1 | United States | 1986–1993 | 500–1500 g | 12/52: 11/110 | Singleton | 1 y | Case-control | Fair | Postnatally caused CP excluded |
| Yoon et al57 1997: 1 | Korea | 1993–1995 | 26–35 | 1/8: 4/75 | Singleton | 6 mo | Retrospective cohort | Fair | May have PIH |
| Cooke58 1990 | United Kingdom | 1980–1986 | <1501 g | 17/81: 6/81 | Both | 2 y | Case-control | Good | |
| CCA in term/ near-term CP | |||||||||
| Neufeld et al45 2005: 2 | United States | 1987–1999 | ≥37 | 7/395: 25/2675 | Singleton | ≤6 y | Case-control | Fair | |
| Wu et al59 2003: 1 | United States | 1991–1998 | ≥36 | 15/106: 9/215 | Singleton | >15 mo | Case-control | Good | Postnatally caused CP excluded |
| Grether and Nelson60 1997: 1 | United States | 1983–1985 | ≥2500 g | 5/46: 5/378 | Singleton | 3 y | Case-control | Good | Postnatally caused CP excluded |
| HCA in preterm CP | |||||||||
| Accordino et al42 2016: 2 | Italy | 2006–2012 | 24–34 | 8/26: 57/142 | Singleton | 1 y | Retrospective | Good | PPROM and PTL |
| Huetz et al33 2016 | France | 2008–2011 | 24–34 | 4/17: 32/179 | Singleton | 2 y | Cohort | Good | |
| Horvath et al61 2012 | Hungary | 2000–2010 | <1500 g | 7/11: 36/130 | NA | >1 y | Cohort | Fair | |
| Redline et al62 2007 | United States | 1992–1995 | <1000 g | 8/18: 61/111 | Singleton | 8 y | Cohort | Good | |
| Vigneswaran et al47 2004: 2 | Australia | — | <1500 g | 22/54: 56/150 | Singleton | 5 y | Case-control | Good | |
| Nelson et al48 2003: 2 | United States | 1988–1994 | <32 | 31/64: 58/107 | Singleton | 4 y | Case-control | Good | Postnatally caused CP excluded |
| Jacobsson et al49 2002: 2 | Sweden | 1983–1990 | <37 | 10/28: 6/45 | Both | >4 y | Case-control | Good | |
| Matsuda et al51 2000: 2 | Japan | 1992–1996 | 26–30 | 8/22: 61/170 | Singleton | >2 y | Case-control | Good | Not matched |
| Redline et al52 2000: 2 | United States | 1983–1991 | <1500 g | 37/60: 35/59 | Singleton | 20 mo | Case-control | Good | |
| Yoon et al53 2000: 2 | Korea | 1993–1995 | ≤35 | 10/12: 44/105 | Singleton | 3 y | Cohort | Good | |
| O’Shea et al56 1998b: 2 | United States | 1986–1993 | 500–1500 g | 14/21: 17/28 | Singleton | 1 y | Case-control | Fair | Postnatally caused CP excluded |
| Yoon et al57 1997: 2 | Korea | 1993–1995 | 26–35 | 6/7: 33/75 | Singleton | 6 mo | Retrospective cohort | Fair | May have PIH |
| HCA in term/ near-term CP | |||||||||
| Wu et al59 2003: 2 | United States | 1991–1998 | ≥36 | 5/19: 1/9 | Singleton | >15 mo | Case-control | Good | |
| Grether and Nelson60 1997: 2 | United States | 1983–1985 | ≥2500 g | 3/46: 3/378 | Singleton | 3 y | Case-control | Good | Postnatally caused CP excluded |
| CCA/HCA in preterm CP | |||||||||
| Costantine et al63 2007 | United States | 1993–2002 | 500 g–1 kg | 11/19: 8/38 | NA | 18–22 mo | Case-control | Good |
PIH, pregnancy-induced hypertension; PPROM, premature rupture of membranes; PTL, preterm labor.
CP in CCA Patients
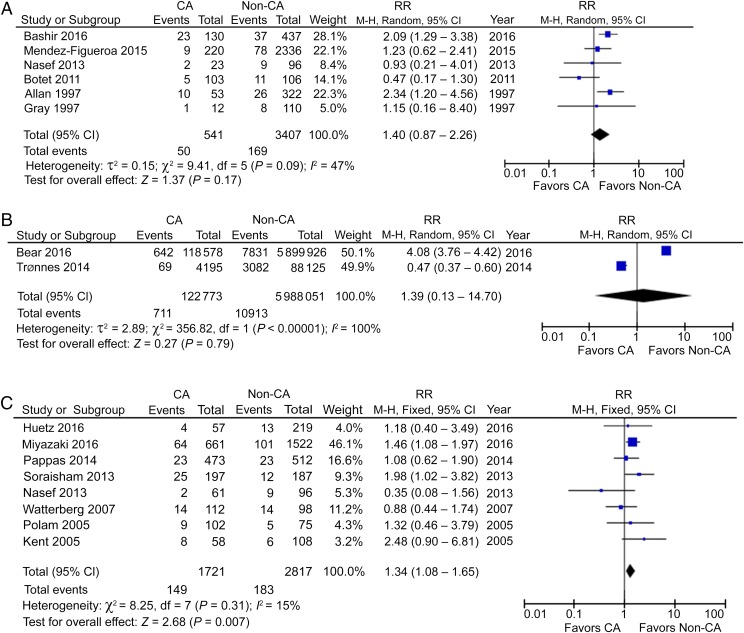
Using a forward approach, 6 cohort studies reported the incidence of CP in preterm CCA patients, including 541 CCA patients and 3407 non-CCA participants and showed no significantly increased risk.25–30 The pooled RR (95% CI) was 1.40 (0.87–2.26), with moderate heterogeneity (I2 = 47%; Fig 2A). Two studies reported CP in a mixed population of preterm and term deliveries in CCA patients, including 122 773 CCA patients and 5 988 051 non-CCA participants,31,32 again with a nonsignificant RR of 1.39 (95% CI: 0.13–14.70) and with high heterogeneity (I2 = 100%; Fig 2B). This analysis would suggest that there may be no relationship between CCA and CP when analyzing data across all stages of pregnancy and that it is inappropriate to report the CA condition in combined gestational stages, which is shown by the high heterogeneity.
FIGURE 2.
A, CP in preterm CCA patients. B, CP in mixed-term CCA patients. C, CP in preterm HCA patients. df, degrees of freedom; M-H, Mantel-Haenszel model.
CP in HCA Patients
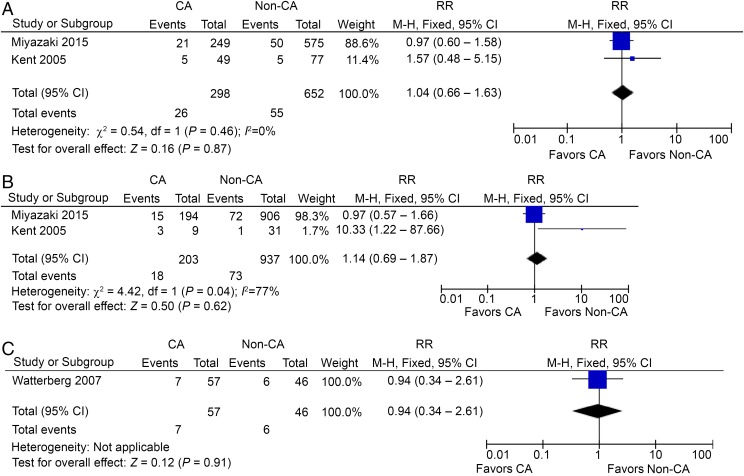
Eight studies reported CP in preterm HCA patients, including 1721 HCA patients and 2817 non-HCA participants.27,33,34,36–40 In contrast to CCA, HCA seemed to be a risk factor for CP in preterm gestation, with a RR of 1.34 (95% CI: 1.08–1.65; P < .01) and with low heterogeneity (I2 = 15%; Fig 2C). However, the incidence of CP in HCA patients is small, only 8.7 per 100 preterm infants compared with 6.5 per 100 non-HCA–exposed infants. Interestingly, the use of prenatal or postnatal steroids was reported in 3 studies, but did not lead to a significant decrease of CP in preterm HCA patients (Figs 3 A–C and 4A).35,38,40
FIGURE 3.
A, CP in preterm HCA patients, prenatal steroids used. B, CP in preterm HCA patients, no prenatal steroids used. C, CP in preterm HCA patients, postnatal steroids used. df, degrees of freedom; M-H, Mantel-Haenszel model.
FIGURE 4.
A, CP in preterm HCA patients, no postnatal steroids used. B, CP in preterm CCA and HCA patients. C, CP in preterm CCA or HCA patients. M-H, Mantel-Haenszel model.
CP in CCA and/or HCA Patients
One study reported CP in preterm patients with both CCA and HCA, including 209 patients and 512 controls.36 The RR was borderline at 1.81 (95% CI: 0.99–3.32; P = .05; Fig 4B). Thus, there is no strong evidence that a combination of CCA and HCA is associated with CP. One study reported CP in preterm patients with CCA or HCA, including 12 patients and 43 controls.41 The RR in this case was not significant at 2.15 (95% CI: 0.60–7.74; Fig 4C), again confirming the view that CCA may not be a risk for preterm CP, as well as the need for definitive criteria for diagnosing CA.
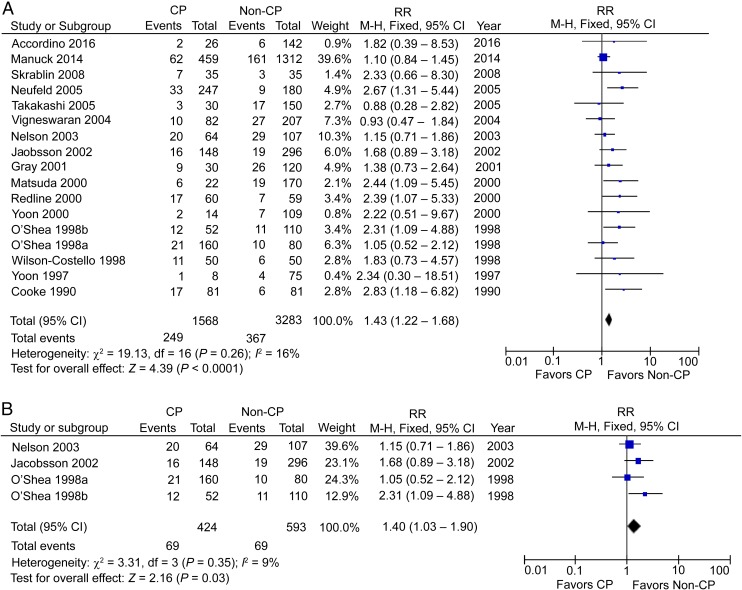
CCA in CP Patients
Using a backward approach, 17 cohort studies reported the incidence of CCA in preterm CP patients, including 1568 CP patients and 3283 non-CP participants.42–58 The RR was significant at 1.43 (95% CI: 1.22–1.68; P < .01), with low heterogeneity (I2 = 21%; Fig 5A). This increased association is in contrast to no significantly increased risk from the analysis using a forward approach. When defined postnatal causes were excluded, we were left with 4 studies with 424 preterm CP patients and 593 controls, which showed a significant increase of CCA in preterm CP patients,48,49,55,56 with an RR of 1.40 (95% CI: 1.03–1.90; P < .05) and low heterogeneity (I2 = 9%; Fig 5B).
FIGURE 5.
A, CCA in preterm CP patients. B, CCA in preterm CP patients, postnatal causes excluded. df, degrees of freedom; M-H, Mantel-Haenszel model.
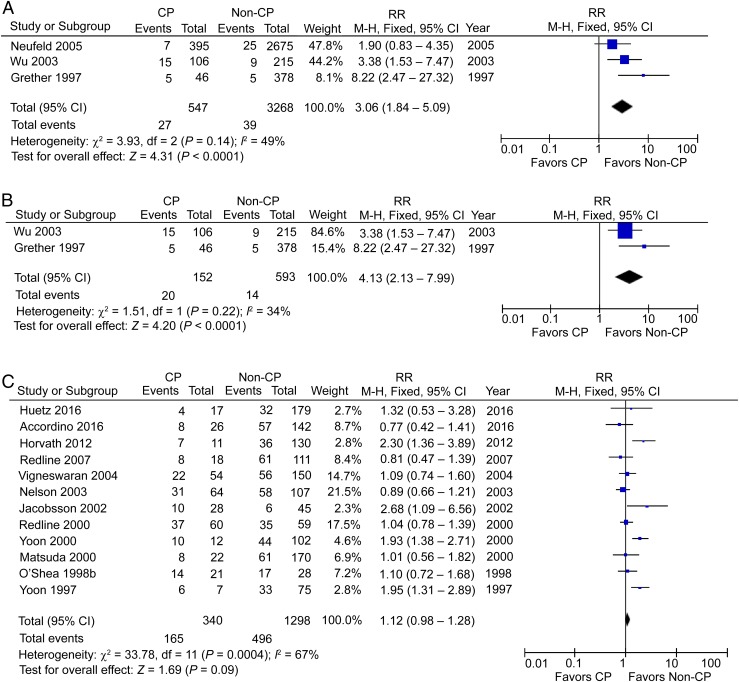
Three studies reported the incidence of CCA in term/near-term CP patients, including 547 CP patients and 3268 non-CP participants.45,59,60 The RR was significantly high at 3.06 (95% CI: 1.84–5.09; P < .01), with medium heterogeneity (I2 = 49%; Fig 6A). Again, when postnatal causes were excluded, 2 studies with 152 term/near-term CP patients and 593 controls showed a significant increase of CCA in term/near-term CP patients,59,60 with an RR of 4.13 (95% CI: 2.13–7.99; P < .01), with medium heterogeneity (I2 = 34%; Fig 6B).
FIGURE 6.
A, CCA in term/near-term CP patients. B, CCA in term/near-term CP patients, postnatal causes excluded. C, HCA in preterm CP patients. df, degrees of freedom; M-H, Mantel-Haenszel model.
HCA in CP Patients
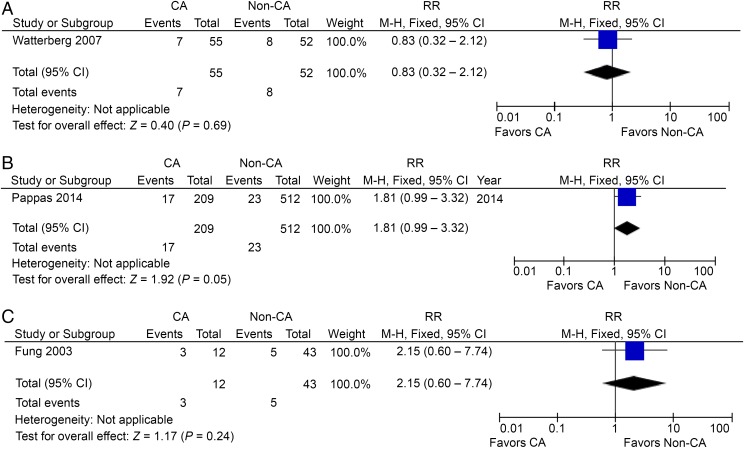
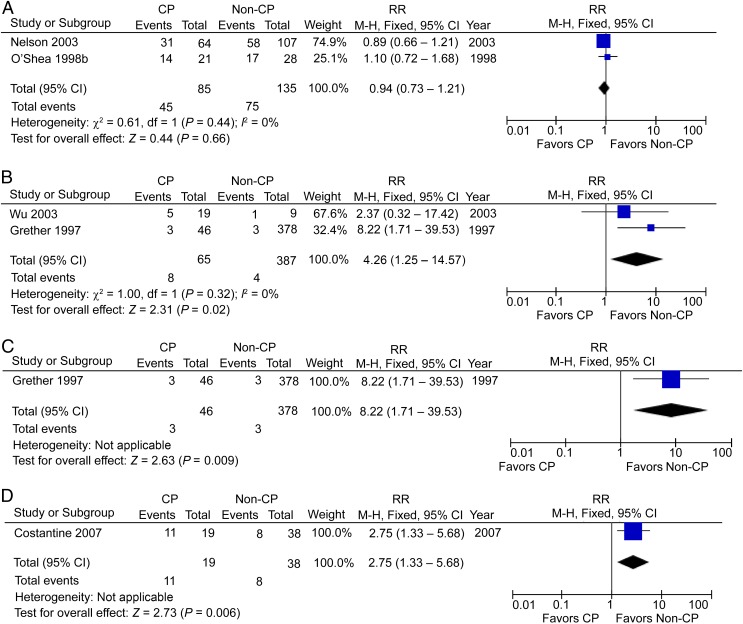
Twelve studies reported the incidence of HCA in preterm CP patients, including 340 CP patients and 1298 non-CP participants,33,42,47–49,51–53,56,57,61,62 but the RR was not significant at 1.12 (95% CI: 0.98–1.28), with high heterogeneity (I2 = 67%; Fig 6C). The absence of association from studies using a backward approach contradicts the conclusion of increased risk in studies using a forward approach. When postnatal causes were excluded, 2 studies with 85 preterm CP patients and 135 controls48,56 showed no significant change with an RR of 0.94 (95% CI: 0.73–1.21; Fig 7A). Two studies reported the incidence of HCA in term/near-term CP patients, including 65 CP patients and 387 non-CP participants,59,60 and the RR in this case was significant at 4.26 (95% CI: 1.25–14.57; P < .05), with low heterogeneity (I2 = 0%; Fig 7B). Again, when postnatal causes were excluded, we were left with 1 study with 46 term/near-term CP patients and 378 controls showing a significant increase of HCA in term/near-term CP patients60 with an RR of 8.22 (95% CI: 1.71–39.53; P < .01; Fig 7C).
FIGURE 7.
A, HCA in preterm CP patients, postnatal causes excluded. B, HCA in term/near-term CP patients. C, HCA in term/near-term CP patients, postnatal causes excluded. D, CCA/HCA in preterm CP patients. df, degrees of freedom; M-H, Mantel-Haenszel model.
CCA or HCA in CP Patients
One study reported CCA or HCA cases in preterm CP patients, including 19 patients and 38 controls,63 with a significant RR of 2.75 (95% CI: 1.33–5.68; P < .01; Fig 7D). One hesitates to come to any conclusion with these small numbers.
Discussion
This meta-analysis reveals the absence of a risk for CP in CCA patients. HCA, on the other hand, seems to be associated with a significant risk for CP, although the incidence of CP in HCA-exposed infants is only 8.6%. This review also points out the less convincing analysis from cohort studies using a backward approach. The fact that there is no increased incidence of CP in CCA patients but a higher incidence of CCA in CP patients perhaps points to another factor (such as prematurity itself) in the causal pathway of CP, perhaps significant only when it coexists with CCA . Statistics derived from a forward approach of studying CA populations have fewer potential sources of bias and confounding than those obtained from a backward approach of studying a CP population. The statistical power of these 2 strategies differs substantially.64 HCA seems to show a stronger risk in the studies that used a forward strategy in the preterm population. Although the number of studies is small, an association is found using a backward approach from studies of the near-term/term population, a group that avoids the potential influence of prematurity. The fact that there is no association of HCA with CP in preterm infants suggests that other factors (including prematurity) probably drown out the small contribution of HCA. The perinatal etiologic risk factors for CP include birth asphyxia, inflammation, restricted growth, birth defects, sex, race, and genetics.65 Treatments in response to CA or preterm labor, such as application of tocolytics, MgSO4, corticosteroids, surfactant, antibiotics, and mode of delivery,58 as well as complications in the pregnant mother12 might also affect the pathogenesis of CP.
There are many possible reasons why CCA is not as strong a risk factor as HCA. Wu10 raised the issue of overdiagnoses of the clinical diagnosis of “chorioamnionitis” in recent years. Maternal fever without maternal inflammation has often been included in the diagnosis of CCA, which then includes a population of fetuses that do not have inflammation. Examples include fever from dehydration and epidural injection. This issue might be solved if all obstetricians followed the recent guidelines of delineating maternal fever alone from triple-I.5 Even if there is maternal inflammation with evidence of high leukocytes >15 000 and definite pus observed from cervical os and high fever, which constitute the criteria of “suspected” triple-I,5 the fetus systematically and the fetal brain may not necessarily be affected by inflammation. The previous definitions of HCA coinciding with the added criteria constituting “confirmed” triple-I,5 Gram positivity, positive culture, and low glucose in amniotic fluid, as well as positive placental pathology may still not be enough to confirm the presence of fetal inflammation. One can speculate about the confounding effects of the immunologic responses and specific microbiomes in the mother, placenta, and fetus. Even with the evidence of fetal involvement in the placenta, only the most severe fetal inflammatory response (ie, subacute necrotizing funisitis and chorionic plate vasculitis with thrombosis) was associated with poor developmental outcome, but this was not the case with the milder stages of fetal inflammatory response.66 Thus, it is possible that with a refined diagnosis of triple-I, clinicians will be able to get a better idea of the risk of CP with inflammation in the mother-infant dyad. It is speculated that the risk of CP is restricted to the population of CA with evidence of severe fetal inflammation and is not present in all cases of confirmed triple-I. Not many hospitals in the United States or Europe have the capability of doing amniotic fluid examination and a quick turnaround of placental pathology. Thus, in the future, there will still be continued confusion about the risk of CP in CCA (now suspected triple-I) and CCA + HCA (confirmed triple-I) populations. The risk of CP in cases of HCA only, without concomitant signs of CCA, is still to be determined.
Other Biases
Most of the studies in Table 1 involved preterm populations of newborns, whereas 7 studies out of the 29 in Table 2 were of term/near-term newborns. According to our analysis, HCA seems to be a risk factor for CP in preterm gestation. However, this is likely due to the large weight in the analysis of the Miyazaki study34 (46.1%). This study does have limitations, such as a retrospective design and a high rate of exclusions due to incomplete data (6316 excluded out of 10 394 cases). The risk of CP is inversely proportional to gestational age and is 60 times higher at <28 weeks of gestation than at term.49 The confounding factor to consider in CA is that the risk of prematurity is higher in cases of CA. More than half of CP cases diagnosed at 1 year of age were free of motor handicap at the age of 7 years.67 A diagnosis made at 2 years of age or later is more reliable than one made before 2 years of age.68 Twin pregnancy was associated with an increased risk of CP.69 Thus, studies confined to singleton pregnancies ought to be compared separately from those with twin pregnancies. Exclusion of certain causes of CP also minimizes the bias in the etiologic study of CA, such as CP caused by child abuse, accidents,31 TORCH (with toxoplasmosis, rubella, cytomegalovirus, and herpes) infection,48 brain malformation, or even prenatal destructive brain lesions.60 The discerning reader will notice that the average relative risk of CP in pooled CCA patients was higher than that of CP in HCA patients. The pooled incidence of CP in preterm CCA was 9.2% vs 5.0% for preterm non-CCA–exposed patients, but the pooled incidence did not reach significance and there was high heterogeneity among enrolled studies, which was not the case for HCA. In any case, the absolute values of CP in both CCA and HCA are still small, suggesting that even HCA constitutes a relatively small risk factor for CP in a preterm infant.
One must also consider the possibility that the incidence of CP may be inversely related to the incidence of death. Investigating the relationship between newborn death and CA from studies with a concomitant report of CP, we found 3 studies that reported the incidence of newborn death before discharge in preterm CCA patients, including 269 CCA patients and 2655 controls,26,27,30 but the RR was not significant at 1.19 (95% CI: 0.80–1.77) and there was high heterogeneity (I2 = 52%; Supplemental Fig 14). Seven studies reported the incidence of newborn death before discharge in preterm HCA patients, including 2825 HCA patients and 4880 controls,27,33,34,36,37,39,40 and in this case, the RR was significant at 1.25 (95% CI: 1.15–1.36; P < .01) and there was low heterogeneity (I2 = 0%; Supplemental Fig 15). However, the study by Pappas et al36 contributed 62% of the weight of the analysis. In this study, extremely premature infants were included, and it is well known that the mortality rate for extremely premature infants is much greater than that of preterm infants. Comparing CCA in preterm CP to CCA in term CP, one finds less of an association with preterm CP (RR: 1.43 vs 3.06). One can speculate that if the premature infants who died had somehow survived, then there could have been a higher rate of CP.
The etiology of maternal inflammation may extend beyond CA. Bear and Wu31 showed that a history of genitourinary and respiratory infections in addition to CCA increases the risk of CP when using a forward approach involving 6 million mother-infant dyads, including preterm and term gestational ages. The RR for CP in 24 414 preterm patients with CCA was 4.1 (95% CI: 3.7–4.5) compared with a RR of 2.01 for 86 335 term CCA patients (95% CI: 1.7–2.4).31 However, no raw data were shown comparing CP in the preterm CCA or non-CCA populations, nor in the term CCA and non-CCA populations, making this study only applicable for mixed-term analysis. When using a backward approach, Bear and Wu31 found an association of CP with either CCA, genitourinary or respiratory infections (incidence of maternal infection in CP vs non-CP, 13.7% vs 5.5%, P < .01). Interestingly, it is not known whether maternal infection other than CA occurred specifically in the second and third trimesters, which raises the issue of a susceptibility to infections or inflammation in mother-infant dyads. Thus, it is possible that a subpopulation of mother-infant dyads has an increased chance of fetal inflammation in CCA. This possibility is supported by the increased RR of 7.0 if there was CCA along with a history of other maternal infections. Furthermore, it has been proposed that alterations in the composition of the microbiota might disturb the human immune system, ultimately leading to altered immune responses that may underlie inflammatory disorders.70
Conclusions
HCA is a risk factor for CP in preterm studies using a forward approach. An association of HCA with CP is found only in studies of near-term/term infants but not in studies of preterm infants using a backward approach. The incidence of CP after HCA is still small. The evidence that CCA is a risk factor for CP is not borne out from studies using a forward approach. An association of CP with CCA is found in studies of both preterm and term infants using a backward approach. Future trials of confirmed triple-I with severe fetal inflammatory involvement need to be carried out.
Glossary
- CA
chorioamnionitis
- CCA
clinical chorioamnionitis
- CI
confidence interval
- CP
cerebral palsy
- HCA
histologic chorioamnionitis
- RR
risk ratio
- triple-I
intrauterine inflammation or infection or both
Footnotes
Dr Shi conceptualized and designed the study, carried out the initial analyses, performed statistical analysis, and drafted the initial manuscript; Dr Ma carried out the initial analyses, performed statistical analysis, and reviewed and revised the manuscript; Drs Luo, Bajaj, Chawla, and Natarajan provided technical support, interpreted data, and critically reviewed the manuscript; Dr Hagberg performed statistical analysis, interpreted data, and critically reviewed the manuscript; Dr Tan obtained funding, conceptualized and designed the study, performed statistical analysis, interpreted data, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by grant R01 NS081936 from National Institute of Neurological Disorders and Stroke, National Institutes of Health (to Dr Tan). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers. 2016;2:15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arneson CL, Durkin MS, Benedict RE, et al. Prevalence of cerebral palsy: Autism and Developmental Disabilities Monitoring Network, three sites, United States, 2004. Disabil Health J. 2009;2(1):45–48 [DOI] [PubMed] [Google Scholar]
- 3.Boulet SL, Boyle CA, Schieve LA. Health care use and health and functional impact of developmental disabilities among US children, 1997-2005. Arch Pediatr Adolesc Med. 2009;163(1):19–26 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment–United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(3):57–59 [PubMed] [Google Scholar]
- 5.Higgins RD, Saade G, Polin RA, et al. ; Chorioamnionitis Workshop Participants . Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol. 2016;127(3):426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauth JC, Gilstrap LC III, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol. 1985;66(1):59–62 [PubMed] [Google Scholar]
- 8.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(suppl 4):S29–S52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):387–392 [DOI] [PubMed] [Google Scholar]
- 10.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8(1):25–29 [DOI] [PubMed] [Google Scholar]
- 11.Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284(11):1417–1424 [DOI] [PubMed] [Google Scholar]
- 12.van Lieshout P, Candundo H, Martino R, Shin S, Barakat-Haddad C. Onset factors in cerebral palsy: a systematic review [published online ahead of print April 1, 2016]. Neurotoxicology. doi:10.1016/j.neuro.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 13.Berger A, Witt A, Haiden N, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37(1):72–78 [DOI] [PubMed] [Google Scholar]
- 14.Kaukola T, Herva R, Perhomaa M, et al. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res. 2006;59(3):478–483 [DOI] [PubMed] [Google Scholar]
- 15.Kim JN, Namgung R, Chang W, et al. Prospective evaluation of perinatal risk factors for cerebral palsy and delayed development in high risk infants. Yonsei Med J. 1999;40(4):363–370 [DOI] [PubMed] [Google Scholar]
- 16.Torfs CP, van den Berg B, Oechsli FW, Cummins S. Prenatal and perinatal factors in the etiology of cerebral palsy. J Pediatr. 1990;116(4):615–619 [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012 [DOI] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute Quality assessment of case-control studies. Available at: www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case-control. Accessed August 30, 2016
- 19.National Heart, Lung, and Blood Institute Quality assessment tool for observational cohort and cross-sectional studies. Available at: www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Accessed August 30, 2016
- 20.Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322(7300):1479–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206 [DOI] [PubMed] [Google Scholar]
- 22.Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24(2):126–151 [DOI] [PubMed] [Google Scholar]
- 23.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055 [DOI] [PubMed] [Google Scholar]
- 24.Cochrane UK The Cochrane Collaboration open learning material. Available at: http://handbook.cochrane.org/chapter_9/9_analysing_data_and_undertaking_meta_analyses.htm. Accessed April 22, 2017
- 25.Bashir RA, Bhandari V, Vayalthrikkovil S, et al. Chorioamnionitis at birth does not increase the risk of neurodevelopmental disability in premature infants with bronchopulmonary dysplasia. Acta Paediatr. 2016;105(11):e506–e512 [DOI] [PubMed] [Google Scholar]
- 26.Mendez-Figueroa H, Abramovici A, O’Neil AE, Dahlke J, Pedroza C, Chauhan S. Chorioamnionitis without and with neonatal sepsis: newborn and infant outcomes. Am J Obstet Gynecol. 2015;212(suppl 1):S318–S319 [Google Scholar]
- 27.Nasef N, Shabaan AE, Schurr P, et al. Effect of clinical and histological chorioamnionitis on the outcome of preterm infants. Am J Perinatol. 2013;30(1):59–68 [DOI] [PubMed] [Google Scholar]
- 28.Botet F, Figueras J, Carbonell-Estrany X, Narbona E. The impact of clinical maternal chorioamnionitis on neurological and psychological sequelae in very-low-birth weight infants: a case-control study. J Perinat Med. 2011;39(2):203–208 [DOI] [PubMed] [Google Scholar]
- 29.Allan WC, Vohr B, Makuch RW, Katz KH, Ment LR. Antecedents of cerebral palsy in a multicenter trial of indomethacin for intraventricular hemorrhage. Arch Pediatr Adolesc Med. 1997;151(6):580–585 [DOI] [PubMed] [Google Scholar]
- 30.Gray PH, Hurley TM, Rogers YM, et al. Survival and neonatal and neurodevelopmental outcome of 24-29 week gestation infants according to primary cause of preterm delivery. Aust N Z J Obstet Gynaecol. 1997;37(2):161–168 [DOI] [PubMed] [Google Scholar]
- 31.Bear JJ, Wu YW. Maternal infections during pregnancy and cerebral palsy in the child. Pediatr Neurol. 2016;57:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Dev Med Child Neurol. 2014;56(8):779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huetz N, Triau S, Leboucher B, et al. Association of severe placental inflammation with death prior to discharge and cerebral palsy in preterm infants. BJOG. 2016;123(12):1956–1963 [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki K, Furuhashi M, Ishikawa K, et al. Impact of chorioamnionitis on short- and long-term outcomes in very low birth weight preterm infants: the Neonatal Research Network Japan. J Matern Fetal Neonatal Med. 2016;29(2):331–337 [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki K, Furuhashi M, Ishikawa K, et al. Long-term outcomes of antenatal corticosteroids treatment in very preterm infants after chorioamnionitis. Arch Gynecol Obstet. 2015;292(6):1239–1246 [DOI] [PubMed] [Google Scholar]
- 36.Pappas A, Kendrick DE, Shankaran S, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014;168(2):137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soraisham AS, Trevenen C, Wood S, Singhal N, Sauve R. Histological chorioamnionitis and neurodevelopmental outcome in preterm infants. J Perinatol. 2013;33(1):70–75 [DOI] [PubMed] [Google Scholar]
- 38.Watterberg KL, Shaffer ML, Mishefske MJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics. 2007;120(1):40–48 [DOI] [PubMed] [Google Scholar]
- 39.Polam S, Koons A, Anwar M, Shen-Schwarz S, Hegyi T. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Arch Pediatr Adolesc Med. 2005;159(11):1032–1035 [DOI] [PubMed] [Google Scholar]
- 40.Kent A, Lomas F, Hurrion E, Dahlstrom JE. Antenatal steroids may reduce adverse neurological outcome following chorioamnionitis: neurodevelopmental outcome and chorioamnionitis in premature infants. J Paediatr Child Health. 2005;41(4):186–190 [DOI] [PubMed] [Google Scholar]
- 41.Fung G, Bawden K, Chow P, Yu V. Chorioamnionitis and outcome in extremely preterm infants. Ann Acad Med Singapore. 2003;32(3):305–310 [PubMed] [Google Scholar]
- 42.Accordino F, Consonni S, Fedeli T, et al. Risk factors for cerebral palsy in PPROM and preterm delivery with intact membranes. J Matern Fetal Neonatal Med. 2016;29(23):3854–3859 [DOI] [PubMed] [Google Scholar]
- 43.Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210(5):426.e1–426.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skrablin S, Maurac I, Banović V, Bosnjak-Nadj K. Perinatal factors associated with the neurologic impairment of children born preterm. Int J Gynaecol Obstet. 2008;102(1):12–18 [DOI] [PubMed] [Google Scholar]
- 45.Neufeld MD, Frigon C, Graham AS, Mueller BA. Maternal infection and risk of cerebral palsy in term and preterm infants. J Perinatol. 2005;25(2):108–113 [DOI] [PubMed] [Google Scholar]
- 46.Takahashi R, Yamada M, Takahashi T, et al. Risk factors for cerebral palsy in preterm infants. Early Hum Dev. 2005;81(6):545–553 [DOI] [PubMed] [Google Scholar]
- 47.Vigneswaran R, Aitchison SJ, McDonald HM, Khong TY, Hiller JE. Cerebral palsy and placental infection: a case-cohort study. BMC Pregnancy Childbirth. 2004;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson KB, Grether JK, Dambrosia JM, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53(4):600–607 [DOI] [PubMed] [Google Scholar]
- 49.Jacobsson B, Hagberg G, Hagberg B, Ladfors L, Niklasson A, Hagberg H. Cerebral palsy in preterm infants: a population-based case-control study of antenatal and intrapartal risk factors. Acta Paediatr. 2002;91(8):946–951 [DOI] [PubMed] [Google Scholar]
- 50.Gray PH, Jones P, O’Callaghan MJ. Maternal antecedents for cerebral palsy in extremely preterm babies: a case-control study. Dev Med Child Neurol. 2001;43(9):580–585 [DOI] [PubMed] [Google Scholar]
- 51.Matsuda Y, Kouno S, Hiroyama Y, et al. Intrauterine infection, magnesium sulfate exposure and cerebral palsy in infants born between 26 and 30 weeks of gestation. Eur J Obstet Gynecol Reprod Biol. 2000;91(2):159–164 [DOI] [PubMed] [Google Scholar]
- 52.Redline RW, Wilson-Costello D, Borawski E, Fanaroff AA, Hack M. The relationship between placental and other perinatal risk factors for neurologic impairment in very low birth weight children. Pediatr Res. 2000;47(6):721–726 [DOI] [PubMed] [Google Scholar]
- 53.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182(3):675–681 [DOI] [PubMed] [Google Scholar]
- 54.Wilson-Costello D, Borawski E, Friedman H, Redline R, Fanaroff AA, Hack M. Perinatal correlates of cerebral palsy and other neurologic impairment among very low birth weight children. Pediatrics. 1998;102(2 pt 1):315–322 [DOI] [PubMed] [Google Scholar]
- 55.O’Shea TM, Klinepeter KL, Dillard RG. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol. 1998;147(4):362–369 [DOI] [PubMed] [Google Scholar]
- 56.O’Shea TM, Klinepeter KL, Meis PJ, Dillard RG. Intrauterine infection and the risk of cerebral palsy in very low-birthweight infants. Paediatr Perinat Epidemiol. 1998;12(1):72–83 [PubMed] [Google Scholar]
- 57.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177(1):19–26 [DOI] [PubMed] [Google Scholar]
- 58.Cooke RW. Cerebral palsy in very low birthweight infants. Arch Dis Child. 1990;65(2):201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290(20):2677–2684 [DOI] [PubMed] [Google Scholar]
- 60.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278(3):207–211 [PubMed] [Google Scholar]
- 61.Horvath B, Grasselly M, Bodecs T, Boncz I, Bodis J. Histological chorioamnionitis is associated with cerebral palsy in preterm neonates. Eur J Obstet Gynecol Reprod Biol. 2012;163(2):160–164 [DOI] [PubMed] [Google Scholar]
- 62.Redline RW, Minich N, Taylor HG, Hack M. Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg). Pediatr Dev Pathol. 2007;10(4):282–292 [DOI] [PubMed] [Google Scholar]
- 63.Costantine MM, How HY, Coppage K, Maxwell RA, Sibai BM. Does peripartum infection increase the incidence of cerebral palsy in extremely low birthweight infants? Am J Obstet Gynecol. 2007;196(5):e6–e8 [DOI] [PubMed] [Google Scholar]
- 64.University of Texas at Austin Department of Mathematics Common mistakes involving power. Available at: www.ma.utexas.edu/users/mks/statmistakes/PowerMistakes.html. Accessed June 30, 2016
- 65.Nelson KB, Blair E. Prenatal factors in singletons with cerebral palsy born at or near term. N Engl J Med. 2015;373(10):946–953 [DOI] [PubMed] [Google Scholar]
- 66.Salas AA, Faye-Petersen OM, Sims B, et al. Histological characteristics of the fetal inflammatory response associated with neurodevelopmental impairment and death in extremely preterm infants. J Pediatr. 2013;163(3):652–657.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson KB, Ellenberg JH. Children who ‘outgrew’ cerebral palsy. Pediatrics. 1982;69(5):529–536 [PubMed] [Google Scholar]
- 68.Kuban KC, Leviton A. Cerebral palsy. N Engl J Med. 1994;330(3):188–195 [DOI] [PubMed] [Google Scholar]
- 69.Yokoyama Y, Shimizu T, Hayakawa K. Prevalence of cerebral palsy in twins, triplets and quadruplets. Int J Epidemiol. 1995;24(5):943–948 [DOI] [PubMed] [Google Scholar]
- 70.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.