Abstract
Regenerative engineering converges tissue engineering, advanced materials science, stem cell science, and developmental biology to regenerate complex tissues such as whole limbs. Regenerative engineering scaffolds provide mechanical support and nanoscale control over architecture, topography, and biochemical cues to influence cellular outcome. In this regard, poly (lactic acid) (PLA)-based biomaterials may be considered as a gold standard for many orthopaedic regenerative engineering applications because of their versatility in fabrication, biodegradability, and compatibility with biomolecules and cells. Here we discuss recent developments in PLA-based biomaterials with respect to processability and current applications in the clinical and research settings for bone, ligament, meniscus, and cartilage regeneration.
Keywords: Poly (lactic acid), PLA, processing, regenerative, engineering, growth factors, small molecules, cells, tissue, bone, ligament, cartilage, meniscus regeneration
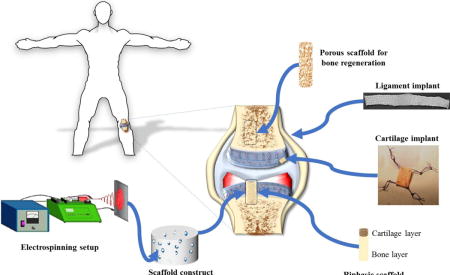
Graphical abstract
A schematic illustrating the application of PLA based biomaterials in the form of sintered microspheres, electrospun nanofibers, porous biphasic, and 3-D braided microfiber scaffolds for bone, cartilage, meniscus, and ligament regeneration.
1. Introduction
Orthopaedics-related medical diagnoses accounted for 225 million visits, costing about 215 billion dollars between the years 2009–2011 [1]. These figures include more than a million total hip and knee replacements, and about 100,000 ligament reconstruction procedures performed annually at a cost of about 25 billion dollars [2, 3]. Current orthopaedic surgical procedures primarily utilize autografts, allografts, and metal and plastic implants [4]. The metal and plastic implants suffer from a variety of challenges such as low fatigue, creep, poor adhesion, and biocompatibility issues with native tissue [5–7]. Similarly, autografts, currently considered as the gold standard, suffer from donor site morbidity, pain, and unavailability of large tissue-volumes [8]. In the case of allografts, donor-site morbidity is not an issue, however, some of its drawbacks includes the risk of communicable diseases, immunogenicity, and inadequate donors [9, 10].
In addition, various biodegradable and biocompatible polymers, of both synthetic and natural origin, have been developed for biomedical applications [11]. Some of these polymeric-materials have found applications in sutures and are fast emerging as implant-material alternatives. Aliphatic polyesters, also known as poly (α-hydroxy esters), are one such bioresorbable and biocompatible group of polymers that have great potential for use in the regeneration of large tissues. This class of polymers include: poly (lactic acid) (PLA), poly (glycolic acid) (PGA), poly (ε-caprolactone) (PCL), poly (dioxanone) (PDO), and poly (trimethylene carbonate) (PTMC) [12, 13]. Within this group, PLA possesses chirality enabling the mid-chain residues to exist in three enantiomeric states, L-Lactide, D-Lactide, and meso-lactide [14]. Of these, the most widely used polylactides are the poly (L-Lactide) (PLLA) and poly (D-Lactide) (PDLA), respectively [13].
PLLA is a slow crystallizing, semi-crystalline polymer with crystallinity, melting and glass transition temperature values ranging from 40–50%, 55–80 °C, and 170–180 °C, respectively [15, 16]. Likewise, PDLA, which is also a semi-crystalline polymer with crystallinity, melting, and glass transition temperature values ranging from 30–45 %, 40–50 °C, and 120–150 °C, respectively [16, 17]. Both PLLA and PDLA have comparable tensile strength (4–8 GPa), elongation at break (1–8%), and tensile strength values (40–70 MPa) [17, 18]. Their slow crystallizing nature predisposes these materials to be typically hard and brittle. The crystallizability of these materials can be improved by processing via isothermal annealing [19], co-polymerizing [20], nucleating by additives [21], and strain induced crystallizing [22]. The random distribution of PLLA and PDLA in PDLLA causes disruption of stereo-regularity, leading to an amorphous poly (D,L-lactic acid) PDLLA [23]. Altering the stereo-regularity of the isomers (PDLLA) is also one way to manipulate the degradation rate of this polymer. In-vivo studies have shown highly-crystalline PLLA to degrade completely in 2–5 years; whereas mostly-amorphous PDLLA loses strength in less than 2 months, and completely degrade within 12 months [12].
The processability, material properties, degradation rates, and tissue compatibility of PLA have been also modulated by copolymerizing it with other monomers resulting in copolymers such as poly (lactic acid-co-glycolic acid) (PLGA), poly (lactic acid-co-caprolactone) (PLCL), poly (lactic acid-co-ethylene glycol) (PLEG), and poly (lactic acid-co-glutamic acid) (PLGM); thus, providing PLA-based biomaterials with tunable-properties for diverse biomedical applications [24–26]. Another advantage with these degradable biomaterials are that unlike non-degradable implant biomaterials, these do not require additional surgery for implant removal [27]. Additionally, the ease of processing PLA based biomaterials by extrusion, injection molding, stretch blow molding, film casting, thermoforming, foaming, fiber spinning, electrospinning, melt electrospinning, and micro- and nano-fabrication techniques into various shapes and sizes have played a critical role in expanding the applications of these materials [28, 29].
In orthopaedic and dental applications, PLA based materials have been extensively used as fixation-devices such as screws, pins, washers, darts, and arrows in reconstructive surgeries including those of the mandibular joint; facelifts; thoracic, hand, leg, finger, and toe fractures; ligament reconstruction procedures; soft and hard tissue fixations; alignment of osteochondral and bone fragments; meniscus repair; and hyaline cartilage fixation [11]. Some of the PLA based implants are shown in Figure 1, and the composition, purpose of those degradable implants are summarized in Table 1. This review summarizes the recent progress in PLA based biomaterials for bone, ligament, cartilage, and meniscus regeneration.
Figure 1. Representative PLA based medical devices currently employed in orthopaedic and dental applications – Biotrak® pins and screws.
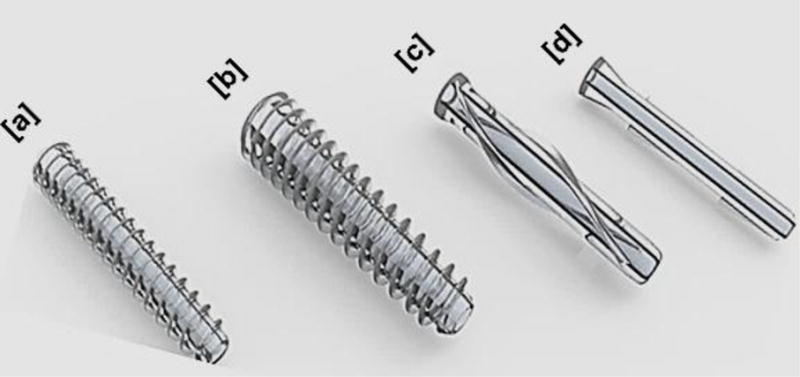
(a) Mini screw used for fixation in treating osteochondral defects and in foot, ankle, and hand surgeries, (b) standard screw used for treating osteochondral defects, osteotomies, and navicular fractures, (c) helical nail used for fixing radial styloid fractures and hammertoes, and (d) pin used for the repair of ulnar styloid fracture. Images reprinted with permission from Acumed LLC.
Table 1.
PLA based commercial medical devices currently used in orthopaedic and dental applications.
| Manufacturer (Country) | Product | Composition | Application |
|---|---|---|---|
| Biomet (USA) | LactoSorb® | PLLA-PGA | Pediatric craniofacial and reconstructive surgery |
| RapidFlap® | PLLA-PGA | Pediatric craniotomy fixation | |
| Lactosorb Distraction Device® | PLLA-PGA | Craniofacial microsomia | |
| Lactosorb Endobrow Screw® | PLLA-PGA | Facelift surgery | |
| LactoSorb SE Delivery® | PLLA-PGA | Restrict screw migration | |
| Takiron (Japan) | Osteotrans-MX® | PLLA-μHA | Bone fracture and fragments fixation; ligaments, and soft tissue fixation |
| Osteotrans-OT® | PLLA-μHA | Ligament reconstruction (ACL, PCL) | |
| Gunze (Japan) | Grand Fix™ | PLLA | Finger and toe fracture fixation; transplanted bone fixation |
| Acumed (USA) | Biotrak® | PLLA | Osteochondral defects; osteotomies; navicular fracture fixation |
| Conmed (USA) | Genesys ™ Matrix Interference Screws | PLDA/β-TCP | ACL/PCL graft fixation |
| Pinn-ACL® Crosspin System | PLLA | ACL reconstruction | |
| BioScrew® Bioab sorbable Interference Screws | PLLA | Graft fixation | |
| EndoPearl® Fixation Device | PLLA | Soft tissue grafts fixation (ACL reconstruction) | |
| BioStinger® Meniscal Fixation System | PLLA | Meniscus repair | |
| Contour™ Meniscus Arrow™ | PLDLA | Meniscus repair | |
| SmartNail® | PLLA | Bone fractures and osteochondral fragments alignment | |
| Arthrex (USA) | Sheathed Bio-Interference Screw | PLLA | Facilitate screw insertion and graft rotation |
| Chondral Dart ™ | PLLA | Osteochondral tear fixation | |
| Trim-It Spin Pin™ | PLLA | Intraosseous ligament and tendon fixation; small bone and soft tissues fixation | |
| Bio-TransFix® Implant | PLLA | Soft tissues; bone-tendon-bone grafts fixation | |
| Stryker (USA) | Biosteon® | PLLA-HA | Ligament reconstruction surgery screw; anchor rotator cuff |
| Bioabsorbable | PLLA | Soft tissue and bone-to-bone grafts fixation | |
| Zimmer (USA | Bio-statak ® | PLLA | Soft tissue fixation |
| Sysorb (Switzerland) | Sysorb® Bioresorbable Interference Screw | PDLA | Juxta-articular graft fixation |
| Depuy (USA) | Rapidsorb™ | PLLA/PGA | Craniofacial fracture repair and reconstruction |
| Biocryl® | PLLA/β-TCP | Soft tissue and bone-to-tendon grafts fixation | |
| Biocryl® Rapide® | PLGA/TCP | Knotless tying (rotator cuff); Bankart repair | |
| Orthomesh® | PLLA | Bone grafts or fragments fixation | |
| Absolute® Interference Screw | PLLA | Soft tissue and bone-bone fixation | |
| BIOINTRAFIX® | PLA/TCP | Tibial fixation (ligament reconstruction) | |
| RIGIDFIX® Cross Pin System | PLLA/β-TCP | ACL reconstruction |
2. Processing
i. Fiber Spinning
An advantage of PLA based biomaterials has been their ability to be fabricated into a variety of structures with the appropriate mechanical properties, topography, geometry, and architecture as required for diverse biomedical applications. One of the oldest methods to fabricate PLA based products has been fiber-spinning from either polymer solution or melt. As PLA is soluble in a wide array of solvents, solution spinning processes has also been widely utilized to fabricate fibers for biomedical applications [30]. Historically, mono- and multi-filament sutures have been prepared from PLA based fibers by spinning; but due to their longer degradation times, other aliphatic polyesters such as PGA have now replaced PLA [30]. In addition, woven, knitted, and braided structures produced from spun fibers have found orthopaedic applications in bone, ligament, and cartilage regeneration (discussed later) [31–33].
PLA based devices currently used for orthopaedic applications (Fig.1, Table 1) are made by rapid processing techniques resulting in poor mechanical properties and crystallinity. PLA based fibers are commonly utilized to enhance the crystallinity and mechanical properties of those orthopaedic fixation devices [34]. While evaluating PLA fibers (self-reinforced) reinforced PLA composites (SR-PLLA) for long term complication and fixation failure rates, Juutilainen et al. noted lower fixation failures (<5%), and higher bone mineral density (BMD) in SR-PLLA composites, compared to metal and unreinforced PLA implants [35]. Similarly, another study showed significant improvements in mechanical properties (tear strength) of PLLA reinforced with PLA fibers (yarns and fabrics) attributed to enhanced interfacial bonding between PLA fibers and PLLA sheets [36]. Besides the linear stresses, orthopaedic devices are also commonly subjected to torsional forces, hence resistance to those forces are also required for low fixation failure rates. A recent study by Wu et al. showed drastic enhancements in the flexural modulus values of PLA composites self-reinforced with braided yarns (3- and 5-direction) [37]. A key notable difference between the studies is that, due to the braided structure of the fibers, modulus values increase significantly throughout the composite structure, thereby increasing the horizon of these composites with enhanced mechanical properties.
The fabrication of SR-PLLA composites is made possible due to the contrasting melting temperatures of the PLLA fibers and the PLLA matrix. But in solution, as both the fibers and matrix are soluble, fabrication of SR-PLLA composites are not possible. However, a recent study demonstrated the possibility of reinforcing an amorphous solution cast PDLLA film with PLLA fibers retaining their architecture. In addition, a 2-fold improvement in the tensile modulus (1.40 vs 0.79 GPA for non-reinforced), and a 3-fold improvement in tensile strength (50 MPa vs 16 MPa) was realized by the authors, thus demonstrating the significance of these PLA fibers in composites for orthopaedic applications [38]. These studies indicate, by judicious selection of matrix/fiber and morphology of the fiber phase, SR-PLLAs can be fabricated using wide array of techniques increasing the application of these devices for orthopaedic applications that require significantly higher modulus and strength.
To improve healing and enhance bony fixation, devices such as interference screws rods, and nails are routinely coated with inorganic materials such as tricalcium phosphate (TCP) or hydroxyapatite (HA), and or antibiotic drugs to prevent osteomyelitis. Compared to PLLA films and sheets, PLA based fibers/rods are more attractive as a carrier for the controlled delivery of these molecules, as these devices possess larger surface area in addition to promoting strong interface between the fiber and matrix. For example, recently Charles et al. showed that PLA composites reinforced with PLLA fibers/HA fabricated by a compaction technique exhibited a linear increase in tensile modulus with increasing HA content (9.7 GPa for 15% HA vs 8.3 GPa for 0% HA) [39]. Likewise, Chen et al. utilized braided and a multilayered PLA fabric to improve the mechanical properties of the otherwise brittle calcium phosphate (CaP) composites [40]. Similarly, incorporation of bioactive glass into SR-PLAs was also observed to result in improved mechanical properties of the composites, making these screws more suitable for intervertebral ossification [41].
Osteomyelitis is a serious condition caused by inflammation in the bone, and it is difficult to treat. Current treatment approaches are based on the use of antibiotics. Similar to the TCP/HA or bioglass coating on PLLA fibers/rods to induce osteogenesis, ciprofloxacin, a commonly used antibiotic have been coated onto PLA rods to treat osteomyelitis. In one study by Veiranto et al., SR-PLA biointerference screws coated with ciprofloxacin elicited a rapid burst followed by which, a controlled delivery of the over a 44-week study was noted [42]. Similar to ciprofloxacin, gentamicin, another commonly used antibiotic for treating osteomyelitis have been frequently embedded in PLA rods, and have shown controlled release, thereby effectively preventing infection to the bone [43]. As gentamicin is a hydrophilic antibiotic molecule, careful selection of the carrier molecule is required for its controlled delivery. In current clinical settings, gentamicin is delivered through PMMA based bone cements to prevent localized infection. But as PMMA is a non-biodegradable, this method requires secondary surgery to remove the PMMA [44]. In this regard, PLA based devices have significant advantages over other biomaterials; primarily by their hydrophobic nature that prevents rapid dissolution of gentamicin, and secondarily by their ability to bind to gentamicin, further delaying the delivery of gentamicin [45, 46]. These studies demonstrate the versatility of PLA based fibers/rods in improving mechanical properties (modulus, strength, wear resistance, flexural modulus), inducing ossification (in conjunction with inorganic materials) and in addition to their capability in preventing osteomyelitis with the incorporation of a suitable antibiotic.
ii. Extrusion and Injection Molding
By far, the extrusion process has been the most important technique to melt process PLA for various orthopaedic applications. Most processing techniques including injection molding, blow molding, and fiber spinning incorporate some of the salient features of the extrusion process [28]. Bioresorbable screws, plates, meshes, rods, and nails currently used in clinical applications (Fig.1, Table 1) have been traditionally made via extrusion and injection molding processes. In addition, extrusion and injection molding processes are also routinely utilized to evaluate new materials for potential clinical applications. For example, it was shown recently by Danoux et al. that composites containing PLA and PLA/nano-hydroxyapatite (n-HA) fabricated by an extrusion process, despite a solid dense structure, demonstrated proliferation of hMSCs in an in vitro and in vivo canine model. In addition, upregulation of alkaline phosphatase (ALP) expression was observed in n-HA containing composite resulting in heterotopic bone formation [47]. Similarly using PLGA, Simpson et al. systematically evaluated the upregulation of rheological, thermal, and mechanical properties by reinforcing with CaCO3, HA, and two bioglass systems prepared by an extrusion process [48]. These results suggest the utility of extrusion as a technique to fabricate dense solid composites that could eventually replace currently used medical device biomaterials.
Extrusion and injection-molding processes predominantly result in dense and solid bulk structures. On the other hand, emerging alternative tissue engineering technologies for orthopaedic applications prefer fabrication techniques such as electrospinning, rapid prototyping, and micro/nano-fabrication techniques, as these techniques give greater control over the micro- and nano-structures of the scaffolds. Sometimes, extrusion is also combined with these emerging techniques to take advantage of multiple processes, resulting in hybrid materials. Rapid prototyping which imbibes the concept of extrusion is now favored for developing complex structures appropriate for tissue regeneration applications.
iii. Rapid Prototyping (RP)
Ever since Giordano and coworkers fabricated complex structures via a layer-by-layer approach called rapid prototyping (RP), interest in applying this technique for tissue engineering applications has grown exponentially [49]. This is partly also because, in recent years, efforts to fabricate tissue engineered scaffold structures using conventional techniques such as solvent casting/particulate leaching, gas foaming, and freeze-drying have been found to be sub-optimal (pore size, mechanical properties, toxicity concerns) for tissue regeneration. Currently, various rapid prototyping techniques such as fused deposition modeling (FDM), meniscus-confined electrodeposition, conformal printing, UV-assisted printing, and solvent-cast printings have been developed with the aid of computer-aided design/manufacturing (CAD/CAM) to fabricate scaffolds that satisfy specific requirements for tissue engineering applications [50].
Due to their suitable mechanical and rheological properties, PLA has been considered as an ideal material for RP process using FDM, conformal printing, and solvent-cast methods. Fused deposition process, a process similar to melt extrusion, has been utilized to fabricate 3-D structures via a layer-by-layer deposition process; by utilizing a robotically-controlled extruder, free standing 3-D structures having fabricated out of PLA [49, 51, 52]. Likewise, by the extrusion of PLA filaments onto a rotating drum, using a conformal printing approach, free standing PLA structures have been also fabricated [53]. Initial concerns with the RP process were if RP could be utilized to (1) prepare tissue engineering scaffolds with the appropriate geometry, (2) embed proteins/growth factors within these scaffolds, and (3) whether the cells would grow on them. These concerns were mitigated by several studies that showed scaffolds could indeed be fabricated with suitable geometry along with the incorporation of growth factors and cells [54–56], and the focus has moved to the dimensional accuracy of these constructs to mimic physiologically-relevant structures.
Several approaches have been reported recently utilizing RP process to fabricate precise scaffolds for tissue regeneration. A recent study reported fabrication of PLA structures by a 3-D printing process that exhibited excellent structural integrity with superior thermo-mechanical characteristics. Such superior structural integrity was achieved by cross-linking the extruded PLA structures by ionizing radiation [57]. Another approach was reported recently by Senatov et al. who reported 3-D printing of dimensionally stable PLA/HA structures which demonstrated a shape-memory effect via multiple compression-heat-compression cycles [58]. This characteristic can be clinically useful as implants are expected to adhere and conform to the site of tissue repair. In addition to improving structural stability as explored by these studies, a complicated set of structures were recently fabricated with high dimensional accuracy for 15 anatomical components of the vertebra [59] offering the possibility of utilizing 3-D printed PLA based materials for regenerating complex tissues. A unique advantage of RP process is their capability to fabricate not only structurally and dimensionally stable structures, but also complex structures comprised of different materials that have suitability for engineering complex tissue such as osteochondral regeneration [60].
iv. Nanofabrication Technologies
a) Nanofibers
Electrospinning as a fiber forming technique was first reported more than a hundred years ago, but interest in those fibers for biomedical applications has emerged only in the past 15 years. Due to their versatility and in-expensive setup costs, it is fast emerging as a technique to prepare fibers with sub-micron diameters [61, 62]. In principle, an electrospinning apparatus consists of (1) a high precision pump to deliver solution, (2) a high voltage generating source, and (3) a collector to collect fibers in the form of a non-woven mat. As the polymer solution or melt is ejected out of the spinneret at a certain rate, the electrical force between the spinneret and the collector causes the formation of spherical droplets at the end of the needle [63]. The droplet then undergoes distortion resulting in the formation of a conical shaped structure, known as a Taylor cone [64]. The Taylor cone thus formed is highly unstable, and as the surface tension is continuously overcome by the electrostatic force, Taylor cone continues to undergo elongation and whipping resulting in the formation of submicron fibers that are collected in the form of a non-woven mat on the collector [65]. By using parallel plates, rotating drum or discs as collectors, the morphology of the resultant fibers can be varied [66]. In addition, the porosity and fiber diameter can be carefully controlled by varying solution properties (concentration, surface tension, molecular weight, additives, conductivity) or processing (potential difference, tip to collector difference, delivery rate, humidity, needle size). For example, by carefully selecting the solvents, the morphology of the electrospun PLA fibers can be varied from porous to smooth [67]. PLA based scaffolds are primarily intended for tissue engineering and drug delivery applications. In addition to tissue engineering applications, PLA based scaffolds have been extensively reported for encapsulating and delivering bioactive agents such as pharmaceutical agents, nanoparticles, anti-microbial, and anti-bacterial agents [68–73]. This is typically accomplished by simply mixing these agents in polymer solution and electrospinning or can be achieved by either co-electrospinning or core-shell electrospinning.
In principle, electrospinning is similar to melt and solution spinning, except that electrospinning utilizes electrostatic force, rather than mechanical extrusion to spin the solution or melt into fibers. Due to the combined effect of solvent evaporation and strong electrical fields between the polymer (solution or melt) and the collector, the polymeric jet undergoes significant elongation, resulting in a drastic reduction in fiber diameters [74]. The resulting low (sub-micron) fiber diameters that mimic extra cellular matrix (ECM) protein structure combined with the high surface area to volume ratio of the electrospun fibers allow cell attachment and easy transport of nutrients and waste have made this process ideal for tissue engineering applications. Our group was the first to evaluate the electrospin PLA nanofibers for skin and cartilage tissue engineering applications [75]. In addition, our group evaluated these biomaterials for several soft and hard tissues, including muscle, ligament, tendon, and bone [76–82]. Despite their capability to mimic the native ECM, typically not seen in scaffolds fabricated by other techniques, few major drawbacks such as the necessity to use a solvent and subsequent removal the applicability of scaffolds made from electrospinning.
To overcome this drawback, currently, melt electrospinning is emerging as an ideal alternative which do not require the use of toxic solvents [83]. Although, first reported by Larrondo and coworkers more than 30 years ago, melt electrospinning process did not find significant advancements primarily due to larger increases in the viscosity resulting in fibers with thicker diameters [84–86]. However, a systemic evaluation indicated applied voltage to play a significant role in the fiber diameters. Another key draw back with melt electrospinning, especially temperature sensitive polymer such as PLA have been their susceptibility to degrade upon thermal exposure [87]. One study investigated the experimental factors that can be attributed to the thermal degradation of PLA including temperature, distance travelled by the jet, type and content of antioxidant. It was found, temperature had a minimal impact on thermal degradation, when compared with the oxidant content or the distance travelled by the jet [88]. As large viscosities lead to thermal degradation of the resultant fibers, one way of overcoming them have been by using biocompatible plasticizer such as PEG. The addition of PEG not only causes reduction in thermal degradation but also enhances the melt electrospinnability and biological performance of PLA composites [89]. Another method was recently reported by Ogata et al., who utilized a laser beam to cause focalized melting of polymer chains in spinning process resulting in sub-micron fiber diameter, but their molar mass still decreased marginally [90].
Despite several drawbacks associated with the process, more recently, several strategies have been developed to make this process more effective for various tissue regeneration application. Direct writing of the electrospun jet, a term coined and extensively reported by Hutmacher and Dalton’s research groups, is one such technique that focuses on directing the melt electrospun jet into localized and targeted collection [91, 92]. This is typically accomplished by an automated lateral translation of the collector with respect to the polymer melt, thus making it possible for precise control of the architecture, similar to what can be achieved by RP process [93]. For example, one study reported the fabrication of a 1 mm × 1 mm × 1mm 3-D structure by carefully stacking new layer atop the previously fabricated layers [94]. Besides such linear 3-D structures, same group have also successfully fabricated 3-D structures with macroscopic pores, patterned and tubular structures that exhibited excellent cellular proliferation, and more importantly that resulted in even distribution of ECM proteins [95–99]. Due to their relative flexibility and thermal stability, most of these studies were performed on PCL. However, few studies were recently reported on PLLA using direct writing approach that explored fabrication of 3-D structures containing n-HA, and another study reported direct writing onto a pork liver demonstrating, although by limited studies, the possibility of melt electrospinning PLLA based fibers for tissue regeneration [29, 100].
b) Nanoparticles
Nanoparticles (NPs) have been classically defined as particles that are within the sub-micron (<1 μm) size range [101]. Based on the mode of loading of the carriers and the drugs, they can be further classified as nanospheres and nanocapsules. Nanospheres are obtained, when the drugs or small molecules are embedded and distributed throughout the matrix. Similarly, nanocapsules are obtained when the drugs or small molecules are confined in a small cavity surrounded by the polymer matrix [102]. Nanoparticles have been considered as superior carriers for proteins, short chain peptides and genes, as they protect the drug from premature degradation and also due to their ability to control the release rate [103, 104]. Furthermore, these nano-carriers, have the potential to permeate biological barriers such as the blood-brain barrier and result in higher cellular uptake compared to micro particles [105].
NPs derived from natural and synthetic polymers have been studied extensively over the past several decades. PLA has been one of the most widely used synthetic polymeric material in the preparation of NPs, due to its excellent biodegradability, non-toxic nature, and biocompatibility [106]. Most importantly, by co-polymerizing lactic acid with a variety of co-monomers such as glycolic acid, ethylene glycol, ε-caprolactone, the degradation rates of the NPs can be modulated [107]. Furthermore, surface properties of the preformed PLA-NPs can be modified by post-processing with PEG, chitosan, dextrans, polyoxamer, polysorbate, and thiol groups to enhance the compatibility and improve the circulation of the NPs in the blood [108–113]. Modulating the surface properties of PLA nanoparticles is attractive as it can help control the cell-material interaction at the polymeric surface [114]. In a recent study, Jain et al., showed that PLA nanoparticles coated with PEG and polysorbate were the least to be taken up by liver and kidney cells in an in-vitro model. This study further demonstrated that by modulating the surface properties, PLA based NPs have the potential for targeted drug delivery [115]. Several methods such as emulsion, nanoprecipitation, salting out, and spray-drying have been reported to encapsulate drugs in in PLA-NPs [116–118]. These studies demonstrate the capability and potential of these nanoparticles to deliver drugs at the site of interest with great temporal and spatial control.
3. The Principles of Regenerative Engineering
After the emergence of tissue engineering as a field, past 30 years has seen significant strides made from simple application of injecting appropriate cells to developing scaffold with topographical, biological, and mechanical cues required to simulate the regeneration of tissues. Tissue engineering, in its infancy focused on utilizing biomaterial to improve or restore the diseased tissue [119]. The paradigm of regenerative engineering, an interdisciplinary field, has emerged from the convergence of tissue engineering, advanced materials science, stem cell science, and developmental biology to regenerate damaged complex tissues [120, 121]. Advanced material science provides scaffolds with the right geometry and architecture to provide adequate mechanical support and modulate the cellular activities by sequestering and presenting the chemical and biochemical cues with precise spatial and temporal control. In parallel, developmental biology provides the understanding of the fundamental processes that drive the development of functional tissue, which may be applied to tissue healing and regeneration. Vital cues provided by developmental biology may need to be recapitulated for complex tissue regeneration beyond critical size defects. In this aspect, growth factors and small molecules (discussed later) play key roles in exercising control over cellular fate [122].
Stem cells play a crucial role in the regenerative engineering as most of the resident cells originate from these cells. Currently, stem cells are being investigated for the repair and regeneration of cartilage, bone, ligament, tendon, and muscle tissue [123]. These cells have the capability to (1) replicate; (2) release bioactive substances such as growth factors, cytokines, and chemokines for the growth and migration of cells, (3) immuno-modulate the tissue environment, allowing the tissue healing; (4) differentiate into the cell phenotype of the tissue of interest [124]. A significant challenge has been to develop suitable carriers to deliver these cells to the injury site to take advantage of their healing potential. Likewise, controlling and tracking the fate of stem cells once delivered are other significant challenges [125]. Beyond its traditional application in tissue engineering, PLA biomaterials offer tremendous potential for use in the regenerative engineering of orthopaedic tissue via innovative application with advanced material fabrication processes, stem cells, and understanding from developmental biology.
4. Bone Regeneration
i. Anatomy
As a dynamic tissue with moderate vasculature, bone provides physical support, locomotion and strength, protects soft tissues, and maintains pH in the body. The bone marrow contains mesenchymal stem cells, calcium and phosphorous ions that are essential for numerous physiological processes [126]. Bone is an organic/inorganic composite organized in a hierarchical structure comprising an organic phase mostly containing type-I collagen (30%) and an inorganic phase containing hydroxyapatite crystals (Fig.2) [127, 128]. In addition to type-I collagen, small amounts (5%) of glycoproteins, glycosaminoglycan, and other proteoglycans are also found in the organic phase. Likewise, small amounts of bicarbonates, citrates, magnesium, potassium and sodium are found in the inorganic phase [129].
Figure 2.
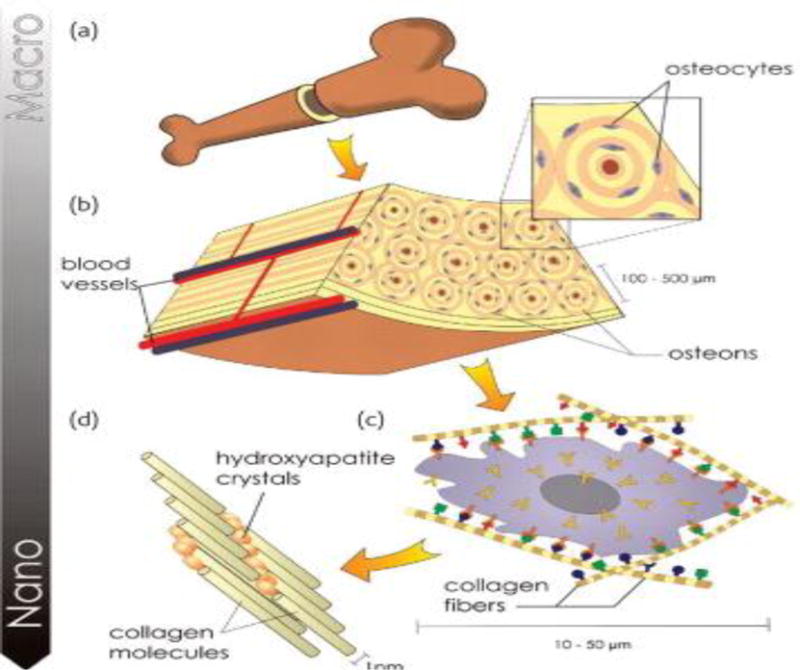
(a) The hierarchical organization of bone structure. (b) Internal structure of the bone is seen with osteons running parallel to the bone structure and centrally-running blood vessels for nutrient and waste transport. (c) Microstructure of the osteon is seen with the constituents of bone extracellular matrix (ECM). (d) Nanostructure of ECM consisting of collagen molecules nucleated with n-HA crystals. Image reproduced with permission from ref [128],copyright Elsevier (2008).
Organic phase (collagen) provides the tensile strength and fracture toughness, whereas the inorganic phase provides the compressive strength to the tissue. The bone structure can be classified by its mechanical properties, geometry, and architecture into cortical (compact) and cancellous (trabecular) bone types. The cortical bone has Young’s modulus values ranging from 17 to 20 GPa and compressive strengths in the range of 130–220 MPa, while trabecular bones has Young’s modulus and compressive strengths in the range of 50–100 MPa, and 5–10 MPa, respectively [130]. The micro/nano sized damage encountered by bone tissue due to routine activities heals by itself via remodeling. In remodeling, cellular components of bone tissue including osteoblasts, osteoclasts, and osteocytes, various growth factors, hormones, and signaling molecules play a key role in establishing the new tissue. The bone formation process, osteogenesis, occur via an endochondral ossification or an intramembranous ossification pathway. Both modes of osteogenesis are highly regulated and share several molecular regulators such as Indian hedgehog, parathyroid hormone related peptides, bone morphogenetic proteins (BMPs), vascular endothelial growth factors (VEGFs) and fibroblast growth factors (FGFs) [131, 132].
ii. Scaffold Based Strategies for Bone Regeneration
Bone tissue undergoes frequent remodeling; however, this process is compromised in individuals with genetic defects, trauma, bone tumors, hormonal imbalance. Current treatment strategies such as allo-, auto-, and xeno-grafts have their own set of complications necessitating the development of biomaterial-based substrates for bone tissue regeneration [133, 134]. The biomaterial based scaffolds intended for bone regeneration must have (1) biodegradable, biocompatible, bio-functional, and non-toxic characteristics; (2) surface epitopes for cells to adhere and proliferate (osteoconductive); (3) degradation rates closely matching the tissue regeneration rates; (4) chemical and physical features, similar to that of native bone tissue (organic/inorganic composite structure); (5) highly interconnected three dimensional pore structure with sufficient pore size (ideal: 300 μm, acceptable: 200 μm) for cells to proliferate, and allow mass transfer (nutrients, and metabolic waste); (7) potential to encapsulate growth factors, stem cells, anti-inflammatory agents, and additional necessary factors (osteogenicity); (8) strong adhesion and bond with the newly formed bone tissue (osteointegrity); (9) ability to recruit progenitor cells and differentiate into desired lineages, and induces new bone growth (osteoinductive).
From a biomaterial standpoint, several materials satisfy most of the requirements in terms of mechanical, chemical, and cell-material interactions. Collagen, for example, would be the most obvious choice as it constitutes the organic phase of the bone tissue [135]. Similarly, ceramics would be another choice as they constitute the inorganic phase of the tissue [136]. However, both have drawbacks: poor processability and brittleness in the case of ceramics, and poor mechanical strength in the case of collagen fibers. Therefore, polymeric biomaterials such as PLA have been extensively investigated for bone and orthopaedic regeneration applications. Since the native bone is a large and three-dimensional tissue (3-D), several fabrication processes such as electrospinning, solvent casting/particulate leaching, gas blowing, microsphere sintering, thermally-induced phase separation, 3-D printing, and self-assembly techniques have been employed to make 3-D scaffolds out of PLA biomaterials. A summary of PLA based scaffolds reported for bone tissue regeneration is shown in Table 2, and representative scaffolds are illustrated in Figure 3.
Table 2.
List of scaffolds that have been reported for bone tissue regeneration.
| Process | Composition | Porosity | Study | Ref |
|---|---|---|---|---|
| Salt leaching | PLLA fiber/PCL | Bimodal (1–10 μm; 100–400 μm) | in-vitro study (Human osteoblasts and MSCs) indicated enhanced osteogenic potential due to higher exposure of bioceramics | [137] |
| Freeze drying | PLLA/Collagen | 400–500 μm | Gradual dexamethasone release from collagen microbeads enhanced the osteogenic differentiation of MSCs | [138] |
| Sugar leaching | PLLA | 200–300μm, and 300–500 μm | Highly porous and interconnected structure made from a non-solvent sugar leaching method resulted in enhanced proliferation of rabbit-MSCs | [139] |
| RGD-g-PLGA/HA-g-PLLA | 100–200μm, >85% porous | RGD peptide grafted porous PLA scaffolds facilitated complete regeneration of bone tissue in a rabbit model. | [140] | |
| Compression molding/particulate leaching | PLLA/β-TCP | 200–400μm, 70% porosity | Porous scaffold (PLA/β-TCP) with high interconnectivity made from organic solvent free technique showed higher cellular adhesion and osteoblasts differentiation in an in vitro model | [141] |
| Particulate leaching | PLGA-g-HA | 152±76 μm, >80% porous | nHA grafted on PLGA scaffolds demonstrated faster and higher mineralization, compared to HA coated scaffolds. | [142] |
| Melt spinning | PLGA-HA | >70% porous | Micro/nonporous scaffold (PLGA/HA) resulted in higher proliferation and differentiation of MSCs. Higher ALP and mineralization was observed in vitro; in addition, rapid bone healing observed in a rabbit model. | [143] |
| Salt and sugar porogen | PLGA | >90% porous | Unique microstructure with pore walls containing microgrooves and micropits controlled the release of BSA, simultaneously facilitating growth of pre-osteoblasts (MC3T3) | [144] |
| Electrospinning | PLGA-nDd | Fiber dia: 270±9nm | Addition of nDd increased the hardness of the PLGA nanofibers and elicited no cytotoxicity. | [145] |
| PLGA-GO | Fiber dia: 0.8–1.5μm | Addition of GO enhanced hydrophilicity, protein binding capability. GO also accelerated cell adhesion, proliferation and differentiation of MSCs into osteogenic lineage. | [146] | |
| PLGA-mSi | >85% porous | Addition of mSi increased cellular adhesion and osteogenic potential of BMSCs. It also facilitated higher loading of BMP-2 and modulated its release. | [147] | |
| PLGA/PCL | Fiber dia: PLGA 2.4±0.66μm | Electrospun biphasic aligned PCL and random PLGA nanofibers facilitated BMSC differentiation into cartilage and osteogenic phenotypes | [148] | |
| PLGA-Willemite | Fiber dia: 300±500 nm | Willemite coating on electrospun PLGA nanofibers had no cytotoxic effects, and facilitated bone regeneration in a rat model. | [149] | |
| Microspheres | PLLA nanofibers/PLLA microspheres | Pore size: 300–355 μm; 425–600 μm; 600–710 μm | Hybrid scaffold (PLLA/HA microspheres and PLLA nanofibers) mimicked the ECM characteristics of the bone. Addition of nanofibers did not hinder the proliferation of murine osteoblasts. | [150] |
| PLLA or PDLLA | Diameter or pore size: not determined | Modification of microspheres by physical adsorption of cationic polymers or addition of copolymer containing PLA and chitosan, enhanced fibroblast attachment and proliferation. | [151] | |
| Hybrid nanofiber (PLLA/PCL)/microsphere (dextran-FGF2) system | Fiber dia:1300±400 nm; 1100±300 nm | Growth factor delivery was modulated by the gradient scaffold with three phases (electrospun nanofiber/microsphere/electrospun nanofiber) making it suitable for interface tissue engineering | [152] | |
| 3-D printing | PLGA/TCP/icarit in | Pore size: 500 μm | Controlled release of icaritin observed from PLGA/HA. The higher loading of icaritin retained the porous structure of the matrix throughout the 12-week study. | [153] |
| PLGA/TCP | Pore size: varying | Controlled porosity and interconnectivity, promoted bone tissue in-growth. Higher bone regeneration observed in these constructs with precise geometry. | [154] | |
| PLGA/nHA/TGF-β1 | Pore size:500 μm | Controlled release of TGF-β from PLGA microspheres modulated bone and cartilage regeneration. | [155] | |
| PLLA | Pre size: varying | The dimensions of 3-D printed vertebrae matched 15 different anatomical features of vertebral body. | [59] |
Figure 3. Scaffolds reported for bone tissue regeneration applications.
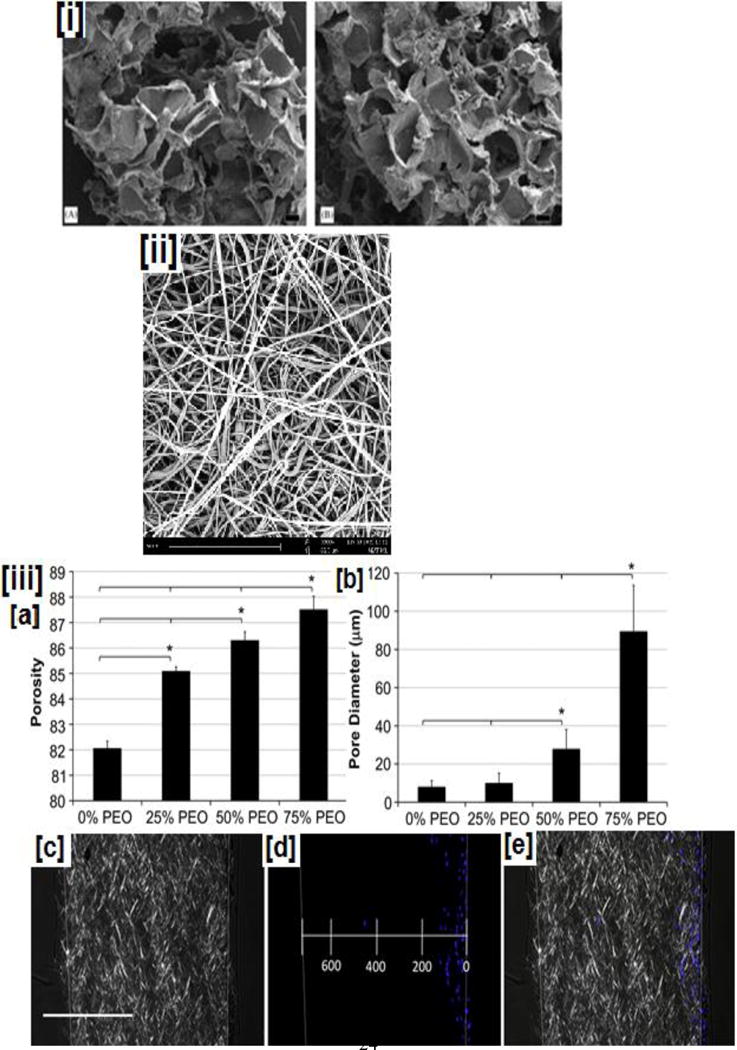
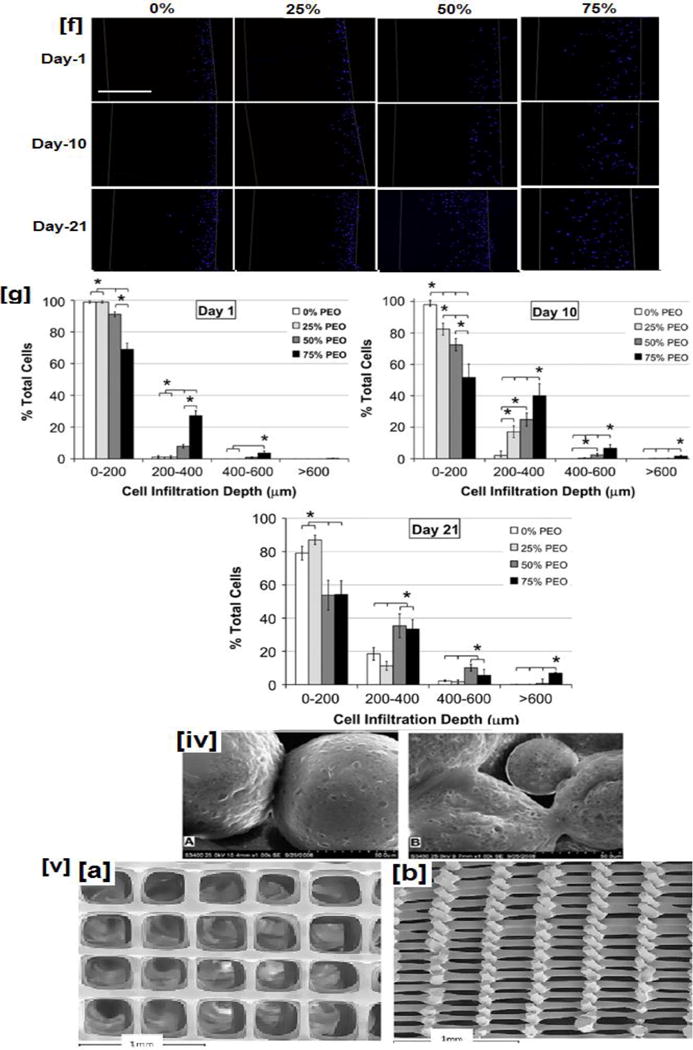
(i) SEM micrographs of (i) PLGA/HA composite obtained from solvent casting/particulate leaching method, (ii) representative electrospun nanofibrous scaffolds, (iii a–b) porosity and pore diameter of electrospun dependence of PLLA nanofibers on the added PEG content, (iii c–e) combined polarized bright field and fluorescent imaging indicate the cellular infiltration through the scaffolds, (iii f) cellular infiltration observed for scaffolds made with varying PEG content at various time points. Higher cellular infiltration is observed in scaffolds containing higher PEG contents even at shorter time points, while scaffolds with lower PEG content exhibit poor cellular infiltration at shorter time points, (iii g) quantification of cellular infiltration through scaffolds made with varying PEG content (iv) PLGA based microspheres: (a). before and (b) 7-days after in-vitro cell culture with osteoblast cells (MG-63), (v) Top (a) and cross sectional view (c) of PLA based scaffolds obtained by nozzle deposition system (3-D printing process). Image (i) reprinted with permission from [137], copyright Elsevier 2006. Image (ii) reproduced with permission from ref [168], copyright American Chemical Society 2015. Images (iii) reproduced with permission from ref [172]. Images (iv) and (v) reprinted with permission from refs [197, 198], copyright Elsevier 2010 and 2013.
PLA based materials, have excellent mechanical properties, processability, biocompatibility, favorable degradation rates, and do not elicit significant inflammatory response. However, their major drawback is the absence of any surface epitopes that could facilitate cell adhesion and proliferation. Several ways have been developed, including the use of plasma treatment, protein adsorption, immobilization of hydrophilic molecules, and surface functionalization with bioactive epitopes to overcome this drawback and to make these materials more conducive for bone regeneration applications[156, 157].
One of the oldest techniques to prepare scaffolds with relatively high porosity and interconnectivity have been by solvent casting/porogen leaching technique (Fig. 3i) [137], This is accomplished by leaching a porogen (salt, saccharides, proteins, or wax) from polymer-porogen blend. Since the 1990’s, both PLLA as well as PLGA scaffolds have been prepared and studied extensively using this technique for bone tissue engineering applications [158, 159]. Key drawbacks of this technique are the use of toxic organic solvents, phase separation of organic/inorganic phases, low thickness (0.5 to 2 mm), and poor cellular infiltration into the interior pores of the scaffolds [160]. A modified approach via thermal phase separation (TIPS) has been extensively studied to prepare polymer solutions at higher temperatures, and induce phase separation between the solids and liquids by lowering the temperature. By removing the liquid phase by freeze-drying or alternative techniques, a large porous structure is formed. Advantages of this method include, uniform and larger pore structures (>100 μm), and better mechanical properties compared to solvent casting-porogen leaching [161, 162].
The necessity for the use of toxic inorganic solvents has been a disadvantage till now; however, several modifications have been proposed using more green solvents such as ethyl acetate (FDA approved food ingredient), dimethyl carbonate, etc., making this process attractive for bone tissue regeneration application [163, 164]. Another approach (gas foaming) employs CO2 or compounds such as ammonium bicarbonate to create pores in the polymeric scaffold structure (Fig.3ii) [137, 165]. This approach completely eliminates the necessity of organic solvents, while resulting in polymer foams with pore sizes of 100 μm and porosity >90% [166, 167]. However, non-uniform pore sizes and poor interconnectivity have been their major drawbacks [167].
Electrospun fibers typically have diameters in the nano-submicron size range and offer a high surface area to volume ratio (Fig.3iii) [168]. In addition, due to their small fiber diameters, they mimic the nano-sized physical characteristics of the ECMs. Growth factors, anti-bacterial drugs, inorganic minerals, and other factors have been embedded and sequestered in the electrospun nanofibrous matrix (for example, using NPs), and delivered appropriately [104, 169, 170]. However, these scaffolds do not possess the appropriate pore size (>200 μm) for osteoblasts/MSCs migration and proliferation. To make the nanofibers more conducive for bone tissue engineering, several methodologies have been explored. In one study, the ECM matrix with its nano-and microstructural architecture was recreated by combining micro- and nanofiber technology[171]. In one study reported by Whited and coworkers, used a three-step methodology to create larger pores while retaining the nanofiber characteristics. In this, initially, electrospun PLLA-PEG nanofibers were obtained followed by the dissolution of the PEG layer. The nanofibers were then subsequently mineralized using an elaborate technique [172]. This methodology holds significant potential as the resulting scaffolds mimic osteogenesis by initially creating a disordered collagen-I nanofibrous matrix followed by mineralization [173]. In this process, active osteoblasts adhered and secreted more ordered/controlled matrix completing the osteogenesis process.
The results confirmed the hypothesis that with higher sacrificial-PEG content, porosity profiles and interconnectivity would support cellular attachment and proliferation through the scaffold. The authors observed a linear increase in porosity with increasing PEG content (Fig 3 (iii a–b)). When visualized by polarized bright field and fluorescent imaging, authors noted significant enhancements in cellular proliferation caused by higher porosity profiles generated by removal of PEG content (Fig 3 (iii c–e)). In addition to cellular proliferation, authors observed that at even shorter time points (1-day), in porous scaffolds, especially those made with 50 and 75% PEG content, 20% of osteoblast-like cells proliferated to 200–400 μm. It was interesting to note in scaffolds made without PEG, cells were not able to proliferate until the next time point (10-days). Finally, even at 10-day time, in 50% and 75% PEG scaffolds, cell proliferation up to 600 μm was possible, and their numbers increased significantly at 21-day point. Furthermore, ALP, an early stage marker for osteoblast attachment was upregulated with higher sacrificial-PEG. Moreover, conclusive evidence was provided by osteocalcin and calcium content, a late-stage marker significantly upregulated indicating the effectiveness of novel porous scaffolds in regenerating bone tissue [172].
Apart from low porosity, lack of bioactive surface epitopes in PLA based biomaterial has been a major drawback for its application in bone regeneration. Incorporation of biological macromolecules such as heparin has been observed to improve surface properties of PLLA. As the incorporated heparin has binding domains for various proteins, growth factors, and bone cells, differentiation of MSCs into osteogenic lineages can be anticipated [128]. More recently, two different approaches were reported by Cui et al [174]., and Dinarvand et al. [175] to address this issue. Cui et al. fabricated PDLLA nanofibers and subsequently functionalized them with various chemical groups (amino-, hydroxyl, and carboxyl-). Upon mineralization from a simulated body fluid, they observed mineralization with smallest crystal size in the scaffolds functionalized with all three groups in a particular ratio of 2:3:5. In addition, higher cell proliferation and osteogenesis was observed in these scaffolds [174]. Dinarvand et al. recently electrospun PLLA scaffolds which was subsequently oxygen plasma treated and mineralized with bioglass (BG), hydroxyapatite (HA), and tricalcium phosphate (TCP) [175]. In addition to plasma treatment and mineralization, the authors studied the bone formation induced by these scaffolds in vivo in a rat model. Post plasma treatment, HA and BG were noted to have a nano-sized coating on the scaffolds; whereas TCP had a microporous structure. Histological, μ-CT, and digital mammography experiments conclusively showed complete regeneration of the bone occurred in the HA-BG-PLLA scaffolds. Similar results were also reported by Andrić et al.[176], who demonstrated that presence of gelatin-PLLA nanofibers not only aided in increased mineralization, but also lead to significantly increased osteoblast attachment, proliferation, and differentiation [176, 177]. These studies cumulatively demonstrate that both porosity as well as surface epitopes are important for successful bone regeneration using PLLA nanofibers.
Another approach to develop scaffolds with high porosity and interconnectivity for bone tissue engineering is by thermal, solvent, and solvent-nonsolvent mediated sintering of microsphere matrices (Fig.3iv) [178]. These sintered matrices, in addition, can also act as a reservoir and delivery vehicles to release bioactive molecules, growth factors, and cytokines required for tissue regeneration [179–183]. Our group has reported preparation of heat, solvent-nonsolvent mediated sintering of several polymeric microspheres [181, 184–193]. In one study, we reported fabrication of orderly packed PLGA sintered microsphere scaffolds with mechanical properties (modulus: ca. 300 MPa) closely matching mid-range values of human trabecular bone [194]. In a sintered matrix, the cells occupy and proliferate through the pores with concurrent matrix degradation providing further volume for new cells to proliferate. However, this technique also suffers from low porosity in the interior of the scaffold resulting in poor nutrient and oxygen supply to cells [195]. To overcome this problem, Amini et al. recently reported scaffolds with pore size gradients that resulting in efficient nutrient and oxygen transfer, enhancing cell survivability [196]. Recent developments in this field include: precise 3-D printing of microspheres with topographies applied to the 3-D sintered microsphere system is the utilization of nanolithography [199] to develop grooves and pits that can not only mimic the ECM’s physical characteristics, but also aid in stem cell differentiation into osteogenic lineage [200].
iii. Nanoparticle-Mediated Bone Tissue Engineering
Nanoparticles (NPs) have been extensively investigated as payload carriers to deliver drugs, proteins, and other bioactive molecules to induce osteogenesis, anti-microbial properties, and enhance mechanical properties of the tissue engineered scaffolds. As bone tissue consists of collagen fibers nucleated with an inorganic phase containing hydroxy apatite particles (Fig.2), initial efforts were focused to replicate this intricate architecture in the scaffold. Nanoparticles of HA, TCP, and bioglass have been well known for their ability to induce osteogenesis, and improve the adhesion, proliferation, and differentiation of osteoblasts and osteoprogenitor cells. In addition, many research groups have demonstrated silver, TiO2, MgO, carbon nanotubes, carbon fibers, alumina, boron nitride nanotubes, cellulose nanocrystals, polyanilines, reduced graphenes, graphene oxides, nano-diamonds, and mesoporous silica-based nanoparticles for their osteogenic properties.
In addition to mechanical stimulation, as bone tissue is a piezoelectric tissue, application of electrical stimulation is thus an innovative way to direct osteogenesis [201]. In fact, some studies have shown the possibility of tissue regeneration with modest electrical stimulation resulting in DNA synthesis and proliferation [202]. A recent study by Cao et al. evaluated the effects of electrical stimulation via electrically conductive aniline pentamer-PLGA composites on bone cells [203]. The study showed, composites containing a minor portion of aniline pentamer (5% by wt) elicited a 50% increase in cell viability at earlier time points (1-day) and a slightly lower (30%) cell viability at longer time points (7-days). In addition to cell viability, they further showed that BMP-2, collagen-I, and osteonectin expression were upregulated at all time points in composites containing aniline pentamers, thereby demonstrating the usefulness of electrical stimulation on osteogenesis [203]. Similarly to this study, Bagchi et al. studied the electrical, mechanical, and chemical properties of three perovskite ceramic filled (calcium-, barium-, and strontium titanate) aliphatic polyester based composites on their capability to induce osteogenesis. The study demonstrated that all the perovskite containing composites exhibited higher conductivities, cell attachment, proliferation, and differentiation of osteoblasts [204]. Similar to AP and ceramics, graphene oxide NPs in PLA matrix was evaluated for their capability to induce cell adhesion and osteoblast differentiation. The study demonstrated excellent attachment due to higher wettability, and differentiation of osteoblasts towards bone formation [205]. These demonstrate by wisely choosing electrically conductive polymer or by adding conductive nanoparticles, osteogenesis can be triggered resulting in neo-tissue formation.
Current strategies to satisfy the mechanical requirements of the scaffolds have been primarily achieved by the incorporation of HA or TCP nanoparticles. However, with concomitant requirements for high porosity, even the addition of such nanoparticles, mechanical properties have still been inferior for cortical bones. In this aspect, as carbon nanotubes (CNT) (modulus >0.5 TPa) and boron nitride-based nanotubes (BNNT) (>0.5 TPa) are few of the strongest materials known to mankind, their application offers significant potential in bone regeneration. One study by Paiyz et al. evaluated the mechanical compatibility and cellular behavior of PLGA microsphere scaffolds reinforced with surface-modified (OH- and COOH-) multi-walled CNTs. They observed a 3-fold improvement in compressive strength and modulus values compared to neat PLGA scaffolds. In addition, no cytotoxic effects by surface modified (OH-) CNTs on PLGA composites was observed. Asides from cytocompatibility, higher cellular proliferation was observed, especially at shorter time points, observed by DNA content. Furthermore, these effects observed in vitro was confirmed by an in vivo rat subcutaneous model that showed delayed of PLGA microparticles due to the presence of MWNTs; however the mechanism of MWNT clearance in vivo was not established [206].
Similar to MWNTs, a study by Lahiri et al. reported the fabrication and subsequently evaluated the osteoblast activity of BNNT reinforced PLLA-PCL nanocomposites [207]. That study demonstrated with the addition of BNNT-NPs, as expected, a 10-fold increase in the modulus values (Fig 4A). But contrastingly, addition of NPs not only elicited cytocompatibility, but in fact, an 8-fold increase in cell viability (Fig 4B). Most important of all, Runx2 expression (Fig.4D), a regulator of osteoblast differentiation tremendously increased (up to 7-fold) with the addition of NPs. [207]. Although, this study particularly report possible reason for such high cell viability and gene expression towards osteoblast differentiation, a recent report suggested the presence of trace boron, and their subsequent interaction with the osteoblasts to cause differentiation of osteoblasts [208]. Boron nitride nanotubes or carbon nanotubes are external agents that trigger osteoblast differentiation of cells by enhancing the mechanical properties of the composite structures. But native bone tissue consists of small amounts of metallic or metal nanoparticles such as cooper, magnesium, zinc, iron, nickel, cobalt, and manganese [209]. As native bone tissue consists of these essential trace elements, few studies have hypothesized that addition of these elements would cause osteogenesis. Although mechanism through which they induce osteogenesis is currently not known, most trace metals are hypothesized to induce angiogenesis, a process interlinked with osteogenesis [209].
Figure 4. Mechanical properties, cell viability, and gene expression (Runx2) of PLLA-PCL scaffolds containing BNNT (2 and 5 wt %).
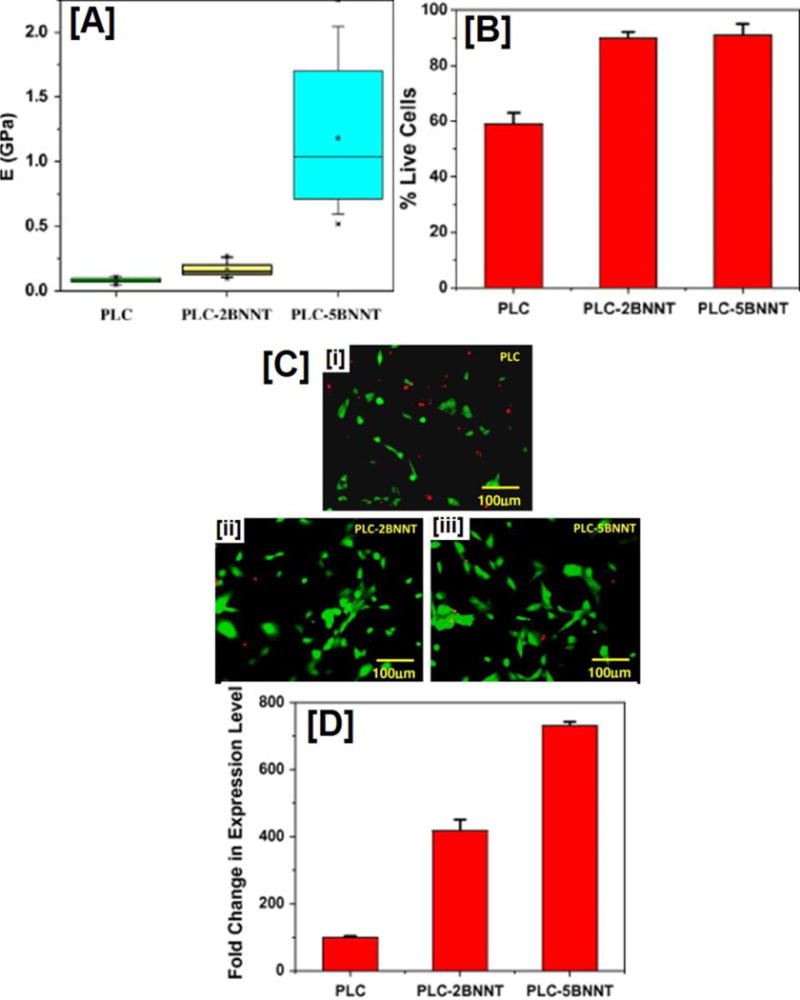
(A) Nano-indentation experiments demonstrate significant increases in modulus values in composites reinforced with BNNTs. The effect is more pronounced in composites containing 5 wt% BNNT. (B and C) quantification and fluorescent images of human osteoblast cells seeded on PLLA-PCL composites with and without BNNTs obtained by live-dead cell assay. Both quantified chart as well as fluorescent image indicate cytocompatibility of cells in scaffolds containing BNNTs; no statistical difference was observed in scaffolds with varying BNNT wt%. Finally, (D) several fold increases in Runx2 expression: a key regulator of osteoblastic differentiation. Images adapted with permission from ref [207], copyright Elsevier Ltd 2010.
In particular, magnesium asides from being a component of bone tissue, have been found to play key role in various physiological functions. Their role in viability of osteoblasts and in inducing osteogenesis was evaluated by Hickey and coworkers, who investigated the adhesion, proliferation, and differentiation of osteoblasts in PLLA-nHA nanocomposites containing MgO NPs. Addition of MgO caused nanoscale roughness, while at microscale level, PLLA control had higher surface roughness (Figs 5A–B). Furthermore, NP size and concentration of NPs were found to have profound impact on the surface roughness of the composites at nanoscale level. Moreover, addition of MgO NPs increased the modulus values of the composites with concomitant decrease in elongation, indicating significant improvements in stiffness of the composites (Fig 5C–D). While, addition of HA in the composites increased both the modulus as well as the elongation values, indicating the ductile-like failure modality. Unlike other NPs (CNTs and BNNTs) which remained in the scaffolds for longer duration, as MgO NPs have faster degradation especially in physiological conditions, two dilemmas existed. Firstly, their effect on cell toxicity and secondly, their effect on pH as they degraded. However, the study showed favorable cell viability and proliferation, in addition to marginal increase in the pH of the solution (Figs.5E–G). Infact, at shorter time points (4h), MgO containing samples elicited 50% and 30% higher cell viability, when compared to neat PLLA and PLLA/20% HA scaffolds, respectively. As with cellular activity, low pH change, especially at longer time points demonstrated low cytotoxic effects [210].
Figure 5. Surface roughness, mechanical properties, cell viability and proliferation, and degradation induced pH changes observed in MgO/HA reinforced PLLA composites.
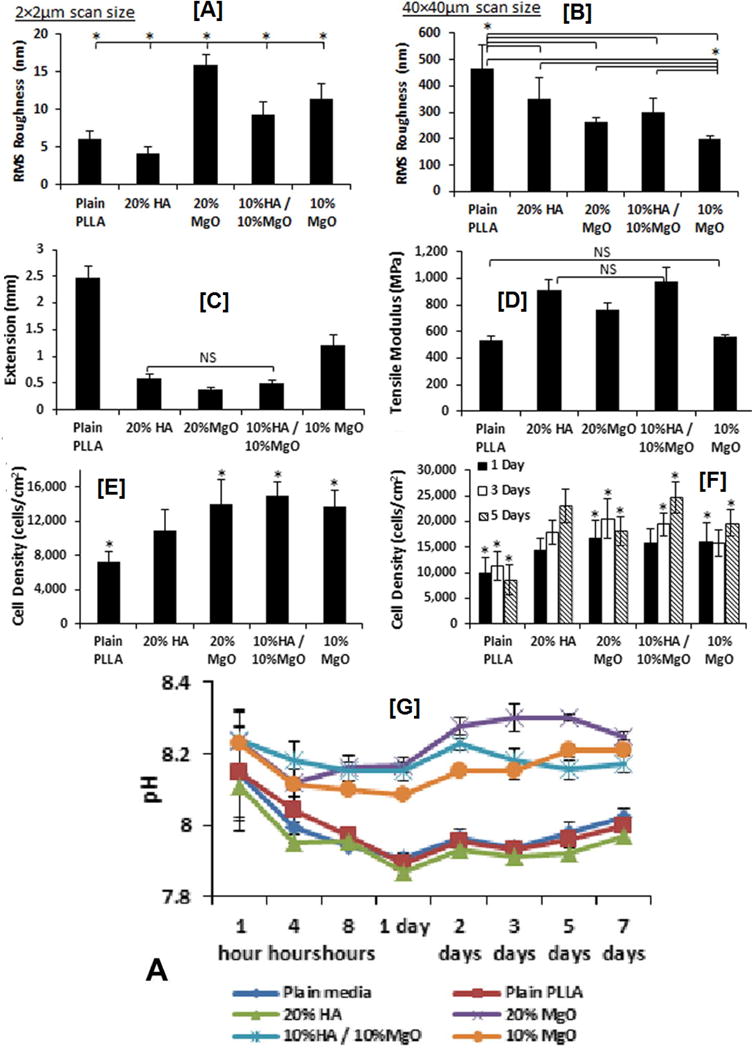
(A) Effect of MgO addition causing surface roughness at nanoscale level, while having minimal effect (B) at microscale level. (C) Lower elongation at failure and higher modulus (D) of composites containing higher loading of MgO indicating higher stiffness, while neat PLLA and 20% HA containing samples exhibit a ductile-like failure. (E) Higher cell viability and proliferation (F) observed in scaffolds containing higher MgO content (20% or 10%MgO/10%HA) compared to neat PLLA scaffolds. (F) pH changes in the cell culture media caused by degrading MgO NPs. While neat PLLA, plain media, and HA containing samples caused the media to turn acidic, MgO containing scaffolds caused sharp decrease at shorter times followed by a marginal increase at longer time points indicating low cytotoxic effects. Images adapted with permission from ref [210] copyright Elsevier Ltd 2015.
Bacterial, microbial, and fungal infections, and bone cancer (osteosarcoma) have been major concerns in the treatment of bone pathology. In addition to silver nanoparticles that have been widely studied for anti-bacterial/microbial applications, graphene oxide [208] and selenium nanoparticles have been demonstrated to be excellent candidates for this purpose [211, 212]. One recent study indicated a significant decrease in long term proliferation of osteoblast-like sarcoma cells, while the proliferation of osteoblasts were two times higher, indicating the dual purpose of these nanoparticles. Additionally such superior performance was realized without the use of chemotherapeutics or any drugs [213]. These studies demonstrated not only their capability to induce osteogenesis via electrical, mechanical stimulation, but also simultaneously provide/stimulate anti-microbial/fungal functionalities to the scaffolds.
iv. Proteins, Growth Factors, and Small Molecules Mediated Bone Regeneration
Bone regeneration requires a carefully orchestrated sequence of events involving osteogenic cells, scaffolds, and growth factors culminating with the vascularization of the newly formed bone tissue. Growth factors have been observed to play a key role in orchestrating the cascade of events from the onset of injury until the formation of tissue. Einhorn and others have carefully investigated this cascade, and have excellently summarized their observations for successful bone regeneration [214–217].
Several growth factors (GFs) such as transformation growth factor-Beta (TGF-β), platelet derived-growth factor (PDGF), bone morphogenetic proteins (BMPs), fibroblast growth factor (FGFs), insulin like growth factors (IGFs), vascular endothelial growth factors (VEGF), and angiopoietins have been implicated to have significant role in the bone remodeling process [215].
BMPs have been the most widely studied GFs for bone regeneration due to their excellent osteoinductive characteristics [218, 219]. Although, they have been approved by FDA, and some in vitro and in vivo studies have indicated their capability to induce bone formation [220], controversy still exists due to their toxicity at the required dosage, and with some studies demonstrating conflicting results [221]. Further, application of individual growth factors has been suboptimal for bone regeneration, as bone formation depends on coordinated events, suggesting the need for several growth factors in combination. Compounding the problem, GFs have a very short life in vivo as they are readily removed, and due to their large size, penetration into the cellular membrane is also low. Controlling the fate of GFs in vivo with temporal and spatial control is key to their utilization in regenerative engineering [222]. To this end, several interesting strategies have been reported. In one study, Su et al. reported incorporation of BMP-2/dexamethasone (Dex)/bovine serum albumin (BSA) in core-shell PLCL nanofibers. Of the several combinations they evaluated, they reported higher stability of BMPs in the core region with Dex contained in the shell region (with PLCL/collagen). The stability and controlled release of BMPs from the core region resulted in higher ALP activity (initial stage marker for osteogenic differentiation), and also osteocalcin expression (late stage marker for osteogenic differentiation) [223]. Likewise, using core-shell nanofibers, Yang and coworkers demonstrated controlled release of BSA from PLA nanofibers. BSA release was observed to be Fickian in nature, and the structure of BSA was retained during the electrospinning process [224]. These studies demonstrated that sequestering GFs in nanofibers had very low impact on the conformation of proteins and growth factors.
A more viable option was demonstrated by Shah and coworkers who showed that by enhancing the interaction between polyelectrolyte coated PLGA scaffolds containing growth factors (BMP-2 and PDGF-BB) fabricated by a layer-by-layer approach, the release of GFs can be temporally modulated to induce osteogenesis. They further showed that with careful arrangement of growth factors, both sequential and simultaneous delivery of GFs can be achieved. In addition, in vivo results indicated complete regeneration of the bone tissue (calvaria) without the necessity of autologous stem cells [225]. Dopamine coating has been widely demonstrated to exhibit higher levels of cellular attachment and proliferation in scaffolds that have significantly high hydrophobicity, and inert materials such as poly tetrafluoroethylene (PTFE), glass, and silicon [226]. Dopamine coating also affords the possibility of immobilizing GFs due to the presence of various surface groups. Shin and coworkers recently utilized a dopamine coated PLCL scaffold for immobilizing VEGF and FGF, and showed that dopamine coating enhanced the immobilization of both the GFs, improving the biochemical activity of human umbilical vein endothelial cells (HUVECS) [227]. In addition, GFs have also been immobilized on biomaterial substrates utilizing benign chemistry such as N-hydroxysuccinimide/N-ethylcarbodiimide or by using spacer molecules such as collagen and heparin [228]. These studies demonstrate significant progress in immobilizing GFs, and presenting these with temporal and spatial control for guiding cell-GF interactions.
Despite progress in GF delivery, the necessity of supra-physiological doses, low permeation into the cells, and higher costs, have caused significant interest in other molecules that function similar to GFs. Smaller therapeutic molecules have the capability to permeate the cell membrane, and can act as signaling molecules in several cell signaling pathways. Furthermore, they have the added advantage of higher stabilities, lower immunogenicity, non-conformation dependent characteristics, and lower costs [229]. Moreover, several extra-and intra-cellular proteins have been reported to act antagonistically to GFs. For example, smad proteins (smurf1, smad 6 and smad7), noggin, and gremlin have been reported to act as negative regulators of the BMP signaling pathway [230]. In orthopaedics, small molecule mediated tissue regeneration are increasingly being reported for bone, cartilage, and ligament regeneration. In a recent study, SVAK-12 has been observed to induce osteogenesis of C2C12 myoblasts by negating the antagonistic smurf-1 pathway [231]. Via a similar mechanism, utilizing a small molecule phenamil, our group recently reported in vitro bone formation through the downregulation of Smurf1 in sintered PLGA microsphere scaffolds [232]. In addition to BMP/smad pathway, several other pathways such as protein kinase A (PKA) simulated by 4-Bnz-cAMP,Wnt, Hh, and BMP/MAPK signaling cascades have also been observed to induce osteogenesis [233–236]. Other small molecules that have been observed to induce bone formation by these pathways include rapamycin, FK-506, and tilorone [237]. Similar to bone tissue regeneration, small molecules also find niches in engineering other orthopaedic tissues such as cartilage and ligament.
5. Ligament Regeneration
i. ACL Anatomy
Ligaments (Fig. 6) are dense, well-organized connective tissues that connect bone to bone. As a connective tissue, ligaments are subjected to and transfers high tensile and torsional loads, thereby mediating normal movement, and further providing stability to the joint [238]. Despite having a thin sheath of synovium, of the four ligaments in the knee, anterior cruciate ligament (ACL) is an avascular tissue [239]. Thus, without external intervention, injuries to ACL typically do not heal on their own. In addition, the ACL deficient knee typically leads to further damage in the meniscus and articular cartilage, resulting in tibial laxity [240, 241]. Due to the avascular nature of ACL and the high failure rates, primary repair by surgical suturing is currently not preferred, and ACL reconstruction using auto- and allo-grafts are the most preferred techniques to treat ACL tear [242]. Similar to bone regeneration, significant issues with auto- and allografts necessitates investigation of biomaterial based strategies for ACL regeneration [243].
Figure 6.
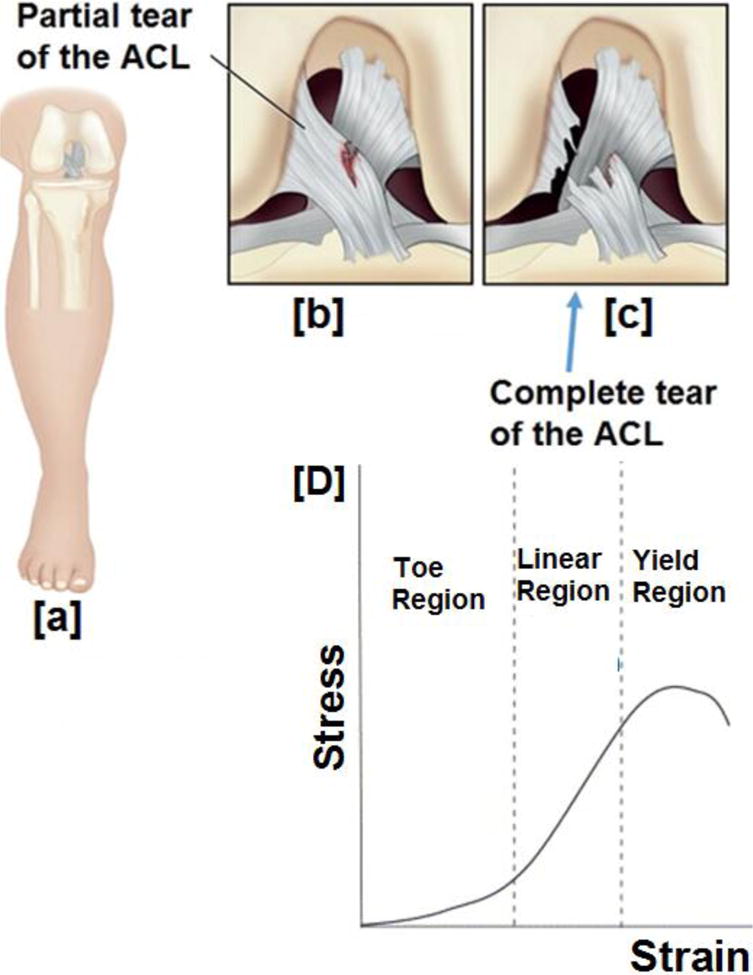
A schematic representation of the ACL progressing to a complete tear (b) partial tear of the ACL caused by an injury, (c) gradually progressing to a complete tear, (D) three stages of behavior encountered by the ligament under mechanical strain. Under strain, there is a toe region where applied strain is not translated to stress because of the straightening of the crimp fibers, while in the linear region, after crimp straightening, applied strain is directly proportional to the stress (Hookean limit). Finally, beyond linear region, ligament yields to undergo rupture. Image (6A–C) modified and reprinted with permission from ref [244], copyright Elsevier (2014). Image 6D reprinted with permission from ref [33]
Strategies to engineer a scaffold suitable for ACL regeneration is hampered by the incapability to mimic the three distinct regions (ligament, fibrocartilage, and bony ends) with unique features present in the ACL tissue. In addition, upon tensile loading, unique crimp pattern present in the ligament causes it to undergo three distinct stress-strain regions before failure. To further add to its complexity, ligaments are comprised of two bundle regions: anteromedial and posterolateral (AM, PL), with each bundle exhibiting distinctive behavior under tension and flexion loads. Thus, strategizing a suitable biomaterial for ACL regeneration is complex. But, based on the physical, chemical, biochemical, and cellular environment of the ACL tissue, an ideal biomaterial for successful ACL regeneration is expected to possess or promote (i) porosities in a gradient fashion to promote ligament-cartilage-bone regeneration; (ii) similar degradation characteristics coinciding with the tissue regeneration; (iii) appropriate biochemical cues (growth factors/small molecules/receptors) to facilitate stem cell differentiation into appropriate lineages; (iv) appropriate biomechanical properties (three zones as shown in Fig.6 D); (v) two bundles (mimicking AM and PL bundles) with crimp pattern; (vi) angiogenesis and provide neovascularization of ACL tissue.
ii. Scaffold Based ACL Regeneration
a) Fiber Based Scaffolds
Similar to bone regeneration, collagen was the first material investigated for ACL regeneration. The choice of collagen was obvious as over 80% (dry mass) of native ACL is comprised of collagen. As was seen with bone regeneration, results were abysmal necessitating search for alternatives. For example, when collagen fibers seeded with ACL fibroblasts were evaluated for ACL regeneration, although the fibroblasts were observed to be viable for 6 months, the collagen scaffold had been completely resorbed by this time point [245, 246]. In addition to collagen, other natural biomaterials such as alginates, chitosan, and silk were also studied. The presence of sericin (in silk) and the poor mechanical properties (of chitosan and alginates) have been their major disadvantage for ACL regeneration [247, 248]. To investigate the feasibility of utilizing biodegradable polymers for ACL regeneration, Bourke et el. evaluated PLLA and poly (Desaminotyrosyl-tyrosine ethyl ester carbonate) (poly DTE carbonate) fibers in an in vitro study for ACL repair. Although PLLA and poly (DTE carbonate) fibers had higher cellular attachment and proliferation, but after 30 weeks of incubation in PBS, only 7% of the initial mechanical strength of PLLA fibers was retained [249]. This was in contrast to a previous study that reported only a marginal decrease in the stiffness of PLLA fibers after 20 weeks of incubation in PBS [250]. One possible reason for the low mechanical properties observed in PLLA fibers was due to the construction technique employed (fiber bundles), resulting in poor transfer of the load.
In our laboratory, to specifically address this issue, we have fabricated 3-D braided scaffolds from PLA based biomaterials with specific geometry and architecture closing mimicking the architecture (intra-articular regions with the femoral and tibial ends) of native ACL tissue. In one study, degradation kinetics, mechanical properties, and cellular performance of these braided scaffolds for ligament regeneration were evaluated [251]. To overcome the drawback of lack of surface epitopes in PLA based materials, in a subsequent in vitro optimization study, Lu et al. studied the temporal, mechanical, cellular response, and degradation properties of braided scaffolds coated with fibronectin (Fn). Due to the fibronectin modification of the PLLA scaffolds, the braided PLLA-Fn scaffolds had higher cell adhesion, long-term cell proliferation, while retaining the degradation rates and higher mechanical properties of PLLA [252]. In addition, a follow-up in vitro study demonstrated braided PLLA-Fn scaffolds to promote larger extracellular matrix production by ACL fibroblasts at longer time points [253]. These studies indicate braided PLLA/PLLA-Fn to be a suitable biomaterial for ACL regeneration applications.
Similar to our approach (Fn coating), several approaches have been investigated to improve cell attachment, proliferation and ECM deposition rates. For example, Sarukawa and coworkers showed that coating PLA fibers with chitosan enhanced the adhesion and proliferation of ACL fibroblast cells. In addition to cell adhesion and proliferation, they further observed chitosan coating improved ECM matrix production [254]. As the selection of appropriate cell source is key for neoligament formation in vivo, one study evaluated ACL fibroblasts and fibroblasts from neighboring connective tissues (MCL, achilles- and patellar tendon) on a 3-D braided PLLA scaffold. The study showed that ACL fibroblasts had remarkable attachment and enhanced matrix production in the scaffolds [255]. Based on promising results from these studies, a 12-week in vivo study was conducted in a rabbit ACL model. At shorter time points (4-weeks), fibrous capsule and tissue infiltration were observed at the periphery of the engineered ligament seeded with ACL cells. But at longer time points, infiltration and strong attachment of cell and collagen-deposition was observed throughout the ligament, further illustrating the potential of braided PLLA fibers for ACL regeneration [256].
In addition to braided PLA based fibers, woven and knitted PLA based scaffolds have also been studied for ACL regeneration [257]. One study showed knitted PLLA yarns to have higher porosities, slower degradation, and structural stability for a long study time (up to 20 weeks). However, knitted PLLA yarns also exhibited lower mechanical properties as they had lesser capability to transfer load. Furthermore, high porosities observed in the knitted scaffolds, caused significant difficulties in cell seeding [31]. Woven scaffolds, on the other hand, reportedly had higher mechanical properties, similar to braided fibers in uniaxial direction, but due to their architecture, lesser cell growth was typically observed in woven constructs [258]. Hence, most preferred textile structure for ligament regeneration are braided structures due to excellent mechanical properties.
As electrospun nanofibers closely mimic the native ECM topography, electrospun nanofibers of PGA, PLGA, PDLLA, PCL, and PLLA have been fabricated and studied in vitro for ligament regeneration. Sudden collapse of nanofibrous structure of PGA and PDLLA at relatively shorter times in in vitro culture, precluded any further evaluation of adhesion/proliferation activity. On the other hand, robust growth of chondrocytes and MSCs were observed throughout the PLLA and PCL scaffolds, signifying the potential of PLLA and PCL nanofibers for ligament regeneration [259]. These results concurred with our microfiber based scaffolds made from aliphatic polyesters. Nanofibers also provide topographical cues, physical stimuli and direct cell growth along fiber surface. This is especially desirable in regeneration of ligament which is comprised of collagen fibers arranged in a hierarchical fashion. To understand the effect of nanofibers in inducing differentiation of MSCs, one study evaluated cellular activities of MSCs on aligned and randomly-oriented PLLA nanofibers for ACL regeneration. They reported, aligned fiber scaffolds to elicit higher cell attachment and proliferation, compared to randomly-oriented nanofibers, which was attributed to the nanoscale architecture. The cellular activity was also greatly enhanced by induced mechanical strain, especially in aligned nanofibers that showed larger matrix deposition. The RT-PCR experiments further illustrated upregulation of scleraxis at all time points. And at longer time-points, scleraxis upregulation was several fold higher than randomly-oriented fibers. This study indicated nanofibers enhanced cell attachment, proliferation, matrix deposition, and can potentially induce differentiation of pluripotent cells into ligamentogenic/tenogenic lineage, provided physical cues are available at the fiber surface [260].
Nanofibers that are typically obtained from electrospinning process are 2-D in nature and thus possess very small thickness. Combining nanofibers with micro-sized matrix to form a biphasic or multiphasic scaffold is an attractive way to utilize the advantages offered by the macro-sized matrix (porosity), while also retaining the ECM-mimicking characteristics of the nanofibers. Several strategies have been reported to combine PLA based micro/nanofibers for ligament/tendon regeneration. One study reported fabrication of PLGA nanofiber/silk microfiber hybrid scaffold joined by an adhesive layer (silk solution), which was then rolled up to form a cylindrical construct. Because of their cylindrical construct, it facilitated excellent proliferation of rabbit-MSCs through the porous regions of the cylindrical constructs [261]. Several other alternatives have also been reported to enhance the potential of these nanofibers in soft tissue regeneration. For example, thicker constructs have been developed by simply rolling or by stacking thinner layers resulting in robust tissue in-growth [262]. Another interesting approach was reported by Barber and coworkers, who developed a hybrid scaffold construct by braiding bundles of electrospun PLLA nanofibers. This approach combined the advantages of braiding and nanofiber structures. In addition, the authors reported a dependence of mechanical properties and MSC differentiation on the number of nanofiber bundles and applied cyclic strain [263]. These strategies efficiently improved the applicability of micro/nanofibers for ligament regeneration, but did not address the fundamental issue of providing a ligament-cartilage-bone fixation. To this extent, several strategies have been reported which will be discussed in the next section.
b) Scaffolds for Ligament-Bone Or Ligament-Cartilage Fixation
The 3-D braided scaffolds (Fig. 7i) developed in our lab provided tissue engineered constructs mimicking regions for ligament and, femoral and tibial insertion ends [264]. In a slightly modified approach, Freeman et al reported a braid-twist design on braided PLLA constructs, which on biomechanical analysis indicated similar tensile behavior to native ACL tissue (Fig 6D). The biomechanical results suggested biomimicry and the possibility of bone fixation; however, in vitro or in vivo experiments were not performed to confirm the hypothesis [33]. These methodologies partially addressed the bone-ligament fixation; however, lower mechanical strength of the constructs observed in a rabbit model indicated poor ligament-cartilage interface [256].
Figure 7. Reported scaffolds for ligament-cartilage-bone regeneration.
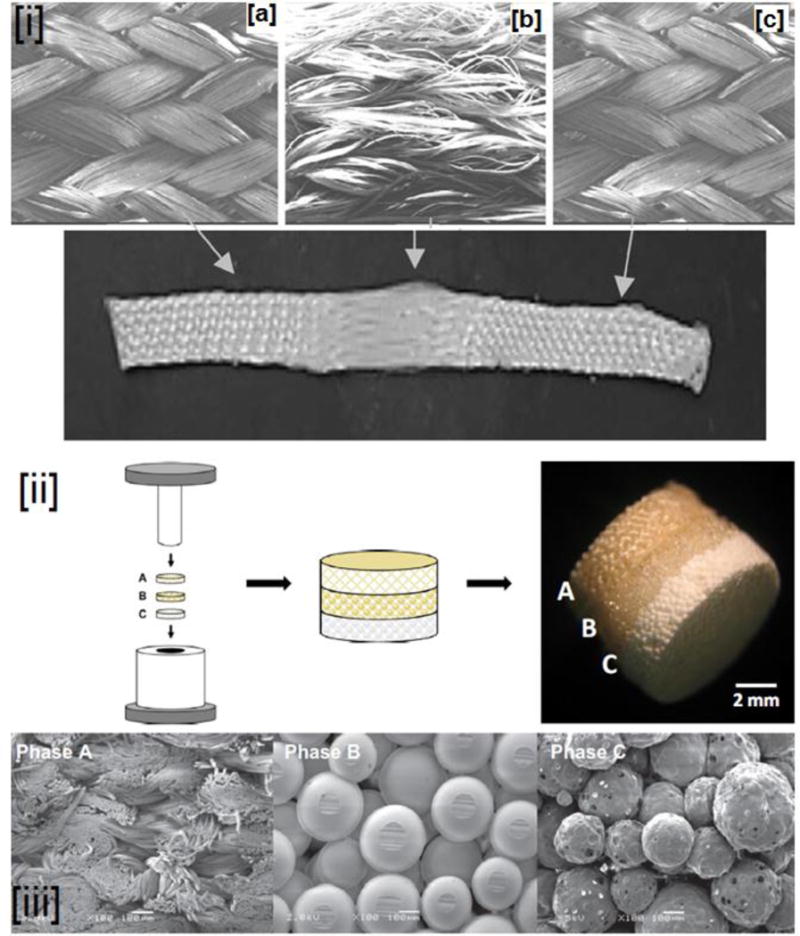
(i) 3-D braided biomimetic scaffold with (a) femoral, (b) intra-articular, and (c) tibial bony ends. The bony ends have higher orientation and the ligament region has lower fiber orientation closely mimicking the physical nature of the ligament, (ii) Triphasic scaffold with each phase mimicking a region (a) ligament, (b) cartilage, and (c) bone, of the ACL. (iii) SEM micrographs of the three phases in the triphasic scaffolds: (a) braided and PLGA fibers sintered with the neighboring phase, (b). Sintered PLGA microspheres, (c). PLGA microspheres with encapsulated bioglass to promote bone formation, (iv). FTIR-imaging of the mineralized and non-mineralized regions of the cartilage region of the ACL (variation of proteoglycan content in this noted). Images (i), (ii) and (iii), modified and reprinted with permission from refs [268, 269] copyright Elsevier (2005), (2015). Image (iv) reprinted with permission from PLOS One (2013) [270].
One approach evaluated PLLA constructs embedded in a gelatin hydrogel matrix containing basic fibroblast growth factor (FGF-β) in an in vivo study. Due to the presence and gradual release of FGF-β, osseous integration which occurred was confirmed by the enhanced production of type-I and type-III collagen. These results were further supported by mechanical testing that showed higher strength and stiffness in the scaffolds that contained bFGF [265]. Similarly, to improve the ligament-cartilage interface, Hayami and colleagues prepared a novel PCL-PDLLA nanofibers embedded in a methacrylated glycol chitosan (MGC) hydrogel system seeded with ligament cells, and their cellular activity observed for 4 weeks. MGC was utilized to mimic the proteoglycan-water phase of the ligament. Significant presence of type-I and III collagen along with decorin was noticed in immunohistochemical evaluation demonstrating the synthesis of cartilage tissue. These studies illustrated strategies that can be utilized to successfully enhance the interface between the cartilage and bone or ligament and cartilage, but not both. These studies further showed that scaffold preparation for ACL regeneration needs to take into account the complex nature of ACL tissue [266, 267].
iii. Scaffolds for Ligament-Cartilage-Bone Regeneration
Engineering connective tissue such as osteochondral have been relatively easier, primarily because of lesser number of phases, when compared to ACL tissue [271]. Based on the physical, mechanical, cellular, and biochemical characteristics, multiphasic scaffolds have been studied for osteochondral regeneration [272]. Initially reported by Yannas’s research group for skin regeneration, such strategies based on collagen-gag scaffolds were further developed for osteochondral regeneration [271, 273]. The multiphasic scaffolds are fabricated by providing variation in the topographical and morphological cues across the scaffold constructs; in addition, biochemical cues are varied by, for example, by utilizing multiple biomaterials, thereby potentially mimicking the complex tissue [274]. Currently, multiphasic and gradient scaffold based approach are commonly employed for periodontal and osteochondral regeneration [275, 276], but reports have been sparse for ligament regeneration.
The first strategy for regenerating ligament-cartilage-bone interface was demonstrated by Spalazzi and coworkers who developed a tri-phasic scaffold (Figs. 7ii–iii). The choice of triphasic scaffold is quite obvious, as each phase in triphasic scaffold can be modelled based on the native ACL tissue components, namely: ligament, cartilage, and bony insertions, respectively. The construct was initially fabricated individually using braided PLGA fibers, PLGA microspheres, and PLGA-bio glass microspheres, respectively (Fig. 7iii). The individual components were then sintered to form a single entity with discrete phases (Fig.7ii) [277]. A co-culture of fibro- and osteoblasts was utilized as a cell source based on a previous experiments that showed temporal and spatial control of cell growth [278]. Gene expression further clarified the presence of type-I collagen in all three phases but at varying proportions (Phase A> Phase B > Phase C), which confirmed the presence of three unique phases: ligament, cartilage, and bone. Additionally, by strategically presenting bioglass in Phase C, and co-culturing fibro- and osteoblasts, ligament/cartilage and bone formation was promoted in Phases A and C, respectively, with a strong interface (Phase B).
In a subsequent study, to account for appropriate cells in Phase-B, the authors employed a tri-culture of fibroblasts, fibrochondrocytes, and osteoblasts. Immunohistochemical analysis and alcian blue staining revealed elevated levels of characteristic markers of cartilage: type-I and type-III collagen, and proteoglycans [279]. Likewise, variation of bone mineral content was noted throughout the scaffold construct. More recently, these results were corroborated using high sensitive imaging technique (Fourier transform infrared spectroscopy-imaging), that showed a gradual increase in the mineral content from the ligament the bone. In addition, an increase of proteoglycan content in the non-mineralized region, and a subsequent gradual decrease in the mineralized region indicated the presence of an interface (Fig. 7 iv) [270]. These results demonstrated that the regenerated tissue is biochemically similar to native ACL tissue, yet its long-term in vivo behavior and mechanical behavior are currently not known. In addition, mechanical evaluation of the construct was not performed, and hence is currently not known.
Similar to Spalazzi’s work, He et al. recently reported a multiphasic scaffold, fabricated by complex processes involving stereo lithography, sintering, and freeze-drying (Fig.8 a) [280]. By using PLGA solutions containing β-TCP at varying proportions, calcified and non- calcified regions of the native ligament was mimicked (lower in non-calcified and higher in calcified region) (middle sections in Figs 8A–B). In principle, the materials chosen (PLA, PLGA, PCL, and β-TCP) to fabricate the scaffolds in this study were similar to those reported by Spalazzi et al (PLGA, bioglass). As both bioglass and β-TCP are known osteoinductive and osteoconductive materials, they are anticipated to elicit similar behavior in in vitro and in vivo conditions. Furthermore, PLGA, PLA, and PCL belong to same group of aliphatic polyesters and are biocompatible. Although, from a materials perspective both the studies are similar, this study more precisely mimics the physical characteristics of the native ACL tissue. Despite its potential, in its current state, this construct had suboptimal porosities in all three phases (ca.100 μm), and a force bearing capability less than the adult native tissue [280].
Figure 8. Schematic of the reported multiphasic scaffold comprising phases for ligament, cartilage, and bone regeneration.
The ligament phase of this scaffold consisted of braided PLGA scaffold, with phase A consisting of PLGA microspheres to promote formation of non-calcified cartilage, and a minor constituent of bioglass added to PLGA microspheres in Phase B to facilitate calcification of cartilage tissue. Phase C consisted of PLGA microspheres with higher concentration of bioglass than Phase B for the promotion of bone regeneration (b) ligament-cartilage-bone interface in native ACL tissue. Images reprinted with permission from ref [280]. Copyright Elsevier 2015.
Yet another approach was reported by Chung et al. who fabricated a triphasic braided fibers comprised of braided porous PLLA fibers and poly (1, 8-octanediol-co-citric acid)–hydroxyapatite nanocomposites (POC–HA). Similar to our approach with braided fibers, in intra-articular region, fibers (PLLA) were loosely braided, and in the femoral and tibial ends, poly (1, 8-octanediol-co-citric acid) was tightly braided. A major difference between our approach and this study is their addition of HA to stimulate osteogenesis in the bony ends. Further functionality tests after in vivo surgery showed rabbits perform nominal tasks, albeit a slight swelling was observed in the reconstructed knee [281]. In addition to multiphasic scaffolds, scaffold with gradient mineralized regions and fiber alignment have been developed using an electrospinning process for ligament-cartilage-bone regeneration. The gradient scaffolds with varying fiber orientations were fabricated by electrospinning solutions containing varying nano-hydroxyapatite (n-HA) crystals for predefined time intervals. The results demonstrated significant variations in the fiber alignment as well as the distribution of n-HA throughout the scaffold, evidenced by fluorescence, thermogravimetric, and wide angle X-ray diffraction analyses. The results suggested that it is possible to sequester appropriate cues (n-HA, in this instance), while also providing physical cues (variations in fiber alignment) by simpler techniques, demonstrating potential for use in complex connective tissue regeneration such as ligaments [282].
Apart from multiphasic scaffolds, gradient scaffolds with appropriate cues such as growth factors have also been developed to generate complex tissue junction. This strategy reported sequestration of biochemical cues (growth factors/small molecules, etc.) into the constructs, and presented these cues with spatial and temporal control. In this study, Singh and coworkers utilized microsphere-based scaffolds to modulate the delivery of bioactive molecules with encapsulated dyes. As a proof-of-concept, they demonstrated the delivery of bioactive molecules with temporal and spatial control from microsphere scaffolds by varying the assembly of microspheres [283]. Utilizing similar systems with encapsulated bioactive molecules, the same research group demonstrated the feasibility of such systems for interface tissue engineering, especially the interface between bone and cartilage [284–287]. Yet another strategy was proposed by Phillips et al. who illustrated strategic immobilization of retrovirus embedded with Runx2 resulting in an enhanced soft-hard tissue interface [288]. But, till date, gradient scaffolds containing biochemical cues have not yet been reported for ligament-cartilage-bone regeneration.
Despite tremendous development in the last decade to fabricate scaffolds for ligament regeneration, a suitable technique has not yet been developed that result in scaffolds with features similar to that of native ACL tissue. From the initial concept of developing a mechanical equivalent of ligament, the stage has now been set for accurate conceptualization of the interface of ligament-bone systems. In this regard, both multiphasic scaffolds as well as gradient scaffolds hold potential as their main focus is the development of whole ligament including the interfacial regions.
6. Cartilage Regeneration
Cartilage tissue supports the body and transmits the applied load. The two most abundant types of cartilage in the body are hyaline cartilage and fibrocartilage. Hyaline cartilage is found at the end of long bones in joints such as the knee, elbow and shoulder, and is also found in the nucleus pulposus of the intervertebral disk. Likewise, fibrocartilage is found in the knee menisci, annulus fibrosus of intervertebral discs of the spine, and pubic symphysis.
One of the first studies to evaluate biomaterials as an alternative for cartilage replacement was reported by Veth et al. who studied a graft of polyurethane-PLLA (PU-PLLA) reinforced with carbon fiber in a canine model [289]. While this composite promoted ingrowth of fibrous tissue, intermittent formation of hyaline cartilage promoted further interest in PLA based biomaterials for meniscus and cartilage reconstruction. In one study, Ike et al. showed that tracheal implantation of a collagen-coated PLA mesh sutured with an autologous periosteal graft leading to the formation of cartilage around the implant [290]. A similar study by Von Schroeder et al. demonstrated the use of porous PLA scaffolds to repair articular cartilage defects in a lapine model [291]. Based on the successes of these early studies, new investigations have reported the use of PLA based scaffolds for the tissue engineering of cartilage and meniscus. A summary of these recent studies done in the last 5 years has been summarized in Table 3.
Table 3.
Recent studies on PLA based materials for articular cartilage regeneration.
| Polymer | Technique used | Cells or factors | In vitro or In vivo | Ref | Conclusions |
|---|---|---|---|---|---|
| PLGA | Solution casting | Chondrocytes | In vitro and In vivo (mice) | Zhang et al., 2012 [292] | Microtubular orientation of the scaffold improved the mechanical properties of the engineered cartilage |
| PLCL/chitosan | Porogen-leaching + freeze extraction + freeze gelation | MSCs | In vitro | Yang et al., 2012 [293] | Chitosan improved cell-seeding in PLCL scaffold |
| PLCL/chitosan | Porogen-leaching + freeze extraction + freeze gelation | Chondrocytes | In vitro | Li et al., 2012 [294] | PLCL cross-linked with chitosan has similar biochemical and viscoelastic properties as native bovine cartilage |
| PLCL/PHBV | Porogen leaching (PLCL), emulsion solvent evaporation (PHBV) | Chondrocytes | In vitro and in vivo (mice) | Li C. et al., 2013 [295] | PHBV microspheres improve the mechanical properties of PLCL |
| PLA | Electrospinning | Chondrocytes | In vitro | Stenhamre et al, 2013[296] | Nano-patterning onto the microfibers influences chondrocyte proliferation |
| PLA | 3D printing | Articular chondrocytes and NuP cells | In vitro | Rosenzweig et al., 2015 [297] | No difference in biomechanical properties of ABS or PLA printed scaffolds but differences in matrix formation. |
i. Knee Articular Cartilage Regeneration
Smooth hyaline cartilage provides a low-friction articulating surface for motion and is divided into superficial, middle, and deep zones (Fig.9) [298]. The superficial zone is composed of chondrocytes and stem cells within a collagen-rich matrix that facilitates bone cartilage repair. The chondrocytes have flattened morphology in the collagen matrix and lie parallel to the articular surface. Lubricants produced by this zone protect the articular surface. The middle and deep zones comprise of proteoglycans (mostly aggrecan) and collagen type II. A tide mark separates the deep zone from the calcified cartilage, with subchondral bone lying below this calcified cartilage. The articular cartilage has capability to withstand high compressive forces and self-repair during mild injuries. However, there are stark limits to the success and homogeneity of this regeneration. This is further complicated by the avascular nature of the tissue, and the replication potential of the resident chondrocytes [299]. Consequently, the degeneration of articular cartilage leads to its vastly damaged composition in the disease of osteoarthritis (OA) [300]. A complication of the OA is the development of an osteochondral lesion [301]. This has established a need to develop treatment for both cartilage and bone loss.
Figure 9. A schematic representation of the synovial joint.
Articular cartilage is illustrated with three zones (deep, middle, and superficial zones), flattened chondrocytes, synovial membrane and subchondral bone.
Osteochondral defects with the PDLGA layer promoting cartilage formation and the calcium sulfate layer promoting bone formation. Although this procedure can be performed in one visit, this system has shown modest improvements in patient outcomes and further clinical trials are needed to investigate its efficacy for cartilage-bone regeneration [302–305].
The strategies currently used in the clinical settings are to treat articular cartilage defects by microfractures, autologous chondrocyte implantation (ACI), and osteoarticular transfer system (OATS) [306–308]. Although these treatments have their advantages, a more convenient treatment technique would (1) be less invasive; (2) be a one-time process; (3) create tissues very similar in composition to native tissue; and (4) has a shorter treatment course [307, 309]. The complexities of hyaline cartilage tissue has been a major obstacle to engineer cartilage. Therefore, some studies have focused on recreating the complex arrangement of zones, cell types, and cell orientation in the body, resulting in a raft of commercially available products, including scaffold-based products [310]. As of now, there are two PLA scaffold-based systems being clinically used for cartilage repair: BioSeed®-C and TRUFIT CB™ systems. The BioSeed®-C 3-D disc from Biotissue Technologies (Fig.10) uses cultivated autologous chondrocytes seeded in 3-D fibrin with a PGA/PLA and polydioxanone (PDO) based scaffold. It is a second generation ACI treatment and has shown significant improvement in patient outcomes for the treatment of post-traumatic OA and focal degenerative cartilage defects [311–313]. Yet, like ACI, this procedure requires two patient visits; the first visit to culture the cells and the second to implant the tissue. Smith and Nephew’s TRUFIT CB™ plug is similar to the OAST system, but is composed of a poly-(D-L-lactide-co-glycolide) (PDLGA) and calcium sulfate bi-layer. The use of Bioseed ®-C is not without limitations. For example, in patients with deep bony lesions, prior spongioplasty is required. In addition, this scaffold may not be suitable for patients who are sensitive to heparin. Another key disadvantage being potential of communicable diseases, and the susceptibility of the product to undergo denaturation when in contact with alcohol.
Figure 10. Arthrotomic implantation of BioSeed®-C.
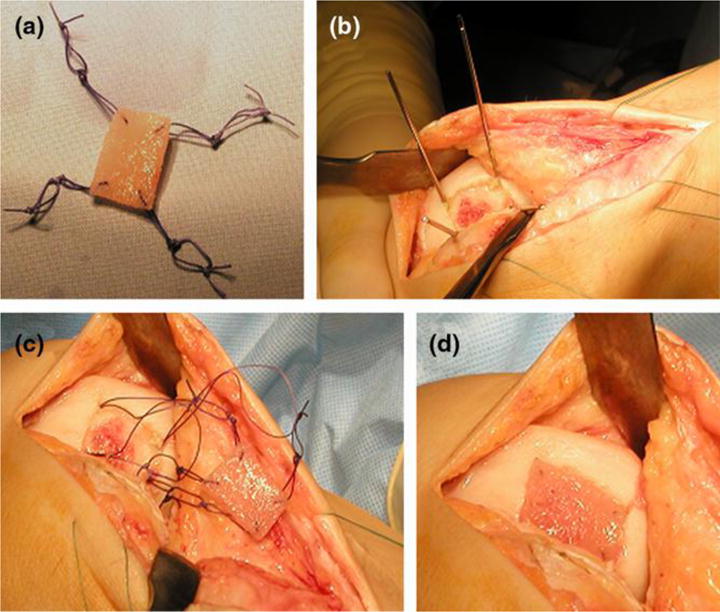
(a) BioSeed®-C was armed in each corner with resorbable threads secured by threefold knots. (b) In every corner of the defect, k-wires were drilled using the inside-out technique. (c) Guiding threads were pulled through the femoral bone using the k-wires, and the knots were guided into the subchondral bone. (d) The knots serve as anchors, seizing the subchondral bone and securely fixing the graft. Images adapted with permission from Kreuz et al. [312]. Copyright Biomed Central 2009.
In light of these disadvantages, several biomaterial-based strategies are currently being investigated to regenerate cartilage and treat osteochondral defects. Some of PLA based in vitro and in vivo studies are summarized in (Table 4). Especially, multiphasic scaffolds based on growth factor gradient, bilayer or tri layers, precultures (prior in vitro cultures), combination of additive manufacturing and traditional systems to make hybrid constructs are being widely studied for regenerating osteochondral defects [314].
Table 4.
List of ongoing studies on PLA based biomaterials for treating osteochondral defects
| Polymer | Technique used | Cells or factors | In vitro or In vivo | Ref | Conclusions |
|---|---|---|---|---|---|
| PLGA/TCP | Suturing of pre-synthesized scaffolds | Autologous chondrocytes and osteoblasts; TGF-β1 | In vivo (mini-pigs) | Cui W. et al., 2011 [315] | Compressive mechanical properties and GAG content on PLGA/TCP composite was higher than PLGA alone. Higher gross grading scale in PLGA/TCP composite demonstrate they are suitable for osteochondral defect |
| PLGA/CoI | Oil-in-water (O/W) single-emulsion method | BMSCs | In vivo (rat) | Yu et al., 2012[316] | PLGA/Col-I microsphere based scaffolds caused more rapid bone repair of fractures in osteoporotic patients |
| PLGA | Salt-leaching technique | None | In vivo (rabbits) | Chang et al., 2012 [317] | PLGA scaffold + early continuous passive motion leads to significant osteochondral regeneration |
| PDLA/PCL | Electrospinning | None | In vitro and in vivo (rat) | Cui Z et al., 2013 [318] | Macroporous PDLA/PCL embedded in a chitosan hydrogel promoted bone formation in an rat model, but did not result in cartilage formation indicating the necessity of chondrocytes for cartilage formation |
| PGA/PLA + PCL/HA | 3 D printing, fused deposition modeling | BMSCs | In vivo (goat) | Ding et al, 2013 [319] | A biphasic scaffold constructed using CAD/CAM technology seeded with chondrocytes and bone marrow stromal cells resulted in the formation of femoral head in a goat model |
| PLGA | Injection molding/particulate leaching | allogenic BMSCs | In vivo (rabbit) | Duan et al., 2014 [320] | Bilayered PLGA scaffold with gradient porosity induces formation of osseous layer in the scaffold with pore size of 300–500 μm; while the cartilage layer formed in the pore size 100–200 μm |
| PGA/PLA | Molding | ASCs | In vivo (pig) | Gong et al., 2014 [321] | PLA/phosphoglycerate scaffold seeded with ASCs differentiated into chondrocytes in weight-bearing areas of porcine articular cartilage defect resulting in neotissue formation. |
| PLGA | Solution-casting/salt-leaching technique | Chondrocytes, ASCs, and BMSCs | In vivo (sheep) | Caminal et al., 2015 [322] | PLGA scaffolds seeded with MSCs, ASCs and chondrocytes were evaluated for their potential to induce differentiation into chondrogenic lineage in an ovine model. MSCs were observed to be the best choice |
In bi-layered approach, most commonly used approach is to utilize softer hydrogels as a base material, with occasional reports of synthetic polymer being used for cartilage section [323]. For bone section, generally used materials are stiffer and stronger such as PLLA, PDLA, PLGA, PCL, coated with osteoinductive materials such as TCP, HA, and bioglass. For detailed information about the scaffold materials currently used/considered can be obtained from excellent reviews by Keeney et al., [324] and more recently by Yousefi et al [325]. In addition to two distinct materials used for mimicking the cartilage and bone phases, a single biomaterial based approach can also be used with varying topography and morphology within the construct. However, due to significant advantage in using multiphasic scaffolds, currently significant research is devoted solely in utilizing multiphasic scaffolds.
As the cellular profiles of the bone and cartilage compartments are fairly unique, a major cause of concern with biphasic scaffolds have been their poor integrity, especially in the cartilage phase. To overcome the poor interface and enhance the stability of these constructs in vivo, fibrin glue, sutures, or chemical dissolution are sometimes used to strengthen the interface between the two phases (Figs. 11 B–C) [60, 326]. Another way to overcome this drawback is to utilize biomaterials of similar composition, for example, aliphatic polyesters in cartilage and bone compartments of osteochondral defects [327]. As pore sizes influence the cell fate, instead of two different biomaterials, PLGA with different pore sizes have shown to be capable of regenerating chondral and bony regions in vivo in as few as 6 weeks. In addition, this study emphasizes the necessity to consider pore size (100–250 μm and 300–450 μm for chondral and bone regions) and cell types (chondrocytes and BMSCs) as a key factor in designing scaffolds for osteochondral regeneration [328]. Similarly, a previous study had also reported the significance of BMSCs in an in vivo study. In that study, combining PLGA with collagen enhanced the mechanical and cell adhesive properties, while simultaneous application of BMSCs facilitated tissue integration and subsequent osteochondral regeneration [323].
Fig 11. Physical composition of the knee joint with individual components. Processing techniques and scaffolds prepared from those techniques.
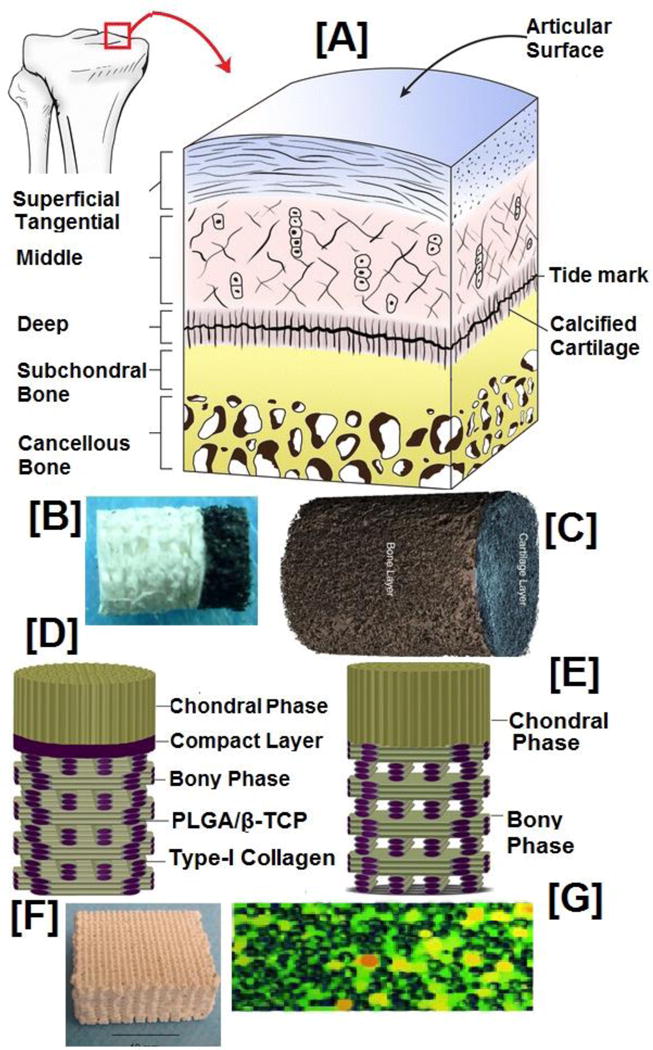
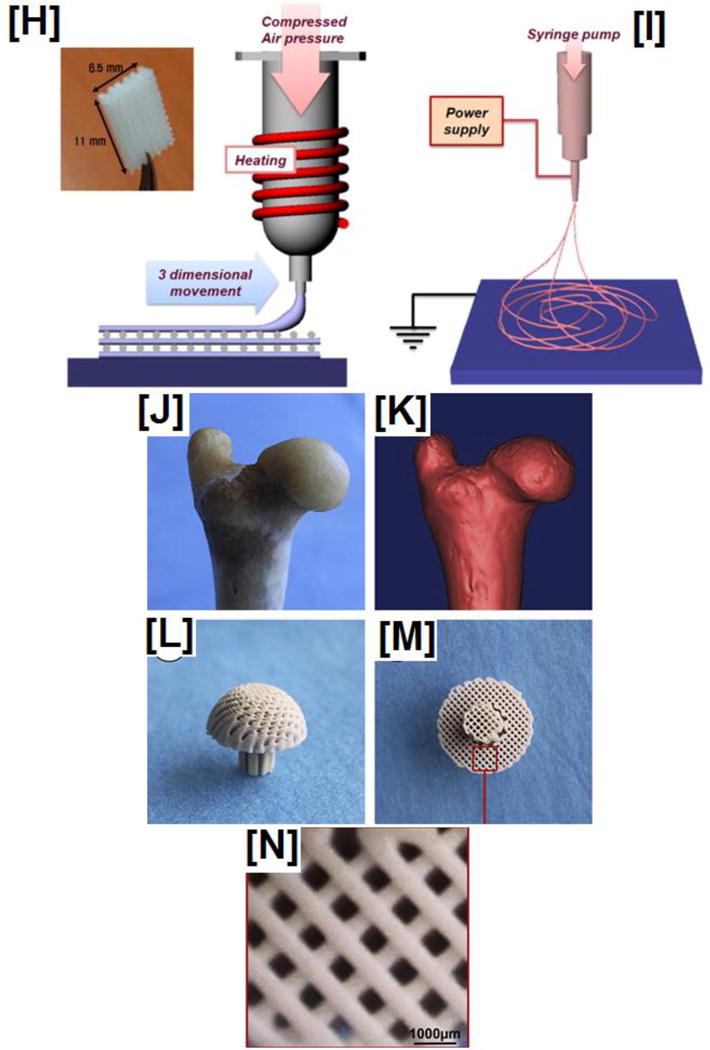
(A) Smooth surface of articular surface is shown with middle and deep zones showing some degree of vascularity. Also shown is a layer of fibrocartilage in the center, progressively forming a bony layer culminating with cancellous bone. (B–C) shows biphasic scaffolds, most widely studied scaffolds for engineering osteochondral regeneration. (D–E) As biphasic scaffolds have been found sub-optimal for regenerating interface between the chondral and osteochondral regions, a modified biphasic scaffolds (multiphasic scaffolds) are current choice of scaffolds that has a separate region between chondral and osteochondral regions that modulates fibrocartilage layer. (F) Shown is sample representation of scaffold intended to regenerating bony region. (G) One way to counter the drawbacks of biphasic scaffold is by utilizing a biphasic scaffold with gradient pore size thereby providing optimal growth of appropriate cells in three different regions (cartilage, calcified cartilage, and subchondral bone). (H–I) schematic of 3-D printing and (melt) electrospinning techniques that can be combined or utilized along to fabricate custom-made scaffolds ideal for osteochondral tissue regeneration. (J–I) Morphological and reconstructed image of goat femoral head. (L–N) 3-D scaffolds developed by 3-D printing demonstrating the possibility of utilizing this technique to fabricate scaffolds for osteochondral regeneration. Images 11A and C; 11B,D,E, and F; G; H and I; J–N adapted and reprinted with permission from ref [319, 330–333]. Copyright Springer 2014, Plos One 2014, Elsevier Ltd 2008, 2013.
A major cause of failure in OC constructs have been their poor regeneration of interface between chondral and subchondral bone layer. Biphasic scaffolds facilitate the formation of two discrete phases with poor formation of interface, that is a calcified cartilage which lies between the hyaline cartilage and subchondral bone [329]. One methodology that is gaining ground is by using a sandwich layer to induce calcified cartilage regeneration. For example, a recent study investigated use of a compact layer containing PLGA/β-TCP to facilitate formation of calcified cartilage (Figs 11D–E). As both bony phase (PLGA wrapped in collagen) and chondral phase (porous PLGA sponge) consisted of PLGA based materials, simple solvent based dissolution enabled formation of dense tri-layered scaffolds. Biomechanical analyses indicated presence of a compact layer enhanced the shear stress and tensile strength due to the compact layer between the two phases. In addition, in vitro results indicated elevated levels of neocartilage GAG and collagen contents to be superior to those scaffolds without the compact layer between the two phases [330]. Another strategy is to fabricate multiphasic constructs based on single biomaterial, but with varying (graded) pore sizes or pore densities or growth factors. As seen by Duan et al [320]., several studies have shown the necessity of changes in pore volume or densities to facilitate appropriate tissue in-growth throughout the scaffolds. For example, Nukavarapu et al. showed that by varying porogen content across the PLGA microsphere based constructs, a gradient in porosity (Fig 11 G) can be induced, and when combined with a hydrogel, significant increase in total DNA content was realized in 14–21 day time points. The PLGA construct, in addition, showed cell viability not only in the periphery, but homogenously throughout the scaffold. Furthermore, mineralization and osteoconductive properties were homogenous throughout the scaffold [332]. A similar study evaluated porous PLGA-based constructs showed pore-size dependent cell behavior. But key differences were larger pore-sizes (350–450 μm) did not elicit significant proliferation of osteoblasts; while smaller sizes showed higher levels of cellular proliferation [334]. Lack of in vivo studies have been major drawbacks of both studies, and hence performance of these constructs in vivo are currently unknown.
Due to complex architecture of the osteochondral region, it is practically not feasible to accommodate all the requirements from an engineering perspective. To overcome this significant drawback, appropriate selection of cell types and growth factors (GFs) are currently pursued to mimic and regenerate the osteochondral region. As several GFs such as BMPs, IGFs, TGF-βs, PRPs, and IGFs, have been implicated in regenerating bone and cartilage by providing biochemical cues to the progenitor cells, applying these GFs can overcome our incapability to accurately mimic native tissue [325, 335]. Mohan and coworkers reported fabrication of multiphasic PLGA microsphere based scaffolds comprised of encapsulated TGF-β1 and BMP-2. The scaffold constructs were fabricated in such a way that a quarter of the construct comprised of cartilage region with TGF-β, and the other quarter, a gradual transition towards the cartilage-bone interface. The remainder of the construct was fabricated exclusively for bony region with BMP-2. When studied in a rabbit femoral condyle model, the authors observed neocartilage formation with GAG contents and thickness similar to that of native tissue. In addition, authors noted significant bone regeneration and mineralization leading to rapid osteochondral regeneration, especially in scaffolds supplemented with n-HA [336].
Although conceptualized in early 2000’s, application of RP (or AM) process to fabricate constructs with high precision for osteochondral regeneration [60], recent focus have been to take advantage of these process to fabricate high precision scaffolds. A schematic of these processes are shown in Fig 11 H–I (3-D printing and melt electrospinning). In this study, using laser imaging technique, femoral head of a goat was mapped, and subsequently reconstructed using CAD/CAM technique (Fig 11 J–K). When the scaffold construct was implanted in vivo in a nude mouse model, the regenerated neocartilage and subchondral bone demonstrated similar histological and biophysical characteristics to native tissue [319]. In addition to their capability to fabricate complex structures with high precision, various studies have also shown their capability to construct scaffolds laden with cells and GFs. Currently most studies on RP and AM process have focused on PCL based scaffolds [337, 338], but with superior properties and their dynamic nature, we can anticipate significant progress in PLA based biomaterials [339] for osteochondral regeneration. This is particular true with evolution of FEA models to predict the mechanical and cell behavior of the constructs, especially a complicated one such as those for osteochondral regeneration. Like AM process, most studies till now have focused on non-PLA based materials, but some studies based on PLA have been reported [340]. This study fabricated and modelled various pore structures (pore, diamond, gyroid, and salt leached) paving way for effectively optimizing the scaffold properties.
i. Knee Meniscus Regeneration
The crescent-shaped structures on the medial and lateral aspects of the knee joint serve several functions in the joint including shock absorption, nutrient provision, and axial load distribution [341]. The meniscus is a fibrocartilage composed chiefly of type I collagen, water, and cells in an extracellular matrix. It is further divided into (i) an outer vascularized red zone, which consists of fibroblast-like cells; and (ii) a poorly vascularized white zone composed of fibrochondrocytes [341, 342]. Like articular cartilage, injury to meniscus shows a poor healing capability, especially in the inner avascularized portion, due to their limited vascularization. Currently, meniscal repair and partial meniscectomy are the two standard surgical treatment techniques recommended for meniscal injury. Clinical and surgical repair of the damaged meniscus presents challenges for physicians and therapists due to factors such as the complexity of the meniscus, advanced patient age, and type of injury/tear [341, 343]. The importance of repairing meniscus is highlighted by the development of articular cartilage loss, consequently leading to osteoarthritic changes [344]. These changes also account for the rapid generation of OA after partial meniscectomies [345].
Meniscus replacement from donor meniscus or artificial scaffold (collagen or synthetic scaffold) is another treatment option for meniscal tears [346]. Given the limitation in availability of donor grafts, the development of artificial scaffolds via regenerative engineering has become more important. One major obstacle to engineer the meniscus is the scarcity of fibrochondrocytes and chondrocytes in the body. A possible solution to this problem would be the use of stem cells, augmented with growth factors to differentiate into the necessary cell types for each zones of the meniscus. Just as with articular cartilage, the complexity of the meniscus serves as a major hurdle to its construction. Similarly, several studies have also been geared towards this goal. In a recent study, Lee and coworkers used a PLA-PCL 3-D printed scaffold loaded with growth factors to synthesize cartilaginous tissue similar to the human meniscus, illustrating the possibility of utilizing synthetic scaffold to regenerate the tissue [347].
There are currently no commercially available PLA based meniscus repair grafts or scaffolds used in clinical settings. Some PLA based systems are currently being investigated for meniscus repair and are summarized in (Table 5).
Table 5.
List of PLA based systems investigated for meniscal repair.
| Technique used | Cells or other factors | In vitro or In vivo | References | Conclusions |
|---|---|---|---|---|
| Solvent casting and particulate leaching | Fibrochondrocytes | In vivo (rabbit) | Esposito et al., 2013 [348] | PLDLA/PCL-T allowed formation of fibrocartilaginous tissue which aids meniscus regeneration |
| 3-D printing | hCTGF and hTGFβ3 | In vivo (sheep) | Lee et al., 2014 [347] | 3-D printed scaffolds loaded with human growth factors formed tissues with different cartilaginous zones similar to human meniscus |
| Electrospinning | Human meniscus cells | In vitro | Baek et al., 2015 [349] | Electrospun materials resulted in cell-based meniscus regeneration. |
| Electrospinning and TIPS | 3T3 Fibroblasts | In vitro | Vaquette et al., 2013 [350] | By combining electrospinning and TIPS technique, drawbacks of the processes (thickness and mechanical properties) are overcome. With mechanical properties in the vicinity of native meniscus tissue, scaffolds might be appropriate for that purpose |
7. Conclusions and Future Directions
The past several decades have seen a rapid progress in many biomedical disciplines including material sciences, life sciences and engineering, resulting in advancements in treatment options for various illnesses and diseases. With the convergence of regenerative engineering as a field, imbibing cues from traditional tissue engineering, advanced material science, stem cell technology, and developmental biology, time has now come for this convergent approach to lead us to providing new solutions for regenerating complex orthopaedic tissues. As the success in regenerating any orthopaedic tissue depends on several factors including the choice of material, fabrication technique, geometry and architecture of the scaffold, in conjunction with other factors, biomaterials are likely to play a significant role in this process. In this regard, as discussed in this review, PLA with its suitable properties, demonstrates suitability for regenerating various orthopaedic tissues including bone, ligament, cartilage, and meniscus.
The main advantage of utilizing a PLA based material has been its versatility to be processed for tissue specific applications. For example, electrospun nanofibers and microsphere sintered scaffolds are more suitable for bone regeneration; similarly, micro/nanofibrous scaffolds are suitable for ligament regeneration. Likewise, injection molded, porogen leached or electrospun scaffolds have been found suitable for cartilage and meniscus regeneration. In this review, we have summarized the state of the art strategies currently utilized for various orthopaedic tissue regeneration. We can now anticipate in the next decade that with further advancements in developmental biology offering us tools and cues which when recapitulated and presented in a sequential fashion to stem cells, using versatile PLA based biomaterials, we may further our capability to regenerate complex orthopaedic tissues such as whole limbs.
Acknowledgments
Funding information
The authors gratefully acknowledge funding from the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, NIH R01AR063698, and NIH DP1 AR068147.
Abbreviations
- PLLA
poly (L-lactic acid)
- PLLA-PGA
copolymer of (L-lactic acid) and (glycolic acid)
- PLDA poly
(D-Lactic acid)
- PLCL
copolymer of (L-lactic acid) and (Ɛ-caprolactone)
- PDLLA
copolymer of (L-lactic acid) and (D-lactic acid)
- SR-PLA
self-reinforced PLA composites
- PLEG
copolymer of (L-lactic acid) and (ethylene glycol)
- PLGM
copolymer of (L-lactic acid) and (glutamic acid)
- PTFE
poly tetrafluoroethylene
- Poly
(DTE carbonate) poly (desaminotyrosyl-tyrosine ethyl ester carbonate)
- POC–HA
poly (18-octanediol-co-citric acid)–hydroxyapatite
- PU-PLLA
poly urethane- PLLA
- PDO
poly (dioxanone)
- MGC
methacrylated glycol chitosan
- μHA
micro particle of hydroxyapatite
- β-TCP
β-tricalcium phosphate
- TCP
tricalcium phosphate
- CaP
Calcium Phosphate
- nDd
nano-diamond
- mSi
mesoporous silica
- GO
graphene oxide
- CNT
carbon nanotube
- BNNT
boron nitride nanotube
- MgO
magnesium oxide
- MgOH
magnesium hydroxide
- SVAK-12
osteoinductive small molecule
- BSA
bovine serum albumin
- RGD
tripeptide containing L-arginine glycine and aspartic acid
- TGF-β1
transforming growth factor-Beta 1
- BMP-2
bone morphogenetic protein-2
- FGF-2
(FGF-β) basic fibroblast growth factor
- hTGF-β3
human-transforming growth factor-Beta 3
- VEGF
vascular endothelial growth factors
- IGF
insulin-like growth factor
- PDGF
platelet derived growth factor
- hCTGF
human connective tissue growth factor
- Dex
dexamethasone
- ACL
anterior cruciate ligament
- PCL
posterior cruciate ligament
- MCL
medial collateral ligament
- AM
anteromedial bundle
- PL
posterolateral bundle
- C2C12
murine myoblast cell line
- ADSC
or ASC adipose-derived stem cells
- C2C12
murine myoblast cell line
- MSC
mesenchymal stem cells
- ALP
alkaline phosphatase expression
- HUVEC
human umbilical vein endothelial cells
- RP
rapid prototyping
- FDM
fused deposition modeling
- AM
additive manufacturing
- CAD/CAM
computer aided design/manufacturing
- ECM
extracellular matrix protein
- BMD
bone mineral density
- TIPS
thermally induced phase separation
- OA
osteoarthritis
- ACI
autologous chondrocyte implantation
- OATS
osteoarticular transfer system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States (BMUS) Third. Rosemont, IL: 2014. Available at http://www.boneandjointburden.org. Accessed 10/14/2015. [Google Scholar]
- 2.Agarwal R, García AJ. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Advanced drug delivery reviews. 2015 doi: 10.1016/j.addr.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K, Goodman SB. Current state and future of joint replacements in the hip and knee. Expert review of medical devices. 2008;5:383–93. doi: 10.1586/17434440.5.3.383. [DOI] [PubMed] [Google Scholar]
- 4.Chang SK, Egami DK, Shaieb MD, Kan DM, Richardson AB. Anterior cruciate ligament reconstruction: allograft versus autograft. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2003;19:453–62. doi: 10.1053/jars.2003.50103. [DOI] [PubMed] [Google Scholar]
- 5.Grood ES, Noyes FR. Cruciate ligament prosthesis: strength, creep, and fatigue properties. J Bone Joint Surg Am. 1976;58:1083–8. [PubMed] [Google Scholar]
- 6.Mascarenhas R, MacDonald PB. Anterior cruciate ligament reconstruction: a look at prosthetics-past, present and possible future. McGill Journal of Medicine: MJM. 2008;11:29. [PMC free article] [PubMed] [Google Scholar]
- 7.Laurencin CT, Ambrosio A, Borden M, Cooper J., Jr Tissue engineering: orthopedic applications. Annual review of biomedical engineering. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Shelton WR, Papendick L, Dukes AD. Autograft versus allograft anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 1997;13:446–9. doi: 10.1016/s0749-8063(97)90122-5. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie JR, Parker RD. Graft selection in anterior cruciate ligament revision surgery. Clinical orthopaedics and related research. 1996;325:65–77. doi: 10.1097/00003086-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2002;18:163–70. doi: 10.1053/jars.2002.30485. [DOI] [PubMed] [Google Scholar]
- 11.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–46. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 12.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Progress in Polymer Science. 2007;32:762–98. [Google Scholar]
- 13.Nair L, Laurencin C. Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. In: Lee K, Kaplan D, editors. Tissue Engineering I. Springer Berlin Heidelberg; 2006. pp. 47–90. [DOI] [PubMed] [Google Scholar]
- 14.Madhavan Nampoothiri K, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresource Technology. 2010;101:8493–501. doi: 10.1016/j.biortech.2010.05.092. [DOI] [PubMed] [Google Scholar]
- 15.Migliaresi C, De Lollis A, Fambri L, Cohn D. The effect of thermal history on the crystallinity of different molecular weight PLLA biodegradable polymers. Clinical Materials. 1991;8:111–8. [Google Scholar]
- 16.Lasprilla AJR, Martinez GAR, Lunelli BH, Jardini AL, Filho RM. Poly-lactic acid synthesis for application in biomedical devices — A review. Biotechnology Advances. 2012;30:321–8. doi: 10.1016/j.biotechadv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Sarasua JR, Arraiza AL, Balerdi P, Maiza I. Crystallinity and mechanical properties of optically pure polylactides and their blends. Polymer Engineering & Science. 2005;45:745–53. [Google Scholar]
- 18.Perego G, Cella GD, Bastioli C. Effect of molecular weight and crystallinity on poly(lactic acid) mechanical properties. Journal of Applied Polymer Science. 1996;59:37–43. [Google Scholar]
- 19.Yasuniwa M, Tsubakihara S, Iura K, Ono Y, Dan Y, Takahashi K. Crystallization behavior of poly(l-lactic acid) Polymer. 2006;47:7554–63. [Google Scholar]
- 20.Dell’Erba R, Groeninckx G, Maglio G, Malinconico M, Migliozzi A. Immiscible polymer blends of semicrystalline biocompatible components: thermal properties and phase morphology analysis of PLLA/PCL blends. Polymer. 2001;42:7831–40. [Google Scholar]
- 21.Narayanan G, Gupta BS, Tonelli AE. Poly(ε-caprolactone) Nanowebs Functionalized with α- and γ-Cyclodextrins. Biomacromolecules. 2014;15:4122–33. doi: 10.1021/bm501158w. [DOI] [PubMed] [Google Scholar]
- 22.Rangari D, Vasanthan N. Study of Strain-Induced Crystallization and Enzymatic Degradation of Drawn Poly(l-lactic acid) (PLLA) Films. Macromolecules. 2012;45:7397–403. [Google Scholar]
- 23.Maurus PB, Kaeding CC. Bioabsorbable implant material review. Operative Techniques in Sports Medicine. 2004;12:158–60. [Google Scholar]
- 24.Makadia H, Siegel S. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers. 2011;3:1377–97. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu T, Wang Y-Y, Yang M, Schneider C, Zhong W, Pulicare S, et al. Biodegradable mucus-penetrating nanoparticles composed of diblock copolymers of polyethylene glycol and poly(lactic-co-glycolic acid) Drug Deliv and Transl Res. 2012;2:124–8. doi: 10.1007/s13346-011-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Deng S, Chen P, Ruan R. Polylactic acid (PLA) synthesis and modifications: a review. Front Chem China. 2009;4:259–64. [Google Scholar]
- 27.Ambrose C, Clanton T. Bioabsorbable Implants: Review of Clinical Experience in Orthopedic Surgery. Annals of Biomedical Engineering. 2004;32:171–7. doi: 10.1023/b:abme.0000007802.59936.fc. [DOI] [PubMed] [Google Scholar]
- 28.Lim LT, Auras R, Rubino M. Processing technologies for poly(lactic acid) Progress in Polymer Science. 2008;33:820–52. [Google Scholar]
- 29.Li X, Liu H, Wang J, Li C. Preparation and characterization of PLLA/nHA nonwoven mats via laser melt electrospinning. Materials Letters. 2012;73:103–6. [Google Scholar]
- 30.Gupta B, Revagade N, Hilborn J. Poly (lactic acid) fiber: an overview. Progress in polymer science. 2007;32:455–82. [Google Scholar]
- 31.Ge Z, Goh JC, Wang L, Tan EP, Lee EH. Characterization of knitted polymeric scaffolds for potential use in ligament tissue engineering. Journal of biomaterials science Polymer edition. 2005;16:1179–92. doi: 10.1163/1568562054798491. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue engineering. 2003;9:431–9. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- 33.Freeman JW, Woods MD, Laurencin CT. Tissue engineering of the anterior cruciate ligament using a braid–twist scaffold design. Journal of Biomechanics. 2007;40:2029–36. doi: 10.1016/j.jbiomech.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tormala P, Rokkanen P, Laiho J, Tamminmaki M, Vainionpaa S. Material for osteosynthesis devices. Google Patents. 1988 [Google Scholar]
- 35.Juutilainen T, Hirvensalo E, Partio E, Pätiälä H, Törmälä P, Rokkanen P. Complications in the first 1043 operations where self-reinforced poly-L-lactide implants were used solely for tissue fixation in orthopaedics and traumatology. International Orthopaedics (SICOT) 2002;26:122–5. doi: 10.1007/s00264-002-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R, Yao D. Preparation of single poly(lactic acid) composites. Journal of Applied Polymer Science. 2008;107:2909–16. [Google Scholar]
- 37.Wu N, Liang Y, Zhang K, Xu W, Chen L. Preparation and bending properties of three dimensional braided single poly (lactic acid) composite. Composites Part B: Engineering. 2013;52:106–13. [Google Scholar]
- 38.Gao C, Meng L, Yu L, Simon GP, Liu H, Chen L, et al. Preparation and characterization of uniaxial poly(lactic acid)-based self-reinforced composites. Composites Science and Technology. 2015;117:392–7. [Google Scholar]
- 39.Charles LF, Kramer ER, Shaw MT, Olson JR, Wei M. Self-reinforced composites of hydroxyapatite-coated PLLA fibers: Fabrication and mechanical characterization. Journal of the Mechanical Behavior of Biomedical Materials. 2013;17:269–77. doi: 10.1016/j.jmbbm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Chen WC, Ko CL, Yang JK, Wu HY, Lin JH. Comparison and preparation of multilayered polylactic acid fabric strengthen calcium phosphate-based bone substitutes for orthopedic applications. Journal of artificial organs: the official journal of the Japanese Society for Artificial Organs. 2015 doi: 10.1007/s10047-015-0863-8. [DOI] [PubMed] [Google Scholar]
- 41.Ella V, Kellomaki M, Tormala P. In vitro properties of PLLA screws and novel bioabsorbable implant with elastic nucleus to replace intervertebral disc. Journal of materials science Materials in medicine. 2005;16:655–62. doi: 10.1007/s10856-005-2537-1. [DOI] [PubMed] [Google Scholar]
- 42.Veiranto M, Törmälä P, Suokas E. In vitro mechanical and drug release properties of bioabsorbable ciprofloxacin containing and neat self-reinforced P(L/DL)LA 70/30 fixation screws. Journal of Materials Science: Materials in Medicine. 2002;13:1259–64. doi: 10.1023/a:1021187331458. [DOI] [PubMed] [Google Scholar]
- 43.Torres-Giner S, Martinez-Abad A, Gimeno-Alcañiz JV, Ocio MJ, Lagaron JM. Controlled Delivery of Gentamicin Antibiotic from Bioactive Electrospun Polylactide-Based Ultrathin Fibers. Advanced Engineering Materials. 2012;14:B112–B22. [Google Scholar]
- 44.Laurencin CT, Gerhart T, Witschger P, Satcher R, Domb A, Rosenberg AE, et al. Bioerodible polyanhydrides for antibiotic drug delivery: in vivo osteomyelitis treatment in a rat model system. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1993;11:256–62. doi: 10.1002/jor.1100110213. [DOI] [PubMed] [Google Scholar]
- 45.Baro M, Sánchez E, Delgado A, Perera A, Évora C. In vitro–in vivo characterization of gentamicin bone implants. Journal of Controlled Release. 2002;83:353–64. doi: 10.1016/s0168-3659(02)00179-7. [DOI] [PubMed] [Google Scholar]
- 46.Shah SR, Tatara AM, D’Souza RN, Mikos AG, Kasper FK. Evolving strategies for preventing biofilm on implantable materials. Materials Today. 2013;16:177–82. [Google Scholar]
- 47.Danoux CB, Barbieri D, Yuan H, de Bruijn JD, van Blitterswijk CA, Habibovic P. In vitro and in vivo bioactivity assessment of a polylactic acid/hydroxyapatite composite for bone regeneration. Biomatter. 2014;4:e27664. doi: 10.4161/biom.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson RL, Nazhat SN, Blaker JJ, Bismarck A, Hill R, Boccaccini AR, et al. A comparative study of the effects of different bioactive fillers in PLGA matrix composites and their suitability as bone substitute materials: A thermo-mechanical and in vitro investigation. Journal of the Mechanical Behavior of Biomedical Materials. 2015;50:277–89. doi: 10.1016/j.jmbbm.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Giordano RA, Wu BM, Borland SW, Cima LG, Sachs EM, Cima MJ. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. Journal of Biomaterials Science, Polymer Edition. 1997;8:63–75. doi: 10.1163/156856297x00588. [DOI] [PubMed] [Google Scholar]
- 50.Farahani RD, Chizari K, Therriault D. Three-dimensional printing of freeform helical microstructures: a review. Nanoscale. 2014;6:10470–85. doi: 10.1039/c4nr02041c. [DOI] [PubMed] [Google Scholar]
- 51.Guo SZ, Gosselin F, Guerin N, Lanouette AM, Heuzey MC, Therriault D. Solvent-Cast Three-Dimensional Printing of Multifunctional Microsystems. Small. 2013;9:4118–22. doi: 10.1002/smll.201300975. [DOI] [PubMed] [Google Scholar]
- 52.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–24. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 53.Vásuez Quintero As, Frolet N, Marki D, Marette A, Mattana G, Briand D, et al. Printing and encapsulation of electrical conductors on polylactic acid (PLA) for sensing applications. Micro Electro Mechanical Systems (MEMS), 2014 IEEE 27th International Conference on: Ieee. 2014:532–5. [Google Scholar]
- 54.Xiong Z, Yan Y, Wang S, Zhang R, Zhang C. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scripta Materialia. 2002;46:771–6. [Google Scholar]
- 55.Yan Y, Xiong Z, Hu Y, Wang S, Zhang R, Zhang C. Layered manufacturing of tissue engineering scaffolds via multi-nozzle deposition. Materials Letters. 2003;57:2623–8. [Google Scholar]
- 56.Yan Y, Wang X, Pan Y, Liu H, Cheng J, Xiong Z, et al. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials. 2005;26:5864–71. doi: 10.1016/j.biomaterials.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 57.Shaffer S, Yang K, Vargas J, Di Prima MA, Voit W. On reducing anisotropy in 3D printed polymers via ionizing radiation. Polymer. 2014;55:5969–79. [Google Scholar]
- 58.Senatov FS, Niaza KV, Zadorozhnyy MY, Maksimkin AV, Kaloshkin SD, Estrin YZ. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. Journal of the Mechanical Behavior of Biomedical Materials. 2016;57:139–48. doi: 10.1016/j.jmbbm.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 59.Ogden K, Ordway N, Diallo D, Tillapaugh-Fay G, Aslan C. Dimensional accuracy of 3D printed vertebra. 2014:903629–8. doi: 10.1007/s10278-015-9803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherwood JK, Riley SL, Palazzolo R, Brown SC, Monkhouse DC, Coates M, et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–51. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 61.Narayanan G, Gupta BS, Tonelli AE. Enhanced mechanical properties of poly (ε-caprolactone) nanofibers produced by the addition of non-stoichiometric inclusion complexes of poly (ε-caprolactone) and α-cyclodextrin. Polymer. 2015;76:321–30. [Google Scholar]
- 62.Narayanan G, Ormond BR, Gupta BS, Tonelli AE. Efficient wound odor removal by β-cyclodextrin functionalized poly (ε-caprolactone) nanofibers. Journal of Applied Polymer Science. 2015;132 [Google Scholar]
- 63.Jiang T, Carbone EJ, Lo KWH, Laurencin CT. Electrospinning of polymer nanofibers for tissue regeneration. Progress in Polymer Science. 2015;46:1–24. [Google Scholar]
- 64.Nair LS, Bhattacharyya S, Laurencin CT. Development of novel tissue engineering scaffolds via electrospinning. Expert opinion on biological therapy. 2004;4:659–68. doi: 10.1517/14712598.4.5.659. [DOI] [PubMed] [Google Scholar]
- 65.Yarin A, Koombhongse S, Reneker DH. Bending instability in electrospinning of nanofibers. Journal of Applied Physics. 2001;89:3018–26. [Google Scholar]
- 66.Arras MM, Grasl C, Bergmeister H, Schima H. Electrospinning of aligned fibers with adjustable orientation using auxiliary electrodes. Science and technology of advanced materials. 2012;13:035008. doi: 10.1088/1468-6996/13/3/035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zamani F, Amani-Tehran M, Latifi M, Shokrgozar MA. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. Journal of Materials Science: Materials in Medicine. 2013;24:1551–60. doi: 10.1007/s10856-013-4905-6. [DOI] [PubMed] [Google Scholar]
- 68.Santos LG, Oliveira DC, Santos MS, Neves LMG, de Gaspi FO, Mendonca FA, et al. Electrospun membranes of poly (lactic acid)(PLA) used as scaffold in drug delivery of extract of Sedum dendroideum. Journal of nanoscience and nanotechnology. 2013;13:4694–702. doi: 10.1166/jnn.2013.7194. [DOI] [PubMed] [Google Scholar]
- 69.Kim HW, Lee HH, Knowles J. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly (lactic acid) for bone regeneration. Journal of Biomedical Materials Research Part A. 2006;79:643–9. doi: 10.1002/jbm.a.30866. [DOI] [PubMed] [Google Scholar]
- 70.Cao L, Duan P-G, Wang H-R, Li X-L, Yuan F-L, Fan Z-Y, et al. Degradation and osteogenic potential of a novel poly (lactic acid)/nano-sized β-tricalcium phosphate scaffold. International journal of nanomedicine. 2012;7:5881. doi: 10.2147/IJN.S38127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Llorens E, Calderón S, del Valle LJ, Puiggalí J. Polybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Materials Science and Engineering: C. 2015;50:74–84. doi: 10.1016/j.msec.2015.01.100. [DOI] [PubMed] [Google Scholar]
- 72.Wang H, Cheng M, Hu J, Wang C, Xu S, Han CC. Preparation and optimization of silver nanoparticles embedded electrospun membrane for implant associated infections prevention. ACS applied materials & interfaces. 2013;5:11014–21. doi: 10.1021/am403250t. [DOI] [PubMed] [Google Scholar]
- 73.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: Optimization of fabrication parameters. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2004;70B:286–96. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 74.Greiner A, Wendorff JH. Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angewandte Chemie International Edition. 2007;46:5670–703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 75.Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. Journal of biomedical materials research. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharyya S, Kumbar SG, Khan YM, Nair LS, Singh A, Krogman NR, et al. Biodegradable polyphosphazene-nanohydroxyapatite composite nanofibers: scaffolds for bone tissue engineering. Journal of biomedical nanotechnology. 2009;5:69–75. doi: 10.1166/jbn.2009.032. [DOI] [PubMed] [Google Scholar]
- 77.Aviss K, Gough J, Downes S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur Cell Mater. 2010;19:193–204. doi: 10.22203/ecm.v019a19. [DOI] [PubMed] [Google Scholar]
- 78.James R, Kumbar S, Laurencin C, Balian G, Chhabra A. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomedical Materials. 2011;6:025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.James R, Toti U, Laurencin C, Kumbar S. Electrospun Nanofibrous Scaffolds for Engineering Soft Connective Tissues. In: Hurst SJ, editor. Biomedical Nanotechnology. Humana Press; 2011. pp. 243–58. [DOI] [PubMed] [Google Scholar]
- 80.Taylor ED, Nair LS, Nukavarapu SP, McLaughlin S, Laurencin CT. Novel Nanostructured Scaffolds as Therapeutic Replacement Options for Rotator Cuff Disease. The Journal of Bone & Joint Surgery. 2010;92:170–9. doi: 10.2106/JBJS.J.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008;29:4100–7. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomedical Materials. 2011;6:025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Detta N, Brown TD, Edin FK, Albrecht K, Chiellini F, Chiellini E, et al. Melt electrospinning of polycaprolactone and its blends with poly(ethylene glycol) Polymer International. 2010;59:1558–62. [Google Scholar]
- 84.Larrondo L, St John Manley R. Electrostatic fiber spinning from polymer melts. III. Electrostatic deformation of a pendant drop of polymer melt. Journal of Polymer Science: Polymer Physics Edition. 1981;19:933–40. [Google Scholar]
- 85.Lyons J, Li C, Ko F. Melt-electrospinning part I: processing parameters and geometric properties. Polymer. 2004;45:7597–603. [Google Scholar]
- 86.Brown TD, Dalton PD, Hutmacher DW. Melt electrospinning today: An opportune time for an emerging polymer process. Progress in Polymer Science [Google Scholar]
- 87.Zhou H, Green TB, Joo YL. The thermal effects on electrospinning of polylactic acid melts. Polymer. 2006;47:7497–505. [Google Scholar]
- 88.Zhao F, Liu Y, Yuan H, Yang W. Orthogonal design study on factors affecting the degradation of polylactic acid fibers of melt electrospinning. Journal of Applied Polymer Science. 2012;125:2652–8. [Google Scholar]
- 89.Yoon YI, Park KE, Lee SJ, Park WH. Fabrication of Microfibrous and Nano-/Microfibrous Scaffolds: Melt and Hybrid Electrospinning and Surface Modification of Poly(L-lactic acid) with Plasticizer. BioMed Research International. 2013;2013:10. doi: 10.1155/2013/309048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogata N, Yamaguchi S, Shimada N, Lu G, Iwata T, Nakane K, et al. Poly(lactide) nanofibers produced by a melt-electrospinning system with a laser melting device. Journal of Applied Polymer Science. 2007;104:1640–5. [Google Scholar]
- 91.Muerza-Cascante ML, Haylock D, Hutmacher DW, Dalton PD. Melt electrospinning and its technologization in tissue engineering. Tissue Engineering Part B: Reviews. 2014;21:187–202. doi: 10.1089/ten.TEB.2014.0347. [DOI] [PubMed] [Google Scholar]
- 92.Bas O, De-Juan-Pardo EM, Chhaya MP, Wunner FM, Jeon JE, Klein TJ, et al. Enhancing structural integrity of hydrogels by using highly organised melt electrospun fibre constructs. European Polymer Journal. 2015;72:451–63. [Google Scholar]
- 93.Woodruff MA, Hutmacher DW. The return of a forgotten polymer—Polycaprolactone in the 21st century. Progress in Polymer Science. 2010;35:1217–56. [Google Scholar]
- 94.Brown TD, Dalton PD, Hutmacher DW. Direct Writing By Way of Melt Electrospinning. Advanced Materials. 2011;23:5651–7. doi: 10.1002/adma.201103482. [DOI] [PubMed] [Google Scholar]
- 95.Brooke LF, Toby DB, Zee U, Dietmar WH, Paul DD, Tim RD. Dermal fibroblast infiltration of poly(ε-caprolactone) scaffolds fabricated by melt electrospinning in a direct writing mode. Biofabrication. 2013;5:025001. doi: 10.1088/1758-5082/5/2/025001. [DOI] [PubMed] [Google Scholar]
- 96.Paul DD, Nanna TJ, Juergen G, Martin M. Patterned melt electrospun substrates for tissue engineering. Biomedical Materials. 2008;3:034109. doi: 10.1088/1748-6041/3/3/034109. [DOI] [PubMed] [Google Scholar]
- 97.Brown TD, Slotosch A, Thibaudeau L, Taubenberger A, Loessner D, Vaquette C, et al. Design and Fabrication of Tubular Scaffolds via Direct Writing in a Melt Electrospinning Mode. Biointerphases. 2012;7:13. doi: 10.1007/s13758-011-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jungst T, Muerza-Cascante ML, Brown TD, Standfest M, Hutmacher DW, Groll J, et al. Melt electrospinning onto cylinders: effects of rotational velocity and collector diameter on morphology of tubular structures. Polymer International. 2015;64:1086–95. [Google Scholar]
- 99.Brown TD, Edin F, Detta N, Skelton AD, Hutmacher DW, Dalton PD. Melt electrospinning of poly(ε-caprolactone) scaffolds: Phenomenological observations associated with collection and direct writing. Materials Science and Engineering: C. 2014;45:698–708. doi: 10.1016/j.msec.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 100.Qin C-C, Duan X-P, Wang L, Zhang L-H, Yu M, Dong R-H, et al. Melt electrospinning of poly(lactic acid) and polycaprolactone microfibers by using a hand-operated Wimshurst generator. Nanoscale. 2015;7:16611–5. doi: 10.1039/c5nr05367f. [DOI] [PubMed] [Google Scholar]
- 101.Reis CP, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 102.Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26:1025–58. doi: 10.1007/s11095-008-9800-3. [DOI] [PubMed] [Google Scholar]
- 103.Mosqueira VCF, Legrand P, Gulik A, Bourdon O, Gref R, Labarre D, et al. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel PEG-modified nanocapsules. Biomaterials. 2001;22:2967–79. doi: 10.1016/s0142-9612(01)00043-6. [DOI] [PubMed] [Google Scholar]
- 104.James R, Deng M, Laurencin C, Kumbar S. Nanocomposites and bone regeneration. Front Mater Sci. 2011;5:342–57. [Google Scholar]
- 105.Hu Q, Gao X, Gu G, Kang T, Tu Y, Liu Z, et al. Glioma therapy using tumor homing and penetrating peptide-functionalized PEG–PLA nanoparticles loaded with paclitaxel. Biomaterials. 2013;34:5640–50. doi: 10.1016/j.biomaterials.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 106.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced drug delivery reviews. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 107.Mohanraj V, Chen Y. Nanoparticles-a review. Tropical Journal of Pharmaceutical Research. 2007;5:561–73. [Google Scholar]
- 108.Avgoustakis K. Pegylated poly (lactide) and poly (lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery. Current drug delivery. 2004;1:321–33. doi: 10.2174/1567201043334605. [DOI] [PubMed] [Google Scholar]
- 109.Babu A, Wang Q, Muralidharan R, Shanker M, Munshi A, Ramesh R. Chitosan Coated Polylactic Acid Nanoparticle-Mediated Combinatorial Delivery of Cisplatin and siRNA/Plasmid DNA Chemosensitizes Cisplatin-Resistant Human Ovarian Cancer Cells. Molecular pharmaceutics. 2014;11:2720–33. doi: 10.1021/mp500259e. [DOI] [PubMed] [Google Scholar]
- 110.Verma MS, Liu S, Chen YY, Meerasa A, Gu FX. Size-tunable nanoparticles composed of dextran-b-poly (D, L-lactide) for drug delivery applications. Nano Research. 2012;5:49–61. [Google Scholar]
- 111.Santander-Ortega M, Jódar-Reyes A, Csaba N, Bastos-González D, Ortega-Vinuesa J. Colloidal stability of pluronic F68-coated PLGA nanoparticles: a variety of stabilisation mechanisms. Journal of colloid and interface science. 2006;302:522–9. doi: 10.1016/j.jcis.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 112.Ren T, Xu N, Cao C, Yuan W, Yu X, Chen J, et al. Preparation and therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. Journal of Biomaterials Science, Polymer Edition. 2009;20:1369–80. doi: 10.1163/092050609X12457418779185. [DOI] [PubMed] [Google Scholar]
- 113.Nobs L, Buchegger F, Gurny R, Allémann E. Surface modification of poly (lactic acid) nanoparticles by covalent attachment of thiol groups by means of three methods. International journal of pharmaceutics. 2003;250:327–37. doi: 10.1016/s0378-5173(02)00542-2. [DOI] [PubMed] [Google Scholar]
- 114.Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2012;4:219–33. doi: 10.1002/wnan.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jain D, Bajaj A, Athawale R, Shrikhande S, Goel PN, Nikam Y, et al. Surface-coated PLA nanoparticles loaded with temozolomide for improved brain deposition and potential treatment of gliomas: development, characterization and in vivo studies. Drug delivery. 2014:1–18. doi: 10.3109/10717544.2014.926574. [DOI] [PubMed] [Google Scholar]
- 116.Yu Y, Ferrari R, Lattuada M, Storti G, Morbidelli M, Moscatelli D. PLA-based nanoparticles with tunable hydrophobicity and degradation kinetics. Journal of Polymer Science Part A: Polymer Chemistry. 2012;50:5191–200. [Google Scholar]
- 117.Moorkoth D, Nampoothiri KM. Synthesis, colloidal properties and cytotoxicity of biopolymer nanoparticles. Appl Biochem Biotechnol. 2014;174:2181–94. doi: 10.1007/s12010-014-1172-z. [DOI] [PubMed] [Google Scholar]
- 118.Valo H, Peltonen L, Vehviläinen S, Karjalainen M, Kostiainen R, Laaksonen T, et al. Electrospray Encapsulation of Hydrophilic and Hydrophobic Drugs in Poly (L-lactic acid) Nanoparticles. Small. 2009;5:1791–8. doi: 10.1002/smll.200801907. [DOI] [PubMed] [Google Scholar]
- 119.O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Materials Today. 2011;14:88–95. [Google Scholar]
- 120.Laurencin CT, Khan Y. Regenerative engineering. CRC Press; 2013. [DOI] [PubMed] [Google Scholar]
- 121.Laurencin C, Nair L. Regenerative Engineering: Approaches to Limb Regeneration and Other Grand Challenges. Regen Eng Transl Med. 2015;1:1–3. doi: 10.1007/s40883-015-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laurencin CT, Khan Y. Regenerative Engineering. Science Translational Medicine. 2012;4:160ed9–ed9. doi: 10.1126/scitranslmed.3004467. [DOI] [PubMed] [Google Scholar]
- 123.Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. International Orthopaedics (SICOT) 2013;37:2491–8. doi: 10.1007/s00264-013-2059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pipino C, Pandolfi A. Osteogenic differentiation of amniotic fluid mesenchymal stromal cells and their bone regeneration potential. World Journal of Stem Cells. 2015;7:681–90. doi: 10.4252/wjsc.v7.i4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kasir R, Vernekar V, Laurencin C. Regenerative Engineering of Cartilage Using Adipose-Derived Stem Cells. Regen Eng Transl Med. 2015;1:42–9. doi: 10.1007/s40883-015-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Copp DH, Shim S. The homeostatic function of bone as a mineral reservoir. Oral Surgery, Oral Medicine, Oral Pathology. 1963;16:738–44. doi: 10.1016/0030-4220(63)90081-1. [DOI] [PubMed] [Google Scholar]
- 127.Venkatesan J, Bhatnagar I, Manivasagan P, Kang K-H, Kim S-K. Alginate composites for bone tissue engineering: A review. International journal of biological macromolecules. 2015;72:269–81. doi: 10.1016/j.ijbiomac.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 128.Stevens MM. Biomaterials for bone tissue engineering. Materials today. 2008;11:18–25. [Google Scholar]
- 129.Ma PX, Zhang R, Xiao G, Franceschi R. Engineering new bone tissue in vitro on highly porous poly (α-hydroxyl acids)/hydroxyapatite composite scaffolds. Journal of biomedical materials research. 2001;54:284–93. doi: 10.1002/1097-4636(200102)54:2<284::aid-jbm16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 130.Sheikh Z, Najeeb S, Khurshid Z, Verma V, Rashid H, Glogauer M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials. 2015;8:5744–94. doi: 10.3390/ma8095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mackie E, Ahmed Y, Tatarczuch L, Chen K-S, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. The international journal of biochemistry & cell biology. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 132.Amini AR, Laurencin CT, Nukavarapu SP. Bone Tissue Engineering: Recent Advances and Challenges. Critical reviews in biomedical engineering. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Review of Medical Devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 134.Deng M, Kumbar SG, Lo KW-H, Ulery DB, Laurencin CT. Novel Polymer-Ceramics for Bone Repair and Regeneration. Recent Patents on Biomedical Engineering. 2011;4:168–84. [Google Scholar]
- 135.Keogh M, O’Brien F, Daly J. A novel collagen scaffold supports human osteogenesis—applications for bone tissue engineering. Cell Tissue Res. 2010;340:169–77. doi: 10.1007/s00441-010-0939-y. [DOI] [PubMed] [Google Scholar]
- 136.López-Álvarez M, Rodríguez-Valencia C, Serra J, González P. Bio-inspired Ceramics: Promising Scaffolds for Bone Tissue Engineering. Procedia Engineering. 2013;59:51–8. [Google Scholar]
- 137.Kim S-S, Sun Park M, Jeon O, Yong Choi C, Kim B-S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 138.Nanda HS, Nakamoto T, Chen S, Cai R, Kawazoe N, Chen G. Collagen microgel-assisted dexamethasone release from PLLA-collagen hybrid scaffolds of controlled pore structure for osteogenic differentiation of mesenchymal stem cells. Journal of Biomaterials Science, Polymer Edition. 2014;25:1374–86. doi: 10.1080/09205063.2014.938980. [DOI] [PubMed] [Google Scholar]
- 139.Vaquette C, Frochot C, Rahouadj R, Wang X. An innovative method to obtain porous PLLA scaffolds with highly spherical and interconnected pores. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;86:9–17. doi: 10.1002/jbm.b.30982. [DOI] [PubMed] [Google Scholar]
- 140.Zhang P, Wu H, Wu H, Lù Z, Deng C, Hong Z, et al. RGD-conjugated copolymer incorporated into composite of poly (lactide-co-glycotide) and poly (L-lactide)-grafted nanohydroxyapatite for bone tissue engineering. Biomacromolecules. 2011;12:2667–80. doi: 10.1021/bm2004725. [DOI] [PubMed] [Google Scholar]
- 141.Zhao X-F, Li X-D, Kang Y-Q, Yuan Q. Improved biocompatibility of novel poly (L-lactic acid)/β-tricalcium phosphate scaffolds prepared by an organic solvent-free method. International journal of nanomedicine. 2011;6:1385. doi: 10.2147/IJN.S20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang P, Hong Z, Yu T, Chen X, Jing X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly (lactide-co-glycolide) and hydroxyapatite surface-grafted with poly (L-lactide) Biomaterials. 2009;30:58–70. doi: 10.1016/j.biomaterials.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 143.Cui L, Zhang N, Cui W, Zhang P, Chen X. A Novel Nano/Micro-Fibrous Scaffold by Melt-Spinning Method for Bone Tissue Engineering. Journal of Bionic Engineering. 2015;12:117–28. [Google Scholar]
- 144.Qu Z, Ding J. Physical modification of the interior surfaces of PLGA porous scaffolds using sugar fibers as template. Journal of Biomaterials Science, Polymer Edition. 2013;24:447–59. doi: 10.1080/09205063.2012.690285. [DOI] [PubMed] [Google Scholar]
- 145.Brady MA, Renzing A, Douglas TE, Liu Q, Wille S, Parizek M, et al. Development of composite poly (lactide-co-glycolide)-nanodiamond scaffolds for bone cell growth. Journal of Nanoscience and Nanotechnology. 2015;15:1060–9. doi: 10.1166/jnn.2015.9745. [DOI] [PubMed] [Google Scholar]
- 146.Luo Y, Shen H, Fang Y, Cao Y, Huang J, Zhang M, et al. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly (lactic-co-glycolic acid) nanofibrous mats. ACS applied materials & interfaces. 2015;7:6331–9. doi: 10.1021/acsami.5b00862. [DOI] [PubMed] [Google Scholar]
- 147.Zhou P, Cheng X, Xia Y, Wang P, Zou K, Xu S, et al. Organic/Inorganic Composite Membranes Based on Poly (L-Lactic-co-Glycolic Acid) and Mesoporous Silica for Effective Bone Tissue Engineering. ACS applied materials & interfaces. 2014;6:20895–903. doi: 10.1021/am505493j. [DOI] [PubMed] [Google Scholar]
- 148.Samavedi S, Vaidya P, Gaddam P, Whittington AR, Goldstein AS. Electrospun meshes possessing region-wise differences in fiber orientation, diameter, chemistry and mechanical properties for engineering bone-ligament-bone tissues. Biotechnology and bioengineering. 2014;111:2549–59. doi: 10.1002/bit.25299. [DOI] [PubMed] [Google Scholar]
- 149.Jamshidi Adegani F, Langroudi L, Ardeshirylajimi A, Dinarvand P, Dodel M, Doostmohammadi A, et al. Coating of electrospun poly (lactic-co-glycolic acid) nanofibers with willemite bioceramic: improvement of bone reconstruction in rat model. Cell biology international. 2014;38:1271–9. doi: 10.1002/cbin.10318. [DOI] [PubMed] [Google Scholar]
- 150.Nelson C, Khan Y, Laurencin CT. Nanofiber–microsphere (nano-micro) matrices for bone regenerative engineering: a convergence approach toward matrix design. Regenerative Biomaterials. 2014;1:3–9. doi: 10.1093/rb/rbu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Privalova A, Markvicheva E, Sevrin C, Drozdova M, Kottgen C, Gilbert B, et al. Biodegradable polyester-based microcarriers with modified surface tailored for tissue engineering. Journal of Biomedical Materials Research Part A. 2015;103:939–48. doi: 10.1002/jbm.a.35231. [DOI] [PubMed] [Google Scholar]
- 152.Selcan Gungor-Ozkerim P, Balkan T, Kose GT, Sezai Sarac A, Kok FN. Incorporation of growth factor loaded microspheres into polymeric electrospun nanofibers for tissue engineering applications. Journal of Biomedical Materials Research Part A. 2014;102:1897–908. doi: 10.1002/jbm.a.34857. [DOI] [PubMed] [Google Scholar]
- 153.Xin-Hui X, Xin-Luan W, Ge Z, Yi-Xin H, Xiao-Hong W, Zhong L, et al. Structural and degradation characteristics of an innovative porous PLGA/TCP scaffold incorporated with bioactive molecular icaritin. Biomedical Materials. 2010;5:054109. doi: 10.1088/1748-6041/5/5/054109. [DOI] [PubMed] [Google Scholar]
- 154.Roy TD, Simon JL, Ricci JL, Rekow ED, Thompson VP, Parsons JR. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. Journal of Biomedical Materials Research Part A. 2003;66:283–91. doi: 10.1002/jbm.a.10582. [DOI] [PubMed] [Google Scholar]
- 155.Castro NJ, O’Brien J, Zhang LG. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale. 2015;7:14010–22. doi: 10.1039/c5nr03425f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakagawa M, Teraoka F, Fujimoto S, Hamada Y, Kibayashi H, Takahashi J. Improvement of cell adhesion on poly (L-lactide) by atmospheric plasma treatment. Journal of Biomedical Materials Research Part A. 2006;77:112–8. doi: 10.1002/jbm.a.30521. [DOI] [PubMed] [Google Scholar]
- 157.Woo KM, Seo J, Zhang R, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007;28:2622–30. doi: 10.1016/j.biomaterials.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ma Z, Gao C, Gong Y, Shen J. Paraffin spheres as porogen to fabricate poly(L-lactic acid) scaffolds with improved cytocompatibility for cartilage tissue engineering. Journal of biomedical materials research Part B, Applied biomaterials. 2003;67:610–7. doi: 10.1002/jbm.b.10049. [DOI] [PubMed] [Google Scholar]
- 159.Gong Y, Ma Z, Zhou Q, Li J, Gao C, Shen J. Poly (lactic acid) scaffold fabricated by gelatin particle leaching has good biocompatibility for chondrogenesis. Journal of Biomaterials Science, Polymer Edition. 2008;19:207–21. doi: 10.1163/156856208783432453. [DOI] [PubMed] [Google Scholar]
- 160.Liao CJ, Chen CF, Chen JH, Chiang SF, Lin YJ, Chang KY. Fabrication of porous biodegradable polymer scaffolds using a solvent merging/particulate leaching method. Journal of biomedical materials research. 2002;59:676–81. doi: 10.1002/jbm.10030. [DOI] [PubMed] [Google Scholar]
- 161.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Annals of Biomedical Engineering. 2004;32:477–86. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 162.Maquet V, Boccaccini AR, Pravata L, Notingher I, Jérôme R. Porous poly (α-hydroxyacid)/Bioglass® composite scaffolds for bone tissue engineering. I: preparation and in vitro characterisation. Biomaterials. 2004;25:4185–94. doi: 10.1016/j.biomaterials.2003.10.082. [DOI] [PubMed] [Google Scholar]
- 163.Ahmadi R, Mordan N, Forbes A, Day R. Enhanced attachment, growth and migration of smooth muscle cells on microcarriers produced using thermally induced phase separation. Acta biomaterialia. 2011;7:1542–9. doi: 10.1016/j.actbio.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 164.Salerno A, Fernández-Gutiérrez M, del Barrio JSR, Pascual CD. Macroporous and nanometre scale fibrous PLA and PLA–HA composite scaffolds fabricated by a bio safe strategy. RSC Advances. 2014;4:61491–502. [Google Scholar]
- 165.Kim S-S, Park MS, Jeon O, Choi CY, Kim B-S. Poly (lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 166.Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(d,l-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–22. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 167.Mikos AG, Temenoff JS. Formation of highly porous biodegradable scaffolds for tissue engineering. 2000 [Google Scholar]
- 168.Narayanan G, Aguda RM, Hartman M, Chung C-C, Boy R, Gupta BS, et al. Fabrication and Characterization of Poly (ε-caprolactone)/α-Cyclodextrin Pseudorotaxane Nanofibers. Biomacromolecules. 2015 doi: 10.1021/acs.biomac.5b01379. [DOI] [PubMed] [Google Scholar]
- 169.Sahoo S, Ang LT, Goh JC, Toh SL. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. Journal of biomedical materials research Part A. 2010;93:1539–50. doi: 10.1002/jbm.a.32645. [DOI] [PubMed] [Google Scholar]
- 170.Zhao R, Li X, Sun B, Tong Y, Jiang Z, Wang C. Nitrofurazone-loaded electrospun PLLA/sericin-based dual-layer fiber mats for wound dressing applications. RSC Advances. 2015;5:16940–9. [Google Scholar]
- 171.Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. Nano- and microfiber combined scaffolds: A new architecture for bone tissue engineering. Journal of Materials Science: Materials in Medicine. 2005;16:1099–104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- 172.Whited BM, Whitney JR, Hofmann MC, Xu Y, Rylander MN. Pre-osteoblast infiltration and differentiation in highly porous apatite-coated PLLA electrospun scaffolds. Biomaterials. 2011;32:2294–304. doi: 10.1016/j.biomaterials.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 173.Boskey AL. Matrix proteins and mineralization: an overview. Connective tissue research. 1996;35:357–63. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- 174.Cui W, Li X, Xie C, Zhuang H, Zhou S, Weng J. Hydroxyapatite nucleation and growth mechanism on electrospun fibers functionalized with different chemical groups and their combinations. Biomaterials. 2010;31:4620–9. doi: 10.1016/j.biomaterials.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 175.Dinarvand P, Seyedjafari E, Shafiee A, Jandaghi AB, Doostmohammadi A, Fathi MH, et al. New approach to bone tissue engineering: simultaneous application of hydroxyapatite and bioactive glass coated on a poly(L-lactic acid) scaffold. ACS Appl Mater Interfaces. 2011;3:4518–24. doi: 10.1021/am201212u. [DOI] [PubMed] [Google Scholar]
- 176.Andric T, Taylor B, Whittington A, Freeman J. Fabrication and Characterization of Three-Dimensional Electrospun Scaffolds for Bone Tissue Engineering. Regen Eng Transl Med. 2015;1:32–41. doi: 10.1002/jbm.a.34045. [DOI] [PubMed] [Google Scholar]
- 177.Andric T, Wright LD, Taylor BL, Freeman JW. Fabrication and characterization of three-dimensional electrospun scaffolds for bone tissue engineering. Journal of biomedical materials research Part A. 2012;100:2097–105. doi: 10.1002/jbm.a.34045. [DOI] [PubMed] [Google Scholar]
- 178.Brown JL, Peach MS, Nair LS, Kumbar SG, Laurencin CT. Composite scaffolds: Bridging nanofiber and microsphere architectures to improve bioactivity of mechanically competent constructs. Journal of Biomedical Materials Research Part A. 2010;95A:1150–8. doi: 10.1002/jbm.a.32934. [DOI] [PubMed] [Google Scholar]
- 179.Borden M, Attawia M, Khan Y, El-Amin SF, Laurencin CT. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. Journal of Bone & Joint Surgery, British Volume. 2004;86-B:1200–8. doi: 10.1302/0301-620x.86b8.14267. [DOI] [PubMed] [Google Scholar]
- 180.Shen FH, Zeng Q, Lv Q, Choi L, Balian G, Li X, et al. Osteogenic differentiation of adipose-derived stromal cells treated with GDF-5 cultured on a novel three-dimensional sintered microsphere matrix. The Spine Journal. 2006;6:615–23. doi: 10.1016/j.spinee.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 181.Lv Q, Nair L, Laurencin CT. Fabrication, characterization, and in vitro evaluation of poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors. Journal of Biomedical Materials Research Part A. 2009;91A:679–91. doi: 10.1002/jbm.a.32302. [DOI] [PubMed] [Google Scholar]
- 182.Lu HH, El-Amin SF, Scott KD, Laurencin CT. Three-dimensional, bioactive, biodegradable, polymer–bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. Journal of Biomedical Materials Research Part A. 2003;64A:465–74. doi: 10.1002/jbm.a.10399. [DOI] [PubMed] [Google Scholar]
- 183.Cushnie EK, Khan YM, Laurencin CT. Amorphous hydroxyapatite-sintered polymeric scaffolds for bone tissue regeneration: Physical characterization studies. Journal of Biomedical Materials Research Part A. 2008;84A:54–62. doi: 10.1002/jbm.a.31380. [DOI] [PubMed] [Google Scholar]
- 184.Borden M, Attawia M, Laurencin CT. The sintered microsphere matrix for bone tissue engineering: in vitro osteoconductivity studies. Journal of biomedical materials research. 2002;61:421–9. doi: 10.1002/jbm.10201. [DOI] [PubMed] [Google Scholar]
- 185.Jabbarzadeh E, Deng M, Lv Q, Jiang T, Khan YM, Nair LS, et al. VEGF-incorporated biomimetic poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100B:2187–96. doi: 10.1002/jbm.b.32787. [DOI] [PubMed] [Google Scholar]
- 186.Amini AR, Adams DJ, Laurencin CT, Nukavarapu SP. Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Engineering Part A. 2012;18:1376–88. doi: 10.1089/ten.tea.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jiang T, Abdel-Fattah WI, Laurencin CT. In vitro evaluation of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials. 2006;27:4894–903. doi: 10.1016/j.biomaterials.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 188.Brown JL, Nair LS, Laurencin CT. Solvent/Non-Solvent Sintering: A Novel Route to Create Porous Microsphere Scaffolds For Tissue Regeneration. Journal of biomedical materials research Part B, Applied biomaterials. 2008;86B:396–406. doi: 10.1002/jbm.b.31033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abdel-Fattah WI, Jiang T, El-Bassyouni GE-T, Laurencin CT. Synthesis, characterization of chitosans and fabrication of sintered chitosan microsphere matrices for bone tissue engineering. Acta Biomaterialia. 2007;3:503–14. doi: 10.1016/j.actbio.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 190.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue Engineering of Bone: Material and Matrix Considerations. The Journal of Bone & Joint Surgery. 2008;90:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 191.Jiang T, Nukavarapu SP, Deng M, Jabbarzadeh E, Kofron MD, Doty SB, et al. Chitosan–poly(lactide-co-glycolide) microsphere-based scaffolds for bone tissue engineering: In vitro degradation and in vivo bone regeneration studies. Acta Biomaterialia. 2010;6:3457–70. doi: 10.1016/j.actbio.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 192.Aravamudhan A, Ramos DM, Nip J, Harmon MD, James R, Deng M, et al. Cellulose and Collagen Derived Micro-Nano Structured Scaffolds for Bone Tissue Engineering. Journal of Biomedical Nanotechnology. 2013;9:719–31. doi: 10.1166/jbn.2013.1574. [DOI] [PubMed] [Google Scholar]
- 193.Kofron MD, Cooper JA, Kumbar SG, Laurencin CT. Novel tubular composite matrix for bone repair. Journal of Biomedical Materials Research Part A. 2007;82A:415–25. doi: 10.1002/jbm.a.31148. [DOI] [PubMed] [Google Scholar]
- 194.Borden M, El-Amin S, Attawia M, Laurencin C. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials. 2003;24:597–609. doi: 10.1016/s0142-9612(02)00374-5. [DOI] [PubMed] [Google Scholar]
- 195.Nukavarapu SP, Amini AR. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE. IEEE; 2011. Optimal scaffold design and effective progenitor cell identification for the regeneration of vascularized bone; pp. 2464–7. [DOI] [PubMed] [Google Scholar]
- 196.Amini A, Nukavarapu S. Oxygen-Tension Controlled Matrices for Enhanced Osteogenic Cell Survival and Performance. Annals of Biomedical Engineering. 2014;42:1261–70. doi: 10.1007/s10439-014-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Verma S, Kumar N. Effect of biomimetic 3D environment of an injectable polymeric scaffold on MG-63 osteoblastic-cell response. Materials Science and Engineering: C. 2010;30:1118–28. [Google Scholar]
- 198.Serra T, Planell JA, Navarro M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomaterialia. 2013;9:5521–30. doi: 10.1016/j.actbio.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 199.Yang SM, Jang SG, Choi DG, Kim S, Yu HK. Nanomachining by colloidal lithography. Small. 2006;2:458–75. doi: 10.1002/smll.200500390. [DOI] [PubMed] [Google Scholar]
- 200.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 201.Fukada E, Yasuda I. On the Piezoelectric Effect of Bone. Journal of the Physical Society of Japan. 1957;12:1158–62. [Google Scholar]
- 202.Blank M, Goodman R. Do electromagnetic fields interact directly with DNA? Bioelectromagnetics. 1997;18:111–5. doi: 10.1002/(sici)1521-186x(1997)18:2<111::aid-bem3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 203.Cao J, Man Y, Li L. Electrical stimuli improve osteogenic differentiation mediated by aniline pentamer and PLGA nanocomposites. Biomedical Reports. 2013;1:428–32. doi: 10.3892/br.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bagchi A, Meka SR, Rao BN, Chatterjee K. Perovskite ceramic nanoparticles in polymer composites for augmenting bone tissue regeneration. Nanotechnology. 2014;25:485101. doi: 10.1088/0957-4484/25/48/485101. [DOI] [PubMed] [Google Scholar]
- 205.Zhang C, Wang L, Zhai T, Wang X, Dan Y, Turng LS. The surface grafting of graphene oxide with poly(ethylene glycol) as a reinforcement for poly(lactic acid) nanocomposite scaffolds for potential tissue engineering applications. J Mech Behav Biomed Mater. 2016;53:403–13. doi: 10.1016/j.jmbbm.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 206.Paiyz EM, Ami RA, Joysurya B, Arellano-Jimenez MJ, Cato TL, Mary MS, et al. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: fabrication, in vitro and in vivo evaluation. Biomedical Materials. 2014;9:035001. doi: 10.1088/1748-6041/9/3/035001. [DOI] [PubMed] [Google Scholar]
- 207.Lahiri D, Rouzaud F, Richard T, Keshri AK, Bakshi SR, Kos L, et al. Boron nitride nanotube reinforced polylactide-polycaprolactone copolymer composite: mechanical properties and cytocompatibility with osteoblasts and macrophages in vitro. Acta Biomater. 2010;6:3524–33. doi: 10.1016/j.actbio.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 208.Li X, Wang X, Jiang X, Yamaguchi M, Ito A, Bando Y, et al. Boron nitride nanotube-enhanced osteogenic differentiation of mesenchymal stem cells. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2015:n/a–n/a. doi: 10.1002/jbm.b.33391. [DOI] [PubMed] [Google Scholar]
- 209.Lakhkar NJ, Lee I-H, Kim H-W, Salih V, Wall IB, Knowles JC. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Advanced Drug Delivery Reviews. 2013;65:405–20. doi: 10.1016/j.addr.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 210.Hickey DJ, Ercan B, Sun L, Webster TJ. Adding MgO nanoparticles to hydroxyapatite-PLLA nanocomposites for improved bone tissue engineering applications. Acta biomaterialia. 2015;14:175–84. doi: 10.1016/j.actbio.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 211.Stevanovic M, Filipovic N, Djurdjevic J, Lukic M, Milenkovic M, Boccaccini A. 45S5Bioglass(R)-based scaffolds coated with selenium nanoparticles or with poly(lactide-co-glycolide)/selenium particles: Processing, evaluation and antibacterial activity. Colloids and surfaces B, Biointerfaces. 2015;132:208–15. doi: 10.1016/j.colsurfb.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 212.Shameli K, Ahmad MB, Yunus WM, Ibrahim NA, Rahman RA, Jokar M, et al. Silver/poly (lactic acid) nanocomposites: preparation, characterization, and antibacterial activity. Int J Nanomedicine. 2010;5:573–9. doi: 10.2147/ijn.s12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stolzoff M, Webster TJ. Reducing bone cancer cell functions using selenium nanocomposites. Journal of Biomedical Materials Research Part A. 2015:n/a–n/a. doi: 10.1002/jbm.a.35583. [DOI] [PubMed] [Google Scholar]
- 214.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. Journal of Cellular Biochemistry. 2003;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 215.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 36:1392–404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 216.Phillips AM. Overview of the fracture healing cascade. Injury. 36:S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 217.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–5. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lu HH, Kofron MD, El-Amin SF, Attawia MA, Laurencin CT. In vitro bone formation using muscle-derived cells: a new paradigm for bone tissue engineering using polymer–bone morphogenetic protein matrices. Biochemical and Biophysical Research Communications. 2003;305:882–9. doi: 10.1016/s0006-291x(03)00858-1. [DOI] [PubMed] [Google Scholar]
- 219.Lo KWH, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Advanced Drug Delivery Reviews. 2012;64:1277–91. doi: 10.1016/j.addr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schofer MD, Roessler PP, Schaefer J, Theisen C, Schlimme S, Heverhagen JT, et al. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PloS one. 2011;6:e25462. doi: 10.1371/journal.pone.0025462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Valdes MA, Thakur NA, Namdari S, Ciombor DM, Palumbo M. Recombinant bone morphogenic protein-2 in orthopaedic surgery: a review. Archives of orthopaedic and trauma surgery. 2009;129:1651–7. doi: 10.1007/s00402-009-0850-8. [DOI] [PubMed] [Google Scholar]
- 222.Cushnie EK, Ulery BD, Nelson SJ, Deng M, Sethuraman S, Doty SB, et al. Simple Signaling Molecules for Inductive Bone Regenerative Engineering. PloS one. 2014;9:e101627. doi: 10.1371/journal.pone.0101627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Su Y, Su Q, Liu W, Lim M, Venugopal JR, Mo X, et al. Controlled release of bone morphogenetic protein 2 and dexamethasone loaded in core-shell PLLACL-collagen fibers for use in bone tissue engineering. Acta biomaterialia. 2012;8:763–71. doi: 10.1016/j.actbio.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 224.Yang Y, Li X, Cui W, Zhou S, Tan R, Wang C. Structural stability and release profiles of proteins from core-shell poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. Journal of Biomedical Materials Research Part A. 2008;86A:374–85. doi: 10.1002/jbm.a.31595. [DOI] [PubMed] [Google Scholar]
- 225.Shah NJ, Hyder MN, Quadir MA, Dorval Courchesne N-M, Seeherman HJ, Nevins M, et al. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proceedings of the National Academy of Sciences. 2014;111:12847–52. doi: 10.1073/pnas.1408035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ku SH, Ryu J, Hong SK, Lee H, Park CB. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials. 2010;31:2535–41. doi: 10.1016/j.biomaterials.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 227.Shin YM, Lee YB, Kim SJ, Kang JK, Park JC, Jang W, et al. Mussel-inspired immobilization of vascular endothelial growth factor (VEGF) for enhanced endothelialization of vascular grafts. Biomacromolecules. 2012;13:2020–8. doi: 10.1021/bm300194b. [DOI] [PubMed] [Google Scholar]
- 228.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Advanced drug delivery reviews. 2012;64:1292–309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Carbone EJ, Rajpura K, Jiang T, Laurencin CT, Lo KW-H. Regulation of bone regeneration with approved small molecule compounds. Advances in Regenerative Biology. 2014;1 [Google Scholar]
- 230.Lo KW-H, Jiang T, Gagnon KA, Nelson C, Laurencin CT. Small-molecule based musculoskeletal regenerative engineering. Trends in biotechnology. 2014;32:74–81. doi: 10.1016/j.tibtech.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kato S, Sangadala S, Tomita K, Titus L, Boden SD. A synthetic compound that potentiates bone morphogenetic protein-2-induced transdifferentiation of myoblasts into the osteoblastic phenotype. Molecular and cellular biochemistry. 2011;349:97–106. doi: 10.1007/s11010-010-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lo KWH, Ulery BD, Kan HM, Ashe KM, Laurencin CT. Evaluating the feasibility of utilizing the small molecule phenamil as a novel biofactor for bone regenerative engineering. Journal of tissue engineering and regenerative medicine. 2014;8:728–36. doi: 10.1002/term.1573. [DOI] [PubMed] [Google Scholar]
- 233.Lo KWH, Kan HM, Ashe KM, Laurencin CT. The small molecule PKA-specific cyclic AMP analogue as an inducer of osteoblast-like cells differentiation and mineralization. Journal of Tissue Engineering and Regenerative Medicine. 2012;6:40–8. doi: 10.1002/term.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.K WH Lo, B D Ulery, Deng M, K M Ashe, C T Laurencin. Current Patents on Osteoinductive Molecules for Bone Tissue Engineering. Recent Patents on Biomedical Engineering. 2011;4:153–67. [Google Scholar]
- 235.Lo KWH, Ashe KM, Kan HM, Lee DA, Laurencin CT. Activation of cyclic amp/protein kinase: A signaling pathway enhances osteoblast cell adhesion on biomaterials for regenerative engineering. Journal of Orthopaedic Research. 2011;29:602–8. doi: 10.1002/jor.21276. [DOI] [PubMed] [Google Scholar]
- 236.Laurencin CT, Ashe KM, Henry N, Kan HM, Lo KWH. Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug discovery today. 2014;19:794–800. doi: 10.1016/j.drudis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lo KW, Ashe KM, Kan HM, Laurencin CT. The role of small molecules in musculoskeletal regeneration. Regenerative medicine. 2012;7:535–49. doi: 10.2217/rme.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ge Z, Yang F, Goh JCH, Ramakrishna S, Lee EH. Biomaterials and scaffolds for ligament tissue engineering. Journal of Biomedical Materials Research Part A. 2006;77A:639–52. doi: 10.1002/jbm.a.30578. [DOI] [PubMed] [Google Scholar]
- 239.Petrigliano FA, McAllister DR, Wu BM. Tissue Engineering for Anterior Cruciate Ligament Reconstruction: A Review of Current Strategies. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2006;22:441–51. doi: 10.1016/j.arthro.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 240.Levine JW, Kiapour AM, Quatman CE, Wordeman SC, Goel VK, Hewett TE, et al. Clinically Relevant Injury Patterns After an Anterior Cruciate Ligament Injury Provide Insight Into Injury Mechanisms. The American Journal of Sports Medicine. 2013;41:385–95. doi: 10.1177/0363546512465167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Quatman CE, Kiapour A, Myer GD, Ford KR, Demetropoulos CK, Goel VK, et al. Cartilage Pressure Distributions Provide a Footprint to Define Female Anterior Cruciate Ligament Injury Mechanisms. The American Journal of Sports Medicine. 2011;39:1706–13. doi: 10.1177/0363546511400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fu FH, Bennett CH, Ma CB, Menetrey J, Lattermann C. Current Trends in Anterior Cruciate Ligament Reconstruction: Part II. Operative Procedures and Clinical Correlations. The American Journal of Sports Medicine. 2000;28:124–30. doi: 10.1177/03635465000280010801. [DOI] [PubMed] [Google Scholar]
- 243.Kwansa AL, Empson YM, Ekwueme EC, Walters VI, Freeman JW, Laurencin CT. Novel matrix based anterior cruciate ligament (ACL) regeneration. Soft Matter. 2010;6:5016–25. [Google Scholar]
- 244.Lewis SL, Dirksen SR, Heitkemper MM, Bucher L. Medical-Surgical Nursing: Assessment and Management of Clinical Problems, Single Volume. Elsevier Health Sciences; 2014. [Google Scholar]
- 245.Dunn MG, Avasarala PN, Zawadsky JP. Optimization of extruded collagen fibers for ACL reconstruction. Journal of biomedical materials research. 1993;27:1545–52. doi: 10.1002/jbm.820271211. [DOI] [PubMed] [Google Scholar]
- 246.Bellincampi LD, Closkey RF, Prasad R, Zawadsky JP, Dunn MG. Viability of fibroblast-seeded ligament analogs after autogenous implantation. Journal of Orthopaedic Research. 1998;16:414–20. doi: 10.1002/jor.1100160404. [DOI] [PubMed] [Google Scholar]
- 247.Majima T, Funakosi T, Iwasaki N, Yamane ST, Harada K, Nonaka S, et al. Alginate and chitosan polyion complex hybrid fibers for scaffolds in ligament and tendon tissue engineering. Journal of orthopaedic science: official journal of the Japanese Orthopaedic Association. 2005;10:302–7. doi: 10.1007/s00776-005-0891-y. [DOI] [PubMed] [Google Scholar]
- 248.Hansson A, Hashom N, Falson F, Rousselle P, Jordan O, Borchard G. In vitro evaluation of an RGD-functionalized chitosan derivative for enhanced cell adhesion. Carbohydrate polymers. 2012;90:1494–500. doi: 10.1016/j.carbpol.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 249.Bourke SL, Kohn J, Dunn MG. Preliminary development of a novel resorbable synthetic polymer fiber scaffold for anterior cruciate ligament reconstruction. Tissue engineering. 2004;10:43–52. doi: 10.1089/107632704322791682. [DOI] [PubMed] [Google Scholar]
- 250.Dürselen L, Dauner M, Hierlemann H, Planck H, Claes LE, Ignatius A. Resorbable polymer fibers for ligament augmentation. Journal of biomedical materials research. 2001;58:666–72. doi: 10.1002/jbm.1067. [DOI] [PubMed] [Google Scholar]
- 251.Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;26:1523–32. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 252.Lu HH, Cooper JA, Jr, Manuel S, Freeman JW, Attawia MA, Ko FK, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805–16. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 253.Laurencin CT, Freeman JW. Ligament tissue engineering: an evolutionary materials science approach. Biomaterials. 2005;26:7530–6. doi: 10.1016/j.biomaterials.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 254.Sarukawa J, Takahashi M, Abe M, Suzuki D, Tokura S, Furuike T, et al. Effects of Chitosan-Coated Fibers as a Scaffold for Three-Dimensional Cultures of Rabbit Fibroblasts for Ligament Tissue Engineering. Journal of Biomaterials Science, Polymer Edition. 2011;22:717–32. doi: 10.1163/092050610X491067. [DOI] [PubMed] [Google Scholar]
- 255.Cooper JA, Jr, Bailey LO, Carter JN, Castiglioni CE, Kofron MD, Ko FK, et al. Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials. 2006;27:2747–54. doi: 10.1016/j.biomaterials.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 256.Cooper JA, Sahota JS, Gorum WJ, Carter J, Doty SB, Laurencin CT. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proceedings of the National Academy of Sciences. 2007;104:3049–54. doi: 10.1073/pnas.0608837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003;9:431–9. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- 258.Karamuk E, Mayer J, Raeber G. Tissue engineered composite of a woven fabric scaffold with tendon cells, response on mechanical simulation in vitro. Composites Science and Technology. 2004;64:885–91. [Google Scholar]
- 259.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta biomaterialia. 2006;2:377–85. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 260.Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, et al. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34:1942–53. doi: 10.1016/j.biomaterials.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sahoo S, Lok Toh S, Hong Goh JC. PLGA nanofiber-coated silk microfibrous scaffold for connective tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2010;95B:19–28. doi: 10.1002/jbm.b.31678. [DOI] [PubMed] [Google Scholar]
- 262.Kim M, Hong B, Lee J, Kim SE, Kang SS, Kim YH, et al. Composite System of PLCL Scaffold and Heparin-Based Hydrogel for Regeneration of Partial-Thickness Cartilage Defects. Biomacromolecules. 2012;13:2287–98. doi: 10.1021/bm3005353. [DOI] [PubMed] [Google Scholar]
- 263.Barber JG, Handorf AM, Allee TJ, Li WJ. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue engineering Part A. 2013;19:1265–74. doi: 10.1089/ten.tea.2010.0538. [DOI] [PubMed] [Google Scholar]
- 264.Lu HH, Cooper JA, Jr, Manuel S, Freeman JW, Attawia MA, Ko FK, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805–16. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 265.Kimura Y, Hokugo A, Takamoto T, Tabata Y, Kurosawa H. Regeneration of anterior cruciate ligament by biodegradable scaffold combined with local controlled release of basic fibroblast growth factor and collagen wrapping. Tissue engineering Part C, Methods. 2008;14:47–57. doi: 10.1089/tec.2007.0286. [DOI] [PubMed] [Google Scholar]
- 266.Qu D, Mosher C, Boushell M, Lu H. Engineering Complex Orthopaedic Tissues Via Strategic Biomimicry. Ann Biomed Eng. 2015;43:697–717. doi: 10.1007/s10439-014-1190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lu H, Subramony S, Boushell M, Zhang X. Tissue Engineering Strategies for the Regeneration of Orthopedic Interfaces. Ann Biomed Eng. 2010;38:2142–54. doi: 10.1007/s10439-010-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Freeman JW, Woods MD, Laurencin CT. Tissue Engineering of the Anterior Cruciate Ligament Using a Braid-Twist Scaffold Design. Journal of biomechanics. 2007;40:2029–36. doi: 10.1016/j.jbiomech.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mosher CZ, Spalazzi JP, Lu HH. Stratified scaffold design for engineering composite tissues. Methods (San Diego, Calif) 2015;84:99–102. doi: 10.1016/j.ymeth.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 270.Spalazzi JP, Boskey AL, Pleshko N, Lu HH. Quantitative Mapping of Matrix Content and Distribution across the Ligament-to-Bone Insertion. PLoS ONE. 2013;8:e74349. doi: 10.1371/journal.pone.0074349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lynn AK, Best SM, Cameron RE, Harley BA, Yannas IV, Gibson LJ, et al. Design of a multiphase osteochondral scaffold. I. Control of chemical composition. Journal of Biomedical Materials Research Part A. 2010;92A:1057–65. doi: 10.1002/jbm.a.32415. [DOI] [PubMed] [Google Scholar]
- 272.Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. Journal of Biomedical Materials Research Part A. 2010;92A:1078–93. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 273.Ellis DL, Yannas IV. Recent advances in tissue synthesis in vivo by use of collagen-glycosaminoglycan copolymers. Biomaterials. 1996;17:291–9. doi: 10.1016/0142-9612(96)85567-0. [DOI] [PubMed] [Google Scholar]
- 274.Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. Multiphasic Scaffolds for Periodontal Tissue Engineering. Journal of Dental Research. 2014;93:1212–21. doi: 10.1177/0022034514544301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Karring T, Nyman S, Gottlow JAN, Laurell L. Development of the biological concept of guided tissue regeneration — animal and human studies. Periodontology 2000. 1993;1:26–35. [PubMed] [Google Scholar]
- 276.Carlo Reis EC, Borges APB, Araújo MVF, Mendes VC, Guan L, Davies JE. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials. 2011;32:9244–53. doi: 10.1016/j.biomaterials.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 277.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue engineering. 2006;12:3497–508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 278.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochemical and biophysical research communications. 2005;338:762–70. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 279.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. Journal of Biomedical Materials Research Part A. 2008;86A:1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 280.He J, Zhang W, Liu Y, Li X, Li D, Jin Z. Design and fabrication of biomimetic multiphased scaffolds for ligament-to-bone fixation. Materials Science and Engineering: C. 2015;50:12–8. doi: 10.1016/j.msec.2015.01.088. [DOI] [PubMed] [Google Scholar]
- 281.Chung EJ, Sugimoto MJ, Koh JL, Ameer GA. A biodegradable tri-component graft for anterior cruciate ligament reconstruction. Journal of Tissue Engineering and Regenerative Medicine. 2014:n/a–n/a. doi: 10.1002/term.1966. [DOI] [PubMed] [Google Scholar]
- 282.He J, Qin T, Liu Y, Li X, Li D, Jin Z. Electrospinning of nanofibrous scaffolds with continuous structure and material gradients. Materials Letters. 2014;137:393–7. [Google Scholar]
- 283.Singh M, Morris CP, Ellis RJ, Detamore MS, Berkland C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue engineering Part C, Methods. 2008;14:299–309. doi: 10.1089/ten.tec.2008.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Dormer NH, Gupta V, Scurto AM, Berkland CJ, Detamore MS. Effect of different sintering methods on bioactivity and release of proteins from PLGA microspheres. Materials Science and Engineering: C. 2013;33:4343–51. doi: 10.1016/j.msec.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bhamidipati M, Sridharan B, Scurto AM, Detamore MS. Subcritical CO2 sintering of microspheres of different polymeric materials to fabricate scaffolds for tissue engineering. Materials Science and Engineering: C. 2013;33:4892–9. doi: 10.1016/j.msec.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mohan N, Gupta V, Sridharan B, Sutherland A, Detamore MS. The potential of encapsulating “raw materials” in 3D osteochondral gradient scaffolds. Biotechnology and Bioengineering. 2014;111:829–41. doi: 10.1002/bit.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mohan N, Gupta V, Sridharan BP, Mellott AJ, Easley JT, Palmer RH, et al. Microsphere-based gradient implants for osteochondral regeneration: a long-term study in sheep. Regenerative medicine. 2015;10:709–28. doi: 10.2217/rme.15.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Phillips JE, Burns KL, Le Doux JM, Guldberg RE, García AJ. Engineering graded tissue interfaces. Proceedings of the National Academy of Sciences. 2008;105:12170–5. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Veth RP, Jansen HW, Leenslag JW, Pennings AJ, Hartel RM, Nielsen HK. Experimental meniscal lesions reconstructed with a carbon fiber-polyurethane-poly(L-lactide) graft. Clin Orthop Relat Res. 1986:286–93. [PubMed] [Google Scholar]
- 290.Ike O, Shimizu Y, Okada T, Ikada Y, Hitomi S. Experimental studies on an artificial trachea of collagen-coated poly(L-lactic acid) mesh or unwoven cloth combined with a periosteal graft. ASAIO transactions/American Society for Artificial Internal Organs. 1991;37:24–6. doi: 10.1097/00002480-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 291.von Schroeder HP, Kwan M, Amiel D, Coutts RD. The use of polylactic acid matrix and periosteal grafts for the reconstruction of rabbit knee articular defects. Journal of biomedical materials research. 1991;25:329–39. doi: 10.1002/jbm.820250305. [DOI] [PubMed] [Google Scholar]
- 292.Zhang Y, Yang F, Liu K, Shen H, Zhu Y, Zhang W, et al. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials. 2012;33:2926–35. doi: 10.1016/j.biomaterials.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 293.Yang Z, Wu Y, Li C, Zhang T, Zou Y, Hui JH, et al. Improved mesenchymal stem cells attachment and in vitro cartilage tissue formation on chitosan-modified poly(L-lactide-co-epsilon-caprolactone) scaffold. Tissue engineering Part A. 2012;18:242–51. doi: 10.1089/ten.tea.2011.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Li C, Wang L, Yang Z, Kim G, Chen H, Ge Z. A viscoelastic chitosan-modified three-dimensional porous poly (L-lactide-co-ε-caprolactone) scaffold for cartilage tissue engineering. Journal of Biomaterials Science, Polymer Edition. 2012;23:405–24. doi: 10.1163/092050610X551970. [DOI] [PubMed] [Google Scholar]
- 295.Chao L, Jingjing Z, Yijiang L, Shamus M, Gilson K, Zigang G. Poly (l-lactide-co-caprolactone) scaffolds enhanced with poly (β-hydroxybutyrate-co-β-hydroxyvalerate) microspheres for cartilage regeneration. Biomedical Materials. 2013;8:025005. doi: 10.1088/1748-6041/8/2/025005. [DOI] [PubMed] [Google Scholar]
- 296.Stenhamre H, Thorvaldsson A, Enochson L, Walkenström P, Lindahl A, Brittberg M, et al. Nanosized fibers’ effect on adult human articular chondrocytes behavior. Materials Science and Engineering: C. 2013;33:1539–45. doi: 10.1016/j.msec.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 297.Rosenzweig DH, Carelli E, Steffen T, Jarzem P, Haglund L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int J Mol Sci. 2015;16:15118–35. doi: 10.3390/ijms160715118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pacifici M. Introduction to the mini-review series “Articular cartilage: Biology, pathology and repair”. Matrix Biology. 2014;39:1. doi: 10.1016/j.matbio.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 299.Sophia Fox AJ, Bedi A, Rodeo SA. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health. 2009;1:461–8. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instructional course lectures. 2005;54:465–80. [PubMed] [Google Scholar]
- 301.Li X, Jin L, Balian G, Laurencin CT, Greg Anderson D. Demineralized bone matrix gelatin as scaffold for osteochondral tissue engineering. Biomaterials. 2006;27:2426–33. doi: 10.1016/j.biomaterials.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 302.Kon E, Filardo G, Perdisa F, Venieri G, Marcacci M. Clinical results of multilayered biomaterials for osteochondral regeneration. Journal of Experimental Orthopaedics. 2014;1:10. doi: 10.1186/s40634-014-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Bekkers JE, Bartels LW, Vincken KL, Dhert WJ, Creemers LB, Saris DB. Articular cartilage evaluation after TruFit plug implantation analyzed by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) Am J Sports Med. 2013;41:1290–5. doi: 10.1177/0363546513483536. [DOI] [PubMed] [Google Scholar]
- 304.Dhollander AAM, Liekens K, Almqvist KF, Verdonk R, Lambrecht S, Elewaut D, et al. A Pilot Study of the Use of an Osteochondral Scaffold Plug for Cartilage Repair in the Knee and How to Deal With Early Clinical Failures. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 28:225–33. doi: 10.1016/j.arthro.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 305.Verhaegen J, Clockaerts S, Van Osch GJVM, Somville J, Verdonk P, Mertens P. TruFit Plug for Repair of Osteochondral Defects—Where Is the Evidence? Systematic Review of Literature. Cartilage. 2014 doi: 10.1177/1947603514548890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tyler TF, Lung JY. Rehabilitation following osteochondral injury to the knee. Current Reviews in Musculoskeletal Medicine. 2012;5:72–81. doi: 10.1007/s12178-011-9108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Oussedik S, Tsitskaris K, Parker D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2015;31:732–44. doi: 10.1016/j.arthro.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 308.Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41:1426–34. doi: 10.1177/0363546513485931. [DOI] [PubMed] [Google Scholar]
- 309.Miller TT. MR imaging of the knee. Sports medicine and arthroscopy review. 2009;17:56–67. doi: 10.1097/JSA.0b013e3181974353. [DOI] [PubMed] [Google Scholar]
- 310.Filardo G, Kon E, Roffi A, Di Martino A, Marcacci M. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2013;29:174–86. doi: 10.1016/j.arthro.2012.05.891. [DOI] [PubMed] [Google Scholar]
- 311.Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis research & therapy. 2007;9:R41. doi: 10.1186/ar2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kreuz PC, Müller S, Ossendorf C, Kaps C, Erggelet C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis research & therapy. 2009;11:R33–R. doi: 10.1186/ar2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kreuz PC, Muller S, Freymann U, Erggelet C, Niemeyer P, Kaps C, et al. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am J Sports Med. 2011;39:1697–705. doi: 10.1177/0363546511403279. [DOI] [PubMed] [Google Scholar]
- 314.Jeon JE, Vaquette C, Klein TJ, Hutmacher DW. Perspectives in Multiphasic Osteochondral Tissue Engineering. The Anatomical Record. 2014;297:26–35. doi: 10.1002/ar.22795. [DOI] [PubMed] [Google Scholar]
- 315.Cui W, Wang Q, Chen G, Zhou S, Chang Q, Zuo Q, et al. Repair of articular cartilage defects with tissue-engineered osteochondral composites in pigs. Journal of Bioscience and Bioengineering. 2011;111:493–500. doi: 10.1016/j.jbiosc.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 316.Yu Z, Zhu T, Li C, Shi X, Liu X, Yang X, et al. Improvement of intertrochanteric bone quality in osteoporotic female rats after injection of polylactic acid-polyglycolic acid copolymer/collagen type I microspheres combined with bone mesenchymal stem cells. Int Orthop. 2012;36:2163–71. doi: 10.1007/s00264-012-1543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chang NJ, Lin CC, Li CF, Wang DA, Issariyaku N, Yeh ML. The combined effects of continuous passive motion treatment and acellular PLGA implants on osteochondral regeneration in the rabbit. Biomaterials. 2012;33:3153–63. doi: 10.1016/j.biomaterials.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 318.Cui Z, Wright LD, Guzzo R, Freeman JW, Drissi HD, Nair LS. Poly(D-lactide)/poly(caprolactone) nanofiber-thermogelling chitosan gel composite scaffolds for osteochondral tissue regeneration in a rat model. Journal of Bioactive and Compatible Polymers. 2013 [Google Scholar]
- 319.Ding C, Qiao Z, Jiang W, Li H, Wei J, Zhou G, et al. Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials. 2013;34:6706–16. doi: 10.1016/j.biomaterials.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 320.Duan P, Pan Z, Cao L, He Y, Wang H, Qu Z, et al. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. Journal of biomedical materials research Part A. 2014;102:180–92. doi: 10.1002/jbm.a.34683. [DOI] [PubMed] [Google Scholar]
- 321.Gong L, Zhou X, Wu Y, Zhang Y, Wang C, Zhou H, et al. Proteomic analysis profile of engineered articular cartilage with chondrogenic differentiated adipose tissue-derived stem cells loaded polyglycolic acid mesh for weight-bearing area defect repair. Tissue engineering Part A. 2014;20:575–87. doi: 10.1089/ten.tea.2013.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Caminal M, Peris D, Fonseca C, Barrachina J, Codina D, Rabanal RM, et al. Cartilage resurfacing potential of PLGA scaffolds loaded with autologous cells from cartilage, fat, and bone marrow in an ovine model of osteochondral focal defect. Cytotechnology. 2015 doi: 10.1007/s10616-015-9842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Chen G, Sato T, Tanaka J, Tateishi T. Preparation of a biphasic scaffold for osteochondral tissue engineering. Materials Science and Engineering: C. 2006;26:118–23. [Google Scholar]
- 324.Keeney M, Pandit A. The osteochondral junction and its repair via bi-phasic tissue engineering scaffolds. Tissue Engineering Part B: Reviews. 2009;15:55–73. doi: 10.1089/ten.teb.2008.0388. [DOI] [PubMed] [Google Scholar]
- 325.Yousefi A-M, Hoque ME, Prasad RGSV, Uth N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. Journal of Biomedical Materials Research Part A. 2015;103:2460–81. doi: 10.1002/jbm.a.35356. [DOI] [PubMed] [Google Scholar]
- 326.Swieszkowski W, Tuan BHS, Kurzydlowski KJ, Hutmacher DW. Repair and regeneration of osteochondral defects in the articular joints. Biomolecular Engineering. 2007;24:489–95. doi: 10.1016/j.bioeng.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 327.Schaefer D, Martin I, Shastri P, Padera RF, Langer R, Freed LE, et al. In vitro generation of osteochondral composites. Biomaterials. 2000;21:2599–606. doi: 10.1016/s0142-9612(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 328.Duan P, Pan Z, Cao L, He Y, Wang H, Qu Z, et al. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. Journal of Biomedical Materials Research Part A. 2014;102:180–92. doi: 10.1002/jbm.a.34683. [DOI] [PubMed] [Google Scholar]
- 329.Yan L-P, Oliveira JM, Oliveira AL, Reis RL. Current Concepts and Challenges in Osteochondral Tissue Engineering and Regenerative Medicine. ACS Biomaterials Science & Engineering. 2015;1:183–200. doi: 10.1021/ab500038y. [DOI] [PubMed] [Google Scholar]
- 330.Da H, Jia S-J, Meng G-L, Cheng J-H, Zhou W, Xiong Z, et al. The Impact of Compact Layer in Biphasic Scaffold on Osteochondral Tissue Engineering. PloS one. 2013;8:e54838. doi: 10.1371/journal.pone.0054838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Atesok K, Doral MN, Karlsson J, Egol KA, Jazrawi LM, Coelho PG, et al. Multilayer scaffolds in orthopaedic tissue engineering. Knee Surgery, Sports Traumatology, Arthroscopy. 2014:1–9. doi: 10.1007/s00167-014-3453-z. [DOI] [PubMed] [Google Scholar]
- 332.Nukavarapu SP, Laurencin CT, Amini AR, Dorcemus DL. Gradient Porous Scaffolds. US20140178455. US Patent. 2013
- 333.Park SH, Kim TG, Kim HC, Yang D-Y, Park TG. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomaterialia. 2008;4:1198–207. doi: 10.1016/j.actbio.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 334.Tang G, Zhang H, Zhao Y, Zhang Y, Li X, Yuan X. Preparation of PLGA Scaffolds with Graded Pores by Using a Gelatin-Microsphere Template as Porogen. Journal of Biomaterials Science, Polymer Edition. 2012;23:2241–57. doi: 10.1163/156856211X614185. [DOI] [PubMed] [Google Scholar]
- 335.Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. International Orthopaedics (SICOT) 2009;34:589–97. doi: 10.1007/s00264-009-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Engineering Part A. 2011;17:2845–55. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- 337.Yeo M, Kim G. Cell-printed hierarchical scaffolds consisting of micro-sized polycaprolactone (PCL) and electrospun PCL nanofibers/cell-laden alginate struts for tissue regeneration. Journal of Materials Chemistry B. 2014;2:314–24. doi: 10.1039/c3tb21163k. [DOI] [PubMed] [Google Scholar]
- 338.Jeon JE, Vaquette C, Theodoropoulos C, Klein TJ, Hutmacher DW. Multiphasic construct studied in an ectopic osteochondral defect model. Journal of The Royal Society Interface. 2014;11 doi: 10.1098/rsif.2014.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Jin-Hyung S, Jung-Seob L, Jong Young K, Dong-Woo C. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. Journal of Micromechanics and Microengineering. 2012;22:085014. [Google Scholar]
- 340.Melchels FPW, Bertoldi K, Gabbrielli R, Velders AH, Feijen J, Grijpma DW. Mathematically defined tissue engineering scaffold architectures prepared by stereolithography. Biomaterials. 2010;31:6909–16. doi: 10.1016/j.biomaterials.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 341.Fox AJS, Bedi A, Rodeo SA. The Basic Science of Human Knee Menisci: Structure, Composition, and Function. Sports Health: A Multidisciplinary Approach. 2012;4:340–51. doi: 10.1177/1941738111429419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Verdonk PCM, Forsyth RG, Wang J, Almqvist KF, Verdonk R, Veys EM, et al. Characterisation of human knee meniscus cell phenotype. Osteoarthritis and Cartilage. 2005;13:548–60. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 343.Bach BR, Jr, Dennis M, Balin J, Hayden J. Arthroscopic Meniscal Repair. J Knee Surg. 2005;18:278–84. doi: 10.1055/s-0030-1248192. [DOI] [PubMed] [Google Scholar]
- 344.Fairbank T. Knee joint changes after meniscectomy. Journal of Bone & Joint Surgery, British Volume. 1948;30:664–70. [PubMed] [Google Scholar]
- 345.Rangger C, Kathrein A, Klestil T, Glötzer W. Partial Meniscectomy and Osteoarthritis. Sports Med. 1997;23:61–8. doi: 10.2165/00007256-199723010-00006. [DOI] [PubMed] [Google Scholar]
- 346.van Tienen TG, Hannink G, Buma P. Meniscus Replacement Using Synthetic Materials. Clinics in Sports Medicine. 2009;28:143–56. doi: 10.1016/j.csm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 347.Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Science Translational Medicine. 2014;6:266ra171–266ra171. doi: 10.1126/scitranslmed.3009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Esposito AR, Moda M, Cattani SM, de Santana GM, Barbieri JA, Munhoz MM, et al. PLDLA/PCL-T Scaffold for Meniscus Tissue Engineering. BioResearch open access. 2013;2:138–47. doi: 10.1089/biores.2012.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Baek J, Chen X, Sovani S, Jin S, Grogan SP, D’Lima DD. Meniscus tissue engineering using a novel combination of electrospun scaffolds and human meniscus cells embedded within an extracellular matrix hydrogel. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2015;33:572–83. doi: 10.1002/jor.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Vaquette C, Cooper-White J. A simple method for fabricating 3-D multilayered composite scaffolds. Acta Biomaterialia. 2013;9:4599–608. doi: 10.1016/j.actbio.2012.08.015. [DOI] [PubMed] [Google Scholar]


