Abstract
In perennial energy crop breeding programmes, it can take several years before a mature yield is reached when potential new varieties can be scored. Modern plant breeding technologies have focussed on molecular markers, but for many crop species, this technology is unavailable. Therefore, prematurity predictors of harvestable yield would accelerate the release of new varieties. Metabolic biomarkers are routinely used in medicine, but they have been largely overlooked as predictive tools in plant science. We aimed to identify biomarkers of productivity in the bioenergy crop, Miscanthus, that could be used prognostically to predict future yields. This study identified a metabolic profile reflecting productivity in Miscanthus by correlating the summer carbohydrate composition of multiple genotypes with final yield 6 months later. Consistent and strong, significant correlations were observed between carbohydrate metrics and biomass traits at two separate field sites over 2 years. Machine‐learning feature selection was used to optimize carbohydrate metrics for support vector regression models, which were able to predict interyear biomass traits with a correlation (R) of >0.67 between predicted and actual values. To identify a causal basis for the relationships between the glycome profile and biomass, a 13C‐labelling experiment compared carbohydrate partitioning between high‐ and low‐yielding genotypes. A lower yielding and slower growing genotype partitioned a greater percentage of the 13C pulse into starch compared to a faster growing genotype where a greater percentage was located in the structural biomass. These results supported a link between plant performance and carbon flow through two rival pathways (starch vs. sucrose), with higher yielding plants exhibiting greater partitioning into structural biomass, via sucrose metabolism, rather than starch. Our results demonstrate that the plant metabolome can be used prognostically to anticipate future yields and this is a method that could be used to accelerate selection in perennial energy crop breeding programmes.
Keywords: 13C, bioenergy, biomarkers, carbohydrates, cell wall, Miscanthus, soluble sugars, starch
Introduction
Miscanthus is a candidate lignocellulosic biofuel crop owing to its high productivity and low chemical input requirements (Visser & Pignatelli, 2001; Somerville et al., 2010). As a C4 grass, it is a close genetic relative of two major biofuel crops, Zea mays (maize) and Saccharum Sp. (sugarcane; Hodkinson et al., 2002). However, currently, the only commercially grown genotype of Miscanthus is a wild accession and not a breeder's line. Therefore, several breeding programmes are now targeting Miscanthus for yield and quality improvement. A major hindrance to the improvement of perennial energy crops through breeding is the long duration for new crosses to reach maturity when they can be assessed for superiority (Purdy et al., 2015). In Miscanthus, this is typically in the region of 4 years from when a seed is planted. There is a pressing need to identify new methods to accelerate the selection of elite crosses in Miscanthus and other perennial species.
In plant science, numerous studies have demonstrated associations between metabolites and various stress conditions such as increases in proline during chilling (Wanner & Junttila, 1999) or increases in jasmonic acid in response to herbivory (Wang & Wu, 2013). In medicine, metabolic biomarkers are used prognostically, that is to anticipate a future outcome in an asymptomatic individual, an example being the measure of blood cholesterol as a predictor of future heart attack risk. However, in plant science, the metabolome has rarely been used to predict future outcomes in crop species (Steinfath et al., 2010). In Arabidopsis thaliana, several studies have successfully correlated biomass with particular metabolites, groupings of metabolites and enzyme activities (when expressed against total protein content; Meyer et al., 2007; Sulpice et al., 2009, 2010, 2013; Scott et al., 2014). By combining a negative correlation with starch and a positive correlation with enzyme activities, approximately a third of the variation in biomass of an Arabidopsis inbred family could be accounted for (Sulpice et al., 2010). A notable example of biomarker identification in a crop species is in potato, where the abundance of glucose and fructose was found to positively correlate with discoloration during frying (low chip quality). When either of these hexoses was used as markers to predict chip quality in new crosses, the correlation (R S) between predicted and measured quality was 0.67 (Steinfath et al., 2010). In a recent study into drought tolerance in rainforest trees, the abundance of nonstructural carbohydrates (NSC) was found to positively correlate with drought tolerance in trees showing natural variation and in those that had been manipulated (O'Brien et al., 2014). These studies show that metabolites can be used as biomarkers to predict biomass, quality traits and stress responses in species as diverse as Arabidopsis, potato and rainforest trees. In all these studies, it was carbohydrates that were successfully used as markers.
We recently showed that two fast‐growing and high‐yielding genotypes of the perennial bioenergy grass, Miscanthus, displayed a distinctive NSC profile compared to two slower growing genotypes and that this phenotype was consistent across 2 years and different environments (Purdy et al., 2015). However, the limited number of genotypes and hybrids used in this study were insufficient to unequivocally determine whether the carbohydrate metabolic profile (‘glycome’) could be used as a biomarker of productivity. The phenotypic attribute so far shown to most strongly correlate with final yield is (log‐transformed) maximum canopy height (R 2 = 0.55; Robson et al., 2013). Therefore, our primary aim with this study was to identify single or multiple metabolic biomarkers that could predict yield in Miscanthus and to determine how the strength of the correlations compared with height as a predictor. Miscanthus is usually harvested at the end of winter when senescence is complete, but we sampled carbohydrates in stems in the middle of UK summer when growth was most rapid. The summer carbohydrate metabolic profile was then used to predict winter yields harvested the following year.
The choice of nonstructural carbohydrates to profile was based on previously observed genotypic differences in abundance and partitioning in four genotypes (Purdy et al., 2014, 2015). Sucrose is the most abundant soluble sugar in Miscanthus, and owing to the close phylogenetic relationship between Miscanthus and sugarcane (Hodkinson et al., 2002), it was an obvious candidate for study in diverse genotypes. Sucrose is formed of a molecule each of glucose and fructose, and relationships between the hexoses and biomass traits had previously been observed (Purdy et al., 2015). Unlike many C3 temperate grasses, C4 species such as Miscanthus do not accumulate fructans (Muguerza et al., 2013) but instead accumulate starch as a transient form of storage carbohydrate (de Souza et al., 2013; Purdy et al., 2014).
To grow, plants must accumulate structural mass, predominantly cellulose and the cell wall hemicellulose polysaccharides. Both starch and cellulose are polymers of glucose, and we hypothesized that rapidly growing genotypes of Miscanthus may be accumulating cellulose more rapidly at the expense of starch biosynthesis, thus explaining the negative relationship between starch and growth observed in our previous study and that of others (Rocher, 1988; Sulpice et al., 2009; Purdy et al., 2014, 2015). Therefore, starch, cellulose and the hemicelluloses were also assayed to assess a potential role as yield biomarkers.
Materials and methods
Mixed population
A total of 244 Miscanthus genotypes were collected and planted as described previously (Allison et al., 2011; Jensen et al., 2011; Robson et al., 2012). From this population, a selection of seven short and 11 tall plants were used in the experiment. A description of the different species is provided in Table 1. Three biological replicates per genotype were harvested from blocks 1, 2 and 3 of the trial.
Table 1.
The species and experimental structure of the mixed population and the mapping family
| Species | n Total | n Tall | n Short |
|---|---|---|---|
| Miscanthus mixed population | |||
| M. sinensis | 10 | 5 | 5 |
| Hybrid | 4 | 4 | 0 |
| M. sacchariflorus | 4 | 2 | 2 |
| Miscanthus mapping family | |||
| M. sinensis | 1 | 1 | 0 |
| Hybrid | 19 | 9 | 10 |
Mapping family
A total of 102 genotypes from a paired cross between a diploid M. sinensis and a diploid M. sacchariflorus were sown from seed in trays in a glasshouse in 2009. In 2010, individual plants were split to form three replicates of each genotype and then planted out into the field in a spaced‐plant randomized block design comprising three replicate blocks. The field site is located 300 m to the south from the mixed population (described above), and therefore, stone content and soil types are as described previously (Allison et al., 2011); however, the field containing the mapping family is on a gentler slope than the mixed population.
Biomass trait measurements
Growth rate: Canopy heights of the selected plants were measured weekly. The values presented are for the 2‐week period surrounding the harvests to give a value of growth rate cm day−1.
Stem height: A single stem that was representative of canopy height was selected for destructive harvest and its height (cm) measured on the day of harvest.
Destructive harvests
A single stem that was representative of canopy height was selected from each plant, cut at a height of 10 cm from the base, measured then flash‐frozen before freeze‐drying. As NSC show diurnal fluctuations in Miscanthus (Purdy et al., 2013), the two sets of plants were harvested on different days so that each harvest could be completed within a 2‐h window at the same time of day (Zt 8–10 of a 16‐h photoperiod). The mixed population was harvested on 04 July 2013, and the mapping family was harvested on 19 July 2013. For the harvesting of the entire mapping family in 2014, each of the three blocks were harvested on consecutive days in July to stay within the 2‐h time window specified above.
Annual yield harvest: The mixed population and mapping family were destructively harvested for yield in March 2014 (following the 2013 growing season), and the mapping family was harvested in Feb 2015 following the 2014 growing season. Biomass was dried to a constant weight, and then, the average DW weight per plant (kg) was calculated.
Nonstructural carbohydrate (NSC) compositional analyses
Soluble sugars and starch were analysed as previously described (Purdy et al., 2014, 2015). Soluble sugar extraction: approximately 20 mg (actual weight recorded) of each cryomilled (6870 Freezer Mill, Spex, Sampleprep, Stanmore, UK) plant tissue sample was weighed into 2‐mL screwcap microcentrifuge tubes. Sugars were extracted four times with 1 mL of 80% (v/v) ethanol and the resulting supernatants pooled; two extractions were at 80 °C for 20 min and 10 min, respectively, and the remaining two at room temperature. A 0.5 mL aliquot of soluble sugar extract and the remaining pellet containing the insoluble fraction (including starch) were dried down in a centrifugal evaporator (Jouan RC 1022, Saint‐Nazaire, France) until all the solvent had evaporated. The dried down residue from the soluble fraction was then resuspended in 0.5 mL of distilled water. Samples were stored at −20 °C for analysis.
Soluble sugar analysis: Soluble sugars of samples extracted in the previous step were quantified enzymatically by the stepwise addition of hexokinase, phosphoglucose isomerase and invertase (Jones et al., 1977). Samples were quantified photometrically (Ultraspec 4000; Pharmacia Biotech, Uppsala, Sweden) by measuring the change in wavelength at 340 nm for 20 min after the addition of each enzyme. Sucrose, glucose and fructose were then quantified from standard curves included on each 96‐well plate.
Starch quantification: Starch was quantified using a modified Megazyme protocol (Megazyme Total Starch Assay Procedure, AOAC method 996.11; Megazyme International, Wicklow, Ireland). Briefly, the dried pellet was resuspended in 0.4 mL of 0.2 m KOH, vortexed vigorously and heated to 90 °C in a water bath for 15 min to facilitate gelatinization of the starch. A total of 1.28 mL of 0.15 m NaOAc (pH 3.8) was added to each tube (to neutralize the sample) before the addition of 20 μL α‐amylase and 20 μL amyloglucosidase (Megazyme International). After incubation at 50 °C for 30 min and centrifugation for 5 min, a 0.02 mL aliquot was combined with 0.6 mL of GOPOD reagent (Megazyme). A total of 0.2 mL of this reaction was assayed photometrically (Ultraspec 4000; Pharmacia Biotech) on a 96‐well microplate at 510 nm against a water‐only blank. Starch was quantified from known standard curves on the same plate. Each sample and standard was tested in duplicate. Each plate contained a Miscanthus control sample of known concentration for both soluble sugars and starch analysis.
Cell wall carbohydrates and lignin
Lignin and matrix polysaccharides were analysed as described by Foster et al. (2010a,b). To quantify matrix polysaccharides, a Dionex ICS‐5000DC (Thermo Scientific, Loughborough, UK) was used. Each chromatographic run contained sets of standards and a dilution series. Lignin quantification followed the method described by Foster et al. (2010a). Crystalline cellulose was analysed by Seaman hydrolysis and subsequent quantification of glucose (Purdy et al., 2014).
Crystalline cellulose
Approximately 60 mg (actual weight recorded) of purified cell wall was hydrolysed with 0.6 mL of 72% H2SO4, vortexed and left to incubate whilst shaking at 200 rpm for 1 h at 30 °C. After incubation, samples were diluted with 16.8 mL of deionized H2O. Tubes were then capped and autoclaved at 121 °C for 1 h. Once cooled, an aliquot of 0.65 mL was neutralized with 30 mg CaCO3 and centrifuged to pellet the CaCO3 and the supernatant was removed to a fresh tube. Glucose was quantified enzymatically as previously described (Purdy et al., 2014). Standards of glucose were treated alongside experimental samples and included on each plate with a duplicated check sample.
The amount of glucose that was derived from hemicellulose (as described above) was subtracted from the total to give a value derived just from the Seaman hydrolysis of crystalline cellulose.
Data modelling
Principal component analysis (PCA) was performed in simca‐p v.11 (Umetrics AB, Malmo, Sweden) on values averaged across all biological replicates (usually three) of each genotype in each sampled population. Data were mean‐centred and scaled to unit variance, and the reported PC was ‘significant’ in the default simca‐p cross‐validation procedure. Machine learning employed the AttributeSelectedClassifier in the Explorer interface of weka v.3.6 (Frank et al., 2004). This weka ‘metaclassifier’ firstly sought an optimal subset of biomass predictors from the individual carbohydrate levels and their derivative metrics (i.e. sums and ratios). This involved testing all potential predictor combinations by the ExhaustiveSearch algorithm with evaluation by CfsSubsetEval. The metaclassifier then trained a support vector regression algorithm, SMOreg, to relate the selected carbohydrate predictors to their associated biomass trait data. The resultant model was evaluated for its accuracy in predicting the relevant biomass values from the carbohydrate metrics of a ‘test’ set of plants. Test data were always completely excluded from model training. Default parameters were used for each algorithm.
13CO2 pulse labelling
Our chamber design and 13C pulse‐labelling approach were similar to previous methods (Hogberg et al., 2008; Subke et al., 2009; Biasi et al., 2012) and applied to a field trial planted in 2010 with triplicate plots of a M. sinensis, a M. sinensis × M. sacchariflorus hybrid (M. x giganteus) and a M. sacchariflorus in a randomized block design. In each replicate plot, square 13C pulse chambers were erected (2 m l, 2 m w, 3 m h) above the crop, resulting in a total tent volume of 12 m3. Aluminium scaffold was used to support plastic polythene film, which allowed 90% of photosynthetically active radiation to enter the chamber. During the 13C pulse, the chamber was sealed at the base. To counter ambient air temperature increases within the chambers, each was cooled using a water cooled, split air conditioner (Andrew Sykes, Wolverhampton, UK).
The 13C pulse labelling was carried out on 23 July 2013 at ca. 08:20 by introducing ca. 6 L of 99% 13C‐atom enriched pure CO2 (CK Gases, Ibstock, UK) in sequential batches after sealing the tent.
13C Harvesting and sample preparation
Pulsed samples were harvested 30 h after labelling. A single marked stem was harvested as previously described. The level of 13C enrichment above natural background 13C levels was determined in the soluble, starch and structural mass, which was extracted as previously described.
Solid sample analysis was performed on a Costech EC S4010 Elemental Analyser (Costech Analytical Technologies Inc, Valencia, CA, USA) coupled to a Picarro G‐2131i Series CRDS analyzer (Picarro Inc, Santa Clara, CA, USA) via a split‐flow interface using a method similar to (2013). Cryomilled samples of ~2 mg were weighed into ultraclean, 6 × 4 mm pressed tin cups (Elemental MicroAnalysis Ltd, Okehampton, UK), crimped and loaded into a Zero N‐Blank, 50 position carousel, autosampler. From the autosampler, samples were dropped at a throughput of 1 every 15 min into a combustion reactor, maintained at a constant 980 °C. Samples undergo flash combustion and thermally decompose. Evolved CO2 was passed through a thermal conductivity detector (TCD) for C detection and then vented through a split‐flow interface to the Picarro CRDS analyzer for 13C analysis. Standard materials covering a representative range of C and δ 13C values were run during each analysis batch, and results were calibrated against these.
13C Calculations
Stable isotope notation
Studies of this kind have generally either expressed 13C enrichment values in δ 13C, a measure of the ratio of 13C and 12C, reported in parts per thousand (‰) relative to a standard value (Pee Dee Belemnite – PDB) or atom %. Outputs from the Picarro 13CO2 analyzer were in standard delta (δ) value notation (δ 13C). δ 13C values are calculated using the following equation:
| (1) |
where 13C/12CPDB is the isotopic ratio of the standard material PDB given as 0.0112372. Results were converted to atom % and then mg g−1 using the following equations:
| (2) |
where AR = 0.011237. The absolute ratio of standard material (PDB) and δ 13C = standard delta value of sample.
| (3) |
Statistical analyses
Differences between genotypes for biomass traits, NSC, structural carbohydrates and block effects were determined from one‐way anova using genotype or block as the treatment factor (P =≤ 0.05). Genotypic differences in the deposition of 13C were determined by one‐way anova using genotype as the treatment factor and an associated Tukey's HSD test. anova, Tukey and Wilcoxon tests were performed using genstat (13th Edition). Differences between genotypes grouped as ‘tall’ or ‘short’ were determined from Student's two‐tailed t‐tests (assuming unequal variance; P =≤ 0.05) using Microsoft Excel. To compare biomass measures and NSC between across years (2013 and 2014), a Pearson correlation was performed to determine similarities in absolute values and a Spearman rank correlation analysis to compare the ordering of genotypes. Both analyses were carried out in sigmaplot 12 (Systat Software, Inc, San Jose, CA, USA).
Results
Two sets of field‐grown plants were studied (Table 1). The ‘mixed population’, from which 18 plants were selected for study in 2013 (their eighth growing season), was comprised of M. sinensis, M. sinensis × M. sacchariflorus hybrids and M. sacchariflorus genotypes. The ‘mapping family’, from which 20 plants were selected for study in 2013 (their fourth growing season), was comprised of M. sinensis × M. sacchariflorus hybrids, plus a single, tall, M. sinensis genotype. Nonstructural and structural carbohydrates and lignin were sampled in July, during the summer growing season. Measures of biomass traits obtained during the summer sampling were stem height, and growth rate over the surrounding two‐week period, whereas annual yield was obtained at harvest after the following winter. Carbohydrate and biomass data for all genotypes are in Tables S1–S3.
Height has been shown to be the trait that best correlates with final yield in Miscanthus (Robson et al., 2013). Therefore, each set of plants was divided into ‘tall’ and ‘short’ classes for comparison of carbohydrate contents. The average heights of plants grouped as short or tall from the mixed population were 79 cm and 151 cm, respectively, and in the mapping family, the average heights of the short and tall classes were 56 cm and 120 cm, respectively (Table S1).
In both sets of plants, the abundance of fructose was significantly greater in the tall plants compared to the short plants, whereas the opposite was true for starch, which was significantly more abundant in the short plants (Fig. 1a and b; Table S2). Glucose, total hexose and total soluble carbohydrates were significantly different between tall and short plants only in the mixed population.
Figure 1.
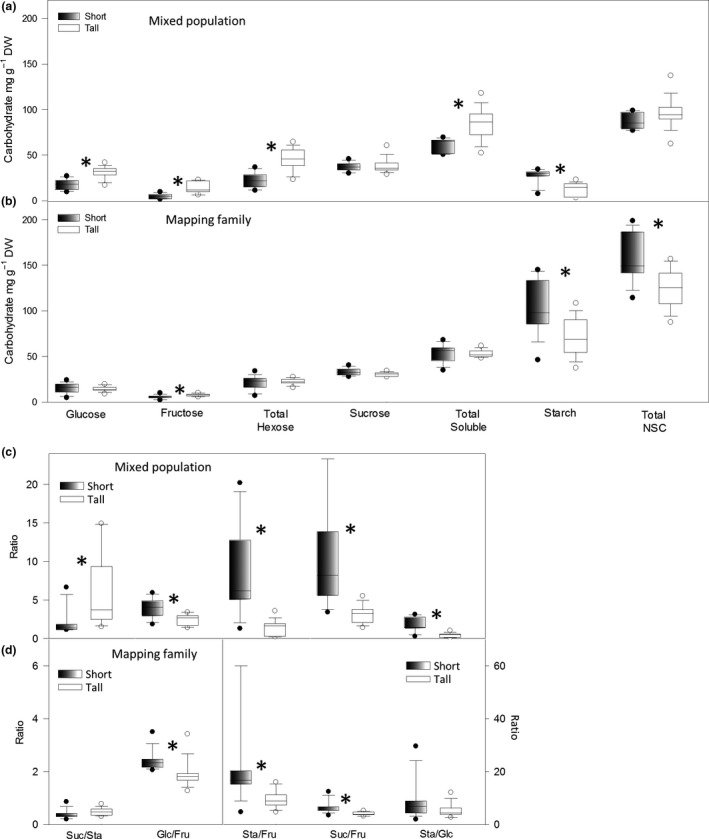
Quantification of NSC in short and tall genotypes of the mixed population (a) and mapping family (b), and ratios between carbohydrate levels in short and tall genotypes of the mixed population (c) and mapping family (d). Circles show outliers. Significant differences between tall and short plants are shown by an asterisk (Student's t‐test assuming unequal variances, P =≤ 0.05). Key: NSC = total nonstructural carbohydrate, Suc/Sta = sucrose‐to‐starch ratio, Glc/Fru = glucose‐to‐fructose ratio, Sta/Fru = starch‐to‐fructose ratio, Suc/Fru = sucrose‐to‐fructose ratio, Sta/Glc = starch‐to‐glucose ratio.
The ratios between different NSC were examined in the short and tall groups, to further investigate the contrasting relationships of fructose and starch with plant height. The greatest difference between tall and short plants was the starch/fructose ratio, which was >fourfold greater in the short plants of the mixed population and >twofold greater in the short plants of the mapping family (Fig. 1c and d). The glucose/fructose and sucrose/fructose ratios were also significantly negatively associated with height in both populations. Differences in the starch/glucose and sucrose/starch ratios of tall and short plants were significant only in the mixed population (Fig. 1c and d).
Significant differences in hemicellulosic glucan were observed between the short and tall plants in both populations, with short plants exhibiting higher glucan levels (Fig. 2a and b). Other significant differences, seen only in the mapping family, were arabinose, galactose and mannose, which were more abundant in short plants, whereas crystalline cellulose and lignin were more abundant in tall plants (Fig. 2b; Table S3b). In the mixed population, these last cell wall components did not show significant height‐associated differences (Fig. 2a; Table S3a), possibly due to a significant effect of the replicate field blocks at the relevant trial site (Table S4). These replicate blocks were arranged parallel to the slope of the hill on which the mixed population was grown (see Materials and Methods ). A block effect was previously reported in this population at the end of the growing season and attributed to differences in wind exposure and water dynamics (Allison et al., 2011). No significant block effects were detected for any of the NSC from either set of plants, or in the cell wall composition of the mapping family (Table S4).
Figure 2.
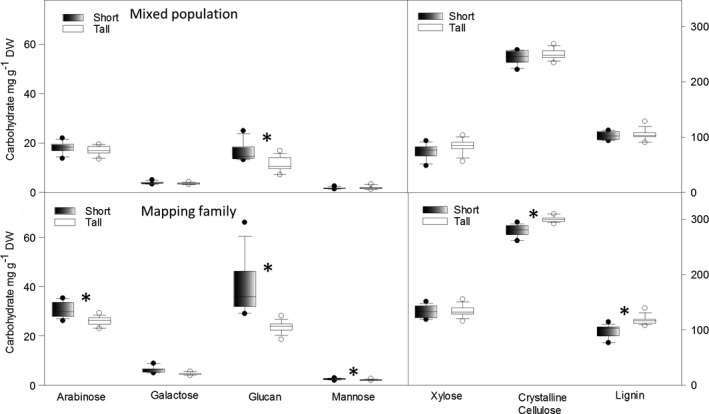
Quantification of structural carbohydrates and lignin in short and tall genotypes of the mixed population (a) and mapping family (b). Circles show outliers. Significant differences between tall and short plants are shown by an asterisk (Student's t‐test assuming unequal variances, P =≤ 0.05).
Having found differences in carbohydrates of height classes of plants selected in 2013, we examined the potential for using carbohydrates to model biomass traits (summer height, summer growth rate and annual yield). To provide more data for this purpose, a much larger selection (102 genotypes) was made in 2014 from the mapping family and similarly analysed for NSC and biomass traits. As an exploratory stage for modelling, we first sought to correlate carbohydrate contents with biomass traits in each set of plants, using Spearman's rank coefficients (R S), which are robust to potential nonlinearity. Of the NSC in the mixed population, both glucose and fructose produced positive correlations with biomass traits, with the strongest correlation being between fructose and stem height (0.91; Fig. 3a). In the mapping family, biomass traits were significantly positively correlated with fructose (correlation coefficients of ~0.5), though not with glucose (Fig. 3b and c). No strong correlation between sucrose and biomass traits was detected in either set (Fig. 3a–c). In contrast, starch negatively correlated with all biomass traits (and with fructose) in both sets of plants (Table S3a–c), the strongest relationship being with growth rate in the mixed population (−0.76). The relationships observed in the mapping family in 2013 and in the extended number of genotypes in 2014 were largely consistent, both showing strong positive correlations of fructose with stem height and yield (~0.5) and strong negative relationships between starch and yield (Fig. 3b and c). Correlations with growth rate, however, were considerably lower in 2014 than 2013 (Fig. 3b and c).
Figure 3.
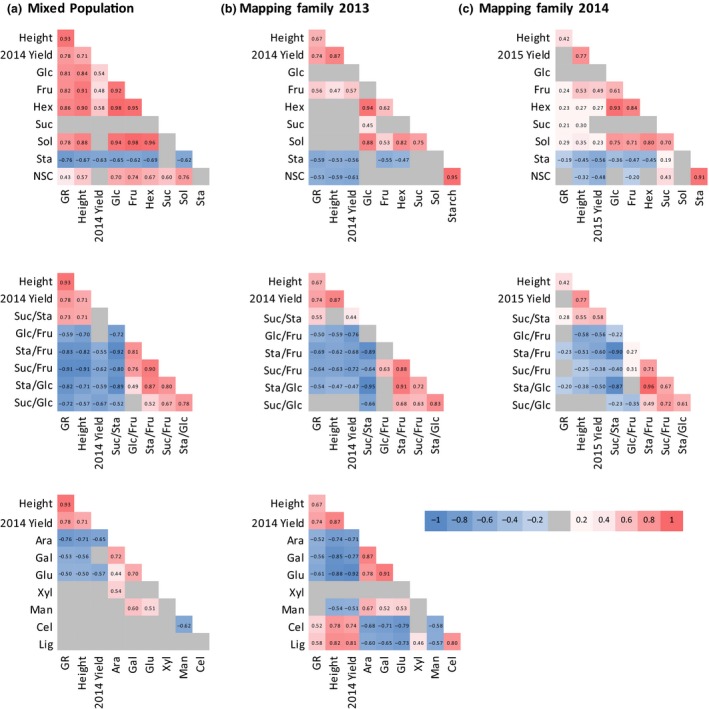
Spearman's rank correlations between biomass parameters, NSC levels (top) and ratios (middle), and cell wall components (bottom) in the Miscanthus mixed population in 2013 (a) and the mapping family in 2013 (b) and 2014 (c). Significant positive correlations are coloured red, and significant negative correlations are coloured blue (P =≤ 0.05). Nonsignificant correlations are coloured grey. Key: Glc = glucose, Fru = fructose, Hex = total hexose, NSC = total nonstructural carbohydrate, Suc = sucrose, Sta = starch, Glc/Fru = glucose‐to‐fructose ratio, Suc/Glc = sucrose‐to‐glucose ratio, Suc/Fru = sucrose‐to‐fructose ratio, Suc/Sta = sucrose‐to‐starch ratio, Sta/Glc = starch‐to‐glucose ratio, Ara = arabinose, Cel = cellulose, Glu = glucan, Gal = galactose, Lig = lignin.
Ratios between NSC produced stronger correlations with biomass traits than did the individual components (Fig. 3a–c, middle row). In both the mixed population and mapping family, significant negative correlations between biomass traits and the starch/fructose and sucrose/fructose ratios were observed. Conversely, positive correlations between the sucrose/starch ratio and biomass traits were observed in both sets of plants (Fig. 3a–c).
In both sets of plants, the matrix polysaccharide components arabinose, galactose and glucan were negatively correlated with biomass traits (Fig. 3a and b, bottom row). The strongest negative correlation in the mixed population was between arabinose and growth rate (−0.76), whilst in the mapping family, matrix glucan correlated negatively with yield (−0.92). In the mapping family, crystalline cellulose and lignin showed positive correlations with biomass traits of 0.5–0.8, whereas these relationships were not observed in the mixed population (Fig. 3a and b).
As similar correlations manifested in the various genotypes, in two different field trial sites and two different years (Fig. 3), it was pertinent to ask whether a ‘carbohydrate phenotype’ related to biomass traits was sufficiently robust to be identifiable across all sampled populations. When the NSC levels (glucose, fructose, sucrose, starch) measured in all populations were subjected to PCA in a unified data set, the first component, PC[1], accounted for 58.3% of overall variance (Fig. 4). The loadings for each NSC on PC[1] identified opposite variations in starch on the one hand, and the hexoses fructose and glucose on the other (Fig. 4a). Thus, PC[1] could be regarded as a composite index of NSC status, such that negative scores on PC[1] were indicative of high starch and low hexoses, and the converse for positive PC[1] scores. Moreover, PC[1] scores of the sampled genotypes showed significant correlations to biomass traits. As seen in Fig. 4b, genotypes with negative PC[1] scores (high starch, low hexoses) tended to be characterized by shorter stature than genotypes with positive scores (low starch, high hexoses). Pearson correlations with PC[1] scores were highly significant (P < 10−12) for height (R, 0.65), yield (R, 0.56) and growth rate (R, 0.62).
Figure 4.
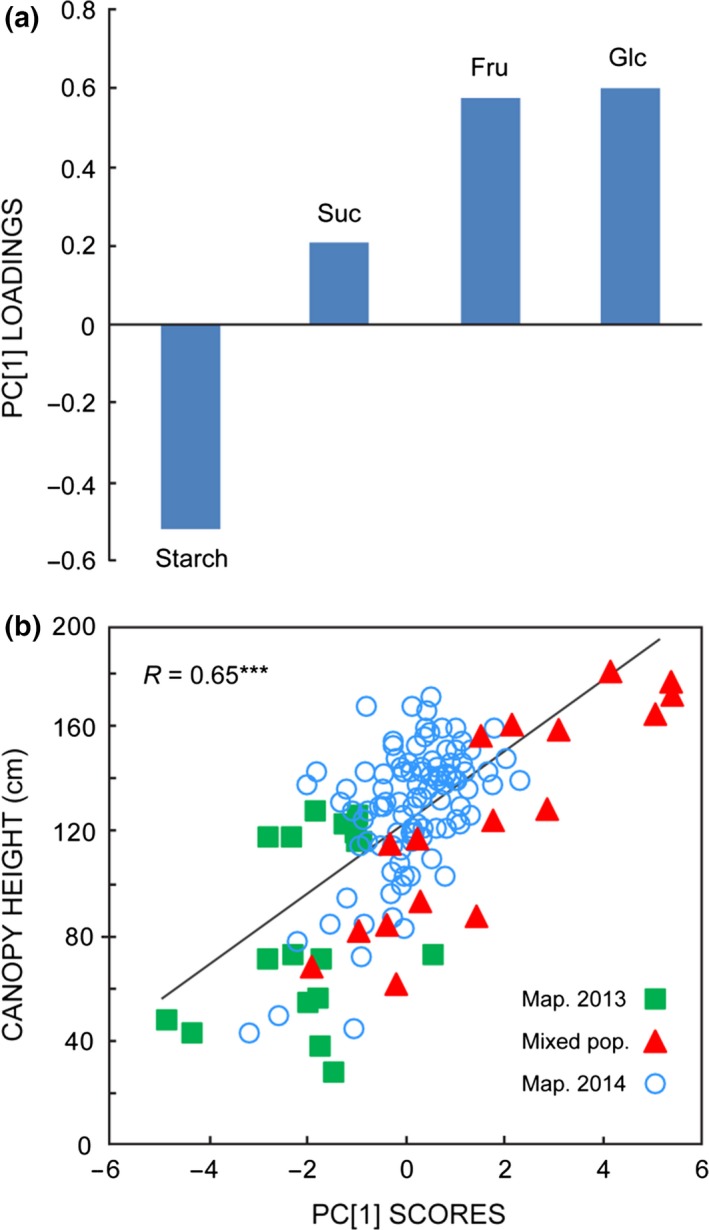
Correlation of multivariate carbohydrate phenotypes and biomass across all experimental populations. Horizontal axis shows scores on PC[1], the major component (58% of variance) from PCA of NSC levels (Glc, Fru, Suc, starch) of each genotype in the mixed population (triangles), the mapping population selected in 2013 (squares) and the extended mapping family analysed in 2014 (open circles). Vertical axis shows mean canopy height of each sampled genotype. R indicates Pearson correlation (***, P < 0.001) between heights and PC[1] scores.
Considering the carbohydrate/biomass correlations in different Miscanthus populations (Fig. 3), and evidence for a biomass‐correlated multivariate ‘carbohydrate phenotype’ across populations (Fig. 4), we sought to test the predictive power of the glycome for biomass traits by multivariate modelling. A machine‐learning approach was chosen to address the complexity of the potentially relevant carbohydrate and genetic data, in the context of the small number and variability of biological replicates typical of a screening trial in the field.
As evident from Fig. 3, a number of potential carbohydrate metrics, including sums and ratios of individual NSC, would be available for inclusion in such a model. However, use of too many of these metrics would be redundant. We therefore used machine learning to identify parsimonious subsets of effective predictors from the following list of 20: glucose, fructose, total hexose, sucrose, total soluble carbohydrates, starch, total NSC, arabinose, galactose, glucan, xylose, mannose, crystalline cellulose, lignin and the ratios sucrose/starch, glucose/fructose, starch/fructose, sucrose/fructose, starch/glucose and sucrose/glucose. For the full list of 20 carbohydrate predictors, 1 048 576 possible combinations needed evaluation. We also fitted models using only the NSC predictors (8192 combinations), and excluding starch, only soluble sugar predictors (256 combinations). Evaluations were performed using a ‘correlation‐based feature selection’ algorithm, CfsSubsetEval, which prefers sets of predictors that have low correlation amongst themselves, but each has high predictive worth (Wang et al., 2005).
A support vector regression algorithm, SMOreg, was ‘trained’ to fit regression models of a given biomass trait using the NSC predictors selected in the above process. Support vector machines were originally developed for classification and may be conceived as mapping to higher dimensional space of a ‘hyperplane’ between two data classes, the ‘support vectors’ being the least‐separated members of the opposite classes. To perform regression, the task is converted to classification by duplicating each y‐axis value by addition and subtraction of a new parameter ε. The hyperplane between the plus‐ε and minus‐ε ‘classes’ is equivalent to a regression function (Li et al., 2009). Regression modelling was investigated for each Miscanthus population by cross‐validation, in which model performance was averaged over nine successive random partitions of the data into training and validation subsets. For each partition, both predictor selection and SMOreg modelling used the training data, and models were tested on the held‐out validation data (Table 2).
Table 2.
Machine‐learning models of biomass traits using carbohydrate data
| A. Canopy height | Information included in models | |||
|---|---|---|---|---|
| Genotype, carbohydrates | Carbohydrates only | |||
| All genotype replicates | All genotype replicates | Averaged by genotype | ||
| Plants | Carbohydrate fractions | R values | ||
| Mixed population | All | 0.92*** | 0.44*** | 0.76*** |
| Nonstructural | 0.81*** | 0.70*** | 0.81*** | |
| Soluble | 0.84*** | 0.52*** | 0.70** | |
| Mapping family 2013 | All carbohydrates | 0.94*** | 0.72*** | 0.77*** |
| Nonstructural | 0.92*** | 0.63*** | 0.68*** | |
| Soluble | 0.93*** | 0.68*** | 0.70*** | |
| Mapping family 2014 | Nonstructural | 0.88*** | 0.61*** | 0.76*** |
| Soluble | 0.86*** | 0.44*** | 0.66*** | |
| Carbohydrate fractions | Constituents common to predictors of all modelsa | |||
| All | Glucan; Fructose (as Fru, Glc/Fru, Hex or Suc/Fru); Glucose (as Glc/Fru, Hex or Sta/Glc); Starch (as Sta, Sta/Glc or Suc/Sta) | |||
| Nonstructural | Fructose (as Fru, Glc/Fru, Hex, NSC or Suc/Fru); Glucose (as Glc/Fru, Hex, NSC, Sta/Glc or Suc/Glc); Starch (as NSC, Sta, Sta/Fru, Sta/Glc or Suc/Sta) | |||
| Soluble | Fructose (as Fru, Glc/Fru, Hex or Suc/Fru) | |||
| B. Harvest yield | Information included in models | |||
|---|---|---|---|---|
| Genotype, carbohydrates | Carbohydrates only | |||
| All genotype replicates | All genotype replicates | Averaged by genotype | ||
| Plants | Carbohydrate fractions | R values | ||
| Mixed populationb | All | 0.79*** | 0.61*** | 0.81*** |
| Nonstructural | 0.79*** | 0.62*** | 0.75*** | |
| Soluble | 0.79*** | 0.62*** | 0.75*** | |
| Mapping family 2013 | All carbohydrates | 0.85*** | 0.77*** | 0.70*** |
| Nonstructural | 0.86*** | 0.65*** | 0.72*** | |
| Soluble | 0.86*** | 0.68*** | 0.72*** | |
| Mapping family 2014 | Nonstructural | 0.75*** | 0.56*** | 0.68*** |
| Soluble | 0.74*** | 0.40*** | 0.58*** | |
| Carbohydrate fractions | Constituents common to predictors of all modelsa | |||
| All | Fructose (as Fru, Glc/Fru or NSC) | |||
| Nonstructural | Fructose (as Fru, Glc/Fru, NSC or Suc/Fru); Glucose (as Glc/Fru, NSC or Suc/Glc); Sucrose (as NSC, Suc/Fru, Suc/Glc or Suc/Sta); Starch (as NSC, Sta or Suc/Sta) | |||
| Soluble | Fructose (as Fru, Glc/Fru or Suc/Fru) | |||
Support vector regression (SMOreg) models were trained using subsets of predictors selected (CfsSubsetEval) for individual correlation with trait but low correlation with each other. Models were evaluated in ninefold cross‐validations. R values indicate Pearson correlation between actual and predicted biomass data.
Full lists in Supplementary Information.
M. sacchariflorus genotype Mb306 was excluded from Table B.
As each experimental population comprised up to 102 genotypes, usually in biological replicates of only three, and distributed in field plots potentially subject to environmental gradients (Table S4), we were interested if genetic structure was detectable in the carbohydrate data. The effects on the SMOreg models of including or excluding genotype information for each replicate plant were therefore examined. Models of height (Table 2a) or harvest yield (Table 2b) were significantly improved (P < 0.05, Wilcoxon tests) by appending genotype information to the carbohydrate predictors. Mean R of predicted vs. actual biomass values improved from 0.59 to 0.89 for height, and from 0.61 to 0.80 for yield, when models had prior knowledge of genotypes. This evidence for genetically conditioned ‘carbohydrate phenotypes’ was supported by the significantly better performance (P < 0.05, Wilcoxon tests) of models constructed on averaged replicates of each genotype (mean R values: 0.73 for height; 0.71 for yield), relative to those based on all replicates (Table 2). This was presumably due to improved signal to noise for each genotype.
Models based on all carbohydrates including cell wall constituents, or on NSC, or on soluble carbohydrates, were all statistically significant (Table 2) and suggested that the more extensive analytical procedures were unlikely to prove essential in screening of Miscanthus populations for biomass potential. The numbers of predictors selected in the machine‐learning models ranged from 4 to 8 for the ‘all carbohydrates models’, 3 to 5 for the ‘NSC models’ and 2 to 4 for the ‘soluble carbohydrates models’, and detailed lists are available in Table S5. For each category of model, Table 2 highlights the carbohydrates that featured in every predictor list, whether as an individual metabolite or in a sum or ratio metric. The single metabolite that featured in every model in all categories was fructose. The other NSCs were prominent in some model categories, but ignored in others, with sucrose featuring the least frequently. Amongst cell wall constituents, glucan was ubiquitous in the all‐carbohydrate models of height (Table 2a), but not yield (Table 2b).
In modelling yield for the mixed population, one M. sacchariflorus genotype, Sac‐2, proved particularly detrimental (without genotype information) and was omitted for Table 2b. Amongst over four hundred plants sampled for this study, the two tallest individuals belonged to Sac‐2, but its yields per unit height were by far the lowest in the mixed population. Inclusion of this outlier genotype in the yield models saw R values for the mixed population (genotypes averaged) fall to 0.62, 0.36 and 0.52 for the all‐carbohydrate, NSC and soluble carbohydrate models, respectively. It was concluded that the traits responsible for the particular morphology of Sac‐2 were not accessible to modelling from carbohydrates.
Biomass and NSC data for the same genotypes in 2013 and 2014 showed high absolute (R) and rank (R S) interyear correlation (Table 3). We therefore investigated whether predictive models relating carbohydrates to biomass traits could be applicable from 1 year to another. Figure 5 shows the application of the machine‐learning regression method to prediction of (a) height, and (b) yield of the mapping family genotypes analysed in 2014, based purely on the measurements of their NSC levels (Table S6). The regression models were pretrained on the NSC and biomass data of the smaller number of mapping genotypes analysed in 2013. Correlations between predicted and actual biomass trait values were highly significant (P < 10−14) for height and yield, with Pearson correlation values of 0.67–0.68 (Fig. 5). Predictions for growth rate data were weaker (R, 0.25; P < 0.05).
Table 3.
Correlations between 2013 and 2014 for biomass traits and nonstructural carbohydrate composition. For biomass traits, N = 102, and for carbohydrates and ratios, N = 20, *P =≤ 0.05 **P= ≤0.01
| Trait | Pearson's (R) | Spearman's Rank (R) | P |
|---|---|---|---|
| Canopy Height | 0.8 | 0.8 | ** |
| Yield | 0.9 | 0.9 | ** |
| Glucose | 0.6 | 0.6 | ** |
| Fructose | 0.8 | 0.7 | ** |
| Hexose | 0.7 | 0.4 | * |
| Sucrose | 0.7 | 0.6 | ** |
| Starch | 0.8 | 0.8 | ** |
| Total NSC | 0.8 | 0.8 | ** |
| Suc/Sta | 0.7 | 0.7 | ** |
| Glc/Fru | 0.7 | 0.8 | ** |
| Sta/Fru | 0.9 | 0.7 | ** |
Figure 5.
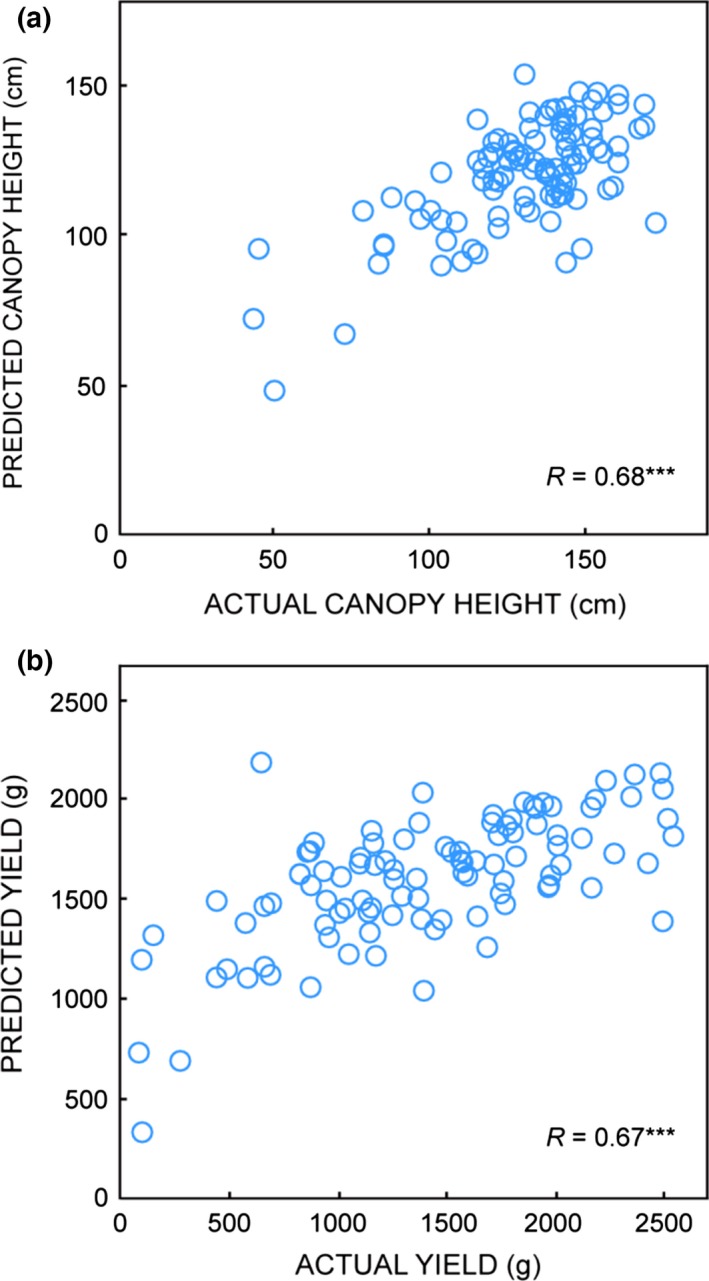
Interyear prediction of biomass traits from carbohydrates. From NSC levels in the 20 genotypes selected in 2013 from the mapping population, machine learning was used to select minimal lists of predictors to model biomass traits of the sampled plants. The machine‐learning models thereby ‘trained’ on the 2013 data were subsequently provided with the NSC data (only) of the 102 mapping family genotypes analysed in 2014 and ‘tested’ for prediction of (a) canopy height, and (b) yield, of the 2014 plants. For both models, the predictor metrics selected by machine learning were as follows: total NSC, and the Glc/Fru and Suc/Fru ratios. R indicates Pearson correlation (***, P < 0.001) between actual and predicted values.
To investigate our hypothesis of a competitive relationship between starch and cellulose biosynthesis, we conducted a 13C‐labelling experiment in the field. We used a M. sinensis genotype (‘Goliath’), which was comparatively slow‐growing, a fast‐growing hybrid genotype phylogenetically similar to Hyb 2 of the mixed population (M. x giganteus), and a M. sacchariflorus genotype phylogenetically similar to Sac‐2. Stems were harvested 30 h after labelling. The slower growing genotype, Goliath, partitioned significantly more pulse‐derived 13C into starch than the fast‐growing hybrid and M. sacchariflorus (Table 4). When analysed by anova, there appeared to be no difference between the genotypes in % deposition into the cell wall even though the mean values were quite different. We considered that the analysis was being skewed by a large amount of variation between replicate in the M. sacchariflorus genotype (Table 4). Therefore, we also performed t‐tests between the three genotypes, which showed that M. x giganteus had deposited significantly more pulse‐derived 13C into the insoluble fraction which would be mainly comprised of cellulose. This approach was also applied on other measurements (such as % soluble), and no additional significant differences between genotypes were observed. The M. sacchariflorus genotype, whilst taller than M. x giganteus, had a slower growth rate at the time of labelling and was not statistically distinct from either of the other genotypes in its pulse‐derived 13C deposition. All three genotypes had accumulated the same total amount of pulse‐derived 13C. Therefore, the observed differences between the hybrid and Goliath were in carbon partitioning rather than capture (Table 4).
Table 4.
Biomass traits and % 13C recovered from the stem 30 h after labelling in three genotypes. N = 3 ± SE. Different letters show significant differences between genotypes according to an anova and associated t‐test. Values in parentheses in the % insoluble column show the additional results of t‐tests between the three genotypes (P =≤ 0.05)
| Stem weight (g DW) | Stem height (cm) | Growth rate (cm day−1) | Pulse derived 13C (mg g−1 DW) | 13C recovered in each fraction as a % of the total | |||
|---|---|---|---|---|---|---|---|
| Soluble | Starch | Insoluble | |||||
| Hybrid | 30.0 ± 3.5 a | 241.0 ± 11.6 a | 2.8 ± 0.4 a | 2.0 ± 0.2 a | 19.0 ± 2.7 a | 18.2 ± 2.8 a | 62.8 ± 0.8 a (a) |
| Goliath | 20.0 ± 1.1 ab | 180.3 ± 2.0 b | 1.0 ± 0.5 b | 2.2 ± 0.4 a | 17.4 ± 2.5 a | 38.2 ± 4.8 b | 44.4 ± 3.2 a (b) |
| M. sacchariflorus | 54.1 ± 10.7 b | 274.3 ± 3.5 c | 1.3 ± 0.3 ab | 1.5 ± 0.2 a | 39.0 ± 12.3 a | 26.3 ± 1.5 a | 34.8 ± 13.8 a (ab) |
In the mapping family in 2013, there was a positive correlation between concentrations of cellulose and fructose, and negative relationships between cellulose and the ratios of glucose/fructose and sucrose/fructose (Fig. 6). However, no negative relationship between starch and cellulose was observed, which did not support our starting hypothesis that a competitive relationship exists between these two polymers of glucose (Fig. 6).
Figure 6.
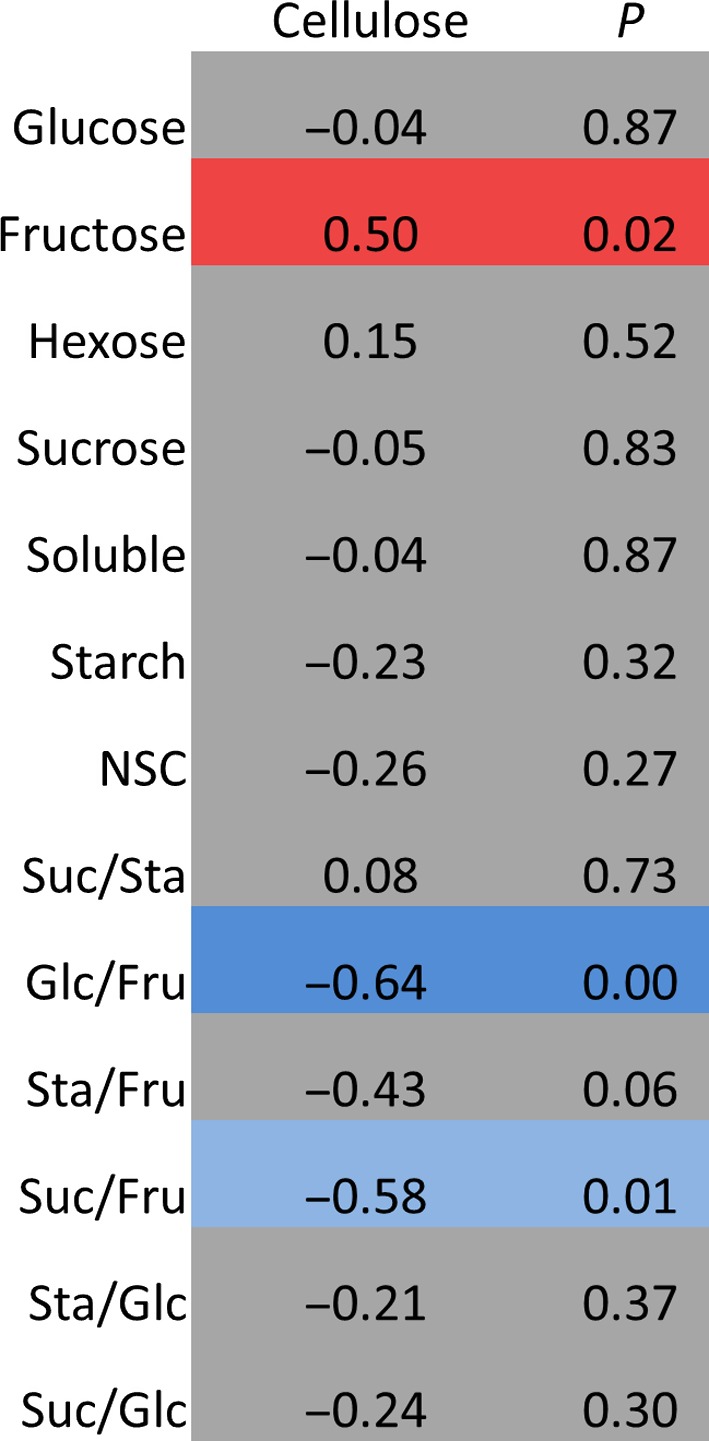
Spearman's rank correlation between cellulose and the NSC levels and ratios in the mapping family in 2013. Significant positive correlations are coloured red, and significant negative correlations are coloured blue (P =≤ 0.05). Nonsignificant correlations are coloured grey.
Discussion
We have demonstrated that the abundance and partitioning of carbohydrates, particularly the NSC, can act as metabolic biomarkers of productivity in diverse genotypes of field‐grown Miscanthus. These findings thereby support other studies that have modelled biomass from metabolites using the model species A. thaliana in controlled environments (Meyer et al., 2007; Sulpice et al., 2009, 2010, 2013; Scott et al., 2010). The present article, by contrast, takes the important step of applying this approach to the field environment using a bioenergy crop species with a limited breeding history. Despite the likely complex impacts of sunlight, temperature and rainfall on the carbohydrate status of the field plants, informative and significant biomass models could be generated.
In our field‐grown Miscanthus, we observed that fructose consistently, positively correlated with yield traits. Fructose is produced exclusively from the metabolism of sucrose by the action of sucrose synthases (SuSy) and invertases, whereas glucose is produced both by the action of invertases (but not SuSy) and the metabolism of starch (Koch, 2004; Smith et al., 2005; Ruan, 2014). Therefore, fructose is a direct indication of sucrose metabolism, whereas glucose provides information about both sucrose and starch metabolism. Whilst our observations are strictly correlative (there is no evidence from our results that a high abundance of fructose specifically causes plants to be taller or higher yielding), there is evidence in the literature that altered partitioning in the ratio of sucrose/starch can follow increases in biomass in other species (Foyer & Ferrario, 1994; Laporte et al., 1997; Signora et al., 1998). For example, sucrose phosphate synthase (SPS) catalyses the penultimate step in the synthesis of sucrose in the cytosol (Signora et al., 1998). In tomato plants engineered to constitutively overexpress maize SPS, biomass was increased up to 100% compared to wild‐type controls (Foyer & Ferrario, 1994; Laporte et al., 1997). Furthermore, when the experiment was replicated in Arabidopsis, the ratio of sucrose to starch increased due to a reduction in starch in the transgenic plants (changes in biomass were not observed in this study; Signora et al., 1998). More recently, it has been found that transgenic expression of Arabidopsis SPS and sucrose phosphate phosphatase also enhanced growth and biomass accumulation in hybrid poplar (Maloney et al., 2015). Conversely, an Arabidopsis double knockout mutant of the major SPS leaf isoforms was strongly impaired in growth and accumulated high levels of starch (Volkert et al., 2014). In the Poaceae, SPS has been of interest as a biochemical marker for complex agronomic traits in several species (Castleden et al., 2004). In rice, an SPS gene coincides with a quantitative trait locus for plant height (Ishimaru et al., 2004; Venu et al., 2014), whilst plants with a maize SPS transgene grew taller (Ishimaru et al., 2004).
Sucrose‐metabolizing enzymes are candidates for explaining the correlation between hexose and biomass. The overexpression of SuSy and UDP‐glucose pyrophosphorylase (either as individual or as double mutants) in transgenic tobacco resulted in an increase in the abundance of hexoses, particularly fructose, a decrease in the glucose/fructose ratio and a concurrent increase in plant biomass (Coleman et al., 2006). Poovaiah et al. (Poovaiah et al., 2015) overexpressed a SuSy transgene in the biofuel feedstock switchgrass (Panicum virgatum), achieving increased height and biomass in some transformants. In Populus alba x grandidentata (hybrid poplar), overexpression of SuSy caused increases in soluble carbohydrates and cellulose, and a decrease in cell wall‐derived arabinose (Coleman et al., 2009). A plasma membrane‐bound isoform of SuSy is thought to transfer UDP‐glucose units directly to the extending glucan chains of cellulose (McFarlane et al., 2014), providing a link between the metabolism of the nonstructural pool and the formation of structural biomass. Some of these results therefore complement our findings using natural variation, in that the highest yielding plants had high hexoses, a low glucose/fructose ratio, higher cellulose and lower arabinose. Therefore, the abundance or activity of enzymes involved in sucrose biosynthesis or metabolism are candidates for the causal basis of our observations on hexoses and biomass.
No negative correlation between cellulose and starch was observed, which did not support our starting hypothesis that a competitive relationship exists. However, significant, negative correlations were observed between cellulose and the ratios of glucose/fructose and sucrose/fructose and a positive relationship between fructose and cellulose. This suggests that when the proportion of fructose is higher (relative to glucose or sucrose), cellulose is also in greater abundance. Cellulose biosynthesis is dependent on sucrose metabolism (Amor et al., 1995; Coleman et al., 2009; Baroja‐Fernandez et al., 2012), and the positive correlation between an increased proportion of fructose and cellulose could be a demonstration of this; as UDP‐glucose units, cleaved from sucrose through the action of SuSy, are transported across the plasma membrane to the extending cellulose chain, an increasing pool of fructose is left behind. Our findings from the 13C‐labelling experiment support this, as the differences in partitioning were observed between the fast‐ and slow‐growing genotype. Therefore, a more rapid rate of growth depends upon a greater accumulation of cellulose, via sucrose metabolism, rather than transient storage as starch.
The strength of the significant correlations found in our study is within the same range as those reported in pairwise analyses of molecular markers and traits. Correlations between short nucleotide polymorphisms (SNPs) and starch quality showed significant correlations (R 2) of 0.17–0.67 in rice. In a human asthma, study correlations between SNPs and lung physiology ranged from (R 2) ~0.3 to 0.9 (Kim & Xing, 2009; Kharabian‐Masouleh et al., 2012). This demonstrates that the strength of the correlation between the starch/fructose ratio (for example) of R = 0.6–0.8 is within a range comparable to molecular markers. Height is the trait that best correlates with yield (R 2 = ~0.55; Robson et al., 2013). Using modelling to combine the strongest biomarkers, the predicted and actual yields of the mapping family in 2014 produced correlations of R = 0.67 (R 2 = 0.44), which is a weaker predictor than height (Robson et al., 2013). However, whilst metabolic profiling was not a stronger predictor of final yield than height alone in Miscanthus, detailed knowledge of the relations of metabolism and biomass accumulation can be expected to yield powerful novel tools to accelerate and enhance energy plant breeding programmes (Robson et al., 2013). For example, in Miscanthus, the juvenile phase severely hinders early phenotypic selection (Robson et al., 2013), but if the metabolic profile could predict mature height in juvenile plants, metabolic biomarkers could then be used in a similar way to molecular markers. To address this hypothesis, the next stage of our experimentation is to screen first‐year seedlings and 1‐year‐old and 2‐year‐old plants to discover at what stage in development the glycome can be used as a biomarker for yield in mature plants. As Miscanthus takes 4 years from sowing seed to reach maturity, if the markers could only be used in the 2nd year of growth, this could reduce screening time by 50%. An alternative scenario in which yield prediction through biomarkers could be highly beneficial is in species such as trees where physical phenotyping is particularly challenging, or in screening for abiotic or biotic stress tolerances. It is also possible that metabolic and molecular markers could be used synergistically in breeding programmes to improve selection.
A concern about the use of metabolic biomarkers is their reliability, given that metabolites are dynamic and their absolute abundances will vary. However, a number of studies have demonstrated that the abundance of NSC and the ratios between different pools is under genetic control (Calenge et al., 2006; Purdy et al., 2015). Furthermore, it is generally accepted (and experimentally demonstrated, e.g. Table 3) that a high‐yielding genotype will consistently produce high yields compared to a low‐yielding type, even though climatic conditions over the course of a whole growing season may vary tremendously from year to year. As yield is implicitly dependent upon the NSC pool to form the structural biomass, it is logical that the NSC composition must also be genetically controlled and similar enough between years and within genotypes to produce consistent results in yield and quality traits. Many of the metabolites measured, such as glucose and most cell wall components, were found to be unreliable for predicting yield when used alone, as they only produced significant relationships with biomass in one or other of the field sites. In contrast, fructose, starch and several of the ratios were found to be consistent indicators of biomass traits in both sets of plants and in both years of study. Therefore, as with molecular markers, it is important to choose robust markers to produce reliable results.
The model generated for the mapping family was based on data from plants in their 3rd and 4th complete growing season in the field. Whilst this is considered a mature crop, it has been shown that yields continue to increase at least until the 5th year of growth (Robson et al., 2013). Therefore, the current model may underestimate yields in subsequent years and have to be reparameterized once the annual increase in yields has plateaued.
In conclusion, our study has shown that fructose and starch positively and negatively correlate with yield traits, respectively. The glycome in the summer growing season can be used as a biomarker to predict future harvest yields in the following year. Plants that partitioned a greater proportion of captured carbon into cellulose rather than starch attained greater biomass. Metabolic biomarker identification may also be an approach that could be adapted for other agronomic traits such as stress tolerance or disease resistance.
Supporting information
Table S1. Biomass traits in the mixed population (a) and mapping family (b). The population consisted of M. sinensis (Sin), Hybrids (Hyb) and M. sacchariflorus (Sac) and the mapping family were all hybrids (M. sacchariflorus × M. sinensis) except a single M. sinensis genotype, Goliath. Statistics show differences between genotypes from anova (P =≤ 0.05). N = 3, ±SE.
Table S2. NSC composition in the mixed population (a) and mapping family (b). All carbohydrates are in mg g−1 DW. Statistics show differences between all genotypes from anova (P =≤ 0.05). N = 3, ±SE.
Table S3. Cell wall composition in a mixed population (a) and mapping family (b). All carbohydrates are in mg g−1 DW. Statistics show differences between all genotypes from anova (P =≤ 0.05). N = 3, ±SE.
Table S4. Block effects across the two trials. For the mixed population N = 18 and for the mapping family N = 19. Statistics (F Pr) show the results of a one‐way anova with block as a treatment factor (Significant differences = ≤ 0.05).
Table S5. Carbohydrate predictors of biomass traits selected by CfsSubsetEval (in the Weka software) for each of the machine learning models in Table 2.
Table S6. NSC composition in the complete mapping family in 2014.
Acknowledgements
This work was supported by the BBSRC Sustainable Bioenergy Centre (BSBEC) grant (BB/G016216/1) working within the BSBEC BioMASS (http://www.bsbec-biomass.org.uk/) Programme of the centre and Ceres inc. and the BBSRC Energy Grasses & Biorefining Institute Strategic Programme (BBS/E/W/00003134) at IBERS and the Ecosystem Land Use Modelling & Soil C Flux Trial (ELUM) at CEH Lancaster and IBERS (ET/I000100/1). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Allison GG, Morris C, Clifton‐Brown J, Lister SJ, Donnison IS (2011) Genotypic variation in cell wall composition in a diverse set of 244 accessions of Miscanthus. Biomass and Bioenergy, 35, 4740–4747. [Google Scholar]
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane‐associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proceedings of the National Academy of Sciences of the United States of America, 92, 9353–9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroja‐Fernandez E, Munoz FJ, Li J et al (2012) Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proceedings of the National Academy of Sciences of the United States of America, 109, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasi C, Pitkamaki AS, Tavi NM, Koponen HT, Martikainen PJ (2012) An isotope approach based on 13C pulse‐chase labelling vs. the root trenching method to separate heterotrophic and autotrophic respiration in cultivated peatlands. Boreal Environment Research, 17, 184–192. [Google Scholar]
- Calenge F, Saliba‐Colombani V, Mahieu S, Loudet O, Daniel‐Vedele F, Krapp A (2006) Natural variation for carbohydrate content in Arabidopsis. Interaction with complex traits dissected by quantitative genetics. Plant Physiology, 141, 1630–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleden CK, Aoki N, Gillespie VJ et al (2004) Evolution and function of the sucrose‐phosphate synthase gene families in wheat and other grasses. Plant Physiology, 135, 1753–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HD, Ellis DD, Gilbert M, Mansfield SD (2006) Up‐regulation of sucrose synthase and UDP‐glucose pyrophosphorylase impacts plant growth and metabolism. Plant Biotechnology Journal, 4, 87–101. [DOI] [PubMed] [Google Scholar]
- Coleman HD, Yan J, Mansfield SD (2009) Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proceedings of the National Academy of Sciences of the United States of America, 106, 13118–13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CE, Martin TM, Pauly M (2010a) Comprehensive compositional analysis of plant cell walls (Lignocellulosic biomass) part I: lignin. Journal of Visualized Experiments, 1745, doi: 10.3791/1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CE, Martin TM, Pauly M (2010b) Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part II: carbohydrates. Journal of Visualized Experiments, 1837, doi: 10.3791/1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Ferrario S (1994) Modulation of carbon and nitrogen‐metabolism in transgenic plants with a view to improved biomass production. Biochemical Society Transactions, 22, 909–915. [DOI] [PubMed] [Google Scholar]
- Frank E, Hall M, Trigg L, Holmes G, Witten IH (2004) Data mining in bioinformatics using Weka. Bioinformatics, 20, 2479–2481. [DOI] [PubMed] [Google Scholar]
- Hodkinson TR, Chase MW, Lledo MD, Salamin N, Renvoize SA (2002) Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL‐F intergenic spacers. Journal of Plant Research, 115, 381–392. [DOI] [PubMed] [Google Scholar]
- Hogberg P, Hogberg MN, Gottlicher SG et al (2008) High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytologist, 177, 220–228. [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Ono K, Kashiwagi T (2004) Identification of a new gene controlling plant height in rice using the candidate‐gene strategy. Planta, 218, 388–395. [DOI] [PubMed] [Google Scholar]
- Jensen E, Farrar K, Thomas‐Jones S, Hastings A, Donnison I, Clifton‐Brown J (2011) Characterization of flowering time diversity in Miscanthus species. GCB Bioenergy, 3, 387–400. [Google Scholar]
- Jones MG, Outlaw WH, Lowry OH (1977) Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiology, 60, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharabian‐Masouleh A, Waters DLE, Reinke RF, Ward R, Henry RJ (2012) SNP in starch biosynthesis genes associated with nutritional and functional properties of rice. Scientific Reports, 2, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Xing EP (2009) Statistical estimation of correlated genome associations to a quantitative trait network. Plos Genetics, 5, e1000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology, 7, 235–246. [DOI] [PubMed] [Google Scholar]
- Laporte MM, Galagan JA, Shapiro JA, Boersig MR, Shewmaker CK, Sharkey TD (1997) Sucrose‐phosphate synthase activity and yield analysis of tomato plants transformed with maize sucrose‐phosphate synthase. Planta, 203, 253–259. [DOI] [PubMed] [Google Scholar]
- Li HD, Liang YZ, Xu QS (2009) Support vector machines and its applications in chemistry. Chemometrics and Intelligent Laboratory Systems, 95, 188–198. [Google Scholar]
- Maloney VJ, Park JY, Unda F, Mansfield SD (2015) Sucrose phosphate synthase and sucrose phosphate phosphatase interact in planta and promote plant growth and biomass accumulation. Journal of Experimental Botany, 66, 4383–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HE, Doring A, Persson S (2014) The cell biology of cellulose synthesis. Annual Review of Plant Biology, 65, 69. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Steinfath M, Lisec J et al (2007) The metabolic signature related to high plant growth rate in Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the United States of America, 104, 4759–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguerza M, Gondo T, Yoshida M, Kawakami A, Terami F, Yamada T, Akashi R (2013) Modification of the total soluble sugar content of the C4 grass Paspalum notatum expressing the wheat‐derived sucrose: sucrose 1‐fructosyltransferase and sucrose: fructan 6‐fructosyltransferase genes. Grassland Science, 59, 196–204. [Google Scholar]
- O'Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A (2014) Drought survival of tropical tree seedlings enhanced by non‐structural carbohydrate levels. Nature Climate Change, 4, 710–714. [Google Scholar]
- Poovaiah CR, Mazarei M, Decker SR, Turner GB, Sykes RW, Davis MF, Stewart CN Jr (2015) Transgenic switchgrass (Panicum virgatum L.) biomass is increased by overexpression of switchgrass sucrose synthase (PvSUS1). Biotechnology Journal, 10, 552–563. [DOI] [PubMed] [Google Scholar]
- Purdy SJ, Maddison AL, Jones LE, Webster RJ, Andralojc J, Donnison I, Clifton‐Brown J (2013) Characterization of chilling‐shock responses in four genotypes of Miscanthus reveals the superior tolerance of M. x giganteus compared with M. sinensis and M. sacchariflorus . Annals of Botany, 111, 999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy SJ, Cunniff J, Maddison AL et al (2014) Seasonal carbohydrate dynamics and climatic regulation of senescence in the perennial grass, Miscanthus. Bioenergy Research, 8, 28–41. [Google Scholar]
- Purdy SJ, Maddison AL, Cunniff J, Donnison I, Clifton‐Brown J (2015) Non‐structural carbohydrate profiles and ratios between soluble sugars and starch serve as indicators of productivity for a bioenergy grass. AoB Plants, 7, plv032, doi: 10.1093/aobpla/plv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P, Mos M, Clifton‐Brown J, Donnison I (2012) Phenotypic variation in senescence in Miscanthus: towards optimising biomass quality and quantity. Bioenergy Research, 5, 95–105. [Google Scholar]
- Robson P, Jensen E, Hawkins S et al (2013) Accelerating the domestication of a bioenergy crop: identifying and modelling morphological targets for sustainable yield increase in Miscanthus. Journal of Experimental Botany, 64, 4143–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher JP (1988) Comparison of carbohydrate compartmentation in relation to photosynthesis, assimilate export and growth in a range of maize genotypes. Australian Journal of Plant Physiology, 15, 677–686. [Google Scholar]
- Ruan YL (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annual Review of Plant Biology, Vol 61, 65, 33–67. [DOI] [PubMed] [Google Scholar]
- Scott IM, Vermeer CP, Liakata M et al (2010) Enhancement of plant metabolite fingerprinting by machine learning. Plant Physiology, 153, 1506–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IM, Ward JL, Miller SJ, Beale MH (2014) Opposite variations in fumarate and malate dominate metabolic phenotypes of Arabidopsis salicylate mutants with abnormal biomass under chilling. Physiologia Plantarum, 152, 660–674. [DOI] [PubMed] [Google Scholar]
- Signora L, Galtier N, Skot L, Lucas H, Foyer CH (1998) Over‐expression of sucrose phosphate synthase in Arabidopsis thaliana results in increased foliar sucrose/starch ratios and favours decreased foliar carbohydrate accumulation in plants after prolonged growth with CO2 enrichment. Journal of Experimental Botany, 49, 669–680. [Google Scholar]
- Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annual Review of Plant Biology, 56, 73–98. [DOI] [PubMed] [Google Scholar]
- Somerville C, Youngs H, Taylor C, Davis SC, Long SP (2010) Feedstocks for Lignocellulosic Biofuels. Science, 329, 790–792. [DOI] [PubMed] [Google Scholar]
- de Souza AP, Arundale RA, Dohleman FG, Long SP, Buckeridge MS (2013) Will the exceptional productivity of Miscanthus x giganteus increase further under rising atmospheric CO2? Agricultural and Forest Meteorology, 171, 82–92. [Google Scholar]
- Steinfath M, Strehmel N, Peters R et al (2010) Discovering plant metabolic biomarkers for phenotype prediction using an untargeted approach. Plant Biotechnology Journal, 8, 900–911. [DOI] [PubMed] [Google Scholar]
- Subke JA, Vallack HW, Magnusson T, Keel SG, Metcalfe DB, Hogberg P, Ineson P (2009) Short‐term dynamics of abiotic and biotic soil 13CO2 effluxes after in situ 13CO2 pulse labelling of a boreal pine forest. New Phytologist, 183, 349–357. [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl ET, Ishihara H et al (2009) Starch as a major integrator in the regulation of plant growth. Proceedings of the National Academy of Sciences of the United States of America, 106, 10348–10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Trenkamp S, Steinfath M et al (2010) Network analysis of enzyme activities and metabolite levels and their relationship to biomass in a large panel of Arabidopsis accessions. Plant Cell, 22, 2872–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Nikoloski Z, Tschoep H et al (2013) Impact of the carbon and nitrogen supply on relationships and connectivity between metabolism and biomass in a broad panel of Arabidopsis accessions. Plant Physiology, 162, 347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venu RC, Ma J, Jia Y et al (2014) Identification of candidate genes associated with positive and negative heterosis in rice. PLoS ONE, 9, e95178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser P, Pignatelli V (2001) Utilization of Miscanthus In: Miscanthus: For Energy and Fibre (ed. Walsh M.), pp. 109–154. Earthscan, London. [Google Scholar]
- Volkert K, Debast S, Voll LM et al (2014) Loss of the two major leaf isoforms of sucrose‐phosphate synthase in Arabidopsis thaliana limits sucrose synthesis and nocturnal starch degradation but does not alter carbon partitioning during photosynthesis. Journal of Experimental Botany, 65, 5217–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wu JQ (2013) The essential role of Jasmonic acid in plant‐herbivore interactions – using the wild tobacco Nicotiana attenuata as a model. Journal of Genetics and Genomics, 40, 597–606. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tetko IV, Hall MA, Frank E, Facius A, Mayer KFX, Mewes HW (2005) Gene selection from microarray data for cancer classification – a machine learning approach. Computational Biology and Chemistry, 29, 37–46. [DOI] [PubMed] [Google Scholar]
- Wanner LA, Junttila O (1999) Cold‐induced freezing tolerance in Arabidopsis. Plant Physiology, 120, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Biomass traits in the mixed population (a) and mapping family (b). The population consisted of M. sinensis (Sin), Hybrids (Hyb) and M. sacchariflorus (Sac) and the mapping family were all hybrids (M. sacchariflorus × M. sinensis) except a single M. sinensis genotype, Goliath. Statistics show differences between genotypes from anova (P =≤ 0.05). N = 3, ±SE.
Table S2. NSC composition in the mixed population (a) and mapping family (b). All carbohydrates are in mg g−1 DW. Statistics show differences between all genotypes from anova (P =≤ 0.05). N = 3, ±SE.
Table S3. Cell wall composition in a mixed population (a) and mapping family (b). All carbohydrates are in mg g−1 DW. Statistics show differences between all genotypes from anova (P =≤ 0.05). N = 3, ±SE.
Table S4. Block effects across the two trials. For the mixed population N = 18 and for the mapping family N = 19. Statistics (F Pr) show the results of a one‐way anova with block as a treatment factor (Significant differences = ≤ 0.05).
Table S5. Carbohydrate predictors of biomass traits selected by CfsSubsetEval (in the Weka software) for each of the machine learning models in Table 2.
Table S6. NSC composition in the complete mapping family in 2014.


