Abstract
In response to starvation, Myxococcus xanthus initiates a developmental program that results in the formation of spore-filled, multicellular fruiting bodies. Many developmentally regulated genes in M. xanthus are transcribed from σ54 promoters, and these genes require enhancer-binding proteins. Here we report the finding of an unusual group of 12 genes encoding σ54-dependent enhancer-binding proteins containing a forkhead-associated (FHA) domain as their N-terminal sensory domain. FHA domains in other proteins recognize phosphothreonine residues. An insertion mutation in one of these genes, Mx4885, caused a cell autonomous aggregation and sporulation defect. In-frame deletion mutants showed that the FHA domain is necessary for proper Mx4885 function. The altered pattern of developmental gene expression in the mutant implied that Mx4885 is on the pathway of response to the morphogenetic C-signal. Immunoblots specific for C-signal and FruA imply that the site of Mx4885 action is downstream of FruA synthesis on the C-signal transduction pathway. Mx4885 may help to coordinate the level of intracellular phosphorylated FruA (FruA-P) with the level of C-signal displayed on the signal donor cell. Because FHA domains respond to phosphothreonine-containing proteins, these results suggest a regulatory link to the abundant Ser/Thr protein kinases in M. xanthus.
Keywords: developmental gene expression, cell–cell signaling, protein kinase
The δ-proteobacterium Myxococcus xanthus executes a 24-h-long program of multicellular development (1). When starved at high cell density on a solid surface, ≈105 cells move by gliding motility to build a fruiting body. Inside the fruiting body, some cells differentiate into dormant myxospores. The developmental program coordinates cell movement with changes in gene expression (2, 3). Developmentally regulated genes are expressed at specific time points during development in an ordered, temporal sequence (4). These genes are also expressed at particular positions within the fruiting body (5, 6). How is the program of temporally and spatially ordered gene expression organized to produce a fruiting body reliably? Cell–cell signals are important, and A-signal and C-signal molecules have been identified chemically (7, 8). A-signal is important during the first few hours of development whereas the processed C-signal, a 17-kDa cell-surface protein that signals by contact, is important for development after 6 h. C-signal is a morphogen that induces rippling, aggregation by streaming, and, later, sporulation, each by progressively higher levels of the signal (9–11). The C-signal also induces expression of most genes turned on after 6 h (12).
Many developmentally regulated genes in M. xanthus are expressed from σ54-dependent promoters (13–17). Such promoters require a specialized transcription factor, called an enhancer-binding activator protein (EBP), in addition to RNA polymerase associated with σ54 (σ54-RNAP) binding at the promoter (18). The EBPs are usually bound to regulatory DNA sequences upstream from the promoters, and DNA bending allows the EBP to interact with σ54-RNAP at the promoter. EBP-catalyzed ATP hydrolysis is required for opening the σ54-RNAP promoter complex to initiate transcription (19–21). Probed by gene knockouts, 10 M. xanthus EBPs have been shown to be required specifically for development (3, 22–29). Other EBP genes have been shown to be required for the heat-shock response (30), and still others have been found for S-motile gliding in swarms (17, 28). The M. xanthus rpoN gene, which encodes σ54 in single copy, is essential not only for normal development but also for growth (31). More than 50 genes appear to encode EBPs in the M. xanthus genome; this is the largest number of EBPs found in any sequenced bacterial genome to date. The abundance of EBPs implies that σ54 is part of a large regulatory network.
Many EBPs participate in signal transduction circuits that respond to environmental cues. EBPs have a common domain organization with a central AAA-ATPase domain responsible for ATP hydrolysis and interaction with σ54, a C-terminal DNA-binding domain, and an N-terminal sensory domain that regulates the ATPase activity of the central domain in response to stimuli (32, 33). The N-terminal sensory domains show the most variation from one EBP to another, and several distinct groups of N-terminal sequences can be recognized (33). Most frequently, a two-component response regulator receiver domain is found there.
Some EBPs, however, have a forkhead-associated (FHA) domain as their N-terminal sensory unit. The FHA domain is shown to be an essential part of the Mx4885 protein, and Mx4885 is the subject of this report. Knockout mutants of Mx4885 show an abnormal pattern of fruiting-body development and developmental gene expression. Both defects are consistent with Mx4885 playing a role in the C-signal transduction pathway. The FHA domain is a phosphothreonine-specific recognition domain involved in specific phosphorylation-dependent protein–protein interactions. Interestingly, this finding suggests a link between σ54-dependent developmental gene expression, C-signaling, and signal transduction pathways involving Ser/Thr protein kinases (STPK) in M. xanthus.
Materials and Methods
M. xanthus Growth, Development, and Strain Construction. CTT medium (34), clone-fruiting (CF) agar (22), glycerol-induced sporulation (35), and photomicroscopic procedures (3) have all been published. M. xanthus strains used in this work are listed in Table 2, which is published as supporting information on the PNAS web site. Plasmids were introduced into M. xanthus by electroporation as described in ref. 22. DK12702 has Mx4885 disrupted by integration of plasmid pJEL4885.8 by homologous recombination. Plasmid construction is detailed in Plasmid Construction in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Immunoblotting and Measurement of β-Galactosidase Activity. Cells were induced to develop on CF agar and harvested at the time indicated, and specific β-galactosidase activity was measured as described in ref. 4. For FruA immunoblots, cells were boiled for 5 min in SDS lysis buffer (36) containing 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, and 0.25 mM phenylmethylsulfonylfluoride, and 4 μg of total protein was separated on a Tris(hydroxymethyl)-aminomethane/SDS/10% polyacrylamide gel. For CsgA immunoblots, cells were boiled for 5 min in Tris(hydroxymethyl)-aminomethane/SDS lysis buffer (37) containing protease inhibitors as described above, and 10 μg of total protein was separated on a Tris(hydroxymethyl)-aminomethane/SDS/10% polyacrylamide gel. Immunoblots were prepared by standard procedures (36) and probed with rabbit anti-FruA serum (38) at a 1:7,000 dilution or rabbit anti-CsgA serum (11) at a 1:5,000 dilution. After reaction with peroxidase-conjugated goat anti-rabbit IgG (Roche Molecular Biochemicals), the blots were developed with the PerkinElmer chemiluminescence reagent. Protein concentrations were determined with Bradford reagent (Bio-Rad) with bovine IgG as standard.
Sequence Analysis. Most of the putative EBPs were discovered by using the PF00158 Pfam motif as described in ref. 3 on the >95% complete M. xanthus M1 genome sequence. The putative EBPs and their surrounding genes were confirmed and analyzed on the complete M. xanthus genome sequence provided by The Institute for Genomic Research. Protein domains were characterized by using the Pfam hidden Markov model (HMM) database (http://pfam.wustl.edu/hmmsearch.shtml). Sequences were aligned by using the clustalw service at the European Bioinformatics Institute (www.ebi.ac.uk/clustalw) and presented by using genedoc (www.psc.edu/biomed/genedoc).
Results
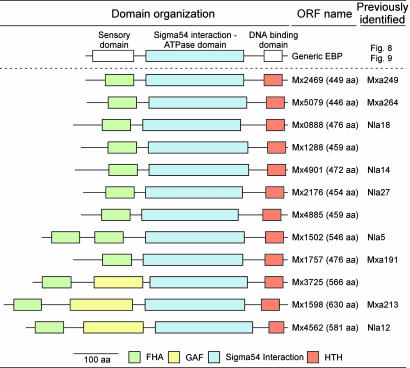
EBPs with FHA Domains. By using the highly conserved central ATPase domain for identification (Fig. 1), we found 52 EBP-like genes in the M. xanthus genome. Alignment of these 52 sequences revealed conserved residues arranged in seven regions as described previously for the central domain of EBPs (see Fig. 8, which is published as supporting information on the PNAS web site) (32). The GAFTGA sequence motif, which is present in genuine σ54-dependent EBPs (33), was found in variant forms in all sequences except ORF Mx3725, where it appears to be absent. Seeking the regulatory functions of these EBPs, we analyzed their domain architecture by using the Pfam HMM database. The majority, 28, of the 52 M. xanthus EBPs were found to have a two-component response regulator receiver domain at their N termini. These domains among others are commonly found in bacterial EBPs (see Fig. 9, which is published as supporting information on the PNAS web site) (33). Twelve potential EBPs in M. xanthus have a FHA domain at their N termini shown in Fig. 1.
Fig. 1.
Domain organization of a generic EBP and of putative FHA-EBPs in M. xanthus. Domains are color-coded as follows: green, FHA domains (Pfam accession no. PF00498); yellow, GAF domains (Pfam accession no. PF00072); blue, σ54-dependent EBP domains (Pfam accession no. PF00158); red, helixturn-helix (HTH) DNA-binding domains (Pfam accession no. PF02954). (Scale bar, 100 aa.)
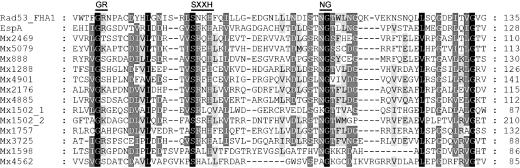
Although FHA domains have been found in several proteins, they are new to EBPs, having only recently been proposed for two EBPs in Pirellula species strain 1 (39). Except for Mx4562, Mx3725, and Mx1598, FHA domains were the only N-terminal domains identified in the 12 sequences of Fig. 1. These three sequences contain a GAF domain in addition to their FHA domain. GAF domains are commonly found in EBPs and are signaling modules that bind small-molecule cofactors (33). Alignment of the 12 sequences with the prototypical FHA domain, RAD53FHA1 from yeast (40, 41) shows that the motifs: G69-R70, S85-XX-H88, and N107-G108 from RAD53FHA1 are well conserved in the M. xanthus sequences (Fig. 2). The FHA domain in RAD53 is a small, phosphothreonine-specific recognition domain, and it is thought to interact with a protein partner in a process regulated by reversible protein phosphorylation (41–44). Finding EBPs with an N-terminal FHA domain in M. xanthus, opens the possibility of interactions with an autophosphorylated STPK. In fact, genes encoding STPKs were found immediately next to four EBP ORFs Mx0888, Mx5079, Mx1288, and Mx4901, and others were found close to ORFs Mx1598 and Mx4562.
Fig. 2.
Amino acid sequence alignment of the FHA domains of the putative M. xanthus EBPs with those of RAD53FHA1 from Saccharomyces cerevisiae (GenBank accession no. A39616) and EspA from M. xanthus (GenBank accession no. AAD47812). The conserved GR, SXXH, and NG motifs are indicated above the alignment. Residues on black, dark gray, and light gray backgrounds indicate 100%, 75%, and 50% amino acid similarity, respectively.
Mx4885 Insertion Mutants Have Developmental Defects. Of the 12 genes that encode putative EBPs with FHA domains, nine have previously been inactivated (Fig. 1). Disruption of the Mx0888 and Mx1598 genes results in abnormal fruiting-body development, but the reason for those defects was not apparent (22, 28). To shed light on the physiological role of the three yet uncharacterized EBP genes, we disrupted each of them by plasmid insertion. Disrupting the Mx1288 and Mx3725 genes revealed no abnormality in vegetative growth or in fruiting-body development under the conditions tested.
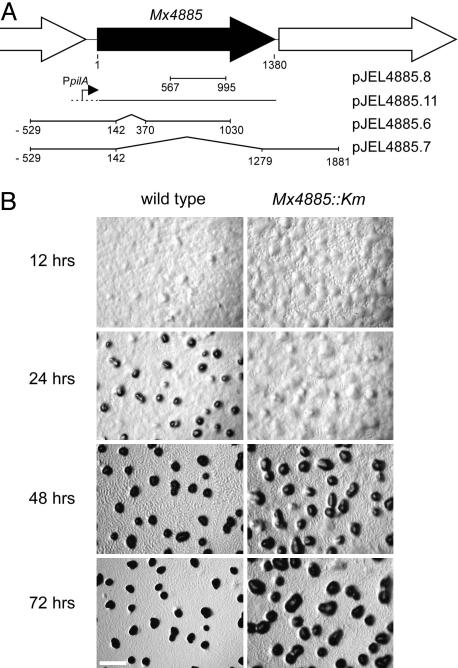
Mx4885 was disrupted by inserting plasmid pJEL4885.8 (Fig. 3A) by means of homologous recombination resulting in strain DK12702 (Mx4885::Km). DK12702 had no obvious growth defects: colony color, morphology, and the rate of spreading (or swarming) on either soft or hard agar were similar to wild type. Development of DK12702 was examined on CF starvation agar, where after 24 h, wild-type cells will have aggregated into translucent mounds (Fig. 3B). During the next 48 h, the wild-type mounds become condensed, darkened, spore-filled fruiting bodies (Fig. 3B). As the wild type aggregated and constructed mature fruiting bodies, rippling was evident at 48 h. Although the insertion mutant Mx4885::Km was able to ripple, it formed abnormal fruiting bodies. By 24 h, the mutant had formed irregular aggregates that were less compact and less dense than those of the wild type (Fig. 3B). Between 24 and 72 h, the mutant aggregates enlarged, but they were always less regular than wild-type fruiting bodies and less compact. Sporulation in the mutant fruiting bodies was reduced 500-fold compared to wild type (Table 1). Mutant cultures could be induced to sporulate by addition of glycerol to aerated liquid cultures like wild-type cells, suggesting that the developmental defect is before the change in cell shape from rod to sphere step that is common to fruiting body and glycerol sporulation (35).
Fig. 3.
Mx4885. (A) Shown is a physical map of the Mx4885 neighborhood. ORFs are indicated by large arrows. The +1 map coordinate is the first nucleotide in the translation start codon of Mx4885, and 1380 is the last nucleotide of the translation stop. Plasmids containing the indicated DNA fragments of the Mx4885 region are shown on the lines below the physical map. (B) Shown is the aggregate morphology of wild type (DK1622) and Mx4885::Km mutant (DK12702) starved on CF agar for the indicated periods of time. (Scale bar, 0.5 mm.)
Table 1. Sporulation frequency.
| Strain* | Genotype | Sporulation frequency |
|---|---|---|
| DK1622 | Wild type | 100† |
| DK12702 | Mx4885::Km | 0.2 ± 0.2 |
| DK12703 | ΔMx488548–426 | 0.6 ± 0.8 |
| DK12704 | ΔMx488548–123 | 0.5 ± 0.5 |
| DK12705 | Mx4885::Km, attB::pJEL4885.11 | 184.5 ± 20.1 |
| DK12706 | Mx4885::Km, attB::pSWU30 | 0.4 ± 0.2 |
Strains are described in Table 2
The frequency was normalized to that of the wild type (DK1622) measured in the same experiment and set at 100%
To investigate whether the developmental sporulation defect caused by the Mx4885::Km insertion could be due to lack of an exchangeable extracellular substance, or signal (24, 27, 28, 45), wild-type cells (DK1622) were mixed with an equal number of mutant cells (DK12702) and the number of spores formed by the DK12702 strain was measured after 72 h of development. However, the experiment gave 0.06 ± 0.03% sporulation of the mutant after codevelopment with wild-type cells. Because there was no more sporulation than the 0.2 ± 0.2% shown for DK12702 alone in Table 1, there is no evidence for a signal defect.
Some aggregation defects have been shown to be consequences of a defective A-engine or S-engine for gliding. This consideration was addressed by constructing Mx4885 S- and Mx4885 A- double mutants; strains with both an A- and an S- mutation are nonspreading and grow as small, smooth-edged colonies (46, 47). However, neither Mx4885 double mutant showed loss of motility, as illustrated in Fig. 10, which is published as supporting information on the PNAS web site. The developmental defect observed for DK12702 is not secondary to a defect in either one of the two gliding engines.
Mx4885 Is Necessary for Fruiting-Body Development. The gene immediately downstream of Mx4885 is oriented in the same direction for transcription as Mx4885, raising the possibility that insertion of the plasmid pJEL4885.8 in Mx4885 (in DK12702) has a polar effect on the expression of the downstream gene (Fig. 3A). To discriminate between inactivation of the Mx4885 gene itself or the downstream gene in the mutant, a copy of the wild-type Mx4885 gene without the downstream gene was placed under the control of the pilA promoter and was added by integrating pJEL4885.11 to give the strain DK12705 (Fig. 3A). The pilA promoter is active during both growth and development (17). As a control, the vector pSWU30 (without the Mx4885 gene) was also introduced into DK12702 to give the strain DK12706. As seen in Fig. 4 and Table 1, when all these strains were induced to develop on CF agar plates in parallel, the abnormal developmental phenotype observed for DK12702 was corrected by the introduction of a single wild-type copy of the Mx4885 gene expressed from the pilA promoter. Introduction of pSWU30 into DK12702 did not have any effect on the phenotype (Fig. 4 and Table 1). These results point to the disruption of Mx4885 itself as the cause behind the developmental failure of DK12702.
Fig. 4.
Aggregation phenotypes of Mx4885 mutants. The strains indicated above the images were starved on CF agar plates for 24 h before photography. The strains used were DK1622 (wild type), DK12702 (Mx4885::Km), DK12703 (ΔMx488548–426), DK12704 (ΔMx488548–123), DK12705 (Mx4885::Km, attB::pJEL4885.11), and DK12706 (Mx4885::Km, attB::pSWU30). (Scale bar, 0.5 mm.)
If the loss of Mx4885 function alone is responsible for the developmental defects in DK12702, then an in-frame deletion mutant of Mx4885 should be defective. An in-frame deletion mutant was constructed by using plasmid pJEL4885.7 (Fig. 3A). DK12703 has an 1,137-bp in-frame deletion within Mx4885 (ΔMx488548–426) that removes codons 48–426 of the Mx4885 ORF. This construction deletes the FHA motif, the entire central domain, and most of the C-terminal DNA-binding domain from the EBP. As shown in Fig. 4, DK12703 displayed abnormal and delayed aggregation and reduced sporulation (Table 1), very much like the Mx4885::Km mutant.
Strain DK12704 suffers a shorter, 228-bp, in-frame deletion of Mx4885 (ΔMx488548–123), which eliminates codons 48–123 of the Mx4885 ORF by using plasmid pJEL4885.6 (Fig. 3A). This construction is expected to delete the FHA domain from the protein while the central and C-terminal domains of the protein remain intact. DK12704 also showed abnormal and delayed aggregation and reduced sporulation, like the ΔMx488548–426 deletion and the Mx4885::Km insertion mutation (Fig. 4 and Table 1). The phenotype of these in-frame deletion mutants establish that the developmental defects observed in DK12702 and DK12703 can be accounted for by loss of function of the Mx4885 gene and its protein product. Because DK12704 is unable to develop, the FHA domain of Mx4885 must be necessary for normal development.
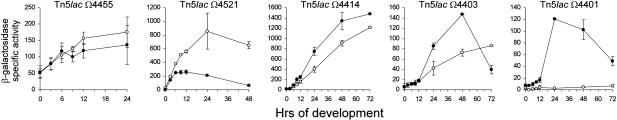
The Mx4885 Mutation Changes Developmental Gene Expression. As previously established, each developmentally regulated Tn5lac reporter fusion increases its expression at some particular time during development in wild-type cells (4). To assess progress through the developmental program and to estimate the time at which Mx4885 is needed for development, expression of five developmentally regulated Tn5lac reporter fusions was compared between the Mx4885::Km insertion mutant and wild-type cells. Expression of the Tn5lac Ω4455 reporter initiates within an hour after onset of starvation in wild type. Induction depends on starvation but not on the A-signal or C-signal (12, 48). In the Mx4885::Km mutant, the expression profile of this fusion was very similar to wild type (Fig. 5, Tn5lac Ω4455), suggesting that the Mx4885::Km mutant has no difficulty before the time of Ω4455 expression and is thus able initially to respond to starvation normally. By contrast, the expression profiles of the other four fusions were significantly altered by the Mx4885::Km mutation. Tn5lac Ω4521 depends on the A-signal for normal expression (48). Because Ω4521 expression is initiated normally, A-signaling appears to be satisfactory. Moreover, bioassays showed normal A-signal production during development from the Mx4885::Km mutant (Fig. 11, which is published as supporting information on the PNAS web site). Nevertheless, Tn5lac Ω4521 expression is much higher in the Mx4885::Km mutantafter 6 h of development (Fig. 5). After 24 h, Mx4885::Km showed a >3-fold higher level of Tn5lac Ω4521 expression than wild type. This effect on Tn5lac Ω4521 expression profile resembles the effect caused by a fruA mutation (38).
Fig. 5.
Effect of the Mx4885 mutation on developmental gene expression. Expression of the indicated Tn5lac reporter fusions in wild-type DK1622 cells (•) and in Mx4885::Km DK12702 cells (○) on CF agar. Culture samples were collected at the indicated time points and assayed for specific activity β-galactosidase, given as nanomoles of o-nitrophenol produced per minute per milligram of total protein.
Three Tn5lac reporter fusions, Ω4414, Ω4403, and Ω4401, depend on C-signaling for normal expression (12). Gene expression in these fusion strains was not induced to wild-type levels when measured in the Mx4885::Km mutant (Fig. 5). In Tn5lac Ω4401, β-galactosidase activity did not rise at all in the Mx4885::Km mutant.
The finding that Mx4885 regulates the expression of these four of five Tn5lac fusions, either directly or indirectly, and the times at which expression differs from wild type, mean that Mx4885 becomes important at ≈6 h of development and remains important thereafter. Even though the Mx4885::Km mutant delayed aggregation, no delay was evident in the expression of β-galactosidase from the Ω4455, Ω4521, Ω4414, or Ω4403 fusions in the Mx4885::Km mutant background (Fig. 5). The level of reporter expression either failed to decrease (Ω4521), was reduced (Ω4414 and Ω4403) or was completely abolished (Ω4401) in Mx4885::Km.
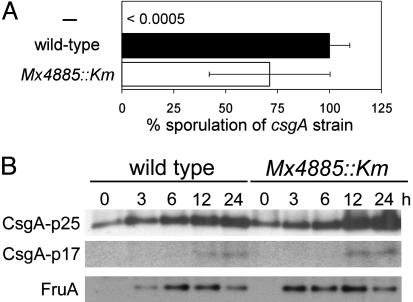
Mx4885 and the C-Signal Transduction Pathway. That the Mx4885::Km mutant expresses three different C-signal-dependent reporters at reduced levels, forms abnormally shaped fruiting bodies, and produces fewer spores points to a defect in the network of C-signaling. To pinpoint the effect of the Mx4885::Km mutation in the C-signaling network, we first assayed the ability of the mutant to present and transmit the C-signal to a C-signal-deficient csgA mutant. Admixed Mx4885::Km cells (DK12702) rescued the sporulation of a csgA mutant (DK5208) as efficiently as did wild-type cells by extracellular complementation (Fig. 6A). Next, C-signal production by the Mx4885::Km mutant was quantified by immunoblots with polyclonal anti-CsgA antibodies. Proteins were isolated from wild-type (DK1622) and Mx4885::Km cells (DK12702) that had been starved on CF medium. p25 and p17 CsgA proteins (11) displayed similar accumulation profiles in the two strains during development (Fig. 6B). p25 CsgA and p17 CsgA represent the cytoplasmic translation product of the csgA gene and the processed, cell-surface, active form of the signal molecule (49). These data show that Mx4885 is not involved in either synthesis or processing of CsgA protein.
Fig. 6.
Assays to find the site of action of Mx4885 in the C-signal transduction pathway. (A) C-signal transmission measured by extracellular complementation for sporulation of the csgA mutant DK5208 by a Mx4885::Km mutant (DK12702; white bar) or wild type (DK1622; black bar). The sporulation efficiency of the csgA test strain is given as the percentage of complementation by wild type. (B) Accumulation of CsgA and FruA proteins measured on immunoblots of Mx4885::Km and wild type. Total cell lysates were prepared from cells at the indicated time of development on CF agar and reacted with polyclonal anti-CsgA or anti-FruA antibodies. In each lane, 10 and 4 μg of total protein was loaded for CsgA and FruA immunoblots, respectively.
Reception of the C-signal increases csgA expression by means of an act gene-dependent positive feedback loop represented in Fig. 7 (9, 24, 45). The number of C-signal molecules increases during development as cells signal each other. An increase in both p25 and the processed p17 is evident in mutant and wild type in Fig. 6B. The increase argues that the mutant can receive C-signal like wild type and that the act feedback loop is normal. In sum, these experiments show that the Mx4885::Km mutant is not defective in production, transmission, reception, or feedback of the C-signal. If the C-signal transduction pathway is correct, Mx4885 must play its role beyond the act feedback loop.
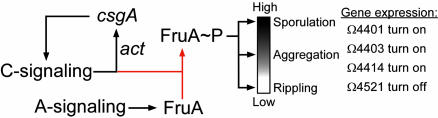
Fig. 7.
A model of the C-signal transduction pathway. C-signaling increases csgA transcription directly or indirectly by means of the proteins of the act operon. A-signal is required for developmental accumulation of the FruA response regulator. Downstream of the act pathway, C-signaling induces phosphorylation of FruA (red lines). Rippling, aggregation, sporulation, and C-signal-dependent gene expression are induced by increasing levels of FruA-P. Different levels are represented by the degree of shading of the open vertical bar. Expression of reporter genes is induced by the corresponding levels of FruA-P. The proposed site of action of Mx4885 is indicated by red lines, which are downstream of the act feedback loop and downstream of FruA accumulation but upstream of FruA-P.
Mx4885 Is Also Beyond FruA Synthesis. The two-component response regulator FruA is required for rippling, aggregation, and sporulation (38, 50, 51). Those three responses are also downstream of the act positive feedback loop in the C-signal transduction pathway that is represented in Fig. 7. Synthesis of FruA protein depends on the A-signal, but synthesis is independent of the C-signal. Instead, reception of C-signal activates FruA posttranslationally, most likely by phosphorylation (50). To investigate the relationship between FruA and Mx4885, the accumulation of FruA protein was examined by using semiquantitative FruA immunoblots. As shown in Fig. 6B, FruA accumulated in the mutant with similar timing and levels as observed in wild type during development on CF agar. Mx4885 therefore has no role in the synthesis of FruA but more likely in the modification that activates it, as indicated by the red line in Fig. 7.
Discussion
Loss of Mx4885 function results in a unique developmental phenotype: normal rippling but abnormal and delayed aggregation, a severe reduction in sporulation, and a novel pattern of developmental gene expression. The earliest change in developmental gene expression was observed at 6 h aftertheinitiation of starvation. Furthermore, expression of all C-signal-dependent gene fusions tested was either reduced or abolished, whereas C-signal-independent fusions were not depressed (Fig. 5). Both the fact that C-signaling gets underway at 6 h as well as the pattern of reporter gene expression in the Mx4885 mutant implicates the C-signal transduction pathway. The mutant produced p25 and p17 CsgA proteins at normal levels throughout development (Fig. 6). Moreover, the Mx4885 mutant was able to complement a C-signal-deficient mutant and rescue its development. Likewise, Mx4885 was shown not to be involved in the developmentally regulated accumulation of FruA, an essential component of the C-signal transduction pathway. Based on these data, we suggest that Mx4885 function lies on the C-signal transduction pathway just downstream of the accumulation of the FruA protein and just downstream of the act feedback loop, as represented in Fig. 7.
C-signaling is thought to ensure the correct temporal and spatial order of rippling, aggregation and sporulation during development by means of an ordered increase in the cellular level of C-signal (9, 10, 11). It has been suggested that the two-component response regulator FruA is activated by phosphorylation in a C-signal-dependent manner (50) whereby a given level of extracellular C-signal level is translated into a specific intracellular level of FruA-P (Fig. 7). The C-signal-dependent events are induced by different levels of FruA-P, as indicated in the model of Fig. 7. Rippling is produced by a low initial level of C-signal and a correspondingly low level of FruA-P. Aggregation then sporulation require higher levels of C-signal and, thus, higher levels of FruA-P. The Mx4885 phenotype could be explained if its level of FruA-P rose only a bit above the rippling level, high enough for streaming to begin but too low for streaming to compact the fruiting body or to induce sporulation. If the function of Mx4885 is to augment the level of FruA-P above the rippling level, loss of that function would give the phenotype observed for the Mx4885 mutant. We suggest that Mx4885 is involved in coordinating the level of FruA-P with that of the extracellular level of C-signal.
Mx4885 encodes one of 52 putative σ54-dependent EBPs found in the M. xanthus genome. It is thus likely that Mx4885 regulates expression of genes transcribed from one or more σ54-dependent promoters and that the products of these genes rather than Mx4885 itself is involved in regulating FruA activity. The Mx4885 protein is a specialized EBP with an FHA domain as its N-terminal sensory domain. Mx4885 and the 11 other EBPs with FHA domains might constitute a new subfamily of EBPs. The presence of the FHA phosphothreonine recognition domain suggests that these EBPs interact with proteins that are themselves regulated by reversible protein phosphorylation by the action of STPKs and phosphatases. The simplest signal transduction pathway involving an FHA–EBP protein would have a direct interaction between the EBP and a cognate STPK. The STPK, having been autophosphorylated in response to a particular stimulus, would then phosphorylate the EBP and activate it to initiate transcription. A precedent is available in Mycobacterium tuberculosis where the FHA domain of the ToxR-like transcriptional regulator EmbR allows the protein to interact with an autophosphylated form of PknH, an STPK, which then catalyzes phosphorylation of EmbR (52). An FHA–EBP might also be activated indirectly by means of an STPK-phosphorylated coactivator protein. In either case, the N-terminal FHA domain of the EBP would regulate its ability to activate transcription in response to the environmental cues detected by the STPK. What those cues may be is not known, but identifying the STPK that is cognate to Mx4885 may lead to their discovery.
Supplementary Material
Acknowledgments
We thank members of the D.K. laboratory for discussions and Monsanto for providing access to their M. xanthus sequence data. Additional sequence data for M. xanthus were obtained from The Institute for Genomic Research web site at www.tigr.org. L.J. was supported by the Carlsberg Foundation, and work in the United States was supported by National Institute of General Medical Sciences Public Health Service Grant GM23441.
Abbreviations: EBP, enhancer-binding activator protein; FHA, forkhead-associated; CF, clone-fruiting; FruA-P, phosphorylated FruA; STPK, Ser/Thr protein kinases.
See Commentary on page 2681.
References
- 1.Reichenbach, H. (1984) in Myxobacteria, ed. Rosenberg, E. (Springer, New York), pp. 1-50.
- 2.Horiuchi, T., Taoka, M., Isobe, T., Komano, T. & Inouye, S. (2002) J. Biol. Chem. 277, 26753-26760. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen, J. S., Jelsbak, L., Jelsbak, L., Welch, R. D., Cummings, C., Goldman, B., Stark, E., Slater, S. & Kaiser, D. (2004) J. Bacteriol. 186, 4361-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroos, L., Kuspa, A. & Kaiser, D. (1986) Dev. Biol. 117, 252-266. [DOI] [PubMed] [Google Scholar]
- 5.Julien, B., Kaiser, A. D. & Garza, A. (2000) Proc. Natl. Acad. Sci. USA 97, 9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sager, B. and Kaiser, D. (1993) Genes Dev. 7, 1645-1653. [DOI] [PubMed] [Google Scholar]
- 7.Kuspa, A., Plamann, L. & Kaiser, D. (1992) J. Bacteriol. 174, 3319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, S. K. & Kaiser, D. (1990) Cell 61, 19-26. [DOI] [PubMed] [Google Scholar]
- 9.Kim, S. K. & Kaiser, D. (1991) J. Bacteriol. 173, 1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, S., Lee, B. U. & Shimkets, L. J. (1992) Genes Dev. 6, 401-410. [DOI] [PubMed] [Google Scholar]
- 11.Kruse, T., Lobedanz, S., Berthelsen, N. M. & Sogaard-Andersen, L. (2001) Mol. Microbiol. 40, 156-168. [DOI] [PubMed] [Google Scholar]
- 12.Kroos, L. & Kaiser, D. (1987) Genes Dev. 1, 840-854. [DOI] [PubMed] [Google Scholar]
- 13.Keseler, I. M. & Kaiser, D. (1995) J. Bacteriol. 177, 4638-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romeo, J. M. & Zusman, D. R. (1991) J. Bacteriol. 173, 2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garza, A. G., Pollack, J. S., Harris, B. Z., Lee, A., Keseler, I. M., Licking, E. F. & Singer, M. (1998) J. Bacteriol. 180, 4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garza, A. G., Harris, B. Z., Pollack, J. S. & Singer, M. (2000) Mol. Microbiol. 35, 812-824. [DOI] [PubMed] [Google Scholar]
- 17.Wu, S. S. & Kaiser, D. (1997) J. Bacteriol. 179, 7748-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck, M., Gallegos, M. T., Studholme, D. J., Guo, Y. & Gralla, J. D. (2000) J. Bacteriol. 182, 4129-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popham, D. L., Szeto, D., Keener, J. & Kustu, S. (1989) Science 243, 629-635. [DOI] [PubMed] [Google Scholar]
- 20.Sasse-Dwight, S. & Gralla, J. D. (1990) Cell 62, 945-954. [DOI] [PubMed] [Google Scholar]
- 21.Wedel, A. & Kustu, S. (1995) Genes Dev. 9, 2042-2052. [DOI] [PubMed] [Google Scholar]
- 22.Gorski, L. & Kaiser, D. (1998) J. Bacteriol. 180, 5896-5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo, D., Wu, Y. & Kaplan, H. B. (2000) J. Bacteriol. 182, 4564-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gronewold, T. M. & Kaiser, D. (2001) Mol. Microbiol. 40, 744-756. [DOI] [PubMed] [Google Scholar]
- 25.Hager, E., Tse, H. & Gill, R. E. (2001) Mol. Microbiol. 39, 765-780. [DOI] [PubMed] [Google Scholar]
- 26.Sun, H. & Shi, W. (2001) J. Bacteriol. 183, 4786-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun, H. & Shi, W. (2001) J. Bacteriol. 183, 6733-6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caberoy, N. B., Welch, R. D., Jakobsen, J. S., Slater, S. C. & Garza, A. G. (2003) J. Bacteriol. 185, 6083-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirby, J. R. & Zusman, D. R. (2003) Proc. Natl. Acad. Sci. USA 100, 2008-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueki, T. & Inouye, S. (2002) J. Biol. Chem. 277, 6170-6177. [DOI] [PubMed] [Google Scholar]
- 31.Keseler, I. M. & Kaiser, D. (1997) Proc. Natl. Acad. Sci. USA 94, 1979-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morett, E. & Segovia, L. (1993) J. Bacteriol. 175, 6067-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studholme, D. J. & Dixon, R. (2003) J. Bacteriol. 185, 1757-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodgkin, J. & Kaiser, D. (1977) Proc. Natl. Acad. Sci. USA 74, 2938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Licking, E., Gorski, L. & Kaiser, D. (2000) J. Bacteriol. 182, 3553-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 37.Schagger, H. & von Jagow, G. (1987) Anal. Biochem. 166, 368-379. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa, M., Fujitani, S., Mao, X., Inouye, S. & Komano, T. (1996) Mol. Microbiol. 22, 757-767. [DOI] [PubMed] [Google Scholar]
- 39.Studholme, D. J. & Dixon, R. (2004) FEMS Microbiol. Lett. 230, 215-225. [DOI] [PubMed] [Google Scholar]
- 40.Stern, D. F., Zheng, P., Beidler, D. R. & Zerillo, C. (1991) Mol. Cell. Biol. 11, 987-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durocher, D. & Jackson, S. P. (2002) FEBS Lett. 513, 58-66. [DOI] [PubMed] [Google Scholar]
- 42.Durocher, D., Henckel, J., Fersht, A. R. & Jackson, S. P. (1999) Mol. Cell 4, 387-394. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann, K. & Bucher, P. (1995) Trends Biochem. Sci. 20, 347-349. [DOI] [PubMed] [Google Scholar]
- 44.Li, J., Lee, G. I., Van Doren, S. R. & Walker, J. C. (2000) J. Cell Sci. 113, 4143-4149. [DOI] [PubMed] [Google Scholar]
- 45.Gorski, L., Gronewold, T. & Kaiser, D. (2000) J. Bacteriol. 182, 2438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodgkin, J. & Kaiser, D. (1979) Mol. Gen. Genet. 171, 167-176. [Google Scholar]
- 47.Hodgkin, J. & Kaiser, D. (1979) Mol. Gen. Genet. 171, 177-191. [Google Scholar]
- 48.Kuspa, A., Kroos, L. & Kaiser, D. (1986) Dev. Biol. 117, 267-276. [DOI] [PubMed] [Google Scholar]
- 49.Lobedanz, S. & Sogaard-Andersen, L. (2003) Genes Dev. 17, 2151-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellehauge, E., Norregaard-Madsen, M. & Sogaard-Andersen, L. (1998) Mol. Microbiol. 30, 807-817. [DOI] [PubMed] [Google Scholar]
- 51.Sogaard-Andersen, L., Slack, F. J., Kimsey, H. & Kaiser, D. (1996) Genes Dev. 10, 740-754. [DOI] [PubMed] [Google Scholar]
- 52.Molle, V., Kremer, L., Girard-Blanc, C., Besra, G. S., Cozzone, A. J. & Prost, J. F. (2003) Biochemistry 42, 15300-15309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.