Abstract
Background
The objective of this study was to describe the first US-based study to use the European Position Paper on Rhinosinusitis (EPOS) criteria to study the prevalence of chronic rhinosinusitis (CRS) in a general population sample.
Methods
A CRS symptom questionnaire was mailed to 23,700 primary care patients from Geisinger Clinic, a health system serving 45 counties in Pennsylvania. CRS cases were categorized into four unique subgroups based on EPOS symptoms: obstruction and discharge with no smell loss or pain/pressure; smell loss without pain/pressure; facial pain and/or pressure without smell loss; and both smell loss and pain/pressure. All cases were required to have nasal obstruction or discharge. Logistic regression was used to evaluate potential factors associated with CRS subgroups.
Results
We found that 11.9% of patients met criteria for CRS. Prevalence peaked at 15.9% between ages 50–59 years and then dropped to 6.8% after age 69. The odds of CRS was higher among patients who were white, younger, smokers, had a history of Medical Assistance, and had other diseases. When CRS subgroups were modeled separately, these associations were no longer significant for some CRS subgroups. Co-morbid diseases were most strongly associated with CRS cases who reported smell loss and facial pain and/or pressure and had the weakest associations with CRS cases who did not report these symptoms.
Conclusions
CRS is a highly prevalent and heterogeneous condition. Differences in risk factors and health outcomes across symptom subgroups may be indicative of differences in etiology that have implications for disease management.
INTRODUCTION
Chronic rhinosinusitis (CRS) is defined by the presence of nasal and sinus symptoms for at least three months accompanied by objective evidence of sinus inflammation documented by either sinus computed tomography (CT) scan or endoscopy. The requirement for objective evidence has been an obstacle to large-scale, population-based studies of CRS. Knowledge of CRS is primarily derived from smaller studies of patients in tertiary healthcare settings [1]. We describe the first large-scale epidemiologic profile of CRS based in the U.S. using consensus criteria developed for epidemiologic studies.
The European Position Paper on Rhinosinusitis (EPOS) created criteria for CRS to be used in epidemiologic studies but without the need for objective evidence. EPOS criteria require nasal and sinus symptoms lasting at least 12 weeks with or without objective evidence of disease [2]. This symptom-based definition has been used to study the prevalence of CRS in Europe (10.9%), China (8%), and Brazil (5.5%), with a two-fold range of estimates [3–5]. The differences in prevalence estimates have been attributed to geographic and demographic variation across studies [3]. To date, there is no estimate for the prevalence of CRS using EPOS criteria in any U.S. population-based sample. The most widely cited study in the U.S. population, based on data from the National Health Interview Study, relied on self-reported doctor’s diagnosis of sinusitis, not CRS, and did not distinguish acute from chronic sinusitis [6].
The Chronic Rhinosinusitis Integrative Studies Program (CRISP) conducted the first US-based study to use the EPOS criteria to study the prevalence of chronic rhinosinusitis (CRS) using a general population sample from Pennsylvania [2]. The goals of this study were to describe the epidemiology of CRS in the general population and to identify associated sociodemographic factors, health behaviors and co-morbidities.
METHODS
Study Overview
A self-administered questionnaire was mailed to a stratified random sample of 23,700 primary care patients from Geisinger Clinic (GC) to obtain data on sociodemographics, sinus symptoms, other relevant symptoms (e.g., headache, fatigue), diagnoses, and diagnostic procedures and treatments for sinus disease. We estimated age- and sex-specific prevalence of EPOS-defined CRS epidemiologic criteria based on symptoms only, hereafter referred to as CRS, and characterized CRS symptom subgroups. The study was approved by Geisinger Health System’s Institutional Review Board.
Study Population and Subject Selection
GC serves more than 400,000 primary care patients across a 45 county area in Pennsylvania. The primary care population is representative of the general population in the region [7]. GC EHR data from 2006 to 2013 were used to categorize all adult primary care patients into one of three groups based on history of sinus-related diagnoses and/or evaluations in the EHR. The “CRS Code” group (n = 13,494) had at least two ICD-9 codes for CRS (International Classification of Disease [ICD]-9 codes 473.x or 471.x) associated with an outpatient, inpatient, or emergency department encounter or had at least one Current Procedural Terminology (CPT) code for sinus computerized tomography (CT), sinus endoscopy, or sinus surgery. The “Asthma/Allergy Code” group (n=49,918) had at least one ICD-9 code for asthma (493.x) or allergic rhinitis (477.x), or a single ICD-9 code for CRS associated with an outpatient, inpatient, or emergency department encounter. The “No Sinus Codes” group consisted of 200,769 patients with no history of asthma, allergic rhinitis, CRS, or sinus-related CPT codes in the record.
A random sample of 23,700 primary care patients, stratified by race (white/non-white) and EHR history (CRS Code, Asthma/Allergy Code, No Sinus Code), was sent the questionnaire. To ensure adequate sample sizes of non-white individuals and individuals with sinus diseases, we oversampled these groups. Sampling fractions included: 100% of individuals in the CRS Code group (n=12,549); 100% of non-white and 9.4% of white individuals in the Asthma/Allergy Code group (n=6093); 40% of non-white and 2% of white individuals in the No Sinus Code group (n=5058) (Table 1).
Table 1.
Characteristics of source population and patients who responded and did not respond to the baseline survey, CRISP epidemiology study, 2014
| Variable | Population eligible for the baseline survey | Population who received baseline survey | Baseline survey responders | Baseline survey non-responders | Responders vs. non-responders (p-value) |
|---|---|---|---|---|---|
|
| |||||
| Number, total | 200,725 | 23,700 | 7,847 | 15,853 | |
|
| |||||
| Age (years)*, mean (SD) | 50.15 (18.45) | 49.39 (17.38) | 55.04 (16.09) | 46.57 (17.33) | <0.0001 |
|
| |||||
| Race/ethnicity, n (%) | <0.0001 | ||||
| White | 192,356 (95.8) | 19,208 (81.0) | 7,095 (90.4) | 12,113 (76.4) | |
| Black | 3,984 (2.0) | 2,171 (9.2) | 342 (4.4) | 1,829 (11.5) | |
| Hispanic | 4,385 (2.2) | 2,321 (9.8) | 410 (5.2) | 1,911 (12.1) | |
|
| |||||
| Sex, n (%) | |||||
| Female | 113,592 (56.6) | 14,157 (59.7) | 4,921 (62.7) | 9,236 (58.3) | <0.0001 |
| Male | 87,133 (43.4) | 9,543 (40.3) | 2,926 (37.3) | 6,617 (41.7) | |
|
| |||||
| Medical record groups**, n (%) | <0.0001 | ||||
| CRS codes | 13,481 (6.7) | 12,549 (53.0) | 4,800 (61.2) | 7,749 (48.9) | |
| Asthma/allergy codes | 49,917 (24.9) | 6,093 (25.7) | 1,843 (23.5) | 4,250 (26.8) | |
| No sinus codes | 137,327 (68.4) | 5,058 (21.3) | 1,204 (15.3) | 3,854 (24.3) | |
|
| |||||
| History of receiving Medical Assistance, n (%) | 27,494 (13.7) | 4,427 (18.7) | 969 (12.4) | 3,458 (21.8) | <0.0001 |
|
| |||||
| Charlson co-morbidity index score, n (%) | |||||
| 0 | 47,153 (23.5) | 4,488 (18.9) | 851 (10.8) | 3,637 (22.9) | |
| 1 | 36,229 (18.1) | 4,802 (20.3) | 1,244 (15.9) | 3,558 (22.4) | |
| 2 | 31,013 (15.4) | 3,898 (16.5) | 1,348 (17.2) | 2,550 (16.1) | |
| 3 | 25,827 (12.9) | 3,258 (13.7) | 1,299 (16.5) | 1,959 (12.4) | <0.0001 |
| 4+ | 60,503 (30.1) | 7,254 (30.6) | 3,105 (39.6) | 4,149 (26.2) | |
Age at median date of survey return dates
Primary care patients selected based on evidence of CRS, asthma, and allergic conditions in electronic health record: high = two or more ICD-9 codes 471.x or 473.x or CPT codes for sinus surgery, sinus endoscopy or sinus CT; moderate = one ICD-9 code for 471.x or 473.x or two or more ICD-9 codes for asthma (493.x) or allergic rhinitis (477.x); low = does not meet criteria for moderate or high.
Development of the CRISP Population Questionnaire
The “CRISP Questionnaire” was the first to assess both past and current symptoms consistent with the EPOS epidemiologic CRS criteria and is self-administered. It was developed by a panel of experts specializing in otolaryngology, allergy, immunology, primary care, survey research, and epidemiology. Questionnaire items were derived from existing instruments or items were created de novo based on the literature and investigator consensus [8–10]. The EPOS criteria require the presence of at least two of four cardinal symptoms of obstruction, anterior/posterior discharge, smell loss, and facial pain/pressure, where at least one symptom is obstruction or discharge, lasting at least 12 weeks [2]. We separated pain/pressure and anterior/posterior discharge symptom categories, creating six questions on the following symptoms: obstruction, post-nasal drip, posterior nasal drainage, facial pain, facial pressure, and smell loss.
The CRISP Questionnaire first asked whether or not participants had ever had each of the six symptoms in their lifetime that lasted three months or more (yes/no). Affirmative responses were followed by questions on persistence of the symptoms over the past three months (never, once in a while, some of the time, most of the time, all of the time). Those respondents who reported one or more symptoms at least most of the time in the last three months were instructed to answer an additional 16 questions about the severity and degree of bother associated with each symptom over the past three months. These questions were followed by questions on symptoms and diagnoses of known or suspected CRS co-morbidities (e.g., allergy, asthma, migraine), CRS treatment history, and sociodemographics.
We pilot-tested the CRISP Questionnaire in 18 otolaryngology clinic patients. Afterwards, a research coordinator conducted a cognitive interview to identify aspects of the survey that were unclear and confirmed that questions were understood. The final survey consisted of 94 questions and required approximately 15–20 minutes to complete (available in online supplemental material).
Patient Recruitment
A pre-notification letter was mailed to all selected individuals in March 2014, two weeks before the CRISP Questionnaire was sent. The questionnaire was mailed with a cover letter and a $1 bill incentive. Those individuals who did not return the questionnaire within six weeks were sent a reminder letter and a second copy of the questionnaire. A third and final reminder and questionnaire were sent to non-respondents three months after the initial mailing.
CRS Classification and Symptom Subgroups
Operational criteria for current CRS, consistent with the EPOS epidemiological definition, required at least two of the six symptoms most or all of the time in the past three months, where at least one of the symptoms was obstruction, anterior nasal discharge, or posterior discharge (post-nasal drip). Past CRS was defined in the same manner and as ever occurring for at least three months, but not in the last three months. Current cases were assigned to one of four subgroups based on frequency of reported symptom combinations as well as on symptoms associated with (CRSwNP) and without (CRSsNP) nasal polyps [11–13]. Per EPOS, all individuals had to report obstruction or discharge (anterior or posterior) and were then assigned by what other symptom(s) was reported: 1) smell loss (SL), but no pain or pressure; 2) pain and/or pressure (PP), but no smell loss; 3) both smell loss and pain and/or pressure (PPSL); and 4) obstruction and discharge only, but no smell loss, pain, or pressure (OBS/DC).
Statistical Analysis
Selection bias was assessed by comparing respondents and non-respondents to the CRISP Questionnaire on EHR data that included age, race/ethnicity, sex, history of receiving Medical Assistance (surrogate for socioeconomic status (SES))[14] the Charlson co-morbidity index [15] and EHR sampling strata. Next, prevalence of CRS and CRS symptoms was estimated using SAS Proc SURVEYFREQ [16] to account for sampling fraction and adjust for differential selection using previously published probabilities and participation weights, a standard methodology for referring back to the source population [17–18].
We conducted unadjusted and adjusted analyses to identify sociodemographic, smoking, and health factors associated with CRS and CRS symptom subgroups. Sociodemographic factors evaluated included sex, race/ethnicity, age, and lifetime Medical Assistance status. Health outcomes included self-reported physician diagnosis of CRS, nasal polyps, asthma, aspirin-intolerant asthma, and hay fever, as well as migraine and fatigue determined by previously validated scales [19]. In unadjusted analysis we used the chi-square test to compare these factors between current CRS and never CRS and across CRS symptom subgroups. In adjusted analysis, we evaluated sociodemographic and health factors separately. For sociodemographic factors we created separate logistic regression models for current CRS (vs. never CRS) and each of the CRS symptom subgroups (vs. never CRS). Each model included sex (male, female); race/ethnicity (white, other), age (linear and quadratic terms to allow for non-linearity), smoking status (current, former, never), and Medical Assistance status (yes, no). To identify health outcomes associated with CRS, we used separate logistic models for each health outcome. Each model included a categorical variable for CRS status (never (reference), OBS/DIS, PP, SL, PPSL) as the primary independent variable and the sociodemographic and smoking variables as covariates.
All modeling was done using SAS Proc SURVEYLOGISTIC procedure, which accounted for the complex sampling design, and utilized sampling and participation weights [17–18]. Weighted analysis balances bias with precision in association estimates; bias is reduced with weighted analysis but precision is also reduced (larger standard errors leading to larger confidence intervals) [17–18]. One of the sampling weights was an order of magnitude larger than any other (weight = 150), because of the under-sampling of white subjects in the “No sinus codes” group. Using this weight severely inflated standard errors, a known consequence of large sampling weights [17–19], so it was truncated to the next largest value (weight = 32), a standard method for dealing with large weights.
Results
The response rate to the mailed questionnaire was 33% (i.e., 7,847 of 23,700 patients) (Table 1). Compared to non-respondents, respondents were significantly more likely to be female, white, have no history of Medical Assistance, have more co-morbidities, and have CRS diagnoses codes or sinus procedures in the EHR (p < 0.0001).
Prevalence of Current CRS, CRS Symptoms, and CRS Subgroups
After weighting for sampling proportions and participation rates, the estimated prevalence of current CRS in the source population was 11.9% and another 17.0% met criteria for past CRS. The prevalence of current increased from 18 to 59 years to a peak of 15.9% and then declined to a low of 6.8% after 69 years of age (p<0.0001) (Figure 1).
Figure 1.
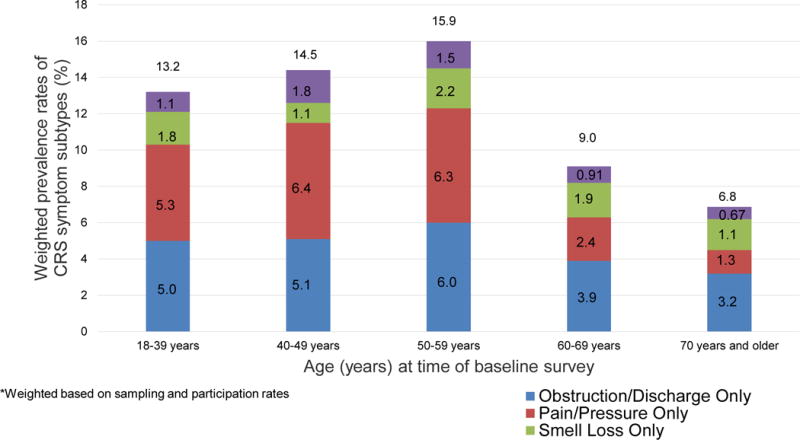
Weighted prevalence (%) of CRS symptom subtypes in a population-based sample by age*
*Weighted based on sampling and participation rates13.2
In order from most to least common, prevalence of current CRS symptoms were post-nasal drip (36.2%), nasal obstruction (27.0%) (Table 2), facial pressure (12.6%), loss of smell (9.4%), facial pain (9.1%), and nasal discharge (6.5%). Facial pain and facial pressure were more prevalent than smell loss up to age 59 years. Among those 70 years and older, smell loss was more prevalent (13.6%) than pain (5.0%), pressure (5.8%), and nasal discharge (6.6%).
Table 2.
| Variable | CRS Symptom
|
|||||
|---|---|---|---|---|---|---|
| Obstruction | Nasal discharge | Post-nasal drip | Loss of smell | Pain | Pressure | |
|
| ||||||
| Overall | 27.0 (24.5–29.4) | 6.5 (5.1–7.8) | 36.2 (33.4–39.0) | 9.4 (7.7–11.0) | 9.1 (7.5–10.7) | 12.6 (10.7–14.4) |
|
| ||||||
| Sex | ||||||
| Male | 29.0 (24.7–33.2) | 6.1 (4.0–8.2) | 33.4 (28.8–37.9) | 9.7 (7.1–12.3) | 5.4 (3.6–7.2) † | 8.8 (6.3–11.2) † |
| Female | 25.9 (22.9–29.0) | 6.7 (4.9–8.4) | 37.7 (34.2–41.2) | 9.2 (7.1–11.3) | 11.0 (8.7–13.2) | 14.5 (12.1–17.0) |
|
| ||||||
| Age (yrs) at baseline survey | ||||||
| < 40 | 30.4 (24.5–36.3) † | 7.1 (4.2–10.0) | 33.8 (27.6–40.1) | 5.7 (2.9–8.4) | 8.2 (5.0–11.3) † | 13.6 (9.3–17.8) † |
| 40–49 | 30.8 (24.4–37.1) | 4.9 (2.5–7.2) | 36.2 (29.3–43.0) | 9.4 (5.2–13.7) | 15.5 (10.2–20.7) | 21.1 (15.3–26.9) |
| 50–59 | 32.2 (27.1–37.4) | 8.9 (5.6–12.1) | 37.4 (32.0–42.8) | 8.3 (5.4–11.2) | 11.2 (7.7–14.7) | 15.1 (11.2–19.0) |
| 60–69 | 20.6 (16.1–25.2) | 3.9 (1.7–6.1) | 42.6 (36.2–49.0) | 10.8 (6.8–14.8) | 5.6 (3.0–8.3) | 7.4 (4.7–10.2) |
| 70+ | 19.0 (13.6–24.3) | 6.6 (3.1–10.2) | 29.2 (22.9–35.4) | 13.6 (8.8–18.3) | 5.0 (1.9–8.2) | 5.8 (2.6–9.0) |
Weighted based on sampling frames and participation rates.
Symptom reported at least most of the time in the last three months
Prevalence differed across characteristic categories (p<0.01)
Of the four symptom subgroups, the OBS/DC subgroup was the most prevalent (4.7%), followed by PP (4.3%), SL (1.8%), and then PPSL (1.2%). PP was more common in women than men (i.e., women: (5.3%, 95% CI: 4.2%–6.5% vs men: 2.5%, 95% CI: 1.6%–3.4%) and prevalence of PP differed by age, declining sharply after 59 years (p<0.0001). Prevalence of the other subgroups did not differ by sex or age.
Sociodemographic Factors Associated with Current CRS and CRS Subgroups
In unadjusted analysis, white race/ethnicity (vs. non-whites), younger age, current and former smoking (vs. never smoker), and a history of Medical Assistance were associated with current CRS (vs. never) (p<0.05). These associations remained in adjusted analysis. (Table 3). When CRS subgroups were modeled separately, these associations were no longer significant for some CRS symptom subgroups. Female sex was only associated with PP (OR, 95% CI) (1.81, 1.32–2.49). White race/ethnicity and younger age were each only associated with PP and OBS/DC. Being a current smoker was associated with PP (1.52, 1.03–2.24) and SL (1.8, 1.01–3.11), but former smoking was only associated with SL (1.90, 1.24–2.89). A history of Medical Assistance was only associated with PP (1.78, 1.20–2.64) and PPSL (3.55, 2.08–6.06), groups reporting pain and/or pressure.
Table 3.
Adjusted associations* (odds ratio, 95% confidence interval) of selected variables with current CRS status among all patients and in symptom subgroups** vs. no history of CRS (n = 3842)
| Variable | Current CRS Symptom Subgroups
|
||||
|---|---|---|---|---|---|
| Current CRS (all) n = 1873 |
OBS/DC n = 619 |
PP n = 689 |
SL n = 331 |
PPSL n = 234 |
|
|
| |||||
| Sex | |||||
| Female vs. male | 1.14 (0.94–1.37) | 0.85 (0.64–1.12) | 1.81 (1.32–2.49) | 1.22 (0.83–1.80) | 0.70 (0.43–1.16) |
|
| |||||
| Race/ethnicity | |||||
| Other vs. white | 0.53 (0.40–0.70) | 0.41 (0.25–0.67) | 0.50 (0.32–0.76) | 0.76 (0.42–1.37) | 0.85 (0.47–1.52) |
|
| |||||
| Age | 0.98 (0.98–0.99) | 0.98 (0.97–0.99) | 0.96 (0.95–0.98) | 1.00 (0.99–1.01) | 0.98 (0.96–1.00) |
|
| |||||
| Age-centered squared | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
|
| |||||
| Smoking status | |||||
| Current vs. never | 1.31 (1.01–1.70) | 0.99 (0.66–1.49) | 1.52 (1.03–2.24) | 1.78 (1.01–3.11) | 1.25 (0.69–2.28) |
| Former vs. never | 1.24 (1.01–1.52) | 0.95 (0.69–1.31) | 1.24 (0.90–1.72) | 1.90 (1.24–2.89) | 1.65 (0.95–2.86) |
|
| |||||
| Medical Assistance | |||||
| Ever vs. never | 1.71 (1.30–2.26) | 1.45 (0.92–2.29) | 1.78 (1.20–2.64) | 1.23 (0.69–2.19) | 3.55 (2.08–6.06) |
Abbreviations: OBS/DC = obstruction and discharge only; PP = pain and/or pressure with at least one cardinal symptom (obstruction and/or discharge); SL = smell loss with at least one cardinal symptom; PPSL = pain and/o pressure, smell loss, and at least one cardinal symptom.
Weighted based on sampling frames and participation rates. Weighted truncated so that the weight of the highest weighted group was weighted at the weight of the next highest weighted subgroup. Adjusted for sex, race/ethnicity, age, age-centered squared, smoking status, medical assistance, and age of onset.
Never: Did not report EPOS CRS symptoms in lifetime; Past: Meet EPOS CRS symptoms in lifetime but not currently; Current: Met EPOS CRS symptoms in the last 3 months;
Associations of CRS with Selected Health Conditions
In adjusted analysis, (Table 4), individuals in each of the CRS subgroups were more likely to report a self-report physician diagnosis of CRS than individuals who did not meet EPOS CRS criteria. Associations were weakest for those with OBS/DC (7.63, 5.44–10.71) and strongest for PPSL (17.67, 10.68–29.24). A similar pattern was observed for associations with self-reported physician diagnosis of nasal polyps, with the weakest associated among those with OBS/DC (3.35, 2.17–5.17) and the strongest for PPSL (6.88, 3.98–11.86). Aspirin-intolerant asthma was associated with all subgroups except for OBS/DC, with the strongest associations again observed for PPSL (5.82, 2.93–11.57). Each CRS symptom subgroup was associated with asthma and hay fever, with little difference across subgroups. Migraine was associated with all symptom subgroups, particularly for CRS that included pain and/or pressure. (PP and PPSL). Odds ratios were more than four times as high in the pain and pressure groups than in CRS without pain or pressure. A similar pattern was observed for severe fatigue.
Table 4.
Adjusted associations* (odds ratio, 95% confidence interval) of CRS symptom subgroups with selected health conditions†
| Dependent Variables | Current CRS Symptom Subgroups (vs. never) | |||
|---|---|---|---|---|
| OBS/DC n = 619 |
PP n = 689 |
SL n = 331 |
PPSL n = 234 |
|
| CRS | 7.63 (5.44–10.71) | 13.23 (9.49–18.43) | 15.62 (10.19–23.95) | 17.67 (10.68–29.24) |
| Nasal polyps | 3.35 (2.17–5.17) | 5.64 (3.80–8.35) | 5.24 (3.25–8.43) | 6.88 (3.98–11.86) |
| Asthma | 1.59 (1.18–2.14) | 1.98 (1.47–2.67) | 1.66 (1.08–2.53) | 1.76 (1.05–2.96) |
| Aspirin-intolerant asthma | 1.49 (0.82–2.72) | 2.74 (1.59–4.69) | 2.94 (1.47–5.88) | 5.82 (2.93–11.57) |
| Hay fever | 2.60 (1.97–3.42) | 2.80 (2.11–3.71) | 2.78 (1.88–4.10) | 3.29 (2.04–5.31) |
| Migraine headache | 2.73 (1.91–3.92) | 10.38 (7.41–14.54) | 2.37 (1.33–4.22) | 16.87 (8.83–32.21) |
| Fatigue, moderate (ref minimal) | 3.13 (2.13–4.60) | 3.18 (1.94–5.22) | 3.00 (1.72–5.26) | 5.86 (2.09–16.45) |
| Fatigue, severe (ref minimal) | 7.64 (5.00–11.68) | 16.81 (10.27–27.52) | 8.79 (4.87–15.88) | 26.68 (9.43–75.49) |
Abbreviations: OBS/DC = obstruction and discharge only; PP = pain and/or pressure with at least one cardinal symptom (obstruction and/or discharge); SL = smell loss with at least one cardinal symptom; PPSL = pain and/o pressure, smell loss, and at least one cardinal symptom.
Weighted based on sampling frames and participation rates. Weighted truncated so that the weight of the highest weighted group was weighted at the weight of the next highest weighted subgroup. Adjusted for sex, race/ethnicity, age, smoking status, and Medical Assistance.
Self-reported doctor diagnosis for all conditions except for migraine and fatigue. Migraine and fatigue were based on previously validated scales.8–9
Discussion
This is the first study in the U.S. to estimate the prevalence of CRS in a population-based study using the EPOS epidemiologic definition for CRS. We found that CRS is highly a prevalent condition, consistent with studies in similar populations outside the U.S. that have used EPOS criteria [5]. Differential age and sex prevalence patterns by symptom subgroups may be indicative of differences in persistence of disease. These subgroups also differed by risk factors and health outcomes, providing some evidence that CRS symptom subgroups may represent distinct disease processes. This work is an important step towards better understanding of the heterogeneity of sinus symptoms in the general population.
Our findings are consistent with the prevalence estimate for CRS in Europe of 10.9% [5] based on a survey of CRS in adults in 12 European countries. As in other studies, we found a higher prevalence of CRS symptoms in women and a decline in prevalence after age 60 years [3–5,20]. There are two possible interpretations for why cross-sectional prevalence estimates decline with age. The most common explanation is that prevalent cases of CRS are remitting faster than new incident cases are occurring [21]. Alternatively, cross-sectional studies represent different age cohorts that may experience differences in exposure to CRS disease mediators that are more intensive for younger age groups than for older age groups. Evidence favors the former explanation. As noted, the age prevalence pattern we observed is consistent with other studies of CRS. Moreover, a number of chronic episodic conditions exhibit a similar age pattern with increasing prevalence to age 40s to 50s and then a decline in prevalence thereafter [22,23].
Our examination of CRS symptom subgroups revealed that prevalence only differs by age for individuals with pain and pressure and no smell loss. While pain and pressure symptoms appear to drop off after middle age, there is gradual decline with age in CRS with smell loss. Differences by symptom subgroups may reflect differences in persistence and remission patterns. Alternatively, smell loss may persist with age, in part, due to age-related decline in olfactory function that is unrelated to sinus disease [24].
Consistent with prior studies, we found CRS to be associated with migraine [25,26]. Potential mechanisms for this association are the crossover interactions of neurogenic and immunogenic inflammation [26]. Alternatively, the association could be due to the overlapping symptoms of these conditions. Migraine is frequently misclassified as sinus headache and frequently accompanied by sinus symptoms [25,26]. In our study, CRS with facial pain and/or pressure has a higher risk of migraine compared to CRS in the absence of these overlapping symptoms. Additionally, the age- and sex-specific prevalence of CRS with pain and pressure mirrored that of migraine; the prevalence of migraine declines after middle age, prevalence is higher in women than in men [27]. Without a clinical exam it is challenging to parse out co-morbid associations from the misclassification of CRS or migraine status.
Smoking was associated with CRS, however, smoking was only predictive of two of the four symptom subgroups. Symptom subgroup differences may be indicative of different pathophysiologic mechanisms that may underlie the different symptom profiles. Prior population-based studies of tobacco use and CRS have produced mixed results [3,5,20]. No prior study has evaluated whether the risk associated with tobacco use differs by symptom profile. Given the differential association of tobacco use with symptom subgroups, conflicting study findings may be due, in part, to differences in the distribution of symptoms in these study populations.
We found an association between a history of receiving Medical Assistance, a surrogate for low family SES, and CRS that was stronger than for all the other variables we examined, including smoking. Notably, this association was strongest for the PPSL group, the group reporting the greatest number of the EPOS symptoms. Similarly, Kilty and colleagues reported that having education less than high school (vs. post-secondary education) and having low income (vs. high) were both associated with higher scores on the sinonasal assessment questionnaire among patients receiving care for CRS from a rhinology clinic [28]. However, a study by Philpott and colleagues of CRS patients from specialty clinics found no association between CRS and social class, index of multiple deprivation, and education.[29] Surrogate measures of SES (i.e. Medical Assistance and income) are not always interchangeable, possibly accounting for conflicting findings. [30] The mechanism of the association between Medical Assistance and CRS severity is unknown, but could be, in part, that Medical Assistance is a surrogate for a number of environmental exposures that differ by SES [31].
The current CRS symptom subgroups were differentially associated with health outcomes, including nasal polyps, aspirin-intolerant asthma, and hay fever, as well as migraine and fatigue. Similar associations have been reported in prior studies of CRS [32–35] but not by symptom status. We found CRS subgroups were most associated with health outcomes when both smell loss and pain and/or pressure were part of the profile, while associations were weakest among CRS profiles without smell loss, pain, or pressure. Differences by subgroup may be evidence of differences in pathophysiology, analogous to differences between CRSwNP and CRSsNP [36]. Alternatively, there may be wide variation in the positive predictive value of various symptom groupings within the EPOS criteria to identify patients with true disease. The positive predictive value of the symptom profile for true disease may be lower among individuals with more common symptoms (e.g., obstruction and discharge) than among individuals who report all of the 6 symptoms of CRS and, thus, may result in the relatively weaker associations that were observed for this group.
Among the strengths of this study was that it was conducted in a primary care patient population. Most of what is known about CRS is based on tertiary care studies that often capture only individuals with the most severe symptoms [1]. Our study was also the first epidemiological study of CRS in the U.S. that classified patients based on EPOS epidemiological criteria. Moreover, it was the first population-based study to evaluate associations of selected factors with CRS subgroups. The varying patterns of associations provide some evidence that the heterogeneity in symptom profiles may represent heterogeneity in the underlying pathologic process consistent with. Recent biomarker studies have shown many distinct endotypes of disease [37]. Future studies could attempt to cross reference the CRS symptom subgroups in our study with the endotypes that emerge in the biomarker studies to begin to relate the inflammatory mediators that drive the distinct symptoms combinations.
This study had a few limitations. First, it was not feasible to obtain CTs from the almost 24,000 patients who received a survey. As a result, our prevalence estimates, like all previously published population-based prevalence estimates, were dependent upon symptom reporting. Second, while our response rate was below 40%, our ability to select patients based on electronic health records allowed us to appropriately account for differential participation rates. Third, EPOS criteria does not specify how frequent sinus symptoms have to have occurred over 12 weeks. Criteria is based on duration of symptoms, not frequency, requiring only that symptoms be present for longer than 12 weeks.2 We required that symptoms be present at least “most of the time” for the last 3 months. By applying a threshold of “most of the time” it is possible that our findings underestimate the prevalence of CRS, but this is not known because prior studies have not consistently measured frequency of symptom occurrence. Finally, the findings of our study should not be interpreted as an estimate of the prevalence of CRS in the U.S., as the study population is not reflective of the U.S. population. However, the GC primary care population is representative of the region’s population, thus our findings provide a valid estimate of the prevalence of CRS in the region studied. [32]
Conclusions
Symptoms compatible with CRS were common but also part of heterogeneous profiles among patients in the general population in central and northeastern Pennsylvania. Prevalence and associations with risk factors and health conditions differed among symptom subgroups. This may be indicative of differences in etiology and natural history that have important implications for targeted disease prevention and management.
Supplementary Material
Acknowledgments
The authors would like to thank Cara Nordberg, MPH, for providing statistical support in the preparation of the manuscript.
Funding: This study was supported in part by Grants R37HL068546 and U19AI106683 (Chronic Rhinosinusitis Integrative Studies Program (CRISP)) from the NIH, and by the Ernest S. Bazley Foundation
Footnotes
Drafting/revision of the manuscript, final approval of the manuscript, interpretation of findings, and agree to be accountable for all aspects of the work: All. Conception and design: AGH, ASS, BKT, RPS, RCK, BSS. Acquisition and analysis: AGH, AJY, WF, AL, BSS.
Conflict of Interest: The authors have no conflicts of interest to report.
References
- 1.Tan BK, Kern RC, Schleimer RP, Schwartz BS. Chronic rhinosinusitis: The unrecognized epidemic. Amer J Respiratory and Critical Care Medicine. 2013;188(100):1275–1277. doi: 10.1164/rccm.201308-1500ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fokkens WJ, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 3.Pilan RR, Pinna F, Bezerra TF, Mori RL, Padua FG, Bento RF, Perez-Novo C, Bachert C, Voegels RL. Prevalence of chronic rhinosinusitis in Sao Paulo. Rhinology. 2012;50:129–138. doi: 10.4193/Rhino11.256. [DOI] [PubMed] [Google Scholar]
- 4.Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ, Zhu DD, Lv W, Lui SX, Li PZ, Ou CQ, Xu G. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70:533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hastan D, Fokkens Wj, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, et al. Chronic rhinosinusitis in Europe – an underestimated disease. A GA2LEN study. Allergy. 2011;66:1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults. National Health Interview Survey. 2012 National Center for Health Statistics. Vital Health Stat. 2014;10(260) [PubMed] [Google Scholar]
- 7.Liu AY, Curriero FC, Glass TA, Stewart WF, Schwartz BS. The contextual influence of coal abandoned mine lands in communities and type 2 diabetes in Pennsylvania. Health Place. 2013;22:115–122. doi: 10.1016/j.healthplace.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J, et al. A self-administered screener for migraine in primary care: The ID migraine validation study. Neurology. 2003;61(3):375–382. doi: 10.1212/01.wnl.0000078940.53438.83. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health. Patient Reported Outcomes Measurement Information System (PROMIS) Fatigue Short Form 8a. 2015 Available: www.assessmentcenter.net [accessed 10 October 2015]
- 10.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, et al. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respi Critical Care Med. 2013;188(3):309–318. doi: 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerji A, Piccirillo JF, Thawley SE, et al. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21:19–26. doi: 10.2500/ajr.2007.21.2979. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya B. Assessing the additional disease burden of polyps in chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2009;118:185–189. doi: 10.1177/000348940911800305. [DOI] [PubMed] [Google Scholar]
- 13.Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology. 2010;48:305–311. doi: 10.4193/Rhin08.137. [DOI] [PubMed] [Google Scholar]
- 14.Casey JA, Curriero FC, Cosgrove SE, Nachman KE, Schwartz BS. High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern Med. 2013;172(21):1980–1990. doi: 10.1001/jamainternmed.2013.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, Gold J. J Clinic Epidemiology. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 16.SAS Institute. The SAS system for Windows. Release 9.2. SAS Institute; Cary, NC: 2011. [Google Scholar]
- 17.Chowdhury S, Khare M, Wolter K. Weight trimming in the National Immunization Survey. Proceedings of the Joint Statistical Meetings Section on Survey Research methods, American Statistical Association. 2007 [Google Scholar]
- 18.Little RJA, Heeringa S, Lepkowski J, Kessler RC. Assessment of weighting methodology for the National Comorbidity Survey. American Journal of Epi. 1997;146(5):439–449. doi: 10.1093/oxfordjournals.aje.a009297. [DOI] [PubMed] [Google Scholar]
- 19.Tustin AW, Hirsch AG, Rasmussen SG, Casey JA, Schwartz BS. Associations between unconventional natural gas development and nasal and sinus, headache, and fatigue symptoms in Pennsylvania. Environmental Health Perspectives. 2016 doi: 10.1289/EHP281. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. The Laryngoscope. 2003;113(7):1199–1205. doi: 10.1097/00005537-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Roy J, Stewart WF. Methods for estimating remission rates from cross-sectional survey data: application and validation using data from a national migraine study. Am J Epidemiol. 2011;173(8):949–55. doi: 10.1093/aje/kwq464. [DOI] [PubMed] [Google Scholar]
- 22.Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Semin Neurol. 2010;30(2):107–19. doi: 10.1055/s-0030-1249220. [DOI] [PubMed] [Google Scholar]
- 23.Gershon A, et al. The course of asthma activity: a population study. J Allergy Clin Immunol. 2012;129(3):679–86. doi: 10.1016/j.jaci.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. The rate of age-related olfactory decline among the general population of older U.S. adults. J Gerontol A Biol Sci Med Sci. 2015;70(11):1435–1441. doi: 10.1093/gerona/glv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eross E, Dodick D, Eross M. The sinus, allergy, and migraine study. Headache. 2007;47(2):213–224. doi: 10.1111/j.1526-4610.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 26.Cady RK, Schreiber CP. Sinus headache or migraine? Neurology. 2002;58(9, suppl 6):S10–S14. doi: 10.1212/wnl.58.9_suppl_6.s10. [DOI] [PubMed] [Google Scholar]
- 27.Lipton R, Bigal M. Migraine: epidemiology, impact, and risk factors for progression. Headache. 2005;45(suppl 1):S3–S13. doi: 10.1111/j.1526-4610.2005.4501001.x. [DOI] [PubMed] [Google Scholar]
- 28.Kilty S, McDonald J, Johnson S, Al-Mutairi Socioeconomic status: a disease modifier of chronic rhinosinusitis. Rhinology. 2011;49(5):533–537. doi: 10.4193/Rhino10.298. [DOI] [PubMed] [Google Scholar]
- 29.Philpott C, Erskine S, Hopkins C, et al. A case-control of medical, psychological, and socioeconomic factors influencing the severity of chronic rhinosinusitis. Rhinology. 2016;54(2):134–140. doi: 10.4193/Rhino15.272. [DOI] [PubMed] [Google Scholar]
- 30.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: One size does not fit all. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 31.Evans GW, Kantrowitz E. Socioeconomic status and health: The potential role of environmental risk exposure. Annual Review of Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- 32.Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. JACI. 2013;131(5):1350–1360. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung S, Chen P, Lin H, Hung S. Comorbidity profile of chronic rhinosinusitis: A population-based study. The Laryngoscope. 2014;12(4):1536–1541. doi: 10.1002/lary.24581. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch AG, Xiaowei Y, Sundaresan A, Tan BK, Schleimer RP, Kern RC, Kennedy TL, Greene JS, Schwartz BS. Five-year risk of incident disease following a diagnosis of chronic rhinosinusitis. Allergy. 2015;70(12):1613–1621. doi: 10.1111/all.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min J, Tan BK. Risk factors for chronic rhinosinusitis. Current Opinion Allergy Clinical Immunology. 2015;15(1):1–13. doi: 10.1097/ACI.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachert C, Zhang N, van Zele T, Gevaert P. Chronic rhinosinusitis: From one disease to different phenotypes. Pediatr Allergy Immunol. 2012;23(Suppl 22):2–4. doi: 10.1111/j.1399-3038.2012.01318.x. [DOI] [PubMed] [Google Scholar]
- 37.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. JACI. 2016;137(5):1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


