Abstract
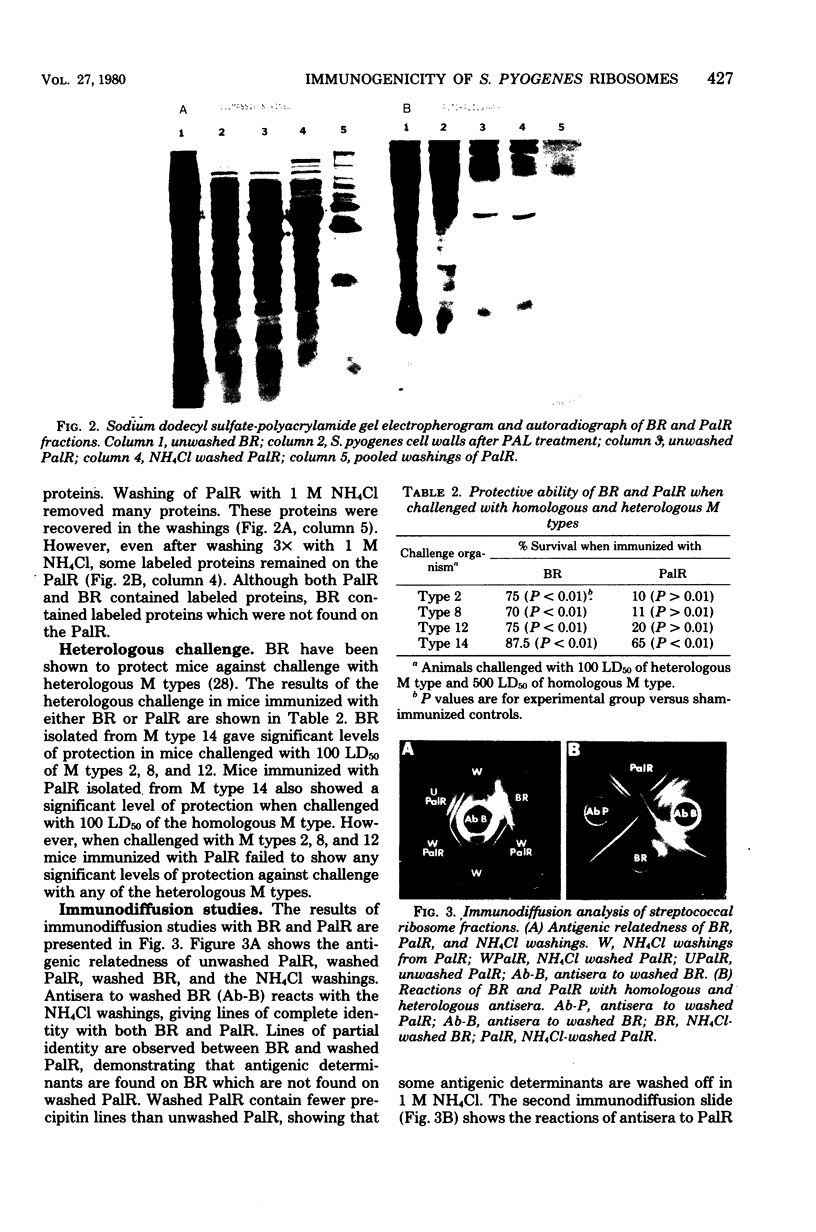
Ribosomal fractions isolated from Streptococcus pyogenes by physical and enzymatic disruption of the cell wall were found to provide protection in mice against challenge with the homologous M type. Although ribosomal fractions isolated by physical disruption of the cells also provided protection against challenge with several heterologous M types, ribosomal fractions from enzymatically lysed cells did not provide protection against any of the heterologous M types. Ribosomes isolated by either method were found to be contaminated with cell surface proteins. Chemical analysis of the ribosomes showed a greater protein:ribonucleic acid ratio in ribosomes from physically disrupted cells than in ribosomes from enzymatically disrupted cells (2:1 versus 1:1). Antisera to ribosomes isolated from physically disrupted cells detected many more antigenic determinants on ribosomes isolated from enzymatically disrupted cells than did the corresponding homologous antisera. Immunodiffusion analysis suggested that ribosomes isolated from physically disrupted cells may contain cell wall antigenic determinants which are present on ribosomes isolated from enzymatically disrupted cells in a partially degraded form. Washing of ribosomes in high-molarity salt solutions suggested that some of the contaminating cell wall proteins are tightly bound to the ribosomes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKULIS S. S., SMITH C., BOLTRALIK J. J., HEYMANN H. STRUCTURE OF STREPTOCOCCAL CELL WALLS. IV. PURIFICATION AND PROPERTIES OF STREPTOCOCCAL PHAGE MURALYSIN. J Biol Chem. 1964 Dec;239:4027–4033. [PubMed] [Google Scholar]
- Corbel M. J. The immunogenic activity of ribosomal fractions derived from Brucella abortus. J Hyg (Lond) 1976 Feb;76(1):65–74. doi: 10.1017/s0022172400054954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit C., Tewari R. P. Immunogenicity of Ribosomal Preparations from Yeast Cells of Histoplasma capsulatum. Infect Immun. 1974 Nov;10(5):1091–1097. doi: 10.1128/iai.10.5.1091-1097.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops P., Prather N. E., Berry J., Ravel J. M. Evidence for an extrinsic immunogen in effective ribosomal vaccines from Salmonella typhimurium. Infect Immun. 1976 Apr;13(4):1184–1192. doi: 10.1128/iai.13.4.1184-1192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R., Gregory B., Naylor J., Actor P. Isolation of protective somatic antigen from Vibrio cholerae (Ogawa) ribosomal preparations. Infect Immun. 1972 Aug;6(2):156–161. doi: 10.1128/iai.6.2.156-161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. I. Immunogenicity of ribosomal fractions isolated from Salmonella typhimurium and Yersinia pestis. Infect Immun. 1972 Jun;5(6):947–952. doi: 10.1128/iai.5.6.947-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. doi: 10.1128/iai.8.3.395-400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOR F. S., COLE R. M. Preparation and antigenicity of M protein released from group A, type 1 streptococcal cell walls by phage-associated lysin. J Exp Med. 1960 Jul 1;112:77–96. doi: 10.1084/jem.112.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M. Studies on the bacteriophages of hemolytic streptococci. II. Antigens released from the streptococcal cell wall by a phage-associated lysin. J Exp Med. 1958 Dec 1;108(6):803–821. doi: 10.1084/jem.108.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieberman M. M. Direct evidence for the presence of lipopolysaccharide components in Pseudomonas ribosomal vaccine. Infect Immun. 1977 Aug;17(2):471–473. doi: 10.1128/iai.17.2.471-473.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M., McKissock D. C., Wright G. L. Passive immunization against Pseudomonas with a ribosomal vaccine-induced immune serum and immunoglobulin fractions. Infect Immun. 1979 Feb;23(2):509–521. doi: 10.1128/iai.23.2.509-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M. Pseudomonas ribosomal vaccines: preparation, properties, and immunogenicity. Infect Immun. 1978 Jul;21(1):76–86. doi: 10.1128/iai.21.1.76-86.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina S., Vas S. I., Robson H. G. Effect of nonspecific stimulation on the defense mechanisms of inbred mice. J Immunol. 1975 Jun;114(6):1720–1725. [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Identification of protective cell surface proteins in ribosomal fractions from Salmonella typhimurium. Infect Immun. 1979 Jun;24(3):808–816. doi: 10.1128/iai.24.3.808-816.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Role of endotoxin contamination in ribiosomal vaccines prepared from Salmonella typhimurium. Infect Immun. 1977 Jul;17(1):98–104. doi: 10.1128/iai.17.1.98-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Variability of protection in inbred mice induced by a ribosomal vaccine prepared from Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):652–659. doi: 10.1128/iai.14.3.652-659.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Schalla W. O., Johnson W. Immunogenicity of ribosomal vaccines isolated from group A, type 14 Streptococcus pyogenes. Infect Immun. 1975 Jun;11(6):1195–1202. doi: 10.1128/iai.11.6.1195-1202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. doi: 10.1128/iai.6.3.377-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen C. L., Johnson W. Humoral immunity to Streptococcus pneumoniae induced by a pneumococcal ribosomal protein fraction. Infect Immun. 1976 Aug;14(2):345–354. doi: 10.1128/iai.14.2.345-354.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Weiss E. Response of mice to injection of ribosomal fraction from group B Neisseria meningitidis. Infect Immun. 1972 Sep;6(3):355–363. doi: 10.1128/iai.6.3.355-363.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Snyder I. S. Protection against pneumococcal infection by a ribosomal preparation. Infect Immun. 1971 Jan;3(1):16–23. doi: 10.1128/iai.3.1.16-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S., Berry L. J. Immunity induced by ribosomal extracts from Staphylococcus aureus. J Reticuloendothel Soc. 1970 Jul;8(1):66–73. [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Failure of synthetic polynucleotides to affect the immunogenicity of mycobacterial ribonucleic Acid and ribosomal protein preparations. Infect Immun. 1971 Jan;3(1):149–153. doi: 10.1128/iai.3.1.149-153.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]