Abstract
Autism spectrum disorder (ASD) is exceptionally heterogeneous in both clinical and physiopathological presentations. Clinical variability applies to ASD-specific symptoms and frequent comorbid psychopathology such as emotional lability (EL). To date, the physiopathological underpinnings of the co-occurrence of EL and ASD are unknown. As a first step, we examined within-ASD inter-individual variability of EL and its neuronal correlates using resting-state functional magnetic resonance imaging (R-fMRI). We analyzed R-fMRI data from 58 children diagnosed with ASD (5–12 years) in relation to the Conners' Parent Rating Scale EL index. We performed both an a priori amygdala region-of-interest (ROI) analysis, and a multivariate unbiased whole-brain data-driven approach. While no significant brain-behavior relationships were identified regarding amygdala intrinsic functional connectivity (iFC), multivariate whole-brain analyses revealed an extended functional circuitry centered on two regions: middle frontal gyrus (MFG) and posterior insula (PI). Follow-up parametric and nonparametric ROI-analyses of these regions revealed relationships between EL and MFG- and PI-iFC with default, salience, and visual networks suggesting that higher-order cognitive and somatosensory processes are critical for emotion regulation in ASD. We did not detect evidence of amygdala iFC underpinning EL in ASD. However, exploratory whole-brain analyses identified large-scale networks that have been previously reported abnormal in ASD. Future studies should consider EL as a potential source of neuronal heterogeneity in ASD and focus on multinetwork interactions.
Keywords: : autism, CWAS, emotion dysregulation, emotional lability
Introduction
Current models of autism spectrum disorder (ASD) highlight its remarkable heterogeneity (Betancur, 2011; Lai et al., 2014; Lecavalier, 2006; Pelphrey et al., 2011). This is evident in regard to both ASD-specific symptoms and comorbid psychopathology (Grzadzinski et al., 2013; Lai et al., 2014). A relevant source of comorbid psychopathology in ASD is emotional lability (EL), which affects nearly 25% of children with ASD and challenges diagnosis and treatment (Simonoff et al., 2012). EL refers to the frequent display of negative emotions, often manifesting as severe temper tantrums in children, which can result in aggressive behavior (Lai et al., 2014). EL and its regulation are thought to be distinct, but intertwined processes (Adolphs et al., 1999; Cole et al., 2004; LeDoux, 1996; Wilensky et al., 2006); increased EL is associated with poor emotion regulation (e.g., Roy et al., 2013). The mechanisms underlying EL in individuals with ASD have not been directly examined (Mazefsky et al., 2013). Yet, identifying the neural mechanisms that place some children with ASD at greater risk for increased EL represents a critical first step toward a finer neurophenotypical characterization of the disorder.
This study aims to characterize the neuronal basis of inter-individual variability in EL among children with ASD. It builds on converging models of emotion regulation in typical development that involve, and extend beyond, the amygdala (Ahmed et al., 2015; Gee et al., 2013; McRae et al., 2012), and the implication of disruptions in overlapping large-scale functional circuitry in ASD (Minshew and Williams, 2007). Most research focusing on the brain circuitry involved in EL in typical (Etkin et al., 2015) and clinical populations (Hulvershorn et al., 2014; Leibenluft, 2011; Shaw et al., 2014) has focused on the amygdala. Notably, amygdalar disruption in ASD has been widely reported (Bachevalier and Loveland, 2006; Dalton et al., 2005; Green et al., 2013; Minshew and Keller, 2010; Monk et al., 2010; Nordahl et al., 2011; Pitskel et al., 2014; Richey et al., 2015; von dem Hagen et al., 2014; Wicker et al., 2008). Specifically, in ASD, the amygdala has been shown to exhibit increased volume growth rate (Mosconi et al., 2009; Nordahl et al., 2011), hyper-activation in task-based face processing (Dalton et al., 2005; Green et al., 2013), and reduced functional connectivity with limbic frontal regions such as orbitofrontal cortex and medial prefrontal cortex during both task-based (Monk et al., 2010) and resting-state functional magnetic resonance imaging (R-fMRI; von dem Hagen et al., 2014). The latter findings suggested the hypothesis that alterations in functional connectivity of the same amygdala circuits involved in typical emotion regulation may also underlie inter-individual differences in EL among children with ASD. This study aims to examine this directly using R-fMRI, an approach increasingly used to characterize intrinsic functional connectivity (iFC) in ASD (Vissers et al., 2012).
Beyond amygdala circuitry, a larger network of fronto-parietal regions has been implicated in EL in typical individuals (Etkin et al., 2015) and clinical populations (e.g., attention-deficit/hyperactivity disorder [ADHD]; Brotman et al., 2010; Posner et al., 2013, 2014). Although studies have examined the response of these regions to emotion regulation tasks in ASD (Pitskel et al., 2014; Richey et al., 2015), and abnormal functional connectivity of this fronto-parietal network has been reported (see review: Geschwind and Levitt, 2007), none have examined whether iFC of these circuits may reflect variability of EL traits in ASD (Kelly et al., 2012). Thus, in addition to the amygdala-based analyses, we explore the larger functional connectome using a novel, whole-brain approach: Multivariate Distance Matrix Regression (MDMR; Shehzad et al., 2014). Combining these complementary analytic strategies (i.e., seed-based and unbiased whole-brain connectivity) allows us to systematically survey the relationship between iFC and inter-individual differences in EL in a relatively large sample of children with ASD.
Methods
Participants
We examined R-fMRI data from 58 children diagnosed with ASD (54 boys; 7–12.9 years) selected from a larger group participating in ongoing studies at the NYU Child Study Center. Upper and lower age boundaries were chosen based on evidence suggesting that EL is relatively stable at this age in typically developing children (Kopp, 2009). A DSM-IV-TR diagnosis of ASD, ability to speak English, and IQ >79 were also required. Participants were excluded if they were taking antipsychotic medications or if they had a chronic neurological or medical condition. Parents/legal guardians and children provided written informed consent and assent, respectively, per procedures approved by the NYU Langone School of Medicine and the NYU Institutional Review Boards.
From an initial sample of 77 children with ASD, we excluded 19 either because of poor amygdala coverage (n = 11) or excessive motion (n = 8); this resulted in a final sample of 58 children with ASD. There were no significant differences between excluded and included participants on age, IQ score, Autism Diagnostic Observation Schedule (ADOS) severity score, or EL (data not shown).
Clinical assessment
Clinicians classified children as having DSM-IV-TR diagnoses of Autistic Disorder (n = 46), Asperger's Disorder (n = 5), or Pervasive Developmental Disorder Not-Otherwise-Specified (n = 7) based on review of the child's history and administration of the ADOS, Module 3 (research reliable n = 57; Lord et al., 2000), and Autism Diagnostic Interview-Revised (ADI-R, research reliable n = 52; Lord et al., 1994). We retrospectively assigned a DSM-5 ASD diagnosis using an approach similar to Huerta et al. (2012) based on review of ADI-R and/or ADOS scores. All but one participant met retrospective DSM-5 criteria.
Psychiatric comorbidity was assessed based on parent semi-structured interview using the Schedule of Affective Disorders and Schizophrenia for Children Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997). IQ was measured using the Differential Ability Scales-second edition (DAS; Elliot, 2007).
EL was assessed using the EL scale of the Conners Parent Rating Scale-Revised-Long Version (CPRS-R-LV; Conners et al., 1998), a widely used, treatment sensitive, and well-normed 80-item instrument assessing psychopathology in children. The EL scale is comprised of three items: “Exhibits temper outbursts,” “Cries often and easily,” and “Mood changes quickly and drastically.” Previous studies have demonstrated associations between scores on this measure and iFC in other clinical populations (Hulvershorn et al., 2014; Posner et al., 2013). Autism severity was assessed using the total score from the Social Responsiveness Scale (SRS; Constantino and Todd, 2003), a 65-item questionnaire measuring ASD severity as a continuous variable. To focus on the specific neural effects of EL, the SRS total score was included in the imaging analyses as a covariate.
MRI acquisition
At least 1 week before imaging, all children were trained with one or more “mock” scan sessions to remain still while watching a movie and a blank screen with motion-tracking feedback was provided. Imaging was performed using the NYU Center for Brain Imaging Siemens Allegra 3.0T Scanner (Siemens; Iselin, NJ). Children completed a 6-min resting scan while looking at a white crosshair on a black screen. The scan comprised 180 contiguous whole-brain functional volumes, acquired using a multi-echo echo planar imaging (EPI) sequence (repetition time = 2000 ms; echo time = 30 ms; flip angle = 90°; 33 slices; matrix = 64 × 64; voxel size = 3 × 3 × 4 mm). Analyses focused on the conventional fully sampled image with echo time of 32.92 ms. To minimize data loss, two EPI sequences were obtained when possible. The first was used for 51 children and the second was used for seven children who moved excessively during the first. For spatial normalization and localization, a high-resolution T1-weighted anatomical image was acquired using a magnetization prepared gradient echo sequence (time to repetition = 2500 ms; echo time = 4.35 ms; inversion time = 900 ms; flip angle = 8; 176 slices, field of view = 256 mm). An eye-tracker was used to monitor whether the child's eyes were open or closed during scans. Most children (n = 52) completed the scan with eyes open; six kept their eyes closed.
MRI analyses
Individual- and group-level analyses were conducted using an alpha version (0.3.4) of The Configurable Pipeline for the Analysis of Connectomes (C-PAC; http://fcp-indi.github.io). C-PAC is a configurable, open-source, Nipype-based (http://nipy.org/nipype), automated processing pipeline for R-fMRI data. Preprocessing consisted of slice time correction (first slice as reference, interleaved acquisitions, Fourier interpolation), three-dimensional motion correction, despiking (removal of extreme time series outliers), spatial smoothing (full-width half maximum = 6 mm), mean-based intensity normalization of all volumes by the same factor, and temporal bandpass filtering (0.01–0.1 Hz). Structural and functional images were registered, coregistered, and normalized to a common stereotaxic space (Montreal Neurological Institute [MNI]) using ANTs software (www.picsl.upenn.edu/ANTS; Avants et al., 2011). Single participant nuisance regression included 24 Friston motion parameters (Friston et al., 1996) and five CompCor signals (Behzadi et al., 2007); all analyses were Gaussian random field (GRF) corrected at p < 0.05, Z > 2.3. Mean frame-wise displacement was computed per Jenkinson et al. (2002) and these values were used as nuisance regressors in group analyses.
Amygdala region-of-interest analysis
At the individual level, we measured the iFC of the amygdala bilaterally, chosen a priori as an region-of-interest (ROI; based on Harvard Oxford Atlas 50% probability as implemented in FSL) as in earlier studies (Hulvershorn et al., 2014). The average time series of all voxels within each ROI was computed, and correlations between this time series and those of all other brain voxels were assessed (GRF correction: p < 0.05, Z > 2.3). This resulted in individual participant-level maps of all voxels exhibiting significant iFC with the amygdala. Group-level analyses were conducted using a random-effects, ordinary least-squares model, including one group mean predictor (ASD), four nuisance covariates (demeaned age, sex, mean frame-wise displacement, and total SRS score) and one covariate of interest (EL from CPRS-R-LV); all were GRF corrected at p < 0.05, Z > 2.3. Total SRS score was included to statistically control for social impairments and isolate the effects of EL.
Multivariate distance matrix regression
For a whole-brain exploration of relationships between EL and iFC, we employed the MDMR method proposed by Shehzad et al. (2014). Briefly, subject-level iFC was first assessed using temporal Pearson correlations at the voxel level at 4 mm3 resolution for computational feasibility. Computations were restricted to voxels falling within a tissue prior gray matter mask provided with FSL (probability > 25%) common to all participants. This resulted in a v × v correlation matrix, where v is the number of 4 mm3 voxels in the 100% group-level whole brain mask (v = 22671). Second, for each voxel, the distance between iFC patterns was calculated (i.e., each voxel's correlation with the rest of the brain) for every possible pairing of participants in the dataset, resulting in an n × n matrix of distances among the 58 participants for each voxel. Third, MDMR tested how well EL explained the distances between participants (nuisance covariates: age, sex, mean frame-wise displacement, and total SRS score). Statistical significance was calculated using a pseudo-F statistic with correction for multiple comparisons. For any region whose iFC was identified as significantly related to EL by MDMR, we conducted follow-up seed-based analyses using ROI methods as described.
As recent work by Eklund et al. (2016) raised concerns about potentially inflated type-I error rates using GRF theory cluster thresholding approaches, we repeated these voxelwise follow-up seed-based analyses with a nonparametric approach using FSL “Randomise” (Winkler et al., 2014). We corrected for multiple comparisons using cluster-based permutation (n = 5000) and set statistical significance at Threshold-Free Cluster Enhancement (TFCE) p < 0.05 to control for family-wise error rate at α = 0.05.
Results
Group characteristics
Table 1 summarizes the demographic and clinical characteristics of the sample. EL T-scores ranged from 41 to 88. Notably, EL ratings for 26 of 58 children (45%) were clinically significant (i.e., scores above 60; see EL scores distributions in Supplementary Figs. S1 and S2; Supplementary Data are available online at www.liebertpub.com/brain). Given prior reports of elevated EL in children with ADHD (Hulvershorn et al., 2014) and that 43% of our ASD sample had comorbid ADHD, we explored whether EL was associated with ADHD in our sample. EL ratings between ASD children with and without ADHD were not statistically significant (M = 54.9 ± 12.7 and M = 61.9 ± 14.6, respectively; t(56) = −1.9, p < 0.06; See Supplementary Fig. S3). As such analyses were conducted across the whole ASD sample, regardless of ADHD comorbidity.
Table 1.
Demographic Summary
| Variable | Mean (SD) |
|---|---|
| Age (years) | 9.7 (1.8) |
| Male, n (%) | 54 (93) |
| Mean frame-wise displacement (mm) | 0.1 (0.08) |
| Full-scale IQ | 108 (17) |
| Nonverbal IQ | 109 (19) |
| Verbal IQ | 106 (15) |
| CPRS-RLV: EL scale (mean T-score) | 58.7 (14.1) |
| SRS (mean total T-score) | 77.6 (11.7) |
| ASD diagnostic subcategory, n (%) | |
| Autism | 46 (79) |
| PDD-NOS | 7 (12) |
| Asperger's syndrome | 5 (8.6) |
| ADOS module 3 (57 research reliable), n (%) | |
| Social-affective | 8 (3.6) |
| Restricted repetitive behaviors | 2.8 (1.5) |
| Total | 10.9 (4.8) |
| Calibrated severity total score | 6.4 (2.1) |
| ADI-R (52 research reliable), n (%) | |
| Reciprocal social interaction | 19.1 (6.2) |
| Communication | 15.1 (3.9) |
| Restricted, repetitive, stereotyped patterns of behavior | 5.9 (3.1) |
| Developmental abnormality before age 36 months | 3.3 (1.3) |
| Comorbid diagnoses, n (%) | |
| ADHD | 25 (43) |
| ODD | 6 (11) |
| Anxiety disorders | 8 (14) |
| Mood disorders | 6 (11) |
| Tic disorder | 8 (14) |
ADHD, attention-deficit/hyperactivity disorder; ADI-R, Autism Diagnostic Interview-Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; EL, emotional lability; ODD, oppositional defiant disorder; PDD-NOS, pervasive developmental disorder, not otherwise specified; SRS, Social Responsiveness Scale.
Ratings of EL positively correlated with total SRS score (r = 0.30, p < 0.02), supporting use of the SRS as a covariate in group analyses to isolate the unique variance explained by EL.
Amygdala ROI
Analyses did not reveal significant associations between amygdala iFC and EL scores.
Multivariate distance matrix regression
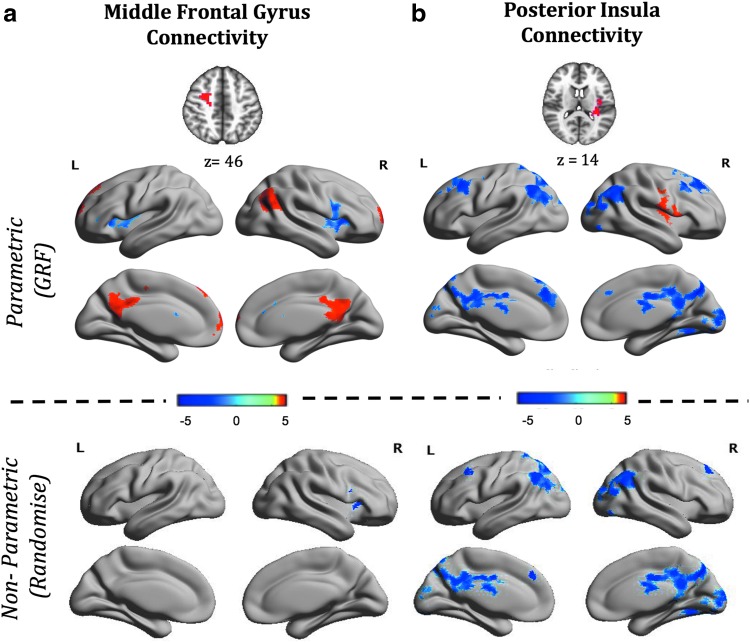
Connectome-wide MDMR analyses indicated that EL scores significantly predicted iFC of middle frontal gyrus (MFG) and left posterior insula (PI). Follow-up seed-based parametric analyses of these regions revealed significant associations between EL scores and MFG iFC with five regions and between EL scores and PI iFC with eight regions (see Fig. 1, Supplementary Table S1, and Supplementary Figs. S1 and S2). Greater EL was associated with greater positive iFC between MFG and right lateral occipital cortex, frontal pole, and precuneus. Conversely, greater EL was associated with reduced positive iFC between MFG and bilateral anterior insula (AI). Further, higher EL scores were associated with greater PI iFC with right central operculum, and lower iFC with bilateral superior frontal gyrus and posterior regions including precuneus, occipital fusiform cortex, bilateral lateral occipital cortex, and lingual gyrus. To verify that the PI iFC with visual cortex was not confounded by including individuals with their eyes closed, we examined the iFC of PI and visual cortex clusters of only those individuals with their eyes open; the resulting pattern was similar to that emerging from primary analyses (Supplementary Fig. S4).
FIG. 1.
Parametric and nonparametric analyses surface maps show regions whose iFC with middle frontal gyrus (a) and posterior insula (b) significantly correlated with parent-rated EL in children with ASD. Clusters whose iFC positively correlated with EL are shown in red, and those negatively correlated are shown in blue. Results from parametric and nonparametric statistical analyses are shown in the top and the bottom panel, respectively. ASD, autism spectrum disorder; EL, emotional lability; iFC, intrinsic functional connectivity. Color images available online at www.liebertpub.com/brain
Seed-based analyses using a nonparametric permutation method revealed similar patterns in respect to the negative correlations between EL and iFC of MFG and PI (Fig. 1, Supplementary Table S1, and Supplementary Fig. S2). Clusters of MFG and PI iFC positively related to EL that were identified using GRF did not survive the TFCE threshold.
To interpret results in the context of known functional brain networks, we computed the percentage of overlap between the voxels in a given cluster identified in our analyses and those of the seven functional cortical networks described by Yeo et al. (2011). Among the voxels in the MFG cluster, 49% were within Yeo's dorsal attention network, the remaining 11.6% were in fronto-parietal network. Additionally, we identified that 64% of the voxels in the clusters whose iFC with MFG were negatively related to EL overlapped with the salience network. We identified that our PI iFC findings negatively related to EL straddled two networks: 32% in default network and 23% in visual network. Finally, while the use of a nonparametric approach appropriately addresses concerns of potential false positive results and thus, applying a more stringent statistical threshold at GRF (Z ≥ 3.1) is overly conservative at this stage of investigation, to enable comparisons and interpretation in future replications we repeated GRF analyses using a threshold of Z ≥ 3.1. As shown in Supplementary Figure S5, results from this more stringent approach are largely similar to those emerging from nonparametric analyses.
Discussion
While clinical evidence suggests that a substantial number of children with ASD demonstrate poor emotional regulation, little is understood about the mechanisms underlying EL in ASD (Mazefsky et al., 2013). Accordingly, we conducted a priori and exploratory analyses of the neural correlates of parent-rated EL in children with ASD. Although a priori analyses of amygdala iFC did not reveal significant brain-behavior relationships with EL, whole brain multivariate analyses identified functional circuitry centered on two main regions, MFG and PI. Parametric and nonparametric approaches to follow-up ROI analyses mostly converged to identify each of these nodes' circuitry in relations to EL. While prior studies have noted altered iFC of both MFG (Jiang et al., 2014; Moseley et al., 2015) and PI (Di Martino et al., 2014; Ebisch et al., 2011) in ASD, our results underscore the role of their circuits in relation to comorbid EL in ASD.
In typical individuals, MFG, a key component of the dorsal attention network, plays an overarching role in emotional, motor, and cognitive self-regulation by directing attention and initiating goal directed behaviors (Depue et al., 2016; Grecucci et al., 2013; Sylvester et al., 2003). In non-ASD children, deficient activation of MFG in response to frustration has been implicated in irritability and EL (Perlman et al., 2015). Our findings highlight the relationship between comorbid EL in ASD and MFG iFC with bilateral dorsal anterior insula (AI). AI is a key region of the salience network, a network thought to integrate external sensory stimuli with internal states (Seeley et al., 2007). While abnormalities in iFC of the MFG and AI in individuals with ASD have been previously reported (Moseley et al., 2015; Uddin et al., 2013) our findings suggest that the interaction between these two nodes and their related broader functional networks may be specifically associated with EL variability. It has been hypothesized that the AI contributes to the allocation of attention to salient stimuli by serving as a regulatory switch between the dorsal attention and default networks (Menon and Uddin, 2010). Results of the GRF analyses in the current study revealed a positive association between EL and MFG iFC with the precuneus, a region within the DN. Taken together these results suggest that higher EL in children with ASD may be associated with stronger iFC between dorsal attention and default networks, perhaps resulting from atypical AI “network switching.” This model should be tested in future large-scale studies designed to examine multinetwork interactions. Given that abnormal iFC in AI has been related to restricted repetitive behaviors/interests (Uddin et al., 2013) in ASD, such multi-network studies should utilize powered explorations of multiple clinical dimensions to disentangle unique neurophenotypic associations to core ASD and associated symptoms.
Our findings relating EL to PI circuitry in ASD underscore the involvement of multiple functional networks beyond those serving higher order cognitive processes. PI is a key component of the sensorimotor network and plays a role in self-regulation (Abram et al., 2015) and emotional experiences by processing interoceptive and exteroceptive sensory information (Chang et al., 2013; Craig, 2009; Nguyen et al., 2016). Intriguingly, the relation between EL and PI iFC with associative visual cortex consistently emerged across parametric and nonparametric analyses. Functional abnormalities in these regions of the visual network, are often reported in ASD and more recent studies have reported atypical iFC of VN in ASD (Chen et al., 2015; Lewis et al., 2014; Nebel et al., 2016). Thus, the relation between sensory processing abnormalities and comorbid EL in a subgroup of individuals diagnosed with ASD should be further explored.
Despite previous work suggesting alterations in amygdala iFC related to emotion processing in ASD (Monk et al., 2010) and other populations (Hulvershorn et al., 2014), we did not find such a relationship among children with ASD. One possibility is that amygdala dysfunction in ASD (Aoki et al., 2015), is not specific to EL variability. Instead, the amygdala is thought to play a fundamental, early role in social and emotional processing, and therefore amygdala iFC abnormalities in ASD may represent impairment in core ASD symptoms; this is consistent with a recent report in preschoolers with ASD (Shen et al., 2016).
Limitations and future directions
Our results should be interpreted considering specific limitations. First, in line with the higher prevalence of boys in ASD, girls were underrepresented in the present sample. Although examining the boys alone did not change the results of our study, we are unable to explore inter-individual variability in the relationship between iFC and EL in ASD relative to gender differences. Since differences in limbic system anatomy and functionality exist between genders (Adinoff et al., 2003), future larger-scale studies of girls and boys are needed to elucidate brain-behavior patterns specific to girls with ASD. Second, although we conducted whole brain voxel-wise analyses, incomplete coverage prevented us from examining ventral aspects of frontal cortex. Prior studies exploring EL in other clinical populations (e.g., ADHD: Posner et al., 2013) have reported a relationship between EL and iFC of ventral frontal regions. Future studies, utilizing improved scan parameters, should seek to explore this relationship in greater depth. Third, our measure of EL is based on parent-report. Future studies should include multiple sources of EL assessment. Lastly, we were unable to directly explore the relationship between mental state (i.e., eyes open/closed implying awake/asleep) and our findings. Future studies should monitor mental state while in the scanner to examine potential dynamic changes in brain-behavior relationships related to this variable.
The explicit goal of this study was to identify neural correlates of EL inter-individual variability within ASD; our study is the first to use R-fMRI methods to explore this relationship. Investigating whether the present findings of EL-iFC relationships are specific to ASD or shared with other typically developing or clinical populations would require inclusion of large community-based samples to optimally capture a distributed range of EL. Further, our study design did not allow us to explore the complex relationship between EL in ASD with and without other comorbidities. For example, 43% of our children were diagnosed with comorbid ADHD, though this was not significantly associated with EL. Dimensional analyses across multiple diagnoses and comorbidities require the inclusion of large and representative sample size.
Conclusions
The present findings enrich our understanding of ASD by showing that in addition to the classically understood variables involved in the disorder (e.g., restricted/repetitive behaviors/interests, social communicative deficits), other behavioral factors such as emotion regulation may contribute to brain-behavior relationships. Our results, if replicated independently, may lead to biologically based subgroups of ASD that involve phenotypic variables not included in the current nosology. Finally, from a methodological perspective, our approach reinforces the potential value of utilizing exploratory whole-brain methods when performing novel analyses.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (K23MH087770 and R01MH105506 to A.D.M; R01MH081218 to F. Xavier Castellanos), from the National Institute of Child Health and Human Development (R01HD065282 to F.X.C.), the Stavros Niarchos Foundation (awarded to F.X.C.), and the National Science Foundation (Graduate Research Fellowship awarded to Randi Bennett). We are grateful to all participants/families who dedicated their time to this study. We thank the research staff of the Phyllis Green Randolph Cowen Institute for Pediatric Neuroscience and the Autism Research Program for their assistance in participant recruitment, assessment, data collection, and data entry over the years. We also thank Drs. F. Xavier Castellanos for his editorial suggestions in the earlier version of this article and Dr. Dorothea Floris for technical support in one aspect of the analyses.
Author Disclosure Statement
A.D.M. is a co-author of the Italian version of the Social Responsiveness Scale, Child Version distributed by Organizzaioni Specialy (OS) in Italy and could receive royalties as a result. The other authors have no financial disclosures to report.
References
- Abram SV, Wisner KM, Grazioplene RG, Krueger RF, MacDonald AW, DeYoung CG. 2015. Functional coherence of insula networks is associated with externalizing behavior. J Abnorm Psychol 124:1079–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Sr., Best SE, Chandler P, Alexander D, Payne K, et al. 2003. Gender differences in limbic responsiveness, by SPECT, following a pharmacologic challenge in healthy subjects. Neuroimage 18:697–706 [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. 1999. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia 37:1111–1117 [DOI] [PubMed] [Google Scholar]
- Ahmed SP, Bittencourt-Hewitt A, Sebastian CL. 2015. Neurocognitive bases of emotion regulation development in adolescence. Dev Cogn Neurosci 15:11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Cortese S, Tansella M. 2015. Neural bases of atypical emotional face processing in autism: a meta-analysis of fMRI studies. World J Biol Psychiatry 16:291–300 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54:2033–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. 2006. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev 30:97–117 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C. 2011. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res 1380:42–77 [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. 2010. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry 167:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. 2013. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 23:739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Keown CL, Jahedi A, Nair A, Pflieger ME, Bailey BA, Muller RA. 2015. Diagnostic classification of intrinsic functional connectivity highlights somatosensory, default mode, and visual regions in autism. Neuroimage Clin 8:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. 2004. Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Dev 75:317–333 [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. 1998. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268 [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. 2003. Autistic traits in the general population: a twin study. Arch Gen Psychiatry 60:524–530 [DOI] [PubMed] [Google Scholar]
- Craig AD. 2009. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70 [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. 2005. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci 8:519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE, Orr JM, Smolker HR, Naaz F, Banich MT. 2016. The organization of right prefrontal networks reveals common mechanisms of inhibitory regulation across cognitive, emotional, and motor processes. Cereb Cortex 26:1634–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, et al. 2014. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 19:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, et al. 2011. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp 32:1013–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 113:7900–7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. 2007. Differential Ability Scales (2nd Edition). San Antonio, TX: Hardcourt Assessment [Google Scholar]
- Etkin A, Buchel C, Gross JJ. 2015. The neural bases of emotion regulation. Nat Rev Neurosci 16:693–700 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. 1996. Movement-related effects in fMRI time-series. Magn Reson Med 35:346–355 [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. 2013. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 33:4584–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. 2007. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 17:103–111 [DOI] [PubMed] [Google Scholar]
- Grecucci A, Giorgetta C, Bonini N, Sanfey AG. 2013. Reappraising social emotions: the role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Front Hum Neurosci 7:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, et al. 2013. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 52:1158–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzadzinski R, Huerta M, Lord C. 2013. DSM-5 and autism spectrum disorders (ASDs): an opportunity for identifying ASD subtypes. Mol Autism 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta M, Bishop SL, Duncan A, Hus V, Lord C. 2012. Application of DSM-5 criteria for autism spectrum disorder to three samples of children with DSM-IV diagnoses of pervasive developmental disorders. Am J Psychiatry 169:1056–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Mennes M, Castellanos FX, Di Martino A, Milham MP, Hummer TA, Roy AK. 2014. Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 53:351–361.e351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841 [DOI] [PubMed] [Google Scholar]
- Jiang YV, Capistrano CG, Palm BE. 2014. Spatial working memory in children with high-functioning autism: intact configural processing but impaired capacity. J Abnorm Psychol 123:248–257 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988 [DOI] [PubMed] [Google Scholar]
- Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. 2012. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn Sci 16:181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB. 2009. Emotion-focused coping in young children: self and self-regulatory processes. New Dir Child Adolesc Dev 2009:33–46 [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Baron-Cohen S. 2014. Autism. Lancet 383:896–910 [DOI] [PubMed] [Google Scholar]
- Lecavalier L. 2006. Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. J Autism Dev Disord 36:1101–1114 [DOI] [PubMed] [Google Scholar]
- LeDoux JE. 1996. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York, NY: Simon & Schuster [Google Scholar]
- Leibenluft E. 2011. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry 168:129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Evans AC, Pruett JR, Botteron K, Zwaigenbaum L, Estes A, et al. 2014. Network inefficiencies in autism spectrum disorder at 24 months. Transl Psychiatry 4:e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. 2000. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223 [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685 [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, White SW. 2013. The role of emotion regulation in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 52:679–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, et al. KN. 2012. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci 7:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Keller TA. 2010. The nature of brain dysfunction in autism: functional brain imaging studies. Curr Opin Neurol 23:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. 2007. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol 64:945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M, et al. 2010. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci 35:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. 2009. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry 66:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley RL, Shtyrov Y, Mohr B, Lombardo MV, Baron-Cohen S, Pulvermuller F. 2015. Lost for emotion words: what motor and limbic brain activity reveals about autism and semantic theory. Neuroimage 104:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, et al. 2016. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol Psychiatry 79:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Breakspear M, Hu X, Guo CC. 2016. The integration of the internal and external milieu in the insula during dynamic emotional experiences. Neuroimage 124(Pt A):455–463 [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, et al. 2011. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A 108:20195–20200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. 2011. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry 52:631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Jones BM, Wakschlag LS, Axelson D, Birmaher B, Phillips ML. 2015. Neural substrates of child irritability in typically developing and psychiatric populations. Dev Cogn Neurosci 14:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel NB, Bolling DZ, Kaiser MD, Pelphrey KA, Crowley MJ. 2014. Neural systems for cognitive reappraisal in children and adolescents with autism spectrum disorder. Dev Cogn Neurosci 10:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Kass E, Hulvershorn L. 2014. Using stimulants to treat ADHD-related emotional lability. Curr Psychiatry Rep 16:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson BS. 2013. Dissociable attentional and affective circuits in medication-naive children with attention-deficit/hyperactivity disorder. Psychiatry Res 213:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey JA, Damiano CR, Sabatino A, Rittenberg A, Petty C, Bizzell J, et al. 2015. Neural mechanisms of emotion regulation in autism spectrum disorder. J Autism Dev Disord 45:3409–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Klein RG, Angelosante A, Bar-Haim Y, Leibenluft E, Hulvershorn L, et al. 2013. Clinical features of young children referred for impairing temper outbursts. J Child Adolesc Psychopharmacol 23:588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E. 2014. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry 171:276–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly C, Reiss PT, Cameron Craddock R, Emerson JW, McMahon K, et al. 2014. A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage 93 Pt 1:74–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Li DD, Keown CL, Lee A, Johnson RT, Angkustsiri K, Rogers SJ, et al. 2016. Functional connectivity of the amygdala is disrupted in preschool-aged children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 55:817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Jones CR, Pickles A, Happe F, Baird G, Charman T. 2012. Severe mood problems in adolescents with autism spectrum disorder. J Child Psychol Psychiatry 53:1157–1166 [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. 2003. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia 41:357–370 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. 2013. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 70:869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. 2012. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev 36:604–625 [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Rowe JB, Baron-Cohen S, Calder AJ. 2014. Direct gaze elicits atypical activation of the theory-of-mind network in autism spectrum conditions. Cereb Cortex 24:1485–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. 2008. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Soc Cogn Affect Neurosci 3:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. 2006. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26:12387–12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. 2014. Permutation inference for the general linear model. Neuroimage 92:381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.