Abstract
Malaria transmission requires that Anopheles mosquitoes ingest Plasmodium gametocyte stages circulating in the human bloodstream. In the context of malaria elimination, understanding the epidemiology of gametocytes relative to all Plasmodium infections and the contribution of asymptomatic and sub-microscopic parasite carriers to the gametocyte reservoir is necessary, especially in low endemic settings with predominance of P.vivax. A 13-month longitudinal study was conducted in two communities (n = 1935 individuals) of Loreto Department, Peru, with five active screenings for Plasmodium infections and gametocyte stages by quantitative real-time PCR (qPCR) and reverse transcription (RT)-qPCR, respectively. Parasite prevalence by qPCR was 7.2% for P.vivax (n = 520/7235; range by survey 6.0%-8.1%) and 3.2% for P.falciparum (n = 235/7235; range by survey 0.4%-7.7%). Sub-microscopic infections accounted for 73.5% of P.vivax (range by survey 60%-89%) and almost the totality of P.falciparum cases. Gametocytes were found in 28.4% P.vivax infections (range by survey 18.7%-34.1%), with a peak of 61.5% in one community at the start of the transmission season. About 59.8% of all P.vivax gametocyte carriers were asymptomatic and 31.9% were sub-microscopic. Age patterns for gametocyte prevalence paralleled asexual stage infections and peaked among >15–25 year old individuals. Asexual parasite density was found to be the strongest predictor for P.vivax gametocyte presence in longitudinal multivariate analysis (odds ratio 2.33 [95% confidence interval 1.96, 2.78]; P<0.001). Despite significant differences in seasonality patterns and P.vivax prevalence found at the local scale, sub-microscopic and asymptomatic infections predominate and contribute significantly to the gametocyte reservoir in different communities of the Peruvian Amazon. Control and elimination campaigns need sensitive tools to detect all infections that escape routine malaria surveillance, which may contribute to maintain transmission in the region.
Author summary
Malaria elimination, i.e. the complete interruption of parasite transmission in a region, is in the agenda of health authorities in countries that achieved substantial reduction of the disease burden in the past decade. However, our understanding of transmission epidemiology for low transmission areas where Plasmodium vivax is endemic, like the Amazon basin, is still limited. In this study, we describe the prevalence and risk factors for carrying the parasite stages that are transmitted to the mosquito vectors, named gametocytes, in 1935 individuals from two communities of the Peruvian Amazon that were regularly screened during 1 year. We report that malaria infections with no clinical symptoms and those with parasite levels below microscopy detection threshold, account for two thirds of all P.vivax infections with gametocytes, and that the highest infection rate is found among young adults. In addition, almost the totality of P.falciparum infections detected was sub-microscopic. Because all these infections escape current malaria surveillance systems -based on passive case detection and/or microscopy diagnosis-, new approaches are necessary to target all infections in order to eliminate the malaria transmission reservoir in Peru.
Introduction
As countries in the Americas develop plans for malaria pre-elimination, a better understanding of Plasmodium transmission epidemiology is necessary to implement effective interventions, in particular for Plasmodium vivax [1,2]. In Peru, similar to other regions in Amazonia, P. vivax is responsible for about 80% of all malaria infections [3], with asymptomatic low-grade parasitemia reported for ≈75% of them [4,5]. Between 2005 and 2011, malaria was reduced by over 50% thanks to intensification of control efforts and the PAMAFRO campaign (Malaria Control Program in Border Areas of the Andean Region), which included active screening and treatment and distribution of insecticide-treated nets [6]. Unfortunately, and coinciding with a reduction of control measures, a steady increase in cases has been reported in the country since 2012 characterized by high levels of transmission heterogeneity at the local scale [6,7]. In this context, accurate description of the human malaria reservoir and the contribution of asymptomatic and sub-microscopic infections to transmission is lacking.
Malaria transmission from the human host to the mosquito vector requires that Anopheles species ingest mature sexual forms of Plasmodium parasites, named gametocytes, during a blood meal [8]. Therefore, gametocyte carriage can be used as indirect estimator of the infectiousness potential of individuals in molecular epidemiology studies (taking into account that mosquito infections may be modulated by inter-individual variation in yet poorly understood vector, parasite and host factors [8,9]). The characterization of the gametocyte reservoir includes the detection of sexual stage infections, their quantification relative to asexual parasites, the identification of factors that determine gametocyte emergence, determining how gametocyte carriage changes over time, or what is the spatial clustering of sexual stage infections.
Most of the current knowledge on Plasmodium gametocyte epidemiology comes from studies on Plasmodium falciparum species. Sexual P.falciparum stages are normally observed in peripheral blood ≈7–12 days after initial asexual infection is established. A recent clinical trials meta-analysis showed that, on average, 12.1% of infections carry gametocytes at enrolment by light microscopy (LM), and that these infections are associated with anaemia, absence of fever and low asexual parasite densities; after treatment, gametocyte rate is higher among individuals taking sulphadoxine pyrimethamine or chloroquine as compared to artemisinin combination therapies (ACT) [10]. Community-based cross-sectional and cohort studies, which take into account asymptomatic infections, have set the proportion of P.falciparum gametocyte carriers in a wide range from ≈10% by LM in Senegal to as high as 61% in Papua New Guinea (PNG) by reverse-transcription quantitative PCR (RT-qPCR) or 70.1% in Burkina Faso by QT-NASBA [4,11–16]. These studies showed that gametocyte carriage is higher in young age groups [11–15], and correlates with asexual parasite densities -when measured by molecular methods [11,15]-, presence of fever or clinical malaria [13–15], haemoglobin C and S variants [16,17], and blood groups O and B [14]. On the other hand, P.vivax transmission epidemiology and the characteristics of its infectious reservoir have been less studied [8]. P.vivax gametocytes are produced shortly after initial asexual wave of parasitemia in the bloodstream, have a shorter lifetime, and are suggested to infect mosquitoes more efficiently than P.falciparum [8,18]. In recent community based surveys, the proportion of infections with P.vivax sexual stages was found to reach >50% by LM [4,19], whereas by RT-qPCR 23.5%-96% of infections carried gametocytes in studies in Oceania and Brazil [15,20,21]. Like for P.falciparum, young age [15,20,22] and high asexual parasite density were described as the main predictors [15,19,21–25]. Low haemoglobin levels and fever have also been linked with P.vivax gametocyte presence [15,23], although equally high rates of gametocytes were reported in symptomatic and asymptomatic infections in Brazil [21,26].
Here, we aim to improve our understanding of Plasmodium gametocyte epidemiology and generate region specific data that can inform health authorities to tailor transmission-blocking strategies to the local context of transmission heterogeneity. A longitudinal cohort study was conducted in two separate communities, where all individuals were regularly screened for asexual and sexual stage infections using molecular tools for one year. The factors that contribute to gametocyte carriage, with a focus on asymptomatic and sub-microscopic carriers, are discussed.
Methods
Study area
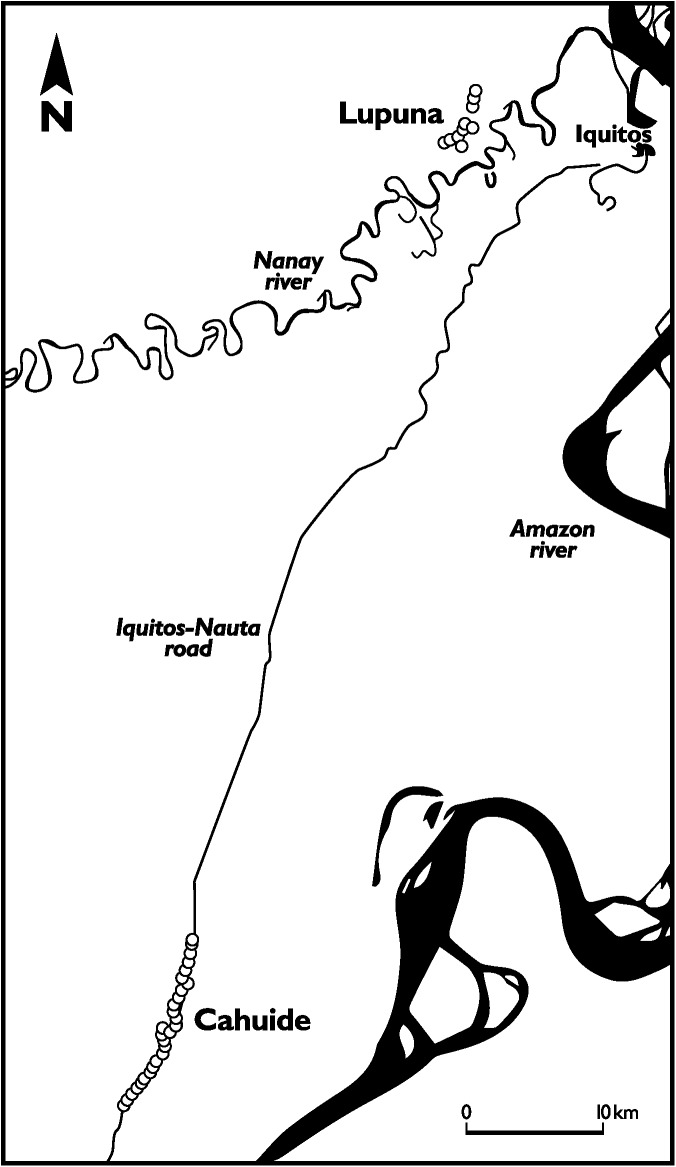
The study was conducted in the communities of Cahuide and Lupuna, Maynas Province, Loreto Department, Peru (Fig 1). The region is characterized by a tropical climate with a mean temperature of 27.5°C. Despite significant transmission heterogeneity, overall malaria is perennial with marked seasonality and a peak in April-June. In 2013, Loreto Department accounted for 89.5% (43284) of all malaria cases in Peru, being 81.9% (35458) caused by P.vivax [27]. Cahuide is a rural community 57 km away from Iquitos city. Houses are scattered along 9 km of the Nauta road, at the intersection with Itaya river. The area is characterized by road-driven deforestation and palm-roof production. Monthly entomological inoculation rate (EIR) range was 0–2.52 infective bites/person/month in 2012 [28]. On the other hand, Lupuna community includes three villages located 500–1000m away from the left bank of river Nanay (Fig 1). Although closer to Iquitos city than Cahuide, the area is only accessible by boat and mainly forested. Population is relatively stable and works in agricultural activities. Monthly EIR range estimates for Lupuna were 0–1.98 in 2012. In both sites Anopheles darlingi is the dominant vector species [28]. Malaria control in the area relies mainly on passive case detection and LM diagnosis performed at health posts. Active case detection campaigns by LM are performed only when outbreaks or unusual rises in case numbers are reported in a community.
Fig 1. Map of the study area.
Location of Cahuide and Lupuna communities relative to Iquitos city, the Amazon and Nanay rivers and Iquitos-Nauta road.
Study design and sample collection
As part of a 3-year prospective population-based cohort study with longitudinal follow-up, we analyzed gametocyte carriage every three months from December 2013 to December 2014 (five surveys). A total of 1935 individuals from 442 households aged >3 months were included. At each survey, all individuals were screened for malaria symptoms and completed a questionnaire with demographic data. Blood smears, filter paper blood spots, and three blood drops (≈50 μl ±10 μl) into 250 μl of RNAprotect stabilizer reagent (Qiagen), were collected from finger pricks. RNAprotect-blood mixture was kept cold and transferred to Iquitos for storage at -20°C. Blood smears were stained with Giemsa and examined for malaria parasites under 700x magnification. Parasite counts per 200 leukocytes were used to estimate parasite density, assuming 8000 leukocytes in 1 μl of blood. All infections diagnosed by LM were treated according to National Guidelines from Peruvian Ministry of Health (MINSA), regardless of symptoms.
Total parasite quantification by qPCR
DNA was extracted from one punch of dried blood spots on filter paper (≈25mm2, ≈7.5 μl) using QIAamp DNA Mini kit (Qiagen) following manufacturer’s instructions, and eluted in 150 μl of AE buffer. Identification and quantification of Plasmodium species was done by real-time qPCR protocol targeting 18S ribosomal genes, adapted from Mangold et al [29]. Briefly, 5 μl of DNA were added to a final reaction volume of 25 μl including 12.5 μl of PerfeCTa SYBR Green FastMix (Quantabio) and 300nM primers PL1473F18 and PL1679R18 (IDT), and run in a CFX Connect thermocycler (Bio-Rad). Species were identified by melting temperature (Tm) curve analysis using CFX Manager software (Bio-Rad), with a Tm of 77°C (±1°C) for P.vivax and 73°C (±1°C) for P.falciparum. Plasmids with species-specific 18S gene inserts were used as controls. The limit of detection was set at the dilution where at least 60% of the replicates were positive, and corresponded to 1 copy/reaction for both species (i.e. 4 copies/μl of blood in study samples). Sample quantification was done using standard curves built from 1:10 plasmid dilution series. Only samples with both valid Ct and Tm values were considered positive.
Gametocyte quantification by RTqPCR
The presence of mature gametocytes in qPCR positive samples was determined by one-step reverse transcription qPCR (RTqPCR), targeting Pfs25 (P.falciparum, PF3D7_1031000) and Pvs25 (P.vivax, PVX_111175) mature gametocyte specific gene transcripts. RNA was extracted from RNAprotect samples using RNeasy Mini Kit columns (Qiagen) and eluted in 50 μl of THE-RNA Storage Solution (Ambion). Contaminant genomic DNA was removed by treatment with TURBO DNAse (Ambion) for 1h at 37°C. Parasite RNA presence was confirmed in a random set of 10% of the samples using RT (Maxima First Strand cDNA Kit, Thermo) and 18S qPCR. Gametocytes RTqPCR was performed in a LightCycler 480 using LightCycler Multiplex RNA Virus Master kit (Roche), with primers and HEX(P.falciparum)- and FAM(P.vivax)-labelled hydrolysis probes from Wampfler et al [30] (IDT). All samples were tested in duplicate reaction and controls without RT enzyme were added to exclude false positives due to the presence of genomic DNA. Analysis was done in LightCycler480 software version 1.5.0. Replicates with Ct difference >1 were repeated. P.falciparum gametocyte densities were quantified using a standard curve generated from in vitro cultured gametocytes. Briefly, P.falciparum 3D7-E5 strain (kindly provided by Dr. Alfred Cortés, ISGlobal, Barcelona) was synchronized with 5% sorbitol and induced for gametocytogenesis by stress with partially spent medium for 2 consecutive days. Asexual stages were removed by 50 mM N-acetyl-glucosamine treatment until day ≈12–14, when mature stage V gametocytes were harvested and concentrated using MACS magnetic separation (LD columns, Milteny Biotec). A 7-point 1:10 dilution series ranging from 100.000 to 0.1 gametocytes/μl was prepared in whole blood and resuspended in RNAprotect as described above for gametocyte density quantification. Due to the lack of P.vivax in vitro culture, P.vivax densities were first estimated from a P.falciparum standard curve quantified using a FAM-labelled Pfs25 probe, and a correction factor for the differential expression of Pvs25 vs Pfs25 was then applied using recently published P.falciparum and P.vivax gametocyte trend lines [15].
Definitions and statistical methods
Clinical malaria was defined as confirmed Plasmodium infection by microscopy and/or qPCR presenting with fever, chills and/or headache at the time of visit, or reporting one of these symptoms during the previous 7 days. Asymptomatic individuals were defined as those with confirmed infection not presenting any of these symptoms at the time of visit or in the past 7 days. Prevalence was defined as the number of parasite carriers out of the total population, whereas the term 'rate' was used to define the proportion of asymptomatic/sub-microcopic/gametocytemic individuals out of the total number of infections. Because only malaria positive samples were processed for RNA-based detection of gametocytes, gametocyte population prevalence is considered an estimate. Incidence of gametocyte carriage was calculated as the number of new sexual stage infections per 1000 person/year. Time-at-risk was calculated as 90 days per each survey in which an individual participated after study initiation. When treatment was reported, 15 days were subtracted as a risk-free time [31]. For individuals positive for gametocytes in two consecutive surveys without reported treatment, only first observation was counted, whereas those positive in two non-consecutive surveys were considered as independent infections. For parasite densities, only results by molecular methods are reported.
Comparisons between demographic categorical variables were done using chi-square or Fisher’s exact test, and age means were compared by t-test. Non-parametric Kruskal-Wallis test was used to compare parasite densities. Multilevel regression models were used to determine risk factors for Plasmodium infection during longitudinal follow-up in Stata software (version 11.0; StataCorp), with each observation nested by individual and individuals grouped by household. Data were set as panel ordered by time of screening. Logistic regression was used for association analysis with parasite prevalence and gametocyte rate, while log transformed parasite densities were analyzed by linear regression. Univariate models were first run including as independent variables demographic data (age, gender, pregnancy status, community of residence, bed net coverage), work status and occupation, house construction materials, clinical data (malaria symptoms, history of malaria in previous year), and 18S copy numbers–the latter for gametocyte associations only-. Multivariate models were built with stepwise forward selection of all co-variables with 5% significance level in univariate analysis plus age. Predictors added in decreasing order of significance were kept if its addition led to a decrease in Akaike Information Criterion value. Overall significance for variables with multiple categories was estimated using Wald test. P-values <0.05 were considered statistically significant. Data were plotted using Prism 7 (GraphPad).
Spatial statistics
Spatial scan analysis was conducted to identify purely spatial (by survey) and spatio-temporal (all surveys) clusters of gametocyte carriers at households or village level. Separate analysis were run for each community among all qPCR-positive individuals in SaTScan 9.4.2 [32]. Briefly, circular windows of multiple sizes containing a maximum of 30% of the population were applied, in which the probability that the observed prevalence is higher than the expected under the hypothesis of no clustering was tested using a Bernoulli distribution model. P-values were computed across 999 Monte-Carlo replications. Because spatial scan statistics may have limitations to identify hotspots in areas where data is distributed linearly like Cahuide [33], autocorrelation analysis was performed in ArcMap 10.4 (ArcGIS, ESRI, [34]) using Getis-Ord Gi* statistic and False Discovery Rate correction for multiple testing. Distance bands were calculated using Incremental Spatial Autocorrelation tool in windows of 25 m and the distance corresponding to first Z-score peak was selected (200m). Results were mapped in QGIS 2.12.
Ethics statement
Written informed consent was obtained from all individuals -or their parents or guardians in the case of minors- before conducting any study activity. The study received ethical approval from the Comité Institucional de Ética, Universidad Peruana Cayetano Heredia (Lima, Peru; code SIDISI 57395), and the Institutional Review Board, Institute of Tropical Medicine (Antwerp, Belgium; reference 1080/16).
Results
Characteristics of study population at screening
Out of 1935 censed individuals, 1369 (71%) participated in ≥4 surveys, 290 (15%) participated in 3 surveys and 276 (14%) in ≤2 surveys (Table 1 and S1 Table). 7265 samples were collected over the study period, with full clinical data records available for 5746 visits. Individuals from Cahuide were younger and reported a significantly higher number of malaria cases in the year prior to study initiation than individuals from Lupuna (Table 1, P<0.001). However, symptomatic malaria by LM was more frequent in Lupuna during the time of study (P<0.001). About 96% of households (425/442) reported having bed nets, either long-lasting insecticide-treated or locally produced using tocuyo textile. Recruitment was significantly lower in March compared to other months (S1 Table). Overall, individuals sampled in this particular survey did not differ in age, gender, occupation or type of housing, but had significantly higher malaria history in the previous year compared to other surveys (P = 0.001). The difference in malaria history between surveys was not observed after stratifying by community (P>0.852).
Table 1. Characteristics of the study cohort at screening (December 2013- December 2014).
| Total | Cahuide | Lupuna | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P-value | |
| Individuals | 1935 | - | 1062 | - | 873 | - | NA |
| Age, mean (95% CI) | 27.8 (4, 67) | 26.0 (4, 65) | 30.2 (5, 70) | <0.001 | |||
| Age | |||||||
| ≤5y | 202 | 10.4 | 123 | 11.6 | 79 | 9.0 | <0.001 |
| >5y - 10y | 286 | 14.8 | 167 | 15.7 | 119 | 13.6 | |
| >10y - 15y | 250 | 12.9 | 161 | 15.2 | 89 | 10.2 | |
| >15y - 25y | 311 | 16.1 | 153 | 14.4 | 158 | 18.1 | |
| >25y | 886 | 45.8 | 458 | 43.1 | 428 | 49.0 | |
| Male/female ratio | 1.1 | - | 1.1 | - | 1.0 | - | 0.555 |
| Work status | |||||||
| Employed | 658 | 34.0 | 348 | 32.8 | 310 | 35.5 | |
| Students or children | 854 | 44.1 | 497 | 46.8 | 357 | 40.9 | |
| Other (including housework) | 423 | 21.9 | 217 | 20.4 | 206 | 23.6 | 0.034 |
| Households | 442 | - | 241 | - | 201 | - | NA |
| House wall materials | <0.001 | ||||||
| Brick, cement | 39 | 8.8 | 10 | 4.2 | 29 | 14.4 | |
| Wood | 344 | 77.8 | 200 | 83.0 | 144 | 71.6 | |
| Palm | 38 | 8.6 | 16 | 6.6 | 22 | 11.0 | |
| Other | 21 | 4.8 | 15 | 6.2 | 6 | 3.0 | |
| Had malaria previous year | 599 | 31.3 | 474 | 44.8 | 125 | 14.6 | <0.001 |
| Number of visitsa | 7265 | - | 4091 | - | 3174 | - | NA |
| Visits with symptomsa,b | 762 | 13.3 | 284 | 9.5 | 478 | 17.3 | <0.001 |
| Clinical malaria casesa,c | |||||||
| P. vivax | 56 | 1.0 | 17 | 0.6 | 39 | 1.4 | 0.734 |
| P. falciparum | 2 | 0.03 | 0 | 0 | 2 | 0.1 | - |
atotal for 5 surveys
bfever, headache and/or chills (among those with full clinical data available at the time of visit and during the previous 7 days)
cby light microscopy at screening. NA, not applicable; CI, confidence interval.
Plasmodium infections (all stages)
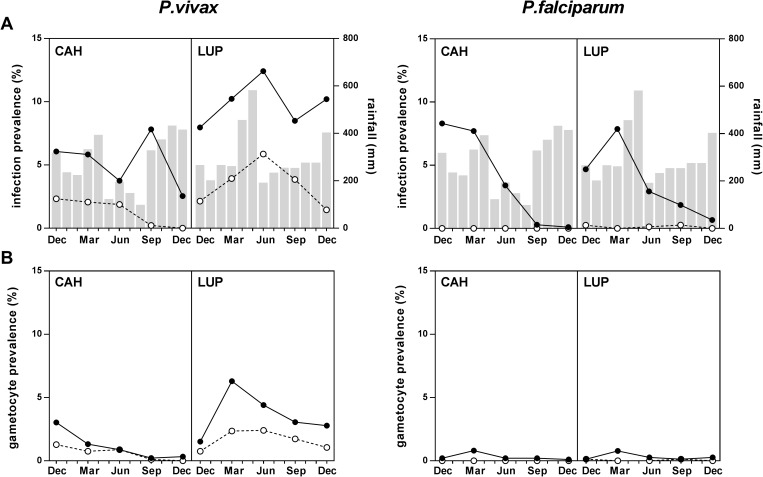
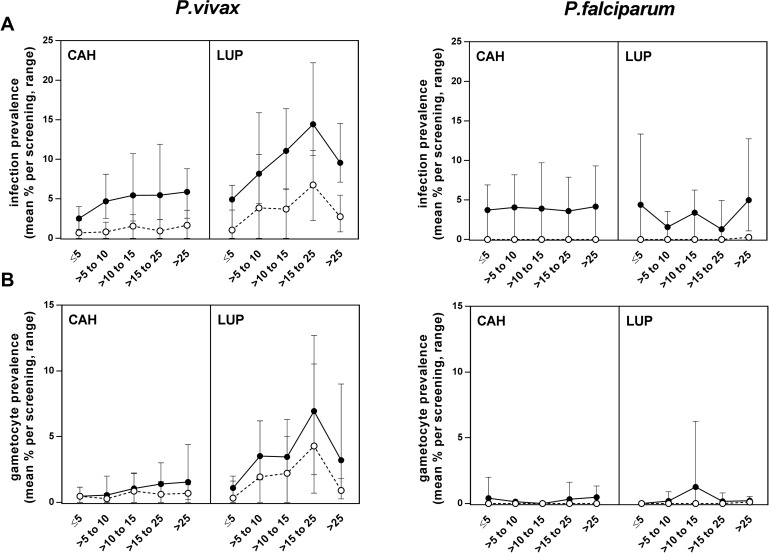
P.vivax was the most common malaria species both by LM (156/7265, 2.1% [range by survey 0.7%-3.7%]) and qPCR (520/7265, 7.2% [6.0%-8.1%]). A total of 432 individuals (22.3% of the population) had a P.vivax infection at some point during follow-up, and 71 (3.7%) were positive in more than one survey. By qPCR, prevalence was higher in Lupuna (mean 9.9% [range by survey 8%-12.4%]) than Cahuide (5.2% [3.8%-7.8%], P<0.001, Fig 2A), with a marked seasonal pattern peaking in June after major rainfall. On the contrary, Cahuide showed an overall decrease in infections despite a peak in September, with no positive blood smears by the end of the follow-up period in December 2014. P.vivax infections increased with age until 25 years old (Fig 3A), and peaked among >15–25 year old in Lupuna (82/545 by qPCR, 15% [range by survey 10.5%-22%], P<0.001; Fig 3A). Overall median P.vivax density was 112 copies of 18S/μl (inter-quartile range [IQR] 44, 536), with higher parasite densities in Lupuna (140 copies/μl [IQR 56, 1016]) than Cahuide (80 copies/μl [IQR 32, 296], P<0.001; S1 Fig).
Fig 2. Plasmodium infections and gametocyte carriage from December 2013 to December 2014.
A) P.vivax and P.falciparum prevalence by LM (white circles) and qPCR (black circles) in Cahuide (CAH) and Lupuna (LUP). Monthly rainfall records are shown in bars, corresponding to station 153-Morallillo (for CAH) and station 154-Iquitos (for LUP; source: http://www.senamhi.gob.pe/ [35]). B) P.vivax and P.falciparum gametocyte prevalence by LM (white circles) and RTqPCR (black circles).
Fig 3. Plasmodium infections and gametocyte carriage by age.
A) P.vivax and P.falciparum prevalence by LM (white circles) or qPCR (black circles) in Cahuide (CAH) and Lupuna (LUP). B) Gametocyte prevalence by LM (white circles) or RTqPCR (black circles). Symbols correspond to the mean of all surveys and error bars indicate minimal and maximal prevalence observed during surveys.
P.falciparum parasites were detected in 5/7265 blood smears and 235/7265 qPCR samples (3.2% [range by survey 0.4–7.7%]), originating from 222 different individuals (11.5% of the population). P.falciparum qPCR infections were more frequent in Cahuide (3.6% [range by survey 0.1%-8%]) than Lupuna (2.8% [0.7–4.7%], P<0.001; Fig 2A), and decreased with time down to an overall prevalence of 0.4% (6/1657) in the last survey. No differences in P.falciparum prevalence were found by age groups in any community (P>0.174, Fig 3A). Median P.falciparum density (28 copies/μl [IQR 12, 56]; S1 Fig) was lower than for P.vivax (P<0.001).
High rates of sub-microscopic and asymptomatic infections were found throughout the study period for both species (Table 2). Sub-microscopic infections accounted for 73% (range by survey 60%-89%) of all P.vivax and 97.8% (88%-100%) of all P.falciparum positive samples. Using the qPCR result for clinical malaria case definition, the number of P.vivax clinical cases increased from 56 (by LM at screening) to 101, and the number of P.falciparum clinical cases from 2 to 30. Still, the large majority of infections detected after qPCR were asymptomatic (77.2% [range by survey 72%-82%] for P.vivax and 82.7% [60%-90%] for P.falciparum). Parasite densities in P.vivax asymptomatic carriers were lower (92 copies/μl [IQR 40, 292]) than those in clinical cases (544 copies/μl [IQR 88, 5712], P<0.001). No difference in parasite densities was found between P.falciparum asymptomatic and symptomatic infections (7 copies/μl [IQR 3, 14]) vs 5 copies/μl [IQR 2, 13], P = 0.364). By community, Cahuide showed significantly higher rates of sub-microscopic infections for both species (P<0.005), as well as higher asymptomatic rates for P.falciparum (P = 0.015; Table 2).
Table 2. Sub-microscopic and asymptomatic Plasmodium infection rates, by qPCR/RTqPCR.
| Total | Cahuide | Lupuna | |||||
|---|---|---|---|---|---|---|---|
| n/N | % (range)a | n/N | % (range)a | n/N | % (range)a | P-value | |
| P.vivax infections | |||||||
| Sub-microscopic rate | 380 / 520 | 73 (60–89) | 172 / 210 | 82 (65–100) | 208 / 310 | 67 (55–86) | 0.005 |
| Sub-microscopic+gametocytemic rate | 76 / 520 | 15 (9–20) | 26 / 210 | 12 (3–33) | 50 / 310 | 16 (10–39) | 0.235 |
| Asymptomatic rateb | 342 / 443 | 77 (72–82) | 139 / 170 | 82 (47–94) | 203 / 273 | 74 (65–87) | 0.071 |
| Asymptomatic+gametocytemic rateb | 73 / 443 | 16 (9–21) | 18 / 170 | 11 (2–24) | 55 / 273 | 20 (17–42) | 0.008 |
| P.falciparum infections | |||||||
| Sub-microscopic rate | 230 / 235 | 98 (88–100) | 147 / 147 | 100 (100–100) | 83 / 88 | 94 (86–100) | 0.003 |
| Sub-microscopic+gametocytemic rate | 16 / 235 | 7 (2–50) | 11 / 147 | 7 (3–100) | 5 / 88 | 6 (0–40) | 0.060 |
| Asymptomatic rateb | 143 / 173 | 83 (60–90) | 87 / 98 | 89 (84–100) | 56 / 75 | 75 (50–89) | 0.015 |
| Asymptomatic+gametocytemic rateb | 12 / 173 | 7 (0–40) | 8 / 98 | 8 (0–100) | 4 / 75 | 5 (0–25) | 0.468 |
a range by survey
bamong those with full clinical data available at the time of visit and during the previous 7 days.
Gametocyte carriage
P.vivax gametocytes were observed in 72 blood smears (1% population prevalence; Fig 2B). By molecular methods, gametocytes were detected in 143 samples (2% estimated population prevalence) originating from 135 different individuals (7%); only 8/135 (6%) individuals carried gametocytes in more than one time-point. The estimated annual incidence for P.vivax gametocyte carriage was 72 sexual-stage infections/1000 person-year (136.2 for Lupuna and 72 for Cahuide). Seasonal trends in gametocyte prevalence differed by community: in Lupuna, gametocytes by RTqPCR peaked in March (8/127, 6.3% est. population prevalence; Fig 2B) and decreased afterwards (21/74, 2.8% est. population prevalence in December), whereas in Cahuide gametocyte prevalence decreased during the whole study period, as did asexual parasitemia (Fig 2B). Age patterns for gametocyte prevalence were similar than those for asexual stage infections, and highest among the >15–25 year old group (Fig 3B). P.vivax gametocyte densities by RTqPCR did not vary by survey (P = 0.261) or community (P = 0.319; S1 Fig). P.falciparum gametocytes were detected in 18 samples by RTqPCR (0.3% population prevalence). Annual incidence was 10.7 cases/1000person-year (11.1 in Cahuide and 10.1 in Lupuna).
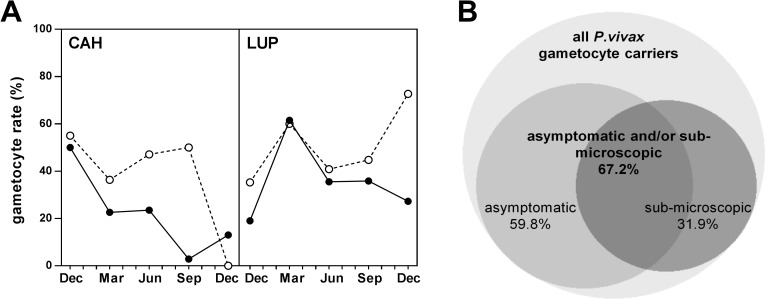
Among positive samples, the proportion of P.vivax infections carrying gametocytes was 46.2% (72/156 [range by survey 42.6%-72.7%]) for blood smears and 28.4% (143/520; 18.7%-34.1%) by RTqPCR (Fig 4A). P.vivax gametocyte rates were higher in Lupuna (35.9% [range by survey 19%-61.5%]) than Cahuide (22.4% [3%-50%], P = 0.001; Fig 4A) and did not vary significantly by age category (P = 0.191). The gametocyte rate for P.falciparum was 7.6% (18/235 [range by survey 3%-50%]) and was similar in both communities and in all age groups (P>0.234).
Fig 4. Proportion of P.vivax infections carrying gametocytes.
A) P.vivax gametocyte rate among all infected individuals (by LM in white circles, or by qPCR/RTqPCR in black circles). B) Venn diagram for the contribution of asymptomatic and sub-microscopic infections to total P.vivax gametocyte reservoir, by qPCR/RTqPCR (built using BioVenn [36]; only samples with complete clinical records were considered).
Sub-microscopic and/or asymptomatic infections with gametocytes were found to constitute a significant proportion of the total P.vivax reservoir, with rates ranging from 9% to 21% overall, and up to 42% for in Lupuna community (Table 2). If only gametocyte positive infections with full clinical data records are taken into account, sub-microscopic and/or asymptomatic infections represent 67.2% (82/122) of the gametocyte reservoir detected by molecular methods (Fig 4B). P.vivax gametocyte density in asymptomatic individuals (8.6 gametocytes/μl [IQR 1.9, 85]) did not differ from that in clinical cases (5.7 gametocytes/μl [IQR 2.0, 133.7], P = 0.696). On the other hand, the P.falciparum gametocyte reservoir was entirely constituted by infections that were either sub-microscopic and/or asymptomatic, although sample size was small (n = 18).
Factors associated with Plasmodium infection and gametocyte carriage
Results for all co-variables associated with P.vivax infection in univariate analysis are provided as S2 Table, and were used to build multivariate models with stepwise forward selection. Final models showed that risk of P.vivax infection was highest in the >15–25 year old group, when compared to children under five (odds ratio, OR 3.1 [95% confidence interval (CI) 1.75, 5.49], P<0.001; Table 3). Other independent risk factors for P.vivax infection included residence in Lupuna, and living in houses built with palm or other materials -a category that includes mainly plastics and corrugated iron-, as compared to concrete or brick houses. Among infected individuals, total parasite density was the main independent predictor for the presence of gametocytes, with a 10% increase in 18S copy numbers associated to double risk of carrying gametocytes (Table 3). In addition, a borderline association towards higher risk of gametocyte carriage was found for Lupuna. Age was not found to be a significant predictor for gametocyte risk in multivariate analysis (P>0.411).
Table 3. Multivariate models for P. vivax infection and gametocyte carriage.
| Parasite prevalence | Gametocyte rate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P-valuea | OR | 95% CI | P-valuea | ||
| Age | ||||||||
| ≤5y | 1 | 1 | ||||||
| >5y - 10y | 2.07 | 1.17, 3.69 | 0.013 | 0.88 | 0.16, 4.91 | 0.887 | ||
| >10y - 15y | 2.64 | 1.47, 4.71 | 0.001 | 1.27 | 0.24, 6.87 | 0.780 | ||
| >15y - 25y | 3.10 | 1.75, 5.49 | <0.001 | 1.99 | 0.39, 10.24 | 0.411 | ||
| >25y | 1.94 | 1.13, 3.32 | 0.016 | (0.001) | 1.58 | 0.32, 7.83 | 0.573 | (0.529) |
| Village | ||||||||
| Cahuide | 1 | 1 | ||||||
| Lupuna | 1.88 | 1.45, 2.44 | <0.001 | 1.87 | 1.01, 3.48 | 0.050 | ||
| House wall | ||||||||
| Brick, cement | 1 | - | - | - | ||||
| Wood | 1.66 | 1.02, 2.70 | 0.040 | - | - | - | ||
| Palm | 2.10 | 1.15, 3.84 | 0.016 | - | - | - | ||
| Other | 3.31 | 1.62, 6.73 | 0.001 | (0.007) | - | - | - | |
| 18S copies/μl (log) | - | - | - | 2.33 | 1.96, 2.78 | <0.001 | ||
| Fever, headache or chills | 2.09 | 1.59, 2.74 | <0.001 | - | - | - | ||
aresult of Wald test shown in brackets. OR, odds ratio; CI, confidence interval.
Models for P.vivax parasite densities showed that 18S copies were positively associated with clinical symptoms (OR 5.93 [95% CI 5.73, 9.17], P<0.001) as well as residence in Lupuna community (OR 1.59 [95% CI 1.05, 2.42], P = 0.028); S3 Table). Parasite density by 18S was the only predictor of gametocyte density (OR 1.16 [95% CI 1.01, 1.32], P = 0.034; S3 Table), which did not differ between children and adults (P>0.188).
Association analysis was not attempted for P.falciparum sexual stage infections due to the low number of cases. Prevalence of P.falciparum asexual infection was associated with the absence of clinical symptoms, whereas no variables were associated with parasite density (S4 Table).
Spatial clusters of P.vivax gametocyte carriage
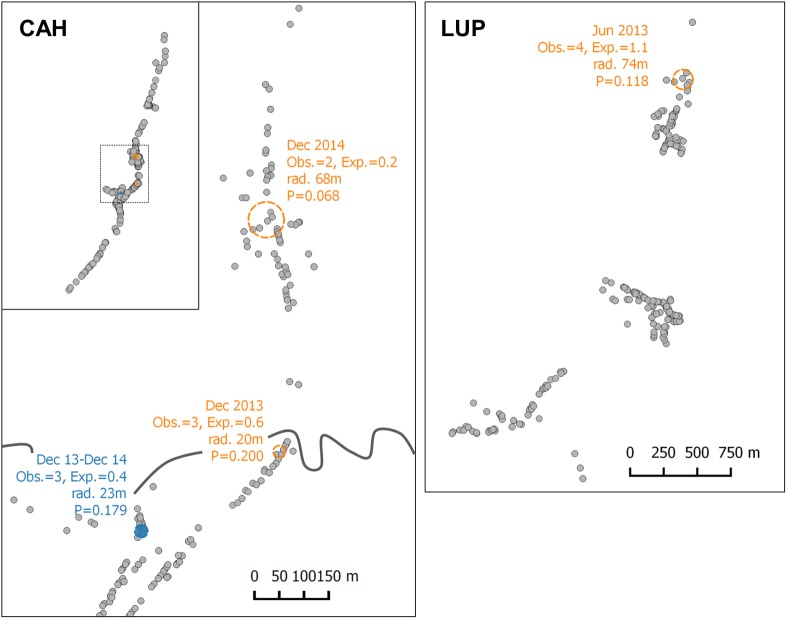
SaTScan analysis could not identify spatial clusters with statistical significance (P<0.05), which may have been limited by the low number of observations per time-point together with uneven household geographical distribution. Four areas showed a higher-than-expected risk with significance thresholds below 20%, including three spatial clusters in Cahuide and Lupuna, and a small spatio-temporal cluster in Cahuide (Fig 5; relative risk range 3.8–21, P-value range 0.068–0.200). Individuals residing inside these areas did not differ in age, gender, type of household, occupation nor clinical characteristics compared to those outside the cluster (P>0.113). No hotspots at household level were identified by Getis-Ord Gi* statistic.
Fig 5. Spatial cluster analysis for P.vivax gametocyte carriage.
Regions with higher-than-expected risk and P<0.200 are shown, based on SaTScan result. Orange circles indicate spatial clusters; blue circle indicates a spatio-temporal cluster.
Discussion
The data presented in this study constitutes the most accurate quantification of the Plasmodium gametocyte reservoir in Peru to date, and the largest conducted in the Amazon region. A previous study in Peru from 2003 using LM alone reported a gametocyte rate of 22% for P.vivax and 53% for P.falciparum [4], as compared to the 46% for P.vivax reported here by LM (almost all P.falciparum infections were sub-microscopic). This increase is in line with the overall reduction in endemicity in the country over the past decade, which may translate into a higher investment in sexual stages to sustain transmission [8]. On the other hand, the average gametocyte rate of 28.4% for P.vivax by RTqPCR compares to community-level data from a similar epidemiological context in Solomon islands (23.5% gametocyte rate in 2012) [20], but is lower than the 49% found in PNG in 2010 or a remarkably high 96% in Brazilian Amazon in 2011–12 [15,21]. Different factors may explain this variability. First, factors attributable to seasonality and study-designs. While the study in PNG was a cross-sectional conducted during the rainy-season, the study from Brazil estimated gametocyte rates from only a subset of surveys and samples (n = 55). Our data covers one full year and, in fact, rates reached as high as 61% when taking into account only the transmission peak in Lupuna community. Second, differences in age distribution of malaria infections between the different locations. The proportion of gametocyte carriers is known to be higher among younger age groups in areas of high malaria transmission, where children have higher asexual densities [8]. Thus it is reasonable to expect an increase in gametocyte rates in a high transmission setting like PNG, where P.vivax infections concentrate in children <12 years old, as opposed to our study[15]. Third, factors attributable to different blood collection and parasite detection methods. On one hand, Barbosa et al processed 200μl of blood in Brazil, as compared to 50 μl blood collections both in the present (estimated volume) and in other studies [15,20,21]. Using high blood volumes can increase the chance to detect low-density gametocytemic samples; moreover, the few negative samples reported in that study had asexual parasite densities below 11 parasites/μl, levels that are highly frequent in our surveys. On the other hand, the performance of the all-stage parasite detection qPCR protocol may affect gametocyte rates by diminishing estimates when a highly sensitive asexual qPCR is used. Because gametocytes often represent only a small proportion of the total parasite biomass, the high rate of sub-microscopic infections found in this area compromises technical sensitivity for gametocyte detection, and may explain why gametocyte rates by RTqPCR were lower than those detected by LM. This effect was particularly relevant for P.falciparum (98% sub-microscopic rate), as the few sexual stage parasites detected were in either submicroscopic or asymptomatic infections. In fact, when only patent infections were taken into account, gametocyte rates by RTqPCR were close to 90%.
Sub-microscopic infections predominated in both communities independently of the observed differences in local transmission patterns. Overall, these results highlight the need of using highly sensitive molecular methods for malaria surveillance in pre-elimination settings. In this context, the relevance of sub-microscopic and asymptomatic infections (i.e. those that would escape routine LM-based diagnostics by field teams) resides largely on whether they contribute to effective mosquito infections and disease transmission. Although mosquito infectivity is known to increase with parasite density [37], P.falciparum infections at sub-microscopic levels have been suggested to account for as much as ≈30% of human-mosquito transmissions in countries like Burkina Faso [12,37,38]. For P.vivax, our understanding of the infectivity of sub-microscopic infections is much more limited, and the few data available has shown some variability, probably due in part to modest sample sizes [18,39]. A study in Brazil in which Anopheles darlingi fed directly on 11 P.vivax sub-microscopic patients found only 2 positive midguts [39], while Vallejo et al showed that 56% of naturally infected P.vivax sub-microscopic carriers from Colombia could infect Anopheles Albimanus, an infectivity rate similar to that of symptomatic carriers albeit with lower number of infected mosquitoes and oocyst counts [18]. Remarkably, the asymptomatic infections in the present study showed median gametocyte densities similar to those in symptomatic individuals. Moreover, a third of all gametocytemic patients were asymptomatic with patent parasitemia. Taken together, the data suggests that a lower success in infectivity of sub-microscopic infections might be compensated by the high frequency of both sub-microscopic and/or asymptomatic carriers (67% of P.vivax and all P.falciparum gametocyte carriers), as well as the relatively high P.vivax gametocyte densities in part of the asymptomatic population. In addition, asymptomatic parasite carriers will contribute to infectiousness for longer periods of time, as these individuals are not seeking treatment [40,41].
In terms of age groups, all those above 5 years of age were found to be more susceptible to both sexual and asexual P.vivax infections in this study, with a peak in >15–25 year old. This parallelism is in agreement with the strong association found between total parasite densities and presence of gametocytes. Conversely, other studies on P.vivax epidemiology have reported that prevalence of sexual stage infections decreases with age [10,15,19,20,23]. On one hand, individuals aged >15 years included here worked mainly in agriculture, forestry and fishing activities, what can increase their exposure to Anopheles darlingi bites during daytime. On the other hand, it is also reasonable to think that the sustained period of low transmission in Peru between 2005–2011 might have impacted immune patterns and shifted the acquisition of immunity towards older ages. This finding is in apparent contradiction with the observed high rates of sub-microscopic infections across all age groups, especially for P.falciparum. However, it has been suggested that after a period of reduction in malaria transmission, hosts might have better control of parasitemia and clinical disease provided that some immunity persists, time before reinfection is extended, and new infections are likely to be monoclonal [42]. Indeed, the study area experienced a reduction of the effective parasite population size due to a bottleneck event as a result of 2005–2011 control programs [43]. Whether age patterns will shift again after the increase in transmission observed since 2012 remains to be determined.
Significant differences in malaria indicators were found at community level, with residence in Lupuna being independently associated with higher infection rates in multivariate models. Differences between Cahuide and Lupuna communities are not surprising given their demographic and geographical characteristics. Whereas Lupuna is formed by forested riverine villages, Cahuide has scattered households, road-driven deforestation and a much more unstable population. Intense malaria control measures were applied in Cahuide in mid-2012 after a malaria outbreak, thus contributing to explain the lower infection prevalence. Furthermore, population genetic studies in the region showed that P.vivax population is highly structured suggesting different interactions with the host at the local scale [43]. The higher transmission in Lupuna was also accompanied by marked seasonality in P.vivax gametocyte carriage, which peaked one trimester before total infections did. However, since the addition of survey variable did not have a significant effect on the fit of multivariate model, differences in gametocyte rates between surveys are likely due to differences in total parasite densities rather than to a true seasonal effect. Overall, the detailed data obtained for two contrasting communities provides clues on what can be expected in other areas sharing similar characteristics and on how targeted interventions could be adapted.
This study has some limitations. On one hand, despite gametocyte carriage is a better indicator of the infectious reservoir than total parasite rates, it still remains indirect as compared to mosquito feedings, which have more control on factors like host and vector immunity. On the other hand, the trimestral sampling strategy allows for epidemiological characterization of gametocyte carriage, but does not allow for accurate calculation of the duration of gametocytemia; future studies on gametocyte dynamics in the area will contribute to answer these questions.
In conclusion, asymptomatic and sub-microscopic infections are significant contributors to the gametocyte reservoir in the Peruvian Amazon, despite the high degree of heterogeneity of transmission at the local scale and throughout the transmission season. Gametocyte prevalence peaks in young adults, but rates relative to asexual stage infections are similar across all age groups. Control and elimination campaigns need sensitive tools to detect infections that would otherwise escape routine malaria surveillance and may contribute to the maintenance of transmission in the Amazon region.
Supporting information
(PDF)
(PDF)
Only significant results are shown (P<0.05).
(PDF)
(PDF)
For univariate analysis only significant results are shown (P<0.05). Association analysis was not attempted for sexual stage infections due to the low number of cases.
(PDF)
A) P.vivax and P.falciparum parasite densities by qPCR as 18S rRNA copy numbers/μl of blood. B) P.vivax and P.falciparum gametocyte density estimates as gametocytes/μl of blood by RTqPCR. Numbers in brackets indicate number of observations at each survey.
(PDF)
Acknowledgments
We sincerely thank all individuals who accepted to participate in the study, as well as field workers, laboratory technicians, nurses, clinicians and data managers for their contribution to this work.
Data Availability
Public availability of data would compromise patient privacy; de-identified data is available, in unrestricted manner, upon request from qualified investigators to Jef Verellen: jverellen@itg.be.
Funding Statement
Funding was provided by the Belgian Directorate-General for Development Cooperation (DGD, http://diplomatie.belgium.be/en/policy/development_cooperation) within DGD-ITM Framework Agreement 3-III, by International Centers for Excellence in Malaria Research (ICEMR) program from National Institute of Allergy and Infectious Diseases (NIAID), United States of America (https://www.niaid.nih.gov/research/icemr-program-overview) and by US Public Health Service Grants U19AI089681 (Joseph M. Vinetz). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bassat Q, Velarde M, Mueller I, Lin J, Leslie T, Wongsrichanalai C, et al. Key Knowledge Gaps for Plasmodium vivax Control and Elimination. Am J Trop Med Hyg. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan-American Health Organization (PAHO). Plan of action for malaria elimination 2016–2020. 68th session of the regional committee of WHO for the Americas. 2016.
- 3.Dirección General de Epidemiología, Ministerio de Salud, Perú. Análisis de la situación de salud del Perú. 2013.
- 4.Branch O, Casapia WM, Gamboa D V, Hernandez JN, Alava FF, Roncal N, et al. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J. 2005;4:27 doi: 10.1186/1475-2875-4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roshanravan B, Kari E, Gilman RH, Cabrera L, Lee E, Metcalfe J, et al. Endemic malaria in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg. 2003;69(1):45–52. [PubMed] [Google Scholar]
- 6.Rosas-Aguirre A, Gamboa D, Manrique P, Conn JE, Moreno M, Lescano AG, et al. Epidemiology of Plasmodium vivax Malaria in Peru. Am J Trop Med Hyg. 2016. October 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosas-Aguirre A, Speybroeck N, Llanos-Cuentas A, Rosanas-Urgell A, Carrasco-Escobar G, Rodriguez H, et al. Hotspots of Malaria Transmission in the Peruvian Amazon: Rapid Assessment through a Parasitological and Serological Survey. PLoS One. 2015;10(9):e0137458 doi: 10.1371/journal.pone.0137458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24(2):377–410. doi: 10.1128/CMR.00051-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone W, Gonçalves BP, Bousema T, Drakeley C. Assessing the infectious reservoir of falciparum malaria: Past and future. Trends Parasitol. 2015;31(7):287–96. doi: 10.1016/j.pt.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 10.WWARN gametocytes study group. Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med. 2016;14(79). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouédraogo AL, Schneider P, De Kruijf M, Nébié I, Verhave JP, Cuzin-Ouattara N, et al. Age-dependent distribution of Plasmodium falciparum gametocytes quantified by PFS25 real-time QT-NASBA in a cross-sectional study in Burkina Faso. Am J Trop Med Hyg. 2007;76(4):626–30. [PubMed] [Google Scholar]
- 12.Ouédraogo AL, Gonçalves BP, Gnémé A, Wenger EA, Guelbeogo MW, Ouédraogo A, et al. Dynamics of the Human Infectious Reservoir for Malaria Determined by Mosquito Feeding Assays and Ultrasensitive Malaria Diagnosis in Burkina Faso. J Infect Dis. 2015;213(1):90–9. doi: 10.1093/infdis/jiv370 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z, Mitchell RM, Kariuki S, Odero C, Otieno P, Otieno K, et al. Assessment of submicroscopic infections and gametocyte carriage of Plasmodium falciparum during peak malaria transmission season in a community-based cross-sectional survey in western Kenya, 2012. Malar J. 2016;15(1):421 doi: 10.1186/s12936-016-1482-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grange L, Loucoubar C, Telle O, Tall A, Faye J, Sokhna C, et al. Risk factors for Plasmodium falciparum gametocyte positivity in a longitudinal cohort. PLoS One. 2015;10(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koepfli C, Robinson LJ, Rarau P, Salib M, Sambale N, Wampfler R, et al. Blood-Stage Parasitaemia and Age Determine Plasmodium falciparum and P. vivax Gametocytaemia in Papua New Guinea. PLoS One. 2015;10(5):e0126747 doi: 10.1371/journal.pone.0126747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouagna LC, Bancone G, Yao F, Yameogo B, Dabire KR, Costantini C, et al. Genetic variation in human HBB is associated with Plasmodium falciparum transmission. Nat Genet. 2010;42(4):328–31. doi: 10.1038/ng.554 [DOI] [PubMed] [Google Scholar]
- 17.Lawaly YR, Sakuntabhai A, Marrama L, Konate L, Phimpraphi W, Sokhna C, et al. Heritability of the human infectious reservoir of malaria parasites. PLoS One. 2010;5(6):e11358 doi: 10.1371/journal.pone.0011358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallejo AF, García J, Amado-Garavito AB, Arévalo-Herrera M, Herrera S. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J. 2016;15(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nacher M, Carrara VI, McGready R, Ashley E, Nguen J V, Thwai KL, et al. Seasonal fluctuations in the carriage of Plasmodium vivax gametocytes in Thailand. Ann Trop Med Parasitol. 2004;98(2):115–20. doi: 10.1179/000349804225003145 [DOI] [PubMed] [Google Scholar]
- 20.Waltmann A, Darcy AW, Harris I, Koepfli C, Lodo J, Vahi V, et al. High Rates of Asymptomatic, Sub-microscopic Plasmodium vivax Infection and Disappearing Plasmodium falciparum Malaria in an Area of Low Transmission in Solomon Islands. PLoS Negl Trop Dis. 2015;9(5):e0003758 doi: 10.1371/journal.pntd.0003758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbosa S, Gozze AB, Lima NF, Batista CL, Bastos M da S, Nicolete VC, et al. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8(8):e3109 doi: 10.1371/journal.pntd.0003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nacher M, Silachamroon U, Singhasivanon P, Wilairatana P, Phumratanaprapin W, Fontanet A, et al. Risk factors for Plasmodium vivax gametocyte carriage in Thailand. Am J Trop Med Hyg. 2004;71(6):693–5. [PubMed] [Google Scholar]
- 23.Karl S, Laman M, Moore BR, Benjamin JM, Salib M, Lorry L, et al. Risk factors for Plasmodium falciparum and Plasmodium vivax gametocyte carriage in Papua New Guinean children with uncomplicated malaria. Acta Trop. 2016;160:1–8. doi: 10.1016/j.actatropica.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 24.Douglas NM, Simpson JA, Phyo AP, Siswantoro H, Hasugian AR, Kenangalem E, et al. Gametocyte dynamics and the role of drugs in reducing the transmission potential of plasmodium vivax. J Infect Dis. 2013;208(5):801–12. doi: 10.1093/infdis/jit261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mckenzie FE, Wongsrichanalai C, Magill AJ, Forney JR, Permpanich B, Lucas C, et al. Gametocytemia in Plasmodium vivax and Plasmodium falciparum infections. J Parasitol. 2006;92(6):1281–5. doi: 10.1645/GE-911R.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima NF, Bastos MS, Ferreira MU. Plasmodium vivax: reverse transcriptase real-time PCR for gametocyte detection and quantitation in clinical samples. Exp Parasitol. 2012;132(3):348–54. doi: 10.1016/j.exppara.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mateo S. Situación epidemiológica de la malaria en el Perú (SE 52–2013). Bol Epidemiol Perú - Minist Salud del Perú. 2013;22 (52):1088–1097. [Google Scholar]
- 28.Moreno M, Saavedra MP, Bickersmith SA, Lainhart W, Tong C, Alava F, et al. Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar J. 2015;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangold KA, Manson RU, Koay ESC, Stephens L, Regner M, Thomson RB, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43(5):2435–40. doi: 10.1128/JCM.43.5.2435-2440.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, et al. Strategies for detection of Plasmodium species gametocytes. PLoS One. 2013;8(9):e76316 doi: 10.1371/journal.pone.0076316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betuela I, Rosanas-Urgell A, Kiniboro B, Stanisic DI, Samol L, de Lazzari E, et al. Relapses contribute significantly to the risk of Plasmodium vivax infection and disease in Papua New Guinean children 1–5 years of age. J Infect Dis. 2012;206(11):1771–80. doi: 10.1093/infdis/jis580 [DOI] [PubMed] [Google Scholar]
- 32.Kulldorff M. A spatial scan statistic. Commun Stat Theory Meth. 1997;26(6):1481–96. [Google Scholar]
- 33.Griffith DA. The Boundary Value Problem in Spatial Statistical Analysis. J Reg Sci. 1983;23(3):377–87. [DOI] [PubMed] [Google Scholar]
- 34.Getis A, Ord JK. The Analysis of Spatial Association by Use of Distance Statistics. Geogr Anal. 1992;24:189–206. [Google Scholar]
- 35.Servicio Nacional de Meteorología e Hidrología del Perú (SENAMHI), Ministerio del Ambiente, Peru: Available from: http://www.senamhi.gob.pe. [Google Scholar]
- 36.Hulsen T, de Vlieg J, Alkema W, Venn J, Chow S, Ruskey F, et al. BioVenn–a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics. 2008;9(1):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouédraogo AL, et al. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife. 2013. January;2:e00626 doi: 10.7554/eLife.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouédraogo AL, Bousema T, Schneider P, de Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One. 2009;4(12):e8410 doi: 10.1371/journal.pone.0008410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42(5):777–9. [DOI] [PubMed] [Google Scholar]
- 40.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11(6):623–39. doi: 10.1586/eri.13.45 [DOI] [PubMed] [Google Scholar]
- 41.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Pereira da Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002;66(6):641–8. [DOI] [PubMed] [Google Scholar]
- 42.Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237 doi: 10.1038/ncomms2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgado-Ratto C, Gamboa D, Soto-Calle VE, Van den Eede P, Torres E, Sánchez-Martínez L, et al. Population Genetics of Plasmodium vivax in the Peruvian Amazon. PLOS Neg Trop Dis. 2016;10(1):e0004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Only significant results are shown (P<0.05).
(PDF)
(PDF)
For univariate analysis only significant results are shown (P<0.05). Association analysis was not attempted for sexual stage infections due to the low number of cases.
(PDF)
A) P.vivax and P.falciparum parasite densities by qPCR as 18S rRNA copy numbers/μl of blood. B) P.vivax and P.falciparum gametocyte density estimates as gametocytes/μl of blood by RTqPCR. Numbers in brackets indicate number of observations at each survey.
(PDF)
Data Availability Statement
Public availability of data would compromise patient privacy; de-identified data is available, in unrestricted manner, upon request from qualified investigators to Jef Verellen: jverellen@itg.be.