ABSTRACT
MGE1 encodes a yeast chaperone involved in Fe-S cluster metabolism and protein import into the mitochondria. In this study, we identified MGE1 as a multicopy suppressor of susceptibility to the antifungal fluconazole in the model yeast Saccharomyces cerevisiae. We demonstrate that this phenomenon is not exclusively dependent on the integrity of the mitochondrial DNA or on the presence of the drug efflux pump Pdr5. Instead, we show that the increased dosage of Mge1 plays a protective role by retaining increased amounts of ergosterol upon fluconazole treatment. Iron metabolism and, more particularly, Fe-S cluster formation are involved in regulating this process, since the responsible Hsp70 chaperone, Ssq1, is required. Additionally, we show the necessity but, by itself, insufficiency of activating the iron regulon in establishing the Mge1-related effect on drug susceptibility. Finally, we confirm a similar role for Mge1 in fluconazole susceptibility in the pathogenic fungi Candida glabrata and Candida albicans.
KEYWORDS: Candida albicans, Candida glabrata, Fe-S cluster, Mge1, Saccharomyces cerevisiae, antifungal susceptibility, fluconazole, iron metabolism, mitochondrial chaperone
IMPORTANCE
Although they are mostly neglected compared to bacterial infections, fungal infections pose a serious threat to the human population. While some of them remain relatively harmless, infections that reach the bloodstream often become lethal. Only a few therapies are available, and resistance of the pathogen to these drugs is a frequently encountered problem. It is thus essential that more research is performed on how these pathogens cope with the treatment and cause recurrent infections. Baker’s yeast is often used as a model to study pathogenic fungi. We show here, by using this model, that iron metabolism and the formation of the important iron-sulfur clusters are involved in regulating susceptibility to fluconazole, the most commonly used antifungal drug. We show that the same process likely also occurs in two of the most regularly isolated pathogenic fungi, Candida glabrata and Candida albicans.
INTRODUCTION
Fungal infections pose a significant threat to the health of humans and other organisms. Some of these infections are superficial and merely impose a mild form of inconvenience to the patient, while others are invasive, causing severe disease and, potentially, death. Once an invasive infection is established, the likelihood of survival for the patient rarely exceeds 50% (1). The gravity of fungal infections and the concomitant importance of searching for new and better antifungal therapies are generally underappreciated. The number of drugs available against fungal infections is limited, and those that are commonly used often suffer from being fungistatic rather than fungicidal (2, 3). The azoles, with fluconazole (flu) being the most studied, comprise one of these commonly used, fungistatic classes of antifungals (4). The azoles target the ergosterol biosynthesis pathway, more particularly, the lanosterol 14α-demethylase (Erg11). This enzyme is essential in Saccharomyces cerevisiae, making the nonfungicidal nature of these drugs paradoxical (5, 6). Resistance to azoles is regularly caused by increased expression of genes encoding efflux pumps, causing overexpression of or altering the target gene by point mutations or generating cellular responses to cope with stress (4). The fungus can, however, also obtain certain transient, metabolic or epigenetic, adaptations that confer decreased susceptibility to the antifungal agent. This slow residual growth at inhibitory concentrations of the drug is called tolerance or trailing growth and hypothetically also generates the time needed for and the possibility of directional selection promoting the acquirement of alterations in the genome, causing resistance (7, 8).
In this project, we isolated MGE1 as a multicopy suppressor of fluconazole susceptibility in S. cerevisiae. Mge1 is a cochaperone for members of the Hsp70 family of chaperones (9, 10), which serve functions in several cellular processes such as protein folding, preventing protein aggregation, protein translocation, targeted degradation, and adjusting the activity of regulatory proteins (11, 12). This cochaperone was discovered as a member of the mitochondrial import system, translocating proteins across the inner membrane into the matrix of the mitochondria (9, 13–15). The Hsp70 molecule involved is Ssc1, which is, like Mge1, an essential protein and is involved in refolding of denatured proteins (9, 16–18). Mge1 also functions as the nucleotide exchange factor of Ssq1, another Hsp70 chaperone, which is involved in the Fe-S cluster biosynthesis pathway (19). Fe-S clusters are essential cofactors involved in redox, catalytic, and regulatory processes, including the regulation of the iron starvation response (20–24). Ssq1 is responsible for transferring the assembled Fe-S cluster from the Isu1 scaffold to the target protein by destabilizing the connection between the cluster and this scaffold (25). In contrast to Ssc1 and Mge1, Ssq1 is not essential because when Ssq1 is depleted, Ssc1 can probably take over part of its function (26). We showed earlier that iron metabolism is involved in regulating susceptibility to fluconazole, since addition of the iron chelator doxycycline to fluconazole-treated Candida albicans and S. cerevisiae cells reduces or even completely abolishes tolerance (7, 27). In this paper, we provide evidence of the involvement of Fe-S cluster metabolism and signaling through the iron regulon in the Mge1-dependent regulation of fluconazole susceptibility in S. cerevisiae. We also demonstrate that this altered susceptibility is accompanied by modulation of the metabolic flux through the ergosterol synthesis pathway. Finally, we show that overexpressing the orthologues of MGE1 in the pathogenic fungi C. glabrata and C. albicans affects fluconazole susceptibility in a similar way. As such, elucidating this apparently conserved fungal mechanism may yield interesting new targets for drug development.
RESULTS
Increased dosage of Mge1 acts as a suppressor of susceptibility to fluconazole in S. cerevisiae.
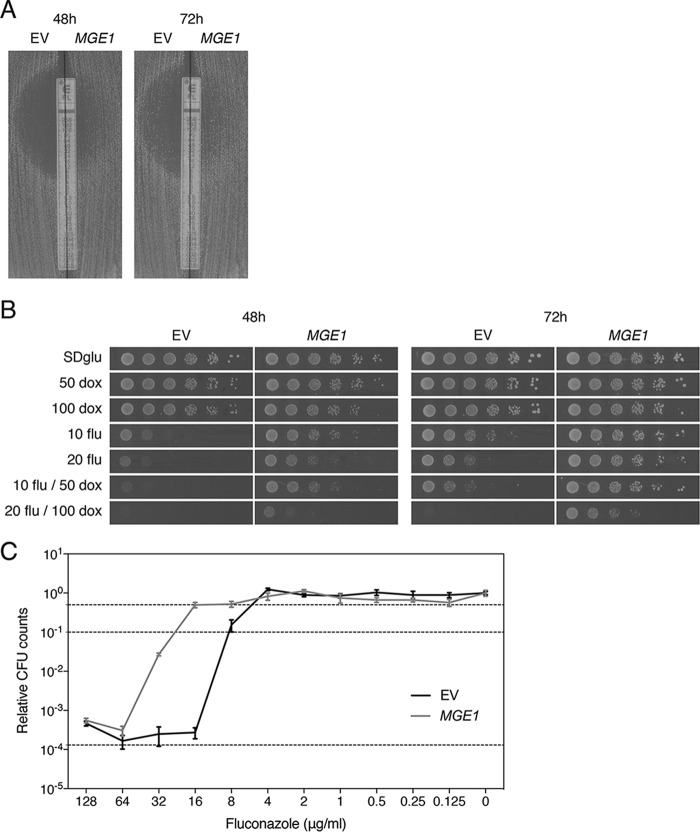
Aiming to identify new regulators of fluconazole susceptibility, we performed a screening of BY4742 transformed with multicopy plasmids, containing parts of the S. cerevisiae genomic library obtained from F. Lacroute (28). To reduce the background growth of the reference strain on the screening medium containing supra-minimum inhibitory concentrations (MICs) of fluconazole, we added the iron chelator doxycycline, for which we and others reported a synergistic effect with fluconazole earlier (7, 27). The resulting reduction of background growth allowed us to more clearly distinguish true multicopy suppressors of fluconazole susceptibility. Using these sensitized screening conditions (10 μg/ml fluconazole and 50 μg/ml doxycycline), we isolated the Hsp70 cochaperone Mge1, next to Erg11, as a dosage-dependent suppressor of susceptibility to fluconazole. We subcloned the MGE1 fragment (containing the promoter, open reading frame [ORF], and terminator) from the pFL44 plasmid into YEPlac195 and verified overexpression in transformants using quantitative reverse transcription-PCR (qRT-PCR), which yielded a fold increase of 11.7 (standard error of the mean [SEM], 1.40) compared to the control strain. This increased expression causes a strong decrease in susceptibility to fluconazole compared to the empty vector control. The improved growth of the transformed BY4742 strain (indicated as MGE1 in all figures) compared to the control (with empty YEPlac195, indicated as EV) was visualized by means of the Etest method and spot assays (Fig. 1A and B). The MICflu of these strains was determined by Etest analyses and broth microdilution assays. All experiments were done with at least three biological repeats, showing consistent results. The MICflu values are depicted in Table 1. We can conclude from these data that overexpression of MGE1 causes a decrease in the susceptibility to fluconazole in S. cerevisiae and that this effect is more clearly visible under sensitized conditions where doxycycline is added to the medium. From the broth microdilution assay, we not only were able to determine the MIC50 and MIC90 of the mutant compared to the control but also defined the effect of the overexpression on the growth at supra-MICs of fluconazole, called tolerance. Figure 1C shows that, although the MIC50 and MIC90 change clearly when MGE1 is overexpressed, there is no significant difference between the colony forming unit (CFU) counts at higher fluconazole concentrations. Therefore, in the following parts of this article, we use only the MICflu as a readout of drug susceptibility.
FIG 1 .
MGE1 overexpression improves growth of the wild-type S. cerevisiae strain on fluconazole. (A) Etest analysis of the overexpression strain (MGE1) and control strain (EV). (B) Serial dilutions of both strains were spotted on SDglu medium containing fluconazole (flu; 10 or 20 μg/ml) and/or doxycycline (dox; 50 or 100 μg/ml). Pictures were taken after 48 and 72 h of incubation at 30°C. (C) Tolerance assay. Data represent dose-response curves determined for both strains, with dotted lines indicating 50% (upper line) and 90% (middle line) growth inhibition and the initial inoculum (lower line). No significant difference was observed in trailing growth between the overexpression strain and control strain (P = 0.731 for 128 μg/ml flu and P = 0.381 for 64 μg/ml flu, tested by two-way ANOVA with Bonferroni correction).
TABLE 1 .
The effect of MGE1 overexpression on the MICflu of several strainsd
| Strain | MICflu (μg/ml) |
||
|---|---|---|---|
| Etest | Broth microdilution assay |
||
| MIC50 | MIC90 | ||
| S. cerevisiae BY4742 EV | 6–8 | 8–16 | 16 |
| S. cerevisiae BY4742 MGE1 | 24–32 | 16–32 | 32–64 |
| S. cerevisiae ira2Δ EV | 4–6 | 8–16 | 8–16 |
| S. cerevisiae ira2Δ MGE1 | 12–16 | 16–32 | 16–32 |
| S. cerevisiae yme1Δ EV | 12–16 | 8–16 | 16–32 |
| S. cerevisiae yme1Δ MGE1 | 32–48 | 16–32 | 32–64 |
| S. cerevisiae opi1Δ EV | 12–16 | 8–16 | 8–16 |
| S. cerevisiae opi1Δ MGE1 | 48–64 | 32–64 | 32–64 |
| S. cerevisiae rho0 EV | 24–32 | 16–32 | 16–32 |
| S. cerevisiae rho0 MGE1 | >256 | 32–64 | 32–64 |
| S. cerevisiae pdr5Δ EV | 0.25 | 0.5–1 | 0.5–1 |
| S. cerevisiae pdr5Δ MGE1 | 0.75 | 0.5–1 | 1–2 |
| S. cerevisiae upc2Δ EV | 4–6 | 4–8 | 4–8 |
| S. cerevisiae upc2Δ MGE1 | 24–32 | 8–16 | 16–32 |
| S. cerevisiae tom70Δ EV | 6–8 | 8–16 | 8–16 |
| S. cerevisiae tom70Δ MGE1 | 16–24 | 16–32 | 32–64 |
| S. cerevisiae ecm10Δ EV | 6–8 | 8–16 | 16–32 |
| S. cerevisiae ecm10Δ MGE1 | 24–32 | 32–64 | 32–64 |
| S. cerevisiae ssq1Δ EV | 2–4a | 2–4a | 2–4a |
| S. cerevisiae ssq1Δ MGE1 | 1–1.5a | —b | —b |
| S. cerevisiae aft1Δ EV | 4–6 | 8–16 | 16 |
| S. cerevisiae aft1Δ MGE1 | 4–6 | 8–16 | 8–16 |
| S. cerevisiae aft2Δ EV | 4–6 | 8–16 | 8–16 |
| S. cerevisiae aft2Δ MGE1 | 32–48 | 16–32 | 32–64 |
| S. cerevisiae BY4742 | 6–8 | 8–16 | 16–32 |
| S. cerevisiae fra1Δ | 6–8 | 8–16 | 16–32 |
| C. glabrata HTL EV | 8 (16–24)c | 2–4 | 4–8 |
| C. glabrata HTL pTDH3-CgMGE1 | 24 (48–64)c | 4–8 | 8–16 |
| C. glabrata HTL pPGK1-CgMGE1 | 16 (48–64)c | 2–4 | 8–16 |
Data were determined after 72 h on SCglu (latter only for Etest).
—, data could not be determined due to low growth.
RPMI medium with 0.2% (or 2%) glucose was used.
Values were determined by Etest and broth microdilution analysis. MGE1, MGE1 overexpression; EV, empty vector control.
Next, we aimed to check the effect of fluconazole on MGE1 expression under our experimental conditions. We performed qRT-PCR experiments on a wild-type BY4742 strain in the absence or presence of 20 μg/ml fluconazole. The expression of the gene decreased 2-fold in the presence of the drug, indicating that Mge1 itself might be a direct or indirect target of fluconazole {relative expression level with SEM, 1 ± 0.046 versus 0.498 ± 0.045 for 0 versus 20 μg/ml fluconazole with P = <0.001 [paired Student’s t test on log2(Y) transformed data]}.
Mge1 can induce fluconazole resistance independently of rho0 formation and Pdr5.
S. cerevisiae cells can lose part or all of their mitochondrial genome, generating so-called rho− or rho0 cells, respectively (29, 30). It has been reported that such cells acquire resistance to certain chemicals such as fluconazole, though the underlying mechanisms are not yet fully known (31). Petite-negative strains contain nuclear mutations that render the loss of (part of) the mitochondrial genome lethal (32). Consequently, these strains cannot form rho0 or rho− cells. To verify whether decreased fluconazole susceptibility of the MGE1 overexpression strain might be caused by increased generation of rho0/− cells, we transformed petite-negative strains with the overexpression vector. We chose three mutants involved in seemingly independent processes. The null mutants of OPI1, IRA2, and YME1 were all discovered to be dependent on mitochondrial DNA (mtDNA) (33, 34). For these strains, the MICflu tests were performed in minimal synthetic defined glucose (SDglu) medium as well as rich yeast extract-peptone-dextrose (YPD) medium, as it has been suggested that some petite-negative strains depend only on their mtDNA in rich medium (34). Figure S1A in the supplemental material and the MICflu values in Table 1 and in Table S4 in the supplemental material show the sustained effect of MGE1 overexpression on growth of the petite-negative strains in the presence of fluconazole, arguing against the hypothesis that rho0/− cells are the sole cause of improved growth on fluconazole. We have to take into account, however, that overexpression of MGE1 could potentially suppress the dependency of the petite-negative mutants on their mtDNA. Nevertheless, overexpression of MGE1 also causes an increase in the MICflu of a rho0 strain, as can be seen in Table 1 and Fig. S1A, confirming our hypothesis more incontestably. Taken together, these data suggest that the decreased susceptibility to fluconazole in MGE1-overexpressing cells is not (solely) caused by increased generation of rho0/− cells.
Etest analysis of the S. cerevisiae strains under study. Pictures were taken after 48 h (or 72 h) of incubation at 30°C. (A) MGE1 overexpression in different petite-negative strains and a rho0 strain, tested on SDglu and YPD media. (B) Deletion of PDR5 in BY4742 and rho0 and MGE1 overexpression in the pdr5Δ strain. (C) MGE1 overexpression compared to EV control results in a upc2Δ strain. (D) tom70Δ strain compared to the BY4742 wild type and MGE1 overexpression in this deletion strain. (E) ecm10Δ strain compared to the BY4742 wild type and MGE1 overexpression in this deletion strain. (F) ssq1Δ strain compared to the BY4742 wild type and MGE1 overexpression in this deletion strain [SC(-URA)glu medium was used here, results seen after 48 and 72 h are shown]. (G) MGE1 overexpression compared to EV control in aft1Δ and aft2Δ strains. (H) fra1Δ strain compared to the BY4742 wild type. Download FIG S1, PDF file, 8 MB (8.2MB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overexpression of genes encoding drug efflux pumps is another well-known method of acquiring resistance to drugs that can penetrate the cell. In Candida species, expression of genes of the CDR and MDR families, encoding ABC transporters and major facilitators, respectively, are often found upregulated in clinically isolated resistant strains (35–37). The orthologue of the C. albicans CDR1 gene in S. cerevisiae is PDR5. It was verified that PDR5 expression is augmented in rho0/− cells compared to rho+ cells. The acquired resistance to several types of chemicals is thought to be caused by this phenomenon (31). Expression levels of PDR5 were higher in the MGE1 overexpression strain, indicating that Pdr5 might have been involved in the increased growth on fluconazole {relative expression level ± SEM for 0 μg/ml fluconazole and EV versus MGE1, 1.000 ± 0.047 versus 1.596 ± 0.165 with P < 0.01; for 20 μg/ml fluconazole and EV versus MGE1, 1.482 ± 0.086 versus 2.494 ± 0.088 with P < 0.001 [Bonferroni-corrected two-way analysis of variance (ANOVA) of log2(Y) transformed data]}. To determine whether this increase was the sole cause of the decreased susceptibility of the MGE1 overexpression strain, we assessed the effect of MGE1 overexpression on the MICflu of the pdr5Δ strain. It can be seen from Table S4 and Fig. S1B that deletion of PDR5 in the rho0 background reduced the MICflu to the same level as deletion of PDR5 in the wild-type BY4742 background, reinforcing the notion that much of the fluconazole resistance of rho0/− cells is due to upregulation of PDR5 expression. Overexpressing MGE1 in a pdr5Δ strain still resulted in a significant increase of the MICflu from 0.25 to 0.75 μg/ml (Table 1; Fig. S1B) indicating that, while increased expression of PDR5 in cells with an elevated dosage of Mge1 may still play a minor role, Mge1 can induce fluconazole resistance independently of the efflux pump.
Overexpression of MGE1 increases the residual amount of ergosterol after treatment with fluconazole independently of the expression of fluconazole-induced ERG genes.
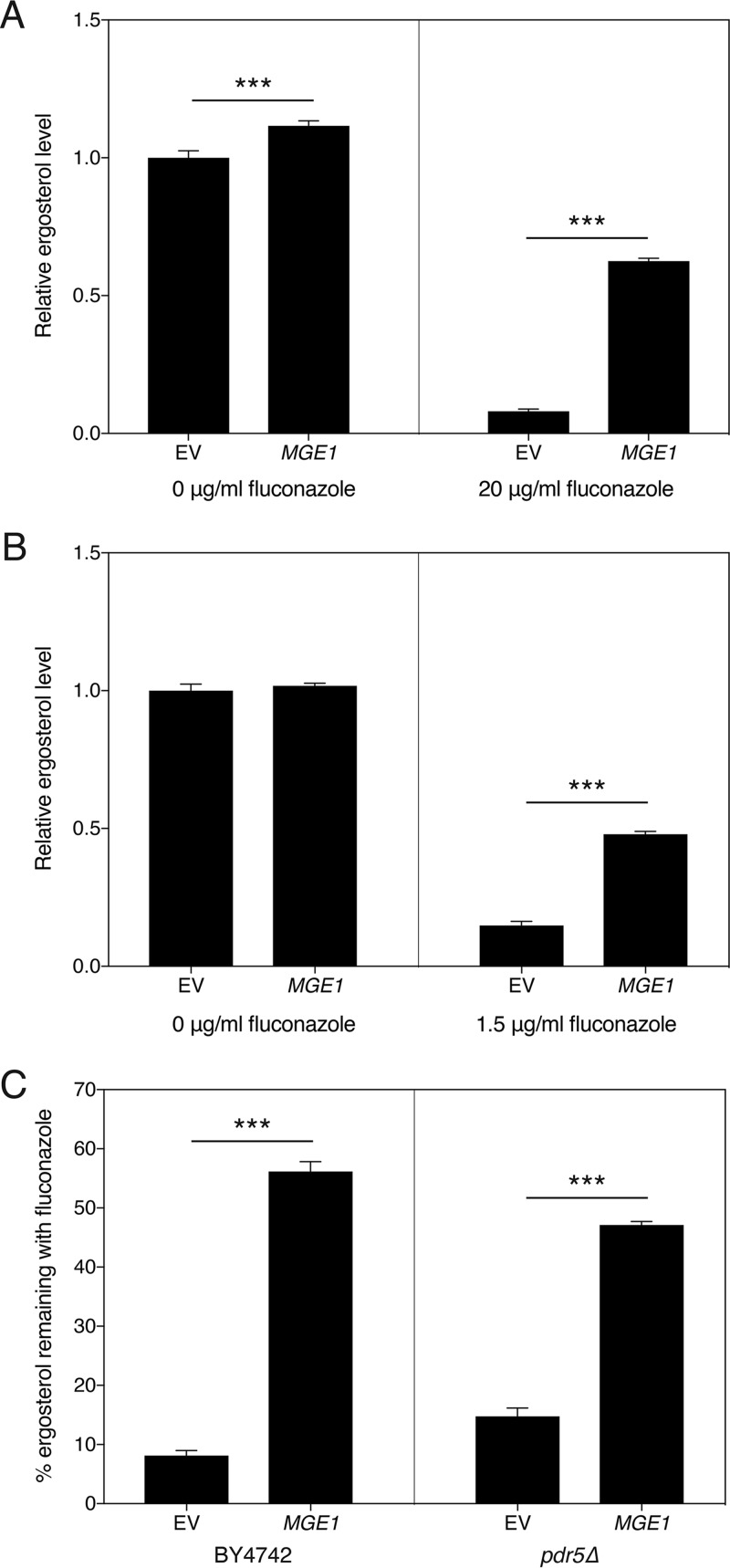
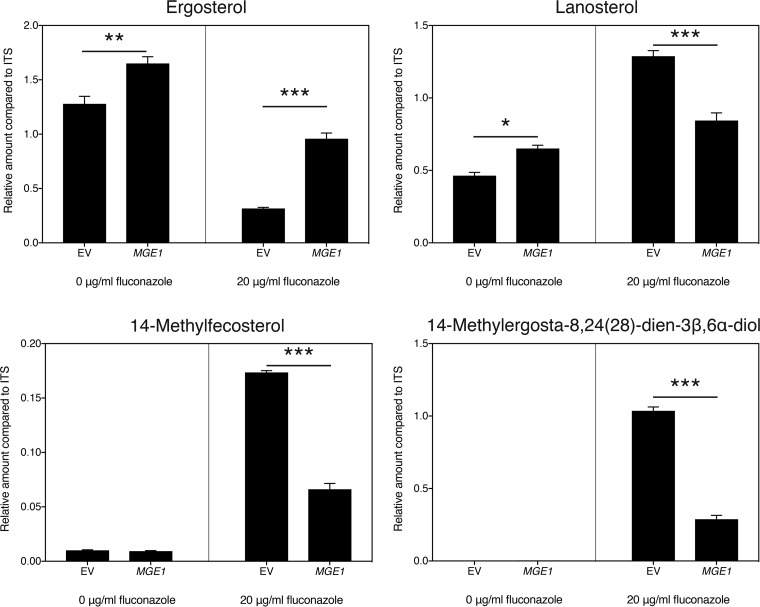
As already shown by Arthington-Skaggs et al. for C. albicans (38), resistance to fluconazole often correlates with higher residual ergosterol levels after drug application. To investigate the possible role of ergosterol in mediating the effect of MGE1 overexpression on fluconazole susceptibility, we measured ergosterol in the overexpression mutant. As can be seen from Fig. 2A, in the absence of fluconazole, there was only a small difference between the control and the strain overexpressing MGE1. Upon treatment with fluconazole, however, the fraction of ergosterol remaining in the mutant was significantly higher than the control (Fig. 2A and C). This phenotype was again independent of Pdr5, since the effect was still visible in the pdr5Δ mutant (Fig. 2B and C). To elucidate how MGE1 overexpression affects sterol synthesis in general, we performed gas chromatography-mass spectrometry (GC-MS) analysis of the sterols isolated from our strains, in the absence and presence of fluconazole (Fig. S2A and B). As fluconazole targets Erg11, lanosterol accumulates and ergosterol levels decrease in the presence of the drug. Under these conditions, lanosterol is also converted to 14-methylfecosterol and ultimately to the toxic compound 14-methylergosta-8,24(28)-dien-3β,6α-diol, which represents an important aspect of the mode of action of the drug (39, 40). Interestingly, we saw that, compared to the control strain, overexpression of MGE1 reduced the metabolic flux that leads to toxic sterol formation, thereby maintaining a higher flux toward ergosterol production (Fig. 3).
FIG 2 .
Ergosterol levels are less affected by fluconazole when MGE1 is overexpressed. S. cerevisiae cells were grown in SDglu medium for 24 h, in the presence or absence of fluconazole. (A and B) Ergosterol levels for transformants in the BY4742 background (A) and pdr5Δ background (B) are displayed. We note that for the pdr5Δ strain, a smaller amount of fluconazole had to be used, due to the increased sensitivity to the drug. The values were calculated relative to the average of the values from the untreated samples. For panels A and B, the interaction between both parameters was statistically significant (P < 0.001). (C) Percentage of residual ergosterol for both backgrounds, after fluconazole treatment. Statistical analysis was conducted by two-way ANOVA with Bonferroni correction (A and B) and an unpaired Student’s t test (C); ***, P < 0.001.
FIG 3 .
MGE1 overexpression alters the level of several sterols. Cells were grown in SDglu medium for 24 h in the presence or absence of fluconazole. Sterol levels were determined by GC-MS and are displayed for ergosterol, lanosterol, 14-methylfecosterol, and 14-methylergosta-8,24(28)-dien-3β,6α-diol. The values were calculated relative to the internal standard (ITS; cholestane). The interaction between the two parameters was significant for each sterol (P < 0.05). Statistical analysis was conducted by two-way ANOVA with Bonferroni correction; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data from other sterols that were detected, but that were generally less abundant or could not be identified, are displayed in Fig. S2A.
Sterols detected by GC-MS in the MGE1 overexpression strain compared to the control. (A) Strains were grown in SDglu medium for 24 h, in the absence or presence of fluconazole. The sterol composition of EV and MGE1 strains was analyzed under both sets of conditions. The abundance of each compound was calculated as the peak area relative to cholestane (internal standard) based on CG-MS analysis of four biological replicates. RT, retention time; RRT, relative retention time. (B) Schematic representation of the main ergosterol biosynthesis pathway (adapted from reference 78), with the sterols that we identified in red. The symbol depicted next to the identified sterol indicates how the fluconazole-induced change in this sterol is altered upon overexpression of MGE1 (only significant changes are shown [tested by two-way ANOVA with Bonferroni correction]). Download FIG S2, PDF file, 0.8 MB (883.3KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
As Erg11 is the target of fluconazole and the point in the sterol synthesis pathway where progress to either ergosterol or the toxic sterol is defined, it seems valid to hypothesize that Erg11 might be the enzyme linking Mge1 to ergosterol. We checked the expression levels and protein levels of ERG11 and the Erg11 protein, respectively, in the mutant and control strains in both the absence and presence of fluconazole. Remarkably, gene expression levels and protein levels remained the same and were reduced, respectively, rather than upregulated in the MGE1 overexpression strain (Fig. S3A and B). For the Western blot analysis, we used an anti-hemagglutinin (anti-HA) antibody and the AFc202 strain, where ERG11 was tagged chromosomally with a 3× HA tag, thus representing native expression. We verified that the MICflu of this mutant is similar to that of the BY4742 wild-type strain, as can be seen in Table S4. Apart from ERG11, other genes encoding ergosterol biosynthesis enzymes have also been shown to be induced upon azole treatment (41–44). Still, we found that overexpression of MGE1 did not significantly upregulate the expression of ERG2, ERG3, ERG4, ERG5, ERG6, ERG7, ERG8, ERG9, ERG12, ERG19, ERG24, or ERG25 under either control or fluconazole-treated conditions (Fig. S3A). As described by MacPherson et al. in 2005 for C. albicans (43), Upc2 confers resistance to antifungals by modulating expression of certain genes involved in ergosterol biosynthesis. We confirm here that Mge1 did not function upstream of Upc2 in increasing the MICflu, since MGE1 overexpression still caused a decrease in fluconazole susceptibility in an upc2Δ strain (Table 1; Fig. S1C). Additionally, Upc2 did not influence MGE1 expression, as can be seen from Fig. S3C. In summary, although Mge1 alters the flux through the sterol synthesis pathway, thereby maintaining increased ergosterol levels and decreasing toxic sterol levels, this does not appear to be mediated by changing the expression level of the fluconazole-dependent genes encoding the main biosynthesis enzymes in this sterol pathway.
Increased dosage of Mge1 does not cause Erg11 levels or ERG-related gene expression to increase. (A) Expression of none of the ERG genes increased significantly when MGE1 was overexpressed. Cells were grown for 24 h in SDglu medium, in the presence or absence of 20 μg/ml fluconazole. The results of qRT-PCR analysis are displayed as the average of log2(Y) transformed values with the SEM. The values were calculated relative to the average of the values from the untreated samples. Statistical analysis was conducted on the transformed values by two-way ANOVA with Bonferroni correction; *, P < 0.05; **, P < 0.01. (B) Erg11 protein levels decrease slightly when MGE1 is overexpressed. Cells from strain AFc202 were grown for 24 h in SDglu medium, in the presence or absence of 20 μg/ml fluconazole. The blots were probed with anti-HA or anti-Pgk1 antibodies (loading control). (C) Expression of MGE1 does not alter significantly in an upc2 Δ strain compared to the wild type. The results of qRT-PCR analysis are displayed as the average of log2(Y) transformed values with the SEM relative to BY4742 values. Statistical analysis was conducted on the transformed values by unpaired Student’s t test. Download FIG S3, PDF file, 0.3 MB (313.9KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The Mge1-dependent decrease in fluconazole susceptibility requires the mitochondrial chaperone Ssq1.
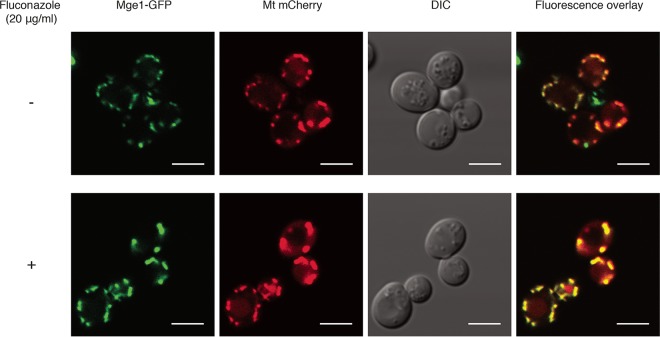
Both known processes involving Mge1, i.e., Fe-S cluster formation and protein import across the inner mitochondrial membrane, are localized to the mitochondria. Although the literature also reports on the localization of Mge1 to this organelle (45), the experimental procedures used always consisted of in vitro rather than in vivo methods. To verify that Mge1 indeed functions inside the mitochondria, in the absence as well as the presence of fluconazole, we checked its localization by fluorescence microscopy. From Fig. 4, it can be seen that Mge1 localized to the mitochondria in the overexpression mutant, under both conditions. This indicates that the function by which Mge1 causes a decrease in the susceptibility to fluconazole must also be confined to this organelle. It was verified that MGE1-GFP overexpression still caused an increased MICflu level (Table S4).
FIG 4 .
Mge1 localizes to the mitochondria. The BY4742 strain expressing both MGE1-GFP and mitochondrially targeted (Mt) mCherry was incubated for 24 h in the absence or presence of 20 μg/ml fluconazole, pictures were taken afterward. The scale bar represents 5 μm. DIC, differential interference contrast.
To further elucidate the mode of action by which Mge1 converts resistance to fluconazole, we postulated that this phenotype is effectuated by either of the downstream Hsp70 proteins. Ssc1 is part of the TIM23 complex spanning the inner mitochondrial membrane and works as an ATPase, providing energy to transport proteins into the mitochondria. A paralog of Ssc1, Ecm10, probably arose through genome duplication (82% amino acid identity) and is thought to have functions that overlap those of Ssc1 (46–48). It has been shown that Ecm10 also interacts with Mge1 (48). Ssq1 shows limited homology with Ssc1 (52% amino acid identity) and plays a role in one of the initial steps of Fe-S cluster formation, together with Mge1 (19). To determine whether the effect of MGE1 overexpression on fluconazole susceptibility operates through Ssc1, Ecm10, or Ssq1, we verified if the resistance phenotype is still observed in mutants with a defect in either of the downstream pathways. Tom70 is part of the translocase of the outer mitochondrial membrane (TOM) complex, playing a role in recognizing and importing mitochondrial proteins (49, 50). Deletion of the TOM70 gene affects protein import into the mitochondria (50). The MICflu of the tom70Δ strain was equal to that of the wild type (Fig. S1D; Table S4), and overexpression of MGE1 in this strain resulted in an increase in the MICflu similar to that seen with the wild type, indicating that full protein import into the mitochondria is not essential for the Mge1-related effect on fluconazole susceptibility (Fig. S1D; Table 1). As mentioned before, Ecm10 is a paralog of Ssc1. Since deletion of ECM10, in contrast to SSC1, is viable, we decided to see if overexpression of MGE1 in this strain would still cause an increase in the MICflu. As can be seen from Fig. S1E and Table S4, the ecm10Δ strain had an MICflu similar to that of the wild-type BY4742 strain. Overexpression of MGE1 in this strain changed this MICflu in the same way as was seen with BY4742 (Fig. S1E; Table 1), implying that Ecm10 is also not involved. Deletion of SSQ1 is viable; therefore, we also tested the MICflu of the ssq1Δ strain and found it to be significantly lower than that of the BY4742 wild-type strain (Fig. S1F; Table S4), in agreement with a previous report by Dagley et al. (51). Overexpression of MGE1 in the ssq1Δ strain yielded remarkably few and slow-growing transformants (our unpublished observations), suggesting that combining a deletion of SSQ1 with overexpression of MGE1 alters the cells’ fitness. Additionally, when MGE1 was overexpressed, the MICflu of the ssq1Δ strain did not increase compared to that of the empty vector control and even displayed a decrease (Fig. S1F; Table 1). This suggests that Ssq1 is necessary to establish the Mge1-mediated effect on fluconazole susceptibility.
Activation of the iron regulon is necessary but not sufficient for Mge1 to exert its effect on fluconazole susceptibility.
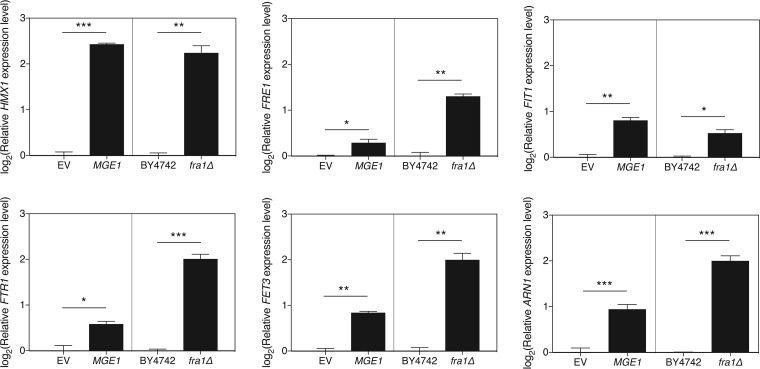
It seems evident, from the literature and previous findings described above, that iron plays a role in regulating susceptibility to fluconazole (7, 27). In an attempt to clarify how this happens and how Mge1 provides a link in this process, we investigated the possible involvement of the iron regulon. Aft1 is a transcriptional regulator which induces transcription of genes involved in the recovery of iron upon iron starvation (22, 23). AFT2 encodes a paralog of AFT1, which arose through gene duplication. The proteins encoded by the two genes have partially overlapping functions, with Aft2 being responsible for the iron metabolism when Aft1 is not present (24). Overexpression of MGE1 still reduced the susceptibility to fluconazole in an aft2Δ strain, but this effect was lost in the aft1Δ strain (Fig. S1G; Table 1). As Aft1 is necessary for the Mge1-related effect, we speculated that, upon overexpression of the cochaperone gene, the expression of iron regulon genes might also be induced. We confirmed this for six iron regulon genes (Fig. 5). Next, we questioned whether mere activation of the iron regulon could explain the Mge1-regulated effect on fluconazole susceptibility or whether this is only part of the mechanism. Fra1 is a negative regulator of the iron regulon. In the presence of an as-yet-unknown signal coming from the Fe-S cluster metabolism in the mitochondria, Fra1 forms a complex with Fra2, Grx3, and Grx4 and inhibits the translocation of Aft1 to the nucleus, thereby inhibiting transcription of the iron regulon genes (52). Deletion of FRA1 was shown to induce the iron regulon, even in the presence of large amounts of iron (52). We confirmed that deletion of FRA1 induced expression of the iron regulon genes in our experimental setup as well. In Fig. 5, we show that the induction of expression in the fra1Δ strain was always similar to or higher than the induction seen upon MGE1 expression, indicating the validity of the comparison. If activation of the iron regulon were the sole mechanism by which MGE1 overexpression leads to fluconazole resistance, the fra1Δ strain should also show an increased MICflu compared to that of the wild-type BY4742 strain. However, Table 1 and Fig. S1H show that this is not the case, indicating the requirement of yet another unknown process to work in conjunction with iron regulon activation in establishing fluconazole resistance downstream of Mge1. Thus, activation of the iron regulon is necessary but is insufficient by itself to induce resistance against fluconazole downstream of Mge1.
FIG 5 .
Expression of typical iron regulon genes increases upon overexpression of MGE1 or deletion of FRA1. Expression of the representative iron regulon genes HMX1, FRE1, FIT1, FTR1, FET3, and ARN1 was analyzed by qRT-PCR. For each gene, the left panel shows the effect of overexpressing MGE1 in the BY4742 strain versus the EV control. The right panel shows comparisons of the levels of gene expression between BY4742 and fra1Δ strains. Results are displayed as the average of log2(Y) transformed values with the SEM. The values were calculated relative to the averages of the values from the respective controls. Statistical analysis was conducted by unpaired Student’s t test with Bonferroni correction; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Increased dosage of Mge1 also acts as a suppressor of susceptibility to fluconazole in C. glabrata.
C. glabrata and C. albicans are two of the most frequently isolated pathogenic fungi in humans (53). For the past few years, C. glabrata infections have been on the rise in northern Europe and the United States, indicating a need for specific research and drug development (54). Its evolutionarily close relationship with S. cerevisiae (55) implies that the phenotype that we observed for S. cerevisiae MGE1 (ScMGE1) overexpression with respect to susceptibility to fluconazole might also apply to MGE1 in C. glabrata. The closest C. glabrata orthologue of S. cerevisiae Mge1 is encoded by CAGL0J03850g, which is indicated as an uncharacterized ORF in the Candida Genome Database (CGD) (56). Comparing the protein sequence of S. cerevisiae Mge1 to that of its orthologue in C. glabrata yielded an amino acid identity of 68%. We thus refer to the C. glabrata orthologue as C. glabrata Mge1 (CgMge1). To investigate the effect of CgMGE1 overexpression on susceptibility to fluconazole, we created two plasmids expressing the CgMGE1 ORF, together with its terminator, from either the CgPGK1 promoter or the CgTDH3 promoter. Overexpression of MGE1 in the transformed 2001HTL strains was verified using qRT-PCR, yielding fold increases of 24.9 (SEM, 5.54) for the CgPGK1 promoter and 42.9 (SEM, 4.0) for the CgTDH3 promoter compared to the control. Both Etest and broth microdilution analyses indicated that overexpression of CgMGE1 in C. glabrata also increased the MICflu (Table 1; Fig. S4A). The microdilution assay was performed on RPMI medium containing 0.2% glucose, while the Etest analysis was performed on RPMI agar plates containing both 0.2% and 2% glucose. The addition of extra glucose generally enhances the ability to visually inspect the MICflu, as formerly shown for C. albicans (57). As with S. cerevisiae, no significant effect of CgMGE1 overexpression on tolerance was observed (Fig. S4B). In summary, these data suggest that, similarly to the situation in S. cerevisiae, CgMge1 plays a role in regulating susceptibility to fluconazole in C. glabrata.
Etest analysis and microdilution assay of CgMGE1 overexpression in the C. glabrata 2001HTL strain. (A) Etest analysis, performed on media containing 0.2% or 2% glucose. Pictures were taken after 48 h of incubation at 37°C. (B) Dose-response curves of all strains, with dotted lines indicating 50% (upper line) and 90% (middle line) growth inhibition and the initial inoculum (lower line). P = 0.807 (128 μg/ml flu, EV versus pPGK1-CgMGE1), P = 0.857 (64 μg/ml flu, EV versus pPGK1-CgMGE1), P = >0.999 (128 μg/ml flu, EV versus pTDH3-CgMGE1), and P = 0.118 (64 μg/ml flu, EV versus pTDH3-CgMGE1). P values were calculated by two-way ANOVA with Bonferroni correction. Download FIG S4, PDF file, 1.6 MB (1.6MB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overexpression of MGE1 affects both resistance and tolerance in C. albicans.
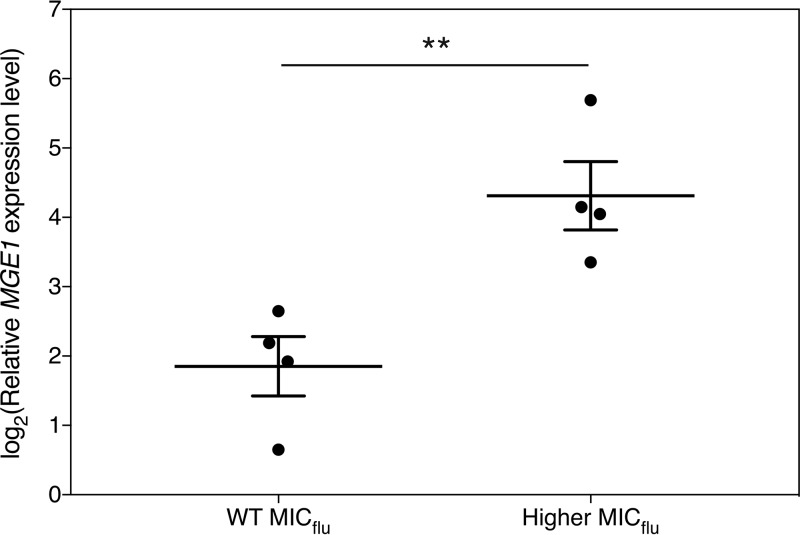
Although the incidence of C. glabrata infections is increasing steadily in certain parts of the world, C. albicans is still the most prevalent cause of Candida infections worldwide (53). The evolutionary distance between this important pathogen and S. cerevisiae is, however, bigger than is the case for C. glabrata, indicating that S. cerevisiae might not be as good a model system for C. albicans as it is for C. glabrata (55). To check whether Mge1 is also involved in fluconazole susceptibility in C. albicans, we generated a plasmid where C. albicans MGE1 (CaMGE1) is under the control of the strong, constitutive CaACT1 promoter. The CIp10 plasmid integrates in the genome at the RP10 locus, where it should stably overexpress CaMGE1 (58). The SC5314 strain was transformed with either the overexpression construct or the empty plasmid as a control. While the control transformants displayed a uniform MICflu phenotype, overexpression of CaMGE1 yielded two phenotypes. One group of transformants did not show an alteration in the MICflu compared to the EV controls, while others showed an increased MICflu which was mainly visible after 24 h of incubation (Fig. S5A and B). We reasoned that this could have been due to different levels of overexpression of CaMGE1, as we also had observed various levels of (over)expression in the past upon transformation of C. albicans (our unpublished observations). It is speculated that this might be due to the high plasticity of the C. albicans genome (59). Here, we confirm a highly variable level of CaMGE1 overexpression in our transformants and demonstrate that the observed variation is largely due to the various results with respect to copy number integration of the plasmid in the genome (Fig. S5A). As can be seen in Fig. 6, the highest CaMGE1 expression levels of the transformants correlated with an increase in the MICflu, indicating that, above a certain threshold of CaMGE1 expression, increased resistance to fluconazole was detected. Intriguingly, for those strains, we found a decrease in tolerance (Fig. S5C). Our results thus indicate that upon (sufficient) overexpression of MGE1, resistance of C. albicans to fluconazole is increased, similarly to the situation in S. cerevisiae and C. glabrata. In contrast to the latter organisms, however, this increased resistance in C. albicans seems to come at the cost of a lower tolerance to the same drug.
FIG 6 .
Overexpression level of CaMGE1 in SC5314 correlates with the MICflu. C. albicans strain SC5314 was transformed with plasmid pLDa01 (CIp10-CaMGE1), and fluconazole sensitivity was determined with the Etest method. Transformants with MICflu values that were similar to or higher than those seen with the EV control strains were obtained. For 4 transformants of each group, CaMGE1 expression was determined by qRT-PCR, and the values were calculated relative to the average of the values from the EV control samples (see Fig. S5). The results are displayed as the average of log2(y) transformed values with the SEM along with the separate data points. The statistical analysis was conducted by unpaired Student’s t test; **, P < 0.01. WT, wild type.
Etest analysis and microdilution assay of CaMGE1 overexpression in the C. albicans SC5314 strain. (A) Eight transformants overexpressing CaMGE1 (LDa01 through LDa08) were analyzed for copy number integration, relative CaMGE1 expression levels, and MICflu category compared to the control strain (LDa09). (B) Etest analysis. Pictures were taken after 24 h of incubation at 37°C. Transformants LDa05 and LDa07 are examples of those with an increase in MICflu and LDa04 and LDa06 as examples of those with an MICflu similar to that of the controls (LDa09). (C) Tolerance assay assessing trailing growth of 3 transformants showing an increased MICflu compared to the average of the values (with SEM) determined for three control strains (LDa09, n = 3). Due to the variance in CaMGE1 overexpression (see panel A), CFU counts of the overexpression transformants are shown individually as well as via the average and SEM of the three strains together. Statistical analysis was conducted by two-way ANOVA with Bonferroni correction. ***, P = <0.001. Dotted lines indicate 50% (upper line) and 90% (middle line) growth inhibition and the initial inoculum (lower line). Download FIG S5, PDF file, 1.4 MB (1.4MB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
In this study, we identified Mge1, a cochaperone involved in Fe-S cluster metabolism and protein import into the mitochondria, as a multicopy suppressor of fluconazole susceptibility (16, 17, 19). When an S. cerevisiae mutant strain grows in the presence of an otherwise inhibitory chemical, it is important to consider increased rho0/− formation and drug efflux as possible modes of action (60). Several groups have already reported on a relation between Mge1, or its downstream chaperones, and mtDNA stability (18, 30, 61, 62). However, we show here that neither loss of the mitochondrial DNA nor drug efflux through Pdr5 can solely account for the increased growth of the MGE1 overexpression strain on fluconazole (33, 34).
The target of fluconazole is Erg11, an enzyme involved in the biosynthesis of ergosterol (5, 6). In this report, we show that ergosterol levels are elevated in an S. cerevisiae strain where MGE1 is overexpressed, a phenomenon which is much more prominent after the addition of fluconazole. It thus seems that Mge1 evokes a protective mechanism by which the cell can retain higher levels of ergosterol upon treatment with fluconazole. A detailed analysis of the sterol spectra of our strains indicated that, upon fluconazole addition, MGE1 overexpression reduces the metabolic flux toward potential toxic sterols, most notably 14-methylergosta-8,24(28)-dien-3β,6α-diol (39, 40). The reduced accumulation of this sterol, together with the retention of more ergosterol upon fluconazole treatment, illustrates how Mge1 reduces susceptibility to the drug. As Erg11 functions at the cross-section between the pathways leading to either ergosterol production or toxic sterol accumulation, we analyzed the abundance of this enzyme but found no increase at the level of either gene expression or protein abundance upon MGE1 overexpression. Analysis of the expression of other ERG genes, known to be regulated by fluconazole, could also not identify a transcriptional mechanism explaining the observed sterol profiles. It is possible that MGE1 overexpression specifically alters the enzyme activity of Erg11 or of other ergosterol biosynthesis enzymes, rather than their expression. It remains unclear how Mge1, operating in the mitochondria, would impact ergosterol biosynthesis, which mainly takes place in the endoplasmic reticulum (ER) (63).
To further elucidate how Mge1 function might be linked to fluconazole susceptibility, we looked at the known Mge1 effectors. We demonstrated the involvement of the Hsp70 chaperone Ssq1, as this chaperone is necessary for the cell to retain its MICflu at the wild-type level and as overexpression of MGE1 in the ssq1Δ strain could not increase fluconazole resistance. Ssq1 is essential for mitochondrial Fe-S cluster metabolism, which somehow functions as an iron-sensing system in the cell, since in the presence of sufficient iron, an inhibitory signal originates from this metabolism and impairs transcription of the iron regulon genes (64). Intriguingly, we found that overexpressing MGE1 in the wild-type strain causes a significant increase in expression of characteristic iron regulon genes. Furthermore, deleting AFT1, the gene encoding the main transcriptional regulator of the iron regulon, impairs the effect of Mge1 on fluconazole susceptibility, indicating the strict dependence of our phenotype on this regulon. It is possible that overloading the cell with Mge1 might impair, rather than increase, the cochaperone’s function. This would then lead to a reduced Fe-S signal, thereby activating the iron regulon and generating fluconazole resistance by modulating sterol synthesis, as shown before under iron-limiting conditions in yeast (65, 66). Several elements argue against such a straightforward mechanism, however. First of all, Schmidt et al. reported that overexpression of MGE1 increases the activity of Ssq1 (67), implying increased rather than impaired Fe-S cluster biogenesis. Second, impairing Ssq1 function does not lead to fluconazole resistance, as we observed that the ssq1Δ strain was more sensitive, and not resistant, to fluconazole. Finally, although we demonstrate the dependency of fluconazole resistance on the activation of the iron regulon, we also clearly show that this is not sufficient, since mere activation of the iron regulon through FRA1 deletion does not cause any change in the MICflu. It thus remains to be investigated how Mge1 activity is linked to the iron regulon on one side and to fluconazole susceptibility on the other side. It is tempting to speculate that increasing Mge1 activity alters the balance in Fe-S cluster proteins in a specific way, causing fluconazole resistance via two separate pathways: by activating the iron regulon and simultaneously by some other, yet-to-be-elucidated mechanism. Future in-depth analysis of the changes in the Fe-S cluster metabolism upon MGE1 overexpression would thus represent a valuable system to elucidate this mechanism. This analysis could pinpoint Fe-S species which regulate the resistance to fluconazole through modulation of ergosterol metabolism, i.e., by reducing toxic sterol production and increasing ergosterol retention. At the same time, as the identities of the specific Fe-S species which are involved in regulating the iron regulon are still unknown at present, such an analysis would also provide crucial information on this topic.
Apart from the observations made in S. cerevisiae, we also validated the Mge1-related effect on fluconazole resistance in the fungal pathogens C. glabrata and C. albicans. Very little is known about Fe-S cluster metabolism in either pathogen. The C. glabrata orthologue of ScSSQ1 is uncharacterized (56). The C. albicans orthologue was characterized recently (68); these researchers confirmed a role for CaSsq1 in iron metabolism and iron regulon modulation. More research is necessary to uncover the exact role of the Mge1-Ssq1 module in regulating the susceptibility of fungal cells to fluconazole. Knowledge of this mechanism could provide novel drug targets which would increase the antifungal potential of azoles in combinatorial therapies.
MATERIALS AND METHODS
Strains and plasmids.
All S. cerevisiae strains used in this study are isogenic with respect to the BY4742 laboratory strain and are listed in Table S1 in the supplemental material. Strain AFc202, carrying a chromosomal 3×HA C-terminal tag at ERG11, was constructed by transforming BY4742 with a PCR fragment obtained using primers listed in Table S2 and plasmid pMPY-3xHA as a template (69). Transformants were allowed to pop out the URA3 marker by homologous recombination, and uracil auxotrophs were selected using 5-fluoroorotic acid (5-FOA). The BY4742 strain was made rho0 by repeated growth in the presence of 25 μg/ml ethidium bromide in minimal medium, as described in reference 70. Deletion of PDR5 in BY4742 and rho0 strains was accomplished by amplification of the hygromycin resistance marker gene from plasmid pFA6a-hphNT1 (71) and consequent transformation. C. glabrata and C. albicans strains used in the experiments are also listed in Table S1. C. albicans strains LDa01 to LDa08 were generated by transforming the StuI-linearized pLDa01 plasmid in SC5314. The LDa09 strain was created similarly by integration of the empty CIp10-NAT1 plasmid. All specific genotypes were checked by diagnostic PCR.
Strains used in this study. Download TABLE S1, PDF file, 0.1 MB (104.7KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used in this study. Download TABLE S2, PDF file, 0.1 MB (85.4KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Plasmids are listed in Table S3. Plasmid pAFc86 contains MGE1 under the control of its promoter and terminator. The SnaBI-XhoI fragment from the Lacroute library plasmid was cloned into YEplac195 linearized using SmaI. Plasmid pESc01 is similar to pAFc86, with fusion of MGE1 to the gene encoding green fluorescent protein [GFP(S65T)]. This plasmid was created by amplification of the MGE1 promoter, the MGE1 ORF, the GFP gene, and the MGE1 terminator and assembly of them in the YEPlac195 plasmid using In-Fusion cloning (Clontech). The mitochondria were marked by transforming a plasmid containing mCherry fused to a mitochondrial targeting sequence in the appropriate strains (72). Plasmids pESg01 and pESg02 were generated by assembling the CgPGK1 promoter or CgTDH3 promoter and the CgMGE1 ORF and its terminator in the pCgACH backbone (73) by In-Fusion cloning. Plasmid CIp10-NAT1 was generated by exchanging the CaURA3 marker together with its promoter and terminator from the CIp10 plasmid (58) for the dominant C. albicans optimized NAT1 gene together with a CaACT1 promoter and terminator (74), using NotI and SpeI. Another CaACT1 promoter and terminator were added in the multiple cloning site, opened with MluI-NheI and XhoI-KpnI, respectively. Plasmid pLDa01 was generated by integrating the CaMGE1 gene in the PstI-ClaI-cut CIp10-NAT1 vector.
Plasmids used in this study. Download TABLE S3, PDF file, 0.1 MB (74.9KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
MICflu values of several strains. Data indicated with a superscript "a" represent the results of overexpression of MGE1-GFP. Data indicated with a superscript "b" were determined after 72 h on SCglu medium (the latter was used only for the Etest; BY4742 had MICflu values of 8 to 12 on this medium). Download TABLE S4, PDF file, 0.1 MB (76KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth conditions: media and chemicals.
S. cerevisiae strains were grown in SDglu, unless stated otherwise. This medium contains 0.17% Difco yeast nitrogen base without amino acids or ammonium sulfate, 0.5% ammonium sulfate, and 2% glucose. Liquid medium was pH adapted to pH 5.5. For solid medium, the pH was set at 6.5 and 1.6% agar was added. Depending on the strain, additional amino acids or nucleotides were added according to the method described in reference 75. For spot assays, fluconazole (F8929; Sigma) and doxycycline (D9891; Sigma) were added to the medium at concentrations of 10 or 20 μg/ml and 50 or 100 μg/ml, respectively. The procedure used to screen for multicopy suppressors of susceptibility to fluconazole-doxycycline was described in reference 7. For some specific experiments, YPD medium (containing 1% yeast extract, 2% peptone, and 2% glucose) was used. C. albicans and C. glabrata strains were pregrown in synthetic complete glucose (SCglu) medium or SC(-HIS)glu medium, composed of SDglu with the addition of complete or drop-out CSM (MP Biomedicals). Assays were carried out in filter-sterilized RPMI 1640 medium with l-glutamine (R6504; Sigma) and buffered with 0.165 M morpholinepropanesulfonic acid at pH 7. Depending on the assay, autoclave-sterilized and precooled agar and/or 1.8% glucose was added to the medium. Cell cultures containing fluconazole or doxycycline were always kept in the dark.
Determination of fluconazole susceptibility: MICflu evaluation, tolerance assays, and spot assays.
To determine the MICflu for the strains, two methods were always used in parallel. In the Etest method (BioMérieux), the MICflu was determined as the concentration of fluconazole where the halo of growth inhibition/retardation intersected with the strip. Overnight cultures were adjusted to an optical density at 600 nm (OD600) of 0.5 in water for S. cerevisiae and an OD600 of 0.2 for C. glabrata and C. albicans and were spread on SDglu or RPMI (with 0.2% or 2% glucose) plates. The strips were placed onto the lawn of cells, and the plates were incubated at 30°C or 37°C for 48 h. Broth microdilution assays were conducted according to the Clinical Laboratory and Standards Institute (CLSI) standard methods (76). Round-bottom, UV-sterilized 96-well microtiter plates were used, where all wells were filled with 0.5 to 2.5 × 103 cells/ml, 0 to 128 μg/ml fluconazole in 1/2 dilutions, and SDglu or RPMI medium (the latter with 0.2% glucose). For C. albicans, the fluconazole dilution series was set between 0 and 32 μg/ml fluconazole. After incubation of the plates at 30°C or 37°C under nonshaking conditions for 48 h, we measured the OD600 of the resuspended cultures in each well to obtain quantitative and objective data. A dose-response curve was created, and the MIC values were calculated by subtracting the background OD values determined for the medium from all measured data points and subsequent normalization to the condition without fluconazole. The concentrations of the drug, between which the relative OD falls below 50 or 10% of the no-drug OD are called the MIC50 and the MIC90, respectively. To evaluate the drug tolerance of our strains, we generated dose-response curves based on CFU counts for the MGE1 overexpression strain and empty vector control. The wells of the broth microdilution assay plate were resuspended, and each culture was diluted and plated. The drug tolerance was determined by checking the CFU counts under the conditions seen with the two highest fluconazole concentrations. For spot assays, overnight cultures were adapted to an OD600 of 1, and 5 serial 1/5 dilutions were spotted. SDglu medium was used with different concentrations of fluconazole and doxycycline. The plates were incubated at 30°C for 48 or 72 h. All experiments were conducted with at least three biological repeats, and representative results are shown.
Sterol measurement.
Sterols were extracted according to the method described in reference 77, with a few adaptations. In summary, cells were grown for 24 h in minimal medium, with or without 20 μg/ml fluconazole. The cells were collected, resuspended in saponification medium, and subjected to vortex mixing. The samples were incubated for 1 h at 80°C, after which 1 ml of water and 4 ml of hexane were added. After mixing, the two layers were allowed to separate. For spectrophotometrical analysis, UV-transmittable 96-well microtiter plates (3635; Costar Corning) were used to allow measurement of the OD281 and OD230. A formula from reference 38 was used to measure the percentages of ergosterol (corrected for cellular wet weight and resuspension volume). For GC-MS analysis, the sterols were extracted twice with hexane, which was then evaporated by vacuum centrifugation. The sterols were resuspended in 100 μl silylating mixture (85432; Sigma) and incubated at room temperature for 30 min. Finally, 500 μl hexane was added and the samples were immediately stored at −20°C for later analysis by GC-MS. One microliter of the sample was injected into a gas chromatograph-mass spectrometer (Shimadzu QP2010 Ultra Plus) equipped with an HP-5ms nonpolar column (Agilent) (30 m in length, 0.25-mm inner diameter [id.]; 0.25-µm thin layer). Helium was used as carrier gas with a flow rate of 1.4 ml/min. Injection was carried out at 250°C in split mode after 1 min and with a ratio of 1:10. The temperature was first held at 50°C for 1 min and then allowed to rise to 260°C at a rate of 50°C/min, followed by a second ramp of 2°C/min until 325°C was reached; that temperature was maintained for 3 min. The mass detector was operated in scan mode (50 to 600 atomic mass units [amu]), using electron impact ionization (70 eV). The temperatures of the interface and detector were 290°C and 250°C, respectively. A mix of linear n-alkanes (from C8 to C40) was injected to serve as external retention index markers. Sterols were identified by their retention time relative to the internal standard (cholestane) and specific mass spectrometric patterns using AMDIS version 2.71. The deconvoluted spectra were matched to GC-MS libraries described in reference 78 and NIST/EPA/NIH version 2011. Analysis was performed by integration over the base ion of each sterol, and abundance was calculated relative to the internal standard, comparing the relative peak areas of the compounds across treatments using two-way ANOVA with Bonferroni correction. Apart from the P values for pairwise comparison, the P values for interaction between the two parameters are also described.
RNA extraction and gene expression analysis by qRT-PCR.
S. cerevisiae strains were grown in SDglu medium at 30°C for 24 h, with or without 20 μg/ml fluconazole. C. glabrata and C. albicans cells were incubated for 8 h at 37°C or 30°C in RPMI medium, with or without 1 μg/ml fluconazole. Cells were washed with ice-cold water, resuspended in TRIzol (Thermo, Fisher), and broken using glass beads and a FastPrep machine (MP Biomedicals). RNA was extracted by respective addition of chloroform and isopropanol and washed three times with 70% ethanol. Equal amounts of RNA were treated with a DNase enzyme (New England Biolabs) and converted to cDNA (iScript cDNA synthesis kit; Bio-Rad). Real-time quantitative PCR (qPCR) reactions were conducted using GoTaq polymerase (Promega) and a StepOnePlus real-time PCR device (Thermo, Fisher). Data were analyzed using qBasePlus software (Biogazelle) (79). Further data analysis and statistics analysis of log2(Y) transformed expression values were performed with Graphpad Prism. Transformation of the data points was performed to enable the use of standard statistical methods. Graphs show the means of the transformed values, together with their SEM. The statistical method used is mentioned under each figure. Copy number analysis of the genomic DNA of transformants was performed by qPCR, as described above.
Western blotting.
S. cerevisiae strains were grown in SDglu medium for 24 h at 30°C, with or without 20 μg/ml fluconazole. Cells were washed with lysis buffer (200 mM sorbitol, 20 mM HEPES–KOH [pH 6.8], 1 mM EDTA, 50 mM potassium acetate and protease inhibitors [Roche]), and glass beads were added to break them using a FastPrep machine. The amount of proteins was quantified using the Pierce protein assay (Thermo, Fisher), and 6 μg was loaded per well on an Invitrogen NuPage Novex bis-Tris gradient gel (4% to 12%). We used anti-HA (12013819001; Roche) and anti-Pgk1 (459250; Invitrogen) as loading controls. The blots were visualized using a FujiFilm LAS-4000 mini system and accompanying software.
Fluorescence microscopy.
To determine the location of Mge1-GFP inside the cell, we used a FluoView FV1000 confocal microscope (Olympus IX81) and its software. We visualized GFP with a 488-nm argon laser and BA505-540 emission filter and mCherry with a 559-nm laser and BA575-675 emission filter. A 60× UPlanSApo (numerical aperture [NA], 1.35) objective lens was used.
ACKNOWLEDGMENTS
We thank Cindy Colombo and Ilse Palmans for technical assistance and Nico Vangoethem for great help with the figures. We thank Hélène Tournu for making the CIp10-NAT1 plasmid. L.D., E.S., A.F., and B.H.-M. designed and executed all experiments. All of us were involved in discussion of the results and revision of the manuscript.
L.D. and E.S. were supported personally by the FWO (Fund for Scientific Research Flanders). We further acknowledge financial project support from the FWO (G.OD48.13), the Research Fund of KU Leuven (GOA/13/006), and the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office (IAP P7/28). Research in the laboratory of K.V. is supported by VIB, AB-InBev-Baillet Latour Fund, FWO, VLAIO, European Research Council (ERC) Consolidator grant CoG682009, and Human Frontier Science Program (HFSP) grant 246 RGP0050/2013.
Footnotes
Citation Demuyser L, Swinnen E, Fiori A, Herrera-Malaver B, Verstrepen K, Van Dijck P. 2017. Mitochondrial cochaperone Mge1 is involved in regulating susceptibility to fluconazole in Saccharomyces cerevisiae and Candida species. mBio 8:e00201-17. https://doi.org/10.1128/mBio.00201-17.
REFERENCES
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. 2010. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 9:719–727. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 3.Roemer T, Krysan DJ. 2014. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:e019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro RS, Robbins N, Cowen LE. 2011. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev 75:213–267. doi: 10.1128/MMBR.00045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanden Bossche H. 1985. Biochemical targets for antifungal azole derivatives: hypothesis on the mode of action. Curr Top Med Mycol 1:313–351. doi: 10.1007/978-1-4613-9547-8_12. [DOI] [PubMed] [Google Scholar]
- 6.Kelly SL, Arnoldi A, Kelly DE. 1993. Molecular genetic analysis of azole antifungal mode of action. Biochem Soc Trans 21:1034–1038. doi: 10.1042/bst0211034. [DOI] [PubMed] [Google Scholar]
- 7.Fiori A, Van Dijck P. 2012. Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis. Antimicrob Agents Chemother 56:3785–3796. doi: 10.1128/AAC.06017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delarze E, Sanglard D. 2015. Defining the frontiers between antifungal resistance, tolerance and the concept of persistence. Drug Resist Updat 23:12–19. doi: 10.1016/j.drup.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Laloraya S, Gambill BD, Craig EA. 1994. A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc Natl Acad Sci U S A 91:6481–6485. doi: 10.1073/pnas.91.14.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verghese J, Abrams J, Wang Y, Morano KA. 2012. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol Mol Biol Rev 76:115–158. doi: 10.1128/MMBR.05018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartl FU, Hayer-Hartl M. 2009. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol 16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 12.Mayer MP, Bukau B. 2005. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolliger L, Deloche O, Glick BS, Georgopoulos C, Jenö P, Kronidou N, Horst M, Morishima N, Schatz G. 1994. A mitochondrial homolog of bacterial GrpE interacts with mitochondrial hsp70 and is essential for viability. EMBO J 13:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laloraya S, Dekker PJ, Voos W, Craig EA, Pfanner N. 1995. Mitochondrial GrpE modulates the function of matrix Hsp70 in translocation and maturation of preproteins. Mol Cell Biol 15:7098–7105. doi: 10.1128/MCB.15.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neupert W, Herrmann JM. 2007. Translocation of proteins into mitochondria. Annu Rev Biochem 76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 16.Miao B, Davis JE, Craig EA. 1997. Mge1 functions as a nucleotide release factor for Ssc1, a mitochondrial Hsp70 of Saccharomyces cerevisiae. J Mol Biol 265:541–552. doi: 10.1006/jmbi.1996.0762. [DOI] [PubMed] [Google Scholar]
- 17.Craig EA, Kramer J, Kosic-Smithers J. 1987. SSC1, a member of the 70-kDa heat shock protein multigene family of Saccharomyces cerevisiae, is essential for growth. Proc Natl Acad Sci U S A 84:4156–4160. doi: 10.1073/pnas.84.12.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda E, Yoshida S, Mitsuzawa H, Uno I, Toh-e A. 1994. YGE1 is a yeast homologue of Escherichia coli grpE and is required for maintenance of mitochondrial functions. FEBS Lett 339:265–268. doi: 10.1016/0014-5793(94)80428-1. [DOI] [PubMed] [Google Scholar]
- 19.Strain J, Lorenz CR, Bode J, Garland S, Smolen GA, Ta DT, Vickery LE, Culotta VC. 1998. Suppressors of superoxide dismutase (SOD1) deficiency in Saccharomyces cerevisiae. Identification of proteins predicted to mediate iron-sulfur cluster assembly. J Biol Chem 273:31138–31144. doi: 10.1074/jbc.273.47.31138. [DOI] [PubMed] [Google Scholar]
- 20.Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Uzarska MA, Webert H, Wilbrecht C, Mühlenhoff U. 2012. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta 1823:1491–1508. doi: 10.1016/j.bbamcr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Lill R. 2009. Function and biogenesis of iron-sulphur proteins. Nature 460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi-Iwai Y, Dancis A, Klausner RD. 1995. AFT1: a mediator of iron regulated transcriptional control in Saccharomyces cerevisiae. EMBO J 14:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi-Iwai Y, Stearman R, Dancis A, Klausner RD. 1996. Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J 15:3377–3384. [PMC free article] [PubMed] [Google Scholar]
- 24.Blaiseau PL, Lesuisse E, Camadro JM. 2001. Aft2p, a novel iron-regulated transcription activator that modulates, with Aft1p, intracellular iron use and resistance to oxidative stress in yeast. J Biol Chem 276:34221–34226. doi: 10.1074/jbc.M104987200. [DOI] [PubMed] [Google Scholar]
- 25.Mühlenhoff U, Gerber J, Richhardt N, Lill R. 2003. Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p. EMBO J 22:4815–4825. doi: 10.1093/emboj/cdg446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schilke B, Forster J, Davis J, James P, Walter W, Laloraya S, Johnson J, Miao B, Craig E. 1996. The cold sensitivity of a mutant of Saccharomyces cerevisiae lacking a mitochondrial heat shock protein 70 is suppressed by loss of mitochondrial DNA. J Cell Biol 134:603–613. doi: 10.1083/jcb.134.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad T, Chandra A, Mukhopadhyay CK, Prasad R. 2006. Unexpected link between iron and drug resistance of Candida spp.: iron depletion enhances membrane fluidity and drug diffusion, leading to drug-susceptible cells. Antimicrob Agents Chemother 50:3597–3606. doi: 10.1128/AAC.00653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stettler S, Chiannilkulchai N, Hermann-Le Denmat S, Lalo D, Lacroute F, Sentenac A, Thuriaux P. 1993. A general suppressor of RNA polymerase I, II and III mutations in Saccharomyces cerevisiae. Mol Gen Genet 239:169–176. [DOI] [PubMed] [Google Scholar]
- 29.Dujon B. 1981. Mitochondrial genetics and functions. Cold Spring Harb Monogr Arch 11:505–635. https://cshmonographs.org/index.php/monographs/article/viewArticle/4239. [Google Scholar]
- 30.Contamine V, Picard M. 2000. Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol Mol Biol Rev 64:281–315. doi: 10.1128/MMBR.64.2.281-315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traven A, Wong JM, Xu D, Sopta M, Ingles CJ. 2001. Interorganellar communication. Altered nuclear gene expression profiles in a yeast mitochondrial dna mutant. J Biol Chem 276:4020–4027. doi: 10.1074/jbc.M006807200. [DOI] [PubMed] [Google Scholar]
- 32.Chen XJ, Clark-Walker GD. 1999. The petite mutation in yeasts: 50 years on. Int Rev Cytol 194:197–238. doi: 10.1016/S0074-7696(08)62397-9. [DOI] [PubMed] [Google Scholar]
- 33.Dunn CD, Lee MS, Spencer FA, Jensen RE. 2006. A genomewide screen for petite-negative yeast strains yields a new subunit of the i-AAA protease complex. Mol Biol Cell 17:213–226. doi: 10.1091/mbc.E05-06-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn CD, Jensen RE. 2003. Suppression of a defect in mitochondrial protein import identifies cytosolic proteins required for viability of yeast cells lacking mitochondrial DNA. Genetics 165:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White TC. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother 41:1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman GH, da Silva Ferreira ME, dos Reis Marques E, Savoldi M, Perlin D, Park S, Godoy Martinez PC, Goldman MH, Colombo AL. 2004. Evaluation of fluconazole resistance mechanisms in Candida albicans clinical isolates from HIV-infected patients in Brazil. Diagn Microbiol Infect Dis 50:25–32. doi: 10.1016/j.diagmicrobio.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Perea S, López-Ribot JL, Wickes BL, Kirkpatrick WR, Dib OP, Bachmann SP, Keller SM, Martinez M, Patterson TF. 2002. Molecular mechanisms of fluconazole resistance in Candida dubliniensis isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother 46:1695–1703. doi: 10.1128/AAC.46.6.1695-1703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ. 1999. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol 37:3332–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson PF, Rose ME, Ellis SW, England H, Kelly SL. 1989. Defective sterol C5-6 desaturation and azole resistance: a new hypothesis for the mode of action of azole antifungals. Biochem Biophys Res Commun 164:1170–1175. doi: 10.1016/0006-291X(89)91792-0. [DOI] [PubMed] [Google Scholar]
- 40.Kelly SL, Lamb DC, Corran AJ, Baldwin BC, Kelly DE. 1995. Mode of action and resistance to azole antifungals associated with the formation of 14 alpha-methylergosta-8,24(28)-dien-3 beta,6 alpha-diol. Biochem Biophys Res Commun 207:910–915. doi: 10.1006/bbrc.1995.1272. [DOI] [PubMed] [Google Scholar]
- 41.Bammert GF, Fostel JM. 2000. Genome-wide expression patterns in Saccharomyces cerevisiae: comparison of drug treatments and genetic alterations affecting biosynthesis of ergosterol. Antimicrob Agents Chemother 44:1255–1265. doi: 10.1128/AAC.44.5.1255-1265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Backer MD, Ilyina T, Ma XJ, Vandoninck S, Luyten WH, Vanden Bossche H. 2001. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob Agents Chemother 45:1660–1670. doi: 10.1128/AAC.45.6.1660-1670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacPherson S, Akache B, Weber S, De Deken X, Raymond M, Turcotte B. 2005. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob Agents Chemother 49:1745–1752. doi: 10.1128/AAC.49.5.1745-1752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimster-Denk D, Rine J, Phillips J, Scherer S, Cundiff P, DeBord K, Gilliland D, Hickman S, Jarvis A, Tong L, Ashby M. 1999. Comprehensive evaluation of isoprenoid biosynthesis regulation in Saccharomyces cerevisiae utilizing the genome reporter matrix. J Lipid Res 40:850–860. [PubMed] [Google Scholar]
- 45.Marada A, Allu PK, Murari A, PullaReddy B, Tammineni P, Thiriveedi VR, Danduprolu J, Sepuri NB. 2013. Mge1, a nucleotide exchange factor of Hsp70, acts as an oxidative sensor to regulate mitochondrial Hsp70 function. Mol Biol Cell 24:692–703. doi: 10.1091/mbc.E12-10-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lussier M, White AM, Sheraton J, di Paolo T, Treadwell J, Southard SB, Horenstein CI, Chen-Weiner J, Ram AF, Kapteyn JC, Roemer TW, Vo DH, Bondoc DC, Hall J, Zhong WW, Sdicu AM, Davies J, Klis FM, Robbins PW, Bussey H. 1997. Large scale identification of genes involved in cell surface biosynthesis and architecture in Saccharomyces cerevisiae. Genetics 147:435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakasegawa Y, Hachiya NS, Tsukita S, Kaneko K. 2003. Ecm10p localizes in yeast mitochondrial nucleoids and its overexpression induces extensive mitochondrial DNA aggregations. Biochem Biophys Res Commun 309:217–221. doi: 10.1016/S0006-291X(03)01548-1. [DOI] [PubMed] [Google Scholar]
- 48.Baumann F, Milisav I, Neupert W, Herrmann JM. 2000. Ecm10, a novel hsp70 homolog in the mitochondrial matrix of the yeast Saccharomyces cerevisiae. FEBS Lett 487:307–312. doi: 10.1016/S0014-5793(00)02364-4. [DOI] [PubMed] [Google Scholar]
- 49.Steger HF, Söllner T, Kiebler M, Dietmeier KA, Pfaller R, Trülzsch KS, Tropschug M, Neupert W, Pfanner N. 1990. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J Cell Biol 111:2353–2363. doi: 10.1083/jcb.111.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hines V, Brandt A, Griffiths G, Horstmann H, Brütsch H, Schatz G. 1990. Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS70. EMBO J 9:3191–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dagley MJ, Gentle IE, Beilharz TH, Pettolino FA, Djordjevic JT, Lo TL, Uwamahoro N, Rupasinghe T, Tull DL, McConville M, Beaurepaire C, Nantel A, Lithgow T, Mitchell AP, Traven A. 2011. Cell wall integrity is linked to mitochondria and phospholipid homeostasis in Candida albicans through the activity of the post-transcriptional regulator Ccr4-Pop2. Mol Microbiol 79:968–989. doi: 10.1111/j.1365-2958.2010.07503.x. [DOI] [PubMed] [Google Scholar]
- 52.Kumánovics A, Chen OS, Li L, Bagley D, Adkins EM, Lin H, Dingra NN, Outten CE, Keller G, Winge D, Ward DM, Kaplan J. 2008. Identification of FRA1 and FRA2 as genes involved in regulating the yeast iron regulon in response to decreased mitochondrial iron-sulfur cluster synthesis. J Biol Chem 283:10276–10286. doi: 10.1074/jbc.M801160200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perlroth J, Choi B, Spellberg B. 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 54.Guinea J. 2014. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20(Suppl 6):5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 55.Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuvéglise C, Talla E, Goffard N, Frangeul L, Aigle M, Anthouard V, Babour A, Barbe V, Barnay S, Blanchin S, Beckerich JM, Beyne E, Bleykasten C, Boisramé A, Boyer J, Cattolico L, Confanioleri F, De Daruvar A, Despons L, Fabre E, Fairhead C, Ferry-Dumazet H, Groppi A, Hantraye F, Hennequin C, Jauniaux N, Joyet P, Kachouri R, Kerrest A, Koszul R, Lemaire M, Lesur I, Ma L, Muller H, Nicaud JM, Nikolski M, Oztas S, Ozier-Kalogeropoulos O, Pellenz S, Potier S, Richard GF, Straub ML. 2004. Genome evolution in yeasts. Nature 430:35–44. doi: 10.1038/nature02579. [DOI] [PubMed] [Google Scholar]
- 56.Skrzypek MS, Binkley J, Binkley G, Miyasato SR, Simison M, Sherlock G. 2017. The Candida Genome Database (CGD): incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res 45:D592–D596. doi: 10.1093/nar/gkw924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Tudela JL, Martinez-Suarez JV. 1994. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob Agents Chemother 38:45–48. doi: 10.1128/AAC.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murad AM, Lee PR, Broadbent ID, Barelle CJ, Brown AJ. 2000. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16:325–327. [DOI] [PubMed] [Google Scholar]
- 59.Selmecki A, Forche A, Berman J. 2010. Genomic plasticity of the human fungal pathogen Candida albicans. Eukaryot Cell 9:991–1008. doi: 10.1128/EC.00060-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hallstrom TC, Moye-Rowley WS. 2000. Multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in Saccharomyces cerevisiae. J Biol Chem 275:37347–37356. doi: 10.1074/jbc.M007338200. [DOI] [PubMed] [Google Scholar]
- 61.Moczko M, Schönfisch B, Voos W, Pfanner N, Rassow J. 1995. The mitochondrial ClpB homolog Hsp78 cooperates with matrix Hsp70 in maintenance of mitochondrial function. J Mol Biol 254:538–543. doi: 10.1006/jmbi.1995.0636. [DOI] [PubMed] [Google Scholar]
- 62.Knight SA, Sepuri NB, Pain D, Dancis A. 1998. Mt-Hsp70 homolog, Ssc2p, required for maturation of yeast frataxin and mitochondrial iron homeostasis. J Biol Chem 273:18389–18393. doi: 10.1074/jbc.273.29.18389. [DOI] [PubMed] [Google Scholar]
- 63.Henneberry AL, Sturley SL. 2005. Sterol homeostasis in the budding yeast, Saccharomyces cerevisiae. Semin Cell Dev Biol 16:155–161. doi: 10.1016/j.semcdb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Chen OS, Crisp RJ, Valachovic M, Bard M, Winge DR, Kaplan J. 2004. Transcription of the yeast iron regulon does not respond directly to iron but rather to iron-sulfur cluster biosynthesis. J Biol Chem 279:29513–29518. doi: 10.1074/jbc.M403209200. [DOI] [PubMed] [Google Scholar]
- 65.Puig S, Askeland E, Thiele DJ. 2005. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120:99–110. doi: 10.1016/j.cell.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan J, McVey Ward D, Crisp RJ, Philpott CC. 2006. Iron-dependent metabolic remodeling in S. cerevisiae. Biochim Biophys Acta 1763:646–651. doi: 10.1016/j.bbamcr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt S, Strub A, Röttgers K, Zufall N, Voos W. 2001. The two mitochondrial heat shock proteins 70, Ssc1 and Ssq1, compete for the cochaperone Mge1. J Mol Biol 313:13–26. doi: 10.1006/jmbi.2001.5013. [DOI] [PubMed] [Google Scholar]
- 68.Dong Y, Zhang D, Yu Q, Zhao Q, Xiao C, Zhang K, Jia C, Chen S, Zhang B, Zhang B, Li M. 2017. Loss of Ssq1 leads to mitochondrial dysfunction, activation of autophagy and cell cycle arrest due to iron overload triggered by mitochondrial iron-sulfur cluster assembly defects in Candida albicans. Int J Biochem Cell Biol 85:44–55. doi: 10.1016/j.biocel.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 69.Schneider BL, Seufert W, Steiner B, Yang QH, Futcher AB. 1995. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- 70.Fox TD, Folley LS, Mulero JJ, McMullin TW, Thorsness PE, Hedin LO, Costanzo MC. 1991. Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol 194:149–165. [DOI] [PubMed] [Google Scholar]
- 71.Janke C, Magiera MM, Rathfelder N, Taxis C, Reber S, Maekawa H, Moreno-Borchart A, Doenges G, Schwob E, Schiebel E, Knop M. 2004. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21:947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- 72.Swinnen E, Wilms T, Idkowiak-Baldys J, Smets B, De Snijder P, Accardo S, Ghillebert R, Thevissen K, Cammue B, De Vos D, Bielawski J, Hannun YA, Winderickx J. 2014. The protein kinase Sch9 is a key regulator of sphingolipid metabolism in Saccharomyces cerevisiae. Mol Biol Cell 25:196–211. doi: 10.1091/mbc.E13-06-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitada K, Yamaguchi E, Arisawa M. 1996. Isolation of a Candida glabrata centromere and its use in construction of plasmid vectors. Gene 175:105–108. doi: 10.1016/0378-1119(96)00132-1. [DOI] [PubMed] [Google Scholar]
- 74.Shen J, Guo W, Köhler JR. 2005. CaNAT1, a heterologous dominant selectable marker for transformation of Candida albicans and other pathogenic Candida species. Infect Immun 73:1239–1242. doi: 10.1128/IAI.73.2.1239-1242.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sherman F. 2002. Getting started with yeast. Methods Enzymol 350:3–41. doi: 10.1016/S0076-6879(02)50954-X. [DOI] [PubMed] [Google Scholar]
- 76.Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed Approved standard. CLSI M27-A3 (28) Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 77.Morio F, Pagniez F, Lacroix C, Miegeville M, Le Pape P. 2012. Amino acid substitutions in the Candida albicans sterol Delta5,6-desaturase (Erg3p) confer azole resistance: characterization of two novel mutants with impaired virulence. J Antimicrob Chemother 67:2131–2138. doi: 10.1093/jac/dks186. [DOI] [PubMed] [Google Scholar]
- 78.Müller C, Binder U, Bracher F, Giera M. 2017. Antifungal drug testing by combining minimal inhibitory concentration testing with target identification by gas chromatography-mass spectrometry. Nat Protoc 12:947–963. doi: 10.1038/nprot.2017.005. [DOI] [PubMed] [Google Scholar]
- 79.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Etest analysis of the S. cerevisiae strains under study. Pictures were taken after 48 h (or 72 h) of incubation at 30°C. (A) MGE1 overexpression in different petite-negative strains and a rho0 strain, tested on SDglu and YPD media. (B) Deletion of PDR5 in BY4742 and rho0 and MGE1 overexpression in the pdr5Δ strain. (C) MGE1 overexpression compared to EV control results in a upc2Δ strain. (D) tom70Δ strain compared to the BY4742 wild type and MGE1 overexpression in this deletion strain. (E) ecm10Δ strain compared to the BY4742 wild type and MGE1 overexpression in this deletion strain. (F) ssq1Δ strain compared to the BY4742 wild type and MGE1 overexpression in this deletion strain [SC(-URA)glu medium was used here, results seen after 48 and 72 h are shown]. (G) MGE1 overexpression compared to EV control in aft1Δ and aft2Δ strains. (H) fra1Δ strain compared to the BY4742 wild type. Download FIG S1, PDF file, 8 MB (8.2MB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sterols detected by GC-MS in the MGE1 overexpression strain compared to the control. (A) Strains were grown in SDglu medium for 24 h, in the absence or presence of fluconazole. The sterol composition of EV and MGE1 strains was analyzed under both sets of conditions. The abundance of each compound was calculated as the peak area relative to cholestane (internal standard) based on CG-MS analysis of four biological replicates. RT, retention time; RRT, relative retention time. (B) Schematic representation of the main ergosterol biosynthesis pathway (adapted from reference 78), with the sterols that we identified in red. The symbol depicted next to the identified sterol indicates how the fluconazole-induced change in this sterol is altered upon overexpression of MGE1 (only significant changes are shown [tested by two-way ANOVA with Bonferroni correction]). Download FIG S2, PDF file, 0.8 MB (883.3KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Increased dosage of Mge1 does not cause Erg11 levels or ERG-related gene expression to increase. (A) Expression of none of the ERG genes increased significantly when MGE1 was overexpressed. Cells were grown for 24 h in SDglu medium, in the presence or absence of 20 μg/ml fluconazole. The results of qRT-PCR analysis are displayed as the average of log2(Y) transformed values with the SEM. The values were calculated relative to the average of the values from the untreated samples. Statistical analysis was conducted on the transformed values by two-way ANOVA with Bonferroni correction; *, P < 0.05; **, P < 0.01. (B) Erg11 protein levels decrease slightly when MGE1 is overexpressed. Cells from strain AFc202 were grown for 24 h in SDglu medium, in the presence or absence of 20 μg/ml fluconazole. The blots were probed with anti-HA or anti-Pgk1 antibodies (loading control). (C) Expression of MGE1 does not alter significantly in an upc2 Δ strain compared to the wild type. The results of qRT-PCR analysis are displayed as the average of log2(Y) transformed values with the SEM relative to BY4742 values. Statistical analysis was conducted on the transformed values by unpaired Student’s t test. Download FIG S3, PDF file, 0.3 MB (313.9KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Etest analysis and microdilution assay of CgMGE1 overexpression in the C. glabrata 2001HTL strain. (A) Etest analysis, performed on media containing 0.2% or 2% glucose. Pictures were taken after 48 h of incubation at 37°C. (B) Dose-response curves of all strains, with dotted lines indicating 50% (upper line) and 90% (middle line) growth inhibition and the initial inoculum (lower line). P = 0.807 (128 μg/ml flu, EV versus pPGK1-CgMGE1), P = 0.857 (64 μg/ml flu, EV versus pPGK1-CgMGE1), P = >0.999 (128 μg/ml flu, EV versus pTDH3-CgMGE1), and P = 0.118 (64 μg/ml flu, EV versus pTDH3-CgMGE1). P values were calculated by two-way ANOVA with Bonferroni correction. Download FIG S4, PDF file, 1.6 MB (1.6MB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Etest analysis and microdilution assay of CaMGE1 overexpression in the C. albicans SC5314 strain. (A) Eight transformants overexpressing CaMGE1 (LDa01 through LDa08) were analyzed for copy number integration, relative CaMGE1 expression levels, and MICflu category compared to the control strain (LDa09). (B) Etest analysis. Pictures were taken after 24 h of incubation at 37°C. Transformants LDa05 and LDa07 are examples of those with an increase in MICflu and LDa04 and LDa06 as examples of those with an MICflu similar to that of the controls (LDa09). (C) Tolerance assay assessing trailing growth of 3 transformants showing an increased MICflu compared to the average of the values (with SEM) determined for three control strains (LDa09, n = 3). Due to the variance in CaMGE1 overexpression (see panel A), CFU counts of the overexpression transformants are shown individually as well as via the average and SEM of the three strains together. Statistical analysis was conducted by two-way ANOVA with Bonferroni correction. ***, P = <0.001. Dotted lines indicate 50% (upper line) and 90% (middle line) growth inhibition and the initial inoculum (lower line). Download FIG S5, PDF file, 1.4 MB (1.4MB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains used in this study. Download TABLE S1, PDF file, 0.1 MB (104.7KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used in this study. Download TABLE S2, PDF file, 0.1 MB (85.4KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Plasmids used in this study. Download TABLE S3, PDF file, 0.1 MB (74.9KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
MICflu values of several strains. Data indicated with a superscript "a" represent the results of overexpression of MGE1-GFP. Data indicated with a superscript "b" were determined after 72 h on SCglu medium (the latter was used only for the Etest; BY4742 had MICflu values of 8 to 12 on this medium). Download TABLE S4, PDF file, 0.1 MB (76KB, pdf) .
Copyright © 2017 Demuyser et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.