Abstract
Synaptic plasticity (e.g. long-term potentiation; LTP) is considered as the cellular correlate of learning. Recent optogenetic studies on memory engram formation assign a critical role in learning to suprathreshold activation of neurons and their integration into active engrams (‘engram cells’). Here we review evidence that ensemble integration may result from LTP, but also from cell-autonomous changes in membrane excitability. We propose that synaptic plasticity determines synaptic connectivity maps, while intrinsic plasticity – possibly separated in time – amplifies neuronal responsiveness and acutely drives engram integration. Our proposal marks a move away from an exclusively synaptocentric toward a non-exclusive, neurocentric view of learning.
Ever since its discovery in the early 70’s, long-term potentiation of synaptic transmission (LTP; Bliss and Lomo, 1973) has been seen as a plausible cellular mechanism underlying information storage and learning. LTP indeed meets the basic requirements that a cellular learning correlate needs to fulfill: a) LTP increases synaptic weights and enhances the probability that an active synaptic input contributes to action potential generation, b) LTP lasts sufficiently long to lay the foundation for stable memories, c) LTP can be input-specific and thus allows for selective information storage, and d) LTP is actively reversed by long-term depression (LTD) enabling bidirectional modification (for reviews on LTP/LTD in different neural circuits, see Pittenger and Kandel, 2003: Aplysia; Malenka and Nicoll, 1993: hippocampus; Singer, 1995: neocortex; Jörntell and Hansel, 2006: cerebellum). However, a preeminent role of LTP – and, in extension, synaptic plasticity – in learning has been challenged based on the argument that properties of LTP do not match crucial properties of learning. Most importantly, learning can result from single experiences, while LTP typically requires repetitive stimulation (for this and other critiques of the synaptic learning theory, see Gallistel and Matzel, 2013; Gallistel and Balsam, 2014).
New technologies – based on the manipulation of neuronal activity using optogenetics – now allow us to monitor and manipulate ‘mnemic traces’ (Semon, 1904), or memory engrams, enabling a critical assessment of established views on the cellular events that underlie memory storage and retrieval (for review, see Tonegawa et al., 2015; Holtmaat and Caroni, 2016). Using these techniques, Roberto Malinow and colleagues were able to show that optogenetic stimulation of auditory inputs to the amygdala, timed to mimic LTD and LTP protocols, successfully inactivates and reactivates fear memories, respectively (Nabavi et al., 2014). This finding is in line with the long-standing notion that synaptic plasticity is a cellular correlate of learning (e.g. blockade of NMDA receptor signaling or CaMKII activation prevents LTP and impairs fear conditioning; Kim et al. 1991; Miller et al., 2002, but see also Gu et al., 2002, for a dissociation between effects on LTP and fear conditioning in a Fragile X syndrome mouse model). The study does not, however, provide a critical test for a general, pan-essential involvement of synaptic plasticity in memory formation.
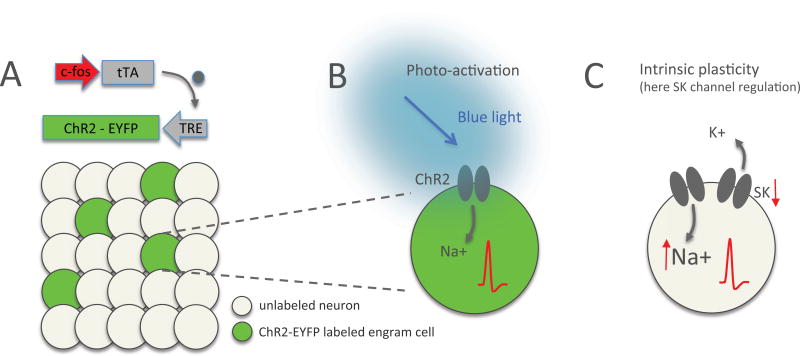
Optogenetics has also been used to study memory storage and retrieval by engram cells. An important first step has been the development of transgenic mice, in which activation of the c-fos promoter is coupled to the expression of the tetracycline-transactivator (tTA; Reijmers et al., 2007). Using this technique, it is possible to label neurons that have been activated (c-fos promoter) during a specific conditioning period (conditioning in the absence of doxycycline; Reijmers et al., 2007). When coupled to the expression of channelrhodopsin 2 and the fluorescent marker EYFP (ChR2-EYFP), an elegant system becomes available that allows for the fluorescence labeling and light-triggered re-activation of neurons that participate in a memory engram (Fig. 1A). This technique has been used to optogenetically retrieve fear memories in the hippocampus. The experiments were designed to obtain an expression of ChR2-EYFP only in those neurons of the dentate gyrus that were active during the conditioning phase and in which the c-fos promoter was activated. Subsequent light stimulation resulted in increased freezing, suggesting a successful, optogenetic retrieval of the fear memory engram (Liu et al., 2012; for review, see Tonegawa et al., 2015). Photo-activation has subsequently been used to create artificial neuronal ensembles in the visual cortex, which can be reactivated upon stimulation of individual neurons participating in the ensemble (Carillo-Reid et al., 2016). The photo-activation of channelrhodopsin opens nonspecific cation conductances that depolarize the labeled neurons and initiates spike firing (Fig. 1B). In these studies, light-triggered memory recall rests on the channelrhodopsin-mediated depolarization that brings the cell closer to spike threshold. Remarkably, in the presence of the protein synthesis inhibitor anisomycin, which blocks the late phase of LTP, photo-activation caused freezing at a rate that was indistinguishable from controls (Ryan et al., 2015). Context-dependent freezing also took place, although at a significantly reduced rate. Of note, anisomycin treatment prevented the strengthening of synaptic weights and the increase in spine density that is observed under control conditions, but unveiled an increase in intrinsic excitability (slope of the spike frequency versus injected current curve; f–i curve) (Ryan et al., 2015). These studies allow for several conclusions of interest. First, the observation that photo-activation alone – in control or anisomycin-treated mice – triggers freezing shows that the ultimately decisive factor in memory recall is the suprathreshold re-activation of participating engram cells. From the perspective of synaptic learning theories, the interpretation of this finding is that under physiological conditions enhanced synaptic weights increase the probability for spike firing in the engram cell, and that photo-stimulation simply bypasses the need for sufficiently strong synaptic drive (see Nabavi et al., 2014; Poo et al., 2016). However, second, freezing also occurs in anisomycin-treated mice when they are placed in the context in which they were conditioned – in the absence of photo-activation, and in the absence of synapse or spine plasticity, but with a significant, >30% increase in excitability present (Ryan et al., 2015).
Figure 1.
Neural engram labeling / re-activation and plasticity. (A) Engram cells can be labeled by expression of ChR2-EYFP after activation of an activity-dependent promoter such as c-fos or arc. Labeled engram cells (green) can be re-activated by photo-stimulation of channelrhodopsin (ChR2). TRE: tetracycline-responsive element; tTA: tetracycline transactivator (grey dot). (B) Photo-activation of a ChR2-expressing neuron with blue light causes influx of Na+ ions and depolarization, which brings the membrane potential closer to the spike threshold and enhances the probability of spike firing. (C) Intrinsic plasticity results from a cell-autonomous modulation that similarly changes the probability of action potential generation. In the example shown here, intrinsic plasticity-related downregulation of calcium-activated, small conductance SK-type K+ channels will reduce the afterhyperpolarization following depolarizing events. As a consequence EPSPs will be enhanced and prolonged and the spike threshold will be reached more easily (EPSP amplification, faster re-depolarization toward threshold and/or reduction of the threshold itself).
Together these findings raise the possibility that non-synaptic, cell-autonomous modulation of neuronal excitability is sufficient for engram integration under some conditions (Fig. 1C) and that, therefore, synaptic plasticity is not essential for the integration process (note that the terms ‘non-synaptic’ and ‘intrinsic’ refer to the expression phase of this form of plasticity; synaptic activation is needed for its induction). An intrinsic plasticity-dependent engram model is reminiscent of a class of so-called ‘constructive’ machine learning algorithms, in which the ability to move artificial neurons in and out of ensembles is used for optimization of multiple-layer networks (e.g. Mezard and Nadal, 1989; Marblestone et al., 2016). Intrinsic plasticity functionally resembles such a mechanism, because it modulates the activity level and output efficacy of the entire neuron rather than selected subsets of its inputs. But is there indeed a physiological equivalent to such operating algorithms used in machine learning? While intrinsic plasticity has been observed in electrophysiological recordings, and has been characterized in some molecular and cellular detail a conceptual framework for its role in learning and memory is still missing. Here, we will summarize what is known about intrinsic plasticity, present first steps toward an ‘intrinsic theory’ of learning, and discuss what experiments are needed to test whether intrinsic plasticity can indeed assume key functions in learning and memory.
We suggest that purely synaptic learning theories are no longer enough to reconcile models of information storage in neural networks with the emerging role of entire neurons (‘engram cells’) in memory engram physiology. We propose an extended learning hypothesis, in which we assign a more broadly defined role in establishing connectivity maps to synapses, and add plasticity of intrinsic excitability as a mechanism for engram integration:
-
1)
The decisive factor in memory engram formation and recall is the activation / integration of participating neurons (‘engram cells’). Two plasticity processes are critically involved, synaptic and intrinsic plasticity.
-
2)
Intrinsic plasticity sets an amplification factor that enhances or lowers synaptic penetrance, and defines the neuron’s presence within a memory engram. Here we introduce the term synaptic penetrance in analogy to genetic penetrance to describe that synaptic weight changes (as in LTP) do not always result in enhanced spike firing, but that other factors, such as intrinsic amplification, provide important co-determinants. Intrinsic plasticity alone can in some conditions mediate engram cell integration, based on pre-existing, but unaltered synaptic connectivity.
-
3)
Synapses play three fundamental roles in learning: a) they convey the specific information contents and input patterns that are to be memorized, b) synaptic plasticity shapes connectivity maps by establishing connection patterns and by assigning synaptic weights, and c) synaptic activity triggers intrinsic plasticity (induction phase) and drives the (re-) activation of memory engrams, albeit without the need for accompanying changes in synaptic weight.
-
4)
From the above it follows that learned information is represented in two different ways in memory engrams: first, by the synaptic inputs that convey information to a neuron and collectively determine the coding identity of this neuron. Second by the neuron itself, whose response threshold and activation characteristics determine its impact on target circuits and the representation weight of the information that it encodes.
Intrinsic plasticity: cellular mechanisms and relevance to learning
In a recent review, we compared molecular signaling cascades involved in LTD and developmental synaptic pruning at synapses in the visual cortex, the cerebellum and at the neuromuscular junction, and found that at each type of synapse the signaling pathways used for developmental and adult plasticity almost completely overlap (Piochon et al., 2016a). This observation suggests that forms of synaptic plasticity found in the adult brain (LTD and LTP) may serve similar functions as their equivalents in the developing brain, namely the weakening / elimination of weak synapses and the strengthening / stabilization of efficient ones. A more general prediction from these findings is that the optimization of neural circuits and synaptic input maps continues into adulthood, and that synaptic plasticity is the cellular tool used to fine-tune these connectivity maps in an experience-dependent way. This prediction is the basis for a core claim of our hypothesis: synaptic plasticity primarily forms and adjusts connectivity maps, and only under specific conditions contributes to acute learning effects.
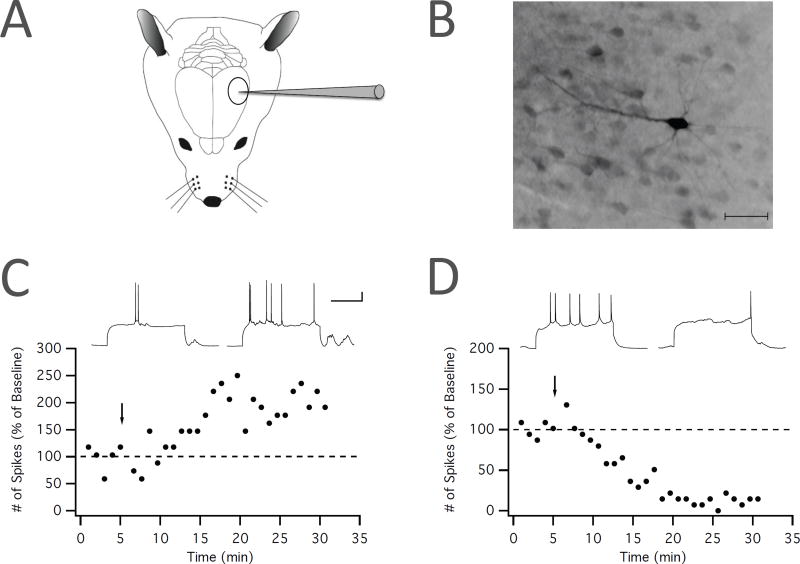
What then is the evidence that intrinsic plasticity provides a crucial cellular learning correlate? Most importantly, intrinsic plasticity has been observed in vivo (see Table 1) and available evidence demonstrates a role in some forms of learning. Using intracellular recordings from layer 5 pyramidal neurons in the motor cortex of anesthetized rats, Stéphane Charpier and his colleagues found that repeated injection of suprathreshold, depolarizing current pulses led to a long-term change in the intrinsic excitability of these neurons. Excitability was determined by injecting current pulses during the test periods before and after tetanization and by measuring the slope of the f– i curve as well as the spike threshold, and was altered in 21/33 recordings. Intrinsic potentiation occurred about twice as often as depression (Paz et at., 2009). Similarly, repeated current injection caused intrinsic potentiation (18/30) or depression (12/30) in layer 5 pyramidal neurons of the barrel cortex of anesthetized rats (Mahon and Charpier, 2012; for example traces from layer 2/3 barrel cortex neurons, see Fig. 2). Importantly, intrinsic plasticity is not an artifact of anesthesia: increases in spontaneous spike firing were observed subsequent to parallel fiber (PF) burst stimulation in Purkinje cells of non-anesthetized, decerebrated rats (Belmeguenai et al., 2010). These studies show that intrinsic plasticity is an activity-dependent phenomenon that can be observed in vivo in cell types as diverse as pyramidal cells and Purkinje cells. They also show that intrinsic plasticity differs from homeostatic plasticity (for review, see Turrigiano, 2011), because in the majority of recordings, neuronal activation causes a further increase in spike firing.
Table 1.
Intrinsic plasticity studied using in vivo electrical or behavioral conditioning. The table summarizes reports of intrinsic plasticity observed during in vivo recordings, or in recordings from slices that were prepared subsequent to in vivo conditioning . Note that this is an incomprehensive selection that is focused on mammalian studies. N.D.: not determined. Question marks in the ‘Mechanism’ column indicate that the cellular mechanisms listed here are suggested by separate studies other than the main reference.
| Circuit | Neuron | Method | Conditioning | Excitability | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Motor cortex (cat) | Neurons | Intracellular recording (awake) | Eyeblink | ↑ | N.D. | Aou et al., 1992 |
| Motor cortex (rat) | LV pyramidal cells | Intracellular recording (anaesthetized) | Intracellular conditioning | ↑↓ | N.D. | Paz et al., 2009 |
| Somatosensory cortex (rat) | LV pyramidal cells | Intracellular recording (anaesthetized) | Intracellular conditioning | ↑↓ | SK-type K current ? (Sourdet et al., 2003) | Mahon & Charpier, 2012 |
| CA1 Hippocampus (rabbit) | Pyramidal cells | Intracellular recording in slice (in vivo conditioning) | Eyeblink | ↑ | SK- or BK- type K current? (McKay et al., 2012 Matthews & Disterhoft, 2016) | Disterhoft et al., 1986 |
| CA1 Hippocampus (rat) | Pyramidal cells | Whole-cell recordings in slice (in vivo conditioning) | Fear conditioning | ↑↓ | N.D. | McKay et al., 2009 |
| Lateral Amygdala (Mouse) | Principal neurons | Whole-cell recordings | Overexpression of CREB or dnKCNQ2 | ↑ | CREB Fear memory allocation during subsequent training | Yiu et al., 2014 |
| Cerebellum (rabbit) | Purkinje cells | Intracellular recording in slice (in vivo conditioning) | Eyeblink | ↑ | A-type K current | Schreurs et al., 1998 |
| Cerebellum (rat) | Purkinje cells | Single-unit extracellular (awake; decerebrated) | Synaptic activation | ↑ | SK-type K current | Belmeguenai et al., 2010 |
Figure 2.
Intrinsic plasticity in the primary somatosensory cortex in vivo. (A) Recording configuration. Whole-cell patch-clamp recordings are performed from L2/3 pyramidal neurons in the barrel cortex of anesthetized rats. (B) Neurons are labeled with neurobiotin (0.2%) for histological identification subsequent to the recordings. The picture shows a neurobiotin-labeled L2/3 pyramidal neuron. Scale bar: 50µm. (C)+(D) Example recordings (H.T. and C.H.; unpublished data) illustrating the bidirectionality of intrinsic excitability changes. Intrinsic plasticity is triggered by repeated injection of depolarizing current pulses (5Hz for 8s). (C) In this example recording, tetanization resulted in an increase in excitability as measured by the number of spikes evoked by test pulses. (D) Example of a neuron, in which the same tetanization protocol resulted in a depression of the spike count. Scale bars: 200ms / 20mV. Arrows indicate the time of tetanization.
Plasticity of the intrinsic membrane excitability of neurons has been discussed for many years as a mechanism that might play a role in learning, possibly complementing forms of synaptic plasticity (for review, see Marder et al., 1996; Hansel et al., 2001; Daoudal and Debanne, 2003; Zhang and Linden, 2003; Frick and Johnston, 2005; Mozzachiodi and Byrne, 2010). Intrinsic plasticity has been described in a variety of preparations, but has been most often examined in recordings from brain slices. The phenomenon itself is less strictly defined than synaptic plasticity and has been probed using various measures, such as spontaneous and evoked spike rates, the spike threshold and the afterhyperpolarization (AHP) amplitude. Similar to synaptic plasticity, intrinsic excitability changes may result from brain-derived neurotrophic factor (BDNF) signaling (Desai et al., 1999; Graves et al., 2016). In contrast to synaptic plasticity, intrinsic plasticity is not mediated by changes in neurotransmitter receptors, but results from modifications of voltage- or calcium-dependent ion channels such as K channels or mixed Na/K channels. For example, forms of intrinsic plasticity have been described that are mediated by changes in A-type K channels (Schreurs et al., 1998; Frick et al., 2004), calcium-activated BK channels (Nelson et al., 2005), calcium-activated SK channels (Sourdet et al., 2003; Lin et al., 2008; Belmeguenai et al., 2010) as well as hyperpolarization-activated HCN channels (Nolan et al., 2003; Fan et al., 2005; Brager and Johnston, 2007). Intrinsic plasticity is not restricted to specific neurons, but has been observed in a variety of invertebrate and vertebrate neurons, including hippocampal and neocortical pyramidal cells (for review, see Zhang and Linden, 2003; Frick and Johnston, 2005; Mozzachiodi and Byrne, 2010). In the cerebellar system, intrinsic plasticity has been found in granule cells (Armano et al., 2000), Purkinje cells (Schreurs et al., 1998; Belmeguenai et al., 2010) and in neurons of the cerebellar nuclei (Aizenman and Linden, 2000) and the vestibular nuclei (Nelson et al., 2005).
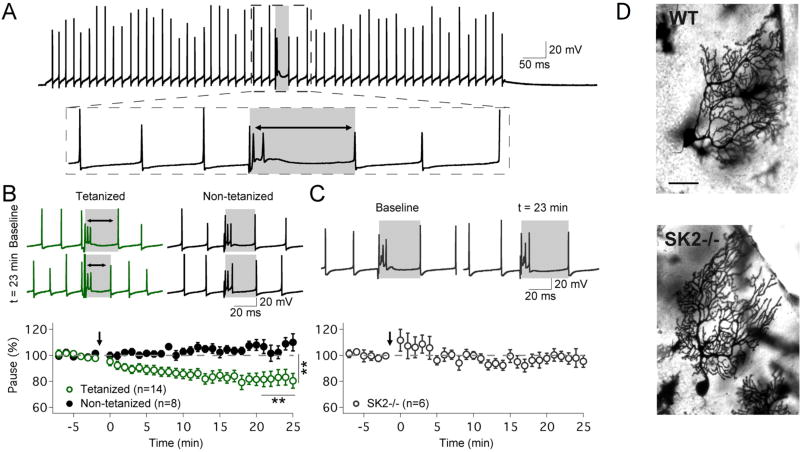
The available data suggest that intrinsic plasticity may serve a range of functions, some of which complement synaptic plasticity, while others point toward roles independent from synaptic plasticity. For example, increases in excitability that are mediated by a downregulation of SK channels fall into both categories. SK channel downregulation amplifies spine calcium transients and enhances the LTP induction probability in pyramidal cells (Stackman et al., 2002; Ngo-Anh et al., 2005; Hammond et al. 2006), while a similar boost of spine calcium signals by SK channel-dependent intrinsic plasticity reduces the probability for LTP induction in Purkinje cells (Belmeguenai et al., 2010). The latter effect likely results from the ‘inverse’ calcium thresholds that govern LTP and LTD induction at PF – Purkinje cell synapses (Coesmans et al., 2004; Piochon et al., 2016b). These examples show that intrinsic plasticity can modify the LTP induction probability in an activity-dependent way. On the other hand, intrinsic plasticity may affect neuronal spike output independent from synaptic plasticity. For example, SK channel-dependent intrinsic plasticity reduces the duration of spike pauses in Purkinje cells and may thus alter the spike output of the cerebellar cortex without any synaptic involvement in the expression phase of this plasticity mechanism (Grasselli et al., 2016; Fig. 3). Finally, SK channel plasticity may alter the spike threshold and thus affect EPSP-spike coupling. This effect demonstrates that it does not have to be either a synaptic or an intrinsic mechanism that allows for specific memory storage, but that both can perfectly complement each other, e.g. when potentiated synapses receive further intrinsic amplification. This phenomenon has been observed in layer 5 pyramidal neurons of the sensorimotor cortex (Sourdet et al., 2003) as well as in cerebellar Purkinje cells (Ohtsuki et al., 2012). Here, we focused on SK channel plasticity as an example to describe various consequences of intrinsic plasticity, because SK channel modulation has been particularly well studied. There are aspects of SK channel plasticity that are not shared by all types of ion channel plasticity: importantly, SK channel deactivation is slow, which is reflected in the SK channel participation in the medium-late component of afterhyperpolarization currents (ImAHP; Bond et al., 2004). This feature enables SK channel plasticity to not only facilitate action potential generation per se (one spike vs. no spike), but also to promote spike burst firing (burst vs. one spike or no spike), which might well be the relevant parameter for integration of neurons into active ensembles. Notably, excitability changes localized to specific dendritic compartments will benefit small groups of synapses (Ohtsuki et al., 2012; for a similar A-type K channel-mediated effect in pyramidal neurons, see Losonzcy et al., 2008). As intrinsic plasticity is – under physiological conditions – driven by synaptic activity (in the induction phase) it is conceivable that local intrinsic plasticity may favor potentiated synapses. In contrast, when a spike threshold shift occurs close the site of action potential generation at or near the soma (e.g. as a result of changes in ion channels that affect the spike threshold, or as a result of changes in the function and/or location of the axon initial segment; see Grubb and Burrone, 2010; Grubb et al., 2011), it will similarly benefit all excitatory synapses that contact that neuron. This ‘somatic’ expression of intrinsic plasticity has been described in multiple studies and can be observed whenever intrinsic plasticity is measured in the test periods before and after tetanization by counting spikes evoked by depolarizing current injections into the soma (e.g. Sourdet et al., 2003; Belmeguenai et al., 2010; Fig. 2).
Figure 3.
Plasticity of pauses in spike firing is mediated by an intrinsic mechanism. (A) Typical trace from an in vitro Purkinje cell recording showing simple spike firing evoked by depolarizing current injection and a complex spike triggered by climbing fiber stimulation. The complex spike pause is measured as the interval between the complex spike onset and the next subsequent simple spike (gray area). (B) Top: typical traces (green) show that 5 Hz injection of depolarizing currents causes a shortening of the spike pause. In contrast, pause duration remains stable in control recordings (black traces). Bottom: time graph showing that the intrinsic plasticity protocol shortens the pause duration, while the pause remains unaltered in non-tetanized cells. (C) In SK2−/− Purkinje cells, no pause plasticity is observed. (D) Golgi staining of Purkinje cells in WT (top) and SK2−/− mice (bottom). Scale bar: 50µm. This figure is reproduced from Grasselli et al. (2016). Copyright 2016 by Elsevier.
Together, these findings show that neurons can modulate their membrane excitability, and that such a modulation is activity-dependent. But is intrinsic plasticity indeed involved in learning? To answer this question, rabbits were subjected to trace eyeblink conditioning, and intracellular recordings were performed from CA1 pyramidal neurons in hippocampal slices that were prepared ≥24hrs after conditioning. In control experiments, recordings were performed from pseudoconditioned and naïve animals. In the conditioned group, neurons showed enhanced excitability and the AHP’s following spike bursts were significantly reduced (Disterhoft et al., 1986; Moyer et al., 1996). Similar observations were made in Purkinje cell recordings in vitro after delay eyeblink conditioning in rabbits. Here, too, neurons in slices prepared from conditioned animals showed enhanced excitability and reduced AHP amplitudes (Schreurs et al., 1998). These observations are not the results of artifacts of the in vitro recording conditions: enhanced excitability subsequent to eyeblink conditioning has also been observed in intracellular recordings from the motor cortex of awake cats (Aou et al., 1992). Together, these findings suggest that intrinsic plasticity exists under physiological conditions, and that it plays a critical role in learning. There indeed seems to be ‘memory from the dynamics of intrinsic membrane currents’ as postulated about 20 years ago by Eve Marder and colleagues (Marder et al., 1996).
Synaptic penetrance as a limiting factor in memory storage
When describing above the four critical components of our intrinsic theory of learning, we introduced the term synaptic penetrance to stress that intrinsic amplification often is required to lift EPSPs – in some cases even at potentiated synapses – above spike threshold. An assumption underlying synaptic learning theories is that LTP – supported by the phenomena of temporal and spatial summation (e.g. Guzman et al., 2016) – will enable synapses to efficiently contribute to spike generation. This argument is based on the necessity that cells participating in a memory engram must show suprathreshold activity. However, recent findings suggest that low synaptic penetrance is not the exception, but is instead frequently found. The problem for locally evoked EPSPs to reach spike threshold is illustrated by the strong attenuation of EPSP amplitudes from their origin in dendritic spines all the way to the soma: using two-photon glutamate uncaging and simultaneous two-photon voltage-sensitive dye recordings from cortical L5 pyramidal neurons, a recent study measured spine potentials in the range of 6.5–30.8mV (average 13.0mV) that evoked average somatic EPSPs of 0.59mV (Acker et al., 2016; for similar results, see Bloodgood et al., 2009; Palmer and Stuart, 2009; Harnett et al., 2012; Popovic et al., 2015). The latter value corresponds well to EPSP amplitudes that were somatically recorded in response to the activation of unitary synapses in the visual cortex (average 0.55mV, range: 0.05 – 2.08mV; Mason et al., 1991) and in the motor cortex (in different cell pairs, average EPSP amplitudes in the range of 1.1 to 1.3mV were reported; Deuchars et al., 1994). While these values are informative, they do not tell us anything about the compound EPSP amplitudes evoked by several synapses under physiological conditions. The relationship between compound EPSPs and the spike probability has been best studied in the primary somatosensory cortex. In L2/3 pyramidal neurons of the rat barrel cortex, the average amplitude of responses to stimulation of the principal whisker has been determined to be 9.1mV. The resting membrane potential was found to be 15–40mV below spike threshold, resulting in an evoked spike rate of 0.031 action potentials per stimulus (Brecht et al., 2003). In L5 pyramidal neurons, the average response amplitude to stimulation of the principal whisker was 5.0mV. As the voltage difference to spike threshold was lower than in L2/3 (average 20.9mV), the evoked spike rate was slightly higher (0.12 action potentials per stimulus; Manns et al., 2004). These results indicate sparse coding in some cortical areas, an observation that has been made in both anaesthetized and awake recordings (Margrie et al., 2002) and suggests low synaptic penetrance. Widespread, non-linear dendritic integration has been demonstrated during active touch, resulting from coincident sensory and motor input (Xu et al., 2012). Such regenerative dendritic events can be predicted to enhance spike output under sparse coding conditions, supporting the notion that low synaptic penetrance can be overcome by intrinsic amplification. Similarly, local intrinsic amplification of EPSPs – resulting from a downregulation of K conductances, or the activation of subthreshold Na conductances (Carter et al., 2012) – provides a cellular mechanism to boost synaptic penetrance and to integrate neurons more efficiently into active ensembles.
Notably, different cortical areas might show different coding characteristics. The findings described above are in stark contrast to the ‘high-input regime’ hypothesis that describes cortical neurons (here in visual cortices) as being bombarded by synaptic input (Shadlen and Newsome, 1998). To some degree the differences between the observations made in the barrel cortex by Sakmann, Brecht and colleagues (using whole-cell patch-clamp recordings) and those made in the visual cortex using extracellular unit recordings (referred to in Shadlen and Newsome, 1998) result from an undersampling bias that is characteristic for extracellular unit recordings, which are ‘blind’ to neurons that are silent or fire spikes at low frequencies (as pointed out in Brecht et al., 2005). However, highly reliable spike firing – in response to preferred direction movement of visual stimuli – has also been found in patch-clamp recordings from the cat primary visual cortex (Priebe and Ferster, 2005), suggesting that the high response rates found in visual cortex cannot be fully explained by differences in the recording technique. It is conceivable that the primary visual cortex is tuned to high synaptic penetrance / intrinsic amplification values, because of the uninterrupted inflow of visual signals that need to be processed, while cortical areas such as the barrel cortex and possibly higher cognitive areas operate using low-input regimes, in which the distinction between spike and no-spike output is a more critical component of information processing. A prediction resulting from these considerations is that intrinsic amplification is not equally critical in all cell types and brain areas.
Features added by intrinsic plasticity
Above we have shown that intrinsic plasticity is a physiologically relevant phenomenon that takes place in the context of learning. What then are the consequences of intrinsic plasticity for learning, and what features does it add to memory engram storage? In the following, we will discuss functional implications of intrinsic plasticity focusing on changes in excitability that take place at or near the soma where they affect the spike output of the entire neuron. Note that for this consideration it is irrelevant whether this form of plasticity is localized to the axon initial segment, the soma, or proximal parts of the dendrites as long as the probability to reach the spike threshold is changed for the majority of synaptic inputs. Intrinsic plasticity – if it occurs at or near the soma and will affect the spike threshold and / or the excitability (slope of the f-i curve) – can be predicted to have the following consequences:
First, intrinsic plasticity will alter the spike firing probability of a neuron and will thus determine its degree of integration into ensembles of synchronously active neurons (see Singer, 1995). In this view, intrinsic plasticity adds an intrinsic amplification factor that acts like a light dimmer adjusting synaptic penetrance via two effects: a) by boosting the response amplitude, and b) by lowering the spike threshold. For example, in recordings from layer 5 pyramidal neurons in the barrel cortex of anesthetized rats, it was found that intrinsic potentiation and depression, respectively, modulate the spike firing threshold by about 1–2mV, suggesting a total dynamic range of up to 4mV (Mahon and Charpier, 2012). Furthermore, enhanced excitability in learning may not only integrate neurons into ensembles, but may also attract memory storage to ensembles that encoded a different memory up to several hours prior (‘memory allocation hypothesis’; Cai et al., 2016).
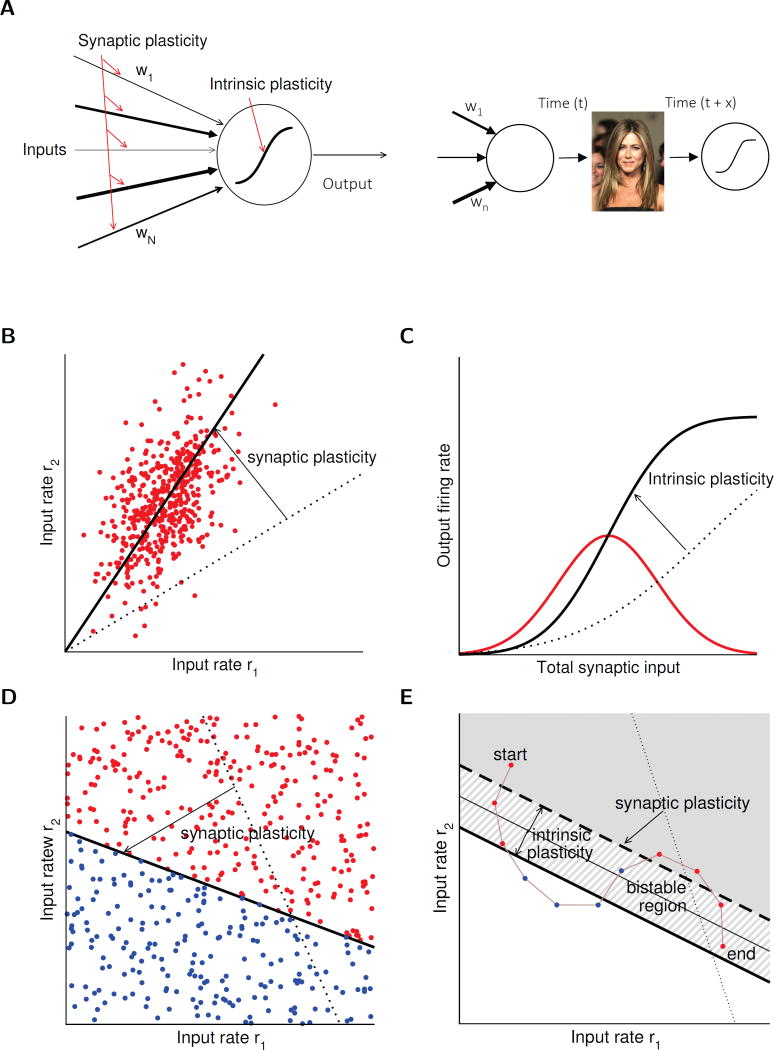
Second, intrinsic plasticity will similarly affect all synaptic inputs onto a neuron (note that this discussion only focuses on excitatory synapses), because all synaptic responses will be further amplified and will more easily reach spike threshold (likewise, intrinsic plasticity can reduce excitability and make it more difficult to reach spike threshold). A consequence of this neuron-wide scaling effect is that all synaptic inputs – independent of their recent potentiation or depression history and their current synaptic weight –share the same amplification fate and will therefore be up- or downregulated together. If a neuron’s synaptic inputs convey largely unrelated information, combinatorial encoding across several neurons will reveal which information content was highlighted and stored, without further consequences of shared intrinsic scaling. However, if a neuron’s synaptic input portfolio is dominated by one or a few sets of synaptic inputs, or if the majority of synapses convey largely related information, intrinsic plasticity and the shared amplification fate of its inputs will define a coding identity of the neuron, i.e. activity of this neuron encodes specific information content. In the extreme case – this is likely to be true for neurons that code for complex feature constellations, such as concept cells, e.g. a ‘Jennifer Aniston neuron’ (Quian Quiroga et al., 2005; for review, see Quian Quiroga, 2012) – synaptic weights and weight ratios are optimally adjusted (Fig. 4A). Here, the advantages of intrinsic plasticity become obvious as intrinsic plasticity enables memory storage without changes in synaptic weight ratios that would disturb proper feature representation.
Figure 4.
Theoretical models of intrinsic and synaptic plasticity. (A) Left: Sketch of a simplified single neuron model showing synaptic weight adjustment by synaptic plasticity. Synaptic input weights are summed linearly and passed through a static non-linearity. Loci of plasticity are indicated in red. Right: Illustration of the hypothesis that the adjustment of synaptic weights (synaptic input map) and the plasticity of the intrinsic amplification factor can be separated in time. The example shown here depicts a face-recognition cell encoding the face of the actress Jennifer Aniston. In such highly specialized ‘concept cells’ synaptic input weights are optimally adjusted so that the neuron encodes the object, or a generalized concept of it. Intrinsic plasticity – occurring at time t+x – may enhance engram representation without altering synaptic weight ratios, but based on pre-existing connectivity. Image copyright: the authors (Editorial use license purchased from Shutterstock, Inc.). (B) – (C) Unsupervised learning. (B) Classic unsupervised synaptic plasticity rules allow a neuron to pick the direction of maximal variance in its inputs. Red dots show inputs drawn from a correlated 2D Gaussian distribution, in the space of two inputs to a neuron. The dotted line shows the initial synaptic weight vector. The total synaptic input to a neuron is given by the dot product between the input vector and the synaptic weight vector. Classic Hebbian synaptic plasticity rules – such as the Oja rule – adjust the weight vector until it picks the direction of maximal variance in the inputs, therefore performing principal component analysis (PCA). (C) The resulting distribution of total synaptic inputs is shown in red. The dotted line shows an initial static transfer function (f-I curve) that maximizes mutual information between neuron output and input. It is proportional to the cumulative distribution function of the inputs (Laughlin, 1981). Intrinsic plasticity could adjust this non-linearity until the transfer function matches the optimal one. (D) – (E) Supervised learning. (D) A classic supervised learning problem: the neuron should separate inputs into two classes (red: neuron should be active; blue: neuron should be inactive). The neuron learns to classify inputs by changing its synapses (modifying the hyperplane that separates active and inactive regions). Intrinsic plasticity can help by adjusting the neuronal threshold that measures the distance of the hyperplane from the origin. Learning can be achieved using the classic perceptron algorithm. (E) In some cases, a standard perceptron algorithm fails. In the example shown here, the neuron should learn a particular sequence of input-output associations (shown by colored dots connected by brown line). The neuron should be active in response to red inputs, but inactive for blue inputs. This example cannot be learned by a standard perceptron, because no straight line separating the blue and red dots exists. However, a bistable neuron can learn this sequence: in the bistable region (hatched region between the two thick black lines) the state of the neuron depends on the initial condition. It is active when it starts from an active state (gray shaded region), and inactive when it starts from an inactive state (white region). Intrinsic plasticity could in principle allow a neuron to become bistable and therefore allow it to solve problems that are not learnable by standard perceptrons (Clopath et al., 2013).
Third, the available data on features of intrinsic plasticity suggest that it may form the basis for learning phenomena that are not as easily explained by synaptic plasticity. Most notably, an important argument against LTP as a learning correlate has been that LTP requires prolonged, repetitive stimulation for its induction, and thus cannot provide a cellular correlate of single experience learning (Gallistel and Matzel, 2013; Gallistel and Balsam, 2014). In slice recordings, LTP induction indeed usually requires stimulation in the range of minutes, although there are types of LTP that can be triggered using activation protocols that last 15–20s (Salin et al., 1996; Frick et al., 2004). Intrinsic plasticity on its own can be evoked with very short activation periods that have not similarly been reported for LTP induction. For example, Purkinje cell intrinsic plasticity results from synaptic or somatic (injection of depolarizing currents) activation periods as short as 3s (Belmeguenai et al., 2010; Ohtsuki et al., 2012). Whether or not this difference in stimulus duration is critical needs to be assessed in future studies, but the available data point toward shorter minimal stimulus periods for intrinsic plasticity, making it a more suitable type of plasticity for single experience learning than LTP. Behavioral learning studies support the claim that intrinsic plasticity can be triggered by single experiences. This has been demonstrated in fear-conditioned rats, which show extinction and reversal of intrinsic excitability changes after a single trial (McKay et al., 2009). Comparing the outcome of a range of tetanization protocols on plasticity in dentate gyrus granule cells, it has recently been shown that intrinsic plasticity results from mild conditioning protocols, while synaptic plasticity emerges when stronger protocols are applied, suggesting that intrinsic plasticity might generally have a lower induction threshold, and might thus be more readily induced, than synaptic plasticity (Lopez-Rojas et al., 2016).
There are additional phenomena in learning that are particularly well explained by intrinsic plasticity as they seem to involve cell-wide rather than spatially restricted changes. One of these is ‘generalization’, which happens in associative conditioning: after acquisition of a conditioned response (CR) and extinction to a specific conditioned stimulus (CS) –unconditioned stimulus (US) pair (e.g. tone and airpuff), subsequent acquisition of a CR using a different CS (e.g. light) occurs faster (for discussion, see Hansel et al., 2001). As generalization can occur with various CS configurations in the first and second training sessions, this phenomenon cannot readily be explained by localized synaptic changes, but is likely related to widespread changes in intrinsic excitability. A second phenomenon that we predict to involve intrinsic plasticity is receptive field / map plasticity. Sensory input maps can change from specific / localized to general / widespread (see examples in Jörntell and Hansel, 2006). It is conceivable that large-scale changes in input maps involve synaptic potentiation, but require intrinsic amplification to reach the dramatic increase in map size that is frequently observed. Both map plasticity and CS generalization seem to benefit from intrinsic amplification, but this hypothesis has not been experimentally tested yet.
Intrinsic plasticity in theoretical models
Theoretical studies have been useful to explore and quantify the potential benefits of intrinsic plasticity for learning. Learning in neural circuits is traditionally categorized as being unsupervised or supervised (Dayan and Abbott, 2001), with different brain regions typically associated with one or the other (Doya, 2000).
In classical unsupervised learning rules, synapses evolve according to the correlation of pre and postsynaptic firing activities, subject to constraints that are needed to avoid uncontrolled growth of weights. Such synaptic plasticity rules have been shown in some instances to be able to maximize the mutual information between synaptic inputs and neuronal output, by selecting the direction in input space that maximizes the variance of total synaptic inputs (Oja, 1982), as shown in Fig. 4B. This concept of information maximization can be extended to intrinsic plasticity, as shown by Stemmler and Koch (1999), and as illustrated in Fig. 4C. Given a particular distribution of total synaptic inputs, Laughlin (1981) showed that a specific input-output transfer function (f-I curve) maximizes the mutual information between inputs and outputs. In the absence of constraints on average firing rates, the distribution of output rates that maximizes information is a uniform distribution, between the minimal (zero) and maximal firing rates. This has the consequence that the f-I curve that maximizes information is proportional to the cumulative distribution function of synaptic inputs. This information maximization principle can be extended to the case when a constraint on average firing rates exists; in this case, the optimal distribution of output rates is an exponential distribution, which leads to a different form of f-I curve. Stemmler and Koch proposed a learning rule that modifies calcium and potassium conductances, such that the f-I curve becomes progressively closer to the optimal one. Such a plasticity mechanism would naturally solve the `penetrance’ problem described above. If initially total synaptic inputs are far below threshold for the vast majority of inputs, such a plasticity mechanism would progressively adjust the threshold and gain of the neuron, until it uses its dynamic range optimally, subject to an average firing rate constraint. These arguments point to a natural division of labor between synaptic and intrinsic plasticity – in the model depicted in Fig. 4B and C, the role of synaptic plasticity is to pick the most informative direction in the high dimensional space of inputs to a neuron, while the role of intrinsic plasticity would then be to ensure that neurons use their dynamic range in an optimal way, given a distribution of synaptic inputs shaped by synaptic plasticity. This complementarity between synaptic and intrinsic plasticity has been further explored in a number of theoretical studies. In particular, Triesch and collaborators showed that in the case of non-Gaussian inputs the interaction between Hebbian synaptic plasticity and intrinsic plasticity allows a neuron to learn sparse directions in the input, and to perform Independent Component Analysis (ICA), both in firing rate models (Triesch, 2007) and in networks of spiking neurons (Savin et al., 2010).
Note that in specific cases, synaptic plasticity could be sufficient to maximize mutual information – in particular, when multiplying all synaptic weights by the same factor can allow the neuron to optimize its dynamic range. Such a global multiplication may be performed by homeostatic plasticity (Turrigiano et al., 1998). However, even in this scenario it might be advantageous to use intrinsic plasticity, since it would be far more economical to adjust only a few parameters characterizing the intrinsic currents of a neuron, rather than all the synaptic weights. Vice versa, in other situations, intrinsic plasticity could be sufficient to optimize the information transmitted by neurons. This would be the case for instance after an initial stage where synaptic plasticity has already picked the most informative direction in the space of inputs. If then the magnitude of these inputs (but not the direction) changes, then intrinsic plasticity could readily adjust the neuronal input-output relationship to restore an optimal dynamic range. Intrinsic plasticity could then be responsible for the adaptive rescaling of the input-output relationship with changes of the statistics of sensory inputs, which has been demonstrated in several sensory systems (see e.g. Brenner et al 2000).
In supervised learning theories, synapses are modified based on pre and post-synaptic activity, but also based on an additional `error signal’. The classic example is learning at the parallel fiber to Purkinje cell synapse, where plasticity depends on the powerful climbing fiber input, which is thought to provide the `error signal’ (Marr, 1969; Albus, 1971). In the simplest possible supervised learning model, a binary output neuron needs to learn to classify all possible input patterns in two classes: those for which it should be active, and those for which it should remain silent (see Fig. 4D). The role of synaptic plasticity is then to adjust synaptic weights until the neuron performs the right classification (see straight line separating the sets of points of different colors in Fig. 4D). In this simple model, the role of intrinsic plasticity would be limited to adjusting the neuronal threshold until the hyperplane separating the two sets of points is at the right distance from the origin, while synaptic plasticity would adjust the direction of this hyperplane. Note however that in this case again, learning can perfectly occur without intrinsic plasticity, since changes in neuronal threshold are equivalent to a uniform change in all synaptic strengths. As in the case of supervised learning, there are however cases where intrinsic plasticity could allow a neuron to learn a classification task that could not be done without such a mechanism. This would be the case when the neuron is no longer binary, but rather its desired outputs are continuous-valued firing rates. In this case, intrinsic plasticity could again be needed in order for the correct input-output classification to be performed. Another example of potential benefits of intrinsic plasticity is shown in Fig. 4E. In this case, the neuron has to learn to be active at specific points in time in an input temporal sequence. In the example shown in this figure, this particular learning problem cannot be solved by a standard perceptron. However, it could be solved by a bistable neuron. For such a neuron, a range of total inputs exists for which it is bistable, and its state for these inputs depends on the initial condition. The bistable zone is the hatched region in between the solid and dashed thick and straight lines in Fig. 4E. Clopath and Brunel (2013) showed that in the case of random correlated sequences, bistability is beneficial provided temporal correlations in the outputs are large enough, and that there exists an optimal size of the bistable range. This size could be adjusted using intrinsic plasticity mechanisms that would regulate intrinsic conductances leading to bistability. Another way to achieve learning of complex input/output relationships is to add neurons until a particular input/output relationship is reached. This class of learning algorithms is known in machine learning as constructive algorithms (see e.g. Mezard and Nadal, 1989). One might speculate that the addition of neurons into an active network could be mediated by intrinsic plasticity. Such mechanism would allow silent neurons to take part in a network whose goal is to solve a particular computational task.
Critical tests for a role of intrinsic plasticity in learning and memory
To establish a significant role of intrinsic plasticity in learning, it needs to be demonstrated that it occurs under physiological conditions relevant to learning, and it needs to be assessed whether intrinsic plasticity is necessary for memory engram storage. The ability of neurons to regulate their intrinsic excitability in an activity-dependent way has been demonstrated in multiple studies that we have discussed above. Most notably, intrinsic plasticity has been found in vivo in the neocortex (Paz et al., 2009; Mahon and Charpier, 2012) and in the cerebellum (Belmeguenai et al., 2010). In the latter study, intrinsic plasticity was observed in nonanesthetized, decerebrated adult rats after synaptic activation, demonstrating that intrinsic plasticity is not restricted to anesthesia conditions, and occurs in response to activation of synaptic inputs. In addition, recent work from Sheena Josselyn, Paul Frankland and colleagues shows that high intrinsic excitability predisposes neurons for allocation to a memory trace (Yiu et al., 2014). This study points toward a relationship between high excitability of a neuron and its assignment as an engram cell, but it remains unclear whether the engram allocation ultimately results from high intrinsic excitability per se or from an enhanced probability for LTP induction at activated synapses.
Up to this point, we have not addressed the important question whether intrinsic plasticity is necessary for learning. The appropriate approach to address the question of necessity is to prevent intrinsic plasticity using pharmacological or genetic approaches and to monitor the effects on learning. There are several studies in which this strategy has indeed been used. In trace eyeblink conditioning, hippocampal pyramidal neurons show a reduction in fast AHPs that is mediated by a downregulation of BK channels (Mathews et al., 2008). It was subsequently shown that BK channel blockade impairs trace eyeblink conditioning (Mathews and Disterhoft, 2009), suggesting that BK channel availability for plasticity-related modulation is a requirement for this type of associative learning. Similarly, pharmacological activation of SK channels – which mediate the medium AHP that is also downregulated in trace eyeblink conditioning (Moyer et al., 1996) – impairs object memory encoding (Vick et al., 2010) and trace eyeblink conditioning (McKay et al., 2012). These studies suggest that pharmacological interference– in ways that occlude or counteract the downregulation of the respective potassium channels – will prevent proper intrinsic plasticity and learning. Purkinje cell intrinsic plasticity depends on the activation of calcineurin (PP2B; Belmeguenai et al., 2010). In mice with a Purkinje cell-specific knockout of PP2B (L7-PP2B) motor learning is affected (Schonewille et al., 2010). However, LTP at PF synapses depends on calcineurin activation as well (Belmeguenai and Hansel, 2005). Thus, the study on learning deficits in L7-PP2B mice was useful to examine consequences of impairment of potentiation (rather than LTD) in cerebellar learning, but does not allow one to distinguish between the need for synaptic and intrinsic potentiation mechanisms, respectively. The experimental isolation of intrinsic plasticity-related deficits is difficult in general, because the pharmacological or genetic interference with intrinsic excitability will alter parameters relevant to synaptic plasticity and spike coding. For example, SK channel modulation / intrinsic plasticity changes spine calcium transients and the probability for LTP induction (Ngo-Anh et al., 2005; Belmeguenai et al., 2010). Future studies will have to attempt to separate the blockade of intrinsic plasticity from interference with LTP and LTD, for example through spatial specificity (e.g. blockade of mechanism underlying excitability changes only in the soma). It will be equally important to attempt to separate blockade of intrinsic plasticity from changes in basic excitability. To this end, upstream signaling factors need to be identified that are involved in intrinsic plasticity, but do not control excitability under baseline conditions and do not interfere with other forms of plasticity.
It can be concluded from these studies that multiple lines of evidence point toward a role of intrinsic plasticity in learning. The evidence showing that intrinsic plasticity is necessary for memory encoding, too, is somewhat weaker. This conclusion results from the observation that it has so far been impossible to fully isolate intrinsic from synaptic plasticity, i.e. to block intrinsic plasticity without interfering with synaptic plasticity as well. It is similarly difficult to block intrinsic plasticity without disturbing neuronal spike firing patterns. These separations will be required to convincingly demonstrate the necessity of intrinsic plasticity in memory encoding and thus should be a priority in future studies. Finally, it should be emphasized that even simple forms of learning are likely based on complex interactions between several types of plasticity, which makes it difficult to assign roles to any specific plasticity mechanism.
In addition to experiments addressing the necessity of intrinsic plasticity for learning, studies are needed that address the questions whether intrinsic plasticity is a long-term phenomenon, and under which conditions it is optimally triggered. We already know that intrinsic excitability changes accompany some forms of learning, and last at least 24hrs after training (Disterhoft et al., 1986; Moyer et al., 1996; Schreurs et al., 1998). It remains possible that excitability returns to naïve pre-training values soon after, which would indicate a more transient role in memory engram (re-) activation. Thus, it will be important to determine whether intrinsic plasticity mechanisms can last sufficiently long to play a role in the various stages of memory processing, including encoding, recall and consolidation. From a conceptual point of view, intrinsic plasticity can be involved in any of these stages to the degree that they require the integration of individual neurons into memory engrams. At any of these stages, the specificity in information processing rests on the connectivity patterns of synapses onto engram cells and between them. Further studies are needed to assess whether the specific roles of synaptic and intrinsic plasticity qualitatively differ between the different stages of memory processing. As at many types of synapses enhanced postsynaptic excitability will increase the probability for LTP induction, it also needs to be investigated how increases in excitability can be curtailed/reversed to avoid run-away activity and enable stabilization. Decreases in intrinsic excitability have been described in the cortex (Paz et al., 2009; Mahon and Charpier, 2012), which in this scenario would serve to homeostatically regulate excitability. Homeostatic plasticity has been described in detail, and can also occur in a cell-autonomous manner (Turrigiano, 2011). It needs to be determined, under which conditions increases and reductions of intrinsic excitability take place.
It will also be crucial to examine induction conditions for intrinsic plasticity, which will offer clues about sensory environments and input constellations that efficiently engage this plasticity mechanism. Induction of intrinsic plasticity will typically involve glutamatergic transmission (note that the term ‘intrinsic’ only refers to the expression, not the induction phase). Indirect evidence suggests that the attentional state of the animal is of importance, too. Layer 5 cortical pyramidal neurons show SK channel-dependent intrinsic plasticity (Sourdet et al., 2003; see also Belmeguenai et al., for similar results from Purkinje cell recordings). SK channels can be inhibited by muscarinic acetylcholine receptors (mAChRs; Buchanan et al., 2010; Giessel and Sabatini, 2010), suggesting that SK channel downregulation may be facilitated when neuronal activation coincides with cholinergic signaling. Thus, it is conceivable that cholinergic modulators – by promoting SK channel plasticity – may set the amplification scale of neurons, affecting their ability to generate spike output on both short and long time scales. This possible role of cholinergic signaling is exciting as it suggests an impact of the attentional state of the animal on memory engram formation and recall, but still awaits experimental verification.
The synapse versus the neuron as the critical site in memory engram storage
The current thinking about learning is dominated by a synaptocentric view, which describes the synapse as the site central to all processes involved in learning and memory. Here, we advocate for an alternative, neurocentric view of memory, which describes the entire neuron with its synaptic input portfolio as the central player in any memory engram (hence the term ‘engram cell’, see Tonegawa et al., 2015). The shift of focus towards entire cells ultimately includes cells with a modulatory function in plasticity, such as astrocytes, which also play a role in learning. This important aspect is beyond the scope of this paper, but excellent reviews on the topic are available (e.g. Singh and Abraham, in press). The neurocentric view of learning presented here is based on the argumentation that a memory engram is defined by the spike output of all participating neurons, a parameter that is efficiently controlled by intrinsic excitability. Activity-dependent intrinsic plasticity modulates excitability and has the capacity to mark engram cells for low-threshold reactivation and possibly burst firing, a feature that promotes memory engram storage and retrieval. Thus, intrinsic excitability can be seen as the ‘currency’ in engram storage, which regulates the synaptic penetrance factor of a particular neuron. A potentially important role in amplification was previously ascribed to intrinsic plasticity (Marder et al., 1996; Hansel et al., 2001; Zhang and Linden, 2003; Frick and Johnston, 2005; Debanne, 2009), but these previous proposals have not as radically abandoned the established view that LTP and LTD are ultimately the sole correlates of memory (‘synaptic memory’). Here, we present a different perspective, which looks at the immediate cellular consequences of synaptic and intrinsic plasticity mechanisms, stripped down from any traditional assumptions about their respective roles in learning. In this view, LTP and LTD change synaptic weights in an experience-dependent manner, and provide the processes needed to establish proper synaptic connectivity between neurons, similar to the synapse stabilization and pruning events that shape neural circuits during development (Piochon et al., 2016a). It remains undisputed that LTP has the potential to contribute to information storage by enhancing the probability that activation of the potentiated synapse(s) contributes to spike firing in the postsynaptic neuron. However, synaptic penetrance is a limiting factor and intrinsic plasticity provides a critical activity- and experience-dependent amplification mechanism that will boost (or negatively regulate) synaptic responses and will change the spike threshold (or the slope of the f-i curve) for all synapses contacting a neuron, or for a subset of these synapses. In the latter case, locally restricted intrinsic excitability changes may further amplify synaptic responses (Losonczy et al., 2008; Ohtsuki et al., 2012), which can be a powerful mechanism, particularly in combination with synapse clustering (Govindarajan et al., 2006; 2011; Kleindienst et al., 2011; Makino and Malinow, 2011; Takahashi et al., 2012). It has recently been demonstrated by one of us that in the cortex information storage is optimized when a large fraction of synapses are silent (Brunel, 2016; see also Brunel et al., 2004). In this scenario, synaptic response amplitudes need to be sufficiently close to spike threshold so that a critical number of coactive synaptic inputs leads to suprathreshold activity. Intrinsic plasticity might well play a role in fine-adjusting the spike threshold.
If LTP and LTD are primarily phenomena concerned with experience-dependent changes in network connectivity, it can be predicted that synaptic and intrinsic components of learning can be separated in time. Prior experience may lead to the formation of proper synaptic connectivity and input maps, and a new learning situation may – at a later time point – trigger intrinsic plasticity and memory engram formation without further changes in synaptic weight. In this functional distinction, LTP/LTD-mediated changes in synaptic connectivity and input maps are part of an active learning process as well, and will not be restricted to early development. For example, it is likely that synaptic re-wiring occurs during associative learning in the adult brain, which typically is a slow process that evolves over several consecutive days. The available data suggest that – in contrast – intrinsic plasticity is a faster phenomenon that may enable acute and, possibly, single-experience learning. This is a plausible scenario as the induction threshold for intrinsic plasticity is lower than that for synaptic plasticity (e.g. Purkinje cell intrinsic plasticity can be triggered within 3s of activation, which has not been shown for any form of synaptic plasticity; Belmeguenai et al., 2010; Ohtsuki et al., 2012; Lopez-Rojas et al., 2016). Thus, intrinsic plasticity may well occur isolated from synaptic plasticity, and may enable an ultra-fast change of intrinsic amplification values (and thus memory engram formation) underlying single-experience learning.
In the considerations detailed above, intrinsic plasticity is described as an independent and crucial mechanism in learning, but still operates by amplifying synaptic responses. On a final note, we would like to point out that changes in intrinsic excitability can also be part of the memory trace without any involvement of synapses (other than in the induction phase). We have recently shown that intrinsic plasticity can alter the duration of spike pauses in Purkinje cells, thus changing the spike burst - pause sequence that is characteristic for the output from cerebellar cortex (Grasselli et al., 2016; Fig. 3). This finding points to an additional aspect of intrinsic plasticity that might play an important role in types of neurons that show high spontaneous spike firing rates. Together, the findings reviewed here suggest that we need to expand the currently dominating, too narrowly focused view on synaptic learning mechanisms to fully appreciate the complexity of important cellular tools that the brain uses for memory storage.
Titley et al. argue that purely synaptic learning theories are not sufficient to explain memory representation in populations of neurons (memory engrams). Instead, engram integration results from intrinsic plasticity of neurons, whose connectivity maps were established by prior synaptic plasticity.
Acknowledgments
We thank David Freedman, Daniel Margoliash and members of the Hansel laboratory for comments. N.B. is supported by the National Science Foundation (NSF IIS-1430296). C.H. is supported by the National Institutes of Health (NS62771).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker CD, Hoyos E, Loew LM. EPSPs measured in proximal dendritic spines of cortical pyramidal neurons. eNeuro. 2016;3:e0050-15.2016. doi: 10.1523/ENEURO.0050-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat. Neurosci. 2000;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- Albus JS. A theory of cerebellar function. Math. Biosciences. 1971;10:25–61. [Google Scholar]
- Aou S, Woody CD, Birt D. Increases in excitability of neurons of the motor cortex of cats after rapid acquisition of eye blink conditioning. J. Neurosci. 1992;12:560–569. doi: 10.1523/JNEUROSCI.12-02-00560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armano S, Rossi P, Taglietti V, D’Angelo E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J. Neurosci. 2000;20:5208–5216. doi: 10.1523/JNEUROSCI.20-14-05208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J. Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, et al. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J. Neurosci. 2010;30:13630–13643. doi: 10.1523/JNEUROSCI.3226-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Giessel AJ, Sabatini BL. Biphasic synaptic calcium influx arising from compartmentalized electrical signals in dendritic spines. PLoS Biol. 2009;7:e1000190. doi: 10.1371/journal.pbio.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, et al. Small conductance Ca2+ -activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J. Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J. Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Roth A, Sakmann B. Dynamic receptive fields of reconstructed pyramidal cells in layers 3 and 2 of rat somatosensory barrel cortex. J. Physiol. 2003;5531:243–265. doi: 10.1113/jphysiol.2003.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Schneider M, Manns ID. Silent neurons in sensorimotor cortices: implications for cortical plasticity. In: Ebner F, editor. Neural Plasticity in Adult Somatic Sensory-Motor Systems. Boca Raton, FL: CRC Press; 2005. pp. 1–19. [Google Scholar]
- Brenner N, Bialek W, de Ruyter van Steveninck R. Adaptive rescaling maximizes information transmission. Neuron. 2000;26:695–702. doi: 10.1016/s0896-6273(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Brunel N, Hakim V, Isope P, Nadal JP, Barbour B. Optimal information storage and the distribution of synaptic weights: perceptron versus Purkinje cell. Neuron. 2004;43:745–757. doi: 10.1016/j.neuron.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Brunel N. Is cortical connectivity optimized for storing information? Nat. Neurosci. 2016;19:749–755. doi: 10.1038/nn.4286. [DOI] [PubMed] [Google Scholar]
- Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV, Mellor JR. Facilitation of long-term potentiation by muscarinic M(1) receptors is mediated by inhibition of SK channels. Neuron. 2010;68:948–963. doi: 10.1016/j.neuron.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai DJ, et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534:115–118. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carillo-Reid L, Yang W, Bando Y, Peterka DS, Yuste R. Imprinting and recalling cortical ensembles. Science. 2016;353:691–694. doi: 10.1126/science.aaf7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BC, Giessel AJ, Sabatini BL, Bean BP. Transient sodium current at subthreshold voltages: activation by EPSP waveforms. Neuron. 2012;75:1081–1093. doi: 10.1016/j.neuron.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C, Nadal JP, Brunel N. Storage of correlated patterns in standard and bistable Purkinje cell models. PLoS Comput Biol. 2012;8:e1002448. doi: 10.1371/journal.pcbi.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn. Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- Dayan P, Abbott L. Theoretical neuroscience: Computational and mathematical modeling of neural systems. MIT Press; [Google Scholar]
- Debanne D. Plasticity of neuronal excitability in vivo. J. Physiol. 2009;58713:3057–3058. doi: 10.1113/jphysiol.2009.175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. BDNF regulates the intrinsic excitability of cortical neurons. Learn. Mem. 1999;6:284–291. [PMC free article] [PubMed] [Google Scholar]
- Deuchars J, West DC, Thomson AM. Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. J. Physiol. 1994;4783:423–435. doi: 10.1113/jphysiol.1994.sp020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, Alkon DL. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr. Opin. Neurobiol. 2000;10:732–739. doi: 10.1016/s0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Fan Y, et al. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat. Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J. Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat. Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Balsam PD. Time to rethink the neural mechanism of learning and memory. Neurobiol. Learn. Mem. 2014;108:136–144. doi: 10.1016/j.nlm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, Matzel LD. The neuroscience of learning: beyond the Hebbian synapse. Annu. Rev. Psychol. 2013;64:169–200. doi: 10.1146/annurev-psych-113011-143807. [DOI] [PubMed] [Google Scholar]
- Giessel AJ, Sabatini BL. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron. 2010;68:963–947. doi: 10.1016/j.neuron.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat. Rev. Neurosci. 2006;7:575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Israely I, Huang SY, Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G, et al. Activity-dependent plasticity of spike pauses in cerebellar Purkinje cells. Cell Reports. 2016;14:2546–2553. doi: 10.1016/j.celrep.2016.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AR, Moore SJ, Spruston N, Tryba AK, Kaczorowski CC. Brain-derived neurotrophic factor differentially modulates excitability of two classes of hippocampal output neurons. J. Neurophysiol. 2016;116:466–471. doi: 10.1152/jn.00186.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Shu Y, Kuba H, Rasband MN, Wimmer VC, Bender KJ. Short- and long-term plasticity at the axon initial segment. J. Neurosci. 2011;31:16049–16055. doi: 10.1523/JNEUROSCI.4064-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, et al. Impaired conditioned fear and enhanced long-term potentiation in Fmr2 knockout mice. J. Neurosci. 2002;22:2753–2763. doi: 10.1523/JNEUROSCI.22-07-02753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman SJ, Schlögl A, Frotscher M, Jonas P. Synaptic mechanisms of pattern completion in the hippocampal CA3 network. Science. 2016;353:1117–1123. doi: 10.1126/science.aaf1836. [DOI] [PubMed] [Google Scholar]
- Hammond RS, et al. Small-conductance Ca2+ -activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J. Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- Harnett MT, Makara JK, Spruston N, Kath WL, Magee JC. Synaptic amplification by dendritic spines enhances input cooperativity. Nature. 2012;491:599–602. doi: 10.1038/nature11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Caroni P. Functional and structural underpinnings of neuronal assembly formation in learning. Nat. Neurosci. 2016;19:1553–1562. doi: 10.1038/nn.4418. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Kim JJ, DeCola JP, Landeira-Fernandez J, Fanselow MS. N-methyl-D-aspartate receptor antagonist APV blocks acquisition but not expression of fear conditioning. Behav. Neurosci. 1991;105:126–133. doi: 10.1037//0735-7044.105.1.126. [DOI] [PubMed] [Google Scholar]
- Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron. 2011;72:1012–1024. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Laughlin S. A simple coding procedure enhances a neuron’s information capacity. Z. Naturforsch. C. 1981;36:910–912. [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat. Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rojas J, Heine M, Kreutz MR. Plasticity of intrinsic excitability in mature granule cells of the dentate gyrus. Sci. Rep. 2016;6:21615. doi: 10.1038/srep21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Mahon S, Charpier S. Bidirectional plasticity of intrinsic excitability controls sensory inputs efficiency in layer 5 barrel cortex neurons in vivo. J. Neurosci. 2012;32:11377–11389. doi: 10.1523/JNEUROSCI.0415-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Malinow R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron. 2011;72:1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends in Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Manns ID, Sakmann B, Brecht M. Sub- and suprathreshold receptive field properties of pyramidal neurons in layers 5A and 5B of rat somatosensory barrel cortex. J. Physiol. 2004;5562:601–622. doi: 10.1113/jphysiol.2003.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marblestone AH, Wayne G, Kording KP. Toward an integration of deep learning and neuroscience. Frontiers Comp. Neurosci. 2016;10:94. doi: 10.3389/fncom.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Abbott LF, Turrigiano GG, Liu Z, Golowasch J. Memory from the dynamics of intrinsic membrane currents. Proc. Natl. Acad. Sci. USA. 1996;93:13481–13486. doi: 10.1073/pnas.93.24.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie TW, Brecht M, Sakmann B. In vivo, low-resistance, recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 2002;444:491–498. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J. Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A, Nicoll A, Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J. Neurosci. 1991;11:72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc. Natl. Acad. Sci. USA. 2008;39:15154–15159. doi: 10.1073/pnas.0805855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E, Disterhoft JF. Blocking the BK channel impedes acquisition of trace eyeblink conditioning. Learn. Mem. 2009;16:106–109. doi: 10.1101/lm.1289809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, Disterhoft JF. Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J. Neurophysiol. 2009;102:2763–2770. doi: 10.1152/jn.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, et al. Increasing SK2 channel activity impairs associative learning. J. Neurophysiol. 2012;108:863–870. doi: 10.1152/jn.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezard M, Nadal JP. Learning in feedforward layered networks: the tiling algorithm. J. Phys. A: Math. Gen. 1989;22:2191–2203. [Google Scholar]
- Miller S, et al. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J. Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, Byrne JH. More than synaptic plasticity: role of non-synaptic plasticity in learning and memory. Trends Neurosci. 33:17–26. doi: 10.1016/j.tins.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, et al. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46:623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, et al. SK channels and NMDA receptors form a Ca2+ -mediated feedback loop in dendritic spines. Nat. Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Nolan MF, et al. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115:551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- Ohtsuki G, Piochon C, Adelman JP, Hansel C. SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron. 2012;75:108–120. doi: 10.1016/j.neuron.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja E. Simplified neuron model as a principal component analyzer. J. Math. Biol. 1982;15:267–273. doi: 10.1007/BF00275687. [DOI] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Membrane potential changes in dendritic spines during action potentials and synaptic input. J. Neurosci. 2009;29:6897–6903. doi: 10.1523/JNEUROSCI.5847-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, et al. Multiple forms of activity-dependent intrinsic plasticity in layer V cortical neurons in vivo. J. Physiol. 2009;58713:3189–3205. doi: 10.1113/jphysiol.2009.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Kano M, Hansel C. LTD-like molecular pathways in developmental synaptic pruning. Nat. Neurosci. 2016a;19:1299–1310. doi: 10.1038/nn.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Titley HK, Simmons DH, Grasselli G, Elgersma Y, Hansel C. Calcium threshold shift enables frequency-independent control of plasticity by an instructive signal. Proc. Natl. Acad. Sci. USA. 2016b;113:13221–13226. doi: 10.1073/pnas.1613897113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Kandel ER. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Phil. Trans. R. Soc Lond. B. 2003;358:757–763. doi: 10.1098/rstb.2002.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM, et al. What is memory? The present state of the engram. BMC Biol. 2016;14:40. doi: 10.1186/s12915-016-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic MA, Carnevale N, Rozsa B, Zezevic D. Electrical behavior of dendritic spines as revealed by voltage imaging. Nat. Commun. 2015;6:8436. doi: 10.1038/ncomms9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron. 2005;45:133–145. doi: 10.1016/j.neuron.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R. Concept cells: the building blocks of declarative memory functions. Nat. Rev. Neurosci. 2012;13:587–597. doi: 10.1038/nrn3251. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Engram cells retain memory under retrograde amnesia. Science. 2015;348:1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Savin C, Joshi P, Triesch J. Independent component analysis in spiking neurons. PLOS Comp. Biol. 2010;6:e1000757. doi: 10.1371/journal.pcbi.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille M, et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and motor learning. Neuron. 2010;67:618–628. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J. Neurosci. 1998;18:5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon R. Die Mneme als erhaltendes Prinzip im Wechsel des organischen Geschehens. Leipzig: Wilhelm Engelmann; 1904. [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- Singh A, Abraham WC. Astrocytes and synaptic plasticity in health and disease. Exp. Brain Res. 2017 Mar 15; doi: 10.1007/s00221-017-4928-1. (in press) [DOI] [PubMed] [Google Scholar]
- Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J. Neurosci. 2003;23:10238–10248. doi: 10.1523/JNEUROSCI.23-32-10238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, et al. Small conductance Ca2+ -activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmler M, Koch C. How voltage-dependent conductances can adapt to maximize the information encoded by neuronal firing rate. Nat Neurosci. 1999;2:521–527. doi: 10.1038/9173. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kitamura K, Matsuo N, Mayford M, Kano M, Matsuki N, Ikegaya Y. Locally synchronized synaptic inputs. Science. 2012;335:353–356. doi: 10.1126/science.1210362. [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Pignatelli M, Roy DS, Ryan TJ. Memory engram storage and retrieval. Curr. Opin. Neurobiol. 2015;35:101–109. doi: 10.1016/j.conb.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Triesch J. Synergies between intrinsic and synaptic plasticity mechanisms. Neural Comp. 2007;19:885–909. doi: 10.1162/neco.2007.19.4.885. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Vick KA, Guidi M, Stackman RW. In vivo pharmacological manipulation of small conductance Ca2+-activated K+ channels influences motor behavior, object memory and fear conditioning. Neuropharmacol. 2010;58:650–659. doi: 10.1016/j.neuropharm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NL, et al. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature. 2012;492:247–251. doi: 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]
- Yiu AP, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat. Rev. Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]