SUMMARY
At least 30 types of retinal ganglion cell (RGC) send distinct messages through the optic nerve to the brain. Available strategies of promoting axon regeneration act on only some of these types. Here we tested the hypothesis that over-expressing developmentally important transcription factors in adult RGCs could reprogram them to a “youthful” growth-competent state and promote regeneration of other types. From a screen of transcription factors, we identified Sox11 as one that could induce substantial axon regeneration. Transcriptome profiling indicated that Sox11 activates genes involved in cytoskeletal remodeling and axon growth. Remarkably, alpha-RGCs, which preferentially regenerate following treatments such as PTEN deletion, were killed by Sox 11 overexpression. Thus, Sox 11 promotes regeneration of non-alpha RGCs, which are refractory to PTEN deletion-induced regeneration. We conclude that Sox11 can reprogram adult RGCs to a growth-competent state, suggesting that different growth-promoting interventions promote regeneration in distinct neuronal types.
INTRODUCTION
Neurons in the adult central nervous system (CNS) regenerate poorly after damage. Both extrinsic inhibitory factors and intrinsic constraints on growth in mature and injured neurons contribute to this regenerative failure. Recent studies have identified several methods that can activate growth-associated signaling pathways and promote neuronal survival and axon regeneration after injury (Bradke et al., 2012; He and Jin, 2016; Crair and Mason, 2016; Benowitz et al., 2017). However, the regeneration effects observed to date are still limited. For example, the activation of mTOR by deleting PTEN or over-expression of osteopontin and insulin-like growth factor (IGF1) promotes the selective regeneration of alpha-RGCs (Duan et al., 2015; Park et al., 2008), which comprise only ~6% of RGCs in intact retinas (Duan et al., 2015; Sanes and Masland, 2015). Thus, new strategies are needed to promote regeneration of other types of RGCs.
We reasoned that manipulation of transcription factors that act as master controlling factors of axon growth during development might represent another avenue for promoting axon regeneration in adults. In fact, previous studies have shown that manipulations of Krüppel-like family of transcription factors (KLFs) promoted axon regeneration (Moore et al., 2009). However, the observed regeneration after KLF manipulations are relatively modest. A possible clue is that KLF expression changes significantly during the early postnatal period, coincident with dramatically decreasing axon growth ability in the neonatal age (Goldberg et al., 2002). Thus, KLFs might maintain an axon growth program once it has been activated by other factors, rather than initiating such a program. Based on this reasoning, we focused here on transcription factors that regulate the early differentiation of RGCs, during which axon growth is initiated (Livesey and Cepko, 2001; Mu and Klein, 2004).
RESULTS
Identification of Sox11 as a regeneration-promoting transcription factor
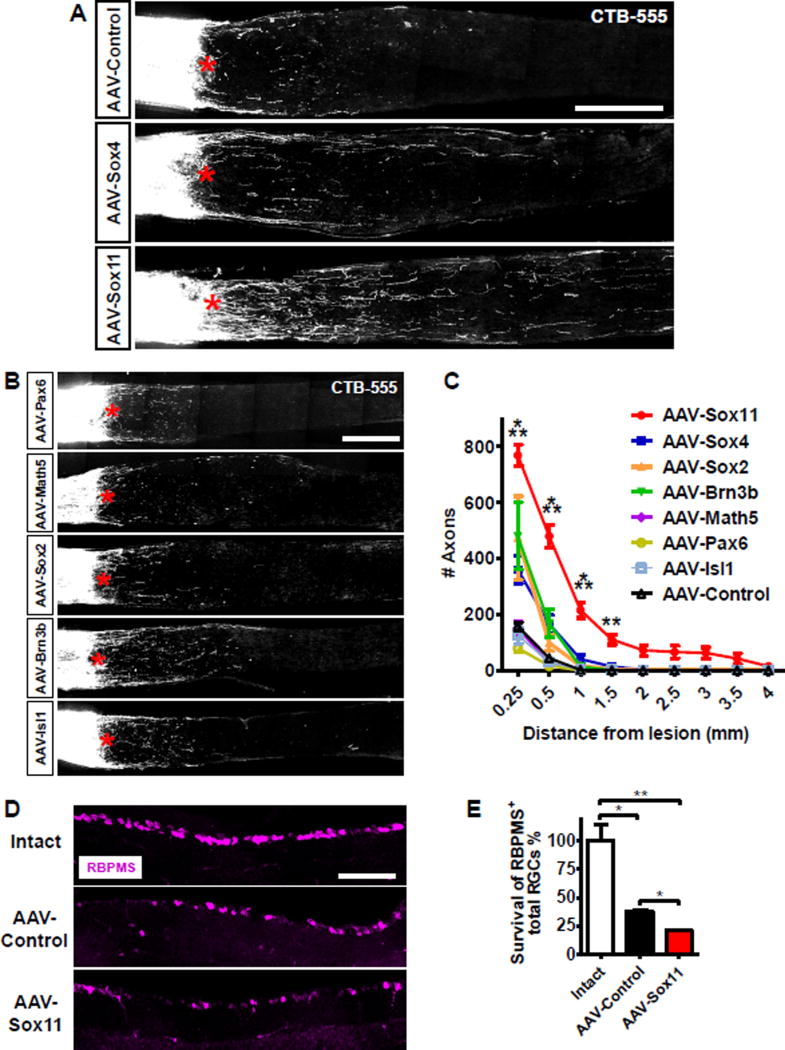
We tested 7 transcription factors for their ability to promote optic nerve regeneration. Genes were delivered by intravitreal injection of AAV serotype 2 vectors (AAV2) to mouse retinas; our previous work showed that this protocol leads to transduction of > 90% of RGCs (Park et al., 2008; Nawabi et al., 2015). As a control, we injected AAV-PLAP (placental alkaline phosphatase), an unrelated protein with no detectable effect on retinal structure or regeneration (Nawabi et al., 2015; Bei et al., 2016). Two weeks after virus injection, the optic nerve was crushed. The 2 weeks after injury, axon regeneration was monitored by an anterograde tracer fluorescent-conjugated cholera toxin subunit B (CTB).
Overexpression of Sox11 significantly increased regeneration of RGC axons (Figures 1A, 1C, S1A, S1B). Sox4, a Sox11 homolog, and Brn3b (Figure 1A–C) also increased axonal regeneration, but these effects did not reach statistical significance. The four other genes screened did not detectably increase regeneration (Figure 1A–C). Interestingly, although some of these factors such as Sox2 have been shown to be able to reprogram terminal differentiated fibroblasts into multipotent neuronal stem cells (Ring et al., 2012), their over-expression failed to promote axon regeneration after injury. We therefore focused on Sox11 in subsequent studies.
Figure 1. Over-expression of Sox11 promotes optic nerve regeneration.
(A, B) Representative images of optic nerve sections showing CTB-labeled axons in wild type mice with intravitreal injections of AAV expressing PLAP, Sox4, and Sox11 (A), and Pax6, Math5, Sox2, Brn3b, and Isl1 (B) at 2 weeks after optic nerve injury. The crush site is indicated with a red asterisk. Scale bars in (A) and (B) represent 250 µm.
(C) Quantification of regenerating axons from A and B. Data are expressed as mean ± SEM (n = 3–12). *** P < 0.001 (ANOVA with Bonferroni posttests; relative to AAV-Control).
(D) Representative retinal sections stained with anti-RBPMS antibodies from intact retina, or the retina at 2 weeks after injury with prior injection of AAVs expressing PLAP (Control) or Sox11. Scale bar represents 100 µm. (E) Quantification as in D. RBPMS-positive cells in the ganglion cell layer were imaged and quantified for two sections per retina, and normalized to length counted. Data are expressed as mean ± SD (n = 3–4). * P < 0.05, ** P < 0.01 (ANOVA with Bonferroni posttests).
In a separate experiment, we found that at 4 weeks after injury, Sox11-induced axons frequently extended more than 4 mm past the crush site at this time (Figure S1C–D). However, Sox11 expression failed to increase neuronal survival and instead modestly decreased RGC survival (Figure 1D and 1E), as revealed by immunostaining retinal sections with antibodies to the pan-RGC marker RBPMS (Rodriguez et al., 2014). Thus in contrast to other interventions such as the deletion of PTEN and/or SOCS3, which increase both neuronal survival and axon regeneration (Park et al. 2008; Sun et al. 2011), Sox11 over-expression promoted axon regeneration but not survival.
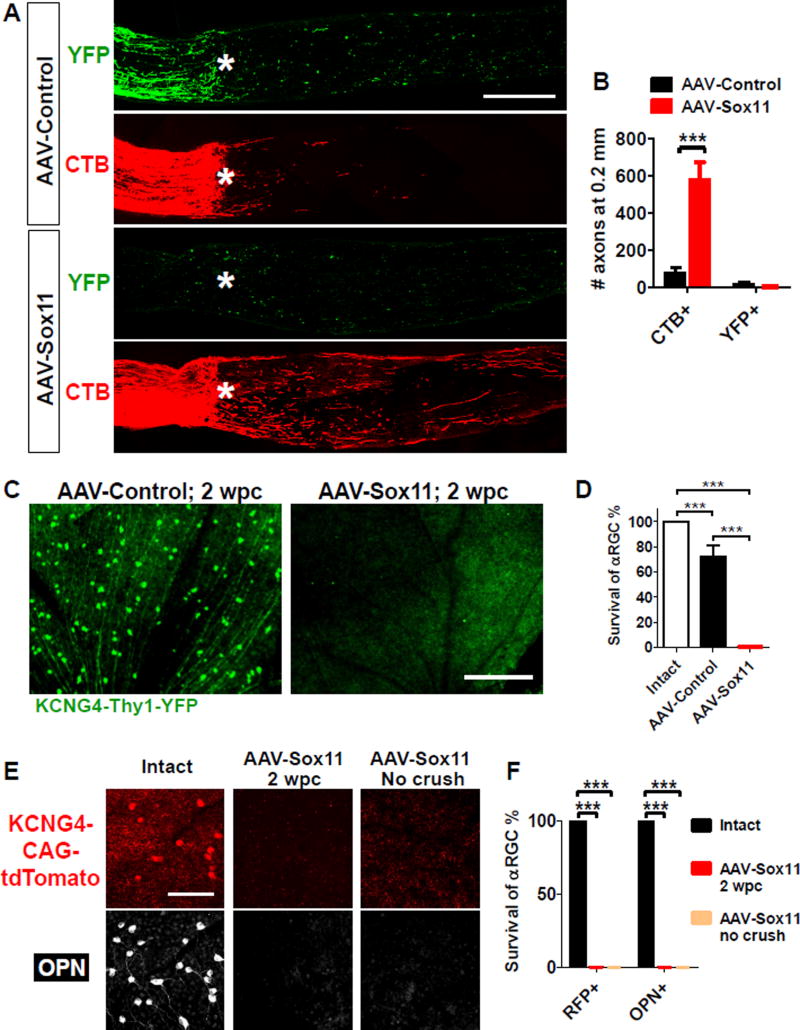
Sox11 kills alpha-RGCs and promotes axon regeneration from non-alpha-RGCs
Recent studies have shown that some interventions such as PTEN inhibition or co-expression of osteopontin and IGF1 selectively promote axon regeneration from alpha-RGCs (Duan et al. 2015). We asked whether the same was true for Sox11. We marked alpha RGCs with YFP using KCNG4-Cre mice crossed with a reporter line Thy1-fl-STOP-fl-YFP (Duan et al., 2015). We injected AAV-PLAP (Control) or AAV-Sox11 into the vitreous bodies of KCNG4-Cre; Thy1-fl-STOP-fl-YFP mice, crushed the optic nerve crush and labeled all regenerating axons with CTB as described above. Although many regenerating CTB+ axons were found in Sox11-treated eyes, none of these were YFP positive (Figure 2A, 2B). In addition, we found a total absence of YFP+ fibers proximal to the crush site whereas YFP+ fibers are evident in the control group (Figure 2A, 2B). Moreover, few YFP-positive RGCs were present in Sox11-overexpressing retinas at 2 weeks after injury (Figure 2C–D).
Figure 2. Sox11 ablates alpha-RGCs and promotes axon regeneration from non-alpha-RGCs.
(A–D) Axon regeneration (A, B) and survival of alpha-RGCs (C, D) in KCNG4-Cre mice crossed with Thy1-fl-STOP-fl-YFP at 2 weeks post crush (wpc). alpha-RGCs and their axons are labeled with YFP. Regenerating axons distal to the crush sites (asterisk) are anterogradely traced by CTB (red). Retina whole-mount staining confirms there is almost complete ablation of YFP-positive alpha RGCs in Sox11-treated retinas (C, D). Scale bars in A and C represent 300 µm.
(E, F) Survival of Sox11-treated alpha-RGCs labeled by either crossing KCNG4-Cre mice with Rosa26-CAG-STOP-tdTomato or staining with an antibody against osteopontin (OPN). 4 weeks after AAV-Sox11 injection, few alpha-RGCs were observed either with optic nerve crush (2 wpc) or without crush. Scale bar in E represents 100 µm. Data are expressed as mean ± SD (n = 3–5). *** P < 0.001 (ANOVA with Bonferroni posttests).
The apparent absence of YFP-labeled alpha-RGCs could be explained either by the down-regulation of their molecular markers (YFP expression from the Thy1 locus) or by cell death. To distinguish between these possibilities, we used two additional markers. First, we used another reporter, Rosa26-CAG-fl-STOP-fl-tdTomato, which expresses the fluorescent protein tdTomato under the control of a constitutive promoter (Madisen et al., 2010). Second, we immunostained retinas with an endogenous marker of alpha-RGCs, osteopontin (Duan et al., 2015). In AAV-PLAP treated eyes, we confirmed the presence of alpha-RGCs through tdTomato expression coincident with osteopontin. In contrast, AAV-Sox11 led to the elimination of tdTomato+ or osteopontin+ RGCs, both in intact retinas and following nerve crush (Figure 2E, 2F). Thus, Sox11 expression likely induces the death of alpha-RGCs. This result implies that axons regenerating after Sox11 over-expression must arise from non-alpha RGCs.
In addition, we tested the effect of Sox11 in transgenic mice overexpressing Bcl2, a well-established anti-apoptotic molecule that increases RGC survival after nerve crush (Bei et al., 2016; Bonfanti et al., 1996). As expected, AAV-Sox11 improved regeneration over AAV-Control in the Bcl2 background (Figure S2A). However, the extent of Sox11-induced regeneration in Bcl2-overexpressing mice was not appreciably different from that observed in wildtype animals (compare Figure S2A to Figure 1C). Further, even with Bcl2 co-treatment, Sox11 still failed to increase RGC survival (Figure S2B) and Bcl2 failed to rescue the Sox11-induced loss of Osteopontin+ alpha-RGCs (Figure S2C).
Intravitreally injected AAV2 vectors can transduce RGCs as well as other retinal cells (Park et al., 2008). To determine whether Sox11 acts directly on RGCs to promote regeneration, we injected AAV2-FLEX-Sox11 into the vitreous bodies of vGlut2-Cre mice, in which Cre is expressed in RGCs selectively (Ellis et al., 2016), and subjected these mice to an optic nerve injury. We found that AAV-FLEX-Sox11 induced regeneration was comparable to those induced by non-conditional AAV-Sox11 (Figure S2D, S2E, as compared with Figure 1A, 1C). Likewise, FLEX-Sox11 reduced pan-RGC survival essentially indistinguishable from non-specific AAV-Sox11 (Figure S2F). Thus, Sox11-induced axon regeneration is likely regulated in a cell autonomous fashion.
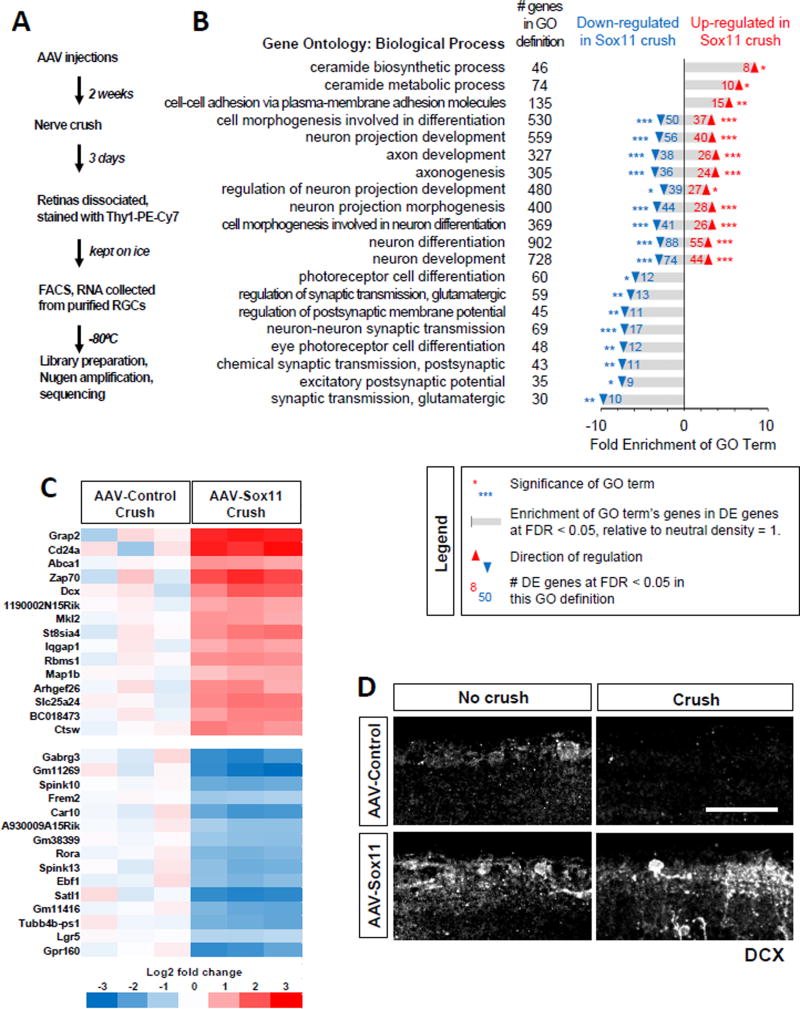
Developmental axon growth programs activated by Sox11 in adult RGCs
To gain mechanistic insights into the effects of Sox11, we performed gene expression profiling analysis in RGCs. To this end, we injected AAV-Sox11 or AAV-PLAP into the vitreous bodies of wild type mice, waited two weeks and performed optic nerve injury. Three days later, we dissociated RGCs, labeled them with antibodies against the RGC-selective marker Thy1, and purified them by FACS (Fig. 3A). By immunostaining, we verified the presence of alpha-RGCs in the samples prepared from both PLAP and Sox11 treated retinas at this time point (Figure S3A, S3B).
Figure 3. Sox11 overexpression induces changes in transcripts with roles in cell stress, axon growth, and other functions.
(A) Overview of RNA-sequencing and analysis.
(B) Partial listing of Gene Ontology (GO) Biological Process terms. GO terms were generated separately for genes up-regulated or down-regulated (thresholded at FDR < 0.05) by AAV-Sox11 relative to AAV-PLAP. After Bonferroni correction, 57 terms were significantly enriched in up-regulated transcripts, and 144 in down-regulated transcripts. This panel shows the 20 GO terms with the largest sum of Fold Enrichment from up- and down-lists, then sorts the terms by direction of enrichment. Numbers in black indicate the overall number of genes in the GO term definition; numbers in blue and red indicate the number of differentially expressed genes at FDR < 0.05 that are listed in the given GO term definition. Bars indicate the Fold Enrichment over the number of genes expected to appear if genes were randomly selected. Asterisks indicate significance of GO term enrichment: *, p < 0.05; **, p < 0.01; ***, p < 0.001. The full list of significant GO terms is provided in Supplemental Table S2, alongside the enrichment scores and p-values.
(C) Heatmap showing a selection of differentially expressed genes between AAV-PLAP and AAV-Sox11 after crush. The 15 most significant genes (by smallest FDR value) are given for genes that are either up-regulated (red) or down-regulated (blue) by AAV-Sox11 relative to control. Color values indicate log2 transforms of expression values as specified in the color key (bottom), where all values are relative to the average expression in the AAV-PLAP Crush group. The columns correspond to individual samples, with each treatment group performed in triplicate.
(D) Retinal sections were immunostained for Doublecortin/DCX in either intact or post-crush samples as indicated. Scale bar: 50 µm.
Messenger RNAs from the purified cells were then used for library preparation and sequencing (Figure 3A, Figure S3). From 26,691 annotated transcripts, we identified 2797 differentially expressed (DE) genes at the threshold of FDR < 0.1 (Figure S3 and Supplemental Table S1). Gene ontology (GO) analysis showed that two groups of similar GO terms account for a large fraction of the up-regulated transcripts (Figure 3B, Supplemental Table S2). The first group is related to the biosynthesis and metabolism of ceramide (Figure 3B). In light of evidence for a role of the ceramide-sphingolipid in cell death (Mencarelli and Martinez-Martinez, 2013), these results might point to a possible mechanistic link to Sox11-triggered death of alpha RGCs. The second group includes axon growth-related cellular functions, such as cell-cell adhesion, cell morphogenesis involved in differentiation, neuron process development and axonogenesis (Figure 3B), suggesting that Sox11 is able to re-activate axon growth programs. On the other hand, the majority of genes down-regulated by Sox11 were associated with synaptic transmission (Figure 3B). As previous studies showed that during development, axonal growth loss is associated with the switching from an axon growth mode in embryonic stages to the synapse growth mode in mature stages (Goldberg et al., 2002; Enes et al., 2010), these results support the model that Sox11 over-expression reprogram adult RGCs into an immature stage.
In further support of the notion that Sox11 activates an axon growth program, we found that among the most significantly altered genes (Figure 3C), several are cytoskeletal regulators: for example, doublecortin (DCX) (Gleeson et al., 1999; Schaar et al., 2004), microtubule-associated protein 1B (MAP1B (Hammarback et al., 1991; Opal et al., 2003), Ras GTPase-activating-like protein IQGAP1 (IQGAP1) (Hart et al., 1996), and Rho Guanine Nucleotide Exchange Factor 26 (ARHGEF26) (Samson et al., 2013). DCX was of particular interest because it is crucial for axon growth and neuronal migration during development (Gleeson et al., 1999; Schaar et al., 2004); it is expressed by newly differentiated neurons and then quickly down-regulated during neuronal maturation (Gleeson et al., 1999). Moreover, we showed recently that over-expression of DCX promotes optic nerve regeneration (Nawabi et al., 2015). We therefore used immunostaining with anti-DCX antibodies to validate the effect of Sox11 on DCX expression. As expected, DCX staining was barely detectable in RGC cell bodies from control uninjured eyes, but Sox11 overexpression increased DCX staining of RGCs in both intact retina and following optic nerve crush (Figure 3D). These results support the idea that Sox11 promotes axon regeneration by activating the axon growth program used during development.
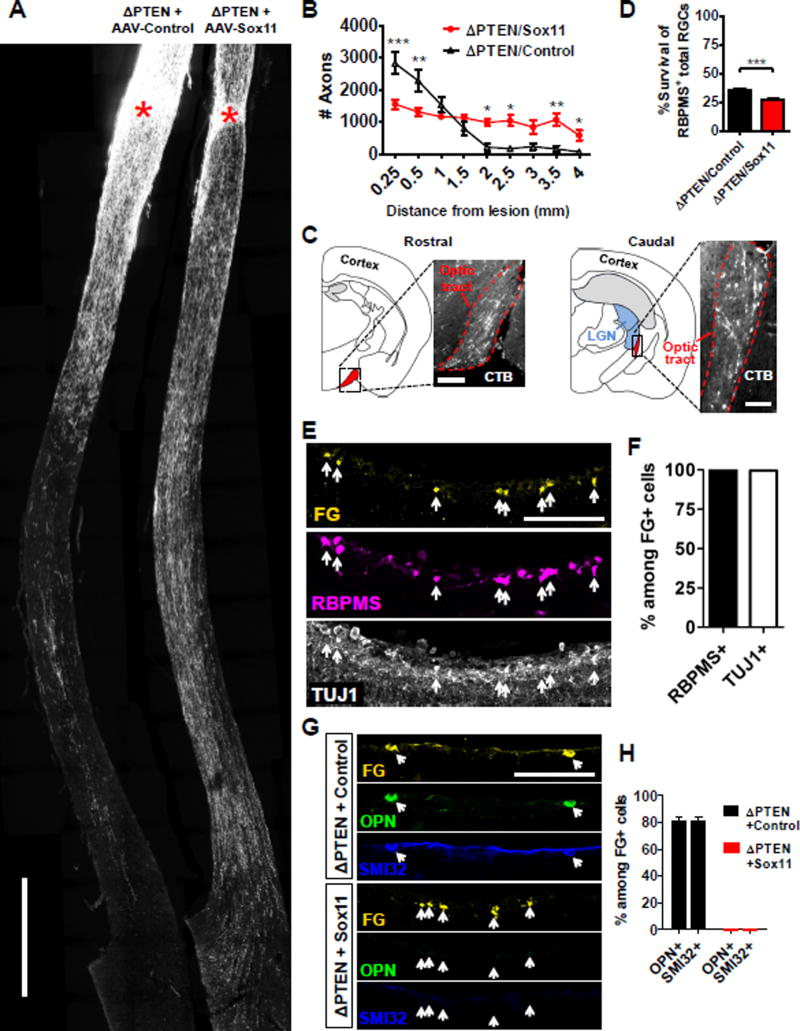
PTEN deletion further enhances the regeneration-promoting effects of Sox11 from non-alpha RGCs
Previous studies showed that PTEN dramatically enhances survival of RGCs, and selectively promotes regeneration of alpha RGCs (Park et al., 2008; Duan et al., 2015). In contrast, results present above show that Sox11 leads to death of alpha RGCs and promotes regeneration of non-alpha RGCs. These distinct effects led us to explore the functional interaction of Sox11 and PTEN deletion. In PTENf/f mice, we deleted PTEN using AAV-Cre mixed with either AAV-PLAP (hereafter “ΔPTEN/Control”), or AAV-Sox11 (hereafter “ΔPTEN/Sox11”). Optic nerves were injured two weeks later and axon regeneration was analyzed by CTB tracing 2 or 7 weeks after injury. In the ΔPTEN/Control group, many regenerating axons project for a relatively short distance (Figure 4A–B for 2 weeks and Figure S4A–B for 7 weeks). In the ΔPTEN/Sox11 group fewer axons regenerated but they grew for much longer distances than those in the ΔPTEN/Control group (Figure S4A, 4B), or in mice with Sox11 over-expression only (Figure 1). By 7 weeks after injury, many regenerating axons were observed in the optic tract after crossing the chiasm (Figure 4A–C); this length of regeneration was seldom observed in mice following either Sox11 expression or PTEN deletion alone. Notably, as in wild-type mice, Sox11 decreased the survival of RBPMS+ RGCs in PTEN-deleted mice (Figures 1 and 4D). The number of Sox11-expressing RGCs and the intensity of the expression were moderately increased in PTEN-deleted retinas as compared with PTEN-preserved ones, when all retinas were treated with AAV-Sox11.
Figure 4. Pro-regenerative effect of Sox11 in non-alpha-RGCs is enhanced by PTEN deletion.
(A) Representative images of optic nerves showing regenerating axons from different groups at 6–7 weeks after injury.
(B) Quantification of regenerating axons shown in (A).
(C) Images showing regenerating axons in ΔPTEN/Sox11-treated mice in the optic tract.
(D) RGC survival results at 6–7 weeks after injury.
(E) Retinal sections from ΔPTEN/Sox11-treated mice with FluoroGold (FG) injection to the distal optic nerve, and stained with RBPMS or TUJ1 antibodies.
(F) Quantification showing percentages of RBPMS- and TUJ1-co-stained cells among FG-positive cells shown in (E).
(G, H) Retinal images (G) and quantification (H) showing co-staining of FG-labeled RGCs with anti-OPN or SMI32. In ΔPTEN-treated animals, 80% of FG-labeled cells are both OPN-positive and SMI32-positive; however, none of the FG-labeled cells are co-stained with either OPN or SMI32 in ΔPTEN/Sox11-treated animals. Scale bar in (A) represents 500 µm. Scale bars in (C), (E) and (G) represent 100 µm. Data in (B) are expressed as mean ± SEM while data in (D), (F) and (H) are expressed as mean ± SD (n = 3–5). Significance levels in (B) are indicated by *, P < 0.05; **, P < 0.01; ***, P < 0.001 by ANOVA with Bonferroni posttests. Significance in (D) is indicated by ***, p < 0.001 by Welch’s t-test.
We next wanted to distinguish whether regeneration induced by ΔPTEN/Sox11 acted upon alpha- or non-alpha-RGCs, and whether Sox11-regulated mechanisms were conserved in ΔPTEN/Sox11 regenerating RGCs. Thus, we developed a protocol to retrogradely label RGCs with regenerating axons by injecting the tracer FluoroGold in the distal optic nerve and then analyzed FluoroGold-positive RGCs in the retinal sections. With this protocol, many RGCs were labeled in intact wild type mice but none of RGCs were labeled in the mice after injury (Figure S4C). In the ΔPTEN/Control group, FluoroGold-positive RGCs were observed across the retina sections. Consistent with previous results (Duan et al., 2015), the majority of these individually examined RGCs (over 200 cells) were alpha-RGCs as they were co-stained with the alpha-RGC marker OPN as well as a second alpha-RGC marker SMI32 (Figure 4G–H).
In the ΔPTEN/Sox11 group, many RGCs were also labeled with FluoroGold (Figure 4E). All these FluoroGold-positive RGCs expressed the RGC markers RBPMS and class III beta-tubulin or TUJ1 (Figure 4E–F), but none were from alpha-RGCs (Figure 4G–H). In fact, few OPN or SMI32-positive RGCs were observed in all ΔPTEN/Sox11-treated retinas, suggesting a near-total loss of alpha-RGCs (Figure S4F). Finally, as expected, many of these FluoroGold-labeled RGCs also expressed DCX (Figure S4D), a young-neuron marker up-regulated by Sox11 alone (Figure 3C and E) and phospho-S6 (Figure S4E), an mTOR-activity indicator up-regulated by PTEN deletion (Park et al., 2008). Together our results indicate that PTEN deletion could further enhance axonal regeneration from non-alpha-RGCs induced by Sox11 over-expression.
DISCUSSION
Repurposing developmental programs for regeneration
Multiple factors have been shown to be key in the differentiation of RGCs during development (Livesey and Cepko, 2001; Mu and Klein, 2004). However, it remains unclear which factors are most relevant to the axon growth program. Our results suggest Sox11 is one such critical factor. Both Sox11 and Sox4 are members of the Sry-related high mobility group (HMG) box (SOX) family of transcription factors which have been implicated as critical regulators of cell fate, differentiation, and survival during development (Sarkar and Hochedlinger, 2013; Chang et al., 2017; Kuwajima et al., 2017). Sox11 promoted strong and significant axonal regeneration, and Sox4 likely promoted regeneration albeit with marginal significance. These results are consistent with the notion that Sox4 and Sox11 have redundant biological effects (Jiang et al., 2013). Previous studies have also implicated Sox11 in promoting regeneration of PNS (Jing et al., 2012; Jankowski et al., 2009; Chandran et al., 2016) and corticospinal axons (Wang et al., 2015). Gene profiling showed Sox11 activates a set of developmental axon growth-related genes in RGCs, consistent with the possibility that it is a master regulator of the axonal growth program. Sox11 also down-regulates genes involved in synaptic transmission and related functions, consistent with previous observations on the development-dependent switching from axon growth mode immature neurons to dendrite/synapse growth mode in mature neurons (Goldberg et al., 2002; Enes et al., 2010). However, it remains to be determined whether the inhibition of dendrite/synapse function is required for Sox11-mediated activation of axon growth program.
Along with its beneficial effect on promoting axon regeneration, Sox11 expression had the distressing effect of leading to the death of nearly all alpha-RGCs. In this regard, our gene profiling studies indeed revealed that Sox11 over-expression could up-regulate the biosynthesis and metabolism of ceramide, a critical regulator of neuronal stress response and cell death (Mencarelli and Martinez-Martinez, 2013). In addition, Welsbie et al show that Sox11 is involved in DLK/MAPK-mediated RGC death (accompanying manuscript). Furthermore, a previous study showed that despite promoting some CST regrowth, Sox11 over-expression resulted in worsening of motor performance outcomes (Wang et al., 2015; Jayaprakash et al., 2016); we speculate that Sox11-mediated cell death might have contributed to such behavioral impairments. On the other hand, it has been shown that, during development, Sox11 and Sox4 are critical for preventing the death of RGC (Jiang et al., 2013), sympathetic (Potzner et al., 2010) and DRG neurons (Lin et al., 2011). How Sox11 shows such differential survival effects on different neuronal types remains unclear. Any attempts to use Sox11 or other reprogramming methods as neural repair strategies (Li and Chen, 2016) will need to take account of these double-edged effects.
Neuronal subtype-specific control of axon regeneration
Our recent studies showed that PTEN deletion or overexpression of osteopontin and IGF1 selectively promotes the axon regeneration from alpha-RGCs (Duan et al., 2015). In view of this result, it was surprising that Sox11 expression both promoted axon regeneration and killed virtually all alpha-RGCs. The implication, which we supported by retrograde labeling from regenerating axons, is that Sox11 promotes regeneration of RGC types that are refractory to the effects of mTOR activation. Moreover, even in combination with mTOR activation (PTEN deletion), Sox11 kills alpha RGCs and promotes regeneration from non-alpha-RGCs. There are at least 30 RGC types in mouse, each with distinct morphological, molecular and functional properties (Sanes and Masland, 2015; Baden et al., 2016). It will be challenging to identify which RGC types are affected by Sox11, but the availability of increasing numbers of markers and transgenic lines (Sanes and Masland, 2015; Dhande and Huberman, 2014) provides a means of addressing this issue.
These results provide new evidence for the idea that distinct neuron types differ in their regenerative responses to certain manipulations (He and Jin, 2016). The presence of resilient and susceptible populations of RGCs is particularly intriguing in this regard, because most RGCs are similar in many respects: their somata are in the ganglion cell layer, their dendrites arborize in the inner plexiform layer, their axons run through the optic nerve to the brain, they are glutamatergic, and they express markers such as RBPMS, Thy1, and Brn3 class transcription factors. These similarities, then, make the remaining differences between RGC types all the more compelling. By comparing RGC types before and after injury, and following interventions such as Sox11 or PTEN deletion it should be possible to identify the critical factors required for survival or regeneration following injury.
STAR*METHODS
Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCE TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
-
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse lines
Mouse husbandry
-
METHOD DETAILS
-
In vivo procedures and reagents
Production of AAVs
Intravitreal injection and optic nerve crush
Retrograde tracing of RGCs
-
Histology
Tissue Preparation
Staining conditions
Antibodies
Microscopy
-
Experimental design
Inclusion and exclusion criteria
Randomization and efforts to minimize systematic errors
Blinding
-
RNA-Seq Preparation
RGC enrichment and RNA isolation
Sequencing reactions
-
-
QUANTIFICATION AND STATIDTICAL ANALYSIS
Quantifications involving regenerating axons
Quantifications involving RGC cell bodies
Statistical testing of axon or RGC cell body quantification
RNA-sequencing analysis
DATA and SOFTWARE AVAILABILITY
STAR*METHODS
CONTACT FOR REAGENTS AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to, and will be fulfilled by, the Lead Contact, Zhigang He (Zhigang.He@childrens.harvard.edu).
EXPERIMENTAL MODELS
Mouse lines
Mouse lines including KCNG4-Cre and Thy1-fl-STOP-fl-YFP were generated and characterized in our laboratories (Duan et al., 2015). KCNG4-Cre was crossed with Thy1-fl-STOP-fl-YFP or Rosa26-CAG-fl-STOP-fl-tdTomato (Jackson Laboratory) to label alpha RGCs. Wild-type mice were purchased from Charles River Laboratories. Bcl2 mouse line was described previously (Bei et al., 2016). Vglut2-Cre mice were obtained from Jackson Laboratory (016963). In experiments where a transgenic mouse line was not specified, mixed-background wildtypes were used.
Mouse husbandry
All experiments were performed in compliance with protocols approved by the IACUC at Boston Children’s Hospital. Mice were given ad libitum access to food and water, and housed in cages under positive-pressure filtered air supplies with bedding changed frequently. Mice were not permitted to breed before or during their inclusion in in vivo experiments.
METHOD DETAILS
In vivo procedures and reagents
Production of AAVs
Vectors of AAV-Pax6 and AAV-Isl1 vectors were made by inserting mouse Pax6 cDNA (Addgene #32932) and mouse Isl1 cDNA (Addgene #32929) respectively into an AAV plasmid consisting of the cytomegalovirus enhancer fused to the chicken beta-actin promoter (CAG promoter) and Woodchuck hepatitis virus Posttranscriptional Regulatory Element (WPRE). The other vectors were assembled similarly, with coding sequences either cloned in-house from cDNAs or directly chemically synthesized. All pAAVs, as listed in the Key Resources Table, were then packaged into AAVs of serotype 2/2 (titers: > 5 × 1012 genome copies per milliliter) by the Boston Children’s Hospital Viral Core.
Intravitreal injection and optic nerve crush
For intravitreal injection, adult animals were anesthetized with ketamine/xylazine (100/10 mg/kg) and then AAV (1–3 µl) or Alex-conjugated cholera toxin beta subunit (CTB-555, 1 mg/ml; 1–2 µl) was injected intravitreally with a fine glass pipette attached to the Hamilton syringe using plastic tubing. CTB-555 injection was performed 2–3 days before euthanasia to trace regenerating RGC axons. For optic nerve crush in anesthetized animals, the optic nerve was accessed intraorbitally and crushed using a pair of Dumont #5 forceps (FST), two weeks after AAV injection. More detailed surgical methods were described by Park et al. (2008) and Bei et al. (2016).
Retrograde tracing of RGCs
Two weeks after optic nerve crush, a cranial opening was created over the frontal cortex and a distal segment of the crushed optic nerve was exposed intracranially through removing overlying brain tissues. 4% FluoroGold (Fluorochrome) was slowly injected into the optic nerve approximately 1–1.5 mm distal to the crush site using a fine glass pipette. Care was taken to minimize passive diffusion of FluoroGold solution back toward the crush site including removing overflowing FluoroGold solution.
Histology
Tissue preparation
Anesthetized animals were transcardially perfused with 4% paraformaldehyde (PFA). Dissected optic nerves, brains, and eyeballs were post-fixed in 4% PFA overnight, and then immersed in sucrose solutions at least two days before embedding and snap-freezing in OCT. Sucrose solutions contained 15% sucrose in PBS for optic nerves, or 30% sucrose in PBS for eyes and brains. Typically, 10–14 µm thick sections were cut for optic nerves, 16–20 µm thick sections for retinas and 25 µm thick sections for mouse brains. Sections were adhered to room-temperature charged microscope slides, dried, and frozen until further processing. Slides were then either washed and mounted for imaging with an anti-fade reagent, for example, for some CTB-traced optic nerves, or further processed for immunohistochemistry. Some retinas were dissected out in toto after post-fixing in PFA, washed with PBS, immunostained, cut radially with scissors to flatten the tissue, and then mounted for imaging.
Staining conditions
For immunohistochemistry, whole mount retinas were generally blocked for one hour in PBS with 5% donkey serum and 0.3% Triton X-100, and then incubated 0.5–2 days at 4 °C in primary antibodies diluted in PBS with 3% donkey serum and 0.3% Triton X-100, followed by treatment of secondary antibodies (Jackson ImmunoResearch or Invitrogen) for 1–2 hours at room temperature after rinsing. Immunohistochemistry on sections was performed generally by blocking with 3% bovine serum albumin and 0.5% Triton X-100 in PBS, incubation with primary antibodies overnight at 4 °C in blocking solution, then incubation with secondary antibodies for 2 hours at room temperature.
Antibodies
Primary antibodies used were: Goat anti-DCX (1:400, Santa Cruz sc8066); chicken anti-GFP (1:1000, Abcam ab13970); rabbit anti-RFP (1:500, Abcam ab34771); goat anti-Osteopontin (1:1000, R&D Systems AF808); rabbit anti-phosphorylated S6 Ser235/236 (1:200, Cell Signaling 4857); rabbit anti-RBPMS (1:500, Abcam ab194213); guinea pig anti-RBPMS (P4-P24) (1:2000, Raygene custom order A008712 to peptide GGKAEKENTPSEANLQEEEVRC); mouse anti-SMI32 (1:1000, BioLegend/Covance SMI-32R); goat anti-Sox11 C-20 (1:200, Santa Cruz sc17347); mouse anti-TUJ1 (1:400, BioLegend 801202); rabbit anti-Tubb3 (1:400, Abcam ab18207). Fluorescent secondary antibodies used were generally from either Invitrogen/Thermo-Fisher Scientific or Jackson ImmunoResearch, raised in either goat or donkey against the primary antibody’s host species, and conjugated to fluorophores of DyLight 405; Alexa Fluor 488; Cy3; or Alexa Fluor 647 as appropriate, and generally used at 1:800 final dilution.
Microscopy
For nerve sections and some whole-mount retinas, individual fluorescent images were acquired using Ultraview Vox Spinning Disk Confocal Microscope (Perkin Elmer) and automatically stitched using Volocity software (Perkin Elmer). For some retina sections, images were taken using epi-fluorescent microscopy with a Nikon TiE Eclipse epifluorescent microscope with automated tiling or Zeiss LSM 700 fluorescent confocal microscopy. Z stacks were projected onto a single plane using either Volocity or ImageJ. Brightness and contrast of the images were adjusted and pseudo-colors applied for presentation. When imaging was taken for quantification, image capture and processing were kept constant.
Experimental design
Inclusion and exclusion criteria
The vast majority of samples in all experiments were included in their respective final datasets. However, occasionally samples were rejected as follows: (1) At the time of sacrifice and dissection, eyes were rejected if lens injury or hardening of the vitreous humor was apparent. This generally resulted in rejection of zero to one retinas and/or optic nerves per experiment. (2) During sample preparation, a minority of samples were damaged from human error, such as cryosectioning severely orthogonal to the intended plane of sectioning, and excluded from further analysis. (3) Microscopy images of retinal or optic nerve sections were excluded if sectioning was orthogonal, similar to criteria 2 above. (4) Once experiments entered statistical analysis, no further samples were excluded by any formal or informal criterion.
Randomization and efforts to minimize systematic errors
Experiments involving mice included both adult males and females, generally between the ages of 6 to 10 weeks, and control and treatment groups were always balanced for female:male ratios, ages, different litters, and/or background as applicable.
Experimental design for histological samples involving surgeries, i.e. injections and nerve crushes, were always carefully balanced for possible unilateral-specific artifacts caused by the surgeon. More specifically, experiments always used one of two strategies: (1) using only one retina and nerve (e.g. the right eye) per mouse, for all treatment groups, or (2) using an equal 50:50 balance of left and right eyes for both treatment groups.
Blinding
Generally, phenotypes were sufficiently obvious and reproducible in the hands of multiple authors that blinding was judged unnecessary for analysis. However, during the initial screens that identified Sox11, blinding was used for our first characterizations of pan-RGC survival. Additionally, for some histological analysis of retinas involving PTEN/Sox11, the results from non-blinded quantifications were repeated by a second investigator who was blinded. In such cases, the second investigator’s conclusions would be considered final.
RNA-seq preparation
RGC enrichment and RNA isolation
Retinas were dissected, dissociated in serum-free media using papain, triturated carefully, then stained with Thy1.2-PE-Cy7 antibody (Affymetrix eBioscience 25–0902-82) and SYTOX live-dead cell stain, then flow sorted on a BD FACS Aria II using an 85 micron nozzle to a goal of approximately 100,000 events. Using the Arcturus PicoPure kit, RGCs identified by FACS were sorted directly into extraction buffer XB and lysed immediately after sorting according to the kit’s instructions. RNA quality was verified with an Agilent BioAnalyzer 2100. All experimental steps through RNA extraction were performed in triplicate for the control and experimental group, with each replicate performed a different day. The samples within a replicate were prepared on the same day, in a different order each replicate, to avoid any systemic errors from differences in timing.
Sequencing reactions
RNA-sequencing was carried out for the RNAs with by the UCLA Neuroscience Genomics Core. Briefly, cDNA was generated using the Ovation RNA-Seq System V2 kit (NuGEN), followed by library preparation using the TruSeq Nano DNA Library Prep kit (Illumina). Samples were sequenced using an Illumina HiSeq-4000 sequencer with 69-base paired end reads resulting in a minimum of 50M reads per sample.
Quantification and Statistical Analysis
Quantifications involving regenerating axons
For quantifying regeneration of axons traced with fluorescent CTB, we took longitudinal sections of optic nerves and counted the total number of CTB+ axons at multiple distances along the optic nerve, anterograde from the crush site. The counts were transformed to a density of axons, then multiplied by the nerve’s approximate cross-sectional area to estimate the total number of axons in each respective nerve. More specifically, we used four sections from each nerve to estimate that nerve’s axon count, and took each estimate as a single biological sample for subsequent statistical testing. For quantification of fluorescent protein co-localization with regenerating axons in optic nerves, axons were counted with their number estimated according to a method described previously (Bei et al. 2016). In analyzing YFP-positive axons, care was taken to exclude fluorescent signals from tissue debris. We confirmed that axon regeneration counts, at several distances from the crush site, followed an approximately normal distribution by the Kolmogorov-Smirnov test (P > 0.1).
Quantification involving RGC cell bodies
For quantification of RGCs in sections, generally 2 sections were quantified per retina for a total quantified ganglion cell layer length of 3 to 4 mm per section. In intact control retinas, generally 300 to 500 RBPMS+ cells were counted per section. The cell count was normalized to the length of GCL counted (measured in ImageJ for every section), and one value was generated per retina for subsequent statistical analysis. For quantification of RGCs in whole mounts, each retina was sampled with generally six to eight fields of view focused on the ganglion cell layer (each ~0.4mm × ~0.4mm). Densities were calculated for each field of view, then averaged to generate one value for each retina for subsequent statistical analysis. We confirmed that RGC survival counts, in our experimental layout, followed an approximately normal distribution by the Kolmogorov-Smirnov test (P > 0.1).
Statistical testing of axon or RGC cell body quantifications
All statistical tests were two-tailed, and the sample size n was defined as the number of individual eyes, retinas, nerves, or mice as appropriate in the experiment. For such quantifications, generally a t-test or ANOVA with post-testing was used for analysis, as detailed in the corresponding figure legends. Asterisks, e.g. *, **, *** indicate significance levels as specified in the corresponding figure legends. Where applicable, graphs indicate the SEM with error bars. Analyses were performed in either PRISM or Excel, and checked against raw data by more than one author.
RNA-sequencing Analysis
Reads were aligned to the latest mouse mm10 reference genome using the STAR (ver 2.4.0) spliced read aligner (Dobin et al., 2013). Read counts for Ensembl genes were generated by HT-seq 0.6.1 (Anders et al., 2015). Transcripts with at least 10 read events in any sample were permitted into the dataset, for a total of 26,691 transcripts. Raw counts were normalized by upper quartile normalization followed by removal of unwanted variation using RUVSeq (Risso et al., 2014). Differentially expressed (DE) genes were obtained using the EdgeR bioconductor R package (Robinson et al., 2009). GO analysis was performed separately for up- and down-regulated gene lists using the Gene Ontology Reference Genome Project’s PANTHER Classification System (pantherdb.org) against the GO biological process complete annotation data set, with Bonferroni correction.
DATA AND SOFTWARE AVAILABILITY
Raw and processed RNA-seq data are deposited to Gene Expression Omnibus (GEO submission GSE87046).
Supplementary Material
Acknowledgments
We thank Drs. Paola Arlotta, Connie Cepko, Thomas Scammell, Clifford Woolf, Y. Peng for advice. This study was supported by NIH fellowship 5T32HL007901-18 (to M.N.), and NIH grant EY026939 (Z.H.), grants from Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (G.C., Z.H.). We thank IDDRC and viral cores supported by the NIH grants P30 HD018655 and P30EY012196 and the UCLA Neuroscience Genomics Core (www.semel.ucla.edu/ungc) supported by P30 NS062691.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information includes four figures and two tables (Table 1: Significant differentially expressed genes, related to Figure 3; Table 2: Gene Ontology list of terms enriched or depleted by AAV-Sox11 versus AAV-control in RGCs, related to Figure 3).
AUTHOR CONTRIBUTIONS
M.N., F.B., J.S., G.C., and Z.H. designed experiments. M.N., F.B., R.K., Q.W., B.B. performed the experiments and analyzed the data. C.W. performed optic nerve crush and AAV injections. M.N., F.B. and Z.H wrote the paper with the inputs from all authors.
References
- Bei F, Lee HHC, Liu X, Gunner G, Jin H, Ma L, Wang C, Hou L, Hensch TK, Frank E, Sanes JR, Chen C, Fagiolini M, He Z. Restoration of Visual Function by Enhancing Conduction in Regenerated Axons. Cell. 2016;164:219–232. doi: 10.1016/j.cell.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz L, He Z, Goldberg JL. Reaching the brain: Advances in optic nerve regeneration. Exp Neurol. 2017;287:365–373. doi: 10.1016/j.expneurol.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou J-C, Maffei L, Rabacchi SA. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci. 1996;16(13):4186–4194. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat. Rev. Neurosci. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Chang KC, Hertz J, Zhang X, Jin XL, Shaw P, Derosa BA, Li JY, Venugopalan P, Valenzuela DA, Patel RD, Russano KR, Alshamekh SA, Sun C, Tenerelli K, Li C, Velmeshev D, Cheng Y, Boyce TM, Dreyfuss A, Uddin MS, Muller KJ, Dykxhoorn DM, Goldberg JL. Novel Regulatory Mechanisms for the SoxC Transcriptional Network Required for Visual Pathway Development. J Neurosci. 2017;37:4967–4981. doi: 10.1523/JNEUROSCI.3430-13.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M, Yekkirala A, Barrett L, Blesch A, Michaelevski I, Davis-Turak J, Gao F, Langfelder P, Horvath S, He Z, Benowitz L, Fainzilber M, Tuszynski M, Woolf CJ, Geschwind DH. A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron. 2016;89:956–970. doi: 10.1016/j.neuron.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Mason CA. Reconnecting Eye to Brain. J Neurosci. 2016;36:10707–10722. doi: 10.1523/JNEUROSCI.1711-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Huberman AD. Retinal ganglion cell maps in the brain: implications for visual processing. Curr Opin Neurobiol. 2014;24:133–142. doi: 10.1016/j.conb.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Qiao M, Bei F, Kim I-J, He Z, Sanes JR. Subtype-Specific Regeneration of Retinal Ganglion Cells following Axotomy: Effects of Osteopontin and mTOR Signaling. Neuron. 2015:1–13. doi: 10.1016/j.neuron.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EM, Gauvain G, Sivyer B, Murphy GJ. Shared and distinct retinal input to the mouse superior colliculous and dorsal lateral geniculate nucleus. J. Neurophysiol. 2016;116:602–610. doi: 10.1152/jn.00227.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enes J, Langwieser N, Ruschel J, Carballosa-Gonzalez MM, Klug A, Traut MH, Ylera B, Tahirovic Sm, Hofmann F, Stein V, Moosmang S, Hentall ID, Bradke F. Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr Biol. 2010;20:1154–1164. doi: 10.1016/j.cub.2010.05.055. [DOI] [PubMed] [Google Scholar]
- Gleeson J, Lin P, Flanagan L, Walsh C. Doublecortin Is a Microtubule-Associated Protein and Is Expressed Widely by Migrating Neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- Hammarback Ja, Obar Ra, Hughes SM, Vallee RB. MAP1B is encoded as a polyprotein that is processed to form a complex N-terminal microtubule-binding domain. Neuron. 1991;7:129–139. doi: 10.1016/0896-6273(91)90081-a. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- He Z, Jin Y. Intrinsic Control of Axon Regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ding Q, Xie X, Libby RT, Lefebvre V, Gan L. Transcription factors SOX4 and SOX11 function redundantly to regulate the development of mouse retinal ganglion cells. J. Biol. Chem. 2013;288:18429–38. doi: 10.1074/jbc.M113.478503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X, Wang T, Huang S, Glorioso JC, Albers KM. The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprr1a. Exp. Neurol. 2012;233:221–32. doi: 10.1016/j.expneurol.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima T, Soares CA, Sitko AA, Lefebvre V, Mason C. SoxC Transcription Factors Promote Contralateral Retinal Ganglion Cell Differentiation and Axon Guidance in the Mouse Visual System. Neuron. 2017;93:1110–1125. doi: 10.1016/j.neuron.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Lee VM, Wang Y, Lin JS, Sock E, Wegner M, Lei L. Sox11 regulates survival and axonal growth of embryonic sensory neurons. Dev. Dyn. 2011;240:52–64. doi: 10.1002/dvdy.22489. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2001;2:109–18. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Hatim A, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Allan R, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli C, Martinez-Martinez P. Ceramide function in the brain: When a slight tilt is enough. Cell. Mol. Life Sci. 2013;70:181–203. doi: 10.1007/s00018-012-1038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, John L, Lemmon VP, Goldberg JL. KLF Family Members Regulate Intrinsic Axon Regeneration Ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Klein WH. A gene regulatory hierarchy for retinal ganglion cell specification and differentiation. Semin. Cell Dev. Biol. 2004;15:115–23. doi: 10.1016/j.semcdb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Nawabi H, Belin S, Cartoni R, Williams PR, Wang C, Latremolière A, Wang X, Zhu J, Taub DG, Fu X, Yu B, Gu X, Woolf CJ, Liu JS, Gabel CV, Steen JA, He Z. Doublecortin-Like Kinases Promote Neuronal Survival and Induce Growth Cone Reformation via Distinct Mechanisms. Neuron. 2015;88:704–719. doi: 10.1016/j.neuron.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal P, Garcia JJ, Propst F, Matilla A, Orr HT, Zoghbi HY. Mapmodulin/Leucine-rich Acidic Nuclear Protein Binds the Light Chain of Microtubule-associated Protein 1B and Modulates Neuritogenesis. J. Biol. Chem. 2003;278:34691–34699. doi: 10.1074/jbc.M302785200. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–6. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potzner MR, Tsarovina K, Binder E, Penzo-Méndez A, Lefebvre V, Rohrer H, Wegner M, Sock E. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development. 2010;137:775–784. doi: 10.1242/dev.042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AR, Müller LP, de SC, Brecha N. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol. 2014;522:1411–1443. doi: 10.1002/cne.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson T, van Buul JD, Kroon J, Welch C, Bakker EN, Matlung HL, van den Berg TK, Sharek L, Doerschuk C, Hahn K, Burridge K. The Guanine-Nucleotide Exchange Factor SGEF Plays a Crucial Role in the Formation of Atherosclerosis. PLoS One. 2013:8. doi: 10.1371/journal.pone.0055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annu. Rev. Neurosci. 2015;38:221–246. doi: 10.1146/annurev-neuro-071714-034120. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Hochedlinger K. The Sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar BT, Kinoshita K, McConnell SK. Doublecortin Microtubule Affinity Is Regulated by a Balance of Kinase and Phosphatase Activity at the Leading Edge of Migrating Neurons. Neuron. 2004;41:203–213. doi: 10.1016/s0896-6273(03)00843-2. [DOI] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner Ba, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–5. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Reynolds A, Kirry A, Nienhaus C, Blackmore MG. Overexpression of sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 2015;35:3139–45. doi: 10.1523/JNEUROSCI.2832-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.