Abstract
Tomato is the world’s second most cultivated vegetable. During cultivation or post-harvest storage, it is susceptible to more than 200 diseases caused by an array of pathogenic fungi, nematodes, bacteria, and viruses. Although wide range of chemical pesticides are currently available to manage plant diseases, continuous application of pesticides not only affect the nutritional contents of tomato but also the texture or productivity of soil. In this context, plant growth promoting bacteria (PGPB) are one of the nature friendly, safe, and effective alternatives for the management of diseases and pathogens of tomato. Currently, numbers of microbes have been used as soil or plant inoculants in different plants including tomato as biocontrol. Besides disease inhibition, these inoculants also act as growth modulators. The present article describes the biocontrol potential of PGPB strains and mechanisms for the diseases management in tomato.
Keywords: Biocontrol, Tomato, Plant growth promoting bacteria (PGPB), Disease management
Introduction
Recently, it has been estimated that huge proportions of vegetable crops get deteriorated annually during growth or post-harvest storage, owing to the diseases caused by fungus, nematodes, bacteria, and viruses. This is one of the major limiting factors influencing the food production and human development over thousands of years (Dun-chun et al. 2016). From last 50 years, application of chemical pesticides has been the prevailing control measure for disease management in crop and vegetables production. The continuous exposure to chemical pesticides such as fungicides and weedicides adversely affect the productivity, texture of soil, nutritional content of vegetables, as well as the health of human being. Due to the hazards associated with chemically synthesized herbicides and pesticides, management of diseases via biological means is the novel emerging technology and gaining importance in better agricultural sustainability.
Tomato (Solanum lycopersicum L.) is the second most important vegetable crop next to potato in the world, with estimated production reaching as 170 million MT in 2014, where China accounts for 31% of the total, followed by USA, India, and Turkey as the major producers (http://www.fao.org/). Apart from being the important vegetable crop worldwide, tomato is also used as a model plant for genetical studies related to fruit quality, stress tolerance (biotic and abiotic), and other physiological traits. This is widely adapted to a variety of agro climate spanning from the tropics to temperate regions (Panthee and Chen 2010). Presently, the production and quality of tomato are known to be largely affected by the pathogens in the field or post-harvest processing (Walker 1971; Ramyabharathi et al. 2012). Disease development during field or/post-harvest storage and shipment without the effective inhibitor of microbial growth results in huge economic loss. Therefore, a critical need of sustainable approach for the plant disease management is necessary. In this context, soil or plant microbial inoculants of plant growth promoting bacteria (PGPB) seem to be promising approach for disease management in different crops and vegetables (Kumar et al. 2015a, c).
Rhizosphere and plant growth promoting bacteria (PGPB) of tomato
Rhizosphere (interface between root and soil) is the most prominent zone for diversified plant–microbial interactions, determined by the root exudates that comprise array of chemical signals, carbon containing metabolites such as shedding of root cells, exudation, secretion, and the leakage of sugars, organic acids, and amino acids in soil matrix (Oku et al. 2012; Kumar et al. 2015b). While rhizospheric microbes mostly pose neutral effect on plants, even though some have positive or negative impact on the host development and health via complex interactions (Glick 2012). Some microorganisms are deleterious as they compete with plants for nutrients or cause disease (soil-borne plant pathogens), while others like mycorrhizal fungi and PGPB support their hosts by mobilizing nutrients, stimulating growth, increasing yield, or reducing biotic and abiotic stresses (Smith and Smith 2011).
Rhizobacteria inhabiting plant roots exert positive effects ranging from the direct ones like modulation of phytohormone levels, phosphate solubilization, ammonia production to the indirect effect like antibiotic, siderophore, and HCN production (Kumar et al. 2014, 2015a). Various species of bacteria like Pseudomonas, Azospirillum, Azotobacter, Klebsiella, Enterobacter, Alcaligenes, Arthrobacter, Burkholderia, Bacillus, and Serratia enhance plant growth and thus act as PGPB (Jasim et al. 2013; Kumar et al. 2014, 2015a, 2016a, b). In case of tomato, several plant growth promoting strains like Pseudomonas fluorescens, Bacillus sp., Azotobacter, Serratia, and Micromonospora (Pastor et al. 2012; Hammami et al. 2013; Babu et al. 2015; Martínez-Hidalgo et al. 2015) are involved in growth promotion as well as disease management.
Important diseases in tomato
Currently, more than 200 pests and diseases have been identified in tomato, causing losses in their production directly or indirectly (Nowicki et al. 2013). Diseases caused by fungi, nematodes, bacteria, and viruses are of the most severe concern in cereal crops and vegetables, which not only affect their nutritional contents, but also human health and overall economy. Some of the most important diseases in tomato caused by fungal pathogens are late blight, Sclerotinia rot, Fusarium wilt, Fusarium crown, and root rot. Late blight caused by the Phytophthora infestans is one of the most destructive diseases of tomato resulting in significant economic loss (20–70%) (Foolad et al. 2008; Nowicki et al. 2012, 2013). Sclerotinia rot, caused by Sclerotinia sclerotiorum, is another one of the important diseases affecting the tomato crop productivity. Wilt, crown, and root rot diseases in tomato caused by Fusarium species have been most intensively studied (Laurence et al. 2014; McGovern 2015). Fusarium wilt is common vascular disease caused by Fusarium oxysporum, resulting in extensive (10–80%) yield loss in many tomato producing countries (Kesavan and Chaudhary 1977). In root rot disease of tomato caused by Fusarium and Phytoptora sp., the plant foliage becomes yellow and wilts, eventually the plant dies. Fusarium crown root disease generally strikes the root system. At present, such pathogens are causing extensive loss to this important vegetable crop in the field and under green house conditions, and remain major limiting factors for tomato production. It is estimated that approximately 45% of the tomato yield has been reduced in India due to Fusarium sp. (Ramyabharathi et al. 2012).
Root-knot caused by the nematode Meloidogyne sp. is the other most devastating and widespread disease in tomato (Hunt and Handoo 2009; Zhou et al. 2016). Nematode not only affects the crop yield directly but also makes the plants more susceptible to fungal and bacterial infections (Ashraf and Khan 2010). In China, it causes up to 30–50% yield reductions of tomato (Yang et al. 2011). This disease also severely reduces productivity of a variety of vegetables and crops worldwide. However, efficient control measures have yet been developed.
Bacterial leaf spot is common bacterial diseases of tomato caused by Xanthomonas campestris. It is highly destructive in both greenhouses as well as in field conditions, causing 10–50% yield loss (Kallo 1991). In India, tomato productivity loss has been estimated to range from 10 to 80% (Sharma and Sharma 2005), whereas annual production loss due to this disease is 10–20%, which may rise to 80% in some cases (Sharma and Sharma 2005; Reddy et al. 2012). Ralstonia solanacearum is the most important soil-borne plant pathogens that cause bacterial wilt in over 200 families of plants, including tomatoes and hampers their production (Huang et al. 2013). Clavibacter michiganensis infection systemically causes wilting and canker on the stem, while blister-like spots are developed in locally infected leaves causing substantial economic loss in tomato production worldwide. C. michiganensis virulence factor plays an important role during blister formation compared to wilting, and also causes local and systemic infection in tomato (Chalupowicz et al. 2016).
Viral disease of tomato includes tomato spotted wilt virus, one of the most important viral diseases which occasionally lead to plant death (Rossello et al. 1993). Tomato yellow leaf curl is another viral disease of cultivated tomato in the tropical and subtropical regions worldwide, and losses up to 100% are most frequent. In many regions, tomato yellow leaf curl is one of the limiting factors in tomato production. The causal agents are a group of Gemini virus species belonging to the genus Begomo virus, all of them named as tomato yellow leaf curl virus. Pepino mosaic virus is the rapidly emerging virus that has established itself as one of the most important viral diseases affecting tomato crops.
PGPB as biocontrol agent in tomato
Some pest management researchers have focused their efforts on developing alternatives to synthetic chemicals for controlling pests and diseases. Among these alternatives to as biological control or biocontrol are referred. The term biocontrol is used not only to control diseases in plants but also disease management practiced during the fruits storage. Plant growth promoting bacteria (PGPB) as biocontrol agents (BCA) have certain advantages over the conventional chemical control methods, because the former are ecofriendly, non-toxic, naturally occurring microorganisms, and their application is sustainable not only for the environment but also to the human health. Another advantage of PGPB as biocontrol agent is the mode of action against the pathogens or the diseases, which also helps in the enhancement of crop growth and yield. The important mechanisms involved in the antagonism by BCA, are the production of antibiotics, cell wall degrading enzymes, bio-surfactants and volatiles, and also induction of systemic resistance (ISR) in plants (Pérez-Montano et al. 2014; Kumar et al. 2015c). PGPBs are also involved in competition for space, nutrients, and stimulation of the plant’s defense capacity (Van der Ent et al. 2008).
Studies on the control of pathogens by rhizobacteria usually focus on pathogenic microorganisms, but they are equally effective against weeds and insects (Flores-Fargas and O’Hara 2006; Siddiqui et al. 2005; Kumar et al. 2015c). The effective control of soil-borne diseases using PGPB has been reported by many authors (Whipps 2001; Lucy et al. 2004; Berg and Smalla 2009). Recently, a large number of bacterial strains have been isolated and identified for their development as biocontrol agents against tomato diseases. Punja et al. (2016) used Bacillus subtilis strain under greenhouse conditions to control the post-harvest fruit infection. B. subtilis strains were also utilized by Kilani-Feki et al. (2016) for the suppression of Botrytis cinerea, the causative agent of tomato fruit rot. Gowtham et al. (2016) utilized ten rhizobacterial strains to manage the Fusarium wilt in tomato, and found that two different strains Bacillus amyloliquefaciens and Ochrobacttrum intermedium significantly inhibited the incidence of wilt and also enhanced the vigor index of seedlings. Abdallah et al. (2016) inoculated seven different endophytic strains isolated from the native Nicotiana glauca plants, and found 94–88% significant reductions in yellowing and wilt symptom, and 95–97.5% in vascular browning. Hammami et al. (2013) screened the effectiveness of Pseudomonas fluorescens strains against different diseases such as damping-off, root rot, stem canker, and leaf blight of tomato. Khan et al. (2012) utilized Paenibacillus lentimorbus strains for controlling early blight disease by Alternaria solani in tomato.
Goudjal et al. (2014) utilized endophytic actinomycetes for the biocontrol of Rhizoctonia solani causing damping-off in tomato. These strains significantly inhibited the pathogen growth, and enhanced the growth parameters of tomato. In recent years, the biocontrol of plant diseases, particularly using the antibiotic metabolites of actinomycetes, has emerged as an alternative to chemical control agents (Huang et al. 2011). The role of actinomycetes in biocontrol of soil-borne plant pathogen has been demonstrated against Fusarium spp. (Gopalakrishnan et al.2011), Phytophthora spp. (Shahidi Bonjar et al. 2006) and Pythium spp. (Hamdali et al. 2008).
Some of the fungal strains have also been used as biocontrol for pathogens and diseases in tomato. Kriaa et al. (2015) isolated glucose oxidase producing Aspergillus tubingensis, which inhibited growth and spore production in Fusarium solani. Trichoderma isolates have also been reported as the potential biocontrol for some fungal pathogens in tomato. You et al. (2016) reported Trichoderma-mediated growth inhibition of Botrytis cinerea, and their application in soils promoted growth of tomato. Some of the important strains of PGPB and their biocontrol potential against the pathogens are described in Table 1.
Table 1.
Biocontrol potential of PGPB against tomato diseases
| Sr. no. | Disease | Disease causing organism | Antagonistic organisms | References |
|---|---|---|---|---|
| 1 | Bacterial speck | Pseudomonas syringae pv. tomato | Pseudomonas sp., Bacillus amyloliquefaciens/methylotrophicus and Pseudomonas veronii | Romero et al. (2016) |
| 2 | Bacterial spot | Xanthomonas campestris | Bacillus subtilis | Abbasi and Weselowski (2015) |
| 3 | Bacterial wilt and canker | Clavibacter michiganenensis | Pseudomonas putida | Aksoy et al. (2017) |
| 4 | Bacterial wilt |
Sclerotinia sclerotiorumm
Fusarium solani Alternaria alternata |
Pseudomonas fluorescens | Hammami et al. (2013) |
| 5 | Bacterial wilt | Ralstonia solanacearum | PGPR | Huang et al. (2013) |
| 6 | Bacterial wilt | Ralstonia solanacearum | Lactic acid bacterium | Konappa et al. (2016) |
| 7 | Bacterial wilt | Ralstonia solanacearum | Ralstonia pickettii QL-A6 | Wei et al. (2013) |
| 8 | Bacterial wilt | Ralstonia solanacearum | Endophytic bacteria | Nawangsih et al. (2011) |
| 9 | Crown and stem rot | Rhizoctonia solani and Sclerotium rolfsii | Burkholderia cepacia T1A-2B and Pseudomonas sp. T4B-2A | De Curtis et al. (2010) |
| 10 | Damping-off | Pythium aphanidermatum and Pythium ultimum | P. corrugata, P. fluorescens, P. marginalis, P. putida, P. syringae,P. viridiflava | Gravel et al. (2005) |
| 11 | Early blight | Alternaria solani | Pseudomonas, Bacillus, Azotobacter, Seeatia | Babu et al. (2015) |
| 12 | Early blight | Alternaria solani | Paenibcillus lentimorbus | Khan et al. (2012) |
| 13 | Fusarium crown and root rot of tomato | Fusarium oxysporum f.sp. radicis-lycopersici | Bacillus megaterium c96 and Burkholderia cepacia c91 | Omar et al. (2006) |
| 14. | Fusarium wilt | Fusarium oxysporum f. sp. lycopersici | Alcaligenes faecalis S18 and Bacillus cereus S42 | Abdallah et al. (2016) |
| 15. | Fusarium wilt | Fusarium oxysporum | Bacillus pumilis | Benhamou et al. (1998) |
| 16. | Fusarium wilt | Fusarium oxysporum | Bacillus amyloliquefaciens | Gowtham et al. (2016) |
| 17. | Fusarium wilt | Fusarium oxysporum | Bacillus amyloliquefaciens | Loganathan et al. (2014) |
| 18. | Fusarium wilt | Fusarium oxysporum | Bacillus subtilis, P. fluorescens | Sundaramoorthy and Balabaskar (2013) |
| 19 | Fusarium wilt | Fusarium oxysporum | P. putida | Srinivasan et al. (2009) |
| 20 | Root rot | Fusarium solani | Aspergillus tubingensis | Kriaa et al. (2015) |
| 21 | Grey mould or fruit rot disease | Botrytis cinerea | Bacillus subtilis V26 | Kilani-Feki et al. (2016) |
| 22 | Grey mould or fruit rot disease | Botrytis cinerea | Trichoderma harzianum | Elad et al. (1993) |
| 23 | Grey mould or fruit rot disease | Botrytis cinerea | Streptomyces sp. | Li et al. (2011) |
| 24 | Grey mould or fruit rot disease | Botrytis cinerea | Candida oleophila | Lima et al. (1997) |
| 25 | Grey mould or fruit rot disease | Botrytis cinerea | Paenibacillus polymyxa | Helbig (2001) |
| 26 | Tomato Rot disease | Rhizoctonia solani | Bacillus subtilis B99-2 | Ma et al. (2015) |
| 27 | Grey mould or fruit rot disease | Botrytis cinerea | Micromonospora | Martínez-Hidalgo et al. (2015) |
| 28 | Grey mould or fruit rot disease | Botrytis cinerea | Bacillus amyloliquefaciens | Mari et al. (1996) |
| 29 | Grey mould or fruit rot disease | Botrytis cinerea | Bacillus subtilis | Hang et al. (2005) |
| 30 | Grey mould or fruit rot disease | Botrytis cinerea | Pseudomonas rhodesiae, Pseudomonas sp., Exiguobacterium sp., Bacillus amyloliquefaciens/methylotrophicus, Pseudomonas veronii, Pseudomonas sp. and Pantoea eucalypti | Romero et al. (2016) |
| 31 | Grey mould or fruit rot disease | Botrytis cinerea | Trichoderma | You et al. (2016) |
| 32 | Post –harvest diseases | Penicillium and Rhizopus | Bacillus subtilis | Punja et al. (2016) |
| 33 | Root-knot disease | Meloidogyne incognita | Bacillus methylotrophicus strain R2-2 and Lysobacter antibioticus strain 13-6 | Zhou et al. (2016) |
| 34 | Stem cankar | Alternaria alternata | P. fluorescens | Pastor et al. (2012) |
| 35 | Tomato bacterial wilt | Ralstonia solanacearum | Streptomyces virginiae Y30 and E36 | Tan et al. (2011) |
| 36 | Vascular wilt and crown root rot | Fusarium oxysporum f. sp. lycopersici and F. oxysporum f. sp. radicis-lycopersici | Pseudomonas fluorescens Pf-5 and SB65, P. corrugata SB40 and Burkholderia Cepacia | Larkin and Fravel (1998) |
Mechanisms of biocontrol by PGPB
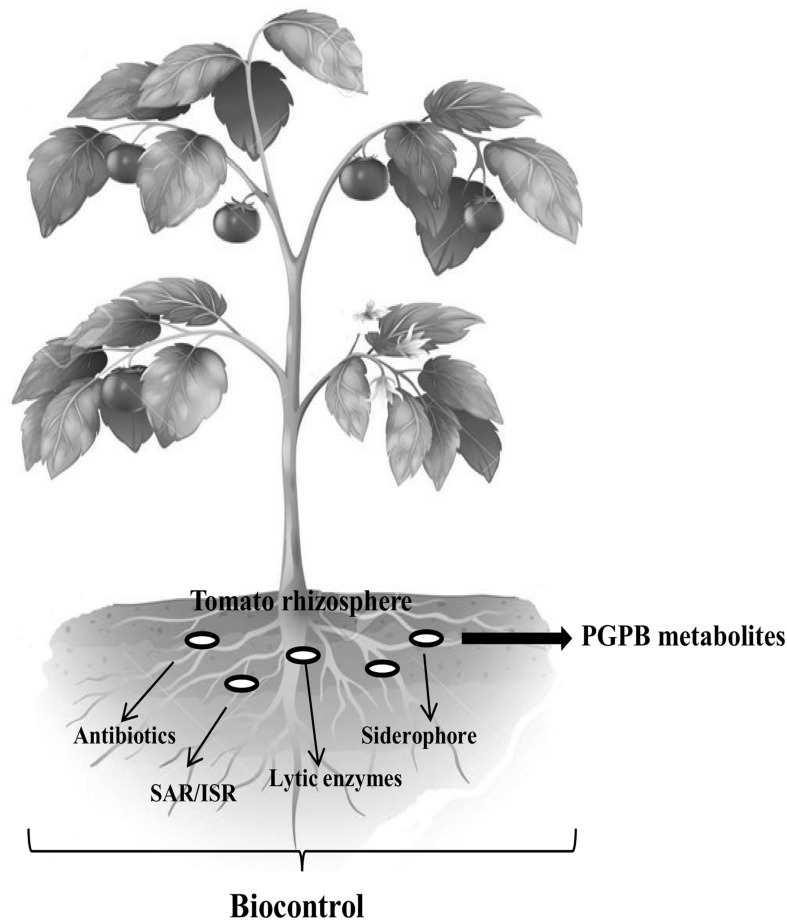
Disease controls through BCA (biocontrols) commonly rely on competition for nutrients and space at the infection site, production of metabolites, and manipulation of bacterial signaling molecules (Kloepper 1993; Wu et al. 2009). In all such cases, pathogens are antagonized by the presence and activities of other organisms they encounter (Fig. 1). The primary mechanism of pathogen suppression via nutrient competition involves the secretion of compounds like siderophores that efficiently sequester iron and deprive the pathogen from this important element (Raaijmakers et al. 2002). Some bacteria inhibit pathogen’s growth by secretion of metabolites that include antibiotics, toxins, surface active compounds (biosurfactant), and cell wall degrading enzymes (Whipps 2001; Compant et al. 2005; Haas and Defago 2005; Kumar et al. 2015c), whereas their specific metabolites also trigger the induction of systemic resistance (Van Loon et al. 1998). It is obvious that several mechanisms of action work simultaneously in many BCA. Some of the general mechanisms regarding biocontrol of tomatoes diseases are briefly discussed below.
Fig. 1.
Overview of plant growth promoting bacteria in disease management of tomato
Production/synthesis of antimicrobial metabolites
Antibiosis is an attractive and a highly effective mode of action as witnessed by BCA in disease suppression, especially in the soil-borne infections in a number of crops (Handelsman and Stab 1996). In general, BCA produce antimicrobial metabolites, the low-molecular weight diverse group of organic compounds, which are deleterious for the growth and metabolic activities of other microorganisms (Fravel 1988; Raaijmakers et al. 2002). In the past decades, a large number of microorganisms, especially the bacterial genera, have been used for the production of metabolic products. Some of the metabolites produced by bacterial BCA have broad spectrum activity and act against various groups of microorganisms (Raaijmakers et al. 2002). Some species of Bacillus and Pseudomonas produce a large number of antimicrobial products which act against pathogenic fungi, nematodes, bacteria, helminths, etc. (Thomashow and Weller 1995; Raaijmakers et al. 2002; Almaghrabi et al. 2013).
Secondary metabolite such as pyrrolnitrin (3-chloro-4-(20-nitro-30-chlorophenyl) pyrrole), produced by different bacterial strains like Pseudomonas (Ligon et al. 2000), Serratia sp. (Kalbe et al. 1996), and B. cepecia (Burkhead et al. 1994), effectively acts against different pathogens. Another metabolite 2,4-diacetylpholoroglucinol (DAPG) produced by Pseudomonas fluorescens CHA0 has been greatly utilized for the suppression of root-knot in tomato (Siddiqui and Shaukat 2003). Phenazine produced by Pseudomonas sp. effectively acts against pathogen Fusarium oxysporum in tomato (Chin-A-Woeng et al. 1998). Production of antimicrobial metabolite is modulated by exogenous and endogenous factors, addition of fertilizers, carbon sources, and minerals (Shanahan et al. 1992; Duffy and Defago 1999). The addition of glucose enhanced production of DAPG in Pseudomonas strains, whereas the supplementation of phosphate fertilizer repressed the process (Duffy and Defago 1999).
Production of cell wall degrading enzymes
Production and secretion of cell wall degrading enzymes are the major mechanisms used by BCA to control soil-borne pathogens (Kobayashi et al. 2002; Kumar et al. 2015c). These enzymes affect the structural integrity of target pathogens cell wall (Budi et al. 2000). Cell wall degrading enzymes secreted by biocontrol strains used β-1, 3-glucanase, chitinase, cellulase, and protease that exert direct inhibitory effect on the hyphal growth of fungal pathogens, and chitinase and β-1, 3-glucanase lyse chitin, insoluble linear polymer of α-1, 4-N-acetylglucosamine of cell wall of pathogens (Labuschagne et al. 2010). Some of the biocontrol strains like P. aeruginosa, and P. fluorescens possess chitinolytic activities that degrade the chitin in the cell wall (Nelson and Sorenson 1999). Someya et al. (2000) reported chitinolytic and antifungal activities of the potent biocontrol strain of S. marcescens B2 that effectively acted against the soil-borne pathogens R. solani and F. oxysporum.
Induced resistance (IR)
Induced resistance (IR) is defined as an enhancement of plant’s defensive capacity against a broad spectrum of pests and pathogens (Ramamoorthy et al. 2001). The elevated resistance is due to an inducing agent like the pathogen or upon exposure to biotic or abiotic stimuli. There are two major types of IR which includes induced systemic resistance (ISR) and systemic acquired resistance (SAR) (Kumar et al. 2015c). Plants acquire enhanced level of resistance to pathogens upon exposure to biotic stimuli provided by many PGPB, which are activated by certain molecules to as elicitors. Elicitors are generally cell wall polysaccharides, salicylic acid, cyclic lipopeptides, signal molecules like N-acyl-homoserine-lactones (AHLs), phytohormones, ethylene, and jasmonic acid (Van loon 2007; Van der Ent et al. 2009; Pérez-Montano et al. 2014). Induced systemic resistance in tomato against Botrytis cinerea involves jasmonic acid signaling as observed during biochar amendments (Mehari et al. 2015). The siderophore, pyocyanin, and pyochelin produced by Pseudomonas species are reported to induce resistance in tomato plants against tobacco mosaic virus (Choudhary et al. 2007). In another study, it was observed that root inoculation of tomato plants with Micromonospora strains effectively reduced leaf infection by the fungal pathogen Botrytis cinerea. The Micromonospora induced defense mechanism upon exposure to pathogen attack has been validated by gene expression studies. Tomato plants treated with PGPR Micromonospora sp. responded in terms of strong and quick induction of jasmonate-regulated defense pathway upon exposure to pathogen (Martínez-Hidalgo et al. 2015).
Future prospective
Food security for the ever increasing human population can be achieved by sustainable management of natural resources. Various studies reported a significant role of PGPB in agricultural management. However, knowledge gap is still underlying plant–microbe interactions under different stress conditions particularly the biotic ones. Knowledge of rhizosphere ecology governing the distribution of pathogens and antagonists may open the door for enhancing biocontrol effectiveness against phytopathogens. Future research demands intensive rhizo-engineering based on favorable identification and partitioning of the novel biomolecules, which might create the unique setting for interactions between plants and microbes. Alternatively, exploration and application of multi strain microbial inoculants over the single strain could be the effective means for disease suppression. In addition, genetic modifications for enhancing the biocontrol efficacy can also be an emerging research field for future disease managements. For instance, the transformation of strains with increased levels of antimicrobials and growth enhancing metabolites can be the better options (Walsh et al. 2001). Temperature-dependent activity can be enhanced by the addition of ice-nucleating PGPB. Furthermore, role of non-symbiotic endophytic PGPB in disease management and growth promotion is very limited. The microbial, biochemical, and molecular study using cutting edge tool may provide in-depth knowledge for better understanding of interactions between the plants and interacting microorganism. For example, root colonization of Pseudomonas fluorescens PICF7, an olive root endophyte triggered expression of genes potentially coding for olive lipoxygenase (LOX-2), phenylalanine ammonia lyase (PAL), acetone cyanohydrin lyase (ACL) in not only the targeted organ (root) but also showed broad pattern of plant defense in terms of tissues (Cabanas et al. 2014). To sum up, future challenge is to improve the efficacy and durability of biocontrol under field conditions. If this is resolved, the efficacy of biocontrol could feasibly be improved through implications of the expertise to develop improved screening protocols, formulations, and application procedures, as well as the innovative integrated disease management practices.
Acknowledgements
Authors are thankful to University Grants Commission and CSIR, New Delhi for granting fellowship in the form of JRF and SRF and also Head, Centre of Advanced Study in Botany, Banaras Hindu University for providing the laboratory facilities.
Compliance with ethical standards
Conflict of interest
None of authors have conflict of interest.
References
- Abbasi PA, Weselowski B. Efficacy of Bacillus subtilis QST 713 formulations, copper hydroxide, and their tank mixes on bacterial spot of tomato. Crop Prot. 2015;74:70–76. doi: 10.1016/j.cropro.2015.04.009. [DOI] [Google Scholar]
- Abdallah RA, Mokni-Tlili S, Nefzi A, Jabnoun-Khiareddine H, Daami-Remadi M. Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Nicotiana glauca organs. Biol Control. 2016;97:80–88. doi: 10.1016/j.biocontrol.2016.03.005. [DOI] [Google Scholar]
- Aksoy H, Kaya Y, Ozturk M, Secgin Z, Onder H, Okumus A. Pseudomonas putida Induced response in phenolic profile of tomato seedlings (Solanum lycopersicum L.) infected by Clavibacter michiganensis subsp. michiganensis. Biol Control. 2017;105:6–12. doi: 10.1016/j.biocontrol.2016.11.001. [DOI] [Google Scholar]
- Almaghrabi OA, Massoud Samia I, Abdelmoneim Tamer S. Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J Biol Sci. 2013;20:57–61. doi: 10.1016/j.sjbs.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf MS, Khan TA. Integrated approach for the management of Meloidogyne javanica on eggplant using oil cakes and biocontrol agents. Arch Phytopathol Plant Prot. 2010;43:609–614. doi: 10.1080/03235400801972434. [DOI] [Google Scholar]
- Babu AN, Jogaiah S, Ito S, Nagaraj AK, Tran LP. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci. 2015;231:62–73. doi: 10.1016/j.plantsci.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Benhamou N, Kloepper JW, Tuzun S. Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: ultra structure and cytochemistry of the host response. Planta. 1998;204:153–168. doi: 10.1007/s004250050242. [DOI] [Google Scholar]
- Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol. 2009;68:1–13. doi: 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- Budi SW, Van TD, Arnould C, Dumas-Gaudot E, Gianinazzi-Pearson V, Gianinazzi S. Hydrolytic enzyme activity of Paenibacillus sp. strain B2 and effects of the antagonistic bacterium on cell integrity of two soil borne pathogenic bacteria. Appl Soil Ecol. 2000;15:191–199. doi: 10.1016/S0929-1393(00)00095-0. [DOI] [Google Scholar]
- Burkhead KD, Schisler DA, Slininger PJ. Pyrrolnitrin production by biological-control agent Pseudomonas cepacia B37w in culture and in colonized wounds of potatoes. Appl Environ Microbiol. 1994;60:2031–2039. doi: 10.1128/aem.60.6.2031-2039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanas CGL, Schilirò E, Corredor AV, Blanco JM. The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces systemic defense responses in aerial tissues upon colonization of olive roots. Front Microbiol. 2014;5:427. doi: 10.3389/fmicb.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupowicz L, Barash I, Reuven M, Dror O, Sharabani G, Gartemann KH, Eichenlaub R, Sessa G, Manulis-Sasson S. Differential contribution of Clavibacter michiganensis virulence factors to systemic and local infection in tomato. Mol Plant Pathol. 2016 doi: 10.1111/mpp.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-A-Woeng TFC, Bloemberg GV, Van der Bij AJ, Van der Drift KMGM, Schripsema J, Kroon B, Scheffer RJ, Keel C, Bakker PAHM, De Bruijn FJ, Thomas-Oates JE, Lugtenberg BJJ. Biocontrol by phenazine-1-carboxamide producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporumf. sp. radicis lycopersici. Mol Plant Microbe Interact. 1998;10:79–86. doi: 10.1094/MPMI.1997.10.1.79. [DOI] [Google Scholar]
- Choudhary DK, Prakash A, Johri BN. Induced systemic resistance (ISR) in plants: mechanism of action. Indian J Microbiol. 2007;47:289–297. doi: 10.1007/s12088-007-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S, Duffy B, Nowak J, Clement C, Barka EA. Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol. 2005;71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Curtis F, Lima G, Vitullo D, De Cicco V. Biocontrol of Rhizoctonia solani and Sclerotium rolfsii on tomato by delivering antagonistic bacteria through a drip irrigation system. Crop Prot. 2010;29(7):663–670. doi: 10.1016/j.cropro.2010.01.012. [DOI] [Google Scholar]
- Duffy BK, Defago G. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl Environ Microbiol. 1999;65:2429–2438. doi: 10.1128/aem.65.6.2429-2438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun-chun HE, Zhan J, Xie L. Problems, challenges and future of plant disease management: from an ecological point of view. J Integ Agri. 2016;15(4):705–715. doi: 10.1016/S2095-3119(15)61300-4. [DOI] [Google Scholar]
- Elad Y, Zimand G, Zaqs Y, Zuriel S. Use of Trichoderma harzianum in combination or alternation with fungicides to control cucumber grey mould (Botrytis cinerea) under commercial greenhouse conditions. Plant Pathol. 1993;42:324–332. doi: 10.1111/j.1365-3059.1993.tb01508.x. [DOI] [Google Scholar]
- Flores-Fargas RD, O’Hara GW. Isolation and characterization of rhizosphere bacteria with potential for biological control of weeds in vine yards. J Appl Microbiol. 2006;100:946–954. doi: 10.1111/j.1365-2672.2006.02851.x. [DOI] [PubMed] [Google Scholar]
- Foolad MR, Merk HL, Ashrafi H. Genetics, genomics and breeding of late blight and early blight resistance in tomato. Crit Rev Plant Sci. 2008;27:75–107. doi: 10.1080/07352680802147353. [DOI] [Google Scholar]
- Fravel DR. Role of antibiosis in the biocontrol of plant diseases. Annu Rev Phytopathol. 1988;26:75–91. doi: 10.1146/annurev.py.26.090188.000451. [DOI] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012 doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Pande S, Sharma M, Humayun P, Keerthi Kiran BK, Sandeep D, et al. Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 2011;30:1070–1078. doi: 10.1016/j.cropro.2011.03.006. [DOI] [Google Scholar]
- Goudjal Y, Toumatiaa O, Yekkoura A, Sabaoua N, Mathieuc F, Zitounia A. Biocontrol of Rhizoctonia solani damping-off and promotion of tomato plant growth by endophytic actinomycetes isolated from native plants of Algerian Sahara. Microbiol Res. 2014;169:59–65. doi: 10.1016/j.micres.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Gowtham HG, Hariprasad P, Nayak SC, Niranjana SR. Application of rhizobacteria antagonistic to Fusarium oxysporum f. sp. lycopersici for the management of Fusarium wilt in tomato. Rhizosphere. 2016;2:72–74. doi: 10.1016/j.rhisph.2016.07.008. [DOI] [Google Scholar]
- Gravel V, Martinez C, Antoun H, Tweddell RJ. Antagonist microorganisms with the ability to control Pythium damping-off of tomato seeds in rockwool. Bio Control. 2005;50(5):771–786. [Google Scholar]
- Haas D, Defago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3(4):307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- Hamdali H, Hafidi M, Virolle MJ, Ouhdouch Y. Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Appl Soil Ecol. 2008;40:510–517. doi: 10.1016/j.apsoil.2008.08.001. [DOI] [Google Scholar]
- Hammami I, Hsouna AB, Hamdi N, Gdoura R, Triki MA. Isolation and characterization of rhizosphere bacteria for the biocontrol of the damping-off disease of tomatoes in Tunisia. C R Biol. 2013;336(11–12):557–564. doi: 10.1016/j.crvi.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Handelsman J, Stab EV. Biocontrol of soilborne plant pathogens. Plant Cell. 1996;8:1855–1869. doi: 10.1105/tpc.8.10.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang NTT, Oh SO, Kim GH, Hur JS, Koh YJ. Bacillus subtilis S1-0210 as a biocontrol agent against Botrytis cinerea in strawberries. Plant Pathol J. 2005;21:59–63. doi: 10.5423/PPJ.2005.21.1.059. [DOI] [Google Scholar]
- Helbig J. Biological control of Botrytis cinerea Pers. Ex Fr. in strawberry by Paenibacillus polymyxa (isolate 18191) J Phytopathol. 2001;149:265–273. doi: 10.1046/j.1439-0434.2001.00609.x. [DOI] [Google Scholar]
- Huang X, Zhang N, Yong X, Yang X, Shen Q. Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol Res. 2011;167:135–143. doi: 10.1016/j.micres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Huang J, Wei Z, Tan S, Mei X, Yin S, Shen Q, Xu Y. The rhizosphere soil of diseased tomato plants as a source for novel microorganisms to control bacterial wilt. Appl Soil Ecol. 2013;72:79–84. doi: 10.1016/j.apsoil.2013.05.017. [DOI] [Google Scholar]
- Hunt DJ, Handoo ZA. Taxonomy, identification and principal species. In: Perry RN, Moens M, Starr JL, editors. Root-knot nematodes. Wallingford: CABI; 2009. pp. 55–97. [Google Scholar]
- Jasim B, Joseph AA, John CJ, Mathew J, Radhakrishnan EK. Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3 Biotech. 2013 doi: 10.1007/s13205-013-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe C, Marten P, Berg G. Strains of the genus Serratia as beneficial rhizobacteria of oilseed rape with antifungal properties. Microbiol Res. 1996;151:433–439. doi: 10.1016/S0944-5013(96)80014-0. [DOI] [PubMed] [Google Scholar]
- Kallo G. Genetic improvement of tomato. Berlin: Springer; 1991. [Google Scholar]
- Kesavan V, Chaudhary B. Screening for resistance to Fusarium wilt of tomato. SABRO J. 1977;9:51–65. [Google Scholar]
- Khan N, Mishra A, Nautiyal CS. Paenibacillus lentimorbus B-30488r controls early blight disease in tomato by inducing host resistance associated gene expression and inhibiting Alternaria solani. Biol Cont. 2012;62:65–74. doi: 10.1016/j.biocontrol.2012.03.010. [DOI] [Google Scholar]
- Kilani-Feki O, Khedher SB, Dammak M, Kamoun A, Jabnoun-Khiareddine H, Daami-Remadi M, Tounsi S. Improvement of antifungal metabolites production by Bacillus subtilis V26 for biocontrol of tomato postharvest disease. Biol Control. 2016;95:73–82. doi: 10.1016/j.biocontrol.2016.01.005. [DOI] [Google Scholar]
- Kloepper JW. Plant growth-promoting rhizobacteria as biological control agents. In: Metting B, editor. Soil microbial technologies. New York: Marcel Dekker; 1993. pp. 255–274. [Google Scholar]
- Kobayashi DY, Reedy RM, Bick JA, Oudemans PV. Characterization of chitinase gene from Stenotrophomonas maltophilia strain 34S1 and its involvement in biological control. Appl Environ Microbiol. 2002;68:1047–1054. doi: 10.1128/AEM.68.3.1047-1054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konappa NM, Maria M, Uzma F, Krishnamurthy S, Nayaka SC, Niranjana SR, Chowdappa S. Lactic acid bacteria mediated induction of defense enzymes to enhance the resistance in tomato against Ralstonia solanacearum causing bacterial wilt. Sci Hortic. 2016;207:183–192. doi: 10.1016/j.scienta.2016.05.029. [DOI] [Google Scholar]
- Kriaa M, Hammami I, Sahnoun M, Azebou MC, Triki MA, Kammoun R. Biocontrol of tomato plant diseases caused by Fusarium solani using a new isolated Aspergillus tubingensis CTM 507 glucose oxidase. C R Biol. 2015;338(10):666–677. doi: 10.1016/j.crvi.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Kumar A, Singh R, Giri DD, Singh PK, Pandey KD. Effect of Azotobacter chroococcum CL13 inoculation on growth and curcumin content of turmeric (Curcuma longa L.) Int J Curr Microbiol App Sci. 2014;3(9):275–283. [Google Scholar]
- Kumar A, Vandana RS, Singh M, Pandey KD. Plant growth promoting rhizobacteria (PGPR). A promising approach for disease management. In: Singh JS, Singh DP, editors. Microbes and environmental management. New Delhi: Studium Press; 2015. pp. 195–209. [Google Scholar]
- Kumar A, Vandana Yadav A, Giri DD, Singh PK, Pandey KD. Rhizosphere and their role in plant–microbe interaction. In: Chaudhary KK, Dhar DW, editors. Microbes in soil and their agricultural prospects. Hauppauge: Nova Science Publisher, Inc; 2015. pp. 83–97. [Google Scholar]
- Kumar V, Kumar A, Pandey KD, Roy BK. Isolation and characterization of bacterial endophytes from the roots of Cassia tora L. Ann Microbiol. 2015;65:1391–1399. doi: 10.1007/s13213-014-0977-x. [DOI] [Google Scholar]
- Kumar A, Singh R, Yadav A, Giri DD, Singh PK, Pandey KD. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech. 2016;6:60. doi: 10.1007/s13205-016-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Vandana Singh M, Singh PP, Singh SK, Singh PK, Pandey KD. Isolation of plant growth promoting rhizobacteria and their impact on growth and curcumin content in Curcuma longa L. Biocatal Agric Biotechnol. 2016;8:1–7. [Google Scholar]
- Labuschagne N, Pretorius T, Idris AM. Plant growth-promoting rhizobacteria as biocontrol agents against soil-borne plant diseases. Microbiol Monogr. 2010;18:211–230. doi: 10.1007/978-3-642-13612-2_9. [DOI] [Google Scholar]
- Larkin RP, Fravel DR. Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 1998;82:1022–1028. doi: 10.1094/PDIS.1998.82.9.1022. [DOI] [PubMed] [Google Scholar]
- Laurence MH, Summerell BA, Burgess LW, Liew ECY. Genealogical concordance phylogenetic species recognition in the Fusarium oxysporum species complex. Fungal Biol. 2014;118:374–384. doi: 10.1016/j.funbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Li QL, Ning P, Zheng L, Huang JB, Li GQ, Hsiang T. Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biol Control. 2011;61:113–120. doi: 10.1016/j.biocontrol.2011.10.014. [DOI] [Google Scholar]
- Ligon JM, Hill DS, Hammer PE, Torkewitz NR, Hofmann D, Kempf HJ, van Pee KH. Natural products with antifungal activity from Pseudomonas biocontrol bacteria. Pest Manag Sci. 2000;56:688–695. doi: 10.1002/1526-4998(200008)56:8<688::AID-PS186>3.0.CO;2-V. [DOI] [Google Scholar]
- Lima G, Ippolito A, Nigro F, Salerno M. Effectiveness of Aureobasidium pullulans and Candida oleophila against post-harvest strawberry rots. Postharvest Biol Technol. 1997;10:169–178. doi: 10.1016/S0925-5214(96)01302-6. [DOI] [Google Scholar]
- Loganathan P, Vigneswaran S, Kandasamy J, Bolan NS. Removal and recovery of phosphate from water using sorption. Crit Rev Environ Sci Technol. 2014;44(8):847–907. doi: 10.1080/10643389.2012.741311. [DOI] [Google Scholar]
- Lucy M, Reed E, Glick BR. Application of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek. 2004;86:1–25. doi: 10.1023/B:ANTO.0000024903.10757.6e. [DOI] [PubMed] [Google Scholar]
- Ma X, Wang X, Cheng J, Nie X, Yu X, Zhao Y, Wang W. Microencapsulation of Bacillus subtilis B99-2 and its biocontrol efficiency against Rhizoctonia solani in tomato. Biol Control. 2015;90:34–41. doi: 10.1016/j.biocontrol.2015.05.013. [DOI] [Google Scholar]
- Mari M, Guizzardi M, Brunelli M, Folchi A. Post-harvest biological control of grey mould (Botrytis cinerea Pers.: Fr.) on fresh-market tomatoes with Bacillus amyloliquefaciens. Crop Prot. 1996;15:699–705. doi: 10.1016/S0261-2194(96)00042-7. [DOI] [Google Scholar]
- Martínez-Hidalgo P, García JM, Pozo MJ. Induced systemic resistance against Botrytis cinerea by Micromonospora strains isolated from root nodules. Front Microbiol. 2015;6:922. doi: 10.3389/fmicb.2015.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern RJ. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 2015 [Google Scholar]
- Mehari ZH, Elad Y, Rav-David D, Graber ER, Harel YM. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil. 2015;395(1–2):31. doi: 10.1007/s11104-015-2445-1. [DOI] [Google Scholar]
- Nawangsih AA, Damayanti I, Wiyono S, Kartika JG. Selection and characterization of endophytic bacteria as biocontrol agents of tomato bacterial wilt disease. Hayati J Biosci. 2011;18:66–70. doi: 10.4308/hjb.18.2.66. [DOI] [Google Scholar]
- Nelson MN, Sorenson J. Chitinolytic activity of Pseudomonas fluorescens isolates from barley and sugar beet rhizosphere. FEMS Microbiol Ecol. 1999;30:217–227. doi: 10.1111/j.1574-6941.1999.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Nowicki M, Foolad MR, Nowakowska M, Kozik EU. Potato and tomato late blight caused by Phytophthora infestans: an overview of pathology and resistance breeding. Plant Dis. 2012;96:4–17. doi: 10.1094/PDIS-05-11-0458. [DOI] [PubMed] [Google Scholar]
- Nowicki M, Kozik EU, Foolad MR (2013) Late blight of tomato. Translational genomics for crop breeding, volume I: biotic stress. 1st edn. Varshney RK, Tuberosa R (eds) Wiley, Hoboken
- Oku S, Komastu A, Tajima T, Nakashimada Y, Kato J. Identification of chemotaxis sensory proteins for aminoacids in Pseudomonas fluorescens Pf0-1 and their involvement in chemo taxis to tomato root exudates and root colonization. Microbes Environ. 2012;27:462–469. doi: 10.1264/jsme2.ME12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar I, O’neill TM, Rossall S. Biological control of Fusarium crown and root rot of tomato with antagonistic bacteria and integrated control when combined with the fungicide carbendazim. Plant Pathol. 2006;55(1):92–99. doi: 10.1111/j.1365-3059.2005.01315.x. [DOI] [Google Scholar]
- Panthee DR, Chen F. Genomics of fungal disease resistance in tomato. Curr Genom. 2010;11:30–39. doi: 10.2174/138920210790217927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor N, Carlier E, Andrés J, Rosas SB, Rovera M. Characterization of rhizosphere bacteria for control of phytopathogenic fungi of tomato. J Environ Manag. 2012;95:S332–S337. doi: 10.1016/j.jenvman.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Pérez-Montano F, Alias-Villegas C, Bellogin RA, del Cerro P, Espuny MR, Jimenez-Guerrero I, Lopez-Baena FJ, Ollero FJ, Cubo T. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res. 2014;169:325–336. doi: 10.1016/j.micres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Punja ZK, Rodriguez G, Tirajoh A. Effects of Bacillus subtilis strain QST 713 and storage temperatures on post-harvest disease development on greenhouse tomatoes. Crop Prot. 2016;84:98–104. doi: 10.1016/j.cropro.2016.02.011. [DOI] [Google Scholar]
- Raaijmakers JM, Vlami M, de Souza JT. Antibiotic production by bacterial biocontrol agent. Anton van Leeuwenhoek. 2002;81:537–547. doi: 10.1023/A:1020501420831. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R. Innduction of systemic resistance by plant growth promoting rhizobacteria in crop against pest and diseases. Crop Prot. 2001;20:1–11. doi: 10.1016/S0261-2194(00)00056-9. [DOI] [Google Scholar]
- Ramyabharathi SA, Meena B, Raguchander T. Induction of chitinase and b-1,3- glucanase PR proteins in tomato through liquid formulated Bacillus subtilis EPCO 16 against Fusarium wilt. J Today’s Biol Sci Res Rev JTBSRR. 2012;1(1):50–60. [Google Scholar]
- Reddy SA, Bagyaraj DJ, Kale RD. Management of tomato bacterial spot caused by Xanthomonas campestris using vermin compost. J Biopest. 2012;5(1):10–13. [Google Scholar]
- Romero FM, Marina M, Pieckenstain FL. Novel components of leaf bacterial communities of field-grown tomato plants and their potential for plant growth promotion and biocontrol of tomato diseases. Res Microbiol. 2016;167(3):222–233. doi: 10.1016/j.resmic.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Rossello MA, Descals E, Cabrer B. Nia epidermoidea, a new marine gasteromycete. Mycol res. 1993;97(1):68–70. doi: 10.1016/S0953-7562(09)81114-3. [DOI] [Google Scholar]
- Shahidi Bonjar GH, Barkhordar B, Pakgohar N, Aghighi S, Biglary S, Rashid Farrokhi P, et al. Biological control of Phytophthora drechsleri Tucker, the causal agent of pistachio gummosis, under greenhouse conditions by use of actinomycetes. Plant Pathol J. 2006;5:20–23. doi: 10.3923/ppj.2006.20.23. [DOI] [Google Scholar]
- Shanahan P, O’Sullivan DJ, Simpson P, Glennon JD, O’Gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992;58:353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RC, Sharma JN. Challenging problems in horticulture and Forest pathology. New Delhi: Indus publishing Company; 2005. [Google Scholar]
- Siddiqui IA, Shaukat SS. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biol Biochem. 2003;35:1615–1623. doi: 10.1016/j.soilbio.2003.08.006. [DOI] [Google Scholar]
- Siddiqui A, Haas D, Heeb S. Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root knot nematode Meloydogyne incognita. Appl Environ Microbiol. 2005;71:5646–5649. doi: 10.1128/AEM.71.9.5646-5649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- Someya N, Kataoka N, Komagata T, Hirayae K, Hibi T, Akutsu K. Biological control of cyclamen soil borne diseases by Serratia marcescens strain B2. Plant Dis. 2000;84:334–340. doi: 10.1094/PDIS.2000.84.3.334. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Gilardi G, Garibaldi A, Gullino ML. Bacterial antagonists from used rockwool soilless substrates suppress fusarium wilt of tomato. J Plant Pathol. 2009;91(1):147–154. [Google Scholar]
- Sundaramoorthy S, Balabaskar P. Biocontrol efficacy of Trichoderma spp. against wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici. J App Biol Biotechnol. 2013;1(03):36–40. [Google Scholar]
- Tan H, Zhou S, Deng Z, He M, Cao L. Ribosomal-sequence-directed selection for endophytic streptomycete strains antagonistic to Ralstonia solanacearum to control tomato bacterial wilt. Biol Control. 2011;59:245–254. doi: 10.1016/j.biocontrol.2011.07.018. [DOI] [Google Scholar]
- Thomashow LS, Weller DM. Current concepts in the use of introduced bacteria for biological disease control. In: Stacey G, Keen N, editors. Plant–microbe interactions. New York: Chapman and Hall; 1995. pp. 187–235. [Google Scholar]
- Van der Ent S, Verhagen BW, Van Doorn R, Bakker D, Verlaan MG, Pel MJ, Joosten RG, Proveniers MC, Van Loon LC, Ton J, Pieterse CM. MYB72 is required in early signaling steps of rhizobacteriainduced systemic resistance in Arabidopsis. Plant Physiol. 2008;146(3):1293–1304. doi: 10.1104/pp.107.113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ent S, Van Wees SCM, Pieterse CMJ. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochem. 2009;70:1581–1588. doi: 10.1016/j.phytochem.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Van Loon LC. Systemic resistance induced by rhizosphere bacteria. Ann Rev Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- Van Loon LC. Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol. 2007;119:243–254. doi: 10.1007/s10658-007-9165-1. [DOI] [Google Scholar]
- Walker JC. Fusarium wilt of tomato. Monogr. 6. St. Paul: APS Press; 1971. [Google Scholar]
- Walsh UF, Morrissey JP, O’Gara F. Pseudomonas for biocontrol of phytopathogens: from functional genomics to commercial exploitation. Curr Opin Biotech. 2001;12:289–295. doi: 10.1016/S0958-1669(00)00212-3. [DOI] [PubMed] [Google Scholar]
- Wei Z, Huang J, Tan S, Mei X, Shen Q, Xu Y. The congeneric strain Ralstonia pickettii QL-A6 of Ralstonia solanacearum as an effective biocontrol agent for bacterial wilt of tomato. Biol Control. 2013;65(2):278–285. doi: 10.1016/j.biocontrol.2012.12.010. [DOI] [Google Scholar]
- Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52(487):511. doi: 10.1093/jexbot/52.suppl_1.487. [DOI] [PubMed] [Google Scholar]
- Wu CH, Bernard SM, Andersen GL, Chen W. Developing microbe–plant interactions for applications in plant-growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microb Biotechnol. 2009;2:428–440. doi: 10.1111/j.1751-7915.2009.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yuan L, Duan Y (2011) The investigation and prevention of tomato root knot nematode in Yunnan Yuanmou. Plant Prot Technol 44–45
- You J, Zhang J, Wu M, Yang L, Chen W, Li G. Multiple criteria-based screening of Trichoderma isolates for biological control of Botrytis cinerea on tomato. Biol Control. 2016;101:31–38. doi: 10.1016/j.biocontrol.2016.06.006. [DOI] [Google Scholar]
- Zhou L, Yuen G, Wang Y, Wei L, Ji G. Evaluation of bacterial biological control agents for control of root-knot nematode disease on tomato. Crop Prot. 2016;84:8–13. doi: 10.1016/j.cropro.2015.12.009. [DOI] [Google Scholar]