Abstract
Reef-building corals form symbiotic relationships with dinoflagellates of the genus Symbiodinium. Symbiodinium are genetically and physiologically diverse, and corals may be able to adapt to different environments by altering their dominant Symbiodinium phylotype. Notably, each coral species associates only with specific Symbiodinium phylotypes, and consequently the diversity of symbionts available to the host is limited by the species specificity. Currently, it is widely presumed that species specificity is determined by the combination of cell-surface molecules on the host and symbiont. Here we show experimental evidence supporting a new model to explain at least part of the specificity in coral–Symbiodinium symbiosis. Using the laboratory model Aiptasia–Symbiodinium system, we found that symbiont infectivity is related to cell size; larger Symbiodinium phylotypes are less likely to establish a symbiotic relationship with the host Aiptasia. This size dependency is further supported by experiments where symbionts were replaced by artificial fluorescent microspheres. Finally, experiments using two different coral species demonstrate that our size-dependent-infection model can be expanded to coral–Symbiodinium symbiosis, with the acceptability of large-sized Symbiodinium phylotypes differing between two coral species. Thus the selectivity of the host for symbiont cell size can affect the diversity of symbionts in corals.
Introduction
Endosymbiotic dinoflagellates of the genus Symbiodinium reside in many cnidarian organisms, such as reef-building coral, sea anemone, jellyfish and hydrocoral (Davy et al., 2012). The Symbiodinium–cnidarian association is a mutualistic symbiotic relationship, with each member supporting the other to survive in an oligotrophic environment. Symbiodinium provide photosynthetically fixed carbon and in return receive inorganic nutrients from the host cnidarian (Yellowlees et al., 2008). Symbiodinium is a key primary producer of coral reefs and the coral–Symbiodinium symbiotic relationship is a cornerstone for biologically diverse coral reef ecosystems.
The first step in initiating a symbiotic relationship is recruiting Symbiodinium into the cnidarian host’s cells. There are two main ways for this to occur; they can be inherited from the parent (vertical transmission) or obtained directly from the environment (horizontal transmission). Vertical transmission occurs in select host taxa (for example, brooding corals) that release eggs or brooded planula larvae only after symbionts have been transferred to the offspring. Horizontal transmission could potentially happen in any host taxon, mainly in the early developing stages but can also occur in the adult stage (Baker, 2003).
Recent molecular and genetic analysis classified Symbiodinium into nine large groups called clades (A–I) with each clade containing multiple phylotypes (Pochon and Gates, 2010). Between Symbiodinium phylotypes, morphological differences can be seen in chromosome number, cell size of both the vegetative and motile phases and in the chloroplast number, size and arrangement (Trench, 1979; LaJeunesse, 2001). This variation leads to physiological differences among phylotypes, including their sensitivity to environmental stresses such as increased seawater temperature or strong light (Tchernov et al., 2004; Takahashi et al., 2008; Takahashi et al., 2013). Hosts can harbor multiple Symbiodinium phylotypes and the dominant Symbiodinium phylotype can vary with changes in the environmental conditions. For the host to survive and adapt to changing conditions, it can be critical that it harbors Symbiodinium phylotypes that are suitable to the new environment (Baker, 2001; Baker et al., 2004; Lewis and Coffroth, 2004; Rowan, 2004; Berkelmans and van Oppen, 2006; Jones et al., 2008; Howells et al., 2012).
The uptake of Symbiodinium from the environment into host cells is restricted by species specificity. Laboratory infection tests using sea fans (Kinzie, 1974), upside-down jellyfish (Fitt, 1985), sea anemone (Schoenberg and Trench, 1980; Belda-Baillie et al., 2002; Rodriguez-Lanetty et al., 2003; Hambleton et al., 2014) and coral (Weis et al., 2001) demonstrated that the host could only establish a symbiotic relationship with specific Symbiodinium phylotypes. Furthermore, the degree of specificity differed among both the host species and the Symbiodinium phylotypes (LaJeunesse, 2002; Baker, 2003), restricting the range of compatible partners. In corals, the in situ symbionts can differ between larvae and adult polyps (Little et al., 2004). Therefore, it has been suggested that species specificity can vary with life stage. However, a study in the sea anemone Aiptasia showed that symbiont specificity did not change with life stage (Hambleton et al., 2014).
In experiments looking at the symbioses between green Hydra and Chlorella (Meints and Pardy, 1980) or marine cnidarians and Symbiodinium (Lin et al., 2000; Wood-Charlson et al., 2006), the pattern recognition receptors on the host cell membrane and the microbe-associated molecular patterns on the symbiont cell surface were proposed to be important in initiating the symbiotic relationship (Davy et al., 2012). It was found that molecular patterns, that is, glycans, differ among Symbiodinium strains (Wood-Charlson et al., 2006; Logan et al., 2010), suggesting that the combination of cell-surface molecules on the host and symbiont could mediate species specificity. However, direct experimental data supporting this hypothesis is still lacking, and the mechanisms associated with cnidarian–algae specificity are still poorly understood.
In this study, we investigated the relationship between infectivity and Symbiodinium cell size using two different types of host: artificially bleached sea anemone (Aiptasia sp.) and aposymbiotic juvenile polyps of two different coral species (Acropora tenuis and Cyphastrea serailia). Our results demonstrated that cell size affected the infectivity of Symbiodinium phylotypes and that the maximum threshold for symbiont cell size differed among coral species.
Materials and methods
Cultures and growth conditions
All Symbiodinium strains used for infection tests were obtained from the National Center for Marine Algae and Microbiota (East Boothbay, ME, USA), CSIRO Australian National Algae Culture Collection (Hobart, TAS, Australia) or the Buffalo Undersea Reef Research Culture Collection (Buffalo, NY, USA). To ensure that cultures were monoclonal, all Symbiodinium phylotypes were subcultured from a single cell isolated by a cell sorter (BD FACS Aria II, BD Biosciences, San Jose, CA, USA). Symbiodinium strains used in this study did not always correspond to the information provided by the culture collection centers, that is, genotype (subclade) of Symbiodinium phylotypes (Supplementary Table S1). To avoid confusion, Symbiodinium phylotypes whose genotype did not match the provided information were renamed (Supplementary Table S1). All Symbiodinium strains used in Figure 6 to examine the relationship between clade and cell size were obtained from Buffalo Undersea Reef Research Culture Collection and categorized according to the genotype information provided by the culture collection; they are all expected to be monoclonal lines.
Symbiodinium cells were grown in filtered (0.22 μm pore filter; Steritop-GP Filter Unit, Merck Millipore, Billerica, MA, USA) artificial seawater (sea salt; Sigma-Aldrich, St Louis, MO, USA) containing Daigo’s IMK medium for marine microalgae (Wako, Osaka, Japan). Symbiodinium cells were grown at a continuous temperature of 23 °C under photosynthetically active radiation at 80 μmol photons m−2 s−1 with 16 h light (white color, fluorescence tubes) in a day. Symbiodinium cells that had reached the mid-logarithmic phase of growth were harvested by centrifugation at 2000 g for 3 min and resuspended in fresh seawater media.
The sea-anemone used in our study is Aiptasia (cf. insignis) isolated from Okinawa (Japan) >10 years ago (Belda-Baillie et al., 2002). This Aiptasia originally harbored a clade B Symbiodinium (GenBank accession number AF289267; Belda-Baillie et al., 2002). Aiptasia polyps used in our study were monoclonal, originating from a single polyp. Aiptasia were cultured in the presence of a mixture of Symbiodinium phylotypes from clades A and B. Aiptasia were grown under the same light and temperature conditions as Symbiodinium using artificial seawater without Daigo’s IMK. Before infection, Aiptasia were artificially bleached (aposymbiotic Aiptasia) by incubating them at 33 °C in complete darkness for 3 weeks and then returned to normal growth temperature for >3 weeks. Seawater was changed every 2 days during bleaching treatment. Bleaching was confirmed by the inability to detect any symbiotic algae under a MZ FLIII fluorescence stereomicroscope (Leica Microsystems, Heerbrugg, Switzerland). Once Aiptasia polyps were bleached, they were cultured separately from other Aiptasia polyps, in the absence of Symbiodinium. Freshly hatched Artemia nauplii were fed to Aiptasia once a week.
A. tenuis egg–sperm bundles were collected from several colonies near the Sesoko marine station, Okinawa, Japan (University of the Ryukyus) in June 2013. C. serailia egg–sperm bundles were collected from several colonies at the Seto Marine Biological Laboratory, Wakayama, Japan (Kyoto University) in July 2013. Bundles collected from several colonies were combined and incubated for a few hours to allow fertilization to occur. Embryos were then transferred to fresh seawater that had been filtered through a 3 μm polypropylene cartridge (TCW-3N-PPS, Advantec, Tokyo, Japan) with a filter housing (1PA, Advantec) or a 0.22 μm filter (Steritop-GP Filter Unit, Merck Millipore). Planula larvae were maintained in filtered seawater at 25 °C until use. Metamorphosis was induced in A. tenuis with 1 μM Hym-248 (Iwao et al., 2002), and juvenile polyps were distributed into six-well plates. For C. serailia, planula larvae were kept in polystyrene boxes as the juvenile polyps did not settle in plastic petri dishes. Peptide treatment, such as Hym-248, was not necessary to induce metamorphosis in C. serailia. All juvenile polyps were cultured in filtered artificial seawater until use.
Measurement of Symbiodinium cell size
The size of Symbiodinium cells was measured with an automated cell counter (Cellometer Auto X4; Nexcelom Bioscience, Lawrence, MA, USA). Symbiodinium cells were collected in the middle of the light period by centrifugation (16 000 g) for 1 min. The collected Symbiodinium cells were resuspended in fresh seawater and mixed by vortexing. After these steps, Symbiodinium cells lost their flagella and were all in the vegetative form (round cell shape without flagella). Measurements were conducted three times using separately cultured cells. Motile and vegetative Symbiodinium cells (Supplementary Figure S1) were photographed using a Leica DM6000B microscope (Leica Microsystems) equipped with a SPOT Flex camera (SPOT Imaging Solutions, Sterling Heights, MI, USA).
Infection of Aiptasia by Symbiodinium
To examine the infectivity of Symbiodinium into Aiptasia, single aposymbiotic polyps were placed in multiple clear plastic cups with 30 ml of artificial seawater and each mixed with different Symbiodinium strains (40 000 cells ml−1). The number of Symbiodinium cells was measured with an automated cell counter (TC20, Bio-Rad, Hercules, CA, USA). Three Aiptasia polyps cultured in separate petri dishes were used for each Symbiodinium strain. Infection of Aiptasia by Symbiodinium was confirmed by monitoring symbiont chlorophyll fluorescence in tentacles using a MZ FLIII fluorescence stereomicroscope (Leica Microsystems) with a GFP2 filter (excitation: 480/40, emission: 510LP) and a digital camera (EOS 600D, Canon, Tokyo, Japan). Infection of Aiptasia polyps was evaluated on days 1, 2, 3, 4, 6, 8, 9, 10, 11, 14, 16, 18, 21, 23, 25 and 35 after mixing with Symbiodinium. Infection was determined by examination in a separate container with fresh seawater to avoid counting the Symbiodinium cells in the media. Aiptasia polyps were then returned to the original seawater containing Symbiodinium cells. The Aiptasia polyp was said to be infected when >30 foci of Symbiodinium cells could be seen within a tentacle.
Incorporation of fluorescent microspheres into Aiptasia
Fluoresbrite carboxylate yellow-green microspheres (2.5% solids-latex, excitation max.=441 nm; emission max.=486 nm) with diameters of 6.3±0.18, 10.4±0.25 and 11.4±0.30 μm (Polysciences, Inc., Warrington, PA, USA) were used to examine the uptake of microspheres into host cells. The 11.4 μm microspheres were custom made. The size of microspheres was verified with an automated cell counter (Cellometer Auto X4). Each suspension of microspheres (~12 000 microspheres) was mixed with 30 fresh A. nauplii and the mixed pellet (approximately 1 mm in diameter) was fed using forceps to individual Aiptasia polyps harboring Symbiodinium (that is, Aiptasia polyps cultured in the presence of a mixture of Symbiodinium phylotypes from clades A and B). Microspheres could be taken into the host cells without mixing with Artemia. However, mixing with Artemia was necessary to ensure that the same number of microspheres was introduced into the stomach in each experiment. Three Aiptasia polyps were used for each microsphere size. The number of microspheres was counted with a TC20 automated cell counter. Incorporation of microspheres into Aiptasia tentacles was confirmed by imaging yellow-green fluorescence using a fluorescence stereomicroscope (see Infection of Aiptasia by Symbiodinium section). Incorporation was evaluated after 6 h, 1 day, 2, 3 and 6 days. The Aiptasia polyp was said to have incorporated microspheres when >30 foci of microspheres could be seen within a tentacle.
To confirm that the microspheres were inside host cells, we examined their localization by laser-scanning confocal microscopy. Fluoresbrite carboxylate yellow-green microspheres (6.3 μm in diameter) were fed to Aiptasia polyps as described above. After 3 days, Aiptasia polyps were incubated in sea water supplemented with 190 mM MgCl2 to stop their movement and then fixed with 10% formalin for 1 h. Fixed Aiptasia polyps were washed with seawater and placed on 0.5% agarose gel on a glass depression slide and covered with a glass cover slip. Images of Aiptasia tentacles were taken using a LSM780 laser-scanning confocal microscope (Zeiss, Oberkochen, Germany) equipped with a 40 × objective (NA=1.1). Fluoresbrite microspheres and Symbiodinium were excited at 488 nm (emission: 499–561 nm) and 633 nm (emission: 648–735 nm), respectively. Transmitted light was collected after excitation at 633 nm. Z-stacks were taken (0.7 μm between each single plane) and processing, including 3D reconstruction and depth indexation, was performed using the Zen 2012 software package (Zeiss).
Infection of corals by Symbiodinium
To examine the infectivity of Symbiodinium into corals, aposymbiotic polyps were mixed with different Symbiodinium strains (40 000 cells ml−1). The number of Symbiodinium cells was measured with a Thoma hemocytometer (SLGC, Tokyo, Japan). Infection of coral polyps by Symbiodinium was evaluated by examining Symbiodinium cells (brownish color) using stereomicroscope (Leica M165C) fitted with a digital camera (Leica MC120HD). Examinations were carried out in fresh seawater to avoid counting the Symbiodinium cells in the media. Polyps were then replaced into the original seawater containing Symbiodinium cells. Polyps were replaced in fresh seawater media containing the Symbiodinium strains (40 000 cells ml−1) every 2 days.
Symbiodinium genotyping
Total DNA was isolated from approximately 5 × 105 cultured Symbiodinium cells using the DNeasy Plant MiniKit (Qiagen, Hilden, Germany). The complete internal transcribed spacer 2 (ITS2) region was amplified using Platinum Taq DNA Polymerase High fidelity (Invitrogen, Carlsbad, CA, USA) using the primers ITSintfor2 (LaJeunesse, 2002) and ITS2rev (Apprill and Gates, 2007). PCR conditions were as described in Apprill and Gates (2007). Amplified products were purified with the QIAquick PCR Purification Kit (Qiagen) and ligated into pGEM-T Easy (Promega, Madison, WI, USA). Plasmids containing inserts were isolated using the QIAGEN Plasmid Mini Kit (Qiagen) and amplified using illustra TempliPhi (GE Healthcare, Piscataway, NJ, USA). DNA was sequenced (6–20 clones per culture) using Big Dye Terminator v. 3.1 (Applied Biosystems, Foster City, CA, USA) and reactions were run on an ABI 3730 sequencer at the Biomolecular Resource Facility (John Curtin School of Medical Research, Australian National University, Acton, ACT, Australia). Sequences were analyzed using DNASTAR (Lasergene, Madison, WI, USA) and MacVector (Accelrys, San Diego, CA, USA) and assigned to clades by performing BLAST searches against the ITS2 sequence database from GeoSymbio (Franklin et al., 2012) or GenBank.
Statistical analysis
The data for Symbiodinium cell size (Figure 1) was analyzed by one-way analysis of variance between the three size groups after the normality was confirmed by Chi-Square Goodness-of-Fit test and the homoscedasticity by Bartlett’s test. When a significant difference was established, Scheffe’s test was employed to determine which group(s) differed. The data of symbiont infectivity (Figure 2c) and symbiont cell size in corals (Supplementary Figure S4) were analyzed between the three size groups using Kruskal–Wallis test. Where a significant difference was established, Steel–Dwass test was employed to determine which group(s) differed.
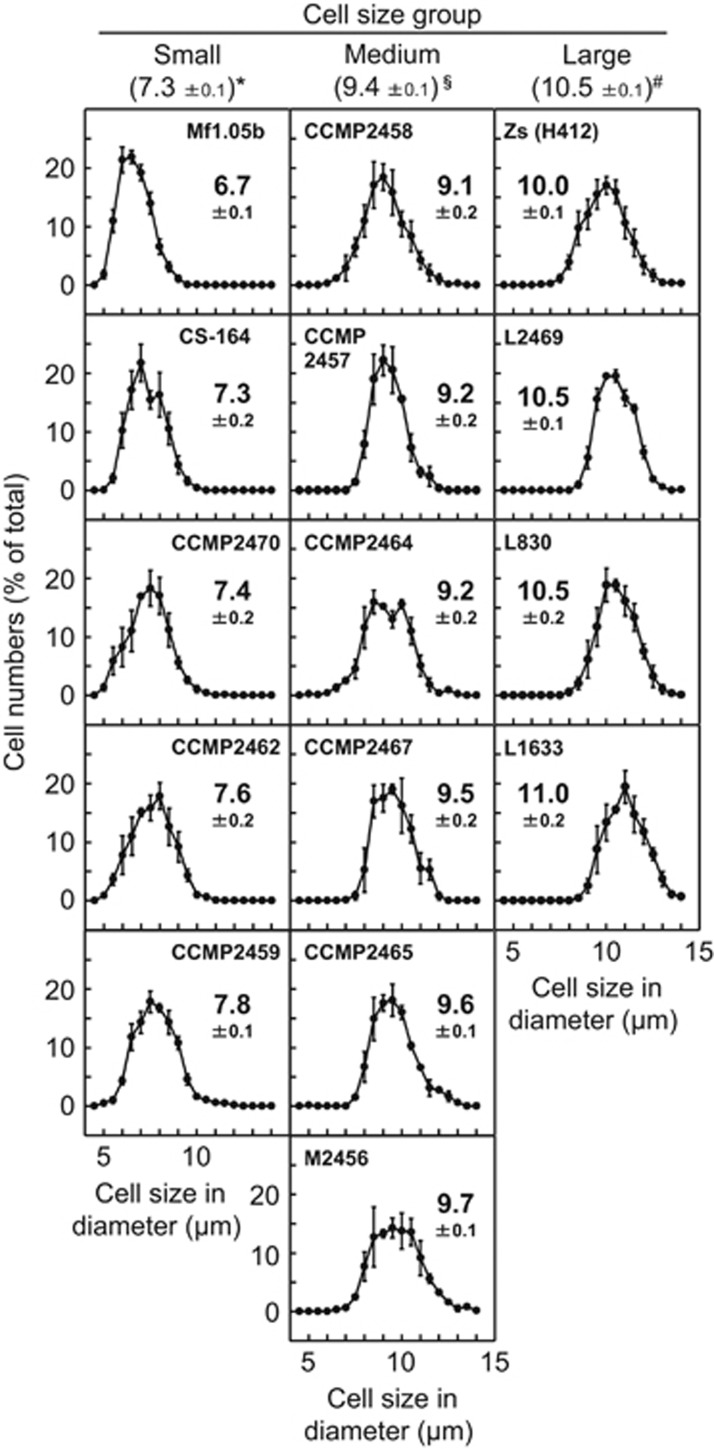
Figure 1.
Distribution of cell size in cultured Symbiodinium strains. The diameter of individual cells of each strain was measured with an automated cell counter (>100 cells in each measurement) and plotted as a percentage of the total sampled population. Numbers besides the plot in each panel show the average cell diameter (μm)±s.e. (n=3). Numbers below the Symbiodinium cell size group headings show the average cell diameter (μm)±s.e. for all strains in that group. Different symbols (*, § and #) indicate significant difference (P<0.01) between groups.
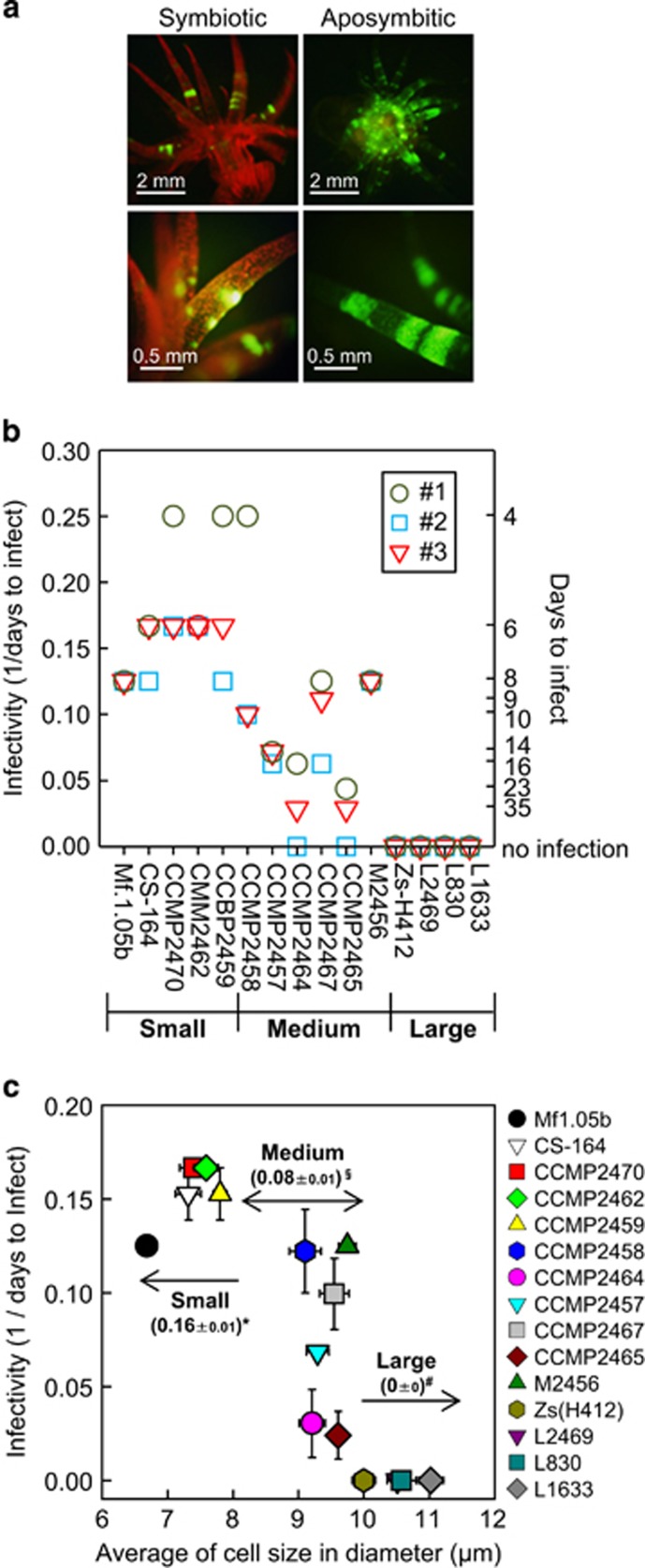
Figure 2.
Infection of different Symbiodinium strains into Aiptasia. (a) Fluorescence stereomicroscope images of Aiptasia anemones with (symbiont) and without (aposymbiont) symbiotic algae Symbiodinium. Red fluorescence is from the chlorophyll in Symbiodinium, while the autofluorescence of Aiptasia is green. (b) Days to infect (the number of days until infection was verified) and infectivity (1/days to infect) are shown on the right and left y axes, respectively. An Aiptasia polyp was said to be infected when >30 foci of Symbiodinium cells could be seen within a tentacle. Three independent experiments (#1, #2 and #3) were carried out in each Symbiodinium strain. (c) Relationship between Symbiodinium cell size and infectivity. Numbers in the panel show the average infectivity (1/days to infect)±s.e. of each size group. Different symbols (*, § and #) indicate significant difference (P<0.01) between groups. Error bars, ±s.e. (n=3).
Results
Large-sized Symbiodinium strains failed to infect Aiptasia
We used cultured Symbiodinium strains isolated from different host taxa, including scleractinian coral, sea anemone, octocoral, jellyfish, zoanthid and giant clams (Supplementary Table S1). Symbiodinium cell size (diameter) was measured using an automatic cell counter. As cell division generally occurs during the dark period (Fitt and Trench, 1983), cell size measurements were conducted in the middle of the light period to avoid counting dividing cells. The average Symbiodinium cell size differed among strains and ranged from 6.7 to 11.0 μm (Figure 1). The maximum and minimum cell sizes also differed among strains but scaled with their average cell size, that is, the maximum and minimum cell sizes were smaller in the smaller Symbiodinium strains (Figure 1). In this study, we classified Symbiodinium strains into three groups based on their average cell diameter: small (<8.0 μm, n=5), medium (8.0–10.0 μm, n=6), and large (>10.0 μm, n=4; Figure 1). This cell size classification was consistent for both the motile (with flagella) and vegetative life stages (Supplementary Figure S1).
We tested the infectivity of each Symbiodinium strain using artificially bleached aposymbiotic Aiptasia anemones. After bleaching, Aiptasia lost all Symbiodinium-associated red chlorophyll fluorescence and showed only its intrinsic green fluorescence (Figure 2a). To test infection efficiency, an individual Aiptasia polyp was separately exposed to a single Symbiodinium strain. Infection by Symbiodinium was monitored by observing the recovery of chlorophyll fluorescence in the host over 35 days (Supplementary Figure S2). An Aiptasia polyp was said to be infected when >30 foci of Symbiodinium cells could be seen within an Aiptasia tentacle. Infection follows the uptake of Symbiodinium cells into the gastrovascular cavity (stomach). All Symbiodinium strains were taken into the stomach within 2 days after inoculation. However, infection was achieved by only 11 of the 15 Symbiodinium stains tested (Figure 2b). The time taken to infect varied considerably between Symbiodinium strains, ranging from 5 to 35 days, consistent with a previous report (Belda-Baillie et al., 2002). Additionally, under our experimental conditions, infection by Symbiodinium CCMP2464 and CCMP2465 was very slow and was only achieved in two of the three replicate experiments (Figure 2b). Aiptasia incubated under the same experimental conditions without Symbiodinium remained bleached after 35 days (Supplementary Figure S2), indicating that there was no Symbiodinium contamination in our bleached polyps or in the seawater.
We next examined the relationship between infectivity and Symbiodinium cell size (Figure 2c). Infectivity was very high for all small Symbiodinium strains while it was lower and considerably more varied for Symbiodinium strains of medium size (Figure 2c). Importantly, all of the Symbiodinium strains that were unable to infect Aiptasia were large (>10 μm; Figure 2c). These results suggest that Symbiodinium cell size is inversely related to infectivity into Aiptasia anemone, that is, the larger the cell size, the lower the infectivity.
Size-dependent uptake of artificial microspheres into host cells
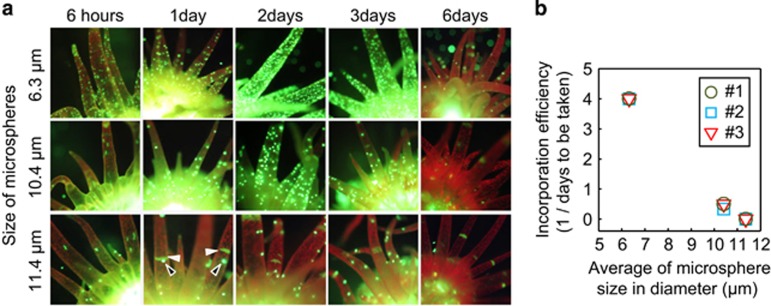
To determine whether cell size is a determinant of symbiont specificity in Aiptasia, we monitored the incorporation of different-sized yellow-green fluorescent microspheres (6.3±0.18, 10.4±0.25, and 11.4±0.30 μm in diameter) into Aiptasia cells (Figure 3). In each experiment, approximately 12 000 microspheres were fed directly into the mouth of individual Aiptasia already harboring Symbiodinium (n=3) and the number of microspheres located in tentacles was monitored for 6 days (Figures 3a and b). An individual Aiptasia polyp was said to have incorporated the microspheres when >30 could be seen within a tentacle. All three Aiptasia polyps tested successfully incorporated 6.3 and 10.4 μm microspheres in 6 h and 2–3 days, respectively. Compared with our infectivity assay results, the microspheres were taken up much faster than the Symbiodinium cells. This is possibly because the microspheres were fed directly into the mouth of the host and were therefore quickly and abundantly taken up into the gastrovascular cavity. Importantly, none of the Aiptasia polyps tested with the 11.4 μm microspheres reached the threshold needed to ensure that they had incorporated the microspheres, although a small number were seen in the tentacles (Figure 3a). Interestingly, all microspheres seen in the tentacles were completely expelled from the polyps after 6 days (Figure 3a). This result indicates that microspheres are not able to be retained in host cells.
Figure 3.
Incorporation of microspheres of different sizes into Aiptasia. (a) Fluorescence stereomicroscopic images of Aiptasia anemones after introducing yellow-green fluorescent microspheres of different sizes (6.3±0.18, 10.4±0.25, and 11.4±0.3 μm in diameter). Microspheres of each size were separately fed into the mouths of anemones and their presence in tentacles was monitored for 6 days. White and black arrow heads indicate autofluorescence of Aiptasia and microspheres, respectively. (b) Relationship between incorporation efficiency (1/the number of days until incorporation was verified) and average microsphere size. An Aiptasia polyp was said to have incorporated microspheres when >30 foci could be seen within a tentacle. Three independent experiments (#1, #2 and #3) were carried out in each size.
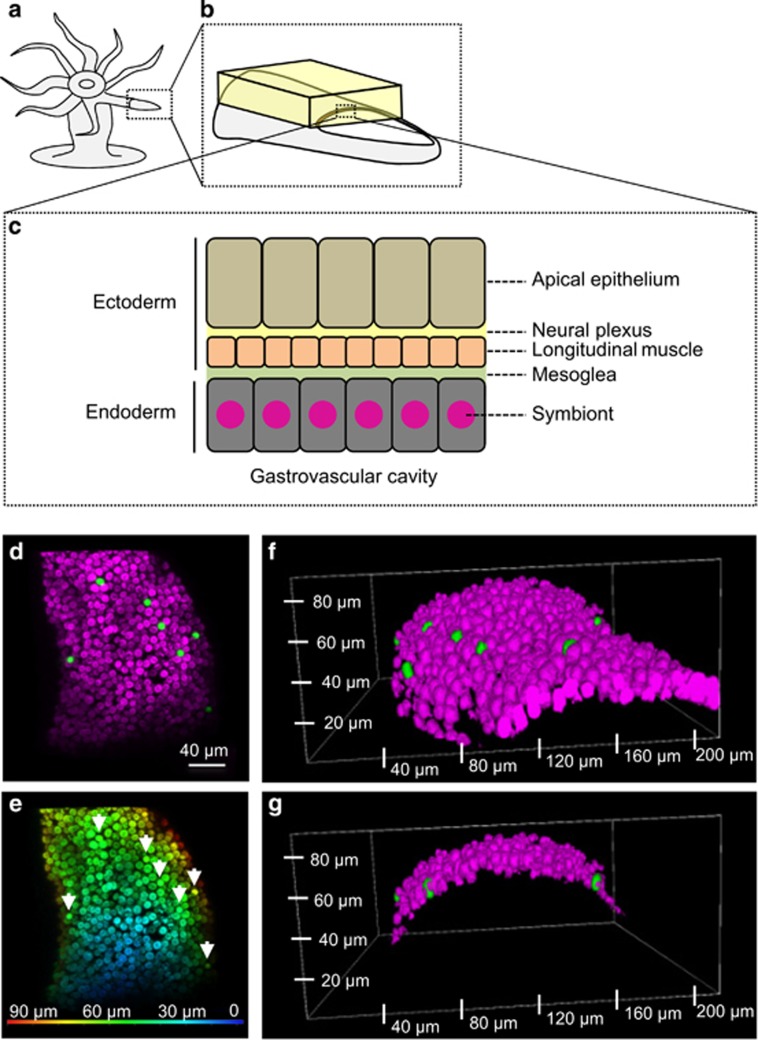
To determine whether the microspheres seen in the tentacles resided within the host cells or the gastrovascular cavity, we looked at the localization of 6.3 μm microspheres with respect to Symbiodinium cells (Figure 4). Aiptasia tentacles have a single layer of endoderm cells that face the gastrovascular cavity and harbor the Symbiodinium symbionts (Figures 4a–c). In our experiment, the fluorescent microspheres were found in the same layer as the symbiont, interspersed between Symbiodinium cells (Figures 4d–g). These results indicated that host endodermal cells were able to take up small microspheres in a similar manner to small Symbiodinium with both localizing to the endodermal cell layer, whereas larger microspheres were inefficiently taken into host cells.
Figure 4.
Incorporation of microspheres into Aiptasia cells. (a–c) Illustration of Aiptasia sample used. (b) The yellow cuboid highlights the volume of tentacle imaged in subsequent panels. (d–g) Confocal microscopic images of an Aiptasia tentacle containing Symbiodinium (magenta) and small-sized (6.3±0.18) microspheres (green). (d) 3D reconstruction of the cuboid seen from the surface of the tentacle. (e) 3D visualization of the same cuboid showing the depth (μm from the surface) of each symbiont or microsphere (arrows). (f) 3D reconstruction of the tentacle (side view). (g) 3D reconstruction cut through two microspheres (side view).
The maximum threshold of symbiont cell size differs among coral species
In our study, all large-sized Symbiodinium strains failed to infect Aiptasia. These strains were originally isolated from a range of host organisms (Supplementary Table S1), including sea anemones (Cyphastrea gigantea, Aiptasia pallida and Aiptasia pulchella) and zoanthids (Zoanthus sociatus) (LaJeunesse, 2001; Santos et al., 2001). However, it cannot be determined whether these Symbiodinium strains were residing within host cells or rather were present on the surface of the organisms at the time of isolation. Nevertheless, some coral species (for example, Pocillopora sp.) have been shown to harbor large-sized Symbiodinium phylotypes (Domotor and Delia, 1986). It is therefore conceivable that the maximum threshold for symbiont cell size differs among host species and that the infectivity of the symbiont is related to both the cell size of the symbiont and the maximum size threshold of the host.
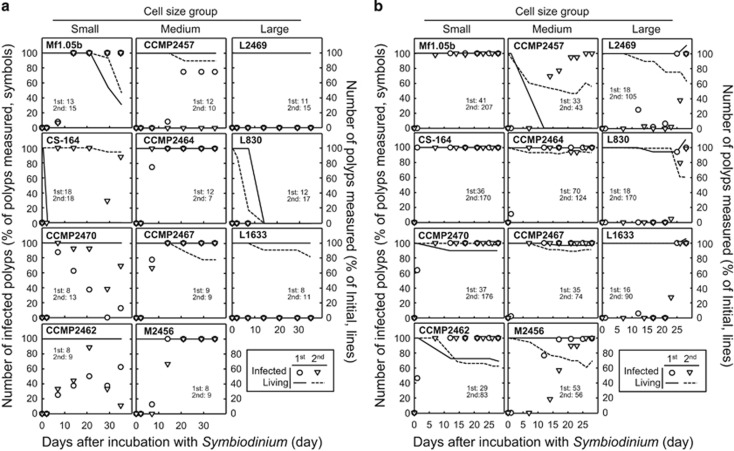
To test our hypothesis, we examined the infectivity of 11 Symbiodinium strains of different cell size using aposymbiotic juvenile polyps from two coral species; A. tenuis and C. serailia. These coral species were chosen as they do not undergo vertical transmission and therefore aposymbiont juvenile polyps can be readily obtained in the laboratory from collected egg–sperm bundles. Individual polyps of each coral species were incubated with a single Symbiodinium strain and infection was visually monitored over 30 days for C. serailia and 35 days for A. tenuis (Supplementary Figure S3). In A. tenuis, 8 of the 11 Symbiodinium strains tested could infect the juvenile polyps (Figure 5a). The three remaining Symbiodinium strains were all large sized. Two of these strains failed to infect after 35 days, and the third one (L830) caused all the polyps to die after 14 days (Figure 5a). These results demonstrated that symbiont cell size also influenced infectivity in corals. In C. serailia, all Symbiodinium strains tested were able to infect the host, including the large-sized strains, albeit their infection being much slower than that of small- and medium-sized strains (Figure 5b). Total infection of all juvenile polyps was achieved in 7 days, 7–25 days, and 25–28 days for small-, medium- and large-sized strains, respectively (Figure 5b). As each Symbiodinium culture contained a range of cell sizes (Figure 1), we isolated symbionts from infected C. serailia and confirmed the presence of the largest cells of the large-sized Symbiodinium strains in the host (Supplementary Figure S4).
Figure 5.
Infection of different Symbiodinium strains into corals. Aposymbiotic juvenile polyps of (a) A. tenuis and (b) C. serailia were incubated with different Symbiodinium strains and monitored for 35 and 30 days, respectively. Number of infected polyps is shown on the left y axes (percentage of total polyps measured, symbols). Number of polyps measured at each time point (percentage of initial, lines) is shown on the right y axes. Only healthy looking polyps were included in the measurements at each time point. A coral polyp was said to be infected when >30 foci of Symbiodinium cells could be seen within a tentacle. Each experiment was carried out twice using two set of polyps in different containers (microplates) and each result is shown separately. First experiment, closed symbols and solids line. Second experiment, open symbols, dashed line. The initial number of polyps used for the first and second experiment is shown in each panel.
Differences in cell size among Symbiodinium clades
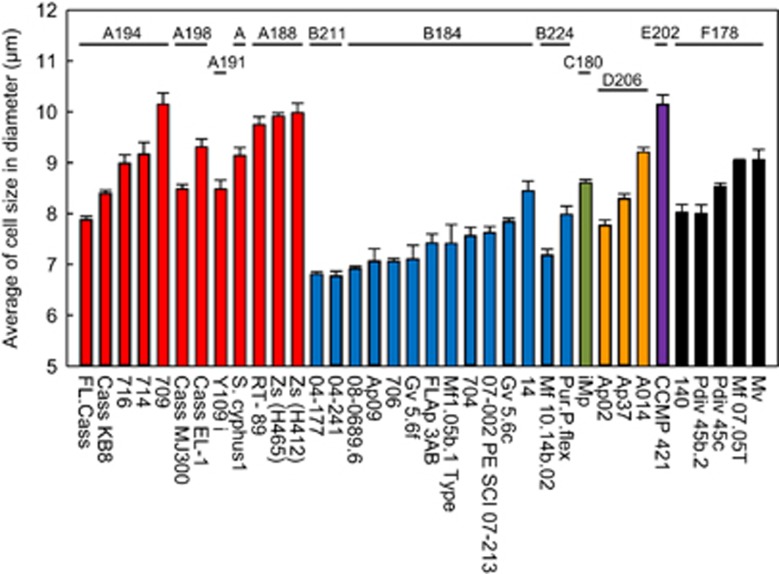
Strikingly, among the Symbiodinium strains used in our infection study, all of the small-sized strains were from clade B, and all of the medium- and large-sized strains were from clade A. This observation supports the previous hypothesis that Symbiodinium cell size is strongly correlated with Symbiodinium genotype (LaJeunesse, 2001; LaJeunesse et al., 2005). To further examine this postulate, we measured the cell size of 12 different clade A and 14 different clade B Symbiodinium strains of known subclade based on chloroplast large subunit (cp23S)-rDNA genotyping (Figure 6). The average cell size differed among Symbiodinium strains of the same clade: ranging from 7.9 to 10.1 μm in clade A and from 6.7 to 8.5 μm in clade B. Furthermore, the cell size significantly differed among Symbiodinium strains of the same subclade (cp23S-rDNA genotype); for instance, within the A194 subclade five phylotypes were measured with sizes ranging from 7.9 to 10.1 μm. Overall, however, clade A Symbiodinium strains were generally larger than clade B Symbiodinium strains (Figure 6). Thus our results suggest that the infectivity of different Symbiodinium strains can be inferred from their genotype. This hypothesis is compatible with previous results showing that clade B (type B1) Symbiodinium infect diverse coral species (LaJeunesse, 2002), whereas clade A (type A2) Symbiodinium have a high selectivity toward host species (LaJeunesse, 2001). Average cell size also varied between Symbiodinium strains from other clades (Figure 6), but their infectivity potential remains to be tested.
Figure 6.
Distribution of Symbiodinium cell size among different phylotypes. The average cell size (diameter) of 36 cultured Symbiodinium strains was measured with an automated cell counter. Details of the cp23S-rDNA phylotypes (A194, A198, A191, A188, B211, B184, B224, C180, D206, E202 and F178) were obtained from the culture collection center (Buffalo Undersea Reef Research Culture Collection). Error bars, ±s.e. (n=3).
Discussion
Symbiont specificity for a host has been proposed to be determined by the combination of cell-surface proteins on both the host cnidarian and the symbiont Symbiodinium during phagocytosis. However, in the present study, we found the infectivity of Symbiodinium strains toward both Aiptasia (Figure 2) and A. tenuis (Figure 5a) correlated with symbiont cell size. Furthermore, experiments testing the incorporation of microspheres into host cells confirmed that the size of the particle influences uptake, while also suggesting that the presence of specific cell surface proteins may not be necessary for this process (Figure 3). We therefore conclude that the infectivity potential of Symbiodinium strains is influenced by their cell size. This result is consistent with our general understanding of the mechanism of phagocytosis, which is known to be affected by the size of the material to be engulfed (Tabata and Ikada, 1988; Champion et al., 2008). However, we still cannot exclude that large-sized Symbiodinium cells may be less well circulated throughout the gastrovascular cavity and therefore have a lower chance of being taken up by the host cells. It should be noted that, in our study, Symbiodinium CCMP2470 could quickly infect A. tenuis but a significant number of the symbionts dissociated from the host after 35 days (Figure 5a). Furthermore, the fluorescent microspheres that were taken into host cells were all expelled in 6 days (Figure 3a). These results suggest that at least one additional mechanism (for example, membrane surface molecules, cell division or the production of energy) is important to engage and maintain a symbiotic relationship and that creating beneficial symbiosis requires more than the sole ability of entering the host cell (Davy et al., 2012).The relationship between symbiont specificity for a host and symbiont cell size has been examined previously in experiments using Aiptasia tagetes (Schoenberg and Trench, 1980) and the upside-down jellyfish Cassiopeia xamachana (Fitt, 1985). In the study using A. tagetes, one of the smallest Symbiodinium strains tested failed to infect. Conversely, in the C. xamachana study, one of the large Symbiodinium strains tested succeeded in infecting. Therefore, while previous data showed a similar trend to that being reported here, a single outlier in each experiment meant there was no clear correlation between infection and size; rather it was suggested that species specificity is not related to symbiont cell size (Schoenberg and Trench, 1980). The reason why these two studies did not find a definitive correlation between infection and size is unclear, but it indicates that additional factors may also affect species specificity and infectivity.
It is conceivable that rapid procurement of new symbionts from the environment could be important for corals to adapt to environmental changes. Previous studies have demonstrated that infection speed differs among Symbiodinium strains (Belda-Baillie et al., 2002). However, the mechanism controlling the infection speed remained unclear. In the present study, we found that infection speed was related to symbiont cell size in both sea anemone (Figure 2c) and corals (Figure 5). This finding was supported by experimental data using microspheres of different sizes; smaller microspheres were taken into host cells significantly faster than larger ones (Figures 3a and b). However, cell size might not be the only factor determining the infection speed, and it is conceivable that cell number (Colley and Trench, 1983) and cell mortality may also influence infection speed. Nevertheless, our findings suggest that corals living in environments maintaining abundant and diverse small Symbiodinium strains, for example, clade B Symbiodinium, may have a greater chance of quickly recruiting new symbionts and therefore adapting to environmental changes.
Our results demonstrated that the maximum threshold of symbiont cell size differs between the two different coral species tested (Figure 5). It is still uncertain what determines the maximum threshold of symbiont cell size in each host species. However, as the endodermal cell size vary from 10 to 25 μm in corals and other anthozoans (Davy et al., 2012), the endodermal cell size in the host could be associated with the maximum threshold of symbiont cell size. We still cannot exclude that differences in the maximum threshold of symbiont cell size is related to the ability of each host to circulate large-sized Symbiodinium cells throughout the gastrovascular cavity.
In coral–Symbiodinium associations, some coral species (generalists) harbor diverse Symbiodinium phylotypes, whereas others (specialists) do not (LaJeunesse, 2002; Baker, 2003). Moreover, some Symbiodinium phylotypes (generalists) can infect diverse host species and others (specialists) do not (LaJeunesse, 2002; Baker, 2003). Our findings suggest a possible selection mechanism of infection that can explain these observations: host species with a higher maximum threshold of symbiont cell size may acquire more diverse Symbiodinium phylotypes, whereas host species with a lower threshold may acquire a more limited range of phylotypes (that is, small-sized Symbiodinium phylotypes). Furthermore, small-sized Symbiodinium phylotypes may infect many host species, whereas large-sized Symbiodinium phylotypes may exhibit a more limited host range (that is, only hosts with a higher maximum threshold for symbiont cell size). However, not all Symbiodinium phylotypes able to infect a host can maintain a symbiotic relationship, for instance, when the environmental conditions are not suitable. Therefore, the combinations of host and symbiont seen in the field are likely to be determined by multiple factors, rather than infectivity alone.
Biologically enriched coral reef ecosystems sustained by healthy coral–Symbiodinium symbiotic associations have been in decline over the past few decades due, at least partially, to thermally induced coral bleaching (Hughes et al., 2003). This decline is expected to continue if global climate change and warming continues as predicted (Hughes et al., 2003). The sensitivity of corals to bleaching under increased seawater temperature differs among coral species (Marshall and Baird, 2000; Loya et al., 2001). However, the bleaching sensitivity is influenced by the dominant in situ Symbiodinium phylotype within the coral (Glynn et al., 2001), suggesting that corals may at least temporarily acclimatize to a warm environment if they possess suitable (that is, heat tolerant) Symbiodinium phylotypes (Baker, 2001; Baker et al., 2004; Lewis and Coffroth, 2004; Rowan, 2004; Berkelmans and van Oppen, 2006; Jones et al., 2008; Howells et al., 2012). As the diversity of symbionts available to each coral host depends on their maximum symbiont cell size threshold (Figure 5), corals with a higher maximum threshold may have more chance to associate with suitable symbionts and therefore adapt to increased seawater temperature.
Acknowledgments
We dedicate this paper to the memory of Sylvain Forêt, whose scientific contribution to coral research, positive spirit and wit will be greatly missed. We thank Mary Alice Coffroth and Andrew Baird for their comments on this study. We also thank William Leggat for his advice on Symbiodinium genotyping, Daryl Webb and Adrienne Hardham for help with microscopy, Harpreet Vohra and Michael Devoy for help with cell sorting, Teresa Neeman for help with statistical analyses, Sara Milward for proof reading and Murray Badger for supporting our project. We also thank the Center of Advanced Microscopy of the Australian National University for access to their confocal microscope. This work was supported by an Australian Research Council Discovery Project grant (no. DP110102364 to ST) and a Grants-in-Aid for Scientific Research grant from the ministry of Education, Culture, Sports, Science and Technology of Japan (no. 15K14611 to ST).
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Apprill AM, Gates RD. (2007). Recognizing diversity in coral symbiotic dinoflagellate communities. Mol Ecol 16: 1127–1134. [DOI] [PubMed] [Google Scholar]
- Baker AC. (2001). Reef corals bleach to survive change. Nature 411: 765–766. [DOI] [PubMed] [Google Scholar]
- Baker AC. (2003). Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34: 661–689. [Google Scholar]
- Baker AC, Starger CJ, McClanahan TR, Glynn PW. (2004). Corals' adaptive response to climate change. Nature 430: 741–741. [DOI] [PubMed] [Google Scholar]
- Belda-Baillie CA, Baillie BK, Maruyama T. (2002). Specificity of a model cnidarian-dinoflagellate symbiosis. Biol Bull 202: 74–85. [DOI] [PubMed] [Google Scholar]
- Berkelmans R, van Oppen MJH. (2006). The role of zooxanthellae in the thermal tolerance of corals: a 'nugget of hope' for coral reefs in an era of climate change. Proc R Soc B Biol Sci 273: 2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Walker A, Mitragotri S. (2008). Role of particle size in phagocytosis of polymeric microspheres. Pharm Res 25: 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley NJ, Trench RK. (1983). Selectivity in phagocytosis and persistence of symbiotic algae by the scyphistoma stage of the jellyfish Cassiopeia xamachana. Proc R Soc B-Biol Sci 219: 61–82. [DOI] [PubMed] [Google Scholar]
- Davy SK, Allemand D, Weis VM. (2012). Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Biol Rev 76: 229–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domotor SL, Delia CF. (1986). Cell-size distributions of zooxanthellae in culture and symbiosis. Biol Bull 170: 519–525. [Google Scholar]
- Fitt WK, Trench RK. (1983). The relation of diel patterns of cell division to diel patterns of motility in the symbiotic dinoflagellate Symbiodinium microadriaticum Freudenthal in culture. New Phytol 94: 421–432. [Google Scholar]
- Fitt WK. (1985). Effect of different strains of the zooxanthella Symbiodinium microadriaticum on growth and survival of their coelenterate and molluscan hosts, Vol. 6. In: Delesalle B, Galzin R and Salvat B (eds). Proceeding of the 5th International Coral Reef Congress; 27 May–1 June 1985; Tahiti, French Polynesia. Antenne Museum-EPHE: Moorea, French Polynesia, pp 131–136.
- Franklin EC, Stat M, Pochon X, Putnam HM, Gates RD. (2012). GeoSymbio: a hybrid, cloud-based web application of global geospatial bioinformatics and ecoinformatics for Symbiodinium-host symbioses. Mol Ecol Resour 12: 369–373. [DOI] [PubMed] [Google Scholar]
- Glynn PW, Maté JL, Baker AC, Calderon MO. (2001). Coral bleaching and mortality in Panama and Ecuador during the 1997-1998 El Niño-Southern oscillation event: spatial/temporal patterns and comparisons with the 1982-1983 event. Bull Mar Sci 69: 79–109. [Google Scholar]
- Hambleton EA, Guse A, Pringle JR. (2014). Similar specificities of symbiont uptake by adults and larvae in an anemone model system for coral biology. J Exp Biol 217: 1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, van Oppen MJH. (2012). Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat Clim Chang 2: 116–120. [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C et al. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301: 929–933. [DOI] [PubMed] [Google Scholar]
- Iwao K, Fujisawa T, Hatta M. (2002). A cnidarian neuropeptide of the GLWamide family induces metamorphosis of reef-building corals in the genus Acropora. Coral Reefs 21: 127–129. [Google Scholar]
- Jones AM, Berkelmans R, van Oppen MJH, Mieog JC, Sinclair W. (2008). A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc R Soc B Biol Sci 275: 1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzie RA. (1974). Experimental infection of aposymbiotic gorgonian polyps with zooxanthellae. J Exp Mar Biol Ecol 15: 335–345. [Google Scholar]
- LaJeunesse TC. (2001). Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the its region: In search of a ‘species’ level marker. J Phycol 37: 866–880. [Google Scholar]
- LaJeunesse TC. (2002). Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141: 387–400. [Google Scholar]
- LaJeunesse TC, Lambert G, Andersen RA, Coffroth MA, Galbraith DW. (2005). Symbiodinium (Pyrrhophyta) genome sizes (DNA content) are smallest among dinoflagellates. J Phycol 41: 880–886. [Google Scholar]
- Lewis CL, Coffroth MA. (2004). The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science 304: 1490–1492. [DOI] [PubMed] [Google Scholar]
- Lin KL, Wang JT, Fang LS. (2000). Participation of glycoproteins on zooxanthellal cell walls in the establishment of a symbiotic relationship with the sea anemone, Aiptasia pulchella. Zool Stud 39: 172–178. [Google Scholar]
- Little AF, van Oppen MJH, Willis BL. (2004). Flexibility in algal endosymbioses shapes growth in reef corals. Science 304: 1492–1494. [DOI] [PubMed] [Google Scholar]
- Logan DDK, LaFlamme AC, Weis VM, Davy SK. (2010). Flow-cytometric characterisation of the cell-surface glycans of symbiotic dinoflagellates (Symbiodinium spp.). J Phycol 46: 525–533. [Google Scholar]
- Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R. (2001). Coral bleaching: the winners and the losers. Ecol Lett 4: 122–131. [Google Scholar]
- Marshall PA, Baird AH. (2000). Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19: 155–163. [Google Scholar]
- Meints RH, Pardy RL. (1980). Quantitative demonstration of cell surface involvement in a plant-animal symbiosis: lectin inhibition of reassociation. J Cell Sci 43: 239–251. [DOI] [PubMed] [Google Scholar]
- Pochon X, Gates RD. (2010). A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai'i. Mol Phylogenet Evol 56: 492–497. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lanetty M, Chang SJ, Song JI. (2003). Specificity of two temperate dinoflagellate-anthozoan associations from the north-western Pacific Ocean. Mar Biol 143: 1193–1199. [Google Scholar]
- Rowan R. (2004). Thermal adaptation in reef coral symbionts. Nature 430: 742–742. [DOI] [PubMed] [Google Scholar]
- Santos SR, Taylor DJ, Coffroth MA. (2001). Genetic comparisons of freshly isolated versus cultured symbiotic dinoflagellates: implications for extrapolating to the intact symbiosis. J Phycol 37: 900–912. [Google Scholar]
- Schoenberg DA, Trench RK. (1980). Genetic variation in Symbiodinium (=Gymnodiniummicroadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. III. Specificity and infectivity of Symbiodinium microadriaticum. Proc R Soc B Biol Sci 207: 445–460. [Google Scholar]
- Tabata Y, Ikada Y. (1988). Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials 9: 356–362. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Whitney S, Itoh S, Maruyama T, Badger M. (2008). Heat stress causes inhibition of the de novo synthesis of antenna proteins and photobleaching in cultured Symbiodinium. Proc Natl Acad Sci USA 105: 4203–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Yoshioka-Nishimura M, Nanba D, Badger MR. (2013). Thermal acclimation of the symbiotic alga Symbiodinium spp. alleviates photobleaching under heat stress. Plant Physiol 161: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernov D, Gorbunov MY, de Vargas C, Yadav SN, Milligan AJ, Häggblom M et al. (2004). Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA 101: 13531–13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trench RK. (1979). Cell biology of plant-animal symbiosis. Annu Rev Plant Physiol Plant Molec Biol 30: 485–531. [Google Scholar]
- Weis VM, Reynolds WS, deBoer MD, Krupp DA. (2001). Host-symbiont specificity during onset of symbiosis between the dinoflagellates Symbodinium spp. and planula larvae of the scleractinian coral Fungia scutaria. Coral Reefs 20: 301–308. [Google Scholar]
- Wood-Charlson EM, Hollingsworth LL, Krupp DA, Weis VM. (2006). Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell Microbiol 8: 1985–1993. [DOI] [PubMed] [Google Scholar]
- Yellowlees D, Rees TAV, Leggat W. (2008). Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ 31: 679–694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.