Abstract
Methamphetamine (Meth) abuse not only increases the risk of human immunodeficiency virus-1 (HIV-1) infection, but exacerbates HIV-1-associated neurocognitive disorders (HAND) as well. The mechanisms underlying the co-morbid effect are not fully understood. Meth and HIV-1 each alone interacts with microglia and microglia express voltage-gated potassium (KV) channel KV1.3. To understand whether KV1.3 functions an intersecting point for Meth and HIV-1, we studied the augment effect of Meth on HIV-1 glycoprotein 120 (gp120)-induced neurotoxic activity in cultured rat microglial cells. While Meth and gp120 each alone at low (subtoxic) concentrations failed to trigger microglial neurotoxic activity, Meth potentiated gp120-induced microglial neurotoxicity when applied in combination. Meth enhances gp120 effect on microglia by enhancing microglial KV1.3 protein expression and KV1.3 current, leading to an increase of neurotoxin production and resultant neuronal injury. Pretreatment of microglia with a specific KV1.3 antagonist 5-(4-Phenoxybutoxy)psoralen (PAP) or a broad spectrum KV channel blocker 4-aminopyridine (4-AP) significantly attenuated Meth/gp120-treated microglial production of neurotoxins and resultant neuronal injury, indicating an involvement of KV1.3 in Meth/gp120-induced microglial neurotoxic activity. Meth/gp120 activated caspase-3 and increased caspase-3/7 activity in microglia and inhibition of caspase-3 by its specific inhibitor significantly decreased microglial production of TNF-α and iNOS and attenuated microglia-associated neurotoxic activity. Moreover, blockage of KV1.3 by specific blockers attenuated Meth/gp120 enhancement of caspase-3/7 activity. Taking together, these results suggest an involvement of microglial KV1.3 in the mediation of Meth/gp120 co-morbid effect on microglial neurotoxic activity via caspase-3 signaling.
Keywords: HIV protein, drug abuse, microglia, voltage-gated K channel, neurodegeneration
Introduction
Methamphetamine (Meth) abuse and human immunodeficiency virus (HIV) type 1 (HIV-1) infection are two major public health problems worldwide. Meth abuse not only increases the risk of HV-1 infection but potentiates HIV-1-associated neurotoxicity as well (Theodore et al., 2007; Theodore et al., 2006; Urbina and Jones, 2004). While a large body of research has investigated the individual effects on the brain, far less has focused on their synergistic influence. Increasing evidence indicates that Meth and HIV-1 appear to produce more severe neuropathology and neurocognitive deficits than each alone (Hoefer et al., 2015; Rippeth et al., 2004; Silverstein et al., 2011). Nevertheless, the mechanisms underlying the co-morbid effect of Meth and HIV-1 are not fully understood. Studies have shown that HIV-1 brain infection causes microglia (brain microglia and macrophages) activation and release of pro-inflammatory molecules leading to the development of HIV-1-associated neurocognitive disorders (HAND) (Gannon et al., 2011; Garvey et al., 2014; Kaul and Lipton, 2006a; Letendre, 2011). Meth abuse exacerbates HAND (Carey et al., 2006; Chana et al., 2006; Kesby et al., 2015b; Silverstein et al., 2012), and such an exacerbation is also associated with microglial neurotoxic activity (Cadet and Krasnova, 2007; Hauser and Knapp, 2014; Silverstein et al., 2011). Thus, microglia appears to be a common target for both Meth and HIV-1.
Microglia, the resident immune competent phagocytic cells in the brain, is the predominant cell type productively infected by HIV-1 (Lipton and Gendelman, 1995). A striking feature in an HIV-1-infected brain is microglial activation, and the activated microglia exhibits neurotoxic activity by releasing a variety of neurotoxic substances leading to neuronal injury and ultimate development of HAND. Multiple lines of evidence have also shown a robust activation of microglia following exposure of rodents to a neurotoxic regimen of Meth (Kuhn et al., 2008; LaVoie et al., 2004; Loftis and Janowsky, 2014). Meth acts on microglia and alters microglial activity and inflammatory signaling cascades, resulting in neuronal damage (Loftis and Janowsky, 2014; Silverstein et al., 2012). How Meth abuse potentiates HIV-1-associated microglial neurotoxic activity remains unclear.
It has been shown that microglia express several types of voltage-gated potassium (Kv) channels including inward rectifier Kir2.1 and outward rectifiers Kv1.5 and Kv1.3. Exposure to a variety of stimuli produces a characteristic pattern of up-regulation of Kv1.3 (Eder, 1998; Fischer et al., 1995; Norenberg et al., 1994; Schilling et al., 2000). Accumulating evidence indicates that microglial Kv1.3 plays an important role in switching microglia from a resting condition to an activation state (Farber and Kettenmann, 2005; Kotecha and Schlichter, 1999). Electrophysiological studies have revealed the presence of large outward rectifying K currents that has been speculated as a sign of microglia activation (Ilschner et al., 1996; Visentin et al., 1995; Visentin and Levi, 1997; Visentin et al., 2001). Activated microglia produces soluble pro-inflammatory molecules resulting in neuronal injury via a process requiring Kv1.3 activity since blockage of microglial Kv1.3 or decrease of Kv1.3 expression attenuates microglia-mediated neurotoxicity (Fordyce et al., 2005; Nutile-McMenemy et al., 2007). We hypothesize that Meth potentiates HIV-1-associated microglial neurotoxic activity via activation of Kv1.3. To test our hypothesis, we studied the co-morbid effects of Meth on HIV-1 envelope glycoprotein 120 (gp120)-associated microglial neurotoxic activity on primary rat neural cell cultures. Our results showed that application of Meth or gp120 each alone at a low concentration had no apparent effect on triggering microglial neurotoxic activity. When applied in combination, however, Meth significantly potentiated gp120-induced microglial neurotoxicity. The Meth potentiation of gp120-associated microglial neurotoxic activity was blocked by specific Kv1.3 channel blockers, suggesting an involvement of Kv1.3 in Meth-mediated potentiation.
Materials and Methods
Materials
HIV-1gp120 MN (product #1021-2) was purchased from Immunodiagnostics, Inc. (Woburn, MA). Aliquots of gp120 were kept as 100nM stock solution at −80°C. The stock solution was diluted to desired concentrations with artificial cerebrospinal fluid (ACSF) 2–5 min before test. (+)methamphetamine was purchased from Sigma (St. Louis, MO, Cat # M-8750) with DEA license # RX0374974. All chemicals, unless otherwise specified, were from Sigma.
Animals
Pregnant female Sprague-Dawley rats used for experiments were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed at constant temperature (22°C) and relative humidity (50%) under a regular light-dark cycle (light on at 7:00 AM and off at 5:00 PM) with free access to food and water. All animal use procedures were strictly reviewed by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center (IACUC No. 13-069-10-EP).
Isolation and culture of microglial and neuronal cells
Microglia and neurons were prepared from the cerebral cortex of postnatal 0–1 day old or 18-day old embryonic Sprague Dawley rats as described previously (Liu et al., 2012). In brief, rat cortical tissues were dissected in cold Hank’s Balanced Salt Solution (HBSS: Mediatech, Inc. Manassas, VA) and digested with 0.25% trypsin and 200 kunitz units/ml DNase (Sigma-Aldrich, St. Louis. MO) in 37 °C for 15 min. The digested tissues were then suspended in cold HBSS and filtrated through 100µM and 40µm pore cellular strainers (BD Bioscience, Durham, NC), respectively. For microglial culture, the isolated cells (25×106) were plated into T75 cm2 flasks in a high-glucose Dulbecco’s modified Eagle’s medium (DMEM) contained 10% fetal bovine serum (FBS), 1x glutaMAX, 1% penicillin/streptomycin (Life Technologies, Grand Island, NY), and 300ng/ml macrophage colony-stimulating factor (M-CSF) supplied by Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center. After 10 days in culture, the flasks were gently shaken and detached cells were collected and seeded on 35 mm2 (2.5×106 cells/dish), 60 mm2 (7.5×106 cells /dish) culture dishes, 12 well (1×106/well) or 96 well plates (0.4×106/well) based on the experimental requirements with M-CSF free DMEM. The suspensory glial cells were removed 1 h after seeding by changing culture media. The resultant cultures were 98–100% microglia as determined by staining with anti-Iba1 (Wako, Richmond, VA), a marker for microglia. For neuronal culture, the isolated cells were seeded in poly-D-lysine-coated plates at a density of 0.05×106 cells/well in 96-well plates, 0.2×106 cells/well in 12-well plates, or 1.0×106 cells/well in 6-well plates. Neuronal cultures were maintained at 37 °C for 10 days in neurobasal medium supplemented with 2% B27, 1% penicillin/streptomycin and 0.25% glutaMAX (all regeants from Life Technologies). The purity of neuronal cells (> 90%) was determined by staining with microtubule-associated protein-2 antibody (MAP-2: 1:1000, Chemicon, Temecula, CA).
Collection of microglial conditioned media
After detachment from flasks, microglial cells were placed in 6 well plates and cultured for 48 h and then treated with Meth or gp120 or both for additional 24 h. After washing three times with PBS, the cells were added with fresh neurobasal media and cultured for another 24 h. The culture supernatants (free of Meth and gp120) were then collected and stored at −80°C freezer for subsequent experimental use.
Electrophysiology
Whole-cell outward K+ currents were recorded from primary rat microglial cultures at room temperature. Pre-treated microglia were perfused with oxygenated (bubbled with 95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM) NaCl 150, KCl 4.5, CaCl2 2, MgCl2 1, HEPES 5, glucose 11, pH 7.3 (adjusted with NaOH) with an osmolarity of 310 mOsm. Patch-clamp electrodes, pulled from borosilicate glass capillaries (WPI, Sarasota, FL) had tip resistance of 4–6Ω when filled with pipette solution containing (mM): KCl 150, MgCl2, CaCl2 1, EGTA 11, HEPES 10, with pH of 7.3 adjusted with KOH. Voltage-dependent currents were evoked by voltage steps (600ms in duration) with the first step from a holding potential of −70mV to −150mV and then to +70mV in a 20mV increments. The seal resistance was 1–10 GΩ. Junction potentials were corrected, and the cell capacitance was compensated (~70%) in most cells. Current signals were amplified with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The current and voltage traces were displayed and recorded in a Dell computer using pClamp 10.1 data acquisition/analysis system. The outward K+ current density (pA/pF) was calculated by dividing the cell capacitance from the peak current generated by a voltage step.
Measurement of Nitric Oxide (NO) and Reactive Oxygen Species (ROS) productions
NO production was estimated by measuring the concentration of nitrite with Griess Reagent System according to the manufacturer’s instructions (Promega, Madison, WI). Supernatants (50µl) collected from pre-treated microglia were mixed with equal volume of the sulfanilamide solution for 10 min followed by 50µl of NED solution and continued incubation for 10 min at room temperature. The optical density of reactants was measured at 520nm and 540nm using an ELISA plate reader. All experiments were repeated at least three times. For analysis of ROS production, 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) kit was used (Life Technologies). Pretreated microglia were cultured with 2µM of CM-H2DCFDA for 1 h at 37 °C. Afterwards, microglia were mounted with ProLong Gold antifade reagent with 4’,6’-diamidino-2-phenylindol (DAPI) staining nuclei (Life Technologies) and visualized by Nikon fluorescent microscope in a 40x objective.
TUNEL staining and MTT assay
Neuronal damage was evaluated using In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, Indianapolis, IN). In brief, neurons growing on poly-D-lysine-coated coverslips (0.2×106 cells/well in a 12-well plate) were treated with conditional media collected from pre-treated microglia at 1:5 dilution for 24 h. Afterwards, neurons were fixed with 4% paraformaldehyde, followed by permeabilizing with 0.1% Triton X-100. The neurons were then processed for TUNEL staining (green) for 1 h at 37 °C and mounted with ProLong Gold antifade reagent with DAPI (Life Technologies). Cells were visualized by Nikon fluorescent microscope in a 40x objective. The percentage of damaged neurons was determined by TUNEL positive cells normalized by total cells. Neuronal damage was also assessed by MTT assay and a reduction of MTT colorimetry was considered as an indicator of cell damage or death. Pre-treated neurons were exposed to fresh neurobasal medium containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (500µg/ml) for 3 h. MTT solution was then replaced with 300µl of dimethyl sphingosine (DMSO, Sigma-Aldrich) for cell lyses, and the optical density (OD) of absorption was measured at 560 nm.
Western blot analysis
Membrane proteins were prepared using a Membrane Protein Extraction Kit (BioVision, Mountain View, CA) according to manufacturer’s instructions. Total proteins were isolated using a RIPA Buffer (Sigma-Aldrich). Twenty microgram membrane proteins or 30µg total proteins were separated by electrophoresis using 4–15% mini-protean TGX precast gel and transferred to polyvinyl difluoride (PVDF) membranes. Membranes were blocked with 5% dry milk in tris-buffered saline (TBS) (all products from Bio-Rad Laboratories, Hercules, CA) and probed overnight at 4 °C with primary-antibodies including rabbit polyclonal KV1.3 (1:100; Alomone Lab, Israel), anti-iNOS, anti-TNF-α, anti-IL-1β antibody (1:100, Abcam, Cambridge, MA), cleaved caspase-3 antibody, caspase-3 rabbit mAb (1:1000; Cell Signaling Technology, Danvers, MA), and anti-mouse β-actin monoclonal antibody (1:10,000, Sigma-Aldrich). Membranes were washed (4 times, 10 min each) in TBS with 0.2% Tween (TBS-T) and incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit, or anti-mouse secondary antibody (1:10,000, Jackson ImmunoResearch Laboratories, West Grove, PA) for 1h at RT. Labeled proteins were visualized by Pierce ECL Western Blotting Substrate (Thermo Scientific, Rockford, IL). Band densities of KV1.3, iNOS, TNF-α, IL-1β were normalized to their p-actin, and cleaved caspase-3 was normalized to caspase-3 in each sample.
Caspase-3/7 activity Assay
Caspase-3/7 activity was evaluated by Caspase-Glo 3/7 assay kit (Promega). In brief, control and pre-treated microglia in a white-walled 96-well luminometer plate were exposed to equal volume of enzyme or vehicle and incubated to equilibrium. After 30 min, 100µl of Caspase-Glo 3/7 reagents were added to each well and gently mixed. Cells were then continuously incubated at room temperature for 6 h or 24 h. The luminescent activity of Caspase-Glo 3/7 was read in a plate-reading luminometer (BioTek Instruments, Inc., Winooski, VT).
Statistical Analysis
Experimental data were expressed as mean ± S.D. unless otherwise indicated. Statistical analyses were performed on SAS® 9.4 using general linear model methods, especially (nested) ANOVA (two-way, three-way or four-way according to the structure of the data) followed by Dunnett’s test for comparing one treatment with the rest (Littell RC et al., 2002). The difference between groups was considered significant when p < 0.05.
Results
Meth enhances gp120-induced microglial neurotoxic activity
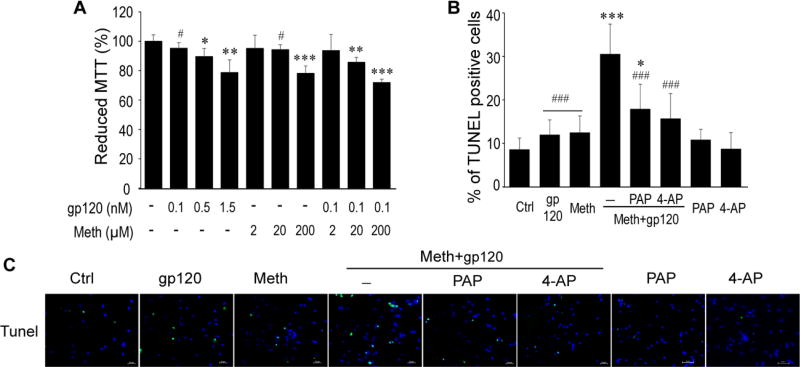
Ample evidence indicate that HIV-1-infected and immune-activated microglia release soluble, neurotoxic molecules leading to the pathogenesis of HAND. The incidence and severity of HAND are increased with concomitant use of Meth, suggesting that Meth may potentiate HIV-induced microglial neurotoxicity. To determine whether Meth potentiates HIV-associated microglial neurotoxic activity, we first collected the supernatants recovered from cultured rat microglia treated with gp120 or Meth at different concentrations, and then tested their effects on cultured rat cortical neurons via MTT assay. As analyzed by two-way ANOVA (gp120, F3,28=24.24, p<0.001; Meth, F3,28=425.20, p<0.001) and shown in Figure (Fig. 1A), addition of gp120 (0.1, 0.5, 1.5nM)- or Meth (2, 20, 200µM)-treated microglial supernatants (1:5 dilution, v/v) each alone to the neuronal cultures for 24 h produced a concentration-dependent decrease of the percentage of surviving neurons (as indicated by MTT reduction) to 95.21 ± 3.93% (p > 0.05, Dunnett’s test, the same in the following ), 89.54 ± 5.50% (p < 0.05) and 78.70 ± 8.61 %(p < 0.01), or to 95.07 ± 8.99% (p > 0.05), 94.25 ± 3.29% (p > 0.05), and 78.13 ± 5.04% (p < 0.01) of control (untreated), respectively. As gp120 0.1 nM and Meth 20µM had no significant effects on neuronal viability when applied alone, we examined if Meth 20µM could potentiate gp120 (0.1nM) effect of microglial neurotoxic activity. Also shown in Fig. 1A (far right), the supernatants recovered from microglia treated with Meth (20µM) and gp120 (0.1 nM) in combination significantly decreased the percentage of surviving neurons (p<0.001), suggesting Meth potentiation of gp120-induced microglial neurotoxic activity. As Meth (20µM) and gp120 (0.1nM) in combination significantly decreased neuronal survival, this combination was used for subsequent experiments.
Fig. 1. KV 1.3 channel is involved in Meth/gp120-induced microglial neuronal injury.
A. MTT assay shows that supernatants collected from microglia treated with gp120 (0.1nM) in presence of Meth (20µM) synergistically enhanced neuronal death. *p < 0.05, **p < 0.01, ***p < 0.001 vs ctrl, #p < 0.05 vs Meth (20µM)+gp120 (0.1nM). Data represent the mean ± SD in three independent experiments and analyzed using two-way ANOVA followed by Dunnett’s test. B and C. Tunel staining reveals PAP (10nM) or 4-AP (0.5µM) significantly decreased Meth+gp120-induced neuronal apoptosis. Scale bar represents 50µM. *p <0.05, ***p < 0.001 vs ctrl, #p < 0.05, ###p <0.001 vs Meth+gp120.
Meth potentiation of gp120 on microglial neurotoxic activity was further examined by TUNEL staining. After addition of the conditioned media recovered from Meth (20µM)-, gp120 (0.1nM)-, or Meth+gp120 (Meth/gp120)-treated microglia to the neuronal cultures for 24h, the TUNEL positive cells were 12.5 ± 3.82%, 11.99 ± 3.42%, or 30.56 ± 6.86%, respectively (Fig. 1B and 1C). To determine if K channel KV1.3 is involved in Meth potentiation of gp120 neurotoxicity, we tested effects of 4-aminopyridine (4-AP), a broad-spectrum blocker of members of KV1 channel family, and PAP, the specific KV1.3 channel blocker, on their blockage of Meth/gp120-mediated neurotoxicity. As shown in Fig. 1B and 1C, pre-treatment of microglia with PAP (10ng/ml) or 4-AP (0.5µM) for 1 h reduced the numbers of Meth/gp120-induced TUNEL positive neurons to 17.91 ± 5.70 (PAP pre-treated) or 15.71 ± 5.76 (4-AP pre-treated), respectively. A four-way ANOVA revealed that all the factors gp120 (F1, 41=4.23, P < 0.05), Meth (F1, 41=5.68, P < 0.05), 4-AP (F1, 41=16.09, P < 0.001) and PAP (F1, 41=16.09, P < 0.001) are statistically significant. Statistical analysis also showed that the interaction of gp120 and meth is significant (F1, 41=16.09, P < 0.01) and positive. Significant results held for three factor interactions gp120 +Meth + 4-AP (F1, 41=16.09, P<0.001) and gp120+Meth+PAP (F1, 41=17.04, P<0.001), further supporting the notion that Meth potentiates gp120-induced microglial neurotoxic activity.
Meth potentiates gp120 enhancement of outward K current and KV1.3 protein expression
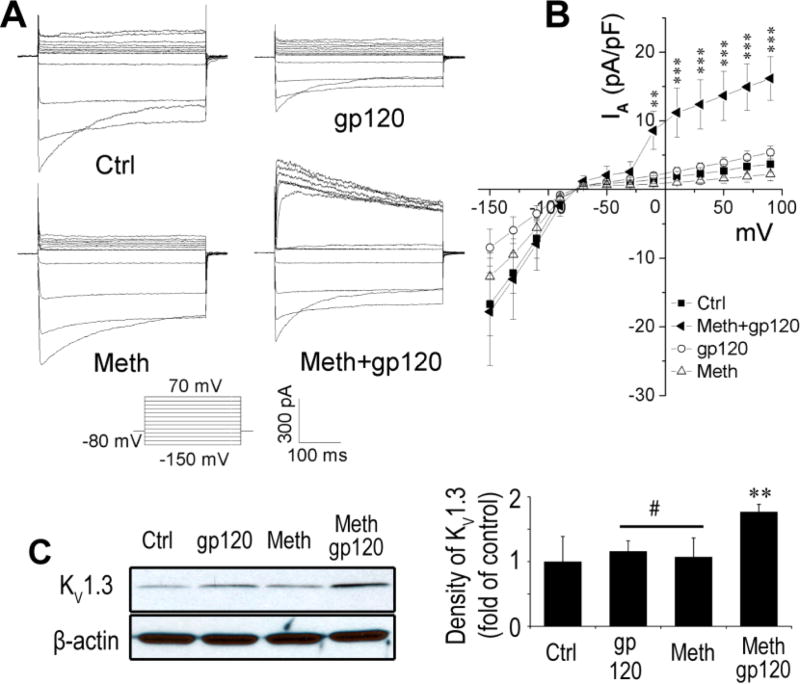
Increasing evidence indicates that microglial K channel Kv1.3 plays an important role in microglial activation (Farber and Kettenmann, 2005; Kotecha and Schlichter, 1999), and activated microglia release neurotoxic molecules leading to neuronal injury. We previously showed that HIV-1 gp120 enhanced Kv1.3 current recorded in cultured rat microglia (Liu et al., 2012). We hypothesize that Meth may potentiate gp120-associated neurotoxicity by augmenting gp120 enhancement of the microglial KV1.3 current. To address this hypothesis, we tested the effect of Meth and gp120 on microglial whole-cell current alone or together. As shown in Fig. 2A and 2B, when microglia were incubated with Meth (20µM) or gp120 (0.1nM) alone for 2 h, there was no significant changes in outward K currents although gp120 showed a decrease of inward currents (statistically not significant). However, the outward K current was significantly increased when Meth and gp120 were added together to microglial culture media, suggesting that Meth potentiates gp120 enhancement on microglial outward K current. To understand if the Meth/gp120-induced increase of outward K current was associated with elevated expression of KV1.3 protein in microglia, we detected the levels of microglial KV1.3 protein expression by Western blot analyses. Microglia treated with Meth (20µM) or gp120 (0.1nM) alone for 24 h exhibited no significant change on the levels of KV 1.3 protein expression with 1.06 ± 0.009 fold of control (Meth) or 0.96 ± 0.008 fold of control (gp120), respectively (Fig. 2C). In contrast, the levels of KV 1.3 protein expression increased to 1.45 ± 0.03 fold of control with the addition of Meth and gp120 together. The difference was statistically significant (p<0.01), suggesting a potential mechanism underlying Meth augmentation of gp120 enhancement of microglial outward K current.
Fig. 2. Bath application of Meth/gp120 enhances KV1.3 currents synergistically in microglia.
A. Outward K+ currents were recorded from microglia under different experimental conditions as indicated. B. Current-voltage (I–V) relationship curves show Meth/gp120 significantly increased Kv current (n = 8, **p < 0.01 vs ctrl, ***p < 0.001 vs ctrl). C. Western blot results illustrate Meth enhancement of gp120-induced microglial KV1.3 protein expression (left) and their corresponding densitometries in bar graph (right) (n = 3, **p < 0.01 vs ctrl, #p < 0.05 vs Meth+gp120.
Meth augments gp120 increase of neurotoxin expression levels in microglia
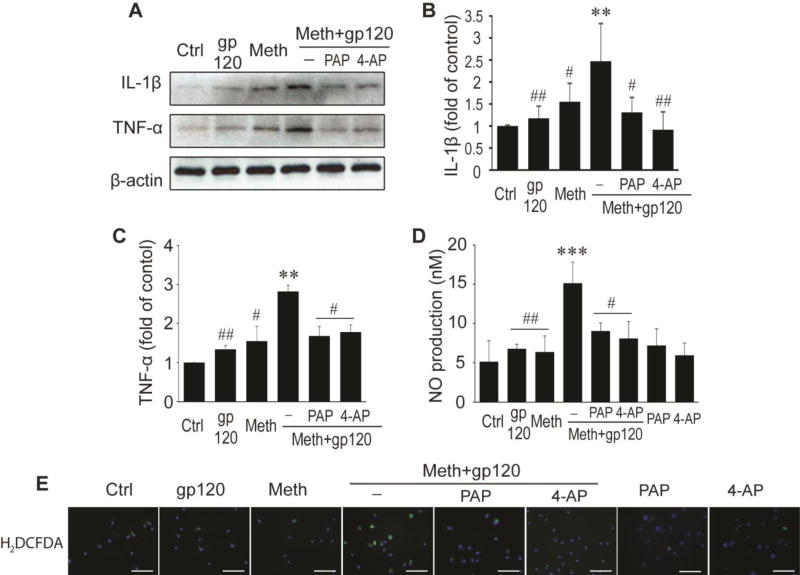
HIV-1-infected and immune activated macrophages and microglia produce pro-inflammatory molecules including cytokines and NO leading to neuronal dysfunction and injury. After demonstration of Meth potentiation of gp120-induced microglial neurotoxicity via KV1.3, we further examined whether Meth augments gp120-associated increase of neurotoxin expression levels in microglia. Western blot analyses showed that addition of Meth and gp120 each alone to microglial culture for 24 h slightly altered expression levels of IL-1β but not TNF-α. Compared with the levels detected from untreated microglia, the fold changes for IL-1β were 1.55 ± 0.42 (Meth) and 1.17 ± 0.28 (gp120), respectively (Fig. 3A – C). In contrast, addition of Meth and gp120 in combination (Meth/gp120) significantly increased the expression levels of both, with fold changes of 2.47 ± 0.86, for IL-1β and 2.82 ± 0.16 for TNF-α, respectively. The Meth/gp120-induced increase of cytokine expression was abrogated by PAP (10nM) or 4-AP (0.5µM). A four-way ANOVA showed that the fold changes mediated by four substances gp120 (F1, 12=29.03, P < 0.001), Meth (F1, 12=46.09, P < 0.001), 4-AP (F1, 12=24.09, P < 0.001) and PAP (F1, 12= 28.97, P < 0.001) are statistically significant, indicating a positive and significant interaction between gp120 and Meth significant (F1, 12= 9.89, P= 0.0084657) and an involvement of microglial KV1.3 in Meth/gp120-associated elevation of cytokine expression in microglia. Treatment of microglia with PAP or 4-AP each alone had no significant effects on TNF-α (1.36 ± 0.41, n=2; or 1.28 ± 0.33, n=2) or IL-1β production (1.62 ± 0.64, n=2 or 1.59 ± 0.47, n=2). In addition to cytokine assays, we also evaluated effects of Meth and gp120 on oxidative stress including NO and ROS productions. The levels of NO in supernatants from microglia treated with Meth/gp120 exhibited a significant increase (15.13 ± 2.70nM) compared with microglia treated with gp120 (6.79 ± 0.59nM) or Meth (6.38 ± 2.04nM) each alone. Addition of KV channel blockers (PAP or 4-AP) before application of Meth/gp120 significantly reduced NO production (gp120+Meth+PAP, F1, 16=8.38, P < 0.05; gp120+Meth+4-AP, F1, 16=7.79, P <0.05). The average concentration was 9.04 ± 1.05nM (PAP pre-treated group) or 8.08 ± 2.65nM (4-AP pre-treated) (Fig. 3D). Treatment of microglia with PAP or 4-AP alone had no significant effect on alteration NO production. Similarly, microglial production of ROS was also detected by CM-H2DCFDA (a ROS detection reagent) immunoreaction. As shown in Fig. 3E, microglia treated with Meth/gp120 largely enhanced ROS production when compared with microglia treated with gp120 or Meth alone. This enhancement was attenuated by KV channel blockers PAP or 4-AP. Collectively, these results strongly support a critical role for KV1.3 in the expression and production of neurotoxins by Meth/gp120-treated microglia.
Fig. 3. Blockage of KV1.3 channel mitigates neurotoxins secreted by Meth/gp120-activated microglia.
A. Western blots show expression of IL-1β, TNF-α, and internal control β-actin. B and C. Increased folds of IL-1β and TNF-α are illustrated on bar graphs. D. Graph shows NO production. E. Immunostaining reveals expression of H2DCFDA, a ROS detection reagent. Images were visualized by fluorescent microscopy at x40 original magnification. Scale bar equals 100 µm. **p < 0.01, or ***p < 0.001 vs ctrl; #p < 0.05, or ##p < 0.01 vs Meth+gp120. Data represent the mean ± SD in three independent experiments.
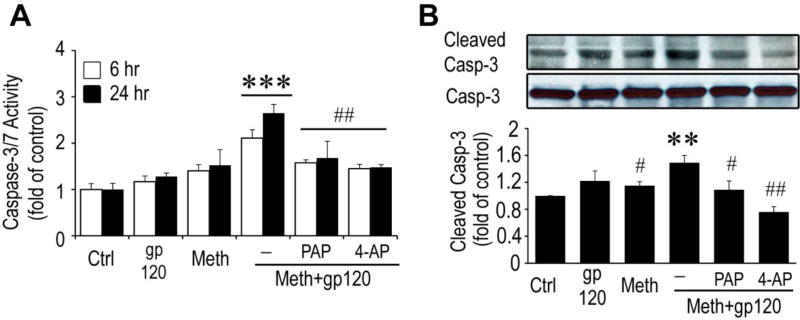
Meth/gp120-induced microglial neurotoxicity requires caspase-3 signaling
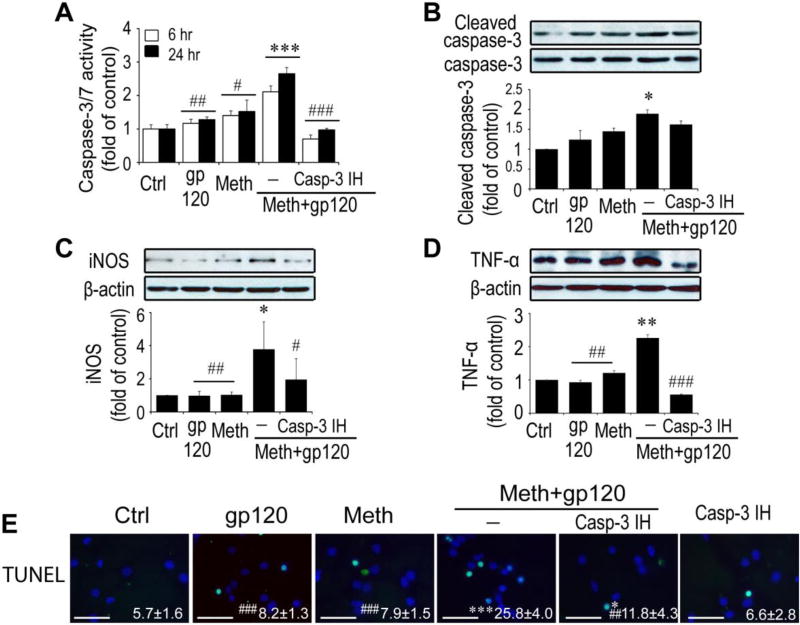
Caspase 3 and 7 are frequent downstream effectors of the caspase cascade and are activated in HAND (Garden et al., 2002) and other neurodegenerative disorders (Hartmann et al., 2000; Su et al., 2000). Recently, Burguillos et al reported a novel function of caspases in regulation of microglial activation and microglia-associated neurotoxicity (Burguillos et al., 2011). They found that activation of caspase-8 and caspase-3/7 cause microglial activation and neuronal injury. To determine whether caspase-3 signaling is involved in Meth/gp120-associated microglial activation, we first exposed microglia to Meth and gp120 each alone or in a combination for 6 h or 24 h and caspase-3/7 activity in microglial cultures was assessed by Luminescent Assay (Promega). As shown in Fig. 4A, a significant increase of DEVDase activity was observed 6 h (F1, 16= 31.83, P = 0.001) and 24 h (F1, 16= 19.20, P < 0.001) after exposure to Meth/gp120. The detected DEVDase activity, primarily caspase 3/7 activity, was in a time dependent manner and reached as high as 2.11 ± 0.18- and 2.65 ± 0.19-fold of control at time points of 6 and 24 h. In contrast, the caspase-3/7 activities at the same two time points were 1.40 ± 0.13- and 1.52 ± 0.34-fold of control, or 1.17 ± 0.12- and 1.27 ± 0.11-fold of control when microglia were treated with gp120 or Meth alone. Since caspase-3/7 activity accounts for both of the caspase-3 and caspase-7 DEVDase activity, we used a specific caspase-3 inhibitor (Casp-3 IH) to confirm the role of caspase-3 in microglial activation. Competitive inhibition of DEVDase activity with Casp-3 IH (2µM) significantly blocked Meth/gp120-induced increase of caspase-3/7 activity (four-way nested ANOVA, 6h, F1, 16= 200.57, P < 0.001; 24h, F1, 16= 88.21, P<0.001), suggesting caspase-3 signaling was involved in Meth/gp120-induced microglial activation. This suggestion was further confirmed by Western blot analyses. In Fig 4B, treatment of microglia with Meth/gp120 for 24 h activated caspase-3 and elevated the level of cleaved caspase-3 expression to 1.89-fold of control. The elevated level of cleaved caspase-3, however, was not mitigated by pre-treatment of microglia with 2µM Casp-3 IH, suggesting that casp-3 IH could inhibit DEVDase activity, but not block Meth/gp120-induced caspase-3 cleavage. To examine if inhibition of caspase-3/7 activity could abrogate Meth/gp120-associated microglial neuronal toxicity, we added Casp-3 IH (2µM) to microglial culture 1 h prior to addition of Meth/gp120. In presence of Casp-3 IH, Meth/gp120 failed to induce microglial production of TNF-α and iNOS (Fig. 4C and 4D). Moreover, neuronal damage induced by culture supernatant collected from Meth/gp120-treated microglia in the presence of Casp-3 IH was significantly (A three-way ANOVA, F1, 10= 35.19, P <0.001) reduced to 11.9 ± 4.3 in comparison to supernatant treated with Meth/gp120 in the absence of Casp-3 IH (25.8 ± 4.0) (Fig. 4E). Taken together, these data suggested an involvement of caspase-3 signaling in Meth/gp120 triggered microglial neurotoxic activity. Although caspase-3 is known as a major executor to induce apoptosis, the Meth/gp120-induced increase in caspase-3/7 activity did not result in microglial cell death within 24 h.
Fig. 4. Meth/gp120-induced neurotoxicity requires caspase-3 signaling.
A. Caspase-3/7 activity was quantified by using caspase-3/7 activity kit (Promega, Madison USA) 6h and 24 after experimental treatments as indicated. Note that Meth and gp120 each alone had minimal effects on caspase-3/7 activity, but they significantly increased caspase-3/7 activity when applied in combination. The Meth+gp120 increase of caspase-3/7 activity was inhibited by Casp-3 IH, demonstrating Meth+gp120 enhancement of microglial caspase-3/7 activity. B, C and D. Western blots illustrate expression of cleaved caspase-3 and its internal control caspase-3 (B), iNOS (C), TNF-α (D), and β-actin of microglia. Increased folds are reflected on bar graphs (lower B, C and D). E. TUNEL staining was visualized by fluorescent microscopy at x100 original magnification. Data represent mean ± SD of values derived from three independent experiments. Statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001 vs ctrl, and # p < 0.05, ##p < 0.01, ###p < 0.001 vs Meth+gp120-treated group. Scale bar equals 50µM.
Involvement of KV1.3 in Meth/gp120 increase of caspase-3 activity in microglia
After demonstration that Meth potentiates gp120 enhancement of KV1.3 protein expression and KV1.3 currents, resulting in microglial activation, subsequent production of neurotoxins and consequent neuronal damage which were associated with an increase of microglial caspase-3 activity, we further investigated the link between KV1.3 channels and caspase cascade on Meth/gp120-induced microglial neurotoxic activity. Either the specific KV1.3 blocker PAP or the broad spectrum KV channel blocker 4-AP was added to the microglial cultures 1 h prior to addition of Meth, gp120 alone or in combination. The Caspase-3/7 activity was measured at 6 and 24 h. While Meth and gp120 each alone had no significant effects on caspase-3/7 activity, Meth/gp120 did produce a significant increase of caspase-3/7 activity that was significantly attenuated by pre-treatment of microglia with KV channel blockers PAP or 4-AP (Fig. 5A), indicating an involvement of KV1.3 in Meth/gp120 increase of caspase-3 activity in microglia. Consistent with this effect, the complementary study of caspase-3 cleavage revealed a similar finding, that is, Meth/gp120 led to an increase of caspase-3 cleavage (Fig. 5B). This enhancement of cleaved caspase-3 expression on microglial cells was largely inhibited by PAP and 4-AP (Fig. 5B, a four-way nested ANOVA, F1,, 12= 15.96, P < 0.001 for PAP, F1, 12=52.12, P < 0.001 for 4-AP), supporting the involvement of KV1.3 in Meth/gp120 increase of caspase-3 activity in microglia.
Fig. 5. KV1.3 modulates caspase 3 activity.
A. Blockage of KV1.3 by PAP (10nM) or 4-AP (0.5µM) significantly decreased Meth/gp120-induced caspase-3/7 activity detected at 6 and 24 h time points. To illustrate Meth and gp120 increase of caspase-3/7 activity in combination and the blockage of Meth+gp120-induced increase by Kv1.3 blockers PAP and 4-AP, the 4 groups of data on the left (Ctrl, gp120, Meth and Meth+gp120) of Fig. 4A were reused in this figure. B. Western blots revealed a cleaved caspase-3 expression (upper). The bar graph (lower) illustrates increased fold after normalization with caspase-3. Data (mean ± SD) represent three experiments and each in triplicates. Statistical significance **p<0.01, ***p<0.001 vs ctrl, and #p < 0.05, ##p <0.01, vs meth+gp120-treated group.
Discussion
It is widely accepted that HIV-1 infection has become a chronic illness with very low levels of viral replication and chronic immune activation when successfully treated with combined anti-retroviral therapy (cART) (Gates and Cysique, 2016; Jaeger and Nath, 2012; Watkins and Treisman, 2015). Despite this promising success, HAND remains prevalent in the HIV-1-infected population (Alfahad and Nath, 2013; Heaton et al., 2011; Simioni et al., 2010). While the underlying mechanisms remain to be determined, several comorbidities have been identified as important risk factors including drug abuse, in particular the abuse of Meth. HIV-1 reproduces in microglia, the brain immune cells, causing brain inflammation and pathogenesis of HAND (Gannon et al., 2011; Kaul and Lipton, 2006b; Letendre et al., 2011; Silverstein et al., 2012). Meth abuse exacerbates HAND (Carey et al., 2006; Chana et al., 2006; Chang et al., 2005; Sekine et al., 2008), and such an exacerbation is also associated with microglial neurotoxic activity (Cadet and Krasnova, 2007; Flora et al., 2003; Nath, 2010; Sekine et al., 2008; Silverstein et al., 2011). To understand how Meth exacerbates HAND via microglia, we investigated Meth potentiation of gp120-induced microglial neurotoxic activity using sub-toxic concentrations of Meth and gp120 that were determined from dose-response studies (Fig. 1A). We also examined involvement of a microglial K channel KV1.3 in Meth- and gp120-associated microglial neurotoxic activity. We found that neither Meth (20µM) nor gp120 (0.1nM) had, when applied each alone, significant effect on inducing microglial neurotoxic activity as determined by analyzing neuronal survival rate after addition of the culture supernatants recovered from Meth- or gp120-treated microglia to the neuronal cultures, respectively. In contrast, the supernatant collected from cultured microglia treated with Meth and gp120 in combination significantly reduced neuronal survival rate, suggesting a synergistic effect of Meth and gp120 or Meth potentiation of gp120 effect on microglial neurotoxic activity. Such synergy/potentiation was associated in parallel with Meth/gp120-induced increase of Kv1.3 expression and outward K+ current and the production of neurotoxic substances in microglial cells. Blockage of microglial Kv1.3 channel by either a specific Kv1.3 antagonist PAP or by a broad spectrum K channel blocker 4-AP significantly attenuated the potentiation of Meth on gp120-induced microglial neurotoxic activity. Further investigations revealed an involvement of caspase3/7 signaling in Meth-associated potentiation/synergy on gp120-induced microglial neurotoxic activity as demonstrated by the results that Meth/gp120-induced increase of caspase3/7 activity, and cleaved caspase 3 was inhibited by either a specific caspase 3 inhibitor or a specific Kv1.3 channel blocker.
Meth is one of the most commonly abused drugs among individuals infected with HIV-1 (Marquez et al., 2009). Ample evidence indicate that Meth abuse increases the risk of HIV-1 infection (Buchacz et al., 2005; Gonzales et al., 2010; Mitchell et al., 2006) and exacerbates the cognitive deficits and neurodegenerative abnormalities in HIV-1-infected patients and animal models (Hoefer et al., 2015; Kesby et al., 2015a; Kesby et al., 2015b). It has also been shown that HIV-1 infection and Meth abuse each alone can cause neuronal injury and cognitive impairments (Chana et al., 2006; Kaul et al., 2001; Larsen et al., 2002; Mattson et al., 2005; Xu et al., 2004). Although HIV-1 does not infect neurons (Lindl et al., 2010), neuronal injury is primarily thought to be mediated by the neurotoxins released from infected cells, mostly resident microglia (Gendelman and Meltzer, 1989; Meltzer and Gendelman, 1992; Xiong et al., 2000). Among the neurotoxins is HIV-1 glycoprotein gp120. The neurotoxic effects of gp120 can be mediated either through direct or indirect mechanisms (Gonzalez-Scarano and Martin-Garcia, 2005; Kaul and Lipton, 2006b). In terms of indirect mechanisms, gp120 has been shown to induce microglial activation and production of neurotoxic substances including, but not limited to, cytokines, chemokines, and nitric oxides (Albright et al., 2001; Lisi et al., 2012; Liu et al., 2012; Walsh et al., 2014). Meth also acts on microglia and alters microglial secretory activity, exacerbating HIV-1/gp120-associated neurotoxicity (Loftis and Janowsky, 2014; Silverstein et al., 2012). Our results revealed that microglia appear to be an intersecting target, as well as an effector, for the two. At a lower concentration, neither gp120 (0.1nM) nor Meth (20 µM) triggered microglial neurotoxic activity. In contrast, Meth potentiated gp120-associated microglial neurotoxic activity when applied in combination. The observed potentiation suggests that Meth and gp120 may induce neural cell injury by acting on microglia, although Meth and gp120 have been reported to act on other types of central nervous system cells as well (Nair et al., 2009; Purohit et al., 2011; Shah et al., 2013; Shah et al., 2012).
Despite mounting evidence that Meth abuse potentiates HIV-1 infection and HIV-1-associated neuropathology, limited studies have been carried out to explore the mechanisms underlying such a potentiation. It has been shown that Meth accelerates HIV infection and synergistically potentiates gp120- and Tat-associated neurotoxicity (Maragos et al., 2002; Nair et al., 2009; Nair and Samikkannu, 2012; Silverstein et al., 2012; Turchan et al., 2001). Multiple mechanisms for Meth-induced potentiation have been proposed including upregulation of HIV-1 co-receptors CXCR4 and CCR5 expression (Nair and Saiyed, 2011; Nair et al., 2009), interaction with NMDA receptors producing excitotoxic neural injury (Aksenov et al., 2012), oxidative stress (Purohit et al., 2011; Shah et al., 2013), and disruption of dopaminergic function (Aksenov et al., 2012; Purohit et al., 2011) and the blood-brain barrier (Mahajan et al., 2008). In this study, we found that Meth and gp120 enhance microglial expression of Kv1.3 channel protein and outward K current leading to an increased microglial production of TNFα, IL-1β, and NO and resultant enhancement of microglial neurotoxic activity. To our knowledge, our results demonstrate for the first time that Meth and gp120 act in a converging way on microglial Kv1.3 to induce their co-morbid effects through microglial activation and increased production of neurotoxins.
It has been demonstrated in animal models of neuroAIDS that gp120 protein may cause cognitive impairment, and Meth enhances such impairment (Henry et al., 2013; Hoefer et al., 2015; Kesby et al., 2015b). A recent cross species study has also demonstrated that HIV-1 in humans and gp120 in animals impaired learning, and Meth exposure exacerbated HIV/gp120-associated neurocognitive deficits in both species (Kesby et al., 2015a). These findings clearly indicate that the gp120 protein may contribute to HAND in humans. Considering the facts that HIV-1 infection becomes, in the era of cART, a chronic illness with very low levels of viral replication in microglia and both Meth and gp120 employed in this study were at sub-toxic doses, the results we observed that Meth potentiation of gp120 on microglial neurotoxic activity may reflect a pathophysiological condition in the brain of a HIV-1-infected Meth user under the cART regimen. Low levels of HIV/gp120 in the brain may or may not induce microglia-associated neurotoxic activity. In the presence of Meth, however, the effect of HIV/gp120 on microglia could be exacerbated. This might explain why HAND remains prevalent even in the era of cART. Nevertheless, our results on Meth potentiation of gp120-associated microglial neurotoxic activity may have implications for HAND pathogenesis in the era of cART.
It is worth pointing out that the subtoxic concentrations of Meth and gp120, determined in a concentration-response study as shown in Fig. 1A, were employed in this study. The reasons for using subtoxic concentrations are: 1) to mimic the disease condition of HAND which becomes a chronic illness with very low levels of viral replication in the era of cART; and 2) to easily examine the potentate/synergistic effects of Meth and gp120 in an in vitro cell model of HAND pathogenesis. As both Meth and gp120 cause microglial cell damage at higher concentrations (Coelho-Santos et al., 2012; Xu et al., 2011), it might be difficult to evaluate the effects of Meth potentiation of gp120-induced microglial neurotoxic activity. The use of subtoxic concentration of gp120 may also reflect the HAND pathogenesis in the cART era that lower levels of viral replication do not produce neural injury, but do cause neural injury in the presence of Meth, a potential mechanism underlying Meth exacerbation of HAND seen in HIV-1-infected patients. It is also worth pointing out that the conditioned media employed in this study was collected 24 h after addition of fresh neurobasal media (see methods). Although this procedure prevents the direct action of Meth and gp120 on neuronal cells, it may remove all the proinflammatory substances released by microglia during the first 24h in culture and contributes to the low (subtoxic) effects of Meth (20µM) or gp120 (0.1nM). Thus, the “subtoxic” concentrations of Meth and gp120 employed in the present study are only applicable for an experimental condition.
In summary, the present study demonstrated that Meth potentiates gp120-induced microglial neurotoxic activity via activation of voltage-gated K channel Kv1.3 and caspase 3/7 signaling. Under experimental conditions, Meth or gp120 each alone at a low concentration had no apparent effect on triggering microglial neurotoxic activity. When applied in combination, however, Meth significantly potentiated gp120-induced microglial neurotoxicity. The Meth potentiation of gp120-associated microglial neurotoxic activity was blocked by specific Kv1.3 channel blockers, suggesting an involvement of Kv1.3 in Meth-mediated potentiation. Considering the scenarios of HIV-1 brain infection plus Meth abuse in the era of cART, when the levels of viral replication are low and long-term use of Meth, the observed Meth potentiation of gp120-associated microglial neurotoxic activity at subtoxic concentrations may represent a potential mechanism for HAND pathogenesis in the era of cART.
Highlights.
Meth potentiates gp120-induced microglial neurotoxic activity.
Meth potentiation of gp120 effect on microglia via K+ channel Kv1.3 and caspase 3/7 signaling.
Present results may imply a potential mechanism for HAND pathogenesis in the era of cART.
Acknowledgments
The authors thank Ms. Robin Taylor for her reading the manuscript. This work was supported by NIH grant R01 NS077873.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. D1/NMDA receptors and concurrent methamphetamine+ HIV-1 Tat neurotoxicity. J Neuroimmune Pharmacol. 2012;7:599–608. doi: 10.1007/s11481-012-9362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright AV, Martin J, O’Connor M, Gonzalez-Scarano F. Interactions between HIV-1 gp120, chemokines, and cultured adult microglial cells. J Neurovirol. 2001;7:196–207. doi: 10.1080/13550280152403245. [DOI] [PubMed] [Google Scholar]
- Alfahad TB, Nath A. Update on HIV-associated neurocognitive disorders. Curr Neurol Neurosci Rep. 2013;13:387. doi: 10.1007/s11910-013-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, Klausner JD. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19:1423–1424. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotox Res. 2007;12:181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10:185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Santos V, Goncalves J, Fontes-Ribeiro C, Silva AP. Prevention of methamphetamine-induced microglial cell death by TNF-alpha and IL-6 through activation of the JAK-STAT pathway. J Neuroinflammation. 2012;9:103. doi: 10.1186/1742-2094-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C. Ion channels in microglia (brain macrophages) Am J Physiol. 1998;275:C327–C342. doi: 10.1152/ajpcell.1998.275.2.C327. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Physiology of microglial cells. Brain Res Brain Res Rev. 2005;48:133–143. doi: 10.1016/j.brainresrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Fischer HG, Eder C, Hadding U, Heinemann U. Cytokine-dependent K+ channel profile of microglia at immunologically defined functional states. Neuroscience. 1995;64:183–191. doi: 10.1016/0306-4522(94)00398-o. [DOI] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp Neurol. 2003;179:60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- Fordyce CB, Jagasia R, Zhu X, Schlichter LC. Microglia Kv1.3 channels contribute to their ability to kill neurons. J Neurosci. 2005;25:7139–7149. doi: 10.1523/JNEUROSCI.1251-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24:275–283. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, Winston A. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28:67–72. doi: 10.1097/01.aids.0000432467.54003.f7. [DOI] [PubMed] [Google Scholar]
- Gates TM, Cysique LA. The Chronicity of HIV Infection Should Drive the Research Strategy of NeuroHIV Treatment Studies: A Critical Review. CNS Drugs. 2016;30:53–69. doi: 10.1007/s40263-015-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Meltzer MS. Mononuclear phagocytes and the human immunodeficiency virus. Curr Opin Immunol. 1989;2:414–419. doi: 10.1016/0952-7915(89)90152-0. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Knapp PE. Interactions of HIV and drugs of abuse: the importance of glia, neural progenitors, and host genetic factors. International review of neurobiology. 2014;118:231–313. doi: 10.1016/B978-0-12-801284-0.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell M, Perry W, Young JW, Minassian A. Behavioral effects of chronic methamphetamine treatment in HIV-1 gp120 transgenic mice. Behav Brain Res. 2013;236:210–220. doi: 10.1016/j.bbr.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer MM, Sanchez AB, Maung R, de Rozieres CM, Catalan IC, Dowling CC, Thaney VE, Pina-Crespo J, Zhang D, Roberts AJ, Kaul M. Combination of methamphetamine and HIV-1 gp120 causes distinct long-term alterations of behavior, gene expression, and injury in the central nervous system. Exp Neurol. 2015;263:221–234. doi: 10.1016/j.expneurol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilschner S, Nolte C, Kettenmann H. Complement factor C5a and epidermal growth factor trigger the activation of outward potassium currents in cultured murine microglia. Neuroscience. 1996;73:1109–1120. doi: 10.1016/0306-4522(96)00107-8. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Nath A. Modeling HIV-associated neurocognitive disorders in mice: new approaches in the changing face of HIV neuropathogenesis. Dis Model Mech. 2012;5:313–322. doi: 10.1242/dmm.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol. 2006a;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006b;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Heaton RK, Young JW, Umlauf A, Woods SP, Letendre SL, Markou A, Grant I, Semenova S. Methamphetamine Exposure Combined with HIV-1 Disease or gp120 Expression: Comparison of Learning and Executive Functions in Humans and Mice. Neuropsychopharmacology. 2015a;40:1899–1909. doi: 10.1038/npp.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S. Cognitive deficits associated with combined HIV gp120 expression and chronic methamphetamine exposure in mice. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2015b;25:141–150. doi: 10.1016/j.euroneuro.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha SA, Schlichter LC. A Kv1.5 to Kv1.3 switch in endogenous hippocampal microglia and a role in proliferation. J Neurosci. 1999;19:10680–10693. doi: 10.1523/JNEUROSCI.19-24-10680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine disposition in the presynaptic process regulates the severity of methamphetamine-induced neurotoxicity. Annals of the New York Academy of Sciences. 2008;1139:118–126. doi: 10.1196/annals.1432.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Experimental neurology. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011;19:137–142. [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, Marquie-Beck J, Navia B. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17:63–69. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome [see comments] N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Lisi L, Tramutola A, De Luca A, Navarra P, Dello Russo C. Modulatory effects of the CCR5 antagonist maraviroc on microglial pro-inflammatory activation elicited by gp120. J Neurochem. 2012;120:106–114. doi: 10.1111/j.1471-4159.2011.07549.x. [DOI] [PubMed] [Google Scholar]
- Littell RC, Stroup WWRJF, editors. SAS for Linear Models. 4. SAS Institute Inc; Gary, NC: 2002. [Google Scholar]
- Liu J, Xu C, Chen L, Xu P, Xiong H. Involvement of Kv1.3 and p38 MAPK signaling in HIV-1 glycoprotein 120-induced microglia neurotoxicity. Cell Death Dis. 2012;3:e254. doi: 10.1038/cddis.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Neuroimmune basis of methamphetamine toxicity. International review of neurobiology. 2014;118:165–197. doi: 10.1016/B978-0-12-801284-0.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Adal A, Qi M, Toh J, Xu G, Prasad PN, Schwartz SA. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008;1203:133–148. doi: 10.1016/j.brainres.2008.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83:955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 2009;21:575–582. doi: 10.1080/09540120802385579. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Meltzer MS, Gendelman HE. Mononuclear phagocytes as targets, tissue reservoirs, and immunoregulatory cells in human immunodeficiency virus disease. Curr Top Microbiol Immunol. 1992;181:239–263. doi: 10.1007/978-3-642-77377-8_9. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Morris SR, Kent CK, Stansell J, Klausner JD. Methamphetamine use and sexual activity among HIV-infected patients in care--San Francisco, 2004. AIDS patient care and STDs. 2006;20:502–510. doi: 10.1089/apc.2006.20.502. [DOI] [PubMed] [Google Scholar]
- Nair MP, Saiyed ZM. Effect of methamphetamine on expression of HIV coreceptors and CC-chemokines by dendritic cells. Life sciences. 2011;88:987–994. doi: 10.1016/j.lfs.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MP, Saiyed ZM, Nair N, Gandhi NH, Rodriguez JW, Boukli N, Provencio-Vasquez E, Malow RM, Miguez-Burbano MJ. Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J Neuroimmune Pharmacol. 2009;4:129–139. doi: 10.1007/s11481-008-9128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MP, Samikkannu T. Differential regulation of neurotoxin in HIV clades: role of cocaine and methamphetamine. Curr HIV Res. 2012;10:429–434. doi: 10.2174/157016212802138742. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Gebicke-Haerter PJ, Illes P. Voltage-dependent potassium channels in activated rat microglia. J Physiol. 1994;475:15–32. doi: 10.1113/jphysiol.1994.sp020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutile-McMenemy N, Elfenbein A, Deleo JA. Minocycline decreases in vitro microglial motility, beta1-integrin, and Kv1.3 channel expression. J Neurochem. 2007;103:2035–2046. doi: 10.1111/j.1471-4159.2007.04889.x. [DOI] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44:102–110. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Schilling T, Quandt FN, Cherny VV, Zhou W, Heinemann U, Decoursey TE, Eder C. Upregulation of Kv1.3 K(+) channels in microglia deactivated by TGF-beta. Am J Physiol Cell Physiol. 2000;279:C1123–C1134. doi: 10.1152/ajpcell.2000.279.4.C1123. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Kumar S, Simon SD, Singh DP, Kumar A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell death & disease. 2013;4:e850. doi: 10.1038/cddis.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Silverstein PS, Kumar S, Singh DP, Kumar A. Synergistic cooperation between methamphetamine and HIV-1 gsp120 through the P13K/Akt pathway induces IL-6 but not IL-8 expression in astrocytes. PloS one. 2012;7:e52060. doi: 10.1371/journal.pone.0052060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Shah A, Gupte R, Liu X, Piepho RW, Kumar S, Kumar A. Methamphetamine toxicity and its implications during HIV-1 infection. J Neurovirol. 2011;17:401–415. doi: 10.1007/s13365-011-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Shah A, Weemhoff J, Kumar S, Singh DP, Kumar A. HIV-1 gp120 and drugs of abuse: interactions in the central nervous system. Curr HIV Res. 2012;10:369–383. doi: 10.2174/157016212802138724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Su JH, Nichol KE, Sitch T, Sheu P, Chubb C, Miller BL, Tomaselli KJ, Kim RC, Cotman CW. DNA damage and activated caspase-3 expression in neurons and astrocytes: evidence for apoptosis in frontotemporal dementia. Experimental neurology. 2000;163:9–19. doi: 10.1006/exnr.2000.7340. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Nath A, Maragos WF. Progress in understanding basal ganglia dysfunction as a common target for methamphetamine abuse and HIV-1 neurodegeneration. Current HIV research. 2007;5:301–313. doi: 10.2174/157016207780636515. [DOI] [PubMed] [Google Scholar]
- Theodore S, Stolberg S, Cass WA, Maragos WF. Human immunodeficiency virus-1 protein tat and methamphetamine interactions. Annals of the New York Academy of Sciences. 2006;1074:178–190. doi: 10.1196/annals.1369.018. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina A, Jones K. Crystal methamphetamine, its analogues, and HIV infection: medical and psychiatric aspects of a new epidemic. Clin Infect Dis. 2004;38:890–894. doi: 10.1086/381975. [DOI] [PubMed] [Google Scholar]
- Visentin S, Agresti C, Patrizio M, Levi G. Ion channels in rat microglia and their different sensitivity to lipopolysaccharide and interferon-gamma. J Neurosci Res. 1995;42:439–451. doi: 10.1002/jnr.490420402. [DOI] [PubMed] [Google Scholar]
- Visentin S, Levi G. Protein kinase C involvement in the resting and interferon-gamma-induced K+ channel profile of microglial cells. J Neurosci Res. 1997;47:233–241. [PubMed] [Google Scholar]
- Visentin S, Renzi M, Levi G. Altered outward-rectifying K(+) current reveals microglial activation induced by HIV-1 Tat protein. Glia. 2001;33:181–190. doi: 10.1002/1098-1136(200103)33:3<181::aid-glia1017>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Walsh JG, Reinke SN, Mamik MK, McKenzie BA, Maingat F, Branton WG, Broadhurst DI, Power C. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology. 2014;11:35. doi: 10.1186/1742-4690-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CC, Treisman GJ. Cognitive impairment in patients with AIDS - prevalence and severity. HIV AIDS (Auckl) 2015;7:35–47. doi: 10.2147/HIV.S39665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Zeng YC, Lewis T, Zheng J, Persidsky Y, Gendelman HE. HIV-1 infected mononuclear phagocyte secretory products affect neuronal physiology leading to cellular demise: relevance for HIV-1-associated dementia. J Neurovirol. 2000;6(Suppl 1):S14–S23. [PubMed] [Google Scholar]
- Xu C, Liu J, Chen L, Liang S, Fujii N, Tamamura H, Xiong H. HIV-1 gp120 enhances outward potassium current via CXCR4 and cAMP-dependent protein kinase a signaling in cultured rat microglia. Glia. 2011;56:997–1007. doi: 10.1002/glia.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Kulkosky J, Acheampong E, Nunnari G, Sullivan J, Pomerantz RJ. HIV-1-mediated apoptosis of neuronal cells: Proximal molecular mechanisms of HIV-1-induced encephalopathy. Proc Natl Acad Sci U S A. 2004;101:7070–7075. doi: 10.1073/pnas.0304859101. [DOI] [PMC free article] [PubMed] [Google Scholar]