Abstract
Hematopoiesis is a balance among quiescence, self-renewal, proliferation, and differentiation, which is believed to be firmly adjusted through interactions between hematopoietic stem and progenitor cells (HSPCs) with the microenvironment. This microenvironment is derived from a common progenitor of mesenchymal origin and its signals should be capable of regulating the cellular memory of transcriptional situation and lead to an exchange of stem cell genes expression. Mesenchymal stem cells (MSCs) have self-renewal and differentiation capacity into tissues of mesodermal origin, and these cells can support hematopoiesis through release various molecules that play a crucial role in migration, homing, self-renewal, proliferation, and differentiation of HSPCs. Studies on the effects of MSCs on HSPC differentiation can develop modern solutions in the treatment of patients with hematologic disorders for more effective Bone Marrow (BM) transplantation in the near future. However, considerable challenges remain on realization of how paracrine mechanisms of MSCs act on the target tissues, and how to design a therapeutic regimen with various paracrine factors in order to achieve optimal results for tissue conservation and regeneration. The aim of this review is to characterize and consider the related aspects of the ability of MSCs secretome in protection of hematopoiesis.
Keywords: Hematopoietic Stem Cell, Cytokine, Mesenchymal Stem Cells, Microvesicle, miRNA
Introduction
Hematopoiesis is a procedure in which hematopoietic stem and progenitor cell (HSPCs) show continued cellular actions, including self-renewal, apoptosis, proliferation, and differentiation into multiple lineages, which creates different types of mature blood cells, as well as sufficient numbers of blood cells required for maintaining homeostasis.1 This process is the result of cooperation between HSPCs and MSCs.2 Different HSPC subpopulations express the CD34 marker, which are the most undifferentiated stem cell type, as well as multipotent progenitors (MPPs) downstream of the differentiation hierarchy with capacity of multilineage production.3 Self-renewal is essential for maintaining the HSPC reconstitution and is therefore a prerequisite for lifelong hematopoiesis.4 Most HSPCs are quiescent and in G0 phase of cell cycle,5,6 and daily hematopoiesis is largely maintained by highly proliferative downstream HSPCs.7 Cellular actions of HSPCs are controlled by both intrinsic cellular factors such as transcriptional regulatory networks, as well as extrinsic cellular factors like growth factors, cytokines, chemokines and microvesicles (MVs); for example, G-CSF, CXCL12, and transforming growth factor-β (TGF-β).8 During embryonic, fetal, and adult life, hematopoiesis depends on a microenvironment involving soluble components and cell-cell interactions. This microenvironment is known as the hematopoietic niche, which is mostly derived from a common progenitor of mesenchymal origin that adjusts the steady HSPC quiescence and activation(Figure 1).9 Stem cells (including HSPCs or MSCs) assure the lifelong regeneration of tissues.10 Research has indicated that the cytokines and growth factors from MSCs exert their advantageous effects on target cells to boost tissue repair and regeneration, including immune response moderation, cell survival, anti-apoptosis, metabolism, proliferation, differentiation, hematopoiesis, angiogenesis, myogenesis, remodeling, wound healing, hair growth, neuroprotection, collateral development, and renal protection.9,11-15 The role of MSCs in support of hematopoiesis has been demonstrated by various studies. Dexter et al for the first time examined the establishment of in vitro culture conditions for long-term bone marrow culture (LTBMC) and showed that an adherent stromal-like culture could support the HSPCs.16 HSPCs are increasingly used for allogeneic and autologous transplantation but recovery of platelets occurs with a lower rate; therefore, several studies have shown that the proliferation of HSPCs in vitro could result in faster recovery after transplantation.17,18 MSCs release many growth factors that stimulate hematopoiesis, prepare a scaffold for hematopoiesis, protect primitive progenitor cells, expand and maintain HSPCs in LTBMC with CD34 hematopoietic progenitor cells (HPCs), supporting both erythroid and myeloid differentiation.19
Figure 1.
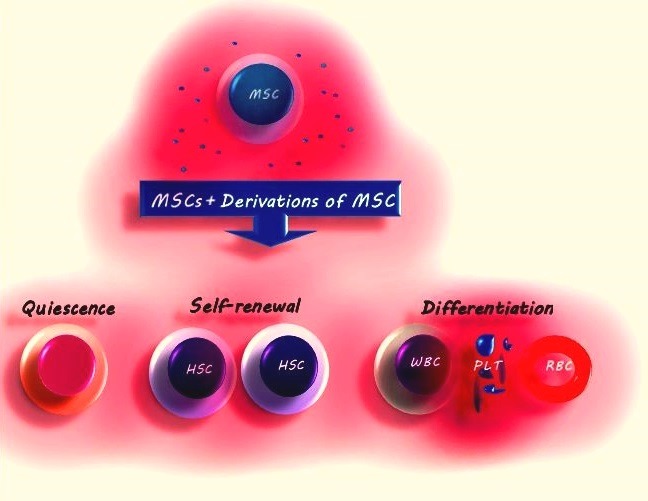
MSCs and their derivatives can regulate the action of HSPCs, such as self-renewal, differentiation, and quiescence
Mesenchymal stem cells (MSCs)
Friedenstein was the first scientist who identified MSCs in bone marrow. He described an undifferentiated heterogeneous subset of cells able to differentiate into mesenchymal lineages, such like osteocytes, adipocytes, and chondrocytes.20,21 MSCs can be isolated from various organs such as bone marrow, liver, adipose tissue, dental pulp, spleen, lung, umbilical cord blood,22-24 normal peripheral blood,25 and during or following normal pregnancy, with or without fetal origin.26,27 MSCs include 0.001%–0.01% of the nucleated cells in human bone marrow.28 The MSCs are largely believed to be derived from mesoderm; notably, the earliest lineage providing MSC-like cells during embryonic body formation is actually Sox1+ neuroepithelium rather than mesoderm, after which these early MSCs are replaced with MSCs from other sources in later processes.29 MSCs have been isolated from fetal blood, liver, and BM in the first-trimester of pregnancy with morphologic, immunophenotypic, and functional characteristics resembling adult-derived MSCs.23 Co-expression of surface markers and adhesion molecules like CD105 (SH2, transforming growth factor-b receptor III), CD73 (SH3&SH4, NT5E), CD90 (thy-1), CD29, CD44, CD106, CD16630 but lack of expression of hematopoietic stem cell markers CD34, CD45, CD117 (cKit), HLA class I, HLA-DR (except for HLA-ABC) and lineage-specific markers are important indicators of MSC immunophenotyping for detection of MSCs.30-32 MSCs have the ability of adhesion to plastic surfaces when cultured ex vivo with spindle-shaped and fibroblast-like morphology.33 MSCs can protect the reconstitution of erythroid, myeloid, lymphoid, and megakaryocytic lineages, which could improve hematopoietic engraftment.34 MSCs with immunosuppressive properties are useful in the treatment of graft versus host disease (GVHD)35 and can function through different ways from cell replacement to secretion of paracrine factors and cytokines.
Hematopoiesis and Hematopoietic Stem and Progenitor Cells (HSPC)
Hematopoiesis is initiated by rare somatic multipotent BM HSPCs and is a continuous process involving a hierarchy of differentiating progenitor cells, as well as production and consumption of mature blood cells that create the hemato-lymphoid system.36 HSPCs in the BM have two unique potentials: generating themselves (self-renewal capacity) and all other blood cells (multi-lineage differentiation capacity), i.e. erythrocytes, megakaryocytes/platelets, B/T lymphocytes, monocytes/macrophages, neutrophils/granulocytes, eosinophils and basophils, such that HSPCs proliferation is associated with their proliferation. The self-renewal capacity is necessary for homeostasis because mature blood cells have a short lifetime.4 HSPCs can be retrieved from BM, umbilical cord blood (UCB), and peripheral blood (PB) by apheresis after mobilizing HSPCs from BM to PB under the effect of granulocyte-colony stimulating factor (G-CSF). HPCs are uni-, bi-, or multi-potent, which have differentiation potential into various types of blood cells with limited self-renewal capacity.37 All functional HSPCs are associated with decreased and absence of expression of cell surface markers naturally detected on differentiating or mature blood cells while displaying Sca1 and c-kit markers. HSPCs can be identified with the absence of all lineage markers (Lin−) using a complex multi-flow cytometric labeling. CD34 is one of the most important markers, which is observed on early progenitor cells but not in mature cells, and CD38 is another surface marker that has been applied in association with CD34 to differentiate between HSPC, multipotent progenitors (CD38−), and committed progenitors (CD38+). Primitive HSPCs are CD34+,CD133+, CD38-, Lin−, Thy-1+(CD90), Sca1+, and c-kit+, while the coexpression of CD34+, CD38−, and CD90−defines MPPs. The expression of CD10 on CD34+ cells defines the lymphoid-committed progenitors and the expression of IL-3αRlo(CD123), CD45RA− as well as CD34+ and CD38+ defines myeloid committed progenitors.38,39
Based on their self-renewal capacity, HSPCs are divided into two categories: LT-HSC (long term-HSC) with high self-renewal ability and ST-HSC (short term-HSC) with limited self-renewal power that are derived from LT-HSCs. ST- HSCs have the potential to differentiate to the common myeloid and lymphoid progenitors (CMP, CLP) and provide hematopoiesis for a short time.21,40 The promotion of cell differentiation is determined by increase in each of the CD13, CD38, CD45 and CD56 markers. Proliferation and differentiation of these cells are regulated by cell interactions, soluble components, intrinsic and extrinsic signals in embryonic yolk sac, placenta, liver, and finally in BM,41-43 respectively. Cell interactions are regulated by various extracellular matrix (ECM) proteins such as secreted growth factors, cytokines, adhesion molecules, MVs, transfer of genetic information, and miRNA.44-46 ECM proteins, as well as MSCs, have effects on the maintenance and differentiation into lineage-committed HSPCs.47
Potential signaling pathways associated with hematopoiesis
Signaling pathways and cellular interactions adjust the BM niche for HSPCs. MSCs produce numerous paracrine agents, and it may be difficult to investigate the mechanisms accountable for the production of distinctive factors.48 Some of these signaling pathways have been demonstrated to be associated with the expression and production of paracrine factors, involving a variety of signaling pathway receptors including Akt, signal transducer and activator of transcription (STAT), Tie2/Ang-1, p38 mitogen-activated protein kinase (MAPK), and tumor necrosis factor (TNF). The study of Gnecchi et al demonstrated that MSCs express and produce paracrine factors that play a role in homing and reduction of apoptosis, including VEGF, FGF-2, Angiopoetin-1 (Ang-1), and hepatocyte growth factor (HGF) from MSCs. These are potential mediators of the impact of Akt-MSC conditional medium and are considerably up-regulated in the Akt-MSCs in response to hypoxia, representing that Akt signaling is critical to the adjustment of the expression of these factors by MSCs.49 CCL5 (RANTES) and CXCL12 chemokines could activate STAT signaling pathways and are implicated in the survival and proliferation of HSPCs. CXCL12 selectively activates STAT-5 whereas CCL5 activates STAT-1, and these two chemokines also activate MAPK signaling pathways.50 HGFs can be divided into two types: upstream and downstream HGFs. The former induce HSPCs proliferation (most are asymmetric divisions), while the latter induce the committed progenitor cells to differentiate.51 The secretion of HGF, VEGF, and IGF-I by MCSs is crucially increased by stimulation with TNF, which is involved in the enhanced activation of p38 MAPK. Inhibition of p38 MAPK signaling significantly decreases the production of HGF, VEGF, and IGF-I. However, p38 MAPK inhibitor by itself has no influence on the production of these factors without TNF stimulation. Research shows that TNF promotes the production of paracrine factors in MSCs through a p38 MAPK-dependent mechanism.52 Also, the expression and production of CXCL-1, interleukin (IL)-6, and IL-8 is reduced through deactivation of p38 MAPK signaling in MSCs.53 p38 MAPKs are involved in the regulation of hematopoiesis, erythropoiesis, and myelopoiesis. p38MAPKs respond to different extracellular stimuli, especially cellular stress, including hypoxia, UV radiation, growth factors, and inflammatory cytokines54 and p38 activation can be induced by erythropoietin (EPO).55
Tie2/Ang-1 signaling pathway has a critical role in the maintenance of HSPCs. The tight adhesive binding of MSCs to HSPCs by Ang-1 ligand and tyrosine kinase receptor (Tie2) allows for a specific population of HSPCs to keep quiescence even in presence of mobilizing factors such as G-CSF, the stimulation of which is involved in the maintenance of LT-HSCs repopulating.56 Similarly, the thrombopoietin (TPO) receptor (c-Mpl) is expressed by a quiescent population of LT-HSCs that are found to be associated with TPO produced by MSCs, and the stimulation of this pathway increases the number of quiescent HSPCs, while its blockage leads to a reduction in LT-HSC.57
Through the production of Notch ligands via Wingless-type (Wnt) pathway, MSCs play a role in HSPCs58 survival and proliferation but inhibits their differentiation. In addition, through Jagged-1/Notch1,2 pathway, MSCs support HSPC self-renewal, which blocks differentiation into MPP and myeloid and monocytic cell lineage. Notch-1 promotes T-cell differentiation versus B-cell differentiation.59,60 Researchers have shown the expression of Notch-1 and Notch-2 by HSPCs, as well as Notch ligands Delta-1 (Dll-1) and Jagged-1 (Jag1) by hMSC.61 Notch-1 plays an important role in the T- versus B-lineage selection of common lymphocyte precursors, but Notch-1 signaling has little role in the myeloid lineage differentiation.62 Further studies demonstrated that Notch-1 signaling increases the generation of precursor cells and inhibits B-cell and myeloid differentiation, inducing T-cells so that the distinctive activation of Notch target genes results from selective activation of various Notch receptors as a result of specific ligand interactions, leading to diverse cellular outcomes.63,64 In addition, cross-talk between pathways such as the Notch and Wnt may lead to synergistic effects. Furthermore, soluble or cell-expressed Jagged-1 induced the expansion of HSPCs in vitro and mediated HSPC hematopoiesis and maintenance.64,65 Wnt/β-catenin signaling by MSC-MVs can improve the expansion of CD34+ cells66 through induced expression of the notch ligands (jagged-1, Dll-1)67,68 or p15INK4b mRNA. Wnt pathway is involved in HSPCs self-renewal, proliferation, repopulating activity or lineage specific differentiation. Wnt pathway is activated by binding two types of receptors: the Frizzled family and a subset of low-density lipoprotein receptor-related protein (LRP) family (LRP-5 or 6). Since Wnt induces HSPC self-renewal in some organs, it enables the in vitro expansion of such cells and maintains their potency to reconstitute the entire cells after transplantation.17,18 Hedgehog signaling pathway modulates the transcription of target genes that affect the quiescence, self-renewal, proliferation, and differentiation of HSPCs. Three distinct ligands, i.e. Desert (Dhh), Indian (Ihh) and Sonic (Shh) Hedgehog exist in humans.
MSCs derivatives
The MSCs represent important components of the microenvironment. They produce a large diversity of cytokines and soluble forms of adhesion molecules, e.g. vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), which regulate hematopoiesis and are effective in homing similar to CXCL12.69-71 MSCs isolated from BM are functionally similar to umbilical cord blood derived MSCs72 and cytokine profile of BM and UCB MSCs is the same.73 A higher number of cytokines are released from placenta-MSC (P-MSCs) than umbilical cord-MSC (u-MSCs).67 The roles of some cytokines include maintaining HSPCs in quiescence, homing or induced self-renewal rather than differentiation. At the beginning of 1996, it was observed that MSCs isolated from human BM expressed and released G-CSF, stem cell factor (SCF), leukemia inhibitory factor (LIF), macrophage-CSF (M-CSF), IL-6, and IL-11 within the in vitro culture medium with a role in the adjustment of the differentiation of cells isolated from BM stroma through receptors related to gp130 and associated with signal transduction pathways.69 Most types of MSCs had a common expression pattern, including GRO-α (growth related oncogene α, CXCL1), IL-8 (CXCL8), and IL-6 that advance differentiation toward the myeloid lineage, as well as macrophage migration inhibitory factor (MIF, GIF, DER6) and Serpin E1 (PAI-1). Monocyte chemotactic protein-1 (MCP-1, CCL2) was expressed in both BM and amniotic MSCs, but the expression of stromal-derived factor-1 (SDF-1 or CXCL-12) involved in the homing and mediating the migration of HSPCs was higher in BM-MSCs.74 CCL2 acts as a strong chemotactic factor for monocytes, eosinophils, basophils, and a subset of T lymphocytes.75 Also, CXCL1, CXCL8, Serpin E1, and GM-CSF play a role in mobilization similar to G-CSF.76 A unique panel of chemokines, including CCR7, CCR9, CXCR4, CXCR5, and CXCR6 are involved in homeostatic leukocyte trafficking and cell compartmentalization within BM and/or in secondary lymphoid organs.77,78
The extracellular vesicles (EVs) derived from MSC are of three main types, including exosomes, microvescicles, and apoptotic bodies. They have different sizes (40–150 nm) and production mechanisms, and their cells of origin are determined by surface markers.79-81 These particles have a vital role in intercellular communication.82 MVs are derived both through outward budding surfaces of activated cells or follow the endosomal membrane formation after fusion of secretory granules with the plasma membrane, so that later exosomes are formed within the endosome and make multi-vesicular bodies (MVB)83-85 of varying size and composition. They often contain a number of factors, which include functional transmembrane proteins, cytoplasmic protein, bioactive lipids, messenger RNAs )mRNAs(, tRNA, and microRNAs, mediating the transfer of these factors to target cells.86 Their RNA is nominated as “exosomal shuttle RNA” (esRNA). microRNAs (miRNA or miR) involve a class of small regulatory non-coding RNAs (19−23 nucleotides) that post-transcriptionally modulate gene expression, playing an important role in normal hematopoiesis by binding to their different target mRNAs.87 miRNAs have been implicated in all phases of hematopoiesis, including preservation of self-renewal and differentiation of HSPCs to mature blood cells, which might moderate cellular action by regulating transactivation, histone modification, DNA methylation, alternative splicing, and other miRNAs.88,89
The therapeutic effects of paracrine mechanisms of MSCs are extremely complex, including numerous cytokines, growth factors, as well as related receptors and signaling molecules with a wide area of biological functions.27 It is necessary to identify the factors involved in the adjustment of expression and production of these paracrine molecules in MSCs to gain an optimal therapeutic result.90 Effects of MSCs derivatives on HSPCs and hematopoiesis are summarized in Table 1.
Table 1. Effects of MSCs derivatives on HSPCs and Hematopoiesis .
| MSCs derivatives | Effects on HSPCs and Hematopoiesis | References |
| SCF TPO CXCL12 flt3l PGE2 miR221, miR451, miR654-3p |
HSPCs expansion and development | 57,133 |
| IL-3, IL-6, IL-11 G-CSF |
HSPCs differentiation | 57 |
| TPO | Early Megakaryocyte differentiation | 95 |
| IL-11 | Platelet formation | 95 |
| IL-6 G-CSF |
Myeloid differentiation | 96,97 |
| IL-6 in combination with SCF Laminin miR-223 |
HSPCs proliferation | 96,97,122,134 |
| SCF/c-kit (CD117) | Myeloid and Erythroid differentiation | 98,99 |
| CXCL8, CXCL12, CXCL16 CCL2, CCL5 CX3CL1 (fractalkine) Flt-3 ligand(FL) SCF, G-CSF, VEGF VCAM-1, E-selectin Collagen I Fibronectin, Laminin, Vimentin miR-126 |
HSPC homing and mobilization | 124-128 |
| CXCL12 (SDF-1) | Early B-cell lymphopoiesis Preservation, Quiescence |
117,118,129-131 |
| VEGF FGF-2 Angiopoetin-1 (Ang-1) |
Homing and reduction apoptosis | 132 |
| IL-10 | Increased CD5+ regulatory B cells generation | 138 |
| IL-6 and M-CSF | Inhibit DCs differentiation | 139 |
| M-CSF, HGF IL-6, IL-10 TNF PGE2 TGF-β COX-1, COX-2 IDO or NO HLA-G5 |
Immunosuppressive and immunomodulation | 140,141,146 |
| IDO | M2 macrophage differentiation | 148 |
| miR-451 | Erythroid differentiation | 89 |
| CEBPA/miR-182 EGR2/miR-150 miR-92, miR-9, miR-150 MPO/ hsa_piR_020814_ DQ598650 |
Apoptosis and differentiation | 88,107,108 |
| hsa_piR_020814_DQ598650 | Regulates MPO synthesis during myeloid differentiation | 106 |
| miR210-5p miR106b-3p miR155-5p |
Inhibited radiation-induced apoptosis of HSPC | 133 |
| miR-21-5p miR-181a-5p miR92a-3p |
Reduction of caspase dependent apoptosis | 88 |
Regulation of HSPCs by MSCs derivatives
Signaling pathways associated with the maintenance and regulation of HSPCs obviously present useful knowledge on new findings in the treatment of various diseases and the developments in large scale preparation of HSPCs for transplantation.39,53,56 Also, the signaling pathways can provide understanding of the cancer stem cells to explore their possible use in treatments. All hematopoietic and immune cells are continuously generated by HSPCs through the intensely organized procedure of hierarchical lineage commitment.4,39 The MSCs represent important components with significant effects on different stages of hematopoiesis. Some of the cytokines released by MSCs are as follows: SCF, LIF, SDF-1, bone morphogenic protein (BMP)-4, Flt-3 ligand(FL), Kit-L, TNF-α, and TGF- β1.69,83,84 Some MSC cytokines can affect the maturation of HPCs, such as granulocyte-macrophage-CSF (GM-CSF), G-CSF, and also IL-1, IL-3, IL-6, IL-7, IL-11, IL-12, IL-14, IL-15 and TPO, as well as FL to promote self-renewal, proliferation, and differentiation of HSPCs.84,91-93 SCF, TPO, and FL are the most potent cytokines for HSPCs expansion. In contrast, IL-3, IL-6, IL-11, and G-CSF have a capacity to produce differentiated cells.57 A unique mix of immobilized ligand Delta1, fibronectin fragments, and cytokines (i.e. TPO, SCF,Flt3 ligand, IL-3, IL-6) led to increase in the number of CD34+ cells after 17 days of culture.94 TPO is important for early megakaryocyte differentiation and is modulated through c-mpl receptor and IL-11, resulting in platelet formation.95 IL-6 and G-CSF are necessary for myeloid differentiation, and IL-6 in combination with SCF can induce considerable proliferation of HSPCs.96,97 SCF/c-kit (CD117) in combination with FL/flt3 supports the preservation, proliferation, and differentiation toward myeloid and erythroid lineages of HSPC, as well as a number of other factors.98-100 BMP promotes blood production during in vitro differentiation. Kaimeng Hu et al showed that u-MSCs could be induced into hematopoietic cells and this differentiation is regulated through overexpression of miR-218 and miR-451 and affects the MITF-HoxB4 pathway.101miR-451 is involved in specific differentiation of HSPC to erythroid lineage,89 as well as MV-mRNA that is involved in the hematopoietic differentiation along with Hexokinase 3 (HK3) and Eosinophil peroxidase (EPX).102 CEBPA/miR-182, EGR2/miR-150 and miR-92, MPO/ hsa_piR_020814_ DQ598650 influence the down-regulated genes and therefore play a crucial role in cell death and differentiation.88 CEBPA-alpha regulates the equivalence between expansion and differentiation within early hematopoietic and myeloid development, which is controlled by miR-182.103-105 The hsa_piR_020814_DQ598650 regulates myeloperoxidase (MPO) synthesis during myeloid differentiation.106miR-150107 and miR-9108 regulate another down-regulated gene, i.e. Early Growth Response 2 (EGR2), which is involved in apoptosis and differentiation.109,110
MSCs also have a principal role in HSPC homing by secreting SDF-1,111 FL, SCF,112 VCAM-1, E-selectin, and collagen I,113 as well as expression of extracellular matrix proteins such as fibronectin, laminin, and vimentin in hematopoietic niche.114-116 The expression of SDF-1 chemokine is influenced by miR-886-3p that targets the 3´untranslated part of SDF-1 mRNA. SDF-1 plays a crucial role in early B-cell lymphopoiesis and hematopoietic regulation.117,118 HSPCs stick to fibronectin through at least two integrin pairs: VLA-4 (a4ß1) and VLA-5 (a5ß1). Fibronectin has either inhibitory or promotion effects on proliferation by inhibiting the G1/S promotion of HSPCs, which seems to be controversial.119-121 Laminin supports HSPCs proliferation and migration122 and chemokines conduct hematopoietic cell trafficking and localization in tissue.123 Several components can induce migration of HSPCs from BM to the peripheral blood. In the clinical setting, G-CSF is the most applicable inducer of HSPC mobilization,124 and miR-126 in EVs of stem cells is required for the adjustment of HSPC mobilization by down-regulation of VCAM-1 on HSPC surface, causing a reduced mobilization response to G-CSF.125
In addition, chemokines released from MSCs such as CCL2, CCL5, CX3CL1 (fractalkine), CXCL8, CXCL12, and CXCL16 can stimulate chemotaxis.126-128 CXCL12 /CXCR4 protects the preservation, homing, quiescence, survival, and HSPCs development;129-131 also, G-CSF, VEGF, and CXCL16 are associated with HSPC homing.132 CX3CL1 protects cell growth, differentiation, and migration.126
BM-MSC-derived EV miRNAs can reduce apoptosis and differentiation of UCB-CD34+ cells.88 Overexpression of MV-miRs such as miR221, miR451, and miR654-3p induced cell development but the overexpression of miR210-5p, miR106b-3p, and miR155-5p inhibited radiation-induced apoptosis of HSPC.133 Luciana De Luca et al. demonstrated that BM-MSC-EVs can influence UCB-CD34+ gene expression model, resulting in the reduction of caspase dependent apoptosis via expression of miR-21-5p, miR-181a-5p, and miR92a-3p, inducing cell survival, inhibiting hematopoietic cell differentiation and boosting their movement to BM. Since these genes encode chemokines and cytokines (and their receptors) involved in the chemotaxis procedure of various BM cells, their potential role in the hematopoietic reconstitution is vital for engraftment.88 miR-223 has a role in HSPC proliferation.134 A study showed that miR-223 was the highly expressed in platelets, peripheral blood mononuclear cells, and their plasma MVs.135
BM-MSC-EVs miRNAs/piRNAs such as miR-21-5p, miR-181a-5p, and miR92a-3p notably reduce the apoptosis pathway and caspase 3/7 activity but miR-27b-3p and miR-10a-5p can reduce CD38 expression or gene expression pattern of up-regulated genes (for example, IL6, CSF2, CCL3) under the regulation of miRNA targeted genes (for example, ZFP36/miR-27b-3p).88
Immunosuppressive Effects of MSCs Derivatives
MSCs have the immunosuppressive potential and can affect both natural and adaptive immunity by cell–cell contact or via secretion of soluble factors; however, the final effects depend on the type and condition of immune cells.136 Friedenstein showed that the transplantation of MSC/marrow stromal cells with HSPCs promotes the recovery of hematopoiesis and replicates the features of BM.137 Peng et al. revealed that MSCs significantly increased the production of CD5+ regulatory B-cells via generation of IL-10.138 MSCs can inhibit DCs differentiation139 through the secretion of IL-6 and M-CSF and can eventually moderate immune responses via generation of growth factors and cytokines, including M-CSF, IL-6, prostaglandin E2 (PGE2), TGF-β, HGF, cyclooxygenase (COX)-1, COX-2, indoleamine 2,3-dioxygenase (IDO), nitric oxide (NO), and HLA-G5.140-142 PGE2 is capable of enhancing self-renewal and proliferation of HSPCs through interaction with Wnt pathway by elevating the β-catenin expression levels.143,144 Also, PGE2 affects macrophages so that MSCs may improve organ function and be effective in treating sepsis.145 TNF, IL-10, IL-6, and PGE2 inhibit DC maturation, T-cell function, as well as activation and proliferation of both B and NK cells.146 Moreover, HLA-G5 release by MSCs suppresses NK-cell and activity of T- and B-cells.142 IL-6 and the intercellular adhesion molecule 1 receptor inhibit T-cells, and have effects on B- cells.147 Increased IDO level is implicated in the differentiation of monocytes toward immune suppressive M2 macrophages, thus promoting the MSC immunosuppressive effect.148 In addition, MSC-EVs have the ability to suppress the maturation and activity of T- and B- cells, as well as differentiation of monocytes to M2-types, which is a result of functional development of CD4+,CD25+, highFoxP3+ regulatory T-cells (Tregs) through different ways, including CCL-1 induction and soluble HLA-G5 release.27,142,149,150 MSCs can inhibit T-cell proliferation, and activated T-cells are arrested in the G0 ⁄ G1 phase.151,152 MSCs express Toll-like receptors (TLRs) such as TLR3 and TLR4, which can inhibit the MSC immune-regulatory action by their ligands through Notch ⁄ Jagged1 signaling.153
Conclusion
BM has received special consideration because it contains MSCs as well as HSPCs. Utilization of MSCs provides for the regeneration of damaged organs with cell-cell contact, soluble factors, and autocrine or paracrine effects promoting their function and preparing considerable therapeutic advantages in different diseases14,32 through cell-free products from hMSCs that are effective on wound healing.154 In vitro expansion of HSPCs for transplantation is an intensive investigation field. The advantages of such investigations include accelerated engraftment, least stem cell harvests, reduced risk of infection, and enhanced effectiveness of genetically modified stem cells.155 The balance between self-renewal and proliferation of HSPCs will be helpful for the improvement of HSPC expansion and BM transplantation. MSCs and their derivatives have a critical role in homing, self-renewal, proliferation, and differentiation of HSPCs. Co-transplantation of MSCs and HSPCs promotes the engraftment of HSPCs and reduces the incidence of GVHD. This enhancement was higher after co-transplantation of HSPCs with GM-CSF and SCF-transfected MSCs, showing that these growth factors have effects on engraftment;156,157 therefore, these MSC cytokines and growth factors exert their advantageous effects on the target cells. Several studies have been conducted for demonstrating some of the effects of MSC on the expansion and differentiation of HSPC. Some studies on the expansion of HSPCs have been mentioned but further studies are required for the effects of MSCs on differentiation of HSPCs, especially the effects of MVs derived from MSCs, and the research for MSCs derivatives is an active subject of investigation. Novel and more sensitive devices and technology are required to discover, identify, and characterize recent MSCs derivatives that are found in low levels or have a labile nature.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
Abbreviations
MSC: Mesenchymal stem cell; HSPC: hematopoietic stem and progenitor cell; HSC: hematopoietic stem cell; MPP: multipotent progenitor; BM: bone marrow; HLA: human leukocyte antigen; GVHD: graft versus host disease; LT-HSC: long term-HSC; ST-HSC: short term-HSC; ECM: extracellular matrix; G-CSF: granulocyte-colony stimulating factor; TPO: Thrombopoietin; MAPK: mitogen-activated protein kinase; EVs: extracellular vesicles; MV: microvesicle; FL: Flt-3 ligand.
References
- 1.Valtieri M, Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008;217(2):296–300. doi: 10.1002/jcp.21521. [DOI] [PubMed] [Google Scholar]
- 2.Chou SH, Lin SZ, Day CH, Kuo WW, Shen CY, Hsieh DJ. et al. Mesenchymal stem cell insights: Prospects in hematological transplantation. Cell Transplant. 2013;22(4):711–21. doi: 10.3727/096368912x655172. [DOI] [PubMed] [Google Scholar]
- 3.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012;10(2):120–36. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Hao S, Chen C, Cheng T. Cell cycle regulation of hematopoietic stem or progenitor cells. Int J Hematol. 2016;103(5):487–97. doi: 10.1007/s12185-016-1984-4. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura-Ishizu A, Takizawa H, Suda T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 2014;141(24):4656–66. doi: 10.1242/dev.106575. [DOI] [PubMed] [Google Scholar]
- 6.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–29. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202(11):1599–611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132(4):631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 10.Wagner W, Horn P, Bork S, Ho AD. Aging of hematopoietic stem cells is regulated by the stem cell niche. Exp Gerontol. 2008;43(11):974–80. doi: 10.1016/j.exger.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50(2):280–9. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Fu X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res. 2012;348(3):371–7. doi: 10.1007/s00441-012-1393-9. [DOI] [PubMed] [Google Scholar]
- 13.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481–92. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 14.Kuroda Y, Kitada M, Wakao S, Dezawa M. Bone marrow mesenchymal cells: How do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp. 2011;59(5):369–78. doi: 10.1007/s00005-011-0139-9. [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, Danieli P, Cervio E. Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol. 2012;57(1):48–55. doi: 10.1016/j.vph.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Dexter TM. Stromal cell associated haemopoiesis. J Cell Physiol Suppl. 1982;1:87–94. doi: 10.1002/jcp.1041130414. [DOI] [PubMed] [Google Scholar]
- 17.Staal FJ, Luis TC. Wnt signaling in hematopoiesis: Crucial factors for self-renewal, proliferation, and cell fate decisions. J Cell Biochem. 2010;109(5):844–9. doi: 10.1002/jcb.22467. [DOI] [PubMed] [Google Scholar]
- 18.Zanette DL, Lorenzi JC, Panepucci RA, Santos AR, Molfetta GA, Araujo AG. et al. Microarray profiles of ex vivo expanded hematopoietic stem cells show induction of genes involved in noncanonical wnt signaling. Genet Mol Res. 2013;12(2):1691–7. doi: 10.4238/2013.May.15.1. [DOI] [PubMed] [Google Scholar]
- 19.Frassoni F, Podesta M, Maccario R, Giorgiani G, Rossi G, Zecca M. et al. Cord blood transplantation provides better reconstitution of hematopoietic reservoir compared with bone marrow transplantation. Blood. 2003;102(3):1138–41. doi: 10.1182/blood-2003-03-0720. [DOI] [PubMed] [Google Scholar]
- 20.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA. et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83–92. [PubMed] [Google Scholar]
- 21.Saleh M, Shamsasanjan K, Movassaghpourakbari A, Akbarzadehlaleh P, Molaeipour Z. The impact of mesenchymal stem cells on differentiation of hematopoietic stem cells. Adv Pharm Bull. 2015;5(3):299–304. doi: 10.15171/apb.2015.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 23.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 24.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13(10):845–53. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA. et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2(6):477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Donoghue K, Choolani M, Chan J, de la Fuente J, Kumar S, Campagnoli C. et al. Identification of fetal mesenchymal stem cells in maternal blood: Implications for non-invasive prenatal diagnosis. Mol Hum Reprod. 2003;9(8):497–502. doi: 10.1093/molehr/gag063. [DOI] [PubMed] [Google Scholar]
- 27.Lotfinegad P, Shamsasenjan K, Movassaghpour A, Majidi J, Baradaran B. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: A hope in cell therapy. Adv Pharm Bull. 2014;4(1):5–13. doi: 10.5681/apb.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pashoutan Sarvar D, Shamsasenjan K, Akbarzadehlaleh P. Mesenchymal stem cell-derived exosomes: New opportunity in cell-free therapy. Adv Pharm Bull. 2016;6(3):293–9. doi: 10.15171/apb.2016.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG. et al. Neuroepithelial cells supply an initial transient wave of msc differentiation. Cell. 2007;129(7):1377–88. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 31.Soncini M, Vertua E, Gibelli L, Zorzi F, Denegri M, Albertini A. et al. Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med. 2007;1(4):296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- 32.Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H. et al. Mesenchymal stem cells: New aspect in cell-based regenerative therapy. Adv Pharm Bull. 2013;3(2):433–7. doi: 10.5681/apb.2013.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 34.Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J. et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: Outcome of a pilot clinical study. Leukemia. 2008;22(3):593–9. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 35.Gurevitch O, Prigozhina TB, Pugatsch T, Slavin S. Transplantation of allogeneic or xenogeneic bone marrow within the donor stromal microenvironment. Transplantation. 1999;68(9):1362–8. doi: 10.1097/00007890-199911150-00024. [DOI] [PubMed] [Google Scholar]
- 36.Potocnik AJ, Kohler H, Eichmann K. Hemato-lymphoid in vivo reconstitution potential of subpopulations derived from in vitro differentiated embryonic stem cells. Proc Natl Acad Sci U S A. 1997;94(19):10295–300. doi: 10.1073/pnas.94.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112(9):3543–53. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11(7):585–93. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 39.Chotinantakul K, Leeanansaksiri W. Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res. 2012;2012:270425. doi: 10.1155/2012/270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol. 2011;12(10):643–55. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein MI, Zhu J, Emerson SG. Molecular pathways regulating the self-renewal of hematopoietic stem cells. Exp Hematol. 2004;32(12):1129–36. doi: 10.1016/j.exphem.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci U S A. 1997;94(8):4080–5. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavian M, Peault B. The changing cellular environments of hematopoiesis in human development in utero. Exp Hematol. 2005;33(9):1062–9. doi: 10.1016/j.exphem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: A disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11(3):156–64. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 45.Taback B, Hoon DS. Circulating nucleic acids and proteomics of plasma/serum: Clinical utility. Ann N Y Acad Sci. 2004;1022:1–8. doi: 10.1196/annals.1318.002. [DOI] [PubMed] [Google Scholar]
- 46.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P. et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mrna and protein delivery. Leukemia. 2006;20(5):847–56. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 47.Lazar-Karsten P, Dorn I, Meyer G, Lindner U, Driller B, Schlenke P. The influence of extracellular matrix proteins and mesenchymal stem cells on erythropoietic cell maturation. Vox Sang. 2011;101(1):65–76. doi: 10.1111/j.1423-0410.2010.01453.x. [DOI] [PubMed] [Google Scholar]
- 48.Reinisch A, Chan SM, Thomas D, Majeti R. Biology and clinical relevance of acute myeloid leukemia stem cells. Semin Hematol. 2015;52(3):150–64. doi: 10.1053/j.seminhematol.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F. et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20(6):661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 50.Janowska-Wieczorek A, Majka M, Ratajczak J, Ratajczak MZ. Autocrine/paracrine mechanisms in human hematopoiesis. Stem Cells. 2001;19(2):99–107. doi: 10.1634/stemcells.19-2-99. [DOI] [PubMed] [Google Scholar]
- 51.Renstrom J, Kroger M, Peschel C, Oostendorp RA. How the niche regulates hematopoietic stem cells. Chem-Biol Interact. 2010;184(1-2):7–15. doi: 10.1016/j.cbi.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R880–4. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 53.Yew TL, Hung YT, Li HY, Chen HW, Chen LL, Tsai KS. et al. Enhancement of wound healing by human multipotent stromal cell conditioned medium: The paracrine factors and p38 MAPK activation. Cell Transplant. 2011;20(5):693–706. doi: 10.3727/096368910x550198. [DOI] [PubMed] [Google Scholar]
- 54.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26(22):3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 55.Nagata Y, Moriguchi T, Nishida E, Todokoro K. Activation of p38 map kinase pathway by erythropoietin and interleukin-3. Blood. 1997;90(3):929–34. [PubMed] [Google Scholar]
- 56.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111(2):492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 57.Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015;43(7):498–513. doi: 10.1016/j.exphem.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Weber JM, Calvi LM. Notch signaling and the bone marrow hematopoietic stem cell niche. Bone. 2010;46(2):281–5. doi: 10.1016/j.bone.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 60.Fujita S, Toguchida J, Morita Y, Iwata H. Clonal analysis of hematopoiesis-supporting activity of human mesenchymal stem cells in association with jagged1 expression and osteogenic potential. Cell Transplant. 2008;17(10-11):1169–79. doi: 10.3727/096368908787236611. [DOI] [PubMed] [Google Scholar]
- 61.Sorrentino BP. Clinical strategies for expansion of haematopoietic stem cells. Nat Rev Immunol. 2004;4(11):878–88. doi: 10.1038/nri1487. [DOI] [PubMed] [Google Scholar]
- 62.MacDonald HR, Wilson A, Radtke F. Notch1 and t-cell development: Insights from conditional knockout mice. Trends Immunol. 2001;22(3):155–60. doi: 10.1016/s1471-4906(00)01828-7. [DOI] [PubMed] [Google Scholar]
- 63.Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99(7):2369–78. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- 64.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human cd34(+)cd38(-) cord blood cells. J Clin Invest. 2002;110(8):1165–74. doi: 10.1172/jci16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S. et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192(9):1365–72. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amirizadeh N, Oodi A, Nikougoftar M, Soltanpour MS. Expression and promoter methylation changes of the p15ink4b during ex vivo cord blood cd34+ cell expansion following co-culture with mesenchymal stromal cells. Hematology. 2013;18(5):260–8. doi: 10.1179/1607845412y.0000000062. [DOI] [PubMed] [Google Scholar]
- 67.Kadekar D, Kale V, Limaye L. Differential ability of mscs isolated from placenta and cord as feeders for supporting ex vivo expansion of umbilical cord blood derived cd34(+) cells. Stem Cell Res Ther. 2015;6:201. doi: 10.1186/s13287-015-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie H, Sun L, Zhang L, Liu T, Chen L, Zhao A. et al. Mesenchymal stem cell-derived microvesicles support ex vivo expansion of cord blood-derived cd34(+) cells. Stem Cells Int. 2016;2016:6493241. doi: 10.1155/2016/6493241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166(3):585–92. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 70.Roberts I. Mesenchymal stem cells. Vox Sang. 2004;87 Suppl 2:38–41. doi: 10.1111/j.1741-6892.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 71.Van Overstraeten-Schlogel N, Beguin Y, Gothot A. Role of stromal-derived factor-1 in the hematopoietic-supporting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76(6):488–93. doi: 10.1111/j.1600-0609.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 72.Panepucci RA, Siufi JL, Silva WA Jr, Proto-Siquiera R, Neder L, Orellana M. et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22(7):1263–78. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 73.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: Functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171(7):3426–34. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 74.Hwang JH, Shim SS, Seok OS, Lee HY, Woo SK, Kim BH. et al. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. 2009;24(4):547–54. doi: 10.3346/jkms.2009.24.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 76.Ratajczak MZ, Kim C. The use of chemokine receptor agonists in stem cell mobilization. Expert Opin Biol Ther. 2012;12(3):287–97. doi: 10.1517/14712598.2012.657174. [DOI] [PubMed] [Google Scholar]
- 77.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 78.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling b lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am J Physiol Cell Physiol. 2014;306(7):C621–33. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 80.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1(1):98–110. [PMC free article] [PubMed] [Google Scholar]
- 81.Andaloussi SEL, Mager I, Breakefield XO, Wood MJA. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–57. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 82.Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176(1):57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 84.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9(6):841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 85.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–9. [PubMed] [Google Scholar]
- 86.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 87.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mrnas are conserved targets of micrornas. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Luca L, Trino S, Laurenzana I, Simeon V, Calice G, Raimondo S. et al. Mirnas and pirnas from bone marrow mesenchymal stem cell extracellular vesicles induce cell survival and inhibit cell differentiation of cord blood hematopoietic stem cells: A new insight in transplantation. Oncotarget. 2016;7(6):6676–92. doi: 10.18632/oncotarget.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Masaki S, Ohtsuka R, Abe Y, Umemura T. Expression analysis of micrornas in erythropoiesis. Rinsho Byori. 2008;56(12):1086–92. [PubMed] [Google Scholar]
- 90. Xiao Y, Li X, Hao H, Cui Y, Chen M, Liu L, et al. Secretome of mesenchymal stem cells. In: Wang S, Zhao RC, editors. Essentials of mesenchymal stem cell biology and its clinical translation. Springer; 2013. P. 33-46.
- 91.Meuleman N, Tondreau T, Ahmad I, Kwan J, Crokaert F, Delforge A. et al. Infusion of mesenchymal stromal cells can aid hematopoietic recovery following allogeneic hematopoietic stem cell myeloablative transplant: A pilot study. Stem Cells Dev. 2009;18(9):1247–52. doi: 10.1089/scd.2009.0029. [DOI] [PubMed] [Google Scholar]
- 92.Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20(3):161–71. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Mishima S, Nagai A, Abdullah S, Matsuda C, Taketani T, Kumakura S. et al. Effective ex vivo expansion of hematopoietic stem cells using osteoblast-differentiated mesenchymal stem cells is CXCL12 dependent. Eur J Haematol. 2010;84(6):538–46. doi: 10.1111/j.1600-0609.2010.01419.x. [DOI] [PubMed] [Google Scholar]
- 94.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Solar GP, Kerr WG, Zeigler FC, Hess D, Donahue C, de Sauvage FJ. et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92(1):4–10. [PubMed] [Google Scholar]
- 96.Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: Molecular mechanisms of action during steady state and 'emergency' hematopoiesis. Cytokine. 2008;42(3):277–88. doi: 10.1016/j.cyto.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heike T, Nakahata T. Ex vivo expansion of hematopoietic stem cells by cytokines. Biochim Biophys Acta. 2002;1592(3):313–21. doi: 10.1016/s0167-4889(02)00324-5. [DOI] [PubMed] [Google Scholar]
- 98.Kimura Y, Ding B, Imai N, Nolan DJ, Butler JM, Rafii S. C-kit-mediated functional positioning of stem cells to their niches is essential for maintenance and regeneration of adult hematopoiesis. PLoS One. 2011;6(10):e26918. doi: 10.1371/journal.pone.0026918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jang YK, Jung DH, Jung MH, Kim DH, Yoo KH, Sung KW. et al. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006;85(4):212–25. doi: 10.1007/s00277-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 100.de Kruijf EJ, Hagoort H, Velders GA, Fibbe WE, van Pel M. Hematopoietic stem and progenitor cells are differentially mobilized depending on the duration of flt3-ligand administration. Haematologica. 2010;95(7):1061–7. doi: 10.3324/haematol.2009.016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu K, Xu C, Ni H, Xu Z, Wang Y, Xu S. et al. Mir-218 contributes to the transformation of 5-Aza/GF induced umbilical cord mesenchymal stem cells into hematopoietic cells through the MITF pathway. Mol Biol Rep. 2014;41(7):4803–16. doi: 10.1007/s11033-014-3351-y. [DOI] [PubMed] [Google Scholar]
- 102.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F. et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–67. doi: 10.1681/asn.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kandilci A, Grosveld GC. Reintroduction of CEBPA in MN1-overexpressing hematopoietic cells prevents their hyperproliferation and restores myeloid differentiation. Blood. 2009;114(8):1596–606. doi: 10.1182/blood-2009-02-205443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fasan A, Haferlach C, Alpermann T, Jeromin S, Grossmann V, Eder C. et al. The role of different genetic subtypes of CEBPA mutated AML. Leukemia. 2014;28(4):794–803. doi: 10.1038/leu.2013.273. [DOI] [PubMed] [Google Scholar]
- 105.Wang X, Huang G, Mei S, Qian J, Ji J, Zhang J. Over-expression of C/EBP-alpha induces apoptosis in cultured rat hepatic stellate cells depending on p53 and peroxisome proliferator-activated receptor-gamma. Biochem Biophys Res Commun. 2009;380(2):286–91. doi: 10.1016/j.bbrc.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 106.Tobler A, Miller CW, Johnson KR, Selsted ME, Rovera G, Koeffler HP. Regulation of gene expression of myeloperoxidase during myeloid differentiation. J Cell Physiol. 1988;136(2):215–25. doi: 10.1002/jcp.1041360203. [DOI] [PubMed] [Google Scholar]
- 107.Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K. et al. MiR-150 promotes gastric cancer proliferation by negatively regulating the pro-apoptotic gene EGR2. Biochem Biophys Res Commun. 2010;392(3):340–5. doi: 10.1016/j.bbrc.2009.12.182. [DOI] [PubMed] [Google Scholar]
- 108.Pospisil V, Vargova K, Kokavec J, Rybarova J, Savvulidi F, Jonasova A. et al. Epigenetic silencing of the oncogenic miR-17-92 cluster during PU1-directed macrophage differentiation. EMBO J. 2011;30(21):4450–64. doi: 10.1038/emboj.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Unoki M, Nakamura Y. EGR2 induces apoptosis in various cancer cell lines by direct transactivation of BNIP3L and BAK. Oncogene. 2003;22(14):2172–85. doi: 10.1038/sj.onc.1206222. [DOI] [PubMed] [Google Scholar]
- 110.Gabet Y, Baniwal SK, Leclerc N, Shi Y, Kohn-Gabet AE, Cogan J. et al. Krox20/EGR2 deficiency accelerates cell growth and differentiation in the monocytic lineage and decreases bone mass. Blood. 2010;116(19):3964–71. doi: 10.1182/blood-2010-01-263830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J. et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106(11):1331–9. doi: 10.1172/jci10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Horwitz EM, Maziarz RT, Kebriaei P. MSCs in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(1 Suppl):S21–9. doi: 10.1016/j.bbmt.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 113.Verfaillie CM. Adhesion receptors as regulators of the hematopoietic process. Blood. 1998;92(8):2609–12. [PubMed] [Google Scholar]
- 114.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T. et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 115.Akbari AA, Mozdarani H, Akhlaghpoor S, Pourfatollah AA, Soleimani M. Evaluation of the homing of human CD34+ cells in mouse bone marrow using clinical mr imaging. Pak J Biol Sci. 2007;10(6):833–42. doi: 10.3923/pjbs.2007.833.842. [DOI] [PubMed] [Google Scholar]
- 116.Zhang C, Zhang X, Chen XH. Granulocyte-colony stimulating factor-mobilized mesenchymal stem cells: A new resource for rapid engraftment in hematopoietic stem cell transplantation. Med Hypotheses. 2011;76(2):241–3. doi: 10.1016/j.mehy.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 117.Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N. et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15(2):323–34. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 118.Pillai MM, Yang X, Balakrishnan I, Bemis L, Torok-Storb B. MiR-886-3p down regulates CXCL12 (SDF1) expression in human marrow stromal cells. PLoS One. 2010;5(12):e14304. doi: 10.1371/journal.pone.0014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hurley RW, McCarthy JB, Verfaillie CM. Direct adhesion to bone marrow stroma via fibronectin receptors inhibits hematopoietic progenitor proliferation. J Clin Invest. 1995;96(1):511–9. doi: 10.1172/jci118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li T, Wu Y. Paracrine molecules of mesenchymal stem cells for hematopoietic stem cell niche. Bone Marrow Res. 2011;2011:353878. doi: 10.1155/2011/353878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prosper F, Verfaillie CM. Regulation of hematopoiesis through adhesion receptors. J Leukoc Biol. 2001;69(3):307–16. [PubMed] [Google Scholar]
- 122.Gu YC, Kortesmaa J, Tryggvason K, Persson J, Ekblom P, Jacobsen SE. et al. Laminin isoform-specific promotion of adhesion and migration of human bone marrow progenitor cells. Blood. 2003;101(3):877–85. doi: 10.1182/blood-2002-03-0796. [DOI] [PubMed] [Google Scholar]
- 123.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 124.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25(2):211–7. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 125.Salvucci O, Jiang K, Gasperini P, Maric D, Zhu J, Sakakibara S. et al. MicroRNA126 contributes to granulocyte colony-stimulating factor-induced hematopoietic progenitor cell mobilization by reducing the expression of vascular cell adhesion molecule 1. Haematologica. 2012;97(6):818–26. doi: 10.3324/haematol.2011.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030–41. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 127.Broxmeyer HE, Kim CH, Cooper SH, Hangoc G, Hromas R, Pelus LM. Effects of CC, CXC, C, and CX3C chemokines on proliferation of myeloid progenitor cells, and insights into SDF-1-induced chemotaxis of progenitors. Ann N Y Acad Sci. 1999;872:142–62; discussion 63. doi: 10.1111/j.1749-6632.1999.tb08460.x. [DOI] [PubMed] [Google Scholar]
- 128.Han ZC, Lu M, Li J, Defard M, Boval B, Schlegel N. et al. Platelet factor 4 and other CXC chemokines support the survival of normal hematopoietic cells and reduce the chemosensitivity of cells to cytotoxic agents. Blood. 1997;89(7):2328–35. [PubMed] [Google Scholar]
- 129.Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: The role of reticular cells. Trends Immunol. 2011;32(7):315–20. doi: 10.1016/j.it.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 130.Luan X, Li G, Wang G, Wang F, Lin Y. Human placenta-derived mesenchymal stem cells suppress T cell proliferation and support the culture expansion of cord blood CD34(+) cells: A comparison with human bone marrow-derived mesenchymal stem cells. Tissue Cell. 2013;45(1):32–8. doi: 10.1016/j.tice.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 131.Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R. et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25(10):2638–47. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- 132.Thevenot PT, Nair AM, Shen J, Lotfi P, Ko CY, Tang L. The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31(14):3997–4008. doi: 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wen S, Dooner M, Cheng Y, Papa E, Del Tatto M, Pereira M. et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30(11):2221–31. doi: 10.1038/leu.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O. et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451(7182):1125–9. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 135.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L. et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3(11):e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305(1):33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 137.Friedenstein AJ, Piatetzky S, II II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–90. [PubMed] [Google Scholar]
- 138.Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L. et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29(3):636–46. doi: 10.1038/leu.2014.225. [DOI] [PubMed] [Google Scholar]
- 139.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood. 2009;113(26):6576–83. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 140.Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164(1):1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35(2):213–21. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 142.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L. et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 143.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–11. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL. et al. Genetic interaction of PGE2 and wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–47. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K. et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012;10(6):709–16. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 147.Rasmusson I, Le Blanc K, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human b cells. Scand J Immunol. 2007;65(4):336–43. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 148.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–95. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 149.Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M. et al. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2015;24(12):2615–27. doi: 10.3727/096368915x687543. [DOI] [PubMed] [Google Scholar]
- 150.Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22(2):369–79. doi: 10.3727/096368911x582769. [DOI] [PubMed] [Google Scholar]
- 151.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P. et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 152.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 153.Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F. et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26(1):279–89. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 154.Mishra PJ, Mishra PJ, Banerjee D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells. 2012;4(5):35–43. doi: 10.4252/wjsc.v4.i5.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brugger W, Mocklin W, Heimfeld S, Berenson RJ, Mertelsmann R, Kanz L. Ex vivo expansion of enriched peripheral blood CD34+ progenitor cells by stem cell factor, interleukin-1 beta (IL-1 beta), IL-6, IL-3, interferon-gamma, and erythropoietin. Blood. 1993;81(10):2579–84. [PubMed] [Google Scholar]
- 156.Han JY, Goh RY, Seo SY, Hwang TH, Kwon HC, Kim SH. et al. Cotransplantation of cord blood hematopoietic stem cells and culture-expanded and GM-CSF-/SCF-transfected mesenchymal stem cells in SCID mice. J Korean Med Sci. 2007;22(2):242–7. doi: 10.3346/jkms.2007.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim DH, Yoo KH, Yim YS, Choi J, Lee SH, Jung HL. et al. Cotransplanted bone marrow derived mesenchymal stem cells (MSC) enhanced engraftment of hematopoietic stem cells in a MSC-dose dependent manner in NOD/SCID mice. J Korean Med Sci. 2006;21(6):1000–4. doi: 10.3346/jkms.2006.21.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]


