ABSTRACT
The susceptibility of colistin-resistant clinical isolates of Klebsiella pneumoniae to ceragenins and antimicrobial peptides (AMPs) suggests that there is little to no cross-resistance between colistin and ceragenins/AMPs and that lipid A modifications are found in bacteria with modest changes in susceptibility to ceragenins and with high levels of resistance to colistin. These results suggest that there are differences in the resistance mechanisms to colistin and ceragenins/AMPs.
KEYWORDS: colistin, resistant, antimicrobial peptides, ceragenin, Gram-negative bacteria
TEXT
The continuous emergence of drug-resistant bacteria has led to dire predictions of a possible “postantibiotic” era in which common infections will not be treatable with the current arsenal of antibiotics (1, 2). Of particular concern are Gram-negative bacteria, because these organisms are inherently resistant to many antibiotics due to the permeability barrier provided by their outer membranes and the efflux pumps located therein (3, 4). To treat infections from Gram-negative bacteria, clinicians are increasingly using colistin, a member of the polymyxin family of antibiotics (5). Colistin is considered the antibiotic of last resort because, while it has side effects, including nephrotoxicity and ototoxicity, it is broadly active against Gram-negative bacteria (6, 7). Isolation of colistin-resistant bacteria in many countries underscores the need for development of novel strategies for targeting Gram-negative bacteria, including drug-resistant strains (8).
Endogenous antimicrobial peptides (AMPs) have played an important role in innate immunity for eons (9), and there have been efforts to use AMPs clinically. Challenges for clinical use of peptide therapeutics include the relatively high costs of large-scale production and the susceptibility of AMPs to degradation by proteases (10). We have developed a class of small molecules, termed ceragenins (Fig. 1), that circumvent these challenges while maintaining the same general mechanism of bactericidal activity of AMPs. Ceragenins can be prepared on a large scale, and because they are not peptide based, they are not substrates for proteases. As mimics of AMPs, ceragenins display broad-spectrum antibacterial activity, including potent antibiofilm activity (11–13). In in vivo studies involving medical devices and bone regrowth, ceragenins are effective in eliminating bacterial challenges, and local administration is well tolerated (14–16).
FIG 1.
Structure of ceragenins CSA-13, CSA-44, CSA-131, CSA-138, and CSA-142.
Consideration of the common structural features of colistin, AMPs, and ceragenins (multiple cationic groups, substantial hydrophobic character, and interaction with bacterial membranes) leads to three questions. (i) Are colistin-resistant bacteria also resistant to AMPs and ceragenins? (ii) Does generation of resistance to colistin occur at the same rate as potential generation of resistance to AMPs and ceragenins? (iii) Since the primary mechanism for bacterial resistance to AMPs and ceragenins is through modification of the lipid A portion of lipopolysaccharide (LPS) (17–19), how important are these modifications in the resistance of Gram-negative bacteria to colistin, AMPs, and ceragenins?
To address these questions, we compared the susceptibility of colistin-resistant clinical isolates of Klebsiella pneumoniae to colistin, representative AMPs (LL-37, cecropin A, and magainin 1), and the representative ceragenins shown in Fig. 1. Our initial focus on K. pneumoniae was due to its known pathogenicity and its ability to transfer resistance genes to other Gram-negative bacteria (20, 21). We and our collaborators previously found ceragenins to be active against other colistin-resistant bacteria (19, 22), and we have extended these observations to clinical isolates of K. pneumoniae using additional, later-generation ceragenins. MICs and minimum bactericidal concentrations (MBCs) were determined using a broth microdilution method (CLSI protocol, with Mueller-Hinton [MH] substituted for cation-adjusted MH) (23). Colistin-resistant clinical isolates of K. pneumoniae gave MICs of 16 to 200 μg/ml with colistin, while a susceptible strain (ATCC 13883) gave an MIC of 2 μg/ml (Table 1). MICs of LL-37 and magainin 1 were relatively high against the reference strain as well as the clinical isolates; MICs were lower and less varied with cecropin A. With the ceragenins, MICs with the susceptible strain were relatively low (1 to 3 μg/ml), and only small changes in MICs were observed with colistin-resistant isolates. MBCs with CSA-44 and CSA-131 were 2 to 10 μg/ml, demonstrating bactericidal rather than bacteriostatic activity, and colistin resistance did not significantly impact the MBCs of the ceragenins.
TABLE 1.
MICs of colistin, AMPs, and CSAs against a standard strain of K. pneumoniae (ATCC 13883) and colistin-resistant clinical isolates
| K. pneumoniae strain | MICs (MBCs) (μg/ml) for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Colistin | LL-37 | Cecropin A | Magainin 1 | CSA-13 | CSA-44 | CSA-131 | CSA-138 | CSA-142 | |
| ATCC 13883 | 2.0 | 32 | 2.0 | 64 | 2.0 | 1.0 (2.0) | 1.0 (2.0) | 3.0 | 3.0 |
| ARLG-1127 | 32 | 64 | 2.0 | 64 | 2.0 | 1.0 (2.0) | 1.0 (2.0) | 2.0 | 2.0 |
| ARLG-1340 | 100 | 100 | NMa | NM | 2.0 | 1.0 (2.0) | 1.0 (6.0) | 3.0 | 4.0 |
| ARLG-1349 | 16 | 64 | 4.0 | 64 | 2.0 | 1.0 (2.0) | 3.0 (4.0) | 3.0 | 8.0 |
| ARLG-1360 | 64 | 100 | 4.0 | 150 | 2.0 | 1.0 (2.0) | 2.0 (6.0) | 6.0 | 6.0 |
| ARLG-1389 | 200 | 100 | 4.0 | 200 | 6.0 | 2.0 (2.0) | 3.0 (10) | 8.0 | 8.0 |
| ARLG-1406 | 64 | 64 | 4.0 | 100 | 3.0 | 1.0 (3.0) | 3.0 (8.0) | 6.0 | 16 |
NM, not measured.
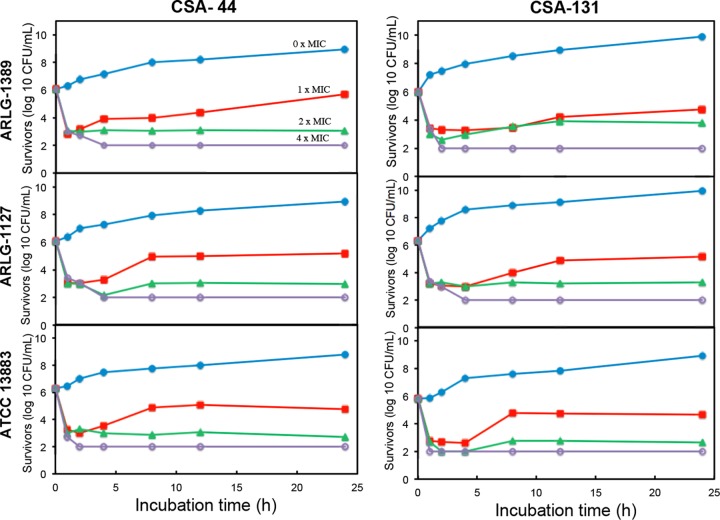
To quantify rates of bactericidal activity, time-kill assays were performed with CSA-44 and CSA-131 against the colistin-resistant strains ARLG-1127 and ARLG-1389 and compared to a susceptible strain of K. pneumoniae (Fig. 2). For these assays, the protocol for MIC measurement was used, and aliquots (10 μl) were removed at varied intervals, plated on nutrient agar, and incubated (24). At 2× MIC for both ceragenins, the inoculum was decreased by at least 3 logs within 2 h. At 4× MIC, the inoculum was decreased to the detection limit (2 logs) within the same time frame. These assays revealed that there are only minor differences in the kinetics of bactericidal activity among the colistin-resistant and colistin-susceptible strains, again suggesting that colistin resistance does not significantly influence susceptibility to ceragenins.
FIG 2.
Rates of bactericidal activity of ceragenins are similar among colistin-resistant and colistin-susceptible strains. Time-kill curves with CSA-44 and CSA-131 against colistin-resistant K. pneumoniae strains (ARLG-1127 and ARLG-1389) and colistin-susceptible strain ATCC 13883. Detection limit is 2 logs (CFU/ml).
We compared the relative rates at which K. pneumoniae (ATCC 13883) and other Gram-negative bacteria (Pseudomonas aeruginosa [ATCC 27853] and Acinetobacter baumannii [ATCC 19606]) become resistant to colistin and ceragenin CSA-131 by serially exposing these organisms to both antimicrobials and monitoring susceptibility. MICs (at 24 h) for the strains were determined, and bacterial populations growing at the highest concentrations of the antimicrobials were used to inoculate fresh medium. This process was repeated every 18 to 24 h. Concentrations of the antimicrobials were adjusted to allow determination of MICs (19). This process was repeated for 10 periods (of 24 h each) with colistin, with MICs rising from 1 to 2 μg/ml to ≥350 μg/ml, and for 30 days with CSA-131. Resulting MICs are shown in Table 2, along with the susceptibility of the resulting bacteria to colistin, CSA-131, and representative AMPs. Serial exposure to colistin, resulting in the generation of highly resistant organisms, caused little or no change in MICs with CSA-131. Some changes in MICs were observed with the AMPs against colistin-resistant organisms (1.3- to 8-fold increases). Serial exposure to CSA-131 resulted in increases of MICs from 1 to 2 μg/ml to 2 to 8 μg/ml and resulted in increased MICs with AMPs (2.3- to 12-fold increases).
TABLE 2.
MICs of colistin, CSA-131, LL-37, magainin 1, and cecropin A with susceptible standard strains of K. pneumoniae, A. baumannii, and P. aeruginosa and with strains serially exposed to colistin or CSA-131
| Strain | MICs (μg/ml) for: |
||||
|---|---|---|---|---|---|
| Colistin | CSA-131 | LL-37 | Magainin 1 | Cecropin A | |
| K. pneumoniae ATCC 13883 | 2.0 | 1.0 | 32 | 64 | 2.0 |
| Serially exposed to colistina | 350 | 1.5 | 64 | 82 | 16 |
| Serially exposed to CSA-131b | 32 | 8.0 | 100 | 150 | 24 |
| A. baumannii ATCC 19606 | 1.0 | 2.0 | 16 | 32 | 4.0 |
| Serially exposed to colistina | 400 | 2.0 | 64 | 100 | 16 |
| Serially exposed to CSA-131b | 32 | 2.0 | 128 | 150 | 32 |
| P. aeruginosa ATCC 27853 | 1.0 | 2.0 | 32 | 64 | 4.0 |
| Serially exposed to colistina | 350 | 2.0 | 64 | 100 | 8.0 |
| Serially exposed to CSA-131b | 32 | 4.0 | 100 | 150 | 16 |
Ten days of exposure.
Thirty days of exposure.
In a previous study, we found that serial exposure of Gram-negative bacteria to ceragenin CSA-13 resulted in increased MICs and modifications to the lipid A portion of lipopolysaccharide (19). Lipid A is a primary target of colistin (25), ceragenins (11), and AMPs (26), and lipid A modifications were observed as mechanisms for the generation of resistance to these antimicrobials (26–30). To determine if colistin resistance and serial exposure to CSA-131 result in comparable lipid A modifications (phosphate ester formation with 4-aminoarabinose and/or ethanolamine), lipid A was isolated from three colistin-resistant clinical isolates of K. pneumoniae, and bacteria were serially exposed to colistin or CSA-131. Lipid A was isolated using the TRI reagent method (31) and analyzed via mass spectrometry (electrospray ionization, negative-ion mode, Agilent 6230 series time-of-flight spectrometer). Lipid A from the parent strain (K. pneumoniae [ATCC 13883]) showed the expected masses lacking 4-aminoarabinose and ethanolamine, while lipid A from each of the clinical isolates and from bacteria serially exposed to colistin or CSA-131 showed these modifications (Table 3 [mass spectra are shown in the supplemental material]), as expected from activation of two-component systems (e.g., PhoP/PhoQ and PmrA/PmrB) (26–30). Additions of fatty acids were also observed. Comparable masses were observed for parent and modified lipid A (27, 32, 33). Susceptibility of these strains to colistin or CSA-131 varies dramatically, and yet there are modifications to lipid A common to these organisms. These modifications to lipid A may impact the activities of colistin and CSA-131 differently; alternatively, there may be other mechanisms of resistance with these organisms (e.g., loss of lipopolysaccharide) (34) that provide high levels of colistin resistance without influencing susceptibility to CSA-131.
TABLE 3.
Masses of isolated lipid A from colistin-susceptible (ATCC 13883) and colistin-resistant strains of K. pneumoniae and bacteria serially exposed to colistin and CSA-131
| K. pneumoniae strain | Observed lipid A mass (m/z) | Mass of parent lipid A structures (m/z) | Additions to parent lipid A structures |
|---|---|---|---|
| ATCC 13883c | 1,795 | ||
| 1,840 | |||
| 1,853 | |||
| 1,910 | |||
| ARLG-1389 | 2,023 | 1,840 | Lauric acid |
| 2,209 | 1,840 | Aminoarabinose and palmitic acid | |
| ARLG-1349 | 2,021 | 1,840 | Lauric acid |
| 2,209 | 1,840 | Aminoarabinose and palmitic acid | |
| ARLG-1360 | 2,034 | 1,910 | Ethanolamine |
| ATCC 13883 serially exposed to colistina | 1,795 | 1,795 | |
| 1,987 | 1,840 | Aminoarabinose and hydroxyl group | |
| ATCC 13883 serially exposed to CSA-131b | 1,840 | 1,840 | |
| 2,021 | 1,840 | Lauric acid | |
| 2,152 | 1,840 | Aminoarabinose and lauric acid |
Ten days of exposure.
Thirty days of exposure.
ATCC 13883 is the fully susceptible strain, so no modifications to lipid A are identified (this is the source of the parent lipid A structures).
Considering the common structural features of colistin, AMPs, and ceragenins, the issue of cross-resistance arises. Among colistin-resistant isolates and strains generated by serial exposure to colistin, MICs increase up to several hundredfold compared to susceptible strains, while up to 5-fold increases in MICs were observed with the AMPs and ceragenins tested. However, in some cases, MICs of ceragenins were the same with both colistin-susceptible and -resistant bacteria. While specific mechanisms for resistance of Gram-negative bacteria to colistin have been identified, multiple mechanisms likely influence resistance (35). At least one of these mechanisms causes very high resistance to colistin but does not appear to impact, to the same extent, susceptibility to AMPs and ceragenins. These observations are consistent with the reported susceptibility of colistin-resistant bacteria to a variety of AMPs (36, 37). The fact that lead ceragenins (CSA-44 and CSA-131) retain bactericidal activity against highly colistin-resistant bacteria provides further support for development of these compounds as broad-spectrum antibacterial agents in multiple potential clinical applications.
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00292-17.
REFERENCES
- 1.Rojas LJ, Salim M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Marshall S, Rudin SD. 2017. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis 64:711–718. doi: 10.1093/cid/ciw805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGann P, Snesrud E, Maybank R, Corey B, Ong AC, Clifford R, Hinkle M, Whitman T, Lesho E, Schaecher KE. 2016. Escherichia coli harboring mcr-1 and blactx-m on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob Agents Chemother 60:4420–4421. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aminlari L, Hashemi MM, Aminlari M. 2014. Modified lysozymes as novel broad spectrum natural antimicrobial agents in foods. J Food Sci 79:R1077–1090. doi: 10.1111/1750-3841.12460. [DOI] [PubMed] [Google Scholar]
- 4.Savage PB. 2001. Multidrug-resistant bacteria: overcoming antibiotic permeability barriers of Gram-negative bacteria. Ann Med 33:167–171. doi: 10.3109/07853890109002073. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng YH, Lin TL, Pan YJ, Wang YP, Lin YT, Wang JT. 2015. Colistin resistance mechanisms in Klebsiella pneumoniae strains from Taiwan. Antimicrob Agents Chemother 59:2909–2913. doi: 10.1128/AAC.04763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim LM, Ly N, Anderson D, Yang JC, Macander L, Jarkowski A, Forrest A, Bulitta JB, Tsuji BT. 2010. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30:1279–1291. doi: 10.1592/phco.30.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castanheira M, Griffin MA, Deshpande LM, Mendes RE, Jones RN, Flamm RK. 2016. Detection of mcr-1 among Escherichia coli clinical isolates collected worldwide as part of the SENTRY antimicrobial surveillance program during 2014-2015. Antimicrob Agents Chemother 60:5623–5624. doi: 10.1128/AAC.01267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirioni O, Giacometti A, Ghiselli R, Bergnach C, Orlando F, Silvestri C, Mocchegiani F, Licci A, Skerlavaj B, Rocchi M. 2006. LL-37 protects rats against lethal sepsis caused by Gram-negative bacteria. Antimicrob Agents Chemother 50:1672–1679. doi: 10.1128/AAC.50.5.1672-1679.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenssen H, Hamill P, Hancock REW. 2006. Peptide antimicrobial agents. Clin Microb Rev 19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai X-Z, Feng Y, Pollard J, Chin JN, Rybak MJ, Bucki R, Epand RF, Epand RM, Savage PB. 2008. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc Chem Res 41:1233–1240. doi: 10.1021/ar700270t. [DOI] [PubMed] [Google Scholar]
- 12.Chin JN, Jones RN, Sader HS, Savage PB, Rybak MJ. 2008. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J Antimicrob Chemother 61:365–370. [DOI] [PubMed] [Google Scholar]
- 13.Niemirowicz K, Surel U, Wilczewska AZ, Mystkowska J, Piktel E, Gu X, Namiot Z, Kułakowska A, Savage PB, Bucki R. 2015. Bactericidal activity and biocompatibility of ceragenin-coated magnetic nanoparticles. J Nanobiotechnology 13:1–11. doi: 10.1186/s12951-014-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindeler A, Nicole Y, Cheng TL, Sullivan K, Mikulec K, Peacock L, Matthews R, Little DG. 2015. Local delivery of the cationic steroid antibiotic CSA-90 enables osseous union in a rat open fracture model of Staphylococcus aureus infection. J Bone Joint Surg Am 97:302–309. doi: 10.2106/JBJS.N.00840. [DOI] [PubMed] [Google Scholar]
- 15.Williams DL, Haymond BS, Beck JP, Savage PB, Chaudhary V, Epperson RT, Kawaguchi B, Bloebaum RD. 2012. In vivo efficacy of a silicone–cationic steroid antimicrobial coating to prevent implant-related infection. Biomaterials 33:8641–8656. doi: 10.1016/j.biomaterials.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinclair KD, Pham TX, Williams DL, Farnsworth RW, Loc-Carrillo CM, Bloebaum RD. 2013. Model development for determining the efficacy of a combination coating for the prevention of perioperative device related infections: a pilot study. J Biomed Mater Res B Appl Biomater 101:1143–1153. doi: 10.1002/jbm.b.32924. [DOI] [PubMed] [Google Scholar]
- 17.Raetz CR, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in Gram-negative bacteria. Annu Rev Biochem 76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunn JS. 2001. Bacterial modification of LPS and resistance to antimicrobial peptides. J Endotoxin Res 7:57–62. [PubMed] [Google Scholar]
- 19.Pollard JE, Snarr J, Chaudhary V, Jennings JD, Shaw H, Christiansen B, Wright J, Jia W, Bishop RE, Savage PB. 2012. In vitro evaluation of the potential for resistance development to ceragenin CSA-13. J Antimicrob Chemother 67:2665–2672. doi: 10.1093/jac/dks276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter SN, Frasson I, Bergo C, Parisi S, Cavallaro A, Palù G. 2011. Transfer of KPC-2 carbapenemase from Klebsiella pneumoniae to Escherichia coli in a patient: first case in Europe. J Clin Microbiol 49:2040–2042. doi: 10.1128/JCM.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goren MG, Carmeli Y, Schwaber MJ, Chmelnitsky I, Schechner V, Navon-Venezia S. 2010. Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg Infect Dis 16:1014–1017. doi: 10.3201/eid1606.091671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vila-Farres X, Callarisa AE, Gu X, Savage PB, Giralt E, Vila J. 2015. CSA-131, a ceragenin active against colistin-resistant Acinetobacter baumannii and Pseudomonas aeruginosa clinical isolates. Int J Antimicrob Agents 46:568–571. doi: 10.1016/j.ijantimicag.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, 7th ed CLSI document M7-A7 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Olajuyigbe OO, Afolayan AJ. 2012. In vitro antibacterial and time-kill evaluation of the Erythrina caffra Thunb. extract against bacteria associated with diarrhoea. ScientificWorldJournal 2012:738314. doi: 10.1100/2012/738314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hood MI, Becker KW, Roux CM, Dunman PM, Skaar EP. 2013. Genetic determinants of intrinsic colistin tolerance in Acinetobacter baumannii. Infect Immun 81:542–551. doi: 10.1128/IAI.00704-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz SM, Ernst RK, Miller SI. 2004. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol 186:575–579. doi: 10.1128/JB.186.2.575-579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llobet E, Martinez-Moliner V, Moranta D, Dahlstrom KM, Regueiro V, Tomas A, Cano V, Perez-Gutierrez C, Frank CG, Fernandez-Carrasco H, Insua JL, Salminen TA, Garmendia J, Bengoechea JA. 2015. Deciphering tissue-induced Klebsiella pneumoniae lipid A structure. Proc Natl Acad Sci U S A 112:E6369–E6378. doi: 10.1073/pnas.1508820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao R, Hu Y, Li Z, Sun J, Wang Q, Lin J, Ye H, Liu F, Srinivas S, Li D. 2016. Dissemination and mechanism for the mcr-1 colistin resistance. PLoS Pathog 12:e1005957. doi: 10.1371/journal.ppat.1005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeaman MR, Yount NY. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 30.Matamouros S, Miller SI. 2015. S. typhimurium strategies to resist killing by cationic antimicrobial peptides. Biochim Biophys Acta 1848:3021–3025. doi: 10.1016/j.bbamem.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi EC, Hackett M. 2000. Rapid isolation method for lipopolysaccharide and lipid A from Gram-negative bacteria. Analyst 125:651–656. doi: 10.1039/b000368i. [DOI] [PubMed] [Google Scholar]
- 32.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clements A, Tull D, Jenney AW, Farn JL, Kim S-H, Bishop RE, McPhee JB, Hancock RE, Hartland EL, Pearse MJ. 2007. Secondary acylation of Klebsiella pneumoniae lipopolysaccharide contributes to sensitivity to antibacterial peptides. J Biol Chem 282:15569–15577. doi: 10.1074/jbc.M701454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffatt JH, Harper M, Harrison P, Hale JDF, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Girardello R, Visconde M, Cayô R, de Figueiredo RCBQ, da Silva Mori MA, Lincopan N, Gales AC. 2017. Diversity of polymyxin resistance mechanisms among Acinetobacter baumannii clinical isolates. Diagn Microbiol Infect Dis 87:37–44. doi: 10.1016/j.diagmicrobio.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Kao C, Lin X, Yi G, Zhang Y, Rowe-Magnus DA, Bush K. 2016. Cathelicidin antimicrobial peptides with reduced activation of Toll-like receptor signaling have potent bactericidal activity against colistin-resistant bacteria. mBio 7:e01418-16. doi: 10.1128/mBio.01418-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conlon JM, Sonnevend A, Pál T, Vila-Farrés X. 2012. Efficacy of six frog skin-derived antimicrobial peptides against colistin-resistant strains of the Acinetobacter baumannii group. Int J Antimicrob Agents 39:317–320. doi: 10.1016/j.ijantimicag.2011.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.